Open Access Article
Open Access ArticleCatalytic polymersomes to produce strong and long-lasting bioluminescence†
Claire Elsa
Meyer
,
Ioana
Craciun
,
Cora-Ann
Schoenenberger
 ,
Riccardo
Wehr
and
Cornelia Gabriela
Palivan
,
Riccardo
Wehr
and
Cornelia Gabriela
Palivan
 *
*
Department of Chemistry, University of Basel, Mattenstrasse 24a, Basel-4002, Switzerland. E-mail: cornelia.palivan@unibas.ch
First published on 14th December 2020
Abstract
Here, we introduce an artificial bioluminescent nanocompartment based on the encapsulation of light-producing enzymes, luciferases, inside polymersomes. We exploit nanocompartmentalization to enhance luciferase stability in a cellular environment but also to positively modulate enzyme kinetics to achieve a long-lasting glow type signal. These features pave the way for expanding bioluminescence to nanotechnology-based applications.
Bioluminescence, described as the production of light resulting from enzyme-catalyzed reactions,1 is distinguished from other spectroscopy methods that rely on fluorescence or absorbance by superior sensitivity and a lower background. Bioluminescent systems possess unique advantages such as no light excitation requirement (as opposed to fluorophores), particularly strong signal output, and low background due to the lack of endogenous bioluminescent reactions in mammalian cells. Bioluminescence has been extensively exploited to develop in vitro and in vivo assays based on the real-time detection of light emitting enzymes (luciferases).2,3 However, because the majority of luciferases have a short half-life or do not produce long-lived light signals, bioluminescence applications are largely restricted to genetically engineered cells with a constant expression of luciferase.4,5 Although high, but short-lived burst signals, i.e. flash-type kinetics afford high sensitivity, they are extremely complex to implement in an assay format, as special logistics and equipment (e.g. injectors) are required to not miss the signal.6
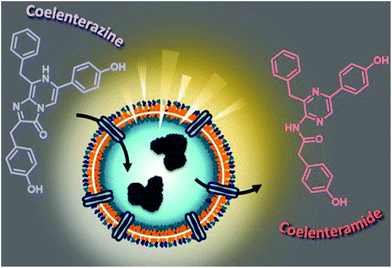
Here, we aim to build a cell-free bioluminescent system with a sustained high luminescence output based on functional catalytic nanocompartments. We encapsulated a specific light-producing enzyme (Gaussia Luciferase, GLuc) inside the aqueous cavity of nano-sized polymeric vesicles (polymersomes), where the enzyme is protected and preserves its activity (Fig. 1). When an appropriate substrate from the environment penetrates the polymersome, GLuc catalyzes the in situ production of bioluminescence, which represents the core functionality of the catalytic nanocompartments (GLuc Ncomp). Polymersomes have been reported to prolong the stability of encapsulated enzymes and increase the probability of enzyme–substrate interactions within the confined space.7 The polymeric membrane is equipped with channel proteins (outer membrane protein F, OmpF) to render the membrane permeable for the passage of substrates and products. However, for channel proteins with low molecular weight cutoffs like OmpF, the diffusion of the substrate towards the polymersome cavity can decrease the in situ activity of the encapsulated enzymes.8,9 In our case, exploiting this diffusion process could allow a modulation of the enzyme kinetics to provide a stable bioluminescent system producing a long-lasting, more convenient to work with light signal, aimed to expand the use of bioluminescence.
Polymersomes, depending on the properties of the copolymers forming the nanocompartment, offer advantages such as biocompatibility,10 low protein binding,11 high blood circulation times,12 stealth and escape from the immune system,11,13 and have thus proven their suitability for numerous applications (imaging, therapeutic, sensing). Compared to their lipid counterparts (liposomes), polymersomes not only possess greater mechanical stability while maintaining a soft architecture, but especially benefit from countless tunability possibilities based on associated chemistry.8,10,14–16 Thus, polymersomes are particularly suitable for the development of cell-free bioluminescent systems aiming at expanding the breadth of bioluminescence applications. We selected a poly(dimethyl siloxane)-block-poly(2-methyl-2-oxazoline) (PDMS-b-PMOXA) polymer for its non-toxicity,10 stealth properties,11,13 short block length permitting membrane protein reconstitution,8,14 self-assembly into polymersomes at high polymer concentration (10 mg mL−1) and protective effect on enzymes from proteolytic degradation to enhance their half-life.17
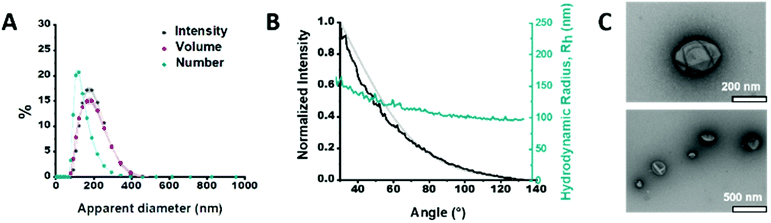
We synthesized the short-chained PDMS25-b-PMOXA10 diblock copolymer via sequential ionic polymerization18 (Fig. S1 and2, ESI†) and built our functional catalytic compartment via film rehydration. The morphology of the resulting GLuc encapsulating polymersomes (GLuc Ncomp) was first characterized by dynamic light scattering (DLS) (Fig. 2A), from which we obtained an average apparent diameter (DH) of 189 ± 57 nm, with a polydispersity index (PDI) of 0.167. Their colloidal stability was also demonstrated as no aggregation was observed. Additional characterization was carried out by static light scattering (SLS) to determine the radius of gyration (Rg = 105 nm). The hydrodynamic radius (Rh = 114 ± 17 nm) was obtained from the DLS profile (Fig. 2B), leading to Rg/Rh = 0.92, consistent with a hollow spherical morphology of Gluc Ncomp.19 Finally, the variation in size of polymersomes as well as their morphology was visualized via transmission electron microscopy (TEM), where we observed the typical collapsed architecture corresponding to soft polymersomes (Fig. 2C). Neither the insertion of OmpF, nor the presence of enzymes inside the cavity of polymersomes affected the morphology or the dispersity of polymersomes, as shown by TEM and light scattering methods (Fig. S3 and4, ESI†). The nanometer size range, high robustness, flexibility and strong colloidal stability of the PDMS-b-PMOXA polymersomes constitute desirable physico-chemical properties for building a versatile nanotechnology-based bioluminescent system.10,15,16
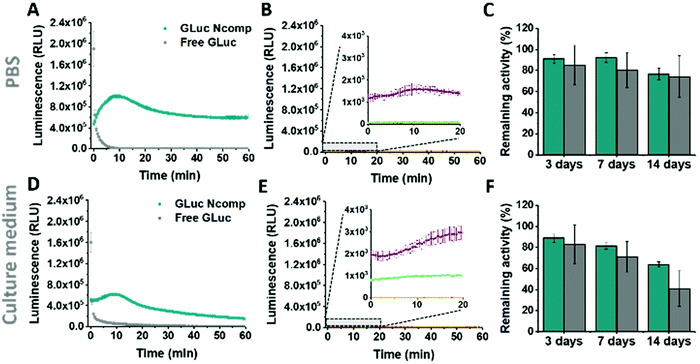
GLuc, the encapsulated enzyme, is a widely used reporter enzyme that we selected for its small size (19.9 kDa), great thermostability, high intensity luminescence output, commercial availability and because it does not require a cofactor.6,20,21 GLuc produces a recordable light via enzymatic oxidation of its coelenterazine substrate, in the form of a flash-type kinetics that implies a strong albeit very prompt and transitory light signal making the detection difficult.6 By encapsulating GLuc within polymersomes (12 ± 5% encapsulation efficiency, ESI†), we aimed at modulating the flash-kinetics to extend the luminescent signal over long periods of time (1 h) and also increase its stability in a cell environment. To investigate the effects of encapsulation on the enzymatic production of light, we compared the bioluminescence emitted by non-encapsulated (free) enzyme to the one produced by encapsulated enzyme (GLuc Ncomp), at equal final concentration (0.2 μg mL−1).8,9,22 The typical flash-type kinetics of free GLuc was confirmed as after a fast transient burst, the signal quickly faded and was no more detectable after 5 min (Fig. 3A). The advantage of encapsulating GLuc inside polymersomes is immediately observable as the enzyme's kinetics is shifted towards a long-lasting light signal: after 1 min the luminescence signal of GLuc Ncomp overtakes that produced by free enzyme (Fig. 3A). The encapsulation of GLuc enables the production of a signal that slowly increases to plateau at maximum intensity reached after 10 min and that is still readily detectable even after 1 hour. The permeability of polymersomes exclusively relies on incorporated transmembrane pores (OmpF), as the compactness of the polymeric membrane is not affected by the insertion of such pore-forming proteins. This long-lasting signal arises from the slow diffusion of the substrate coelenterazine through the OmpF pore to reach the encapsulated GLuc. In fact, OmpF has a molecular cutoff of 600 Da allowing smaller molecules such as coelenterazine (423.46 Da) to enter in the cavity of impermeable PDMS-b-PMOXA polymersomes.23,24 The resulting diffusion effect is the limiting factor for encapsulated enzyme efficiency, especially in our case as coelenterazine is uncharged and thus, its diffusion does not benefit from the attractive interactions that arise between zwitterionic molecules and charged residues of OmpF.25 The diffusion of bulky substrates through OmpF pores decreases the activity of encapsulated enzymes, so it is often perceived as a drawback for catalytic compartments, e.g. in applications like the in situ production of drugs.8 Here, this drawback is turned into an advantage as the slow diffusion of coelenterazine though OmpF enables a slow yet steady supply of substrate for the encapsulated enzyme as compared to free enzyme, resulting in the production of a persisting detectable signal (for at least one hour). This can be of particular advantage in applications like bioluminescence imaging as optical imagers equipped with charge-coupled device (CCD) cameras sum the photons detected during the measurement which results in the amplification of signal over the duration of recording.3 Moreover, in contrast to flash-type signals, such long-lasting luminescence offers greater accuracy as the maximum intensity signal occurs within a plateau. Long-lasting luminescence has already proven its attractiveness as it is akin to the glow-type luminescence of other kinds of luciferases (NanoLuc, Firefly Luciferase), which are particularly sought out for long-term recording in luminescence-based assays.2,20 While these enzymes have the appropriate glow-type luminescence, they often show poor stability and their expression depends on transfected cells, which again limits potential applications.2,20
The same flash-to-glow switch of kinetics of GLuc Ncomp is maintained in cell medium (Fig. 3D) even if the signal intensity is reduced compared to PBS for both free and encapsulated GLuc. This could emerge from the higher viscosity of the cell medium that slows down the diffusion of the substrate but also from the slightly higher background luminescence obtained due to greater auto-oxidation of coelenterazine in cell medium (Fig. 3B–E and Fig. S5, 6, ESI†).26 A similar background is obtained for GLuc Ncomp without OmpF in the presence of coelenterazine, corroborating the absence of free or exposed enzymes (Fig. 3B–E). However, such backgrounds are negligible compared to the high luminescence signal obtained for free and encapsulated GLuc. Also, enzymatically-triggered coelenterazine oxidation can be exploited to develop redox sensors, as shown for our GLuc Ncomp in presence of ascorbic acid (Fig. S7, ESI†). It should be noticed that our system is scalable as this strong light signal results from a diluted sample of only 50 μL (0.5 mg mL−1 of GLuc Ncomp resulting in 0.2 μg mL−1 of GLuc). Therefore, both concentrations and volumes can be easily increased especially using our polymer that self-assembles at high concentrations, to reach even higher signals (2 times higher for twice more concentrated GLuc Ncomp) (Fig. S8, ESI†).
Considering the importance of system stability for storage and applicability reasons, we showed activity retention of free and encapsulated GLuc up to 2 weeks of incubation in PBS and cell medium, at 4 °C and 37 °C (Fig. 3C–F and Fig. S9–11, ESI†). Furthermore, we demonstrated the protein repellence of our system as GLuc Ncomp did not substantially lose activity after 2 weeks of incubation in culture medium. This indicates that encapsulated enzymes are protected but also well supplied with substrate, which implies that OmpF is not obstructed as the passage of substrate is maintained. This preservation of activity of GLuc Ncomp reflects that protein adsorption (from the cell medium containing 10% fetal bovine serum) on the surface of polymersomes is predominantly absent and thus confirms the protein repellence of PMOXA based on its non-ionic nature.11,13 Our system, by means of its stability and protein repellence possesses a certain potency that is essential for developing translational applications.
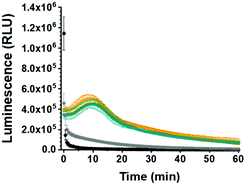
To further probe the applicability of our system in a biological environment, we incubated GLuc Ncomp in the presence of cells (MCF-7) for 3 days and subsequently recorded their activity in the cell supernatant. GLuc Ncomp showed a signal similar to the one obtained in culture medium alone (Fig. 4 and Fig. S12, ESI†), indicating that the activity is not affected by the presence of cells. In contrast, free GLuc showed decreased signal intensity in presence of cells compared to culture medium alone (Fig. 4 and Fig. S13, ESI†), corroborating its premature degradation under biological conditions.6 The encapsulation within polymersomes improves the stability of GLuc by protection from harmful external milieu, e.g. proteolytic degradation (Fig. S14, ESI†).9 Such results highlight the advantage of GLuc Ncomp compared to free enzymes that are more likely to be degraded under biological conditions. Thus, by encapsulating the enzyme our polymersome-based system makes do without the cell's continuous supply of luciferase and thereby warrants exploiting bioluminescence entirely cell-free. In addition, inside polymersomes, the shift to an extended, glow-type kinetics of Gaussia Luciferase is maintained under different conditions (with and without cells) which corroborates the integrity of the polymeric membrane, so the robustness of polymersomes even in the environment of cells.8–10,14–16 Additionally, GLuc Ncomps are not cytotoxic as the same cell viability is obtained in presence of GLuc Ncomp, free GLuc or PBS (Fig. S12, ESI†). Thus, our GLuc Ncomp system is of particular interest for biological applications as it is compatible with cells under physiological conditions and provides enhanced enzyme stability compared to free enzymes.
Conclusions
Here, we present the first artificial bioluminescent nanocompartment with high potential as cell-compatible yet cell-independent bioluminescent system that overcomes the need for using cells transfected with luciferase gene constructs. We also pinpoint that the diffusion of coelenterazine into the nanocompartment, afforded by the membrane insertion of biopores, can be exploited to achieve more suitable kinetics. By means of polymersomes confining Gaussia Luciferase, our system enables a favorable switch in kinetics towards a glow-type luminescence to reach long-lasting but still powerful light signal. Compartmentalization not only allows for tuning signal output but also stabilizes the encapsulated enzyme in a cell environment. Thus, our system overcomes the restrictions arising from enzyme degradation and those related to quick fading of the signal, which positions these bio-hybrid catalytic nanocompartments as potent, cell-free alternative. Plus, the intrinsic properties (e.g. robustness, non-toxicity) and tunability of polymersomes could be an attractive extension that could be exploited for developing in vitro assays (redox sensing applications)27 or in vivo preclinical imaging methods (e.g. detection and targeting of tumours). This new bioluminescent nanosystem, based on an artificial bio-hybrid nanocompartment, is expected to overcome cell-related restrictions and thereby greatly expand the usage of bioluminescence in biomedical domains.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the Swiss National Science Foundation, NCCR-MSE and the University of Basel. The authors thank Andrea Belluati for providing OmpF and Davy Daubian for SLS templates.References
- R. T. Sadikot and T. S. Blackwell, Proc. Am. Thorac. Soc., 2005, 2, 537–540 CrossRef CAS.
- D. M. Close, T. Xu, G. S. Sayler and S. Ripp, Sensors, 2010, 11, 180–206 CrossRef.
- S. M. Coleman and A. McGregor, Future Virol., 2015, 10, 169–183 CrossRef CAS.
- A. E. Masser, G. Kandasamy, J. M. Kaimal and C. Andréasson, Yeast, 2016, 33, 191–200 CrossRef CAS.
- B. A. Tannous, Nat. Protoc., 2009, 4, 582–591 CrossRef CAS.
- S. S. El-Amouri, P. Cao, C. Miao and D. Pan, Mol. Biotechnol., 2013, 53, 63–73 CrossRef CAS.
- P. Baumann, M. Spulber, O. Fischer, A. Car and W. Meier, Small, 2017, 13, 1603943 CrossRef.
- C. E. Meyer, J. Liu, I. Craciun, D. Wu, H. Wang, M. Xie, M. Fussenegger and C. G. Palivan, Small, 2020, 16, 1906492 CrossRef CAS.
- A. Belluati, I. Craciun, J. Liu and C. G. Palivan, Biomacromolecules, 2018, 19, 4023–4033 CrossRef CAS.
- C. G. Palivan, R. Goers, A. Najer, X. Zhang, A. Car and W. Meier, Chem. Soc. Rev., 2016, 45, 377–411 RSC.
- X. Zhang and P. Zhang, Curr. Med. Chem., 2017, 13, 124–129 CAS.
- T. Anajafi and S. Mallik, Ther. Delivery, 2015, 6, 521–534 CrossRef CAS.
- A. Najer, D. Wu, D. Vasquez, C. G. Palivan and W. Meier, Nanomedicine, 2013, 8, 425–447 CrossRef CAS.
- F. Itel, A. Najer, C. G. Palivan and W. Meier, Nano Lett., 2015, 15, 3871–3878 CrossRef CAS.
- J. Liu, I. Craciun, A. Belluati, D. Wu, S. Sieber, T. Einfalt, D. Witzigmann, M. Chami, J. Huwyler and C. G. Palivan, Nanoscale, 2020, 12, 9786–9799 RSC.
- K. Kiene, S. H. Schenk, F. Porta, A. Ernst, D. Witzigmann, P. Grossen and J. Huwyler, Eur. J. Pharm. Biopharm., 2017, 119, 322–332 CrossRef CAS.
- P. Tanner, O. Onaca, V. Balasubramanian, W. Meier and C. G. Palivan, Chem. – Eur. J., 2011, 17, 4552–4560 CrossRef CAS.
- D. Wu, M. Spulber, F. Itel, M. Chami, T. Pfohl, C. G. Palivan and W. Meier, Macromolecules, 2014, 47, 5060–5069 CrossRef CAS.
- D. Daubian, J. Gaitzsch and W. Meier, Polym. Chem., 2020, 11, 1237–1248 RSC.
- C. G. England, E. B. Ehlerding and W. Cai, Bioconjugate Chem., 2016, 27, 1175–1187 CrossRef CAS.
- A. W. Al-Ani, L. Zhang, L. Ferreira, L. Turyanska, T. D. Bradshaw and N. R. Thomas, Nanomedicine, 2019, 20, 102005 CrossRef CAS.
- T. Yu, J. R. Laird, J. A. Prescher and C. Thorpe, Protein Sci., 2018, 27, 1509–1517 CrossRef CAS.
- G. Kefala, C. Ahn, M. Krupa, L. Esquivies, I. Maslennikov, W. Kwiatkowski and S. Choe, Protein Sci., 2010, 19, 1117–1125 CrossRef CAS.
- C. Edlinger, T. Einfalt, M. Spulber, A. Car, W. Meier and C. G. Palivan, Nano Lett., 2017, 17, 5790–5798 CrossRef CAS.
- E. M. Nestorovich, C. Danelon, M. Winterhalter and S. M. Bezrukov, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 9789–9794 CrossRef CAS.
- H. Zhao, T. C. Doyle, R. J. Wong, Y. Cao, D. K. Stevenson, D. Piwnica-Worms and C. H. Contag, Mol. Imaging., 2004, 3, 43–54 CrossRef CAS.
- H.-Y. Li, X.-M. Zheng, M.-X. Che and H.-Y. Hu, PLoS One, 2012, 7, e35628 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Methods and synthesis, NMR, GPC, TEM, DLS and SLS results. See DOI: 10.1039/d0nr07178a |
| This journal is © The Royal Society of Chemistry 2021 |