Chemo- and regioselective hydroformylation of alkenes with CO2/H2 over a bifunctional catalyst†
Abstract
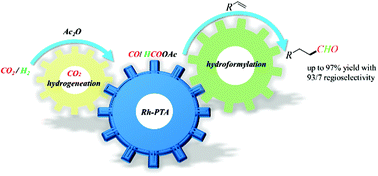
As is well known, CO2 is an attractive renewable C1 resource and H2 is a cheap and clean reductant. Combining CO2 and H2 to prepare building blocks for high-value-added products is an attractive yet challenging topic in green chemistry. A general and selective rhodium-catalyzed hydroformylation of alkenes using CO2/H2 as a syngas surrogate is described here. With this protocol, the desired aldehydes can be obtained in up to 97% yield with 93/7 regioselectivity under mild reaction conditions (25 bar and 80 °C). The key to success is the use of a bifunctional Rh/PTA catalyst (PTA: 1,3,5-triaza-7-phosphaadamantane), which facilitates both CO2 hydrogenation and hydroformylation. Notably, monodentate PTA exhibited better activity and regioselectivity than common bidentate ligands, which might be ascribed to its built-in basic site and tris-chelated mode. Mechanistic studies indicate that the transformation proceeds through cascade steps, involving free HCOOH production through CO2 hydrogenation, fast release of CO, and rhodium-catalyzed conventional hydroformylation. Moreover, the unconventional hydroformylation pathway, in which HCOOAc acts as a direct C1 source, has also been proved to be feasible with superior regioselectivity to that of the CO pathway.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...