Open Access Article
Open Access ArticleDesign of Co1Al3(OH)m/carbon nitride hybrid nanostructures for enhanced capacitive energy storage in an alkaline electrolyte†
Prajnashree
Panda
,
Ranjit
Mishra
 ,
Sonali
Panigrahy
and
Sudip
Barman
,
Sonali
Panigrahy
and
Sudip
Barman
 *
*
School of Chemical Sciences, National Institute of Science Education and Research (NISER), HBNI, Bhubaneswar, Orissa-752050, India. E-mail: sbarman@niser.ac.in; Tel: +91-674-2494183
First published on 15th September 2021
Abstract
Over the past few years, layered double hydroxide (LDH) nanostructures have attracted the attention of the scientific community owing to their facile synthesis, interesting structure and morphology, and have been promising in the field of energy storage applications. In this work, we have synthesized CoAl LDH over a graphitic carbon nitride (CNx) surface by varying the ratio of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al and among them, the Co1Al3(OH)m/CNx composite was found to have the maximum electrochemical behaviour for supercapacitor application in alkaline electrolytes. Interestingly, it exhibits a 3D nano flower-like structure which provides a high specific capacitance (Cs) value of 138 mA h g−1 (1000 F g−1) at 1 A g−1 current density and cyclic durability of approximately 84.46% after 4500 cycles at 10 A g−1 current density. In addition, we obtained a specific capacitance of 71.5 F g−1 at 1 A g−1 current density along with long-term cyclic stability for the asymmetric supercapacitor (ASC) Co1Al3(OH)m/CNx//AC assembled with Co1Al3(OH)m/CNx and activated carbon (AC) as the positive and negative electrodes respectively. Furthermore, an energy density of 22.35 W h kg−1 is obtained at 750.2 W kg−1 power density in ASC. The enhanced charge storage property of the aforementioned material can be attributed to the high surface area of the composite and the synergetic interaction between CNx and CoAl-LDH. Moreover, this facile synthesis method is promising for designing a novel and cost-effective electrode material for supercapacitor applications.
Al and among them, the Co1Al3(OH)m/CNx composite was found to have the maximum electrochemical behaviour for supercapacitor application in alkaline electrolytes. Interestingly, it exhibits a 3D nano flower-like structure which provides a high specific capacitance (Cs) value of 138 mA h g−1 (1000 F g−1) at 1 A g−1 current density and cyclic durability of approximately 84.46% after 4500 cycles at 10 A g−1 current density. In addition, we obtained a specific capacitance of 71.5 F g−1 at 1 A g−1 current density along with long-term cyclic stability for the asymmetric supercapacitor (ASC) Co1Al3(OH)m/CNx//AC assembled with Co1Al3(OH)m/CNx and activated carbon (AC) as the positive and negative electrodes respectively. Furthermore, an energy density of 22.35 W h kg−1 is obtained at 750.2 W kg−1 power density in ASC. The enhanced charge storage property of the aforementioned material can be attributed to the high surface area of the composite and the synergetic interaction between CNx and CoAl-LDH. Moreover, this facile synthesis method is promising for designing a novel and cost-effective electrode material for supercapacitor applications.
Introduction
The depletion of fossil fuels and the rising environmental concerns have made the development of sustainable and clean energy sources crucial.1–3 The ever-increasing demand for modern electric vehicles and electronic devices promotes the growth of highly efficient energy storage devices with high energy density as well as high power density. Out of several energy storage devices, supercapacitors have gained enormous attention owing to their long life cycles, high power density and safe operation.2,4 Compared to conventional secondary batteries, supercapacitors offer promising power efficiencies to meet the demands of practical applications. Based on the energy storage mechanism, general supercapacitors are classified as electrical double-layer capacitors (EDLCs) and pseudocapacitors.5,6 In fact, EDLCs work through the adsorption of electrolytic ions on the electrode surface and pseudocapacitors work on the basis of the existence of faradaic redox reactions during the charge–discharge process.7,8 Examples of EDLCs include carbon-based materials, while pesudocapacitors include metal oxides, hydroxides and sulfides etc.9,10 Therefore designing better supercapacitors relies on developing electrode materials with noticeable capacity values.In electrochemical applications, layered double hydroxides (LDHs) have appeared as promising materials towards applications like water electrolysis and supercapacitors.2,11 LDHs are composed of both divalent and trivalent metal cations with a general formula of [M(II)1−xM(III)x(OH)2]x+[An−]x/n·zH2O, where A is an anion. Also, LDHs can intercalate and exchange anions. Since LDHs can provide many electrochemical active sites, they are potential materials that could improve the capacity of modern electrochemical capacitors. However, the low rate of diffusion of mass and electron transfer restricts the high charge–discharge capability of LDHs.1 Several efforts have been made to design LDH-based supercapacitor electrodes using non-precious metals, and different strategies such as designing a porous morphology, controlling the size of the nanocomposite, amorphization of materials, synthesizing oxygen-deficient materials or defect-rich materials, and tuning the composition of LDH have been adopted for enhanced capacitive behaviour.12 Previously it has been reported that the molar ratio of trivalent and divalent ions affects the morphology, structure and charge storage capacity of LDHs. Recently, Wu et al. used NiAl-LDH as an electrode material for a supercapacitor and investigated the impact of change in the molar feeding ratio of Ni and Al on the capacitive performance of the LDH.13 The results revealed that the LDH with a Ni/Al ratio of 3 provides the maximum specific capacitance value along with a stable lifecycle. The introduction of aluminium broadened the inter-layer spacing and improved the ion diffusion kinetics of LDH. Recently some other reports have also explored the fact that the change in molar ratio of Co/Ni can change the morphology of the NiCo LDH.14 Moreover tuning of the metal ion composition in LDHs can be crucial for improving the capacitive behaviour of the material and the electronic behaviour of active electrode materials can be enhanced via doping low-cost other metals like Al and Cu etc.
In addition, the performance of LDHs can be optimized via preparing hybrid composites with two-dimensional carbon-based materials (graphene, carbon nanotube and carbon nitride etc.) which can provide better charge transfer, electronic conductivity and high surface area to the composite material arising from the synergistic interactions between the components.9 Recently nitrogen-doped carbon materials have gained attention for energy storage applications, as the presence of nitrogen provides electron pairs to the carbon materials which results in enhanced electronic conductivity.15 Recently Tian et al. synthesized a hybrid composite of NiAl LDH and nitraime-N doped graphene which showed an enhanced capacitive performance.16 The presence of N improved the electrostatic interaction between carbon sheets and NiAl LDH and also enhanced the amount of Ni(III) ion in the material. The composite material provided increased electrochemical active sites and better electron transport during the redox reaction at the electrode–electrolyte interface. Also graphitic carbon nitride could specifically enhance the pseudocapacitive property of composites through its high nitrogen content providing surface polarity and better electron donor capacity. Although few reports of CNx supported LDHs have been published in recent times, there is a need for research on CNx supported hybrid nanocomposites for supercapacitor application.17
In this work, we propose a single step one pot synthesis of Co1Al3(OH)m/CNx composite for supercapacitor application. The growth and in situ nucleation of Co1Al3(OH)m over carbon nitride results in the formation of a 3D nanoflower type structure with a smooth surface. The Co1Al3(OH)m/CNx composite showed a superior specific capacitance value along with long term cyclic stability.
Experimental
Materials
Al (NO3)3·9H2O (aluminum(III)nitrate nonahydrate) and Co (NO3)2·6H2O (cobalt(II)nitrate hexahydrate) were purchased from Merck (India). Hexamethylenetetramine (HMT), NH2CONH2 (Urea) and KOH (potassium hydroxide) were obtained from Thermo Fisher scientific India. NH4F (Ammonium fluoride) was purchased from HI Media India. All the chemicals were used directly without any additional purification. Deionized water (DI H2O) was obtained from an ultrafiltration system (Milli-Q) at room temperature with a resistivity of 19.0 MΩ cm.Synthesis of CNx
Carbon nitride was synthesized from urea using a modified procedure similar to the reported literature.18,19 5 g of urea was taken in a porcelain crucible and covered with a Petri dish followed by heating at a temperature of 500 °C for three hours in a muffle furnace. The resulting yellow colored product was washed several times with ethanol to remove any unwanted residues and allowed to dry to obtain the desired product.Synthesis of Co1Alδ(OH)m/CNx composites
In a typical procedure, 35 mg of CNx was added to a beaker containing 20 mL of DI H2O and allowed to sonicate for 15 min. Into this, Co(NO3)2·6H2O and Al(NO3)3·9H2O were added varying in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in order to attain a total molarity of 40 mM. To this above mixture solution, 214 mmol of HMT and 135 mmol of NH4F were added and stirred to get a homogeneous mixture. Then the mixture solutions were sealed into a 50 mL Stainless steel autoclave reactor and allowed to heat at 120 °C for 6 h. After the heating was completed, the autoclave reactor was allowed to cool naturally to room temperature. Then the obtained precipitates were centrifuged at 1000 rpm and washed with DI H2O followed by ethanol several times to obtain Co1Alδ(OH)m/CNx. The Co1Alδ(OH)m/CNx was named according to their Co/Al molar ratio. Also only Co1Al3(OH)m was synthesized using a procedure the same as the above except using CNx.
4 in order to attain a total molarity of 40 mM. To this above mixture solution, 214 mmol of HMT and 135 mmol of NH4F were added and stirred to get a homogeneous mixture. Then the mixture solutions were sealed into a 50 mL Stainless steel autoclave reactor and allowed to heat at 120 °C for 6 h. After the heating was completed, the autoclave reactor was allowed to cool naturally to room temperature. Then the obtained precipitates were centrifuged at 1000 rpm and washed with DI H2O followed by ethanol several times to obtain Co1Alδ(OH)m/CNx. The Co1Alδ(OH)m/CNx was named according to their Co/Al molar ratio. Also only Co1Al3(OH)m was synthesized using a procedure the same as the above except using CNx.
Electrode fabrication
Fabrication of a working electrode was carried out on a piece of Ni foam with an area of 1 × 1 cm2. In order to wipe off the oxide layers, the electrode was washed in 3 M HCl for 30 min and then cleaned with DI H2O and then ethanol followed by drying. Homogenous slurry was prepared using a polyvinylidene fluoride (PVDF) binder in N-methyl-2-pyrrolidone (NMP), conductive carbon (CC) and active materials in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 weight percent and coated on the 1 × 1 cm2 Ni foam and dried. The weight of the active material on the Ni foam in a single electrode was 1 mg. For designing ASC, active material was used as the cathode, activated carbon (AC) as the anode and cellulose paper as the separator. Before the electrochemical performance the separator was soaked in 2 M KOH. From the calculation, mass loading of positive and negative electrodes were taken to be 1 mg and 5.4 mg and the weight ratio of positive and negative electrodes was found to be 0.185.
80 weight percent and coated on the 1 × 1 cm2 Ni foam and dried. The weight of the active material on the Ni foam in a single electrode was 1 mg. For designing ASC, active material was used as the cathode, activated carbon (AC) as the anode and cellulose paper as the separator. Before the electrochemical performance the separator was soaked in 2 M KOH. From the calculation, mass loading of positive and negative electrodes were taken to be 1 mg and 5.4 mg and the weight ratio of positive and negative electrodes was found to be 0.185.
Results and discussion
Structural and morphological characterizations
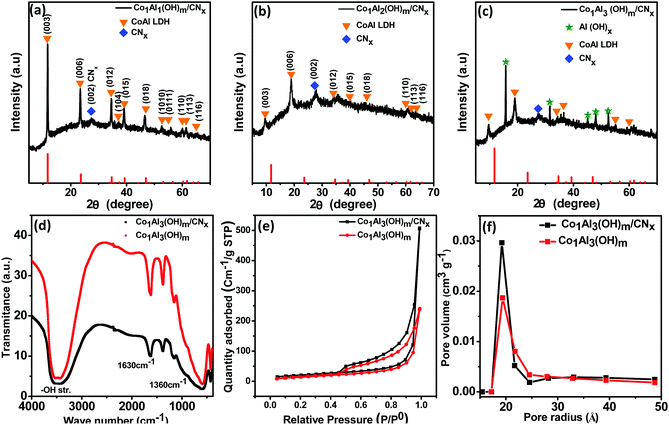
The crystal structure of as synthesized Co1Alδ(OH)m/CNx (δ = 1–4), Al(OH)x/CNx and Co(OH)2/CNx composites were characterized by using powder X-ray diffraction (p-XRD). Fig. 1(a–c) show the diffraction pattern of Co1Al1(OH)m/CNx, Co1Al2(OH)m/CNx and Co1Al3(OH)m/CNx composites respectively. The XRD peaks of Co1Al1(OH)m/CNx and Co1Al2 (OH)m/CNx composite at 11.74°, 23.6°, 34.67°, 37.39°, 39.32°, 46.92°, 60.37°, 61.69°, and 65.7° correspond to the (003), (006), (012), (104), (015), (018), (110), (113), and (116) planes of CoAl LDH structure (PDF 04-014-8855) and the peak at 27.3° corresponds to the (002) plane of CNx. The (003) diffraction peak at a 2θ value of 11.74° corresponding to the d-spacing of 0.75 nm indicates the presence of CO32− ions and H2O molecules in the interlayer space.20 As displayed in Fig. 1(c), in the XRD pattern of Co1Al3(OH)m/CNx along with the peaks of LDH structure, additional peaks of Al(OH)X were observed. On moving from Co1Al1(OH)m/CNx to Co1Al3(OH)m/CNx, the left shift of the XRD peak corresponding to the (003) plane is due to the increasing amount of Co/Al ratio.12,13,21The peak corresponding to the (003) plane moves from a 2θ value of 11.74° to 9.62° and 9.5° for Co1Al1(OH)m/CNx, Co1Al2(OH)m/CNx, and Co1Al3(OH)m/CNx respectively. This indicates that with an increasing amount of Co/Al ratio the interlayer spacing increases.13 In the case of Co1Al3(OH)m/CNx the peaks at 2θ values of 15.79°, 31.56°, 45.08°, 48° and 52.59° correspond to the (111), (222), (422), (511) and (440) plane of Al(OH)x (PDF-00-060-0273). The XRD pattern of CNx is shown in Fig. S1 (ESI†) which shows a typical diffraction peak at a 2θ value of 27.3° indexed to the plane (002) of graphitic carbon nitride. The p-XRD patterns of only Al(OH)x/CNx, Co(OH)2/CNx and Co1Al3(OH)m are shown in Fig. S2(a, b) and S3 (ESI†) respectively. The average grain size was calculated by using Scherrer's equation, D = (kλ)/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where D is the grain size, k is the shape factor with a typical value of 0.94, λ is the X-ray wavelength (0.154 nm), β is the full width at half maximum (fwhm), and θ is Bragg's angle. The average crystallite sizes of the Co1Al1(OH)m/CNx, Co1Al2(OH)m/CNx and Co1Al3(OH)m/CNx, composite materials are found to be 35.05, 24.40 and 15.05 nm respectively. Fig. 1(d) shows the Fourier transform infrared (FTIR) spectra of Co1Al3(OH)m and Co1Al3(OH)m/CNx composites. A broad peak at around 3485 cm−1 in both the samples is due to the structural –OH group stretching vibrations of water molecules. The band at 1630 cm−1 is due to the bending vibrations of H2O and the band at 1360 cm−1 indicates the presence of the interlayered CO32− ion (C–O vibrations). The absorption bands below a wavelength of 800 cm−1 indicates the stretching as well as bending modes of the metal oxygen (M–O) bond in the hydrotalcite structure22 of LDH.
θ), where D is the grain size, k is the shape factor with a typical value of 0.94, λ is the X-ray wavelength (0.154 nm), β is the full width at half maximum (fwhm), and θ is Bragg's angle. The average crystallite sizes of the Co1Al1(OH)m/CNx, Co1Al2(OH)m/CNx and Co1Al3(OH)m/CNx, composite materials are found to be 35.05, 24.40 and 15.05 nm respectively. Fig. 1(d) shows the Fourier transform infrared (FTIR) spectra of Co1Al3(OH)m and Co1Al3(OH)m/CNx composites. A broad peak at around 3485 cm−1 in both the samples is due to the structural –OH group stretching vibrations of water molecules. The band at 1630 cm−1 is due to the bending vibrations of H2O and the band at 1360 cm−1 indicates the presence of the interlayered CO32− ion (C–O vibrations). The absorption bands below a wavelength of 800 cm−1 indicates the stretching as well as bending modes of the metal oxygen (M–O) bond in the hydrotalcite structure22 of LDH.
The specific surface area (SSA) as well as the porosity are considered as crucial aspects for the improvement of electrochemical activities of the supercapacitor electrode materials. The N2 adsorption–desorption isotherms were used to determine the SSA as well as the average pore size distribution (PSD) of Co1Al3(OH)m/CNx and Co1Al3(OH)m composites. Fig. 1(e) represents a type IV adsorption/desorption isotherm with an obvious type-H3 hysteresis loop (P/P0 > 0.4) indicating the typical mesoporous nature17,23 of the Co1Al3(OH)m/CNx composite. Total BET SSA values of Co1Al3(OH)m/CNx and Co1Al3(OH)m composites were found to be 72.78 m2 g−1 and 52.051 m2 g−1 which indicates that the SSA for Co1Al3(OH)m/CNx is much higher than that of Co1Al3(OH)m. This high SSA of the Co1Al3(OH)m/CNx composite is believed to provide a large electrolyte–electrode interface for accumulation of charge electrostatically and the transportation of ions are facilitated by increasing electrical contact as well as by shortening the diffusion path.17 The average pore radius of the samples are determined from the Barrett–Joyner–Halenda (BJH) pore size distribution analysis and the average pore radius of the composites are lying below 40 Å. The average pore radius of the Co1Al3(OH)m/CNx and Co1Al3(OH)m composites are found to be 19.36 Å and 19.2 Å respectively which indicates the Co1Al3(OH)m composite has smaller mesopores than the Co1Al3(OH)m/CNx composite which is shown in Fig. 1(f). From the above obtained results, it can be stated that the introduction of CNx in the LDH plays a vital role for the improvement of the dispersibility of LDH as well as the formation of the mesopore structure of the Co1Al3(OH)m/CNx composite24 which also suggests that the participation of the small amount of CNx relieves the agglomeration in the LDH layers.25
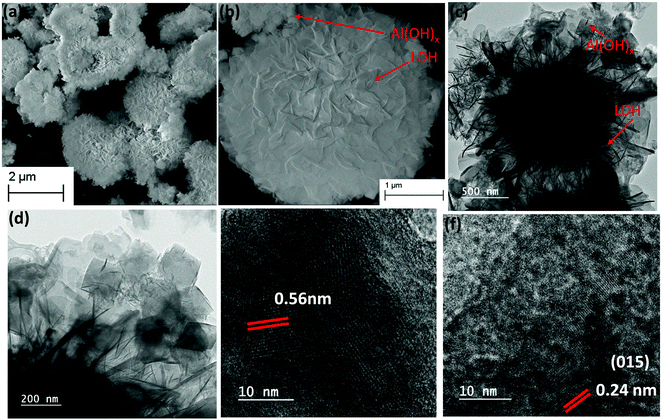
The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis were performed to characterize the morphological features of the Co1Al3(OH)m/CNx composite at different magnifications. Fig. 2(a and b) show the FESEM images of Co1Al3(OH)m/CNx. The 2D nanosheets were self-assembled to form a 3D nanoflower type structure with a smooth surface to provide sufficient surface area which allows an easy passage of electrolytes through the nanosheets resulting in high capacitive performance.26 From the EDAX analysis, the presence of all the elements in Co1Alδ(OH)m/CNx (δ = 1–4) were confirmed. Fig. S4(a–f) (ESI†) presents the FESEM image and corresponding elemental mappings of C, N, Co, Al and O which clearly shows the uniform distribution of elements over the nanocomposite structure of Co1Al3(OH)m/CNx and Fig. S4(g) (ESI†) shows the corresponding EDAX spectra. From elemental mapping, the percentages of elements were calculated and are shown in Fig. S4(h) (ESI†). The atomic percentage of Al and Co were found to be 15.70% and 5.40% respectively with a ratio of 2.9 which is close to the precursor ratio taken during synthesis. The atomic percentage of Co and Al present in all the composites of Co1Alδ(OH)m/CNx (δ = 1–4) are shown in Table S1 (ESI†) which is in good accordance with the amount of precursor used during synthesis.
SEM images of CNx are shown in Fig. S5(a and b) (ESI†) which shows the sheet type morphology of CNx. Fig. 2(c and d) represent the low-resolution transmission electron microscopy images of the Co1Al3(OH)m/CNx composite which shows the presence of nanosheets of LDH structure along with some amount of cubic aluminium hydroxides distributed over the sheets. High resolution transmission electron microscopy (HRTEM) images of Co1Al3(OH)m/CNx are displayed in Fig. 2(e and f) and the lattice fringes with an interplanar distance of 0.56 nm and 0.24 nm were indexed to the (111) and (015) planes of aluminium hydroxide and CoAl LDH structure respectively, which is well consistent with the p-XRD data.
ICP-OES measurement was carried out to determine a more accurate composition of the composite and to calculate the metal ion content of Co1Al3(OH)m/CNx. The Co1Al3(OH)m/CNx composite contains 23.3% of Al and 7.9% of Co while the Al/Co atomic ratio was obtained to be 2.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which is very close to the SEM EDAX analysis data of Co1Al3(OH)m/CNx. In addition to this, the CHN analysis of CNx and Co1Al3(OH)m/CNx composite was performed to determine the percentage of nitrogen and carbon present in it. From the CHN analysis the N/C ratio in only CNx is found to be 1.75 where for the Co1Al3(OH)m/CNx composite the N/C ratio was 1.68. The ratio of N/C in CNx was higher than in the Co1Al3(OH)m/CNx composite which might be because of the loss of nitrogen-containing groups during hydrothermal synthesis.3
1 which is very close to the SEM EDAX analysis data of Co1Al3(OH)m/CNx. In addition to this, the CHN analysis of CNx and Co1Al3(OH)m/CNx composite was performed to determine the percentage of nitrogen and carbon present in it. From the CHN analysis the N/C ratio in only CNx is found to be 1.75 where for the Co1Al3(OH)m/CNx composite the N/C ratio was 1.68. The ratio of N/C in CNx was higher than in the Co1Al3(OH)m/CNx composite which might be because of the loss of nitrogen-containing groups during hydrothermal synthesis.3
X-ray photoelectron spectroscopy (XPS) measurements were obtained for better analysis of the surface oxidation state of Co1Al3(OH)m/CNx composites. XPS studies were carried out using monochromatic Mg Kα as a source. The XPS spectra were charge corrected with respect to the C 1s peak. Fig. 3(a) presents the XPS survey scan of the Co1Al3(OH)m/CNx composite that confirms the presence of Co, Al, O, N and C elements with binding energies ranging from 0 to 800 eV. The high resolution XPS spectra of Co 2p are shown in Fig. 3(b) which appears in the spectrum as a doublet of 2p3/2 and 2p1/2. The 2p3/2 and 2p1/2 spectra of Co were decomposed to four peaks. The Co 2p3/2 peak was deconvoluted into two peaks at 780.48 eV and 782.24 eV corresponding to Co2+ and Co3+ oxidation states. An additional peak at 789.2 eV is a satellite peak. Similarly Co 2p1/2 was also deconvoluted into two peaks at 796.03 eV and 797.5 eV due to the presence of the Co2+ and Co3+ oxidation state. This confirms the coexistence of Co2+ and Co3+ species. The relative percentage area of Co+2 and Co+3 and the atomic ratio of Co+2/Co+3 for 2p3/2 and 2p1/2 are provided in Table S2 (ESI†). The atomic ratio of Co+2/Co+3 was obtained by integrating the area of deconvoluted Co+2 and Co+3 peaks and was found to be nearly 1.95. It has been reported that the presence of Co3+ enhances the conductivity of the CoAl LDH structure. Hence the presence of Co3+ could be one of the possible factors for better electrochemical behaviour of the electrode material. The high resolution XPS spectra of Al 2p is provided in Fig. 3(c) and a singlet peak centred at 74.28 eV can be ascribed to the Al3+ oxidation state. The XPS spectra of O 1s is given in Fig. 3(d) and it is deconvoluted into three peaks centred at 530.54, 531.9 and 532.72 eV which can be assigned to the metal–hydroxyl bond, adsorbed H2O and C–O bond respectively. The high resolution 1s XPS peaks of carbon, shown in Fig. 3(e), can be deconvoluted into three peaks. The peak at 284.2 eV is because of the C–C bond whereas the peaks at 285.5 eV and 286.9 eV are because of C–OH bonds and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds indicating the presence of an CO32− intercalated anion in the LDH12. Fig. 3(f) displays the high resolution N 1s XPS spectra which can be deconvoluted into three different peaks. The peak position at 398.3 eV refers to graphitic nitrogen whereas the peaks at 398.9 eV and 402.2 eV are assigned for pyridinic nitrogen and pyrrolic nitrogen respectively.27,28
C bonds indicating the presence of an CO32− intercalated anion in the LDH12. Fig. 3(f) displays the high resolution N 1s XPS spectra which can be deconvoluted into three different peaks. The peak position at 398.3 eV refers to graphitic nitrogen whereas the peaks at 398.9 eV and 402.2 eV are assigned for pyridinic nitrogen and pyrrolic nitrogen respectively.27,28
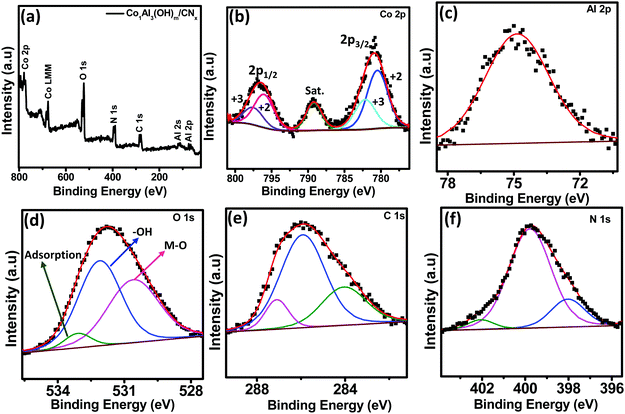 | ||
| Fig. 3 (a) XPS survey spectrum of the Co1Al3(OH)m/CNx composite. High resolution XPS spectra of (b) Co 2p, (c) Al 2p, (d) O 1s, (e) C 1s and (f) N 1s respectively. | ||
Electrochemical analysis
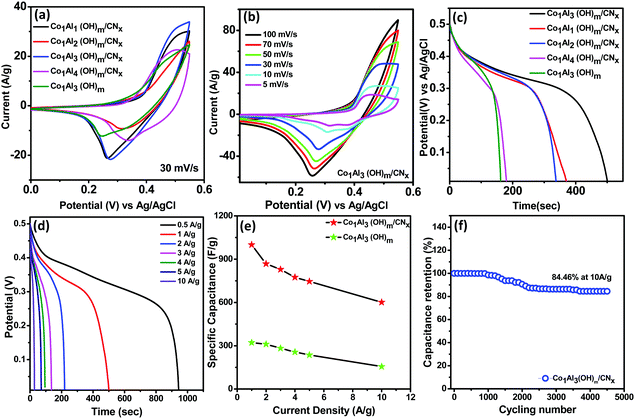
The electrochemical measurements of Co1Alδ(OH)m/CNx (δ = 1–4) and Co1Al3(OH)m composites were investigated in a standard three-electrode system through cyclic voltammetry (CV), galvanostatic charge discharge (GCD) and electrochemical impedance spectroscopy (EIS) measurements at a voltage range of 0–0.55 V in 2 M KOH. The preparation method for the electrodes is briefly explained in the electrode fabrication section. Fig. 4(a) displays the comparison of the CV curve of Co1Alδ(OH)m/CNx (δ = 1–4) and only Co1Al3(OH)m at a sweep rate of 30 mV s−1. All the CV curves exhibit similar shapes and for each CV cycle there is a pair of redox peaks which is due to the reversible reaction occurring between Co+2 to Co+3 and vice-versa present in Co1Al3(OH)m LDH. The CV integral area for the Co1Al3(OH)m/CNx composite is much larger than other composites. The Co1Al3(OH)m/CNx composite shows reversible cathodic and anodic peaks at 0.47 and 0.27 V respectively which are shifted from the initial value compared to the Co1Al3(OH)m composite. This shift in peak position may be attributed due to the presence of CNx.20 The redox peaks for Co1Al3(OH)m/CNx are because of the faradaic redox reaction occurring between LDH–Co–OH and LDH–Co–O–O–H which can be represented as follows.13,17,29,30| LDH–Co (OH)2 + OH−↔ LDH–CoOOH + H2O + e− |
| LDH–CoOOH + OH− ↔ LDH–CoO2 + H2O + e− |
During the redox reaction, Al+3 is non electroactive but this promotes the oxidation of Co+2 to Co+3 which improves the electrochemical activity of the Co1Al3(OH)m/CNx composite.29,31
Due to the introduction of Al+3 in LDH, the crystallinity and hydrophilicity of LDHs are improved which is useful to improve the charge transport and utilization of electrolytes.29,32,33
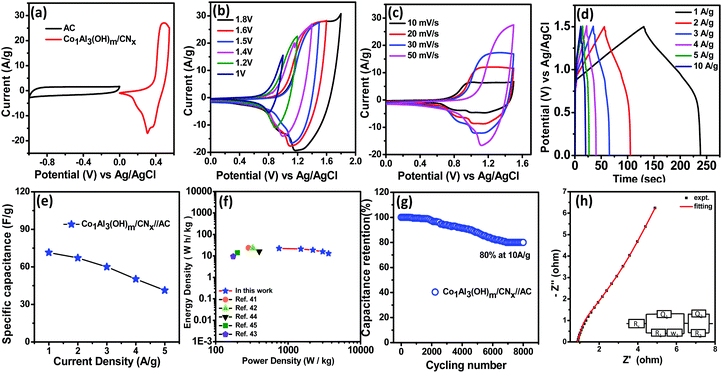
The CV curves of the Co1Al3(OH)m/CNx composite at different sweep rates from 5 mV s−1 to 100 mV s−1 in a voltage window varying from 0–0.55 V are displayed in Fig. 4(b). With increase in scan rate, the potential difference between the cathodic and anodic peak increases34,35 and the current also increases gradually with an increase in sweep rate indicating the good electrochemical responses of the Co1Al3(OH)m/CNx composite.36 The area under the CV curve at different sweep rates for Co1Al3(OH)m/CNx is much larger than that for only Co1Al3(OH)m which indicates that Co1Al3(OH)m/CNx shows a higher specific capacitance.34 The specific capacitance value for the Co1Al3(OH)m/CNx composite was found to be 821.81 F g−1 and 594.73 F g−1 at a sweep rate of 5 mV s−1 and 10 mV s−1 respectively and for Co1Al3(OH)3 it was found to be 611.87 F g−1 and 549.09 F g−1 which are calculated from the area under the CV curve. The presence of CNx promotes the SSA as well as conductivity of the Co1Al3(OH)m/CNx composite enhancing the electrochemical behaviour of the material. High SSA is one of the important factors for increased faradaic reaction between the electrolyte and electrode material and the increase in conductivity promotes the transfer of electrons in the redox reaction. Moreover the synergic interaction between LDH and CNx promotes the electrochemical performance25 of the Co1Al3(OH)m/CNx composite. Table S3 (ESI†) presents the comparison of the electrochemical performance of the Co1Al3(OH)m/CNx composite with previously reported literature. Fig. 4(c) presents the comparison of the non-linear GCD curves of Co1Alδ(OH)m/CNx (δ = 1–4) and only Co1Al3(OH)m in the potential window of 0–0.5 V at 1 A g−1 current density. In order to show the electrochemical contribution of bare Ni foam, GCD measurements were carried out which shows a negligible contribution towards the electrochemical performance as shown in Fig. S6 (ESI†). From the discharge curve, the specific capacitance of Co1Alδ(OH)m/CNx (δ = 1–4) composites and Co1Al3(OH)m were calculated to be 102.8 mA h g−1 (739 F g−1), 93.7 mA h g−1 (674.18 F g−1), 138 mA h g−1 (1000 F g−1), and 50 mA h g−1 (321 F g−1) respectively under a 1 A g−1 current density which implies the specific capacitance of the hybrid materials are enhanced compared to other control samples and also pure Co1Al3(OH)m. The non-linear GCD profiles of the composite obtained at 1 to 10 A g−1 demonstrate the faradaic behaviour of the electrodes.22,26Fig. 4(d) shows the GCD curve of the Co1Al3(OH)m/CNx composite at a current density ranging from 0.5 to 10 A g−1 and the corresponding specific capacitance was calculated to be 138 mA h g−1 (1000 F g−1), 122 mA h g−1 (884 F g−1), 96 mA h g−1 (690.66 F g−1) and 78 mA h g−1 (560 F g−1) under 1, 2, 5 and 10 A g−1 current densities respectively. The specific capacitance value increases with a decrease in current density as the diffusion of electrolyte ions gain access to the maximum electrode surface area at a low current density resulting in a high specific capacitance. In the case of higher current density, the decrease in capacitance is due to the reduction in effective interaction between the electrolyte ions and electrode.25 The composite Co1Al3(OH)m/CNx retains 69% of its initial specific capacitance at a current density of 5 A g−1.
Fig. 4(e) shows the specific capacitance (F g−1) plot as a function of current densities (A g−1) for Co1Al3(OH)m/CNx and Co1Al3(OH)m composites. The specific capacitance of only Co1Al3(OH)m is lower than that of Co1Al3(OH)m/CNx and the high specific capacitance of Co1Al3(OH)m/CNx correlates with the CV results, high SSA and average pore size distribution results. Fig. 4(f) represents the cyclic durability of the single electrode which shows an excellent 84.46% capacitance retention of thte initial value after 4500 cycles at a current density of 10 A g−1.
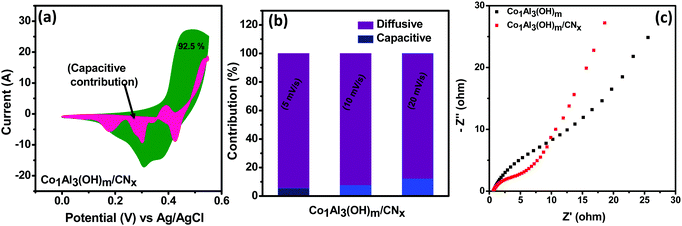
In order to give a better explanation for the charge storage mechanism of the electrode material, differentiation of capacitive contribution and diffusive contribution to the total capacitance is important. Capacitive current arises from the electrical double layer (surface ion adsorption/desorption process) which is directly proportional to the sweep rate while diffusion-controlled current arises from the diffusion of electrolyte ions from and into the electrode.37 At a fixed potential (V), the current (i) can be calculated by using the following eqn (1)11,38–40
| i(V) = k1v + k2v1/2 | (1) |
EIS measurements were performed under open circuit potential in the frequency window of 100 kHz to 0.1 Hz for better evaluation of the electrochemical performance of the Co1Al3(OH)m/CNx composite. The Nyquist plot for Co1Al3(OH)m/CNx and Co1Al3(OH)m are shown in Fig. 5(c). The Co1Al3(OH)m/CNx composite shows a lower equivalent series resistance (Rs) value of 0.64 Ω and also a lower charge transfer resistance value than Co1Al3(OH)m (Rs = 0.67 Ω). The more vertical nature of the EIS plot of Co1Al3(OH)m/CNx than only Co1Al3(OH)m in the low frequency region signifies that the Co1Al3(OH)m/CNx composite has a high electrochemical capacitive performance compared with Co1Al3(OH)m.
Before assembling the asymmetric supercapacitor (ASC), the capacitive performance of activated carbon (AC) was evaluated as AC was used as the negative electrode in the device. Fig. 6(a) represents the CV curves of AC at different sweep rates while GCD curves at different current densities under a voltage range of 0 to −1 V are provided in Fig. 6(b). The rectangular shape of the CV curves having no redox peaks and the symmetrical GCD curves indicate the EDLC type behaviour of AC17. From the CV curves, the specific capacitance values for AC were found to be 260 F g−1 and 220 F g−1 at sweep rates of 10 and 100 mV s−1 respectively while from the GCD curve, it was found to be 135 F g−1 at a current density of 1 A g−1.
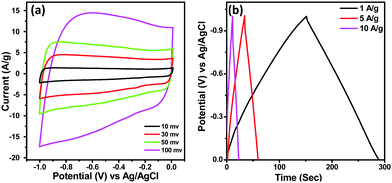 | ||
| Fig. 6 (a) CV curves of AC at different sweep rates and (b) GCD plot of AC at different current densities. | ||
For the practical application of the Co1Al3(OH)m/CNx composite, an asymmetric supercapacitor (ASC) device was assembled with Co1Al3(OH)m/CNx and AC as the cathode and anode respectively. All the electrochemical measurements of the ASC device were performed in 2 M KOH. Fig. 7(a) shows the CV curves of both AC and Co1Al3(OH)m/CNx at a scan rate of 10 mV s−1. Fig. 7 (b) shows the CV curves of the ASC device measured by varying the potential range from 1 V to 1.8 V. With an increase in potential from 1 V to 1.6 V, the nature of the CV curve does not change, which indicates that the ASC device can work steadily in the potential range of 1.6 V. Fig. 7(c) shows the CV curves in the voltage window of 0–1.6 V for the ASC device at different sweep rates.1 At different sweep rates, the nature of the CV curves are similar which indicates the good rate capability of the device.
Fig. 7(d) represents the galvanostatic charge discharge curves at current densities ranging from 1 to 5 A g−1 current density. The specific capacitance value for the ASC device was found to be 71.5, 67.13, 43.84, 36.37 and 29.47 F g−1 at 1,2,3,4 and 5 A g−1 current density respectively which indicates the ∼41.21% retention of the initial specific capacitance at a current density of 5 A g−1. The specific capacitance vs current density for Co1Al3(OH)m/CNx//AC ASC is plotted in Fig. 7(e). As the energy density and power density are important parameters for supercapacitors, their evaluation is very much important. Fig. 7(f) represents the Ragone plot for Co1Al3(OH)m/CNx//AC which shows the variation of energy density with change in power density. An energy density value of 22.35 W h kg−1 was at a power density of 750.2 W kg−1 while the power density was found to be 3613.36 W kg−1 at an energy density of 12.89 W h kg−1 which is much higher than the LDH or Co-based ASC devices reported in the literature such as NixCo1−x LDH–ZTO//AC (23.7 W h kg−1, 284.2 W kg−1),41 NiCo2O4–rGO//AC (23.32 W h kg−1, 324.9),42 NiCo2O4/MnO2//AG (9.4 W h kg−1, 175 W kg−1),43 Co–Fe LDHs-CFC//AC (16.1 W h kg−1, 399 W kg−1),44 and C–MnO212 h (14 W h kg−1, 200 W kg−1).45 The cycling stability of the Co1Al3(OH)m/CNx//AC ASC device was performed at a current density of 10 A g−1 for 8000 cycles and the capacitance retention was found to be 80% which is shown in Fig. 7(g). EIS spectra were measured in the frequency range of 0.1 Hz to 100 kHz and Fig. 7(h) presents the EIS plot of Co1Al3(OH)m/CNx//AC ASC along with the equivalent circuit. Rs, Rct, CPE and W represent the equivalent series resistance, charge transfer resistance, constant phase element and Warburg impedance respectively. The Rs for ASC was obtained to be 0.89 Ω and the Rct value was found to be 1.18 Ω which was calculated by fitting the experimental data with an equivalent circuit. The low value of Rct signifies the low resistance and high charge transfer property of the material.
The high capacitance of the Co1Al3(OH)m/CNx composite can be attributed to the following factors:
(i) The presence of CNx in the Co1Al3(OH)m LDH composite improves the dispensability of the LDH and hence improves the charge transfer property of the material which in turn improves the electrochemical performance.1,46–49 It prevents the rapid agglomeration of layered materials and hence provides high structural and chemical stability during the electrochemical process.1,46,48
(ii) The introduction of Al in the LDH increases the formation of more Co3+ ions in the interlayer of the LDH composite which enhances the conductivity and electrochemical performance of the material.29–31,33
(iii) The synergetic interaction of the CNx and Co1Al3(OH)m LDH is another possible factor for increased electrochemical performances of the Co1Al3(OH)m/CNx electrode material where CNx provides a large active surface area for effective transfer of electrons and restricts the stacking of the LDH.25 Hence the interfacial contact can be enhanced by anchoring CNx on the layered material resulting in fast and smooth ion diffusion through the layered structure.
(iv) The high SSA of the Co1Al3(OH)m/CNx composite compared to Co1Al3(OH)m provides a large interface between electrolyte–electrode for electrostatic charge accumulation and it facilitates the transport of ions by increasing the electrical contact as well as by shortening the diffusion path.17
Conclusions
In this work we have synthesised Co1Al3(OH)m/CNx composites by one-pot hydrothermal synthesis method for supercapacitor applications. The as-synthesised composite shows a 3D nanoflower type structure with a smooth surface which is believed to improve the electrochemical performance of the composite. The maximum specific capacitance for Co1Al3(OH)m/CNx was obtained to be 138 mA h g−1 (1000 F g−1) at a current density of 1 A g−1 (approximately 3 times higher than that of Co1Al3(OH)m) and capacitance retention of 84.46% even after 4500 cycles. Furthermore the ASC provides an energy density of 22.35 W h kg−1 at a power density of 750.2 W kg−1 with 80% capacitive retention even after 8000 cycles at a current density of 10 A g−1. The synergetic interaction between CNx and Co1Al3(OH)m LDH provides a large electroactive surface providing faster ion diffusion through the LDH structure. Moreover the Co1Al3(OH)m/CNx composite with a superior capacitance along with long term stability makes it a promising electrode material for supercapacitor applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful to NISER and the Department of Atomic Energy (DAE), India for financial support.References
- C. Jing, B. Dong and Y. Zhang, Energy Environ. Mater., 2020, 3, 346–379 CrossRef CAS.
- X. Li, D. Du, Y. Zhang, W. Xing, Q. Xue and Z. Yan, J. Mater. Chem. A, 2017, 5, 15460–15485 RSC.
- T. Bhowmik, R. Mishra and S. Barman, Energy Fuels, 2021, 35, 5206–5216 CrossRef CAS.
- N. L. Wulan Septiani, Y. V. Kaneti, K. B. Fathoni, J. Wang, Y. Ide, B. Yuliarto, Nugraha, H. K. Dipojono, A. K. Nanjundan, D. Golberg, Y. Bando and Y. Yamauchi, Nano Energy, 2020, 67, 104270 CrossRef CAS.
- K.-B. Wang, Q. Xun and Q. Zhang, EnergyChem, 2020, 2, 100025 CrossRef.
- Y. Li, J. Henzie, T. Park, J. Wang, C. Young, H. Xie, J. W. Yi, J. Li, M. Kim, J. Kim, Y. Yamauchi and J. Na, Bull. Chem. Soc. Jpn., 2019, 93, 176–181 CrossRef.
- K.-B. Wang, R. Bi, Z.-K. Wang, Y. Chu and H. Wu, New J. Chem., 2020, 44, 3147–3167 RSC.
- J. Tang, R. R. Salunkhe, H. Zhang, V. Malgras, T. Ahamad, S. M. Alshehri, N. Kobayashi, S. Tominaka, Y. Ide, J. H. Kim and Y. Yamauchi, Sci. Rep., 2016, 6, 30295 CrossRef CAS PubMed.
- R. Mishra, P. Panda and S. Barman, New J. Chem., 2021, 45, 5897–5906 RSC.
- J.-H. Cha, E. B. Park, S. W. Han, Y. D. Kim and D.-Y. Jung, Chem. – Asian J., 2019, 14, 446–453 CrossRef CAS PubMed.
- C. Li, Y. Zhou, X. Li, H. Wang, P. Huo and X. Wang, Appl. Surf. Sci., 2021, 536, 147780 CrossRef CAS.
- G. Wang and Z. Jin, J. Mater. Chem. C, 2021, 9, 620–632 RSC.
- G. Li, X. Zhang, D. Qiu, Z. Liu, C. Yang, C. B. Cockreham, B. Wang, L. Fu, J. Zhang, B. Sudduth, X. Guo, H. Sun, Z. Huang, J. Qi, J. Sun, S. Ha, Y. Wang and D. Wu, Adv. Electron. Mater., 2019, 5, 1900215 CrossRef.
- T. Wang, S. Zhang, X. Yan, M. Lyu, L. Wang, J. Bell and H. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 15510–15524 CrossRef CAS PubMed.
- X. Xu, J. Tang, H. Qian, S. Hou, Y. Bando, M. S. A. Hossain, L. Pan and Y. Yamauchi, ACS Appl. Mater. Interfaces, 2017, 9, 38737–38744 CrossRef CAS PubMed.
- H. Tian, W. Bao, Y. Jiang, L. Wang, L. Zhang, O. Sha, C. Wu and F. Gao, Chem. Eng. J., 2018, 354, 1132–1140 CrossRef CAS.
- S. Sanati and Z. Rezvani, Chem. Eng. J., 2019, 362, 743–757 CrossRef CAS.
- Y. Zheng, Z. Zhang and C. Li, J. Photochem. Photobiol., A, 2017, 332, 32–44 CrossRef.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC.
- T. M. Masikhwa, M. J. Madito, D. Y. Momodu, J. K. Dangbegnon, O. Guellati, A. Harat, M. Guerioune, F. Barzegar and N. Manyala, RSC Adv., 2016, 6, 46723–46732 RSC.
- H. Wang, X. Xiang and F. Li, J. Mater. Chem., 2010, 20, 3944–3952 RSC.
- J. Fang, M. Li, Q. Li, W. Zhang, Q. Shou, F. Liu, X. Zhang and J. Cheng, Electrochim. Acta, 2012, 85, 248–255 CrossRef CAS.
- W. Yang, Z. Gao, J. Wang, J. Ma, M. Zhang and L. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 5443–5454 CrossRef CAS PubMed.
- L. Zhang, M. Ou, H. Yao, Z. Li, D. Qu, F. Liu, J. Wang, J. Wang and Z. Li, Electrochim. Acta, 2015, 186, 292–301 CrossRef CAS.
- B. Patil, C. Park and H. Ahn, RSC Adv., 2019, 9, 33643–33652 RSC.
- T. Li, G. H. Li, L. H. Li, L. Liu, Y. Xu, H. Y. Ding and T. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 2562–2572 CrossRef CAS PubMed.
- M. Arif, G. Yasin, M. Shakeel, M. A. Mushtaq, W. Ye, X. Fang, S. Ji and D. Yan, Mater. Chem. Front., 2019, 3, 520–531 RSC.
- M. Arif, G. Yasin, M. Shakeel, X. Fang, R. Gao, S. Ji and D. Yan, Chem. – Asian J., 2018, 13, 1045–1052 CrossRef CAS PubMed.
- J. Li, P. Zhang, X. Zhao, L. Chen, J. Shen, M. Li, B. Ji, L. Song, Y. Wu and D. Liu, J. Colloid Interface Sci., 2019, 549, 236–245 CrossRef CAS PubMed.
- E. Scavetta, B. Ballarin, M. Gazzano and D. Tonelli, Electrochim. Acta, 2009, 54, 1027–1033 CrossRef CAS.
- Q. Zhang, B. Zhao, J. Wang, C. Qu, H. Sun, K. Zhang and M. Liu, Nano Energy, 2016, 28, 475–485 CrossRef.
- X. Wang, Y. Lin, Y. Su, B. Zhang, C. Li, H. Wang and L. Wang, Electrochim. Acta, 2017, 225, 263–271 CrossRef CAS.
- L. Qian, Z. Lu, T. Xu, X. Wu, Y. Tian, Y. Li, Z. Huo, X. Sun and X. Duan, Adv. Energy Mater., 2015, 5, 1500245 CrossRef.
- A. Zhang, C. Wang, Q. Xu, H. Liu, Y. Wang and Y. Xia, RSC Adv., 2015, 5, 26017–26026 RSC.
- I. Shakir, M. Shahid, U. A. Rana, I. M. A. Nashef and R. Hussain, Electrochim. Acta, 2014, 129, 28–32 CrossRef CAS.
- C. Jing, Y. Huang, L. Xia, Y. Chen, X. Wang, X. Liu, B. Dong, F. Dong, S. Li and Y. Zhang, Appl. Surf. Sci., 2019, 496, 143700 CrossRef CAS.
- K. Wang, R. Bi, M. Huang, B. Lv, H. Wang, C. Li and H. Wu, Inorg. Chem., 2020, 59, 6808–6814 CrossRef CAS PubMed.
- Q. Yang, Y. Liu, L. Xiao, M. Yan, H. Bai, F. Zhu, Y. Lei and W. Shi, Chem. Eng. J., 2018, 354, 716–726 CrossRef CAS.
- K. Wang, Q. Li, Z. Ren, C. Li, Y. Chu, Z. Wang, M. Zhang, H. Wu and Q. Zhang, Small, 2020, 16, 2001987 CrossRef CAS PubMed.
- K. Wang, S. Wang, J. Liu, Y. Guo, F. Mao, H. Wu and Q. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 15315–15323 CrossRef CAS PubMed.
- X. Wang, A. Sumboja, M. Lin, J. Yan and P. S. Lee, Nanoscale, 2012, 4, 7266–7272 RSC.
- X. Wang, W. S. Liu, X. Lu and P. S. Lee, J. Mater. Chem., 2012, 22, 23114–23119 RSC.
- M. Kuang, Z. Q. Wen, X. L. Guo, S. M. Zhang and Y. X. Zhang, J. Power Sources, 2014, 270, 426–433 CrossRef CAS.
- K. Ma, J. P. Cheng, F. Liu and X. Zhang, J. Alloys Compd., 2016, 679, 277–284 CrossRef CAS.
- V. J. Mane, D. B. Malavekar, S. B. Ubale, V. C. Lokhande and C. D. Lokhande, Inorg. Chem. Commun., 2020, 115, 107853 CrossRef CAS.
- J. Wu, Q. e. Zhang, J. Wang, X. Huang and H. Bai, Energy Environ. Sci., 2018, 11, 1280–1286 RSC.
- S. Mondal, U. Rana and S. Malik, Chem. Commun., 2015, 51, 12365–12368 RSC.
- M. Ghaemmaghami and R. Mohammadi, Sustainable Energy Fuels, 2019, 3, 2176–2204 RSC.
- Y. Luo, Y. Yan, S. Zheng, H. Xue and H. Pang, J. Mater. Chem. A, 2019, 7, 901–924 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ma00665g |
| This journal is © The Royal Society of Chemistry 2021 |