Open Access Article
Open Access ArticleBiogenic colourants in the textile industry – a promising and sustainable alternative to synthetic dyes†
Richard
Fried
 a,
Ilinca
Oprea
b,
Karin
Fleck
*b and
Florian
Rudroff
a,
Ilinca
Oprea
b,
Karin
Fleck
*b and
Florian
Rudroff
 *a
*a
aInstitute for Applied Synthetic Chemistry, TU Wien, Getreidemarkt 9, OC-163, 1060 Vienna, Austria. E-mail: florian.rudroff@tuwien.ac.at
bVTL GmbH, Rudolf-von-Alt-Platz 4/13, 1030 Vienna, Austria. E-mail: karin.fleck@viennatextilelab.at
First published on 19th November 2021
Abstract
The textile and dyeing industry has shown dramatic growth alongside synthetic chemistry in the last century. With increased operations and economic importance, challenges also emerged, including environmental pollution, work safety, and consumer health. Hence, a change of perspectives in the industry is on the rise, and environmentally-friendly solutions for safer processes and more circularity are urgently sought-after. A possible solution for the pressing issue of textile dyeing is found in the use of natural colourants obtained in fermentation processes. The structural diversity of biogenic dyes is potentially high enough to pose an alternative on a broad front. Matching fundamental properties concerning dyeing, (bio)synthesis and toxicity give an impression of the advantages of developing this natural alternative. In this contribution, a comparison between biologically-derived dyes and their synthetic counterparts is conducted on several levels. The most important topics for the future are outlined. Significant challenges and potential solutions that limit the implementation of biogenic dyes into the textile dyeing value chain are discussed. Biotechnology offers numerous methods to increase reliable production or engineer molecular properties elegantly. The biggest advantage from a Green Chemistry perspective is the possibility to switch to renewable starting materials and obtain more biodegradable dyes. A more sustainable alternative in textile dyeing is afoot. It needs to be embraced and developed to provide the world population with a sustainable way into the future.
Introduction
Colour is a beautiful feature of clothes and objects around us. In early times, colour was reserved for kings and aristocrats, dyeing their robes with the most precious substances such as Tyrian purple, produced from sea snails. For most of the time in history, natural dyes were used for colouring textiles. Plant parts or insects were the primary sources of dyes. The indigo plant supplied blue, cochineal insects red and Persian berries yellow while dyeing methods hardly developed over centuries.1However, the former luxury topic has turned into a global problem. With the development of synthetic chemistry, the first synthetic textile dye (purple Mauveine) was discovered.2 From the invention of the first synthetic dyes in the 1850s, chemical and dyeing industries developed side by side at an unpreceded pace. Different substance classes were developed, dyeing procedures for every textile material were found, and every hue was possible to achieve. Industrialisation increased production volumes as well as pollution of ecosystems. One of the first manufacturing plants for synthetic alizarin increased its production volume from one ton in 1869 to 435 tonnes only four years later.2 One hundred years later, world production of dyes was estimated to be over 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year.3 Nowadays colour is available to everyone, basically unlimitedly, and over 1 million tonnes are produced annually.4
000 tonnes per year.3 Nowadays colour is available to everyone, basically unlimitedly, and over 1 million tonnes are produced annually.4
The possibilities we have now come at a low price but high chemical expenses. Chemical spending for different textile processing steps (e.g. desizing, scouring, colour levelling, mordanting) is enormous as consumer demand is high on, for example, quality and variety. There was no alternative to synthetic dyes that could compete in availability, variety of colours and price. In the past, different alternative natural sources were highlighted (animal, mineral, plant, microbial origin) as opposed to petrochemical origin.5 Moreover, plants are again investigated as biological sources for textile dyes, but their key disadvantages prevail. Seasonal and geographical variance in availability, time and space consuming production, low yields hinder their large-scale application.5b Exactly these challenges could be ideally tackled by biotechnological production of dyes with microorganisms. Since the sharp advances in biotechnology, microbial sources for dyes may become a feasible alternative that could transform dyeing processes and the supply chain for a more environmentally friendly future.
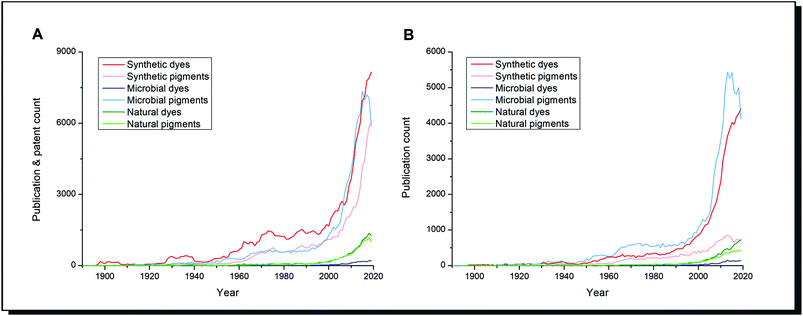
Scientific literature reflects this highly increased importance of research on dyes and alternatives (Fig. 1). The number of publications on natural dyes and pigments steadily increased over the last 20 years. In contrast, the research interest on dyes and pigments from microorganisms grew exponentially alongside synthetic dyes/pigments. When patent literature is excluded (Fig. 1B), microbial pigments can be considered the most important research subject since the 1960s among the search terms mentioned. The difference between dyes and pigments is expressed in the Colour Index by the Society of Dyers and Colourists. Dyes are soluble molecules that temporarily lose any crystalline arrangement of molecules in the application process. In contrast, pigments are insoluble in the vehicle of application so that a particulate structure is retained throughout the colouration process.6 It should be pointed out that in the biological literature, these definitions are not strictly applied. The term “microbial pigment” is preferred over “microbial dye”, as shown in Fig. 1. It refers to any coloured biological metabolite, and usually, no difference is made between pigments and dyes.
Dyes and pigments from microbial or plant origin continue to be a promising topic in science. Recent publications provide a good overview on the topic, sometimes with an impressive collection of molecular structures, but lack a detailed description of the available structures and their direct comparison to synthetic dyes.5c,130 Applications of biogenic dyes were mainly considered in the food industry and medicine fields, which have been treated extensively in the past literature. Food grade dyes were discussed for their use in aliments, exhibiting intense colour, antioxidant properties or nutritional value. Challenges and possible solutions were highlighted mainly in regard to the efficient production of dyes.8 In pharmaceutical applications, microbial dyes were considered not for their dyeing capability, but several “anti”-properties, such as antimicrobial, anticancer or antiinflammatory activity.7c,9 Almost exclusively, applications other than textile dyeing were focussed on in recent literature. Examples of dyes from fungal origin applied in textile dyeing have been compiled, only briefly touching essential topics related to application such as toxicity testing.10 Most of the literature on biogenic dyes belongs to the microbiology field, and a chemistry perspective is largely missing. When speaking about textile dyeing, properties of the molecules and Green Chemistry aspects have to be considered. Undesired bioactive properties, toxicity, bio-accumulation, ecotoxicity or carcinogenicity are critical in assessing the suitability of a particular substance for a large-scale application such as textile dyeing. No detailed consideration of these topics was found in the literature so far and was thus treated in the present publication. Problems like the use of hazardous chemicals, poor treatment of waste water, and resulting environmental pollution are driven by textile processing and leather manufacturing, dyeing, and tanning. This is an essential aspect of the environmental impact of dyeing and shall not be forgotten. The dyeing of leather is more complex due to the fibrous protein network that adds additional parameters to the equation, such as pore size, fibre density, secondary structures of collagen fibres and non-collagenous materials. More dyeing auxiliaries are necessary to ensure the distribution and adsorption of dyes in the three-dimensional protein matrix.11 Same as for textiles, the treatment of leather processing wastewater is complex due to the high chemical load and persistent nature of the compounds. Wastewater treatment technologies and strategies are an active research topic.12 Considering the extent of this topic, the present review has to be focused on textile dyeing only. The interested reader is referred to a basic review on leather dyeing using dyes of fungal origin.13
Discussing chemical structures of microbial dyes, analysing toxicity and Green Chemistry related properties or specific challenges and considerations for textile application will give readers insight into substances available as alternatives for textile dyeing.
According to recent market research, about two-thirds of dyes are used in the textile field, which had a considerably heavy environmental impact, as detailed in the next section.14 Thus, from an environmental point of view, the textile industry is probably the most exciting field for the application of microbial dyes. The European Commission laid out a long-term chemical strategy, following the European Green Deal,15 a growth strategy for a sustainable and circular economy in Europe. The EU chemicals policy shall continue to develop the continent ‘towards a toxic-free environment’. Besides moving the economy towards sustainable and circular operation, the protection of health and the environment was highlighted. Sustainable and safe-by-design chemicals shall achieve a toxic-free environment, among other measures.16 Improvement in a highly impacting industry branch like the textile industry is of outstanding interest and importance. One of the most significant environmental polluters to date is the textile industry. Hazardous chemicals are used in the production and dyeing of textiles originating from petrochemical feedstock. These include the dyes themselves, next to agents for fabric softening, colour levelling, fixation or preservation. Especially the dyeing process creates billions of tons of toxic wastewater that may end up in the environment due to inadequate treatment, as NGO reports revealed a few years ago.17 Due to poor treatment of wastewater and/or recalcitrant nature of dyestuff, high amounts of dyes and process chemicals were detected in effluents. Estimated 280![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of textile dyes are discharged in dye house effluents per year, a number being cited for over 20 years.4,18 Data on aquatic toxicology on numerous synthetic dyes can be found in Tkaczyk et al. 103 of 140 dyes listed were classified in one of the acute toxicity categories defined by the OECD.4 Even small amounts of dyes in water (below 1 mg L−1) absorb sunlight and significantly disturb the transparency of water bodies. As a result, the photosynthesis of water organisms and other light-driven processes may be impaired.4,19 Upon environmental degradation of dyes, harmful breakdown products may be formed. Azodyes may be cleaved by microbial azoreductases liberating possible cancerogenic aromatic amines. Triphenylmethane dyes may be reduced to their lipophilic leuco form, which facilitates bioaccumulation. This mode of action led to the formation of chemicals that may migrate from plants and fish into tertiary consumers (human).4,20
000 tons of textile dyes are discharged in dye house effluents per year, a number being cited for over 20 years.4,18 Data on aquatic toxicology on numerous synthetic dyes can be found in Tkaczyk et al. 103 of 140 dyes listed were classified in one of the acute toxicity categories defined by the OECD.4 Even small amounts of dyes in water (below 1 mg L−1) absorb sunlight and significantly disturb the transparency of water bodies. As a result, the photosynthesis of water organisms and other light-driven processes may be impaired.4,19 Upon environmental degradation of dyes, harmful breakdown products may be formed. Azodyes may be cleaved by microbial azoreductases liberating possible cancerogenic aromatic amines. Triphenylmethane dyes may be reduced to their lipophilic leuco form, which facilitates bioaccumulation. This mode of action led to the formation of chemicals that may migrate from plants and fish into tertiary consumers (human).4,20
The dyeing process impacts the environment, the health and the well-being of workers and consumers. The workforce in the textile industry is based on a majority of female workers, especially in some Asian countries like Bangladesh, the percentage of women employed in the industry is above 80%.21 The conditions for these workers include long working hours, low salaries and dangerous or toxic work environments.22 Although general safety at workplaces increased in recent decades, effects on people's health were reported in many studies. Links between occupation in the textile business and increased occurrence of different cancer types could also be established.23 Occupational asthma, rhinitis, and contact urticaria from oxidative hair dyes were described in hairdressers in a metastudy in Finland.24 This turns contact with dyes and other chemicals from the dyeing process into an active health issue that must be tackled.
Circularity and ecological footprint have become more important in the textile industry.5c,25 Dyes from biological origin may pose a promising alternative for this application.
Microorganisms found suitable answers to almost any physiological or environmental stress condition throughout evolution by developing secondary metabolites. These substances are not continuously produced and are not essential for the organism. Specific genes or gene clusters are activated in microorganisms under specific conditions or upon experiencing a trigger, leading to the production of specialised small organic compounds. Both small molecules and biosynthetic genes of secondary metabolism were investigated with a great interest for different fields. In medicine, special attention is paid to bioactive molecules. Red-coloured prodigiosin (31) from Serratia marcescens was found to have cytotoxic properties against various cancer cell lines while low or no toxicity to healthy cells.26 In a combined therapy approach, it also showed potent cytotoxicity against triple-negative breast cancer cells.27 Food industry is looking for alternatives to synthetic food dyes. One food-grade example is astaxanthin (10) from Haematococcus pluvialis, which was approved for use in fish and animal foods.8a Anti-oxidant and UV-protective properties also make secondary microbial metabolites attractive for the cosmetic industry. Melanin (28) from Halomonas venusta was successfully tested as a photoprotective ingredient in sunscreen.28 Metabolites from cyanobacteria showed potential application as UV-protectant (scytonemin, mycosporine-like amino acids) or moisturiser (extracellular polymeric substance sacran).29 From this point of view, it becomes clear that is it crucial to explore microbial sources for new metabolites.30
When producing small organic molecules as potential dye alternatives in biological systems, many features of bio-manufacturing can be realised. In general, biotechnological processes show advantages compared to synthetic ones by the use of renewable feedstock, mild process conditions, largely environmentally benign or easy-to-treat waste. Typical feedstocks for synthetic dyes are petroleum-based chemicals like benzene, ethylene, propylene, and the resulting platform chemicals aniline, phenol, ethylene glycol, and vinyl chloride. Biological systems use higher oxidised feedstock such as sugars (glucose, xylose), alcohols (ethanol, glycerol) or carboxylic acids (lactic acid, succinic acid, furan dicarboxylic acid), which may be obtained from renewable resources or by fermentation processes.31 The ideal dye would be a stable and essentially inert molecule with high affinity or strong bonding to the textile fibre. This would mean no loss in dyeing or washing processes and no adverse effects for humans or other organisms.
This review compares chemically and biologically derived dyes in terms of the production process, accessible chemical structures, and molecule properties. Further, the main challenges for the application of microbial dyes to textile dyeing will be compiled. Opportunities, challenges and risks of applying coloured secondary metabolites to textiles will be highlighted and critically discussed. The awareness for applying secondary metabolites for unconventional purposes will be increased, so progress toward global sustainable manufacturing can be made.
Chemically vs. biologically derived dyes
Accessible chemical structures and synthesis
A detailed compilation of synthetic dyes and their structural classes can be found in, e.g. Tkaczyk et al.4 Among the many classes of synthetic dyes developed, the most relevant ones are shown in Fig. 2. From these, azo dyes are the most important, representing more than 50% of all textile dyes in use, followed by anthraquinones and phthalocyanines.32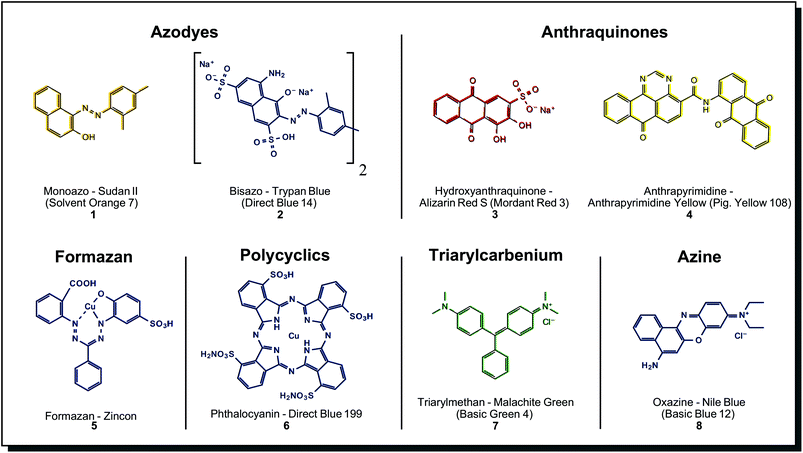 | ||
| Fig. 2 Substance classes and structures of selected synthetic dyes. The vast majority of textile dyes belong to azodyes, anthraquinones and phthalocyanines. | ||
Azo dyes can be further divided into mono-, bis-, tris- and poly azo compounds. Azo dyes are formed from a diazo component (e.g. an aromatic amine) and a coupling component (e.g. a naphthylamine). An enormous variety of azo dyes can be created by combining these components in a ‘mix and match’ fashion. Fig. 2 shows two simple examples, the solvent dye Sudan II (1) and the water-soluble dye Trypan Blue (2). There are many possibilities for derivatisation and property engineering, so almost the complete range of colour hues is covered. Azo dyes, in general, show bright and intense colours, which makes them highly cost-effective, but dyeing properties like wash or light-fastness are usually only moderate. Blocking groups may be inserted between two chromophores in a molecule, enabling intramolecular colour mixing (e.g. for green colour). Also, the affinity for textiles and wash fastness may be increased.32 The latter properties can be improved by so-called reactive azo dyes. They carry a reactive functional group such as chlorotriazine or vinyl sulphone. In an alkaline environment, reactive azo dyes covalently bond to the nucleophilic functional groups on the fibre. In comparison with direct dyes, reactive dyes have less complex chemical structures, and the resulting colours after dyeing are brighter.33 In metal-complex azo dyes, the dye component chelates with the metal ion on the fibre to form highly stable salts. Aluminium, iron, copper, and chromium are used to obtain a multitude of shades and outstanding light-fastness values, but the brightness is lower than for the non-metallised dye. A planar structure is generally observed in Cu2+ azo-dye complexes, while Cr3+ and Co3+ complexes exhibit a non-planar structures.32,34
Heterocyclic diazo components (with S or N heterocycles) are applied to obtain brighter dyes with higher colour fastness. Azo dyes are available for dyeing all natural or synthetic textile materials. Cellulosic fibres such as cotton and animal fibres such as wool and silk are usually dyed using reactive azo dyes. For dyeing cellulose acetate, polyamides, polyesters, and polyacrylonitrile dispersed azo dyes are more commonly used.35 No equivalent azo structures are known to date to exist in nature.36
Anthraquinones have a more complex chemical core motif, and therefore, the synthesis is more sophisticated. The three-ring core structure is assembled from benzene derivatives or phthalic anhydride. A limited number of positions on the skeleton reduce the variety of derivatives. Sulfonic acid or nitro groups are introduced by electrophilic substitution and are the basis for further derivatisation efforts. Possibilities are alkylation, halogenation, hydroxylation, amination. Replacement reactions yield N-alkyls, amides, quaternary ammonium salts or anthraquinone dimers.37 Alizarin Red S (3), the water-soluble synthetic derivative of naturally occurring alizarin, is shown in Fig. 2 as an example. In synthetic anthraquinones, the skeleton may also be modified with condensed (hetero)cycles (imidazole, oxa/thiazol), giving benzanthrones, pyrazoloanthrones, anthrapyrimidines, indanthrones (phenazine substructure) or anthanthrones. Anthrapyrimidine Yellow (4) is a member of these more complex derivatives, representing a fast-dyeing pigment. Both simpler and more complex derivatives of base anthraquinones are abundantly found among microorganisms, especially in fungi (see Fig. 4 for more details). Benzodifuranes are counted as anthraquinones, although they present different core structures37 and are prepared from hydroquinones and arylacetic acids. These substances are designed initially as tools for investigating serotonin receptors and do not appear in nature.38 The closest natural match may be azaphilone structures found in fungi see Fig. 4. Anthraquinones are mainly used to dye cellulosic fibres by vat dyeing or synthetic fibres like polyester, polyamide and acetate by disperse dyeing. Also, wool and silk can be dyed with acid anthraquinone dyes, which obtain blue shades that are not possible with azo dyes.37 Its fastness properties are excellent – especially lightfastness, but they show decreased brightness and lower tinctorial strength, making them less cost-effective than azo dyes.39 Anthraquinones are mostly confined to red and blue colours, whereas anthraquinone derivatives show yellow-orange-brown-olive colours. Benzodifuranes offer hues across the whole spectrum.36
Formazan dyes are closely related to azo dyes, as they can be synthesised by coupling a diazonium salt with a hydrazone but are regarded differently enough to form a separate dye class. These nitrogen-rich structures quickly form complexes with metal ions, see Cu2+-binding zincon (5). From the 1950s, formazans were used for textile dyeing on wool, polyester and cellulose fibres. Typical colours are blue, greenish-blue, orange and red. Reactive blue formazan dyes are important components for trichromic dyeing with red and yellow azo dyes.34 Tetrazolium salts and formazans are often jointly mentioned. By reduction of colourless tetrazolium salt, the corresponding intensely coloured formazan can be obtained. This reaction may be catalysed by biological reduction processes so that tetrazolium salts/formazans serve as biological redox indicators and are used for the detection of cell viability.40 Derivatives with both electron donor and electron acceptor groups were investigated for their application in photonics and light-emitting diodes.40c
Phthalocyanines are dyes not found in nature, although their cyclic structure reminds of porphyrins (tetrapyrroles). They are synthesised from phthalocyanide or phthalic anhydride in one step at >180 °C and easily form metal complexes. Among those, copper complexes show the best combinations of properties. A typical example is Direct Blue 199 (6), which is depicted in Fig. 2. Several derivatisation methods, like alkyl/aryl substitutions, halogenation or sulfonation, are performed. However, none of these dramatically impact colour appearance.41 Because of this reason, phthalocyanines are mostly limited to blue and green, including brilliant turquoise and green hues that are unique among synthetic dyes.42 Only a few colours outside this range could be achieved, for example, in phthalocyanine-porphyrin hybrid molecules. Red, brown and purple examples were described.43 Overall, the dyes are thermally and chemically very stable, and the colours are bright and tinctorially strong, so they are considered cost-effective. Challenges with phthalocyanines are their low solubility in polar and apolar solvents. Sufficient solubility is only possible in acids. In application, phthalocyanines are often formed from precursors directly on the textile fibres, mainly used on synthetic ones. For dyeing cotton, sulfonic acid salt derivatives are applied.40a
The last examples in Fig. 2 belong to other synthetic dyes of less importance for textile dyeing, such as the triarylcarbenium Malachite Green (7) and the azine Nile Blue (8).
Biogenic dyes are coloured small molecules naturally produced by microorganisms. The structural diversity is similarly high as for synthetic dyes. An extensive compilation of biogenic dye structures can be found in Ramesh et al.,7c and others.7a,9a,30,44 Classification attempts in recent literature mainly were made from a biological perspective, focused on certain substance classes only or were not satisfyingly consistent. In this review, a solid classification of microbial dyes attempts to show the interested reader what is chemically possible among coloured biological molecules. In the first step, chromophores can be divided into hydrocarbon-based structures and heterocycle-based structures. Further separation into subclasses is indicated in the following paragraphs and respective figures. Strikingly, the classes anthraquinones, indoles, and azines can be found in natural and synthetic dyes.
Polyenes show typical conjugated double bonds as the chromophore. The molecules may be derived from isoprene building blocks or not. Fig. 3 shows a selection of such terpenoids and non-terpenoids. The major subgroup is the well-known carotenoids with more than 750 compounds found among microorganisms such as microalgae (Haematococcus pluvialis, Dunaliella salina), fungi (Blakeslea trispora), bacteria (Brevundimonas species), plants (Adonis species) and insects (spider mite).45 Furthermore, they can be divided into purely hydrocarbon structures (carotenes) or oxygenated structures (xanthophylls) and are coloured in yellow, orange and red.46 In the cellular metabolism, structures evolve from linear molecules without end groups like lycopene to molecules with cyclic hydrocarbon end groups and later to oxygenated or derivatised end groups instead. These structures may be aromatic rings or monounsaturated cyclohexene rings as present in β-carotene (9). Oxygenated rings may contain keto, epoxy or hydroxy groups as in astaxanthin (10), and derivatised end groups may feature methyl ethers, acetylation or glycosylation.46 Carotenoid tetraterpenes can be differentiated from azulenes (11), representing sesquiterpenes with an interesting cycloheptatriene-cyclopentadiene scaffold on the blue end of the spectrum.47 Also non-terpenoid polyenes exist such as granadaene (12). Synthetic polyenes are rather called (poly)methine dyes when applied in textile dyeing. They are characterised by a system of conjugated double bonds with an odd number of carbon atoms connecting two possibly charge-carrying terminal groups. Their importance is very low among synthetic dyes, with a few examples used for dyeing polyacrylonitrile or polyester material.40a
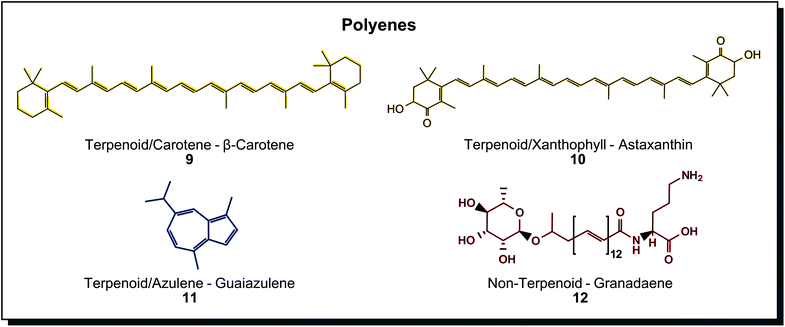 | ||
| Fig. 3 Polyene structures found in microorganisms are primarily derived from terpenoid biosynthesis. | ||
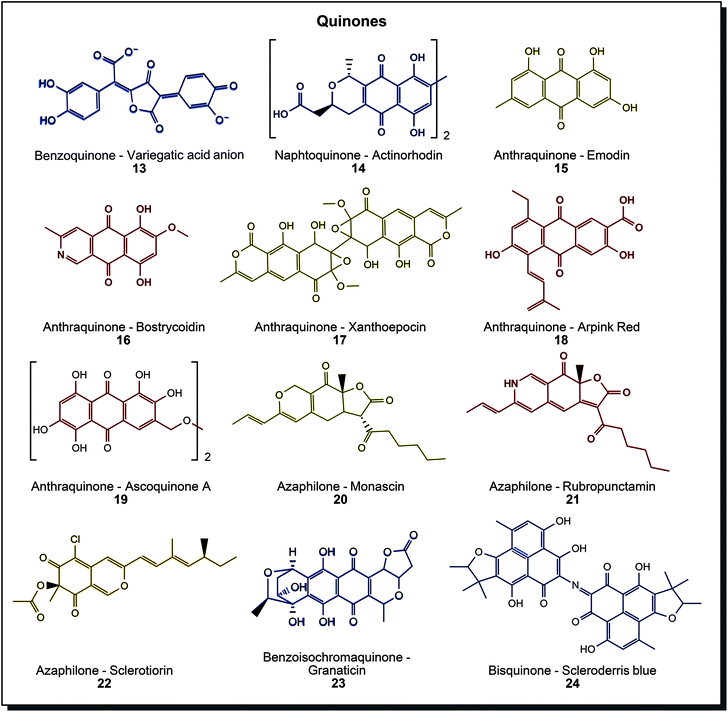
Quinones are a diverse group of compounds with several subclasses and colours spanning across the entire visible spectrum. Fig. 4 sketches the considerable diversity that is found in nature. The benzenoid/chinoid chromophore can be extended by one or two aromatic rings, giving benzoquinones, naphthoquinones or anthraquinones, see (13) to (15). Further extension on the aromatic rings is possible, such as hydroxy/methoxy groups, alkyl chains or annealed heterocycles. Notably, many derivatives of anthraquinones are found in microorganisms. Hydroxylated methyl species such as emodin (15), heterocyclic bostrycoidin (16), epoxide containing xanthoepocin (17), the commercialised alkenyl- and carboxy-functionalised Arpink Red (18) and species with ether-bridged dimers such as ascoquinone A (19) can be found in different members of Aspergillus, Fusarium or Penicillium.44c Azaphilones form a subclass, also typically found in fungal organisms. Especially the genus Monascus is known for its variety in these yellow to red structures 20 and 21, but also different azaphilones can be found (22). Originally used in Asia for food purposes (production of red rice), these dyes were also investigated for the dyeing of fabrics.48 A significant drawback is the coproduction of mycotoxins from most organisms.49 Several Talaromyces species also produce azaphilones but without mycotoxin formation. Intense research is being conducted on this genus.50 The pyran-benzofuran substructure in azaphilones may be the most similar natural counterpart to benzodifuran synthetic dyes. Other structures in this diverse class are benzoisochromanequinones such as blue granaticin (23) or bisquinones with imine linkage as found in scleroderris blue (24).44a As mentioned above, anthraquinone synthetic dyes are mainly used to dye synthetic fibres like polyester, polyamide and acetate or natural ones like cellulosics, wool and silk. Microbial quinones were tested quite extensively on different materials already. Anthraquinones dyed wool/silk with good rub and wash fastness values in a 70 °C dyeing bath and cotton under the same conditions with similar values for perspiration and rub fastness, but poor values for light and wash fastness.51 Azaphilones were used to dye cotton and wool with mordanting, achieving fair to good colour fastness values.10,52
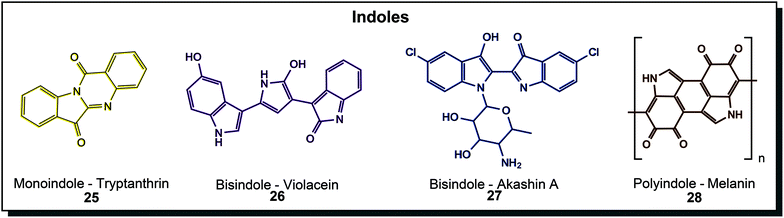
Indoles are well-known structural motifs with colouring properties because of their conjugated double bonds but do not exhibit high diversity as polyenes or quinones. Natural derivatives can be grouped according to the number of indole units incorporated, as depicted in Fig. 5. Tryptanthrin (25) is a yellow monoindole that presents a simple quinazolinone derivative of indole. It was discovered in the yeast Candida lipolytica and dyer's plants such as Chinese woad.53 Research interest focuses on various biological activities, including anti-protozoan and anti-tumour activity.54 Bisindole substances are the most important in this class. First of all, the blue indigo (chemical substance name is actually indigotin), which was produced from plants for centuries, was also found in microorganisms. Some hydrocarbon-degrading organisms, such as certain Pseudomonas species, have the ability to produce indigo from indole.55 This is possible because the specialised naphthalene dioxygenase or styrene monooxygenase enzymes also accept indole and turn it into indoxyl, leading to indigo formation.56 Purple violacein (26) is probably the most extensively treated microbial dye in the literature. Its unique bisindole-pyrroline architecture is created from the amino acid tryptophan by Chromobacterium, Janthinobacterium and many more like the marine Pseudoalteromonas.57 It is mainly studied because of its bioactive properties but also dyeing experiments were conducted.58 An N-cycloalkyl derivative of indigo, akashin A (27), was found in marine Streptomyces and showed cytotoxic activity.44a Another highly abundant indole structure is the indole polymeric structure melanin (28) which can be mainly found in yeasts and fungi such as Exophiala, Cryptococcus, Trametes or Aspergillus (brown to black colour eponymous for Aspergillus niger) but also in Streptomyces bacteria. They can be further divided into eumelanin, phaeomelanin and allomelanin, which has strong protective effects, primarily against UV radiation, reactive oxygen species or chemical stresses.30a,44c Indigo and its derivatives are used as a vat dye for cotton and animal fibres such as wool and silk.40a While tryptanthrin was solely investigated for its bioactive properties, other molecules from this class were used in dyeing experiments. Violacein (26) was applied to polyamide by different dip-dyeing methods achieving uniform dyeing in different purple hues.58 Melanin (28) dyed cotton fabric when culture supernatant of the native producer Streptomyces glaucescens was used.59
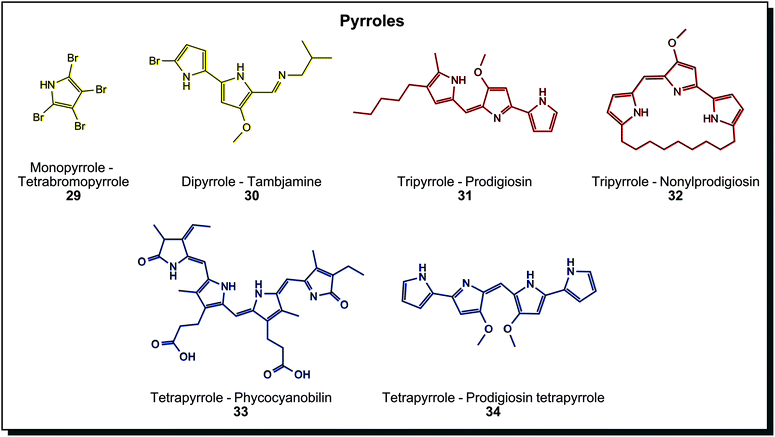
Pyrrole containing dyes are again abundant in marine microorganisms and can be grouped by their number of pyrrole units. Fig. 6 shows a selection with an increasing unit count. Yellow monopyrrole tetrabromopyrrole (29) and dipyrrole tambjamine (30) are found in Pseudoalteromonas species.7c The brightly red tripyrrole prodigiosin (31) from Serratia marcescens is one of the most investigated microbial dyes in science to date. Derivatives with linear alkyl chains, annealed cycloalkyls, and cyclised variants exist (32), and all of them show various biological activities (antibiotic, anti-cancer, among others).60 Prodigiosin-producing bacteria growing on bread or communion wafers may be the reason for numerous “blood miracles” in the Catholic Church, reported throughout the centuries.61 Tetrapyrroles are usually degradation products of heme in animals, but also bacterial tetrapyrroles are known. Phycocyanobilin (33) is a blue light-harvesting molecule in Cyanobacteria. Bactobilin, another bacterial tetrapyrrole from Propionibacterium shermanii is a similar but presumably more stable variant of phycocyanobilin. Both are not biologically active, in contrast to the blue prodigiosin tetrapyrrole (34) from marine Hahella.44a Due to increasing conjugated double bonds in different pyrroles, the colour range covers comprehensive parts of the visible spectrum. Dyeing tests were performed with prodigiosin (31), for example, on silk or acryl. Thereby the antibacterial properties of the dye could be transferred to the fabric.62 In fact, tests of dyeing fabrics with prodigiosin were already carried out years earlier than the invention of synthetic textile dyes. Bartolomeo Bizio, the discoverer of the first prodigiosin-producing organism, used ethanol extracts of Serratia to dye silk and wool but was not successful enough to pursue it further.63
Azines are again more abundant metabolites in colours ranging across the whole spectrum. Hundreds of natural derivatives were discovered mainly in Pseudomonas or Streptomyces, and examples are shown in Fig. 7. Pyocyanin (35) from Pseudomonas aeruginosa is the prime example. Derivatives on the tricyclic core scaffold are manifold, including amidated chlororaphine (36), N-oxidised iodinin (37) or simple hydroxylated, carboxylated, aminated and/or methoxylated species. Also, more complex dimers exist, such as 39 and 40. Cytotoxic properties are shared among biological azines.7c,44a Molecules may contain a varying number of conjugated double bonds, making them appear in colour hues across the visible spectrum. Phenazines are the most abundant subclass of azines found in microorganisms. A few examples of oxazines or thiazines, such as the cytotoxic phenoxazine strepoxazin A, exist in marine Streptomyces species.64 Dyeing experiments were conducted with pyorubin and oxychlororaphine extracted from Pseudomonas species. Silk was dyed after pre-mordanting and retained antimicrobial activity.65 Interestingly, the very first synthetic dye is a phenazine derivative, mauveine A (38). In synthetic dyes, oxazine and thiazine dyes exist beside phenazines. They are applied to leather and cotton as direct dyes or synthetic fibres such as polyacrylonitrile, nylon or polyester as basic dyes and as a reactive dye for wool and silk.40a
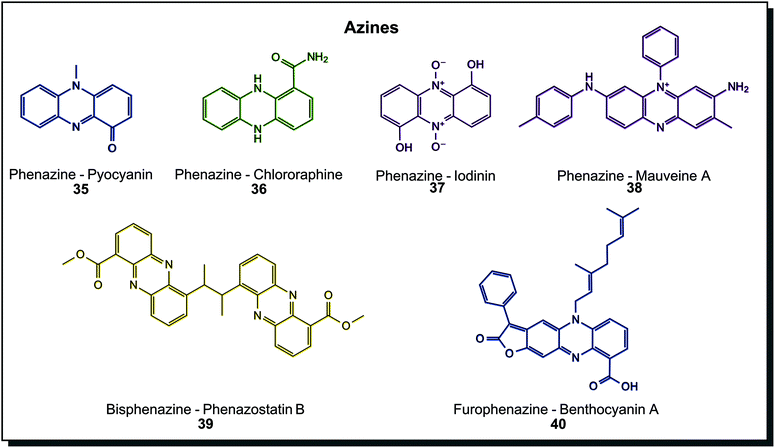 | ||
| Fig. 7 Azine structures found in microorganisms. The first synthetic dye, mauveine A is included for comparison. | ||
Other N-heterocycles are relatively rare structural elements in biogenic dyes, albeit not less attractive, as Fig. 8 demonstrates. Rubrolone A (41) is a red product of polyketide synthesis. Investigations are directed towards cardioprotective properties.66 Indigoidine (42) is the prime example here, exhibiting an indigo-like blue hue. Non-ribosomal peptide synthases produce this glutamate-derived metabolite in particular bacterial species, such as Arthrobacter, Erwinia or Corynebacterium. In vivo functions were described as protection against environmental conditions such as oxidative stress or low temperatures.30a,67 Other members in this group are derived from different metabolic pathways. Dyeing was investigated with indigoidine from recombinant Corynebacterium glutamicum compared to indigo in a solvent dyeing process on cotton. Results showed similar colourimetric properties, indicating promising application as a sustainable dye.68
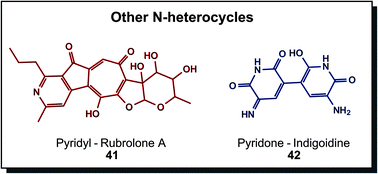 | ||
| Fig. 8 Biogenic nitrogen-containing heterocycles that are not part of previous groups show highly substituted ring structures. | ||
Biosynthesis
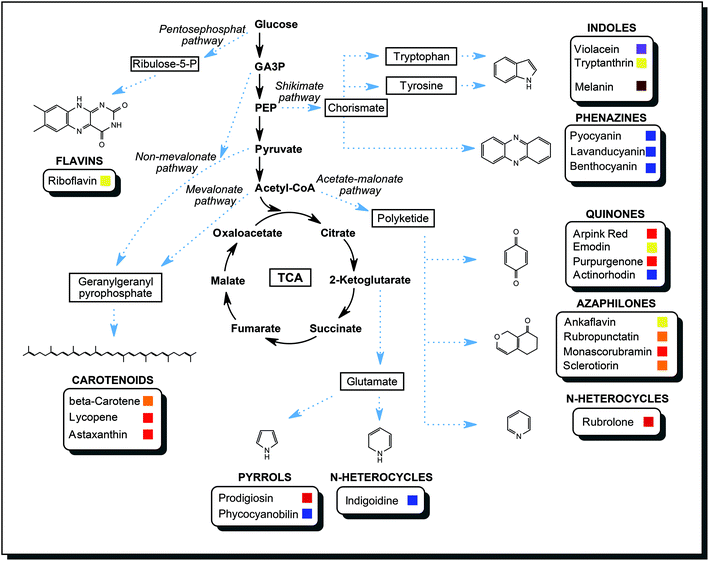
In the course of the primary metabolism, microorganisms produce energy and build up the biomass from carbon and nitrogen sources they consume. Glycolysis and citrate cycle make available important intermediates and products of primary metabolism: sugar phosphates, amino acids, fatty acids, lipids, organic acids. These are the compounds that are starting materials available for secondary metabolism. An extension to a primary metabolic pathway provides the cell with functional secondary metabolites to counter various stress conditions. A gene cluster for a pathway of secondary metabolism is activated upon a suitable external signal. Common external triggers are environmental stresses such as changes in temperature, pH, redox conditions, light or competition for nutrients by other organism species. The cell needs a specific response, so biosynthetic enzymes are produced. The flux of certain primary metabolites is then directed towards the secondary metabolite. As an example, the biosynthesis of azaphilones is introduced briefly. Usually, the end product of glycolysis, acetyl-coenzyme A, mainly enters the citrate cycle, which supplies the cell with reducing equivalents for energy generation and biochemical precursors. Activation of the acetate-malonate pathway creates an additional sink for acetyl-coenzyme A, and a reduced amount is available for the citrate cycle. Instead, acetyl-CoA is converted into polyketides, which are aliphatic structures with multiple carbonyl groups. Polyketide synthases are specialised multi-domain enzymes that modify such a polyketide with a series of reaction steps, yielding the desired azaphilone with defined biological functions.69By the same principle, metabolic intermediates are directed into other pathways to fuel the production of other secondary metabolites. As described in the previous section, a variety of chemical structures were discovered in microorganisms. No single organism is capable of producing all classes of metabolites mentioned. Fig. 9 compiled many different pathways described in the literature to highlight the versatility and the biosynthetic power of microorganisms.60,70
Properties
Besides the basic properties concerning dyeing, which were already described in the section of chemical structures, green chemistry related properties such as toxicity, bioaccumulation, ecotoxicity, or cancerogenicity must be considered for a proper sustainability evaluation. Neither synthetic nor biogenic dyes can be evaluated and categorised generally, but discrimination between dye classes must be made. The effects of synthetic dyes on organisms and the environment were investigated on numerous occasions. Depending on the dye class, acute toxicity, irritation (skin, respiratory), mutagenic and cancerogenic effects were observed.20,23,24 Microbial metabolites were often found to have bioactive properties, as they are designed by nature as functional tools for microorganisms. Ramesh et al. compiled an extensive list of bioactive properties,7c like antimicrobial effects (e.g. antibacterial, antifungal, antiviral), found in pyrrole metabolites, anthraquinones, indoles or phenazines and various quinone species. Furthermore, cytotoxic activity, including anticancer, was observed among pyrroles like prodigiosin (33), indoles like violacein (26), phenazines and several quinones of the azaphilone subgroup. Especially metabolites from marine organisms are rich in bioactive properties.70cThe research on biogenic dye substances is focused on the medical field and biological activities. Little information can be found regarding biodegradability or ecotoxicity. As a workaround, the biodegradability of dyes can be predicted from database tools. The EPA CompTox Chemistry dashboard represents such a database that combines in vivo toxicology data from different scientific sources and can predict data for substances where there are no tests yet. Also, data like biodegradation half-life or bioconcentration factor can be predicted.71 Such estimates are called “read-across” and are becoming more and more popular to fill gaps in environmental and health impact data. Uncertainty has to be managed to give reliable data.72
Table 1 and ESI† present sustainability-related data such as acute toxicity, predicted biodegradability half-life and observed bioactivity in standardised assays for biogenic and synthetic dyes. In Table S2† the data for synthetic dyes were obtained in the same way to allow direct comparison.
| Class/name | CAS no. | Toxicity test | Value acute toxicity | Test species | Pred. biodegrad. t1/2 (days) | Confidence level in OPERA modela | Bioassay activity | Colour index no. |
|---|---|---|---|---|---|---|---|---|
| a Predicted biodegradability half-life comes with a confidence level index (ranging from 0 to 1) to decide about the reliability of this prediction. | ||||||||
| Polyenes | ||||||||
| β-Carotene (9) | 7235-40-7 | LD50 | >5000 mg kg−1 | Rat (oral)78 | 74.4 | 0.517 | Binding solute carrier transporter, antiviral, antioxidant | C.I. Food Orange 5 |
| Astaxanthin (10) | 472-61-7 | LD50 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 000 mg kg−1 |
Rat (oral)79 | 44.4 | 0.514 | — | — |
| Azulene | 275-51-4 | LD50 | 108 mg kg−1 | Mouse (intra-peritoneal)131 | 8.58 | 0.553 | — | — |
| Azulene | 275-51-4 | LD50 | >3000 mg kg−1 | Mouse (oral)131 | — | — | ||
| Guaiazulene (11) | 489-84-9 | LD50 | 525 mg kg−1 | Mouse (intra-peritoneal)80 | 2.50 | 0.730 | Binding nuclear receptors and histone enzymes | — |
| Guaiazulene (11) | 489-84-9 | LD50 | 1220 mg kg−1 | Mouse (oral)80 | ||||
Toxicity is evaluated by acute, chronic or cytotoxicity tests on bacteria, algae, invertebrates, fish or rodents. The Globally Harmonised System GHS considers results in acute toxicity tests for lethal dose LD50 (oral) as harmful (category 4) if they are in the range of 300–2000 mg kg−1 and toxic below 300 mg kg−1 (category 1–3).73 Results of aquatic tests are considered hazardous to the aquatic environment (category 3) below 100 mg L−1 and acute toxic (category 1–2) in the range of 10–100 mg kg−1.74 This is applied to tests for lethal concentration LC50 (fish 96 hours), effective concentration EC50 (crustaceans 48 hours) and effective concentration of growth rate reduction ErC50 tests (algae 72 hours). Results for the inhibitory concentration for microorganisms IC50 below 100 mg L−1 are considered a toxic effect.75
Toxicity may be mediated by the dye molecule itself or by breakdown products thereof (metabolites). In the case of azo dyes, several aromatic amines used as diazo or coupling components were found to be potent carcinogens, such as benzidine or o-toluidine.75 The same is true for anthraquinones. Primary amino groups or methylamino groups are features of molecules with higher cancerogenic properties.75 Phthalocyanines were described as largely non-toxic due to their low solubility and non-bioavailability, although at higher concentrations, the copper ions from the Cu–phthalocyanine complex exhibit toxic effects.41 In general, metal ions such as cobalt, nickel or chromium from metal-complex dyes pose a significant issue in water pollution and aquatic toxicity.75 Chavan gave an overview of studies on different toxicity types. From 4461 synthetic dyes tested for LD50 acute toxicity using animals, only 8% were classified as harmful or poisonous. In contrast, aquatic toxicity was found to be more widespread. In LC50 tests using fish, 30% of 3000 synthetic dyes were classified as acute toxic according to OECD definition.75,76
Such extensive evaluation is not available for biogenic dyes. Data on typical examples from different biogenic dye classes can be found in Table 1 and the ESI.† For polyenes (Table 1), substances β-carotene (9) and astaxanthin (10) may be regarded as non-toxic; on the other hand, azulenes highly depend on the test performed. Some toxicity results in Table S1† fall in a range that is considered harmful, some even below. LD50 values below 300 mg kg−1 were found for some derivatives of pyrroles, phenazines, azulenes and violacein (26). It has to be noted that results depend on the organism tested or the type of test used.4 This can especially be seen for violacein (26) or emodin (15). Violacein did not show an effect at problematic concentrations to the fungus P. capsici (EC50 560 mg L−1) but did so when injected into mice (LD50 100 mg kg−1). Toxicity of emodin showed a significant difference when administered orally (LD50 1000 mg kg−1) or by injection (LD50 35 mg kg−1) to mice.
For biogenic dyes, the literature only mentions the (assumed) better biodegradability of biogenic dyes compared to synthetic dyes.8a,10 This assumption can be regarded as justified; however, reliable and comprehensive data is missing on this topic. The degradation of selected phenazines by microbial action was investigated in more detail but not in a wastewater treatment environment. Rhodococcus sp. degraded phenazine-1-carboxylic acid into a dihydroxylated product with a newly discovered dioxygenase enzyme. The antimicrobial activity of phenazine-1-carboxylic acid was strongly decreased by converting it into dihydroxyphenazine.88 Microbial degradation of blue pyocyanin (35) was also investigated more closely. It serves the bacterium Pseudomonas aeruginosa as a virulence factor and biofilm mediator. The newly discovered enzyme pyocyanin demethylase removed the methyl group to leave hydroxy phenazine. Biofilm formation was disrupted, and sessile conditions of the bacteria were disturbed.89
Predicted biodegradation half-lives (t1/2) in Table 1 and ESI Tables S1 and S2† illustrate the impact on water bodies if dyes should end up there. Relatively high values were found for carotenoids, the quinones actinorhodin (14) and emodin (15), the pyridine derivative rubrolone A (41) and the phenazine benthocyanin (40). Other substances like monascin (20), prodigiosin (31), pyocyanin (35) or azulene were predicted to be easily biodegraded. The values for confidence levels are mediocre, so conclusions have to be drawn with caution. In conclusion, biogenic dyes show better predicted biodegradability than synthetic dyes. Bioassays indicated binding to certain enzymes or receptors for many biogenic dyes. Among synthetic dyes, many showed acute toxicity and, most notably, disruptive endocrine activity by binding to androgen receptors.
Bioproduction of ‘critical’ synthetic dye alternatives
On many occasions, the advantages of bioproduction have been described, including mild reaction conditions during fermentation, possible use of renewable starting material, unmatched selectivity of many enzymes/whole-cell production systems and generation of environmentally benign waste. Table 2 provides an overview of biotechnological production processes for dyes found in the literature, and some examples shall be discussed in this section. A comparison of biotechnological processes was made to plant-derived or synthetic ones where data was found. In many cases, dyes are unique to microorganisms and have not yet been found in plants. Indigo is one of the few cases for which three types of production at a larger scale can be compared: plant-based, synthetic and biotechnological production.| Compound | Production system | Yield | Total prod. scale in EU according to ECHA | Resources, chemicals used | Process steps | Waste generated | Ref. |
|---|---|---|---|---|---|---|---|
| a Used for analytical assays only. | |||||||
| Polyenes | |||||||
| β-Carotene (9) | Dunaliella algae | 5% w/w of biomass | 1–10 t a−1 | Sea water salts (NaCl, NO3, SO4, PO4) | Growth14 days, carotenogenesis, freeze-dry cells or extraction with edible oils/solvents | High salt solution, solid cell mass | ECHA99 |
| Astaxanthin (10) | Haematococcus pluvialis | 2% w/w of biomass | 1–10 t a−1 | Fresh water | Growth, carotenogenesis, freeze dry or cell disruption/extraction | Solid cell mass | ECHA99 |
| Carotenoids | Rhodotorula marina | 0.4 mg g−1 cells | Non-industrial, batch | Water, minimal medium, spent brewer's yeast, raw glycerol, petrol ether | Preculture and fermentation at 30 °C, centrifugation, bead grinding, solvent extraction | Aqueous medium, solid cell mass, solvent waste | 100 |
| Indoles | |||||||
| Indigo | Plant | 1% of plant mass | Today non-industrial | Water, H2SO4 | Plant material fermented in water for 15 h to cleave indican glucoside, purification by precipitation from dilute H2SO4, oxidation in air | Plant biomass, dilute acid | 90 and 91 |
| Indigo | Synthetic | 90% of aniline | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t a−1 (global) 000 t a−1 (global) |
Formaldehyde, cyanide, NaNH2, KOH/NaOH solvent | Reaction at 200 °C, oxidation and filtration | Aniline derivatives due to decomposition, alkaline wastewater | 90, 91b and 103 |
| Indigo | Recombinant E. coli | 74 mol% of tryptophane/15 mol% of glucose (23 g L−1) | Non-industrial, fed-batch | Water, complex medium, DMSOa | Preculture and fermentation at 30 °C, centrifugation, extraction from biomass | Aqueous medium, solid cell mass, solvent waste | 104 |
| Indigo | Recombinant E. coli | 37 mol% of tryptophane (0.9 g L−1) | Non-industrial, batch | Water, yeast extract medium, DMSOa | Preculture and fermentation at 30 °C, centrifugation, extraction from biomass | Aqueous medium, solid cell mass, solvent waste | 93 |
| Violacein (26) | Janthinobacterium lividum | 3 mol% of glycerol (0.1 g L−1 pure) | Non-industrial, fed-batch | Water, complex medium, methanol | Preculture and fermentation at 25 °C, centrifugation, extraction from biomass | Aqueous medium, solid cell mass, solvent waste | 58 |
| N-Heterocycles | |||||||
| Rubrolone A (41) | Engineered Streptomyces albus | 0.01 mol% of lactose (0.01 g L−1) | Non-industrial, batch | Water, complex medium, adsorbent resin, methanol | Preculture and fermentation at 28 °C, centrifugation, solid phase extraction, solvent extraction | Aqueous medium, solid cell mass, solvent waste | 66 |
| Indigoidine (42) | Recombinant Rhodotorula toruloides | 14 mol% of glucose (18 g L−1) | Non-industrial, fed-batch | Water, defined medium, DMF | Preculture and fermentation at 25 °C, centrifugation, extraction from biomass | Aqueous medium, solid cell mass, solvent waste | 96 |
| Pyrroles | |||||||
| Prodigiosin (31) | Engineered Serratia marcescens | 35 mol% of sucrose (16 g L−1) | <1 t a−1 | Water, complex medium, ethanol | Preculture and fermentation at 28 °C, centrifugation, extraction from biomass | Aqueous medium, solid cell mass, solvent waste | 95 |
| Azines | |||||||
| Pyocyanin (35) | Pseudomonas aeruginosa | 0.1 mol% of glycerol (0.02 g L−1) | Non-industrial, batch | Water, complex medium, HCl, chloroform | Preculture and fermentation at 30 °C, centrifugation, acidic extraction from supernatant | Aqueous medium, solid cell mass, solvent waste | 97a |
| Pyocyanin (35) | Pseudomonas aeruginosa | 3.3 mg L−1 from maise processing wastewater | Non-industrial, batch | Maise wastewater, flocculation agent, HCl, chloroform | Preculture and fermentation at 30 °C, centrifugation, extraction from supernatant | Aqueous medium, solid cell mass, solvent waste | 101 |
| Pyocyanin (35) | Synthetic | 29 mol% of aniline/fluoro-nitrobenzene | Non-industrial, batch + flow | Water, CaCO3, EtOAc. Triphenyl-phosphane, toluene, acidic resin, MeOH, NEt3. PhCl2, Me2SO4 | Microwave react. at 120 °C, flash chrom. Microwave react. at 250 °C, flash chrom. Flow react. in PhCl2 at 110 °C, crystallisation. Flow react. in water with irradiation, quenching in acidic resin column, capture on C18 material. | Solvent waste, halogenated waste, silica gel | 98 |
| Hydroxy-phenazine | Engineered Pseudomonas chlororaphis | 1 mol% of glycerol (0.45 g L−1) | Non-industrial, batch | Water, complex medium, HCl, ethylacetate | Preculture and fermentation at 28 °C with induction, centrifugation, acidify and extraction from supernatant | Aqueous medium (acidic), solid cell mass, solvent waste | 97b |
| Hydroxy-phenazine | Synthetic | 26 mol% of aniline/fluoro-nitrobenzene | Non-industrial, batch + flow | Water, CaCO3, EtOAc. Triphenyl-phosphane, toluene, acidic resin, MeOH, NEt3. PhCl2, Me2SO4 | Same as above. Last step: quench in basic resin column. | Solvent waste, halogenated waste, silica gel | 98 |
Historically indigo was extracted from plants, requiring minimal chemical spending (sulfuric acid, water) and high land use (7000 km2 for 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t around 1900 in India) for small yields.90 Soon after the establishment of the petrochemical industry, indigo was mainly produced synthetically. Good yields (up to 90%) were obtained from aniline starting material under harsh reaction conditions (e.g. 200 °C, KOH/NaOH solvent, formaldehyde).91 From the 1980s, the production of indigo from indole or tryptophan was investigated with recombinant microorganisms. Gene technology, enzyme and metabolic engineering enabled biotechnological production in an aqueous medium.55,92E. coli expressing a recombinant flavin monooxygenase produced indigo from tryptophan in synthetic medium with a yield of 37 mol% based on tryptophan.93 Although this work was performed at a 3000 L pilot scale, commercialisation is still waiting due to difficulties in product separation from biomass and treatment of large volumes of by-products. Recently, the former most expensive dye in the world, Tyrian purple, was produced by genetically engineered E. coli.94 Formation of this brominated indigo species was achieved by expressing a recombinant flavin-containing tryptophan halogenase, an L-tryptophan indole-lyase (tryptophanase) and a flavin monooxygenase. These enzymes achieved the bromination of tryptophan, cleavage of the bromoindole unit and dimerization to Tyrian purple by oxidation, respectively. Also, other non-natural halogenated indigo species could be produced using tryptophan halogenases with different regioselectivities.94 Besides indigo, also other dyes were isolated in biotechnological processes. Prodigiosin (31) was produced in an engineered Serratia marcescens with 35 mol% yield using carbon source sucrose (16 g L−1 concentration in medium).95 Recombinant Rhodotorula toruloides yielded blue-coloured indigoidine (42) with 14 mol% on glucose or 18 g L−1 in medium.96
000 t around 1900 in India) for small yields.90 Soon after the establishment of the petrochemical industry, indigo was mainly produced synthetically. Good yields (up to 90%) were obtained from aniline starting material under harsh reaction conditions (e.g. 200 °C, KOH/NaOH solvent, formaldehyde).91 From the 1980s, the production of indigo from indole or tryptophan was investigated with recombinant microorganisms. Gene technology, enzyme and metabolic engineering enabled biotechnological production in an aqueous medium.55,92E. coli expressing a recombinant flavin monooxygenase produced indigo from tryptophan in synthetic medium with a yield of 37 mol% based on tryptophan.93 Although this work was performed at a 3000 L pilot scale, commercialisation is still waiting due to difficulties in product separation from biomass and treatment of large volumes of by-products. Recently, the former most expensive dye in the world, Tyrian purple, was produced by genetically engineered E. coli.94 Formation of this brominated indigo species was achieved by expressing a recombinant flavin-containing tryptophan halogenase, an L-tryptophan indole-lyase (tryptophanase) and a flavin monooxygenase. These enzymes achieved the bromination of tryptophan, cleavage of the bromoindole unit and dimerization to Tyrian purple by oxidation, respectively. Also, other non-natural halogenated indigo species could be produced using tryptophan halogenases with different regioselectivities.94 Besides indigo, also other dyes were isolated in biotechnological processes. Prodigiosin (31) was produced in an engineered Serratia marcescens with 35 mol% yield using carbon source sucrose (16 g L−1 concentration in medium).95 Recombinant Rhodotorula toruloides yielded blue-coloured indigoidine (42) with 14 mol% on glucose or 18 g L−1 in medium.96
Although some biotechnological processes show already decent yields, Table 2 indicates that other syntheses of biogenic dyes show rather low productivities. This issue has to be tackled with molecular biology methods and will be discussed in the next section. Azines like pyocyanin (35) and hydroxyphenazine showed low yields in wild-type or engineered Pseudomonas.97 In contrast, synthetic processes reach higher yields for these substances, even though more process steps and energy are needed.98 This fact directly points to the great potential that stems from biotechnological processes: sustainability. Probably the most significant advantage compared to the production of dyes from petrochemicals is the use of renewable starting materials. This fact allows the integration of dye production into a biorefinery scaffold and, therefore, into circular economy processes. Yield may not be similarly high as with the usage of defined carbon sources like glucose. Still, the drastic reduction in medium costs and development towards circular processes can make this replacement option worth investigating. Different classes of biogenic dyes were already produced using low-cost industrial by-products as a nutrient source in the medium. Blue bispyridone indigoidine (42) was obtained by the genetically engineered fungus Rhodotorula toruloides. When using different lignocellulose hydrolysates as the sole carbon source, a production titre of 0.3 g L−1 could be reached. In contrast, in the same report fed-batch fermentation in synthetic medium with high glucose content achieved 18 g L−1.96 Carotenoids like β-carotene (9) or astaxanthin (10) are produced industrially mainly by algae in open ponds or photobioreactors with moderate yields around 5 wt%.99 But also Rhodotorula yeast is suited for this task, using low-cost carbon and nitrogen source for fermentation such as raw glycerol and spent brewer's yeast. With this setup, Rodrigues et al. obtained a mixture of four carotenes with a total concentration of 0.4 mg g−1 cell mass which was higher than any other carotene yield from Rhodotorula species until then.100 Pyocyanin (37) was produced by Pseudomonas aeruginosa grown solely on maise processing wastewater. After optimisation using a response surface methodology, the dye yield was improved to 3.3 mg L−1.101
Renewable feedstock is one and waste generation another significant advantage. Aqueous growth medium may be disposed into the sewers after the inactivation of microorganisms. Solid cell residues can be used as fertilisers or are even suited as animal feed. Residues with high salinity or acidic/basic pH however, would need more thorough treatment.102
Challenges for application of biogenic dyes
In the past, many contributions treated challenges that arise with the large-scale production of dyes by fermentation. Examples include the use of cheap production medium, filamentous growth of many fungi, a potential co-production of mycotoxins or product yield. Molecular biology provides excellent methods for dealing with these.8a,c,10,105 Many challenges discussed in the following section have still to be tackled and solved in the future.Finding suitable coloured metabolites and conditions of production
Although a plethora of possible biogenic dyes are described in the literature, finding new coloured substances and isolating them is not a straightforward task. Secondary metabolites are only produced under particular conditions. In standard lab conditions, an estimated 90% of putative biosynthetic gene clusters remain silent.105 Investigation of production conditions of biogenic dyes often lacks a level of detail. The influence of physical and chemical growth parameters such as temperature, pH, aeration conditions, redox conditions, light, carbon and nitrogen sources have been widely acknowledged. However, a detailed and systematic study of all parameters is time-consuming and costly.44c,106Without DNA sequence data, such studies have to be carried out exploratively using the “one strain many compounds” approach. In this strategy, microorganisms are subjected to several growth conditions, and metabolic profile changes are determined. Changes were even observed with different shaking speeds or vessel types (e.g. altered shear forces). Such screenings should be carried out in a high-throughput fashion, for example, by cultivating microbes in microtiter plates.107 In combination with optimisation by response surface methodology (RSM), statistically valid models for metabolite production can be obtained.108
Alternatively, if the DNA sequence data is available, genomic data mining can be performed. Comparison to annotated sequence data with known functions can not only confirm the existence of hidden biosynthetic gene clusters in the target organism's DNA but also identify them. In this way, the product substance class may be predicted and favourable production conditions inferred. Follow-up studies may be carried out much more targeted than with the “one strain many compounds” approach. For example, in the genetic analysis of a newly isolated Antarctic bacterium, five biosynthetic gene clusters were identified; two were found to be of terpene type. Their products were predicted by comparing the clusters, gene by gene, with their most similar matches from databases, which resulted in a possible C50 β-cyclic carotenoid product. Preliminary characterisation of extracts after microorganism cultivation by UV-vis and mass spectroscopy supported this prediction.109
Industrial production
Once new potential biogenic gene clusters have been identified, they can be transferred into industrial production hosts. Thereby, a complex activation and prior knowledge of growth and dye-production conditions can be circumvented. Heterologous expression of gene clusters may not succeed right away, as cofactors, pathway regulation, or essential genes outside the gene cluster can cause problems with the efficient production of the desired secondary metabolite. Many challenges and possible strategies solutions were shown in a recent publication on biotechnological production under time pressure.110 In this work, ten more or less complex molecules, from APIs (pacidamycin) to platform chemicals (carvone, THF), were targeted to be produced biotechnologically in a 3-months pressure test. Each of the target molecules had faced particular challenges along the usual path of biotechnological production – biosynthetic pathway construction, cloning into a host organism, expression of pathway enzymes and production. The study compiled strategies from bioinformatics, molecular biology, organic chemistry and biochemistry to solve the aforementioned challenges in a short timeframe. In cases of problematic pathway regulation, the gene cluster have to be redesigned. The DNA may be codon-optimised for the individual host organism, genes may be reorganised into artificial operons, native repressor or regulating DNA sequence may be removed, and inducible promoters may be added. In the pacidamycin example, functional gene expression was achieved by replacing an activator gene from the gene cluster with an activator gene from the heterologous host under a host-specific promoter.110 Additionally, inhibitory effects of the recombinantly produced molecule on the host organism have to be considered. Metabolic regulatory functions or bioactive properties may pose a challenge for high-yield production. This issue was addressed in the recombinant production of prodigiosin (31). Besides the large size and complexity of the gene cluster to be cloned, the antimicrobial activity of the desired product was identified as a challenge in that work. The heterologous production host Pseudomonas putida was chosen over Bacillus subtilis and Escherichia coli due to its non-susceptibility to the antimicrobial. In fact, the prodigiosin-producing mutant showed no difference in growth behaviour compared to the wild-type P. putida.111Colour range
Accessible biogenic dye structures cover only the integral colour spectrum (yellow, orange, red, blue, purple, but hardly any green), and synthetic dyes exhibit a much broader colour range as chemical synthesis opened the door to virtually any hue in the visible spectrum. Additionally, synthetic dyes offer a continuous colour palette, as can be seen on manufacturers’ websites.112 Biogenic dyes in comparison have a more “patchy” spectrum which nevertheless provides excellent opportunities for engineering.8cMany chemical and biological methods are available for creating analogues of natural products, which may alter the properties of biogenic dye molecules, including the colour.113 These methods encompass the well-known semi-synthetic approach,114 precursor-directed biosynthesis,115 mutasynthesis with or without post-biosynthetic modification,116 combinatorial biosynthesis117 and chemogenetics,118 from which two easy to use methods are described in more detail.
Precursor-directed biosynthesis is the first step in obtaining non-natural variants of metabolites. No genetic engineering is required. Novel derivatives of the pathway products can be obtained just by exploiting the tolerance of biosynthetic enzymes to non-natural precursor molecules (substrate promiscuity). Precursors are supplied to the organism and incorporated into the product. As precursor derivatives compete with natural ones, a mixture of products is obtained. Violacein (26) formation in Chromobacterium and other species should be mentioned as a natural example of precursor-directed biosynthesis. This biogenic dye molecule is formed from the precursor L-tryptophane. The final enzyme of the secondary metabolite pathway is the NADPH-dependent monooxygenase VioC. It can accept two different pathway intermediates, leading to violacein (26) as major and deoxyviolacein as a minor natural product (Fig. 10A).119 Natural precursor-directed biosynthesis, in this case, yields molecules with a different colour. Violacein appears blue with an absorption maximum at 572 nm, whereas deoxyviolacein shows purple colour with its maximum at 560 nm. Also, acid/alkali tolerance and antitumour activity of the substances were found to be significantly different.120 Mutasynthesis is a similar strategy that involves partial knockout of the biosynthetic pathway. One building block for the final product cannot be produced any longer but is exogenously provided to the organism in the form of a non-natural derivative. The final product is thus obtained as a derivative of the natural analogue. This approach also takes advantage of substrate promiscuity of enzymes but avoids the formation of non-derivatised products. The biosynthetic pathway for prodigiosin (33) consists of two parallel formations of precursors, a monopyrrole unit from 2-octenal and a bipyrrole unit from L-proline. In a final step, they are condensed by the membrane-bound synthetase PigC, forming the tripyrrole prodigiosin.121 Klein et al. engineered this pathway by knocking out the enzymatic steps responsible for monopyrrole formation. Supplying the organism with non-natural monopyrrole derivatives resulted in the formation of prodigiosin derivatives with altered colour and bioactive properties (Fig. 10B).116
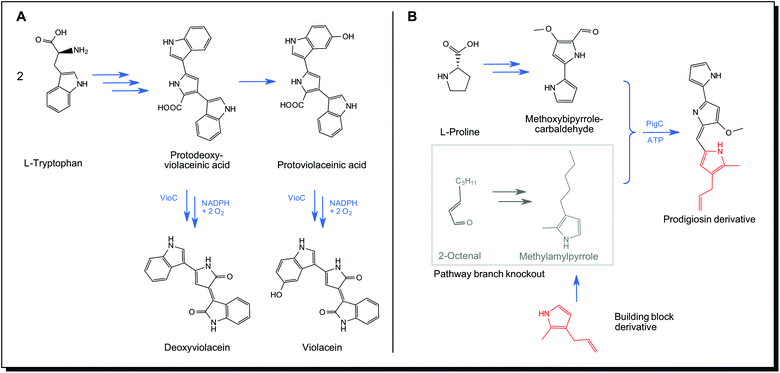 | ||
| Fig. 10 Natural substrate promiscuity of monooxygenase VioC in violacein biosynthesis pathway (A), mutasynthesis in prodigiosin pathway according to Klein et al. by exploiting substrate promiscuity of prodigiosin synthetase PigC116 (B). NADPH – reduced Nicotinamide adenine dinucleotide phosphate, ATP – Adenosine triphosphate. | ||
Colourimetry
Another essential aspect of this topic concerns colour measurement. In the scientific literature, the colour of natural dyes is usually characterised by spectroscopic analysis (UV-vis spectrum, absorption maximum, extinction coefficient).44a In the industry, the colour space is measured differently, depending instead on the application. Devices that produce colours electronically (monitors, scanners) use the additive colour space (RGB, red, green and blue). In contrast, ink-jet printing is associated with the subtractive colour space (CMYK, cyan, yellow, magenta and black). The International Commission of Illumination (CIE) developed a device-independent colour space, CIE L*a*b*. It defines colours in the dimensions lightness (L*), red-green value (a*) and blue-yellow value (b*).122 “Pantone” and “Color Atlas” provide frameworks for standardising dye colours for the industry. These measurements characterise the final appearance of a dyed textile, depending on the concentration of the dye in use or the fabric type, among others. On the other hand, spectroscopy characterises the inherent properties of the dye molecule, whereas colourimetry assesses the final dyeing result. For a quantitative comparison of the available colour range between synthetic and biogenic dyes, comparable measurements have to be available first. Unfortunately, colourimetric data for biogenic dyes are largely unavailable. Only specific publications carry out colourimetric measurements according to industry practice. Ren et al. characterised acrylic fabric dyed with aqueous prodigiosin (31) nano-suspension by colourimetry and colour fastness to washing, rubbing and perspiration.62b Räisänen investigated emodin (15) and other fungal anthraquinones in dyeing/printing of cotton and linen with mordanting and obtained excellent results for dye uptake rates, colourimetric properties and good scores in fastness tests.123 Several authors analysed dyeing with raw extracts of unspecified microbial dyes. De Santis et al. investigated the colourimetric properties of red and orange Monascus purpureus dyes with different mordanting methods on wool.48 Yan et al. used an unidentified yellow fungal pigment on silk and wool with different mordanting.124 In general, the interest in dyeing with microbial-derived molecules is growing; hence, more investigations of different structure classes are expected to follow. The study situation seems to be a spotted landscape at the moment. Given the countless parameters of the textile dyeing process (e.g. dye method, pre/post-mordanting, temperature, pH value, liquor ratio) that all may influence colourimetric and colour fastness properties, more systematic tests are required. A comparison of the performance of different dyes in the same dyeing method or vice versa will ensure better comparability of results.Downstream processing
One of the key challenges in the biotechnological production of dyes is downstream processing. The dyes have to be isolated from the fermentation media as well as from the biomass. Furthermore, they need to be separated from the cell-derived metabolites. Efficient production is of course, the basis of a successful process, but less was talked about the isolation of the product from the fermentation broth. Selectively separating the dyes from cell mass or aqueous medium is still a big challenge and is needed to facilitate the subsequent purification significantly. Kalra et al. discussed extraction methods for intracellular dyes with regard to green chemistry aspects. Extraction assisted by ultrasound, microwave or pulsed electric fields increase extraction efficiency at low temperatures, reducing energy usage. Extraction by pressurised liquid, ionic liquids or supercritical fluids reduces solvent consumption or applies new and tuneable affinities of extractants, increasing the efficiency of the process. The authors concluded that there is no generally applicable isolation method for dyes.106Little attention has been paid to using a solid phase for the extraction of dyes, similar to the concept of ‘in situ product recovery’. This topic was reviewed by Dafoe et al., also giving a possible explanation for the limited application of the technique. Resins have to be selected for each task, which is done mainly by trial and error. Only a few attempts exist to make rational resin selection by first principles considerations.125 Performing an isolation step via solid-phase extraction lead to less solvent and time consumption for the extraction. A recent example of in situ product recovery in dye fermentation was presented by Domröse et al. Prodigiosin (31) was produced in a heterologous organism and was isolated from the culture by a polyurethane solid-phase. The dye is primarily located intracellularly, but a fraction diffuses into the liquid medium. They reasoned that the solid phase acted as a possible product sink, shifting the intra/extracellular partitioning equilibrium. Additionally, the productivity of the culture was found to be enhanced by using the solid phase.111
An interesting example of secondary metabolite isolation was presented by Bailey et al. The authors successfully applied natural plant spores as isolation material for the extraction of a light-sensitive polyene antibiotic from fermentation. The porous structure of the spores encapsulated the compound of interest and provided photoprotection.126 No generally applicable method for extraction was shown here either, but these resins exist in a wide range of polarities or functionalities. A material selective enough for a particular task can most likely be found.
Undesired properties of biogenic compounds
Many biogenic dyes show certain properties that may impede their application as textile colourants. Firstly, they often display bioactive properties like antibacterial, antifungal, anticancer activities, which should be avoided in everyday application. Toxicity data and bioactivity assay results from Table 1 suggest that this may be an issue, but safe molecules can be found among biogenic dyes. Careful consideration herein should prevent regrettable substitutions.Many biogenic dyes exhibit bioactive properties, of which antimicrobial and cytotoxic effects may be the most concerning. Cytotoxic dyes do not exhibit the initially proposed ideal inertness, rather posing hazards to workers and consumers. If antimicrobial substances are used in the scale that textile dyes necessitate, the threat of resistance development becomes imminent. This usage would go against current efforts to ensure the efficacy of antimicrobial drugs. The engineering of molecules should offer solutions for such issues. Each class of biogenic dyes provides different derivatives with altered properties. If no suitable natural molecule can be found, biological derivatization methods can be applied to obtain non-natural derivatives. Herein, different approaches can engineer and remove unfavourable properties of dyes.75 Adding water-soluble functional groups to a dye molecule not only improves its applicability in aqueous dyeing baths but also facilitates its excretion from cells, whereby bioaccumulation can be significantly reduced. Primarily used functional groups are sulfonic acids, carboxylic acids, hydroxy groups and quaternary ammonium salts. An increase in size (molar weight) and polarity of the dye molecule strongly impedes transport across the cell membrane. The genotoxic potential is thus decreased as the molecule cannot reach the DNA in the cell anymore. Replacement of problematic building blocks with safer alternatives also reduces toxic impact. Innovative azo dyes, for example, contain benzimidazole amines instead of potential cancerogenic aromatic amines. In metal-complex dyes, aquatic toxicity may be lowered if iron instead of a more concerning metal (e.g. Cu) is used.75 This strategy may not always be applied, especially if a particular colour has to be achieved. Additionally, as dye complexes with different metals give different colours, dye structure may be altered.127
Other challenges for biogenic dyes could be limited stability in the conventional dyeing process or low water solubility. Studies reported insufficient stability to high temperatures or extreme pH values faced in the dyeing process.50 Typical conditions in dyeing processes range from 40 °C–90 °C and pH 3–10 for up to 60 min.123,128 New ways of dye production also offer opportunities for novel applications, preferably oriented in a sustainable direction. Indigo derived from biotechnological production provides an excellent example for a more sustainable process. Herein the indigo precursor, indican is produced in recombinant E. coli. Indican, naturally found in plants, represents the activated form of indole protected with a sugar moiety. In the dyeing process, the sugar protecting group of the water-soluble indicant is cleaved off enzymatically, and indigo is formed in situ on the fibre. No reducing agent or base is required as in the traditional vat dyeing process.129
Outlook
Facing the enormous effects of synthetic dyes on the environment and the human population and following political decisions on stricter environmental legislation, such as the EU Green Deal, humanity is searching for a feasible alternative to replace problematic dyes gradually. Biogenic dyes pose a promising alternative in textile dyeing, as comparison on different levels were shown in this review. Microorganisms can provide a diverse choice of chemical structures with important colours available. Gaps in the colour palette may be closed by employing biochemical derivatization or molecular biology methods. Production is possible in large-scale without suffering typical disadvantages of plant dyes. Moreover, a renewable feedstock may be used to close the circular economy loop on particular organic waste streams. Isolation of dyes from biomass is challenging, but various methods are already at hand to achieve this with only reasonable effort. However, low yields are still a significant obstacle for the feasible production of biogenic dyes. Biotechnology, molecular biology and bioinformatics offer numerous tools to discover dyes and engineer organisms for improved properties. Dyeing experiments with fungal or bacterial dyes have been carried out for years. Also, colourimetric measurements have started to be used for efficient comparison with industrial standards. Toxicology data sets are certainly not completed and have to be accomplished when dyeing development yields various derivatives with altered properties. The most significant advantage from a green chemistry perspective is the possibility to switch to renewable starting materials and improve biodegradability. Prediction results on biodegradability are promising in favour of biogenic dyes, although confidence levels are low which has to be taken into account. The future of dyeing is bio-based, as more and more scientists, designers and policy makers work on a sustainable transition of our colourful life.Conflicts of interest
VTL GmbH has commercial interest in dyes from microbial origin.Acknowledgements
Funding from the Austrian Forschungsförderungsgesellschaft (FFG, project #25838178) is gratefully acknowledged.References
- P. J. T. Morris and A. S. Travis, Am. Dyest. Rep., 1992, 81, 59–107 CAS.
- R. D. Welham, J. Soc. Dyers Colour., 1963, 79, 146–152 CrossRef CAS.
- K. Hunger, in Industrial Dyes, ed. K. Hunger, Wiley VCH, 1st edn, 2002, pp. 1–12 Search PubMed.
- A. Tkaczyk, K. Mitrowska and A. Posyniak, Sci. Total Environ., 2020, 717, 137222–137241 CrossRef CAS PubMed.
- (a) S. Sánchez-Muñoz, G. Mariano-Silva, M. O. Leite, F. B. Mura, M. L. Verma, S. S. da Silva and A. K. Chandel, in Biotechnological Production of Bioactive Compounds, ed. M. L. Verma and A. K. Chandel, Elsevier B.V., 2020, ch. 11, pp. 327–361 Search PubMed; (b) L. Nambela, L. V. Haule and Q. Mgani, J. Cleaner Prod., 2020, 246, 119036–119049 CrossRef CAS; (c) M. Yusuf, M. Shabbir and F. Mohammad, Nat. Prod. Bioprospecting, 2017, 7, 123–145 CrossRef CAS PubMed.
- Society of Dyers and Colourists and AATCC, Definitions of a dye and a pigment, https://colour-index.com/definitions-of-a-dye-and-a-pigment, (accessed 5.5.2021).
- (a) M. P. N. Rao, M. Xiao and W. J. Li, Front. Microbiol., 2017, 8, 1113–1125 CrossRef; (b) O. V. Singh, Bio-pigmentation and Biotechnological Implementations, 2017 CrossRef; (c) C. Ramesh, N. V. Vinithkumar, R. Kirubagaran, C. K. Venil and L. Dufosse, Microorganisms, 2019, 7, 186–231 CrossRef CAS.
- (a) T. Sen, C. J. Barrow and S. K. Deshmukh, Front. Nutr., 2019, 6, 7–20 CrossRef PubMed; (b) L. Dufossé, J. Food Compos. Anal., 2018, 69, 156–161 CrossRef; (c) C. K. Venil, L. Dufossé and P. R. Devi, Front. Sustain. Food Syst., 2020, 4, 100–116 CrossRef; (d) P. S. Nigam and J. S. Luke, Curr. Opin. Food Sci., 2016, 7, 93–100 CrossRef.
- (a) R. Mumtaz, S. Bashir, M. Numan, Z. K. Shinwari and M. Ali, Curr. Microbiol., 2019, 76, 783–790 CrossRef CAS; (b) M. Numan, S. Bashir, R. Mumtaz, S. Tayyab, N. U. Rehman, A. L. Khan, Z. K. Shinwari and A. Al-Harrasi, 3 Biotech, 2018, 8, 207–221 CrossRef.
- C. K. Venil, P. Velmurugan, L. Dufosse, P. R. Devi and A. V. Ravi, J. Fungi, 2020, 6, 68–90 CrossRef CAS.
- N. R. Singha, P. K. Chattopadhyay, A. Dutta, M. Mahapatra and M. Deb, J. Mol. Liq., 2019, 293, 111470 CrossRef.
- E. Hansen, P. Monteiro de Aquim and M. Gutterres, J. Environ. Manage., 2021, 294, 113003 CrossRef CAS.
- W. F. Fuck, A. Brandelli and M. Gutterres, J. Am. Leather Chem. Assoc., 2018, 113, 299–310 CAS.
- Grand View Research, Dyes & Pigments Market Size, Share & Trends Analysis Report, 2021–2028, https://www.grandviewresearch.com/industry-analysis/dyes-and-pigments-market, (accessed 8.4.2021).
- European Commission, Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions, 2019, COM(2019) 640 final.
- European Commission, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, 2020, COM/2020/667 final.
- Greenpeace International, Dirty Laundry - Unravelling the corporate connections to toxic water pollution in China, 2012 Search PubMed.
- (a) R. Maas and S. Chaudhari, Process Biochem., 2005, 40, 699–705 CrossRef CAS; (b) F. Sosath, Biologisch-chemische Behandlung von Abwässern der Textilveredelung mit Reaktivfarbstoffen, VDI-Verlag, Düsseldorf, 1999 Search PubMed.
- R. Javaid and U. Y. Qazi, Int. J. Environ. Res. Public Health, 2019, 16, 2066–2092 CrossRef CAS PubMed.
- S. Varjani, P. Rakholiya, H. Y. Ng, S. You and J. A. Teixeira, Bioresour. Technol., 2020, 314, 123728–123735 CrossRef CAS PubMed.
- M. Rock, in Organising Labour in Globalising Asia, ed. A. Brown and J. Hutchison, Taylor & Francis, London, 1st edn, 2001, ch. 2, pp. 28–49 Search PubMed.
- L. G. Gallagher, W. Li, R. M. Ray, M. E. Romano, K. J. Wernli, D. L. Gao, D. B. Thomas and H. Checkoway, Am. J. Ind. Med., 2015, 58, 267–275 CrossRef PubMed.
- Z. Singh and P. Chadha, J. Occup. Med. Toxicol., 2016, 11, 39–44 CrossRef.
- E. Helaskoski, H. Suojalehto, H. Virtanen, L. Airaksinen, O. Kuuliala, K. Aalto-Korte and M. Pesonen, Ann. Allergy Asthma Immunol., 2014, 112, 46–52 CrossRef CAS.
- Y. Yan, C. Wang, D. Ding, Y. Zhang, G. Wu, L. Wang, X. Liu, C. Du, Y. Zhang and C. Zhao, Acta Ecol. Sin., 2016, 36, 119–125 CrossRef.
- B. Davient, J. P. Z. Ng, Q. Xiao, L. Li and L. Yang, Front. Oncol., 2018, 8, 573–585 CrossRef.
- M. M. Anwar, M. Shalaby, A. M. Embaby, H. Saeed, M. M. Agwa and A. Hussein, Sci. Rep., 2020, 10, 14706–14720 CrossRef CAS.
- N. Poulose, A. Sajayan, A. Ravindran, T. V. Sreechithra, V. Vardhan, J. Selvin and G. S. Kiran, J. Photochem. Photobiol., B, 2020, 205, 111816–111824 CrossRef CAS.
- P. Derikvand, C. A. Llewellyn and S. Purton, Eur. J. Phycol., 2016, 52, 43–56 CrossRef.
- (a) W. Sajjad, G. Din, M. Rafiq, A. Iqbal, S. Khan, S. Zada, B. Ali and S. Kang, Extremophiles, 2020, 24, 447–473 CrossRef PubMed; (b) A. Nawaz, R. Chaudhary, Z. Shah, L. Dufosse, M. Fouillaud, H. Mukhtar and I. U. Haq, Microorganisms, 2020, 9, 11–35 CrossRef.
- E. de Jong and G. Jungmeier, in Industrial Biorefineries & White Biotechnology, 2015, pp. 3–33 Search PubMed.
- R. J. Chudgar, J. Oakes and U. b. Staff, in Kirk–Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, 2014, pp. 1–81 Search PubMed.
- (a) D. M. Lewis, in Handbook of Textile and Industrial Dyeing, ed. M. Clark, Woodhead Publishing, 2011, pp. 303–364 Search PubMed; (b) K. Hunger, Industrial Dyes - Chemistry, Properties, Applications, WILEY-VCH Verlag GmbH, 2003 Search PubMed.
- K. Grychtol and W. Mennicke, in Ullmann's Encyclopedia of Industrial Chemistry, 2000 Search PubMed.
- S. Benkhaya, S. M'Rabet and A. El Harfi, Heliyon, 2020, 6, 1–26 CrossRef PubMed.
- K. Hunger, P. Gregory, P. Miederer, H. Berneth, C. Heid and W. Mennicke, in Industrial Dyes, ed. K. Hunger, Wiley VCH, 1st edn, 2002, ch. 2, pp. 13–112 Search PubMed.
- H.-S. Bien, J. Stawitz and K. Wunderlich, in Ullmann's Encyclopedia of Industrial Chemistry, 2000, pp. 514–578 Search PubMed.
- S. L. Greene, in Novel Psychoactive Substances, ed. P. I. Dargan and D. M. Wood, Academic Press, Boston, 1st edn, 2013, pp. 383–392 Search PubMed.
- A. Gürses, M. Açıkyıldız, K. Güneş and M. S. Gürses, in Dyes and Pigments, ed. A. Gürses, M. Açıkyıldız, K. Güneş and M. S. Gürses, Springer International Publishing, Cham, 2016, pp. 31–45 Search PubMed.
- (a) K. Hunger, R. Hamprecht, P. Miederer, C. Heid, A. Engel, K. Kunde, W. Mennicke and J. Griffiths, in Industrial Dyes, ed. K. Hunger, Wiley VCH, 1st edn, 2002, ch. 3, pp. 113–338 CrossRef; (b) K. M. Joo, S. Kim, Y. J. Koo, M. Lee, S. H. Lee, D. Choi and K. M. Lim, Toxicol. In Vitro, 2019, 60, 412–419 CrossRef CAS; (c) C. G. Hızlıateş, J. Heterocycl. Chem., 2019, 56, 2017–2026 CrossRef.
- G. Löbbert, in Ullmann's Encyclopedia of Industrial Chemistry, 2000, pp. 181–213 Search PubMed.
- S. Zhang, H. Tappe, W. Helmling, P. Mischke, K. Rebsamen, U. Reiher, W. Russ, L. Schläfer and P. Vermehren, in Ullmann's Encyclopedia of Industrial Chemistry, 2000, pp. 1–20 Search PubMed.
- (a) P. M. Burnham, M. J. Cook, L. A. Gerrard, M. J. Heeney and D. L. Hughes, Chem. Commun., 2003, 2064–2065 RSC; (b) F. Alkorbi, A. Diaz-Moscoso, J. Gretton, I. Chambrier, G. J. Tizzard, S. J. Coles, D. L. Hughes and A. N. Cammidge, Angew. Chem., Int. Ed., 2021, 60, 7632–7636 CrossRef CAS PubMed.
- (a) A. G. Newsome, C. A. Culver and R. B. van Breemen, J. Agric. Food Chem., 2014, 62, 6498–6511 CrossRef CAS PubMed; (b) J. Lebeau, M. Venkatachalam, M. Fouillaud, T. Petit, F. Vinale, L. Dufosse and Y. Caro, J. Fungi, 2017, 3, 34–54 CrossRef; (c) A. C. Lagashetti, L. Dufosse, S. K. Singh and P. N. Singh, Microorganisms, 2019, 7, 604–639 CrossRef CAS.
- (a) J. Alcaino, M. Baeza and V. Cifuentes, Subcell. Biochem., 2016, 79, 3–33 CAS; (b) N. Fang, C. Wang, X. Liu, X. Zhao, Y. Liu, X. Liu, Y. Du, Z. Zhang and H. Zhang, Trends Food Sci. Technol., 2019, 92, 162–171 CrossRef CAS.
- R. Alvarez, B. Vaz, H. Gronemeyer and A. R. de Lera, Chem. Rev., 2014, 114, 1–125 CrossRef CAS PubMed.
- T. O. Leino, A. Turku, J. Yli-Kauhaluoma, J. P. Kukkonen, H. Xhaard and E. A. A. Wallen, Eur. J. Med. Chem., 2018, 157, 88–100 CrossRef CAS.
- D. De Santis, M. Moresi, A. M. Gallo and M. Petruccioli, J. Chem. Technol. Biotechnol., 2005, 80, 1072–1079 CrossRef CAS.
- S. A. Mapari, U. Thrane and A. S. Meyer, Trends Biotechnol., 2010, 28, 300–307 CrossRef CAS.
- L. Morales-Oyervides, J. P. Ruiz-Sanchez, J. C. Oliveira, M. J. Sousa-Gallagher, A. Mendez-Zavala, D. Giuffrida, L. Dufosse and J. Montanez, Biotechnol. Adv., 2020, 43, 107601–107623 CrossRef CAS PubMed.
- (a) D. Sharma, C. Gupta, S. Aggarwal and N. Nagpal, Indian J. Fibre Text. Res., 2012, 37, 68–73 CAS; (b) S. Devi and P. Karuppan, Indian J. Fibre Text. Res., 2015, 40, 315–319 Search PubMed.
- P. Velmurugan, M.-J. Kim, J.-S. Park, K. Karthikeyan, P. Lakshmanaperumalsamy, K.-J. Lee, Y.-J. Park and B.-T. Oh, Fibers Polym., 2010, 11, 598–605 CrossRef CAS.
- Y. Jahng, Arch. Pharm. Sci. Res., 2013, 36, 517–535 CrossRef CAS PubMed.
- R. Kaur, S. K. Manjal, R. K. Rawal and K. Kumar, Bioorg. Med. Chem., 2017, 25, 4533–4552 CrossRef CAS.
- X. Han, W. Wang and X. Xiao, Chin. J. Biotechnol., 2008, 24, 921–926 CrossRef CAS.
- A. N. Fabara and M. W. Fraaije, Appl. Microbiol. Biotechnol., 2020, 104, 925–933 CrossRef CAS PubMed.
- N. Duran, G. Z. Justo, M. Duran, M. Brocchi, L. Cordi, L. Tasic, G. R. Castro and G. Nakazato, Biotechnol. Adv., 2016, 34, 1030–1045 CrossRef CAS PubMed.
- M. Kanelli, M. Mandic, M. Kalakona, S. Vasilakos, D. Kekos, J. Nikodinovic-Runic and E. Topakas, Front. Microbiol., 2018, 9, 1495–1507 CrossRef PubMed.
- S. Y. Ahn, S. Jang, P. Sudheer and K. Y. Choi, Int. J. Mol. Sci., 2021, 22, 2413–2426 CrossRef CAS.
- N. R. Williamson, P. C. Fineran, F. J. Leeper and G. P. Salmond, Nat. Rev. Microbiol., 2006, 4, 887–899 CrossRef CAS PubMed.
- A. Furstner, Angew. Chem., Int. Ed., 2003, 42, 3582–3603 CrossRef.
- (a) Y. Ren, J. Gong, R. Fu, J. Zhang, K. Fang and X. Liu, J. Cleaner Prod., 2018, 201, 648–656 CrossRef CAS; (b) Y. Ren, R. Fu, K. Fang, R. Xie, L. Hao, W. Chen and Z. Shi, J. Cleaner Prod., 2021, 281, 125295–125303 CrossRef CAS.
- J. W. Bennett and R. Bentley, Adv. Appl. Microbiol., 2000, 47, 1–32 CAS.
- C. Cheng, E. M. Othman, A. Fekete, M. Krischke, H. Stopper, R. Edrada-Ebel, M. J. Mueller, U. Hentschel and U. R. Abdelmohsen, Tetrahedron Lett., 2016, 57, 4196–4199 CrossRef CAS.
- R. Saranya, J. Jayapriya and A. Tamilselvi, Color. Technol., 2012, 128, 440–445 CAS.
- Y. Yan, J. Yang, Z. Yu, M. Yu, Y. T. Ma, L. Wang, C. Su, J. Luo, G. P. Horsman and S. X. Huang, Nat. Commun., 2016, 7, 13083–13092 CrossRef CAS PubMed.
- P. A. Day, M. S. Villalba, O. M. Herrero, L. A. Arancibia and H. M. Alvarez, Antonie Van Leeuwenhoek, 2017, 110, 415–428 CrossRef CAS.
- M. R. Ghiffary, C. P. S. Prabowo, K. Sharma, Y. Yan, S. Y. Lee and H. U. Kim, ACS Sustainable Chem. Eng., 2021, 9, 6613–6622 CrossRef CAS.
- C. Chen, H. Tao, W. Chen, B. Yang, X. Zhou, X. Luo and Y. Liu, RSC Adv., 2020, 10, 10197–10220 RSC.
- (a) J. Avalos, J. Pardo-Medina, O. Parra-Rivero, M. Ruger-Herreros, R. Rodriguez-Ortiz, D. Hornero-Mendez and M. C. Limon, J. Fungi, 2017, 3, 39–54 CrossRef PubMed; (b) W. Chen, R. Chen, Q. Liu, Y. He, K. He, X. Ding, L. Kang, X. Guo, N. Xie, Y. Zhou, Y. Lu, R. J. Cox, I. Molnar, M. Li, Y. Shao and F. Chen, Chem. Sci., 2017, 8, 4917–4925 RSC; (c) M. Fouillaud, M. Venkatachalam, E. Girard-Valenciennes, Y. Caro and L. Dufosse, Mar. Drugs, 2016, 14, 64–127 CrossRef; (d) T. Hoshino, Appl. Microbiol. Biotechnol., 2011, 91, 1463–1475 CrossRef CAS PubMed.
- A. J. Williams, C. M. Grulke, J. Edwards, A. D. McEachran, K. Mansouri, N. C. Baker, G. Patlewicz, I. Shah, J. F. Wambaugh, R. S. Judson and A. M. Richard, J. Cheminf., 2017, 9, 61–87 Search PubMed.
- K. Blackburn and S. B. Stuard, Regul. Toxicol. Pharmacol., 2014, 68, 353–362 CrossRef PubMed.
- United Nations, Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 2017, pp. 115–126 Search PubMed.
- United Nations, Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 2017, pp. 219–242 Search PubMed.
- R. B. Chavan, in Handbook of Textile and Industrial Dyeing, ed. M. Clark, Woodhead Publishing, 2011, pp. 515–561 Search PubMed.
- C. Desai, Colourage, 1992, 39, 51–54 CAS.
- M. H. G. Berntssen, R. Hannisdal, L. Buttle, R. Hoogenveen, M. Mengelers, B. G. H. Bokkers and M. J. Zeilmaker, Food Addit. Contam., Part A, 2018, 35, 1484–1496 CrossRef CAS PubMed.
- Sigma-Aldrich, Safety Data Sheet beta-carotene, https://www.sigmaaldrich.com/AT/en/sds/sigma/c9750, (accessed 14.8.2021).
- T. Niu, J. Zhou, F. Wang, R. Xuan, J. Chen, W. Wu and H. Chen, Regul. Toxicol. Pharmacol., 2020, 115, 104695–104702 CrossRef CAS PubMed.
- (a) H. Yamasaki, S. Irino, A. Uda, K. Uchida, H. Ohno, N. Saito, K. Kondo, K. Jinzenji and T. Yamamoto, Folia Pharmacol. Jpn., 1958, 54, 362–377 CrossRef CAS; (b) H. Yamasaki, K. Kondo, T. Uda, T. Yamamoto and K. Endo, Acta Med. Okayama, 1961, 15, 347–366 CAS.
- International Agency for Research on Cancer (IARC), in IARC monographs on the evaluation of carcinogenic risks to humans, ed. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Lyon, 2008 Search PubMed.
- (a) K. Piaskowski, R. Swiderska-Dabrowska and P. K. Zarzycki, J. AOAC Int., 2018, 101, 1371–1384 CrossRef CAS PubMed; (b) S. Ledakowicz and K. Pazdzior, Molecules, 2021, 26, 870–914 CrossRef CAS.
- D. Bhatia, N. R. Sharma, J. Singh and R. S. Kanwar, Crit. Rev. Environ. Sci. Technol., 2017, 47, 1836–1876 CrossRef CAS.
- R. Sarnthima, S. Khammuang and J. Svasti, Biotechnol. Bioprocess Eng., 2009, 14, 513–522 CrossRef CAS.
- R. G. Saratale, G. D. Saratale, D. C. Kalyani, J. S. Chang and S. P. Govindwar, Bioresour. Technol., 2009, 100, 2493–2500 CrossRef CAS.
- H. Ali, Water, Air, Soil Pollut., 2010, 213, 251–273 CrossRef CAS.
- D. A. Yaseen and M. Scholz, Int. J. Environ. Sci. Technol., 2018, 16, 1193–1226 CrossRef.
- W. Hui, L. Xiaoan, W. Chenglong, Z. Mingliang, K. Zhijian, J. Wankui, Z. Yidong, Q. Jiguo and H. Qing, Sci. Total Environ., 2020, 706, 135726–135736 CrossRef.
- K. C. Costa, N. R. Glasser, S. J. Conway and D. K. Newman, Science, 2017, 355, 170–173 CrossRef CAS PubMed.
- E. Steingruber, in Ullmann's Encyclopedia of Industrial Chemistry, 2004, vol. 19, pp. 55–63 Search PubMed.
- (a) R. B. Chavan, in Denim, Elsevier Ltd, 2015, pp. 37–67 Search PubMed; (b) R. Paul, R. S. Blackburn and T. Bechtold, in Ullmann's Encyclopedia of Industrial Chemistry, 2021, pp. 1–16 Search PubMed.
- G. H. Han, H.-J. Shin and S. W. Kim, Enzyme Microb. Technol., 2008, 42, 617–623 CrossRef CAS.
- G. H. Han, S. E. Bang, B. K. Babu, M. Chang, H.-J. Shin and S. W. Kim, Process Biochem., 2011, 46, 788–791 CrossRef CAS.
- J. Lee, J. Kim, J. E. Song, W. S. Song, E. J. Kim, Y. G. Kim, H. J. Jeong, H. R. Kim, K. Y. Choi and B. G. Kim, Nat. Chem. Biol., 2020, 104–112 CAS.
- X. Pan, C. Sun, M. Tang, C. Liu, J. Zhang, J. You, T. Osire, Y. Sun, Y. Zhao, M. Xu, T. Yang and Z. Rao, Front. Bioeng. Biotechnol., 2019, 7, 367–380 CrossRef PubMed.
- (a) M. Wehrs, J. M. Gladden, Y. Liu, L. Platz, J.-P. Prahl, J. Moon, G. Papa, E. Sundstrom, G. M. Geiselman, D. Tanjore, J. D. Keasling, T. R. Pray, B. A. Simmons and A. Mukhopadhyay, Green Chem., 2019, 21, 6027–6029 RSC; (b) M. Wehrs, J. M. Gladden, Y. Liu, L. Platz, J.-P. Prahl, J. Moon, G. Papa, E. Sundstrom, G. M. Geiselman, D. Tanjore, J. D. Keasling, T. R. Pray, B. A. Simmons and A. Mukhopadhyay, Green Chem., 2019, 21, 3394–3406 RSC.
- (a) M. Ozdal, S. Gurkok, O. G. Ozdal and E. B. Kurbanoglu, Biocatal. Agric. Biotechnol., 2019, 22, 101365–101370 CrossRef; (b) K. Liu, H. Hu, W. Wang and X. Zhang, Microb. Cell Fact., 2016, 15, 131–142 CrossRef PubMed.
- F. B. Mortzfeld, J. Pietruszka and I. R. Baxendale, Eur. J. Org. Chem., 2019, 5424–5433 CrossRef CAS.
- L. Dufossé, P. Galaup, A. Yaron, S. M. Arad, P. Blanc, K. N. C. Murthy and G. A. Ravishankar, Trends Food Sci. Technol., 2005, 16, 389–406 CrossRef.
- T. A. Rodrigues, T. A. Schueler, A. J. R. d. Silva, E. F. C. Sérvulo and F. J. S. Oliveira, Braz. J. Chem. Eng., 2019, 36, 99–107 CrossRef CAS.
- F. J. Bacame-Valenzuela, J. A. Perez-Garcia, M. L. Figueroa-Magallon, F. Espejel-Ayala, L. A. Ortiz-Frade and Y. Reyes-Vidal, Microorganisms, 2020, 8, 1559–1575 CrossRef CAS PubMed.
- L. Hossain and M. S. Khan, Water, 2020, 12, 2760–2792 CrossRef CAS.
- D. Philips, J. Soc. Dyers Colour., 2008, 112, 183–186 CrossRef.
- A. Berry, T. C. Dodge, M. Pepsin and W. Weyler, J. Ind. Microbiol., 2002, 28, 127–133 CrossRef CAS PubMed.
- M. E. Pailliè-Jiménez, P. Stincone and A. Brandelli, Front. Sustain. Food Syst., 2020, 4, 590439–590446 CrossRef.
- R. Kalra, X. A. Conlan and M. Goel, Front. Chem., 2020, 8, 369–391 CrossRef CAS.
- S. Romano, S. A. Jackson, S. Patry and A. D. W. Dobson, Mar. Drugs, 2018, 16, 244–272 CrossRef PubMed.
- M. Venkatachalam, A. Shum-Cheong-Sing, L. Dufosse and M. Fouillaud, Microorganisms, 2020, 8, 711–729 CrossRef CAS PubMed.
- L. Liao, S. Su, B. Zhao, C. Fan, J. Zhang, H. Li and B. Chen, Mar. Drugs, 2019, 17, 388–402 CrossRef CAS PubMed.
- A. Casini, F. Y. Chang, R. Eluere, A. M. King, E. M. Young, Q. M. Dudley, A. Karim, K. Pratt, C. Bristol, A. Forget, A. Ghodasara, R. Warden-Rothman, R. Gan, A. Cristofaro, A. E. Borujeni, M. H. Ryu, J. Li, Y. C. Kwon, H. Wang, E. Tatsis, C. Rodriguez-Lopez, S. O'Connor, M. H. Medema, M. A. Fischbach, M. C. Jewett, C. Voigt and D. B. Gordon, J. Am. Chem. Soc., 2018, 140, 4302–4316 CrossRef CAS PubMed.
- A. Domrose, A. S. Klein, J. Hage-Hulsmann, S. Thies, V. Svensson, T. Classen, J. Pietruszka, K. E. Jaeger, T. Drepper and A. Loeschcke, Front. Microbiol., 2015, 6, 972–981 Search PubMed.
- Archroma, Archroma Colourfinder, https://colorfinder.archroma.com/, (accessed 14.8.2021).
- R. J. M. Goss, S. Shankar and A. A. Fayad, Nat. Prod. Rep., 2012, 29, 870–889 RSC.
- B. Ruan, K. Pong, F. Jow, M. Bowlby, R. A. Crozier, D. Liu, S. Liang, Y. Chen, M. L. Mercado, X. Feng, F. Bennett, D. von Schack, L. McDonald, M. M. Zaleska, A. Wood, P. H. Reinhart, R. L. Magolda, J. Skotnicki, M. N. Pangalos, F. E. Koehn, G. T. Carter, M. Abou-Gharbia and E. I. Graziani, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 33–38 CrossRef CAS.
- Z. L. Reitz and A. Butler, Chem. Commun., 2020, 56, 12222–12225 RSC.
- A. S. Klein, A. Domrose, P. Bongen, H. U. C. Brass, T. Classen, A. Loeschcke, T. Drepper, L. Laraia, S. Sievers, K. E. Jaeger and J. Pietruszka, ACS Synth. Biol., 2017, 6, 1757–1765 CrossRef CAS PubMed.
- M. Cummings, A. D. Peters, G. F. S. Whitehead, B. R. K. Menon, J. Micklefield, S. J. Webb and E. Takano, PLoS Biol., 2019, 17, 1–34 CrossRef.
- C. J. Magnus, P. H. Lee, J. Bonaventura, R. Zemla, J. L. Gomez, M. H. Ramirez, X. Hu, A. Galvan, J. Basu, M. Michaelides and S. M. Sternson, Science, 2019, 364, 1–8 CrossRef.
- K. Shinoda, T. Hasegawa, H. Sato, M. Shinozaki, H. Kuramoto, Y. Takamiya, T. Sato, N. Nikaidou, T. Watanabe and T. Hoshino, Chem. Commun., 2007, 4140–4142 RSC.
- P. X. Jiang, H. S. Wang, S. Xiao, M. Y. Fang, R. P. Zhang, S. Y. He, K. Lou and X. H. Xing, Appl. Microbiol. Biotechnol., 2012, 94, 1521–1532 CrossRef CAS PubMed.
- S. R. Chawrai, N. R. Williamson, T. Mahendiran, G. P. C. Salmond and F. J. Leeper, Chem. Sci., 2012, 3, 447–454 RSC.
- A. Collis and J. Wilson, JAIC, 2012, 9, 20–31 Search PubMed.
- R. Räisänen, Color. Technol., 2019, 135, 22–31 Search PubMed.
- B. Yan, M. Yang, Q. Zhou, T. Xing, G. Chen and J. Sheng, Color. Technol., 2019, 135, 267–274 CAS.
- J. T. Dafoe and A. J. Daugulis, Biotechnol. Lett., 2014, 36, 443–460 CrossRef CAS PubMed.
- C. S. Bailey, J. S. Zarins-Tutt, M. Agbo, H. Gao, A. Diego-Taboada, M. L. Gan, R. B. Hamed, E. R. Abraham, G. Mackenzie, P. A. Evans and R. J. M. Goss, Chem. Sci., 2019, 10, 7549–7553 RSC.
- G. Hussain, N. Abass, G. Shabir, M. Athar, A. Saeed, R. Saleem, F. Ali and M. A. Khan, J. Appl. Res. Technol., 2017, 15, 346–355 CrossRef.
- L. Xia, A. Wang, Y. Wang, C. Zhang, Y. Wang, S. Zhou, Z. Fu, H. Zhao, C. Ding and W. Xu, Green Chem., 2021, 23, 796–807 RSC.
- T. M. Hsu, D. H. Welner, Z. N. Russ, B. Cervantes, R. L. Prathuri, P. D. Adams and J. E. Dueber, Nat. Chem. Biol., 2018, 14, 256–261 CrossRef CAS.
- F. Alihosseini, Plant-based compounds for antimicrobial textiles, Antimicrobial Textiles, ed. Gang Sun, Woodhead Publishing, 2016, pp. 155–195 Search PubMed.
- F. A. Andersen, Int. J. Toxicol., 1999, 18, 27–32 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc02968a |
| This journal is © The Royal Society of Chemistry 2022 |