Open Access Article
Open Access ArticleContinuous dimethyldioxirane generation for polymer epoxidation†
Grace P.
Ahlqvist
 ,
Eileen G.
Burke‡
,
Jeremiah A.
Johnson
,
Eileen G.
Burke‡
,
Jeremiah A.
Johnson
 * and
Timothy F.
Jamison
* and
Timothy F.
Jamison
 *
*
Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA, USA. E-mail: jaj2109@mit.edu; tfj@mit.edu
First published on 19th January 2021
Abstract
Post-polymerization modification of commodity polymers yields new applications for materials already produced industrially. Incorporation of small amounts of epoxides into unsaturated polymers such as polybutadiene expands their use for grafting and compatibilization applications, but controlled epoxidation of these polymers in a safe, scalable manner presents a challenge. Herein we describe the development of a reactor for the continuous flow generation and use of dimethyldioxirane (DMDO) and its application to the low-level epoxidation of unsaturated polymers. A continuous stirred tank reactor (CSTR) prevents reactor clogging by allowing solid precipitates to settle, enabling the pumping of a homogeneous solution of oxidant. Modification of relative concentrations, flow rates, and temperatures achieves variable epoxidation levels. This method has been demonstrated on gram scale.
Post-polymerization modification vastly expands the properties and applications of simple commodity polymers such as polyolefins.1,2 Polymer epoxidation can alter properties such as solubility, aggregation, and thermal stability, as well as providing functional handles for further modification.3–5 Low epoxidation levels are particularly useful for compatibilization and grafting applications, as minimal backbone alteration preserves polymer properties while providing functional handles for novel modifications. For example, pure poly(lactic acid) (PLA) is brittle at room temperature, but can be strengthened by blending with rubber. However, since PLA is immiscible with common rubbers like polybutadiene (PBD), modifications allowing reactive compatibilization improve the properties of the resultant polymer blend.6 Sun et al. report PLA toughening from acrylonitrile-butadiene-styrene (ABS) rubber containing a small (1%) amount of glycidyl methacrylate (GMA), which contains epoxides that react with PLA end groups.7 Similar effects have been observed for PLA toughened with methyl methacrylate-butadiene block copolymers with added GMA.8
Direct use of epoxidized PBD removes the need for copolymerization with a compatibilizer like GMA, and low-level epoxidation of polybutadiene is particularly valuable, as high epoxidation levels cause an increase in glass transition temperature that negatively impact the toughness of the resulting PLA blends.9 Unfortunately, typical peracid epoxidation methods3,4 necessitate storage and use of hydrogen peroxide or an organic peroxide reagent, which is costly and hazardous on scale.10 Safe, sustainable epoxidation methods are desirable for the large-scale production of polymer with low-level epoxide incorporation.
To circumvent the issues of storing hydrogen peroxide solutions, dimethyldioxirane (DMDO) can be employed. DMDO epoxidation has been demonstrated for unsaturated polymers in batch.11–14 DMDO is generated from acetone and solid, commercially available Oxone® (active ingredient potassium monoperoxysulfate) in a buffered aqueous solution.15 Due to the inherent instability and hazards of storage and use of organic peroxides, DMDO is typically prepared in situ or immediately before use. Its preparation is low-yielding and requires a hazardous distillation procedure to isolate it as a solution in acetone.16,17 On-demand generation of DMDO is desirable for large-scale applications, such as polymer oxidation at industrial scale, to avoid the hazards of using large amounts of organic peroxides.
Reports of dioxirane generation in continuous flow are promising, as synthesis in flow is ideally suited for on-demand generation and use of hazardous reagents.18,19 The first use of DMDO continuous flow was reported in 2018 by McCluskey et al., who epoxidized a variety of alkenes in 60–98% yield.20 However, the use of acetone as solvent limits the generality of this method for substrates such as higher olefin polymers that are insoluble in acetone. Methyl(trifluoromethyl)dioxirane (TFDO) has also been generated in continuous flow and used for aliphatic C–H oxidation.21 Unfortunately, the use of acetone to replace trifluoroacetone in the system reportedly caused precipitation. A robust, scalable protocol for DMDO generation and use in continuous flow for epoxidation of nonpolar polymers has yet to be developed.
We envisioned a modular continuous flow reactor in which DMDO would be generated, reacted with a polymer dissolved in organic solvent, and then quenched at the output. This system design both minimizes the amount of DMDO present at any one time in the system, and allows tuning of experimental variables such as temperature and residence time so different levels of epoxide incorporation can be targeted. In particular, we focused on low (<20%) levels of epoxide incorporation for the purposes of this investigation, due to the compatibilization and grafting applications mentioned above.
Our initial design plan combined three inputs (Oxone® solution, base solution, and acetone solution) in a cross-mixer to make DMDO, and then added the polymer solution at a T-mixer (Fig. S1, ESI†). However, we observed rapid formation of white solids that led to clogging in this system. We hypothesized that the solid precipitation was due to the high concentrations of inorganic salts combined with acetone at the cross-mixer. Oxone® is a triple salt, and it contains one mole of less soluble, inactive salts (KHSO4, K2SO4) for every one mole of active salt (KHSO5). We sought a method to mix the reagents for DMDO formation that would allow a homogeneous oxidant solution to be pumped forward without clogging due to the precipitation of undesired salts.
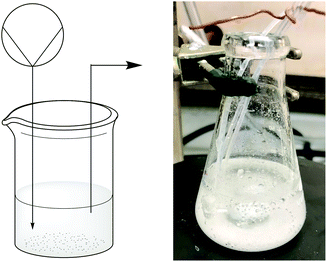
Handling solid–liquid mixtures in continuous flow is an ongoing challenge in the field.22 To address this challenge in our system, we designed a simple continuous stirred tank reactor (CSTR) that would pre-mix the reagents to produce DMDO while allowing the undesired solids to settle to the bottom (Fig. 1).
Reagents were pumped via syringe pump into a small tank (a flask, stoppered syringe, test tube, or other vessel of similar size). Under slow stirring, the undesired solids settled to the bottom of the reactor. The liquid phase of the mixture, containing a solution of water, acetone, base, Oxone®, and DMDO, could then be pumped forward to react with the polymers. We quickly learned that filtration was necessary to ensure that finely divided solids did not clog the tubing; a simple piece of filter paper tied to the tubing using Teflon tape was sufficient for this purpose (see Fig. S2 in ESI†). Using common materials (flasks, filter paper, Teflon tape), a simple CSTR efficiently pre-mixed the reagents for DMDO formation while preventing solids from clogging narrow-diameter tubing and fittings.
DMDO is typically generated using NaHCO3 as a base to buffer the aqueous solution, as DMDO is formed most efficiently at neutral or slightly alkaline pH.15 However, we observed that using sodium bicarbonate to generate DMDO in flow caused precipitation of sodium salts and evolution of carbon dioxide gas. Excess precipitation of salts caused clogging, while gas evolution and cavitation from clogging caused inconsistencies in residence time due to the formation of gas slugs in the plug flow reactor. While the CSTR improves solid handling, generating less solid overall aids in the performance of the system over long runs and avoids wasting reagents that are added but precipitate out due to solubility issues. Thus, we sought to identify a superior base for the generation of DMDO in continuous flow (Table 1).
| Entry | Base | pHa | Gas evolutionb | Solid formationb |
|---|---|---|---|---|
| a pH in CSTR tested by pH strip after system equilibration. b Gas and solid observed and compared qualitatively. | ||||
| 1 | 0.7 M Na2CO3 | 5.0 | High | High |
| 2 | 0.5 M K2HPO4 | 5.5 | Low | Low |
| 3 | 1.0 M K2HPO4 | 6.0 | Medium | Medium |
| 4 | 0.5 M K3PO4 | 6.0 | Low | Low |
| 5 | 1.0 M K3PO4 | 7.0 | Medium | Medium |
While 1.0 M K3PO4 yielded the highest pH at steady state (entry 5), further reactions with this base concentration showed no epoxidation, possibly due to the degradation of Oxone®. Thus, 0.5 M K3PO4 (entry 4) was chosen, yielding a pH of 6.0 in the CSTR and producing a minimal amount of solid and gaseous byproducts.
We then modified the setup to contain only two inputs rather than three to use fewer pumps and minimize the amount of excess salt introduced into the system. Our goal was to combine the base and acetone reagents into one solution. Due to the limited solubility of inorganic bases in acetone, high concentrations of base in acetone–water mixtures induced phase separation in the stock solutions. After screening for stability of the base–acetone mixture and pH of the resultant DMDO solution, a solution of 0.33 M K3PO4 in a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mixture of water and acetone was chosen as the ideal balance for mixing with equal amounts of a 0.8 M Oxone® solution.
30 mixture of water and acetone was chosen as the ideal balance for mixing with equal amounts of a 0.8 M Oxone® solution.
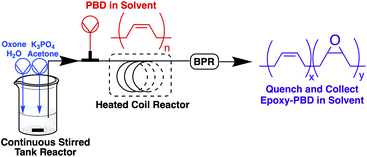
With conditions in hand to prepare DMDO in continuous flow, we combined the DMDO stream with liquid PBD dissolved in an organic solvent. A flow reactor was constructed using a peristaltic pump to pump DMDO from the CSTR to the rest of the system (Fig. 2).
The DMDO solution was combined with the polymer solution using a T-mixer and the biphasic mixture was pumped forward to react in a coil of perfluoroalkoxy polymer (PFA) tubing heated by an oil bath. The system was maintained at a constant pressure using a back-pressure regulator (BPR), allowing for superheating of the solution above the boiling point of acetone.
With a well-functioning flow reactor in hand, we screened solvents and concentrations to determine their influence on the epoxidation of PBD (Table 2).
| Entry | Solvent | [PBD] (g L−1) | Epoxidation (%) |
|---|---|---|---|
Reaction conditions: 70 °C, tR 10 min, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 flow rate ratio of DMDO solution to PBD solution. 1 flow rate ratio of DMDO solution to PBD solution. |
|||
| 1 | Dichloromethane | 30 | 4 |
| 2 | Dichloromethane | 10 | 11 |
| 3 | Toluene | 30 | 3 |
| 4 | Toluene | 10 | 5 |
| 5 | Ethyl Acetate | 10 | 14 |
We observed the expected increase in epoxidation with decreased concentration of PBD at the same DMDO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBD flow rate ratio. Although PBD is less soluble in ethyl acetate than in toluene or dichloromethane, environmental considerations and epoxidation yield favored the use of ethyl acetate for further investigation.
PBD flow rate ratio. Although PBD is less soluble in ethyl acetate than in toluene or dichloromethane, environmental considerations and epoxidation yield favored the use of ethyl acetate for further investigation.
We then screened residence time, temperature, and PBD concentration to determine conditions that could provide a variety of epoxidation yields (Fig. 3).
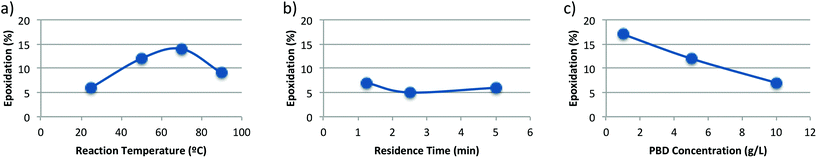 | ||
| Fig. 3 Effect of (a) temperature, (b) residence time, and (c) polymer concentration on epoxide incorporation. Unless otherwise noted, conditions are 25 °C, 5 min tR, and 10 g L−1 PBD in EtOAc. Epoxide incorporation calculated by quantitative NMR, see section 3.1 in ESI† for epoxidation calculations and section 4.1† for additional experimental data. | ||
Gentle heating increased the extent of epoxidation, but higher heat caused a decrease in epoxidation (Fig. 3a). We hypothesize that this decrease is due to instability of DMDO, which is typically stored and used below room temperature.15 Decreasing the residence time from 5 minutes to 1.25 minutes did not impact epoxidation yield in the coil reactor, possibly also due to the limited stability of DMDO (Fig. 3b). Reducing the PBD concentration led to an expected increase in epoxidation (Fig. 3c). The various conditions screened using PFA coil reactors yielded epoxy-PBD with up to 17% epoxidation. Repeated experiments showed reproducible epoxidation values (see section 4.2 in ESI† for details).
We next expanded the substrate scope to include higher molecular weight (MW) PBD. Our previous work used a viscous liquid PBD (average Mn of 1530–2070 g mol−1), while solid polymers with much higher MW are preferred for many industrial applications. We subjected higher MW PBD (average Mw 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–300
000–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) to similar epoxidation conditions as the low MW PBD. Solubility limitations required the use of toluene as solvent, which allowed use of higher concentrations of PBD. Under similar conditions as low MW PBD, the high MW PBD was also successfully epoxidized at various levels using different concentrations of PBD (Table 3). GPC and NMR data indicates clean epoxidation of these polymers without backbone degradation or loss of molecular weight (Fig. 4).
000 g mol−1) to similar epoxidation conditions as the low MW PBD. Solubility limitations required the use of toluene as solvent, which allowed use of higher concentrations of PBD. Under similar conditions as low MW PBD, the high MW PBD was also successfully epoxidized at various levels using different concentrations of PBD (Table 3). GPC and NMR data indicates clean epoxidation of these polymers without backbone degradation or loss of molecular weight (Fig. 4).
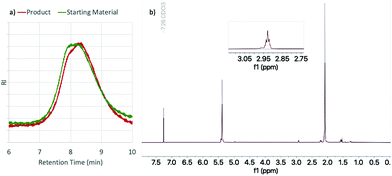 | ||
| Fig. 4 (a) GPC and (b) NMR data for HMW PBD starting material (green/gray) and product (red). See section 7 in ESI† for additional data. | ||
| Entry | [PBD] (g L−1) | t R (min) | Epoxidation (%) |
|---|---|---|---|
Reaction conditions: Room temperature, toluene, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 flow rate ratio of DMDO solution to PBD solution. 1 flow rate ratio of DMDO solution to PBD solution. |
|||
| 1 | 30 | 1 | 1 |
| 2 | 10 | 1.25 | 3 |
| 3 | 1 | 1.25 | 22 |
We also investigated the epoxidation of Z-polyisoprene (PIP, Mw 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), a common unsaturated polymer that is the primary constituent of natural rubber and has desirable properties after epoxidation.23 PIP proved a more accessible substrate, yielding more than twice the epoxide incorporation as PBD under the same reaction conditions (Table 4). This difference is likely due to the greater substitution, and therefore greater nucleophilicity, of the PIP alkene. Indeed, DMDO epoxidation of PIP in the presence of PBD has been achieved in batch due to the chemoselectivity for greater alkene substitution.11 Importantly, GPC data (section 7, ESI†) for all three polymers evaluated show that the epoxidized polymers have similar GPC traces to their non-epoxidized precursor, indicating that the flow method does not mechanically or chemically deteriorate the polymer backbone.
000 g mol−1), a common unsaturated polymer that is the primary constituent of natural rubber and has desirable properties after epoxidation.23 PIP proved a more accessible substrate, yielding more than twice the epoxide incorporation as PBD under the same reaction conditions (Table 4). This difference is likely due to the greater substitution, and therefore greater nucleophilicity, of the PIP alkene. Indeed, DMDO epoxidation of PIP in the presence of PBD has been achieved in batch due to the chemoselectivity for greater alkene substitution.11 Importantly, GPC data (section 7, ESI†) for all three polymers evaluated show that the epoxidized polymers have similar GPC traces to their non-epoxidized precursor, indicating that the flow method does not mechanically or chemically deteriorate the polymer backbone.
| Entry | Polymer | [Polymer] (g L−1) | t R (min) | Epoxidation (%) |
|---|---|---|---|---|
Reaction conditions: Room temperature, ethyl acetate, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 flow rate ratio of DMDO solution to polymer solution. 1 flow rate ratio of DMDO solution to polymer solution. |
||||
| 1 | Low MW PBD | 10 | 1.25 | 7 |
| 2 | PIP | 10 | 1.25 | 17 |
We next worked to produce a larger sample of epoxidized polymer to demonstrate the reaction on gram scale. A new flow reactor was constructed using peristaltic pumps for all reagents, circumventing the limited volume of syringe pumps to allow longer run times and therefore production of larger samples (Fig. S3, ESI†). Reaction time was decreased slightly from 1.25 min to 1 min and flow rates were increased to a total of 2 mL min−1 with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMDO to polymer solution ratio. The larger system was used to produce a 1.28 g sample of 1% epoxidized low MW PBD in a total run-time of 90 minutes (see ESI section 5.4† for full experimental details). These larger-scale experiments correspond to over 0.8 g of product per hour in a benchtop reactor without distilling DMDO or producing large quantities of peroxides at one time. The low polymer concentrations required for higher epoxidation samples constrain the throughput of those polymers in this reactor, but a larger-scale flow reactor, albeit outside the scope of this investigation, would alleviate this constraint.
1 DMDO to polymer solution ratio. The larger system was used to produce a 1.28 g sample of 1% epoxidized low MW PBD in a total run-time of 90 minutes (see ESI section 5.4† for full experimental details). These larger-scale experiments correspond to over 0.8 g of product per hour in a benchtop reactor without distilling DMDO or producing large quantities of peroxides at one time. The low polymer concentrations required for higher epoxidation samples constrain the throughput of those polymers in this reactor, but a larger-scale flow reactor, albeit outside the scope of this investigation, would alleviate this constraint.
A safe and operationally simple continuous flow method for the generation of DMDO has been developed using a bench-scale CSTR. The reactor design prioritizes usability and safety by limiting the amount of DMDO generated at one time and using simple parts. This reactor has been applied to the oxidation of unsaturated polymers, including PBD and PIP. The system described herein provides advantages for the safety, scalability, and modularity of polyolefin epoxidation. Further investigations may include increased scale and throughput and targeting higher epoxide incorporation for different applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank DOW Chemical Company for research funding and Dr Patrick Heider (DOW) for helpful discussions. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1122374 (GPA).References
- E. Blasco, M. B. Sims, A. S. Goldmann, B. S. Sumerlin and C. Barner-Kowollik, Macromolecules, 2017, 50, 5215–5252 CrossRef CAS.
- K. A. Günay, P. Theato and H.-A. Klok, J. Polym. Sci., Part A: Polym. Chem., 2017, 51, 5215–5252 Search PubMed.
- J. H. Bradbury and M. C. S. Perera, Ind. Eng. Chem. Res., 1988, 27, 2196–2203 CrossRef CAS.
- J. C. Brosse, I. Campistron, D. Derouet, A. E. Hamdaoui, S. Houdayer, D. Reyx and S. Ritoit-Gillier, J. Appl. Polym. Sci., 2000, 78, 1461–1477 CrossRef CAS.
- J.-P. Dilcher, H. Jürgens and G. A. Luinstra, Multi-Component and Sequential Reactions in Polymer Synthesis, Springer International Publishing, Cham, 2015, pp. 163–201 Search PubMed.
- K. Hamad, M. Kaseem, M. Ayyoob, J. Joo and F. Deri, Prog. Polym. Sci., 2018, 85, 83–127 CrossRef CAS.
- S. Sun, M. Zhang, H. Zhang and X. Zhang, J. Appl. Polym. Sci., 2011, 122, 2992–2999 CrossRef CAS.
- Y. Hao, H. Liang, J. Bian, S. Sun, H. Zhang and L. Dong, Polym. Int., 2014, 63, 660–666 CrossRef CAS.
- Y. Wang, Z. Wei, X. Leng, K. Shen and Y. Li, Polym. J., 2016, 92, 74–83 CAS.
- H. P. L. Gemoets, Y. Su, M. Shang, V. Hessel, R. Luque and T. Noël, Chem. Soc. Rev., 2016, 45, 83–117 RSC.
- R. B. Grubbs, M. E. Broz, J. M. Dean and F. S. Bates, Macromolecules, 2000, 33, 2308–2310 CrossRef CAS.
- M. M. A. Nikje, S. Motahari, M. Haghshenas and R. K. Sanami, J. Macromol. Sci., Part A: Pure Appl.Chem., 2006, 43, 1205–1214 CrossRef.
- M. M. A. Nikje, A. Rafiee and M. Haghshenas, Des. Monomers Polym., 2006, 9, 293–303 CrossRef CAS.
- M. M. A. Nikje and Z. Mozaffari, Des. Monomers Polym., 2008, 11, 271–281 CrossRef.
- J. K. Crandall, R. Curci, L. D'Accolti and C. Fusco, Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2005 Search PubMed.
- H. Mikula, D. Svatunek, D. Lumpi, F. Glocklhöfer, C. Hametner and J. Fröhlich, Org. Process Res. Dev., 2013, 17, 350–357 CrossRef.
- D. F. Taber, P. W. DeMatteo and R. A. Hassan, Org. Synth., 2013, 90, 350–357 CrossRef CAS.
- M. Movsisyan, E. I. P. Delbeke, J. K. E. T. Berton, C. Battilocchio, S. V. Ley and C. V. Stevens, Chem. Soc. Rev., 2016, 45, 4892–4928 RSC.
- M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef CAS.
- P. J. Cossar, J. R. Baker, N. Cain and A. McCluskey, R. Soc. Open Sci., 2018, 5, 171190 CrossRef.
- M. Lesieur, C. Battilocchio, R. Labes, J. Jacq, C. Genicot, S. V. Ley and P. Pasau, Chem. – Eur. J., 2018, 1203–1207 Search PubMed.
- R. L. Hartman, Org. Process Res. Dev., 2012, 16, 870–887 CrossRef CAS.
- Y. Heping, L. Sidong and P. Zheng, J. Therm. Anal. Calorim., 1999, 58, 293–299 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0py01676d |
| ‡ Present address: 3M Company, 2501 Hudson Rd, Maplewood, MN 55144. |
| This journal is © The Royal Society of Chemistry 2021 |