Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterising and communicating the potential hazard posed by potentially toxic elements in indoor dusts from schools across Lagos, Nigeria†
Abimbola O.
Famuyiwa
 *ab and
Jane A.
Entwistle
*ab and
Jane A.
Entwistle
 b
b
aDepartment of Science Laboratory Technology, Moshood Abiola Polytechnic, Abeokuta, Ogun State P.M.B 2210, Nigeria. E-mail: abimbola.famuyiwa@gmail.com
bDepartment of Geography and Environmental Sciences, Northumbria University, Newcastle Upon Tyne NE1 8ST, UK
First published on 22nd April 2021
Abstract
Ambient and indoor air pollution results in an estimated 7 million premature deaths globally each year, representing a major contemporary public health challenge, but one poorly quantified from a toxicological and source perspective. Indoor exposure represents possibly the greatest potential overall exposure, yet our indoor environments are still poorly understood, modelled and characterized. In rapidly growing cities, such as Lagos, Nigeria, environmental monitoring can play an important role in establishing baseline data, monitoring urban pollution trends and in environmental education. Classroom dust samples were collected from 40 locations from across the twenty local government areas (LGAs) of Lagos, in June 2019. The aim of the study was to assess the potential hazard posed by PTE in indoor dusts and to develop a suitable risk communication strategy to inform and educate the public, promoting environmental health literacy. Concentrations of total PTE in indoor dusts were assessed using Energy Dispersive X-Ray Fluorescence (ED-XRF) spectrometry. Oral bioaccessibility determinations using the unified BARGE method, and analysis by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) were also performed on the dust samples to determine the fraction available for absorption in the gastrointestinal tract. Results showed that the indoor dust samples were largely uncontaminated, with only few exceptions (2 samples). Enrichment factor pollution trend for the total PTE concentrations was in the order of Pb > Zn > U > Cr > Cu > Ba > Mn > V > As > Cd > Ni > Al. Source apportionment studies using factor analysis suggests concentrations of Al, As, Fe, Mn, Ni, and U may be influenced largely by lithogenic factors, while Cd, Cu and Pb originated principally from anthropogenic sources. Chromium, V and Zn appear to originate from mixed sources of both lithogenic and anthropogenic origin. Our oral bioaccessibility determinations indicate that the assumption of 100% bioavailability based on pseudototal or total concentrations would overestimate the hazard potential of PTE in these indoor dusts. Zinc was the most bioaccessible PTE (mean of 88%), with Mn (57%), Pb (48%), Ba (48%), Al (41%), As (37%), Cu (36%), Ni (28%), Cr (10%) and Fe (7%) the least bioaccessible. Human health risk assessment, for both children and adults using the bioaccessible fraction, showed values to be within acceptable risk levels.
Environmental significanceExposure to particulate matter can initiate or enhance disease in humans, yet the nature of the hazard that indoor dust presents remains poorly characterized from a toxicological and a source perspective. Our research provides key information about the spatial distributions and concentrations of contaminants in indoor dusts from school environments in Lagos, Nigeria, providing geochemical baseline information and source characterization of 12 Potentially Toxic Elements (PTE). A range of contamination assessment criteria were employed, as well as a modified approach incorporating oral bioaccessibility measurements into human exposure risk models. The online ‘dust atlas’ and ancillary supporting information enables participants to interactively view the levels of PTE in indoor dusts across all participating regions and the range of practical actions to improve indoor environmental quality. |
1. Introduction
Urbanization and industrialization have contributed to increased pollution levels in cities, including the contamination of environment media such as soil, dusts, and particulate matter (PM) with potentially toxic elements, PTE.1 Particulate matter can pose serious risks to human health and an increasing number of associations are now being reported between air particulates and a broad number of disease outcomes.2 Ambient and indoor air pollution results in an estimated 7 million premature deaths globally each year,3 representing a major contemporary public health challenge. Health impacts include increased incidences of strokes,4 Alzheimer's,5 respiratory disease,6 cardiovascular disease7 and cancer.8Dusts contain harmful agents, including metals/metalloids, organic substances, microbes and allergens. Indoor dust is a crucial, often ignored, part of the daily exposure to PM given the amount of time people spend indoors (in homes, offices and schools); estimated to be about 88% of the day for adults and 71–79% of the day for children.9 Potential sources of dust in the indoor environment include ingress of ambient particles, cooking, smoking, cleaning, wear and tear, and soils transported on clothes and footwear. However, incidental or deliberate ingestion of indoor dust can be an important non-dietary pathway by which PTE get into human body, especially children. Involuntary inhalation of resuspended dust particles in the indoor environment coupled with dermal contact are other PTE exposure pathways to humans.10
It is increasingly recognized that the human health risk associated with PM is not solely related to the physical abundance of the PM, or total or pseudototal PTE concentration, but with the biologically available portion of the contaminant.11 Bioaccessibility is a surrogate in vitro estimation of bioavailability. Oral bioaccessibility of a contaminant is the fraction extractable in the human gastro-intestinal (GI) system.12 Indeed, a significant portion of the PM inhaled also reaches the GI system through particle clearance mechanisms in the human pulmonary system.13 In addition to lung clearance mechanisms, indoor dust has a direct oral ingestion pathway to the GI system through hand-to-mouth activity. When these fractions reach the GI tract the PM reacts with gastric juices and the PTE released represent the orally available (bioaccessible) fraction.14
The 2019 United Nations world population statistics revealed that more than half of the world population growth will occur in Africa between now and the year 2050.15 Environmental degradation, with accompanying health impacts, may be one of the resultant effects of the projected population growth, and associated urbanization and industrialization. Several studies which include oral bioaccessibility and risk assessment of PTE in indoor dusts have been reported from a range of countries globally,16–21 however previous studies on PM in Nigeria have largely focused on the outdoor environment, with soil and street dusts the prime research focus.22,23 Recent relevant PM concerns for the Nigerian community include release and resuspension of mining and smelting sources, power generation emissions, traffic, and major fire incidents.24 Lagos is Nigeria's most populous city and seventh fastest growing city in the world.25 Lagos, like many other urban centres across Nigeria, has experienced an unprecedented increase in urban migration, but one amplified by the fact that it serves as the commercial nerve centre of Nigeria.10 Lagos State Government estimated the population of Lagos as 17.5 million during a parallel census conducted in 2006 with over 12 million people living in the urban areas. A more recent account reported its population as 21 million, making it the largest city in Africa.26 In comparison to most developed countries, developing countries typically lack suitable public and environmental health standards and thus residential, community and educational indoor environments can be a source of unacceptable exposure to PM and to PTE in these indoor dusts. This problem can be exacerbated due to the lack of, or poor implementation of, environmental sanitation policies. The aim of this current study is to (i) assess the potential hazard posed by PTE in indoor dusts from schools across Lagos and, (ii) to develop and implement a risk communication strategy to help inform and educate the public around the potential hazards of PM, promoting environmental health literary to reduce exposure. Specific objectives include: (1) to assess the level of PTE in indoor dusts collected from nursery and primary schools in all 20 local government areas of Lagos state, (2) application of an oral bioaccessibility procedure to indoor dusts (250 μm fraction) to determine the fraction of PTE available for absorption, (3) assessment of the human health risk of PTE in classroom dust to children and adults in Lagos environs, and (4) dissemination of the research findings to the local communities in an accessible format. To widen the reach of our environmental health literacy message the focus of our indoor dust collection was on schools.
Equipping the next generation, and more broadly the wider public, with better information about dust-related hazards in their local environmental has the potential to improve the health and wellbeing of communities by empowering citizens to take ownership of response strategies.
2. Materials and methods
2.1 Sampling
Dust samples were collected from 40 locations from across the twenty local government areas (LGAs) of Lagos. The sampling campaign was conducted in June 2019 and two schools were sampled in each of the LGAs. Classroom dusts were collected using a combination of short sweeping brush and/or long handled broom to sweep together dust from across the room to be sampled. Floor dust from five to ten classrooms (according to both the size of the school and the volume of dust available) across an individual school were bulked together to obtain a composite sample for that school. All samples were stored in polythene bags, labelled and taken to the Lagos State Environmental Protection Agency (LASEPA) laboratory for further processing. Samples were air-dried, refuse and small stones were removed, and samples stored prior to transportation to Northumbria University, Newcastle upon Tyne, England UK for subsequent processing.2.2 Sample preparation and analysis
Samples were sieved through 250 μm plastic mesh and approximately 4.0 g of the resultant PM (weighed to 4 decimal places) was made into pellets with a Cereox binder (Fluxana, Borschelstr, Germany) in ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for total metal determination analysis using an Energy Dispersive X-Ray Fluorescence (ED-XRF) spectrometer (manufactured by Spectro Xepos Kleve, Germany). The equipment uses an adaptive excitation, advance tube design and high count throughput detection system which allows for low limits of detection for a wide range of elements.
1 for total metal determination analysis using an Energy Dispersive X-Ray Fluorescence (ED-XRF) spectrometer (manufactured by Spectro Xepos Kleve, Germany). The equipment uses an adaptive excitation, advance tube design and high count throughput detection system which allows for low limits of detection for a wide range of elements.
The in vitro oral bioaccessibility extraction protocol employed in this work was based on the unified BARGE method (UBM).27 This in vitro extraction method has been widely employed and validated for oral bioaccessibility measurements in soil and dusts.28–30 The simulated digestive fluids were prepared and stored in the refrigerator 1 day before extraction commences.31 Approximately 0.6 g (weighed to 4 decimal places) of dust sample was weighed into individual polycarbonate extraction tubes and mixed with 9.0 ml of saliva. After a quick manual shaking (10 seconds), 13.5 ml of gastric solution was pipetted and added. The pH of the solution was adjusted to 1.20 ± 0.05 with HCl (37%). The tubes were shaken using an end-over-end rotator inserted into a thermostatic water bath (@ 37 °C) for 1 hour and then centrifuged at 4500 g for 15 minutes. The supernatant was thereafter carefully pipetted and was acidified using HNO3 and stored in a refrigerator prior to inductively coupled plasma optical emission spectrometry (ICP-OES) using a PerkinElmer Optima 8000 (configured with software Winlab 32 for ICP, version 5.5), Waltham Massachusetts USA.
Determination of the pseudo-total metal concentration was undertaken using a microwave digestion technique. Approximately 0.50 g (weighed to 4 decimal places) of dust was weighed into Teflon digestion vessels and 12.0 ml of aqua-regia mixture was added. The sample was heated in a temperature-controlled microwave digestion system (CEM MARS 6™, Buckingham UK). After digestion, any violent chemical reactions were allowed to subside as the vessels cooled to near room temperature. Digests were filtered and diluted using deionized water and stored in a refrigerator prior to ICP-OES analysis.
2.3 Quality control
The potential of ED-XRF to quantify a wide range of elements is well known, but the number actually determined is dependent upon the calibration standards used, and the elements they are quantified for. For the ED-XRF analysis, calibration for major, minor and trace elements was performed by measuring a series of fourteen certified reference materials (CRMs: GBW 7308, 7310, 7313, 7401, 7402, 7403, 7404, 7405, 7406, 7407, 7411, 7603, SRM 2710A, BCR 146R; further details of each CRM are provided in ESI Table 1†). By comparing certified concentrations with our determined ED-XRF concentrations we calculated individual elemental correction factors which were then applied to ‘calibrate’ the dust data. Good agreement between certified and determined values was obtained for all of our PTEs (regression R2 values all exceeded 93%), and individual element correction factors ranged from 0.812 for Ba to 1.15 for Cd.For improved short-term precision (repeatability), each pellet is rotated during acquisition of the spectra in the Xepos, and as part of our quality control procedures we additionally ran selected sample pellets three times to monitor the % relative standard deviation (% RSD). Where sample concentrations were above 1.0 mg kg−1, % RSDs ranged from <1% to 10% for all of the elements of interest. Instrument limits of detection (LOD, determined for a pure quartz sample) are: Al 27 mg kg−1; As 0.1 mg kg−1; Ba 2.0 mg kg−1; Cd 0.2 mg kg−1; Cr 0.2 mg kg−1; Cu 0.5 mg kg−1; Fe 1.2 mg kg−1; Mn 0.2 mg kg−1; Ni 1.0 mg kg−1; Pb 0.2 mg kg−1; V 0.3 mg kg−1; Zn 0.2 mg kg−1, and U 0.2 mg kg−1, respectively, with all determinations made using the instruments TurboQuant II software (Spectro Analytical Instruments, XRF-113-AB).32
All sample extractions were run in triplicate with procedural blanks (i.e. reagents only, used for blank subtraction and to monitor for any contamination introduced during the laboratory processing) included in each batch. For ICP-OES analysis of the extracts, calibration curves were prepared for the PTE under investigation, using aqueous standards across the calibration range from 0 to 100 mg L−1. The ICP-OES six-point calibration curves produced linear graphs over the determined ranges, with regression coefficient (R2) data of at least 0.998 for all PTE analysed. An internal standard solution (indium) was used to monitor and correct for instrument drift and 2% nitric acid was used to achieve appropriate dilutions. Instrument drift was additionally monitored by analysing calibration standards after every tenth sample measurement. BGS 102 guidance material was used to determine the accuracy of the determinations in the UBM procedure33 (Table 1), and BCR 143R for the aqua-regia digests (Table 2).
| Al | As | Ba | Cd | Cr | Cu | Fe | Mn | Ni | Pb | V | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 5 | Determined mean | 2500 ± 198 | 5.5 ± 0.51 | 118 ± 6.73 | 3.54 ± 10.6 | 36.7 ± 2.58 | 5.82 ± 1.41 | 545 ± 299 | 3470 ± 268 | 13.1 ± 1.34 | 17.1 ± 1.53 | 1.0 ± 1.24 | 46.2 ± 3.49 |
| n = 50 | Reported data33 | 2568 ± 245 | 3.9 ± 0.36 | 72.2 ± 6.02 | 0.24 ± 0.03 | 36.7 ± 2.48 | 8.5 ± 1.03 | 854 ± 237 | 2979 ± 294 | 13.0 ± 1.27 | 15.2 ± 2.97 | 6.1 ± 0.91 | 41.5 ± 4.45 |
| % recovery | 97 | 140 | 163 | NIL | 100 | 68 | 64 | 117 | 101 | 112 | 16 | 111 |
| Cd | Cr | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|
| Determined mean (n = 6) | 65.1 ± 4 | 458 ± 69 | 783 ± 120 | 272 ± 13 | 160 ± 7 | 1060 ± 120 |
| BCR 143R certificate values | 72.0 ± 1.8 | 426 ± 12 | 858 ± 11 | 296 ± 4 | 174 | 1063 ± 16 |
| % Recovery | 90 | 109 | 91 | 92 | 92 | 100 |
2.4 Pollution indices
A wide array of methods for determining metal enrichment have been reported in the literature.34–36 All come with their own advantages and disadvantages associated with their implementation. Given these statistical approaches have typically been applied to media and settings other than indoor PM we took a pragmatic approach and selected enrichment factors as one of the more commonly applied approaches.35Enrichment factor (EnF) of PTE in the indoor dust samples were calculated using eqn (1):
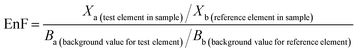 | (1) |
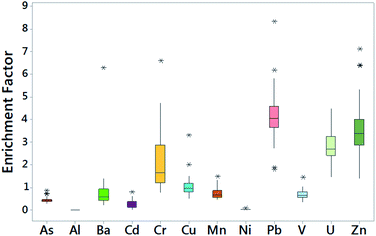
Zhang and Liu41 noted that EnF values between 0.5 and 1.5 indicate PTE is entirely from natural origins whereas EnF values greater than 1.5 suggests source/s are more likely to be anthropogenic. Five contamination categories have been proposed to indicate the degree of pollution: no or minimal enrichment (EnF < 2); moderate enrichment (EnF = 2–5); significant enrichment (EnF = 5–20); very high enrichment (EnF = 20–40); extremely high enrichment (EnF > 40).
2.5 Health risk assessment
The difficultly of obtaining standardised, representative indoor dust samples is well acknowledged in the literature, and dust metal concentrations (as an amount of metal per given quantity of dust; mg kg−1) are not the same measurements as dust metal loadings (quantity of dust on the area sampled; μg m−2).20,42 The dust samples collected in this study were bulk, composite, floor sweepings.Whilst dust metal loadings are important for assessing potential exposure to indoor dust, the resultant concentration data provides us with a useful indicator of the presence of different sources of metals across the Lagos schools investigated. This concentration data has also been shown to be of use for comparing indoor dust with outdoor sources such as street dust and soil.43,44 Where only metal concentration data exist (without loadings data), the approach commonly adopted by other studies is based on the health risk assessment method developed by United States Environmental Protection Agency.45–49
Human exposure to PTE can occur via three main routes; (a) inhalation of particulates through the nose and mouth (b) dermal absorption adhered to exposed skin (c) through ingestion of particles. For this study, we computed the carcinogenic and non-carcinogenic risks of PTE exposure to two age groups; children and adults.
The average daily dose values for the three main exposure routes were calculated as follows:
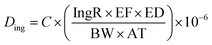 | (2) |
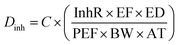 | (3) |
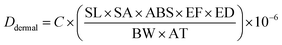 | (4) |
C (exposure point concentration, mg kg−1) in eqn (2)–(4) is an estimate of reasonable maximum exposure, but in this current study is replaced with bioaccessible concentration values (mg kg−1) which represents the fraction that is available for absorption in the gastro intestinal tract of humans. For non-carcinogenic risk estimation each PTE average daily dose was divided with corresponding reference dose (RfD) to yield a hazard quotient (HQ) as shown in eqn (5). The hazard index (HI) was estimated to be equal to the sum of the HQs calculated. The HI index evaluated the overall potential for non-carcinogenic risks to man from the three exposure routes considered (eqn (6)). If the HI value is greater than 1, there is a probability of non-carcinogenic risks occurrence in a lifetime. Carcinogenic risk is the probability of an individual developing any type of cancer during a lifetime exposure. Risk was evaluated by multiplying the average daily dose values against oral slope factor (SF) (eqn (7)).
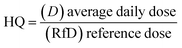 | (5) |
| HI = ∑HQi | (6) |
| CR = D × SF | (7) |
Only As, Cr, Ni and Pb were considered since oral slope factors are only available for these elements. Calculated cancer risks above 1 × 10−4 were considered unacceptable and risks below 1 × 10−6 were considered not to have the capability of triggering any health effect, while values between 10−6 to 10−4 were considered to be within acceptable limits.23
2.6 Statistical analyses
Microsoft office excel package 2016 was used for the computation of PTE concentration measurements and simple descriptive statistical analysis such as standard deviation, mean, and human health risk calculations. Factor analysis (FA) was carried out to predict the possible sources of each PTE studied. Factor analysis was conducted using the principal component extraction method thereby reducing the dimensionality of the data set and then describing the variability among observed correlated variables. The components of the FA were transformed using varimax rotation and Kaiser normalization after analysis.52 Minitab software version 18.0 was used for the aforementioned exploratory and multivariate statistical analysis.2.7 Public focused dissemination activity
As part of our strategy to communicate the potential hazards associated with indoor dust, a short, bespoke report has been prepared for each school, setting that school's dust results within the wider context of the indoor dust data available for Lagos (the reporting template is included in ESI†), as well as key strategies on how to reduce personal exposure. To further promote public awareness of indoor environmental quality issues, and wider access to these data, we worked with Apperception-Group, LLC, and colleagues at Indiana University-Purdue University, USA (Gabe Filippelli), and Macquarie University, Australia (Mark Taylor), to develop an online resource, accessible at https://mapmyenvironment.com.53 Data visualisations are “double-jittered” to ensure individual sample locations are not specifically identified. Liu et al.54 found that an interactive portal was important to the success of citizen science projects, especially one that evolves and provides users with access to dynamic information. The MapMy Environment site continuously updates to display global data on environmental quality indicators, alongside plain language information and videos to enhance the environmental health literacy of global citizen.3. Results and discussion
3.1 Total dust metal concentrations
The summary statistics of PTE concentration in the indoor dusts (determined by ED-XRF) are presented in Table 3, with the full dataset presented in ESI Tables 2 and 3.† Apart from Pb, no guidance or regulatory limits exist for the concentration of these PTE in indoor dusts. To provide some context for the range of total PTE concentrations observed we have included selected soil guideline values (for soil in residential areas) for three countries that highlight the range of international values reported (Table 3). Indeed, the outdoor environment typically plays a significant role in the indoor environment and research by Oomen and Lijzen (2004)55 and Van Holderbeke et al. (2008),56 amongst others, report that the contribution of exterior soil to interior house dust ranges from 8% to >80% for Pb. The USEPA's recent review of dust Pb hazard standards lowered the floor Pb loading threshold from 40 to 10 μg per square foot.57 This threshold of 10 μg per square foot is a combination of dust mass and Pb concentration in the dust, so does not readily translate into a Pb concentration in bulk sampled vacuum dust. However, for comparison purposes, initial work by Pat Rasmussen (personal communication), based on extensive investigations on house dusts20,43,44 suggests a threshold of 10 μg per square foot approximately equates to a median Pb concentration of 800 ppm.| Al | As | Ba | Cd | Cr | Cu | Fe | Mn | Ni | Pb | U | V | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a https://www.esdat.net/environmentalstandards/canada/soil/rev_soil_summary_tbl_7.0_e.pdf. b https://www.miljodirektoratet.no/globalassets/publikasjoner/klif2/publikasjoner/andre/1691/ta1691.pdf. c Contaminated Land: Applications in Real Environments (CLAIRE) consortium, SP1010 Final Project Report (Revision 2). Development of Category 4 Screening Levels for Assessment of Land Affected by Contamination. DEFRA R&P Project Report, 2014a. d V. Alekseenko, A. Alekseenko, The abundances of chemical elements in urban soils, Journal of Geochemical Exploration, 2014, volume 147, part B, pages 245–249. e https://pubs.geoscienceworld.org/gsa/gsabulletin/article/72/2/175/5262/Distribution-of-the-Elements-in-Some-Major-Units. | |||||||||||||
| Minimum | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.4 | 97.4 | <LOD | 39.8 | 9.6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
106 | 10.5 | 14.9 | 2.2 | 15.9 | 66.9 |
| Mean | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.3 | 254 | 0.5 | 130 | 28.1 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
368 | 20.9 | 47.4 | 6.1 | 52.4 | 208 |
| Median | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
3.2 | 212 | 0.4 | 76.9 | 23.0 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
339 | 20.1 | 43.7 | 5.5 | 49.4 | 163 |
| Max | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
4.8 | 1560 | 1.3 | 606 | 110 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
764 | 40.3 | 114 | 15.0 | 103 | 622 |
| SD | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.96 | 233 | 0.29 | 114 | 17.6 | 8530 | 141 | 6.34 | 20.1 | 2.76 | 22.4 | 129 |
| Canada SGVa | 12 | 10 | 0.4 | 63 | 50 | 140 | 200 | ||||||
| Norway SGVb | 12 | 12.2 | 75 | 150 | 90 | 90 | 300 | ||||||
| CLEA, UKc | 40 | 150 | 310 | ||||||||||
| Earth's crustd | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.7 | 650 | 0.13 | 83 | 47 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1000 | 58 | 16 | 90 | 83 | |
| Continental Shalee | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13 | 580 | 0.3 | 45 | 45 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
850 | 68 | 20 | 3.7 | 130 | 95 |
Aluminium, Ba and V concentrations were compared against the typical background soil concentration. None of these three PTE (Al, Ba and V) were elevated when compared to these typical soil concentrations/crustal abundances. This suggests, levels of Al, Ba and V may largely be from natural sources, as anthropogenic contributions are not pronounced considering comparisons with respective background values.
Cadmium measurements ranged from below the LOD (0.1 mg kg−1) to 1.3 mg kg−1, with a mean concentration of 0.5 mg kg−1. None of the Cd measurements exceeded the Norwegian and Canadian soil guideline values (SGVs) but nearly all of the sample measurements were higher than the typical soil background value. The highest Cd measurement in a previous study of residential soils from Lagos and Ibadan (Nigeria) was 2.8 mg kg−1, with an average concentration of 0.94 mg kg−1,58 and indicated a similar range of Cd measurements to this current study.
Iron and Mn are major components of soil minerals and have been noted to be abundant elements in the earth crust.59 Our Fe and Mn concentrations were within typical soil concentration ranges, with all measurements for both of these PTE falling below the earth's crust and continental shale values respectively. Our dust samples were similarly not contaminated with Cu and Ni, with concentrations below typical soil concentrations and the reported SGVs (Table 3).
Zinc, Pb and Cr displayed a rather different behaviour among the PTE. Nearly all of the Zn concentration measurements in the dust samples exceeded the background values (99%) and about 33% were higher in value than Canadian SGV; some multiple times higher. Several samples did exhibit slightly elevated Cr and Pb concentrations when compared to the typical background concentrations (earth crust and continental shale values), but only Cr had dust samples which exceeded the reported SGVs.
To provide further context for the data, PTE in indoor house dusts from selected studies are reported (Table 4). Direct comparisons however are again somewhat limited as these studies focus on house dust, and differences in sample preparation and analysis also exist. However, our Lagos indoor school dust samples typically had PTE concentrations that fell within the mean/geomean data reported in these other studies (Table 4). Our observed As concentrations ranged between 1.4–4.8 mg kg−1; comparable to concentrations measured in classrooms and offices from Ogun state, Nigeria by Olujimi et al.10 and below representative countries soil guideline values (Table 3). Our Cr concentrations were also largely comparable with those data reported in Table 4. An average concentration of Cr in cement typically ranges from 179–257 mg kg−1.60 The aging concrete (cement) floors within the classrooms may have contributed to Cr concentrations measured in the samples. Whilst the majority of PTE we investigated in this study did not indicate elevated concentrations when compared to our selected SGVs (with the exception of Zn and Cr), many were higher than those measured in other Nigerian cities. For instance, Cd, Cu, Fe, Mn and Pb measurements in Ilorin city,61 Makurdi,62 and Kano city63 (other cities in Nigeria) were all lower than concentrations measured in this current study. Lagos is the commercial nerve centre of the country and over sixty percent of all industrial activities in Nigeria are domiciled in Lagos. The huge industrialization and urbanization in Lagos may have contributed to the higher PTE load in our indoor dusts in comparison to these other Nigerian cities. Indeed, in a recent study in the Niger delta area of Nigeria,64 classroom dusts collected from schools along major roads with high traffic density showed elevated PTE concentrations. In keeping with this study, several of our sampled schools were located in close proximity to busy markets (e.g. Ojuwoye and Balogun markets in Mushin, and Lagos Island LGA), and so elevated PTE might be expected in our indoor dust samples attributed to traffic sources. The high influx of vehicles, emissions from small and medium scale enterprises, alternative power supply (use of gasoline or diesel electricity generators) and artisan workshops around the vicinities of our sampled schools may all have contributed to the PTE load in the dust samples. Activities in the local vicinity will influence the PTE load in indoor dusts. Potentially toxic elements find their way into buildings through a range of mechanisms, such as backtracking of particles on people and animals, as airborne dust particles (such as through open windows), as well as from numerous indoor sources, such as building materials, furnishings and consumer products. Darus et al.71 noted that indoor dust or particle load can be associated with street dust or soil and are transported via wind blow through building openings and by the movement of occupants in and out of the building. On average, the As, Cd, Cu, Ni, Pb and Zn contents obtained in this study on Lagos were lower, and in some instances multiple times lower, than ones reported for Xian, China;1 Guangzhou, China;67 Istanbul Turkey;68 Japan,69 Stratoni Greece;70 (see Table 4). The higher PTE levels in indoor dust from these cities, compared to that of this study (Lagos), are likely connected to the long histories of industrialization and urbanization in these countries. In contrast, the industrialization of Lagos only started in the post-independence era (1960 to 70 s).72 Of particular concern is the apparent build-up of PTE in the Lagos environment over a relatively short period of time, as observed when we compare our data (on samples from 2019) with those of a previous study across Lagos24 from 2012 (although we acknowledge that these were on a different set of 20 public primary schools). Making such data publicly available can raise awareness of environmental issues and drive appropriate mitigation strategies.
| As | Cd | Cr | Cu | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|
| Ogun state, Nigeria10 | 0.96–5.33 | 82.9–8595 | 17.7–105 | 2.37–250 | 81.7–600 | 2.37–58.4 | 3.80–127 | 15.2–480 |
| Niger-delta Nigeria45 | 0.00–22.8 | 0.00–1.12 | 1.33–153 | 0.31–2.60 | 0.00–34.6 | 0.06–1.63 | 5.00–149 | |
| Lagos, Nigeria24 | 0.01–8.28 | 3.45–17.4 | 0.06–82.5 | |||||
| Xian, China1 | 6.0–38.3 | 88.9–751 | 33.2–164 | 398–824 | 20.5–104 | 87.5–593 | 184–1838 | |
| Sri Serdang, Malaysia49 | 0.4–2.65 | 6.44–86.5 | 0.17–68.3 | |||||
| Canada20 | 0.1–153 | 0.5–223 | 0.5–2930 | 24–4880 | 17.3–2300 | 14.2–7800 | 144–6630 | |
| Port Harcourt, Nigeria65 | 0.07–1.53 | 0.53–3.52 | 19–45.2 | 10.3–25.4 | ||||
| Kumasi, Ghana66 | BDL | 0.00–33.2 | 0.00–41.9 | |||||
| Guangzhou, China67 | 3.30–155 | 355–2745 | 17.7–281 | 178–3394 | 331–3928 | |||
| Istanbul, Turkey68 | 0.4–20 | 2.8–460 | 62–1800 | 8.0–1300 | 120–2600 | 3.0–300 | 210–2800 | |
| Japan69 | 0.18–5.62 | 14.8–285 | 66.8–7720 | 38.6–679 | 11.9–3730 | 188–5920 | ||
| Stratoni, Greece70 | 3–28 | 71–8390 | 390–6920 | |||||
| This study | 1.4–4.8 | 0–1.3 | 39.8–606 | 9.6–110 | 106–764 | 10.5–40.3 | 14.9–114 | 66.9–622 |
3.2 Contamination assessment
To quantify levels of PTE enrichment in the indoor dusts investigated, enrichment factors were calculated (Fig. 1). The degree of enrichment was estimated using Fe as the reference element and its average continental shale value as the background level. In the current study, the mean EnF pollution trend for the PTE studied was in the order of Pb (4.3) > Zn (3.6) > U (2.9) > Cr (2.2) > Cu (1.1) > Ba (0.84) > Mn (0.78) > V (0.7) > As (0.5) > Cd (0.3) > Ni (0.1) >Al (<0.001). Aluminium, As, Ba, Cu, Mn, Ni and V EnF values were below the limit of contamination of 1.5 suggesting no enrichment or minimal enrichment in a few cases. Exceptions included Ba with an EnF of 6.30 in sample A1. Ba and its compound have been noted to have a variety of uses including getters in electronic tubes, colorants in paints, rodenticides and X-ray contrast medium.73 Although there were no obvious sources for this enrichment in the indoor dust from the Amowo-Odofin area of Lagos.Moderate enrichment of Cu was found in samples A2 (3.31), A19 (1.51) and A39 (2.02). Anthropogenic Cu sources have been linked to car components, tyre abrasion, corrosion of galvanized metal parts in cars, lubricants, engine wear, brake dust and thrust bearings.74 So, it is not surprising to see enrichment of Cu in a number of samples taken from our urban schools, it is perhaps more surprising that we do not see this moderate enrichment more widespread across the sample set.
The Pb, U and Zn EnF values all indicated moderate to significant enrichment, with the majority of the Cr samples (63%) also indicating either moderate or significant enrichment. Lead and Zn enrichment in indoor dusts have been linked to biomass burning, vehicular traffic and industrial activities18 whilst releases from mill tailings, combustion of coal and other fuels, mining of phosphate ores, use of phosphate fertilizers and emissions from nuclear plants have variously been blamed for the anthropogenic loading of U in soils.75 Although we can rule out a nuclear source, no nuclear power plants exist in Lagos, the combustion of coal and other fuels are likely a key contributor to the widespread enrichment of Pb, Zn and U in our indoor school dusts.
3.3 Source apportionment
A wide number of methods for determining source apportionment of total elemental concentrations data have been reported in the literature43,55 and here we employ factor analysis (FA). Such source identification tools allow for the prediction or determination of the contribution of possible pollution sources to total amount of PTE. Results of varimax rotated factor loadings, communalities, Eigen values and percentage variance of PTE in the dust samples are presented in Table 5. Results showed PTE groupings in three components which accounted for 75% of the total data variation.| Variable | Factor 1 | Factor 2 | Factor 3 | Communality |
|---|---|---|---|---|
| As | 0.748 | −0.283 | −0.151 | 0.662 |
| Al | 0.832 | 0.068 | 0.161 | 0.722 |
| Ba | 0.080 | −0.261 | −0.544 | 0.370 |
| Cd | −0.095 | −0.872 | −0.104 | 0.780 |
| Cr | 0.122 | −0.271 | 0.858 | 0.825 |
| Cu | 0.252 | −0.832 | −0.009 | 0.756 |
| Fe | 0.786 | −0.481 | 0.154 | 0.873 |
| Mn | 0.737 | −0.012 | −0.073 | 0.548 |
| Ni | 0.855 | −0.327 | 0.199 | 0.877 |
| Pb | 0.468 | −0.793 | 0.155 | 0.871 |
| V | 0.739 | −0.093 | 0.630 | 0.952 |
| U | 0.780 | −0.252 | 0.020 | 0.672 |
| Zn | 0.661 | −0.657 | 0.097 | 0.877 |
| Variance | 5.0467 | 3.1490 | 1.5910 | 9.7868 |
| % Var. | 38.8 | 24.2 | 12.2 | 75.3 |
Factor 1 was dominated by Al, As, Fe, Mn, Ni, V and U accounting for 39% of the total variance. This suggests concentrations of these elements may be influenced by a common factor or source, which given the crustal abundance of many of these elements is likely to be the local lithology of the study area. Indeed, previous studies have observed this PTE grouping and their association with natural sources.18
The second component explains 24% of the total variance and was negatively loaded on Cd, Cu and Pb. The close association between these elements in urban soils and dusts have been observed and identified as ‘typical urban metals’.76 Indeed, these PTE are typically associated with releases from leaded gasoline, braking, engine wear and other traffic-related activities,68,69 and have been reported to have their sources from pesticides, coal and biomass burning, sewage sludge, and industrial emissions.18 Factor 2 thus suggests PTE originating predominantly from anthropogenic sources.
The third component explains 12% of the total variance and is primarily loaded on Cr, with a slightly lower loading on V (<0.700 but >0.600). As factor 1 has been associated with natural origins for the different PTE, and factor 2 as predominantly anthropogenic sources, factor 3 may suggest a more mixed source. Here, we should also note that Zn occurs, at moderate loadings on both factors 1 and 2 (0.661 on factor 1 and −0.657 on factor 2; Table 5). It is likely that Cr, V and Zn are indicating a mixed origin, rather than dominated by either natural or anthropogenic sources. Geochemical association of Cr with Fe and Mn hydroxides and silicate minerals may suggest a natural source, while urban sources of Cr include cigarette smoke and ash, abrasion of stainless steel, cooking pans, yellow/red/green chromium-bearing paints as well as chrome plated fixtures and fittings.77 Urban anthropogenic releases of V are typically small in comparison to lithogenic releases, with oxides and hydroxides of Fe identified as a common primary source of V, but the increased presence/use of V has been reported within urban areas.78 Zinc occurs naturally in the earth's crust with trace levels found in water, air, soil and food, but contamination often occurs in relation to industrial processes, and industrial wastes. Compounds containing Zn are commonly found in association with anthropogenic activity, as Zn is used in wood preservatives, fabric dyes, in paints, and is typically associated with the construction and the automobile industry.18
Based on the findings of our multivariate analyses we allocated each PTE to one of three groups, identifying the dominant source for that PTE in indoor school dusts across Lagos:
A predominantly lithogenically derived group: Al, As, Fe, Mn, Ni, U.
A predominantly anthropogenically derived group: Cd, Cu and Pb.
A highly mixed origin group: Cr, V and Zn.
3.4 Oral bioaccessibility
A subset of indoor dust samples (n = 8) were investigated for oral bioaccessibility (ESI Table 3†). The subset of selected samples for oral bioaccessibility measurements were chosen based on values obtained from enrichment factor calculations. Samples with high EnF values (higher concentration values) were selected for this purpose.Bioaccessibility in this study is expressed as metal solubilized in the UBM stomach phase relative to the pseudototal concentration and expressed on percentage basis (see eqn (8)):
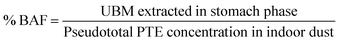 | (8) |
Vanadium, Cd were below detection in the UBM extract and are not considered further. Of the remaining PTE investigated, Fe, Cr and Ni were the least bioaccessible PTE, with mean% BAF of 7%, 10% and 28% respectively. This indicated only a small fraction of these PTE would be available in the case of accidental ingestion.
Copper (36%), As (37%) and Al (41%) indicated higher mean bioaccessible concentrations in the indoor dusts, while Pb (48%), Ba (48%) and Mn (57%) were higher again, with mean bioaccessible concentrations of close to 50% or above. Zinc was the most bioaccessible PTE (mean of 88%), with bioaccessibility of around 100% in half of the indoor dust samples studied, suggesting that the simulated UBM gastric solution was as efficient in solubilizing Zn as the aqua-regia. Assuming 100% bioavailability based on pseudototal or total concentrations would overestimate the risk of potential of these PTE.
Our observed trend in indoor dust bioaccessibility was thus, in ascending order, Fe < Cr < Ni < Cu < As < Al < Ba < Pb < Mn < Zn. Such a trend is largely comparable to the order for mean values of bioaccessibility of the elements observed in urban street dusts from a number of studies (e.g. street dust from Nanjing, China: Cd > Zn > Pb ≈ Mn > As > Cu > Ni > V > Cr ≈ Fe80). Indeed, mean Pb bioaccessibility is commonly reported to be around 50% across urban soils and street dusts (e.g. 47% bioaccessibility in urban street dusts Nanjing,79 China, 53% in urban street dusts of Newcastle upon Tyne, UK80) with typically low Cr bioaccessibility reported (e.g. 4% in some urban soils81). Studies on indoor dusts have similarly reported high bioaccessibility of Zn, Mn and Al.19 About 80 to 90% of Zn content were bioaccessible in household dusts of Estarreja, Portugal.18
Whilst we acknowledge the challenges in comparing bioaccessibility extraction test results due to the array of extraction protocols employed, differences in sample composition, environmental media (rural, urban, street dust, indoor dust, sediments etc.), geological profile of study areas etc. there do appear to be common trends in PTE bioaccessibility reported in the literature that are also evidenced in our school dusts.
3.5 Implications to human health
Results obtained for human health risk assessment (both carcinogenic and non-carcinogenic risks) for this current study are presented in Table 6. For this current study, total or pseudototal concentrations (mg kg−1) were replaced with bioaccessible concentrations since it has been established (at least for Pb, As and Cd) that the later tends to be a more realistic assessment of what is actually bioavailable in the blood stream of humans; thereby USEPA equation models were modified to accommodate this observation. Other studies have reported approximately 30% in the reduction of aggregated hazard quotients and cancer risks results obtained for assessment of human exposure to dusts when total concentrations were replaced by bioaccessible concentrations.80 Exposures to PTE in indoor dusts studied were within acceptable limits for both carcinogenic and non-carcinogenic risks for both age groups considered (children and adult). Ingestion appeared to be most significant route of exposure for both categories of age groups, followed by dermal contact and inhalation in that order. Among the PTE studied, Cr was the largest contributor for non-carcinogenic risks for both children and adult followed by Ni, Pb, Al, As, Zn, Mn and Cu in that descending order. The hazard index (HI) which is summation of hazards quotients for ingestion, inhalation and dermal contact was less than 1 which suggests both children and adults are not at susceptible to non-carcinogenic risks. The only exception to this is HI index value for Cr in children which exceeded 1. Children prone to pica tendencies or geophagy may be at risk of Cr toxicity, however, speciation studies on the indoor dust samples may be required in future studies to ascertain the form in which Cr (either Cr III or Cr VI) exists in the dust samples. Results of this current study are consistent with other previous work on indoor dusts, urban soils, urban street dust.| HQing | HQinh | HQder | ∑HI children | HQing | HQinh | HQder | ∑HI adult | CR children | CR adult | |
|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1.70 × 10−2 | 7.24 × 10−6 | 5.76 × 10−2 | 7.50 × 10−2 | 2.20 × 10−3 | 2.17 × 10−6 | 3.64 × 10−3 | 5.85 × 10−3 | ||
| As | 3.97 × 10−2 | 5.21 × 10−10 | 3.96 × 10−3 | 4.37 × 10−2 | 5.05 × 10−3 | 1.56 × 10−10 | 2.50 × 10−4 | 5.31 × 10−3 | 1.68 × 10−6 | 8.19 × 10−7 |
| Cd | 6.92 × 10−2 | 4.53 × 10−9 | 1.08 × 10−1 | 1.77 × 10−1 | 8.79 × 10−3 | 1.36 × 10−9 | 5.81 × 10−4 | 9.37 × 10−3 | ||
| Cr | 8.22 × 10−3 | 1.61 × 10−10 | 1.09 × 105 | 1.10 × 103 | 1.04 × 10−3 | 4.84 × 10−11 | 6.90 × 10−2 | 7.00 × 10−2 | 4.57 × 10−6 | 1.42 × 10−6 |
| Cu | 2.36 × 10−3 | 7.86 × 10−3 | 1.02 × 10−2 | 3.01 × 10−4 | 4.97 × 10−4 | 7.97 × 10−4 | ||||
| Mn | 7.72 × 10−3 | 1.42 × 10−8 | 2.56 × 10−2 | 3.33 × 10−2 | 9.82 × 10−4 | 4.25 × 10−9 | 1.62 × 10−3 | 2.60 × 10−3 | ||
| Ni | 1.71 × 10−3 | 2.09 × 10−7 | 1.42 × 10−1 | 1.43 × 10−1 | 2.18 × 10−4 | 6.26 × 10−8 | 8.98 × 10−3 | 9.19 × 10−3 | 5.86 × 10−6 | 1.82 × 10−6 |
| Pb | 4.68 × 10−4 | 1.33 × 10−1 | 1.33 × 10−1 | 5.95 × 10−5 | 8.42 × 10−3 | 8.48 × 10−3 | 4.42 × 10−7 | 1.37 × 10−7 | ||
| Zn | 5.65 × 10−3 | 1.87 × 10−2 | 2.44 × 10−2 | 7.19 × 10−4 | 1.18 × 10−3 | 1.90 × 10−3 |
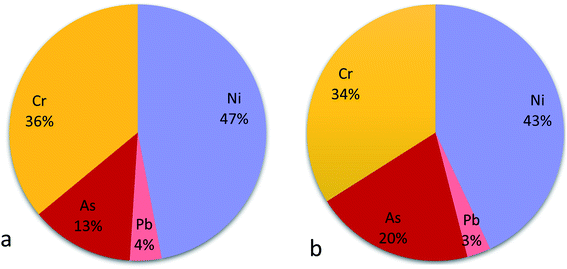
Since not all PTE are a cancer risk, only As, Cr, Ni and Pb were additionally considered for carcinogenic risks assessment, using the slope factors for these PTE available in literature. Contributions of each PTE to possible carcinogenic and non-carcinogenic risks are presented in Fig. 2 and 3. Cr and Ni were the prominent among the PTE considered. Chromium was the largest contributor to non-cancer risks (70% in children and 67% in adult; Fig. 2), whilst Ni was the largest contributor to the total cancer risk (with 47% in children and 43% in adults; Fig. 3). The mean cancer risks were within acceptable limit of no probable carcinogenic effects (10−4 to 10−6).
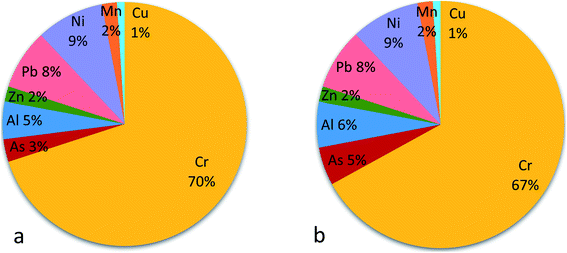 | ||
| Fig. 2 Pie chart showing contributions of PTE to noncarcinogenic risks to children (a) and adult (b). | ||
4. Conclusion
The average concentrations of PTE measured in our Lagos indoor dusts were higher than those reported from other Nigerian cities, including those of a previous study conducted in 2012,22 suggesting PTE build up in the Lagos environment in recent years. Indeed, the pollution assessment tools we employed (EnF and factor analysis) suggest anthropogenic contributions dominate the enrichment of certain PTE in the indoor dusts (i.e. Cd, Cu, Pb, and to a lesser extent Zn). However, to set this within a broader context, then the determined PTE concentrations were lower than soil guideline values established across a range of countries (e.g. UK, Norway and Canada), and the calculated risks factors (cancer and non-cancer risks) were within acceptable limits for both children and adults. Our oral bioaccessibility determinations indicated that an assumption of 100% bioavailability, as commonly employed in risk assessment protocols based on pseudototal or total concentrations would overestimate the hazard potential of PTE in these indoor dusts. Whilst Zn was the most bioaccessible PTE (mean of 88%), all of the other PTE had oral bioaccessibility determinations significantly lower than 100% (ranging from 57% for Mn down to only 7% for Fe).Human health risk assessment, for both children and adults using the bioaccessible fraction, showed values to be within acceptable risk levels, however children appeared to be more susceptible to non-carcinogenic and carcinogenic risks than adults since all hazard quotient values, HI and carcinogenic risks evaluation in children exceeded that of adults for all PTE.
To maintain the PTE content in Lagos indoor dusts as low as practicable it is important that environmental laws are strictly adhered to and enforced, in tandem with ongoing improvements in the regulatory environment. Increasing levels of environmental awareness potentially empowers citizens to take actions to reduce exposure to indoor contaminants. An integral part of this project was public dissemination of our research findings to enhance the environmental health literacy of the staff and pupils within our participant schools.
As far as possible, ‘plain english’ was used to feedback results to the schools, with data made available on an interactive site at https://mapmyenvironment.com. Embedding citizen engagement is now acknowledged and accepted as an integral part of the research process as the potential exists for wider environmental benefits which result from a more environmentally aware society.
Funding
Dr Abimbola Famuyiwa is grateful to the schools who took part and to the Analytical Chemistry Trust Fund of Royal Society of Chemistry, UK for providing the grant (Developing world scholarship) for this work, as part of a visiting international scholarship at Northumbria University, UK. J. Entwistle acknowledges funding from the Natural Environment Research Council (Research Grant NE/T004401/1) to support development of https://mapmyenvironment.com, the online portal used to display the Lagos data.Conflicts of interest
There are no conflicts to declare.References
- H. Chen, X. Lu and Y. Chang, et al., Heavy metal contamination in dust from kindergartens and elementary schools in Xian, China, Environ. Earth Sci., 2013, 71, 22701–22709 Search PubMed.
- F. J. Kelly and J. C. Fussell, Air pollution and public health: emerging hazards and improved understanding of risk, Environ. Geochem. Health, 2015, 37(4), 631–649 CrossRef CAS PubMed.
- World Health Organization, W. H. O Burden of disease from Household Air Pollution, http://www.who.int/phe/health_topics/outdoorair/databases/FINAL_HAP_AAP_BoD_24March2014.pdf, accessed 12/01/2021 Search PubMed.
- V. L. Feigin, G. A. Roth and M. Naghavi, et al., Global burden of stroke and risk factors in 188 countries, during 1990 & 2013: a systematic analysis for the Global Burden of Disease Study 2013, Lancet Neurol., 2016, 15(9), 913–924 CrossRef.
- B. A. Maher, A. M. Imad and M. Ahmed, et al., Magnetite pollution nanoparticles in the human brain, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(39), 10797–10801 CrossRef CAS PubMed.
- M. Pirani, N. Best and M. Blangiardo, et al., Analysing the health effects of simultaneous exposure to physical and chemical properties of airborne particles, Environ. Int., 2015, 79, 56–64 CrossRef CAS PubMed.
- N. J. Anderson, R. Strader and C. I. Davidson, Airborne reduced nitrogen: ammonia emissions from agriculture and other sources, Environ. Int., 2003, 29, 277–286 CrossRef CAS PubMed.
- World Health Organization, Outdoor air pollution a leading environmental cause of cancer deaths, 2017, available from: http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/news/news/2013/10/outdoor-air-pollution-a-leading-environmental-cause-of-cancer-deaths, accessed 12/12/2020 Search PubMed.
- J. A. Entwistle, A. S. Hursthouse and P. A. Marinho Reis, et al., Metalliferous Mine Dust: Human Health Impacts and the Potential Determinants of Disease in Mining Communities, Curr. Pollut. Rep., 2019, 5, 67–83 CrossRef CAS.
- O. Olujimi, O. Steiner and W. Goessler, Pollution indexing and health risk assessments of trace elements in indoor dusts from classrooms, living rooms and offices in Ogun State Nigeria, J. Afr. Earth Sci., 2015, 101, 396–404 CrossRef CAS.
- M. H. Zia, E. E. Codling and K. G. Scheckel, et al., In vitro and in vivo approaches for the measurement of oral bioavailability of lead (Pb) in contaminated soils: a review, Environ. Pollut., 2011, 159(10), 2320–2327 CrossRef CAS PubMed.
- J. A. H. Alpofaed, C. M. Davidson and D. Littlejohn, Oral bioaccessibility tests to measure potentially toxic elements in inhalable particulate matter collected during routine air quality monitoring, Anal. Methods, 2016, 8, 5466 RSC.
- F. Kasturya, E. Smitha and R. R. Karnab, et al., Methodological factors influencing inhalation bioaccessibility of metal(loid)s in PM2.5 using simulated lung fluid, Environ. Pollut., 2018, 241, 930–937 CrossRef PubMed.
- N. I. Elom, J. Entwistle and J. R. Dean, Human health risk from Pb in urban street dust in northern UK cities, Environ. Chem. Lett., 2014, 12, 209–218 CrossRef CAS.
- United Nations, Department of Economic and Social Affairs, Population Dynamics, 2018 Revision of World Urbanization Prospects, https://population.un.org/wup/, accessed 20/01/2020 Search PubMed.
- T. Falta, A. Limbeck, G. Koellensperger and S. Hann, Bioaccessibility of selected trace metals in urban PM2.5 and PM10 samples: a model study, Anal. Bioanal. Chem., 2008, 390(4), 1149–1157 CrossRef CAS PubMed.
- G. M. Filippelli, J. Adamic and D. Nichols, et al., Mapping the Urban Lead Exposome: A Detailed Analysis of Soil Metal Concentrations at the Household Scale Using Citizen Science, Int. J. Environ. Res. Public Health, 2018, 15, 1531 CrossRef PubMed.
- A. P. Reis Marinho, M. Cave and A. J. Sousa, et al., Lead and zinc concentrations in household dust and toenails of the residents (Estarreja, Portugal): a source-pathway-fate model, Environ. Sci.: Processes Impacts, 2018, 20(9), 1210–1224 RSC.
- A. Turner and K. H. Ip, Bioaccessibility of metals in dust from the indoor environment: application of a physiologically based extraction test, Environ. Sci. Technol., 2007, 41(22), 7851–7856 CrossRef CAS PubMed.
- P. E. Rasmussen, C. Levesque and M. Chenier, et al., Canadian House Dust Study: population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes, Sci. Total Environ., 2013, 9, 443–520 Search PubMed.
- C. Puls, A. Limbeck and S. Hann, Bioaccessibility of palladium and platinum in urban aerosol particulates, Atmos. Environ., 2012, 55, 213–219 CrossRef CAS.
- D. O. Olukanni and D. O. Adeoye, Heavy Metal Concentrations in Road Side Soils from Selected Locations in the Lagos Metropolis, Nigeria, Int. J. Eng. Technol., 2012, 1743–1751 Search PubMed.
- A. O. Famuyiwa, C. M. Davidson and A. O. Oyeyiola, et al., Pollution characteristics and health risk assessment of potentially toxic elements in school playground soils: a case study of Lagos, Nigeria, Hum. Ecol. Risk Assess., 2019, 25, 1729–1744 CrossRef CAS.
- O. E. Popoola, O. Bamgbose and O. J. Okonkwo, et al., Heavy metals content in classroom dusts of some public primary schools in Metropolitan Lagos, Nigeria, Res. J. Environ. Earth Sci., 2012, 4, 460–465 CAS.
- Top 10 Fastest Growing Cities In The World, 2014, available from: http://omglist.com/travel/top-10-fastest-growing-cities-in-the-world/, accessed 13/07/2020 Search PubMed.
- Lagos population, 2019, available from http://worldpopulationreview.com/world-cities/lagos-population/13/07/2020 Search PubMed.
- J. Wragg, M. Cave and N. Basta, et al., An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil, Sci. Total Environ., 2011, 409(19), 4016–4030 CAS.
- A. Okorie, J. Entwistle and J. R. Dean, Estimation of daily intake of potentially toxic elements from urban street dust and the role of oral bioaccessibility testing, Chemosphere, 2012, 86(5), 460–467 CrossRef CAS PubMed.
- S. Denys, J. Caboche and K. Tack, et al., In vivo validation of the unified BARGE method to assess the bioaccessibility of arsenic, antimony, cadmium, and lead in soils, Environ. Sci. Technol., 2012, 46(11), 6252–6260 CrossRef CAS PubMed.
- J. Li, K. Li and X. Cui, et al., In vitro bioaccessibility and in vivo relative bioavailability in 12 contaminated soils: method comparison and method development, Sci. Total Environ., 2015, 532, 812–820 CrossRef CAS PubMed.
- A. Pelfrêne and F. Douay, Assessment of oral and lung bioaccessibility of Cd and Pb from smelter-impacted dust, Environ. Sci. Pollut. Res., 2018, 25(4), 3718–3730 CrossRef PubMed.
- Spectro Analytical Instruments. An application brief from Spectro Analytical Instruments, XRF-113-AB, https://extranet.spectro.com/-/media/9B656726-B9E4-4910-89EF-64949FDFA493.pdf, accessed 12-08-2020 Search PubMed.
- E. M. Hamilton, T. S. Barlow, C. J. B. Gowing and M. J. Watts, Bioaccessibility performance data for fifty-seven elements in guidance material BGS 102, Microchem. J., 2015, 123, 131–138 CrossRef CAS.
- Y. Faiz, M. Tufail and M. T. Javed, Road dust pollution of Cd, Cu, Ni, Pb and Zn along Islamabad expressway, Pakistan, Microchem. J., 2009, 92, 186–192 CrossRef CAS.
- S. Jahan and V. Strezov, Comparison of pollution indices for the assessment of heavy metals in the sediments of seaports of NSW, Australia, Pollut. Bull., 2018, 128, 295–306 CrossRef CAS PubMed.
- A. Rehmana, G. Liu and B. Yousafa, et al., Characterizing pollution indices and children health risk assessment of potentially toxic metal(oid)s in school dust of Lahore, Pakistan, Ecotoxicol. Environ. Saf., 2020, 190, 110059 CrossRef PubMed.
- J. Iqbal and M. H. Shah, Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan, J. Hazard. Mater., 2011, 192, 887–898 CrossRef CAS PubMed.
- O. F. Olorundare, K. OIpinmoroti, A. V. Popoola and J. G. Ayenimo, Anthropogenic Influence on Selected Heavy Metal Contamination of Urban Soils of Akure City, Nigeria, Soil Sediment Contam., 2011, 20, 509–524 CrossRef CAS.
- X. Jiao, Y. Teng and Y. Zhan, et al., Soil heavy metal pollution and risk assessment in Shenyang industrial district, Northeast China, PLoS One, 2015, 10, e0127736 CrossRef PubMed.
- K. K. Turekian and K. H. Wedepohl, Distribution of the Elements in Some Major Units of the Earth's Crust, Geol. Soc. Am. Bull., 1961, 72, 175–192 CrossRef CAS.
- J. Zhang and C. L. Liu, Riverine Composition and Estuarine Geochemistry of Particulate Metals in China—Weathering Features, Anthropogenic Impact and Chemical Fluxes, Estuarine, Coastal Shelf Sci., 2002, 54, 1051–1070 CrossRef CAS.
- W. Butte and B. Heinzow, Pollutants in House Dust as Indicators of Indoor Contamination, Rev. Environ. Contam. Toxicol., 2002, 175, 1–46 CAS.
- P. E. Rasmussen, K. S. Subramanian and B. J. Jessiman, A multi-element profile of house dust in relation to exterior dust and soils in the city of Ottawa, Canada, Sci. Total Environ., 2001, 267, 125–140 CrossRef CAS PubMed.
- P. E. Rasmussen, S. Beauchemin and M. Nugent, et al., Influence of matrix composition on bioaccessible copper, zinc and nickel in urban residential dust and soil, Hum. Ecol. Risk Assess., 2008, 14(2), 351–371 CrossRef CAS.
- K. H. Kim, E. Kabir and S. Kabir, A review on the human health impact of airborne particulate matter, Environ. Int., 2015, 74, 136–143 CrossRef CAS PubMed.
- USEPA (United States Environmental Protection Agency), Human Health Evaluation Manual, in Risk Assessment Guidance for Superfund, vol. l. Office of Solid Waste and Emergency Response, EPA/540/1e89/002, 1989 Search PubMed.
- USEPA (United States Environmental Protection Agency), Soil Screening Guidance: Technical Background Document, Office of Solid Waste and Emergency Response, EPA/540/R-95/128, 1996 Search PubMed.
- USEPA (United States Environmental Protection Agency), Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites, Office of Solid Waste and Emergency Response, OSWER 9355.4e24, 2001 Search PubMed.
- USEPA, Risk assessment guidance for superfund, in Part A: Human Health Evaluation Manual; Part E, Supplemental Guidance for Dermal Risk Assessment; Part F, Supplemental Guidance for Inhalation Risk Assessment, 2011a, vol. I Search PubMed.
- Exposure Factors Handbook Chapter 5 (Update): Soil and Dust Ingestion, 2017, https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=337521, accessed 12/05/2020 Search PubMed.
- https://www.worldlifeexpectancy.com/nigeria-life-expectancy, accessed 01/02/2021.
- N. Rafique and S. R. Tariq, Distribution and source apportionment studies of heavy metals in soil of cotton/wheat fields, Environ. Monit. Assess., 2016, 188, 309 CrossRef PubMed.
- Map My Environment, Urban Environmental Health - Map My Environment, https://www.mapmyenvironment.com, accessed: 26/09/2020 Search PubMed.
- H. Liu, M. Kobernus and D. Broday, et al., A conceptual approach to a citizens' observatory – supporting community-based environmental governance, Environ. Health, 2014, 13, 107 CrossRef PubMed.
- A. G. Oomen and J. P. A. Lijzen, Relevancy of Human Exposure Via House Dust to the Contaminants Lead and Asbestos, RIVM report no. 711701037, 2004, http://rivm.openrepository.com/rivm/bitstream/10029/9070/1/711701037.pdf, accessed 07/8/2020 Search PubMed.
- M. Van Holderbeke, C. Cornelis, J. Bierkens and R. Torfs, Review of the Soil Ingestion Pathway in Human Exposure Assessment. Study in Support of the BeNeKempen Project—Subproject on Harmonization of the Human Health Risk Assessment Methodology, Final Report, VITO, Mechelen, Belgium, 2008, 2008/IMS/R/015, https://www.researchgate.net/profile/Johan_Bierkens/publication/227096808_Exposure_Through_Soil_and_Dust_Ingestion/links/00b4951b850b013a28000000.pdf, accessed 07/8/2020 Search PubMed.
- USEPA, Review of the Dust-Lead Hazard Standards and the Definition of Lead-Based Paint, FRL-9976-04, EPA-HQ-OPPT-2018-0166, 2018, https://www.federalregister.gov/documents/2018/07/02/2018-14094/review-of-the-dust-lead-hazard-standards-and-the-definition-of-lead-based-paint, accessed 07/8/2020 Search PubMed.
- A. A. Adeyi and B. A. Babalola, Lead and Cadmium Levels in Residential Soils of Lagos and Ibadan, Nigeria, J. Health Pollut., 2017, 7(13), 42–55 CrossRef PubMed.
- A. Hunt, D. L. Johnson, J. Brooks and D. A. Griffith, Risk remaining from fine particle contaminants after vacuum cleaning of hard floor surfaces, Environ. Geochem. Health, 2008, 30(6), 597–611 CrossRef CAS PubMed.
- A. Eštoková, L. Palaščákováa, E. Singovszkáb and M. Holub, Analysis of the chromium concentrations in cement materials, Procedia Eng., 2012, 42, 124–130 Search PubMed.
- F. A. Adekola and O. O. Dosunmu, Heavy metal determination in household dusts from Ilorin city, Nigeria, NISEB J., 2001, 1, 217–221 Search PubMed.
- S. E. Ishaq, U. A. Itodo and K. A. Julius, Assessment of Heavy Metals in Indoor Settled Harmattan Dust from the University of Agriculture Makurdi, Nigeria, Open J. Air Pollut., 2015, 4(4), 198–207 CrossRef.
- S. O. Sanni, Determination of heavy metals (Pb, Cu, Cd) in dust from selected household in Kano city, Nigeria, Int. J. Adv. Acad. Res., 2019, 5(1), 1–11 Search PubMed.
- C. M. A. Iwegbue, E. C. Oliseyenum and B. S. Martincigh, Spatio-temporal distribution of metals in household dust from rural, semi-urban and urban environments in the Niger Delta, Nigeria, Environ. Sci. Pollut. Res. Int., 2017, 16, 14040–14059 CrossRef PubMed.
- V. Olua, P. K. Chukwuemeka and E. O. Nwaichi, Heavy metals content and health risk assessment of classroom corner dusts in selected primary schools in Rivers state, Nigeria, J. Environ. Pollut. Hum. Health, 2018, 6(4), 138–147 CrossRef.
- M. A. Nkansah, J. R. Fianko, S. Mensah, M. Debrah and G. W. Francis, Determination of heavy metals in dust from selected nursery and kindergarten classrooms within the Kumasi metropolis of Ghana, Cogent Chem., 2015, 1, 1 Search PubMed.
- J. Zheng, K. Chen, X. Yan and S. Chen, et al., Metals in food, house dust, and water from an e-waste recycling area in South China and the potential risk to human health, Ecotoxicol. Environ. Saf., 2013, 96, 205–212 CrossRef CAS PubMed.
- P. B. Kurt-Karakus, Determination of heavy metals in indoor dust from Istanbul, Turkey: estimation of the health risk, Environ. Int., 2012, 50, 47–55 CrossRef CAS PubMed.
- J. Yoshinaga, K. Yamasaki, A. Yonemura and Y. Ishibashi, et al., Lead and other elements in house dust of Japanese residences – Source of lead and health risks due to metal exposure, Environ. Pollut., 2014, 189, 223–228 CrossRef CAS PubMed.
- A. Argyraki, Garden soil and house dust as exposure media for lead uptake in the mining village of Stratoni, Greece, Environ. Geochem. Health, 2014, 36, 677–692 CrossRef CAS PubMed.
- F. M. Darus, R. A. Nasir and S. M. Sumari, et al., Heavy Metals Composition of Indoor Dust in Nursery Schools Building, Procedia Soc. Behav. Sci., 2012, 38, 169–175 CrossRef.
- D. W. Dagogo, Nigerian Industrial Development between 1943 and 2013: Challenges and Opportunities, Int. Rev. Res. Emerg. Mark. Glob. Econ., 2014, 1(3), 132–148 Search PubMed.
- G. H. Ong, C. K. Yap and M. Mahmood, et al., Barium Levels in Soils and Centella asiatica, Trop. Life Sci. Res., 2013, 24(1), 55–70 CAS.
- O. A. Al-Khashman, Heavy metal distribution in dust, street dust and soils from the work place in Karak Industrial Estate, Jordan, Atmos. Environ., 2004, 38(39), 6803–6812 CrossRef CAS.
- S. J. Kaishwa, E. M. Marwa, J. J. Msaky and W. N. Mwakalasya, Uranium natural levels in soil, rock and water: assessment of the quality of drinking water in Singida Urban District, Tanzania, J. Water Health, 2018, 16(4), 542–548 CrossRef CAS PubMed.
- M. Biasioli, R. Barberis and F. Ajmone-Marsan, The influence of a large city on some soil properties and metals content, Sci. Total Environ., 2006, 356(1), 154–164 CrossRef CAS PubMed.
- D. Hou, D. O'Connor, P. Nathanail, L. Tian and Y. Ma, Integrated GIS and multivariate statistical analysis for regional scale assessment of heavy metal soil contamination: a critical review, Environ. Pollut., 2017, 231, 1188–1200 CrossRef CAS PubMed.
- I. Guagliardi, D. Cicchella and R. De Rosa, et al., Geochemical sources of vanadium in soils: evidences in a southern Italy area, J. Geochem. Explor., 2018, 184, 358–364 CrossRef CAS.
- X. Hu, Y. Zhang and J. Luo, et al., Bioaccessibility and health risk of arsenic, mercury and other metals in urban street dusts from a mega-city, Nanjing, China, Environ. Pollut., 2011, 159, 1215–1221 CrossRef CAS PubMed.
- A. Okorie, J. Entwistle and J. R. Dean, Estimation of daily intake of potentially toxic elements from urban street dust and the role of oral bioaccessibility testing, Chemosphere, 2012, 86(5), 460–467 CrossRef CAS PubMed.
- A. Broadway, M. R. Cave and J. Wragg, et al., Determination of the bioaccessibility of chromium in Glasgow soil and the implications for human health risk assessment, Sci. Total Environ., 2010, 409(2), 267–277 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0em00445f |
| This journal is © The Royal Society of Chemistry 2021 |