Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Protein from renewable resources: mycoprotein production from agricultural residues†
Thomas
Upcraft
a,
Wei-Chien
Tu
a,
Rob
Johnson
 b,
Tim
Finnigan
b,
Nguyen
Van Hung
b,
Tim
Finnigan
b,
Nguyen
Van Hung
 c,
Jason
Hallett
c,
Jason
Hallett
 *a and
Miao
Guo
*a and
Miao
Guo
 *ad
*ad
aDepartment of Chemical Engineering, Imperial College London, SW7 2AZ, UK. E-mail: j.hallett@imperial.ac.uk; miao.guo@kcl.ac.uk
bQuorn Foods, Station Road, Stokesley, North Yorkshire TS9 7AB, UK
cInternational Rice Research Institute, Los Baños, Laguna 4031, Philippines
dDepartment of Engineering, King's College London, WC2R 2LS, UK
First published on 24th June 2021
Abstract
Globally, one in nine people suffer from undernourishment with evidence that this number is increasing. Additionally, due to the projected 50% increase in global population, the demand in worldwide animal-sourced protein is expected to double by 2050. Furthermore, not only are global animal protein supply chains susceptible to the effects of climate change, but they are also a significant contributor to greenhouse gas emissions and require large areas of arable land. Single cell proteins (SCPs) are an alternative protein source that offer a potential route to reduce the environmental impact of global protein consumption. One such SCP is Fusarium venenatum, which is a strain of mycoprotein widely sold under the brand name Quorn and is produced through the fermentation of the microorganism on glucose. However, this glucose still has a significant arable land-use burden associated with it. In order to mitigate this burden, sugars derived from agricultural lignocellulosic residues could be used, however, additional processing steps are required. In this work, exploratory research on fermentation of F. venenatum on sugars derived from lignocellulosic residues is presented. The food-grade ionic liquid [Ch][HSO4] was employed in combination with food-grade Celluclast 1.5L to extract glucose from rice straw residues, which resulted in an overall glucose yield of 42.4% compared to using non-food certified ionic liquid [TEA][HSO4], which yielded 92.8%. Based on these results, a Techno-Economic Analysis (TEA) and Life Cycle Assessment (LCA) were conducted after synthesising a biorefinery process model. TEA modelling outcomes highlighted that the crude mycoprotein paste product could be produced for $5.04 kg−1 ($40.04 per kg-protein). By conducting a retro-techno-economic analysis, it was shown that there is reasonable scope for reducing this price further by improving saccharification yields and utilising feedstocks with high cellulose contents. Furthermore, LCA results demonstrated significant sustainability benefits of lignocellulosic-derived mycoprotein, with greenhouse gas emissions less than 14% that of protein from beef. However, the most significant advantage of this technology is the minimal dependence on arable land compared to animal-sourced and plant-sourced proteins such as beef and tofu.
1. Introduction
The projected 50% increase in global population combined with global south growth is expected to bring over a 50% rise in food demand by 2050 with global animal-sourced protein demand estimated to nearly double.1 Despite the efforts to tackle malnutrition, one in nine people (821 million) in the world are still undernourished2 and current trends suggest that global malnourishment is increasing. Furthermore, the positive feedback loops posed by the effects of climate change3 may risk severe disruption to agricultural activities and threaten protein security as an estimated 56% of global protein nutrition is derived from crops in which the protein content is particularly sensitive to elevated CO2 levels.4 Furthermore, plant-sourced and animal-sourced protein contributes significantly to greenhouse gas (GHG) emissions and is highly dependent on arable lands. Notably, the global livestock sector is responsible for substantial GHG emissions and is responsible for 30% of terrestrial land use for grazing and compound feeding.5 Research has emerged in recent years on alternative protein sources with minimal environmental damage, in particular food-grade single cell proteins (SCPs). SCPs refer to the protein derived from microorganisms, such as bacteria, algae and fungi that can be used as a protein supplement for human consumption or animal feeds. Animal-feed SCP research has utilised feed-safe waste such as agro-industrial residues; sugarcane molasses,6 soy molasses,7 wheat bran,8,9 starch processing wastes9,10 and mixed-food industry wastes.11 Whereas, food-grade SCP are mainly sourced from arable-land-dependent food crops due to strict regulatory requirements12 and predominantly used as food-supplements rather than meat-substitutes. Saccharomyces cerevisiae and Pichia jadinii are examples of successful SCPs produced from starch-derived glucose for human consumption. There has been an increasing research interest in alternative non-food feedstocks such as carbon dioxide13 which were investigated for SCP for human consumption on a technical level.13,14 However, food-grade SCP production from non-crop feedstocks, which are independent of arable lands and offer low-carbon solutions, remains an open area for investigation.This study presents exploratory research on mycoprotein derived from food-grade lignocellulosic agricultural residues, which offers potential sustainable solutions for future protein security. Mycoprotein, developed in response to global protein deficiency since 1967, is derived from the fermentation of glucose with Fusarium venenatum A3/5 and has been produced by Marlow Foods Ltd under the trade name Quorn™ since 1985, with current annual sales over £200m.14,15 Containing high protein (45% mass) and essential amino acids (44% of total protein), mycoprotein offers a range of positive attributes compared with animal-sourced protein such as favourable fatty acid profile and high fibre content. However, its starch-derived glucose sourced from food crops is still dependent on arable land. Alternatively, lignocellulosic-sourced sugar offers a promising solution. Cellulose the most abundant organic material on earth, can be derived from agricultural residues and other food-safe lignocellulosic resources such as rice straw. Rice as a staple crop feeds over 45% of the world's population.16 With continuous global growth in rice demand, there is a corresponding increase in rice straw production. Much of this straw is deemed as waste and is burned, leading to severe health complications, adverse impacts to the atmospheric quality and soil pollution.17 Several pretreatment technologies have been investigated to fractionate lignocellulose enabling glucose production, amongst which, the use of ionic liquids (ILs) is one of the most promising technologies due to its high sugar release.18 Furthermore, the use of promising low-cost food-grade ILs may eliminate many hurdles relating to food safety and enabling lignocellulosic-SCP. Despite the advancements in lignocellulosic fractionation and biochemical production, no publicly available research has been found on food-grade lignocellulosic-glucose extraction for SCP production.
For establishing such a process at industrial scale, biorefinery modelling is an important area of research, however, biorefinery models with IL pretreatment are a scarcity. Baral and Shah19 investigated IL pretreatment for ethanol production and concluded that IL recovery and cost were significant economic factors. SCP production modelling also represents an open research area with very few published studies focusing on feed-grade SCP. Strong, Kalyuzhnaya20 discussed the potential for a methanotroph-based multiple product biorefinery which considered protein production via a biogas-derived methane-fed fermentation. Their modelling suggested a technically feasible system producing 0.54 tonnes of SCP per tonne of methane feed. Molitor, Mishra13 presented an economic viability study for a two-stage power-to-protein process which fixed CO2 in the presence of electrolysis-derived H2 in the first stage, followed by S. cerevisiae fermentation on the acetate effluent from stage A. The biorefinery process proposed by Aggelopoulos, Katsieris11 for solid state fermentation of food industry wastes to feed SCP revealed economically competitive waste-derived SCP (∼$460–550 per tonne vs. $425 per tonne (soybean meal)).21 However, a research gap remains on modelling of lignocellulosic-derived food-grade single cell protein.
In this work, through both experimental investigation and modelling, we investigate, for the first time, the technical feasibility and sustainability of lignocellulosic-SCP, in particular, lignocellulosic-mycoprotein production. We employ food-grade ionic liquids for sugar extraction from rice straw to assess the pretreatment performance of the ionic liquid. Our experimental data then forms the basis of the biorefinery model developed to assess the techno-economic feasibility and environmental sustainability of the process. Finally, the model was probed to highlight the key variables for process optimisation and intensification. The novelty of this exploratory research is based on the particular application of food-grade ionic liquids and enzyme in the treatment of rice straw with the aim of valorisation of the recovered fermentable sugar through producing mycoprotein for human consumption.
2. Methodology
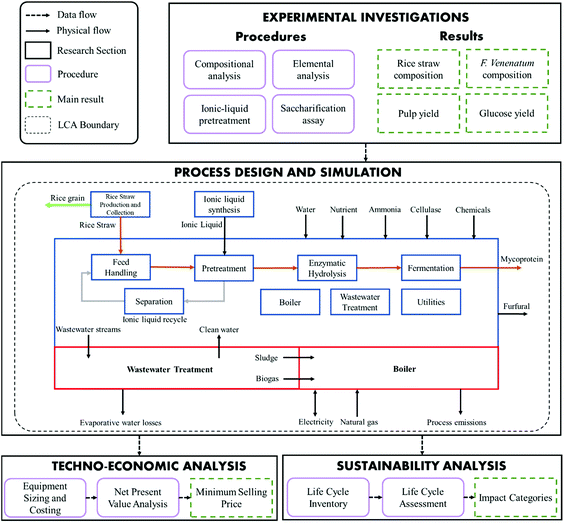
We experimentally investigated food-grade lignocellulosic sugar production from rice straw using the food-safe ionic liquid, [Ch][HSO4], and cellulase enzyme Celluclast 1.5L where a non-food grade ionic liquid ([TEA][HSO4]) and enzyme (Novozyme CELLIC® CTEC 2) were used as a benchmark. The methodology of this paper (Fig. 1) consists of laboratory experiments and modelling. The data from the experimental work formed the basis of the process design and modelling. The developed modelling framework consists of three components: process design and simulation, techno-economic analysis, and sustainability performance.The process was synthesised in the process simulation software Aspen Plus V9. Coupling Aspen Plus with Microsoft Excel and MATLAB, the economic performance of the process including capital and operating costs were evaluated. Subsequentially, the minimum selling price of the protein product was determined as part of the economic evaluation of the process. Finally, a life cycle assessment of lignocellulosic-mycoprotein was conducted in the software SimaPro to evaluate the sustainability performance.
2.1 Experimental
IonoSolv fractionation experiments were performed in triplicates, where both ionic liquids ([Ch][HSO4] and [TEA][HSO4]) were investigated. Each fresh straw sample equivalent to 1 g oven dry weight (ODW) with 9 g ionic liquid was placed into a 15 mL pressure tube and thoroughly mixed using a vortex mixer. Pressure tubes were placed in the oven at 150 °C for 1 hour. After cooling for 15–20 min to room temperature, the sample was transferred to a 50 ml centrifuge tube and washed with absolute ethanol 4 times. At each wash cycle, 40 ml ethanol was added to each tube; after mixing with the vortex mixer, they were rested at room temperature for one hour. Next, the samples were centrifuged (VWR Mega Star 3.0 centrifuge) at 3000 rpm for 50 minutes. Supernatants were removed at each wash whereas pellets (i.e. pretreated pulp) remained in the centrifuge tube. After 4 washes the pulp was transferred to a Whatman cellulose thimble for soxhlet extraction with 150 ml of absolute ethanol at 135 °C for 20 hours in a fume hood. After the completion of the extraction the ethanol was removed, and the contents of the thimbles were transferred to a pre-weighed 50 ml centrifuge tube for two water-wash cycles. Water and pulp mixture were centrifuged at 3000 rpm for 50 minutes in each cycle. The water was removed via decanting and the tube containing pulp was weighed to determine the moisture content of the pulp and the cellulose pulp recovery yields calculated.
2.2. Process design and simulation
Based on our experimental results supplemented by publicly available data, we modelled a lignocellulosic-mycoprotein biorefinery with process simulation software Aspen Plus V9. The production capacity was based on an estimate of the current annual production capacity obtained from literature13,26 and information regarding the capacity increase due to new investments at the production site.27 Overall, the expected capacity, and thus the production rate used in this work is 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year. The process design and simulation aimed to understand the technical feasibility to scale-up mycoprotein production using food-grade lignocellulosic sugar derived from rice straw. As shown in Fig. 1, the process was decomposed into distinct processing areas, of which, pretreatment, separation and fermentation are a primary focus of this model whilst the other sections are adapted from previous work.28 The design for each processing area is detailed in result section 3.2. The origin of the physical properties for the compounds used in this work is described in (ESI 5.2†). Four scenarios were developed based on the experimental data in which either the ionic liquid or cellulase differed in each case ([TEA][HSO4], [Ch][HSO4] and Cellic CTEC 2, Celluclast 1.5L).
000 tonnes per year. The process design and simulation aimed to understand the technical feasibility to scale-up mycoprotein production using food-grade lignocellulosic sugar derived from rice straw. As shown in Fig. 1, the process was decomposed into distinct processing areas, of which, pretreatment, separation and fermentation are a primary focus of this model whilst the other sections are adapted from previous work.28 The design for each processing area is detailed in result section 3.2. The origin of the physical properties for the compounds used in this work is described in (ESI 5.2†). Four scenarios were developed based on the experimental data in which either the ionic liquid or cellulase differed in each case ([TEA][HSO4], [Ch][HSO4] and Cellic CTEC 2, Celluclast 1.5L).
2.3 Economic evaluation
We used the Minimum Selling Price (MSP) to assess the economic viability of the process to produce lignocellulosic-mycoprotein from rice straw. The capital costs, denoted as Fixed Capital Investment (FCI), were determined based on detailed sizing and costing of each process section described in the process area (ESI S2.1†). The input and output streams of reagents, utilities, products and byproducts contribute to the annual operating costs (ESI S2.2†). To determine the MSP accurately, we split the process into upstream and downstream segments. For the ‘upstream’ process, which involves pretreatment of rice straw, enzymatic hydrolysis and subsequent fermentation to mycoprotein paste and the auxiliary facilities, we calculated a MSP of the paste (MSPpaste) by finding the MSP of the paste that makes the upstream Net Present Value (NPV) 0 (ESI S2.4. and S2.5†). As the mycoprotein paste is not the final supermarket product, and in the interest of finding the true cost of lignocellulosic-mycoprotein to the consumer, we pursued an estimate of the MSP of the final product (MSPproduct) by analysing the published Quorn revenue figures. Furthermore, we made a prediction of the current production price of the mycoprotein paste using the same NPV analysis based on several assumptions relating to the input materials to the process. These are summarised in ESI S2.6.†The process design for the conversion of rice straw to mycoprotein paste excludes the subsequent texturisation steps required to produce the final consumer product. In this study, the lignocellulosic mycoprotein paste was modelled as the crude protein and compared to other crude protein sources, namely, livestock. The crude protein contents of mycoprotein and live-stock-sourced protein are given in ESI S2.7.†
To investigate the effects of certain process parameters, economic assumptions, a sensitivity analysis was carried out considering an upper and a lower bound of the design space. The bounds of most process parameters were selected based on representative literature data; whereas for the price of cellulase enzyme and rice straw were varied ±20% of the base-case value.
2.4 Surrogate-based retro-techno-economic analysis
In this study, a novel surrogate-based retro-techno-economic analysis (RTEA) was developed to identify the feasible design space for performance optimisation of the lignocellulosic-mycoprotein process. Reversing the TEA process i.e. determination of economic key performance indicators (KPI), RTEA29–31 allows the declaration of a minimum acceptable KPI, in order to identify the combination and value of operating conditions and process targets (feasible design space) that would result in the desired KPI e.g. MSP. Thus, RTEA offers a powerful tool to provide performance targets enabling process design to effectively screen alternatives and focusing on performance-limiting steps. RTEA has been applied in previous research to equation-orientated simulators29–31 in which the economic model is implemented explicitly in equation form, and the model converges only when all equations are satisfied. In this study, the simulation and design were conducted with a sequential-modular programme, where iterative procedures are used to converge the flowsheet. Thus, we employed a novel RTEA approach. Kriging surrogate models32 were constructed using the Surrogate Modeling (SUMO) Toolbox in Matlab.33 A latin hypercube sampling method with corner points was selected as the initial design where the sample space was constructed with a combination of input variables (cellulase dosage, rice straw cellulose and xylan composition, and saccharification yield) and the output variable was the MSP. In all cases, the feedstock flowrate was fixed at 378![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year.
000 tonnes per year.
2.5 Life cycle assessment (LCA)
To assess the cradle-to-factory gate environmental sustainability of the lignocellulosic-mycoprotein process, we conducted a life cycle assessment using SIMAPRO V9. The input-output flows derived from process simulation were fed into the LCA as inventory data. The inputs and assumptions for the Life Cycle Inventory (LCI) are detailed in ESI S3.† Through contributional analyses, LCA comparison and sensitivity analyses, we aimed to (a) identify the main environmental contributors and performance-limiting processes and outline potential improvements, (b) compare lignocellulosic-mycoprotein with food-crop derived mycoprotein and traditional protein sources, highlighting benefits and drawbacks, and (c) investigate the most sensitive parameters on the environmental performance of the lignocellulosic-mycoprotein system.The system boundary defined in this ‘cradle-to-gate’ study is shown by the black dashed line in Fig. 1 and includes the biorefinery process, converting rice straw to mycoprotein, as well as cultivation of rice, and ionic liquid synthesis. The functional unit was defined as 1 kg mycoprotein paste at biorefinery gate, with a solids content of 25% based on a production capacity of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year. We applied an economic allocation approach where multiple-products occur at feedstock cultivation (rice grain and straw) and lignocellulosic-mycoprotein production stages (mycoprotein paste and furfural). A problem oriented (midpoint) approach ReCiPe 2016 Midpoint (hierarchist version) was adopted as the baseline Life Cycle Impact Analysis (LCIA) method. Under this approach, the impact categories of acidification, climate change, depletion of abiotic resources, ecotoxicity, eutrophication, human toxicity, ozone layer depletion, particulate matter, and photochemical oxidation. Land use consumption characterisation methods are detailed in ESI S3.1† to account for arable and pastureland occupation by arable crop cultivation and livestock grazing/feeding to meet protein demand including animal-sourced and plant-based protein.
000 tonnes per year. We applied an economic allocation approach where multiple-products occur at feedstock cultivation (rice grain and straw) and lignocellulosic-mycoprotein production stages (mycoprotein paste and furfural). A problem oriented (midpoint) approach ReCiPe 2016 Midpoint (hierarchist version) was adopted as the baseline Life Cycle Impact Analysis (LCIA) method. Under this approach, the impact categories of acidification, climate change, depletion of abiotic resources, ecotoxicity, eutrophication, human toxicity, ozone layer depletion, particulate matter, and photochemical oxidation. Land use consumption characterisation methods are detailed in ESI S3.1† to account for arable and pastureland occupation by arable crop cultivation and livestock grazing/feeding to meet protein demand including animal-sourced and plant-based protein.
Sensitivity analyses were conducted to understand the implications of varying process parameters and economic assumptions on LCIA results. The LCA results of lignocellulosic-mycoprotein derived from rice straw was compared to potential lignocellulosic-mycoprotein derived from other sources, namely, miscanthus, switchgrass and corn stover to highlight sustainability improvements that could be attained by switching feedstock.
3. Results
3.1 Experimental results – food-grade lignocellulosic glucose extraction from rice straw
Compositional analyses were performed to determine the lignin, cellulose and hemicellulose content of the straw samples from rice varieties cultivated at IRRI Philippines. Three rice varieties (Rc 25, Rc 442, and Rc 400) were selected from an initial screening to represent high and low grain yield varieties (ESI S4.1†). Their chemical composition is presented in Table S13.† The cellulose and hemicellulose components vary between 25.25–27.55% and 14.8–17.3% respectively, which represents the sugar release potential from ionic liquid pretreatment and subsequent enzymatic hydrolysis.A high pulp recovery was observed from the rice straw pre-treated with [Ch][HSO4] ranging between 70.9–74.0% (Fig. 2A) (Table S14†). The high recovery rates compared to [TEA][HSO4] (50.3 ± 1.3%) indicated a lower delignification and hemicellulose removal achieved by [Ch][HSO4], which was confirmed by compositional analysis of the pulp (Table S15†). Delignification with [Ch][HSO4] was only 37.5% compared to 68.4% with [TEA][HSO4]. Hemicellulose removal was also less, at 80.3% for [Ch][HSO4] as opposed to 87.9% for [TEA][HSO4]. Additionally, using [Ch][HSO4] resulted in a cellulose loss in the pulp of 29.3% compared to 12.3% using [TEA][HSO4]. The lower delignification and hemicellulose removal combined with a loss of cellulose have a direct effect on subsequent enzymatic saccharification and glucose yields which was confirmed in the treated saccharified samples. Saccharification of the [TEA][HSO4] treated Rc 400 sample resulted in over double the glucose yield (92.8 ± 1.7%) compared to the [Ch][HSO4] treated samples (42.4 ± 1.8%). However, for rice varieties, pretreatment with either ionic liquid improves the saccharification yield compared to the untreated sample significantly (glucose yield increases by 10%–67%) (Table S16†).
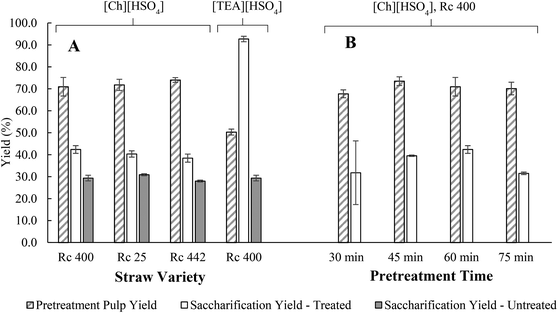 | ||
| Fig. 2 Yields from ionic liquid pretreatment and subsequent saccharification of rice straw varieties Rc 400, Rc 25 and Rc 442 (A) and time course experiments on rice straw variety Rc 400 to determine the optimum time for pretreatment with [Ch][HSO4] at 150 C (B). Pretreatment pulp yield = recovery of solid fraction from pretreatment; saccharification yield = glucose yields from untreated straw and pre-treated samples with food-grade choline hydrogen sulphate [Ch][HSO4] and non-food grade triethylamine hydrogen sulphate [TEA][HSO4]. Food-grade Celluclast 1.5L used for saccharification. Detailed data given in ESI Tables S14, S16 and S17.† | ||
Of the three varieties pretreated with [Ch][HSO4], Rc 400, which presented the highest glucan content, lowest ash, and lignin content of the three varieties, showed the lowest pulp recovery yield. Rc 400 straw was selected for time course experiments (Fig. 2B) to understand the limiting step for food-grade lignocellulosic sugar release, including the performance of [Ch][HSO4] with varying reaction time and performance of Celluclast 1.5L. The sample was pretreated with [Ch][HSO4] under constant temperature at 150 °C for 30, 45, 60, and 75 min. Rc 400 showed 67–74% cellulose pulp recovery yield (Fig. 2B). Further saccharification experiments using Celluclast 1.5L delivered 31.5–42.4% glucose release from Rc 400 straw. The highest pulp (73.5 ± 2.0%) and glucose yields (42.4 ± 1.8%) were observed at 45 min and 60 min respectively (Table S17†).
Overall, our results highlight the importance of pretreatment in lignocellulosic-sugar production, where the ionic liquid performance plays a significant role. The performance between [Ch][HSO4] and [TEA][HSO4] on Rc 400 fractionation shows that the ionic liquid is a driving factor for glucose yield in this study, where a significant difference in glucose yields was observed for the [TEA][HSO4]-pretreated and [Ch][HSO4]-pretreated Rc 400 Straw (92.7 ± 1.2% and 42.4 ± 1.8% respectively). Similar glucose yields were found for different cellulase enzyme (97.8 ± 1.7% and 92.7 ± 1.2% for Cellic® CTEC2 and Celluclast 1.5L respectively).
3.2 Process design
Based on our experimental work, we modelled a lignocellulosic-mycoprotein biorefinery (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year) using Aspen Plus V9. The design overview is presented in Fig. 3 and below with more detail in (ESI S5†).
000 tonnes per year) using Aspen Plus V9. The design overview is presented in Fig. 3 and below with more detail in (ESI S5†).
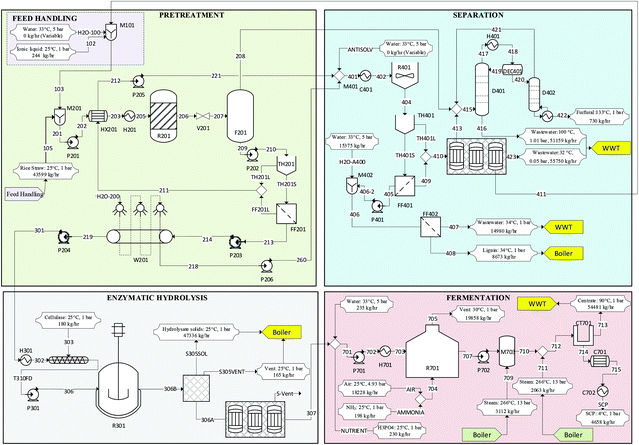 | ||
| Fig. 3 Overview of lignocellulosic-mycoprotein process with feed handling, pretreatment, enzymatic hydrolysis, separation, and fermentation shown in detail. A stream table is provided (Table S18†) in the ESI. For sizing and costing of labelled process equipment, refer to Table S19.† | ||
The IL rich stream is treated through a three-effect evaporator to reduce the water content to 20%. The vapour stream from the first effect is mixed with the vapours from the pretreatment flash. This combined stream is the feed to the furfural recovery section. The vapours from the second and third effects are sent to wastewater treatment.
A minimum-boiling azeotrope, which exists between water and furfural at 97.78 °C (1 bar), complicates the recovery of furfural. However, the presence of acetic acid acts as an entrainer itself to facilitate distillation via heteroazeotropic distillation by taking advantage of the vapour–liquid–liquid equilibrium formed between the 3 components. Previous work has investigated this separation problem,43,44 achieving a 95% pure furfural product. In this case 99% purity is desired, therefore an extra column was employed. The sequence used in this work involved a first column of 7 stages with no reboiler where the distillate splits into a fufural rich phase and a water rich phase in the decanter, from which the fufural rich phase is sent to a smaller column (5 stages) where the purity is increased from 95% to 99% and is recovered in the bottoms. The distillate is recycled to the first column. There is an opportunity for recovery of the acetic acid with an alternative distillation configuration, however, Galeotti, Jirasek44 concluded that it was not economically justified.
3.3. Lignocellulosic mycoprotein viability
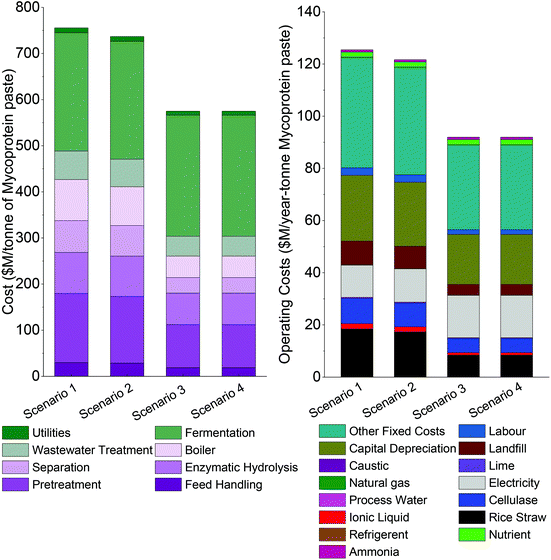
Based on the experimental data, pretreatment with [Ch][HSO4] for 1 hour, and saccharification with Celluclast 1.5L were selected to be the base case food-grade scenario (scenario 1) for the lignocellulosic-mycoprotein biorefinery model. This scenario was compared to the three other combinations of ionic liquid ([Ch][HSO4] and [TEA][HSO4]) and cellulase (Celluclast 1.5L and Cellic CTec 2).The fixed capital investment for scenarios 1, 2, 3 and 4 is $756m, $737m, $575m and $575m respectively (Fig. 4A and Table S26†), and total annual operating costs are $125m, $122m, $92m and $92m respectively (Fig. 4B and Table S27†). Overall, these costs are largely dependent on the pretreatment performance, with significant economic improvements due to the lower pulp yield (thus greater lignin and hemicellulose removal), which facilitates saccharification to glucose. The choice of enzyme also affects costs, with the food-grade CELLIC® CTEC2 enzyme scenarios being economically inferior to the equivalent non-food grade cellulase scenarios, however, the effect is less pronounced than the degree of pretreatment.
The MSPpaste reflects the trend in costs, with the resulting prices of $5.04 kg−1, $4.95 kg−1, $3.78 kg−1 and $3.78 kg−1 for scenarios 1–4 respectively. This reveals that significant improvements in the MSPpaste can be achieved with selection of a high performance ionic liquid solvent. However, of greatest interest is the present possibility of lignocellulosic-mycoprotein production, in which case, food-grade reagents are a necessity, therefore, scenario 1 is the focus of our analyses.
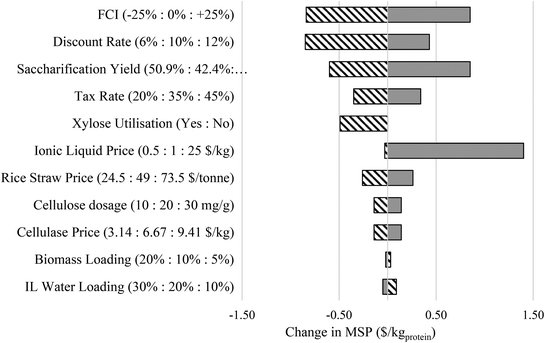 | ||
| Fig. 5 Sensitivity analysis of the minimum selling price (MSP $ kg−1) of lignocellulosic-mycoprotein paste to a set of process parameters and economic assumptions (shown on the y-axis). Exact values given in Table S28.† | ||
The process variables with the greatest impact are the saccharification yield and the potential utilisation of xylose in tandem with glucose by F. venenatum A3/5. The scenario with greater glucose saccharification yield is modelled to represent the improvement in food grade ionic liquid performance or enhancement in cellulase activity. Xylose utilisation represents a promising future scenario with an increased carbon utilisation efficiency by F. venenatum A3/5 where the strain is capable of growth on a mixed sugar substrate derived from lignocellulosic resources. This alters the aim of the pretreatment process design and ionic liquid selection. For the case in which only glucose can be utilised by F. venenatum A3/5, pretreatment should aim to remove both lignin and hemicellulose using high-performing IL e.g. [TEA][HSO4]. However, if xylose can be utilised, pretreatment should target lignin removal and limit hemicellulose loss which can be achieved by the low-performing food grade [Ch][HSO4]. Our modelling results suggest the potential degree of process improvement and highlight the future research frontier for lignocellulosic mycoprotein technology, i.e. ionic liquid performance and F. venenatum A3/5 growth on a wider variety of sugar substrates.
The impacts of rice straw prices on the MSPproduct suggest the importance of feedstock screening in particular, considering the dependence of agro-crops on environmental variables (e.g. climate). Therefore, an ideal feedstock would be the lignocellulosic plant species with resilient traits towards environmental change and other extreme events. The ionic liquid price has been factored in with a range of $0.5 kg−1 and $25 kg−1 to represent the general cost of various ionic liquids. The extent to which the MSP is affected by ionic liquid price is reduced with ionic liquid recycling. However, at a high price of $25 kg−1, solvent costs could hinder economic feasibility significantly. Fortunately, protic ionic liquids, as demonstrated in our study, offer a cost-effective alternative to costly ionic liquids.46–48 Finally, benefiting from the high recycle rate of ionic liquids, biomass loading of the solvent has a negligible effect on the MSP, water loading of the solvent has a greater effect, however, this model does not account for any change in pretreatment performance due to the change in water present, as has been found in experimental studies.
3.4 Surrogate-based retro-techno-economic analysis
A surrogate-based RTEA was performed to highlight the feasible design space of lignocellulosic-mycoprotein processes, where key variables such as biomass composition, ionic liquid selectivity, cellulase dosage, and carbon source utilization by F. venenatum were investigated. Kriging surrogate models were constructed by running the model with 54 samples spanning the design space and the corresponding resulting MSP. The surrogate models were then used to conduct the RTEA. The training data and model details can be found in ESI S29–S32.† The results of the RTEA are shown in Fig. 6.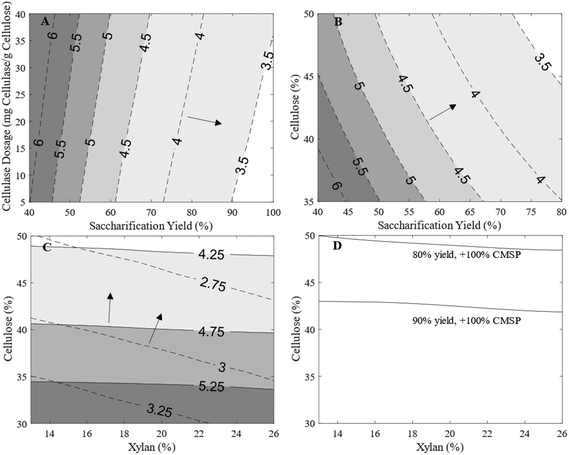 | ||
| Fig. 6 Results of the retro-techno-economic analysis (RTEA). A surrogate model of the process was created for certain input variables to find the response of the minimum selling price (MSP) for a change in the input variables. Figures (A–C) are for a two variable RTEA in which the variables on the axes were varied within their displayed ranges. The contour lines for A, B and C show a number of MSP targets ($ kg−1) and the arrow represents the direction of improvement of the MSP. The solid lines of (C) are for the scenario where cellulose and xylan composition of rice straw is varied where xylose cannot be utilised by F. venenatum. The dashed lines represent the scenario in which xylose can be utilised by F. venenatum. Finally, (D) is a three variable RTEA where rice straw cellulose and xylan composition were varied as well as the saccharification yield of cellulose and xylan to glucose and xylose respectively. For this scenario, F. venenatum utilisation of xylose was assumed. The solid contour lines show the cellulose and xylose compositions required at selected saccharification yield (80% and 90%) to achieve an MSP than is no more than 100% of the predicted current minimum selling price (CMSP – see ESI S2.6† for derivation). 100% increase was chosen as there is no combination of variables (feasible region) capable of achieving the CMSP. | ||
In Fig. 6A, contours representing different MSP prices are shown for combinations of saccharification yield and cellulase dosage. The primary driver for economic improvement in this scenario is the saccharification yield. Trivially, for a single cellulase mixture, as dosage increases one would expect the saccharification yield to increase. However, in this case, Fig. 6A can be used as a tool to cross-reference with existing cellulase formulations to identify alternatives that can be used to reduce MSP.
Significant improvements in the MSP can be achieved by combining a high cellulose composition and saccharification yield (Fig. 6B), which maximises the glucose yield per unit of feedstock. Fig. 6B also offers a tool to quickly screen potential alternative feedstocks and ionic liquid pretreatment combinations based on experimental results in literature to highlight their potential for implementation in the lignocellulosic-mycoprotein process to reduce the MSP.
Fig. 6C shows the effects of varying feedstock cellulose and xylan composition and implication of carbon use efficiency by F. venenatum, two scenarios were modelled, where maximised carbon utilisation can be achieved if F. venenatum A3/5 is capable of growth on both lignocellulosic glucose and xylose (dashed line) in contrast to glucose utilisation only (solid line). For the case of xylose utilisation, instead of xylan being a hindrance on economic performance, it becomes desirable for a feedstock to maximise cellulose and xylan composition. Our experimental results suggested the importance of IL selection for lignocellulosic glucose extraction, where food-grade IL [Ch][HSO4] pretreatment showed low xylose and lignin removal efficiency in comparison to [TEA][HSO4]. However, such a strategy for xylose utilisation through appropriate strain selection offers a potential pathway for large improvements in the MSP.
Finally, a three variable RTEA was conducted where saccharification yield was also investigated alongside feedstock cellulose and xylan composition (Fig. 6D) as these are the most promising parameters to reduce the MSP. In this scenario xylose utilisation by F. venenatum was also assumed. The (predicted) current minimum selling price (CMSP) of the paste (see ESI S2.6† for derivation) was used as a benchmark to judge how competitive lignocellulosic-mycoprotein could be. No feasible region could be determined for achieving the CMSP. Therefore, the RTEA was run for a 100% increase of the CMSP. This was selected as it was deemed to be potentially acceptable by consumers for a product that could be produced with a reduced environmental impact (to be discussed later). As can be seen, even at a modest 80% saccharification yield, high cellulose feedstocks could be used to achieve that is no greater than twice the CMSP. If 90% yields could be obtained, then feedstocks with a cellulose content greater than 43% would be promising.
Overall, our RTEA modelling results suggest the potential degree of process improvement through improvement of certain process variables and highlight the future research frontier for lignocellulosic mycoprotein technology, i.e. ionic liquid performance and F. venenatum A3/5 growth on a wider variety of sugar substrates.
3.5 Comparative protein sources
In this study, the mycoprotein paste was modelled as the crude protein and compared to other crude protein sourced from livestock. Therefore, lignocellulosic-mycoprotein was compared both economically and environmentally to livestock at the slaughterhouse gate.From an economic perspective (Fig. 7), lignocellulosic-mycoprotein is comparable to Lamb and Beef on a mass basis, however on a protein basis (Table S7†) the cost of lignocellulosic-mycoprotein is approximately twice that of beef, and almost 5 times more expensive than chicken. At supermarket prices, the predicted consumer price of the final mycoprotein product ($21.80 kg−1/$173.02 per kg-protein) follows a similar trend compared to the cost of livestock protein (Table S8†). On the other hand, when we compare this price to an average price across a range of current Quorn products, the cost of lignocellulosic-mycoprotein is predicted to be around 1.9 times the current average Quorn supermarket selling price.
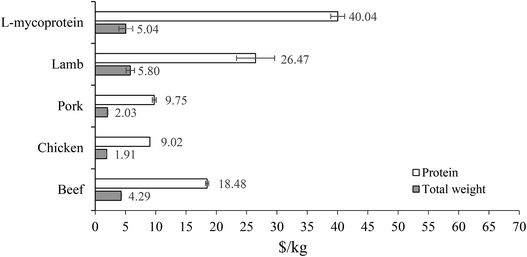 | ||
| Fig. 7 Comparison of minimum selling price (MSP) of lignocellulosic-mycoprotein (L-mycoprotein) to other protein sources on a basis of their production costs per kg protein (white bar) and kg product (grey bar). Error bars for comparative protein sources are standard deviation of monthly prices over a 12-month period (June 2019–May 2020). For L-mycoprotein, error bar represents the largest price change from responses to sensitivity analysis inputs (Table S28†). | ||
3.6. Life cycle assessment
Based on experimental data and process simulation (inventory detailed in ESI 3.2†), life cycle assessment has been conducted to investigate the environmental profiles of lignocellulosic mycoprotein. As shown in Fig. 8A, from a sustainability perspective, lignocellulosic-mycoprotein delivers significant advantages over beef in all impact categories except water consumption and delivers similar environmental footprint to chicken on global warming and terrestrial acidification. However, in comparison to animal-sourced protein and plant-based protein (Tofu), lignocellulosic-mycoprotein shows significant environmental benefits in arable land-use. Thus, lignocellulosic mycoprotein offers a promising protein solution which is largely independent of arable land in comparison to land-demanding protein sourced from animal and arable crops (e.g. meat and tofu).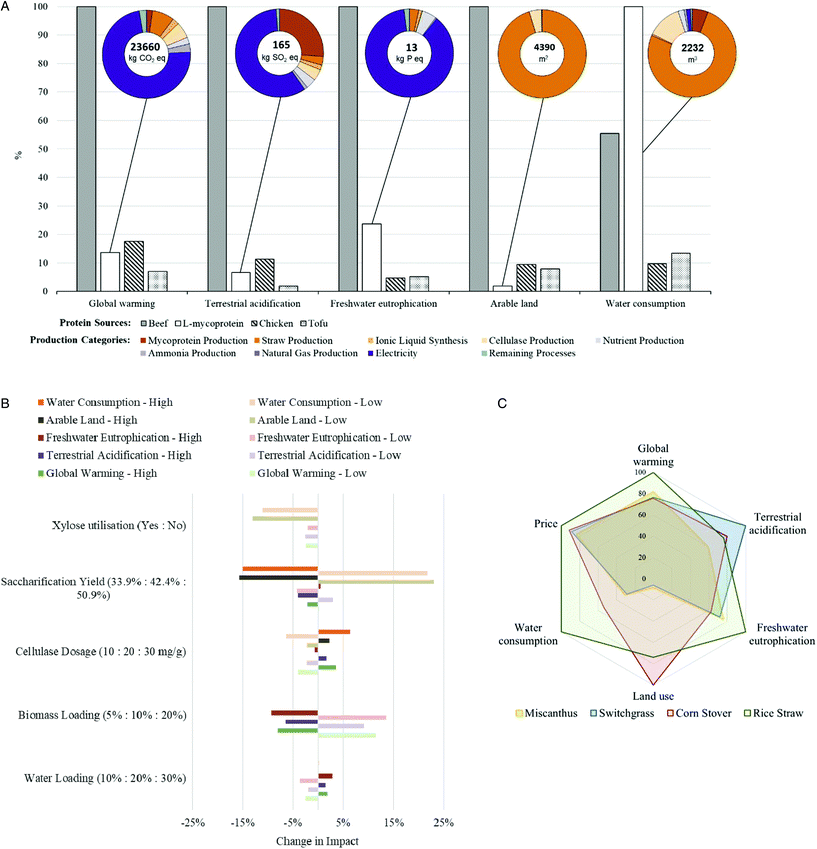 | ||
| Fig. 8 (A) LCIA comparison of lignocellulosic-mycoprotein (L-mycoprotein) vs. animal-sourced protein (beef and chicken) and plant-based protein (Tofu) on 5 impact categories; global warming, terrestrial acidification, freshwater eutrophication, arable land, and water consumption (Table S33†). LCIA results have been normalised to 100%. The breakdown of the process contribution to each impact category for lignocellulosic-mycoprotein is shown in the circular charts (Table S34†). (B) Sensitivity analysis for process assumptions used in Fig. 5 compared to the base case scenario (Tables S35 and S36). ‘High’ and ‘Low’ values of impact categories represent the bounds of the sensitivity range tested (i.e. water consumption – high represents the water consumption for the higher value of each sensitivity parameter shown on the y-axis). (C) LCIA comparison of lignocellulosic-mycoprotein from rice straw compared to three other feedstocks: switchgrass, miscanthus and corn stover. ReCiPe2016 Midpoint (H) V1.02, economic allocation, functional unit = 1 tonne protein. | ||
The performance-limiting steps over the lignocellulosic-mycoprotein life cycle vary between impact category. Straw production dominates the impacts on water consumption (75.7%), due to rice irrigation. A less water-demanding feedstock would offer a mitigation solution to reduce water consumption.
For global warming, total GHGs of 516 kg CO2 eq. per tonne protein are attributed to process emissions, after accounting for biogenic emissions (ESI S.8†), that is to say, 516 kg CO2 eq. per tonne of is emitted that does not originate from biogenic sources, representing only 2.4% of overall contribution to global warming. The main contributor is the emissions due to external electricity use and production representing 72.9% of emissions. Therefore, a potential decarbonisation solution would be to integrate renewable energy resources in lignocellulosic mycoprotein system to target over a third of energy-related emissions.
For terrestrial acidification the largest contributions to overall acidification are electricity and process emissions which contribute 58.4% and 26.3% respectively. In the case of marine eutrophication, 67.58% is due to straw production.
In terms of arable land, over 95% is associated with rice straw production which is due to allocating some of the rice growth burden to the rice straw itself (1.56% of total impact of rice production). However, as the straw is considered a waste product of rice growth, it is worth considering the change in impact if no burden is allocated to the straw. Therefore, sensitivity analyses were performed to understand the implications of the allocation approach on LCA results. With zero burden of rice production allocated to rice straw, a significant reduction of the impact of lignocellulosic mycoprotein was observed on land-use (209 ha per tonne-protein), significantly less than the 4390 ha per tonne-protein when the straw is still considered to have an impact associated with its production. This analysis highlights the difficulties in assigning an exact arable land consumption due to allocation of burden to byproducts. Furthermore, the allocation sensitivity analysis also revealed a significant reduction in water consumption (542 m3 water per tonne-protein, 76% reduction) (Fig. S5†) due to water use in the rice production stage being mitigated in a zero burden scenario. This result highlights the advantage of utilising lignocellulosic feedstocks compared to starch-derived glucose, as much of the impact from crop growth is mitigated.
Finally, to understand the sensitivity of the LCA results due to process parameters, a sensitivity analysis was conducted for a range of process parameters (Fig. 8B). The results revealed that across all impact categories, the maximum variation is +23/−16% of the base case scenario. Improvements in saccharification yield, and xylose utilisation would provide the greatest benefits to the sustainability of the lignocellulosic mycoprotein process due primarily to large reductions in feedstock required to produce the same output of protein. Although the electricity grid mix was not considered in the sensitivity analysis, based on the assumed electricity production mix (natural gas – 20.47%, coal – 41.55%, oil – 3.95%, nuclear – 11.27%, hydroelectric – 18.46%, wind – 3.22%, biomass – 0.42%, biogas – 0.31%, peat – 0.03% (Table S9†)), this work can be used as a baseline to understand how the sustainability of the process may change depending on the location of its implementation or changes in the grid mix due to future shifts in electricity generation sources. With the continuing progress towards an increasing share of renewably-sourced electricity, a significant improvement in the sustainability could be attained. On the other hand, if this technology is to be implemented in parts of the world still heavily reliant on fossil fuel resources, the sustainability of this process could be reduced. Therefore, future work should address the uncertainty regarding location-based parameters, such as the electricity grid mix, and their effect on the sustainability of the technology.
Lignocellulosic mycoprotein performance could be further optimised by utilising alternative lignocellulosic resources instead of rice straw (Fig. 8C). Benefiting from higher cellulose composition, three other feedstocks (switchgrass, miscanthus and corn stover) delivered an improved MSP between 83–91% that of rice-straw derived mycoprotein (Table S38†). However, significant reductions in water consumption would be realised for these alternative feedstocks due to their less water demanding growth. Alternative lignocellulosic resources (miscanthus and switchgrass) offer significant reduced arable land use compared with lignocellulosic agricultural residues. Finally, improvements in GHGs can be achieved with alternative feedstocks. Overall, non-food crops (miscanthus and switchgrass) represent environmentally competitive lignocellulosic resources for future exploratory research on lignocellulosic mycoprotein.
4. Conclusion
This study presents a new lignocellulosic mycoprotein solution, which has been demonstrated as a potential technically viable and environmentally sustainable meat substitute. A considerable amount of cellulose resources are available globally, offering potential underutilised resources for lignocellulosic mycoprotein production. Assuming the estimated annual global availability of rice straw of 520 million tonnes,49 8 million tonnes of rice straw-derived mycoprotein could be produced, which would represent 4% of the current predicted global protein demand.1Our experimental results suggested the importance of ionic liquid pretrement in lignocellulosic mycoprotein production. In contrast with choice of cellulase enzyme, the ionic liquid produced greater impacts on the glucose release. Non food-grade IL [TEA][HSO4] outperformed its food-grade counterpart ([Ch][HSO4]), leading to significantly higher (over 50%) glucose release. The food-safety of [TEA][HSO4] is unknown but potentially can be certified as food-grade ionic liquid.
Modelling results demonstrate that the untextured lignocellulosic mycoprotein paste could be produced at a competitive price of $5.04 kg−1 ($40.02 per kg-protein). Although more expensive than meat-protein, lignocellulosic-mycoprotein offers significant potential for improving the sustainability of protein for human consumption. Lignocellulosic mycoprotein offers decarbonisation potential in comparison with protein derived from beef. However, the highlight of this technology is the minimum arable land usage required due to the main feedstock being agricultural residue.
This work can be seen as an initial study highlighting from both an economical and sustainability perspective the potential for this technology. Further work in this area is needed before full commercialisation could be envisaged. Primarily, further process optimisation should be undertaken from both an experimental and computational perspective. For example, increasing pretreatment yields through improving IL performance or selection of the ionic liquid/biomass combination, optimised separation design for cost-effective recovery of the ionic liquid by exploring alternative separation technologies and specifications. Ionic liquid has been researched as a promising pretreatment technology due to the customisable nature of the IL (selecting the cation–anion pair) and process safety offered by their low volatility. Notably, the food-grade ionic liquid (choline hydrogen sulphate) investigated in our research, can also be used to enhance F. venenatum fermentation; thus there exists a scope for future in-depth research to optimise the separation through synergistic process integration. Furthermore, instead of ILs, alternative food-safe pretreatment technologies offer another research frontier for future investigation.
Overall, our research suggests that lignocellulosic mycoprotein sourced from food-safe lignocellulose represents a transformative solution to animal and plant-sourced proteins which are carbon-intensive and natural resource-demanding. Both animal sourced and plant sourced proteins not only cause significant environmental concerns on climate change and arable land use, but also are constrained by long-production cycles; thus, their supply chains are vulnerable to global arable land scarcity and extreme events e.g. public health emergency. In contrast, lignocellulosic mycoprotein not only enables decarbonised protein supply, which is largely independent of arable land, but also offers future protein security due to its manufacturing in controlled environments and very short production cycles.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would also like to acknowledge the Research England GCRF for providing financial support for research under the programme ‘Production of single-cell proteins from agricultural and forestry waste’. We would also like to acknowledge the UK Engineering and Physical Sciences Research Council (EPSRC) for providing financial support for research under the DTP programme ‘Systems modelling of ionic liquid biorefining – lignocellulosic mycoprotein process design’ and project ‘Resilient and Sustainable Biorenewable Systems Engineering Model’ [EP/N034740/1].In addition, the enzyme samples and data support from Novozymes A/S are hugely appreciated. The authors would also like to express gratitude to International Rice Research Institute (IRRI) for providing the rice straw samples in our collaborative research.
References
- M. Henchion, M. Hayes, A. M. Mullen, M. Fenelon and B. Tiwari, Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium, Foods, 2017, 6(7), 53 CrossRef PubMed
.
-
FAO, IFAD, UNICEF, WFP and WHO. The State of Food Security and Nutrition in the World 2019, FAO, Rome, 2019 Search PubMed
.
-
I. C. Prentice, S. Willams and P. Friedlingstein, Biosphere feedbacks and climate change, Grantham Insitute, 2015 Search PubMed
.
- M. R. Smith and S. S. Myers, Impact of anthropogenic CO2 emissions on global human nutrition, Nat. Clim. Change, 2018, 8(9), 834–839 CrossRef CAS
.
- M. Herrero, B. Henderson, P. Havlík, P. K. Thornton, R. T. Conant and P. Smith,
et al., Greenhouse gas mitigation potentials in the livestock sector, Nat. Clim. Change, 2016, 6(5), 452–461 CrossRef
.
- J. Yan, B. Han, X. Gui, G. Wang, L. Xu and Y. Yan,
et al., Engineering Yarrowia lipolytica to Simultaneously Produce Lipase and Single Cell Protein from Agro-industrial Wastes for Feed, Sci. Rep., 2018, 8(1), 758 CrossRef PubMed
.
- Y. Gao, D. Li and Y. Liu, Production of single cell protein from soy molasses using Candida tropicalis, Ann. Microbiol., 2011, 62(3), 1165–1172 CrossRef
.
- F.-u.-N. Yunus, M. Nadeem and F. Rashid, Single-cell protein production through microbial conversion of lignocellulosic residue (wheat bran) for animal feed, J. Inst. Brew., 2015, 121(4), 553–557 CrossRef CAS
.
- B. Liu, J. Song, Y. Li, J. Niu, Z. Wang and Q. Yang, Towards industrially feasible treatment of potato starch processing waste by mixed cultures, Appl. Biochem. Biotechnol., 2013, 171(4), 1001–1010 CrossRef CAS PubMed
.
- B. Liu, Y. Li, J. Song, L. Zhang, J. Dong and Q. Yang, Production of single-cell protein with two-step fermentation for treatment of potato starch processing waste, Cellulose, 2014, 21(5), 3637–3645 CrossRef CAS
.
- T. Aggelopoulos, K. Katsieris, A. Bekatorou, A. Pandey, I. M. Banat and A. A. Koutinas, Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production, Food Chem., 2014, 145, 710–716 CrossRef CAS PubMed
.
- A. Ritala, S. T. Hakkinen, M. Toivari and M. G. Wiebe, Single Cell Protein-State-of-the-Art, Industrial Landscape and Patents 2001-2016, Front. Microbiol., 2017, 8, 2009 CrossRef
.
- B. Molitor, A. Mishra and L. T. Angenent, Power-to-protein: converting renewable electric power and carbon dioxide into single cell protein with a two-stage bioprocess, Energy Environ. Sci., 2019, 12, 3515–3521 RSC
.
- M. G. Wiebe, Myco-protein from Fusarium venenatum: a well-established product for human consumption, Appl. Microbiol. Biotechnol., 2002, 58(4), 421–427 CrossRef CAS
.
- Quorn. Quorn Foods sees 16% global growth in 2017 2018 [available from: https://www.quorn.co.uk/company/press/quorn-foods-global-growth-in-2017.
- IRRI. Our Work: International Rice Research Institute; 2019 [available from: https://www.irri.org/our-work/research.
-
P. Kumar and L. Joshi, Pollution Caused by Agricultural Waste Burning and Possible Alternate Uses of Crop Stubble: A Case Study of Punjab, 2013, pp. 367–385 Search PubMed
.
- W. Den, V. K. Sharma, M. Lee, G. Nadadur and R. S. Varma, Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals, Front. Chem., 2018, 6, 141 CrossRef PubMed
.
- N. R. Baral and A. Shah, Techno-economic analysis of cellulose dissolving ionic liquid pretreatment of lignocellulosic biomass for fermentable sugars production, Biofuels, Bioprod. Biorefin., 2016, 10(1), 70–88 CrossRef CAS
.
- P. J. Strong, M. Kalyuzhnaya, J. Silverman and W. P. Clarke, A methanotroph-based biorefinery: Potential scenarios for generating multiple products from a single fermentation, Bioresour. Technol., 2016, 215, 314–323 CrossRef CAS PubMed
.
- Animal feed prices. In: Department for Environment FRA, editor. https://www.gov.uk/government/statistical-data-sets/animal-feed-prices2020.
- D. J. M. Hayes, Development of near infrared spectroscopy models for the quantitative prediction of the lignocellulosic components of wet Miscanthus samples, Bioresour. Technol., 2012, 119, 393–405 CrossRef CAS PubMed
.
- D. J. M. Hayes. Personal communication., 2019.
- F. J. Gschwend, A. Brandt, C. L. Chambon, W. C. Tu, L. Weigand and J. P. Hallett, Pretreatment of Lignocellulosic Biomass with Low-cost Ionic Liquids, J. Visualized Exp., 2016, 114 Search PubMed
.
- F. J. V. Gschwend, F. Malaret, S. Shinde, A. Brandt-Talbot and J. P. Hallett, Rapid pretreatment of Miscanthus using the low-cost ionic liquid triethylammonium hydrogen sulfate at elevated temperatures, Green Chem., 2018, 20(15), 3486–3498 RSC
.
-
D. Moore, G. D. Robson and A. P. J. Trinci, in 21st Century Guidebook to Fungi, Cambridge University Press, Cambridge, 2nd edn, 2020 Search PubMed
.
- THE WORLD'S BIGGEST MEAT ALTERNATIVE PRODUCTION FACILITY OPENS IN THE HEART OF THE NORTH EAST [press release]. 15th November 2018 2018.
-
R. Davis, L. Tao, C. Scarlata, E. C. D. Tan, J. Ross and J. Lukas, et al., Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons, National Renewable Energy Laboratory, 2015 Search PubMed
.
- A. A. Longati, A. R. A. Lino, R. C. Giordano, F. F. Furlan and A. J. G. Cruz, Defining research & development process targets through retro-techno-economic analysis: The sugarcane biorefinery case, Bioresour. Technol., 2018, 263, 1–9 CrossRef CAS PubMed
.
- M. G. Brondi, A. M. Elias, F. F. Furlan, R. C. Giordano and C. S. Farinas, Performance targets defined by retro-techno-economic analysis for the use of soybean protein as saccharification additive in an integrated biorefinery, Sci. Rep., 2020, 10(1), 7367 CrossRef CAS PubMed
.
- F. F. Furlan, C. B. B. Costa, A. R. Secchi, J. M. Woodley and R. C. Giordano, Retro-Techno-Economic Analysis: Using (Bio)Process Systems Engineering Tools To Attain Process Target Values, Ind. Eng. Chem. Res., 2016, 55(37), 9865–9872 CrossRef CAS
.
- D. G. Krige, A statistical approach to some basic mine valuation problems on the Witwatersrand, J. South. Afr. Inst. Min. Metall., 1951, 52(6), 119–139 Search PubMed
.
- D. Gorissen, K. Crombecq, I. Couckuyt, T. Dhaene and P. Demeester, A Surrogate Modeling and Adaptive Sampling Toolbox for Computer Based Design, J. Mach. Learn. Res., 2010, 11, 2051–2055 Search PubMed
.
-
J. R. Hess, C. T. Wright, K. L. Kenney and E. M. Searcy, Uniform-Format Solid Feedstock Supply System: A Commodity-Scale Design to Produce an Infrastructure-Compatible Bulk Solid from Lignocellulosic Biomass - Executive Summary, Laboratory IN, 2009 Search PubMed
.
- K. L. Kenney, W. A. Smith, G. L. Gresham and T. L. Westover, Understanding biomass feedstock variability, Biofuels, 2014, 4(1), 111–127 CrossRef
.
- S. Harun, V. Balan, M. S. Takriff, O. Hassan, J. Jahim and B. E. Dale, Performance of AFEX™ pretreated rice straw as source of fermentable sugars: the influence of particle size, Biotechnol. Biofuels, 2013, 6, 40 CrossRef CAS PubMed
.
-
D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu and A. Aden, et al., Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. National Renewable Energy Laboratory, 2011 Search PubMed
.
- A. Brandt-Talbot, F. J. V. Gschwend, P. S. Fennell, T. M. Lammens, B. Tan and J. Weale,
et al., An economically viable ionic liquid for the fractionation of lignocellulosic biomass, Green Chem., 2017, 19(13), 3078–3102 RSC
.
-
H. Sixta, Handbook of Pulp, Wiley, 2006 Search PubMed
.
-
D. Moore, G. D. Robson and A. P. J. Trinci, 21st Century Guidebook to Fungi, Cammbridge University Press, Cambridge, UK, 2011 Search PubMed
.
- H. J. Vogel, A Convenient Growth Medium for Neurospora crassa, Microb. Genet. Bull., 1956, 13, 42–47 Search PubMed
.
- D. Klein-Marcuschamer, B. A. Simmons and H. W. Blanch, Techno-economic analysis of a lignocellulosic ethanol biorefinery with ionic liquid pre-treatment, Biofuels, Bioprod. Biorefin., 2011, 5(5), 562–569 CrossRef CAS
.
- L. Fele and V. Grilc, Separation of Furfural from Ternary Mixtures, J. Chem. Eng. Data, 2003, 48(3), 564–570 CrossRef CAS
.
- N. Galeotti, F. Jirasek, J. Burger and H. Hasse, Recovery of Furfural and Acetic Acid from Wood Hydrolysates in Biotechnological Downstream Processing, Chem. Eng. Technol., 2018, 41(12), 2331–2336 CrossRef CAS PubMed
.
-
W. D. Seider, D. R. Lewin, J. D. Seader, S. Widagdo, R. Gani and K. M. Ng. Product and process design principles : synthesis, analysis, and evaluation, 2017 Search PubMed
.
- A. George, A. Brandt, K. Tran, S. M. S. N. S. Zahari, D. Klein-Marcuschamer and N. Sun,
et al., Design of low-cost ionic liquids for lignocellulosic biomass pretreatment, Green Chem., 2015, 17(3), 1728–1734 RSC
.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Inexpensive ionic liquids: [HSO4]−-based solvent production at bulk scale, Green Chem., 2014, 16(6), 3098–3106 RSC
.
- H. Baaqel, I. Díaz, V. Tulus, B. Chachuat, G. Guillén-Gosálbez and J. P. Hallett, Role of life-cycle externalities in the valuation of protic ionic liquids – a case study in biomass pretreatment solvents, Green Chem., 2020, 22(10), 3132–3140 RSC
.
-
N. Van Hung, M. C. Maguyon-Detras, M. V. Migo, R. Quilloy, C. Balingbing and P. Chivenge, et al., Rice Straw Overview: Availability, Properties, and Management Practices, in Sustainable Rice Straw Management, ed. M. Gummert, N. V. Hung, P. Chivenge and B. Douthwaite, Springer International Publishing, Cham, 2020. p. 1–13 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc01021b |
| This journal is © The Royal Society of Chemistry 2021 |