Core–shell gold nanorod@mesoporous-MOF heterostructures for combinational phototherapy†
Zehao
Zhou
ab,
Jian
Zhao
bc,
Zhenghan
Di
bc,
Bei
Liu
b,
Zhaohui
Li
 d,
Xuemin
Wu
d,
Xuemin
Wu
 *a and
Lele
Li
*a and
Lele
Li
 *bc
*bc
aInnovation Center of Pesticide Research, Department of Applied Chemistry, College of Science, China Agricultural University, Beijing, 100193, China. E-mail: wuxuemin@cau.edu.cn
bCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190 China. E-mail: lilele@nanoctr.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
dCollege of Chemistry, Zhengzhou University, Zhengzhou 450001, China
First published on 7th December 2020
Abstract
Despite the increasing usage of porphyrinic metal–organic frameworks (MOFs) for combination therapy, the controlled encapsulation of inorganic nanoparticle-based therapeutics into such MOFs with specific structures has remained a major obstacle for improved tumor therapy. Here, we report the synthesis of a mesoporous MOF shell on the surface of gold nanorods (AuNRs), wherein a single AuNR is captured individually in single-crystalline MOFs with a controlled crystallographic orientation, for combinational phototherapy against solid tumors. The core–shell heterostructures have the benefits of a mesoporous structure and photoinduced singlet oxygen generation behavior characterized by the porphyrinic MOF shell, together with the plasmonic photothermal conversion characteristic of AuNRs. We demonstrated that the AuNR@MOF nanoplatform enables an efficient tumor treatment strategy by combining photodynamic therapy and photothermal therapy. We should emphasize that such systems could have applications beyond the field of cancer therapy, like plasmonic harvesting of light energy to induce and accelerate catalytic reactions within MOFs and multifunctional nanocarriers for agricultural formulations.
Introduction
Due to their high spatiotemporal selectivity, light-triggered therapies (phototherapies) such as photodynamic therapy (PDT) and photothermal therapy (PTT) show great potential for disease treatment.1–5 Metal–organic frameworks (MOFs), constructed by the coordination-mediated assembly of metal ions/clusters and organic linkers,6–9 have been extensively explored for applications in catalysis,10–16 gas storage/separation17–20 and cancer therapy.21–24 Recently, porphyrinic nanoscale MOFs (nMOFs)25–29 have shown great potential as nanophotosensitizers (nPSs) for PDT.30–39 The use of extra nanocarriers can be circumvented in such structures, thereby resulting in a high loading capacity of porphyrinic PSs.30,31 In addition, the unique porous structures of nMOFs not only prevent the aggregation-induced quenching effect of PSs, but also allow for more opportunity to react with molecular oxygen nearby, leading to enhanced PDT efficacy over traditional nPSs.31 However, these nMOF-based PDT systems are limited by the requirement of visible light to excite the porphyrins, which hindered in vivo and clinical applications due to poor tissue penetration. To tackle these issues, we recently reported the synthesis of heterostructures composed of porphyrinic nMOFs and lanthanide-doped upconversion nanoparticles for near-infrared (NIR) light-induced PDT.40–42 Alternatively, many efforts have been focused on the incorporation of additional therapeutic modalities into the nMOF-based PDT platforms to improve their anti-tumor outcomes.43–49 In particular, bifunctional PTT/PDT agents are more promising for oncotherapies due to their enhanced efficacy.50–54Recently, we reported the trace water mediated growth of oriented single-crystalline mesoporous MOFs on the surface of gold nanorods (AuNRs).55 Herein, we explore the potential of the core–shell AuNR@MOF heterostructures with an individual AuNR as the core and mesoporous porphyrinic MOFs as the shell for combinational PDT and PTT against tumors (Scheme 1). Because of their outstanding photothermal conversion ability, AuNRs have been widely used as photothermal agents.56–60 We demonstrate that combining porphyrinic MOFs and plasmonic AuNRs into a single heterostructure enables efficient wavelength-dependent light harvesting for photothermal conversion and singlet oxygen production. Furthermore, AuNR@MOFs show more efficient antitumor efficacy compared with those using PDT or PTT alone both in vitro and in vivo.
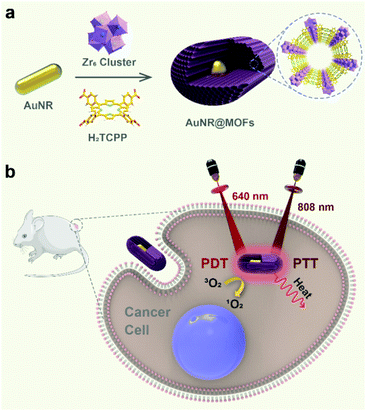 | ||
| Scheme 1 (a) Schematic illustration of the synthesis of AuNR@MOFs and (b) the use of AuNR@MOFs for dual light-activated combinational phototherapy. | ||
Results and discussion
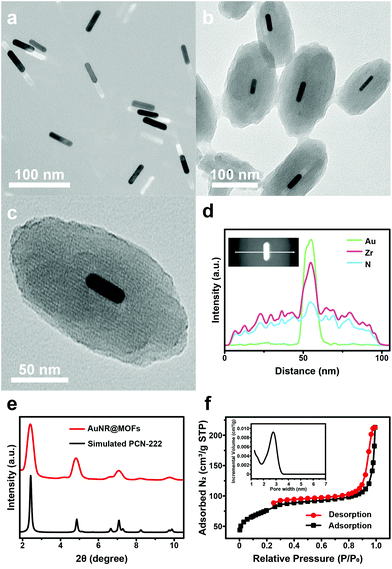
Transmission electron microscopy (TEM) showed that the as-synthesized AuNRs were well dispersed with an average size of ∼10.0 × 41.4 nm (width × length) (Fig. 1a). In order to stabilize AuNRs in the growth solution of MOFs, the capping ligands (cetyltrimethylammonium bromide) of AuNRs were exchanged with polyvinylpyrrolidone (PVP). The PVP-coated AuNRs (PVP-AuNRs) were stable in either the aqueous phase or the N,N-dimethylformamide (DMF) phase (Fig. S1†). Then, MOFs were grown on PVP-AuNRs in the presence of benzoic acid (BA), zirconyl chloride octahydrate (ZrOCl2·8H2O), 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (H2TCPP) and trace amounts of water (1% v/v). Upon reaction in DMF at 90 °C for 3 h, purple heterostructures were obtained after centrifugation and washing with DMF.TEM images of the products showed that AuNRs were coated with spindle-like MOFs, resulting in core–shell heterostructures with an average size of ∼102.7 × 186.8 nm (width × length) (Fig. 1b and Fig. S2†). The high-resolution TEM (HRTEM) image indicates that AuNR@MOFs possessed a single-crystalline and highly oriented mesoporous structure (Fig. 1c). Further evidence for the formation of the core–shell structure is provided by characterization with the energy-dispersive X-ray spectroscopy (EDS) line scan (Fig. 1d) and high-angle annular dark-field scanning transmission electron microscopy energy-dispersive X-ray spectroscopy (HAADF-STEM-EDS) element mapping (Fig. S3†). Then, the crystal structure of AuNR@MOFs was evaluated by small-angle X-ray scattering (SAXS) and powder X-ray diffraction (PXRD). The SAXS pattern of AuNR@MOFs shows a perfect match with the standard pattern of PCN-222 MOFs (Fig. 1e), confirming that the shell of AuNR@MOFs is PCN-222 MOFs. In the PXRD pattern of AuNR@MOFs, all the visible peaks can be indexed to the face-centered cubic (fcc) Au (111), (200), (220) and (311) face (Fig. S4†). These results confirmed that AuNR@MOFs contained highly crystalline AuNRs coated with PCN-222 MOF shells. In addition, nitrogen (N2) adsorption and desorption curves verified the mesoporous structure of AuNR@MOFs (Fig. 1f). The pore size calculated by the DFT method is up to 2.73 nm. It is noted that AuNR@MOFs could maintain a monodisperse status in water over 24 h (Fig. S5†), which means that the nanoparticles (NPs) possess relative stability.
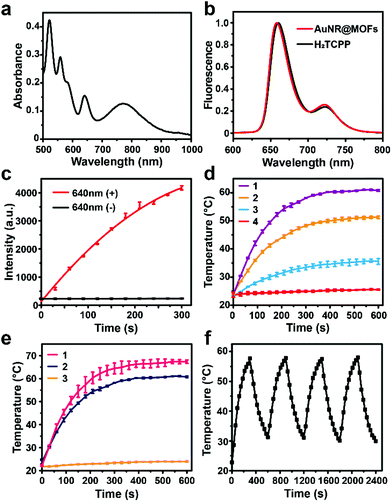
The UV-vis absorption spectrum of AuNR@MOFs exhibits the representative absorption peaks of both PCN-222 MOFs and AuNRs (Fig. 2a). The bands at 522, 558, 580 and 640 nm could be attributed to the typical four Q-bands of porphyrinic MOFs, while the peak at 810 nm was assigned to the longitudinal surface plasmon resonance (LSPR) of AuNRs. For the fluorescence spectra, AuNR@MOFs show quite the same tendency as H2TCPP under 420 nm excitation (Fig. 2b), indicating that the optical properties of the porphyrin-based ligands are maintained.
The singlet oxygen (1O2) generation induced by AuNR@MOFs was then evaluated by using an 1O2 indicator (SOSG), which can be oxidized by 1O2 to increase their fluorescence. Upon exposure to LED light (640 nm, 20 mW cm−2), the fluorescence intensity of SOSG in the solution of AuNR@MOFs increased with increasing irradiation time, yet remained unchanged without irradiation (Fig. 2c). As a control, PVP-coated AuNRs did not show any effect for light-triggered 1O2 production (Fig. S6†). We also found that the singlet oxygen generation efficiency of AuNR@MOFs was similar to that of MOF NPs (Fig. S7†). Next, we investigated the photothermal performance of AuNR@MOFs by irradiating them with an 808 nm NIR laser at varied power densities (Fig. 2d). Laser irradiation increased the solution temperature rapidly during the first 3 min and then the temperature reached a plateau within 5 min. The plateau temperature increased from 35 to 60 °C as the irradiance power was increased from 0.25 to 0.75 W cm−2. In contrast, the temperature of MOF NPs exhibited no obvious change upon 808 nm irradiation (Fig. 2e), indicating that the presence of AuNRs in the heterostructure can efficiently convert NIR light into thermal energy. To evaluate the photothermal stability of AuNR@MOFs, the recycling temperature variations of the solution of AuNR@MOFs were measured under NIR irradiation for 5 min followed by cooling to room temperature. AuNR@MOFs exhibited no significant deterioration in the photothermal capability during the four irradiation/cooling cycles (Fig. 2f), highlighting their potential as a durable photothermal agent. Furthermore, the photothermal conversion efficiency of AuNR@MOFs was calculated as 22.5% (Fig. S8†), which indicated that the as-prepared AuNR@MOFs had relatively high photothermal conversion efficiency.
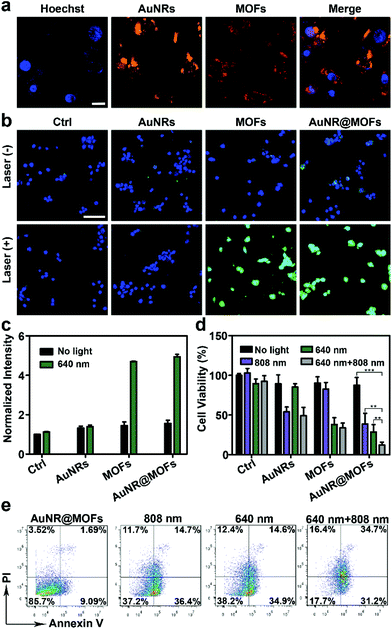
Inspired by the wavelength-dependent photoinduced cytotoxic 1O2 generation and photothermal effect of AuNR@MOFs, we explored their use for combined PDT and PTT. To assess the cellular uptake of AuNR@MOFs, we incubated them with 4T1 breast cancer cells at 37 °C for 2 h. The resulting cells exhibited remarkable fluorescence of MOFs, which colocalized well with the light scattering signals from AuNRs (Fig. 3a), indicating the efficient internalization of the heterostructures by the tumor cells. We then examined intracellular 1O2 generation by AuNR@MOFs using dichlorofluorescein diacetate (DCF-DA), which can generate green fluorescence upon oxidation into dichlorofluorescein (DCF) by 1O2. Confocal fluorescence images show that strong green fluorescence was emitted from the 4T1 cells treated with AuNR@MOFs or MOF NPs under 640 nm irradiation, whereas negligible fluorescence was observed in the cells that were treated with PVP-AuNRs followed by 640 nm irradiation (Fig. 3b). The heterostructure-mediated 1O2 production inside the cells was further quantitatively measured by flow cytometry assays (Fig. 3c). The cells treated with AuNR@MOFs and 640 nm irradiation showed 3.3-fold higher DCF fluorescence when compared with the cells incubated with AuNR@MOFs but without irradiation. In addition, control studies using PVP-AuNRs under 640 nm irradiation exerted much less effect on 1O2 production.
AuNR@MOFs showed good biocompatibility when examined by the CCK-8 assay (Fig. S9†). Then, the photoinduced cell damage was evaluated (Fig. 3d). Treatment with only irradiation or AuNR@MOFs resulted in insignificant effects on the cell viability, suggesting the negligible cytotoxicity of irradiation and AuNR@MOFs to 4T1 cells. In contrast, significant cytotoxicity was observed after treatment with AuNR@MOFs under 640 nm or 808 nm irradiation, confirming their effect as PDT and PTT agents, respectively. Notably, the incubation of the cells with AuNR@MOFs following both 640 nm and 808 nm irradiation led to much higher cytotoxicity than that of the cells treated with AuNR@MOFs under only one-wavelength light irradiation, indicating that an enhanced potency could be achieved through the combination of PTT and PDT. The other control groups including the 4T1 cells with AuNRs + 640 nm + 808 nm and MOF NPs + 640 nm + 808 nm exhibited moderate effects on the cell viability. The results were further confirmed by the Annexin V-FITC/PI apoptosis detection assay (Fig. 3e). AuNR@MOFs with both 640 nm and 808 nm irradiation evoked the least level of healthy cells (17.7%) among all the groups.
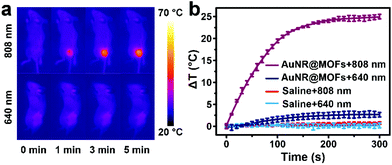
Encouraged by the good performance of this system in vitro, we further carried out in vivo experiments to investigate its anti-tumor activity. To explore the hyperthermia in tumors, 4T1 breast tumor-bearing mice were treated with AuNR@MOFs and subjected to 808 nm (0.5 W cm−2) or 640 nm (0.1 W cm−2) irradiation at 2 h post-injection. The tumor-site temperature, recorded using an infrared thermal camera, rapidly increased by ∼25 °C after 5 min of 808 nm irradiation, while a slight temperature increase was observed under 640 nm irradiation (Fig. 4a and b). In comparison, the controls with saline showed a negligible temperature change under 640 or 808 nm irradiation (Fig. 4b).
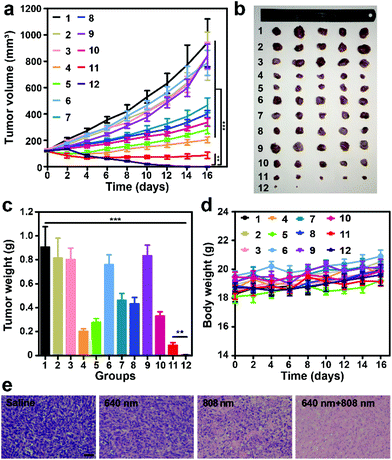
We then investigated the photoinduced antitumor efficacy of AuNR@MOFs. As shown in Fig. 5a, there was no statistically significant difference in the final tumor size or weight for mice in the groups of saline, saline + 640 nm + 808 nm, and AuNR@MOFs, indicating that these treatments had no effect on tumor growth. The treatment of AuNRs + 640 nm + 808 nm or nMOFs + 640 nm + 808 nm had moderate antitumor capability. The tumor growth of mice treated with AuNR@MOFs + 808 nm and AuNR@MOFs + 640 nm was efficiently delayed due to PTT and PDT effects, respectively, while in the group treated with AuNR@MOFs + 808 nm + 640 nm, the tumors were completely eradicated without further reoccurrence after the treatment, indicating that the combination of PTT and PDT was more effective than either modality alone (Fig. 5b and c). The treatment showed no significant change in mice body weight (Fig. 5d). The hematoxylin and eosin (H&E) staining results further confirmed that the combined treatment led to highly significant necrosis of tumor cells compared to the control groups (Fig. 5e and Fig. S10†).
Conclusions
In conclusion, we report the synthesis of porphyrinic MOF-based heterostructures for combinational tumor therapy. The heterostructure was obtained with high yields through a straightforward approach. The integration of plasmonic and porphyrinic components in one mesoscopic construct enabled wavelength-dependent light harvesting for photothermal conversion and singlet oxygen generation. We demonstrated that the heterostructure represents a promising platform to combine PDT and PTT for enhanced tumor treatment. Currently, most MOFs reported possess pores in the microporous range that are inaccessible to larger molecular catalysts or drugs. In contrast, mesoporous MOFs in our system will enable the accommodation of large functional molecules, expanding their applications in photocatalysis, drug delivery, and agriculture.Experimental
Chemicals
Cetyltrimethylammonium bromide (CTAB, ≥99%) was bought from Acros. Gold(III) chloride trihydrate (HAuCl4·3H2O, 99%) was bought from Macklin. N,N-Dimethylformamide (DMF) was bought from J&K. 5,10,15,20-Tetrakis(4-carboxyphenyl)porphyrin (H2TCPP) was bought from TCI. Benzoic acid (BA, 99.5%), silver nitrate (AgNO3, 99.8%), and sodium borohydride (NaBH4, 98%) were purchased from Aladdin. Polyvinylpyrrolidone (PVP, M.W. = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), L-ascorbic acid (AA, ≥99%), and zirconyl chloride octahydrate (ZrOCl2·8H2O, 98%) were purchased from Sigma-Aldrich. Deionized water (Millipore Milli-Q grade) with a resistivity of 18.2 MΩ was used. All chemicals were used as received without further purification.
000), L-ascorbic acid (AA, ≥99%), and zirconyl chloride octahydrate (ZrOCl2·8H2O, 98%) were purchased from Sigma-Aldrich. Deionized water (Millipore Milli-Q grade) with a resistivity of 18.2 MΩ was used. All chemicals were used as received without further purification.
Instrumentation
Transmission electron microscopy (TEM) images were obtained on an HT7700 transmission electron microscope (Hitachi Co. Ltd, Japan) with an accelerating voltage of 120 kV. UV-vis spectra were recorded on a Hitachi 5300 spectrophotometer (Hitachi Co. Ltd, Japan). Fluorescence spectra were recorded on a Hitachi F-4600 spectrometer (Hitachi Co. Ltd, Japan). Powder X-ray diffraction patterns were measured on a D/MAX-TTRIII(CBO) X-ray diffractometer (Rigaku Co. Ltd, Japan) at 40 kV and 200 mA. Small angle X-ray diffraction patterns were measured on a Xeuss SAXS/WAXS system (XENOCS Co. Ltd, France). Infrared-radiation (IR) thermal images were monitored through an E40 infrared-radiation thermal camera (FLIR Systems Inc., America). Confocal microscopy images were obtained using a Zeiss LSM 710 confocal microscope (Carl Zeiss Co. Ltd, Germany). Flow cytometry analysis was performed on a BD Accuri™ C6 flow cytometer (BD Biosciences Co. Ltd, America).Synthesis of AuNR@MOFs
The gold nanorods were prepared via the well-established seed-mediated growth method.61–63 AuNR@MOFs were synthesized according to a reported approach.55 Typically, a 20 μL volume of a concentrated aqueous solution of PVP-AuNRs (0.45 μM) was added to 2 mL of a DMF solution containing H2TCPP (2.0 mg, 2.5 μmol), ZrOCl2·8H2O (6.0 mg, 18.6 μmol) and benzoic acid (56 mg, 0.46 mmol) in a 5 mL round-bottom flask. After brief sonication, the mixture was heated at 90 °C for 5 h. Finally, the products were collected by centrifugation, washed with DMF three times and re-dispersed in 1 mL DMF for further use. Pure MOF NPs were synthesized under the same reaction conditions but without adding the concentrated aqueous solution of PVP-AuNRs.Singlet oxygen generation
SOSG stock solution (5 μL, 5 mM) was added into AuNR@MOF solution (1 mL, 5 μg mL−1). The solution was kept in the dark for a while, and then irradiated with a 640 nm laser (0.1 W cm−2). The fluorescence of SOSG was measured.Cell culture and intracellular ROS detection
Murine 4T1 breast cancer cells were seeded in chamber slides (for imaging) or 6-well plates (for flow cytometry) at a density of 4 × 105 cells per well in RPMI 1640 supplemented with 10% FBS and 1% penicillin–streptomycin at 37 °C in a CO2 incubator, and grown overnight to allow for cell adherence. The medium was replaced with fresh Opti-MEM containing AuNR@MOFs, AuNRs, and MOF NPs (MOFs equivalent 10 μg mL−1). After 2 h of incubation in the dark, the cells were washed to remove excess materials. Fresh culture medium containing DCF-DA (10 μM) was added. After incubation for 30 min in the dark, the cells were washed and irradiated with a 640 nm laser for 5 min (0.1 W cm−2). The post-treatment cells were administered for fluorescence imaging and flow cytometric analysis.Cell apoptosis assessment
4T1 cells were seeded in 6-well plates at a density of 4 × 105 cells per well for 24 h. The medium was replaced with fresh Opti-MEM medium containing different samples (MOFs equivalent 20 μg mL−1 and AuNRs equivalent 9 × 10−11 mol L−1). After 2 h of incubation, the cells were washed and replaced with fresh culture medium. The cells were irradiated with a 640 nm laser (0.1 W cm−2, 5 min), an 808 nm NIR laser (0.5 W cm−2, 5 min) or both. After another 6 h of incubation, the cells were stained with Annexin V and PI using the Annexin V-APC Apoptosis Detection Kit (BioLegend) according to the manufacturer's instructions for flow cytometric analysis.Cell viability assay
Cells were seeded in a 96-well plate at a density of 2 × 104 cells per well beforehand. After 24 h of incubation, the cells were treated with different samples (MOFs equivalent 20 μg mL−1) for 2 h, and then washed and replaced with fresh culture medium. After irradiation with the indicated light as mentioned above, the cells were maintained for another 24 h. Finally, 100 μL fresh culture medium containing 10% CCK-8 was added to each well for 2 h, and the absorbance was measured at 450 nm using an Epoch2 microplate reader. The cells treated with PBS served as a control.In vivo tumor therapy
All animal experiments were performed in compliance with the relevant laws or guidelines of laboratory animals of the Ministry of Science and Technology of the People's Republic of China (Beijing), and were approved by the Institutional Animal Care and Use Committee (IACUC) of the National Center for Nanoscience and Technology (NCNST). The female BALB/c mice (17–19 g) brought from Beijing Vital River Laboratory Animal Technology Co. Ltd were used to set up the breast tumor model. 4T1 tumor cells (1 × 106 cells per 100 μL, PBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Matrigel = 1
Matrigel = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)) were subcutaneously inoculated in the back of the mouse. Once the tumor volume reached 50–100 mm3, the mice were randomly divided into 12 groups for the different treatments (1: Saline, 2: Saline + 640 nm + 808 nm, 3: PVP-AuNRs, 4: PVP-AuNRs + 808 nm, 5: PVP-AuNRs + 640 nm + 808 nm, 6: MOF NPs, 7: MOF NPs + 640 nm, 8: MOF NPs + 640 nm + 808 nm, 9: AuNR@MOFs, 10: AuNR@MOFs + 640 nm, 11: AuNR@MOFs + 808 nm, and 12: AuNR@MOFs + 640 nm + 808 nm) (MOFs equivalent 12 mg kg−1 and AuNRs equivalent 5.62 × 10−11 mol kg−1). For the PTT and PDT treatment, light irradiation was performed at the tumor site (808 nm, 0.5 W cm−2, 5 min; 640 nm, 0.1 W cm−2, 10 min) 2 h post-injection. Then, the tumor volume and the body weight were measured every other day. The tumor volume was calculated using the following equation: tumor volume = length × width × width/2.
1 (v/v)) were subcutaneously inoculated in the back of the mouse. Once the tumor volume reached 50–100 mm3, the mice were randomly divided into 12 groups for the different treatments (1: Saline, 2: Saline + 640 nm + 808 nm, 3: PVP-AuNRs, 4: PVP-AuNRs + 808 nm, 5: PVP-AuNRs + 640 nm + 808 nm, 6: MOF NPs, 7: MOF NPs + 640 nm, 8: MOF NPs + 640 nm + 808 nm, 9: AuNR@MOFs, 10: AuNR@MOFs + 640 nm, 11: AuNR@MOFs + 808 nm, and 12: AuNR@MOFs + 640 nm + 808 nm) (MOFs equivalent 12 mg kg−1 and AuNRs equivalent 5.62 × 10−11 mol kg−1). For the PTT and PDT treatment, light irradiation was performed at the tumor site (808 nm, 0.5 W cm−2, 5 min; 640 nm, 0.1 W cm−2, 10 min) 2 h post-injection. Then, the tumor volume and the body weight were measured every other day. The tumor volume was calculated using the following equation: tumor volume = length × width × width/2.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the NSFC (No. 21822401, 21771044, and 21805060) and the Young Thousand Talents Program.Notes and references
- C. Feng, J. Ouyang, Z. Tang, N. Kong, Y. Liu, L. Fu, X. Ji, T. Xie, O. C. Farokhzad and W. Tao, Matter, 2020, 3, 127–144 CrossRef.
- J. Ouyang, X. Ji, X. Zhang, C. Feng, Z. Tang, N. Kong, A. Xie, J. Wang, X. Sui, L. Deng, Y. Liu, J. S. Kim, Y. Cao and W. Tao, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 28667–28677 CrossRef CAS PubMed.
- B. Liu, R. Ma, J. Zhao, Y. Zhao and L. Li, Sci. China: Chem., 2020, 63, 1490–1497 CrossRef CAS.
- Z. Di, B. Liu, J. Zhao, Z. Gu, Y. Zhao and L. Li, Sci. Adv., 2020, 6, eaba9381 CrossRef CAS PubMed.
- J. Ouyang, C. Feng, X. Ji, L. Li, H. K. Gutti, N. Y. Kim, D. Artzi, A. Xie, N. Kong, Y. N. Liu, G. J. Tearney, X. Sui, W. Tao and O. C. Farokhzad, Angew. Chem., Int. Ed., 2019, 58, 13405–13410 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- C. He, D. Liu and W. Lin, Chem. Rev., 2015, 115, 11079–11108 CrossRef CAS PubMed.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- A. J. Howarth, Y. Liu, P. Li, Z. Li, T. C. Wang, J. T. Hupp and O. K. Farha, Nat. Rev. Mater., 2016, 1, 15018 CrossRef CAS.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC.
- L. Ma, J. M. Falkowski, C. Abney and W. Lin, Nat. Chem., 2010, 2, 838–846 CrossRef CAS PubMed.
- D. Feng, Z. Y. Gu, J. R. Li, H. L. Jiang, Z. Wei and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed.
- T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982–5993 RSC.
- T. A. Goetjen, J. Liu, Y. Wu, J. Sui, X. Zhang, J. T. Hupp and O. K. Farha, Chem. Commun., 2020, 56, 10409–10418 RSC.
- W. Zhao, G. Li and Z. Tang, Nano Today, 2019, 27, 178–197 CrossRef CAS.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- J. R. Li, J. Sculley and H. C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed.
- J. R. Li, J. Yu, W. Lu, L. B. Sun, J. Sculley, P. B. Balbuena and H. C. Zhou, Nat. Commun., 2013, 4, 1538 CrossRef PubMed.
- X. Wang, W. Xu, J. Gu, X. Yan, Y. Chen, M. Guo, G. Zhou, S. Tong, M. Ge, Y. Liu and C. Chen, Nanoscale, 2019, 11, 17782–17790 RSC.
- C. He, K. Lu, D. Liu and W. Lin, J. Am. Chem. Soc., 2014, 136, 5181–5184 CrossRef CAS PubMed.
- X. Lian, Y. Huang, Y. Zhu, Y. Fang, R. Zhao, E. Joseph, J. Li, J. P. Pellois and H. C. Zhou, Angew. Chem., Int. Ed., 2018, 57, 5725–5730 CrossRef CAS PubMed.
- G. Lan, K. Ni and W. Lin, Coord. Chem. Rev., 2019, 379, 65–81 CrossRef CAS PubMed.
- H. Zhang, Q. Zhang, C. Liu and B. Han, Biomater. Sci., 2019, 7, 1696–1704 RSC.
- S. Yuan, J. S. Qin, J. Li, L. Huang, L. Feng, Y. Fang, C. Lollar, J. Pang, L. Zhang, D. Sun, A. Alsalme, T. Cagin and H. C. Zhou, Nat. Commun., 2018, 9, 808 CrossRef PubMed.
- D. Feng, W. C. Chung, Z. Wei, Z. Y. Gu, H. L. Jiang, Y. P. Chen, D. J. Darensbourg and H. C. Zhou, J. Am. Chem. Soc., 2013, 135, 17105–17110 CrossRef CAS PubMed.
- H. J. Son, S. Jin, S. Patwardhan, S. J. Wezenberg, N. C. Jeong, M. So, C. E. Wilmer, A. A. Sarjeant, G. C. Schatz, R. Q. Snurr, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 862–869 CrossRef CAS PubMed.
- D. E. Williams, J. A. Rietman, J. M. Maier, R. Tan, A. B. Greytak, M. D. Smith, J. A. Krause and N. B. Shustova, J. Am. Chem. Soc., 2014, 136, 11886–11889 CrossRef CAS PubMed.
- J. Park, Q. Jiang, D. Feng, L. Mao and H. C. Zhou, J. Am. Chem. Soc., 2016, 138, 3518–3525 CrossRef CAS PubMed.
- K. Lu, C. He, N. Guo, C. Chan, K. Ni, R. R. Weichselbaum and W. Lin, J. Am. Chem. Soc., 2016, 138, 12502–12510 CrossRef CAS PubMed.
- G. Lan, K. Ni, Z. Xu, S. S. Veroneau, Y. Song and W. Lin, J. Am. Chem. Soc., 2018, 140, 5670–5673 CrossRef CAS PubMed.
- J. Park, D. Feng, S. Yuan and H. C. Zhou, Angew. Chem., Int. Ed., 2015, 54, 430–435 CrossRef CAS PubMed.
- K. Lu, C. He and W. Lin, J. Am. Chem. Soc., 2015, 137, 7600–7603 CrossRef CAS PubMed.
- G. Lan, K. Ni, R. Xu, K. Lu, Z. Lin, C. Chan and W. Lin, Angew. Chem., Int. Ed., 2017, 56, 12102–12106 CrossRef CAS PubMed.
- K. Lu, C. He and W. Lin, J. Am. Chem. Soc., 2014, 136, 16712–16715 CrossRef CAS PubMed.
- R. Freund, U. Lachelt, T. Gruber, B. Ruhle and S. Wuttke, ACS Nano, 2018, 12, 2094–2105 CrossRef CAS.
- K. Ni, T. Luo, G. Lan, A. Culbert, Y. Song, T. Wu, X. Jiang and W. Lin, Angew. Chem., 2020, 59, 1108–1112 CrossRef CAS PubMed.
- M. Lismont, L. Dreesen and S. Wuttke, Adv. Funct. Mater., 2017, 27, 1606314 CrossRef.
- K. Ni, T. Aung, S. Li, N. Fatuzzo, X. Liang and W. Lin, Chem, 2019, 5, 1892–1913 CAS.
- Y. Li, Z. Di, J. Gao, P. Cheng, C. Di, G. Zhang, B. Liu, X. Shi, L. D. Sun, L. Li and C. H. Yan, J. Am. Chem. Soc., 2017, 139, 13804–13810 CrossRef CAS PubMed.
- C. Liu, B. Liu, J. Zhao, Z. Di, D. Chen, Z. Gu, L. Li and Y. Zhao, Angew. Chem., 2020, 59, 2634–2638 CrossRef CAS.
- Y. Shao, B. Liu, Z. Di, G. Zhang, L. D. Sun, L. Li and C. H. Yan, J. Am. Chem. Soc., 2020, 142, 3939–3946 CrossRef CAS PubMed.
- J. Liu, Y. Yang, W. Zhu, X. Yi, Z. Dong, X. Xu, M. Chen, K. Yang, G. Lu, L. Jiang and Z. Liu, Biomaterials, 2016, 97, 1–9 CrossRef CAS PubMed.
- Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang, Y. Huang, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 12, 651–661 CrossRef CAS PubMed.
- X. Cai, B. Liu, M. Pang and J. Lin, Dalton Trans., 2018, 47, 16329–16336 RSC.
- Y. Liu, W. Hou, L. Xia, C. Cui, S. Wan, Y. Jiang, Y. Yang, Q. Wu, L. Qiu and W. Tan, Chem. Sci., 2018, 9, 7505–7509 RSC.
- H. Min, J. Wang, Y. Qi, Y. Zhang, X. Han, Y. Xu, J. Xu, Y. Li, L. Chen, K. Cheng, G. Liu, N. Yang, Y. Li and G. Nie, Adv. Mater., 2019, 31, 1808200 CrossRef PubMed.
- K. Zhang, X. Meng, Y. Cao, Z. Yang, H. Dong, Y. Zhang, H. Lu, Z. Shi and X. Zhang, Adv. Funct. Mater., 2018, 28, 1804634 CrossRef.
- Z. He, Y. Dai, X. Li, D. Guo, Y. Liu, X. Huang, J. Jiang, S. Wang, G. Zhu, F. Zhang, L. Lin, J. J. Zhu, G. Yu and X. Chen, Small, 2019, 15, 1804131 CrossRef PubMed.
- L. Luo, W. Sun, Y. Feng, R. Qin, J. Zhang, D. Ding, T. Shi, X. Liu, X. Chen and H. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 12591–12599 CrossRef PubMed.
- K. Hu, L. Xie, Y. Zhang, M. Hanyu, Z. Yang, K. Nagatsu, H. Suzuki, J. Ouyang, X. Ji, J. Wei, H. Xu, O. C. Farokhzad, S. H. Liang, L. Wang, W. Tao and M. R. Zhang, Nat. Commun., 2020, 11, 2778 CrossRef CAS PubMed.
- X. Ji, Y. Kang, J. Ouyang, Y. Chen, D. Artzi, X. Zeng, Y. Xiao, C. Feng, B. Qi, N. Y. Kim, P. E. Saw, N. Kong, O. C. Farokhzad and W. Tao, Adv. Sci., 2019, 6, 1901211 CrossRef CAS PubMed.
- Y. Huang, N. He, Y. Wang, D. Shen, Q. Kang, R. Zhao and L. Chen, J. Mater. Chem. B, 2019, 7, 1149–1159 RSC.
- W. T. Huang, M. H. Chan, X. Chen, M. Hsiao and R. S. Liu, Theranostics, 2020, 10, 782–796 CrossRef CAS PubMed.
- Z. Zhou, M. Li, J. Zhao, Z. Di, C. Di, B. Liu, C. Zhang, C. H. Yan and L. Li, Chem. Commun., 2018, 54, 8182–8185 RSC.
- W. Zhang, Y. Wang, X. Sun, W. Wang and L. Chen, Nanoscale, 2014, 6, 14514–14522 RSC.
- Y. Huang, Q. Liu, Y. Wang, N. He, R. Zhao, J. Choo and L. Chen, Nanoscale, 2019, 11, 12220–12229 RSC.
- H. Chen, T. Ming, L. Zhao, F. Wang, L. D. Sun, J. Wang and C. H. Yan, Nano Today, 2010, 5, 494–505 CrossRef CAS.
- N. Goswami, F. Lin, Y. Liu, D. T. Leong and J. Xie, Chem. Mater., 2016, 28, 4009–4016 CrossRef CAS.
- J. Jin, M. Ovais and C. Chen, Nano Today, 2018, 22, 83–99 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065–4067 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- E. Carbo-Argibay, B. Rodriguez-Gonzalez, J. Pacifico, I. Pastoriza-Santos, J. Perez-Juste and L. M. Liz-Marzan, Angew. Chem., Int. Ed., 2007, 46, 8983–8987 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nr07681c |
| This journal is © The Royal Society of Chemistry 2021 |