Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Au101–rGO nanocomposite: immobilization of phosphine-protected gold nanoclusters on reduced graphene oxide without aggregation†
Hanieh
Mousavi
 a,
Yanting
Yin
b,
Liam
Howard-Fabretto
b,
Shailendra Kumar
Sharma
a,
Yanting
Yin
b,
Liam
Howard-Fabretto
b,
Shailendra Kumar
Sharma
 c,
Vladimir
Golovko
c,
Vladimir
Golovko
 c,
Gunther G.
Andersson
c,
Gunther G.
Andersson
 b,
Cameron J.
Shearer
b,
Cameron J.
Shearer
 *a and
Gregory F.
Metha
*a and
Gregory F.
Metha
 *a
*a
aDepartment of Chemistry, University of Adelaide, Adelaide SA 5005, Australia. E-mail: cameron.shearer@adelaide.edu.au; greg.metha@adelaide.edu.au
bFlinders Centre for NanoScale Science and Technology, Flinders University, Adelaide SA 5001, Australia
cThe MacDiarmid Institute for Advanced Materials and Nanotechnology, School of Physical and Chemical Sciences, University of Canterbury, Christchurch 8140, New Zealand
First published on 7th January 2021
Abstract
Graphene supported transition metal clusters are of great interest for potential applications, such as catalysis, due to their unique properties. In this work, a simple approach to deposit Au101(PPh3)21Cl5 (Au101NC) on reduced graphene oxide (rGO) via an ex situ method is presented. Reduction of graphene oxide at native pH (pH ≈ 2) to rGO was performed under aqueous hydrothermal conditions. Decoration of rGO sheets with controlled content of 5 wt% Au was accomplished using only pre-synthesised Au101NC and rGO as precursors and methanol as solvent. High resolution scanning transmission electron microscopy indicated that the cluster size did not change upon deposition with an average diameter of 1.4 ± 0.4 nm. It was determined that the rGO reduction method was crucial to avoid agglomeration, with rGO reduced at pH ≈ 11 resulting in agglomeration. X-ray photoelectron spectroscopy was used to confirm the deposition of Au101NCs and show the presence of triphenyl phosphine ligands, which together with attenuated total reflectance Fourier transform infrared spectroscopy, advocates that the deposition of Au101NCs onto the surface of rGO was facilitated via non-covalent interactions with the phenyl groups of the ligands. Inductively coupled plasma mass spectrometry and thermogravimetric analysis were used to determine the gold loading and both agree with a gold loading of ca. 4.8–5 wt%. The presented simple and mild strategy demonstrates that good compatibility between size-specific phosphine protected gold clusters and rGO can prevent aggregation of the metal clusters. This work contributes towards producing an agglomeration-free synthesis of size-specific ligated gold clusters on rGO that could have wide range of applications.
1 Introduction
In recent decades, gold clusters (AuNCs) with sizes of less than 2 nm have been attracting increased attention due to their unique properties compared to larger gold nanoparticles and bulk gold.1,2 Size-specific clusters can offer high atomic efficiency due to high surface to-volume ratio with most atoms being at or close to the surface. Also, the strongly size-dependent electronic properties of ultra-small metal clusters offer the opportunity to tune their reactivity by choice of appropriate size and composition.3,4 Furthermore, such clusters are small enough to perform high level DFT calculations,5–7 which are crucial for comprehensive understanding of the catalytic mechanisms needed for the development of superior catalysts.8 Therefore, size-specific clusters are considered as a platform for investigation of catalysts at the atomic level.9 However, AuNCs need to be protected and stabilized with ligands during their synthesis to stop uncontrolled particle growth and aggregation.10 Triphenylphosphine (PPh3) ligands are often employed in the synthesis of phosphine-protected gold clusters as this approach allows fabrication of ultra-small clusters. Importantly, good control over the cluster metal core size can allow narrow particle size distributions (as in the case of Au101(PPh3)21Cl5), while many other clusters are synthesised with atomic precision (i.e. no size distribution with all species having exact same number of metal atoms as in the case of Au9(PPh3)8(NO3)3). In addition, the electronic structure and reactivity of the clusters could be improved due to electronic and steric factors imposed by the phosphine ligands.11 As a result, phosphine-protected gold clusters formulated as Aun(PPh3)m, such as Au9(PPh3)8(NO3)3 and Au101(PPh3)21Cl5, have been shown to exhibit catalytic activity in a wide range of reactions, such as hydrogenation of terminal alkynes into alkenes,12 CO oxidation,13,14 styrene oxidation to benzaldehyde15 and styrene epoxide,16 and benzyl alcohol oxidation.17,18 However, the size-specific catalytic activity of AuNCs has often been difficult to determine experimentally as clusters are prone to aggregation either during deposition or activation on the support,19,20 or deactivation of the catalyst following initial reaction.21 Therefore, methods for the fabrication of gold cluster-based catalysts resilient to aggregation are in high demand in nanomaterials,22 catalysis4,16,23,24 and sensing communities.25,26It has been shown that the successful deposition of AuNCs on surfaces depends on various factors, such as the type of support and ligand, synthetic conditions and cluster–support interactions.9 Therefore, understanding these factors is crucial in the design of deposition method.
It has been demonstrated that small metal nanoparticles (not size specific) such as Ag,27 Pd28 and Au,21,29,30 can be immobilized on reduced graphene oxide (rGO).31 rGO has a two-dimensional structure with extraordinary properties, such as high electrical conductivity, large surface area and high charge mobility.32 Moreover, rGO has the potential to be involved in non-covalent interactions with clusters through π–π stacking with the protecting organic ligands, such as PPh3.33
There are a few recent examples of nanocomposites containing small gold nanoparticles (not size specific) and rGO supports, yet most of the previously used synthesis methods involve the use of harsh reagents, such as ammonia34,35 and hydrazine hydrate.30 For these examples, the gold nanoparticles are formed in situ during nanocomposite synthesis. Furthermore, to the best of our knowledge, research on size-specific triphenylphosphine-protected AuNCs supported on rGO has not been reported.
Herein, we present a simple and rapid method to produce a nanocomposite based on pre-synthesised AuNCs and rGO without any additional pre-treatment of this support via an ex situ method at room temperature (RT). The reduction of GO occurs in the absence of ammonia in aqueous media; rGO is consequently washed and suspended in methanol. The PPh3 ligands act as tethers creating a strong interaction with rGO and enabling uniform distribution of Au101NCs on the surface of rGO without any aggregation even at the relatively high metal loading used here. The properties and formation mechanism of the Au101NCs–rGO nanocomposite are discussed.
2 Experimental section
2.1 Reagents and materials
All chemicals used as received throughout the study, unless otherwise stated: natural graphite flakes (Uley, Eyre Peninsula, South Australia), 98% sulfuric acid (H2SO4, RCI Labscan), 85% phosphoric acid (H3PO4, Chem-Supply), 70% nitric acid (HNO3, Chem-Supply), 32% hydrochloric acid (HCl, RCI Labscan), 30% hydrogen peroxide (H2O2, Chem-Supply), potassium permanganate (KMNO4, Merck), methanol (CH3OH, Merk, Analysis Grade), high-purity Milli-Q water (18.2 MΩ cm at 25 °C), 99% triphenylphosphine (PPh3, Merck), and gold single component standard ICP (TraceCERT, Merck, 999 mg L−1), 68 Component ICP-MS Standard (High Purity Standards, HPS, 10 μg mL−1).2.2 Preparation of graphene oxide (GO)
GO was prepared using the improved Hummers' method.36 Natural graphite flakes were ground and sieved to a particle size < 150 μm. A mixture of concentrated H2SO4 and H3PO4 with 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (by volume) was cooled down to 4 °C and then slowly poured into a mixture of graphite powder (3.0 g) and KMnO4 (18.0 g). The mixture was heated up to 50 °C and magnetically stirred for 12 h, followed by cooling down to room temperature. The mixture was poured onto ice (∼200 mL) in the presence of 30% H2O2 (3 mL). Subsequently, the resulting material was magnetically stirred for 2 h to ensure sufficient exfoliation to GO. The product was then washed multiple times with MilliQ water until pH ∼ 2 and centrifuged at 4000 rpm for 30 min (Sigma 2-16 P) to recover GO solid product during each wash before re-dispersing it in the fresh portion of MilliQ by sonication (Elmasonic P).
1 ratio (by volume) was cooled down to 4 °C and then slowly poured into a mixture of graphite powder (3.0 g) and KMnO4 (18.0 g). The mixture was heated up to 50 °C and magnetically stirred for 12 h, followed by cooling down to room temperature. The mixture was poured onto ice (∼200 mL) in the presence of 30% H2O2 (3 mL). Subsequently, the resulting material was magnetically stirred for 2 h to ensure sufficient exfoliation to GO. The product was then washed multiple times with MilliQ water until pH ∼ 2 and centrifuged at 4000 rpm for 30 min (Sigma 2-16 P) to recover GO solid product during each wash before re-dispersing it in the fresh portion of MilliQ by sonication (Elmasonic P).
2.3 Preparation of reduced graphene oxide (rGO)
Reduction of GO was carried out using the hydrothermal method at 190 °C for 12 h in a 500 mL Teflon-lined autoclave.37 The reduction suspensions were prepared using GO aqueous solution (8.3 mg mL−1) in MilliQ water. Due to the acidic process of graphite oxidation, this solution had a low pH ≈ 2 after diluting with water. The resultant black product was then collected by centrifugation at 4000 rpm and the supernatant liquid decanted away. The remaining material was first washed multiple times with MilliQ water (as described in 2.2 above) until neutral pH (Mettler-Toledo FG2 pH meter) of supernatant liquid was achieved. The obtained product was then washed three times with methanol in a similar manner. In order to obtain a stable suspension, the rGO was suspended in methanol (7.32 mg mL−1) via sonication in an ice-bath using probe sonication (Branson 450 Digital Sonifier, 400 W) for 10 minutes at 60% power to obtain a well-dispersed rGO suspension. Obtained suspensions were kept in the fridge and did not show any sign of flocculation for over a year. For comparison experiments, the pH of GO was adjusted employing ammonia to 11 and the resulting solution was hydrothermally treated and processed in the same manner described above.2.4 Preparation of Au101NC–rGO nanocomposite
The procedure for fabrication of rGO sheets with controlled 5 wt% Au loading of Au101NCs via ex situ method is schematically presented in Fig. 1.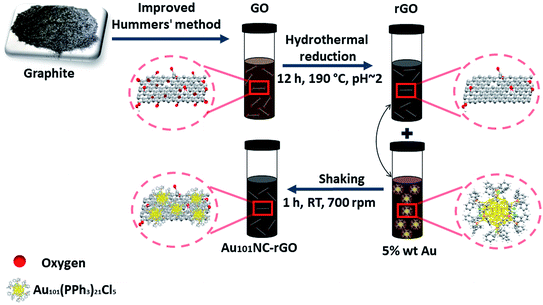 | ||
| Fig. 1 Schematic of synthesis of Au101NC–rGO showing oxidation of graphite to GO, hydrothermal reduction in acidic aqueous media to create rGO and mixing with Au101NC in methanol to form Au101NC–rGO. | ||
Au101(PPh3)21Cl5 was synthesized as reported by Hutchison and co-workers.38 5 mg of Au101(PPh3)21Cl5 was dispersed in 5 mL of methanol via bath sonication (Elmasonic P) for 10 min at RT to obtain a homogeneous dispersion. The obtained suspension was then wrapped immediately with aluminium foil to minimize the effect of light on the Au101NC and was kept in the fridge.
Typically, to make 5 mg Au101NC–rGO nanocomposite with 5 wt% Au loading in 1.5 mL methanol, 0.32 mL of the as-obtained Au101NC dispersion (which corresponds to 0.25 mg non-ligated or ∼0.32 mg ligated Au101) was added slowly dropwise to the magnetically stirred as-synthesized rGO dispersion (4.68 mg in 0.64 mL) at RT and made up to 1.5 mL with methanol. Then, it was wrapped immediately with aluminium foil to minimize the effect of light, followed by mixing using an orbital shaker (THERMOstar) for 1 h at RT at 700 rpm. Obtained product was kept in the fridge; the dispersion did not show signs of flocculation over six months.
2.5 Preparation of PPh3–rGO composite (control samples)
The procedure to make 5 mg PPh3–rGO composite in 1.5 mL methanol, containing the same quantity of PPh3 as the 5 wt% Au nanocomposites, is similar to the preparation of Au101NC–rGO nanocomposite described in Section 2.4. The only difference is that a solution of PPh3 (2.7 mg) in methanol (5 mL) was made. An aliquot (0.13 mL) of as-obtained PPh3 solution containing ∼0.07 mg PPh3 was added slowly dropwise to the as-synthesized rGO dispersion (4.93 mg in 0.673 mL) at RT and made up to 1.5 mL with methanol.2.6 Characterization
rGO, Au101NC and Au101NC–rGO nanocomposite were analysed by different characterization techniques including attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), transmission electron microscopy (TEM), scanning electron microscopy (SEM), high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), inductively coupled plasma mass spectrometry (ICP-MS), X-ray photoelectron spectroscopy (XPS), ultraviolet-visible absorption spectroscopy (UV-Vis) and thermogravimetric analysis (TGA). Detailed descriptions of the characterization protocols are given in the ESI.†3 Results and discussion
3.1 Characterization of rGO
rGO was prepared by hydrothermal reduction in aqueous solution (pH ∼2) and redispersed in methanol. Methanol was chosen as the solvent because it is compatible with a range of AuNCs including Au101NCs. Characterisation was completed to confirm reduction of GO and morphology of rGO flakes. GO and rGO display a set of distinctive spectroscopic characteristics which distinguish them. UV-vis absorption spectroscopy was employed to confirm the reduction of GO; the spectra of GO and rGO are shown in Fig. 2(a). The spectrum of GO shows a strong maximum at 232 nm, which can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C π–π* transitions, and a weak shoulder at 300 nm due to C
C–C π–π* transitions, and a weak shoulder at 300 nm due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O n–π* transitions.39 After the reduction of GO, the strong absorption peak at 232 nm shifts to 270 nm due to the restoration of the sp2 network, as commonly observed during GO reduction.40
O n–π* transitions.39 After the reduction of GO, the strong absorption peak at 232 nm shifts to 270 nm due to the restoration of the sp2 network, as commonly observed during GO reduction.40
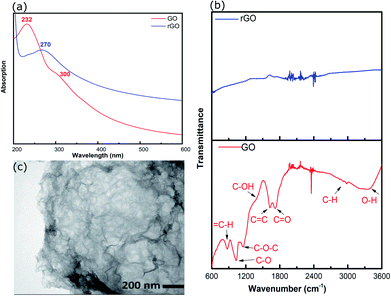 | ||
| Fig. 2 (a) UV-vis spectra of GO and rGO, (b) ATR-FTIR spectra of GO and rGO, and (c) TEM image of rGO. | ||
ATR-FTIR was applied to identify functional groups on GO and rGO and determine the degree of oxidation/reduction; the spectra of GO and rGO are presented in Fig. 2(b). The GO exhibited several characteristic absorption bands due to oxygen-containing functional groups. These peaks decreased dramatically in intensity, or even disappeared, due to the reduction of GO, which is consistent with previous reports.36,41,42
The morphology of rGO was investigated using TEM. The TEM image of rGO in Fig. 2(c) displays an ultrathin sheet with folds and scrolls at the edges and a variety of ripples and wrinkles on the surface.
3.2 Characterization of Au101NC
As will be seen later, it is important to fully characterise the gold cluster, including the PPh3 ligands. The ATR-FTIR spectra of Au101NC and PPh3 are shown in Fig. S3.† The absorption bands at 690, 740, 1000–1100, 1430 and 1475 cm−1 (pink boxes) contain the signatures of PPh3. The bands at 690, 1100, 1430, and 1475 cm−1 correspond to the C–C asymmetric stretch of phenyl groups attached directly to P (P–C(Ph)), and the peak at 740 cm−1 can be assigned to the CH(Ph) (out-of-plane) vibration.45 Other bands revealed at 1000, 1020 and 1070 cm−1 are attributed to ring breathing of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph) and CH(Ph) (in-plane) vibrations respectively.46 Overall, the spectra indicate the presence of PPh3 ligands attached to the Au101 core.
C(Ph) and CH(Ph) (in-plane) vibrations respectively.46 Overall, the spectra indicate the presence of PPh3 ligands attached to the Au101 core.
The TGA results (in N2) for the Au101NC and PPh3 are given in Fig. S4.† It is seen that PPh3 weight loss occurs between 110–245 °C, while the complete removal of PPh3 ligands from the Au101NC core occurs between 150 °C and 350 °C. This shift to higher temperature indicates that PPh3 is strongly attached to the gold cluster core. For Au101NC, the weight loss showed a percentage loss of 24.1%, which is close to the theoretical amount of 22.2% (loss of PPh3 and Cl), based on the chemical formula Au101(PPh3)21Cl5.38
The UV-vis absorption spectrum of Au101NC in methanol is shown in Fig. 3(a) (lower trace). It is clearly seen that there is no localised surface plasmon resonance (LSPR) absorption, indicating that the clusters are non-metallic and retain the small size of Au101NC, less than 2 nm.18 To compare with an agglomerated form of Au101NC, the dispersion was left under ambient conditions (including light) for 2 weeks and the UV-vis absorption was re-measured. As seen in the upper trace of Fig. 3(a), the appearance of a LSPR band at 533 nm indicates the formation of gold nanoparticles greater than 2 nm.18
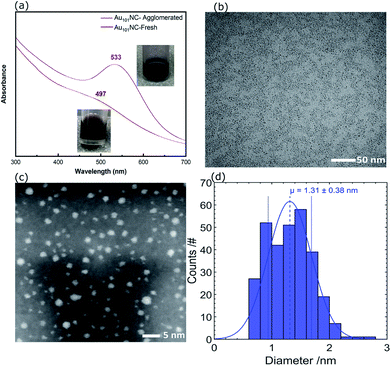 | ||
| Fig. 3 (a) UV-vis spectra of agglomerated and fresh Au101NC in methanol, (b) TEM image, (c) HAADF-STEM, and (d) size distribution histogram of Au101NC dropcast onto TEM grid from methanol solution. | ||
For further investigation, the size distribution of Au101NC dispersed in methanol was analysed by TEM; a representative image is shown in Fig. 3(b). The image shows a homogenous and narrow distribution of Au101NC. Additionally, high resolution HAADF-STEM image and size-distribution histogram, shown in Fig. 3(c) and (d), respectively, indicate an average diameter of 1.3 ± 0.4 nm. It is important to note that 26% of the clusters have diameters smaller than 1 nm and 3% larger than 2 nm, in agreement with previously reported values (1.5 ± 0.4 nm).38 This will be further addressed when discussing the XPS results (vide infra).
3.3 Characterization of Au101NC–rGO nanocomposite
The composite of Au101NC–rGO was prepared by gently mixing methanol suspensions of rGO and Au101NC in the dark at room temperature. This mild procedure was chosen to reduce the likelihood of AuNCs agglomerating and forming nanoparticles (>2 nm) during deposition. Characterization of the nanocomposite, Au101NC–rGO, was performed to determine structural features, morphology, composition, degree of agglomeration, and to provide an evaluation of the interactions between the AuNCs and rGO.Initial experiments were conducted using AuNCs deposited onto rGO produced by hydrothermal reduction subjected to ammonia (pH ∼11). This is the most common hydrothermal reduction method for GO.34,35 As can be seen in Fig. 4(a), this results in significant non-homogeneity and agglomeration of AuNCs. The gold particle size histogram (Fig. S5†) was found to have a log-normal distribution with mode of 2.57 nm (mean = 3.5 ± 2.6). Therefore, an investigation of the influence of pH (used during reduction of GO) on the gold particle size upon deposition of AuNCs was conducted. It was observed that Au101NCs are sensitive to aggregation at basic pH with Au101NC solutions turning blue (indicating agglomeration) upon addition of small amounts of dilute NaOH to Au101NC dispersion in water. In comparison, when adding a small amount of HCl to Au101NC in water we observed no colour change. We also determined that Au101NC solutions have a natural acidic pKa from dispersion in water. Consequently, the TEM image in Fig. 4(b) shows that when GO is reduced at low pH (∼2), deposition of Au101NC results in material with ultra-small gold particles with narrow particle size distribution decorating the surface of rGO. This simple change to the GO reduction procedure clearly results in a marked improvement in nanocomposite preparation. A full spectroscopic comparison of rGO (UV-Vis, FTIR, Raman, XPS) is shown in the ESI,† with the major difference being the presence of nitrogen in the rGO reduced in basic conditions. We believe the basic surface groups on rGO are causing the agglomeration of gold. All subsequent experiments focus on rGO produced at low pH.
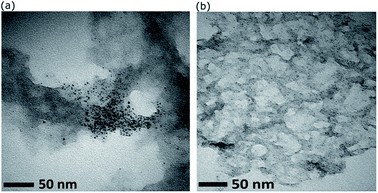 | ||
| Fig. 4 TEM images showing the effect of pH on size and distribution of Au101NC on rGO reduced hydrothermally at (a) pH ∼ 11, and (b) pH ∼ 2. | ||
Representative high resolution HAADF-STEM images for the as-prepared Au101NC–rGO nanocomposite (with rGO made at low pH) are presented in Fig. 5(a–d) and the size-distribution histogram is shown in Fig. 5(e). The Au101NCs have average diameter of 1.4 ± 0.4 nm, including about 8% of gold particles with diameter greater than 2 nm, indicating that the deposition procedure has not significantly changed the cluster size (compare to Fig. 3(c and d)). In comparison, earlier reports on Au101NCs deposited on supports such as TiO2,18 SiO2,18 WO3,47 and activated carbon48 mention gold particle sizes of 2.0–2.7, ∼3.6, ∼2.2, and ∼2.6 nm, respectively, indicating agglomeration of Au101NCs on these supports. In addition, HAADF-STEM elemental mapping images obtained using energy-dispersive X-ray spectroscopy (EDX) collected at each pixel in the image (Fig. 5(f)) confirms that the ∼1.4 nm sized features observed are composed of gold. STEM-EDX elemental mapping for P is more difficult to determine since the P Kα peak overlaps with the more intense Au Mα line. Nevertheless, it is possible to show that P is also co-located on the cluster cores, as shown in the STEM-EDX map and spectrum in Fig. S6.†
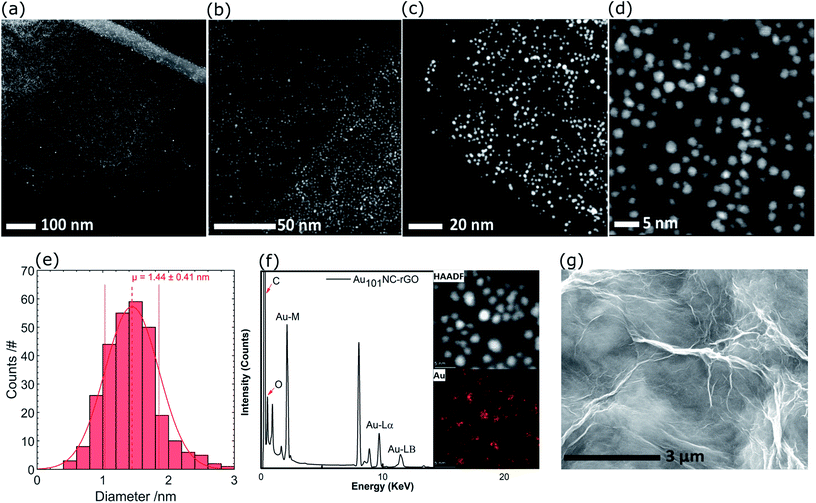 | ||
| Fig. 5 (a–d) HAADF-STEM images of Au101NC–rGO with different magnifications, (e) Au particle size distribution histogram, (f) HAADF and elemental mapping of Au, and (g) SEM image of Au101NC–rGO. | ||
The SEM of Au101NC–rGO nanocomposite shown in Fig. 5(g) is very similar to that seen for rGO (Fig. S7†). The similarity of the support morphology indicates that the Au101NC has integrated uniformly with the rGO to form the Au101NC–rGO nanocomposite without affecting the morphology of the rGO support.
ICP-MS of Au and P was used to determine the loading of AuNCs onto rGO. Instead of completing acid digestion of the Au101NC–rGO nanocomposite (with concomitant matrix dissolution problems), the residual solvent (i.e. after forming the nanocomposite) was analysed, which is a commonly used method.49 This yielded the amount of Au and P not adsorbed to rGO, which is then used to calculate a wt% loading. 99.3 ± 0.5% of Au was adsorbed while 85.2 ± 4.0% of P was adsorbed. This equates to an Au wt% loading of 4.9 wt% (Table 1).
| Characterization | % Au adsorbed | % P adsorbed | Au loading (wt%) |
|---|---|---|---|
| ICP-MS | 99.3 ± 0.5 | 85.2 ± 4.0 | 4.9 ± 0.5 |
| TGA | 94.0 ± 0.1 | — | 4.7 ± 0.1 |
Interestingly, the higher residual% P than % Au in the solvent after Au101NCs deposition, corresponds to approximately 4 extra PPh3 ligands remaining in solution per Au cluster, indicating that there are ∼17 PPh3 ligands which could be still attached to each Au101NC on rGO (P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au of 0.17).
Au of 0.17).
To evaluate the interaction of Au101NC with the rGO surface, a combined study using XPS, ATR-FTIR, TGA, and UV-vis was employed.
Samples of Au101NC, rGO and Au101NC–rGO nanocomposite were investigated by XPS to determine the elemental composition and chemical environment. Based on our previous XPS studies of Au clusters on various supports,19 the degree of agglomeration of Au101NCs to bulk-like large nanoparticles (i.e. >2 nm) can also be estimated. The XPS survey spectrum of rGO (Fig. 6(a)-lower trace) revealed two main peaks, C 1s and O 1s. The XPS survey spectrum of the Au101NC–rGO nanocomposite, Fig. 6(a)-upper trace, shows peaks due to Au 4f as well as C 1s and O 1s.
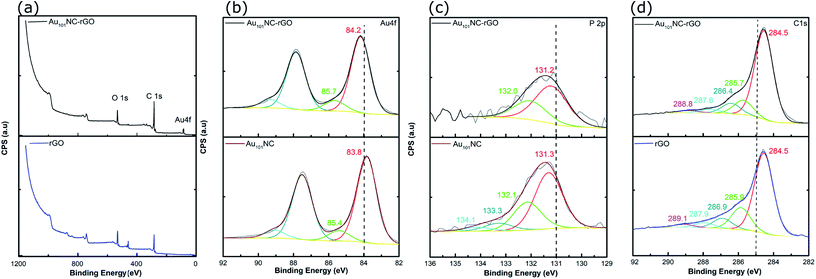 | ||
| Fig. 6 (a) Overview of XPS spectra of rGO and Au101NC–rGO (b) Au 4f spectra of Au101NC and Au101NC–rGO, (c) P 2p spectra of Au101NC and Au101NC–rGO, and (d) C 1s spectra of rGO and Au101NC–rGO. | ||
Within the Au 4f region of Au101NC in Fig. 6(b)-lower trace, there are two sets of doublets, due to the 4f7/2 and 4f5/2 spin–orbit components that are separated by 3.7 eV. The Au 4f7/2 component shows a major peak at 84.2 ± 0.54 eV, and a minor peak at 85.7 eV ± 0.58 eV. We have previously referred to these peaks as the low binding peak (LBP) and high binding peak (HBP), respectively; the former is specifically due to Au101NC and the latter is due to smaller clusters perhaps even Au1 (a by-product of the cluster synthesis).19 The presence of smaller clusters is also seen in the images and histogram from the HAADF-STEM, shown in Fig. 3(d). It is not clear if these smaller clusters are present in the prepared Au101NC or whether they occur upon dissolution in the methanol. The Au 4f region of the Au101NC–rGO nanocomposite, Fig. 6(b)-upper trace, has a similar set of features to that observed for pure, unsupported Au101NCs; interestingly both peaks show a ∼0.4 eV shift towards higher binding energy upon deposition on rGO. It is also evident that the smaller Au clusters are present in the nanocomposite in approximately the same % of the total gold population (11.1% and 12.9%, respectively, see Table S1†).
Analysis of the P 2p region for the pure, unsupported cluster, Au101NC, (Fig. 6(c)-lower trace), shows a broad peak which was fit to two sets of doublets, due to the 2p3/2 and 2p1/2 spin–orbit components that are separated by 0.87 eV. The P 2p3/2 component shows a major peak at 131.3 ± 0.55 eV, due to triphenylphosphine bound to the gold cluster core, and a minor peak at 133.3 eV, which is possibly a trace amount of triphenylphosphine oxide. The P 2p region of the Au101NC–rGO nanocomposite (upper trace) shows only a single doublet with P 2p3/2 at 131.2 ± 0.64 eV, indicating that the triphenylphosphine ligands remain attached to the Au cluster after deposition onto rGO.38,50 The P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au atomic ratio is 0.26 ± 0.04 for Au101NC and 0.25 ± 0.04 for Au101NC–rGO, further indicating that the number of ligands remaining attached to the gold cluster gold is relatively unchanged upon deposition onto rGO (theoretical value based on the formulae Au101(PPh3)21Cl5 is 0.21). This is similar to the ICPMS results (vide supra), which showed a P
Au atomic ratio is 0.26 ± 0.04 for Au101NC and 0.25 ± 0.04 for Au101NC–rGO, further indicating that the number of ligands remaining attached to the gold cluster gold is relatively unchanged upon deposition onto rGO (theoretical value based on the formulae Au101(PPh3)21Cl5 is 0.21). This is similar to the ICPMS results (vide supra), which showed a P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio of 0.17.
Au ratio of 0.17.
The C 1s region of rGO and Au101NC–rGO nanocomposite can be used to determine the chemical nature of carbon in the system. The spectrum of rGO, Fig. 6(d)-lower trace, shows five peaks centred at 284.5, 285.9, 286.9, 287.9 and 289.1 eV, which can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C (sp2), C–C (sp3), C–OH, C–O–C/C
C–C (sp2), C–C (sp3), C–OH, C–O–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.43,44 In the Au101NC–rGO nanocomposite (upper trace), the same five components are observed, indicating that the relative abundance of the different carbon groups in rGO and Au101NC–rGO are very similar. The fraction of each functional group present in rGO, Au101NC, and Au101NC–rGO nanocomposite, and the analysis from deconvolution of C 1s, O 1s, Au 4f and P 2p spectra are shown in Tables S1 and S2.†
O, respectively.43,44 In the Au101NC–rGO nanocomposite (upper trace), the same five components are observed, indicating that the relative abundance of the different carbon groups in rGO and Au101NC–rGO are very similar. The fraction of each functional group present in rGO, Au101NC, and Au101NC–rGO nanocomposite, and the analysis from deconvolution of C 1s, O 1s, Au 4f and P 2p spectra are shown in Tables S1 and S2.†
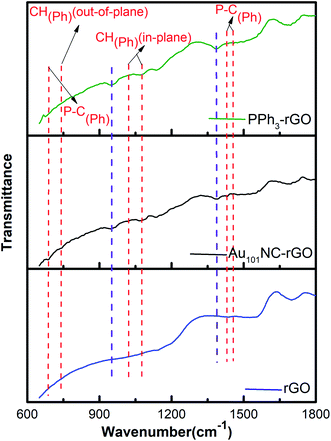
ATR-FTIR study was also undertaken to monitor any functional group changes upon interaction of Au101NCs with the rGO surface. The ATR-FTIR spectra of rGO, Au101NC–rGO, and PPh3–rGO are presented in Fig. 7(a). The red dashed lines indicate features arising from the PPh3 ligands. The major PPh3 features remain in the Au101NC–rGO although they have diminished in intensity compared to the spectra of pure PPh3 and Au101NC (Fig. S3†) due to the low ligand content of the composite (<1%). The observation of phenyl peaks confirms the presence of ligands from Au101NC on the surface of rGO, which supports assignment to PPh3 of the P 2p peak observed in XP spectrum. Furthermore, the Au101NC–rGO spectrum reveals new, albeit weak, peaks at ∼1390 and ∼950 cm−1 (purple dashed lines). These new absorption peaks are difficult to assign with confidence, but the peak at 950 cm−1 is in the general region for C–O–C(aryl) and P–O–C(aryl).51 They are not observed in Au101NC nor rGO but are seen in the spectrum of PPh3–rGO and therefore potentially arise from an interaction of the PPh3 ligands with rGO.
The UV-vis spectra of PPh3, PPh3–rGO and Au101NC–rGO are presented in Fig. 8(a). The spectrum of PPh3 (inset) exhibits two peaks at 227 and 260 nm, which are assigned to the n–σ* transition from P atoms and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C π–π* transition in the aromatic ring, respectively.52 As discussed above, the rGO spectrum has a strong absorption peak centred at 270 nm (Fig. 2(a)). When combined (i.e. PPh3–rGO), the features merge to form a shoulder at 230 nm and a narrower peak at 270 nm. The position shift of the PPh3 related peaks could be due to the π–π interaction between the ligand and rGO.52 The spectrum of Au101NC–rGO shows similar absorption bands to PPh3–rGO, although the peak at 270 nm is more intense but less distinct. Again, this suggests the formation of non-covalent interactions between the PPh3 ligands and rGO in the Au101NC–rGO nanocomposite. The lack of an LSPR peak near 520 nm in Au101NC–rGO confirms that there is no agglomeration of AuNCs deposited onto rGO made under low pH conditions, as seen in the HAADF-STEM images. Stability of the clusters was tested by measuring the UV-vis absorbance after 1 month (stored in dark, −10 °C) and no LSPR was observed (Fig. S10†), in comparison a sample deliberately agglomerated showed a clear LSPR at 550 nm.
C π–π* transition in the aromatic ring, respectively.52 As discussed above, the rGO spectrum has a strong absorption peak centred at 270 nm (Fig. 2(a)). When combined (i.e. PPh3–rGO), the features merge to form a shoulder at 230 nm and a narrower peak at 270 nm. The position shift of the PPh3 related peaks could be due to the π–π interaction between the ligand and rGO.52 The spectrum of Au101NC–rGO shows similar absorption bands to PPh3–rGO, although the peak at 270 nm is more intense but less distinct. Again, this suggests the formation of non-covalent interactions between the PPh3 ligands and rGO in the Au101NC–rGO nanocomposite. The lack of an LSPR peak near 520 nm in Au101NC–rGO confirms that there is no agglomeration of AuNCs deposited onto rGO made under low pH conditions, as seen in the HAADF-STEM images. Stability of the clusters was tested by measuring the UV-vis absorbance after 1 month (stored in dark, −10 °C) and no LSPR was observed (Fig. S10†), in comparison a sample deliberately agglomerated showed a clear LSPR at 550 nm.
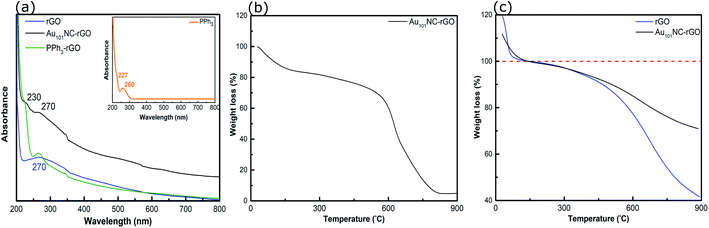 | ||
| Fig. 8 (a) UV-vis spectra of rGO, PPh3–rGO and Au101NC–rGO, (b) TGA curve of Au101NC–rGO (in air), and (c) TGA curves of rGO and Au101NC–rGO (in N2). Insert in (a) shows the UV-vis spectrum of PPh3. | ||
The TGA of Au101NC–rGO nanocomposite (in air), shown in Fig. 8(b), was conducted to determine the mass loading of Au from the residual weight. The residual mass was 4.7 w% which we assign to the mass of Au in the sample. The mass loading is similar to the desired loading (5 wt%) and the loading determined from ICP-MS (4.9 wt%), Table 1.
TGA traces for rGO and the Au101NC–rGO nanocomposite, obtained under N2 flow, are presented in Fig. 8(c). Both samples display a rapid, initial mass loss up to 150 °C due to solvent adsorbed between the rGO sheets, which complicates comparison. Therefore, the % weight loss is set to 100% at this temperature. Both samples exhibit weight loss with two slopes (after solvent loss). The first weight loss occurs due to removal of PPh3 ligands on Au101NC–rGO (Fig. S4†), and labile functional groups on the rGO. The second weight loss occurs due to the loss of other functional groups from the rGO structure. At 900 °C, the overall weight loss for rGO is 58% (i.e. 42% remaining) and for the Au101NC–rGO nanocomposite is 29 ± 5% (i.e. 71 ± 5% remaining). The extra mass remaining in the Au101NC–rGO exceeds the mass of Au in the sample (5%). Therefore, it appears that the presence of Au101NC prevents the loss of some rGO functional groups. This again supports our assumption of a non-covalent interaction between the phenyl groups of the PPh3 and the rGO support to stabilise the composite.
The combination of information from the characterisation techniques applied give insight on the interaction between Au101NC and rGO. Greater than 99% of the pre-synthesised AuNCs were incorporated into the rGO composite (ICPMS) at a relatively high loading of 5 wt% Au. The deposition proceeded quickly under mild deposition conditions which indicates that there is a high affinity between rGO and the Au101NC. XPS and ATR-FTIR show that the PPh3 ligands remain within the composite after attachment and are therefore most likely responsible for the interaction. With each Au101NC containing ca. 17 PPh3 ligands remaining (based on the ICP-MS estimate) there are 51 phenyl groups decorating each gold cluster. Therefore, π–π stacking and hydrophobic non-covalent interactions between ligands and graphitic structures within rGO are expected to be driving the formation of the nanocomposite. There is a large body of literature also supporting this interaction between aryl ligands such as PPh3 and sp2 hybridised carbon such as rGO.53,54
We anticipate other rGO reduction methods (thermal reduction, chemical, electrochemical) may also be amenable to forming composites with PPh3 ligated metal clusters although our findings suggest some guidelines. Any method which uses a high pH or may impart basic functional groups should be avoided as we have observed this will lead to agglomeration of the clusters (Fig. 4). Reduction process should be chosen such that the rGO is highly soluble (to aid in dispersion and reduce stacking) in a good solvent for the cluster and any further processing step (e.g. high boiling point and high volatility solvents should be avoided due to difficult processing). Finally, rGO flake size (width and stacking), and degree of reduction should be optimised for any final application in which conductivity, transparency, and dispersion are expected to have an effect.
The simple and mild strategy presented here demonstrates an excellent compatibility between size-specific triphenylphosphine protected gold clusters and rGO which can prevent aggregation of the metal clusters. We expect that this procedure can be applied to any PPh3 ligated cluster such as Au11(PPh3)8Cl3,55 Au9(PPh3)8(NO3)3,56 and Au8(PPh3)8(NO3)2 (ref. 19) as well as triphenylphosphine stabilised clusters of other metals on rGO in order to form agglomeration-resistant cluster–rGO composites, which could have diverse applications.
4 Conclusion
In summary, a method was developed for the deposition of the triphenylphosphine-protected Au101NCs onto rGO. The results from a wide range of characterization techniques verify the agglomeration-free loading of Au101NC with narrow particle size distribution (1.4 ± 0.4 nm) onto the rGO sheets. This has been achieved by a simple modification to the hydrothermal reduction of GO to use aqueous medium at low pH. We hypothesise that the PPh3 ligands play an important role in formation composite acting as a non-covalent linking agent resulting in uniform deposition of Au101NCs without aggregation.The proposed methodology provides an easy and convenient avenue towards the preparation of rGO-based nanocomposites with other size-specific phosphine-ligated metal clusters.
Conflicts of interest
The authors have no conflicts to declare.Acknowledgements
This work was supported by funding from The Australian Solar Thermal Research Initiative (ASTRI) program, which, is supported by the Australian Government, through the Australian Renewable Energy Agency (ARENA). HM wishes to acknowledge the University of Adelaide for the awarding of a postgraduate scholarship. The authors acknowledge the facilities of Microscopy Australia under the National Collaborative Research Infrastructure Strategy, at the South Australian Regional Facility, Adelaide Microscopy (University of Adelaide) and Flinders Microscopy and Microanalysis, (Flinders University). In particular, for the Raman measurements, we acknowledge the scientific and technical assistance of Dr Christopher Gibson. We thank Dr Ashley Slattery for STEM analysis. We also acknowledge Dr D.J. Osborn for the analysis of the EDX mapping images. CJS acknowledges funding support from both The University of Adelaide Beacon Research Fellowship (2018–2019) and The Australian Research Council (ARC) for a Future Fellowship (FT190100854).References
- D. A. Pichugina, N. E. Kuz'menko and A. F. Shestakov, Russ. Chem. Rev., 2015, 84, 1114–1144 CrossRef CAS
.
- M. Zhou, A. Zhang, Z. Dai, Y. P. Feng and C. Zhang, J. Phys. Chem. C, 2010, 114, 16541–16546 CrossRef CAS
.
- A. B. Hungria, R. Raja, R. D. Adams, B. Captain, J. M. Thomas, P. A. Midgley, V. Golovko and B. F. G. Johnson, Angew. Chem., 2006, 45, 4782–4785 CrossRef CAS
.
- B. G. Donoeva, D. S. Ovoshchnikov and V. B. Golovko, ACS Catal., 2013, 3, 2986–2991 CrossRef CAS
.
- J. F. Alvino, T. Bennett, D. Anderson, B. Donoeva, D. Ovoshchnikov, R. H. Adnan, D. Appadoo, V. Golovko, G. Andersson and G. F. Metha, RSC Adv., 2013, 3, 22140–22149 RSC
.
- T. Bennett, R. H. Adnan, J. F. Alvino, V. Golovko, G. G. Andersson and G. F. Metha, Inorg. Chem., 2014, 53, 4340–4349 CrossRef CAS
.
- R. White, T. Bennett, V. Golovko, G. G. Andersson and G. F. Metha, ChemistrySelect, 2016, 1, 1163–1167 CrossRef CAS
.
- P. Poldorn, Y. Wongnongwa, S. Namuangruk, N. Kungwan, V. B. Golovko, B. Inceesungvorn and S. Jungsuttiwong, Appl. Catal., A, 2020, 595, 117505 CrossRef CAS
.
- A. Halder, L. A. Curtiss, A. Fortunelli and S. Vajda, J. Chem. Phys., 2018, 148, 110901 CrossRef
.
- M. N. Golosnaya, D. A. Pichugina and N. E. Kuz’menko, Struct. Chem., 2019, 30, 501–507 CrossRef CAS
.
- J. C. Burgos, S. M. Mejía and G. F. Metha, ACS Omega, 2019, 4, 9169–9180 CrossRef CAS
.
- G. Li and R. Jin, J. Am. Chem. Soc., 2014, 136, 11347–11354 CrossRef CAS
.
- O. Lopez-Acevedo, K. A. Kacprzak, J. Akola and H. Häkkinen, Nat. Chem., 2010, 2, 329–334 CrossRef CAS
.
- W. Liu, Y. F. Zhu and Q. Jiang, J. Phys. Chem. C, 2010, 114, 21094–21099 CrossRef CAS
.
- Y. Pei, N. Shao, Y. Gao and X. C. Zeng, ACS Nano, 2010, 4, 2009–2020 CrossRef CAS
.
- M. Turner, V. B. Golovko, O. P. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. Johnson and R. M. Lambert, Nature, 2008, 454, 981–983 CrossRef CAS
.
- J. Kilmartin, R. Sarip, R. Grau-Crespo, D. Di Tommaso, G. Hogarth, C. Prestipino and G. Sankar, ACS Catal., 2012, 2, 957–963 CrossRef CAS
.
- R. H. Adnan, G. G. Andersson, M. I. Polson, G. F. Metha and V. B. Golovko, Catal. Sci. Technol., 2015, 5, 1323–1333 RSC
.
- D. P. Anderson, J. F. Alvino, A. Gentleman, H. A. Qahtani, L. Thomsen, M. I. J. Polson, G. F. Metha, V. B. Golovko and G. G. Andersson, Phys. Chem. Chem. Phys., 2013, 15, 3917–3929 RSC
.
- J.-Y. Ruzicka, F. Abu Bakar, C. Hoeck, R. Adnan, C. McNicoll, T. Kemmitt, B. C. Cowie, G. F. Metha, G. G. Andersson and V. B. Golovko, J. Phys. Chem. C, 2015, 119, 24465–24474 CrossRef CAS
.
- C. Wang, N. Li, Q. Wang and Z. Tang, Nanoscale Res. Lett., 2016, 11, 336 CrossRef
.
- M. F. Lynam, N.-J. Ke, S. J. Bradley, T. Nann, A. Neiman, R. J. Reeves, A. J. Downard, V. B. Golovko and M. W. Allen, APL Mater., 2019, 7, 022518 CrossRef
.
- T. Tsukuda, Bull. Chem. Soc. Jpn., 2012, 85, 151–168 CrossRef CAS
.
- Y. Liu, H. Tsunoyama, T. Akita, S. Xie and T. Tsukuda, ACS Catal., 2011, 1, 2–6 CrossRef CAS
.
- M. Z. Ahmad, V. B. Golovko, R. H. Adnan, F. Abu Bakar, J.-Y. Ruzicka, D. P. Anderson, G. G. Andersson and W. Wlodarski, Int. J. Hydrogen Energy, 2013, 38, 12865–12877 CrossRef CAS
.
- M. Z. Ahmad, A. Z. Sadek, M. H. Yaacob, D. P. Anderson, G. Matthews, V. B. Golovko and W. Wlodarski, Sens. Actuators, B, 2013, 179, 125–130 CrossRef CAS
.
- G. Liu, Y. Wang, X. Pu, Y. Jiang, L. Cheng and Z. Jiao, Appl. Surf. Sci., 2015, 349, 570–575 CrossRef CAS
.
- G. Darabdhara, M. A. Amin, G. A. M. Mersal, E. M. Ahmed, M. R. Das, M. B. Zakaria, V. Malgras, S. M. Alshehri, Y. Yamauchi, S. Szunerits and R. Boukherroub, J. Mater. Chem. A, 2015, 3, 20254–20266 RSC
.
- Z. Song, W. Li, F. Niu, Y. Xu, L. Niu, W. Yang, Y. Wang and J. Liu, J. Mater. Chem. A, 2017, 5, 230–239 RSC
.
- H. Yin, H. Tang, D. Wang, Y. Gao and Z. Tang, ACS Nano, 2012, 6, 8288–8297 CrossRef CAS
.
- R. M. Del Castillo and L. E. Sansores, Eur. Phys. J. B, 2015, 88, 248 CrossRef
.
- M. Hu, Z. Yao and X. Wang, Ind. Eng. Chem. Res., 2017, 56, 3477–3502 CrossRef CAS
.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Chem. Rev., 2016, 116, 5464–5519 CrossRef CAS
.
- Y. Zhou, Q. Bao, L. A. L. Tang, Y. Zhong and K. P. Loh, Chem. Mater., 2009, 21, 2950–2956 CrossRef CAS
.
- C. Bosch-Navarro, E. Coronado, C. Martí-Gastaldo, J. F. Sánchez-Royo and M. G. Gómez, Nanoscale, 2012, 4, 3977–3982 RSC
.
- M. Aunkor, I. Mahbubul, R. Saidur and H. Metselaar, RSC Adv., 2016, 6, 27807–27828 RSC
.
- W. I. Hayes, P. Joseph, M. Z. Mughal and P. Papakonstantinou, J. Solid State Electrochem., 2015, 19, 361–380 CrossRef CAS
.
- W. W. Weare, S. M. Reed, M. G. Warner and J. E. Hutchison, J. Am. Chem. Soc., 2000, 122, 12890–12891 CrossRef CAS
.
- J. N. Ding, Y. B. Liu, N. Y. Yuan, G. Q. Ding, Y. Fan and C. T. Yu, Diamond Relat. Mater., 2012, 21, 11–15 CrossRef CAS
.
- A. T. Habte and D. W. Ayele, Adv. Mater. Sci. Eng., 2019, 2019, 9 Search PubMed
.
- D. Konios, M. M. Stylianakis, E. Stratakis and E. Kymakis, J. Colloid Interface Sci., 2014, 430, 108–112 CrossRef CAS
.
- H.-L. Guo, X.-F. Wang, Q.-Y. Qian, F.-B. Wang and X.-H. Xia, ACS Nano, 2009, 3, 2653–2659 CrossRef CAS
.
- M. T. H. Aunkor, I. M. Mahbubul, R. Saidur and H. S. C. Metselaar, RSC Adv., 2016, 6, 27807–27828 RSC
.
- A. Ellis, A. Al-deen, H. Dalal and G. Andersson, J. Phys. Chem. C, 2013, 117, 21312–21319 CrossRef CAS
.
- H. G. M. Edwards, I. R. Lewis and P. H. Turner, Inorg. Chim. Acta, 1994, 216, 191–199 CrossRef CAS
.
- R. Faggiani, H. E. Howard-Lock, C. J. L. Lock and M. A. Turner, Can. J. Chem., 1987, 65, 1568–1575 CrossRef CAS
.
- D. S. Ovoshchnikov, B. G. Donoeva, B. E. Williamson and V. B. Golovko, Catal. Sci. Technol., 2014, 4, 752–757 RSC
.
- S. Ghadamgahi, B. E. Williamson and V. B. Golovko, Catal. Lett., 2016, 146, 1027–1032 CrossRef CAS
.
- W. Kurashige, Y. Mori, S. Ozaki, M. Kawachi, S. Hossain, T. Kawawaki, C. J. Shearer, A. Iwase, G. F. Metha, S. Yamazoe, A. Kudo and Y. Negishi, Angew. Chem., 2020, 59, 7076–7082 CrossRef CAS
.
- P. A. Grutsch, M. V. Zeller and T. P. Fehlner, Inorg. Chem., 1973, 12, 1431–1433 CrossRef CAS
.
-
D. R. Lide, CRC Handbook of Chemistry and Physics, Taylor & Francis, 84th edn, 2003 Search PubMed
.
- S. Bouchareb, R. Doufnoune, F. Riahi, H. Cherif-Silini and L. Belbahri, Mater. Chem. Phys., 2020, 243, 122598 CrossRef CAS
.
- C. J. Shearer, A. Cherevan and D. Eder, Adv. Mater., 2014, 26, 2295–2318 CrossRef CAS
.
- Y. Mu, H. Liang, J. Hu, L. Jiang and L. Wan, J. Phys. Chem. B, 2005, 109, 22212–22216 CrossRef CAS
.
- L. Wang, J. Peng, Z. Tang, X. Kang, M. Fu and S. Chen, Appl. Catal., A, 2018, 557, 1–6 CrossRef CAS
.
- H. S. Al Qahtani, G. F. Metha, R. B. Walsh, V. B. Golovko, G. G. Andersson and T. Nakayama, J. Phys. Chem. C, 2017, 121, 10781–10789 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00927j |
| This journal is © The Royal Society of Chemistry 2021 |