Open Access Article
Open Access ArticleInvestigating the origin and tissue concentration of polycyclic aromatic hydrocarbons in seafood and health risk in Niger Delta, Nigeria
Udeme Sunday
Udofia
a,
Charles
Ameh
b,
Eula
Miller
c and
Mandu Stephen
Ekpenyong
 *c
*c
aDepartment of Food Science and Technology, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria
bCollege of Applied Food Sciences and Tourism, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria
cDepartment of Nursing, Manchester Metropolitan University, Manchester, M15 6GX, UK. E-mail: M.Ekpenyong@mmu.ac.uk
First published on 18th October 2021
Abstract
The origin, tissue concentration, and health risk of polycyclic aromatic hydrocarbons (PAHs) contaminants in three economically important species of seafood, including catfish (Chrysichthys nigrodigitatus), prawns (Macrobrachium macrobrachium), and periwinkles (Tympanotonus fuscatus) from the crude oil-impacted Niger Delta region, were investigated. The concentrations of PAHs were measured by coupled gas chromatography-mass spectrometry after repeated extraction by ultrasonication in hexane and cleaning up in silica gel. The origin of PAHs was deduced using established mathematical protocols. Health risk from the consumption of contaminated seafood was evaluated for 60 kg bodyweight individuals at a fish consumption rate of 36.94 g per person per day. Different tissue concentrations of PAHs residues at low, moderate, and chronic levels were revealed. Mean total PAHs varied from 4.55 to 6.36 mg kg−1 in catfish, 4.61 to 7.75 mg kg−1 in prawns, and 4.91 to 6.14 mg kg−1 in periwinkles. The tissue concentrations were high above PM2.5, enough to suspect PAHs-related health risk, especially among residents who consume a large quantity of seafood. Carcinogenic PAHs index, benzo[a]pyrene, varied from below instrument detection (<0.01) to 0.29 mg kg−1. The estimated carcinogenic potency equivalent concentrations (PEC) of PAH varied from 0.653 to 2.153 above the screening value (SV), 0.01624 in the three species investigated. Mathematical evaluation and dominant tissue concentration of high molecular weight PAHs in all the seafood investigated showed pyrogenic origin of PAHs.
Environmental significanceThe concentration of carcinogenic polycyclic aromatic hydrocarbons (PAHs) in body tissues, as revealed by the mass spectroscopic evaluation of seafood in Niger Delta, is chronically high above the legal limit considered safe for human consumption. The potential health risk of PAHs exposure is cancer. Food consumption has been shown to be the main source of polycyclic aromatic hydrocarbon (PAHs) intake, thus highlighting the importance of research on PAHs in food and the development of mitigation strategies to reduce their contents in food. Unfortunately, there is inadequate reporting of data in Africa regarding tissue concentration and health risk from the ingestion of PAHs contaminated food. Information on the origin and their formation in seafood is also lacking, thus limiting mitigation options to prevent risk to humans and aquatic animals. Also, the nonexistence of a threshold for the cancer end point and the duration for actual manifestation of cancer in humans, especially from dietary exposure, need further study. The absence of a database on cancer demography in Nigeria also limits investigation on the spread and formation; thus, efforts to provide a standard regulatory agency guideline to be updated and integrated into state regulatory processes through routine training programs are difficult. The present study has helped to bridge the knowledge-gap in the origin, tissue concentration, and health risk from the consumption of PAHs-contaminated seafood in the Niger Delta region of Nigeria. |
Introduction
The Niger Delta (N'Delta) region of Nigeria is predominantly coastal with vast rural communities. Due to decades of crude oil exploration, almost the entire ecosystem has been substantially degraded by petroleum. Oil spills, gas flaring, illegal bunkering activities, and refining of crude oil locally known as ‘kpo’ fire are almost unavoidable. Cleanup efforts are often inadequate, resulting in the loss of delicate ecosystems as well as fisheries and farmland. The luxuriant mangrove forests, swamps, and rivers that once supported healthy human life and vibrant ecosystem have been disastrously impacted, leaving the rural peasant farmers with no alternative means of livelihood. As an effect, land, water, plants, animals, and fish are polluted and have been rendered unsafe for human consumption despite their many benefits. Food consumption has been shown to be the main source of polycyclic aromatic hydrocarbon (PAHs) intake, thus highlighting the importance of research on PAHs in food and the development of mitigation strategies to reduce their contents in food.1 Extensive studies on oil spills and the occurrence of PAHs in different environmental media including fish and shellfish have been reported in the N'Delta region.2,3 Unfortunately, only very few studies have highlighted the public health consequences of exposure to PAHs. Negative concomitant effects of oil spills on fish production in the N'Delta region from 1981 to 2015 has been reported.4 The observed increased concentration of total PAHs in the tissues of Littorina littorea, Crassostrea virginica, and Periophthalmus koeleuteri has also been reported.3 Also, varying concentrations of PAHs in fish tissues and health risks in the N'Delta region of Nigeria have been reported for C. gariepinus, T. zilli, E. fimbriata, and S. scombrus.5–8Polycyclic aromatic hydrocarbons (PAHs), a group of complex hydrocarbons that are formed during the incomplete burning of oil, gas, coal, wood, garbage, or other organic substances, such as tobacco and charbroiled meat,9 are present in petroleum and constitute hazardous components of oil spills in the marine ecosystem.
Seafood quality is associated with marine environment quality. Seafood and fish can be tainted and contaminated from being exposed to PAHs present in water and sediments due to atmospheric pollution or oil spills. When PAHs and NPAHs (nitro-polycyclic aromatic hydrocarbons) are contained within particulate matter with an aerodynamic diameter of 2.5 μm (PM2.5) or less, the particulate matter is categorized as a group 1 contaminant (carcinogenic to humans).10 Under the safe drinking water Act, the U.S. Environmental Protection Agency set legal maximum limits of polycyclic aromatic hydrocarbons on the level of benzo[a]pyrene in drinking water (0.2 μg L−1).11
The carcinogenic effect of PAHs has been adequately tested on experimental animals with benzo[a]pyrene (PAH marker) using dietary administration. The levels of PAHs in foods are reduced to as low as reasonably achievable (ALARA) following the nonexistence of threshold effects.1 In 2008, benzo[a]pyrene was considered an inadequate marker of PAH contamination in food; rather, the sum of benzo[a]pyrene, benzo[a]anthracene, chrysene, and benzo[b]fluoranthene (collectively called PAH4) was adopted as a suitable indicator of the total PAHs contamination in food12 and the maximum legal limits set. Naturally, the average background PAH in uncooked food (including fish) is usually 0.01 to 1 μg kg−1.13
Oil spills, gas flaring, and well fires in the N'Delta region are almost unavoidable with enormous consequences. At present, there are about 606 oilfields (355 onshore and 251 offshore),14 and over 178 flare points9 in the N'Delta region, which collectively flare about 1 billion standard cubic feet (SCF) of gas with about 65% of the flare points located offshore.15 Daily, about 45.8 billion kilowatts of heat is discharged into the atmosphere from 1.8 billion cubic feet of gas in the N'Delta region, leading to temperatures that render large areas uninhabitable.16 Frequent pipeline leakages and vandalism have resulted in the burning of viable mangrove habitat and vegetation, and engulfed animals within the area.17 The United Nations Development Programme18 estimated that Nigeria discharges 75% of the gas it produces more than any other country in the world, significantly contributing to hydrocarbon pollution.
Although human health has not been considered to be at risk, the possible consequences of bioaccumulation in body tissues cannot be ignored, especially in coastal communities where the consumption and exposure of aquatic lives to oil spills is more pronounced. Polycyclic aromatic hydrocarbons readily accumulate in the fatty tissues of fish following uptake and the lipophilic nature of PAHs.19 The primary endpoint considered when studying the potential health risk of hydrocarbons is cancer. Human exposure to PAHs has been associated with increased risk of cancer in several tissues such as lung, bladder, stomach, and skin depending on the mode of exposure and the form of PAH.20 Also, elevated levels of cancer have been reported in populations close to oil fields than those far from them.21 Therefore, maintaining clean environmental quality is crucial for several socio-economic reasons.22 For instance, loss of confidence in seafood safety can impact the seafood market.
The importance of seafood safety cannot be ignored in international fish trade especially with its recent expansion in the world market.23 Fish is an important part of household diet in many countries around the world. In Nigeria, fish makes up about 40% of the country's protein intake, with household consumption at 13.3 kg per capita (36.94 g per person per day),24 compared with the world's average of 20.3 kg per capita per year.24 More than 80% of Nigeria's total domestic fish are produced by artisanal small-scale fishers from the coastal communities of N'Delta.24 Polycyclic aromatic hydrocarbons have been extensively researched in foods and environmental media but very few in seafood. Furthermore, literature is available mostly for the investigation of PAHs in developed countries but not much in Africa. The few studies reported on the concentration of PAHs in seafood25 are on smoked fish. Information on the origin, tissue concentration, and health risk associated with PAHs and their formation in seafood is lacking. Due to the inadequate reporting of data, the present study, therefore, sought to provide reliable data on PAHs in seafood with regards to tissue concentration, origin, and health risk in the N'Delta region, with the view of providing mitigation options that will prevent risk to humans and aquatic animals. The nonexistence of a threshold for the cancer end point and the duration for the manifestation of PAHs-related cancer, especially from dietary exposure, should be investigated. A reliable public-based cancer demography in Nigeria needs to be studied to understand the spread and the possible causes.
Materials and methods
Study area
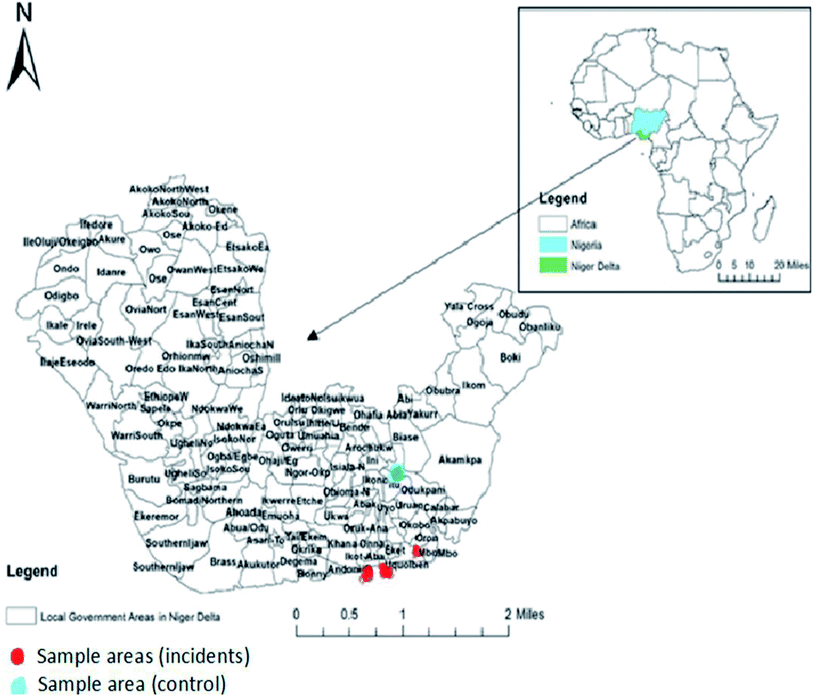
The area under study lies within the coastal area of Akwa Ibom State in N'Delta, which is the Southern coastal morphological unit in Nigeria. This coastal system lies between longitude 07° 35′ 00′′E and longitude 8° 30′ 20′′E (Fig. 1 and 2). The sampling stations were selected from crude oil impacted rivers (estuaries) of ‘Iko’ in Eastern Obolo Local Govt. Area, ‘Mkpanak’ in Ibeno Local Govt. Area, and ‘Ibaka’ in Mbo Local Govt. Area, and one reference river of ‘Ayadehe’ in Itu Local Govt. Area. The study area is characterized by ‘pronounced’ fishing activities and history of frequent oil spill incidents from offshore oil activities and gas flaring; hence it was chosen for this study (Table 1). The three species of seafood selected were based on economic importance, habitat utilization, and feeding strategies.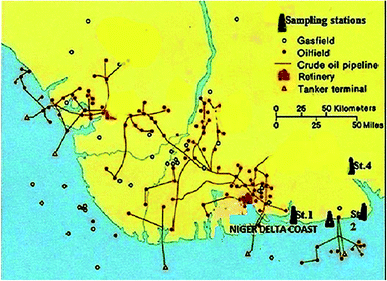 | ||
| Fig. 1 Map of the Niger Delta coastal area showing oil and gas fields and sampling stations (about 606 oilfields – 355 onshore, 251 offshore, and 178 gas flare points). Source: Nigerian oil and gas (1997) and Anifowose et al.,14 (2014). | ||
| Stations | Sample codes | Coordinates | Landmark features |
|---|---|---|---|
| a Sample identification (samples with codes 1, 2, and 3 are from incident zones, while codes 4 are from the control zones): UC1, UC2, UC3, UC4 – Catfish, US1, US2, US3, US4 – Periwinkle, UP1, UP2, UP3, UP4 – Prawn. | |||
| Station 1 | |||
| Iko River (Eastern Obolo LGA) | UC1 | N 04° 31′ 18.6′′ | Oil wells and oil pipelines |
| UP1 | E 007° 45′ 15.9′′ | Commercial fishing | |
| US1 | 19 m above sea level | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Station 2 | |||
| Mkpanak river (Ibeno LGA) | UC2 | N 04° 33′ 22.3′′ | Mobil oil operation. Qua |
| UP2 | E 007° 57′ 12.9′′ | Iboe oil terminal | |
| US2 | 19 m above sea level | Oil pipelines and wells | |
| Commercial fishing | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Station 3 | |||
| Ibaka river (Mbo LGA) | UC3 | N 04° 38′ 19.8′′ | Commercial fishing |
| UP3 | E 008° 18′ 32.5′′ | Petrol platform | |
| US3 | 18 m above sea level | Oil pipelines | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Station 4 | |||
| Ayadehe river (Itu LGA) | UC4 | N 05° 10′ 42.2′′ | Commercial fishing |
| UP4 | E 008° 03′ 54.1′′ | Local market | |
| US4 | 21 m above sea level | ||
Samples collection
A total of 120 mature and marketable sizes seafood samples were collected randomly, 30 samples per zone, from different sampling points across 4 zones using fishing nets and baskets for this investigation. This number of samples was thought to be sufficient, considering the absence of an official sample size for different fish species in the study area and the potential variability of PAH toxicity to individual fish species. The length of fish, measured from the tip of the mouth to the tail end with a ruler, was found to be 40 ± 0.50 cm for catfish, 16 ± 0.20 cm for prawns, and 4 ± 0.20 cm for periwinkles. The corresponding masses were 1.1 ± 0.10 kg for catfish, 0.09 ± 0.02 kg for prawns, and 0.01 ± 0.01 kg for periwinkles. UC1, UC2, UC3 are catfish from incident zones while UC4 are catfish from the control zone. All samples were preserved separately in pollution-free icepack coolers and taken to the laboratory for various analyses “as-is”.Determining the lipid content of tissues
The lipid content of each sample was determined in two steps. The lipids were first extracted from samples by continuous solvent extraction using a Soxhlet apparatus and then quantified by gravimetric analysis as follows.23 In brief, about 5 g of each sample, wrapped in filter paper (Whatman), was extracted continuously using petroleum ether (200 mL) over 4 h. The residue was removed and reserved for crude fiber analysis. The extract was concentrated to dryness and dried further in an oven for 3 min at 60 °C. The dry samples were then cooled to room temperature in a desiccator, reweighed a few times until a constant mass was achieved, and then the mass of the lipids was determined by difference of mass as follows.| % Lipids = ((W2 − W1)/(weight of sample)) × 100% | (1) |
Determination of tissue moisture content
The moisture content of the tissues was determined according to a previously published method.16 In brief, 5 g of each sample was dried in a moisture can of known mass at 105 °C in an oven for 3 h initially, then to constant mass thereafter. Mass loss due to moisture was then determined as percent difference. Thus,| % Lipids = ((W2 − W3)/(W2 − W1)) × 100% | (2) |
Determination of tissue PAHs concentration
Sample preparation, extraction, and cleanup
All samples were processed under fume hoods to limit exposure to exogenous sources. Glassware were washed before use with n-hexane and dried in an oven at 105 °C. Other materials were previously washed with ultrapure water and acetone. Before extraction, the seafood samples were removed from the icepack coolers, thawed, and cleaned separately under tap water, and the muscular tissues were removed and ground for homogeneity using a mortar and pestle. About 10 g of each homogenized seafood sample was extracted with 30 mL hexane and DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) by the sonication bath method at 35 °C for 25 min. The cleanup process as described in the United States Environmental Protection Agency (USEPA) method 3630C was employed. Exactly 250 mL of the extract sample was spiked with 1 mL of the surrogate standard p-terphenyl-d14 (2 ng μL−1), and 30 mL of DCM was added. The sample was agitated in a digital shaker for 6 h, followed by shaking in an ultrasonic bath for a further 5 min to extract any organic pollutants that may have been adsorbed on to the wall of the flask.
1, v/v) by the sonication bath method at 35 °C for 25 min. The cleanup process as described in the United States Environmental Protection Agency (USEPA) method 3630C was employed. Exactly 250 mL of the extract sample was spiked with 1 mL of the surrogate standard p-terphenyl-d14 (2 ng μL−1), and 30 mL of DCM was added. The sample was agitated in a digital shaker for 6 h, followed by shaking in an ultrasonic bath for a further 5 min to extract any organic pollutants that may have been adsorbed on to the wall of the flask.
The mixture was then transferred into a separation funnel and left for 5 min to separate the water from the organic solvent layer. The bottom DCM layer containing the hydrocarbons was decanted into a 250 mL round-bottomed flask, and the extraction was repeated three times. The combined extract was then allowed to mix with granule-activated copper overnight to remove any sulphur contaminants, the extract was passed through a glass column containing 5 g anhydrous Na2SO4 (activated at 400 °C for 4 h before use) to remove any residual water, and then concentrated to 3 mL using a rotary evaporator (RE52-1, PEC Medical USA). Cyclohexane (10 mL) was then added as an exchange solvent, and the extract was concentrated to 2 mL by rotary evaporator. The extract was then passed through a glass column containing 5 g activated silica gel (previously activated by heating at 200 °C for 16 h before use) and 1 g of anhydrous Na2SO4 to clean up all non-hydrocarbons.
The PAH fractions were then eluted at the rate of 2 mL min−1 using a 30 mL mixture of DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pentane (2
pentane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). Next, elution was done using a 20 mL mixture of DCM
3 v/v). Next, elution was done using a 20 mL mixture of DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pentane (2
pentane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). The combined extract was concentrated to 2 mL using a rotary evaporator. The concentrated extract was finally reduced to 1 mL under a gentle stream of nitrogen gas. All the sample extracts were kept in amber glass vials at −4 °C until analyzed according to 1.1 of US EPA Method 8270 (PAH only): GC analysis of PAHs on SLB®-5ms26 and using an Agilent 7890/5975C GC/MSD, which was previously calibrated with PAH standards under specific conditions.
3 v/v). The combined extract was concentrated to 2 mL using a rotary evaporator. The concentrated extract was finally reduced to 1 mL under a gentle stream of nitrogen gas. All the sample extracts were kept in amber glass vials at −4 °C until analyzed according to 1.1 of US EPA Method 8270 (PAH only): GC analysis of PAHs on SLB®-5ms26 and using an Agilent 7890/5975C GC/MSD, which was previously calibrated with PAH standards under specific conditions.
Analyses of sample extract
Briefly, the MSD was first test run and standardized with DCM blank (1 μL). Then, 1 μL of the extract was injected into the injection port. A fused silica TR-5ms capillary column (30 m × 0.25 mm i.d.) with a film thickness of 0.25 μm (Thermo Fisher, USA) was used in the column separation. High-purity helium (99.9%) was used as the carrier gas, makeup gas, and purge gas at flow rates of 0.8, 35, and 30 mL min−1, respectively. The gas chromatogram was operated in split-less mode and separation was conducted with the oven temperature programmed as follows: initial setting at 50 °C (2 min hold), ramped to 310 °C at 24 °C min−1 (for 2 min), and, finally, to 320 °C at 5 °C min−1 (10 min hold). The injector was held at 250 °C and the detector maintained at 340 °C. The external standard calibration comprising 17 PAH standards was used to extrapolate the identity and quantity of each component peak in the sample chromatogram. In the integration events, the following conditions were ensured: slope sensitivity (3.7936), peak width (0.4248), area reject (3.1407), height reject (0.4037), and shoulders (off).Analytical quality control
A 2000 μg mL−1 stock solution of the 17 PAHs mixture was used for external calibrations. To determine the efficiency of extraction for the target compounds, recovery studies were conducted by introducing known concentrations of standard PAH mixtures to selected analyzed samples and the whole procedure of analysis from extraction to cleanup was repeated. The matrix effect was evaluated by spiking the fish extract sample in the concentration range (2, 10, 50, 100, and 500 μg mL−1) used for calibration. PAH working (spiked) standards, surrogate standards, and internal standard solutions were included in each batch of samples to ensure the integrity of the analytical method and the corresponding results. The limit of detection (LOD) and limit of quantitation (LOQ) were estimated according to the following equations.| LOD = Xb1 + 3Sb1, |
| LOQ = Xb1 + 10Sb1, |
Determination of PAHs origin
The origin of tissue PAHs in this study was established by subjecting the data to mathematical evaluation. Thus, the ratio of the concentration of phenanthrene/anthracene (Phe/Ant) > 10 indicates petrogenic origin of PAHs, while the concentration of Phe/Ant < 10 indicates pyrogenic origin of PAHs.28 The ratio of fluoranthene/pyrene (Flur/Py) > 1 indicates pyrogenic origin, while Flur/Py < 1 indicates petrogenic origin. The ratio of low molecular weight/high molecular weight (LMW/HMW) PAHs >1 indicates petrogenic origin, while LMW/HMW < 1 indicates pyrogenic origin.29 The ratio of Ant/(Ant + Phe) < 0.1 indicates petrogenic origin, while Ant/(Ant + Phe) > 0.1 indicates pyrogenic origin; BaA/(BaA + Chr) < 0.2 indicates petrogenic origin, while BaA/(BaA + Chr) between 0.2 to 0.35 indicates either pyrogenic or petrogenic origin, and BaA/(BaA + Chr) > 0.35 indicates pyrogenic origin.30Statistical analysis of data
Triplicate measurements were done and the results were reported as mean and standard deviation. Data generated from the study were subjected to analysis of variance (ANOVA) and Tukey–Kramer multiple comparison tests to determine if there was any statistically significant difference for the PAH concentrations within and between the samples means at 5% confidence level. Differences with p < 0.05 were considered to be statistically significant. All statistical analyses were performed with SPSS software version 16.0 (SPSS Inc., Chicago).Health risk from the consumption of PAHs-contaminated fish
The United State Environmental Protection Agency (USEPA) guidelines31 were used21 for determining the human health risk from the ingestion of PAHs-contaminated fish. By this method, benzo[a]pyrene is used as a marker for the occurrence and effect of carcinogenic PAHs in foods, and the overall carcinogenic health risk from the measured PAHs was estimated based on the toxicity equivalence factors (TEFs) derived from the cancer potencies of individual PAH compounds relative to the cancer potency of benzo[a]pyrene.The product of the PAH concentration (μg g−1) and its TEF gives a benzo[a]pyrene equivalent concentration (BaPeq) for each PAH. All the individual benzo[a]pyrene equivalents were summed up to give a carcinogenic potency equivalent concentration (PEC) of all the PAHs according to eqn (3).32
| PEC = total ∑(TEF × concentration) | (3) |
Potency equivalent concentration values were then compared with a screening value for carcinogenic PAHs. The screening value is calculated from eqn (4).
| SV = [(RL/OSF) × BW]/CR | (4) |
Screening value (SV) is the threshold concentration of total PAHs/or chemicals in edible fish tissue that is of potential public health concern; BW is the average human body weight (g) and was set to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g (60 kg) for the adult population; CR is the consumption rate (g per day). The fish consumption rate was set at 36.94 g per person per day.33 RL is the maximum acceptable risk level (dimensionless), and was set to 1 × 10−5 (U.S. EPA, 2000) so that the maximum risk would be one additional cancer death per 100
000 g (60 kg) for the adult population; CR is the consumption rate (g per day). The fish consumption rate was set at 36.94 g per person per day.33 RL is the maximum acceptable risk level (dimensionless), and was set to 1 × 10−5 (U.S. EPA, 2000) so that the maximum risk would be one additional cancer death per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons if an adult weighing 60 kg consumed 36.94 g of fish daily with the same measured concentrations of PAHs for 70 years; OSF (oral slope factor) is an estimate of the increased cancer risk from oral exposure to a dose (of carcinogenic PAHs) of 1 mg per kg per day for a lifetime of 70 years. The OSF can be multiplied by an estimate of lifetime exposure (in mg per kg per day) to estimate the lifetime cancer risk.33
000 persons if an adult weighing 60 kg consumed 36.94 g of fish daily with the same measured concentrations of PAHs for 70 years; OSF (oral slope factor) is an estimate of the increased cancer risk from oral exposure to a dose (of carcinogenic PAHs) of 1 mg per kg per day for a lifetime of 70 years. The OSF can be multiplied by an estimate of lifetime exposure (in mg per kg per day) to estimate the lifetime cancer risk.33
Results and discussion
PAHs concentration in tissues
The occurrence of PAHs in seafood tissues is an indication of contamination in coastal water in the study area. The present study showed varying concentration of PAHs, lipids, and moisture content in the tissues of seafood investigated. The spike recoveries of PAHs ranged from 70 to 92% and the limit of detection of PAHs varied from < 0.01 to < 0.03 mg kg−1 fw (Table 5).The method adopted for tissue PAHs measurement in seafood produced characteristics that met the requirement of the European Union Commission Regulation 836/2011 (recovery between 50 to 120%) for the PAH4 group (benzo[a]pyrene, benzo[a]anthracene, benzo[b]fluoranthene, and chrysene). PAH concentrations in the present study were compared with previously observed PAH in fish and with legal maximum limits set by EU via Commission Regulation No. 835/2011, Commission Regulation No. 1881/2006 (as amended) for polycyclic aromatic hydrocarbons in certain foodstuffs including 6.0 μg kg−1 fw for benzo[a]pyrene, 35.0 μg kg−1 fw for the sum of PAH4 in smoked bivalve molluscs, 2.0 μg kg−1 fw for benzo[a]pyrene for the muscle meat of smoked fish and smoked fishery products, 12.0 μg kg−1 fw for sum of PAH4 for smoked crustaceans, and 2 μg kg−1 fw for benzo[a]pyrene in fish, which are considered safe for human consumption.34 Also, the WHO maximum permissible limit of PAHs in fish and shellfish, i.e., 0.001 μg g−1,35 was compared.
The present study showed significant (p < 0.05) differences in the tissue concentration of PAHs in catfish (Table 2), prawns (Table 3), and periwinkles (Table 4). The total concentration of PAHs (∑PAHs) in the tissues of catfish, periwinkles, and prawns from the control zones (UC4, US4, and UP4) were higher than the total concentration in samples from incident zones. This suggested sources other than the suspected oil spills for the PAHs contamination of seafood. There was elevated concentration of PAHs in prawns' tissue than in catfish or periwinkles tissues across the zones. The concentrations of PAHs in the fish samples did not follow any particular trend across the zones. The absence or rather low detection of certain PAHs in the seafood tissues may be attributed to their rapid depuration or biotransformation. The concentration of PAHs observed in catfish, prawn, and periwinkles significantly exceeded the average background PAH (0.01 μg kg−1 to 1.0 μg kg−1) in uncooked foods1 including seafood. The concentration of PAH markers (benzo[a]pyrene and sum of the PAH4 group) varied significantly higher in prawns (0.03 to 0.14 mg kg−1 fw for benzo[a]pyrene and 0.1 to 0.12 mg kg−1 fw for the sum of PAH4) than the EU regulatory limit of maximum level of 12.0 μg kg−1 fw for the sum of PAH4 for smoked crustaceans in Commission Regulation (EU) No 835/2011. The EU legal limit for benzo[a]pyrene for fish considered safe for human consumption is 2 μg kg−1 (0.002 mg kg−1) fw. This level of concentration of PAH markers in the seafood tissues of the present study far exceeded the maximum limit set by EU Commission Regulation 1881/2006 (as amended) for PAHs in some foods. Elevated levels of indeno[1,2,3-c,d]pyrene than other PAH congeners was observed in all the samples across the zones. In a similar study, Nwaichi and Ntorgbo also observed elevated levels of indeno[1,2,3-c,d]pyrene levels in Crassostrea virginica, Littorina littorea, and Periophthalmus koeleuteri from Sime, Iko, and Kporghor locations of N'Delta, which exceeded the European Union (EU) limit of 5 μg kg−1 wet wt.3 Indeno[1,2,3-c,d]pyrene is one of the most suitable indicators for PAHs contamination in food.1 Following a single topical application, 100 μg benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, and indeno[1,2,3-c,d]pyrene were reported to bind to DNA in CD-l mouse skin (Weyand et al. 1987).48 The relative extent of binding was benzo[b]fluoranthene > benzo[j]fluoranthene > benzo[k]fluoranthene > indeno[1,2,3-c,d]pyrene. The covalent binding of chemicals to DNA can result in strand breaks and DNA damage, ultimately leading to mutations. The incidence of forestomach tumors (papillomas and carcinomas) in mice was reported to relate to the duration of oral exposure to benzo[a]pyrene, following intermediate-duration administration of dietary benzo[a]pyrene at various doses up to 250 ppm (33.3 mg per kg per day) for 30–197 days (Neal and Rigdon 1967).49 Dominant concentration of high molecular weight (HMW) PAHs was also observed in all the seafood samples analyzed. The findings from the present study showed increased total PAHs in seafood than those by3 WHO reported mean total PAHs levels in the tissues of fish in the N'Delta region ranging from below detection (ND) limit to 22.400 μg kg−1 fw in Littorina littorea, ND to 87.400 μg kg−1 fw in Crassostrea virginica and from ND to 171.000 μg kg−1 fw in Periophthalmus koeleuteri. High molecular weight (HMW) PAHs were also found to be predominant in samples compared to low molecular weight (LMW) PAHs. Higher tissue levels of PAHs were also reported by ref. 5–8. The observed PAHs levels in the present study, however, exceeded the reported measurable low levels in fresh fish and seafood studied in some communities of N'Delta.36 The most frequently detected PAHs in all the seafood tissues analyzed were dibenz[a,h]anthracene, benzo[g,h,i]perylene, indeno[1,2,3-c,d] pyrene, benzo[a]pyrene, acenaphthene, and 2-methylnaphthalene. The total PAHs (∑PAHs) concentrations reported in the present study are within the range reported in similar studies around the world, including 0.432 to 14.939 mg kg−1 dry wt. for various seafood species in the Iragi marine waters,37 and 23.90 to 57.90 mg kg−1 dry wt. for fish in the Red Sea coast of Yemen.38 Common among the present and previous studies are elevated tissue concentrations of high molecular weight (HMW) PAHs than low molecular weight (LMW) PAHs in seafood. The mean tissue concentration of low molecular weight (LMW) PAHs in samples across the zones varied from below instrument detection (ND) to 0.98 mg kg−1 fw, while high molecular weight PAHs (flur, b[a]a, chry, b[b]f, b[k]f, b[a]p, db[a,h]a, and in[1,2,3-c,d]p) also varied from ND to 3.80 mg kg−1 fw in samples across zones. The study showed that seafood in the N'Delta region is chronically contaminated with total PAHs at levels (4.55 to 7.75 mg kg−1 fw) considered unsafe for human consumption. The total PAHs levels obtained in fish from this study were higher than 45.9–171.9 μg kg−1 (0.0459–0.1719 mg kg−1) reported in seafood from Niger Delta coastal waters3 and 48.75–166.79 μg kg−1 reported in fish from Ghana coastal waters.39 The PAHs levels in seafood were significantly higher than the recommended levels considered safe for human consumption by WHO and the EU. The EU recommended limit for b[a]p (PAHs marker) for fish considered safe for human consumption is 2 μg kg−1 (0.002 mg kg−1) fw. The study also showed that tissue concentrations of PAHs in all the studied seafood far exceeded the FAO/WHO guidelines for concentrations of PAHs in food (0.001 mg kg−1 fw) considered safe for human consumption.27 Lower concentrations of PAHs from non-oil impacted areas of Badagry Creek and Ologe Lagoon were observed in C. nigrodigitatus and M. macrobrachion, which were below the WHO maximum permissible limit of 0.001 μg g−1 in fish and shellfish.14 Elevated levels of ∑PAHs have been reported in seafood tissues from oil-impacted areas around the world such as 251 μg g−1 dry wt. for pearl oyster, 173 to 846 μg g−1 dry wt for rock oyster in the United Arab Emirates,40 and from 91.32 mg kg−1 to 1154.45 mg kg−1 in the Arabian Gulf.33 Also, the PAH profile in crab (Ocypode africana) samples from the shoreline of the N'Delta areas, crab droppings, Donax acutangulus, and Tympanotonus fuscatus were reported to be below the limit of detection in all the stations despite the high level of total petroleum hydrocarbon (TPH) observed in the fauna.41 The concentration of b[a]p detected in periwinkles at US1 and US2 varied from 0.07 mg kg−1 fw to 0.29 mg kg−1 fw above the EU recommended limit (0.002 mg kg−1) fw for fish considered safe for human consumption. Bivalve mollusks such as mussels and oysters can filter large volumes of water and accumulate high molecular mass PAHs but are not capable of metabolizing all PAHs efficiently.42 The absence or variation in the tissue concentrations of PAHs in seafood in the N'Delta region and around the world may be attributed to a number of factors such as route and duration of exposure, uptake capacity or lipophilicity of tissues, environmental factors, differences in species age and sex, and exposure to other xenobiotics.
| PAHs (mg kg−1 fresh wt) | Samples (N = 10) | TEFs (WHO/IPCS 1998) | |||
|---|---|---|---|---|---|
| UC1 | UC2 | UC3 | UC4 | ||
| a Results are means and standard deviations of triplicate measurements. UC1, UC2, UC3 are catfish from incident zones while UC4 are catfish from the control zone. ND = below detection limit (<0.01 to < 0.03). TEFs = Toxicity equivalent factors. MC = Moisture content. Values with different alphabet superscripts in the same rows are significantly (p < 0.05) different. | |||||
| Naphthalene | ND | ND | ND | 0.76a ± 0.00 | — |
| 2-Methylnaphthalene | 0.86a ± 0.00 | 0.18 ± 0.00c | 0.17 ± 0.01d | 0.76b ± 0.00 | — |
| Acenaphthylene | ND | ND | ND | 0.04a ± 0.00 | — |
| Acenaphthene | 0.33b ± 0.00 | 0.98a ± 0.01 | 0.98a ± 0.00 | 0.21c ± 0.01 | — |
| Fluorene | 0.05bc±0.01 | 0.06b ± 0.01 | ND | 0.81a ± 0.42 | — |
| Phenanthrene | ND | ND | ND | ND | — |
| Anthracene | ND | ND | ND | ND | — |
| Fluoranthene | ND | ND | ND | ND | — |
| Pyrene | ND | ND | ND | ND | — |
| Benz[a]anthracene | ND | ND | ND | ND | 0.1 |
| Chrysene | ND | ND | ND | ND | 0.01 |
| Benzo[b]fluoranthene | ND | ND | ND | ND | — |
| Benzo[k]fluoranthene | ND | ND | ND | ND | 0.1 |
| Benzo[a]pyrene | ND | ND | ND | ND | 1 |
| Dibenz[a,h]anthracene | 1.08a ± 0.00 | 0.64b ± 0.01 | 0.51d ± 0.00 | 0.55c ± 0.00 | 1.0 |
| Benzo[g,h,i]perylene | 0.23b ± 0.01 | 0.08c ± 0.00 | 0.23b ± 0.00 | 0.27a ± 0.00 | 0.01 |
| Indeno[1,2,3-c,d]pyrene | 2.75c ± 0.00 | 3.80a ± 0.00 | 2.66c ± 0.00 | 2.96b ± 0.00 | 0.1 |
| ∑PAHs | 5.30 | 5.74 | 4.55 | 6.36 | |
| ∑PAH4 | — | — | — | — | |
| PEC | 1.357 | 1.021 | 0.778 | 0.849 | |
| SV (μg kg −1 ) | 0.1624 | 0.1624 | 0.1624 | 0.1624 | |
| % Lipid | 2.33 a ± 1.33 | 1.47 a ± 0.47 | 2.46 a ± 0.40 | 2.57 a ± 0.49 | |
| %MC | 76.53 a ± 1.42 | 74.67 ab ± 1.80 | 72.14 b ± 1.61 | 74.89 ab ± 1.51 | |
| LMW/HMW | 0.31 | 0.27 | 0.34 | 0.68 | |
| PAHs (mg kg−1 fresh wt) | Samples (N = 10) | TEFs (WHO/IPCS 1998) | |||
|---|---|---|---|---|---|
| UP1 | UP2 | UP3 | UP4 | ||
| a Results are means and standard deviations of triplicate measurements. ND = below detection (<0.01 to < 0.03) TEFs = toxicity equivalent factors. PEC = carcinogenic potency equivalent concentration. SV = screening (threshold) value. UP1, UP2, UP3 are prawns from incident zones while UP4 are prawns from the control zone. MC = moisture content. Values with different alphabet superscripts in the same rows are significantly different. | |||||
| Naphthalene | ND | ND | ND | 1.10 ± 0.00a | — |
| 2-Methylnaphthalene | 1.21c ± 0.00 | 0.17d ± 0.00 | 1.63a ± 0.01 | 1.24b ± 0.01 | — |
| Acenaphthylene | ND | ND | 0.06b ± 0.00 | 0.13a ± 0.00 | — |
| Acenaphthene | 0.16b ± 0.00 | 0.17b ± 0.01 | 0.37a ± 0.00 | 0.12c ± 0.01 | — |
| Fluorene | 0.06c ± 0.01 | 0.22a ± 0.01 | 0.22a ± 0.00 | 0.10b ± 0.00 | — |
| Phenanthrene | 0.08a ± 0.01 | 0.09a ± 0.00 | 0.09a ± 0.00 | 0.08a ± 0.00 | — |
| Anthracene | ND | 0.09a ± 0.00 | 0.09a ± 0.00 | 0.09a ± 0.00 | — |
| Fluoranthene | ND | ND | ND | ND | — |
| Pyrene | ND | ND | ND | ND | — |
| Benzo[a]anthracene | ND | ND | ND | ND | 0.1 |
| Chrysene | ND | ND | ND | ND | 0.01 |
| Benzo[b]fluoranthene | 0.05c ± 0.00 | 0.05c ± 0.00 | 0.07b ± 0.00 | 0.13a ± 0.00 | 0.1 |
| Benzo[k]fluoranthene | 0.16a ± 0.00 | 0.03c ± 0.00 | 0.05b ± 0.00 | 0.15a ± 0.01 | 0.01 |
| Benzo[a]pyrene | 0.07b ± 0.00 | 0.05c ± 0.00 | 0.03d ± 0.00 | 0.14a ± 0.00 | 1 |
| Dibenz[a,h]anthracene | 0.54d ± 0.00 | 1.41a ± 0.01 | 0.65c ± 0.01 | 0.81b ± 0.00 | 1.0 |
| Benzo[g,h,i]perylene | 0.40d ± 0.01 | 3.03a ± 0.01 | 1.34b ± 0.00 | 0.42c ± 0.00 | 0.01 |
| Indeno[1,2,3-c,d]pyrene | 1.79d ± 0.01 | 2.38c ± 0.00 | 2.76a ± 0.00 | 2.70b ± 0.00 | 0.1 |
| ∑PAHs | 4.61 | 7.75 | 7.39 | 7.24 | |
| ∑PAH4 | 0.12 | 0.1 | 0.1 | 0.27 | |
| PEC | 0.6385 | 1.5194 | 0.7247 | 0.9957 | |
| SV (μg kg −1 ) | 0.1624 | 0.1624 | 0.1624 | 0.1624 | |
| % Lipid | 2.40 a ± 1.04 | 2.39 a ± 1.01 | 2.68 a ± 0.74 | 2.47 a ± 0.25 | |
| %MC | 72.91 c ± 0.82 | 75.56 b ± 1.68 | 75.67 a ± 0.59 | 70.63 d ± 0.97 | |
| Phen/Anth | 0.08 | 1.00 | 1.00 | 0.89 | |
| LMW/HMW | 0.502 | 0.106 | 0.502 | 0.657 | |
| Ant/(Ant + Phe) | — | 0.5 | 0.5 | 0.47 | |
| PAHs (mg kg−1 fresh wt) | Samples (N = 10) | TEFs (WHO/IPCS 1998) | |||
|---|---|---|---|---|---|
| US1 | US2 | US3 | US4 | ||
| a Results are means and standard deviations of triplicate measurements. ND = below detection limit <0.01 to <0.03. TEFs = Toxicity equivalent factors. PEC = carcinogenic potency equivalent concentration. SV = screening (threshold) value. US1, US2, US3 are periwinkles from incident zones while US4 are periwinkles from the control zone. MC = moisture content. Values with different alphabet superscripts in rows are significantly different. | |||||
| Naphthalene | ND | ND | ND | 1.01 ± 0.00a | — |
| 2-Methylnaphthalene | 0.17c ± 0.01 | 0.48b ± 0.01 | 0.17c ± 0.00 | 1.25a ± 0.01 | — |
| Acenaphthylene | ND | 0.04b ± 0.00 | ND | 0.10a ± 0.01 | — |
| Acenaphthene | 0.12c ± 0.00 | 0.10d ± 0.00 | 0.21b ± 0.00 | 0.70a ± 0.01 | — |
| Fluorene | 0.32a ± 0.00 | ND | ND | ND | — |
| Phenanthrene | 0.08a ± 0.00 | 0.05b ± 0.01 | 0.09a ± 0.01 | ND | — |
| Anthracene | ND | ND | ND | ND | — |
| Fluoranthene | ND | 0.05a ± 0.00 | ND | ND | — |
| Pyrene | ND | 0.05a ± 0.00 | ND | ND | — |
| Benz[a]anthracene | ND | ND | ND | ND | 0.1 |
| Chrysene | ND | ND | ND | ND | 0.01 |
| Benzo[b]fluoranthene | ND | 0.28a ± 0.01 | ND | ND | 0.1 |
| Benzo[k]fluoranthene | ND | 0.05a ± 0.01 | ND | ND | 0.1 |
| Benzo[a]pyrene | 0.07b ± 0.01 | 0.29a ± 0.01 | ND | ND | 1 |
| Dibenz[a,h]anthracene | 0.55c ± 0.01 | 1.60a ± 0.00 | 0.60a ± 0.00 | 0.17b ± 0.00 | 1.0 |
| Benzo[g,h,i]perylene | 1.33a ± 0.00 | 0.35d ± 0.01 | 1.03c ± 0.00 | 1.31b ± 0.01 | 0.01 |
| Indeno[1,2,3-c,d]pyrene | 1.35d ± 0.01 | 2.24b ± 0.00 | 2.66a ± 0.01 | 1.65c ± 0.01 | 0.1 |
| ∑PAHs | 5.11 | 5.60 | 4.91 | 6.14 | |
| ∑PAH4 | 0.07 | 0.29 | — | — | |
| PEC | 0.7683 | 2.1505 | 0.8763 | 0.3481 | |
| SV (μg kg −1 ) | 0.1624 | 0.1624 | 0.1624 | 0.1624 | |
| % Lipid | 2.17 a ± 0.71 | 2.51 a ± 0.13 | 2.17 a ± 0.71 | 1.63 a ± 0.51 | |
| %MC | 73.43 a ± 0.97 | 74.30 a ± 1.14 | 76.46 a ± 0.98 | 76.90 a ± 1.77 | |
| LMW/HMW | 0.16 | 0.04 | 0.07 | 0.85 | |
| PAH compounds | Matrix | LOD (mg kg−1) | LOQ (mg kg−1) | r 2 | % Recovery range |
|---|---|---|---|---|---|
| Nap | Fish | 0.02 | 0.07 | 1.86 | 88.5–90.5 |
| 2MNap | Fish | 0.01 | 0.03 | 0.65 | 80.1–83.0 |
| Acy | Fish | 0.01 | 0.03 | 0.49 | 70.6–73.1 |
| Ace | Fish | 0.01 | 0.03 | 0.67 | 91.3–92.0 |
| Flu | Fish | 0.01 | 0.03 | 0.74 | 90.8–92.1 |
| Phe | Fish | 0.03 | 0.08 | 1.34 | 91.3–93.4 |
| Ant | Fish | 0.02 | 0.07 | 1.57 | 76.1–80.0 |
| Flurt | Fish | 0.01 | 0.03 | 0.66 | 80.8–82.2 |
| Pyr | Fish | 0.02 | 0.07 | 1.43 | 99.0–101.1 |
| B[a]a | Fish | 0.02 | 0.07 | 0.97 | 91.7–92.3 |
| Chr | Fish | 0.03 | 0.08 | 0.65 | 89.6–90.2 |
| B[b]f | Fish | 0.02 | 0.07 | 0.46 | 84.2–84.6 |
| B[k]f | Fish | 0.03 | 0.08 | 0.37 | 83.4–83.6 |
| B[a]p | Fish | 0.03 | 0.08 | 0.92 | 82.8–84.2 |
| D[a,h]a | Fish | 0.01 | 0.03 | 0.48 | 94.0–94.8 |
| B[g,h,i]p | Fish | 0.01 | 0.03 | 1.44 | 90.8–91.4 |
| In[1,2,3-c,d]p | Fish | 0.01 | 0.03 | 1.97 | 88.1–89.0 |
PAHs are readily absorbed in tissues of fish and shellfish because of their lipophilic nature. It could be expected that the higher the tissue lipid, the higher the PAHs concentration. However, this was not the case in the present study. There was no correlation between the concentrations of PAHs and tissue lipid of fish under study. The lipid content ranged from 1.47 to 2.57% for catfish, 2.39 to 2.68% for prawns, and 1.63 to 2.57% for periwinkles, while total PAHs (∑PAHs) range from 4.55 to 6.36 mg kg−1 fw for catfish, 4.61 to 7.75 mg kg−1 fw for prawns, and 4.91 to 6.14 mg kg−1 fw for periwinkles, with no particular trend across the four zones. Dietary fat levels have been shown to have an effect on the disposition and toxicity of PAHs. The metabolism of benzo[a]pyrene in hepatocytes in vitro from rats fed high-fat (as corn oil) diets was decreased.43 This effect was not due to a decrease in the activity of AHH. The authors postulated that the high-fat diets allowed benzo[a]pyrene, which is highly lipophilic, to become sequestered in lipid droplets and, therefore, become inaccessible to the P-450 enzymes. The moisture content (MC) varied from 70.63% to 76.90% in all the seafood samples across the four zones. This result agrees with the general report that the composition of most fish falls in the range of about 0.2 to 20% lipid, 14 to 20% protein, 60 to 80% moisture, and 1.0 to 1.8% ash.44 Therefore, tissue concentration of PAHs in seafood in the present study showed no correlation with the lipid and moisture composition of the seafood.
Origin of PAHs detected in seafood
PAHs is known to originate from three sources—diagenic, petrogenic, and pyrogenic.Diagenic are natural PAHs generated by biological processes (retene and perylene are examples of PAHs that may be from diagenic or petrogenic sources). Petrogenic PAHs are typically formed from petroleum and fossil fuels (these typically include many alkylated PAHs). Pyrogenic PAHs are products of incomplete combustion (typically the biggest component of most urban and industrial samples).
In the present study, the PAHs detected in the tissues of catfish, prawns, and periwinkles were pyrogenic, based on mathematical evaluation. The ratio of LMW/HMW < 1 was evaluated for all the seafood samples across the zones. This finding was in agreement with the fingerprinting test on water and sediments of the ‘Post-Oil Spill Impact Assessment Study’ in the N'Delta region, which showed that the pristine/phytane ratios that normally indicate the petrogenic origin of hydrocarbons were nil. The petrogenic origin is usually indicated by a pristine/phytane ratio of 3.0.41 The observation was also in agreement with the report3 on the pyrogenic origin of tissue PAHs in fish. The tissue concentration of PAHs varied widely with molecular weight, which is in agreement with report on the assessment on PAHs levels in some finsh and seafood from different coastals water in the Niger Delta.3 Studies have shown that larger concentrations of LMW PAHs (e.g., acenaphthene and fluorene) most often occur in sample matrices contaminated with naturally occurring PAHs (i.e., petrogenic and biogenic origins), while PAHs from combustion processes (i.e., pyrogenic origin) often contain elevated concentrations of HMW PAHs (e.g., fluoranthene and pyrene) and fewer LMW PAHs.38 This confirmed the fact that PAHs with HMW are typically generated by high-temperature combustion processes40 such as gas flaring and burning oil wells. The overall mean concentration of PAHs in prawns across the zones ranged from below detection (ND) to 3.03 mg kg−1 fw for high molecular weight (HMW) PAHs, and from ND to 1.63 mg kg−1 fw for LMW PAHs.
Potential health risk of PAHs consumption
Cancer potency evaluations of environmental media are a necessary component of cancer risk assessments. A number of PAHs have shown carcinogenic effects in experimental animals, and it has been concluded that benzo[a]pyrene is carcinogenic to humans (group 1).9 The concern, therefore, is about their presence in food and health risk from ingestion. Although the estimated household fish consumption rate of 36.94 g per person per day in Nigeria is exceedingly less than the USEPA-recommended fish consumption rate of 142.2 g per person per day for subsistence consumers29 and the world's average fish consumption rate of 20.3 kg per capita per year,24 the estimated carcinogenic potency equivalent concentrations (PEC) of PAHs from fish consumption was higher than the screening value (SV) for about 5 to 8 times for catfish, 4 to 9 times for prawns, and 2 to 13 times for periwinkles. This indicates significant cancer risk potential (CRP) of PAHs from the consumption of seafood in the N'Delta region. In a similar study,5 the estimated cumulative excess cancer risk (ECR) for E. fimbriata and C. gariepinus and the PAH4 index for all the assessed smoked fish species from markets in southern Nigeria exceeded the threshold values, indicating potential carcinogenic risk from consumption. Mixtures of diagenic PAHs are generally not considered to have health impacts on people at environmental exposures levels. Short-term environmental exposures to petrogenic and pyrogenic PAHs can lead to tissue irritation (e.g., skin, respiratory, eyes, and gastrointestinal),45 while long-term exposures can lead to liver damage, hematological effects, and reproductive and developmental difficulties in animals. In addition to irritation, decreased fertility, developmental neurological effects, and renal toxicity have been demonstrated in laboratory animals exposed to relatively high levels of PAHs.45 The most studied endpoint for long term PAH exposure is cancer. Individual carcinogenic PAHs (cPAHs) have been shown to have different cancer potencies and may induce different types of cancer in laboratory animals (e.g., oral exposure to benzo[a]pyrene or dibenzo[a,h]pyrene predominantly results in gastrointestinal tract cancers or lung cancer, respectively).46Conclusion
Exposure and health risk from PAHs can be prevented by adopting appropriate control measures coupled with the awareness of the ways that PAHs are formed in food. Foodborne illness data are scarce and often under reported. Understanding the linkage between the origin, formation, and ‘food vehicle’ is important for finding the mitigation strategy to PAHs contamination and the associated health risk.The present study showed elevated tissue concentration of polycyclic aromatic hydrocarbons in seafood in the N'Delta region far above the legal maximum limits allowed for food safety. Mathematical evaluation and dominant concentration of high molecular weight PAHs revealed that the origin of PAHs in the present study was mainly pyrogenic. This was in agreement with the previous studies in the area.3,5,8,46,47 The carcinogenic potency equivalent concentrations (PEC) exceeded the screening value (SV) in the samples analyzed, showing that the consumption of seafood at the rate of 36.94 g per person per day or higher poses serious health risks. It is necessary to regularly monitor PAHs and other contaminants levels in the aquatic environment even when everything appears to be normal so as to provide mitigation options that will prevent risk to humans and aquatic animals. A comprehensive database and standard regulatory agency guidelines relating to seafood safety should be developed, updated, and integrated into Nigeria regulatory processes through routine and uniform training programs. Specialized test instruments that can quantitatively detect at the zero level any traces of chemical contaminants in samples are required. The mechanism of pyrogenic PAHs uptake by aquatic organisms requires further investigation. The duration for the manifestation of PAHs-related tumors from dietary exposure should be investigated. Also, the need for a comprehensive database on PAHs and the health risk on humans or organisms at the cellular and biochemical levels require investigation.
Author contributions
Udeme Udofia: conceptualization of the research idea, data curation, funding, investigation, methodology, formal analysis, and original draft. Charles Ameh: for the project administration, software, and data analysis. Eula Miller: original draft, and review and editing. Mandu Ekpenyong: resources, original draft, and review and editing.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
We wish to acknowledge Michael Okpara University of Agriculture for providing us with the equipment to carry out this study.References
- Science Committee on Food, Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food. Brussels, Belgium, 2002 December, http://ec.europa.eu/food/fs/sc/scf/out153_en.pdf, accessed 15.07.2018 Search PubMed.
- K. W. Nkpaa, M. O. Wegwu and E. B. Essien, Assessment of polycyclic aromatic hydrocarbons (PAHs) levels in two commercially important fish species from crude oil polluted waters of Ogoniland and their carcinogenic health risks, J. Environ. Earth Sci., 2013, 3(8), 128–137 Search PubMed.
- E. O. Nwaichi and S. A. Ntorgbo, Assessment of PAHs levels in some fish and seafood from different coastal waters in the Niger Delta, Toxicol. Rep., 2016, 3, 167–172 CrossRef CAS PubMed.
- E. S. Osuagwu and E. Olaifa, Effects of oil spills on fish production in the Niger Delta, PLoS One, 2018, 13(10), e0205114 CrossRef PubMed.
- I. Tongo, O. Ogbeide and L. Ezemonye, Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria, Toxicol. Rep., 2017, 4, 55–61 CrossRef CAS PubMed.
- D. H. Phillips, Polycyclic aromatic hydrocarbons in the diet, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 1999, 443(1–2), 139–147 CrossRef CAS.
- L. M. Palm, D. Carboo, O. P. Yeboah, W. J. Quasie, M. A. Gorleku and A. Darko, Characterization of polycyclic aromatic hydrocarbons (PAHs) present in smoked fish from Ghana, Adv. J. Food Sci. Technol., 2011, 3(5), 332–338 CAS.
- B. O. Silva, O. T. Adetunde, T. O. Oluseyi, K. O. Olayinka and B. I. Alo, Polycyclic aromatic hydrocarbons (PAHs) in some locally consumed fishes in Nigeria, Afr. J. Food Sci., 2011 Jul 31, 5(7), 384–391 Search PubMed.
- Agency for Toxic Substances and Disease Registry, Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs), U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, 1995 Search PubMed.
- World Health Organization, Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide, World Health Organization, 2006 Search PubMed.
- C. Ris, US EPA health assessment for diesel engine exhaust: a review, Inhalation Toxicol., 2007, 19(sup1), 229–239 CrossRef CAS PubMed.
- European Food Safety Authority (EFSA), Polycyclic Aromatic Hydrocarbons in Food-Scientific Opinion of the Panel on Contaminants in the Food Chain, EFSA J., 2008, 6(8), 724 CrossRef.
- L. C. Obara and E. Nangih, Accounting practices and performance of oil and gas industry (upstream sector) in Nigeria: An empirical analysis, Int. J. Acad. Res. Account. Financ. Manag. Sci., 2017, 7(2), 215–222 Search PubMed.
- B. Anifowose, D. Lawler, D. van der Horst and L. Chapman, Evaluating interdiction of oil pipelines at river crossings using Environmental Impact Assessments, Area, 2014, 46(1), 4–17 CrossRef.
- T. Agbola and T. A. Olurin, Landuse and Landcover change in the Niger delta, Excerpts from a Research Report presented to the Centre for Democracy and Development, 2003 Search PubMed.
- Z. Nenibarini, Impacts of Extractive industries on the Biodiversity of the Niger Delta National Workshop on Coastal and Marine Biodiversity Management, 2004 Search PubMed.
- United Nations Development Programs, Niger Delta Human Development Report, 2011 Search PubMed.
- R. Van der Oost, H. Heida, A. Opperhuizen and N. P. Vermeulen, Interrelationships between bioaccumulation of organic trace pollutants (PCBs, organochlorine pesticides and PAHs), and MFO-induction in fish, Comp. Biochem. Physiol., C: Comp. Pharmacol., 1991, 100(1–2), 43–47 CrossRef CAS.
- O. C. Adeigbe and A. A. Rahaman, Polycyclic Aromatic Hydrocarbons (PAHs) and Carcinogenic Polycyclic Aromatic Hydrocarbons (cPAHs); Implications for Groundwater and Stream qualities around Mamu Coal Exposure in Okaba-Odagbo Mining District, Ankpa, North-Central Nigeria, Pet. Coal, 2020, 62(3), 845–857 CAS.
- A. K. Hurtig and M. San Sebastián, Geographical differences in cancer incidence in the Amazon basin of Ecuador in relation to residence near oil fields, Int. J. Epidemiol., 2002, 31(5), 1021–1027 CrossRef PubMed.
- A. R. Price, C. R. Sheppard and C. M. Roberts, The Gulf: its biological setting, Mar. Pollut. Bull., 1993, 27, 9–15 CrossRef.
- C. Sheppard, M. Al-Husiani, F. Al-Jamali, F. Al-Yamani, R. Baldwin, J. Bishop, F. Benzoni, E. Dutrieux, N. K. Dulvy, S. R. Durvasula and D. A. Jones, The Gulf: a young sea in decline, Mar. Pollut. Bull., 2010, 60(1), 13–38 CrossRef CAS PubMed.
- L. Ababouch, Market-based standards and certification in aquaculture, Farming the Waters for People and Food, 2010, p. 525 Search PubMed.
- Food and Agriculture Organization (FAO), The State of World Fisheries and Aquaculture, FAO, Rome, Italy, 2004 Search PubMed.
- A. B. Dauda, I. Natrah, M. Karim, M. S. Kamarudin and A. H. Bichi, African catfish aquaculture in Malaysia and Nigeria: Status, trends and prospects, Fish. Aquacult. J., 2018, 9(1), 1–5 Search PubMed.
- Nos C. Sephadex® G-25.
- A. Shrivastava and V. B. Gupta, Methods for the determination of limit of detection and limit of quantitation of the analytical methods, Chron. Young Sci., 2011, 2(1), 21–25 CrossRef.
- G WHO, World Health Organization International Program on Chemical Safety, 1994 Search PubMed.
- K. C. Cheung, H. M. Leung, K. Y. Kong and M. H. Wong, Residual levels of DDTs and PAHs in freshwater and marine fish from Hong Kong markets and their health risk assessment, Chemosphere, 2007, 66(3), 460–468 CrossRef CAS PubMed.
- G. De Luca, A. Furesi, R. Leardi, G. Micera, A. Panzanelli, P. C. Piu and G. Sanna, Polycyclic aromatic hydrocarbons assessment in the sediments of the Porto Torres Harbor (Northern Sardinia, Italy), Mar. Chem., 2004, 86(1–2), 15–32 CrossRef CAS.
- B. Vrana, A. Paschke and P. Popp, Polyaromatic hydrocarbon concentrations and patterns in sediments and surface water of the Mansfeld region, Saxony-Anhalt, Germany, J. Environ. Monit., 2001, 3(6), 602–609 RSC.
- I. C. Nisbet and P. K. Lagoy, Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs), Regul. Toxicol. Pharmacol., 1992, 16(3), 290–300 CrossRef CAS PubMed.
- M. Tobiszewski and J. Namieśnik, PAH diagnostic ratios for the identification of pollution emission sources, Environ. Pollut., 2012, 162, 110–119 CrossRef CAS PubMed.
- U. Varanasi, J. E. Stein and M. Nishimoto, Biotransformation and Disposition of Polycyclic Aromatic Hydrocarbons (PAH) in Fish, Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment, CRC Press, Inc., Boca Raton Florida, 1989, pp. 93–149, 20 fig, 15 tab, 171 ref., NOAA Contract Y 01-CP-40507, 1989 Search PubMed.
- S. A. Adeyeye, O. T. Bolaji, T. A. Abegunde and F. Idowu-Adebayo, Polycyclic aromatic hydrocarbon profile, chemical composition and acceptability of Suya (a West African grilled meat), Polycyclic Aromat. Compd., 2020, 1 Search PubMed.
- World Health Organization, Guidelines for Drinking-Water Quality: Second Addendum, vol. 1, Recommendations, 2008 Search PubMed.
- United Nations Development Programs, Niger Delta Human Development Report, 2011 Search PubMed.
- B. Yan, L. A. Benedict, D. A. Chaky, R. F. Bopp and T. A. Abrajano, Levels and patterns of PAH distribution in sediments of the New York/New Jersey harbor complex, Northeast. Geol. Environ. Sci., 2004, 26(1–2), 113–122 Search PubMed.
- H. T. Al-Saad, H. M. Bedair, H. M. Heba and M. K. Zukhair, Sources of polycyclic aromatic hydrocarbons (PAHs) in fish samples from the North-West Arabian Gulf and the Red Sea coast of Yemen, Mesopot. J. Mar. Sci., 2006, 21(1), 1–12 Search PubMed.
- E. Nyarko and B. E. Klubi, Polycyclic aromatic hydrocarbons (PAHs) levels in two commercially important fish species from the coastal waters of Ghana and their carcinogenic health risks, West Afr. J. Appl. Ecol., 2011, 19(1), 53–66 Search PubMed.
- I. Tolosa, S. J. De Mora, S. W. Fowler, J. P. Villeneuve, J. Bartocci and C. Cattini, Aliphatic and aromatic hydrocarbons in marine biota and coastal sediments from the Gulf and the Gulf of Oman, Mar. Pollut. Bull., 2005, 50(12), 1619–1633 CrossRef CAS PubMed.
- W. Guo, M. He, Z. Yang, C. Lin, X. Quan and H. Wang, Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China, Chemosphere, 2007, 68(1), 93–104 CrossRef CAS PubMed.
- R. G. Harvey, Environmental Chemistry of PAHs. PAHs and Related Compounds, 1998, pp. 1–54 Search PubMed.
- J. Zaleski, G. Y. Kwei, R. G. Thurman and F. C. Kauffman, Suppression of benzo[a]pyrene metabolism by accumulation of triacylglycerols in rat hepoatacytes: effect of high-fat and food-restricted diets, Carcinogenesis, 1991, 12(11), 2073–2079 CrossRef CAS PubMed.
- B. J. Efiuvwevwere and L. O. Amadi, Effects of preservatives on the bacteriological, chemical, and sensory qualities of mangrove oyster (Crassostrea gasar), Br. J. Appl. Sci. Technol., 2015, 5(1), 76 CrossRef.
- S. Kelley, J. Meinert and C. Williams, Review of the Minnesota Department of Health Contaminants of Emerging Concern Program Process for Selecting Chemicals, 2016 Search PubMed.
- European Commission, Commission Regulation (EU) No 836/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs, Off. J. Eur. Communities: Legis., 2011, 215, 4–8 Search PubMed.
- C. M. Weyand, J. Goronzy and C. G. Fathman, Modulation of CD4 by antigenic activation., J. Immunol., 1987, 138(5), 1351–1354 CAS.
- J. Neal and R. H. Rigdon, Gastric tumors in mice fed benzo (a) pyrene: a quantitative study, Tex. Rep. Biol. Med., 1967, 25(4), 553–557 CAS.
| This journal is © The Royal Society of Chemistry 2021 |