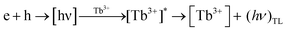Open Access Article
Open Access ArticleDevelopment of LiMgBO3:Tb3+ as a new generation material for thermoluminescence based personnel neutron dosimetry†
Meghnath
Sen
ab,
Rakesh
Shukla
c,
Nimai
Pathak
bd,
Kaustava
Bhattacharyya
 bc,
V.
Sathian
a,
Probal
Chaudhury
ab,
Mukund S.
Kulkarni
be and
Avesh K.
Tyagi
bc,
V.
Sathian
a,
Probal
Chaudhury
ab,
Mukund S.
Kulkarni
be and
Avesh K.
Tyagi
 *bc
*bc
aRadiation Safety Systems Division, Bhabha Atomic Research Centre, Mumbai-400085, India
bHomi Bhabha National Institute, Anushaktinagar, Mumbai-40094, India
cChemistry Division, Bhabha Atomic Research Centre, Mumbai-400085, India
dRadiochemistry Division, Bhabha Atomic Research Centre, Mumbai-400085, India
eHealth Physics Division, Bhabha Atomic Research Centre, Mumbai-400085, India. E-mail: aktyagi@barc.gov.in; Tel: +0091-22-2559-5330
First published on 5th April 2021
Abstract
Personnel neutron dosimetry is of utmost importance due to high relative biological effectiveness (RBE) of neutrons compared with other ionizing radiation. Thermoluminescence (TL) is one of the most widely used techniques in this vital application where LiF:Mg,Ti is recognized as the benchmark material used world-over. However, its complex TL glow curve, complicated treatment procedure and loss of sensitivity upon reuse demand the development of new and more efficient materials. The present work reports the systematic development of the LiMgBO3:Tb3+ phosphor for personnel neutron dosimetry applications along with detailed structural characterizations. The neutron irradiated TL study on LiMgBO3:Tb3+ reveals a simple TL glow curve compared with the very complex pattern of LiF:Mg,Ti. The TL sensitivity for neutrons and the n/γ dose discrimination capability of LiMgBO3:Tb3+ is found to be 2.2 times and 4.5 times higher, respectively, compared with that of the standard material. Also, the TL response up to 105 mSv of neutron dose shows excellent linearity and, most importantly, the fading of the TL signal even up to 90 days of storage is less than 10%. These features meet the ISO-21909 criteria for the practical applicability of this material as a TL based personnel neutron dosimeter. The detailed Photoluminescence (PL) emission and lifetime along with Electron Paramagnetic resonance (EPR) studies were carried out to delineate the underlying TL behaviour through identifying different neutron induced defect centres. Moreover, the trap parameters have been calculated using different methods in order to understand the TL kinetics.
1. Introduction
Thermoluminescence (TL) is one of most widely used research techniques applied in several fields viz. persistent luminescence, band gap engineering, photosynthesis and, most importantly, radiation dosimetry etc.1–6 Among several types of ionizing radiation, the most important one is neutron radiation as it has the highest relative biological effectiveness (RBE) over other radiation types. TL has been successfully utilised over the years as one of the personnel neutron dosimetry techniques which, however, is very complex and poses great challenges. This is mainly because neutrons have a wide range of energy and their interaction cross section is highly dependent upon their energy. Moreover, the presence of gamma radiation along with neutrons in most workplaces makes the situation most critical. Considering these intricacies, so far, mainly LiF based materials have been used for this application. Doped LiF was reported for TL dosimetry applications by Cameron in 1961.7 After that, the pair of TLD-600 (6LiF:Mg,Ti) and TLD-700 (7LiF:Mg,Ti) was successfully introduced for personnel neutron monitoring.8–10 LiF:Mg,Cu,P has also been studied for the said purpose by several researchers.11,12 However, these dosimeters suffer from many disadvantages, such as their complex glow curve structure, complicated annealing procedure, loss of sensitivity upon reuse and retention of the residual signals.13,14 Therefore, there is great scope for the development of new and more efficient materials for TL based personnel neutron dosimetry. Over the years, enormous research has been devoted towards borate based materials owing to their ease of preparation, large band gap and excellent luminescence efficiency.15 Considering these, several researchers in the recent past have worked specifically on the development of borate based TL materials as an alternative to the existing LiF:Mg,Ti neutron dosimeter.16–20 However, the majority of these studies lack systematic investigations viz. neutron dose linearity within the personnel dosimetry region, fading studies, minimum detectable dose measurements etc. and, most importantly, the physical understanding of the observed neutron induced TL behaviour. In view of the above, the present paper focused on the synthesis, detailed structural characterization and TL based personnel neutron dosimetry studies of the novel LiMgBO3:Tb3+ phosphor along with the in-depth understanding of the TL behaviour supported by neutron irradiated PL and EPR investigations. LiMgBO3 is one of the lightest candidates of the mixed metal borate family (MM′BO3 where M = Li, Na, K; M′ = Be, Mg, Ca, Sr and Ba) where the main building block is a planar [BO3]3− unit which offers highly localized valence electrons, anisotropic polarizability, high optical quality and low wavelength absorption.21,22 These features have attracted researchers to study their detailed crystal structure and applications as deep UV NLO (non-linear optical) materials and in phosphor converted solid state lighting.23–26 The crystal structure of LiMgBO3 was first reported by Lehman et al.27 in 1962. In the recent past, various researchers have reported un-doped or doped LiMgBO3 for its structural and electrical properties, luminescence properties, and applications as coloured chromophores and in red-light emitting diodes.28–32 However, although there are reports on the TL based dosimetry studies on LiMgBO3 for beta- and gamma-radiation, its applications for neutron dosimetry have not been studied so far.33,34 Recently TL based personnel neutron dosimetry study was reported for LiMgBO3:Dy3+ but the low neutron sensitivity and high fading of this phosphor have limited its practical use.35 Therefore, aiming at better neutron dosimetry properties, and based on previous research experiences, the present paper investigated the novel LiMgBO3:Tb3+ phosphor (using the Tb3+ dopant instead of Dy3+) which not only overcomes the above-mentioned shortcomings but also provides very promising results which might become a potential alternative to the existing LiF:Mg,Ti dosimeter.36–38 The systematic basis for the selection of LiMgBO3 as the host material for TL based personnel neutron dosimetry has been discussed in detail in Text-S1 (ESI†). On the other hand, the Tb3+ dopant was selected considering two major potent features: first, it is well known for its potential green light emission characteristics where the majority of photomultiplier tubes used in TL readers have very good sensitivity.39–41 Second, and most importantly, the Tb3+ dopant usually generates deep trap centres in the host materials giving rise to a reasonably high temperature and stable TL glow peak which is very desirable for luminescence based dosimetry applications.42,43 LiMgBO3:Tb3+ was synthesized by the sol–gel method, and Rietveld refinement of the XRD pattern enabled to obtain the crystal structure of the compound. Diffuse Reflectance UV-visible (DRUV-vis) spectroscopy study revealed LiMgBO3 as a good insulator with a high optical band gap of 6.3 eV, an essential criterion for TL based dosimetry applications. Infra-red spectra (IR), SEM and EDS measurements were carried out to identify the functional groups, crystal morphology and the presence of constituent elements in the material, respectively. Photoluminescence (PL) study proved LiMgBO3:Tb3+ to be a potential green emitting phosphor from the emission characteristics of the Tb3+ dopant, which is also responsible for the glow curve in the TL study. PL lifetime studies of the un-irradiated samples explained the distribution of Tb3+ among different crystallographic sites whereas PL lifetime studies for neutron irradiated compounds were correlated (for the first time to the best of our knowledge) to the origin of the TL glow curve and their relative contributions towards the total TL intensity. The effect of neutron- and gamma-irradiation on the PL emission properties was also studied thoroughly. Electron Paramagnetic Resonance (EPR) study helped to understand the nature of defect centres generated upon irradiation with neutron- and gamma-radiation, based on which a possible mechanism for the TL emission of LiMgBO3:Tb3+ has been established. Additionally, various kinetic parameters viz. the activation energy (E), frequency factor (s) and order of kinetics (b) responsible for the TL properties have been estimated with two different methodologies and the results were found to be in good agreement. Therefore, a combination of these studies on LiMgBO3:Tb3+ not only provides a new strategy towards the rational development of new TL materials for personnel neutron dosimetry applications but will also benefit greatly understanding the impact of neutron radiation on the photophysical properties of phosphor materials.2. Experimental section
2.1 Starting chemicals
Lithium acetate di-hydrate [CH3COOLi·2H2O, AR], magnesium nitrate hexa-hydrate [Mg(NO3)2·6H2O, AR] and boric acid [H3BO3, 99.95%]. Citric acid, CA [C6H8O7, AR], ethylene glycol, EG [(CH2OH)2, AR], nitric acid [HNO3, AR], terbium oxide [Tb4O7, 99.9%], deionized H2O.2.2 Synthesis
Synthesis of the phosphor LiMgBO3:xTb3+ (x = 0, 0.01, 0.02, 0.03, 0.04 and 0.06 mol) was carried out by sol–gel method. The stoichiometric amounts of CH3COOLi·2H2O and Mg(NO3)2·6H2O were weighed and dissolved in slightly acidic deionised water in a beaker (here beaker no. 1). Required amount of Tb4O7 was dissolved in dil. HNO3 medium in beaker no. 2. In another beaker, no. 3, an aqueous solution of H3BO3 was also prepared to the required concentration. All the stock solutions were kept overnight which resulted in clear solutions. After that, the H3BO3 and Tb4O7 solutions from beaker nos. 2 and 3 were poured into beaker 1 under continuous slow stirring. Beaker 1 was then allowed to boil slowly on the hotplate under continuous stirring. The temperature of the solution was kept fixed at 353 K. Meanwhile, solution of the required amount of citric acid (CA) was also prepared in beaker no. 4. After that, CA and EG were added to the mixed metal nitrate (MN) solution in beaker 1 very slowly by keeping the MN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA and MN
CA and MN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG ratio at 1
EG ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 respectively under continuous stirring. After a while, brown nitrous fumes emanated and a viscous gel was formed, which upon further heating produced a yellowish white coloured foamy product. It was then dried at 473 K for 6 h, which gave rise to a very fine powder on crushing. Next, the powders were ground well using a grey agate mortar and pestle before being transferred into an alumina boat. Calcination of the powder was done in three steps viz. at 723 K, 1073 K and 1123 K, respectively for 6 hours (heating rate 5 K min−1) at each temperature, and in the final step a white colored compound was obtained. During the interval of each step, the intermediate product was ground thoroughly and then kept for heating at the next higher temperature. All products were then washed with hot de-ionized water to remove any boron oxide impurities. Last, the materials were annealed at 573 K for 30 min before conducting the characterizations.
2 respectively under continuous stirring. After a while, brown nitrous fumes emanated and a viscous gel was formed, which upon further heating produced a yellowish white coloured foamy product. It was then dried at 473 K for 6 h, which gave rise to a very fine powder on crushing. Next, the powders were ground well using a grey agate mortar and pestle before being transferred into an alumina boat. Calcination of the powder was done in three steps viz. at 723 K, 1073 K and 1123 K, respectively for 6 hours (heating rate 5 K min−1) at each temperature, and in the final step a white colored compound was obtained. During the interval of each step, the intermediate product was ground thoroughly and then kept for heating at the next higher temperature. All products were then washed with hot de-ionized water to remove any boron oxide impurities. Last, the materials were annealed at 573 K for 30 min before conducting the characterizations.
2.3 Instrumentation and irradiation details
The phase pure formation of the synthesized materials was characterized by powder X-Ray diffraction (XRD) using monochromatic Cu-Kα radiation (λ = 1.5406 Å) on a Rigaku smart lab diffractometer. An SNE4500 instrument was used to record the SEM micrographs. A Bruker Nano GmbHX Flash detector 410-M (Berlin, Germany) was used for recording the EDS spectrum. UV-visible measurements were carried out on a two-beam spectrometer (V-670, JASCO) with a diffuse reflectance (DR) attachment having an integration sphere coated with barium sulphate (BaSO4) as a reference. A Nicolet (Madison, WI, USA) 6700 FTIR spectrometer equipped with both mercury cadmium telluride (MCT) and DTGS (Deuterated Triglycine Sulphate) detectors was used to record the FT-IR spectra. PL measurements were carried out using an Edinburgh CD-920 unit with M 300 monochromators. F-900 software from Edinburgh Analytical Instruments, UK was used for data analysis. A Xenon flash with a frequency of 100 Hz was utilized to record the emission spectra. The PL lifetime measurements were carried out based on the Time-correlated Single-Photon Counting (TCSPC) technique. EPR experiments were carried out using a Bruker EMX (micro) 10/12 spectrometer operating at X-band frequency (9.4218 GHz) equipped with 100 kHz field modulation and phase sensitive detection. Diphenyl picrylhydrazyl (DPPH) was used for calibration of g-values of the paramagnetic species. A lexsyg smart automatic TL/OSL reader, Freiberg, Germany was used to measure all the thermoluminescence (TL) data using an IRSL-TL filter (Schott-BG-39-3 mm). The reader used a standard bi-alkali cathode PMT (HAMAMATSU). All the neutron irradiations were carried out at the Standard thermal Assembly in Graphite [STAG] facility which is a primary standard for the thermal neutron fluence rate. Six 241Am-Be neutron sources were kept inside a cubic assembly made of graphite. Thermal neutrons are produced by the moderation of fast neutrons through interaction with graphite. Irradiation with gamma rays during sensitivity comparisons was carried out using a 60Co source from Co-machine (Theratron 780E, Kirloskar), RSSD, BARC. The distance between the centre of the source and the sample was 1 meter. The samples were sandwiched between two build up sheets (6 mm thick sheet in the front side facing the radiation and 20 mm thick sheet in the back side) made of perspex. The activity of the source was 4181 Ci as on the date of experiment and the field size was 10 mm × 10 mm. For EPR studies the irradiation with gamma rays (dose = 8.2 kGy) was performed in gamma chamber-1200 (Board of Radiation and Isotope Technology, Mumbai, India) where 60Co was used as the source and the dose rate was 20 Gy min−1.3. Results and discussion
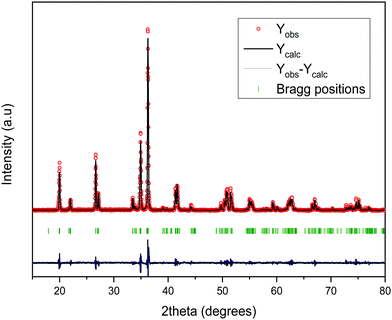
3.1 Structural characterization
| Molecular formula | LiMgBO3 |
| Molecular weight | 485.54 |
| Space group | C2/c |
| Unit cell parameters: | |
| a (Å) | 5.1568(2) |
| b (Å) | 8.8858 (4) |
| c (Å) | 9.9128 (3) |
| β (°) | 91.236 (3) |
| Volume (Å3) | 454.12 (3) |
| Density (calculated) (g/cc) | 2.63 |
| Refinement | Rietveld refinements (Fullprof-2K) |
| (Rodriguez-Caravjal, 2000) | |
| Profile | Pseudo-Voigt |
| Goodness-of-fit (χ2) | 2.58 |
| R p | 16.0 |
| R wp | 20.8 |
| R F | 11.6 |
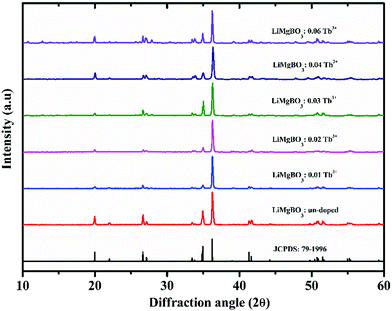
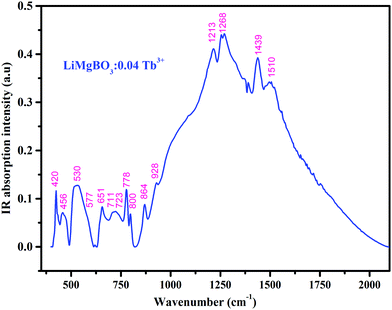
In order to investigate the crystal size and surface morphology of the different compositions of LiMgBO3:Tb3+, Scanning Electron Microscopy (SEM) images were recorded as given in Fig. S2 (ESI†). These revealed that the materials were microcrystalline in nature with irregular shapes. Energy Dispersive Spectroscopy (EDS) studies as shown in Fig. S3 (ESI†) were carried out to investigate the elemental compositions in the matrix. The presence of Mg and O was confirmed by their characteristic peaks at 1.25 keV and 0.52 keV, respectively. The inset curve shows the EDS pattern of un-doped and Tb3+ doped samples, which clearly indicated the characteristic Tb peak at 6.3 keV in the doped sample whereas the same is absent for the un-doped material. Being low-Z elements, Li and B could not be detected whereas the Gold (Au) peak was observed due to the coating of the samples. To characterize the coordination environment of the synthesized LiMgBO3:Tb3+, room temperature IR spectra were recorded as shown in Fig. 4.
The spectrum showed the presence of about 15 different active IR bands in the range 400–2000 cm−1, which matched very well with different modes as reported in the literature.45–48 The bands within 1000–1550 cm−1 (1213 cm−1, 1268 cm−1 and 1439 cm−1 and 1510 cm−1) were attributed to the asymmetric stretching vibrations of the B–O bond in BO3 whereas, the band at 864 cm−1 is due to the symmetric stretching of the B–O bond in BO3. The IR bands within 650–850 cm−1 (711 cm−1, 723 cm−1, 778 cm−1 and 800 cm−1) arise due to the out-of-plane bending vibrations of the B-O bond and those within 500–600 cm−1 (530 cm−1, 577 cm−1 and 651 cm−1) correspond to the in-plane deformation modes of the B–O bond. Based on these active bands it was confirmed that the triangular [BO3]3− group is present in the crystal environment which is one of the main building blocks of the material.49 Also, the bands at 420 cm−1 and 456 cm−1 refer to the Mg–O and Li–O bonds respectively.
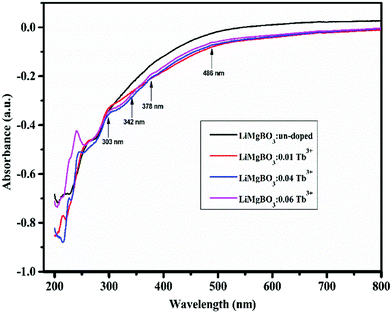
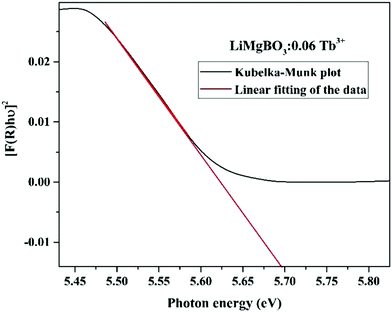
Some of the observable peaks for the doped samples are at 486 nm, 378 nm, 342 nm and 303 nm, which might be attributed to transitions from ground state 7F6 to the higher excited levels, namely, 5D4, (5G6, 5D3), (5L7,8,5G3), and 4f8 → 4f7 5d1 of Tb3+ ions respectively.50 The optical direct band gap (Eg) values of the LiMgBO3:xTb3+ (x = 0, 0.01, 0.04 and 0.06) compounds were estimated from the DRS spectra by using the Kubelka–Munk function, the detailed procedure for which is given in Text-S2 (ESI†).51 Accordingly, the band gap of all compositions was calculated as given in Table S1 (ESI†) whereas the representative Kubelka–Munk plot for one composition (LiMgBO3:0.06Tb3+) is shown in Fig. 6. The band gap of the un-doped LiMgBO3 was found to be 6.3 eV, indicating it to be a good insulator which is one of the most desirable properties for a TL material.52 It is worth mentioning that, this feature of the title compound may also find potential applications as a deep UV NLO material. It was also observed that, as the concentration of the Tb3+ ion was increased from x = 0 to x = 0.06 the band gap also decreased significantly. In the case of LiMgBO3:0.06Tb3+ the band gap was found to be 5.69 eV, which almost matches with the Dy3+ doped LiMgBO3.53 However, the calculated band gap by ab initio techniques in previous literature reported shows a discrepancy with respect to the presently reported values.32 The electronic band structure calculation there revealed that the valence band (VB) was dominated by O ‘2p’ orbitals with some weak B ‘2p’ orbital contribution and only a small amount of Li ‘2s’ and Mg ‘3s’ orbital contributions. The conduction band (CB) was mainly composed of B ‘2p’ and O ‘2p’ orbitals with only small Li and Mg orbital contributions. Remarkably, hybridization between B ‘2p’ and O ‘2p’ orbitals occurred through B–O bonding.32 Here, the potential replacement of Mg2+ by Tb3+ in the LiMgBO3 lattice (as discussed in Section 3.1.3) might have formed a hybridization between valence (5d/4f) orbitals and the valence O ‘2p’ orbital to form a mid-band bap state above the Fermi level similar to as shown earlier where Mn2+ replaced Li+ in the same LiMgBO3 lattice.32 In the present scenario, the Tb3+ doping in the LiMgBO3 lowers the band gap as a function of concentration. Similar observations were also reported by other researchers.54–56
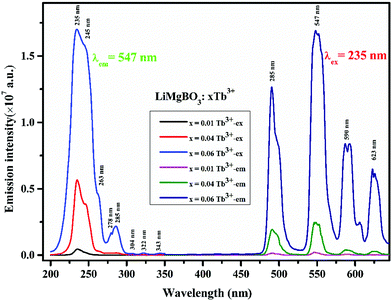
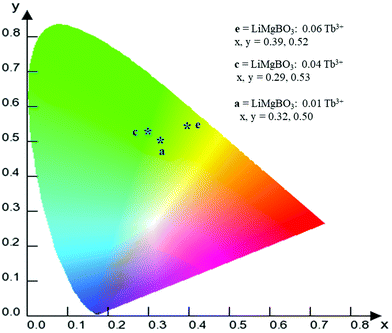
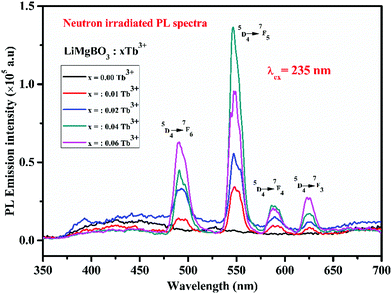
There are different possibilities for the excitation of Tb3+ions, which are (i) direct excitation of Tb3+ ions through f–f transition and f–d transition, (ii) charge transfer transition (CT), and (iii) excitation through defect assisted energy transfer.57 The excitation spectra of all the compounds showed intense peaks at around 235 nm which can be attributed to the charge transfer transition from the ‘p’ orbital of oxygen to the vacant ‘f’ orbital of Tb3+, while the peak at 245 nm can be attributed to the spin allowed f–d transition of the Tb3+ ion.58 Feeble excitation bands at 285, 304, 322, and 340 nm can be assigned to 7F6 → 9E, 5F5, 5H6 and 5H7 respectively.59,60 All the emission spectra were recorded at the 235 nm excitation wavelength, which showed four strong and characteristic emission lines of the Tb3+ ion, viz. 489, 547, 590 and 623 nm which were attributed to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4 and 5D4 → 7F3 transitions, respectively. The PL emission intensity was highest for the 547 nm excitation band for all the compounds, which can be attributed to the magnetic dipole transition (MDT) of the Tb3+, and such transitions are least affected by the crystal field strength. On the other hand, the emission band at 489 nm was attributed to the electric dipole transition (EDT) of Tb3+, which is sensitive to local structure. The PL emission intensity was found to be highest at x = 0.06 Tb3+. The calculated CIE color coordinate of the title compound, as shown in Fig. 8, clearly indicated that it is also a potential green emitting phosphor that could be highly useful in phosphor converted LEDs, and which also reveals the multifunctional nature of the material.
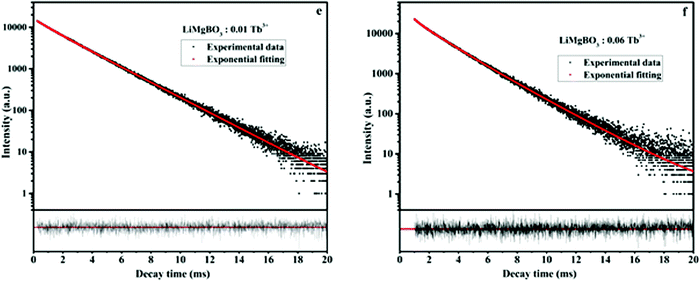
Photoluminescence lifetime measurements were carried out in order to investigate the local surroundings of the Tb3+ ions and the number of Tb3+ components existing in different lattice environments. The lifetime measurements were carried out at 235 nm excitation and 545 nm emission wavelengths. It was observed that the decay curves as shown in Fig. 9 were best fitted by the following the bi-exponential equation:
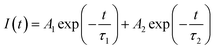 | (1) |
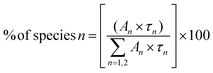 | (2) |
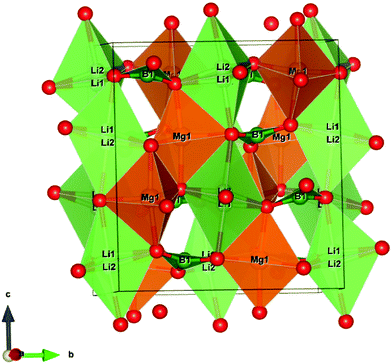
The lifetime values of the un-irradiated LiMgBO3:xTb3+ compounds, as given in Table 2, indicate that the Tb3+ ions have two different local structures. Either Tb3+ ions exist in two different lattice sites or they may exist in a similar lattice site but with different defect surroundings. The difference in ionic radii between Mg2+ [(0.72 Å), (VI)] and Tb3+ [(0.92 Å), (VI)] is greater compared with that between Li+ [(0.76 Å), (VI)] and Tb3+ and thus Tb3+ ions are more likely to go to the Li+ site. However, there will be a charge difference of ‘+2’ and hence there will be creation of new negatively charged cationic vacancies required for the charge balance. These defects may induce distortion into the matrix and may affect the neighboring lattice sites significantly. Thus, there will be an interplay of two factors which will play an important role in deciding the distribution of Tb3+ ions among the two different lattice sites: the higher charge difference at the Li+-site and the size difference at the Mg2+-site. The crystal structure of LiMgBO3 showed that, there are two different Li+ sites and one Mg2+ site, and all of them have a five-coordinated environment. The five coordinated Mg2+ sites roughly form trigonal bipyramidal polyhedra. However, the Li sites have a distorted trigonal bipyramidal configuration since both the Li atoms are five-coordinated by four close O atoms and one distant O atom. Since f–f transitions of Tb3+ are spin- and parity-forbidden and become allowed in an asymmetric environment, the short lifetime value can be attributed to Tb3+ occupying these distorted Li sites. Further, the charge difference of ‘+2’ will create additional defect centers which will induce more distortion. The fact that the fraction of the short-lived component is very low (≤10%) suggests that Tb3+ does not prefer to occupy the Li+ site. On the contrary, the long-lived component with a higher percentage must be due to Tb3+ ions at the more symmetric Mg2+ site. However, the higher intensity of the MD line at 545 nm in the emission spectra for all the compounds suggests that the Tb3+ ions are preferably occupying the more symmetrical Mg2+ site, since the ED line is only allowed in the case of a more asymmetric environment. Therefore, the two different lifetime values might be due to two different Tb3+ ion situated at crystallographically identical Mg2+ sites but with different defect centers surrounding them. Some defect centers may act as an electron trap state which increases the lifetime value while others provide a nonradiative pathways to the excited state and thus reduce the lifetime value.61 Moreover, the distance of such defect centers from the lattice sites also exerts an impact on the excited states. Thus, the short-lived component might be close to a defect center while the long-lived component might be far from such defect centers.62 It is important to note that the short life time value makes a low contribution in samples where the concentration of the Tb3+ dopant is low, while it is the reverse when the concentration of Tb3+ ion is increased. Therefore, it is plausible to infer that, at a low dopant level, the defect centers are present in very low amounts since the amount of charge imbalance in the matrix is low. However, with an increase in the concentration of Tb3+ ions, the overall number of defect centers created in the matrix must be increased due to higher amount of charge imbalance which results in the higher percentage of short-lived species. It might be noted that the values of the short-lived and long-lived components are also different at higher dopant levels, which might be because of the different location of such defect centers with respect to the Tb3+ ion.
| LiMgBO3:xTb3+ | Value of τ1 (ms) | Contribution (%) | Value of τ2 (ms) | Contribution (%) |
|---|---|---|---|---|
| x = 0.01 | 1.136 | 8 | 2.389 | 92 |
| x = 0.04 | 2.041 | 85 | 2.814 | 15 |
| x = 0.06 | 2.301 | 84 | 2.679 | 16 |
3.2 Application of LiMgBO3:Tb3+ as personnel neutron dosimeter
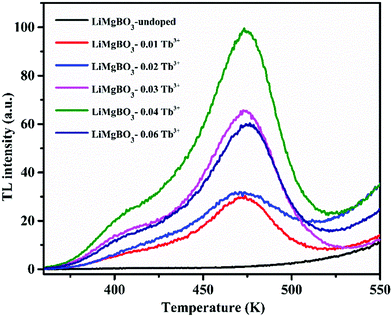
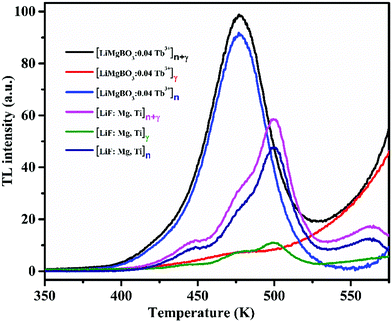
Fig. 10 also indicated that the TL intensity gradually increased as the concentration of dopant ion is increased from 0.01 Tb3+ to 0.04 Tb3+ where it reaches maximum and begins to decrease on further increase in the concentration until 0.06 Tb3+, as also shown in Fig. S4 (ESI†). The above observation may be explained as follows: the irradiation of LiMgBO3:xTb3+ with neutrons resulted in the creation of high linear energy transfer (LET) charged particles followed by the formation of electron–hole pairs which were trapped by the respective trap centres present in the matrix. These electrons and holes upon heating during TL measurements recombined at luminescence centres resulting in radiative photons which further excited the nearby Tb3+ ions giving rise to TL emission. As the concentration of Tb3+ increased, a greater number of Tb3+ ions were excited which led to the increase in the TL intensity subsequently. However, after the peak dopant concentration (0.04 Tb3+), the overall TL intensity was found to decrease due to the concentration quenching phenomenon.64,65 Therefore, 0.04 Tb3+ was found to be the optimum dopant concentration in terms of the maximum TL intensity among the studied compositions, and further neutron dosimetry studies were carried out using this composition unless otherwise specified. Further, the neutron induced TL sensitivities of the presently developed LiMgBO3:0.04Tb3+ and the commercially available LiF:Mg,Ti dosimeter were compared. Equal amounts of both dosimeters were subjected to a neutron dose of 21.7 mSv and the TL glow curves were recorded after 15 days of storage which almost nullified the effect of the initial fading of the TL signal of both the materials. A total of eight samples were irradiated: four samples each from the LiMgBO3:0.04Tb3+ and LiF:Mg,Ti dosimeters. Two samples from each dosimeter were irradiated by being kept inside a 1 mm thick cadmium (Cd) disc which will absorb all the thermal neutrons and will allow the gamma radiation emanating from 241Am-Be neutron source to pass through. Thus, these samples will have a TL response (Rγ) mainly for gamma radiation whereas the other two samples from each dosimeter were irradiated without the Cd box, which will have a response for both neutron- and gamma-radiation (Rn+γ). Therefore, the net neutron response was obtained by subtracting the average values of Rγ from those of Rn+γ. The comparison between the TL glow curves of LiMgBO3:0.04Tb3+ and LiF:Mg,Ti is given in Fig. 11. It shows that the TL glow curve of the presently developed material is very simple compared with the complex and multipeak pattern of 6/7LiF:Mg,Ti. The most important observation to note here is that the neutron induced net TL sensitivity of LiMgBO3:0.04Tb3+ is about 2.2 times higher than that of LiF:Mg,Ti when the area under the TL glow curve of both the dosimeters was compared. The high neutron sensitivity of LiMgBO3:0.04Tb3+ compared with LiF:Mg,Ti may partly be attributed to the presence of both Li and B in the LiMgBO3:0.04Tb3+, which create a larger number of effective carriers responsible for the TL sensitivity upon interaction with neutrons compared with that of LiF:Mg,Ti where only Li is present in the host matrix. On the other hand, it is well known that the neutron field is mostly associated with gamma radiation in workplace environments. Therefore, a comparison of the TL response of both the dosimeters was also done by irradiating with gamma rays. To do this, both the samples were irradiated with a 2000 mGy gamma dose from a 60Co source and the corresponding TL glow curves are shown in Fig. S5 (ESI†). It is shown that LiMgBO3:0.04Tb3+ has two broad TL glow peaks along with an additional small peak lying at 408 K, 463 K and 360 K, respectively. The comparisons of the TL sensitivity have been summarized in Table 3 where the TL intensity was taken as the area under the full TL glow peak of both the dosimeters. It was observed that the TL intensity of LiMgBO3:0.04Tb3+ is almost half compared with LiF:Mg,Ti for the same gamma dose. However, the most important observation here is that the ratio of TL response per unit neutron dose to the TL response per unit gamma dose is about 4.5 times greater for LiMgBO3:0.04Tb3+ than that of LiF:Mg,Ti. This suggests that LiMgBO3:0.04Tb3+ has a greater n/γ dose discrimination capability in comparison to LiF:Mg,Ti dosimeter which is highly advantageous in mixed field environments.
| Data type | LiMgBO3:Tb3+ | LiF:Mg,Ti | ||
|---|---|---|---|---|
| Neutron dose (21.7 mSv) | Gamma dose (2000 mGy) | Neutron dose (21.7 mSv) | Gamma dose (2000 mGy) | |
| TL intensity [AUC × 105 mg−1] | (6.82 ± 0.12) | (11.7 ± 0.73) | (3.08 ± 0.04) | (23.7 ± 1.42) |
| TL intensity [AUC × 104 mg−1 per dose] | (3.14 ± 0.06) | (0.06 ± 0.004) | (1.42 ± 0.02) | (0.12 ± 0.01) |
| TL intensity for neutron [LiMgBO3:Tb3+]/[LiF:Mg,Ti] | (2.2 ± 0.05) | |||
| TL intensity for gamma [LiMgBO3:Tb3+]/[LiF:Mg,Ti] | (0.5 ± 0.04) | |||
| TL intensity [neutron dose (mSv)]/[gamma dose (mGy)] | (54 ± 3) | (12 ± 1) | ||
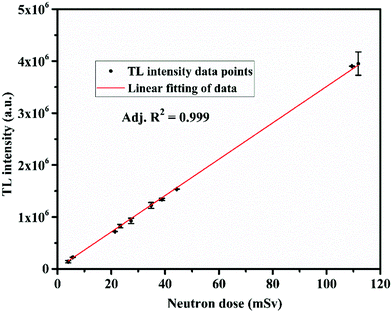
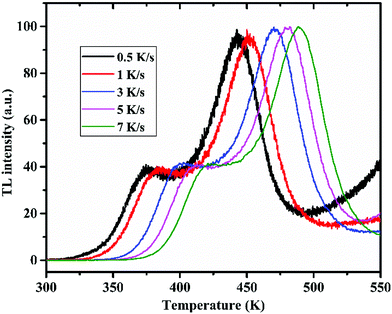
One of the most important properties for any TL material to be applicable for personnel neutron dosimetry is that its TL response should be linear up to a neutron dose of 100 mSv, as per ISO-21909.66 In order to carry out the study, equal weights of the annealed powder samples (2 mg) of LiMgBO3:0.04Tb3+ were taken in stainless cups and spread uniformly over the surface. The samples were kept for irradiation at the central cavity of the STAG facility.63 The dose rate for thermal neutrons at the irradiation location was 0.23 mSv h−1 on the date of the experiment, which was calculated by multiplying the thermal neutron fluence rate and the corresponding conversion factor as given in ISO-8529-3.67 Initially all the samples were kept at the irradiation chamber together and then removed at different time intervals and subsequently they were kept inside a light-tight box. The TL readout of the stored samples was carried out after almost the same latency period such that the fading of the TL signal does not have any effect on the TL intensity. The corresponding TL glow curves of the samples for different neutron doses are shown in Fig. S6 (ESI†). The important feature observed from the glow curves is that the temperature corresponding to the maximum TL intensity (Tm) remained unchanged even at a very high neutron dose of 105 mSv. This indicates that the TL process is obeying first order kinetics. However, detailed analysis will follow later. Fig. 12 depicts the neutron dose linearity curve where the area under the dosimetric glow peak was taken as the TL response.
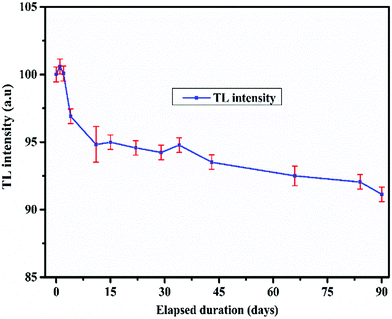
It is clearly seen that the TL response up to the neutron dose of 105 mSv showed excellent linearity with an adjusted R2 = 0.999 which satisfies the ISO-21909 criteria. The minimum detectable dose (MDD) is another highly important parameter for a TL based neutron dosimeter which signifies the ability of the material to measure the lowest possible neutron dose. Based on the variations in the background, the MDD values for both the LiMgBO3:0.04Tb3+ and LiF:Mg,Ti dosimeters were estimated as three times the standard deviation (σ) of the background TL signal. Four samples each from both the LiMgBO3:0.04Tb3+ and LiF:Mg,Ti dosimeters were taken. The background TL readings of each un-irradiated sample were recorded. The mean and standard deviations were evaluated for both dosimeters. The neutron irradiated net TL intensity was obtained from the corresponding TL measurements (with respect to Fig. 11 as was mentioned earlier in the neutron sensitivity comparison of both the dosimeters). The MDD was found to be about 5 μSv and 6 μSv for LiMgBO3:0.04Tb3+ and LiF:Mg,Ti respectively, which is shown in Table S2 (ESI†). This suggests that the MDD of the presently developed material is comparable is to the existing dosimeter. Another most important and desirable feature is that the fading of the neutron induced TL signal must be negligibly small for its practical use in dosimetry applications. According to ISO-21909, the fading must be less than 10% for a storage period of 90 days.66 To investigate such a stringent performance of the developed LiMgBO3:0.04Tb3+, equal amounts of samples were irradiated for same neutron dose of 10 mSv. After removal, all the samples were kept in a light-tight box in a plastic cover. The TL readings were recorded at different time intervals spanning up to 90 days from the date of the removal from the irradiation chamber, as shown in Fig. 13.
The area under the dosimetric TL glow peak was taken as the signal and each data point is the average of three different samples. It was observed that about 6% of the TL signal fades within a storage period of the first 10 days and the remaining 3% signal fades over a span of 80 days, leading to maximum fading of about 9% in 90 days which satisfies the ISO-21909 criteria.
3.3 Understanding TL behaviour
The PL intensity of the 547 nm peak increases from x = 0.01 Tb3+ to x = 0.04 Tb3+ and then decreases on further increases in the dopant concentration (x = 0.06 Tb3+). This might also be due to the concentration quenching phenomenon as described earlier in TL.64,65 However, instead of heating in the TL study, UV light acts here as the excitation source because of which the generated electron and hole pairs followed by neutron irradiation, get de-trapped and recombined similar to TL which in turn excites the Tb3+ ions resulting in PL emission. Among the neutron irradiated samples, highest PL intensity was obtained for the LiMgBO3:0.04Tb3+ sample. Further, to study the effect of neutron- and gamma-irradiation on the PL emission property, the LiMgBO3:xTb3+ (x = 0.04 and 0.06) samples were irradiated with both neutron- and gamma-radiation. Interestingly, it was observed that the PL intensity of both the neutron- and gamma-irradiated samples decreased significantly from that of the un-irradiated ones as shown in Fig. 15.
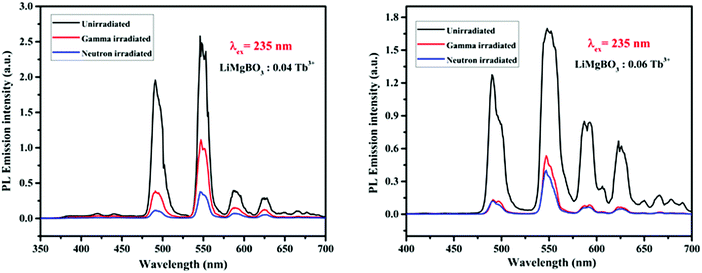 | ||
| Fig. 15 Comparison of PL emission intensity for un-irradiated, gamma- and neutron-irradiated LiMgBO3:xTb3+ (x = 0.04 and 0.06). | ||
Moreover, the extent of decrease in the PL intensity is greater in the case of neutron-irradiated samples than that for the gamma-irradiated ones. This phenomenon may be explained as follows: as the present compound consists of both Li and B atoms, both of which have a very high absorption cross section for thermal neutrons; in turn, high-LET charged particles were generated leading to the creation of several defect centres which exert a severe impact on the matrix and might lead to the pathways favouring the non-radiative processes during the emission from the excited states to ground states of Tb3+. On the other hand, the interaction of gamma radiation with the matrix creates only secondary electrons, which are relatively low LET particles, and thereby the extent of impact is less and there is a lower creation of defect centres, thereby the decrease in PL intensity is low compared with that by neutron irradiation. However, there was no observable change in the wavelength of the emission lines for both the irradiated samples. The lifetime properties of these irradiated compounds were also studied. It is worth mentioning here that the defect centres created in the irradiated samples were mainly due to radicals, while those for un-irradiated compounds were mostly related to various lattice site vacancies. Thus, the lifetime values of the former must be different from those of the latter ones. In irradiated samples it was also found that the decay profiles as shown in Fig. 16 followed bi-exponential behaviour and two lifetime values were obtained, which were completely different from the respective un-irradiated samples.
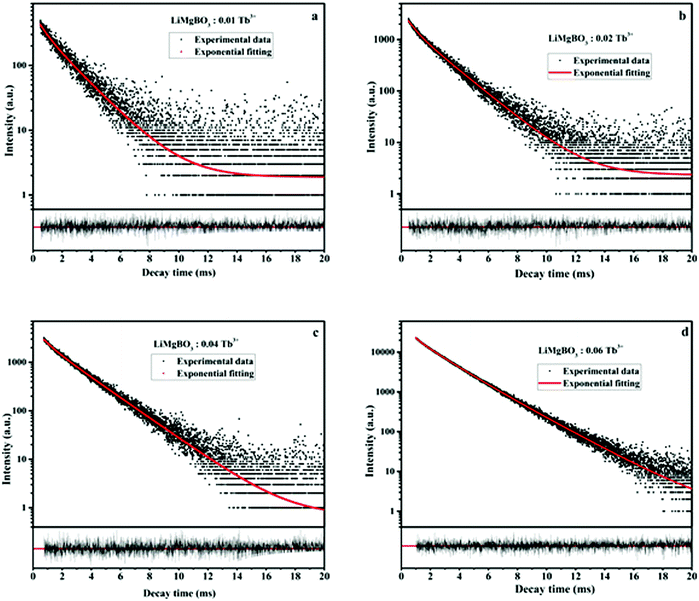 | ||
| Fig. 16 Lifetime plot of neutron irradiated LiMgBO3:xTb3+ (a–d: x = 0.01, 0.02, 0.04 & 0.06) samples. | ||
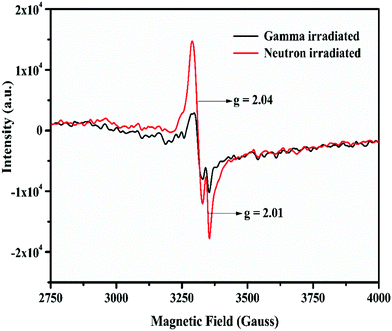
It was observed from Table 4 that the short-lived component has a lower value than that of the un-irradiated samples, and the fraction of the long-lived component is always higher. Interestingly, the neutron irradiated TL study revealed that there were two different glow peaks at two different temperatures, which indicated that there are two different types of trap centres. Thus, the two different lifetime values have been linked to these two trap states. The long-lived component with the higher contribution (%) is linked to the deep trap state which is responsible for the TL glow curve at higher temperatures (Tm = 475 K), while the short-lived component with the lower contribution (%) is linked to a shallow trap state which is responsible for the TL glow peak at low temperatures (Tm = 408 K). Further, the relative contributions in terms of the area under the two different TL glow peaks of LiMgBO3:0.04Tb3+ were also estimated after irradiating with different neutron doses as shown in Table 5. Remarkably, it was found that the relative contributions of both the low- and high-temperature TL glow peaks matched very well with those of the short-lived and long-lived components, respectively, which again justifies the correlation of lifetime values to the different TL glow peaks. Therefore, the PL lifetime measurement of the neutron irradiated samples can be a great tool in order to explain the generation of various TL glow peaks and their relative contributions towards the total TL intensity. On the other hand, the lifetime values for neutron- and gamma-irradiated samples were also compared as shown in Table S3 (ESI†). It was observed that the lifetime values were low for neutron irradiated samples compared with the gamma irradiated ones. Further the fraction of the long-lived component is relatively higher compared with the short-lived component when the samples were irradiated with neutrons. On the other hand, for gamma irradiated samples, such a difference between the two components is comparatively low. Lastly, in order to provide more concrete evidence about the evolution of different defect centers, the room temperature EPR study of neutron- and gamma-irradiated LiMgBO3:0.04Tb3+ was carried out. The un-irradiated samples did not show any EPR signals which indicated the absence of any paramagnetic centers prior to irradiation. Fig. 17 shows the EPR spectra of gamma- and neutron-irradiated LiMgBO3:0.04Tb3+ samples.
| LiMgBO3:xTb3+ | Value of τ1 (ms) | Contribution (%) | Value of τ2 (ms) | Contribution (%) |
|---|---|---|---|---|
| x = 0.01 | 0.592 | 9 | 1.892 | 90 |
| x = 0.02 | 0.546 | 11 | 1.877 | 89 |
| x = 0.04 | 0.623 | 7 | 2.075 | 93 |
| x = 0.06 | 0.870 | 13 | 2.091 | 87 |
From the spectra, two well-resolved resonance signals were found at g = 2.01 and g = 2.04 respectively. Referring to the literature these signals were attributed to the [BO3]2− and [O2]− radicals, respectively, both of which act as possible hole trap centers.68–70 Thermoluminescence (TL) emission was mainly caused by the radiative recombination of holes and electrons which are thermally released from the corresponding trap centers. In the present case, that the existence of the electrons trapped at an anion vacancy [Ov]− could not be detected may be because of the very low concentration or large line width of the resonance signal.71,72 Based on the generation of the above trap centers, a possible mechanism for the generation of TL in LiMgBO3:Tb3+ has been established. Upon irradiation of the material with neutron- or gamma-radiation, different defect centres viz. [BO3]2− and [O2]− and [Ov]− were created. When heated during TL readout, the hole trap centre releases holes which recombine with the electrons emitted from the electron trap centre, resulting in the emission of radiative photons from the luminescence centres. The energy released here excites the energy levels of the adjacent Tb3+ ions present in the material. The excited Tb3+ ions decay through their characteristic emission of light, giving rise to the resulting TL glow peaks. The mechanism may be given as follows:
| O2− → O22− + h |
| BO32− → BO33− + h |
| Ov− → Ov + e |
Another observable feature in Fig. 17 is that the intensity of the EPR signal is greater in the case of the neutron-irradiated sample than it is for the gamma-irradiated one. This might be due to the large thermal neutron absorption cross sections of both Li and B present in the host matrix, which create large numbers of defects compared with the gamma irradiation. Moreover, the EPR intensity at the g = 2.04 signal being the highest must be responsible for the high temperature TL glow peak, whereas the lower intensity signal at g = 2.01 may be corelated to the low temperature TL peak. Therefore, the combination of neutron irradiated PL and EPR studies provided lots of valuable and innovative insights which helped to understand the origin of TL glow peaks, their relative intensities, and the underlying TL mechanism of the LiMgBO3:Tb3+ phosphor.
3.4 Determination of TL based kinetic parameters
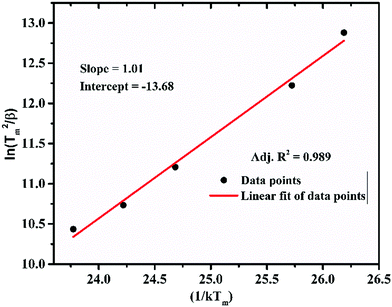
To understand the kinetics of the neutron irradiated TL glow curve of the LiMgBO3:Tb3+ phosphor it was very important to estimate various kinetic parameters viz. the activation energy (E), frequency factor (s) and order of kinetics (b). Therefore, two different methods viz. the peak shape and variable heating rate were applied.It was observed that, the Tm shifted towards higher temperature as the heating rate was increased. This was attributed to the fact that the time spent by the material at a temperature is higher at the lower heating rate than it is at higher heating rates. Therefore, the thermal release at that particular temperature is greater for a low heating rate. However, as the heating rate increases, the time spent is lower, which needs a higher temperature for the same amount of thermal release from the material. This led to the shifting of the TL glow peaks to the higher temperature side. However, the shape of the glow curve was unchanged. Following the relation between Tm and β from the first derivative of the Randall–Wilkins equation as given in Text-4 (ESI†), Hoogenstraaten (1958) proposed to estimate the value of ‘E’ and ‘s’ from the plot of ln(Tm2/β) against (1/kTm) which gives a straight line whose slope is equal to E and the intercept is equal to 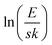 .74,75 For LiMgBO3:0.04Tb3+, the linear fitting of the plot of ln(Tm2/β) against (1/kTm), as shown in Fig. 19, gives the slope and intercept of 1.01 and −13.68 respectively. The activation energy (E) and frequency factor (s) obtained are 1.01 eV and 1.02 × 1010 s−1, respectively. Further, E and s values were also estimated following the methodology of using two different heating rates (β1 and β2), proposed by Booth and Bohun as given in Text S5 (ESI†).76,77 The results for the LiMgBO3:0.04Tb3+ are depicted in Table S5 (ESI†) and show that E varied from 0.92 to 1.02 eV and that s varied between 0.1 × 1010 and 6.6 × 1010 s−1. The average value for ‘E’ and ‘s’ was found to be 0.98 ± 0.04 eV and (7.1 ± 4.8) ×109 s−1 respectively. Therefore, it is found that the activation energy and the frequency factor obtained from the different methods agree very well. The most important conclusion is that the TL kinetics of the material follow a first order relationship as obtained from Chen's peak shape method of analysis, which is also supported by the results obtained from the variation of the TL glow peaks with different neutron doses.
.74,75 For LiMgBO3:0.04Tb3+, the linear fitting of the plot of ln(Tm2/β) against (1/kTm), as shown in Fig. 19, gives the slope and intercept of 1.01 and −13.68 respectively. The activation energy (E) and frequency factor (s) obtained are 1.01 eV and 1.02 × 1010 s−1, respectively. Further, E and s values were also estimated following the methodology of using two different heating rates (β1 and β2), proposed by Booth and Bohun as given in Text S5 (ESI†).76,77 The results for the LiMgBO3:0.04Tb3+ are depicted in Table S5 (ESI†) and show that E varied from 0.92 to 1.02 eV and that s varied between 0.1 × 1010 and 6.6 × 1010 s−1. The average value for ‘E’ and ‘s’ was found to be 0.98 ± 0.04 eV and (7.1 ± 4.8) ×109 s−1 respectively. Therefore, it is found that the activation energy and the frequency factor obtained from the different methods agree very well. The most important conclusion is that the TL kinetics of the material follow a first order relationship as obtained from Chen's peak shape method of analysis, which is also supported by the results obtained from the variation of the TL glow peaks with different neutron doses.
4. Conclusion
In summary, a highly efficient and near tissue equivalent LiMgBO3:Tb3+ phosphor has been developed for the potential application in the personnel neutron dosimetry. The DRUV-Vis study proved that it as a wide band gap (6.3 eV) material. PL emission study of the un-irradiated compounds revealed it to be a strong green emitting phosphor and the corresponding lifetime study explained the distribution of Tb3+ in the host matrix. The neutron-irradiated PL lifetime study was remarkably correlated with the origin of different TL glow peaks and their relative contributions. EPR study revealed the generation of different defect centres upon neutron- and gamma-irradiation, based on which the underlying TL mechanism has been established. The neutron induced TL study of LiMgBO3:Tb3+ showed a simple glow curve compared with the complex pattern of the existing LiF:Mg,Ti dosimeter. On comparison, it was found that the neutron-induced TL sensitivity and the n/γ dose discrimination capability of LiMgBO3:Tb3+ is 2.2 times and 4.5 times higher, respectively, than that of the standard LiF:Mg,Ti dosimeter. Moreover, the presently developed material showed an excellent neutron dose linearity up to 105 mSv and it has less than 10% fading of the TL signal even up to a 90 day storage period. These features meet the ISO 21909 criteria for its practical applicability as a TL based personnel neutron dosimeter. Therefore, the present work provides a great boost towards the systematic development of new and efficient materials along with the in-depth understanding of dosimetry properties to mitigate the huge scarcity of TL materials for neutron dosimetry applications. It is strongly believed that the presently developed LiMgBO3:Tb3+ can be a potential alternative to the existing LiF:Mg,Ti dosimeter for personnel neutron dosimetry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are highly thankful to Suresh Babu R. M. Director, Health Safety and Environment Group for his support in carrying out these works. Thanks to Fuel Chemistry Division, BARC for the help in recording the SEM and EDS data. Also special thanks to Radiological Standards group, RSS, RSSD, BARC and for providing the standard LiF:Mg,Ti powder and support during the gamma irradiations of the samples in 60Co machine. The work was funded by Bhabha Atomic research Centre (BARC), Department of Atomic Energy, India.References
- F. Daniels, C. Boyd and D. Saunders, Science, 1953, 117, 343–349 CrossRef CAS PubMed.
- S. W. S. McKeever, Thermoluminescence of Solids, Cambridge, UK, 1985 Search PubMed.
- S. Zhang, Z. Song, S. Wang, Z. Wang, F. Wang and Q. Liu, J. Mater. Chem. C, 2020, 8, 4956–4964 RSC.
- T. Lyu and P. Dorenbos, Chem. Mater., 2020, 32, 1192–1209 CrossRef CAS.
- G. Repettoa, J. L. Zuritaa, M. Roncelb and J. M. Ortega, Aquat. Toxicol., 2015, 158, 88–97 CrossRef PubMed.
- J. F. Fowler, E. Shuttleworth, V. Svarcer, J. T. White and C. J. Karzmark, Nature, 1965, 207, 997–998 CrossRef CAS PubMed.
- J. R. Cameron, F. Daniels, N. M. Johnson and G. Kenney, Science, 1961, 134, 233 CrossRef PubMed.
- K. Ayyangar, A. R. Lakshmanan, B. Chandra and K. Ramadas, Phys. Med. Biol., 1974, 19, 665–676 CrossRef CAS PubMed.
- M. Budzanowski, P. Bilski, T. Niewiadomski, B. Brugkhardtanf and E. Piesch, Radiat. Prot. Dosim., 1993, 47, 419–423 CrossRef CAS.
- A. R. Lakshmanan, Nucl. Tracks, 1982, 6, 59–78 CAS.
- Y. S. Horowitch and B. B. Shachar, Radiat. Prot. Dosim., 1988, 23, 401–404 CrossRef.
- M. Budzanowski and B. Burghhardt, Radiat. Meas., 1995, 24, 445–448 CrossRef CAS.
- Y. S. Horowitch, Radiat. Meas., 1993, 47, 135–141 Search PubMed.
- J. I. Lee, J. S. Yang, J. L. Kim, A. S. Pradhan, J. D. Lee, K. S. Chung and H. S. Choe, Appl. Phys. Lett., 2006, 89, 094110 CrossRef.
- Y. Y. Liang and Z. H. Liu, J. Mater. Res., 2016, 31, 1433–1439 CrossRef CAS.
- A. C. Fernandes, M. Osvay, J. P. Santos, V. Holovey and M. Ignatovych, Radiat. Meas., 2008, 43, 476–479 CrossRef CAS.
- S. İflazoğlu, A. Yilmaz, V. E. Kafadar and A. N. Yazici, J. Therm. Anal. Calorim., 2018, 133, 1327–1333 CrossRef.
- S. İflazoğlua, A. Yilmaz, V. E. Kafadar, M. Topaksu and A. N. Yazici, Appl. Radiat. Isot., 2019, 147, 91–98 CrossRef PubMed.
- O. Annalakshmi, M. T. Jose, B. Venkatraman and G. Amarendra, Mater. Res. Bull., 2014, 50, 494–498 CrossRef CAS.
- I. Kawamura, H. Kawamoto, Y. Fujimoto, M. Koshimizu, G. Okada, Y. Koba, R. Ogawara, T. Yanagida and K. Asai, J. Ceram. Soc. Jpn., 2019, 127, 663–668 CrossRef CAS.
- L. Wu, J. C. Sun, Y. Zhang, S. F. Jin, Y. F. Kong and J. J. Xu, Inorg. Chem., 2010, 49, 2715–2720 CrossRef CAS PubMed.
- C. T. Chen, Y. Wang, B. Wu, K. Wu, W. Zeng and L. Yu, Nature, 1995, 373, 322–324 CrossRef CAS.
- S. Wang, N. Ye and G. Zou, Inorg. Chem., 2014, 53, 2742–2748 CrossRef CAS PubMed.
- P. Becker, Adv. Mater., 1998, 10, 979–992 CrossRef CAS.
- W. Hongping, Y. Hongwei, Z. Yang, X. Hou, X. Su, S. Pan, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2013, 135(11), 4215–4218 CrossRef PubMed.
- Z. Jiyou, Z. Ya, H. Shruti, Z. Weiren, W. Jun and B. Jakoah, Chem. Mater., 2020, 32, 882–888 CrossRef.
- H. A. Lehmann, H. Schadow and H. J. Papenfuss, Z. Anorg. Allg. Chem., 1962, 314, 159–166 CrossRef CAS.
- K. H. Prasad, S. Subramanian, T. N. Sairam, G. Amarendra, E. S. Srinadhu and N. Satyanarayana, J. Alloys Compd., 2017, 718, 459–470 CrossRef.
- L. Zhijuan, M. Fuwang, Z. Xinguo and Z. Liya, J. Lumin., 2014, 151, 47–51 CrossRef.
- S. Tamilarasan, S. Laha, S. Natarajan and J. Gopalakrishnan, Eur. J. Inorg. Chem., 2016, 288–293 CrossRef CAS.
- A. K. Bedyal, A. K. Kunti, S. G. Menon, V. Kumar and H. C. Swart, J. Alloys Compd., 2020, 830, 154622 CrossRef CAS.
- L. Wu, Y. Baia, L. Wua, H. Yia, X. Zhanga, L. Zhanga, Y. Konga, Y. Zhangb and J. Xu, Dalton Trans., 2018, 47, 13094–13105 RSC.
- S. N. Menon, S. Kadam, S. Watanabe, T. K. G. Rao, M. S. Kulkarni and D. A. R. Babu, J. Lumin., 2015, 167, 140–155 CrossRef CAS.
- M. M. Yerpude, V. Chopra, N. S. Dhoble, R. M. Kadam, A. R. Krupski and S. J. Dhoble, Luminescence, 2019, 1, 1–12 Search PubMed.
- M. Sen, R. Shukla, V. Sathian, M. S. Kulkarni and A. K. Tyagi, Ceram. Int., 2020, 46, 20236–20242 CrossRef CAS.
- D. I. Shahare, B. T. Deshmuk, S. V. Moharil, S. M. Dhopte, P. L. Muthal and V. K. Kondawa, Phys. Status Solidi, 1994, 141, 329–334 CrossRef CAS.
- L. C. Oliveira, E. G. Yukihara and O. Baffa, Sci. Rep., 2016, 6, 24348 CrossRef CAS PubMed.
- D. Wang, B. A. Doull, L. C. Oliveira and E. G. Yukihara, RSC Adv., 2013, 3, 26127 RSC.
- X. Huang, J. Liang, S. Rtimi, B. Devakumar and Z. Zhang, Chem. Eng. J., 2021, 405, 126950 CrossRef CAS.
- S. Wang, B. Devakumar, Q. Sun, J. Liang, L. Sun and X. Huang, J. Mater. Chem. C, 2020, 8, 4408–4420 RSC.
- L. Sun, B. Devakumar, J. Liang, S. Wang, Q. Suna and X. Huang, J. Mater. Chem. C, 2019, 7, 10471–10480 RSC.
- P. P. Kulkarni, K. H. Gavhane, M. S. Bhadane, V. N. Bhoraskar, S. S. Dahiwale and S. D. Dhole, Mater. Adv., 2020, 1, 1113–1124 RSC.
- M. Prokić and E. G. Yukihara, Radiat. Meas., 2008, 43, 463–466 CrossRef.
- J. Rodriguez-Carvajal, A Program for Rietveld, Profile Matching and Integrated Intensity Refinements for X-ray and Neutron Data, Version 1.6, Laboratoire Leon Brillouin, Gif sur Yvette, France, 2001 Search PubMed.
- A. Belkebir, P. Tarte, A. Rulmont and B. Gilbert, New J. Chem., 1996, 20, 311–316 CAS.
- A. Rulmont and M. Almou, Spectrochim. Acta, 1989, 45A, 603–610 CrossRef CAS.
- Z. Wang, M. Zhang, S. L. Pan, Y. Wang, H. Zhang and Z. Chen, Dalton Trans., 2014, 43, 2828–2834 RSC.
- L. Wu, J. C. Sun, Y. Zhang, S. F. Jin, Y. F. Kong and J. J. Xu, Inorg. Chem., 2010, 49, 2715–2720 CrossRef CAS PubMed.
- R. Ma, L. Shao, K. Wu, M. Lao, M. Shui, C. Chen, D. Wang, N. Long, Y. Ren and J. Shu, Ceram. Int., 2013, 39, 9309–9317 CrossRef CAS.
- A. Dehelean, S. Rada, M. Zagrai, R. Suciu and C. Molnar, Anal. Lett., 2020, 54, 88–97 CrossRef.
- A. E. Morales, E. S. Mora and U. Pal, Rev. Mex. Fis., 2007, 53, 18–22 CAS.
- V. Kortov, Radiat. Meas., 2007, 42, 576–581 CrossRef CAS.
- A. K. Bedyal, V. Kumar, R. Prakash, O. M. Ntwaeaborwa and H. C. Swart, Appl. Surf. Sci., 2015, 329, 40–46 CrossRef CAS.
- A. D. Sontakke, K. Biswas and K. Annapurna, J. Lumin., 2009, 129, 1347–1355 CrossRef CAS.
- A. Wagh, Y. Raviprakash, V. Upadhyaya and S. D. Kamath, Spectrochim. Acta, Part A, 2015, 151, 696–706 CrossRef CAS PubMed.
- H. Guo, Y. Wang, Y. Gong, H. Yin, Z. Mo, Y. Tang and L. Chi, J. Alloys Compd., 2016, 686, 635–640 CrossRef CAS.
- V. S. Kavitha, R. R. Krishnan, R. S. Sreedharan, K. Suresh, C. K. Jayasankar and V. P. M. Pillai, J. Alloys Compd., 2019, 788, 429–445 CrossRef CAS.
- S. Xu, L. Panlai, Z. Wang, T. Li, Q. Bai, J. Sun and Z. Yanga, J. Mater. Chem. C, 2015, 3, 9112–9121 RSC.
- W. Carnall, P. Fields and K. Rajnak, Int. J. Chem. Phys., 1968, 49, 4447 CrossRef CAS.
- J. Chrysochoos and A. Evers, Spectrosc. Lett., 1973, 6, 203–216 CrossRef CAS.
- P. Das, N. Pathak, B. Sanyal, S. Dash and R. M. Kadam, J. Alloys Compd., 2019, 810, 151906 CrossRef CAS.
- S. K. Gupta, N. Pathak and R. M. Kadam, J. Lumin., 2016, 169, 106–114 CrossRef CAS.
- A. Kannan, P. S. Rao, R. N. Sachadev, V. V. Shaha, D. Sharma and P. K. Srivastava, BARC/I992/E/O48, https://inis.iaea.org/collection/NCLCollectionStore/Public/24/067/24067417.pdf?r=1&r=1, 1992.
- D. L. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063 CrossRef CAS.
- P. D. Johnson and F. E. Williams, J. Chem. Phys., 1950, 18, 1477 CrossRef CAS.
- ISO 21909, First edition, International Standard, Passive personal neutron dosimeters: Performance and test requirements, 2005 (E).
- ISO 8529-3, Calibration of area and personal dosimeters and determination of their response as a function of neutron energy and angle of incidence, 1998 (E).
- N. K. Porwal, R. M. Kadam, T. K. Seshagiri, V. Natarajan, A. R. Dhobale and A. G. Page, Radiat. Meas., 2005, 40, 69–75 CrossRef CAS.
- M. Anitha, M. Mohapatra, R. M. Kadam, T. K. Seshagiri, A. K. Tyagi and V. Natarajan, J. Mater. Res., 2006,(21), 1117–1123 CrossRef CAS.
- C. Naccache, P. Meriaudeau, M. Che and A. J. Tench, Trans. Faraday Soc., 1971, 67, 506 RSC.
- X. Koschnick, J.-M. Spaeth and S. R. Eachus, J. Phys.: Condens. Matter, 1992, 4, 3015–3029 CrossRef.
- T. H. Pawlik, V. Dierolf and J.-M. Spaeth, J. Phys.: Condens. Matter, 1997, 9, 1857–1862 CrossRef CAS.
- R. Chen, J. Electrochem., 1969, 116, 1254–1257 Search PubMed.
- R. Chen, J. Mater. Sci., 1976, 11, 1521–1541 CrossRef CAS.
- W. Hoogenstraaten, Philips Res. Rep., 1958, 13, 515–562 CAS.
- A. H. Booth, Can. J. Chem., 1954, 32, 214 CrossRef CAS.
- A. Bohun, Czech. J. Phys., 1954, 4, 91 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00737d |
| This journal is © The Royal Society of Chemistry 2021 |