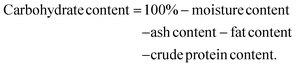Open Access Article
Open Access ArticleEnriching street-vended zobo (Hibiscus sabdariffa) drink with turmeric (Curcuma longa) to increase its health-supporting properties
Folake
Idowu-Adebayo
 ab,
Mary J.
Toohey
a,
Vincenzo
Fogliano
ab,
Mary J.
Toohey
a,
Vincenzo
Fogliano
 a and
Anita R.
Linnemann
*a
a and
Anita R.
Linnemann
*a
aFood Quality and Design Group, Department of Agrotechnology and Food Sciences, Wageningen University and Research Centre, Wageningen, The Netherlands
bDept of Food Science and Technology, Federal University Oye-Ekiti, Nigeria. E-mail: anita.linnemann@wur.nl
First published on 21st December 2020
Abstract
Street-vended foods are cheap, readily available and have been currently identified as possible means for micronutrient fortification in an effort to prevent malnutrition in developing countries. The effect of enriching street-vended zobo drink (Hibiscus sabdariffa) with turmeric (Curcuma longa) was studied to assess the potential to increase health-supporting properties for its consumers. Two processing methods were tested: boiled turmeric root in zobo and addition of fresh turmeric paste to zobo in different concentrations. Vitamin C in turmeric-fortified zobo ranged from 496–725 μg per 100 mL, delphinidin-3-sambubioside from 52–69 mg per 100 mL, and cyanidin-3-sambubioside from 21–27 mg per 100 mL. Micronutrients ranged from 10.9–14 mg L−1 and 2.19–2.67 mg L−1 for iron and zinc, respectively. Folic acid, vitamin C, anthocyanins and iron showed the highest amounts in the 2% boiled turmeric zobo samples. Ferulic acid (0.16–2.03 mg per 100 mL), and chlorogenic acid (20–24 mg per 100 mL) did not show the same statistically significant improvement for 2% boiled turmeric-fortified zobo. The zobo samples with turmeric paste consistently had lower values of vitamins, polyphenols and minerals in comparison with the boiled turmeric-fortified zobo samples. Turmeric-fortified zobo can play a role in a healthy diet by its health-supporting properties. Consumption of a typical one serving of 500 mL (representative packaged bottle size of zobo drink by the street vendors in Nigeria) of turmeric-fortified zobo would contribute 63–88% DV and 18–23% DV of iron and zinc. Overall, fortification with boiled turmeric improves the antioxidant and nutritional quality of zobo, specifically regarding vitamin C, delphinidin-3-sambubioside and iron.
1. Introduction
Street foods provide a high percentage of daily energy, ranging from 13% to 50% in both children and adults, in developing countries.1 A popular street food drink produced and sold in Nigeria is zobo, which is produced from dried hibiscus (Hibiscus sabdariffa L.) calyces, and optional flavorings such as garlic, ginger, and fruit.2 Similar to fruit juices, zobo is commonly consumed for its affordability, nutritive value and refreshing quality.Hibiscus is an underutilized crop grown in Africa, Asia, Australia, Central America and Europe.3 In English, it is commonly known as roselle, and has various local names depending on the continent. In Africa, Burkina Faso, Côte d'Ivoire and Mali, it is referred to as dabileni. It is known as karkade in Sudan and Egypt (where it is also called “drink of the Pharaohs”). It is commonly known as karkanji, wonjo, zobo and bissap in Chad, Gambia, Nigeria and Senegal, respectively. In Asia, it is named mei gui qie, karkade and krajeap in China, Saudi Arabia and Thailand, respectively. It is recognized as asam belanda, asam susur, asam paya or ribena, in Malaysia. The Indian subcontinent calls it by many tribal names such as Indian sorrel, mesta, lal ambari, patwa, amta and amti. It is known as rosella in Australia and flor de Jamaica in Mexico. In Europe, it is referred to as Congo, Jamaica and karkade in France, Spain and Switzerland, respectively.3–6 The many names for this crop reflect its ubiquity and popularity.
Scientific studies have revealed that zobo calyces are rich in flavonoids, minerals, organic acids, vitamins and polyphenol compounds, which all contribute to the nutritive value and biological activity of the drink.7–9 The two major anthocyanin compounds in H. sabdariffa calyces are delphinidin-3-sambubioside and cyanidin-3-sambubioside. These anthocyanins have been reported as unstable and likely to degrade during the preparation of the drink. However, processing conditions such as extraction temperature, protection from UV light, and addition of food-grade organic acid have been reported to impact anthocyanin stability.10 Recent study characterized the physiochemical properties and non-volatiles of H. sabdariffa calyces cultivated in Australia, China, Chad, Malaysia, Mexico, Nigeria, Sudan and Thailand.3 They concluded that variation found in the characterization of the H. sabdariffa calyces grown in different countries were mainly due to the practice of sun drying. Sun drying is a traditional processing method to obtain dried H. sabdariffa calyces that are easy to transport and store in developing countries, including Nigeria.
Due to zobo's popularity, any nutritional improvement of the drink is likely to benefit many people in Nigeria, where malnutrition is a major concern.11 One approach to further augment zobo would be through food-to-food fortification, which is a solution for the population without changing food consumption patterns in a radical way.12 Fortification of the zobo drink with another food ingredient that is rich in bioactive compounds could also improve the stability of the anthocyanin content, based on reports that the structure of the organic acid and food matrix in which it is combined would have a strong effect on the stability of the anthocyanins.10
Turmeric (Curcuma longa) can be used in zobo to fortify and potentially improve the antioxidant and nutritional quality of the drink. It is a shallow-rooted crop that is commonly grown throughout Nigeria, making it production and sales regionally and economically beneficial.13 Turmeric contains curcumin, a well-known polyphenol with many acclaimed health benefits in addition to anti-inflammatory and antioxidant properties that have been researched extensively.14 In addition to curcumin, turmeric has been found to contain other antioxidants, such as ferulic acid.15 Ferulic acid exhibits anti-inflammatory, antidiabetic, and anticancer activity. In addition, ferulic acid has protective effects on organs, tissues, and cells of the cardiovascular system and skin.16
In this study, turmeric was added to zobo in different concentrations using two processing methods: boiling of turmeric root in the zobo and addition of fresh turmeric paste to the final zobo drink. The research aimed at determining if addition of turmeric could improve the antioxidant and nutritional profile of zobo drink. Overall, the effect of processing on the antioxidant and nutritional quality of turmeric-fortified zobo was established.
2. Materials and methods
2.1. Materials
2.2. Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 by weight, in a large pot for five min. The pot was then removed from the heat source and allowed to soak overnight.
25 by weight, in a large pot for five min. The pot was then removed from the heat source and allowed to soak overnight.
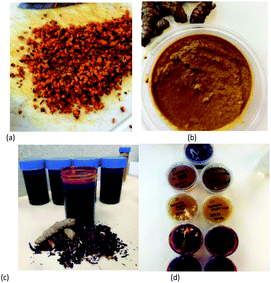
Next, the beverage was sieved. The final beverage samples are shown in Fig. 1c.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
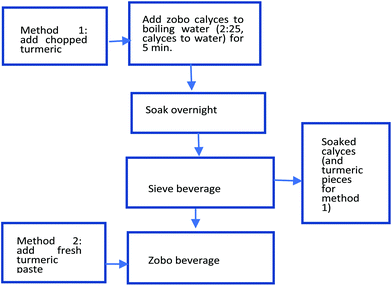
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 by weight. The zobo with boiled turmeric was prepared by boiling H. sabdariffa calyces with finely chopped turmeric root pieces for five min, followed by soaking at room temperature overnight and sieving in the morning. The turmeric pieces were added at either 2% or 6% of the weight of the water during the addition. The zobo with fresh turmeric paste was made using the same method as the zobo control, but with the addition of either 2% or 6% turmeric paste after the overnight soak and sieving. A schematic representation is presented in Fig. 2.
25 by weight. The zobo with boiled turmeric was prepared by boiling H. sabdariffa calyces with finely chopped turmeric root pieces for five min, followed by soaking at room temperature overnight and sieving in the morning. The turmeric pieces were added at either 2% or 6% of the weight of the water during the addition. The zobo with fresh turmeric paste was made using the same method as the zobo control, but with the addition of either 2% or 6% turmeric paste after the overnight soak and sieving. A schematic representation is presented in Fig. 2.
2.3. Chemical analysis
2.3.1.1. pH. The pH of the zobo samples was measured with a pH meter (pHenomenal VWr pH 1000 L).
2.3.2.1. Moisture content. The moisture content was determined following the method described by Ekanem.17
2.3.2.2. Ash content. The ash content was measured by heating the sample in a Carbolite CSF1100 ashing oven at 550 °C for 6 h, after the method of Ekanem.17
The ash content was calculated with the formula below.
2.3.2.3. Crude protein content. The protein content was analysed using LECO CN 628 Dumas analyser, Germany. Freeze-dried samples and blank samples with cellulose (Sigma Aldrich 310697, US) were weighed in tin cups (Interscience, Belgium) and put in a sample tray. The samples were then oxidised in the Dumas metal column filled with chemicals at high temperature and water filters in a separation column, on which nitrogen was separated and calculated into protein content. The standard nitrogen to protein conversion factor, 6.25, was used to calculate the protein concentration.18
As all food fractions should add up to 100%, the carbohydrate content was calculated by the formula:17
2.3.4.1. Folic acid. Folic acid concentration was determined by HPLC as described by Chen et al.20 A Thermo Scientific (Germany) Ultimate 3000 HPLC machine with RS diode array detector was used. Modifications made were: folic acid standards were prepared in KH2PO4 buffer at pH 8–9. Alltech prevail C18 5u column (4.6 × 250 mm) was used. The samples were prepared by sonication of freeze-dried samples for 5 h at 40 °C. Centrifugation followed at 4000 rpm for seven min. Samples were then filtered into amber HPLC vials. Quantification based on measurement of peak areas was taken with UV detection at 283 nm for 25 min.
2.3.4.2. Vitamin C. Ascorbic acid was determined as described by Hernández et al.21 using a Thermofisher HPLC (Thermo Scientific, Germany). The samples containing zobo were diluted twice, while samples containing just water and turmeric were not diluted. A Varia Polaris 5 C18-A column (4.6 × 150 mm) was used.
2.3.5.1. Delphidin-3-sambubioside and cyanidin-3-sambubioside. Delphidin-3-sambubioside and cyanidin-3-sambubioside were determined using HPLC (Thermo Scientific, Germany). Zobo samples were prepared according to the previously mentioned protocol in 2.2.3. The zobo and water samples were extracted using 0.1% HCl in methanol. 5 mL of sample was combined with 5 mL of extraction liquid in a 15 mL greiner tube and shaken for one h at maximum speed. All samples were then centrifuged at 3000 rpm for 30 min. Next, samples were filtered using 0.45 μm RC filters into amber HPLC vials. HPLC analysis utilized 1% formic acid in Milli-Q® water as eluent A and 100% methanol as eluent B. The gradient elution started with 5% (B), which increased to 60% (0–20 min) and then to 100% (20–25 min). The conditions were held at 100% for 5 min prior to returning to 5% (30–31 min) with a final isocratic run of 5% B (31–35 min). The flow rate was 1 ml min−1 and the injection volume was 20 μl. A Varian Polaris 5 C18-A column (5 μm, 4.6 × 150 mm) was used. Calibration curves for both delphidin-3-sambubioside and cyanidin-3-sambubioside were made by making a stock solution of 1 mg mL−1 in methanol. The stock solutions were stored at −20 °C. The calibration curve consisted of seven calibration standards: 17.19–34.38–68.75–137.5–275–550 (μg ml−1) of delphidin-3-sambubioside and cyanidin-3-sambubioside. The UV-Vis spectrum of 510 nm was used for analyzing data from chromatograms.
2.4. Statistical analysis
Data were analysed using Microsoft Office 2016, Chromeleon 7 and IBM SPSS Statistics. Analysis was performed in triplicate with three independent batches for all samples except otherwise stated in section 2.3. The differences between the samples were determined using paired t-tests between batches of the same sample and assumed equal variance t-tests (two-sample) between different sample means. To determine if data was normally distributed, the test of Shapiro–Wilk was used with p < 0.05, where H0 = data is normally distributed and Ha = data is not normally distributed.3. Results and discussion
3.1. pH
| Samples | pH | Moisture | Ash | Protein | Carbohydratesa |
|---|---|---|---|---|---|
| Significant differences (p < 0.05) in the same column are indicated with different superscripts.a The carbohydrate content was calculated from the other fractions to have an indication of the carbohydrate content of our samples. | |||||
| Control (zobo with 0% turmeric) | 2.4 ± 0.01B | 96.2 ± 0.29A | 0.47 ± 0.03A | 0.18 ± 0.01B | 3.1 |
| 2% boiled turmeric in zobo | 2.4 ± 0.03ABC | 95.9 ± 0.45AB | 0.54 ± 0.06A | 0.23 ± 0.01A | 3.4 |
| 6% boiled turmeric in zobo | 2.6 ± 0.02AC | 95.8 ± 0.18AB | 0.56 ± 0.06A | 0.22 ± 0.01A | 3.4 |
| 2% fresh turmeric paste in zobo | 2.4 ± 0.01C | 95.9 ± 0.15AB | 0.45 ± 0.04A | 0.18 ± 0.03AB | 3.5 |
| 6% fresh turmeric paste in zobo | 2.5 ± 0.02A | 95.8 ± 0.24B | 0.47 ± 0.05A | 0.20 ± 0.04AB | 3.5 |
An higher pH, namely 3.6, was found by Ekanem17 using a lower ratio of hibiscus calyces (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). Furthermore in his study the zobo was boiled for a longer time (40 min) and not soaked overnight. This could influence the composition of the extracts, thus influencing the pH. Adesokan et al.23 reported an even higher pH of 3.94. Their preparation method used a ratio of 15
25). Furthermore in his study the zobo was boiled for a longer time (40 min) and not soaked overnight. This could influence the composition of the extracts, thus influencing the pH. Adesokan et al.23 reported an even higher pH of 3.94. Their preparation method used a ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, much higher than the ratio used in this research. Additionally, the calyces were added to boiling water and only allowed to soak for 15 min. Consequently, the higher pH in literature might be due to the shorter time the hibiscus calyces were kept in the water. Letting the zobo soak overnight allows for a longer extraction time, in which more acid compounds might be dissolved, thus resulting in the lower pH found in our study. The slight increase in pH by adding turmeric to zobo is unlikely to significantly affect product properties.
25, much higher than the ratio used in this research. Additionally, the calyces were added to boiling water and only allowed to soak for 15 min. Consequently, the higher pH in literature might be due to the shorter time the hibiscus calyces were kept in the water. Letting the zobo soak overnight allows for a longer extraction time, in which more acid compounds might be dissolved, thus resulting in the lower pH found in our study. The slight increase in pH by adding turmeric to zobo is unlikely to significantly affect product properties.
3.2. Proximate composition
3.3. Mineral content
Iron and zinc deficiencies are globally predominant, classified 9th and 11th, respectively, in the category of the main risk factors for world-wide problem of ailment and they mostly occur in developing nations.29 The amount of iron and zinc in the water used for zobo preparation was negligible: −0.01 and 0.02 mg L−1 for iron and zinc, respectively. The iron and zinc in the turmeric-fortified zobo were from both the hibiscus calyces and turmeric. The average amounts of iron and zinc in our samples ranged between 10–14 mg L−1 and 2.1–2.7 mg L−1, respectively with the highest values of iron and zinc recorded in 2% and 6% boiled turmeric in zobo respectively. These ranges are within the interval reported in literature.30,31 In children aged 2 to 5 years, the daily recommended intake of iron is 8 mg and the daily recommended intake of zinc is 6 mg.32,33 Consumption of a typical one serving of 500 mL (representative packaged bottle size of zobo drink by the street vendors) of turmeric-fortified zobo would contribute 63–88% DV and 18–23% DV of iron and zinc respectively. In agreement to the recent literature34 on the use of nutrition information and understanding of percentage Daily Value (DV) based on the health Canada campaign message (“5% DV or less is a little; 15% DV or more is a lot”), thus, drinking turmeric-fortified zobo would contribute significantly towards the required intake of iron and zinc.3.4. Vitamins
Vitamin B9, or folate, is synthetically produced as folic acid for food fortification and supplementation. Folic acid was detected in all samples containing zobo, whereas no folic acid was found in the turmeric in water samples. Folic acid ranged from 256–301 μg per 100 mL (Table 2). The Recommended Dietary Allowance (RDA) for adults is 400 μg day−1, with pregnant and lactating women needing an additional 100–200 μg day−1.35 Further 400 μg day−1 of synthetic folic acid from either supplements or fortified-foods is recommended for females capable of becoming pregnant to lessen the risk of the neural tube defects (NTD).36 Drinking turmeric-fortified zobo would contribute in excess to the recommended daily intake of folic acid as one serving contains 320–376% DV, 213–251% DV and 160–188% DV for adults, lactating and women capable of becoming pregnant recommended daily intake respectively. Zobo is usually sold by street vendors in 500 mL bottles in Nigeria. Hence, drinking a half bottle (250 mL) is enough to exceed the daily required amount of folic acid for all ages and genders. Adequate daily intake of folic acid is important to prevent neural tube defects, which are four times more likely to occur in developing countries, such as Nigeria, than in developed countries.37| Samples | Ferulic acid (mg per 100 mL) | Chlorogenic acid (mg per 100 mL) | Cyanidin-3-sambubioside (mg per 100 mL) | Delphinidin-3-sambubioside (mg per 100 mL) | Folic acid (μg per 100 mL) | Ascorbic acid (μg per 100 mL) |
|---|---|---|---|---|---|---|
| Different letters represent significant differences within the same column (p < 0.05). ND represents not detected and NA represent not applicable. | ||||||
| Control (zobo with 0% turmeric) | 1.9 ± 0.6C | 22.3 ± 2.1AB | 23.0 ± 5.4A | 58.1 ± 13.3A | 295 ± 36AB | 577 ± 59A |
| 2% boiled turmeric in zobo | 1.5 ± 0.1C | 24.1 ± 3.3A | 26.9 ± 0.9 B | 68.8 ± 2.2B | 301 ± 37A | 725 ± 84B |
| 6% boiled turmeric in zobo | 1.5 ± 0.3C | 22.7 ± 1.9AB | 24.2 ± 1.8A | 60.3 ± 5.1A | 297 ± 27A | 535 ± 146AC |
| 2% fresh turmeric paste in zobo | 2.0 ± 0.8C | 21.1 ± 1.2BC | 22.1 ± 5.4A | 55.6 ± 12.9A | 256 ± 51B | 533 ± 48A |
| 6% fresh turmeric paste in zobo | 1.8 ± 0.6C | 20.0 ± 1.4C | 20.6 ± 5.1A | 52.2 ± 12.6A | 276 ± 87AB | 496 ± 75C |
| 2% boiled turmeric in water | 0.2 ± 0.1A | ND | NA | NA | ND | ND |
| 6% boiled turmeric in water | 0.7 ± 0.3B | ND | NA | NA | ND | ND |
| 2% fresh turmeric paste in water | ND | ND | NA | NA | ND | ND |
| 6% fresh turmeric paste in water | ND | ND | NA | NA | ND | ND |
Vitamin C was measured as the total amount of ascorbic acid. The four control samples containing turmeric in water resulted in no detectable peaks, corroborating that turmeric does not contain ascorbic acid.38 The amount of vitamin C in the samples ranged from 496–725 μg per 100 mL (Table 2). Samples fortified with 6% turmeric paste had the lowest value with no significant difference with the zobo boiled with 6% turmeric root. However, the value found in the control sample (0% turmeric) was lower than in other scientific articles, which ranged from 1500–3000 μg per 100 mL.2,39 The difference in vitamin C content could be due to the process applied to dry the H. sabdariffa calyces used for production of the zobo, as vitamin C can be lost through heating.40 Additionally, the quantity of H. sabdariffa calyces used for the production of zobo also has an effect, as research has shown that vitamin C content increases when the amount increases from 2% calyces to 15% calyces in water.39 In this study, the quantity of H. sabdariffa calyces used in zobo was 8%. According to the previous research, increasing H. sabdariffa calyces above the 8% used in our study could increase the total ascorbic acid content. The vitamin C content in the sample with 2% boiled turmeric in zobo was significantly higher than in all other samples, showing a similar pattern as for the anthocyanin in the samples (Table 2). Ascorbic acid was not detectable in the four control samples containing turmeric in water. The highest value reported in 2% boiled turmeric in zobo, which was significantly different from the control (zobo with 0% turmeric), could be attributed to the effect of food-to-food fortification,41 as the effect of a bioactive may differ between the whole food (matrix effect) and the isolated compound.42,43 Research has also shown that a heat treatment, such as boiling, can release bound antioxidant principles from turmeric, resulting in a higher ascorbic acid content.44 Other studies, focusing on curcumin, have suggested that the activity following the heating step could be a result of the degradation products, which provide similar activity.45 Vitamin C contents in 6% boiled turmeric in zobo appeared to be significantly lower than the vitamin C content found in 2% boiled turmeric in zobo, which we suspect to be due to aggregation and/or precipitation at a higher concentration. The Recommended intake for vitamin C is 75 mg for women and 90 mg for men.46 For children, the RDA is 40 mg, which is necessary to prevent wasting in sub-Saharan Africa.47 The vitamin C content in one serving (one bottle of 500 mL) of the 2% boiled turmeric zobo could moderately34 contribute 5% DV, 4% DV and 9% DV for women (75 mg), men (90 mg) and children (40 mg) recommended intake respectively. Addition of fruit juices, common in zobo processing, could increase the overall content of vitamin C.2
3.5. Anthocyanins
Cyanidin-3-sambubioside has been identified as one of the two major anthocyanin components of H. sabdariffa.3,10 However, it has not been determined if the addition of turmeric affects the anthocyanin content. Cyanidin-3-sambubioside was present in all zobo samples in a range of 21–27 mg per 100 mL (Table 2).It accounts for approximately 30% of the total anthocyanin content in turmeric-fortified zobo, which is similar to values reported in previous research. An increase in the amount of calyces used for production and boiling time of the turmeric-fortified zobo beverage could potentially result in a higher quantities of total anthocyanins found in this study.
Turmeric-fortified zobo processed with 2% boiled turmeric is significantly higher in Cyanidin-3-sambubioside than all other samples. This is in line with the theory explained in the vitamin C section, stating that heat treatment of turmeric can impact other compounds. As turmeric alone contains no anthocyanins, heat treatment cannot release bound anthocyanin principles. Rather, the heat treatment can result in degradation products that act similarly to anthocyanins.45
Delphinidin-3-sambubioside is the other primary anthocyanin in H. sabdariffa.3,10 Delphinidin-3-sambubioside in the turmeric-fortified zobo is present in a range of 52–69 mg per 100 mL (Table 2). Delphinidin-3-sambubioside constitutes approximately 70% of the total anthocyanins, across all samples. This result correlates with previous research, which has shown that delphinidin-3-sambubioside accounts for between 75–85% of the total anthocyanins in zobo.48,49
The effect of turmeric processing has similar outcomes for both delphinidin-3-sambubioside and cyanidin-3-sambubioside. The highest amount of delphinidin-3-sambubioside was recorded for 2% boiled turmeric in zobo, as 69 mg per 100 mL was significantly higher than the contents of all other samples. In fresh calyces of H. sabdariffa, both delphinidin-3-sambubioside and cyanidin-3-sambubioside have concentrations more than double of that found in the turmeric-fortified zobo beverage.48 The significant loss of anthocyanins is most likely due to heat degradation during drying.50 Juhari et al.3 confirmed that variation in the anthocyanin content of H. sabdariffa calyces grown in different countries was mainly due to sun drying.
Addition of food-grade organic acids has been reported to improve anthocyanin stability.10 These findings were corroborated by the increase in the anthocyanin content of the 2% boiled turmeric zobo. This increased amount of anthocyanins could contribute to the anti-inflammatory, visual and neurological-health improving properties of turmeric-fortified zobo.51,52
3.6. Ferulic and chlorogenic acid
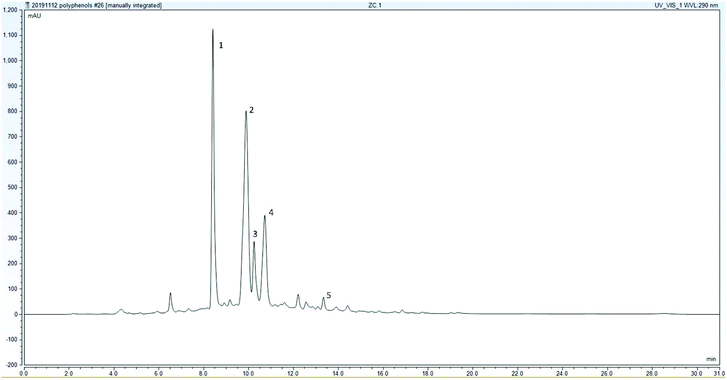
H. sabdariffa contains several polyphenols: chlorogenic acid, hydroxycitric acid, protocatechuic acid, quercetin, ferulic acid, and caffeic acid.22,53,54 In an exploratory trial at the beginning of this study, the peaks with the largest area were determined to identify the polyphenols present in the highest concentrations. The polyphenols analyzed via HPLC for identification were: caffeic acid, ferulic acid, protocatechuic acid, gallic acid, quercetin, catechin, rutin, naringin, benzoic acid, and chlorogenic acid. Five major peaks (Fig. 3) were consistently found in either zobo or boiled turmeric in water, namely ferulic acid, chlorogenic acid, two anthocyanin peaks, and a major polyphenol peak that remained unknown. The anthocyanin peaks were characterized by their high UV maximum (515 nm). These peaks were identified as cyanidin-3-sambubioside and delphinidin-3-sambubioside. The two other main peaks were phenolic acids, namely ferulic acid and chlorogenic acid.The zobo and the turmeric-fortified zobo did not differ in the quantities of ferulic acid they contained. This demonstrates that turmeric, whether boiled or in paste form, does not affect the total ferulic acid in turmeric-fortified zobo in the quantities that we tested. The only two samples with significantly lower amounts were the water samples with boiled turmeric. Yet, when 6% of turmeric was added instead of 2%, the total ferulic acid content significantly increased. This shows that more heat degradation products are formed with a higher amount of added turmeric. According to Vankar et al.,44 the boiling of turmeric could potentially release bound antioxidant principles, which could allow for a detectable amount of ferulic acid. Other research has also shown that total phenolic content can increase after heat treatment due to the inactivation of polyphenol oxidase.55 This supports the findings that turmeric paste in water has no detectable ferulic acid or phenolics. In the zobo samples, most of the ferulic acid originated from H. sabdariffa, making the differences between boiled turmeric and turmeric paste insignificant. The processing of turmeric had no impact on the overall ferulic acid content, making the addition of turmeric not an extra source of ferulic acid in zobo.
4. Conclusions
The present study contributes to the potential utilization of turmeric root in the production of street-vended zobo drink. The results show that the antioxidant and nutritional quality of zobo (H. sabdariffa) drink can be improved by the way of processing (heating/boiling) and the addition of a second ingredient (i.e. turmeric) which could be easily adopted by the street vendors in Nigeria. Nowadays there is an increasing awareness of the relation between food intake and health, which has boosted the demand for health-supporting food products by consumers. Ascorbic acid, cyanidin-3-sambubioside, delphinidin-3-sambubioside, ferulic acid, and chlorogenic acid were present in turmeric-fortified zobo. Overall, the addition of 2% boiled turmeric in street-vended zobo could contribute beneficially to the Nigerian diet, due to the increased content of vitamin C and anthocyanins. Thus, by inclusion of turmeric in zobo production street-vendors can prepare what consumers want to be able to make healthier food choices. Presently the Nigerian Government is promoting the turmeric crop, which is relatively cheap and widely available within the country, hence making turmeric-fortified zobo affordable for the public.The heat treatment applied in our study resulted in increased concentrations of vitamin C and anthocyanins. Further research is needed to determine the binding, precipitation, or aggregation effects that play a role at higher concentrations of boiled turmeric (6%) in zobo. We followed the traditional processing methods of the zobo street vendors in this study by boiling the zobo with the turmeric. Addition of turmeric powder to zobo instead of turmeric paste could be another processing method that warrants follow-up research. Studies of the effect of turmeric-fortification on other street-vended drinks like soymilk, which is rich in protein, are also recommended to determine the influence of a different food matrix.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the Tertiary Education Trust Fund (TETFund) of Nigeria, 2015 Academic Staff Training and Development intervention for Federal University Oye-Ekiti (FUOYE), Nigeria.References
- N. P. Steyn, Z. Mchiza, J. Hill, Y. D. Davids, I. Venter, E. Hinrichsen, M. Opperman, J. Rumbelow and P. Jacobs, Nutritional contribution of street foods to the diet of people in developing countries: a systematic review, Public Health Nutr., 2014, 17, 1363–1374 CrossRef
.
- S. C. Izah, L. A. Orutugu and L. T. Kigigha, A review of the quality assessment of zobo drink consumed in Nigeria, ASIO J. Microbiol. Food Sci. Biotechnol. Innovations, 2015, 1, 34–44 Search PubMed
.
- N. H. Juhari, W. L. P. Bredie, T. B. Toldam-Andersen and M. A. Petersen, Characterization of Roselle calyx from different geographical origins, Food Res. Int., 2018, 112, 378–389 CrossRef CAS
.
- G. Riaz and R. Chopra, A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L, Biomed. Pharmacother., 2018, 102, 575–586 CrossRef CAS
.
- A. Bechoff, M. Cissé, G. Fliedel, A.-L. Declemy, N. Ayessou, N. Akissoe, C. Touré, B. Bennett, M. Pintado and D. Pallet, Relationships between anthocyanins and other compounds and sensory acceptability of Hibiscus drinks, Food Chem., 2014, 148, 112–119 CrossRef CAS
.
- N. Mohd-Esa, F. S. Hern, A. Ismail and C. L. Yee, Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds, Food Chem., 2010, 122, 1055–1060 CrossRef CAS
.
- I. Jabeur, E. Pereira, L. Barros, R. C. Calhelha, M. Soković, M. B. P. Oliveira and I. C. Ferreira, Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents, Food Res. Int., 2017, 100, 717–723 CrossRef CAS
.
- Y. Xu, D. Hu, T. Bao, J. Xie and W. Chen, A simple and rapid method for the preparation of pure delphinidin-3-O-sambubioside from Roselle and its antioxidant and hypoglycemic activity, J. Funct. Foods, 2017, 39, 9–17 CrossRef CAS
.
- G. Mercado-Mercado, F. J. Blancas-Benitez, G. R. Velderrain-Rodríguez, E. Montalvo-González, G. A. González-Aguilar, E. Alvarez-Parrilla and S. G. Sáyago-Ayerdi, Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.), J. Funct. Foods, 2015, 18, 171–181 CrossRef CAS
.
- I. F. Pérez-Ramírez, E. Castaño-Tostado, J. A. Ramírez-de León, N. E. Rocha-Guzmán and R. Reynoso-Camacho, Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage, Food Chem., 2015, 172, 885–892 CrossRef
.
- A. O. Omotayo, Correlates of hunger severity and food intake among rural household in Nigeria: Facing the key challenges of the 21st century, Socioeconomica, 2018, 7, 53–76 Search PubMed
.
-
O. Dary and R. Hurrell, Guidelines on food fortification with micronutrients, World Health Organization, Food and Agricultural Organization of the United Nations, Geneva, 2006 Search PubMed
.
- J. Nwaekpe, H. Anyaegbunam, B. Okoye and G. Asumugha, Promotion of turmeric for the food/pharmaceutical industry in Nigeria, J. Exp. Agric. Int., 2015, 335–341 CAS
.
-
A. Trifan, A. C. Aprotosoaie and A. Miron, Curcumin in Food, Handbook of Dietary Phytochemicals, 2019, pp. 1–44 Search PubMed
.
- N. A. Siddiqui, Evaluation of thermo sensitivity of curcumin and quantification of ferulic acid and vanillin as degradation products by a validated HPTLC method, Pak. J. Pharm. Sci., 2015, 28, 299–305 CAS
.
- E. de Oliveira Silva and R. Batista, Ferulic acid and naturally occurring compounds bearing a feruloyl moiety: a review on their structures, occurrence, and potential health benefits, Compr. Rev. Food Sci. Food Saf., 2017, 16, 580–616 CrossRef
.
- J. O. Ekanem, Microbial, sensory and nutritional properties of laboratory prepared sorrel (zobo) drinks fortified with spices and sugar, J. Global Biosci., 2018, 7, 5573–5584 Search PubMed
.
- S. G. Sáyago-Ayerdi, S. Arranz, J. Serrano and I. Goñi, Dietary fiber content and associated antioxidant compounds in roselle flower (Hibiscus sabdariffa L.) beverage, J. Agric. Food Chem., 2007, 55, 7886–7890 CrossRef
.
-
E. Temminghoff and V. Houba, Plant analyses procedures, Kluwer Academic Publishers, Dodrecht, The Netherlands, 2004 Search PubMed
.
- C.-H. Chen, Y.-H. Yang, C.-T. Shen, S.-M. Lai, C.-M. J. Chang and C.-J. Shieh, Recovery of vitamins B from supercritical carbon dioxide-defatted rice bran powder using ultrasound water extraction, J. Taiwan Inst. Chem. Eng., 2011, 42, 124–128 CrossRef CAS
.
- Y. Hernández, M. G. Lobo and M. González, Determination of vitamin C in tropical fruits: A comparative evaluation of methods, Food Chem., 2006, 96, 654–664 CrossRef
.
- R. Beltrán-Debón, C. Alonso-Villaverde, G. Aragonès, I. Rodríguez-Medina, A. Rull, V. Micol, A. Segura-Carretero, A. Fernández-Gutiérrez, J. Camps and J. Joven, The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans, Phytomedicine, 2010, 17, 186–191 CrossRef
.
- I. Adesokan, O. Abiola, M. Adigun and O. Anifowose, Analysis of quality attributes of Hibiscus sabdariffa (zobo) drinks blended with aqueous extract of ginger and garlic, Afr. J. Food Sci., 2013, 7, 174–177 CrossRef CAS
.
- P. Adeniji, Nutritional, Sensory and Microbiological Quality Assessment of fortified Zobo Drink: A Home-Prepared Traditional Nigerian Beverage, J. Nutr. Food Sci., 2017, 7, 2 Search PubMed
.
- A. Adelekan, N. Arisa, A. Alamu, Y. Adebayo and G. Popoola, Production and acceptibility of fruits enhanced zobo drink, Food Sci. Technol. Lett., 2014, 5, 046–051 Search PubMed
.
- O. Ezearigo, P. Adeniji and F. Ayoade, Screening of natural spices for improving the microbiological, nutritional and organoleptic qualities of the Zobo drink, J. Appl. Biosci., 2014, 76, 6397–6410 CrossRef
.
- A. Ikpeama, G. Onwuka and C. Nwankwo, Nutritional composition of Tumeric (Curcuma longa) and its antimicrobial properties, Int. J. Sci. Eng. Res., 2014, 5, 1085–1089 Search PubMed
.
- I. Chattopadhyay, K. Biswas, U. Bandyopadhyay and R. K. Banerjee, Turmeric and curcumin: Biological actions and medicinal applications, Curr. Sci., 2004, 87, 44–53 CAS
.
- H. Shumoy, S. Lauwens, M. Gabaza, J. Vandevelde, F. Vanhaecke and K. Raes, Traditional fermentation of tef injera: Impact on in vitro iron and zinc dialysability, Food Res. Int., 2017, 102, 93–100 CrossRef CAS
.
- S. Cid-Ortega and J. Guerrero-Beltrán, Roselle calyces (Hibiscus sabdariffa), an alternative to the food and beverages industries: a review, J. Food Sci. Technol., 2015, 52, 6859–6869 CrossRef CAS
.
- N. M. Karaaslan, A comprehensive study about Hibiscus sabdariffa leaves: antioxidant activity, polyphenol profile and macro-and micro-element content, Chem. Pap., 2019, 73, 791–799 CrossRef CAS
.
- Voedingscentrum, Journal, Ijzer, https://www.voedingscentrum.nl/encyclopedie/foliumzuur.aspx, 2018.
- Voedingscentrum, Journal, Zink, https://www.voedingscentrum.nl/encyclopedie/ijzer.aspx, 2018.
- B. Cormier, L. Vanderlee and D. Hammond, Use of Nutrition Information and Understanding of” Percent Daily Value” on Nutrition Facts Tables: Evaluating the Impact of a National Public Education Campaign among Youth and Young Adults in Canada, Can. J. Diet. Pract. Res., 2019, 80, 200–204 CrossRef
.
- L. B. Bailey and J. F. Gregory III, Folate metabolism and requirements, J. Nutr., 1999, 129, 779–782 CrossRef CAS
.
- J. L. Simpson, L. B. Bailey, K. Pietrzik, B. Shane and W. Holzgreve, Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part I–folate, vitamin B12, vitamin B6, J. Matern.-Fetal Neonat. Med., 2010, 23, 1323–1343 CrossRef CAS
.
- T. A. Lawal and A. O. Adeleye, Determinants of folic acid intake during preconception and in early pregnancy by mothers in Ibadan, Nigeria, Pan Afr. Med. J., 2014, 19, 113 Search PubMed
.
- G. S. Kumar, H. Nayaka, S. M. Dharmesh and P. Salimath, Free and bound phenolic antioxidants in amla (Emblica officinalis) and turmeric (Curcuma longa), J. Food Compos. Anal., 2006, 19, 446–452 CrossRef CAS
.
- A. Obadina and O. Oyewole, Assessment of the antimicrobial potential of roselle juice (zobo) from different varieties of roselle calyx, J. Food Process. Preserv., 2007, 31, 607–617 CrossRef CAS
.
- S. Fasoyiro, S. Babalola and T. Owosibo, Chemical composition and sensory quality of fruit-flavoured roselle (Hibiscus sabdariffa) drinks, World J. Agric. Sci., 2005, 1, 161–164 Search PubMed
.
- U. Onoja, P. Akubor, D. Gernar and C. Chinmma, Evaluation of complementary food formulated from local staples and fortified with calcium, iron and zinc, J. Nutr. Food Sci., 2014, 4, 1–6 Search PubMed
.
- P. J. Moughan, Holistic properties of foods: a changing paradigm in human nutrition, J. Sci. Food Agric., 2020, 100, 5056–5063 CrossRef CAS
.
- N. A. Nasef, S. M. Loveday, M. Golding, R. N. Martins, T. M. Shah, M. Clarke, J. Coad, P. J. Moughan, M. L. Garg and H. Singh, Food matrix and co-presence of turmeric compounds influence bioavailability of curcumin in healthy humans, Food Funct., 2019, 10, 4584–4592 RSC
.
- V. Tiwari, R. Shanker, J. Srivastava and P. S. Vankar, Change in antioxidant activity of spices-turmeric and ginger on heat treatment, Electron. J. Environ., 2006, 5, 1313–1317 CAS
.
- L. Shen, H.-H. Jiang and H.-F. Ji, Is boiled food spice curcumin still biologically active? An experimental exploration, Food Nutr. Res., 2018, 62, 1–8 Search PubMed
.
- R. A. Jacob and G. Sotoudeh, Vitamin C function and status in chronic disease, Nutr. Clin. Care, 2002, 5, 66–74 CrossRef
.
- D. K. Matilsky, K. Maleta, T. Castleman and M. J. Manary, Supplementary feeding with fortified spreads results in higher recovery rates than with a corn/soy blend in moderately wasted children, J. Nutr., 2009, 139, 773–778 CrossRef CAS
.
- A. Piovesana, E. Rodrigues and C. P. Z. Noreña, Composition analysis of carotenoids and phenolic compounds and antioxidant activity from hibiscus calyces (Hibiscus sabdariffa L.) by HPLC–DAD–MS/MS, Phytochem. Anal., 2019, 30, 208–217 CrossRef CAS
.
- P. J. Tsai, J. Mclntosh, P. Pearce, B. Camden and B. R. Jordan, Anthocyanin and Antioxidant capacity in roselle (Hibiscus sabdariffa L.) extract, Food Res. Int., 2002, 35, 351–356 CrossRef CAS
.
- L. Havlíková and K. Míková, Heat stability of anthocyanins, Z. Lebensm.-Unters. Forsch., 1985, 181, 427–432 CrossRef
.
- H. E. Khoo, A. Azlan, S. T. Tang and S. M. Lim, Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits, Food Nutr. Res., 2017, 61, 1361779 CrossRef
.
- T. Sogo, N. Terahara, A. Hisanaga, T. Kumamoto, T. Yamashiro, S. Wu, K. Sakao and D. X. Hou, Anti–inflammatory activity and molecular mechanism of delphinidin 3–sambubioside, a Hibiscus anthocyanin, BioFactors, 2015, 41, 58–65 CrossRef CAS
.
- I. Da-Costa-Rocha, B. Bonnlaender, H. Sievers, I. Pischel and M. Heinrich, Hibiscus sabdariffa L.–A phytochemical and pharmacological review, Food Chem., 2014, 165, 424–443 CrossRef CAS
.
- S. Kakkar and S. Bais, A review on protocatechuic acid and its pharmacological potential, ISRN Pharmacol., 2014, 2014, 952943 Search PubMed
.
- A. Prathapan, M. Lukhman, C. Arumughan, A. Sundaresan and K. Raghu, Effect of heat treatment on curcuminoid, colour value and total polyphenols of fresh turmeric rhizome, Int. J. Food Sci. Technol., 2009, 44, 1438–1444 CrossRef CAS
.
- J. Wang, X. Cao, H. Jiang, Y. Qi, K. L. Chin and Y. Yue, Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simultaneous determination five major antioxidant compounds by LC-Q-TOF-MS, Molecules, 2014, 19, 21226–21238 CrossRef
.
| This journal is © The Royal Society of Chemistry 2021 |