Rocaglamide and silvestrol: a long story from anti-tumor to anti-coronavirus compounds
Göran
Schulz†
 a,
Catherine
Victoria†
a,
Catherine
Victoria†
 a,
Andreas
Kirschning
a,
Andreas
Kirschning
 *a and
Eike
Steinmann
b
*a and
Eike
Steinmann
b
aInstitute of Organic Chemistry, Leibniz University Hannover, Schneiderberg 1B, 30167 Hannover, Germany. E-mail: andreas.kirschning@oci.uni-hannover.de
bDepartment of Molecular and Medical Virology, Ruhr-University Bochum, Universitätsstrasse 150, 44801 Bochum, Germany
First published on 23rd July 2020
Abstract
Covering: up to the beginning of 2020
Many natural substances have been transformed again and again with regard to their pharmaceutical-medical potential, including new members of a growing class of natural products, the flavaglines. Important representatives are rocaglamide and silvestrol, isolated from the Aglaia species, which are highlighted here. These products started as potential anti-tumor agents five decades ago and have recently proved to be very promising antiviral agents, especially against RNA viruses. Today they are discussed as potential starting compounds for developing drug candidates and therapeutics.
Introduction
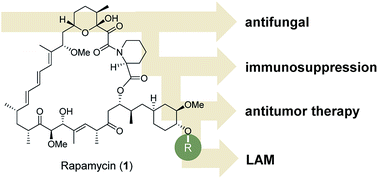
Besides their pharmaceutical relevance in many therapeutic areas, a remarkable aspect of natural products is the fact that they can address different biological targets and thus have been employed occasionally for completely different medical applications. However, the full biomedical potential of individual natural products is often unravelled over a long period of time.1Rapamycin 1 is a famous and telling example: it was discovered more than thirty years ago from Streptomyces hygroscopicus in a soil sample from the island of Rapa Nui.2 It showed antifungal activities but subsequently it was found to have immunosuppressive properties (Fig. 1). Under the name sirolimus, it is used to coat coronary stents and in prevention of organ transplant rejection, particularly in kidney transplants by inhibiting activation of T cells and B cells. In 1999 it was approved by the FDA and marketed under the trade name Rapamune. Rapamycin and its analogues (rapalogs) are used as inhibitors of the mammalian target of rapamycin (mTOR), a serine/threonine-specific protein kinase.
Later, it was found that rapamycin also inhibits cell growth in tumor cell lines.3 Hence, several rapamycin analogues were developed for treating breast and other cancers. Due to partial mTOR inhibition, rapalogs do not show sufficient activity for achieving a broad and robust anticancer effect, at least when used in monotherapy.4 Lately, it was established that rapamycin can be used in the treatment of lymphangioleiomyomatosis (LAM), a rare, progressive and systemic disease that typically results in cystic lung destruction. Rapamycin has become the first drug approved to treat this disease.5
In this highlight, we reveal the story of the rocaglates, a group of natural products that began almost half a century ago as promising anti-cancer agents and recently gained attention as agents against emerging RNA viruses like SARS-CoV-2. The reader is referred to a comprehensive review to obtain a more complete overview of this class of secondary plant metabolites.6
Rocaglamide and silvestrol – two natural products with a long history
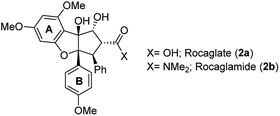
Besides the rocaglates, the aglain derivates and the aglaforbesin derivates belong to the natural product class of the flavaglines. These natural products originate from several species of the genus Aglaia (Meliaceae), a species of trees that grow in subtropical and tropical forests of Southeast Asia, Northern Australia and the Pacific region.7 It began with the analysis of alcoholic extracts of Aglaia elliptifolia as early as in 1975. These samples exerted significant activity against P-388 lymphatic leukemia in CDF1 mice and inhibitory activity in vitro against cells derived of human epidermoid carcinoma of the nasopharynx (KB cells).8 Almost a decade later, the bioactive substance responsible for the extract's antileukemic effect was isolated and its chemical structure was elucidated.9a King et al. named this first member of the flavaglines that commonly bear a 1H-cyclopenta[b]benzofuran core rocaglamide (2b; Fig. 2). It was postulated, that they are biosynthetically derived from two precursors, cinnamic acid derivatives and a flavonoid core.9bSince then, more than 100 flavaglines have been reported.6b The promising biological potential of rocaglamide initiated synthetic programmes that culminated in the first total synthesis by Trost et al. in 1990. This work also established the absolute configuration of rocaglamide.10
The second decade of research on the flavaglines saw the disclosure of more biological properties. For example, in 1992 Wiriyachitra and co-workers described the insecticidal effect of rocaglamide against the variegated cutworm, Peridroma saucia.11 Moreover, an antifungal effect of different flavaglines, isolated from Aglaia odorata, A. elaeagnoidea, and A. edulis were found at the turn of the century.12 Inspired by the use of crude extracts from different Aglaia species as anti-inflammatory remedies in traditional medicine in several Southeast Asian countries, Proksch and co-workers investigated various natural rocaglamides for their anti-inflammatory properties. It was shown that these inhibit NF-κB activation in T cells with very high effectiveness.13a As a large number of flavaglines with significantly different biological properties and varying efficacy were uncovered, first relationships between structure and activity could be drawn in 1999.13b
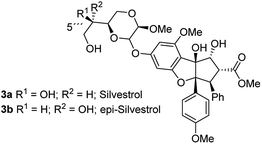
As evidence of the structure–activity relationship was emerging, a very prominent member of this class of compounds, flavagaline and its 5′′′-epimer was isolated from Aglaia foveolata in the year 2004.14a Structurally, silvestrol (3a) and epi-silvestrol (3b) contain the unusual dioxanyloxy unit that is attached to the phenyl ring A (Fig. 3). Both epimers are more cytotoxic in in vitro tests such as breast (MCF-7, ED50 = 1.5 nM for 3; 5.5 nM for 3b), prostate (LNCaP, ED50 = 1.5 nM for 3a; 3 nM for 3b), lung (Lu1, ED50 = 1.2 nM for 3a; 3.8 nM for 3b) and colon (HT-29, ED50 = 0.7 nM for 3a; 2.29 μM for 3b) cancer cell lines than rocaglamide (ED50 = 5.0 nM against HT-29) and other analogues that lack the dioxanyloxy moiety.14
Silvestrol (3a) was also subjected to in vivo studies in the hollow fiber test and in the murine P-388 leukemia model. These studies revealed a particularly pronounced activity against the human prostate cancer line LNCaP.14a
Originally, silvestrol was validated as an anticancer lead that included studies on its mode of action. In 2006, Swanson et al. demonstrated that silvestrol significantly induces alterations of 20 apoptosis and cell cycle-related genes in LNCaP cancer cells.15 It showed cytotoxic effects in LNCaP cells by blocking the cell cycle at the G2/M control point. This is an important barrier for tumor formation in healthy cells as replication of cells with DNA damage is prevented.15
It took 26 years after the rocaglamide discovery that a new therapeutic door was opened for other flavaglines for their antiviral properties. As part of a study that described the cytotoxic activities of newly discovered flavaglines isolated from Aglaia foveolata, another producer of silvestrol, the authors noted that desacetylpyramidaglain C (4; Fig. 4) exerts moderate antiviral activity against the herpes simplex virus type 1 (HSV-1). Remarkably, this plant metabolite also showed a moderate antibacterial effect against the Mycobacterium tuberculosis H37Ra.16
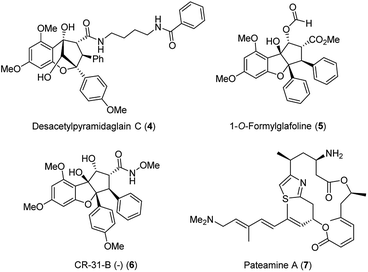 | ||
| Fig. 4 Structures of desacetylpyramidaglain C (4), 1-O-formylglafoline (5), of rocaglate derivative CR-31-B (−) (6) and of panteamine A (7). | ||
This finding redirected the focus of flavaglines and breathed another biomedical life into them from 2008 onwards. This appears to be surprising in light of a report from the year 1983, which disclosed that the extracts of Aglaia roxburghiana var. beddomei showed antiviral activities.17 However, at that time no link was established with the natural product class of the flavaglines and research was not extended to this potential medicinal use.
A series of publications rapidly followed that widened and deepened the understanding of these antiviral properties and the biological target of selected flavaglines. Silvestrol (3a) and 1-O-formylglafoline (5) were further studied and were shown to stimulate eIF4Af-RNA clamping.18 The eukaryotic initiation factor 4A (eIF4A) is an ATP-dependent RNA helicase, responsible for unwinding the secondary structure of mRNAs. Flavaglines force an engagement between eIF4A and RNA and prevent eIF4A from participating in the ribosome-recruitment step of translation (Fig. 5). The effect is comparable for the one established with the macrocyclic mixed polyketide–peptide pateamine A (7),19 but in case of silvestrol the toxicity is reduced compared to this “gold standard”. At this point one has to raise the question, why silvestrol (3a) initially was studied for anti-tumor therapy despite its low cytotoxic properties. The stimulation of RNA binding of eIF4Ac by silvestrol is cap-dependent by inhibiting protein synthesis without observable cell apoptosis in many tumor and cancer cell lines such as breast (MDA-MB-231) and prostate (PC-3) cancer cell lines, angiogenic stimulated HUVEC and xenografts tumor cells.20
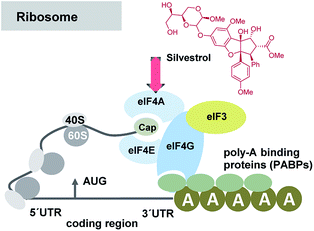 | ||
| Fig. 5 Simplified presentation of elements involved in the initiation of eukaryotic translation and site of interaction of silvestrol (3a). Several eukaryotic initiation factors (eIFs) are involved in the recruitment of the 43S PIC to the mRNA template. It is facilitated by eIF-4F, a complex consisting of the mRNA 5′-cap-binding subunit (eIF-4E), a large scaffolding protein (eIF-4G) and a RNA helicase (eIF-4A). eIF-4G also interacts with the poly(A)-binding protein (PABP), which associates with the mRNA 3′ poly(A) tail, to cause mRNA circularization to stabilize mRNAs and bolster translation.21 | ||
Since virus replication relies on the host translation machinery and thus on elF4A, it is not surprising that silvestrol and other flavaglines show antiviral activity. Reduction of translation leads to a decrease of viral capsid formation and several host-factors activities. E.g. for the hepatitis E virus these include RNAses and proteasomal, lysosomal or autophagosomal proteins.22 In 2018 it was reported that silvestrol inhibits hepatitis E virus (HEV) replication in vitro and in vivo.21 As HEV also upregulates Major Vault Protein expression (MVP, virus-inducible host-defense mechanism) in the host, it is worth noting that silvestrol significantly decreases the amount of MVP in HEV-positive cells.22 Silvestrol is considered as pangenotypic anti-HEV agent with high potency, due to its capability to inhibit the replication of different HEV genotypes at a low nanomolar concentration. Combined treatment of viral infections with ribavirin, a nucleoside inhibitor, revealed an additive effect. Mechanistically both molecules have different modes of action, which is important from a therapeutic point of view when considering the problem of resistance.23
A year earlier, the excellent broadband antiviral activity of silvestrol (3a) was further substantiated by the finding that this activity extends to the highly pathogenic Ebola virus,24 as well as corona-, Zika- and picornaviruses without marked cytotoxic effects for primary cell lines (Huh-7 and MRC-5).25 Here, silvestrol (3a) inhibits protein expression and formation of viral replication/transcription complexes. The reduced potency towards picornaviruses was rationalized in that translation is mediated by an internal ribosomal entry site mechanism (IRES).
That year a synthetic rocaglate CR-31-B (−) (6) was studied as potent broad-spectrum antiviral agent in primary cells and in an ex vivo bronchial epithelial cell system. The derivative is able to inhibit the replication of corona-, Zika-, Lassa- and Crimean Congo hemorrhagic fever viruses. As in several other studies with flavagines before, the cytotoxicity was determined to be at low nanomolar concentrations.26a–c
In contrast to silvestrol,22 the effect of CR-31-B (−) (6) on hepatitis E virus (HEV) is less pronounced.27 HEV has a polypurine-free 5′-UTR, however, it is predicted to still fold into a stable hairpin structure. Crystal structure analysis of human eIF4A bearing a polypurine RNA and RocA suggested that purine bases are important for π–π interactions with rings A and B of rocaglate (Fig. 2).28 Importantly, in the absence of polypurine, silvestrol can still clamp RNA and the authors hypothesised that the unique dioxane moiety has to be made responsible.
Noteworthy, silvestrol also displayed antiviral activity against human coronaviruses (CoV) like HCoV-E229 and MERS-CoV, implying potential broad-spectrum antiviral activity against this virus family.25 Given the diversity of CoV strains in zoonotic reservoirs with epidemic potential, as most recently exemplified by the newly emerged SARS-CoV-2, the causative agent of COVID-19, broadly active antivirals are clearly required to rapidly respond to new CoV outbreaks. Earlier this year the simplified derivative of silvestrol CR-31-B (6) has been found to be an attractive starting point to optimise antiviral agents with therapeutic potential against SARS-CoV-2.29,30 Moreover, as a host targeting agent with potential broad-spectrum antiviral activity, silvestrol has a high genetic barrier for development of resistance and can help to maximise pandemic preparedness during future outbreaks.
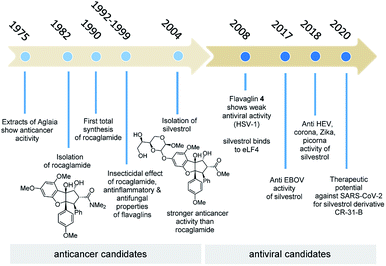
The natural products rocaglamid (2b) and silvestrol (3a) have a long history of academic interest. In 1975, the same year in which rapamycin, the starting point of our journey, was found, two reports on the anticancer effect of extracts from Aglaia species were published for the first time,8 the starting point of an exciting scientific and biomedical journey (Fig. 6). In the first phase of this journey, which lasted almost 30 years, the focus was on their anticancer properties, while the second part of the story is strongly related to the antiviral properties of these two flavaglines and several synthetic derivatives. The importance of natural products for medicinal chemistry and drug development has diminished over the last three decades. An often overlooked reason of this development has been covered in this highlight. Natural products are evolutionary optimised ligands for natural receptor macromolecules, which makes them highly attractive for pharmaceutical research. The time that is sometimes needed to find the right therapeutic application can span over several decades and often the story is not straightforward (Fig. 6).
Silvestrol and synthetic analogues may be another example of such a natural product success story which hopefully will end up in a new broadband antiviral drug. The current pandemic situation of COVID-19 will likely intensify research on nature-derived lead structures with potential antiviral properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Bundesministerium für Bildung und Forschung (BMBF, project SILVIR: 16GW0202).Notes and references
- N. E. Thomford, D. A. Senthebane, A. Rowe, D. Munro, P. Seele, A. Maroyi and K. Dzobo, Int. J. Mol. Sci., 2018, 19, 1578 CrossRef.
- S. N. Sehgal, H. Baker and C. Vézina, J. Antibiot., 1975, 727–732 CrossRef CAS.
- S. Vignot, S. Faivre, D. Aguirre and E. Raymond, Ann. Oncol., 2005, 16, 525 CrossRef CAS.
- (a) S. Brachmann, C. Fritsch, S.-M. Maira and C. García-Echeverría, Curr. Opin. Cell Biol., 2009, 21, 194–198 CrossRef CAS; (b) Y.-J. Zhang, Y. Duan and X. F. S. Zheng, Drug Discovery Today, 2011, 16, 325–331 CrossRef CAS; (c) S. A. Wander, B. T. Hennessy and J. M. Slingerland, J. Clin. Invest., 2011, 121, 1231–1241 CrossRef CAS.
- F. X. McCormack, Y. Inoue, J. Moss, L. G. Singer, C. Strange, K. Nakata, A. F. Barker, J. T. Chapman, M. L. Brantly, J. M. Stocks, K. K. Brown, J. P. Lynch 3rd, H. I. Goldberg, L. R. Young, B. W. Kinder, G. P. Downey, E. J. Sullivan, T. V. Colby, R. T. McKay, M. M. Cohen, L. Korbee, A. M. Taveira-DaSilva, H. S. Lee, J. P. Krischer and B. C. Trapnell, N. Engl. J. Med., 2011, 364, 1595–1606 CrossRef CAS.
- (a) L. Pan, J. L. Woodard, D. M. Lucas, J. R. Fuchs and A. D. Kinghorn, Nat. Prod. Rep., 2014, 31, 924–939 RSC; (b) S. S. Ebada, N. Lajkiewicz, J. A. Porco, M. Li-Weber and P. Proksch, Prog. Chem. Org. Nat. Prod., 2011, 94, 1–58 CAS.
- C. M. Pannell, in Tree Flora of Sabah and Sarawak, ed. E. Soepadmo, L. G. Saw, R. C. K. Chung and R. Kiew, Ampang Press Sdn Bhd, Kuala Lumpur, Malaysia, 2007, pp. 24–107 Search PubMed.
- M. L. King, H. C. Ling, C. B. Wang and S. C. Leu, Med. Sci., 1975, 1, 11 Search PubMed.
- (a) M. L. King, C.-C. Chiang, H.-C. Ling, E. Fujita, M. Ochiai and A. T. McPhail, J. Chem. Soc., Chem. Commun., 1982, 1150–1151 RSC; (b) B. W. Nugroho, R. A. Edrada, V. Wray, L. Witte, G. Bringmann, M. Gehling and P. Proksch, Phytochemistry, 1999, 51, 367–376 CrossRef CAS.
- B. M. Trost, P. D. Greenspan, B. V. Yang and M. G. Saulnier, J. Am. Chem. Soc., 1990, 112, 9022–9024 CrossRef CAS.
- C. Satasook, M. B. Isman and P. Wiriyachitra, Pestic. Sci., 1992, 36, 53–58 CrossRef CAS.
- D. Engelmeier, F. Hadacek, T. Pacher, S. Vajrodaya and H. Greger, J. Agric. Food Chem., 2000, 48, 1400–1404 CrossRef CAS.
- (a) B. Baumann, F. Bohnenstengel, D. Siegmund, H. Wajant, C. Weber, I. Herr, K.-M. Debatin, P. Proksch and T. Wirth, J. Biol. Chem., 2002, 277, 44791–44800 CrossRef CAS; (b) F. I. Bohnenstengel, K. G. Steube, C. Meyer, B. W. Nugroho, P. D. Hung, L. C. Kiet and P. Proksch, Z. Naturforsch., C: J. Biosci., 1999, 54, 55–60 CAS.
- (a) B. Y. Hwang, B.-N. Su, H. Chai, Q. Mi, L. B. S. Kardono, J. J. Afriastini, S. Riswan, B. D. Santarsiero, A. D. Mesecar, R. Wild, C. R. Fairchild, G. D. Vite, W. C. Rose, N. R. Farnsworth, G. A. Cordell, J. M. Pezzuto, S. M. Swanson and A. D. Kinghorn, J. Org. Chem., 2004, 69, 3350–3358 CrossRef CAS; (b) L. Pan, L. B. S. Kardono, S. Riswan, H. Chai, E. J. Carcache de Blanco, C. M. Pannell, D. D. Soejarto, T. G. McCloud, D. J. Newman and A. D. Kinghorn, J. Nat. Prod., 2010, 73, 1873 CrossRef CAS; (c) L. Pan, U. M. Acuña, J. Li, N. Jena, T. N. Ninh, C. M. Pannell, H. Chai, J. R. Fuchs, E. J. Carcache de Blanco, D. D. Soejarto and A. D. Kinghorn, J. Nat. Prod., 2013, 76, 394 CrossRef CAS . The name silvestrol is the result of a mistake, since the authors initially assumed that they isolated the product from Aglaia silvestris (ref. 13b).
- Q. Mi, S. Kim, B. Y. Hwang, B.-N. Su, H. Chai, Z. H. Arbieva, A. D. Kinghorn and S. M. Swanson, Anticancer Res., 2006, 26, 3349–3356 CAS.
- N. Joycharat, H. Greger, O. Hofer and E. Saifaha, Phytochemistry, 2008, 69, 206–211 CrossRef CAS.
- O. P. Babbar, M. N. Joshi and B. L. Chowdhury, Indian J. Exp. Biol., 1983, 21, 637–638 CAS.
- M.-E. Bordeleau, F. Robert, B. Gerard, L. Lindqvist, S. M. H. Chen, H.-G. Wendel, B. Brem, H. Greger, S. W. Lowe, J. A. Porco Jr and J. Pelletier, J. Clin. Invest., 2008, 118, 2651–2660 CAS.
- M.-E. Bordeleau, R. Cencic, L. Lindqvist, P. Northcote, G. Wagner and J. Pelletier, Cell Chem. Biol., 2006, 13, 1287–1295 CAS.
- R. Cencic, M. Carrier, G. Galicia-Vázquez, M.-E. Bordeleau, R. Sukarieh, A. Bourdeau, B. Brem, J. G. Teodoro, H. Greger, M. L. Tremblay, J. A. Porco Jr and J. Pelletier, PLoS One, 2009, 4, e5223 CrossRef.
- Further details on eukaryotic translation see: M. Bhat, N. Robichaud, L. Hulea, N. Sonenberg, J. Pelletier and I. Topisirovic, Nat. Rev. Drug Discovery, 2015, 14, 261–278 CrossRef CAS.
- M. Glitscher, K. Himmelsbach, K. Woytinek, R. Johne, A. Reuter, J. Spiric, L. Schwaben, A. Grünweller and E. Hildt, Viruses, 2018, 10, 301 CrossRef.
- D. Todt, N. Moeller, D. Praditya, V. Kinast, M. Friesland, M. Engelmann, L. Verhoye, I. M. Sayed, P. Behrendt, V. L. Dao Thi, P. Meuleman and E. Steinmann, Antiviral Res., 2018, 157, 151–158 CrossRef CAS.
- N. Biedenkopf, K. Lange-Grünweller, F. W. Schulte, A. Weißer, C. Müller, D. Becker, S. Becker, R. K. Hartmann and A. Grünweller, Antiviral Res., 2017, 137, 76 CrossRef CAS.
- C. Müller, F. W. Schulte, K. Lange-Grünweller, W. Obermann, R. Madhugiri, S. Pleschka, J. Ziebuhr, R. K. Hartmann and A. Grünweller, Antiviral Res., 2018, 150, 123–129 CrossRef.
- (a) F. Elgner, C. Sabino, M. Basic, D. Ploen, A. Grünweller and E. Hildt, Viruses, 2018, 10, 149 CrossRef; (b) S. Günther, M. Asper, C. Röser, L. K. S. Luna, C. Drosten, B. Becker-Ziaja, P. Borowski, H. M. Chen and R. S. Hosmane, Antiviral Res., 2004, 63, 209–215 CrossRef; (c) S. Ölschläger, M. Gabriel, J. Schmidt-Chanasit, M. Meyer, E. Osborn, N. G. Conger, P. F. Allan and S. Günther, J. Clin. Virol., 2011, 50, 90–92 CrossRef.
- C. Müller, W. Obermann, F. W. Schulte, K. Lange-Grünweller, L. Oestereich, F. Elgner, M. Glitscher, E. Hildt, K. Singh, H.-G. Wendel, R. K. Hartmann, J. Ziebuhr and A. Grünweller, Antiviral Res., 2020, 175, 104706 CrossRef.
- S. Iwasaki, W. Iwasaki, M. Takahashi, A. Sakamoto, C. Watanabe, Y. Shichino, S. N. Floor, K. Fujiwara, M. Mito, K. Dodo, M. Sodeoka, H. Imataka, T. Honma, K. Fukuzawa, T. Ito and N. T. Ingolia, Mol. Cell, 2019, 73, 738–748 CrossRef CAS.
- G. Li and E. De Clercq, Nat. Rev. Drug Discovery, 2020, 3, 149–150 CrossRef.
- Y. Zhou, Y. Hou, J. Shen, Y. Huang, W. Martin and F. Cheng, Cell Discovery, 2020, 6, 14 CrossRef CAS.
Footnote |
| † These authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2021 |