Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isolation and characterization of extracellular vesicles for clinical applications in cancer – time for standardization?
Nikki
Salmond
 and
Karla C.
Williams
and
Karla C.
Williams
 *
*
University of British Columbia, Faculty of Pharmaceutical Sciences, Vancouver, V6T 1Z3, Canada. E-mail: karla.williams@ubc.ca
First published on 16th February 2021
Abstract
Extracellular vesicles (EVs) are nanometer sized lipid enclosed particles released by all cell types into the extracellular space and biological fluids in vivo, and into cell culture media in vitro. An important physiological role of EVs is cell–cell communication. EVs interact with, and deliver, their contents to recipient cells in a functional capacity; this makes EVs desirable vehicles for the delivery of therapeutic cargoes. In addition, as EVs contain proteins, lipids, glycans, and nucleic acids that reflect their cell of origin, their potential utility in disease diagnosis and prognostication is of great interest. The number of published studies analyzing EVs and their contents in the pre-clinical and clinical setting is rapidly expanding. However, there is little standardization as to what techniques should be used to isolate, purify and characterize EVs. Here we provide a comprehensive literature review encompassing the use of EVs as diagnostic and prognostic biomarkers in cancer. We also detail their use as therapeutic delivery vehicles to treat cancer in pre-clinical and clinical settings and assess the EV isolation and characterization strategies currently being employed. Our report details diverse isolation strategies which are often dependent upon multiple factors such as biofluid type, sample volume, and desired purity of EVs. As isolation strategies vary greatly between studies, thorough EV characterization would be of great importance. However, to date, EV characterization in pre-clinical and clinical studies is not consistently or routinely adhered to. Standardization of EV characterization so that all studies image EVs, quantitate protein concentration, identify the presence of EV protein markers and contaminants, and measure EV particle size and concentration is suggested. Additionally, the use of RNase, DNase and protease EV membrane protection control experiments is recommended to ensure that the cargo being investigated is truly EV associated. Overall, diverse methodology for EV isolation is advantageous as it can support different sample types and volumes. Nevertheless, EV characterization is crucial and should be performed in a rigorous manor.
Introduction to extracellular vesicles
EV biogenesis
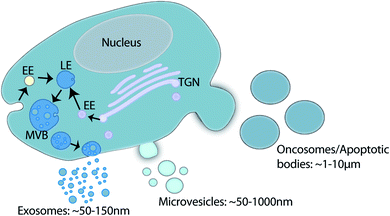
Extracellular vesicles (EVs) are lipid membrane enclosed nano-sized particles released into the extracellular environment and biological fluids by virtually every cell line and cell type. The term EV is used to encompass a large family of vesicles including, exosomes, microvesicles, oncosomes and apoptotic bodies.1 Exosome (∼30–150 nm) biogenesis occurs within the late endosome through inward membrane budding and fission which generates intraluminal vesicles and creates a structure classically described as the multivesicular body (MVB). Fusion of the MVB at the plasma membrane releases intraluminal vesicles into the extracellular environment as exosomes.1–3 Microvesicle (∼50–1000 nm), oncosome (∼1–10 μm), and apoptotic body (∼1–5 μm) biogenesis occurs via direct membrane budding and fission at the plasma membrane.1,4 Oncosome biogenesis has been associated with cancer cells,5–7 and apoptotic bodies are released by cells undergoing apoptosis (Fig. 1).8,9EV content and function
EVs contain ribonucleic acid (RNA),10–13 deoxyribonucleic acid (DNA),14,15 proteins,6,16 lipids,17–20 metabolites21,22 and glycans23–25 from the cell of origin. Although originally thought to be trash containers for cellular waste removal,26 work over the last decade has highlighted the diverse and important functions of EVs in cell–cell communication, maintaining homeostasis, and in multiple pathological conditions including cancer.27,28 After release into the extracellular space, or into biological fluids (i.e. blood, urine, saliva, breast milk),29–31 EVs are taken up by recipient cells and deliver their functional protein and nucleic acid contents to alter the recipient cell phenotype.27 This exchange of information between cells not only occurs locally between neighboring cells, but also with cells at distant sites of the body after transport in biological fluids such as blood.32–36 EVs have a well-established role in physiological processes such as coordinating the immune response,37 coagulation38 and angiogenesis.39 Just as EVs are effective in delivering cargo to recipient cells to support healthy homeostasis, EVs from diseased cells also deliver their contents to recipient cells promoting multifarious phenotypic changes locally and regionally at distant organs. EVs have been found to have important roles in neurodegenerative,40 cardiovascular,41 and inflammatory42 disease states amongst many.EV function in cancer
A rapidly expanding field of EV research is that of EVs in cancer biology. EVs released by cancer cells have demonstrated roles in all stages of cancer progression and metastasis. However, as the focus of this review is on EV developments in the clinical setting we will only briefly describe some highlights on EV functions in cancer and direct readers to comprehensive reviews by others detailing EV-based cell-to-cell communication,28,43,44 immune modulation,45–48 drug resistance,49 and the pre-metastatic niche in cancer.33The first report suggesting a role for EVs in neoplastic cell function was published in 1981.50 Following this, some of the first discoveries on EV function in cancer identified immunomodulatory roles for EVs. In 1998, a pivotal study by Zitvogel, L. et al. isolated tumor antigen-presenting exosomes from dendritic cells pulsed with tumor antigens and demonstrated their immunostimulatory effect which decreased tumor growth, and in some instances even resulted in tumor eradication.51 Furthermore, it was shown that tumor-derived EVs transfer tumor antigens to dendritic cells and stimulate antitumor effects.52 These early studies introduced the field to the possibility that EVs could be used in a therapeutic capacity for cancer treatment. Whilst the immunostimulatory properties of EVs can increase tumor recognition, studies focused on cancer cell derived EVs have also detailed potent immunosuppression of T-cells,53,54 Natural Killer cells,55 promotion of myeloid suppressor cell differentiation56 and pro-tumorigenic differentiation of macrophages.57 Taken together, all these studies highlight the complex and diverse actions of EVs.
Cancer cell derived EVs not only modulate the immune system but also support the transfer of EV nucleic acid and protein contents between neoplastic cells to promote tumorigenic phenotypes. For example, it has been observed that mutant Epidermal Growth Factor Receptor (EGFR) protein can be transferred to neighboring cells via cancer cell derived EVs and promote a tumorigenic phenotype in recipient cells.6 Additionally, EVs released by glioblastoma cells deliver protein and RNA cargoes to recipient cells to promote angiogenesis and tumorigenesis.12 Cancer cell EVs support not only local cell-to-cell communication, but also act on cells at distant sites. This was elegantly shown by Zomer, et al. (2015) using intravital in vivo imaging to show the transfer of cre recombinase containing EVs from tumor cells to less malignant recipient cre reporter cells both locally and systemically. Not only was cre transferred in a functional capacity, but less malignant recipient cells started to display increased migratory and metastatic phenotypes demonstrating EV mediated transfer of tumorigenic phenotypes between different cell populations.32 Additionally, melanoma derived EVs educate bone marrow progenitor cells towards a pro-metastatic phenotype, and promote vascular leakiness at pre-metastatic sites, thus, playing a role in pre-metastatic niche formation.36 Pancreatic cancer cell derived EVs have been found to promote fibrotic pre-metastatic environment formation in the liver through education of kupffer cells and recruitment of macrophages.35 Interestingly, EV priming of the metastatic niche is thought to depend upon specific integrin expression on cancer cell derived EVs; integrins appear to impart a tropism of EVs to specific extracellular matrices within organs and can be predictive of metastatic site.35 These studies are a few of many which highlight the functional role of cancer derived EVs and support the notion of a unique molecular profile which could potentially be used for disease detection, monitoring and prognosis.
EVs as non-invasive biomarkers in cancer
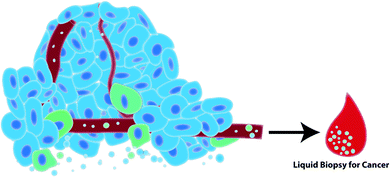
Growing interest in the use of EVs for biomedical research has led to an explosion of EV-based publications aiming to develop liquid biopsies for cancer. EVs act as windows of information about the cell from which they derived in their nucleic acid, protein and lipid signatures. In cancer, cells often exhibit unique nucleic acid/protein/lipid profiles that should be reflected in the EVs that those cells release. For example, proteomic analysis identified epithelial mesenchymal transition (EMT) associated proteins in EVs released by metastatic bladder cancer cells but not non-metastatic bladder cancer cells.58 Additionally, alterations and mutations in cancer cell DNA can be detected in the EVs released by cancer cells.14,15,59 This suggests that interrogation of EV content can be exploited for cancer diagnosis and prognostication. EVs can be readily detected in biological fluids such as blood, urine and saliva which further supports the use of EVs as ideal biomarker candidates in the development of minimally invasive testing platforms for cancer (Fig. 2).29–31,60 Cargo stability is another advantage of EVs. EVs are enclosed by a lipid bilayer which protects its nucleic acid and protein contents from degradation in the circulation and during isolation and storage.61,62 Importantly, these attributes support the retrospective isolation and analysis of EVs from biobanked biological samples in pre-clinical biomarker discovery phases of research.EVs as therapeutics in cancer
Autologous and HEK293 cell line derived EVs are seemingly immunologically and toxicologically inert.63,64 These properties alongside the ability of EVs to be taken up by and deliver functional cargoes to recipient cells locally and at distant sites in the body, make EVs attractive candidates to use as therapeutic delivery vehicles. This field of research explores EV loading with therapeutic cargoes by engineering cell lines or through EV manipulation post-isolation. Whilst most clinical studies use autologous cell derived EVs, pre-clinical studies use cell line derived EVs for proof of principle and concept development. In order to use EVs for clinical applications the isolation and characterization of EVs needs to be carefully planned and controlled.In this review, we assess the different EV isolation and characterization techniques employed in biomarker and therapeutic development studies and discuss their utility in pre-clinical and clinical studies.
Isolation and characterization of extracellular vesicles
EV isolation
For EV use in clinical applications, the isolation strategy needs careful consideration since it directly effects the EV population isolated and therefore the study outcome. The isolation and purification of EVs from biological fluids and cell culture supernatants can be achieved using several different techniques. Important considerations in method selection include the fluid from which EVs are to be isolated (cell culture supernatant, blood, urine etc.), the volume of fluid from which EVs will be isolated, and the desired EV purity. EV purity is of critical importance for use as a clinical therapeutic to ensure engineered EVs are pure and free of contaminating proteins and nucleic acids that could have a negative impact upon clinical administration. Additionally, separation of EVs away from other proteins and nucleic acids ensures that biological effects of therapeutic vesicles are attributed to EV enclosed/associated cargoes and not co-purified contaminants. However, for clinical biomarker studies, depending on what is being studied EV purity may be less of a concern. Analysis of select biomarkers via sequencing, Enzyme Linked Immunosorbent Assay (ELISA), or nanoscale flow cytometry would be less dependent on purity and more concerned with quantity. On the other hand biomarker discovery studies would require a high level of purity and thorough characterization of EVs before proceeding to validation studies and clinical applications.A classical and commonly used EV isolation technique is differential ultracentrifugation. Often considered the “gold standard” for EV isolation, this technique uses increasing centrifugation force to remove cells and debris from cell culture supernatant or biological fluid (300g & 2500g), pellet large EVs (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g), and finally small EVs (100
000g), and finally small EVs (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g/200
000g/200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g).65 Results from the literature, however, bring in to question the reproducibility of studies using this isolation method. Potential causes for this include rotor use (swing bucket versus fixed angle),66 sample viscosity,67 and tube k-factor.66 EV preparations using high centrifugal forces can contain contaminating particles from protein aggregation and other contaminants. Ultracentrifugation alone cannot remove contaminating lipoproteins from biological samples, such as blood, unless used in conjunction with a gradient and/or other chromatography techniques.68–70 Sucrose or iodixonal density gradients can be used to separate EVs from contaminating proteins, whereby solutions of increasing concentrations of sucrose or iodixonal are layered on top of one another to create a density gradient. Lipid encapsulated EVs applied to the bottom of the gradient float upwards during ultracentrifugation (200
000g).65 Results from the literature, however, bring in to question the reproducibility of studies using this isolation method. Potential causes for this include rotor use (swing bucket versus fixed angle),66 sample viscosity,67 and tube k-factor.66 EV preparations using high centrifugal forces can contain contaminating particles from protein aggregation and other contaminants. Ultracentrifugation alone cannot remove contaminating lipoproteins from biological samples, such as blood, unless used in conjunction with a gradient and/or other chromatography techniques.68–70 Sucrose or iodixonal density gradients can be used to separate EVs from contaminating proteins, whereby solutions of increasing concentrations of sucrose or iodixonal are layered on top of one another to create a density gradient. Lipid encapsulated EVs applied to the bottom of the gradient float upwards during ultracentrifugation (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g overnight) according to their density allowing separation of EV populations from contaminating proteins.65,71,72 Whilst a useful technique for laboratory based research, ultracentrifugation lacks practicality in a clinical setting due to time intensive preparation, significant equipment needs and poor scope for high throughput scalability.
000 × g overnight) according to their density allowing separation of EV populations from contaminating proteins.65,71,72 Whilst a useful technique for laboratory based research, ultracentrifugation lacks practicality in a clinical setting due to time intensive preparation, significant equipment needs and poor scope for high throughput scalability.
On the other hand, ultrafiltration techniques, such as tangential or sequential flow filtration, support rapid EV isolation from large volumes of cell culture supernatants or biological fluids. In tangential flow filtration, EV containing solutions flow over a membrane filter with a defined molecular weight cut-off (commonly 500 kDa) to permit small particles, such as proteins and liquid, to flow through whilst retaining EVs in the retentate. This process supports the concentration of large or small volumes of liquid whilst capturing EVs. However, the technique yields EVs with high protein contamination and there are concerns of the impact the filtration membrane might have upon EV integrity. Where high purity is required, tangential flow filtration would need to be used in conjunction with a second technique to improve purity, such as size exclusion chromatography.73–76 Alternatively, sequential filtration uses a combination of three filtration steps to isolate EVs devoid of contaminating proteins. First, dead end filtration is used to remove cells and debris. This is followed by tangential flow filtration to concentrate the sample and retain EVs as described above. Finally filtration occurs through a track edged membrane of increasing pore sizes (50–200 nm) to isolate and fractionate EVs by size.76
Size exclusion chromatography is an efficient chromatography technique that separates particles based on size. This has been adapted to separate and purify EVs from proteins in complex biological samples. When biological fluids such as blood plasma/serum is applied to a sepharose size exclusion column, differential exclusion causes EVs to elute first and separately from proteins which get trapped in the resin pores and only begin to elute in later fractions.77 However, size exclusion chromatography technology cannot efficiently separate EVs from lipoproteins of similar size when used to purify EVs from plasma or serum. For complete lipoprotein removal a mixture of isolation and purification methods would need to be carried out including density gradient ultracentrifugation followed by size exclusion chromatography.68 Other chromatography techniques that have been developed for EV purification include affinity purification and ion exchange chromatography. Membrane affinity purification methods such as exoEasy spin columns can isolate EVs from biological samples, however purity may be sub-optimal in comparison to size exclusion chromatography.78 Affinity based methods have also been optimized using the calcium sensitive, phosphatidylserine binding protein Tim4. EVs bound to Tim4 can subsequently be simply released by addition of calcium chelators.79 Other immuno-affinity capture agents can include heparin, tetraspanins and Epithelial Cell Adhesion Molecule (EpCAM).80–82 However the disadvantage of immuno-affinity capture is that only select populations of EVs are purified and EVs can be difficult to remove from the substrate without very harsh conditions such as low pH. A final chromatography method that is proving to be efficient for the isolation of EVs from cell culture supernatants in a scalable and efficient manner is anion exchange chromatography.83,84 Negatively charged EVs bind to positively charged columns and EVs are then eluted from the column using increasing concentrations of salt. Using anion exchange chromatography EVs can be isolated from 1 liter of cell culture conditioned media within 2 hours with minimal user input.83 This approach to EV isolation demonstrates scalability and rapid isolation suggesting it may have promise in supporting the use of EVs as therapeutics.
A popular method for isolation of EVs from clinical biological samples is by precipitation using commercially available reagents. The precipitation of EVs using polyethylene glycol (PEG),85 or commercially available reagents such as exoquick86 allows EVs to be pelleted by centrifugation at lower speeds, removing the need for time consuming and equipment dependent ultracentrifugation. EVs can be captured from small volumes of biological fluids, or larger volumes of pre-concentrated biological fluids/cell culture supernatants. Although very user friendly and amenable for use with a large number of biological samples, some reports suggest that EV purity after precipitation can be low as precipitation can also pellet proteins and lipoproteins.86,87 A second purification step after precipitation may be necessary to isolate a pure preparation of EVs. Additionally, precipitation reagents remaining in EV preparations can affect recipient cell viability EV and biological activity.88,89
Finally microfluidic chips are an emerging technology useful for the capture and analysis of EVs from small volumes of clinical samples and show promise for liquid biopsy diagnosis of disease. Microfluidic devices have been engineered for immuno-capture using tumor specific antigens or other markers of interest. For example, Human Epidermal Growth Factor Receptor (HER2) and Prostate Specific Antigen (PSA) positive tumor derived EVs have been captured on chips employing nanoshearing fluid flow. Captured EVs can then be quantified by a colorimetric reaction.90 Others have isolated and then eluted EVs for downstream analysis. For instance, EGFR wild type or EGFRvIII EVs can be isolated and quantified from glioblastoma patient plasma. Additionally EVs have been eluted from a chip and used for further in-depth RNA sequencing of EGFRvIII EVs.91 An alternative chip device approach used EpCAM aptamers to capture EVs, and electro-oxidation of metal nanoparticles to detect specific epitopes present upon captured EVs (specifically EpCAM and Prostate Specific Membrane Antigen (PSMA)). Oxidation of metal particles provides an electro chemical peak that can be used as a read out for quantification of captured EVs.92 Microfluidic chips have also been designed with specific size thresholds to capture tumor derived microvesicles. The EVs travel through the microfluidic chip and are eluted from different ports (dependent on size) for further downstream processing.93Table 1 summarizes the pros and cons of current popular EV isolation techniques.
| Isolation | Pros | Cons |
|---|---|---|
| Ultracentrifugation | Well characterized and common technique. Isolates and separates large EVs from small EVs | Time consuming. Significant equipment needs. Contaminating proteins and lipoproteins are not removed |
| Gradient | Separates EVs from contaminant proteins and some lipoproteins | Time consuming. Significant equipment needs. Further purification steps may be needed for complete lipoprotein removal |
| Ultrafiltration | Rapid isolation of EVs from large volumes | Contaminating proteins/lipoproteins are not removed |
| Size exclusion chromatography | Rapid isolation of EVs from small volumes of biological samples or from larger volumes that have been pre-concentrated | High-throughput scalability low. Further purification needed for removal of lipoproteins |
| Affinity | Rapid isolation | Only isolates very specific EV populations. Difficult to remove from beads intact |
| Anion exchange chromatography | Rapid isolation of EVs from large volumes of cell culture media. Early evidence suggests high purity | Necessity of further purification steps is to be determined |
| Precipitation | Rapidly isolate EVs from biological samples. High-throughput scalability | Pre-concentration needed for large volumes. Further purification often needed to remove contaminating proteins and lipoproteins |
| Microfluidic chips | Rapid processing from small volumes of biological samples. High-throughput scalability | Engineering and fabrication of chips; not readily commercially available |
EV characterization
Equally as important as selecting a method for EV isolation and purification is the characterization of EVs before use in downstream assays. There is an array of different methods that can be used to validate EV size, concentration, purity, and biomarker presence. Western blotting is a standard method used whereby probing for different EV markers in conjunction with a biomarker of interest can be carried out. EV markers that can be used include CD63, CD81, CD9, ALIX, TSG101, Flotillin and Annexins amongst many others.72 These markers do not differentiate between different EV subtypes but do confirm the presence of EVs. Western blots can, to some extent, also be useful in the analysis of EV purity. Western blotting for contaminant proteins such as cell organelle specific proteins and albumin which should be absent from EV preparations, can be useful to determine the extent of protein contamination in the EV preparation.72 To analyze the concentration and size of EVs there are several technologies available such as nanoparticle tracking analysis (NTA) and tunable resistive pulse sensing.94–97 Although useful, these technologies cannot differentiate between EVs, protein aggregates or lipoproteins and as such quantify all small particles in a solution. Dynamic light scattering (DLS) can be used to determine the size distribution of EVs but not their concentration. Accurate data is dependent upon the use of monodisperse particulate solutions and again the technology cannot differentiate between EVs, proteins lipoproteins or other particulate matter.98 To determine EV preparation purity, a method has been developed whereby the calculated value from the ratio of EV particle concentration to protein concentration can be used to estimate EV preparation purity.99To visualize EVs electron microscopy imaging is routinely performed to assess EV morphology and quality as well as any other co-isolated contaminates such as protein, DNA, lipoprotein, or virus.100,101 Immunogold electron microscopy can be used to stain EVs for EV markers or biomarker, typically a protein, of interest.65 EVs can also be analyzed using flow cytometry whereby EVs are adhered to magnetic/latex beads and stained using lipid dyes or antibodies to EV markers.102,103 More recently nanoscale flow cytometry technology has been developed which allows for analysis of individual EVs by staining them with antibodies against biomarkers of choice, without the need to adhere them onto beads. Nanoscale flow cytometry has shown great promise for analyzing EVs, particularly in complex biofluids, but is still in its infancy of development. Proper instrumentation, antibodies and controls must be used to ensure accurate EV detection.104–109
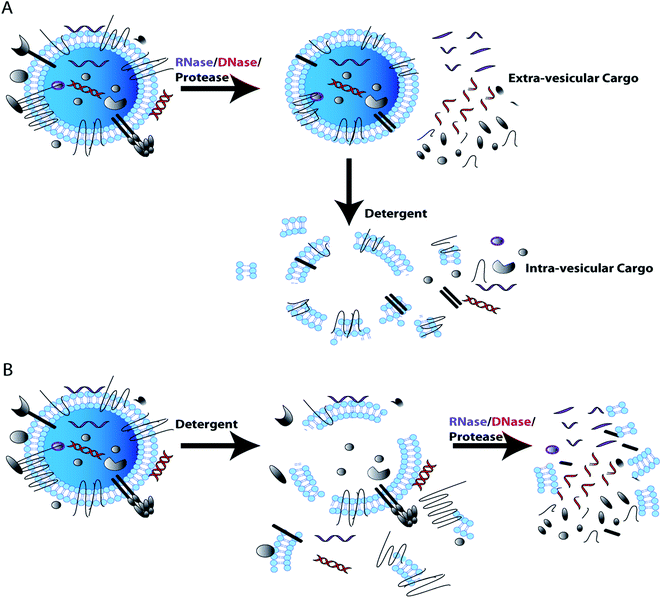
In characterizing EV content, it is important to show that the RNA/DNA/protein of interest is truly EV associated and not simply co-purified with EVs. For example, albumin and lipoproteins are abundantly present in plasma and serum and co-isolate with EVs by most currently used isolation techniques.68,69,86,87 Soluble proteins and DNA associated with the outer EV membranes have also been shown to have utility in diagnostics. However, membrane associated particles may be more difficult to replicate in cross validation studies.107,110–112 To validate nucleic acids/proteins of interest are encapsulated by EV membranes, EVs can be treated with RNase and/or DNase or protease with and without tritonX-100/detergent lysis. If the cargo is detected when EVs are treated with RNase/DNase/protease, but are not detected if detergent lysis of EVs is followed by RNase/DNase/protease treatment, this indicates that the cargo resides within EVs and is protected by the EV membrane. If the cargo is not detected when intact EVs are treated with RNase/DNase, this indicates that the cargo is not protected by the EV membrane and is most likely an EV contaminant that co-isolated with EVs. Protease treatment of EVs will also remove transmembrane and membrane associated proteins from the EV surface as well as co-isolated contaminant proteins. Additional controls can support the validation of EV membrane proteins exclusively associated the external EV surface. For example immuno-gold electron microscopy enables direct visualization of protein localization within the EV preparation.65 These simple RNase/DNase/protease EV membrane protection control experiments can determine whether the cargo of interest is EV associated or an experimental contaminant (Fig. 3).72
Overall, the choice of EV isolation and characterization methods are very important for determining that the EVs being studied are bona-fide and pure. If EVs are impure – there is a chance that the results obtained in an experiment are not necessarily directly attributed to the isolated EVs but in fact due to co-isolated contaminants. This, in particular, could make cross validation studies difficult and reduce reproducibility of studies. Additionally if the EV concentration used is inaccurately reported then the data interpretation and comparison to other studies can be ineffective. Adherence of the scientific community to performing and reporting on EV characterization and control experiments in pre-clinical and clinical studies ensures that published EV data is accurate, easily interpreted, and reproducible.
Extracellular vesicles and their clinical applications
Pre-clinical cancer EV diagnostic and prognostic biomarker studies
Numerous studies have investigated EV number and EV protein or nucleic acid content as diagnostic and prognostic biomarkers in several different types of cancer. For instance, it has been demonstrated that elevated levels of circulating plasma EVs correlates with poor prognosis of colorectal cancer patients,113 whereas low levels of circulating plasma EVs is a poor prognostic indicator of esophageal cancer.114 More commonly investigated is the association of EV contents with cancer diagnosis and prognosis. For example, EV associated double stranded DNA fragments isolated from cancer cell culture supernatant and the blood of tumor bearing mice can be used to detect mutated oncogenes.14 Furthermore, in prostate cancer, EV associated DNA reflects gene copy number alterations that are commonly associated with prostate cancer metastasis and, interestingly, most of the EV associated DNA was exclusive to the large EVs/oncosomes.59Instead of DNA, many research groups investigate the association of different RNA species present in EVs with cancer diagnosis and prognosis. Elevated levels of heterogeneous nuclear ribonucleoprotein H1 messenger RNA (mRNA) is detected in serum derived EVs of hepatocellular carcinoma patients115 and elevated human telomerase reverse transcriptase mRNA in circulating serum EVs has shown promise as a pan-cancer marker.116 RNA silencing microRNAs (miRNAs) are present in abundance inside EVs and many studies have found that miRNAs can act as diagnostic and/or prognostic indicators of several types of cancer. Pre-clinical studies have shown diagnostic and prognostic value of EV miRNAs in renal,117 non-small-cell lung,118,119 glioma,120 hepatocellular,121,122 ovarian,123,124 pancreatic125,126 and colorectal cancers127,128 to name only a few. Additionally, EV associated long non-coding RNAs (lncRNA) are also proving useful for cancer diagnosis and prognostication in pre-clinical studies. EV associated lncRNA-p21 is elevated in the urine of prostate cancer patients and can distinguish between benign disease and cancer.129 Plasma EV associated lncRNA UEGC1 shows promise as a diagnostic for early stage gastric cancer,130 and serum derived EV-associated HOTTIP may be useful as a diagnostic and prognostic indicator of gastric cancer.131 Finally, lncRNA MALAT1 is elevated in serum EVs from non-small-cell lung cancer patients.132 The less common family of circular RNAs is becoming increasingly recognized to be associated with EVs and potentially useful as a cancer biomarker.133 In fact, the presence of circular RNA-PDE8A is associated with pancreatic cancer diagnosis and progression.134
Finally, proteins either encapsulated by or associated with EV membranes can also be used for biomarker discovery. For example, circulating EV associated Copine III has been found to have diagnostic and prognostic significance for colorectal cancer.135 Melanoma biomarkers MIA and S100B are found on serum EVs and are predictive of diagnosis and prognosis of melanoma.136 Ascites derived EV associated E-cadherin has diagnostic utility in ovarian cancer and serum EV associated EphrinA2 may aid diagnosis of prostate cancer.137,138
There is a large number of research studies published regarding the analysis of EVs as biomarkers for cancer. To assess the EV isolation and characterization methods employed by the large number of EV studies, we selected studies based on a previous meta-analysis study that looked at the clinical significance of EVs in cancer.139 This study narrowed down the available selection of cancer EV biomarker literature between 2010–September 2018. The authors did a literature search using the key words ‘exosome and cancer and diagnosis or prognosis’. The authors then reviewed all literature and included only studies that involved EVs in cancer patient biofluids with at least 10 patients and matched controls being used. The biomarker had to have clinical significance and reporting of specificity and sensitivity for diagnostic markers, and confidence interval reported for prognostic markers. The literature search was narrowed down to include 60 studies. We further narrowed these studies down to only include studies that used at least 20 patient samples and matched controls. We then used the identical search terms and found that since September 2018 an additional 448 reports have been published. Using the same parameters but increasing the sample size required to n ≥ 20 patient samples and n ≥ 20 matched controls, we narrowed this down further and identified an additional 38 studies into our analysis. We use a total of 88 studies here to determine the EV characterization and isolation methods commonly used in pre-clinical biomarker discovery studies (Table 2).
| EV pre-clinical cancer biomarkers | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker (type) | Biofluid | EV isolation | EV characterization | Reference | |||||||||
| UC | Gradient | Precipitation | Other | EM/imaging | NTA/DLS | WB | Flow | Protein | RNase/DNase/protease | Other | |||
| HOTTIP (lncRNA) | Serum | ✓ | Y | Y | Y | Y | 140 | ||||||
| miR-30c-5p (miRNA) | Urine | ✓ | Y | Y | Y | 141 | |||||||
| LINC02418 (lncRNA) | Serum | ✓ | Y | Y | Y | Y | 142 | ||||||
| miRNA-320d (miRNA) | Serum | ✓ | Y | Y | Y | 143 | |||||||
| Hsa-circ-0065149 (circRNA) | Plasma | ✓ | 144 | ||||||||||
| miR-1246 (miRNA) | Serum | ✓ | Y | Y | 145 | ||||||||
| miR-21 & MMP1 (miRNA & protein) | Urine | ✓ | Y | Y | 146 | ||||||||
| miR-181b-5p (miRNA) | Ascites | ✓ | 147 | ||||||||||
| miR-21& miR-92a (miRNA) | Plasma | ✓ | Y | 148 | |||||||||
| miR-19b1-5p, 21-5p, 136-5p, 139-5p, 210-3p (miRNAs) | Urine | ✓ | 149 | ||||||||||
| GNAQ-6:1 (lncRNA) | Serum | ✓ | Y | Y | Y | Y | 150 | ||||||
| GPC-1 (protein) | Serum | ✓ | ✓ | 151 | |||||||||
| Hsa-circ-0004771 (circRNA) | Serum | ✓ | Y | Y | Y | Y | 152 | ||||||
| miR-150-5p & miR-99b-5p (miRNA) | Serum | ✓ | Y | Y | Y | Y | 153 | ||||||
| Pcsk2-2:1 (lncRNA) | Serum | ✓ | Y | Y | Y | 154 | |||||||
| 5 lncRNAs (lncRNA) | Serum | ✓ | Y | Y | Y | 155 | |||||||
| 4 miRNAs (miRNA) | Serum | ✓ | Y | 156 | |||||||||
| H19 (lncRNA) | Serum | ✓ | Y | Y | Y | Y | 157 | ||||||
| lncSLC2A12-10:1 (lncRNA) | Plasma | ✓ | Y | Y | Y | Y | 158 | ||||||
| 8 miRNAs (miRNA) | Plasma | ✓ | Y | Y | Y | Y | Y | 159 | |||||
| miR-1910p-3p (miRNA) | Serum | ✓ | Y | Y | Y | 160 | |||||||
| Circ-PNN (circRNA) | Serum | ✓ | Y | Y | Y | Y | 161 | ||||||
| TBILA & AGAP2-AS1 (lncRNA) | Serum | ✓ | Y | Y | Y | Y | Y | 162 | |||||
| miR-210 (miRNA) | Serum | ✓ | Y | 163 | |||||||||
| HULC (lncRNA) | Serum | ✓ | 164 | ||||||||||
| miR-320d (miRNA) | Serum | ✓ | Y | 165 | |||||||||
| CEBPA-AS1 (lncRNA) | Plasma | ✓ | Y | 166 | |||||||||
| miR-378 (miRNA) | Serum | ✓ | 167 | ||||||||||
| miR-874 (miRNA) | Serum | ✓ | 168 | ||||||||||
| miR-10b-5p (miRNA) | Serum | ✓ | Y | 169 | |||||||||
| 8 miRNAs (miRNA) | Ascites | ✓ | Y | 170 | |||||||||
| miR-17-5p (miRNA) | Serum | ✓ | Y | Y | Y | 171 | |||||||
| GAS5 (lncRNA) | Serum | ✓ | Y | Y | Y | Y | 172 | ||||||
| FGB & FGG (protein) | Plasma | ✓ | Y | Y | Y | 173 | |||||||
| *c-MET & PDL1 (protein) | Serum | ✓ | 174 | ||||||||||
| H19 (lncRNA) | Serum | ✓ | Y | Y | Y | Y | 175 | ||||||
| miR-4525, miR-451a& miR-21 (miRNA) | Plasma | ✓ | Y | 176 | |||||||||
| PCAT-1, UBC1 & SNHG16 (lncRNA) | Serum | ✓ | Y | Y | Y | Y | Y | 177 | |||||
| miR-454-3p (miRNA) | Serum | ✓ | 178 | ||||||||||
| alpha-2-HS-(glycoprotein) & extracellular matrix protein 1 (protein) | Serum | ✓ | Y | Y | Y | Y | 179 | ||||||
| miR-210 (microRNA) | Serum | ✓ | Y | Y | 180 | ||||||||
| 16 lipids (lipid) | Plasma | ✓ | Y | 181 | |||||||||
| PTENP1 (lncRNA) | Plasma | ✓ | Y | Y | Y | Y | 182 | ||||||
| KRAS (DNA) | Plasma | ✓ | 183 | ||||||||||
| miR-122, miR-125b, miR-145, miR-192, miR-194, miR-29a, miR-17-5p, and miR-106a (miRNA) | Serum | ✓ | Y | Y | 184 | ||||||||
| lncRNA PRINS (lncRNA) | Serum | ✓ | 185 | ||||||||||
| miR-200b (miRNA) | Plasma | ✓ | Y | Y | 123 | ||||||||
| Copine III (protein) | Plasma | ✓ | Y | Y | Y | Y | 135 | ||||||
| miR-122-5p, miR-125b-5p, miR-192-5p, miR-193b-3p, miR-221-3p and miR-27b-3p (miRNA) | Plasma | ✓ | 186 | ||||||||||
| TACSTD2 (protein) | Urine | ✓ | Y | Y | Y | Y | 187 | ||||||
| ENST00000 | |||||||||||||
| 588480.1/517758.1 (lncRNAs) | Bile | ✓ | Y | Y | Y | 188 | |||||||
| CRNDE-h (lncRNAs) | Serum | ✓ | Y | Y | Y | Y | 189 | ||||||
| miR-21 (miRNAs) | Serum | ✓ | 190 | ||||||||||
| 91H (lncRNAs) | Serum | ✓ | Y | Y | Y | 191 | |||||||
| miR-4772-3p (miRNA) | Serum | ✓ | Y | Y | Y | 128 | |||||||
| miR-19a (miRNA) | Serum | ✓ | ✓ | Y | 127 | ||||||||
| miR-548c-5p (miRNA) | Serum | ✓ | 192 | ||||||||||
| miR-200 family (miRNA) | Plasma | ✓ | Y | Y | 193 | ||||||||
| miRNA-21 (miRNA) | Plasma | ✓ | Y | 194 | |||||||||
| miR-6869-5p (miRNA) | Serum | ✓ | 195 | ||||||||||
| miR-6803-5p (miRNA) | Serum | ✓ | 196 | ||||||||||
| EVs | Plasma | ✓ | Y | Y | 114 | ||||||||
| lncUEGC1 (lncRNA) | Plasma | ✓ | ✓ | Y | Y | Y | Y | Y | 130 | ||||
| miR-423-5p (miRNA) | Serum | ✓ | Y | Y | Y | Y | 197 | ||||||
| miR-23b (miRNA) | Plasma | ✓ | Y | 198 | |||||||||
| miR-451 (miRNA) | Serum | ✓ | 199 | ||||||||||
| RNU-1 (sncRNA) | Serum | ✓ | Y | Y | Y | Y | Y | 200 | |||||
| miR-301a (miRNA) | Serum | ✓ | Y | 120 | |||||||||
| miR-21 (miRNA) | Serum | ✓ | 201 | ||||||||||
| hnRNPh1 (mRNA) | Serum | ✓ | 115 | ||||||||||
| ENSG00000258332.1 & LINC00635 (lncRNA) | Serum | ✓ | 202 | ||||||||||
| miR-125b (miRNA) | Serum | ✓ | Y | Y | Y | Y | 121 | ||||||
| miR-638 (miRNA) | Serum | ✓ | 122 | ||||||||||
| miR-93 (miRNA) | Serum | ✓ | Y | 203 | |||||||||
| LINC00161 (lncRNAs) | Serum | ✓ | Y | Y | Y | Y | 204 | ||||||
| miR21 (miRNA) & HOTAIR (lncRNA) | Serum | ✓ | Y | Y | Y | 205 | |||||||
| MIA & S100B (protein) | Serum | ✓ | Y | Y | Y | 136 | |||||||
| MALAT-1 (lncRNAs) | Serum | ✓ | Y | Y | Y | Y | 132 | ||||||
| miR-451a (miRNA) | Plasma | ✓ | Y | 119 | |||||||||
| miR-373, 200a, 200b & 200c (miRNAs) | Serum | ✓ | Y | Y | 124 | ||||||||
| miR-451a (miRNA) | Plasma | ✓ | Y | 206 | |||||||||
| miR-191, 21 & 451a (miRNAs) | Serum | ✓ | 125 | ||||||||||
| Glypican-1 (proteoglycan) | Serum | ✓ | ✓ | Y | Y | Y | Y | 207 | |||||
| miR-125b-5p (miRNA) | Plasma | ✓ | 186 | ||||||||||
| p21 (lncRNAs) | Urine | ✓ | 129 | ||||||||||
| EphrinA2 (protein) | Serum | ✓ | Y | Y | Y | 137 | |||||||
| SChLAP-1 (lncRNAs) | Plasma | ✓ | Y | Y | Y | Y | 208 | ||||||
| miR-1290 & 375 (miRNA) | Plasma | ✓ | 209 | ||||||||||
| Total: 88 | 23 | 3 | 60 | 6 | 52 | 34 | 45 | 4 | 31 | 12 | 2 | ||
Analysis of the EV isolation and characterization techniques used by 88 pre-clinical biomarker discovery studies revealed that the most common EV isolation method used is precipitation (60/88) and ultracentrifugation (23/88). Whilst ultracentrifugation is not a scalable or time efficient technique to use in a diagnostic clinical setting, EV precipitation is an excellent technique to use for isolation of EVs from large numbers of small volume biological fluids. However, due to reported impurities of EV preparations after some precipitation techniques,86,87 careful characterization of EVs needs to be done to ensure the biomarker of interest is actually EV associated. The most popular EV characterization methods used by this set of studies was electron microscopy/imaging (52/88), followed by western blot for EV markers (45/88), protein concentration determination (31/88) and nanoparticle tracking analysis/dynamic light scattering (34/88). The Minimal Information for Study of Extracellular Vesicles (MISEV) 2018 guidelines suggest that studies should carry out the following characterization steps: analysis of single EVs by two techniques (such as electron microscopy/imaging AND NTA/DLS) evaluation of EV marker and contaminant proteins (western blot/flow analysis), quantification of EV preparation (protein concentration/protein concentration![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) particle concentration ratio for example).72 Most studies carried out at least one or more of the EV characterization techniques but only 18 completed all recommended characterization steps. Surprisingly, there were 27 studies out of 88 that did not do any form of EV characterization. For some studies this may be due to previous publications reporting the EV isolation and characterization. Even in these cases it would still be beneficial to provide some quality control EV characterization in their current publications and clearly direct the reader to previous publications on characterization studies. Very few (12/88) of the analyzed studies included an RNase, DNase or protease EV membrane protection control experiments to determine whether the EV associated cargo/biomarker was in fact EV enclosed/associated and not a co-isolated contaminant. During the discovery phase of biomarker development, this is an important control experiment to include to present clear data that could be used to move forward to a clinical setting.
particle concentration ratio for example).72 Most studies carried out at least one or more of the EV characterization techniques but only 18 completed all recommended characterization steps. Surprisingly, there were 27 studies out of 88 that did not do any form of EV characterization. For some studies this may be due to previous publications reporting the EV isolation and characterization. Even in these cases it would still be beneficial to provide some quality control EV characterization in their current publications and clearly direct the reader to previous publications on characterization studies. Very few (12/88) of the analyzed studies included an RNase, DNase or protease EV membrane protection control experiments to determine whether the EV associated cargo/biomarker was in fact EV enclosed/associated and not a co-isolated contaminant. During the discovery phase of biomarker development, this is an important control experiment to include to present clear data that could be used to move forward to a clinical setting.
Clinical translation of EV diagnostic and prognostic biomarker studies in cancer
While many studies have investigated new cancer biomarkers in pre-clinical settings, there are only a handful of biomarker tests that have been current good manufacturing practice (cGMP) approved and only one has been approved for use by clinicians (Table 3). Exosome Diagnostics, Inc. has developed workflows to isolate cell free DNA and EV associated DNA/mRNA from plasma using a cGMP clinically certified process Exolution™ Plus. The DNA and EVs are then subject to lysis and quantitative RT-PCR (qRTPCR) analysis of EGFR mutations. This has clinical utility for lung cancer patients to direct patient treatment strategies.210–212 The same company has developed an additional cGMP and clinically approved technology – ExoPro urine clinical sample concentrator kit – to isolate EVs from urine by ultrafiltration centrifugation and to extract EV associated RNA. QRTPCR was used to analyze the expression of a set of genes (PCA3, SPDEF, ERG) and found to have utility as non-invasive test to risk stratify men with suspected prostate cancer and improve the identification of individuals with clinically significant disease.213–215| Clinical/cGMP approved cancer EV biomarker studies | ||||
|---|---|---|---|---|
| Biomarker | Isolation | Characterization | Test | In clinic? |
| EGFR mutation in NSCLC in plasma217 | Exolution™ plus: isolated EV and cell free DNA. cGMP | WB, NTA, SEM characterization218 | qRTPCR for EGFR mutations | No |
| EGFR T790M NSCLC in plasma212 | Exolution™ plus: isolated EV and cell free DNA cGMP | WB, NTA, SEM characterization218 | qRTPCR for EGFR mutations | No |
| EGFR activating/resistance mutation detection NSCLC in plasma210 | Exolution™ plus: isolated EV and cell free DNA. cGMP | WB, NTA, SEM characterization218 | qRTPCR for EGFR mutations | No |
| ExoDX™ prostate intelliscore in urine213–215 | EXOPRO urine clinical sample concentrator kit. cGMP | Characterized in pre-clinical studies | qRTPCR | Yes |
Why are there so many pre-clinical studies identifying new cancer biomarkers and so few are getting to clinic? There are several reasons why a bottle neck exists. These include lack of implementation of robust methodologies that comply with good laboratory practice (GLP) and cGMP protocols that are also approved for use in the clinic.216 Currently, Exosome Diagnostics, Inc. uses their cGMP and clinical laboratory approved technologies to isolate EVs from prostate cancer patient urine and non-small cell lung carcinoma patient plasma. In order to reduce the bottle neck, there is a need for the development of a simple EV isolation protocol that is cGMP approved and can be used widely in a clinical setting to support the analysis of multiple clinical samples in a high through put manner. Whilst reagents have been developed that are commonly used in pre-clinical biomarker discovery studies and are also GLP and cGMP compliant, such as exoquick (EV precipitation reagent), there is conflicting information in the literature regarding the efficiency of precipitation in isolating pure EV preparations from biological samples.78,86–88
Pre-clinical development of EV therapeutic delivery vehicles for cancer
Many research groups are developing therapeutic EVs that can be used as delivery vehicles of small molecules (RNA, DNA, proteins and pharmacological agents) for cancer treatment (Table 4). EVs loaded with small molecules can deliver chemotherapeutics such as paclitaxel and doxorubicin to cancer cells and reduce off target effects on non-specific organs.219–222 Cargo loading of small molecules can be achieved through manual loading of EVs post-EV isolation by incubation, electroporation or sonication. It has also been demonstrated that cells can be engineered to load oncolytic virus into EVs. EVs loaded with oncolytic virus and paclitaxel increased antitumor effects both in vitro and in vivo as compared to paclitaxel loaded EVs alone.223 For these systems, EV contaminating proteins must be assessed in addition to an assessment of efficient removal of excess drug; this is essential to allow accurate control of dosage and drug delivery.| EVs as therapeutic delivery vehicles | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cargo (target/drug) | EV isolation | EV characterization | Reference | ||||||||||
| DC/UC | UF | Gradient/SC | Precipitation | SEC | EM/imaging | NTA/DLS | WB | Flow | Protein | DNase/RNase/protease | Detergent | ||
| Small molecule (doxorubicin) | ✓ | ✓ | ✓ | Y | Y | N/A | 219 | ||||||
| Small molecule (doxorubicin) | ✓ | Y | Y | Y | N/A | 220 | |||||||
| Small molecule (Piclitaxel) | ✓ | ✓ | Y | Y | Y | Y | N/A | 221 | |||||
| Small molecule (piclitaxel) | ✓ | ✓ | Y | Y | Y | Y | N/A | 222 | |||||
| Oncolytic virus and piclitaxel | ✓ | Y | N/A | 223 | |||||||||
| siRNA (Kras) | ✓ | ✓ | Y | Y | Y | Y | RNase, proteinase K | Y | 224 | ||||
| siRNA (Rad51, Rad52) | ✓ | Y | Y | Y | Y | 225 | |||||||
| siRNA (PLK-1) | ✓ | Y | 226 | ||||||||||
| miRNA & siRNA (Let7, VEGF) | ✓ | ✓ | Y | Y | Y | RNase | 227 | ||||||
| miRNA (mir-134) | ✓ | ✓ | Y | Y | Y | RNase | 228 | ||||||
| miRNA (mir-31, mir-451a) | ✓ | Y | Y | Y | Y | Y | RNase | Y | 240 | ||||
| gRNA (reporter) | ✓ | ✓ | Y | Y | Y | Y | RNase, proteinase K | Y | 231 | ||||
| DNA (Cas9/gRNA – RUNX2 & CTNNB1) | ✓ | Y | Y | Y | Y | DNase, proteinase K | 233 | ||||||
| DNA (Cas9/gRNA – PARP1) | ✓ | ✓ | Y | Y | Y | Y | DNase | 232 | |||||
| Protein & gRNA (Cas9/gRNA RNP – HIV LTR) | ✓ | Y | Y | 234 | |||||||||
| Protein/mRNA (p53/Cas9 gRNA RNP) | ✓ | ✓ | Y | Y | Y | 238 | |||||||
| Protein (Bax/srlkB/cre) | ✓ | ✓ | Y | Y | Y | 237 | |||||||
| Protein (ww-cre) | ✓ | ✓ | Y | Y | Y | Proteinase K | Y | 239 | |||||
| Total: 18 | 12 | 5 | 4 | 6 | 3 | 12 | 16 | 13 | 2 | 14 | 8 | 4 | |
EVs can be loaded with small interfering RNA (siRNA) and miRNA to manipulate gene expression in cancer cells and reduce tumorigenic phenotypes. For example, siRNA to kRASG12D has been packaged into foreskin fibroblast-derived EVs by electroporation. Intraperitoneal injection of kRASG12D siRNA loaded EVs resulted in regression of not only the primary pancreatic tumor, but also the metastatic tumors.224 Loaded EVs delivering siRNA against Rad51 (ref. 225) and PLK1 (ref. 226) to cancer cells in vitro reversed cancer phenotypes. In another example, EVs were both targeted to nucleoilin (which is over-expressed on breast cancer cell membranes) and loaded with VEGF siRNA and Let-7 miRNA to initiate anti-tumor activity in vitro and in vivo.227 Rather than electroporation, another study used engineered cell lines to overexpress miRNAs of interest which passively load into EVs during biogenesis.228
An alternative way to manipulate cancer cell gene expression is to edit the genome directly using CRISPR/Cas9.229,230 This technology has the potential to treat any disease with a genetic basis including cancer, however, it requires an appropriate vehicle for its delivery to diseased cells. Guide RNA can be loaded into EVs using simple over-expression and delivered in a functional capacity to recipient reporter cells in vitro.231 Other methods have loaded DNA plasmids encoding both the Cas9 protein and the guide RNA into isolated EVs by electroporation232 or lipofectamine hybridosome formation233 to deliver gene editing machinery into cancer cells and mesenchymal stem cells, respectively. Alternatively, Cas9 protein forms a ribonuclease protein complex (RNP) with guide RNA, this RNP can be loaded into EVs by rapalog induced dimerization of two fusion proteins: membrane associated mCherry picker-DMRA and Cas9-DMRC. Co-expression of vesicular stomatitis virus G protein (VSVg) induces budding of a unique form of EVs called ‘Gesicles’. Rapalog induced dimerization of DMRA and DMRC localizes Cas9-gRNA RNP to the membrane in the vicinity of EV biogenesis ensuring its incorporation into the specialized Gesicles.234 Alternative dimerisation systems have also been designed and used to load Cas9-gRNA into EVs.235,236
Another heterodimerisation system uses light to load protein cargo into EVs. The two fusion proteins, CD9-CIBN and cargo-Cry2, dimerize in the presence of blue light. Dimerization of cargo protein to membrane localized tetraspanin CD9, leads to incorporation of cargo protein into EVs during biogenesis. This system can deliver proteins such as tumor suppressor Bax, Srlkb and Cre to recipient cells in vitro and in vivo.237 Another study used Arrestin domain containing protein 1 (ARRDC1) which localizes to the plasma membrane (cytosolic side) and recruits ESCRT machinery to initiate ARRDC1-mediated microvesicle budding (ARMMs). Fusion of tumor suppressor p53 with ARRDC1 led to incorporation of functional ARRDC1-p53 into ARRMS.238 Finally, tagging of cargo proteins such as Cre with a WW-tag drives the proteins ubiquitination and localization to endosomal membranes where WW-tagged proteins are incorporated into intraluminal vesicles during exosome biogenesis. Exosome loaded WW-Cre was functionally delivered in vivo.239 The various approaches that can be used to load therapeutic cargoes into EVs both pre- and post-isolation are summarized in a diagram in Fig. 4.
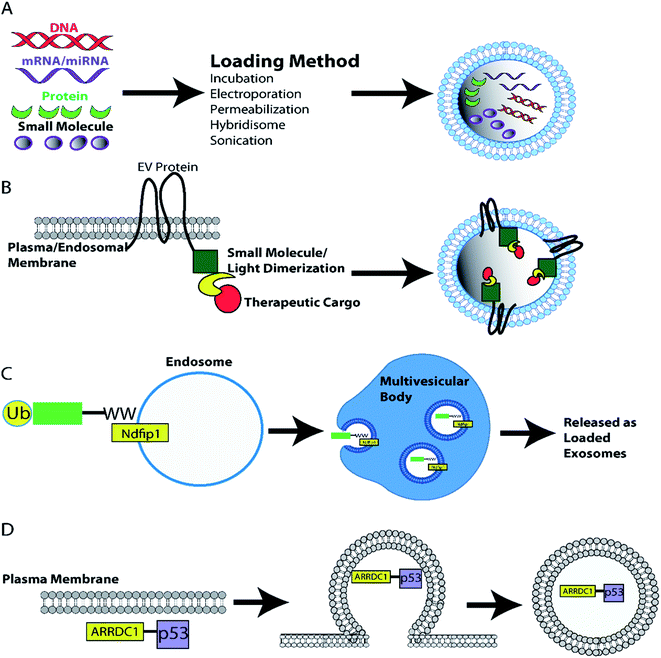 | ||
| Fig. 4 EV loading with therapeutic cargoes. (A) EVs can be loaded with therapeutic cargoes post-isolation using techniques such as incubation, electroporation, sonication, hybridization with lipofectamine, and permeabilization. Alternatively, cell lines can be engineered to load EVs with protein cargoes during biogenesis. (B) Generation of EV marker and cargo fusion proteins enables light/ligand induced dimerization loading of EVs as dimerization localizes the desired cargo to the plasma/endosomal membrane – the site of EV biogenesis.234,237 (C) Alternatively WW-tagged proteins promotes loading of ubiquitinated cargo into endosomal intraluminal vesicles and eventual release as exosomes.239 (D) Furthermore, the ARRDC1-p53 fusion protein recruits ESCRT machinery to the plasma membrane inducing p53 filled EV ARMM budding from the plasma membrane.238 | ||
Diverse types of cargo have been loaded into EVs and the techniques/strategies used vary from study to study. Table 4 details the cargo loading approaches used in pre-clinical therapeutic EV studies and the EV characterization techniques performed. The majority of studies used differential ultracentrifugation to isolate EVs (12/18). The second most common technique used to isolate EVs was EV precipitation reagents such as exoquick or PEG, or in one case immunoprecipitation (6/18). In some studies that used precipitation, a second EV purification procedure was carried out. The majority of studies that did not use a second purification technique after precipitation included RNase/DNase/protease EV membrane protection control experiments. Less than half of the studies characterized isolated EVs with imaging, NTA/DLS, protein quantification AND western blot or flow cytometry for EV markers (8/18), and 4/18 used only one or two of the MISEV recommended EV characterization techniques. Over half of the eligible reviewed studies carried out, RNase/DNase/protease EV membrane protection control experiments (8/13), and 4 studies use detergent lysis of EVs in conjunction with the EV membrane protection control experiment. These control experiments are important to ensure that the therapeutic cargo of interest is incorporated within the EV lumen and not delivered to recipient cells as a co-isolated contaminating protein or nucleic acid.
Clinical translation of EVs as therapeutic vehicles for cancer
Clinical trials have been conducted using dendritic-cell derived exosomes (Dex) pulsed with tumor antigens to stimulate an anti-tumor immune response.241,242 Second generation Dex derived from interferon (IFN)-γ-maturated dendritic cells (IFN-γ-Dex) have also been used in a phase II clinical trial on non-small cell lung cancer (NSCLC) patients.243 As an alternative approach, EVs derived from tumor ascites have been used in clinical trials for immunotherapy in colorectal cancer.244 To date, these studies represent the majority of published clinical trials that have used EVs as therapeutic delivery vehicles to treat cancer. There are many studies in progress/recruiting, the results of these will be expected over the coming years.The use of EVs as therapeutic delivery vehicles requires large scale manufacturing of clinical grade EV doses. Several papers have been published describing safe GMP of clinical grade EVs (Table 5). In the first protocol developed in 2002, monocyte derived dendritic cell conditioned media was filtered to remove cells and debris and concentrated using tangential flow filtration (TFF) with a 500 MWCO hollow fiber membrane. EVs were then purified using sucrose density cushion and ultracentrifugation and finally diafiltrated into a physiological buffer using TFF and filter sterilized using a 0.22 μm filter pore size. The use of TFF in the protocol allows for processing of large volumes of cell culture supernatant in a time efficient manner and the multi-step process maximizes the purity of EVs.75 This protocol has been commonly used and adapted in subsequent clinical trials241–243 and GMP development studies.245 However, both the original and subsequent studies did not carry out extensive EV characterization and relied predominantly upon ELISA and flow cytometry.
| EVs as therapeutic vehicles in the clinic | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EV | EV purification | EV characterization | Reference | Clinical phase | |||||||||||
| DC/UC | UF | Gradient | Precipitation | SEC | EM/imaging | NTA/DLS | WB | ELISA | Protein | Flow | DNase/RNase/protease | Detergent | |||
| a Studies used protocols formulated/adapted from Lamparski et al., 2002.75 | |||||||||||||||
| Dendritic cell derived EVs | ✓ | ✓ | Y | Y | Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
75 | GMPa | ||||||||
| Dendritic cell EVs | ✓ | ✓ | Y | 241 | Phase, 1 CTa | ||||||||||
| Colorectal cancer ascites EVs | ✓ | ✓ | Y | Y | Y | 244 | Phase, 1 CT | ||||||||
| Bone marrow derived MSC EVs | ✓ | Y | Y | Y | Y | RNase, | Y | 248 | GMP | ||||||
| Mesenchymal stromal cell derived EV | ✓ | Y | Y | Y | RNase, proteinase K | 247 | GMP | ||||||||
| Hek293 EVs | ✓ | ✓ | ✓ | Y | Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Y | Y | Y | Y | 246 | GMP | ||||
| Dendritic cell derived EVs | ✓ | ✓ | Y | 242 | Phase, 1 CTa | ||||||||||
| Dendritic cell derived EVs | ✓ | ✓ | Y | Y | 243 | Phase, 2 CTa | |||||||||
| Total: 8 | 4 | 5 | 5 | 0 | 1 | 3 | 3 | 3 | 4 | 6 | 4 | 2 | 1 | ||
Another study used a multi-step process to generate GMP compliant EV preparations from HEK293 cells. This study used a combination of filtration, TFF and size exclusion chromatography to generate GMP compliant EVs.246 The EVs were also thoroughly characterized using EM, ELISA, western blot and flow cytometry. Other GMP compliant EV purification studies generated clinical grade EVs using ultracentrifugation and/or gradients.244,247,248 The problem with ultracentrifugation and gradients is that they are time consuming and not as scalable as other techniques in the clinical setting if large numbers of EV doses are required to be manufactured. Overall 5/8 studies used ultrafiltration and 8/8 used ultracentrifugation and/or gradient.
Compared to pre-clinical studies, thorough characterization of EVs in most clinical studies is minimal. Only 3/8 studies showed EV structure by electron microscopy and 3/8 characterized particle concentration and size by NTA/DLS. However, 7/8 characterized EVs by western blot/flow, 4/8 used ELISA and 6/8 quantified protein. None of the studies carried out all characterization steps recommended by MISEV guidelines.72 Additionally, the RNase/DNase/protease EV membrane protection control experiments are rarely carried out (2/8). Although these studies may have been following protocols that have previously been developed and optimized, researchers should consider including more EV characterization and quality control datasets within clinical and cGMP compliant studies and/or provide clear reference to the publication that contains the EV characterization.
The majority of clinical studies using EVs as therapeutics use autologous primary human cells however some GMP studies have explored the purification of clinical grade EVs from cell lines. This is a stark contrast to pre-clinical studies where the majority of studies use human cell lines to generate therapeutic delivery vehicles that can be used in vitro and in vivo. The use of autologous EVs in pre-clinical research is time consuming and costly. Clinical trials show a clear preference for the use of autologous EVs to prevent any immunogenic side effects. However, current research suggests that cell line derived EVs are non-toxic and non-immunogenic in vivo.63,64 Further research needs to be conducted to understand the safety and clinical applicability of EVs derived from immortalized cell lines.
The need for standardization of extracellular vesicle isolation and characterization protocols for clinical applications
Our review of the literature investigates the most common EV isolation and characterization techniques that are used by pre-clinical and clinical studies. The review has uncovered important points regarding what we should be doing as a scientific community when isolating and characterizing our EVs.Identification of the optimal EV isolation protocol for pre-clinical studies is essential and dependent upon the fluid and volume from which EVs are being isolated. We conclude that it is possible to use any isolation technique deemed necessary (most frequently used was ultracentrifugation and precipitation) as long as characterization steps are employed: electron microscopy (or alternative), western blot/flow for EV markers and contaminants, EV preparation quantitation (protein concentration quantification for example) and nanoparticle tracking analysis (or alternative technology). In addition, the number of studies employing RNase/DNase/protease EV membrane protection control experiments were surprisingly low. Performing these simple experiments, would provide strong evidence that the biomarker being investigated or the therapeutic cargo loaded is in fact EV-associated/enclosed. Thorough EV characterization and EV membrane protection control experiments are important for the identification of bona fide EV cargoes; particularly given that other particles in biological fluids likely reside in EV preparations. These other particles may also have biomarker utility as has been shown for the extracellular particle termed exomere.249,250 Exomeres lack a lipid bilayer and likely arise through a distinct and different biogenesis pathway from EVs. Exomeres have a physiological role and have demonstrated potential as biomarkers for cancer.249,250 It is likely that all extracellular particles (such as EVs and exomeres) have potential to support the development of new diagnostic and prognostic testing. However, thorough characterization of the particle that one is working with will improve our understanding of the cargoes that they carry and the biological functions that they may have, enhance our understanding of small particles, and support study reproducibility.
For EV isolation in a clinical setting more cGMP approved technologies for small scale and large scale EV isolation need to be developed. Large scale isolation of EV therapeutic delivery vehicles in the clinic use multi-step methods to isolate pure EVs. However most methods include an ultracentrifugation and/or gradient step which are not easily scaled up and therefore these methods may not be suitable for large scale isolation of EV therapeutics in the clinic. Ultrafiltration technologies such as TFF can concentrate and isolate EVs at a large scale, but a second purification step is necessary. Therefore, other EV purification strategies that can isolate EVs in a large scale, quickly with minimal hands on input need to be developed. EV characterization in clinical studies often relies upon past publications using the same or similar isolation strategy. Improved reporting on past study EV characterization and inclusion of EV quality control and characterization would be beneficial in clinical studies.
Conclusion
Is there a need for EV purification and characterization standardization? One could argue that there is not a need for standardization of EV purification protocols for pre-clinical and clinical studies, as long as purified EVs are thoroughly characterized and controlled. There is plenty of room for technology development in terms of EV isolation and purification for biomarker analysis and for therapeutic delivery vehicle development. MISEV recommend that EVs are characterized on the single molecule level using two techniques (electron microscopy/other imaging techniques and by a particle analysis technology such as NTA/DLS), analyzed for EV markers and absence of contaminants (western blot/flow cytometry), and that the EV preparation should be quantitated (protein concentration or protein concentration to particle number ratio).72 Currently a large proportion of pre-clinical and clinical studies still do not fully characterize the isolated EVs. EV characterization should be standardized and become routine within the field. Additionally, the use of the RNase/DNase/protease EV membrane protection control experiment should also become a new routine standard control experiment included in all EV biomarker and therapeutic loading studies. Without these simple controls, there is no way the reader can know if the cargo/biomarker of interest reported by the study is actually EV associated or is rather a co-isolated contaminant.Author contributions
NS and KCW conceptualized review. NS drafted the manuscript. NS and KCW reviewed and edited the manuscript. KCW generated manuscript figures.Conflicts of interest
There are no conflicts of interest to declareAcknowledgements
This work was supported by the Department of Defense Congressionally Directed Medical Research Programs, Breast Cancer Research Program (W81XWH-16-1-0525) and the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants program. K. C. W. holds a Tier 2 Canada Research Chair and a Michael Smith Foundation for Health Research Scholar award.References
- G. van Niel, G. D'Angelo and G. Raposo, Shedding light on the cell biology of extracellular vesicles, Nat. Rev. Mol. Cell Biol., 2018, 19, 213–228, DOI:10.1038/nrm.2017.125.
- B. T. Pan, K. Teng, C. Wu, M. Adam and R. M. Johnstone, Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes, J. Cell Biol., 1985, 101, 942–948, DOI:10.1083/jcb.101.3.942.
- C. Harding, J. Heuser and P. Stahl, Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes, J. Cell Biol., 1983, 97, 329–339, DOI:10.1083/jcb.97.2.329.
- C. Tricarico, J. Clancy and C. D'Souza-Schorey, Biology and biogenesis of shed microvesicles, Small GTPases, 2017, 8, 220–232, DOI:10.1080/21541248.2016.1215283.
- D. Di Vizio, et al., Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease, Am. J. Pathol., 2012, 181, 1573–1584, DOI:10.1016/j.ajpath.2012.07.030.
- K. Al-Nedawi, et al., Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells, Nat. Cell Biol., 2008, 10, 619–624, DOI:10.1038/ncb1725.
- T. H. Lee, et al., Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular 'debris', Semin. Immunopathol., 2011, 33, 455–467, DOI:10.1007/s00281-011-0250-3.
- G. K. Atkin-Smith, et al., A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure, Nat. Commun., 2015, 6, 7439, DOI:10.1038/ncomms8439.
- M. Hristov, W. Erl, S. Linder and P. C. Weber, Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro, Blood, 2004, 104, 2761–2766, DOI:10.1182/blood-2003-10-3614.
- H. Valadi, et al., Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nat. Cell Biol., 2007, 9, 654–659, DOI:10.1038/ncb1596.
- E. N. Nolte-'t Hoen, et al., Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions, Nucleic Acids Res., 2012, 40, 9272–9285, DOI:10.1093/nar/gks658.
- J. Skog, et al., Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers, Nat. Cell Biol., 2008, 10, 1470–1476, DOI:10.1038/ncb1800.
- J. Ratajczak, et al., Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery, Leukemia, 2006, 20, 847–856, DOI:10.1038/sj.leu.2404132.
- B. K. Thakur, et al., Double-stranded DNA in exosomes: a novel biomarker in cancer detection, Cell Res., 2014, 24, 766–769, DOI:10.1038/cr.2014.44.
- C. Kahlert, et al., Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer, J. Biol. Chem., 2014, 289, 3869–3875, DOI:10.1074/jbc.C113.532267.
- C. Thery, et al., Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles, J. Immunol., 2001, 166, 7309–7318, DOI:10.4049/jimmunol.166.12.7309.
- J. F. Brouwers, et al., Distinct lipid compositions of two types of human prostasomes, Proteomics, 2013, 13, 1660–1666, DOI:10.1002/pmic.201200348.
- R. A. Haraszti, et al., High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources, J. Extracell. Vesicles, 2016, 5, 32570, DOI:10.3402/jev.v5.32570.
- K. Trajkovic, et al., Ceramide triggers budding of exosome vesicles into multivesicular endosomes, Science, 2008, 319, 1244–1247, DOI:10.1126/science.1153124.
- A. Llorente, et al., Molecular lipidomics of exosomes released by PC-3 prostate cancer cells, Biochim. Biophys. Acta, 2013, 1831, 1302–1309, DOI:10.1016/j.bbalip.2013.04.011.
- X. Luo, M. An, K. C. Cuneo, D. M. Lubman and L. Li, High-Performance Chemical Isotope Labeling Liquid Chromatography Mass Spectrometry for Exosome Metabolomics, Anal. Chem., 2018, 90, 8314–8319, DOI:10.1021/acs.analchem.8b01726.
- M. Clos-Garcia, et al., Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression, J. Extracell. Vesicles, 2018, 7, 1470442, DOI:10.1080/20013078.2018.1470442.
- C. Williams, et al., Assessing the role of surface glycans of extracellular vesicles on cellular uptake, Sci. Rep., 2019, 9, 11920, DOI:10.1038/s41598-019-48499-1.
- C. Williams, et al., Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives, J. Extracell. Vesicles, 2018, 7, 1442985, DOI:10.1080/20013078.2018.1442985.
- Y. Liang, et al., Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment, J. Biol. Chem., 2014, 289, 32526–32537, DOI:10.1074/jbc.M114.606269.
- R. M. Johnstone, M. Adam, J. R. Hammond, L. Orr and C. Turbide, Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes), J. Biol. Chem., 1987, 262, 9412–9420 CrossRef CAS.
- M. Mathieu, L. Martin-Jaular, G. Lavieu and C. Thery, Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication, Nat. Cell Biol., 2019, 21, 9–17, DOI:10.1038/s41556-018-0250-9.
- J. Maia, S. Caja, M. C. Strano Moraes, N. Couto and B. Costa-Silva, Exosome-Based Cell-Cell Communication in the Tumor Microenvironment, Front. Cell Dev. Biol., 2018, 6, 18, DOI:10.3389/fcell.2018.00018.
- M. P. Caby, D. Lankar, C. Vincendeau-Scherrer, G. Raposo and C. Bonnerot, Exosomal-like vesicles are present in human blood plasma, Int. Immunol., 2005, 17, 879–887, DOI:10.1093/intimm/dxh267.
- C. Lasser, et al., Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages, J. Transl. Med., 2011, 9, 9, DOI:10.1186/1479-5876-9-9.
- T. Pisitkun, R. F. Shen and M. A. Knepper, Identification and proteomic profiling of exosomes in human urine, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 13368–13373, DOI:10.1073/pnas.0403453101.
- A. Zomer, et al., In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior, Cell, 2015, 161, 1046–1057, DOI:10.1016/j.cell.2015.04.042.
- I. Wortzel, S. Dror, C. M. Kenific and D. Lyden, Exosome-Mediated Metastasis: Communication from a Distance, Dev. Cell, 2019, 49, 347–360, DOI:10.1016/j.devcel.2019.04.011.
- A. Hoshino, et al., Tumour exosome integrins determine organotropic metastasis, Nature, 2015, 527, 329–335, DOI:10.1038/nature15756.
- B. Costa-Silva, et al., Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver, Nat. Cell Biol., 2015, 17, 816–826, DOI:10.1038/ncb3169.
- H. Peinado, et al., Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET, Nat. Med., 2012, 18, 883–891, DOI:10.1038/nm.2753.
- G. Raposo, et al., B lymphocytes secrete antigen-presenting vesicles, J. Exp. Med., 1996, 183, 1161–1172, DOI:10.1084/jem.183.3.1161.
- I. Del Conde, C. N. Shrimpton, P. Thiagarajan and J. A. Lopez, Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation, Blood, 2005, 106, 1604–1611, DOI:10.1182/blood-2004-03-1095.
- A. Brill, O. Dashevsky, J. Rivo, Y. Gozal and D. Varon, Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization, Cardiovasc. Res., 2005, 67, 30–38, DOI:10.1016/j.cardiores.2005.04.007.
- J. Howitt and A. F. Hill, Exosomes in the Pathology of Neurodegenerative Diseases, J. Biol. Chem., 2016, 291, 26589–26597, DOI:10.1074/jbc.R116.757955.
- A. Ibrahim and E. Marban, Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology, Annu. Rev. Physiol., 2016, 78, 67–83, DOI:10.1146/annurev-physiol-021115-104929.
- B. D. Chan, et al., Exosomes in Inflammation and Inflammatory Disease, Proteomics, 2019, 19, e1800149, DOI:10.1002/pmic.201800149.
- A. Becker, et al., Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis, Cancer Cell, 2016, 30, 836–848, DOI:10.1016/j.ccell.2016.10.009.
- C. Ciardiello, et al., Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer, Int. J. Mol. Sci., 2016, 17, 175, DOI:10.3390/ijms17020175.
- F. Fanini and M. Fabbri, Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art, Semin. Cell Dev. Biol., 2017, 67, 23–28, DOI:10.1016/j.semcdb.2016.12.004.
- C. Sheehan and C. D’Souza-Schorey, Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer, J. Cell Sci., 2019, 132(20) DOI:10.1242/jcs.235085.
- P. D. Robbins and A. E. Morelli, Regulation of immune responses by extracellular vesicles, Nat. Rev. Immunol., 2014, 14, 195–208, DOI:10.1038/nri3622.
- D. W. Greening, S. K. Gopal, R. Xu, R. J. Simpson and W. Chen, Exosomes and their roles in immune regulation and cancer, Semin. Cell Dev. Biol., 2015, 40, 72–81, DOI:10.1016/j.semcdb.2015.02.009.
- N. M. Namee and L. O'Driscoll, Extracellular vesicles and anti-cancer drug resistance, Biochim. Biophys. Acta, Rev. Cancer, 2018, 1870, 123–136, DOI:10.1016/j.bbcan.2018.07.003.
- E. G. Trams, C. J. Lauter, N. Salem Jr and U. Heine, Exfoliation of membrane ecto-enzymes in the form of micro-vesicles, Biochim. Biophys. Acta, 1981, 645, 63–70, DOI:10.1016/0005-2736(81)90512-5.
- L. Zitvogel, et al., Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes, Nat. Med., 1998, 4, 594–600, DOI:10.1038/nm0598-594.
- J. Wolfers, et al., Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming, Nat. Med., 2001, 7, 297–303, DOI:10.1038/85438.
- A. J. Abusamra, et al., Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis, Blood Cells, Mol., Dis., 2005, 35, 169–173, DOI:10.1016/j.bcmd.2005.07.001.
- A. Clayton, S. Al-Taei, J. Webber, M. D. Mason and Z. Tabi, Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production, J. Immunol., 2011, 187, 676–683, DOI:10.4049/jimmunol.1003884.
- C. Liu, et al., Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function, J. Immunol., 2006, 176, 1375–1385, DOI:10.4049/jimmunol.176.3.1375.
- X. Xiang, et al., Induction of myeloid-derived suppressor cells by tumor exosomes, Int. J. Cancer, 2009, 124, 2621–2633, DOI:10.1002/ijc.24249.
- T. Cooks, et al., Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246, Nat. Commun., 2018, 9, 771, DOI:10.1038/s41467-018-03224-w.
- D. K. Jeppesen, et al., Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors, Proteomics, 2014, 14, 699–712, DOI:10.1002/pmic.201300452.
- T. Vagner, et al., Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma, J. Extracell. Vesicles, 2018, 7, 1505403, DOI:10.1080/20013078.2018.1505403.
- K. B. Johnsen, J. M. Gudbergsson, T. L. Andresen and J. B. Simonsen, What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer, Biochim. Biophys. Acta, Rev. Cancer, 2019, 1871, 109–116, DOI:10.1016/j.bbcan.2018.11.006.
- Q. Ge, et al., miRNA in plasma exosome is stable under different storage conditions, Molecules, 2014, 19, 1568–1575, DOI:10.3390/molecules19021568.
- Y. Cheng, Q. Zeng, Q. Han and W. Xia, Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes, Protein Cell, 2019, 10, 295–299, DOI:10.1007/s13238-018-0529-4.
- X. Zhu, et al., Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells, J. Extracell. Vesicles, 2017, 6, 1324730, DOI:10.1080/20013078.2017.1324730.
- A. F. Saleh, et al., Extracellular vesicles induce minimal hepatotoxicity and immunogenicity, Nanoscale, 2019, 11, 6990–7001, 10.1039/c8nr08720b.
- C. Thery, S. Amigorena, G. Raposo and A. Clayton, Isolation and characterization of exosomes from cell culture supernatants and biological fluids, Curr. Protoc. Cell Biol., 2006, 3(3.22) DOI:10.1002/0471143030.cb0322s30.
- A. Cvjetkovic, J. Lotvall and C. Lasser, The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles, J. Extracell. Vesicles, 2014, 3 DOI:10.3402/jev.v3.23111.
- F. Momen-Heravi, et al., Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles, Front. Physiol., 2012, 3, 162, DOI:10.3389/fphys.2012.00162.
- N. Karimi, et al., Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins, Cell. Mol. Life Sci., 2018, 75, 2873–2886, DOI:10.1007/s00018-018-2773-4.
- B. W. Sodar, et al., Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection, Sci. Rep., 2016, 6, 24316, DOI:10.1038/srep24316.
- Y. Yuana, J. Levels, A. Grootemaat, A. Sturk and R. Nieuwland, Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation, J. Extracell. Vesicles, 2014, 3 DOI:10.3402/jev.v3.23262.
- K. Li, D. K. Wong, K. Y. Hong and R. L. Raffai, Cushioned-Density Gradient Ultracentrifugation (C-DGUC): A Refined and High Performance Method for the Isolation, Characterization, and Use of Exosomes, Methods Mol. Biol., 2018, 1740, 69–83, DOI:10.1007/978-1-4939-7652-2_7.
- C. Thery, et al., Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, J. Extracell. Vesicles, 2018, 7, 1535750, DOI:10.1080/20013078.2018.1535750.
- R. A. Haraszti, et al., Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity, Mol. Ther., 2018, 26, 2838–2847, DOI:10.1016/j.ymthe.2018.09.015.
- G. Corso, et al., Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography, Sci. Rep., 2017, 7, 11561, DOI:10.1038/s41598-017-10646-x.
- H. G. Lamparski, et al., Production and characterization of clinical grade exosomes derived from dendritic cells, J. Immunol. Methods, 2002, 270, 211–226, DOI:10.1016/s0022-1759(02)00330-7.
- M. L. Heinemann and J. Vykoukal, Sequential Filtration: A Gentle Method for the Isolation of Functional Extracellular Vesicles, Methods Mol. Biol., 2017, 1660, 33–41, DOI:10.1007/978-1-4939-7253-1_4.
- A. N. Boing, et al., Single-step isolation of extracellular vesicles by size-exclusion chromatography, J. Extracell. Vesicles, 2014, 3 DOI:10.3402/jev.v3.23430.
- R. Stranska, et al., Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma, J. Transl. Med., 2018, 16, 1, DOI:10.1186/s12967-017-1374-6.
- W. Nakai, et al., A novel affinity-based method for the isolation of highly purified extracellular vesicles, Sci. Rep., 2016, 6, 33935, DOI:10.1038/srep33935.
- L. Balaj, et al., Heparin affinity purification of extracellular vesicles, Sci. Rep., 2015, 5, 10266, DOI:10.1038/srep10266.
- B. J. Tauro, et al., Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes, Methods, 2012, 56, 293–304, DOI:10.1016/j.ymeth.2012.01.002.
- M. P. Oksvold, A. Neurauter and K. W. Pedersen, Magnetic bead-based isolation of exosomes, Methods Mol. Biol., 2015, 1218, 465–481, DOI:10.1007/978-1-4939-1538-5_27.
- N. Heath, et al., Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography, Sci. Rep., 2018, 8, 5730, DOI:10.1038/s41598-018-24163-y.
- M. Kosanovic, B. Milutinovic, S. Goc, N. Mitic and M. Jankovic, Ion-exchange chromatography purification of extracellular vesicles, Biotechniques, 2017, 63, 65–71, DOI:10.2144/000114575.
- A. K. Ludwig, et al., Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales, J. Extracell. Vesicles, 2018, 7, 1528109, DOI:10.1080/20013078.2018.1528109.
- M. Ding, et al., Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling, Anal. Bioanal. Chem., 2018, 410, 3805–3814, DOI:10.1007/s00216-018-1052-4.
- M. Macias, et al., Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis, Clin. Chem. Lab. Med., 2019, 57, 1539–1545, DOI:10.1515/cclm-2018-1297.
- A. Gamez-Valero, et al., Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles' characteristics compared to precipitating agents, Sci. Rep., 2016, 6, 33641, DOI:10.1038/srep33641.
- L. Paolini, et al., Residual matrix from different separation techniques impacts exosome biological activity, Sci. Rep., 2016, 6, 23550, DOI:10.1038/srep23550.
- R. Vaidyanathan, et al., Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing, Anal. Chem., 2014, 86, 11125–11132, DOI:10.1021/ac502082b.
- E. Reategui, et al., Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles, Nat. Commun., 2018, 9, 175, DOI:10.1038/s41467-017-02261-1.
- Y. G. Zhou, et al., Interrogating Circulating Microsomes and Exosomes Using Metal Nanoparticles, Small, 2016, 12, 727–732, DOI:10.1002/smll.201502365.
- S. M. Santana, M. A. Antonyak, R. A. Cerione and B. J. Kirby, Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations, Biomed. Microdevices, 2014, 16, 869–877, DOI:10.1007/s10544-014-9891-z.
- R. A. Dragovic, et al., Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis, Nanomedicine, 2011, 7, 780–788, DOI:10.1016/j.nano.2011.04.003.
- J. de Vrij, et al., Quantification of nanosized extracellular membrane vesicles with scanning ion occlusion sensing, Nanomedicine, 2013, 8, 1443–1458, DOI:10.2217/nnm.12.173.
- J. C. Akers, et al., Comparative Analysis of Technologies for Quantifying Extracellular Vesicles (EVs) in Clinical Cerebrospinal Fluids (CSF), PLoS One, 2016, 11, e0149866, DOI:10.1371/journal.pone.0149866.
- S. L. Maas, M. L. Broekman and J. de Vrij, Tunable Resistive Pulse Sensing for the Characterization of Extracellular Vesicles, Methods Mol. Biol., 2017, 1545, 21–33, DOI:10.1007/978-1-4939-6728-5_2.
- R. Szatanek, et al., The Methods of Choice for Extracellular Vesicles (EVs) Characterization, Int. J. Mol. Sci., 2017, 18(6), 1153, DOI:10.3390/ijms18061153.
- J. Webber and A. Clayton, How pure are your vesicles?, J. Extracell. Vesicles, 2013, 2 DOI:10.3402/jev.v2i0.19861.
- Y. Yuana, et al., Cryo-electron microscopy of extracellular vesicles in fresh plasma, J. Extracell. Vesicles, 2013, 2 DOI:10.3402/jev.v2i0.21494.
- L. G. Rikkert, R. Nieuwland, L. Terstappen and F. A. W. Coumans, Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent, J. Extracell. Vesicles, 2019, 8, 1555419, DOI:10.1080/20013078.2018.1555419.
- A. Clayton, et al., Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry, J. Immunol. Methods, 2001, 247, 163–174, DOI:10.1016/s0022-1759(00)00321-5.
- H. Suarez, et al., A bead-assisted flow cytometry method for the semi-quantitative analysis of Extracellular Vesicles, Sci. Rep., 2017, 7, 11271, DOI:10.1038/s41598-017-11249-2.
- A. Morales-Kastresana, et al., High-fidelity detection and sorting of nanoscale vesicles in viral disease and cancer, J. Extracell. Vesicles, 2019, 8, 1597603, DOI:10.1080/20013078.2019.1597603.
- J. P. Nolan and J. C. Jones, Detection of platelet vesicles by flow cytometry, Platelets, 2017, 28, 256–262, DOI:10.1080/09537104.2017.1280602.
- G. C. t. Brittain, et al., A Novel Semiconductor-Based Flow Cytometer with Enhanced Light-Scatter Sensitivity for the Analysis of Biological Nanoparticles, Sci. Rep., 2019, 9, 16039, DOI:10.1038/s41598-019-52366-4.
- N. Salmond, K. Khanna, G. R. Owen and K. C. Williams, Nanoscale flow cytometry for immunophenotyping and quantitating extracellular vesicles in blood plasma, Nanoscale, 2021, 13, 2012–2025, 10.1039/d0nr05525e.
- A. Morales-Kastresana, et al., Labeling Extracellular Vesicles for Nanoscale Flow Cytometry, Sci. Rep., 2017, 7, 1878, DOI:10.1038/s41598-017-01731-2.
- J. A. Welsh, et al., MIFlowCyt-EV: a framework for standardized reporting of extracellular vesicle flow cytometry experiments, J. Extracell. Vesicles, 2020, 9, 1713526, DOI:10.1080/20013078.2020.1713526.
- J. C. Gross, V. Chaudhary, K. Bartscherer and M. Boutros, Active Wnt proteins are secreted on exosomes, Nat. Cell Biol., 2012, 14, 1036–1045, DOI:10.1038/ncb2574.
- E. Lazaro-Ibanez, et al., DNA analysis of low- and high-density fractions defines heterogeneous subpopulations of small extracellular vesicles based on their DNA cargo and topology, J. Extracell. Vesicles, 2019, 8, 1656993, DOI:10.1080/20013078.2019.1656993.
- E. I. Buzas, E. A. Toth, B. W. Sodar and K. E. Szabo-Taylor, Molecular interactions at the surface of extracellular vesicles, Semin. Immunopathol., 2018, 40, 453–464, DOI:10.1007/s00281-018-0682-0.
- J. Silva, et al., Analysis of exosome release and its prognostic value in human colorectal cancer, Genes Chromosomes Cancer, 2012, 51, 409–418, DOI:10.1002/gcc.21926.
- Y. Matsumoto, et al., Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma, Oncol. Rep., 2016, 36, 2535–2543, DOI:10.3892/or.2016.5066.
- H. Xu, X. Dong, Y. Chen and X. Wang, Serum exosomal hnRNPH1 mRNA as a novel marker for hepatocellular carcinoma, Clin. Chem. Lab. Med., 2018, 56, 479–484, DOI:10.1515/cclm-2017-0327.
- H. Goldvaser, et al., Characterisation of blood-derived exosomal hTERT mRNA secretion in cancer patients: a potential pan-cancer marker, Br. J. Cancer, 2017, 117, 353–357, DOI:10.1038/bjc.2017.166.
- N. Fujii, et al., Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma, Oncotarget, 2017, 8, 109877–109888, DOI:10.18632/oncotarget.22436.
- Q. Liu, et al., Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer, Oncotarget, 2017, 8, 13048–13058, DOI:10.18632/oncotarget.14369.
- R. Kanaoka, et al., Usefulness of Plasma Exosomal MicroRNA-451a as a Noninvasive Biomarker for Early Prediction of Recurrence and Prognosis of Non-Small Cell Lung Cancer, Oncology, 2018, 94, 311–323, DOI:10.1159/000487006.
- F. Lan, et al., Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma, Cell. Oncol., 2018, 41, 25–33, DOI:10.1007/s13402-017-0355-3.
- W. Liu, et al., Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma, OncoTargets Ther., 2017, 10, 3843–3851, DOI:10.2147/OTT.S140062.
- M. Shi, et al., Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma, J. Cell. Biochem., 2018, 119, 4711–4716, DOI:10.1002/jcb.26650.
- C. Pan, et al., Exosomal microRNAs as tumor markers in epithelial ovarian cancer, Mol. Oncol., 2018, 12, 1935–1948, DOI:10.1002/1878-0261.12371.
- X. Meng, et al., Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer, Oncotarget, 2016, 7, 16923–16935, DOI:10.18632/oncotarget.7850.
- T. Goto, et al., An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker, BMC Cancer, 2018, 18, 116, DOI:10.1186/s12885-018-4006-5.
- R. Que, G. Ding, J. Chen and L. Cao, Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma, World J. Surg. Oncol., 2013, 11, 219, DOI:10.1186/1477-7819-11-219.
- T. Matsumura, et al., Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer, Br. J. Cancer, 2015, 113, 275–281, DOI:10.1038/bjc.2015.201.
- C. Liu, et al., Serum exosomal miR-4772-3p is a predictor of tumor recurrence in stage II and III colon cancer, Oncotarget, 2016, 7, 76250–76260, DOI:10.18632/oncotarget.12841.
- M. Isin, et al., Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease, Front. Genet., 2015, 6, 168, DOI:10.3389/fgene.2015.00168.
- L. Y. Lin, et al., Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer, Mol. Canc., 2018, 17, 84, DOI:10.1186/s12943-018-0834-9.
- R. Zhao, et al., Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer, Mol. Canc., 2018, 17, 68, DOI:10.1186/s12943-018-0817-x.
- R. Zhang, et al., Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer, Biochem. Biophys. Res. Commun., 2017, 490, 406–414, DOI:10.1016/j.bbrc.2017.06.055.
- Y. Li, et al., Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis, Cell Res., 2015, 25, 981–984, DOI:10.1038/cr.2015.82.
- Z. Li, et al., Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer, Canc. Lett., 2018, 432, 237–250, DOI:10.1016/j.canlet.2018.04.035.
- B. Sun, et al., Circulating exosomal CPNE3 as a diagnostic and prognostic biomarker for colorectal cancer, J. Cell. Physiol., 2019, 234, 1416–1425, DOI:10.1002/jcp.26936.
- E. Alegre, et al., Circulating melanoma exosomes as diagnostic and prognosis biomarkers, Clin. Chim. Acta, 2016, 454, 28–32, DOI:10.1016/j.cca.2015.12.031.
- S. Li, et al., Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer, J. Cancer, 2018, 9, 2659–2665, DOI:10.7150/jca.25201.
- M. K. S. Tang, et al., Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface, Nat. Commun., 2018, 9, 2270, DOI:10.1038/s41467-018-04695-7.
- C. H. Wong and Y. C. Chen, Clinical significance of exosomes as potential biomarkers in cancer, World J Clin Cases, 2019, 7, 171–190, DOI:10.12998/wjcc.v7.i2.171.
- F. Oehme, et al., Low level of exosomal long non-coding RNA HOTTIP is a prognostic biomarker in colorectal cancer, RNA Biol., 2019, 16, 1339–1345, DOI:10.1080/15476286.2019.1637697.
- S. Song, et al., Urinary exosome miR-30c-5p as a biomarker of clear cell renal cell carcinoma that inhibits progression by targeting HSPA5, J. Cell. Mol. Med., 2019, 23, 6755–6765, DOI:10.1111/jcmm.14553.
- Y. Zhao, et al., Long noncoding RNA LINC02418 regulates MELK expression by acting as a ceRNA and may serve as a diagnostic marker for colorectal cancer, Cell Death Dis., 2019, 10, 568, DOI:10.1038/s41419-019-1804-x.
- Y. Tang, et al., Tumor-derived exosomal miRNA-320d as a biomarker for metastatic colorectal cancer, J. Clin. Lab. Anal., 2019, 33, e23004, DOI:10.1002/jcla.23004.
- Y. Shao, et al., Hsa_circ_0065149 is an Indicator for Early Gastric Cancer Screening and Prognosis Prediction, Pathol. Oncol. Res., 2020, 26, 1475–1482, DOI:10.1007/s12253-019-00716-y.
- Y. Shi, et al., Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer, Int. J. Clin. Oncol., 2020, 25, 89–99, DOI:10.1007/s10147-019-01532-9.
- W. Ando, et al., Novel breast cancer screening: combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis, Sci. Rep., 2019, 9, 13595, DOI:10.1038/s41598-019-50084-5.
- J. Yun, et al., Exosomal miR-181b-5p Downregulation in Ascites Serves as a Potential Diagnostic Biomarker for Gastric Cancer-associated Malignant Ascites, J. Gastric. Cancer, 2019, 19, 301–314, DOI:10.5230/jgc.2019.19.e27.
- N. Soeda, et al., Plasma exosome-encapsulated microRNA-21 and microRNA-92a are promising biomarkers for the prediction of peritoneal recurrence in patients with gastric cancer, Oncol Lett, 2019, 18, 4467–4480, DOI:10.3892/ol.2019.10807.
- G. Gullu Amuran, et al., Urinary micro-RNA expressions and protein concentrations may differentiate bladder cancer patients from healthy controls, Int. Urol. Nephrol., 2020, 52, 461–468, DOI:10.1007/s11255-019-02328-6.
- S. Li, et al., Exosomal long noncoding RNA lnc-GNAQ-6:1 may serve as a diagnostic marker for gastric cancer, Clin. Chim. Acta, 2020, 501, 252–257, DOI:10.1016/j.cca.2019.10.047.
- E. Buscail, et al., High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery, Cancers, 2019, 11 DOI:10.3390/cancers11111656.
- B. Pan, et al., Identification of Serum Exosomal hsa-circ-0004771 as a Novel Diagnostic Biomarker of Colorectal Cancer, Front. Genet., 2019, 10, 1096, DOI:10.3389/fgene.2019.01096.
- Y. J. Zhao, et al., Circulating Exosomal miR-150-5p and miR-99b-5p as Diagnostic Biomarkers for Colorectal Cancer, Front. Oncol., 2019, 9, 1129, DOI:10.3389/fonc.2019.01129.
- C. Cai, et al., Serum Exosomal Long Noncoding RNA pcsk2-2:1 As A Potential Novel Diagnostic Biomarker For Gastric Cancer, OncoTargets Ther., 2019, 12, 10035–10041, DOI:10.2147/OTT.S229033.
- Y. Zhang, et al., Identification of an exosomal long non-coding RNAs panel for predicting recurrence risk in patients with colorectal cancer, Aging, 2020, 12, 6067–6088, DOI:10.18632/aging.103006.
- S. Tang, et al., Combination of Four Serum Exosomal MiRNAs as Novel Diagnostic Biomarkers for Early-Stage Gastric Cancer, Front. Genet., 2020, 11, 237, DOI:10.3389/fgene.2020.00237.
- G. Zhong, et al., Determination of Serum Exosomal H19 as a Noninvasive Biomarker for Breast Cancer Diagnosis, OncoTargets Ther., 2020, 13, 2563–2571, DOI:10.2147/OTT.S243601.
- P. Zheng, et al., Plasma Exosomal Long Noncoding RNA lnc-SLC2A12-10:1 as a Novel Diagnostic Biomarker for Gastric Cancer, OncoTargets Ther., 2020, 13, 4009–4018, DOI:10.2147/OTT.S253600.
- M. Liang, et al., A Panel of Plasma Exosomal miRNAs as Potential Biomarkers for Differential Diagnosis of Thyroid Nodules, Front. Genet., 2020, 11, 449, DOI:10.3389/fgene.2020.00449.
- B. Wang, et al., Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-kappaB signaling pathway, Canc. Lett., 2020, 489, 87–99, DOI:10.1016/j.canlet.2020.05.038.
- Y. Xie, et al., RNA-Seq Profiling of Serum Exosomal Circular RNAs Reveals Circ-PNN as a Potential Biomarker for Human Colorectal Cancer, Front. Oncol., 2020, 10, 982, DOI:10.3389/fonc.2020.00982.
- Y. Tao, et al., Exploration of Serum Exosomal LncRNA TBILA and AGAP2-AS1 as Promising Biomarkers for Diagnosis of Non-Small Cell Lung Cancer, Int J Biol Sci, 2020, 16, 471–482, DOI:10.7150/ijbs.39123.
- F. Lan, X. Yue and T. Xia, Exosomal microRNA-210 is a potentially non-invasive biomarker for the diagnosis and prognosis of glioma, Oncol Lett, 2020, 19, 1967–1974, DOI:10.3892/ol.2020.11249.
- K. Takahashi, et al., Circulating extracellular vesicle-encapsulated HULC is a potential biomarker for human pancreatic cancer, Cancer Sci., 2020, 111, 98–111, DOI:10.1111/cas.14232.
- W. Li, et al., Downregulation of serum exosomal miR-320d predicts poor prognosis in hepatocellular carcinoma, J. Clin. Lab. Anal., 2020, 34, e23239, DOI:10.1002/jcla.23239.
- H. Y. Piao, S. Guo, Y. Wang and J. Zhang, Exosomal Long Non-Coding RNA CEBPA-AS1 Inhibits Tumor Apoptosis and Functions as a Non-Invasive Biomarker for Diagnosis of Gastric Cancer, OncoTargets Ther., 2020, 13, 1365–1374, DOI:10.2147/OTT.S238706.
- Y. Zhang and H. Xu, Serum exosomal miR-378 upregulation is associated with poor prognosis in non-small-cell lung cancer patients, J. Clin. Lab. Anal., 2020, 34, e23237, DOI:10.1002/jcla.23237.
- N. Zhang, et al., Reduced serum exosomal miR-874 expression predicts poor prognosis in colorectal cancer, Eur Rev Med Pharmacol Sci, 2020, 24, 664–672, DOI:10.26355/eurrev_202001_20043.
- H. J. Cho, et al., Serum Exosomal MicroRNA, miR-10b-5p, as a Potential Diagnostic Biomarker for Early-Stage Hepatocellular Carcinoma, J Clin Med, 2020, 9 DOI:10.3390/jcm9010281.
- B. Roman-Canal, et al., EV-Associated miRNAs from Peritoneal Lavage are a Source of Biomarkers in Endometrial Cancer, Cancers, 2019, 11, 839, DOI:10.3390/cancers11060839.
- Y. Zhang, Y. Zhang, Y. Yin and S. Li, Detection of circulating exosomal miR-17-5p serves as a novel non-invasive diagnostic marker for non-small cell lung cancer patients, Pathol., Res. Pract., 2019, 215, 152466, DOI:10.1016/j.prp.2019.152466.
- C. Li, et al., Tumor-derived exosomal lncRNA GAS5 as a biomarker for early-stage non-small-cell lung cancer diagnosis, J. Cell. Physiol., 2019, 234, 20721–20727, DOI:10.1002/jcp.28678.
- M. Kuang, et al., FGB and FGG derived from plasma exosomes as potential biomarkers to distinguish benign from malignant pulmonary nodules, Clin. Exp. Med., 2019, 19, 557–564, DOI:10.1007/s10238-019-00581-8.
- A. Lux, C. Kahlert, R. Grutzmann and C. Pilarsky, c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer, Int. J. Mol. Sci., 2019, 20, 3305, DOI:10.3390/ijms20133305.
- J. Wang, K. Yang, W. Yuan and Z. Gao, Determination of Serum Exosomal H19 as a Noninvasive Biomarker for Bladder Cancer Diagnosis and Prognosis, Med. Sci. Monit., 2018, 24, 9307–9316, DOI:10.12659/MSM.912018.
- S. Kawamura, et al., Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21 in portal vein blood is a high-sensitive liquid biomarker for the selection of high-risk pancreatic ductal adenocarcinoma patients, J Hepatobiliary Pancreat Sci, 2019, 26, 63–72, DOI:10.1002/jhbp.601.
- S. Zhang, et al., Evaluation of serum exosomal LncRNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer, J. Cell. Mol. Med., 2019, 23, 1396–1405, DOI:10.1111/jcmm.14042.
- N. Shao, et al., miR-454-3p Is an Exosomal Biomarker and Functions as a Tumor Suppressor in Glioma, Mol. Cancer Ther., 2019, 18, 459–469, DOI:10.1158/1535-7163.MCT-18-0725.
- L. Niu, et al., Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer, Cancer Sci., 2019, 110, 433–442, DOI:10.1111/cas.13862.
- X. Wang, et al., Serum exosomal miR-210 as a potential biomarker for clear cell renal cell carcinoma, J. Cell. Biochem., 2018 DOI:10.1002/jcb.27347.
- T. W. M. Fan, et al., Exosomal lipids for classifying early and late stage non-small cell lung cancer, Anal. Chim. Acta, 2018, 1037, 256–264, DOI:10.1016/j.aca.2018.02.051.
- R. Zheng, et al., Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression, Mol. Canc., 2018, 17, 143, DOI:10.1186/s12943-018-0880-3.
- V. Bernard, et al., Circulating Nucleic Acids Are Associated With Outcomes of Patients With Pancreatic Cancer, Gastroenterology, 2019, 156, 108–118, DOI:10.1053/j.gastro.2018.09.022.
- X. Xue, Y. Zhao, X. Wang, L. Qin and R. Hu, Development and validation of serum exosomal microRNAs as diagnostic and prognostic biomarkers for hepatocellular carcinoma, J. Cell. Biochem., 2019, 120, 135–142, DOI:10.1002/jcb.27165.
- L. Sedlarikova, et al., Circulating exosomal long noncoding RNA PRINS-First findings in monoclonal gammopathies, Hematol Oncol, 2018, 36, 786–791, DOI:10.1002/hon.2554.
- X. Zhou, et al., Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: A miRNA expression analysis, Gene, 2018, 673, 181–193, DOI:10.1016/j.gene.2018.06.037.
- C. L. Chen, et al., Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients, J. Proteome Res., 2012, 11, 5611–5629, DOI:10.1021/pr3008732.
- X. Ge, et al., The diagnostic/prognostic potential and molecular functions of long non-coding RNAs in the exosomes derived from the bile of human cholangiocarcinoma, Oncotarget, 2017, 8, 69995–70005, DOI:10.18632/oncotarget.19547.
- T. Liu, et al., Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer, Oncotarget, 2016, 7, 85551–85563, DOI:10.18632/oncotarget.13465.
- R. Uratani, et al., Diagnostic Potential of Cell-Free and Exosomal MicroRNAs in the Identification of Patients with High-Risk Colorectal Adenomas, PLoS One, 2016, 11, e0160722, DOI:10.1371/journal.pone.0160722.
- T. Gao, et al., Exosomal lncRNA 91H is associated with poor development in colorectal cancer by modifying HNRNPK expression, Canc. Cell Int., 2018, 18, 11, DOI:10.1186/s12935-018-0506-2.
- Z. Y. Peng, R. H. Gu and B. Yan, Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer, J. Cell. Biochem., 2018 DOI:10.1002/jcb.27291.
- S. Santasusagna, et al., Prognostic Impact of miR-200 Family Members in Plasma and Exosomes from Tumor-Draining versus Peripheral Veins of Colon Cancer Patients, Oncology, 2018, 95, 309–318, DOI:10.1159/000490726.
- M. Tsukamoto, H. Iinuma, T. Yagi, K. Matsuda and Y. Hashiguchi, Circulating Exosomal MicroRNA-21 as a Biomarker in Each Tumor Stage of Colorectal Cancer, Oncology, 2017, 92, 360–370, DOI:10.1159/000463387.
- S. Yan, et al., MicroRNA-6869-5p acts as a tumor suppressor via targeting TLR4/NF-kappaB signaling pathway in colorectal cancer, J. Cell. Physiol., 2018, 233, 6660–6668, DOI:10.1002/jcp.26316.
- S. Yan, et al., Exosomal miR-6803-5p as potential diagnostic and prognostic marker in colorectal cancer, J. Cell. Biochem., 2018, 119, 4113–4119, DOI:10.1002/jcb.26609.
- H. Yang, et al., Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer, Mol. Carcinog., 2018, 57, 1223–1236, DOI:10.1002/mc.22838.
- Y. Kumata, et al., Exosomeencapsulated microRNA23b as a minimally invasive liquid biomarker for the prediction of recurrence and prognosis of gastric cancer patients in each tumor stage, Oncol. Rep., 2018, 40, 319–330, DOI:10.3892/or.2018.6418.
- F. Liu, Z. Bu, F. Zhao and D. Xiao, Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells, Cancer Sci., 2018, 109, 65–73, DOI:10.1111/cas.13429.
- L. Manterola, et al., A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool, Neuro Oncol., 2014, 16, 520–527, DOI:10.1093/neuonc/not218.
- W. Liu, S. Chen and B. Liu, Diagnostic and prognostic values of serum exosomal microRNA-21 in children with hepatoblastoma: a Chinese population-based study, Pediatr. Surg. Int., 2016, 32, 1059–1065, DOI:10.1007/s00383-016-3960-8.
- H. Xu, Y. Chen, X. Dong and X. Wang, Serum Exosomal Long Noncoding RNAs ENSG00000258332.1 and LINC00635 for the Diagnosis and Prognosis of Hepatocellular Carcinoma, Cancer Epidemiol. Biomark. Prev., 2018, 27, 710–716, DOI:10.1158/1055-9965.EPI-17-0770.
- X. Xue, X. Wang, Y. Zhao, R. Hu and L. Qin, Exosomal miR-93 promotes proliferation and invasion in hepatocellular carcinoma by directly inhibiting TIMP2/TP53INP1/CDKN1A, Biochem. Biophys. Res. Commun., 2018, 502, 515–521, DOI:10.1016/j.bbrc.2018.05.208.
- L. Sun, et al., Serum and exosome long non coding RNAs as potential biomarkers for hepatocellular carcinoma, J. Cancer, 2018, 9, 2631–2639, DOI:10.7150/jca.24978.
- J. Wang, et al., Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma, Med. Oncol., 2014, 31, 148, DOI:10.1007/s12032-014-0148-8.
- K. Takahasi, et al., Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma, J Hepatobiliary Pancreat Sci, 2018, 25, 155–161, DOI:10.1002/jhbp.524.
- S. A. Melo, et al., Glypican-1 identifies cancer exosomes and detects early pancreatic cancer, Nature, 2015, 523, 177–182, DOI:10.1038/nature14581.
- Y. H. Wang, et al., Tumor-Derived Exosomal Long Noncoding RNAs as Promising Diagnostic Biomarkers for Prostate Cancer, Cell. Physiol. Biochem., 2018, 46, 532–545, DOI:10.1159/000488620.
- X. Huang, et al., Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer, Eur. Urol., 2015, 67, 33–41, DOI:10.1016/j.eururo.2014.07.035.
- E. Castellanos-Rizaldos, et al., Exosome-based detection of activating and resistance EGFR mutations from plasma of non-small cell lung cancer patients, Oncotarget, 2019, 10, 2911–2920, DOI:10.18632/oncotarget.26885.
- A. K. Krug, et al., Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma, Ann. Oncol., 2018, 29, 700–706, DOI:10.1093/annonc/mdx765.
- E. Castellanos-Rizaldos, et al., Exosome-Based Detection of EGFR T790M in Plasma from Non-Small Cell Lung Cancer Patients, Clin. Cancer Res., 2018, 24, 2944–2950, DOI:10.1158/1078-0432.CCR-17-3369.
- M. J. Donovan, et al., A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result, Prostate Cancer Prostatic Dis., 2015, 18, 370–375, DOI:10.1038/pcan.2015.40.
- J. McKiernan, et al., A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2-10 ng ml−1 at Initial Biopsy, Eur. Urol., 2018, 74, 731–738, DOI:10.1016/j.eururo.2018.08.019.
- J. McKiernan, et al., A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy, JAMA Oncol, 2016, 2, 882–889, DOI:10.1001/jamaoncol.2016.0097.
- A. Yekula, et al., From laboratory to clinic: Translation of extracellular vesicle based cancer biomarkers, Methods, 2020, 177, 58–66, DOI:10.1016/j.ymeth.2020.02.003.
- A. K. Krug, et al., Improved EGFR mutation detection using combined exosomal RNA and circulating tumor DNA in NSCLC patient plasma, Ann. Oncol., 2018, 29, 2143, DOI:10.1093/annonc/mdy261.
- L. Mohrmann, et al., Liquid Biopsies Using Plasma Exosomal Nucleic Acids and Plasma Cell-Free DNA Compared with Clinical Outcomes of Patients with Advanced Cancers, Clin. Cancer Res., 2018, 24, 181–188, DOI:10.1158/1078-0432.CCR-17-2007.
- Y. Tian, et al., A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy, Biomaterials, 2014, 35, 2383–2390, DOI:10.1016/j.biomaterials.2013.11.083.
- C. Schindler, et al., Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency, PLoS One, 2019, 14, e0214545, DOI:10.1371/journal.pone.0214545.
- H. Saari, et al., Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells, J. Controlled Release, 2015, 220, 727–737, DOI:10.1016/j.jconrel.2015.09.031.
- M. S. Kim, et al., Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells, Nanomedicine, 2016, 12, 655–664, DOI:10.1016/j.nano.2015.10.012.
- M. Garofalo, et al., Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment, J. Controlled Release, 2018, 283, 223–234, DOI:10.1016/j.jconrel.2018.05.015.
- S. Kamerkar, et al., Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer, Nature, 2017, 546, 498–503, DOI:10.1038/nature22341.
- T. A. Shtam, et al., Exosomes are natural carriers of exogenous siRNA to human cells in vitro, Cell Commun. Signaling, 2013, 11, 88, DOI:10.1186/1478-811X-11-88.
- K. A. Greco, et al., PLK-1 Silencing in Bladder Cancer by siRNA Delivered With Exosomes, Urology, 2016, 91, 241–247, DOI:10.1016/j.urology.2016.01.028.
- Y. Wang, et al., Nucleolin-targeted Extracellular Vesicles as a Versatile Platform for Biologics Delivery to Breast Cancer, Theranostics, 2017, 7, 1360–1372, DOI:10.7150/thno.16532.
- K. O'Brien, et al., miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity, Oncotarget, 2015, 6, 32774–32789, DOI:10.18632/oncotarget.5192.
- L. Cong, et al., Multiplex genome engineering using CRISPR/Cas systems, Science, 2013, 339, 819–823, DOI:10.1126/science.1231143.
- P. Mali, et al., RNA-guided human genome engineering via Cas9, Science, 2013, 339, 823–826, DOI:10.1126/science.1232033.
- O. G. de Jong, et al., A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA, Nat. Commun., 2020, 11, 1113, DOI:10.1038/s41467-020-14977-8.
- S. M. Kim, et al., Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting, J. Controlled Release, 2017, 266, 8–16, DOI:10.1016/j.jconrel.2017.09.013.
- Y. Lin, et al., Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs, Adv Sci, 2018, 5, 1700611, DOI:10.1002/advs.201700611.
- L. A. Campbell, et al., Gesicle-Mediated Delivery of CRISPR/Cas9 Ribonucleoprotein Complex for Inactivating the HIV Provirus, Mol. Ther., 2019, 27, 151–163, DOI:10.1016/j.ymthe.2018.10.002.
- Y. Ye, et al., An engineered exosome for delivering sgRNA:Cas9 ribonucleoprotein complex and genome editing in recipient cells, Biomater. Sci., 2020, 8, 2966–2976, 10.1039/d0bm00427h.
- P. Gee, et al., Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping, Nat Commun, 2020, 11, 1334, DOI:10.1038/s41467-020-14957-y.
- N. Yim, et al., Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module, Nat. Commun., 2016, 7, 12277, DOI:10.1038/ncomms12277.
- Q. Wang, et al., ARMMs as a versatile platform for intracellular delivery of macromolecules, Nat. Commun., 2018, 9, 960, DOI:10.1038/s41467-018-03390-x.
- U. Sterzenbach, et al., Engineered Exosomes as Vehicles for Biologically Active Proteins, Mol. Ther., 2017, 25, 1269–1278, DOI:10.1016/j.ymthe.2017.03.030.
- M. A. C. Pomatto, et al., Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs, Mol. Ther.--Methods Clin. Dev., 2019, 13, 133–144, DOI:10.1016/j.omtm.2019.01.001.
- M. A. Morse, et al., A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer, J. Transl. Med., 2005, 3, 9, DOI:10.1186/1479-5876-3-9.
- B. Escudier, et al., Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial, J. Transl. Med., 2005, 3, 10, DOI:10.1186/1479-5876-3-10.
- B. Besse, et al., Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC, Oncoimmunology, 2016, 5, e1071008, DOI:10.1080/2162402X.2015.1071008.
- S. Dai, et al., Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer, Mol. Ther., 2008, 16, 782–790, DOI:10.1038/mt.2008.1.
- H. Navabi, et al., Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial, Blood Cells, Mol., Dis., 2005, 35, 149–152, DOI:10.1016/j.bcmd.2005.06.008.
- D. C. Watson, et al., Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes, J. Extracell. Vesicles, 2018, 7, 1442088, DOI:10.1080/20013078.2018.1442088.
- K. Pachler, et al., A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles, Cytotherapy, 2017, 19, 458–472, DOI:10.1016/j.jcyt.2017.01.001.
- M. Mendt, et al., Generation and testing of clinical-grade exosomes for pancreatic cancer, JCI Insight, 2018, 3, e99263, DOI:10.1172/jci.insight.99263.
- H. Zhang, et al., Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nat. Cell Biol., 2018, 20, 332–343, DOI:10.1038/s41556-018-0040-4.
- Q. Zhang, et al., Transfer of Functional Cargo in Exomeres, Cell reports, 2019, 27, 940–954, DOI:10.1016/j.celrep.2019.01.009.
| This journal is © The Royal Society of Chemistry 2021 |