Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective suppression of {112} anatase facets by fluorination for enhanced TiO2 particle size and phase stability at elevated temperatures†
Emerson C.
Kohlrausch
ab,
Roberto
dos Reis
 c,
Rhys W.
Lodge
c,
Rhys W.
Lodge
 b,
Isabel
Vicente
d,
Alexandre G.
Brolo
b,
Isabel
Vicente
d,
Alexandre G.
Brolo
 e,
Jairton
Dupont
e,
Jairton
Dupont
 a,
Jesum
Alves Fernandes
a,
Jesum
Alves Fernandes
 *b and
Marcos. J. L.
Santos
*b and
Marcos. J. L.
Santos
 *a
*a
aInstituto de Química – UFRGS, 91501-970, Porto Alegre, RS, Brazil. E-mail: mjls@ufrgs.br
bSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK. E-mail: jesum.alvesfernandes@nottingham.ac.ik
cDepartment of Materials Science and Engineering, Northwestern University, Evanston, Illinois 60208, USA
dUnitat de Tecnologíe Químiques, EURECAT, Tarragona, 43007 Spain
eDepartment of Chemistry, University of Victoria, P. O. Box 3065, V8W 3V6, BC, Canada
First published on 3rd September 2021
Abstract
Generally, anatase is the most desirable TiO2 polymorphic phase for photovoltaic and photocatalytic applications due to its higher photoconductivity and lower recombination rates compared to the rutile phase. However, in applications where temperatures above 500 °C are required, growing pure anatase phase nanoparticles is still a challenge, as above this temperature TiO2 crystallite sizes are larger than 35 nm which thermodynamically favors the growth of rutile crystallites. In this work, we show strong evidence, for the first time, that achieving a specific fraction (50%) of the {112} facets on the TiO2 surface is the key limiting step for anatase-to-rutile phase transition, rather than the crystallite size. By using a fluorinated ionic liquid (IL) we have obtained pure anatase phase crystallites at temperatures up to 800 °C, even after the crystallites have grown beyond their thermodynamic size limit of ca. 35 nm. While fluorination by the IL did not affect {001} growth, it stabilized the pure anatase TiO2 by suppressing the formation of {112} facets on anatase particles. By suppressing the {112} facets, using specific concentrations of fluorinated ionic liquid in the TiO2 synthesis, we controlled the anatase-to-rutile phase transition over a wide range of temperatures. This information shall help synthetic researchers to determine the appropriate material conditions for specific applications.
Introduction
Surface chemistry plays a crucial role in the properties of solid-state materials, especially in nanoparticles where high surface-area-to-volume ratios amplify the unique physicochemical characteristics of highly energetic surface atoms.1 Significant efforts have been devoted to controlling nanoparticle facets to produce materials with predictable optical, electrical, and catalytic properties.2–4 The surface energy of these facets contributes to the minimization of the total surface energy of the particle during crystal growth, which can be controlled, to a certain degree, by certain synthetic approaches.5–7One of the most explored approaches to control the size, shape, and catalytic properties of TiO2 is through the incorporation of dopants, such as transition metals (Fe, Ni, Co) or non-metals (N, F, S).8–16 The effect of fluorine on the size, phase, and morphology of TiO2 particles has been widely explored and hydrofluoric acid has been found to be the key reactant to obtain nanoparticles with their surface dominated by exposed {001} facets.17 Urea and EDTA have also been found to drive the formation of TiO2 nanoparticles with large {001} facets.18–20 Adjusting the fluoride/titanium molar ratio controlled the size of TiO2 sheets with predominant {001} facets which was shown to improve the photodegradation efficiency of organic dyes.21 {001} facets on uniform anatase TiO2 single crystals were also selectively grown using hydrofluoric acid as a morphology controlling agent.22 More recently, another study of TiO2 growth using HF showed that whilst fluorine was responsible for the anatase morphology with exposed low-index facets, H+ was responsible for increasing the percentage of {001} facets.23 When TiO2 was synthesized in fluorinated ionic liquids, it preferentially formed the brookite phase, rather than anatase, above a certain concentration of [BF4]− anions. Subsequent thermal treatment led to a transition from brookite to anatase that was accompanied by the release of fluorine, through which the authors concluded that fluorine was responsible for stabilizing the brookite phase.24
Of the notable facets of anatase, the {112} facet is important because it plays a crucial role in the anatase-to-rutile phase transitions. Penn et al. have demonstrated that rutile nucleate at {112} twin anatase interfaces.25 Recently, Zhu et al.26 have studied the pathways of surface restructuring, showing that {112} undergoes reconstruction that can lead to a new phase propagating into the anatase bulk, while the reconstruction of (001), (100), (101), and other planes only takes place at the surface. Therefore, they concluded that only the {112} in anatase is responsible for the initial nucleation that will allow anatase-to-rutile phase transition.
Furthermore, the TiO2 crystallite size is dependent on the thermodynamic stability of each polymorphic phase. For crystallites smaller than 35 nm, the anatase phase is more stable than rutile, and an anatase-to-rutile phase transition only starts after the thermodynamic critical size is reached (>35 nm) which usually occurs at temperatures above 500 °C.27–30 However, the growth of pure anatase phase TiO2 nanoparticles larger than 35 nm at temperatures above 500 °C has yet to be reported as they are thermodynamically unfavorable.31–33
In this work, we attempted to synthesize pure anatase phase TiO2 nanoparticles larger than 35 nm in a fluorinated ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate (BMIm·BF4), at temperatures up to 800 °C and compare their efficiency with that of pure TiO2 nanoparticles when applied to dye-sensitized solar cells (DSSCs). By using XRD diffraction patterns from thermally treated nanoparticles in a wide range of temperature, we have used the Wulff construction to extract meaningful information about facet growth on the TiO2 particles.34–36
Experimental section
Synthesis of TiO2
TiO2 nanoparticles were synthesized by adding acetic acid (5.7 mL) to titanium isopropoxide (15 mL) under constant stirring at 25 °C.37 The solution was stirred for 15 min and poured into deionized water (70 mL). The mixture was stirred for one hour at room temperature to complete the hydrolysis. The ionic liquid (IL) BMIm·BF4 (1% or 10% w/w, with respect to deionized water) was then added to the solution, in addition to nitric acid (63%, 1 mL), before being stirred for 8 hours at 80 °C. Finally, the mixture was transferred to an autoclave and heated at 230 °C for 12 hours. All samples were subsequently rinsed with water (3 × 50 mL) followed by ethanol (3 × 50 mL). The samples were labelled as TiO2 (no addition of IL), TiO2/IL 1% (1% (w/w) of the ionic liquid) and TiO2/IL 10% (10% (w/w) of the ionic liquid). The samples were thermally treated at 300, 400, 500, 600, 700, 800 and 900 °C for 3 hours in air.Characterization
The morphology, size and structural characteristics of the as-synthesized TiO2 nanoparticles were observed by transmission electron microscopy performed with a Libra Zeiss 120 and a Philips CM300. SEM images were obtained using a JEOL 7100F Field-Emission Gun Scanning Electron Microscope (FEG-SEM). A working distance of 10 mm was maintained with acquisitions utilizing a beam voltage of 15 kV. For analysis, a small amount of the sample was deposited onto double-sided carbon tape mounted on a stub, followed by sputter-coating with iridium (5 nm thickness) to make the sample conductive. X-ray powder diffraction (XRD) patterns were obtained using a Siemens D5000 diffractometer with Cu-Kα radiation (λ = 1.5418 Å) in a 2θ range from 10 to 90° with a step size of 0.05° and time of 1 s per step.Wulff grain construction
The Wulff grain construction was obtained by inputting the Miller indices and respective crystallite size for the {hkl} plane families {101}, {103}, {004} and {112} from the anatase phase with the auxiliary of VESTA software. The size of each family's planes was obtained using the Scherrer equation from XRD patterns obtained in the present work. Wulff construction was performed using the atomic position set and the space group of the anatase structure I41/amd, no. 141. The unit cell is defined by the lattice vectors a and c and contains two TiO2 units with Ti ions at 4b Wyckoff positions (0, 1/4, 3/8) and (0, 3/4, 5/8) and O ions at 8e Wyckoff positions (0, 1/4, u), (0, 3/4, 1/4 + u), (1/2, 1/4, −u + 1/2) and (1/2, 3/4, 1/4 − u).38,39DSSC assembly and measurements
The procedure used to assemble the DSSCs followed a previous literature method.37 The characterization and performance of the DSSCs were evaluated by current versus potential measurements, and carried out using a 300 W xenon arc lamp and an AM1.5 filter. The power of the simulated light was calibrated to 100 mW cm−2 and recorded by a picoamperimeter (Keithley, model 2400).Results and discussion
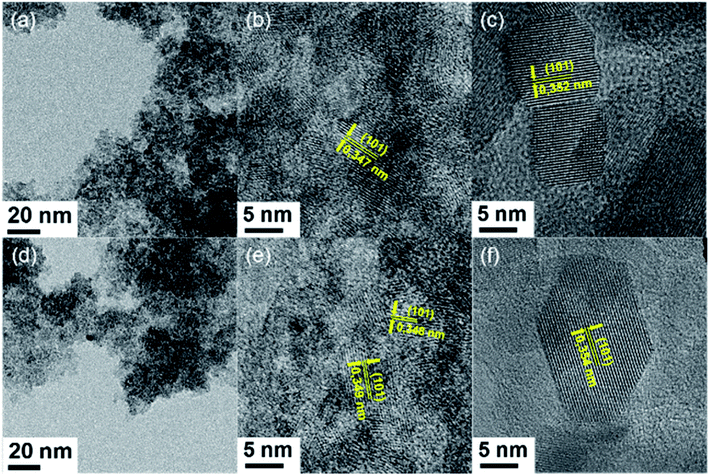
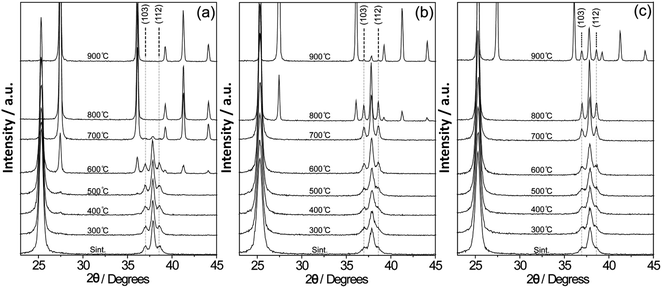
HR-TEM investigations of TiO2, TiO2/IL 1% and TiO2/IL 10% were carried out after different synthetic steps. TEM images were acquired for all samples after the hydrolysis step and, while no formation of anatase seeds was observed from TiO2, Fig. 1 shows the formation of anatase nanoseeds from TiO2/IL 1% and TiO2/IL 10% (Fig. 1a, b and d, e, respectively).40–43 After the autoclave step at 230 °C, crystalline particles were obtained for all the samples, and no significant variation in morphology, crystalline structure, or size distribution was observed (Fig. S1 and S2†).To track TiO2 phase transitions, and to evaluate the thermal stability of the anatase crystals, the samples were thermally treated at 300, 400, 500, 600, 700, 800 and 900 °C (Fig. 2 and S3†). For TiO2, the phase transition of anatase-to-rutile began at 400 °C with a small diffraction peak at ca. 27.4°. As the temperature rose to 600 °C, a sharper, defined diffraction peak at ca. 27.4° was observed (Fig. 2a). The calculated TiO2 crystallite sizes at 600 °C were 24.4 and 36.2 nm for anatase and rutile, respectively (Fig. 3b and Table S1†). A complete conversion to rutile took place between 700 and 800 °C resulting in rutile crystallites 43.0 nm in size. At 700 °C, only a small number of anatase particles with a crystallite size of 28.2 nm were observed. For TiO2/IL 1% (Fig. 2b), the anatase-to-rutile phase transition started at ca. 800 °C with anatase presenting a crystallite size of 42.8 nm and an incomplete conversion of anatase-to-rutile being observed even at 900 °C with anatase crystallites of 47.6 nm still present (Table S1†). In the TiO2/IL 10% sample (Fig. 2c), the anatase-to-rutile phase transition only started at 900 °C and again showed anatase crystallites (47.5 nm) beyond their thermodynamic limit of 35 nm. Raman spectroscopy measurements revealed that the temperature required to promote the solid-to-solid phase transition from anatase-to-rutile was dependent on the concentration of fluorine (Fig. S4†). For TiO2 thermally treated at temperatures higher than 600 °C, the rutile phase was predominant over anatase; however, for TiO2/IL 1% this predominance was only observed at 900 °C and for TiO2/IL 10% anatase was the main phase even at 900 °C. UV-Vis measurements showed a significant redshift for the fluorinated samples, when compared with TiO2, at the temperature at which the phase transition took place (Fig. S5†).44–47
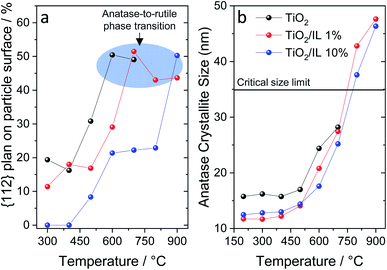 | ||
| Fig. 3 (a) Percentage of {112} planes on the particle surface as a function of temperature highlighting the suppression of this facet by the IL compared to pure TiO2. The data were extracted from the Wulff construction (see details in ESI Table S7†). (b) Anatase crystallite size for all samples, from the {101} planes (Table S3†), as a function of the temperature highlighting the crystallite critical size limit. | ||
As discussed earlier, the anatase-to-rutile phase transition temperature can be affected or controlled by how efficiently {112} twin boundaries interact with each other.25,26 Therefore, we evaluated the effect of fluorine on the {112} facet growth by comparing the equivalent diffraction peaks related to the {112} plane at 38.60° and to the {103} plane at 36.95° (PDF number # 21-1272) (see Tables S2 and S3†).48 For the fluorine–TiO2 samples, the relative areas of the diffraction peaks ({112} and {103}) became nearly equivalent only at temperatures at which the material was about to undergo the anatase-to-rutile phase transition, while for pristine TiO2 they remained similar to those for the synthesis at 600 °C. These results demonstrated that fluorine plays a critical role in suppressing the formation of {112} planes, thus delaying the anatase-to-rutile transition. The crystallite sizes for the {101}, {103}, {004} and {112} planes for TiO2, TiO2/IL 1% and TiO2/IL 10% are presented in Tables S4–S6.† Although many reports in the literature have shown the use of fluoride to drive the preferential growth of {001} facets in TiO2,22,49,50 in the present work we have no clear evidence about the influence of the fluorinated ionic liquid on the {001} planes. In fact, neither hydrofluoric acid nor titanium tetrachloride was used in the synthesis and the F/Ti molar ratio in both samples was smaller than that commonly used to drive the preferential growth of {001} facets. We suggest that the amount of fluorine used to obtain TiO2/IL 1% and TiO2/IL 10% is enough to suppress the {112} planes, but not to affect the {001} growth.
Wulff grain construction was performed to confirm the effect of fluorine on the {112} plane at different temperatures (Fig. 3a and S6, and Tables S7–S10†).51 Wulff principles are widely used in grain growth to determine which crystal geometrical shape has the minimum surface energy. The morphological equilibrium of the crystal corresponds to the minimization of the surface energy; from a thermodynamic perspective, the equilibrium crystal shape corresponds to the lowest free energy of the particle under specific conditions.52,53Fig. 3a shows the Wulff construction and highlights the {112} content, as a percentage, at which the anatase-to-rutile phase transition started. For pristine TiO2, the contribution of the {112} facets increased from ca. 15% to 50% from 400 to 600 °C. TiO2/IL 1% presented a similar trend, albeit from 500 to 700 °C instead. For TiO2/IL 10%, three prominent features were observed: (i) the complete suppression of the {112} facets up to 400 °C; (ii) a contribution of only 20–25% of the {112} facets up to 800 °C; and (iii) a ca. 50% contribution at 900 °C, the temperature at which the anatase-to-rutile phase transition started for TiO2/IL 10%. Additionally, Fig. 3b shows the crystallite sizes obtained from XRD measurements at different temperatures, highlighting the size limit within which anatase is thermodynamically stable. While a maximum size of ca. 28.2 nm was observed for TiO2, the samples modified with fluorinated ionic liquid, TiO2/IL 1% and TiO2/IL 10%, exhibited crystallites of ca. 48 nm that surpass the thermodynamic limit. These results strongly suggested that achieving a specific fraction of the {112} facets might be the threshold limiting step for anatase-to-rutile phase transition and not the crystallite size itself.
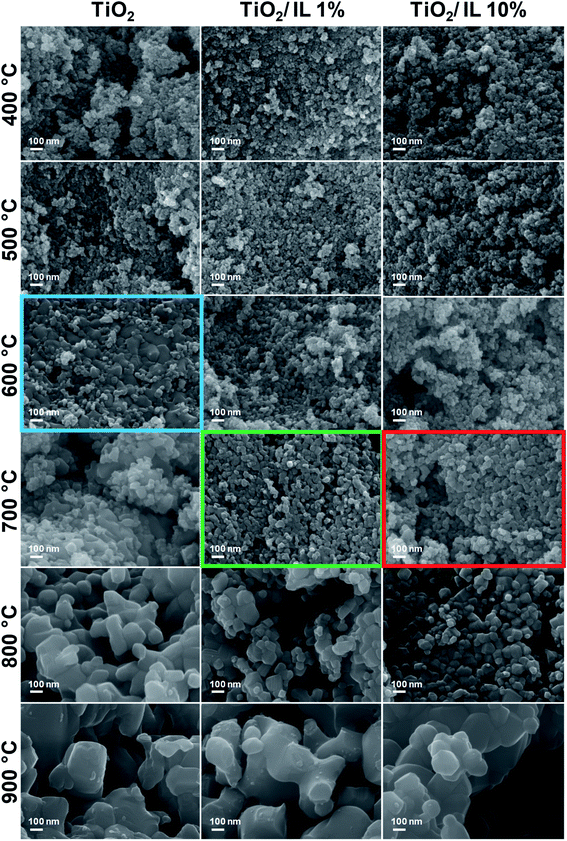
FEG-SEM of TiO2, TiO2/IL 1% and TiO2/IL 10% was performed to investigate the morphological changes before and after thermal treatment (Fig. 4). For pristine TiO2, a sharp change in nanoparticle morphology was observed at 600 °C (blue box, Fig. 4), at which the sintering of relatively small TiO2 nanoparticles led to the formation of much larger nanoparticles; this agreed with the anatase-to-rutile phase transition observed in XRD and Raman spectroscopy measurements. However, for TiO2/IL 1% and TiO2/IL 10% the sintering and formation of larger anatase nanoparticles were observed at 700 °C (green and red boxes, respectively, Fig. 4), which was a lower temperature than that observed for the anatase-to-rutile transition by XRD and Raman spectroscopy. In contrast to some previous reports, this demonstrated that the anatase-to-rutile phase transition limiting step is directly related to {112} facet concentration rather than a critical crystallite size of 35 nm.
EDX spectroscopy and XPS (Fig. S7–S10 and Table S3†) revealed the presence of fluorine in the samples prepared with ionic liquid prior to and after thermal treatment, while neither boron nor nitrogen were observed. HR-XPS of the F 1s region from the as-synthesized TiO2/IL 1% and TiO2/IL 10% showed a peak at 684.6 eV which was associated with adsorbed F− ions on the TiO2 surface (Fig. S10b and S10c†).54 However, after thermal treatment at 500 °C, an additional peak at 687.8 eV was observed which was attributed to the incorporation of fluorine atoms into the TiO2 structure (Figure S10e and S10f†). This suggested that the fluorine ions were coordinating to surface Ti4+ and Ti3+ ions, impacting the anatase-to-rutile phase transition.55,56
Photocurrent versus voltage (I–V) measurements of DSSCs assembled with TiO2, TiO2/IL 1%, and TiO2/IL 10% were carried out (Fig. S11†) and the following parameters were measured from the assembled devices: the short-circuit current (Isc), open-circuit voltage (Voc), Fill Factor (FF) and efficiency (η) (Table 1). The power conversion efficiencies (under AM 1.5G illumination) of standard TiO2, TiO2/IL 1%, and TiO2/IL 10% were 5.50%, 5.96%, and 6.35%, respectively. The 15% enhancement in efficiency found for TiO2/IL 10%, when compared to TiO2, can be ascribed to the red-shift shown by the fluorinated samples. Additionally, it could be due to the suppression of rutile phase growth for the TiO2 fluorinated samples during the sintering of the device assembly (Fig. S12†).57 This may lead to a decrease in the density of defects in the fluorinated samples when compared to pristine TiO2, which can act as recombination centers for photogenerated electron–hole pairs.58–60
| Sample | I sc (mA) | V oc (V) | I max V max | FF | η |
|---|---|---|---|---|---|
| TiO2 | 14.50 | 0.71 | 5.50 | 55% | 5.50% |
| TiO2/IL 1% | 14.75 | 0.71 | 5.96 | 57% | 5.96% |
| TiO2/IL 10% | 15.12 | 0.73 | 6.35 | 58% | 6.35% |
Conclusions
An ionic liquid (BMIm·BF4) was used in the hydrothermal synthesis of TiO2, resulting in fluorinated anatase phase nanoparticles greater in size than the previously determined 35 nm size limit. Fluorine favored the formation of the anatase phase at very low temperatures and contributed to the maintenance of this phase by hindering the growth of the {112} planes. This delayed the anatase-to-rutile phase transition, requiring much higher temperatures than usually observed for TiO2. Wulff construction along with analyses by XPS, XRD, and Raman spectroscopy showed that the {112} facet played the main role in the anatase-to-rutile phase transition of TiO2. These results strongly suggested that achieving a specific fraction of the {112} facets might be the threshold limiting step for anatase-to-rutile phase transition. Our findings demonstrate that the formation of {112} facets on the TiO2 surface can be finely tuned by altering the concentration of fluorinated ionic liquid in the TiO2 synthesis, enabling precise control of the anatase-to-rutile phase transition for a wide range of temperatures. DSSCs assembled with mesoporous layers based on TiO2/IL 1% and TiO2/IL 10% presented an improvement of the photocurrent and fill factor, and 15% greater efficiency when compared to pristine TiO2.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant 408182/2016-4), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul and CAPES. JAF would like to acknowledge the University of Nottingham Beacons of Excellence: Propulsion Futures & Green Chemicals and EPSRC: LiPPS XPS system, and EP/K005138/1 “University of Nottingham Equipment Account” for providing financial support for this work and the Nanoscale and Microscale Research Centre (University of Nottingham, UK) for access to XPS facilities.References
- K. Jacobs, D. Zaziski, E. C. Scher, A. B. Herhold and A. P. Alivisatos, Activation Volumes for Solid-Solid Transformations in Nanocrystals, Science, 2001, 293, 1803–1806 CrossRef CAS PubMed.
- D. Zhang, G. Li, X. Yang and J. C. Yu, A micrometer-size TiO2 single-crystal photocatalyst with remarkable 80% level of reactive facets, Chem. Commun., 2009, 4381–4383 RSC.
- R. Shi and Y. Chen, Controlled Formation of Defective Shell on TiO2 (001) Facets for Enhanced Photocatalytic CO2 Reduction, ChemCatChem, 2019, 11, 2270–2276 CrossRef CAS.
- G. Liu, H. G. Yang, J. Pan, Y. Q. Yang, G. Q. Lu and H. Cheng, Titanium dioxide crystals with tailored facets, Chem. Rev., 2014, 114(19), 9559–9612 CrossRef CAS PubMed.
- K. Qi, D. Li, J. Fu, L. Zhu, X. Duan, Q. Qin, G. Wang and W. Zheng, Elucidating ionic liquid environments that affect the morphology of TiO2 nanocrystals: a DFT+D study, J. Phys. Chem. C, 2014, 118, 23320–23327 CrossRef CAS.
- J. Joo, B. Y. Chow, M. Prakash, E. S. Boyden and J. M. Jacobson, Face-selective electrostatic control of hydrothermal zinc oxide nanowire synthesis, Nat. Mater., 2011, 10, 596–601 CrossRef CAS PubMed.
- A. S. Barnard and L. A. Curtiss, Prediction of TiO2 Nanoparticle Phase and Shape Transitions Controlled by Surface Chemistry, Nano Lett., 2005, 5, 1261–1266 CrossRef CAS PubMed.
- M. Batzill, E. H. Morales and U. Diebold, Influence of Nitrogen Doping on the Defect Formation and Surface Properties of TiO2 Rutile and Anatase, Phys. Rev. Lett., 2006, 96, 26103 CrossRef PubMed.
- H. Zhang and J. F. Banfield, Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Insights from TiO2, J. Phys. Chem. B, 2000, 104, 3481–3487 CrossRef CAS.
Insights from TiO2, J. Phys. Chem. B, 2000, 104, 3481–3487 CrossRef CAS. - M. Pal, J. G. Serrano, P. Santiago and U. Pal, Size-Controlled Synthesis of Spherical TiO2 Nanoparticles:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Morphology, Crystallization, and Phase Transition, J. Phys. Chem. C, 2007, 111, 96–102 CrossRef CAS.
Morphology, Crystallization, and Phase Transition, J. Phys. Chem. C, 2007, 111, 96–102 CrossRef CAS. - H. Zhang and J. F. Banfield, Size Dependence of the Kinetic Rate Constant for Phase Transformation in TiO2 Nanoparticles, Chem. Mater., 2005, 17, 3421–3425 CrossRef CAS.
- D. Li, H. Wang, D. Xiao, M. Song, B. Legg and J. Chun, Investigating the magnitude and source of orientation-dependent interactions between TiO2 crystal surfaces, Nanoscale, 2017, 9, 10173–10177 RSC.
- D. Majumder and S. Roy, Non-fluorinated synthesis of anatase TiO2 with dominant {001} facets: influence of faceted structures on formaldehyde sensitivity, New J. Chem., 2017, 41, 7591–7597 RSC.
- J. Pan, G. Liu, G. Q. Lu and H. M. Cheng, On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals, Angew. Chem., 2011, 50, 2133–2137 CrossRef CAS PubMed.
- M. Bellardita, C. Garlisi, A. M. Venezia, G. Palmisano and L. Palmisano, Influence of fluorine on the synthesis of anatase TiO2 for photocatalytic partial oxidation: are exposed facets the main actors?, Catal. Sci. Technol., 2018, 8, 1606–1620 RSC.
- X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, Synthesis of Titania Nanosheets with a High Percentage of Exposed (001) Facets and Related Photocatalytic Properties, J. Am. Chem. Soc., 2009, 131, 3152–3153 CrossRef CAS PubMed.
- D. Zhang, G. Li, X. Yang and C. J. Yu, A micrometer-size TiO2 single-crystal photocatalyst with remarkable 80% level of reactive facets, Chem. Commun., 2009, 29, 4381–4383 RSC.
- X. Han, X. Wang, S. Xie, Q. Kuang, J. Ouyang, Z. Xie and L. Zheng, Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets, RSC Adv., 2012, 2, 3251–3253 RSC.
- F. Lin, Y. Chen, L. Zhang, D. Mei, L. Kovarik, B. Sudduth, H. Wang, G. Gao and Y. Wang, Single-Facet Dominant Anatase TiO2 (101) and (001) Model Catalysts to Elucidate the Active Sites for Alkanol Dehydration, ACS Catal., 2020, 10, 4268–4279 CrossRef CAS.
- Y. Wu, F. Gao, H. Wang, L. Kovarik, B. Sudduth and Y. Wang, Probing Acid–Base Properties of Anatase TiO2 Nanoparticles with Dominant {001} and {101} Facets Using Methanol Chemisorption and Surface Reactions, J. Phys. Chem. C, 2021, 125(7), 3988–4000 CrossRef CAS.
- Z. Tan, K. Sato, S. Takami, C. Numako, M. Umetsu, K. Soga, M. Nakayama, R. Sasaki, T. Tanaka, C. Ogino, A. Kondo, K. Yamamoto, T. Hashishin and S. Ohara, Particle size for photocatalytic activity of anatase TiO2 nanosheets with highly exposed {001} facets, RSC Adv., 2013, 3, 19268–19271 RSC.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Anatase TiO2 single crystals with a large percentage of reactive facets, Nature, 2008, 453, 638–641 CrossRef CAS PubMed.
- T. Butburee, P. Kotchasarn, P. Hirunsit, Z. Sun, Q. Tang, P. Khemthong, W. Sangkhun, W. Thongsuwan, P. Kumnorkaew, H. Wang and K. Faungnawakij, New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance, J. Mater. Chem. A, 2019, 7, 8156 RSC.
- P. Voepel, C. Seitz, J. M. Waack, S. Zahn, T. Leichtwei, A. Zaichenko, D. Mollenhauer, H. Amenitsch, M. Voggenreiter, S. Polarz and B. M. Smarsly, Peering into the Mechanism of Low-Temperature Synthesis of Bronze-type TiO2 in Ionic Liquids, Cryst. Growth Des., 2017, 17, 5586–5601 CrossRef CAS.
- R. L. Penn and J. F. Banfield, Formation of rutile nuclei at anatase {112} twin interfaces and the phase transformation mechanism in nanocrystalline titania, Am. Miner., 1999, 84, 871–876 CrossRef CAS.
- S.-C. Zhu, S.-H. Xie and Z.-P. Liu, Nature of Rutile Nuclei in Anatase-to-Rutile Phase Transition, J. Am. Chem. Soc., 2015, 137, 11532–11539 CrossRef CAS PubMed.
- N. Satoh, T. Nakashima and K. Yamamoto, Metastability of anatase: size dependent and irreversible anatase-rutile phase transition in atomic-level precise titania, Sci. Rep., 2013, 3, 1959 CrossRef PubMed.
- H. Wu, Y. Yang, Y. Ou, B. Lu, J. Li, W. Yuan, Y. Wang and Z. Zhang, Early Stage Growth of Rutile Titania Mesocrystals, Cryst. Growth Des., 2018, 18(8), 4209–4214 CrossRef CAS.
- R. D. Shannon and J. A. Pask, Kinetics of the Anatase-Rutile Transformation, J. Am. Ceram. Soc., 1965, 48, 391–398 CrossRef CAS.
- A. A. Gribb and J. F. Banfield, Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2, Am. Mineral., 1997, 82, 717–728 CAS.
- X. Mettan, J. Jaćimović, O. S. Barišić, A. Pisoni, I. Batistić, E. Horváth, S. Brown, L. Rossi, P. Szirmai, B. Farkas, H. Berger and L. Forró, Tailoring thermal conduction in anatase TiO2, Commun. Phys., 2019, 2, 123 CrossRef.
- X. Bai, T. Li, U. Gulzar, E. Venezia, L. Chen, S. Monaco, Z. Dang, M. Prato, S. Marras, P. Salimi, S. Fugattini, C. Capiglia and R. P. Zaccaria, Towards enhanced sodium storage of anatase TiO2via a dual-modification approach of Mo doping combined with AlF3 coating, Nanoscale, 2020, 12, 15896–15904 RSC.
- P. Mazzolini, P. Gondoni, V. Russo, D. Chrastina, C. S. Casari and A. Li Bassi, Tuning of Electrical and Optical Properties of Highly Conducting and Transparent Ta-Doped TiO2 Polycrystalline Films, J. Phys. Chem. C, 2015, 119(13), 6988–6997 CrossRef CAS.
- E. Ringe, R. P. Van Duyne and L. D. Marks, Wulff Construction for Alloy Nanoparticles, Nano Lett., 2011, 11(8), 3399–3403 CrossRef CAS PubMed.
- C. Boukouvala and E. Ringe, Wulff-Based Approach to Modeling the Plasmonic Response of Single Crystal, Twinned, and Core–Shell Nanoparticles, J. Phys. Chem. C, 2019, 123(41), 25501–25508 CrossRef CAS PubMed.
- S. Yang, B. X. Yang, L. Wu, Y. H. Li, P. Liu, H. Zhao, Y. Y. Yu, X. Q. Gong and H. G. Yang, Nat. Commun., 2014, 5, 5355 CrossRef CAS PubMed.
- S. Ito, T. Murakami, P. Comte, P. Liska, C. Gratzel, M. Nazeeruddin and M. Gratzel, Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%, Thin Solid Films, 2008, 516, 4613–4619 CrossRef CAS.
- K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Cryst., 2011, 44, 1272–1276 CrossRef CAS.
- N. Rahimi, R. A. Pax and E. MacA Gray, Review of functional titanium oxides. I: TiO2 and its modifications, Prog. Solid State Chem., 2016, 44, 86–105 CrossRef CAS.
- E. Binetti, A. Panniello, R. Tommasi, A. Agostiano, S. Fantini, M. L. Curri and M. Striccoli, Interaction of TiO2 Nanocrystals with Imidazolium-Based Ionic Liquids, J. Phys. Chem. C, 2013, 117, 12923–12929 CrossRef CAS.
- T. Alammar, H. Noei, Y. Wang and A. V. Mudring, Mild yet phase-selective preparation of TiO2 nanoparticles from ionic liquids – a critical study, Nanoscale, 2013, 5, 8045–8055 RSC.
- X. Duan, J. Ma, J. Lian and W. Zheng, The art of using ionic liquids in the synthesis of inorganic nanomaterials, CrystEngComm, 2014, 16(13), 2550–2559 RSC.
- P. Voepel and B. M. Smarsly, Synthesis of Titanium Oxide Nanostructures in Ionic Liquids, Z. Anorg. Allg. Chem., 2017, 643(1), 3–13 CrossRef CAS.
- S. Winardi, R. R. Mukti, K. P. Kumar, J. Wang, W. Wunderlich and T. Okubo, Critical Nuclei Size, Initial Particle Size and Packing Effect on the Phase Stability of Sol-Peptization-Gel-Derived Nanostructured Titania, Langmuir, 2010, 26(7), 4567–4571 CrossRef CAS PubMed.
- M. Fernández-García, X. Wang, C. Belver, J. C. Hanson and J. A. Rodriguez, Anatase-TiO2 Nanomaterials:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Morphological/Size Dependence of the Crystallization and Phase Behavior Phenomena, J. Phys. Chem. C, 2007, 111, 674–682 CrossRef.
Morphological/Size Dependence of the Crystallization and Phase Behavior Phenomena, J. Phys. Chem. C, 2007, 111, 674–682 CrossRef. - D. Casotti, M. Ardit, R. Dinnebier, M. Dondi, F. Matteucci, I. Zama and G. Cruciani, Limited Crystallite Growth upon Isothermal Annealing of Nanocrystalline Anatase, Cryst. Growth Des., 2015, 15(5), 2282–2290 CrossRef CAS.
- Y. Wang, B. Wen, A. Dahal, G. A. Kimmel, R. Rousseau, A. Selloni, N. G. Petrik and Z. Dohnálek, Binding of Formic Acid on Anatase TiO2(101), J. Phys. Chem. C, 2020, 124(37), 20228–20239 CrossRef CAS.
- F. Scarpelli, T. F. Mastropietro, T. Poerio and N. Godbert, Mesoporous TiO2 Thin Films: State of the Art, Titan. Dioxide Mater. A Sustain. Environ., 2018, 57–80 CAS.
- F. Pellegrino, E. Morra, L. Mino, G. Martra, M. Chiesa and V. Maurino, Surface and Bulk Distribution of Fluorides and Ti3+ Species in TiO2 Nanosheets: Implications on Charge Carrier Dynamics and Photocatalysis, J. Phys. Chem. C, 2020, 124(5), 3141–3149 CrossRef CAS.
- W. Yang, W. Xu, Y. Wang, D. Chen, X. Wang, Y. Cao, Q. Wu, J. Tu and C. Zhen, Photoelectrochemical Glucose Biosensor Based on the Heterogeneous Facets of Nanocrystalline TiO2/Au/Glucose Oxidase Films, ACS Applied Nano Materials, 2020, 3(3), 2723–2732 CrossRef CAS.
- D. González, B. Camino, J. Heras-Domingo, A. Rimola, L. Rodríguez-Santiago, X. Solans-Monfort and M. Sodupe, BCN-M: A Free Computational Tool for Generating Wulff-like Nanoparticle Models with Controlled Stoichiometry, J. Phys. Chem. C, 2020, 124(1), 1227–1237 CrossRef.
- P. L. Hansen, J. B. Wagner, S. Helveg, J. R. Rostrup-Nielsen, B. S. Clausen and H. Topsøe, Atom-Resolved Imaging of Dynamic Shape Changes in Supported Copper Nanocrystals, Science, 2002, 295, 2053–2055 CrossRef CAS PubMed.
- F. Lai, Y. Chen and H. Guo, Surface energies of non-centrosymmetric nanocrystals by the inverse Wulff construction method, Phys. Chem. Chem. Phys., 2019, 21, 16486–16496 RSC.
- M. V. Dozzi and E. Selli, Doping TiO2 with p-block elements: Effects on photocatalytic activity, J. Photochem. Photobiol. C: Photochem. Rev., 2013, 14, 13–28 CrossRef CAS.
- J. C. Yu, J. Yu, W. Ho, Z. Jiang and L. Zhang, Effects of F− Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders, Chem. Mater., 2002, 14, 3808–3816 CrossRef CAS.
- E. M. Samsudin, S. B. A. Hamid, J. C. Juan, W. J. Basirun and G. Centi, Synergetic effects in novel hydrogenated F-doped TiO2 photocatalysts, Appl. Surf. Sci., 2016, 370, 380–393 CrossRef CAS.
- V. Mansfeldova, M. Zlamalova, H. Tarabkova, P. Janda, M. Vorokhta, L. Piliai and L. Kavan, Work Function of TiO2 (Anatase, Rutile, and Brookite) Single Crystals: Effects of the Environment, J. Phys. Chem. C, 2021, 125(3), 1902–1912 CrossRef CAS.
- A. M. Czoska, S. Livraghi, M. Chiesa, E. Giamello, S. Agnoli, G. Granozzi, E. Finazzi, C. Di Valentin and G. Pacchioni, The Nature of Defects in Fluorine-Doped TiO2, J. Phys. Chem. C, 2008, 112, 8951–8956 CrossRef CAS.
- J. Yu, Y. Yang, R. Fan, L. Li and X. Li, Rapid Electron Injection in Nitrogen- and Fluorine-Doped Flower-Like Anatase TiO2 with {001} Dominated Facets and Dye-Sensitized Solar Cells with a 52% Increase in Photocurrent, J. Phys. Chem. C, 2014, 118(17), 8795–8802 CrossRef CAS.
- T. Su, Y. Yang, Y. Na, R. Fan, L. Li, L. Wei, B. Yang and W. Cao, An Insight Into the Role of Oxygen Vacancy in Hydrogenated TiO2 Nanocrystals in the Performance of Dye Sensitized Solar Cells, ACS Appl. Mater. Interfaces, 2015, 7, 3754–3761 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Fig. S1–S12 and Tables S1–S11, which provide more details and comprehensive image analysis. See DOI: 10.1039/d1na00528f |
| This journal is © The Royal Society of Chemistry 2021 |