Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Norditerpenoid alkaloids from Aconitum and Delphinium: structural relevance in medicine, toxicology, and metabolism
Ashraf M. A.
Qasem
 ,
Ziyu
Zeng
,
Ziyu
Zeng
 ,
Michael G.
Rowan
and
Ian S.
Blagbrough
,
Michael G.
Rowan
and
Ian S.
Blagbrough
 *
*
Department of Pharmacy and Pharmacology, University of Bath, Bath BA2 7AY, UK. E-mail: prsisb@bath.ac.uk
First published on 12th October 2021
Abstract
Covering: 77 A.D. up to 2020
Norditerpenoid alkaloids (NDA), typically N-ethylpiperidine containing C19 or C18 natural product diterpenes, are hexacycles with several contiguous often oxygenated stereocentres. As a function of their structural complexity, they display important pharmacological activities. The processed plants are used as important folk drugs and four NDAs have now been clinically approved. Many metabolism studies on Aconitum alkaloids have been reported as the understanding of their biotransformation in living systems and in cell-free systems is important for the development of these alkaloids as drugs. This Highlight sets out the missing links in NDA biosynthesis, their biological applications, SAR, toxicity, metabolism, and analytical studies.
1 Introduction to norditerpenoid alkaloids (NDA)
The majority of norditerpenoid alkaloids (NDA, C18- or C19-diterpenoid alkaloids) are isolated from the genera Aconitum and Delphinium (larkspur), Consolida, and Spirea,1 and the whole plants are lethal, e.g., Delphinium has been extensively reported as responsible for poisoning livestock in North America leading to financial losses.2 NDA display important pharmacological activities. Especially, they have been used as insecticides for two thousand years. At the time of the eruption of Mount Vesuvius covering Pompeii and Herculaneum, Pliny the Elder reported: Delphinium seeds, pounded and extracted into wine (ethanol), rid the head and body of lice.3Delphinium seeds were even reportedly issued to British soldiers at the battle of Waterloo (still for the treatment of body lice).4 Therefore, if the NDA dose is appropriately adjusted, it is of no surprise that they have been known to be widely used as folk medicines, not least earning the trivial (?) name Wolf's bane, but perhaps more accurate is the descriptive name Monkshood. They are deadly poisons to mammals, even on simple handling if the skin is broken, e.g., if it has been scratched.NDA have a complex highly oxygenated hexacyclic structure (Fig. 1A),5,6 A/B/C/D/E/F rings are 6/7/5/6/6/5-membered rings respectively. The substituent at position 14 is commonly drawn going under the C15–C16 C–C bond, but our single-crystal X-ray diffraction (SXRD) data show that in reality it lies above that bond (Fig. 1C), although it is in the α-orientation.5 Also, the C7 substituent in the lycoctonine-type NDA is usually assigned in the β-orientation while the SXRD shows that it cannot be axially β-orientated like C6 and C8 substituents, but it is equatorial β-orientated and it is better to draw it in the plane of the paper (Fig. 1).5 This attention to molecular detail is important when considering the drug-like physical and chemical properties of such molecules and their binding modes at their target receptors and ion channels as conformation is an important determinant of biological activity.
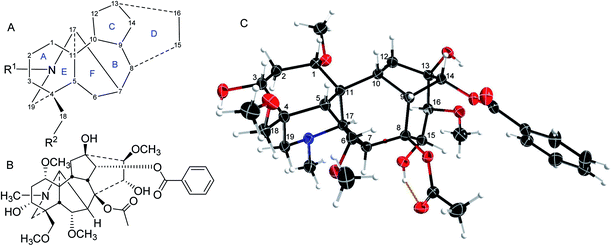 | ||
| Fig. 1 (A) The NDA numbering system; (B) mesaconitine (1); (C) SXRD data of mesaconitine (1).5 | ||
2 Missing links in NDA biosynthesis
The biosynthesis of C19 and C18 NDA starts from the precursor isopentenyl pyrophosphate (IPP) which is produced from the mevalonate and the methylerythritol (MEP) pathways (Fig. 2). Allylic isomerisation converts IPP into dimethylallyl PP (DMAPP) by isopentenyl diphosphate isomerase (IPP isomerase). Geranylgeranyl pyrophosphate synthase converts DMAPP with 3 IPP units into geranylgeranyl pyrophosphate and then ent-copalyl diphosphate is produced from GGPP by ent-copalyl diphosphate synthase.1ent-Cpp is further cyclised to ent-kaurane, which leads into veatchine- and napelline-type alkaloids, and ent-atisane which leads into atisine- and denudatine-type alkaloids. ent-Kaurane and ent-atisane are the only two precursors for the C19 and C18 NDA biosynthesis. ent-Kaurene synthase mediates the conversion of ent-cpp into ent-kaurene and then ent-kaurenal is produced from ent-kaurene by ent-kaurene oxidase (KO, CYP701A).1 Amination by L-serine aminotransferase happens to ent-kaurane-type to produce veatchine-type which rearrange to napelline-type. While L-serine aminotransferase amination of ent-atisane-type results in atisine-type which rearranged to denudatine-type diterpenoid alkaloids.1,7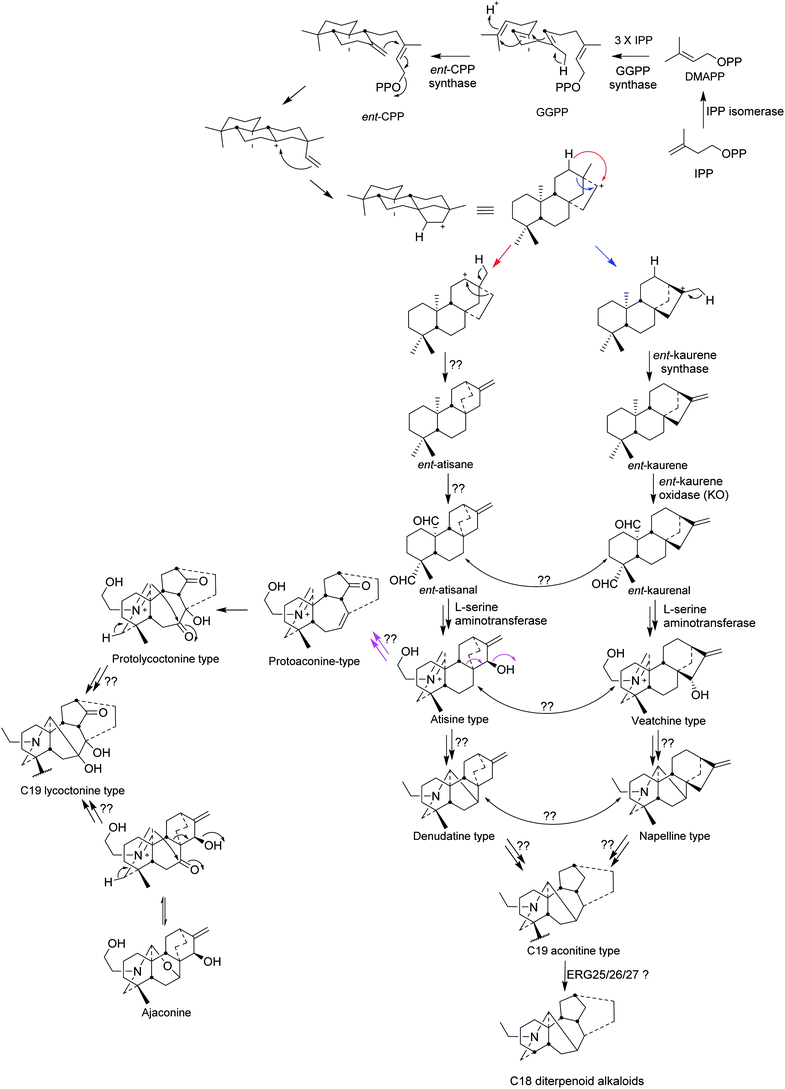 | ||
| Fig. 2 Biosynthesis of C19 lycoctonine type, C19 aconitine type, and C18 diterpenoid alkaloids.1,7 | ||
The production of ent-atisane and ent-atisanal from ent-cpp remains unclear. There could be rearrangement between atisine- and veatchine types and between denudatine- and napelline types, but there is no clear evidence for this. The wide distribution of denudatine- and napelline NDA in Aconitum species supports their position in the biosynthesis of C19 aconitine type NDA. The C19 lycoctonine type NDA are likely to be formed from protoaconine type rather than by the direct rearrangement of the atisine type.7 In addition, the high occurrence of ajaconine in Delphinium species supports the idea that it could be the most important precursor of the lycoctonine type,7 where scientific results are still missing. The rearrangement of C20 DA to C19 NDA happens through oxidative cleavage (loss of the exo-methylene) and the latter can undergo oxidative loss of one carbon by a different mechanism to generate the C18 NDA (Fig. 2).1,7 The loss of the C-18 carbon atom from C-4 may indeed follow the established pathway for the removal of one or two methyl groups at C-4α in ergosterol (ERG) catalysed sequentially by the enzymes ERG25/26/27.
3 Medicinal applications of Aconitum and Delphinium in current TCM
Clinical applications of Aconitum in traditional medicinal systems are found worldwide, particularly in Eurasia.8 In Table 1 are highlighted some Traditional Chinese Medicine (TCM) uses.9–12 The key processes for the raw dried main roots of A. carmichaelli Debx. and A. kusnezoffii Reichb. are boiling in water or steaming (usually known as Pao’Zhi), and then slicing. The slices are boiled again thus detoxifying to obtain the decoction, or mixed with excipients, e.g., honey pills for oral use, which are used clinically as an oral analgesic for rheumatism, joint pain, and several other different pains.| Plant material | Use | Ref. |
|---|---|---|
| Aconitum root (tuber) | Anti-rheumatic and analgesic | 9 |
| No specific species was mentioned | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| A. carmichaelli Debx. | ||
| Dry main root (Chuan'wu) | Normally use after processing as analgesic | 10 |
| Processed main root (Zhi‘chuan’wu) | Normally used as decoction, as anti-rheumatic and analgesic for joint pain | 10 |
| Processed side root (Fu'zi) | Normally used as decoction, as analgesic and tonic | 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| A. kusnezoffii Reichb. | ||
| Dry main root (Cao'wu) | Normally use after processing as analgesic | 10 |
| Tincture used externally as anti-rheumatic | 11 | |
| Processed main root (Zhi‘cao’wu) | Normally used as decoction, as anti-rheumatic and analgesic for joint pain | 10 |
| Dry leaves (Cao‘wu’ye) | Normally used as round pills, used as anti-inflammatory antipyretics, and analgesic; | 10 |
| Clinically used for abdominal pain, diarrhoea, headache, and toothache | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| A. tanguticum (Maxim.) Stapf. and A. naviculare (Bruhl.) Stapf. | ||
| Dry whole plant (Tang‘gu’te Wu'tou) | Used as anti-inflammatory, antipyretic, and wound-healing-promoting agent | 12 |
| Clinically used for fever, hepatitis, cholecystitis, gastritis, skin ulcers and sores, and for bites (by insects, snakes etc.) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| A. flavum Hand.-Mazz. and A. pendulum Busch. | ||
| Processed main root (Tie‘bang’chui) | Used as anti-inflammatory, analgesic, and sedation | 12 |
| Clinically used for skin ulcers and sores, leprosy, and mania | ||
| Dry seedlings (seedlings of Tie‘bang’chui) | Used as anti-inflammatory, antipyretic, and analgesic | 12 |
| Clinically used for fever, ‘flu, skin ulcers, and sores | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| D. kamaonense Huth var. glabrescens (W. T. Wang) W. T. Wang | ||
| Dry above-ground parts (Zhan‘mao’cui'que) | Used as anti-inflammatory and analgesic | 12 |
| Clinically used for diarrhoea and dysentery, also externally used on skin ulcers and sores | ||
In TCM, there are many formulae which are mixtures of Aconitum with other plants. Si'ni decoction is an official formula in the Chinese Pharmacopoeia used as a tonic. Si'ni decoction is composed of the processed side-root of A. carmichaelii (fu'zi), Glycyrrhiza uralensis, and Zingiber officinalis. The diester NDA are hydrolysed during the decoction process to afford the monoester NDA (vide infra 5.2 where metabolism is discussed).
The medicinal application of Delphinium is popular in the Unani medicine system, a traditional medicinal system applied across nearly the whole of South and Central Asia, particularly in India and Pakistan. The species D. denudatum Wall, known as Jadwar, has even been described as one of the most important drugs in the Unani medicine system. Its roots are reported to be used for the treatment of aconite poisoning, brain diseases, fungal infection, piles, and toothache.13
4 NDA in current medicine and their structure–activity relationship (SAR) studies
Despite their similar sounding trivial names and being based on the same hexacyclic skeleton, many NDA do not have the same oxygen substitution pattern and this structural change may be one of the reasons for their different pharmacological properties.4.1 Aconitine type
Mesaconitine (1), aconitine (2), and hypaconitine (3)14 (Fig. 3) are cardiotoxins,15,16 interacting with cardiac voltage-sensitive sodium channels (VSSCs) and maintaining them in an open conformation,15,17,18 introducing arrhythmia,19,20 whereby these alkaloids are highly toxic, e.g., LD50 (aconitine 2, mice, subcutaneous, mg kg−1) = 0.12–0.20.17,21 The potent, indeed lethal cardiotoxin aconitine (2) was first discovered in 1833 by P. L. Geiger from A. napellus.22,23 These NDA (1–3) act at VSSCs in the central nervous system (CNS) and muscle tissues exhibiting analgesic activity.24 The NDA (1–3) with 8-OAc or other fatty acid ester sidechains are also known as lipo-alkaloids.25 The primary functional subunit of VSSCs is the α-subunit, and this subunit contains four domains (I–IV). There are at least five reported toxin binding sites on domain I, and the lipophilic NDA, e.g., mesaconitine (1) and aconitine (2) that carry ester groups 8-OAc and 14-OBz, can bind on the neurotoxin receptor 2 that is located in the transmembrane region, and this binding leads to VSSCs remaining in the open-state.26 The boiling process in the preparation of Aconitum roots hydrolyses the highly toxic diester NDA, e.g., aconitine 2 to less toxic monoester NDA, e.g., 14-OBz-aconine 4. Structure–activity relationship (SAR) studies show that further hydrolysis produces alkaloids lacking an ester group and which are even less toxic, e.g., aconine 5, but the analgesic activities are also lost.17,18,27 When the NDA bear different oxygenated substituents, e.g. 3-O-acetylaconitine (6) and crassicauline A (7, also known as bulleyaconitine A), they may interact with the different subdomains on the α-subunit and this results in decreased toxicity as evidenced by LD50 (mice; subcutaneous; mg kg−1) = 0.87–1.40 and 0.92, respectively.17,19,21 These NDA (6, 7) exhibit non-addictive potent analgesic activity ED50 (mice; hotplate; mg kg−1) = 0.16 and 0.087, respectively. Although the clinical use of the lipo-alkaloids as diester (crassicauline A, 7) or even triesters (3-O-acetylaconitine 6) is debatable as they are toxic,17 3-O-acetylaconitine (6) and crassicauline A (7) were introduced in the 1980s into clinical use in China as analgesic agents for long-term treatment.28–30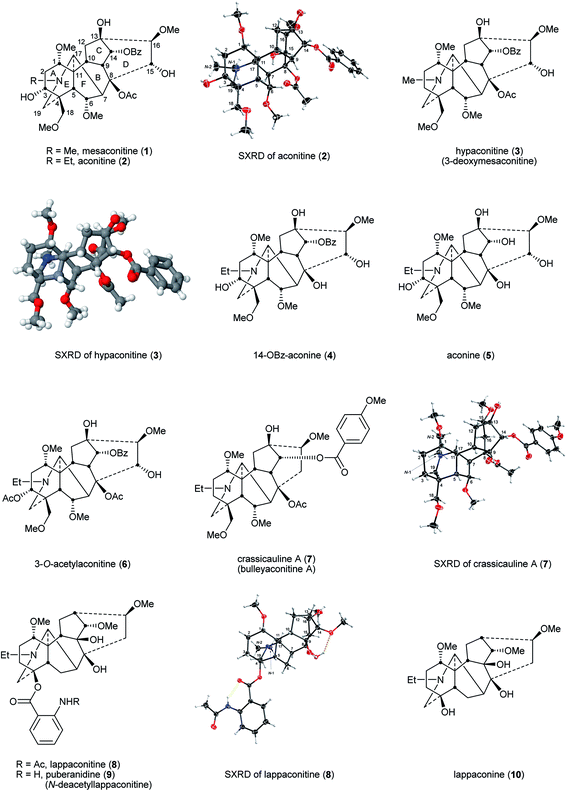 | ||
| Fig. 3 Mesaconitine (1), aconitine (2), hypaconitine (3), and their related NDA. SXRD of hypaconitine (3) is reprinted with permission from ref. 14. Copyright 2012 ACS. | ||
4.2 Lappaconitine type
Lappaconitine (8), the first C18 NDA to be reported, was isolated from A. septentrionale Koelle by H. V. Rosendahl in 1895.31 The most successful medical application of an NDA to date is Allapinin, the hydrobromide salt of lappaconitine (8) as it is a VSSC blocker.17,32–34 It has been used clinically in Russia since 1987 as an anti-arrhythmic drug.35–39 This compound also shows a potent non-addictive analgesic property, and recent studies reported that this bioactivity is related to a decrease of expression and sensitisation of the P2X3 receptors on mice dorsal root ganglion (DRG) neurons.40,41 A naturally occurring analogue of lappaconitine (8), puberanidine (9, N-deacetyllappaconitine), exhibits similar biological activities. Indeed, it is an N-deacetyl metabolite of lappaconitine (8) found in the urine of certain drug users.42 Toxicity studies on these two compounds revealed that their LD50 (mice; subcutaneous; mg kg−1) were 11.7 and 36.4, respectively which, due to their lower toxicity, are significantly higher than that of aconitine (2) given above (mice, subcutaneous, mg kg−1 = 0.12–0.20).17,21 Structurally, lappaconitine (8) and puberanidine (9) are monoesters and they have a smaller chance to bind to neurotoxin receptor 2 in the transmembrane region in comparison with more lipophilic aconitine (2), thus, these compounds are less toxic. When these NDA lack lipophilic ester groups, their (toxic) activities on VSSCs are less, whereas the analgesic effects on the CNS remain, e.g., as demonstrated by lappaconine (10).344.3 Lycoctonine type
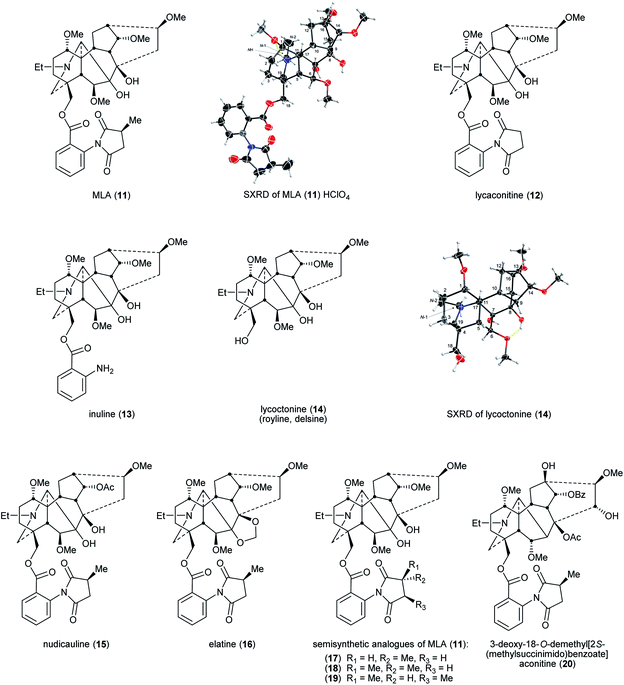
Lycoctonine (14) (Fig. 4) was first reported in 1865 isolated from A. lycoctonum L.43 and lycaconitine (15) was first reported in 1884 also from A. lycoctonum L.44 Methyllycaconitine (MLA, 11) was first reported by Manske in 1938 from D. brownii Rydb.45 In 1943, Goodson reported its exact formula (Fig. 3).46 MLA (11) is one of the most potent competitive antagonists of α7-nicotinic acetylcholine receptors (nAChRs)47 with highly selective targeting of the snake venom toxin α-bungarotoxin (α-BgTx) binding sites.48,49A rare pharmaceutical preparation of MLA (11), mellictin, has been reported in clinical use for the treatment of Parkinson's disease and cerebral palsy at least in Uzbekistan and Kyrgyzstan.36 The 2S-(methylsuccinimido)benzoate moiety was the key for this compound to exhibit high affinity. Compared with MLA (11) IC50 = 7.6 ± 3.4 × 10−9 M (mice brain neuronal α7 nAChR), the IC50 of lycaconitine (12) on α7 nAChR was 6.8 ± 0.9 × 10−6 M as the methyl group on the succinimide is removed, significantly lowering affinity. The IC50 of inuline (13) is similarly modest 1.6 ± 0.6 × 10−6 M due to the removal of the essential methylsuccinimide group. When the entire (methylsuccinimido)benzoate ester is cleaved, the affinity of lycoctonine (14, which is also known as royline and delsine50) has sharply decreased, the IC50 increasing through the μM range to 1.0 ± 0.1 × 10−5 M, which indicates that this neopentyl alcohol derived from MLA (11) has essentially lost all affinity for the α7 nAChR.51 For NDA substituted with 18-O-2S-(methylsuccinimido)benzoates, e.g., nudicauline (16) and elatine (17), their IC50 remained at the nM level as MLA (11), 1.7 ± 0.7 × 10−9 M and 6.1 ± 1.5 × 10−9 M, respectively. Also, Carroll and co-workers reported that stereochemical modification on the succinimide ring of MLA (11), e.g., changing the chirality of the succinimide methine substituted with a methyl from S to R decreases the affinity for α7 nAChR, e.g., semisynthetic analogues (17–19).52
The pharmacophore in MLA (11)51 for nAChR incorporates the key distance between the N-atom of the piperidine E-ring (ring labelling see Fig. 1) and the ester carbonyl of 18-O-2S-(methylsuccinimido)benzoate. Aconitine (2) contains no such distance and therefore it shows only modest affinity for nAChR. However, after the 3-OH and the O-methyl of 18-OMe were specifically removed chemically and then 2S-(methylsuccinimido)benzoic acid was esterified with the unmasked 18-neopentyl alcohol, the product 3-deoxy-18-O-demethyl[2S-(methylsuccinimido) benzoate]aconitine (20) exhibited high affinity for α7 nAChR at the same (low) nM level as that of MLA (11).53
The complex structure of such hexacyclic NDA secondary metabolites make them potential drugs, at an appropriate dose, rather than toxins. Their biophysical properties show that they are well absorbed in mammals and therefore they possess good properties for orally delivered human medicines leading to good distribution in the body. NDA are therefore prime candidates for aspects of computational drug design and development, starting from natural product leads (from sustainable sources) and progressing in particular using computers to assess the (bio)physical properties and data sets, e.g., Aconitum and Delphinium alkaloids: “Drug-likeness” descriptors related to toxic mode of action.54 Such QSAR studies of large datasets of NDA aim to discriminate between “drugs” and “non-drugs”. These calculations have been proven to give reliable results on specific NDA as to whether they are more likely to be a poison or a drug. Rasulev and co-workers constructed QSAR models with “drug-likeness” descriptors discussed in terms of the mode of toxic action exhibited by the selected set of 95 NDA.54 The antiarrhythmic NDA, e.g. lappaconitine (8), the curare-like NDA, e.g. MLA (11), and aconitine-like NDA were studied using a range of typical “drug-likeness” descriptors. The molecular size descriptors (differences in positive and negative regression terms of MWt and Ghose-Grippen molar refractivity, MR) were identified as those most related to toxic mode of action. Preliminary theoretical absorption, distribution, metabolism, and excretion (ADME) properties of the NDA gave promising data. However, NDA showing the higher desirable therapeutic activity are still those of high toxicity. Dose remains crucial and therefore further pharmacological studies should be carried out.
4.4 Delphinium (larkspur) toxicity
Larkspur toxicity varies as a function of, e.g., species, stage of growth, plant part, environment, and the individual NDA.55 There are many important structural features contributing to the toxicity of larkspur NDA. The ionized nitrogen plays a major role in the interaction with nAChR. The ester moiety in MLA-type (methylsuccinimidoanthranoyl lycoctonine, MSAL) plays an important role in neuromuscular blockade (at α1 nAChR). In addition, a C14 substituent has a profound biological effect. This important functionality was reported in many studies which established that the electronic or the stereochemical factors of the C14 substituent affect the affinity for nAChR. The order of activity at C14 was determined to be acetate > hydroxyl > methoxy > carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). In contrast, the observed effect of substituents at C1 and C16 is: methoxy > hydroxyl > acetate. The opposite biological effects are caused by substituents at C1 and C16, and C14.55–60
O). In contrast, the observed effect of substituents at C1 and C16 is: methoxy > hydroxyl > acetate. The opposite biological effects are caused by substituents at C1 and C16, and C14.55–60
Livestock intoxication by larkspur is a function of the cattle breed and their genetics also affect the susceptibility to the intoxication. Age is another factor where young heifers are more susceptible than mature cows. Cattle sex is also important. Heifers are more prone to the toxicity than steers and bulls. Of course, the plant plays an important role where the MSAL and non-MSAL alkaloid concentration and composition, which depend on the population, species, climate, and the year, affect the cattle toxicity and the amount needed from the plant to develop clinical signs of toxicity.61,62 The 7,8-methylenedioxy-NDA are the least toxic alkaloids. The lycoctonine-type is twice as toxic, but is still considered to be of relatively low toxicity. The MSAL alkaloids, e.g. MLA (11), are 10-times more toxic than any other tested alkaloid.56 The MSAL alkaloid concentration in tall larkspur is the major contributor to livestock poisoning. An investigation of the SAR of NDA toxicity found that 7,8-methylenedioxy-NDA exacerbates the toxicity of the MSAL alkaloids. The exact molecular mechanism of this toxicity is not known, but a possible explanation is that such substituted NDA can act as co-agonists on nAChR, enhancing the activity at the receptors.63
5 Metabolism of NDA
As set out above, Aconitum preparations are widely used in TCM and a few NDA have been clinically approved: 3-O-acetylaconitine (6), crassicauline A (7), lappaconitine (8), and MLA (11). The metabolism of these alkaloids has been studied extensively in vitro in cell-free systems (microsomes) (Table 2) and in vivo in animals and humans.| NDA | Metabolic enzymes | Ref. |
|---|---|---|
| RLM | ||
| Aconitine (2) | Major CYP3A | 64 |
| Minor CYP1A1/2 | 64 | |
| Crassicauline A (7) | Major CYP3A and 2C | 67 |
| Minor CYP2D and 2E1 | 67 | |
| Least CYP1A2 | 67 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| HLM | ||
| Aconite diesters | Major CYP3A | 68 |
| Minor other CYPs | 68 | |
| Mesaconitine (1) | Major CYP3A4/5 | 66 |
| Minor CYP2C8/9, 2D6 | 66 | |
| Aconitine (2) | Major CYP3A4/5 | 65 |
| Minor CYP2C8/9, 2D6 | 65 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| HIM | ||
| Aconite diesters | Major CYP3A | 68 |
5.1 Cell-free systems – microsomal studies
Metabolism of aconitine (2) in rat liver microsomes (RLM) was studied and it was found to be metabolised through hydrolysis, demethylation, N-deethylation, and dehydrogenation. Several CYP450 inhibitors were used to determine which isoform was involved in the metabolism; CYP3A and/or CYP1A1/2 inducers or inhibitors will affect the metabolism of aconitine (2).64 Metabolic studies using human liver microsomes (HLMs), where the liver cells were obtained from donors aged between 21–76, showed that the main metabolic pathways are hydroxylation, dehydrogenation, O-demethylation, di-O-demethylation and/or N-deethylation. CYP3A5 and 2D6 mediate the di-O-demethylation and the hydroxylation, CYP3A4/5, 2D6, and 2C9 the N-deethylation, and CYP3A4/5 the dehydrogenation.65Metabolism of mesaconitine (1) using the above HLM obtained from male donors, was found to be conducted mainly through demethylation, hydroxylation, dehydrogenation, and demethylation-dehydrogenation. It was found that CYP3A4/5, 2C8, and 2D6 mediated the demethylation pathway; CYP3A4/5 the hydroxylation and dehydrogenation; CYP3A4/5, 2C8/9, and 2D6 the demethylation-dehydrogenation.66
Metabolism of crassicauline A (bulleyaconitine A, 7) by RLM results showed that the main metabolic pathways were hydroxylation, deacetylation, O-demethylation, di-O-demethylation and/or N-deethylation, and deacetylation-dehydration. CYP3A and 2C mediated all pathways, CYP3A, 2C, and 2D contributed to the hydroxylation, CYP3A, 2C and 2E1 the demethylation, CYP3A, 2C, 2D, and 2E1 the N-deethylation, deacetylation and deacetylation-dehydration.67
The metabolism of A. carmichaelii Debx. roots' individual diesters, mesaconitine (1), aconitine (2), and hypaconitine (3), and monoesters, benzoylmesaconine, benzoylaconine, and benzoylhypaconine NDA by HLM and human intestinal microsomes (HIM), obtained from mixed gender donors, was studied. It was found that demethylation and dehydrogenation were the main metabolic pathways, and N-deethylation was observed in aconitine (2) and benzoylaconine. Hydroxylation was detected in mesaconitine (1) and hypaconitine (3). Deoxygenation was observed in aconitine (2). Subsequent metabolism was observed in diesters more than monoesters to form, e.g., di-O-demethylated, didehydrogenated, and demethylated-dehydrogenated metabolites.68 The amount/concentration of HLM metabolites were higher than HIM metabolites as HLM catalytic capacity is higher and could be due to other CYPs alongside CYP3A that could play a role in the metabolism which was reported previously.65,66 Glucuronidation followed phase I metabolism, but no conjugated metabolites were found in HIM and HLM incubations.68
Metabolism of lappaconitine (8) by HLM and RLM showed that N-deacetylation, hydroxylation, and O-demethylation were the main metabolic pathways whereas N-deethylation and hydrolysis contributed to a lesser extent. Subsequent metabolism was observed, and many metabolites of metabolites were generated from combinations of the previously mentioned pathways. The O-demethylated metabolites were present in the order 16-O-demethyl- > 14-O-demethyl- > 1-O-demethyl-lappaconitine. It was found that HLM metabolism generated more metabolites than RLM.69
5.2 Animal studies
A study showed that G. uralensis and Z. officinalis enhance the absorption of A. carmichaelii monoesters and reduce the half-life by increasing the excretion or speeding up the metabolism of other active compounds.70In vivo metabolic studies of Si'ni decoction70 in rats urine showed that hydrolysis and demethylation were the main metabolic pathways of diterpenoid alkaloids.71In vivo study of pure aconitine (2) metabolism in rats and rabbits stomach showed that the biotransformation was mainly through oxidation (+16 Da) resulting in hydroxylation, deoxygenation (−16 Da) in dehydroxylation, O-demethylation (−14 Da), and di-O-demethylation and/or N-deethylation (−28 Da). Ester exchange at C8 was also observed as some fatty acyl chains replaced the acetyl moiety to form lipo-aconitines (Fig. 5). The metabolic profile from rats and rabbits stomach is similar, but there are fewer metabolites in rats.72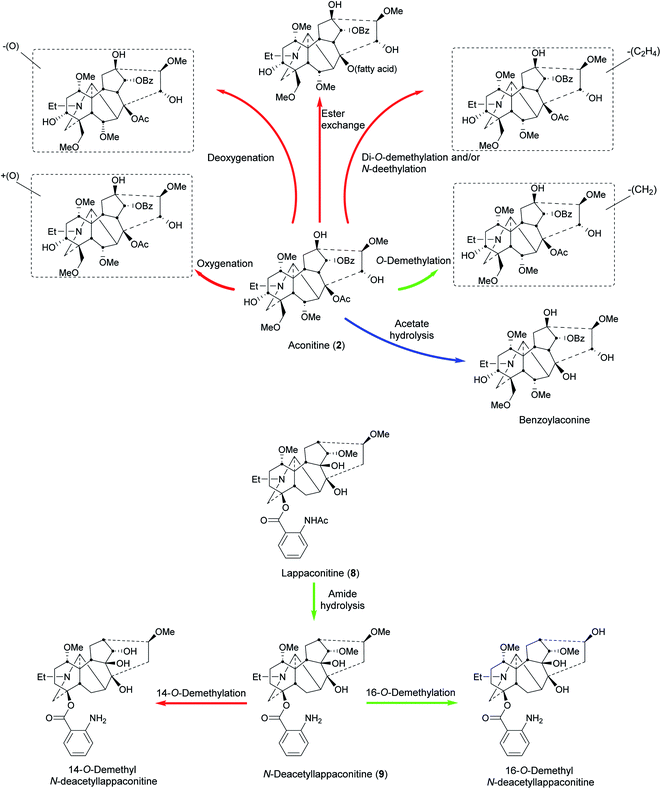 | ||
| Fig. 5 Metabolism of aconitine (2)72,78 and lappaconitine (8)77,79 in humans (blue), animals (red), and pathways common in both (green). | ||
The metabolism of crassicauline A (bulleyaconitine A, 7) (p.o. and i.v.), which is used clinically in China, has been studied in rat urine and faeces. The amount recovered unchanged was higher in the i.v. group compared to that in the p.o. group.73 A hydroxylated metabolite was observed in MS (+16 Da). From the fragmentation analysis, it was concluded that the hydroxylation was probably at C15 to give deoxyjesaconitine, but this conclusion requires scientific confirmation.73
Qi–Li–Qiang–Xin (QLQX) is a TCM prescription composed of eleven herbal medicines, which can interfere with the metabolism of each other. One of them is Radix Aconiti Lateralis which is also known as fu'zi (the side roots of A. carmichaelii). The metabolism of QLQX capsules (p.o.) in rats' plasma, urine, and faeces was studied. Diester NDA were not detected in the preparation and the metabolic pathways according to the metabolites identified mainly in urine and plasma were: hydrolysis, hydroxylation, methylation, O-demethylation, dehydrogenation, and dehydration. In addition, no NDA phase II metabolites were detected.74 Similarly, the pharmacokinetics of multiple NDA, e.g., mesaconitine, aconitine, hypaconitine, and benzoylmesaconitine, have been reported following the administration of the TCM Zhenwu Tang and Radix Aconiti Lateralis Praeparata extracts to rats with quantitative analysis by HPLC-MS/MS.75 Likewise, the simultaneous determination and the pharmacokinetics of six NDA in rat plasma have been reported following the oral administration of Radix Aconiti extracts.76 The analytical technique of SPE-HPLC-MS/MS is sensitive and accurate. These results provide a scientific basis for a metabolism study of Aconitum NDA in humans. Therefore, they pave the way for studies in to the clinical uses of Aconitum NDA and its extracts.
The metabolism of lappaconitine (8) HBr (Allapinin) was studied in rats after i.v. administration. Deacetyllappaconitine (9), 14-O-demethyl-deacetyllappaconitine, and 16-O-demethyl-deacetyllappaconitine, were detected in rat urine in addition to unmetabolized drug (8). Their ratio was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 respectively after 24 h. As lappaconitine (8) concentration decreased with time, deacetyllappaconitine (9) increased due to metabolism by N-deacetylation. Also, as the amount of deacetyllappaconitine (9) decreased, 14-O-demethyl- and 16-O-demethyl-deacetyllappaconitine increased (Fig. 5).77 Another study in rats showed that N-deacetylation, O-demethylation, and hydroxylation are the main metabolic pathways, where 16-O-demethyl-deacetyllappaconitine and 5′-OH-lappaconitine are the major metabolites.69
4 respectively after 24 h. As lappaconitine (8) concentration decreased with time, deacetyllappaconitine (9) increased due to metabolism by N-deacetylation. Also, as the amount of deacetyllappaconitine (9) decreased, 14-O-demethyl- and 16-O-demethyl-deacetyllappaconitine increased (Fig. 5).77 Another study in rats showed that N-deacetylation, O-demethylation, and hydroxylation are the main metabolic pathways, where 16-O-demethyl-deacetyllappaconitine and 5′-OH-lappaconitine are the major metabolites.69
5.3 Human studies
The metabolism of aconite NDA was studied in the urine of females who had been given p.o. decoctions of a prescription consisting of 10 g Cao'wu (processed roots of A. kusnezoffii Reichb) and 10 g Chuan'wu (processed roots of A. carmichaelii Debx.). In addition to the parent alkaloids, mesaconitine (1), aconitine (2), and hypaconitine (3), there were three hydrolysed metabolites, benzoylmesaconine, benzoylaconine, and benzoylhypaconine, and two O-demethylated metabolites, which were assigned as 16-O-demethylaconitine and 16-O-demethylhypaconitine78 by analogy.77 The monoesters may not only be derived by metabolism as the processed roots contain substantial amounts of them.Lappaconitine (8) metabolism was studied in the urine of men who had received i.m. injections of lappaconitine (8) HBr (Allapinin). In addition to the parent alkaloid, N-deacetyllappaconitine (9) and 16-O-demethyl-deacetyllappaconitine were detected (Fig. 5). In 24 h collected urine, the ratio of lappaconitine (8), the N-deacetyl-, and 16-O-demethyl-deacetyllappaconitine was 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively,79 different from the rat metabolism ratio (i.v. administered).77 The high ratio of lappaconitine (8) compared to its metabolites is after i.m. administration which (like i.v.) allows the drug to bypass the liver, thereby avoiding first-pass (through the liver) metabolism. In addition, Allapinin (8 as an HBr salt) is likely to be more water soluble and potentially rapidly excreted unchanged in the urine. The amounts of lappaconitine (8) and its metabolites detected did not show significant difference upon potential hydrolysis after incubation (in vitro) with the enzymes β-glucuronidase and sulfatase. This indicates that conjugation with glucuronic or (PAPS, the biological equivalent precursor for) sulfuric acid was not a major metabolic pathway.79
1 respectively,79 different from the rat metabolism ratio (i.v. administered).77 The high ratio of lappaconitine (8) compared to its metabolites is after i.m. administration which (like i.v.) allows the drug to bypass the liver, thereby avoiding first-pass (through the liver) metabolism. In addition, Allapinin (8 as an HBr salt) is likely to be more water soluble and potentially rapidly excreted unchanged in the urine. The amounts of lappaconitine (8) and its metabolites detected did not show significant difference upon potential hydrolysis after incubation (in vitro) with the enzymes β-glucuronidase and sulfatase. This indicates that conjugation with glucuronic or (PAPS, the biological equivalent precursor for) sulfuric acid was not a major metabolic pathway.79
5.4 Metabolic effects on biological activity
The high toxicity of Aconitum alkaloids is due mainly to the benzoyl ester at C14 and C8 acetate. C1, C6, C16, C18 methoxyl groups, and C13 hydroxyl also contribute to their toxicity.17,19 Hydrolysis of Aconitum alkaloids results in monoester alkaloids which have lower toxicity. In addition, demethoxylation and dehydration aid in lowering the toxicity. The formation of lipo-aconitines in rat and rabbit stomachs72 is an important biotransformation as they show an anti-inflammatory effect which is variable according to the length and degree of unsaturation of the fatty acids.80 The metabolic processes convert Aconitum alkaloids into more polar metabolites, and consequently their excretion will be promoted and their retention in the body will be shorter. Such conversion can be considered as a natural response to reduce the toxicity of these alkaloids.66The metabolism of lappaconitine (8) is mainly through deacetylation to yield N-deacetyllappaconitine (9) which shows higher biological activity than the parent compound.18 It has been proposed that lappaconitine (8) does not act directly on the CNS, but is converted in vivo into the active metabolite N-deacetyllappaconitine (9),81 and the activity can be considered as a sum of the parent compound and this metabolite.82 The number of metabolites of lappaconitine (8)69 was much higher than of mesaconitine (1), aconitine (2), and hypaconitine (3),64 and the lower toxicity of lappaconitine (8) compared with other Aconitum alkaloids17 can be attributed to this extensive metabolism, at least in rats.69 It is obvious that the liver is the main metabolic site, but the stomach and intestinal metabolism cannot be ignored as sites of metabolic detoxification.
6 Analysis of NDA
Aconitum alkaloids vary widely in their chemical structures and substitution patterns as well as being well known for their bioactivity and toxicity. Naturally occurring analogues with closely similar structures or positional isomers are widespread in herbs of the Aconitum genus. It is still challenging to identify rapidly unknown compounds by MS, especially positional isomers. MS is also used in the monitoring and detection of NDA metabolites, often achieved using LC-ESI-MS due to its sensitivity. It is important to understand the MS fragmentation of the parent alkaloids as the metabolites follow them in their fragmentation patterns. The fragmentation ions of aconitine (2)68,72 and lappaconitine (8)69 skeletons (as representative aconitine type and lappaconitine type NDA) show that such NDA undergo cleavage of the benzoate or acetate ester side chain, loss of water, and loss of methanol. These fragmentations involve typically two sequential cleavages of O-methyl ethers occurring with one or even two dehydration steps, in either order.Due to the lack of major MS fragmentations of the NDA skeleton, the actual position of the CYP-catalysed metabolic reactions e.g., O-demethylation, hydroxylation, can be difficult to pinpoint. In addition, it is difficult in some cases to determine if there is one metabolic process or a combination of more than one e.g., di-O-demethylation vs. N-deethylation. In such cases, the MS fragment analysis can indicate a metabolic position, but it needs to be confirmed using other analytical techniques.
F. Gao and co-workers continue to report new NDA, most recently from the roots of A. novoluridum and from the aerial parts of A. apetalum. The former provided 3 novolunines A, B, and C where the structures were established using spectral data analysis.83 The latter afforded 5 apetalrines A–E based on N-acylated 2-aminobenzoyl esters and also structurally identified following detailed spectral analysis.84 Ablajan et al. reported 5 previously undescribed DA, barpuberudine and barpubesines A–D, and two interesting novel C18 NDA, barpubenines A–B, isolated from the whole plant of A. barbatum var. puberulum Ledeb. The possible rearrangement of the C18 NDA structures led to a discussion of their probable biosynthetic pathway.85 The global profiling of NDA in A. stapfianum and analysis of the basis of its use for detoxification of fu'zi has been reported.86 The analysis was performed by UPLC/Q-TOF-(ESI)-MS with fragmentation patterns from MS/MS spectra as this is a method of high sensitivity, selectivity, and analytical speed.86
Our double Mannich route87,88 and the elegant studies of Brimble and co-workers89–93 afford a rapid and efficient entry to small molecule NDA analogues, i.e., without completing a total or even a formal synthesis of lycoctonine (14), inuline (13), or MLA (11). These studies are an important foundation for the next phase of this research. The regioselective O-demethylation of aconitine (2)94 has enabled its regioselective anthranoylation95 leading to such NDA analogues and thereby swapping from the VSSC to the nAChR series of important biological activities.53 Our studies on deuteriation96 led to the tritiation of the key ligand MLA (11).49,97,98 A huge amount of important pharmacology has been uncovered using this biological tool.
The through-space 1H NMR effect of steric compression by the lone-pair electrons of O- and N-atoms, with an associated deshielding effect, has been shown in synthetic [3.3.1]oxa- and azabicycles.99 Likewise the relevance of determining the crystal or solution protonated [3.3.1]azabicycle conformation, true-boat/true-chair conformation in the crystal lattice, but in solution the conformation is true-chair/true-chair.99
Moving from bicycles to hexacycles, conformational analyses have very recently been reported of several pharmacologically important NDA in crystal and in solution states.100 Indeed, crystal data of 8 NDA (4 free bases and 4 salts) were obtained, in which crassicauline A (7), aconitine (2) HCl, and methyllycaconitine (11) HClO4 have been reported for the first time together with the comprehensive 1D/2D NMR spectroscopies of 7 NDA.100 The A/E-rings of NDA-free bases exist in twisted-chair/twisted-chair conformations, with examples of the 1H NMR effect of steric compression shown in the A-ring. The A/E-rings of NDA salts adopt boat/chair crystal conformations, as do the protonated synthetic [3.3.1]azabicycle. However, in aqueous solutions at physiological pH, this protonated analogue adopts a true chair/true chair conformation where the NDA salts retain their twisted boat/twisted chair.100
15N NMR analysis showed that the 15N chemical shift of 1-OMe NDA increases (electron density decreases) upon protonation6 due to H-bonding between 1-OMe and N–H+ which stabilize the twisted boat-twisted chair conformation of the AE bicycle.100 Such an increase in the 15N chemical shift has been observed in the 1-αOH NDA free bases where ring A adopts a twisted boat conformation due to intramolecular H-bonding.5,6 These results prove that the analysis of the N-electron density of piperidine containing alkaloids is useful for solution conformational analysis.
7 Conclusions and future prospects
Although currently studied and, if starting with Pliny the Elder,3 now for 2000 years, research in this area of phytochemistry continues apace. New C19 and C18 NDA continue to be discovered and their biosynthesis will be better understood with analysis at the genetic level. These natural products and their analogues and semi-synthetic variants exhibit a wide range of important biological activities. SAR studies have a focus of discovering NDA with low toxicity and a wider therapeutic window. There are many metabolic studies on Aconitum alkaloids, but to-date none has been reported on Delphinium. NDA are excellent proving grounds for fundamental aspects of analytical chemistry.All of these continuing studies turn out to be based on the stellar contributions of Professor S. William Pelletier (1924–2004) who thought deeply and published widely in this important research area.
8 Conflicts of interest
The authors declare no competing financial interest.9 Acknowledgements
We thank Zarqa University, Jordan, for the Studentship to Ashraf M. A. Qasem. We thank the University of Bath for the partial support of Dr Ziyu Zeng.10 References
- Y. Shen, W.-J. Liang, Y.-N. Shi, E. J. Kennelly and D.-K. Zhao, Nat. Prod. Rep., 2020, 37, 763–796 RSC.
- D. Cook, J. A. Pfister, J. R. Constantino, J. M. Roper, D. R. Gardner, K. D. Welch, Z. J. Hammond and B. T. Green, J. Agric. Food Chem., 2015, 63, 1220–1225 CrossRef CAS PubMed.
- G. Plinius, Naturalis Historia, Book 23/37, Ch. XIII, 77 A.D., pp. 426–427 Search PubMed.
- M. H. Benn and J. M. Jacyno, Alkaloids: Chem. Biol. Perspect., 1983, vol. 1, 153–210 Search PubMed.
- Z. Zeng, A. M. A. Qasem, G. Kociok-Köhn, M. G. Rowan and I. S. Blagbrough, RSC Adv., 2020, 10, 18797–18805, 10.1039/d0ra03811c.
- Z. Zeng, A. M. A. Qasem, T. J. Woodman, M. G. Rowan and I. S. Blagbrough, ACS Omega, 2020, 5, 14116–14122, DOI:10.1021/acsomega.0c01648.
- F.-P. Wang and Q.-H. Chen, The C19-Diterpenoid Alkaloids, in The Alkaloids: Chemistry and Biology, ed. G. A. Cordell, Elsevier, Amsterdam, 2010, vol. 69, pp. 362–369 and references cited therein Search PubMed.
- G. H. Zhou, L. Y. Tang, X. D. Zhou, T. Wang, Z. Z. Kou and Z. J. Wang, J. Ethnopharmacol., 2015, 160, 173–193 CrossRef CAS PubMed.
- G. Gu, A. Dubreuil and X. Dubreuil, The Divine Farmer's Classic of Materia Medica (200–250 A.D.), Foreign Languages Press, Beijing, 2015 Search PubMed.
- Chinese Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China, vol. 1, China Medical Science Press, Beijing, 2020, edn, in Chinese Search PubMed.
- Chinese Pharmacopoeia Commission, People's Republic of China Ministry of Health, Drug Standards: Pharmaceutics of Chinese Medicine Prescriptions, vol. 8, Beijing, 1993, edn, in Chinese Search PubMed.
- Chinese Pharmacopoeia Commission, People's Republic of China Ministry of Health, Drug Standards: Tibetan Medicine, vol. 1, Beijing, 1995, edn, in Chinese Search PubMed.
- S. Rahman, R. Ali Khan and A. Kumar, BMC Complementary Altern. Med., 2002, 2, 6 CrossRef PubMed.
- B. Jiang, et al. , J. Nat. Prod., 2012, 75, 1145–1159 CrossRef CAS.
- Y. T. Tai, C. P. Lau, K. Young and P. H. But, Lancet, 1992, 340, 1254–1256 CrossRef CAS.
- X.-X. Liu, X.-X. Jian, X.-F. Cai, R.-B. Chao, Q.-H. Chen, D.-L. Chen, X.-L. Wang and F.-P. Wang, Chem. Pharm. Bull., 2012, 60, 144–149 CrossRef CAS PubMed.
- A. Ameri, Prog. Neurobiol., 1998, 56, 211–235 CrossRef CAS.
- A. Ameri, Brain Res., 1997, 769, 36–43 CrossRef CAS PubMed.
- F. Dzhakhangirov, M. Sultankhodzhaev, B. Tashkhodzhaev and B. Salimov, Chem. Nat. Compd., 1997, 33, 190–202 CrossRef CAS.
- J. Friese, J. Gleitz, U. T. Gutser, J. F. Heubach, T. Matthiesen, B. Wilffert and N. Selve, Eur. J. Pharmacol., 1997, 337, 165–174 CrossRef CAS.
- D. Y. Zhu, D. L. Bai and X. C. Tang, Drug Dev. Res., 1996, 39, 147–157 CrossRef.
- W. R. Dunstan and W. H. Ince, J. Chem. Soc., Trans., 1891, 59, 271–287 RSC.
- L. H. Keith and S. W. Pelletier, in Chemistry of the Alkaloids, ed. S. W. Pelletier, Van Nostrand Reinhold, New York, 1970, pp. 549–590 Search PubMed.
- Y. Li, Y. X. Li, M. J. Zhao, A. Yuan, X. H. Gong, M. J. Zhao and C. Peng, Eur. J. Drug Metab. Pharmacokinet., 2017, 42, 441–451 CrossRef CAS PubMed.
- M. Murayama, T. Mori, H. Bando and T. Amiya, J. Ethnopharmacol., 1991, 35, 159–164 CrossRef CAS.
- M. E. Adams and B. M. Olivera, Trends Neurosci., 1994, 17, 151–155 CrossRef CAS.
- T. F. Li, N. Gong and Y. X. Wang, Front. Pharmacol., 2016, 7, 367 Search PubMed.
- D. X. Lu, X. Guo and X. C. Tang, Acta Pharmacol. Sin., 1988, 9, 216–220 CAS.
- C. F. Wang, P. Gerner, S. Y. Wang and G. K. Wang, Anesthesiology, 2007, 107, 82–90 CrossRef CAS PubMed.
- H. Q. Zhu, J. Xu, K. F. Shen, R. P. Pang, X. H. Wei and X. G. Liu, Exp. Neurol., 2015, 273, 263–272 CrossRef CAS.
- H. V. Rosendahl, J. Pharm., 1896, 4, 262–266 CAS.
- J. Singhuber, M. Zhu, S. Prinz and B. Kopp, J. Ethnopharmacol., 2009, 126, 18–30 CrossRef PubMed.
- N. Mollov, M. Tada and L. Marion, Tetrahedron Lett., 1969, 10, 2189–2192 CrossRef.
- F.-P. Wang, Q.-H. Chen and X.-T. Liang, The C18-Diterpenoid Alkaloids, in The Alkaloids: Chemistry and Biology, ed. G. A. Cordell, Elsevier, Amsterdam, 2009, vol. 67, ch. 1, pp. 1–73 Search PubMed.
- Y. V. Vakhitova, E. I. Farafontova, R. Y. Khisamutdinova, V. M. Yunusov, I. P. Tsypysheva and M. S. Yunusov, Russ. J. Bioorg. Chem., 2013, 39, 92–101 CrossRef CAS.
- D. E. Zaurov, I. V. Belolipov, A. G. Kurmukov, I. S. Sodombekov, A. A. Akimaliev and S. W. Eisenman, in Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan, ed. S. W. Eisenman, D. E. Zaurov and L. Struwe, Springer, New York, 2013, ch. 5, pp. 15–273 Search PubMed.
- M. S. Yunusov, Russ. Chem. Bull., 2011, 60, 633–638 CrossRef CAS.
- T. M. Gabbasov, E. I. Andrianova, S. F. Petrova, S. P. Ivanov, E. M. Tsyrlina, A. Z. Sadikov, S. S. Sagdullaev and M. S. Yunusov, Chem. Nat. Compd., 2020, 56, 767–770 CrossRef CAS.
- I. B. Chernikova, T. M. Gabbasov, E. M. Tsyrlina and M. S. Yunusov, Russ. Chem. Bull., 2021, 70, 515–519 CrossRef CAS.
- S. Ou, Y. D. Zhao, Z. Xiao, H. Z. Wen, J. Cui and H. Z. Ruan, Neurochem. Int., 2011, 58, 564–573 CrossRef CAS PubMed.
- J. S. S. Quintans, A. R. Antoniolli, J. R. G. S. Almeida, V. J. Santana and L. J. Quintans, Basic Clin. Pharmacol. Toxicol., 2014, 114, 442–450 CrossRef CAS.
- F. M. Xie, H. C. Wang, J. H. Li, H. L. Shu, J. R. Jiang, J. P. Chang and Y. Y. Hsieh, Biomed. Chromatogr., 1990, 4, 43–46 CrossRef CAS.
- V. F. Hubschmann, Schweizerische Wochenschrift für Pharm, 1865, 3, 269–271 Search PubMed.
- V. G. Dragendorff and H. Spohn, Am. J. Pharm, 1884, 8, 44 Search PubMed.
- R. H. F. Manske, Can. J. Res., 1938, 16, 57–60 CrossRef.
- J. A. Goodson, J. Chem. Soc., 1943, 139–141 RSC.
- S. Wonnacott, E. X. Albuquerque and D. Bertrand, Methods Neurosci., 1993, 263–275 CAS.
- G. E. Barrantes, A. T. Rogers, J. Lindstrom and S. Wonnacott, Brain Res., 1995, 672, 228–236 CrossRef CAS.
- A. R. L. Davies, D. J. Hardick, I. S. Blagbrough, B. V. L. Potter, A. J. Wolstenholme and S. Wonnacott, Neuropharmacology, 1999, 38, 679–690 CrossRef CAS.
- O. E. Edwards and L. Marion, Can. J. Chem., 1954, 32, 1146–1148 CrossRef CAS.
- D. J. Hardick, I. S. Blagbrough, G. Cooper, B. V. L. Potter, T. Critchley and S. Wonnacott, J. Med. Chem., 1996, 39, 4860–4866 CrossRef CAS PubMed.
- F. I. Carroll, W. Ma, H. A. Navarro, P. Abraham, S. A. Wolckenhauer, M. Damaj and B. R. Martin, Bioorg. Med. Chem., 2007, 15, 678–685 CrossRef CAS PubMed.
- D. J. Hardick, G. Cooper, T. Scott-Ward, I. S. Blagbrough, B. V. L. Potter and S. Wonnacott, FEBS Lett., 1995, 365, 79–82 CrossRef CAS.
- M. A. Turabekova, B. F. Rasulev, F. N. Dzhakhangirov and S. I. Salikhov, Environ. Toxicol. Pharmacol., 2008, 25, 310–320 CrossRef CAS PubMed.
- J. D. Olsen, G. D. Manners and S. W. Pelletier, Collect. Bot., 1990, 19, 141–152 CrossRef.
- G. D. Manners, K. E. Panter, M. H. Ralphs, J. A. Pfister, J. D. Olsen and L. F. James, J. Agric. Food Chem., 1993, 41, 96–100 CrossRef CAS.
- C. F. Kukel and K. R. Jennings, Can. J. Physiol. Pharmacol., 1994, 72, 104–107 CrossRef CAS PubMed.
- G. D. Manners, K. E. Panter and S. W. Pelletier, J. Nat. Prod., 1995, 58, 863–869 CrossRef CAS.
- G. D. Manners, K. E. Panter, J. A. Pfister, M. H. Ralphs and L. F. James, J. Nat. Prod., 1998, 61, 1086–1089 CrossRef CAS PubMed.
- K. E. Panter, G. D. Manners, B. L. Stegelmeier, S. Lee, D. R. Gardner, M. H. Ralphs, J. A. Pfister and L. F. James, Biochem. Syst. Ecol., 2002, 30, 113–128 CrossRef CAS.
- B. T. Green, J. W. Keele, G. L. Bennett, D. R. Gardner, C. A. Stonecipher, D. Cook and J. A. Pfister, Toxicon, 2019, 165, 31–39 CrossRef CAS PubMed.
- B. T. Green, J. W. Keele, D. R. Gardner, K. D. Welch, G. L. Bennett, D. Cook, J. A. Pfister, T. Z. Davis, C. A. Stonecipher, S. T. Lee and B. L. Stegelmeier, J. Anim. Sci., 2019, 97, 1424–1432 CrossRef PubMed.
- K. D. Welch, B. T. Green, D. R. Gardner, D. Cook, J. A. Pfister and K. E. Panter, J. Anim. Sci., 2012, 90, 2394–2401 CrossRef CAS.
- Y. Wang, S. Wang, Y. Liu, L. Yan, G. Dou and Y. Gao, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2006, 844, 292–300 CrossRef CAS PubMed.
- L. Tang, L. Ye, C. Lv, Z. Zheng, Y. Gong and Z. Liu, Toxicol. Lett., 2011, 202, 47–54 CrossRef CAS PubMed.
- L. Ye, L. Tang, Y. Gong, C. Lv, Z. Zheng, Z. Jiang and Z. Liu, Xenobiotica, 2011, 41, 46–58 CrossRef CAS.
- Y. Bi, X. Zhuang, H. Zhu, F. Song, Z. Liu and S. Liu, Biomed. Chromatogr., 2015, 29, 1027–1034 CrossRef CAS PubMed.
- M. Zhang, C.-S. Peng and X.-B. Li, Toxicol. In Vitro, 2017, 45, 318–333 CrossRef CAS PubMed.
- S. Yang, H. Zhang, R. C. Beier, F. Sun, X. Cao, J. Shen, Z. Wang and S. Zhang, J. Pharm. Biomed. Anal., 2015, 110, 1–11 CrossRef CAS.
- H. Zhang, M. Liu, W. Zhang, J. Chen, Z. Zhu, H. Cao and Y. Chai, Biomed. Chromatogr., 2015, 29, 1076–1083 CrossRef CAS PubMed.
- G. Tan, M. Liu, X. Dong, S. Wu, L. Fan, Y. Qiao, Y. Chai and H. Wu, J. Pharm. Biomed. Anal., 2014, 96, 187–196 CrossRef CAS PubMed.
- Z. Sui, N. Li, Z. Liu, J. Yan and Z. Liu, Xenobiotica, 2013, 43, 628–635 CrossRef CAS.
- X. Fan, S. S. Yin, X. J. Li, K. Yang, L. Xu and K. Lan, Eur. J. Drug Metab. Pharmacokinet., 2017, 42, 857–869 CrossRef CAS.
- W. J. Yun, Z. H. Yao, C. L. Fan, Z. F. Qin, X. Y. Tang, M. X. Gao, Y. Dai and X. S. Yao, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, 1090, 56–64 CrossRef CAS.
- Y. Liu, H. Sun, C. Li, Z. Pu, Z. Wu, M. Xu, X. Li, Y. Zhang, H. Li, J. Dong, R. Bi, H. Xie and D. Liang, Xenobiotica, 2021, 51, 345–354 CrossRef CAS PubMed.
- B. Fan, S. Xu, J. Bi, S. Huang, Z. Zu and C. Qian, ACS Pharmacol. Transl. Sci., 2021, 4, 118–127 CrossRef CAS PubMed.
- F. Xie, H. Wang, H. Shu, J. Li, J. Jiang, J. Chang and Y. Hsieh, J. Chromatogr. B: Biomed. Sci. Appl., 1990, 526, 109–118 CrossRef CAS.
- H. G. Zhang, Y. Sun, M. Y. Duan, Y. J. Chen, D. F. Zhong and H. Q. Zhang, Toxicon, 2005, 46, 500–506 CrossRef CAS.
- F. Xie, H. Wang, J.-H. Li, H.-L. Shu, J.-R. Jiang, J.-P. Chang and Y.-Y. Hsieh, Biomed. Chromatogr., 1990, 4, 43–46 CrossRef CAS PubMed.
- B. Borcsa, U. Widowitz, D. Csupor, P. Forgo, R. Bauer and J. Hohmann, Fitoterapia, 2011, 82, 365–368 CrossRef CAS PubMed.
- X. Guo and X. C. Tang, Acta Pharmacol. Sin., 1990, 11, 107–112 CAS.
- M. S. Yunusov, Russ. Chem. Bull., 2011, 60, 633–638 CrossRef CAS.
- J. Lu, J.-B. Xu, X. Li, X.-L. Zhou, C. Zhang and F. Gao, Chem. Pharm. Bull., 2021, 69, 811–816 CrossRef CAS PubMed.
- L.-X. Wan, J.-F. Zhang, Y.-Q. Zhen, L. Zhang, X. Li, F. Gao and X.-L. Zhou, J. Nat. Prod., 2021, 84, 1067–1077 CrossRef CAS.
- N. Ablajan, B. Zhao, J.-Y. Zhao, B.-L. Wang, S. S. Sagdullaev and H. A. Aisa, Phytochemistry, 2021, 181, 112567, DOI:10.1016/j.phytochem.2020.112567.
- S. Liu, C. Lai, Y. Long, W. Yang, Q. Ren, L. Huang and J. Chen, J. Chromatogr. A, 2021, 1652, 462362 CrossRef CAS PubMed.
- P. A. Coates, I. S. Blagbrough, M. G. Rowan, B. V. L. Potter, D. P. J. Pearson and T. Lewis, Tetrahedron Lett., 1994, 35, 8709–8712 CrossRef CAS.
- G. Grangier, W. J. Trigg, T. Lewis, M. G. Rowan, B. V. L. Potter and I. S. Blagbrough, Tetrahedron Lett., 1998, 39, 889–892 CrossRef CAS.
- D. Barker, M. A. Brimble, M. McLeod, G. P. Savage and D. J. Wong, J. Chem. Soc., Perkin Trans. 1, 2002, 924–931 RSC.
- C. Brocke, M. A. Brimble, D. S.-H. Lin and M. D. McLeod, Synlett, 2004, 2359–2363 CAS.
- M. A. Brimble and C. Brocke, Eur. J. Org. Chem., 2005, 2385–2396 CrossRef CAS.
- K. J. Goodall, D. Barker and M. A. Brimble, Synlett, 2005, 1809–1827 CAS.
- K. J. Goodall, M. A. Brimble and D. Barker, Magn. Reson. Chem., 2006, 44, 980–983 CrossRef CAS PubMed.
- I. S. Blagbrough, D. J. Hardick, S. Wonnacott and B. V. L. Potter, Tetrahedron Lett., 1994, 35, 3367–3370 CrossRef CAS.
- D. J. Hardick, I. S. Blagbrough, S. Wonnacott and B. V. L. Potter, Tetrahedron Lett., 1994, 35, 3371–3374 CrossRef CAS.
- D. J. Hardick, I. S. Blagbrough and B. V. L. Potter, J. Am. Chem. Soc., 1996, 118, 5897–5903 CrossRef CAS.
- P. Whiteaker, A. R. L. Davies, M. J. Marks, I. S. Blagbrough, B. V. L. Potter, A. J. Wolstenholme, A. C. Collins and S. Wonnacott, Eur. J. Neurosci., 1999, 11, 2689–2696 CrossRef CAS.
- R. J. Lind, D. J. Hardick, I. S. Blagbrough, B. V. L. Potter, A. J. Wolstenholme, A. R. L. Davies, M. S. Clough, F. G. P. Earley, S. E. Reynolds and S. Wonnacott, Insect Biochem. Mol. Biol., 2001, 31, 533–542 CrossRef CAS.
- Z. Zeng, G. Kociok-Köhn, T. J. Woodman, M. G. Rowan and I. S. Blagbrough, ACS Omega, 2021, 6, 12769–12786 CrossRef CAS.
- Z. Zeng, G. Kociok-Köhn, T. J. Woodman, M. G. Rowan and I. S. Blagbrough, Eur. J. Org. Chem., 2021, 2169–2179 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |