A review of the properties and applications of bioadhesive hydrogels
Yingshuo
Xiong
 ,
Xiaoran
Zhang
,
Xintao
Ma
,
Wenqi
Wang
,
Feiyan
Yan
,
Xiaohan
Zhao
,
Xiaoxiao
Chu
*,
Wenlong
Xu
,
Xiaoran
Zhang
,
Xintao
Ma
,
Wenqi
Wang
,
Feiyan
Yan
,
Xiaohan
Zhao
,
Xiaoxiao
Chu
*,
Wenlong
Xu
 * and
Changmei
Sun
*
* and
Changmei
Sun
*
School of Chemistry and Materials Science, Ludong University, Yantai 264025, China. E-mail: 3498@ldu.edu.cn; sunchangmei0535@126.com
First published on 17th May 2021
Abstract
Due to their outstanding properties, bioadhesive hydrogels have been extensively studied by researchers in recent years. By designing the properties of bioadhesive hydrogels, they can be reasonably applied to biomedicine. Therefore, this practicality has prompted the birth of many bioadhesive hydrogels with excellent properties. In this review, by studying the progress in the field of bioadhesive hydrogels, we summarized the properties of hydrogels in bioadhesion, including adhesion, biocompatibility, degradability and antibacterial properties. In addition, we also summarized the applications of bioadhesive hydrogels in wound dressings, tissue repair, cell adhesion and wearable sensors. Finally, we summarized and outlooked the research on bioadhesive hydrogels, hoping to provide a valuable reference for the progress in the field of bioadhesive hydrogels.
1. Introduction
Bioadhesion refers to the phenomenon of natural or synthetic materials adhering to biological surfaces. It can also refer to the use of bioadhesives to bond two surfaces together.1 The current research on bioadhesion includes many aspects such as cell adhesion,2 mucosal adhesion,3 and bioadhesives.4 Experiments have shown that a bioadhesive polymer should have similar physical and chemical properties to the human body, so that it will not produce any adverse reactions when in contact with the human body.4 Hydrogels contain a large amount of water with a three-dimensional network structure,5–7 which has similar physical and biological properties to the extracellular matrix, and hence they can be considered as ideal bioadhesives.8,9 Bioadhesive hydrogels have both bioadhesive properties and controllable polymer properties, so they are favored by many scientists.3,10,11 Studies have shown that although bioadhesion may cause biological pollution problems, its beneficial effects are still dominant.1,12 The knowledge of adhesion was brought to bioadhesion by Baier et al. in 1968.13 Later, with the continuous study of the factors affecting the surface properties of bioadhesion, the adhesion mechanism,14 and the relationship between polymer properties and the adhesion of hydrogels,3,15 people had a deeper understanding of bioadhesion. Many research groups reviewed its applications in mucosal adhesion,16 bioadhesives17 and other fields, and put forward many challenges. This points to a future research direction for people. At present, bioadhesive hydrogels have been used in many fields of biomedicine, such as implant scaffolds in the field of tissue engineering,18 mucosal adhesives to extend the administration site time,19 and bioadhesives instead of seam needles to reduce infection.11For bioadhesive hydrogels, understanding their adhesive properties is the key. In recent decades, researchers have conducted more detailed studies on their performance.1 At present, the theoretical explanations of common biological adhesion phenomena include electronic theory, adsorption theory, diffusion theory, wetting theory, and fracture theory.20,21 Palacio and Bhushan pointed out that there are many factors affecting adhesion, which can be divided into surface morphological effects, chemical interactions, physiological factors, and physical and mechanical effects.1 In recent years, with the research on natural biological adhesion mechanisms (reversible dry adhesion, reversible wet adhesion and permanent adhesion),22 many bionic adhesion surfaces have been designed, which are expected to solve problems such as wet tissue adhesion.23–25 The polymer materials commonly used to prepare bioadhesive hydrogels include natural materials such as gelatin,26 pectin,27 and sodium alginate,28 and synthetic materials such as polyacrylic acid29–31 and sodium carboxymethyl cellulose.32 These materials themselves or after modification often have excellent properties such as biocompatibility, degradability, and antibacterial properties.26,33 By combining different materials, hydrogels can be endowed with versatility to meet different requirements.33–36 Generally speaking, an ideal bioadhesive hydrogel should have excellent biocompatibility, that is it should not produce any adverse effects when in contact with the human body.37 In addition, degradability and antibacterial properties are also extremely important in some applications.4,38 For example, when used as a bioadhesive instead of stitches, antibacterial properties can prevent inflammation from occurring when in contact with cells or tissues,38 and degradability can be effective to avoid problems such as residue and infection of the adhesive after use.4 Adhesive hydrogels with these properties can not only play a role in tissue repair and wound healing, but also promote cell proliferation and the formation of specific cells to apply for cell therapy.2,11,39 In addition, bioadhesive hydrogels can also be designed as wearable sensors for human movement monitoring.40,41
Studies have shown that the properties of bioadhesive hydrogels can be designed according to their practical applications.35,41 Therefore, it is necessary to conduct more detailed studies on these properties in order to provide a basis for the development and application of new bioadhesive hydrogels. In this review, we mainly introduced the possible properties of hydrogels used in bioadhesion, including adhesion, biocompatibility, degradability and antibacterial properties. In addition, we also summarized the research progress in bioadhesive hydrogels for wound dressing, tissue repair, cell adhesion and wearable sensors. Finally, we summarized and outlooked the research on bioadhesive hydrogels.
2. Properties of bioadhesive hydrogels
2.1. Adhesion properties
Tissue adhesives have attracted more and more attention in tissue repair,42 drug delivery,43 wound dressing44 and bioelectronics,45 mainly because they have significant advantages such as simple operation, effective sealing of air and body fluid leakage, and no need for disassembly.42 However, some tissue adhesives have certain disadvantages, for example, fibrin glues have weak adhesion to tissues,46 and cyanoacrylate adhesives may cause cytotoxic reactions.47 On balance, hydrogels have great potential for bioadhesion due to their excellent adhesion properties and biocompatibility. The following paragraphs will introduce the mechanism of enhanced adhesion between hydrogels and biological tissue.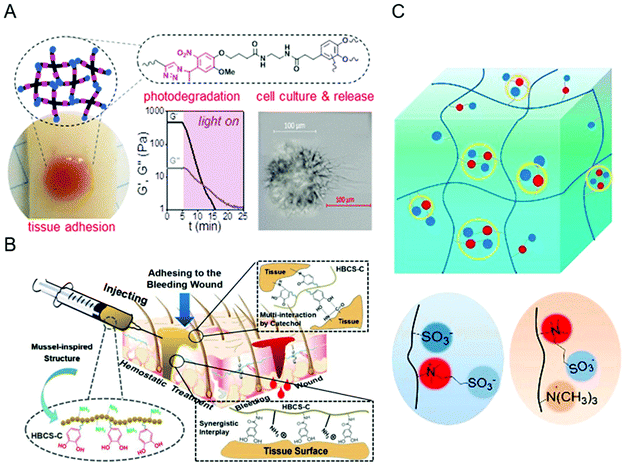 | ||
| Fig. 1 Mechanism diagram of the biological adhesion of hydrogels through weak interactions. (A) Hydrogels form adhesion through hydrogen bonds and hydrophobic interactions; Figure reproduced with permission from American Chemical Society.50 (B) Hydrogels form adhesion through multiple weak interactions between catechol/amino groups and tissues; Figure reproduced with permission from American Chemical Society.51 (C) Schematic diagram of –N+(R)3 and –SO3− on the hydrogel network. Figure reproduced with permission from American Chemical Society.45 | ||
 | ||
| Fig. 2 The mechanism diagram of the biological adhesion of the hydrogel through covalent bonds. (A) Schematic illustration of a potential mechanism of the bioadhesion. Figure reproduced with permission from American Chemical Society.42 (B) Schematic illustration of the liquid bandage: the NB-CMC/CMC photoresponsive hydrogel as a first-aid tissue adhesive. Figure reproduced with permission from Wiley.44 (C) Schematic illustration of the mussel-inspired PLGA/ALG-CHO-catechol adhesive injectable hydrogels.54 | ||
There are three main ways to enhance the adhesion strength of bioadhesive hydrogels: weak interaction forces (hydrogen bond, van der Waals force, etc.), covalent bonds and topological adhesion. Besides, the addition of “intermediate connectors” and mechanical interlock to the hydrogels can also enhance the adhesion strength of biological tissues and hydrogels. The representative bonds of various bond types are listed in Table 1. Although commonly used bioadhesive materials have many advantages, they have problems such as long curing time, weak bonding strength, and susceptibility to infection and contamination.42,65 In contrast, hydrogels with bioadhesive functions are a better choice in biomedicine.
2.2. Biocompatibility
In 2010, Kohane and Langer redefined biocompatibility as “an expression of the benignity of the relation between a material and its biological environment”.68 Studies have shown that ideal bioadhesive materials should have good biocompatibility.4,69,70 That is, when biological materials contact the human body, the host response and material response must be maintained at acceptable levels, and the use of these biological materials must be safe and effective.37 Materials lacking biocompatibility have toxic effects on the human body, and are prone to adverse biological reactions, such as inflammation, immunological reaction and immunotoxicity.4,37,68,70 In addition, the host immune system may have a latent or innate immune response to the biological material, which may prevent biological materials from performing their functions. Therefore, the biocompatibility of the materials used must be improved and evaluated.In recent years, hydrogels as a good biocompatible material have been widely used in the field of bioadhesion.18,56,71 In the past, people commonly used hydrogel adhesives including cyanoacrylate adhesives,72 fibrin glue,73 and gelatin-resorcinol-formaldehyde adhesives,74 which can bond well with the biological tissues. However, their cytotoxicity and the risk of viral infection limit their application in clinical medicine.4,75 Therefore, many materials with good biocompatibility have been developed, such as chitosan,76,77 cellulose,78 hyaluronic acid,42 alginate,28 silk fibroin,79 and polyethylene glycol (PEG).80 These materials do not easily cause inflammation and immune response due to their non-immune, non-antigenic and protein rejection properties. These materials do not easily cause inflammation, but may cause problems such as reduced functionality during processing.18 In order to realize the long-term coexistence of the bioadhesive hydrogel and tissues, many researchers also modified the surface of raw materials to improve their biological functions.34 Common modification methods include graft polymerization,81–83 covalent coupling,84–86 plasma treatment,87 and electrostatic spinning technology.88 For example, experiments show that using a Schiff base reaction in situ cross-linking oxidized hydroxyethyl starch (O-HES) and modified carboxymethyl chitosan (M-CMCS) can avoid the use of potentially toxic initiators and form a better biocompatible hydrogel (Fig. 3A).89 Using electrospun fiber technology to blend calcium phosphate nanoparticles (CaPs) with gelatin-methacryloyl (GelMA), a hybrid hydrogel fiber with good biocompatibility can be constructed (Fig. 3B).90 Atmospheric pressure plasma jet (APPJ) can locally activate the polymer surface to make it moderately hydrophilic to achieve the best biocompatibility (Fig. 3C).91 These results show that the modified material exhibits higher biocompatibility compared with the unmodified surface and is able to better integrate with the biological tissues. In addition, scientists have also developed biomimetic strategies, such as mussels (Fig. 3D) and gecko-inspired hydrogel adhesives based on different skeleton polymers.23,92,93 These nature-inspired adhesives have higher biocompatibility in the human body and are expected to eliminate potential immune responses.4 Of course, research on the biocompatibility of bioadhesive hydrogels is far from enough, there are many other biomaterial combinations waiting to be explored. In the future, people should create more materials that can control inflammation and immune response to reduce patient rejection and protect people's lives.
 | ||
| Fig. 3 Some examples of methods to improve biocompatibility. (A) In situ cross-linking of O-HES and M-CMCS; Figure reproduced with permission from American Chemical Society.89 (B) Preparation of biocompatible hybrid hydrogel fibers by electrospinning technology; Figure reproduced with permission from Elsevier.90 (C) APPJ technology activates polymer surfaces; Figure reproduced with permission from American Chemical Society.91 (D) Imitation mussel adhesion. Figure reproduced with permission from Elsevier.23 | ||
In order to ensure that the bioadhesive hydrogel does not have any toxicity when in contact with the human body and reduce the possibility of immune response and rejection when the host is in contact with biological materials, it is necessary to conduct a biocompatibility assessment.4 At present, a series of biocompatibility evaluation methods are mainly used including in vivo animal experiments59 and in vitro cytotoxicity tests, such as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay,81 cell counting kit-8 (CCK-8) assay,35 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay,94 Alamar Blue® assay,33 live/dead® viability/cytotoxicity kit assay,92 blood compatibility test (hemolysis test34 and coagulation test95), etc. But these methods are not perfect at present and the main reason is that the in vitro and in vivo experiments using animals as models cannot truly simulate the application of biological materials in the human body.18 In addition, the risks of mutagenicity and carcinogenesis cannot be detected through early clinical experiments, and long-term research and investigation is needed.4
In summary, it can be seen that many efforts have been made to improve the biocompatibility of bioadhesive hydrogels, but there are many factors affecting biocompatibility, and there are still many uncertainties in the mechanism and conditions of hydrogel biocompatibility. We should invest more time to understand biocompatibility so that it can be better used in the field of bioadhesion.
2.3. Degradability
Degradability is a basic requirement for tissue adhesives, and permanent adhesion may cause infection, secondary damage after surgery, and thrombosis problems,96,97 and degradable hydrogels have the potential to solve these problems. When applied to the clinic, the degradation products should be biocompatible,98,99 noncytotoxic,100 and can be excreted through the respiratory system or secretory system. According to different sources of degradable hydrogels, it can be divided into natural degradable hydrogels and synthetic degradable hydrogels.Natural degradable hydrogels can be composed of chitosan,101 hyaluronic acid,102 gelatin103 and so on. Chitosan is a polysaccharide composed of glucosamine as the basic unit (Fig. 4A). It has a structure of polyamino and polyhydroxyl groups, and can form hydrogen bonds with groups such as amino groups on the surface of tissue to produce adhesion.80 Chitosan can be triggered by heat or pH to produce gelation, and is degraded by lysozyme and chitosanase in the body.104 The degradable adhesive hydrogel based on chitosan has great application value in the field of biomedicine.33 Hyaluronic acid is a disaccharide unit glycosaminoglycan composed of D-glucuronic acid and N-acetylglucosamine (Fig. 4B), which can form hydrogels by thermal response,105 enzymatic cross-linking,106 Schiff base reaction107 and Michael addition.108 Hyaluronic acid is biocompatible, and can be broken down into small molecules of sugars and amino acids in the body.102 Therefore, hyaluronic acid hydrogels can be widely used as tissue adhesives in the field of biomedical and tissue engineering drug/gene delivery systems.42,102 Gelatin, as a macromolecular hydrophilic colloid, is a protein obtained by partial hydrolysis of collagen, and has good biodegradability and biocompatibility. Crosslinked gelatin can be rapidly hydrolyzed by protease K.109 Therefore, gelatin-based degradable adhesive hydrogels are a promising tissue adhesive that can be widely used as a biological material for a variety of drugs and medical applications.110
 | ||
| Fig. 4 Chemical structure of (A) chitosan, (B) hyaluronic acid, and (C) ester bond modified PEG; (D) Schematic of applying a PEG-based degradable adhesive to the tissue. Figure reproduced with permission from American Chemical Society. https://pubs.acs.org/doi/10.1021/am504566v. Further permissions related to the material excerpted should be directed to the ACS.80 | ||
However, the natural degradable hydrogels formed in this way lack sufficient mechanical properties and require long degradation time,102 which affect their application as biomaterials. Compared with natural degradable polymers, synthetic degradable polymers not only have outstanding mechanical properties, but also have a controllable degradation rate.100,111,112 Generally, ester bonds,80 disulfide bonds96 or enzymatically degradable structures113 are introduced into the hydrogel network to synthesize degradable polymers, and the decomposition of these structures endows the hydrogel degradability. PEG is widely used in the field of biomedicine because of its good biocompatibility, but its chain is difficult to degrade in vivo.114 In the preparation process, PEG can be modified into macromolecules with ester bonds (Fig. 4C), and then cross-linked to form a degradable hydrogel. As shown in Fig. 4D, the four-arm PEG is terminally modified with glutaric acid and dopamine.80 PEG and glutaric acid are connected by an ester bond, and the breakage of the ester bond leads to degradation of the hydrogel. Dopamine contains catechol groups, which can be oxidized to form highly active quinones with intermolecular cross-linking ability. Quinones can react with functional groups on the surface of biological tissues (–NH2 and –SH) to produce adhesion properties. In addition, disulfide bonds can be introduced into the gel network by means of cross-linking agents96 or grafting.115 The presence of cysteine containing sulfhydryl groups in the human body can break the disulfide bonds and cause hydrogel degradation. In addition, enzymatically degradable structures such as amino acids116 or polypeptides113 are added to the hydrogel network to make the hydrogel network have biodegradable properties. The hydrogel formed in this way can not only control the rate of degradation,113 but also the degraded product has low cytotoxicity and has potential applications in the field of tissue adhesives.
2.4. Antibacterial property
Bioadhesive hydrogels have many applications in drug delivery,117–119 tissue repair,44,120,121 and wound dressing.60,122,123 Therefore, bioadhesive hydrogels need to have antibacterial properties to solve wound infection problems. However, most of the bioadhesive hydrogels do not possess antibacterial properties.124–128 Generally, antibacterial agents such as antibiotics,124,126 metal ions or oxides,127,129 antimicrobial peptides (AMP)128,130 and quaternary ammonium compounds125 are added to improve their antibacterial properties.The introduction of antibiotics is a common method to prevent bacterial infections. Antibiotics can enter bacterial cells and bind to specific targets to disrupt protein synthesis.131 Therefore, antibiotics are generally introduced into the drug-loaded bioadhesive hydrogels and can be released sustainably to achieve long-acting therapeutic effects. This performance renders the adhesive hydrogel with antibiotics to have excellent effects on bacterial infections in heart surgery124 and ophthalmology treatments,132 respectively. In addition, adhesives with targeting effects can accurately deliver drugs to infected wounds and achieve precise treatment. An adhesive hydrogel embedded with cephalexin has a targeting effect and can treat infectious cellulitis.126 Metal ions including Ag+ and Zn2+ are well-known antibacterial drugs,127,133,134 which have been widely used in wound treatment. Metal ions can interact with the bacterial cell membrane and penetrate the cell wall to cause the loss of bacterial protein and DNA, leading to bacterial cell death.129,135 Adhesive hydrogels with antibacterial active metal nanoparticles can be applied to wound dressing.127 Because most of the metal ions are cytotoxic, rare earth ions with lower genotoxicity such as terbium ions (Tb3+) are added to the adhesive hydrogel, which can be used to treat chronic wound infections (Fig. 5A).131 The introduction of other antibacterial agents in bioadhesive hydrogels, such as quaternary ammonium compounds, peptides and phenols, can also prevent infection. The introduction of quaternized chitosan (QCS) (Fig. 5B)33 and phenols (Fig. 5C)136 renders the adhesive hydrogel able to promote wound healing. For example, chitosan can form QCS with a quaternary ammonium salt group (QAS) and inactivate bacteria through electrostatic interactions.137 The introduction of peptides128 and truffle acid (UA)138 can make the adhesive hydrogel play a role in preventing postoperative infection.
 | ||
| Fig. 5 (A) Schematic showing the preparation of antibacterial hydrogels containing Tb3+ and the application for treating infected chronic wounds. Figure reproduced with permission from Royal Society of Chemistry.131 (B) Traces of wound bed closure within 15 days of each treatment with the QCS adhesive hydrogel. Figure reproduced with permission from Elsevier.33 (C) Schematic diagram of tannin-containing hydrogels acting on wound closure and healing. Figure reproduced with permission from Royal Society of Chemistry.136 | ||
In summary, the introduction of the above-mentioned antibacterial agents can endow the adhesive hydrogel with antibacterial properties, but the use of antibiotics can lead to bacterial resistance,139 and metal ions are usually cytotoxic.140 Improvements can be made by designing innovative antibacterial hydrogel wound dressing systems, such as making programmable antibacterial hydrogels or designing a wound dressing integrated with smart electronics, which are expected to provide early infection diagnosis through real-time monitoring and on-demand treatment.
3. Applications of the bioadhesive hydrogel
3.1. Wound dressing
Due to the shortcomings of poor air permeability and inability to promote wound healing, traditional wound dressings (such as gauze, cotton pads and synthetic fibers) have always had various problems.141 A wound dressing hydrogel can provide a moist environment for the wound, absorb tissue exudate and possesses good biocompatibility and adhesion. It is considered to be a new type of wound dressing with practical prospects.33,131,142When the human body encounters trauma, the bleeding should be stopped first, otherwise it will cause shock or even death due to excessive blood loss. However, uncontrollable bleeding is a major problem during surgery and after major trauma. The bioadhesive hydrogel that can quickly polymerize at the wound, reducing blood loss from the wound (Fig. 6A).143 While reducing blood loss, blood clotting factors and platelets need to accumulate in the wound to stop bleeding. Due to their unique adhesion and absorption characteristics, hydrogels have been extensively studied in hemostasis.144 The hydrogel with a porous structure has a very good effect on hemostasis, because the dense porous hydrogel can absorb the water in the serum, and it is easy to locally concentrate the clotting factors, red blood cells and platelets,145 which can make the blood clot faster. Meanwhile, the bioadhesive function of the hydrogel can prevent the wound from cracking again and effectively accelerate the hemostasis of the wound.146
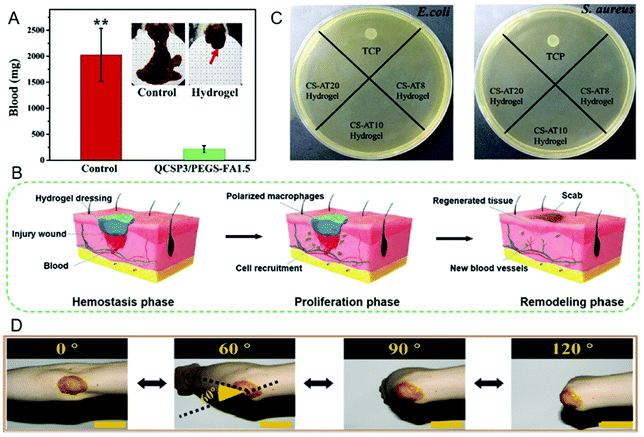 | ||
| Fig. 6 (A) Hydrogels can significantly reduce wound blood loss. Figure reproduced with permission from Elsevier.143 (B) Hydrogels can play an active role in all stages of wound healing. Figure reproduced with permission from Wiley.67 (C) Hydrogels can inhibit the proliferation of Escherichia coli and Staphylococcus aureus. Figure reproduced with permission from American Chemical Society.156 (D) When the hydrogel is applied to the elbow, the experimenter can freely bend the elbow without any resistance. Figure reproduced with permission from Elsevier.33 | ||
The ability to promote wound healing is one of the characteristics of hydrogels as a wound dressing (Fig. 6B).67 Stable adhesion to the wound is the prerequisite for the hydrogel to promote wound healing. For this purpose, people have been inspired by many adhesive organisms in nature and have prepared a series of hydrogel adhesives.146–148 When the hydrogel adheres to the wound, some of its own characteristics can promote wound healing. For example, due to the network structure and water binding capacity of the hydrogel, it can promote the exchange of oxygen and nutrients, and provide a humid environment for the migration of epidermal cells,149 and the drugs and cytokines in the hydrogel can promote wound healing. In addition, biocompatible hydrogels can also provide a good foundation for bioadhesion. For example, glycoproteins play a vital role in the extracellular matrix and can promote wound healing.150 Inspired by this, researchers have developed a hydrogel with a chemical composition similar to natural glycoprotein molecules.67,151 The good biocompatibility makes this hydrogel effectively promote wound healing.
Skin wounds are very susceptible to bacterial infections, which not only hinder wound healing, but also lead to systemic complications.152 This requires wound dressings to protect the wound from bacterial infections. Bioadhesive hydrogels with antibacterial properties have great advantages in wound healing. Hydrogels can promote the gas exchange between the wound and the outside world, and inhibit the reproduction of anaerobic bacteria in the wound.153–155 Antibacterial hydrogels can inhibit the growth of a variety of bacteria (such as Escherichia coli, Staphylococcus aureus, etc.) and provide a sterile environment for wound healing (Fig. 6C).156 In addition, due to the biocompatibility of the antibacterial hydrogel, it can only kill bacteria without cytotoxicity.131 When the antibacterial hydrogel is used for bioadhesion, it can provide an antibacterial barrier to protect the wound from bacterial infection,35 thereby achieving a better wound healing effect.
Wounds on stretchable parts of the body often result in delayed healing and poor healing due to frequent exercise. However, the mechanics of ordinary wound dressings is not suitable for wounds, and they show poor curative effect and short life span when used. When a hydrogel with appropriate mechanical strength is applied to bioadhesion, it can promote wound healing in stretchable parts of the body (ankle, elbow, knee, wrist, etc.), avoiding the problem of poor wound healing caused by stretching (Fig. 6D).33 After the wound has healed abnormally, it is easy to form scars and continues to affect human activities.157 The hydrogel with proper mechanical strength can keep the wound in a natural state and avoid scars after healing.158 The mechanical strength of the hydrogel is the guarantee for its use as a wound dressing. Therefore, it is necessary for the hydrogel to have suitable mechanical strength25,159 to have better practicability in terms of bioadhesion.
In summary, hydrogels have great advantages as wound dressings, and they also have broad practical prospects. The development of hydrogels with excellent properties can effectively promote the development of biomedicine. However, ordinary hydrogels cannot be used as wound dressings due to the complicated process, long polymerization time, and weak mechanical strength. These problems of hydrogels can be improved by preparing multi-network hydrogels and adding cross-linking agents. However, due to various problems such as difficult to design schemes and uncontrollable conditions in the experiment, it is still challenging to develop suitable hydrogels as wound dressings.
3.2. Tissue repair
The traditional suturing or stapling method used in tissue repair will not only cause secondary injury, but also face the problem of stitch removal.160 There are risks of bleeding, foreign body invasion and bacterial infection in the wound after surgery. As a material with good biocompatibility and degradability,96 hydrogels can be applied to tissue repair by adjusting the adhesion properties.The bioadhesive hydrogel shows unique advantages in cartilage, oral and corneal repair, and treatment of myocardial infarction.70,161–163 Hydrogels are especially widely used in cartilage tissue repair. Catecholamines, especially polydopamine, have strong and durable surface adhesion. Phenolic components produced by plants, such as caffeic acid, have more advantages than dopamine. For example, a nanoclay-organic hydrogel bone sealant (NoBS) with excellent adhesion properties was assembled by phytochemical-grafted chitosan (PGC) and nanosilicates (NCs) through interactions of catechol groups in phytochemicals, which can promote bone regeneration.121 Smoothened agonist (SAG) is a model drug, which can evaluate NoBS’ drug delivery system. The strong and durable surface adhesion of the catechol group renders NoBS with good adhesion ability, but a growth factor of SAG was needed in this process to activate hedgehog signaling and osteogenesis, thereby promoting bone regeneration (Fig. 7A). The addition of activating substances makes the tissue repair process more complicated. The emergence of the polydopamine–chondroitin sulfate–polyacrylamide hydrogel (PDA–CS–PAM) has solved this problem.164 Chondroitin sulfate (CS) is the main component of body tissues. However, the natural CS hydrogel is limited in cartilage regeneration due to its poor mechanical properties and short duration in the body. In addition, due to its negative charge and lack of adhesion motifs, CS shows weak affinity to some cells. With the help of abundant active catechol groups on PDA, the PDA–CS complex is formed through the self-assembly of PDA and CS. The PDA–CS complex is uniformly incorporated into the elastic hydrogel network, endowing the hydrogel with good cell affinity and tissue adhesion properties, and promoting cell adhesion and tissue integration. This hydrogel creates a growth factor-free bionic microenvironment for cartilage cell growth and cartilage regeneration, and provides inspiration for the development of growth factor-free biomaterials for cartilage repair (Fig. 7B). Compared with single hydrogels, blended hydrogels have more development prospects. Agarose and silk fibroin blended hydrogels well prove this point of view.120 AG hydrogels have certain mechanical strength in cartilage repair, but their cell adhesion and cell proliferation abilities are weak, while SF from mulberry (Bombyx mori) and non-mulberry (Antheraea assamensis) has better cell adhesion abilities. The arginine–glycine–aspartic acid (RGD) is an integrin binding site that facilitates cell attachment, and the adhesion of SF is derived from the RGD tripeptide structure of Antheraea assamensis. When the cartilage tissue is repaired, the two hydrogels are combined to obtain a blended hydrogel with good mechanical strength and adhesion properties. Combining different hydrogels through physical and chemical interactions to prepare blended hydrogels has great development prospects in cartilage tissue repair.
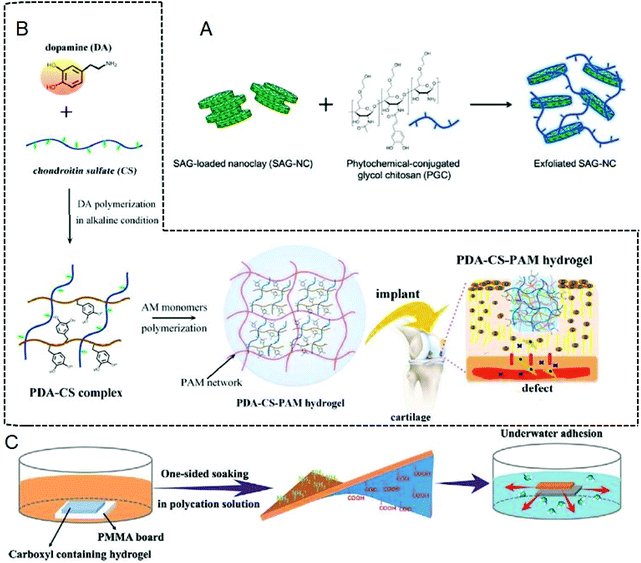 | ||
| Fig. 7 (A) Schematic diagram of NoBS. Figure reproduced with permission from Wiley.121 (B) Schematic diagram of PDA–CS–PAM. Figure reproduced with permission from American Chemical Society.164 (C) Schematic diagram of the Janus hydrogel formed by immersing one side of a carboxyl-containing self-adhesive hydrogel in a polycation solution and complexing with the gradient polyelectrolyte. Figure reproduced with permission from Wiley.169 | ||
The adhesion of hydrogels in the oral cavity,165 cornea166 and treatment of myocardial infarction59,167 is gradually becoming common. In recent years, people have used dental implants to replace missing teeth, which has increased the number of people suffering from peri-implant diseases (PIDs). Antimicrobial peptide hydrogels (GelAMP) improve the treatment of PIDs.162 GelAMP is prepared by mixing cationic AMP and photocrosslinkable gelatin methacryl hydrogels, which can strongly adhere to hard and soft oral surfaces for the treatment of skin diffusion. This strong adhesive hydrogel is cured by dental light to prevent infection and promote bone healing. In corneal tissue repair, the hydrogel used for repair is required to have high adhesion, cohesion and regeneration. In order to meet the demand, a highly biocompatible and transparent bioadhesive for corneal reconstruction was designed.163 This bioadhesive hydrogel uses GelCORE for corneal regeneration, and can effectively seal corneal defects and induce matrix regeneration. In addition, the modification of the adhesive can maintain proper biodegradability and high cell compatibility in vitro. Apart from this, GelCORE's in situ photopolymerization allows the hydrogel to bioadhere according to the geometry required by the defect. These advantages render GelCORE as a promising corneal repair bioadhesive. The application of hydrogel in myocardial infarction has solved many difficult problems. Fullerol nanoparticles are introduced into alginate to prepare a bioadhesive hydrogel, which serves as a cell delivery vehicle to solve the problems during cardiac repair.168 This kind of hydrogel combines with stem cells to render it easy for reactive oxygen species to attach to the surface of fullerene nanoparticles, thereby increasing the retention and survival rate of implanted stem cells, and promoting the adhesion of stem cells to the extracellular matrix of heart tissue. The stem cells attached to the hydrogel can fully develop their differentiation potential and promote the recovery of cardiac function.
Although hydrogel adhesives are useful for tissue repair, they may cause trouble, for example, there are still many difficulties in adhering to human wet tissues. Thus, researchers have developed a variety of bioadhesive hydrogels for bonding wet tissues.169,170–174 The hydrogel is usually modified with adhesive groups and optimized network structures to enhance tissue repair in a wet environment. For example, the catechol-modified hyaluronic acid-catechol (HA-CA) hydrogel can adhere to the surface of various types of materials including wet tissue.172 This hydrogel can be applied to minimally invasive cell therapy. In addition, the hydrogel prepared based on the reaction of thiourea-catechol shows great potential in wet adhesion,173,174 and gelation enables the formation of interfacial covalent bonds between the stretchable hydrogel and the tissue surface, which can significantly enhance interfacial adhesion. This hydrogel shows great potential in promoting the healing of gastric ulcers in pigs. There is also a mussel-like hydrogel obtained by controlling the dimerization of catechol, which also shows strong adhesion to wet biological tissues, and provides a way for us to solve underwater adhesion.171 In addition, most adhesives can not only adhere postoperative wounds together, but also the other normal tissues nearby the wound. Cui et al. proposed a Janus hydrogel with a unique adhesion mechanism to solve this problem.169 On one hand, the polycation solution and the catechol group in the mussel foot proteins (mfps) with opposite charges can induce phase separation to reduce the interface energy of the mfps, thereby removing water to achieve wet adhesion. On the other hand, one side (top side) of the carboxyl polymer hydrogel is immersed in a polycation solution to neutralize the carboxyl group, losing its adhesion property, while the other side (bottom side) maintains the adhesion property (Fig. 7C). Therefore, the Janus hydrogel adhesive can be fabricated with different adhesion strength of the two sides, which can achieve desired tissue adhesion. There is no doubt that this double-sided hydrogel has great development prospects.
The application of the bioadhesive hydrogel overcomes the shortcomings of traditional sutures, which solves many problems in tissue repair. However, in the development process, hydrogels also show some shortcomings. In terms of postoperative anti-adhesion and non-flat complex geometric shape tissue repair,175 hydrogels still have great potential for development.
3.3. Cell adhesion
In recent years, the research of hydrogels in the field of cell adhesion has attracted extensive attention because hydrogels have strong adhesive properties with degradability, and cells can adhere to the hydrogel for proliferation and diffusion.176 Now hydrogels have become important adhesives for cell adhesion.177The regulation of cell adhesion strength is helpful for medical treatment, so it is very important in the application of cell adhesion. Researchers are committed to regulate the strength of cell adhesion by physical or chemical methods, such as electrostatic interaction and forming chemical bonds.178,179 For example, Ming et al. designed a hydrogel with positive charges by the addition of a photosensitive cationic monomer, which could form electrostatic interaction with the surface of the protein to enhance the cell adhesion strength (Fig. 8A).178 Xu et al. fabricated an injectable polypeptide hydrogel, which can provide a scaffold simulating extracellular matrix for organisms by dynamically regulating cell adhesion.179 The cell adhesive RGD binds to the hydrogel network through a disulfide bond, which can form stable adhesion between the cells and the hydrogel.179 In addition to weak interactions and chemical bonds, the hydrophilicity or hydrophobicity can also regulate the cell adhesion strength. Ajiro et al. designed double network (DN) hydrogels, which were prepared using secondary N-vinylacetamide (NVA) in PNVA gels (NVA/NVA DN).180 During the secondary polymerization of the hydrogel, the external contact surface is more hydrophilic due to the second glass contact effect, which increases the adhesion between the hydrogel and cells. Therefore, the cell adhesion strength can be regulated by changing the surface characteristics and internal crosslinking density of the gel.180
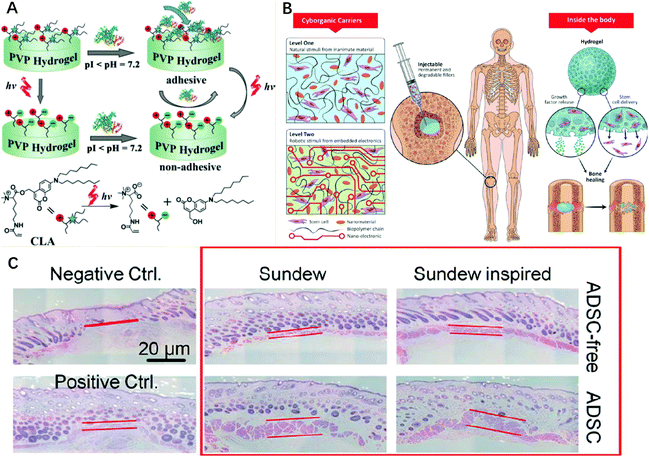 | ||
| Fig. 8 (A) The phototriggered charge changes to combine electrostatic adsorption, and ultimately achieves controlled cell adhesion by protein guidance. Figure reproduced with permission from Wiley.178 (B) Schematic diagram of the ability of the composite and nanoengineered biomaterials to induce stem cell bone regeneration in natural and factor-free environments. Figure reproduced with permission from American Chemical Society.185 (C) The wounds treated with sundew-derived and sundew-inspired adhesive hydrogels have a higher rehabilitation rate than the untreated groups. Figure reproduced with permission from American Chemical Society.2 | ||
One of the most common application fields of cell adhesion is to promote cell proliferation.181 Because the unique network structure of hydrogels is similar to the environment in the human body, it is conducive to promote cell proliferation.182,183 Moreover, it can cause cell adherent growth in hydrogels and accelerate the cell growth rate, thus effectively promoting the healing of wounds and tissue regeneration. For example, notch is a substance that can regulate the proliferation of cardiomyocytes, which can be combined with hydrogels to promote cell proliferation,184 and accelerates the healing rate of cardiac wounds during surgery.185 Cell adhesion can also promote the formation of specific cells, especially in the treatment of cartilage, spinal cord and other specific cell formation and regeneration (Fig. 8B).185 The unique 3D network structure of hydrogels provides a suitable environment for cell adhesion and differentiation at the injured site.186 Hydrogels play an important role in biological adhesion because of their ability to proliferate cells and promote specific cell formation, which can be used for cell therapy. For example, Sun et al. prepared a sundew-inspired adhesive hydrogel, in which sundew is a drug that contains wound healing factors. The hydrogel is a complex network composed of nanofibers and nanoparticles and contains a high water content and has strong adhesion properties. The wound healing factors can be transferred through cell adhesion, so the sundew-inspired adhesive hydrogels realize the function of hydrogels for cell therapy (Fig. 8C).2 Thus, differentiation and attachment of fibroblast-like cells can be promoted. In addition, cell adhesion can also be used in heart disease to achieve cell therapy.187 Phelps et al. prepared a maleimide crosslinked polyethylene glycol (PEG-MAL) hydrogel, which could deliver therapeutic proteins to the postinfarct cardiac extracellular matrix proteins to provide sites for cell adhesion and affect cellular behavior. The results show that the hydrogel has the property of improving cell adhesion and maintains high cell survival rate function.188 The cell therapy of the PEG-MAL hydrogel in heart repair surgery was realized.
There are no doubt hydrogels play an important role in the application of cell adhesion, however, it is undeniable that hydrogels used for cell adhesion still have some unavoidable problems, for example, cell adhesion still needs to be improved with poor bone conductivity.189,190 Therefore, relevant research still needs to be explored.
3.4. Wearable sensor
Wearable sensors play a vital role in personalized healthcare, clinical diagnosis and physical monitoring.40,191 Strain sensors composed of metal and semiconductor films exhibit brittleness and rigidity, low stretchability and high modulus, and therefore cannot seamlessly adhere to the tissue surface of the human body.192,193 In order to solve this problem, biopolymer-based hydrogels have been developed as sustainable building blocks for wearable sensors because of their high stretchability, high strain sensitivity, adhesion and autonomous self-repair capabilities.194 However, most sensors require additional tape or bandages to attach to the skin or clothing, which makes the sensor unable to detect weak signals and greatly hinders the application in wearable devices.195 Therefore, strong and long-lasting adhesion on the skin surface is essential for many hydrogel-based wearable sensors.196,197Hydrogel wearable sensors can exhibit adhesion performance to the skin surface by designing an appropriate interface interaction. Weak interactions such as electrostatic interaction, hydrophobic interaction, and hydrogen bonds can be applied for the adhesion of hydrogel wearable sensors.35,198,199 Similarly, the adhesion properties can also be achieved through the synergistic effect of a variety of weak interactions.41,200,201 In addition to weak interactions, the covalent interaction between the hydrogel and the skin surface can also make it sticky, for example, a mussel-like wearable sensor can provide better adhesion performance through covalent interaction with the skin surface.202–204
The hydrogel wearable sensor can be attached to the skin to monitor various human movements and tiny physiological signals in real time, so it needs to have excellent sensitivity.205,206 The introduction of conductive polymers such as graphene and MXene into the hydrogel can increase the conductivity of the wearable sensor and further improve the sensitivity.207,208 With the increase of using time and frequency, the adhesion ability of the wearable hydrogel sensor may gradually decrease, and the response signal may also gradually disappear. Therefore, it is urgent to improve the performance of the hydrogel wearable sensor.203 Some hydrogel sensors based on catechol groups generally show more excellent adhesion performance. Due to the dynamic redox balance between catechol groups and quinones, the hydrogel wearable sensors have reversible adhesion properties (Fig. 9A).207 Adding nanomaterials with redox activity to the hydrogel containing a large amount of catechol groups can also endow the hydrogel wearable sensor reversible adhesion performance (Fig. 9B).199 This excellent adhesion performance greatly improves the application market of wearable sensors. The self-adhesive properties of hydrogel sensors are often ineffective for damp, greasy and sweaty human skin. Sweating hinders the interaction between the hydrogel and the interface, building a strong adhesion system in oil and water is a problem that hydrogel sensors need to solve at present. Silk fibroin is a biologically sustainable and biodegradable material, it is usually used to construct bioadhesive hydrogels. In the presence of water, silk fibroin has a Young's modulus similar to that of skin.209,210 The silk fibroin-based hydrogel wearable sensor can maintain excellent adhesion performance even in the case of sweating, and can perform dynamic physiological tests.211 Besides, inspired by the structure of DNA, some nucleobase-based hydrogels exhibit adhesion properties in various solvents, even in the case of strenuous exercise and sweating, it can maintain strong adhesion performance and detect subtle physiological signals accurately.212 However, in a cold environment, the adhesion and toughness of hydrogels may disappear.213,214 To solve this defect, nucleobase pairs can be introduced into the hydrogel, even in the harsh temperature environment of −20 °C to 80 °C, the hydrogel has excellent adhesion and stability, and can be effectively attached to the wet skin of the human body and used to monitor various human movements including talking, nodding and bending various joints, which improves the applicability of the wearable sensor in harsh environments (Fig. 9C).215
 | ||
| Fig. 9 Methods to improve the performance of the wearable hydrogel sensors. (A) The dynamic redox between catechol and quinone endow the hydrogel with reversible adhesion properties: (a) mussel bond mechanism; (b) redox environment inside the hydrogel and maintain long-term adhesion of catechol groups; (c) interactions between the hydrogel and various substrates: hydrogen bond, coordination bond, π–π stacking, etc. Figure reproduced with permission from Wiley.207 (B) Hydrogels containing clay nanoplatelets increase the adhesion performance: (a) preparation of the hydrogel; (b) the reversible adhesion cycle of the hydrogel on the substrate. Figure reproduced with permission from Elsevier.199 (C) Hydrogel wearable sensor with adhesion performance in a cold environment: (a) preparation of the wearable sensor hydrogel. (b) Hydrogel performance at −20 °C in a cold environment. (c) The hydrogel wearable sensor detects weak signals from the body. Figure reproduced with permission from Elsevier.215 | ||
Polymer hydrogels can exhibit great extensibility and diversified functions and adhesive performance is an important condition for hydrogel wearable sensors. Hydrogels need to have good adhesion to biological tissues, and sensors with excellent adhesion properties can not only extend the service life, but also can withstand extreme conditions of use. Therefore, the development of conductive hydrogels with tissue adhesion is of special significance, and the development of hydrogel sensors with excellent adhesion properties is an important step in expanding its applicable fields.
4. Conclusions
Bioadhesion undoubtedly plays an important role in the field of biomedicine. In recent years, hydrogels have been developed rapidly in bioadhesion because of their excellent properties, including adhesion, biocompatibility, degradability and antibacterial properties. The adjustable adhesion strength of the hydrogel allows it to act on multiple parts of the body as needed, and the biocompatibility of the hydrogel prevents it from having adverse effects on the body, which hopefully leads to the further development of hydrogels in biomedicine. The degradable hydrogel can be removed from the wound as needed, and the degradation process is simple, which provides a new idea for the post-treatment of the wound. The antibacterial properties of the hydrogel can protect wounds from bacterial infections and provide a good channel for solving the problem of bacterial resistance. Therefore, bioadhesive hydrogels have broad application prospects in biomedicine. When hydrogels are used as wound dressings, their appropriate mechanical strength can be applied to stretchable parts of the body and can accelerate wound hemostasis and promote wound healing. In the field of tissue repair, hydrogels can effectively close wounds by virtue of their excellent adhesion, thereby promoting tissue regeneration. In terms of cell adhesion, hydrogels can adjust the adhesion strength of cells and promote the formation of specific cells to use for cell therapy. As a wearable sensor, hydrogels can effectively transmit and detect physiological signals generated by the body. This article summarizes the properties of hydrogels and their applications in the field of bioadhesion, which can provide inspiration for the design of hydrogels and support the development of hydrogels in bioadhesion.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The work is financially supported by the National Natural Science Foundation of China (no. 22001107) and the Natural Science Foundation of Shandong Province (ZR2020ME066).Notes and references
- M. L. Palacio and B. Bhushan, Philos. Trans. R. Soc., A, 2012, 370, 2321–2347 Search PubMed.
- L. Sun, Y. Huang, Z. Bian, J. Petrosino, Z. Fan, Y. Wang, K. H. Park, T. Yue, M. Schmidt, S. Galster, J. Ma, H. Zhu and M. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 2423–2434 Search PubMed.
- N. A. Peppas and J. J. Sahlin, Biomaterials, 1996, 17, 1553–1561 Search PubMed.
- W. Zhu, Y. J. Chuah and D. A. Wang, Acta Biomater., 2018, 74, 1–16 Search PubMed.
- Y. Li, H. Wang, Y. Niu, S. Ma, Z. Xue, A. Song, S. Zhang, W. Xu and C. Ren, ChemistrySelect, 2019, 4, 14036–14042 Search PubMed.
- C. Zhu, Y. Xia, Y. Zai, Y. Dai, X. Liu, J. Bian, Y. Liu, J. Liu and G. Li, Chem. Eng. J., 2019, 369, 863–873 Search PubMed.
- Z. Li, C. Cui, Z. Zhang, X. Meng, Q. Yan, J. Ouyang, W. Xu, Y. Niu and S. Zhang, ChemistrySelect, 2019, 4, 7838–7843 Search PubMed.
- J. Xu, Y. Liu and S. H. Hsu, Molecules, 2019, 24, 3005 Search PubMed.
- Z. Sun, Z. Li, K. Qu, Z. Zhang, Y. Niu, W. Xu and C. Ren, J. Mol. Liq., 2021, 325, 115254 Search PubMed.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 Search PubMed.
- L. Zhang, M. Liu, Y. Zhang and R. Pei, Biomacromolecules, 2020, 21, 3966–3983 Search PubMed.
- A. K. Halvey, B. Macdonald, A. Dhyani and A. Tuteja, Philos. Trans. R. Soc., A, 2019, 377, 20180266 Search PubMed.
- R. E. Baier, E. G. Shafrin and W. A. Zisman, Science, 1968, 162, 1360–1368 Search PubMed.
- H. Park and J. R. Robinson, Pharm. Res., 1987, 4, 457–464 Search PubMed.
- S.-H. S. Leung and J. R. Robinson, J. Controlled Release, 1988, 5, 223–231 Search PubMed.
- S.-H. S. Leung and J. R. Robinson, ACS Symp. Ser., 1991, 467, 350–366 Search PubMed.
- N. A. Peppas, J. Controlled Release, 1985, 2, 257–275 Search PubMed.
- S. Naahidi, M. Jafari, M. Logan, Y. Wang, Y. Yuan, H. Bae, B. Dixon and P. Chen, Biotechnol. Adv., 2017, 35, 530–544 Search PubMed.
- R. Shaikh, T. R. Raj Singh, M. J. Garland, A. D. Woolfson and R. F. Donnelly, J. Pharm. BioAllied Sci., 2011, 3, 89–100 Search PubMed.
- P. B. Geraghty, D. Attwood, J. H. Collett, H. Shama and Y. Dandiker, Biomaterials, 1997, 18, 63–67 Search PubMed.
- Y. Mao and L. Anand, J. Mech. Phys. Solids, 2018, 115, 30–53 Search PubMed.
- P. M. Favi, S. Yi, S. C. Lenaghan, L. Xia and M. Zhang, J. Adhes. Sci. Technol., 2014, 28, 290–319 Search PubMed.
- D. W. R. Balkenende, S. M. Winkler and P. B. Messersmith, Eur. Polym. J., 2019, 116, 134–143 Search PubMed.
- G. S. Watson, D. W. Green, L. Schwarzkopf, X. Li, B. W. Cribb, S. Myhra and J. A. Watson, Acta Biomater., 2015, 21, 109–122 Search PubMed.
- A. Ma, G. Wang, Z. Yang, L. Bai, H. Chen, W. Wang, H. Yang, D. Wei and L. Yang, Chem. Eng. J., 2020, 385, 123962 Search PubMed.
- O. Pinkas, D. Goder, R. Noyvirt, S. Peleg, M. Kahlon and M. Zilberman, Acta Biomater., 2017, 51, 125–137 Search PubMed.
- P. A. Markov, N. S. Krachkovsky, E. A. Durnev, E. A. Martinson, S. G. Litvinets and S. V. Popov, J. Biomed. Mater. Res., Part A, 2017, 105, 2572–2581 Search PubMed.
- T. Chen, Y. Chen, H. U. Rehman, Z. Chen, Z. Yang, M. Wang, H. Li and H. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 33523–33531 Search PubMed.
- A. Li, Y. Jia, S. Sun, Y. Xu, B. Minsky, M. A. C. Stuart, H. Cölfen, R. v. Klitzing and X. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 10471–10479 Search PubMed.
- J. Wang, D. Yang, M. Chen, B. Liu, H. Chen, H. Xu, W. Wang and L. Bai, Anal. Methods, 2018, 10, 526–532 Search PubMed.
- H. Chen, Y. Liang, M. Wang, P. Lv and Y. Xuan, Chem. Eng. J., 2009, 147, 297–301 Search PubMed.
- G. O. Akalin and M. Pulat, J. Nanomater., 2018, 2018, 1–12 Search PubMed.
- J. Qu, X. Zhao, Y. Liang, T. Zhang, P. X. Ma and B. Guo, Biomaterials, 2018, 183, 185–199 Search PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. Madanchi, H. A. M. Aliabadi and A. Maleki, Carbohydr. Polym., 2020, 248, 116802 Search PubMed.
- Z. Li, W. Xu, X. Wang, W. Jiang, X. Ma, F. Wang, C. Zhang and C. Ren, Eur. Polym. J., 2021, 146, 110253 Search PubMed.
- W. Xu, Y. Hong, Y. Hu, J. Hao and A. Song, ChemPhysChem, 2016, 17, 2157–2163 Search PubMed.
- D. F. Williams, Biomaterials, 2008, 29, 2941–2953 Search PubMed.
- J. Guo, W. Sun, J. P. Kim, X. Lu, Q. Li, M. Lin, O. Mrowczynski, E. B. Rizk, J. Cheng, G. Qian and J. Yang, Acta Biomater., 2018, 72, 35–44 Search PubMed.
- S. Tarafder, G. Y. Park, J. Felix and C. H. Lee, Acta Biomater., 2020, 117, 77–92 Search PubMed.
- W. Wu and H. Haick, Adv. Mater., 2018, 30, 1705024 Search PubMed.
- Z. Li, X. Meng, W. Xu, S. Zhang, J. Ouyang, Z. Zhang, Y. Liu, Y. Niu, S. Ma, Z. Xue, A. Song, S. Zhang and C. Ren, Soft Matter, 2020, 16, 7323–7331 Search PubMed.
- D. Zhou, S. Li, M. Pei, H. Yang, S. Gu, Y. Tao, D. Ye, Y. Zhou, W. Xu and P. Xiao, ACS Appl. Mater. Interfaces, 2020, 12, 18225–18234 Search PubMed.
- D. Das and S. Pal, RSC Adv., 2015, 25014–25050, 10.1039/C4RA16103C.
- Y. Ma, J. Yao, Q. Liu, T. Han, J. Zhao, X. Ma, Y. Tong, G. Jin, K. Qu, B. Li and F. Xu, Adv. Funct. Mater., 2020, 30, 2001820 Search PubMed.
- J. Wang, L. Wang, C. Wu, X. Pei, Y. Cong, R. Zhang and J. Fu, ACS Appl. Mater. Interfaces, 2020, 12, 46816–46826 Search PubMed.
- D. H. Sierra, J. Biomater. Appl., 1993, 7, 309–352 Search PubMed.
- P. Karami, C. Wyss, A. Khoushabi, A. Schmocker, M. Broome, C. Moser, P.-E. Bourban and D. Pioletti, ACS Appl. Mater. Interfaces, 2018, 10, 38692–38699 Search PubMed.
- M. Mehdizadeh and J. Yang, Macromol. Biosci., 2013, 13, 271–288 Search PubMed.
- Y. Gao, J. Chen, X. Han, Y. Pan, P. Wang, T. Wang and T. Lu, Adv. Funct. Mater., 2020, 30, 2003207 Search PubMed.
- M. Villiou, J. I. Paez and A. d. Campo, ACS Appl. Mater. Interfaces, 2020, 12, 37862–37872 Search PubMed.
- Y. Shou, J. Zhang, S. Yan, P. Xia, P. Xu, G. Li, K. Zhang and J. Yin, ACS Biomater. Sci. Eng., 2020, 6, 3619–3629 Search PubMed.
- X. Su, Y. Luo, Z. Tian, Z. Yuan, Y. Han, R. Dong, L. Xu, Y. Feng, X. Liu and J. Huang, Mater. Horiz., 2020, 7, 2651–2661 Search PubMed.
- F. Nishio, I. Hirata, K. Nakamae, K. Tsuga and K. Kato, Int. J. Adhes. Adhes., 2021, 104, 102746 Search PubMed.
- S. Yan, W. Wang, X. Li, J. Ren, W. Yun, K. Zhang, G. Li and J. Yin, J. Mater. Chem. B, 2018, 6, 6377–6390 Search PubMed.
- J. Chen, J. Yang, L. Wang, X. Zhang, B. C. Heng, D. A. Wang and Z. Ge, Bioact. Mater., 2021, 6, 1689–1698 Search PubMed.
- S. Huang, X. Kong, Y. Xiong, X. Zhang, H. Chen, W. Jiang, Y. Niu, W. Xu and C. Ren, Eur. Polym. J., 2020, 141, 110094 Search PubMed.
- Z. Bao, M. Gao, Y. Sun, R. Nian and M. Xian, Mater. Sci. Eng., C, 2020, 111, 110796 Search PubMed.
- P. Heidarian, A. Z. Kouzani, A. Kaynak, M. Paulino, B. Nasri-Nasrabadi, A. Zolfagharian and R. Varley, Carbohydr. Polym., 2020, 231, 115743 Search PubMed.
- Y. Hong, F. Zhou, Y. Hua, X. Zhang, C. Ni, D. Pan, Y. Zhang, D. Jiang, L. Yang, Q. Lin, Y. Zou, D. Yu, D. E. Arnot, X. Zou, L. Zhu, S. Zhang and H. Ouyang, Nat. Commun., 2019, 10, 2060 Search PubMed.
- Z. Zhang, X. Wang, Y. Wang and J. Hao, Biomacromolecules, 2018, 19, 980–988 Search PubMed.
- Z. Li, F. Zhou, Z. Li, S. Lin, L. Chen, L. Liu and Y. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 25194–25202 Search PubMed.
- J. Yang, R. Bai and Z. Suo, Adv. Mater., 2018, 30, 1800671 Search PubMed.
- L. C. Bradley, N. D. Bade, L. M. Mariani, K. T. Turner, D. Lee and K. J. Stebe, ACS Appl. Mater. Interfaces, 2017, 9, 27409–27413 Search PubMed.
- R. Michel, M. Roquart, E. Llusar, F. Gaslain, S. Norvez, J. S. Baik, G.-R. Yi, M. Manassero and L. Corté, ACS Appl. Bio Mater., 2020, 3, 8808–8819 Search PubMed.
- X. Li, D. Xu, H. Wang, C. Gong, H. Li, Y. Huang, S. Long and D. Li, Macromol. Rapid Commun., 2020, 41, 2000127 Search PubMed.
- F. Yu, X. Cao, J. Du, G. Wang and X. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 24023–24031 Search PubMed.
- Z. Feng, Q. Su, C. Zhang, P. Huang, H. Song, A. Dong, D. Kong and W. Wang, Adv. Funct. Mater., 2020, 30, 2006454 Search PubMed.
- D. S. Kohane and R. Langer, Chem. Sci., 2010, 1, 441–446 Search PubMed.
- A. P. Duarte, J. F. Coelho, J. C. Bordado, M. T. Cidade and M. H. Gil, Prog. Polym. Sci., 2011, 37, 1031–1050 Search PubMed.
- E. S. Sani, A. Kheirkhah, D. Rana, Z. Sun, W. Foulsham, A. Sheikhi, A. Khademhosseini, R. Dana and N. Annabi, Sci. Adv., 2019, 5, eaav1281 Search PubMed.
- W. Xu, Y. Hong, A. Song and J. Hao, Colloids Surf., B, 2020, 185, 110567 Search PubMed.
- F. J. Papatheofanis, J. Biomed. Mater. Res., 1989, 23, 661–668 Search PubMed.
- M. D. Thomas and E. MacGillivray, J. Cardiovasc. Surg., 2003, 18, 480–485 Search PubMed.
- T. Kunihara, K. Iizuka, S. Sasaki, N. Shiiya, F. Sata and Y. Matsui, Eur. J. Cardiothorac. Surg., 2009, 36, 962–966 Search PubMed.
- F. Scognamiglio, A. Travan, I. Rustighi, P. Tarchi, S. Palmisano, E. Marsich, M. Borgogna, I. Donati, N. de Manzini and S. Paoletti, J. Biomed. Mater. Res., Part B, 2016, 104, 626–639 Search PubMed.
- M. Kim, Y. Ahn, K. Lee, W. Jung and C. Cha, Carbohydr. Polym., 2020, 229, 115538 Search PubMed.
- G. Li, Q. Wen, T. Zhang and Y. Ju, J. Appl. Polym. Sci., 2013, 127, 2690–2697 Search PubMed.
- G. Xiao, Y. Wang, H. Zhang, L. Chen and S. Fu, Carbohydr. Polym., 2019, 218, 68–77 Search PubMed.
- J.-W. Seo, H. Kim, K. Kim, S. Q. Choi and H. J. Lee, Adv. Funct. Mater., 2018, 28, 1800802 Search PubMed.
- Y. Liu, H. Meng, S. Konst, R. Sarmiento, R. Rajachar and B. P. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 16982–16992 Search PubMed.
- G. Li, Y. Meng, L. Guo, T. Zhang and J. Liu, J. Biomed. Mater. Res., Part A, 2014, 102, 2163–2172 Search PubMed.
- X. Zhang and G. Wu, Polymers, 2018, 10, 1171 Search PubMed.
- M. Sun, J. Deng, Z. Tang, J. Wu, D. Li, H. Chen and C. Gao, Colloids Surf., B, 2014, 122, 134–142 Search PubMed.
- W. Kong, Y. Gao, Q. Liu, L. Dong, L. Guo, H. Fan, Y. Fan and X. Zhang, Int. J. Biol. Macromol., 2020, 160, 1201–1211 Search PubMed.
- L. Münster, Z. Capáková, M. Fišera, I. Kuřitka and J. Vícha, Carbohydr. Polym., 2019, 218, 333–342 Search PubMed.
- M. Chen, D. Fan, S. Liu, Z. Rao, Y. Dong, W. Wang, H. Chen, L. Bai and Z. Cheng, Polym. Chem., 2019, 10, 503–511 Search PubMed.
- H. Bakhshandeh, M. Soleimani, S. S. Hosseini, H. Hashemi, I. Shabani, A. Shafiee, A. H. Nejad, M. Erfan, R. Dinarvand and F. Atyabi, Int. J. Nanomed., 2011, 6, 1509–1515 Search PubMed.
- H. Yu, Y. Jia, C. Yao and Y. Lu, Int. J. Pharm., 2014, 469, 17–22 Search PubMed.
- J. Li, F. Yu, G. Chen, J. Liu, X. L. Li, B. Cheng, X. M. Mo, C. Chen and J. F. Pan, ACS Appl. Mater. Interfaces, 2020, 12, 2023–2038 Search PubMed.
- W. Liu, W. Bi, Y. Sun, L. Wang, X. Yu, R. Cheng, Y. Yu and W. Cui, Mater. Sci. Eng., C, 2020, 110, 110670 Search PubMed.
- S. K. Alavi, O. Lotz, B. Akhavan, G. Yeo, R. Walia, D. R. McKenzie and M. M. Bilek, ACS Appl. Mater. Interfaces, 2020, 12, 38730–38743 Search PubMed.
- X. Jing, H. Y. Mi, Y. J. Lin, E. Enriquez, X. F. Peng and L. S. Turng, ACS Appl. Mater. Interfaces, 2018, 10, 20897–20909 Search PubMed.
- S. Liu, Z. Rao, R. Wu, Z. Sun, Z. Yuan, L. Bai, W. Wang, H. Yang and H. Chen, J. Agric. Food Chem., 2019, 67, 1061–1071 Search PubMed.
- A. Shagan, W. Zhang, M. Mehta, S. Levi, D. S. Kohane and B. Mizrahi, Adv. Funct. Mater., 2019, 30, 1900998 Search PubMed.
- M. Lopez-Carrizales, E. Mendoza-Mendoza, R. D. Peralta-Rodriguez, M. A. Perez-Diaz, D. Portales-Perez, M. Magana-Aquino, A. Aragon-Pina, R. Infante-Martinez, E. D. Barriga-Castro, R. Sanchez-Sanchez, G. A. Martinez-Castanon and F. Martinez-Gutierrez, Colloids Surf., B, 2020, 196, 111292 Search PubMed.
- H. Yang, C. Li, J. Tang and Z. Suo, ACS Appl. Bio Mater., 2019, 2, 1781–1786 Search PubMed.
- L. Li, C. Wang, Q. Huang, J. Xiao, Q. Zhang and Y. Cheng, J. Mater. Chem. B, 2018, 6, 2474–2480 Search PubMed.
- W. Wang, S. Liu, B. Chen, X. Yan, S. Li, X. Ma and X. Yu, Biomacromolecules, 2019, 20, 3672–3683 Search PubMed.
- X. Li, B. Zou, N. Zhao, C. Wang, Y. Du, L. Mei, Y. Wang, S. Ma, X. Tian, J. He, A. Tong, L. Zhou, B. Han and G. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 24469–24479 Search PubMed.
- M. Mehdizadeh, H. Weng, D. Gyawali, L. Tang and J. Yang, Biomaterials, 2012, 33, 7972–7983 Search PubMed.
- Z. Zeng, X. M. Mo, C. He, Y. Morsi, H. El-Hamshary and M. El-Newehy, J. Mater. Chem. B, 2016, 4, 5585–5592 Search PubMed.
- D. Bermejo-Velasco, S. Kadekar, M. V. Tavares da Costa, O. P. Oommen, K. Gamstedt, J. Hilborn and O. P. Varghese, ACS Appl. Mater. Interfaces, 2019, 11, 38232–38239 Search PubMed.
- Y. C. Chan, J. S. Choi, Y. J. Jung and Y. W. Cho, J. Mater. Chem. B, 2014, 2, 201–209 Search PubMed.
- N. Bhattarai, J. Gunn and M. Zhang, Adv. Drug Delivery Rev., 2010, 62, 83–99 Search PubMed.
- L. Mayol, F. Quaglia, A. Borzacchiello, L. Ambrosio and M. Rotonda, Eur. J. Pharm. Biopharm., 2008, 70, 199–206 Search PubMed.
- F. Lee, J. E. Chung and M. Kurisawa, J. Controlled Release, 2009, 134, 186–193 Search PubMed.
- T. Ito, I. P. Fraser, Y. Yeo, C. B. Highley, E. Bellas and D. S. Kohane, Biomaterials, 2007, 28, 1778–1786 Search PubMed.
- A. Skardal, J. Zhang and G. D. Prestwich, Biomaterials, 2010, 31, 6173–6181 Search PubMed.
- T. Chen, D. A. Small, M. K. McDermott, W. E. Bentley and G. F. Payne, Biomacromolecules, 2003, 4, 1558–1563 Search PubMed.
- J. I. Kang and K. M. Park, J. Mater. Chem. B, 2021, 1503–1520, 10.1039/d0tb02582h.
- W. Hu, Z. Zhang, S. Lu, T. Zhang, N. Zhou, P. Ren, F. Wang, Y. Yang and Z. Ji, Biomater. Sci., 2018, 6, 3030–3041 Search PubMed.
- Z. Shao, X. Hu, W. Cheng, Y. Zhao, J. Hou, M. Wu, D. Xue and Y. Wang, Nanoscale, 2020, 12, 18771–18781 Search PubMed.
- Y. Lee, J. W. Bae, D. H. Oh, K. M. Park, Y. W. Chun, H. J. Sung and K. D. Park, J. Mater. Chem. B, 2013, 1, 2407–2414 Search PubMed.
- H. Jiang, S. Qin, H. Dong, Q. Lei, X. Su, R. Zhuo and Z. Zhong, Soft Matter, 2015, 11, 6029–6036 Search PubMed.
- P. M. Kharkar, K. L. Kiick and A. M. Kloxin, Polym. Chem., 2015, 6, 5565–5574 Search PubMed.
- C. E. Brubaker and P. B. Messersmith, Biomacromolecules, 2011, 12, 4326–4334 Search PubMed.
- Z. Sun, C. Song, C. Wang, Y. Hu and J. Wu, Mol. Pharmaceutics, 2020, 17, 373–391 Search PubMed.
- L. H. Fu, C. Qi, M. G. Ma and P. Wan, J. Mater. Chem. B, 2019, 7, 1541–1562 Search PubMed.
- D. Das and S. Pal, RSC Adv., 2015, 5, 25014–25050 Search PubMed.
- Y. P. Singh, N. Bhardwaj and B. B. Mandal, ACS Appl. Mater. Interfaces, 2016, 8, 21236–21249 Search PubMed.
- C. S. Lee, H. S. Hwang, S. Kim, J. Fan, T. Aghaloo and M. Lee, Adv. Funct. Mater., 2020, 30, 2003717 Search PubMed.
- Y. Gao, H. Du, Z. Xie, M. Li, J. Zhu, J. Xu, L. Zhang, J. Tao and J. Zhu, J. Mater. Chem. B, 2019, 7, 3644–3651 Search PubMed.
- L. Wang, X. Zhang, K. Yang, Yu V. Fu, T. Xu, S. Li, D. Zhang, L.-N. Wang and C.-S. Lee, Adv. Funct. Mater., 2020, 30, 1904156 Search PubMed.
- M. N. Sundaram, V. K. Kaliannagounder, V. Selvaprithiviraj, M. K. Suresh, R. Biswas, A. K. Vasudevan, P. K. Varma and a. R. Jayakumar, ACS Sustainable Chem. Eng., 2018, 6, 7826–7840 Search PubMed.
- J. Liao and Z. Ye, Electrochim. Acta, 2018, 259, 626–636 Search PubMed.
- S. Salatin and M. Jelvehgari, Drug Dev. Ind. Pharm., 2020, 46, 1318–1333 Search PubMed.
- X. Ke, Z. Dong, S. Tang, W. Chu, X. Zheng, L. Zhen, X. Chen, C. Ding, J. Luo and J. Li, Biomater. Sci., 2020, 8, 4346–4357 Search PubMed.
- R. Wang, D.-l. Xu, L. Liang, T.-t. Xu, W. Liu, P.-k. Ouyang, B. Chi and H. Xu, RSC Adv., 2016, 6, 8620–8627 Search PubMed.
- C.-C. Liu, H. Xu, L. Wang and X. Qin, Acta Metall. Sin., 2016, 30, 36–44 Search PubMed.
- Y. Yan, Y. Li, Z. Zhang, X. Wang, Y. Niu, S. Zhang, W. Xu and C. Ren, Colloids Surf., B, 2021, 202, 111682 Search PubMed.
- Y. Wang, Y. Lu, J. Zhang, X. Hu, Z. Yang, Y. Guo and Y. Wang, J. Mater. Chem. B, 2019, 7, 538–547 Search PubMed.
- I. A. Khalil, B. Saleh, D. M. Ibrahim, C. Jumelle, A. Yung, R. Dana and N. Annabi, Biomater. Sci., 2020, 8, 5196–5209 Search PubMed.
- J. Xie, Y. Zuo, J. Lv, T. Jiang, C. Liu, H. Xu, L. Wang, S. Gao, C. Ma and J. Jin, Mater. Technol., 2018, 33, 532–542 Search PubMed.
- S. N. Ramirez-Barron, S. Sanchez-Valdes, R. Betancourt, C. A. Gallardo, B. Puente-Urbina, O. S. Rodriguez-Fernández, M. G. Carneiro-da Cunha, M. T. dos Santos-Correia and Z. V. Sanchez-Martinez, Int. J. Adhes. Adhes., 2021, 104, 102749 Search PubMed.
- X. Han, X. Ji, M. Zhao and D. Li, Surf. Coat. Technol., 2020, 397, 126020 Search PubMed.
- X. Du, Y. Hou, L. Wu, S. Li, A. Yu, D. Kong, L. Wang and G. Niu, J. Mater. Chem. B, 2020, 8, 5682–5693 Search PubMed.
- J. Hoque, R. G. Prakash, K. Paramanandham, B. R. Shome and J. Haldar, Mol. Pharmaceutics, 2017, 14, 1218–1230 Search PubMed.
- C. Pagano, M. R. Ceccarini, P. Calarco, S. Scuota, C. Conte, S. Primavilla, M. Ricci and L. Perioli, Colloids Surf., B, 2019, 178, 488–499 Search PubMed.
- H. A. Khan, A. Ahmad and R. Mehboob, Asian Pac. J. Trop. Biomed., 2015, 5, 509–514 Search PubMed.
- Y. Zhong, H. Xiao, F. Seidi and Y. Jin, Biomacromolecules, 2020, 21, 2983–3006 Search PubMed.
- L. Shi, Y. Zhao, Q. Xie, C. Fan, J. Hilborn, J. Dai and D. A. Ossipov, Adv. Healthcare Mater., 2018, 7, 1700973 Search PubMed.
- H. Chen, X. Xing, H. Tan, Y. Jia, T. Zhou, Y. Chen, Z. Ling and X. Hu, Mater. Sci. Eng., C, 2017, 70, 287–295 Search PubMed.
- X. Zhao, H. Wu, B. Guo, R. Dong, Y. Qiu and P. X. Ma, Biomaterials, 2017, 122, 34–47 Search PubMed.
- E. L. Park, J. B. Ulreich, K. M. Scott, N. P. Ullrich, J. A. Linehan, M. H. French, W. Y. Ho, M. J. White, J. R. Talley, A. M. Fellah and S. Ramakumar, J. Urol., 2004, 172, 2446–2450 Search PubMed.
- A. M. Behrens, M. J. Sikorski, T. Li, Z. J. Wu, B. P. Griffith and P. Kofinas, Acta Biomater., 2014, 10, 701–708 Search PubMed.
- S. Li, N. Chen, X. Li, Y. Li, Z. Xie, Z. Ma, J. Zhao, X. Hou and X. Yuan, Adv. Funct. Mater., 2020, 30, 2000130 Search PubMed.
- D. Gan, W. Xing, L. Jiang, J. Fang, C. Zhao, F. Ren, L. Fang, K. Wang and X. Lu, Nat. Commun., 2019, 10, 1487 Search PubMed.
- J. Deng, Y. Tang, Q. Zhang, C. Wang, M. Liao, P. Ji, J. Song, G. Luo, L. Chen, X. Ran, Z. Wei, L. Zheng, R. Dang, X. Liu, H. Zhang, Y. S. Zhang, X. Zhang and H. Tan, Adv. Funct. Mater., 2019, 29, 1809110 Search PubMed.
- Y. S. Zhang and A. Khademhosseini, Science, 2017, 356, eaaf3627 Search PubMed.
- J. E. Hudak and C. R. Bertozzi, Chem. Biol., 2014, 21, 16–37 Search PubMed.
- F. Fu, Z. Chen, Z. Zhao, H. Wang, L. Shang, Z. Gu and Y. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 5900–5905 Search PubMed.
- W. A. Sarhan, H. M. Azzazy and I. M. El-Sherbiny, ACS Appl. Mater. Interfaces, 2016, 8, 6379–6390 Search PubMed.
- J. Boateng and O. Catanzano, J. Pharm. Sci., 2015, 104, 3653–3680 Search PubMed.
- D. R. Griffin, W. M. Weaver, P. O. Scumpia, D. Di Carlo and T. Segura, Nat. Mater., 2015, 14, 737–744 Search PubMed.
- M. Yadollahi, H. Namazi and M. Aghazadeh, Biol. Macromol., 2015, 79, 269–277 Search PubMed.
- R. Dong, X. Zhao, B. Guo and P. X. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 17138–17150 Search PubMed.
- U. H. Choudry, S. L. Moran, S. Li and S. Khan, Plast. Reconstr. Surg., 2007, 119, 1852–1857 Search PubMed.
- S. Li, L. Wang, W. Zheng, G. Yang and X. Jiang, Adv. Funct. Mater., 2020, 30, 2002370 Search PubMed.
- D. Fan, W. Wang, H. Chen, L. Bai, H. Yang, D. Wei, L. Yang, Z. Xue and Y. Niu, New J. Chem., 2019, 43, 3099–3110 Search PubMed.
- J. C. Wheat and J. S. Wolf Jr., Urol. Clin. North Am., 2009, 36, 265–275 Search PubMed.
- P. Behrendt, Y. Ladner, M. J. Stoddart, S. Lippross, M. Alini, D. Eglin and A. R. Armiento, Am. J. Sports Med., 2020, 48, 210–221 Search PubMed.
- E. S. Sani, R. P. Lara, Z. Aldawood, S. H. Bassir, D. Nguyen, A. Kantarci, G. Intini and N. Annabi, Matter, 2019, 1, 926–944 Search PubMed.
- Y. Shu, T. Hao, F. Yao, Y. Qian, Y. Wang, B. Yang, J. Li and C. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6505–6517 Search PubMed.
- L. Han, M. Wang, P. Li, D. Gan, L. Yan, J. Xu, K. Wang, L. Fang, C. W. Chan, H. Zhang, H. Yuan and X. Lu, ACS Appl. Mater. Interfaces, 2018, 10, 28015–28026 Search PubMed.
- J. H. Ryu, J. S. Choi, E. Park, M. R. Eom, S. Jo, M. S. Lee, S. K. Kwon and H. Lee, J. Controlled Release, 2020, 317, 57–66 Search PubMed.
- M. W. Grinstaff, Biomaterials, 2007, 28, 5205–5214 Search PubMed.
- A. Hasan, A. Khattab, M. A. Islam, K. A. Hweij, J. Zeitouny, R. Waters, M. Sayegh, M. M. Hossain and A. Paul, Adv. Sci., 2015, 2, 1500122 Search PubMed.
- T. Hao, J. Li, F. Yao, D. Dong, Y. Wang, B. Yang and C. Wang, ACS Nano, 2017, 11, 5474–5488 Search PubMed.
- C. Cui, T. Wu, X. Chen, Y. Liu, Y. Li, Z. Xu, C. Fan and W. Liu, Adv. Funct. Mater., 2020, 30, 2005689 Search PubMed.
- H.-H. Park, M. Seong, K. Sun, H. Ko, S. M. Kim and H. E. Jeong, ACS Macro Lett., 2017, 6, 1325–1330 Search PubMed.
- P. Zhao, K. Wei, Q. Feng, H. Chen, D. S. H. Wong, X. Chen, C. C. Wu and L. Bian, Chem. Commun., 2017, 53, 12000–12003 Search PubMed.
- J. Shin, J. S. Lee, C. Lee, H.-J. Park, K. Yang, Y. Jin, J. H. Ryu, K. S. Hong, S.-H. Moon, H.-M. Chung, H. S. Yang, S. H. Um, J.-W. Oh, D.-I. Kim, H. Lee and S.-W. Cho, Adv. Funct. Mater., 2015, 25, 3814–3824 Search PubMed.
- K. Wei, X. Chen, P. Zhao, Q. Feng, B. Yang, R. Li, Z. Y. Zhang and L. Bian, ACS Appl. Mater. Interfaces, 2019, 11, 16328–16335 Search PubMed.
- X. Xu, X. Xia, K. Zhang, A. Rai, Z. Li, P. Zhao, K. Wei, L. Zou, B. Yang, W.-K. Wong, P. W.-Y. Chiu and L. Bian, Sci. Transl. Med., 2020, 12, eaba8014 Search PubMed.
- S. Bian, Z. Zheng, Y. Liu, C. Ruan, H. Pan and X. Zhao, J. Mater. Chem. B, 2019, 7, 6488–6499 Search PubMed.
- H. Zhang, A. Patel, A. K. Gaharwar, S. M. Mihaila, G. Iviglia, S. Mukundan, H. Bae, H. Yang and A. Khademhosseini, Biomacromolecules, 2013, 14, 1299–1310 Search PubMed.
- I. A. Dang, A. Kousar, J. Liu, V. Mukwaya, C. Zhao, F. Wang, L. Hou and C. L. Feng, ACS Appl. Mater. Interfaces, 2019, 11, 28657–28664 Search PubMed.
- Z. Ming, X. Ruan, C. Bao, Q. Lin, Y. Yang and L. Zhu, Adv. Funct. Mater., 2017, 27, 1606258 Search PubMed.
- Q. Xu, Z. Zhang, C. Xiao, C. He and X. Chen, Biomacromolecules, 2017, 18, 1411–1418 Search PubMed.
- H. Ajiro, J. Watanabe and M. Akashi, Biomacromolecules, 2008, 9, 426–430 Search PubMed.
- S. Pacelli, P. Paolicelli, S. Petralito, S. Subham, D. Gilmore, G. Varani, G. Yang, D. Lin, M. A. Casadei and A. Paul, ACS Appl. Bio Mater., 2020, 3, 945–951 Search PubMed.
- K. A. Gerbin, K. A. Mitzelfelt, X. Guan, A. M. Martinson and C. E. Murry, Mol. Ther. – Methods Clin. Dev., 2020, 17, 986–998 Search PubMed.
- N. F. Truong, E. Kurt, N. Tahmizyan, S. C. Lesher-Perez, M. Chen, N. J. Darling, W. Xi and T. Segura, Acta Biomater., 2019, 94, 160–172 Search PubMed.
- C. M. Gonzalez-Henriquez, S. C. Galleguillos-Guzman, M. A. Sarabia-Vallejos, A. Santos-Coquillat, E. Martinez-Campos and J. Rodriguez-Hernandez, Mater. Sci. Eng., C, 2019, 103, 109872 Search PubMed.
- M. Hasany, A. Thakur, N. Taebnia, F. B. Kadumudi, M.-A. Shahbazi, M. K. Pierchala, S. Mohanty, G. Orive, T. L. Andresen, C. B. Foldager, S. Yaghmaei, A. Arpanaei, A. K. Gaharwar, M. Mehrali and A. Dolatshahi-Pirouz, ACS Appl. Mater. Interfaces, 2018, 10, 34924–34941 Search PubMed.
- V. Moulisova, S. Poveda-Reyes, E. Sanmartin-Masia, L. Quintanilla-Sierra, M. Salmeron-Sanchez and G. G. Ferrer, ACS Omega, 2017, 2, 7609–7620 Search PubMed.
- S. Bhutani, A. L. Y. Nachlas, M. E. Brown, T. Pete, C. T. Johnson, A. J. Garcia and M. E. Davis, ACS Biomater. Sci. Eng., 2018, 4, 200–210 Search PubMed.
- E. A. Phelps, N. O. Enemchukwu, V. F. Fiore, J. C. Sy, N. Murthy, T. A. Sulchek, T. H. Barker and A. J. Garcia, Adv. Mater., 2012, 24, 64–70 Search PubMed.
- L. Li, Y. Zhang, J. Mu, J. Chen, C. Zhang, H. Cao and J. Gao, Nano Lett., 2020, 20, 4298–4305 Search PubMed.
- X. Zhang, Y. He, P. Huang, G. Jiang, M. Zhang, F. Yu, W. Zhang, G. Fu, Y. Wang, W. Li and H. Zeng, Composites, Part B, 2020, 197, 108183 Search PubMed.
- J. Kim, A. S. Campbell, B. E.-F. de Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 Search PubMed.
- S. Wagner and S. Bauer, MRS Bull., 2012, 37, 207–213 Search PubMed.
- W. Jayathilaka, K. Qi, Y. Qin, A. Chinnappan, W. Serrano-Garcia, C. Baskar, H. Wang, J. He, S. Cui, S. W. Thomas and S. Ramakrishna, Adv. Mater., 2019, 31, 1805921 Search PubMed.
- L. Wang, T. Xu and X. Zhang, TrAC, Trends Anal. Chem., 2021, 134, 116130 Search PubMed.
- N. Rajabi, M. Kharaziha, R. Emadi, A. Zarrabi, H. Mokhtari and S. Salehi, J. Colloid Interface Sci., 2020, 564, 155–169 Search PubMed.
- L. Han, L. Yan, M. Wang, K. Wang, L. Fang, J. Zhou, J. Fang, F. Ren and X. Lu, Chem. Mater., 2018, 30, 5561–5572 Search PubMed.
- L. Han, K. Liu, M. Wang, K. Wang, L. Fang, H. Chen, J. Zhou and X. Lu, Adv. Funct. Mater., 2018, 28, 1704195 Search PubMed.
- J. Chen, Q. Peng, T. Thundat and H. Zeng, Chem. Mater., 2019, 31, 4553–4563 Search PubMed.
- X. Di, Y. Kang, F. Li, R. Yao, Q. Chen, C. Hang, Y. Xu, Y. Wang, P. Sun and G. Wu, Colloids Surf., B, 2019, 177, 149–159 Search PubMed.
- Q. Zhang, X. Liu, L. Duan and G. Gao, Chem. Eng. J., 2019, 365, 10–19 Search PubMed.
- X. Wei, K. Ma, Y. Cheng, L. Sun, D. Chen, X. Zhao, H. Lu, B. Song, K. Yang and P. Jia, ACS Appl. Polym. Mater., 2020, 2, 2541–2549 Search PubMed.
- S. Liu, R. Zheng, S. Chen, Y. Wu, H. Liu, P. Wang, Z. Deng and L. Liu, J. Mater. Chem. C, 2018, 6, 4183–4190 Search PubMed.
- X. Zhang, J. Chen, J. He, Y. Bai and H. Zeng, J. Colloid Interface Sci., 2021, 585, 420–432 Search PubMed.
- C. Xie, X. Wang, H. He, Y. Ding and X. Lu, Adv. Funct. Mater., 2020, 30, 1909954 Search PubMed.
- R. Lv, Z. Bei, Y. Huang, Y. Chen, Z. Zheng, Q. You, C. Zhu and Y. Cao, Macromol. Rapid Commun., 2020, 41, 1900450 Search PubMed.
- Q. Zhang, X. Liu, L. Duan and G. Gao, J. Mater. Chem. A, 2020, 8, 4515–4523 Search PubMed.
- D. Gan, Z. Huang, X. Wang, L. Jiang, C. Wang, M. Zhu, F. Ren, L. Fang, K. Wang, C. Xie and X. Lu, Adv. Funct. Mater., 2019, 30, 1907678 Search PubMed.
- J. Zhang, L. Wan, Y. Gao, X. Fang, T. Lu, L. Pan and F. Xuan, Adv. Electron. Mater., 2019, 5, 1900285 Search PubMed.
- C. J. Bettinger, K. M. Cyr, A. Matsumoto, R. Langer, J. T. Borenstein and D. L. Kaplan, Adv. Mater., 2007, 19, 2847–2850 Search PubMed.
- B. Zhu, H. Wang, W. R. Leow, Y. Cai, X. J. Loh, M.-Y. Han and X. Chen, Adv. Mater., 2016, 28, 4250–4265 Search PubMed.
- H. Yang, S. Ji, I. Chaturvedi, H. Xia, T. Wang, G. Chen, L. Pan, C. Wan, D. Qi, Y.-S. Ong and X. Chen, ACS Mater. Lett., 2020, 2, 478–484 Search PubMed.
- X. Liu, Q. Zhang, L. Duan and G. Gao, Adv. Funct. Mater., 2019, 29, 1900450 Search PubMed.
- H. Gao, Z. Zhao, Y. Cai, J. Zhou, W. Hua, L. Chen, L. Wang, J. Zhang, D. Han, M. Liu and L. Jiang, Nat. Commun., 2017, 8, 15911 Search PubMed.
- F. Chen, D. Zhou, J. Wang, T. Li, X. Zhou, T. Gan, S. Handschuh-Wang and X. Zhou, Angew. Chem., Int. Ed., 2018, 57, 6568–6571 Search PubMed.
- X. Liu, Q. Zhang and G. Gao, Chem. Eng. J., 2020, 394, 124898 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |