Why analysing microplastics in floodplains matters: application in a sedimentary context
Simone
Lechthaler
 *ab,
Verena
Esser
b,
Holger
Schüttrumpf
a and
Georg
Stauch
b
*ab,
Verena
Esser
b,
Holger
Schüttrumpf
a and
Georg
Stauch
b
aInstitute of Hydraulic Engineering and Water Resource Management, RWTH Aachen University, Mies-van-der-Rohe-Straße 17, 52074 Aachen, Germany. E-mail: lechthaler@iww.rwth-aachen.de; Fax: +49 0241 80-25750; Tel: +49 0241 80-25266
bDepartment of Geography, Chair of Physical Geography and Geoecology, RWTH Aachen University, Wüllnerstraße 5b, 52062 Aachen, Germany
First published on 27th November 2020
Abstract
Microplastics in the environment are a relatively new form of anthropogenic contamination. Right now, the research focus is on the detection of microplastic accumulation in different environmental compartments and understanding the processes that have led to its transport. Detailed information on microplastics in floodplain areas and their distribution in depth are still missing to better understand accumulation points. Therefore, this study presents on the one hand microplastic detection in fluvial sediments from nine sampling sites along a river course. Polymers were determined with infrared spectroscopy and additional sedimentary analysis of the grain size and heavy metal concentration was performed. In total, there was less microplastic in the upper than in the lower river course and slip-off slopes were identified as accumulation hotspots also in deeper sediment layers. Mostly, microplastic particles were detected in fine sediment and heavy metal concentrations along the river were similar to those of microplastics. On the other hand, besides the spatial distribution of microplastics and accumulation in floodplain areas, microplastic analysis offered information in a sedimentary context. Sedimentation rates (0.29–4.00 cm a−1) and patterns between temporal deposition and microplastic polymers were identified. The basis for the development of a dating method by detection of MPs in sediments was thus established. Microplastics as a contaminant provide, in addition to the identification of deposition areas, further data in a temporal and sedimentary perspective.
Environmental significanceMicroplastics as anthropogenic contaminants are mainly transported in fluvial systems and accumulate in fluvial sediments which thus act as sink. With the detection of microplastics there, sedimentation patterns can be identified to understand not only fluvial processes that influence the accumulation and remobilisation but also the life cycle of microplastics within the environment. Furthermore, microplastics as contaminants especially in floodplains can be used in a wider, sedimentary context, similar to other anthropogenic contaminants such as heavy metals, to reconstruct sedimentation there. In this manner, not only processes can be analysed but also the effects can be defined and used. Therefore, microplastics are a relatively new and important part of environmental processes that need to be investigated. |
1 Introduction
Microplastics (MP), defined as plastic particles with d < 5 mm,1 are ubiquitous in the environment and have already been detected in remote mountain areas and arctic deep sea sediments.2,3 Plastic particles have polluted most marine habitats,4 and since the 1970s, plastic has been identified as a buoyant pollutant in the open ocean.5,6 One advantage of plastic is its persistence; yet, the long residence times of the material soon become a disadvantage when plastic enters the environment.7 Although a high contamination of MPs has already been measured in different areas,3,8,9 the mass production of plastic and therefore accompanying environmental pollution started only in the 1940s–1950s.10–13 This time, here 1950 was chosen, can be set as point zero for a possible, following deposition of MPs in the environment.Fluvial systems have been identified as main pathways for MPs of different sources such as littering (the intentional disposal of waste in the environment), washing effluents, tyre abrasion, material loss from industries or landfills. Within the life cycle of MPs, especially sediments in fluvial and marine systems act as (temporal) sinks.14 The densities of MPs vary greatly from 11 kg m−3 up to 2300 kg m−3, while sediments have an average density of 2650 kg m−3.15,16 These densities indicate that MPs behave differently from sediments in riverine processes in terms of various parameters such as flow velocities and turbulences. Apart from density, the MP size and the grain size of a sediment influence transport and sedimentation processes. Waldschläger and Schüttrumpf (2019) already showed that the settling and rise velocities and the erosion behaviour of MPs in aquatic environments differ from those of the sediment and that MPs are therefore more mobile than sediment grains.17,18
However, sediments are major sinks in fluvial systems for MPs and other contaminants such as heavy metals.19,20 During transport in rivers, contaminants accumulate in fluvial sediments from riverbeds and floodplain areas.21,22 Furthermore, in aquatic and terrestrial areas, heavy metals can accumulate directly on MPs, which, in turn, can act as a vector.23–26 Like MPs, heavy metals are persistent pollutants and can be remobilised, transported and accumulated in sediments; unlike MPs that are transported with fine sediment without any adsorbance, heavy metals can be adsorbed on the fine sediment fraction.27–30 Sediment-bound heavy metals also accumulate in floodplain areas and therefore can be used as additional indicators to identify anthropogenic contamination,31 and provide information about geochronological deposition.20,32
Floodplains are characterised by inundation during floods and by deposition of fluvial sediments and different contaminants. Thus, they offer flood induced stratigraphic records and are particularly suitable sites to detect anthropogenic contaminants that are transported in the river and accumulated there.13,20 It is already assumed that the highest MP concentrations do not occur in-channel but on floodplain areas.33 So far, floodplains have been insufficiently studied in terms of MP abundance from source to mouth and in depth since only one 2018 study34 and one 2020 study13 have considered MPs in floodplain areas. In contrast, this study focuses on the further application of MP detection in fluvial systems and additionally analyses grain sizes and heavy metals to gain a holistic view of anthropogenic contaminants in connection with fluvial sediments. This was also the second time that depths >25 cm were sampled and analysed for MPs.13,35 All in all, aquatic environments, including marine and lacustrine areas, provide ideal conditions for (heavy) metals and also organic pollutants to be incorporated and permanently fixed in sediments offering records on various influences.30
A further application of MP detection in sediment is the connection to temporal sediment deposition as the detection process is easier to realise compared to other methods. Hence, MPs are discussed to be a new time marker of the Anthropocene.36,37 The coherence between the deposition of plastics (including MPs) and a time reference (earliest possible deposition after 1950) has already been mentioned in different publications but have not yet been confirmed by sampling and related analysis.13,36–41 Thus, sediments record a man-induced contamination in a specific period of time.30 Regarding plastics (and MPs), the year of polymer development can additionally be seen as the maximum deposition time for each particle that has been detected in a sediment layer, since they can be listed chronologically: polyethylene (PE) 1933, polyamide (PA) 1934, polystyrene (PS) 1937, cellulose acetate (CA, cigarette filters) 1938, polyvinyl chloride (PVC, water pipes) 1940, expanded polystyrene (EPS) 1954, polypropylene (PP) 1959 and polyethylene terephthalate (PET) 1973 (see Fig. 8).7,37
Therefore, if MPs in fluvial sediments are applied as a marker they can be used to investigate the following three aspects and related issues in fluvial systems:
1. The occurrence of MPs in the sediment along the river course in a spatial and temporal scale: where along the river course and in which sediment depths in floodplains was MP detected?
2. The characteristics of the grain size regarding MPs in the sediment samples: is there a correlation between the sediment grain size and MP in the floodplain sediment?
3. The relation between heavy metals and MPs in the sediment samples in a spatial context: is the abundance of MPs and heavy metals in the sediment comparable?
The added value of MP detection indicates temporal classification, including an approximate, possible deposition after 1950, with which sedimentation rates can be determined and which also provides information about the transport and deposition processes of MP in fluvial systems. With the investigation of the sediment grain size and heavy metals, additional information about sediments with high accumulation rates can be obtained and a comparison of the transport and deposition of MPs can be drawn on heavy metal concentrations. By detecting MPs in fluvial sediments, which may not include all types of polymers depending on the density, these aspects can be transferred to any river to fill knowledge gaps and better understand the life cycle of MPs. Compared to heavy metals, it can be assumed, that MPs have no natural or geogenic background.13 Since MPs have so far only been regarded as a contaminant in the environment, this study shows a more far-reaching application in a sedimentary context.
2 Materials and methods
2.1 Study area
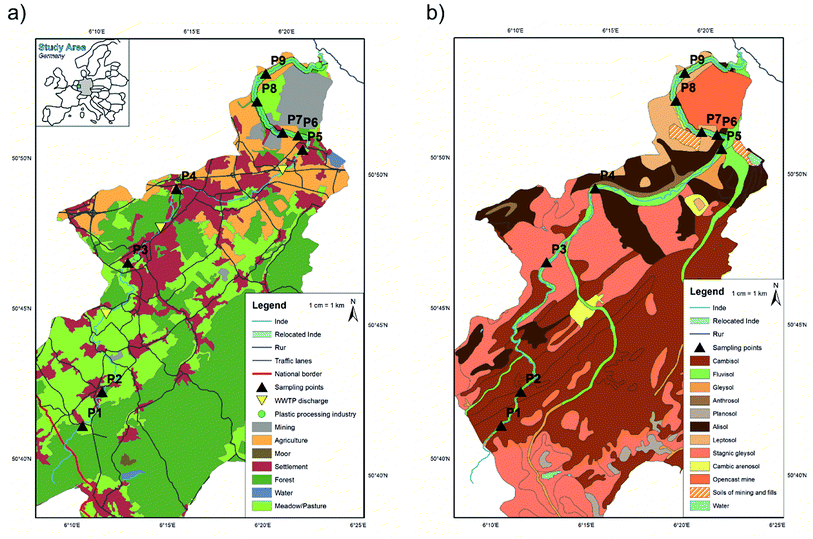
The Inde River is 54.1 km long and located in North-Rhine Westphalia, Germany with a source region located in Belgium. The catchment area is about 344 km2 with a mean discharge (MQ) of 2.8 m3 s−1 (1965–2017).42 The river was chosen because of anthropogenic influences where MPs (littering, car tyres, washing fibres etc.) can enter the fluvial system on different transport paths (e.g. surface runoff, and wastewater treatment plant (WWTP) discharge) (Fig. 1a). Today, the catchment area is dominated by forest areas (45.2%), followed by grassland (21.5%), settlement and industrial areas (15.7%) and agricultural areas (10.5%).43 Low MP concentrations in the upper part and high concentrations in the lower part were expected due to different landcovers throughout the river catchment. Sampling points were selected accordingly.The lower part of the river was artificially relocated in 2005 due to nearby opencast mining activity (see Fig. 1 ‘Relocated Inde’). This stretch ranges from a river kilometre of 41.1 to the river's mouth at a kilometre of 54.1. Since the properties of the material used for building the riverbed and floodplain areas were known (loess and gravel; d15 of 0.06 mm, d50 of 0.4 mm, and d90 of 3 mm),31 the thickness of the accumulated layer within the relocated river section can be defined (here sites P6–P9). A previous contamination of this material with MPs cannot be entirely excluded.
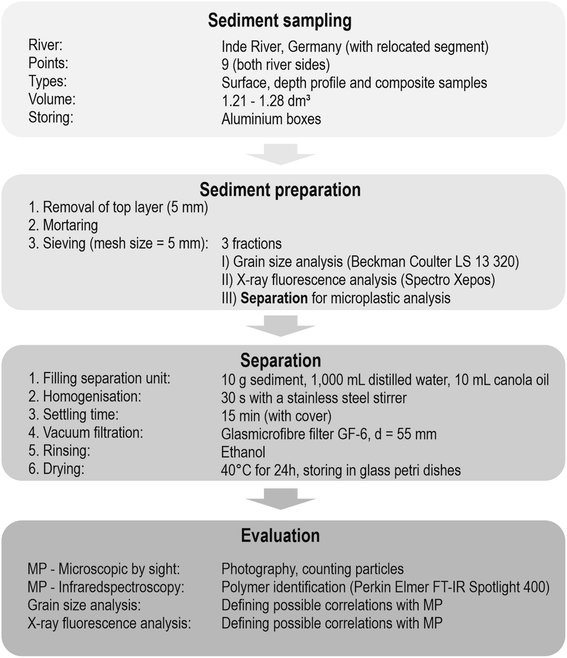
The methodological procedure was divided into four steps: sampling, sediment preparation, separation and evaluation (Fig. 2). With this methodological procedure larger particles (0.5 mm ≤ d < 5 mm) were considered for the detection of MPs.
2.2 Sediment sampling
The samples were taken from nine sampling sites along the river course. If possible, both sites of the rivers were sampled and 15 floodplain areas were analysed in total. Regarding the soil type (Fig. 1b), all samples contained sediment from fluvisol.At each sampling site three different sampling types were applied: composite, depth profile and surface samples (cf.Fig. 3). Composite sediment samples were taken from the riverbed to see if MPs have been transported in the river and sedimented on the shores. To include the impact of previous flood events on the sedimentation of MPs, the floodplain area was analysed by taking samples from depth profiles. For this purpose, a trench was prepared within the embankment of the floodplain, a sampling track was created and the samples were taken from the top to bottom at 10 cm intervals. Only the top layer was sampled in smaller intervals (5 cm, see Fig. 3) to capture possible recent short-term events more accurately. Thus, 15 depth profiles were sampled in total, eight of them in cut-off slopes and seven in slip-off slopes, whereby the following depths were obtained: 20 cm (n = 1), 30 cm (n = 3), 40 cm (n = 6), 60 cm (n = 2), 70 cm (n = 1), 90 cm (n = 1) and 110 cm (n = 1). These different sampling depths were determined by the on-site conditions at the different sampling sites. Additionally, surface samples (upper 5 cm) were taken to define the flooding area. Depending on the morphology at each site, one to three surface samples were taken. All samples were stored in aluminium boxes with a volume of 1.21–1.28 dm3, which were each completely filled with sediment. Aluminium boxes and stainless steel spatulas and ladles were used for sampling to prevent a possible cross contamination from the sampling and storing equipment. In the whole Inde catchment 135 samples were collected, 100 from depth profile sediments, 25 from surface sediments and 10 from riverbed sediments (composite samples).
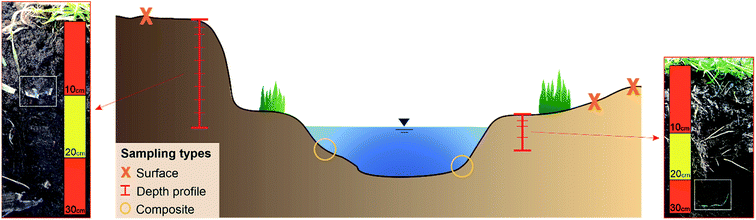 | ||
| Fig. 3 Schematic illustration of a sampling point with the three different sampling types and two depth profiles (not scaled) where larger plastic items have been detected. | ||
2.3 Sediment preparation
All sediment samples were dried after collection. Since the aluminium boxes were opened for drying, the top layer (5 mm) with possible cross contamination was removed and therefore left out of the analysis. In addition, during the whole preparation and separation process all used materials were pre-rinsed and cotton coats were used to avoid cross-contamination.The sediment was homogenised in a mortar and three parts of each sample were separately prepared for grain size analysis (GSA), X-ray fluorescence analysis (XRF) and MP analysis. The grain size was measured with a laser diffracter (Beckman Coulter LS 13320). This method is well established in research due to the fast and precise generation of results.44 The laser diffracter measures grain sizes in a range of 0.04–2000 μm by putting the sample material into the suspension whereby it circulates within the sample cell. To ensure that each sediment grain is detected, one measurement value per second is generated based on the refraction of the laser beam at the sediment grains. After 90 seconds a mean grain size distribution can be calculated.45 A detailed description of the procedure of XRF can be found in Esser et al. (2020).46 For MP analysis, the previously mortarised sediment was sieved (mesh size = 5 mm). It needs to be mentioned that due to mortaring it is possible that larger plastic particles break down to smaller ones. However, it is important to separate aggregated materials.
2.4 Separation
Separation is necessary to extract MPs from sediment samples and 10 g of each sediment sample, analogous to other sediment sampling for MP analysis,15,47,48 were used for separation, representing a random sample. It must be clearly highlighted, that the low sediment amount thus presents only a limited insight into a possible MP contamination but the sediment volume was determined by the applied methodology of oil extraction in conjunction with a plastic free separation unit, which has already been extensively validated.49 In this method the volume of the sediment to be separated and analysed is hence limited to 10 g.The separation unit, a Sediment-Microplastic-Isolator (SMI, detailed described in Lechthaler et al. 2020 (ref. 49)), was filled with sediment (10 g), distilled water (1000 mL) and canola oil (10 mL). The SMI was replicated in aluminium as described by Coppock et al. (2017)50 and is therefore plastic free. Sediment, distilled water and canola oil were homogenised with a stainless steel stirrer manually for 30 seconds. During the following settling time of 15 minutes an aluminium cover was used for the SMI to prevent cross contamination. Afterwards, the gate valve that separates the lower part with sediment and distilled water from the upper part with the canola oil layer and possible MP particles was closed. Due to the lipophilic characteristics of plastic, the MP particles concentrate in the oil layer. This setup and as well as the separation efficiency for different MP types has already been shown in preliminary tests.49 The upper part was vacuum filtered right from the SMI, so no further separation step was necessary that could have led to a loss of material. Glassmicrofibre filters (MN GF-6, d = 55 mm, pore size = 0.6 μm) were used for vacuum filtration. After filtration, the filters were rinsed with ethanol (96%) to avoid interference frequency in further spectroscopic analysis.51 The filters were stored at 40 °C for 24 hours in a drying oven and afterwards placed in glass petri dishes for microscopic and infrared analysis.
2.5 Evaluation
After separation, each sample was microscopically analysed to detect MPs with a lower size limit of 0.5 mm. Thereby, typical characteristics, such as no cellular or organic structure, shininess, unnatural colours or reflective properties,52–54 were used for MP identification. With the visual identification of particles in a size range of 0.5–5 mm, the probability of misidentification is assumed to be lower than in a size range <0.5 mm. Therefore, and in order to obtain information on the shape and colour, microscopic analysis was firstly carried out and all suspected MP particles were photographed. For additional polymer identification particles were analysed with Fourier-transform infrared spectroscopy (FT-IR) by using an NIR Spectrometer System of Perkin Elmer FT-IR Spotlight 400 along an attenuated total reflection (ATR) Imaging Accessory with a Germanium crystal and a Mercury Cadmium Telluride detector (MCT). Since MP particles had to be removed from the sample for the measurements, only half of all microscopically detected polymers could be identified with FT-IR (similar to Lusher et al. 2013 (ref. 55)). Thus, a theoretical error ratio (relative abundance of not identified MP particles) and a verification rate (relative abundance of properly identified MP particles) have been calculated. The corrected MP quantification (MPcorr) was used for further analysis and as basis for correlation and MP load development. In addition to MP analysis, GSA and XRF were performed for each sediment sample.3 Results and discussion
In 69 of all 135 sediment samples MPs were visually identified in a size range of 0.5–5.0 mm (in total 176 particles), which therefore only represents particles in this size category and leaves out possible smaller MPs. 51.9% of the identified shapes were films, 30.4% fragments and 8.9% each fibres and pellets. The predominant colour of all MPs was white (47.7%), and other determined colours were transparent (26.9%), blue (11.5%), green (6.9%), red (3.8%), black (2.3%) and yellow (0.8%). The observations of films as the predominant shape and white as the most detected colour are similar to those in other studies and especially to MPs detected in fluvial sediments.13,15,56 Nevertheless, it must be pointed out that despite the large number of samples (n = 135), the amount of sediment per sample was very small at (10 g) and the results consequently represent only a fraction.MP contamination in this study area can be traced back to different sources. Since no biological effects are taken into consideration, MPs are referred to as a contaminant.57 During sampling, numerous articles of plastic waste in various sizes were found within different layers of the depth profiles (cf.Fig. 3). Such waste can enter the river by wind or surface runoff, be transported in water, fragmented to smaller sizes (MP) and deposited in floodplains as a result of flooding. Since plastic packaging represented the main part of detected waste, littering can be seen as a major source. For sampling sites outside from settlements or walking paths, fluvial transport and deposition can be confirmed as the predominant processes. Other possible sources of MPs are wastewater discharges from WWTPs (e.g. care products, and fibres), and surface runoffs from streets, industry areas and settlement areas (e.g. tyre abrasion, pellets) that enter the river system. There are two plastic processing industries along the river and MP pellets were only found at sampling points located downstream of the first plastic industrial site. The second one is situated directly before the river's mouth, where no samples were taken (see Fig. 1a).
3.1 Data correction
The total number of MP particles was 176 of which only 88 were measured with FT-IR, since not all particles could be removed from the sample and in some samples several MP particles were made of the same material and therefore only one was measured. Of these 88 measured particles, 28 were identified in 19 samples as synthetic polymers with a probability ≥70% (identical to that observed by Thompson et al. 2004 (ref. 58) and Lusher et al. 2013 (ref. 55)) matching with the spectra of the FT-IR library. Since the library contained only the most common polymers, this led to a theoretical error ratio of 68.2% (perror). The most common polymer was PE (67.9%), other measured polymers were PS, PET, PP, PVC and polymethyl methacrylate (PMMA) (cf.Table 1).| Samples | Absolute [n] | Relative [%] | |
|---|---|---|---|
| Total number of samples | 135 | 100 | |
| Contaminated samples | (M) | 66 | 48.9 |
| Uncontaminated samples | (M) | 69 | 51.1 |
| Contaminated samples | (IR) | 19 | 14.1 |
| Uncontaminated samples | (IR) | 116 | 85.9 |
| MP Particles | Absolute [n] | Relative [%] | |
|---|---|---|---|
| Total number of MP particles | (M) | 176 | 100 |
| Measured number of MP particles | (IR) | 88 | 50.0 |
| Number of ‘polymers ≥70%’ | (IR) | 28 | 31.8 |
| Number of ‘no polymers <70%’ | (IR) | 60 | 68.2 |
| Polymers | Absolute [n] | Relative [%] | |
|---|---|---|---|
| PE | (IR) | 19 | 67.9 |
| PS | (IR) | 3 | 10.7 |
| PET | (IR) | 3 | 10.7 |
| PP | (IR) | 1 | 3.6 |
| PVC | (IR) | 1 | 3.6 |
| PMMA | (IR) | 1 | 3.6 |
The parameter MPcorr, [n kg−1] was calculated for each sample using this theoretical error ratio perror to define the load of MP particles based on the number of identified polymers:
| MPcorr = MPtotal × perror with |
MPtotal [n kg−1]: total number of microscopically identified MPs.
p error [%]: theoretical error ratio of polymer identification.
Since MPcorr is a percentage of the corrected number of identified MP particles, this value is given as a decimal number.
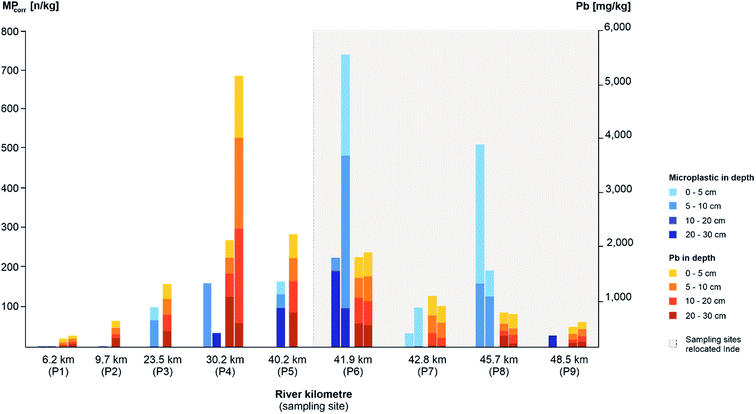
3.2 Microplastics along the river course
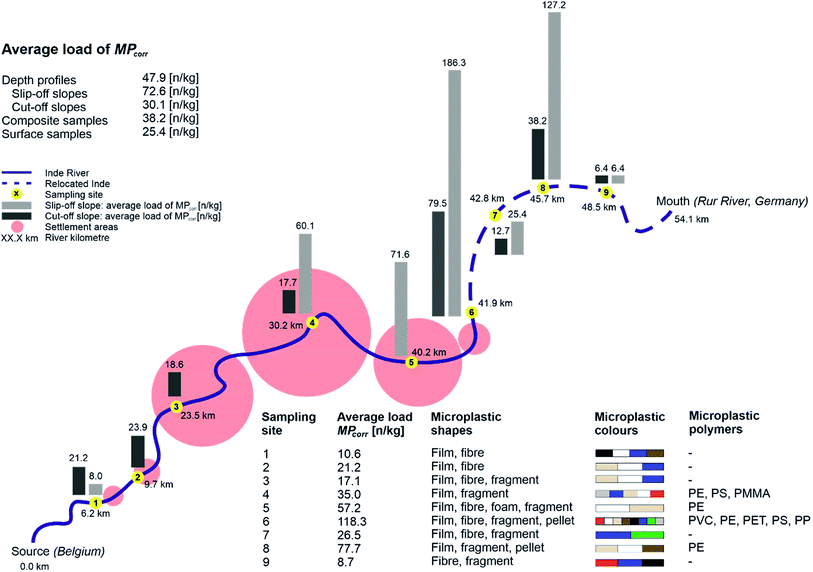
As a first step, the MP load was compared between the different sampling areas and types regarding depth profiles and surface samples in the floodplain area and composite samples from the river bed. Therefore, average concentrations of MPcorr for all samples and average values for each sampling type were calculated by multiplying the microscopically detected MP particles per sample (MPtotal) with the theoretical error ratio perror.The highest average detection of MPs was determined within all samples from depth profiles (MPcorr = 47.9 n kg−1), followed by all composite samples from the river bed (MPcorr = 38.2 n kg−1) and the lowest contamination from all surface samples (MPcorr = 25.4 n kg−1) (cf.Fig. 4). Since there is less MP in sediments from the river bed than in the floodplain area, fluvial transported MPs accumulate especially in floodplain areas outside which the surface samples were taken. In floodplains, remobilisation due to flow velocities and sediment cover is less likely compared to the river bed where a constant flow is present and the bed substrate is affected by every rise of the water level and especially by erosion during flood events. A comparison with other MP contaminations in floodplain areas is currently hardly possible since no mean concentrations have been identified,34 different size classes of plastics were analysed,13 and still mainly river bed sediments have been investigated.33 Since floodplains are seen as accumulation areas with higher concentrations of MPs, there is an even more important and further need for research in this field.
There is a continuous increase of MPcorr from the source up to the relocated part (P1–P5) regarding all sampling types (10.6–57.2 n kg−1) with the following increase for P6 (118.3 n kg−1), caused by high accumulation, and decrease within P7 to P9 within the relocated segment (cf.Fig. 4). Furthermore, the MPcorr values of the first three sampling points (P1–P3) show within all samples only contaminations <100 n kg−1 per sample. This indicates a significantly lower absolute MP load in the upper than in the lower part which correlates with the anthropogenic impact.
On closer look at depth profiles, MPcorr increases from P1 up to P6 and decreases afterwards similar to the average concentrations at each sampling site as described before (cf.Fig. 4: Ø load MPcorr). However, when considering the load within each depth profile, no depth correlation could be identified although more particles had been expected in the upper layers due to the increase in plastic utilisation. One possible explanation could be the limited amount of sediment, that has been analysed. With a separate consideration of the slopes, where the depth profile samples were taken, there is a higher MPcorr concentration in the samples from slip-off slopes than in cut-off slopes. This observation is similar to other studies with comparable results,59,60 and indicates that all slope sediments there are very young, since MPs were detected in all of them. Lower MP levels in cut-off slopes can be explained by higher flow velocities preventing accumulation there while possible lateral erosion can be neglected due to straightening and bank stabilisation along the Inde river.46
At the Inde River, the continuous increase of MPs along the river course was shown with a following decrease within the last three sampling sites (cf.Fig. 4). This can be explained by a remobilisation of already deposited MPs and an overall very high and young sedimentary activity, especially in the relocated segment, which has already been demonstrated.31
Here, two dominant processes are responsible for MP accumulation in fluvial systems. On the one hand MPs can enter the river anytime and anywhere and on the other hand previously deposited MPs can be released anywhere and at any time, too, based on flow-related transport, remobilisation and deposition. Overall, the higher loads of MPs on the slip-off slopes and in total within the lower course reflect fluvial induced accumulation and show that floodplains act as (temporal) sinks. Therefore, MPs there can be used in a temporal context to define sedimentation rates.
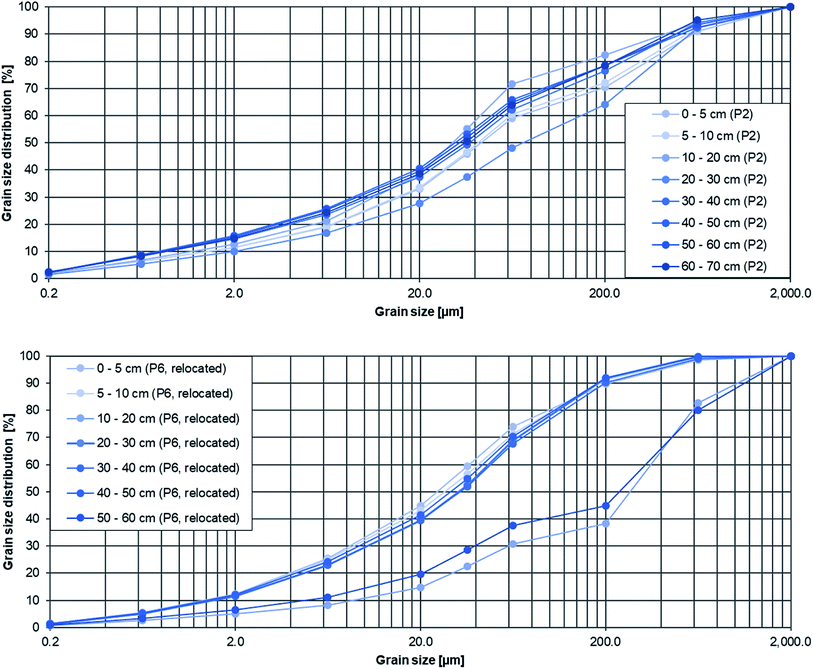
3.3 Sedimentations rates
Sedimentation rates describe the average thickness of floodplain accumulation induced by flooding over time, e.g. per year, and describe sediment activity along a river course. Thus, floodplains act as flood retention areas and the sediment accumulation there is more event-related than continuous. It needs to be mentioned that the layer thickness of 10 cm was chosen without considering potential flood layers. Since floodplains act as (temporal) sinks for anthropogenic contaminants and sediments from fluvial systems, trace elements were already used to define sedimentation rates and analyse floodplain geochronology.20,32 Here, sedimentation rates were determined by two different methods depending on the location at the Inde river. On the one hand MPs were used for detection and on the other hand the grain size was the critical calculation factor. Fig. 5 shows the typical grain size distributions of depth samples from two sampling points, showing differences within the profile from the relocated Inde (d50 = 25.36–324.57 μm, cf.Fig. 5 bottom) and similar distributions from the other river segment (d50 = 30.91–70.96 μm, cf.Fig. 5 top).Within the sampling sites 1–5 MPs were used as a maker and applied to define sedimentation rates by connecting the sediment depth to where MPs were found with the period since MPs could have entered the environment. This period started with the beginning of plastic mass production and is about 70 years (1950–2020). Thus, sedimentation rates srMP [cm a−1] were calculated by relating the sediment depth with MPs to the reference period of 70 years using the following formula:
| srMP = dMP/tMP with |
d MP [cm] sediment depth containing MPs.
t MP [a] reference period of MPs (70 years).
Although the migration and vertical transport of MPs by biological organisms, e.g. earthworms, or by seepage water are possible, it can be assumed that the chosen layer thickness is bigger than potential transport capacity or possible infiltration.61,67 Furthermore, it can be considered, that the transport ability of larger plastic particles is smaller which thus needs to be addressed as limited evidence of the detected MP particles in this study. Despite these considerations, it cannot totally be excluded that particles migrated based on these processes.
The reference period for sedimentation was 15 years, 2005 to 2020, for the relocated Inde since the relocation was carried out in 2005. Accordingly, the sedimentation rates were determined without using MPs as a marker. The artificial sediment layers there have a significantly larger grain size compared to the flood sediment and could be easily identified by GSA (see Fig. 5 bottom, 40–50 cm depth, d50 = 287.45 μm, 50–60 cm depth, d50 = 324.57 μm). Therefore, the thickness of the total accumulated sediment layer since 2005 was used as the sediment depth for calculating the sedimentation rates srGSA [cm a−1] using the following formula:
| srGSA = dFS/tr with |
d FS [cm] Sediment depth of the flood sediment (determined with GSA).
t r [a] Reference period of relocation (15 years).
The sedimentation rates calculated with the two described methods are listed in Table 2. The rates range between 0.29 and 4.00 cm a−1 along the whole river course. In the relocated part the sedimentation rates were significantly higher and at sampling site 6 the highest rate with 4.00 cm a−1 was determined. Also the highest average MPcorr load (cf.Fig. 4) was detected there The Inde River without the relocated part had a mean sedimentation rate of 0.8 cm a−1, whereas the relocated Inde had a significantly higher mean sedimentation rate of 1.92 cm a−1. This indicates the high sediment activity which can be explained by the used building material there. Hence, the upper course is rather characterised by erosion, whereas in the lower course accumulation is predominant, which is reflected in the MP load along the river. On average, there is slightly more sedimentation at slip-off slopes (1.44 cm a−1) compared to cut-off slopes (1.41 cm a−1).
| Sampling site | Sedimentation rate [cm a−1] | Cut-off slope | Slip-off slope |
|---|---|---|---|
| 1 | srMP | 0.29 | 0.29 |
| 2 | srMP | 1.00 | — |
| 3 | srMP | 1.57 | — |
| 4 | srMP | 0.43 | 0.43 |
| 5 | srMP | — | 2.00 |
| 6 | srGSA | 2.67 | 4.00 |
| 7 | srGSA | 2.00 | 2.00 |
| 8 | srGSA | 1.33 | 0.67 |
| 9 | srGSA | 2.00 | 0.67 |
3.4 Heavy metal concentration
Since heavy metals as anthropogenic contaminants are transported in river systems as well as MPs and accumulate due to the adsorption at fine sediments in fluvial sediments too, XRF was carried out with all the taken sediment samples to determine the concentrations of different heavy metals, such as copper, zinc or lead. Compared to the MPcorr values, similarities and differences within fluvial processes and morphodynamics should be identified. For further analysis, lead (Pb) concentrations were used, since Gao et al. (2019)62 showed the highest adsorbance of Pb compared to copper (Cu) and cadmium (Cd) by MPs (PVC, PP and PE). The original and relocated part of the Inde River was considered separately, since based on the applied surface material, slope, lateral and depth erosion occurs and acts as an additional sediment source downstream of the river which results in dilution effects.31To study MPcorr and Pb along the river course, the first 30 cm of each depth profile was considered, as this depth was present in all profiles. Comparable to MPcorr, the mean value of Pb significantly increases from P1 up to P5, whereby the load is equally distributed in the different layers (0–30 cm) while MPs focus on the upper 10 cm (cf.Fig. 6). The contamination levels are determined by the location of its sources.46 In the relocated segment, MPcorr still increases in the first sampling site (P6) compared to the previous sampling sites but decreases afterwards while there is a decline of Pb in the whole relocated Inde. Similar to sediment-bound heavy metal processes, MPs had accumulated in the relocated river segment during the last 15 years and due to erosion and a low natural geogenic background these loads have been diluted.31
To figure out if there is a correlation between MPs and Pb regarding the different sampling depths, hypotheses were tested for the depths 0–5 cm, 5–10 cm, 10–20 cm and 20–30 cm. A statistically significant correlation was shown only for the layer from 10 to 20 cm (Pearson's t-test, p < 0.05, n = 15). The correlation coefficient (r = 0.63) presented a medium correlation which implies a similar contamination of Pb and MPs in this sediment layer. Furthermore, it was tested if there are correlations of MPs and Pb within the other sampling types (surface samples, and composite samples) but none could be identified. It can be assumed that the distribution patterns of MPs and Pb are comparable based on a similar transport behaviour. The expected missing correlation of MPs and Pb could be explained by different sources and their different locations along the river. Furthermore, the main time period when contaminants entered the environment differs: coal mining, ore mining and industrialisation, began more than 500 years earlier than plastic processing industries were developed.10,46 To support the statement of a different temporal occurrence of heavy metals, MPcorr was also correlated with copper (Cu) and zinc (Zn) as these two heavy metals are very common in the Inde catchment area for industrial reasons.46 Since no correlations were available here either, an influence of the different time periods and source types of heavy metals and MPs on the deposition processes can be assumed.
All in all, the contamination course of MPs and Pb along the Inde River shows a very similar trend concerning increase and decrease, although no significant statistical correlations were found and the consistent trend is therefore an increase up to the relocation with a subsequent decrease first for Pb and then for MPs, too.
3.5 Grain size analysis
The grain size of the sediment influences the accumulation of MPs and is seen as a critical proxy for it.63 Enders et al. (2019)63 already showed that with increasing median grain size the MP abundance decreases.In this study, the grain size varied along the river course especially within the depth profiles with similar grain size distributions in the beginning (P1–P5) and greater differences in the relocated part (P6–P9), based on the artificial sediment layer that was used for the relocation there (cf.Fig. 5). The median grain size d50 differed from 30.9 μm up to 477.0 μm (P1–P5) and 28.1 μm up to 551.0 μm (P6–P9) whereby finer sediments were present mainly in the upper layers, for example at cut-off slopes of P4: 77.9 μm (0–5 cm), 50.8 μm (5–10 cm), 171.0 μm (10–20 cm) and 253.3 μm (20–30 cm).
The results show an enrichment of MPcorr within fine sediments. 44.2% of all samples from depth profiles have a median grain size <63 μm (fine sediment fraction) and these samples contain 41.3% of all detected MP particles (cf.Fig. 7, red marking). This means that MPs are mainly transported with fine sediments before deposition, which is a similar observation to that reported by Enders et al. (2019)63 although it needs to be mentioned that estuarine sediments were analysed there.
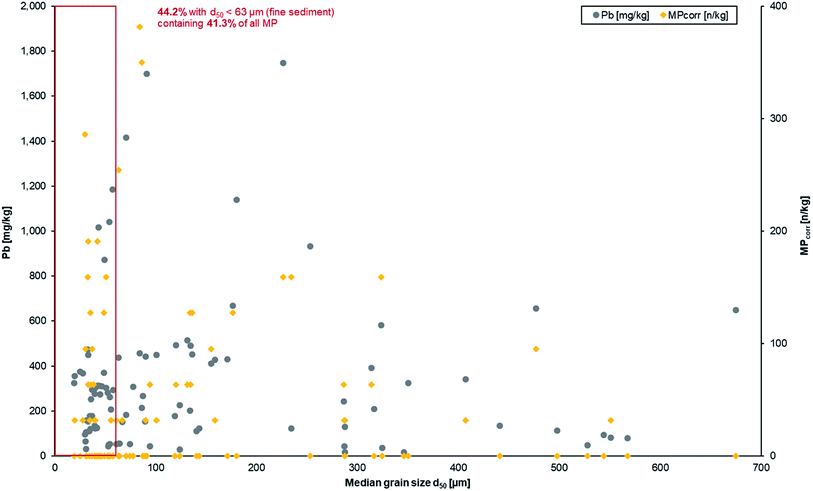 | ||
| Fig. 7 Heavy metal and MP contamination as a function of the median grain size from all depth profile samples. | ||
The results of the Pb load in the fluvial sediments were included within the graphical illustration to see if similar or different deposition patterns occur (cf.Fig. 7). It becomes obvious that Pb mainly accumulates in the fine sediment fraction (corresponds to 44.2% of the sediment), too, what is additionally demonstrated by the mean value of Pb in fine sediment (299.5 mg kg−1) whereas the average load in all sediment samples was only slightly higher (335.2 mg kg−1). This can be explained by binding of heavy metals on fine sediment fractions as a general and common process.27–30 Since heavy metals as anthropogenic contaminants have already been used as deposition markers in sediments for floodplain geochronology, the transferability to MP is given.20,32
In addition, correlation analyses were conducted under the hypothesis whether there is a statistical correlation between median grain size d50 and MPcorr in the different sediment layers and sample types, but none could be identified. A possible explanation is that MPs are more portable than sediment due to their lower densities.18 Furthermore, the settling and rise velocities as well as erosion behaviour of MPs differ significantly from those of sediments.17,18 Although the same processes (transport, deposition, and erosion) are predominant for MPs and sediment in fluvial systems, they are influenced by different variables concerning the density, shape and size of which the conditions need to be determined more precisely for further research.
Overall, a large amount of MP and Pb contamination is located within fine sediments which can be explained by similar transport mechanisms and resulting accumulation sites. Since MPs and sediments are not identical and only similar with regard to their transport behaviour in fluvial systems, MPs seem to be more mobile than transported sediment while Pb is sediment bound. Conversely, the detection of MPs can also indicate fine sediment accumulation. Based on the analogies of heavy metals and MPs, MPs can also be used in a further temporal context which is similar to the application of heavy metals.20,32
3.6 Sediment dating with microplastic detection and polymer identification
Besides the use of MPs as a marker to define fluvial morphological processes, the detection of plastic in sediments enable an adoption of it as a time reference for the deposition of corresponding sediment layers, too. The correlation between MP detection and sediment deposition was already mentioned by Martin et al. (2017),38 where all sediment samples with MPs were characterised as ‘modern’ deposition after 1950. In this study, the detection of MPs is firstly seen as evidence of sedimentation after 1950 which was shown by determining sedimentation rates.In addition, here, for the first time, the occurrence of MPs in sediment layers was exemplarily chronologically linked to the corresponding year of polymer development. Although, there are more detailed questions to answer concerning infiltration by seepage water after deposition of MPs induced by flooding and relocation by soil organisms, which can have an influence on the position and depth of MPs in the sediment layer,61,64–67 this was performed with one of the depth profiles within the relocated Inde (see Fig. 8). Thus, the aim was not to show the method of using MPs to date sediment layers as applicable, but only to point out possible connections in order to work on validation and verifiability in the future.
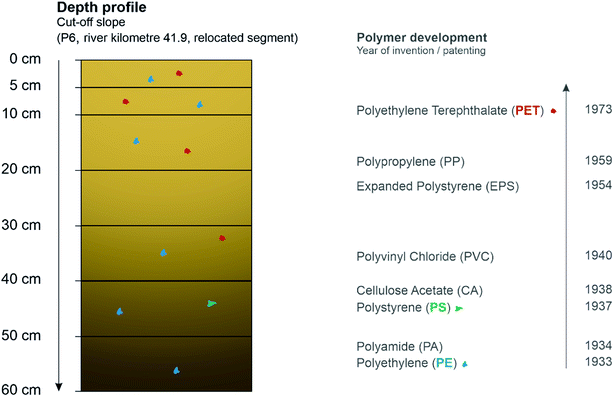 | ||
| Fig. 8 Depth profile at a cut-off slope with detected polymers (coloured) and the associated time scale37 of polymer development (CA for cigarette filters and PVC for water pipes). | ||
The results show that there is a connection between the time, not exactly the year when the polymers have been developed, and the primary deposition in the layers: younger materials, such as PET, patented in 1973, was predominantly found in the upper layers, while in the deepest layer at 60 cm depth only PE was found that was already developed in 1933. PS, first invented in 1937, could only be detected at a section of 40–50 cm. The presence of PE in 6 of 7 layers can be explained by the associated possibility of the environmental entry over a longer period compared to PS or PET. The missing polymer determination within the layer from 20–30 cm can be explained by the size limitations of the methodology since the microscopic analysis showed different MP particles in this layer, too. To conclude, this profile from the relocated Inde shows that within 15 years MPs already accumulated up to a depth of 60 cm which proves the widespread distribution and subsequent enrichment of MPs in the environment.
The connection between different MP polymers in sediment layers and time reference in deposition can be determined as that the older the polymer the deeper the sediment layers it can be found in. In the future, based on the detection of MPs in a sediment layer, deposition after 1950 could thus be proven and therefore provide a dating basis in addition or combination to other methods.
4 Conclusion
The detection and application of MPs in floodplains have shown continuous contamination there and thus proved floodplain areas to be (temporal) sinks for MPs which emphasises the importance of this research area. Furthermore, similar depositions of the heavy metal Pb were shown as well as a prior accumulation of MPs and Pb in fine sediments. By identifying sedimentation rates and creating connections between deposition and the polymer type, the added value of MP detection as a marker in floodplains was demonstrated.Slip-off slopes as hotspots of MP accumulation have been identified by sampling and analysing fluvial sediment from source to mouth along the river. An absolute lower MP load in the upper than in the lower river course was observed, resulting from an increase in settlement areas and other possibilities for MPs to enter the river. Furthermore, sedimentation rates were determined by the detection of MPs, which is a new and innovative method.
Particularly concerning time relating information, MPs and polymers allow a more precise dating of floodplain sediments, because of the temporal delimitation by polymers and a possible additional temporal assignment in the case of determining additives within the detected MP. Compared to heavy metals as markers for sediment dating, MPs offer the possibility of reconstructing sedimentation within the last 70 years, where heavy metals may not have occurred in such quantities due to a change in industrial processes. Furthermore, with MPs one material is focused while heavy metals offer the possibility of many different substances which may be distributed variably. With the evidence of MPs in sediment, the maximum sedimentation time from 1950 is given. This information can be used for all areas where sediment deposits and accumulates. In particular, lake sediments as temporary sinks,68 where no remobilisation takes place, could provide a good data basis for the validation of a dating methodology with MPs. In summary, MPs could be a complement to the application of heavy metals in sedimentary contexts especially concerning floodplains with a focus on recent deposits.
To make this dating method more precise, further sampling, if possible also of larger amounts of sediment, and more precise analysis of MPs will be necessary to detect and identify all polymers. Furthermore, also additives may offer more information on time related deposition. Since some additives are already prohibited and were therefore only in use for a certain period of time, a related time period is given. Azodicarboxylic acid diamide has been already prohibited since 2005 especially for materials in the food sector as well as flame retardants octa- and pentabromodiphenyl ether and short-chain chlorinated paraffins have been prohibited since 2004.69 Thus, these additives could, if detected in found plastics, give an indication of deposition prior to this ban.
In future research work, the newly presented basis for determining sedimentation rates will be examined more closely and compared with that of other methods. In addition, the analysis of MPs will be extended to validate the dating approach. Furthermore, in view of future application, two aspects are important. On the one hand, if sources and entry paths of MPs will cause a further continuous input into fluvial systems, this approach will also be applicable in the future. On the other hand, if a decrease of such input is assumed, plastic detection can be assigned to an even more temporarily limited and precise period. In conclusion, MPs as an environmental contaminant in sediments is very suitable and functional as a marker to evaluate fluvial activity as well as anthropogenic influences.
Funding source
PhD Scholarship: RWTH Scholarships for Doctoral Students.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The corresponding author gratefully acknowledges the funding of RWTH Scholarships for Doctoral Students and the support of the staff members of the Laboratory of Physical Geography and Geoecology at RWTH Aachen University as well as the support of Jan Schwarzbauer and Olga Konechnaya (Laboratory for Organic-Geochemical Analysis at RWTH Aachen University) during the FT-IR measurements and the support of Felix Schreiber in sample preparation and analysis.References
- C. Arthur, J. Baker and H. Bamford, Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris. Sept. 9-11, 2008, NOAA Technical Memorandum NOS-OR&R-30, 2009 Search PubMed.
- S. Allen, D. Allen, V. R. Phoenix, G. Le Roux, P. Durántez Jiménez, A. Simonneau, S. Binet and D. Galop, Atmospheric transport and deposition of microplastics in a remote mountain catchment, Nat. Geosci., 2019, 71, 299 Search PubMed.
- M. Bergmann, V. Wirzberger, T. Krumpen, C. Lorenz, S. Primpke, M. B. Tekman and G. Gerdts, High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory, Environ. Sci. Technol., 2017, 51, 11000–11010 CrossRef CAS PubMed.
- L. van Cauwenberghe, A. Vanreusel, J. Mees and C. R. Janssen, Microplastic pollution in deep-sea sediments, Environ. Pollut., 2013, 182, 495–499 CrossRef CAS.
- E. J. Carpenter and K. L. Smith Jr, Plastics on the Sargasso Sea Surface//Plastics on the Sargasso sea surface, Science, 1972, 175, 1240–1241 CrossRef CAS PubMed.
- J. B. Colton, B. R. Burns and F. D. Knapp, Plastic particles in surface waters of the northwestern atlantic, Science, 1974, 185, 491–497 CrossRef PubMed.
- A. Andrady, Microplastics in the marine environment, Mar. Pollut. Bull., 2011, 1596–1605 CrossRef CAS PubMed.
- C. M. Free, O. P. Jensen, S. A. Mason, M. Eriksen, N. J. Williamson and B. Boldgiv, High-levels of microplastic pollution in a large, remote, mountain lake, Marine Pollution Bulletin, 2014, 85, 156–163 CrossRef CAS.
- G. V. B. Ferreira, M. Barletta, A. R. A. Lima, S. A. Morley, A. K. S. Justino and M. F. Costa, High intake rates of microplastics in a Western Atlantic predatory fish, and insights of a direct fishery effect, Environ. Pollut., 2018, 236, 706–717 CrossRef CAS PubMed.
- D. Barnes, F. Galgani, R. Thompson and M. Barlaz, Accumulation and Fragmentation of Plastic Debris in Global Environments, Philosophical Transaction of the Royal Society Biological Sciences, 2009, 1985–1998 Search PubMed.
- W. Courtene-Jones, B. Quinn, C. Ewins, S. F. Gary and B. E. Narayanaswamy, Consistent microplastic ingestion by deep-sea invertebrates over the last four decades (1976-2015), a study from the North East Atlantic, Environ. Pollut., 2019, 244, 503–512 CrossRef CAS.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, use, and fate of all plastics ever made, Science advances, 2017, 3, e1700782 CrossRef PubMed.
- C. J. Weber and C. Opp, Spatial patterns of mesoplastics and coarse microplastics in floodplain soils as resulting from land use and fluvial processes, Environ. Pollut., 2020, 267, 115390 CrossRef CAS PubMed.
- K. Waldschläger, S. Lechthaler, G. Stauch and H. Schüttrumpf, The way of microplastic through the environment – Application of the source-pathway-receptor model (review), Science of the Total Environment, 2020, 136584 CrossRef PubMed.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Microplastics in the marine environment: a review of the methods used for identification and quantification, Environ. Sci. Technol., 2012, 46, 3060–3075 CrossRef CAS PubMed.
- K. Enders, R. Lenz, C. A. Stedmon and T. G. Nielsen, Abundance, size and polymer composition of marine microplastics ≥10μm in the Atlantic Ocean and their modelled vertical distribution, Mar. Pollut. Bull., 2015, 100, 70–81 CrossRef CAS.
- K. Waldschläger and H. Schüttrumpf, Effects of Particle Properties on the Settling and Rise Velocities of Microplastics in Freshwater under Laboratory Conditions, Environ. Sci. Technol., 2019, 53, 1958–1966 CrossRef PubMed.
- K. Waldschläger and H. Schüttrumpf, Erosion Behavior of Different Microplastic Particles in Comparison to Natural Sediments, Environ. Sci. Technol., 2019, 53, 13219–13227 CrossRef PubMed.
- N. N. Phuong, L. Poirier, F. Lagarde, A. Kamari and A. Zalouk-Vergnoux, Microplastic abundance and characteristics in French Atlantic coastal sediments using a new extraction method, Environ. Pollut., 2018, 243, 228–237 CrossRef CAS PubMed.
- R. Aalto and C. A. Nittrouer, 210Pb geochronology of flood events in large tropical river systems, Philos. Trans. R. Soc., A, 2012, 370, 2040–2074 CrossRef CAS PubMed.
- D. O'Connor, D. Hou, Y. S. Ok, J. Mulder, L. Duan, Q. Wu, S. Wang, F. M. G. Tack and J. Rinklebe, Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review, Environ. Int., 2019, 126, 747–761 CrossRef PubMed.
- L. Nizzetto, G. Bussi, M. Futter, D. Butterfield and P. Whitehead, A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments, Environ. Sci.: Processes Impacts, 2016, 1050–1059 RSC.
- D. Brennecke, B. Duarte, F. Paiva, I. Caçador and J. Canning-Clode, Microplastics as vector for heavy metal contamination from the marine environment, Estuarine, Coastal Shelf Sci., 2016, 178, 189–195 CrossRef CAS.
- J. Prunier, L. Maurice, E. Perez, J. Gigault, A.-C. Pierson Wickmann, M. Davranche and A. T. Halle, Trace metals in polyethylene debris from the North Atlantic subtropical gyre, Environ. Pollut., 2019, 245, 371–379 CrossRef CAS PubMed.
- J. Maršić-Lučić, J. Lušić, P. Tutman, D. Bojanić Varezić, J. Šiljić and J. Pribudić, Levels of trace metals on microplastic particles in beach sediments of the island of Vis, Adriatic Sea, Croatia, Mar. Pollut. Bull., 2018, 137, 231–236 CrossRef PubMed.
- J. Wang, J. Peng, Z. Tan, Y. Gao, Z. Zhan, Q. Chen and L. Cai, Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals, Chemosphere, 2017, 171, 248–258 CrossRef CAS PubMed.
- M. Brinkmann, K. Eichbaum, M. Reininghaus, S. Koglin, U. Kammann, L. Baumann, H. Segner, M. Zennegg, S. Buchinger, G. Reifferscheid and H. Hollert, Towards science-based sediment quality standards-Effects of field-collected sediments in rainbow trout (Oncorhynchus mykiss), Aquat. Toxicol., 2015, 166, 50–62 CrossRef CAS PubMed.
- U. Förstner, W. Salomons and P. Mader, Heavy Metals. Problems and Solutions, Springer, Berlin, Heidelberg, 1995 Search PubMed.
- H. Schüttrumpf, M. Brinkmann, C. Cofalla, R. M. Frings, S. U. Gerbersdorf, M. Hecker, S. Hudjetz, U. Kammann, G. Lennartz, S. Roger, A. Schäffer and H. Hollert, A new approach to investigate the interactions between sediment transport and ecotoxicological processes during flood events, Environ. Sci. Eur., 2011, 23, 943 Search PubMed.
- B. Westrich and U. Förstner, Sediment Dynamics and Pollutant Mobility in Rivers. An Interdisciplinary Approach, Springer-Verlag Berlin Heidelberg, Berlin, Heidelberg, 2007 Search PubMed.
- A.-L. Maaß, V. Esser, R. Frings, F. Lehmkuhl and H. Schüttrumpf, A decade of fluvial morphodynamics: relocation and restoration of the Inde River (North-Rhine Westphalia, Germany), Environ. Sci. Eur., 2018, 30, 1–21 CrossRef.
- M. Buchty-Lemke, L. Hagemann, A.-L. Maaß, H. Schüttrumpf, J. Schwarzbauer and F. Lehmkuhl, Floodplain chronology and sedimentation rates for the past 200 years derived from trace element gradients, organic compounds, and numerical modeling, Environ. Earth Sci., 2019, 78, 1129 Search PubMed.
- J. Tibbetts, S. Krause, I. Lynch and G. Sambrook Smith, Abundance, Distribution, and Drivers of Microplastic Contamination in Urban River Environments, Water, 2018, 10, 1597 CrossRef CAS.
- M. Scheurer and M. Bigalke, Microplastics in Swiss Floodplain Soils, Environ. Sci. Technol., 2018, 52, 3591–3598 CrossRef CAS PubMed.
- C. J. Weber, C. Weihrauch, C. Opp and P. Chifflard, Investigating microplastic dynamics in soils: Orientation for sampling strategies and sample pre-procession, Land Degrad. Dev., 2020, 4, fsw173 Search PubMed.
- P. L. Corcoran, C. J. Moore and K. Jazvac, An anthropogenic marker horizon in the future rock record, GSAT, 2014, 24, 4–8 CrossRef.
- J. Zalasiewicz, C. N. Waters, J. A. Ivar do Sul, P. L. Corcoran, A. D. Barnosky, A. Cearreta, M. Edgeworth, A. Gałuszka, C. Jeandel, R. Leinfelder, J. R. McNeill, W. Steffen, C. Summerhayes, M. Wagreich, M. Williams, A. P. Wolfe and Y. Yonan, The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene, Anthropocene, 2016, 13, 4–17 CrossRef.
- J. Martin, A. Lusher, R. C. Thompson and A. Morley, The Deposition and Accumulation of Microplastics in Marine Sediments and Bottom Water from the Irish Continental Shelf, Sci. Rep., 2017, 1–9, 10772 CrossRef PubMed.
- P. L. Corcoran, T. Norris, T. Ceccanese, M. J. Walzak, P. A. Helm and C. H. Marvin, Hidden plastics of Lake Ontario, Canada and their potential preservation in the sediment record, Environ. Pollut., 2015, 204, 17–25 CrossRef CAS PubMed.
- A. Ockelford, A. Cundy and J. E. Ebdon, Storm Response of Fluvial Sedimentary Microplastics, Sci. Rep., 2020, 10, 1865 CrossRef CAS PubMed.
- D. K. Schneiderman and M. A. Hillmyer, 50th Anniversary Perspective : There Is a Great Future in Sustainable Polymers, Macromolecules, 2017, 50, 3733–3749 CrossRef CAS.
- ELWAS-WEB, Elektronisches wasserwirtschaftliches Verbundsystem für die Wasserwirtschaftsverwaltung in NRW, http://www.elwasweb.nrw.de, accessed 23 March 2020 Search PubMed.
- N. R. W. Mkulnv, Maas Süd NRW Steckbriefe der Planungseinheiten in den nordrhein-westfälischen Anteilen von Rhein, Weser, Ems und Maas. Bewirtschaftungsplan 2016-2021 Oberflächengewässer und Grundwasser Teileinzugsgebiet Maas/Maas Süd NRW. Ministerium für Klimaschutz, Umwelt, Landwirtschaft, Natur-und Verbraucherschutz des Landes Nordrhein-Westfalen, Düsseldorf, 2015 Search PubMed.
- S. Blott and K. Pye, Particle size distribution analysis of sand-sized particles by laser diffraction: an experimental investigation of instrument sensitivity and the effects of particle shape: particle size distribution analysis of sands by laser difraction, Sedimentology, 2006, 671–685 CrossRef.
- P. Schulte, F. Lehmkuhl, F. Steininger, D. Loibl, G. Lockot, J. Protze, P. Fischer and G. Stauch, Influence of HCI pretreatment and organo-mineral complexes on laser diffraction measurement of loess-paleosol-sequences, Catena, 2016, 392–405 CrossRef CAS.
- V. Esser, M. Buchty-Lemke, P. Schulte, L. S. Podzun and F. Lehmkuhl, Signatures of recent pollution profiles in comparable central European rivers – Examples from the international River Basin District Meuse, Catena, 2020, 193, 104646 CrossRef CAS.
- D. K. Kanhai, C. Johansson, J. Frias, K. Gardfeldt, R. C. Thompson and I. O'Connor, Deep sea sediments of the Arctic Central Basin: A potential sink for microplastics, Deep Sea Res., Part I, 2019, 145, 137–142 CrossRef.
- S. Lechthaler, R. Dolny, V. Spelthahn, J. Pinnekamp and V. Linnemann, Sampling concept for microplastics in combined sewage-affected freshwater and freshwater sediments, Fundam. Appl. Limnol., 2019, 37–48 Search PubMed.
- S. E. Lechthaler, L. Hildebrandt, G. Stauch and H. Schüttrumpf, Canola Oil Extraction in Conjunction with a Plastic Free Separation Unit Optimises Microplastics Monitoring in Water and Sediment, Anal. Methods, 2020, 5128–5139 RSC.
- R. Coppock, M. Cole, P. Lindeque, A. Queiros and T. Galloway, A small-scale, portable method for extracting microplastics from marine sediments, Environ. Pollut., 2017, 829–837 CrossRef CAS PubMed.
- E. M. Crichton, M. Noël, E. A. Gies and P. S. Ross, A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments, Anal. Methods, 2017, 9, 1419–1428 RSC.
- F. Noren, Small Plastic Particles in Coastal Swedish Waters, KIMO Sweden, Lysekil, 2007 Search PubMed.
- D. K. Kanhai, R. Officer, O. Lyashevska, R. C. Thompson and I. O'Connor, Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean, Mar. Pollut. Bull., 2017, 115, 307–314 CrossRef PubMed.
- A. L. Lusher, I. L. N. Bråte, K. Munno, R. R. Hurley and N. A. Welden, Is It or Isn't It: The Importance of Visual Classification in Microplastic Characterization, Appl. Spectrosc., 2020, 3702820930733 Search PubMed.
- A. L. Lusher, M. McHugh and R. C. Thompson, Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel, Mar. Pollut. Bull., 2013, 67, 94–99 CrossRef CAS PubMed.
- V. C. Shruti, M. P. Jonathan, P. F. Rodriguez-Espinosa and F. Rodríguez-González, Microplastics in freshwater sediments of Atoyac River basin, Sci. Total Environ., 2019, 654, 154–163 CrossRef CAS PubMed.
- A. Borja and M. Elliott, So when will we have enough papers on microplastics and ocean litter?, Mar. Pollut. Bull., 2019, 146, 312–316 CrossRef CAS PubMed.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. G. John, D. McGonigle and A. E. Russell, Lost at sea: where is all the plastic?, Science, 2004, 304, 838 CrossRef CAS PubMed.
- T. Mani, A. Hauk, U. Walter and P. Burkhardt-Holm, Microplastics profile along the Rhine River, Sci. Rep., 2015, 1–7, 17988 Search PubMed.
- M. Heß, P. Diehl, J. Mayer, H. Rahm, W. Reifenhäuser, J. Stark and J. Schwaiger, Mikroplastik in Binnengewässern Süd- und Westdeutschlands. Bundesländerübergreifende Untersuchungen in Baden-Württemberg, Bayern, Hessen, Nordrhein-Westfalen und Rheinland-Pfalz. Teil 1: Kunststoffpartikel in der oberflächennahen Wasserphase, Karlsruhe, 2018 Search PubMed.
- M. C. Rillig, L. Ziersch and S. Hempel, Microplastic transport in soil by earthworms, Sci. Rep., 2017, 7, 1362 CrossRef PubMed.
- F. Gao, J. Li, C. Sun, L. Zhang, F. Jiang, W. Cao and L. Zheng, Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment, Mar. Pollut. Bull., 2019, 144, 61–67 CrossRef CAS PubMed.
- K. Enders, A. Käppler, O. Biniasch, P. Feldens, N. Stollberg, X. Lange, D. Fischer, K.-J. Eichhorn, F. Pollehne, S. Oberbeckmann and M. Labrenz, Tracing microplastics in aquatic environments based on sediment analogies, Sci. Rep., 2019, 9, 15207 CrossRef CAS PubMed.
- S. Maaß, D. Daphi, A. Lehmann and M. C. Rillig, Transport of microplastics by two collembolan species, Environ. Pollut., 2017, 225, 456–459 CrossRef PubMed.
- D. O'Connor, S. Pan, Z. Shen, Y. Song, Y. Jin, W.-M. Wu and D. Hou, Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles, Environ. Pollut., 2019, 249, 527–534 CrossRef PubMed.
- S. Frei, S. Piehl, B. S. Gilfedder, M. G. J. Löder, J. Krutzke, L. Wilhelm and C. Laforsch, Occurrence of microplastics in the hyporheic zone of rivers, Sci. Rep., 2019, 9, 15256 CrossRef CAS PubMed.
- K. Waldschläger and H. Schüttrumpf, Infiltration Behaviour of Microplastic Particles with Different Densities, Sizes and Shapes – From Glass Spheres to Natural Sediments, Environ. Sci. Technol., 2020, 54, 9366–9373 CrossRef PubMed.
- R. Castaneda, S. Avlijas, A. Simard and A. Ricciardi, Microplastic pollution in St. Lawrence River sediments, Can. J. Fish. Aquat. Sci., 2014, 71, 1767–1771 CrossRef CAS.
- R.-D. Maier and M. Schiller, Handbuch Kunststoff-Additive, Hanser, München, 4th edn, 2016 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |