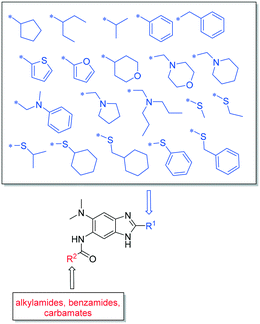
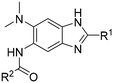
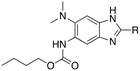
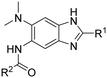
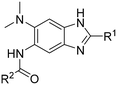
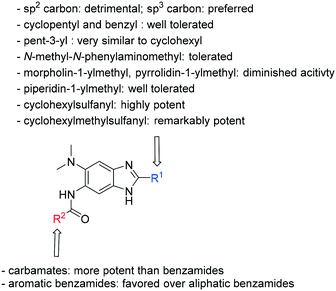
Structure–activity relationship studies on 2,5,6-trisubstituted benzimidazoles targeting Mtb-FtsZ as antitubercular agents†
Krupanandan
Haranahalli
ab,
Simon
Tong
b,
Saerom
Kim
b,
Monaf
Awwa
b,
Lei
Chen
b,
Susan E.
Knudson
c,
Richard A.
Slayden
c,
Eric
Singleton
d,
Riccardo
Russo
d,
Nancy
Connell
de and
Iwao
Ojima
 *ab
*ab
aInstitute of Chemical Biology and Drug Discovery, Stony Brook University, Stony Brook, NY, USA. E-mail: iwao.ojima@stonybrook.edu
bDepartment of Chemistry, Stony Brook University, Stony Brook, NY, USA
cDepartment of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, Colorado 80523-1682, USA
dDepartment of Medicine, Center for Emerging and Re-emerging Pathogens, Rutgers University, Newark, New Jersey 07103, USA
eDepartment of Physiology, Rutgers University, Newark, New Jersey 07103, USA
First published on 16th October 2020
Abstract
Filamenting temperature sensitive protein Z (FtsZ) is an essential bacterial cell division protein and a promising target for the development of new antibacterial therapeutics. As a part of our ongoing SAR studies on 2,5,6-trisubstituted benzimidazoles as antitubercular agents targeting Mtb-FtsZ, a new library of compounds with modifications at the 2 position was designed, synthesized and evaluated for their activity against Mtb-H37Rv. This new library of trisubstituted benzimidazoles exhibited MIC values in the range of 0.004–50 μg mL−1. Compounds 6b, 6c, 20f and 20g showed excellent growth inhibitory activities ranging from 0.004–0.08 μg mL−1. This SAR study has led to the discovery of a remarkably potent compound 20g (MIC 0.0039 μg mL−1; normalized MIC 0.015 μg mL−1). Our 3DQSAR model predicted 20g as the most potent compound in the library.
Introduction
Tuberculosis (TB) an infectious disease caused by Mycobacterium tuberculosis (Mtb), was one of the top 10 killers claiming 1.6 million lives in 2017 alone.1,2 Globally, an estimated 10 million new TB cases were reported in 2017.1 TB being the most common opportunistic infection in HIV-positive patients, resulted in 0.3 million deaths in 2017.1,3 Nearly one fourth of the world's population is afflicted with latent TB.4 Once an individual is infected with TB, there is a 10% probability of developing an active infection.1With the increase in emergence of drug resistant strains of TB, there is an urgent need for newer drugs with novel mechanisms of action.5 In the past decade, 3 new drugs bedaquiline, delamanid and pretomanid have been approved to treat resistant forms of TB.6–8 Although recently there has been progress in antitubercular drug discovery, the newer drugs are associated with safety concerns. Bedaquiline and delamanid are known to cause QT prolongation, which if left unmonitored can lead to cardiac arrest.9,10 It is imperative that patients receiving either of these two medications need to monitored very closely and for the same reason, their combined use has not yet been recommended by WHO.10 Pretomanid is associated with side effects such as nerve damage, hypoglycemia and lower respiratory tract infections.11 Pretomanid has been approved by FDA only in cases of pulmonary XDR-TB and treatment intolerant or non-responsive MDR-TB in combination with other anti-TB drugs.12
With the increase in the number of cases of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) and new drugs displaying life threatening side effects, there is a dire need for the development of new anti-tubercular agents with novel targets, that would not only make it difficult for the bacteria to develop resistance, but are also safe. In this context, filamentous temperature sensitive protein Z (FtsZ), a bacterial cell division protein seems to be a promising target.13,14 FtsZ, a guanosine triphosphate (GTP) dependent prokaryotic cell division protein, is a homolog of eukaryotic tubulin.15 In the presence of GTP, FtsZ first polymerizes into protofilaments which then assemble to form a highly dynamic Z-ring at the center of the bacterial cell.16,17 With the aid of other cell division proteins the Z-ring then forms a septum, which eventually results in cytokinesis.18
From a drug discovery perspective, targeting FtsZ offers several advantages. First, FtsZ is highly conserved among prokaryotes.19 Thus, developing agents that target FtsZ could potentially lead to broad spectrum antibiotics. Second, FtsZ and tubulin share only ∼7% sequence identity but high structural similarity.20,21 This would help design inhibitors that specifically target FtsZ and reduce the chances of toxicity to mammalian cells. Lastly, since there are no FtsZ inhibitors as drugs in the market, there are less chances of cross resistance within the drug resistant bacterial species that already exist.22
Libraries of 2,5,6- and 2,5,7-trisubstituted benzimidazoles have been designed and synthesized as Mtb-FtsZ inhibitors in our laboratories, and evaluated for their antitubercular (anti-TB) activities against Mtb-H37Rv strain.13,14,23 In general, 2,5,6-trisubstituted benzimidazoles exhibited better anti-TB activities than the 2,5,7-trisubstituted counterparts.14 Some of the lead 2,5,6-trisubstituted benzimidazoles, including SB-P17G-C2, SB-P17G-A20, SB-P17G-A38 and SB-P17G-A42 (Fig. 1), exhibited excellent MIC values (0.06–0.31 μg mL−1) against Mtb H37Rv.13,24 Selected lead compounds were found to inhibit the assembly of Mtb FtsZ in a dose dependent manner by increasing the GTPase activity.13,14 When evaluated their efficacies in vivo against immune compromised GKO mice, SB-P17G-A38 and SB-P17G-A42 reduced the bacterial load in the lung by 5.7–6.3 log10 CFU and in the spleen by 3.9–5.0 log10 CFU, respectively.24
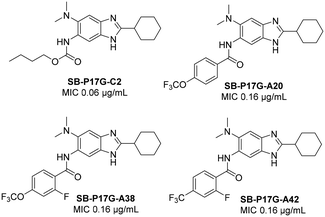 | ||
| Fig. 1 Lead compounds arising from 2,5,6-trisubstituted benzimidazole libraries previously studied.13,24 | ||
Our previous structure activity relationship (SAR) studies on these 2,5,6-trisubstituted benzimidazoles for optimization of potency and metabolic properties were conducted on the 5 and 6 positions, wherein a cyclohexyl group was kept at the 2 position intact. In our present SAR study, we fixed the 6 position as a dimethylamino group and investigated the effects of various substituents at the 2 and 5 positions on the potency and pharmacological properties (Fig. 2).
Results and discussion
Chemical synthesis
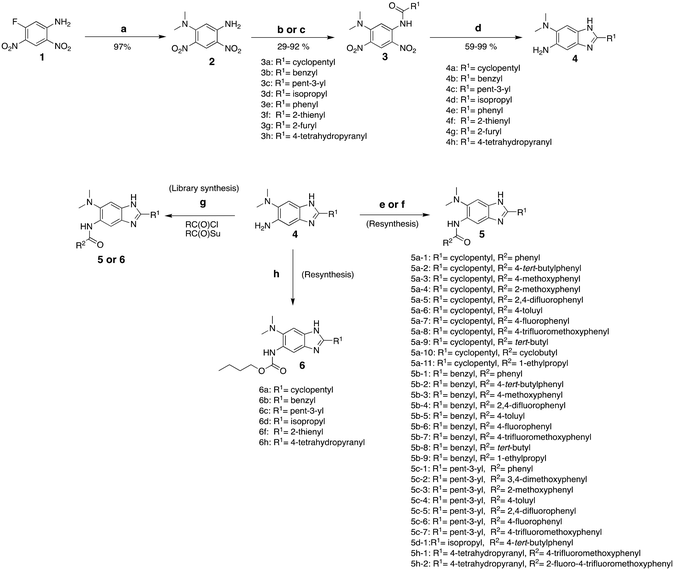
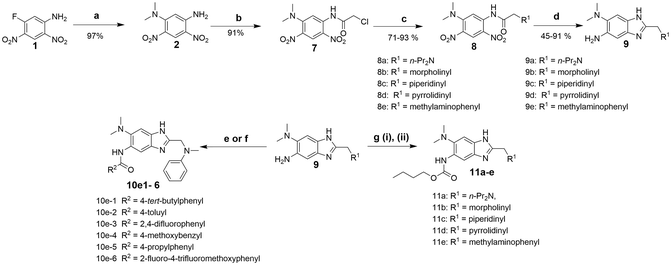
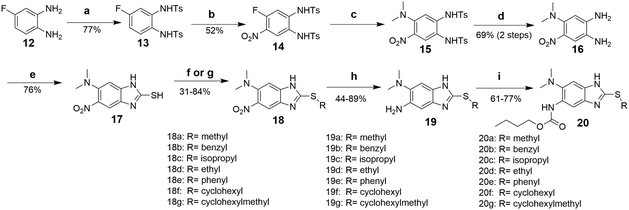
Synthesis of 2,5,6-trisubstituted benzimidazoles with modifications at the 2 position are outlined in Schemes 1–3. Compounds shown in Scheme 1 were synthesized by the method previously described.13,14 The 5-aminobenzimidazoles 4 were further derivatized with 35 different acyl chlorides, hydroxysuccinimide esters and chloroformates (1.1 eq.) in dichloromethane in 96 well plates. The plates were gently shaken at room temperature for 24 h, followed by treatment with aminomethylated polystyrene resin EHL/2% DVB (200–400 mesh) (10 equiv.) in order to scavenge any unreacted acyl chlorides, succinate esters or chloroformates. Then, the reaction mixture was filtered to give 245 novel 2,5,6-trisubstituted benzimidazoles, 5 and 6. In the case of trisubstituted benzimidazoles with N,N-dialkylaminomethyl substitution at the 2-position (Scheme 2), commercially available 2,4-dinitro-5-fluoroaniline (1) was subjected to nucleophilic aromatic substitution with dimethylamine to give 5-dimethylaminodinitroaniline (2) in 97% yield. Acylation of 2 with chloroacetyl chloride afforded 1-chloromethylcarboxamido-5-N,N-dimethylamino-2,4-dinitrobenzene (7) in 91% yield. Nucleophilic substitution of 7 with a variety of dialkylamines afforded compounds 8a–e in 71–93% yields. This was followed by one-pot reduction and cyclization using stannous chloride dihydrate and 4 M hydrochloric acid to give the key intermediates 9a–e. Then, 5-aminobenzimidazoles 9a–e were converted to either benzamides 10 or carbamates 11. For the synthesis of trisubstituted benzimidazoles with 2-sulfanyl substitution (Scheme 3), commercially available 1,2-diamino-4-fluorobenzene 12 was protected, using p-toluenesulfonyl chloride to yield 13 in 77% yield, and the subsequent nitration of 13 afforded 14 in 52% yield. Aromatic nucleophilic substitution of 14 with dimethylamine gave 15. Deprotection of the tosyl groups in 15 afforded 1,2-diamino-4-dimethylamino-5-nitrobenzene (16) in 69% yield for two steps. The reaction of 16 with carbon disulfide to give 6-dimethylamino-5-nitro-2-thio-1H-benzo[d]imidazole (17) in 76% yield. Compound 17 was alkylated, using a variety of alkyl or aromatic halides, to afford 18a–g in 31–84% yields. The reduction of 18a–g with stannous chloride dihydrate gave 19a–g in 44–89% yields. Finally, the amine moiety of 19a–g was converted to the corresponding n-butyl carbamates 20a–g in 61–77% yields.Evaluation of antibacterial activities against Mtb H37Rv and SAR analysis
The library of 2,5,6-trisubstiutted benzimidazoles 5 and 6 (245 compounds) were screened against Mtb H37Rv at 5 μg mL−1 using microplate alamar blue assay (MABA).25,26 Out of 245 compounds, 53 of them showed MIC ≤ 5 μg mL−1. Furthermore, when 37 out of the 53 hits were resynthesized in analytically pure form and examined for their accurate MIC values, there were a few compounds that exhibited higher MIC values than the initial cut off of 5 μg mL−1. This could be attributed to the fact that the actual weight and purity of test compounds are not very accurate in a 96-well plate and could also be due to false positives in high throughput (HTP) screening. As Table 1 shows, the resynthesized compounds showed MIC values ranging from 0.078 to 50 μg mL−1 [note: MIC values were determined by two different laboratories using MABA with slightly different protocols, “A” and “B”. Thus, Table 1 shows normalized MIC values with asterisks and also lists the original values in parentheses with superscript “b” marks].| Compound | R1 | R2 | MICa | Compound | R1 | R2 | MICa |
|---|---|---|---|---|---|---|---|
| MIC: minimum concentration of the compound required to inhibit growth of 99% of Mtb H37Rv cells. a MIC determined by protocol A. See Experimental section. b MIC determined by protocol B. See Experimental section. c Normalized MIC from the MIC determined by protocol B to the MIC by protocol A. | |||||||
| SB-P17G-C2 |
|

|
0.06 | 5c-3 |
|
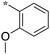
|
1.56 |
| (0.008b) | |||||||
| 0.064c | |||||||
| SB-P17G-A20 |
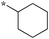
|
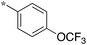
|
0.16 | 5c-4 |
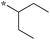
|
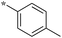
|
0.50 |
| SB-P17G-A38 |
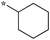
|
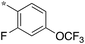
|
0.16 | 5c-5 |
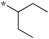
|
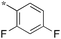
|
0.71 |
| (0.019b) | |||||||
| 0.15c | |||||||
| SB-P17G-A42 |
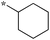
|
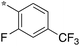
|
0.16 | 5c-6 |
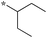
|
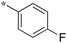
|
0.78 |
| 5a-1 |
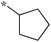
|
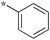
|
1.56 | 5c-7 |
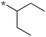
|
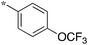
|
0.31 |
| 5a-2 |
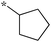
|
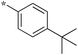
|
0.78 | 5d-1 |
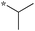
|
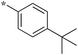
|
0.78 |
| 5a-3 |
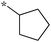
|
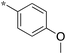
|
1.25 | 5h-1 |
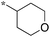
|
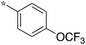
|
>10 |
| 5a-4 |
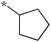
|
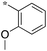
|
12.5 | 5h-2 |
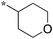
|
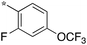
|
10 |
| 5a-5 |
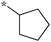
|
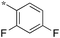
|
0.625 | 6a |
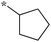
|

|
0.31 |
| 5a-6 |
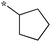
|
|
0.625 | 6b |
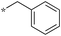
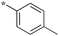
|

|
<0.078 |
| 5a-7 |
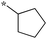
|
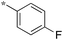
|
0.78 | 6c |
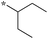
|

|
0.078 |
| 5a-8 |
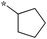
|
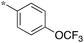
|
1.56 | 6d |
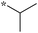
|

|
1.25 |
| 5a-9 |
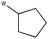
|
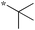
|
50 | 6f |
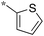
|

|
>10 |
| 5a-10 |
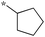
|
|
>10 | 6h |
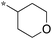
|

|
>2.5 |
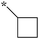
| 5a-11 |
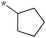
|
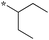
|
>50 | 10e-1 |
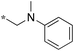
|
|
2.5 |
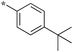
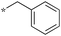
| 5b-1 |
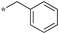
|
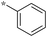
|
1.25 | 10e-2 |
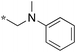
|
|
1.25 |
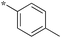
| 5b-2 |
|
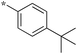
|
0.33 | 10e-3 |
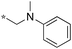
|
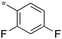
|
1.25 |
| 5b-3 |
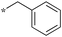
|
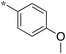
|
1.25 | 10e-4 |
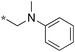
|
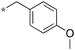
|
>10 |
| 5b-4 |
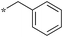
|
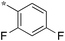
|
0.63 | 10e-5 |
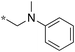
|
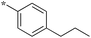
|
5 |
| 5b-5 |
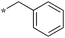
|
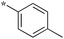
|
1.56 | 10e-6 |
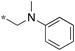
|
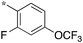
|
0.29c |
| (0.19b) | |||||||
| 5b-6 |
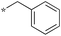
|
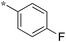
|
0.63 | 11a |
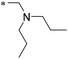
|

|
0.16 |
| 5b-7 |
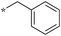
|
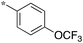
|
1.25 | 11b |
|

|
2.4c |
| (1.6b) | |||||||
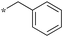
| 5b-8 |
|
|
>10 | 11c |
|

|
0.14c |
| (0.09b) | |||||||
| 5b-9 |
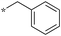
|
|
>10 | 11d |
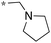
|

|
2.4c |
| (1.6b) | |||||||
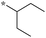
| 5c-1 |
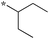
|
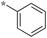
|
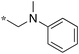
1.56 | 11e |
|
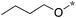
|
0.16 |
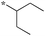
| 5c-2 |
|
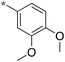
|
12.5 | ||||
Preliminary SAR of the hits from the library indicated that cyclopentyl, pent-3-yl, isopropyl and benzyl substituents at the 2 position were preferred. None of the compounds bearing a phenyl or 2-furyl substituents at the 2 position made the cut-off line in the initial screening. Although there was one hit with thien-2-yl substituent at the 2 position, this compound 6f showed poor cell growth inhibitory activity (MIC >10 μg mL−1) when assayed with the resynthesized compound. These results indicate that an sp3 hybridized carbon is preferred over an sp2 carbon at the 2 position.
Also, compounds bearing substituted benzamides at the 5 position fared better than their aliphatic amide counter parts.
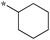
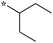
Compounds bearing a cyclopentyl substituent at the 2 position (5a-1–5a-8, 6a exhibited good inhibitory activity. The MIC values of trisubstituted benzimidazoles with pent-3-yl substituent at the 2 position (5c-1–5c-7, c6b) were comparable to the previously reported compounds bearing a cyclohexyl substituent at the 2 position.13 These results suggest that pent-3-yl group is a close mimic of the cyclohexyl group at the 2 position. Compounds with isopropyl substitution at the 2 position (-15d, 6d) were found to be less active than the ones with pent-3-yl substituent, suggesting the importance of the carbon chain length. In the case of 4-tetrahydropyranyl substituent at the 2 position (5h-1, 5h-2 and 6h), there was a significant loss of inhibitory activity when compared to the cyclohexyl counterparts, indicating that the presence of an oxygen atom within the cyclohexyl moiety is unfavorable.
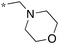
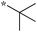
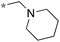
Next, we examined the effect of N,N-dialkylaminomethyl substitution at the 2-position. All the compounds were evaluated for their activity against Mtb H37Rv using MABA.26,27 Compounds with an N,N-methyl(phenyl)aminomethyl group at the 2 position (10e-1–10e-5, 11e) were far less active compared to ones with a pent-3-yl group with an exception of 10e-6. Compound with N,N-dipropylaminomethyl (11a) showed a high activity (MIC 0.16 μg mL−1). Morpholin-4-ylmethyl (11b) and pyrrolidin-1-ylmethyl (11d) groups at the 2 position resulted in diminished activities. 2,5,6-Trisubstituted benzimidazole with a n-butoxycarbonylamino group at the 5 position and a piperidin-1-ylmethyl group at the 2 position (11c) showed a high activity (MIC 0.09 μg mL−1; normalized MIC 0.14 μg mL−1).
In order to further diversify our library, a small series of 2,5,6-trisusbstituted benzimidazoles with alkyl/arylsulfanyl groups at the 2 position and n-butyl carbamate at the 5 position were designed and synthesized. All these sulfide-containing compounds were evaluated for their activities against Mtb H37Rv using MABA.26,27 Results are summarized in Table 2.
| Compound | R1 | MICa |
|---|---|---|
| a MIC determined by protocol A. See Experimental section. b MIC determined by protocol B. See Experimental section. c Normalized MIC from the MIC determined by protocol B to the MIC by protocol A. | ||
| SB-P17G-C2 |
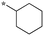
|
0.06 |
| (0.008b) | ||
| 20a |
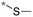
|
9.6c |
| (1.28b) | ||
| 20b |
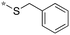
|
0.31 |
| 20c |
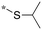
|
0.31 |
| 20d |
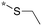
|
1.2c |
| (0.16b) | ||
| 20e |
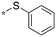
|
0.31 |
| 20f |
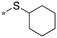
|
<0.031c |
| (<0.0081b) | ||
| 20g |
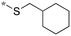
|
0.015c |
| (0.0039b) | ||
Compounds 20b and 20e, bearing a benzylsulfanyl and a phenylsulfanyl groups, respectively, exhibited good activity (MIC 0.31 μg mL−1). In the 2-alkylsulfanyl series of analogs, the size of alkyl groups is critical to their activities. Thus, cyclohexyl analog 20f exhibited high activity (MIC <0.0081 μg mL−1; normalized MIC <0.031 μg mL−1) and isopropyl analog 20c retained good activity (MIC 0.31 μg mL−1), while ethyl analog 20d displayed moderate activity (normalized MIC 1.2 μg mL−1), a significant decrease in activity was observed for the methyl analog 20a (normalized MIC 9.6 μg mL−1). The introduction of a methylene group between the sulfur and cyclohexyl unexpectedly increased the activity. Thus, cyclohexylmethylsulfanyl analog 20g exhibited remarkably enhanced activity (MIC 0.0039 μg mL−1; normalized MIC 0.015 μg mL−1), which marks the highest inhibitory activity so far to date.
| Entry | Compound | R1 | R2 | MICa (μg mL−1) | WI38 % inhibition at 20 μMd | HepG2 % inhibition at 20 μMd |
|---|---|---|---|---|---|---|
| a MIC determined by protocol A. See Experimental section. b MIC determined by protocol B. See Experimental section. c Normalized MIC from the MIC determined by protocol B to the MIC by protocol A. d Average value of MTT assay in triplicate. e Enhancement of cell growth was observed. | ||||||
| 1 | SB-P17G-A38 |
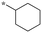
|
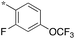
|
0.16 | 78 | 57 |
| 2 | 20g |
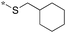
|

|
0.015c | 52 | 0 |
| (0.0039b) | ||||||
| 3 | 20f |
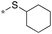
|

|
<0.031a,c | 87 | 50 |
| (<0.0081b) | ||||||
| 4 | 6b |
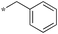
|

|
<0.078 | 93 | 80 |
| 5 | 6c |
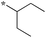
|

|
0.078 | 77 | 32 |
| 6 | 11c |
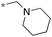
|

|
0.14c | 60 | 0e |
| (0.09b) | ||||||
| 7 | 11a |
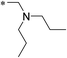
|

|
0.16 | 4.0 | 0e |
| 8 | 11e |
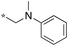
|

|
0.16 | 96 | 54 |
| 9 | 10e-6 |
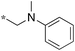
|
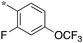
|
0.29 | 59 | 30 |
| 10 | 6a |
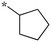
|

|
0.31 | 86 | 42 |
Computational analysis of SAR and toxicity prediction
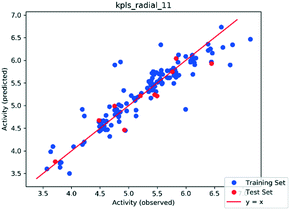 | ||
| Fig. 3 Plot of experimental vs. predicted activities for the training set and test set compounds (AutoQSAR). | ||
Next, we examined the validity of this 3D-QSAR model by applying it for the prediction of the MIC values of the top 12 and other two new benzimidazoles in the present work, as well as four of the lead compounds from our previously published work. The results are summarized in Table 4. As Table 4 shows, the predicted MIC values are all very close to those determined experimentally, except for 20f (entry 6).
| Entry | Compound | R1 | R2 | MICa | Predicted MIC | Clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
|---|---|---|---|---|---|---|
| a MIC determined by Protocol A. See Experimental Section. b MIC determined by Protocol B. See Experimental Section. c Normalized MIC from the MIC determined by Protocol B to the MIC by Protocol A. | ||||||
| 1 | SB-P17G-C2 |
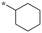
|

|
0.06 | 0.16 | 4.456 |
| 2 | SB-P17G-A20 |
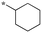
|
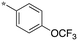
|
0.16 | 0.24 | 4.936 |
| 3 | SB-P17G-A38 |
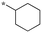
|
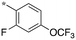
|
0.16 | 0.28 | 4.669 |
| 4 | SB-P17G-A42 |
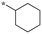
|
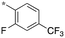
|
0.16 | 0.21 | 4.524 |
| 5 | 20g |
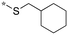
|

|
0.015c | 0.03 | 5.675 |
| (0.0039b) | ||||||
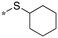
| 6 | 20f |
|

|
0.031 | 0.62 | 5.056 |
| (<0.0081b) | ||||||
| 7 | 6b |
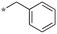
|

|
<0.078 | 0.17 | 3.903 |
| 8 | 6c |
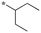
|

|
0.078 | 0.08 | 4.321 |
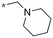
| 9 | 11c |
|

|
0.14c | 0.22 | 3.093 |
| (0.09b) | ||||||
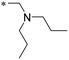
| 10 | 11a |
|

|
0.16 | 0.26 | 4.016 |
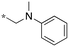
| 11 | 11e |
|

|
0.16 | 0.13 | 3.999 |
| 12 | 10e-6 |
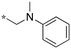
|
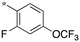
|
0.29c | 0.10 | 4.212 |
| (0.19b) | ||||||
| 13 | 5c-7 |
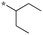
|
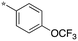
|
0.31 | 0.43 | 4.801 |
| 14 | 6a |
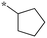
|

|
0.31 | 0.60 | 3.897 |
| 15 | 20b |
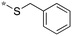
|

|
0.31 | 0.31 | 4.593 |
| 16 | 20c |
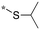
|

|
0.31 | 0.68 | 3.863 |
| 17 | 20e |
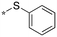
|

|
0.31 | 0.67 | 4.804 |
| 18 | 5b-2 |
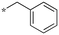
|
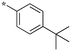
|
0.33 | 0.33 | 5.181 |
| 19 | 20d |
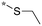
|

|
1.2c | 0.5 | 3.554 |
| (0.16b) | ||||||
| 20 | 20a |
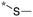
|

|
9.6c | 3.96 | 3.025 |
| (1.28b) | ||||||
It should be noted that the most potent compound, 20g, in this series, is actually predicted as the best compound (entry 5). The results clearly indicate that the prediction based on the AutoQSAR model developed in the present work can be used for the selection of promising compounds from the designed compounds in silico for chemical synthesis and biological evaluations. Table 4 also lists calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values using the ChemDraw Pro program. All compounds show the Clog
P values using the ChemDraw Pro program. All compounds show the Clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 3–5, which are in a good range in the Lipinski's rule of 5.30
P values of 3–5, which are in a good range in the Lipinski's rule of 5.30
We also carried out computational analysis of toxicity prediction using pKCSM program (Table S1†), as well as docking analysis of compound 20g in the putative binding site of Mtb FtsZ (Fig. S1†). This information is available in the ESI.†
Conclusions
A library of 2,5,6-trisubstituted benzimidazoles with a variety of modifications at the 2 position were synthesized and tested for their antitubercular activities against Mtb H37Rv. Among the compounds with different functional groups at the 2 position, those having a butyl carbamate at the 5 position resulted in the most active compound within each series (6a, 6b, 6c, 6d, 11e), following a trend similar to our previous series of 2,5,6-trisubstituted benzimidazoles with a cyclohexyl substitution at the 2 position. Functional groups with an sp3 carbon at the 2 position were preferred over ones with an sp2 carbon. From this series, we identified 13 compounds (5c-7, 6a, 6b, 6c, 10e-6, 11a, 11c, 11e, 20b, 20c, 20e, 20f and 20g) that were highly potent. Overall SAR of 2,5,6-trisusbstituted benzimidazoles is summarized in Fig. 4. We built a robust 3DQSAR model by following up Li's model,29 and used this updated model to predict the MIC of the top 13 compounds. Since almost all of the predicted MIC values are very close to those determined experimentally, this model can be used for the selection of promising compounds from the designed compounds in silico for chemical synthesis and biological evaluations in the future. Compound 20g was found to be the most potent compound (MIC 0.0039 μg mL−1; normalized MIC 0.015 μg mL−1) from the current series, both experimentally and computationally from the 3DQSAR model. Not only was 20g the most potent compound of the series, but also it was more potent than the previous lead SB-P17G-C2 (MIC 0.06 μg mL−1). Also, the cytotoxicity (IC50) of 20g is found to be ca. 20 μM, which indicates the therapeutic index (IC50/MIC) of 20g is >100. Based on our current results, systematic SAR study on 2-alkylsulfanylbenzimidazoles with varying substituents at the 5 and 6 positions is clearly warranted.Experimental section
General methods
Melting points were measured on a Thomas Hoover Capillary melting point apparatus and are uncorrected. NMR spectra were recorded on a Bruker Ascend 700 spectrometer operating at 700 MHz for 1H and 175 MHz for 13C, a Bruker 500 Advance spectrometer operating at 500 MHz and 125 MHz for 1H and 13C, respectively, or a Bruker 400 Nanobay spectrometer operating at 400 MHz, 100 MHz, and 376 MHz for 1H, 13C, and 19F, respectively or a Bruker 300 Nanobay spectrometer operating at 300 MHz of 1H. Chemical shifts were referenced to the residual proton and carbon-13 peaks of solvents used for 1H and 13C NMRs, respectively (1H: CDCl3, δ 7.26; 13C: CDCl3, δ 77.23; 1H: DMSO-d6, δ 2.50; 13C: DMSO-d6, δ 39.51). Signals are listed in ppm, and multiplicity identified as s (singlet), bs (broad singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiplet); J -coupling constants in Hz, and integration. Low resolution mass spectrometry (LRMS) analysis was carried out on an Agilent LC-MSD mass spectrometer and high resolution mass spectrometry (HRMS) analysis was carried out on an Agilent LC-UV-TOF mass spectrometer at the Institute of Chemical Biology and Drug Discovery, Stony Brook. Purity of the synthesized compounds was determined by a Shimadzu LC-2010A HT series HPLC assembly or Agilent 1100 series HPLC assembly. Four analytical conditions were used and noted as a part of the characterization data for synthesized compounds. HPLC (1): adsorbosphere silica 5 μm, 250 mm Å ∼ 4.6 mm column, isopropanol and hexanes, flow rate of 1 mL min−1, t = 0–40 min, gradient of 5–50% isopropanol. (2): Kinetex PFP, 2.6 μm, 4.6 mm Å ∼ 100 mm column, isopropanol and hexanes, flow rate of 0.3 mL min−1,, t = 0–25 min, gradient of 5–95% isopropanol. (3): Kinetex PFP, 2.6 μm, 4.6 mm Å ∼ 100 mm column, isopropanol and hexanes, flow rate of 0.3 mL min−1, t = 0–20 min, gradient of 5–80% isopropanol. (4): Kinetex PFP, 2.6 μm, 4.6 mm Å ∼ 100 mm column, isopropanol and hexanes, flow rate of 0.3 mL min−1, t = 0–30 min, gradient of 5–80% isopropanol. Measurements were made at 254 and 303 nm.Materials
All air- and moisture-insensitive reactions were carried out under an ambient atmosphere, magnetically stirred, and monitored by thin layer chromatography (TLC) using Agilent Technologies TLC plates pre-coated with 250 μm thickness silica gel 60 F254 plates and visualized by fluorescence quenching under UV light. Flash chromatography was performed on SiliaFlash® Silica Gel 40–63 μm 60 Å particle size using a forced flow of eluent at 0.3–0.5 bar pressure. All air- and moisture-sensitive manipulations were performed using oven-dried glassware using the standard Schlenk techniques under nitrogen. Diethyl ether and tetrahydrofuran (THF) were distilled from deep purple sodium benzopheone ketyl. Dichloromethane (DCM), chloroform and acetonitrile were dried over calcium hydride and distilled. DCM was degassed via three freeze-pump-thaw cycles. All other chemicals were used as received. All deuterated solvents were purchased from Cambridge Isotope Laboratories. 5-N,N-Dimethylamino-2,4-dinitroaniline (2) was synthesized using the procedure previously published by us.13 4-Fluoro-1,2-di(4-methylbenzenesulfonamido)benzene (13) and 4-fluoro-5-nitro-1,2-di(4-methylbenzene-sulfonamido)benzene (14) were synthesized by literature methods.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AcOEt
1 AcOEt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes as eluent to afford 5a-1 as an off-white solid (134 mg, 94% yield); mp 176–180 °C; 1H NMR (500 MHz, CDCl3) δ 1.46–1.48 (m, 2 H), 1.62–1.68 (m, 2 H), 1.83–1.96 (m, 4 H), 2.72 (s, 6 H), 3.09 (quintet, J = 8.5 Hz, 1 H), 7.52–7.58 (m, 3 H), 7.61 (s, 1 H), 7.97 (d, J = 7.1 Hz, 2 H), 8.94 (s, 1 H), 9.98 (s, 1 H), 11.66 (s, 1 H); 13C NMR (125 MHz, CDCl3) δ 25.5, 32.5, 39.9, 46.1, 101.8, 110.6, 127.1, 128.9, 129.0, 131.8, 131.9, 135.8, 139.3, 139.9, 159.74, 165.7; HRMS (ESI-TOF) m/z calcd for C21H24N4OH+: 349.2023, found: 349.2013 (Δ = 2.78 ppm); HPLC (1): t = 10.0 min, purity >96%.
hexanes as eluent to afford 5a-1 as an off-white solid (134 mg, 94% yield); mp 176–180 °C; 1H NMR (500 MHz, CDCl3) δ 1.46–1.48 (m, 2 H), 1.62–1.68 (m, 2 H), 1.83–1.96 (m, 4 H), 2.72 (s, 6 H), 3.09 (quintet, J = 8.5 Hz, 1 H), 7.52–7.58 (m, 3 H), 7.61 (s, 1 H), 7.97 (d, J = 7.1 Hz, 2 H), 8.94 (s, 1 H), 9.98 (s, 1 H), 11.66 (s, 1 H); 13C NMR (125 MHz, CDCl3) δ 25.5, 32.5, 39.9, 46.1, 101.8, 110.6, 127.1, 128.9, 129.0, 131.8, 131.9, 135.8, 139.3, 139.9, 159.74, 165.7; HRMS (ESI-TOF) m/z calcd for C21H24N4OH+: 349.2023, found: 349.2013 (Δ = 2.78 ppm); HPLC (1): t = 10.0 min, purity >96%.
The same procedure was used for the synthesis and characterization of 5a-3, 5a-7, 5a-8, 5b-1, 5b-3, 5b-6, 5b-7, 5c-1, 5c-2, 5h-1, 5h-2 and 10e-6.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AcOEt
1 AcOEt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes as eluent to afford 5a-2 as beige solid (132 mg, 80% yield); mp 207–210 °C; 1H NMR (400 MHz, CDCl3) δ 1.38 (s, 9 H), 1.65–1.70 (m, 2 H), 1.82–1.85 (m, 2 H), 1.87–1.93 (m, 4 H), 2.73 (s, 6 H), 3.03–3.12 (quintet, J = 8.4 Hz, 1 H), 7.56 (d, J = 8.4 Hz, 2 H), 7.61 (s, 1 H), 7.91 (d, J = 8.4 Hz, 2 H), 8.96 (s, 1 H), 9.96 (s, 1 H), 11.39 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 25.6, 31.4, 32.5, 35.2, 39.9, 46.2, 101.7, 110.4, 126.0, 127.0, 129.1, 131.9, 133.1, 139.2, 139.9, 155.5, 159.6, 165.9; HRMS (ESI-TOF) m/z calcd for C25H32N4OH+: 405.2649, found: 405.2641 (Δ = 1.87 ppm). HPLC (1): t = 9.98 min, purity >99%.
hexanes as eluent to afford 5a-2 as beige solid (132 mg, 80% yield); mp 207–210 °C; 1H NMR (400 MHz, CDCl3) δ 1.38 (s, 9 H), 1.65–1.70 (m, 2 H), 1.82–1.85 (m, 2 H), 1.87–1.93 (m, 4 H), 2.73 (s, 6 H), 3.03–3.12 (quintet, J = 8.4 Hz, 1 H), 7.56 (d, J = 8.4 Hz, 2 H), 7.61 (s, 1 H), 7.91 (d, J = 8.4 Hz, 2 H), 8.96 (s, 1 H), 9.96 (s, 1 H), 11.39 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 25.6, 31.4, 32.5, 35.2, 39.9, 46.2, 101.7, 110.4, 126.0, 127.0, 129.1, 131.9, 133.1, 139.2, 139.9, 155.5, 159.6, 165.9; HRMS (ESI-TOF) m/z calcd for C25H32N4OH+: 405.2649, found: 405.2641 (Δ = 1.87 ppm). HPLC (1): t = 9.98 min, purity >99%.
The same procedure was used for the synthesis and characterization of compounds 5a-4, 5a-5, 5a-6, 5a-9, 5a-10, 5a-11, 5b-2, 5b-4, 5b-5, 5b-8, 5b-9, 5c-3, 5c-4, 5c-5, 5c-6, 5c-7, 5d-1, 10e-1, 10e-2, 10e-3, 10e-4 and 10e-5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AcOEt
1 AcOEt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes as eluent to afford 6a as a white solid (98 mg, 70% yield); mp 174–175 °C; 1H NMR (500 MHz, CDCl3) δ 0.95 (t, J = 7.4 Hz,3 H), 1.39–1.47 (m, 2 H), 1.63–1.71 (m, 4 H), 1.75–1.82 (m, 2 H), 1.87–1.95 (m, 2 H), 2.07–2.13 (m, 2 H), 2.62 (s, 6 H), 3.23 (quintet, J = 8.4 Hz,1 H,), 4.18 (t, J = 6.7 Hz, 2 H), 7.5 (s, 1 H), 8.19 (s, 2 H), 9.99 (s, 1 H); 13C NMR (125 MHz, CDCl3) δ 14.0, 19.3, 25.7, 31.3, 32.5, 39.8, 45.8, 65.1, 99.7, 110.7, 129.5, 131.3, 138.8, 154.4, 158.7; HRMS (ESI-TOF) m/z calcd for C19H28N4O2H+: 345.2285, found: 345.2279 (Δ = 1.87 ppm). HPLC (2): t = 22.1 min, purity >96%.
hexanes as eluent to afford 6a as a white solid (98 mg, 70% yield); mp 174–175 °C; 1H NMR (500 MHz, CDCl3) δ 0.95 (t, J = 7.4 Hz,3 H), 1.39–1.47 (m, 2 H), 1.63–1.71 (m, 4 H), 1.75–1.82 (m, 2 H), 1.87–1.95 (m, 2 H), 2.07–2.13 (m, 2 H), 2.62 (s, 6 H), 3.23 (quintet, J = 8.4 Hz,1 H,), 4.18 (t, J = 6.7 Hz, 2 H), 7.5 (s, 1 H), 8.19 (s, 2 H), 9.99 (s, 1 H); 13C NMR (125 MHz, CDCl3) δ 14.0, 19.3, 25.7, 31.3, 32.5, 39.8, 45.8, 65.1, 99.7, 110.7, 129.5, 131.3, 138.8, 154.4, 158.7; HRMS (ESI-TOF) m/z calcd for C19H28N4O2H+: 345.2285, found: 345.2279 (Δ = 1.87 ppm). HPLC (2): t = 22.1 min, purity >96%.
Compounds 6b–6d, 6f, 6h, 11a–11e and 20a–20h were synthesized in a similar manner and characterized.
Protocol B:27 Each compound was dissolved in DMSO at a final concentration of 12 mg mL−1 and serial dilutions were performed to generate test concentrations ranging from 32 mg mL−1 to 0.488 ng mL−1. M. tuberculosis strain H37Rv at the midlogarithmic stage of growth (OD580 = 0.4) was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, and 0.1 mL was added to each well of a 96-well plate along with 0.1 mL of test compound solution. After 6 days of incubation at 37 °C, Alamar blue (Invitrogen) reagent was added along with 12.5 mL of 20% Tween 80 (Sigma) to evaluate bacterial cell viability. Plates were scanned 24 h later at 570 nm with a reference wavelength of 600 nm utilizing a Biotek Instruments ELX 808. Inoculum control wells of untreated H37Rv were used to create a survival-inhibition curve with each assay. Rifampicin was used as a positive control (MIC = 0.0125–0.05 μg mL−1).
100, and 0.1 mL was added to each well of a 96-well plate along with 0.1 mL of test compound solution. After 6 days of incubation at 37 °C, Alamar blue (Invitrogen) reagent was added along with 12.5 mL of 20% Tween 80 (Sigma) to evaluate bacterial cell viability. Plates were scanned 24 h later at 570 nm with a reference wavelength of 600 nm utilizing a Biotek Instruments ELX 808. Inoculum control wells of untreated H37Rv were used to create a survival-inhibition curve with each assay. Rifampicin was used as a positive control (MIC = 0.0125–0.05 μg mL−1).
Author contributions
K. H.: experimental design and synthesis, data collections, data analysis, manuscript preparation; S. T. design, synthesis, data collections, partial manuscript preparation; S. K.: 3D-QSAR model building and analysis, pkCSM analysis, data collections, partial manuscript preparation; M. A.: molecular docking analysis, partial 3D-QSAR model building; L. C.: cytotoxicity assay; S. E. K., E. S. and R. R. MABA assay, MIC determination; R. A. S. and N. C. supervision of MABA assays. I. O.: research design, supervision of whole project, manuscript preparation.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research is supported by grants from the National Institutes of Health (Grant AI078251 to I. O. and Grant AI082164 to R. A. S.). The authors thank Adam Taouil for his technical assistance on computational analyses and Dr. Bela Ruzsicska, Institute of Chemical Biology and Drug Discovery, Stony Brook University for HRMS analyses.Notes and references
- Global Tuberculosis Report, W.H.O, 2019 Search PubMed.
- S. J. Berthel, C. B. Cooper and N. Fotouhi, in Annual Reports in Medicinal Chemistry, Academic Press, 2019, DOI:10.1016/bs.armc.2019.05.004.
- M. Sester, F. van Leth, J. Bruchfeld, D. Bumbacea, D. M. Cirillo, A. G. Dilektasli, J. Domínguez, R. Duarte, M. Ernst, F. O. Eyuboglu, I. Gerogianni, E. Girardi, D. Goletti, J.-P. Janssens, I. Julander, B. Lange, I. Latorre, M. Losi, R. Markova, A. Matteelli, H. Milburn, P. Ravn, T. Scholman, P. M. Soccal, M. Straub, D. Wagner, T. Wolf, A. Yalcin and C. Lange, Am. J. Respir. Crit. Care Med., 2014, 190, 1168–1176 CrossRef
.
- A. Cohen, V. D. Mathiasen, T. Schön and C. Wejse, Eur. Respir. J., 2019, 1900655, DOI:10.1183/13993003.00655-2019
.
- U. B. Karale, V. S. Krishna, E. V. Krishna, A. S. Choudhari, M. Shukla, V. R. Gaikwad, B. Mahizhaveni, S. Chopra, S. Misra, D. Sarkar, D. Sriram, V. N. A. Dusthackeer and H. B. Rode, Eur. J. Med. Chem., 2019, 178, 315–328 CrossRef CAS
.
- A. Bahuguna and D. S. Rawat, Med. Res. Rev., 2019, 40, 263–292 CrossRef
.
- A. S. Pym, A. H. Diacon, S.-J. Tang, F. Conradie, M. Danilovits, C. Chuchottaworn, I. Vasilyeva, K. Andries, N. Bakare, T. De Marez, M. Haxaire-Theeuwes, N. Lounis, P. Meyvisch, B. Van Baelen, R. P. G. van Heeswijk and B. Dannemann, Eur. Respir. J., 2016, 47, 564 CrossRef CAS
.
- J. D. Szumowski and J. B. Lynch, Drug Des., Dev. Ther., 2015, 9, 677–682 CAS
.
- J. Jones, V. Mudaly, J. Voget, T. Naledi, G. Maartens and K. Cohen, BMC Infect. Dis., 2019, 19, 544 CrossRef
.
- Y. Li, F. Sun and W. Zhang, Drug Dev. Res., 2019, 80, 98–105 CrossRef CAS
.
-
TB Alliance, Pretomanid and the BPal Regimen, https://www.tballiance.org/access/pretomanid-and-bpal-regimen, 2019 Search PubMed
.
-
TB Alliance, FDA Approves New Treatment for Highly Drug-Resistant Forms of Tuberculosis, https://www.tballiance.org/news/fda-approves-new-treatment-highly-drug-resistant-forms-tuberculosis, (accessed August 14, 2019) Search PubMed
.
- D. Awasthi, K. Kumar, S. E. Knudson, R. A. Slayden and I. Ojima, J. Med. Chem., 2013, 56, 9756–9770 CrossRef CAS
.
- K. Kumar, D. Awasthi, S.-Y. Lee, I. Zanardi, B. Ruzsicska, S. Knudson, P. J. Tonge, R. A. Slayden and I. Ojima, J. Med. Chem., 2011, 54, 374–381 CrossRef CAS
.
- K. Haranahalli, S. Tong and I. Ojima, Bioorg. Med. Chem., 2016, 24, 6354–6369 CrossRef CAS
.
- N. W. Goehring and J. Beckwith, Curr. Biol., 2005, 15, R514–R526 CrossRef CAS
.
- A. K. W. Leung, E. Lucile White, L. J. Ross, R. C. Reynolds, J. A. DeVito and D. W. Borhani, J. Mol. Biol., 2004, 342, 953–970 CrossRef CAS
.
- J. Errington, R. A. Daniel and D.-J. Scheffers, Microbiol. Mol. Biol. Rev., 2003, 67, 52 CrossRef CAS
.
- W. Margolin, Nat. Rev. Mol. Cell Biol., 2005, 6, 862–871 CrossRef CAS
.
- A. Mukherjee and J. Lutkenhaus, J. Bacteriol., 1994, 176, 2754–2758 CrossRef CAS
.
- E. Nogales, S. G. Wolf and K. H. Downing, Nature, 1998, 391, 199–203 CrossRef CAS
.
- S. Tripathy and S. K. Sahu, Bioorg. Chem., 2019, 91, 103169 CrossRef CAS
.
- B. Park, D. Awasthi, S. R. Chowdhury, E. H. Melief, K. Kumar, S. E. Knudson, R. A. Slayden and I. Ojima, Bioorg. Med. Chem., 2014, 22, 2602–2612 CrossRef CAS
.
- S. E. Knudson, D. Awasthi, K. Kumar, A. Carreau, L. Goullieux, S. Lagrange, H. Vermet, I. Ojima and R. A. Slayden, J. Antimicrob. Chemother., 2015, 70, 3070–3073 CrossRef CAS
.
- L. Collins and S. G. Franzblau, Antimicrob. Agents Chemother., 1997, 41, 1004–1009 CrossRef CAS
.
- M. Sarcina and C. W. Mullineaux, FEMS Microbiol. Lett., 2000, 191, 25–29 CrossRef CAS
.
- S. Ekins, Robert C. Reynolds, H. Kim, M.-S. Koo, M. Ekonomidis, M. Talaue, Steve D. Paget, Lisa K. Woolhiser, Anne J. Lenaerts, Barry A. Bunin, N. Connell and Joel S. Freundlich, Chem. Biol., 2013, 20, 370–378 CrossRef CAS
.
- D. E. V. Pires, T. L. Blundell and D. B. Ascher, J. Med. Chem., 2015, 58, 4066–4072 CrossRef CAS
.
- D. Li, B. Chi, W.-W. Wang, J.-M. Gao and J. Wan, Med. Chem. Res., 2016, 26, 153–169 CrossRef
.
- S. L. Dixon, J. Duan, E. Smith, C. D. Von Bargen, W. Sherman and M. P. Repasky, Future Med. Chem., 2016, 8, 1825–1839 CrossRef CAS
.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3–25 CrossRef CAS
.
- R. Hinkens, P. J. Leveque, D. Castelet and J. Nasielski, Heterocycles, 1987, 26, 2433–2442 CrossRef
.
- M. E. Boyne, T. J. Sullivan, C. W. amEnde, H. Lu, V. Gruppo, D. Heaslip, A. G. Amin, D. Chatterjee, A. Lenaerts, P. J. Tonge and R. A. Slayden, Antimicrob. Agents Chemother., 2007, 51, 3562–3567 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization data for all key synthetic intermediates; 1H and 13C NMR spectra of all new 2,5,6-trisubstituted benzimidazoles. See DOI: 10.1039/d0md00256a |
| This journal is © The Royal Society of Chemistry 2021 |