Open Access Article
Open Access ArticleSingle-step ball milling synthesis of highly Li+ conductive Li5.3PS4.3ClBr0.7 glass ceramic electrolyte enables low-impedance all-solid-state batteries†
Marvin
Cronau
,
Marvin
Szabo
and
Bernhard
Roling
 *
*
Department of Chemistry, Philipps-Universität Marburg, Hans-Meerwein-Strasse 4, 35032, Marburg, Germany. E-mail: roling@staff.uni-marburg.de
First published on 11th November 2021
Abstract
A single-step high-energy ball milling process was used for the synthesis of a Li5.3PS4.3ClBr0.7 glass ceramic. This material exhibits a Li+ conductivity of 5.2 mS cm−1, the highest value reported so far at room temperature for a Li+ solid electrolyte pellet prepared without any annealing step. We demonstrate the utilization of this electrolyte in an all-solid-state battery exhibiting a composite cathode impedance of only 5 Ω cm2 at room temperature.
All-solid-state batteries (ASSBs) have come into focus in recent years as energy storage devices with energy densities potentially exceeding those of conventional lithium-ion batteries (LIBs). However, in practice, severe problems still have to be solved. In order to reach high energy densities, it is indispensable to use lithium as anode and to build composite cathodes with high active material loading in the range of 85 wt%.1,2 In order to achieve fast ion transport inside the cathode despite the high active material loading, the solid electrolyte should have a high ionic conductivity and should form a continuous phase with low-tortuosity ion transport pathways.
The currently most promising group of SEs are sulphide-based materials. Recently, Lee et al. built an ASSB with a capacity retention of over 80% after 1000 cycles using microcrystalline argyrodite-type Li6PS5Cl materials as electrolyte.2 Most sulphide-based SEs are synthesized via a two-step or even three-step synthesis. First the starting materials are mixed and pre-treated in a ball mill. Afterwards, the resulting powder is filled into a quartz ampoule, and the quartz ampoule is heated up to a temperature (powder annealing), at which the material is partially crystallized (glass ceramic) or completely crystallised (microcrystalline material). The powder is then ground and pressed to a pellet (cold-pressed pellet), in some cases followed by pellet annealing. Pellet annealing leads usually to a considerable enhancement of the ionic conductivity due to reduced grain boundary resistances.
Argyrodite-type Li6PS5Cl reaches ionic conductivity values up to 6 mS cm−1 after pellet annealing.3–16 Although this value is sufficient for building working ASSBs, some pellet-annealed, fully microcrystalline SEs with a significantly higher conductivity have been discovered. One way to increase the Li+ conductivity is cation doping of the argyrodites. For instance, Li6.6P0.4Ge0.6S5I exhibits a Li+ conductivity of 5.4 mS cm−1 after powder annealing and cold pressing in comparison to Li6PS5I with only 1.3 μS cm−1. After pellet annealing, the conductivity of Li6.6P0.4Ge0.6S5I increases to 18.4 mS cm−1.17 Li6.6Si0.6Sb0.4S5I outperforms Li6.6P0.4Ge0.6S5I with 14.8 mS cm−1 for the cold-pressed pellet and 24 mS cm−1 for the annealed pellet.18 However, materials containing tetrel atoms are chemically less stable than the parent compound Li6PS5Cl.19–21
Another promising approach to boost the Li+ conductivity is the synthesis of halide-rich Li6−xPS5−xHal1+x (Hal = Cl, Br) solid electrolytes.22,23 For example, the reported Li+ conductivities of Li5.5PS4.5Cl1.5 and Li5.5PS4.5Br1.5 after pellet annealing reach values of about 11 and 12 mS cm−1, respectively, while the conductivity of the recently synthesized mixed-halide microcrystalline argyrodite Li5.3PS4.3ClBr0.7 reaches 24 mS cm−1.24–27
In general, two-step or three-step syntheses are well suited for lab-scale production, but not for commercial large-scale production.28 Furthermore, high-temperature annealing (around 500–600 °C) of SE particles inside the composite electrodes of ASSBs is not possible due to the limited thermal stability of active material particles, like NMC.29 Therefore, the development of highly conductive SE materials, which reach high ionic conductivities without using high-temperature annealing steps during synthesis is of utmost importance.
Herein, we present a single-step ball milling synthesis of a glass ceramic (GC) Li5.3PS4.3ClBr0.7 powder. After cold pressing, the resulting pellet reaches the highest Li+ conductivity reported so far for Li+ solid electrolytes prepared without any annealing step. The simple synthesis in comparison to the materials mentioned above, see Fig. 1, and the already demonstrated long-term stability of glass ceramic solid electrolytes in ASSBs30 makes GC Li5.3PS4.3ClBr0.7 a viable candidate for the use in ASSBs. We demonstrate that the integration of GC Li5.3PS4.3ClBr0.7 into an In/LiCoO2 solid-state battery leads to remarkably low cathode impedance and overall battery impedances.
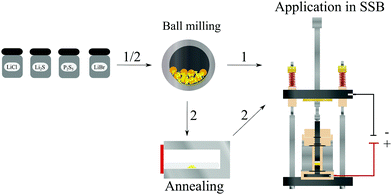 | ||
| Fig. 1 Illustration of different synthesis routes for solid electrolytes and of a home-made cell setup for the characterisation of all solid-state batteries (ASSBs). | ||
Stoichiometric amounts of the precursors were filled into a zirconia pot with 10 zirconia balls (10 mm diameter) and ball milled for 8.25 hours (850 rpm). In the case of argyrodites, this synthesis route leads in general to nanocrystallites in an amorphous matrix, with low volume fractions of the amorphous phase in the range of 10–15 vol%.27,31–33 The resulting powder (Fig. 2) was used as received without any heat treatment (a detailed description of the synthesis can be found in the ESI†). The X-ray powder diffractogram shows the typical nanocrystalline pattern (average particle size about 18 nm) of many argyrodite-type glass ceramic electrolytes.
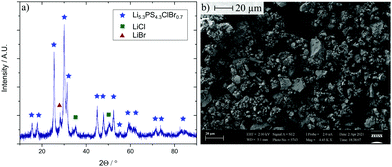 | ||
| Fig. 2 (a) X-Ray diffraction pattern of the SE. (b) Scanning electron microscopy image (magnification: 5000) of the synthesized powder. | ||
For the fabrication of pellets for Li+ conductivity measurements, a fabrication pressure of 490 MPa was used inside a CompreDrive laboratory press (rhd instruments, Darmstadt, Germany). The impedance measurements were carried out by means of an Autolab PGSTAT302N (Metrohm Autolab, Utrecht, Netherlands) under an applied stack pressure of 100 MPa. The impedance spectra were taken in a frequency range from 105–101 Hz and in a temperature range from 30–85 °C with an applied rms AC voltage of 10 mV. Since reported Li+ conductivity values depend strongly on the fabrication pressure and stack pressure, we used identical pressure conditions for the fabrication of four solid electrolyte pellets and the subsequent ionic conductivity measurements. These solid electrolytes are: (i) GC Li6PS5Cl prepared by a single-step ball milling synthesis; (ii) microcrystalline (μC) Li6PS5Cl with applied powder annealing at 550 °C; (iii) GC Li5.5PS4.5Cl1.5 prepared by a single-step ball milling synthesis; (iv) GC Li5.3PS4.3ClBr0.7 prepared by a single-step ball milling synthesis.
Fig. 3 shows an Arrhenius plot of the Li+ conductivity of all materials at 30 °C. With 5.2 mS cm−1, the ionic conductivity of Li5.3PS4.3ClBr0.7 clearly exceeds those of the other materials, and even that of powder-annealed Li6PS5Cl.
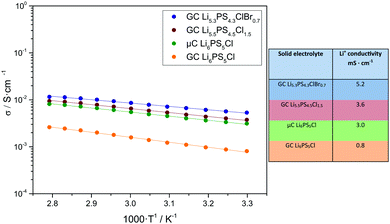 | ||
| Fig. 3 Arrhenius plots of the Li+ conductivity σ of different solid electrolytes (left, temperature range 30 to 85 °C) and table with Li+ ionic conductivity at 30 °C (right). | ||
To demonstrate the performance of the new glass ceramic Li5.3PS4.3ClBr0.7 electrolyte in an ASSB, the electrolyte was used for the preparation of the separator layer as well as for the preparation of composite cathodes with LiNbO3-coated LiCoO2 active material particles and carbon fibers as conductive additive (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
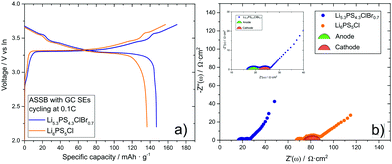
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 wt%). Indium foil was used as anode, which was pressed for one minute with 298 MPa onto the separator layer to form a good anode/separator contact. The lab-scale ASSB was characterized in a home-made cell setup with pressure control, see Fig. 1. For the battery cycling experiments, a pressure of 98 MPa was applied, and the battery was cycled at room temperature with a rate of 0.1 C between the cutoff voltages 2.1 V and 3.7 V. Fig. 4(a) shows the first charge and the first discharge voltage profile of the battery. With a discharge capacity of 147 mA h g−1, the battery exploits the full capacity of LiCoO2. For comparison, an ASSB was built with non-annealed Li6PS5Cl in the composite cathode and in the separating SE layer, which reaches only a capacity of 137 mA h g−1. Impedance spectra of the two ASSBs after the first discharge cycle are shown in Fig. 4(b). The higher ionic conductivity of GC Li5.3PS4.3ClBr0.7 as compared to GC Li6PS5Cl manifest in a lower high-frequency resistance of the GC Li5.3PS4.3ClBr0.7-based battery, which can be identified with the separator resistance, and in a smaller second semicircle, which is usually attributed to the cathode impedance. The cathode impedance is determined by the ion transport resistance of the solid electrolyte within the composite cathode as well as by the charge transfer resistance at the interfaces between the active material particles and the solid electrolyte particles.34 The fast ion transport in the GC Li5.3PS4.3ClBr0.7-based cathode leads to a cathode impedance value of only 5 Ω cm2, which is one of the lowest values reported so far at room temperature.35
3 wt%). Indium foil was used as anode, which was pressed for one minute with 298 MPa onto the separator layer to form a good anode/separator contact. The lab-scale ASSB was characterized in a home-made cell setup with pressure control, see Fig. 1. For the battery cycling experiments, a pressure of 98 MPa was applied, and the battery was cycled at room temperature with a rate of 0.1 C between the cutoff voltages 2.1 V and 3.7 V. Fig. 4(a) shows the first charge and the first discharge voltage profile of the battery. With a discharge capacity of 147 mA h g−1, the battery exploits the full capacity of LiCoO2. For comparison, an ASSB was built with non-annealed Li6PS5Cl in the composite cathode and in the separating SE layer, which reaches only a capacity of 137 mA h g−1. Impedance spectra of the two ASSBs after the first discharge cycle are shown in Fig. 4(b). The higher ionic conductivity of GC Li5.3PS4.3ClBr0.7 as compared to GC Li6PS5Cl manifest in a lower high-frequency resistance of the GC Li5.3PS4.3ClBr0.7-based battery, which can be identified with the separator resistance, and in a smaller second semicircle, which is usually attributed to the cathode impedance. The cathode impedance is determined by the ion transport resistance of the solid electrolyte within the composite cathode as well as by the charge transfer resistance at the interfaces between the active material particles and the solid electrolyte particles.34 The fast ion transport in the GC Li5.3PS4.3ClBr0.7-based cathode leads to a cathode impedance value of only 5 Ω cm2, which is one of the lowest values reported so far at room temperature.35
In conclusion, we synthesized the glass ceramic Li5.3PS4.3ClBr0.7 electrolyte in a single-step synthesis without any annealing step and integrated this electrolyte in a low-impedance ASSB. To our knowledge, cold-pressed GC Li5.3PS4.3ClBr0.7 exhibits the highest Li+ conductivity (5.2 mS cm−1) reported so far for electrolytes prepared without annealing steps and surpasses that of annealed microcrystalline Li6PS5Cl (3 mS cm−1). The cathode impedance of our lab-scale ASSB was only 5 Ω cm2, one of lowest values achieved so far at room temperature. Using a thinner GC Li5.3PS4.3ClBr0.7-based separator layer would further lower the total impedance to values of 30–40 Ω cm2. Building an ASSB with an area of some 1000 cm2, like in commercial Li+ ion batteries,36 would then result in a battery resistance in the range of 10 mΩ, which is similar to that of such commercial Li+ ion batteries. Together with the already reported favourable pressure-dependent properties,37 and the high long-term stability of glass ceramic SE in ASSBs,30 our results give strong indication that GC Li5.3PS4.3ClBr0.7 prepared by a simple one-step synthesis is a viable candidate for next-generation batteries. In future work, milling protocols for reducing the energy consumption should be developed, e.g. by reducing the rotational speed and by using balls with optimized diameter.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Toyota Motor Corporation for providing LiCoO2.References
- J. Janek and W. G. Zeier, A solid future for battery development, Nat. Energy, 2016, 1, 16141 CrossRef.
- Y.-G. Lee, S. Fujiki, C. Jung, N. Suzuki, N. Yashiro, R. Omoda, D.-S. Ko, T. Shiratsuchi, T. Sugimoto, S. Ryu, J. H. Ku, T. Watanabe, Y. Park, Y. Aihara, D. Im and I. T. Han, High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes, Nat. Energy, 2020, 5, 299–308 CrossRef CAS.
- S. Boulineau, M. Courty, J.-M. Tarascon and V. Viallet, Mechanochemical synthesis of Li-argyrodite Li6PS5X (X = Cl, Br, I) as sulfur-based solid electrolytes for all solid-state batteries application, Solid State Ionics, 2012, 221, 1–5 CrossRef CAS.
- S. Choi, J. Ann, J. Do, S. Lim, C. Park and D. Shin, Application of Rod-Like Li6PS5Cl Directly Synthesized by a Liquid Phase Process to Sheet-Type Electrodes for All-Solid-State Lithium Batteries, J. Electrochem. Soc., 2019, 166, A5193–A5200 CrossRef CAS.
- J.-M. Doux, Y. Yang, D. H. S. Tan, H. Nguyen, E. A. Wu, X. Wang, A. Banerjee and Y. S. Meng, Pressure effects on sulfide electrolytes for all solid-state batteries, J. Mater. Chem. A, 2020, 8, 5049–5055 RSC.
- G. Liu, W. Weng, Z. Zhang, L. Wu, J. Yang and X. Yao, Densified Li6PS5Cl Nanorods with High Ionic Conductivity and Improved Critical Current Density for All-Solid-State Lithium Batteries, Nano Lett., 2020, 20, 6660–6665 CrossRef CAS PubMed.
- P. R. Rayavarapu, N. Sharma, V. K. Peterson and S. Adams, Variation in structure and Li+-ion migration in argyrodite-type Li6PS5X (X = Cl, Br, I) solid electrolytes, J. Solid State Electrochem., 2012, 16, 1807–1813 CrossRef CAS.
- N. C. Rosero-Navarro, A. Miura and K. Tadanaga, Preparation of lithium ion conductive Li6PS5Cl solid electrolyte from solution for the fabrication of composite cathode of all-solid-state lithium battery, J. Sol-Gel Sci. Technol., 2019, 89, 303–309 CrossRef CAS.
- S. Wang, Y. Zhang, X. Zhang, T. Liu, Y.-H. Lin, Y. Shen, L. Li and C.-W. Nan, High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes Prepared via Optimized Sintering Processes for All-Solid-State Lithium–Sulfur Batteries, ACS Appl. Mater. Interfaces, 2018, 10, 42279–42285 CrossRef CAS PubMed.
- M. Xuan, W. Xiao, H. Xu, Y. Shen, Z. Li, S. Zhang, Z. Wang and G. Shao, Ultrafast solid-state lithium–ion conductor through alloying induced lattice softening of Li6PS5Cl, J. Mater. Chem. A, 2018, 6, 19231–19240 RSC.
- C. Yu, S. Ganapathy, J. Hageman, L. van Eijck, E. R. H. van Eck, L. Zhang, T. Schwietert, S. Basak, E. M. Kelder and M. Wagemaker, Facile Synthesis toward the Optimal Structure-Conductivity Characteristics of the Argyrodite Li6PS5Cl Solid-State Electrolyte, ACS Appl. Mater. Interfaces, 2018, 10, 33296–33306 CrossRef CAS PubMed.
- C. Yu, L. van Eijck, S. Ganapathy and M. Wagemaker, Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li–ion solid state batteries, Electrochim. Acta, 2016, 215, 93–99 CrossRef CAS.
- S. Yubuchi, S. Teragawa, K. Aso, K. Tadanaga, A. Hayashi and M. Tatsumisago, Preparation of high lithium-ion conducting Li6PS5Cl solid electrolyte from ethanol solution for all-solid-state lithium batteries, J. Power Sources, 2015, 293, 941–945 CrossRef CAS.
- J. Zhang, H. Zhong, C. Zheng, Y. Xia, C. Liang, H. Huang, Y. Gan, X. Tao and W. Zhang, All-solid-state batteries with slurry coated LiNi0.8Co0.1Mn0.1O2 composite cathode and Li6PS5Cl electrolyte: Effect of binder content, J. Power Sources, 2018, 391, 73–79 CrossRef CAS.
- Y. J. Nam, D. Y. Oh, S. H. Jung and Y. S. Jung, Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry- and slurry-mixing processes, J. Power Sources, 2018, 375, 93–101 CrossRef CAS.
- D. H. Kim, D. Y. Oh, K. H. Park, Y. E. Choi, Y. J. Nam, H. A. Lee, S.-M. Lee and Y. S. Jung, Infiltration of Solution-Processable Solid Electrolytes into Conventional Li–Ion-Battery Electrodes for All-Solid-State Li-Ion Batteries, Nano Lett., 2017, 17, 3013–3020 CrossRef CAS PubMed.
- M. A. Kraft, S. Ohno, T. Zinkevich, R. Koerver, S. P. Culver, T. Fuchs, A. Senyshyn, S. Indris, B. J. Morgan and W. G. Zeier, Inducing High Ionic Conductivity in the Lithium Superionic Argyrodites Li6+xP1−xGexS5I for All-Solid-State Batteries, J. Am. Chem. Soc., 2018, 140, 16330–16339 CrossRef CAS PubMed.
- L. Zhou, A. Assoud, Q. Zhang, X. Wu and L. F. Nazar, New Family of Argyrodite Thioantimonate Lithium Superionic Conductors, J. Am. Chem. Soc., 2019, 141, 19002–19013 CrossRef CAS PubMed.
- S. Wenzel, S. Randau, T. Leichtweiß, D. A. Weber, J. Sann, W. G. Zeier and J. Janek, Direct Observation of the Interfacial Instability of the Fast Ionic Conductor Li10GeP2S12 at the Lithium Metal Anode, Chem. Mater., 2016, 28, 2400–2407 CrossRef CAS.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, High-power all-solid-state batteries using sulfide superionic conductors, Nat. Energy, 2016, 1, 16030 CrossRef CAS.
- P. Bron, B. Roling and S. Dehnen, Impedance characterization reveals mixed conducting interphases between sulfidic superionic conductors and lithium metal electrodes, J. Power Sources, 2017, 352, 127–134 CrossRef CAS.
- P. Adeli, J. D. Bazak, K. H. Park, I. Kochetkov, A. Huq, G. R. Goward and L. F. Nazar, Boosting Solid-State Diffusivity and Conductivity in Lithium Superionic Argyrodites by Halide Substitution, Angew. Chem., Int. Ed., 2019, 58, 8681–8686 CrossRef CAS PubMed.
- Z. Zhang, L. Wu, D. Zhou, W. Weng and X. Yao, Flexible Sulfide Electrolyte Thin Membrane with Ultrahigh Ionic Conductivity for All-Solid-State Lithium Batteries, Nano Lett., 2021, 21, 5233–5239 CrossRef CAS PubMed.
- P. Wang, H. Liu, S. Patel, X. Feng, P.-H. Chien, Y. Wang and Y.-Y. Hu, Fast Ion Conduction and Its Origin in Li6–xPS5–xBr1+x, Chem. Mater., 2020, 32, 3833–3840 CrossRef CAS.
- X. Feng, P.-H. Chien, Y. Wang, S. Patel, P. Wang, H. Liu, M. Immediato-Scuotto and Y.-Y. Hu, Enhanced ion conduction by enforcing structural disorder in Li-deficient argyrodites Li6−xPS5−xCl1+x, Energy Storage Mater., 2020, 30, 67–73 CrossRef.
- S. V. Patel, S. Banerjee, H. Liu, P. Wang, P.-H. Chien, X. Feng, J. Liu, S. P. Ong and Y.-Y. Hu, Tunable Lithium-Ion Transport in Mixed-Halide Argyrodites Li6–xPS5–xClBrx: An Unusual Compositional Space, Chem. Mater., 2021, 33, 1435–1443 CrossRef CAS.
- W. D. Jung, J.-S. Kim, S. Choi, S. Kim, M. Jeon, H.-G. Jung, K. Y. Chung, J.-H. Lee, B.-K. Kim, J.-H. Lee and H. Kim, Superionic Halogen-Rich Li-Argyrodites Using In Situ Nanocrystal Nucleation and Rapid Crystal Growth, Nano Lett., 2020, 20, 2303–2309 CrossRef CAS PubMed.
- J. Wu, S. Liu, F. Han, X. Yao and C. Wang, Lithium/Sulfide All-Solid-State Batteries using Sulfide Electrolytes, Adv. Mater., 2021, 33, 2000751 CrossRef CAS PubMed.
- H. Konishi, T. Yuasa and M. Yoshikawa, Thermal stability of Li1−yNixMn(1−x)/2Co(1−x)/2O2 layer-structured cathode materials used in Li–Ion batteries, J. Power Sources, 2011, 196, 6884–6888 CrossRef CAS.
- S. Wang, W. Zhang, X. Chen, D. Das, R. Ruess, A. Gautam, F. Walther, S. Ohno, R. Koerver, Q. Zhang, W. G. Zeier, F. H. Richter, C.-W. Nan and J. Janek, Influence of Crystallinity of Lithium Thiophosphate Solid Electrolytes on the Performance of Solid-State Batteries, Adv. Energy Mater., 2021, 2100654 CrossRef CAS.
- P. Heitjans, M. Masoud, A. Feldhoff and M. Wilkening, NMR and impedance studies of nanocrystalline and amorphous ion conductors: lithium niobate as a model system, Faraday Discuss., 2007, 134, 67–82 RSC.
- D. Prutsch, S. Breuer, M. Uitz, P. Bottke, J. Langer, S. Lunghammer, M. Philipp, P. Posch, V. Pregartner, B. Stanje, A. Dunst, D. Wohlmuth, H. Brandstätter, W. Schmidt, V. Epp, A. Chadwick, I. Hanzu and M. Wilkening, Nanostructured Ceramics: Ionic Transport and Electrochemical Activity, Z. Phys. Chem., 2017, 231(7-8), 1361–1405 CrossRef CAS.
- M. Wilkening, V. Epp, A. Feldhoff and P. Heitjans, Tuning the Li Diffusivity of Poor Ionic Conductors by Mechanical Treatment: High Li Conductivity of Strongly Defective LiTaO3 Nanoparticles, J. Phys. Chem. C, 2008, 112, 9291–9300 CrossRef CAS.
- M. Kroll, M. Duchardt, S. L. Karstens, S. Schlabach, F. Lange, J. Hochstrasser, B. Roling and U. Tallarek, Sheet-type all-solid-state batteries with sulfidic electrolytes: Analysis of kinetic limitations based on a cathode morphology study, J. Power Sources, 2021, 505, 230064 CrossRef CAS.
- Y. Han, S. H. Jung, H. Kwak, S. Jun, H. H. Kwak, J. H. Lee, S.-T. Hong and Y. S. Jung, Single- or Poly-Crystalline Ni-Rich Layered Cathode, Sulfide or Halide Solid Electrolyte: Which Will be the Winners for All-Solid-State Batteries?, Adv. Energy Mater., 2021, 11, 2100126 CrossRef CAS.
- F. Sälzer, L. Pateras Pescara, F. Franke, C. Müller, J. Winkler, M. Schwalm and B. Roling, Assessing the Ion Transport Properties of Highly Concentrated Non-Flammable Electrolytes in a Commercial Li–Ion Battery Cell, Batteries Supercaps, 2020, 3, 117–125 CrossRef.
- M. Cronau, M. Szabo, C. König, T. B. Wassermann and B. Roling, How to Measure a Reliable Ionic Conductivity? The Stack Pressure Dilemma of Microcrystalline Sulfide-Based Solid Electrolytes, ACS Energy Lett., 2021, 6, 3072–3077 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00784j |
| This journal is © The Royal Society of Chemistry 2021 |