Sulfate assisted synthesis of α-type nickel hydroxide nanowires with 3D reticulation for energy storage in hybrid supercapacitors†
Wei
Li
 *ab,
Zhongzheng
Huang
ab,
Yaduo
Jia
ab,
Yunlong
Cui
ab,
Peng
Shi
c,
Tingting
Li
ab,
Hongwei
Yue
ab,
Jinxiao
Wang
ab,
Weiwei
He
*ab,
Zhongzheng
Huang
ab,
Yaduo
Jia
ab,
Yunlong
Cui
ab,
Peng
Shi
c,
Tingting
Li
ab,
Hongwei
Yue
ab,
Jinxiao
Wang
ab,
Weiwei
He
 ab and
Xiaojie
Lou
*c
ab and
Xiaojie
Lou
*c
aKey Laboratory of Micro-Nano Materials for Energy Storage and Conversion of Henan Province, Institute of Surface Micro and Nano Materials, College of Chemical and Materials Engineering, Xuchang University, Henan 461000, P. R. China. E-mail: liwei034@126.com
bHenan Joint International Research Laboratory of Nanomaterials for Energy, P. R. China
cFrontier Institute of Science and Technology, and State Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an, 710049, P. R. China. E-mail: xlou03@mail.xjtu.edu.cn
First published on 16th November 2021
Abstract
Nickel hydroxide, as a high high-efficiency electroactive material, is a promising electroactive material for advanced hybrid supercapacitors due to its high theoretical capacity, low-cost, and good thermal and chemical stabilities. Although various nanostructures of nickel hydroxide have been widely reported for energy storage devices, such as nanoparticles, nanosheets, nanoflowers, and so on, nickel hydroxide with one-dimensional nanowire morphology has been rarely investigated until now. Meanwhile, previous reports have shown that the energy storage performance of α-Ni(OH)2 is superior to other types of nickel hydroxides. Based on the above points of view, α type Ni(OH)2 nanowires with three-dimensional (3D) reticulation containing SO42− (abbreviated as NSOH NWs) were successfully obtained by a sulfate assisted hydrothermal approach in the present work and further employed as an electroactive material in hybrid supercapacitors (HSCs). The formation mechanism of the NSOH and the effects of SO42− are also discussed. Thanks to its novel 3D porous morphology and α-type phase structure, the as-prepared NSOH NW electrode presents a high specific capacity of 246.3 mA h g−1 at 1 A g−1, along with a good cycling stability of 91.7% after 4000 cycles. Meanwhile, the as-assembled NSOH NWs//AC HSCs can provide a high energy density of 59.8 W h kg−1 at a power density of 830.3 W kg−1. The HSCs also show 91.5% specific capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. All above results manifest that the proposed NSOH NWs with 3D reticulation have significant potential for advanced HSC applications.
000 cycles. All above results manifest that the proposed NSOH NWs with 3D reticulation have significant potential for advanced HSC applications.
1. Introduction
Energy storage and conversion devices with high power density and energy density, long service life and environmental friendliness are the inevitably required for modern social and economic development. As an efficient chemical energy storage device, supercapacitors have been attracting increasing attention on account of their intrinsic advantages, such as relatively fast charging–discharging capability and good cycling stability.1,2Until now, although carbon-based supercapacitors have been commercially available, their relatively low energy density (usually lower than 10 W h g−1) still severely limits their large scale application in the mainstream energy storage fields.3,4 The emergence of hybrid supercapacitors (HSCs) provides a new route to further enhance the energy density of supercapacitors. By combining a high-energy battery electrode material with a high-power electric double layer capacitance (EDLC) electrode material, the energy density and voltage of the hybrid supercapacitor can be greatly improved to further satisfy the demands of industrial application.5,6 It is well known that the electrode materials are the key factors that affect the electrochemical energy storage properties of the supercapacitor. Currently, the research studies on electrode materials for HSCs mainly concentrate on three types: transition metal oxides, conductive polymers, and porous carbon materials. Among them, given the variable valence states of their cation, regulable micro–nano morphology and suitable crystal structure, transition metal oxides have been widely studied in recent years. In particular, nickel hydroxide based electroactive materials (such as Ni(OH)2, NiO, Ni–S NiSe, and Ni based MOF etc.) are excellent potential candidates for HSCs owing to their easy availability, high theoretical capacity, low-cost, and good thermal and chemical stabilities.7–10 However, large volume expansion/contraction and the poor ion diffusion of bulk nickel hydroxide materials in the fast charging/discharging process lead to electrical contact failure between electroactive materials and the current collector, further causing its poor cycling stability and low specific capacity.
Over the past few years, many studies have shown that the electrochemical energy storage performance of nickel hydroxides is closely associated with their micro–nanomorphology and crystal structure.11–15 That is, the suitable porous micro–nanomorphology of the electroactive materials is conducive to enhance ion diffusion ability and crystal structure stability during the electrochemical redox process. In particular, compared with bulk materials, one-dimensional (1D) nanostructures have smaller longitudinal volume changes and provide sufficient space in transverse volume changes during the charging–discharging process due to the uniquely porous micro–nanomorphology, which is not only conducive to the release of stress generated and the improvement of the cycling stability of the electrode electroactive materials, but also beneficial to provide sufficient contact between the electroactive site and the electrolyte, further facilitating fast ion diffusion in the mass transfer process of electrochemical redox reaction.16–18 Meanwhile, the crystal structure of nickel hydroxide also has a momentous influence on its electrochemical energy storage performance. Generally, α-Ni(OH)2 possesses superior energy storage performance to β-Ni(OH)2. It can provide a higher theoretical capacity than the β-Ni(OH)2/β-NiOOH redox couple as a result of its exceeding +3 average oxidation state in γ-NiOOH and the existence of Ni4+ defects. In addition, the α-Ni(OH)2/γ-NiOOH redox couple can undergo reversible electrochemical redox at lower potentials than β-Ni(OH)2.1921
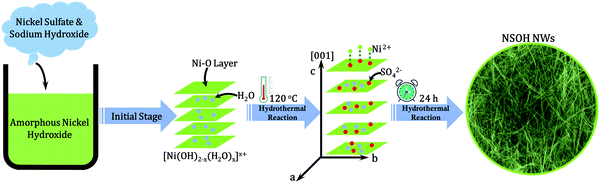
Herein, through introducing a sulfate group between the layers of nickel hydroxide, novel α-type NSOH NWs with 3D reticulation were efficiently synthesized via a facile one-step hydrothermal reaction, as shown in Fig. 1. The effects of different reaction temperatures on the morphology of the nanowires were also investigated. Benefiting from the 3D porous reticulation morphology and α-type crystal structure, the as-prepared NSOH NWs present a high specific capacity of 246.3 mA h g−1 at a current density of 1 A g−1 and an excellent cycling stability of maintaining 91.7% of the initial capacity after 4000 cycles in a three-electrode cell. Furthermore, NSOH NWs//AC HSCs were successfully assembled, which demonstrates a high energy density of 59.8 W h kg−1 at 830.3 W kg−1. The results show that the as-prepared NSOH NW electrode shows potential for applications in HSCs and also provide an effective approach to build 1D nickel based electroactive materials for energy storage.
2. Experimental section
All the reagents and chemicals in the experiments were not further depurated. Nickel Foam was purchased from Shenzhen Lvchuang Electronic Technology Co. Ltd, and nickel sulfate hexahydrate, nickel chloride and sodium hydroxide were purchased from Sinopharm Chemical Reagent Co., Ltd. Polyvinylidene fluoride (PVDF) was purchased from Solvay (Shanghai) Co., Ltd. The water used in all the experiments was depurated using a Millipore water purification equipment.2.1. Synthesis of nickel hydroxide NWs
In a typical experiment, nickel sulfate hexahydrate (5 mmol) and sodium hydroxide (5 mmol) were completely dissolved in 40 ml of pure water to form two transparent solutions by magnetic stirring, respectively. After this, the two solutions were vigorous mixed and allowed to sufficiently react with each other for 20 min to obtain a green precursor suspension. Then, the precursor suspension was transferred to a 100 ml polytetrafluoroethylene reaction kettle lined, sealed and heated at 120 °C for 24 h. After the reaction was completed, the products were washed several times with pure water and ethanol, respectively; then the products were freeze-dried under vacuum conditions for 24 h. For comparison, samples were also prepared at different reaction temperatures of 100 °C to 200 °C using the same method. In addition, pure Ni(OH)2 is also obtained at 120 °C by the same method without the assistance of sulfate.2.2 Materials characterization
X-ray diffraction (XRD) patterns were obtained using an X-ray diffractometer (Bruker, D8-Adrance, Cu-Kα) at the voltage and current of 40 kV and 40 mA, respectively. Scanning electron microscopy (SEM) and electron dispersive X-ray spectroscopy (EDS) were acquired using a Nova nanoSEM 450 (Thermo Fisher FEI) instrument equipped with an EDS system (OXFORD X-MAX50). The detailed morphology and crystal structure were observed using transmission electron microscopy (TEM, JEOL JEM-2100F) operated at 200 kV. The chemical bond and functional groups of the products were detected using Fourier transform infrared spectroscopy (FTIR, Nicolet 6700, Thermo Scientific). The analysis of the specific surface area and pore size distribution was performed using an isotherm nitrogen-adsorption instrument (Bel Japan, BELSORP-MINIII).2.3 Electrochemical measurements
Electrochemical energy storage performance of the single electrode and HSCs was studied in an electrochemical workstation (Princeton Applied Research, PARSTAT 3000A-DX), including cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS). The cycling stability was measured using a battery testing system (NEWARE CT-4008). In the three-electrode cell, the Pt foil, NSOH NW electrode, Hg/HgO electrode were, respectively, used as the counter electrode, working electrode, and reference electrode. All electrochemical tests were performed using 6 M KOH aqueous solution.3. Results and discussion
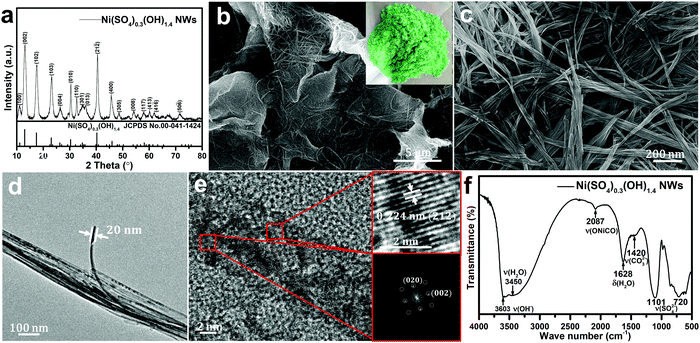
Fig. 2a shows the typical XRD pattern of the as-prepared nickel hydroxide at a reaction temperature of 120 °C. The XRD peak positions do not agree with the two typical Ni(OH)2 phases, α or β-Ni(OH)2. On the contrary, the XRD pattern of the as-prepared nickel hydroxide is in a good agreement with the standard Ni(SO4)0.3(OH)1.4 with a monoclinic structure (JCPDS No. 00-041-1424, abbreviated as NSOH), belonging to paraotwayite-type α-Ni(OH)2. It is worth noting that the intensity of the (h0l) diffraction peaks is higher than that of the (010) and (21![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) crystal plane, suggesting that the as-prepared NSOH product may possess an anisotropic nanostructure. As the a and c axes is larger than the b axis in a unit cell, an enhanced crystal structure growth kinetics along [010] crystal orientation can be expected. The detailed nanostructure of the NSOH products was characterized by SEM. Fig. 2b and c show the typical SEM images and optical photograph of the NSOH product by a simple hydrothermal reaction at 120 °C for 24 h. The 3D reticular architecture with wrinkled lamellar morphologies assembled from interwoven NSOH microwires is shown in Fig. 2b and c, and the average diameter of the interwoven microwires is about 1–2 μm. Each microwire is composed of several nanowires (NWs) connected with each other horizontally, as shown in Fig. 2c. In addition to this, no other secondary nanostructure was found, such as nanoparticles or nanosheets. A detailed micro–nanomorphology of the NSOH NWs was acquired from TEM bright field images (Fig. 2d), which further indicates that the NSOH microwires are assembled from several interconnecting NSOH NWs with a diameter of about 20 nm. The 1D nanostructure also indicates the selective growth behavior of the NSOH NWs. The HRTEM images and their corresponding Fourier patterns suggest a high degree of crystallization of the NSOH NWs (Fig. 2e); the single set of Fourier patterns also clearly manifests single crystallinity of the NSOH NWs. The growth direction of the NSOH NWs is found to be along [010], in accord with the XRD analysis (Fig. 2a). In addition, NSOH products were also obtained at different reaction temperatures using the same method. The SEM images indicate that all the samples have the same 3D reticular architecture under the wide reaction temperature range from 100 °C to 200 °C, as shown in Fig. S1 (ESI†). The result also demonstrate the temperature stability of the hydrothermal method in this work. As a comparison, pure Ni(OH)2 is also obtained at 120 °C by the same method without the assistance of sulfate, that replaced nickel sulfate with nickel chloride. The corresponding SEM images are shown in Fig. S2 (ESI†). Meanwhile, the chemical bond and functional groups of the as-prepared NSOH NWs are characterized by FITR (Fig. 2f). The absorption bands at 3450 and 1628 cm−1 respectively correspond to the O–H stretching and bending vibration modes of the absorbed H2O from the NSOH NW surface. The absorption peak at 3603 cm−1 is related to the bending vibration of O–H bond from the interlayer region of the NSOH NWs.22 The existence of CO32− absorption peak was observed at 1420 cm−1, which may be caused by the CO2 in air dissolved in the solution of hydrothermal reaction. The O–Ni–CO vibration is also detected at 2083 cm−1, in connection with CO32−. The intercalated SO42− anions in NSOH NWs generally generate characteristic vibrations in the range of 650–1200 cm−1. The two absorption peaks located at 1101 and 720 cm−1 in Fig. 2f can, respectively, belong to HSO42− and SO42− vibrations.23 This also suggests that the interlayer H2O in NSOH NWs is probably also bonded with SO42− anions. The FTIR result indicates that SO42− directly exists in the as-prepared NSOH crystal structure. The intrinsic vibrational feature analysis is also consistent with the results of XRD. Fig. 3 shows the EDS spectrum of the NSOH NWs, in which two elements, Ni and S, are detected, and the atomic ratio of Ni and S is about 18.21
) crystal plane, suggesting that the as-prepared NSOH product may possess an anisotropic nanostructure. As the a and c axes is larger than the b axis in a unit cell, an enhanced crystal structure growth kinetics along [010] crystal orientation can be expected. The detailed nanostructure of the NSOH products was characterized by SEM. Fig. 2b and c show the typical SEM images and optical photograph of the NSOH product by a simple hydrothermal reaction at 120 °C for 24 h. The 3D reticular architecture with wrinkled lamellar morphologies assembled from interwoven NSOH microwires is shown in Fig. 2b and c, and the average diameter of the interwoven microwires is about 1–2 μm. Each microwire is composed of several nanowires (NWs) connected with each other horizontally, as shown in Fig. 2c. In addition to this, no other secondary nanostructure was found, such as nanoparticles or nanosheets. A detailed micro–nanomorphology of the NSOH NWs was acquired from TEM bright field images (Fig. 2d), which further indicates that the NSOH microwires are assembled from several interconnecting NSOH NWs with a diameter of about 20 nm. The 1D nanostructure also indicates the selective growth behavior of the NSOH NWs. The HRTEM images and their corresponding Fourier patterns suggest a high degree of crystallization of the NSOH NWs (Fig. 2e); the single set of Fourier patterns also clearly manifests single crystallinity of the NSOH NWs. The growth direction of the NSOH NWs is found to be along [010], in accord with the XRD analysis (Fig. 2a). In addition, NSOH products were also obtained at different reaction temperatures using the same method. The SEM images indicate that all the samples have the same 3D reticular architecture under the wide reaction temperature range from 100 °C to 200 °C, as shown in Fig. S1 (ESI†). The result also demonstrate the temperature stability of the hydrothermal method in this work. As a comparison, pure Ni(OH)2 is also obtained at 120 °C by the same method without the assistance of sulfate, that replaced nickel sulfate with nickel chloride. The corresponding SEM images are shown in Fig. S2 (ESI†). Meanwhile, the chemical bond and functional groups of the as-prepared NSOH NWs are characterized by FITR (Fig. 2f). The absorption bands at 3450 and 1628 cm−1 respectively correspond to the O–H stretching and bending vibration modes of the absorbed H2O from the NSOH NW surface. The absorption peak at 3603 cm−1 is related to the bending vibration of O–H bond from the interlayer region of the NSOH NWs.22 The existence of CO32− absorption peak was observed at 1420 cm−1, which may be caused by the CO2 in air dissolved in the solution of hydrothermal reaction. The O–Ni–CO vibration is also detected at 2083 cm−1, in connection with CO32−. The intercalated SO42− anions in NSOH NWs generally generate characteristic vibrations in the range of 650–1200 cm−1. The two absorption peaks located at 1101 and 720 cm−1 in Fig. 2f can, respectively, belong to HSO42− and SO42− vibrations.23 This also suggests that the interlayer H2O in NSOH NWs is probably also bonded with SO42− anions. The FTIR result indicates that SO42− directly exists in the as-prepared NSOH crystal structure. The intrinsic vibrational feature analysis is also consistent with the results of XRD. Fig. 3 shows the EDS spectrum of the NSOH NWs, in which two elements, Ni and S, are detected, and the atomic ratio of Ni and S is about 18.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.13, which basically matches with the NSOH chemical composition. The EDS result further proves that the presence of SO42− anions in the interlayer region of the as-prepared NSOH NWs. Besides, the signals of the Al and C elements could be attributable to the conductive tapes and Al holder (Fig. 3e).
5.13, which basically matches with the NSOH chemical composition. The EDS result further proves that the presence of SO42− anions in the interlayer region of the as-prepared NSOH NWs. Besides, the signals of the Al and C elements could be attributable to the conductive tapes and Al holder (Fig. 3e).
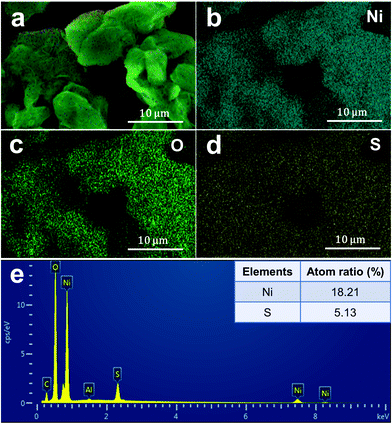 | ||
| Fig. 3 EDX elemental analysis of the NSOH NWs. (a) Composite map; (b) Ni distribution; (c) O distribution; (d) S distribution, and (e) the atom ratio of Ni and S. | ||
The formation of NSOH NWs is closely related to the presence of SO42− anions from NiSO4; SO42− anions can accelerate the crystal growth in one direction in the dissolution–crystallization process. Therefore, the reasons for the formation of nanowire structures may be as follows, and the related schematic representation of hydrothermal reactions of the NSOH NWs is also shown in Fig. 1. Generally speaking, Ni(OH)2 belongs to a layered hexagonal structure, the OH− layers grow along the a axis and stack along the c axis. At the initial reaction stage, amorphous nickel hydroxide prepared before the hydrothermal reaction begins to form a layered structure with primary crystal properties. However, the low OH− anion concentration (the ratio of the Ni2+ and OH− is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the reaction solution facilitates the formation of the layer structured Ni(OH)2−x with OH− defects, in which the layer spacing is close to that of β-Ni(OH)2. In addition, due to hydrogen bonds between H2O and OH−, water molecules are easily inserted into the interlayer space of Ni(OH)2−x, resulting in an increase of layer spacing in Ni(OH)2−x, denoted as [Ni(OH)2−x(H2O)x]x+. Ascribed to the insufficient OH− anions, SO42− anions can be substituted and inserted into the Ni(OH)2−x interlayer region to maintain the charge-balance and stability of the crystal structure of the formed nickel hydroxide under electrostatic interactions, resulting in further widening the layer spacing in Ni(OH)2−x. In addition, SO42− in the solution could combine with Ni2+ ions on the surface and form new Ni(OH)2−x layers. This process is continuously repeated, and the resulting Ni(OH)2−x crystals continue to grow along the c axis of the hexagonal structure, eventually forming the nanowire structure.
1) in the reaction solution facilitates the formation of the layer structured Ni(OH)2−x with OH− defects, in which the layer spacing is close to that of β-Ni(OH)2. In addition, due to hydrogen bonds between H2O and OH−, water molecules are easily inserted into the interlayer space of Ni(OH)2−x, resulting in an increase of layer spacing in Ni(OH)2−x, denoted as [Ni(OH)2−x(H2O)x]x+. Ascribed to the insufficient OH− anions, SO42− anions can be substituted and inserted into the Ni(OH)2−x interlayer region to maintain the charge-balance and stability of the crystal structure of the formed nickel hydroxide under electrostatic interactions, resulting in further widening the layer spacing in Ni(OH)2−x. In addition, SO42− in the solution could combine with Ni2+ ions on the surface and form new Ni(OH)2−x layers. This process is continuously repeated, and the resulting Ni(OH)2−x crystals continue to grow along the c axis of the hexagonal structure, eventually forming the nanowire structure.
As mentioned previously, the suitable porous micro–nanomorphology of the electroactive materials has important influence on energy storage performance. Therefore, the nitrogen adsorption/desorption isotherms were obtained (Fig. 4a) to calculated the specific surface area and pore size distribution of the NSOH NWs, respectively, through the BET method and the BJH model. As shown in Fig. 4a the adsorption–desorption profile of the as-prepared NSOH NWs has no obvious inflexion point, undoubtedly belonging to type III isotherm with H3 hysteresis loops. The NSOH NWs possess a high specific surface area of 64.48 m2 g−1 by the BET method. The pore size distribution (Fig. 4b) is absolutely within the mesopore range using the BJH model, which is the main channel of electrolyte transport and beneficial to the full immersion of the NSOH NWs in the electrolyte and accelerates ion diffusion rate in the electrode/electrolyte interface. Hence the combination of the α-type crystal phase and mesoporosity in NSOH NWs with a 3D reticulation can have a significant contribution for high electrochemical performances.
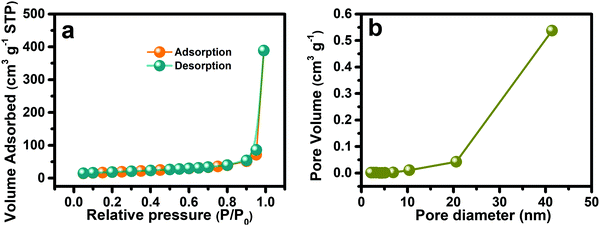 | ||
| Fig. 4 (a) Nitrogen adsorption–desorption isotherm curve; and (b) the pore size distribution of the as-prepared NSOH NWs. | ||
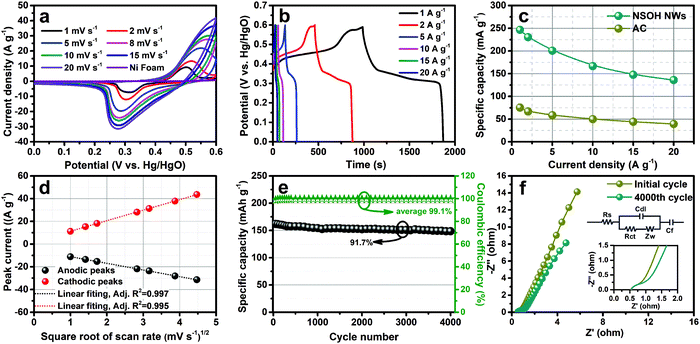
A three-electrode cell was firstly employed to measure the electrochemical energy storage performance of the as-prepared NSOH NW electrode materials, which was synthesized at a reaction temperature of 120 °C for 24 h, as shown in Fig. 5. All the CV curves in Fig. 5a under the scan rates of 1–20 mV s−1 exhibit a pair of typical redox current peaks located at around 0.3 and 0.5 V range from the potential window of 0–0.6 V; their shapes are obviously distinguishable from the rectangular shape of the porous carbon-based EDLC, relating to the reversible redox reaction of the Ni2+/Ni3+ couples, further demonstrating the good battery-type properties of the NSOH NW electrode. Furthermore, the redox current peaks of the NSOH NW electrode also respectively result in a shift to higher and lower potentials with increase in the scan rate. This is because of the ion diffusion-controlled electrochemical reaction processes, which are more obvious at a higher scan rate. Besides, the redox current peak shapes have no obvious aberration with the scan rate increase, indicating the good rate capability and excellent reversibility of the as-prepared NSOH NW electrode. The CV curve of Ni foam (current collector) is also tested at 10 mV s−1 (Fig. 5a). Compared with NSOH NWs, the contribution of nickel foam to capacity is completely negligible. In order to accurately understand the specific capacity of the NSOH NW electrode material. The GCD curves were obtained under various current densities in the range of 1–20 A g−1, as shown in Fig. 5b. The distinct potential plateaus observed in the GCD curves are in agreement with the redox current peaks of the CV curves, further testifying a battery-type feature in NSOH NWs. Furthermore, the symmetric shapes of all the GCD curves indicate the good redox reversibility during the electrochemical GCD process. The specific capacity was calculated using formula S1 (ESI†) and plotted in Fig. 5c. Promisingly, the specific capacity of the as-prepared NSOH NW electrode has a high value of 246.3 mA h g−1 at a current density of 1 A g−1. Even if the current density is up to 20 A g−1, the specific capacity still remains at 135.8 mA h g−1. The decrease in specific capacity with increase in the discharge current can be attributed to the increase of polarization and the insufficient utilization of electroactive materials at higher current density. It is worth noting that the capacity of the NSOH NW electrode is higher than those of nickel hydroxide-based materials reported previously (Table 1). As a comparison, the capacity of activated carbon (AC) is also given in Fig. 5c. In addition to this, the CV and GCD curves of the NSOH NWs and Ni(OH)2 obtained without the assistance of sulfate are shown in Fig. S3 (ESI†). It is obvious that NSOH has better energy storage performance than pure Ni(OH)2 obtained by the same method without sulfate.
| Materials | Current density | Specific capacity | Method | Ref. |
|---|---|---|---|---|
| CoMoO4@Ni(OH)2 | 1 A g−1 | 173.1 mA h g−1 (1236 F g−1, 0.5 V) | Hydrothermal | 24 |
| Ni(OH)2/NCDs | 1 A g−1 | 190.1 mA h g−1 (1711.2 F g−1, 0.4 V) | Hydrothermal | 25 |
| Ni(OH)2/Ni(PO3)2 | 1 A g−1 | 164.1 mA h g−1 (1477 F g−1, 0.4 V) | Electrodeposition | 26 |
| CoP@Ni(OH)2·0.75H2O | 1 A g−1 | 168.6 mA h g−1 | Electrodeposition | 27 |
| FeCo2S4@Ni(OH)2 | 1 A g−1 | 239.7 mA h g−1 | Electrodeposition | 28 |
| Cu7S4@Ni(OH)2 | 1 A g−1 | 134.1 mA h g−1 (482.6 C g−1) | Hydrothermal | 29 |
| CoS2@Ni(OH)2 | 1 A g−1 | 206.4 mA h g−1 (743 C g−1 at) | Electrodeposition | 30 |
| NSOH NWs | 1 A g−1 | 246.3 mA h g−1 | Hydrothermal | This work |
Diffusion kinetic characterization and fitting result are also shown in Fig. 5d. The almost linear relationship between the redox current peaks and the square root of the scan rate further demonstrates the ion diffusion-controlled electrochemical process, also showing the battery-type faradaic reactions on Ni2+/Ni3+ in NSOH NW electrode materials, completely different from the EDLC of the porous carbon-based materials. Cycling stability of the NSOH NW electrode was assessed through continuous GCD test at a high current density of 10 A g−1. The initial specific capacity (166.7 mA h g−1) gradually decreases and holds at 152.8 mA h g−1 from the first cycle to the 4000th cycle (Fig. 5e), which is 91.7% of the specific capacity retention at the 4000th cycle. The GCD curves before and after cycles in Fig. S4 (ESI†) are also plotted for comparison. The morphology of the NSOH NWs after cycling is detected by SEM (Fig. S5) (ESI†). Although the surface of the nanowires becomes rough, they still retain the 3D reticulation nanostructure, which further indicates the good structural stability of the NSOH NWs after the GCD process. The average coulombic efficiency of 99.2% also confirms that the NSOH electrode has high electrochemical reversibility during the cycling process.
Furthermore, the EIS of the NiSOH NW electrode before and after 4000 cycles was also acquired under an open circuit potential in the frequency range of 1 to 104 Hz (Fig. 5f). The Nyquist plots before and after the cycles consist of an inconspicuous semicircle and an inclined linear segment in the high and low frequency regions, respectively. Thereinto, the intercept of the Nyquist plots on the real axis in the high-frequency region is described as series Ohm resistance (Rs), and the semicircle diameter is charge transfer resistance (Rct), and the sloped line is specified as Warburg resistance (Zw), characterizing the ion diffusion-controlled processes. The equivalent circuit (the inset of Fig. 4f) is employed to fit the impedance spectra. According to the fitting results, the Rs of the NSOH NW electrode before and after cycling was nearly unchanged and estimated to be around 0.55 Ω, demonstrating the small internal resistance after cycles. In addition to this, the difference is that Rct increased from 0.73 Ω of the initial state electrode to 1.32 Ω after 4000 cycles, which may be caused by the electrical contact failure between NSOH NWs and the collector during the continuous cycling test. The increase of Rct after 4000 cycles indicate the more difficult faradaic redox reactions. Furthermore, Zw has a lower slope after cycle, demonstrating the ion diffusion resistance of the electrode increases in the cycling process. In addition, Cdl and Cf in equivalent circuit, respectively, represent the EDLC and the limited capacitance. Therefore, the increase in Rct and Zw are the important reasons that lead to the specific capacity loss in the GCD cycling process.
The superior energy storage performance of the NSOH NWs mentioned above may be attributed to the following: firstly, the porous 3D reticular architecture of the as-prepared NSOH NWs could provide lots of reactive sites on the surface, which can help the electroactive material to effective contact the electrolyte, further shorten path of the ion diffusion, and promote more Ni ions to participate in the electrochemical reaction during the GCD process. Secondly, a larger interlayer spacing (>0.75 nm) and a more disorderly feature, due to the insertion of the SO42− anions, also promote the ion diffusion within the bulk phase of the α-type NSOH NWs.21 Meanwhile, the α-Ni(OH)2/γ-NiOOH redox couple can undergo reversible electrochemical redox at lower potentials than β-Ni(OH)2.20 Moreover, because of its exceeding +3 average oxidation state in γ-NiOOH and existence of the Ni4+ defects, it provides a higher theoretical capacity than the β-Ni(OH)2/β-NiOOH redox couple.31 Therefore, the α-type NSOH NWs possess superior electrochemical energy storage properties.
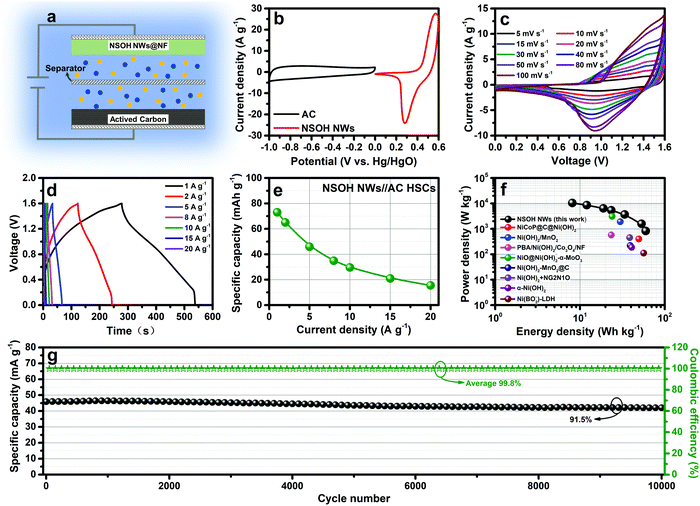
To evaluate the energy storage potential of the NSOH NW electrode in a HSC device, NSOH NWs//AC HSCs were assembled, which respectively employed the NSOH NWs and AC as electrodes, as illustrated in Fig. 6a. Beforehand, the balance of the stored charge between the NSOH NW positive electrode (+) and the AC negative electrode (−) is optimized to improve energy storage properties during HSC assembly. The energy storage performance of a single AC electrode in a three-electrode cell is presented in Fig. S6 (ESI†). A specific capacity of 75 mA h g−1 is achieved for the AC electrode at a current density of 1 A g−1 in a three-electrode cell (Fig. S6c, ESI†). Thus, the optimized mass ratio of the NSOH NWs to the AC is calculated to be approximately 0.3 using formula S3 (ESI†). In addition, the working voltage of the NSOH NWs//AC HSCs is determined to be 1.6 V (Fig. 6b), according to the potential windows of the NSOH NW electrode (0–0.6 V) and AC (−1 to 0 V) measured at 10 mV s−1. The energy storage performance of the NSOH NWs//AC HSCs was evaluated (Fig. 6c–g). The CV curves of the NSOH NWs//AC HSCs were firstly acquired under 5–100 mV s−1 (Fig. 6c). Due to the synthetic contribution of the EDLC of the AC and battery-type redox capacity of the as-prepared NSOH NWs, all the curves exhibit similar geometrical shapes. There is no obvious aberration in the redox current peak shapes with the scan rate ranging from 5 to 100 mV s−1, demonstrating the good electrochemical adaptability between the NSOH NW electrode and the AC electrode in the rapid redox process. The GCD curves of the NSOH NWs//AC HSCs under 1 to 20 A g−1 are plotted in Fig. 6d. The nonlinear feature of the GCD curves could be attributed to the faradaic redox reaction taking place in the NSOH NW electrode of the HSCs. The nearly symmetric GCD curves indicate a small voltage drop at different current densities, also demonstrating high coulombic efficiency and good redox reversibility in NSOH NWs//AC HSCs. The calculated specific capacities of the HSCs based on the total mass of the NSOH NWs and AC electroactive materials are also plotted in Fig. 6e, according to the discharge curves. A high specific capacity of 73 mA h g−1 is obtained at 1 A g−1; the capacity still retains 15.5 mA h g−1 at 20 A g−1. The energy density and power density at 1 A g−1 were calculated to be 59.8 W h kg−1 and 830.3 W kg−1, respectively, using formula S4 and S5 (ESI†) (Fig. 6f), which are superior to those of the previously reported nickel hydroxide-based HSCs, such as NiCoP@C@Ni(OH)2//AC (49.5 W h kg−1 at 399.8 W kg−1),32 Ni(OH)2/MnO2//AC HSCs (29.9 W h kg−1 at 1900 W kg−1),33 PBA/Ni(OH)2/Co3O4/NF//AC HSCs (23.4 W h kg−1 at 570 W kg−1),34 Ni(OH)2–MnO2@C/NF//AC HSCs (39.1 W h kg−1 at 221.4 W kg−1),35 Ni(OH)2 + NG2N1O//AC HSCs (38.64 W h kg−1 at 450 W kg−1),36 α-Ni(OH)2//AC HSCs (40.66 W h kg−1 at 187.06 W kg−1),37 and Ni(BO2−)–LDH//AC HSCs (56.5 W h kg−1 at 111 W kg−1).38 Furthermore, the energy density still remained at 8.1 W h kg−1 at a power density of 10472.3 W kg−1, suggesting the promising applications in HSCs. The specific energy density of the NSOH NWs//AC HSCs and the previously reported nickel hydroxide-based HSCs are plotted for comparison using a Ragone plots (Fig. 6e). Subsequently, the capacity retention of 91.5% could still be obtained after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1. This result demonstrates the good cycling performance of the as-assembled NSOH NWs//AC HSCs, as shown in Fig. 6g. During cycling stability test, the average coulombic efficiency was up to 99.8%, exhibiting excellent electrochemical reversibility and effective utilization of NSOH NW electroactive material of the as-assembled NSOH NWs//AC HSCs.
000 cycles at 5 A g−1. This result demonstrates the good cycling performance of the as-assembled NSOH NWs//AC HSCs, as shown in Fig. 6g. During cycling stability test, the average coulombic efficiency was up to 99.8%, exhibiting excellent electrochemical reversibility and effective utilization of NSOH NW electroactive material of the as-assembled NSOH NWs//AC HSCs.
4. Conclusions
In summary, the NSOH NWs with 3D reticulation exhibiting high energy storage performance have been efficiently synthesized via a one-step hydrothermal process, and high energy density HSCs has been achieved. The 3D reticulation micro–nanomorphology of the NSOH NWs shows little change over a broad temperature range of 100–200 °C, demonstrating a good temperature stability of the hydrothermal method in this work. Thanks to its novel 3D porous morphology and α-type phase structure, the as-prepared NSOH NWs show a high specific capacity of 246.3 mA h g−1 at 1 A g−1. However, after 4000 cycles, the specific capacity retention still holds at 91.7%. Meanwhile, a HSC was fabricated based on NSOH NWs and AC, and could provide a high energy density of 59.8 W h kg−1 and a high-power density of 10472.3 W kg−1 at power density of 830.3 W kg−1 and energy density of 8.1 W h kg−1, respectively. The 91.5% specific capacity retention also shows a good cycling stability of the NSOH NWs//AC HSCs after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. All the above results manifest that the proposed NSOH NWs with 3D reticulation have significant potential for advanced HSC applications.
000 cycles. All the above results manifest that the proposed NSOH NWs with 3D reticulation have significant potential for advanced HSC applications.
Author contributions
Wei Li conceived and designed the research. Wei Li, Zhongzheng Huang, Yaduo Jia, and Yunlong Cui synthesized the materials and evaluated the electrochemical performance. Wei Li, Peng Shi, Tingting Li, Hongwei Yue and Xiaojie Lou conducted the discussion and analysis. Weiwei He provided material characterization support. Wei Li and Xiaojie Lou co-wrote the paper.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Education Department of Henan Province (20A150037), Key R&D and Promotion Special Project of Henan province (Scientific and Technological Project, 212102210610). National Natural Science Foundation of China (51772238, 41372055, 51502229, 11474357, 11804289 and 51902281), the CSS project (YK2015-0602006), the Fundamental Research Funds for the Central Universities, and the World-Class Universities (Disciplines), and the Characteristic Development Guidance Funds for the Central Universities. The Program for Innovative Research Team (in Science and Technology) in University of Henan Province (19IRTSTHN026) and the Zhongyuan Thousand Talents Project (204200510016).References
- W. Raza, F. Ali, N. Raza, Y. Luo, K.-H. Kim, J. Yang, S. Kumar, A. Mehmood and E. E. Kwon, Recent advancements in supercapacitor technology, Nano Energy, 2018, 52, 441–473 CrossRef CAS.
- Y. Wang, L. Zhang, H. Hou, W. Xu, G. Duan, S. He, K. Liu and S. Jiang, Recent progress in carbon-based materials for supercapacitor electrodes: a review, J. Mater. Sci., 2021, 56, 173–200 CrossRef CAS.
- M. Vijayakumar, A. Bharathi Sankar, D. Sri Rohita, T. N. Rao and M. Karthik, Conversion of biomass waste into high performance supercapacitor electrodes for real-time supercapacitor applications, ACS Sustainable Chem. Eng., 2019, 7, 17175–17185 CrossRef CAS.
- J. Pokharel, A. Gurung, A. Baniya, W. He, K. Chen, R. Pathak, B. S. Lamsal, N. Ghimire and Y. Zhou, MOF-derived hierarchical carbon network as an extremely-high-performance supercapacitor electrode, Electrochim. Acta, 2021, 394, 139058 CrossRef CAS.
- Y. Gogotsi and R. M. Penner, Energy storage in nanomaterials – capacitive, pseudocapacitive, or battery-like?, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS PubMed.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Battery-supercapacitor hybrid devices: recent progress and future prospects, Adv. Sci., 2017, 4, 1600539 CrossRef PubMed.
- S. Najib and E. Erdem, Current progress achieved in novel materials for supercapacitor electrodes: mini review, Nanoscale Adv., 2019, 1, 2817–2827 RSC.
- W. Li, S. Wang, L. Xin, M. Wu and X. Lou, Single-crystal β-NiS nanorod arrays with a hollow-structured Ni3S2 framework for supercapacitor applications, J. Mater. Chem. A, 2016, 4, 7700–7709 RSC.
- D. Van Lam, M. Sohail, J.-H. Kim, H. J. Lee, S. O. Han, J. Shin, D. Kim, H. Kim and S.-M. Lee, Laser synthesis of MOF-derived Ni@Carbon for high-performance pseudocapacitors, ACS Appl. Mater. Interfaces, 2020, 12, 39154–39162 CrossRef CAS PubMed.
- C. Young, J. Kim, Y. V. Kaneti and Y. Yamauchi, One-step synthetic strategy of hybrid materials from bimetallic metal–organic frameworks for supercapacitor applications, ACS Appl. Energy Mater., 2018, 1, 2007–2015 CrossRef CAS.
- S. Kumar, G. Saeed, L. Zhu, K. N. Hui, N. H. Kim and J. H. Lee, 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review, Chem. Eng. J., 2021, 403, 126352 CrossRef CAS.
- K. S. Kumar, N. Choudhary, Y. Jung and J. Thomas, Recent advances in two-dimensional nanomaterials for supercapacitor electrode applications, ACS Energy Lett., 2018, 3, 482–495 CrossRef CAS.
- P. Mei, Y. V. Kaneti, M. Pramanik, T. Takei, Ö. Dag, Y. Sugahara and Y. Yamauchi, Two-dimensional mesoporous vanadium phosphate nanosheets through liquid crystal templating method toward supercapacitor application, Nano Energy, 2018, 52, 336–344 CrossRef CAS.
- N. L. Wulan Septiani, Y. V. Kaneti, K. B. Fathoni, J. Wang, Y. Ide, B. Yuliarto, Nugraha, H. K. Dipojono, A. K. Nanjundan, D. Golberg, Y. Bando and Y. Yamauchi, Self-assembly of nickel phosphate-based nanotubes into two-dimensional crumpled sheet-like architectures for high-performance asymmetric supercapacitors, Nano Energy, 2020, 67, 104270 CrossRef CAS.
- A. E. Allah, J. Wang, Y. V. Kaneti, T. Li, A. A. Farghali, M. H. Khedr, A. K. Nanjundan, B. Ding, H. Dou, X. Zhang, B. Yoshio and Y. Yamauchi, Auto-programmed heteroarchitecturing: Self-assembling ordered mesoporous carbon between two-dimensional Ti3C2Tx MXene layers, Nano Energy, 2019, 65, 103991 CrossRef CAS.
- W. Li, M. Wu, P. Shi, T. Li, H. Yue, Z. Dong, Y. Gao and X. Lou, Enhanced energy storage performance of advanced hybrid supercapacitors derived from ultrafine Ni–P@Ni nanotubes with novel three-dimensional porous network synthesized via reaction temperatures regulation, Electrochim. Acta, 2020, 331, 135440 CrossRef CAS.
- W. Li, T. Chen, A. Li, P. Shi, M. Wu, T. Li, H. Yue, Y. Chen, B. Huang and X. Lou, High energy density hybrid supercapacitors derived from novel Ni3Se2 nanowires in situ constructed on porous nickel foam, Inorg. Chem. Front., 2021, 8, 1093–1101 RSC.
- J. Gu, L. Sun, Y. Zhang, Q. Zhang, X. Li, H. Si, Y. Shi, C. Sun, Y. Gong and Y. Zhang, MOF-derived Ni-doped CoP@C grown on CNTs for high-performance supercapacitors, Chem. Eng. J., 2020, 385, 123454 CrossRef CAS.
- M. Aghazadeh, M. Ghaemi, B. Sabour and S. Dalvand, Electrochemical preparation of α-Ni(OH)2 ultrafine nanoparticles for high-performance supercapacitors, J. Solid State Electrochem., 2014, 18, 1569–1584 CrossRef CAS.
- R. Barnard, C. F. Randell and F. L. Tye, Studies concerning charged nickel hydroxide electrodes I. Measurement of reversible potentials, J. Appl. Electrochem., 1980, 10, 109–125 CrossRef CAS.
- T. N. Ramesh and P. V. Kamath, Synthesis of nickel hydroxide: effect of precipitation conditions on phase selectivity and structural disorder, J. Power Sources, 2006, 156, 655–661 CrossRef CAS.
- T. Gao and B. P. Jelle, Paraotwayite-type α-Ni(OH)2 nanowires: structural, optical, and electrochemical properties, J. Phys. Chem. C, 2013, 117, 17294–17302 CrossRef CAS.
- D. Sun, J. Zhang, H. Ren, Z. Cui and D. Sun, Influence of OH− and SO42− anions on morphologies of the nanosized nickel hydroxide, J. Phys. Chem. C, 2010, 114, 12110–12116 CrossRef CAS.
- X. Wang, F. Rong, F. Huang, P. He, Y. Yang, J. Tang and R. Que, Facile synthesis of hierarchical CoMoO4@Ni(OH)2 core-shell nanotubes for bifunctional supercapacitors and oxygen electrocatalysts, J. Alloys Compd., 2019, 789, 684–692 CrossRef CAS.
- Z. Ji, N. Li, Y. Zhang, M. Xie, X. Shen, L. Chen, K. Xu and G. Zhu, Nitrogen-doped carbon dots decorated ultrathin nickel hydroxide nanosheets for high-performance hybrid supercapacitor, J. Colloid Interface Sci., 2019, 542, 392–399 CrossRef CAS PubMed.
- J. Hao, X. Zou, L. Feng, W. Li, B. Xiang, Q. Hu, X. Liang and Q. Wu, Facile fabrication of core-shell structured Ni(OH)2/Ni(PO3)2 composite via one-step electrodeposition for high performance asymmetric supercapacitor, J. Colloid Interface Sci., 2021, 583, 243–254 CrossRef CAS PubMed.
- W. Liu, F. Zhu, Y. Liu and W. Shi, Hierarchical CoP@Ni(OH)2·0.75H2O core-shell nanosheet arrays on carbon cloth for high-performance supercapacitors, J. Colloid Interface Sci., 2020, 578, 1–9 CrossRef CAS PubMed.
- S. Zhou, Y. Liu, M. Yan, L. Sun, B. Luo, Q. Yang and W. Shi, Design of FeCo2S4@Ni(OH)2 core-shell hollow nanotube arrays on carbon paper for ultra-high capacitance in supercapacitors, Electrochim. Acta, 2020, 349, 136337 CrossRef CAS.
- Y. Zhou, S. Zhao, X. Yu, Y. Li, H. Chen and L. Han, Metal–organic framework templated fabrication of Cu7S4@Ni(OH)2 core–shell nanoarrays for high-performance supercapacitors, Inorg. Chem. Front., 2020, 7, 427–436 RSC.
- X. Luo, J. Shao, P. He, M. Zhong, Q. Wang, K. Li and W. Zhao, Metal organic framework derived CoS2@Ni(OH)2 core-shell structure nanotube arrays for high-performance flexible hybrid supercapacitors, Electrochim. Acta, 2020, 354, 136679 CrossRef CAS.
- M. Aghazadeh, M. Ghaemi, B. Sabour and S. Dalvand, Electrochemical preparation of α-Ni(OH)2 ultrafine nanoparticles for high-performance supercapacitors, J. Solid State Electrochem., 2014, 8, 1569–1584 CrossRef.
- Q. Zong, H. Yang, Q. Wang, Q. Zhang, Y. Zhu, H. Wang and Q. Shen, Three-dimensional coral-like NiCoP@C@Ni(OH)2 core-shell nanoarrays as battery-type electrodes to enhance cycle stability and energy density for hybrid supercapacitors, Chem. Eng. J., 2019, 361, 1–11 CrossRef CAS.
- L. Tian, K. Xia, S. Wu, Y. Cai, H. Liu, X. Jing, T. Yang, D. Chen, X. Bai, M. Zhou and L. Li, Rationally design of 2D branched Ni(OH)2/MnO2 hybrid hierarchical architecture on Ni foam for high performance supercapacitors, Electrochim. Acta, 2019, 307, 310–317 CrossRef CAS.
- D. Xiong, C. Lu, C. Chen, J. Wang, J. Chen, F.-Y. Yi and X. Ma, The design and fabrication of ultrahigh-performance supercapacitor electrodes from bimetallic PBA/Ni(OH)2/Co3O4/NF quaternary hybrid nanocomposites, Mater. Chem. Front., 2021, 5, 1388–1397 RSC.
- J. Li, W. Cao, N. Zhou, F. Xu, N. Chen, Y. Liu and G. Du, Hierarchically nanostructured Ni(OH)2–MnO2@C ternary composites derived from Ni-MOFs grown on nickel foam as high-performance integrated electrodes for hybrid supercapacitors, Electrochim. Acta, 2020, 343, 136139 CrossRef CAS.
- K. K. Upadhyay, N. Bundaleska, M. Abrashev, N. Bundaleski, O. M. N. D. Teodoro, I. Fonseca, A. M. de Ferro, R. P. Silva, E. Tatarova and M. F. Montemor, Free-standing N-Graphene as conductive matrix for Ni(OH)2 based supercapacitive electrodes, Electrochim. Acta, 2020, 334, 135592 CrossRef CAS.
- Y. Xin, X. Dai, G. Lv, X. Wei, S. Li, Z. Li, T. Xue, M. Shi, K. Zou, Y. Chen and Y. Liu, Stability-Enhanced α-Ni(OH)2 Pillared by Metaborate Anions for Pseudocapacitors, ACS Appl. Mater. Interfaces, 2021, 13, 28118–28128 CrossRef CAS PubMed.
- Z. Xiao, P. Liu, J. Zhang, H. Qi, J. Liu, B. Li, X. Sun, Q. Zhang, C. Wei and L. Wang, Pillar-Coordinated Strategy to Modulate Phase Transfer of α-Ni(OH)2 for Enhanced Supercapacitor Application, ACS Appl. Energy Mater., 2020, 3, 5628–5636 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1qm01298c |
| This journal is © the Partner Organisations 2022 |