Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of industrial processing and storage procedures on oxysterols in milk and milk products†
D.
Risso‡
 *a,
V.
Leoni‡
b,
C.
Fania
b,
M.
Arveda
a,
L.
Falchero
a,
M.
Barattero
c,
A.
Civra
d,
D.
Lembo
d,
G.
Poli
d and
R.
Menta
a
*a,
V.
Leoni‡
b,
C.
Fania
b,
M.
Arveda
a,
L.
Falchero
a,
M.
Barattero
c,
A.
Civra
d,
D.
Lembo
d,
G.
Poli
d and
R.
Menta
a
aSoremartec Italia Srl, Ferrero Group, Alba, CN, Italy. E-mail: davide.risso@ferrero.com; Tel: +390173313214
bLaboratory of Clinical Chemistry, Hospital of Desio and Monza, ASST-Monza, School of Medicine and Surgery, University of Milano Bicocca, Italy
cInalpi SpA, Via Cuneo, 38, 12033 Moretta, CN, Italy
dDepartment of Clinical and Biological Sciences, University of Torino, San Luigi Hospital, 10043, Orbassano, Torino, Italy
First published on 17th December 2020
Abstract
Oxysterols are products of enzymatic and/or chemical cholesterol oxidation. While some of the former possess broad antiviral activities, the latter mostly originate from the deterioration of the nutritional value of foodstuff after exposure to heat, light, radiation and oxygen, raising questions about their potential health risks. We evaluated the presence of selected oxysterols in bovine colostrum and monitored the evolution of their cholesterol ratio throughout an entire industrial-scale milk production chain and after industrially employed storage procedures of milk powders. We report here for the first time the presence of high levels of the enzymatic oxysterol 27-hydroxycholesterol (27OHC) in concentrations of antiviral interest in bovine colostrum (87.04 ng mL−1) that decreased during the first postpartum days (56.35 ng mL−1). Of note, this oxysterol is also observed in milk and milk products and is not negatively affected by industrial processing or storage. We further highlight an exponential increase of the non-enzymatic oxysterols 7β-hydroxycholesterol (7βOHC) and 7-ketocholesterol (7KC) in both whole (WMPs) and skimmed milk powders (SMPs) during prolonged storage, confirming their role as reliable biomarkers of cholesterol oxidation over time: after 12 months, 7βOHC reached in both SMPs and WMPs amounts that have been found to be potentially toxic in vitro (265.46 ng g−1 and 569.83 ng g−1, respectively). Interestingly, industrial processes appeared to affect the generation of 7βOHC and 7KC differently, depending on the presence of fat in the product: while their ratios increased significantly after skimming and processing of skimmed milk and milk products, this was not observed after processing whole milk and milk cream.
Introduction
Interest towards oxysterols has attracted two main audiences in food science. Food technologists have studied non-enzymatic oxysterols since they mainly originate from industrial processing of cholesterol-containing animal products (e.g. meat, fish, eggs, and milk) and storage procedures that can decrease the nutritional properties of foodstuffs.1,2 These include various processes that are generally applied before food is consumed such as heating, spray-drying, and irradiation or exposure to light and oxygen during storage that may trigger cholesterol oxidation. Nutritionists have focused on both enzymatic and non-enzymatic oxysterols because they mediate many pathophysiological functions including cellular toxicity and inhibition of DNA synthesis, but also regulate a variety of cell functions including innate and adaptative immunity and some possess broad antiviral activities.3–5Over the last few years, growing evidence is clearly indicating how only oxysterols of enzymatic origin and in particular the side chain oxysterols 25-hydroxycholesterol and 27-cholesterol may have a pleiotropic physiological role.6,7 Non-enzymatic oxysterols, in particular 7-ketocholesterol (7KC) and 7β-hydroxycholesterol (7βOHC), have been defined as accurate markers of cholesterol auto-oxidation, as their concentration heavily increases during inflammation and other processes characterized by cell and tissue oxidative stress.8–10 In this relation, it is worth outlining that cholesterol oxidation products (COPs) often measured in foodstuffs belong to the not enzymatic oxysterol sub-family.
Total COPs in foods amount, on average, to 1% of the cholesterol content: however, this number may be as high as over 10%, depending on the processing conditions (e.g. heating temperature, length of heating time, UV irradiation, UHT, desiccation), storage time, and type of packaging.11 Even a storage time as short as ten hours has been shown to affect the generation of COPs and the decrease of the antioxidant potential of the product.12 Fresh foods, because of their low oxygen content, have been reported to contain very low levels of cholesterol oxides. Very early analyses claimed their complete absence in fresh milk, attributing their generation only to oxygen exposure, pH decrease, and technological activities.1,2 However, a few more recent studies, with the development of more sensible methods for cholesterol oxides determination, have disclosed their presence in raw milk, both of cow and human origin.6,13 While earlier studies reported that no or only limited amounts of oxysterols were formed during heating of milk under commercial pasteurization or UHT-treatments, a more recent study has shown that pasteurization and UHT treatments increase the amount of COPs significantly, up to 58% in bovine milk and 42% in caprine milk, when compared to raw milk.13–15 These discrepancies may be attributed, at least in part, to the lack of a generally accepted standardized method for determining COPs in different food matrices. The variability of indicators such as linearity range, detection limit, repeatability and recovery rate of the various analytical methods that have been developed for quantifying COPs in food surely increases entropy. Because of their presence at very low concentration and their overall unstable nature, COPs could be degraded easily or, at the contrary, artifacts may be formed during their extraction.2 In addition, it has been shown that non-negligible amounts of cholesterol are present in feedstuffs and can influence the lipid portion of the derived foods. Therefore, the presence and quantity of cholesterol-derived compounds in milk and derived products may also be affected by the sterol profile of the animal feed.16
The major oxysterol species found in animal products are 7α-hydroxycholesterol (7αOHC), 7β-hydroxycholesterol (7βOHC), 7-ketocholesterol (7KC) 5,6β-epoxycholesterol (β-epoxy), 5,6α-epoxycholesterol (α-epoxy), cholestan-3β,5α,6β-triol (Triol), 25-hydroxycholesterol (25OHC).13,17,18 Some of these compounds have received particular attention due to their potential toxicity: in particular, 7βOHC and 7kC have been reported to be important indicators of the storage conditions of liquid milk, milk powders, butter and egg-derived products, highlighting their role as biomarkers of cholesterol oxidation.1,12,19 25OHC, the only side chain oxysterols measured so far in dairy products and at the same time the only side chain oxysterol also generated by auto-oxidation, has gained recent interest because of its broad-spectrum antiviral activity.20,21 In this regard, in spite of the emerging role of another oxysterol of strictly enzymatic production, i.e.27-hydroxycholesterol (27OHC) in the innate immunity and antiviral defenses,5,6,22–24 to date no data have been published on its presence, or absence, in commonly consumed food product. Of note, 27OHC is the quantitatively most represented oxysterol in human blood25,26 and shows a remarkably high concentration in the human colostrum.6 Moreover, of extreme importance is the very recent discovery that 27OHC inhibits in vitro SARS-CoV-2 and one of the common cold agents HCoV-OC43 and is markedly decreased in COVID-19.27
Because of both potential health risks and advantages of oxysterols, their formation and presence in foods have been the subject of many studies. In the scientific literature, however, most of the available information on food products derives from analyses performed on commercially available samples that have undergone unknown processing (temperature, length of processing, oxygen exposure), transportation, and packaging conditions. No comprehensive report on the generation and variation of oxysterol ratios following an industrial milk production chain step-by-step with stable, replicable, processing conditions is to date available.
For these reasons, we analyzed the content of cholesterol and selected oxysterols in colostrum, milk and milk products collected throughout the entire production chain and across key processing treatments (i.e. fresh raw milk, pasteurized, concentrated, skimmed, transformed in milk cream and anhydrous fat, spray-dried) and after prolonged storage of milk powders (i.e. fresh, 6 and 12 months old) of an Italian dairy supplier. The aim of the study was to further explore the role of both enzymatic (i.e. 27OHC) and non-enzymatic (i.e. 7βOHC and 7KC) oxysterols, although keeping a net distinction between the two sub-classes, as nutritional markers and quality tools to monitor cholesterol oxidation in milk and milk products, of use to the food and ingredient industry.
7βOHC and 7KC were selected as the most trustable indices of cholesterol autoxidation not only because the most represented in both liquid and powdered milk and derivatives,12,13,17,18 but also because much less affected by the quite complex analytical determination procedures than other oxysterols such as β-epoxides.28 Moreover, a large body of literature specifically points to the potential broad cytotoxicity of 7βOHC and 7KC, when present in excess amounts.10
27OHC was chosen in light of its solely enzymatic production and because of its promising emerging nutritional properties, as discussed above. In addition, to our knowledge, its presence in foodstuff still remains to be examined. Other oxysterols such as 25OHC and 7αOHC are in fact of both enzymatic and non-enzymatic origin,29–31 and have already been recorded, although at low levels, in milk and dairy products.2,13,17
Materials and methods
Milk and milk samples
Different bovine milk and milk products were included in this study. Firstly, three samples of bovine colostrum were freshly collected from an Italian farm on days 1, 2, 3 postpartum. A series of 16 milk and milk products were then collected throughout an entire industrial-scale production of milk and anhydrous fat (Tetrapak, Sweden) from a customized manufacturing plant of the Italian dairy producer Inalpi SpA (Table 1): replicate analyses of aliquots of four different production batches were performed for each collected sample and the reported dry matter, milk solids and fat content values are based on 500 routine analyses. The collected samples belonged to the following steps of the production chain: fresh raw whole-milk, skimmed milk, raw milk cream, pasteurized standardized whole and skimmed milk, concentrated whole and skimmed milk treated at higher temperature, pasteurized milk cream, anhydrous milk fat, fresh spray-dried whole (WMP) and skimmed milk powder (SMP), stored WMP and SMP (6 and 12 months).| Milk and derivates (N = 4 for each sample) | Dry matter, total solids (%) | Milk solids, non-fat (%) | Fat content (%) |
|---|---|---|---|
| Fresh raw whole-milk, unprocessed | 12.0–13.5 | 8.6–9.3 | 3.4–4.2 |
| Skimmed milk, prepasteurized | 8.6–8.9 | 8.6–8.9 | 0.01–0.02 |
| Milk cream, prepasteurized | 43–45 | 3.0 | 40–42 |
| Whole-milk, standardized, pasteurized at standard parameters (72 °C for 15 s) | 11.2–12.2 | 8–8.6 | 3.2–3.6 |
| Whole-milk, concentrated and treated at higher temperature (85 °C for 40 s) | 51–52 | 36.7–38.4 | 13.2–14.2 |
| Skimmed milk, pasteurized at standard parameters (72 °C for 15 s) | 8.5–9.6 | 8.5–9.5 | 0.07–0.10 |
| Skimmed milk, concentrated and treated at higher temperature (85 °C for 40 s) | 51–52 | 50.6–51.6 | 0.35–0.45 |
| Milk cream, pasteurized at standard parameters (85–90 °C for 15 s) | 43–45 | 3.0 | 40–42 |
| Anhydrous Milk Fat | 99.8–99.9 | 0.1 | 99.8 |
| Whole-milk powder (WMP), fresh | 97–98 | 71–72 | 26–27 |
| WMP, shelf-life 6 months | 97–98 | 71–72 | 26–27 |
| WMP, shelf-life 12 months | 97–98 | 71–72 | 26–27 |
| Skimmed-milk powder (SMP), fresh | 96.5–97.5 | 95.5–96.8 | Max 1.5 (0.7–1) |
| SMP, shelf-life 6 months | 96.5–97.5 | 95.5–96.8 | Max 1.5 (0.7–1) |
| SMP, shelf-life 12 months | 96.5–97.5 | 95.5–96.8 | Max 1.5 (0.7–1) |
Industrial sampling and processing
Fresh raw milk and anhydrous milk fat were collected from different Italian farms (≈400), mainly breeding Italian Friesian cows (70%), Italian Red Pied (18%), and other dairy breeds (12%). After being stored at 4 °C for max 48 hours, milk was then first skimmed by centrifugation and, depending on the type of production (i.e. whole or skimmed), reconstituted and standardized with milk fat and lactose. Both skimmed and whole milk were then processed under different conditions, namely standard legal pasteurization (STD, 72 °C for 15 s) and pre-heating treatment before concentration at 51–52% milk solids (HT, 85 °C for 40 s for whole milk, 85 °C for 190 s for skimmed). Fifteen milliliters samples were collected in disposable sterile Eppendorf tubes, immediately placed at −50 °C and transported at 4 °C, before being stored at −80 °C until tested (within 48 hours after collection) in order to minimize every degeneration process. Skimmed milk (SMP) and whole milk powders (WMP) were produced after the higher-temperature treatment by two subsequent vacuum evaporations, first using Mechanical Vapor Recompression (MVR), involving the use of a compressor, and then Thermal Vapor Recompression (TVR) performed by steam ejector to reach total milk solids of 48% and 51%, respectively to reach a whey protein nitrogen index (WPNI) between 2.0 and 2.5 for SMP and 3.0 and 3.5 for WMP. Concentrated liquid milks were spray-dried using industrial conditions and equipment. Drying was performed in an industrial spray-drying tower and using high-pressure nozzle technology, operating with an inlet air temperature of 190–220 °C, depending on the production with an outlet temperature of 70–75 °C for WMP and 80–85 °C for SMP. The moisture content after this stage is around 3.8–4.0. The powders were further dried in a vibrating drying belt to get a final moisture of 2.0–3.0 for WMP and 2.5–3.5 for SMP. Five grams samples of SMPs and WMPs were collected right after drying belt stage in disposable sterile Eppendorf tubes immediately after production and stored in the dark at 4 °C until tested (within 48 hours after collection). In addition, SMP and WMP from different batches but produced under the same industrial conditions were collected after storage at 20 ± 2 °C and <65% RH for 6 and 12 months in the dark under non-vacuum conditions. Centrifuged milk cream 40% was also used to produce anhydrous milk fat (99.8%). Cream was pasteurized in a plate heat exchanger and further concentrated to obtain 78% fat. Thus, the concentrated cream was homogenized for phase inversion and fat globules disruption, following a further concentration to 99.5% fat and a final polishing step and a vacuum treatment to obtain anhydrous milk fat (99.8% fat). Milk cream before and after pasteurization at standard parameters (85–90 °C for 15 s) and anhydrous milk fat were also collected in disposable Eppendorf tubes, immediately placed at −50 °C, and transported at 4 °C, before being stored at −80 °C until tested (within 48 hours after collection).Cholesterol and oxysterols determination
To a screw-capped vial sealed with a Teflon septum, 1 ml of milk or colostrum, 1 ml of a 100 mg mL−1 distilled water suspended powered milk or 250 mL of cream or anhydrous butter were added together with 50 μg of epicoprostanol (Sigma) 50 ng of 7β-hydroxycholesterol-25, 26, 26, 26, 27, 27, 27-d7 (d7-7βOHC, Avanti Polar Lipids Inc. USA, SKU 700044P), 50 ng of 7-ketocholesterol-25, 26, 26, 26, 27, 27, 27-d7 (d7-7KC, Avanti Polar Lipids Inc. USA, SKU: 700046P), 50 ng of 27-hydroxycholesterol −25, 26, 26, 26, 27, 27-d6 (d6-27OHC, Avanti Polar Lipids Inc. USA, SKU: 700059P) as internal standards, 50 μl of butylatedhydroxytoluene (BHT, 5 g L−1) and 50 μl of K3-EDTA (10 g L−1) to prevent auto-oxidation. Each vial was flushed with argon for 10 min to remove air. Alkaline hydrolysis was allowed to proceed at 4 °C overnight with magnetic stirring in the presence of ethanolic 2 M potassium hydroxide solution. After hydrolysis, the sterols were extracted twice with 5 ml of cyclohexane. 3 ml of the cyclohexane extract were used for cholesterol analysis. The oxysterols were separated from cholesterol and sterols by elution of the remaining 7 ml on SPE cartridge (SI 100 mg columns, Isolute) with isopropanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 30
hexane 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v. The organic solvents were evaporated under a gentle stream of argon and converted into trimethylsilyl ethers with 100 μL of BSTFA (60 °C for 60 min). Analysis was performed by gas chromatography – isotope dilution mass spectrometry (GC-MS) with a B-XLB column (30 m × 0.25 mm i.d. × 0.25 μm film thick-ness, J&W Scientific Alltech, Folsom, CA, USA.) in a HP 6890 Network GC system (Agilent Technologies, USA) connected with a direct capillary inlet system to a quadruple mass selective detector HP5975B inert MSD (Agilent Technologies, USA). GC system was equipped with a HP 7687 series autosamplers and HP 7683 series injectors (Agilent Technologies, USA). The oven temperature program was as follows: initial temperature of 180 °C was held for 1 min, followed by a linear ramp of 20 °C min−1 to 270 °C, and then a linear ramp of 5 °C min−1 to 290 °C, which was held for 11 min. Helium was used as carrier gas at a flow rate of 1 ml min−1 and 1 μL of sample was injected in splitless mode. Injection was carried at 250 °C with a flow rate of 20 ml min−1. Transfer line temperature was 290 °C. Filament temperature was set at 150 °C and quadrupole temperature at 220 °C according with the manufacturer indication. Mass spectrometric data were acquired in selected ion monitoring mode (OTMSi-ethers) at m/z = 463 (M+-90) for 7β-hydroxycholesterol-d7, m/z = 456 (M+-90) for 7β-hydroxycholesterol (Sigma-Aldrich Inc. USA, SKU: H6891), m/z = 479 (M+-90) for 7-ketocholesterol-d7, m/z = 472 (M+-90) for 7-ketocholesterol (Sigma-Aldrich Inc. USA, SKU: C2394), m/z = 462 (M+-90) for 27-hydroxycholesterol-d6 and m/z = 456 (M+-90) for 27-hydroxycholesterol (Avanti Polar Lipids Inc. USA, SKU: 700021P). Peak integration was performed manually, and oxysterols were quantified from selected-ion monitoring analysis against internal standards (available in the ESI, as Table S1†) using standard curves for the listed sterols.32–35
70 v/v. The organic solvents were evaporated under a gentle stream of argon and converted into trimethylsilyl ethers with 100 μL of BSTFA (60 °C for 60 min). Analysis was performed by gas chromatography – isotope dilution mass spectrometry (GC-MS) with a B-XLB column (30 m × 0.25 mm i.d. × 0.25 μm film thick-ness, J&W Scientific Alltech, Folsom, CA, USA.) in a HP 6890 Network GC system (Agilent Technologies, USA) connected with a direct capillary inlet system to a quadruple mass selective detector HP5975B inert MSD (Agilent Technologies, USA). GC system was equipped with a HP 7687 series autosamplers and HP 7683 series injectors (Agilent Technologies, USA). The oven temperature program was as follows: initial temperature of 180 °C was held for 1 min, followed by a linear ramp of 20 °C min−1 to 270 °C, and then a linear ramp of 5 °C min−1 to 290 °C, which was held for 11 min. Helium was used as carrier gas at a flow rate of 1 ml min−1 and 1 μL of sample was injected in splitless mode. Injection was carried at 250 °C with a flow rate of 20 ml min−1. Transfer line temperature was 290 °C. Filament temperature was set at 150 °C and quadrupole temperature at 220 °C according with the manufacturer indication. Mass spectrometric data were acquired in selected ion monitoring mode (OTMSi-ethers) at m/z = 463 (M+-90) for 7β-hydroxycholesterol-d7, m/z = 456 (M+-90) for 7β-hydroxycholesterol (Sigma-Aldrich Inc. USA, SKU: H6891), m/z = 479 (M+-90) for 7-ketocholesterol-d7, m/z = 472 (M+-90) for 7-ketocholesterol (Sigma-Aldrich Inc. USA, SKU: C2394), m/z = 462 (M+-90) for 27-hydroxycholesterol-d6 and m/z = 456 (M+-90) for 27-hydroxycholesterol (Avanti Polar Lipids Inc. USA, SKU: 700021P). Peak integration was performed manually, and oxysterols were quantified from selected-ion monitoring analysis against internal standards (available in the ESI, as Table S1†) using standard curves for the listed sterols.32–35
Replicated analysis of 1 mL of whole-milk, standardized, pasteurized at standard parameters (n = 12) resulted in an interassay CV as 3.87% for 7βOHC, 3.97% for 7KC and 3.13% for 27OHC, respectively. Whole milk pasteurized at standard temperature was added with 25 ng of 7βOHC, 7KC and 27OHC, and analyzed as previously described (n = 3 independent experiments). Mean recovery (measured/expected %) 102.7% for 7βOHC, 98.3% for 7KC and 101% for 27OHC, respectively.
Statistical analysis
Continuous variables were inspected and tested to determine whether distributions were normal by Kolmogorov–Smirnov normality test, expressed as mean ± SD and compared using ANOVA with the Scheffé post-test for parametric data, with Holm-Sidak method for All Pirewise Multiple Comparison. Comparison between two groups was performed with two tailed Student's t-test, and values for statistical significance were set at p < 0.05. All analyses were performed with Sigmastat 3.5 (SigmaAldrich, St Louis, MO, USA). Because of all the different intermediate steps of which an industrial-scale milk production line is composed, most of the analyzed different milk and milk finished and semi-finished products varied in terms of fat percentage, cholesterol and dry matter content (Table 1). Being oxysterols products of the chemical and/or enzymatic oxidation of cholesterol, we expressed our results as ratios of oxysterols/cholesterol (ng μg−1), for a more accurate assessment of the effects of different processing and storage procedures.Results and discussion
Oxysterols in bovine colostrum
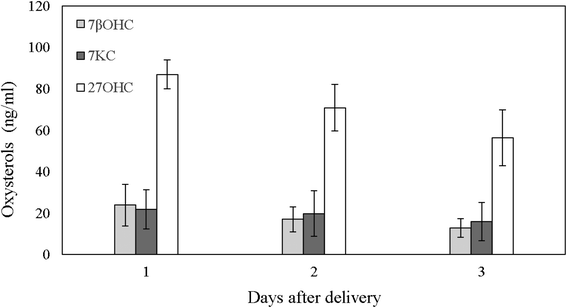
The first analyses carried out were those on the oxysterols content of bovine colostrum, primed by a recent report illustrating the presence of these compounds in the human colostrum, intermediate and mature milk.6 As reported in Fig. 1, the bovine colostrum, as in the case of the human one, showed a marked peak concentration of 27OHC since the first day after delivery (87.04 ± 7.04 ng mL−1), rapidly lowering in the following few days and decreasing by day 3 postpartum to 56.35 ± 13.50 ng mL−1 (P = 0.038; Fig. 1). These concentrations have been shown on in vitro cell lines to efficiently inhibit the replication of different viruses, including human rotavirus, the agent of gastroenteritis in infants.6,36 7βOHC and 7KC, although at significantly lower quantities than 27OHC (P < 0.001; Fig. 1), were also present in bovine colostrum, without showing a reduction between postpartum days (P = 0.247 and P = 0.777, respectively; Fig. 1), as also reported for human colostrum.6 Of note, cholesterol in bovine colostrum showed a progressive reduction from 609.33 ± 111.2 μg mL−1 of day one to 287.45 ± 61.87 μg mL−1 of day three after delivery (P = 0.015), as also observed in human colostrum.6Oxysterols in milk and milk products: effects of processing
| Oxysterolsc | Type of industrial processing | Storage | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Pasteurized ST, standardized | HT treatment, concentrated | Concentrated | Spray-dried | 6 months | 12 months | |||||||||
| WM | SM | MC | WM | SM | MC | WM | SM | AMF | WMP | SMP | WMP | SMP | WMP | SMP | |
| a Values are expressed as ng oxysterol per μg cholesterol and are means ± SD of four different production batches. Values within a row with different lowercase superscripts indicate statistically significant differences at α = 0.05. b ST: Standard temperature; HT: higher temperature; WM: whole milk; SM: skimmed milk; MC: milk cream; AMF: anhydrous milk fat; WMP: whole-milk-powder; SMP: skimmed milk powder. c 7βOHC: 7β-hydroxycholesterol; 7KC: 7-ketocholesterol; 27OHC: 27-hydroxycholesterol. | |||||||||||||||
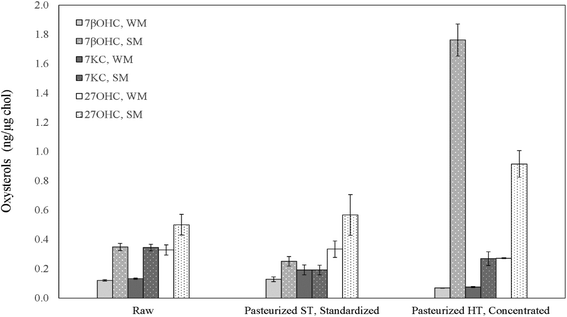
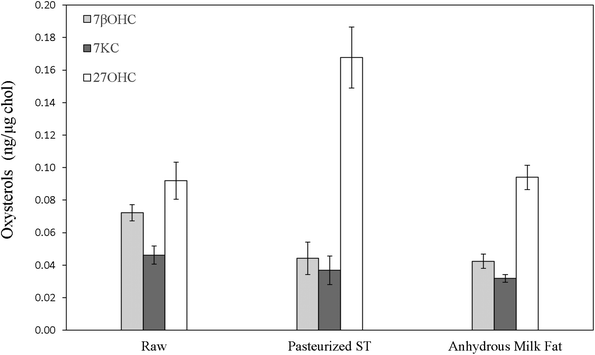
| 7βOHC | 0.12 ± 0.01a | 0.35 ± 0.02b | 0.07 ± 0.01c | 0.13 ± 0.02a | 0.25 ± 0.03bc | 0.04 ± 0.02c | 0.07 ± 0ab | 1.76± 0.11bd | 0.04 ± 0c | 0.04 ± 0ac | 0.74± 0.13be | 0.20± 0.02ad | 0.84± 0.06bd | 0.67± 0.03ae | 1.3± 0.08be |
| 7KC | 0.13 ± 0.01a | 0.34 ± 0.02b | 0.05 ± 0.01c | 0.19 ± 0.03ab | 0.19 ± 0.03bc | 0.04 ± 0.01c | 0.08 ± 0ac | 0.27 ± 0bd | 0.03 ± 0c | 0.05 ± 0ad | 0.46± 0.05be | 0.08± 0.01ae | 0.31± 0.02be | 0.20± 0.01ae | 0.39± 0.03be |
| 27OHC | 0.33 ± 0.03a | 0.50 ± 0.07b | 0.09 ± 0.01c | 0.33 ± 0.06a | 0.57 ± 0.14b | 0.17 ± 0.02cd | 0.27 ± 0ab | 0.92± 0.09bc | 0.09 ± 0.01ce | 0.16 ± 0.01ac | 0.29± 0.06bd | 0.28± 0.02ad | 0.20± 0.03bd | 0.17± 0.01ac | 0.23± 0.04bd |
Oxysterols in WMPs and SMPs: effects of storage
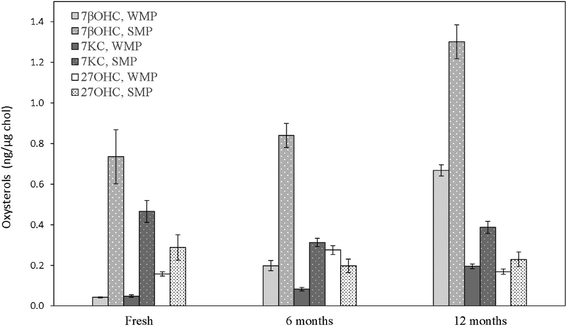
Fresh WMP contained an average 898.41 ± 32.04 μg g−1 cholesterol. Although the cholesterol content after spray-drying increased by roughly 14 times compared to pasteurized whole-milk, the increase in the three measured oxysterols did not follow the same increase ratio, exhibiting, on average, only about a 5-fold increase and reaching absolute 7βOHC, 7KC and 27OHC amounts of 37.63 ± 3.78, 43.71 ± 6.22 and 141.21 ± 5.78 ng mg−1, respectively (P < 0.05). These concentrations are comparable to the ones detected within the physiological range in human peripheral blood of normocholesterolemic adult subjects.43 In SMP, the residual cholesterol level was quantified as an average of 161.69 ± 15.78 μg g−1. Differently from WMP, although the cholesterol content after spray-drying of pasteurized skimmed milk increased by approximately 7 times, the three measured oxysterols were found to increase by roughly 14 times, reaching absolute values of 117.61 ± 13.59, 74.53 ± 1.83 and 46.34 ± 9.56 ng mg−1 (P < 0.05; Table 2). As in liquid milk, SMPs contained noticeably higher R7βOHC (P = 0.029), R7KC (P = 0.029) and R27OHC (P = 0.006) than WMPs (P < 0.05; Fig. 4, Table 2), a result that finds confirmation in previous studies.2,41,447βOHC and 7KC were found to be particularly effective predictors of storage time in WMP: after 6 months, the increase of their ratio was 5 (P < 0.001) and 1.6-fold (P < 0.001), respectively (Fig. 4, Table 2), in agreement with previous findings defining them as effective and reliable markers of autoxidation across storage time in powdered foodstuff.19,41,44 After 12 months, their amounts further increased by 16.8 (P < 0.001) and 4-fold (P < 0.001), respectively (Fig. 4, Table 2). A similar trend was identified in SMPs, with R7βOHC increasing over time (P < 0.001; Fig. 4, Table 2), with a difference regarding R7KC, which did not show a clear trend over time (P > 0.05; Fig. 4, Table 2). Of notable importance is the fact that the absolute content of 7βOHC at 12 months reached 265.46 ± 22.42 ng g−1 and 569.83 ± 42.28 ng g−1 in SMPs and WMPs, respectively: amounts that have been found to be potentially toxic in several in vitro and in vivo experimental models.10,36,45 Interestingly, R27OHC showed an increase across storage time in WMPs, with a peak at 6 months (P < 0.001). In SMPs we did not observe a reduction R27OHC over time (P > 0.05; Fig. 4, Table 2). Although this is the first study to test for the presence of 27OHC in bovine milk products, other enzymatic or semi-enzymatic oxysterols have been reported to show a non-linear increase with storage time, suggesting that prolonged exposure to oxygen may not represent a strong driver of generation of these enzymatic compounds.2,40,44
Conclusions
Although earlier studies reported a complete absence of oxysterols in unprocessed products, showing their occurrence only after exposure to oxygen, pH decrease, and technological activities, our study highlights their presence in fresh milk and milk products, in line with more recent evidence. Remarkably, we found the oxysterol of enzymatic origin 27OHC in concentrations provided with antiviral potential in bovine colostrum and in lower but still nutritionally relevant amounts in mature milk, which were not affected by industrial processing. Furthermore, we stressed the role of the non-enzymatic 7βOHC and 7KC as a duo of reliable biomarkers of cholesterol oxidation, thus pointing to quality deterioration indicators of prolonged storage of milk powders under non-vacuum conditions.Overall, we highlighted how the measurement of a selected subset of enzymatic (i.e. 27OHC) and non-enzymatic (i.e. 7βOHC and 7KC) oxysterols during and after a milk production chain could represent a useful tool to monitor and increase the commercial and nutritional value of milk and milk products under certain processing conditions and storage procedures. For a more comprehensive approach, we acknowledge the need for future studies to also address the determination and comparative evaluation of additional oxysterols, such as 7αOHC, epoxides and triol, to explore their possible inclusion in such tool.
Author contributions
Conceptualization, D. R., V. L., G. P., R. M.; data curation, D. R., V. L., G. P., C. F., M. A., L. F., A. C., M. B., D. L.; formal analysis, D. R., V. L., G. P., C. F.; methodology, D. R., V. L., G. P.; project administration, D. R., G. P., R. M.; resources, M. A., L. F., M. B., D. L., A. C., G. P., R. M., supervision, G. P., R. M.; validation D. R., G. P., R. M.; visualization, D. R., A. C., G. P.; writing—original draft, D. R., V. L., G. P.; writing—review & editing, D. R., V. L., G. P., R. M., M. A., C. F. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
D. Risso, M. Arveda, L. Falchero and R. Menta are full-time employees of Soremartec Italia Srl, Alba (CN, Italy). M. Barattero is an employee of Inalpi SpA Moretta (CN), Italy.Acknowledgements
The research was carried out thanks to the financial support of Soremartec Italia Srl, Alba (CN, Italy). The authors would like to thank Azienda Agricola Abbà Pietro & Figli in Favria (TO) for kindly providing the colostrum samples.References
- G. P. Savage, P. C. Dutta and M. T. Rodriguez-Estrada, Cholesterol oxides: their occurrence and methods to prevent their generation in foods, Asia Pac. J. Clin. Nutr., 2002, 11, 72–78 CrossRef CAS.
- R. Sieber, Oxidised cholesterol in milk and dairy products, Int. Dairy J., 2005, 15, 191–206 CrossRef CAS.
- G. Poli, F. Biasi and G. Leonarduzzi, Oxysterols in the pathogenesis of major chronic diseases, Redox Biol., 2013, 1, 125–130 CrossRef CAS.
- B. Vurusaner, G. Leonarduzzi, P. Gamba, G. Poli and H. Basaga, Oxysterols and mechanisms of survival signaling, Mol. Aspects Med., 2016, 49, 8–22 CrossRef CAS.
- D. Lembo, V. Cagno, A. Civra and G. Poli, Oxysterols: an emerging class of broad spectrum antiviral effectors, Mol. Aspects Med., 2016, 49, 23–30 CrossRef CAS.
- A. Civra, V. Leoni, C. Caccia, S. Sottemano, P. Tonetto, A. Coscia, C. Peila, G. E. Moro, P. Gaglioti, E. Bertino, G. Poli and D. Lembo, Antiviral oxysterols are present in human milk at diverse stages of lactation, J. Steroid Biochem. Mol. Biol., 2019, 193, 105424 CrossRef CAS.
- A. Civra, M. Colzani, V. Cagno, R. Francese, V. Leoni, G. Aldini, D. Lembo and G. Poli, Modulation of cell proteome by 25-hydroxycholesterol and 27-hydroxycholesterol: A link between cholesterol metabolism and antiviral defense, Free Radicals Biol. Med., 2020, 149, 30–36 CrossRef CAS.
- C. Zerbinati and L. Iuliano, Cholesterol and related sterols autoxidation, Free Radicals Biol. Med., 2017, 111, 151–155 CrossRef CAS.
- B. Sottero, D. Rossin, E. Staurenghi, P. Gamba, G. Poli; and G. Testa, Omics analysis of oxysterols to better understand their pathophysiological role, Free Radicals Biol. Med., 2019, 144, 55–71 CrossRef CAS.
- A. Vejux, D. Abed-Vieillard, K. Hajji, A. Zarrouk, J. J. Mackrill, S. Ghosh, T. Nury, A. Yammine, M. Zaibi, W. Mihoubi, H. Bouchab, B. Nasser, Y. Grosjean and G. Lizard, 7-Ketocholesterol and 7β-hydroxycholesterol: In vitro and animal models used to characterize their activities and to identify molecules preventing their toxicity, Biochem. Pharmacol., 2020, 173, 113648 CrossRef CAS.
- S. J. Hur, G. N. Park and S. T. Joo, Formation of cholesterol oxidation products (COPs) in animal products, Food Control, 2007, 18, 939–947 CrossRef CAS.
- D. Cais-Sokolińska and M. Rudzińska, Short communication: Cholesterol oxidation products in traditional buttermilk, J. Dairy Sci., 2018, 101, 3829–3834 CrossRef.
- J. M. Pikul, J. Rudzinska, A. Teichert, R. Lasik, R. Danków and R. Przybylski, Cholesterol oxidation during storage of UHT-treated bovine and caprine milk, Int. Dairy J., 2013, 30, 29–32 CrossRef CAS.
- M. Z. Cleveland and N. D. Harris, Oxidation of cholesterol in commercially processed cow's milk., J. Food Prot., 1987, 50, 867–871 CrossRef CAS.
- B. D. Sander, P. B. Addis, S. W. Park and D. E. Smith, Quantification of cholesterol oxidation products in a variety of foods, J. Food Prot., 1989, 52, 109–114 CrossRef CAS.
- G. Simonetti, P. Di Filippo, D. Pomata, C. Riccardi, F. Buiarelli, E. Sonego and F. Castellani, Characterization of seven sterols in five different types of cattle feedstuffs, Food Chem., 2021, 340, 127926 CrossRef CAS.
- M. Calderόn-Santiago, M. A. Peralbo-Molina, F. Priego-Capote and M. D. Luque de Castro, Cholesterol oxidation products in milk: Processing formation and determination, Eur. J. Lipid Sci. Technol., 2012, 114, 687–694 CrossRef.
- A. Gorassini, G. Verardo, S. C. Fregolent and R. Bortolomeazzi, Rapid determination of cholesterol oxidation products in milk powder-based products by reversed phase SPE and HPLC-APCI-MS/MS, Food Chem., 2017, 230, 604–610 CrossRef CAS.
- M. F. Caboni, E. Boselli, M. C. Messia, V. Velazco, A. Fratianni, G. Panfili and E. Marconi, Effect of processing and storage on the chemical quality markers of spray-dried whole egg, Food Chem., 2005, 92, 293–303 CrossRef CAS.
- S. Y. Liu, R. Aliyari, K. Chikere, G. Li, M. D. Marsden, J. K. Smith, O. Pernet, H. Guo, R. Nusbaum, J. A. Zack, A. N. Freiberg, L. Su, B. Lee and G. Cheng, Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol, Immunity, 2013, 38, 92–105 CrossRef CAS.
- M. Blanc, W. Y. Hsieh, K. A. Robertson, K. A. Kropp, T. Forster, G. Shui, P. Lacaze, S. Watterson, S. J. Griffiths, N. J. Spann, A. Meljon, S. Talbot, K. Krishnan, D. F. Covey, M. R. Wenk, M. Craigon, Z. Ruzsics, J. Haas, A. Angulo, W. J. Griffiths, C. K. Glass, Y. Wang and P. Ghazal, The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response, Immunity, 2013, 38, 106–118 CrossRef CAS.
- Y. Son, S. M. Kim, S. A. Lee, S. K. Eo and K. Kim, Oxysterols induce transition of monocytic cells to phenotypically mature dendritic cell-like cells, Biochem. Biophys. Res. Commun., 2013, 438, 161–168 CrossRef CAS.
- S. Gargiulo, P. Gamba, G. Testa, D. Rossin, F. Biasi, G. Poli and G. Leonarduzzi, Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability, Aging Cell, 2015, 14, 569–581 CrossRef CAS.
- T. Willinger, Oxysterols in intestinal immunity and inflammation, J. Intern. Med., 2019, 285, 367–380 CrossRef CAS.
- I. Björkhem, V. Leoni and S. Meaney, Genetic connections between neurological disorders and cholesterol metabolism, J. Lipid Res., 2010, 51, 2489–2503 CrossRef.
- V. Leoni and C. Caccia, Oxysterols as biomarkers in neurodegenerative diseases, Chem. Phys. Lipids, 2011, 164, 515–524 CrossRef CAS.
- A. Marcello, A. Civra, R. Milan Bonotto, L. Nascimento Alves, S. Rajasekharan, C. Giacobone, C. Caccia, R. Cavalli, M. Adami, P. Brambilla, D. Lembo, G. Poli and V. Leoni, The cholesterol metabolite 27-hydroxycholesterol inhibits SARS-CoV-2 and is markedly decreased in COVID-19 patients, Redox Biol., 2020, 36, 101682 CrossRef CAS.
- S. Dzeletovic, O. Breuer, E. Lund and U. Diczfalusy, Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry, Anal. Biochem., 1995, 225(1), 73–80 CrossRef CAS.
- L. Iuliano, Pathways of cholesterol oxidation via non-enzymatic mechanisms, Chem. Phys. Lipids, 2011, 164, 457–468 CrossRef CAS.
- O. Breuer and I. Björkhem, Simultaneous quantification of several cholesterol autoxidation and monohydroxylation products by isotope-dilution mass spectrometry, Steroids, 1990, 55(4), 185–192 CrossRef CAS.
- C. Helmschrodt, S. Becker, J. Schröter, M. Hecht, G. Aust, J. Thiery and U. Ceglarek, Fast LC–MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque, Clin. Chim. Acta, 2013, 425, 3–8 CrossRef CAS.
- J. Acimovic, A. Lövgren-Sandblom, K. Monostory, D. Rozman, M. Golicnik, D. Lutjohann and I. Björkhem, Combined gas chromatographic/mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877(22), 2081–2086 CrossRef CAS.
- D. Lütjohann, I. Björkhem, S. Friedrichs, A. Kerksiek, A. Lövgren-Sandblom, W. J. Geilenkeuser, R. Ahrends, I. Andrade, D. Ansorena, I. Astiasarán, L. Baila-Rueda, B. Barriuso, S. Becker, L. Bretillon, R. W. Browne, C. Caccia, U. Ceglarek, A. Cenarro, P. J. Crick, G. Fauler, G. Garcia-Llatas, R. Gray, W. J. Griffiths, H. Gylling, S. Harding, C. Helmschrodt, L. Iuliano, H. G. Janssen, P. Jones, L. Kaipiainen, F. Kannenberg, M. J. Lagarda, V. Leoni, A. M. Lottenberg, D. S. MacKay, S. Matysik, J. McDonald, M. Menendez-Carreño, S. B. Myrie, V. Sutti Nunes, R. E. Ostlund, E. Polisecki, F. Ramos, T. C. Rideout, E. J. Schaefer, G. Schmitz, Y. Wang, C. Zerbinati, U. Diczfalusy and H. F. Schött, First international descriptive and interventional survey for cholesterol andnon-cholesterol sterol determination by gas- and liquid-chromatography-Urgent need for harmonisation of analytical methods, J. Steroid Biochem. Mol. Biol., 2019, 190, 115–125 CrossRef.
- D. Lütjohann, I. Björkhem, S. Friedrichs, A. Kerksiek, W. J. Geilenkeuser, A. Lövgren-Sandblom, D. Ansorena, I. Astiasarán, L. Baila-Rueda, B. Barriuso, L. Bretillon, R. W. Browne, C. Caccia, A. Cenarro, P. J. Crick, G. Fauler, G. García-Llatas, W. J. Griffiths, L. Iuliano, M. J. Lagarda, V. Leoni, A. M. Lottenberg, S. Matysik, J. McDonald, T. C. Rideout, G. Schmitz, V. S. Nunes, Y. Wang, C. Zerbinati, U. Diczfalusy and H. F. Schött, International descriptive and interventional survey for oxycholesteroldetermination by gas- and liquid-chromatographic methods, Biochimie, 2018, 153, 26–32 CrossRef.
- V. Leoni, T. Nury, A. Vejux, A. Zarrouk, C. Caccia, M. Debbabi, A. Fromont, R. Sghaier, T. Moreau and G. Lizard, Mitochondrial dysfunctions in 7-ketocholesterol-treated158N oligodendrocytes without or with α-tocopherol: Impacts on the cellular profil of tricarboxylic cycle-associated organic acids, long chain saturated and unsaturated fatty acids, oxysterols, cholesterol and cholesterol precursors, J. Steroid Biochem. Mol. Biol., 2017, 169, 96–110 CrossRef CAS.
- A. Civra, V. Cagno, M. Donalisio, F. Biasi, G. Leonarduzzi, G. Poli and D. Lembo, Inhibition of pathogenic non-enveloped viruses by 25-hydroxycholesterol and 27-hydroxycholesterol., Sci. Rep., 2014, 4, 7487 CrossRef CAS.
- V. Piironen, J. Toivo and A. M. Lampi, New data for cholesterol contents in meat, fish, milk, eggs and their products consumed in Finland, J. Food Compos. Anal., 2002, 15, 705–713 CrossRef CAS.
- C. Y. Tai, Y. C. Chen and B. H. Chen, Analysis, formation and inhibition of cholesterol oxidation products in food: An overview (Part II), J. Food Drug Anal., 2000, 8, 1–5 CAS.
- A. Obara, M. Obiedziński and T. Kołczak, The effect of water activity on cholesterol oxidation in spray- and freeze-dried egg powders, Food Chem., 2006, 95, 173–179 CrossRef CAS.
- S. G. Anema, Effect of milk solids concentration on whey protein denaturation, particle size changes and solubilization of casein in high-pressure-treated skim milk, Int. Dairy J., 2007, 18, 228–235 CrossRef.
- A. J. Angulo, J. M. Romera, M. Ramirez and A. Gil, Determination of cholesterol oxides in dairy products. Effect of storage conditions, J. Agric. Food Chem., 1997, 45, 4318–4323 CrossRef CAS.
- J. Nourooz-Zadeh and L. Appelqvist, Cholesterol oxides in Swedish foods and food ingredients: Butter and cheese, J. Am. Oil Chem. Soc., 1988, 65, 1635–1641 CrossRef CAS.
- V. Leoni, C. Mariotti, L. Nanetti, E. Salvatore, F. Squitieri, A. R. Bentivoglio, M. Bandettini di Poggio, S. Piacentini, D. Monza, M. Valenza, E. Cattaneo and S. Di Donato, Whole body cholesterol metabolism is impaired in Huntington's disease, Neurosci. Lett., 2011, 494(3), 245–249 CrossRef CAS.
- J. Nourooz-Zadeh and L. Appelqvist, Cholesterol oxides in Swedish foods and food ingredients: milk powder products, J. Food Sci., 1988, 53(1), 74–79 CrossRef CAS.
- A. Anderson, A. Campo, E. Fulton, A. Corwin, W. G. Jerome 3rd and M. S. O'Connor, 7-Ketocholesterol in disease and aging, Redox Biol., 2020, 29, 101380 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo02462g |
| ‡ These authors equally contributed to the work. |
| This journal is © The Royal Society of Chemistry 2021 |