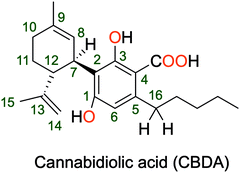
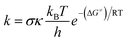Is cannabidiolic acid an overlooked natural antioxidant? Insights from quantum chemistry calculations†
Houssem
Boulebd

Laboratory of Synthesis of Molecules with Biological Interest, University of Frères Mentouri Constantine 1, Constantine, Algeria. E-mail: boulebd.houssem@umc.edu.dz
First published on 16th November 2021
Abstract
Cannabidiolic acid (CBDA) is one of the most abundant phytocannabinoids found in cannabis. This compound has demonstrated interesting biological activities, including anti-cancer, anti-inflammatory, anti-emetic, and anti-convulsant. However, due to increased emphasis on the research of its derivative, cannabidiol, its potential pharmacological effects have been hidden for years. In this contribution, a systematic study of the antioxidant capacity of CBDA was conducted using advanced quantum chemistry methods. Different reaction mechanisms at all potential positions were examined for the reaction of CBDA with the hydroperoxyl radical (HOO˙) and nitrogen dioxide (NO2). The influence of the physiological environment and the acid–base equilibrium were also taken into account. CBDA has been shown to be a good HOO˙ and NO2 scavenger in physiological polar media with rate constants of 2.40 × 106 and 1.30 × 106 M−1 s−1, respectively. This efficiency is mainly due to the increased activity of its dianionic form. When compared to some common antioxidants, the HOO˙ scavenging activity of CBDA is higher than that of Trolox and BHT (butylated hydroxytoluene), and suggests that CBDA is a promising radical scavenger, especially in polar environments.
1. Introduction
Phytochemicals are essential to all living organisms. These natural compounds are essential to humans, whether they are drugs, vitamins, tinctures, beauty products, or even toxins. In terms of therapeutic benefit, phytocannabinoids rank first among this seemingly limitless family of compounds. Phytocannabinoids are molecules that are mostly found in cannabis plants and interact with the endocannabinoid system.1 Humans have been using these molecules for thousands of years, primarily for therapeutic purposes. In just a few decades of research, scientists have discovered more than 100 cannabinoids in the cannabis plant alone.2 The cannabis plant produces cannabinoids in the form of carboxylic acids, which are then converted into neutral form by light and heat. Among the isolated cannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD) are by far the most well-known and widely used molecules in cannabis.3–5 These compounds have the special characteristic of mimicking the effects of endocannabinoids in our bodies and activating our internal health systems. THC is a psychoactive molecule that can partially mimic the natural neurotransmitter “anandamide” and thus activates the CB1 and CB2 receptors, whereas CBD is a non-psychoactive molecule that can modulate the effects of THC.6,7 THC and CBD have both attracted the attention of researchers in recent years due to their remarkable therapeutic potential.Cannabidiolic acid is another molecule abundant in the Cannabis plant (CBDA, Fig. 1).8 CBDA is the precursor of CBD and the primary cannabinoid in hemp seed oil. Due to the intense scientific interest in its derivative, CBD, the therapeutic potential of this molecule has been kept hidden for many years.9 This can be seen by comparing the number of articles published in recent years dealing with CBD, which totals 3687, to those dealing with CBDA, which totals no more than 153 papers (Fig. S1, ESI†). Takeda, S. et al. revealed the first useful evidence regarding the therapeutic potential of CBDA in 2008, demonstrating that CBDA can selectively inhibit cyclooxygenase (COX-2) with a 9-fold higher activity than the inhibition of COX-1, which gives it anti-inflammatory capacity.10 Following that, more extensive research on the anti-inflammatory activity of CBDA was conducted. CBDA, alone or in combination with THC, has been shown in a rodent model of acute carrageenan-induced inflammation in the rat hind paw to have anti-inflammatory and anti-hyperalgesic effects.11 Other studies have shown that administering a low dose of CBDA to rats significantly reduced yawning while strengthening the anti-nausea effect of ondansetron. This suggests that combining CBDA and ondansetron may be more effective in treating acute nausea in chemotherapy patients.12 The anticancer potential of CBDA has been demonstrated against a highly aggressive triple-negative breast cancer cell line (MDA-MB-231). CBDA has the ability to suppress COX-2 mRNA expression as well as the migration of breast cancer cells.13–15 CBDA prevents vomiting in shrews and nausea in rats more effectively than cannabidiol while also increasing 5-HT1A receptor activity. As a consequence, CBDA has the potential to be used as a treatment for nausea and vomiting, particularly in anticipatory disease, which currently has no drug for its treatment.16 A recent study demonstrated in vivo that the bioavailability of CBDA and THCA is significantly better than that of their metabolites CBD and THC.17 All of these findings clearly show that CBDA is a chemical whose bioactivity and pharmacological efficacy have not been thoroughly investigated. Thus, maximizing its full potential and deepening our understanding of its medicinal efficacy should be taken into consideration.
The in vitro evaluation of the antioxidant effects of phytocannabinoids has received a lot of attention, but often without defining their mode of action at the molecular level. CBD, one of the most studied molecules, has received special attention in recent years.18 Experiments have shown that CBD can have a direct impact on the components of the redox system as well as indirectly interacting with other molecular targets related to the components of the redox system to regulate the redox state. Furthermore, it should be noted that there are very few valuable mechanistic studies on the antioxidant action of this molecule. On the other hand, there is only one study to our knowledge that has examined the antioxidant properties of isolated CBDA. It has been reported that cannabinoid acids, including CBDA, present an antioxidant potential greater than that of their neutral counterparts as well as Trolox in a basic or neutral environment (ORAC and CUPRAC assays).19 In this study, it was also shown that the studied cannabinoids are more effective than Trolox in reducing radicals by electron transfer via the SET mechanism (ABTS, CUPRAC, and FRAP assays). This experimental study suggests that CBDA may be an effective natural antioxidant; therefore, further research on its antioxidant properties is required. In the present contribution, we examined in detail both the antioxidant properties and the mechanism of action of CBDA using advanced quantum chemistry methods. All conceivable antiradical mechanisms were considered at all possible sites of CBDA. The influence of physiological conditions, as well as the acid–base equilibrium, were also taken into account. Furthermore, the overall reactivity of CBDA was compared to that of recognized antioxidants.
2. Computational details
To evaluate the antioxidant properties of CBDA, we carried out a detailed theoretical study on the thermodynamics and the kinetics of its reactivity towards the HOO˙ radical and NO2. These two radicals are moderately reactive and have been recommended as an ideal choice for studying antioxidant activity.20,21 The HOO˙ radical is a typical ROS (reactive oxygen species) and it is the most basic of the peroxyl radicals (ROO˙) that are the main targets of phenolic antioxidants.22,23 NO2 is an RNS (reactive nitrogen species) that plays a major role in nitrogen stress.24 Studying these two compounds may provide insight into the reactivity of other important ROS/RNS. The three main mechanisms of antioxidant activity, HAT (hydrogen atom transfer), RAF (radical adduct formation), and SET (single electron transfer) have been studied.25,26 HAT and RAF can take place in both polar and lipid environments, while SET is only possible in polar environments.27,28 However, the SET of an undissociated OH group is often infeasible.29,30 It is therefore significant only for the deprotonated OH groups. The following equations summarize the three mechanisms studied for CBDA.| CBDA(O/C−H) + R˙ → CBDA(O/C)˙ + RH (HAT)CBDA + R˙ → [CBDA + R]˙ (RAF)CBDA(O−H) → CBDA(O)− + H+; CBDA(O)− + R˙ → CBDA(O)˙ + R− (SET) |
All computations in this work were performed using the Gaussian 09 suite of programs.31 The DFT (density functional theory) method using the M06-2X functional32 and the 6-311++G(d,p) basis set was employed for all calculations. The M06-2X functional is one of the most reliable methods to study thermodynamics and kinetics of radical reactions.33,34 The ground and transition states were confirmed by the number of imaginary frequencies (IF): no IF for the ground states and only one for the transition states. The solvation model density (SMD) approach was used to predict the solvent effects of water and pentyl ethanoate, which is frequently used to evaluate the radical scavenging activity of antioxidants with low inaccuracies when compared to experimental data (kcalc/kexp ratio = 1–2.9).20,35–38
The kinetic calculations were carried out following the QM-ORSA (quantum mechanics-based test for overall free radical scavenging activity) methodology.20,21,39 The rate constant (k) was calculated using the standard transition state theory (TST) and a 1 M standard state at 298.15 K according to the equation below:38,40–45
3. Results and discussion
3.1. Analysis of molecular geometry and acid–base equilibrium
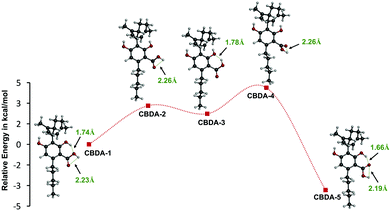
In the initial step, the most stable conformation of CBDA was determined since the reactivity of the different groups is significantly influenced by the surrounding environment. As shown in Fig. 2, CBDA adopts five low-energy conformations stabilized by at least one hydrogen bond. Conformers 1 and 5 have two hydrogen bonds and are more stable compared to the other three conformers. The carbonyl of the carboxyl group of conformer 5 is located between two OH groups and forms two hydrogen bonds with distances of 1.66 and 2.19 Å. While in conformer 1 the carboxyl group is in the opposite orientation and the molecule has two hydrogen bonds of 1.74 and 2.23 Å between O–H⋯O–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯O–H, respectively. By comparison, conformer 5 is characterized by the shortest intramolecular hydrogen bonds and it is more stable than conformer 1 by about 3.3 kcal mol−1. Therefore, only conformer 5 was considered in the following thermodynamic and kinetic calculations.
O⋯O–H, respectively. By comparison, conformer 5 is characterized by the shortest intramolecular hydrogen bonds and it is more stable than conformer 1 by about 3.3 kcal mol−1. Therefore, only conformer 5 was considered in the following thermodynamic and kinetic calculations.
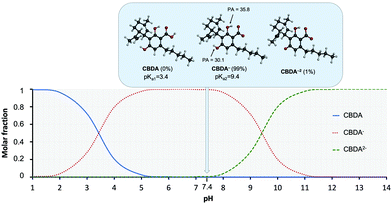
The acid–base equilibrium is another important factor to consider, particularly when studying the effects of the polar physiological environment. At physiological pH, the different OH or COOH groups can deprotonate, which leads to changes in the electronic characteristics and molecular geometry. This can significantly influence the antioxidant capacity. Since the experimental pKa values of CBDA have not yet been determined, we used an approved theoretical approach to predict the pKa values of CBDA.49 The obtained results are depicted in Fig. 3. It is clear from Fig. 3 that in solution at physiological pH most of the CBDA is in the monoanionic state (CBDA−), but a small fraction of the dianionic state (CBDA−2) also exists. Following the SET mechanism, these dianionic states are known to be very reactive, even in small proportions, and can be the driving force behind the antioxidant capacity of a molecule.29,50 The dianionic state of CBDA was therefore also considered for the SET mechanism in water. While only the monoanionic state was considered for the RAF and HAT mechanisms.
3.2. Analysis of reactivity under physiological conditions
The reactivity of CBDA towards reactive species was first evaluated by calculating the Gibbs free energy of all conceivable pathways at all possible sites. The obtained results in polar and lipid media are reported in Fig. 4. In a pentyl ethanoate solution, the reaction of CBDA with both HOO˙ and NO2 was found to be exergonic only via the HAT mechanism at the 7CH position (ΔG = −7.4 and −11.1 kcal mol−1); however, the reaction with HOO˙ at the 1OH position is almost isergonic (ΔG = 2.2 kcal mol−1) and therefore was also taken into account in the kinetic study. In water, CBDA appears to be more reactive than in the lipid-like environment. In this medium, the HAT mechanism is possible for the two studied radicals at both the 7CH and 1OH sites. The SET mechanism is also a conceivable pathway with NO2. Among the various reactions, the SET mechanism with NO2 in water had the lowest ΔG value (−17.9 kcal mol−1); however, the same reaction is exergonic with HOO˙ (ΔG = 3.1 kcal mol−1). The difference in reactivity between these two radicals can be explained by their different electronegativity. As it is well known that the SET process is very fast and can occur even with positive ΔG values, it has been taken into account in the kinetic analysis for both HOO˙ and NO2.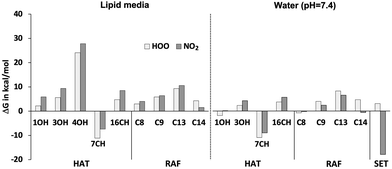 | ||
| Fig. 4 Thermodynamic analysis of the reactions of CBDA with HOO˙ and NO2 in water and pentyl ethanoate. | ||
Regarding the RAF-type reactions, it is well established that reactions with quasi-isergonic behavior are reversible under physiological conditions and are not effective in inhibiting free radicals.51,52 As can be seen from Fig. 4, the computed ΔG values for the RAF reactions range from −0.7 to 10.6 kcal mol−1, which indicates that this mechanism cannot take place for the scavenging of HOO˙ and NO2 by CBDA in physiological environments.
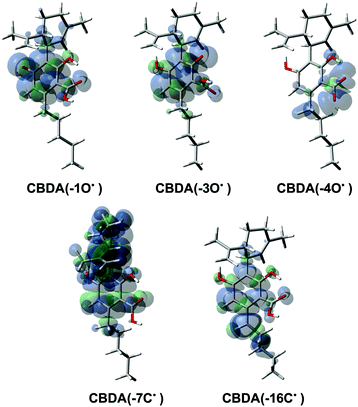
The BDE values of the different OH and CH groups are shown in Table 1. As is illustrated, the calculated BDEs have a similar order in the two studied media. The lowest BDE value was determined for the 7CH group, which is in good agreement with the calculated ΔG values. For the OH groups, the BDE of 1OH is slightly lower than that of 3OH and is significantly lower than that of 4OH. The spin density distributions of each radical product in the gas phase, as shown in Fig. 5, can be used to explain these observations. As can be seen, the resulting radical from the 7CH group has the most distributed spin density, which explains its low BDE value. The radicals formed by the two phenolic groups (1OH and 3OH) as well as the benzylic group 16CH have nearly identical spin density distributions. However, the BDE values of these three groups are not similar. The difference in reactivity between the OH groups can be attributed to the stability of the formed radical product. The radical formed by 3OH is less stable than that formed by 1OH due to the electronic repulsion between the single electron and the adjacent carbonyl. The difference in reactivity observed for the CH group can be explained by the increased electronegativity of the oxygen atom relative to the carbon atom, which further stabilizes the free radical product. On the other hand, the most localized spin density is obtained after the abstraction of a proton from the carboxyl group, which is consistent with its high BDE value.
| Media | State | Position | BDE |
|---|---|---|---|
| P. ethanoate | CBDA | 1OH | 89.7 |
| 3OH | 92.9 | ||
| 4OH | 110.9 | ||
| 7CH | 75.8 | ||
| 16CH | 91.0 | ||
| Water | CBDA− | 1OH | 85.0 |
| 3OH | 88.7 | ||
| 7CH | 75.1 | ||
| 16CH | 89.5 |
3.3. Kinetic studies under physiological conditions
The kinetics of the reactions that show exergonic and almost isergonic behaviors were calculated according to the QM-ORSA protocol20,21,39 and are reported in Table 2. The transition states of the HAT process in the two studied media are shown in Fig. 6. The activation Gibbs reaction-free energies (ΔG#), as well as the obtained tunneling corrections, are also reported in Table 2.| Radical | Solvent | Mechanism | State | ΔG≠ | κ | k app | f | k f | Γ | k overall | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Mole fraction. b k f = f.kapp. c The nuclear reorganization energy (λ). | |||||||||||
| HOO˙ | PE | HAT | 1OH | CBDA | 19.2 | 134.2 | 6.10 × 101 | — | — | 4 | 1.36 × 103 |
| 7CH | 16.7 | 372.3 | 1.30 × 103 | — | — | 96 | |||||
| Water | SET | CBDA−2 | 5.9 | 16.7c | 2.40 × 108 | 0.01 | 2.40 × 106 | 100 | 2.40 × 106 | ||
| HAT | 1OH | CBDA− | 18.9 | 795.6 | 6.70 × 101 | 0.99 | 6.63 × 101 | 0 | |||
| 7CH | 15.6 | 19.6 | 4.20 × 103 | 4.16 × 103 | 0 | ||||||
| NO2 | PE | HAT | 7CH | CBDA | 18.6 | 1.4 | 2.10 × 100 | — | — | 100 | 2.10 × 100 |
| Water | SET | CBDA−2 | 6.3 | 5.8c | 1.30 × 108 | 0.01 | 1.30 × 106 | 100 | 1.30 × 106 | ||
| HAT | 1OH | CBDA− | 16.4 | 40.8 | 3.19 × 101 | 0.99 | 3.16 × 101 | 0 | |||
| 7CH | 14.1 | 2.0 | 5.70 × 102 | 5.64 × 102 | 0 | ||||||
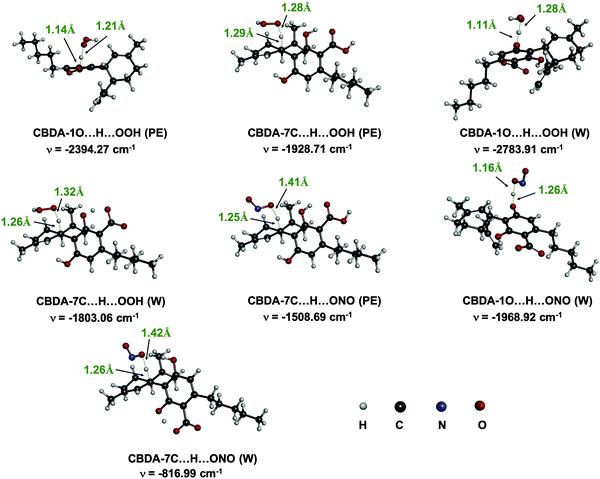 | ||
| Fig. 6 Optimized transition states of the reactions of CBDA with HOO˙ and NO2 in water and pentyl ethanoate. | ||
As can be seen from Table 2, the calculated activation Gibbs free energies range from 5.9 to 19.2 kcal mol−1. The SET process from the dianionic state in water yields the lowest values for both HOO˙ and NO2 (ΔG≠ = 5.9 and 6.3 kcal mol−1, respectively). Regarding the HAT process, the calculated ΔG≠ of the reaction of the 7CH group is lower than that of the 1OH group in the two studied environments, which is in agreement with the thermodynamic analysis.
The overall rate constants calculated in the two studied media clearly show that the reaction of CBDA with HOO˙ or NO2 in water is significantly faster than the reaction in pentyl ethanoate. This difference in reactivity is primarily due to the increased activity of the dianionic form of CBDA (CBDA−2), which is present in a minor proportion in water (about 1%). The SET mechanism was found to be dominant in the inhibition of both HOO˙ and NO2 in water with branching ratios of about 100%. This is consistent with the experimental study of A. L. Dawidowicz et al., which demonstrated that the antioxidant properties of CBDA are more relevant in electron transfer-based assays such as ABTS, CUPRAC, and FRAP than in hydrogen transfer-based assays such as DPPH.19 Similar results were also found in previous work on other phenolic compounds.53–55 For the HAT mechanism, the reaction at the 7CH group was found to be significantly faster than that at the 1OH group in the two studied environments. In pentyl ethanoate, for example, the reaction with HOO˙ at 7CH is about 20 times faster than that at 1OH. This unexpected result can be explained by examining the potential energy surface of the CBDA + HOO˙ reaction in pentyl ethanoate, as shown in Fig. 7. As can be observed, the HAT reaction at both the 7CH and 1OH groups is a typical radial reaction. It proceeds through two intermediate phases, the RC (reactant complex) and the PC (product complex), as well as one TS (transition state), before reaching the final products (P). All of the species involved in the reaction, including RC, TS, PC, and P, have a lower energy at 7CH than that at 1OH. For example, the energy of RC and PC produced by the reaction at 7CH is, respectively, 6.6 and 9.2 kcal mol−1 lower than that produced by the reaction at 1OH. These findings explain why the reaction at 7CH has faster kinetics than that at 1OH.
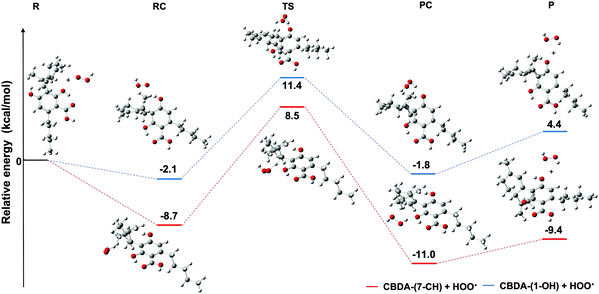 | ||
| Fig. 7 Potential energy surface of the reaction of CBDA with HOO˙ in pentyl ethanoate via the HAT mechanism at 1OH and 7CH. | ||
CBDA has comparable rate coefficients for scavenging the HOO˙ radical and NO2 in water (2.40 × 106 and 1.30 × 106 M−1 s−1, respectively). However, in the lipid-like environment, the reaction with HOO˙ is significantly faster (about 2800 times). Analysis of the ΔG≠ and κ values of each reaction could explain the observed variation in reactivity. As shown in Table 2, the ΔG≠ of the CBDA + HOO˙ reaction is about 2 kcal mol−1 lower than that of the CBDA + NO2 reaction, which allows easier formation of the transition state. Furthermore, the κ value in the first reaction (372.3) is significantly higher than that in the second reaction (1.4), which also plays an important role in the reaction kinetics.
The estimated overall rate coefficients of the interaction of CBDA with the HOO˙ radical were compared to those obtained for known antioxidants using a similar procedure. According to the data obtained, the CBDA + HOO˙ reaction in water is slightly slower than that of ascorbic acid (koverall = 9.97 × 107 M−1 s−1, M052X/6-311++G(d,p)),20 daphnetin (koverall = 1.51 × 107 M−1 s−1),29 and Artepillin C (koverall = 9.94 × 107 M−1 s−1),30 but faster than that of Trolox (koverall = 1.30 × 105 M−1 s−1),56 BHT (koverall = 2.51 × 105 M−1 s−1),57 thymol (koverall = 9.60 × 105 M−1 s−1, M052X/6-311++G(d,p)),50 and melatonin (koverall = 3.11 × 102 M−1 s−1, M052X/6-311++G(d,p)).58 In pentyl ethanoate, CBDA inhibits the hydroperoxyl radical slightly slower than Trolox (koverall = 1.00 × 105 M−1 s−1)56 and BHT (koverall = 1.70 × 104 M−1 s−1),57 but is as fast as ascorbic acid (koverall = 5.71 × 103 M−1 s−1, M052X/6-311++G(d,p)),20 carnosic acid (koverall = 4.73 × 106 M−1 s−1),59 and daphnetin (koverall = 3.21 × 103 M−1 s−1),29 and is significantly faster than Artepillin C (koverall = 4.14 × 100 M−1 s−1),30 thymol (koverall = 4.50 × 101 M−1 s−1, M052X/6-311++G(d,p)),50 and syringic acid (koverall = 5.30 × 100 M−1 s−1).56 All of these findings indicate that CBDA is a potent natural antioxidant, especially in polar environments.
4. Conclusion
The antioxidant capacity of CBDA has been studied under physiological conditions. Different reaction processes and sites, as well as the pH in aqueous solution, have all been explored. It was found that the environment has a significant impact on the antioxidant activity of this compound, which turns out to be significantly greater in aqueous solution than in a pentyl ethanoate solution. At physiological pH, CBDA predominantly exists in the monoanionic form (99%), however, a small proportion of the dianionic form may also exist (1%). It has been demonstrated that the latter defines the antioxidant properties of CBDA in water via the SET mechanism. On the other hand, CBDA reacts exclusively via the HAT mechanism at 7CH in pentyl ethanoate. In comparison, the reactivity of CBDA towards free radicals in polar media is higher than that of common antioxidants such as Trolox and BHT, and indicates that CBDA is a promising antioxidant. The data presented here shed new light on the understanding of the antioxidant properties of CBDA and thus promote the use of this compound as a natural antioxidant.Conflicts of interest
The author declares no competing interests.Acknowledgements
We gratefully acknowledge the MESRS (Ministère de l'Enseignement Supérieur et de la Recherche Scientifique, Algeria), the DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique, Algeria), and the HPC resources of UCI-UFMC (Unité de Calcul Intesif of the university Fréres Mentouri Constantine 1).References
- V. Di Marzo and F. Piscitelli, Neurotherapeutics, 2015, 12, 692–698 CrossRef CAS.
- M. A. ElSohly, M. M. Radwan, W. Gul, S. Chandra and A. Galal, in Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa, ed. A. D. Kinghorn, H. Falk, S. Gibbons and J. I. Kobayashi, Springer International Publishing, Cham, 2017, pp. 1–36 DOI:10.1007/978-3-319-45541-9_1.
- E. Russo and G. W. Guy, Med. Hypotheses, 2006, 66, 234–246 CrossRef CAS.
- C. J. Fowler, Clin. Pharmacol. Ther., 2015, 97, 587–596 CrossRef CAS.
- S. C. Britch, S. Babalonis and S. L. Walsh, Psychopharmacology, 2021, 238, 9–28 CrossRef CAS PubMed.
- P. Pacher, N. M. Kogan and R. Mechoulam, Annu. Rev. Food Sci. Technol., 2020, 60, 637–659 CAS.
- R. J. M. Niesink and M. van Laar, Front. Psychiatry, 2013, 4, 130 Search PubMed.
- V. Brighenti, F. Pellati, M. Steinbach, D. Maran and S. Benvenuti, J. Pharm. Biomed., 2017, 143, 228–236 CrossRef CAS PubMed.
- M. Formato, G. Crescente, M. Scognamiglio, A. Fiorentino, M. T. Pecoraro, S. Piccolella, M. Catauro and S. Pacifico, Molecules, 2020, 25(11), 2638 CrossRef CAS.
- S. Takeda, K. Misawa, I. Yamamoto and K. Watanabe, Drug Metab. Dispos., 2008, 36, 1917 CrossRef CAS.
- E. M. Rock, C. L. Limebeer and L. A. Parker, Psychopharmacology, 2018, 235, 3259–3271 CrossRef CAS.
- E. M. Rock and L. A. Parker, Br. J. Pharmacol., 2013, 169, 685–692 CrossRef CAS PubMed.
- S. Takeda, S. Okajima, H. Miyoshi, K. Yoshida, Y. Okamoto, T. Okada, T. Amamoto, K. Watanabe, C. J. Omiecinski and H. Aramaki, Toxicol. Lett., 2012, 214, 314–319 CrossRef CAS.
- M. Suzuki, S. Takeda, H. Okazaki, K. Watanabe, M. Takiguchi and H. Aramaki, Nat. Prod. Commun., 2017, 12(5), 759–761 CrossRef.
- S. Takeda, H. Okazaki, E. Ikeda, S. Abe, Y. Yoshioka, K. Watanabe and H. Aramaki, J. Toxicol. Sci., 2014, 39, 711–716 CrossRef CAS PubMed.
- D. Bolognini, E. M. Rock, N. L. Cluny, M. G. Cascio, C. L. Limebeer, M. Duncan, C. G. Stott, F. A. Javid, L. A. Parker and R. G. Pertwee, Br. J. Pharmacol., 2013, 168, 1456–1470 CrossRef CAS.
- A. R. Petrovici, N. Simionescu, A. I. Sandu, V. Paraschiv, M. Silion and M. Pinteala, Antioxidants, 2021, 10(5), 738 CrossRef CAS.
- S. Atalay, I. Jarocka-Karpowicz and E. Skrzydlewska, Antioxidants, 2020, 9(1), 21 CrossRef CAS PubMed.
- A. L. Dawidowicz, M. Olszowy-Tomczyk and R. Typek, Fitoterapia, 2021, 152, 104915 CrossRef CAS PubMed.
- A. Galano and J. R. Alvarez-Idaboy, J. Comput. Chem., 2013, 34, 2430–2445 CrossRef CAS PubMed.
- A. Galano and J. R. Alvarez-Idaboy, Int. J. Quantum Chem., 2019, 119, e25665 CrossRef.
- T. Masuda, K. Yamada, T. Maekawa, Y. Takeda and H. Yamaguchi, Food Sci. Technol., 2006, 12, 173–177 CAS.
- A. D. N. J. de Grey, DNA Cell Biol., 2002, 21, 251–257 CrossRef CAS PubMed.
- L. A. Ridnour, D. D. Thomas, D. Mancardi, M. G. Espey, K. M. Miranda, N. Paolocci, M. Feelisch, J. Fukuto and D. A. Wink, Biol. Chem., 2004, 385, 1–10 CAS.
- A. Galano, G. Mazzone, R. Alvarez-Diduk, T. Marino, J. R. Alvarez-Idaboy and N. Russo, Annu. Rev. Food Sci. Technol., 2016, 7, 335–352 CrossRef CAS PubMed.
- M. Leopoldini, N. Russo and M. Toscano, Food Chem., 2011, 125, 288–306 CrossRef CAS.
- Y. Xue, Y. Liu, Y. Xie, C. Cong, G. Wang, L. An, Y. Teng, M. Chen and L. Zhang, Phytochemistry, 2020, 179, 112393 CrossRef CAS.
- I. Amine Khodja and H. Boulebd, Mol. Diversity, 2021, 25, 279–290 CrossRef CAS.
- H. Boulebd and I. Amine Khodja, Phytochemistry, 2021, 189, 112831 CrossRef CAS PubMed.
- H. Boulebd, A. Mechler, N. T. Hoa, P. C. Nam, D. T. Quang and Q. V. Vo, New J. Chem., 2021, 45, 7774–7780 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, A. F. I. H. P. Hratchian, J. Bloino, G. Zheng, M. H. J. L. Sonnenberg, M. Ehara, K. Toyota, J. H. R. Fukuda, M. Ishida, T. Nakajima, Y. Honda, H. N. O. Kitao, T. Vreven, J. A. Montgomery Jr, F. O. J. E. Peralta, M. J. Bearpark, J. Heyd, K. N. K. E. N. Brothers, V. N. Staroverov, R. Kobayashi, K. R. J. Normand, A. P. Rendell, J. C. Burant, J. T. S. S. Iyengar, M. Cossi, N. Rega, N. J. Millam, J. E. K. M. Klene, J. B. Cross, V. Bakken, C. Adamo, R. G. J. Jaramillo, R. E. Stratmann, O. Yazyev, R. C. A. J. Austin, C. Pomelli, J. W. Ochterski, K. M. R. L. Martin, V. G. Zakrzewski, G. A. Voth, J. J. D. P. Salvador, S. Dapprich, A. D. Daniels, J. B. F. Ö. Farkas, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- A. Galano and J. R. Alvarez-Idaboy, J. Comput. Chem., 2014, 35, 2019–2026 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2008, 112, 1095–1099 CrossRef CAS.
- J. R. l. Alvarez-Idaboy and A. Galano, J. Phys. Chem. B, 2012, 116, 9316–9325 CrossRef CAS.
- M. E. Alberto, N. Russo, A. Grand and A. Galano, Phys. Chem. Chem. Phys., 2013, 15, 4642–4650 RSC.
- Q. V. Vo, M. V. Bay, P. C. Nam and A. Mechler, J. Phys. Chem. B, 2019, 123, 7777–7784 CrossRef CAS.
- H. Boulebd, I. Amine Khodja, M. V. Bay, N. T. Hoa, A. Mechler and Q. V. Vo, J. Phys. Chem. B, 2020, 124, 4123–4131 CrossRef CAS.
- S. G. Chiodo, M. Leopoldini, N. Russo and M. Toscano, Phys. Chem. Chem. Phys., 2010, 12, 7662–7670 RSC.
- M. G. Evans and M. Polanyi, Trans. Faraday Soc., 1935, 31, 875–894 RSC.
- H. Eyring, J. Chem. Phys., 1935, 3, 107–115 CrossRef CAS.
- D. G. Truhlar, W. L. Hase and J. T. Hynes, J. Phys. Chem. A, 1983, 87, 2664–2682 CrossRef CAS.
- T. Furuncuoglu, I. Ugur, I. Degirmenci and V. Aviyente, Macromolecules, 2010, 43, 1823–1835 CrossRef CAS.
- E. Vélez, J. Quijano, R. Notario, E. Pabón, J. Murillo, J. Leal, E. Zapata and G. Alarcón, J. Phys. Org. Chem., 2009, 22, 971–977 CrossRef.
- E. Dzib, J. L. Cabellos, F. Ortíz-Chi, S. Pan, A. Galano and G. Merino, Int. J. Quantum Chem., 2019, 119, e25686 CrossRef.
- E. Pollak and P. Pechukas, J. Am. Chem. Soc., 1978, 100, 2984–2991 CrossRef CAS.
- A. Fernández-Ramos, B. A. Ellingson, R. Meana-Pañeda, J. M. Marques and D. G. Truhlar, Theor. Chem. Acc., 2007, 118, 813–826 Search PubMed.
- C. Eckart, Phys. Rev., 1930, 35, 1303 CrossRef CAS.
- A. M. Rebollar-Zepeda, T. Campos-Hernández, M. T. Ramírez-Silva, A. Rojas-Hernández and A. Galano, J. Chem. Theory Comput., 2011, 7, 2528–2538 CrossRef CAS.
- H. Boulebd, Phytochemistry, 2021, 184, 112670 CrossRef CAS.
- V. H. Uc, J. R. Alvarez-Idaboy, A. Galano and A. Vivier-Bunge, J. Phys. Chem. A, 2008, 112, 7608–7615 CrossRef CAS.
- A. Galano, J. R. Alvarez-Idaboy and M. Francisco-Márquez, J. Phys. Chem. B, 2011, 115, 13101–13109 CrossRef CAS.
- A. Galano, J. R. Alvarez-Idaboy, M. Francisco-Márquez and M. E. Medina, Theor. Chem. Acc., 2012, 131, 1173 Search PubMed.
- C. Caicedo, C. Iuga, R. Castañeda-Arriaga and J. R. Alvarez-Idaboy, RSC Adv., 2014, 4, 38918–38930 RSC.
- A. Amić, D. Milenković, Z. Marković, D. Cagardová, J. Rodríguez-Guerra Pedregal and J. M. Dimitrić Marković, New J. Chem., 2021, 45, 7977–7986 RSC.
- Q. V. Vo, M. V. Bay, P. C. Nam, D. T. Quang, M. Flavel, N. T. Hoa and A. Mechler, J. Org. Chem., 2020, 85, 15514–15520 CrossRef CAS.
- H. Boulebd, Int. J. Chem. Kinet., 2021, 1–8, DOI:10.1002/kin.21540.
- A. Galano, Phys. Chem. Chem. Phys., 2011, 13, 7178–7188 RSC.
- H. Boulebd, Phytochemistry, 2021, 192, 112950 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nj04771j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |