 Open Access Article
Open Access ArticleEffect of solute polarity on extraction efficiency using deep eutectic solvents†
Odeh A. O.
Alshammari
,
Ghazi A. A.
Almulgabsagher
,
Karl S.
Ryder
 and
Andrew P.
Abbott
and
Andrew P.
Abbott
 *
*
School of Chemistry, University of Leicester, Leicester, LE1 7RH, UK. E-mail: apa1@le.ac.uk
First published on 21st June 2021
Abstract
While ionic liquids and deep eutectic solvents, DESs, have been extensively used for natural product extraction relatively little is known about the factors affecting extraction efficiency. In this study, 7 simple solutes are extracted into 4 DESs at two temperatures and the thermodynamics of phase transfer are determined. It was found that solutes which are able to form hydrogen bond are more successfully extracted into the DES phase from cyclohexane. For less polar solutes, the extracting DES has a more pronounced effect on extraction efficiency with liquids of a lower surface tension being more effective. With polar solutes the effect of the DES is less pronounced. It is shown that the Gibbs energy of extraction is proportional to the pKa of the solute demonstrating the importance of hydrogen bonding in solute partition. The study was extended to 5 phenolic compounds commonly found in olive oil and again the extraction efficiency was shown to be related to the solute pKa. The green metrics for the extraction of a range of solutes were determined and shown in some cases to be superior to molecular solvents. The energy consumption of extraction was shown to be comparatively small even when mechanically assisted.
1 Introduction
The 2019 the natural product extraction market was estimated to be approximately $23.7 bn and projected to be worth $59.4 bn by 2025.1 This has been driven partly by the move away from synthetic flavours and fragrances towards plant extracts particularly for the food, drink and cosmetic industries. A small but nevertheless important sector is the extraction of antioxidants and most importantly phenolic derivatives which was estimated to be approximately $1.3 bn in 2018 with an 7.2% expected annual growth from 2019–2025.2 There are a range of natural products which contain phenolic antioxidants such as fruits, vegetables, herbs and spices. In plants they fulfil the important role of protection against ozone and ultraviolet damage.3–5 Many natural products are extracted using organic solvents, particularly alcohols. While the move to less toxic solvents is important, there are still concerns over the energy requirements due to the need for multiple extractions and solvent evaporation.The amphiphilic nature of ionic liquids makes them good candidates for the extraction of a range of materials ranging from minerals to natural products. Several groups have demonstrated the use of ionic liquids for extraction of a wide range of solutes but issues such as toxicity, cost and low vapour pressure make solute separation difficult.6 It is also not particularly suitable for natural product extraction due to the need to ensure no ionic liquid residues are left in the extract. Several groups have therefore extended these ideas to deep eutectic solvents which are mixtures of quaternary ammonium salts with hydrogen bond donors.7 These have similar tunability of polarity but cost and toxicity are less of an issue even though they still have similar issues of low vapour pressure. The use of DESs for natural product extraction has recently been reviewed.8–10 Recent work by a French company Naturex uses a process called Eutectys™, where the DES is used as a carrier of the extract in the final product so benign DESs have been developed for this purpose.11 One example of a relatively benign DES is an equimolar mixture of betaine and lactic acid. The company has used this approach to create a range of plant extracts with antioxidant properties and they showed that the extracts actually have a greater efficacy in the DES than from an alcoholic solution.
While numerous groups have studied extraction, relatively few have attempted to understand the factors affecting product partition. A recent study was carried out to test whether DESs would be suitable for extraction of sulfur containing compounds from mineral oils for the specific application of desulfurisation of diesel fuel.12 The thermodynamics of extraction were determined for the first time and it was shown that the partition of thiophene had a small positive Gibbs energy and this was found to be due to the endothermic enthalpy of transfer. By measuring the partition coefficient in a variety of DESs it was found that this was controlled mostly by the enthalpy of cavity formation. The entropy of transfer into the DES was positive showing that it became less structured because it was breaking up the hydrogen bonding network of the DES.12 The partitioning of thiophene into DESs followed the trend in surface tension which is the energy required to break the molecules in the liquid apart. This is related to the energy required to make a cavity in the liquid. Liquid–liquid extraction requires a cavity to be made in the receiving liquid and the energy of this cavity formation has to be smaller than the enthalpy of solvation of the solute for spontaneous extraction to occur.
In the current study, the thermodynamics for solute extraction of a range of compounds from alkanes is studied. The polarity of 7 similarly sized solutes with significantly different polarities was compared. The study was then extended to 5 antioxidant solutes commonly extracted from olive oil to see if the same trends occurred. Finally these data were compared to a variety of literature studies and the Green Chemistry metrics were applied to determine whether the use of DESs could be comparable with standard molecular extraction solvents.
2. Experimental
All materials and reagents employed in this work were used as received and their sources and purities are listed in Table 1.| Compound | Source and purity |
|---|---|
| Choline chloride | Sigma-Aldrich ≥99% |
| Ethylene glycol (EG) | Sigma-Aldrich ≥99% |
| Butanoic acid | Sigma-Aldrich ≥99% |
| Pentanol | Sigma-Aldrich ≥99% |
| Ethyl acetate | Sigma-Aldrich ≥99% |
| 2-Pentanone | Sigma-Aldrich ≥99% |
| Cyclohexane | Sigma-Aldrich ≥99% |
| Urea | Sigma-Aldrich ≥99% |
| Phenol | Sigma-Aldrich ≥99% |
| Benzylalcohol | Alfa Aesar 99% |
| Cyclohexanol | Fisher chemical >98% |
| Oxalic acid dihydrate | Fisher >99% |
| Glycerol | Fisher >99% |
| Tyrosol | Alfa Aesar, 98% |
| Vanillic acid | Alfa Aesar 98% |
| Ferulic acid | Acros organics 99% |
| p-Coumaric acid | Sigma-Aldrich ≥98% |
| Apigenin | Sigma-Aldrich 97% |
| Triolein | Sigma-Aldrich >65% |
The deep eutectic solvents were prepared using similar approaches described by literature methods.13–15 The hydrogen bond donor (HBD) and salt were mixed in the given molar ratio Oxaline (1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 oxalic acid dehydrate), Reline (1 ChCl
1 oxalic acid dehydrate), Reline (1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 urea), Ethaline (1 ChCl
2 urea), Ethaline (1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 EG) and Glyceline (1 ChCl
2 EG) and Glyceline (1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glycerol). The mixture was stirring in a flask and placed on a hot plate, magnetic stirrer at ca. 80 °C and 500 rpm for at least 3 hours until a colourless homogeneous liquid was formed. The liquids were all stored in an oven at 50 °C.
2 glycerol). The mixture was stirring in a flask and placed on a hot plate, magnetic stirrer at ca. 80 °C and 500 rpm for at least 3 hours until a colourless homogeneous liquid was formed. The liquids were all stored in an oven at 50 °C.
Solutions of butanoic acid (0.12 mol kg−1 in 2.0 g cyclohexane), 1-pentanol (0.14 mol kg−1 in 2.0 g cyclohexane), 2-pentanone (0.04 mol kg−1 in 2.0 g cyclohexane), ethyl acetate (0.03 mol kg−1 in 2.0 g cyclohexane), phenol (0.02 mol kg−1 in 2.0 g cyclohexane) benzyl alcohol (0.03 mol kg−1 in 2.0 g cyclohexane) and cyclohexanol (0.04 mol kg−1 in 2.0 g cyclohexane) were prepared, then extracted at two different temperatures (25 and 40 °C) with 2.0 g of DES, at a stirring rate of 500 rpm for 120 min. The concentration of these solutes were determined using GC-FID with a fused silica capillary column (PE Elite-5, 29.45 m long, 0.25 mm in diameter) connected to gas chromatograph (PerkinElmer Autosystem XL) using the Totalchrom software. The operational temperature of the FID was 320 °C, and that of the injector was 310 °C. For the first three minutes, the temperature of column was set at 50 °C, increased to 300 °C at a rate of 15 °C min−1, kept at 300 C for 2 min. Helium was the carrier gas at a flow rate of 1 ml min−1. See ESI† for calibration curves. The concentrations of phenolic compounds tyrosol, vanillic acid, ferulic acid, p-coumaric acid and apigenin were 4.16 × 10−5, 3.57 × 10−5, 3.88 × 10−5, 3.84 × 10−5 and 3.00 × 10−5 mol kg−1 in 2.0 g triolein respectively. The phenolic compounds were then transferred to the DESs following the similar approach above mentioned. Concentrations for these solutes were determined using UV–vis spectroscopy using a Mettler Toledo UV5Bio UV/visible scan spectrophotometer with the cell path length was 10mm.
3 Results and discussion
The four aliphatic solutes chosen were butanoic acid, 1-pentanol, ethyl acetate and 2-pentanone as they all have approximately the same size but clearly the first two are strong hydrogen bond donors whereas the last two are significantly weaker. These were compared with three cyclic alcohols, phenol, benzyl alcohol and cyclohexanol which also have similar sizes but different pKa values. These groups are commonly found in a variety of bioactive compounds extracted from plants.16–18 The partition coefficients of these solutes into four DESs; Ethaline, Glyceline, Oxaline and Reline were determined. The 4 DESs possess different physical properties especially viscosity, surface tension and polarity, which influence the extraction efficiency. The physical properties of the DESs vary according to their composition (HBA and HBD) and the values are given in the literature.12The extraction efficiencies of these 7 solutes were measured at two temperatures and the data are presented in Table 2 in terms of partition coefficients (Kp) and in Table S2† (ESI†) in terms of extraction efficiency with errors analysis.
| DES | K p at | ΔG (kJ mol−1) at | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) at 25 °C | ||
|---|---|---|---|---|---|---|
| 25 °C | 40 °C | 25 °C | 40 °C | |||
| 1-Pentanol pK a = 16.8 RMM = 88.15 g mol −1 | ||||||
| Ethaline | 1.22 | 0.95 | −0.50 | +0.11 | −12.71 | −44.41 |
| Oxaline | 0.71 | 0.80 | +0.82 | +0.57 | +5.95 | +17.20 |
| Glyceline | 0.43 | 0.38 | +2.04 | +2.50 | −7.28 | −31.28 |
| Reline | 0.31 | 0.27 | +2.84 | +3.37 | −7.57 | −34.97 |
| 2-Pentanone pK a = 19.6 RMM = 86.13 g mol −1 | ||||||
| Ethaline | 0.02 | 0.04 | +9.44 | +8.41 | +30.01 | +69.01 |
| Oxaline | 0.17 | 0.20 | +4.38 | +4.08 | +9.66 | +17.83 |
| Glyceline | 0.14 | 0.20 | +4.71 | +4.11 | +16.71 | +40.27 |
| Reline | 0.12 | 0.23 | +5.07 | +3.80 | +30.47 | +85.21 |
| Ethyl acetate pK a = 25 RMM = 88.11 g mol −1 | ||||||
| Ethaline | 0.10 | 0.20 | +5.50 | +4.03 | +38.40 | +110.30 |
| Oxaline | 0.16 | 0.22 | +4.51 | +3.86 | +17.40 | +43.25 |
| Glyceline | 0.09 | 0.18 | +5.86 | +4.40 | +34.93 | +97.54 |
| Reline | 0.10 | 0.22 | +5.62 | +3.91 | +39.58 | +113.94 |
| Phenol pK a = 10 RMM = 94.11 g mol −1 | ||||||
| Ethaline | — | — | — | — | — | — |
| Glyceline | 15.9 | 15 | −6.85 | −7.04 | −3.01 | +12.88 |
| Benzyl alcohol pK a = 15.4 RMM = 108.14 g mol −1 | ||||||
| Ethaline | 6.47 | 6.12 | −4.62 | −4.71 | −2.87 | +5.87 |
| Glyceline | 5.36 | 4.75 | −4.15 | −4.05 | −6.24 | −7.01 |
| Cyclohexanol pK a = 16 RMM = 100.16 g mol −1 | ||||||
| Ethaline | 4.53 | 3.86 | −3.74 | −3.51 | −8.27 | −15.20 |
| Glyceline | 2.51 | 2.09 | −2.28 | −1.91 | −9.46 | −24.09 |
| Thiophene pK a = 33 RMM = 84.14 g mol −1 | ||||||
| Ethaline | 0.3 | 0.35 | +3 | +2.6 | +13.2 | +34 |
| Oxaline | 0.1 | 0.16 | +5.6 | +4.8 | +31.2 | +85.9 |
| Glyceline | 0.09 | 0.14 | +6 | +5 | +35 | +97.4 |
| Reline | 0.06 | 0.14 | +7.2 | +5.04 | +70.8 | +213.5 |
3.1 Thermodynamics and partition coefficient determination
The first part of the study investigated the effect of the functional group type on the thermodynamics of solute extraction. The partition coefficient of the compound between the DESs and the organic phases was studied as a function of temperature and Kp was determined at 298 and 313 K: | (1) |
The thermodynamic parameters were then determined using the following equations:19
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp Kp | (2) |
 | (3) |
| ΔS = (ΔH − ΔG)/T | (4) |
where, R is the gas constant and T is the absolute temperature.
To transfer a solute from one phase to another, there are a variety of enthalpic and entropic terms to be considered. The enthalpic term will be made up of the energy required to create a cavity (hole) in the accepting solvent, which will be endothermic due to the need to break up the strong solvent–solvent interactions. When the solute is solvated the enthalpy of solvation is usually exothermic as the solute interacts with the solvent, in the case of DESs usually through hydrogen bonding. The enthalpy of transfer will depend upon the relative solvent–solvent–solute interactions. The entropic term will mainly depend on how the solute breaks up the structure of the DES.
The transfer of butanoic acid from cyclohexane to the DES phase shows no signs of butanoic acid remaining in the cyclohexane phase after extraction in any of the DESs tested. This is not surprising as butanoic acid is a stronger hydrogen bond donor than ethylene glycol, urea or glycerol and is similar to oxalic acid. The butanoic acid will therefore partition to the DES presumably because it has a strong interaction with the chloride anion of the DESs. The experiment was repeated with Ethaline containing different ratios of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG; 1 ChCl
EG; 1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EG, 1 ChCl
3 EG, 1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 EG and 1 ChCl
4 EG and 1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 EG and in each case all the butanoic acid was extracted into the DES showing that hydrogen bonding dominates extraction.
5 EG and in each case all the butanoic acid was extracted into the DES showing that hydrogen bonding dominates extraction.
Table 2 shows the thermodynamic data for the partitioning of pentanol in the 4 DESs. It can be seen that the partition coefficients are slightly larger than those previously observed for thiophene12 which is not surprising as pentanol is a better hydrogen bond donor. For Ethaline, Glyceline and Reline the enthalpy of transfer is slightly exothermic whereas for Oxaline it is endothermic. This is logical as pentanol will have similar interactions with chloride as glycerol, urea and ethylene glycol. Oxalic acid will have larger interactions with chloride than pentanol so the enthalpy of mixing is understandably endothermic.
ΔG is negative for extracting pentanol of using Ethaline as an extractor solvent, showing that the process is spontaneous, so the equilibrium mostly lies towards the solute being in the DES layer. The process is however nonspontaneous using Oxaline, Reline and Glyceline as the extraction liquid (Table 2). It can be concluded that for Oxaline hole-formation dominates and partition is driven by the increase in entropy by disrupting the structure of the DES whereas for the other DESs it is the enthalpy of solvation which dominates, driven by the formation of hydrogen bonds as the entropy change is negative (the system becomes more ordered).
The thermodynamics of pentanol extraction are only slightly affected by the type of DES, unlike the extraction of thiophene previously reported.12 This shows that the contribution of hole formation is small compared to the enthalpy of solvation for more polar solutes which is dominated by hydrogen bonding. This is an important conclusion as it shows that the DES type is important for non-polar solutes but less important for polar solutes.
Table 2 also shows the thermodynamic data for the partitioning of 2-pentanone and ethyl acetate separately in the 4 DESs. The data confirm that the transfer of both solutes from cyclohexane to the DESs is controlled by the enthalpy of solvation. The process is nonspontaneous with the solute being predominantly in the alkane layer. This is not surprising as the solutes are poor hydrogen bond donors. The data also show that the change in entropy of partition to the DESs is positive which would also seem logical as both solutes disrupt the structure of the ordered DES, which emphasise that it is the formation of voids that limits partition and it is the increase in entropy that drives partition. This also supports the above conclusion about the importance of solvent polarity when extracting non-polar solutes.
Given that the above data show the extraction efficiency depends on the polarity of the functional group, it was decided to fine-tune the polarity of solutes with an OH moiety to see how the acidity of the proton affected the partition coefficient. To this end the extraction of phenol, benzyl alcohol and cyclohexanol into Ethaline and Glyceline were studied. These solutes are clearly able to form a hydrogen bond with the chloride of the DES which is expected to be larger than that between the chloride and the HBD (ethylene glycol or glycerol) of the DES. The acidity of the proton can be determined from the pKa of the solute and these are listed in Table 2.
For phenol, GC-FID showed that > 99% of the solute was extracted by Ethaline at both temperatures. In Glyceline which has higher viscosity, surface tension and lower hole radius than Ethaline, the percentage extracted was also high but slightly lower than with Ethaline (Table 2). Benzyl alcohol extracted well into both Ethaline and Glyceline however not as well as phenol. This supports the hypothesis that the hydrogen bonding ability of the solute is important in its ability to be extracted. The larger pKa of benzyl alcohol compared to phenol (15.4 vs. 10.0) confirms that it should be a less strong hydrogen bond donor. Table 2 showed that cyclohexanol was again extracted well by both DESs but again less than phenol and benzyl alcohol which fits with its lower pKa of 16.0.
The thermodynamic data in Table 2 confirm that the extraction process for all –OH containing solutes is spontaneous as the value of ΔG < 0 at both temperatures. Therefore, the equilibrium lies towards the solutes being in the DES phase. The data also show that the enthalpy of transfer cannot be calculated for phenol using Ethaline which extensively extracted the solute however, it is fair to assume that it is exothermic. The same results in terms of spontaneity for phenol can be also seen for both benzyl alcohol and cyclohexanol. Interestingly the enthalpy of transfer to the DES is still endothermic showing that entropy is still driving the transfer of all of these cyclic alcohols to the polar DES phase.
These data show categorically, for the first time, that for a given DES, the partition coefficient of the solute can be related to the polarity of the solute. Non-polar solutes are driven by entropy change and polar solutes are driven by enthalpy changes i.e. their ability to hydrogen bond to the DES suggesting that it is the chloride which is pulling the solute into the DES.
As a crude assumption, the ability of a solute to hydrogen bond with a solvent could be predicted from the pKa of the solute. Fig. 1 shows the Gibbs Energy of transfer for the solutes listed in Table 2 from cyclohexane into Ethaline (black squares) as a function of solute pKa. It can be seen that there is a relatively good correlation between these two parameters showing that for polar solutes it is the hydrogen bonding ability of the solute which controls the partitioning into the DES. Clearly the polarisability of the solute will also be important but these can only be obtained semi-empirically and can only be applied to liquid solutes. This can be seen for the three cyclic alcohols in Fig. 1 which have negative Gibbs Energy of transfer which will be more polarisable and deviate from the more linear correlation of the other solutes.
 | ||
| Fig. 1 Plot of ΔG values as a function of pKa for the solutes listed in Tables 2 and 3 solutes from cyclohexane (black squares) and Triolein (red circles) to Ethaline. Data: 1 Phenol, 2 Benzyl alcohol, 3 Cyclohexanol, 4 1-Pentanol, 5 2-Pentanone, 6 Ethyl acetate, 7 Benzothiophene, 8 Thiophene, 9 Tyrosol, 10 p-coumaric, 11 Vanilic, 12 Ferulic, 13 Apigenin. | ||
3.2 Natural product extraction
Phenolic compound extraction from natural products is an important area as these compounds are commonly used as antioxidants. Numerous methods are used to extract phenolic compounds from plant oils including solvent extraction and chromatography. The solvent and energy considerations will depend on which product stream is used for the extraction.20 DESs have previously been used for the extraction of phenolic compounds from olive oil.21 These studies are difficult to compare quantitatively due to the variability of the active ingredients in samples so for the purposes of this study a synthetic olive oil analogue was made containing five phenolic compounds; tyrosol, p-coumaric acid, vanilic acid, ferulic acid and apigenin in triolein, a synthetic triglyceride (2,3-bis[[(Z)-octadec-9-enoyl]oxy]propyl (Z)-octadec-9-enoate). These solutes were chosen due to their importance as antioxidants but also their ease of quantification using UV–vis spectroscopy. The structures molecular weights, pKa and melting points of the solutes are shown in Fig. 2.The aim was to assess the extraction efficiency of those phenolic compounds using Ethaline and comparing the method and system used here to other studies. The thermodynamics for the extraction of the phenolic compounds from the oil phase was also studied. From the data in Fig. 2 it would be expected that the solutes with carboxylic acid functionalities and therefore the lower pKas would be preferentially extracted.
The thermodynamics of phase transfer for the extraction of the phenolic compounds listed in Fig. 2 from triolein to Ethaline were calculated at two temperatures. The data in Table 3 shows that all solutes partition more favourably in the Ethaline layer than the oil phase. Table 3 shows that good extraction yield was obtained for all phenolic compounds, but the order of extraction efficiency was vanilic acid > p-coumaric acid > ferulic acid > tyrosol > apigenin. All of the solutes in Fig. 2 have a lower pKa than ethylene glycol and so it would be expected that all of them should form hydrogen bonds more readily with the chloride of the DES than ethylene glycol. The high extraction efficiency is not surprising as the phenolic acids have two types of HBD functionalities namely carboxylic acid and hydroxyl groups. It is however noted that despite apigenin having more hydroxyl groups than tyrosol, the former was transferred with lower extraction efficiency but this can be accounted for in terms of the differences in their molecular weights and melting points.
| Phenolics | K p at | ΔG (kJ mol−1) at | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | pKa | ||
|---|---|---|---|---|---|---|---|
| 25 °C | 40 °C | 25 °C | 40 °C | ||||
| Tyrosol | 3.84 | 12.85 | −3.33 | −6.64 | +62.43 | 221 | 10.2 |
| p-Coumaric acid | 5.48 | 62.36 | −4.21 | −10.75 | +125.63 | 436 | 4 |
| Vanilic acid | 6.86 | 21.29 | −4.78 | −7.95 | +58.27 | 212 | 4.16 |
| Ferulic acid | 4.20 | 28.74 | −3.55 | −8.73 | +99.42 | 346 | 4 |
| Apigenin | 2.09 | 2.76 | −1.82 | −2.64 | +14.39 | 54 | 6.63 |
The negative ΔG values may be expected given that most of the compounds have relatively low pKa values. The data also shows that the solutes partition more favourably into the DES at higher temperature showing that the enthalpy of transfer is positive which is beneficial for extraction as temperature is an easy parameter to improve solute partitioning.
The ΔG values were compared with those calculated in Table 2 and plotted as a function of pKa (Fig. 1 (red circles)) and it can be seen that all data follow an approximate trend. The correlation shows that hydrogen bonding is an important factor controlling partitioning of solutes into DESs however the diversity of solute properties such as size and melting point will also be important. It should also be remembered that the solutes transferred in this section were from triolein rather than a cyclohexane.
It has previously been shown that larger solutes partition less favourably into DESs due to the energy needed to create a larger cavity to accommodate the solute.12 While acid–base (dipole–dipole and ion–dipole) interactions will be important they are not the only factors governing solvent-solute interactions and therefore solubility. Fig. 2 is however important as it will give a guide to the types of compounds which will be preferentially extracted.
Table 4 analyses literature data for the extraction of similar compounds to Table 3 from olive oil into DESs. Direct comparison of all the data is not possible due to the different conditions of each study, however Table 4 shows that within each study higher partition coefficients were obtained for solutes with lower pKa values.21–24 This is the first time that this general prediction has been highlighted.
| Study | DESs | Extracted phenolics | pKa | Yield (%)d |
|---|---|---|---|---|
| a Choline chloride: lactic acid. b Choline chloride: glycerol. c Choline chloride: ethylene glycol. d Yield (%) = (mass of the specific phenolic compound/the total phenol compound mass) × 100. | ||||
| García et al.21 | Acid baseda | Hydroxytyrosol | 9.45 | 44 |
| Oleacein | 9.7 | 2.8 | ||
| Oleocanthal | 10 | 10 | ||
| Tyrosol | 10.2 | 31.6 | ||
| Alcohol basedb | Hydroxytyrosol | 9.45 | 2.4 | |
| Oleacein | 9.7 | 2.5 | ||
| Oleocanthal | 10 | 47 | ||
| Tyrosol | 10.2 | 36 | ||
| Chanioti et al.24 | Acid baseda | Oleuropein | 4.9 | 91 |
| Rutin | 6.4 | 6.4 | ||
| Hydroxytyrosol | 9.4 | 1.3 | ||
| Alcohol basedb | Oleuropein | 4.9 | 84 | |
| Rutin | 6.4 | 10 | ||
| Hydroxytyrosol | 9.4 | 3.4 | ||
| Fanali et al.23 | Acid baseda | Oleuropein aglycon | 4.95 | 27 |
| Lygstroside aglycon | 4.95 | 24 | ||
| Hydroxytyrosol | 9.45 | 7.5 | ||
| Tyrosol | 10.2 | 16 | ||
| Alcohol basedc | Oleuropein aglycon | 4.95 | 26.5 | |
| Lygstroside aglycon | 4.95 | 28 | ||
| Hydroxytyrosol | 9.45 | 11 | ||
| Tyrosol | 10.2 | 10 | ||
| Bonacci et al.22 | Acid baseda | Demethyloleuropein | 4.20 | 16 |
| Oleuropein | 4.95 | 83 | ||
| Oleacein | 9.7 | 0.7 | ||
| Alcohol basedb | Demethyloleuropein | 4.20 | 2.6 | |
| Oleuropein | 4.95 | 96 | ||
| Oleacein | 9.7 | 1.2 | ||
While the primary aim of this study was to determine the factors affecting extraction rather than to optimise the process, the extraction yield, the effect of time, stirring and extractant volume were optimised and the results are shown in the ESI (Table S3†).
3.3 Green chemistry metrics
Ionic liquids and DESs are not inherently green and their toxicity has been quantified.25,26 They can however be tuned for a given application to minimise toxicological issues. It has been highlighted that the two components of a DES can react together particularly when the hydrogen bond donor is acidic and the quaternary ammonium salt has an OH functionality.27 Although this is only a real issue under anhydrous conditions at higher temperature. It should however be noted that toxicity is often associated with lack of reactivity and bioaccumulation so there is a dichotomy between solvent stability and environmental compatibility. To meaningfully assess the impact of using DESs compared to conventional solvent processes it is important to fully assess appropriate metrics including an energy comparison.In the following section, 3 metrics were chosen to compare the data from this study with those from the literature; yield (Y), reaction mass efficiency (RME) and environment factor (E-F). These are defined by the following equations:
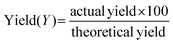 | (5) |
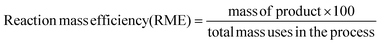 | (6) |
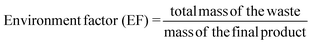 | (7) |
In addition to the metrics, reaction conditions such as extraction temperature, the amount of energy and cost to complete the reaction and the cost of the solvent can be also included in assessing different extraction reactions. These parameters can provide a more comprehensive assessment of the cost-effectiveness and the “greenness” of the extraction reactions. A typical weakness of Green metric assessment is the setting of meaningful and reliable benchmarks. While numerous processes exist all use different starting materials.20 For the purposes of this study the bench marks set in previous DES extraction studies will be used as these do at least use the same starting materials.21,24
Extreme care must be applied when calculating process metrics as they are extremely dependent on the assumptions made when defining inputs and outputs, particularly when classifying what is a waste product. It is important to compare like processes and since the literature has focussed on different sources and extracts this comparison will only consider tyrosol and hydroxytyrosol as the extracted products as they are common to all studies and are some of the most abundant extracts in all studies.28,29 A second and more difficult assumption is what to consider as waste. Initially it is assumed that all extraction solvents including the starting material (natural product) will be waste. None of the literature studies recycle the solvent and the difficulty with comparing the data for the Eutectys approach is that the solvent is part of the product but not an active ingredient. It should, however, be noted that Naturex claim that the DES increase the activity of the active ingredient compared to alcoholic solutions could be used to justify their inclusion as process products.11
For the purposes of this comparison the extraction of tyrosol from triolein using Ethaline was compared to two other studies that used DESs and conventional solvent to extract phenolic compounds from olive products. A study by García et al. extracted tyrosol using glycerol and lactic acid based DESs from extra virgin olive oil.21 The authors also used conventional solvents such as water and methanol/water mixture using the same extraction conditions. Another study by Chanioti et al. used similar extraction solvents but extracted phenolic compounds from olive pomace which is the remainder of the olive once the oil has been pressed from the original berry.24 The pomace will have different types and quantities of compounds than the extra virgin oil component as it contains skin and pits. The study by Chanioti et al. used alternative techniques to increase yields including homogenate-assisted extraction (HAE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE) and high hydrostatic pressure assisted extraction (HHPAE).24
Table 5 lists the percentage yield (Y%), reaction mass efficiency (RME%) and environment-factor (EF) metrics calculated from the three studies listed above. The yield percentage achieved in the current study was higher than most other solvents systems reported by the other two studies. This is partly down to the reaction conditions and experimental designs. In the current study the tyrosol content of the triolein was known so the yield calculation is accurate. The study by García et al.21 determined the tyrosol yield using a literature tyrosol content for olive oil (250 mg kg−1).30 A similar approach was used by Chanioti et al.24 using a literature value of 2800 mg kg−1 for tyrosol in olive pomace.31
| Study | Substrate | Extraction solvent/method | Y (%) | RME% | EF |
|---|---|---|---|---|---|
| a Assuming the DES is part of the active product and not a waste material. | |||||
| This study | Triolein | Ethaline (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a/25 °C) 1a/25 °C) |
79.3 | 0.06 (100.2)a | 1606 (1.0)a |
| García et al.21 | Olive oil | Glycerol-DES | 7.3 | 0.4 | 276 |
| Lactic acid-DES | 55 | 2.8 | 36 | ||
| 80% methanol | 11.5 | 0.6 | 162 | ||
| Water | 5.1 | 0.3 | 395 | ||
| Chanioti et al.24 | Olive pomace | Glycerol DES + HAE | 38.9 | 7.0 | 14.3 |
| Lactic acid DES + HAE | 73.9 | 13.6 | 7.3 | ||
| Water + HAE | 8.6 | 1.8 | 56.3 | ||
| 70% ethanol + HAE | 11.4 | 2.7 | 37.7 | ||
| Glycerol DES + UAE | 38.6 | 6.9 | 14.4 | ||
| Lactic acid DES + UAE | 38.2 | 7.1 | 14.2 | ||
| Water + UAE | 8.2 | 1.7 | 58.7 | ||
| 70% ethanol + UAE | 34.6 | 8.0 | 12.4 | ||
| Glycerol DES + HHPAE | 32.1 | 5.8 | 17.3 | ||
| Lactic acid DES + HHPAE | 91.8 | 16.9 | 5.9 | ||
| Water + HHPAE | 8.9 | 1.9 | 54.0 | ||
| 70% ethanol + HHPAE | 14.6 | 3.4 | 29.5 | ||
The glycerol and lactic acid DESs are more viscous and have higher surface tensions which will affect the thermodynamics of extraction. The experiments were also carried out for shorter time periods so it may be expected that the extraction yields will be lower. The true unknown tyrosol content in the samples of olive oil and olive pomace used by García et al. and Chanioti et al. also make direct comparison difficult.21,24 The result for the lactic acid DES using HHPAE shows very high yields, which demonstrates that the assumed tyrosol content for olive pomace must be approximately valid.
Table 5 shows that tyrosol extraction using Ethaline produces a higher yield than aqueous solutions or water-methanol mixtures. Tyrosol is relatively hydrophobic so it is unsurprising that it poorly partitions into water and water-methanol mixtures. All of the DESs show enhanced extraction capability compared to aqueous solutions even when mechanical action is used to enhance yields. This shows the importance of the amphiphillic nature of the DES. While the yields extracted by the current study are high, the relative mass efficiencies and environmental factors are low which are due to the experimental set up and the comparatively high ratio of DES to triolein. What the above experiments do show is that extraction using DESs is more successful using liquids with lower surface tensions and lower viscosities, at higher temperature.
If the Eutectys™ approach is used and the extract stays in the DES, then the DES is not considered a waste but rather becomes a product. By doing this the RME and EF for the first entry in Table 5 changes from 0.06 to 100.2 and 1606 to 1.0 which are significant improvements over current molecular solvents. The same would be the case for the other DES studies in Table 5.
García et al. showed that both DESs used for extraction demonstrates improved RME and EF values compared to the Ethaline data. Clearly Ethaline would not be a practical solvent due to the toxicity of ethylene glycol but it shows the importance of the HBD in the DES for the thermodynamics of extraction. The lactic-acid based DES used by García show around 50 times better extraction efficiency (RME) and 4.5 lower waste generation (EF) compared to 80% methanol under the same conditions. The same is true in Chanioti method,24 in which DES-based solvent demonstrate better extraction efficiency and lower waste generation than conventional solvents.
The data in Table 5 show that the study by Chanioti et al. was able to significantly increase the RME values and decrease the EF values by increasing the mass transport through mechanically assisted extraction.24 The combination of lactic-acid based DES with high hydrostatic pressure – assisted extraction (HHPAE) showed the best extraction efficiency and the least waste generation followed by the lactic acid based DES using homogenate assisted extraction (HAE). Extraction using olive pomace is clearly advantageous as it has a much lower value than that using extra virgin olive oil but it also has potentially higher loadings of some of the phenolic compounds.
In addition to the green metrics, an important parameter to compare is the energy usage of the different systems. Therefore, the energy and cost of the extraction method were quantified by the reaction temperature, reaction time, and heat capacity of the solvent. Energy consumption in the Triolein–Ethaline extraction system includes both the thermal energy necessary to heat the extraction system and the electrical energy required to run the magnetic stirrer during extraction. Full methods and data are contained in the ESI.† The data show that despite the high viscosity of the DESs, extraction of phenolic compounds can be carried out with decreased energy compared with molecular solvents even when mechanically assisted extraction was carried our due to the higher extraction efficiency. Table S4† shows that the total thermal energy consumption is negligible in comparison to the energy required for the assistant extraction method (i.e. stirring, shaking or homogenisation) in all three studies and these are even more insignificant compared to the cost of the extraction liquid.
As for the Chanioti method,24 the data in Table S4† shows that the phenolic compounds were extracted at the same conditions in terms of time and temperature using HAE and UAE extraction systems. It was however found that the energy costs £0.67 kg−1 of substrate using HAE compared to the obvious lower cost of £0.39 kg−1 using UAE. The data also show that the energy required to extract tyrosol from the oil phase is lower using UAE < HAE ≈ HHPAE extraction techniques which were 0.53, 0.91 and 0.92 kW h respectively. Therefore, although HHPAE show the highest yield and RME and it is also showing the lowest EF value, it consumed higher energy and it is the most expensive extraction system, although it does lead to significantly higher yields.
In general, it has been seen that using DESs to extract tyrosol from an oil phase resulted in reducing the waste generation with a high extraction yield of the target compared to the conventional methods and solvents. These results can be further enhanced using the extraction systems including HAE and HHPAE which require low energy and cost. This approach of natural product extraction therefore only really lends itself to the Eutectys method proposed by Naturex due to the low vapour pressure of the DES and the difficulty in removing it from the extract.11
4 Conclusion
This study investigated the partitioning of solutes of different polarity; butanoic acid, 1-pentanol, 2-pentanone, ethyl acetate phenol, benzyl alcohol and cyclohexanol in 4 DESs, Ethaline, Glyceline, Oxaline and Reline. Partition into DESs is greatest where the enthalpy of solvation is exothermic. This clearly occurs when the solute is strongly hydrogen bonding and to achieve this the hydrogen bond formed between the solute and the chloride of the DES will have to be larger than that between the chloride and the HBD of the DES. It was shown that the DES type had a stronger effect on the extraction of the less polar solutes (ketone and ester) whereas the more polar solutes were less strongly affected by the DES type. It was also shown that there was a correlation between the Gibbs energy of phase transfer and the pKa of the solute showing the importance of hydrogen bonding in the extraction process.These ideas were tested on natural product extraction using tyrosol, p-coumaric acid, vanilic acid, ferulic acid and apigenin in triolein, a synthetic triglyceride. It was found that the solutes with the lower pKa values were extracted more efficiently. This was also shown to be the case for 4 literature studies which extracted phenolic compounds from olive products.
Finally, green chemistry metrics of the extraction reaction studied here were compared with two similar published studies. The metrics assessed were yield, reaction mass efficiency (RME) and environment factor (EF) and these were assessed not only for DESs but also with other traditional solvents. The study also investigated mechanically assisted methods in terms of their cost, energy required and their effectiveness. It was found that DESs showed increased extraction efficiency compared with molecular solvents. RME and EF parameters were shown to be lower using Ethaline, however but this was mostly due differences in experimental conditions. Assuming that the DES was a product rather than a waste using the Eutectys™ method led to RME and EF parameters which were far superior to molecular solvents. The study also showed that mechanically assisted extraction could significantly improve efficiency without a significant increase in process energy or cost.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the Saudi Arabian Cultural Bureau and the University of Ha'il for a studentship (OAOA).References
- Markets and Markets, Plant Extracts Market by Type (Phytomedicines & Herbal Extracts, Essential Oils, Spices, Flavors & Fragrances), Application (Pharmaceuticals & Dietary Supplements, Food & Beverages, Cosmetics), Source, and Region - Global Forecast to 2025, https://www.marketsandmarkets.com/Market-Reports/plant-extracts-market-942.html.
- Research & markets, Polyphenols Market Size, Share & Trends Analysis Report By Product (Grape Seed, Green Tea, Cocoa), By Application (Beverages, Food, Feed, Dietary Supplements, Cosmetics) and Segment Forecasts, 2019–2025.
- J. Jiang and Y. L. Xiong, Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review, Meat Sci., 2016, 120, 107–117 CrossRef CAS.
- S. Bansal, S. Choudhary, M. Sharma, S. S. Kumar, S. Lohan, V. Bhardwaj, N. Syan and S. Jyoti, Tea: A native source of antimicrobial agents, Food Res. Int., 2013, 53, 568–584 CrossRef CAS.
- B. Dimitrios, Sources of natural phenolic antioxidants, Trends Food Sci. Technol., 2006, 17, 505–512 CrossRef CAS.
- B. Tang, W. Bi, M. Tian and K. Ho Row, Application of ionic liquid for extraction and separation of bioactive compounds from plants, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 904, 1–21 CrossRef CAS PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep eutectic solvents (DESs) and their applications, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. Hae Choi, Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents, J. Nat. Prod., 2013, 76, 2162–2173 CrossRef CAS.
- B. Tang, H. Zhang and K. H. Row, Application of deep eutectic solvents in the extraction and separation of target compounds from various samples, J. Sep. Sci., 2015, 38, 1053–1064 CrossRef CAS.
- L. Duan, L.-L. Dou, L. Guo, P. Li and E.-H. Liu, Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products, Sustainable Chem. Eng., 2016, 4, 2405–2411 CrossRef CAS.
- A. Lavaud, M. Laguerre, S. Birtic, A. S. Fabiano Tixier, M. Roller, F. Chemat and A. C. Bily, Eutectic extraction solvents, extraction methods by eutectigenesis using said solvents, and extracts derived from said extraction methods, PatentWO2016162703A1, 2016 Search PubMed.
- A. P. Abbott, A. Y. Al-Murshedi, O. A. Alshammari, R. C. Harris, J. H. Kareem, I. B. Qader and K. S. Ryder, Thermodynamics of Phase Transfer for Polar Molecules from Alkanes to Deep Eutectic Solvents, Fluid Phase Equilib., 2017, 448, 99–104 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. Rasheed and V. Tambyrajah, Novel Solvent Properties of Choline Chloride/Urea Mixtures, Chem. Commun., 2003, 70–71 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, Deep Eutectic Solvents Formed Between Choline Chloride and Carboxylic Acids, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- A. P. Abbott, P. M. Cullis, M. J. Gibson, R. C. Harris and E. Raven, Extraction of glycerol from biodiesel into a eutectic based ionic liquid, Green Chem., 2007, 9, 868–872 RSC.
- S. Sasidharan, Y. Chen, D. Saravanan, K. M. Sundram and L. Y. Latha, Extraction, Isolation And Characterization Of Bioactive Compounds From Plants’ Extracts, Afr. J. Tradit., Complementary Altern. Med., 2011, 8, 1–10 CAS.
- L. Zhang, A. S. Ravipati, S. R. Koyyalamudi, S. C. Jeong, N. Reddy, P. T. Smith, J. Bartlett, K. Shanmugam, G. Münch and M. J. Wu, Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds, J. Agric. Food Chem., 2011, 59, 12361–12367 CrossRef CAS.
- N. Dudareva, A. Klempien, J. K. Muhlemann and I. Kaplan, Biosynthesis, function and metabolic engineering of plant volatile organic compounds, New Phytol., 2013, 198, 16–32 CrossRef CAS PubMed.
- P. Atkins and J. de Paula, Physical Chemistry, Oxford University Press, Oxford, 2014 Search PubMed.
- E. Frankel, A. Bakhouche, J. Lozano-Sánchez, A. Segura-Carretero and A. Fernández-Gutiérrez, Literature Review on Production Process To Obtain Extra Virgin Olive Oil Enriched in Bioactive Compounds. Potential Use of Byproducts as Alternative Sources of Polyphenols, J. Agric. Food Chem., 2013, 61, 5179–5188 CrossRef CAS PubMed.
- A. García, E. Rodríguez-Juan, G. Rodríguez-Gutiérrez, J. J. Rios and J. Fernández-Bolaños, Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs), Food Chem., 2016, 197, 554–561 CrossRef PubMed.
- S. Bonacci, M. L. Di Gioia, P. Costanzo, L. Maiuolo, S. Tallarico and M. Nardi, Antioxidants, 2020, 9, 513 CrossRef CAS.
- C. Fanali, S. Della Posta, L. Dugo, M. Russo, A. Gentili, L. Mondello and L. De Gara, Application of deep eutectic solvents for the extraction of phenolic compounds from extra–virgin olive oil, Electrophoresis, 2020, 41, 1752–1759 CrossRef CAS PubMed.
- S. Chanioti and C. Tzia, Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques, Innovative Food Sci. Emerging Technol., 2018, 48, 228–239 CrossRef CAS.
- Y. Zhao, J. Zhao, Y. Huang, Q. Zhou, X. Zhang and S. Zhang, Toxicity of ionic liquids: Database and prediction via quantitative structure–activity relationship method, J. Hazard. Mater., 2014, 278, 320–329 CrossRef CAS PubMed.
- P. de Morais, F. Gonçalves, J. A. P. Coutinho and S. P. M. Ventura, Ecotoxicity of Cholinium-Based Deep Eutectic Solvents, ACS Sustainable Chem. Eng., 2015, 3, 3398–3404 CrossRef CAS.
- N. Rodriguez Rodriguez, A. van den Bruinhorst, L. J. Kollau, M. C. Kroon and K. Binnemans, Degradation of Deep-Eutectic Solvents Based on Choline Chloride and Carboxylic Acids, ACS Sustainable Chem. Eng., 2019, 7, 11521–11528 CrossRef CAS.
- S. Bulotta, M. Celano, S. M. Lepore, T. Montalcini, A. Pujia and D. Russo, Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases, J. Transl. Med., 2014, 12, 219 CrossRef.
- J. Fernández-Bolaños, B. Felizón, M. Brenes, R. Guillén and A. Heredia, Hydroxytyrosol and tyrosol as the main compounds found in the phenolic fraction of steam-exploded olive stones, J. Am. Oil Chem. Soc., 1998, 75, 1643–1649 CrossRef.
- C. Romero and M. Brenes, Analysis of total contents of hydroxytyrosol and tyrosol in olive oils, J. Agric. Food Chem., 2012, 60, 9017–9022 CrossRef CAS.
- I. Cea Pavez, J. Lozano-Sánchez, I. Borrás-Linares, H. Nuñez, P. Robert and A. Segura-Carretero, Obtaining an extract rich in phenolic compounds from olive pomace by pressurized liquid extraction, Molecules, 2019, 24, 3108 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc01747k |
| This journal is © The Royal Society of Chemistry 2021 |

