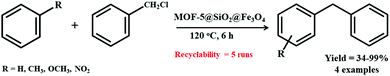Open Access Article
Open Access ArticleMagnetic metal–organic framework composites: structurally advanced catalytic materials for organic transformations
Sneha
Yadav
,
Ranjana
Dixit
,
Shivani
Sharma
,
Sriparna
Dutta
,
Kanika
Solanki
and
Rakesh K.
Sharma
 *
*
Green Chemistry Network Centre, Department of Chemistry, University of Delhi, NewDelhi-110007, India. E-mail: rksharmagreenchem@hotmail.com; Fax: +91-011-27666250; Tel: +91-011-276666250
First published on 15th February 2021
Abstract
Over the past two decades, metal–organic framework (MOF) research has been a rising star in modern materials chemistry and engineering that has contributed immensely to advancing the frontiers of science. Amalgamation of MOFs with magnetic nanostructures represents a newer promising trend that allows researchers to design complex hybrid catalytic materials with enhanced separability, reusability and activity for desired organic reactions in comparison to the individual counterparts. There are a series of meritorious examples in the literature wherein magnetic MOF composites have been successfully employed as versatile catalytic systems. Thus, the present review article aims to present a compendious account of all those magnetic MOF composite-based catalytic materials reported to boost several organic transformations, including oxidation, hydrogenation, coupling, condensation, esterification and multicomponent reactions. A progressive trend of increasing applications of these magnetic MOF composites in the area of photocatalysis and biocatalysis has also been incorporated. Further, the synthetic routes employed to date for the fabrication of magnetic MOF composites depending upon mutual interactions that exist between the magnetic nanoparticles and the MOF during unification have been illustrated in detail. It is further anticipated that this review will not only enlighten the readers about magnetic MOF composites but also assist researchers in designing novel functionalized catalysts for sustainable development.
1. Introduction
Nature is without doubt the mastermind behind all evolutionary processes. Inspired by nature's mastery to construct complex systems for performing advanced functions, researchers have been able to make impressive developments in the fabrication of porous crystalline polymers, the synthesis of which is governed by the well-defined principles of coordination chemistry. In this regard, metal–organic frameworks (MOFs), consisting of spatially assembled multifunctional organic linkers and inorganic secondary building units (metal clusters) to yield a highly organized 3-D crystalline lattice, offer massive design space for resourceful purposes.1–4 The availability of a virtually infinite choice of spacers to build MOFs offers commercial opportunities to imaginative chemists that harness their versatile chemistries in a plethora of potential applications, including water remediation, catalysis, gas storage and separation, photoluminescence, photocatalysis, sensors, drug storage and delivery, imaging, molecular sensing, gas capture and degradation of harmful chemical warfare agents.5–12Amongst them, catalysis represents one of the most distinct and dynamic arenas for MOFs. The catalytic behavior of MOFs arises from both their metal centers and organic linkers. Broadly, three different types of active site in MOFs act as reaction chambers: (i) coordinatively unsaturated metal centers that coordinate with the substrate and catalyze the reaction, (ii) the second type of site involves encapsulation or incorporation of active catalytic species inside the pores of MOF for synergistically enhanced catalysis, and (iii) functionalized ligands present in the framework to initiate the reaction.13–15 MOFs have several other remarkable features that contribute to cooperative catalysis, such as high density and spatially separated catalytic active sites. The interior of MOFs along with the pore size and void volume can also be modified for size-selective catalysis. Further, high porosity and surface area in MOFs along with permeable channels promote facile adsorption and mass transport of reactants, which permits sufficient interaction of substrates with catalytic sites located within the cavities.16–20 Besides this, the structural tunability of MOFs allows insertion of additional functional moieties via post-synthetic modifications, which are desirable for improved catalytic efficiency in a variety of organic transformations.21–23
Though MOFs constitute a burgeoning genre of porous substructures that continues to expand at a progressive rate, there are some issues related to the instability of MOFs that have always been under scrutiny by chemists, impeding their utilization in realistic applications. MOFs possess low chemical, thermal as well as hydrothermal stability, as a result of which the structural framework is more likely to decompose under acidic, basic or moist conditions.24–26 Further, MOFs synthesized via traditional routes possess feeble mechanical strength, thereby making them less competitive in comparison to commercial catalysts when utilized under harsh reactive conditions.27,28 Therefore, it is highly imperative to design stable MOF catalysts with superior features for bulk chemistry.
In this regard, researchers have come up with a promising and well-designed scientific approach that integrates MOFs with extrinsic functional systems, thereby fostering the development of an entirely newfangled category of hybrid MOF composites. Some notable review articles highlighting this aspect have been reported by several research groups.29–34 Further, the synergistic coupling of peculiar features of MOF (structural tunability, high porosity and ordered crystalline pores) with functional materials (possessing unique magnetic, catalytic, optical, thermal stability, extremely high surface area) render the newly designed composite materials with unprecedented hallmarks that simultaneously overcome the drawbacks associated with the discrete components. To date, several hybrid MOFs with sophisticated architectures have been reported, wherein magnetic nanoparticles (MNPs), graphene oxide, silica, alumina, titanium oxide, carbon nanotubes, quantum dots, polymers have been incorporated resulting in high-performance composites with enhanced chemical and physical properties.35–44
In particular, MNPs possessing large surface area to volume ratio, low toxicity, biocompatibility and high saturation magnetization value have been gaining increasing attention from the scientific community. To date, a variety of magnetic metal oxide nanoparticles (NPs), such as Fe3O4, α-Fe2O3, γ-Fe2O3, CoFe2O4, NiFe2O4, MnFe2O4, CuFe2O4 and ZnFe2O4, have been successfully synthesized and reported in the literature.45–47 The synergistic integration of MOFs with superparamagnetic metal oxide NPs not only offers a high surface area but also leads to increased thermal stability and significant enhancement in catalytic activity.48–51 Besides this, sufficiently high saturation magnetization value of magnetic MOF composite materials offers precise positioning with rapid and facile separation under the influence of an external magnet from complicated matrices with improved efficiency. Indeed, magnetic separation appears to be a robust, reliable, highly effective and effortless catalyst recovery tool as compared to the traditional time- and energy-intensive separation methods, including filtration and centrifugation.52–54 In fact, in chemical and manufacturing industries, quick separation and reusability of catalytic material are some of the most sought-after objectives from an environmental and economic point of view.55 In view of the aforementioned benefits, a myriad of magnetic MOF composites have been utilized by several research groups in many industrially significant organic transformations, including oxidation, hydrogenation, coupling, condensation, and esterification. These studies reveal that such magnetic MOF composites have been recognized as a highly versatile catalytic material for many organic reactions with remarkable efficacy in terms of excellent product yield, simple work-up procedure, and facile recoverability and reusability.56–58 Although a few remarkable reviews have been penned in the literature on magnetic MOF composites,30,31 so far none of the reviews have shed extensive light on their catalytic applications.
1.1 Scope of the review
Considering the necessity of an inclusive, encyclopedic and futuristic review on magnetic MOF composite-based catalysts, this review attempts to assimilate all the scattered research on this quintessential subject that focuses on the competent synthetic strategies for fabricating magnetic MOF composites, paying particular attention to the applications in the field of catalysis. Furthermore, a detailed discussion of literature reports based on magnetic MOF composite-catalyzed industrially significant organic transformations, such as oxidation, hydrogenation, coupling, condensation, esterification, photocatalysis and biocatalysis, has been provided. Further, significant challenges encountered in this flourishing research area and opportunities in diverse innovative applications have also been elucidated in the concluding note. It is anticipated that this review will not only act as a scientific introduction and holy grail to the newcomers but also as a reference guide for experienced investigators and scientists. Moreover, we envisage that this review will provide sparkling ideas for the design and fabrication of functional magnetic MOF composite materials that will assist researchers and chemists to come up with several new catalytic protocols that can be commercialized in the near future for sustainable development.2. Fabrication of magnetic MOF composites
2.1 Synthesis of magnetic nanoparticles (MNPs)
The synthesis of magnetic NPs represents the primary step towards the fabrication of the desired composite materials. A comprehensive literature survey reveals that magnetic nanoparticles such as Fe3O4, α-Fe2O3, γ-Fe2O3, CoFe2O4, MnFe2O4, NiFe2O4, MgFe2O4, CuFe2O4 and ZnFe2O4 have been successfully integrated with MOFs to afford magnetic MOF composites.59–68 The synthetic methodology employed while preparing magnetic nanoparticles is ascertained to have a profound effect on the shape, size, morphology and surface chemistry of the resulting nanostructures, which further escalates their utilization in diversified fields. Numerous preparative techniques, such as co-precipitation,69 solvothermal and hydrothermal,70 thermal decomposition,71 sonochemical,72 sol–gel,73 electrochemical,74 microwave-assisted,75 bio-inspired (microbial- and plant-mediated)76 and microemulsion,77 are very well described in several reviews documented in the literature.46,78–80In recent years, several sophisticated neoteric routes, including template-assisted,81 laser pyrolysis,82 nano-imprint lithography,83 continuous and microwave flow,84,85 atomic layer deposition (ALD),86 gas aggregation87 and solution combustion,88 have been intensively explored for rendering a wide array of magnetic metal oxide nanostructures, offering excellent reproducibility along with systematic control over the size and shape in comparison to traditional routes.
Template-assisted synthesis involves utilization of a pre-existing sacrificial template, such as surfactants, polymers, biomolecules, structure-directing agents, silica, carbon or inorganic frameworks, to direct the growth of nanostructures. This template is eventually removed either at high temperature or by employing acidic or basic conditions to generate the final nanostructures. Weller and co-workers designed a versatile protocol for the synthesis of iron oxide (maghemite) nanorods using iron oleate as the precursor salt while water and ethanol act as the soft template.89
Use of flow reactors has been recognized as an outstanding technique for producing high-purity nanoparticles with a short reaction duration. It offers additional benefits of uniform heating, mixing control, ease of scalability and good reproducibility for batch synthesis. In this perspective, Gao and co-workers reported a continuous-flow synthetic technique for acquiring biocompatible PEGylated Fe3O4 nanoparticles after pyrolyzing Fe(acac)3 in anisole at elevated temperatures in the presence of α,ω-dicarboxyl-terminated polyethylene glycol and oleylamine.90
Lately, an extended LaMer's mechanistic approach (involving steady-state growth conditions) has been employed by Huber and co-workers for producing highly crystalline magnetic nanoparticles by simply monitoring the reaction time period along with the volume and rate at which the iron oleate precursor was added to the reaction medium.91 The Verdaguer research group utilized a continuous laser-induced pyrolysis method devoid of surfactants and additives for preparing ultrasmall iron oxide nanoparticles possessing spheroid morphology from an iron pentacarbonyl precursor.92
Nowadays, integrated approaches like microwave-assisted solvothermal, co-precipitation-assisted hydrothermal, co-precipitation with microwave-assisted hydrothermal and template-assisted combustion have also gained significant momentum for synthesizing high-quality magnetic nanoparticles with phenomenal properties.93–96 For instance, Manukyan et al. took advantage of the template-assisted redox-combustion route for fabricating hematite nanoparticles, wherein ferric nitrate was employed as the iron precursor, ammonium nitrate as the oxidizer, glycine as fuel along with mesoporous silica as the template.97 Within a short span of time, the precursors were readily converted into ultrasmall crystalline hematite nanoparticles at elevated temperatures.
2.2 Synthetic approaches for magnetic MOF composites
Knowledge or understanding of the growth mechanisms that form the foundation of magnetic MOF composites is crucial for devising novel MOF-based heterostructures oriented towards specific applications. Therefore, various approaches that stimulate the formation of magnetic MOF composites have been reviewed in detail in this section. Depending on the type of dynamic interactions existing between the MNPs and MOFs, four approaches (layer-by-layer, embedding, encapsulation and mixing) have been deduced so far that facilitate their integration (Scheme 1). Furthermore, depending upon the synthetic strategy applied, the features of magnetic MOF composites can be tailored accordingly for particular organic transformations. Table 1 compiles some of the representative magnetic MOF composites reported in the literature and synthesized via different approaches.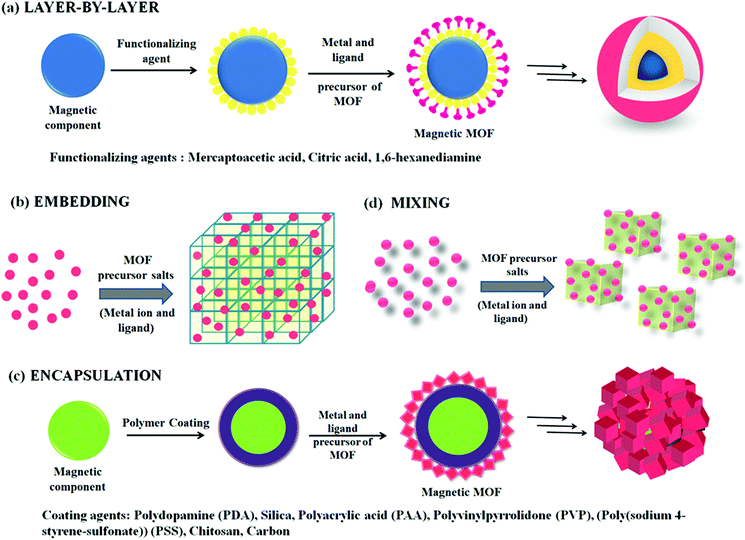 | ||
| Scheme 1 Illustration showing different approaches for the synthesis of magnetic MOF composites. (a) Layer-by-layer; (b) embedding; (c) encapsulation; and (d) mixing. | ||
| S. no. | Approach | Magnetic MOF composite | Synthetic conditions | Composite description | Application | Ref. |
|---|---|---|---|---|---|---|
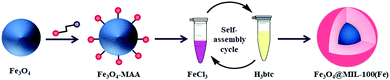
| 1 | Layer-by-layer | Fe3O4@MIL-100(Fe) | Fe3O4 + MAA + FeCl3·6H2O + H3btc + EtOH + 70 °C, 30 min for repeated cycles | Core–shell structure in which Fe3O4 core coated with MIL-100(Fe) shell | Phosphopeptide enrichment | 98 |
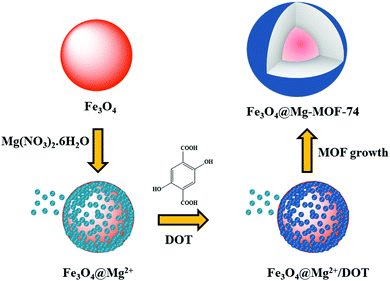
| 2 | Layer-by-layer | Fe3O4@Mg-MOF-74 | Citrate-coated Fe3O4 + Mg(NO3)2 + 2,5 dihydroxyterephthalic acid + DMF + sonicated (30 min), heated in autoclave at 125 °C for 5 h | Core–shell structure having Fe3O4 core and Mg-MOF-74 shell | Glycopeptide enrichment | 101 |
| 3 | Layer-by-layer | Fe3O4-NH2@MOF-235 | 1,6-Hexanediamine-functionalized Fe3O4 + FeCl3·6H2O + H2BDC + DMF + heated in autoclave at 80 °C for 24 h | MOF-235 shell on Fe3O4 | Adsorptive removal of benzoylurea insecticides | 102 |
| 4 | Layer-by-layer | CoFe2O4@MIL-100(Fe) | CoFe2O4 + MAA + FeCl3·6H2O + H3BTC + EtOH + 70 °C, 30 min | Core–shell microstructure in which MIL-100(Fe) layer coated on CoFe2O4 | Adsorptive removal of arsenic | 103 |
| 5 | Layer-by-layer | Fe3O4/Cu3(BTC)2 | Fe3O4 + MAA + Cu(OAc)2 + H3BTC + EtOH, stirred for 2 h | Fe3O4 covered by Cu3(BTC)2 shell | Adsorptive removal of methylene blue | 104 |
| 6 | Layer-by-layer | Magnetic PCN-250 | Citrate-modified Fe3O4 + Fe2Co(μ-3-O)(CH3COO)6 metal clusters + 3,3′,5,5′-azobenzenetetracarboxylic acid + mixed with vortex mixer + exposed to magnetic field for 1.5 h | Growth of MOF crystals around MNPs | CO2 capture and release | 107 |
| 7 | Layer-by-layer | Fe3O4@MIL-100(Fe) | Fe3O4 + MAA + FeCl3·6H2O + H3BTC + EtOH + 70 °C, 30 min for multiple cycles | Core–shell Fe3O4@MIL-100(Fe) | Catalysis (Claisen–Schmidt condensation reaction) | 108 |
| 8 | Embedding | Fe3O4/Cu3(BTC)2 | Fe3O4 nanorods + H3BTC solution of (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) DMF/EtOH + Cu(OAc)2 + 70 °C for 4 h 1) DMF/EtOH + Cu(OAc)2 + 70 °C for 4 h |
Fe3O4 nanorods interspersed within MOF matrix | Drug delivery | 109 |
| 9 | Embedding | Fe3O4@MIL-101 | Bare MIL-101(Cr) MOF + FeCl2 and FeCl3 + stirred in N2 atmosphere for 1 h + NH3 | Fe3O4 NPs incorporated into the matrix of MIL-101 | Catalysis (benzyl alcohol oxidation) | 110 |
| 10 | Embedding | Fe3O4/MIL-96(Al) | Fe3O4 + DMF–H2O + aluminium nitrate +H3BTC + refluxing conditions at 140 °C for 36 h | Spindle and granular morphology of MIL-96(Al) and Fe3O4 respectively along with the uniform embedding of Fe3O4 within MIL-96(Al) | Pb(II) adsorption | 111 |
| 11 | Embedding | Magnetic Fe-BTC | Fe3O4 + neat ground with H3BTC + ground with FeCl3 in EtOH–H2O | Fe3O4 NPs interspersed in the Fe-BTC matrix | Drug delivery | 112 |
| 12 | Embedding | Magnetic MIL-101-SO3H | Bare MIL-101(Cr) MOF + FeCl2 and FeCl3 + stirred in N2 atmosphere for 1 h + NH3 + ClSO3H in CHCl3 + stirred for 2 h | Fe3O4 NPs incorporated in the pores of MIL-101 | Catalysis (synthesis of 1,3,5-triarylbenzenes and 2,4,6-triaryl pyridines) | 113 |
| 13 | Embedding | Magnetic Mg-MOF-74 | Fe3O4 + Mg(NO3)3 + 2,5 dihydroxyterephthalic acid mixed in 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1 DMF/EtOH/H2O, heated in an autoclave at 125 °C for 20 h 1:1 DMF/EtOH/H2O, heated in an autoclave at 125 °C for 20 h |
Fe3O4 NPs incorporated within matrix of Mg-MOF-74 | CO2 capture and release | 114 |
| 14 | Embedding | Fe3O4@IRMOF-3 | Zn(NO3)2 + 2-aminoterephthalic acid (NH2-H2BDC) + Fe3O4 suspension in DMF–EtOH + PVP + heated in autoclave for 4 h at 100 °C | Fe3O4 NPs incorporated within Zn-based MOF | Drug delivery and MRI contrast agent | 115 |
| 15 | Embedding | Fe3O4@ZIF-8 | Zn(NO3)2 + 2-methylimidazole + Fe3O4, stirred for 30 min | Fe3O4 NPs embedded within ZIF-8 | Adsorption and separation of UO22+ | 116 |
| 16 | Embedding | PFe3O4@NH2-MIL-125 (Ti) where P = polymer capped Fe3O4 | PFe3O4 NPs + DMF-MeOH + titanium isopropoxide + 2-aminoterephthalic acid + heated at 160 °C for 12 h | PFe3O4 NPs present on surface and inner shell cavity of NH2-MIL-125 | Pb(II) adsorption | 117 |
| 17 | Embedding | Fe3O4@UiO-66@PPI (PPI = poly(propyleneimine)) | (a) Fe3O4 + ZrCl4 + terephthalic acid + DMF + heated at 130 °C for 12 h | UiO-66 coated on Fe3O4 NPs | Adsorption of direct and acid dyes | 118 |
| (b) Fe3O4@UiO-66 + PPI + MeOH, r.t., 2 h | ||||||
| 18 | Embedding | CoFe2O4/TMU-17-NH2 | CoFe2O4 + zinc nitrate + 2-aminoterephthalic acid and 1,4 bis(4-pyridyl)-2,3-diaza-2,3-butadiene + DMF + heated at 90 °C in autoclave for 3 days | CoFe2O4 NPs embedded inside the matrix of TMU-17-NH2 | Catalysis (dihydropyrimidine synthesis) | 119 |
| 19 | Embedding | Hollow magnetic Fe3O4@NH2-MIL-101(Fe) | Hollow Fe3O4 + ferric chloride + 2-aminoterephthalic acid + stirred at 70 °C for 4 h | Fe3O4 incorporated in NH2-MIL-101(Fe) | Phosphate removal | 120 |
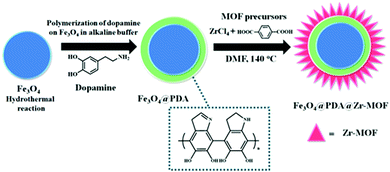
| 20 | Encapsulation | Fe3O4@PDA@Zr-MOF | Fe3O4 + PDA + DMF + ZrCl4 + H2BDC, stirred at 140 °C, 20 min | Core–shell–shell morphology in which Fe3O4 core covered with intermediate PDA shell and outer Zr-MOF shell | Phosphopeptide enrichment | 133 |
| 21 | Encapsulation | Fe3O4@SiO2@Zr-MOF | Fe3O4 + TEOS + DMF + ZrCl4 + 2-aminoterephthalic acid + stirred at 120 °C for 6 h | Core–shell architecture with Fe3O4 core and UiO-66-NH2 shell | Removal of pharmaceutical compounds | 134 |
| 22 | Encapsulation | ZIF-8@CoFe2O4 | CoFe2O4 + PVP + MeOH + 2-methylimidazole + Zn(NO3)2 + reflux, 70 °C for 20 h | Core–shell ZIF-8@CoFe2O4 having CoFe2O4 core and ZIF-8 shell | Adsorptive removal of Congo Red and Basic Red-2 dye | 136 |
| 23 | Encapsulation | Fe3O4/Fe-MIL-101 | PAA-modified Fe3O4 + PVP + FeCl3·6H2O + H2BDC + DMF + stirred at 110 °C for 24 h | Fe3O4 encapsulated within Fe-MIL-101 MOF | Oxidation of alcohols and epoxidation of olefins | 137 |
| 24 | Encapsulation | Fe3O4@PDA@[Cu3(btc)2] | Fe3O4 + PDA + EtOH + Cu(OAc)2 + H3btc, stirred at 70 °C | Fe3O4@PDA wrapped with small crystals of Cu3(btc)2 layer | Carrier for enzyme immobilization | 138 |
| 25 | Encapsulation | Fe3O4@PDA@UiO-66-NH2 | Fe3O4 + PDA + DMF + ZrCl4 + NH2-H2BDC, 120 °C, 45 min | UiO-66-NH2 shell grafted on Fe3O4@PDA (core–shell) microspheres | Glycopeptide and phosphopeptide enrichment | 139 |
| 26 | Encapsulation | MagNP@PDA@ZIF-8 | Fe3O4 + PDA + MeOH + Zn(NO3)2 + 2-methylimidazole, r.t., 12 h | ZIF-8 shell deposited on MagNP@PDA (core–shell) NPs | 4-Nitrophenol reduction and methylene blue degradation | 141 |
| 27 | Encapsulation | Fe3O4@SiO2@UiO-67 | Fe3O4 + TEOS + DMF + ZrCl4 + biphenyl-4,4′-dicarboxylic acid, heated at 120 °C for 24 h in autoclave | Core–shell–shell morphology having Fe3O4 core, SiO2 as intermediate shell and UiO-67 as outer shell | Organophosphorous pesticide removal | 142 |
| 28 | Encapsulation | Fe3O4@SiO2@MOF | Fe3O4 + TEOS + DMF + ZrCl4 + H2BDC + 1,4-phenylenebisboronic acid, stirred at 120 °C, 4 h | Zr-MOF coated on Fe3O4@SiO2 (core–shell) | Glycopeptide and phosphopeptide enrichment | 143 |
| 29 | Encapsulation | Fe3O4@MIL-101(Fe) | PVP-modified Fe3O4 + FeCl3·6H2O + H2BDC + DMF + heated in autoclave at 110 °C for 20 h | Core–shell structure with Fe3O4 as core and MIL-101(Fe) shell | Catalytic oxidation of Acid Orange 7 | 144 |
| 30 | Encapsulation | Fe3O4@PVP-PEI@MOF-PBA | Fe3O4 + PVP + PEI (polyetherimide) + Fe(NO3)3 + 1,4-phenylenebisboronic acid (PBA) + CH3CN + DMF + 120 °C, 2 h | MOF shell on PVP–PEI coated Fe3O4 NPs | Glycoprotein capture and release | 145 |
| 31 | Encapsulation | Fe3O4@P4VP@MIL-100(Fe) | Fe3O4 + P4VP + FeCl3·6H2O + H3BTC, 70 °C, 30 min for 20 cycles | MIL-100(Fe) shell on Fe3O4@P4VP (core–shell) microspheres | Catalysis (selective oxidation of alcohols) | 146 |
| 32 | Encapsulation | Fe3O4@ZIF-8 | Fe3O4 +PSS + MeOH + Zn(NO3)2 + 2-methylimidazole + stirred at 50 °C for 3 h | Uniform ZIF-8 shell on Fe3O4 microspheres | Catalysis (Knoevenagel condensation reaction) | 147 |
| 33 | Encapsulation | Fe3O4/Cu3(btc)2 | PAA-modified Fe3O4 + Cu(OAc)2 + H3btc + 70 °C, 60 min for 10 cycles | Cu3(btc)2 shell on Fe3O4 core | Catalysis (oxidation of alcohols and olefins) | 148 |
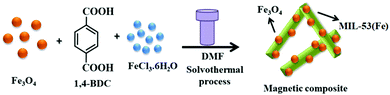
| 34 | Mixing | Fe3O4/MIL-53(Fe) | Fe3O4 + DMF + FeCl3 + H2BDC + 150 °C, 12 h in autoclave | Fe3O4 anchored on MIL-53(Fe) microrods | Photocatalytic degradation of rhodamine B and p-nitrophenol | 149 |
| 35 | Mixing | HPU-13@Fe3O4 | Fe3O4 + CuSO4 + 2-(5-pyridin-4-yl-2H-[1,2,4]triazol-3-yl)-pyrimidine EtOH:H2O, 160 °C for 72 h in autoclave | Fe3O4 coated on rod-like crystals of HPU-13 | Cr(VI) removal | 150 |
| 36 | Mixing | Fe3O4/MIL-100(Fe) | MIL-100(Fe) + FeCl3 + ethylene glycol + 200 °C, 10 h in autoclave | MIL-100(Fe) decorated with Fe3O4 NPs | Adsorptive removal of rhodamine B | 151 |
| 37 | Mixing | NH2-MIL-88B(Fe)/Fe3O4 | FeCl3 + DMF + NH2-H2BDC + Fe3O4 + 170 °C, 24 h in autoclave | Fe3O4 deposited on NH2-MIL-88B(Fe) possessing bipyramidal hexagonal prism like morphology | Enzyme immobilization | 152 |
| 38 | Mixing | MOF-199/Fe3O4 | Fe3O4, (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) DMF 1) DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH + Cu(OAc)2 + H3BTC + triethylamine, 5 h EtOH + Cu(OAc)2 + H3BTC + triethylamine, 5 h |
Fe3O4 NPs attached on surface of MOF-199 | Neonicotinoid insecticide removal | 153 |
| 39 | Mixing | CoFe2O4/Mn-BDC | CoFe2O4 + DMF-MeOH + MnCl2 + H2BDC + 120 °C, 24 h in autoclave | Mn-BDC microflakes decorated with CoFe2O4 NPs | Catalysis (multicomponent click reaction) | 154 |
Another well-known and illustrious Cu3(BTC)2 MOF with magnetic functionality was fabricated by Qiu and co-workers by utilizing MAA as a functionalizing agent.99 Firstly, the synthesized MAA-functionalized Fe3O4 nanospheres were alternatively dispersed in an ethanolic solution of copper acetate and H3BTC at 25 °C. In this report, the authors demonstrated that the thickness of the MOF shell has a profound and direct relationship with the assembly cycle number; therefore, it is essential to control the thickness of the shell precisely while synthesizing core–shell heterostructures. Fig. 1 depicts the TEM images of core–shell structured Fe3O4@[Cu3(BTC)2] obtained after different assembly cycles. The authors further stated that functionalization of ferrite NPs before the assembly process was crucial as grafting of carboxylic moieties using MAA on ferrite NPs instigated the binding of copper ions to the COO– groups, which later bind with the BTC units.
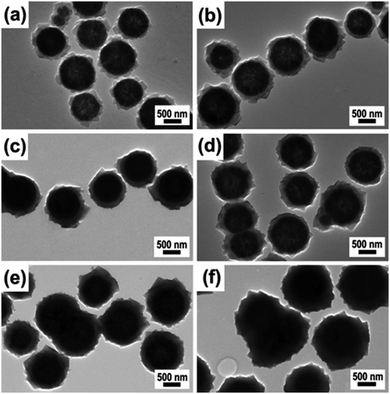 | ||
| Fig. 1 TEM images of core–shell Fe3O4@[Cu3(BTC)2] architecture obtained after (a) 10, (b) 20, (c) 25, (d) 30, (e) 40, and (f) 50 assembly cycles. Reproduced with permission from ref. 99. Copyright 2012 Royal Society of Chemistry. | ||
Literature reports suggest that citric acid is another impressive functionalizing agent that has been employed recurrently by various research groups for modifying the surface of MNPs. Recently, Lv and his research group explored the fabrication of a HKUST-1@Fe3O4-based bi-enzyme system consisting of glucose oxidase (GOx) and horseradish peroxidase (HRP) for cascade catalysis.100 Citric acid-functionalized ferrite NPs were prepared and dispersed in an aqueous solution of copper acetate and H3BTC alternatively to form HKUST-1@Fe3O4 NPs by stirring at room temperature for 8 h. By following the aforementioned procedure, some more layers of HKUST-1 were introduced on citric acid-modified ferrite NPs.
In addition, fabrication of another magnetic MOF composite was beautifully illustrated by Zhou's research group, wherein carboxyl moiety-enriched Fe3O4 nanospheres were coated with a Mg-MOF-74 shell consisting of magnesium ions and a 2,5-dihydroxyterephthalate (DOT) ligand to form Fe3O4@Mg-MOF-74 (Scheme 3).101 Plentiful carboxyl functionalities existing on the surface of ferrite NPs served as an initiator for the subsequent growth of Mg-MOF-74, which was dispersed alternatively in a dimethylformamide (DMF) solution of magnesium ions and 2,5-dihydroxyterephthalic acid (DOT) for 30 min.
In a different publication, Liang et al. constructed a fusiform-like Fe3O4-NH2@MOF-235 composite for the adsorption of insecticides.102 A one-pot in situ solvothermal approach entailing concomitant addition of FeCl3, ethylene glycol and 1,6-hexanediamine was utilized to obtain NH2-functionalized Fe3O4 NPs. The subsequent addition of MOF precursors, i.e., ferric chloride and benzene-1,4-dicarboxylic acid or H2BDC, to Fe3O4-NH2 NPs under solvothermal conditions accelerated the chelation of NH2 functionalities with Fe3+ ions that further merged with H2BDC to yield a shell of MOF-235.
This layer-by-layer approach has also been successfully exploited for fabricating several other magnetic MOF composites, including CoFe2O4@MIL-100(Fe), Fe3O4/ZIF-8@GOx, Fe3O4@MIL-100(Fe)/βCD, and Fe3O4@UiO-66, that further find significant applications in several emerging fields.103–108
The fabrication of a novel magnetic MOF composite, Fe3O4/Cu3(BTC)2, by incorporating Fe3O4 nanorods within the nanocrystals of a 3D Cu3(BTC)2 MOF was explored by Qiu and co-workers.109 In order to achieve the aforesaid objective, the authors explored the possibility of designing two different Fe3O4/Cu3(BTC)2 composites with dissimilar concentrations of ferrite nanorods. For this, an ethanolic suspension of Fe3O4 nanorods (10 mL and 5 mL, designated as 1 and 2, respectively) was initially added into a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF/ethanol solution of H3BTC under heating at 70 °C after which aqueous copper acetate was introduced into the above system and finally heated at 70 °C for 4 h. Microscopic techniques such as TEM (Fig. 2) unveiled the embedding of Fe3O4 nanorods within the MOF matrix.
1 DMF/ethanol solution of H3BTC under heating at 70 °C after which aqueous copper acetate was introduced into the above system and finally heated at 70 °C for 4 h. Microscopic techniques such as TEM (Fig. 2) unveiled the embedding of Fe3O4 nanorods within the MOF matrix.
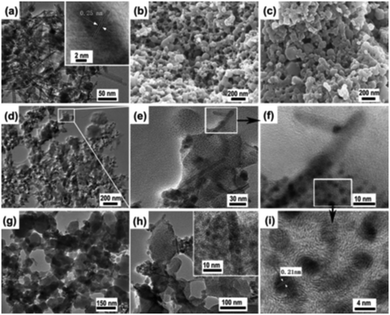 | ||
| Fig. 2 TEM images of Fe3O4 NPs (a), Fe3O4/Cu3(BTC)2 1 (d–f and i) and 2 (g and h), and SEM images of 1 (b) and 2 (c). Reproduced with permission from ref. 109. Copyright 2011 Royal Society of Chemistry. | ||
Quite astonishingly, the protocols reported till now for magnetic MOF composites involve the addition of MNPs to an aqueous solution of MOF salts, wherein in situ formation of the MOF takes place. Nevertheless, Saikia et al. for the very first time prepared bare MIL-101(Cr) MOF and then added this MOF into an aqueous solution containing FeCl2 and FeCl3 salts to obtain the magnetic nanocomposite Fe3O4@MIL-101.110 Furthermore, MIL-101(Cr) possessing a structurally massive surface area enables superior dispersion and incorporation of magnetite NPs on its surface.
Mehdinia et al. fabricated a magnetic Fe3O4/MIL-96(Al)-based framework trap for the adsorption of Pb2+ from aqueous media.111 A hydrothermal approach was employed wherein pre-formed Fe3O4 NPs were mixed in DMF–H2O solution containing MOF salts, i.e. aluminium nitrate and H3BTC, under refluxing conditions. The authors explicated two probable mechanisms through which Fe3O4 was embedded within the MIL-96(Al) (Fig. 3A). The first mechanism was based on the fact that Al3+, having Lewis acidic character with vacant d orbitals, effectively formed coordination bonds with the OH groups of the Fe3O4 NPs and further nucleation resulted in growth of MIL-96(Al) crystals around the Fe3O4. The alternative mechanistic approach encompassed coordination of H3BTC ligand with Fe3+ sites on Fe3O4 and heat-impelled refluxing conditions drove the growth of MIL-96(Al) crystals all around the Fe3O4. The latter mechanism was further supported by the emergence of an Fe–O–C peak in the high-resolution XPS spectrum ascribed to bond formation between oxygen atoms of the carboxylic moieties of the ligand and Fe3+ ions of the Fe3O4. Additionally, TEM and SEM analysis (Fig. 3B) also divulged the spindle and granular morphology of MIL-96(Al) and Fe3O4, respectively, along with the uniform embedding of Fe3O4 in MIL-96(Al).
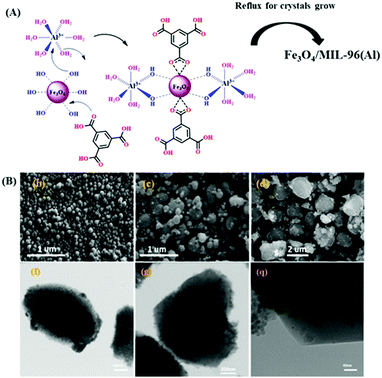 | ||
| Fig. 3 (A) Figure depicting probable mechanisms involved in the embedding of Fe3O4 in MIL-96(Al). (B) SEM micrographs of (b) Fe3O4 NPs and (c) MIL-96(Al). FE-SEM image of (d) Fe3O4/MIL-96(Al) and (f, g and q) TEM micrographs of Fe3O4/MIL-96(Al). Reproduced with permission from ref. 111. Copyright (2018) American Chemical Society. | ||
Bellusci and his research group illustrated the earliest example of a magnetic MOF composite fabricated using a mechanochemical approach.112 The first step towards accomplishing the desired objective involved the synthesis of Fe3O4 NPs from ferric chloride and sodium hydroxide using sodium chloride as a dispersing agent via a ball milling method. Afterwards, the authors smartly employed a neat grinding method for modifying the surface of the MNPs using H3BTC ligand. Later on, a liquid-assisted milling method was applied in which ferric chloride was ground with water and ethanol to get the final magnetic MOF (Fe3O4@Fe-BTC) composite. In this way, magnetic iron oxide NPs were easily embedded in the framework matrix of iron(III) carboxylate.
Similarly, numerous other magnetic MOF composites have been synthesized utilizing this approach.113–120
Very recently, a fascinating magnetic MOF composite exhibiting unprecedented adsorption efficacy towards salicylic acid and acetylsalicylic acid was presented by Yang and co-workers.134 A magnetic UiO-66-NH2 MOF composite with Zr as the metal node and 2-aminoterephthalic acid as the organic linker was designed wherein the elementary step involved the coating of Fe3O4 NPs with a layer of silica using TEOS. Thereafter, Fe3O4@SiO2 and ZrCl4 were ultrasonically dispersed in DMF and mixed with a separately prepared DMF solution of 2-aminoterephthalic acid. The subsequent heating of the resulting solution with constant stirring at 120 °C for 6 h yielded Fe3O4@SiO2@Zr-MOF. The protective coating of silica not only protected the magnetic core from undergoing corrosion but also promoted the controlled growth of Zr-MOF onto Fe3O4via chelating interactions between the –OH groups of the silica and the Zr4+ ions of the MOF.
In an attempt to provide an encouraging platform for targeted delivery of anticancer drugs, Sahu and co-workers nicely delineated the preparation of a nanoscale MOF that incorporated fabrication of MOF and folic acid in a one-pot process.135 A strikingly different methodology of combining IRMOF-3 and chitosan-coated ferrite NPs was presented here. The coating of Fe3O4 NPs with OCMC (O-carboxymethyl chitosan) not only prevented their agglomeration but also rendered uniform growth of the MOF shell. In the final step, folic acid and IRMOF-3 were integrated by mixing zinc nitrate, NH2-H2BDC (or NH2-BDC) in DMF–ethanol solution containing PVP, folic acid and OCMC-coated Fe3O4 NPs, and finally heated in an autoclave at 100 °C for 6 h.
Zhang et al. prepared a core–shell MOF composite for the solid-phase extraction of Congo Red and Basic Red 2 dyes, wherein cobalt ferrite NPs were enclosed within zeolitic imidazolate framework (ZIF)-8 shells.136 However, prior to covering with the MOF shell, the cobalt ferrite NPs were initially coated with a layer of PVP polymer and then dispersed in the methanolic solution of zinc nitrate and 2-methylimidazole with further refluxing at 70 °C for 20 h to form the ZIF-8@CoFe2O4 core–shell magnetic composite (wherein CoFe2O4 acted as the core component and ZIF-8 behaved like a shell).
Wang and co-workers demonstrated the fabrication of a magnetic Fe3O4/Fe-MIL-101 composite via a two-step encapsulation process.137 In order to accomplish the desired objective, the authors prepared PAA-modified magnetic iron oxide NPs using a facile co-precipitation technique that prevented their agglomeration. In the next step, PAA-modified Fe3O4 NPs acted as seeds and were coated with PVP to ensure continuous and smooth growth of MOF crystals. The encapsulation strategy generated highly stable as well as greatly dispersed Fe3O4 NPs efficiently embodied in the MOF heterostructure.
Working on similar lines, several research groups have synthesized Fe3O4@PDA@Cu3(BTC)2, Fe3O4@P4VP@MIL-100(Fe), Fe3O4@PDA@UiO-66-NH2, Fe3O4@SiO2@UiO-67, Fe3O4@MIL-101(Fe), Fe3O4@PVP-PEI@MOF-PBA, etc. using an encapsulation strategy and their efficacy has been successfully explored in the field of catalysis, detection, sensing and water remediation.138–148
Likewise, Wang and co-workers synthesized a novel magnetic HPU-13@Fe3O4 composite by mixing of Fe3O4 with CuSO4 and 2-(5-pyridin-4-yl-2H-[1,2,4]triazol-3-yl)-pyrimidine as the organic linker for the selective adsorption of Cr(VI) from aqueous solution.150 The developed composite appeared in rod-like crystals of HPU-13 having uniformly dispersed Fe3O4 NPs (Fig. 4).
 | ||
| Fig. 4 SEM micrographs of HPU-13@Fe3O4. Reproduced with permission from ref. 150. Copyright (2018) American Chemical Society. | ||
3. Characterization
Magnetic MOF composites can be characterized by various physicochemical analytical techniques, such as powder X-ray diffraction (PXRD), nitrogen adsorption–desorption isotherms, Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), vibrating sample magnetometry (VSM), inductively coupled plasma optical emission spectroscopy (ICP-OES), single-crystal X-ray diffraction, nuclear magnetic resonance (NMR) and aqueous stability tests. PXRD bestows significant knowledge related to the chemical composition, crystallography and phase purity of the prepared composites. The phase purity of MOFs can also be effectively validated by matching the XRD pattern recorded experimentally with the simulated pattern derived from single-crystal X-ray data. Nitrogen (N2) adsorption–desorption isotherms are used to determine the porosity, surface area, intrinsic pore volume as well as apparent pore size distributions of MOFs and magnetic materials. FTIR represents an indispensable technology to gain substantial information about the functional moieties present in the MOFs and MNPs. Besides, the changes and the occurrence of certain stretching frequencies in the FTIR spectra of the magnetic MOF composites help to deduce the type of bonding or the interactions that exist between the MOF and the magnetic material. TGA is performed to determine the thermal stability of the MOF and that of the MNPs too. Magnetic MOF composites offer certain applications in the field of catalysis and storage; therefore, their aqueous stability is also of prime importance. SEM is considered to be a wonderful technique for measuring various properties of magnetic MOF composites, such as their crystal size, morphology and elemental composition. Furthermore, SEM equipped with EDS reveals both quantitative as well as qualitative information about the involved metals and their distribution. VSM analysis is carried out to acquire information related to the magnetic characteristics of the resulting magnetic MOF composites. ICP-OES is executed to analyze the metal content and pristine nature of the sample. NMR spectroscopy is carried out to determine the purity of the MOF and the ratio of the linkers/ligands involved in its synthesis. Single-crystal XRD is considered to be one of the highly sophisticated techniques to unambiguously determine the structure of a MOF. It gives absolute structural information about the material.4. Applications of magnetic MOF composites
4.1 Catalytic systems for various organic transformations
Catalysis has been long considered a driver of innovation and progression and even today it continues to preserve its conspicuous position oriented towards fostering development in contemporary society. It is a foundation pillar of concrete modernization and holds significant potential for improvising antiquated or mature technology, thereby enhancing economic viability in chemical manufacturing industries along with ameliorating environmental impact.155,156 Moreover, with the advent of a novel class of recyclable heterogeneous materials, namely metal–organic frameworks, catalysis has reached new heights owing to their fascinating properties. In recent years, the role of supported MOFs has received substantial interest from the scientific fraternity for mediating a broad array of organic transformations. Several studies have been reported wherein MOFs have been effectively utilized as catalysts for a wide variety of reactions.18,157–160 Nevertheless, separation of the catalyst from reaction mixture post-catalysis requires additional processing steps, as a consequence of which core–shell structured MOF composites bearing magnetic functionality have particularly grabbed the attention of material researchers. The combination of MOFs and magnetic materials is of huge interest because the flexibility of these cooperative functionalities allows their precise positioning and collection and also enables various applications within miniaturized platforms. Apart from that, superparamagnetic behavior facilitates rapid separation of catalysts from the solution media via an external static magnetic field. Driven by the constant demand for energy conservation, there has been an upsurge in interest towards the use of magnetic MOF composites as catalysts for diverse industrially challenging and demanding reactions (Fig. 5). | ||
| Fig. 5 Role of magnetic MOF composites as efficient catalysts for diverse industrially significant transformations. | ||
Nowadays, aerobic transition-metal-catalyzed selective oxidation of alcohols has been gaining huge momentum in fine and industrial organic chemistry. To date, several transition metal-based homogeneous catalysts, such as Cu, Mn, Co and Fe, have been utilized for the oxidation reactions.161,162 However, the difficulties in separation and recoverability hamper their large-scale applicability in industrial processes. To address these issues, Saikia et al. fabricated a Fe3O4@MIL-101(Cr) hybrid for carrying out the solvent-free oxidation of benzyl alcohol to benzaldehyde in the presence of tert-butyl hydroperoxide (TBHP).110 The experimental results revealed that the developed catalytic system exhibited 44% conversion and 98% selectivity towards the formation of benzaldehyde. In fact, a wide range of substituted benzyl alcohols were efficiently converted into the substituted benzaldehyde moieties. Moreover, the catalyst being magnetic in nature was facilely recovered and recycled for three consecutive runs without undergoing any deterioration and deactivation.
The concept of magnetic MOF composites was very well utilized by the Wang research group, wherein they fabricated a Fe3O4/Fe-MIL-101 catalyst for the oxidation of alcohols and epoxidation of olefins.137 Oxidation of alcohols was successfully executed in the presence of an O2 balloon as the sole oxidant, TEMPO as the co-catalyst and KNO2 as an additive. Additionally, the resulting material unveiled impressive activity as a wide series of primary, secondary, acrylic and allylic alcohols underwent reaction smoothly under the optimized reaction conditions with excellent conversion percentage and selectivity. Strikingly, it was observed that the polarity of the solvent significantly affected the conversion percentage during the oxidation of alcohols. Apart from this, the designed catalyst exhibited superior performance in the epoxidation of olefins. Indeed, cyclic olefins such as cyclopentene, cyclooctene and cyclododecene were efficaciously transformed into the preferred epoxides under ambient reaction parameters, i.e., at room temperature, and with short reaction times. Overall, the developed catalyst generated the desired products with high conversion percentages along with excellent selectivity.
Core–shell-structured magnetic MOF composites have emerged as an attractive platform for accomplishing various organic reactions. Recently, Yang and co-workers proposed an efficient strategy for developing a core–shell Fe3O4/Cu3(BTC)2 catalyst, which displayed tremendous performance in the TEMPO-mediated aerobic oxidation of alcohols at 75 °C (Fig. 6).148 Fortunately, owing to the nanosized porous structure of the multifunctional MOF, effective adsorption of the organic reagents occurred, which intensified the contact between the active Cu2+ sites and the reactant molecules, thereby refining the escalated catalytic potential of the designed material. Some of the key intriguing characteristics of the developed methodology were negligible leaching of active species, easy magnetic recoverability of the catalyst along with good recyclability for five successive runs.
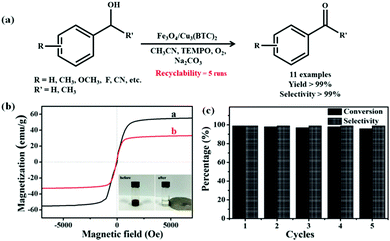 | ||
| Fig. 6 (a) Scheme depicting Fe3O4/Cu3(BTC)2-catalyzed oxidation of alcohols. (b) VSM curves of Fe3O4 and Fe3O4/Cu3(BTC)2 at 25 °C. (c) Recyclability runs of Fe3O4/Cu3(BTC)2 catalyst in the oxidation of benzyl alcohol. Reproduced with permission from ref. 148. Copyright (2016) John Wiley and Sons. | ||
Another important investigation was made by Shu and co-workers.146 The authors designed a core–shell Fe3O4@P4VP@MIL-100(Fe) hybrid and evaluated its catalytic potential in the molecular O2-assisted selective oxidation of alcohols (Scheme 6). The intricately designed catalyst uncovered impressive potential in the concerned oxidation reaction. The hybrid catalyst showed promising prospects of facile recovery and exceptional recyclability for ten consecutive cycles without any considerable deterioration in its structural integrity and activity.
 | ||
| Scheme 6 Illustration showing aerobic oxidation of aromatic alcohols catalyzed by Fe3O4@P4VP@MIL-100(Fe). | ||
Selective oxidation of saturated C–H bonds also symbolizes a major organic transformation in the fine and chemical industries as it invariably leads to the generation of numerous oxidized products that find extensive applications in the agricultural and domestic sectors. One of its key reactants is benzylic C–H bonds, i.e., the benzylic hydrocarbon can be converted into corresponding alcohols, aldehydes or ketones, which serve as resourceful intermediates in the production of versatile generic drugs, pharmaceuticals, dyes and solvents. However, the extremely high activation energy of the inert sp3 C–H bond has been identified as the foremost reason why its oxidation is quite challenging at lower temperatures. Numerous homogeneous catalysts have been reported in the literature for the oxidation of benzylic C–H bonds; however, they suffer from difficulty in the recycling of the catalyst. Besides, several solid heterogeneous catalysts based on mesoporous MnCeOx, Au–Pd/C, CuFe/γ-Al2O3, and MIL-101(Cr) have been availed for the concerned reaction.163–166 Nevertheless, these protocols are accompanied with high energy inputs, elevated reaction temperature and O2 pressure, longer reaction times along with unrestrained selectivity and low conversion percentage. In view of the above-mentioned problems, Wang and co-workers presented the fabrication route for a hybrid magnetic MOF composite for the selective oxidation of benzylic C–H bonds using TBHP as the oxidant (Scheme 7).48 The idea of covalently binding the active catalytic MOF nanostructure onto the magnetic component played a pivotal role in this report. The authors directly synthesized MOF nanolayers onto carboxyl-functionalized MNPs. Initially, carboxyl-functionalized MNPs capped with PVP were synthesized. Eventually, addition of copper ions and H3BTC to the solution mixture led to the in situ generation of a HKUST framework onto ferrite NPs. The developed chemical bonding between the copper ions and carboxylic groups was perceived to be the premier reason behind the MOF nanolayers remaining intact on the MNPs and also the integrity of the nanomaterial being maintained for repetitive catalytic cycles. The synthesized magnetic HKUST-1@Fe3O4 core–shell nanomaterial exhibited incredible catalytic performance towards benzylic hydrocarbon oxidation with 99% conversion and selectivity. The diligently constructed material proved to be a stepping stone in the designing of MOF-based catalysts for continuous magnetic fluid bed purposes.
During the past few years, selective oxidation of benzylic C–H bonds has reportedly been carried out using peroxide oxidants like TBHP and hydrogen peroxide (H2O2). Nonetheless, these oxidizing agents are toxic and corrosive to equipment. Besides, tert-butanol formed after the utilization of TBHP gets left over in the reaction and demands further separation. Thus, use of molecular oxygen as a cheap and clean oxidant is highly demanded from an economic and environmental point of view. In this regard, Wang and Gao along with their research group rationally designed a Cu–CuFe2O4@HKUST-1 heterostructure for carrying out selective oxidation of benzylic C–H bonds (Scheme 8).167 For this, Cu–CuFe2O4 NPs were initially synthesized using a solvothermal approach. The Cu(0) part of the Cu–CuFe2O4 NPs acted as the key source of copper, which after the addition of an ethanolic solution of H3BTC got readily transformed into a MOF shell.
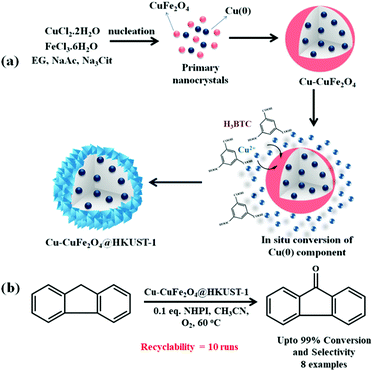 | ||
| Scheme 8 (a) Scheme depicting formation mechanism of Cu–CuFe2O4@HKUST-1 heterostructure. (b) Cu–CuFe2O4@HKUST-1-catalyzed selective oxidation of benzylic C–H bond. | ||
Morphological and elemental characterization of the hybrid heterostructure was successfully accomplished through TEM and HAADF-STEM analysis (Fig. 7). Notably, the resulting multifaceted heterostructure possessing the integrated benefits of the Cu–CuFe2O4 core and the MOF shell displayed distinct potential in the molecular O2 and N-hydroxyphthalimide (NHPI)-aided selective oxidation of benzylic C–H bonds using fluorenone as a representative substrate. Conversion as well as selectivity of 99% were certainly achieved with acetonitrile as the solvent at 60 °C. Furthermore, it was disclosed that during the catalytic procedure, the Cu–CuFe2O4 component delivered multitudinous active sites while the MOF shell, i.e., HKUST-1, performed the role of attracting and preconcentrating the molecular oxygen. Besides, the protocol was also extended for executing selective oxidation of numerous substituted benzyl C–H bonds. In addition, the magnetic core imparted sufficient stability and durability to the developed catalyst, as a result of which the catalyst was applied for 10 successive runs and did not exhibit any marked deterioration in catalytic efficacy.
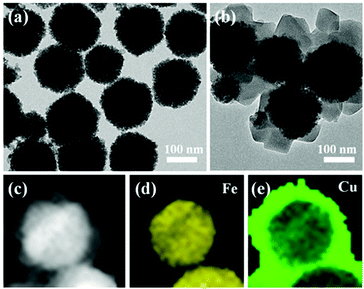 | ||
| Fig. 7 TEM micrographs of (a) Cu–CuFe2O4 and (b) Cu–CuFe2O4@HKUST-1. HAADF-STEM micrograph of (c) Cu–CuFe2O4@HKUST-1 and EDS mapping of (d) Fe and (e) Cu in the heterostructures. Reproduced with permission from ref. 167. Copyright (2017) American Chemical Society. | ||
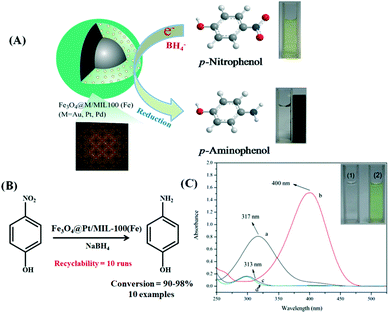 | ||
| Fig. 8 (A) Diagrammatic and photographic presentation showing Fe3O4@M/MIL-100(Fe) (M = Au, Pt, Pd)-catalyzed reduction of nitrophenol. (B) Fe3O4@Pt/MIL-100(Fe)-mediated reduction of p-nitrophenol to p-aminophenol. (C) UV-visible spectra of p-nitrophenol before (a) and after (b) addition of NaBH4. (c) Spectrum obtained after complete reduction using catalyst and (d) spectrum of pure p-aminophenol. Reproduced with permission from ref. 177. Copyright 2014 Royal Society of Chemistry. | ||
Another Au–Fe3O4@MIL-100(Fe)-based hybrid magnetic MOF composite for executing NaBH4-assisted 4-nitrophenol reduction was developed by Zhu et al.178 In the synthesized composite, Au–Fe3O4 acted as the superparamagnetic core and Au NPs were sandwiched betwixt the magnetic component and the porous MOF shell. As manifested from TEM analysis (Fig. 9), Au NPs were uniformly dispersed on ferrite NPs with subsequent development of a porous MOF shell outside the Au–Fe3O4 particles. The synthesized porous magnetic material displayed exceptional activity even at room temperature. Furthermore, a time-dependent UV-visible study as well as the related spectra were obtained for examining the reduction kinetics. Moreover, results acquired from the extended X-ray absorption fine structure (XAFS) revealed MIL-100(Fe) to have higher or more prominent acidic character as a consequence of significant electron-donation phenomenon occurring from the MIL-100(Fe) to the Au NPs. The aforesaid process further enabled hydrogen molecule activation via Lewis acid–base interactions and facilitated faster production of 4-aminophenol.
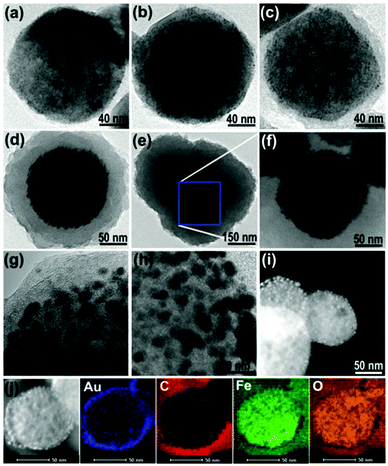 | ||
| Fig. 9 TEM micrographs of (a) Au–Fe3O4 and core–shell Au–Fe3O4@MIL-100(Fe) obtained after (b) 5, (c) 10, (d) 20, and (e and f) 30 cycles. HRTEM and HAADF-STEM images of Au–Fe3O4@MIL-100(Fe) after (g and i) 10 and (h and j) 20 cycles along with elemental mapping for Au, C, Fe and O elements in the composite. Reproduced with permission from ref. 178. Copyright 2015 Royal Society of Chemistry. | ||
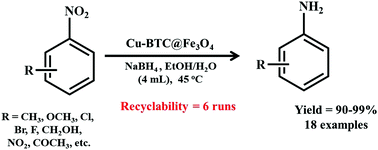
On similar grounds, a remarkable Cu-BTC@Fe3O4 composite for accomplishing NaBH4-mediated nitroarene reduction was synthesized by Zhang and Wang along with their research group (Scheme 9).179 A mixture of ethanol–water in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion was found to be the best solvent for achieving the complete transformation of nitrobenzene into aniline. Notably, the developed composite exhibited broad substrate scope and afforded the desired aniline derivatives in surprisingly good yields. Furthermore, the composite offered the additional advantage of recoverability and reusability for six multiple cycles via external magnetic forces.
1 proportion was found to be the best solvent for achieving the complete transformation of nitrobenzene into aniline. Notably, the developed composite exhibited broad substrate scope and afforded the desired aniline derivatives in surprisingly good yields. Furthermore, the composite offered the additional advantage of recoverability and reusability for six multiple cycles via external magnetic forces.
Bian and co-workers smartly took advantage of a step-by-step assembly strategy for fabricating a core–shell Fe3O4@PDA–Pd@[Cu3(btc)2] composite for the reduction of 4-nitrophenol.49 The prudently designed composite consisted of a Fe3O4 core functionalized with polydopamine (PDA), an intermediate layer of Pd NPs and an outer or exterior shell of MOF. Advanced microscopic techniques such as TEM and HRTEM (Fig. 10) revealed the uniform and homogeneous dispersion of Pd NPs both on the interior and the exterior, i.e., on the surface of the PDA layer. The composite exhibited outstanding catalytic performance in the reduction of 4-nitrophenols and its excellent activity was accredited to the unique hierarchical core–shell structure of the composite. Existence of the PDA layer with numerous catechol and amine groups and strong affinity towards organic molecules endowed the resultant material with good dispersibility in water and ethanol and rendered Pd-active sites accessible to the reactants. Besides, the MOF shell with added benefits of porosity facilitated the specific adsorption of the reactants through electrostatic, van der Waals and π–π stacking interactions, thereby enhancing the contact between the reactants and the active species. Thus, the synergistic unification of each component played a decisive role in boosting the catalytic activity of the designed composite.
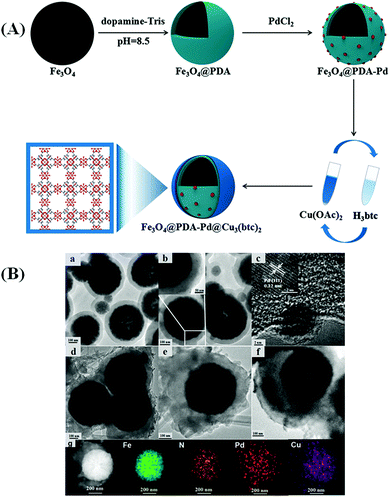 | ||
| Fig. 10 (A) Fabrication route for Fe3O4@PDA–Pd@Cu3(btc)2. (B) TEM micrographs of (a) Fe3O4@PDA, (b) Fe3O4@PDA–Pd, (d and e) Fe3O4@PDA–Pd@Cu3(btc)2 when n = 5 and (f) Fe3O4@PDA–Pd@Cu3(btc)2 when n = 15, (c) HRTEM micrograph of Fe3O4@PDA–Pd, and (g) STEM image of Fe3O4@PDA–Pd@Cu3(btc)2 when n = 5 along with elemental mapping of Fe, N, Pd and Cu in the nanocomposite. Reproduced with permission from ref. 49. Copyright (2018) John Wiley and Sons. | ||
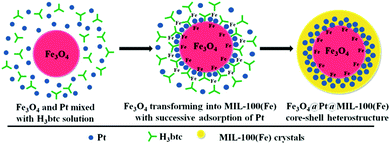
Very recently, Chen et al. designed a multifunctional [Fe3O4@Pt@MIL-100(Fe)] MNP–MOF composite by metamorphosing Fe3O4 nanospheres into MIL-100(Fe) and in situ encapsulating Pt NPs between the Fe3O4 core and the MIL-100(Fe) shell for the reduction of p-nitrophenols.180 The core–shell heterostructure was fabricated by separately synthesizing Fe3O4 and PVP-modified Pt NPs, which were then consequently mixed with H3btc under mechanical stirring and finally heated in an oven at 80 °C for 6 h (Scheme 10). During the course of the reaction, Pt NPs were first adsorbed onto the surface of the Fe3O4 and the oxidative dissolution of the Fe3O4 NPs in the mildly acidic conditions (formed after the dissociation of H3btc) simultaneously generated Fe3+ ions, which further got coordinated with the carboxylate groups of the btc ligand, eventually leading to the formation of MIL-100(Fe) crystals. Meanwhile, some of the residual Pt NPs after being adsorbed on the Fe3O4 core also got adsorbed on the incessantly forming surface of the MIL-100(Fe) shell. The formation mechanism of the heterostructure was also deeply studied by carefully monitoring the morphologies obtained after discrete reaction periods. Synergistic cooperation between the Pt NPs and the MIL-100(Fe) shell (by imparting sufficient docking positions for p-nitrophenol) in the core–shell-structured composite led to astonishing results in the formation of p-aminophenol.
Chang et al. instantiated the fabrication of a magnetic MOF composite embedded with Ag NPs for establishing the reduction of 4-nitrophenol.181 The functional composite was prepared by initiating the layer-by-layer growth of an MIL-100(Fe) shell on a carboxyl-modified Fe3O4 core under mechanical agitation. The next step involved the preparation of Ag NPs via Co-60 gamma radiation-mediated reduction protocol and their uniform dispersion within the pores of MIL-100(Fe). The reduction processes of 4-nitrophenol with NaBH4 were monitored continuously using UV-visible spectroscopy. A representative reaction entailing Fe3O4@MIL-100(Fe) without Ag was conducted and exhibited no significant change in peak intensity in UV analysis, thereby further signifying the crucial role of Ag NPs in nitrophenol reduction. The designed catalyst exhibited marvelous catalytic potency in the concerned reaction along with superb recyclability.
The polymerization of styrene via semihydrogenation of phenylacetylene has been extensively studied by the scientific fraternity. During the transformation, removal of even trace quantities of phenylacetylene represents the most crucial parameter that needs to be taken care of as it results in the poisoning of the catalyst. This reaction is generally carried out in semibatch stirred tank reactors. Recently, Zhou and Fang along with their research group synthesized a series of Pd-based magnetic core–shell catalysts, such as Fe3O4@SiO2/Pd, Fe3O4@AlOOH/Pd, Fe3O4@TiO2/Pd, Fe3O4@Cu3(BTC)2/Pd and Fe3O4@ZIF-8/Pd with Pd loading of 0.5 wt%.182
Physicochemical characterization techniques like TEM and FTIR validated that the designed catalysts possessed core–shell morphological architecture. Besides this, X-ray photoelectron spectroscopic analysis of the catalyst validated the existence of the PdO2 group rather than Pd(0) or PdO as the active component involved in the semihydrogenation process. Amongst all the catalysts investigated for the concerned semihydrogenation process, the Fe3O4@ZIF-8/Pd composite in the presence of ethanol gave the highest conversion percentage of 99.5 along with 90.5% selectivity towards styrene formation (Fig. 11). The magnificent activity was attributed to the influential electronic effects arising as a result of the donation of electrons from the ZIF-8 to PdO2. Moreover, after the successful formation of styrene, its desorption from the catalytic sites suppressed the further hydrogenation process.
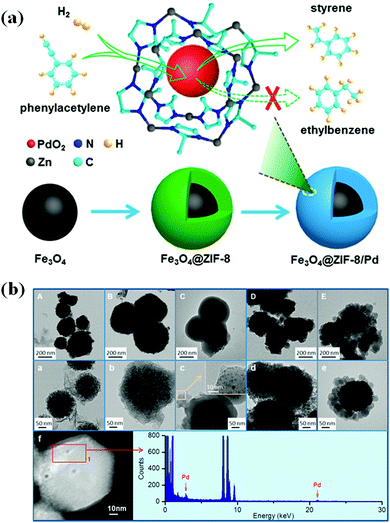 | ||
| Fig. 11 (a) Fe3O4@ZIF-8/Pd-catalyzed semihydrogenation of phenylacetylene to styrene. (b) HRTEM micrographs of support material (A–E) and catalyst (a–e). (A, a) Fe3O4@AlOOH and Fe3O4@AlOOH/Pd, (B, b) Fe3O4@TiO2 and Fe3O4@TiO2/Pd, (C, c) Fe3O4@SiO2 and Fe3O4@SiO2/Pd, (D, d) Fe3O4@Cu3(BTC)2 and Fe3O4@Cu3(BTC)2/Pd, (E, e) Fe3O4@ZIF-8 and Fe3O4@ZIF-8/Pd, and (f) HAADF-STEM and HAADF-EDS of Fe3O4@ZIF-8/Pd. Reproduced with permission from ref. 182. Copyright (2017) American Chemical Society. | ||
Nowadays, there has been a paradigm shift towards constructing hollow MNPs@MOF composites. Owing to their high surface areas, significant lower density and efficient mass transfer, these hollow “yolk–shell” or “reverse bumpy ball” type composites are widely employed as catalysts for the synthesis of complex organic moieties. In this context, Zhao, Chen and research group saliently designed magnetic hollow double-shell-structured Pd/MOF nanospheres through a step-by-step assembly for the liquid-phase-assisted hydrogenation of styrene, cis-stilbene and tetraphenylethylene at room temperature (Fig. 12).183 In an attempt at fabricating the desired catalyst, polystyrene-co-acrylic acid nanospheres were first synthesized as a support material for depositing magnetic iron oxide NPs on its surface. After that, Fe3O4/polystyrene (PS) nanospheres were covered with an outer shell of ZIF-8 followed by their successive encapsulation with Pd NPs in the interior part of ZIF-8 via a wet impregnation method to form Fe3O4/PS@Pd/ZIF-8. In the next step, using the same solution of zinc nitrate and 2-methylimidazole, another outer shell of ZIF-8 was coated on the already synthesized sample to form Fe3O4/PS@Pd/ZIF-8@ZIF-8 nanospheres. In the final step, the polystyrene-co-acrylic acid nanosphere template was removed to form hollow void nFe3O4@Pd/ZIF-8@ZIF-8 nanospheres. This novel architecture displayed outstanding efficacy in the hydrogenation of styrene, wherein it achieved a conversion percentage of 100 within a reaction time of 5 min along with excellent recyclability. It was ascertained that the internal hollow cavity played a dominant factor in accelerating the diffusion rate of reactants, thereby enhancing the overall catalytic activity.
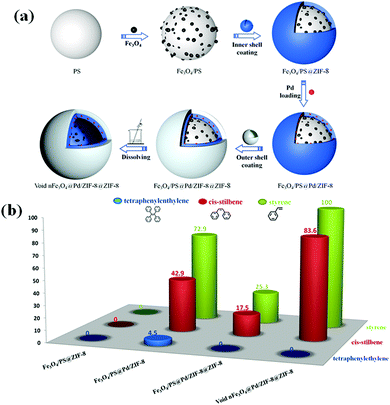 | ||
| Fig. 12 (a) Diagrammatic representation showing synthesis of void nFe3O4@Pd/ZIF-8@ZIF-8. (b) Liquid-phase hydrogenation of styrene, cis-stilbene, and tetraphenylethylene catalyzed by Fe3O4/PS@ZIF-8, Fe3O4/PS@Pd/ZIF-8, Fe3O4/PS@Pd/ZIF-8@ZIF-8 and void nFe3O4@Pd/ZIF-8@ZIF-8 [reaction time: styrene (5 min), cis-stilbene (6 h) and tetraphenylethylene (12 h)]. Reproduced with permission from ref. 183. Copyright (2019) American Chemical Society. | ||
These transformations involve the coupling of two molecular fragments via the aid of a catalyst and most often result in the formation of stereospecific and regioselective products.184–187 The persistent development in this transformation can be directly observed from the continual rise in the number of publications each year. Despite remarkable success, ample reports present in literature demonstrate the use of homogeneous transition metal complexes as catalysts in these reactions. Nevertheless, transition-metal-catalyzed reactions require an additional step of removing the leached metal ion and ligand after its decomposition from the reaction mixture. Besides, removal of trace amounts of transition metal to avoid product contamination is very crucial in the pharmaceutical and medicinal sectors. Therefore, the development of heterogeneous catalysts appears to be a viable solution for these reactions. The incessant expansion in these protocols has completely revolutionized the landscape of synthetic organic chemistry for the generation of natural products, organic materials and polymers. Recently, many magnetic MOF composite-based catalysts have been fabricated as outstanding reagents for accomplishing successful formation of C–C, C–N and C–S bonds in a simplistic and reliable manner.
Suzuki–Miyaura reaction involving coupling between aryl halides and arylboronic acids holds great promise for advancing synthetic methods in practical organic chemistry. In a quest to develop a novel catalyst that manifests ultrahigh efficacy for Suzuki–Miyaura coupling between aryl halides and arylboronic acids, a Fe3O4@PDA–Pd@[Cu3(btc)2] composite was designed by a research group.49 The catalytic performance of the synthesized composite was explored by taking bromobenzene and phenylboronic acid as model substrates in the presence of K2CO3 and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ethanol–water as the optimized base and solvent, respectively (Scheme 11). The reported material was thereafter expanded for executing coupling between diverse aryl halides and arylboronic acids, which further uncovered excellent outcomes. In fact, encouraged by the obtained results, the catalyst was found to be active even towards coupling of highly challenging and perplexing aryl chlorides under ambient conditions. A fascinating attribute of the protocol was that because the catalyst is magnetic in nature, it could be recycled and reused eight times without undergoing any decomposition or decline in its catalytic activity.
1 ratio of ethanol–water as the optimized base and solvent, respectively (Scheme 11). The reported material was thereafter expanded for executing coupling between diverse aryl halides and arylboronic acids, which further uncovered excellent outcomes. In fact, encouraged by the obtained results, the catalyst was found to be active even towards coupling of highly challenging and perplexing aryl chlorides under ambient conditions. A fascinating attribute of the protocol was that because the catalyst is magnetic in nature, it could be recycled and reused eight times without undergoing any decomposition or decline in its catalytic activity.
Li et al. synthesized a MOF-5@SiO2@Fe3O4 heterostructure for the Friedel–Crafts alkylation between benzyl chloride and toluene (Scheme 12).188 A simple solvothermal reaction resulted in the formation of magnetic Fe3O4 particles followed by their subsequent encapsulation within a SiO2 layer via hydrolysis technique. Finally, an in situ strategy was utilized for coating MOF-5 on SiO2@Fe3O4 particles. Initial screening was carried out by synthesizing catalysts with varying contents of MOF-5, i.e., 15.5 wt% MOF-5@SiO2@Fe3O4, 26.8 wt% MOF-5@SiO2@Fe3O4, 42.3 wt% MOF-5@SiO2@Fe3O4 and 59.4 wt% MOF-5@SiO2@Fe3O4. The synthesized 26.8 wt% MOF-5@SiO2@Fe3O4 catalyst possessing plentiful active sites achieved 97% conversion in solvent-free conditions with greater selectivity towards the p-isomer instead of the o-isomer within 6 h at 120 °C. Moreover, the catalyst unveiled superb durability and recyclability for five consecutive runs.
A recent example of utilization of a magnetic MOF composite in the arena of catalysis was reported by Ji and co-workers.189 For the very first time, a MIL-53(Al)@SiO2@Fe3O4 catalyst was in situ fabricated with different MIL-53(Al) contents by encapsulating a shell of MIL-53(Al) onto SiO2@Fe3O4 particles under heating at 220 °C in an autoclave for 72 h. Friedel–Crafts acylation between 2-methylindole and benzoyl chloride was chosen as the representative reaction for assessing the performance of the developed catalyst (Scheme 13). Close inspection of the assimilated results indicated that the catalyst possessed great surface area, apposite superparamagnetism and good catalytic activity and selectivity. MIL-53(Al)@SiO2@Fe3O4 composite with 38.8% MIL-53(Al) content as a catalyst presented 98% conversion of 2-methylindole along with the selective formation of 3-acetylindole and N-acetylindole with 81% and 18%, respectively. Easy separability and reusability of the catalyst for five repeated cycles provided apparent certification of the immensely high activity and durability of the composite. Moreover, the catalyst was capable of maintaining over 90% 2-methylindole conversion and 80% selectivity towards 3-acetylindole even after five cycles, thereby proving its effectiveness after repeated reactions.
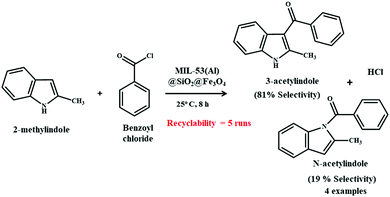 | ||
| Scheme 13 Friedel–Crafts acylation between 2-methyl indole and benzoyl chloride mediated by MIL-53(Al)@SiO2@Fe3O4. | ||
Indeed it is splendid to mention the work carried out by Zhang et al., where the authors smartly exploited an in situ self-assembled strategy for synthesizing a MIL-100(Fe)@SiO2@Fe3O4 composite.190 Primarily, to obtain SiO2@Fe3O4 particles, magnetic particles were synthesized via a solvothermal technique and thereafter modified with a silica layer to inhibit agglomeration and decomposition. The final catalyst was obtained by wrapping of pre-formed uniform SiO2@Fe3O4 particles with a MIL-100(Fe) framework through the self-assembly of Fe(NO3)3 and H3BTC under refluxing conditions at 100 °C for 8 h and subsequently the catalytic activity of the core–shell-structured material was investigated in the liquid-phase acetalization processes involving benzaldehyde and glycol. The coupling reaction involving a 0.55 molar ratio of benzaldehyde to glycol proceeded well at 80 °C and was found to attain conversion of 73.2% within 2 h. It was concluded that the composite possessing copious accessible Lewis acidic sites unveiled magnificent catalytic activity towards the acetalization reaction.
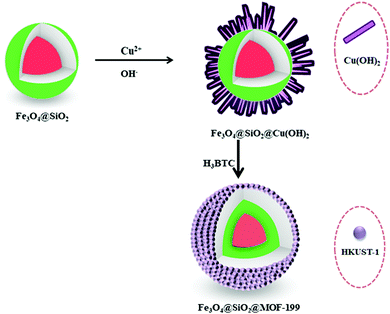
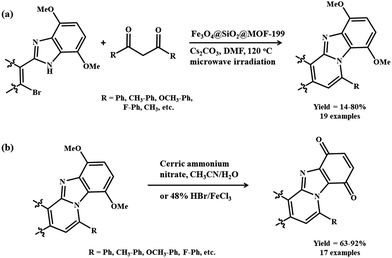
Considering the biological and optical activities of binuclear isoquinoline and pyridine-fused benzimidazoles as anticancer, antitumor and anti-proliferative agents, Cho and colleagues described an efficient synthesis of these motifs by Fe3O4@SiO2@MOF-199.191 This magnetic MOF composite was prepared by growing a Cu(OH)2 shell on Fe3O4@SiO2, and then the Cu(OH)2 was transformed into a Cu-BTC shell with the subsequent addition of H3BTC ligand (Scheme 14). The microwave-assisted reaction between 2-(2-bromoaryl)-4,7-dimethoxy-1-H-benzo[d]imidazoles and (Z)-2-(2-bromovinyl)-4,7-dimethoxy-1H-benzo[d]imidazoles with 1,3-diketones led to the formation of binuclear N-fused scaffolds 8,11-dimethoxybenzo[4,5]imidazo-[2,1-a]isoquinolines and 6,9-dimethoxybenzo[4,5]imidazo-[1,2-a]pyridines (Scheme 15a). Mechanistic studies revealed that the reaction proceeded via C–C coupling, deacylation and finally cyclocondensation. The products obtained were subsequently oxidized in the presence of ceric ammonium nitrate in acetonitrile/H2O or HBr/FeCl3 in H2O to afford binuclear isoquinoline- and pyridine-fused benzimidazole-4,7-diones (Scheme 15b). Furthermore, from the viewpoint of energy-efficient and environmentally sound protocols, the designed methodology appeared to be an attractive platform for the synthesis of various bioreductive quinone based drugs.
Furthermore, the Heck–Mizoroki coupling reaction between olefins and aryl or vinyl halides for constructing C–C bonds has played a crucial role in modern synthetic chemistry. In this connection, the Nuri research group devised a recyclable amine-modified magnetic MIL-101-Pd-based MOF composite for the Heck–Mizoroki reaction (Scheme 16).192 The catalyst preparation route began with the synthesis of amine (1,6-hexanediamine)-functionalized Fe3O4 NPs, which were eventually integrated with Cr-based MIL-101-NH2 MOF under solvothermal conditions with heating in an autoclave at 218 °C for 18 h.
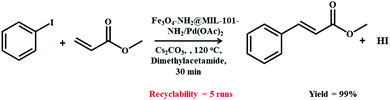 | ||
| Scheme 16 Fe3O4-NH2@MIL-101-NH2/Pd(OAc)2-mediated Heck–Mizoroki coupling reaction between iodobenzene and methyl acrylate. | ||
However, before loading Pd(OAc)2, the obtained Fe3O4-NH2@MIL-101-NH2 MOF was first activated by dispersion in dichloromethane (DCM). With the assistance of the magnetic MOF composite-based catalyst, cross-coupling between iodobenzene and methyl acrylate in dimethylacetamide as the solvent proceeded smoothly at 120 °C. In spite of the fact that the authors could not explore the substrate scope, the catalyst synthetic strategy offers promising potential for the further development of magnetic MOF composite-based catalysts.
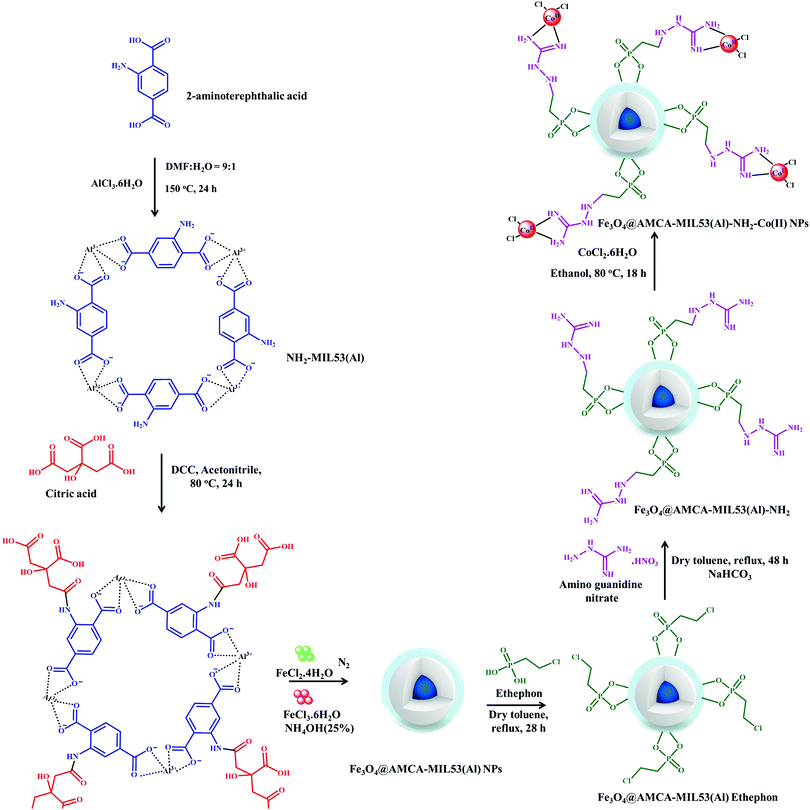
The heteroatom C–N and C–S cross-coupling reactions have emerged as indispensable tools in the construction of a diverse range of products, having extensive utility in biological, pharmaceutical, agrochemical, biochemical and material sectors. The conventional methods employed by various research groups are highly discouraging owing to the involvement of harsh reaction conditions, such as use of strong reducing agents, polar solvents and elevated temperatures. Recently, the possibility of modifying the surface of a MOF, i.e., NH2-MIL53(Al), to obtain Fe3O4@AMCA-MIL53 (Al)-NH2-CoII NPs was explored by Akhlaghinia and co-workers in C–N and C–S bond-forming reactions.193 In an attempt to obtain the desired catalyst, NH2-MIL53(Al) was synthesized using a one-pot solvothermal strategy and for further modifications it was subsequently activated in the presence of dicyclohexylcarbodiamide (DCC) and citric acid in acetonitrile to form AMCA-MIL53(Al). The resulting material was then integrated with the magnetic component under mechanical stirring at r.t., followed by functionalization with 2-chloroethylphosphonic (Ethephon) and amino guanidine nitrate to form Fe3O4@AMCA-MIL53(Al)-Ethephon and Fe3O4@AMCA-MIL53(Al)-NH2 composites, respectively. The product obtained was subsequently boiled in an ethanolic solution of cobalt chloride to form Fe3O4@AMCA-MIL53(Al)-NH2-Co(II) NPs (Scheme 17).
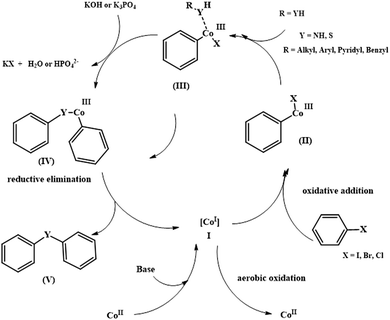
Structural analysis was conducted well using various analytical and spectroscopic techniques, such as FTIR, TEM, FE-SEM, BET, XRD and VSM analysis. The catalytic efficiency of the prudently constructed catalyst was investigated in the solventless C–N cross-coupling of iodobenzene with aniline (Scheme 18a) and C–S cross-coupling of iodobenzene with thiophenol (Scheme 18b). The protocol exhibited wide substrate scope, high functional group tolerance, exceptional yields and good recyclability owing to the magnetic nature of the catalyst. Scheme 19 depicts the synthetic pathway of the cross-coupling reactions, which begins with the in situ reduction of Co(II) species (present in Fe3O4@AMCA-MIL53 (Al)-NH2-CoII) to Co(I) in the presence of base (KOH/K3PO4). In the next step, oxidative addition of aryl halide to Co(I) generates intermediate (II), which later on coordinates with heteroatom to afford intermediate (IV). Meanwhile, intermediate (IV) undergoes reductive elimination to yield the anticipated products in excellent yields along with the regeneration of Co(II) from Co(I). The high catalytic performance of the catalyst was further accredited to its nanosized crystalline nature and simultaneous existence of active cobalt species. Furthermore, to assess the heterogeneous nature of the catalyst, various experiments like hot filtration and poisoning tests were carried out.
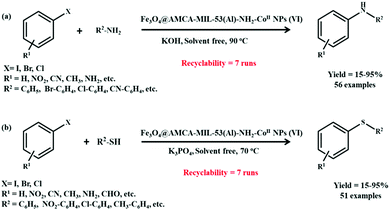 | ||
| Scheme 18 (a) Fe3O4@AMCA-MIL53 (Al)-NH2-CoII-catalyzed C–N cross-coupling reaction (b) Fe3O4@AMCA-MIL53 (Al)-NH2-CoII-catalyzed C–S cross-coupling reaction. | ||
In this direction, a novel bifunctional catalyst entailing use of a magnetic MIL-101-SO3H MOF composite was reported for the preparation of 1,3,5-triarylbenzene scaffolds (Scheme 20a) from acetophenone as the precursor reagent and 2,4,6-triarylpyridine moieties (Scheme 20b) obtained via coupling between acetophenone, aromatic aldehydes and ammonium acetate.113 In both the reactions, wide arrays of functional groups were well tolerated and the targeted products were obtained in splendid yields. Scheme 21 illustrates the mechanistic route through which cooperative interaction between the magnetic MIL-101-SO3H-based catalyst and the substrates furnish 1,3,5-triphenylbenzene motifs. The initial step involves the protonation of acetophenone through acidic sites present in MIL-101-SO3H to generate the enol form. The subsequent reaction between the protonated forms (A and B) and further dehydration leads to the formation of the α,β-unsaturated carbonyl moiety C, which again activates by the catalyst and undergoes reaction with another protonated acetophenone to form D. The compound D then undergoes dehydrogenation and prototrophic shift followed by electrocyclization and dehydrogenation to yield the desired product.
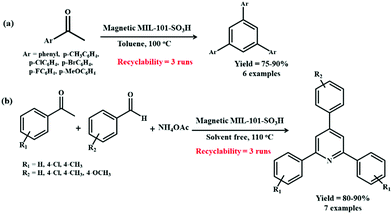 | ||
| Scheme 20 (a) Preparation of 1,3,5-triarylbenzenes mediated by magnetic MIL-101-SO3H catalyst. (b) Preparation of 2,4,6-triaryl pyridines mediated by magnetic MIL-101-SO3H catalyst. | ||
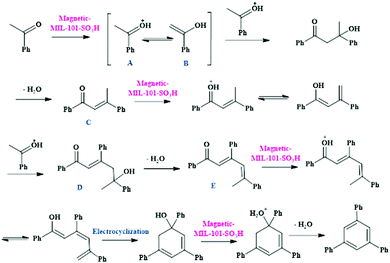 | ||
| Scheme 21 Mechanistic pathway for the synthesis of 1,3,5-triphenylbenzene motifs in the presence of magnetic MIL-101-SO3H composite. | ||
A synthetic pathway involving the critical role of MIL-101-SO3H towards accelerating the formation of 2,4,6-triaryl pyridines is demonstrated in Scheme 22. The scheme depicts the protonation of acetophenone via magnetic MIL-101-SO3H catalyst to produce the enol form, which undergoes nucleophilic addition with benzaldehyde to afford product C through Aldol condensation. Meanwhile, the enamine moiety generated after reaction between ammonium acetate and acetophenone interacts with Cvia Michael addition to form complex E, which eventually cyclizes and undergoes oxidation to form 2,4,6-triphenylpyridines. The synergistic effect between the Lewis acidic sites of CrIII and the active sites of Fe3O4 NPs was uncovered to have a positive and profound influence on the reaction kinetics. Additionally, the Brønsted acidic sites, due to the SO3H groups, were also responsible for achieving exceptional catalytic performance. Some of the startling attributes of the projected catalytic protocol encompassed high efficiency, good chemical stability, high recyclability, high functional group tolerance, magnetic retrievability and high product yield. All these features collectively rendered the proposed methodology to be highly beneficial from economic and environmental concerns.
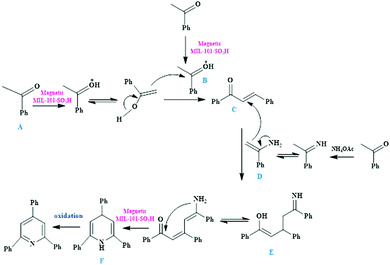 | ||
| Scheme 22 Mechanistic pathway for the synthesis of 2,4,6-triaryl pyridines in the presence of magnetic MIL-101-SO3H composite. | ||
Given the tremendous potential of multicomponent coupling reactions in shaping our current industrial and academic sectors, Zhang et al. recently designed a magnetic NiFe2O4@MOF-5 heterostructure for executing successful solventless multicomponent coupling between benzaldehyde, indole, and kojic acid to yield 2-substituted alkyl and aryl(indolyl) kojic acid derivatives (Scheme 23).197 The final catalyst was synthesized in three steps having a NiFe2O4 magnetic core, zinc ions as connectors and terephthalic acid (H2BDC) as linkers. Firstly, NiFe2O4 NPs prepared via a co-precipitation method were eventually functionalized using sodium citrate and later on the MOF-5 layer was grown onto the citrate-functionalized NPs after the NiFe2O4 particles were heated in an autoclave containing a mixture of zinc nitrate and H2BDC in DMF at 130 °C for 24 h.
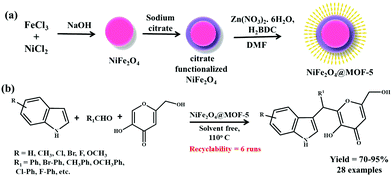 | ||
| Scheme 23 (a) Synthesis of NiFe2O4@MOF-5. (b) NiFe2O4@MOF-5-mediated coupling reaction between aldehyde, indole and kojic acid. | ||
The MOF-assimilated magnetic component possessed sufficient magnetic susceptibility to permit facile separation of the catalyst from the reaction media via external magnetic forces rather than cumbersome filtration and centrifugation techniques. Divergent and distinctive aldehyde and indole moieties were coupled effectively to afford 2-substituted alkyl and aryl(indolyl)kojic acid scaffolds in astonishing and remarkable yields. Scheme 24 portrays a mechanistic route that highlights the synergistic interplay of the NiFe2O4@MOF-5-based catalyst and substrates in stimulating the multicomponent reaction. The reaction commences with the activation of the carbonyl group of benzaldehyde via interaction with NiFe2O4@MOF-5, which further reacts with kojic acid to form intermediate I. This intermediate then undergoes dehydration to give intermediate II, which further interacts with indole through conjugate addition and subsequent enolization yields the final product.
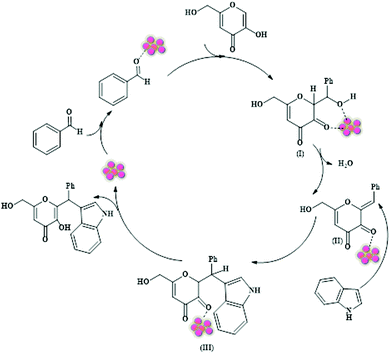 | ||
| Scheme 24 NiFe2O4@MOF-5-catalyzed fabrication of 2-substituted alkyl and aryl(indolyl) kojic acid moieties. | ||
Similarly, a magnetic CoFe2O4/TMU-17-NH2 MOF composite fabricated via an embedding approach was successfully investigated as a catalyst in the multicomponent coupling between 1,3-diketone, aromatic aldehydes and urea or thiourea to form bis-3,4-dihydropyrimidin-2(1H)-one and 3,4-dihydropyrimidin-2(1H)-one derivatives.119 The reaction proceeded smoothly under mild conditions, without the use of solvent and completed within 10–30 min with good to excellent yields.
A magnetically retrievable CoFe2O4@SiO2@NH2@Cu(5-NIPA) heterostructure possessing immense thermal stability was fabricated via a covalent bonding strategy, which exhibited unprecedented catalytic activity in the multicomponent oxidative coupling between primary alkyl/aryl amines, substituted anilines and sodium azides to form 2-substituted benzimidazole moieties (Fig. 13).56 The authors proposed a possible mechanistic route (Scheme 25) in which cooperative interaction between CoFe2O4@SiO2@NH2@Cu(5-NIPA) and reactants successfully afforded 2-substituted benzimidazoles in good to excellent conversion percentages. Oxidation of benzylamine generates an imine moiety, which further reacts with aryl amine to produce an intermediate complex that coordinates with Cu (present in CoFe2O4@SiO2@NH2@Cu(5-NIPA)). Meanwhile, acetic acid reacts with sodium azide to generate hydrazoic acid, which in the presence of TBHP forms N3 radical, which consequently interacts with the Cu-MOF coordinated complex. The subsequent single electron transfer process followed by loss of N2, intramolecular oxidative cyclization and aromatization forms the anticipated benzimidazole moiety. The protocol permitted the fruitful synthesis of a diverse range of benzimidazoles with good conversion percentages. The plentiful coordinatively unsaturated metal centers inside the MOF structure was asserted to be the prime parameter behind the impressive catalytic potential of the designed composite. Besides, the incorporation of green reaction conditions (water as solvent), wide substrate applicability and the excellent recyclability and durability of the catalyst owing to its magnetic characteristics made the protocol highly valuable for addressing industrial and environmental concerns.
 | ||
| Fig. 13 (a) CoFe2O4@SiO2@NH2@Cu(5-NIPA)-catalyzed multicomponent coupling reaction to afford benzimidazole scaffolds. (b) TGA curve of CoFe2O4@SiO2@NH2@Cu(5-NIPA). (c) Recyclability studies for CoFe2O4@SiO2@NH2@Cu(5-NIPA)-mediated benzimidazole synthesis. Reproduced with permission from ref. 56. Copyright (2018) American Chemical Society. | ||
Synthesis of S-aryl phosphorothioates via phosphorylation of aryl thiols has been rated as one of the most poignant transformations in organic synthesis as the resulting products find extensive usage as versatile intermediates in the generation of biologically active compounds. For accomplishing the preferred phosphorylation process, these protocols employ chlorine or bromine along with aryl thiols, which are highly toxic along with foul smelling. Additionally, the reported methodologies not only incorporate homogeneous transition metal salts but also involve unfavourable starting reagents and additives. A recent report wherein phosphorothiolation of aryl boronic acids was carried out using sulfur powder persuaded Zhang and co-workers to formulate a three-component coupling reaction between aniline as the aryl source, H-phosphonates [P(O)H] and sulfur powder to form C(aryl)–S–P bonds catalyzed by a Cu-BTC@Fe3O4 composite (Scheme 26).198 The catalyst was fabricated via a secondary growth approach in which carboxyl-modified Fe3O4 NPs were added into an ethanol–water solution containing copper ions and PVP followed by the stepwise addition of more copper ions and H3BTC under mechanical stirring for 12 h. Proper nucleation and growth of MOF onto Fe3O4 NPs was controlled by fine-tuning the precursor reagent ratios and the manner or series in which reagents were added. In the presence of the developed Cu-BTC@Fe3O4 composite, a broad array of anilines (to in situ generate aryl diazonium salts) and H-phosphonates underwent reaction smoothly to afford the desired scaffolds in remarkable yields. Furthermore, the magnetic retrievability of the catalyst favored its repetitive use for six repeated runs and without any considerable decrease in the catalytic performance.
Very recently, Sharma and co-workers fabricated a CoFe2O4/Mn-BDC hybrid heterostructure for carrying out multicomponent click coupling of alkyl/aryl halides, terminal alkynes and sodium azide to afford 1,4-disubstituted 1,2,3-triazoles (Scheme 27).154 As substantiated through SEM and TEM analysis (Fig. 14), the composite synthesized by a mixing approach contained Mn-BDC possessing microflake morphology with homogeneous dispersion of CoFe2O4 NPs. The developed catalyst, apart from presenting impressive catalytic performance towards the synthesis of a broad array of 1,4-disubstituted triazoles, was also magnetically retrievable and recyclable for five runs, which further added mystique to the designed methodology. Scheme 28 elucidates the crucial role of CoFe2O4/Mn-BDC in promoting the multicomponent click coupling to afford 1,2,3-triazole scaffolds under ambient reaction conditions. The primary step entails the coordination of Mn species (present in CoFe2O4/Mn-BDC) with a terminal alkyne to generate Mn-coordinated complex I. Meanwhile, the in situ formed alkyl azide reacts with complex I and subsequently undergoes 1,3-dipolar cycloaddition to generate the final product.
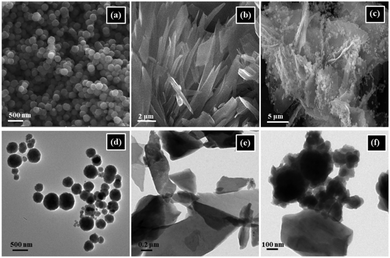 | ||
| Fig. 14 SEM micrographs of (a) CoFe2O4, (b) Mn-BDC and (c) CoFe2O4/Mn-BDC. TEM micrographs of (d) CoFe2O4, (e) Mn-BDC and (f) CoFe2O4/Mn-BDC. Reproduced with permission from ref. 154. Copyright (2020) American Chemical Society. | ||
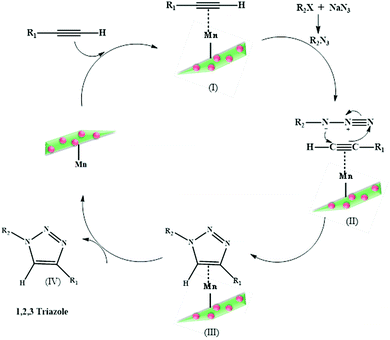 | ||
| Scheme 28 Scheme depicting CoFe2O4/Mn-BDC-mediated preparation of 1,4-disubstituted 1,2,3-triazoles. | ||
Benzodiazepine moieties encompassing a triazole framework are biologically active heterocyclic compounds with immense pharmaceutical significance. In view of this, CuFe2O4@MIL-101(Cr) was lately investigated as a recyclable material in the synthesis of benzodiazepines comprising a triazole skeleton in the eco-friendly solvent of water (Scheme 29).199 Chalcones with acetylene groups, o-phenylene diamine and substituted phenyl azides were taken as multicomponent reaction partners to afford the desired benzodiazepines in excellent yields. The engaged protocol was found to be effective even for gram scale synthesis. Furthermore, magnetic retrievability and reusability for six consecutive runs without any significant decrement in catalytic performance were some added benefits of the methodology.
Another research group led by Ge also reported the Knoevenagel reaction between benzaldehyde and cyanoacetamide using a potent Fe3O4/ZIF-8 catalyst.201 The synthesis of the Fe3O4/ZIF-8 composite was an encapsulation process compelled by the electrostatic interactions between the positive components of ZIF-8 and the negatively charged Fe3O4 (Scheme 30a). Moreover, by simply adjusting the amount of Fe3O4 and Zn(NO3)2, the particle size and surface charge of the overall composite was controlled effectively. The high catalytic activity of the designed composite in the Knoevenagel reaction was attributed to the existence of active basic sites on its external surface (Scheme 30b).
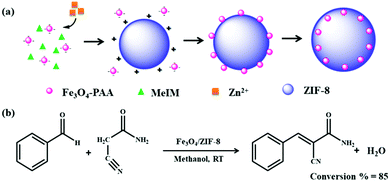 | ||
| Scheme 30 (a) Formation pathway of Fe3O4/ZIF-8. (b) Fe3O4/ZIF-8-mediated Knoevenagel condensation of benzaldehyde and cyanoacetamide. | ||
In this context, a prolific attempt was made by Schneider et al., wherein the authors synthesized a porous Fe3O4@ZIF-8 composite by dispersing smaller sized citrate-modified Fe3O4 NPs within the cavities or pores of the ZIF-8.202 The carboxyl functionalities on Fe3O4 interacted with Zn2+ ions coordinated with 2-methylimidazole, eventually driving the growth of ZIF-8 around the Fe3O4 core. Microscopic studies (Fig. 15) divulged the formation of rhombic dodecahedral shaped ZIF-8 with spherical Fe3O4 NPs assimilated into the framework. The catalytic potency of developed architecture was assessed in the Knoevenagel condensation between benzaldehyde and malononitrile using toluene as a solvent at room temperature (Scheme 31). The protocol was even further stretched to Cu2+-doped Fe3O4/ZIF-8 for catalyzing the cycloaddition reaction between phenyl azides and terminal acetylenes to afford triazole scaffolds.
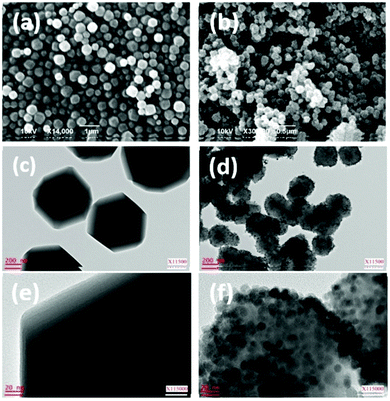 | ||
| Fig. 15 SEM micrographs of (a) ZIF-8 and (b) Fe3O4@ZIF-8. TEM micrographs of (c and e) ZIF-8 and (d and f) Fe3O4@ZIF-8. Reproduced with permission from ref. 202. Copyright 2015 Royal Society of Chemistry. | ||
The Li research group prepared a Fe3O4/IRMOF-3 composite and analyzed its catalytic potential in the Knoevenagel condensation of benzaldehyde and ethyl cyanoacetate.203 The material was synthesized by coating Fe3O4 with a layer of PVP that, apart from acting as a promoter, also ensured implicit encapsulation of Fe3O4 within the MOF matrix. Electrostatic interactions between the Zn2+ ions of the MOF and the pyrrolidone groups of PVP in conjunction with hydrophobic interactions between PVP and the organic linker of the MOF served as the basis for the growth of the overall composite. The authors further illuminated the fact that this MOF-based catalyst attained a marvelous ethyl cyanoacetate conversion of 98.3% within 4 h.
Another magnetically recyclable Fe3O4@UiO-66-NH2 composite possessing a core–shell structure was employed in the Knoevenagel condensation between benzaldehyde and ethyl cyanoacetate.204 The developed catalyst was found to be highly active in the concerned reaction due to the bifunctional characteristics of UiO-66-NH2 along with the superior mass transfer phenomenon commendably eventuating due to its nanosized core–shell morphology. Moreover, the catalyst showed appreciable recycling stability for four consecutive runs due to facile recovery via magnetic forces. In fact, the protocol was further extended for the reaction between numerous larger aromatic aldehydes, like 1-naphthaldehyde and 9-anthraldehyde with ethyl cyanoacetate.
Likewise, another research group also explored Fe3O4@MIL-100(Fe)-NH2 as a potent catalyst in the Knoevenagel condensation between ethyl cyanoacetate and benzaldehyde.205 The high catalytic efficiency of the catalyst was attributed not only to the simultaneous presence of Lewis acidic metal sites and amino groups but also due to the existence of a mesoporous MOF shell.
Recently, a one-pot sequential deacetalization-Knoevenagel cascade condensation reaction was reported by Matsuoka and co-workers using Fe3O4@HKUST-1 as the catalyst.206 The catalyst was fabricated by embedding Fe3O4 NPs into the copper-based ceramic material Cu2(OH)3NO3. At room temperature, this ceramic material in an ethanolic solution of H3BTC was converted into HKUST-1. The cascade reaction between benzaldehyde dimethylacetal and malononitrile occurred smoothly at 363 K in the presence of 1,4-dioxane as the solvent with the successful formation of benzylidene malononitrile. It is worth noting here that the Brønsted and Lewis acidic sites along with coordinatively unsaturated metal centers inside the structure of MOF were mainly responsible for its high catalytic activity. In fact, the newly developed catalyst (Fe3O4@HKUST-1) exhibited high catalytic performance in comparison to the traditional heterogeneous catalysts such as alumina, SiO2 and MgO in the concerned reaction. The authors further explored the scope and versatility of the fabricated magnetic MOF composite towards the hydrogenation of olefin-like 1-octene. For this, Pd NPs were incorporated by mixing in Fe3O4@Cu2(OH)3NO3 solution and with the successive addition of linker (BTC), Pd/Fe3O4@HKUST-1 composite was formed which resulted in octane formation with 98% yield.
The Claisen–Schmidt condensation between a nucleophilic ketone donor and an electrophilic aldehyde acceptor is also considered to be a powerful multicomponent reaction that finds extensive usage in medicinal and synthetic organic chemistry. This reaction generates numerous pharmacologically significant compounds that possess anticancer, anti-inflammatory and anti-malarial properties. Chalcones, an important structural motif in organic synthesis and a progenitor of biologically active scaffolds such as flavonoids, benzothiazepines and pyrazolines can be synthesized via this reaction in acidic or basic conditions.207,208 In view of this, a magnetically recyclable core–shell Fe3O4@MIL-100(Fe) heterostructure fabricated by utilizing a multistep assembly strategy was explored for its ability to catalyze the Claisen–Schmidt reaction affording chalcone motifs (Scheme 32).108 Under optimal reaction conditions and taking benzaldehyde and acetophenone as model substrates, the catalyst demonstrated noteworthy catalytic activity. The high activity was further attributed to the exceptionally high surface area, well-defined pore size and Lewis acidic sites in the structure of the overall composite.
Coumarins, a significant class of oxygen heterocycles, have garnered increasing attention in the agrochemicals, insecticides, cosmetics and fragrance industries. Coumarin and its derivatives possess numerous biological activities, such as anticancer, antimicrobial, anti-HIV, and antithrombotic, that are in high demand in the pharmaceutical sector. Owing to the involvement of milder reaction conditions, simple starting reagents, and shorter reaction times, these motifs can be synthesized easily via Pechmann condensation reaction.209,210 Taking into account the aforementioned benefits offered by these moieties, Ji and co-workers fabricated a series of newer Cu-BTC@SiO2@Fe3O4 materials via ultrasonic-assisted method by varying the mass percent of Cu-BTC in the composite and subsequently utilized them as catalysts in the Pechmann reaction between 1-naphthol and ethyl acetoacetate (Scheme 33).211 Amongst the various synthesized catalysts, 50.8% Cu-BTC@SiO2@Fe3O4 unveiled an outstanding conversion of 96% along with 98% selectivity. Besides this, the ease of catalyst separation and reusability for five consecutive runs without any appreciable loss in efficiency further enhanced the significance of this protocol.
Discovered in 1850, the Strecker reaction represents one of the most efficient strategies in the synthesis of α-amino nitriles. It is a multicomponent reaction in which an amine group, a carbonyl moiety and alkaline metal cyanides effectively cross-couple to form the desired biologically important synthones.212 Considering the sizeable interest in these molecules in synthetic organic chemistry, a porous magnetic Fe3O4/MIL-101(Fe) material was examined as a potent catalyst towards accomplishing three-component Strecker condensation of aldehydes/ketones, amines and trimethylsilyl cyanide to afford α-amino nitriles (Scheme 34).213 A broad range of substituted aldehydes and substituted anilines reacted smoothly in ethanol at room temperature. The protocol generated highly demanded products in exceptional yields, further highlighting the presence of Lewis acidic sites in the catalyst, which were responsible for its high activity.
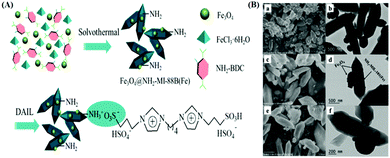 | ||
| Fig. 16 (A) Illustration showing synthesis of ionic liquid-based magnetic MOF composite DAIL-Fe3O4@NH2-MIL-88(Fe). (B) SEM and TEM micrographs of Fe3O4@NH2-MIL-88(Fe) (a and b), DAIL-Fe3O4@NH2-MIL-88(Fe) (c and d) and recovered DAIL-Fe3O4@NH2-MIL-88(Fe) (e and f). Reproduced with permission from ref. 214. Copyright (2016) American Chemical Society. | ||
Another neoteric magnetic MOF composite as a potential prospect for carrying out esterification reaction was delineated by Wang et al.215 The Fe3O4/MIL-100(Fe) heterostructure was fabricated under ambient reaction conditions, i.e., low temperature without the need for HF (hydrofluoric acid) and high pressure. The catalytic efficiency of the resulting composite was investigated in the esterification of rosin with glycerol. Rosin majorly consists of tricyclic diterpene resin acid and its esterification with alcohols is considered to be highly challenging in industry. Surprisingly, the designed catalyst achieved a notable rosin conversion of 94.8% at 240 °C within a 2.5 h reaction time. Elimination of free, adsorbed water molecules from the structure of Fe3O4/MIL-100(Fe) significantly boosted its catalytic efficiency, as evident through the experimental results. Besides, the magnetic retrievability of the catalyst permitted its facile removal via an external magnet, which further enhanced the economic competitiveness of the protocol.
4.2 Photocatalysts
Technological changes and innovation are the thrust of industrialization and development but it comes at the cost of a mounting and invisible water pollution crisis. Water is not only crucial for human survival but also forms the basis of certain socio-economic developments. The persistent cocktail of pollutants like dyes, pesticides, aromatic hydrocarbons and pharmaceuticals are to blame for the water quality deterioration. Thus, the ever-increasing release of these pollutants into the water streams is of prime international concern and has attracted the attention of the scientific fraternity. The discharged wastewater not only damages the ecosystem but also causes serious harm to mankind and aquatic life. Therefore, the need of the hour is to design lucrative, eco-friendly and sustainable technologies that completely remove or degrade these organic pollutants from water. Much of the research endeavors of scientists working in the bourgeoning arena of environmental remediation have been directed in this area.216,217 In this regard, magnetic MOF composite-based catalysts have appeared as a viable solution for accomplishing the proper and absolute degradation of organic pollutants into non-toxic groups. In light of this, Cai and co-workers fabricated a yolk–shell structured composite in which core–shell Pd@Fe3O4 NPs were wrapped with a hollow Fe-MOF shell under solvothermal conditions with heating at 100 °C for 6 h.218 They subsequently investigated its catalytic prospects in the H2O2-assisted Fenton-like degradation of chlorophenols and phenols from water samples. Use of HR-TEM indicated the uniform distribution of Fe3O4 and Pd NPs in the Fe-MOF shell. It was further revealed that the presence of cavities in the yolk–shell structure of Pd@Fe3O4@MOF enabled a faster reactant diffusion rate, thereby enhanced the overall catalytic efficiency of the composite. The reactive oxygen species in the sample were detected using electron spin resonance spin-trap and continuous-flow chemiluminescence techniques. Besides, the outstanding catalytic activity of the composite was ascribed to the faster and continuous production of hydroxyl radicals, which led to the complete degradation and mineralization of pollutants.Nevertheless, use of solar energy irradiation has emerged as a potentially promising solution for tackling water pollution-related issues. Since Fujishima and Honda reported the earliest example of an artificial photocatalytic system, numerous photoactive materials, such as ZnO, CdS, and ZnS, have been reported for the photodegradation of organic pollutants.219 Nonetheless, these conventional inorganic semiconductor-based photomaterials not only suffer from low solar energy conversion efficiency, photocorrosion, and agglomeration but also lead to difficulty in separation and recycling. In recent years, use of MOFs as active photocatalysts has garnered considerable attention from scientists across the globe as evident from some critical reviews already reported in the literature.12,26 In order to drive photocatalytic reactions, different functional units are incorporated into MOFs, which apart from harvesting light energy also simultaneously catalyze the reaction. MOFs offer an additional advantage of excitation via metal-oxo clusters and ligand-to-metal charge transfer.220–222 Furthermore, integrating the superb properties of MOFs with magnetic materials smartly overcomes the post-separation problems via magnetic attraction.
Considering the immense benefits of MOFs in the field of photocatalysis, Qiu and co-workers reported a novel and magnetic recyclable Fe3O4@MIL-100(Fe)-based photocatalyst (Fig. 17A) and studied its photocatalytic activity in methylene blue degradation.223 Firstly, ferrite NPs prepared solvothermally were modified using MAA. Then, MAA-functionalized ferrite NPs were dispersed in a solution containing ferric chloride and H3BTC under ultrasonication. After repeating a number of cycles, core–shell-structured Fe3O4@MIL-100(Fe) was obtained. SEM and TEM analysis clearly showed the spherical and monodisperse nature of the ferrite NPs (Fig. 17B). Additionally, these micrographs also revealed the core–shell morphological structure of the synthesized nanocatalyst as having a ferrite core and a MOF shell. The results disclosed the astonishing photocatalytic activity of Fe3O4@MIL-100(Fe) in both visible and UV-visible irradiation (Scheme 35). It was perceived that the photodegradation efficiency of methylene blue increased when Fe3O4@MIL-100(Fe) was used in conjunction with H2O2. The obtained results were ascribed to the electron acceptor properties of H2O2 suppressing the electron–hole pair recombination tendency and increasing the overall photodegradation efficiency. MIL-100(Fe) functioned like a semiconductor as it possesses an empty d metal orbital that mixes with the LUMO of the organic ligand to yield the conduction band. The overall mechanism was inferred on the basis of the electron excitation from valence to conduction band on irradiation with light and holes got automatically generated in the valence band. The photoinduced transference of electrons transformed H2O2 into hydroxyl radicals (OH), thereby hampering the recombination phenomenon of electrons and holes. The generated holes and hydroxyl radicals altogether possessed strong oxidizing ability and further reacted to oxidize adsorbed methylene molecules. Additionally, the designed composite presented favorable magnetic properties, as a consequence of which it was facilely separated via an external magnet, thus making it an admirable prospect for developing active photocatalysts working within the visible light range for organic pollutant degradation.
 | ||
| Fig. 17 (A) Schematic representation showing construction of Fe3O4@MIL-100(Fe)-based photocatalyst. (B) SEM and TEM micrographs of core–shell Fe3O4@MIL-100(Fe) acquired after 50 cycles. Reproduced with permission from ref. 223. Copyright 2013 Royal Society of Chemistry. | ||
 | ||
| Scheme 35 Diagrammatic representation exhibiting MIL-100(Fe) photocatalyst-assisted methylene blue (MB) degradation along with electron transfer phenomenon under light irradiation. | ||
A new multifunctional MOF heterostructure (MHMC) comprising of 1-D MIL-53(Fe) microrods and Fe3O4 nanospheres was fabricated and achieved impressive photocatalytic performance for the degradation of Rhodamine B and p-nitrophenol.149 H2O2-assisted photodegradation studies were conducted using a 500 W halogen tungsten lamp in a cylindrical Pyrex vessel reactor. Experimental results revealed that with the addition of just a small amount of H2O2, the developed heterostructure resulted in outstanding photocatalytic efficiency for Rhodamine B and p-nitrophenol degradation under visible light irradiation. The results with MOF-based materials were much superior to those with Fe2O3- and Fe3O4-based photocatalysts, further providing a perfect exemplar for large-scale industrial applications.
Amongst emerging contaminants, diclofenac sodium (DCF), a non-steroidal anti-inflammatory drug (NSAID), has been massively sold across the globe. Due to its high consumption, it is often detected at high levels in surface, ground and waste water. Considering the alarming situation, effective materials are required for its complete elimination from water streams. Within this perspective, Hou et al. described the fabrication of an Fe3O4@MIL-100(Fe) composite for the adsorptive removal and photocatalytic degradation of diclofenac sodium from water.224 The overall composite was synthesized by taking Fe3O4 NPs as the metal antecedent and H3BTC as the organic linker. After irradiating the overall solution by microwave at 150 °C for 30 min, an orange-brown solid material was formed, which after washing with water yielded the final Fe3O4@MIL-100(Fe). The developed material not only demonstrated outstanding adsorption capacity for DCF, but also resulted in its 99% degradation with H2O2 under visible light. The high photocatalytic efficacy was accredited to the distinctive structure of Fe3O4@MIL-100(Fe) and the simultaneous presence of H2O2. The metal centers or nodes present in the MOF behaved as semiconductor dots while the charges (holes and electrons) formed in the separation state in visible light migrated to the surface of the MOF. The as produced electrons and holes further reacted with DCF and catalyzed its degradation. It is worth appreciating that the authors proposed a plausible degradation route for diclofenac degradation using the Fe3O4@MIL-100(Fe)/H2O2 system in visible light.
In another report, the He research group described the one-pot fabrication of a magnetic MIL-100(Fe) composite at room temperature.225 The catalytic potency of the resulting material was evaluated in the industrially demanding degradation of sodium sulfadiazine. Degradation experiments were conducted in the presence of H2O2 under a solar simulator with a 300 W xenon lamp. The experimental results highlighted the fact that the morphology of the designed catalyst, i.e., Fe3O4 nanorods, considerably accelerated its catalytic efficiency. Besides this, the designed photocatalyst was facilely retrieved and recycled for five photodegradation runs without undergoing any diminution in its activity. Thus, the saliently designed magnetic MOF composites that combine activity, selectivity and magnetic retrievability on a single platform appear to be a practicable and sustainable solution for large-scale environmental remediation. Song et al. for the very first time introduced a ternary Fe3O4@Au@MIL-100(Fe) catalyst for the photoinduced oxidation of 3,3,5,5-tetramethylbenzidine (TMB) and o-phenylene diamine.226 Fabrication of the overall catalyst commenced with the seed-mediated growth of Au NPs on amine-terminated Fe3O4 NPs (generated using APTES), which after modification with MAA were repeatedly dispersed in FeCl3 and H3BTC solution for 10 cycles to produce Fe3O4@Au@MIL-100(Fe) composite (Scheme 36). The H2O2-mediated photocatalytic oxidation of both the substrates was successfully accomplished via the aid of ascorbic acid and photoirradiation for 60 sec. Furthermore, the complete catalytic process was effectively monitored through UV-visible and surface-enhanced Raman scattering (SERS) spectroscopic studies. Additionally, comparative studies devoid of a photo-assisted route for the oxidation of TMB were also conducted and splendidly highlighted the enhanced and accelerated oxidation rate achieved in the case of the photoinduced process.
 | ||
| Scheme 36 Schematic representation showing fabrication pathway for magnetic MOF composite-based nanocatalyst. | ||
4.3 Biocatalysts
The development of greener and environmentally sound synthetic protocols is one of the major goals to be accomplished in modern organic synthesis. Use of natural enzymes as biocatalysts for catalyzing various organic reactions appears to be a rational solution for switching towards energy-efficient and sustainable processes. Biocatalysis using enzymes is an attractive strategy that not only combines high selectivity and reactivity on a single platform but also minimizes energy input and waste. However, working with natural enzymes in organic solvents is not only challenging but also sometimes problematic. Besides this, poor thermal stability, short-term operational stability, and difficulty in recovering these enzymes further aggravate the problem to a large extent.227 Thus, immobilization of enzymes onto solid support materials, such as magnetic MOF composites, has proven to be an appealing strategy that smartly outwits the shortcomings associated with working with natural enzymes. The encapsulation or immobilization methodology renders the resulting material with enhanced activity and stability necessary to withstand the reaction conditions during catalytic processes.It is worth mentioning here that Bradshaw and co-workers designed a synthetic route for stabilizing functional molecules such as Candida antarctica lipase B (CalB) enzyme within hierarchically porous MOF capsules.228 In this approach, both UiO-66 and Fe3O4 functionalized with heptanoic acid were synthesized using a post-ligand exchange strategy. Afterwards, Hep-UiO-66 and Hep-Fe3O4 were dispersed under ultrasonication in liquid paraffin and then mixed with agarose solution in PBS (phosphate-buffered saline) under stirring for 2 min. It was perceived that both UiO-66 and Fe3O4 acted as templates for stabilizing agarose hydrogel droplets around which another shell of ZIF-8 was deposited. A secondary ZIF-8 shell was then formed by immersing UiO-66/Fe3O4/agarose solution in an isopropanol solution of zinc nitrate and 2-methylimidazole and keeping the stock solution at −20 °C for 2 h. The aforesaid protocol was repeated again using 2-butanol instead of isopropanol, which led to the formation of a dense ZIF-8 microcapsule shell on the exterior of the UiO-66/Fe3O4 hydrogel core (Scheme 37).
 | ||
| Scheme 37 Illustration depicting fabrication of magnetic MOF composite-based microcapsules. Reproduced with permission from ref. 228. Copyright 2015 Royal Society of Chemistry. | ||
The hydrogel core served the purpose of encapsulating enzymes required to carry out biocatalysis while the MNPs resulted in facile separation via external magnetic forces. Various biomolecules or enzymes, such as green fluorescent protein (GFP), Candida antarctica lipase B (CalB) and galactosidase, were encapsulated by the authors in agarose hydrogels before establishment of the shell. The catalytic potential of CalB loaded on microcapsules was investigated in size-selective biocatalytic transesterification between pairs of small substrates (1-butanol and vinyl acetate to form butyl acetate) and pairs of large substrates (3-(4-hydroxyphenyl)propan-1-ol and vinyl laurate to form 3-(4-hydroxyphenyl)propyl dodecanoate). The presented structural magnetic MOF–enzyme composite paved the way for designing such newer materials with wider prospects in chemoenzymatic catalysis, drug delivery, bioseparation and biodegradation fields.
Lv et al. also fabricated three recyclable multienzyme magnetic MOF composites by employing a layer-by-layer assembly approach in which glucose oxidase (GOx) and horseradish peroxidase (HRP) were spatially confined within the pores of a magnetic HKUST-1 MOF heterostructure.100 The catalytic potency of three designed nanocomposites, GOx@HRP@HKUST-1@Fe3O4, GOx-HRP@HKUST-1@Fe3O4 and HRP@GOx@HKUST-1@Fe3O4, was further evaluated in the cascade catalysis of glucose and o-phenylenediamine as representative substrates. In the cascade reaction, GOx first executed glucose oxidation to gluconic acid and H2O2 followed by the decomposition of H2O2 into water and oxygen mediated by HRP. Amongst the three systems, GOx@HRP@HKUST-1@Fe3O4 exhibited the highest catalytic activity with 100% glucose conversion within 15 h while HRP@GOx@HKUST-1@Fe3O4 demonstrated higher thermal and chemical stability. Besides, magnetic retrievability and high operational stability were some of the fascinating features of the designed magnetic MOF–enzyme composite that make it particularly attractive for use in artificial biomimetic reactor-driven processes in intracellular and extracellular biosynthesis.
Zheng and his colleagues synthesized a novel porous magnetic MOF–enzyme composite using a modular-induced defect-formation approach.229 In this, Fe3O4 NPs synthesized via a solvothermal approach were first coated with PDA to induce the growth of the UiO-66-NH2 MOF shell by the addition of ZrCl4 as the metal progenitor, 2-aminoterephthalic acid as the ligand and dodecanoic acid (DA) as the modulator. The subsequent heat treatment at 100 °C for 3 h and 130 °C for 24 h generated Fe3O4@MOF, which after the elimination of the competitive ligand, i.e., dodecanoic acid, under HCl treatment at 90 °C for 24 h led to the formation of hierarchical Fe3O4@MOF with numerous mesopores (Fig. 18). Once the core–shell Fe3O4@MOF was synthesized, an enzyme amidase was covalently immobilized on it using phosphate buffer and glutaraldehyde as the cross linking agent with continuous stirring at 25 °C for 3 h.
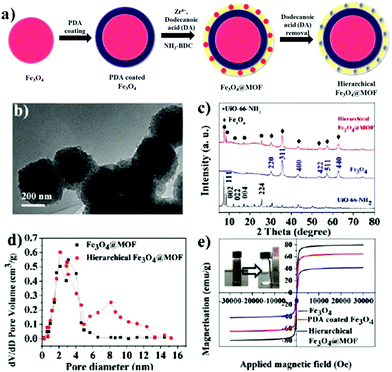 | ||
| Fig. 18 (a) Fabrication of hierarchical Fe3O4@MOF, (b) TEM image of Fe3O4@MOF, (c) XRD patterns, (d) pore size distribution and (e) VSM curves of synthesized materials. Reproduced with permission from ref. 229. Copyright 2019 Royal Society of Chemistry. | ||
The developed catalyst was systematically characterized using sophisticated XRD, TEM, N2 adsorption–desorption and VSM techniques. The catalytic potency of the resulting material was investigated in the synthesis of (S)-4-fluorophenylglycine from varying concentrations of N-phenylacetyl-4-fluorophenylglycine. When the reaction was performed at pH 9 for 2 h at 40 °C and at variable substrate concentrations of 60, 80 and 100 mM, a good conversion of 49.9% with 99.9% enantiomeric excess (ee) was obtained. Apart from this, the authors also prepared a sequence of controlled magnetic MOF composites, namely Fe3O4@[Cu3(btc)2], Fe3O4@MIL-100(Fe) and Fe3O4@ZIF-8, and examined their enzyme loading potential under similar conditions. High thermal and storage stability, good catalytic activity, better organic solvent tolerance, strong magnetic responsiveness, reusability and retention of relative activity up to 98.2% even after 15 runs further added to the significance of the protocol.
5. Conclusions and future outlook
Designing intriguing and multifunctional architectures such as magnetic MOF composites is a burgeoning field of importance. The superb integration of MNPs with the remarkable characteristics of MOFs promotes the successful fabrication of newer synthetic composites that possess innovative, additive and complementarily enhanced properties in comparison to the one-component counterparts. Despite a short history, impressive advancements related to the construction of magnetic MOF composites have been witnessed during the past few years. Furthermore, magnetic MOF composites have evolved as a brilliant star in modern material science and engineering owing to their broad and extensive applications in catalysis, environmental remediation, drug delivery, sensing and device fabrication. Amongst various arenas, catalysis represents one of the most vibrant and dynamic sectors for magnetic MOF composites and has become an exciting research area. Driven by the global drive towards energy miniaturization, particular efforts of the research fraternity are made towards designing novel magnetic MOF composites as effectual catalysts for several industrially significant organic transformations. This review showcased scientific research-based progression attained in the domain of magnetic MOF composites by compiling all the routes employed to date for their fabrication. The synthetic section provided a detailed overview of these methodologies, including layer-by-layer, embedding, encapsulation and mixing along with a brief description of characterizations. Reports on the utilization of magnetic MOF composites for successfully establishing a broad array of organic reactions, including oxidation, hydrogenation, coupling, condensation, esterification and multicomponent coupling, have also been summarized. In addition, recent progress in the budding and proliferating field of photocatalysis and biocatalysis has also been reviewed. It is noteworthy that promising catalytic efficacy with facile recovery and recyclability has been achieved on a single platform by the design of these phenomenal magnetic MOF composites. Although striking and noteworthy results have been achieved in terms of their fabrication, characterization and catalytic applications, a few obstacles still need to be conquered to unveil their full potential in other industrially important processes. Firstly, magnetic MOF composites with ameliorated water stability, shelf life and recyclability are highly pertinent in industrial settings while at the same moment reaction kinetics in magnetic MOF composite-catalyzed processes also warrant deep investigation. Furthermore, intricate synthetic procedures, expensive organic ligands and lower production yields make the overall protocols highly exorbitant and thus confine their utility to laboratory scale. Henceforth, their translation from laboratory to industrial scale requires the earnest efforts of material engineers. Taking sustainability into account, future research should focus upon optimization of synthetic routes so that magnetic MOF composites can be prepared in a simpler, greener and more lucrative manner. Additionally, an in-depth understanding of their formation processes is highly crucial. Though this challenge is not trivial, cutting-edge systematic studies related to the fundamental growth mechanism are imperative for scrutinizing structure–property relationships as well as for engineering complex magnetic MOF composites that find extensive uses in multifaceted applications. We foresee that computational modeling and simulation studies will also help in understanding and predicting the interaction of magnetic materials with the organic and inorganic components of MOF, which will further help in elucidating the exact mechanism responsible for their formation. Besides, advanced spectroscopic techniques like synchrotron X-ray absorption spectroscopy (STXM) and synchrotron X-ray photoelectron spectroscopy (XPS) in combination with in-operando catalytic experiments can further help in probing the behavior of the active molecular species in the composite material. Magnetic MOF composites is an evolving field with enticing prospects and interfacing it with several frontier areas of biology, medicine, energy, and the environment will certainly broader its horizon in the future. It is believed that with the collective and joint efforts of chemists and industrial engineers, these versatile and hybrid materials could offer advanced platforms for future innovative applications in the areas of imaging, drug delivery, luminescent materials, micro-optics, micro-electronics, device fabrication and clinical therapies. Furthermore, it has been rightly said that “Rome was not built in a day”, so the scientific community needs to devote immense efforts and additional time to unleash the concealed fascinating properties of magnetic MOF composites that may provide new perspectives for future societal development.Author contributions
The manuscript was written by Sneha Yadav and Dr Ranjana Dixit. Editing of the manuscript was done by Shivani Sharma, Sriparna Dutta and Kanika Solanki. Prof. Rakesh K. Sharma designed the framework of the review article, edited the manuscript and provided constructive suggestions. All authors have read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Sneha Yadav gratefully acknowledges the Council of Scientific & Industrial Research, New Delhi, India for the award of a Senior Research Fellowship.Notes and references
- D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O’Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257–1283 RSC.
- K. E. Cordova and O. M. Yaghi, Mater. Chem. Front., 2017, 1, 1304–1309 RSC.
- N. R. Catarineu, A. Schoedel, P. Urban, M. B. Morla, C. A. Trickett and O. M. Yaghi, J. Am. Chem. Soc., 2016, 138, 10826–10829 CrossRef CAS.
- D. Zacher, R. Schmid, C. Woell and R. A. Fischer, Angew. Chem., Int. Ed., 2011, 50, 176–199 CrossRef CAS.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- A. U. Czaja, N. Trukhan and U. Müller, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC.
- A. Aijaz, T. Akita, N. Tsumori and Q. Xu, J. Am. Chem. Soc., 2013, 135, 16356–16359 CrossRef CAS.
- P. Hu, J. V. Morabito and C.-K. Tsung, ACS Catal., 2014, 4, 4409–4419 CrossRef CAS.
- H. Fei, M. D. Sampson, Y. Lee, C. P. Kubiak and S. M. Cohen, Inorg. Chem., 2015, 54, 6821–6828 CrossRef CAS.
- X. Zhu, B. Li, J. Yang, Y. Li, W. Zhao, J. Shi and J. Gu, ACS Appl. Mater. Interfaces, 2015, 7, 223–231 CrossRef CAS.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS.
- A. López-Magano, A. Jiménez-Almarza, J. Alemán and R. Mas-Ballesté, Catalysts, 2020, 10, 720 CrossRef.
- M. Liu, J. Wu and H. Hou, Chem. – Eur. J., 2019, 25, 2935–2948 CAS.
- A. Dhakshinamoorthy, Z. Li and H. Garcia, Chem. Soc. Rev., 2018, 47, 8134–8172 RSC.
- V. Pascanu, G. González Miera, A. K. Inge and B. n. Martín-Matute, J. Am. Chem. Soc., 2019, 141, 7223–7234 CrossRef CAS.
- J. Gascon, A. Corma, F. Kapteijn and F. X. Llabres i Xamena, ACS Catal., 2014, 4, 361–378 CrossRef CAS.
- S. M. Rogge, A. Bavykina, J. Hajek, H. Garcia, A. I. Olivos-Suarez, A. Sepúlveda-Escribano, A. Vimont, G. Clet, P. Bazin, F. Kapteijn, M. Daturi, E. V. Ramos-Fernandez, F. X. Llabrés i Xamena, V. V. Speybroeck and J. Gascon, Chem. Soc. Rev., 2017, 46, 3134–3184 RSC.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011–6061 RSC.
- R. J. Kuppler, D. J. Timmons, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D. Young, D. Yuan, D. Zhao, W. Zhuang and H.-C. Zhou, Coord. Chem. Rev., 2009, 253, 3042–3066 CrossRef CAS.
- Q. Yang, Q. Xu and H.-L. Jiang, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- J.-S. Qin, S. Yuan, C. Lollar, J. Pang, A. Alsalme and H.-C. Zhou, Chem. Commun., 2018, 54, 4231–4249 RSC.
- S. Yang, L. Peng, D. T. Sun, M. Asgari, E. Oveisi, O. Trukhina, S. Bulut, A. Jamali and W. L. Queen, Chem. Sci., 2019, 10, 4542–4549 RSC.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Commun., 2017, 53, 10851–10869 RSC.
- L. Chen and Q. Xu, Matter, 2019, 1, 57–89 CrossRef.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang and P. Zhang, Adv. Mater., 2018, 30, 1704303 CrossRef.
- X. Deng, Z. Li and H. García, Chem. – Eur. J., 2017, 23, 11189–11209 CrossRef CAS.
- N. C. Burtch, J. Heinen, T. D. Bennett, D. Dubbeldam and M. D. Allendorf, Adv. Mater., 2018, 30, 1704124 CrossRef.
- Y. J. Ma, X. X. Jiang and Y. K. Lv, Chem. – Asian J., 2019, 14, 3515–3530 CrossRef CAS.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- M. Aghayi-Anaraki and V. Safarifard, Eur. J. Inorg. Chem., 2020, 1916–1937 CrossRef CAS.
- R. Ricco, L. Malfatti, M. Takahashi, A. J. Hill and P. Falcaro, J. Mater. Chem. A, 2013, 1, 13033–13045 RSC.
- P. Silva, S. M. Vilela, J. P. Tomé and F. A. A. Paz, Chem. Soc. Rev., 2015, 44, 6774–6803 RSC.
- G. M. Espallargas and E. Coronado, Chem. Soc. Rev., 2018, 47, 533–557 RSC.
- P. Falcaro, R. Ricco, A. Yazdi, I. Imaz, S. Furukawa, D. Maspoch, R. Ameloot, J. D. Evans and C. J. Doonan, Coord. Chem. Rev., 2016, 307, 237–254 CrossRef CAS.
- R. Lin, L. Ge, H. Diao, V. Rudolph and Z. Zhu, J. Mater. Chem. A, 2016, 4, 6084–6090 RSC.
- C. Jo, H. J. Lee and M. Oh, Adv. Mater., 2011, 23, 1716–1719 CrossRef CAS.
- J. Nan, X. Dong, W. Wang, W. Jin and N. Xu, Langmuir, 2011, 27, 4309–4312 CrossRef CAS.
- A. Huang, W. Dou and J. r. Caro, J. Am. Chem. Soc., 2010, 132, 15562–15564 CrossRef CAS.
- S. Zhang, Q. Yang, W. Wang, C. Wang and Z. Wang, J. Agric. Food Chem., 2016, 64, 2792–2801 CrossRef CAS.
- X. Qiu, X. Wang and Y. Li, Chem. Commun., 2015, 51, 3874–3877 RSC.
- L.-Q. Yu and X.-P. Yan, Chem. Commun., 2013, 49, 2142–2144 RSC.
- J. K. Bristow, K. T. Butler, K. L. Svane, J. D. Gale and A. Walsh, J. Mater. Chem. A, 2017, 5, 6226–6232 RSC.
- Y. Yang, F. Xia, Y. Yang, B. Gong, A. Xie, Y. Shen and M. Zhu, J. Mater. Chem. B, 2017, 5, 8600–8606 RSC.
- T. Wehner, K. Mandel, M. Schneider, G. Sextl and K. Müller-Buschbaum, ACS Appl. Mater. Interfaces, 2016, 8, 5445–5452 CrossRef CAS.
- L. M. Rossi, N. J. Costa, F. P. Silva and R. Wojcieszak, Green Chem., 2014, 16, 2906–2933 RSC.
- S. Singamaneni, V. N. Bliznyuk, C. Binek and E. Y. Tsymbal, J. Mater. Chem., 2011, 21, 16819–16845 RSC.
- N. A. Frey, S. Peng, K. Cheng and S. Sun, Chem. Soc. Rev., 2009, 38, 2532–2542 RSC.
- Y. Chen, X. Huang, X. Feng, J. Li, Y. Huang, J. Zhao, Y. Guo, X. Dong, R. Han, P. Qi, Y. Han, H. Li, C. Hu and B. Wang, Chem. Commun., 2014, 50, 8374–8377 RSC.
- R. Ma, P. Yang, Y. Ma and F. Bian, ChemCatChem, 2018, 10, 1446–1454 CrossRef CAS.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- I. Ahmed and S. H. Jhung, Mater. Today, 2014, 17, 136–146 CrossRef CAS.
- M. Iranmanesh and J. Hulliger, Chem. Soc. Rev., 2017, 46, 5925–5934 RSC.
- P. Falcaro, F. Lapierre, B. Marmiroli, M. Styles, Y. Zhu, M. Takahashi, A. J. Hill and C. M. Doherty, J. Mater. Chem. C, 2013, 1, 42–45 RSC.
- G. M. Lari, G. Pastore, M. Haus, Y. Ding, S. Papadokonstantakis, C. Mondelli and J. Pérez-Ramírez, Energy Environ. Sci., 2018, 11, 1012–1029 RSC.
- M. Babazadeh, R. Hosseinzadeh-Khanmiri, J. Abolhasani, E. Ghorbani-Kalhor and A. Hassanpour, RSC Adv., 2015, 5, 19884–19892 RSC.
- R. K. Sharma, S. Yadav, S. Sharma, S. Dutta and A. Sharma, ACS Omega, 2018, 3, 15100–15111 CrossRef CAS.
- G. Gao, J.-Q. Di, H.-Y. Zhang, L.-P. Mo and Z.-H. Zhang, J. Catal., 2020, 387, 39–46 CrossRef CAS.
- A. Ghorbani-Choghamarani and Z. Taherinia, ACS Comb. Sci., 2020, 22, 902–909 CrossRef CAS.
- C. S. Yan, H. Y. Gao, L. F. Ma, L. L. Dang, L. Zhang, P. P. Meng and F. Luo, J. Mater. Chem. A, 2016, 4, 13603–13610 RSC.
- W. Li, K. Wang, X. Yang, F. Zhan, Y. Wang, M. Liu, X. Qiu, J. Li, J. Zhan, Q. Li and Y. Liu, Chem. Eng. J., 2020, 379, 122256 CrossRef CAS.
- Y. Xiong, F. Ye, C. Zhang, S. Shen, L. Su and S. Zhao, RSC Adv., 2015, 5, 5164–5172 RSC.
- T. Saemian, M. Gharagozlou, M. H. Sadr and S. Naghibi, Polyhedron, 2019, 174, 114163 CrossRef CAS.
- M. Attia, N. McMahon, H. Li, R.-B. Lin, F. DeLuna, Y. Shi, B. Chen and J. Y. Ye, J. Solid State Chem., 2020, 283, 121127 CrossRef CAS.
- L. Nirumand, S. Farhadi, A. Zabardasti and A. Khataee, J. Taiwan Inst. Chem. Eng., 2018, 93, 674–685 CrossRef CAS.
- F. Siadatnasab, S. Farhadi, A.-A. Hoseini and M. Sillanpää, New J. Chem., 2020, 44, 16234–16245 RSC.
- L. Melag, M. M. Sadiq, K. Konstas, F. Zadehahmadi, K. Suzuki and M. R. Hill, RSC Adv., 2020, 10, 40960–40968 RSC.
- M. Heydari, M. Gharagozlou, M. Ghahari and S. Naghibi, Appl. Organomet. Chem., 2020, 34, e5994 CrossRef CAS.
- S. Bahrani, M. Ghaedi, K. Dashtian, A. Ostovan, M. J. K. Mansoorkhani and A. Salehi, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1067, 45–52 CrossRef CAS.
- J. J. Lenders, C. L. Altan, P. H. Bomans, A. Arakaki, S. Bucak, G. de With and N. A. Sommerdijk, Cryst. Growth Des., 2014, 14, 5561–5568 CrossRef CAS.
- H.-J. Song, X.-H. Jia and X.-Q. Zhang, J. Mater. Chem., 2012, 22, 22699–22705 RSC.
- N. R. Jana, Y. Chen and X. Peng, Chem. Mater., 2004, 16, 3931–3935 CrossRef CAS.
- R. Abu-Much and A. Gedanken, J. Phys. Chem. C, 2008, 112, 35–42 CrossRef CAS.
- H. Cui, Y. Liu and W. Ren, Adv. Powder Technol., 2013, 24, 93–97 CrossRef CAS.
- T. J. LaTempa, X. Feng, M. Paulose and C. A. Grimes, J. Phys. Chem. C, 2009, 113, 16293–16298 CrossRef CAS.
- O. Pascu, E. Carenza, M. Gich, S. n. Estradé, F. Peiró, G. Herranz and A. Roig, J. Phys. Chem. C, 2012, 116, 15108–15116 CrossRef CAS.
- T. Parandhaman, N. Pentela, B. Ramalingam, D. Samanta and S. K. Das, ACS Sustainable Chem. Eng., 2017, 5, 489–501 CrossRef CAS.
- K. Wongwailikhit and S. Horwongsakul, Mater. Lett., 2011, 65, 2820–2822 CrossRef CAS.
- W. Wu, C. Z. Jiang and V. A. Roy, Nanoscale, 2016, 8, 19421–19474 RSC.
- Q. Zhang, X. Yang and J. Guan, ACS Appl. Nano Mater., 2019, 2, 4681–4697 CrossRef CAS.
- R. K. Sharma, S. Dutta, S. Sharma, R. Zboril, R. S. Varma and M. B. Gawande, Green Chem., 2016, 18, 3184–3209 RSC.
- Y. Liu, J. Goebl and Y. Yin, Chem. Soc. Rev., 2013, 42, 2610–2653 RSC.
- O. Bomatí-Miguel, L. Mazeina, A. Navrotsky and S. Veintemillas-Verdaguer, Chem. Mater., 2008, 20, 591–598 CrossRef.
- B. S. Kwon, W. Zhang, Z. Li and K. M. Krishnan, Adv. Mater. Interfaces, 2015, 2, 1400511 CrossRef.
- T. Togashi, S. Takami, K. Kawakami, H. Yamamoto, T. Naka, K. Sato, K. Abe and T. Adschiri, J. Mater. Chem., 2012, 22, 9041–9045 RSC.
- C. Blanco-Andujar, D. Ortega, P. Southern, Q. Pankhurst and N. Thanh, Nanoscale, 2015, 7, 1768–1775 RSC.
- Y. Zhang, M. Liu, B. Peng, Z. Zhou, X. Chen, S.-M. Yang, Z.-D. Jiang, J. Zhang, W. Ren and Z.-G. Ye, Sci. Rep., 2016, 6, 1–8 CrossRef CAS.
- A. Shelemin, O. Kylián, J. Hanuš, A. Choukourov, I. Melnichuk, A. Serov, D. Slavínská and H. Biederman, Vacuum, 2015, 120, 162–169 CrossRef CAS.
- H. Aali, S. Mollazadeh and J. V. Khaki, Ceram. Int., 2018, 44, 20267–20274 CrossRef CAS.
- H. Kloust, R. Zierold, J.-P. Merkl, C. Schmidtke, A. Feld, E. Pöselt, A. Kornowski, K. Nielsch and H. Weller, Chem. Mater., 2015, 27, 4914–4917 CrossRef CAS.
- M. Jiao, J. Zeng, L. Jing, C. Liu and M. Gao, Chem. Mater., 2015, 27, 1299–1305 CrossRef CAS.
- E. C. Vreeland, J. Watt, G. B. Schober, B. G. Hance, M. J. Austin, A. D. Price, B. D. Fellows, T. C. Monson, N. S. Hudak, L. Maldonado-Camargo, A. C. Bohorquez, C. Rinaldi and D. L. Huber, Chem. Mater., 2015, 27, 6059–6066 CrossRef CAS.
- R. Costo, V. Bello, C. Robic, M. Port, J. F. Marco, M. Puerto Morales and S. Veintemillas-Verdaguer, Langmuir, 2012, 28, 178–185 CrossRef.
- Z. Kozakova, I. Kuritka, N. E. Kazantseva, V. Babayan, M. Pastorek, M. Machovsky, P. Bazant and P. Sáha, Dalton Trans., 2015, 44, 21099–21108 RSC.
- X. Jing, T. Liu, D. Wang, J. Liu and L. Meng, CrystEngComm, 2017, 19, 5089–5099 RSC.
- D. Bonvin, J. A. Bastiaansen, M. Stuber, H. Hofmann and M. M. Ebersold, RSC Adv., 2017, 7, 55598–55609 RSC.
- M. Ognjanović, B. Dojčinović, M. Fabian, D. M. Stanković, J. F. Mariano and B. Antić, Ceram. Int., 2018, 44, 13967–13972 CrossRef.
- K. V. Manukyan, Y.-S. Chen, S. Rouvimov, P. Li, X. Li, S. Dong, X. Liu, J. K. Furdyna, A. Orlov, G. H. Bernstein, W. Porod, S. Roslyakov and A. S. Mukasyan, J. Phys. Chem. C, 2014, 118, 16264–16271 CrossRef CAS.
- Y. Chen, Z. Xiong, L. Peng, Y. Gan, Y. Zhao, J. Shen, J. Qian, L. Zhang and W. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 16338–16347 CrossRef CAS.
- F. Ke, L.-G. Qiu, Y.-P. Yuan, X. Jiang and J.-F. Zhu, J. Mater. Chem., 2012, 22, 9497–9500 RSC.
- S. Chen, L. Wen, F. Svec, T. Tan and Y. Lv, RSC Adv., 2017, 7, 21205–21213 RSC.
- J. Li, J. Wang, Y. Ling, Z. Chen, M. Gao, X. Zhang and Y. Zhou, Chem. Commun., 2017, 53, 4018–4021 RSC.
- H. Duo, X. Lu, S. Wang, L. Wang, Y. Guo and X. Liang, New J. Chem., 2019, 43, 12563–12569 RSC.
- J.-C. Yang and X.-B. Yin, Sci. Rep., 2017, 7, 1–15 CrossRef.
- X. Zhao, S. Liu, Z. Tang, H. Niu, Y. Cai, W. Meng, F. Wu and J. P. Giesy, Sci. Rep., 2015, 5, 1–10 CAS.
- C. Hou, Y. Wang, Q. Ding, L. Jiang, M. Li, W. Zhu, D. Pan, H. Zhu and M. Liu, Nanoscale, 2015, 7, 18770–18779 RSC.
- I. A. Senosy, Z.-H. Lu, T. M. Abdelrahman, M.-N. O. Yang, H.-M. Guo, Z.-H. Yang and J.-H. Li, Environ. Sci.: Nano, 2020, 7, 2087–2101 RSC.
- Y. Tao, G. Huang, H. Li and M. R. Hill, ACS Sustainable Chem. Eng., 2019, 7, 13627–13632 CrossRef CAS.
- F. Ke, L.-G. Qiu and J. Zhu, Nanoscale, 2014, 6, 1596–1601 RSC.
- F. Ke, Y.-P. Yuan, L.-G. Qiu, Y.-H. Shen, A.-J. Xie, J.-F. Zhu, X.-Y. Tian and L.-D. Zhang, J. Mater. Chem., 2011, 21, 3843–3848 RSC.
- M. Saikia, D. Bhuyan and L. Saikia, New J. Chem., 2015, 39, 64–67 RSC.
- A. Mehdinia, D. Jahedi Vaighan and A. Jabbari, ACS Sustainable Chem. Eng., 2018, 6, 3176–3186 CrossRef CAS.
- M. Bellusci, P. Guglielmi, A. Masi, F. Padella, G. Singh, N. Yaacoub, D. Peddis and D. Secci, Inorg. Chem., 2018, 57, 1806–1814 CrossRef CAS.
- M. B. Boroujeni, A. Hashemzadeh, M.-T. Faroughi, A. Shaabani and M. M. Amini, RSC Adv., 2016, 6, 100195–100202 RSC.
- H. Li, M. M. Sadiq, K. Suzuki, R. Ricco, C. Doblin, A. J. Hill, S. Lim, P. Falcaro and M. R. Hill, Adv. Mater., 2016, 28, 1839–1844 CrossRef CAS.
- A. R. Chowdhuri, D. Bhattacharya and S. K. Sahu, Dalton Trans., 2016, 45, 2963–2973 RSC.
- X. Min, W. Yang, Y.-F. Hui, C.-Y. Gao, S. Dang and Z.-M. Sun, Chem. Commun., 2017, 53, 4199–4202 RSC.
- S. Venkateswarlu, A. Panda, E. Kim and M. Yoon, ACS Appl. Nano Mater., 2018, 1, 4198–4210 CrossRef CAS.
- H. S. Far, M. Hasanzadeh, M. S. Nashtaei, M. Rabbani, A. Haji and B. Hadavi Moghadam, ACS Appl. Mater. Interfaces, 2020, 12, 25294–25303 CrossRef CAS.
- M. Yadollahi, H. Hamadi and V. Nobakht, Appl. Organomet. Chem., 2019, 33, e4629 CrossRef.
- Y. Li, Q. Xie, Q. Hu, C. Li, Z. Huang, X. Yang and H. Guo, Sci. Rep., 2016, 6, 1–11 CrossRef.
- Y. Yan, Y. Lu, B. Wang, Y. Gao, L. Zhao, H. Liang and D. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 26539–26545 CrossRef CAS.
- Y. Yan, Z. Zheng, C. Deng, X. Zhang and P. Yang, Chem. Commun., 2013, 49, 5055–5057 RSC.
- R. K. Sharma, R. Gaur, M. Yadav, A. Goswami, R. Zbořil and M. B. Gawande, Sci. Rep., 2018, 8, 1–12 CrossRef.
- D. Zhang, R. Chung, A. B. Karki, F. Li, D. P. Young and Z. Guo, J. Phys. Chem. C, 2010, 114, 212–219 CrossRef CAS.
- H.-Y. Lee, S.-H. Lee, C. Xu, J. Xie, J.-H. Lee, B. Wu, A. L. Koh, X. Wang, R. Sinclair and S. X. Wang, Nanotechnology, 2008, 19, 165101 CrossRef.
- Y. Zhang, J.-Y. Liu, S. Ma, Y.-J. Zhang, X. Zhao, X.-D. Zhang and Z.-D. Zhang, J. Mater. Sci.: Mater. Med., 2010, 21, 1205–1210 CrossRef CAS.
- H. Liu, P. Hou, W. Zhang and J. Wu, Colloids Surf., A, 2010, 356, 21–27 CrossRef CAS.
- G. Unsoy, S. Yalcin, R. Khodadust, G. Gunduz and U. Gunduz, J. Nanopart. Res., 2012, 14, 1–13 CrossRef.
- L. Zang, J. Qiu, X. Wu, W. Zhang, E. Sakai and Y. Wei, Ind. Eng. Chem. Res., 2014, 53, 3448–3454 CrossRef CAS.
- R. Liu, Y. Guo, G. Odusote, F. Qu and R. D. Priestley, ACS Appl. Mater. Interfaces, 2013, 5, 9167–9171 CrossRef CAS.
- R. Batul, T. Tamanna, A. Khaliq and A. Yu, Biomater. Sci., 2017, 5, 1204–1229 RSC.
- J. Manna, S. Akbayrak and S. Özkar, Appl. Catal., B, 2017, 208, 104–115 CrossRef CAS.
- M. Zhao, C. Deng and X. Zhang, Chem. Commun., 2014, 50, 6228–6231 RSC.
- R. Zhang, Z. Wang, Z. Zhou, D. Li, T. Wang, P. Su and Y. Yang, Ind. Eng. Chem. Res., 2019, 58, 3876–3884 CrossRef CAS.
- A. R. Chowdhuri, T. Singh, S. K. Ghosh and S. K. Sahu, ACS Appl. Mater. Interfaces, 2016, 8, 16573–16583 CrossRef CAS.
- Y. Xu, J. Jin, X. Li, Y. Han, H. Meng, J. Wu and X. Zhang, J. Sep. Sci., 2016, 39, 3647–3654 CrossRef CAS.
- Z. Jin, Y. Luan, M. Yang, J. Tang, J. Wang, H. Gao, Y. Lu and G. Wang, RSC Adv., 2015, 5, 78962–78970 RSC.
- M. Zhao, X. Zhang and C. Deng, Chem. Commun., 2015, 51, 8116–8119 RSC.
- Y. Xie and C. Deng, Sci. Rep., 2017, 7, 1–8 CrossRef.
- J. Zhou, Y. Liang, X. He, L. Chen and Y. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 11413–11421 CrossRef CAS.
- J. Zhou, P. Wang, C. Wang, Y. T. Goh, Z. Fang, P. B. Messersmith and H. Duan, ACS Nano, 2015, 9, 6951–6960 CrossRef CAS.
- Q. Yang, J. Wang, X. Chen, W. Yang, H. Pei, N. Hu, Z. Li, Y. Suo, T. Li and J. Wang, J. Mater. Chem. A, 2018, 6, 2184–2192 RSC.
- B. Luo, Q. Chen, J. He, Z. Li, L. Yu, F. Lan and Y. Wu, ACS Sustainable Chem. Eng., 2019, 7, 6043–6052 CrossRef CAS.
- X. Yue, W. Guo, X. Li, H. Zhou and R. Wang, Environ. Sci. Pollut. Res., 2016, 23, 15218–15226 CrossRef CAS.
- Q. Yang, Y. Zhu, B. Luo, F. Lan, Y. Wu and Z. Gu, Nanoscale, 2017, 9, 527–532 RSC.
- Z. Miao, X. Shu and D. Ramella, RSC Adv., 2017, 7, 2773–2779 RSC.
- T. Zhang, X. Zhang, X. Yan, L. Kong, G. Zhang, H. Liu, J. Qiu and K. L. Yeung, Chem. Eng. J., 2013, 228, 398–404 CrossRef CAS.
- J. Li, H. Gao, L. Tan, Y. Luan and M. Yang, Eur. J. Inorg. Chem., 2016, 4906–4912 CrossRef CAS.
- C. Zhang, L. Ai and J. Jiang, J. Mater. Chem. A, 2015, 3, 3074–3081 RSC.
- H. Li, Q. Li, X. He, N. Zhang, Z. Xu, Y. Wang and Y. Wang, Cryst. Growth Des., 2018, 18, 6248–6256 CrossRef CAS.
- H. Liu, X. Ren and L. Chen, J. Ind. Eng. Chem., 2016, 34, 278–285 CrossRef CAS.
- A. Samui, A. R. Chowdhuri, T. K. Mahto and S. K. Sahu, RSC Adv., 2016, 6, 66385–66393 RSC.
- X. Cao, G. Liu, Y. She, Z. Jiang, F. Jin, M. Jin, P. Du, F. Zhao, Y. Zhang and J. Wang, RSC Adv., 2016, 6, 113144–113151 RSC.
- S. Yadav, S. Sharma, S. Dutta, A. Sharma, A. Adholeya and R. K. Sharma, Inorg. Chem., 2020, 59, 8334–8344 CrossRef CAS.
- M. G. Quesne, F. Silveri, N. H. De Leeuw and C. R. A. Catlow, Front. Chem., 2019, 7, 182 CrossRef CAS.
- L. Rogers and K. F. Jensen, Green Chem., 2019, 21, 3481–3498 RSC.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, ACS Catal., 2018, 9, 1081–1102 CrossRef.
- D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Catal. Sci. Technol., 2011, 1, 856–867 RSC.
- Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC.
- O. A. Kholdeeva and O. V. Zalomaeva, Coord. Chem. Rev., 2016, 306, 302–330 CrossRef CAS.
- Z. Guo, B. Liu, Q. Zhang, W. Deng, Y. Wang and Y. Yang, Chem. Soc. Rev., 2014, 43, 3480–3524 RSC.
- P. Zhang, H. Lu, Y. Zhou, L. Zhang, Z. Wu, S. Yang, H. Shi, Q. Zhu, Y. Chen and S. Dai, Nat. Commun., 2015, 6, 1–10 Search PubMed.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, J. Catal., 2012, 289, 259–265 CrossRef CAS.
- L. Kesavan, R. Tiruvalam, M. H. Ab Rahim, M. I. bin Saiman, D. I. Enache, R. L. Jenkins, N. Dimitratos, J. A. Lopez-Sanchez, S. H. Taylor and D. W. Knight, Science, 2011, 331, 195–199 CrossRef CAS.
- F. Wang, J. Xu, X. Li, J. Gao, L. Zhou and R. Ohnishi, Adv. Synth. Catal., 2005, 347, 1987–1992 CrossRef CAS.
- S. Fan, W. Dong, X. Huang, H. Gao, J. Wang, Z. Jin, J. Tang and G. Wang, ACS Catal., 2017, 7, 243–249 CrossRef CAS.
- J. Schneidewind, R. Adam, W. Baumann, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 1890–1893 CrossRef CAS.
- W. H. Bernskoetter and N. Hazari, Acc. Chem. Res., 2017, 50, 1049–1058 CrossRef CAS.
- J. A. Osborn, F. Jardine, J. F. Young and G. Wilkinson, J. Chem. Soc. A, 1966, 1711–1732 RSC.
- R. Crabtree, Acc. Chem. Res., 1979, 12, 331–337 CrossRef CAS.
- D. Wang and D. Astruc, Chem. Rev., 2015, 115, 6621–6686 CrossRef CAS.
- S. Werkmeister, J. Neumann, K. Junge and M. Beller, Chem. – Eur. J., 2015, 21, 12226–12250 CrossRef CAS.
- V. Y. Doluda, A. Filatova, E. Sul’man, V. Matveeva, S. Mikhailov, A. Sidorov and Y. Y. Kosivtsov, Catal. Ind., 2018, 10, 328–334 CrossRef.
- C. S. Couto, L. M. Madeira, C. P. Nunes and P. Araújo, Chem. Eng. Technol., 2015, 38, 1625–1636 CrossRef CAS.
- R. Xu, S. Chakraborty, S. M. Bellows, H. Yuan, T. R. Cundari and W. D. Jones, ACS Catal., 2016, 6, 2127–2135 CrossRef CAS.
- H.-j. Zhang, X.-y. Niu, J. Hu, C.-l. Ren, H.-l. Chen and X.-g. Chen, Catal. Sci. Technol., 2014, 4, 3013–3024 RSC.
- F. Ke, L. Wang and J. Zhu, Nanoscale, 2015, 7, 1201–1208 RSC.
- S. Yang, Z. H. Zhang, Q. Chen, M. Y. He and L. Wang, Appl. Organomet. Chem., 2018, 32, e4132 CrossRef.
- X. Chen, Y. Zhang, Y. Zhao, S. Wang, L. Liu, W. Xu, Z. Guo, S. Wang, Y. Liu and J. Zhang, Inorg. Chem., 2019, 58, 12433–12440 CrossRef CAS.
- S. Chang, C. Liu, Y. Sun, Z. Yan, X. Zhang, X. Hu and H. Zhang, ACS Appl. Nano Mater., 2020, 3, 2302–2309 CrossRef CAS.
- L. Yang, Y. Jin, X. Fang, Z. Cheng and Z. Zhou, Ind. Eng. Chem. Res., 2017, 56, 14182–14191 CrossRef CAS.
- Y. Zhong, Y. Mao, S. Shi, M. Wan, C. Ma, S. Wang, C. Chen, D. Zhao and N. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 32251–32260 CrossRef CAS.
- A. Biffis, P. Centomo, A. Del Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249–2295 CrossRef CAS.
- C. M. Volla, I. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390–2431 CrossRef CAS.
- P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS.
- L. Xue and Z. Lin, Chem. Soc. Rev., 2010, 39, 1692–1705 RSC.
- Q. Li, S. Jiang, S. Ji, D. Shi and H. Li, J. Porous Mater., 2015, 22, 1205–1214 CrossRef CAS.
- S. Jiang, J. Yan, F. Habimana and S. Ji, Catal. Today, 2016, 264, 83–90 CrossRef CAS.
- Y. Hu, S. Zheng and F. Zhang, Front. Chem. Sci. Eng., 2016, 10, 534–541 CrossRef CAS.
- T. D. Diep, P. D. Q. Dao and C. S. Cho, Eur. J. Org. Chem., 2019, 4071–4079 CrossRef CAS.
- A. Nuri, N. Vucetic, J.-H. Smått, Y. Mansoori, J.-P. Mikkola and D. Y. Murzin, Catal. Lett., 2020, 150, 2617–2629 CrossRef CAS.
- A. Mohammadinezhad and B. Akhlaghinia, New J. Chem., 2019, 43, 15525–15538 RSC.
- A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef CAS.
- S. Zhi, X. Ma and W. Zhang, Org. Biomol. Chem., 2019, 17, 7632–7650 RSC.
- M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547–4592 RSC.
- H.-Y. Zhang, X.-P. Hao, L.-P. Mo, S.-S. Liu, W.-B. Zhang and Z.-H. Zhang, New J. Chem., 2017, 41, 7108–7115 RSC.
- L. Wang, S. Yang, L. Chen, S. Yuan, Q. Chen, M.-Y. He and Z.-H. Zhang, Catal. Sci. Technol., 2017, 7, 2356–2361 RSC.
- A. Gupta, F. K. Sarkar, R. Sarkar, R. Jamatia, C. Y. Lee, G. Gupta and A. K. Pal, Appl. Organomet. Chem., 2020, 34, e5782 CrossRef CAS.
- Q. Li, S. Jiang, S. Ji, M. Ammar, Q. Zhang and J. Yan, J. Solid State Chem., 2015, 223, 65–72 CrossRef CAS.
- F. Pang, M. He and J. Ge, Chem. – Eur. J., 2015, 21, 6879–6887 CrossRef CAS.
- A. Schejn, T. Mazet, V. Falk, L. Balan, L. Aranda, G. Medjahdi and R. Schneider, Dalton Trans., 2015, 44, 10136–10140 RSC.
- W. Li, G. Li and D. Liu, RSC Adv., 2016, 6, 94113–94118 RSC.
- Y. Zhang, T. Dai, F. Zhang, J. Zhang, G. Chu and C. Quan, Chin. J. Catal., 2016, 37, 2106–2113 CrossRef CAS.
- Y. Zhang, F. Zhang, X. Zhang, Y. Xu, X. Qi and C. Quan, Chem. Res. Chin. Univ., 2018, 34, 655–660 CrossRef CAS.
- T. Toyao, M. J. Styles, T. Yago, M. M. Sadiq, R. Riccò, K. Suzuki, Y. Horiuchi, M. Takahashi, M. Matsuoka and P. Falcaro, CrystEngComm, 2017, 19, 4201–4210 RSC.
- E. Rafiee and F. Rahimi, Monatsh. Chem., 2013, 144, 361–367 CrossRef CAS.
- M. R. Sazegar, S. Mahmoudian, A. Mahmoudi, S. Triwahyono, A. A. Jalil, R. R. Mukti, N. H. N. Kamarudin and M. K. Ghoreishi, RSC Adv., 2016, 6, 11023–11031 RSC.
- N. H. Jadhav, S. S. Sakate, N. K. Rasal, D. R. Shinde and R. A. Pawar, ACS Omega, 2019, 4, 8522–8527 CrossRef CAS.
- N. G. Khaligh, Catal. Sci. Technol., 2012, 2, 1633–1636 RSC.
- Q. Li, S. Jiang, S. Ji, D. Shi, J. Yan, Y. Huo and Q. Zhang, Ind. Eng. Chem. Res., 2014, 53, 14948–14955 CrossRef CAS.
- H. Yan, J. S. Oh, J.-W. Lee and C. E. Song, Nat. Commun., 2012, 3, 1–7 Search PubMed.
- M. M. Mostafavi and F. Movahedi, Appl. Organomet. Chem., 2018, 32, e4217 CrossRef.
- Z. Wu, C. Chen, H. Wan, L. Wang, Z. Li, B. Li, Q. Guo and G. Guan, Energy Fuels, 2016, 30, 10739–10746 CrossRef CAS.
- D. Zhou, X. Chen, B. Liang, X. Fan, X. Wei, J. Liang and L. Wang, Microporous Mesoporous Mater., 2019, 289, 109615 CrossRef CAS.
- E. M. Dias and C. Petit, J. Mater. Chem. A, 2015, 3, 22484–22506 RSC.
- L. Yu, H. Wang, Y. Zhang, B. Zhang and J. Liu, Environ. Sci.: Nano, 2016, 3, 28–44 RSC.
- H. Niu, Y. Zheng, S. Wang, L. Zhao, S. Yang and Y. Cai, J. Hazard. Mater., 2018, 346, 174–183 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- S. Abedi and A. Morsali, ACS Catal., 2014, 4, 1398–1403 CrossRef CAS.
- J. Cai, J.-Y. Lu, Q.-Y. Chen, L.-L. Qu, Y.-Q. Lu and G.-F. Gao, New J. Chem., 2017, 41, 3882–3886 RSC.
- C. Yang, X. You, J. Cheng, H. Zheng and Y. Chen, Appl. Catal., B, 2017, 200, 673–680 CrossRef CAS.
- C.-F. Zhang, L.-G. Qiu, F. Ke, Y.-J. Zhu, Y.-P. Yuan, G.-S. Xu and X. Jiang, J. Mater. Chem. A, 2013, 1, 14329–14334 RSC.
- S. Li, J. Cui, X. Wu, X. Zhang, Q. Hu and X. Hou, J. Hazard. Mater., 2019, 373, 408–416 CrossRef CAS.
- H. Tian, J. Peng, Q. Du, X. Hui and H. He, Dalton Trans., 2018, 47, 3417–3424 RSC.
- X. Ma, S. Wen, X. Xue, Y. Guo, J. Jin, W. Song and B. Zhao, ACS Appl. Mater. Interfaces, 2018, 10, 25726–25736 CrossRef CAS.
- S. Neupane, K. Patnode, H. Li, K. Baryeh, G. Liu, J. Hu, B. Chen, Y. Pan and Z. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 12133–12141 CrossRef CAS.
- J. Huo, J. Aguilera-Sigalat, S. El-Hankari and D. Bradshaw, Chem. Sci., 2015, 6, 1938–1943 RSC.
- C. Lin, K. Xu, R. Zheng and Y. Zheng, Chem. Commun., 2019, 55, 5697–5700 RSC.
| This journal is © The Royal Society of Chemistry 2021 |