Open Access Article
Open Access ArticleIndole fused heterocycles as sensitizers in dye-sensitized solar cells: an overview
P. R.
Nitha
ab,
Suraj
Soman
 *ab and
Jubi
John
*ab and
Jubi
John
 *ab
*ab
aChemical Sciences and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Thiruvananthapuram 695019, India. E-mail: suraj@niist.res.in; jubijohn@niist.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
First published on 23rd August 2021
Abstract
The past three decades have witnessed extensive research in developing a range of non-metallic organic dyes for dye sensitized solar cells (DSSCs). Dyes occupy a prominent position among components in DSSCs, and organic dyes have emerged as the most promising candidate for DSSCs due to their performance, ease of synthesis, stability, tunability, low cost and eco-friendly characteristics. In addition to this, so far, the best and highest performing DSSCs reported in the literature use metal-free organic dyes. Organic dyes also provide flexibility to be used along with alternate new generation cobalt and copper electrolytes. Among various organic dyes, heterocycles, mainly N- and S-containing, have found immense applications as sensitizers. Indole fused heterocycles were used by different research groups in their dye designs, mainly as a donor and π-spacer. The planarity of these electron-rich fused indole systems is advantageous as it helps to initiate a more prominent ICT transition in dye molecules. In addition, the possibility for selective functionalization of N-atoms with long or branched alkyl chains prevents the aggregation of the sensitizer, increasing the solubility and is effective in custom design dyes which are in turn capable of preventing back electron transfer (recombination). Fused indole moieties utilized in the design of sensitizers are stable and offer easy synthesis. In the present review, we examine different indole fused heterocycles as building blocks for sensitizers used in DSSCs.
Introduction
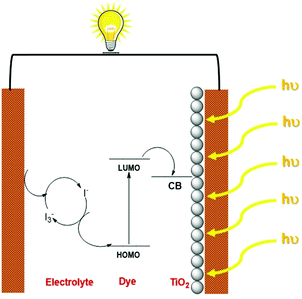
Fossil fuel-based resources have been primarily satisfying the energy demands of humankind for more than a century. The ever-increasing energy demands that are fuelled by the growing human population have contributed to environmental issues with the depletion of conventional resources, necessitating the need for research into developing efficient methods to harness alternative energy sources.1 Solar energy is considered one of the most promising alternatives that could sustainably provide inexhaustible energy.2 The stepping stone for photovoltaic technology was laid with the demonstration of the first silicon-based solar cells by Bell laboratories, which exhibited an efficiency of ∼6%. Since then, the technology has witnessed many advancements in introducing thin films in the late 1970's and finally reaching up to the third-generation solar cells.3 The third-generation photovoltaics consist of dye-sensitized solar cells, organic solar cells, quantum dot solar cells and perovskite solar cells.4 Though their efficiencies are still lagging behind the conventional silicon-based devices, the lower fabrication cost of these devices along with lesser environmental impact are promising factors that urge the scientific community to carry out research and technological advancements in third-generation photovoltaics.5Dye sensitized solar cell research got momentum in 1991 with the pioneering work carried out by Brian O'Regan and Michael Gratzel, where they used Ru metal complex sensitized nanocrystalline TiO2 film, generating a power conversion efficiency of 7%.6 DSSCs have many attractive features, including lower fabrication cost and short payback time with minimum environmental hazards.7 In addition, they can be designed for both outdoor as well as indoor light harvesting.8 DSSC consists of three major components, photoelectrode, electrolyte and a counter electrode. The dye sensitized mesoporous semiconductor layer coated on a conductive glass/plastic substrate collectively acts as the photoanode. The electrolyte is responsible for both regeneration of the dye as well as charge transport to the counter electrode. Liquid electrolytes which consist of redox mediators in organic solvents are typically used in DSSCs. To address the leakage and device lifetime issues originating from the usage of liquid electrolytes, efforts are being made to explore quasi-solid electrolytes and solid conductors. The counter electrode is composed of transparent conductive oxides (ITO/FTO) coated with catalysts like platinum for fast electron transfer reactions. The mechanism of current generation with DSSCs starts with the absorption of light by the dye sensitizers that are adsorbed on the semiconductor surface. These photoexcited electrons are then injected into the conduction band of the semiconductor, which diffuses through the conductive substrate and finally reaches the counter electrode. The oxidized dye molecules are subsequently regenerated by the redox mediators present in the electrolyte, which are then reduced at the counter electrode. This cycles continues generating current without net chemical change in the system (Scheme 1).9
Unlike conventional PV devices, DSSCs excel with the advantage that different components are carrying out light absorption, charge generation, and charge transport. This opens up the broader possibility of achieving higher photovoltaic performance by selectively optimizing each component used in the device. The sensitizer represents the core unit in DSSCs, responsible for light absorption and electron injection into the semiconductor layer. An ideal sensitizer is supposed to display panchromatic absorption with a higher molar extinction coefficient. The optimum redox potential of the dye energy levels (HOMO–LUMO) is necessary to achieve efficient electron injection into the semiconductor conduction band and to realize effective regeneration of the dye ground state by the redox electrolyte. The sensitizer should also possess features such as suitable binding groups (–COOH, –PO3H2) to anchor onto the semiconductor surface and also needs to be engineered in such a way to minimize aggregation on the semiconductor. The prevention of dye aggregation is highly desirable to reduce recombination losses and increase the open circuit potential of the devices, which can also contribute to the stability of the device as a whole.10
Ruthenium-based sensitizers dominated the first two decades in DSSC research since their inception in the early nineties due to their broad absorption and higher power conversion efficiencies and reached up to a PCE of 11.5% using a conventional iodide/triiodide electrolyte.11 Though these sensitizers appear to be feasible for practical applications, with progressing research, a lower molar extinction coefficient of metal complexes along with the scarcity of Ru have slowly paved the way for the advent of metal-free sensitizers. Additionally, the introduction of alternate cobalt and copper electrolyte further encouraged the scientific community to expand the research on organic dyes, which are most suitable with alternate electrolytes. The introduction of metal-free sensitizers has also opened up an arsenal of strategies to develop more efficient devices at a lower cost and in an eco-friendly manner.12 Metal-free sensitizers generally display high molar extinction coefficients, but the narrow absorption of many sensitizers results in serious concern over light harvesting. The emergence of a co-sensitization strategy helped to alleviate this limitation by realizing panchromatic absorption through the sensitization of a combination of different dyes having complementary absorption.13 This also reduced the possibility for dye aggregation. The strongest side of organic sensitizers involves the flexibility in tuning the chemical structure with the help of the well-established synthetic strategies.14 According to the recent reports, the PCE of DSSCs based on single metal free organic sensitizers has reached 13.6% using a cobalt electrolyte, 11.7 for solid state DSSCs and 32% for indoor light harvesting. The co-sensitization strategy could realize 14.3% PCE using a cobalt based electrolyte.15
Molecular engineering of dyes deals with designing systems with potential light-harvesting ability over the entire visible region with proper energetics that realizes electron transfer from the excited state of the dye to the metal oxide upon light absorption. The most widely employed molecular architecture is the donor–π spacer–acceptor (D–π–A) strategy.16 Anchoring groups are supposed to be bifunctional, serving the purposes of adsorption as well as electron acceptance. Although many new anchoring groups have emerged, a cyanoacrylate group is generally found to outperform others because of its agreement with the two functions mentioned above. The strong electron withdrawing nature of the cyanoacrylate moiety facilitates the broadening of the absorption spectra of molecules and the strong adsorption capability to the semiconductor surface increases the injection ability and device lifetime. A wide variety of choices are present for both donor and π-spacer moieties. The derivatives of arylamine, carbazole, coumarins and phenothiazine are some of the popular donor groups of continued interest used in DSSCs.17 The π-linkers are also of paramount importance in tuning the communication between donor and acceptor units, thereby increasing the light-harvesting ability of the dyes. Though thiophene, furan, benzene and oligothiophenes have been used extensively as π-spacers in dyes, fused ring planar systems with strategies to prevent π–π aggregation have not been explored to the full extent.18 Apart from modulating each building block of a sensitizer, considerable effort was also laid in engineering new dye design strategies, which resulted in the introduction of architectures like D–A–π–A, D–D–π–A, A–π–D–π–A and D–π–A–A.19
The present review attempts to consolidate and analyse dye sensitizers which utilize fused indole units as components in sensitizers used in DSSCs. These indole-fused heterocycles are found to exhibit more electron-donating ability than those with indole in conjugation with expanded π-systems.20 Another advantage of the fused indole systems is the better planarity they possess, inducing better donor–acceptor interaction leading to improved PV performance. The possibility of functionalization of the N-atom with long or branched alkyl chains is another advantage of indole-based heterocyclic systems that increase the solubility and prevent aggregation of the sensitizer. The majority of fused indoles were used as a donor moiety in the sensitizers, and very few units were used as π-spacers. The indole fused systems explored as building blocks in DSSC sensitizers include indoloquinoxaline (IQ), indolocarbazole, indoloindole, benzothienoindole, indenoindole, triazatruxene, thienoindole, tetraindole, dithienopyrroloindole, fluorenylindolenine, and indole–imidazole. The exploration of these systems as donor and π-spacers are investigated fitting in different molecular architectures. This will help the scientific community further re-engineer these heterocycles with excellent optoelectronic properties in line with the requirements of the photovoltaic applications.
I. Indoloquinoxaline based sensitizers for DSSCs
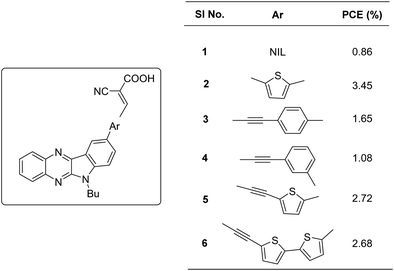
Indolo[2,3-b]quinoxaline (IQ), a built-in donor–acceptor chromophore, consists of an electron-rich indole moiety fused with an electron-deficient quinoxaline unit. The condensation of isatin with o-phenylenediamine can efficiently synthesize these planar heteroarenes.21 By appropriately functionalizing the indole and quinoxaline motifs with electron-donating or electron-withdrawing groups, the electronic properties of this chromophore can be custom-tuned in line with the applications. It has been documented that the donating ability of the indole motif and the donor–acceptor interaction with the quinoxaline unit is enhanced by functionalizing the indole core with electron-donating groups.22The first utilization of this scaffold as a building block in DSSCs was carried out by Venkateswararao et al.23 They constructed dyes 2–6, which are having IQ as an electron donor and cyanoacrylic acid as an anchoring unit (Fig. 1). The sensitizers differed in the π-spacer employed, which were either phenyl or thiophene fragments. Dye 1, which lacks any π-spacer, delivered the least efficiency of 0.86%. Among the remaining sensitizers, 6 showed a red shifted and distinct ICT band implying more effective conjugation. Two dye baths were used to fabricate devices, one with DCM and the other with a combination of CH3CN/tert-butanol/DMSO (3.5/3.5/3, v/v). All the sensitizers exhibited better efficiencies in the latter case. The changes in PCE were mainly caused by significant changes in the photocurrent generated. Though ICT was more prominent in the case of 6, a relatively high degree of planarity might have caused aggregation of the dyes leading to decreased light harvesting. Dye 2 with simple thiophene as the π-spacer outperformed other dyes with PCE of 3.45% with Jsc of 9.29 mA cm−2 and Voc of 579 mV. Other dyes showcased PCE in the order 6 > 5 > 3 > 4 (Table 1). The introduction of acetylene was not found to be beneficial for transmitting charges in the current design.
| Sensitizer | J sc (mA cm−2) | V oc (mV) | FF | PCE (%) | Electrolyte | Coadsorbent (concentration) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 2.66 | 500 | 0.64 | 0.86 | I−/I3− | — | 23 |
| 2 | 9.29 | 579 | 0.64 | 3.45 | I−/I3− | — | 23 |
| 3 | 4.43 | 543 | 0.68 | 1.65 | I−/I3− | — | 23 |
| 4 | 2.73 | 593 | 0.67 | 1.08 | I−/I3− | — | 23 |
| 5 | 7.38 | 568 | 0.65 | 2.72 | I−/I3− | — | 23 |
| 6 | 7.77 | 562 | 0.62 | 2.68 | I−/I3− | — | 23 |
| 7 | 16.0 | 708 | 0.67 | 7.62 | I−/I3− | — | 24 |
| 8 | 14.8 | 701 | 0.63 | 6.48 | I−/I3− | — | 24 |
| 9 | 14.1 | 742 | 0.67 | 7.03 | I−/I3− | — | 24 |
| 10 | 8.9 | 676 | 0.68 | 4.10 | I−/I3− | — | 25 |
| 11 | 14.0 | 705 | 0.69 | 6.82 | I−/I3− | — | 25 |
| 12 | 15.3 | 757 | 0.71 | 8.28 | I−/I3− | — | 25 |
| 13 | 14.2 | 745 | 0.71 | 7.56 | I−/I3− | — | 25 |
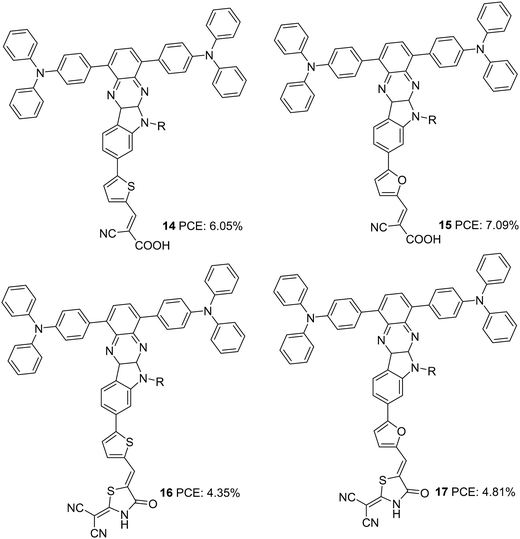
| 14 | 11.9 | 797 | 0.64 | 6.05 | I−/I3− | — | 26 |
| 15 | 12.9 | 817 | 0.67 | 7.09 | I−/I3− | — | 26 |
| 16 | 9.03 | 707 | 0.68 | 4.35 | I−/I3− | — | 26 |
| 17 | 9.71 | 724 | 0.68 | 4.81 | I−/I3− | — | 26 |
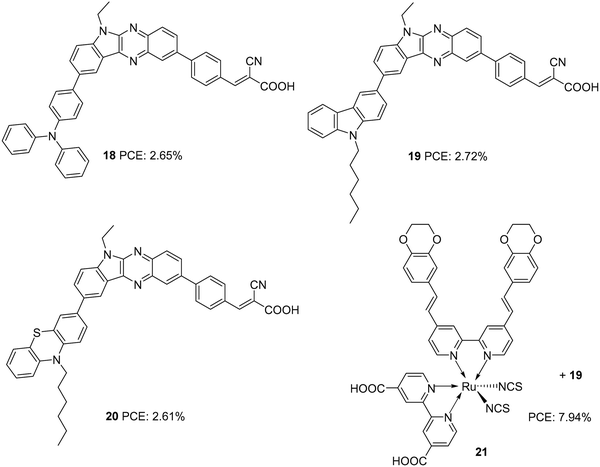
| 18 | 5.56 | 653 | 0.72 | 2.65 | I−/I3− | CDCA (10 mM) | 27 |
| 19 | 5.68 | 622 | 0.73 | 2.72 | I−/I3− | CDCA (10 mM) | 27 |
| 20 | 5.99 | 618 | 0.69 | 2.61 | I−/I3− | CDCA (10 mM) | 27 |
| 21 | 19.0 | 649 | 0.61 | 7.46 | I−/I3− | CDCA (20 mM) | 27 |
| 19/21 | 19.37 | 654 | 0.63 | 7.94 | I−/I3− | CDCA (20 mM) | 27 |
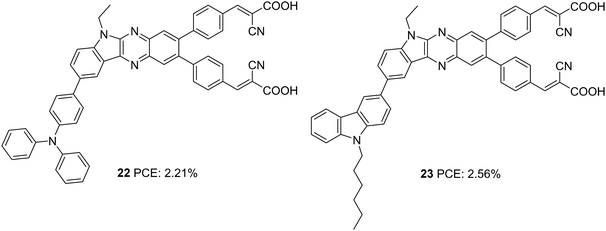
| 22 | 4.73 | 637 | 0.73 | 2.21 | I−/I3− | CDCA (10 mM) | 28 |
| 23 | 5.08 | 676 | 0.74 | 2.56 | I−/I3− | CDCA (10 mM) | 28 |
| 22/21 | 17.79 | 683 | 0.67 | 8.16 | I−/I3− | CDCA (20 mM) | 28 |
| 23/21 | 18.24 | 688 | 0.69 | 8.67 | I−/I3− | CDCA (20 mM) | 28 |
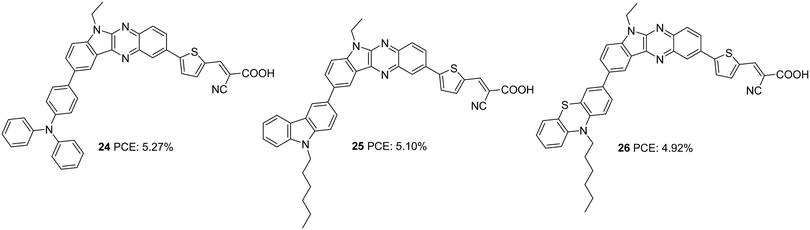
| 24 | 11.10 | 676 | 0.70 | 5.27 | I−/I3− | CDCA (10 mM) | 29 |
| 25 | 11.29 | 657 | 0.69 | 5.10 | I−/I3− | CDCA (10 mM) | 29 |
| 26 | 11.84 | 638 | 0.65 | 4.92 | I−/I3− | CDCA (10 mM) | 29 |
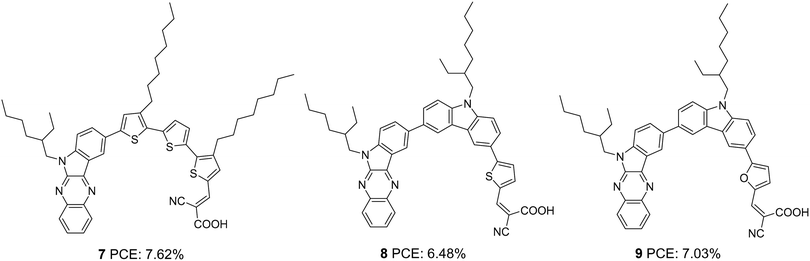
Later, Qian et al. developed three D–π–A dyes having indolo[2,3-b]quinoxaline and cyanoacrylic acid as donor and acceptor groups, respectively.24 Sensitizers 7, 8, and 9 differ in the selection of conjugated spacers, which were oligothiophene, thienylcarbazole, and furylcarbazole, respectively (Fig. 2). The dyes exhibited efficiencies in the order 7 > 9 > 8 (Table 1). The maximum efficiency of 7.62% was delivered by 7, mainly contributed by the significantly larger short circuit current density (Jsc = 16 mA cm−2) of this dye. The electron-rich, oligothiophene π-bridge makes the absorption spectra of 7 more red-shifted with an absorption maximum at 480 nm for the ICT band followed by 8 and 9. The IPCE performance of the devices is following the absorption behaviour of the dyes. While 7 shows broad absorption from 400 to 770 nm, in the case of 8 and 9, the absorption furnishes onset at 700 and 690 nm, respectively illustrating the trend in the dyes' Jsc and light-harvesting ability. Though lower current density was delivered by 9, the higher open circuit potential (Voc = 742 mV) helped 9 outperform 8.
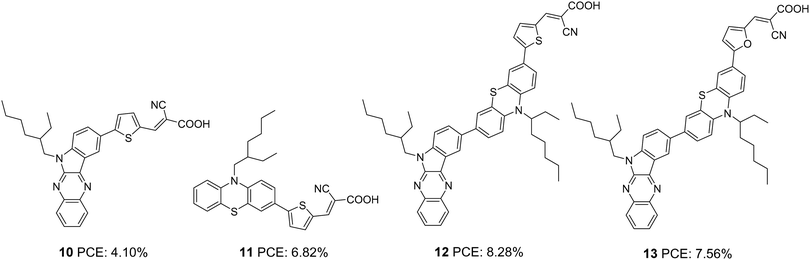
Soon after, the authors used the same scaffold to realize D–D–π–A and D–π–A systems.25 In D–D–π–A design, indoloquinoxaline was used as the primary donor and phenothiazine was used as an auxiliary donor, cyanoacrylic acid as an acceptor and thiophene/furan as a π-bridge to afford 12 and 13, respectively (Fig. 3). These dyes were then compared with 10 and 11, which were D–π–A dyes based on indoloquinoxaline and phenothiazine, respectively, as donors. Among the dyes, 12 having D–D–π–A design was found to outperform the rest. Sensitizer 12 excelled with an efficiency of 8.28% followed by 13 with 7.56%. Compared to furan, the electron richness of thiophene was aiding good ICT transitions reducing the HOMO–LUMO gap for 12, which resulted in more red-shifted and enhanced absorption spectra for 12 compared to that of 13 with furan as a π-spacer. The IPCE spectra of the dyes show a similar trend, with 12 giving over 60% IPCE value from 359 to 600 nm with maximum absorption of 86% at 490 nm. This illustrates the reason for the highest light-harvesting ability and Jsc (15.3 mA cm−2) for 12. The Voc values obtained are in the order 12 > 13 > 11 > 10 (Table 1).
Later in 2017, the authors utilized indoloquinoxaline as an acceptor in a (D)2–A–π–A dye design in which two triphenylamine groups were used as two branches of the primary donor unit. The dyes 14–17 differed in the π-bridges (furan and thiophene) and acceptor groups (cyanoacrylic acid and 2-(1,1-dicyanomethylene)rhodanine or DCRD) (Fig. 4).26 These dyes exhibited efficiencies in the range of 4.55% to 7.09%. Dyes 14 and 15, which had cyanoacrylic acid as the acceptor group, outperformed the corresponding dyes having DCRD as the acceptor unit. Though the absorption spectra of 16 and 17 were much red-shifted compared to 14 and 15, higher dye loading of the latter resulted in larger Jsc values. This is also apparent from the IPCE spectra. Though the spectra of 14 and 15 are blue-shifted with 30 and 20 nm differences respectively with respect to 17, the increase in Jsc value contributed to a better PV performance. Dye 15 could deliver over 60% IPCE value from 400 to 600 nm with a maximum of 83% at 450 nm, resulting in a Jsc value of 12.9 mA cm−2 (Table 1). While comparing the π-spacers, furan substituted sensitizers 15 and 17 were found to deliver better efficiencies than their thiophene substituted counterparts. While 15 delivered a PCE of 7.09% with the highest Jsc and Voc values, 14 slightly lagged with a power conversion efficiency of 6.05%. DCRD substituted dyes 16 and 17 showcased relatively poor performances with a PCE of 4.55% and 4.81%, respectively.
Later, Su and co-workers introduced sensitizers 18–20 based on new molecular architecture, D–D|A–π–A (Fig. 5).27 Here, D|A represents the fused donor–acceptor unit, indolo[2,3-b]quinoxaline. The effect of additional donors was investigated systematically by introducing triphenylamine, carbazole and phenothiazine donors to the indole unit. More significant dye loading in devices based on 18 resulted in effective monolayer formation on the TiO2 surface, thereby preventing recombination effectively. This contributed to the highest open-circuit potential for devices fabricated using 18. When it comes to photocurrent, the hexyl chains incorporated on the end donors of 19 and 20 helped decrease aggregations, contributing to improved Jsc. Maximum efficiency was delivered by 19 (2.72%), followed by 18 (2.65%) and 20 (2.61%). Later, these new generation D–D/A–π–A organic dyes were used successfully as co-sensitizers to improve the PCE of conventional Ru dye (21). The device fabricated with 21 alone showed an efficiency of 7.46%. Co-sensitization of 18–20 improved the efficiency in all three cases with a maximum of 7.94%, when 19 was employed as a co-sensitizer with 21 (Table 1). An increment in open-circuit potential was observed with the co-sensitization approach, which could be attributed to the improved surface coverage of TiO2, resulting in retardation of aggregation and recombination. When it comes to photocurrent, only the device with 19 as a co-sensitizer showed an increment in current density from 19.00 mA cm−2 to 19.37 mA cm−2. More significant dye loading in the remaining cases might have resulted in competitive absorption at the overlapping regions between the Ru dye and organic co-sensitizer.
Later, Su et al. introduced a di-branched di-anchoring approach to the previous molecular architecture to develop D–D|A–(π–A)2 dyes 22 and 23, which differed in additional donors between triphenylamine and carbazole, respectively (Fig. 6).28 The performances were also compared with devices based on 19. The dianchoring approach was found to help form adequate surface coverage, which is also evident from the higher dye loading present in these dyes. This could cause an increment in Voc of dianchored dyes compared to 19 due to minimal recombination. Among the dianchored dyes, 23 with hexyl chain incorporated carbazole as the secondary donor exhibited the highest Voc. Dye 19 excelled when photocurrent is taken into account, which even contributed to higher PCE compared to the rest. The downfall in light-harvesting efficiency of 22–23 was brought about by the increased dihedral angle and strain induced by the di-anchoring branches. A more prominent donating ability of carbazole caused a slightly higher increment in Jsc of 23 compared to 22. Co-sensitization of these dyes with 21 could enhance the efficiency with the highest PCE of 8.67% for 23, followed by 13 (8.16%) and 19 (7.50%). While the considerably improved Voc contributed the increment in device performance of co-sensitized 22-23, a slight increment in current density for 19 caused a corresponding increment in PCE.
In the subsequent work, the π-spacer in 18–20 was changed to thiophene from benzene, and the performance of the sensitizers (24–26) was evaluated under full sun illumination (Fig. 7).29 The dyes exhibited efficiencies in the range 4.92–5.27%, which was higher than previously reported sensitizers having phenyl as a spacer. Dye 24, having triphenylamine as a donor, displayed the maximum PCE of 5.27% with a Voc of 0.67 V and FF of 70.1 with improved Jsc of 11.10 mA cm−2 (Table 1). Higher dye loading seems to be responsible for improving FF and open-circuit potential for the triphenylamine donor dye 24. Though the highest photocurrent was observed for 26 due to its increased light-harvesting ability, comparatively lower values obtained for the rest of the parameters contributed towards inferior PCE of 4.92% for 26. Co-sensitization of 21 with these dyes also resulted in improved Voc and FF. In addition, the competition for light absorption resulted in a considerable reduction of photocurrent, leading to net poor performance for the co-sensitized device compared to the individual dyes.
From Table 1, it is clear that indoloquinoxaline is a potential scaffold for dye sensitizers in DSSCs. The highest efficiency achieved so far using IQ based sensitizer is 8.2%, where the IQ unit and π-spacer (thiophene) is attached to either end of the auxiliary donor (phenothiazine) with cyanoacrylic acid as the acceptor unit. In the same architecture itself, optimum tuning of auxiliary donors and π-spacers along with the alkyl groups could render sensitizers capable of delivering more than 10% PCE. From the reported sensitizers using IQ, it is impossible to generalize suitable π-spacers for the system that could change depending on the donor attached to it and the IQ position (indole/quinoxaline end) to which it is attached. Many studies were not carried out in this direction of anchoring units, opening up further possibilities towards efficient IQ-based devices.
II. Indolocarbazole based sensitizers for DSSCs
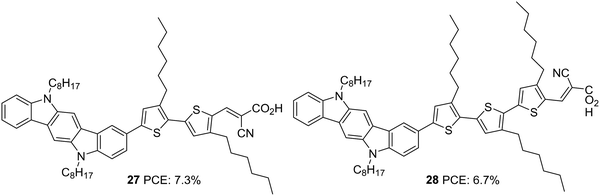
Indolocarbazole, especially indolo[3,2-b]carbazole isomer, is a linear pentacene with two N-atoms with the possibility of introducing alkyl chains of any length requirement either to improve solubility or to prevent back electron transfer. When compared to carbazole, indolocarbazole possesses better energetics, improved electron-donating capabilities and superior absorption profiles. These characteristics find indolocarbazoles a unique position among various applications involving organic thin-film transistors, organic light-emitting diodes and photovoltaics.30 Indolocarbazoles can be prepared either by Fischer indole synthesis of 1,4-bis(2-phenylhydrazono)cyclohexane or by a Cadogen reaction of appropriate nitroarenes.31Indolocarbazole was first used as a donor in DSSCs by Zang et al. (Fig. 8).32 They synthesized dyes 27 and 28, which differ in the number of thiophene groups incorporated as the π-linker. While a red shift in the absorption profile was observed for 28 when adsorbed on TiO2, higher electron injection efficiency was obtained for 27 sensitized devices. The trade-off between the two factors renders 28 with slightly higher Jsc compared to that of 27. The difference in efficiencies of the two dyes were also brought about by the fill factor. While 27 possesses a FF of 0.67, the larger molecular size of 28 resulted in a FF of 0.62 (Table 2). This resulted in a higher PCE of 7.3% for 27 and 6.7% for 28.
| Sensitizer | J sc (mA cm−2) | V oc (mV) | FF | PCE (%) | Electrolyte | Coadsorbent (concentration) | Ref. |
|---|---|---|---|---|---|---|---|
| a TiO2 films were made with 4 µm thick scattering layer. b TiO2 films were made with 8 µm thick scattering layer. | |||||||
| 27 | 15.4 | 710 | 0.67 | 7.3 | I−/I3− | — | 32 |
| 28 | 15.5 | 700 | 0.62 | 6.7 | I−/I3− | — | 32 |
| 29 | 11.95 | 768 | 0.66 | 6.09 | I−/I3− | — | 33 |
| 30 | 11.57 | 707 | 0.68 | 5.55 | I−/I3− | — | 33 |
| 31 | 9.40 | 644 | 0.68 | 4.11 | I−/I3− | — | 33 |
| 32 | 13.96 | 674 | 0.68 | 6.40 | I−/I3− | — | 33 |
| 33 | 10.28 | 713 | 0.67 | 4.90 | I−/I3− | — | 33 |
| 34 | 10.40 | 747 | 0.70 | 5.43 | I−/I3− | — | 35 |
| 34 | 12.92 | 710 | 0.66 | 6.01 | I−/I3− | — | 35 |
| 35 | 13.44 | 752 | 0.65 | 6.53 | I−/I3− | — | 35 |
| 35 | 16.41 | 706 | 0.64 | 7.49 | I−/I3− | — | 35 |
| 36 | 14.91 | 651 | 0.67 | 6.48 | I−/I3− | — | 35 |
| 36 | 15.88 | 620 | 0.68 | 6.64 | I−/I3− | — | 35 |
| 37 | 12.6 | 729 | 0.68 | 6.25 | I−/I3− | — | 36 |
| 38 | 15.2 | 745 | 0.71 | 8.09 | I−/I3− | — | 36 |
| 39 | 13.9 | 738 | 0.68 | 6.98 | I−/I3− | — | 36 |
| 40 | 14.1 | 757 | 0.71 | 7.58 | I−/I3− | — | 36 |
| 41 | 5.69 | 750 | 0.72 | 3.11 | I−/I3− | CDCA (1 mM) | 37 |
| 42 | 5.09 | 750 | 0.74 | 2.83 | I−/I3− | — | 37 |
| 43 | 3.89 | 610 | 0.69 | 1.65 | I−/I3− | — | 37 |
| 44 | 10.16 | 710 | 0.71 | 5.12 | I−/I3− | — | 38 |
| 45 | 12.85 | 720 | 0.69 | 6.34 | I−/I3− | — | 38 |
| 44/45 | 13.38 | 740 | 0.71 | 7.03 | I−/I3− | — | 38 |
| 46 | 12.45 | 690 | 0.70 | 6.02 | I−/I3− | — | 39 |
| 47 | 10.73 | 731 | 0.74 | 5.78 | I−/I3− | — | 40 |
| 48 | 9.81 | 680 | 0.78 | 5.23 | I−/I3− | — | 40 |
| 49 | 10.95 | 754 | 0.72 | 5.97 | I−/I3− | — | 40 |
| 50 | 7.05 | 690 | 0.53 | 2.56 | I−/I3− | — | 41 |
| 51 | 9.78 | 660 | 0.57 | 3.68 | I−/I3− | — | 41 |
| 52 | 4.04 | 620 | 0.64 | 1.59 | I−/I3− | — | 41 |
| 53 | 7.57 | 640 | 0.53 | 2.5 | I−/I3− | — | 41 |
| 54 | 12.16 | 560 | 0.69 | 4.68 | I−/I3− | CDCA (10 mM) | 42 |
| 55 | 11.43 | 610 | 0.69 | 4.66 | I−/I3− | CDCA (1 mM) | 42 |
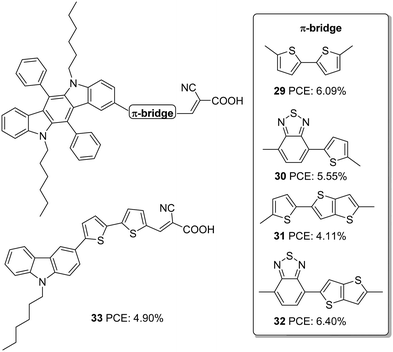
Cai et al. designed four dyes based on 5,7-dihexyl-6,12-diphenyl-5,7-dihydroindolo[2,3-b]carbazole (DDC) with benzothiadiazole (or thiophene) and thieno[3,2-b]thiophene (TT) (or thiophene) as the π-spacer and 2-cyanoacrylic acid as an acceptor (Fig. 9).33 Along with the high electron-donating ability, the fused carbazole systems also contribute towards improved π-conjugation, which will be advantageous in promoting the ICT and the photostability of the system. The two phenyl rings integrated on the donor DDC unit, and the alkyl groups on the nitrogen atom effectively reduced the aggregation and improved the lifetime for devices fabricated with these dyes. The devices were also subjected to comparison with carbazole based D–π–A sensitizer 33. The molar extinction coefficients of both ICT and π–π transition bands display significant enhancement in 29–32 compared to 33 indicating the improved light-harvesting ability of the new fused conjugated donor. Except for 31, the Jsc value for all other dyes was higher than 33. This is apparent from the IPCE spectra, where the performance follows the order 32 > 29 > 30 > 33 > 31, which is consistent with the dye loading present in the fabricated devices. Though the Voc value of 32 (674 mV) was not very high compared to 31 (768 mV), 32 exhibited the highest efficiency of 6.4% among these sensitizers with a Jsc of 13.96 mA cm−2 and FF of 0.68 (Table 2). The photostability evaluation of the dyes by adopting the methods of Katoh and co-workers revealed that the new donor is effective in stabilizing the cation formed after irradiation of light compared to the carbazole based dye. The benzothiadiazole containing dyes possess more stability which is consistent with the previous reports.34 The results also paved the way to the observation that TT was also beneficial for contributing towards photostability. Compound 32, having both BTD and TT as a π-spacer, exhibited maximum photostability, while 29 was least stable among the indolocarbazole based dyes. All the dyes were also found to be thermally stable.
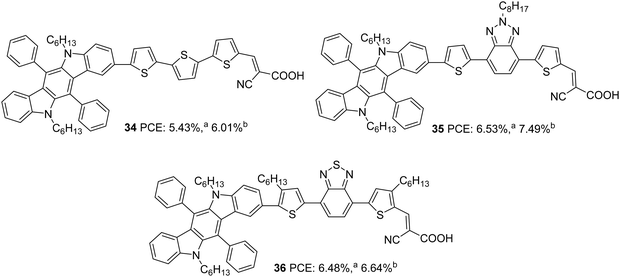
The same group further attempted to introduce a bridge with extended conjugation to widen the absorption spectra of the indolocarbazole dyes (Fig. 10).35 Among the dyes, 36, which contains benzothiadiazole as an auxiliary acceptor along with alkylated thiophenes flanked on both sides, showed maximum red-shifted spectra followed by 35 having alkyl-substituted benzothiadiazole as an auxiliary acceptor and 34 with simple D–π–A architecture having ter-thiophene as a spacer with a difference of 78 and 50 nm, respectively, compared to 36. The device efficiency was tested under two conditions. In one case, the TiO2 films were made of 3 µm thickness with 13 nm sized nanoparticles (TSP) and scattering layer of 4 µm thickness, and in the second case, the TiO2 films were made of 3 µm thickness with 13 nm sized nanoparticles (TSP) and scattering layer of 8 µm thickness. Though higher dye loading is possible in the second case, a thick scattering layer can cause chances of recombination. This resulted in a trend of increase in current density and decrease in Voc for all the dyes fabricated with an 8 µm scattering layer when compared to those of 3 µm thickness. A trade-off between these two factors leads to higher PCE with a thicker scattering layer for all the dyes. In the first condition, the highest IPCE value was obtained for 36 with an absorption maximum of 81.2% at 460 nm followed by 35 (77.2% at 500 nm) and 34 (69.9% at 520 nm). A more comprehensive absorption profile for 36 contributed towards the highest Jsc value of 14.91 mA cm−2. The least Jsc value of 10.40 mA cm−2 was obtained for 34 due to the narrow IPCE spectra resulting from a lower absorption profile (Table 2). When devices were fabricated using an 8 µm scattering layer, dye 35 showed improved IPCE profile and Jsc value, while dye 36 resulted in lower molar extinction coefficients, low dye loading amount, and low electron injection yield. The trend in Voc was the same in both the conditions with the least value delivered by 36. While 35 excelled with the highest PCE of 7.49%, 34 delivered a lower photovoltaic performance of 6.01%. The stability studies reinforced the observation that BTD units are capable of increasing the photo-stability of dyes.
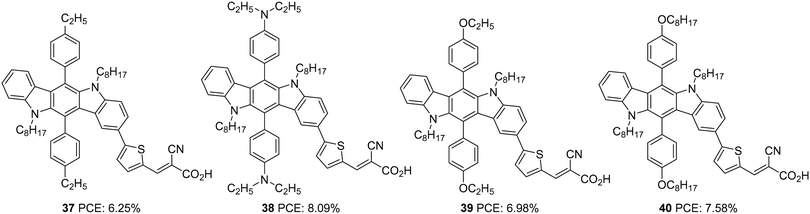
Later in 2016, Qian et al. investigated the same donor groups in a new photosensitizer design. They synthesized four dyes utilizing modified donor units (Fig. 11) consisting of indolo[3,2-b]carbazole as the primary donor and groups such as ethylbenzene, N,N-diethylaniline, ethyloxybenzene, and octyloxybenzene grafted to indolo[3,2-b]carbazole as secondary donor groups, thiophene as the π-conjugated linker, and 2-cyanoacylic acid unit as the electron acceptor/anchoring group.36 The secondary donor groups assist in improving the donating strength of the system, but it was also highly beneficial for reducing the aggregation and recombination. Thus the nonplanar secondary groups could enhance the photovoltage of the device. The IPCE performance of the dyes parallels with the electron-donating ability of the secondary donor. The most robust donor, N,N-diethylaniline attached dye 38, displayed a broad IPCE response and highest Jsc of 15.2 mA cm−2 followed by 40, 39 and 37 (Table 2). The efficiency of the devices also follows the same trend, with 30 having the highest PCE of 8.09%, and the most deficient performance was showcased by 37 with 6.25%.
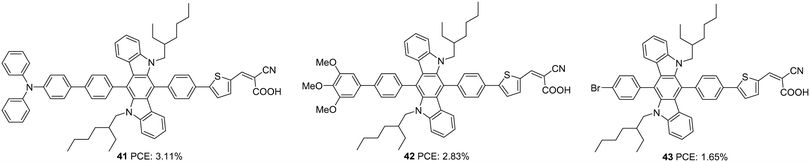
Later, Xiao et al. also employed 6,12-diphenylindolo[3,2-b]carbazole as auxiliary donors in D–D–π–A dyes.37 The sensitizers 41, 42 and 43 differ in the donor groups: triphenylamine, trimethoxyphenyl and trimethoxybromine, respectively (Fig. 12). Unlike the previous work, the additional donor and π-spacers were appended to the end of the phenylene groups attached to the indolo[3,2-b]carbazole moiety. The ICT absorption bands of these compounds did not show much difference compared to the π–π* transition, which can be attributed to the interrupted charge transfer in the molecules resulting from the non-planar conformation of the attached phenyl groups. The absorption behaviour of the compounds was consistent with the electron-donating ability of the secondary donor. The triphenylamine donor in 41, which has a higher electron-donating ability, contributed to the broader absorption and higher molar extinction coefficient for 41, followed by 42 and 43. Though these dyes could deliver reasonably good Voc (0.61–0.75 V) and FF (0.69–0.74) using I−/I3− electrolyte, a relatively low value of Jsc resulted in moderate PCE (1.65–3.11%). Dye 41 yielded the highest efficiency of 3.11% followed by 42 and 43 (Table 2).
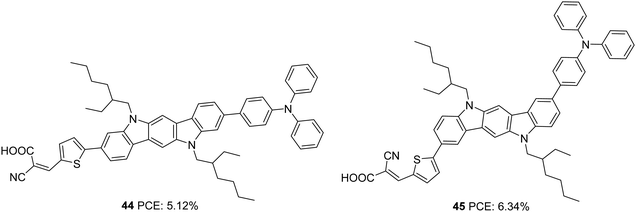
Further investigations by the same group to increase the photoconversion efficiency of 41 resulted in the synthesis of 44 and 45 where a triphenylamine donor was attached to indolo[3,2-b]carbazole via the ninth and eighth positions, respectively (Fig. 13).38 Co-planarity and π-electron delocalization were found to improve, which is apparent from the absorption profile of the new dyes 44 and 45. Dye 45, where the phenylene/thiophene rings were connected onto the para positions of the N-atom, showed more red-shifted absorption with an absorption maximum at 500 nm, whereas absorption of 44 was blue-shifted by 38 nm. While 44 showed improvement in PCE when it was used in conjunction with CDCA (10 mM), 45 responded in a reverse manner. This can be accounted for by the molecular orientation of the dyes when adsorbed on TiO2. Dye 44 adopted a perpendicular orientation to the substrate plane. Somewhat hindered conformation of 45 resulted in more inclination of the dye towards the TiO2 surface. This resulted in fewer aggregations and recombinations, making 45 capable of delivering more photocurrent even with a lower dye loading of 3.64 × 10−7 mol cm−2 compared to 4.54 × 10−7 mol cm−2 for 44 and without co-adsorbent CDCA. Photosensitizer 45 outperformed 44 with a Jsc of 12.85 mA cm−2, Voc of 0.72 V, FF of 0.69 and PCE of 6.34% (Table 2). The co-sensitization of 44/45 further improved the performance to 7.03%.
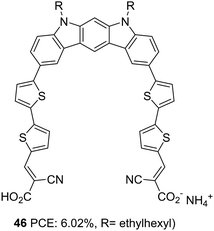
Indolo[2,3-b]carbazoles were rarely explored for optoelectronic applications due to a lack of efficient synthetic strategies. Su et al. could establish new strategies for synthesising indolo[2,3-b]carbazoles and their utilization to construct DSSC sensitizers with a PCE of up to 6.02%.39 The curved molecular conformation of indolo[2,3-b]carbazole dye synthesized by Su et al. (46), along with its rigid and planar nature, offer the possibility to be explored as a di-anchor dye. The sensitizer 46 adopts A–π–D–π–A architecture in which bithiophene is introduced as the π-bridge between the electron-rich indolo[2,3-b]carbazole core and cyanoacrylic acid acceptor unit (Fig. 14). FTIR analysis of the pristine 46 and the compound loaded TiO2 film reveals the involvement of both the carboxylic group in anchoring the dye on the TiO2 surface. This is further confirmed by the Deacon Philips rule, where a frequency difference of 224 cm1 suggests a bidentate binding mode for 46. An efficient electron transfer from the HOMO of 46/(TiO2)70 (indolo[2,3-b]carbazole) to the LUMO of 46/(TiO2)70 (TiO2 nano cluster) was illustrated using computational analysis.
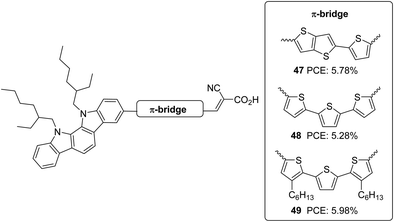
Indolo[2,3-a]carbazole was first introduced in DSSCs by Zhang et al.40 Taking this new system as a donor and cyanoacrylic acid as an acceptor, they studied the effect of conjugate mode of π-spacers such as thienyl-thieno[2,3-b]thiophene and terthiophene in photovoltaic performance (Fig. 15). To control dye aggregation and prevent recombination, alkyl-substituted terthiophene was employed as a π-spacer to construct 49. Sensitizers 47 and 49 outperformed 48 in Jsc and Voc. 48 gave an efficiency of 5.28%. The spatial effect of hexyl groups on the π-backbone of 49 resulted in the highest efficiency of 5.98% and the highest photocurrent and photovoltage, followed by 47 with 5.78% PCE (Table 2).
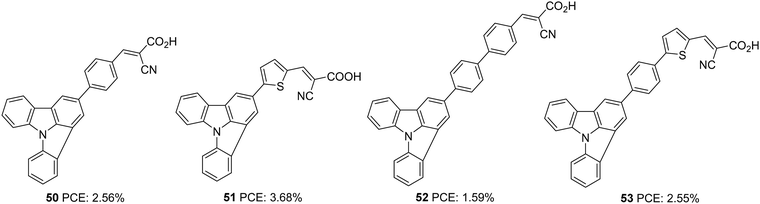
Indolo[3,2,1-jk]carbazole is another positional isomer of indolocarbazole where indole is fused with the carbazole moiety in a slightly strained manner. The system was found to be thermostable and showcase strong electron-donating ability, which resulted in its application as a charge transport material and conducting film material. Luo et al. utilized this core for developing a sensitizer for DSSCs by integrating it as a donor in a D–π–A architecture (Fig. 16).41 They developed four devices based on the dyes 50–53, which exhibited PCE in the range 1.59–3.68%. Among the dyes, 51 outperformed others with a PCE of 3.68% (Table 2). With its lower stabilization energy, thiophene enabled the system to have more delocalization and efficient light-harvesting ability. This can be accounted for by the enhanced photocurrent in dyes 51 and 53 using thiophene as the π-linker. Low spectral coverage leads to decreased performance in 50 and 52. Even an attempt to increase the π-bridge conjugation by introducing the phenyl group in 52 was not found to be an effective strategy to increase the PCE.
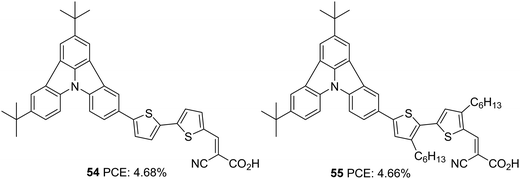
Further attempts were carried out to investigate the effect of π-linkers on the performance of indolo[3,2,1-jk]carbazole based dyes by Cao et al. (Fig. 17).42 Dyes 54 and 55 incorporate bisthiophene and unsubstituted bisthiophene, respectively as π-linkers. The slightly twisted conformation of 55 resulted in lower dye loading on TiO2 than 54, resulting in a slight increment in the Jsc value for 54, the reverse trend of what was observed for Voc. The significant improvement in Jsc of the 54 based device, when adsorbed with CDCA, illustrates the aggregation tendency of these sensitizers on TiO2. While the 54 based devices delivered PCE of 4.68% in the presence of 10 mM CDCA, dye 55 could yield 4.66% with 1 mM CDCA (Table 2).
Among different isomers of indolocarbazole, indolo[3,2-b]carbazole is the most explored isomer for DSSC applications. The comparison of efficiency versus structure reveals that to improve the device's performance, systematic investigation of the position of attachment of the π-spacer and the additional donor on indolo[3,2-b]carbazole is also needed, along with the integration of suitable donor and spacer groups. From the reported data so far, the substitution of donor and spacer units at the second and eighth positions of indolo[3,2-b]carbazole is more effective than substitution at the ninth and third positions. When the donor group was changed to DDC, the additional phenyl groups integrated at the sixth and twelfth positions were found to prevent recombination while preserving the donating ability and the backbone's planarity. However, attempts to increase the light-harvesting by allowing modifications at these phenyl groups could not lead to realize photovoltaic performance as expected due to the non-planar conformation of the phenyl groups as seen in the case of dyes 41 and 42.
III. Triazatruxene based sensitizers for DSSCs
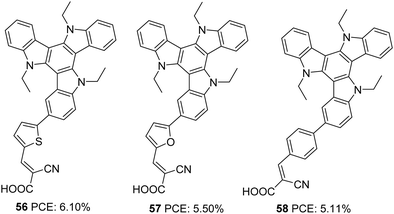
Triazatruxene (TAT) is an expanded π-conjugated system with good electron-donating capacity, consisting of three indole units combined using one benzene ring. Due to its favourable features such as electron richness and rigid π-extended structure, it has found application in various optoelectronic fields, like organic field-effect transistors (OFETs), organic light-emitting diodes (OLEDs), two-photon absorption (TPA) materials, non-linear optics and liquid crystal displays.43 The most widely used method for the synthesis of TAT is by the reaction between indole and indolone in the presence of bromine and POCl3.44The first attempt to use TAT as a donor in DSSCs was reported by Qian et al.45 They succeeded in developing three D–π–A dyes (56, 57, 58) with variable π-spacers achieving more than 5% efficiency. These dyes were made using TAT as the donor and cyanoacrylic acid as the acceptor/anchoring group (Fig. 18). The system's efficiency was studied by changing the π-spacers (thiophene 56, furan 57 and benzene 58). Though high open-circuit voltage was found in dye 58, with a reduced current density, devices fabricated with 58 only realized a low PCE of 5.11%. The highest efficiency of 6.1% was contributed by the device fabricated using dye 56. The higher PCE resulted from higher current density showcased by 56, which was also evident from the IPCE response (Table 3). Better electron delocalization and superior electron-donating capabilities render the TAT system improved light-harvesting behaviour to be used as an efficient sensitizer in DSSCs.
| Sensitizer | J sc (mA cm−2) | V oc (mV) | FF | PCE (%) | Electrolyte | Coadsorbent (concentration) | Ref. |
|---|---|---|---|---|---|---|---|
| a ssDSSC. | |||||||
| 56 | 14.7 | 670 | 0.62 | 6.10 | I−/I3− | — | 45 |
| 57 | 13.6 | 654 | 0.62 | 5.50 | I−/I3− | — | 45 |
| 58 | 11.6 | 686 | 0.64 | 5.11 | I−/I3− | — | 45 |
| 59 | 5.89 | 582 | 0.72 | 2.47 | I−/I3− | CDCA (10 mM) | 46 |
| 60 | 8.33 | 617 | 0.70 | 3.60 | I−/I3− | CDCA (10 mM) | 46 |
| 61 | 14.97 | 793 | 0.63 | 7.5 | I−/I3− | CDCA (5 mM) | 47 |
| 62 | 16.45 | 707 | 0.62 | 7.15 | I−/I3− | CDCA (5 mM) | 47 |
| 63 | 13.46 | 775 | 0.62 | 6.50 | I−/I3− | CDCA (5 mM) | 47 |
| 64 | 15.89 | 743 | 0.62 | 7.26 | I−/I3− | CDCA (5 mM) | 47 |
| 65 | 11.4 | 722 | 0.68 | 5.55 | I−/I3− | CDCA (0.4 mM) | 48 |
| 66 | 13.2 | 741 | 0.68 | 6.65 | I−/I3− | CDCA (0.4 mM) | 48 |
| 67 | 11.0 | 849 | 0.53 | 5.2 | I−/I3− | CDCA (0.5 mM) | 49 |
| 68 | 20.73 | 956 | 0.69 | 13.6 | [Co(bpy)3]2+/3+ | CDCA (2 mM) | 50 |
| 69 | 20.57 | 887 | 0.70 | 12.8 | [Co(bpy)3]2+/3+ | CDCA (2 mM) | 50 |
| 68 | 9.9 | 952 | 0.70 | 6.6 | [Co(bpy)3]2+/3+ | CDCA (2 mM) | 51 |
| 69 | 8.4 | 945 | 0.69 | 5.4 | [Co(bpy)3]2+/3+ | CDCA (2 mM) | 51 |
| 70 | 11.94 | 808 | 0.74 | 7.20 | [Co(bpy)3]2+/3+ | CDCA (2 mM) | 52 |
| 71 | 15.1 | 966 | 0.70 | 10.2 | [Co(bpy)3]2+/3+ | — | 53 |
| 72 | 13.4 | 934 | 0.69 | 8.6 | [Co(bpy)3]2+/3+ | — | 53 |
| 73 | 16.92 | 926 | 0.75 | 11.7 | [Co(bpy)3]2+/3+ | — | 54 |
| 74 | 15.98 | 911 | 0.73 | 10.6 | [Co(bpy)3]2+/3+ | — | 54 |
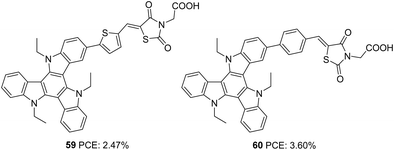
As an extension of the previous experiments, the effect of rhodanine-3-acetic acid as an electron-withdrawing/acceptor group was studied by the same group (Fig. 19).46 Though much broader absorption was obtained for dyes with rhodanine-3-acetic acid, the performance of these dyes was inferior to the dyes being synthesized using cyanoacrylic acid as the electron-withdrawing group. This is attributed to the interruption of electron transfer from the dyes to the semiconductor by the broken (NCH2COOH) conjugation in rhodanine-3-acetic acid. Instead of displaying broader absorption spectra with higher ε, devices fabricated using 59 showed lower current density. The trend can be rationalized from the LUMO energy level of the dyes, which are in the order of 60 (−1.16 V) >59 (−1.02 V), indicating more effective electron injection from 60 to the semiconductor. Thus 60 could yield a higher PCE of 3.60% with a short-circuit photocurrent density of 8.33 mA cm−2, an open-circuit photovoltage of 617 mV, and a fill factor of 0.70 (Table 3).
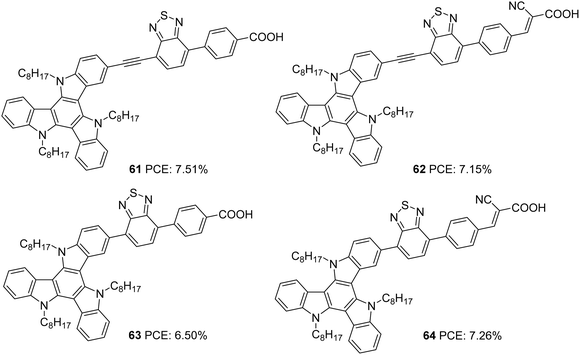
Triazatruxene was incorporated as a donor in D–A–π–A based sensitizers by Pan and co-workers in 2018 (Fig. 20).47 These sensitizers employed benzothiadiazole as an auxiliary acceptor, and they introduced variation in the acceptors (carboxylic acid and cyanoacrylic acid), and the connectivity between the donor and auxiliary acceptor was modified using single bond and ethynyl linkages. The introduction of the ethynyl linker and cyanoacrylic acid was found to increase the conjugation of the dye molecules. Dyes 62 and 64 having cyanoacrylic acid as the acceptor group showed better light-harvesting capability with broader absorption and higher IPCE value. These observations were also reflected in the Jsc value of the corresponding sensitizers. Another critical parameter that determines the efficiency of the system is the Voc. Dyes 61 and 63 with a single carboxylic acid acceptor exhibited higher photovoltage compared to the rest. Among the two sets of D–A–π–A sensitizers having a triazatruxene donor (single bonded and ethynyl bridged), dyes 61 and 64 showed maximum efficiencies of 7.51% and 7.26%, respectively, which indicates that a delicate balance between Jsc and Voc is essential to obtain higher power conversion efficiencies (Table 3).
Qian and co-workers used triazatruxene as a secondary donor to construct porphyrin-based sensitizers (Fig. 21).48 Triazatruxene was attached directly to the meso-position of the porphyrin ring, and two variants were synthesized by changing the acceptor groups (carboxylic acid and cyanoacrylic acid). When cyanoacrylic acid was employed as an acceptor group, improvement was found in the dye's light-harvesting ability, which is evident from the broader and enhanced IPCE spectra resulting in a higher Jsc value dye (Table 3). The Voc value also follows the same trend, which resulted in a maximum PCE of 6.05% for 66. The efficiencies of both dyes were found to improve when co-adsorbent chenodeoxycholic acid (CDCA) was used, which implies the possibility of intermolecular aggregation of the dyes on the TiO2 surface.
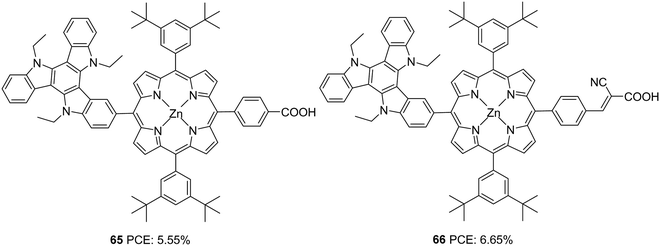 | ||
| Fig. 21 Photosensitizers 65–66 with a triazatruxene moiety as a secondary donor and porphyrin ring as a primary chromophore. | ||
Qin et al. also incorporated TAT into the meso-position of the porphyrin chromophore to obtain 67 (Fig. 22).49 Dye 67, which was used in ssDSSC, with spiro-MeOTAD being the HTM, showcased an efficiency of 5.2% in the presence of co-adsorbent CDCA.
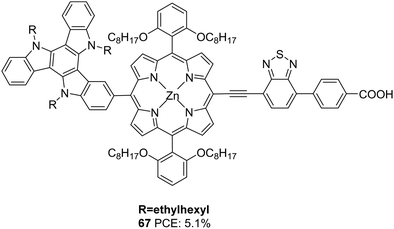 | ||
| Fig. 22 Photosensitizer 67 with a triazatruxene moiety as a secondary donor and porphyrin ring as the primary chromophore. | ||
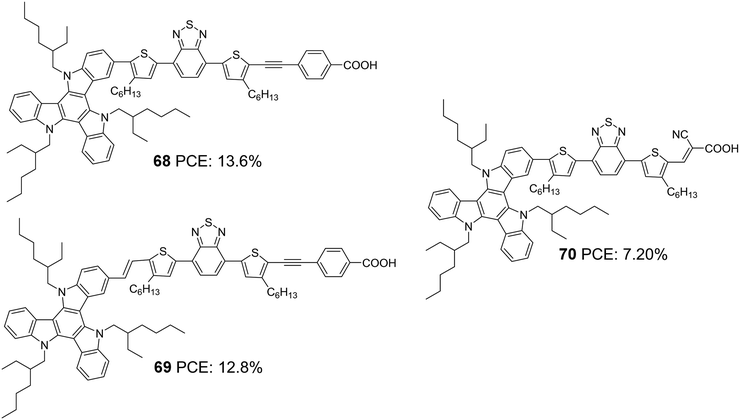
The most efficient DSSC's based on TAT molecules were reported by Zhang and co-workers.50 They systematically modified the D–π–A backbone and developed two dyes employing TAT as a donor, 4,7-bis(4-hexylthiophen-2-yl)benzo[c][1,2,5]-thiadiazole (BTBT) as a π-bridge and 4-ethynyl benzoic acid (EBA) as an acceptor, which differs in the linkage between the donor and π-bridge (Fig. 23). The study aimed to evaluate the effect of the rigid single bond and flexible z-type double bond on various parameters that determine the efficiency of the device. The optimized devices based on 68 and 69 achieved PCEs of 13.6% and 12.8%, respectively, using cobalt-based redox electrolyte ([Co(bpy)3]2+/3+). Though the double bond was found to widen and enhance the absorption of the molecules, the Jsc value for 68 was found to be higher than 69, which resulted from the more significant dye loading observed for the former. Devices fabricated using 68 also showcased better open-circuit potential (956 mV) than 69 (887 mV) (Table 3). The efficient electron injection in 68, evident from the femtosecond transient absorption and up-conversion fluorescence studies, reinforces the observation mentioned earlier. Dye 68 and 69 were also applied to ssDSSCs using spiro-OmeTAD as the hole transporting material (HTM).51 Longer electron lifetime and high regeneration efficiency caused 68 to outperform 69 with a PCE of 6.6%. Dye 69 exhibited a PCE of 5.4% (Table 3).
In order to probe the effect of the rigid 4-ethynylbenzoic acid acceptor group in 68, dye 70 was synthesized by the same group (Fig. 23) with Z-type cyanoacrylic acid and used to fabricate devices using a cobalt-based redox electrolyte ([Co(bpy)3]2+/3+).52 Higher dye loading, better light-harvesting ability and improved electron injection efficiency made 68 deliver the highest efficiency of 13.4%, while there was a drastic drop in PCE for 70, which could only afford a PCE of 7.2% (Table 3). This again clearly illustrates that rigid structures are pertinent when it comes to designing sensitizers that will be beneficial for reducing energy loss during electron injection, leading to improved current density and photovoltage. Fine-tuning of the molecular backbone without compromising the energetics is highly required to realize higher PCE.
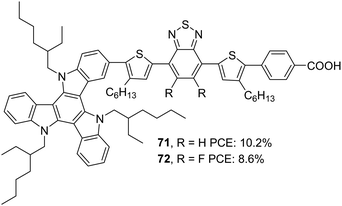
Later, Li et al. tried to improve the efficiency of 68 by developing two modified dyes 71 and 72 using a TAT donor (Fig. 24).53 While benzothiadiazole (BT) functions as the auxiliary acceptor in 71, BT was replaced with difluorobenzo[c][1,2,5]thiadiazole (DFBT) in 72. The attempt to introduce fluorine on BT was justified by lowering the LUMO level of the molecule by the electron-withdrawing (inductive) effect of fluorine. The electron-donating mesomeric effect was found to dominate the former. This renders 72 with a large band gap and blue-shifted absorption spectrum compared to that of 71. The higher dye loading in 71 (1.4 times) and wider absorption band compensated the reduction in molar extinction coefficient, resulting in a higher Jsc of 15.1 mA cm−2. This ended up in maximum efficiency of 10.2% for 71 and a lower efficiency of 8.6% for 72 using cobalt-based redox electrolyte ([Co(bpy)3]2+/3+). (Table 3).
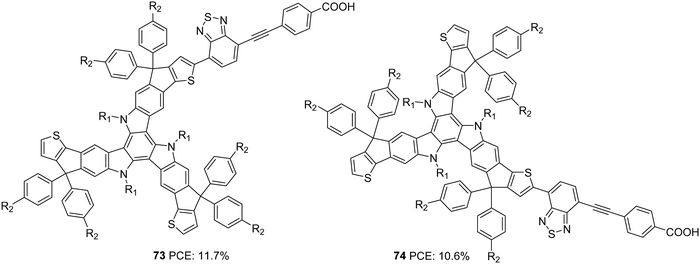
Yao et al. found that introducing bulky groups on TAT successfully hindered dye aggregation and recombination (Fig. 25).54 They synthesized two modified TAT sensitizers (73 and 74) having a more conjugated TAT donor unit. Their attempt resulted in a larger Voc for 73 (926 mV) and 74 (911 mV). Higher dye loading in 73 compared to 74 allowed adequate coverage of the semiconductor surface, abating the chances of dark current formation (recombination), leading to higher Voc. DFT studies also revealed more planar conformation for 74 than 73, which confirms the slightly red-shifted absorption profile of 74. It was also observed that there was an upshift in HOMO level for 74, which affected the regeneration rate of the dye adversely. Poor regeneration and lower dye loading resulted in lower current density and Voc for 74 compared to 73. Thus, PCEs of 11.7% and 10.6% were obtained for 73 and 74, respectively (Table 3).
Triazatruxene was incorporated as a donor in D–π–A dyes and studies were carried out to evaluate the effect of different π-spacers and anchoring groups. When it comes to the anchoring group, cyanoacrylic acid has proved to outperform other acceptor units (rhodanine-3-acetic acid, carboxylic acid) in terms of light-harvesting and electron injection capability, which is consistent with the many reports available. Though devices based on thiophene bridged dye 56 could showcase higher PCE than those of phenyl substituted dye 58, this trend reverses when the anchoring group was changed to rhodanine-3-acetic acid. Devices fabricated with phenyl bridged dye 60 delivered higher PCE with higher Jsc and Voc compared to those of 59 with a thiophene spacer. This implies that selecting a suitable π-spacer depends on the other components of the molecular architecture. The introduction of an auxiliary acceptor improved the photovoltaic parameters, and the highest efficiency achieved so far using metal-free dyes is from devices based on 68 (13.6%) where BTBT was used as the π-bridge (Table 3). The takeaway from the consecutive studies performed by Zang and co-workers is that molecular engineering has to be carried out in such a way as to reduce energy loss during electronic transitions, which could, in turn, lead to efficient PCE.
IV. Indeno[1,2-b]indole based sensitizers for DSSCs
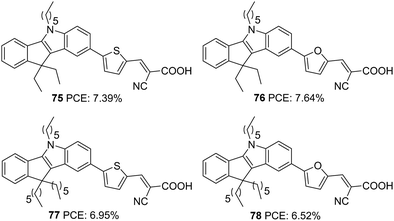
Indeno[1,2-b]indole consists of an indole unit fused with an indene moiety, making it planar and electron-rich with efficient electron delocalization. In addition to the alkylation of the N-atom in the tetracene, the indene unit also offers the flexibility to be alkylated. This possibility has a positive effect on the photovoltaic performance as the presence of multiple alkyl groups increases the solubility of indeno[1,2-b]indole based dyes and prevents their aggregation. At the same time, the presence of alkyl groups is also effective in blocking the approach of the oxidized species coming close to the semiconductor and thereby improving lifetime. The well-established synthetic route towards preparing this tetracene is by the Fisher indole synthesis involving indanone and phenyl hydrazine.55The first report on the use of indeno[1,2-b]indole as a donor in a DSSC came in 2016 by Qian et al.56 They designed four dyes (Fig. 26) based on D–π–A design, and the basic skeleton employs indeno[1,2-b]indole as a donor and cyanoacrylic acid as the acceptor. Tuning of device performance was carried out by changing the π bridges (furan and thiophene) and introducing alkyl groups (ethyl and hexyl) on the indene ring. The attached alkyl groups were at a particular angle with the molecular plane, which was beneficial for reducing aggregation of dyes and thereby increasing the PCE. All four dyes exhibited good power conversion efficiency in the range 6.52–7.64%. Among these dyes, the sensitizer 76, featuring furan as the π-spacer and ethyl groups as the alkyl chain, contributed the highest PCE of 7.64% with a Jsc of 15.8 mA cm−2 and a Voc of 763 mV (Table 4).
| Sensitizer | J sc (mA cm−2) | V oc (mV) | FF | PCE (%) | Electrolyte | Coadsorbent (concentration) | Ref. |
|---|---|---|---|---|---|---|---|
| 75 | 15.6 | 710 | 0.67 | 7.39 | I−/I3− | — | 56 |
| 76 | 15.8 | 763 | 0.63 | 7.64 | I−/I3− | — | 56 |
| 77 | 14.6 | 742 | 0.64 | 6.95 | I−/I3− | — | 56 |
| 78 | 13.7 | 733 | 0.65 | 6.52 | I−/I3− | — | 56 |
| 79 | 11.0 | 813 | 0.70 | 6.29 | I−/I3− | — | 57 |
| 80 | 4.23 | 700 | 0.75 | 2.22 | I−/I3− | — | 57 |
| 81 | 7.10 | 695 | 0.73 | 3.60 | I−/I3− | — | 57 |
| 82 | 11.9 | 707 | 0.64 | 5.41 | I−/I3− | — | 57 |
| 83 | 10.4 | 843 | 0.66 | 5.74 | I−/I3− | CDCA (3 mM) | 58 |
| 84 | 13.3 | 796 | 0.65 | 6.86 | I−/I3− | CDCA (3 mM) | 58 |
| 85 | 14.1 | 829 | 0.68 | 7.99 | I−/I3− | CDCA (3 mM) | 58 |
| 84/85 | 14.7 | 819 | 0.70 | 8.37 | I−/I3− | — | 58 |
| 86 | 18.25 | 707 | 0.68 | 8.74 | I−/I3− | — | 59 |
| 87 | 20.08 | 703 | 0.64 | 8.98 | I−/I3− | — | 59 |
| 88 | 12.01 | 837 | 0.69 | 6.92 | I−/I3− | — | 59 |
| 86/88 | 18.24 | 787 | 0.67 | 9.56 | I−/I3− | CDCA (4 mM) | 59 |
| 89 | 11.59 | 840 | 0.66 | 6.43 | [Co(phen)3]2+/3+ | — | 60 |
| 90 | 6.84 | 770 | 0.63 | 3.31 | [Co(phen)3]2+/3+ | — | 60 |
| 91 | 12.43 | 829 | 0.65 | 6.69 | [Co(phen)3]2+/3+ | — | 60 |
| 92 | 13.52 | 855 | 0.64 | 7.40 | [Co(phen)3]2+/3+ | — | 60 |
Qian and co-workers later designed three more dyes with indeno[1,2-b]indole as the donor to investigate the role of different acceptor groups on the photovoltaic performance of the devices.57 The synthesized D–π–A dyes had indeno[1,2-b]indole as a donor and benzene as the π-bridge. The dyes differed in the acceptor groups incorporated, which were cyanoacrylic acid, rhodanine-3-acetic acid and 2-(1,1-dicyanomethylene)rhodanine (DCRD), respectively (79, 80 and 81) (Fig. 27). The results revealed better PCE for devices fabricated using dye having cyanoacrylic acid as the acceptor over the other two groups. Though dye 79 showed blue-shifted absorption, it contributed towards the highest efficiency of 6.29% with better Jsc and Voc compared to other dyes. To assess the effect of different π-spacers on the DCRD acceptor, dye 82 was synthesized with thiophene as the π-bridge. The introduction of thiophene increased the efficiency of 81 from 3.60% to 5.41%, illustrating the importance of tuning the π-spacer of the sensitizer (Table 4) while changing the acceptor functionality in a way to achieve higher PCE.
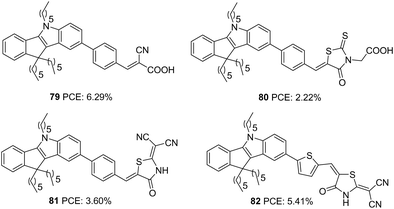 | ||
| Fig. 27 Photosensitizers 79–82 with an indeno[1,2-b]indole moiety as a donor and variable acceptor groups. | ||
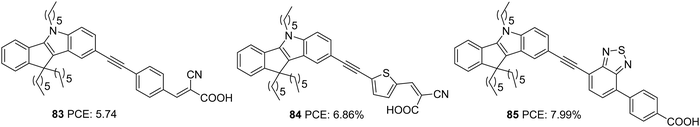
Yan et al. later designed and synthesized indeno[1,2-b]indole based D–π–A dyes with extended conjugation of the π-spacer by introducing an ethynyl group and variation of the π-spacer and auxiliary acceptor. To this end, they constructed D–π–A dyes, which employ indeno[1,2-b]indole as the donor and cyanoacrylic acid as the acceptor group (Fig. 28).58 The dyes 83 and 84 vary in the π-bridge between benzene and thiophene, respectively. They also incorporated the ethynyl group as the linker between the donor and π-spacer to decrease the repulsion and increase the conjugation of the dye molecules. A third dye, 85 with benzothiadiazole as the auxiliary acceptor, was developed to compare with the previous set realizing D–π–A architecture. The dye with an auxiliary acceptor (85) was found to deliver the highest efficiency with the highest Jsc and Voc. Among the D–π–A dyes, thiophene substituted dye 84 showed the highest efficiency with significantly improved current density, though Voc was highest for the benzene substituted dye 83. Co-sensitization of 84 and 85 outperformed all other dyes delivering an efficiency of 8.37% (Table 4). This is attributed to the increment in the Jsc value of the device.
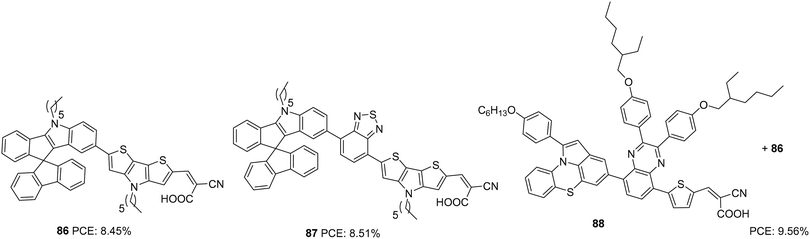
Dai and co-workers first synthesized indeno[1,2-b]indole-spirofluorene (IISF) and used it as a donor unit in DSSC dyes. This molecular engineering was carried out to combine the electron-donating ability of the indenoindole with the steric effect of the spirofluorene.59 They were successful in developing two dyes, 86 and 87, with IISF as the donor, dithieno[3′,2′-b:2′,3′-d]pyrrole (DTP) as the π-bridge and cyanoacrylic acid as an acceptor (Fig. 29). The dye 87 also employs 2,1,3-benzothiadiazole (BTD) as an auxiliary acceptor. Both 86 and 87 delivered efficiencies above 8%, which was further enhanced with the combined effect of co-adsorption with CDCA and co-sensitization with 88. The highest efficiency of 9.56% was obtained from the co-sensitization of 86 with 88 in the presence of co-adsorbent, CDCA (Table 4).
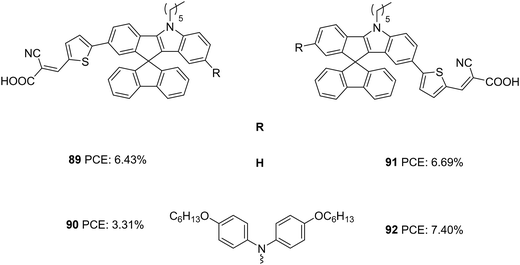
Later the same group synthesized two sets of dyes based on IISF to study the effect of position of attachment of the π-spacer to IISF (indole/indene end) and the effect of the additional donors (Fig. 30).60 The D–π–A dyes 89 and 91 consist of IISF as the donor, thiophene as the π-spacer and cyanoacrylic acid as the acceptor. While thiophene is attached to the indene ring in 89, dye 91 has the π-spacer attached to the indole end. An additional donor group (hexyloxydiphenylamine) was attached to 89 to obtain 90, and dye 92 is the latter's regioisomer. Dyes with additional donors exhibited bathochromic as well as intensified spectra compared to D–π–A dyes. Among the two regioisomers, the one with the π-spacer attached to the indole end of IISF is seen to facilitate more ICT transition. The IPCE spectra of 90 and 92 showed wider but downshifted absorption behaviour than 89 and 91. A higher adsorption angle of 89 (41.83°) and 91 (42.33°) helped them to achieve higher dye loading of 115.83 and 118.14 nmol cm−2, respectively. The bulkier donor group in 90 and 92 caused decreased dye loading compared to their D–π–A counterpart, but 92 managed to obtain 77% dye loading of 91 due to its larger adsorption angle (49.5°). Though dye loading was lower for 92 (90.97 nmol cm−2) than 89 and 91, the broader absorption could compensate for it having the highest Jsc (13.52 mA cm−2). While 89 and 91 exhibited comparable Jsc of 11.59 and 12.43 mA cm−2, respectively, 90 delivered the least Jsc value of 6.84 mA cm−2. Apart from the lower dye loading, the weak driving force for degeneration also caused the downfall in current density for 90. This was again illustrated by changing alternate electrolytes for device fabrication which are having lower oxidation potential than [Co(phen)3]2+/3+([0.56 V for Co(bpy)3]2+/3+, and 0.43 V for [Co(dmbpy)3]2+/3+). The current density of 90 increased in the order [Co(dmbpy)3]2+/3+ > [Co(bpy)3]2+/3 > [Co(phen)3]2+/3+. While the bulky hexyloxy diphenyl helped 92 alleviate recombination and achieve higher Voc, the same group adversely affected the Voc in 90 by breaking the compact layer formed by dye 89. A trade-off between Jsc and Voc in 89 and 91 lead to comparable efficiencies for them. While 92 showcased the highest PCE of 7.40%, dye 90 delivered the lowest PCE of 6.84%. Co-sensitization of 92 was carried out with 89/91. In both cases, increment in current density was observed, 91/92 being the combination with the highest PCE (8.32%)
On comparing the sensitizers 77, 78 and 79 having the same dye skeleton and differing only in their π-spacer, sensitizer 77 having thiophene as the π-spacer outperformed the rest of the dyes with higher current density. Though sensitizer 79 has higher Voc, the least PCE was delivered due to lower current density. A triple bond was introduced between the indenoindole unit and π-spacer in 77 and 79 to furnish 84 and 83. Though the motive was to increase the conjugation and PCE of the devices, it was found to showcase low PCE compared to its predecessor. Though the new structure could bring about an increment in Voc, current density followed the reverse trend.
V. Thieno[3,2-b]indole (TI) and thieno[2,3-b]indole based sensitizers for DSSCs
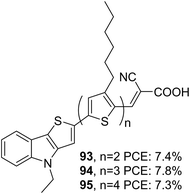
In thieno[3,2-b]indole (TI), an electron-rich system like thiophene is fused to the five-membered ring of the indole moiety at the 2–3 positions. It has been proved that thieno[3,2-b]indole is a better donor than both indole and carbazole units. The introduction of thieno[3,2-b]indole as a component in the dye design would certainly improve photovoltaic performance due to the co-planarity and strong electron-donating ability of these heteroacenes. The thieno[3,2-b]indole moiety can be easily synthesized using a Cadogen reaction of suitable functionalized 2-(2-nitrophenyl)thiophene.61The first report of a thieno[3,2-b]indole based DSSC was reported by Zang et al. in 2010.62 They synthesized three dyes (93, 94 and 95) employing 4-ethyl-4H-thieno[3,2-b]indole moiety as an electron donor, n-hexyl substituted oligothiophene units as a π-spacer and cyanoacrylic acid as an electron acceptor/anchoring group (Fig. 31). A comparison was also made with dyes having N-ethyl carbazole, which consists of the same dye skeleton.63 The electron lifetime measurement values obtained for the TI based devices are lower than their parent D–π–A carbazole dyes, leading to lower Voc for these devices, having the least value of 660 mV for the 95 based device. According to the DFT calculations, the dihedral angle between the thienyl group and the donor part is found to be less for TI based sensitizers in comparison to that of carbazole dyes. This increase in planarity and better electron-donating capability is reflected in the broader absorption spectra and higher ε values displayed by dyes employing thieno[3,2-b]indole as the donor unit. This resulted in an increment in current density, which was in line with the increment in the number of thiophene groups in the π-backbone. Among the TI dyes, 94 exhibited a higher PCE of 7.8%, and the minor performance was delivered by 95 (7.3%) (Table 5). An increase in the number of thiophene moieties resulted in higher HOMO levels for 95. Though this tendency could produce low bandgap sensitizers with better light-harvesting, a decrease in driving force for dye regeneration and the chances of recombination of injected electrons with the oxidized dyes may be responsible for decelerating the performance of 95.
| Sensitizer | J sc (mA cm−2) | V oc (mV) | FF | PCE (%) | Electrolyte | Coadsorbent (concentration) | Ref. |
|---|---|---|---|---|---|---|---|
| 93 | 13.8 | 700 | 0.77 | 7.4 | I−/I3− | — | 62 |
| 94 | 14.6 | 700 | 0.76 | 7.8 | I−/I3− | — | 62 |
| 95 | 15.0 | 660 | 0.74 | 7.3 | I−/I3− | — | 62 |
| 96 | 16.84 | 810 | 0.72 | 9.83 | [Co(bpy)3]2+/3+ | CDCA (20 mM) | 64 |
| 97 | 18.35 | 804 | 0.75 | 11.04 | [Co(bpy)3]2+/3+ | CDCA (20 mM) | 64 |
| 98 | 19.39 | 825 | 0.74 | 11.84 | [Co(bpy)3]2+/3+ | CDCA (20 mM) | 64 |
| 99 | 16.39 | 834 | 0.75 | 10.2 | [Co(bpy)3]2+/3+ | HC-A1 (0.6 mM) | 65 |
| 100 | 17.15 | 839 | 0.74 | 10.5 | [Co(bpy)3]2+/3+ | HC-A1 (0.6 mM) | 65 |
| 101 | 17.12 | 849 | 0.73 | 10.6 | [Co(bpy)3]2+/3+ | HC-A1 (0.6 mM) | 65 |
| 102 | 17.49 | 898 | 0.72 | 11.4 | [Co(bpy)3]2+/3+ | HC-A1 (0.6 mM) | 65 |
| 103 | 15.62 | 759 | 0.76 | 9.05 | [Co(bpy)3]2+/3+ | HC-A1 (6 mM) | 66 |
| 104 | 16.42 | 846 | 0.77 | 10.69 | [Co(bpy)3]2+/3+ | HC-A1 (6 mM) | 66 |
| 105 | 16.50 | 847 | 0.77 | 10.80 | [Co(bpy)3]2+/3+ | HC-A1 (6 mM) | 66 |
| 106 | 1.06 | 490 | 0.73 | 0.37 | [Co(bpy)3]2+/3+ | — | 68 |
| 107 | 3.2 | 360 | 0.69 | 0.79 | [Co(bpy)3]2+/3+ | — | 68 |
| 108 | 19.0 | 590 | 0.56 | 6.3 | [Co(bpy)3]2+/3+ | — | 69 |
| 109 | 19.9 | 390 | 0.44 | 3.4 | [Co(bpy)3]2+/3+ | — | 69 |
| 110 | 4.7 | 470 | 0.61 | 1.3 | [Co(bpy)3]2+/3+ | — | 69 |
| 111 | 6.6 | 370 | 0.56 | 1.4 | [Co(bpy)3]2+/3+ | — | 69 |
The role of thieno[3,2-b]indole (TI) as a π-spacer was demonstrated successfully by Kim and co-workers.64 In their initial work, they designed and synthesized D–A–π–A sensitizers using TI (97, 98) as a π-spacer and a comparative investigation was made between dyes with thieno[3,2-b]benzothiophene (TAB) as a π-spacer (96) (Fig. 32). The more electron-donating nature of TI could induce effective charge transfer in 97 and 98 with resultant wider absorption behaviour and ε-values compared to those of 96. These molecules also exhibited effective electron injection efficiency, with 98 being the best performer with improved light-harvesting ability. The IPCE performance of the dyes parallels these observations leading to the highest Jsc for 98 (19.39 mA cm−2) followed by 97 (18.35 mA cm−2) and 96 (16.84 mA cm−2). The hexyl chain was found to minimize the dye aggregations effectively in 98 with lesser recombination, which resulted in a higher Voc (825 mV) for the same (Table 5). The trends described above in device parameters ended in highest PCE for 98 (11.84%) followed by 97 (11.04%) and 96 (9.83%) using CDCA co-adsorbent and cobalt electrolyte ([Co(bpy)3]2+/3+).
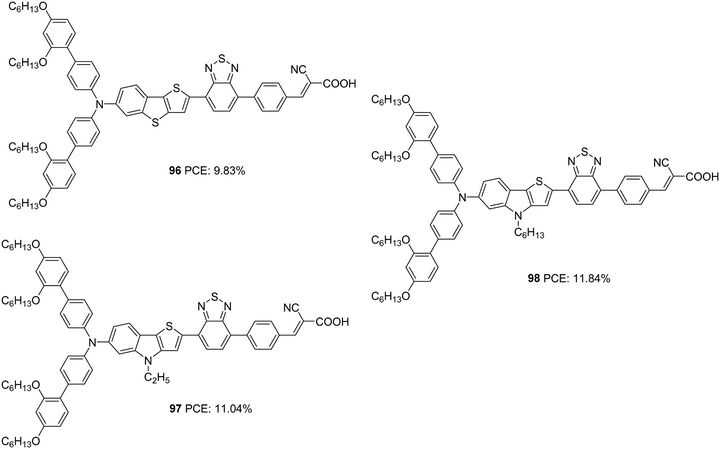 | ||
| Fig. 32 Photosensitizers with a thieno[3,2-b]indole moiety (97, 98) and thieno[3,2-b] benzothiophene (96) as a π-spacer. | ||
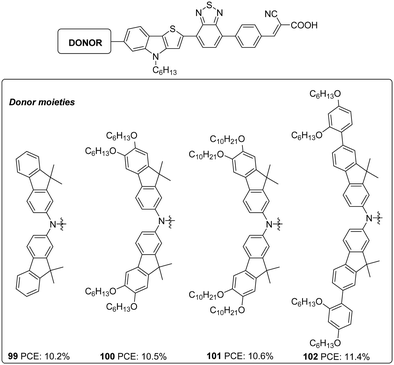
Later the same group synthesized 99–102. The objective was to reduce the aggregation-induced recombination occurring in devices fabricated with 98. The donor group in 98 was substituted with fluorenyl derivatives with the other building blocks remaining unchanged using D–A–π–A architecture with BTD as an auxiliary acceptor and cyanoacrylic acid as an acceptor/anchoring group (Fig. 33).65 The synthesized dyes 99–102 employ bis(9,9-dimethyl-9H-fluoren-2-yl)amino (FA),[20] bis(6,7-bis(hexyloxy)-9,9-dimethyl-9H-fluoren-2-yl)amino (HFA), bis(6,7-bis(decyloxy)-9,9-dimethyl-9H-fluoren-2-yl)amino (DFA), and bis(7-(2,4-bis(hexyloxy)phenyl)-9,9-dimethyl-9H-fluoren-2-yl)amine (BBFA) groups respectively as donors. To encounter solubility problems associated with the fluorene derivatives, all the devices were made with a change in dipping solvent resulting in lower PCE (10.5%, Jsc: 16.67 mA cm−2, Voc: 840 mV, FF: 0.75) for 98 than previously reported. All the devices were found to exhibit improved performance when the electrolyte was changed from iodide/triiodide to cobalt-based electrolyte ([Co(bpy)3]2+/3+) and in the presence of HC-A1 as the co-adsorbent. The alkoxy, as well as phenyloxy substituted fluorene derivatives (100–102), were found to be more effective both in red shifting the absorption spectrum as well as in preventing recombination, taking advantage of the electron-donating as well as the bulkiness of the donor groups, when compared to FA substituted dye 99. This lead to higher current density and photocurrent for 100–102 resulting in higher PCE. Though 99 does not have additional alkoxy substitutions integrated on it, the absorption profile shows a redshift when compared to those of 98 having BBPA (bis(2′,4′-bis(hexyloxy)-[1,1′-biphenyl]-4-yl)amino) as the donor. This absorption behaviour can be accredited to a lack of electronic communication between the alkoxy groups and the tertiary amine in BBPA based dye 98. A higher molar extinction coefficient of 98 contributed towards higher current density compared to 99. The bulkier BBPA also rendered 98 to have higher Voc and hence higher PCE when compared to those of 99. The device fabricated based on 102 showcased the highest efficiencies of 11.4%, and 99 delivered the least efficiency of 10.2% (Table 5).
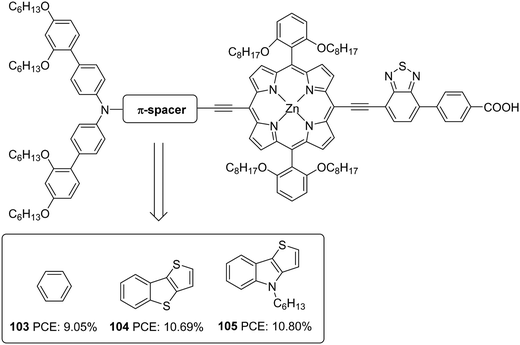
The superior performance of TI over TAB as a π-conjugator was again illustrated by Ji et al. (Fig. 34).66 To this end, two porphyrin based D–π–A dyes (D-ethynyl-zinc porphyrinyl-ethynyl-benzothiadiazole-acceptor) with extended conjugation at the donor sites were constructed. The dyes 104 and 105 differ in the auxiliary spacer between thieno[3,2-b]benzothiophene (TBT) and 4-hexyl-4H-thieno[3,2-b]indole, and were also subjected to a comparison with phenylethylene based dye 103 from earlier work.67 The dihedral angle between the donor part and π-spacer showed increment when the phenyl group in 103 was replaced with TI and TAB units with more conjugation. The trend observed in Voc was following the increase in dihedral angle (105 > 104 > 103), which might have helped prevent the recombination effectively. The more electron-donating TI and TAB groups could also bring changes in the light-harvesting ability of the dyes. In the Q-bands, significant changes were observed, having a more expansive and intensified absorption profile for 104–105, leading to current densities in the order 105 > 104 > 103. The more planar, electron-donating nature of the TI group along with the incorporated hexyl functionality contributed towards the best efficiency of 10.80% for 105 with higher Jsc (16.50 mA cm−2) and Voc (0.847 V) (Table 5) in the presence of HC-A1 as the co-adsorbent, using [Co(bpy)3]2+/3+ (bpy = 2,2′-bipyridine) redox electrolyte.
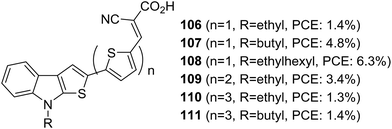
Thieno[2,3-b]indole was applied as a building block in DSSCs for the first time by Irgashev et al. (Fig. 35).68,69 They introduced a new synthetic route for constructing thieno[3,2-b]indole from 1-alkylisatin and 2-acetylthiophene. Among the dyes, 108 with thiophene as the π-bridge and ethyl hexyl as the alkyl group contributed towards the highest PCE of 6.3%. The bithiophene and terthiophene groups were entertaining aggregation of dyes which resulted in more recombination. Except for ethyl hexyl, other smaller alkyl groups like butyl and ethyl were not found to obstruct the dye aggregation, resulting in the downfall in efficiency using these spacers.
Thienoindoles have proved to have the potential to function both as donor as well as π-spacer units. Systematic engineering of different π-spacers and auxiliary donors to this moiety could bring impressive PCE in the future. In this line, dye designs that incorporate a TI unit with other indole fused systems open promising alternatives.
VI. Indolo[3,2-b]indole based sensitizers for DSSCs
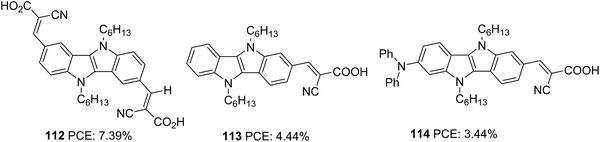
Indolo[3,2-b]indoles70 (IID) which consists of a central pyrrolopyrrole ring fused with two benzene rings on both sides, are considered as good electron donors with impressive hole-transporting properties. The planar nature of IID with a C2h symmetry facilitates better intermolecular interactions, which are beneficial for improving the hole transporting properties of these systems in the solid-state. In addition to these advantages, the two alkylation sites also make IID a potential candidate in various solution-processable optoelectronic applications such as organic thin film transistors, heterojunction solar cells, and organic light-emitting diodes.71The first attempt to incorporate IID as a building block in a DSSC was carried out by Ruangsupapichat et al. (Fig. 36).72 They developed dyes that fall in three categories: double acceptor system (112), donor–acceptor system (113), and donor–π spacer–acceptor (114). While IID functions as a donor in 112 and 113, it assumes the linker function in 114. The additional acceptor group and donor moieties in 112 and 114 helped to redshift the absorption spectra of the compounds by 30 nm and 10 nm, respectively with respect to 113. The PCE of the dyes was in the range 3–7%. The difference in PCE of the dyes resulted from the difference in Jsc values. The highest Jsc of 14.56 mA cm−2 of 112 contributed towards achieving the highest efficiency of 7.39%. This resulted from the extra electron extraction pathways provided by the additional electron anchoring groups present in the molecule. The device fabricated with dye 114, having an additional diphenylamine donor group, showcased the minor performance with only 3.44% PCE. According to the DFT studies, HOMO and LUMO energy levels of dye 114 are primarily localized on diphenylamine donors and cyanoacetic acid. Whereas in 112, a good overlap of the HOMO and LUMO levels may favour effective electron injection to the semiconductor's conduction band. Less effective electron injection in 114 maybe lowering the Jsc (8.25 mA cm−2) and thus the efficiency of the device (Table 6).
| Sensitizer | J sc (mA cm−2) | V oc (mV) | FF | PCE (%) | Electrolyte | Coadsorbent (concentration) | Ref. |
|---|---|---|---|---|---|---|---|
| 112 | 14.56 | 740 | 0.68 | 7.39 | I−/I3− | — | 72 |
| 113 | 10.09 | 660 | 0.66 | 4.44 | I−/I3− | — | 72 |
| 114 | 8.25 | 620 | 0.68 | 3.44 | I−/I3− | — | 72 |
| 115 | 11.59 | 638 | 0.70 | 5.20 | I−/I3− | — | 73 |
| 116 | 17.04 | 718 | 0.64 | 7.86 | I−/I3− | — | 73 |
| 117 | 5.51 | 620 | 0.69 | 2.38 | I−/I3− | CDCA (20 mM) | 74 |
| 118 | 5.22 | 570 | 0.67 | 2.00 | I−/I3− | CDCA (20 mM) | 74 |
| 119 | 4.60 | 540 | 0.69 | 1.71 | I−/I3− | CDCA (20 mM) | 74 |
| 120 | 14.55 | 671 | 0.69 | 6.73 | I−/I3− | — | 76 |
| 121 | 14.83 | 672 | 0.71 | 7.03 | I−/I3− | — | 76 |
| 122 | 16.65 | 691 | 0.69 | 8.00 | I−/I3− | — | 76 |
| 123 | 16.74 | 705 | 0.70 | 8.32 | I−/I3− | — | 76 |
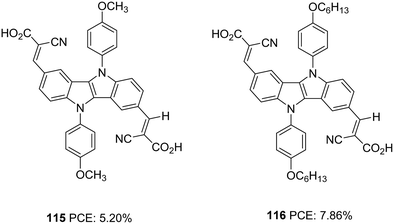
The same group further attempted to improve the efficiency of A–D–A dye 99 by introducing extra donor methoxyphenyl (115) and hexyloxyphenyl (116) moieties at the N,N′-positions of the IID core (Fig. 37).73 The dyes show involvement of both the carboxyl groups on anchoring but differ significantly in their performance. Dye 116 outperformed 112 and 115 in Jsc (17.04 mA cm−2) and Voc (718 mV) values leading to a higher PCE of 7.86%. Dye 115 exhibited 5.20% PCE with Jsc of 11.59 mA cm−2 and Voc of 638 mV. While the introduction of a longer alkyl chain in 116 was found to minimise recombination effectively, it also helped to improve the homogeneity of dye adsorption on the semiconductor surface. Broader absorption and higher dye loading amount improved the current density for 116.
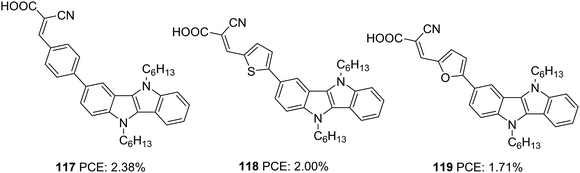
Our group also tried to explore the potential of IID as a donor unit towards the development of D–π–A dyes by introducing an additional π-spacer between the IID donor and cyanoacrylic acid acceptor and also by varying π-linkers between benzene, thiophene and furan (Fig. 38, 117, 118, 119).74 The donor unit IID was synthesized utilizing the methodology we developed in our laboratory, which involves a sequential multicomponent and oxidation approach.75 The absorption spectra of 119 showed more red-shifted and intensified spectra followed by 118 and 117 in order. Nevertheless, the current density and Voc produced by the compounds follow the reverse trend. To investigate the degree of conjugation, molecular geometry analysis of the dyes was also carried out. The acceptor group was found to be almost coplanar with the IID in all the dyes. But the dihedral angle between the donor and π-spacer increases in the order 119 (1.66°) < 118 (21.99°) < 117 (34.48°). Though the more planar geometry of 119 could help red shift the absorption spectra of the dye, it may also be entertaining the aggregation between dye molecules, which could eventually lead to more recombination. A slightly twisted conformation of the benzene substituted dye could prevent the approach of oxidized species from the electrolyte to come close to the semiconductor and the formation of π-stacks, thereby decreasing the chance of recombination. The addition of CDCA enhanced the PCE in all the cases. To utilize the different absorption profile showcased by 119 where recombination destroyed the PCE, we custom designed BID dyes which will be discussed in detail in the next section.
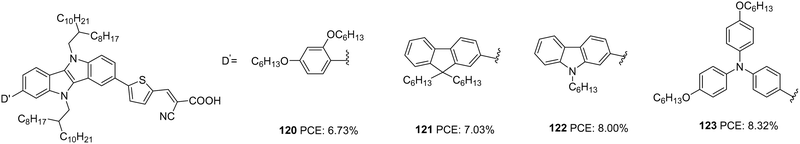
Wang et al. synthesized four D–D–π–A dyes based on IID as the primary donor, thiophene as π-spacer and cyanoacrylic acid as an acceptor.76 Dyes 120–123 contain hexyloxyphenyl, fluorene, carbazole and hexyloxytriphenylamine, respectively, as the auxiliary donor (Fig. 39). The current density and open-circuit potential followed the same trend, following the electron-donating ability and steric hindrance of the auxiliary donors. The bulkier and more electron-donating triphenylamine made 123 outperform other dyes with PCE of 8.32% with the highest Jsc of 16.74 mA cm−2 and Voc of 705 mV. Other dyes exhibited efficiency in the order 122 > 121 > 120 (Table 6).
VII. Benzothieno[3,2-b]indole based sensitizers for DSSCs
Benzothieno[3,2-b]indole (BID) is another unique tetracene with a benzothiophene moiety fused to indole. This heteroacene has found applications in medicinal chemistry but is seldom used in materials science and optoelectronic applications.77–79 Like what we discussed for indolo[3,2-b]indole; this fused indole moiety is also a planar conjugated system with the potential to be used both as a donor and a π-spacer. In addition, the appropriate functionalization of the N-atom offers flexibility to engineer the BID core with features that render better solubility and will also prevent aggregation of the system, arresting recombination.As a continuation to our previous studies with indolo[3,2-b]indole, the donor group was changed to dodecyl chain functionalized benzothieno[3,2-b]indole. Here also the newly developed methodology was employed for the synthesis of the BID core. The devices developed based on dyes 124 (phenyl spacer), 125 (thiophene spacer) and 126 (furan spacer) were found to showcase their best efficiency in the absence of any co-adsorbent. The addition of CDCA as a co-adsorbent decreased the photovoltaic performances of the devices. This illustrates the role of longer alkyl chains (dodecyl) in reducing aggregation of dyes and preventing back electron transfer. Unlike our observation with indolo[3,2-b]indole based dyes, furan substituted dye 126 outperformed the other two sensitizers with 4.11% efficiency with the highest Jsc and Voc (Table 7 and Fig. 40).80
| Sensitizer | J sc (mA cm−2) | V oc (mV) | FF | PCE (%) | Electrolyte | Coadsorbent (concentration) | Ref. |
|---|---|---|---|---|---|---|---|
| 124 | 2.86 | 590 | 0.69 | 1.16 | I−/I3− | — | 79 |
| 125 | 6.51 | 652 | 0.73 | 3.10 | I−/I3− | — | 79 |
| 126 | 8.38 | 671 | 0.73 | 4.11 | I−/I3− | — | 79 |
| 127 | 12.1 | 750 | 0.64 | 5.79 | I−/I3− | — | 81 |
| 128 | 13.0 | 762 | 0.65 | 6.46 | I−/I3− | — | 81 |
| 129 | 14.0 | 725 | 0.68 | 6.90 | I−/I3− | — | 82 |
| 130 | 13.5 | 680 | 0.67 | 6.15 | I−/I3− | — | 82 |
| 131 | 10.5 | 650 | 0.65 | 4.43 | I−/I3− | — | 82 |
| 132 | 14.2 | 753 | 0.71 | 7.59 | I−/I3− | — | 82 |
| 133 | 13.0 | 640 | 0.72 | 5.99 | I−/I3− | — | 82 |
| 134 | 12.6 | 652 | 0.70 | 5.75 | I−/I3− | CDCA (0.3 mM) | 83 |
| 135 | 13.8 | 657 | 0.71 | 6.42 | I−/I3− | CDCA (0.3 mM) | 83 |
| 136 | 8.98 | 643 | 0.70 | 4.05 | I−/I3− | CDCA (0.3 mM) | 83 |
| 137 | 12.1 | 633 | 0.70 | 5.37 | I−/I3− | CDCA (0.3 mM) | 83 |
| 138 | 2.19 | 700 | 0.59 | 1.09 | I−/I3− | — | 84 |
| 139 | 12.56 | 780 | 0.62 | 6.04 | I−/I3− | — | 84 |
| 140 | 3.92 | 680 | 0.53 | 1.42 | I−/I3− | — | 84 |
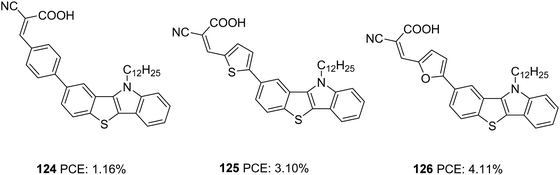 | ||
| Fig. 40 Molecular structures of photosensitizers 124–126 with benzothieno[3,2-b]indole as the donor. | ||
VIII. Tetraindole based sensitizers for DSSCs
The synthesis of tetraindole was first reported in 2006, which involved a one-pot tetramerization of indolin-2-one mediated by phosphoryl chloride.81 This polyacene has four indole units fused to cyclooctatetraene, which acts as a promising donor motif for solar cell applications. In addition, the unique structural characteristics of this saddle-shaped scaffold will also prevent self-assembly of the dyes on the semiconductor surface.Tetraindole found a place in DSSCs through the work of Qian and co-workers.82 The D–π–A dyes 127 and 128 use thiophene and bithiophene, respectively, as π-bridges (Fig. 41). The dyes exhibit improved open-circuit potential benefitting from the saddle like structure with flanked octyl alkyl chains of the new donor moiety. The sensitizer 128 exhibited a broader ICT band and better light-harvesting ability, which is evident from the broader and enhanced response of the IPCE spectra. 128 delivered a maximum efficiency of 6.21% with the highest Jsc (13.0 mA cm−2) and Voc (762 mV), whereas dye 127 could only deliver a PCE of 5.79% with Jsc of 12.1 mA cm−2 and Voc of 750 mV. The reduced aggregation behaviour of these dye was further illustrated by the co-adsorption experiments using CDCA. Both dyes exhibited lower efficiencies in the presence of CDCA, the reduction mainly arose from lower Jsc values (Table 7). Decreased dye loading in the presence of CDCA can account for the decrease in photocurrent and hence the overall efficiency.
IX. Dithienopyrroloindole based sensitizers for DSSCs
Optimization of the π-bridge is also as important as other building blocks in a photosensitizer to realize improved PCE. Rigidified aromatics occupy a prominent space among various conjugated functionalities in this regard due to their better charge transfer ability, high molar extinction coefficient and reduced reorganization energies. The compound 4,5-dihexyl-4,5-dihydrothieno[2″,3″:4′,5′]pyrrolo[2′,3′:4,5]thieno[3,2-b]indole (DPTI) as a π-spacer was first employed by Zang et al. to construct photosensitizers 129, 131–133 (Fig. 42).83 To compare the efficiency of a dithienopyrroloindole (DTP) unit as a spacer, dye 130 was synthesized. Dye 129 outperformed 130 with improved light-harvesting ability and open-circuit potential, indicating the importance of DPTI over DTP. Dyes 131 and 132 were designed to investigate the effect of additional donor and alkylated spacer units on the performance of DPTI based dye. The introduction of an additional donor unit in 131 caused a negative shift in HOMO compared to 129, whereas a positive shift in LUMO energy level was observed in 132 without much change in HOMO energy level. This low driving force for dye regeneration, enhanced recombination of electrons from the semiconductor with the oxidized dye and lowest dye loading may be responsible for the least efficiency of 4.4% for 131. The broader spectra of 132 with its twisted conformation induced by the 3-hexylthiophene could deliver a maximum efficiency of 7.59%. In dye 133, the DPTI unit assumes both the function of a donor and spacer, the indole ring acting as the donor and DTP as the spacer. The conjugated donor–π-spacer structure allows better delocalization of the HOMO and LUMO orbitals over the entire molecule and facilitates good charge transfer from donor to acceptor. Though it managed to perform better than 131, the lack of additional donor decreased the charge separation and electron injection yield leading to lower photocurrent and an efficiency of 5.99%, lower than the rest of the dyes.X. Fluorenylindolenine donor-based dyes for DSSCs
Jayaraj and co-workers synthesized four novel unsymmetrical squaraine dyes employing fluorenylindolenine as donor 134–137 (Fig. 43).84 Apart from increasing the conjugation and NIR light-harvesting capability of the molecules, this skeleton also helps to modulate the charge recombination by incorporating an out of plane alkyl chain on sp3 carbon and in-plane alkyl chain on the nitrogen. While 134 and 135 are composed of an indole unit toward the anchoring side with an N-methylated and N-hexylated fluorenylindolenine, 136 and 137 contained a benzo[e]indole unit toward the anchoring side with an N-methylated and N-hexylated donor, respectively. Though the latter set showcased more red-shifted spectra, they exhibited slightly lower efficiency. The difference in PCE of the devices might be due to the difference in dipole moment of the dyes. The calculated dipole moments are in the order 135 (10.6) > 134 (10.0 D) > 136 (7.4 D) > 137 (7.3 D). The non-directionality induced by the benz[e]indole group in 136–137 may be responsible for lowering the dipole moment and electron injection efficiency. The larger dipole moment exerted by dyes 134–135 on TiO2 may also be helping to upshift the conduction band of TiO2, which is evident from the higher Voc observed for these dyes. In both sets of dyes, the hexyl substituted dyes were found to outperform the methylated dye. All the dyes exhibited an increment in PCE when dye and CDCA were used in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratios, the effect majorly contributed by the increase in current density. It is already known that squaraines are prone to aggregation. Adding CDCA helps to reduce this aggregation leading to improved PCE.
3 ratios, the effect majorly contributed by the increase in current density. It is already known that squaraines are prone to aggregation. Adding CDCA helps to reduce this aggregation leading to improved PCE.
XI. Indole–imidazole donor dyes for DSSC
An indole–imidazole fused system was incorporated as an auxiliary acceptor (AN) by Ramasami to construct three dyes with different molecular architectures: 138 (D–AN(π–A)–D), 139 (D–AN–(DA2)–D) and 140 (D–AN–D(A)–AN–D) (Fig. 44).85 Dye 139 showed maximum efficiency with the highest current density and photovoltage. Along with the increment in molar extinction coefficient and red-shifted absorption, the dianchoring approach also facilitated adequate surface coverage and helped to reduce back electron transfer between the semiconductor surface and electrolyte. The minimum reorganization energy calculated for the same also implies efficient charge injection to the semiconductor for these dyes. The photovoltaic parameters of DSSCs 124–140 are given in Table 7.Conclusions and future perspectives
Engineering sensitizers have got their prime importance when efficiency enhancement of DSSCs is concerned. Electron rich fused heterocycles have proved their efficacy as donors as well as π-spacers in the sensitizer design. Indole fused aromatic systems have also been utilized to this end, effectively realizing efficient DSSCs. These systems were found to exhibit more electron-donating ability than indole in conjugation with expanded π-systems. Though the planarity could induce ICT in molecules, a trade-off between light harvesting and dye aggregations leads to a shortfall of the power conversion efficiency in many instances. The strategic introduction of bulky substituents on the donor side and the π-conjugator helped abate these situations to some extent. Another notable advantage is the ease of synthesising fused indole heterocyclic motifs with functionalities required for further transformations. In addition, the stability of these fused heterocycles has contributed to enhancing the device lifetime.The most efficient DSSC (PCE: 13.6%) with a fused indole based sensitizer was reported with dye 68, which had a triazatruxene moiety as a donor unit. There are still a plethora of easily accessible fused indole heterocyclic moieties with the required structural features that can act as suitable donors and π-spacers in sensitizer designs. One of the simplest fused indole heteroacenes used as a sensitizer building block is thieno[3,2-b]indole, which gave DSSCs up to 11% efficiency. Likewise, pyrrolo[3,2-b]indole can be utilized, resulting in high performing DSSCs. The class of indole fused tetraacenes such as indoloindoles, benzothienoindoles and benzofuroindoles, which are excellent donors, are seldom used building blocks in sensitizer designs. These tetraacenes can exist as different isomers based on the position of fusion, and these isomers differ in their structure and properties and these have all the structural and electronic characteristics to act as good π-spacers. Moreover, these heteroacenes can be used in alternate and new sensitizer designs such as D–A–π–A or (D)2–A–π–A, resulting in highly efficient DSSCs. As part of our continued interest in this area, we focus on developing new and simpler methodologies to access indole fused heterocycles and extend our research detailed in Section VI and VII by incorporating additional donors and auxiliary acceptors to the D–π–A architecture being optimized.
The challenging task in realizing efficient DSSCs is to prevent back electron transfer/recombination. Along with the proper molecular design and keeping a delicate balance between electronic and steric factors, importance must also be given to the electrolyte used. Significant improvement in photovoltaic parameters and the devices' performance is reported for 98 to 102 when the electrolyte was changed from I−/I3− to [Co(bpy)3]2+/3+. Most of the sensitizers mentioned in the review use conventional iodide/triiodide electrolyte. This indicates that more promising results could be obtained in the future using appropriate alternate cobalt and copper electrolytes with these fused heterocyclic dyes. In addition, these fused heterocycles can serve as excellent co-sensitizers along with other dyes tapping the visible absorption for indoor photovoltaic applications. With continuing efforts to develop stable, easily accessible and efficient sensitizers for DSSCs, we hope significant advancements will be made with fused indole heterocyclic moieties in the near future.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank DST for DST-SERB Project [DST/SERB/F/481]. NPR thanks CSIR for the research fellowship. NPR and JJ also thanks CSIR (HCP-029) for financial assistance. SS would like to thank SERB CRG project (CRG/2020/001406) and CSIR-FIRST (MLP65) project. SS also acknowledges CSIR Mission (HCP30) and FTT (MLP38) Projects for financial support.References
- X. Chen, C. Li, M. Grätzel, R. Kostecki and S. S. Mao, Nanomaterials for renewable energy production and storage, Chem. Soc. Rev., 2012, 41, 7909–7937 RSC
.
-
(a) N. Armaroli and V. Balzani, The future of energy supply: Challenges and opportunities, Angew. Chem., Int. Ed., 2007, 46, 52–66 CrossRef CAS PubMed
; (b) R. Eisenberg and D. G. Nocera, Preface: Overview of the forum on solar and renewable energy, Inorg. Chem., 2005, 44, 6799–6801 CrossRef CAS PubMed
.
-
(a) W. A. Badawy, A review on solar cells from Si-single crystals to porous materials and quantum dots, J. Adv. Res., 2015, 6, 123–132 CrossRef CAS PubMed
; (b) S. Sharma, K. K. Jain and A. Sharma, Solar cells: In research and applications—a review, Mater. Sci. Appl., 2015, 06, 1145–1155 CAS
.
-
(a) M. Grätzel, Solar energy conversion by dye-sensitized photovoltaic cells, Inorg. Chem., 2005, 44, 6841–6851 CrossRef PubMed
; (b) I. Chung, B. Lee, J. He, R. P. H. Chang and M. G. Kanatzidis, All-solid-state dye-sensitized solar cells with high efficiency, Nature, 2012, 485, 486–489 CrossRef CAS PubMed
; (c) Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Bulk heterojunction solar cells: Morphology and performance relationships, Chem. Rev., 2014, 114, 7006–7043 CrossRef CAS PubMed
; (d) I. Mora-Seró, Current challenges in the development of quantum dot sensitized solar cells, Adv. Energy Mater., 2020, 10, 1–6 Search PubMed
; (e) O. E. Semonin, J. M. Luther and M. C. Beard, Quantum dots for next-generation photovoltaics, Mater. Today, 2012, 15, 508–515 CrossRef CAS
; (f) M. A. Green, A. Ho-Baillie and H. J. Snaith, The emergence of perovskite solar cells, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS
; (g) R. Rajeswari, M. Mrinalini, S. Prasanthkumar and L. Giribabu, Emerging of inorganic hole transporting materials for perovskite solar cells, Chem. Rec., 2017, 17, 681–699 CrossRef CAS PubMed
.
- G. Gokul, S. C. Pradhan and S. Soman, Dye sensitized solar cells as potential candidates for indoor/diffused light harvesting applications: From BIPV to self powered IoTs, Adv. Sol. Energy Res., 2019, 281–316 Search PubMed
.
- B. O'Regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 1991, 353, 737–740 CrossRef
.
-
(a) J. Gong, J. Liang and K. Sumathy, Review on dye-sensitized solar cells (DSSCs): Fundamental concepts and novel materials, Renewable Sustainable Energy Rev., 2012, 16, 5848–5860 CrossRef CAS
; (b) Z. Ning, Y. Fu and H. Tian, Improvement of dye-sensitized solar cells: What we know and what we need to know, Energy Environ. Sci., 2010, 3, 1170–1181 RSC
; (c) T. Bessho, S. M. Zakeeruddin, C. Y. Yeh, E. W. G. Diau and M. Grätzel, Highly efficient mesoscopic dye-sensitized solar cells based on donor-acceptor-substituted porphyrins, Angew. Chem., Int. Ed., 2010, 49, 6646–6649 CrossRef CAS PubMed
; (d) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Dye-sensitized solar cells, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed
; (e) H. S. Jung and J. K. Lee, Dye sensitized solar cells for economically viable photovoltaic systems, J. Phys. Chem. Lett., 2013, 4, 1682–1693 CrossRef CAS PubMed
.
-
(a) H. Michaels, M. Rinderle, R. Freitag, I. Benesperi, T. Edvinsson, R. Socher, A. Gagliardi and M. Freitag, Dye-sensitized solar cells under ambient light powering machine learning: Towards autonomous smart sensors for the internet of things, Chem. Sci., 2020, 11, 2895–2906 RSC
; (b) E. Tanaka, H. Michaels, M. Freitag and N. Robertson, Synergy of co-sensitizers in a copper bipyridyl redox system for efficient and cost-effective dye-sensitized solar cells in solar and ambient light, J. Mater. Chem. A, 2020, 8, 1279–1287 RSC
; (c) Y. Cao, Y. Liu, S. M. Zakeeruddin, A. Hagfeldt and M. Grätzel, Joule, 2018, 2, 1108–1117 CrossRef CAS
; (d) S. Sasidharan, S. Soman, S. C. Pradhan, K. N. N. Unni, A. A. P. Mohamed, B. N. Nair and H. U. N. Saraswathy, New J. Chem., 2017, 41, 1007–1016 RSC
; (e) M. Freitag, J. Teuscher, Y. Saygili, X. Zhang, F. Giordano, P. Liska, J. Hua, S. M. Zakeeruddin, J. E. Moser, M. Grätzel and A. Hagfeldt, Dye-sensitized solar cells for efficient power generation under ambient lighting, Nat. Photonics, 2017, 11, 372–378 CrossRef CAS
; (f) S. Venkatesan, W. H. Lin, H. Teng and Y. L. Lee, High-efficiency bifacial dye-sensitized solar cells for application under indoor light conditions, ACS Appl. Mater. Interfaces, 2019, 11, 42780–42789 CrossRef CAS PubMed
.
-
(a) J. M. Ji, H. Zhou and H. K. Kim, Rational design criteria for D–π–A structured organic and porphyrin sensitizers for highly efficient dye-sensitized solar cells, J. Mater. Chem. A, 2018, 6(30), 14518–14545 RSC
; (b) W. Wei, K. Sun and Y. H. Hu, An efficient counter electrode material for dye-sensitized solar cells – flower-structured 1T metallic phase MoS2, J. Mater. Chem. A, 2016, 4(32), 12398–12401 RSC
; (c) W. Wei, K. Sun and Y. H. Hu, Direct conversion of CO2 to 3D graphene and its excellent performance for dye-sensitized solar cells with 10% efficiency, J. Mater. Chem. A, 2016, 4(31), 12054–12057 RSC
; (d) D. Wang, W. Wei and Y. H. Hu, Highly efficient dye-sensitized solar cells with composited food dyes, Ind. Eng. Chem. Res., 2020, 59(22), 10457–10463 CrossRef CAS
.
-
(a) Q. Huaulmé, V. M. Mwalukuku, D. Joly, J. Liotier, Y. Kervella, P. Maldivi, S. Narbey, F. Oswald, A. J. Riquelme, J. A. Anta and R. Demadrille, Photochromic dye-sensitized solar cells with light-driven adjustable optical transmission and power conversion efficiency, Nat. Energy, 2020, 5, 468–477 CrossRef
; (b) A. Gopi, S. Lingamoorthy, S. Soman, K. Yoosaf, R. Haridas and S. Das, Modulating FRET in organic–inorganic nanohybrids for light harvesting applications, J. Phys. Chem. C, 2016, 120, 26569–26578 CrossRef CAS
; (c) M. V. Vinayak, M. Yoosuf, S. C. Pradhan, T. M. Lakshmykanth, S. Soman and K. R. Gopidas, A detailed evaluation of charge recombination dynamics in dye solar cells based on starburst triphenylamine dyes, Sustainable Energy Fuels, 2018, 2, 303–314 RSC
; (d) H. Iftikhar, G. G. Sonai, S. G. Hashmi, A. F. Nogueira and P. D. Lund, Progress on electrolytes development in dye-sensitized solar cells, Materials, 2019, 12, 1998–2065 CrossRef CAS PubMed
; (e) S. C. Pradhan, A. Hagfeldt and S. Soman, Resurgence of DSCs with copper electrolyte: a detailed investigation of interfacial charge dynamics with cobalt and iodine based electrolytes, J. Mater. Chem. A, 2018, 6, 22204–22214 RSC
; (f) S. Soman, Y. Xie and T. W. Hamann, Cyclometalated sensitizers for DSSCs employing cobalt redox shuttles, Polyhedron, 2014, 82, 139–147 CrossRef CAS
; (g) S. Soman, M. A. Rahim, S. Lingamoorthy, C. H. Suresh and S. Das, Strategies for optimizing the performance of carbazole thiophene appended unsymmetrical squaraine dyes for dye-sensitized solar cells, Phys. Chem. Chem. Phys., 2015, 17, 23095–23103 RSC
; (h) M. V. Vinayak, T. M. Lakshmykanth, M. Yoosuf, S. Soman and K. R. Gopidas, Effect of recombination and binding properties on the performance of dye sensitized solar cells based on propeller shaped triphenylamine dyes with multiple binding groups, Sol. Energy, 2016, 124, 227–241 CrossRef CAS
; (i) Q. Wali, A. Fakharuddin and R. Jose, Tin oxide as a photoanode for dye-sensitised solar cells: Current progress and future challenges, J. Power Sources, 2015, 293, 1039–1052 CrossRef CAS
.
-
(a) K. L. Wu, W. P. Ku, J. N. Clifford, E. Palomares, S. Te Ho, Y. Chi, S. H. Liu, P. T. Chou, M. K. Nazeeruddin and M. Grätzel, Harnessing the open-circuit voltage via a new series of Ru(II) sensitizers bearing (iso-)quinolinyl pyrazolate ancillaries, Energy Environ. Sci., 2013, 6, 859–870 RSC
; (b) Z. She, Y. Cheng, L. Zhang, X. Li, D. Wu, Q. Guo, J. Lan, R. Wang and J. You, Novel ruthenium sensitizers with a phenothiazine conjugated bipyridyl ligand for high-efficiency dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2015, 7, 27831–27837 CrossRef CAS PubMed
; (c) L. Han, A. Islam, H. Chen, C. Malapaka, B. Chiranjeevi, S. Zhang, X. Yang and M. Yanagida, High-efficiency dye-sensitized solar cell with a novel co-adsorbent, Energy Environ. Sci., 2012, 5, 6057–6060 RSC
.
-
(a) Y. S. Yen, H. H. Chou, Y. C. Chen, C. Y. Hsu and J. T. Lin, Recent developments in molecule-based organic materials for dye-sensitized solar cells, J. Mater. Chem., 2012, 22, 8734–8747 RSC
; (b) A. Mishra, M. K. R. Fischer and P. Büuerle, Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS PubMed
; (c) A. Carella, F. Borbone and R. Centore, Research progress on photosensitizers for DSSC, Front. Chem., 2018, 6, 1–24 CrossRef PubMed
.
-
(a) J. J. Cid, J. H. Yum, S. R. Jang, M. K. Nazeeruddin, E. Martínez-Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Molecular cosensitization for efficient panchromatic dye-sensitized solar cells, Angew. Chem., Int. Ed., 2007, 46, 8358–8362 CrossRef CAS PubMed
; (b) N. V. Krishna, J. V. S. Krishna, M. Mrinalini, S. Prasanthkumar and L. Giribabu, Role of co-sensitizers in dye-sensitized solar cells, ChemSusChem, 2017, 10, 4668–4689 CrossRef CAS PubMed
.
-
(a) B. Hemavathi, V. Jayadev, P. C. Ramamurthy, R. K. Pai, K. N. Narayanan Unni, T. N. Ahipa, S. Soman and R. G. Balakrishna, Variation of the donor and acceptor in D–A–π–A based cyanopyridine dyes and its effect on dye sensitized solar cells, New J. Chem., 2019, 43, 15673–15680 RSC
; (b) B. Hemavathi, V. Jayadev, S. C. Pradhan, G. Gokul, K. Jagadish, G. K. Chandrashekara, P. C. Ramamurthy, R. K. Pai, K. N. Narayanan Unni, T. N. Ahipa, S. Soman and R. Geetha Balakrishna, Aggregation induced light harvesting of molecularly engineered D–A–π–A carbazole dyes for dye-sensitized solar cells, Sol. Energy, 2018, 174, 1085–1096 CrossRef CAS
; (c) T. Maeda, T. V. Nguyen, Y. Kuwano, X. Chen, K. Miyanaga, H. Nakazumi, S. Yagi, S. Soman and A. Ajayaghosh, Intramolecular exciton-coupled squaraine dyes for dye-sensitized solar cells, J. Phys. Chem. C, 2018, 122, 21745–21754 CrossRef CAS
; (d) V. Nikolaou, A. Charisiadis, S. Chalkiadaki, I. Alexandropoulos, S. C. Pradhan, S. Soman, M. K. Panda and A. G. Coutsolelos, Enhancement of the photovoltaic performance in D3A porphyrin-based DSCs by incorporating an electron withdrawing triazole spacer, Polyhedron, 2018, 140, 9–18 CrossRef CAS
; (e) J. S. Panicker, B. Balan, S. Soman and V. C. Nair, Understanding structure–property correlation of metal free organic dyes using interfacial electron transfer measurements, Sol. Energy, 2016, 139, 547–556 CrossRef CAS
; (f) S. Soman, S. C. Pradhan, M. Yoosuf, M. V. Vinayak, S. Lingamoorthy and K. R. Gopidas, Probing recombination mechanism and realization of marcus normal region behavior in DSSCs employing cobalt electrolytes and triphenylamine dyes, J. Phys. Chem. C, 2018, 122, 14113–14127 CrossRef CAS
; (g) M. Yoosuf, S. C. Pradhan, S. Soman and K. R. Gopidas, Triple bond rigidified anthracene–triphenylamine sensitizers for dye-sensitized solar cells, Sol. Energy, 2019, 188, 55–65 CrossRef CAS
.
-
(a) L. Zhang, X. Yang, W. Wang, G. G. Gurzadyan, J. Li, X. Li, J. An, Z. Yu, H. Wang, B. Cai, A. Hagfeldt and L. Sun, 13.6% Efficient organic dye-sensitized solar cells by minimizing energy losses of the excited state, ACS Energy Lett., 2019, 4, 943–951 CrossRef CAS
; (b) W. Zhang, Y. Wu, H. W. Bahng, Y. Cao, C. Yi, Y. Saygili, J. Luo, Y. Liu, L. Kavan, J. E. Moser, A. Hagfeldt, H. Tian, S. M. Zakeeruddin, W. H. Zhu and M. Grätzel, Comprehensive control of voltage loss enables 11.7% efficient solid-state dye-sensitized solar cells, Energy Environ. Sci., 2018, 11, 1779–1787 RSC
; (c) Y. Cao, Y. Liu, S. M. Zakeeruddin, A. Hagfeldt and M. Gratzel, Direct contact of selective charge extraction layers enables high-efficiency molecular photovoltaics, Joule, 2018, 2, 1108–1117 CrossRef CAS
; (d) K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J. Fujisawa and M. Hanaya, Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes, Chem. Commun., 2015, 51, 15894–15897 RSC
.
-
(a) L. Alibabaei, J. H. Kim, M. Wang, N. Pootrakulchote, J. Teuscher, D. Di Censo, R. Humphry-Baker, J. E. Moser, Y. J. Yu, K. Y. Kay, S. M. Zakeeruddin and M. Grätzel, Molecular design of metal-free D–π–A substituted sensitizers for dye-sensitized solar cells, Energy Environ. Sci., 2010, 3, 1757–1764 RSC
; (b) A. Yella, R. Humphry-Baker, B. F. E. Curchod, N. Ashari Astani, J. Teuscher, L. E. Polander, S. Mathew, J. E. Moser, I. Tavernelli, U. Rothlisberger, M. Grätzel, M. K. Nazeeruddin and J. Frey, Molecular engineering of a fluorene donor for dye-sensitized solar cells, Chem. Mater., 2013, 25, 2733–2739 CrossRef CAS
.
-
(a) J. Wang, K. Liu, L. Ma and X. Zhan, Triarylamine: Versatile platform for organic, dye-sensitized, and perovskite solar cells, Chem. Rev., 2016, 116, 14675–14725 CrossRef CAS PubMed
; (b) K. Hara, K. Sayama, Y. Ohga, A. Shinpo, S. Suga and H. Arakawa, A coumarin-derivative dye sensitized nanocrystalline TiO2 solar cell having a high solar-energy conversion efficiency up to 5.6%, Chem. Commun., 2001, 569–570 RSC
; (c) Z. S. Wang, K. Hara, Y. Dan-oh, C. Kasada, A. Shinpo, S. Suga, H. Arakawa and H. Suglhara, Photophysical and (photo)electrochemical properties of a coumarin dye, J. Phys. Chem. B, 2005, 109, 3907–3914 CrossRef CAS PubMed
; (d) B. Liu, R. Wang, W. Mi, X. Li and H. Yu, Novel branched coumarin dyes for dye-sensitized solar cells: Significant improvement in photovoltaic performance by simple structure modification, J. Mater. Chem., 2012, 22, 15379–15387 RSC
; (e) M. Marszalek, S. Nagane, A. Ichake, R. Humphry-Baker, V. Paul, S. M. Zakeeruddin and M. Grätzel, Tuning spectral properties of phenothiazine based donor-π-acceptor dyes for efficient dye-sensitized solar cells, J. Mater. Chem., 2012, 22, 889–894 RSC
; (f) S. H. Kim, H. W. Kim, C. Sakong, J. Namgoong, S. W. Park, M. J. Ko, C. H. Lee, W. I. Lee and J. P. Kim, Effect of five-membered heteroaromatic linkers to the performance of phenothiazine-based dye-sensitized solar cells, Org. Lett., 2011, 13, 5784–5787 CrossRef CAS PubMed
; (g) K. Srinivas, C. R. Kumar, M. A. Reddy, K. Bhanuprakash, V. J. Rao and L. Giribabu, D–π–A organic dyes with carbazole as donor for dye-sensitized solar cells, Synth. Met., 2011, 161, 96–105 CrossRef CAS
.
-
(a) Y. Liu, J. He, L. Han and J. Gao, Influence of the auxiliary acceptor and π-bridge in carbazole dyes on photovoltaic properties, J. Photochem. Photobiol., A, 2017, 332, 283–292 CrossRef CAS
; (b) K. Hara, Z. S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-Oh, C. Kasada, A. Shinpo and S. Suga, Oligothiophene-containing coumarin dyes for efficient dye-sensitized solar cells, J. Phys. Chem. B, 2005, 109, 15476–15482 CrossRef CAS PubMed
; (c) J. Liu, X. Yang, A. Islam, Y. Numata, S. Zhang, N. T. Salim, H. Chen and L. Han, Efficient metal-free sensitizers bearing circle chain embracing π-spacers for dye-sensitized solar cells, J. Mater. Chem. A, 2013, 1, 10889–10897 RSC
; (d) L. Huang, P. Ma, G. Deng, K. Zhang, T. Ou, Y. Lin and M. S. Wong, Novel electron-deficient quinoxalinedithienothiophene- and phenazinedithienothiophene-based photosensitizers: The effect of conjugation expansion on DSSC performance, Dyes Pigm., 2018, 159, 107–114 CrossRef CAS
.
-
(a) Y. Wu and W. Zhu, Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC
; (b) W. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z. S. Wang and H. Tian, Organic D–A–π–A solar cell sensitizers with improved stability and spectral response, Adv. Funct. Mater., 2011, 21, 756–763 CrossRef CAS
; (c) W. Li, Y. Wu, Q. Zhang, H. Tian and W. Zhu, D–A–π–A featured sensitizers bearing phthalimide and benzotriazole as auxiliary acceptor: Effect on absorption and charge recombination dynamics in dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2012, 4, 1822–1830 CrossRef CAS PubMed
; (d) Y. Wu, W. H. Zhu, S. M. Zakeeruddin and M. Grätzel, Insight into D–A–π–A structured sensitizers: A promising route to highly efficient and stable dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2015, 7, 9307–9318 CrossRef CAS PubMed
; (e) J. He, Y. Liu, J. Gao and L. Han, New D–D–π–A triphenylamine-coumarin sensitizers for dye-sensitized solar cells, Photochem. Photobiol. Sci., 2017, 16, 1049–1056 CrossRef CAS PubMed
; (f) W. Sharmoukh, J. Cong, J. Gao, P. Liu, Q. Daniel and L. Kloo, Molecular engineering of D–D–π–A-based organic sensitizers for enhanced dye-sensitized solar cell performance, ACS Omega, 2018, 3, 3819–3829 CrossRef CAS PubMed
; (g) V. Cuesta, M. Vartanian, P. de la Cruz, R. Singhal, G. D. Sharma and F. Langa, Comparative study on the photovoltaic characteristics of A–D–A and D–A–D molecules based on Zn-porphyrin; a D–A–D molecule with over 8.0% efficiency, J. Mater. Chem. A, 2017, 5, 1057–1065 RSC
; (h) K. S. V. Gupta, T. Suresh, S. P. Singh, A. Islam, L. Han and M. Chandrasekharam, Carbazole based A–π–D–π–A dyes with double electron acceptor for dye-sensitized solar cell, Org. Electron., 2014, 15, 266–275 CrossRef CAS
.
-
(a) I. Cho, S. K. Park, B. Kang, J. W. Chung, J. H. Kim, K. Cho and S. Y. Park, Design, synthesis, and versatile processing of indolo[3,2-b]indole-based π-conjugated molecules for high-performance organic field-effect transistors, Adv. Funct. Mater., 2016, 26, 2966–2973 CrossRef CAS
; (b) Y. Y. Lai, J. M. Yeh, C. E. Tsai and Y. J. Cheng, Synthesis, molecular and photovoltaic properties of an indolo[3,2-b]indole-based acceptor–donor–acceptor small molecule, Eur. J. Org. Chem., 2013, 5076–5084 CrossRef CAS
; (c) H. Jiang, H. Zhao, K. K. Zhang, X. Chen, C. Kloc and W. Hu, High-performance organic single-crystal field-effect transistors of indolo[3,2-b]carbazole and their potential applications in gas controlled organic memory devices, Adv. Mater., 2011, 23, 5075–5080 CrossRef CAS PubMed
.
- For the synthesis of indolo[2,3-b]quinoxaline see: G. M. Badger and P. J. Nelson, Polynuclear heterocyclic systems. Part XIV. Indoloquinoxalines, J. Chem. Soc., 1962, 3926–3931 RSC
.
- K. R. J. Thomas and P. Tyagi, Synthesis, Spectra, and Theoretical Investigations of the Triarylamines Based on 6H-Indolo[2,3-b]quinoxaline, J. Org. Chem., 2010, 75, 8100–8111 CrossRef CAS PubMed
.
- A. Venkateswararao, P. Tyagi, K. R. J. Thomas and P. Chen, Organic dyes containing indolo [ 2, 3- b] quinoxaline as a donor: synthesis, optical and photovoltaic properties, Tetrahedron, 2014, 70, 6318–6327 CrossRef CAS
.
- X. Qian, H. H. Gao, Y. Z. Zhu, L. Lu and J. Y. Zheng, 6H-Indolo[2,3-b]quinoxaline-based organic dyes containing different electron-rich conjugated linkers for highly efficient dye-sensitized solar cells, J. Power Sources, 2015, 280, 573–580 CrossRef CAS
.
- X. Qian, X. Wang, L. Shao, H. Li, R. Yan and L. Hou, Molecular engineering of D–D–π–A type organic dyes incorporating indoloquinoxaline and phenothiazine for highly efficient dye-sensitized solar cells, J. Power Sources, 2016, 326, 129–136 CrossRef CAS
.
- X. Qian, X. Lan, R. Yan, Y. He, J. Huang and L. Hou, T-shaped (D)2–A–π–A type sensitizers incorporating indoloquinoxaline and triphenylamine for organic dye-sensitized solar cells, Electrochim. Acta, 2017, 232, 377–386 CrossRef CAS
.
- R. Su, L. Lyu, M. R. Elmorsy and A. El-Shafei, Novel metal-free organic dyes constructed with the D–D|A–π–A motif: Sensitization and co-sensitization study, Sol. Energy, 2019, 194, 400–414 CrossRef CAS
.
- R. Su, S. Ashraf, L. Lyu and A. El-shafei, Tailoring dual-channel anchorable organic sensitizers with indolo[2,3-b]quinoxaline moieties: Correlation between structure and DSSC performance, Sol. Energy, 2020, 206, 443–454 CrossRef CAS
.
- R. Su, L. Lyu, M. R. Elmorsy and A. El-Shafei, Structural studies and photovoltaic investigation of indolo[2,3:B] quinoxaline-based sensitizers/co-sensitizers achieving highly efficient DSSCs, New J. Chem., 2020, 44, 2797–2812 RSC
.
-
(a) N. X. Hu, S. Xie, Z. Popovic, B. Ong, A. M. Hor and S. Wang, 5,11-Dihydro-5,11-di-1-naphthylindolo[3,2-b]carbazole:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Atropisomerism in a novel hole-transport molecule for organic light-emitting diodes, J. Am. Chem. Soc., 1999, 121, 5097–5098 CrossRef CAS
Atropisomerism in a novel hole-transport molecule for organic light-emitting diodes, J. Am. Chem. Soc., 1999, 121, 5097–5098 CrossRef CAS ; (b) S. Wakim, J. Bouchard, M. Simard, N. Drolet, Y. Tao and M. Leclerc, Organic microelectronics:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Design, synthesis, and characterization of 6,12-dimethylindolo[3,2-b]carbazoles, Chem. Mater., 2004, 16, 4386–4388 CrossRef CAS
Design, synthesis, and characterization of 6,12-dimethylindolo[3,2-b]carbazoles, Chem. Mater., 2004, 16, 4386–4388 CrossRef CAS ; (c) Y. Li, Y. Wu, S. Gardner and B. S. Ong, Novel peripherally substituted indolo[3,2-b]carbazoles for high-mobility organic thin-film transistors, Adv. Mater., 2005, 17, 849–853 CrossRef CAS
; (d) Y. Wu, Y. Li, S. Gardner and B. S. Ong, Indolo[3,2-b]carbazole-based thin-film transistors with high mobility and stability, J. Am. Chem. Soc., 2005, 127, 614–618 CrossRef CAS PubMed
; (e) P. T. Boudreault, S. Wakim, N. Blouin, M. Simard, C. Tessier, Y. Tao, M. Leclerc and V. La, Synthesis, characterization, and application of indolo[3,2-b]carbazole semiconductors, J. Am. Chem. Soc., 2007, 129, 9125–9136 CrossRef CAS PubMed
.
- For the synthesis of indolocarbazole see:
(a) B. Robinson, The Fischer indolisation of cyclohexane-1,4-dione bisphenylhydrazone, J. Chem. Soc., 1963, 3097–3099 RSC
; (b) J. I. G. Cadogan, M. Carmeron-Wood, R. K. Makie and R. I. J. Searle, The reactivity of organophosphorus compounds. Part XIX. Reduction of nitro-compounds by triethyl phosphite: A convenient new route to carbazoles, indoles, indazoles, triazoles, and related compounds, J. Chem. Soc., 1965, 4831 RSC
.
- X. H. Zhang, Z. S. Wang, Y. Cui, N. Koumura, A. Furube and K. Hara, Organic sensitizers based on hexylthiophene-functionalized indolo[3, 2-b]carbazole for efficient dye-sensitized solar cells, J. Phys. Chem. C, 2009, 113, 13409–13415 CrossRef CAS
.
- S. Cai, G. Tian, X. Li, J. Su and H. Tian, Efficient and stable DSSC sensitizers based on substituted dihydroindolo[2,3-b]carbazole donors with high molar extinction coefficients, J. Mater. Chem. A, 2013, 1, 11295–11305 RSC
.
- Y. Wu and W. Zhu, Organic sensitizers from D–π–A to D–A–π–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC
.
- S. Cai, X. Hu, G. Tian, H. Zhou, W. Chen, J. Huang, X. Li and J. Su, Photo-stable substituted dihydroindolo[2,3-b]carbazole-based organic dyes: Tuning the photovoltaic properties by optimizing the π structure for panchromatic DSSCs, Tetrahedron, 2014, 70, 8122–8128 CrossRef CAS
.
- X. Qian, L. Shao, H. Li, R. Yan, X. Wang and L. Hou, Indolo[3,2-b]carbazole-based multi-donor–π–acceptor type organic dyes for highly efficient dye-sensitized solar cells, J. Power Sources, 2016, 319, 39–47 CrossRef CAS
.
- Z. Xiao, Y. Di, Z. Tan, X. Cheng, B. Chen and J. Feng, Efficient organic dyes based on perpendicular 6,12-diphenyl substituted indolo[3,2-b] carbazole donor, Photochem. Photobiol. Sci., 2016, 15, 1514–1523 CrossRef CAS PubMed
.
- Z. Xiao, B. Chen, Y. Di, H. Wang, X. Cheng and J. Feng, Effect of substitution position on photoelectronic properties of indolo[3,2-b]carbazole-based metal-free organic dyes, Sol. Energy, 2018, 173, 825–833 CrossRef CAS
.
- J. Y. Su, C. Y. Lo, C. H. Tsai, C. H. Chen, S. H. Chou, S. H. Liu, P. T. Chou and K. T. Wong, Indolo[2,3-b]carbazole synthesized from a double-intramolecular Buchwald–Hartwig reaction: Its application for a dianchor DSSC organic dye, Org. Lett., 2014, 16, 3176–3179 CrossRef CAS PubMed
.
- H. Zhang, Z. E. Chen, J. Hu and Y. Hong, Novel metal-free organic dyes containing linear planar 11,12-dihydroindolo[2,3-a]carbazole donor for dye-sensitized solar cells: Effects of π spacer and alkyl chain, Dyes Pigm., 2019, 164, 213–221 CrossRef CAS
.
- C. Luo, W. Bi, S. Deng, J. Zhang, S. Chen, B. Li, Q. Liu, H. Peng and J. Chu, Indolo[3,2,1-jk] carbazole derivatives-sensitized solar cells: Effect of π-bridges on the performance of cells, J. Phys. Chem. C, 2014, 118, 14211–14217 CrossRef CAS
.
- W. Cao, M. Fang, Z. Chai, H. Xu, T. Duan, Z. Li, X. Chen, J. Qin and H. Han, New D–π–A organic dyes containing a tert-butyl-capped indolo[3,2,1-jk]carbazole donor with bithiophene unit as π-linker for dye-sensitized solar cells, RSC Adv., 2015, 5, 32967–32975 RSC
.
- X. C. Li, C. Y. Wang, W. Y. Lai and W. Huang, Triazatruxene-based materials for organic electronics and optoelectronics, J. Mater. Chem. C, 2016, 4, 10574–10587 RSC
and references cited therein.
- For the synthesis of triazatruxene see:
(a) J. Bergman and N. Eklund, Synthesis of 2,2′-biindolyls by coupling reactions, Tetrahedron, 1980, 36, 1439–1443 CrossRef CAS
; (b) J. Bergman and N. Eklund, Synthesis and studies of tris-indolobenzenes and related compounds, Tetrahedron, 1980, 36, 1445–1450 CrossRef CAS
; (c) M. A. Eissenstat, M. R. Bell, T. E. D’Ambra, E. J. Alexander, S. J. Daum, J. H. Ackerman, M. D. Gruett, V. Kumar, K. G. Estep, E. M. Olefirowicz, J. R. Wetzel, M. D. Alexander, J. D. Weaver, D. A. Haycock, D. A. Luttinger, F. M. Casiano, S. M. Chippari, J. E. Kuster, J. I. Stevenson and S. J. Ward, Aminoalkylindoles: Structure–activity relationships of novel cannabinoid mimetics, J. Med. Chem., 1995, 38, 3094–3105 CrossRef CAS PubMed
.
- X. Qian, Y. Z. Zhu, J. Song, X. P. Gao and J. Y. Zheng, New donor-π-acceptor type triazatruxene derivatives for highly efficient dye-sensitized solar cells, Org. Lett., 2013, 15, 6034–6037 CrossRef CAS PubMed
.
- X. Qian, L. Lu, Y. Z. Zhu, H. H. Gao and J. Y. Zheng, Triazatruxene-based organic dyes containing a rhodanine-3-acetic acid acceptor for dye-sensitized solar cells, Dyes Pigm., 2015, 113, 737–742 CrossRef CAS
.
- B. Pan, Y. Z. Zhu, D. Ye, F. Li, Y. F. Guo and J. Y. Zheng, Effects of ethynyl unit and electron acceptors on the performance of triazatruxene-based dye-sensitized solar cells, New J. Chem., 2018, 42, 4133–4141 RSC
.
- X. Qian, R. Yan, L. Shao, H. Li, X. Wang and L. Hou, Triindole-modified push–pull type porphyrin dyes for dye-sensitized solar cells, Dyes Pigm., 2016, 134, 434–441 CrossRef CAS
.
- P. Qin, P. Sanghyun, M. I. Dar, K. Rakstys, H. ElBatal, S. A. Al-Muhtaseb, C. Ludwig and M. K. Nazeeruddin, Weakly conjugated hybrid zinc porphyrin sensitizers for solid-state dye-sensitized solar cells, Adv. Funct. Mater., 2016, 26, 5550–5559 CrossRef CAS
.
- L. Zhang, X. Yang, W. Wang, G. G. Gurzadyan, J. Li, X. Li, J. An, Z. Yu, H. Wang, B. Cai, A. Hagfeldt and L. Sun, 13.6% Efficient organic dye-sensitized solar cells by minimizing energy losses of the excited state, ACS Energy Lett., 2019, 4, 943–951 CrossRef CAS
.
- L. Zhang, X. Yang, B. Cai, H. Wang, Z. Yu and L. Sun, Triazatruxene-based sensitizers for highly efficient solid-state dye-sensitized solar cells, Sol. Energy, 2020, 212, 1–5 CrossRef CAS
.
- L. Zhang, X. Yang, S. Li, Z. Yu, A. Hagfeldt and L. Sun, Electron-withdrawing anchor group of sensitizer for dye-sensitized solar cells, cyanoacrylic acid, or benzoic acid?, Sol. RRL, 2020, 4, 1900436 CrossRef CAS
.
- S. Li, X. Yang, L. Zhang, J. An, B. Cai and X. Wang, Effect of fluorine substituents on benzothiadiazole-based D–π–A′–π–A
photosensitizers for dye-sensitized solar cells, RSC Adv., 2020, 10, 9203–9209 RSC
.
- Z. Yao, X. Liao, Y. Guo, H. Zhao, Y. Guo, F. Zhang, L. Zhang, Z. Zhu, L. Kloo, W. Ma, Y. Chen and L. Sun, Exploring overall photoelectric applications by organic materials containing symmetric donor isomers, Chem. Mater., 2019, 31, 8810–8819 CrossRef CAS
.
- For the synthesis of indeno[1,2-b]indole see: D. W. Brown, M. F. Mahon, A. Ninan, M. Sainsbury and H. G. Shertzer, The Fischer indolisation reaction and the synthesis of dihydroindenoindoles, Tetrahedron, 1993, 49, 8919–8932 CrossRef CAS
.
- X. Qian, R. Yan, C. Xu, L. Shao, H. Li and L. Hou, New efficient organic dyes employing indeno[1,2-b]indole as the donor moiety for dye-sensitized solar cells, J. Power Sources, 2016, 332, 103–110 CrossRef CAS
.
- X. Qian, R. Yan, Y. Hang, Y. Lv, L. Zheng, C. Xu and L. Hou, Indeno[1,2-b]indole-based organic dyes with different acceptor groups for dye-sensitized solar cells, Dye. Pigment., 2017, 139, 274–282 CrossRef CAS
.
- R. Yan, X. Qian, Y. Jiang, Y. He, Y. Hang and L. Hou, Ethynylene-linked planar rigid organic dyes based on indeno[1,2-b]indole for efficient dye-sensitized solar cells, Dyes Pigm., 2017, 141, 93–102 CrossRef CAS
.
- P. P. Dai, Y. Z. Zhu, Q. L. Liu, Y. Q. Yan and J. Y. Zheng, Novel indeno[1,2-b]indole-spirofluorene donor block for efficient sensitizers in dye-sensitized solar cells, Dyes Pigm., 2020, 175, 108099 CrossRef CAS
.
- P. P. Dai, J. Han, Y. Z. Zhu, Y. Q. Yan and J. Y. Zheng, Orientation-dependent effects of indeno[1,2-b]indole-spirofluorene donor on photovoltaic performance of D–π–A and D–D–π–A sensitizers, J. Power Sources, 2021, 481, 228901 CrossRef CAS
.
- X. Zhou, J. Lu, H. Huang, Y. Yun, Z. Li and F. You, Dyes and pigments thieno[3,2-b]indole (TI) bridged A–π–D–π–A small molecules: Synthesis, characterizations and organic solar cell applications, Dyes Pigm., 2019, 160, 16–24 CrossRef CAS
.
- X. Zhang, Y. Cui, R. Katoh, N. Koumura and K. Hara, Organic dyes containing thieno[3,2-b]indole donor for efficient dye-sensitized solar cells, J. Phys. Chem. C, 2010, 114, 18283–18290 CrossRef CAS
.
- N. Koumura, Z. S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, Alkyl-functionalized organic dyes for efficient molecular photovoltaics, J. Am. Chem. Soc., 2006, 128, 14256–14257 CrossRef CAS PubMed
.
- Y. K. Eom, S. H. Kang, I. T. Choi, Y. Yoo, J. Kim and H. K. Kim, Significant light absorption enhancement by a single heterocyclic unit change in the π-bridge moiety from thieno[3,2-b]benzothiophene to thieno[3,2-b]indole for high performance dye-sensitized and tandem solar cells, J. Mater. Chem. A, 2017, 5, 2297–2308 RSC
.
- J. M. Ji, H. Zhou, Y. K. Eom, C. H. Kim and H. K. Kim, 14.2% efficiency dye-sensitized solar cells by co-sensitizing novel thieno[3,2-b]indole-based organic dyes with a promising porphyrin sensitizer, Adv. Energy Mater., 2020, 10, 2000124 CrossRef CAS
.
- J. M. Ji, S. H. Kim, H. Zhou, C. H. Kim and H. K. Kim, D–π–A-structured porphyrins with extended auxiliary π-spacers for highly efficient dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2019, 11, 24067–24077 CrossRef CAS PubMed
.
- S. H. Kang, S. Y. Jung, Y. W. Kim, Y. K. Eom and H. K. Kim, Exploratory synthesis and photovoltaic performance comparison of D–π–A structured Zn-porphyrins for dye-sensitized solar cells, Dye. Pigment., 2018, 149, 341–347 CrossRef CAS
.
- R. A. Irgashev, A. A. Karmatsky, S. A. Kozyukhin, V. K. Ivanov, A. Sadovnikov, V. V. Kozik, V. A. Grinberg, V. V. Emets, G. L. Rusinov and V. N. Charushin, A facile and convenient synthesis and photovoltaic characterization of novel thieno[2,3-b]indole dyes for dye-sensitized solar cells, Synth. Met., 2015, 199, 152–158 CrossRef CAS
.
- R. A. Irgashev, A. A. Karmatsky, G. A. Kim, A. A. Sadovnikov, V. V. Emets, V. A. Grinberg, V. K. Ivanov, S. A. Kozyukhin, G. L. Rusinov and V. N. Charushin, Novel push-pull thieno[2,3-b]indole-based dyes for efficient dye-sensitized solar cells (DSSCs), ARKIVOC, 2017, 34–50 Search PubMed
.
- For the synthesis of indolo[3,2-b]indole see:
(a) S. A. Samsoniya and M. V. Trapaidze, The chemistry of indoloindoles, Russ. Chem. Rev., 2007, 76, 313 CrossRef CAS
and references cited therein; (b) A. N. Grinev and S. Y. Ryabova, New method for the synthesis of indolo [3,2-b] indole derivatives, Chem. Heterocycl. Compd., 1982, 18, 153 CrossRef
; (c) P. Kaszynski and D. A. Dougherty, Synthesis and properties of diethyl 5,10-dihetera-5,10-dihydroindeno[2,1-a]indene-2,7-dicarboxylates, J. Org. Chem., 1993, 58, 5209 CrossRef CAS
; (d) L. Qiu, C. Yu, N. Zhao, W. Chen, Y. Guo, X. Wan, R. Yang and Y. Liu, An expedient synthesis of fused heteroacenes bearing a pyrrolo[3,2-b]pyrrole core, Chem. Commun., 2012, 48, 12225–12227 RSC
; (e) L. Qiu, X. Wang, N. Zhao, S. Xu, Z. An, X. Zhuang, Z. Lan, L. Wen and X. Wan, Reductive ring closure methodology toward heteroacenes bearing a dihydropyrrolo[3,2-B]pyrrole core: Scope and limitation, J. Org. Chem., 2014, 79, 11339–11348 CrossRef CAS PubMed
; (f) M. A. Truong and K. Nakano, Synthesis of benzofuro- and indolo[3,2-b]indoles via palladium-catalyzed double N-arylation and their physical properties, J. Org. Chem., 2015, 80, 11566–11572 CrossRef CAS PubMed
; (g) H. E. Ho, K. Oniwa, Y. Yamamoto and T. Jin, N-Methyl transfer induced copper-mediated oxidative diamination of alkynes, Org. Lett., 2016, 18, 2487–2490 CrossRef CAS PubMed
; (h) L. H. Leijendekker, J. Weweler, T. M. Leuther and J. Streuff, Catalytic reductive synthesis and direct derivatization of unprotected aminoindoles, aminopyrroles, and iminoindolines, Angew. Chem., Int. Ed., 2017, 56, 6103–6106 CrossRef CAS PubMed
.
-
(a) M. M. Murray, D. A. Kaisaki, W. Chang, D. A. Dougherty and P. Kaszynski, Prototypes for the polaronic ferromagnet. Synthesis and characterization of high-spin organic polymers, J. Am. Chem. Soc., 1994, 116, 8152–8161 CrossRef CAS
; (b) J. Sim, K. Do, K. Song, A. Sharma, S. Biswas, G. D. Sharma and J. Ko, D–A–D–A–D push pull organic small molecules based on 5,10-dihydroindolo[3,2-b]indole (DINI) central core donor for solution processed bulk heterojunction solar cells, Org. Electron., 2016, 30, 122–130 CrossRef CAS
; (c) I. Cho, S. K. Park, B. Kang, J. W. Chung, J. H. Kim, K. Cho and S. Y. Park, Design, synthesis, and versatile processing of indolo[3,2-b]indole-based π-conjugated molecules for high-performance organic field-effect transistors, Adv. Funct. Mater., 2016, 26, 2966–2973 CrossRef CAS
; (d) Y. Y. Lai, J. M. Yeh, C. E. Tsai and Y. J. Cheng, Synthesis, molecular and photovoltaic properties of an indolo[3,2-b]indole-based acceptor–donor–acceptor small molecule, Eur. J. Org. Chem., 2013, 5076–5084 CrossRef CAS
.
- N. Ruangsupapichat, M. Ruamyart, P. Kanchanarugee, C. Boonthum, N. Prachumrak, T. Sudyoadsuk and V. Promarak, Toward rational design of metal-free organic dyes based on indolo[3,2-b]indole structure for dye-sensitized solar cells, Dyes Pigm., 2018, 151, 149–156 CrossRef CAS
.
- C. Ruamyart, P. Chasing, T. Sudyoadsuk, V. Promarak and N. Ruangsupapichat, Double anchor indolo[3,2-b]indole-derived metal-free dyes with extra electron donors as efficient sensitizers for dye-sensitized solar cells, New J. Chem., 2021, 45, 7542–7554 RSC
.
- P. V. Santhini, V. Jayadev, S. C. Pradhan, S. Lingamoorthy, P. R. Nitha, M. V. Chaithanya, R. K. Mishra, K. N. Narayanan Unni, J. John and S. Soman, Indolo[3,2-b]indole donor-based D–π–A dyes for DSCs: Investigating the role of π-spacers towards recombination, New J. Chem., 2019, 43, 862–873 RSC
.
-
(a) P. V. Santhini, S. A. Babu, A. Krishnan, R. E. Suresh and J. John, Heteroannulation of 3-nitroindoles and 3-nitrobenzo[b]thiophenes: A multicomponent approach toward pyrrole-fused heterocycles, Org. Lett., 2017, 19, 2458–2461 CrossRef CAS PubMed
; (b) P. V. Santhini, R. Akhil Krishnan, S. A. Babu, B. S. Simethy, G. Das, V. K. Praveen, S. Varughese and J. John, One-pot MCR-oxidation approach toward indole-fused heteroacenes, J. Org. Chem., 2017, 82, 10537–10548 CrossRef CAS PubMed
.
- J. Wang, S. Liu, K. Chang, Q. Liao, S. Li, H. Han, Q. Li and Z. Li, Synergy effect of electronic characteristics and spatial configurations of electron donors on photovoltaic performance of organic dyes, J. Mater. Chem. C, 2020, 8, 14453–14461 RSC
.
- For the synthesis of benzothieno[3,2-b]indole see:
(a) T. Sugahara, K. Murakami, H. Yorimitsu and A. Osuka, Palladium-catalyzed amination of aryl sulfides with anilines, Angew. Chem., Int. Ed., 2014, 53, 9329–9333 CrossRef CAS PubMed
; (b) Y. Huang, D. Wu, J. Huang, Q. Guo, J. Li and J. You, Use of the Wilkinson catalyst for the ortho-C–H heteroarylation of aromatic amines: Facile access to highly extended π-conjugated heteroacenes for organic semiconductors, Angew. Chem., Int. Ed., 2014, 53, 12158–12162 CrossRef CAS PubMed
; (c) N. Kamimoto, D. Schollmeyer, K. Mitsudo, S. Suga and S. R. Waldvogel, Palladium-catalyzed domino C–H/N–H functionalization: An efficient approach to nitrogen-bridged heteroacenes, Chem. – Eur. J., 2015, 21, 8257–8261 CrossRef CAS PubMed
; (d) H. Huang, P. Dang, L. Wu, Y. Liang and J. Liu, Copper-catalyzed synthesis of benzo[b]thiophene-fused imidazopyridines via the cleavage of C–H bond and C–X bond, Tetrahedron Lett., 2016, 57, 574–577 CrossRef CAS
; (e) X. Zhao, Q. Li, J. Xu, D. Wang, D. Zhang-Negrerie and Y. Du, Cascade synthesis of benzothieno[3,2-b]indoles under oxidative conditions mediated by CuBr and tert-butyl hydroperoxide, Org. Lett., 2018, 20, 5933–5937 CrossRef CAS PubMed
; (f) X. H. Shan, B. Yang, J. P. Qu and Y. B. Kang, CuSO4-Catalyzed dual annulation to synthesize O, S or N-containing tetracyclic heteroacenes, Chem. Commun., 2020, 56, 4063–4066 RSC
.
-
(a) Q. Ji, J. Gao, J. Wang, C. Yang, X. Hui, X. Yan, X. Wu, Y. Xie and M. W. Wang, Benzothieno[3,2-b]indole derivatives as potent selective estrogen receptor modulators, Bioorg. Med. Chem. Lett., 2005, 15, 2891–2893 CrossRef CAS PubMed
; (b) S. A. Al-Trawneh, M. M. El-Abadelah, J. A. Zahra, S. A. Al-Taweel, F. Zani, M. Incerti, A. Cavazzoni and P. Vicini, Synthesis and biological evaluation of tetracyclic thienopyridones as antibacterial and antitumor agents, Bioorg. Med. Chem., 2011, 19, 2541–2548 CrossRef CAS PubMed
; (c) M. Ning, C. Zhou, J. Weng, S. Zhang, D. Chen, C. Yang, H. Wang, J. Ren, L. Zhou, C. Jin and M. W. Wang, Biological activities of a novel selective oestrogen receptor modulator derived from raloxifene (Y134), Br. J. Pharmacol., 2007, 150, 19–28 CrossRef CAS PubMed
.
- R. K. Konidena, K. H. Lee, J. Y. Lee and W. P. Hong, A new benzothienoindole-based bipolar host material for efficient green phosphorescent organic light-emitting diodes with extremely small efficiency roll-off, Org. Electron. Phys., Mater. Appl., 2019, 70, 211–218 CAS
.
- P. R. Nitha, V. Jayadev, S. C. Pradhan, V. Divya, C. H. Suresh, J. John, S. Soman and A. Ajayaghosh, Regulating back electron transfer through donor and π-spacer alterations in benzothieno[3,2-b]indole-based dye-sensitized solar cells, Chem. – Asian J., 2020, 15, 3503–3512 CrossRef CAS PubMed
.
- For the synthesis of tetraindole see:
(a) H. Hiyoshi, T. Sonoda and S. Mataka, Synthesis of cyclic novel symmetric cyclic indole-tetramers, Heterocycles, 2006, 68, 763–769 CrossRef CAS
; (b) F. Wang, X. C. Li, W. Y. Lai, Y. Chen, W. Huang and F. Wudl, Synthesis and characterization of symmetric cyclooctatetraindoles: Exploring the potential as electron-rich skeletons with extended π-systems, Org. Lett., 2014, 16, 2942–2945 CrossRef CAS PubMed
.
- X. Qian, H. H. Gao, Y. Z. Zhu, B. Pan and J. Y. Zheng, Tetraindole-based saddle-shaped organic dyes for efficient dye-sensitized solar cells, Dye. Pigment., 2015, 121, 152–158 CrossRef CAS
.
- H. Cheng, Y. Wu, J. Su, Z. Wang, R. P. Ghimire, M. Liang, Z. Sun and S. Xue, Organic dyes containing indolodithienopyrrole unit for dye-sensitized solar cells, Dyes Pigm., 2018, 149, 16–24 CrossRef CAS
.
- R. Bisht, V. Sudhakar, M. Fairoos, M. Kavungathodi, N. Karjule and J. Nithyanandhan, Fused fluorenylindolenine donor based unsymmetrical squaraine dyes for dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2018, 10, 26335–26347 CrossRef CAS PubMed
.
- S. Ramasamy, ScienceDirect organic photosensitizers containing fused indole- imidazole ancillary acceptor with triphenylamine donor moieties for efficient dye-sensitized solar cells, Int. J. Hydrogen Energy, 2021, 46, 3475–3483 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2021 |