Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solid waste-derived carbon nanomaterials for supercapacitor applications: a recent overview
Gaurav
Tatrari
,
Manoj
Karakoti
,
Chetna
Tewari
,
Sandeep
Pandey
,
Bhashkar Singh
Bohra
,
Anirban
Dandapat
 and
Nanda Gopal
Sahoo
and
Nanda Gopal
Sahoo
 *
*
Prof. Rajendra Singh Nanoscience and Nanotechnology Centre, Department of Chemistry, DSB Campus, Kumaun University, Nainital, Uttarakhand, India. E-mail: ngsahoo@yahoo.co.in
First published on 20th January 2021
Abstract
Universal solid waste management and its hazardous effects on the ecology, ecosystem, and the global human health index are some of the major issues that are currently threatening our future. The overproduction of solid waste materials is mainly the consequence of human population explosion and extraordinary increase in global economy along with global productivity. Due to the rapid growth in global population and the related demands, the production of solid waste materials is increasing day by day. In order to have a better solution to solid waste management, the best ethical way is to convert solid waste materials into valuable carbon-based nanomaterials (CNMs) such as CNT, graphene, and carbon quantum dots (CQDs), which can further work as precursors in energy storage devices. This critical review summarizes the recent progress in the structural forms and current situational analysis of universal solid waste based CNMs. It also includes broadly discussed issues, starting with the current waste situation analysis of global energy crisis and the production of graphene, including its counterparts, as the precursors for supercapacitor applications. A paradigm study of various solid waste derived CNMs with their significance, which, in particular, depends upon the types of CNMs and the classification of solid waste derived CNMs on the basis of different types of pore size and pore distribution, has been carried out. The various structural modifications and their effects on the electrochemical properties have been discussed thoroughly for supercapacitor applications including their future possibilities and scope. The review outlines the crucial mechanism followed during the charge storage of different kinds of supercapacitors and various electrochemical calculations involved during the performance testing of the fabricated device. We hope that the target audience, belonging to the energy or material science field, may acquire relevant information from this review, which may further help them to design futuristic studies in the field.
1. Introduction
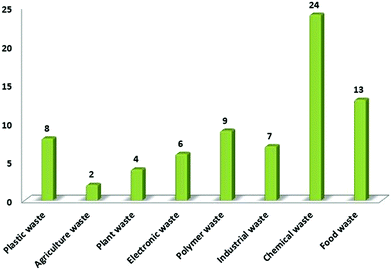
Global population explosion has led an the exponential and unorthodox growth in universal development and thus, the related demands are also increasing day by day, which lead to an exponential increase in the production of different kinds of solid wastes. According to some recent scientific articles, globally, a total of 7 to 8 billion tons solid waste is being produced every year, which has several harmful environmental and health effects, which create a huge challenge for the scientific community.1 Waste plastic typically weighs 10 wt% of the global waste generated per year.2 In the last 50 years, universal contamination due to plastic waste has increased dramatically to its extreme level, mainly due to its applications in various industries, such as packaging, building and construction, transportation, automobile energy storage devices, and electronic goods. In 2011, universal plastic production reached at the highest record value of 280 million tons, to which 23% was contributed by China alone, followed by 21% from the European Union, 16% from USA, and 5% from Japan.3 Solid biomass and agricultural wastes generate many toxins on burning that are usually released into the atmosphere. The nature of emission mainly depends upon the kind of feedstock of biomass and their types. Several pollutants such as nitrogen oxide, carbon monoxide, and sulfur dioxide are commonly released in the atmosphere. Waste biomass causes forest fires and can also increase the global production of greenhouse gases by 25 to 30% each year.4–10 According to some reports, universal crops yield about 146 billion tons of solid waste every year11 and the household waste is about 100 billion tons.12 The proper management of such a huge amount of solid waste is itself a big environmental issue.13 To overcome the situation with great success, scientific development needs an innovative approach, which can solve and manage the utilization of such wastes by adopting some advanced scientific technologies. In this regard, the existence of nano-science and nano-technology has been a huge boon for the scientific community, which can work simultaneously as the lifeline for the sustainable management of several universal obligations, including solid waste management and the universal energy crisis. Accordingly, waste management technologies need to be innovated so that the problem of solid waste managements can be dealt with comprehensively. In addition, the major problem in front of the scientific community is to develop a new pathway for the production of eco-friendly and cost-effective energy storage devices to deal with the energy crisis of the world. Meanwhile, dealing with these global issues, several scientific communities have been able to report some distinct researches, including efficient waste management and cost-effective production of carbon nanomaterials (CNMs), for e.g., carbon nanotubes (CNT), graphene, and carbon quantum dots (CQDs), for various industrial applications, especially in the field of energy. CNMs are carbon-based nanomaterials having extensive potential for various applications. Thus, the generated solid waste can be utilized by increasing the production of various CNMs from solid waste materials. Among CNMs, CNTs, CQDs, and graphene have some exceptional properties, which includes a huge aspect ratio, supreme electrical properties, exceptional mechanical strength, and superior thermal conductivity.14–17 The research articles published during 2014–2019 on the conversion of solid wastes into CNMs and related applications such as supercapacitors, solar cells, fuel cells, drug delivery, and bioimaging are outlined in Fig. 1. It clearly shows the extensive research that is taking place in the field of waste management and solid waste based CNMs. It also represents the growing futuristic interest among the scientific community for solid waste based CNMs. The evaluated data was searched in Feb. 2020 with the help of Web of Science. The successive increment in per year research publications on solid waste based CNMs, especially for their use in the field of energy, i.e., supercapacitor applications, show the increasing number of publications per year (Fig. 1).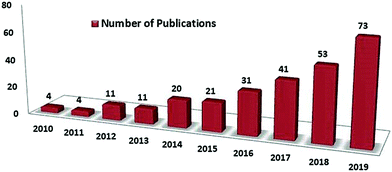 | ||
| Fig. 1 Number of publications per year reported on waste based CNMs from 2010 to 2019 (using Web of Science database; date of search Feb. 2020). | ||
Fig. 2 clearly shows the current trends and research direction involving different kinds of solid waste based CNMs, which indicates the role of waste management in futuristic development and innovations. The presence of high carbon content in solid waste materials allows the scientific community to convert them into different CNMs such as graphene, CNT, and CQDs; further, modified and doped nanomaterials have been used as materials in energy appliances extensively by various researchers in the last few years.
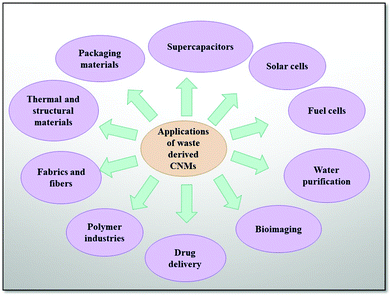
The applications of CNMs in various fields of science and technology are due to their respective excellent properties and surface modifications along with the specific properties, which have also been assisted thoroughly in various applied fields (Fig. 3), such as supercapacitor materials,18–20 solar cells,21–23 as a battery's electrode,24,25 catalyst supports systems for proton exchange membrane fuel cells,26–28 and also to develop polymer nanocomposites for various applications,29–31i.e., in drug delivery, bioimaging,32–34 medicinal biology,35–38 aerospace,39,40 and new age weapon technology.41,42
Numerous theoretical approaches have been made by researchers for the analysis of waste based CNMs utilized in various applications by functionalization and doping possibilities with various metals,43,44 metal oxide,45,46 non-metals,47,48 or by strong surface interactions with organic reagents,49,50 conductive polymer incorporation,51,52etc. Moreover, it has been reported that functionalized CNMs are stronger candidates for various uses, in comparison to non-functionalized ones, as they have aggregation problems.53,54 Based on the structure and elemental doping, CNMs have been vigorously used in electrode materials of batteries, supercapacitors, and fuel cells for various biomedical applications.
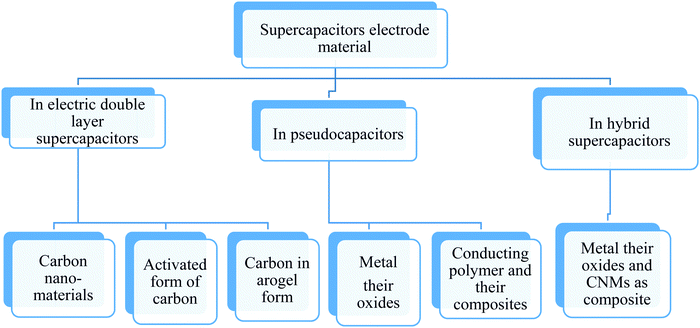
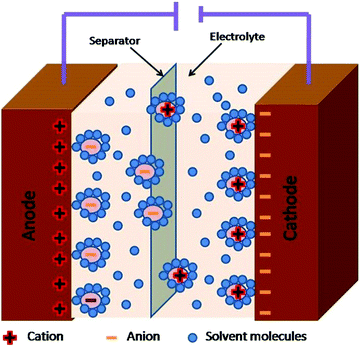
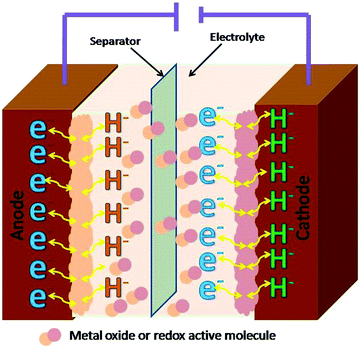
Supercapacitors have been sub-divided as the electrochemical double layer (EDLC) and pseudo supercapacitors.55,56 The use of CNMs as electrode materials for EDLC and pseudo supercapacitors have been rising since the last decade due to the exuberant power density and excessive energy density they produce, especially for various energy applications in comparison to conventional batteries and Li-ion batteries.57–59 In EDLC, the electrical energy is stored through the electrostatic accumulation of charge and in pseudocapacitors through reversible reactions. The electrical conductivity, theoretical specific surface area, geometrical configurations, porous nature, and variations in mass densities excessively affect the performance of supercapacitors.
Nowadays, structural modifications are also building up some hype in the field; as recently found, flexible supercapacitors are emerging as a winner in comparison to conventional fixed-batch devices as they have been successfully implemented in various devices such as in portable electronic devices, electrical vehicles, and memory backup systems.60–62 Thus, by such a theoretical evaluation of universal waste management situations and universal energy requirements, it can be outlined that the solid waste based CNMs are the best way to design proficient technology having economic and ecofriendly benefits.
This review highlights the remarkable properties of various solid waste based CNMs and their successive utilization in energy storage applications. The recent development and innovations to deal with universal solid waste management and innovative structural modifications of CNMs to the enhance electrochemical performance of the fabricated device based on their unique properties are also included. In conjunction, the specific study and performance about cost-effective supercapacitors with specific reference to their structural modifications, degree of carbonizations, pore structure, surface properties, doping, and variations in mass densities have been discussed. The review also includes various scientific methodologies followed during performance testing and the instrumentation required. In addition, various new developments have been discussed in universal waste management and universal energy crisis to make this review a valuable tool for the future perspective of mankind.
2. Solid waste as a universal problem
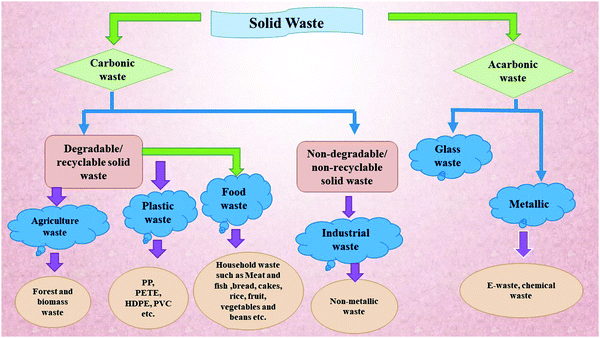
Solid waste has emerged as a universal problem mainly due to its harmful environmental aspects and health hazardous consequences. Continued population growth and their increasing universal demands are the main reason behind the overproduction of various solid wastes such as waste plastics, agriculture waste, chemical waste, paper waste, industrial waste, and electronic waste. Solid waste has emerged as an extremely dangerous factor that affects the human health and the environment simultaneously. It causes diseases such as cancer, skin disease, respiratory disease, birth defects, and reproductive disorders.63,64 According to Biggeri et al.,65 the risk of having lung cancer increases on decreasing the distance of the source from the incinerator. Comba et al.66 reported a case-controlled study on the effect of solid waste materials on human health. They reported a significant risk of cancer and other diseases in residents living within 2 km of the solid waste dumping sites.66 On the other hand, Zambon et al.67 reported a clear association in between soft tissue sarcoma and dioxin exposure at the municipal solid waste incinerators site. Several other diseases have also been reported as the side effects of solid waste sites,68 which includes cancer, stomach ache, liver damage, and lung cancer. Hsiue et al. reported a study on the respiratory health issues of children living in polluted areas, where a total of 384 children were chosen from polluted areas. The successive decrement in relative pulmonary function was reported for most of the children.69 Similarly, a comprehensive study was done by Miyake et al.70 on school children. They observed a relationship amongst the frequency of allergic sicknesses and symptoms in children with the distance from the polluted areas, where the children studying at schools nearer to incineration plants were reported to have more frequent headaches and wheezing. Along with this, poor waste management is also a crucial factor in polluting the oceans, blocking drains, triggering flooding, and harming animals that eat waste naively, thus disturbing environmental and economic development.Table 1 depicts the contribution of various provinces worldwide in the production of waste plastic and Fig. 4 shows the distribution of solid waste in India. According to the World Bank's report waste 2.0, universally, 2.01 billion tonnes of solid waste is generated annually, in which at least 33% is not environment friendly. According to the World Bank report, rapid urbanization, population explosion, and economic growth will push universal solid wastes so as to increase their content by 70% in the coming 30 years by generating 3.40 billion tonnes of solid waste annually. Waste plastic alone makes up to 90% of marine junk and chokes most of the oceans. In the year 2016, 242 million tonnes of plastic waste was generated globally, which is equivalent to 24 trillion 500 mm 10 g plastic bottles. The water volume of such bottle could fill up 2400 Olympic stadiums, 4.8 million Olympic size swimming pools, or 40 billion bathtubs. This is also the weight of 3.4 million adult blue whales or 1376 Empire State Buildings.75 It can be concluded that solid waste has emerged as a huge potential problem universally and the scientific community. In order to view the situation more clearly, universal solid wastes can be classified in to two subheadings, viz., carbonic and non-carbonic solid wastes. Fig. 5 shows the classification of different solid wastes present in the global perspective. In depth, the current universal solid waste situation and its distribution scenario depicts the urgency for its sustainable management. The section given below explains the possible application potentials of solid waste materials and their derivatives for the sustainable management of universal ecology.
| S. no. | Province | Plastic production in % |
|---|---|---|
| 1. | China | 23 |
| 2. | Europe | 21 |
| 3. | Rest Asia | 20 |
| 4. | Middle east Africa | 7 |
| 5. | Latin America | 5 |
| 6. | Japan | 5 |
| 7. | CIS | 3 |
| 8. | NAFA | 20 |
| 9. | USA | 16 |
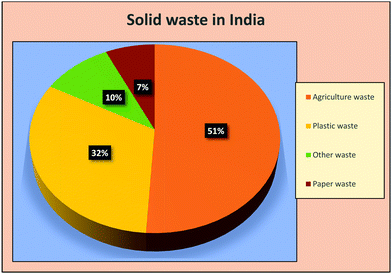 | ||
| Fig. 4 The distribution of solid waste in India.72–74 | ||
3. Solid wastes as precursors of various CNMs
Solid waste includes waste plastic, agricultural waste, municipal waste, paper waste, polymeric wastage, and industrial waste, most of which consist of carbonic skeletons as the structural framework, thus enabling nano-science to operate at the ground level. On the other hand, the proficiency to operate the activation can alter the structural morphology of the carbonic skeleton, mostly depending upon the processing methodology. Fig. 6 shows different kinds of solid waste materials that are used as the precursors of CNMs.There are various methodologies to avail the conversion of carbonic waste into CNMs, which include chemical vapour deposition (CVD), physical vapour deposition (PVD), organic redox methodologies (e.g., modified Hummers’ method), and pyrolytic heating. Every methodology (Fig. 7) has its own specificity for producing different carbon nanomaterials (CNMs), which mainly depend upon specific surface reactions and variation of reagents. However, in the pyrolysis process, the heating temperature, pressure, atmosphere, and scan rate matters the most, which alters the formations of layers and variations in CNMs. This review summarizes the solid waste to CNMs conversion strategies, their application potential in supercapacitors, and other morphological effects affecting the electrochemical performance of the fabricated supercapacitor device.
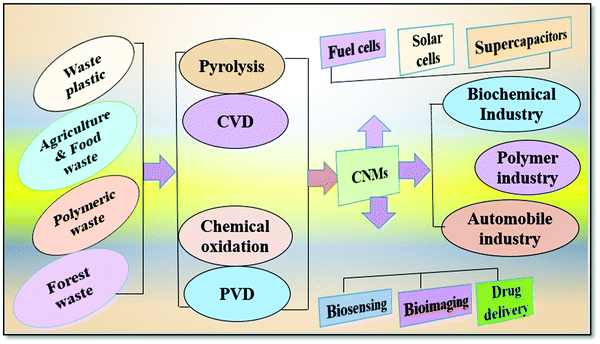 | ||
| Fig. 7 Schematic representation showing various synthetic pathways and industrial uses of different waste derived CNMs. | ||
3.1. Solid waste to graphene
Mostly, solid waste consists of a carbonic framework of atoms having millions of layers; thus, it can be converted into graphene. Graphene is a single layer of graphite that has a 2D skeleton and is the thinnest known allotropic form of carbon, with the highest specific surface area, excellent electrical and thermal conductivity, surpassing the mechanical strength76–79 due to C–C sigma covalent linkage, which makes graphene a brilliant candidate for its use in various applications.Essawy et al. have synthesized graphene from waste plastic bottles containing PET plastic bottles. The plastic wastes were crushed firstly, and sieved to get micron-size particles, then the sieved residue (2 g) was placed inside the stainless-steel autoclave reactor (SS316), which was placed inside the center of an electric furnace at 800 °C for 1 h and finally cooled the sample, which was characterized as graphene.80 Gong et al. have used the up-cyclization process for the conversion of plastic waste into graphene. They have reported a simple method of synthesizing graphene flakes with high yield by introducing organically modified catalyst (OMMT), which was used as a degradation agent for waste polypropylene (PP) to graphene conversion at 700 °C inside the tube furnace under an inert environment.81
Fig. 8 displays the synthesis of porous graphitic carbon from natural cotton and the conversion of reeds into interconnected porous carbon nanosheets. In the first case, high temperature carbonization, followed by the activation through sodium metal ion and further modifying it with an organic molecule, were performed to manufacture porous graphitic carbon from natural cotton.82 On the other hand, Fig. 8b depicts the processing of cellulosic reed by the hydrothermal approach, followed by carbonization and activation to produce interconnected porous carbon nanosheets.83
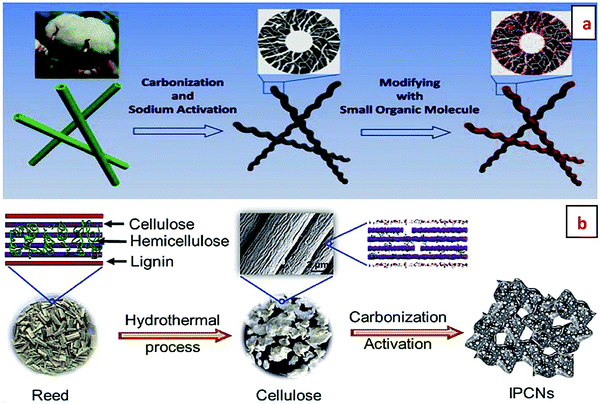 | ||
| Fig. 8 Schematic illustration for (a) the synthesis of porous graphitic carbon from natural cotton.82 Reproduced by permission of Elsevier, and (b) the conversion of reeds into interconnected porous carbon nanosheets.83 Reproduced by permission of RSC. | ||
Kamali et al. have reported the pyrolysis of waste plastic bottles in a tube furnace at 1300 °C with the heating rate of 10 °C min−1, followed by for overnight air drying with heating at 80 °C, which converts waste plastics into the graphene nanostructure.84 Recently, Wen et al. unveiled the conversion of waste plastic into graphene nanostructure using organically-modified montmorillonite (OMMT) clay as the catalyst and solvolytic KOH as the activation agent. OMMT was added in the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in the mixture at 190 °C for 5 min, followed by carbonization in a conventional quartz tube reactor for 10 min at 700 °C to obtain the resultant CNM/MMT composite. Further, washing with nitric acid and HF delivers the purified form of graphene. However, for obtaining potassium-doped CNMs, the activation process followed by heating at 850 °C temperature and KOH solution as the activator were used.85
5 in the mixture at 190 °C for 5 min, followed by carbonization in a conventional quartz tube reactor for 10 min at 700 °C to obtain the resultant CNM/MMT composite. Further, washing with nitric acid and HF delivers the purified form of graphene. However, for obtaining potassium-doped CNMs, the activation process followed by heating at 850 °C temperature and KOH solution as the activator were used.85
Recently, Pandey et al. reported a robust and innovative approach to deal with waste plastic problem by the mass conversion of waste plastic into graphene sheets, following the sophisticated two-step pyrolysis process (Fig. 9). In the first step, slow pyrolysis was carried out in a primary reactor at 400 °C with a heating rate of 5 °C min−1. At this temperature, lower hydrocarbons were released in the form of fuels and gases. In the second step, high temperature pyrolysis at 750 °C was conducted with the heating rate of 10 °C min−1 in an inert environment. The final product was confirmed to be graphene nano-sheets.86 In another report, Tewari et al. reported an eco-friendly and green approach for the synthesis of potassium (K)-doped graphene oxide from the agricultural waste of oak seeds via solvothermal process using ethyl alcohol and distilled water as green solvents. The internal part of oak seeds was used and heated at 120 °C for 4 h inside the oven until a brown residue was obtained. The brownish residue was again crushed and mixed in 30 mL distilled water, followed by the stirring of 30 min, has been discussed. Finally, filtration was done by using 0.2-micron pore-sized nylon filter paper. The obtained filtered liquid was dried at 100 °C and thus the obtained material was identified as potassium-doped graphene oxide.87
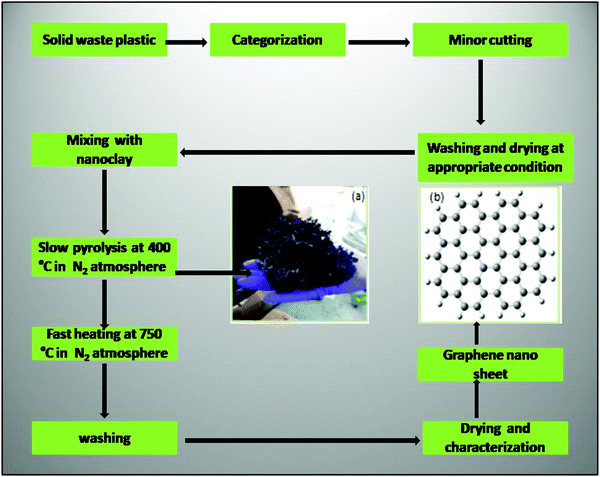 | ||
| Fig. 9 Flow chart for the conversion of solid plastic into CNMs. (a) The carbon material obtained from the low temperature pyrolysis of waste plastic, (b) Skeleton structure of the graphene nanosheets’ high temperature pyrolysis.86 Reproduced by permission of Elsevier. | ||
The conversion of halogen based solid waste materials, especially PTFE, PVDF, and PVC into nano-porous carbon, using zinc powder as a hard template, was reported by Chen et al.88 The mass ratio between the plastics and zinc powder as well as the carbonization temperature play a crucial role in determining the carbonic structure and the resultant electrochemical performances. The optimized product was obtained by carbonizing polytetrafluoroethene and zinc powder (mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at 700 °C under the inert atmosphere of argon.88
3) at 700 °C under the inert atmosphere of argon.88
The conversion of solid waste into CNMs followed the CVD process, as reported by various research groups. Briefly, properly washed solid waste materials were placed into a tube furnace having high temperature and an inert atmosphere. Further, the scan rate of 150/25 sccm acted as a very effective parameter for the synthesis of graphene. Thus, the treated solid waste was processed at 1050 °C for 2 hours in the tube furnace just to remove the presence of oxide functionalities; finally, the annealing process resulted in few layered graphene. The scan rates of the gases may be one of the factors behind the good graphitization of solid waste materials at such a high temperature.89
Papon et al. reported the pyrolysis of carbonic waste in quartz furnace using Cu foils and a heating range of 1050–1070 °C with a specific gas flow of 100 square cubic centimeters of hydrogen in both the pre-annealing and annealing processes. They found that the gas flow of 98 sccm for argon and 2.5 sccm for hydrogen produces good quality graphene.90 Cui et al. reported the CVD method for the production of graphene from waste plastic, with argon and hydrogen as the continuously flowing gases. They used various waste plastics, such as PET, PVC, PP, PMMA, and PS and Ni foil as the catalyst. Ni foil was initially heated in an Ar/H2 atmosphere at 1050 °C for half an hour to remove the oxides from the Ni surface. Thereafter, the main annealing process was carried out by following the CVD method at 1050 °C to produce free-standing graphene foil.91 Sharma et al. have used the solid waste plastic as a precursor for the production of graphene by the CVD methodology; further, Raman and other characterization techniques were used to identify the prepared product as graphene.92
3.2. Solid waste to CNT
Solid waste can also be converted into carbon nanotubes (CNT) by variations in the temperature, pressure, and scan rates of the pyrolysis unit. On the basis of their structural alignment, CNT is of two types: single walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). The former one has a cylindrical roll of graphene sheets with in-phase and out-phase bonding of sp2 carbon atoms.93,94 SWCNTs are categorized in accordance to their structural patterns, conductivity, and chirality basis into metallic SWCNTs, semiconductor SWCNTs, and semi-metallic SWCNTs.94,95 The co-dimensional arrangement of SWCNTs leads to MWCNTs, whereas the difference of diameter is also huge for MWCNTs in compared to SWCNTs.96 The stronger carbon–carbon sigma bonds lead to molecular stiffness and toughness of the CNTs.97Ganesh et al. reported waste plastic to CNTs conversion by using Ni/Mo/MgO as the catalyst. The specific activity of three components Ni, Mo, and MgO was measured and found to be interdependent. Nonetheless, the effect of Ni was found to be the most efficient one. The catalyst having a composition of Ni0.8Mo0.1MgO0.1 produces optimum yields of CNT.98
Mishra et al. used thermal heating for the conversion of municipality solid waste into CNTs using Ni as the catalyst. They used 10 g of PP as the precursor and 1 mg of Ni as the catalyst and further introduced them into a tube furnace under 10 sccm of hydrogen along with 90 sccm for argon. The temperature of the furnace ranged from 600 to 800 °C and the product obtained at 800 °C was found to be the purest.99 Similarly, the pyrolytic approach for the production of CNTs from solid waste plastic has been explained by Pol et al. where they used 1 g polyethene waste with cobalt acetate as the catalyst placed into an autoclave in nitrogen atmosphere. The furnace was raised to the temperature of 700 °C and they reported 0.4 g CNTs as the final product along with emissions of some LPG gases, which were said to be responsible for the generation of pressure inside the vessel.100
As reported by Wu et al., the gasification of waste PP leads the formation of hydrogen gas and CNTs as major products using either Ni/Ca–Al and or Ni/Zn–Al as the catalyst. However, an enhanced amount of hydrogen was yielded by Ni/Zn–Al compared to that of Ni/Ca–Al, while Ni/Ca–Al provided higher amounts of CNTs than that in the case of Ni/Zn–Al from a fixed amount of polypropylene.101 Nickel dioxides, trioxide, and hydroxides along with organically modified clay (OMMT) were used by Jiang et al. for the qualitative formation of MWCNTs at a temperature of 630–830 °C.102 They reported a variation in the quality of CNTs due to slight variations in the temperature range and OMMT clays concentration along with Ni-based catalysts.102 One-pot direct synthesis was used by Gong et al., where heteroatom-based carbonization played a very crucial role, specially using nickel trioxide and copper chloride as the catalysts. The direct effect on the morphology and quantity of CNTs has been observed with the implementation of molar variations of each catalyst and raw materials.103
CNTs having a diameter in the range of 10–40 nm were obtained by the upcycling of waste carbonaceous material at the temperature of 450 °C and inert helium atmosphere.104 Curved CNTs were synthesized using Ni-plates as outstanding decomposition catalysts by Mukhopadhyay et al.104 Zhuo et al. reported the conversion of waste plastic bottle into CNTs via the up-cyclisation process, where PE and PET bottles were used under high temperature pyrolytic up-cyclization procedure, which included three steps.105 In the first step, chipped plastic bottles were placed at the temperature of 600–1000 °C under nitrogen atmosphere and lateral stage feedstock pyrolyzates were introduced into the secondary reactor with venture setup, which is the synthetic reactor, while O2 was introduced to balance the presence of the lower ratio between carbon and oxygen in the feedstock. Finally, in the third step, the introduction of catalytic substrate positioned in the direction of gaseous flow has been operated.105
Similarly, the conversion of waste PP into CNT was done by Liu et al. through binary synthetic pyrolytic approach in a screw kiln reactor. They reported pyrolysis at 1023 °C in the presence of zeolite-based catalyst for the decomposition of plastic into a moving bed reactor with an inert atmosphere. The synthesis of MWCNTs at 723–1123 °C temperatures in the presence of nickel oxide as the catalyst has also been reported, having a maximum yield of MWCNTs at 973 °C.106 These studies showed that the nature and proportions of the catalyst play a key role in the development of CNTs from waste plastic. Some research studies have also established Ni as an excellent catalyst and excellent promoter for CNT production. On the other hand, the optimum quality of MWCNTs was obtained by varying the concentrations organic and inorganic catalysts and the corresponding temperature. Researchers have revealed that the optimum molar ratios of carbon and nickel can also enhance the production of CNTs, where click chemistry plays a very crucial role. A molar ratio of 0.125 for the Cl/Ni complex results in excellent yield for CNT production.104
The implementation of different bed reactors and high temperatures for the synthesis of CNTs have been reported by various researchers, where the venture setup of the reactor was stated as the specific reactor setup, which can control the production of CNTs with specific flow rates of O2 for oxidation. The temperature of 973 K was specified as the maximum production temperature for multiwalled CNTs, mostly due to the distinct folding of the layers at such a high temperature. The slow or sudden rise of temperature with catalytic eminence and types of reactors could be the possible reason behind the changes in production and qualities of CNTs.
3.3. Waste to carbon quantum dots
CQDs have some interesting properties, such as exciting photoluminescence behavior, optically stable nature, biocompatibility, and low toxicity, which have attracted researchers worldwide.107–110 Various methodologies, i.e., arc-discharge, laser ablation, electrochemical oxidation and microwave heating, and hydrothermal methodology, have been reported for the preparation of CQDs from waste materials.111,112In general, there are two methodologies for the synthesis of quantum dots, which includes top-down and bottom-up approaches, as depicted in Fig. 10. In the bottom-up approach, smaller molecules aggregate to form larger ones, while in the top-down approach, large molecules are broken down into their smaller counterparts for the synthesis of quantum dots.113,114 The synthetic methods follow either of these two broad categories. In top-bottom and bottom-top methods, however, the synthesis of quantum dots (QDs) from solid waste materials is usually processed through the top-bottom approach.
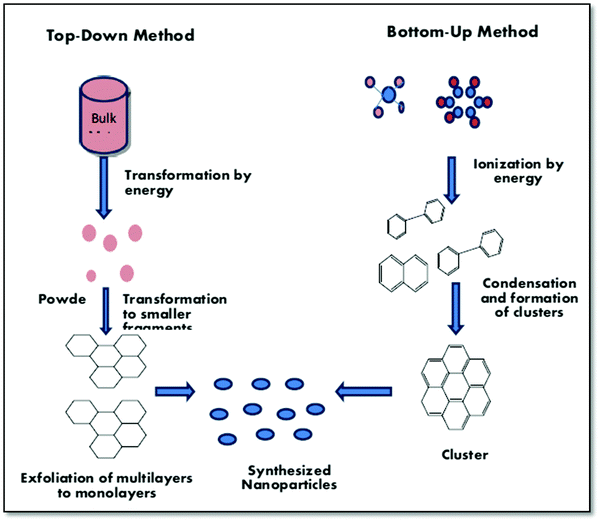 | ||
| Fig. 10 Schematic representation of the bottom-up and top-down approach for the synthesis of quantum dots.113 Reproduced by permission of Elsevier.114 Reproduced by permission of MDPI. | ||
Wei et al. reported the synthesis of CQDs from waste paper by a simple green hydrothermal methodology, where they chopped waste paper and sonicated it with distilled water for 30 min, then transferred it into a Teflon-lined stainless-steel reactor of 40 mL at 180 °C for 10 h. A dark brown precipitate was collected by centrifugation and the obtained residue was finally dialyzed for 48 h with distilled water for further purification.115
Hu et al. reported the synthesis of CQDs from waste plastic bags by hydrothermal treatment in H2O2 solution, where small pieces of plastic waste were poured into a 50 mL stainless steel autoclave having 30 mL H2O2 solution at 180 °C for 12 h.116 Further, centrifugation was performed at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to remove the presence of various impurities, followed by dialysis for 3 days under a solution of deionized water to obtain the pure CQDs. 6 mg of CQDs was dissolved in 100 mL PBS buffer solution and then ferrate ions (0.1 mL) were added into the CQDs (0.9 mL) dispersion to record the fluorescent emission spectra with the excitation wavelength of 320 nm.116
000 rpm for 10 min to remove the presence of various impurities, followed by dialysis for 3 days under a solution of deionized water to obtain the pure CQDs. 6 mg of CQDs was dissolved in 100 mL PBS buffer solution and then ferrate ions (0.1 mL) were added into the CQDs (0.9 mL) dispersion to record the fluorescent emission spectra with the excitation wavelength of 320 nm.116
The conversion of polyolefins into fluorescent CQDs by the green hydrothermal approach was also carried out by Kumari et al. A combination of green hydrothermal approach and chemical oxidation process produced CQDs with a very high quantum yield of 4.84%.117
Park et al. reported a simple sustainable large-scale approach to synthesize CQDs, where they converted 100 kg of food waste into water soluble graphene quantum dots. The food waste (100 kg) in 500 L 10% ethanol was treated at 40 kHz ultrasound for 45 min, followed by centrifugation at speed of 4500 rpm for 5 min to obtain uniformity in the particle size and shape, while a 0.22 μm filtration membrane was utilized in the final purification of the G dots. The band at 400–470 nm confirms the synthesis of photo-luminescent G dots (Fig. 11).118
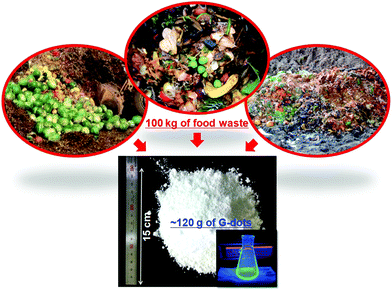 | ||
| Fig. 11 Schematic description of the large-scale synthesis of G-dots. These nano-dots represent efficient transition from large food waste to valuable carbon-based nanomaterials.118 Reproduced by permission of American Chemical Society. | ||
According to Thambiraj et al., the green synthesis of CQDs can be achieved from sugarcane bagasse by chemical oxidation, followed by the high temperature exfoliation of sugarcane biomass, where they dried sugarcane biomass in sunlight for a week, then processed it for combustion at 60 °C in an open atmosphere. After that, the residue was mixed into 200 mL toluene and stirred thoroughly for 24 h at room temperature; further sonication was done for 1 h along with centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min at room temperature, while ethanol was used for the dilution of the obtained liquid.119
000 rpm for 30 min at room temperature, while ethanol was used for the dilution of the obtained liquid.119
CQDs were also prepared from orange waste peels via the hydrothermal carbonization method at mild temperature (180 °C). Firstly, the orange peels were washed and oven dried at 150 °C for 10 h, followed by washing with sulphuric acid (0.1 M), and then oxidized by sodium hypochlorite solution at room temperature for 4 hours.120 Finally, the oxidized peels were placed under hydrothermal conditions in a Teflon-lined autoclave at 180 °C for 12 h to obtain the desired CQDs. In a similar way, Tyagi et al. also obtained CQDs from lemon peel following the hydrothermal approach at 200 °C temperature121 (Fig. 12).
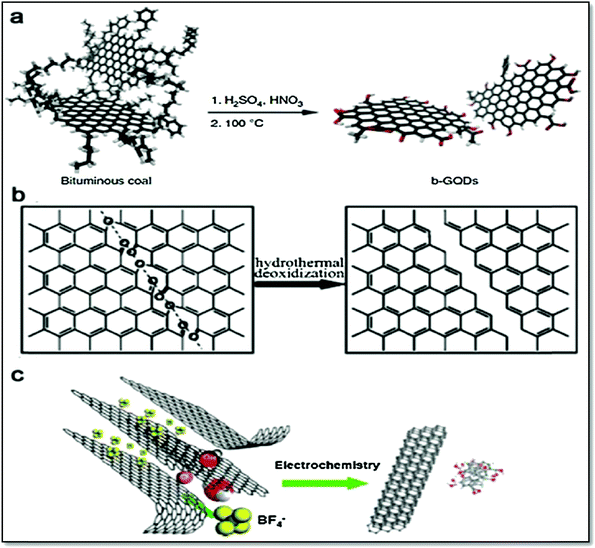 | ||
| Fig. 12 (a) Microwave synthesis of GQDs, (b) GQDs synthesis by exfoliation of CNTs, (c) GQDs synthesis by exfoliation of graphite.122 Reproduced by permission of Elsevier. | ||
As observed in previous reports, most of the researchers have followed the hydrothermal approach for the synthesis of quantum dots from various agricultural wastes. The yield and properties of the developed CQDs mainly depend on the parameters of the manufacturing method such as centrifugation speed, concentration of solid waste, oxidation time, and temperature of the hydrothermal reactor. The time duration of the hydrothermal reaction also plays a crucial role that affects the synthesis and quality of CQDs. Moreover, the variation of different oxidizing agents and solvents affects the optical properties of the synthesized CQDs. The tabulation of the above reported data on the synthetic procedure and the conditions for the development of different CNMs using solid waste as the precursor has been summarized in Table 2.
| S. no. | Precursor | Synthesis method | Synthesis conditions | Derived carbon nanomaterials | Ref. |
|---|---|---|---|---|---|
| 1. | PET bottles | Controlled pyrolysis | Wastes containing stainless steel autoclave reactor was placed at 800 °C temperature for 1 h | Graphene | 80 |
| 2. | Polypropylene (PP) waste | Controlled pyrolysis | Inside the tube furnace at 700 °C using OMMT catalyst and inert environment | Graphene | 81 |
| 3. | Waste plastics | Controlled pyrolysis | Heated at 1300 °C with the heating rate of 10 °C min−1 using inert environment | Graphene | 84 |
| 4. | Waste plastic | Pyrolysis | In quartz tube reactor using OMMT catalyst at 700 °C. for 10 min followed by washing with nitric acid and HF | Graphene | 85 |
| 5. | Waste plastic | Pyrolysis | Two step pyrolysis; firstly, slow pyrolysis at 400 °C with heating rate of 5 °C min−1 and then fast pyrolysis at 750 °C with the heating rate of 10 °C min−1 | Graphene | 86 |
| 6. | Agriculture waste | Solvothermal | Internal part of oak seeds was heated at 120 °C for 4 h | K-Doped graphene oxide | 87 |
| 7. | PTFE, PVDF, PVC | Template carbonization | Carbonizing the plastic and zinc powder in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mass ratio at 700 °C under argon atmosphere 3 mass ratio at 700 °C under argon atmosphere |
Nano-porous carbon | 88 |
| 8. | Solid waste | CVD | Scan rate of 150/25 sccm and annealing at 1050 °C for 2 h | CNMs | 89 |
| 9. | Carbonic waste | Controlled pyrolysis | Calcined at 1050–1070 °C under the gas flow of 98 sccm for argon and 2.5 sccm for hydrogen in a Cu foil | Graphene | 90 |
| 10. | Waste plastic | CVD | Annealing at 1050 °C in an Ar/H2 atmosphere for 30 min | Graphene | 91 |
| 11. | Waste plastic | CVD | Annealed within a Cu substrate at 1100 °C for 1 h under 100 sccm flow for H2 | Graphene | 92 |
| 12. | Waste plastic | Pyrolysis | Shredded polypropylene waste was pyrolized in presence of Ni/Mo/MgO catalyst at 800 °C for 10 min | CNTs | 98 |
| 13. | Polypropylene | Controlled pyrolysis | Pyrolysis at 600 to 800 °C using Ni catalyst under inert environment | CNTs | 99 |
| 14. | Polyethene | Thermal dissociation | HDPE containing 20 wt% cobalt acetate catalyst was placed inside an closed autoclave and heated at 700 °C | MWCNTs | 100 |
| 15. | Polypropylene | Pyrolysis | Gasification at 800 °C using Ni/Ca–Al and or Ni/Zn–Al as catalyst | CNTs | 101 |
| 16. | Solid waste | Catalytic combustion | Heat-treated at 630–830 °C in presence of nickel compounds and OMMT as catalyst under inert environment | MWCNTs | 102 |
| 17. | Waste carbonaceous material | Controlled pyrolysis | Pyrolysis at 450 °C within Ni-plates under inert atmosphere | CNTs | 104 |
| 18. | PE, PET bottles | Pyrolysis | Three step pyrolytic up-cyclization into tube furnace under nitrogen environment at 600–1000 °C temperature | CNTs | 105 |
| 19. | PP waste | Pyrolysis | Two stage pyrolysis: catalytic pyrolysis (550–750 °C) of PP over HZSM-5 zeolite in a screw kiln reactor and the subsequent catalytic decomposition (500–800 °C) of pyrolysis gases over a nickel catalysts in a moving-bed reactor | MWCNTs | 106 |
| 20. | Paper waste | Hydrothermal | Treatment of the wastes in a Teflon-lined stainless-steel reactor at 180 °C for 10 h | CQDs | 113 |
| 21. | Waste plastic | Hydrothermal | 5 wt% of H2O2 solution at 180 °C for 12 h | CQDs | 116 |
| 22. | Polyolefins | Pyrolysis and hydrothermal | Firstly, pyrolysis of waste polyolefin followed by hydrothermal process at 120 °C for 12 h | CQDs | 117 |
| 23. | Food waste | Sonochemical | 5 wt% of waste in 10% ethanol was sonicated at 40 kHz for 45 min followed by centrifugation at speed of 4500 rpm for 5 min | G dots | 118 |
| 24. | Sugarcane bagasse | Chemical oxidation | Sugarcane biomass was dried in sunlight for a week followed by the combustion at 60 °C in an open atmosphere to form carbon. Then the carbon was added in toluene and stirred for 24 h | CQDs | 119 |
| 25. | Orange waste peels | Hydrothermal | In a Teflon lined autoclave at 180 °C for 12 h followed by sonication for 1 h | CQDs | 120 |
| 26. | Lemon peel | Hydrothermal | In a Teflon lined autoclave at 200 °C for 12 h | CQDs | 121 |
It can be clearly visualized from Table 2 that for the synthesis of graphene and CNTs, usually, pyrolysis and CVD processes are followed, while quantum dots are synthesized via the hydrothermal methodology. The comparison in the structure and properties of CNMs derived from solid wastes and conventional graphite precursors is not dependent on the carbon precursors rather the presence of some impurities in the wastes that create some defects, such as edge dislocation and point dislocation in the case of waste-derived CNMs.86 The traditional Hummers’ method synthesizes graphene, which usually appears in the oxide form (i.e., graphene oxide), is brownish in colour. On the other hand, pyrolysis and CVD process provide graphene that usually possesses lower oxidative functionality due to high heating rates and pressure. Based on the analysis from the references in Table 2, it is observed that the slow rate of pyrolysis, quantity of the catalysts, and the temperature of annealing are the key factors for the steady conversion of carbonic solid waste materials into CNMs. These observations not only give us a clue about the role of the catalyst for the decomposition of the carbonic layers but also offers a handful of information about the role of high temperature degradations on the formations of graphene. In the CVD based methods, the quality of the CNMs is strongly correlated to the processing temperatures and flow rates of hydrogen/argon. In the CVD growth phase, the optimum rate of gas flow per sccm with a particular time span is necessary for the development of graphene foil (GF). Overall, the quality of graphene mainly depends upon the atomic composition of solid waste materials, amount of catalyst, pyrolytic temperature, and gas flow rates. Graphene obtained by pyrolysis and CVD procedures is mainly obtained in its 2–3 layered form. It is may be due to the process of high temperature exfoliation of carbonic form, which allows van der Waals forces of attractions between the layers of carbon to get distorted; thus, the separation of carbonic layers becomes easier. Furthermore, the excessive presence of heteroatoms, metal ions, and metal oxides can influence the purity of synthesized graphene.
4. Structural and morphological properties
Structural properties such as the degree of carbonization or graphitization, pore size, pore volume, and their surface morphologies are strongly correlated to the electrochemical performance of the fabricated device. The doping of metals and heteroatoms can also control the structural and morphological properties to some extent.4.1. Degree of carbonization
The degree of carbonization is very crucial for the electrochemical study of solid waste based CNMs. The degree of carbonization alters the rate capacity, comprehensive resistance, power density, and power delivery along with the cyclic stability and energy efficiency. Carbonization improves the hydrophilicity for aqueous electrolytes, which mainly enhances the diffusion of ions along with the transport number, which enhances the capacitive performance of the fabricated devices.123 The low resistivity suggests high conductivity in the sample associated with a high degree of carbonization or carbonic activation.But as the carbonization and graphitization increase, the relative pore volume and the structure gets distorted due to smaller specific area. Thus, in order to maximize the electrochemical potential, we need to find the compromising link in between the degree of carbonization and the pore structure. High temperature pyrolysis in an inert atmosphere usually reduces the functional moieties of the material, which can somehow limit the reach of the exact surface area for the corresponding material. The transition metal and their oxide based catalytic degradation of solid waste materials is reported as one of the efficient methods for considerable carbonization or graphitization for high performance electrochemical studies. Sun et al. derived two-dimensional porous carbon nanosheets having an excellent surface area for electrochemical studies by using coconut shell as the raw material.123 Here, FeCl3 and ZnCl2 were used to fix the appropriate ionic composition by using them as degradation and activation catalysts, respectively. Thus, the obtained carbon nanomaterial showed an excellent surface area of 1874 m2 g−1 and good electrical performance of 268 F g−1 at 1 A g−1 using KOH as the electrolyte. Zhang et al. reported carbonization using K4Fe(CN)6 by the chemical activation of hierarchical carbon nanotubes, which further offered good ionic diffusion and excellent conductivity.124 The activated carbonic nanotubes were further loaded with MnO2 and the resultant hybrid supercapacitor showed huge electrochemical capacitance of 550.8 F g−1 at a current density of 2 A g−1, with 62% retention at the current density of 50 A g−1. Interestingly, 90% capacitance retention was reported even after 5000 cycles for the same device.
Carbonization at a temperature 1000 °C or above results in excellent conductivity; thus, the prepared CNMs usually act as excellent candidates for semiconductors, supercapcitor electrodes, and temperature-resistant catalyst carrier. Typically, the conductivity increases up to 1000 °C temperature due to the relative decrease in the CNMs diameter and increase in the surface area. On the other hand, the favorite alignment of the carbonic layers having a flat surface rises significantly, the cavity volume declines efficiently along with the increase in the crystallite dimension. Consequently, with the increase in the reaction temperature, the CNMs structure commences to change into flake-type during the dehydrogenation reaction.125 Higher temperature can significantly increase the conductivity. Thus, the CNMs carbonized at 1000 °C have excellent ‘degree of carbonization’. At higher temperature range, the outflow and volatilization of atoms creates pores on the CNMs surface, which makes these CNMs excellent candidates for different electrochemical applications. The increase in the carbonization temperature leads to a denser structure at the nanoscale with a reduced diameter. This specifies that carbonic weight percentage, in such cases, increases with increasing carbonization temperature, resulting in progressive graphitization at higher temperature. CNMs at higher temperature range show significantly higher conductivity in comparison to CNMs obtained at lower temperature, mostly due to the availability of moving electrons.125
4.2. Porosity & surface framework
Solid waste derived CNMs are usually formed with unique structural framework, which include spherical tubes, hierarchical spheres, honeycomb lattice of carbon, carbon onion, and vertical flower. On the other hand, the electrical performance of the supercapacitors usually increases with increasing specific surface area of the active material with some diversions.In addition to the surface area, the size of the pores, their distribution, and pore volume are also important factors that directly affect the possible electrochemical performance of the device (Fig. 13). The presence of macropores (i.e., size >50 nm) can perform as the storage sites for different metal or non-metallic ions, whereas mesopores (2–50 nm) can offer channels for charge transfer. Micropores ensure high specific surface area and contribute to the electrical double layer capacitance. To obtain the optimized performance, researchers are synthesizing hierarchically porous CNMs, which contain both the mesopores and micropores by adjusting the experimental parameters, such as the amount of the actuating reagent,126–128 pyrolysis temperature and pressure,129–133 and activating reagents.134–139
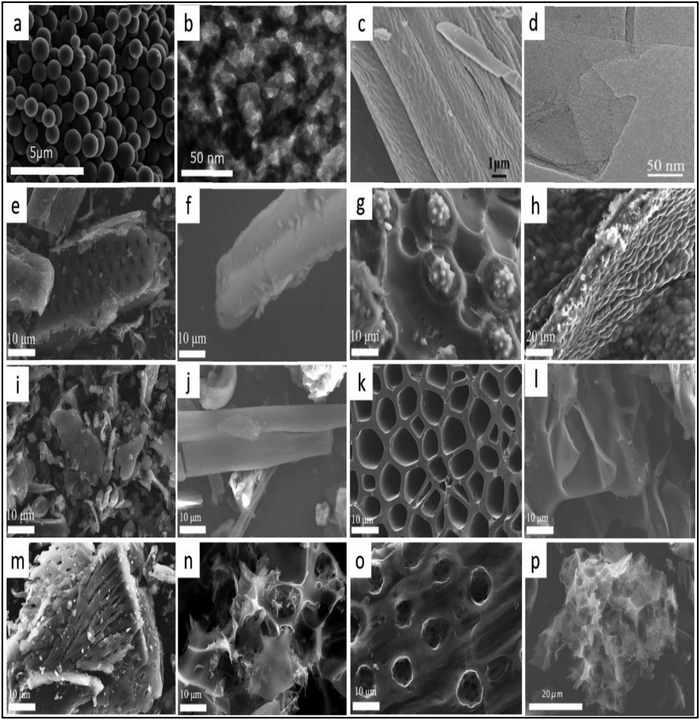 | ||
| Fig. 13 Electron microscopic images of (a and b) fermented rice derived porous carbon spheres,132,140 Reproduced by permission of Elsevier and RSC, (c) dandelion derived active carbon,132,141 Reproduced by permission of Elsevier, (d) coconut derived porous graphene nanosheets,132,142 Reproduced by permission of Elsevier and RSC, (e) cotton stalk, (i) carbonized cotton stalk, (m) activated cotton stalk,132,143 Reproduced by permission of Elsevier, (f) Saussurea involucrata stalk, (j) carbonized Saussurea involucrata stalk, (n) activated Saussurea involucrata stalk. SEM images of (g) fresh coconut shell, (k) after the carbonization of the coconut shell and (o) activated coconut shell. The SEM image of algae (h), carbonized algae (l), activated algae (p).132,144 Reproduced by permission of Elsevier and RSC. | ||
Recently, highly microporous activated carbon was synthesized from cotton stalk by Ma et al.143 They altered the mixing proportion of KOH and carbon and highly ordered, microporous activated carbon (HOMAC) was obtained using the KOH carbon ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The synthesized microporous material-based supercapacitor device showed good specific capacitance of 322 F g−1 along with an energy density of 33.91 kg−1 using H2SO4 as the liquid electrolyte.
4. The synthesized microporous material-based supercapacitor device showed good specific capacitance of 322 F g−1 along with an energy density of 33.91 kg−1 using H2SO4 as the liquid electrolyte.
Cui et al. reported the synthesis of porous 3D carbon aerogel by taking proliferate-green-tide in the pyrolysis process, followed by appropriate chemical activation.145 The variations in the temperature for the activation process works as the regulator of the resulting pore structure and pore sizes, i.e., macro, meso, and micropores. The obtained material showed an excellent surface area of 2200 m2 g−1 and a good specific capacitance of 260.6 F g−1 in the liquid electrolyte, whereas 92% retention for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles was reported for the same device. They also checked the effects of different activation agents on the porosity and porous nature of the carbonic nanomaterials using CaCl2, NaCl, ZnCl2, KCl, K2FeO4, and H3PO4. Thangavel et al. explained the relationship between the electrochemical performance and the designed structure of the carbonic materials with different activators such as KOH, ZnCl2, and H3PO4.146 They showed very strong correlation between the activating agents and the structural variations of different pores. The KOH activated material showed hierarchical, open, and large pores. The pores with ZnCl2 were invisible and the sample activated with H3PO4 showed pores embedded into different cavities.
000 cycles was reported for the same device. They also checked the effects of different activation agents on the porosity and porous nature of the carbonic nanomaterials using CaCl2, NaCl, ZnCl2, KCl, K2FeO4, and H3PO4. Thangavel et al. explained the relationship between the electrochemical performance and the designed structure of the carbonic materials with different activators such as KOH, ZnCl2, and H3PO4.146 They showed very strong correlation between the activating agents and the structural variations of different pores. The KOH activated material showed hierarchical, open, and large pores. The pores with ZnCl2 were invisible and the sample activated with H3PO4 showed pores embedded into different cavities.
Wang et al. synthesized activated porous material from cornstalk biomass, where they used neutral salts NaCl and KCl as the activating agents during the process.147Fig. 14 represents the schematic illustration of the whole process. Similarly, sulphur and nitrogen co-doped porous carbon was synthesized using ginkgo leaf waste by Zheng et al.148 They believed that chemical activation led to the hierarchical structure of mesoporous/microporous CNMs.
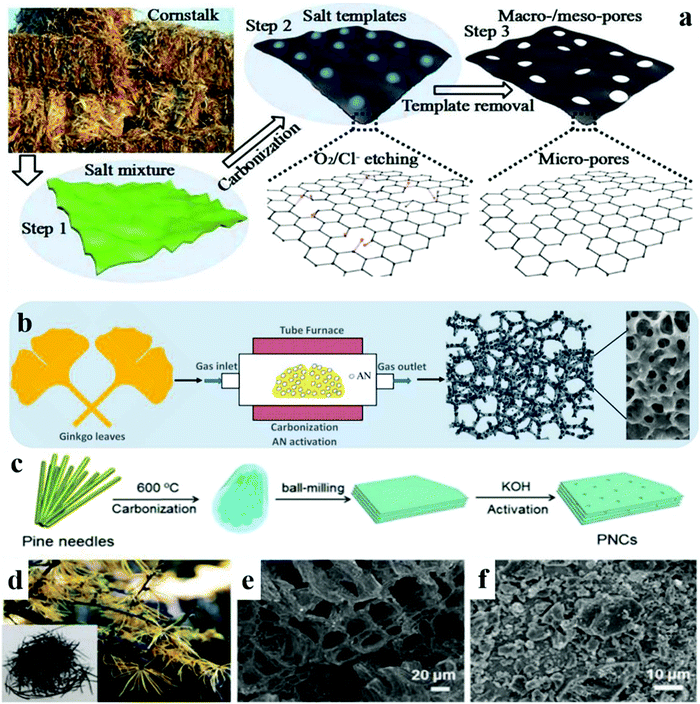 | ||
| Fig. 14 (a) Synthesis of the meso and macroporous structure from cornstalk,132,147 Reproduced by permission of Elsevier and RSC. (b) Synthesis of hierarchically porous carbon from ginkgo leaf,132,148 Reproduced by permission of Elsevier and ACS. (c) Synthesis of porous carbon from pine needles. (d) Image of pine needles. SEM images of activation at (e) 600 °C, (f) 900 °C.132,149 Reproduced by permission of Elsevier and RSC. | ||
The structural framework of porous carbon can be better described as zero dimensional (0D), one dimensional (1D), two dimensional (2D), and three dimensional (3D) materials. The direct correlation between the pore size and distance of ionic transport is a very crucial factor for the enhancement of the capacitors’ performance. Recently, various structures have been reported for solid-derived CNMs, such as 0D hollow structure, 1D nanofibers, 1D nanotubes, 2D nanosheets, and 3D hierarchical framework, which are discussed in the section below.
Gao et al. reported the synthesis of hollow 0D spheres using fermented rice as the precursor. The reported hollow 0D carbon sphere showed a huge specific surface area of 2106.0 m2 g−1 along with a pore volume of 1.1 cm3 g−1.140 The special hollow skeleton of carbon is usually obtained by activating agents such as ZnCl2, which usually transfers ions from the electrolyte to the electrode quickly (Fig. 13a and b). The fabricated device showed a good specific capacitance of 219 F g−1 at a high current density of 15 A g−1. Usually, the interconnected hollow 0D carbon spheres skeleton provides continually open ionic transfer and ionic diffusion at the activated surface area. It accesses and insures good chemical activation, and charging/discharging during the electrochemical performance testing of the fabricated device.
Here, the potential flow and its rate, and the quantity of the solution along with the length of the whole device work as crucial factors to adjust the structure of carbonic nanofibers. Carbon fibers were reported with polymer incorporation in most of the studies, especially via the cross-linking framework. Mainly, polymers are used to enhance the viscosity of carbon fibers in the electrochemical spinning methodology. Recently, Tao et al. reported the liquification of walnut shells by phenol along with the addition of polyvinyl alcohol for one hour during the spinning process.153 They quoted increased fluidic activity of the solution by the addition of polyvinyl alcohol; the process resulted in the synthesis of 1D carbon fibers having a diameter of 280 nm. Thus, the obtained walnut shell/PVA nanofiber was further used for supercapacitor device fabrication and showed excellent specific capacitance of 380 mA h g−1 at 0.03 A g−1 with good cyclic stability.
Liu et al. reported the synthesis of 1D porous nanofiber using lotus seed pods as the precursor through the activated carbonization method.154 The fabricated devices showed good energy density of 13 W h kg−1 and a power density of 260 W kg−1 in the two-electrode system. A good precursor solid waste can have a hollow framework as that in cotton and willow catkins for the synthesis of 1D nanofibers. On the basis of various reported articles, cotton fibre could be used as the best candidate for the synthesis of 1D fibers via thermal cracking and the pyrolysis approach. Similarly, willow catkins and poplar are used for the synthesis of porous nanotubes via the pyrolytic approach, whereas raw dandelion produces 1D tubular nanofibers.141 The framework can be retained via carbonization, which increases the surface and interaction of ions. Hollow nanotubes with good porosity usually consist of 20 to 50 μm structural length, where tubes are found to be interconnected. Overall, both 1D nanofibers and the nanotubular structure suggest a new route for charge transfer along with minimizing the ionic diffusion during the electrochemical activation process.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The fabricated device showed an energy density of 22 W h kg−1 along with 96% capacitance retention for 10
000 cycles. The fabricated device showed an energy density of 22 W h kg−1 along with 96% capacitance retention for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Cornstalk waste biomass based activated carbon nanomaterials were prepared by using K4[Fe(CN)6] as a catalyst during pyrolysis.156 Wang et al. used hemp fibers to design the synthesis of graphene-shaped 2D nanomaterial. The diameter of hemp fibers was in between 10 nm to 30 nm and is specially made up of multi-level cellulose and lignin framework. The hemp usually has inherent landscapes of being incorporated internally in 2 dimensions, which can be further utilized to be linked with different materials via activation and carbonization. Zheng et al. reported the synthesis of ultrathin 2D CNM for electrochemical performance testing taking glucose as the precursor material.157 KOH was used for the chemical activation of the synthesized 2D nanomaterial. Thus, the fabricated device showed excellent capacitance of 257 F g−1 at 0.5 A g−1 with 72% retention of capacitance at a current density of 100 A g−1. The good percentage of micropores and mesopores produces a huge surface area of 2600 m2 g−1. Thus, the obtained material is clearly responsible for efficient high electrochemical performance of the fabricated device. Usually, the layers alignment and their incorporation in the metal ions is responsible for fastening of ionic diffusion, which results in an enhancement in electrochemical capacitance.
000 cycles. Cornstalk waste biomass based activated carbon nanomaterials were prepared by using K4[Fe(CN)6] as a catalyst during pyrolysis.156 Wang et al. used hemp fibers to design the synthesis of graphene-shaped 2D nanomaterial. The diameter of hemp fibers was in between 10 nm to 30 nm and is specially made up of multi-level cellulose and lignin framework. The hemp usually has inherent landscapes of being incorporated internally in 2 dimensions, which can be further utilized to be linked with different materials via activation and carbonization. Zheng et al. reported the synthesis of ultrathin 2D CNM for electrochemical performance testing taking glucose as the precursor material.157 KOH was used for the chemical activation of the synthesized 2D nanomaterial. Thus, the fabricated device showed excellent capacitance of 257 F g−1 at 0.5 A g−1 with 72% retention of capacitance at a current density of 100 A g−1. The good percentage of micropores and mesopores produces a huge surface area of 2600 m2 g−1. Thus, the obtained material is clearly responsible for efficient high electrochemical performance of the fabricated device. Usually, the layers alignment and their incorporation in the metal ions is responsible for fastening of ionic diffusion, which results in an enhancement in electrochemical capacitance.
Feng and group reported the unique synthesis of polyporous 3D carbonic structure by using waste bagasse as the precursor for hydrothermal method, followed by subsequent chemical activation. The huge surface area and good porosity behavior is reported for porous 3D framework that showed a surface area of 2296 m2 g−1. The fabricated device showed a specific capacitance of 320 F g−1 at 0.5 A g−1 with 71% retention of capacitance at 50 A g−1. Recently, Hao et al. synthesized 3D CNMs using bagasse as the raw material.158 The fabricated supercapacitor device delivered good specific capacitance of 142 F g−1 at 0.5 A g−1. The prepared device showed 94% retention of capacitance over 5000 cycles. Similarly, Fan et al. reported the synthesis of honeycomb-shaped CNMs using wheat flour waste as the raw material via pyrolysis approach along with chemical activation by KOH.159 3D hierarchical and porous framework produced a large surface area of 1313 m2 g−1 and displayed excellent specific capacitance of 473 F g−1 at 0.5 A g−1 using 6 M KOH as the liquid electrolyte. Recently, activated carbon was derived from cotton stalk, Saussurea involucrata stalk, coconut shell, and algae, and is depicted in Fig. 13e–h.
The pores in the structure of cotton stalks were found to have a diameter of 2 μm, while the stalks of Saussurea involucrata have a high aspect ratio and the coconut shell showed long fibrous and denser particle distribution on the surface. Clear changes in their structural morphologies have been observed after carbonization (Fig. 13i–l).142 The SEM images of the CNMs after the activation process are shown in Fig. 13m–p. Fig. 13m depicts the presence of clear vacancies in cotton stalk derived 3D CNMs.142 Similarly, CNMs derived from Saussurea stalk displays a distinctive hierarchical porous carbonic structure. The activated carbon obtained from cotton stalk showed the capacitance of 254 F g−1 at a current density of 0.2 A g−1, in comparison to that of the hierarchical porous carbon nanomaterial synthesized from Saussure stalk, which displayed a specific capacitance of 322 F g−1 at 0.2 A g−1 current density. The CNMs derived from coconut shell displayed an electrochemical capacitance of 157 F g−1 at a current density of 0.2 A g−1. It also showed 100% retention of capacitance over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at the current density of 1 A g−1. The hierarchical porous carbonic network is crucial for ionic diffusion and transportation along with charge storage. The electrochemical capacitance of 283 F g−1 was reported for the hierarchical 3D porous network at a current density of 0.2 A g−1. These results indicate the advantages of porous fibrous CNMs for utilization in supercapacitor application.
000 cycles at the current density of 1 A g−1. The hierarchical porous carbonic network is crucial for ionic diffusion and transportation along with charge storage. The electrochemical capacitance of 283 F g−1 was reported for the hierarchical 3D porous network at a current density of 0.2 A g−1. These results indicate the advantages of porous fibrous CNMs for utilization in supercapacitor application.
The internal framework is usually interrelated with the intrinsic structure of the precursor materials. For example, cotton stalk-derived porous CNM exhibits similar morphological features (Fig. 13e and m).142Table 3 depicts the variations in the pore sizes of different solid waste derived materials and the cyclic stability of the respective capacitor device.
| S. no. | Carbonic bio-waste as the precursor | Pore volume of the CNMs (cm3 G−1) | Stability (cycle Life) | Ref. |
|---|---|---|---|---|
| 1. | Acacia based gum | 0.99 | 1000 | 159 |
| 2. | Sugarcane bio-waste | 0.81 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
160 |
| 3. | Flax | — | 1000 | 161 |
| 4. | Bio-waste of corncob | 1.95 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
162 |
| 5. | Beans of coffee | 0.57 | 2000 | 163 |
| 6. | Coconut outer shell | 1.21 | 2000 | 164 |
| 7. | Rice plants husk | 1.07 | 1000 | 165 |
| 8. | Oil Palm | 0.02 | 2500 | 166 |
| 9. | Carrageenan | 1.43 | 1000 | 167 |
| 10. | Shaddok peelings | 0.49 | 132 | 168 |
The mesoporous nanomaterials usually have a cylindrical pore surface and a wormhole-like structure. Based on this, it can be said that ion transportation usually takes place through the pores in electric double layer (EDLC) capacitors. But because of the very smaller pore size of the micropores, the electrolyte ions can form an electrical wire in the cylindrical capacitor (EWCC) network.169 The electrochemical transportation of ions for the process of charging and discharging is mainly dependent upon the relative pore size, pore shape, and their distribution.170 The hierarchical and porous carbonic network in 0D, 1D, 2D, and three-dimensional lattice usually tend to shorten the diffusion distance for ions travelling from electrolyte to electrodes and ultimately minimizes the diffusion time.
However, the control and selectivity of CNMs synthesis is especially dependent on the processing temperature, pressure and catalyst used during the process (pyrolysis or CVD). For the synthesis of graphene, degradation catalyst such as OMMT and ZSM are specially used due to their efficiency for CNMs production, ease of removal, and lesser consumption during the processing and manufacturing of graphene. However, during the synthesis of CNTs, catalysts, especially nickel, aluminum, cobalt, and other transition metal ions are used owing to their tendency of easy electron liberation during the high temperature exfoliation process, which might intercalate into the cluster of the carbonic layers, resulting in the easy exfoliation of the carbonic layers.
4.3. Surface properties
Precisely, the surface area, porous structure, and other morphological features signify the electrochemical potential of solid waste derived CNMs, while the porous nature of activated CNMs can be easily monitored via chemical doping and activating by different methodologies that somehow modulates the variations in the porous nature, viz., meso, micro, and macro. On the other hand, thermal heating, as well as chemical and acidic treatments can adjust the position and performances of the surface functional groups. The adjustment of the functional moieties does alter many morphological features of solid waste derived materials, which commonly include electrical and mechanical properties.132,171The doping of various metallic and non-metallic elements such as transition metals, p-block gases, and heavy metals usually signifies the surface porosity and surface morphology including surface area and electrochemical conductance of the agricultural waste based carbonic nano-materials.172 Recently, researchers are focusing on the synthesis of CNMs by using solid waste materials as the precursors and their further doping via heteroatoms to obtain a visible separation in between the layers of synthesized CNMs or to intercalate the metal ions for obtaining a more acute porous nature for the enhancement of electrochemical properties.132,171 Zhang et al. synthesized oxygen-containing carbon nanomaterials using biological gelatin as the precursor, having a layered morphology.172 The synthesized carbonic clustered nanomaterial showed excellent electrochemical specific capacitance of 276.6 F g−1 at current density of 1 A g−1. The electrochemical performance remained intact and retained almost 73% of the capacitance at 100 A g−1. They outlined the efficiency of oxygen as the modulator for the electrochemical properties.172 Similarly, nitrogen is another heteroatom that is extensively studied for the device fabrication of various electrochemical studies. The Doping of nitrogen is reported to increase the conductivity of carbon nanomaterials and enhances the specific capacitance of the resulting material-based supercapacitor device. This may be due to the presence of lone pair of electrons in the nitrogen atom, which is available for bonding and conduction purpose, thus increasing the conductivity and electrochemical performance of the resulting CNMs. Nitrogen usually exists in three different structural carbonic species, i.e., pyridine nitrogen, pyrrole nitrogen, and quaternary nitrogen. The ring atoms of pyridine nitrogen are reported to exist in sp2 hybridization and the lone pair of electrons, due to the aromatic nature of ring, projects themselves outside the ring. This enhances the electrochemical properties of the doped carbonic materials. On the other hand, the pyrrole nitrogen ring has no aromatic character and the lone pairs are delocalized, which also manage to enhance the conductive property of the doped carbon nanomaterials. On the other hand, the quaternary nitrogen atom can only enhance the conductivity by the ionization of the carbonic electrode.171 Recently, Hou et al. reported the synthesis of hierarchically thin activated carbon nanomaterial using raw silk as the precursor, where the chemical activation and carbonization procedures was followed thoroughly for the enhancement of electrochemical conductivity and the performance of the electrode material.173 A nitrogen rich mesoporous carbonic cluster having a huge specific surface area of 1030 m2 g−1 was synthesized by Zhou et al., which showed 15.0 nm diameter of pore size along with 7 wt% of nitrogen as the doped atom. The synthesized material showed excellent specific capacitance of 264 F g−1.174 Cellulosic precursor based porous carbon material was synthesized by Lu et al. They reported microcrystalline structured CNM, along with intrinsic nitrogen doping.175 The hierarchical porous structure along with high nitrogen content is more responsible for charge storage via pseudo storage mechanism. Thus, the synthesized material showed exceptional electrochemical capacitance of 426 F g−1 with excellent cyclic stability.175 3D oxygen and nitrogen doped carbon nanosheets were reported by Liu et al. by taking waste wood as the precursor. They followed the methodology of chemical oxidation, bio-swelling, and low temperature pyrolysis for the synthesis of the material.176
Similarly, some other heteroatoms such as phosphorous, boron, and sulphur are also reported as electrochemical performance booster for CNMs by enhancing their surface area and adding the pseudo capacitive property. Ling et al. reported a flexible device with co-doping of boron and nitrogen through annealing process.177 Here, boric acid was used as the source of boron doping. The fabricated device showed excellent specific capacitance of 268 F g−1 at 0.1 A g−1 along with an extended lifetime 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–15
000–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and 105–113% capacity retention. Dandelion fluff derived porous and hierarchical carbon nanotubes with good composition of boron and nitrogen was reported by Zhao et al.178 The excellent ionic diffusion and fast charge transfer mechanism along with a very stable framework of electrons was reported for the fabricated device. The hierarchical CNTs showed good energy density of 12.2 W h L−1 and excellent power density of 699.8 W L−1. Huang et al. reported phosphorous-doped porous carbon nanomaterial using coffee waste materials as the precursor. The chemical activation of CNM was done with the help of H3PO4.179 Electrochemical performance testing was performed by using 6 M KOH as the electrolyte for the process. The energy density of 15 W h kg−1 was reported at a good power density of 75 W kg−1. A sulphur and nitrogen co-doped hierarchical and porous activated carbon-based device was fabricated by Xu et al., using amino acid rich bean shells along with some vitamins as precursor material.180 The bean was pyrolyzed at a high temperature, i.e., at 800 °C for 2 h and then activated via organic reaction at high temperature along with an inert atmosphere of nitrogen with a constant heating rate of 3 °C min−1. They reported that sulphur enhances the space through the exclusive sorption of ions, whereas nitrogen enhances the electrical conductivity and wettability for the fabricated electrode. The specific electrochemical capacitance of 202 F g−1 at 0.5 A g−1 current density was reported by taking 6 M KOH as the electrolyte. The heteroatoms used in all the above reports are either directly derived from parts of the solid waste materials or incorporated by any activation method. These evaluations concluded that heteroatom doping in CNMs usually enhances the electrochemical performance of the device.
000 cycles and 105–113% capacity retention. Dandelion fluff derived porous and hierarchical carbon nanotubes with good composition of boron and nitrogen was reported by Zhao et al.178 The excellent ionic diffusion and fast charge transfer mechanism along with a very stable framework of electrons was reported for the fabricated device. The hierarchical CNTs showed good energy density of 12.2 W h L−1 and excellent power density of 699.8 W L−1. Huang et al. reported phosphorous-doped porous carbon nanomaterial using coffee waste materials as the precursor. The chemical activation of CNM was done with the help of H3PO4.179 Electrochemical performance testing was performed by using 6 M KOH as the electrolyte for the process. The energy density of 15 W h kg−1 was reported at a good power density of 75 W kg−1. A sulphur and nitrogen co-doped hierarchical and porous activated carbon-based device was fabricated by Xu et al., using amino acid rich bean shells along with some vitamins as precursor material.180 The bean was pyrolyzed at a high temperature, i.e., at 800 °C for 2 h and then activated via organic reaction at high temperature along with an inert atmosphere of nitrogen with a constant heating rate of 3 °C min−1. They reported that sulphur enhances the space through the exclusive sorption of ions, whereas nitrogen enhances the electrical conductivity and wettability for the fabricated electrode. The specific electrochemical capacitance of 202 F g−1 at 0.5 A g−1 current density was reported by taking 6 M KOH as the electrolyte. The heteroatoms used in all the above reports are either directly derived from parts of the solid waste materials or incorporated by any activation method. These evaluations concluded that heteroatom doping in CNMs usually enhances the electrochemical performance of the device.
5. Effects of CNMs structure on electrochemical properties
Carbon nanomaterials have become distinct materials in the field of science and technology so far due to their extraordinary properties starting from the electrical, mechanical, and thermal properties.181–184 On the other hand, some properties such as chemical stability, light weighted structure, excellent optical transparency, and extraordinary mechanical flexibility are responding good to the new technologies in various respective fields, such as in energy application, i.e., solar cells, supercapacitors, fuel cells, Li-ion batteries, and in the biomedical field, i.e., drug delivery, bioimaging, and biosensors.185–190 The carbon nanomaterials such as graphene, CQD, and CNT have been synthesized by various research groups, while using expanded graphite in various solid waste materials as starting raw materials. The variations in their physiochemical and mechanical properties have some extraordinary effects in addition. So, for much a deeper understanding of their potential use, it becomes very important to discuss their electrochemical properties in detail.The solid waste derived carbon nano-materials (CNMs) have vast variations in their electrochemical properties especially due to the variation in the specific surface area and the morphological behavior, while most of the structural properties of CNMs vary along with the variation in the structural framework of the precursor solid waste materials. Morphological expansion and changes in the carbonic framework result in the variation of the electronic conduction inside the layers of graphitic component, which is an appropriate reason behind the difference in the qualitative and conductometric behavior of various carbon nano-materials, such as graphene, CNT, and GQDs.
Lian et al. fabricated a capacitor using polyethylene derived hierarchical porous carbon nanomaterials with the incorporation of ammonia. The obtained activated hierarchical porous carbonic framework was reported with exceptional high surface area and contributes in subsequent capacitance along with a high energy density of 43 W h kg−1.191
Chen et al. reported porous carbonic material by using straw cellulose waste as the precursor for supercapacitor applications. Thus, the obtained porous carbonic material was activated by sodium hydroxide and showed an excellent rate performance of 281.32 F g−1 using 6 M KOH as the electrolyte at 0.5 A g−1. The fabricated device showed 92.93% cyclic stability with 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles rate at 1 A g−1 and an energy density of 8.67 W h kg−1 along with a power density of 3.50 kW kg−1 using 6 M KOH solution as an electrolyte.192
000 cycles rate at 1 A g−1 and an energy density of 8.67 W h kg−1 along with a power density of 3.50 kW kg−1 using 6 M KOH solution as an electrolyte.192
Recently, Peng et al. derived porous nitrogen doped carbon nanosheets using waste pomelo mesocarps as the precursor along with simultaneous activation and carbonization. The developed material showed excellent surface area of 974.6 m2 g−1 and the fabricated supercapacitor device showed excellent specific capacitance of 245 F g−1 at 0.5 A g−1 in 2 mol L−1 KOH electrolyte. Moreover, the supercapacitor possesses an energy density of 14.7 W h kg−1 at 90 W kg−1 power density, which was operated in the potential range of 1.8 V in aqueous Na2SO4 electrolyte.193 Li et al. reported mangosteen peel derived 3D porous carbonic structure, where they concluded its excellent CO2 absorbing capacity with high specific capacitance.194 They reported an increased surface area, i.e., 1270 m2 g−1 by following hydrothermal methodology in alkaline solution, which showed the excellent capacity of CO2 capture 6.93 and 4.77 mmol g−1 at 0 °C and 25 °C, respectively.194 At last, the electrochemical performance testing of the material showed the capacitance of 240 F g−1 using 6 M KOH in 1 A g−1 solution.194
Zhang et al. fabricated a supercapacitor device using hierarchically microporous carbonic material on nickel foam. They reported the high specific capacitance of 1601.6 F g−1 at 2 A g−1 current density for a capacitor with a specific molar ratio, i.e., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of nickel and cobalt.195
1 of nickel and cobalt.195
Recently, W. Xing et al. developed hierarchical porous carbons material for an ultra-capacitor electrode. The chemical activation of the material resulted in the variation in its pore size and thus the reported capacitance was calculated to be about 180 F g−1 at 1 Hz.196
The electrochemical device was fabricated using biomass waste derived carbon nanomaterials for symmetrical supercapacitors by Deng et al.197 They reported doping with multiple heteroatoms with a relative increase in the surface area along with the hierarchical porosity. They also reported the excellent specific capacitance of 412 F g−1 in 6 M KOH electrolyte using a current density of 0.5 A g−1. Energy density up to 28.78 W h kg−1 and the power density of 300 W kg−1 at the voltage of 2.0 V in 1 M Na2SO4 as an electrolyte was reported along with the cycling stability of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and 92.9% capacitance retention for the same.197
000 cycles and 92.9% capacitance retention for the same.197
The doping of non-metals, especially p-block elements into carbonic nanomaterials, usually increases the specific charge holding capacity along with electron donation ability of the surface, which somehow alters the electrochemical to thermal properties related to carbonic nanomaterials. The variations in the specific capacitance along with the energy and power density of different electrode materials is mostly due to the alteration in the surface area and the surface behavior of carbon nanomaterials.
Some structural modifications such as hierarchical structure, porous surface, 3D porous modifications, and doping by various different organic and inorganic reagents including metals and non-metals have increased the chances of conduction of electrons within the layer of carbonic framework. This has become a new route to enhance the electrochemical performance of the supercapacitors using 3D porous CNMs as the electrode material.
Hierarchical porous CNMs incorporating heteroatoms such as nitrogen and sulphur possess exceptionally high surface area and high energy density due to the availability of adequate sites for electron capture and release during the redox reaction or double layer formation.190 However, the porous carbon materials doped by alkali metal ions such as sodium and potassium also possess higher capacitance, basically due to the ease of electron conduction for charging and charge storage processes. Alkali metals are electron donors, which make them viable donors. In addition, the porous nature of carbonic materials helps in the easy capture of electrons because of the available capture sites developed due to the good porosity.192,193 The hierarchical, porous, and 3D CNMs, especially those deposited on metal foam (such as nickel, aluminum, or cobalt), hold huge specific capacitance and energy density, may be due to the ease of ionic movability during the formation of the double layer and during the redox reactions. Moreover, this discussion concludes that the hierarchically porous surface and 3D porous CNMs show are almost similar in specific capacitance; however, the reasons for their charge storage mechanics lies in the dispensation of electrons around the web of carbonic layers, totally depending upon the ease of electron availability, surface area, movability of ions, and the formation of a double layer.
When considering the synthetic methods for CNMs, the desired structure and properties are largely dependent upon the size, shape, foreign element doping, and surface functional groups of the CNMs. Although the properties of the synthesized CNMs does not much dependent on the carbon precursors, however, the presence of foreign elements/compounds during the synthetic procedure could help in the doping and surface functionalities, which strongly influenced the properties of the CNMS. The CNMs derived from solid waste can have metals or phytochemical as impurities, which actually help in modifying their properties. However, some undesired contamination in the CNMs might also decrease their performances compared to purer one.198 Among solid waste materials, plastics are loaded with fillers, pigments, metals, agriculture, and food waste, mostly consisting of natural pigments doped with different metal depending on their natural precursors.198 These impurities could stay on the developed CNMs and may positively or negatively affect the electrochemical performances of the developed materials.
6. Solid waste derived CNMs in supercapacitors
As observed in the above studies, the properties of solid waste derived CNMs are strongly dependent on the raw material and experimental conditions. They show outstanding electrical conductivity and very high specific surface area, which facilitate their application as supercapacitor device fabrication materials. Supercapacitors are classified as electrochemical storage devices, which can store and deliver energy at a very faster rate and offer very high current in a short period. Henceforth, they are frequently applied in electric vehicles, uninterruptible power supplies (UPS), and memory backups in IT systems and in various other fields. An overview of carbon materials (CNMs) utilized in different types of supercapacitors is given in Fig. 15.Different surface structure, variation in the quality of CNMs, i.e., thermo-chemical stability, structural and chemical properties, etc., are the main parameters behind the variations in the electrochemical properties. These materials can be further used as catalytic surfaces for electrode development materials. Graphene itself has an outstanding electrical conductance to charge mobility (>200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1),199–201 reactive chemical inertness,200 and also excellent mechanical strength (>1 TPa).202 However, the use of CNMs in various energy applications have been reported by various groups, such as electrode materials, catalytic materials for fuel cells, active layer for solar cells, and Li-ion batteries.
000 cm2 V−1 s−1),199–201 reactive chemical inertness,200 and also excellent mechanical strength (>1 TPa).202 However, the use of CNMs in various energy applications have been reported by various groups, such as electrode materials, catalytic materials for fuel cells, active layer for solar cells, and Li-ion batteries.
The enormously high surface area of CNMs plays a crucial role in the conductivity, capacitance, and storage properties. The conductivity behavior of CNMs and the diffusion rates of the ions are mostly the responsible factors for the storage quality of ultracapacitors. Material development part for supercapacitors is mainly composed of two parts, the first one of carbon nano-materials, while the other part includes different metals, metal oxides, and their polymer composites. Table 4 depicts the various types of supercapacitors and materials used for device fabrication.
| S. no. | Type of supercapacitor | Electrode material | Charge storage mechanism | Merits and demerits |
|---|---|---|---|---|
| 1. | Electrochemical double layer capacitor (EDLC) | Activated carbon nano-materials | Non-faradaic charge storage | Low specific capacitance & energy density, fare cyclic stability |
| 2. | Pseudocapacitors | Different metal oxides and polymer composite having redox property | Redox electrochemical reactions with faradaic current | Low-rate capability and high-power density, high energy density along with excellent specific capacitance and good cyclic stability |
| 3. | Hybrid capacitors | For asymmetric hybrid supercapacitors anode is made up of the same material as in case of pseudo-capacitance ultracapacitors, while cathode is usually made up of activated carbon nano materials | Anodic reaction is redox reaction while cathode follows electrochemical double layer mechanism | Show high energy density and high-power density with relatively good cyclic stability |
| For symmetric hybrid ultracapacitors the metal oxide with activated CNMs or polymer matrix with activated carbon nano-composites | Redox reactions and electrochemical double layer mechanism is followed. | High energy density with moderate cyclic stability. |
Zequine et al.203 have reported the flexible supercapacitor device based on bamboo fibers for high temperature supercapacitor applications. They have developed high-performance flexible supercapacitors electrode from carbonized bamboo fibers with specific capacitances of 510 F g−1 at 0.4 A g−1 with excellent energy density of 54 W h kg−1. The fibers displayed excellent charge storage stability without degradation up to 5000 charge–discharge cycles. The fabricated supercapacitor device was reported to have an aerial capacitance of 1.55 F cm−2 at room temperature. On bending, the fabricated electrode device showed an excellent charge storage capacity with 65% improvement in the performance at 70 °C compared to that at 10 °C.203
Mishra et al.203 used polypropylene plastic waste for the synthesis of MWCNTs at 800 °C by chemical vapor deposition (CVD) in the presence of Ni as the catalyst. The devices showed the specific capacitance of 59 F g−1 with a scan rate of 5 mV s−1 in 1 N KOH solution.204
According to Zhi et al.,205 activated carbon for supercapacitor applications can be obtained from petroleum-based precursors, which is prepared by the pyrolysis of raw materials along with chemical activation. Statistical multiple linear regression and stepwise regression methods have been employed to investigate the dependence of specific capacitance on the porosity of activated carbon. Zhi et al. reported that the specific capacitance is dependent on the micropores and their volume while it is independent of the mesopore volume. Nonetheless, the volume ratio of mesopore/micropore dominates over the absolute volume of the mesopores.206
Waste polystyrene derived 3D porous CNMs were synthesized via Friedel–Crafts reaction using polystyrene waste as the carbon source. It is believed that high cross linkage and excessive bonding between the carbonyl group and linear polystyrenes provides a good pathway for building a hierarchical porous morphology. Thus, the obtained 3D porous structure showed a large specific surface area along with high capacitance and excellent thermo-chemical stability for ultracapacitors.204 On the other hand, waste paper based flexible supercapacitors with their design, applications, and fabrication have been reviewed by Zhang et al.207
According to Wen et al,208 nano-sized carbon black having nickel oxide in the form of Ni2O3 works as a brilliant candidate for synergistically catalyzing the carbonization of waste polyolefin for supercapacitor applications. Polypropylene (PP), polyethylene (PE), and polystyrene (PS) were dehydrogenated into CNTs by using carbon black along with nickel particle as the catalyst and the developed materials showed higher specific capacitance as compared to commercially available CNTs.
Puthusseri et al.209 developed Li-based capacitor materials from a waste paper basket using hydrothermal methodology, followed by high temperature pyrolysis. The materials showed a very high surface area (2341 m2 g−1) and hierarchical pore size distributions, where the micro and mesopores are distributed ideally to be function as high-performance supercapacitors. Ion liquid gel polymer was used as the electrolyte for the performance testing of the fabricated device, which showed a power density of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 and an energy density of 31 W h kg−1 along with a good energy storage of 61 W h kg−1.209
000 W kg−1 and an energy density of 31 W h kg−1 along with a good energy storage of 61 W h kg−1.209
Dongale et al. demonstrated the modeling of cyclic voltammetry using Artificial Neural Network (ANN), as shown in Fig. 16a. The Levenberg–Marquart propagation algorithm alongside with sigmoid function was used to optimize the power of ANNs. The model established good behavior with an error rate up to 1.24%, 1.03%, 0.87%, 1.17%, and 1.28% for differently deposited MnO2.210 Wiegert et al. thoroughly evaluated the charging process of the supercapacitor and showed excellent performance with a correlation coefficient of 0.95 (Fig. 16b).211 Zhu et al. observed 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 evaluations for the electrochemical capacitance of the carbonic supercapacitors from almost 1000 articles. They identified five variables, which affects the capacitance of the supercapacitors such as the surface area, pore size, ID/IG ratio, doping, and voltage window.212
000 evaluations for the electrochemical capacitance of the carbonic supercapacitors from almost 1000 articles. They identified five variables, which affects the capacitance of the supercapacitors such as the surface area, pore size, ID/IG ratio, doping, and voltage window.212
 | ||
| Fig. 16 (a) Multilayer supercapacitor model,132 reproduced by permission of Elsevier. (b) Expected cycle life at a discharge current of 2.0 A,132,211 reproduced by permission of Elsevier. (c) Artificial model for supercapacitor, (d) importance of inputs and output.132,144 Reproduced by permission of Elsevier and RSC. | ||
Ma's et al. also predicted the ANN model for the energy storage of electrode devices (Fig. 16c and d) based on the sets of 200 biomass derived carbon nanomaterials and their electrochemical properties. They showed that the specific capacitance can be simulated by features such as aspect ratio, cellulose content, surface area, pore size and volume, internal resistance, and conductance. Random algorithm was used for the calculation of the relative influence on the structural features and the specific capacitance. Machine-based learning predicted the relationship between the morphological features and the performance of available databases. They concluded that the surface area, resistance, pore size, and volume are the utmost significant variables, having a weight of 30.1%, 19.4%, and 18.7%, respectively, which affect the electrochemical performance of supercapacitors.144
Bridged 3D graphene blocks were used as supercapacitor electrode materials by Zhi et al.,205 where they reported the existence of Coulombic interaction between CNTs and graphene oxide sheets the as results of KOH activation. Wong et al. reported the intercalation of CNTs into graphene, which provided a huge surface area and as the result, the obtained material gained high electrical conductivity (39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 S m−1) at the mass density of 1.06 g cm−3. The energy density of 117.2 W h L−1 or 110.6 W h kg−1 and power density of 400 kW kg−1 was reported based on the mass density of the active material.213
400 S m−1) at the mass density of 1.06 g cm−3. The energy density of 117.2 W h L−1 or 110.6 W h kg−1 and power density of 400 kW kg−1 was reported based on the mass density of the active material.213
Table 5 depicts the various agricultural waste derived CNMs and their activities in supercapacitor applications. The data shows specific capacitance, current density, and surface area, and strongly depends on the externally activating agents. The reported data indicates the presence of huge surface area with a microporous and meso porous structure, 3D-GO, and 3D-RGO, 2D nanomaterials, hierarchical pore size distributions, variations in mass loadings, flexibility of device along with other structural outlining of morphological variations works as the stimulating agent for enhancement of capacitive properties. On the other hand, the activation achieved by some alkali metal ions works as an inclusive agent for the electrochemical performance of ultracapacitor electrodes. The variation in the structure and states, e.g., hydrogels and flexible arrangements, of electrodes have a huge effect on the outcomes of the electrochemical performances of supercapacitors.
| S. no | Raw materials | Activating agent | Specific surface area (m2 g−1) | Specific capacitance (F g−1) | Current density (A g−1) | Rate performance | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | Coconut shells | K2CO3 | 1506 | 91.15 | 0.2 | 89% at 2 A g−1 | 214 |
| 2. | Wheat hay | CaCl2 | 892 | 275 | 0.2 | 81% at 8 A g−1 | 215 |
| 3. | Corn-shoot | NaCl, KCl | 1588 | 407 | 1 | 60% at 20 A g−1 | 216 |
| 4. | Cotton shoot | KOH | 1964 | 254 | 0.2 | 87% at 20 A g−1 | 143 |
| 5. | Lotus vessel | 1015 | 1340 | 0.5 | 67% at 20 A g−1 | 217 | |
| 6. | Flowers of Bougainvillea | 850 | 458 | 2.28 | 22% at 5.8 A g−1 | 218 | |
| 7. | Flowers of Rose | KOH | 1911 | 208 | 0.5 | 52% at 20 A g−1 | 219 |
| 8. | Rice shell | KOH | 3120 | 315 | 0.1 | 60% at 50 A g−1 | 220 |
| 9. | Fish scale | KOH | 962 | 306 | 1 | 78% at 200 A g−1 | 221 |
| 10 | Rice straw | KOH | 1122 | 337 | 1 | 83% at 20 A g−1 | 222 |
7. Electrochemical performance testing
Typically, the electrochemical performance of supercapacitors is evaluated by using either the three-electrode or the two-electrode system. The three-electrode system is based only on the testing of the minimum amount of active materials by the screening electrode. On the other hand, the two-electrode system is a kind of prototype of the original supercapacitor, where performance is evaluated under ideal conditions.7.1. Three-electrode system
This system is generally used for the study of the electrochemical behavior of active materials. The system consists of a working electrode, a reference electrode, and a counter electrode, which are connected with a potentiostat (Fig. 17). The potentiostat is used for controlling the potential of electrodes; it records the change in the current of the electrode concerning the potential. It also controls the current that is passing through an electrode and then records the potential change concerning the current.223,224The working electrode is prepared by the coating of a sticky paste or ink of active materials over the glassy carbon or platinum metal. First, the active material is uniformly dispersed in the appropriate solvent and then coated over the pre-polished working electrode via drop-casting. Further, a small amount of Nafion is added over deposited ink deposition, which prevents the diffusion of ink into the electrolyte. On the other hand, a reference electrode sets up the base potential in the three-electrode system by attaining an unchanged potential. There are different reference electrodes that are used in three-electrode systems such as the silver chloride electrode (Ag/AgCl), saturated calomel electrode (SCE), and normal hydrogen electrode (NHE). The final part includes a counter electrode that establishes the balance in the reactions happening at the working electrode by tuning its potential. The CE is generally made of some inert material such as platinum meshes and graphite rods.
7.2. Two electrode system
This system is the test fixture configurations for material testing, which can mimic the unit cell and is able to match the performance of packaged supercapacitor cells, thus giving exact characteristics of the active materials.225 In the two electrode-system, the two active materials coated electrodes are used, which acted as the cathode and anode. These electrodes are electronically separated from each other by the separators, which avoid short circuit. Also, metal plates, graphite sheets, carbon cloth, and carbon fibers are used as current collectors, which act as a substrate for active materials and are used to establish a connection with a potentiostat. The cathode and anode are generally made by a coating of sticky paste/ink of the active materials (carbon nanomaterials, conducting polymers, and metals oxide) over the current collectors. The active materials may be the same or different for both the electrodes (Fig. 18).7.3. Techniques for the evaluation of performance of the supercapacitor
The performance of supercapacitors is generally measured in terms of specific capacitance (C) in Farads (F), which is directly related to the amount of energy stored. This can be evaluated by cyclic voltammetry, galvanostatic charging/discharging, electrochemical impedance, and spectroscopic techniques. Apart from the specific capacitance, power, and energy density along with cyclic stabilities are also an important parameter for performance testing of the supercapacitor.The specific capacitance is measured from the current at the center point of potential range (I) where the CV curve is divided into the two equal halves and scan rate (v) by using the following equation.
Conversely, specific capacitance can be evaluated from different equations for pseudocapacitors since pseudocapacitance arises through redox or faradaic charge transfer reaction. Thus, the CV curve deviated from the rectangular shape and shows oxidation and reduction peaks. Hence, the average specific capacitance is measured using voltammetric charge integrated from the CV curve, according to the following equation.
where, f (in Hz) and m are the frequency and mass, respectively, of the active materials coated on the electrode.
According to the theoretical concept, in the Nyquist plot, the ideal capacitor exhibits a parallel line to the imaginary axis. In reality, the plot exhibits a line with an inclined angle between 450 and 900 to the real axis, which arose due to the ion diffusion mechanism between Warburg diffusion and pseudocapacitance.228,229 This kind of shift from ideal to real by parallel line arises due to the different diffusion depth in the pores and faradaic reaction at the electrodes, which generate abnormal capacitance and pseudocapacitance.230,231
where, C, I, and dV/dt are the capacitance, applied current, and slope of the discharging curve, respectively.
On the other hand, the non-linear curve suggested the pseudocapacitance, which arose due to faradaic reaction and the capacitance can be calculated for the following equation without the use of the slope.232
where C is the specific capacitance evaluated from the CV or GCD and ΔV is the operating voltage.
Alternatively, the power density (PD) determines the quickest possibility to deliver the power by the supercapacitor under a constant current. This is expressed in watt per kilogram. The PD of a supercapacitor is evaluated from the following expression.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, in which one cycle corresponds to the one charge–discharge cycle at a constant current density for the cyclic stability generally performed under the GCD technique at a constant current density. The cyclic stability is important for supercapacitors because a long cyclic run gradually damages the electrode along with the corrosion of its components, which reduced the capacitance and increases the ESR. Therefore, reducing the overall electrochemical performance of the supercapacitor. This helps in the identification of materials that can perform or not in real application fields. Also, the EDLCs-based materials such as activated carbon and carbon nanomaterials show excellent cyclic performance in comparison to the pseudocapacitive materials.
000 cycles, in which one cycle corresponds to the one charge–discharge cycle at a constant current density for the cyclic stability generally performed under the GCD technique at a constant current density. The cyclic stability is important for supercapacitors because a long cyclic run gradually damages the electrode along with the corrosion of its components, which reduced the capacitance and increases the ESR. Therefore, reducing the overall electrochemical performance of the supercapacitor. This helps in the identification of materials that can perform or not in real application fields. Also, the EDLCs-based materials such as activated carbon and carbon nanomaterials show excellent cyclic performance in comparison to the pseudocapacitive materials.
7.4. Charge storage mechanism of supercapacitors
The supercapacitor has two type of charge storage mechanisms, which depends upon the way of energy stored, i.e., electrical double-layer capacitors (EDLCs), where the capacitance arises by pure electrostatic charge gathering at the interface of electrode and electrolyte and pseudocapacitors, where the capacitance arises due to the fast and reversible redox reaction in active electrode materials.Conversely, the discharging process is just the reverse of the charging process. Such a mechanism of electrical charge generation at the interface of the electrode and electrolyte suggested that the electrolyte concentration is always constant despite the charging and discharging process. Therefore, energy is accumulated at the interface of the electrode and electrolyte.
The amount of energy stored in EDLCs is calculated in the same way as that used in the conventional capacitor by using the following equation.
The carbon nanomaterials such as graphene and CNTs are used in the pseudocapacitor either in the functionalized form or in the composite form with conducting polymer and metal oxides. The developed composite showed higher performance as compared to their native devices. In particular, the metal oxide-based electrode materials show higher specific capacitance and energy density but these are not economically viable for commercial production and also have lower stability and complexity in porosity tailoring along with low conductivity, which has a certain effect on the power density and rate capability performance of the pseudocapacitors.249 Conversely, the conducting polymer shows good pseudocapacitive behavior due to their faster charging/discharging rate, appropriate structures, lower cost, and faster doping and undoping process. However, the slow diffusion rate of the electrolytes ions in the polymer network and the volume expansion of the polymers at the time of charging and discharging, reduce their electrochemical performance.250 To overcome these glitches, the composites of metal oxides with conductive polymers and carbon nanomaterials have been developed. Such composites can achieve high energy density without compromising their power density because of the involvement of both EDLC (carbon nanomaterials materials) as well as pseudocapacitance (metal oxide and conducting polymers). Besides, these composites-based pseudocapacitors also showed longer life cycles.
8. Conclusion and future aspects
The universal status of various solid wastes has come to the time where we can only control waste by its proper utilizations. On the other hand, the foundation of a connection between material science and human world is one of the greatest achievements of nanotechnology, which that can not only deal with such a big environmental issue but also it can make a balance between science and human world. Due to the presence of exceptional carbon contents in various solid waste materials, they can be converted into CNMs, which have distinct incorporative qualities to be applied in various fields, allowing the CNMs and their composite to exhibit their transitional properties.During the last three decades, various efforts have been made by scientists and researchers to balance the relationship between the waste material based CNMs and various materialistic applications of nanoscience and nanotechnology. The balancing of such a huge issue not only possesses the characteristic that can boost various industries but it can easily provide a myriad of new opportunities for every field of science and technology, which can play a crucial role in the development of human society for decades.
The utilization of waste materials into various CNMs and their further use into polymer nanocomposites, energy storage, energy conservation, drug delivery, and aerospace has increased the waste-based CNMs importance for mankind. Considering the importance of waste materials as promising candidates for the production of CNMs, this review article is an attempt to archive the very recent developments in the field. It includes the utilization of various kinds of waste materials to produce different CNMs with controllable shape, morphologies, physical and chemical properties along with their application potential, methodological changes and others. The structural necessities and other aspects are thoroughly discussed along with their opportunity to regulate the electrochemical to organo-mechanical properties. This review gives an idea about the comparative studies of different waste based CNMs. The review not only clarifies the current universal waste status but also extends the strategies for the production of CNMs with a clearer image of their importance in human life has been also explained.
The waste based CNMs used in different supercapacitors have been justified starting from preliminary to higher studies. While the structural analogy and various methodology have been explained for the justifications of best suitable materials for specific applications. The variations in pore size, pore volume and structure, carbonization, pyrolysis temperature, catalyst compositions and its effects along with the effects of surface morphological changes have been discussed thoroughly. Beside these important aspects, there are some associated drawbacks with these waste based CNMs. For example, the presence of various metals can act as a lethal inclusion for applicative point of view and the toxicity associated with them also play a critical role in many applications. This review highlights different scientific statics that are required to accomplish high specific capacitance and it also includes diverse technical specifications, along with various parameters required to remark on the relative potential of the fabricated device.
The modest synthesis methods, with ecofriendly benefits and cost-effective methodology, are appropriate for the mass scale production of CNMs from solid waste materials. Furthermore, courtesy should be made on the synthesis of different CNMs based composites. The electrochemical performance of 3D porous CNMs and their composites with transition metals and metal oxides might be projected as better alternates for energy storage materials as they have more mobile and sufficient electronic transition. However, to date, there are various studies on the synthesis of CNMs and their doping with heteroatoms, metal ions, metal oxides, and polymers; however, the relation between the mechanical bonding and physical modification of CNMs needs to be examined in depth with the help of computational studies. This will not only be helpful in visualizing the doping patterns but also induce improvement in mechanics and in determining the novel design for the synthesis and manufacture of 3D CNMs. Further, the combination of 3D CNMs with photoluminous nanomaterials, viz., metallic quantum dots and G-dots will explore the potential into some other fields such as bioimaging, energy conservation, and water purification.
The dispersive properties of CNMs play a crucial role in elevating some of the properties such as electrochemical, elasticity and mechanical properties, etc., whereas the mass production of single layer graphene sheets along with good thermal stability is a huge challenge in front of the scientists. The economic production of CNMs is an essential factor for real life applications. Much advancement is needed for the bulk scale production of high quality porous CNMs, which is to be applied in various fields. The absorption of these CNMs into bulk scale industries can become a very impulsive step for the growth of universal economy. However, before processing at the industrial scale, very concise and extensive study should be made on the labor and maintenance cost, resource type, their availability, reproducibility, and associated disadvantages.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Mission on Himalayan Studies (NMHS), GBPNIHE, Almora, India (GBPNI/NMHS-2019-20/MG), and Department of Science and Technology (DST-FIST), Delhi, India.References
- M. Ripa, G. Fiorentino, H. Giani, A. Clausen and S. Ulgiati, Appl. Energy, 2017, 186, 211–225 CrossRef CAS.
- H. Zhou, A. Meng, Y. Long, Q. Li and Y. Zhang, Renewable Sustainable Energy Rev., 2014, 36, 107–122 CrossRef CAS.
- C. Zhuo and Y. A. Levendis, J. Appl. Polym. Sci., 2014, 131, 1–14 CrossRef.
- G. Berndes, Global Environ. Change., 2002, 12, 253–271 CrossRef.
- P. Singh Nee Nigam and A. Pandey, Biotechnol. Agro-Industrial Residues Util. Util. Agro-Residues, 2009, pp. 1–466 Search PubMed.
- J. Cook and J. Beyea, Biomass Bioenergy, 2000, 18, 441–455 CrossRef CAS.
- M. Balat, Energy Sources, Part B, 2007, 2, 167–181 CrossRef.
- T. Searchinger, R. Heimlich, R. A. Houghton, F. Dong, A. Elobeid, J. Fabiosa, S. Tokgoz, D. Hayes and T. H. Yu, Science, 2008, 319, 1238–1240 CrossRef CAS.
- N. Moreira, Sci. News, 2005, 168, 218 CrossRef.
- M. Parikka, Biomass Bioenergy, 2004, 27, 613–620 CrossRef.
- F. Abnisa and W. M. A. Wan Daud, Energy Convers. Manage., 2014, 87, 71–85 CrossRef CAS.
- S. Gillet, M. Aguedo, L. Petitjean, A. R. C. Morais, A. M. Da Costa Lopes, R. M. Łukasik and P. T. Anastas, Green Chem., 2017, 19, 4200–4233 RSC.
- G. A. Ferrero, A. B. Fuertes and M. Sevilla, J. Mater. Chem. A, 2015, 3, 2914–2923 RSC.
- Z. Spitalsky, D. Tasis, K. Papagelis and C. Galiotis, Prog. Polym. Sci., 2010, 35, 357–401 CrossRef CAS.
- J. M. Carlton, J. H. Adams, J. C. Silva, S. L. Bidwell, H. Lorenzi, E. Caler, J. Crabtree, S. V. Angiuoli, E. F. Merino, P. Amedeo, Q. Cheng, R. M. R. Coulson, B. S. Crabb, H. A. Del Portillo, K. Essien, T. V. Feldblyum, C. Fernandez-Becerra, P. R. Gilson, A. H. Gueye, X. Guo, S. Kang’A, T. W. A. Kooij, M. Korsinczky, E. V. S. Meyer, V. Nene, I. Paulsen, O. White, S. A. Ralph, Q. Ren, T. J. Sargeant, S. L. Salzberg, C. J. Stoeckert, S. A. Sullivan, M. M. Yamamoto, S. L. Hoffman, J. R. Wortman, M. J. Gardner, M. R. Galinski, J. W. Barnwell and C. M. Fraser-Liggett, Nature, 2008, 455, 757–763 CrossRef CAS.
- N. G. Sahoo, S. Rana, J. W. Cho, L. Li and S. H. Chan, Prog. Polym. Sci., 2010, 35, 837–867 CrossRef CAS.
- X. L. Xie, Y. W. Mai and X. P. Zhou, Mater. Sci. Eng., R, 2005, 49, 89–112 CrossRef.
- X. Luo, Q. Yang, Y. Dong, X. Huang, D. Kong, B. Wang, H. Liu, Z. Xiao and L. Zhi, J. Mater. Chem. A, 2020, 8, 17558–17567 RSC.
- J. Liang, H. Zhao, L. Yue, G. Fan, T. Li, S. Lu, G. Chen, S. Gao, A. M. Asiri and X. Sun, J. Mater. Chem. A, 2020, 8, 16747–16789 RSC.
- Y. Yang, Y. X. Liu, Y. Li, B. W. Deng, B. Yin and M. B. Yang, J. Mater. Chem. A, 2020, 8, 17257–17265 RSC.
- S. You, X. Xi, X. Zhang, H. Wang, P. Gao, X. Ma, S. Bi, J. Zhang, H. Zhou and Z. Wei, J. Mater. Chem. A, 2020, 8, 17756–17764 RSC.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS.
- A. Izadi-Najafabadi, S. Yasuda, K. Kobashi, T. Yamada, D. N. Futaba, H. Hatori, M. Yumura, S. Iijima and K. Hata, Adv. Mater., 2010, 22, 235–241 CrossRef.
- K. Liu, M. Wu, H. Jiang, Y. Lin and T. Zhao, J. Mater. Chem. A, 2020, 8, 18802–18809 RSC.
- G. Li, Y. Wang, H. Guo, Z. Liu, P. Chen, X. Zheng, J. Sun, H. Chen, J. Zheng and X. Li, J. Mater. Chem. A, 2020, 8, 16920–16925 RSC.
- J. Zhang and X. S. Zhao, ChemSusChem, 2012, 5, 818–841 CrossRef CAS.
- F. Paquin, J. Rivnay, A. Salleo, N. Stingelin and C. Silva, J. Mater. Chem. C, 2015, 3, 10715–10722 RSC.
- Q. Cao, H. S. Kim, N. Pimparkar, J. P. Kulkarni, C. Wang, M. Shim, K. Roy, M. A. Alam and J. A. Rogers, Nature, 2008, 454, 495–500 CrossRef CAS.
- M. Jung, J. Kim, J. Noh, N. Lim, C. Lim, G. Lee, J. Kim, H. Kang, K. Jung, A. D. Leonard, J. M. Tour and G. Cho, IEEE Trans. Electron Devices, 2010, 57, 571–580 CAS.
- X. Li, F. Gittleson, M. Carmo, R. C. Sekol and A. D. Taylor, ACS Nano, 2012, 6, 1347–1356 CrossRef CAS.
- S. R. Sivakkumar and D.-W. Kim, J. Electrochem. Soc., 2007, 154, A134 CrossRef CAS.
- H. Wu, K. Wang, Y. Meng, K. Lu and Z. Wei, J. Mater. Chem. A, 2013, 1, 6366–6372 RSC.
- G. Liu, H. Shen, J. Mao, L. Zhang, Z. Jiang, T. Sun, Q. Lan and Z. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 6909–6914 CrossRef CAS.
- H. Hu, J. Yu, Y. Li, J. Zhao and H. Dong, J. Biomed. Mater. Res., Part A, 2012, 100A, 141–148 CrossRef CAS.
- C. Wang, J. Li, C. Amatore, Y. Chen, H. Jiang and X.-M. Wang, Angew. Chem., 2011, 123, 11848–11852 CrossRef.
- Z. Liu, X. Li, S. M. Tabakman, K. Jiang, S. Fan and H. Dai, J. Am. Chem. Soc., 2008, 130, 13540–13541 CrossRef CAS.
- D. Pissuwan, S. M. Valenzuela and M. B. Cortie, Trends Biotechnol., 2006, 24, 62–67 CrossRef CAS.
- G. Pagona and N. Tagmatarchis, Curr. Med. Chem., 2006, 13, 1789–1798 CrossRef CAS.
- O. Gohardani, M. C. Elola and C. Elizetxea, Prog. Aerosp. Sci., 2014, 70, 42–68 CrossRef.
- T. Natsuki, T. Tsuchiya, Q. Q. Ni and M. Endo, Carbon, 2010, 48, 4362–4368 CrossRef CAS.
- A. L. Ramaswamy, P. Kaste, A. W. Miziolek, B. Homan, S. Trevino and M. A. O’Keefe, Am. Chem. Soc., 2005, 180–197 CAS.
- N. M. Barkoula, B. Alcock, N. O. Cabrera and T. Peijs, Polym. Polym. Compos., 2008, 16, 101–113 CAS.
- Y. Zhao, R. Nakamura, K. Kamiya, S. Nakanishi and K. Hashimoto, Nat. Commun., 2013, 4, 1–7 Search PubMed.
- K. Tarawneh and N. Al-Aqtash, J. Comput. Theor. Nanosci., 2013, 10, 2080–2087 CrossRef CAS.
- D. Yu, E. Nagelli, F. Du and L. Dai, J. Phys. Chem. Lett., 2010, 1, 2165–2173 CrossRef CAS.
- K. R. S. Chandrakumar, K. Srinivasu and S. K. Ghosh, J. Phys. Chem. C, 2008, 112, 15670–15679 CrossRef CAS.
- Z. Yang, H. Nie, X. Chen, X. Chen and S. Huang, J. Power Sources, 2013, 236, 238–249 CrossRef CAS.
- B. R. Sathe, X. Zou and T. Asefa, Catal. Sci. Technol., 2014, 4, 2023–2030 RSC.
- W. K. Maboya, N. J. Coville and S. D. Mhlanga, S. Afr. J. Chem., 2016, 69, 15–26 CrossRef CAS.
- A. J. Clancy, M. K. Bayazit, S. A. Hodge, N. T. Skipper, C. A. Howard and M. S. P. Shaffer, Chem. Rev., 2018, 118, 7363–7408 CrossRef CAS.
- Z. Zhao, M. Li, L. Zhang, L. Dai and Z. Xia, Adv. Mater., 2015, 27, 6834–6840 CrossRef CAS.
- J. D. Ryan, A. Lund, A. I. Hofmann, R. Kroon, R. Sarabia-Riquelme, M. C. Weisenberger and C. Müller, ACS Appl. Energy Mater., 2018, 1, 2934–2941 CrossRef CAS.
- R. Pang, M. Li and C. Zhang, Talanta, 2015, 131, 38–45 CrossRef CAS.
- E. Flahaut, L. Evariste, L. Gauthier, C. Larue, C. Line, E. Meunier and F. Mouchet, Techniques de l’Ingénieur, 2018, 1–34 Search PubMed.
- T. Sato, Y. Suda, H. Uruno, H. Takikawa, H. Tanoue, H. Ue, N. Aoyagi, T. Okawa and K. Shimizu, J. Phys.: Conf. Ser., 2012, 352, 012032 CrossRef.
- R. K. Emmett, M. Karakaya, R. Podila, M. R. Arcila-Velez, J. Zhu, A. M. Rao and M. E. Roberts, J. Phys. Chem. C, 2014, 118, 26498–26503 CrossRef CAS.
- Z. L. Xu, J. K. Kim and K. Kang, Nano Today, 2018, 19, 84–107 CrossRef CAS.
- W. Lu, High performance batteries with carbon nanomaterials and ionic liquids, US Pat. 8236446, ADA Technologies Inc., 2012 Search PubMed.
- H. K. Liu, G. X. Wang, Z. Guo, J. Wang and K. Konstantinov, J. Nanosci. Nanotechnol., 2006, 6, 1–15 CrossRef CAS.
- T. Chen and L. Dai, Mater. Today, 2013, 16, 272–280 CrossRef CAS.
- B. K. Kim, V. Chabot and A. Yu, Electrochim. Acta, 2013, 109, 370–380 CrossRef CAS.
- A. Lund, N. M. van der Velden, N. K. Persson, M. M. Hamedi and C. Müller, Mater. Sci. Eng., R, 2018, 126, 1–29 CrossRef.
- B. L. Johnson and C. DeRosa, Rev. Environ. Health, 1997, 12, 235–251 CAS.
- A. C. Upton, T. Kneip and P. Toniolo, Annu. Rev. Public Health, 1989, 10, 1–25 CrossRef CAS.
- A. Biggeri, F. Barbone, C. Lagazio, M. Bovenzi and G. Stanta, Environ. Health Perspect., 1996, 104, 750–754 CrossRef CAS.
- P. Comba, V. Ascoli, S. Belli, M. Benedetti, L. Gatti, P. Ricci and A. Tieghi, Occup. Environ. Med., 2003, 60, 680–683 CrossRef CAS.
- P. Zambon, P. Ricci, E. Bovo, A. Casula, M. Gattolin, A. R. Fiore, F. Chiosi and S. Guzzinati, Environ. Health: Global Access Sci. Source, 2007, 6, 1–10 CrossRef.
- P. Elliott, G. Shaddick, I. Kleinschmidt, D. Jolley, P. Walls, J. Beresford and C. Grundy, Br. J. Cancer, 1996, 73, 702–710 CrossRef CAS.
- T. R. Hsiue, S. S. Lee and H. I. Chen, Chest, 1991, 100, 698–702 CrossRef CAS.
- Y. Miyake, A. Yura, H. Misaki, Y. Ikeda, T. Usui, M. Iki and T. Shimizu, Eur. J. Epidemiol., 2005, 20, 1023–1029 CrossRef CAS.
- C. Zhuo and Y. A. Levendis, J. Appl. Polym. Sci., 2014, 131, 1–14 CrossRef.
- C. S. Burke, E. Salas, K. Smith-Jentsch and M. A. Rosen, Macrocognition Metrics Scenar. Des. Eval. Real-World Teams, 2012, pp. 29–43 Search PubMed.
- C. S. Burke, E. Salas, K. Smith-Jentsch and M. A. Rosen, Macrocognition Metrics Scenar. Des. Eval. Real-World Teams, 2012, pp. 29–43 Search PubMed.
- I. J. Ahluwalia and U. Patel, Indian Counc. Res. Int. Econ. Relations, 2018, pp. 1–48 Search PubMed.
- D. M. C. Chen, B. L. Bodirsky, T. Krueger, A. Mishra and A. Popp, Environ. Res. Lett., 2020, 15(7), 074021 CrossRef CAS.
- F. Akbar, M. Kolahdouz, S. Larimian, B. Radfar and H. H. Radamson, J. Mater. Sci.: Mater. Electron., 2015, 26, 4347–4379 CrossRef CAS.
- J. M. Allen, T. C. Vincent and K. B. Richard, Chem. Rev., 2010, 110, 132–145 CrossRef.
- E. Pop, V. Varshney and A. K. Roy, MRS Bull., 2012, 37, 1273–1281 CrossRef CAS.
- T. V. Cuong, V. H. Pham, Q. T. Tran, J. S. Chung, E. W. Shin, J. S. Kim and E. J. Kim, Mater. Lett., 2010, 64, 765–767 CrossRef CAS.
- N. A. El Essawy, S. M. Ali, H. A. Farag, A. H. Konsowa, M. Elnouby and H. A. Hamad, Ecotoxicol. Environ. Saf., 2017, 145, 57–68 CrossRef CAS.
- J. Gong, J. Liu, X. Wen, Z. Jiang, X. Chen, E. Mijowska and T. Tang, Ind. Eng. Chem. Res., 2014, 53, 4173–4181 CrossRef CAS.
- H. Wang, H. Yi, C. Zhu, X. Wang and H. J. Fan, Nano Energy, 2015, 13, 658–669 CrossRef CAS.
- Z. Bi, Q. Kong, Y. Cao, G. Sun, F. Su, X. Wei, X. Li, A. Ahmad, L. Xie and C. M. Chen, J. Mater. Chem. A, 2019, 7, 16028–16045 RSC.
- A. R. Kamali, J. Yang and Q. Sun, Appl. Surf. Sci., 2019, 476, 539–551 CrossRef CAS.
- Y. Wen, K. Kierzek, X. Chen, J. Gong, J. Liu, R. Niu, E. Mijowska and T. Tang, Waste Manage., 2019, 87, 691–700 CrossRef CAS.
- S. Pandey, M. Karakoti, S. Dhali, N. Karki, B. SanthiBhushan, C. Tewari, S. Rana, A. Srivastava, A. B. Melkani and N. G. Sahoo, Waste Manage., 2019, 88, 48–55 CrossRef CAS.
- C. Tewari, G. Tatrari, M. Karakoti, S. Pandey, M. Pal, S. Rana, B. SanthiBhushan, A. B. Melkani, A. Srivastava and N. G. Sahoo, Mater. Sci. Eng., C, 2019, 104, 109970 CrossRef CAS.
- X. Y. Chen, L. X. Cheng, X. Deng, L. Zhang and Z. J. Zhang, Ind. Eng. Chem. Res., 2014, 53, 6990–6997 CrossRef CAS.
- L. Cui, X. Wang, N. Chen, B. Ji and L. Qu, Nanoscale, 2017, 9, 9089–9094 RSC.
- R. Papon, C. Pierlot, S. Sharma, S. M. Shinde, G. Kalita and M. Tanemura, Phys. Status Solidi B, 2017, 254, 1–7 CrossRef.
- L. Cui, X. Wang, N. Chen, B. Ji and L. Qu, Nanoscale, 2017, 9, 9089–9094 RSC.
- S. Sharma, G. Kalita, R. Hirano, S. M. Shinde, R. Papon, H. Ohtani and M. Tanemura, Carbon, 2014, 72, 66–73 CrossRef CAS.
- Z. Baig, O. Mamat and M. Mustapha, Crit. Rev. Solid State Mater. Sci., 2018, 43, 1–46 CrossRef CAS.
- V. N. Popov, Mater. Sci. Eng., R, 2004, 43, 61–102 CrossRef.
- N. Zhang, Y. Zhang, M. Q. Yang, Z. R. Tang and Y. J. Xu, J. Catal., 2013, 299, 210–221 CrossRef CAS.
- O. G. Apul, Q. Wang, Y. Zhou and T. Karanfil, Water Res., 2013, 47, 1648–1654 CrossRef CAS.
- J. Appenzeller, J. Knoch, R. Martel, V. Derycke, S. J. Wind and P. Avouris, IEEE Trans. Nanotechnol., 2002, 1, 184–188 Search PubMed.
- G. Bajad, V. Guguloth, R. P. Vijayakumar and S. Bose, Fullerenes, Nanotubes, Carbon Nanostruct., 2016, 24, 162–169 CrossRef CAS.
- N. Mishra, G. Das, A. Ansaldo, A. Genovese, M. Malerba, M. Povia, D. Ricci, E. Di Fabrizio, E. Di Zitti, M. Sharon and M. Sharon, J. Anal. Appl. Pyrolysis, 2012, 94, 91–98 CrossRef CAS.
- V. G. Pol and P. Thiyagarajan, J. Environ. Monit., 2010, 12, 455–459 RSC.
- C. Wu, Z. Wang, L. Wang, P. T. Williams and J. Huang, RSC Adv., 2012, 2, 4045–4047 RSC.
- Z. Jiang, R. Song, W. Bi, J. Lu and T. Tang, Carbon, 2007, 45, 449–458 CrossRef CAS.
- J. Gong, J. Liu, L. Ma, X. Wen, X. Chen, D. Wan, H. Yu, Z. Jiang, E. Borowiak-Palen and T. Tang, Appl. Catal., B, 2012, 117–118, 185–193 CrossRef CAS.
- K. Mukhopadhyay, A. Koshio, N. Tanaka and H. Shinohara, Jpn. J. Appl. Phys., 1998, 37, L1257 CrossRef CAS.
- C. Zhuo, J. O. Alves, J. A. S. Tenorio and Y. A. Levendis, Ind. Eng. Chem. Res., 2012, 51, 2922–2930 CrossRef CAS.
- J. Liu, Z. Jiang, H. Yu and T. Tang, Polym. Degrad. Stab., 2011, 96, 1711–1719 CrossRef CAS.
- M. Hassan, E. Haque, K. R. Reddy, A. I. Minett, J. Chen and V. G. Gomes, Nanoscale, 2014, 6, 11988–11994 RSC.
- H. Sun, N. Gao, K. Dong, J. Ren and X. Qu, ACS Nano, 2014, 8, 6202–6210 CrossRef CAS.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J. J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- S. Kim, S. W. Hwang, M. K. Kim, D. Y. Shin, D. H. Shin, C. O. Kim, S. B. Yang, J. H. Park, E. Hwang, S. H. Choi, G. Ko, S. Sim, C. Sone, H. J. Choi, S. Bae and B. H. Hong, ACS Nano, 2012, 6, 8203–8208 CrossRef CAS.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J. J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960–9963 CrossRef CAS.
- M. Kaur, M. Kaur and V. K. Sharma, Adv. Colloid Interface Sci., 2018, 259, 44–64 CrossRef CAS.
- Y. Du, P. Xiao, J. Yuan and J. Chen, Coatings, 2020, 109, 892 CrossRef.
- J. Wei, X. Zhang, Y. Sheng, J. Shen, P. Huang, S. Guo, J. Pan, B. Liu and B. Feng, New J. Chem., 2014, 38, 906–909 RSC.
- Y. Hu, J. Yang, J. Tian, L. Jia and J. S. Yu, RSC Adv., 2014, 4, 47169–47176 RSC.
- A. Kumari, A. Kumar, S. K. Sahu and S. Kumar, Sens. Actuators, B, 2018, 254, 197–205 CrossRef CAS.
- S. Y. Park, H. U. Lee, E. S. Park, S. C. Lee, J. W. Lee, S. W. Jeong, C. H. Kim, Y. C. Lee, Y. S. Huh and J. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 3365–3370 CrossRef CAS.
- S. Thambiraj and D. R. Shankaran, Appl. Surf. Sci., 2016, 390, 435–443 CrossRef.
- A. Prasannan and T. Imae, Ind. Eng. Chem. Res., 2013, 52, 15673–15678 CrossRef CAS.
- A. Tyagi, K. M. Tripathi, N. Singh, S. Choudhary and R. K. Gupta, RSC Adv., 2016, 6, 72423–72432 RSC.
- P. Jegannathan, A. TermehYousefi, M. S. A. Karim and N. A. Kadri, Probl. Biocybern. Biomed. Eng., 2018, 38, 481–497 CrossRef.
- L. Sun, C. Tian, M. Li, X. Meng, L. Wang, R. Wang, J. Yin and H. Fu, J. Mater. Chem. A, 2013, 1, 6462–6470 RSC.
- X. Zhang, K. Zhang, H. Li, Q. Cao, L. Jin and P. Li, J. Power Sources, 2017, 344, 176–184 CrossRef CAS.
- C. K. Liu, Y. Feng, H. J. He, J. Zhang, R. J. Sun and M. Y. Chen, Mater. Des., 2015, 85, 483–486 CrossRef CAS.
- F. Paquin, J. Rivnay, A. Salleo, N. Stingelin and C. Silva, J. Mater. Chem. C, 2015, 3, 10715–10722 RSC.
- H. Chen, F. Yu, G. Wang, L. Chen, B. Dai and S. Peng, ACS Omega, 2018, 3, 4724–4732 CrossRef CAS.
- X. Q. Lin, Q. F. Lü, Q. Li, M. Wu and R. Liu, ACS Omega, 2018, 3, 13283–13289 CrossRef CAS.
- Y. Zhang, L. Liu, P. Zhang, J. Wang, M. Xu, Q. Deng, Z. Zeng and S. Deng, Chem. Eng. J., 2019, 355, 309–319 CrossRef CAS.
- J. Xia, N. Zhang, S. Chong, D. Li, Y. Chen and C. Sun, Green Chem., 2018, 20, 694–700 RSC.
- M. Karnan, K. Subramani, N. Sudhan, N. Ilayaraja and M. Sathish, ACS Appl. Mater. Interfaces, 2016, 8, 35191–35202 CrossRef CAS.
- J. Wang, X. Zhang, Z. Li, Y. Ma and L. Ma, J. Power Sources, 2020, 451, 227794 CrossRef CAS.
- O. Norouzi, S. Jafarian, F. Safari, A. Tavasoli and B. Nejati, Bioresour. Technol., 2016, 219, 643–651 CrossRef CAS.
- Y. Huang, Y. Liu, G. Zhao and J. Y. Chen, J. Mater. Sci., 2017, 52, 478–488 CrossRef CAS.
- C. Wang, D. Wu, H. Wang, Z. Gao, F. Xu and K. Jiang, J. Power Sources, 2017, 363, 375–383 CrossRef CAS.
- Y. Gong, D. Li, C. Luo, Q. Fu and C. Pan, Green Chem., 2017, 19, 4132–4140 RSC.
- M. Zhang, Y. Li, H. Si, B. Wang and T. Song, Int. J. Electrochem. Sci., 2017, 12, 7844–7852 CrossRef CAS.
- X. Tian, H. Ma, Z. Li, S. Yan, L. Ma, F. Yu, G. Wang, X. Guo, Y. Ma and C. Wong, J. Power Sources, 2017, 359, 88–96 CrossRef CAS.
- A. K. Mondal, K. Kretschmer, Y. Zhao, H. Liu, C. Wang, B. Sun and G. Wang, Chem. – Eur. J., 2017, 23, 3683–3690 CrossRef CAS.
- S. Gao, Y. Chen, H. Fan, X. Wei, C. Hu, H. Luo and L. Qu, J. Mater. Chem. A, 2014, 2, 3317–3324 RSC.
- C. Wang, J. Huang, H. Qi, L. Cao, Z. Xu, Y. Cheng, X. Zhao and J. Li, J. Power Sources, 2017, 358, 85–92 CrossRef CAS.
- L. Sun, C. Tian, M. Li, X. Meng, L. Wang, R. Wang, J. Yin and H. Fu, J. Mater. Chem. A, 2013, 1, 6462–6470 RSC.
- X. Tian, H. Ma, Z. Li, S. Yan, L. Ma, F. Yu, G. Wang, X. Guo, Y. Ma and C. Wong, J. Power Sources, 2017, 359, 88–96 CrossRef CAS.
- J. Wang, Z. Li, S. Yan, X. Yu, Y. Ma and L. Ma, RSC Adv., 2019, 9, 14797–14808 RSC.
- J. Cui, Y. Xi, S. Chen, D. Li, X. She, J. Sun, W. Han, D. Yang and S. Guo, Adv. Funct. Mater., 2016, 26, 8487–8495 CrossRef CAS.
- R. Thangavel, K. Kaliyappan, H. V. Ramasamy, X. Sun and Y. S. Lee, ChemSusChem, 2017, 10, 2805–2815 CrossRef CAS.
- C. Wang, D. Wu, H. Wang, Z. Gao, F. Xu and K. Jiang, J. Mater. Chem. A, 2018, 6, 1244–1254 RSC.
- L. Zheng, S. Wang, Y. Yang, X. Fu, T. Jiang and J. Yang, ACS Omega, 2019, 4, 5904–5914 CrossRef CAS.
- G. Zhu, L. Ma, H. Lv, Y. Hu, T. Chen, R. Chen, J. Liang, X. Wang, Y. Wang, C. Yan, Z. Tie, Z. Jin and J. Liu, Nanoscale, 2017, 9, 1237–1243 RSC.
- D. Yan, C. Yu, D. Li, X. Zhang, J. Li, T. Lu and L. Pan, J. Mater. Chem. A, 2016, 4, 11077–11085 RSC.
- R. R. Gaddam, D. Yang, R. Narayan, K. V. S. N. Raju, N. A. Kumar and X. S. Zhao, Nano Energy, 2016, 26, 346–352 CrossRef CAS.
- T. E. Rufford, D. Hulicova-Jurcakova, K. Khosla, Z. Zhu and G. Q. Lu, J. Power Sources, 2010, 195, 912–918 CrossRef CAS.
- L. Tao, Y. Huang, Y. Zheng, X. Yang, C. Liu, M. Di, S. Larpkiattaworn, M. R. Nimlos and Z. Zheng, J. Taiwan Inst. Chem. Eng., 2019, 95, 217–226 CrossRef CAS.
- B. Liu, X. Zhou, H. Chen, Y. Liu and H. Li, Electrochim. Acta, 2016, 208, 55–63 CrossRef CAS.
- C. Long, X. Chen, L. Jiang, L. Zhi and Z. Fan, Nano Energy, 2015, 12, 141–151 CrossRef CAS.
- L. Wang, G. Mu, C. Tian, L. Sun, W. Zhou, P. Yu, J. Yin and H. Fu, ChemSusChem, 2013, 6, 880–889 CrossRef CAS.
- X. Zheng, W. Lv, Y. Tao, J. Shao, C. Zhang, D. Liu, J. Luo, D. W. Wang and Q. H. Yang, Chem. Mater., 2014, 26, 6896–6903 CrossRef CAS.
- P. Hao, Z. Zhao, J. Tian, H. Li, Y. Sang, G. Yu, H. Cai, H. Liu, C. P. Wong and A. Umar, Nanoscale, 2014, 6, 12120–12129 RSC.
- Y. Fan, P. Liu, B. Zhu, S. Chen, K. Yao and R. Han, Int. J. Hydrogen Energy, 2015, 40, 6188–6196 CrossRef CAS.
- W. Gu and G. Yushin, Wiley Interdiscip. Rev.: Energy Environ., 2014, 3, 424–473 CAS.
- S. He and W. Chen, J. Power Sources, 2015, 294, 150–158 CrossRef CAS.
- C. Zhang, Z. Geng, M. Cai, J. Zhang, X. Liu, H. Xin and J. Ma, Int. J. Hydrogen Energy, 2013, 38, 9243–9250 CrossRef CAS.
- S. I. Mussatto, E. M. S. Machado, S. Martins, J. A. Teixeira, J. Fermoso, O. Mašek, F. Codignole Luz, M. Volpe, L. Fiori, A. Manni, S. Cordiner, V. Mulone, V. Rocco, S. Park, S. J. Kim, K. C. Oh, L. Cho, M. J. Kim, I. S. Jeong, C. G. Lee, D. H. Kim, N. Kondamudi, S. K. Mohapatra, M. Misra, D. Pujol, C. Liu, J. Gominho, M. À. Olivella, N. Fiol, I. Villaescusa, H. Pereira, T. M. Mata, A. A. Martins, N. S. Caetano, C. L. Mendoza Martinez, E. P. Alves Rocha, A. de C. Oliveira Carneiro, F. J. Borges Gomes, L. A. RibasBatalha, E. Vakkilainen, M. Cardoso and E. B. Conference, Bioresour. Technol., 2018, 256, 11757–11760 Search PubMed.
- K. Yang, J. Peng, C. Srinivasakannan, L. Zhang, H. Xia and X. Duan, Bioresour. Technol., 2010, 101, 6163–6169 CrossRef CAS.
- X. He, P. Ling, M. Yu, X. Wang, X. Zhang and M. Zheng, Electrochim. Acta, 2013, 105, 635–641 CrossRef CAS.
- G. A. M. Ali, S. A. B. A. Manaf, A. Kumar, K. F. Chong and G. Hegde, J. Phys. D: Appl. Phys., 2014, 47, 495307 CrossRef.
- Y. Fan, X. Yang, B. Zhu, P. F. Liu and H. T. Lu, J. Power Sources, 2014, 268, 584–590 CrossRef CAS.
- K. L. Hong, L. Qie, R. Zeng, Z. Q. Yi, W. Zhang, D. Wang, W. Yin, C. Wu, Q. J. Fan, W. X. Zhang and Y. H. Huang, J. Mater. Chem. A, 2014, 2, 12733–12738 RSC.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- D. W. Wang, F. Li, M. Liu, G. Q. Lu and H. M. Cheng, J. Phys. Chem. C, 2008, 112, 9950–9955 CrossRef CAS.
- C. Wang, Y. Xiong, H. Wang, C. Jin and Q. Sun, J. Mater. Chem. A, 2017, 5, 15759–15770 RSC.
- L. L. Zhang, H. H. Li, Y. H. Shi, C. Y. Fan, X. L. Wu, H. F. Wang, H. Z. Sun and J. P. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 4233–4241 CrossRef CAS.
- J. Hou, C. Cao, F. Idrees and X. Ma, ACS Nano, 2015, 9, 2556–2564 CrossRef CAS.
- D. D. Zhou, W. Y. Li, X. L. Dong, Y. G. Wang, C. X. Wang and Y. Y. Xia, J. Mater. Chem. A, 2013, 1, 8488–8496 RSC.
- H. Lu, L. Zhuang, R. R. Gaddam, X. Sun, C. Xiao, T. Duignan, Z. Zhu and X. S. Zhao, J. Mater. Chem. A, 2019, 7, 22579–22587 RSC.
- M. Liu, K. Zhang, M. Si, H. Wang, L. Chai and Y. Shi, Carbon, 2019, 153, 707–716 CrossRef CAS.
- Z. Ling, Z. Wang, M. Zhang, C. Yu, G. Wang, Y. Dong, S. Liu, Y. Wang and J. Qiu, Adv. Funct. Mater., 2016, 26, 111–119 CrossRef CAS.
- J. Zhao, Y. Li, G. Wang, T. Wei, Z. Liu, K. Cheng, K. Ye, K. Zhu, D. Cao and Z. Fan, J. Mater. Chem. A, 2017, 5, 23085–23093 RSC.
- C. Huang, T. Sun and D. Hulicova-Jurcakova, ChemSusChem, 2013, 6, 2330–2339 CrossRef CAS.
- H. Xu, C. Wu, X. Wei and S. Gao, J. Mater. Chem. A, 2018, 6, 15340–15347 RSC.
- E. P. Randviir, D. A. C. Brownson and C. E. Banks, Mater. Today, 2014, 17, 426–432 CrossRef CAS.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270–274 CrossRef CAS.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J. H. Ahn, P. Kim, J. Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS.
- S. Bae, H. Kim, Y. Lee, X. Xu, J. S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. Ri Kim, Y. Il Song, Y. J. Kim, K. S. Kim, B. Özyilmaz, J. H. Ahn, B. H. Hong and S. Iijima, Nat. Nanotechnol., 2010, 5, 574–578 CrossRef CAS.
- T. Hasan, V. Scardaci, P. H. Tan, F. Bonaccorso, A. G. Rozhin, Z. Sun and A. C. Ferrari, Molecular- and Nano-Tubes, 2011 Search PubMed.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867–884 RSC.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS.
- M. A. Garakani, S. Abouali, Z. L. Xu, J. Huang, J. Q. Huang and J. K. Kim, J. Mater. Chem. A, 2017, 5, 3547–3557 RSC.
- W. G. Chong, J. Q. Huang, Z. L. Xu, X. Qin, X. Wang and J. K. Kim, Adv. Funct. Mater., 2016, 27(4), 1604815 CrossRef.
- Y. Lian, M. Ni, Z. Huang, R. Chen, L. Zhou, W. Utetiwabo and W. Yang, Chem. Eng. J., 2019, 366, 313–320 CrossRef CAS.
- Z. Chen, X. Wang, B. Xue, Q. Wei, L. Hu, Z. Wang, X. Yang and J. Qiu, ChemSusChem, 2019, 12, 1390–1400 CrossRef CAS.
- H. Peng, G. Ma, K. Sun, Z. Zhang, Q. Yang and Z. Lei, Electrochim. Acta, 2016, 190, 862–871 CrossRef CAS.
- Y. Li, X. Wang and M. Cao, J. CO2 Util., 2018, 27, 204–216 CrossRef CAS.
- Z. Zhang, Y. Liu, Z. Huang, L. Ren, X. Qi, X. Wei and J. Zhong, Phys. Chem. Chem. Phys., 2015, 17, 20795–20804 RSC.
- W. Xing, S. Z. Qiao, R. G. Ding, F. Li, G. Q. Lu, Z. F. Yan and H. M. Cheng, Carbon, 2006, 44, 216–224 CrossRef CAS.
- P. Deng, S. Lei, W. Wang, W. Zhou, X. Ou, L. Chen, Y. Xiao and B. Cheng, J. Mater. Sci., 2018, 53, 14536–14547 CrossRef CAS.
- R. Jalili, D. Esrafilzadeh, S. H. Aboutalebi, Y. M. Sabri, A. E. Kandjani, S. K. Bhargava, E. D. Gaspera, T. R. Gengenbach, A. Walker, Y. Chao and C. Wang, Nat. Commun., 2018, 9, 1–13 CrossRef CAS.
- B. Campbell and J. Manning, Rise Vict. Cult. Microaggressions, Safe Spaces, New Cult. Wars, 2018, pp. 1–265 Search PubMed.
- D. Prasai, J. C. Tuberquia, R. R. Harl, G. K. Jennings, B. R. Rogers and K. I. Bolotin, ACS Nano, 2012, 6, 4540 CrossRef CAS.
- A. S. Mayorov, R. V. Gorbachev, S. V. Morozov, L. Britnell, R. Jalil, L. A. Ponomarenko, P. Blake, K. S. Novoselov, K. Watanabe, T. Taniguchi and A. K. Geim, Nano Lett., 2011, 11, 2396–2399 CrossRef CAS.
- A. K. Mondal, K. Kretschmer, Y. Zhao, H. Liu, H. Fan and G. Wang, Microporous Mesoporous Mater., 2017, 246, 72–80 CrossRef CAS.
- C. Zequine, C. K. Ranaweera, Z. Wang, S. Singh, P. Tripathi, O. N. Srivastava, B. K. Gupta, K. Ramasamy, P. K. Kahol, P. R. Dvornic and R. K. Gupta, Sci. Rep., 2016, 6, 1–10 CrossRef.
- N. Mishra, S. Shinde, R. Vishwakarma, S. Kadam, M. Sharon and M. Sharon, AIP Conf. Proc., 2013, 1538, 228–236 CrossRef CAS.
- M. Zhi, F. Yang, F. Meng, M. Li, A. Manivannan and N. Wu, ACS Sustainable Chem. Eng., 2014, 2, 1592–1598 CrossRef CAS.
- Y. Zhang, Z. Shen, Y. Yu, L. Liu, G. Wang and A. Chen, J. Mater. Sci., 2018, 53, 12115–12122 CrossRef CAS.
- Y. Z. Zhang, Y. Wang, T. Cheng, W. Y. Lai, H. Pang and W. Huang, Chem. Soc. Rev., 2015, 44, 5181–5199 RSC.
- X. Wen, X. Chen, N. Tian, J. Gong, J. Liu, M. H. Rümmeli, P. K. Chu, E. Mijiwska and T. Tang, Environ. Sci. Technol., 2014, 48, 4048–4055 CrossRef CAS.
- D. Puthusseri, V. Aravindan, B. Anothumakkool, S. Kurungot, S. Madhavi and S. Ogale, Small, 2014, 10, 4395–4402 CAS.
- T. D. Dongale, P. R. Jadhav, G. J. Navathe, J. H. Kim, M. M. Karanjkar and P. S. Patil, Mater. Sci. Semicond. Process., 2015, 36, 43–48 CrossRef CAS.
- T. Weigert, Q. Tian and K. Lian, J. Power Sources, 2011, 196, 4061–4066 CrossRef CAS.
- S. Zhu, J. Li, L. Ma, C. He, E. Liu, F. He, C. Shi and N. Zhao, Mater. Lett., 2018, 233, 294–297 CrossRef CAS.
- S. I. Wong, J. Sunarso, B. T. Wong, H. Lin, A. Yu and B. Jia, J. Power Sources, 2018, 396, 182–206 CrossRef CAS.
- J. Xia, N. Zhang, S. Chong, D. Li, Y. Chen and C. Sun, Green Chem., 2018, 20, 694–700 RSC.
- Z. Kang, C. Wu, L. Dong, W. Liu, J. Mou, J. Zhang, Z. Chang, B. Jiang, G. Wang, F. Kang and C. Xu, ACS Sustainable Chem. Eng., 2019, 7, 3364–3371 CrossRef CAS.
- C. Wang, D. Wu, H. Wang, Z. Gao, F. Xu and K. Jiang, J. Mater. Chem. A, 2018, 6, 1244–1254 RSC.
- C. Jing, Y. Huang, L. Xia, Y. Chen, X. Wang, X. Liu, B. Dong, F. Dong, S. Li and Y. Zhang, Appl. Surf. Sci., 2019, 496, 143700 CrossRef CAS.
- A. Jain, C. Xu, S. Jayaraman, R. Balasubramanian, J. Y. Lee and M. P. Srinivasan, Microporous Mesoporous Mater., 2015, 218, 55–61 CrossRef CAS.
- C. Zhao, Y. Huang, C. Zhao, X. Shao and Z. Zhu, Electrochim. Acta, 2018, 291, 287–296 CrossRef CAS.
- S. Dong, X. He, H. Zhang, X. Xie, M. Yu, C. Yu, N. Xiao and J. Qiu, J. Mater. Chem. A, 2018, 6, 15954–15960 RSC.
- M. Liu, J. Niu, Z. Zhang, M. Dou and F. Wang, Nano Energy, 2018, 51, 366–372 CrossRef CAS.
- S. Liu, Y. Zhao, B. Zhang, H. Xia, J. Zhou, W. Xie and H. Li, J. Power Sources, 2018, 381, 116–126 CrossRef CAS.
- B. K. Kim, S. Sy, A. Yu and J. Zhang, Handb. Clean Energy Syst., 2015, pp. 1–25 Search PubMed.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294–1301 RSC.
- J. Zhang and X. S. Zhao, ChemSusChem, 2012, 5, 818–841 CrossRef CAS.
- C. G. Cameron, Springer Handbooks, 2017, vol. 38, pp. 563–589 Search PubMed.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- W. Sun, R. Zheng and X. Chen, J. Power Sources, 2010, 195, 7120–7125 CrossRef CAS.
- L. Cui, J. Li and X. G. Zhang, J. Appl. Electrochem., 2009, 39, 1871–1876 CrossRef CAS.
- H. Liu, P. He, Z. Li, Y. Liu and J. Li, Electrochim. Acta, 2006, 51, 1925–1931 CrossRef CAS.
- H. K. Song, H. Y. Hwang, K. H. Lee and L. H. Dao, Electrochim. Acta, 2000, 45, 2241–2257 CrossRef CAS.
- B. K. Kim, V. Chabot and A. Yu, Electrochim. Acta, 2013, 109, 370–380 CrossRef CAS.
- N. Zhao, S. Wu, C. He, C. Shi, E. Liu, X. Du and J. Li, Mater. Lett., 2012, 87, 77–79 CrossRef CAS.
- G. Guidi, T. M. Undeland and Y. Hori, IEEJ Trans. Ind. Appl., 2008, 128, 418–423 CrossRef.
- S. S. Williamson, P. A. Cassani, S. Lukic and B. Blunier, Energy storage, Elsevier Inc., 3rd edn, 2011 Search PubMed.
- L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- P. Simon, Nat. Mater., 2008, 7, 845–854 CrossRef CAS.
- Z. Tan, G. Chen and Y. Zhu, Nanocarbons Adv. Energy Storage, 2015, 1, 211–225 CAS.
- M. Aghazadeh, M. Hosseinifard, B. Sabour and S. Dalvand, Appl. Surf. Sci., 2013, 287, 187–194 CrossRef CAS.
- R. Kumar, R. K. Singh, P. K. Dubey, D. P. Singh and R. M. Yadav, ACS Appl. Mater. Interfaces, 2015, 7, 15042–15251 CrossRef CAS.
- F. Zhou, Q. Liu, J. Gu, W. Zhang and D. Zhang, J. Power Sources, 2015, 273, 945–953 CrossRef CAS.
- S. Sahoo and A. K. Satpati, J. Electroanal. Chem., 2017, 801, 416–424 CrossRef CAS.
- L. Feng, Y. Zhu, H. Ding and C. Ni, J. Power Sources, 2014, 267, 430–444 CrossRef CAS.
- A. M. Abioye, Z. A. Noorden and F. N. Ani, Electrochim. Acta, 2017, 225, 493–502 CrossRef CAS.
- C. Wang, J. Xu, M. F. Yuen, J. Zhang, Y. Li, X. Chen and W. Zhang, Adv. Funct. Mater., 2014, 24, 6372–6380 CrossRef CAS.
- X. Zhu, H. Dai, J. Hu, L. Ding and L. Jiang, J. Power Sources, 2012, 203, 243–249 CrossRef CAS.
- Y. Yan, B. Li, W. Guo, H. Pang and H. Xue, J. Power Sources, 2016, 329, 148–169 CrossRef CAS.
- Q. Yang, R. Bi, K. Chuen Yung and M. Pecht, Electrochim. Acta, 2017, 231, 125–134 CrossRef CAS.
- Q. Abbas, R. Raza, I. Shabbir and A. G. Olabi, J. Sci.: Adv. Mater. Devices, 2019, 4, 341–352 Search PubMed.
- H. P. Wu, D. W. He, Y. S. Wang, M. Fu, Z. L. Liu, J. G. Wang and H. T. Wang, Proc. – 2010 8th Int. Vac. Electron Sources Conf. Nanocarbon, IVESC 2010 NANOcarbon 2010, 2010, pp. 465–466.
| This journal is © The Royal Society of Chemistry 2021 |