Open Access Article
Open Access ArticleDeep-bed filters as post-treatment for ozonation in tertiary municipal wastewater treatment: impact of design and operation on treatment goals†
Daniel
Sauter
a,
Agata
Dąbrowska
b,
Robert
Bloch
a,
Michael
Stapf
c,
Ulf
Miehe
 c,
Alexander
Sperlich
c,
Alexander
Sperlich
 a,
Regina
Gnirss
a and
Thomas
Wintgens
*de
a,
Regina
Gnirss
a and
Thomas
Wintgens
*de
aBerliner Wasserbetriebe, Neue Juedenstr. 1, 10179 Berlin, Germany
bFaculty of Chemistry, Adam Mickiewicz University, ul. Uniwersytetu Poznanskiego 8, 61-614 Poznań, Poland
cKompetenzzentrum Wasser Berlin, Cicerostr. 24, 10709 Berlin, Germany
dInstitut für Siedlungswasserwirtschaft, RWTH Aachen University, Mies-van-der-Rohe-Str. 1, 52074 Aachen, Germany. E-mail: wintgens@isa.rwth-aachen.de
eSchool of Life Sciences, Institute for Ecopreneurship, University of Applied Sciences and Arts Northwestern Switzerland, Hofackerstrasse 40, 4132 Muttenz, Switzerland
First published on 25th November 2020
Abstract
Ozonation followed by biological post-treatment is an established technology for abatement of organic micropollutants (OMP) from municipal wastewater. Although the necessity of biological post-treatment for oxidation by-product (OBP) removal is widely accepted, there is still discussion about the appropriate design and operation. The presented pilot-study investigates the impact of filter material and contact time on the removal efficiency of bulk organics, OMP, and OBP in three different deep-bed filters operated in parallel as post-treatment after ozonation (biological activated carbon (BAC) filter, dual-media filter sand/BAC and dual-media filter sand/anthracite). The use of BAC instead of non-adsorptive filter material resulted in higher removal of DOC and dissolved oxygen which indicates increased biological activity. Moreover, both BAC containing filters showed additional removal for a number of OMP even at high treated bed volumes of >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 whereas no removal was observed in the sand/anthracite filter. Analysis of N-nitrosodimethylamine (NDMA) and several carbonyl compounds revealed a clear formation of these biodegradable OBP during ozonation. A strong correlation was found between carbonyl formation and the specific ozone dose. Removal of OBP in the sand/BAC and the sand/anthracite filter was tested at different empty bed contact times (EBCT). While NDMA was efficiently removed independent of EBCT changes, there was a slightly negative impact of shorter EBCT on the reduction of carbonyl compounds. Furthermore, it was demonstrated that the integration of enhanced phosphorus removal into post-treatment is feasible with relatively low efforts by inline coagulant dosing (FeCl3) in the filter influent.
000 whereas no removal was observed in the sand/anthracite filter. Analysis of N-nitrosodimethylamine (NDMA) and several carbonyl compounds revealed a clear formation of these biodegradable OBP during ozonation. A strong correlation was found between carbonyl formation and the specific ozone dose. Removal of OBP in the sand/BAC and the sand/anthracite filter was tested at different empty bed contact times (EBCT). While NDMA was efficiently removed independent of EBCT changes, there was a slightly negative impact of shorter EBCT on the reduction of carbonyl compounds. Furthermore, it was demonstrated that the integration of enhanced phosphorus removal into post-treatment is feasible with relatively low efforts by inline coagulant dosing (FeCl3) in the filter influent.
Water impactDeep-bed filtration is the most common technology for biological post-treatment after ozonation in tertiary wastewater treatment. This long-term pilot-study shows that the choice of filter material and empty bed contact time are important for an optimised removal of organic contaminants and that an integration of enhanced phosphorus removal in post-treatment by coagulant dosing is feasible without drawbacks. |
1 Introduction
Anthropogenic organic micropollutants (OMP) are detected in surface waters worldwide in the range of ng L−1 to μg L−1.1 Introduction of OMP into surface waters can be attributed to a large extent to the discharge of municipal wastewater treatment plant effluents.2 Only a part of the wide spectrum of chemicals is sufficiently removed by conventional activated sludge systems.3,4 Despite their low concentrations, OMP can pose a potential risk for aquatic ecosystems.5,6 Moreover, they can be critical if receiving waters serve as resources for drinking water production. Natural barriers in the drinking water treatment train like managed aquifer recharge cannot guarantee full removal of OMP.7Ozonation for removal of OMP from municipal wastewater has been studied extensively in the last years and it developed to an established technology with several full-scale applications already implemented, mainly in Switzerland and Germany.8,9 It is generally accepted that ozone treatment requires biological post-treatment in order to degrade potentially toxic and/or carcinogenic oxidation by-products (OBP) formed in the oxidation process. However, there is still discussion about the appropriate design and operation of such a post-treatment step. Numerous systems have been studied for post-treatment such as fixed bed bioreactors,9 moving bed bioreactors,9,10 constructed wetlands,11 integrated solutions with ozonation as an intermediate step in the activated sludge process12 or deep-bed filters. Latter option is most commonly applied and uses either non-adsorptive filter materials such as sand8,9 or granular activated carbon9,13 (GAC).
The above-mentioned post-treatment options have an impact on several different water quality parameters but a uniform approach for assessment of these processes has still not been established. Considering the concept of biological post-treatment after ozonation, efficient removal of biodegradable OBP can be regarded as the minimum requirement for a suitable solution. Typical representatives of biodegradable OBP are carbonyl compounds which are mainly formed by oxidation of bulk organic matter and often associated with toxic effects.14,15 Also, potentially carcinogenic nitrosamines such as N-nitrosodimethylamine (NDMA) can be formed during ozone treatment in presence of specific precursors like secondary and tertiary amines.16 However, NDMA can be degraded in biological-post-treatment.9
Beyond OBP removal, treatment goals of post-treatment can differ depending on the site-specific requirements (e.g. targeting additional OMP removal). While OMP abatement by ozonation is a selective process, an additional purpose of post-treatment can be to complement the ozone action mode and widen the spectrum of abated substances. Relevant additional removal of OMP has only been reported for GAC containing deep-bed filters that were either operated as primarily adsorptive treatment steps or as biological activated carbon (BAC) filters.9,13,17 This also applies to transformation products of individual OMP which are often only slightly modified (e.g. formation of N-oxides) and thus do not exhibit an improved biodegradability compared to their parent compounds.18,19 For post-treatment with BAC filtration, substantial OMP removal was still observed at high treated bed volumes (BV) of approx. 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 leading the authors of the study to consider the involvement of biotransformation processes.9 Long-term water quality gains without a frequent exchange of the costly GAC material would make BAC filters a promising compromise between non-adsorptive filters and primarily adsorptive GAC filters.
000 leading the authors of the study to consider the involvement of biotransformation processes.9 Long-term water quality gains without a frequent exchange of the costly GAC material would make BAC filters a promising compromise between non-adsorptive filters and primarily adsorptive GAC filters.
The combination of ozonation and subsequent biological treatment also reduces concentrations of bulk organic matter by first enhancing its bioavailability in the oxidation process and subsequently degrading the bioavailable carbon source in the post-treatment. While the removal of the biodegradable/assimilable fraction of organic matter (usually measured as BDOC or AOC) after ozonation is an important aim for drinking water applications in order to guarantee biological stability in the water distribution network,20 its relevance in advanced wastewater treatment is the prevention of oxygen consumption in the receiving water. Moreover, organic bulk parameters can be used as a simple indicator for efficiency comparison of different post-treatment technologies.
A common treatment goal in advanced wastewater treatment is an enhanced phosphorus removal, typically realized as a coagulation/filtration step. If back-washable deep-bed filters are applied, enhanced phosphorus removal can be integrated with relatively low efforts by installing a coagulant dosing station in the filter influent.21
The present pilot-study investigates ozonation followed by a single-media BAC filter, a dual-media sand/BAC filter, and a dual-media sand/anthracite filter (non-adsorptive), all operated in parallel. Both dual-media filters are equipped with coagulant dosing in the filter influent for additional phosphorus removal. The removal efficiency for OBP (carbonyl compounds and NDMA), OMP, and bulk organics with different filter materials (non-adsorptive anthracite vs. BAC) and different operational conditions (influence of contact time) is the main focus of this study. In addition, the operation of the post-treatment as a coagulation/filtration step for enhanced phosphorus removal is evaluated.
2 Material and methods
2.1 Secondary effluent quality
The pilot-plant was installed at the municipal WWTP Schoenerlinde, north of Berlin, which represents a conventional secondary treatment. After mechanical pre-treatment, aerated sand/grease trap, and primary sedimentation, wastewater passes activated sludge treatment with pre-denitrification. Phosphorus is removed by enhanced biological treatment, complemented by coagulant dosing in the activated sludge process. Final treatment takes place in secondary clarifiers. The secondary effluent quality for relevant parameters is summarised in Table 1. The selected OMP are typical representatives of compounds with a fast (diclofenac), moderate (benzotriazole), and slow (metformin) reaction with ozone. An overview of all monitored OMP and their reactivity with ozone is given in Table S1.†| Parameter | Unit | Mean ± standard deviation (n = number of data) |
|---|---|---|
| DOC | mg L−1 | 10.0 ± 1.2 (n = 42) |
| UVA254 | 1/m | 24.6 ± 2.1 (n = 42) |
| COD | mg L−1 | 32.4 ± 5.2 (n = 41) |
| Total phosphorus (Ptot) | mg L−1 | 0.6 ± 0.4 (n = 26) |
| TSS | mg L−1 | 7.0 ± 4.2 (n = 34) |
| T | °C | 18.6 ± 3.9 (n = 44) |
| Diclofenac | μg L−1 | 3.8 ± 0.9 (n = 41) |
| Benzotriazole | μg L−1 | 11.6 ± 1.8 (n = 42) |
| Metformin | μg L−1 | 0.46 ± 0.3 (n = 41) |
2.2 Pilot-plant
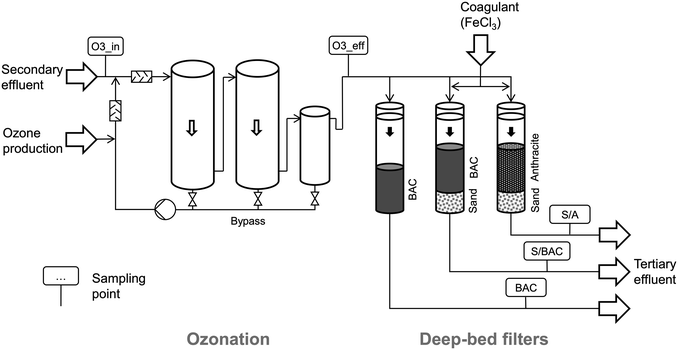
As shown in Fig. 1 the pilot-scale treatment of secondary effluent consists of an ozonation unit (flow rate approx. 7 m3 h−1) followed by different deep-bed filtration steps for post-treatment that are operated in parallel.Based on previous experiences a specific applied ozone dose of 0.7 mg O3/mg DOC was targeted for efficient OMP degradation.9,22 Ozone dose control via online measurement of the UV absorbance at 254 nm (UVA254) in the influent and effluent was applied.23 Ozonation was operated with a target UVA254 reduction of 47% since this value was determined to correspond with the target ozone dose in Schoenerlinde secondary effluent.24 Online ozone measurement in the influent and the offgas enables the ozone unit to calculate the actual amount of dosed ozone via mass balancing. The calculated specific ozone dose was 0.65 ± 0.09 mg O3 per mg DOC (arithmetic mean ± standard deviation) and hence, slightly lower than originally targeted. The ozone generator (GSO40, Xylem Inc.) is supplied with oxygen by three pressure swing adsorption units (Topaz Ultra, Chart Industries Inc.). Ozone is dosed into a recirculated sidestream by a venturi injection system and subsequently mixed with the mainstream. Two stainless steel ozone reactors connected in series, with a volume of 2 m3 each, enable a hydraulic retention time of >15 min for reaction of ozone. The maximum treatment capacity of the ozone unit is 15 m3 h−1.
Ozonation effluent is distributed to the inlets of the three identically constructed deep-bed filter columns with a diameter of 0.3 m each. An overflow keeps the water level in the supernatant of each filter and hence the filtration pressure constant. The first filter represents a widely used solution for biological post-treatment after ozonation, a single-media filter, filled with GAC (BAC filter). The other two filters additionally aim at the enhanced removal of phosphorus, besides biological post-treatment. Hence, they are operated as coagulation/filtration steps with prior ferric chloride (Stockmeier Chemie, Germany) dosing. Due to the increased solids load originating from coagulation, the filters are designed as dual-media filters, with sand in the lower layer for safe particle retention. While in one filter the upper layer consists of anthracite (S/A filter), GAC with comparable grain size is used in the other one (S/BAC filter). BAC and S/BAC filter were commissioned with fresh GAC and operated within this study until approx. 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV (corresponds to approx. 1.5 years of pilot-operation).
000 BV (corresponds to approx. 1.5 years of pilot-operation).
For coagulation in the dual-media filters S/A and S/BAC, a stock solution (0.19 kg Fe per L) was dosed inline in front of static mixers into the filter influents. Since ortho-phosphate (o-PO4-P) was not monitored online, a constant coagulant dose of 1.8 mg Fe per L was applied and could not be adapted to variations of phosphorus concentrations, as it would be the case at full-scale. The dose was calculated for annual mean phosphorus concentrations in the secondary effluent from 2016 (o-PO4-P = 0.2 mg L−1, Ptot = 0.4 mg L−1) based on the desired molar ratio of 4.4 mol Fe per mol o-PO4-P. As phosphorus concentrations during the study period were higher than in 2016 (Table 1), the molar ratio fell below the target value of 4.4 mol Fe per mol o-PO4-P for several samplings.
Filter backwash was carried out daily on weekdays, first with air (2 min, 60 m h−1) and then with water (8 min, 60 m h−1). At the beginning of each filtration cycle filtration rates were adjusted with variable area flow meters to approximately 10 m h−1 during phase 1 and 5 m h−1 during phase 2 in order to reach target EBCT of 7.5 and 15 min, respectively. Actual EBCT and BV were calculated based on cumulative filtrate volumes measured by water meters in the filter effluents. Calculation of EBCT and BV relates to the upper filter layer only. Due to inattention during filter backwash, ∼15% of the GAC layer from the BAC filter (at ∼9000 BV) and ∼8% from the S/BAC (at ∼35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV) filter were flushed out. After these incidents, EBCT and BV calculations were adjusted accordingly.
000 BV) filter were flushed out. After these incidents, EBCT and BV calculations were adjusted accordingly.
Details on filter media and operational parameters are given in Table 2. The discrepancy between target EBCT and actual EBCT is based on deviations of the variable area flowmeter and the water meter measurement.
| Parameter | BAC | S/BAC | S/A |
|---|---|---|---|
| Upper layer | |||
| Filter mediaGrain size | 1.2 m GAC (Jacobi, Aquasorb 2000)1.4–2.4 mm | 1.2 m GAC (Jacobi, Aquasorb 2000)1.4–2.4 mm | 1.2 m hydro-anthracite1.4–2.5 mm |
| Effective size d10 | 1.6 mm | 1.6 mm | 1.5 mm |
| Uniformity coefficient | <1.4 | <1.4 | <1.4 |
| Lower layer | |||
| Filter media | — | 0.6 m filter sand | 0.6 m filter sand |
| Grain size | — | 0.7–1.25 mm | 0.7–1.25 mm |
| Effective size d10 | — | 0.74 mm | 0.74 mm |
| Uniformity coefficient | — | 1.38 | 1.38 |
| Phase 1 | |||
| Target EBCT | 7.5 min | 7.5 min | 7.5 min |
| Actual EBCT | 8.5 ± 3.0 min | 9.8 ± 2.9 min | 9.9 ± 1.9 min |
| Treated bed volumes | 0–33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0–28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0–27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Target coagulant dose | — | 1.8 mg Fe per L | 1.8 mg Fe per L |
| Phase 2 | |||
| Target EBCT | 15 min | 15 min | 15 min |
| Actual EBCT | 12.2 ± 3.2 min | 15.5 ± 3.8 min | 16.1 ± 3.0 min |
| Treated bed volumes | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–53 000–53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–53 000–53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–49 000–49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Target coagulant dose | — | 1.8 mg Fe per L | 1.8 mg Fe per L |
Short-term changes of the described operational parameters were done for the experiments on impacting factors of OBP formation and removal presented in chapter 3.3.
2.3 Sampling
Regular sampling campaigns were conducted to monitor the treatment efficiency of the pilot system over time. To compensate diurnal concentration changes, samples were taken as 24 h composite samples, using 10 L glass bottles. Automatic samplers with a cooling system were used for ozonation influent and effluent. Filter effluent samples were collected with a multichannel peristaltic pump and bottles were stored in cooled boxes during sampling.Special sampling campaigns were conducted for OBP that required temporary adjustment of operational parameters. Different specific ozone doses were tested (each dose for approx. 2 h), in order to investigate the ozone dose dependency of carbonyl formation. In another campaign, filtration rates were varied (each filtration rate for one day), to examine the influence of EBCT on OBP degradation. Sampling campaigns for OBP were carried out as grab samplings in order to minimise storage times and hence potential biotransformation of the target compounds in the sample. Furthermore, copper sulfate was added as a biocide to aldehyde/ketone samples due to their high biodegradability.
For all samplings, a time offset according to the hydraulic retention time of the respective treatment process was considered in order to receive corresponding influent and effluent samples.
2.4 Analytical methods
Analysis of OMP was carried out with high performance liquid chromatography followed by tandem-mass spectrometry (HPLC-MS/MS) according to DIN 38407-F47 and DIN 38407-F36. For industrial chemicals benzotriazole and tris(1-chloro-2-propyl) phosphate (TCPP) automatic sample preparation was carried out by Thermo Fisher Scientific (Dreieich, Germany) EQuan Max online solid-phase extraction with a Hypersil Gold™ aQ column (12 μm, 2.1 × 20 mm) and an injection volume of 1 mL. The subsequent LC-MS/MS system consisted of an UltiMate 3000 HPLC (analytical column: ACQUITY UPLC HSS T3, 1.8 μm, 2.1 × 50 mm, Waters, Eschborn, Germany) and a Q Exactive™ high resolution mass spectrometer (Thermo Fisher Scientific, Dreieich, Germany). All other analysed OMP where measured on a Waters (Eschborn, Germany) ACQUITY HPLC system (analytical column: ACQUITY UPLC HSS T3, 1.8 μm, 2.1 × 100 mm, Waters, Eschborn, Germany) with direct injection (injection volume: 50 μL) followed by Xevo TQ-S tandem mass spectrometer (Waters, Eschborn, Germany). Water with 0.1% formic acid and methanol were used as eluents in all applied LC methods.
NDMA samples were pre-treated in a solid-phase extraction step (combination of octadecyl-modified silica gel and carbonaceous sorbent) before analysis with gas chromatography followed by quadrupole MS according to a literature protocol.16 LOQ for NDMA was 5 ng L−1.
2.5 Data processing
When the effluent concentration of a process was below LOQ for a certain compound its removal or relative effluent concentration (c/c0) was only calculated if the influent concentration was at least 5 times the LOQ. Otherwise, the data was not taken into account to minimise errors based on false assumptions. If the influent concentration condition was fulfilled removal or relative effluent concentration was calculated using ½ LOQ as effluent concentration. Thus, the highest possibly committed error amounts to ±10% removal or relative effluent concentration, respectively.All boxplot figures are defined as follows. The lower and upper boundary of the box represents the 25th and 75th percentile, respectively. The intermediate line in the box marks the median value. Minimum and maximum values are illustrated by the whiskers, as long as they are not excluded as outliers. Values are defined as outliers if their distance to the upper or lower boundary of the box is bigger than 1.5 times the interquartile range (IQR: distance between 25th and 75th percentile).25
3 Results and discussion
3.1 Organic bulk parameters
The results for organic bulk parameters DOC and UVA254 under steady-state conditions are shown in Fig. 2. It was found that after an initial breakthrough of both parameters in the BAC containing filters a constant level of removal was reached from approx. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV onwards (Fig. S1†). All data before that were excluded from the box-plots in Fig. 2. The observed behaviour is typical for BAC filter operation which can be divided in the first phase of adsorption (breakthrough), the second phase of concurrent adsorption and biodegradation (transition from breakthrough to steady-state), and the third phase of biodegradation (steady-state).26 Other pilot and full-scale studies on BAC filtration of wastewater treatment plant effluents with and without prior ozonation confirmed the establishment of biological degradation processes in the filters.17,21
000 BV onwards (Fig. S1†). All data before that were excluded from the box-plots in Fig. 2. The observed behaviour is typical for BAC filter operation which can be divided in the first phase of adsorption (breakthrough), the second phase of concurrent adsorption and biodegradation (transition from breakthrough to steady-state), and the third phase of biodegradation (steady-state).26 Other pilot and full-scale studies on BAC filtration of wastewater treatment plant effluents with and without prior ozonation confirmed the establishment of biological degradation processes in the filters.17,21
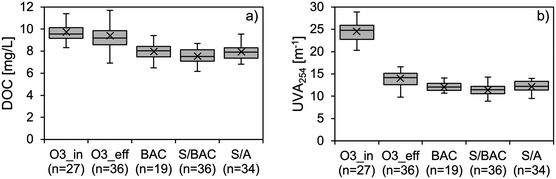 | ||
Fig. 2 Box-plots for a) DOC and b) UVA254 concentrations at different sampling points. All data from the operation before 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV were excluded. n is the number of data points. 000 BV were excluded. n is the number of data points. | ||
DOC and UVA254 exhibited different behaviour during ozonation. While mean DOC reduction was marginal (5%), UVA254 was reduced by 44% on average. Low DOC removal confirms that reaction with ozone at applied doses mostly leads to partial oxidation and not to mineralisation of bulk organic matter. The strong decrease of UV254 can be mainly attributed to the oxidation of aromatic compounds, which typically absorb light in this range of wavelengths. The direct reaction of ozone specifically attacks these electron-rich moieties via ozonide formation and subsequent cleavage of the C–C bond.14 Aldehydes, ketones, and carboxylic acids are typical products of this reaction14 and their formation was confirmed by several studies on ozonation of drinking water or wastewater.15,27–29 They can therefore be considered oxidation by-products of the bulk organic matter that need to be removed by subsequent biological filtration.30 The formation of these compounds and their fate during post-treatment were also investigated in this study and will be discussed separately in chapter 3.3.
Laboratory results for UVA254 showed that the target UVA254 reduction of 47% during ozonation was not completely reached. This might be explained by deviations between the UVA254 probes used onsite for online-monitoring and the photometer used for laboratory analysis. Despite regular cleaning, online probes are more prone to biofouling due to constant wastewater exposition. Furthermore, influent and effluent are measured with two separate probes which produces additional potential for errors. The overestimation of UVA254 removal by the online probes was also in line with the slight ozone underdosing (0.65 instead of 0.7 mg O3 per mg DOC).
The post-treatment steps BAC, S/BAC, and S/A removed DOC on average by 11%, 19%, and 15%, respectively. The better performance of the dual-media filters S/BAC and S/A compared to BAC is likely to result mainly from coagulant dosing in the influent. It is well known that, besides removing phosphate from water, the coagulation process with ferric chloride also causes a reduction of DOC.31,32 The slightly higher EBCT in the upper layer of the dual-media filters during both phases of operation and the extra contact time in the sand layer might have also led to additional biodegradation of DOC. Despite nearly identical operational conditions, DOC removal in the S/BAC filter was more pronounced than in the S/A. This indicates that biological activity is higher on BAC compared to anthracite. Results for UVA254 (Fig. 2) and COD (Fig. S3†) were analogous to DOC and hence, confirm the described findings. With dissolved oxygen (DO) concentrations mainly above 20 mg L−1 after ozonation, redox conditions were strictly aerobic. Hence, the predominant biodegradation mechanism for organic matter is aerobic respiration, using oxygen as the final electron acceptor. Anaerobic respiration processes with other electron acceptors such as nitrate, ferric iron, or sulfate are negligible under the described conditions.33 DOC removal in the filters based on biodegradation should therefore be closely connected to DO consumption (ΔDO). Indeed, average ΔDO in the S/BAC filter was 1.8 mg L−1 higher than in the S/A (Fig. S3†), which supports the assumption of higher biological activity on BAC than on anthracite.
In accordance with these results, other studies also observed better removal of biodegradable organic matter on BAC than on non-adsorptive filter materials.34–36 Multiple explanations for the benefits of BAC have been reported. The porous structure of GAC media provides a significantly higher specific surface area and increased roughness of the surface, compared with compact grains of anthracite or sand.35,36 The rough surface facilitates the attachment of microorganisms and hence, microbial colonization.36 The high specific surface area of GAC plays an important role for the adsorptive removal of organic contaminants, but it is not fully available for biofilm growth. Typically, a high portion of the total surface area is formed by mesopores (2–50 nm) and micropores (<2 nm), which are not accessible for bacteria, due to their size.37 Since the grain size distribution is the same in the S/BAC and the S/A filter, it can be assumed that the total surface area available for biofilm growth is also similar. However, there is a strong interaction between sorption processes on GAC and the attached biofilm that promote an efficient removal of organic compounds. While microorganisms on non-adsorptive media only have access to the organic substrate that intrudes the biofilm by diffusive transport from the water, biomass attached to GAC can additionally degrade organic matter adsorbed to the outer activated carbon surface.26 Thus, the biofilm on GAC has an enhanced availability of organic substrate. Moreover, biodegradation of adsorbed compounds regenerates adsorption sites on the GAC surface and thereby prolongs its adsorptive lifetime.26 These biodegradation processes are usually incomplete and lead to the formation of smaller biotransformation products. Due to their reduced size, these molecules are then capable of diffusing into the micropore system of the activated carbon and adsorb there again. If the concentration on the outer GAC surface decreases sufficiently, the biotransformation products in the micropores are desorbed and transported back to the outer surface, where a further degradation by the biofilm takes place.26
3.2 Organic micropollutants
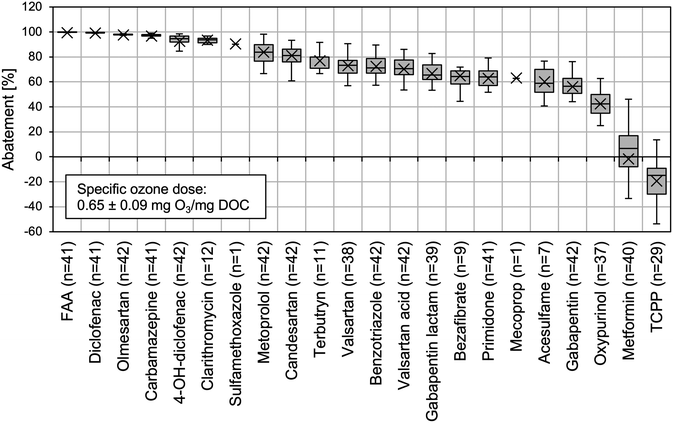
As suggested by previous studies, it is useful to categorise OMP based on their kO3 values as fast reacting (kO3 > 104 M−1 s−1), moderately reacting (kO3 = 102–104 M−1 s−1) and slowly reacting compounds (kO3 < 102 M−1 s−1).22,39,40 Table S1† shows the second-order rate constants and the respective categorisation for the investigated OMP. Indeed, all fast reacting OMP (4-formylaminoantipyrine (FAA), diclofenac, carbamazepine, clarithromycin, sulfamethoxazole) were degraded by more than 90% on average during ozonation. For olmesartan and 4-OH-diclofenac, kO3 values were not found in literature. However, it is likely that they are >104 M−1 s−1 since the mean abatement of the compounds was also above 90%. In the case of 4-OH-diclofenac, a similar behaviour as diclofenac can be expected due to its almost identical chemical structure (one additional hydroxy group). However, it is likely that they are >104 M−1 s−1 since the mean abatement of the compounds was also above 90%. In the case of 4-OH-diclofenac, a similar behaviour as diclofenac can be expected due to its almost identical chemical structure (one additional hydroxy group).
The degradation efficiency for OMP with a moderate reactivity with ozone (metoprolol, candesartan, benzotriazole, bezafibrate, gabapentin) ranged from over 80% on average for metoprolol down to approx. 40% for gabapentin (kO3 = 2.2 × 102 M−1 s−1). In contrast to fast reacting OMP, their abatement is strongly influenced by the reaction with OH-radicals, besides the direct reaction with ozone.41 The kOH values of the compounds are similar, ranging from 7.3 × 109 M−1 s−1 to 9.1 × 109 M−1 s−1. Hence, it could be observed as expected that for most of the OMP, degradation efficiency correlated with their kO3 values. Only bezafibrate stayed behind the expected reduction. With a kO3 of 5.9 × 103 M−1 s−1, an abatement similar to candesartan (kO3 = 5.6 × 103 M−1 s−1) would be assumed. A comparable study confirmed a similar degradation for bezafibrate and candesartan during ozone treatment.9 The discrepancy in the present study might be explained by the small number of samplings that could be used for evaluation of the respective treatment efficiency (n = 9), due to low influent concentrations often close to LOQ.
The abatement during ozonation for slowly reacting OMP (valsartan, primidone, mecoprop, acesulfame, metformin, TCPP) lay between approx. 70% on average for valsartan and below 0% for TCPP. The concentration increase of TCPP could not be explained within this study. To the best of our knowledge a formation of TCPP during ozone treatment has not been reported yet and is therefore unlikely. For the slowly reacting compounds, the reaction with OH-radicals is the dominating degradation mechanism.41 Consequently, the abatement in this group of OMP was determined to a large extent by their kOH values (kOH for TCPP was not available), and not with kO3 values. Compounds with high kOH values can even be degraded efficiently (e.g. valsartan with kOH = 1010 M−1 s−1), despite their low direct reactivity with ozone. Consequently, a clear separation of moderately and slowly reacting OMP in terms of abatement efficiency is not possible. One approach for a more precise prediction of degradation based on second-order reaction rate constants, is a further sub-division of slowly reacting compounds into kOH < 5 × 109 M−1 s−1 and kOH ≥ 5 × 109 M−1 s−1.41
For the remaining compounds gabapentin lactam, oxypurinol, tertbutryn, and valsartan acid, literature data on reaction kinetics was not available. Based on their medium abatements between 40% and 80%, a high reactivity with ozone (kO3 > 104 M−1 s−1) can be excluded. However, a further classification as moderately or slowly reacting OMP is not possible, as described above.
Among the compounds with lower elimination efficiency were gabapentin and oxypurinol. This result is of particular concern because the two compounds can be critical for drinking water production downstream of the WWTP. Due to their persistence in the environment, both have been found in different compartments of the water cycle including ground and drinking water.43–45 There is limited data on toxicity for oxypurinol and gabapentin. However, health-related indication values for drinking water that were defined by the German Environment Agency, have been temporarily exceeded in one of Berlin's waterworks in the past.43 Hence, efficient removal of these compounds during advanced wastewater treatment is an important goal, especially for oxypurinol with its high secondary effluent concentration of 35 μg L−1.
Relative effluent concentrations of the S/A filter remained at approx. 100% over the whole testing period for all monitored OMP, demonstrating that no relevant additional removal took place. These findings are in accordance with other comparable studies where post-treatment in non-adsorptive filters did not contribute to OMP removal.9,13,35 In contrast, other studies that worked with longer contact times did report biodegradation in tertiary filtration under oxic conditions (without previous ozonation) for benzotriazole, TCPP, gabapentin, and metoprolol.21,47,48 Hence, there is potential for additional OMP removal during post-treatment under appropriate conditions. It is likely that the EBCT of approx. 16 min in the S/A filter was insufficient for biological processes. Another reason that could hinder biotransformation of OMP, is the formation of readily biodegradable organic matter during ozonation which is preferentially degraded by microorganisms. Carbon limitation can substantially promote OMP removal in biological systems, as long as the removal mechanism is not based on a co-metabolic reaction.49
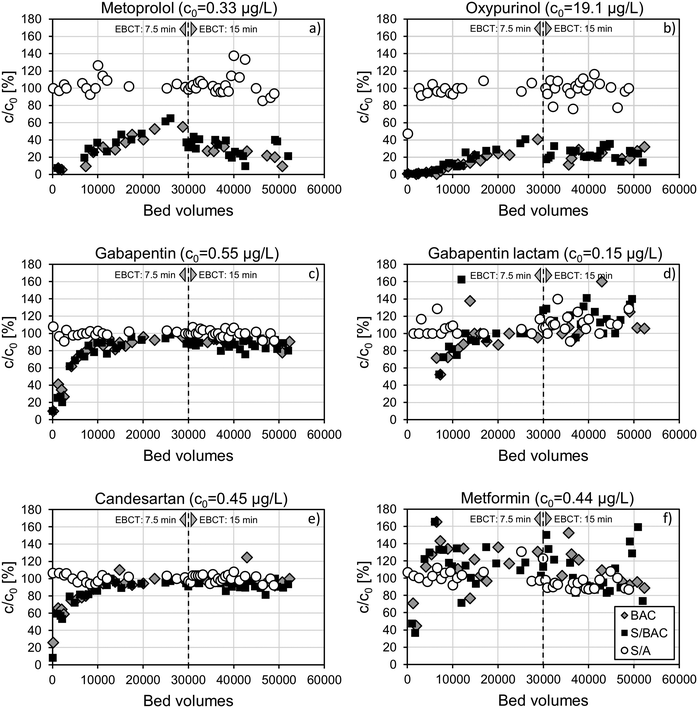
At the beginning of the operation, GAC containing filters BAC and S/BAC showed very similar breakthrough behaviour for most OMP. Low initial relative effluent concentrations gradually increased with advancing BV. Typically, breakthrough curves in GAC adsorbers keep rising until they asymptotically converge towards complete breakthrough (c/c0 = 100%). If biotransformation processes are involved besides adsorption, breakthrough curves should stabilise at a level of c/c0 < 100%.26
The breakthrough behaviour of combined adsorption and potential biotransformation was observed for metoprolol, oxypurinol, gabapentin, benzotriazole, primidone, valsartan, and TCPP (Fig. 4a–c and S2a–d†). For most substances, the change of target EBCT from 7.5 min to 15 min at ∼33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV for the BAC filter and 28
000 BV for the BAC filter and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV for the S/BAC filter is clearly visible as a drop in the breakthrough curve. Extension of EBCT improves kinetic conditions for both, adsorption and biodegradation. The steady OMP removal from approx. 30
000 BV for the S/BAC filter is clearly visible as a drop in the breakthrough curve. Extension of EBCT improves kinetic conditions for both, adsorption and biodegradation. The steady OMP removal from approx. 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV onwards until the end of the testing period (∼53
000 BV onwards until the end of the testing period (∼53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV) points at the involvement of biological transformation processes besides adsorption during BAC filtration. In that sense, the first 30
000 BV) points at the involvement of biological transformation processes besides adsorption during BAC filtration. In that sense, the first 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV can be considered as an acclimation period for OMP removal. The levels of steady-state abatement varied a lot between the compounds. Well adsorbing compounds metoprolol, oxypurinol, and benzotriazole exhibited the highest removal. Excluding all data below 30
000 BV can be considered as an acclimation period for OMP removal. The levels of steady-state abatement varied a lot between the compounds. Well adsorbing compounds metoprolol, oxypurinol, and benzotriazole exhibited the highest removal. Excluding all data below 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV, median abatements of these OMP in the S/BAC filter amounted to 69%, 76%, and 91%, respectively. Benzotriazole and metoprolol are known to be biodegradable under oxic conditions and behaved similarly in another study, where they were still well abated in a loaded GAC filter with 50
000 BV, median abatements of these OMP in the S/BAC filter amounted to 69%, 76%, and 91%, respectively. Benzotriazole and metoprolol are known to be biodegradable under oxic conditions and behaved similarly in another study, where they were still well abated in a loaded GAC filter with 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV subsequent to ozone treatment.9 In contrast, oxypurinol degradation was only reported under strongly reducing conditions during river bank filtration.50 A pilot study in drinking water treatment also observed steady removal of oxypurinol in a GAC adsorber with >35
000 BV subsequent to ozone treatment.9 In contrast, oxypurinol degradation was only reported under strongly reducing conditions during river bank filtration.50 A pilot study in drinking water treatment also observed steady removal of oxypurinol in a GAC adsorber with >35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV but assumed that it was based on adsorption.43 Further operation of the filters will reveal if breakthrough of oxypurinol will continue at a later stage or if removal will stay steady due to biotransformation. As explained in section 3.2.1, the removal of oxypurinol is an important treatment goal, due to its potential negative impact on drinking water production, downstream of the WWTP. Considering only data after acclimation, the combined treatment with ozone and S/BAC would reach an efficient removal of 86%, whereas the combination of ozone and S/A would result in 42% removal. Among the moderately and poorly adsorbing OMP gabapentin, valsartan, primidone, and TCPP, steady-state abatement was lower. Median removal efficiencies in the S/BAC filter above 30
000 BV but assumed that it was based on adsorption.43 Further operation of the filters will reveal if breakthrough of oxypurinol will continue at a later stage or if removal will stay steady due to biotransformation. As explained in section 3.2.1, the removal of oxypurinol is an important treatment goal, due to its potential negative impact on drinking water production, downstream of the WWTP. Considering only data after acclimation, the combined treatment with ozone and S/BAC would reach an efficient removal of 86%, whereas the combination of ozone and S/A would result in 42% removal. Among the moderately and poorly adsorbing OMP gabapentin, valsartan, primidone, and TCPP, steady-state abatement was lower. Median removal efficiencies in the S/BAC filter above 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV for these compounds were 13%, 18%, 24%, and 32% respectively. Biological removal of gabapentin in BAC filters could be expected as its biodegradability in different kinds of filter systems has been documented in a number of studies.21,35,42,46,48 However, the achieved abatement stayed behind the results of GAC filters with similar EBCT.21,44 Once again, higher concentrations of biodegradable organic carbon formed during prior ozone treatment could be one explanation for the comparatively low gabapentin removal. Moreover, one study suggested that high OMP influent concentrations also contribute to an enhanced removal, as increased gabapentin abatement was observed after a concentration rise in the influent.21 A recent study revealed that gabapentin lactam is the major biological transformation product of gabapentin and that it exhibits a higher persistence to biodegradation than the original compound.51 Indeed, there was no evidence for gabapentin-lactam biodegradation in BAC filters and a slight formation was noticed after the acclimation period (Fig. 4d). Valsartan reached full breakthrough in the S/BAC filter with shorter EBCT and started to be steadily removed after extending EBCT. This behavior clearly points at biotransformation of valsartan in the GAC filter bed, which is confirmed by another study on tertiary GAC filtration.21 However, it is likely that part of the removed valsartan is biologically transformed into valsartan acid.52 Due to high valsartan acid concentrations in the filter influent compared to valsartan (more than 10 times higher), the analytical accuracy does not allow for relating partial valsartan removal to valsartan acid formation. Removal of valsartan acid did not take place after the initial adsorptive phase of filter operation anymore, which demonstrated that valsartan acid was persistent to further biotransformation. This is in disagreement with several studies that reported efficient biodegradation of valsartan acid under oxic conditions.42,46,50 While two studies worked with hydraulic retention times in the range of days in soil column experiments,46,50 the third study achieved ∼50% biodegradation with only 15 min EBCT in a GAC adsorber fed with drinking water.42 Thus, contact time cannot be the only driver for biological valsartan acid removal but other factors such as carbon source availability seem to play an essential role. Several studies found that the compound primidone was not biodegradable, neither in GAC filters21 nor in natural systems,53 whereas significant removal was reported during infiltration in the hyporheic zone of a wastewater impacted river under oxic/suboxic conditions.54 Hence, biological transformation of primidone is possible and potentially explains the observed removal in the S/BAC filter. Inconsistent results for biodegradation of TCPP in tertiary wastewater treatment were found in literature. While removal of approx. 50% (median) was observed in a retention soil filter subsequent to a WWTP,47 very low biological abatement of TCPP was found in BAC filtration as post-treatment after ozonation.9,55 Contact time seems to play an important role since TCPP had already converged towards complete breakthrough during the first phase of operation (target EBCT 7.5 min) and steady removal established only after doubling EBCT.
000 BV for these compounds were 13%, 18%, 24%, and 32% respectively. Biological removal of gabapentin in BAC filters could be expected as its biodegradability in different kinds of filter systems has been documented in a number of studies.21,35,42,46,48 However, the achieved abatement stayed behind the results of GAC filters with similar EBCT.21,44 Once again, higher concentrations of biodegradable organic carbon formed during prior ozone treatment could be one explanation for the comparatively low gabapentin removal. Moreover, one study suggested that high OMP influent concentrations also contribute to an enhanced removal, as increased gabapentin abatement was observed after a concentration rise in the influent.21 A recent study revealed that gabapentin lactam is the major biological transformation product of gabapentin and that it exhibits a higher persistence to biodegradation than the original compound.51 Indeed, there was no evidence for gabapentin-lactam biodegradation in BAC filters and a slight formation was noticed after the acclimation period (Fig. 4d). Valsartan reached full breakthrough in the S/BAC filter with shorter EBCT and started to be steadily removed after extending EBCT. This behavior clearly points at biotransformation of valsartan in the GAC filter bed, which is confirmed by another study on tertiary GAC filtration.21 However, it is likely that part of the removed valsartan is biologically transformed into valsartan acid.52 Due to high valsartan acid concentrations in the filter influent compared to valsartan (more than 10 times higher), the analytical accuracy does not allow for relating partial valsartan removal to valsartan acid formation. Removal of valsartan acid did not take place after the initial adsorptive phase of filter operation anymore, which demonstrated that valsartan acid was persistent to further biotransformation. This is in disagreement with several studies that reported efficient biodegradation of valsartan acid under oxic conditions.42,46,50 While two studies worked with hydraulic retention times in the range of days in soil column experiments,46,50 the third study achieved ∼50% biodegradation with only 15 min EBCT in a GAC adsorber fed with drinking water.42 Thus, contact time cannot be the only driver for biological valsartan acid removal but other factors such as carbon source availability seem to play an essential role. Several studies found that the compound primidone was not biodegradable, neither in GAC filters21 nor in natural systems,53 whereas significant removal was reported during infiltration in the hyporheic zone of a wastewater impacted river under oxic/suboxic conditions.54 Hence, biological transformation of primidone is possible and potentially explains the observed removal in the S/BAC filter. Inconsistent results for biodegradation of TCPP in tertiary wastewater treatment were found in literature. While removal of approx. 50% (median) was observed in a retention soil filter subsequent to a WWTP,47 very low biological abatement of TCPP was found in BAC filtration as post-treatment after ozonation.9,55 Contact time seems to play an important role since TCPP had already converged towards complete breakthrough during the first phase of operation (target EBCT 7.5 min) and steady removal established only after doubling EBCT.
Among the OMP with combined adsorption and potential biotransformation, compounds with a higher adsorption affinity to GAC also reached a more efficient steady-state removal. As discussed in section 3.1 there is a close interaction between adsorption and biotransformation on the GAC surface. The immobilisation of OMP by adsorption makes biotransformation processes on the GAC surface independent of contact time.26 Thus, it is consistent that better adsorption comes along with better biotransformation for not readily biodegradable compounds. Also, it explains why biotransformation of OMP was observed in the BAC filters, but not in the S/A filter. A prediction of the behaviour based on distribution coefficients log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 was not possible. For instance, metoprolol was removed significantly better than primidone, although their distribution coefficients indicate the opposite (log
D7.4 was not possible. For instance, metoprolol was removed significantly better than primidone, although their distribution coefficients indicate the opposite (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4(metoprolol) = −0.4, log
D7.4(metoprolol) = −0.4, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4(valsartan) = 1.1). Other factors and chemical properties such as the molecule size also play an important role. Since the biofilm strongly reduces convective transport to the GAC surface, diffusion is the dominant mass transport process.26 The diffusive transport preferentially permits small molecules to pass the biofilm and adsorb onto the GAC.26
D7.4(valsartan) = 1.1). Other factors and chemical properties such as the molecule size also play an important role. Since the biofilm strongly reduces convective transport to the GAC surface, diffusion is the dominant mass transport process.26 The diffusive transport preferentially permits small molecules to pass the biofilm and adsorb onto the GAC.26
Typical behaviour of a purely adsorptive removal was observed for candesartan, gabapentin lactam, and valsartan acid (Fig. 4d and e and S2e†). After a gradually decreasing removal efficiency at the beginning of the pilot operation, full breakthrough was reached at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30
000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 BV. In the case of gabapentin lactam, even a slight formation was observed after full breakthrough, most likely due to biotransformation of other sartans.52
000 BV. In the case of gabapentin lactam, even a slight formation was observed after full breakthrough, most likely due to biotransformation of other sartans.52
Metformin was the only compound that was not removed at all in the BAC filters from the beginning on (Fig. 4f). It is known that biotransformation of metformin by activated sludge treatment is possible, leading to the formation of guanylurea.56 However, the short contact time in the filters compared to conventional activated sludge treatment is most likely the reason, why metformin was not affected by BAC filtration. A biotransformation of metformin, immobilised on the GAC surface by adsorption, can also not be expected, since it is extremely polar (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 = −5.6) and does not adsorb in relevant amounts.
D7.4 = −5.6) and does not adsorb in relevant amounts.
3.3 Formation of OBP during ozonation and their fate during post-treatment
Aldehydes, ketones, and carboxylic acids are typical OBP formed by the reaction of ozone with both, OMP and effluent organic matter.15,28 Since concentrations of effluent organic matter (mg L−1 range) are typically several orders of magnitude higher than of OMP (ng L1–μg L−1 range) formation of these compounds is expected to be higher compared with other transformation products that result from the reaction with specific precursors. Indeed, a sampling campaign at different specific ozone doses revealed a clear, ozone dose dependent concentration increase of carbonyl compounds (Δc) during ozonation, as depicted in Fig. 5 and S4.† The carbonyl formation varied a lot between the investigated compounds. At a specific ozone dose of 0.67 mg O3 per mg DOC, it ranged from <1 μg L−1 for propanal and methylglyoxal up to >10 μg L−1 for formaldehyde and heptanal. Other studies confirmed formaldehyde as a major carbonyl by-product from wastewater ozonation.15,28 As shown in Fig. 5 and S4,† the formation of some of the compounds correlates well with the applied specific ozone dose (R2 > 0.8 for formaldehyde, acetaldehyde, butanal, heptanal, nonanal, and glyoxal). Since there is an excess of effluent organic matter and the reaction is expected to be limited by the availability of ozone the observed trend for aldehyde formation in dependence of the ozone concentration seems logical. However, for other investigated carbonyl compounds (propanal, hexanal, methylglyoxal, acetone) the correlation was not observed or not as pronounced (Fig. S4†). Interestingly, the best correlation with the ozone dose (R2 = 0.98) was found for the total aldehyde concentration (sum of all measured carbonyl compounds) which reveals that the overall potential for aldehyde formation at a given ozone dose is valid even though unpredictable shifts between concentrations of single compounds might occur. These results are in accordance with literature where a strong correlation of total aldehydes and the specific ozone dose was observed (R2 = 0.99).15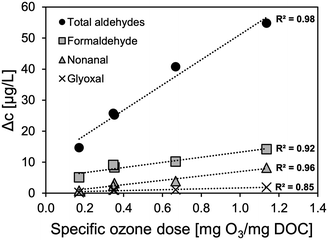 | ||
| Fig. 5 Aldehyde formation (Δc) during ozone treatment at different specific ozone doses (nitrite corrected). | ||
The influence of the ozone dose on NDMA formation was not investigated within this study because it has been previously reported that there is no correlation.8,9,22 The variation of specific precursor concentrations over time has a higher impact on NDMA formation than the ozone dose.
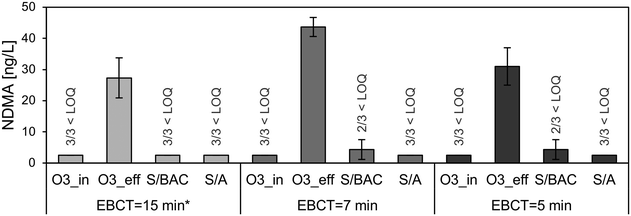
The formation of OBP during ozone treatment and their removal during post-treatment in dual-media filters applying different EBCT or filtration rates, respectively, were investigated. As shown in Fig. 6, a total aldehyde formation of up to approx. 40–100 μg L−1 was observed at specific ozone doses between approx. 0.7 and 1 mg O3 per mg DOC (the increased ozone doses were not intended but due to fouling on the UVA254 probes for ozone dose control). With an EBCT of approx. 15 min (corresponds to a filtration rate of 5 m h−1) the carbonyl compounds were removed by 88% and 87% in the S/BAC and the S/A filter, respectively. When filters were operated at higher filtration rates and hence lower contact times, a decrease of removal was observed. An EBCT of approx. 7 min (corresponds to filtration rate of 10 m h−1) resulted in a reduction of total aldehydes concentration by 74% and 75% in the S/BAC and S/A filter, respectively. A further decrease of EBCT down to 5 min (corresponds to filtration rate of 13 m h−1) again lowered the removal performance in the S/BAC filter a degradation of 68% was observed, while in the S/A filter removal decreased to 51%. The results demonstrate that changes of EBCT within the tested range affect total aldehyde removal efficiency in the deep-bed filters. In full-scale applications, deep-bed filters are often operated with EBCT that are constantly changing as a function of diurnal flow variations of the WWTP (especially with rain events). Thus, it might be relevant to take decreased removal performance at low EBCT into account for the dimensioning of deep-bed filters in post-treatment applications. Many studies on biological filtration after ozonation have been conducted with typical EBCT of ≥10 min.9,13,17,57 The lower end of EBCT conditions that occur in full-scale wastewater systems during rain events and dry weather peaks were rarely investigated.58 More research in that field is needed in order to provide solid information on a wider spectrum of compounds for improved dimensioning of post-treatment filters in the future. The effect of filter material played a minor role for carbonyl compound degradation. Both filter materials, BAC and anthracite, proved to be suitable for removing well degradable carbonyl compounds, although at the lowest EBCT the S/BAC slightly outperformed the S/A filter, likely due to higher biological activity.
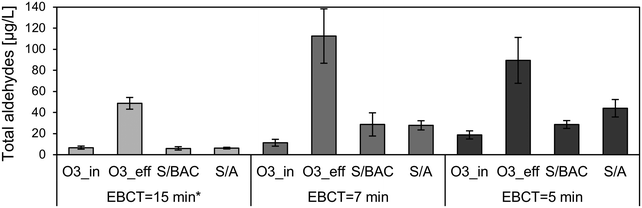 | ||
| Fig. 6 Mean values with standard deviation (n = 3) of total aldehyde concentrations at different sampling points with decreasing EBCT. *S/BAC = 16 min, S/A = 15 min. Mean specific ozone doses: 0.65 (15 min), 1.05 (7 min), and 1.04 mg O3 per mg DOC (5 min). Figures for individual compounds are shown in Fig. S5–S11.† | ||
As depicted in Fig. 7, NDMA concentrations of up to approx. 40 ng L−1 were found after ozone treatment while influent concentrations were always below LOQ. Post-treatment with both dual-media filters removed the formed NDMA again, reaching concentrations below LOQ for almost all samplings. Hence, a high removal performance was achieved independent of the choice of filter material or EBCT within the investigated range. However, another study observed a strong decline of NDMA removal in a BAC filter and an anthracite filter after reducing EBCT from 10 min to 2 min,58 which shows that a further decrease of EBCT could have a negative effect on performance.
3.4 Combination of nutrient removal and post-treatment
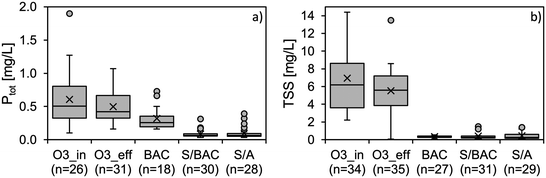
As depicted in Fig. 8, ozonation did not have a significant effect on Ptot or TSS. The marginal decrease of both parameters can be attributed to sedimentation effects in the ozone contactors. With filter influent concentrations (= O3_eff) of Ptot up to 1 mg L−1, the constant coagulant dose of 1.8 mg Fe per L resulted in molar ratios below the target value of 4.4 mol Fe per mol o-PO4-P in several samplings. However, the dual-media filters exhibited efficient removal. The 75th percentile concentrations of Ptot in both filters effluents were <0.1 mg L−1. A reliable compliance with an effluent concentration of 0.1 mg L−1 is feasible, if the coagulant dose is controlled as a function of the current phosphorus concentration in the filter influent. In full-scale coagulation/filtration systems this is state-of-the-art. The BAC filter also slightly reduced Ptot concentrations although coagulant dosing was not applied. Here, the removal is linked to the retention of phosphorus containing TSS in the filter bed. As also shown in Fig. 8, all filters reached TSS effluent concentrations of mostly <1 mg L−1. A similar study with a GAC containing coagulation/filtration step without previous ozonation also reached effluent concentrations predominantly below 0.1 mg L−1 Ptot.21 It can be concluded that the use of GAC as filter material is equally suitable for coagulation/filtration when compared with typically applied materials like sand or anthracite. The results also show that the ozone process does not adversely affect the coagulation/filtration step. Neither does the coagulation process have negative effects on the filters' function as biological post-treatment considering the results for OBP, OMP, and bulk organics.Results for nitrogen species NH4-N and NO3-N are shown in Fig. S12 and S13.† Residual NH4-N in the secondary effluent was not affected by the ozone treatment but further removed during deep-bed filtration, most likely by nitrification. NO3-N concentrations remained constant throughout the whole treatment process. Nitrification of NH4-N does not result in a visible concentration increase of NO3-N, since NH4-N concentrations are approx. 100 times lower than for NO3-N and the effect is marginal. The high DO concentrations after ozonation do not allow for a NO3-N reduction in the filters by denitrifying bacteria since their development requires anoxic redox-conditions. A recent study on an ozone/BAC treatment system demonstrated that external carbon source dosing in the filter influent can lead to efficient NO3-N removal during post-treatment, as the carbon source quickly consumes DO and then acts as an electron donor for denitrifying bacteria.57
4 Conclusions
The present study demonstrates that design and operational aspects have to be taken into account for an optimised post-treatment with deep-bed filters after ozonation. The use of BAC instead of non-adsorptive filter material like anthracite is more suitable for promoting metabolic activity and hence, improves aerobic degradation of organic matter. Furthermore, post-treatment with BAC filters allows for additional removal of several OMP, whereas non-adsorptive filters do not contribute to OMP removal. Since OMP removal in the BAC filters remained relatively stable even after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 treated BV the involvement of biological processes besides sorption is assumed. The filters of this study will be further operated in order to investigate their long-term performance and possibly verify the assumption. The primary function of biological post-treatment after ozonation – the efficient removal of biodegradable OBP – can be achieved independent of the choice of filter material. A change of EBCT (5–15 min) or filtration rate (5–13 m h−1), respectively, has a limited impact on the efficient removal of OBP within the tested range. However, the slight decrease of total aldehydes removal at short EBCT suggests that a further reduction of EBCT should be avoided. Tertiary treatment applications that additionally aim for enhanced phosphorus removal can integrate an inline coagulant dosing in the influent of the deep-bed filter without affecting its function as biological post-treatment.
000 treated BV the involvement of biological processes besides sorption is assumed. The filters of this study will be further operated in order to investigate their long-term performance and possibly verify the assumption. The primary function of biological post-treatment after ozonation – the efficient removal of biodegradable OBP – can be achieved independent of the choice of filter material. A change of EBCT (5–15 min) or filtration rate (5–13 m h−1), respectively, has a limited impact on the efficient removal of OBP within the tested range. However, the slight decrease of total aldehydes removal at short EBCT suggests that a further reduction of EBCT should be avoided. Tertiary treatment applications that additionally aim for enhanced phosphorus removal can integrate an inline coagulant dosing in the influent of the deep-bed filter without affecting its function as biological post-treatment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was conducted within the AquaNES project which received funding by the European Union‘s Horizon 2020 research and innovation programme [grant agreement no. 689450].References
- Y. Luo, W. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai, J. Zhang, S. Liang and X. C. Wang, A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment, Sci. Total Environ., 2014, 473–474, 619–641, DOI:10.1016/j.scitotenv.2013.12.065.
- B. Petrie, R. Barden and B. Kasprzyk-Hordern, A review on emerging contaminants in wastewaters and the environment, Water Res., 2015, 72, 3–27, DOI:10.1016/j.watres.2014.08.053.
- P. Falås, A. Wick, S. Castronovo, J. Habermacher, T. A. Ternes and A. Joss, Tracing the limits of organic micropollutant removal in biological wastewater treatment, Water Res., 2016, 95, 240–249, DOI:10.1016/j.watres.2016.03.009.
- A. S. Stasinakis, N. S. Thomaidis, O. S. Arvaniti, A. G. Asimakopoulos, V. G. Samaras, A. Ajibola, D. Mamais and T. D. Lekkas, Contribution of primary and secondary treatment on the removal of benzothiazoles, benzotriazoles, endocrine disruptors, pharmaceuticals and perfluorinated compounds in a sewage treatment plant, Sci. Total Environ., 2013, 463–464, 1067–1075, DOI:10.1016/j.scitotenv.2013.06.087.
- K. Fent, A. A. Weston and D. Caminada, Ecotoxicology of human pharmaceuticals, Aquat. Toxicol., 2006, 76, 122–159, DOI:10.1016/j.aquatox.2005.09.009.
- S. Jobling, R. Williams, A. Johnson, A. Taylor, M. Gross-Sorokin, M. Nolan, C. R. Tyler, R. van Aerle, E. Santos and G. Brighty, Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations, Environ. Health Perspect., 2006, 114(Suppl 1), 32–39, DOI:10.1289/ehp.8050.
- B. Wiese, G. Massmann, M. Jekel, T. Heberer, U. Dünnbier, D. Orlikowski and G. Grützmacher, Removal kinetics of organic compounds and sum parameters under field conditions for managed aquifer recharge, Water Res., 2011, 45, 4939–4950, DOI:10.1016/j.watres.2011.06.040.
- S. G. Zimmermann, M. Wittenwiler, J. Hollender, M. Krauss, C. Ort, H. Siegrist and U. von Gunten, Kinetic assessment and modeling of an ozonation step for full-scale municipal wastewater treatment, Water Res., 2011, 45, 605–617, DOI:10.1016/j.watres.2010.07.080.
- M. Bourgin, B. Beck, M. Boehler, E. Borowska, J. Fleiner, E. Salhi, R. Teichler, U. von Gunten, H. Siegrist and C. S. McArdell, Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments, Water Res., 2018, 129, 486–498, DOI:10.1016/j.watres.2017.10.036.
- H. El-Taliawy, M. E. Casas and K. Bester, Removal of ozonation products of pharmaceuticals in laboratory Moving Bed Biofilm Reactors (MBBRs), J. Hazard. Mater., 2018, 347, 288–298, DOI:10.1016/j.jhazmat.2018.01.002.
- F. Sharif, Use of Ozonation and Constructed Wetlands to Remove Contaminants of Emerging Concern from Wastewater Effluent, PhD thesis, Arizona State University, 2013 Search PubMed.
- C. Baresel, J. Malmborg, M. Ek and R. Sehlén, Removal of pharmaceutical residues using ozonation as intermediate process step at Linköping WWTP, Sweden, Water Sci. Technol., 2016, 73, 2017–2024, DOI:10.2166/wst.2016.045.
- G. Knopp, C. Prasse, T. A. Ternes and P. Cornel, Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters, Water Res., 2016, 100, 580–592, DOI:10.1016/j.watres.2016.04.069.
- Y. Lee and U. von Gunten, Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent, Environ. Sci.: Water Res. Technol., 2016, 2, 421–442, 10.1039/C6EW00025H.
- Z. Yan, Y. Zhang, H. Yuan, Z. Tian and M. Yang, Fish larval deformity caused by aldehydes and unknown byproducts in ozonated effluents from municipal wastewater treatment systems, Water Res., 2014, 66, 423–429, DOI:10.1016/j.watres.2014.08.019.
- C. Lee, C. Schmidt, J. Yoon and U. von Gunten, Oxidation of N-nitrosodimethylamine (NDMA) precursors with ozone and chlorine dioxide, Environ. Sci. Technol., 2007, 41, 2056–2063, DOI:10.1021/es062484q.
- J. Reungoat, B. I. Escher, M. Macova, F. X. Argaud, W. Gernjak and J. Keller, Ozonation and biological activated carbon filtration of wastewater treatment plant effluents, Water Res., 2012, 46, 863–872, DOI:10.1016/j.watres.2011.11.064.
- U. Hübner, U. von Gunten and M. Jekel, Evaluation of the persistence of transformation products from ozonation of trace organic compounds - a critical review, Water Res., 2015, 68, 150–170, DOI:10.1016/j.watres.2014.09.051.
- I. Zucker, H. Mamane, A. Riani, I. Gozlan and D. Avisar, Formation and degradation of N-oxide venlafaxine during ozonation and biological post-treatment, Sci. Total Environ., 2018, 619–620, 578–586, DOI:10.1016/j.scitotenv.2017.11.133.
- S. Velten, M. Boller, O. Köster, J. Helbing, H.-U. Weilenmann and F. Hammes, Development of biomass in a drinking water granular active carbon (GAC) filter, Water Res., 2011, 45, 6347–6354, DOI:10.1016/j.watres.2011.09.017.
- J. Altmann, D. Rehfeld, K. Träder, A. Sperlich and M. Jekel, Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal, Water Res., 2016, 92, 131–139, DOI:10.1016/j.watres.2016.01.051.
- J. Hollender, S. G. Zimmermann, S. Koepke, M. Krauss, C. S. McArdell, C. Ort, H. Singer, U. von Gunten and H. Siegrist, Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration, Environ. Sci. Technol., 2009, 43, 7862–7869, DOI:10.1021/es9014629.
- M. Stapf, U. Miehe and M. Jekel, Application of online UV absorption measurements for ozone process control in secondary effluent with variable nitrite concentration, Water Res., 2016, 104, 111–118, DOI:10.1016/j.watres.2016.08.010.
- M. Stapf, Internal study on ozonation of wastewater treatment plant effluents in Berlin, not published, KompetenzZentrum Wasser Berlin gGmbH, Berlin, 2016 Search PubMed.
- M. Komorowski, D. C. Marshall, J. D. Salciccioli and Y. Crutain, Secondary Analysis of Electronic Health Records, Exploratory Data Analysis, Cham, CH, 2016 Search PubMed.
- D. R. Simpson, Biofilm processes in biologically active carbon water purification, Water Res., 2008, 42, 2839–2848, DOI:10.1016/j.watres.2008.02.025.
- A. Dabrowska, B. Kasprzyk-Hordern and J. Nawrocki, Aldehydes formation during water disinfection by ozonation and chlorination process, Global NEST J., 2005, 7, 61–71 Search PubMed.
- C. Liu, X. Tang, J. Kim and G. V. Korshin, Formation of aldehydes and carboxylic acids in ozonated surface water and wastewater, Chemosphere, 2015, 125, 182–190, DOI:10.1016/j.chemosphere.2014.12.054.
- J. Nawrocki, J. Świetlik, U. Raczyk-Stanisławiak, A. Dąbrowska, S. Biłozor and W. Ilecki, Influence of Ozonation Conditions on Aldehyde and Carboxylic Acid Formation, Ozone: Sci. Eng., 2003, 25, 53–62, DOI:10.1080/713610650.
- U. von Gunten, Ozonation of drinking water, Water Res., 2003, 37, 1443–1467, DOI:10.1016/S0043-1354(02)00457-8.
- J. Haberkamp, A. S. Ruhl, M. Ernst and M. Jekel, Impact of coagulation and adsorption on DOC fractions of secondary effluent and resulting fouling behaviour in ultrafiltration, Water Res., 2007, 41, 3794–3802, DOI:10.1016/j.watres.2007.05.029.
- J. Altmann, F. Zietzschmann, E.-L. Geiling, A. S. Ruhl, A. Sperlich and M. Jekel, Impacts of coagulation on the adsorption of organic micropollutants onto powdered activated carbon in treated domestic wastewater, Chemosphere, 2015, 125, 198–204, DOI:10.1016/j.chemosphere.2014.12.061.
- A.-K. Ghattas, F. Fischer, A. Wick and T. A. Ternes, Anaerobic biodegradation of (emerging) organic contaminants in the aquatic environment, Water Res., 2017, 116, 268–295, DOI:10.1016/j.watres.2017.02.001.
- K. E. Greenstein, J. Lew, E. R. V. Dickenson and E. C. Wert, Investigation of biotransformation, sorption, and desorption of multiple chemical contaminants in pilot-scale drinking water biofilters, Chemosphere, 2018, 200, 248–256, DOI:10.1016/j.chemosphere.2018.02.107.
- J. Reungoat, B. I. Escher, M. Macova and J. Keller, Biofiltration of wastewater treatment plant effluent, Water Res., 2011, 45, 2751–2762, DOI:10.1016/j.watres.2011.02.013.
- H. Wang, L. Ho, D. M. Lewis, J. D. Brookes and G. Newcombe, Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins, Water Res., 2007, 41, 4262–4270, DOI:10.1016/j.watres.2007.05.057.
- M. Rattier, J. Reungoat, W. Gernjak, A. Joss and J. Keller, Investigating the role of adsorption and biodegradation in the removal of organic micropollutants during biological activated carbon filtration of treated wastewater, J. Water Reuse Desalin., 2012, 2, 127–139, DOI:10.2166/wrd.2012.012.
- Y. Lee and U. von Gunten, Oxidative transformation of micropollutants during municipal wastewater treatment, Water Res., 2010, 44, 555–566, DOI:10.1016/j.watres.2009.11.045.
- U. Hübner, U. Miehe and M. Jekel, Optimized removal of dissolved organic carbon and trace organic contaminants during combined ozonation and artificial groundwater recharge, Water Res., 2012, 46, 6059–6068, DOI:10.1016/j.watres.2012.09.001.
- K. Hellauer, D. Mergel, A. Ruhl, J. Filter, U. Hübner, M. Jekel and J. Drewes, Advancing Sequential Managed Aquifer Recharge Technology (SMART) Using Different Intermediate Oxidation Processes, Water, 2017, 9, 221, DOI:10.3390/w9030221.
- Y. Lee, L. Kovalova, C. S. McArdell and U. von Gunten, Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent, Water Res., 2014, 64, 134–148, DOI:10.1016/j.watres.2014.06.027.
- M. M. Huber, S. Canonica, G.-Y. Park and U. von Gunten, Oxidation of pharmaceuticals during ozonation and advanced oxidation processes, Environ. Sci. Technol., 2003, 37, 1016–1024 CrossRef CAS.
- A. Sperlich, M. Harder, F. Zietzschmann and R. Gnirss, Fate of Trace Organic Compounds in Granular Activated Carbon (GAC) Adsorbers for Drinking Water Treatment, Water, 2017, 9, 479, DOI:10.3390/w9070479.
- P. van Baar, Entwicklung und Anwendung von UHPLC-MS Verfahren für organische Spurenstoffe zur Bewertung der Sicherheit der Rohwasserressourcen der Wasserwerke der Stadt Berlin, 2015 Search PubMed.
- J. Funke, C. Prasse, C. Lütke Eversloh and T. A. Ternes, Oxypurinol - A novel marker for wastewater contamination of the aquatic environment, Water Res., 2015, 74, 257–265, DOI:10.1016/j.watres.2015.02.007.
- J. P. Pocostales, M. M. Sein, W. Knolle, C. von Sonntag and T. C. Schmidt, Degradation of Ozone-Refractory Organic Phosphates in Wastewater by Ozone and Ozone/Hydrogen Peroxide (Peroxone), Environ. Sci. Technol., 2010, 44, 8248–8253, DOI:10.1021/es1018288.
- A. F. Brunsch, T. L. Ter Laak, E. Christoffels, H. H. M. Rijnaarts and A. A. M. Langenhoff, Retention soil filter as post-treatment step to remove micropollutants from sewage treatment plant effluent, Sci. Total Environ., 2018, 637–638, 1098–1107, DOI:10.1016/j.scitotenv.2018.05.063.
- J. Müller, J. E. Drewes and U. Hübner, Sequential biofiltration - A novel approach for enhanced biological removal of trace organic chemicals from wastewater treatment plant effluent, Water Res., 2017, 127, 127–138, DOI:10.1016/j.watres.2017.10.009.
- T. Rauch-Williams, C. Hoppe-Jones and J. E. Drewes, The role of organic matter in the removal of emerging trace organic chemicals during managed aquifer recharge, Water Res., 2010, 44, 449–460, DOI:10.1016/j.watres.2009.08.027.
- V. Burke, L. Schneider, J. Greskowiak, P. Baar, A. Sperlich, U. Dünnbier and G. Massmann, Trace Organic Removal during River Bank Filtration for Two Types of Sediment, Water, 2018, 10, 1736, DOI:10.3390/w10121736.
- N. Henning, U. Kunkel, A. Wick and T. A. Ternes, Biotransformation of gabapentin in surface water matrices under different redox conditions and the occurrence of one major TP in the aquatic environment, Water Res., 2018, 137, 290–300, DOI:10.1016/j.watres.2018.01.027.
- K. Nödler, M. Tsakiri and T. Licha, The impact of different proportions of a treated effluent on the biotransformation of selected micro-contaminants in river water microcosms, Int. J. Environ. Res. Public Health, 2014, 11, 10390–10405, DOI:10.3390/ijerph111010390.
- U. Hass, U. Duennbier and G. Massmann, Occurrence and distribution of psychoactive compounds and their metabolites in the urban water cycle of Berlin (Germany), Water Res., 2012, 46, 6013–6022, DOI:10.1016/j.watres.2012.08.025.
- J. L. Schaper, W. Seher, G. Nützmann, A. Putschew, M. Jekel and J. Lewandowski, The fate of polar trace organic compounds in the hyporheic zone, Water Res., 2018, 140, 158–166, DOI:10.1016/j.watres.2018.04.040.
- Y. Sun, B. Angelotti, M. Brooks, B. Dowbiggin, P. J. Evans, B. Devins and Z.-W. Wang, A pilot-scale investigation of disinfection by-product precursors and trace organic removal mechanisms in ozone-biologically activated carbon treatment for potable reuse, Chemosphere, 2018, 210, 539–549, DOI:10.1016/j.chemosphere.2018.06.162.
- B. A. J. Poursat, R. J. M. van Spanning, M. Braster, R. Helmus, P. de Voogt and J. R. Parsons, Biodegradation of metformin and its transformation product, guanylurea, by natural and exposed microbial communities, Ecotoxicol. Environ. Saf., 2019, 182, 109414, DOI:10.1016/j.ecoenv.2019.109414.
- Z. Zhang, J. F. King, A. Szczuka, Y.-H. Chuang and W. A. Mitch, Pilot-scale ozone/biological activated carbon treatment of reverse osmosis concentrate, Environ. Sci.: Water Res. Technol., 2020, 6, 1421–1431, 10.1039/D0EW00013B.
- F. Bacaro, E. Dickenson, R. A. Trenholm and D. Gerrity, N-Nitrosodimethylamine (NDMA) formation and mitigation in potable reuse treatment trains employing ozone and biofiltration, Environ. Sci.: Water Res. Technol., 2019, 5, 713–725, 10.1039/C8EW00926K.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ew00684j |
| This journal is © The Royal Society of Chemistry 2021 |