 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of fungal beta-glucans on health – a systematic review of randomized controlled trials
Marigoula
Vlassopoulou
 ab,
Mary
Yannakoulia
a,
Vasiliki
Pletsa
b,
Georgios I.
Zervakis
c and
Adamantini
Kyriacou
ab,
Mary
Yannakoulia
a,
Vasiliki
Pletsa
b,
Georgios I.
Zervakis
c and
Adamantini
Kyriacou
 *a
*a
aDepartment of Nutrition and Dietetics, Harokopio University, 70 El. Venizelou Str., Kallithea 176 76, Greece. E-mail: mkyriacou@hua.gr
bInstitute of Chemical Biology, National Hellenic Research Foundation, 48 Vas. Constantinou Ave., Athens 116 35, Greece
cLaboratory of General and Agricultural Microbiology, Department of Crop Science, Agricultural University of Athens, 75 Iera Odos Str., Athens 118 55, Greece
First published on 2nd March 2021
Abstract
Introduction: Beta-glucans are polysaccharides that exhibit a wide range of biological properties as a result of their varying chemical composition. Like all dietary fibers, they avoid catabolism in the upper gastrointestinal tract, and they reach the large intestine undigested. There, they undergo fermentation by the gut microbiota, a process that has potential beneficial effects for the host. The aim of this systematic review is to assess the effects of consumption of beta-(1 → 3,1 → 6)-D-glucans, naturally found in the cell walls of fungi, on health outcomes. Methods: A comprehensive literature search was performed on PubMed, Cochrane Library and Web of Science to retrieve studies that applied randomized controlled trials (RCTs) to investigate the impact of exclusive oral administration of fungal beta-glucans in any form and at any dosage to healthy subjects or patients. Results: Thirty-four RCTs, of the 917 records retrieved in total, met the eligibility criteria and are included in the present review. The sources of fungal beta-glucans were Saccharomyces cerevisiae, Aureobasidium pullulans, Pleurotus ostreatus, Lentinula edodes and Ganoderma lucidum, and the dosage of supplementation ranged from 2.5 to 1000 mg daily for up to 6.5 months. The primary physiological outcome of the majority of the interventions was immunomodulation, which resulted in (a) strengthened immune defense that reduces the incidence and symptoms of cold, flu and other respiratory infections and (b) improvement of allergic symptoms. However, the findings on the induction of immune response alterations were inconsistent at the cellular and molecular levels. Another aspect is psychological wellbeing, as the cohorts that received the polysaccharides of interest reported improvement in their mood states as well as amelioration of overall wellbeing. At the same time, it might also be useful as a complementary agent to patients undergoing cancer therapies. Furthermore, supplements containing beta-(1 → 3,1 → 6)-D-glucan administered to overweight/obese adults might have the potential to decrease comorbid conditions associated with obesity. Notably, no adverse event causally related to glucans was recorded. Conclusions: Supplementation with beta-(1 → 3,1 → 6)-D-glucans is well-tolerated, and health-promoting properties are manifested primarily through the potentiation of the immune system. More studies are required to confirm their additional beneficial effects, to establish the optimal dose, and to reveal the underlying molecular mechanisms.
Introduction
Beta-glucans are a heterogeneous group of glucose polymers that exhibit a wide range of biological properties as a result of their diverse morphology.1 They are categorized as dietary fibers since they escape digestion by human gastric enzymes and reach the large intestine undigested.2 Then, they undergo fermentation by the gut microbiota with potential beneficial effects for the host. The cereal-derived beta-(1 → 3/1 → 4)-D-glucans exert cardioprotective effects predominantly through the enhanced control of hyperlipidemia, hypertension, weight and glycemic response.3–6On the other hand, fungal beta-glucans, and more specifically beta-(1 → 3,1 → 6)-D-glucans, are naturally found in the cell walls of Ascomycota and Basidiomycota7 and have not been extensively studied so far. The content of beta-glucans in yeasts and filamentous fungi varies depending on the species.8In vitro and in vivo experiments showed that fungal beta-(1 → 3,1 → 6)-D-glucans induce alterations in the composition of the gut microbiota, favoring the species that promote the host's health9,10 and that they exhibit immunomodulatory,11–13 anti-tumor,11,12 antimicrobial,14 antioxidative14 and radioprotective7 effects. The aim of the present systematic review was to summarize the results of the randomized and controlled clinical trials (RCTs) and to evaluate the effect of yeast- or mushroom-derived beta-(1 → 3,1 → 6)-D-glucan supplementation on health outcomes.
Materials and methods
Literature search
A comprehensive literature search was performed using three search engines, namely PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library (https://www.cochranelibrary.com/) and Web of Science (webofknowledge.com/) to access the topic relevant databases, following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines15 (Fig. 1). The keywords used were (a) ((((((beta-glucan) OR β-glucan) OR (beta-(1-3),(1-6)-D-glucan)) OR lentinan) OR pleuran) NOT (((((beta-(1-3),(1-4)-D-glucan)) OR intravenous) OR spray) OR cream)) in Pubmed, along with additional restrictions for the article type (clinical trial), the species (humans) and the language (English), (b) (TS = (clinical trial AND (beta-glucan OR β-glucan OR lentinan OR pleuran OR “beta-(1-3),(1-6)-D-glucan”) NOT (intravenous OR spray OR cream OR “beta-(1-3),(1-4)-D-glucan”))) and language: (English) and document types: (article) in Web of Science, and (c) beta-glucan OR β-glucan OR “beta-(1-3),(1-6)-D-glucan” OR lentinan OR pleuran NOT (“beta-(1-3),(1-4)-D-glucan” OR intravenous OR spray OR cream) in title abstract keyword of the trials section, in Cochrane Library.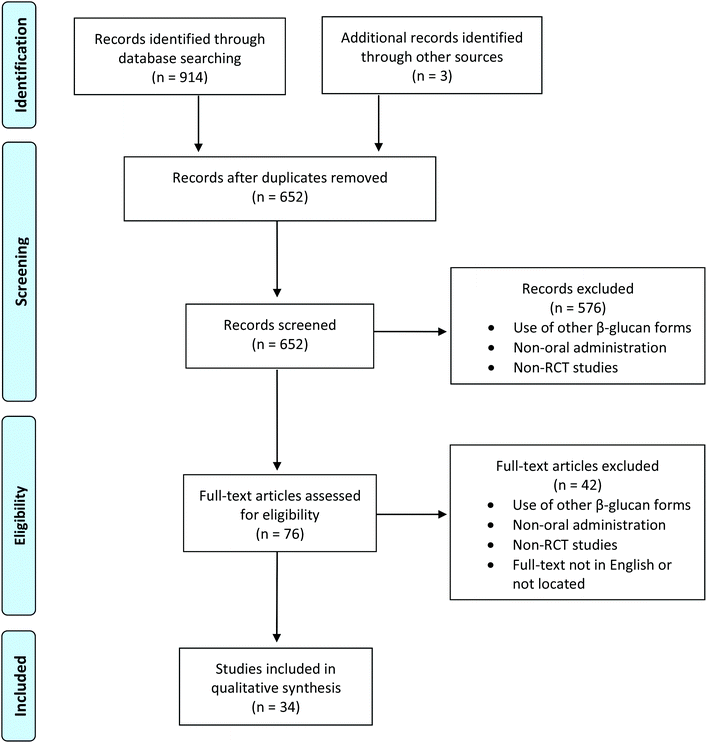 | ||
| Fig. 1 Flowchart of the selection process, following the PRISMA guidelines.15 | ||
Study selection
All records identified through database searching were screened for duplicates and subsequently, their abstracts were screened according to specified eligibility criteria. Firstly, only RCTs were considered. Secondly, the aim was to retrieve studies that investigated the impact of exclusive oral intake of beta-(1 → 3,1 → 6)-D-glucans with no dosage restriction through supplements. Hence, studies using any other form of beta-glucan or administration method (i.e., intravenous, nasal spray, and cream applied on the skin) were excluded. Furthermore, the results were restricted to studies in humans involving healthy individuals or patients with no age restriction. In addition, records were considered relevant if their full-text publication was available in English. For the records that passed this screening, full-text articles were assessed for eligibility and were included in the review.Results
Characteristics of included studies
In total 917 records were retrieved from PubMed (n = 283), Cochrane Library (n = 535), Web of Science (n = 96) and other sources (https://www.researchgate.net/ and https://agris.fao.org/; n = 3); after removing the duplicates (n = 265), the title and abstract items were screened in 652 articles (Fig. 1). From them, 576 were excluded due to the use of other beta-glucan forms, non-oral administration of glucans or lack of the control group and/or randomization in the study design. Seventy-six records were selected for full-text revision to assess the eligibility according to the aforementioned screening criteria, a process that is necessary for cases in which information relevant to the criteria was not provided in the title and/or abstract of the article; 34 of them were RCTs, were performed from 2005 to 2020, met the eligibility criteria and thus were included in the present systematic review. The characteristics of the included studies are summarized in Tables 1–3. Most of the trials used yeast-derived beta-glucans; the sources of extraction for either soluble or insoluble beta-(1 → 3,1 → 6)-D-glucan fractions were the yeasts Saccharomyces cerevisiae and Aureobasidium pullulans (26 studies) and the mushrooms Pleurotus ostreatus, Lentinula edodes and Ganoderma lucidum (8 studies). The daily dosage varied from 2.5 mg to 1 g and was administered typically via capsules (commercially available supplements Imunoglukan®, Wellmune WGP®, Yestimun®, Lentinex®, and Imuneks®) for a duration ranging from 4 days to 26 weeks. Participants’ age also varied across trials, from children to older adults (>65 years old). Healthy individuals and patients suffering from a disease or having a particular medical history (respiratory tract infections, allergies, cancer, and obesity) were included as volunteers.| Study | Subjects | Sample size (M/F) | Age (years) | Supplement | Glucan source | Dosage (mg day−1) | Placebo control | Duration | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| RTI: respiratory tract infection, URTI: upper respiratory tract infection, AECOPD: acute exacerbation of chronic obstructive pulmonary disease. | |||||||||
| A. Healthy | |||||||||
Feldman et al., 2009![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
Healthy | 40 [27 completed the trial] (12/28) | 18–65 | Wellmune WGP® | S. cerevisiae | 500 | Rice flour | 12 weeks | • Similar incidence of symptomatic RTIs |
| • Intervention group did not miss any days of work/school due to colds, while the placebo group missed an average of 1.38 days | |||||||||
| • Significantly improved quality of life score | |||||||||
| • Significantly lower average fever score | |||||||||
| • No adverse events or safety concerns | |||||||||
Graubaum et al., 2012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
Healthy | 100 (42/58) | ≥18 | Yestimun® | S. cerevisiae | 900 | Microcrystalline cellulose | 26 weeks | • Significantly fewer subjects with incidences of common cold and significantly fewer infections during the most intense infection season |
| • Significantly reduced typical cold symptoms (“sore throat and/or difficulty swallowing”, “hoarseness and/or cough” and “runny nose”) | |||||||||
| B. Athletes | |||||||||
Bergendiova et al., 2011![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
Healthy top-level athletes | 50 (26/24) | ∼24 | Imunoglukan capsules | P. ostreatus | 200 | Capsules without beta-glucan | 3 months | • reduced incidence of URTI symptoms |
| • Circulating NK cells increased | |||||||||
| • Phagocytosis remained stable in contrast to placebo group's significant reduction | |||||||||
Mah et al., 2020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
Marathon runners | 202 (100/102) | 18–65 | Wellmune in dairy-based beverage | S. cerevisiae | 250 | No beta-glucan added in the beverage | 91 days | • Decreased URTI symptomatic days, severity of specific URTI symptoms and missed post-marathon workout days due to URTI |
| • No significant changes in average duration and number of URTI episodes | |||||||||
Talbott & Talbott, 2009![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
Marathon runners | 75 (35/40) | 36 ± 9 (18–53) | Wellmune WGP® | S. cerevisiae | 250 or 500 | Rice flour | 1 month | • Significantly fewer URTI symptoms and better overall health |
| • Decreased confusion, fatigue, tension and anger, and increased vigor | |||||||||
| C. Older adults | |||||||||
Fuller et al., 2017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
Healthy | 100 [49 completed the trial] | 50–70 | Wellmune WGP® | S. cerevisiae | 250 | Rice flour | 90 days | • Decreased occurrence of URTIs |
| • Decreased number of symptom days | |||||||||
| • No significant difference in symptom severity | |||||||||
| • LPS-stimulated blood from participants showed an increase in interferon-γ and a smaller decrease in monokine induced by interferon-γ | |||||||||
| • No difference in serum and non-stimulated blood cytokines and chemokines or in salivary immunoglobulin A | |||||||||
Gaullier et al., 2011![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
Healthy | 42 [41 completed the trial] | >65 | Lentinex® tablets | L. edodes (shiitake) | 2,5 | Cellulose | 6 weeks | • Increased number of circulating B-cells |
| • No significant difference in the number of NK cells between groups; other factors of the immune response (immunoglobulins, complement proteins, cytokines) were not altered | |||||||||
| D1. History of respiratory health issues – adults | |||||||||
Auinger et al., 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
Healthy with recurring common cold | 162 (50/112) | 43.2 ± 15.7 | Yestimun® | S. cerevisiae | 900 | Maltodextrin | 4 months | • Reduced number and severity of symptomatic common cold infections |
| • Reduced sleep difficulties caused by cold episode | |||||||||
| • Efficacy of beta-glucan rated better than the placebo both by physicians and participants | |||||||||
Dharsono et al., 2019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
Healthy with ≥3 URTIs during the previous year | 291 [281 completed the trial] | 18–70 | Yestimun® | S. cerevisiae | 900 | Maltodextrin | 16 weeks | • Similar incidence and global severity of URTIs |
| • Reduced severity of physical URTI symptoms during the first week of an episode | |||||||||
| • Significant increase in the joy subscore of the Perceived Stress Questionnaire (PSQ20) | |||||||||
| • Reduction of systolic and diastolic blood pressure | |||||||||
Fuller et al., 2012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
Healthy with ≥1 cold during the previous year | 100 (50/50) | 18–30 (1 subject 50 y.o.) | Wellmune WGP® | S. cerevisiae | 250 | Rice flour | 90 days | • Decreased total number of days with URTI symptoms |
| • Significantly improved ability to “breathe easily” | |||||||||
| • No significant difference in the other URTI severity scores | |||||||||
| • No difference in cytokines and chemokines at study entry or day 90 | |||||||||
| • Lower monocyte chemotactic protein-1 during the URTI | |||||||||
Sun & Zhao, 2019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
Individuals with severe acute AECOPD under mechanical ventilation treated with inhalation of the corticosteroid budesonide | 72 | Middleaged adults | Lentinan | L. edodes (shiitake) | 500 | No administration | 4 days | • Improved clinical efficacy of budesonide |
| • Significantly lower airway pressure and shorter time of mechanical ventilation and stay in the intensive care unit | |||||||||
| • Significantly lower plasma levels of adiponectin, D-dimer, IL-17 and high-sensitivity C-reactive protein | |||||||||
| • Significantly lower pressure of CO2 and higher partial O2 pressure | |||||||||
| • Elevated proportions of CD3+ and CD4+ T-cells and decreased proportion of CD8+ T-cells | |||||||||
| D2. History of respiratory health issues – children | |||||||||
Jesenak et al., 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
Children with >5 RTIs during the previous year | 175 [158 completed the trial] (97/78) | 5.65 ± 2.39 | Imunoglukan P4H® syrup | P. ostreatus | 10/5 kg (270 ± 250 mg) | No pleuran in syrup | 6 months | • Significantly fewer subjects with incidences of respiratory infections |
| • Significantly decreased frequency of flu and flu-like disease and number of lower respiratory tract infections; statistically significant modulation of humoral and cellular immunity | |||||||||
Meng et al., 2016![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
Children with URTI history in the past three months | 175, [156 evaluated] (73/83) | 1–4 | Wellmune® dissolved in water | S. cerevisiae | 35 or 75 | No beta-glucan in treatment | 3 months | • Lower incidence of common childhood infectious illness episodes |
| • Beta-glucan was well tolerated | |||||||||
Richter et al., 2015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
Children with chronic respiratory problems | 77 (34/43) | 10.3 ± 2.1 | Insoluble glucan #300 | S. cerevisiae | 100 | Same looking pill | 4 weeks | • Beta-glucan stimulated physical endurance in children with respiratory problems and via stabilization of the sIgA levels, contributed to their mucosal immunity |
Vetvicka et al., 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
Children with chronic respiratory problems | 40 (16/24) | 10.7 ± 2.3 (8–12) | Insoluble glucan #300 | S. cerevisiae | 100 | Same looking pill | 4 weeks | • Significant increase in production of all tested antibodies (salivary IgG, IgA and IgM) |
| • Stimulation of mucosal immunity of children with chronic respiratory problems | |||||||||
| E. Mental health – stressed subjects | |||||||||
Talbott & Talbott, 2010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
Moderately to highly stressed subjects | 150 (45/105) | 18–65 (39 ± 11) | Wellmune WGP® | S. cerevisiae | 250 or 500 | Rice flour | 4 weeks | • Fewer URTI symptoms and better overall health |
| • Increased vigour, and decreased tension, fatigue and confusion based on the Profile of Mood States (POMS) assessment | |||||||||
Talbott & Talbott, 2012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
Moderately stressed women | 77 (0/77) | 41 ± 11 (18–65) | Wellmune WGP® | S. cerevisiae | 250 | Rice flour | 3 months | • Reduced URTI symptoms |
| • Better overall well-being and global mood state | |||||||||
| • Superior mental/physical energy levels | |||||||||
| Study | Subjects | Sample size (M/F) | Age (years) | Supplement | Glucan source | Dosage (mg day−1) | Placebo control | Duration | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| A. Healthy | |||||||||
Leentjens et al., 2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
Healthy subjects | 15 (15/0) | 19–24 | Water-insoluble beta-glucan #300, Biothera | S. cerevisiae | 1000 | No administration | 1 week | • Beta-glucan barely detectable in serum |
| • Neither cytokine production nor microbicidal activity of leukocytes were affected | |||||||||
| B. Athletic subjects | |||||||||
Bobovčák et al., 2010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
Healthy regularly trained elite athletes | 20 (16/4) | 20–25 | Imunoglukan capsules | P. ostreatus | 100 | Fructose | 2 months | • Protection against exercise-induced reduction in natural killer (NK) cell activity |
Carpenter et al., 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
Recreationally active healthy subjects | 60 (29/31) | ∼22.5 | Wellmune WGP® | S. cerevisiae | 250 | Rice flour | 10 days | • Altered typical post-exercise innate immune response: increased potential of blood leucocytes for the production of IL-2, IL-4, IL-5 and IFN-γ |
| • Increased total and pro-inflammatory monocyte concentrations after exercise | |||||||||
| • Increased LPS-stimulated cytokines (IL-2, IL-4, IL-5 and IFN-g) before exercise | |||||||||
| • Increased plasma cytokine (IL-4, IL-5, IL-7, IL-8, IL-10 and IFN-g) concentrations after exercise | |||||||||
McFarlin et al., 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
Subjects undergoing intense exercise | 182 (96/86) | 18–46 | Soluble or insoluble beta-glucan | S. cerevisiae | 250 | Rice flour | 1 month | • 37% reduction in the number of cold/flu symptom days post-marathon |
| 60 (29/31) | 18–35 | 10 days | • 32% increase in salivary IgA at 2 h after exercise | ||||||
Zabriskie et al., 2020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
Recreationally active adults | 31 (16/15) | 29.9 ± 7.7 | Yestimun® | S. cerevisiae | 250 | Maltodextrin | 13 days | • Lower concentrations of serum pro-inflammatory cytokines (MIP-1, IL-8, MCP-1, and TNF-α) |
| • Stable vigor scores 72 h after exercise | |||||||||
| C. Children | |||||||||
Henao et al., 2018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
Healthy children | 167 [124 evaluated] | 3–5 | Yogurt enriched with beta-glucans | G. lucidum | 350 | No beta-glucan added yogurt | 12 weeks | • Significantly higher absolute count of peripheral blood total lymphocytes (CD3+, CD4+, and CD8+ T cells) |
| • Safe and well-tolerated | |||||||||
| • No abnormal increases in serum creatinine or hepatic aminotransferases occurred, and adherence was higher than 90% in the intervention groups | |||||||||
| • No significant difference in NK count, CD4/CD8 ratio, sIgA and cytokine amounts | |||||||||
Jesenak et al., 2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
Children with recurrent respiratory tract infections | 175 (97/78) | 2–10 | Imunoglukan P4H® syrup | P. ostreatus | 10/5 kg | No pleuran in syrup | 6 months | • Potential suppressive effect on the markers of allergic inflammation in peripheral blood (reduction of peripheral blood eosinophilia and stabilisation of the levels of total IgE in serum), especially in atopic subjects. |
| D. Allergic subjects | |||||||||
Jippo et al., 2015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
Subjects with a history of cedar pollinosis | 65 (50/15) | 22–62 | Water-soluble, low-MW beta-glucan | A. pullulans (black yeast) | 150 | No beta-glucan added to the beverage | 2 months | • Significantly lower prevalence of sneezing, nose-blowing, tears and hindrance to the activities of daily living |
Kirmaz et al., 2005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
Subjects with seasonal allergic rhinitis | 24 (13/11) | ≥18 | Imuneks® capsule | S. cerevisiae | 20 | (No information) | 3 months (out of the pollen season) | • Decrease of Th2-originated IL-4 and IL-5 levels in NLF nasal lavage fluid |
| • Increased Th1-originated IL-12 levels | |||||||||
| • No change in IFN-c levels | |||||||||
| • Decreased percentage of eosinophils in the NLF, but not in the peripheral blood | |||||||||
Talbott et al., 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
Ragweed allergy sufferers | 48 (17/31) | 36 ± 9 (18–53) | Wellmune WGP® | S. cerevisiae | 250 | Rice flour | 4 weeks | • Improved allergy (nasal, non-nasal and eye) symptoms |
| • Improved overall physical health, and emotional well-being [increased vigor, physical health, energy and emotional well-being, and reduced tension, depression, anger, fatigue, confusion and sleep problems; improved quality of life (QOL) and global mood state] | |||||||||
| E. Other conditions | |||||||||
Truong et al., 2019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
Osteoarthritis patients | 100 [82 completed the trial] | 18–80 | Capsules containing polycan | A. pullulans (black yeast) | 50 | Capsules without polycan | 12 weeks | • Significant reduction in the total osteoarthritis symptoms questionnaire (WOMAC) score after treatment |
| • Significant reduction in the frequency of rescue medication | |||||||||
| • No significant changes in hematology and biochemistry parameters or health indices | |||||||||
Urbancikova et al., 2020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
HSV-1 positive patients (herpes labialis) | 90 [77 com-pleted the preventive phase] (37/53) | Active 25.3 ± 2.3; placebo 17.4 ± 1.5 (all >6 y.o.) | Imunoglukan P4H® ACUTE! & imunoglukan P4H® capsules | P. ostreatus | 300 for 10 days, then 100 for 120 days | Capsules without beta-glucan | 130 days (10 + 120) | • Significantly shorter duration of herpes simplex symptoms |
| • Lower duration and severity of respiratory symptoms, significant difference in cough | |||||||||
| • No significant side effects | |||||||||
| Study | Subjects | Sample size (M/F) | Age (years) | Supplement | Glucan source | Dosage (mg day−1) | Placebo control | Duration | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| A. Overweight/obese | |||||||||
Mosikanon et al., 2017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
Overweight/obese | 44 (13/31) | 21–65 | Beta-glucan capsules | S. cerevisiae | 477 mg for 14 days, then 954 mg for 28 days | Rice flour | 6 weeks | • No significant difference in triglyceride, cholesterol, lipid profile, liver and renal function, or energy and nutrient intake compared; increased IL-10 |
| • Reduced IL-6 and TNF-α | |||||||||
| • Modulation of pro-cytokines that accelerate overweight/obese comorbidities | |||||||||
| • Reduced blood pressure | |||||||||
| • Reduced waist circumference | |||||||||
Strączkowski et al., 2018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
Obese during weight loss | 52 (27/25) | ≥18 | Beta-glucan preparation | S. cerevisiae | 500 | No administration | 12 weeks | • No metabolic or anti-inflammatory effects |
| B. Cancer patients | |||||||||
| Ostadrahimi et al., 2014a45 | Breast cancer patients undergoing chemotherapy | 30 (0/30) | 28–65 | Imuneks® capsules | S. cerevisiae | 20 | Same shape and size capsules without beta-glucan | 3 weeks | • Less pronounced decrease in white blood cell counts |
| • Increased IL-12 serum levels | |||||||||
| • Decreased IL-4 serum levels | |||||||||
| Ostadrahimi et al., 2014b44 | Breast cancer patients undergoing chemotherapy | 30 (0/30) | 28–65 | Soluble beta-glucan capsules | S. cerevisiae | 20 | Same shape and size capsules without beta-glucan | 3 weeks | • No significant change in global health status/QoL score |
| • Significantly decreased symptom scale\items score | |||||||||
| • No significant change in functional scale score | |||||||||
Yenidogan et al., 2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
Breast carcinoma patients, planned to undergo mastectomy | 130 (0/130) | ∼50 | B-glucan capsules | S. cerevisiae | 20 | Capsules without beta-glucan | 10 days | • Lower daily drainage volumes between days 2 and 8 post-mastectomy and earlier removal of drains |
| • Significantly lower TNF-a and IL-6 levels on days 1 and 2 | |||||||||
All of the trials included in the present review were randomized and placebo-controlled, as this was one of the eligibility criteria. Regarding the blinding, the vast majority of the 34 trials were double-blind (31 trials), one was single-blind,16 one open-label,17 and one of unknown blinding status.18
Clinical outcomes
The majority of the studies focused on the effect of oral administration of fungal beta-glucans on immunomodulation and the potential immune system – the strengthening properties of these polysaccharides. This effect was predominantly demonstrated through the reduction of incidence and symptoms of common cold, flu and upper respiratory tract infections (RTIs) in general, as observed in subjects with or without chronic respiratory problems (Table 1). In trial participants with a history of recurring RTIs, the positive effects on the immune response to RTIs during beta-glucan supplementation included the reduced number,19 severity19–22 and duration23 of symptomatic common cold infections. Additionally, supplementation reduced sleeping difficulties caused by the cold episode19 and improved the ability to “breathe easily”.23 The outcomes of trials in individuals with no RTI history were in agreement with the aforementioned results regarding the reduction of RTI incidence24,25 and the severity of typical symptoms such as “sore throat and/or difficulty swallowing”, “hoarseness and/or cough”, “runny nose”25 and average fever score.26 There were also reports of better overall wellbeing.21,22Even though there is some dispersed evidence of similar incidence23,26 and severity20,24 of RTIs among the outcomes of trials that used yeast-derived beta-glucans, the positive effect of mushroom-derived beta-glucans in general on the body's potential to defend against invading pathogens is supported by the majority of the retrieved studies at the clinical level. In contrast, the results appear to be incoherent at the molecular and cellular levels. Gaullier et al.27 observed that a dosage as low as 2.5 mg day−1 of lentinan (beta-glucan from L. edodes – “shiitake mushroom”), which is significantly lower than what was used in most of the other trials, increased the number of circulating B-cells in older adults, but showed no significant difference in the number of NK cells or other factors of the immune response (immunoglobulins, complement proteins, and cytokines). However, Leentjens et al. (2014)17 showed that even a daily administration of 1000 mg of yeast-derived beta-glucan for seven days to 15 young and healthy male adults altered neither the beta-glucan plasma levels nor the cytokine production (TNF-a, IL-6, IL-10, IL-1b, IL-17, IL-22, and IFN-c) by leukocytes that were ex vivo stimulated by various stimuli.17 Along the same lines, the microbicidal activity was the same as that of the control group.17 Likewise, studies reported no difference in cytokines,23,24 chemokines23 or in salivary IgA24 at the end of the intervention, although an increase in interferon-γ24 has been detected in the intervention group, as well as lower levels of monocyte chemotactic protein-1 during the RTI, compared to the counts of the control group.23
Vulnerable populations at risk of RTIs
Children are usually among the vulnerable groups that are susceptible to various respiratory threats (Tables 1 and 2). Trials in children with a history of recurrent RTIs showed that fewer volunteers encountered episodes of common childhood infectious illnesses related to the respiratory system in the intervention group compared to the placebo.28,29 Furthermore, children affected by RTIs while receiving beta-glucan had a significantly lower frequency and duration of such incidents. Jesenak et al. (2013)28 detected statistically significant modulation of specific humoral and cellular immunity parameters in this group and in NK cells. Other studies reported the stimulation of physical endurance in children with respiratory problems30 and potentiation of their mucosal immunity via stabilization of salivary IgA,30,31 IgG, and IgM antibody levels.31 Accordingly, a study on healthy children found that administration of yogurt enriched with beta-glucan from G. lucidum increased the frequency of peripheral blood total lymphocytes (CD3+, CD4+, and CD8+ T cells), which are critical elements for the body's defense against infectious threats.32Another group that is more susceptible to RTIs compared to the general population includes individuals undergoing intense physical training, like elite athletes and marathon runners (Tables 1 and 2). Beta-glucans were found to reduce the incidence33 and duration34,35 of RTI symptoms. At the cellular level, they demonstrated the prevention of exercise-induced reduction in the natural killer (NK) cell activity36,37 and numbers.36 Furthermore, post-exercise, beta-glucan supplementation increased the levels of salivary IgA, a marker of mucosal immunity improvement,34 as well as the potential of blood leucocytes to produce a range of cytokines (IL-4, IL-5, IL-7, IL-8, IL-10 and IFN-γ).38 Moreover, it lowered the concentrations of serum pro-inflammatory cytokines (MIP-1, IL-8, MCP-1, and TNF-α).39
Beta-glucans have been co-administered with medication as conventional therapy for respiratory diseases. Sun & Zhao (2019)40 administered 500 mg of lentinan to individuals with severe acute exacerbation of chronic obstructive pulmonary disease (AECOPD) under mechanical ventilation, who were treated, in parallel, with inhalation of the corticosteroid budesonide (Table 1). Researchers reported improved clinical efficacy of budesonide inhalation when combined with lentinan treatment.
Allergies
Apart from the immune response to pathogens, supplementation with beta-(1 → 3,1 → 6)-D-glucans modulates allergic reactions (Table 2). Ragweed allergy sufferers41 and subjects with a history of cedar pollinosis16 reported alleviation of allergy symptoms such as sneezing, nose-blowing and tears. Furthermore, their overall physical health, quality of life (QOL)41 and daily functionality16 were improved. At the cellular and molecular levels, there is evidence supporting the potential suppressive effect on allergic inflammation markers. A RCT in children suggested the effectiveness of fungal beta-glucan supplementation in children with atopy through the reduction of peripheral blood eosinophilia and stabilization of the total IgE level in serum.42 In addition, the administration of a low dose of 20 mg day−1 of yeast-derived beta-glucan to adults with seasonal allergic rhinitis for three months out of the pollen season resulted in a decrease in the levels of the Th2-originated cytokines IL-4 and IL-5 and an increase in Th1-originated IL-12 levels in nasal lavage fluid, as well as a decrease in the nasal lavage fluid percentage.43Other areas of action
Three studies investigated the effect of yeast-derived beta-glucan administration to women diagnosed with breast carcinoma (Table 3). Daily supplementation of 20 mg of beta-glucan during the interval between two chemotherapy courses (21 days) resulted in alleviation of body symptoms such as fatigue, nausea, vomiting, pain, dyspnea, insomnia and appetite loss,44 and in beneficial changes in several blood and biochemical markers (IL-12, IL-4, and whole blood cells);45 however, it did not improve the quality of life or everyday functioning.44 Furthermore, beta-glucan supplementation to breast cancer patients after mastectomy decreased drain discharges between days 2 and 8 post-mastectomy and the drains were removed significantly earlier compared to the placebo group.46 The same study recorded significantly lower TNF-a and IL-6 levels in the serum of breast cancer patients who were administered beta-glucan.46Beta-glucan supplementation to obese subjects had unclear results (Table 3). Strączkowski et al.18 found that after 500 mg day−1 intake for 12 weeks no metabolic or anti-inflammatory effects were exhibited during weight loss. On the other hand, Mosikanon et al.,47 who administered 477 mg day−1 for 14 days and then 954 mg day−1 for 28 days to overweight and obese individuals, found that although there was no significant difference in the lipid profile, liver and renal function, or dietary intake when compared to the control group, beta-glucan might have the potential to decrease comorbid conditions associated with obesity. Specifically, it reduced blood pressure and waist circumference, and modulated the inflammatory markers associated with the development of comorbidities.
Another aspect of potential beneficial effects of beta-glucan consumption is mental health, with relevant studies reporting improvement of mood state and amelioration of overall well-being. RCTs with daily supplementation with one or two capsules of Wellmune WGP® (250 mg or 500 mg) for four weeks detected increased healthy subjects’ ratings of vigor and decreased tension, depression, anger, fatigue, and confusion.33,41 Moreover, this yeast-derived beta-glucan improved global mood states as well as physical health, energy, and emotional well-being.41 Consistent with these results were the outcomes of trials studying the effect of the same beta-glucan supplement on moderately to highly stressed subjects for one and three months, and showed similar changes in psychological and emotional factors.21,22 In addition, people with a history of RTIs who received beta-glucan supplements for four months, reported a significant increase in the joy subscore of the Perceived Stress Questionnaire (PSQ20).20
In terms of anti-viral defense, HSV-1 (herpes labialis virus) positive patients who received beta-glucan derived from P. ostreatus reported a significantly shorter duration of herpes simplex symptoms48 (Table 2).
Another population that could potentially be assisted by beta-glucan supplementation are osteoarthritis patients (Table 2). In a trial investigating this hypothesis, individuals suffering from osteoarthritis received A. pullulans-derived beta-glucan for 12 weeks and reported a significant reduction in the respective symptoms, as well as a significant reduction in the frequency of rescue medication.49
Discussion
The results of the present systematic review of RCTs indicate a potentiation effect of oral supplementation of fungal beta-glucans on the immune defense system. The outcomes of 17 studies demonstrated that individuals receiving beta-glucan supplementation had stronger defense against upper respiratory tract infections, whereas 8 trials studying immune system decline after intense exercise in recreationally active subjects and elite athletes found that beta-glucans reduced this adverse effect. Furthermore, beta-glucans had beneficial effects in seasonal allergy sufferers, HSV-1 positive patients and osteoarthritis patients, all of whom reported alleviated symptoms following supplementation. In trials investigating alterations in overall wellbeing, beta-glucan supplementation led to improvement of mood state and emotional well-being, enhanced decrease in obesity-associated comorbid conditions and amelioration of adverse side-effects of the treatment of cancer patients undergoing chemotherapy or mastectomy.Notably, none of the included studies reported adverse effects causally related to beta-glucan supplementation. The treatment was well tolerated in all the different populations, regardless of variations in age, sex and health status. This is an important observation that favors the use of beta-glucan supplements for many purposes, as it is supported by a significant number of studies with great variability in population. This extends even in cases of patients who receive these supplements in co-administration with other medicines prescribed for specific conditions, as seen in breast cancer,44–46 osteoarthritis,49 chronic obstructive pulmonary disease40 and HSV-1 positive48 patients.
Interestingly, age does not seem to be a factor that affects the overall efficacy of beta-glucan supplementation in immunological potentiation. Although most studies were performed in subjects of all age groups, two included only older adults24 and six dealt with children;24 the respective results demonstrated that both of these population groups could benefit from the administration of beta-glucans.
By examining the outcomes of the studies that did not record differences in certain parameters of interest between the intervention and placebo adult groups, it was noticed that this only occurred in trials where yeast- and not mushroom-derived beta-glucans were used, and in doses that did not exceed 500 mg day−1.
The heterogeneity of the studies in this review did not allow us to perform a meta-analysis. This heterogeneity is observed in the age, sex, health status and size of the sample population, as well as in the source of beta-glucans, the daily administered dose and the duration of supplementation. Another limitation of the conducted studies is that they aimed at evaluating the effects of dietary supplementation, which can be affected by an individual's eating behaviour. The aforementioned properties of beta-glucans are manifested primarily as a result of their fermentation by the gut microbiota that have a complex role in human health and can be highly affected by the dietary intake of an individual. This is a parameter that was not monitored in any of the included trials and in most of them diet was ad-libitum. Furthermore, the majority of the trials lack a follow-up assessment of the effects of beta-glucan supplements. Only three studies17,28,42 performed follow-up testing after a certain period of time from the completion of the intervention.
Conclusions
Oral supplementation with beta-(1 → 3,1 → 6)-D-glucans in humans is well-tolerated and demonstrates health-promoting properties, primarily through the potentiation of the immune system, with the most prominent of them being the prophylactic effect against the occurrence and severity of upper respiratory tract infections. Further investigation is required in order to determine other potential beneficial effects of these fibers, to unravel the molecular mechanisms behind their impact on physical health, and to establish the optimal administration parameters and source of extraction. In addition, the observed beneficial immunomodulatory effect of beta-glucans on coping with respiratory infections should be studied on distinct bacterial and viral causes of infection, such as SARS-CoV-2.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research has been co-financed by the EU and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation (Call RESEARCH-CREATE-INNOVATE, project code T1EDK-03404).References
- S. Rahar, G. Swami, N. Nagpal, M. A. Nagpal and G. S. Singh, Preparation, characterization, and biological properties of beta-glucans, J. Adv. Pharm. Technol. Res., 2011, 2, 94–103 CrossRef CAS PubMed.
- J. Slavin, Fiber and prebiotics: mechanisms and health benefits, Nutrients, 2013, 5, 1417–1435 CrossRef CAS PubMed.
- Y. Wang, S. V. Harding, S. J. Thandapilly, S. M. Tosh, P. J. H. Jones and N. P. Ames, Barley beta-glucan reduces blood cholesterol levels via interrupting bile acid metabolism, Br. J. Nutr., 2017, 118, 822–829 CrossRef CAS PubMed.
- A. Frid, A. Tura, G. Pacini and M. Ridderstrale, Effect of Oral Pre-Meal Administration of Betaglucans on Glycaemic Control and Variability in Subjects with Type 1 Diabetes, Nutrients, 2017, 9, 1004 CrossRef PubMed.
- K. C. Maki, R. Galant, P. Samuel, J. Tesser, M. S. Witchger, J. D. Ribaya-Mercado, J. B. Blumberg and J. Geohas, Effects of consuming foods containing oat beta-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure, Eur. J. Clin. Nutr., 2007, 61, 786–795 CrossRef CAS PubMed.
- S. Aoe, Y. Ichinose, N. Kohyama, K. Komae, A. Takahashi, D. Abe, T. Yoshioka and T. Yanagisawa, Effects of high beta-glucan barley on visceral fat obesity in Japanese individuals: A randomized, double-blind study, Nutrition, 2017, 42, 1–6 CrossRef CAS PubMed.
- F. M. Zhu, B. Du, Z. X. Bian and B. J. Xu, Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities, J. Food Compos. Anal., 2015, 41, 165–173 CrossRef CAS.
- O. Rop, J. Mlcek and T. Jurikova, Beta-glucans in higher fungi and their health effects, Nutr. Rev., 2009, 67, 624–631 CrossRef PubMed.
- J. Perry and W. Ying, A Review of Physiological Effects of Soluble and Insoluble Dietary Fibers, J. Nutr. Food Sci., 2016, 06, 476 Search PubMed.
- E. K. Mitsou, G. Saxami, E. Stamoulou, E. Kerezoudi, E. Terzi, G. Koutrotsios, G. Bekiaris, G. I. Zervakis, K. C. Mountzouris, V. Pletsa and A. Kyriacou, Effects of Rich in Beta-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study, Molecules, 2020, 25, 2806 CrossRef CAS PubMed.
- X. Li, J. Wang, W. Wang, C. Liu, S. Sun, J. Gu, X. Wang, D. Boraschi, Y. Huang and D. Qu, Immunomodulatory activity of a novel, synthetic beta-glucan (beta-glu6) in murine macrophages and human peripheral blood mononuclear cells, PLoS One, 2013, 8, e80399 CrossRef CAS PubMed.
- S. Baldassano, G. Accardi and S. Vasto, Beta-glucans and cancer: The influence of inflammation and gut peptide, Eur. J. Med. Chem., 2017, 142, 486–492 CrossRef CAS PubMed.
- H. Stier, V. Ebbeskotte and J. Gruenwald, Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan, Nutr. J., 2014, 13, 38 CrossRef PubMed.
- I. Giavasis, Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals, Curr. Opin. Biotechnol., 2014, 26, 162–173 CrossRef CAS PubMed.
- D. Moher, L. Shamseer, M. Clarke, D. Ghersi, A. Liberati, M. Petticrew, P. Shekelle, L. A. Stewart and P.-P. Group, Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement, Syst. Rev., 2015, 4, 1 CrossRef PubMed.
- T. Jippo, T. Suzuki, H. Sato, Y. Kobayashi and M. Shigekawa, Water-soluble low-molecular-weight β-(1, 3–1, 6) D-Glucan inhibit cedar pollinosis, Funct. Foods Health Dis., 2015, 5, 80–88 CrossRef CAS.
- J. Leentjens, J. Quintin, J. Gerretsen, M. Kox, P. Pickkers and M. G. Netea, The effects of orally administered Beta-glucan on innate immune responses in humans, a randomized open-label intervention pilot-study, PLoS One, 2014, 9, e108794 CrossRef PubMed.
- M. Strączkowski, A. Nikołajuk, R. Majewski, R. Filarski, M. Stefanowicz, N. Matulewicz and M. Karczewska-Kupczewska, The effect of moderate weight loss, with or without (1,3)(1,6)-beta-glucan addition, on subcutaneous adipose tissue inflammatory gene expression in young subjects with uncomplicated obesity, Endocrine, 2018, 61, 275–284 CrossRef PubMed.
- A. Auinger, L. Riede, G. Bothe, R. Busch and J. Gruenwald, Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body's defence against pathogens: a double-blind, randomized, placebo-controlled, multicentric study in healthy subjects, Eur. J. Nutr., 2013, 52, 1913–1918 CrossRef CAS PubMed.
- T. Dharsono, K. Rudnicka, M. Wilhelm and C. Schoen, Effects of Yeast (1,3)-(1,6)-Beta-Glucan on Severity of Upper Respiratory Tract Infections: A Double-Blind, Randomized, Placebo-Controlled Study in Healthy Subjects, J. Am. Coll. Nutr., 2019, 38, 40–50 CrossRef PubMed.
- S. Talbott and J. Talbott, Beta 1,3/1,6 glucan decreases upper respiratory tract infection symptoms and improves psychological well-being in moderate to highly-stressed subjects, Agro Food Ind. Hi-Tech, 2010, 21, 21–24 Search PubMed.
- S. M. Talbott and J. A. Talbott, Baker's Yeast Beta-Glucan Supplement Reduces Upper Respiratory Symptoms and Improves Mood State in Stressed Women, J. Am. Coll. Nutr., 2012, 31, 295–300 CrossRef CAS PubMed.
- R. Fuller, H. Butt, P. S. Noakes, J. Kenyon, T. S. Yam and P. C. Calder, Influence of yeast-derived 1,3/1,6 glucopolysaccharide on circulating cytokines and chemokines with respect to upper respiratory tract infections, Nutrition, 2012, 28, 665–669 CrossRef CAS PubMed.
- R. Fuller, M. V. Moore, G. Lewith, B. L. Stuart, R. V. Ormiston, H. L. Fisk, P. S. Noakes and P. C. Calder, Yeast-derived beta-1,3/1,6 glucan, upper respiratory tract infection and innate immunity in older adults, Nutrition, 2017, 39–40, 30–35 CrossRef CAS PubMed.
- R. Busch, H. Stier and J. Gruenwald, A Double-Blind, Randomized, Placebo-Controlled Nutritional Study Using an Insoluble Yeast Beta-Glucan to Improve the Immune Defense System, Food Nutr. Sci., 2012, 03, 738–746 Search PubMed.
- S. Feldman, H. I. Schwartz, D. S. Kalman, A. Mayers, H. M. Kohrman, R. Clemens and D. R. Krieger, Randomized phase II clinical trials of wellmune WGP[R] for immune support during cold and flu season, J. Appl. Res., 2009, 9, 30 CAS.
- J. M. Gaullier, J. Sleboda, E. S. Ofjord, E. Ulvestad, M. Nurminiemi, C. Moe, A. Tor and O. Gudmundsen, Supplementation with a soluble beta-glucan exported from Shiitake medicinal mushroom, Lentinus edodes (Berk.) singer mycelium: a crossover, placebo-controlled study in healthy elderly, Int. J. Med. Mushrooms, 2011, 13, 319–326 CrossRef CAS PubMed.
- M. Jesenak, J. Majtan, Z. Rennerova, J. Kyselovic, P. Banovcin and M. Hrubisko, Immunomodulatory effect of pleuran (beta-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections, Int. Immunopharmacol., 2013, 15, 395–399 CrossRef CAS PubMed.
- F. Meng, Bakers Yeast Beta-Glucan Decreases Episodes of Common Childhood Illness in 1 to 4 Year Old Children during Cold Season in China, J. Nutr. Food Sci., 2016, 6, 518 Search PubMed.
- J. Richter, V. Svozil, V. Král, L. Rajnohová Dobiášová and V. Vetvicka, β-glucan affects mucosal immunity in children with chronic respiratory problems under physical stress: clinical trials, Ann. Transl. Med., 2015, 3, 52 Search PubMed.
- V. Vetvicka, J. Richter, V. Svozil, L. Rajnohová Dobiášová and V. Král, Placebo-Driven Clinical Trials of Transfer Point Glucan #300 in Children with Chronic Respiratory Problems: Antibody Production, Am. J. Immunol., 2013, 9, 43–47 CrossRef.
- S. L. D. Henao, S. A. Urrego, A. M. Cano and E. A. Higuita, Randomized Clinical Trial for the Evaluation of Immune Modulation by Yogurt Enriched with beta-Glucans from Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), in Children from Medellin, Colombia, Int. J. Med. Mushrooms, 2018, 20, 705–716 CrossRef PubMed.
- S. Talbott and J. Talbott, Effect of BETA 1, 3/1, 6 GLUCAN on Upper Respiratory Tract Infection Symptoms and Mood State in Marathon Athletes, J. Sports Sci. Med., 2009, 8, 509–515 Search PubMed.
- B. K. McFarlin, K. C. Carpenter, T. Davidson and M. A. McFarlin, Baker's Yeast Beta Glucan Supplementation Increases Salivary IgA and Decreases Cold/Flu Symptomatic Days After Intense Exercise, J. Diet. Suppl., 2013, 10, 171–183 CrossRef CAS PubMed.
- E. Mah, V. N. Kaden, K. M. Kelley and D. J. Liska, Soluble and Insoluble Yeast beta-Glucan Differentially Affect Upper Respiratory Tract Infection in Marathon Runners: A Double-Blind, Randomized Placebo-Controlled Trial, J. Med. Food, 2020, 23, 416–419 CrossRef CAS PubMed.
- K. Bergendiova, E. Tibenska and J. Majtan, Pleuran (beta-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes, Eur. J. Appl. Physiol., 2011, 111, 2033–2040 CrossRef CAS PubMed.
- M. Bobovčák, R. Kuniaková, J. Gabriž and J. Majtán, Effect of Pleuran (beta-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes, Appl. Physiol., Nutr., Metab., 2010, 35, 755–762 CrossRef PubMed.
- K. C. Carpenter, W. L. Breslin, T. Davidson, A. Adams and B. K. McFarlin, Baker's yeast β-glucan supplementation increases monocytes and cytokines post-exercise: implications for infection risk?, Br. J. Nutr., 2013, 109, 478–486 CrossRef CAS PubMed.
- H. A. Zabriskie, J. C. Blumkaitis, J. M. Moon, B. S. Currier, R. Stefan, K. Ratliff, P. S. Harty, R. A. Stecker, K. Rudnicka, R. Jager, M. D. Roberts, K. Young, A. R. Jagim and C. M. Kerksick, Yeast Beta-Glucan Supplementation Downregulates Markers of Systemic Inflammation after Heated Treadmill Exercise, Nutrients, 2020, 12, 1144 CrossRef CAS PubMed.
- J. Sun and G. Zhao, Clinical effects of lentinan combined with budesonide inhalation in treating acute exacerbation of chronic obstructive pulmonary disease under mechanical ventilation, Exp. Ther. Med., 2019, 17, 1503–1508 CAS.
- S. M. Talbott, J. A. Talbott, T. L. Talbott and E. Dingler, beta-Glucan supplementation, allergy symptoms, and quality of life in self-described ragweed allergy sufferers, Food Sci. Nutr., 2013, 1, 90–101 CrossRef CAS PubMed.
- M. Jesenak, M. Hrubisko, J. Majtan, Z. Rennerova and P. Banovcin, Anti-allergic effect of Pleuran (beta-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections, Phytother. Res., 2014, 28, 471–474 CrossRef PubMed.
- C. Kirmaz, P. Bayrak, O. Yilmaz and H. Yuksel, Effects of glucan treatment on the Th1/Th2 balance in patients with allergic rhinitis: a double-blind placebo-controlled study, Eur. Cytokine Network, 2005, 16, 128–134 CAS.
- A. Ostadrahimi, A. Esfahani, M. Asghari Jafarabadi, J. Eivazi Ziaei, A. Movassaghpourakbari and N. Farrin, Effect of Beta glucan on quality of life in women with breast cancer undergoing chemotherapy: a randomized double-blind placebo-controlled clinical trial, Adv. Pharm. Bull., 2014, 4, 471–477 Search PubMed.
- A. Ostadrahimi, J. E. Ziaei, A. Esfahani, M. A. Jafarabadi, A. Movassaghpourakbari and N. Farrin, Effect of beta glucan on white blood cell counts and serum levels of IL-4 and IL-12 in women with breast cancer undergoing chemotherapy: a randomized double-blind placebo-controlled clinical trial, Asian Pac. J. Cancer Prev., 2014, 15, 5733–5739 CrossRef PubMed.
- E. Yenidogan, G. G. Akgul, M. A. Gulcelik, S. Dinc, M. K. Colakoglu and H. A. Kayaoglu, Effect of beta-glucan on drain fluid and amount of drainage following modified radical mastectomy, Adv. Ther., 2014, 31, 130–139 CrossRef CAS PubMed.
- K. Mosikanon, D. Arthan, A. Kettawan, R. Tungtrongchitr and P. Prangthip, Yeast β-Glucan Modulates Inflammation and Waist Circumference in Overweight and Obese Subjects, J. Diet. Suppl., 2017, 14, 173–185 CrossRef CAS PubMed.
- I. Urbancikova, D. Hudackova, J. Majtan, Z. Rennerova, P. Banovcin and M. Jesenak, Efficacy of Pleuran (β-Glucan from Pleurotus ostreatus) in the Management of Herpes Simplex Virus Type 1 Infection, Evid. Based Complement. Alternat. Med., 2020, 2020, 8562309 Search PubMed.
- T. T. T. Truong, J. M. Lim, H. R. Cho, Y. S. Kim, D. G. Dao, Q. H. Tran and J. S. Choi, A Double-Blind, Randomized Controlled 12-Week Follow-Up Trial to Evaluate the Efficacy and Safety of Polycan in Combination with Glucosamine for the Treatment of Knee Osteoarthritis, Evid. Based Complement. Alternat. Med., 2019, 2019, 9750531 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |





