Aryl acyl peroxides for visible-light induced decarboxylative arylation of quinoxalin-2(1H)-ones under additive-, metal catalyst-, and external photosensitizer-free and ambient conditions†
Long-Yong
Xie
a,
Sha
Peng
a,
Li-Hua
Yang
a,
Cun
Peng
a,
Ying-Wu
Lin
 b,
Xianyong
Yu
c,
Zhong
Cao
b,
Xianyong
Yu
c,
Zhong
Cao
 d,
Yu-Yu
Peng
d and
Wei-Min
He
d,
Yu-Yu
Peng
d and
Wei-Min
He
 *a
*a
aDepartment of Chemistry and Bioengineering, Hunan University of Science and Engineering, Yongzhou 425100, China
bSchool of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
cSchool of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China. E-mail: weiminhe2016@yeah.net
dHunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha, 410114, China
First published on 23rd October 2020
Abstract
Aryl radicals were generated for the first time from cheap and easily available aryl acyl peroxides in eco-friendly ethyl acetate under ambient conditions and visible-light illumination in the absence of any additive, metal catalyst, or external photosensitizer. The present arylation of quinoxalin-2(1H)-ones was chemo- and regioselective, and provided good access to various 3-arylquinoxalin-2(1H)-ones.
Introduction
Carboxylic acids, as a class of fundamental and critical compounds, are commonly used in almost all fields of chemical science, especially fine chemistry and pharmaceutical chemistry. Given their low cost, nontoxicity and abundant availability advantages, the utilization of carboxylic acid derivatives as radical precursors for the production of value-added chemicals has emerged as a powerful approach in organic synthesis.1 Diacyl peroxides are frequently used as free-radical precursors, as they can not only generate oxygen-centered radicals (acyloxyl radicals)2 and carbon-centered radicals (alkyl and aryl radicals),3 but also be easily prepared through the oxidation of carboxylic acids with H2O2. Although significant progress in the utilization of diacyl peroxides as radical precursors has been achieved, the vast majority of these reactions require a high reaction temperature (>100 °C) and/or transition-metal catalysts, which greatly limits their practical applications.In recent decades, visible-light photocatalysis has become an increasingly important subject in synthetic chemistry.4 Indeed, photocatalysis has been considered to meet the requirements of green chemistry. However, in most cases, precious or toxic transition metals, or ecologically harmful photocatalysts are necessary for the process of photocatalysis, which brings about cumbersome separation and purification processes, and this not only produces waste, but also increases production costs and leads to environmental problems. Therefore, it is necessary to develop new visible light induced reactions with no external photocatalysts according to the requirements of green chemistry.
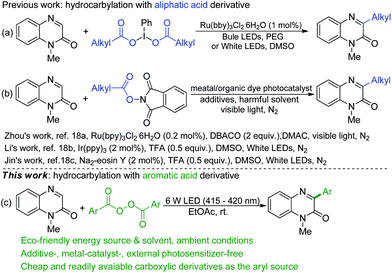
Quinoxalin-2(1H)-one is an important structural motif and is found in plenty of natural products and biologically active compounds.5 Over the past few years, a series of efficient protocols for the preparation of C3-functionalized quinoxalin-2(1H)-ones have been established,6 including alkylation,7 arylation,8 alkoxylation,9 acylation,10 amination,11 amidation,12 cyanation,13 fluoromethylation,14 sulfenylation,15 and phosphonation16 reactions. Due to the valuable biological and pharmaceutical properties of 3-arylquinoxalin-2(1H)-ones, considerable efforts have been devoted to eco-friendly construction of such motifs, especially in pharmaceutical chemistry. In contrast to the wealth of literature data on using carboxylic derivatives phenyliodine(III) dicarboxylates17 (Scheme 1a) and N-hydroxyphthalimide esters18 (Scheme 1b) as alkyl radical precursors in the photocatalytic functionalization of quinoxalin-2(1H)-ones, the usage of aryl acyl peroxides as aryl radical precursors in photocatalysis, particularly in the functionalization of quinoxalin-2(1H)-ones, is still unreported.
Under the guidance of green chemistry principles,19 we herein disclose an eco-friendly visible-light-induced decarboxylative arylation of quinoxalin-2(1H)-ones with aryl acyl peroxides for the construction of various 3-arylquinoxalin-2(1H)-ones in eco-friendly ethyl acetate at room temperature. To the best of our knowledge, this is the first example of the visible-light induced arylation reaction with carboxylic derivatives as aryl sources in a green solvent under additive-, metal-catalyst-, and external photosensitizer-free conditions.
Results and discussion
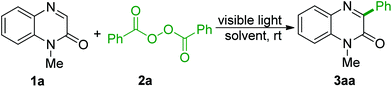
We started our investigation by employing the N-methyl-3-phenylquinoxalin-2(1H)-one (1a) and dibenzoyl peroxide (BPO, 2a) as the template substrates (Table 1). Conducting the reaction in MeCN under the irradiation of a 6 W LED (400–405 nm) at room temperature gave the target product 3aa in 16% NMR yield (Table 1, entry 1). Subsequently, a series of reaction solvents were examined, and the results revealed that the reaction medium had a significant influence on the transformation (entries 2–7). The eco-friendly ethyl acetate20 was found to be the most suitable reaction medium for the current transformation (entry 3), while other solvents gave lower yields or a trace amount of product 3aa. Screening of visible-light sources showed that the wavelength of the LED had a significant impact on the reaction efficiency (entries 8–13). Performing the reaction with the irradiation wavelength in the range of 415–420 nm delivered an 84% NMR yield of 3aa (entry 11). No transformation was observed when the reaction was carried out in the absence of visible light (entry 14). Performing the arylation reaction under a nitrogen atmosphere did not influence the reaction outcome (entry 15). No improvement in the yield was observed by prolonging the irradiation time to 12 h (entry 16).| Entry | Light source | Solvent | Yieldb |
|---|---|---|---|
| a Conditions: Unless otherwise specified, the reactions were carried out in a quartz tube in the presence of 1a (0.1 mmol), 2a (0.15 mmol), and solvent (0.5 mL) under a 6 W LED for 3 h. b Estimated by 1H NMR using diethyl phthalate as an internal reference. c Under a nitrogen atmosphere. d 12 h. NR: no reaction. | |||
| 1 | 400–405 nm | CH3CN | 16 |
| 2 | 400–405 nm | DCE | 28 |
| 3 | 400–405 nm | EtOAc | 75 |
| 4 | 400–405 nm | EtOH | 7 |
| 5 | 400–405 nm | THF | 34 |
| 6 | 400–405 nm | DMSO | Trace |
| 7 | 400–405 nm | H2O | NR |
| 8 | 390–395 nm | EtOAc | 64 |
| 9 | 395–400 nm | EtOAc | 66 |
| 10 | 410–415 nm | EtOAc | 76 |
| 11 | 415–420 nm | EtOAc | 84 |
| 12 | 420–425 nm | EtOAc | 74 |
| 13 | 440–445 nm | EtOAc | 32 |
| 14 | Dark conditions | EtOAc | NR |
| 15c | 415–420 nm | EtOAc | 84 |
| 16d | 415–420 nm | EtOAc | 83 |
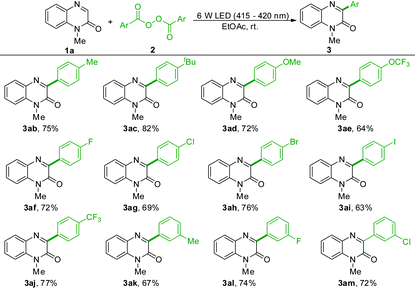
With the optimal general procedure in hand (Table 1, entry 11), the generality of decarboxylative arylation was investigated with respect to both quinoxalin-2(1H)-ones and aryl acyl peroxides. Quinoxalinones modified with alkyl and phenyl groups of various chain lengths at the nitrogen atom reacted with 2a efficiently and delivered the target products (3aa–3ka) in good yields. A variety of important functional groups, such as alkenyl (3ea), alkynyl (3fa), ester (3ga), 2-oxo-2-phenylethyl (3ha), Boc (3ia) and tosyl (3ja), were well tolerated in the current reaction, enabling further possible functionalization at these positions. However, no reaction was observed when 1-(methylsulfonyl)quinoxalin-2(1H)-one was used as the substrate. Plenty of mono- and disubstituted-quinoxalinones worked well in the current protocol, providing the target products (3la–3wa) in moderate to good yields. Among these 3-phenylquinoxalinone derivatives, the halogen-containing products could be further functionalized for the late-stage construction of complex organic compounds. In addition, the quinoxalinone without the N-protected group was also a suitable substrate for the present reaction and delivered the product 3xa in 78% yield.
Subsequently, the scope of aryl acyl peroxides was investigated. As shown in Table 2, aryl acyl peroxides containing a series of functional groups at the para-position of the phenyl ring ranging from electron-donating groups such as alkyl (3ab and 3ac) and alkoxy (3ad and 3ae) to electron-withdrawing groups such as halogens (3af–3ai) and trifluoromethyl (3aj) underwent the reaction smoothly to give the corresponding products in 63–82% yields. Among these products, the resulting products containing halogens such as chlorine, bromine and iodine could be available for further functionalization. Moreover, the current protocol was also well tolerated with various substitutions at the meta positions of the phenyl ring of peroxides, providing the desired products in good yields (3ak–3am). 2-Methylbenzoic peroxyanhydride gave no desired products, presumably due to the steric hindrance. Only a trace amount of the alkylated product was observed when using lauroyl peroxide as the alkylating agent.
| a Conditions: 1 (0.3 mmol), 2 (0.45 mmol), EtOAc (1.5 mL), a 6 W LED (415–420 nm), rt. |
|---|
|
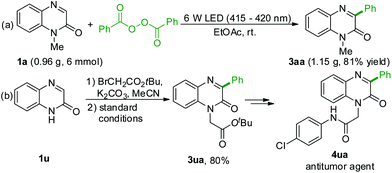
Once the substrate scope of this decarboxylative arylation was established, it was important to investigate its practical applicability. Performing the reaction of quinoxalin-2(1H)-one 1a (6 mmol) under the optimal conditions gave the target product 3aa in 81% yield (Scheme 2a). In addition, substrate 1u underwent one-pot N-alkylation and decarboxylative arylation delivering the desired product 3ua, which could be easily transformed into the antitumor agent 4ua (Scheme 2b).
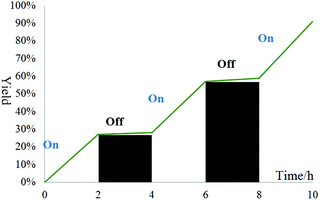
The visible-light irradiation on–off experiment of the present reaction revealed that the current reaction was seriously suppressed in the absence of visible light irradiation (Fig. 1). These experimental results demonstrate the dependence of the reaction on light.
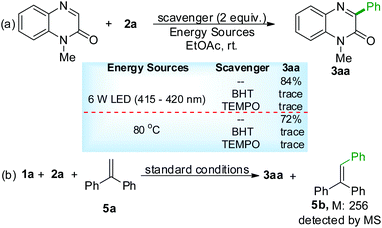
To better understand the reaction mechanism, some control experiments were conducted. When 2 equiv. of TEMPO or BHT were added to the reaction system, the expected transformation was fully inhibited with only a trace amount of 3aa generated under visible-light radiation or traditional heating conditions (Scheme 3a). The treatment of BPO with 1,1-diphenylethylene (5a, 2 equiv.) under the irradiation of a 6 W LED (415–420 nm) at ambient temperature gave triphenylethene (5b), revealing that the phenyl radical might be involved in this process (Scheme 3b). The results of UV-Vis absorption spectroscopy and fluorescence quenching experiment (for details see the ESI†) suggested that both the substrate 1a and product 3aa could absorb visible light, and they may serve as photosensitizes.
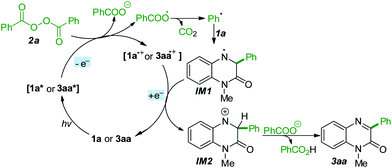
Based on these experimental results and related literature precedents,21 a possible reaction mechanism is depicted in Scheme 4. The quinoxalin-2(1H)-one (1a) was firstly excited with visible light to form the excited 1a*, which next underwent a single electron transfer (SET) process with BPO (2a) to generate 1a radical cation, a benzoate anion and a benzoate radical. The benzoate radical was easily converted into a phenyl radical, which reacted with ground-state 1a to afford the N-centered radical IM1. Subsequently, a second SET process occurred between the radical IM1 and 1a radical cation, leading to the formation of cationic intermediate IM2 and ground-state 1a. Eventually, the product 3aa and benzoic acid were formed by the deprotonation of intermediate IM2 with the assistance of the benzoate anion.
Conclusions
In conclusion, we have established, for the first time, a facile and eco-friendly visible-light initiated decarboxylative arylation of quinoxalin-2(1H)-ones with aryl acyl peroxides as aryl radical precursors. In sharp contrast to the previous radical functionalizations with carboxylic acid derivatives as radical precursors, the present reaction was promoted by visible light in the absence of any additive, metal catalyst, or external photosensitizer. A wide range of 3-arylquinoxalin-2(1H)-ones (36 examples) were chemo- and regioselectively synthesized with good yields in eco-friendly ethyl acetate at ambient temperature under mild conditions. The reaction has the advantages of easy to obtain raw materials, low cost, clean and mild reaction conditions, simple operation steps and strong scalability, so it is expected to be an ideal method for the synthesis of pharmaceuticals and other fine chemicals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the Hunan Provincial Natural Science Foundation of China (No. 2020JJ5203 and 2019JJ20008).Notes and references
- N. Rodríguez and L. J. Goossen, Chem. Soc. Rev., 2011, 40, 5030 RSC.
- (a) C. Qian, D. Lin, Y. Deng, X.-Q. Zhang, H. Jiang, G. Miao, X. Tang and W. Zeng, Org. Biomol. Chem., 2014, 12, 5866 RSC; (b) Z. Zhou, J. Cheng and J.-T. Yu, Org. Biomol. Chem., 2015, 13, 9751 RSC; (c) X. Yao, X. Weng, K. Wang, H. Xiang and X. Zhou, Green Chem., 2018, 20, 2472 RSC; (d) V. Botla, N. Pilli and C. Malapaka, Green Chem., 2019, 21, 1735 RSC.
- (a) C. Pan, J. Zhu, R. Chen and J.-T. Yu, Org. Biomol. Chem., 2017, 15, 6467 RSC; (b) R. Wei, H. Xiong, C. Ye, Y. Li and H. Bao, Org. Lett., 2020, 22, 3195 CrossRef CAS; (c) X. Zhu, M. Su, Q. Zhang, Y. Li and H. Bao, Org. Lett., 2020, 22, 620 CrossRef CAS.
- (a) Y. Kong, W. Xu, F. Ye and J. Weng, Chin. J. Org. Chem., 2019, 39, 3065 CrossRef CAS; (b) J. Xuan, X.-K. He and W.-J. Xiao, Chem. Soc. Rev., 2020, 49, 2546 RSC; (c) X. Mi, Y. Kong, J. Zhang, C. Pi and X. Cui, Chin. Chem. Lett., 2019, 30, 2295 CrossRef CAS; (d) D.-Q. Dong, L.-X. Li, G.-H. Li, Q. Deng, Z.-L. Wang and S. Long, Chin. J. Catal., 2019, 40, 1494 CrossRef CAS; (e) Y. Lv, P. Bao, H. Yue, J.-S. Li and W. Wei, Green Chem., 2019, 21, 6051 RSC; (f) W.-B. He, L.-Q. Gao, X.-J. Chen, Z.-L. Wu, Y. Huang, Z. Cao, X.-H. Xu and W.-M. He, Chin. Chem. Lett., 2020, 31, 1895 CrossRef CAS; (g) S. He, X. Chen, F. Zeng, P. Lu, Y. Peng, L. Qu and B. Yu, Chin. Chem. Lett., 2020, 31, 1863 CrossRef CAS; (h) S. Peng, Y.-W. Lin and W.-M. He, Chin. J. Org. Chem., 2020, 40, 541 CrossRef; (i) L.-Y. Xie, Y.-S. Liu, H.-R. Ding, S.-F. Gong, J.-X. Tan, J.-Y. He, Z. Cao and W.-M. He, Chin. J. Catal., 2020, 41, 1168 CrossRef CAS; (j) L. Wang, M. Zhang, Y. Zhang, Q. Liu, X. Zhao, J.-S. Li, Z. Luo and W. Wei, Chin. Chem. Lett., 2020, 31, 67 CrossRef CAS.
- S. Leilei, Z. Hua, W. Jifeng and L. Xun, Mini-Rev. Org. Chem., 2015, 12, 96 Search PubMed.
- P. Miao, J. Zhu, J. Yuan, L. Yang, Y. Xiao and C. Zhang, Chin. J. Org. Chem., 2019, 39, 1529 CrossRef.
- (a) J. Yuan, J. Fu, J. Yin, Z. Dong, Y. Xiao, P. Mao and L. Qu, Org. Chem. Front., 2018, 5, 2820 RSC; (b) L. Yang, P. Gao, X.-H. Duan, Y.-R. Gu and L. N. Guo, Org. Lett., 2018, 20, 1034 CrossRef CAS; (c) W. Wei, L. Wang, H. Yue, P. Bao, W. Liu, C. Hu, D. Yang and H. Wang, ACS Sustainable Chem. Eng., 2018, 6, 17252 CrossRef CAS; (d) F. Lian, K. Xu, W. Meng, H. Zhang, Z. Tan and C. Zeng, Chem. Commun., 2019, 55, 14685 RSC; (e) W. Zhang, Y.-L. Pan, C. Yang, X. Li and B. Wang, Org. Chem. Front., 2019, 6, 2765 RSC; (f) L. Wang, J. Zhao, Y. Sun, H.-Y. Zhang and Y. Zhang, Eur. J. Org. Chem., 2019, 2019, 6935 CrossRef CAS; (g) Y.-F. Si, X.-L. Chen, X.-Y. Fu, K. Sun, X. Song, L.-B. Qu and B. Yu, ACS Sustainable Chem. Eng., 2020, 8, 10740 CAS; (h) Y. Gao, Z. Wu, L. Yu, Y. Wang and Y. Pan, Angew. Chem., Int. Ed., 2020, 59, 10859 CrossRef CAS; (i) J. Shen, J. Xu, L. Huang, Q. Zhu and P. Zhang, Adv. Synth. Catal., 2020, 362, 230 CrossRef CAS; (j) S. Jin, H. Yao, S. Lin, X. You, Y. Yang and Z. Yan, Org. Biomol. Chem., 2020, 18, 205 RSC; (k) X.-K. He, J. Lu, A.-J. Zhang, Q.-Q. Zhang, G.-Y. Xu and J. Xuan, Org. Lett., 2020, 22, 5984 CrossRef CAS.
- (a) K. Yin and R. Zhang, Org. Lett., 2017, 19, 1530 CrossRef CAS; (b) K. Yin and R. Zhang, Synlett, 2018, 14, 597 Search PubMed; (c) S. Paul, J. H. Ha, G. E. Park and Y. R. Lee, Adv. Synth. Catal., 2017, 359, 1515 CrossRef CAS; (d) L. Wang, P. Bao, W. Liu, S. Liu, C. Hu, H. Yue, D. Yang and W. Wei, Chin. J. Org. Chem., 2018, 38, 3189 CrossRef CAS; (e) Y.-Y. Jiang, G.-Y. Dou, L.-S. Zhang, K. Xu, R. D. Little and C.-C. Zeng, Adv. Synth. Catal., 2019, 361, 5170 CrossRef CAS; (f) S. Paul, H. D. Khanal, C. D. Clinton, S. H. Kim and Y. R. Lee, Org. Chem. Front., 2019, 6, 231 RSC.
- (a) X. Jiang, L. Yang, Z. Ye, X. Du, L. Fang, Y. Zhu, K. Chen, J. Li and C. Yu, Eur. J. Org. Chem., 2020, 2020, 1687 CrossRef CAS; (b) X. Xu, C. Xia, X. Li, J. Sun and L. Hao, RSC Adv., 2020, 10, 2016 RSC; (c) L. Zhao, L. Wang, Y. Gao, Z. Wang and P. Li, Adv. Synth. Catal., 2019, 361, 5363 CrossRef CAS; (d) J. Xu, H. Yang, H. Cai, H. Bao, W. Li and P. Zhang, Org. Lett., 2019, 21, 4698 CrossRef CAS; (e) Q. Yang, X. Han, J. Zhao, H.-Y. Zhang and Y. Zhang, J. Org. Chem., 2019, 84, 11417 CrossRef CAS.
- (a) X. Zeng, C. Liu, X. Wang, J. Zhang, X. Wang and Y. Hu, Org. Biomol. Chem., 2017, 15, 8929 RSC; (b) J.-W. Yuan, J.-H. Fu, S.-N. Liu, Y.-M. Xiao, P. Mao and L.-B. Qu, Org. Biomol. Chem., 2018, 16, 3203 RSC; (c) J. Lu, X.-K. He, X. Cheng, A.-J. Zhang, G.-Y. Xu and J. Xuan, Adv. Synth. Catal., 2020, 362, 2178 CrossRef; (d) P. Bao, F. Liu, Y. Lv, H. Yue, J.-S. Li and W. Wei, Org. Chem. Front., 2020, 7, 492 RSC.
- (a) W. Wei, L. Wang, P. Bao, Y. Shao, H. Yue, D. Yang, X. Yang, X. Zhao and H. Wang, Org. Lett., 2018, 20, 7125 CrossRef CAS; (b) Q. Yang, Z. Yang, Y. Tan, J. Zhao, Q. Sun, H.-Y. Zhang and Y. Zhang, Adv. Synth. Catal., 2019, 361, 1662 CrossRef CAS; (c) K.-J. Li, K. Xu, Y.-G. Liu, C.-C. Zeng and B.-G. Sun, Adv. Synth. Catal., 2019, 361, 1033 CrossRef CAS; (d) T. Guo, C.-C. Wang, X.-H. Fu, Y. Liu and P.-K. Zhang, Org. Biomol. Chem., 2019, 17, 3333 RSC; (e) J.-W. Yuan, J.-L. Zhu, H.-L. Zhu, F. Peng, L.-Y. Yang, P. Mao, S.-R. Zhang, Y.-C. Li and L.-B. Qu, Org. Chem. Front., 2020, 7, 273 RSC; (f) Y. Li, M. Gao, L. Wang and X. Cui, Org. Biomol. Chem., 2016, 14, 8428 RSC; (g) Q. Yang, Y. Zhang, Q. Sun, K. Shang, H.-Y. Zhang and J. Zhao, Adv. Synth. Catal., 2018, 360, 4509 CrossRef CAS.
- (a) J. Yuan, J. Zhu, J. Fu, L. Yang, Y. Xiao, P. Mao, X. Du and L. Qu, Org. Chem. Front., 2019, 6, 925 RSC; (b) J. Yuan, S. Liu, Y. Xiao, P. Mao, L. Yang and L. Qu, Org. Biomol. Chem., 2019, 17, 876 RSC; (c) J.-W. Yuan, J.-L. Zhu, B. Li, L.-Y. Yang, P. Mao, S.-R. Zhang, Y.-C. Li and L.-B. Qu, Org. Biomol. Chem., 2019, 17, 10178 RSC.
- J. Wang, B. Sun, L. Zhang, T. Xu, Y. Xie and C. Jin, Org. Chem. Front., 2020, 7, 113 RSC.
- (a) S. Liu, Y. Huang, F.-L. Qing and X.-H. Xu, Org. Lett., 2018, 20, 5497 CrossRef CAS; (b) L. Wang, Y. Zhang, F. Li, X. Hao, H.-Y. Zhang and J. Zhao, Adv. Synth. Catal., 2018, 360, 3969 CrossRef CAS; (c) L. Wang, H. Liu, F. Li, J. Zhao, H.-Y. Zhang and Y. Zhang, Adv. Synth. Catal., 2019, 361, 2354 CrossRef CAS; (d) Z. Wei, S. Qi, Y. Xu, H. Liu, J. Wu, H. Li, C. Xia and G. Duan, Adv. Synth. Catal., 2019, 361, 5490 CrossRef CAS; (e) G. Hong, J. Yuan, J. Fu, G. Pan, Z. Wang, L. Yang, Y. Xiao, P. Mao and X. Zhang, Org. Chem. Front., 2019, 6, 1173 RSC; (f) G.-Y. Dou, Y.-Y. Jiang, K. Xu and C.-C. Zeng, Org. Chem. Front., 2019, 6, 2392 RSC; (g) Y. Gao, L. Zhao, T. Xiang, P. Li and L. Wang, RSC Adv., 2020, 10, 10559 RSC; (h) W. Zhang, X.-X. Xiang, J. Chen, C. Yang, Y.-L. Pan, J.-P. Cheng, Q. Meng and X. Li, Nat. Commun., 2020, 11, 638 CrossRef CAS.
- (a) J. Zhou, P. Zhou, T. Zhao, Q. Ren and J. Li, Adv. Synth. Catal., 2019, 361, 5371 CrossRef CAS; (b) Q.-H. Teng, Y. Yao, W.-X. Wei, H.-T. Tang, J.-R. Li and Y.-M. Pan, Green Chem., 2019, 21, 6241 RSC.
- (a) M. Gao, Y. Li, L. Xie, R. Chauvin and X. Cui, Chem. Commun., 2016, 52, 2846 RSC; (b) K.-J. Li, Y.-Y. Jiang, K. Xu, C.-C. Zeng and B.-G. Sun, Green Chem., 2019, 21, 4412 RSC.
- (a) L.-Y. Xie, L.-L. Jiang, J.-X. Tan, Y. Wang, X.-Q. Xu, B. Zhang, Z. Cao and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 14153 CrossRef CAS; (b) W. Xue, Y. Su, K.-H. Wang, R. Zhang, Y. Feng, L. Cao, D. Huang and Y. Hu, Org. Biomol. Chem., 2019, 17, 6654 RSC.
- (a) L. Liu, N. Pan, W. Sheng, L. Su, L. Liu, J. Dong, Y. Zhou and S.-F. Yin, Adv. Synth. Catal., 2019, 361, 4126 CrossRef CAS; (b) H. Zhang, J. Xu, M. Zhou, J. Zhao, P. Zhang and W. Li, Org. Biomol. Chem., 2019, 17, 10201 RSC; (c) Z. Yan, B. Sun, X. Zhang, X. Zhuang, J. Yang, W. Su and C. Jin, Chem. - Asian J., 2019, 14, 3344 CrossRef CAS.
- (a) Z. Cao, Q. Zhu, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2019, 30, 2132 CrossRef CAS; (b) K.-J. Liu, J.-H. Deng, J. Yang, S.-F. Gong, Y.-W. Lin, J.-Y. He, Z. Cao and W.-M. He, Green Chem., 2020, 22, 433 RSC; (c) K.-J. Liu, J.-H. Deng, T.-Y. Zeng, X.-J. Chen, Y. Huang, Z. Cao, Y.-W. Lin and W.-M. He, Chin. Chem. Lett., 2020, 31, 1868 CrossRef CAS; (d) L.-Y. Xie, Y.-S. Bai, X.-Q. Xu, X. Peng, H.-S. Tang, Y. Huang, Y.-W. Lin, Z. Cao and W.-M. He, Green Chem., 2020, 22, 1720 RSC.
- The efficiency of the photocatalytic arylation was strongly affected by the solvents. However, the detailed reason is still unclear at the present stage. Further studies on the detailed reaction mechanism are currently in progress.
- (a) D. Li, N. Xu, Y. Zhang and L. Wang, Chem. Commun., 2014, 50, 14862 RSC; (b) L. Zou, P. Li, B. Wang and L. Wang, Chem. Commun., 2019, 55, 3737 RSC; (c) L. Zou, L. Wang, L. Sun, X. Xie and P. Li, Chem. Commun., 2020, 56, 7933 RSC; (d) J. Yuan, S. Liu and L. Qu, Adv. Synth. Catal., 2017, 359, 4197 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc02844d |
| This journal is © The Royal Society of Chemistry 2021 |