Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microfluidic synthesis of protein-loaded nanogels in a coaxial flow reactor using a design of experiments approach†
Zoe
Whiteley‡
a,
Hei Ming Kenneth
Ho‡
 a,
Yee Xin
Gan
a,
Luca
Panariello
a,
Yee Xin
Gan
a,
Luca
Panariello
 b,
Georgios
Gkogkos
b,
Asterios
Gavriilidis
b,
Georgios
Gkogkos
b,
Asterios
Gavriilidis
 b and
Duncan Q. M.
Craig
b and
Duncan Q. M.
Craig
 *a
*a
aSchool of Pharmacy, University College London, 29-39 Brunswick Square, London WC1N 1AX, UK. E-mail: duncan.craig@ucl.ac.uk
bDepartment of Chemical Engineering, University College London, Torrington Place, WC1E 7JE, UK
First published on 18th February 2021
Abstract
Ionic gelation is commonly used to generate nanogels but often results in poor control over size and polydispersity. In this work we present a novel approach to the continuous manufacture of protein-loaded chitosan nanogels using microfluidics whereby we demonstrate high control and uniformity of the product characteristics. Specifically, a coaxial flow reactor (CFR) was employed to control the synthesis of the nanogels, comprising an inner microcapillary of internal diameter (ID) 0.595 mm and a larger outer glass tube of ID 1.6 mm. The CFR successfully facilitated the ionic gelation process via chitosan and lysozyme flowing through the inner microcapillary, while cross-linkers sodium tripolyphosphate (TPP) and 1-ethyl-2-(3-dimethylaminopropyl)-carbodiimide (EDC) flowed through the larger outer tube. In conjunction with the CFR, a four-factor three-level face-centered central composite design (CCD) was used to ascertain the relationship between various factors involved in nanogel production and their responses. Specifically, four factors including chitosan concentration, TPP concentration, flow ratio and lysozyme concentration were investigated for their effects on three responses (size, polydispersity index (PDI) and encapsulation efficiency (% EE)). A desirability function was applied to identify the optimum parameters to formulate nanogels in the CFR with ideal characteristics. Nanogels prepared using the optimal parameters were successfully produced in the nanoparticle range at 84 ± 4 nm, showing a high encapsulation efficiency of 94.6 ± 2.9% and a high monodispersity of 0.26 ± 0.01. The lysis activity of the protein lysozyme was significantly enhanced in the nanogels at 157.6% in comparison to lysozyme alone. Overall, the study has demonstrated that the CFR is a viable method for the synthesis of functional nanogels containing bioactive molecules.
1. Introduction
The use of microfluidics for the manufacture of drug delivery systems is at a relatively early stage yet is arguably showing great promise.1–4 The term refers to the manipulation of fluid flow at a microscopic scale, allowing flow characteristics to be finely controlled for both rapid and homogeneous mixing. Microfluidics was first employed as an approach to microanalysis but rapidly became recognized as a versatile means of both analysis and production.5 Indeed, the miniaturisation of the manufacturing process enables portability, efficiency of processing with reduced reagent volumes, low variation between batches and the potential for scale-up where formulations may be optimised at low volumes and the process subsequently scaled up through parallelisation and increased device density.6 Advantages such as continuous manufacturing and low instrumental footprints can be achieved at the same time as improving the speed of reactions, increasing throughput and enhancing reproducibility of formulations,7 making the technology extremely attractive to the pharmaceutical industry.Nanogels are described as dispersions of hydrogel nanoparticles based on crosslinked polymeric networks8 and their properties render them suitable for numerous drug delivery applications. Nanogels share many advantages with other nanocarrier systems such as aiding in the passage across biological membranes, large surface areas, potential for modification and derivatisation, avoidance of rapid renal clearance and increasing circulation half-life. Nanogels may also confer several other intrinsic advantages which render them attractive drug delivery candidates. Given their hydrophilicity, they can encapsulate a range of hydrophilic molecules, and their surrounding polymer network provides loaded therapeutics with a protective environment against degradation.9 Nanogels have a high capacity to hold water and an ability to swell in aqueous media, thus allowing imbibition of large quantities of water whilst preserving the internal cross-linked polymeric matrix.10 Often formed under mild aqueous conditions, these systems are ideal for the encapsulation of many biological molecules such as genes, proteins and peptides.11 Natural polymers are appealing for the formulation of nanogels given their biodegradability and biocompatibility.12 Chitosan is the natural polymer of choice in this study as its cationic nature allows complexation of its amine groups to negatively charged cross-linkers such as sodium tripolyphosphate (TPP) by ionic gelation.
At present, techniques relating to the synthesis of nanogels focus largely around ionic gelation alongside self-assembly and emulsification. Ionic gelation is one of the most widely used methods for fabricating nanogels, and refers to the use of polyelectrolytes forming cross-links in the presence of ions.13 Although simple and rapid, this technique is typically difficult to control given the instant assembly of polymer and cross-linkers, though certain factors have been identified to control the process such as; mass ratio of polymer to cross linker, temperature, charge density of cross-linker and polymer and also the pH.14 Ionic gelation is usually a batch process resulting therefore in batch to batch variations in physiochemical properties such as particle size, polydispersity, surface charge and drug release profiles,15–17 which is unfavourable for nanomedicines requiring a high level of quality control. There is therefore a compelling argument to consider the use of microfluidic approaches as an alternative and continuous means of producing nanogels of uniform size and architecture.
Microfluidic approaches have indeed been previously explored for this application, including a study by Bazban-Shotorbani et al.18 where alginate nanogels were formed by ionic gelation using hydrodynamic flow-focussing microchips to cross-link a core stream of calcium chloride and a sheath stream of alginate. This work was followed by Mahmoudi et al.19 who also synthesised alginate nanogels on microchips, where fine-tuning of the flow rate ratio allowed control over the size of nanogels produced. Huang et al.20 used the microchip platform to ensure controlled mixing and fabrication of hyaluronic acid based nanogels via photo-click cross-linking. Majedi et al.21 produced hydrophobically modified chitosan nanoparticles via self-assembly for the encapsulation of hydrophobic anticancer agents, where a T-shaped microfluidic chip was used to achieve particle synthesis. Efforts to produce chitosan/TPP nanogels using microfluidic have more recently been explored, where for example Chiesa et al.22 successfully formulated chitosan nanoparticles using a staggered herringbone micromixer in order to address the lack of reproducibility via batch production methods. In the formation of chitosan/ATP nanoparticles, Pessoa et al.23 observed channel obstruction in the microchips, whereby they implemented a central aqueous stream to allow for a large diffusion length for molecular diffusion, thus mitigating the fouling issues. Here we introduce the use of a coaxial flow reactor (CFR), whereby a core and sheath flow can be controlled by adjusting the flow rates and flow ratios accordingly. One of the main potential advantages of using a CFR in comparison to a microchip is that the event fouling may be greatly reduced; in the CFR the core solution is surrounded completely by the sheath flow and hence the reaction interface is shielded from the channel walls,24 giving a 3D flow-focussing effect. To our knowledge, this approach has not yet been implemented for the formation of nanogels and more specifically for chitosan/TPP nanogels. Rhee et al.25 compared the use of both 2D and 3D flow focussing devices for the production of polymeric nanoparticles and found that the 3D flow-focussing device consistently produced nanoparticles of smaller size and higher monodispersity which may be attributed to the greater efficiency of mixing. CFRs are also relatively simple to assemble, inexpensive and offer the ability to easily change the outer tube or inner capillary should any fouling occur, whereas serious fouling in a microchip often requires the fabrication of a completely new device. The use of CFRs in the literature is sparse and relates mainly to the synthesis of nanoparticles from silver,24,26 titania,27 iron oxide28 and polymeric nanoparticles,3,4,25,29 though the potential of such reactors and indeed microfluidics in general has yet to be explored fully in the pharmaceutical arena. Many of the current microfluidic devices used in the fabrication of pharmaceutical nanoparticles and nanogels are made from polydimethylsiloxane (PDMS)2,30 which usually swells in contact with organic solvents, resulting in the potential adsorption of small molecules into the channel walls.4 Further deposition of polymer on the channel wall may result in changes to the device structure and consequent negative effects on the mixing efficiency and robustness of nanoparticle production inside the device.
In the field of nanofabrication, it is not uncommon to find that several experimental factors can influence the properties of the resulting particles. Design of Experiments (DoE) is a technique which can be implemented to identify variables having a significant effect on response properties.31 In particular, response surface methodology (RSM) is able to identify important interactions between factors which are typically overlooked by ‘one variable at a time’ (OVAT) approaches.32 Using RSM requires fewer experiments to be carried out than a full factorial model and is capable of determining more effects than in OVAT approaches, including the interactions between factors and quadratic effects. Therefore, significant conclusions can be drawn whilst minimising cost and limiting wastage of reagents.
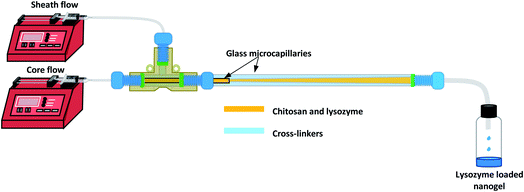
In the present study, DoE is used to optimise the nanogel fabrication process, reduce the number of experiments conducted and identify the optimal experimental conditions. The CFR is designed to add control to the fabrication of nanogels allowing regulation over particle size, polydispersity index (PDI) and encapsulation efficiency (% EE). Using the CFR, a core solution of chitosan and lysozyme is fed through an inner microcapillary and a solution of sodium triphosphate (TPP) and 1-ethyl-2-(3-dimethylaminopropyl)-carbodiimide (EDC) cross-linkers comprise the sheath flow through the outer glass tube, creating a 3D flow-focussing profile. TPP was used to form nanogels by ionic gelation, and the EDC is subsequently used to affect covalent cross-links in the nanogels.33 A four-factor three-level face-centered central composite design (CCD) was used to run a series of 27 experiments varying the chitosan, TPP and lysozyme concentrations and also the flow ratio, which is a ratio between the flow rates of the core and sheath solutions. The CFR set-up and the implementation of a dilute outer sheath stream allows us to mitigate the effect of fouling highlighted by Pessoa et al.23 which is a fundamental obstacle in enabling microfluidics to be widely used in formulating chitosan/TPP nanogels. The study aims to yield particles of a size below 100 nm, with a PDI below or equal to 0.3 and an % EE as close to 100% as possible, and so a desirability function was applied where each response was transformed into an individual desirability function in order to find the optimum conditions for successful nanogel production.
2. Methods
2.1. Materials
Medium molecular weight chitosan, TPP, EDC, lysozyme from chicken egg white (∼70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mg−1) and Micrococcus lysodeikticus ATCC no. 4698 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glacial acetic acid and sodium hydroxide were of analytical grade and obtained from Fisher Scientific (Waltham, MA, USA). Ultrapure water was obtained from PURELAB® Chorus 2+ machine (ELGA LabWater, High Wycombe, UK). All solutions were filtered prior to entering the CFR through a 0.22 μm syringe filter unit from Merck Millipore (Billerica, MA, USA). Pierce bicinchoninic acid (BCA) protein assay kit was purchased from Thermo Fisher (Waltham, MA, USA). Amicon Ultra Diafiltration tubes (0.5 mL), with molecular weight cut-off of 30 kDa, were purchased from Merck Millipore (Billerica, MA, USA). The 0.595 mm ID glass microcapillary used to fabricate the CFR was purchased from Cole-Parmer (St Neots, UK), the 1.6 mm ID glass tube was purchased from VWR International Ltd (Lutterworth, UK). Polyether ether alkene (PEEK) T-junctions and perfluoroalkoxy alkane (PFA) tubing were purchased from Gilson Scientific (Middleton, WI, USA), while nuts and ferrules were purchased from Fisher Scientific (Waltham, MA, USA).
000 U mg−1) and Micrococcus lysodeikticus ATCC no. 4698 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glacial acetic acid and sodium hydroxide were of analytical grade and obtained from Fisher Scientific (Waltham, MA, USA). Ultrapure water was obtained from PURELAB® Chorus 2+ machine (ELGA LabWater, High Wycombe, UK). All solutions were filtered prior to entering the CFR through a 0.22 μm syringe filter unit from Merck Millipore (Billerica, MA, USA). Pierce bicinchoninic acid (BCA) protein assay kit was purchased from Thermo Fisher (Waltham, MA, USA). Amicon Ultra Diafiltration tubes (0.5 mL), with molecular weight cut-off of 30 kDa, were purchased from Merck Millipore (Billerica, MA, USA). The 0.595 mm ID glass microcapillary used to fabricate the CFR was purchased from Cole-Parmer (St Neots, UK), the 1.6 mm ID glass tube was purchased from VWR International Ltd (Lutterworth, UK). Polyether ether alkene (PEEK) T-junctions and perfluoroalkoxy alkane (PFA) tubing were purchased from Gilson Scientific (Middleton, WI, USA), while nuts and ferrules were purchased from Fisher Scientific (Waltham, MA, USA).
2.2. Fabrication of coaxial flow reactor
The CFR as shown in Fig. 1 was designed in order to create a 3D flow-focussing profile. The reactor was assembled by fixing the smaller microcapillary of ID 0.595 mm and length 54 mm, into a larger glass tube with an ID of 1.6 mm and length 162 mm. The two tubes were fitted to a PEEK T-junction whereby chitosan and lysozyme of varying concentrations in accordance with the CCD were used as the core solution flowing through the inner microcapillary.2.3. Experimental design
A systematic optimisation approach was employed in this study to determine the optimal nanogel fabrication parameters, with reference to three key parameters of nanogels including Z-average particle size (hydrodynamic particle size), PDI, and % EE. Based on our preliminary findings, four variables including chitosan concentration, TPP concentration, flow ratio and lysozyme concentration were considered important and were thus selected as the studied factors. We deliberately maintained the pH throughout the study at pH 5 in order to preserve the activity of the lysozyme and simultaneously dissolve the chitosan. A range of nanogels were prepared in the CFR by varying these factors in line with the DoE model.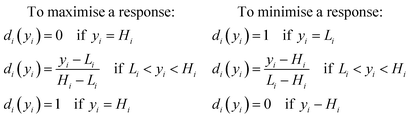 | (1) |
When a product or a process has multiple quality characteristics, simultaneous consideration of all responses is important to determine the optimal conditions of the independent variables. Thus, an overall desirability (D) was calculated, as the geometric mean of the combined individual desirabilities, as shown in eqn (2), where all responses are weighted equally. The operating condition with highest overall desirability was determined as the optimal conditions for nanogel fabrication in the CFR, which was identified via JMP 15.
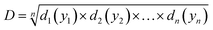 | (2) |
A final experimental run was conducted in triplicate using this optimal condition to validate the experimental design. The predicted values of response variables obtained from the respective polynomial equations were compared to the experimental values with the percentage error calculated.
2.4. Synthesis of nanogels in CFR
The core and sheath solutions were prepared in accordance with the CCD matrix in Table 2. Chitosan solutions of concentrations between 0.1–0.3% (w/v) were prepared by dissolving chitosan in 1% (v/v) aqueous acetic acid solution and heating to approximately 60 °C under stirring until fully dissolved. Solutions were adjusted to pH 5 with diluted sodium hydroxide solution, to enable the amine groups of chitosan to be sufficiently positively charged, whilst also preserving the stability of lysozyme. Lysozyme was added to the chitosan solution to achieve final concentrations ranging from 0.1–0.3% (w/v), forming the core solution for the CFR. TPP was prepared in various concentrations between 0.01–0.02% (w/v) and EDC was added to this solution to obtain a fixed concentration of 0.5% (w/v) to allow its presence in excess and formed the sheath flow solution. The sheath flow was maintained at a constant flow rate of 160 μL min−1 and the core flow rate was varied to achieve differing flow ratios (core/sheath flow rate) between 0.1–0.3 to meet the DoE matrix requirements. A flow ratio of 0.3, for example, would represent a flow rate of 48 μL min−1 for the core solution and 160 μL min−1 for the sheath flow. All experiments were performed at ambient temperature.| Run | Independent variables | Dependent variables | Additional measurements (not included in DoE model) | |||||
|---|---|---|---|---|---|---|---|---|
| Chitosan conc. (% w/v) | TPP conc. (% w/v) | Flow ratio | Lysozyme conc. (% w/v) | Particle size (nm) | PDI | % EE | Zeta potential (mV) | |
| a Excluded in the CCD model. | ||||||||
| 1 | 0.3 | 0.010 | 0.3 | 0.1 | 63 | 0.818 | 91.2 | 16.0 |
| 2 | 0.1 | 0.020 | 0.3 | 0.3 | 113 | 0.199 | 88.1 | 20.8 |
| 3 | 0.2 | 0.015 | 0.3 | 0.2 | 103 | 0.295 | 97.1 | 23.1 |
| 4 | 0.1 | 0.010 | 0.3 | 0.1 | 105 | 0.249 | 94.3 | 23.0 |
| 5 | 0.2 | 0.015 | 0.2 | 0.2 | 117 | 0.205 | 95.5 | 21.4 |
| 6 | 0.1 | 0.015 | 0.2 | 0.2 | 118 | 0.203 | 72.6 | 21.7 |
| 7 | 0.2 | 0.015 | 0.2 | 0.1 | 131 | 0.213 | 79.1 | 23.1 |
| 8 | 0.2 | 0.020 | 0.2 | 0.2 | 123 | 0.251 | 83.1 | 21.8 |
| 9 | 0.3 | 0.010 | 0.3 | 0.3 | 46 | 0.675 | 96.5 | 16.2 |
| 10 | 0.1 | 0.020 | 0.1 | 0.3 | 1567a | 0.474 | 31.3 | 21.3 |
| 11 | 0.1 | 0.010 | 0.1 | 0.3 | 129 | 0.19 | 62.4 | 18.3 |
| 12 | 0.3 | 0.020 | 0.3 | 0.1 | 168 | 0.198 | 91.0 | 23.4 |
| 13 | 0.3 | 0.015 | 0.2 | 0.2 | 216 | 0.163 | 93.5 | 23.2 |
| 14 | 0.3 | 0.020 | 0.3 | 0.3 | 147 | 0.248 | 96.8 | 16.7 |
| 15 | 0.2 | 0.015 | 0.1 | 0.2 | 134 | 0.203 | 80.1 | 21.1 |
| 16 | 0.3 | 0.010 | 0.1 | 0.1 | 100 | 0.21 | 75.9 | 21.3 |
| 17 | 0.1 | 0.010 | 0.3 | 0.3 | 64 | 0.299 | 96.6 | 19.8 |
| 18 | 0.1 | 0.010 | 0.1 | 0.1 | 188 | 0.257 | 17.2 | 20.9 |
| 19 | 0.3 | 0.020 | 0.1 | 0.1 | 238 | 0.368 | 54.3 | 20.2 |
| 20 | 0.1 | 0.020 | 0.1 | 0.1 | 5237a | 0.425 | 23.9 | 20.1 |
| 21 | 0.1 | 0.020 | 0.3 | 0.1 | 133 | 0.209 | 83.8 | 24.2 |
| 22 | 0.2 | 0.010 | 0.2 | 0.2 | 77 | 0.29 | 95.5 | 21.0 |
| 23 | 0.2 | 0.015 | 0.2 | 0.3 | 105 | 0.223 | 95.0 | 22.6 |
| 24 | 0.3 | 0.020 | 0.1 | 0.3 | 125 | 0.222 | 86.9 | 17.3 |
| 25 | 0.2 | 0.015 | 0.2 | 0.2 | 111 | 0.199 | 95.4 | 22.2 |
| 26 | 0.2 | 0.015 | 0.2 | 0.2 | 99 | 0.223 | 95.6 | 22.0 |
| 27 | 0.3 | 0.010 | 0.1 | 0.3 | 221 | 0.154 | 91.8 | 23.4 |
| Model | Source of variation | Degrees of freedom | Sum of squares | Mean square | F value | Prob. > F | Significance | R 2 (adj R2) |
|---|---|---|---|---|---|---|---|---|
| Z-Average particle size | Model | 4 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
6886 | 4.87 | 0.0066 | Significant | 0.493 |
| Residual | 20 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
1413 | (0.392) | ||||
| Lack of fit | 9 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
1202 | 0.76 | 0.6560 | Not significant | ||
| Pure error | 11 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
1587 | |||||
| PDI | Model | 7 | 0.54 | 0.077 | 19.9 | <0.001 | Significant | 0.880 |
| Residual | 19 | 0.07 | 0.0039 | (0.836) | ||||
| Lack of fit | 7 | 0.04 | 0.0064 | 2.65 | 0.0660 | Not significant | ||
| Pure error | 12 | 0.03 | 0.0024 | |||||
| % EE | Model | 5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 860 |
2172 | 16.0 | <0.001 | Significant | 0.792 |
| Residual | 21 | 2856 | 136.0 | (0.792) | ||||
| Lack of fit | 9 | 1893 | 210.3 | 2.62 | 0.0612 | Not significant | ||
| Pure error | 12 | 962.9 | 80.24 |
2.5. Synthesis of nanogels by stirring approach
The optimal nanogel formulation as identified in the DoE model was also produced in batch by a conventional stirring method for nanogel production. Nanogels were made in 3 mL batches where the volumes of the TPP/EDC solution and the chitosan/lysozyme solution used were 2.3 mL and 0.7 mL respectively, in order to replicate the volume ratio used in the CFR when the flow ratio is 0.3. The TPP/EDC solution was added to a glass vial and placed under stirring at 600 rpm. The second solution of chitosan and lysozyme at pH 5 was added to the glass vial dropwise over a period of 39 seconds to mimic the mixing time in the CFR at the flow ratio of 0.3. The mixing in the vial was allowed to continue for 14.4 minutes to simulate the time that would be required for collection of the same volume (3 mL) in the microfluidic reactor.2.6. Characterisation of lysozyme-loaded nanogels
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The solutions were then centrifuged at a rotational speed of 14
000). The solutions were then centrifuged at a rotational speed of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 30 minutes at room temperature using a mini centrifuge (SciSpin Micro Centrifuge, SciQuip, Wem, UK). The unencapsulated lysozyme in the nanogel suspension was determined by bicinchoninic acid (BCA) assay and % EE was calculated using eqn (3). The experiment was performed in triplicate and the results presented as mean value ± standard deviation.
000 × g for 30 minutes at room temperature using a mini centrifuge (SciSpin Micro Centrifuge, SciQuip, Wem, UK). The unencapsulated lysozyme in the nanogel suspension was determined by bicinchoninic acid (BCA) assay and % EE was calculated using eqn (3). The experiment was performed in triplicate and the results presented as mean value ± standard deviation.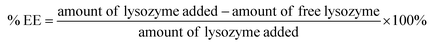 | (3) |
For the drug loading (% DL), the nanogels produced using the optimum formulation were freeze-dried for a duration of 48 hours. A sample of freeze-dried nanogels was taken from each replicate and a 2 mg mL−1 suspension obtained. The concentration of lysozyme in the nanogels was measured using a BCA assay and % DL was calculated using eqn (4).
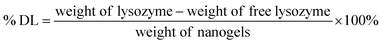 | (4) |
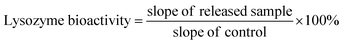 | (5) |
3. Results and discussion
3.1. CCD development
Using the experimental design matrix, twenty-seven formulations of nanogels were prepared with the corresponding independent and dependent variables presented in Table 1. Responses were fitted with a polynomial model, which includes individual independent terms, their interaction and quadratic terms. The polynomial equation is comprised of coefficients β0 to β14 and the terms, as shown in eqn (6). Factors estimated to have an effect were selected by the stepwise regression for each individual response. If the lack of fit test was significant, terms with the highest p-value were further excluded from the model until the lack of fit test became insignificant. Two outliers were detected according to Cook's distances (data not shown) in the model for Z-average particle size, and thus these points were removed from the training set.| Y = β0 + β1A + β2B + β3C + β4D + β5AB + β6AC + β7AD + β8BC + β9BD + β10CD + β11A2 + β12B2 + β13C2 + β14D2 | (6) |
| Factor | Optimal value | Response | Predicted value | Experimental value | Mean difference (%) |
|---|---|---|---|---|---|
| Chitosan conc.% (w/v) | 0.154 | Particle size (nm) | 84.5 | 84.0 ± 4.0 | 0.6 |
| TPP conc.% (w/v) | 0.015 | PDI | 0.193 | 0.261 ± 0.007 | 35.2 |
| Flow ratio | 0.3 | EE (%) | 99.1 | 94.6 ± 2.9 | 4.5 |
| Lysozyme conc.% (w/v) | 0.3 | Zeta potential (mV) | N/A | 19.9 ± 0.6 | N/A |
| DL (%) | N/A | 73.1 ± 1.8 | N/A |
To validate the accuracy of the model in predicting the optimal conditions, an additional experimental run was carried out at the optimal running conditions. The experimental results measured were 84.0 ± 4 nm (Z-average particle size), 0.261 ± 0.007 (PDI), 94.6 ± 2.9% (% EE) as reported in Table 3 and the profile in Fig. S1.† A good correlation between the predicted and experimental responses indicated that the models for Z-average particle size and % EE have sufficient accuracy in predicting the optimal condition. However, the larger mean difference between the measured and predicted PDI may indicate that other factors not included in this model might also affect the PDI of the nanogels.
The percentage drug loading was performed on the optimum formulation of nanogels and found to be 73.1% ± 1.8% (w/w). Nanogels have repeatedly demonstrated their ability to accommodate a high drug loading efficiency, often achieved by physical entrapment of drugs38 or in the case of biological agents, high loading is attained by electrostatic, van-der Waals and/or hydrophobic interactions with the polymeric network interactions.39 Hydrophilic nanogels typically show a high drug loading capacity given that in their unloaded state they hold a high-water capacity, which provides sufficient space for large quantities of biological agents to be loaded.39
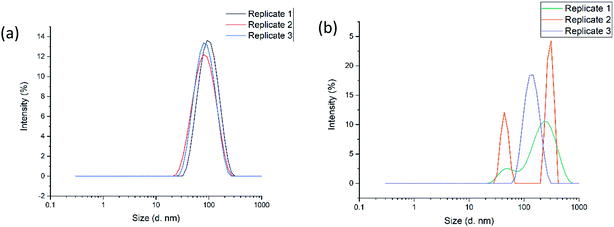
The optimal formulation for the nanogels as determined by the DoE model, was carried out both in the CFR and also by a conventional stirring approach for comparison. It is evident from Fig. 2(a) that nanogels produced in the CFR are composed of only one size population, as only one single peak was observed for each of the three replicates tested. In Fig. 2(b) the three replicates tested for nanogels produced by a conventional stirring approach have multiple populations of sizes given the various peaks that can be seen. This highlights that the CFR is a reproducible method for formulating the nanogels in this study, whereas the stirring approach in batch yields high levels of size variation. The average size of the nanogels produced in the CFR was 84.0 ± 4.0 nm with a PDI of 0.261 ± 0.007, whereby the batch produced nanogels had a size of 319.1 ± 307.5 nm with a high PDI of 0.492 ± 0.446. Such results indicate that the stirring approach in batch is less able to control the particle size and reduce the polydispersity of the nanogels in this study. The large variation between the three replicates, as indicated by the standard deviation, also shows that the formulation is not reproducible when using this approach.
3.2. Effects of factors on responses
The model terms were selected in stepwise regression, wherein the terms were chosen based on the estimated effect only. However, not all of the effects for these selected terms were statistically significant at the 95% confidence interval (p < 0.05) and at that particular degree of freedom despite the ANOVA result for the overall model being statistically significant. Therefore, the effect of the individual, interaction and quadratic terms selected in the CCD models were also evaluated and are reported in Table 4. The interaction of chitosan concentration and lysozyme concentration (AD), and the interaction between TPP concentration and lysozyme concentration (BD), quadratic effects of flow ratio (C2) and lysozyme concentration (D2) were not found to affect any of the properties of nanogels and thus are not represented in Table 4.| Factors | Z-Average particle size | PDI | EE% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sum of squares | F-Ratio | p-Value > F | Sum of squares | F-Ratio | p-Value > F | Sum of squares | F-Ratio | p-Value > F | |
| a p-Value < 0.05. b p-Value < 0.01. | |||||||||
| A | 331.350 | 0.234 | 0.6336 | 0.017 | 4.351 | 0.0507 | 2394.089 | 17.61 | 0.0004b |
| B | 9102.017 | 6.438 | 0.0196a | 0.017 | 4.303 | 0.0519 | |||
| C | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703.230 703.230 |
11.107 | 0.0033a | 0.026 | 6.763 | 0.0176a | 5394.835 | 39.67 | <0.001b |
| D | 1009.053 | 7.42 | 0.0127a | ||||||
| AB | 0.080 | 20.694 | 0.0002b | ||||||
| AC | 0.118 | 30.479 | <0.001b | 1626.711 | 11.96 | 0.0024b | |||
| BC | 0.217 | 56.072 | <0.001b | ||||||
| CD | 435.453 | 3.20 | 0.0880 | ||||||
| A 2 | 5510.756 | 3.898 | 0.0623 | ||||||
| B 2 | 0.066 | 16.918 | 0.0006b | ||||||
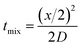 | (7) |
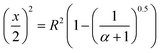 | (8) |
The mixing times have been computed using a typical diffusion coefficient of 10−9 m2 s−1 for TPP in water. This is a significant assumption, as the chitosan inner stream is more viscous than water, hence one could argue that the diffusion coefficient is lower. The viscosity of the chitosan solutions was found to be approximately two times larger than water (see Table S2†). Using the highest viscosity (i.e., 2.8 cP) and considering that the diffusivity is inversely proportional to the viscosity, we would get a diffusivity coefficient of 3.6 × 10−10 m2 s−1, resulting in a range of mixing time between 42 and 109 s. Considering the uncertainties in the value of the diffusion coefficient of TPP in water and the assumptions of the equations above, these calculations suggest that the ratio tmix/τ ∼ 1. Given this result, it could be argued that the reaction may not reach completion inside the flow rector. One would expect then that this would be cause of irreproducibility and poor particle size distribution, as sample collection at the reactor outlet is a rather uncontrolled process, which however does not appear to be the case.
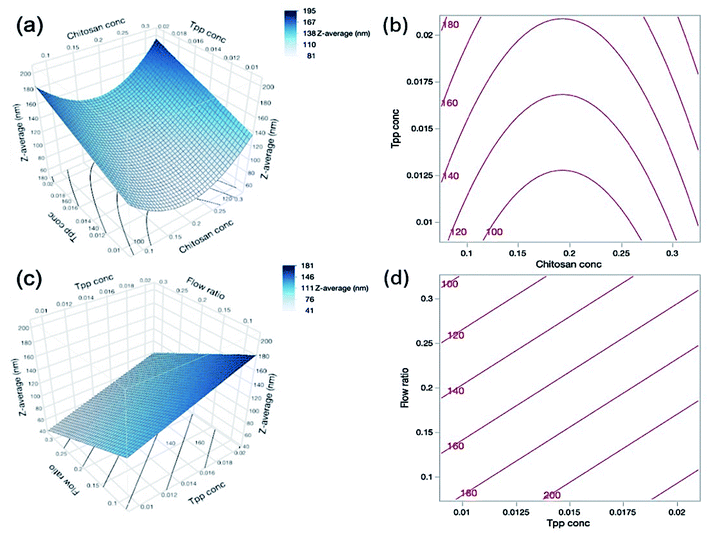
As can be seen in Table 4, both the TPP concentration and flow ratio were shown to be statistically significant factors influencing the nanogel size. TPP is the anionic cross-linker of choice in this study and is able to complex with the cationic charge of chitosan. Eqn (9) shows the positive effect of TPP concentration (B) on nanogel size, and so as the TPP concentration increases, so too does the particle size; we suggest that this is because the increased concentration allows for additional cross-links to form within the nanogel structure, thus forming larger particles. Fig. 3(c and d) depict the effect of both TPP concentration and flow ratio on the particle size.
| Size (nm) = 217.7222 − 1205.0000A + 4926.6667B − 323.5556C + 3130.0000A2 | (9) |
According to eqn (9), the flow ratio (C) had a negative value, indicating that an increase in flow ratio results in a decrease in the particle size. At higher flow ratios the flow rate of the core solution is increased whilst the sheath flow remains constant, and so more chitosan and lysozyme are added to the formulation. Therefore, the additional positive charge of the chitosan and lysozyme may allow further charge interaction and condensation of the nanogel particles resulting in the decreased size.
Furthermore, as the flow ratio increases the ratio of chitosan and TPP is also increased, which has been shown in the literature to vastly affect the particle size. Koukaras et al.43 observed that the particle size decreased to a minimum of 340 nm at a chitosan/TPP ratio of 4/1 and increased thereafter. This is in agreement with Zhang et al.44 who achieved a decrease in particle size with increasing ratio until 5/1 where particles were at a minimum of 109 ± 4 nm, and particles increased in size thereafter. This indicates that although in this work the chitosan concentration was statistically insignificant on the particle size, the flow ratio was significant and therefore there may be an optimum flow ratio for the formation of nanogels in the same way that there is an optimum chitosan/TPP ratio when using standard mixing techniques. The effect of chitosan concentration and TPP concentration on the particle size is depicted in Fig. 3(a and b). It is interesting to note, that despite increases in the chitosan concentration with increasing flow ratios, there was no fouling in the reactor and at the tip of the inner microcapillary throughout all experiments.
| PDI = 0.3284 + 0.7117A − 56.6056B + 2.1598C − 141.6250AB + 8.5938AC − 233.1250BC + 4182.2222B2 | (10) |
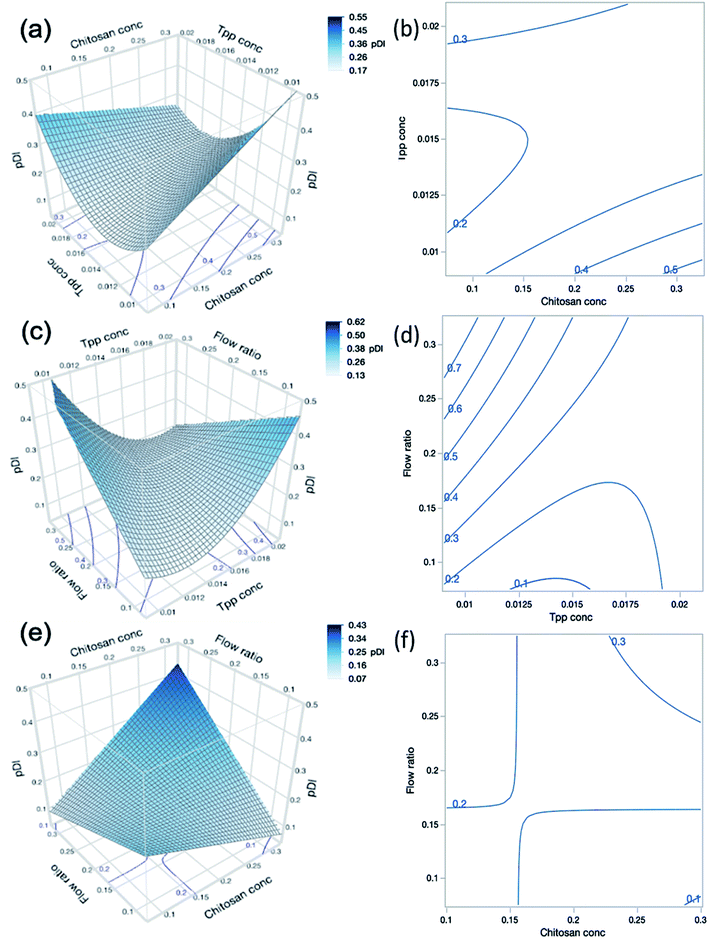
As shown in Table 1, the CFR successfully formulated nanogels with a PDI below 0.3 for 22 out of 27 experimental runs, with PDI values reaching as low as 0.154. Where the highest PDI values of 0.818 for run 1 and 0.675 for run 9 were obtained, both of these formulations also had the lowest zeta potential at +16.0 and +16.2 mV respectively. This may indicate that the particles are more likely to aggregate given the insufficient charge repulsion between nanoparticles leading to a higher propensity for aggregation. Both run 1 and run 9 also had the lowest level of TPP present in the formulation at 0.01% (w/v) and the highest level of chitosan at 0.3% (w/v) which may infer that there was an insufficient amount of cross-linker to allow adequate particle formation to occur. Such findings are also confirmed by the results presented in Table 4 which show that the PDI was significantly influenced by the interaction between chitosan concentration (A) and TPP concentration (B) where this interaction had a p-value less than 0.0002. This is demonstrated in contour plots shown in Fig. 4(a), which shows that at lower TPP concentrations PDI increases with increasing chitosan concentrations. In contrast, at higher TPP concentrations, an increase in the concentration of chitosan led to lower PDI values. It is suggested that as more chitosan becomes available to form cross-links with TPP, nanogels with greater uniformity in size distribution could be obtained as the chitosan–TPP ratio has been optimised. It is possible that optimum parameters for the size and PDI of nanogels using the CFR system differs, as can be observed from the difference in the optimum for size and PDI in Fig. 3(a) and 4(a) respectively. The overall desirability calculation is therefore used to determine a balance between optimum parameters required for size, PDI and % EE.
Additionally, only flow ratio (C) amongst all individual factors exhibited a statistically significant effect on the PDI (p < 0.05) as shown in Table 4. An increased flow ratio signifies a higher flow rate of the core solution whilst the sheath flow rate remained constant. This therefore causes an increase in the overall flow rate and an increase in the mixing time, as discussed previously in Section 3.2.1. The increased mixing time with higher flow ratios causes an increase in PDI, which may indicate that the residence time is not high enough to accommodate the requirement for increased mixing and thus the mixing may be incomplete at higher flow ratios.
As shown in Fig. 4(c), there is an interaction effect of chitosan concentration and flow ratio, whereby at the low concentrations of chitosan, increasing the flow ratio leads to a decrease in PDI. This is due to the fact that at low chitosan concentration and low flow ratio simultaneously, there is an insufficient amount of chitosan in the system to adequately cross-link particles hence leading to larger variations in particle sizes and high PDI values. However, as the flow ratio increases, a sufficient amount of chitosan is introduced into the system to cross-link with TPP and thus the PDI reduces. The cross-interactions between chitosan concentration, TPP concentration and flow ratio (AB, AC and BC) also exhibited statistically significant effects on the PDI of the nanogels (p < 0.0001).
It should also be noted that the quadratic effect of TPP concentration (B2) was also statistically significant on PDI (p = 0.0006), indicating that as TPP concentration increases, PDI decreases until a minimum point before increasing again. This again indicates that there is an optimum concentration of TPP required for the nanogel formation. If the TPP level is too low there is an insufficient amount to form adequate cross-linking in the nanogels, and when the level is too high this may introduce aggregation due to inter-particular cross-links.
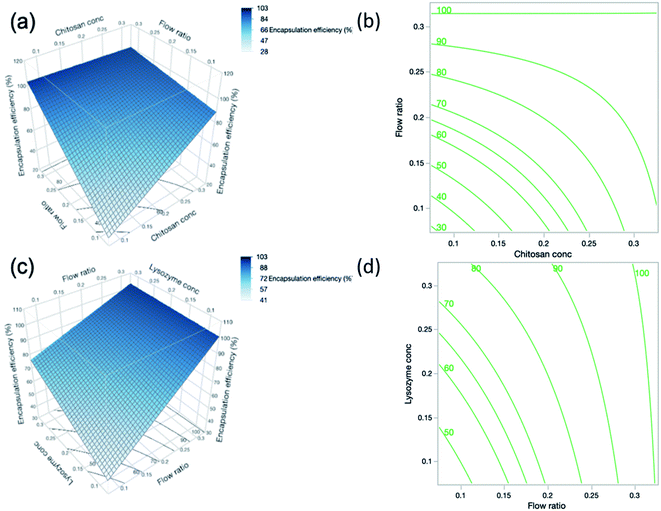
The interaction between chitosan concentration and flow ratio on % EE was also statistically significant (p = 0.0024). These findings are reflected in the surface profile and contour plot in Fig. 5(a and b) respectively, showing that the % EE increased with chitosan concentration and flow ratio, and then levelled off at high chitosan concentration and flow ratio. This observation is similar to that reported by Deng et al.46 who found an initial increase in % EE. However, this holds true until a certain concentration of chitosan, beyond which % EE decreased. When the concentration of chitosan further increases, the greater viscosity of the polymer may hinder the diffusion of lysozyme into the nanoparticle system in order to be encapsulated.
The concentration of lysozyme also had a strong influence on % EE and the positive relationship is indicated by the sign of the coefficient in eqn (11). As lysozyme concentration increases, % EE increases. This trend was demonstrated in DoE runs 19 and 24, where the smallest lysozyme concentration of 0.1% (w/v) (run 19) yielded a % EE of 54.3% and increasing lysozyme concentration to 0.3% (w/v) (run 24) gave a higher % EE of 86.9% despite otherwise having identical formulations. By increasing the lysozyme concentration, more nucleation seeds become available for nanogel growth to occur and hence there may be a higher number of particles which are able to encapsulate more lysozyme. In contrast, the interaction effect between flow ratio and lysozyme concentration shown in Fig. 5(c and d) was deemed statistically insignificant according to the statistical analysis.
| EE% = −53.6944 + 316.9903A + 479.1222C + 179.2097D − 1008.3125AC − 521.6875CD | (11) |
3.3. Fourier-transform infrared spectroscopy
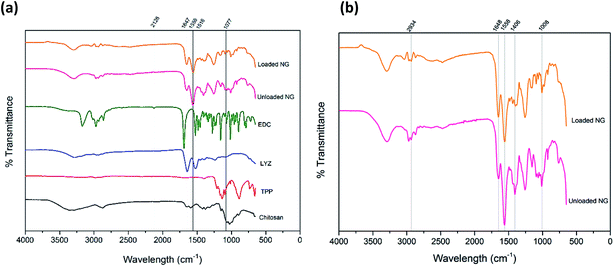
Fig. 6(a) shows the FTIR spectrum for both unloaded and loaded chitosan nanogels along with corresponding individual components of EDC, lysozyme, TPP and chitosan for reference, whilst Fig. 6(b) has been provided for a detailed comparison between the unloaded and loaded nanogels. The broad bands at 3295 cm−1 in the spectra of both loaded and unloaded nanogels can be attributed to the stretching vibration of N–H overlapped with the O–H stretching vibration.47–49 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group from the amide structure of acetylated portions of chitosan can be seen at 1647 cm−1 in the nanogels and the second amide peak at 1558 cm−1 due to N–H bending and C–N stretching which was also observed by Wu et al.50 Given that such peaks are present in loaded and unloaded gels, may indicate that nanogels retain their structure upon incorporation of lysozyme.50 The peaks at 1406 cm−1 in the nanogel spectra are likely due to O–H bending in the structure of chitosan. The peak present in nanogels at 1008 cm−1 can be attributed to the C–O stretching of groups present in the ether group of chitosan and has shifted from 1077 cm−1 in the spectrum of chitosan alone. At 1258 cm−1 the sharp peak present in the nanogels is indicative of the P
O group from the amide structure of acetylated portions of chitosan can be seen at 1647 cm−1 in the nanogels and the second amide peak at 1558 cm−1 due to N–H bending and C–N stretching which was also observed by Wu et al.50 Given that such peaks are present in loaded and unloaded gels, may indicate that nanogels retain their structure upon incorporation of lysozyme.50 The peaks at 1406 cm−1 in the nanogel spectra are likely due to O–H bending in the structure of chitosan. The peak present in nanogels at 1008 cm−1 can be attributed to the C–O stretching of groups present in the ether group of chitosan and has shifted from 1077 cm−1 in the spectrum of chitosan alone. At 1258 cm−1 the sharp peak present in the nanogels is indicative of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in TPP within the nanogel structure, albeit shifted from 1209 cm−1 in TPP alone as a result of the interaction with chitosan. The peaks at 1647 cm−1 and 1516 cm−1 for lysozyme can be attributed to the amide peaks I and II respectively.51
O bond in TPP within the nanogel structure, albeit shifted from 1209 cm−1 in TPP alone as a result of the interaction with chitosan. The peaks at 1647 cm−1 and 1516 cm−1 for lysozyme can be attributed to the amide peaks I and II respectively.51
For the TPP spectra, findings were in agreement with those of Loutfy et al.47 where in our study, stretching vibrations can be seen at 1209 cm−1 assigned to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, a peak around 1136 cm−1 attributed to the O–P
O bond, a peak around 1136 cm−1 attributed to the O–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and a peak around 886 cm−1 belonging to the stretching vibration of the PO3 group. Such peaks can be seen in the nanogel formulations, though given the low quantity of TPP in the optimum formulation they are much weaker than in TPP alone. The peak at 2126 cm−1 in the EDC spectra is characteristic of the carbodiimide group (N
O group and a peak around 886 cm−1 belonging to the stretching vibration of the PO3 group. Such peaks can be seen in the nanogel formulations, though given the low quantity of TPP in the optimum formulation they are much weaker than in TPP alone. The peak at 2126 cm−1 in the EDC spectra is characteristic of the carbodiimide group (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), this is absent in both the unloaded and loaded nanogels. The absence of the carbodiimide group in the nanogel formulation is representative of the reaction of EDC and nitrogen whereby the absence of a carboxylic acid group in the chitosan is due to the direct reaction between the free amine groups of chitosan and the EDC, forming a covalent bond.52 A summary of the peaks and their attributions can be found in Table S3.† In conclusion, the FTIR confirms the presence of the individual components and the lack of structural change of the nanogels upon encapsulation and loading of lysozyme.
N), this is absent in both the unloaded and loaded nanogels. The absence of the carbodiimide group in the nanogel formulation is representative of the reaction of EDC and nitrogen whereby the absence of a carboxylic acid group in the chitosan is due to the direct reaction between the free amine groups of chitosan and the EDC, forming a covalent bond.52 A summary of the peaks and their attributions can be found in Table S3.† In conclusion, the FTIR confirms the presence of the individual components and the lack of structural change of the nanogels upon encapsulation and loading of lysozyme.
3.4. Shape and morphology of the nanogels
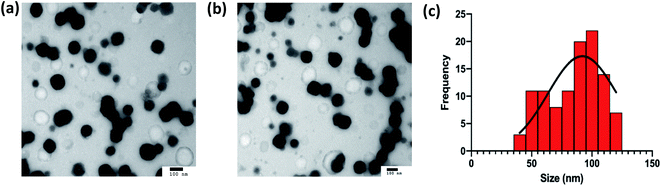
The nanogels observed were largely spherical in shape, as observed in Fig. 7(a). The average diameter of the optimal formulation of nanogels is measured to be 92.0 ± 28.5 nm (n = 107) using ImageJ, which is in agreement with the value observed by DLS of 84.4 ± 4 nm albeit with a smaller standard deviation around the mean. The difference may arise from the fact that the size measured in the image was the diameter at the dry state, while the size measured in DLS was hydrodynamic size, of which particles were suspended in water with an assumption of the particles being spherical. The wide size distribution demonstrated on the TEM images may indicate that the drying stage required to prepare the TEM samples was not sufficient as some of the nanogels may not be fully dried and may appear larger than others. The drying of the nanogels can also allow aggregates to form, which may appear as larger particles on the TEM as observed in Fig. 7(a and b) thus contributing to the wide size distribution seen.3.5. Lysozyme activity assay
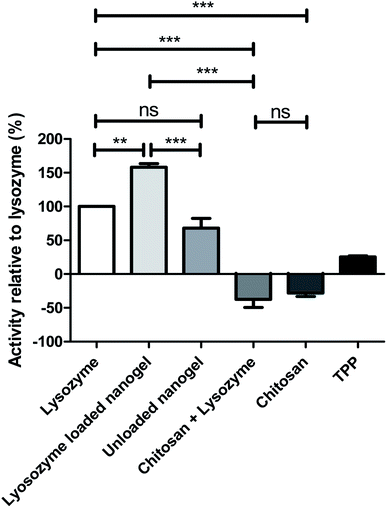
The lysozyme activity assay was carried out on the optimum formulation in order to assess both the activity of the protein after processing in the CFR and encapsulation within nanogels. The rate of lysis of Micrococcus lysodeikticus cells was measured after exposure to native lysozyme compared to individual components of the nanogels and lysozyme loaded nanogels as can be seen in Fig. 8. Chitosan alone, and chitosan in combination with lysozyme exhibited negative activity values, indicating that bacterial growth continues and thus such components have little or no activity against the bacterial cell walls of Micrococcus lysodeikticus cells. Positive values were observed for TPP, unloaded nanogels and lysozyme loaded nanogels, indicating that these components are active on the lysis of the bacterial cell wall. Interestingly, individual components of the formulation have negligible effect in comparison to native lysozyme, whereas the unloaded nanogel and the loaded nanogels show an activity of 68.4% and 157.6% respectively. The activity of the lysozyme was therefore enhanced greatly in the nanogel formulation in comparison to lysozyme alone as the control represented at 100% in Fig. 8. The unloaded nanogels still exert an activity against Micrococcus lysodeikticus cells at 68.4% that of the lysozyme control. This effect can be explained in the study by Atay et al.53 who found that when chitosan is used in low concentrations, such as in the nanogels formulated in the CFR given the dilution from the sheath flow, the chitosan is able to bind to the negatively charged cell surface and disrupt the cell wall causing leakage of intracellular components and consequently rapid cell death. However, in the chitosan solution and chitosan and lysozyme combination where chitosan was present at higher concentrations, the chitosan may simply coat the cell surface and prevent leakage of intracellular components. The high encapsulation efficiency of the nanogels may also contribute to concentrating the lysozyme at the site of action thus allowing it to exert its activity on the bacterial cell wall.4. Conclusions
In this study, chitosan nanogels loaded with the protein lysozyme were successfully synthesised using microfluidics in a coaxial flow reactor (CFR). Given that microfluidics is a somewhat new technique in the field of nanogels, a face-centered central composite design (CCD) was employed to determine the interaction of various factors involved in nanogel production and their responses. The four investigated factors were chitosan, lysozyme, tripolyphosphate (TPP) concentrations and flow ratio, and their effects on the particle size (Z-average particle size), polydispersity (PDI) and encapsulation efficiency (% EE) were established. Following the application of a desirability function to find a set of operating conditions which yield nanogels with desired characteristics, nanogels were produced with the optimum conditions (chitosan concentration 0.154% (w/v), TPP concentration 0.015% (w/v), flow ratio 0.3 and lysozyme concentration of 0.3% (w/v)). The predictability of the experimental model was subsequently determined by comparing the predicted and experimental values of all measured outcomes. The Z-average particle size of the optimum nanogel formulation was predicted to be 84.5 nm and the experimental value was 84.0 ± 4.0 nm, the PDI predicted to be 0.193 and the obtained value was 0.261 ± 0.007, and the % EE predicted to be 99.1% and an actual of 94.6 ± 2.9% was achieved. Overall, this shows that the Design of Experiments (DoE) model was able to achieve a high level of predictability for the properties of nanogels, and in combination with the CFR successfully enabled the production of nanosized, highly monodisperse nanogels with the ability to encapsulate a significant amount of lysozyme. Using response surface methodology (RSM), we were able to ascertain significant interactive effects between factors, in particular for PDI and % EE. The mixing time in the CFR was calculated, leading to the ratio between mixing time and residence time, tmix/τ ∼ 1. This would suggest that the CFR, operated in the conditions explored here, provides controlled and reproducible mixing, rather than fast mixing, enabling reproducible synthesis of chitosan nanogels. Furthermore, the use of this system prevents the lysozyme to experience high shear during the loading process, hence preserving its biological functions.Importantly, a lack of fouling was observed throughout the study when using the CFR system, this being a common obstacle when using microfluidics with polymeric materials. This was achieved through the use of glass microcapillaries and a 3D flow-focussing system which is able to shield the reaction interface from the channel wall. The CFR enabled the core flow of polymer to be surrounded completely by the sheath flow of aqueous cross-linker solution to produce controlled mixing and consequently nanogels of high monodispersity, adequate size, and high encapsulation efficiency. The current ionic gelation technique for nanogel production lacks adequate control over product characteristics due to inefficient mixing and batch-to-batch variations. The CFR therefore offers a novel approach to formulating nanogels loaded with therapeutic agents, where mixing is controlled and homogeneous, products are reproducible between batches and scale-up in the pharmaceutical industry is possible. Although the use of microfluidics and particularly the CFR have not yet been extensively explored for drug delivery purposes, such techniques allow the fine tuning of parameters and ultimately production of nanogels with precise characteristics.
Conflicts of interest
No conflict of interest to declare.Acknowledgements
This work was also supported by the EPSRC Centre in Doctoral Training for Nanomedicine and Advanced Therapeutics [EP\L01646X] awarded to HMKH and the EPSRC [EP/R513143/1] studentship awarded to ZW. We would also like to acknowledge financial and intellectual support from the Interreg 2 Seas Programme 2014–2020 co-funded by the European Regional Development Fund under subsidy contract 2S01-059_IMODE. The authors would like to thank Dr Andrew Weston for the TEM imaging in this study.References
- F. Araújo, et al., In vivo dual-delivery of glucagon like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP4) inhibitor through composites prepared by microfluidics for diabetes therapy, Nanoscale, 2016, 8, 10706–10713 RSC.
- S. Bazban-Shotorbani, E. Dashtimoghadam, A. Karkhaneh, M. M. Hasani-Sadrabadi and K. I. Jacob, Microfluidic Directed Synthesis of Alginate Nanogels with Tunable Pore Size for Efficient Protein Delivery, Langmuir, 2016, 32, 4996–5003 CrossRef CAS.
- R. Karnik, et al., Microfluidic Platform for Controlled Synthesis of Polymeric Nanoparticles, Nanoletters, 2008, 8, 2906–2912 CrossRef CAS.
- D. Liu, et al., A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties, Adv. Mater., 2015, 27, 2298–2304 CrossRef CAS.
- G. M. Whitesides, The origins and the future of microfluidics, Nature, 2006, 442, 368–373 CrossRef CAS.
- S. Garg, G. Heuck, S. Ip and E. Ramsay, Microfluidics: a transformational tool for nanomedicine development and production, J. Drug Targeting, 2016, 24, 821–835 CrossRef CAS.
- P. Cui and S. Wang, Application of micro fluidic chip technology in pharmaceutical analysis: a review, J. Pharm. Anal., 2019, 9, 238–247 CrossRef.
- I. Neamtu, A. G. Rusu, A. Diaconu, L. E. Nita and A. P. Chiriac, Basic concepts and recent advances in nanogels as carriers for medical applications, Drug Delivery, 2017, 24, 539–557 CrossRef CAS.
- D. Li, C. F. van Nostrum, E. Mastrobattista, T. Vermonden and W. E. Hennink, Nanogels for intracellular delivery of biotherapeutics, J. Controlled Release, 2017, 259, 16–28 CrossRef CAS.
- H. K. S. Yadav, N. Anwar, A. Halabi and G. A. Alsalloum, Nanogels as Novel Drug Delivery Systems - A Review Properties of Nanogels, Insights in Pharma Research, 2017, 1–8 Search PubMed.
- J. Zhang, A. Sen, E. Cho, J. Soo Lee and K. Webb, Poloxamine/fibrin hybrid hydrogels for non-viral gene delivery, J. Tissue Eng. Regener. Med., 2014, 11, 246–255 CrossRef CAS.
- Y. Li, D. Maciel, J. Rodrigues, X. Shi and H. Tomás, Biodegradable polymer nanogels for drug/nucleic acid delivery, Chem. Rev., 2015, 115, 8564–8608 CrossRef CAS.
- A. C. Lima, P. Sher and J. F. Mano, Production methodologies of polymeric and hydrogel particles for drug delivery applications, Expert Opin. Drug Delivery, 2012, 9, 231–248 CrossRef CAS.
- F. S. El-banna, M. E. Mahfouz, S. Leporatti, M. El-Kemary and N. A. N. Hanafy, Chitosan as a natural copolymer with unique properties for the development of hydrogels, Appl. Sci., 2019, 9, 1–11 Search PubMed.
- E. Chiesa, et al., The microfluidic technique and the manufacturing of polysaccharide nanoparticles, Pharmaceutics, 2018, 10, 267 CrossRef CAS.
- E. Dashtimoghadam, H. Mirzadeh, F. A. Taromi and B. Nyström, Microfluidic self-assembly of polymeric nanoparticles with tunable compactness for controlled drug delivery, Polymer, 2013, 54, 4972–4979 CrossRef CAS.
- D. Liu, H. Zhang, F. Fontana, J. T. Hirvonen and H. A. Santos, Current developments and applications of microfluidic technology toward clinical translation of nanomedicines, Adv. Drug Delivery Rev., 2018, 128, 54–83 CrossRef CAS.
- S. Bazban-Shotorbani, E. Dashtimoghadam, A. Karkhaneh, M. M. Hasani-Sadrabadi and K. I. Jacob, Microfluidic Directed Synthesis of Alginate Nanogels with Tunable Pore Size for Efficient Protein Delivery, Langmuir, 2016, 32, 4996–5003 CrossRef CAS.
- Z. Mahmoudi, et al., Promoted chondrogenesis of hMCSs with controlled release of TGF-β3 via microfluidics synthesized alginate nanogels, Carbohydr. Polym., 2020, 229, 115551 CrossRef CAS.
- K. Huang, et al., Small, Traceable, Endosome-Disrupting, and Bioresponsive Click Nanogels Fabricated via Microfluidics for CD44-Targeted Cytoplasmic Delivery of Therapeutic Proteins, ACS Appl. Mater. Interfaces, 2019, 11, 22171–22180 CrossRef CAS.
- F. S. Majedi, et al., Microfluidic assisted self-assembly of chitosan based nanoparticles as drug delivery agents, Lab Chip, 2013, 1, 204–207 RSC.
- E. Chiesa, et al., Staggered herringbone microfluid device for the manufacturing of chitosan/TPP nanoparticles: systematic optimization and preliminary biological evaluation, Int. J. Mol. Sci., 2019, 20, 1–22 Search PubMed.
- A. C. S. N. Pessoa, C. C. Sipoli and L. G. De La Torre, Effects of diffusion and mixing pattern on microfluidic-assisted synthesis of chitosan/ATP nanoparticles, Lab Chip, 2017, 17, 2281–2293 RSC.
- R. Baber, L. Mazzei, N. T. K. Thanh and A. Gavriilidis, An engineering approach to synthesis of gold and silver nanoparticles by controlling hydrodynamics and mixing based on a coaxial flow reactor, Nanoscale, 2017, 9, 14149–14161 RSC.
- M. Rhee, et al., Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels, Adv. Mater., 2011, 23, 79–83 CrossRef.
- R. Baber, L. Mazzei, N. T. K. Thanh and A. Gavriilidis, Synthesis of silver nanoparticles in a microfluidic coaxial flow reactor, RSC Adv., 2015, 5, 95585–95591 RSC.
- M. Takagi, T. Maki, M. Miyahara and K. Mae, Production of titania nanoparticles by using a new microreactor assembled with same axle dual pipe, Chem. Eng. J., 2004, 101, 269–276 CrossRef CAS.
- A. Abou Hassan, O. Sandre, V. Cabuil and P. Tabeling, Synthesis of iron oxide nanoparticles in a microfluidic device: preliminary results in a coaxial flow millichannel, Chem. Commun., 2008, 1783–1785, 10.1039/b719550h.
- J. M. Lim, et al., Ultra-high throughput synthesis of nanoparticles with homogeneous size distribution using a coaxial turbulent jet mixer, ACS Nano, 2014, 8, 6056–6065 CrossRef CAS.
- X. Li, M. Aghaamoo, S. Liu, D. H. Lee and A. P. Lee, Lipoplex-Mediated Single-Cell Transfection via Droplet Microfluidics, Small, 2018, 1802055 CrossRef.
- S. Kollipara, G. Bende, S. Movva and R. Saha, Application of rotatable central composite design in the preparation and optimization of poly(lactic-co-glycolic acid) nanoparticles for controlled delivery of paclitaxel, Drug Dev. Ind. Pharm., 2010, 36, 1377–1387 CrossRef CAS.
- G. D. Bowden, B. J. Pichler and A. Maurer, A Design of Experiments (DoE) Approach Accelerates the Optimization of Copper-Mediated 18F-Fluorination Reactions of Arylstannanes, Sci. Rep., 2019, 9, 1–10 CrossRef CAS.
- I. Dmour and M. O. Taha, Novel nanoparticles based on chitosan-dicarboxylate conjugates via tandem ionotropic/covalent crosslinking with tripolyphosphate and subsequent evaluation as drug delivery vehicles, Int. J. Pharm., 2017, 529, 15–31 CrossRef CAS.
- E. C. Harrington, The desirability function, Industrial Quality Control, 1965, vol. 21, pp. 494–498 Search PubMed.
- G. Derringer and R. Suich, Simultaneous optimization of several response variables, J. Qual. Technol., 1980, 12, 214–219 CrossRef.
- R. Ghaderi and J. Carlfors, Biological activity of lysozyme after entrapment in poly(d,l-lactide-co-glycolide)-microspheres, Pharm. Res., 1997, 14, 1556–1562 CrossRef CAS.
- J. Xie, W. J. Ng, L. Y. Lee and C. H. Wang, Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray, J. Colloid Interface Sci., 2008, 317, 469–476 CrossRef CAS.
- F. Pinelli, Ó. F. Ortolà, P. Makvandi, G. Perale and F. Rossi, In vivo drug delivery applications of nanogels: a review, Nanomedicine, 2020, 15, 2707–2727 CrossRef CAS.
- A. V. Kabanov and S. V. Vinogradov, Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities, Angew. Chem., Int. Ed. Engl., 2009, 48, 5418–5429 CrossRef CAS.
- A. Abou-Hassan, et al., Fluorescence confocal laser scanning microscopy for Ph mapping in a coaxial flow microreactor: application in the synthesis of superparamagnetic nanoparticles, J. Phys. Chem. C, 2009, 113, 18097–18105 CrossRef CAS.
- G. Soni and K. S. Yadav, Nanogels as potential nanomedicine carrier for treatment of cancer: a mini review of the state of the art, Saudi Pharm. J., 2016, 24, 133–139 CrossRef.
- M. Vicario-de-la-Torre and J. Forcada, The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy, Gels, 2017, 3, 16 CrossRef.
- E. N. Koukaras, S. A. Papadimitriou, D. N. Bikiaris and G. E. Froudakis, Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate, Mol. Pharm., 2012, 9, 2856–2862 CrossRef CAS.
- H. Zhang, M. Oh, C. Allen and E. Kumacheva, Monodisperse chitosan nanoparticles for mucosal drug delivery, Biomacromolecules, 2004, 5, 2461–2468 CrossRef CAS.
- H. Zhang, Y. Zhai, J. Wang and G. Zhai, New progress and prospects: the application of nanogel in drug delivery, Mater. Sci. Eng., C, 2016, 60, 560–568 CrossRef CAS.
- Q. Y. Deng, C. R. Zhou and B. H. Luo, Preparation and characterization of chitosan nanoparticles containing lysozyme, Pharm. Biol., 2006, 44, 336–342 CrossRef CAS.
- S. A. Loutfy, et al., Synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles: in vitro liver cancer model, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2016, 7, 035008 Search PubMed.
- L. Qi, Z. Xu, X. Jiang, C. Hu and X. Zou, Preparation and antibacterial activity of chitosan nanoparticles, Carbohydr. Res., 2004, 339, 2693–2700 CrossRef CAS.
- Y. Xu and Y. Du, Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles, Int. J. Pharm., 2003, 250, 215–226 CrossRef CAS.
- T. Wu, et al., Integration of lysozyme into chitosan nanoparticles for improving antibacterial activity, Carbohydr. Polym., 2017, 155, 192–200 CrossRef CAS.
- M. Najafi, J. Chery and M. M. Frey, Functionalized electrospun poly(vinyl alcohol) nanofibrous membranes with poly(methyl vinyl ether-alt-maleic anhydride) for protein adsorption, Materials, 2018, 11, 1002 CrossRef.
- F. Costa, S. Maia, J. Gomes, P. Gomes and M. C. L. Martins, Characterization of hLF1-11 immobilization onto chitosan ultrathin films, and its effects on antimicrobial activity, Acta Biomater., 2014, 10, 3513–3521 CrossRef CAS.
- H. Y. Atay, Antibacterial Activity of Chitosan-Based Systems, in Functional Chitosan, Springer, 2019, pp. 457–489 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na01051k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |