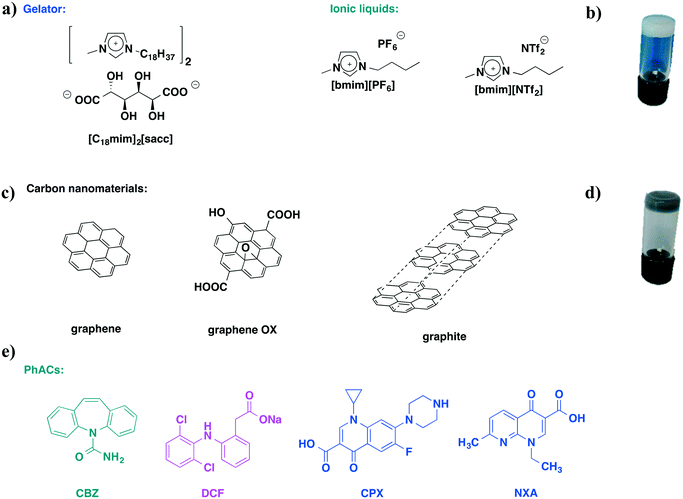
Carbon-based ionic liquid gels: alternative adsorbents for pharmaceutically active compounds in wastewater†
Carla
Rizzo
 ,
Salvatore
Marullo
,
Salvatore
Marullo
 and
Francesca
D'Anna
and
Francesca
D'Anna
 *
*
Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche, Sezione di Chimica, Viale delle Scienze, Università degli Studi di Palermo, Palermo 90128, Italy. E-mail: francesca.danna@unipa.it
First published on 9th December 2020
Abstract
With the aim of obtaining adsorbent systems to be used for the removal of pharmaceutically active compounds (PhACs) from wastewater, some hybrid ionic liquid gels (HILGs) were obtained from the combination of ionic liquid-based supramolecular gels and carbon materials, like graphite, graphene and graphene oxide (graphene OX). The properties of HILGs were investigated by determining their gel–sol transition temperature and rheological features. They were tested for the removal of PhACs belonging to different pharmaceutical classes, like antibiotics, antidepressants, anti-inflammatory. In particular, the removal of carbamazepine (CBZ), diclofenac sodium salt (DCF), ciprofloxacin (CPX) and nalidixic acid (NXA) alone or in combination was performed. Adsorption tests were carried out with the aim of identifying how different operational parameters, like the time of contact, the PhAC concentration, the volume of treated wastewater and the use under dynamic or static conditions, affect the removal efficiency of the soft materials investigated. Furthermore, the possibility to recycle and restore the sorbent systems for further reuse was also considered. Finally, the most efficient system was also tested in real apparatuses, like a dialysis membrane and columns. HILGs proved to be promising soft materials for addressing the target issue, revealing easy to handle sorbent systems with broad applicability in the field of environmental remediation.
Environmental significanceContamination of water effluents by pharmaceutically active compounds (PhACs) represents a major issue in wastewater treatment, due to their large consumption and structural variety. Carbon materials, mainly used for their removal, have the drawback of leaching in treated waters. To overcome this, soft materials, obtained by confining graphite, graphene and graphene oxide in the 3D network of ionic liquid gels (ILGs), prove a valuable option. Herein ILGs and, in particular, the ones formed with graphite, the cheapest material, exhibit high adsorption efficiency towards PhACs, selectivity and recyclability. They can be reused for eight cycles, without intermediate washing and loss in performance. After partial restoration, they can be reused for further nine cycles, lowering the environmental impact of the process. |
Introduction
Since water is the most important resource for human and environment life, water contamination is one of the most relevant issues of modern society. Several pollutants, derived from both industrial and domestic areas, contribute to wastewater contamination. Among the most common pollutants, heavy metals, organic dyes and biologically active species have been considered.1,2 These contaminants can be present at high concentrations especially in industrial effluents; consequently their detection and water purification follow standardized methods and procedures.However, besides the above mentioned contaminants, the presence of pharmaceutically active compounds (PhACs) has also recently attracted the attention of a small community of researchers.3 Indeed, the massive use of pharmaceutics in prevention, control and cure of diseases can cause a consequent PhAC water contamination, affecting not only the pharmaceutical industry but also domestic wastewater. This phenomenon is essentially due to the relative stability of PhACs that can be only partially assimilated by humans. Hence, the unmetabolized part of PhACs, eliminated from the organism, can enter domestic wastewater systems. In addition, the large use of PhACs in manufacturing processes, agricultural fields and animal feeding operations also contributes to the pollution of wastewater, surface water, groundwater and in some cases even of drinking water.4
The reason behind this capillary contamination has to be found also in the huge variety of PhACs actually existing which poses several issues in using selective systems to detect and remove the contaminants.5 In addition, the low concentration at which PhACs are usually present in polluted water is also a limitation. Nevertheless, even at low concentrations many pharmaceuticals can have acute or chronic toxicity to fish and invertebrates.6 This is the reason why the level of health risks associated with the exposure to PhACs in drinking water has to be constantly reviewed by researchers as, unfortunately, the chemical persistence and the microbial resistance of PhACs are still not completely known. Hence, solving the issue of PhAC contamination is urgent and of health security concern; however its complexity results in few reports so far reported.
Nanotechnologies and adsorption processes seem to be powerful approaches to address PhAC contamination.7 Nanoadsorbents are organic or inorganic materials of nanometric size that have high porosity and a large surface area. These peculiarities, associated with an opportune functionalization of the surface, endow nanomaterials with efficient performance for the adsorption of pollutants from wastewater.8 Particular attention has been recently focused on carbon nanomaterials, especially because the existence of several allotropes of carbon, such as carbon nanotubes (CNTs), fullerene, graphene, and graphene oxide (GO), allows their use depending on the characteristics of the system that has to be processed and the problem that has to be faced.5 Adsorption in the presence of carbonaceous materials is essentially governed by hydrophobic effects, π–π stacking, hydrogen bonding and electrostatic interactions.9 For example, different classes of PhACs have been selectively adsorbed on CNTs having different morphologies, structures and properties. In particular, the adsorption process proceeded via multiple mechanisms, depending on the different geometric shapes, functional groups, and substituents of both CNTs and PhACs, with a pivotal role played by pH and ionic strength.10 To this aim, pH was the major factor influencing the adsorption of antibiotics in the presence of the nanocomposite obtained from biochar and clay mineral11 and of non-steroidal anti-inflammatory drugs in the presence of magnetic CNTs.12 In both cases, the process was driven by surface functional group interactions among the nanocomposites and adsorbate.
Graphene and graphene oxide have been also successfully applied for PhAC adsorption thanks to the large extension of the π-surface.13,14 Interestingly, a previously reported work outlines the correlation occurring among the removal ability of different PhACs by several carbon allotropes and the order of their surface areas and micropore volumes.15
In spite of the great adsorption ability of carbonaceous nanomaterials against PhACs, the high cost, the poor separation properties and the possible release in water, after treatment, represent serious limitations to their real application in purification systems.5
This issue can be overcome by immobilizing carbon materials in other 3D materials, fabricating new hybrid composites or including them inside a semisolid matrix, like gels. In particular, some hybrid aerogels or hydrogels have been used as efficient adsorbents for PhACs by virtue of their highly porous structure.16,17 These graphene-doped gels integrate the advantages of chemical resistance and excellent mechanical properties of graphene with the porous structure and simple hand-ability of the gel, making them potential systems for environmental applications. The gels used in this area are essentially formed from biopolymers as inexpensive, highly biocompatible and biodegradable materials.18,19 Nevertheless, to provide higher swelling ability and mechanical performance to biopolymer gels,20 it was essential to combine CNTs, graphene oxide and sodium alginate to obtain a triple-structure gel with good adsorption ability.21
A different way to design strong and efficient hybrid gels could be derived from the combination of carbon nanomaterials with supramolecular gels. These soft materials are formed from low molecular weight molecules, able to self-assemble building a 3D network in which a solvent can be trapped in.22 In the last few years, this class of physical gels, held together thanks to non-covalent interactions,23 has been efficiently applied for environmental remediation.24,25 Particular attention has to be devoted to the ones formed in non-conventional solvents like ionic liquids (ILs) and deep eutectic solvents (DESs).26 These solvents have low vapor pressure and flammability that allowed them to fit the definition of eco-compatible solvents with respect to conventional organic ones. Thanks to these properties, ILs, DESs and gels formed in these solvents have been recently used also for environmental remediation,27–30 allowing the effect that the remediation process itself can have on the environment to be decreased. On this subject, they proved very efficient in the removal of dyes. In particular, supramolecular ionic liquid gels (ILGs), based on imidazolium surfactants, have shown very high affinity for anionic dyes with removal efficiency higher than 80%. This parameter also stayed constant after 25 cycles of reuse.31 More recently, Fe–TAML has been entrapped in basic ionic liquid silica gels and ionic liquid silica spheres. The obtained systems were successfully used to decolorize dye polluted wastewater by using a combination of dye adsorption and catalytic degradation.32 However, these kinds of materials have been seldom used for PhAC wastewater remediation. In particular, a series of DESs have been efficiently tested, using the liquid–liquid extraction method for the removal of ciprofloxacin,33 but this procedure can easily suffer from water contamination after treatment. Conversely, a series of hybrid ionic liquid gels (HILGs) with CNTs, graphite and graphene have been tested for the removal of ciprofloxacin and nalidixic acid.34 Although the results obtained were encouraging, a wider range of PhACs needs to be tested as a consequence of their structural diversity and new HILGs deserve the possibility to be tested for the cause.
Recently, the properties of some sugar-derived organic salts forming hydro-, organo- and ionic liquid gels have been studied. In particular, hydrogels proved to be excellent adsorbents for Cr(VI) from wastewater, while in comparison ILGs possessed a lower removal efficiency, ascribed to the lower affinity of Cr(VI) for the IL environment.35 However, considering the organic nature of PhACs and their possible affinity for an IL-based system as well as taking advantage of the well-known ability of carbon nanomaterials for PhAC removal, the use of sugar-derived HILGs for PhAC adsorption from wastewater was considered as an option worthy of investigation.
For these reasons, in the present work the ILG matrix is composed of 1-methyl-3-octadecyl imidazolium saccharate, [C18mim]2[sacc], as a gelator, and some of the most commonly used ILs, namely 1-butyl-3-methyl imidazolium hexafluorophosphate [bmim][PF6] and 1-butyl-3-methyl imidazolium N,N-bis(trifluoromethanesulfonyl)imide [bmim][NTf2] (Scheme 1a). The above gels were chosen on the grounds of their good mechanical properties,35 which are an essential requirement for their application in adsorption processes. Both ILs used are generally claimed as hydrophobic ILs and this should give a reasonable warranty of low leaching in the aqueous phase. Furthermore, as a consequence of the presence of a relatively short alkyl chain, they should exhibit low toxicity.36
HILGs were obtained by adding graphene, graphene oxide (graphene OX) and graphite (Scheme 1c). Graphene with a large surface area allows an easy contact with pollutants. In addition to this property, the presence of functional groups on the surface of graphene oxide should allow a higher affinity for PhACs. Finally, the comparison with graphite, a carbon material in bulk form, can give an idea of the interactions involved in the adsorption process. Among the carbon materials tested, graphite was the cheapest and its use perfectly meets the criteria of sustainability.
The gel–sol transition temperatures, Tgel, and rheological properties of both ILGs and HILGs were investigated. The removal efficiency of all the gels was tested against three classes of PhACs, antiepileptic/antidepressant, anti-inflammatory and antibiotics, as they are frequently present as mixtures in wastewater. In particular, some of the most commonly used PhACs, such as carbamazepine (CBZ), diclofenac sodium salt (DCF), ciprofloxacin (CPX), and nalidixic acid (NXA), have been chosen (Scheme 1e).
The adsorption capacity of each gel was proven over each PhAC. Then, the gel with the best performance was selected to study the different aspects of the adsorption process, like the kinetics of adsorption of each PhAC, the possibility to recycle and regenerate the gel, and the possibility to perform adsorption tests under static and dynamic conditions. Furthermore, the effect of wastewater volume and PhAC concentration and the possibility of using real sets, such as a dialysis membrane or a series of columns, were also considered. Finally, the adsorption capacity of the gel over a mixture of PhACs was also analysed.
The results obtained allow us to have an exhaustive overview on the removal efficiency of several HILGs over different PhAC water pollutants. The use of several experimental conditions also warrants good results in real apparatuses, encouraging the application of these supramolecular hybrid gels as sorbents to clean wastewater.
Materials and methods
Chemicals
The gelator [C18mim]2[sacc] was synthesized as previously reported.35Graphene, graphene OX, graphite, nalidixic acid, carbamazepine, diclofenac sodium salt, ciprofloxacin, 5-hydroxymethylfurfural (5-HMF), ethyl lactate, ethyl acetate, dimethyl carbonate, 1-octanol, isoamyl alcohol, methanol and 2-methyl-tetrahydrofuran (2-Me-THF) were purchased from commercial sources and used without further purification. ILs, such as [bmim][PF6] and [bmim][NTf2], have been purchased from a commercial source, before use they were dried at 60 °C under high vacuum and stored in a desiccator under an argon atmosphere.
Gel formation
The gelator at 5 wt% and carbon materials at 0.1 wt% were dissolved in a minimal amount of MeOH (≈ 1 mL for 100 mg of gelator) with the aid of an ultrasonic bath (power of 200 W and frequency of 45 kHz). The mixture was then evaporated to remove the solvent residue, obtaining in this way a homogeneous solid mixture of the gelator and nanocomposite in an appropriate ratio.The solid mixture was weighed in a screw-capped vial (diameter 1 cm) together with the appropriate IL (≈ 250 mg), and the mixture was dispersed under ultrasonication, for 10 min. Then, it was heated at 80 °C for 1 h, under magnetic stirring. Afterwards, the mixture was kept at 4 °C overnight to allow gel formation.
The tube inversion test was used to assess gel formation; when the vial was turned upside down no flow of the mixture was observed.22
T gel determination
T gel was determined by the falling-drop method. The vial containing the gel was immersed and turned upside down in a water bath, and the bath temperature was gradually increased, with a rate of 2 °C min−1, until the first drop of the gel fell. Tgel values were reproducible within 1 °C.Whenever possible, Tgel was also confirmed by the lead ball method. A lead ball (weighing 46.23 mg and 2 mm in diameter) was placed on top of the gel, and the vial was immersed in a water bath. The bath temperature was gradually increased (2 °C min−1) until the gel melted and the lead ball reached the bottom of the vial. Tgel values were reproducible within 1 °C.
Rheological measurements
Rheological measurements were recorded on an ARES G2 (TA Instruments) strain-controlled rheometer using a plate–plate (PP 25-2) tool. The sample was placed between the shearing plates of the rheometer. Rheological measurements, such as strain and frequency sweeps, were recorded three times on three different aliquots of gels. Strain sweeps were recorded at a fixed angular frequency (1 rad s−1) and frequency sweeps at a fixed strain of 0.04%. These values were chosen as falling within the linear viscoelastic region (LVR) of gels. All measurements were performed at 25 °C.PhAC adsorption
PhAC water solutions, having a concentration equal to 1.8 × 10−4 M, were used to test gel's removal efficiency, with the only exception of ciprofloxacin (1.4 × 10−4 M) due to its low solubility in water. Usually, stock solutions of PhACs were prepared in MeOH, and the volume of the co-solvent did not exceed the 10% of total volume in the final water solution used.The removal of PhACs was firstly tested in vials. In particular, 300 μL of a PhAC aqueous solution was cast on top of 250 mg of gels at 5% wt of the gelator and 0.1% wt of the carbon material. The RE was estimated from the aqueous solution, withdrawing the solutions from the gel at a fixed interval of time and analyzing them through UV-vis spectroscopy. The final concentration was calculated according to the Beer–Lambert law (A = εbc, where A is the absorbance of the PhAC, ε is the molar extinction coefficient, expressed in mol L−1 cm−1, b is the path length of the incident light, cm, and c is the concentration of the PhAC in solution, mol L−1). The molar extinction coefficient of the PhAC aqueous solution with a b of 0.2 cm is: 1456 mol L−1 cm−1 for nalidixic acid (A316nm), 5639 L−1 cm−1 for ciprofloxacin (A273nm), 2574 mol L−1 cm−1 for carbamazepine (A280nm) and 2461 L−1 cm−1 for diclofenac sodium salt (A275nm). The final concentration of the PhAC in solution was obtained using the Beer–Lambert equation.
Kinetics of adsorption
The time required by gels to remove the maximum amount of each PhAC was determined, by performing kinetic experiments. To this aim, different gel samples (250 mg; 5% wt of the gelator and 0.1% wt of the carbon material) were prepared and put into contact with 0.3 mL of PhAC for suitable times of contact. The PhAC concentration was determined by UV-vis measurements by using the Beer–Lambert equation.Gel recycling and regeneration
Recycling of the adsorbing gel was carried out by loading the suitable gel with the PhAC solution (0.6 mL cast on 500 mg of gel) as previously described. After 5 h, the aqueous solution was removed and replaced with a fresh batch of 0.6 mL of a PhAC solution. After each cycle, the gel maintained its characteristics, as evidenced by the tube inversion test.The regeneration of the gel was performed by back-washing 500 mg of polluted gel after 9 cycles of adsorption. The contact between the gel and the solvent, selected on the grounds of environmental sustainability and the ability to solubilize only the PhAC and not the gel, allowed PhAC desorption using at least 3 cycles of contact with 0.6 mL of fresh desorption solvent. In the case of 2-Me-THF, the regeneration was performed both after one cycle of adsorption and after 9 cycles of adsorption. The contact between the gel and 2-Me-THF allowed the desorption of PhAC. The regeneration of the gel after one cycle of adsorption required 3 × 0.6 mL washings with 5 min of contact, while the regeneration of the gel after 9 cycles required 7 × 0.6 mL washings with 10 min of contact. After regeneration, the gel was heated at 90 °C and stored overnight at 4 °C to allow its recovery to further reuse it for new adsorption cycles.
Adsorption in dynamic systems
The removal efficiency of the gels was also investigated at 5 and 24 h in a dialysis membrane, which was filled with 1 g of gel and immersed in 20 mL of carbamazepine (1.8 × 10−4 M) water solution. A membrane dialysis with a cut off of 12/14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Dalton and a diameter of 14.3 mm was used.
000 Dalton and a diameter of 14.3 mm was used.
In addition, three syringes with a capacity of 1 mL were filled with 0.5 g of gel and the RE% after that 1 mL of PhAC solution was flowed through the three syringes was determined. To block the column, cotton was used and the adsorption capacity of cotton was checked and it was equal to zero. The contact time between the gel and solution was 5 min, after which a pressure was applied to allow the flow of the aqueous solution. The latter was firstly analyzed to check the RE and after passing through the second column and so on.
The removal efficiency of the gel under dynamic conditions was tested using an incubating mini-shaker with a stirring rate ranging from 0 up to 800 rpm. The same test was performed by applying on the gel different volumes of PhAC solutions. In particular, 0.6, 1.2, 2.4 and 3.6 mL of aqueous solution were cast on 500 mg of gels and the RE% was determined under static and dynamic conditions.
Adsorption of mixtures of PhACs
Several mixtures of PhAC solutions were prepared to test the adsorption capacity of the gel. In particular, a mixture of nalidixic acid, carbamazepine and diclofenac sodium salt and three binary mixtures of two PhACs were prepared with each component present at a concentration of 1.8 × 10−4 M. 0.5 mL of each mixture were cast on 250 mg of gel and after 24 h of contact, the mixture was spectrophotometrically analyzed.Release of HILG components in water or in desorption solvents
To test the release of the gelator and IL in water, 300 μL of D2O were cast on 250 mg of graphite/PF6 and graphite/NTf2 gels. After 5 h, the supernatant solution was analyzed via1H NMR. The 1H NMR spectra were recorded by using 5-HMF as a reference compound. The reference signals did not superimpose with those of the gelators or ILs, so it was possible to determine the amount of component released by comparing the peak areas of the reference and gel components.The same procedure was performed to test the release of gel components in the desorption solvents. In particular, to record 1H NMR, the solution extracted using 2-Me-THF was evaporated and dissolved in CD3OD together with 5-HMF. Meanwhile, to record the 1H NMR for the solution extracted using ethyl lactate, DMSO-d6 and 5-HMF were used as standards, due to the high boiling point of ethyl lactate.
To test the release of graphite from the HILG, turbidity measurements were performed by recording the UV spectra of the supernatant water solution after 5 h of contact with the HILG and of a water suspension containing 0.1 wt% of graphite. Absorbance values at 400 nm gave turbidity results.37
Results and discussion
Hybrid ionic liquid gel properties
[C 18 mim] 2 [sacc] forms white opaque ILGs (Scheme 1b) in the selected ILs as previously reported.35 On the other hand, to obtain homogeneous HILGs, a solid mixture of the gelator and carbon material was previously prepared. This solid mixture was subsequently dispersed in the IL with the aid of ultrasound and heated under magnetic stirring at 80 °C. The mixture, cooled at 4 °C, formed homogeneous dark ionic liquid gels stable under the tube inversion test (Scheme 1d).22The gelator and carbon material concentrations were chosen on the grounds of the best performances of the gels obtained from previous studies.34,35 In particular, 5 wt% of gelator and 0.1 wt% of carbon material were used. These concentrations allowed the combination of the good chemical–physical properties of the gels, mainly derived from the gelator, with the minimal amount of carbon material. Considering that the gelator and IL cation are the same in each case, for simplicity, from now on gels will be indicated as carbon material/IL anion, e.g.graphene/PF6.
Taking into consideration that gels were designed to perform adsorption processes in environmental remediation, we firstly assessed their Tgel and rheological parameters (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = G′′/G′ and values of γ at G′ = G′′ for gels investigated at 5 wt% gelator and 0.1 wt% of carbon material concentrations at 25 °C. For rheological parameters, error limits are based on the average of three different measurements with different aliquots of gels
δ = G′′/G′ and values of γ at G′ = G′′ for gels investigated at 5 wt% gelator and 0.1 wt% of carbon material concentrations at 25 °C. For rheological parameters, error limits are based on the average of three different measurements with different aliquots of gels
| Entry | Gel | T gel (°C)a | G′ (Pa)b | G′′ (Pa)b | tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δb δb |
Crossover point (Pa)c |
|---|---|---|---|---|---|---|
| a T gel determined with the falling drop method, reproducible within 1 °C. b Values reported in the linear viscoelastic region, at ω = 1 rad s−1 and γ = 0.04%. c Values reported at ω = 1 rad s−1. | ||||||
| 1 | None/PF6 (ref. 35) | 61 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 3400 500 ± 3400 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ± 400 400 ± 400 |
0.47 ± 0.05 | 79 ± 5 |
| 2 | Graphite/PF6 | 59 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 9000 200 ± 9000 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ± 4000 300 ± 4000 |
0.22 ± 0.06 | 8.9 ± 0.1 |
| 3 | Graphene/PF6 | 60 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 1600 200 ± 1600 |
4100 ± 800 | 0.20 ± 0.05 | 32 ± 8 |
| 4 | None/NTf2 (ref. 35) | 38 | 3100 ± 900 | 1180 ± 120 | 0.37 ± 0.03 | 8 ± 1 |
| 5 | Graphite/NTf2 | 35 | 620 ± 140 | 263 ± 30 | 0.49 ± 0.05 | 23 ± 1 |
| 6 | Graphene/NTf2 | 34 | 770 ± 100 | 400 ± 70 | 0.51 ± 0.05 | 40 ± 6 |
The temperature of the gel–sol transition was, generally, poorly affected by the presence of carbon materials, as previously observed for other HILGs.34 For HILGs in [bmim][PF6], the Tgel stayed constant both in the presence of graphite and graphene, while a modest decrease in Tgel was determined only for graphene OX/PF6 with a Tgel of 56 °C with respect to 61 °C of the corresponding ILG. On the other hand, [bmim][NTf2]-HILGs were more sensitive to the presence of carbon materials. Indeed, a decrease in Tgel values was already observed in hybrid gels formed in the presence of graphite and graphene (35 and 34 °C for graphite and graphene-based HILGs, respectively vs. 38 °C for the corresponding ILG). Once again, the lowest Tgel was determined for graphene OX/NTf2 (32 °C vs. 38 °C of the corresponding ILG).
Then, in general, analysis of Tgels demonstrates that carbon materials do not prevent ionic liquid gel formation, but in contrast to what was previously observed for other hybrid supramolecular gels,34,38 they do not improve gel response from a thermal point of view. According to previous reports for graphene and graphite hybrid gels, π–π stacking interactions may be predominant in gel network formation,39 and the thermal stability stayed constant or decreased with respect to pure ILGs. Differently, the presence of hydrophilic functional groups on the graphene moiety surface has a more marked negative effect on the ionic liquid gel thermal stability. Probably, the possibility to establish hydrogen bond interactions both with the IL and gelator cation weakens the supramolecular interactions held inside the 3D network of the gel.
To understand the effect of the carbon materials on the ionic liquid gel rheological properties, strain and frequency sweep measurements were performed and compared with the ones of pure gels. These measurements ensure the gel-like behaviour of materials, analysing the evolution of storage, G′, and viscous moduli, G′′, as a function of the strain or frequency applied. For both measurements, the intermediate rheological behaviour between solids and liquids, typical of gels, was observed (Fig. 1 and S1†).
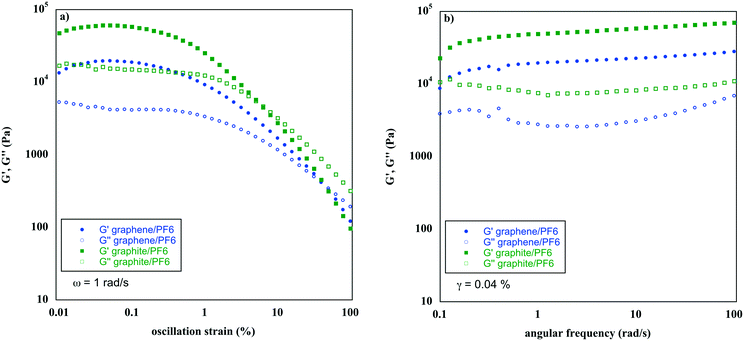 | ||
| Fig. 1 a) Strain and b) frequency sweep of hybrid ionic liquid gels at 5 wt% of gelator and 0.1 wt% of carbon material. | ||
Analysis of Fig. 1a evidences how for low values of strain, G′ is higher than G′′, indicating a solid-like behaviour, until reaching the so-called “crossover point”. This is the value of strain at which G′ is equal to G′′ and after which an inversion of moduli occurs, indicating a liquid-like behaviour. Once known, the linear visco-elastic region (LVR) of the material, i.e. the plateau region where G′ is higher than G′′, allows the analysis of moduli variations as a function of the angular frequency, at a fixed strain. Fig. 1b shows that, in the range of frequencies investigated, G′ is higher than G′′ and both moduli are independent from frequency.
Different from what was observed for Tgel values, the rheological properties were drastically affected by the presence of carbon materials (Table 1). However, a different behaviour was detected depending on the gelation solvent. For gels in [bmim][PF6], the addition of graphite contributed to the reinforcement of the gel (Table 1, entry 2 vs. 1). Indeed, G′ is much larger than that of the corresponding pure gel and also, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, the ratio between G′′ and G′, indicates the higher stiffness of the gel. As is known, the lower the value of tan
δ, the ratio between G′′ and G′, indicates the higher stiffness of the gel. As is known, the lower the value of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, the higher the stiffness, giving an idea of the strength of colloidal forces held in the gel network. Conversely, the presence of carbon nanomaterials, like graphene, caused a decrease in the magnitude of G′, with respect to that of the pure gel. However, according to previous reports,40 and in agreement with previous data, graphene/PF6 presents a lower tan
δ, the higher the stiffness, giving an idea of the strength of colloidal forces held in the gel network. Conversely, the presence of carbon nanomaterials, like graphene, caused a decrease in the magnitude of G′, with respect to that of the pure gel. However, according to previous reports,40 and in agreement with previous data, graphene/PF6 presents a lower tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, confirming the occurrence of stronger colloidal forces in the hybrid than in pure gels.
δ, confirming the occurrence of stronger colloidal forces in the hybrid than in pure gels.
The situation is inverted for [bmim][NTf2] gels, as the hybrid gels present lower rheological performances with respect to the pure ones, as accounted for by the G′ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values. In this case, the presence of carbon materials weakens the ILGs, even if the crossover point of HILGs occurred at higher strain values with respect to the pure gels.
δ values. In this case, the presence of carbon materials weakens the ILGs, even if the crossover point of HILGs occurred at higher strain values with respect to the pure gels.
The presence of carbon materials usually reinforces the gel structure. However, as for Tgels, in the case of ILs with a low cross-linking ability such as [bmim][NTf2], it seems to induce a destabilizing effect.
Then, different from what was detected for Tgels, the rheological performance heavily depends on the nature of the IL anion. The solvents used show small differences in the anion coordination ability (β = 0.207 and 0.243 for [bmim][PF6] and [bmim][NTf2], respectively),41 but they exhibit significantly differences in viscosity (η = 450 and 52 for [bmim][PF6] and [bmim][NTf2], respectively)42 and in the cross-linking ability of the anion, which according to previous reports,43 decreases going from [bmim][PF6] to [bmim][NTf2]. Data collected show that a decrease in the latter two mentioned parameters negatively affects the occurrence of mixed gelator–solvent–carbon material interactions that are essential to create a stronger 3D network in the HILGs.
Adsorption of PhACs
Initially, all the gels were tested as adsorbents after 3 h of contact with the PhAC solution. These preliminary analyses were performed under static conditions by simply casting 0.3 mL of PhAC aqueous solution on 250 mg of gel, at 25 °C. After 3 h, the solution was spectrophotometrically analysed to determine the PhAC concentration, thanks to the calibration curves previously determined. The removal efficiency (RE) of the gels was calculated according to eqn (1):| RE = 100·(C0 − Ci/C0) | (1) |
| Entry | Gel | RE (%) NXA | RE (%) CPX | RE (%) CBZ | RE (%) DCF |
|---|---|---|---|---|---|
| 1 | None/PF6 | 12% | 0% | 87% | 41% |
| 2 | Graphene/PF6 | 45% | 20% | 92% | 49% |
| 3 | Graphite/PF6 | 39% | 24% | 90% | 51% |
| 4 | Graphene OX/PF6 | >99% | 46% | 69% | 19% |
| 5 | None/NTf2 | 10% | 0% | 83% | 41% |
| 6 | Graphene/NTf2 | 43% | 20% | 85% | 43% |
| 7 | Graphite/NTf2 | 53% | 29% | 75% | 53% |
| 8 | Graphene OX/NTf2 | >99% | 53% | 57% | 0% |
In general, the removal efficiency of ILGs depends on the nature of the PhAC used. Indeed, in both cases, poor RE values were measured for antibiotic drugs, NXA and CPX (entries 1 and 5, Table 2), while more significant values were collected as far as CBZ and DCF were considered. The above values were not affected by the nature of the IL solvent.
The performances of pure ILGs were enhanced by the presence of carbon materials, most especially for CPX and NXA. However, different effects deriving from the nature of the carbon materials were detected depending on the nature of the solvent used.
In particular, graphene/PF6 and graphite/PF6 HILGs have a similar RE (entries 2 and 3, Table 2), whereas for [NTf2−]-based gels, graphite endows the soft material with a better performance than the one detected for graphene-based gels (entries 6 and 7, Table 2) with the only exception of CBZ.
A completely different scenario is observed for graphene OX gels, as with respect to pure gels, they show a better affinity for antibiotics and, in particular NXA was totally removed (entries 4 and 8, Table 2). On the other hand, as far as CBZ and DCF are concerned, going from pure to hybrid gels, a significant decrease in RE values was detected (entries 1 and 4, 5 and 8, Table 2). These results give an idea about the selectivity that HILGs can exhibit as a function of the carbon material dispersed in the gel matrix, opening the use of a suitable hybrid ionic liquid gel depending on the needs.10
Nevertheless, among the gels, graphite/PF6 seems to be the most suitable to use as a model adsorbent, presenting excellent or fair RE for all selected PhACs. On the other hand, [bmim][PF6], as a gelation solvent, warrants better chemical–physical properties than [bmim][NTf2] gels, allowing a better resistance of the gel, useful for gel recycle.
Besides physico-chemical properties, considering the environmental application of these materials, the toxicity effect must also be considered, to better deal with issues due to possible release of material components in the treated wastewater. On this subject, it is widely known that the toxicity of the ILs, generally, depends on the microorganism on which it is tested. In particular, [bmim][PF6] proved biocompatible when tested on Acetobacter,44L. kefir,45S. termitida46 and E. coli.47 Further analyses on this latter evidenced no significant effects in 4 h and a moderate decrease of membrane integrity after 5 h.48 On the other hand, [bmim][PF6] proved biocompatible in the presence of C. parapsilosis49and C. albicans.50 In the light of these observations, we are reasonably confident that [bmim][PF6] is a good compromise between toxicity and efficiency.
Although the RE values obtained with both graphene and graphene OX HILGs are in some cases comparable to the ones collected using graphite gels, the graphite-based soft material should be preferred on the grounds of its low cost. This is the reason why graphite/PF6 was used as the model sorbent system and to have further insights on its features, information about morphology was gained by recording SEM images (Fig. S2†). The xerogel obtained, by washing a thin layer of the gel with acetone, exhibited a highly entangled fibrous structure in which the carbon material was uniformly disperse.
To further analyse the properties of the model adsorption system, component release in water of HILGs was investigated via1H NMR and UV spectroscopy. Firstly, the integrity of the gel after 5 h of contact with PhAC water solution was observed, as reported in Fig. S3a.†
The release in water of graphite from the HILG was not observed, as the supernatant water solution was limpid. The supernatant water sample was compared to a water suspension of graphite at the same concentration used in the HILG (0.1 wt%), (Fig. S3b–e†). In addition, turbidity measurements revealed that high turbidity is detected only for the graphite water suspension and not for the supernatant water solution cast on the HILG, 0.3 and 0.01 a.u., respectively (Fig. S4†).37
On the other hand, to test the gelator and IL release in water, the 1H NMR spectra of D2O after 5 h of contact with HILGs were obtained; 5 h was the time needed to reach the maximum RE (see later). In all cases, no gelator release has been observed in the 1H NMR spectra (Fig. S5 and S6†) in agreement with what was previously observed.35 Meanwhile, small amounts of ILs were released from both graphite/PF6 and graphite/NTf2. In particular, 3% and 1.5% of [bmim][PF6] and [bmim][NTf2] were released, respectively (Fig. S5 and S6†). However, these quantities should be in line with toxicological data previously reported.48 In addition, the pH of the water supernatant solution was neutral, indicating that no significant amounts of HF formed due to the presence of the PF6 anion in water. Probably, the additional supramolecular interactions within the HILG help to stabilize all the gel components in the hybrid materials, making it more difficult for leaching to occur.
Kinetics of adsorption
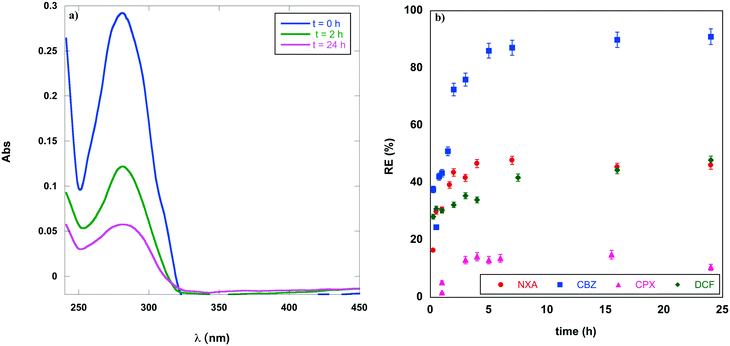
Bearing in mind the previous analysis, clearly indicating the high selectivity of HILGs for CBZ and the above considerations about the benefit derived from the use of graphite/PF6, the kinetics of PhAC adsorption were measured using batch experiments (Fig. 2). In Fig. 2a, the evolution of some UV spectra of PhAC solution, before and after contact with the gel, shows the decrease of the absorption band as a function of time.The plot of REs as a function of time (Fig. 2b) for all the PhACs confirms that CBZ is the most adsorbed contaminant. The equilibrium value for graphite/PF6 in the presence of CBZ was reached in 5 h with a RE of 90%. On the other hand, the limiting adsorption value was reached more rapidly for NXA and DCF, 2 and 3 h, respectively, but with a RE of 45%, in both cases significantly lower than that of CBZ. Finally, the maximum of CPX adsorption is obtained in 3 h, but it is only equal to 20%.
The above results shed light on the significant effect that the pollutant nature exerts on the adsorption process. The pollutants considered are all structurally characterized by the presence of a π-surface, having different extension and functional groups able to establish hydrogen bond interactions. A further distinction can be made on the grounds of their acidic (CPX and NXA) or basic nature (CBZ and DCF). The adsorption rate changes in the order: CBZ > NXA > DCF > CPX, demonstrating that the extension of the π-surface area (increasing from DFC to CBZ) plays a more significant role with respect to the nature of the PhAC and the ability to establish hydrogen bonds. The relevance of this latter factor can be ruled out on the grounds of the very low affinity of the HILG towards CPX, notwithstanding the presence on its structure of different hydrogen bond donor and acceptor groups. The hydrogen bond donor ability of PhACs plays a significant role only in the case of graphene OX gels. Indeed, these materials show the highest affinity for NXA and CPX, presenting carboxylic acid functionalities able to interact with multiple hydrophilic functional groups on graphene OX and allowing the establishment of multiple hydrogen bond interactions among the PhACs and gel matrix.21
Recycle and regeneration of gel
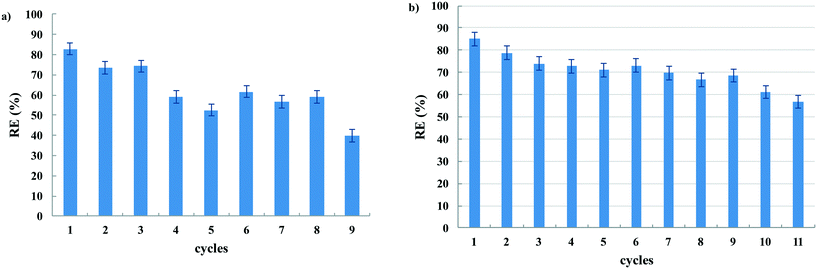
Considering the selectivity of graphite/PF6 through the adsorption of CBZ, subsequent tests based on the possible use of this gel under different adsorption conditions and set ups were carried out.Firstly, the possibility to recycle the gel matrix after the adsorption was taken into account. To this aim, the adsorption of CBZ after 5 h, the time needed to reach the maximum adsorption, was determined. Then, the solution was withdrawn and a fresh solution of CBZ was cast on the same gel until the RE significantly decreased (Fig. 3a and Table S1†).
A gradual decrease in RE was observed during the 4th and the 5th cycles of adsorption. However, even after these cycles, the gel kept its adsorption ability with a RE of 60% until the 8th cycle, after which it drastically decreased down to 40%.
Considering that the concentration of 1.8 × 10−4 M corresponds to 42 mg L−1 CBZ, after the first cycle of adsorption, the gel adsorbs 34 mg L−1 CBZ. However, the recycle of the same gel for 9 times allows the adsorption of 235 mg L−1 CBZ and this represents a huge advantage in terms of the uptake ability of the gel.
To reduce the environmental footprint, the possibility to regenerate the gel was investigated. To this aim, particular attention was devoted to the nature of the solvents used for contaminant desorption as they were chosen among the eco-compatible ones, according to the GSK selection guide.51 However, the desorption solvents should also be able to solubilize CBZ and not the gel; for example acetone, ethyl acetate and dimethyl carbonate could not be used due to the immediate solubilisation of the HILG. Meanwhile, 2-Me-THF, ethyl lactate, 1-octanol and isoamyl alcohol were tested, even if the last two suffer from lower solubilisation ability of CBZ. All these four solvents kept the gel integrity during and after the desorption process, as shown in Fig. S10.† A particular case was that of 2-Me-THF as melting of the gel occurred during the process, but a clear liquid–liquid phase separation allowed to proceed in the desorption process. In addition, the subsequent heating of the gel allowed its reformation for further reuse.
With the aim of decreasing the amount of solvent used for gel regeneration, the desorption process was carried out on the gel recycled 9 times, which was able to adsorb 235 mg L−1 CBZ. Among the solvents tested, the best extraction efficiency of CBZ was obtained in 2-Me-THF and in ethyl lactate (80 and 58% of CBZ desorbed, Table S2†). Nevertheless, in the case of ethyl lactate after the third cycle of desorption, a decrease in gel volume has been observed. For this reason, we recorded the 1H NMR spectra of the solutions resulting from the extraction of the gel with 2-Me-THF and ethyl lactate (Fig. S8 and S9†). Like in water solution, the HILG released only part of IL, 17 and 20% for 2-ME-THF and ethyl lactate, respectively.
Even if 2-Me-THF is flammable and causes a partial release of the IL, it allowed the extraction of a larger amount of CBZ and, deriving from biomass conversion, can be considered safer than other organic solvents. For these reasons, 2-Me-THF can be considered the best solvent for the desorption process. On the other hand, 1-octanol and isoamyl alcohol allowed the recovery of just 11 and 25% of CBZ, respectively (Table S2†).
In addition, after the desorption of 80% of CBZ from the gel (7 × 0.6 mL of 2-Me-THF), the same gel was used to adsorb again fresh solutions of CBZ with good RE for further 11 cycles (Fig. 3b). The decrease in efficiency was less prominent than in the first recycling experiment. Indeed, after a first decrease in the third cycle, the RE values stayed constant at 70% until the 9th cycle. However, in the 11th cycle, it was slightly lower than 60%. The good performance of the restored gel gives further support to the hypothesis that CBZ adsorption is mainly driven by π–π interactions.
The regeneration of the gel was also analysed after one cycle of adsorption. For a gel adsorbing CBZ with a RE of 85%, a PhAC desorption of 79% was determined (3 × 0.6 mL of 2-Me-THF). After washing, the gel was able to adsorb again CBZ with a RE of 85%.
Probably, the residual amount of aromatic compounds in the regenerated gel, better than the corresponding clean one, is able to establish the above interactions favoring the adsorption of a larger amount of PhAC.
Influence of PhAC initial concentration and volume
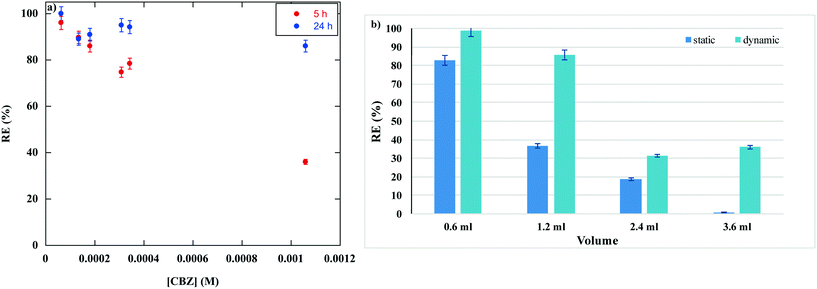
The RE of graphite/PF6 was also tested as a function of different initial concentrations of CBZ, in the range from 6 × 10−5 to 1 × 10−3 M (Table S3†). The RE value was determined after 5 and 24 h of contact time. In both cases, the RE equilibrium value was reached if a concentration of CBZ equal to 1.8 × 10−4 M was used (Fig. 2b). Data collected are displayed in Fig. 4a.The data obtained evidence that, at 5 h, the RE gradually decreased on increasing the initial concentration of CBZ, indicating a lower efficiency of the gel to adsorb a larger amount of the PhAC in the selected time. We obtained similar results by performing dye adsorption from wastewater, using both ILGs and eutectogels,28,29 allowing the identification of the above behaviour as typical for such a kind of soft material. However, a deep analysis of data collected also shed light on the impact that the time of contact has on the adsorption efficiency. Indeed, on raising the concentration, the decrease in RE value was more significant for a contact time of 5 h rather than 24 h, clearly indicating that for larger initial concentrations, the time needed to reach the maximum adsorption is shifted to longer intervals, but the RE of graphite/PF6 is still retained.
The adsorption process could be also influenced by static or dynamic conditions of batch experiments.33 To understand if this factor also affected the RE of graphite/PF6, adsorption experiments were carried out on an incubator, using a stirring ramp, for a time of contact of 5 h and using a CBZ concentration of 1.8 × 10−4 M (Table S4†). The RE values increased as a function of the stirring rate of the system going from 86 up to 96% under static and dynamic conditions (400 rpm), respectively. The above result is different from the one reported for PhAC liquid–liquid extraction performed in the presence of deep eutectic solvents, evidencing a negligible effect of the stirring speed on the extraction efficiency.33 Probably, in the case of the gel phase, the dynamic system allowed an easy access to all adsorption sites of the gel, favouring the process.
Based on these results, the RE of graphite/PF6 was also determined, increasing the volume of CBZ solution (1.8 × 10−4 M) both under static and dynamic conditions (Fig. 4b and Table S5†). The RE was drastically affected by the volume of PhAC solution cast on the gel, especially under static conditions. In particular, the RE value halved passing from 0.6 to 1.2 mL and from 1.2 to 2.4 mL, while in the presence of 3.6 mL of CBZ solution the RE was really low.
However, the drop in RE with the volume increase was much less relevant under dynamic conditions. Indeed at 800 rpm, the RE decreased from 98% to 86%, passing from 0.6 mL to 1.2 mL, but this still represents a significant adsorption efficiency. The further increase in volume induced a drastic decrease in RE down to 31%. However, it also remained constant by using 3.6 mL of solution, confirming a better response of the gel to adsorption under dynamic conditions.
Adsorption in real apparatuses
The removal efficiency of the gel was also analysed in two real apparatuses: a series of columns and a dialysis membrane (Fig. 5). | ||
| Fig. 5 Adsorption of CBZ (1.8 × 10−4 M) a) on a series of syringes filled with graphite/PF6 and b) using a dialysis membrane. | ||
As shown in Fig. 5a, three syringes were loaded with the same amount of graphite/PF6 gel (0.5 g) and 1 mL of CBZ solution was eluted in sequence. The RE was determined after each elution and it was equal to 41%, 53% and 65%, respectively. The RE gradually increased from the first to the third elution and the final RE is competitive with respect to the batch/static system, considering that the contact between the solution and gel is 5 min in each case.
Differently, when 1 g of graphite/PF6 gel was used in a dialysis membrane immersed in 20 mL of CBZ (Fig. 5b), the RE was equal to 31% and 27% at 5 h and 24 h, respectively, and it did not increase even at a longer time of contact. Probably, this apparatus suffered from both the large volume of solution and the static conditions, which had negative effects on the adsorption process as previously analysed.
Mixtures of PhACs
In a real sample, wastewater is often polluted by more than one PhAC; for this reason the RE of graphite/PF6 was also tested over a mixture of PhACs. To this aim, an aqueous solution of CBZ, NXA and DFC at a concentration of 1.8 × 10−4 M was cast on the gel for 24 h. Due to the low adsorption selectivity of the gel for CPX, this PhAC was not considered in the mixture.The UV spectra of the mixture of PhACs before and after adsorption on the gel are reported in Fig. 6. The UV bands of CBZ and DFC presenting maxima at 280 and 275 nm are superimposed in the mixture spectra (Fig. 6a), whereas the NXA band is recognizable at 316 nm.
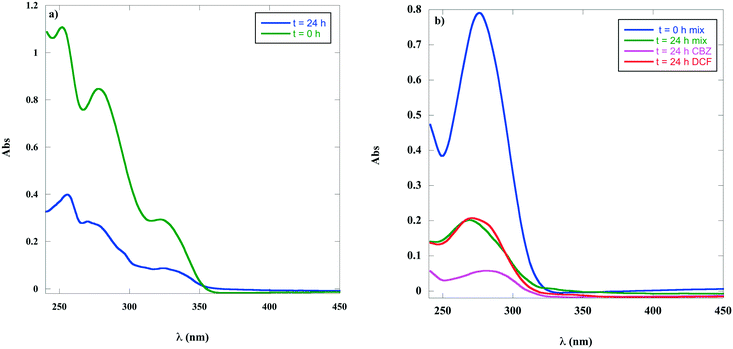 | ||
| Fig. 6 UV spectra at t = 0 and after 24 h of contact with graphite/PF6 of a) CBZ, DCF and NDA mixture, b) CBZ and DCF mixture and of single PhACs at 24 h. | ||
At the end of the contact time, graphite/PF6 showed a good adsorption efficiency for NXA (71%), which was higher than the one measured for the solution of the pure PhAC (46%) and a modest removal ability for CBZ and DFC, which was found to be globally equal to 58% (Table S6†). The above value was slightly higher than the one detected for DFC alone (45%), but significantly lower with respect to the RE measured for a single solution of CBZ (90%).
We are aware of the fact that, in the mixture, multiple interactions can occur among PhACs, not only on the grounds of their aromatic nature that can favour the occurrence of π–π interactions, but also on the basis of their acidic or basic nature that can induce a neutralization process or the formation of ion pairs.
To have more insights on the nature of the interactions occurring between acidic and basic PhACs, binary mixtures NXA/CBZ and NXA/DFC were cast on the graphite/PF6 gel. Unfortunately, in both cases after 24 h, the UV band at 280 nm, corresponding to basic PhACs, is partially hidden by the second maximum of NXA at 250 nm. Consequently, the RE of basic PhACs cannot be quantified, even if the band is clearly halved with respect to the initial mixture before the contact with the gel (Fig. S4†). Differently, adsorption of NXA was evaluated from the second absorption band occurring at 316 nm and it gave RE values of 43 and 77% in the presence of DFC and CBZ, respectively. Then, with respect to the NXA solution, adsorption of this contaminant in the mixture remained practically unchanged in the presence of DFC, but significantly improved in the presence of CBZ. The above results account for the occurrence of significant interactions only between CBZ and NXA. This can be understood taking into consideration the PhAC structures. As previously stated, besides acid–base interactions, π–π stacking can also operate in the aqueous solution. These latter prove to be more significant in the presence of more extended π-systems. Consequently, in our system, they should be strengthened in the presence of CBZ which has a more extended π-surface with respect to DFC, accounting for the observed effect.
Conclusions
Combining properties of some ILGs with those of carbon materials, like graphite, graphene and graphene oxide, some HILGs were obtained, with the aim of designing new sorbent systems for the removal of PhACs from wastewater. The majority of soft materials obtained exhibited good performance towards the removal of different classes of contaminants, like antidepressants, anti-inflammatory and antibiotics.With respect to use as solid sorbents, the incorporation of carbon materials in the ILG matrix prevents their leaching in the treated wastewater. On the other hand, the IL leaching also proves low.
Among the carbon materials tested, the addition of a highly available and cheap material, like graphite, endowed the gels with a removal efficiency comparable to the one detected in the presence of more costly ones, like graphene or graphene OX. This is the reason why, according to sustainability criteria, the gel graphite/PF6 was used as the testing element to dissect all factors acting on the performance of the adsorption systems and to evaluate the application prospects of these systems.
A deep investigation of the adsorption process proved that it is driven by π–π interactions, as accounted for by the high selectivity exhibited towards CBZ, the largest π-conjugated contaminant tested in this work. Hydrogen bond interactions become relevant only in the presence of graphene OX, which shares hydrophilic groups on its surface for the interaction with efficient hydrogen bond donors PhACs, like NXA and CPX.
Analysis of sorbent systems' performance as a function of different operational parameters evidences that they work better with small volumes of diluted samples and the raise in PhAC concentration induces a parallel increase in the time needed to achieve the maximum uptake.
Different from previous reports about liquid–liquid extraction of PhACs using DESs,33 HILGs exhibited better removal efficiency under dynamic conditions with a contaminant uptake increase in parallel with the stirring rate.
Different attempts were carried out for restoring the HILGs. Among the solvents tested, selected on the grounds of GSK solvent selection guides,51 2-Me-THF gave the best results. From an environmental point of view, the key aspect of the used HILGs stands on the possibility of reusing them for 8 cycles without any intermediate washing. Interestingly, the polluted gel can be restored. The washing procedure induces a modest leaching of the IL in 2-Me-THF. However, the restored gel can be reused for at least 11 cycles and it shows better performance than the pure gel, probably as a consequence of the higher concentration of the nanocomposite. Further investigation will be performed to lower the IL leaching during the desorption process.
From an application point of view, HILGs can be used as column stationary phases. The sequential elution on three columns allowed to achieve a RE of 60% towards CBZ, in a total contact time of 15 minutes, which appears competitive with the equilibration time needed in the vial (∼5 h). Furthermore, good results were also collected when mixtures of contaminants were considered, further strengthening the application prospects of the systems analysed.
Unfortunately, comparison with other adsorbent systems is extremely hard, as hybrid materials previously studied are really different from the HILGs here reported. In addition, the PhACs used as model pollutants are quite limited. Then, this is one of the few studies in which adsorbents can be efficiently used to remove different PhACs alone or in mixture. This approach has been used for carbon nanomaterials alone,10,15 but not for hybrid supramolecular gels.
Funding sources
This study was funded by the University of Palermo FFR 2017.Conflicts of interest
There are no conflicts to declare.References
- S. S. Fiyadh, M. A. AlSaadi, W. Z. Jaafar, M. K. AlOmar, S. S. Fayaed, N. S. Mohd, L. S. Hin and A. El-Shafie, Review on heavy metal adsorption processes by carbon nanotubes, J. Cleaner Prod., 2019, 230, 783–793 CrossRef CAS.
- J. Núñez, M. Yeber, N. Cisternas, R. Thibaut, P. Medina and C. Carrasco, Application of electrocoagulation for the efficient pollutants removal to reuse the treated wastewater in the dyeing process of the textile industry, J. Hazard. Mater., 2019, 371, 705–711 CrossRef.
- C. Gadipelly, A. Pérez-González, G. D. Yadav, I. Ortiz, R. Ibáñez, V. K. Rathod and K. V. Marathe, Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse, Ind. Eng. Chem. Res., 2014, 53, 11571–11592 CrossRef CAS.
- T. A. der Beek, F.-A. Weber, A. Bergmann, S. Hickmann, I. Ebert, A. Hein and A. Küster, Pharmaceuticals in the environment—Global occurrences and perspectives, Environ. Toxicol. Chem., 2016, 35, 823–835 CrossRef.
- Z. Cai, A. D. Dwivedi, W.-N. Lee, X. Zhao, W. Liu, M. Sillanpää, D. Zhao, C.-H. Huang and J. Fu, Application of nanotechnologies for removing pharmaceutically active compounds from water: development and future trends, Environ. Sci.: Nano, 2018, 5, 27–47 RSC.
- A. B. A. Boxall, M. A. Rudd, B. W. Brooks, D. J. Caldwell, K. Choi, S. Hickmann, E. Innes, K. Ostapyk, J. P. Staveley, T. Verslycke, G. T. Ankley, K. F. Beazley, S. E. Belanger, J. P. Berninger, P. Carriquiriborde, A. Coors, P. C. Deleo, S. D. Dyer, J. F. Ericson, F. Gagné, J. P. Giesy, T. Gouin, L. Hallstrom, M. V. Karlsson, D. G. J. Larsson, J. M. Lazorchak, F. Mastrocco, A. McLaughlin, M. E. McMaster, R. D. Meyerhoff, R. Moore, J. L. Parrott, J. R. Snape, R. Murray-Smith, M. R. Servos, P. K. Sibley, J. O. Straub, N. D. Szabo, E. Topp, G. R. Tetreault, V. L. Trudeau and G. Van Der Kraak, Pharmaceuticals and personal care products in the environment: what are the big questions?, Environ. Health Perspect., 2012, 120, 1221–1229 CrossRef.
- C. Santhosh, V. Velmurugan, G. Jacob, S. K. Jeong, A. N. Grace and A. Bhatnagar, Role of nanomaterials in water treatment applications: A review, Chem. Eng. J., 2016, 306, 1116–1137 CrossRef CAS.
- A. M. Awad, R. Jalab, A. Benamor, M. S. Nasser, M. M. Ba-Abbad, M. El-Naas and A. W. Mohammad, Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview, J. Mol. Liq., 2020, 301, 112335 CrossRef CAS.
- S. Zhang, T. Shao, H. S. Kose and T. Karanfil, Adsorption of Aromatic Compounds by Carbonaceous Adsorbents: A Comparative Study on Granular Activated Carbon, Activated Carbon Fiber, and Carbon Nanotubes, Environ. Sci. Technol., 2010, 44, 6377–6383 CrossRef CAS.
- H. Zhao, X. Liu, Z. Cao, Y. Zhan, X. Shi, Y. Yang, J. Zhou and J. Xu, Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes, J. Hazard. Mater., 2016, 310, 235–245 CrossRef CAS.
- A. Ashiq, B. Sarkar, N. Adassooriya, J. Walpita, A. U. Rajapaksha, Y. S. Ok and M. Vithanage, Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media, Chemosphere, 2019, 236, 124384 CrossRef CAS.
- A. H. El-Sheikh, R. F. Qawariq and J. I. Abdelghani, Adsorption and magnetic solid-phase extraction of NSAIDs from pharmaceutical wastewater using magnetic carbon nanotubes: Effect of sorbent dimensions, magnetite loading and competitive adsorption study, Environ. Technol. Innov., 2019, 16, 100496 CrossRef.
- A. Amiri, M. Mirzaei and S. Derakhshanrad, A nanohybrid composed of polyoxotungstate and graphene oxide for dispersive micro solid-phase extraction of non-steroidal anti-inflammatory drugs prior to their quantitation by HPLC, Microchim. Acta, 2019, 186, 534 CrossRef.
- F. Yu, J. Ma and D. Bi, Enhanced adsorptive removal of selected pharmaceutical antibiotics from aqueous solution by activated graphene, Environ. Sci. Pollut. Res., 2015, 22, 4715–4724 CrossRef CAS.
- F.-f. Liu, J. Zhao, S. Wang, P. Du and B. Xing, Effects of Solution Chemistry on Adsorption of Selected Pharmaceuticals and Personal Care Products (PPCPs) by Graphenes and Carbon Nanotubes, Environ. Sci. Technol., 2014, 48, 13197–13206 CrossRef CAS.
- J. Ma, Y. Sun, M. Zhang, M. Yang, X. Gong, F. Yu and J. Zheng, Comparative Study of Graphene Hydrogels and Aerogels Reveals the Important Role of Buried Water in Pollutant Adsorption, Environ. Sci. Technol., 2017, 51, 12283–12292 CrossRef CAS.
- J. Ma, M. Yang, F. Yu and J. Zheng, Water-enhanced Removal of Ciprofloxacin from Water by Porous Graphene Hydrogel, Sci. Rep., 2015, 5, 13578 CrossRef.
- N. Kumar, H. Mittal, S. M. Alhassan and S. S. Ray, Bionanocomposite Hydrogel for the Adsorption of Dye and Reusability of Generated Waste for the Photodegradation of Ciprofloxacin: A Demonstration of the Circularity Concept for Water Purification, ACS Sustainable Chem. Eng., 2018, 6, 17011–17025 CrossRef CAS.
- G. Liu, Z. Zhu, Y. Yang, Y. Sun, F. Yu and J. Ma, Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater, Environ. Pollut., 2019, 246, 26–33 CrossRef CAS.
- S. Thakur, S. Pandey and O. A. Arotiba, Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue, Carbohydr. Polym., 2016, 153, 34–46 CrossRef CAS.
- J. Ma, Z. Jiang, J. Cao and F. Yu, Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions, Chemosphere, 2020, 242, 125188 CrossRef CAS.
- P. Terech and R. G. Weiss, Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels, Chem. Rev., 1997, 97, 3133–3160 CrossRef CAS.
- N. M. Sangeetha and U. Maitra, Supramolecular gels: Functions and uses, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- J. Y. C. Lim, S. S. Goh, S. S. Liow, K. Xue and X. J. Loh, Molecular gel sorbent materials for environmental remediation and wastewater treatment, J. Mater. Chem. A, 2019, 7, 18759–18791 RSC.
- B. O. Okesola and D. K. Smith, Applying low-molecular weight supramolecular gelators in an environmental setting – self-assembled gels as smart materials for pollutant removal, Chem. Soc. Rev., 2016, 45, 4226–4251 RSC.
- P. C. Marr and A. C. Marr, Ionic liquid gel materials: applications in green and sustainable chemistry, Green Chem., 2016, 18, 105–128 RSC.
- F. Billeci, F. D'Anna, H. Q. N. Gunaratne, N. V. Plechkova and K. R. Seddon, “Sweet” ionic liquid gels: materials for sweetening of fuels, Green Chem., 2018, 20, 4260–4276 RSC.
- S. Marullo, A. Meli, N. T. Dintcheva, G. Infurna, C. Rizzo and F. D'Anna, Environmentally Friendly Eutectogels Comprising l-amino Acids and Deep Eutectic Solvents: Efficient Materials for Wastewater Treatment, ChemPlusChem, 2020, 85, 301–311 CrossRef CAS.
- C. Rizzo, S. Marullo, P. R. Campodonico, I. Pibiri, N. T. Dintcheva, R. Noto, D. Millan and F. D'Anna, Self-Sustaining Supramolecular Ionic Liquid Gels for Dye Adsorption, ACS Sustainable Chem. Eng., 2018, 6, 12453–12462 CrossRef CAS.
- M. Kuddushi, J. Mata and N. Malek, Self-Sustainable, self-healable, Load Bearable and Moldable stimuli responsive ionogel for the Selective Removal of Anionic Dyes from aqueous medium, J. Mol. Liq., 2020, 298, 112048 CrossRef CAS.
- N. Cheng, Q. Hu, Y. Guo, Y. Wang and L. Yu, Efficient and Selective Removal of Dyes Using Imidazolium-Based Supramolecular Gels, ACS Appl. Mater. Interfaces, 2015, 7, 10258–10265 CrossRef CAS.
- P. McNeice, A. Reid, H. T. Imam, C. McDonagh, J. D. Walby, T. J. Collins, A. C. Marr and P. C. Marr, Designing Materials for Aqueous Catalysis: Ionic Liquid Gel and Silica Sphere Entrapped Iron-TAML Catalysts for Oxidative Degradation of Dyes, Environ. Sci. Technol., 2020, 54, 14026–14035 CrossRef.
- C. Florindo, F. Lima, L. C. Branco and I. M. Marrucho, Hydrophobic Deep Eutectic Solvents: A Circular Approach to Purify Water Contaminated with Ciprofloxacin, ACS Sustainable Chem. Eng., 2019, 7, 14739–14746 CrossRef CAS.
- C. Rizzo, S. Marullo, N. T. Dintcheva and F. D'Anna, Carbon Nanomaterial Doped Ionic Liquid Gels for the Removal of Pharmaceutically Active Compounds from Water, Molecules, 2019, 24, 2788 CrossRef.
- C. Rizzo, J. L. Andrews, J. W. Steed and F. D'Anna, Carbohydrate-supramolecular gels: Adsorbents for chromium(VI) removal from wastewater, J. Colloid Interface Sci., 2019, 548, 184–196 CrossRef CAS.
- S. P. F. Costa, A. M. O. Azevedo, P. C. A. G. Pinto and M. L. M. F. S. Saraiva, Environmental Impact of Ionic Liquids: Recent Advances in (Eco)toxicology and (Bio)degradability, ChemSusChem, 2017, 10, 2321–2347 CrossRef CAS.
- H. Kitano, K. Tachimoto and Y. Anraku, Functionalization of single-walled carbon nanotube by the covalent modification with polymer chains, J. Colloid Interface Sci., 2007, 306, 28–33 CrossRef CAS.
- C. Rizzo, F. Arcudi, L. Đorđević, N. T. Dintcheva, R. Noto, F. D'Anna and M. Prato, Nitrogen-Doped Carbon Nanodots-Ionogels: Preparation, Characterization, and Radical Scavenging Activity, ACS Nano, 2018, 12, 1296–1305 CrossRef CAS.
- S. Bhattacharya and S. K. Samanta, Soft-Nanocomposites of Nanoparticles and Nanocarbons with Supramolecular and Polymer Gels and Their Applications, Chem. Rev., 2016, 116, 11967–12028 CrossRef CAS.
- Y. Chen, L. Chen, H. Bai and L. Li, Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification, J. Mater. Chem. A, 2013, 1, 1992–2001 RSC.
- L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Solvent–solute interactions in ionic liquids, Phys. Chem. Chem. Phys., 2003, 5, 2790–2794 RSC.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation, Green Chem., 2001, 3, 156–164 RSC.
- A. J. McLean, M. J. Muldoon, C. M. Gordon and I. R. Dunkin, Bimolecular rate constants for diffusion in ionic liquids, Chem. Commun., 2002, 1880–1881 RSC.
- X.-T. Wang, D.-M. Yue, M.-H. Zong and W.-Y. Lou, Use of Ionic Liquid To Significantly Improve Asymmetric Reduction of Ethyl Acetoacetate Catalyzed by Acetobacter sp. CCTCC M209061 Cells, Ind. Eng. Chem. Res., 2013, 52, 12550–12558 CrossRef CAS.
- H. Pfruender, M. Amidjojo, F. Hang and D. Weuster-Botz, Production of Lactobacillus kefir cells for asymmetric synthesis of a 3,5-dihydroxycarboxylate, Appl. Microbiol. Biotechnol., 2005, 67, 619–622 CrossRef CAS.
- M. Matsumoto, K. Mochiduki and K. Kondo, Toxicity of ionic liquids and organic solvents to lactic acid-producing bacteria, J. Biosci. Bioeng., 2004, 98, 344–347 CrossRef CAS.
- H. Pfruender, R. Jones and D. Weuster-Botz, Water immiscible ionic liquids as solvents for whole cell biocatalysis, J. Biotechnol., 2006, 124, 182–190 CrossRef CAS.
- K. S. Egorova and V. P. Ananikov, Ionic liquids in whole-cell biocatalysis: a compromise between toxicity and efficiency, Biophys. Rev., 2018, 10, 881–900 CrossRef CAS.
- B.-B. Zhang, J. Cheng, W.-Y. Lou, P. Wang and M.-H. Zong, Efficient anti-Prelog enantioselective reduction of acetyltrimethylsilane to (R)-1-trimethylsilylethanol by immobilized Candida parapsilosis CCTCC M203011 cells in ionic liquid-based biphasic systems, Microb. Cell Fact., 2012, 11, 108 CrossRef CAS.
- D. S. Zampieri, B. R. S. de Paula, L. A. Zampieri, J. A. Vale, J. A. R. Rodrigues and P. J. S. Moran, Enhancements of enantio and diastereoselectivities in reduction of (Z)-3-halo-4-phenyl-3-buten-2-one mediated by microorganisms in ionic liquid/water biphasic system, J. Mol. Catal. B: Enzym., 2013, 85–86, 61–64 CrossRef CAS.
- R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Expanding GSK's solvent selection guide – embedding sustainability into solvent selection starting at medicinal chemistry, Green Chem., 2011, 13, 854–862 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en01042a |
| This journal is © The Royal Society of Chemistry 2021 |