Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Manganese dioxide nanosheet coated carbon cloth as a multifunctional interlayer for advanced lithium–sulfur batteries†
Huayun
Liu
,
Hao
Cheng
 ,
Han
Jin
,
Cheng
Gao
,
Peng
Zhang
* and
Miao
Wang
,
Han
Jin
,
Cheng
Gao
,
Peng
Zhang
* and
Miao
Wang
 *
*
Zhejiang Province key Laboratory of Quantum Technology and Device, Department of Physics, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: miaowang@zju.edu.cn
First published on 13th January 2021
Abstract
Carbonized cotton cloth decorated with manganese dioxide (MnO2) nanosheets is developed as a multifunctional interlayer. MnO2 and conductive carbon cloth exhibit dual functions for better electrochemical capability; the batteries with MCC–PP deliver an initial discharge capacity of 843.4 mA h g−1 at 1 C with a capacity decay of 0.063% per cycle after 300 cycles.
Lithium–sulfur (Li–S) batteries are expected to be a promising substitute for rechargeable lithium-ion batteries due to their high theoretical specific capacity (1675 mA h g−1) and energy density (2600 W h Kg−1),1 as well as environmentally friendly feature and lower cost. However, there are still some challenges that hinder the implementation of lithium–sulfur batteries. Typically, these problems mainly revolve around the sulfur cathode in three aspects: (1) lithium polysulfide intermediates inevitably dissolve in the electrolyte, which can result in a serious “shuttle effect”;2 (2) nearly non-conductive sulfur (S) and lithium sulfide (Li2S) constantly lead to poor utilization of active materials during cycling; and (3) the density distinction between sulfur (2.03 g cm−3) and lithium sulfide (1.66 g cm−3) causes uncontrollably strong volume expansion (up to 80%) during lithiation.3
To greatly overcome these challenges, numerous efforts have been made in terms of sulfur composites, electrolytes, lithium metal anodes, separators and multi-functional interlayers to improve the performance of Li–S batteries.4–10 Among various studies, inserting a multifunctional interlayer of carbon materials such as multi-walled carbon nanotubes,11 porous carbon,12 and carbon fiber cloth13 between the sulfur cathode and the PP separator has been proposed, which has been proved to be effective in enhancing the sulfur utilization and capturing polysulfides.14,15 However, since there is no chemical affinity between non-polar carbon materials and polar polysulfides, the suppression ability towards the “shuttle effect” is inefficient during long-term discharging and charging cycles.16 Therefore, some specific structures, which can form chemical interactions between carbon materials and polysulfides, should be designed to impede the dissolution of lithium polysulfides. Extensive studies have shown that many polar metal oxides (MnO2,17 TiO2,18 Co3O4,19 V2O520 and Al2O321) can form strong chemical bonds with lithium polysulfides to suppress the “shuttle effect” effectively. Particularly, MnO2 is a promising functional material to capture and immobilize polysulfides due to its strong chemical absorption properties, which can greatly improve the performance of Li–S batteries.
Inspired by this point, carbon cloth (CC) decorated with MnO2 nanosheet arrays (denoted as MCC) is developed as a bifunctional interlayer to inhibit the “shuttle effect” and promote the redox reaction kinetics. Carbon cloth was simply prepared by the carbonization of waste cotton cloth, and then used as a substrate to deposit MnO2 nanosheets by a facile hydrothermal method (Fig. S1a, ESI†). This barrier layer of MnO2 can form strong chemical absorption with polysulfides to suppress the “shuttle effect” (Fig. S1b, ESI†). Meanwhile, the dense carbon cloth matrix delivers a faster route for electron transfer and remarkably mitigates the volume expansion of the cathode.22 As a result, the battery with MCC–PP delivers an initial discharge capacity of 843.4 mA h g−1 and maintains 682.6 mA h g−1 after 300 cycles at 1 C.
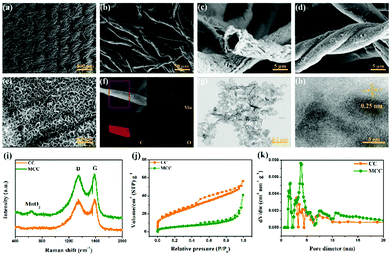
Fig. 1 exhibits the structures and phase compositions of our samples. As seen from Fig. 1a–c, the CC has a braided structure constituted by many hollow carbon fibers with a diameter of about 5 μm. The conductive carbon cloth matrix with a dense structure plays an important role in mitigating the volume expansion for sulfur as well as delivers a fast route for electron transfer. There may be some sizing resins on the surface of raw CC, while it became smoother after the acid oxidation for 12 h (Fig. S2, ESI†). After depositing MnO2 on CC (Fig. 1d and e), we can observe numerous nanosheet arrays which uniformly grow on the CC, interlinking with each other and forming an ordered arrangement. Energy dispersive spectroscopy (EDS) mapping of the MCC composites is depicted in Fig. 1f, demonstrating a uniform distribution of the Mn and O elements throughout the CC. The typical TEM and HRTEM images of MnO2 obtained from MCC composites after intense ultrasonic vibration in ethyl alcohol for 5 h are presented in Fig. 1g and h. The MnO2 nanosheets are thin and the lattice spacing is around 0.25 nm, well consistent with the (100) plane of the birnessite-type MnO2 phase.23Fig. 1i discloses the Raman spectra of the CC and MCC composites. For CC, the two peaks exist at 1350.2 and 1581.9 cm−1 and are in accordance with the defected and graphitized carbon, indicating a D band and a G band, respectively. For MCC composites, there is a new peak originating from MnO2 at about 650 cm−1. Moreover, the ID/IG peak ratio of CC (0.96) increases to 1.02 after the deposition of MnO2, interpreting that the sufficient disorders and defects can supply substantial active sites and strengthen the multi-electronic delivery.24 The results of the nitrogen adsorption–desorption isotherms and the pore size distributions are shown in Fig. 1j and k. After the loading of MnO2 on the CC, the Brunauer–Emmett–Teller (BET) surface area decreases from 73.1 m2 g−1to 18.2 m2 g−1. The pore size distribution of MCC is mainly in the range of 2–4 nm while the pore sizes of CC are mainly 3–5 nm. The distribution of MnO2 can accelerate the redox reaction during operation, and at the same time the satisfactory surface area and the pore volume are vital for supplying ample active sites.
Crystalline structures of the CC and MCC compounds were determined through the X-ray diffraction patterns illustrated in Fig. S3a (ESI†). The two broad diffraction peaks of CC at 23.1° and 43.9° refer to the (002) and (100) planes of the amorphous carbon, respectively.25 MCC composites display three new diffraction peaks at 12.0°, 25.2° and 37.3°, which are indexed to the (001), (002), and (111) planes, respectively, of birnessite-type MnO2 (JCPDS no. 80-1098).26 The (002) plane peak of MnO2 shows weak intensity and overlaps with the diffraction peak of CC probably because of the lower loaded amount of MnO2. The thermogravimetric analysis (TGA) curve was evaluated to obtain the weight ratio of MnO2 on CC (Fig. S3b, ESI†), demonstrating that the MCC composites contain a loading mass for MnO2 of about 38.57%. X-ray photoelectron spectroscopy (XPS) was employed to study the elemental compositions and functional groups of the MCC composites. The spectrum of the MCC composites presented in Fig. S4a (ESI†) exhibits three peaks at 642.1, 530.1 and 248.0 eV, corresponding to Mn, O and C, respectively.27 The C 1s spectrum (Fig. S4b, ESI†) evidences three peaks at 284.5, 286.0 and 288.3 eV corresponding to C–C or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O and C
C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.28 The spectrum of O 1s also shows three peaks located at 529.8 eV (C
O, respectively.28 The spectrum of O 1s also shows three peaks located at 529.8 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Mn
O, Mn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 531.3 eV (C–OH) and 532.8 eV (C–O) (Fig. S4c, ESI†).29
O), 531.3 eV (C–OH) and 532.8 eV (C–O) (Fig. S4c, ESI†).29
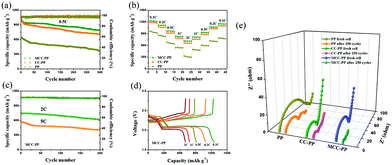
Fig. 2 shows a comparison of the electrochemical performances of the batteries with PP, CC–PP and MCC–PP, respectively. As shown in Fig. 2a, the initial discharge capacity of the batteries with MCC–PP is 1013.6 mA h g−1 at 0.5 C, which is about the same as batteries with CC–PP (993.4 mA h g−1) while evidently higher than those with PP (642.9 mA h g−1), representing the contribution of the carbon cloth interlayer with a high conductivity in improving the charge transfer. After 300 cycles, the batteries with MCC–PP maintain a capacity of 788.6 mA h g−1 and a capacity retention rate of 77.85%. This retention capacity is higher than those of the batteries with CC–PP (662.8 mA h g−1) and PP (362.7 mA h g−1). Additionally, the batteries with MCC–PP possess a higher coulombic efficiency (98%), which remains almost unchanged after 300 cycles. Besides, from the morphologies and EDS detection signals (Fig. S5, ESI†), the anode surface of the batteries with MCC–PP is found to be smoother than other anodes with CC–PP and PP. The EDS detection signals indicate that the anode with PP expresses the strongest sulfur signal, while the anode with MCC–PP is detected by the weakest sulfur signal. This confirms that the MCC–PP interlayer is highly effective for suppressing the polysulfide migration during cycling. When the current rate increases to 1 C, as shown in Fig. S6 (ESI†), the batteries with MCC–PP still exhibit the best performance of an initial discharge capacity of 843.4 mA h g−1 and it remains at 682.6 mA h g−1 after 300 cycles with a lower capacity fading of 0.063% per cycle. Importantly, the batteries with MCC–PP also show good cycling electrochemical performances even at large rates of 2 and 5 C, owing to the synergistic function of MnO2 and CC in the absorption and re-utilization toward polysulfides (Fig. 2c). The rate performances of different batteries are illustrated in Fig. 2b. We can observe that the batteries with MCC–PP have much better rate capability than those with CC–PP and PP at current rates of 0.1, 0.2, 0.5, 1 and 2 C. Meanwhile, for the batteries with MCC–PP, the discharge capacities can nearly recover when the rate is restored, suggesting excellent stability and rate capabilities.
Typical discharge–charge profiles at different rates for the batteries with MCC–PP are displayed in Fig. 2d, and the curves present two typical voltage plateaus even at a large current density of 2 C, further revealing an excellent reversibility of the batteries with MCC–PP. The CV curves of the batteries are shown in Fig. S7 (ESI†). The curves exhibit two obvious reduction peaks in the cathode scan, which denote the conversion of sulfur to long-chain lithium polysulfides (Li2Sn, 4 ≤ n < 8) and short-chain sulfide species (Li2S2, Li2S).11 In the anodic scan, the oxidation peak at about 2.4 V is attributed to the transformation from polysulfides to solid sulfur. Compared with the cells with PP and CC–PP, the one with MCC–PP displays a higher peak current and a lower anodic oxidation potential, which indicates the enhanced chemical reaction kinetics owing to the existence of manganese dioxide.
To further study the influence of different interlayers on the internal resistance, electrochemical impedance spectroscopy (EIS) tests were conducted (Fig. 2e). For the fresh cells, the Rct values of cells with CC–PP (29 Ω) and MCC–PP (47 Ω) are evidently lower than those of the batteries with PP (83 Ω), demonstrating the positive efficacy of the high conductive carbon cloth interlayer for the charge transfer. However, the initial Rct value of MCC–PP is slightly larger than that of CC–PP due to the lower conductive properties of MnO2, which can be illustrated by the resistance measurements of CC and MCC (Fig. S8, ESI†). After 250 cycles at 0.2 C, the Rct values of the batteries all significantly decrease since the active materials are well redistributed and contacted with the electrolyte, which accelerates the charge transfer.30 The minimum Rct value of MCC after cycling once more confirms the enhanced redox kinetics and the increased utilization of polysulfides due to the existence of MnO2.
To intuitively observe the trapping ability of the MCC composites towards polysulfides, the adsorption test was researched (Fig. S9, ESI†). The Li2S6 solution containing MCC composites fades from yellow to light yellow after 12 h. By contrast, the solution containing CC shows no obvious color change compared to the blank Li2S6 solution, indicating the excellent adsorption ability of the MCC composites toward polysulfides. MCC composites with or without the adsorption test were characterized by XPS. For the pristine MCC composites (Fig. 3a), the Mn 2p3/2 spectrum exhibits an Mn2+ peak at 640.6 eV. Obviously, we can see a maximum peak at 642.1 eV which results from the Mn4+ valent state as an emergence of characteristic multiplet and agrees with the two satellite peaks at 643.4 and 644.9 eV.28 In contrast, the MCC composites after permeation tests exhibit an enhanced signal from the Mn2+ ions located at 640.8 eV, accompanied by the decreased contribution from the Mn4+ ions, which may be the redox reaction between Mn4+ and Li2S6 (Fig. 3b). For the S 2p spectrum of MCC-Li2S6 (Fig. 3c), there exists two peaks at 163.5 eV and 164.6 eV, which can be attributed to the terminal (S−1t) and bridging sulfur (S0b) atoms, respectively.31 The other two peaks at 168.4 and 169.3 eV are consistent with the polythionate complex, implying the interaction and strong chemical bonds between MnO2 and polysulfides.19 At the same time, the peaks of S−1t and S0b for MCC-Li2S6 are both higher than those of CC-Li2S6, corresponding to the strong chemical interaction between MnO2 and Li2S6.32
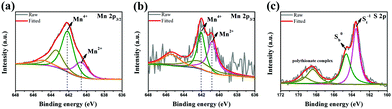 | ||
| Fig. 3 Mn 2p3/2 XPS spectra of MCC composites (a) before and (b) after soaking tests; (c) the S 2p XPS spectrum of MCC composites after the soaking tests. | ||
In summary, a multifunctional CC interlayer decorated with MnO2 nanosheets is successfully designed to improve the performances effectively of Li–S batteries. The conductive CC serves as a physical barrier and provides a fast pathway for electron transportation. MnO2 can form chemical bonding with polysulfides, which can capture the polysulfide intermediates from the cathode and significantly favor the recycling of active materials. A significantly improved performance of capacity, rate and cycling stability for Li–S batteries with MCC–PP is accomplished. A retained capacity of 682.6 mA h g−1 is achieved after 300 cycles with a capacity decay of only 0.063% per cycle at 1 C. This work may offer some new insights into the designs to inhibit the shuttle effect, which can contribute to the multifunctional interlayer in-depth studies of advanced-performance rechargeable batteries.
Author contributions
Huayun Liu: methodology, investigation, software, and writing-draft. Hao Cheng: validation, formal analysis, visualization, writing-review, and data curation. Han Jin: validation, formal analysis, and writing-editing. Cheng Gao: data curation. Peng Zhang: writing-review and writing-editing. Miao Wang: resources, writing-review, writing-editing, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 61471317).Notes and references
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS.
- Y.-X. Yin, S. Xin, Y.-G. Guo and L.-J. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS.
- B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691–714 CrossRef CAS.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4301 CrossRef CAS.
- S. S. Zhang, J. Power Sources, 2013, 231, 153–162 CrossRef CAS.
- L. Suo, Y.-S. Hu, H. Li, M. Armand and L. Chen, Nat. Commun., 2013, 4, 1481 CrossRef.
- K. Yan, Z. Lu, H.-W. Lee, F. Xiong, P.-C. Hsu, Y. Li, J. Zhao, S. Chu and Y. Cui, Nat. Energy, 2016, 1, 16010 CrossRef CAS.
- Y.-X. Yao, X.-Q. Zhang, B.-Q. Li, C. Yan, P.-Y. Chen, J.-Q. Huang and Q. Zhang, InfoMat, 2020, 2, 379–388 CrossRef CAS.
- Y. Liang, C.-Z. Zhao, H. Yuan, Y. Chen, W. Zhang, J.-Q. Huang, D. Yu, Y. Liu, M.-M. Titirici, Y.-L. Chueh, H. Yu and Q. Zhang, InfoMat, 2019, 1, 6–32 CrossRef CAS.
- B.-Q. Li, L. Kong, C.-X. Zhao, Q. Jin, X. Chen, H.-J. Peng, J.-L. Qin, J.-X. Chen, H. Yuan, Q. Zhang and J.-Q. Huang, InfoMat, 2019, 1, 533–541 CrossRef CAS.
- Y.-S. Su, Y. Fu, T. Cochell and A. Manthiram, Nat. Commun., 2013, 4, 2985 CrossRef.
- Y. Zhong, X. Xia, S. Deng, J. Zhan, R. Fang, Y. Xia, X. Wang, Q. Zhang and J. Tu, Adv. Energy Mater., 2018, 8, 1701110 CrossRef.
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, Nat. Energy, 2016, 1, 16132 CrossRef CAS.
- Z. Wei, Y. Ren, J. Sokolowski, X. Zhu and G. Wu, InfoMat, 2020, 2, 483–508 CrossRef CAS.
- C. Zhang, L. Cui, S. Abdolhosseinzadeh and J. Heier, InfoMat, 2020, 2, 613–638 CrossRef CAS.
- H. Tang, W. Li, L. Pan, K. Tu, F. Du, T. Qiu, J. Yang, C. P. Cullen, N. McEvoy and C. Zhang, Adv. Funct. Mater., 2019, 29, 1901907 CrossRef.
- Z. Li, J. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 12886–12890 CrossRef CAS.
- Z. Xiao, Z. Yang, L. Wang, H. Nie, M. E. Zhong, Q. Lai, X. Xu, L. Zhang and S. Huang, Adv. Mater., 2015, 27, 2891–2898 CrossRef CAS.
- J. Pu, Z. Shen, J. Zheng, W. Wu, C. Zhu, Q. Zhou, H. Zhang and F. Pan, Nano Energy, 2017, 37, 7–14 CrossRef CAS.
- W. Li, J. Hicks-Garner, J. Wang, J. Liu, A. F. Gross, E. Sherman, J. Graetz, J. J. Vajo and P. Liu, Chem. Mater., 2014, 26, 3403–3410 CrossRef CAS.
- Z. Zhang, Y. Lai, Z. Zhang, K. Zhang and J. Li, Electrochim. Acta, 2014, 129, 55–61 CrossRef CAS.
- H. Tang, W. Li, L. Pan, C. P. Cullen, Y. Liu, A. Pakdel, D. Long, J. Yang, N. McEvoy, G. S. Duesberg, V. Nicolosi and C. Zhang, Adv. Sci., 2018, 5, 1800502 CrossRef.
- X. Liang, C. Hart, Q. Pang, A. Garsuch, T. Weiss and L. F. Nazar, Nat. Commun., 2015, 6, 5682 CrossRef.
- L. Peng, Y. Liang, H. Dong, H. Hu, X. Zhao, Y. Cai, Y. Xiao, Y. Liu and M. Zheng, J. Power Sources, 2018, 377, 151–160 CrossRef CAS.
- S. He and W. Chen, J. Power Sources, 2015, 294, 150–158 CrossRef CAS.
- A. Boisset, L. Athouel, J. Jacquemin, P. Porion, T. Brousse and M. Anouti, J. Phys. Chem. C, 2013, 117, 7408–7422 CrossRef CAS.
- L. Ni, G. Zhao, G. Yang, G. Niu, M. Chen and G. Diao, ACS Appl. Mater. Interfaces, 2017, 9, 34793–34803 CrossRef CAS.
- X. Wang, G. Li, J. Li, Y. Zhang, A. Wook, A. Yu and Z. Chen, Energy Environ. Sci., 2016, 9, 2533–2538 RSC.
- S. Yuan, Z. Guo, L. Wang, S. Hu, Y. Wang and Y. Xia, Adv. Sci., 2015, 2, 1500071 CrossRef.
- X. Li, Q. Zhu, W. Guo and Q. Lu, J. Electroanal. Chem., 2019, 840, 144–152 CrossRef CAS.
- S. Rehman, T. Tang, Z. Ali, X. Huang and Y. Hou, Small, 2017, 13, 1700087 CrossRef.
- Y. Wei, Y. Tao, C. Zhang, J. Wang, W. Qiao, L. Ling and D. Long, Electrochim. Acta, 2016, 188, 385–392 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00793e |
| This journal is © The Royal Society of Chemistry 2021 |