Environment-friendly and efficient synthesis of 2-aminobenzo-xazoles and 2-aminobenzothiazoles catalyzed by Vitreoscilla hemoglobin incorporating a cobalt porphyrin cofactor†
Abstract
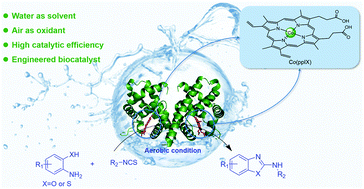
In this study, an environment-friendly and efficient artificial Vitreoscilla hemoglobin (VHb) for the synthesis of 2-aminobenzoxazoles and 2-aminobenzothiazoles has been reported. We demonstrate an expression-based porphyrin substitution strategy to produce VHb containing cobalt porphyrin instead of native hemin, which can catalyze the oxidative cyclization of corresponding 2-aminobenzoxazoles and 2-aminobenthiazoles with up to 97% yield and 4850 catalytic turnovers in water under aerobic conditions. Hence, we provide a green and mild method for the synthesis of 2-aminobenzoxazoles and 2-aminobenzothiazoles. In addition, we indicate the value of porphyrin ligand substitution as a strategy to tune and enhance the catalytic properties of hemoproteins in non-natural reactions.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...