Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent progress in the toxicity of nitric oxide-releasing nanomaterials
Joana Claudio
Pieretti
a,
Milena Trevisan
Pelegrino
a,
Ariane
Boudier
 b and
Amedea Barozzi
Seabra
b and
Amedea Barozzi
Seabra
 *a
*a
aCenter for Natural and Human Sciences (CCNH), Federal University of ABC (UFABC), Av. dos Estados 5001, CEP 09210-580, Santo André, SP, Brazil. E-mail: amedea.seabra@ufabc.edu.br
bUniversité de Lorraine, CITHEFOR, F-54000 Nancy, France
First published on 4th October 2021
Abstract
Nitric oxide (NO) is a signaling molecule controlling important biological processes. Depending on its concentration, location and cellular environment, NO can have protective or toxic effects. As NO is a free radical, several classes of NO donors/generators have been prepared and combined with nanomaterials, in particular, with polymeric nanoparticles. Engineered nanoparticles are attractive nanocarriers extensively used in biomedical applications, particularly, in cancer biology due to their ability to promote a site-target therapeutic effect, with minimum side effects to health tissues. NO-releasing nanoparticles can have direct toxic effects on tumor cells, or it can promote cancer cell sensitization for traditional cancer treatments. The combination of NO-releasing nanoparticles with conventional anticancer therapies is a promising approach in the reversion of multidrug resistance (MDR) cells. This review presents and discusses the recent progress in the cytotoxicity (tumoral and non-tumoral cell lines) of NO-releasing polymeric and/or polymer-coated nanomaterials and the in vivo biocompatibility of NO-releasing nanoparticles. Moreover, the ability of these nanoparticles to combat MDR, their mechanisms of toxicity and drawbacks are also discussed.
1. Introduction: the biological importance of nitric oxide
Nitric oxide (NO) is a small signaling molecule associated with several cellular functions.1–3 NO is relatively a simple molecule, composed only of a covalent bond between nitrogen and oxygen atoms with an unpaired electron on the antibonding 2π*y, therefore, a free radical.4 Until the 1970s, NO was majorly known as an atmospheric pollutant and a toxic agent.2,5,6 However, the Nobel Prize of Medicine and Physiology awarded to Louis J. Ignarro, Robert F. Furchgott and Ferid Murad for identifying NO as an EDRF (endothelium derived relaxing factor) sheds light on the importance of this signaling molecule in several physiological and pathophysiological processes.7–11 In 1992, NO was elected the ‘molecule of the year’ by the journal Science due to the increasing number of published articles regarding its impactful cellular effects.12NO is endogenously synthesized by the action of cluster catalytic enzymes named nitric oxide synthase (NOS). The NOS enzyme family includes three isoforms: (i) endothelial (eNOS), (ii) neuronal (nNOS) and (iii) inducible (iNOS).1,13,14 Both eNOS and nNOS synthesize NO at lower amounts for a longer time-period in the presence of calcium, while iNOS produces NO only when induced by an external signal at higher concentrations for a longer time frame.1,15,16 All members of the NOS enzyme family catalyze the oxidation of L-arginine to L-citrulline with the formation of one NO molecule.4,16
In cancer biology, NO serves multiple functions, being considered an anti- and pro-apoptotic agent, promoting vasodilation, angiogenesis and interactions with macrophages.4,6,14,15,17 For instance, a member of the NOS family, eNOS, in endothelium has been linked to a prognostic factor for patients with stomach adenocarcinoma (STAD).13 In this sense, NO is well recognized for playing a dual role in cancer cells, having deleterious or beneficial features.2,6,18 In some studies, NO or NOS levels are correlated with tumor suppression; however, in other studies, they are correlated with tumor progression and metastasis.1,2,18 NO interactions with cellular targets can vary based on the spatial and temporal distribution of NO influx within tumor cells and tumor microenvironments.17,19 Thus, the key factors linked to modulating the dichotomous effect of NO are the amount of NO released, the time-period and the spatial distribution of this signaling molecule in tumor tissues.20 In addition, the tumor microenvironment and heterogeneity also play an important role in the dichotomous effect of NO.1 Thus, for proposing the use of exogenous NO in cancer cells/tissues, it is of fundamental importance to tailor the desired amount of NO released to the target site of application. To this end, the combination of NO donors with nanomaterials represents an important approach.3,9,21,22
Tumor tissue has unique vascular characteristics such as hypervasculature, irregular morphology and enhanced permeability.23 Tumor tissue recovery of some substances from tumor interstitium into lymphatics is slow; this effect is observed for macromolecules, lipids and nanoparticles.22,23 Therefore, nanoparticles naturally remain within tumor tissue for a longer time-period, compared with other drugs; this is a well-known phenomenon called the enhanced permeability and retention (EPR) effect.22,23 EPR is one of the reasons to explain why nanomaterials are intensively developed for cancer treatment.24 Interestingly, NO has been linked to mediate the enhanced permeability. Indeed, the NO scavenger and NOS inhibitor suppressed the EPR effect in mice with sarcoma solid tumor.23,25
NO donors are a class of molecules capable of mimicking NO flux conditions and modulating their concentration.3,17 Due to the NO instability, NO donors have been successfully used to exogenously deliver NO to a target site, in different biomedical applications.19,26 The most common classes of NO donors are sodium nitroprusside, organic nitrite and nitrate, furoxans, benzofuroxans, diazeniumdiolates (NONOates), S-nitrosothiols (RSNOs), Roussin's black salt (RBS) and metal nitrosyl complexes.3,17,27–31 The molecular representations of commonly used NO donors are shown in Fig. 1. NONOates and RSNOs are highlighted as the most used NO donors in oncological therapeutic applications due to the NO release features and feasibility to be produced.3,32,33 NONOate releases NO only in the presence of protons and can be classified according to its secondary amine structure, which varies its half-life.3,21,33 In RSNOs, NO is covalently bound to a sulfur group (RS), and NO is spontaneously released from the homolytic S–N cleavage.26,27 RSNOs is also involved in the S-transnitrosation reaction that has been linked to be a relevant step in the downward signaling route created by NO.19,26,34–36 Another commonly used NO donor is the RBS, which is a metal-nitrosyl complex that releases NO after illumination.31,37
Although NO donors have allowed the use of exogenous NO in biomedical applications, these molecules have limitations in reaching the NO concentrations spatially and temporally.26,27 In order to overcome this challenge, the combination of nanomaterials and NO has been extremely successful.3,27,32,38–40 Nanomaterials have been applied to cancer diagnosis and treatment with positive results.23,41,42 Cancer is a leading cause of death worldwide; its treatment usually involves surgical resection, radiation and chemotherapy.43 Although several treatments are conventionally used with certain degree of success, there are some drawbacks such as collateral effects and multi-drug resistant cancers.3,18,22,30,40
In this sense, the combination of nanomaterials and NO donors can be used alone or with chemotherapy or radiotherapy agents.3,27,43 This combination is cooperative; nanoparticles are carriers for NO allowing it to reach the target site at suitable concentrations. In addition, as a vasodilator, NO can increase tumor permeability allowing nanoparticle accumulation at the tumor site.23 All these features together can provide an alternative strategy to overcome the setbacks of traditional cancer treatment.
In this scenario, the scope of this review is to point out the recent advances in the design of nanomaterials capable of releasing NO and their activity against cancer cell lines, highlighting their efficiency against multi-drug resistant tumor, in vivo results, and selective toxicity. We aimed to overview the recent publications available on reliable platforms such as Web of Science and PubMed from the last five years (2016–2021), focusing on NO-releasing polymeric nanomaterials and/or polymer-coated nanomaterials.
2. Cytotoxicity of NO-releasing nanoparticles against cancer and non-cancerous cells
A few years after NO was defined as the endothelium-derived relaxing factor (EDRF),7,44 NO was described as an important signaling molecule, involved in cytotoxic mechanisms, demonstrating relevant cytoprotective properties.5,45 Regarding its cell cytotoxicity, NO has been reported as a possible modulator of apoptosis, angiogenesis, invasion, and metastasis, and also as a regulator of the cell cycle.46 The cytotoxic effect of NO on tumor cells relies, mainly, on the suppression of DNA synthesis and cellular respiration, shifting iron metabolism, modulation of apoptosis by activating caspase family proteases, upregulation of p53 protein and other apoptosis-related proteins.46 More recently, NO has also been mentioned as a sensitizing agent, demonstrating promising cytotoxic effects against tumoral cells when allied to traditional therapeutic approaches (chemotherapy and radiotherapy).3,47 As previously mentioned, NO is a relatively unstable molecule, therefore, NO donors have been employed as a strategy to promote an effective delivery. More recently, the combination of NO donors with nanoparticles has improved the use of NO as an antitumoral therapeutic strategy.3In this scenario, NO has been allied to different kinds of nanoparticles, ranging from metallic nanoparticles, as gold nanoparticles (AuNPs), silver nanoparticles (AgNPs), and quantum dots (QDs);48 metal oxide nanoparticles, such as superparamagnetic iron oxide nanoparticles (SPIONs); zeolites and other metal–organic frameworks;49 porous nanomaterials, as silica nanoparticles (SiO2);50 to polymeric nanoparticles.51–54 Depending on the nature of the nanoparticles, they can have direct cytotoxicity, or they can act as nanocarriers of active drugs, enhancing the intratumoral penetration, thus promoting a more efficient drug delivery.55 Generally, an empty nanocarrier itself does not present a toxic or cytotoxic effect, being only a nanovehicle for improving drug delivery. When compared to control cells or in vivo models, empty nanoparticles should demonstrate similar patterns, as no antitumoral effect should be promoted. For instance, poly(lactic-co-glycolic acid)-based nanoparticles (PLGA NPs), approved by the US Food and Drug Administration (FDA) for medical use, demonstrate promising potential to be combined with different drugs, other nanoparticles or molecules, since these nanoparticles do not demonstrate significant cytotoxic effects without the mentioned combinations.56 In contrast, there are some types of nanoparticles that present intrinsic properties that may lead to significant cytotoxic potential, as well as contributing to the diagnosis, such as theragnostic nanoparticles.57 AuNPs, for example, exhibit unique optical and electronic properties that make them suitable for cell and/or tumor imaging, besides presenting size- and shape-dependent toxicity against cancerous and non-cancerous cells.58,59 AgNPs demonstrate higher cytotoxicity against cancer cells when compared to AuNPs, drastically reducing cell viability, as demonstrated for colon cancer cells, regulating redox processes and the cell cycle, and mediating cell apoptosis and DNA disfunction.60
Moreover, AgNPs may demonstrate a synergic toxic effect in combination with NO.54 For instance, it is well-known that AgNPs may cause toxicity due to the ability to generate reactive nitrogen and oxygen species, leading to oxidative damage in mammalian cells and/or microorganisms.61 Similar to AgNPs, NO sub-products, such as peroxynitrite (ONOO−), are also key players for increasing the oxidative stress and causing cellular damage.62 NO rapidly reacts with superoxide (O2˙−) leading to the formation of harmful peroxynitrite, described as follows:
| NO˙ + O2˙− → ONOO− | (1) |
In this section, we aim to overview the recent progress in the cytotoxicity promoted by NO-releasing polymeric nanoparticles and/or polymer-coated nanoparticles and their interference in cellular mechanisms, which might be directly related to the NO delivered, or to the combination of the NO donor with the nanoparticles and/or other chemotherapeutic drugs.
2.1 Cytotoxicity of NO-releasing polymeric nanoparticles against tumoral and non-tumoral cell lines
Polymer-based nanoparticles have stood out in nanomedicine and drug delivery, mostly due to their synthesis facility, high biocompatibility, and the possibility of achieving different designs such as polymeric nanocapsules and/or nanospheres, micelles, and liposomes.60 The last one stands out for being approved by the FDA and being used for delivering the chemotherapeutic doxorubicin (DOX), improving its efficacy.63 In recent years, NO has been combined with different polymeric nanoparticles in order to promote or potentiate cytotoxic effects against tumoral cells.42 PLGA NPs containing the NO donor dinitrosyl iron complex were developed to promote a sustained NO release, regarding the concentration, localization, and duration.64 Nanoparticles 120 nm in size were responsible for promoting the EPR effect in tumor cells/tissues. When comparing free NO and nanoencapsulated NO at a final concentration of 20 μmol L−1, a more pronounced cytotoxic effect was observed for the free NO donor. The inferior cytotoxicity of encapsulated NO was evidenced by a 2-fold increase in the IC50 in comparison to free NO IC50, resulting from a slow NO release in the cell media. The mentioned in vitro results are important to critically analyze the importance of evaluating different parameters (i.e. blood compatibility, NO life-time in blood circulation, targeting efficiency and tissue accumulation) when it comes to NO delivery. Regarding the cytotoxic effect of nanoencapsulated NO, an improved cytotoxicity against tumoral cell lines is usually expected. Although, despite observing a higher IC50 for NO after encapsulation, a prolonged circulation of the encapsulated NO donor after intravenous administration was verified, with an outstanding 25-fold increase of the NO donor half-life and a higher accumulation in liver tumoral tissue, when compared to the free NO donor. Similar patterns were observed for RSNO (1–10 mmol L−1) encapsulated in poly(propylene sulfide) nanoparticles, in which no significant cytotoxicity was observed for the encapsulated molecule, but a 2-fold increase in the accumulation in the targeted tissue was verified in vivo, with no apparent signs of inflammation.65In contrast, other combinations have demonstrated potential cytotoxic effects against tumoral cell lines.30,51,66 RSNO encapsulated in chitosan nanoparticles (CS NPs), with an average hydrodynamic size of 108 nm, has demonstrated a sustained NO release for 10 h, at a concentration of 50 mmol L−1, in which NO should demonstrate significant cytotoxic effects.51 The potential cytotoxic effect of RSNO encapsulated in CS NPs was confirmed against different tumoral cell lines, hepatocellular carcinoma (HepG2), skin melanoma (B16-F10), chronic myeloid leukemia (K562), and vincristine resistant chronic myeloid leukemia (Lucena 1), compared with non-tumoral melanocyte (Melan-A) cells. A cell viability reduction was observed in all the evaluated concentrations (5–40 μg mL−1), although the resultant IC50 demonstrated a higher toxicity against B16-F10 and HepG2 cell lines, when compared to non-tumoral Melan-A cells. Leukemic cells demonstrated to be less sensible to NO-releasing CS NPs. Lucena 1 exhibited the highest IC50, which was expected as it is characterized as a resistant cell lineage derived from K562. When comparing the pair B16-F10 and Melan-A (both skin cells), a 2-fold selectivity for the tumoral cell-line reinforces the potential of NO-releasing CS NPs in the treatment of tumors. The reported cytotoxicity involved different mechanisms, such as caspase-dependent apoptosis, which was associated with oxidative stress promoted by NO, along with an increased superoxide production in the mitochondria and the oxidation of thiol containing proteins.67 Interestingly, tyrosine nitration and cysteine S-nitrosation in cellular proteins were also observed, demonstrating the involvement of the delivered NO in different cell mechanisms.
Similar results were reported for RSNO-loaded CS NPs functionalized with hyaluronic acid (HA).66 Cytotoxic results were verified for all the tested concentrations (5–200 μg mL−1), achieving 0% of cell viability at the highest evaluated concentration. Interestingly, the HA did not contribute to the cytotoxicity, although it might promote a higher selectivity for cells that overexpresses CDD4 protein.66 Another targeted nanomaterial was reported by Liu and coworkers, in which molecular imprinted nanoparticles (MIPs) were designed to target tumor that overexpresses sialic acid.30 The cytotoxicity was evaluated against different tumoral cell lines, such as HepG2 cells and mammary cancer cells (MCF-7), and compared to that against non-tumoral hepatocellular cells. The NO-releasing nanoparticles demonstrated selectivity for sialic acid overexpressing cells (HepG2 and MCF-7), evidenced by a higher cellular uptake and cytotoxicity against these cells when compared to non-tumoral hepatocellular cells, with low sialic acid expression. Interestingly, HepG2 cells that survived NO-releasing MIPs were re-cultured and treated with the nanoparticles, and no acquired resistance was observed. This is an important result for future studies with NO-releasing nanoparticles, as it is directly related to NO chemical biology. NO and its sub-products are known to cause oxidative and nitrosative stress and DNA deamination, besides interacting with different proteins, comprehending a complex mechanism that may hinder the development of resistance mechanisms in tumoral cells.30,68
Despite focusing on NO, other gasotransmitters also play important biomedical roles, including cytotoxicity against tumoral cells.69 Indeed, a more recent strategy has demonstrated promising results by combining two different gasotransmitters, NO and hydrogen sulfide (H2S), co-delivered by a polymeric nanoparticle based on carboxyl-functionalized mPEG-PLGH-thiobenzamide [(methoxy poly(ethylene glycol-b-lactic-co-glycolic-co-hydroxymethyl propionic acid)–thiobenzamide)], PTA copolymer.70 H2S is produced in mammalian cells mainly by three different enzymes: cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (MST).71 Biological pathways occur through sulfhydration, a post-translational modification of specific protein cysteines.69 Regarding antitumoral properties, H2S suppresses cellular bioenergetics, reduces the intracellular levels of antioxidant glutathione, and modulates p53 and nuclear factor-κB (NF-κB) pathways.72 When cells were treated with NO/H2S-releasing nanoparticles, an enhanced angiogenesis was observed, resulting from the NO/H2S crosstalk.70 Moreover, these nanoparticles demonstrated high biocompatibility in different cell types. Thus, the combination of NO/H2S in nanomaterials represents a new approach in biomedical applications, and further studies are welcome. The crosstalk between NO and H2S, and its biological responses, is under intensive investigation.73
2.2 Cytotoxicity of NO-releasing polymer-functionalized nanoparticles against tumoral and non-tumoral cell lines
Polymer-coated metal or metal-oxide nanoparticles have also been reported as promising templates for delivering NO.22,48,74 The surface functionalization enables the delivery of therapeutics, such as NO or traditional chemotherapeutics, and also promotes targeted delivery to cancerous cells.74,75 Among the different nanoparticles, AuNPs have been one of the most employed in drug delivery.50 Similar to what was demonstrated for CS NPs, AuNPs were functionalized with hyaluronic acid for targeting CDD4 protein expressed by breast tumoral cells and the consequent lung metastasis. Furthermore, AuNPs were functionalized to deliver the chemotherapeutic paclitaxel (PTX), indocyanine green and NO, from a nitrate ester modified hyaluronic acid. The combined nanomaterials (AuNPs, PTX, indocyanine green and nitrate ester modified hyaluronic acid) exhibited the lowest IC50 against breast cancer cell lines (4T1) when compared to controls (nanoparticles without indocyanine green, and free PTX), evidencing a synergistic effect of PTX, indocyanine green, and NO delivered by AuNPs. The NO role was even more evident in apoptosis analysis, in which the necrotic and apoptotic 4T1 cells reached 52% when treated with AuNPs, PTX, indocyanine green and nitrate ester modified hyaluronic acid, in comparison to 40.9% for AuNPs without hyaluronic acid and NO coating, suggesting an important role in drug internalization combined with NO cytotoxic effects.50The apoptotic and necrotic effect of NO was also evident on osteosarcoma cells (MG63) when combined with hybrid nanoparticles based on SPIONs and AgNPs.74 Apoptotic and necrotic cells comprehended 25% of the cell population after 24 h of treatment with hybrid nanoparticles based on SPIONs and AgNPs, coated with NO-releasing chitosan, at a concentration of 100 μg mL−1, which corroborated with a pronounced cell viability reduction. Moreover, it was possible to observe the interference in the cell cycle, inducing a cycle arrest at the S-phase. Thus, it is evident that NO plays an important role against tumoral cells, combined or not with other therapeutics. Still it is important to better comprehend the cytotoxic mechanism of NO against different cell lines, as well as the innovative proposed combinations with active nanomaterials and/or drugs.
3. Cytotoxicity of NO-releasing nanoparticles against multidrug resistant cancer cells
As reported in the previous section, when evaluating the potential cytotoxicity of NO-releasing nanoparticles against different cell lines, resistant cells demonstrated higher IC50 when treated with RSNO–CS NPs,51 compared with no resistant cells, confirming the necessity to employ higher dosages against these cell lines. We have recently published a review focused on the overview of recent innovative combinations of NO and nanomaterials, allied or not to chemotherapeutics, that represent a potential approach to overcome resistance mechanisms.3 An illustrative scheme shown in Fig. 2 describes the possible resistance mechanisms that can be reverted by nanoparticles combined with NO donors.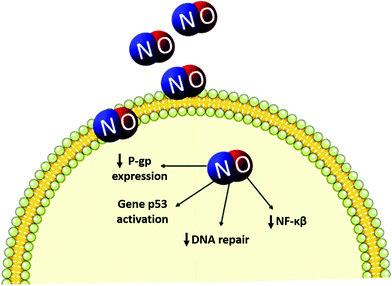 | ||
| Fig. 2 Schematic representation of NO interference in resistance mechanisms. NO has demonstrated to promote a decrease in the expression of NF-κβ, responsible for the expression of anti-apoptotic genes, in addition to promote a decrease in DNA repair and in the expression of proteins responsible for the efflux of chemotherapeutic agents, such as glycoprotein-P (P-gp).3,76 | ||
Recent publications point to the increasing strategy of employing NO donors as a cell sensitizer agent for reverting resistance mechanisms.58,77–80 Important results were observed for a NONOate combined with pH sensitive liposomes and PTX, which facilitated tumor cell uptake of both compounds. In vitro experiments with adenocarcinoma cells (A549/T) confirmed a higher cytotoxicity for the designed combination when compared to controls. Some mechanisms have correlated NO release to the downregulation of P-gp protein through the inhibition in MDR-1 mRNA levels.77 A similar design was reported by Wu and coworkers, combining an important RSNO, S-nitrosoglutathione (GSNO), with a vastly used chemotherapeutic drug, DOX, in polymeric block nanoparticles based on poly(ethylene glycol) and poly(propylene sulfide) (PEG–PPS NPs).80 Even at the lowest evaluated concentration (1 μg mL−1 of DOX), a statistically significant increase in the cytotoxicity against HepG2 cells was observed for the encapsulated drug combined with GSNO. When comparing tumoral to non-tumoral cells, the cytotoxicity of the nanomaterials was 37-fold higher, evidencing a high selectivity; this is of great importance when it comes to chemotherapeutic drugs. Through apoptosis analysis, it was evident that GSNO encapsulated in PEG–PPS NPs at a concentration of 25 μmol L−1 already interferes with cell mechanisms and leads to a small percentage of apoptotic cells. Although, when combined with DOX and the PEG–PPS NPs, a synergistic effect was observed by reaching almost 100% of late apoptotic cells, in contrast with 23.6% for DOX encapsulated nanoparticles and 2.41% for GSNO encapsulated nanoparticles. With these data, it is clear that a NO donor can sensitize the resistant cancerous cells, promoting a pronounced effect of chemotherapeutics.
For micelle nanoparticles with a similar design, combined with DOX, a pattern similar to the one previously discussed was observed when evaluated against MCF-7 cells.78 Micelles presenting 130 nm were obtained from a biodegradable polycarbonate-based copolymer, tailored for NO release from a cyclic nitrate trimethylene carbonate monomer. Once more, NO was correlated with the decrease of P-gp protein expression in a dose-dependent manner, enhancing the chemotherapeutic efficiency. Inorganic nanoparticles, such as silica nanoparticles (230 nm), loaded with NO and combined with DOX, reinforced the mechanism of P-gp expression decrease when employed against MCF-7 cells.58 It is already clear that NO has an important involvement in the decrease in the expression of P-gp.
Different designs of nanoparticles, drug combinations, and component concentrations may lead to different cytotoxic effects and biochemical mechanisms. Some issues are not as clear as the decrease of P-gp expression and should be further investigated. Do these combinations initiate inflammatory processes? Are there any DNA damages? What is the ideal proportion for promoting apoptosis and/or necrosis? What is the redox balance in MDR cells treated with NO-releasing nanoparticles? Is there any influence in enzymatic activities? To answer these questions, we therefore expect to encourage researchers to further investigate the mechanism of NO in MDR reversal/sensitization, as it is a promising advance in the area of chemotherapy.
3.1 Combination of NO-releasing nanomaterials with traditional anti-cancer therapies
Recently, in cancer treatment, the combination of chemotherapy or radiotherapy with NO donors is a promising strategy that might overcome drug resistance. Furthermore, the addition of nanomaterials allied to NO donors may enhance this positive effect. Nanomaterials can load either NO donors/generators or chemotherapeutic drugs. Interestingly, in clinical applications, administration of NO donors at subtoxic doses along with suboptimal doses of chemotherapeutics can lead to additive or synergistic effects, which are suitable for overcoming chemoresistance.47 Such combinations (NO donors, nanomaterials and chemo/radio therapies) can also activate the anti-tumor immune response.Chemotherapy remains restricted by poor drug delivery efficacy due to the heterogenous nature of tumor and the development of tumor resistance.81 To overcome these issues, considering combined approaches, versatile nanomaterials have been prepared for cancer treatment. In particular, chemotherapeutic drugs can be allied to NO donors and nanomaterials. DOX and NO donor (RSNO) were loaded in mesoporous silica nanoparticles.22 The obtained nanomaterials enhanced tumor penetration allowing a localized released of DOX and NO. NO released reacts with superoxide anion yielding the powerful oxidant peroxynitrite, under pathological conditions. This process promotes protein nitration and oxidation, lipid peroxidation, DNA damage. Moreover, peroxynitrite activates pro-matrix metalloproteinases into active matrix metalloproteinases, which degrade collagen in the tumor extracellular matrix. In other words, NO contributes to disintegrate tumor matrix collagen improving nanoparticle penetration.22
A nanocarrier containing a NO donor (N-NO moiety of a polymer) and paclitaxel was prepared.82 The polymeric micelles (hydrodynamic diameter of 137 nm) were able to spontaneously release NO, under physiological conditions, and demonstrated toxic effects against the MDR ovarian cancer cell line OVCAR-8/ADR. Higher cytotoxicity was found for micelles containing both the NO donor and paclitaxel, compared with micelles containing only individual drugs. Moreover, these nanoparticles were found in tumor tissue for more than 48 h.82 Hyaluronan and keratin nanogels containing the NO donor and DOX (average size of 60 nm) demonstrated significant and improved toxicity towards the breast cancer 4T1 cell line, which expresses high levels of CD44 receptors.83 The natural polysaccharide hyaluronic acid specifically binds to CD44 receptors, which overexpresses on the cytomembrane of tumor cells. The nanomaterials increased intracellular NO levels, which in turn sensitizes cancer cells enhancing the efficacy of DOX. Thus, in addition to EPR effects, the presence of hyaluronic acid in the nanomaterials enhances the tumor targetability.
In addition to chemotherapeutics allied to NO in nanomaterials, irradiation can also be employed. High energy ionizing radiation in radiotherapy treatment damages DNA and other biomolecules causing cell death through photon–biomolecule interactions or by the generation of oxygen and nitrogen radical species, including hydroxyl radicals and nitrogen dioxide. However, as solid tumors are often oxygen deficient, tumor resistance to radiation can be observed. In this tumor hypoxic microenvironment, NO has been reported to act as a radiosensitizer, enhancing the radical damage of biomolecules.84
Recently, NO-releasing (nitrosylated maytansinoid containing poly(lactide-co-glycolic)-block-poly(ethylene glycol)) nanoparticles were used to sensitize non-small cell lung carcinoma to radiotherapy. Nanoparticles were efficiently delivered to tumors thorough the EPR effect, where upon irradiation, oxidative stress and NO release were generated.85 In addition, soft X-ray (45 kVp, 0.18–0.85 mGy) was used in combination with a NaYF4:Gd/Tb lanthanide scintillator nanorod as a light transducer for on-demand NO release (from Roussin's black salt) gas-sensitized cancer therapy.86 This combined therapy can reach up to 3 cm in tumor tissue, making it suitable for solid tumors.
AuNPs were functionalized with nitroimidazole, a cell penetrating peptide (CPP) and poly(ethylene glycol) (PEG), for a combined cancer radiosensitization approach.47 Nitroimidazole releases nitrite ions, NOx species, while CPP induce nucleus internalization of the nanomaterials. Nitrite ions are reported to be easily reduced to NO, especially in a hypoxic and/or acidic tumor microenvironment.87 The nanomaterials acted in the sensitization of cancer cells upon radiation with clinically used X-ray (6 MeV X-rays). Interestingly, nitrite ion release is trigged by X-ray radiation, and no release was observed without radiation. In vitro studies with the epidermoid carcinoma cell line A-431 demonstrated that such nanoparticles combined with radiation have enhanced toxicity. The improved radiotherapy effect can be associated with the action of both nitrite ion/NO in the oxidative damage and the actions of AuNPs themselves in the production of ROS.42
Recently, a soft X-ray luminescence nanotransducer (down to 0.9 mGy) integrating ZnGa2O4:Mn nanoparticles with Roussin's black salt (as a light trigged NO donor) was prepared. The authors reported an efficient NO release even after 40 min after stopping the radiation with a deep tumor tissue penetration. Similarly, NaYbF4:Tm@NaYF4:Yb/Er upconversion nanoparticles containing Roussin's black salt releasing NO upon near-infrared radiation (980 nm) were reported.88 This combined therapy is able to either release high amounts of NO (for direct toxic effects) or release low concentrations of NO for the modulation of P-gp to overcome multi-drug resistance. Bismuth sulfide nanoparticles/bis-N-nitroso nanocomposites were prepared to release NO upon near-infrared radiation (808 nm).89
Other versatile strategies have been developed in recent years to overcome drug resistance in combined therapies involving NO donors, nanomaterials and chemo/radio therapeutic agents. Overall, the combination of light irradiation with nanomaterials and NO donors facilitates the spatiotemporal trigger of drug release and allows a targeted therapy. These nanomaterials act as smart materials particularly designed to target tumor cells/tissues. Thus, the tumor microenvironment is of fundamental importance for the design of these nanomaterials. In this sense, near infrared lasers were used in combination with hyaluronidase-triggered sized shrinkable hyaluronic acid shells, containing NO donor and DOX.81 Upon systemic administration, larger size nanoparticles preferably accumulate in the tumor site due to the EPR effect and CD44-targeting.90 At the tumor site, small size dendrimeric prodrug DOX and NO are formed via enzymatic degradation enhancing the deep tumor penetration of the active drugs, increasing targetable-toxic effects (Fig. 3).81
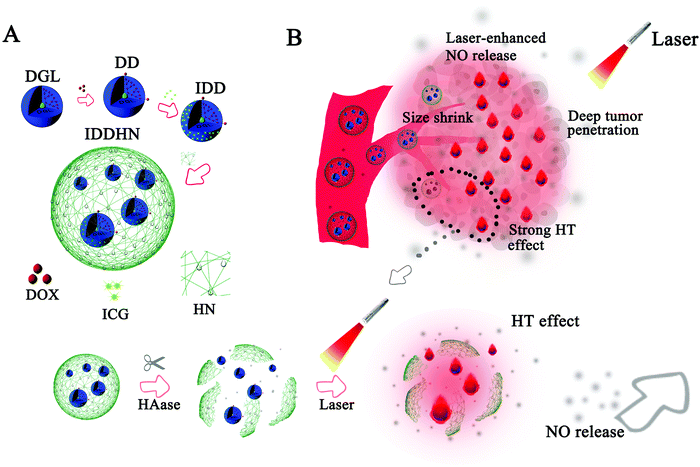 | ||
| Fig. 3 Schematic representation of the combination of hyaluronidase-triggered sized shrinkable hyaluronic acid shells, containing NO donor and DOX (A). (B) Schematic illustration of the synergistic effects upon laser irradiation for deep tumor penetration and therapy effects. Reproduced from ref. 81 with permission from Elsevier. | ||
NaYF4:Yb,Er upconversion nanoparticles (75 nm) conjugated with the natural polymer chitosan and loaded with the photo-NO donor Roussin's black salt and DOX were prepared.91 Under acid conditions, usually found in a solid tumor microenvironment, the entrapped DOX is released from swollen nanomaterials. The enhanced toxic effect demonstrated on the colorectal carcinoma cell line (CT26 cells) was attributed to the combined therapy of NO and DOX released from the nanomaterials. As expected, the simultaneous release of NO and DOX promoted a synergistic antitumor effect. The concentration range of NO release was found to be up to 2 μmol L−1. These effects were attributed to the fact that low doses of NO mediate a P-gp-increased DOX uptake, while high doses of NO have a direct toxic effect in coordination with DOX.91 A similar approach was designed by combing NO-releasing platinum prodrugs and micelles in a photoresponsive therapy.92 Similar to DRX, platinum prodrugs, including cisplatin, have been clinically used as chemotherapeutic agents. Once internalized by cells, NO showing controlled release upon light irradiation at concentrations requires sensitization of cancer cells (human breast carcinoma cells, HCT-116 and MCF-7 cell lines). This process led to the cleavage of photolabile hydrophobic groups releasing platinum(IV) prodrugs. Thus, NO enhanced the chemotherapy efficacy by inactivating P-glycoproteins and MDR-associated proteins, depleting glutathione (that inactivates cisplatin), impairing hypoxia-induced factors, and inhibiting nuclear factor kappa B (NF-κB), overcoming drug resistance.93
4. In vivo biocompatibility of NO-releasing NPs or NO-producing NPs
Concerning the published in vivo studies, two main strategies of NO release are currently described. The NP can release NO when designed from synthesized pro-drugs (mainly from RSNO). NO can be produced by a biosynthesis based on NO synthases viaL-arginine brought by NPs or by a catalytic effect using copper NPs on circulating endogenous RSNO. The claimed therapeutic indications are anticancer or antibacterial effects or the study of a new implanted medical device. The recent papers are reported and compared in Table 1.| Mean of NO release | NPs | Animal species | Administered dosage | In vivo toxicity | Ref. |
|---|---|---|---|---|---|
| a General toxicity includes measure of body weight. b General signs of toxicity (behavior, dizziness, respiratory distress). c Embryonic mortality, morphology modifications or behavior modifications. d Histopathological studies were performed on major organs (heart, liver, spleen, lungs, kidneys). e Biochemistry analyses on blood. f On the coagulation system. g On the hepatic function. h On the renal function. | |||||
| From synthesized pro-drugs | NPs made of NO-releasing tert-dodecane S-nitrosothiol encapsulated into polystyrene-maleic acid. | BALB/c female mice implanted with mouse 4T1 mammary carcinoma cells | Intra-tumor or intravenous NPs (1 mg kg−1 each time) at day 0 day 8 | General toxicity:a no toxicity | 94 |
| SNAP loaded pegylated liposomes | Pancreatic cancer cells (PSCs/PCs) implanted nude mice | NP injected intravenously (10 mg kg−1 SNAP equivalent) | General toxicity:a no toxicity | 54 | |
| Histopathological studies:d no major abnormalities | |||||
| Polyprodrug nanoparticles (reductive-sensitive NP releasing NO, ONOO− and cisplatine in the cytoplasm) | Adenocarcinomic human alveolar basal epithelial cells (A549) and cisplatin-resistant (A549/DDP) tumor-bearing mice | Intravenous injection every 3 days for a total of five injections with a dose of NP equivalent of 5 mg cisplatin per kg. | General toxicity:a no toxicity | 95 | |
| Histopathological studies:d no major abnormalities | |||||
| Biochemistry analyses:efgh minimal changes but an overall good biocompatibility and little side effects | |||||
| Sodium nitroprusside and docetaxel doped mesoporous Prussian blue NPs | BALB/c mice bearing breast cancer (4T1) tumors | Intravenous injection of NPs 100 μL, 5 mg kg−1 of docetaxel or equivalent dose every two days | General toxicity:a no toxicity | 96 | |
| Histopathological studies:d no major abnormalities | |||||
| Nitrosylated maytansinoid loaded poly(lactide-co-glycolic)-block-poly(ethylene glycol) NPs | Albino BALB/c mice | Intravenous administration of 260.8 nmol kg−1 of NPs equivalent to 0.2 mg nitrosylated maytansinoid per kg | General toxicity:ab no toxicity | 85 | |
| Histopathological studies:d no major abnormalities | |||||
| Biochemistry analyses:efgh within normal values. | |||||
| Phototriggered NO nanogenerators loaded with doxorubicin | BALB/c nude mice implanted with multidrug-resistant breast cancer (MCF-7/ADR) cells | Intravenous injection of a dose equivalent to doxorubicin of 5 mg kg−1 (100 μL) | General toxicity:a no toxicity | 97 | |
| NPs loaded with doxorubicin, indocyanine green and NO donor | BALB/c mice implanted with (4T1) breast tumor cells | Intravenous injection of equivalent of 2.0 mg indocyanine green per kg and 2.8 mg doxorubicine per kg, 300 mL every four days for 4 cycles | General toxicity:a no toxicity | 81 | |
| Histological studies:d no major abnormalities | |||||
| Polymer NPs loaded in protoporphyrin (PpIX)-based NO donors | Multidrug-resistant breast cancer (MCF-7/ADR) tumor-bearing BALB/c nude mice | Intravenous injection of NPs at a dose of 5 mg PpIX per kg, repeated every 3 days | General toxicity:a no toxicity | 98 | |
| Histopathological studies:d no major abnormalities | |||||
| Biochemistry studies:gh within normal values | |||||
| S-Nitrosated human serum albumin dimer and albumin bound paclitaxel NPs | BALB/c mice implanted with (SUIT2) pancreatic cancer cells | Intravenous injection of nab-paclitaxel (20 mg kg−1) with dimer (1.95 mmol kg−1) | General toxicity:a no toxicity | 99 | |
| Hollow double-layer imprinted polymer NP loaded in S-nitrosothiols or RSNO loaded NPs | Liver cancer (HepG2) bearing BALB/c mice | Intravenous injection of NPs 5 mg kg−1 | General toxicity:a no toxicity except for the group treated with RSNO NPs which showed a weight loss and a physical decline. | 21 | |
| Histological studies:d no major abnormalities except for RSNO NP treated mice with a slight inflammatory in the kidney, and a serious inflammatory and an alveolar membrane thickening in the lung | |||||
| S-Nitrosocaptopril NP | Zebrafish embryos (Danio rerio, wild type, 5D-tropical strain) | S-Nitrosocaptopril NP diluted from 0.016 to 250 ppm in fish water in 150 μL | General toxicity:c no toxicity | 100 | |
| GSH-responsive NO donor-modified NPs loaded with 10-hydroxycamptothecin (HCPT) | Multidrug-resistant breast cancer (MCF-7/ADR) tumor-bearing BALB/c mice | Intravenous injection of 100 μL of NPs equivalent of HCPT: 5 mg kg−1, NO: 2.8 mg kg−1 every four days | General toxicity:a no toxicity except for mice treated with unformulated HCPT | 101 | |
| Histopathological studies:d no major abnormalities except for the group treated with unformulated HCPT with serious damages of livers and kidneys | |||||
| S-Nitrosocompound loaded pluronic-stabilized, poly(propylene sulfide)-core NPs | C57BL/6J mice | Intradermal injection of 30 μL of NP | General toxicity: no local nor systemic inflammation due to the injection of NPs | 65 | |
| Histopathological studies: increase in lymph node size (but not for spleen) with SNO-NP but not with SNAP, explained by dramatic increase in resident immune cell frequencies. | |||||
| Biochemistry: hepatic function: unchanged levels of plasma alanine transaminase and aspartate transaminase | |||||
| Photothermally triggered nitric oxide nanogenerator targeting type IV pili | P. aeruginosa-infected BALB/c mice | Subcutaneous injection of 200 μL, 80 μg mL−1 of NP on day 1, 3 and 5 | General toxicity: no significant toxicity noticed | 80 | |
| Histopathological studies: no significant statistical difference in major organs (heart, liver, spleen, lung, kidney) | |||||
| Paclitaxel loaded modified S-nitrosoalbumin | BALB/c mice | Intravenously administrated with NPs (200 μL, Ptx: 2 mg mL−1, NO: 2 mM) on days 0, 2, 4, and 6 for four total treatments. | Histopathological studies: no observable pathological abnormalities found in major organs | 102 | |
| Biochemistry: serum biochemical detection and whole blood analysis, platelet counts in whole blood and tail bleeding time showed no alteration | |||||
| From biosynthesis based on NOS | Polyion complex micelles based on poly(ethylene glycol)-block-poly(L-arginine) and chondroïtine sulfate | BALB/c mice, male injected with (C26) adenocarcinoma cells | Intravenous route of 16 mg kg−1 on arginine basis and up to 4 injection each 24 h | General toxicity: no difference in the body weight even after four intravenous injections of the PIC micelles | 103 |
| Erythrocyte membrane-enveloped proteinic nanoparticles with a biomimic nitric oxide synthase (NOS) (co-loading of L-arginine (LA) and photosensitizer IR783) | Uterine cervical cancer cells (U14) tumor-bearing Kunming mice | Intravenous injection of 0.1 mL every two days and the dose of IR783 and LA equal to 0.6 mg kg−1 and 0.9 mg kg−1 | Histopathologcal studies: the analyses of the major organs (heart, liver, spleen, kidney, lungs) revealed no overt histological change and function damages | 104 | |
| NP composed of upconversion nanoparticles, porphyrinic MOFs doped with L-arginine and incorporated into matrices | BALB/c mice infected with S. aureus in a wound | Circular wounds of 4 mm covered by membranes | General toxicity: no fluctuation of mice weight | 105 | |
| From a catalytic activity on RSNO | Coating of extra-corporeal circuits made of copper NPs catalyzing NO release from GSNO | New Zealand white rabbits equipped with arteriovenous extracorporeal circuits | Extra-corporeal circulation with surfaces releasing NO with a flux of ca. 1 to 10 × 10−10 mol cm−2 min−1 | General toxicity: only copper nanoparticles combined with GSNO loop showed no clot formation. | 106 |
| During the entire 4 h procedure, Cu GSNO loops maintained higher platelet counts compared to other test loops or both NO-releasing loops (GSNO and Cu GSNO), all 3 rabbits survived the 4 h procedure | |||||
| Oxygenation system via a veno-venous extracorporeal membrane based on PDMS hollow fibers embedded with copper NPs and supplied with an infusion of the NO donor S-nitroso-N-acetyl-penicillamine | Miniature artificial lung (MAL) attached to Montadale sheep in parallel in a veno-venous extracorporeal membrane oxygenation circuit | Each device received a SNAP infusion of 0.12 lmol min−1 and catalytic generation of NO at 3 ± 0 × 10−10 mol cm−2 min−1 by Cu–PDMS fibers | General toxicity: mean arterial blood pressure and heart rate were stable and within normal ranges during the testings. | 107 | |
| Biochemistry analyses: parameters of coagulation and inflammation remained stable during the testings | |||||
| Keratin/doxorubicin complex nanoparticles releasing NO from endogenous GSNO | Nude mice bearing (A549) tumors | Intravenous injection of 500 mg of NP per kg mouse | General toxicity: body weight of NP group displayed a slight decrease non-significant, interpreted as a non-severe toxic side effect | 108 | |
| Histopathological studies: no significant histological modifications for the heart, liver, and kidney | |||||
| Hemolysis study: no hemolysis induced | |||||
The main animal species used to study the NO in vivo effects are mainly mammals and especially mice, except one study in which Zebrafish embryos were used.101 The mice usually bear tumors which are implanted before testing the NPs. Tests on zebrafishes are often used in the literature to assess the toxicity or the safety of compounds, drugs or even nanoparticles.109 Moreover, the regulatory agencies, FDA and European Medicines Agency (EMA), accept these data for the approval of investigative new drugs. Globally, the articles concerning the biocompatibility of NO-releasing/producing NPs reported a general good tolerance of the treatment by the animals.
Usually, besides the general signs of toxicity, an exploration of the possible disturbance of the biochemical parameters is performed. After an animal is sacrificed, a histopathological study is often performed on main organs. The NO-releasing/producing NPs are generally well-tolerated; only two articles reported toxicity.21,63 Toxicity can be induced by the NPs and/or by the release or the production of NO. NO, the gaseous transmitter with pleiotropic activities in the organisms, at uncontrolled concentrations, may induce a great variety of toxic side effects (inflammation, respiratory problems, modification in heart rate and blood pressure).110 This can be counterbalanced by the very short half-life of NO and consequently, its limited action zone.104 In parallel, after their parenteral administration, which is the most studied administration route, NPs rapidly interact with immune cells and are distributed to reticuloendothelial organs (liver, spleen, lungs).111 The properties of NO and NPs together explain why, besides the general indicators of toxicity (body weight, general behavior of animals), other physiological and biochemical parameters must be explored to assess the potential systemic toxicity of nanoarchitectures. Interactions with immune cells, with or without an observable inflammation, were observed with some particles loaded with an RSNO.21,63 In their work,21 Liu and colleagues explained their observation by high release of high concentrations of NO in an immoderate manner, but the inflammatory effect could also be exacerbated by the nanoparticulate form.
All these in vivo studies can be considered as very preliminary toxicological studies and lay the bases for a more complete work. Indeed, to go further into the development of a drug candidate for human use in medicine, the regulatory agencies impose many constraints to ensure the final safety of the drug.112 For example, toxicology tests must be performed on rodent and non-rodent species according to the Good Laboratory Practices (GLP). The toxicity must be evaluated for a long term: the International Conference on Harmonization (ICH) guidance recommends 9-month chronic toxicity studies on nonrodents.112
5. Final remarks: drawbacks, challenges and perspectives
As represented and discussed in this work, the recent progress in the design of various promising nanoplatforms of controlled release and therapeutic levels of NO at the desirable site of application has opened up new avenues in this interesting research field. Intensive and fruitful research has made several advances in the preparation of smart and versatile nanomedicines that can release NO on-demand in biomedical applications highlighting NO-sensitized synergistic cancer therapy. NO-releasing nanomaterials can have a direct cytotoxic effect (for high NO concentrations) or can sensitize cancer cells (for low NO concentrations, combined with other therapy, such as chemotherapy or radiotherapy). The combined therapy significantly improved the response over monotherapy in solid tumors.47 For instance, in radiotherapy, NO enhances DNA damage and inhibits DNA repair by acting as an oxygen-like radiosensitizer in hypoxic tumors.73The driving force to deeper evaluate the effects of NO-releasing nanomedicines in combination with traditional anti-cancer therapies is mainly based on the systemic toxicity of these traditional drugs and the increasing problem of MDR. NO-releasing nanomedicine is a promising candidate to mitigate the side effects of chemo/radio therapies. More effort has been made in the design of smart NO donors that can sensitively generate NO or NO-related species upon exogenous/endogenous stimuli at the desired site of application (tumor tissue), with minimum side effects to normal tissues.3,74 Undoubtedly, nanotechnology has significantly contributed to the preparation of a variety of smart and efficient multifunctional NO-containing nanostructures in several biomedical applications, including cancer biology. Although several advances have been achieved in this topic, as presented in this work, some issues still need to be further explored: (i) the in-depth mechanism of NO inducing cell death, and more importantly, cancer cell sensitization should be investigated by using genetic and molecular approaches; (ii) development of stable nanomedicines at room temperature that can release NO in a controlled manner upon external or internal stimuli; (iii) further studies (in vitro, in vivo and clinical) of multitherapy by combining NO donors and/or traditional chemotherapeutic drugs, and/or radiotherapy and/or X-ray are still strongly encouraged to be developed; (iv) strategies to enhance the deep penetration of NO and/or chemotherapeutic drugs still need to the further developed, by considering the tumor microenvironment.
Moreover, a great challenge in the biomedical applications of NO donors is still the ability to accurately measure and real-time monitor the NO generation from nanomedicines at the target site. To this end, several important and different experimental techniques should be considered. An exactly controlled NO release profile from nanomaterials is of fundamental importance to avoid a burst in the NO release and poisoning. Further in vivo studies are required and the fate of each component in the nanomaterials should be monitored in the biological system, along with the studies of biocompatibility, biosafety and biodistribution prior to clinical applications. Finally, we expect that this work brings new avenues in the development of integrated nanostructures form combined therapeutic modalities allowing a successful and safe usage of NO-sensitized synergist effects.
Authors’ contribution
All authors have contributed equally to writing – review and editing – preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review and commentary or revision – including pre- or post-publication stages.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We appreciate the support from CNPq (404815/2018-9, 313117/2019-5) and FAPESP (2018/08194-2, 2019/07766-5, 2018/03646-2, 2020/03646-2).References
- J. Hickok and D. Thomas, Curr. Pharm. Des., 2010, 16, 381–391 CrossRef CAS PubMed.
- S. Habib and A. Asif, Indian J. Clin. Biochem., 2011, 26, 3–17 CrossRef CAS PubMed.
- J. C. Pieretti, M. T. Pelegrino, M. H. M. Nascimento, G. R. Tortella, O. Rubilar and A. B. Seabra, Biochem. Pharmacol., 2020, 113740 CrossRef CAS PubMed.
- M. Valko, D. Leibfritz, J. Moncol, M. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef CAS PubMed.
- D. A. Wink, L. Ridnour, S. Hussain and C. Harris, Nitric oxide, 2008, 19, 65–67 CrossRef CAS PubMed.
- L. B. Kreuzer and C. K. Parel, Science, 1971, 173, 47 CrossRef PubMed.
- L. J. Ignarro, G. M. Buga, K. S. Wood, R. E. Byrns and G. Chaudhuri, Proc. Natl. Acad. Sci. U. S. A., 1987, 24, 9265–9269 CrossRef PubMed.
- T. Infante, D. Costa and C. Napoli, Angiology, 2021, 72, 411–425 CrossRef CAS PubMed.
- J. Mintz, V. Rosete, K. Shah, G. Goldstein, J. M. Hare, R. Ramasamy and H. Arora, Vaccines, 2021, 9, 94 CrossRef CAS PubMed.
- F. Lisi, A. N. Zelikin and R. Chandrawati, Adv. Sci., 2021, 8, 2003895 CrossRef CAS PubMed.
- W. Hu, J. Shi, J. Zhang, Y. Wang, Y. Gua and Z. Zhang, Adv. Ther., 2021, 100032 Search PubMed.
- D. Koshland, Science, 1992, 258, 5090 Search PubMed.
- D. Zou, Z. Li, F. Lv, Y. Yang, C. Yang, J. Song, Y. Chen, Z. Jin, J. Zhou, Y. Jiang, Y. Ma, Z. Jing, Y. Tang and Y. Zhang, Front. Oncol., 2021, 11, 1–14 Search PubMed.
- S. Kobayashi, T. Homma and J. Fujii, Biochem. Biophys. Rep., 2021, 26, 100942 Search PubMed.
- J. Scicinski, B. Oronsky, S. Ning, S. Knox, D. Peehl, M. M. Kim, P. Langecker and G. Fanger, Redox Biol., 2015, 6, 1–8 CrossRef CAS PubMed.
- C. Gokmenoglu, N. Ozmeric, C. Sungur, R. Bildik, I. Erguder and S. Elgun, Arch. Oral Biol., 2018, 85, 207–211 CrossRef CAS PubMed.
- R. Y. S. Cheng, D. Basudhar, L. A. Ridnour, J. L. Heinecke, A. H. Kesarwala, S. Glynn, C. H. Switzer, S. Ambs, K. Miranda and D. A. Wink, Nitric oxide, 2014, 43, 17–28 CrossRef CAS PubMed.
- B. Bonavida and G. Hermes, Redox Biol., 2015, 6, 486–494 CrossRef CAS PubMed.
- M. T. Pelegrino, A. Paganotti, A. B. Seabra and R. Weller, Histochem. Cell Biol., 2020, 0123456789 Search PubMed.
- M. T. Pelegrino, J. C. Pieretti, G. Nakazato, M. C. Gonçalves, J. C. Moreira and A. B. Seabra, Nitric oxide, 2021, 106, 24–34 CrossRef CAS PubMed.
- Z. Q. Liu, J. Wang, P. Zhang, Z. Zhang, D. Guo and X. Yang, Colloids Surf., B, 2019, 173, 356–365 CrossRef PubMed.
- X. Dong, H. J. Liu, H. Y. Feng, S. C. Yang, X. L. Liu, X. Lai, Q. Lu, J. F. Lovell, Z. Chen and C. Fang, Nano Lett., 2019, 19, 997–1008 CrossRef CAS PubMed.
- H. Maeda, C. Noguchi, K. Sato and T. Akaike, J. Cancer Red., 1994, 85, 331–334 CAS.
- Z. Fang, Y. Shen and D. Gao, New J. Chem., 2021, 45, 4534–4544 RSC.
- M. Feelisch and K. Olson, Nitric Oxide, 2013, 35, 2–4 CrossRef CAS PubMed.
- M. G. Oliveira, Basic Clin. Pharmacol. Toxicol., 2016, 119, 49–56 CrossRef PubMed.
- A. B. Seabra and N. Durán, Med. Chem., 2016, 17, 216–223 Search PubMed.
- L. Keefer, Curr. Top. Med. Chem., 2005, 5, 625–636 CrossRef CAS PubMed.
- Y. Lan, X. Zhu, M. Tang, Y. Wu, J. Zhang, J. Liu and Y. Zhang, Nanoscale, 2020, 12, 7875–7887 RSC.
- T. Liu, Z. Qiao, J. Wang, P. Zhang, Z. Zhang, D.-S. Guo and X. Yang, Colloids Surf., B, 2018, 173, 356–365 CrossRef PubMed.
- L. Chen, H. Qianjun, L. Minyi, X. Liwei, S. Kun, T. Liwei, J. Zhaokui, W. Tianfu and Q. Zhiyong, ACS Appl. Mater. Interfaces, 2017, 9, 36473–36477 CrossRef CAS PubMed.
- J. F. Quinn, M. Whittaker and T. Davis, J. Controlled Release, 2015, 205, 190–205 CrossRef CAS PubMed.
- B. Li, Y. Ming, Y. Liu, H. Xing, R. Fu, Z. Li, R. Ni, L. Li, D. Duang, J. Xu, C. Li, M. Xiang, H. Song and J. Chen, Front. Pharmacol., 2020, 11, 923 CrossRef CAS PubMed.
- P. Ford and I. Lorkovic, Chem. Rev., 2002, 102, 993–1018 CrossRef CAS PubMed.
- S. Wynia-Smith and B. Smith, Nitric oxide, 2017, 63, 52–60 CrossRef CAS PubMed.
- J. Jahnová, L. Luhová and M. Petřivalský, Plants, 2019, 8, 1–19 CrossRef PubMed.
- A. Janczyk, A. Wolnicka-Glubisz, A. Chmura, M. Elas, Z. Matuszak, G. Stochel and K. Urbanska, Nitric Oxide, 2004, 10, 42–50 CrossRef CAS PubMed.
- A. B. Seabra, G. Justo and P. S. Haddad, Biotechnol. Adv., 2015, 33, 1370–1379 CrossRef CAS PubMed.
- H. Duong, Z. Kamarudin, R. B. Erlich, Y. Li, M. W. Jones, M. Kavallaris, C. Boyer and T. Davis, Chem. Commun., 2013, 49, 4190–4192 RSC.
- P. Couvreur, Adv. Drug Delivery Rev., 2013, 65, 21–23 CrossRef CAS PubMed.
- D. Brambilla, P. Luciani and J. P. Leroux, J. Controlled Release, 2014, 190, 9–14 CrossRef CAS PubMed.
- A. B. Seabra, G. Z. Justo and P. S. Haddad, Biotechnol. Adv., 2015, 33, 1370–1379 CrossRef CAS PubMed.
- F. Liu, J. Lou and D. Hristov, Nanoscale, 2017, 9, 14627–14634 RSC.
- L. J. Ignarro, Nitric Oxide Biology and Pathobiology, Academic Press, San Diego, 2000 Search PubMed.
- K.-D. Kröncke, K. Fehsel and V. Kolb-Bachofen, Nitric Oxide, 1997, 1, 107–120 CrossRef PubMed.
- S. K. Choudhari, S. Korde, M. Chaudhary, S. Bagde, A. R. Gadbail and V. Joshi, World J. Surg. Oncol., 2013, 11, 118 CrossRef PubMed.
- B. Bonavida, Biochem. Pharmacol., 2020, 113913 CrossRef CAS PubMed.
- L. Tan, A. Wan and H. Li, ACS Appl. Mater. Interfaces, 2021, 13, 18392 CrossRef CAS PubMed.
- M. Neidrauer, U. K. Ercan, A. Bhattacharyya, J. Samuels, J. Sedlak, R. Trikha, K. A. Barbee, M. S. Weingarten and S. G. Joshi, J. Med. Microbiol., 2014, 63, 203–209 CrossRef CAS PubMed.
- B. Xiao, P. S. Wheatley, X. Zhao, A. J. Fletcher, S. Fox, A. G. Rossi, I. L. Megson, S. Bordiga, L. Regli, K. M. Thomas and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 1203–1209 CrossRef CAS PubMed; R. Liu, W. Xiao, C. Hu, R. Xie and H. Gao, J. Controlled Release, 2018, 278, 127–139 CrossRef PubMed.
- M. T. Pelegrino, L. C. Silva, C. M. Watashi, P. S. Haddad, T. Rodrigues and A. B. Seabra, J. Nanopart. Res., 2017, 19, 57 CrossRef.
- M. C. Santos, A. B. Seabra, M. T. Pelegrino and P. S. Haddad, Appl. Surf. Sci., 2016, 367, 26–35 CrossRef CAS.
- W. R. Rolim, J. C. Pieretti, D. L. S. Renó, B. A. Lima, M. H. M. Nascimento, F. N. Ambrosio, C. B. Lombello, M. Brocchi, A. C. S. De Souza and A. B. Seabra, ACS Appl. Mater. Interfaces, 2019, 11, 6589–6604 CrossRef CAS PubMed.
- X. Chen, F. Jia, Y. Li, Y. Deng, Y. Huang, W. Liu, Q. Jin and J. Ji, Biomaterials, 2020, 246, 119999 CrossRef CAS PubMed.
- S. Rezvantalab, N. I. Drude, M. K. Moraveji, N. Güvener, E. K. Koons, Y. Shi, T. Lammers and F. Kiessling, Front. Pharmacol., 2018, 9, 1260 CrossRef CAS PubMed.
- V. S. Madamsetty, A. Mukherjee and S. Mukherjee, Front. Pharmacol., 2019, 10, 1264 CrossRef CAS PubMed.
- M. Enea, E. Pereira, J. Costa, M. E. Soares, D. D. da Silva, M. de Lourdes Bastos and H. F. Carmo, Toxicol. In Vitro, 2020, 105046 Search PubMed.
- J. Guo, K. Rahme, Y. He, L.-L. Li, J. Holmes and C. O’Driscoll, Int. J. Nanomed., 2017, 12, 6131–6152 CrossRef CAS PubMed.
- S. Gurunathan, M. Qasim, C. Park, H. Yoo, J.-H. Kim and K. Hong, Int. J. Mol. Sci., 2018, 19, 2269 CrossRef PubMed.
- K. M. El-Say and H. S. El-Sawy, Int. J. Pharm., 2017, 528, 675–691 CrossRef CAS PubMed.
- I. Inkielewicz-Stepniak, M. J. Santos-Martinez, C. Medina and M. W. Radomski, Int. J. Nanomed., 2014, 9, 1677–1687 Search PubMed.
- T. A. Heinrich, R. S. da Silva, K. M. Miranda, C. H. Switzer, D. A. Wink and J. M. Fukuto, Br. J. Pharmacol., 2013, 169, 1417–1429 CrossRef CAS PubMed.
- S. A. Abraham, D. N. Waterhouse, L. D. Mayer, P. R. Cullis, T. D. Madden and M. B. Bally, Liposomes, 2005, 391, 71–97 CAS.
- Y. C. Sung, P. R. Jin, L. A. Chu, F.-F. Hsu, M.-R. Wang, C.-C. Chang, S.-J. Chiou, J. T. Qiu, D.-Y. Gao, C.-C. Lin, Y.-S. Chen, Y.-C. Hsu, J. Wang, F.-N. Wang, P.-L. Yu, A.-S. Chiang, A. Y.-T. Wu, J. J.-S. Ko, C. P.-K. Lai, T.-T. Lu and Y. Chen, Nat. Nanotechnol., 2019, 14, 1160–1169 CrossRef CAS PubMed.
- A. Schudel, L. F. Sestito and S. N. Thomas, J. Biomed. Mater. Res., Part A, 2018, 106, 1463–1475 CrossRef CAS PubMed.
- M. T. Pelegrino, C. Baldi, A. Souza and A. Seabra, J. Phys.: Conf. Ser., 2019, 012019 CrossRef CAS.
- L. S. Ferraz, C. M. Watashi, C. Colturato-Kido, M. T. Pelegrino, E. J. Paredes-Gamero, R. B. Weller, A. B. Seabra and T. Rodrigues, Mol. Pharmaceutics, 2018, 15, 1160–1168 CrossRef CAS PubMed.
- E. M. Hetrick, J. H. Shin, N. A. Stasko, C. B. Johnson, D. A. Wespe, E. Holmuhamedov and M. H. Schoenfisch, ACS Nano, 2008, 2, 235–246 CrossRef CAS PubMed.
- C. Szabo, Nat. Rev. Drug Discovery, 2016, 15, 185–203 CrossRef CAS PubMed.
- J. Lee, C. Yang, S. Ahn, Y. Choi and K. Lee, Biomater. Sci., 2021, 9, 5150–5159 RSC.
- C. Szabo, Nat. Rev. Drug Discovery, 2007, 6, 917–935 CrossRef CAS PubMed.
- C. Szabo, C. Coletta, C. Chao, K. Módis, B. Szczesny, A. Papapetropoulos and M. R. Hellmich, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12474–12479 CrossRef CAS PubMed.
- J. C. Pieretti, C. V. C. Junho, M. S. Carneiro-Ramos and A. B. Seabra, Pharmacol. Res., 2020, 161, 105121 CrossRef CAS PubMed.
- J. C. Pieretti, M. C. Gonçalves, G. Nakazato, A. C. S. de Souza, A. Boudier and A. B. Seabra, J. Mater. Sci.: Mater. Med., 2021, 32, 23 CrossRef CAS PubMed.
- A. L. Tessaro, A. Fraix, A. C. Pedrozo da Silva, E. Gazzano, C. Riganti and S. Sortino, Nanomaterials, 2019, 9, 823 CrossRef CAS PubMed.
- E. Hays and B. Bonavida, Antioxidants, 2019, 8, 1397–1406 CrossRef PubMed.
- M. Chen, F. Song, Y. Liu, J. Tian, C. Liu, R. Li and Q. Zhang, Nanoscale, 2019, 11, 3814–3826 RSC.
- S. Gao, W. Zhang, R. Wang, S. P. Hopkins, J. C. Spagnoli, M. Racin, L. Bai, L. Li, W. Jiang, X. Yang, C. Lee, K. Nagata, E. W. Howerth, H. Handa, J. Xie, Q. Ma and A. Kumar, ACS Nano, 2020, 14, 1468–1481 CrossRef CAS PubMed.
- A. Wilson, V. Menon, Z. Khan, A. Alam, L. Litovchick and V. Yakovlev, Redox Biol., 2019, 24, 101169 CrossRef CAS PubMed.
- W. Wu, M. Chen, T. Luo, Y. Fan, J. Zhang, Y. Zhang, Q. Zhang, A. Sapin-Minet, C. Gaucher and X. Xia, Acta Biomater., 2020, 103, 259–271 CrossRef CAS PubMed.
- C. Hu, X. Cun, S. Ruan, R. Liu, W. Xiao, X. Yang, Y. Yang, C. Yang and H. Gao, Biomaterials, 2018, 168, 64–75 CrossRef CAS PubMed.
- W. Fan, B. C. Yung and X. Chen, Angew. Chem., Int. Ed., 2018, 57, 8383–8394 CrossRef CAS PubMed.
- Z. Sun, Z. Yi, X. Cui, X. Chen, W. Su, X. Ren and X. Li, Nanoscale, 2018, 10, 12109 RSC.
- E. I. Azzam, J. P. Jay-Gerin and D. Pain, Cancer Lett., 2012, 327, 48 CrossRef CAS PubMed.
- S. Gao, W. Zhang, R. Wang, S. P. Hopkins, J. C. Spagnoli, M. Racin, L. Bai, L. Li, W. Jiang, X. Yang, C. Lee, K. Nagata, E. W. Howerth, H. Handa, J. Xie, Q. Ma and A. Kumar, ACS Nano, 2020, 14, 1468–1481 CrossRef CAS PubMed.
- M. Jiang, Z. Xue, Y. Li, H. Liu, S. Zeng and J. Hao, Nanoscale Horiz., 2020, 5, 268–273 RSC.
- A. B. Seabra, M. Oullet, M. Antonic, M. N. Chretien and A. M. English, Nitric oxide, 2013, 35, 116–122 CrossRef CAS PubMed.
- X. Zhang, G. Tian, W. Yin, L. Wang, X. Zheng, L. Yan, J. Li, H. Su, C. Chen, Z. Gu and Y. Zhao, Adv. Funct. Mater., 2015, 25, 3049–3056 CrossRef CAS.
- X. Zhang, J. Du, Z. Guo, J. Yu, Q. Gao, W. Yin, S. Zhu, Z. Gu and Y. Zhao, Adv. Sci., 2019, 6, 1801122 CrossRef PubMed.
- M. Gotte and G. Yip, Cancer Res., 2006, 66, 10233–10237 CrossRef PubMed.
- L. Tan, R. Huang, X. Li, S. Liu and Y. M. Shen, Acta Biomater., 2017, 57, 498–510 CrossRef CAS PubMed.
- S. Pramanick, J. Kim, J. Kim, G. Saravanakumar, D. Park and W. J. Kim, Bioconjugate Chem., 2018, 29, 885–897 CrossRef CAS PubMed.
- J. Kim, B. C. Yung, W. J. Kim and X. Chen, J. Controlled Release, 2017, 263, 223–230 CrossRef CAS PubMed.
- H. Alimoradi, K. Greish, A. Barzegar-Fallah, L. Alshaibani and V. Pittala, Int. J. Nanomed., 2018, 13, 7771–7787 CrossRef CAS PubMed.
- C. Chu, X. Lyu, Z. Wang, H. Jin, S. Lu, D. Xing and X. Hu, Chem. Eng. J., 2020, 402, 126125 CrossRef CAS.
- T. Feng, J. Wan, P. Li, H. Ran, H. Chen, Z. Wang and L. Zhang, Biomaterials, 2019, 214, 119213 CrossRef PubMed.
- R. Guo, Y. Tian, Y. Wang and W. Yang, Adv. Funct. Mater., 2017, 27, 1606398 CrossRef.
- X. Huang, F. Xu, H. Hou, J. Hou, Y. Wang and S. Zhou, Nano Res., 2019, 12, 1361–1370 CrossRef CAS.
- R. Kinoshita, Y. Ishima, V. T. G. Chuang, H. Nakamura, J. Fang, H. Watanabe, T. Shimizu, K. Okuhira, T. Ishida, H. Maeda, M. Otagiri and T. Maruyama, Biomaterials, 2017, 140, 162–169 CrossRef CAS PubMed.
- B. Mordorski, R. Pelgrift, B. Adler, A. Krausz, A. B. da Costa Neto, H. Liang, L. Gunther, A. Clendaniel, S. Harper, J. M. Friedman, J. D. Nosanchuk, P. Nacharaju and A. J. Friedman, Nanomedicine, 2015, 11, 283–291 CrossRef CAS PubMed.
- X. Niu, J. Cao, Y. Zhang, X. Gao, M. Cheng, Y. Liu, W. Wang and Z. Yuan, Nanomedicine, 2019, 20, 102015 CrossRef CAS PubMed.
- Y. Xu, J. Liu, Z. Liu, H. Ren, J. Yong, W. Li, H. Wang, Z. Yang, Y. Wang, G. Chen and X. Li, ACS Nano, 2020, 14, 9780–9795 CrossRef CAS PubMed.
- S. Kudo and Y. Nagasaki, J. Controlled Release, 2015, 217, 256–262 CrossRef PubMed.
- Z. Ma, S. Liu, Y. Ke, H. Wang, R. Chen, Z. Xiang, Z. Xie, Q. Shi and J. Yin, Biomaterials, 2020, 255, 120141 CrossRef CAS PubMed.
- J. Sun, Y. Fan, W. Ye, L. Tian, S. Niu, W. Ming, J. Zhao and L. Ren, Chem. Eng. J., 2020, 417, 128049 CrossRef.
- M. E. Douglass, M. J. Goudie, J. Pant, P. Singha, S. Hopkins, R. Devine, C. W. Schmiedt and H. Handa, ACS Appl. Bio Mater., 2019, 2, 2539–2548 CrossRef CAS PubMed.
- A. Lai, C. T. Demarest, C. C. Do-Nguyen, R. Ukita, D. J. Skoog, N. M. Carleton, K. A. Amoako, P. J. Montoya and K. E. Cook, Acta Biomater., 2019, 90, 122–131 CrossRef CAS PubMed.
- Y. Li, J. Lin, X. Zhi, P. Li, X. Jiang and J. Yuan, Mater. Sci. Eng., C, 2018, 91, 606–614 CrossRef CAS PubMed.
- C. Chakraborty, A. R. Sharma, G. Sharma and S.-S. Lee, J. Nanobiotechnol., 2016, 14, 65 CrossRef PubMed.
- R. S. Ronson, M. Nakamura and J. Vinten-Johansen, Cardiovasc. Res., 1999, 44, 47–59 CrossRef CAS PubMed.
- N. Khlebtsov and L. Dykman, Chem. Soc. Rev., 2011, 40, 1647–1671 RSC.
- M. M. Dotzel, Fed. Regist., 1999, 64, 34259–34260 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |