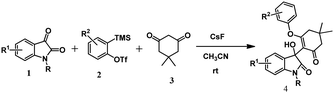
One-pot synthesis of 3-substituted-3-hydroxyindolin-2-ones: three component coupling of N-protected isatin, aryne precursor and 1,3-cyclodione under metal-free conditions†
Hemanta
Hazarika
ab,
Kangkana
Chutia
a,
Babulal
Das
c and
Pranjal
Gogoi
 *ab
*ab
aApplied Organic Chemistry Group, Chemical Science and Technology Division, CSIR-North East Institute of Science and Technology, Jorhat 785006, India. E-mail: gogoipranj@yahoo.co.uk; gogoipranj@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, UP 201002, India
cDepartment of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India
First published on 13th November 2021
Abstract
An aryne-based synthetic protocol has been developed for the synthesis of 3-substituted-3-hydroxy-indolin-2-ones. A wide variety of 3-hydroxyindolin-2-ones were synthesized in good yields under metal-free conditions via three component coupling of N-protected isatin, aryne precursor, and 1,3-cyclodione. One of our synthesized compounds has been unambiguously confirmed by single crystal XRD analysis. Further, treating the synthesized 3-hydroxyindolin-2-one derivatives with an inorganic base at high temperature leads to an interesting o-arylated product of 1,3-cyclohexandione.
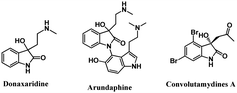
Indolin-2-one derivatives are important structural motifs found in many biologically active compounds. They have been playing an essential role in the pharmaceutical industry.1 Indolin-2-ones substituted at the C-3 position by other functional groups are widely found in various natural products and exhibit diverse biological properties.2 The activities of these molecules mostly reside at the substitution at the C-3 quaternary center.3 In particular, 3-substituted-3-hydroxyindolin-2-ones are one of them, exhibiting interesting biological properties and found in several biologically active compounds.4–6 As a result, many research groups have been working on this area and exploring new analogues of biological interest. Here, a few examples of natural products containing 3-substituted-3-hydroxyindolin-2-one framework are shown in Fig. 1.
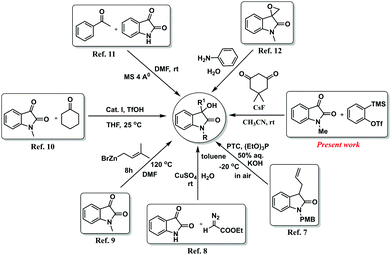
Owing to their significance in medicinal chemistry and pharmaceuticals, numerous noteworthy methods, including catalyst-mediated and catalyst-free, have been reported for the synthesis of 3-substituted-3-hydroxyindolin-2-ones. Regarding the catalyst-mediated synthesis of these important scaffolds, Itoh and co-workers described a method for the synthesis of 3-hydroxy-2-oxindoles via catalytic asymmetric hydroxylation of oxindoles by molecular oxygen using a phase-transfer catalyst (PTC).7 Later on, Hu and co-workers reported a CuSO4-catalyzed three-component reaction of α-diazo ester, water, and isatin for the synthesis of 3-hydroxyindolin-2-one derivatives.8 Again, another convenient method has been developed by Zhao and his co-workers for the synthesis of 3-prenyl-3-hydroxy-2-oxindoles.9 This transformation was achieved by zinc-mediated C-3 α-prenylation of isatin with prenyl bromide. Recently, Pericas group developed another aldol type reaction between cyclic ketones and isatins using C2-symmetric chiral bifunctional triamine (Cat. I) catalyst for the synthesis of 3-hydroxyindolin-2-one derivatives.10 On the other hand, the Yuan group reported a catalyst-free method for the synthesis of 3-substituted-3-hydroxyindolin-2-ones.11 They have accomplished this via aldol condensation of ketones and isatins under mild reaction conditions in DMF using molecular sieve 4 Å as an additive. In addition to those, Nair's group added another interesting method for the synthesis of 3-hydroxy-3-aminomethylindolin-2-one derivatives under catalyst-free conditions using H2O as solvent.12 Although there are several efficient ways for the synthesis of 3-hydroxyindolin-2-one derivatives, most of the reported methods require a base, metal-catalyst or metal-complex, or other additives. Furthermore, catalyst-free methods for the synthesis of this important scaffold are rare. Although the reported synthetic approaches have gained substantial progress, the development of new and efficient synthetic methods for new molecules with an enhanced scope is still highly anticipated. Synthetic methodologies described by different groups along with our approach for the synthesis of 3-substituted-3-hydroxyindolin-2-ones are shown in Fig. 2.
On another note, recent developments in synthetic organic chemistry have been attributed towards the transition metal-free reactions because of their advantages over transition metal-catalyzed reactions.13 The main disadvantages of transition metal catalyzed reactions include their sensitivity towards moisture,14 less abundance in nature, high cost, and waste generation after the reaction.
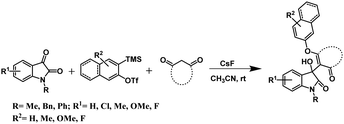
Moreover, it is difficult to separate the residual transition metals present in trace amounts from the desired products, which makes unavoidable issues for drug candidates. Regarding the transition metal-free organic transformations, aryne having distinct electronic properties has been used as a versatile organic synthon for various metal-free organic transformations.15 However, generation of aryne via Kobayashi's protocol16 is extensively recognized and widely used for aryne-based synthetic routes for the synthesis of heterocycles17 and complex natural products.18 Although there are several aryne-based synthetic methods, recent approaches have been preferably dedicated towards transition-metal-free reactions. For instance, some of the transition metal-free aryne-based methods are cycloaddition reactions,19 multicomponent couplings (MCCs),20 and insertion reactions.21 Considering the importance of transition-metal free reactions and in continuation of our ongoing research work on aryne chemistry,22 we came up with a transition metal-free synthetic protocol for the synthesis of 3-substituted-3-hydroxyindolin-2-ones from N-protected isatin, aryne precursors, and 1,3-cyclodione (Scheme 1). To the best of our knowledge, there is no such report on the synthesis of 3-substituted-3-hydroxyindolin-2-ones using aryne intermediate.
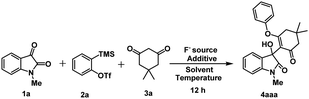
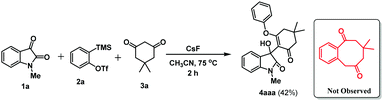
At the outset, our estimated reaction was performed using 1-methyl isatin 1a (0.5 mmol), 2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2a (0.5 mmol), dimedone 3a (0.5 mmol), and KF (3.0 equiv.) as fluoride source in CH3CN at room temperature for 12 h. Under these reaction conditions, we obtained our expected product 4aaa in 47% yield (Table 1, entry 1). To improve the efficiency of our reaction strategy, some other fluoride sources, such as CsF and TBAF, were also tested under the same reaction conditions (Table 1, entries 2 and 3). The reaction offers trace amounts of 4aaa using TBAF, whereas CsF improves the reaction yield affording 76% of 4aaa. For our curiosity, additive 18-crown-6 (1.0 equiv.) along with KF (3.0 equiv.) as well as CsF (3.0 equiv.) were investigated under similar reaction conditions; however, no improvement of yield was observed (Table 1, entry 4 and 5). In addition to those optimization studies, we also varied the amount of the fluoride source; however, the yield of the product 4aaa was encouraging (Table 1, entries 6 and 7). After having the optimized fluoride source as CsF, we next screened some other reaction media. In this regard, THF, toluene, and a mixture of CH3CN and toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were investigated as reaction media; however, the desired product 4aaa was obtained in a lower yield. These experiments highlight the superior properties of CH3CN as the reaction medium (Table 1, entries 8–10). Notably, on increasing the reaction temperature to 50 °C, the yield of our desired product was decreased (Table 1, entry 11).
1) were investigated as reaction media; however, the desired product 4aaa was obtained in a lower yield. These experiments highlight the superior properties of CH3CN as the reaction medium (Table 1, entries 8–10). Notably, on increasing the reaction temperature to 50 °C, the yield of our desired product was decreased (Table 1, entry 11).
| Sl. no. | F− source (equiv.) | Additive (1 equiv.) | Solvent | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Conditions: 1-methyl isatin 1a (0.5 mmol), o-silyl aryl triflate 2a (0.5 mmol), dimedone 3a (0.5 mmol), fluoride source (1.5 to 2.5 mmol), additive (0.5 mmol), solvent (3 mL) stirred. b Isolated yield; ND: not detected. | |||||
| 1 | KF (3.0) | — | CH3CN | rt | 47 |
| 2 | CsF (3.0) | — | CH 3 CN | rt | 76 |
| 3 | TBAF (3.0) | — | CH3CN | rt | Trace |
| 4 | KF (3.0) | 18-Crown-6 | CH3CN | rt | 57 |
| 5 | CsF (3.0) | 18-Crown-6 | CH3CN | rt | 73 |
| 6 | CsF (1.0) | — | CH3CN | rt | 52 |
| 7 | CsF (5.0) | — | CH3CN | rt | 74 |
| 8 | CsF (3.0) | — | THF | rt | Trace |
| 9 | CsF (3.0) | — | Toluene | rt | 45 |
| 10 | CsF (3.0) | — | CH3CN/toluene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 66 |
| 11 | CsF (3.0) | — | CH3CN | 50 | 46 |
| 12 | — | — | CH3CN | rt | ND |
As a control experiment, we also carried an additional reaction in the absence of a fluoride source; however, no product formation was observed (Table 1, entry 12).
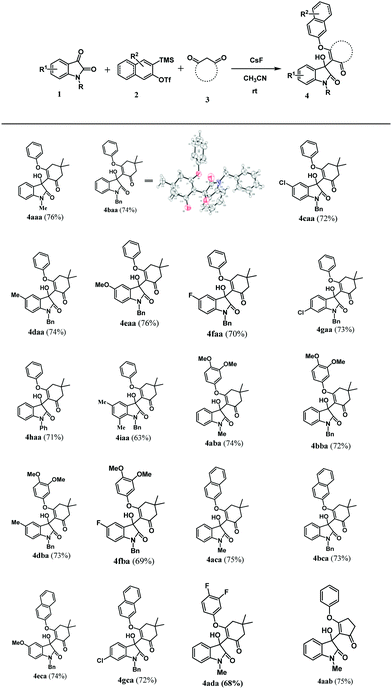
After optimizing the reaction conditions, we successively explored the substrate scope for our synthetic protocol. At first, we treated 2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2a and dimedone 3a with a variety of N-protected isatins 1. A diverse set of 3-substituted-3-hydroxyindolin-2-ones were obtained in good to moderate yields, and the results are summarized in Table 2. As shown in Table 2, various N-protected isatins bearing both electron donating and electron withdrawing groups at different positions on the phenyl ring smoothly participated in our synthetic protocol and provided corresponding 3-substituted-3-hydroxyindolin-2-ones in good yields (Table 2, 4aaa–4haa). However, isatin without the protecting group does not lead to the desired product.
| a Conditions: N-Protected isatin 1 (0.5 mmol), Aryne precursor 2 (0.5 mmol), 1,3-cyclodione 3 (0.5 mmol), CsF (1.5 mmol), CH3CN (3 mL) stirred at room temperature for 12 h. |
|---|
|
N-Protected isatin having a substitution at 5 and 7 positions of the phenyl ring was also tested for our synthetic protocol, and it gives the corresponding 3-substituted-3-hydroxyindolin-2-one 4iaa with a slightly lower yield. To further explore the substrate scope for our synthetic protocol, other symmetrical aryne precursors such as 4,5-dimethoxy-2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2b, 3-(trimethylsilyl)-2-naphthyl trifluoromethanesulfonate 2c, and 4,5-difluoro-2-(trimethylsilyl)phenyltrifluoromethanesulfonate 2d were also examined, and a series of 3-substituted-3-hydroxyindolin-2-ones were synthesized in good yields (Table 2, 4aba–4ada). All the synthesized 3-substituted-3-hydroxyindolin-2-ones were characterized using 1H NMR, 13C NMR, and HRMS analyses. Additionally, one of our synthesized compounds 4baa has been unambiguously confirmed by X-ray single crystal analysis (details are in ESI†).23 In addition to these substrate scope experiments, cyclopentane-1,3-dione instead of dimedone was considered as the third component with 1-methylisatin 1a and aryne precursor 2a under the optimized reaction conditions. As shown in Table 2, it resulted in the desired product 4aab in 75% yield. Moreover, 5-phenyl-1,3-cyclohexandione and 5-methyl-1,3-cyclohexandiones were also used as third components instead of dimedone.
These two active methylene components led to a complex mixture of products, which were difficult to isolate into individual products. As a result, these substrates were not considered for further investigations. Besides these 1,3-cyclodiones, 2,4-pentanedione was planned to explore as acyclic 1,3-dione for our synthetic protocol. This diketone gave 3-hydroxy-3-methyl-3,4-dihydronaphthalen-1(2H)-one as a sole product instead of 3-substituted-3-hydroxyindolin-2-one under the optimized reaction conditions.24 Further, Meldrum's acid and acetone were also examined as ketone substrates for our synthetic protocol; however, no desired products were observed under the optimized reaction conditions.
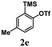
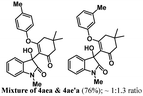
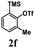
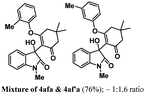
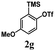
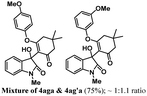
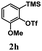
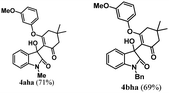
Encouraged by the results of symmetrical aryne precursors, some unsymmetrical aryne precursors were also examined for our synthetic protocol. Unsymmetrical benzyne precursors such as 4-methyl-2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2e, 2-methyl-6-(trimethylsilyl)phenyl trifluoromethanesulfonate 2f, and 4-methoxy-2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2g were treated with 1-methyl isatin 1a and dimedone 3a under the optimized reaction conditions (Table 3). Benzyne precursors 2e, 2f, and 2g provided the corresponding 3-substituted-3-hydroxyindolin-2-ones 4aea & 4ae′a, 4afa & 4af′a and 4aga & 4ag′a as mixture of regioisomers in good yields. These regioisomers were difficult to separate into their individual isomers, and they were represented as mixtures. The ratios of their mixtures were characterized by NMR, and their proportions were evaluated from 1H NMR analysis (details are in ESI†). Moreover, 3-methoxy-2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2h was also exposed to N-protected isatin 1a and 1b along with dimedone 3a under the optimized reaction conditions. Notably, they afforded desired products with single regioisomers 4aha and 4bha in 71% and 69% yields, respectively.
| Entry | Benzyne precursors | 3-Substituted-3-hydroxyindolin-2-ones |
|---|---|---|
| a Conditions: N-protected isatin 1 (0.5 mmol), benzyne precursor 2 (0.5 mmol), dimedone 3 (0.5 mmol), CsF (1.5 mmol), CH3CN (3 mL) stirred at room temperature for 12 h. | ||
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
During the investigation of the substrate scope, in some cases, an interesting side-product was observed after prolonged reaction time. Isolation and characterization of the side product encouraged us to further investigate our strategy. In this regard, we planned to treat our synthesized 3-substituted-3-hydroxyindolin-2-one 4aaa independently in CH3CN at 60 °C for another 6 h.
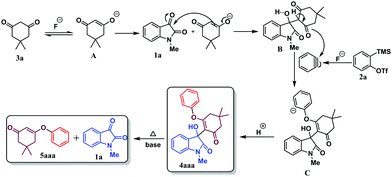
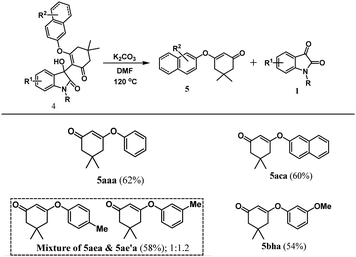
Under these reaction conditions, an interesting O-arylated product 1,3-cyclohexanedione 5aaa was isolated in 18% yield. The yield of the side product 5aaa derived from 4aaa was increased to 42%, when 3-substituted-3-hydroxyindolin-2-one was treated in DMF at 120 °C for 2 h. To further investigate, the product 4aaa was treated with a base K2CO3 in DMF at 120 °C for 2 h. Under these reaction conditions, the O-arylated product 1,3-cyclohexanedione 5aaa was isolated in 62% yield along with the starting material N-protected isatin 1. Various synthesized 3-substituted-3-hydroxyindolin-2-ones 4 were investigated under these conditions, and some O-arylated 1,3-cyclohexanediones (Table 4, 5aaa, 5aca, and 5bha) were derived. Additionally, regioisomeric mixture of 4aea & 4ae′a were also treated under these conditions, which resulted in the formation of regioisomers 5aea & 5ae′a in 58% yield.
| a Conditions: 3-substituted-indolin-2-ones 4 (0.2 mmol), K2CO3 (0.6 mmol), and DMF (2 mL) stirred at 120 °C for 2 h. |
|---|
|
To gain insight into the reaction mechanism, a few independent experiments were performed. Initially, all three components were treated under the reported conditions of Mehta and Srihari.25 In this experiment, 1-methyl isatin 1a, benzyne precursor 2a, and dimedone 3a were treated with CsF in CH3CN at 75 °C for 2 h. Under these reaction conditions, the reported product benzocyclooctanone was not observed. However, we isolated 3-substituted-3-hydroxyindolin-2-one 4aaa in 42% yield (Scheme 2).
Based on the previously reported methods,10,11,26 and some of our independent experiments, we proposed here a probable reaction mechanism for our synthetic protocol. As shown in Scheme 3, initially, in the presence of F− ion, dimedone 3a and 1-methyl isatin 1a undergo aldol condensation reaction to generate the intermediate B. The intermediate B then undergoes nucleophilic attack to in situ generated benzyne16,21d to form the anion intermediate C. Finally, the intermediate C undergoes protonation in the presence of residual water22g,i to give the desired product 4aaa.
Again, in the presence of an inorganic base, 1-methyl isatin 1a gets eliminated from 4aaa to give the product 5aaa at a high reaction temperature.
In summary, we have developed a transition metal-free synthetic protocol for the one-pot synthesis of 3-substituted-3-hydroxyindolin-2-ones. A broad range of 3-hydroxy-indolin-2-one derivatives was synthesized in good yields by three components coupling of N-protected isatins, aryne precursors, and 1,3-cyclodiones. Our one-pot synthetic strategy leads to the formation one C–C and one C–O bond in a single operation under metal-free conditions. This efficient synthetic protocol leads to a new route for the synthesis of 3-hydroxyindolin-2-one derivatives in a convenient manner. Moreover, interesting O-arylated products of 1,3-cyclohexandiones were obtained by treating the synthesized 3-substituted-3-hydroxyindolines in the presence of an inorganic base at high temperatures.
Experimental section
General
All reactions involving oxygen- or moisture-sensitive compounds were carried out under an argon atmosphere using oven-dried or flame-dried glassware. All other solvents and reagents were purified according to standard procedures or were used as received from Aldrich, Acros, TCI, Merck, and Spectrochem. Reactions were monitored by thin-layer chromatography (TLC) using aluminium-backed silica gel plates (0.2 mm thickness); the chromatograms were visualized with ultraviolet light (254 nm). Flash column chromatography was performed with silica gel 60 (230–400 mesh). HRMS data were recorded by electrospray ionization with a Q-TOF mass analyzer.General procedure for the synthesis of 3-substituted-3-hydroxyindolin-2-ones
An oven-dried round bottomed flask (50 mL capacity) equipped with a magnetic stir bar was evacuated and purged with argon. N-Protected isatin (0.5 mmol, 1.0 equiv.), aryne precursor (0.5 mmol, 1 equiv.), 1,3-cyclodione (0.5 mmol, 1.0 equiv.), CsF (1.5 mmol, 3 equiv.), and CH3CN (3 mL) were added successively at room temperature. The reaction mixture was stirred at room temperature for 12 h. Water (10 mL) was added to the reaction mixture, and the organic layer was extracted with EtOAc (2 × 20 mL). The combined organic phases were washed with brine and dried over sodium sulfate. The solvent was removed, and the residue was purified by column chromatography on silica gel using hexane/ethyl acetate as an eluent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.61 (br s, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.24–7.29 (m, 3H), 7.12 (t, J = 7.4 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 6.70–6.72 (m, 3H), 3.01 (s, 3H), 2.53 (d, J = 16.2 Hz, 1H), 2.37 (d, J = 16.2 Hz, 1H), 2.32 (d, J = 17.7 Hz, 1H), 1.97 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 0.98 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 170.3, 152.4, 143.4, 132.4, 129.8, 129.2, 125.7, 123.2, 122.8, 120.2, 107.9, 76.5, 51.6, 40.6, 32.7, 28.6, 26.9, 26.0; IR (CHCl3): 3429, 2958, 2930, 1728, 1489, 1353, 1227, 754 cm−1; HRMS (+ESI) calcd for C23H22NO3 [(M-H2O) + H]+: 360.1600; found: 360.1607.
3) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.61 (br s, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.24–7.29 (m, 3H), 7.12 (t, J = 7.4 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 6.70–6.72 (m, 3H), 3.01 (s, 3H), 2.53 (d, J = 16.2 Hz, 1H), 2.37 (d, J = 16.2 Hz, 1H), 2.32 (d, J = 17.7 Hz, 1H), 1.97 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 0.98 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 170.3, 152.4, 143.4, 132.4, 129.8, 129.2, 125.7, 123.2, 122.8, 120.2, 107.9, 76.5, 51.6, 40.6, 32.7, 28.6, 26.9, 26.0; IR (CHCl3): 3429, 2958, 2930, 1728, 1489, 1353, 1227, 754 cm−1; HRMS (+ESI) calcd for C23H22NO3 [(M-H2O) + H]+: 360.1600; found: 360.1607.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; mp 150–151 °C; 1H NMR (500 MHz, CDCl3): δ 7.74 (br s, 1H), 7.37 (dd, J1 = 0.9 Hz, J2= 7.4 Hz, 1H), 7.25–7.28 (m, 2H), 7.08–7.19 (m, 5H), 6.99–7.05 (m, 3H), 6.72 (d, J = 7.7 Hz, 2H), 6.59 (d, J = 7.8 Hz, 1H), 4.83 (d, J = 15.8 Hz, 1H), 4.68 (d, J = 15.8 Hz, 1H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 1.98 (d, J = 17.7 Hz, 1H), 1.07 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.7, 152.4, 142.7, 135.5, 132.6, 129.8, 129.1, 128.3, 127.1, 125.9, 123.3, 122.9, 120.9, 109.2, 76.5, 51.6, 43.9, 40.8, 32.7, 28.8, 26.9; IR (CHCl3): 3430, 2961, 2935, 1740, 1493, 1358, 1234, 760 cm−1; HRMS (+ESI) calcd for C29H26NO3 [(M-H2O) + H]+: 436.1913; found: 436.1909.
3) as the eluent; mp 150–151 °C; 1H NMR (500 MHz, CDCl3): δ 7.74 (br s, 1H), 7.37 (dd, J1 = 0.9 Hz, J2= 7.4 Hz, 1H), 7.25–7.28 (m, 2H), 7.08–7.19 (m, 5H), 6.99–7.05 (m, 3H), 6.72 (d, J = 7.7 Hz, 2H), 6.59 (d, J = 7.8 Hz, 1H), 4.83 (d, J = 15.8 Hz, 1H), 4.68 (d, J = 15.8 Hz, 1H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 1.98 (d, J = 17.7 Hz, 1H), 1.07 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.7, 152.4, 142.7, 135.5, 132.6, 129.8, 129.1, 128.3, 127.1, 125.9, 123.3, 122.9, 120.9, 109.2, 76.5, 51.6, 43.9, 40.8, 32.7, 28.8, 26.9; IR (CHCl3): 3430, 2961, 2935, 1740, 1493, 1358, 1234, 760 cm−1; HRMS (+ESI) calcd for C29H26NO3 [(M-H2O) + H]+: 436.1913; found: 436.1909.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent; mp 181–182 °C; 1H NMR (500 MHz, CDCl3): δ 7.82 (br s, 1H), 7.34 (d, J = 2.1 Hz, 1H), 7.30 (t, J = 7.8 Hz, 2H), 7.21 (t, J = 7.4 Hz, 1H), 7.04–7.13 (m, 6H), 6.76 (d, J = 7.7 Hz, 2H), 6.50 (d, J = 8.3 Hz, 1H), 4.82 (d, J = 15.9 Hz, 1H), 4.66 (d, J = 15.9 Hz, 1H), 2.55 (d, J = 16.2 Hz, 1H), 2.41 (d, J = 16.3 Hz, 1H), 2.30 (d, J = 17.7 Hz, 1H), 2.02 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 1.01 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.8, 171.1, 152.2, 141.3, 135.0, 134.1, 129.9, 128.9, 128.4, 128.1, 127.3, 127.1, 126.1, 123.9, 120.9, 110.3, 76.5, 51.5, 44.1, 40.8, 32.7, 28.5, 27.3; IR (CHCl3): 3425, 2953, 2923, 1720, 1475, 1349, 1220, 750 cm−1; HRMS (+ESI) calcd for C29H25NO3Cl [(M-H2O) + H]+: 470.1523; found: 470.1519.
3) as eluent; mp 181–182 °C; 1H NMR (500 MHz, CDCl3): δ 7.82 (br s, 1H), 7.34 (d, J = 2.1 Hz, 1H), 7.30 (t, J = 7.8 Hz, 2H), 7.21 (t, J = 7.4 Hz, 1H), 7.04–7.13 (m, 6H), 6.76 (d, J = 7.7 Hz, 2H), 6.50 (d, J = 8.3 Hz, 1H), 4.82 (d, J = 15.9 Hz, 1H), 4.66 (d, J = 15.9 Hz, 1H), 2.55 (d, J = 16.2 Hz, 1H), 2.41 (d, J = 16.3 Hz, 1H), 2.30 (d, J = 17.7 Hz, 1H), 2.02 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 1.01 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.8, 171.1, 152.2, 141.3, 135.0, 134.1, 129.9, 128.9, 128.4, 128.1, 127.3, 127.1, 126.1, 123.9, 120.9, 110.3, 76.5, 51.5, 44.1, 40.8, 32.7, 28.5, 27.3; IR (CHCl3): 3425, 2953, 2923, 1720, 1475, 1349, 1220, 750 cm−1; HRMS (+ESI) calcd for C29H25NO3Cl [(M-H2O) + H]+: 470.1523; found: 470.1519.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; mp 195–196 °C; 1H NMR (500 MHz, CDCl3): δ 7.65 (br s, 1H), 7.28 (t, J = 7.8 Hz, 2H), 7.01–7.29 (m, 7H), 6.91 (d, J = 7.9 Hz, 1H), 6.76 (d, J = 7.8 Hz, 2H), 6.48 (d, J = 7.9 Hz, 1H), 4.81 (d, J = 15.8 Hz, 1H), 4.65 (d, J = 15.8 Hz, 1H), 2.55 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 2.29 (s, 3H), 1.99 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.0, 170.6, 152.4, 140.3, 135.6, 132.5, 132.4, 129.8, 129.3, 128.2, 127.1, 127.0, 125.8, 124.0, 120.9, 108.9, 76.6, 51.6, 43.9, 40.8, 32.6, 28.6, 27.1, 20.9; IR (CHCl3): 3430, 2960, 2932, 1731, 1491, 1355, 1230, 758 cm−1; HRMS (+ESI) calcd for C30H28NO3 [(M-H2O) + H]+: 450.2069; found: 450.2069.
3) as the eluent; mp 195–196 °C; 1H NMR (500 MHz, CDCl3): δ 7.65 (br s, 1H), 7.28 (t, J = 7.8 Hz, 2H), 7.01–7.29 (m, 7H), 6.91 (d, J = 7.9 Hz, 1H), 6.76 (d, J = 7.8 Hz, 2H), 6.48 (d, J = 7.9 Hz, 1H), 4.81 (d, J = 15.8 Hz, 1H), 4.65 (d, J = 15.8 Hz, 1H), 2.55 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 2.29 (s, 3H), 1.99 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.0, 170.6, 152.4, 140.3, 135.6, 132.5, 132.4, 129.8, 129.3, 128.2, 127.1, 127.0, 125.8, 124.0, 120.9, 108.9, 76.6, 51.6, 43.9, 40.8, 32.6, 28.6, 27.1, 20.9; IR (CHCl3): 3430, 2960, 2932, 1731, 1491, 1355, 1230, 758 cm−1; HRMS (+ESI) calcd for C30H28NO3 [(M-H2O) + H]+: 450.2069; found: 450.2069.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent; mp 175 °C; 1H NMR (500 MHz, CDCl3): δ 7.71 (br s, 1H), 7.26–7.29 (m, 2H), 7.18–7.21 (m, 1H), 7.14 (d, J = 7.3 Hz, 2H), 7.09 (d, J = 7.3 Hz, 1H), 7.05 (d, J = 7.6 Hz, 2H), 6.99 (d, J = 2.6 Hz, 1H), 6.76 (d, J = 7.8 Hz, 2H), 6.66 (dd, J1 = 2.6 Hz, J2 = 8.5 Hz, 1H), 6.48 (d, J = 8.5 Hz, 1H), 4.79 (d, J = 15.8 Hz, 1H), 4.66 (d, J = 15.8 Hz, 1H), 3.74 (s, 3H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 1.99 (d, J = 17.6 Hz, 1H), 1.08 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 170.8, 156.2, 152.4, 136.3, 135.6, 133.8, 129.9, 128.4, 127.2, 127.1, 125.9, 121.0, 113.7, 110.6, 109.7, 76.9, 55.8, 51.6, 44.1, 40.9, 32.7, 28.8, 27.1; IR (CHCl3): 3431, 2959, 2932, 1729, 1493, 1354, 1231, 756 cm−1; HRMS (+ESI) calcd for C30H28NO4 [(M-H2O) + H]+: 466.2018; found: 466.2016.
3) as eluent; mp 175 °C; 1H NMR (500 MHz, CDCl3): δ 7.71 (br s, 1H), 7.26–7.29 (m, 2H), 7.18–7.21 (m, 1H), 7.14 (d, J = 7.3 Hz, 2H), 7.09 (d, J = 7.3 Hz, 1H), 7.05 (d, J = 7.6 Hz, 2H), 6.99 (d, J = 2.6 Hz, 1H), 6.76 (d, J = 7.8 Hz, 2H), 6.66 (dd, J1 = 2.6 Hz, J2 = 8.5 Hz, 1H), 6.48 (d, J = 8.5 Hz, 1H), 4.79 (d, J = 15.8 Hz, 1H), 4.66 (d, J = 15.8 Hz, 1H), 3.74 (s, 3H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 1.99 (d, J = 17.6 Hz, 1H), 1.08 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 170.8, 156.2, 152.4, 136.3, 135.6, 133.8, 129.9, 128.4, 127.2, 127.1, 125.9, 121.0, 113.7, 110.6, 109.7, 76.9, 55.8, 51.6, 44.1, 40.9, 32.7, 28.8, 27.1; IR (CHCl3): 3431, 2959, 2932, 1729, 1493, 1354, 1231, 756 cm−1; HRMS (+ESI) calcd for C30H28NO4 [(M-H2O) + H]+: 466.2018; found: 466.2016.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent; mp 178–179 °C; 1H NMR (500 MHz, CDCl3): δ 7.74 (br s, 1H), 7.23 (t, J = 7.8 Hz, 2H), 7.12–7.15 (m, 1H), 7.03–7.06 (m, 4H), 6.98 (t, J = 7.4 Hz, 2H), 6.73–6.77 (m, 1H), 6.68 (d, J = 7.8 Hz, 2H), 6.42 (dd, J1 = 4.06 Hz, J2 = 8.6 Hz, 1H), 4.75 (d, J = 15.8 Hz, 1H), 4.59 (d, J = 15.9 Hz, 1H), 2.48 (d, J = 16.2 Hz, 1H), 2.32 (d, J = 16.2 Hz, 1H), 2.24 (d, J = 17.7 Hz, 1H), 1.93 (d, J = 17.7 Hz, 1H), 1.01 (s, 3H), 0.94 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 171.1, 159.3 (d, J = 241.3 Hz), 152.2, 2 × 138.6, 135.2, 129.9, 128.4, 127.2, 127.1, 126.1, 120.9, 115.2 (d, J = 23.5 Hz), 111.4 (d, J = 24.7 Hz), 109.8 (d, J = 7.8 Hz), 76.6, 51.5, 44.1, 40.8, 32.6, 28.6, 27.1; IR (CHCl3): 3421, 2945, 2922, 1715, 1477, 1348, 1219, 743 cm−1; HRMS (+ESI) calcd for C29H25NO3F [(M-H2O) + H]+: 454.1818; found: 454.1821.
3) as eluent; mp 178–179 °C; 1H NMR (500 MHz, CDCl3): δ 7.74 (br s, 1H), 7.23 (t, J = 7.8 Hz, 2H), 7.12–7.15 (m, 1H), 7.03–7.06 (m, 4H), 6.98 (t, J = 7.4 Hz, 2H), 6.73–6.77 (m, 1H), 6.68 (d, J = 7.8 Hz, 2H), 6.42 (dd, J1 = 4.06 Hz, J2 = 8.6 Hz, 1H), 4.75 (d, J = 15.8 Hz, 1H), 4.59 (d, J = 15.9 Hz, 1H), 2.48 (d, J = 16.2 Hz, 1H), 2.32 (d, J = 16.2 Hz, 1H), 2.24 (d, J = 17.7 Hz, 1H), 1.93 (d, J = 17.7 Hz, 1H), 1.01 (s, 3H), 0.94 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 171.1, 159.3 (d, J = 241.3 Hz), 152.2, 2 × 138.6, 135.2, 129.9, 128.4, 127.2, 127.1, 126.1, 120.9, 115.2 (d, J = 23.5 Hz), 111.4 (d, J = 24.7 Hz), 109.8 (d, J = 7.8 Hz), 76.6, 51.5, 44.1, 40.8, 32.6, 28.6, 27.1; IR (CHCl3): 3421, 2945, 2922, 1715, 1477, 1348, 1219, 743 cm−1; HRMS (+ESI) calcd for C29H25NO3F [(M-H2O) + H]+: 454.1818; found: 454.1821.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent; mp 155–157 °C; 1H NMR (500 MHz, CDCl3): δ 7.71 (br s, 1H), 7.27–7.31 (m, 3H), 7.21 (t, J = 7.4 Hz, 1H), 7.04–7.14 (m, 5H), 6.98 (dd, J1 = 1.8 Hz, J2 = 7.9 Hz, 1H), 6.76 (d, J = 7.7 Hz, 2H), 6.59 (d, J = 1.4 Hz, 1H), 4.78 (d, J = 15.9 Hz, 1H), 4.65 (d, J = 15.9 Hz, 1H), 2.54 (d, J = 16.2 Hz, 1H), 2.38 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 1.99 (d, J = 17.7 Hz, 1H), 1.07 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 2 × 170.9, 152.2, 144.0, 134.9, 134.7, 131.0, 129.9, 128.5, 127.4, 127.1, 126.1, 124.3, 122.7, 120.9, 109.7, 75.9, 51.5, 44.1, 40.8, 32.7, 28.7, 26.9; IR (CHCl3): 3424, 2956, 2928, 1723, 1482, 1350, 1221, 749 cm−1; HRMS (+ESI) calcd for C29H25NO3Cl [(M-H2O) + H]+: 470.1523; found: 470.1501.
3) as eluent; mp 155–157 °C; 1H NMR (500 MHz, CDCl3): δ 7.71 (br s, 1H), 7.27–7.31 (m, 3H), 7.21 (t, J = 7.4 Hz, 1H), 7.04–7.14 (m, 5H), 6.98 (dd, J1 = 1.8 Hz, J2 = 7.9 Hz, 1H), 6.76 (d, J = 7.7 Hz, 2H), 6.59 (d, J = 1.4 Hz, 1H), 4.78 (d, J = 15.9 Hz, 1H), 4.65 (d, J = 15.9 Hz, 1H), 2.54 (d, J = 16.2 Hz, 1H), 2.38 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 1.99 (d, J = 17.7 Hz, 1H), 1.07 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 2 × 170.9, 152.2, 144.0, 134.9, 134.7, 131.0, 129.9, 128.5, 127.4, 127.1, 126.1, 124.3, 122.7, 120.9, 109.7, 75.9, 51.5, 44.1, 40.8, 32.7, 28.7, 26.9; IR (CHCl3): 3424, 2956, 2928, 1723, 1482, 1350, 1221, 749 cm−1; HRMS (+ESI) calcd for C29H25NO3Cl [(M-H2O) + H]+: 470.1523; found: 470.1501.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent; mp 151 °C; 1H NMR (500 MHz, CDCl3): δ 7.84 (br s, 1H), 7.43 (d, J = 7.4 Hz, 1H), 7.27–7.31 (m, 5H), 7.16–7.20 (m, 2H), 7.01–7.11 (m, 3H), 6.75 (d, J = 7.3 Hz, 2H), 6.67 (d, J = 7.8 Hz, 1H), 2.56 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.35 (d, J = 17.7 Hz, 1H), 2.01 (d, J = 17.6 Hz, 1H), 1.09 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.4, 170.7, 152.4, 143.5, 134.3, 132.4, 129.9, 129.3, 129.1, 127.7, 126.4, 125.9, 123.7, 123.4, 120.7, 109.4, 109.3, 76.8, 51.7, 40.8, 32.8, 28.8, 26.9; IR (CHCl3): 3430, 2959, 2932, 1729, 1490, 1353, 1228, 755 cm−1; HRMS (+ESI) calcd for C28H24NO3[(M-H2O) + H]+: 422.1756; found: 422.1741.
3) as eluent; mp 151 °C; 1H NMR (500 MHz, CDCl3): δ 7.84 (br s, 1H), 7.43 (d, J = 7.4 Hz, 1H), 7.27–7.31 (m, 5H), 7.16–7.20 (m, 2H), 7.01–7.11 (m, 3H), 6.75 (d, J = 7.3 Hz, 2H), 6.67 (d, J = 7.8 Hz, 1H), 2.56 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.35 (d, J = 17.7 Hz, 1H), 2.01 (d, J = 17.6 Hz, 1H), 1.09 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.4, 170.7, 152.4, 143.5, 134.3, 132.4, 129.9, 129.3, 129.1, 127.7, 126.4, 125.9, 123.7, 123.4, 120.7, 109.4, 109.3, 76.8, 51.7, 40.8, 32.8, 28.8, 26.9; IR (CHCl3): 3430, 2959, 2932, 1729, 1490, 1353, 1228, 755 cm−1; HRMS (+ESI) calcd for C28H24NO3[(M-H2O) + H]+: 422.1756; found: 422.1741.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent; mp 216 °C; 1H NMR (500 MHz, CDCl3): δ 7.66 (br s, 1H), 7.29 (t, J = 7.9 Hz, 2H), 7.19 (t, J = 7.4 Hz, 1H), 7.05–7.08 (m, 6H), 6.81 (d, J = 7.6 Hz, 2H), 6.72 (s, 1H), 5.02 (d, J = 17.0 Hz, 1H), 4.94 (d, J = 17.0 Hz, 1H), 2.55 (d, J = 16.1 Hz, 1H), 2.40 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 17.6 Hz, 1H), 2.26 (s, 3H), 2.07 (s, 3H), 2.03 (d, J = 17.6 Hz, 1H), 1.09 (s, 3H), 1.02 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 176.3, 170.5, 152.6, 138.3, 137.7, 133.5, 132.5, 129.8, 128.4, 126.7, 125.8, 125.7, 122.1, 120.9, 119.5, 76.1, 51.7, 45.2, 40.9, 32.7, 28.7, 27.2, 20.7, 18.5; IR (CHCl3): 3432, 2960, 2933, 1730, 1491, 1355, 1230, 756 cm−1; HRMS (+ESI) calcd for C31H30NO3 [(M-H2O) + H]+: 464.2226; found: 464.2228.
3) as eluent; mp 216 °C; 1H NMR (500 MHz, CDCl3): δ 7.66 (br s, 1H), 7.29 (t, J = 7.9 Hz, 2H), 7.19 (t, J = 7.4 Hz, 1H), 7.05–7.08 (m, 6H), 6.81 (d, J = 7.6 Hz, 2H), 6.72 (s, 1H), 5.02 (d, J = 17.0 Hz, 1H), 4.94 (d, J = 17.0 Hz, 1H), 2.55 (d, J = 16.1 Hz, 1H), 2.40 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 17.6 Hz, 1H), 2.26 (s, 3H), 2.07 (s, 3H), 2.03 (d, J = 17.6 Hz, 1H), 1.09 (s, 3H), 1.02 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 176.3, 170.5, 152.6, 138.3, 137.7, 133.5, 132.5, 129.8, 128.4, 126.7, 125.8, 125.7, 122.1, 120.9, 119.5, 76.1, 51.7, 45.2, 40.9, 32.7, 28.7, 27.2, 20.7, 18.5; IR (CHCl3): 3432, 2960, 2933, 1730, 1491, 1355, 1230, 756 cm−1; HRMS (+ESI) calcd for C31H30NO3 [(M-H2O) + H]+: 464.2226; found: 464.2228.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 132–133 °C; 1H NMR (500 MHz, CDCl3): δ 7.66 (br s, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.25–7.29 (m, 1H), 7.05 (t, J = 7.5 Hz, 1H), 6.70–6.74 (m, 2H), 6.29 (d, J = 8.3 Hz, 1H), 6.20 (s, 1H), 3.84 (s, 3H), 3.76 (s, 3H), 3.04 (s, 3H), 2.53 (d, J = 16.2 Hz, 1H), 2.36 (d, J = 16.2 Hz, 1H), 2.29 (d, J = 17.7 Hz, 1H), 1.99 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 0.97 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.0, 170.9, 149.4, 146.7, 146.0, 143.4, 132.6, 129.2, 123.2, 122.7, 111.7, 110.9, 108.0, 104.3, 76.4, 55.9, 51.5, 40.3, 32.6, 28.7, 26.9, 26.0; IR (CHCl3): 3433, 2960, 2933, 1733, 1495, 1353, 1228, 755 cm−1; HRMS (+ESI) calcd for C25H26NO5 [(M-H2O) + H]+: 420.1811; found: 420.1806.
1) as the eluent; mp 132–133 °C; 1H NMR (500 MHz, CDCl3): δ 7.66 (br s, 1H), 7.36 (d, J = 7.3 Hz, 1H), 7.25–7.29 (m, 1H), 7.05 (t, J = 7.5 Hz, 1H), 6.70–6.74 (m, 2H), 6.29 (d, J = 8.3 Hz, 1H), 6.20 (s, 1H), 3.84 (s, 3H), 3.76 (s, 3H), 3.04 (s, 3H), 2.53 (d, J = 16.2 Hz, 1H), 2.36 (d, J = 16.2 Hz, 1H), 2.29 (d, J = 17.7 Hz, 1H), 1.99 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 0.97 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.0, 170.9, 149.4, 146.7, 146.0, 143.4, 132.6, 129.2, 123.2, 122.7, 111.7, 110.9, 108.0, 104.3, 76.4, 55.9, 51.5, 40.3, 32.6, 28.7, 26.9, 26.0; IR (CHCl3): 3433, 2960, 2933, 1733, 1495, 1353, 1228, 755 cm−1; HRMS (+ESI) calcd for C25H26NO5 [(M-H2O) + H]+: 420.1811; found: 420.1806.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 181–182 °C; 1H NMR (500 MHz, CDCl3): δ 7.74 (br s, 1H), 7.37 (dd, J1 = 0.9 Hz, J2 = 7.4 Hz, 1H), 6.99–7.18 (m, 7H), 6.72 (d, J = 8.7 Hz, 1H), 6.62 (d, J = 7.8 Hz, 1H), 6.31 (dd, J1 = 2.3 Hz, J2 = 8.6 Hz, 1H), 6.18 (s, 1H), 4.89 (d, J = 15.8 Hz, 1H), 4.68 (d, J = 15.8, 1H), 3.85 (s, 3H), 3.83 (s, 3H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 2.01, (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.2, 171.3, 149.6, 146.9, 146.1, 142.9, 135.5, 132.9, 129.1, 128.3, 127.2, 127.1, 123.3, 122.8, 112.4, 111.0, 109.3, 104.9, 76.5, 56.1, 56.0, 51.6, 44.0, 40.6, 32.6, 28.8, 26.9; IR (CHCl3): 3442, 2963, 2936, 1728, 1493, 1360, 1230, 757 cm−1; HRMS (+ESI) calcd for C31H30NO5 [(M-H2O) + H]+: 496.2124; found: 496.2125.
1) as the eluent; mp 181–182 °C; 1H NMR (500 MHz, CDCl3): δ 7.74 (br s, 1H), 7.37 (dd, J1 = 0.9 Hz, J2 = 7.4 Hz, 1H), 6.99–7.18 (m, 7H), 6.72 (d, J = 8.7 Hz, 1H), 6.62 (d, J = 7.8 Hz, 1H), 6.31 (dd, J1 = 2.3 Hz, J2 = 8.6 Hz, 1H), 6.18 (s, 1H), 4.89 (d, J = 15.8 Hz, 1H), 4.68 (d, J = 15.8, 1H), 3.85 (s, 3H), 3.83 (s, 3H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 17.7 Hz, 1H), 2.01, (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.2, 171.3, 149.6, 146.9, 146.1, 142.9, 135.5, 132.9, 129.1, 128.3, 127.2, 127.1, 123.3, 122.8, 112.4, 111.0, 109.3, 104.9, 76.5, 56.1, 56.0, 51.6, 44.0, 40.6, 32.6, 28.8, 26.9; IR (CHCl3): 3442, 2963, 2936, 1728, 1493, 1360, 1230, 757 cm−1; HRMS (+ESI) calcd for C31H30NO5 [(M-H2O) + H]+: 496.2124; found: 496.2125.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 142–144 °C; 1H NMR (500 MHz, CDCl3): δ 7.65 (br s, 1H), 7.19 (s, 1H), 7.16 (d, J = 7.4 Hz, 2H), 7.09 (d, J = 7.2 Hz, 1H), 7.05 (d, J = 7.4 Hz, 2H), 6.94 (d, J = 7.9 Hz, 1H), 6.73 (d, J = 8.7 Hz, 1H), 6.50 (d, J = 7.9 Hz, 1H), 6.36 (d, J = 8.6 Hz, 1H), 6.23 (s, 1H), 4.86 (d, J = 15.8 Hz, 1H), 4.66 (d, J = 15.8 Hz, 1H), 3.86 (s, 3H), 3.74 (s, 3H), 2.55 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 18.0 Hz, 1H), 2.28 (s, 3H), 2.02 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 171.2, 149.6, 146.9, 146.2, 140.4, 135.7, 132.8, 132.4, 129.4, 128.3, 127.2, 127.1, 124.1, 112.5, 111.0, 109.1, 105.0, 76.6, 56.1, 55.9, 51.6, 44.1, 40.6, 32.6, 28.7, 27.2, 21.0; IR (CHCl3): 3433, 2959, 2932, 1729, 1493, 1355, 1228, 756 cm−1; HRMS (+ESI) calcd for C32H32NO5[(M-H2O) + H]+: 510.2280; found: 510.2285.
1) as the eluent; mp 142–144 °C; 1H NMR (500 MHz, CDCl3): δ 7.65 (br s, 1H), 7.19 (s, 1H), 7.16 (d, J = 7.4 Hz, 2H), 7.09 (d, J = 7.2 Hz, 1H), 7.05 (d, J = 7.4 Hz, 2H), 6.94 (d, J = 7.9 Hz, 1H), 6.73 (d, J = 8.7 Hz, 1H), 6.50 (d, J = 7.9 Hz, 1H), 6.36 (d, J = 8.6 Hz, 1H), 6.23 (s, 1H), 4.86 (d, J = 15.8 Hz, 1H), 4.66 (d, J = 15.8 Hz, 1H), 3.86 (s, 3H), 3.74 (s, 3H), 2.55 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.31 (d, J = 18.0 Hz, 1H), 2.28 (s, 3H), 2.02 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 171.2, 149.6, 146.9, 146.2, 140.4, 135.7, 132.8, 132.4, 129.4, 128.3, 127.2, 127.1, 124.1, 112.5, 111.0, 109.1, 105.0, 76.6, 56.1, 55.9, 51.6, 44.1, 40.6, 32.6, 28.7, 27.2, 21.0; IR (CHCl3): 3433, 2959, 2932, 1729, 1493, 1355, 1228, 756 cm−1; HRMS (+ESI) calcd for C32H32NO5[(M-H2O) + H]+: 510.2280; found: 510.2285.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 157–158 °C; 1H NMR (500 MHz, CDCl3): δ 7.84 (br s, 1H), 7.11–7.15 (m, 4H), 7.05–7.07 (m, 2H), 6.81–6.84 (m, 1H), 6.73 (dd, J1 = 2.0 Hz, J2 = 8.7 Hz, 1H), 6.50–6.53 (m, 1H), 6.34 (d, J = 8.5 Hz, 1H), 6.24 (s, 1H), 4.87 (d, J = 15.8 Hz, 1H), 4.65 (d, J = 15.8 Hz, 1H), 3.85 (s, 3H), 3.74 (s, 3H), 2.54 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.32 (d, J = 17.7 Hz, 1H), 2.03 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 1.01 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 171.7, 171.6, 159.2 (d, J = 241.3 Hz), 149.6, 147.0, 145.9, 138.7, 134.1 (d, J = 7.3 Hz), 128.3, 127.2, 127.1, 115.2 (d, J = 23.4 Hz), 112.3, 111.4 (d, J = 24.8 Hz), 111.0, 109.8 (d, J = 7.8 Hz), 104.8, 76.5, 56.0, 55.9, 51.4, 44.1, 40.5, 32.5, 28.6, 27.1; IR (CHCl3): 3428, 2957, 2930, 1726, 1485, 1351, 1223, 753 cm−1; HRMS (+ESI) calcd for C31H29NO5F [(M-H2O) + H]+: 514.2030; found: 514.2009.
1) as the eluent; mp 157–158 °C; 1H NMR (500 MHz, CDCl3): δ 7.84 (br s, 1H), 7.11–7.15 (m, 4H), 7.05–7.07 (m, 2H), 6.81–6.84 (m, 1H), 6.73 (dd, J1 = 2.0 Hz, J2 = 8.7 Hz, 1H), 6.50–6.53 (m, 1H), 6.34 (d, J = 8.5 Hz, 1H), 6.24 (s, 1H), 4.87 (d, J = 15.8 Hz, 1H), 4.65 (d, J = 15.8 Hz, 1H), 3.85 (s, 3H), 3.74 (s, 3H), 2.54 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.32 (d, J = 17.7 Hz, 1H), 2.03 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 1.01 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 171.7, 171.6, 159.2 (d, J = 241.3 Hz), 149.6, 147.0, 145.9, 138.7, 134.1 (d, J = 7.3 Hz), 128.3, 127.2, 127.1, 115.2 (d, J = 23.4 Hz), 112.3, 111.4 (d, J = 24.8 Hz), 111.0, 109.8 (d, J = 7.8 Hz), 104.8, 76.5, 56.0, 55.9, 51.4, 44.1, 40.5, 32.5, 28.6, 27.1; IR (CHCl3): 3428, 2957, 2930, 1726, 1485, 1351, 1223, 753 cm−1; HRMS (+ESI) calcd for C31H29NO5F [(M-H2O) + H]+: 514.2030; found: 514.2009.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 7.9 Hz, 1H), 7.76 (d, J = 8.9 Hz, 1H), 7.71 (d, J = 7.9 Hz, 1H), 7.60 (br s, 1H), 7.47–7.52 (m, 2H), 7.40 (d, J = 6.8 Hz, 1H), 7.25–7.28 (m, 1H), 7.14 (s, 1H), 7.08 (t, J = 7.5 Hz, 1H), 6.88 (dd, J1 = 1.8 Hz, J2 = 8.8 Hz, 1H), 6.70 (d, J = 7.8 Hz, 1H), 3.00 (s, 3H), 2.56 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.36 (d, J = 17.7 Hz, 1H), 2.01 (d, J = 17.7 Hz, 1H), 1.07 (s, 3H), 0.97 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.4, 150.1, 143.5, 133.6, 132.6, 131.2, 130.1, 129.3, 127.8, 127.4, 127.0, 125.9, 123.4, 122.9, 119.8, 117.2, 108.1, 76.6, 51.7, 40.9, 32.8, 28.7, 27.0, 26.1; IR (CHCl3): 3435, 2958, 2931, 1730, 1491, 1355, 1229, 755 cm−1; HRMS (+ESI) calcd for C27H24NO3 [(M-H2O) + H]+: 410.1756; found: 410.1754.
3) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 7.9 Hz, 1H), 7.76 (d, J = 8.9 Hz, 1H), 7.71 (d, J = 7.9 Hz, 1H), 7.60 (br s, 1H), 7.47–7.52 (m, 2H), 7.40 (d, J = 6.8 Hz, 1H), 7.25–7.28 (m, 1H), 7.14 (s, 1H), 7.08 (t, J = 7.5 Hz, 1H), 6.88 (dd, J1 = 1.8 Hz, J2 = 8.8 Hz, 1H), 6.70 (d, J = 7.8 Hz, 1H), 3.00 (s, 3H), 2.56 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.36 (d, J = 17.7 Hz, 1H), 2.01 (d, J = 17.7 Hz, 1H), 1.07 (s, 3H), 0.97 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.4, 150.1, 143.5, 133.6, 132.6, 131.2, 130.1, 129.3, 127.8, 127.4, 127.0, 125.9, 123.4, 122.9, 119.8, 117.2, 108.1, 76.6, 51.7, 40.9, 32.8, 28.7, 27.0, 26.1; IR (CHCl3): 3435, 2958, 2931, 1730, 1491, 1355, 1229, 755 cm−1; HRMS (+ESI) calcd for C27H24NO3 [(M-H2O) + H]+: 410.1756; found: 410.1754.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; mp 139 °C; 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 7.9 Hz, 1H), 7.74(d, J = 8.7 Hz, 1H), 7.70 (d, J = 7.9 Hz, 1H), 7.46–7.52 (m, 2H), 7.41 (dd, J1 = 1.0 Hz, J2 = 7.3 Hz, 1H), 7.13–7.16 (m, 4H), 7.02–7.16 (m, 1H), 6.90–6.97 (m, 3H), 6.85 (dd, J1 = 1.4 Hz, J2 = 8.7 Hz, 1H), 6.61 (d, J = 7.7 Hz, 1H), 4.79 (d, J = 15.8 Hz, 1H), 4.69 (d, J = 15.8 Hz, 1H), 2.57 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.35 (d, J = 17.7 Hz, 1H), 2.01 (d, J = 17.6 Hz, 1H), 1.06 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.2, 170.8, 149.9, 142.9, 135.5, 133.6, 132.8, 131.2, 130.1, 129.2, 128.3, 127.8, 127.4, 127.2, 127.1, 127.0, 126.0, 123.4, 122.9, 120.2, 117.9, 109.3, 76.6, 51.7, 44.0, 41.1, 32.8, 28.8, 26.9; IR (CHCl3): 3440, 2962, 2933, 1730, 1492, 1355, 1230, 757 cm−1; HRMS (+ESI) calcd for C33H28NO3 [(M-H2O) + H]+: 486.2069; found: 486.2072.
3) as the eluent; mp 139 °C; 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 7.9 Hz, 1H), 7.74(d, J = 8.7 Hz, 1H), 7.70 (d, J = 7.9 Hz, 1H), 7.46–7.52 (m, 2H), 7.41 (dd, J1 = 1.0 Hz, J2 = 7.3 Hz, 1H), 7.13–7.16 (m, 4H), 7.02–7.16 (m, 1H), 6.90–6.97 (m, 3H), 6.85 (dd, J1 = 1.4 Hz, J2 = 8.7 Hz, 1H), 6.61 (d, J = 7.7 Hz, 1H), 4.79 (d, J = 15.8 Hz, 1H), 4.69 (d, J = 15.8 Hz, 1H), 2.57 (d, J = 16.2 Hz, 1H), 2.39 (d, J = 16.2 Hz, 1H), 2.35 (d, J = 17.7 Hz, 1H), 2.01 (d, J = 17.6 Hz, 1H), 1.06 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.2, 170.8, 149.9, 142.9, 135.5, 133.6, 132.8, 131.2, 130.1, 129.2, 128.3, 127.8, 127.4, 127.2, 127.1, 127.0, 126.0, 123.4, 122.9, 120.2, 117.9, 109.3, 76.6, 51.7, 44.0, 41.1, 32.8, 28.8, 26.9; IR (CHCl3): 3440, 2962, 2933, 1730, 1492, 1355, 1230, 757 cm−1; HRMS (+ESI) calcd for C33H28NO3 [(M-H2O) + H]+: 486.2069; found: 486.2072.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; mp 122–123 °C; 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 7.9 Hz, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.72 (d, J = 7.9 Hz, 1H), 7.47–7.53 (m, 2H), 7.18 (s, 1H), 7.13 (d, J = 7.3 Hz, 2H), 7.04 (d, J = 2.3 Hz, 1H), 6.88–6.97 (m, 4H), 6.67 (dd, J1 = 2.4 Hz, J2 = 8.5 Hz, 1H), 6.49 (d, J = 8.5 Hz, 1H), 4.77 (d, J = 15.8 Hz, 1H), 4.66 (d, J = 15.8 Hz, 1H), 3.76 (s, 3H), 2.57 (d, J = 16.2 Hz, 1H), 2.34–2.41 (m, 2H), 2.03 (d, J = 17.5 Hz, 1H), 1.07 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.0, 170.9, 156.2, 149.9, 136.3, 135.6, 133.8, 133.6, 131.3, 130.1, 128.3, 127.8, 127.5, 127.2, 127.1, 127.0, 126.0, 120.3, 117.9, 113.7, 110.7, 109.7, 76.9, 55.8, 51.6, 44.1, 41.1, 32.8, 28.8, 27.0; IR (CHCl3): 3441, 2963, 2933, 1729, 1490, 1355, 1227, 756 cm−1; HRMS (+ESI) calcd for C34H30NO4 [(M-H2O) + H]+: 516.2175; found: 516.2165.
3) as the eluent; mp 122–123 °C; 1H NMR (500 MHz, CDCl3): δ 7.83 (d, J = 7.9 Hz, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.72 (d, J = 7.9 Hz, 1H), 7.47–7.53 (m, 2H), 7.18 (s, 1H), 7.13 (d, J = 7.3 Hz, 2H), 7.04 (d, J = 2.3 Hz, 1H), 6.88–6.97 (m, 4H), 6.67 (dd, J1 = 2.4 Hz, J2 = 8.5 Hz, 1H), 6.49 (d, J = 8.5 Hz, 1H), 4.77 (d, J = 15.8 Hz, 1H), 4.66 (d, J = 15.8 Hz, 1H), 3.76 (s, 3H), 2.57 (d, J = 16.2 Hz, 1H), 2.34–2.41 (m, 2H), 2.03 (d, J = 17.5 Hz, 1H), 1.07 (s, 3H), 1.00 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.0, 170.9, 156.2, 149.9, 136.3, 135.6, 133.8, 133.6, 131.3, 130.1, 128.3, 127.8, 127.5, 127.2, 127.1, 127.0, 126.0, 120.3, 117.9, 113.7, 110.7, 109.7, 76.9, 55.8, 51.6, 44.1, 41.1, 32.8, 28.8, 27.0; IR (CHCl3): 3441, 2963, 2933, 1729, 1490, 1355, 1227, 756 cm−1; HRMS (+ESI) calcd for C34H30NO4 [(M-H2O) + H]+: 516.2175; found: 516.2165.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; mp 195–196 °C; 1H NMR (500 MHz, CDCl3): δ 7.84 (d, J = 7.8 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 7.9 Hz, 1H), 7.48–7.55 (m, 2H), 7.32 (d, J = 7.9 Hz, 1H), 7.19 (s, 1H), 7.12 (d, J = 7.2 Hz, 2H), 7.02 (dd, J1 = 1.8 Hz, J2 = 7.9 Hz, 1H), 6.89–6.98 (m, 4H), 6.62 (d, J = 1.8 Hz, 1H), 4.76 (d, J = 15.8 Hz, 1H), 4.65 (d, J = 15.8 Hz, 1H), 2.56 (d, J = 16.2 Hz, 1H), 2.33–2.40 (m, 2H), 2.02 (d, J = 17.6 Hz, 1H), 1.06 (s, 3H), 0.98 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.9, 149.8, 144.1, 134.9, 134.8, 133.6, 131.3, 131.1, 130.2, 128.4, 127.8, 127.5, 127.3, 127.2, 127.1, 126.1, 124.4, 122.8, 120.2, 118.1, 109.8, 76.0, 51.6, 44.2, 41.1, 32.9, 28.7, 26.9; IR (CHCl3): 3431, 2960, 2932, 1728, 1490, 1354, 1227, 753 cm−1; HRMS (+ESI) calcd for C33H27NO3Cl [(M-H2O) + H]+: 520.1679; found: 520.1692.
3) as the eluent; mp 195–196 °C; 1H NMR (500 MHz, CDCl3): δ 7.84 (d, J = 7.8 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 7.9 Hz, 1H), 7.48–7.55 (m, 2H), 7.32 (d, J = 7.9 Hz, 1H), 7.19 (s, 1H), 7.12 (d, J = 7.2 Hz, 2H), 7.02 (dd, J1 = 1.8 Hz, J2 = 7.9 Hz, 1H), 6.89–6.98 (m, 4H), 6.62 (d, J = 1.8 Hz, 1H), 4.76 (d, J = 15.8 Hz, 1H), 4.65 (d, J = 15.8 Hz, 1H), 2.56 (d, J = 16.2 Hz, 1H), 2.33–2.40 (m, 2H), 2.02 (d, J = 17.6 Hz, 1H), 1.06 (s, 3H), 0.98 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.9, 149.8, 144.1, 134.9, 134.8, 133.6, 131.3, 131.1, 130.2, 128.4, 127.8, 127.5, 127.3, 127.2, 127.1, 126.1, 124.4, 122.8, 120.2, 118.1, 109.8, 76.0, 51.6, 44.2, 41.1, 32.9, 28.7, 26.9; IR (CHCl3): 3431, 2960, 2932, 1728, 1490, 1354, 1227, 753 cm−1; HRMS (+ESI) calcd for C33H27NO3Cl [(M-H2O) + H]+: 520.1679; found: 520.1692.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; 1H NMR (400 MHz, CDCl3): δ 7.27 (d, J = 7.9 Hz, 1H), 7.18–7.22 (m, 2H), 6.96–7.04 (m, 2H), 6.66 (d, J = 7.7 Hz, 1H), 6.53–6.58 (m, 1H), 6.44–6.47 (m, 1H), 2.96 (s, 3H), 2.47 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 16.2 Hz, 1H), 2.24 (d, J = 17.7 Hz, 1H), 1.88 (d, J = 17.6 Hz, 1H), 1.03 (s, 3H), 0.92 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 203.2, 174.9, 169.0, 150.7(d, J = 206.5 Hz), 150.5 (d, J = 204.6 Hz), 148.3 (d, J = 8.1 Hz), 147.1 (d, J = 12.4 Hz), 143.5, 132.4, 129.6, 123.4 (d, J = 31.9 Hz), 119.9, 117.9 (d, J = 18.9 Hz), 116.4, 110.3 (d, J = 19.8 Hz), 108.3, 76.5, 51.8, 40.7, 33.0, 28.8, 27.2, 26.3; IR (CHCl3): 3430, 2958, 2930, 1731, 1496, 1350, 1226, 753 cm−1; HRMS (+ESI) calcd for C23H20NO3F2 [(M-H2O) + H]+: 396.1411; found: 396.1409.
1) as the eluent; 1H NMR (400 MHz, CDCl3): δ 7.27 (d, J = 7.9 Hz, 1H), 7.18–7.22 (m, 2H), 6.96–7.04 (m, 2H), 6.66 (d, J = 7.7 Hz, 1H), 6.53–6.58 (m, 1H), 6.44–6.47 (m, 1H), 2.96 (s, 3H), 2.47 (d, J = 16.1 Hz, 1H), 2.31 (d, J = 16.2 Hz, 1H), 2.24 (d, J = 17.7 Hz, 1H), 1.88 (d, J = 17.6 Hz, 1H), 1.03 (s, 3H), 0.92 (s, 3H); 13C NMR (101 MHz, CDCl3): δ 203.2, 174.9, 169.0, 150.7(d, J = 206.5 Hz), 150.5 (d, J = 204.6 Hz), 148.3 (d, J = 8.1 Hz), 147.1 (d, J = 12.4 Hz), 143.5, 132.4, 129.6, 123.4 (d, J = 31.9 Hz), 119.9, 117.9 (d, J = 18.9 Hz), 116.4, 110.3 (d, J = 19.8 Hz), 108.3, 76.5, 51.8, 40.7, 33.0, 28.8, 27.2, 26.3; IR (CHCl3): 3430, 2958, 2930, 1731, 1496, 1350, 1226, 753 cm−1; HRMS (+ESI) calcd for C23H20NO3F2 [(M-H2O) + H]+: 396.1411; found: 396.1409.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 195–196 °C; 1H NMR (400 MHz, CDCl3): δ 7.42 (dd, J1 = 7.3 Hz, J2 = 1.3 Hz, 1H), 7.24–7.35 (m, 4H), 7.08 (td, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 6.86–6.90 (m, 3H), 6.79 (d, J = 7.8 Hz, 1H), 3.17 (s, 3H), 2.52–2.56 (m, 2H), 2.31–2.38 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 206.6, 182.7, 174.6, 153.1, 143.3, 130.8, 129.9, 129.7, 126.6, 123.9, 123.0, 120.7, 118.7, 108.3, 75.1, 34.1, 26.2, 25.4; IR (CHCl3): 3431, 2956, 2929, 1730, 1491, 1350, 1225, 753 cm−1; HRMS (+ESI) calcd for C20H16NO3 [(M-H2O) + H]+: 318.1130; found: 318.1133.
1) as the eluent; mp 195–196 °C; 1H NMR (400 MHz, CDCl3): δ 7.42 (dd, J1 = 7.3 Hz, J2 = 1.3 Hz, 1H), 7.24–7.35 (m, 4H), 7.08 (td, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 6.86–6.90 (m, 3H), 6.79 (d, J = 7.8 Hz, 1H), 3.17 (s, 3H), 2.52–2.56 (m, 2H), 2.31–2.38 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 206.6, 182.7, 174.6, 153.1, 143.3, 130.8, 129.9, 129.7, 126.6, 123.9, 123.0, 120.7, 118.7, 108.3, 75.1, 34.1, 26.2, 25.4; IR (CHCl3): 3431, 2956, 2929, 1730, 1491, 1350, 1225, 753 cm−1; HRMS (+ESI) calcd for C20H16NO3 [(M-H2O) + H]+: 318.1130; found: 318.1133.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; mp 130–132 °C; 1H NMR (500 MHz, CDCl3): δ 7.64 (br s, 2H), 7.35 (d, J = 7.4 Hz, 2H), 7.25 (t, J = 7.7 Hz, 2H), 7.13 (t, J = 7.9 Hz, 1H), 7.02–7.06 (m, 4H), 6.97 (d, J = 7.4 Hz, 1H), 6.70 (d, J = 7.7 Hz, 2H), 6.59 (d, J = 8.1 Hz, 2H), 6.51 (s, 1H), 6.49 (s, 1H), 3.01 (s, 6H), 2.49–2.53 (m, 2H), 2.31–2.38 (m, 4H), 2.29 (s, 3H), 2.28 (s, 3H), 1.97 (d, J = 17.7 Hz, 2H), 1.07 (s, 3H), 1.01 (s, 3H), 0.97 (s, 3H), 0.96 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 175.0, 170.7, 170.5, 152.4, 150.2, 143.4, 140.1, 135.5, 132.5, 130.2, 129.4, 129.1, 126.4, 2 × 123.2, 122.7, 120.7, 120.0, 117.2, 2 × 107.9, 76.5, 76.4, 51.6, 51.5, 40.6, 40.5, 32.7, 32.6, 28.6, 28.5, 2 × 26.9, 26.0, 25.9, 21.1, 20.7; IR (CHCl3): 3430, 2959, 2931, 1728, 1489, 1354, 1228, 752 cm−1; HRMS (+ESI) calcd for C24H24NO3 [(M-H2O) + H]+: 374.1756; found: 374.1757.
3) as the eluent; mp 130–132 °C; 1H NMR (500 MHz, CDCl3): δ 7.64 (br s, 2H), 7.35 (d, J = 7.4 Hz, 2H), 7.25 (t, J = 7.7 Hz, 2H), 7.13 (t, J = 7.9 Hz, 1H), 7.02–7.06 (m, 4H), 6.97 (d, J = 7.4 Hz, 1H), 6.70 (d, J = 7.7 Hz, 2H), 6.59 (d, J = 8.1 Hz, 2H), 6.51 (s, 1H), 6.49 (s, 1H), 3.01 (s, 6H), 2.49–2.53 (m, 2H), 2.31–2.38 (m, 4H), 2.29 (s, 3H), 2.28 (s, 3H), 1.97 (d, J = 17.7 Hz, 2H), 1.07 (s, 3H), 1.01 (s, 3H), 0.97 (s, 3H), 0.96 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 175.0, 170.7, 170.5, 152.4, 150.2, 143.4, 140.1, 135.5, 132.5, 130.2, 129.4, 129.1, 126.4, 2 × 123.2, 122.7, 120.7, 120.0, 117.2, 2 × 107.9, 76.5, 76.4, 51.6, 51.5, 40.6, 40.5, 32.7, 32.6, 28.6, 28.5, 2 × 26.9, 26.0, 25.9, 21.1, 20.7; IR (CHCl3): 3430, 2959, 2931, 1728, 1489, 1354, 1228, 752 cm−1; HRMS (+ESI) calcd for C24H24NO3 [(M-H2O) + H]+: 374.1756; found: 374.1757.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the eluent; mp 177–178 °C; 1H NMR (500 MHz, CDCl3): δ 7.98 (br s, 1H), 7.65 (br s, 1H), 7.34–7.36 (m, 2H), 7.24–7.27 (m, 2H), 7.10–7.15 (m, 4H), 7.02–7.05 (m, 3H), 6.97 (d, J = 7.4 Hz, 1H), 6.71 (d, 2H), 6.51 (s, 1H), 6.49 (s, 1H), 3.01 (s, 6H), 2.51–2.56 (m, 2H), 2.33–2.38 (m, 4H), 2.29 (s, 3H), 2.28 (s, 3H), 1.97 (d, 2H), 1.08 (s, 3H), 1.06 (s, 3H), 0.97 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 175.3, 174.9, 171.0, 170.5, 152.3, 2 × 143.4, 140.1, 132.6, 132.5, 131.2, 129.4, 129.1, 127.3, 126.4, 126.0, 123.2, 122.8, 122.7, 120.7, 117.2, 107.9, 107.8, 76.7, 76.5, 51.6, 51.4, 40.5, 40.4, 32.7, 28.7, 26.9, 26.0, 25.9, 21.1; IR (CHCl3): 3430, 2959, 2931, 1728, 1489, 1354, 1228, 752 cm−1; HRMS (+ESI) calcd for C24H24NO3 [(M-H2O) + H]+: 374.1756; found: 374.1755.
3) as the eluent; mp 177–178 °C; 1H NMR (500 MHz, CDCl3): δ 7.98 (br s, 1H), 7.65 (br s, 1H), 7.34–7.36 (m, 2H), 7.24–7.27 (m, 2H), 7.10–7.15 (m, 4H), 7.02–7.05 (m, 3H), 6.97 (d, J = 7.4 Hz, 1H), 6.71 (d, 2H), 6.51 (s, 1H), 6.49 (s, 1H), 3.01 (s, 6H), 2.51–2.56 (m, 2H), 2.33–2.38 (m, 4H), 2.29 (s, 3H), 2.28 (s, 3H), 1.97 (d, 2H), 1.08 (s, 3H), 1.06 (s, 3H), 0.97 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 175.3, 174.9, 171.0, 170.5, 152.3, 2 × 143.4, 140.1, 132.6, 132.5, 131.2, 129.4, 129.1, 127.3, 126.4, 126.0, 123.2, 122.8, 122.7, 120.7, 117.2, 107.9, 107.8, 76.7, 76.5, 51.6, 51.4, 40.5, 40.4, 32.7, 28.7, 26.9, 26.0, 25.9, 21.1; IR (CHCl3): 3430, 2959, 2931, 1728, 1489, 1354, 1228, 752 cm−1; HRMS (+ESI) calcd for C24H24NO3 [(M-H2O) + H]+: 374.1756; found: 374.1755.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.65 (br s, 1H), 7.58 (br s, 1H), 7.35 (d, J = 7.4 Hz, 2H), 7.24–7.27 (m, 2H), 7.15 (t, J = 8.2 Hz, 1H), 7.05 (t, J = 7.5 Hz, 2H), 6.77 (d, J = 8.9 Hz, 2H), 6.69–6.73 (m, 3H), 6.65 (d, J = 8.7 Hz, 2H), 6.29 (d, J = 7.8 Hz, 1H), 6.22 (s, 1H), 3.77 (s, 3H), 3.73 (s, 3H), 3.03 (s, 6H), 2.49–2.55 (m, 2H), 2.25–2.38 (m, 4H), 2.00 (d, J = 17.7 Hz, 1H), 1.95 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 1.07 (s, 3H), 0.97 (s, 3H), 0.96 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.2, 174.9, 171.0, 170.2, 160.7, 157.3, 153.5, 145.9, 143.5, 143.4, 132.6, 132.5, 130.2, 129.3, 129.2, 123.3, 122.8, 121.4, 114.7, 112.4, 111.5, 108.0, 107.9, 106.0, 76.6, 76.5, 55.5, 55.4, 51.7, 51.6, 40.5, 40.4, 32.7, 32.6, 2 × 28.6, 27.1, 26.9, 26.1, 26.0; IR (CHCl3): 3433, 2960, 2932, 1730, 1492, 1355, 1230, 753 cm−1; HRMS (+ESI) calcd for C24H24NO4 [(M-H2O) + H]+: 390.1705; found: 390.1703.
1) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.65 (br s, 1H), 7.58 (br s, 1H), 7.35 (d, J = 7.4 Hz, 2H), 7.24–7.27 (m, 2H), 7.15 (t, J = 8.2 Hz, 1H), 7.05 (t, J = 7.5 Hz, 2H), 6.77 (d, J = 8.9 Hz, 2H), 6.69–6.73 (m, 3H), 6.65 (d, J = 8.7 Hz, 2H), 6.29 (d, J = 7.8 Hz, 1H), 6.22 (s, 1H), 3.77 (s, 3H), 3.73 (s, 3H), 3.03 (s, 6H), 2.49–2.55 (m, 2H), 2.25–2.38 (m, 4H), 2.00 (d, J = 17.7 Hz, 1H), 1.95 (d, J = 17.7 Hz, 1H), 1.08 (s, 3H), 1.07 (s, 3H), 0.97 (s, 3H), 0.96 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.2, 174.9, 171.0, 170.2, 160.7, 157.3, 153.5, 145.9, 143.5, 143.4, 132.6, 132.5, 130.2, 129.3, 129.2, 123.3, 122.8, 121.4, 114.7, 112.4, 111.5, 108.0, 107.9, 106.0, 76.6, 76.5, 55.5, 55.4, 51.7, 51.6, 40.5, 40.4, 32.7, 32.6, 2 × 28.6, 27.1, 26.9, 26.1, 26.0; IR (CHCl3): 3433, 2960, 2932, 1730, 1492, 1355, 1230, 753 cm−1; HRMS (+ESI) calcd for C24H24NO4 [(M-H2O) + H]+: 390.1705; found: 390.1703.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 132 °C; 1H NMR (500 MHz, CDCl3): δ 7.52 (br s, 1H), 7.27 (d, J = 6.9 Hz, 1H), 7.17 (t, J = 7.3 Hz, 1H), 7.07 (t, J = 7.9 Hz, 1H), 6.96 (t, J = 7.1 Hz, 1H), 6.63 (d, J = 6.2 Hz, 2H), 6.21 (d, J = 6.8 Hz, 1H), 6.14 (s, 1H), 3.64 (s, 3H), 2.94 (s, 3H), 2.44 (d, J = 16.1 Hz, 1H), 2.24–2.30 (m, 2H), 1.93 (d, J = 17.7 Hz, 1H), 0.99 (s, 3H), 0.89 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 170.2, 160.6, 153.3, 143.4, 132.4, 130.1, 129.2, 123.1, 122.7, 112.2, 111.4, 107.9, 105.9, 76.4, 55.3, 51.6, 40.4, 32.6, 28.6, 26.9, 25.9; IR (CHCl3): 3433, 2960, 2932, 1730, 1492, 1355, 1230, 753 cm−1; HRMS (+ESI) calcd for C24H24NO4[(M-H2O) + H]+: 390.1705; found: 390.1707.
1) as the eluent; mp 132 °C; 1H NMR (500 MHz, CDCl3): δ 7.52 (br s, 1H), 7.27 (d, J = 6.9 Hz, 1H), 7.17 (t, J = 7.3 Hz, 1H), 7.07 (t, J = 7.9 Hz, 1H), 6.96 (t, J = 7.1 Hz, 1H), 6.63 (d, J = 6.2 Hz, 2H), 6.21 (d, J = 6.8 Hz, 1H), 6.14 (s, 1H), 3.64 (s, 3H), 2.94 (s, 3H), 2.44 (d, J = 16.1 Hz, 1H), 2.24–2.30 (m, 2H), 1.93 (d, J = 17.7 Hz, 1H), 0.99 (s, 3H), 0.89 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 174.9, 170.2, 160.6, 153.3, 143.4, 132.4, 130.1, 129.2, 123.1, 122.7, 112.2, 111.4, 107.9, 105.9, 76.4, 55.3, 51.6, 40.4, 32.6, 28.6, 26.9, 25.9; IR (CHCl3): 3433, 2960, 2932, 1730, 1492, 1355, 1230, 753 cm−1; HRMS (+ESI) calcd for C24H24NO4[(M-H2O) + H]+: 390.1705; found: 390.1707.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)as the eluent; mp 155–156 °C; 1H NMR (500 MHz, CDCl3): δ 7.66 (br s, 1H), 7.36 (dd, J1 = 0.8 Hz, J2 = 7.4 Hz, 1H), 7.10–7.16 (m, 5H), 7.04–7.06 (m, 2H), 7.01 (dt, J1 = 0.9 Hz, J2 = 7.6 Hz, 1H), 6.71–6.73 (m, 1H), 6.59 (d, J = 7.8 Hz, 1H), 6.32 (d, J = 7.8 Hz, 1H), 6.21 (s, 1H), 4.84 (d, J = 15.8 Hz, 1H), 4.70 (d, J = 15.8 Hz, 1H), 3.71 (s, 3H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.1 Hz, 1H), 2.34 (d, J = 17.7 Hz, 1H), 2.02 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.6, 160.8, 153.5, 142.9, 135.6, 132.8, 130.2, 129.2, 128.4, 127.3, 127.2, 123.4, 122.9, 113.1, 111.9, 109.3, 106.7, 76.5, 55.5, 51.7, 44.1, 40.8, 32.7, 28.8, 26.9; IR (CHCl3): 3436, 2962, 2935, 1731, 1493, 1357, 1232, 756 cm−1; HRMS (+ESI) calcd for C30H28NO4 [(M-H2O) + H]+: 466.2018; found: 466.2027.
1)as the eluent; mp 155–156 °C; 1H NMR (500 MHz, CDCl3): δ 7.66 (br s, 1H), 7.36 (dd, J1 = 0.8 Hz, J2 = 7.4 Hz, 1H), 7.10–7.16 (m, 5H), 7.04–7.06 (m, 2H), 7.01 (dt, J1 = 0.9 Hz, J2 = 7.6 Hz, 1H), 6.71–6.73 (m, 1H), 6.59 (d, J = 7.8 Hz, 1H), 6.32 (d, J = 7.8 Hz, 1H), 6.21 (s, 1H), 4.84 (d, J = 15.8 Hz, 1H), 4.70 (d, J = 15.8 Hz, 1H), 3.71 (s, 3H), 2.55 (d, J = 16.1 Hz, 1H), 2.38 (d, J = 16.1 Hz, 1H), 2.34 (d, J = 17.7 Hz, 1H), 2.02 (d, J = 17.7 Hz, 1H), 1.09 (s, 3H), 0.99 (s, 3H); 13C NMR (126 MHz, CDCl3): δ 175.1, 170.6, 160.8, 153.5, 142.9, 135.6, 132.8, 130.2, 129.2, 128.4, 127.3, 127.2, 123.4, 122.9, 113.1, 111.9, 109.3, 106.7, 76.5, 55.5, 51.7, 44.1, 40.8, 32.7, 28.8, 26.9; IR (CHCl3): 3436, 2962, 2935, 1731, 1493, 1357, 1232, 756 cm−1; HRMS (+ESI) calcd for C30H28NO4 [(M-H2O) + H]+: 466.2018; found: 466.2027.
General procedure for the synthesis of O-arylated 1,3-cyclohexanediones
An oven-dried round bottomed flask (25 mL capacity) equipped with a magnetic stir bar was evacuated and purged with argon. 3-Substituted-3-hydroxyindolin-2-one (0.2 mmol, 1 equiv.), K2CO3 (0.6 mmol, 3 equiv.), and DMF (2 mL) were added successively at room temperature. The reaction mixture was stirred at 120 °C for 2 h. Water (10 mL) was added to the reaction mixture, and the organic layer was extracted with EtOAc (2 × 10 mL). The combined organic phases were washed with brine and dried over sodium sulfate. The solvent was removed, and the residue was purified by column chromatography on silica gel using hexane/ethyl acetate as an eluent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 70–71 °C; 1H NMR (500 MHz, CDCl3): δ 7.37–7.40 (m, 2H), 7.22–7.26 (m, 1H), 7.03 (dd, J1 = 1.1 Hz, J2 = 8.9 Hz, 2H), 5.11 (s, 1H), 2.52 (s, 2H), 2.24 (s, 2H), 1.15 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 199.4, 176.7, 152.8, 129.9, 126.1, 121.3, 104.9, 104.8, 50.6, 42.3, 32.7, 28.3; IR (CHCl3): 3063, 2959, 1661, 1616, 1587, 1489, 1370, 1204, 1164, 1022, 765, 582 cm−1; HRMS (+ESI) calcd for C14H17O2 [M + H]+: 217.1229; found: 217.1220.
1) as the eluent; mp 70–71 °C; 1H NMR (500 MHz, CDCl3): δ 7.37–7.40 (m, 2H), 7.22–7.26 (m, 1H), 7.03 (dd, J1 = 1.1 Hz, J2 = 8.9 Hz, 2H), 5.11 (s, 1H), 2.52 (s, 2H), 2.24 (s, 2H), 1.15 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 199.4, 176.7, 152.8, 129.9, 126.1, 121.3, 104.9, 104.8, 50.6, 42.3, 32.7, 28.3; IR (CHCl3): 3063, 2959, 1661, 1616, 1587, 1489, 1370, 1204, 1164, 1022, 765, 582 cm−1; HRMS (+ESI) calcd for C14H17O2 [M + H]+: 217.1229; found: 217.1220.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 93–95 °C; 1H NMR (500 MHz, CDCl3): δ 7.77–7.80 (m, 2H), 7.71 (d, J = 7.6 Hz, 1H), 7.41–7.44 (m, 3H), 7.09 (dd, J1 = 2.3 Hz, J2 = 8.8 Hz, 1H), 5.08 (s, 1H), 2.50 (s, 2H), 2.19 (s, 2H), 1.09 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 199.4, 176.7, 150.3, 133.9, 131.4, 130.2, 127.8, 127.5, 126.9, 125.9, 120.6, 118.4, 105.1, 50.7, 42.4, 32.8, 28.3; IR (CHCl3): 3065, 2959, 1663, 1618, 1589, 1489, 1373, 1206, 1164, 1025, 765, 585 cm−1; HRMS (+ESI) calcd for C18H19O2 [M + H]+: 267.1385; found: 267.1382.
1) as the eluent; mp 93–95 °C; 1H NMR (500 MHz, CDCl3): δ 7.77–7.80 (m, 2H), 7.71 (d, J = 7.6 Hz, 1H), 7.41–7.44 (m, 3H), 7.09 (dd, J1 = 2.3 Hz, J2 = 8.8 Hz, 1H), 5.08 (s, 1H), 2.50 (s, 2H), 2.19 (s, 2H), 1.09 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 199.4, 176.7, 150.3, 133.9, 131.4, 130.2, 127.8, 127.5, 126.9, 125.9, 120.6, 118.4, 105.1, 50.7, 42.4, 32.8, 28.3; IR (CHCl3): 3065, 2959, 1663, 1618, 1589, 1489, 1373, 1206, 1164, 1025, 765, 585 cm−1; HRMS (+ESI) calcd for C18H19O2 [M + H]+: 267.1385; found: 267.1382.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.23–7.26 (m), 7.17 (d), 7.04 (d), 6.89–6.91 (m), 6.81–6.83 (m), 5.12 (s), 5.11 (s), 2.51 (s), 2.50 (s), 2.35 (s), 2.34 (s), 2.24 (s), 2.23 (s), 1.14 (s), 1.13 (s); 13C NMR (126 MHz, CDCl3): δ 199.5, 199.4, 176.9, 176.8, 152.7, 150.5, 140.3, 135.7, 130.4, 129.6, 126.8, 121.9, 120.9, 118.2, 104.8, 104.7, 50.7, 42.3, 32.7, 28.3, 21.2, 20.8; IR (CHCl3): 3062, 2960, 1660, 1617, 1587, 1488, 1371, 1204, 1163, 1024, 765, 584 cm−1; HRMS (+ESI) calcd for C15H19O2 [M + H]+: 231.1385; found: 231.1383.
1) as the eluent; 1H NMR (500 MHz, CDCl3): δ 7.23–7.26 (m), 7.17 (d), 7.04 (d), 6.89–6.91 (m), 6.81–6.83 (m), 5.12 (s), 5.11 (s), 2.51 (s), 2.50 (s), 2.35 (s), 2.34 (s), 2.24 (s), 2.23 (s), 1.14 (s), 1.13 (s); 13C NMR (126 MHz, CDCl3): δ 199.5, 199.4, 176.9, 176.8, 152.7, 150.5, 140.3, 135.7, 130.4, 129.6, 126.8, 121.9, 120.9, 118.2, 104.8, 104.7, 50.7, 42.3, 32.7, 28.3, 21.2, 20.8; IR (CHCl3): 3062, 2960, 1660, 1617, 1587, 1488, 1371, 1204, 1163, 1024, 765, 584 cm−1; HRMS (+ESI) calcd for C15H19O2 [M + H]+: 231.1385; found: 231.1383.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent; mp 89–90.0 °C; 1H NMR (500 MHz, CDCl3): δ 7.28 (t, J = 6.4 Hz, 1H), 6.78 (dd, J1 = 2.4 Hz, J2 = 8.4 Hz, 1H), 6.62 (dd, J1 = 2.1 Hz, J2 = 8.0 Hz, 1H), 6.57 (t, J = 2.3 Hz, 1H), 5.17 (s, 1H), 3.79 (s, 3H), 2.51 (s, 2H), 2.24 (s, 2H), 1.14 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 199.4, 176.5, 160.9, 153.7, 130.4, 113.4, 111.7, 107.4, 104.9, 55.4, 50.7, 42.3, 32.7, 28.3; IR (CHCl3): 3066, 2962, 1663, 1616, 1590, 1489, 1373, 1208, 1168, 1026, 769, 586 cm−1; HRMS (+ESI) calcd for C15H19O3 [M + H]+: 247.1334; found: 247.1330.
1) as the eluent; mp 89–90.0 °C; 1H NMR (500 MHz, CDCl3): δ 7.28 (t, J = 6.4 Hz, 1H), 6.78 (dd, J1 = 2.4 Hz, J2 = 8.4 Hz, 1H), 6.62 (dd, J1 = 2.1 Hz, J2 = 8.0 Hz, 1H), 6.57 (t, J = 2.3 Hz, 1H), 5.17 (s, 1H), 3.79 (s, 3H), 2.51 (s, 2H), 2.24 (s, 2H), 1.14 (s, 6H); 13C NMR (126 MHz, CDCl3): δ 199.4, 176.5, 160.9, 153.7, 130.4, 113.4, 111.7, 107.4, 104.9, 55.4, 50.7, 42.3, 32.7, 28.3; IR (CHCl3): 3066, 2962, 1663, 1616, 1590, 1489, 1373, 1208, 1168, 1026, 769, 586 cm−1; HRMS (+ESI) calcd for C15H19O3 [M + H]+: 247.1334; found: 247.1330.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Director, CSIR-NEIST, Jorhat, India for his interest in this work and facilities. HH acknowledge the DST, New Delhi, India for DST-Inspire fellowship grants.Notes and references
- (a) C. R. Edwankar, R. V. Edwankar, O. A. Namjoshi, S. K. Rallapalli, J. Yang and J. M. Cook, Curr. Opin. Drug Discovery Dev., 2009, 12, 752 CAS; (b) Shaveta, A. Singh, M. Kaur, S. Sharma, R. Bhatti and P. Singh, Eur. J. Med. Chem., 2014, 77, 185 CrossRef CAS PubMed; (c) B. Biersack and R. Schobert, Curr. Drug Targets, 2012, 13, 1705 CrossRef CAS PubMed.
- (a) H. Qiu, M. Li, L. Q. Jiang, F. P. Lv, L. Zan, C. W. Zhai, M. P. Doyle and W. H. Hu, Nat. Chem., 2012, 4, 733 CrossRef CAS PubMed; (b) I. Ammetto, T. Gasperi, A. M. Loreto, A. Migliorini, F. Palmarelli and P. A. Tardella, Eur. J. Org. Chem., 2009, 6189 CrossRef CAS.
- (a) N. Kamakine, Y. Takaishi, G. Honda, M. Ito, Y. Takeda, O. K. Kodzhimatov and O. Ahurmetov, Nat. Med., 2005, 59, 45 Search PubMed; (b) J. I. Jimenez, U. Huber, R. E. Moore and G. M. L. Patterson, J. Nat. Prod., 1999, 62, 569 CrossRef CAS PubMed; (c) T. Kagata, S. Saito, H. Shigemori, A. Ohsaki, H. Ishiyama, T. Kubota and J. Kobayashi, J. Nat. Prod., 2006, 69, 1517 CrossRef CAS PubMed.
- For selected examples, see: (a) R. R. Goehring, Y. P. Sachdeva, J. S. Pisipati, M. C. Sleevi and J. F. Wolfe, J. Am. Chem. Soc., 1985, 107, 435 CrossRef CAS; (b) H. B. Rasmussen and J. K. MacLeod, J. Nat. Prod., 1997, 60, 1152 CrossRef CAS; (c) J. Kohno, Y. Koguchi, M. Nishio, K. Nakao, M. Juroda, R. Shimizu, T. Ohnuki and S. Komatsubara, J. Org. Chem., 2000, 65, 990 CrossRef CAS.
- (a) Y. Koguchi, J. Kohno, M. Nishio, K. Takahashi, T. Okuda, T. Ohnuki and S. Komatsubara, J. Antibiot., 2000, 53, 105 CrossRef CAS; (b) Y. Q. Tang, I. Sattler, R. Thiericke, S. Grabley and X. Z. Feng, Eur. J. Org. Chem., 2001, 261 CrossRef CAS; (c) J. Nagamine, R. Nagata, H. Seki, N. Nomura-Akimaru, Y. Ueki, K. Kumagai, M. Taiji and H. Noguchi, J. Endocrinol., 2001, 171, 481 CAS.
- (a) R. Raj, J. Gut, P. J. Rosenthal and V. Kumar, Bioorg. Med. Chem. Lett., 2014, 24, 756 CrossRef CAS; (b) K. Kumar, S. Carrère-Kremer, L. Kremer, Y. Guèrardel, C. Biot and V. Kumar, Organometallics, 2013, 32, 5713 CrossRef CAS; (c) R. Raj, V. Sharma, M. J. Hopper, N. Patel, D. Hall, L. A. Wrischnik, K. M. Land and V. Kumar, Med. Chem. Res., 2014, 23, 3671 CrossRef CAS; (d) M. J. Spallek, D. Riedel, F. Rominger, A. S. K. Hashmi and O. Trapp, Organometallics, 2012, 31, 1127 CrossRef CAS.
- D. Sano, K. Nagata and T. Itoh, Org. Lett., 2008, 10, 1593 CrossRef CAS.
- C. Jing, T. Shi, D. Xing, X. Guo and W.-H. Hu, Green Chem., 2013, 15, 620 RSC.
- L.-M. Zhao, A.-L. Zhang, J.-H. Zhang, H.-S. Gao and W. Zhou, J. Org. Chem., 2016, 81, 5487 CrossRef CAS.
- S. Cañellas, P. Alonso and M. À. Pericàs, Org. Lett., 2018, 20, 4806 CrossRef.
- W.-B. Chen, Y.-H. Liao, X.-L. Du, X.-M. Zhang and W.-C. Yuan, Green Chem., 2009, 11, 14656 Search PubMed.
- M. Chouhan, K. R. Senwar, R. Sharma, V. Grover and V. A. Nair, Green Chem., 2011, 13, 2553 RSC.
- (a) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS; (b) A. Z. Halimehjani, S. Shokrgozar and P. Beier, J. Org. Chem., 2018, 83, 5778 CrossRef CAS PubMed; (c) N. E. Leadbeater and M. Marco, Angew. Chem., Int. Ed., 2003, 42, 1407 CrossRef CAS; (d) V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957 RSC; (e) D. Arunprasath, B. B. Devi and G. Sekar, Adv. Synth. Catal., 2019, 361, 117 CrossRef; (f) F. Lv, B. Tang, E. Hao, Q. Liu, H. Wang and L. Jiao, Chem. Commun., 2019, 55, 1639 RSC; (g) R. Zhang, S. Jin, Q. Liu, S. Lin and Z. Yan, J. Org. Chem., 2018, 83, 13030 CrossRef CAS PubMed; (h) K. Kisiel, J. Brześkiewicz, R. Loska and M. Mąkosza, Adv. Synth. Catal., 2019, 361, 1641 CrossRef CAS.
- For high tolerance against moisture, see: (a) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (b) P. M. Stein, M. Rudolph and A. S. K. Hashmi, Adv. Synth. Catal., 2021, 363, 1 CrossRef.
- A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC.
- Y. Himeshima, T. Sonoda and H. Kobayashi, Chem. Lett., 1983, 1211 CrossRef CAS.
- T. Kaicharla and A. T. Biju, in Green Synthetic Approaches for Biologically Relevant Heterocycles, ed. G. Brahmachari, Elsevier, Amsterdam, 2014, p. 45 Search PubMed.
- (a) H. Yoshida, M. Watanabe, J. Ohshita and A. Kunai, Chem. Commun., 2005, 3292 RSC; (b) S. S. Bhojgude, D. R. Baviskar, R. G. Gonnade and A. T. Biju, Org. Lett., 2015, 17, 6270 CrossRef CAS PubMed; (c) S. S. Bhojgude, T. Roy, R. G. Gonnade and A. T. Biju, Org. Lett., 2016, 18, 5424 CrossRef CAS; (d) M. M. Ahire, R. Khan and S. B. Mhaske, Org. Lett., 2017, 19, 2134 CrossRef CAS PubMed; (e) S. S. Bhojgude, T. Kaicharla and A. T. Biju, Org. Lett., 2013, 15, 5452 CrossRef CAS PubMed; (f) A. Bhunia, D. Porwal, R. G. Gonnade and A. T. Biju, Org. Lett., 2013, 15, 4620 CrossRef CAS PubMed; (g) A. Bhunia, T. Roy, P. Pachfule, P. R. Rajamohanan and A. T. Biju, Angew. Chem., Int. Ed., 2013, 52, 10040 CrossRef CAS; (h) T. Kaicharla, S. S. Bhojgude and A. T. Biju, Org. Lett., 2012, 14, 6238 CrossRef CAS; (i) S. S. Bhojgude, T. Kaicharla, A. Bhunia and A. T. Biju, Org. Lett., 2012, 14, 4098 CrossRef CAS PubMed.
- (a) J. Li, N. Wang, C. Li and X. Jia, Org. Lett., 2012, 14, 4994 CrossRef CAS; (b) M. Thangaraj, S. S. Bhojgude, R. H. Bisht, R. G. Gonnade and A. T. Biju, J. Org. Chem., 2014, 79, 4757 CrossRef CAS PubMed; (c) Y. Tao, F. Zhang, C.-Y. Tang, X.-Y. Wu and F. Sha, Asian J. Org. Chem., 2014, 3, 1292 CrossRef CAS; (d) S. S. Bhojgude, M. Thangaraj, E. Suresh and A. T. Biju, Org. Lett., 2014, 16, 3576 CrossRef CAS; (e) X. Zhong, J. Lv and S. Luo, Org. Lett., 2015, 17, 1561 CrossRef CAS; (f) F. Sha, Y. Tao, C.-Y. Tang, F. Zhang and X.-Y. Wu, J. Org. Chem., 2015, 80, 8122 CrossRef CAS PubMed; (g) Y. Chen and M. C. Willis, Org. Lett., 2015, 17, 4786 CrossRef CAS.
- (a) E. Yoshioka, S. Kohtani and H. Miyabe, Angew. Chem., Int. Ed., 2011, 50, 6638 CrossRef CAS; (b) E. Yoshioka, M. Nishimura, T. Nakazawa, S. Kohtani and H. Miyabe, J. Org. Chem., 2015, 80, 8464 CrossRef CAS.
- (a) F.-L. Liu, J.-R. Chen, Y.-Q. Zou, Q. Wei and W.-J. Xiao, Org. Lett., 2014, 16, 3768 CrossRef CAS PubMed; (b) J. Wallbaum, P. G. Jones and D. B. Werz, J. Org. Chem., 2015, 80, 3730 CrossRef CAS PubMed; (c) M. Pawliczek, L. K. B. Garve and D. B. Werz, Org. Lett., 2015, 17, 1716 CrossRef CAS PubMed; (d) H.-Y. Li, L.-J. Xing, M.-M. Lou, H. Wang, R.-H. Liu and B. Wang, Org. Lett., 2015, 17, 1098 CrossRef CAS.
- (a) K. Neog, A. Borah and P. Gogoi, J. Org. Chem., 2016, 81, 11971 CrossRef CAS; (b) K. Neog, D. Dutta, B. Das and P. Gogoi, Org. Lett., 2017, 19, 730 CrossRef CAS PubMed; (c) K. Neog, B. Das and P. Gogoi, Org. Biomol. Chem., 2018, 16, 3138 RSC; (d) A. Sharma and P. Gogoi, ChemistrySelect, 2017, 2, 11801 CrossRef CAS; (e) A. Sharma and P. Gogoi, Org. Biomol. Chem., 2019, 17, 333 RSC; (f) K. Neog, D. Dutta, B. Das and P. Gogoi, Org. Biomol. Chem., 2019, 17, 6450 RSC; (g) H. Hazarika, K. Neog, A. Sharma, B. Das and P. Gogoi, J. Org. Chem., 2019, 84, 5846 CrossRef CAS; (h) A. Sharma and P. Gogoi, Org. Biomol. Chem., 2019, 17, 9014 RSC; (i) H. Hazarika and P. Gogoi, Org. Biomol. Chem., 2020, 18, 2727 RSC.
- CCDC 2098054 (compound 4baa)†.
- K. Okuma, R. Itoyama, A. Sou, N. Nagahora and K. Shioj, Chem. Commun., 2012, 48, 11145 RSC.
- R. Samineni, P. Srihari and G. Mehta, Org. Lett., 2016, 18, 2832 CrossRef CAS.
- B. Liang, S. Kalidindi, J. A. Porco Jr and C. R. J. Stephenson, Org. Lett., 2010, 12, 572 CrossRef CAS.
- Z.-F. Xu, C.-X. Cai, M. Jiang and J.-T. Liu, Org. Lett., 2014, 16, 3436 CrossRef CAS PubMed.
- D. H. R. Barton, J.-P. Finet, J. Khamsi and C. Pichon, Tetrahedron Lett., 1986, 27, 3619 CrossRef CAS.
- D. H. R. Barton, J.-C. Blazejewski, B. Charpiot and W. B. Motherwe, J. Chem. Soc., Chem. Commun., 1981, 10, 503 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR and HRMS spectra of all products are available. CCDC 2098054. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1nj04295e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2022 |