 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvothermal preparation of spherical Bi2O3 nanoparticles uniformly distributed on Ti3C2Tx for enhanced capacitive performance†
Tao
Li
ab,
Xuefeng
Chang
ab,
Lifang
Mei
ab,
Xiayun
Shu
*ab,
Jidong
Ma
c,
Li
Ouyang
ab and
Siyong
Gu
 *c
*c
aMechanical and Automotive Engineering, Xiamen University of Technology, Xiamen, Fujian 361024, P. R. China. E-mail: shuxiayun@xmut.edu.cn
bInstitute of Precision Actuation and Transmission, Xiamen, Fujian 361024, P. R. China
cThe Key Laboratory for Power Metallurgy Technology and Advanced Materials of Xiamen, Xiamen, Fujian 361024, P. R. China. E-mail: gu-siyong@163.com
First published on 5th August 2021
Abstract
Ti3C2Tx is a promising new two-dimensional layered material for supercapacitors with good electrical conductivity and chemical stability. However, Ti3C2Tx has problems such as collapse of the layered structure and low pseudocapacitance. In this paper, we propose Bi2O3–Ti3C2Tx nanocomposites prepared by a solvothermal method, study the impact of Bi2O3 loading on the phase state and microstructure, and evaluate the electrochemical performance of Bi2O3–Ti3C2Tx. Studies have shown that spherical Bi2O3 particles were uniformly dispersed in the interlayer and surface of Ti3C2Tx, which enlarged the interlayer spacing of the Ti3C2Tx and increased the pseudocapacitance. When the mass percentage of Bi2O3 and Ti3C2Tx was 30% (TB30), the specific capacity of TB30 was as high as 183 F g−1 at a current density of 0.2 A g−1, which was about 2.8 times that of Ti3C2Tx (TB0). Moreover, a typical asymmetric supercapacitor device assembled with TB0 as the positive electrode and TB30 as the negative electrode exhibited a high energy density of 3.92 W h kg−1 and a maximum power density of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 and maintained 77.4% of the initial capacitance after 5000 cycles at a current density of 2 A g−1. Therefore, the Bi2O3–Ti3C2Tx as the negative electrode of supercapacitor has broad application prospects in the field of energy storage.
000 W kg−1 and maintained 77.4% of the initial capacitance after 5000 cycles at a current density of 2 A g−1. Therefore, the Bi2O3–Ti3C2Tx as the negative electrode of supercapacitor has broad application prospects in the field of energy storage.
1. Introduction
With the rapid development of the global economy, fossil fuel consumption continues to increase, causing serious environmental pollution. The search for efficient, clean and sustainable renewable energy and energy storage technologies has become an important research area.1 At present, common electrochemical energy conversion and storage technologies include lithium-ion batteries, fuel cells, and supercapacitors. Supercapacitors have wide application potential because of their combined advantages of high power density, fast charging and discharging, long cycle lifetime and environmental friendliness.2–4The performance of supercapacitors is mainly influenced by the electrolyte, separator, electrode materials and packaging technology, among which the electrode materials determine the energy density.5,6 Different electrode materials correspond to different energy storage mechanisms. Supercapacitors can be divided into electrochemical double-layer capacitors (EDLCs) and pseudocapacitors (PCs). EDLCs rely on the electrostatic interactions between the electrode and electrolyte to store charge, and PCs rely on the highly reversible redox reaction on the electrode surface to store energy.7,8 The ideal electrode material should have a large specific surface area, large interlayer spacing, excellent hydrophilicity, great stability, good conductivity and high pseudocapacitance.
Common electrode materials include carbon materials, metal oxide materials and conductive polymer materials.9–13 Ti3C2Tx is a new class of electrode material with high specific surface area, high hydrophilicity, good electrical conductivity and high chemical stability, with a layered structure similar to graphene, and has received widespread attention since its discovery in 2011.14 There are surface defects and porous structure after the corrosion of Ti3C2Tx by HF. The defects would be beneficial for increasing the contact area with the electrolyte. Therefore, the surface defects and porous structure provide large accessible surface area for cation intercalation15 and abundant active sites for the oxygen reduction reactions,16 facilitates easy charge-carrier transport, leading to enhanced electrochemical performance. These characteristics provide high electrode double-layer capacitance, pseudocapacitance, and reaction planes, making Ti3C2Tx suitable as an electrode material for supercapacitors. However, studies have found that the collapse of the layered structure and the small pseudocapacitance are the main factors limiting the performance of Ti3C2Tx electrode materials.17,18
An effective way to improve the performance is to introduce pseudocapacitive materials such as MnO2,19 TiO2,20 and polypyrrole21 into the Ti3C2Tx interlayer.22 Enlarging the interlayer spacing of Ti3C2Tx makes it conducive for a variety of ions, such as Na+, K+, NH4+, to reversibly enter the material to achieve charge storage and better pseudocapacitance.12,28 For instance, Yuan et al.19 successfully introduced MnO2, with good pseudocapacitive performance, into the Ti3C2Tx layers, which effectively prevented layer stacking and exhibited higher specific surface area than Ti3C2Tx. The MnO2/Ti3C2Tx showed a specific capacitance of 254 F g−1 (0.5 A g−1). Zhu et al.21 studied a new way to enhance structural stability and interlayer spacing of Ti3C2Tx by forming a freestanding and conductive thin film through intercalating polypyrrole into layered Ti3C2Tx electrodes modified by polypyrrole. The results showed that due to the modification of polypyrrole, the surface capacitance of Ti3C2Tx reached 203 mF cm−2, and after long term cycling (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), there was no obvious capacity degradation.
000 cycles), there was no obvious capacity degradation.
Bismuth oxide (Bi2O3) is a pseudocapacitive material with excellent comprehensive performance owing to its high theoretical capacitance (1370 F g−1), suitable potential window, low cost and non-toxicity.23 Recent studies have shown that Bi2O3 can be used as an electrode material for supercapacitors. Furthermore, its electrochemical performance has been improved by combining with other electrode materials based on carbon and transition metal oxides, such as carbon nanotubes,24 graphene25 and MnO2.26 Thus, we expect that Bi2O3 would show an affinity for Ti3C2Tx-based materials, which has not yet been reported in literature. In this work, we report a series of novel Bi2O3–Ti3C2Tx nanocomposites prepared by a facile solvothermal reaction. Bi2O3 was evenly loaded onto the interlayer and surface of Ti3C2Tx, which successfully increased the interlayer spacing, stabilized the layered structure, and significantly improved the electrochemical performance. This work demonstrates that Bi2O3–Ti3C2Tx nanocomposites have an enhanced performance compared to pure Ti3C2Tx
2. Experimental section
2.1 Fabrication procedures
| Sample | Ti3C2Tx (g) | Bi(NO3)3·5H2O (g) | (CH2OH)2 (mL) | C2H6O (mL) | Annotation |
|---|---|---|---|---|---|
| TB0 | 0.5 | 0 | 13 | 52 | 0% |
| TB10 | 0.5 | 0.12 | 13 | 52 | 10% |
| TB20 | 0.5 | 0.26 | 13 | 52 | 20% |
| TB30 | 0.5 | 0.45 | 13 | 52 | 30% |
| TB40 | 0.5 | 0.69 | 13 | 52 | 40% |
2.2 Materials characterization
The crystal phases of the products were analyzed by X-ray diffraction (XRD) with Cu Kα radiation (Rigaku, D/max-RB12, Japan). X-ray photoelectron spectroscopy (XPS, PHI-5300) was used to investigate the chemical valence composition of the surface elements of the product. The morphology and particle size distribution of the as-prepared samples were investigated using field emission scanning electron microscopy (SEM, Zeiss, ULTRA 55, German). Transmission electron microscopy (TEM, FEI, Tecnai G2 F20, USA) was used to investigate the high resolution topography and electron diffraction.2.3 Electrochemical performance measurements
All electrochemical performance measurements were carried out on the electrochemical workstation (CHI760E, Shanghai Chenhua). The working electrode was prepared with TBn and acetylene black, which were ground and mixed uniformly. The mixture was added to an NMP solution with a PVDF content of 60% for ultrasonic dispersion to form a slurry, where the mass ratio of TBn, acetylene black and PVDF was 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The slurry was uniformly coated onto the nickel current collector foam and dried at 80 °C for 12 h. The loading of TBn was about 2 mg cm−2.
1. The slurry was uniformly coated onto the nickel current collector foam and dried at 80 °C for 12 h. The loading of TBn was about 2 mg cm−2.
The electrochemical performance of the single electrodes was evaluated by the typical three-electrode system configuration. The 6 M KOH solution served as the electrolyte, with Ag/AgCl as the reference electrode, the platinum sheet as the counter electrode, and TBn as the working electrode. Cyclic voltammetry (CV) curves were collected at different scan rates of 5, 10, 20, 50, 100, and 200 mV s−1, within a voltage window range of −1 to −0.4 V. Galvanostatic charge/discharge (GCD) curves were measured at different current densities of 0.2, 0.5, 1, 2, 5 and 10 A g−1. Alternating current electrochemical impedance spectroscopy (EIS) was performed between 0.01−100000 Hz at open circuit voltage with an amplitude of 5 mV.
The specific capacity (Cm) values of the working electrodes determined from CV were calculated according to eqn (1), and the Cm derived from GCD was calculated according to eqn (2).
 | (1) |
 | (2) |
Asymmetric supercapacitors (ASC) using the two-electrode system were used for evaluating the electrochemical performance. The outer shell consisted of a CR2032 battery case; the anode and cathode used were TB0 and TB30, respectively, with 6 M KOH as the electrolyte and a polypropylene separator. In order to balance the charge of the two electrodes, the best mass ratio of the anode and the cathode were calculated according to eqn (3).
 | (3) |
 | (4) |
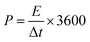 | (5) |
3. Results and discussion
3.1 Morphology and structure
Fig. 2(a) shows typical XRD patterns of TB0–TB40. The diffraction peak at 8.8° corresponds to the (002) plane of Ti3C2Tx.27 Notably, the Ti3C2Tx (002) peaks of the TB10, TB20, TB30 and TB40 were found to gradually shift significantly to the left with the increase in Bi2O3. This result indicates that the interlayer spacing of Ti3C2Tx tends to increase due to the successful intercalation of Bi2O3 among the layers of Ti3C2Tx. Diffraction peaks of Bi2O3 (ICDD#: 71-2274) were clearly visible in the spectra of TB10–TB40.29,30 As the Bi2O3 content increases, the diffraction peak intensity of Bi2O3 was seen to gradually increase. In addition, TB10 can be indexed to the diffraction peaks of metallic Bi (ICDD#: 05-0519),31 which is attributed to the carbothermal reduction of a small amount of free C atoms in Ti3C2Tx and Bi2O3 in N2 atmosphere during the heat treatment.32 Therefore, it can be inferred that the following reactions occur: | (6) |
 | (7) |
 | ||
| Fig. 2 (a) XRD patterns, (b) XPS survey scan, and (c) Bi 4f high-resolution spectra of TB0−TB40. (d) The mass ratio as a function of the Bi/Ti and Bi/C atomic ratios. | ||
To further explore the elemental composition of all the samples, XPS analyses were then carried out for TB0–TB40. The XPS survey spectra in Fig. 2(b) show the presence of Ti, C, Bi, and O in the TB10–TB40. The Bi 4f spectra of TB0–TB40 are shown in Fig. 2(c), which exhibit binding energies at 159 eV and 164.3 eV corresponding to the characteristic peaks of Bi 4f7/2 and Bi 4f5/2, suggesting the presence of Bi2O3.33 The bare Bi2O3 responds to characteristic Bi 4f7/2 and 4f5/2 XPS peaks at 158.7 and 164.0 eV, respectively, acquired by the National Institute of Standards and Technology (NIST) XPS database. The negatively charged functional groups, such as O–, OH–, tend to attract electrons from bridged Bi cations that change the chemical environment and relative electronic distribution of Bi element, resulting in the peak shift of Bi 4f7/2 and 4f5/2 XPS peaks to the higher binding energy in Bi2O3–Ti3C2Tx nanocomposite.41 Such a phenomenon demonstrates the chemical bonding between Bi2O3 nanoparticles and Ti3C2Tx sheets in Bi2O3–Ti3C2Tx composite material. The peak intensities increase with the increasing Bi2O3–Ti3C2Tx mass ratio, from 10% to 40%, consistent with the XRD results in Fig. 2(a). The appearance of the characteristic peaks of F and O can be attributed to the corrosion of Ti3C2Tx by HF to produce a small amount of functional groups such as –F, –OH and –O.34,35 The C 1s and O 1s high-resolution spectra of TB0−TB40 are provided in Fig. S1 in the ESI† for comparision. Fig. S1(a)† shows the high-resolution C 1s XPS spectra of the TB0–TB40. C 1s spectrum of pristine Ti3C2Tx can be fitted into four peaks, corresponding to Ti–C–O at 282.2 eV, C–C/C–H at 284.6 eV, C–O at 286.4 eV and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 288.1 eV, respectively.19 After the solvothermal reaction, In the spectra of the composite samples, the Ti–C–O bonds disappear and new Ti–C peaks appear at 281.2 eV. As for the O 1s XPS spectrum of pristine Ti3C2Tx in Fig. S1(b),† the peaks located at 529.8 eV, 530.4 eV, 531.2 eV, 532 eV and 533.6 eV are attributed to Ti–O, TiO2, C–Ti–O, C–Ti–OH and adsorbed H2O, respectively.23 The new Bi–O peaks appear at 530.1 eV and the concentration of C–Ti–OH and C–Ti–O in TB10–TB40 decreased after the solvothermal, indicating that more O combined to form Bi–O bonds. The atomic ratios of Bi, Ti and C in TB0–TB40 are shown in Fig. 2(d). The Bi/Ti and Bi/C ratios tend to increase with the increasing mass of Bi2O3, consistent with the XRD and XPS results in Fig. 2(a and b).
O at 288.1 eV, respectively.19 After the solvothermal reaction, In the spectra of the composite samples, the Ti–C–O bonds disappear and new Ti–C peaks appear at 281.2 eV. As for the O 1s XPS spectrum of pristine Ti3C2Tx in Fig. S1(b),† the peaks located at 529.8 eV, 530.4 eV, 531.2 eV, 532 eV and 533.6 eV are attributed to Ti–O, TiO2, C–Ti–O, C–Ti–OH and adsorbed H2O, respectively.23 The new Bi–O peaks appear at 530.1 eV and the concentration of C–Ti–OH and C–Ti–O in TB10–TB40 decreased after the solvothermal, indicating that more O combined to form Bi–O bonds. The atomic ratios of Bi, Ti and C in TB0–TB40 are shown in Fig. 2(d). The Bi/Ti and Bi/C ratios tend to increase with the increasing mass of Bi2O3, consistent with the XRD and XPS results in Fig. 2(a and b).
Fig. 3 shows the morphologies of the as-prepared Ti3AlC2 and TB0–TB40. Ti3AlC2 has a ternary layered structure with a particle size of about 8 μm, as shown in Fig. 3(a). After the etching treatment to remove Al from Ti3AlC2 by HF,36 Ti3C2Tx, with a unique accordion structure and a clean surface without impurities, was obtained, as shown in Fig. 3(b). Fig. 3(c–f) displays the structure and morphology of TB10–TB40, in which a large number of spherical Bi2O3 nanoparticles were found uniformly dispersed in the interlayer and on the surface of Ti3C2Tx. A small amount of spherical Bi2O3 nanoparticles were found to have adhered on the surface of the Ti3C2Tx (TB10) in Fig. 3(c). A large number of tiny spherical Bi2O3 nanoparticles were found to be distributed between the layers of Ti3C2Tx (TB20, TB30), as shown in Fig. 3(d and e); this can effectively prevent layer stacking and collapse, exhibit higher specific surface area and improve the performance of the electric double layer capacitance.19,37 However, when the loading of Bi2O3 continues to increase, the surface of Ti3C2Tx (TB40) was found to be covered by numerous coarse spherical Bi2O3 nanoparticles and the lamellar structure of Ti3C2Tx was no longer obvious, as shown in Fig. 3(f).
To further investigate the composition and microstructure of the Bi2O3–Ti3C2Tx, the representative nanocomposite, TB30, was characterized by TEM, HAADF-STEM, elemental mapping for Ti, C, O, Bi and HRTEM, as shown in Fig. 4. Fig. 4(a–c) indicates that a large number of spherical Bi2O3 nanoparticles and a small number of coarse spherical Bi2O3 nanoparticles were distributed on the Ti3C2Tx. Spherical Bi2O3 nanoparticles, with an average diameter of 10–50 nm, were distributed between the layers of Ti3C2Tx and coarse spherical Bi2O3 nanoparticles, with an even particle size of around 80–200 nm, were found on the surface of Ti3C2Tx. The particle-size distribution graph has been shown in Fig. S2.† The spherical Bi2O3 nanoparticles have distinct characteristics of high dispersibility and uniformity, consistent with the SEM results. Fig. 4(d) shows lattice fringe spacings of 0.325 nm and 0.938 nm, which correspond to the (−121) plane of Bi2O3 and (002) plane of Ti3C2Tx,38,39 respectively. Significant contact was achieved between the Bi2O3 and Ti3C2Tx for more efficient electron transfer, improving the performance of electrochemistry. The spherical Bi2O3 nanoparticles were found to be covered by an amorphous Bi2O3 film with a thickness of about 2 nm, which is consistent with the findings by Wu et al.24 In addition, Fig. S3† shows a lattice fringe spacing of 0.328 nm, which corresponds to the (012) plane of metallic Bi.40 This proves that TB30 contains a small amount of Bi metal, which is attributed to the Bi2O3 reduced to metallic Bi by free C in Ti3C2Tx, consistent with the XRD results in Fig. 1(a).
 | ||
| Fig. 4 (a) TEM image, (b) HAADF-STEM image, (c) Ti, C, O, Bi elemental mapping images, and (d) HRTEM image of TB30. | ||
3.2 Electrochemical performance
Fig. 5(a and b) show the CV curves of TB0 and TB30, respectively, at different scan rates. As shown in Fig. 5(a), the curves of TB0 look approximately like a symmetrical rectangle, which can be attributed to the double electrode layer capacitance of Ti3C2Tx. All the CV curves of TB0 show a similar symmetrical rectangle with the increase in scan rate, indicating superior rate performance of TB0.42 The CV curves of TB30 show a prominent redox peak,43 which can be attributed to the introduction of Bi2O3, according to the following reactions: | (8) |
 | (9) |
 | ||
| Fig. 5 CV curves of (a) TB0 and (b) TB30 at different rates. (c) CV curves of TB0−TB40 at a scan rate of 5 mV s−1. | ||
The current at the redox peak tends to slightly shift with the increase of the scan rate due to the slightly reduced conductivity resulting from the interfacial resistance between the electrode surface and the electrolyte solution, suggesting a better rate performance of TB30.44 The CV curves of TB0−TB40 obtained at the scan rate of 5 mV s−1 are shown in Fig. 5(c). It can be clearly seen that the CV curves of TB10, TB20, TB30 and TB40 show stronger redox peak currents and a larger integral than TB0, indicating the superior capacitor properties of the Bi2O3–Ti3C2Tx hybrid electrode. The best capacitance performance is obtained with TB30, i.e., when the mass percentage of Bi2O3 and Ti3C2Tx is 30%.
Fig. 6(a and b) show the GCD curves of TB0 and TB30, respectively, at various current densities. The GCD curves of TB0 present a typical symmetrical triangle, while those of TB30 are asymmetrical with voltage plateaus. The shape of the two curves is significantly different, which can be attributed to the pseudocapacitive characteristics of Bi2O3.43,45 The GCD curves of TB0–TB40 obtained at a current density of 0.2 A g−1 are shown in Fig. 6(c). It can be seen that the discharge time becomes longer with the increase of Bi2O3 loading. The discharge time of TB10, TB20, TB30 and TB40 was found to be longer than that of TB0, which indicates a high specific capacitance due to the presence of Bi2O3. The specific capacitance was calculated according to eqn (2); the specific capacitance of TB30 was found to be 2.8 times greater than that of TB0. In addition, the lower capacity of TB40 compared to TB30 can be ascribed to the fact that too many coarse spherical Bi2O3 nanoparticles cover the surface of Ti3C2Tx, which impedes the transport of ions in the electrolyte and weakens the conductivity of Ti3C2Tx.
Fig. 6(d) shows the variations in specific capacitance of TB0–TB40 with current density, which were calculated from the GCD curves according to eqn (2). The specific capacitance values of TB0–TB40 at a current of 0.2 A g−1 were found to be 64.8, 91, 136.1, 183, 164.1 F g−1, respectively. The specific capacitance value of the TB30 is much higher than that of all the other samples at different current densities and is attributed to a large number of tiny spherical Bi2O3 nanoparticles distributed on the surface and layers of Ti3C2Tx. The spherical Bi2O3 nanoparticles tend to enlarge the spacing between the Ti3C2Tx layers, promote electron transfer, and shorten the diffusion path of ions in the electrolyte. In addition, the TB30 still exhibits a capacity of 129.7 F g−1 (about 70.9% retention) even at a current density of 1 A g−1, indicating an excellent rate capability for TB30. The specific capacitance calculated at 10 A g−1 is 34.6% of the specific capacitance calculated at 0.2 A g−1, and can be attributed to the insufficient redox reaction in the active material at high current densities, resulting in a decrease in specific capacitance.46 The comparison of those Ti3C2Tx-based materials is shown in Table 2.15,20,22,51 It was clear that the performance of Bi2O3–Ti3C2Tx (TB30) electrode in this work was higher than those of reported materials. Meanwhile, as shown in Fig. 7(e), the TB30 exhibits acceptable electrochemical stability with the capacitive retention still as high as 79.6% even after 5000 cycles at 5 A g−1, and can be attributed to the expansion and contraction of Bi2O3 during the insertion and embedding of electrolyte ions by charging and discharging, resulting in a decrease in the capacity.
Nyquist impedance plots of TB0–TB40 are shown in Fig. 6(f). The equivalent series resistance (ESR) of electrodes, which is related to the ionic resistance of the electrolyte, the internal resistance of the active material and the current collector, and the interface contact resistance between the electrode and the electrolyte impedance of the electrolyte,47 depends on the intercept of the semicircle at the X-axis in the high frequency range. It can be seen that the ESR values of TB0–TB40 are 0.87, 0.79, 0.82, 0.73 and 0.80 Ω, respectively. Among them, TB30 has the smallest ESR. The charge transfer resistance (Rct) depends on the arc diameter of the semicircle.48 The Rct values of TB10, TB20, TB30 and TB40 are much larger than that of TB0, which can be attributed to the poor conductivity of Bi2O3. Simultaneously, the increase of resistance reveals the successful incorporation of Bi2O3. The linear region in the low frequency range is related to the diffusion resistance of the ions in the electrolyte.49 The linear part of TB30 is closest to the vertical, indicating that the electrolyte ion diffusion has the lowest resistance. The above results prove that TB30 has good electrochemical capacitance behavior, consistent with the results shown in Fig. 6(d).
In order to further study the Bi2O3–Ti3C2Tx nanocomposite for practical applications, an asymmetric supercapacitor (ASC) was constructed,50 with TB0 and TB30 as the positive and negative electrodes, respectively. Fig. 7(a) shows the CV curves of the TB0//TB30 ASC at different scan rates. It can be seen that the shape of the CV curves of the TB0//TB30 ASC remained the same at different scan rates, implying excellent rate capacity and good reversibility, indicating the good synergistic effect of the electrochemical behavior of both TB0 and TB30 electrodes. The GCD curves of TB0//TB30 ASC at various current densities are shown in Fig. 7(b). The non-linearity of the curve is due to the redox reaction that occurred during the charging and discharging process. The specific capacitance of the TB0//TB30 ASC at different current densities in Fig. 7(c), indicates that the capacitive retention is still as high as 79.1% even after the current density is increased from 0.2 A g−1 to 1 A g−1, and the specific capacitance is reduced from 28.2 F g−1 to 22.3 F g−1. In addition, the specific capacitance calculated at 10 A g−1 is 11 F g−1 with a capacitive retention of 39%, which is attributed to the insufficient redox reaction in the active material at high current density, resulting in a decrease in specific capacitance. This is consistent with the trend of the capacitive retention of TB30. Meanwhile, as shown in Fig. 7(d), the TB0//TB30 ASC exhibits acceptable electrochemical stability with the capacitive retention still as high as 77.4% even after 5000 cycles at 2 A g−1 and higher than those of reported devices such as Ni(OH)2-CNT//Bi–Bi2O3-CNT based ASC (72.9%/1000 cycles at 1 A g−1),24 PPy-Ti3C2Tx//PPy-Ti3C2Tx based SC (73.68%/4000 cycles at 1 A g−1).52 To further illustrate the overall electrochemical characteristics of the TB0//TB30 ASC, a Ragone plot of the TB0//TB30 ASC was carried out and compared with that of other reported devices based on the a two-electrode configuration in Fig. 7(d) and Table 3.53–58 The highest energy density of the TB0//TB30 ASC is 3.92 W h kg−1 at a power density of 720 W kg−1, and the highest power density of the TB0//TB30 ASC is 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 at an energy density of 1.53 W h kg−1. The energy density and power density of our device is higher than that of many reported Ti3C2Tx-based and carbon-based SC and ASC devices. The Fig. 7(f) shows that three TB0//TB30 ASC coin cells in series could power a green light-emitting diode (LED, 3 V, 20 mA). Based on above mentioned results, it can be conclued that Bi2O3–Ti3C2Tx is a promising electrode material for supercapacitors in the future.
000 W kg−1 at an energy density of 1.53 W h kg−1. The energy density and power density of our device is higher than that of many reported Ti3C2Tx-based and carbon-based SC and ASC devices. The Fig. 7(f) shows that three TB0//TB30 ASC coin cells in series could power a green light-emitting diode (LED, 3 V, 20 mA). Based on above mentioned results, it can be conclued that Bi2O3–Ti3C2Tx is a promising electrode material for supercapacitors in the future.
4. Conclusions
In this study, we prepared Bi2O3–Ti3C2Tx nanocomposites by a solvothermal method. The spherical Bi2O3 nanoparticles were uniformly distributed on the surface and layers of Ti3C2Tx, which effectively prevented layer stacking and increased the inter-layer spacing. When the mass percentage of Bi2O3 and Ti3C2Tx was 30% (TB30), the best capacitance performance was obtained. The specific capacitance of TB30 was 2.8 times that of TB0 at 0.2 A g−1, which was attributed to the outstanding pseudocapacitance of Bi2O3 and the improvement of layer stacking of Ti3C2Tx. However, too many coarse spherical Bi2O3 nanoparticles tend to cover the surface of Ti3C2Tx (TB40), impeding the transport of ions in the electrolyte and weakening the electrochemical performance. The asymmetric supercapacitor device assembled with TB0 as the positive electrode and TB30 as the negative electrode displayed a high energy density of 3.92 W h kg−1 and a maximum power density of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1, and maintained 77.4% of the initial capacitance after 5000 cycles at a current density of 2 A g−1. This work shows that Bi2O3–Ti3C2Tx as a negative material has excellent prospects in high-performance electrochemical energy storage devices like supercapacitors.
000 W kg−1, and maintained 77.4% of the initial capacitance after 5000 cycles at a current density of 2 A g−1. This work shows that Bi2O3–Ti3C2Tx as a negative material has excellent prospects in high-performance electrochemical energy storage devices like supercapacitors.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province (2020J01288) and the National Natural Science Foundation of China (51975501).Notes and references
- A. Gonzalez, E. Goikolea, J. A. Barrena and R. Mysyk, Renewable Sustainable Energy Rev., 2016, 58, 1189–1206 CrossRef CAS.
- A. Afir, S. M. H. Rahman, A. T. Azad, J. Zaini, M. A. Islan and A. K. Azad, Journal of Energy Storage, 2019, 25, 100852 CrossRef.
- Y. Liu, Z. Wang, Y. Zhong, X. Xu, J. M. Veder, M. R. Rowles, M. Saunders, R. Ran and Z. Shao, Chem. Eng. J., 2020, 390, 124645 CrossRef CAS.
- Y. Liu, S. P. Jiang and Z. Shao, Mater. Today Adv., 2020, 7, 100072 CrossRef.
- L. Chen, Energy Environ. Sci., 2017, 8, 1777–1783 RSC.
- C. Liu, J. Wang, J. Li, M. Zeng, R. Luo, J. Shen, X. Sun, W. Han and L. Wang, ACS Appl. Mater. Interfaces, 2016, 11, 7194–7204 CrossRef.
- Y. Shao, M. F. El-Kady, J. Sun, Y. Li, Q. Zhang, M. Zhu, H. Wang, B. Dunn and R. B. Kaner, Chem. Rev., 2018, 118, 9233–9280 CrossRef CAS PubMed.
- Z. Wang, Z. Xu, H. Huang, X. Chu, Y. Xie, D. Xiong, C. Yan, H. Zhao, H. Zhang and W. Yang, ACS Nano, 2020, 14, 4916–4924 CrossRef CAS.
- C. Yi, J. Zou, H. Yang and X. Leng, Trans. Nonferrous Met. Soc. China, 2018, 10, 1980–2001 CrossRef.
- L. Zhang, D. Huang, N. Hu, C. Yang, M. Li, H. Wei, Z. Yang, Y. Su and Y. Zhang, J. Power Sources, 2017, 342, 1–8 CrossRef CAS.
- B. Shen, X. Zhang, R. Guo, J. Lang, J. Chen and X. Yan, J. Mater. Chem. A, 2016, 21, 8180–8189 RSC.
- Z. Lin, D. Sun, Q. Huang, J. Yang, M. W. Barsoum and X. Yan, J. Mater. Chem. A, 2015, 27, 14096–14100 RSC.
- F. Beguin, V. Presser, A. Balducci and E. Frackowiak, Adv. Mater., 2014, 14, 2219–2251 CrossRef PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- Y. Tang, J. Zhu, C. Yang and F. Wang, J. Electrochem. Soc., 2016, 163, A1975–A1982 CrossRef CAS.
- C. Su, Y. Liu, Z. Luo, J. Veder, Y. Zhong, S. P. Jiang and Z. Shao, Chem. Eng. J., 2021, 406, 126883 CrossRef CAS.
- M. R. Lukatskaya, S. Bak, X. Yu, X. Yang, M. W. Barsoum and Y. Gogotsi, Adv. Energy Mater., 2015, 5, 1500589 CrossRef.
- H. Li, Y. Hou, F. Wang, M. R. Lohe, X. Zhuang, L. Niu and X. Feng, Adv. Energy Mater., 2017, 7, 1601847 CrossRef.
- W. Yuan, L. Cheng, B. Zhang and H. Wu, Ceramurgia Int., 2018, 44, 17539–17543 CrossRef CAS.
- J. Zhu, Y. Tang, C. Yang, F. Wang and M. Cao, J. Electrochem. Soc., 2016, 163, A785–A791 CrossRef CAS.
- M. Zhu, Y. Huang, Q. Deng, J. Zhou, Z. Pei, Q. Xue, Y. Huang, Z. Wang, H. Li, Q. Huang and C. Zhi, Adv. Energy Mater., 2016, 6, 1600969 CrossRef.
- M. Zhao, C. E. Ren, Z. Ling, M. R. Lukatskaya, C. Zhang, K. L. Van Aken, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2015, 27, 339–345 CrossRef CAS PubMed.
- S. Yang, L. Qian, Y. Ping, H. Zhang, J. Li, B. Xiong, P. Fang and C. He, Ceramurgia Int., 2021, 47, 8290–8299 CrossRef CAS.
- H. Wu, J. Guo and D. A. Yang, J. Mater. Sci. Technol., 2020, 47, 169–176 CrossRef.
- A. Deepi, G. Srikesh and A. S. Nesaraj, Nano-Struct. Nano-Objects, 2018, 15, 10–16 CrossRef CAS.
- Z. A. Shaikh, P. V. Shinde, S. F. Shaikh, A. M. Al-Enizi and R. S. Mane, Solid State Sci., 2020, 102, 106158 CrossRef CAS.
- A. Feng, Y. Yu, Y. Wang, F. Jiang, Y. Yu, M. Le and L. Song, Mater. Des., 2017, 114, 161–166 CrossRef CAS.
- O. Mashtalir, M. R. Lukatskaya, A. I. Kolesnikov, E. Raymundo-Piñero, M. Naguib, M. W. Barsoum and Y. Gogotsi, Nanoscale, 2016, 8, 9128–9133 RSC.
- S. Sood, A. Umar, S. Kumar Mehta and S. Kumar Kansal, Ceramurgia Int., 2015, 41, 3355–3364 CrossRef CAS.
- K. V. Mishchenko, K. B. Gerasimov and Y. M. Yukhin, Mater. Today: Proc., 2020, 25, 391–394 CAS.
- L. Qu, Z. Luo and C. Tang, Mater. Res. Bull., 2013, 48, 4601–4605 CrossRef CAS.
- W. Chen, X. Pan and X. Bao, J. Am. Chem. Soc., 2007, 129, 7421–7426 CrossRef CAS PubMed.
- R. Liu, L. Ma, G. Niu, X. Li, E. Li, Y. Bai and G. Yuan, Adv. Funct. Mater., 2017, 27, 1701635 CrossRef.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS PubMed.
- F. Chang, C. Li, J. Yang, H. Tang and M. Xue, Mater. Lett., 2013, 109, 295–298 CrossRef CAS.
- F. Wang, C. Yang, C. Duan, D. Xiao, Y. Tang and J. Zhu, J. Electrochem. Soc., 2014, 162, B16–B21 CrossRef.
- H. Xu, D. Zheng, F. Liu, W. Li and J. Lin, J. Mater. Chem. A, 2020, 8, 5853–5858 RSC.
- F. Qin, H. Zhao, G. Li, H. Yang, J. Li, R. Wang, Y. Liu, J. Hu, H. Sun and R. Chen, Nanoscale, 2014, 6, 5402 RSC.
- W. Feng, H. Luo, S. Zeng, C. Chen, L. Deng, Y. Tan, X. Zhou, S. Peng and H. Zhang, Mater. Chem. Front., 2018, 2, 2320–2326 RSC.
- M. Chen, Y. Li, Z. Wang, Y. Gao, Y. Huang, J. Cao, W. Ho and S. Lee, Ind. Eng. Chem. Res., 2017, 56, 10251–10258 CrossRef CAS.
- Z. Deng, T. Liu, T. Chen, J. Jiang, W. Yang, J. Guo, J. Zhao, H. Wang and L. Gao, ACS Appl. Mater. Interfaces, 2017, 9, 12469–12477 CrossRef CAS PubMed.
- M. Zhang, X. Chen, J. Sui, B. S. Abraha, Y. Li, W. Peng, G. Zhang, F. Zhang and X. Fan, Inorg. Chem. Front., 2020, 7, 1205–1211 RSC.
- V. Vivier, A. Regis, G. Sagon, J. Y. Nedelec, L. T. Yu and C. Cachet-Vivier, Electrochim. Acta, 2001, 46, 907–914 CrossRef CAS.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed.
- S. Yang, L. Qian, Y. Ping, H. Zhang, J. Li, B. Xiong, P. Fang and C. He, Ceramurgia Int., 2021, 47, 8290–8299 CrossRef CAS.
- Z. Pan, F. Cao, X. Hu and X. Ji, J. Mater. Chem. A, 2019, 7, 8984–8992 RSC.
- X. Zhang, X. Liu, R. Yan, J. Yang, Y. Liu and S. Dong, J. Mater. Chem. C, 2020, 8, 2008–2013 RSC.
- H. Tang, J. Wang, H. Yin, H. Zhao, D. Wang and Z. Tang, Adv. Mater., 2015, 27, 1117–1123 CrossRef CAS PubMed.
- B. Ahmed, D. H. Anjum, Y. Gogotsi and H. N. Alshareef, Nano Energy, 2017, 34, 249–256 CrossRef CAS.
- S. Venkateshalu and A. N. Grace, Electrochim. Acta, 2020, 341, 136035 CrossRef CAS.
- D. Antiohos, K. Pingmuang, M. S. Romano, S. Beirne, T. Romeo, P. Aitchison, A. Minett, G. Wallace, S. Phanichphant and J. Chen, Electrochim. Acta, 2013, 101, 99–108 CrossRef CAS.
- D. Wei, W. Wu, J. Zhu, C. Wang, C. Zhao and L. Wang, J. Electroanal. Chem., 2020, 877, 114538 CrossRef CAS.
- Z. Wang, Z. Xu, H. Huang, X. Chu, Y. Xie, D. Xiong, C. Yan, H. Zhao, H. Zhang and W. Yang, ACS Nano, 2020, 14, 4916–4924 CrossRef CAS.
- Y. Liu, Z. Wang, Y. Zhong, X. Xu, J. M. Veder, M. R. Rowles, M. Saunders, R. Ran and Z. Shao, Chem. Eng. J., 2020, 390, 124645 CrossRef CAS.
- Y. Liu, S. P. Jiang and Z. Shao, Mater. Today Adv., 2020, 7, 100072 CrossRef.
- A. M. Navarro-Suárez, K. L. Van Aken, T. Mathis, T. Makaryan, J. Yan, J. Carretero-González, T. Rojo and Y. Gogotsi, Electrochim. Acta, 2018, 259, 752–761 CrossRef.
- W. Wu, S. Lin, T. Chen, L. Li, Y. Pan, M. Zhang, L. Wu, H. Gao and X. Zhang, J. Alloys Compd., 2017, 729, 1165–1171 CrossRef CAS.
- Y. Tang, J. Zhu, C. Yang and F. Wang, J. Electrochem. Soc., 2016, 163, A1975–A1982 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00443c |
| This journal is © The Royal Society of Chemistry 2021 |




