Symmetric cytotoxic trimeric and dimeric indole alkaloids isolated from Bousigonia angustifolia†
Abstract
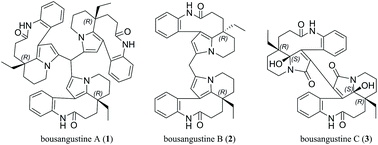
A symmetric monoterpenoid indole alkaloid trimer, bousangustine A, is reported for the first time, as well as the dimeric alkaloids bousangustines B and C; these were isolated from the trunks of Bousigonia angustifolia. The structures, which feature a symmetric 6/9/5/6 ring system, were elucidated using comprehensive spectroscopic analysis. Their absolute configurations were determined via X-ray crystal diffraction, as well as computational chemistry. They can be constructed through Friedel–Crafts and free radical reactions, respectively. These compounds exhibited significant cytotoxicity against tumor cells.
- This article is part of the themed collection: FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...