Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interactions with freshwater biofilms cause rapid removal of common herbicides through degradation – evidence from microcosm studies†
Maria Alexandra
Bighiu
 * and
Willem
Goedkoop
* and
Willem
Goedkoop
Department of Aquatic Sciences and Assessment, Swedish University of Agricultural Sciences, Uppsala, Sweden. E-mail: maria.bighiu@slu.se
First published on 14th December 2020
Abstract
We investigated the role of periphyton biofilms for the fate of three common herbicides, i.e. bentazone, metazachlor and metribuzin, at low, environmental levels and 100 times higher, during a 16 days laboratory experiment. We found that herbicide water concentrations were stable during the first 8 days, whereas substantial declines (>78%) occurred between days 8–16 for all three herbicides. These rapid declines were explained only to a small extent (<8% of the total herbicide loss) by biofilm sorption. As herbicide concentrations in light and dark treatments without biofilms were similar, and the applied light regimen did not cover the UV-spectrum, herbicide photolysis was ruled out as a possible explanation for the observed declines. Furthermore, based on the compounds' characteristics, also volatilization was judged negligible. Therefore, we conjecture that the observed declines in herbicides were due to biodegradation and subsequent evasion of 14CO2 that was driven by enzymatic action from heterotrophic microbes. We reason that heterotrophic microbes used herbicide molecules as labile organic C-sources during C-limitation. Future studies should identify the microbial communities and genes involved in biodegradation in order to understand better the role of biofilms for the self-purification of surface waters.
Environmental significanceBiofilms are complex communities of bacteria, algae, fungi and other microorganisms that grow on virtually all submerged surfaces and fulfil key ecological roles such as primary production and carbon and nutrient cycling. Thus, biofilms have high biological activity and availability of sorption sites, which make them important drivers of the environmental fate of contaminants. In this study we focus on herbicides, which are the largest group of pesticides commonly found in surface waters. Our findings show that biofilms rapidly mineralize the herbicides bentazone, metazachlor and metribuzin, rather than accumulate them for longer periods, which underlines the importance of biofilms in the self-purification of surface waters. |
Introduction
Freshwater periphyton biofilms are complex assemblages of algae, bacteria, fungi, protozoans, and meiofauna embedded in a matrix of extracellular polymeric substances, EPS.1 Biofilms are sites of high biological activity and play important roles in primary production,1 as a basal food resource for higher trophic levels,2 and for carbon and nutrient cycling in freshwater ecosystems.3 Biofilm EPS consists of microbial polysaccharides, lipids, proteins, nucleic acids, and heteropolymers that are essential for biofilm integrity and stability.4 This EPS, as well as the microbial cells in biofilms, represent a large surface area with efficient sorption sites for both heavy metals5,6 and organic contaminants.7,8 For example, Tien and Chen9 found that copper, nickel, chromium and lead were enriched by a factor ranging 1.6 × 10−5 to 7.15 × 10 −5 L kg−1 in biofilms, while Rooney et al.8 reported bioconcentration factors (BCFs) between 12 and 6864 for 20 organic pesticides in biofilms. As the detection frequency of current-use pesticides in biofilms was 4-fold larger than in sediments, while also better reflecting ecological risks (e.g. for invertebrate communities), Mahler et al.10 suggested that pesticide monitoring should also involve biofilm as a complement to sediment. These studies emphasize the key role of biofilms for the fate of pesticides in aquatic ecosystems.Modern pesticides are used in crop protection worldwide, with almost 6 million tons of active ingredients applied in 2017, of which 7% (by weight) were herbicides.11 Through leaching or spray drift, pesticides commonly enter surface waters where they pose a risk to non-target aquatic organisms.12 For example, pesticides negatively affect the abundance of invertebrates,13 as well as the density, antioxidant defence and photosynthetic efficiency of diatoms,14 although the latter may reflect short-term inhibition.15 Herbicides are commonly more water-soluble than insecticides and fungicides and thus comprise a large fraction of the pesticides frequently detected in surface water monitoring.16,17 Herbicides in surface waters are submitted to both abiotic (e.g., photolysis, hydrolysis) and biotic (microbial) degradation, the latter usually being quantitatively more important. Microbial degradation of pesticides includes the enzymatic transformation by heterotrophic microbes via conjugation and complexation, resulting in compounds that have either increased or decreased persistence.18 Heterotrophic microbes degrade pesticides as these are relatively labile molecules that constitute a source of organic carbon, nutrients, and other elements necessary for growth19. Knowledge of herbicide behaviour and fate in aquatic ecosystems is important, especially as their use is expected to increase with climate change20 and a growing human population.21
In this laboratory study, we assess the role of periphyton biofilms for the fate of common herbicides in inland surface waters. We hypothesized that the herbicide water concentrations would be slowly declining during our 16 d experiment, driven by sorption to biofilms. We also hypothesized that the high biological activity in biofilms could contribute to accelerating the degradation of sorbed herbicides. We envision that our results provide an insight in the sorption characteristics of herbicides to biofilms and their behaviour and fate in freshwater ecosystems.
Materials and methods
Herbicide selection
Our study addressed the fate of metazachlor, metribuzin and bentazone, three herbicides that are commonly found in water from agricultural streams.16,17 These compounds are commonly applied in cultures of rapeseed, potatoes and carrots to prevent weed growth. Metazachlor is an inhibitor of cell division, while bentazone and metribuzin are photosynthesis inhibitors. Beside their common occurrence in inland waters, these herbicides were selected based on their similar and low log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values and their availability as 14C-labeled standards. Applied exposure concentrations were similar to those found in European inland waters,16,22 as well as 100-fold higher (referred to as ‘low’, and ‘high’ concentrations, respectively, Table 1).
Kow values and their availability as 14C-labeled standards. Applied exposure concentrations were similar to those found in European inland waters,16,22 as well as 100-fold higher (referred to as ‘low’, and ‘high’ concentrations, respectively, Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow (octanol–water partitioning coefficient)
Kow (octanol–water partitioning coefficient)
| Herbicide properties | Metazachlor | Metribuzin | Bentazone | Unit |
|---|---|---|---|---|
| Group | Chloroacetamide | Triazinone | Benzothiazinone | — |
| CAS nr | 67129-08-2 | 21087-64-9 | 25057-89-0 | — |
| Position of 14C label | Phenyl-U-14C | Ring-6-14C | Carbonyl-14C | — |
| Purity | 98.39 | 99.65 | 99.82 | % |
| Specific activity | 5.5 | 1.85 | 1.85 | MBq mmol−1 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow Kow |
2.13 | 1.54 | 2.34 | — |
| K foc | 79.6 | 37.9 | 59.6 | — |
| DT50 in water | 216 | 41 | 80 | days |
| Water solubility | 450 | 10700 | 7112 | mg L−1 |
| Henry's law constant | 5.90 × 10−5 | 2.00 × 10−5 | 7.20 × 10−5 | Pa m3 mol−1 |
| EC50 | 16.2 | 26.6 | 10100 | μg L−1 |
| Low treatment | 5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
0.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
1.5 | μg L−1 |
| High treatment | 540 | 62 | 150 | μg L−1 |
Experiment description
An inoculum of epilithic biofilm was collected from mesotrophic Lake Erken (59°50′15.6′′N 18°38′06.1′′E) by brushing the upper sides of a number of submersed cobbles collected in the littoral zone and transported back to the laboratory in a cooling box. This inoculum was used to grow biofilms on unglazed ceramic tiles (3 × 3 cm) covered with L16V medium25 at 12 ± 0.2 °C and 16 h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h light
8 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle with a light intensity of 924 ± 144 lux. The L16V-medium is a broad synthetic medium that has been used for culturing multiple species of algae belonging to major taxonomic groups (e.g. Ahlgren et al. 1990
dark cycle with a light intensity of 924 ± 144 lux. The L16V-medium is a broad synthetic medium that has been used for culturing multiple species of algae belonging to major taxonomic groups (e.g. Ahlgren et al. 1990![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26). After 9 weeks of growth, biofilms were well-established, and microscopic analysis showed that green algae made up 90% of the total biovolume, while diatoms and cyanobacteria accounted for 8% and 1%, respectively (detailed in15). These biofilms were used for exposure to pesticides as described below.
26). After 9 weeks of growth, biofilms were well-established, and microscopic analysis showed that green algae made up 90% of the total biovolume, while diatoms and cyanobacteria accounted for 8% and 1%, respectively (detailed in15). These biofilms were used for exposure to pesticides as described below.
At the start of the experiment (t = 0) four biofilm-covered tiles each were placed in experimental units (i.e. 1 L glass beakers, n = 4) containing 0.25 L of autoclaved L16V medium with additions of 14C-labeled herbicides (IZOTOP Institute of Isotopes Co. Ltd., Budapest, Hungary). Blanks (n = 4) were set up similarly, but did not receive herbicide additions. Experimental units were placed randomly on a table and provided with continuous, gentle aeration using glass Pasteur pipettes and aquaria pumps. After 0.5, 2, 4, 8 hours and then after 1, 2, 4, 8, 12, and 16 days, 500 μL water samples were collected with an automated pipette from each of the experimental units and transferred to 20 mL scintillation vials. Biofilm samples were collected from by removing single tiles from the same experimental units after 1, 8 and 16 days. Biofilms were detached from tiles with rubber cell scrapers, transferred to 20 mL scintillation vials, suspended in 2 mL of tissue solubilizer (Soluene 350, PerkinElmer), placed in an oven at 60 °C for 4 h and allowed to cool to room temperature.
All samples then received 10 mL of scintillation cocktail (Ultima Gold®, PerkinElmer) and were kept in the darkness at room temperature for 24 h to obtain stable scintillation readings. Scintillation counts were made for at least 5 minutes or 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CPM using a Beckman LS 6000TA-liquid scintillation counter. Quench corrections were done using internal standards ratios (Perkin Elmer). Disintegration rates for samples were corrected for the background values obtained for corresponding blanks. The QA/QC was performed using Internal Standard Kit, 14C-W for aqueous samples and results are expressed as disintegrations per minute (DPM) and corrected for evaporation. Evaporation was quantified by weighing the experimental units before and after each sampling. Sorption (in)to biofilm is defined here as the ratio between the herbicide concentration in the biofilm and that measured in water at the start (expressed as %). We thus consider the whole biofilm, and do not distinguish among the different sorption/uptake mechanisms (e.g., diffusion, sorption to EPS or cellular uptake, etc). Herbicide loss from the experimental units was calculated as the difference between the DPM measured in water at the start and the DPM at the end (biofilm + water). Blanks (no herbicides added) were used to quantify background radiation. To facilitate comparisons of our results with other studies, concentrations are also presented as μg L−1 and μg kg−1, back calculated from DPM based on the specific activity of each compound (Table 1), using eqn (1) and (2).
000 CPM using a Beckman LS 6000TA-liquid scintillation counter. Quench corrections were done using internal standards ratios (Perkin Elmer). Disintegration rates for samples were corrected for the background values obtained for corresponding blanks. The QA/QC was performed using Internal Standard Kit, 14C-W for aqueous samples and results are expressed as disintegrations per minute (DPM) and corrected for evaporation. Evaporation was quantified by weighing the experimental units before and after each sampling. Sorption (in)to biofilm is defined here as the ratio between the herbicide concentration in the biofilm and that measured in water at the start (expressed as %). We thus consider the whole biofilm, and do not distinguish among the different sorption/uptake mechanisms (e.g., diffusion, sorption to EPS or cellular uptake, etc). Herbicide loss from the experimental units was calculated as the difference between the DPM measured in water at the start and the DPM at the end (biofilm + water). Blanks (no herbicides added) were used to quantify background radiation. To facilitate comparisons of our results with other studies, concentrations are also presented as μg L−1 and μg kg−1, back calculated from DPM based on the specific activity of each compound (Table 1), using eqn (1) and (2).
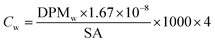 | (1) |
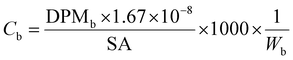 | (2) |
DPMw is the upscaled disintegrations-per-minute from scintillation counting for 250 mL of water (the volume of the test units), corrected for blanks, evaporation, previous sampling and external standard recovery; ×4 is the multiplication factor for the conversion of the volume in our experimental units (250 mL) to litres.
DPMb is the upscaled disintegrations-per-minute from scintillation counting for the whole biofilm from one tile, corrected for external standard recovery;
1.67 × 10−8 is the conversion factor from DPM to MBq;
SA is the specific activity of the herbicide in MBq mg−1;
W b is the biofilm wet weight in kg.
In an additional set up, treatments with autoclaved L16V medium lacking biofilms were deployed in dark and light (629 ± 34 lux) conditions, respectively, to quantify the photolysis of herbicides. For this, water samples were analysed for pesticide concentrations at the start (day 0) and at the end (day 16) of the experiment by LC-MS according to the standard methods US EPA 535![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 and US EPA 1694.28 LC-MS was used in this experiment instead of scintillation counting as all 14C-labelled herbicides had been used in the sorption experiment. The nutrient concentrations in the blanks were determined at start and on day 8, by the standard methods SS-EN ISO 6878:2005 mod., Bran Luebbe, Method No G-175-96 for AAIII (Total-P) and SS EN 12260:2004 (Total-N).
27 and US EPA 1694.28 LC-MS was used in this experiment instead of scintillation counting as all 14C-labelled herbicides had been used in the sorption experiment. The nutrient concentrations in the blanks were determined at start and on day 8, by the standard methods SS-EN ISO 6878:2005 mod., Bran Luebbe, Method No G-175-96 for AAIII (Total-P) and SS EN 12260:2004 (Total-N).
Data analysis
Pearson's correlations were used to assess relationships between herbicides' concentrations in water and in biofilms, as well as between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow and mean biofilm sorption. Repeated measures ANOVAs were run to test for effects of herbicide start concentrations and time on log-transformed herbicide sorption to biofilms, and Tukey HSD-tests were used for post-hoc pairwise comparisons. Both tests were also used for investigating differences in biofilm sorption between compounds. Normality of residuals was assessed from normal quantile plots.
Kow and mean biofilm sorption. Repeated measures ANOVAs were run to test for effects of herbicide start concentrations and time on log-transformed herbicide sorption to biofilms, and Tukey HSD-tests were used for post-hoc pairwise comparisons. Both tests were also used for investigating differences in biofilm sorption between compounds. Normality of residuals was assessed from normal quantile plots.
Results
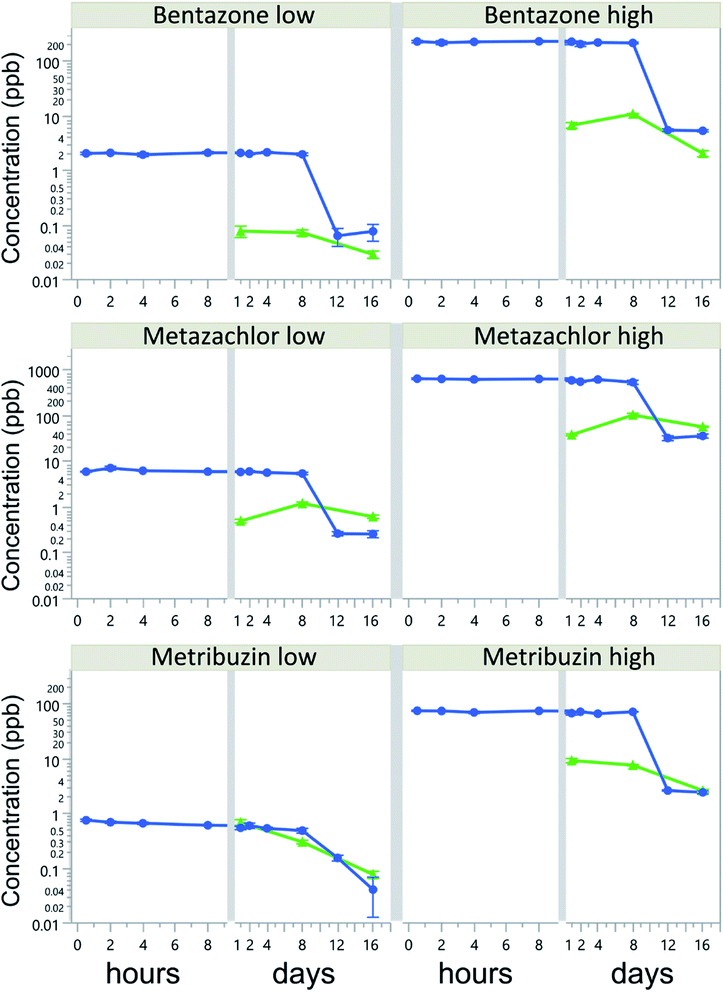
Herbicide water concentrations in all treatments decreased over time, but without a corresponding concentration increase in the biofilms. Herbicide water concentrations were rather constant during the first 8 days of the experiment, with a CV of 2 to 22%, but rapidly dropped by 78–98% of the initial concentration after 12 days (Fig. 1). The most dramatic decline was observed in the bentazone-high treatment, where water concentrations dropped from 150.5 to 5.4 μg L−1 between days 8 and 12. By the end of the experiment (16 d), water concentrations of all herbicides had dropped by more than 94% of their initial concentrations.Biofilm sorption was generally low, on average less than 0.16 ± 0.18% of initially added concentrations. The highest average biofilm sorption was 0.51%, reached after 1 day in the metribuzin-low treatment. Biofilm sorption of bentazone-low and metribuzin decreased linearly over time, whereas for bentazone-high and metazachlor there was an increase in sorption from day 1 to day 8, followed by a decrease between days 8 and 16 (Table S2†). The initial herbicide concentration had a significant effect on the biofilm sorption of metazachlor and metribuzin, but not of bentazone (Table 2). Most notably, metribuzin sorption was 5 times higher at the low concentration than at the high concentration (Table S2†). Moreover, in the low-concentration treatments, metribuzin sorption to biofilms was on average 17-times higher than that of bentazone and 4-times higher than that of metazachlor (p < 0.0001 for both), despite the much lower initial concentrations (3-fold and 7-fold lower, respectively). In the high-concentration treatments, metazachlor sorption exceeded that of bentazone and metribuzin (p < 0.0001 and p = 0.035, respectively), whereas metribuzin sorption was higher than that of bentazone (p < 0.0001). Although these differences were significant, sorption to biofilms was generally very low and accounted for only a small fraction of the total herbicide losses from the water.
| Herbicide | Concentration, df = 1 | Time, df = 2 | Concentration × time, df = 2 | |||
|---|---|---|---|---|---|---|
| p value | F ratio | p value | F ratio | p value | F ratio | |
| Bentazone | n.s. | <0.0001 | 23.56 | n.s. | ||
| Metribuzin | <0.0001 | 228.34 | <0.0001 | 172.11 | 0.0012 | 12.52 |
| Metazachlor | 0.0119 | 12.67 | <0.0001 | 127.28 | n.s. | |
The hydrophobicity of the herbicides (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) decreased their average sorption to biofilms, albeit not significantly (r = 0.99, p = 0.0591). The largest fraction (i.e., 8% of total loss) was recorded in metazachlor-high on day 8 of the experiment. By the end of the experiment (day 16), biofilm sorption accounted for at most 0.01, 0.08 and 0.09% of the total loss of bentazone, metazachlor and metribuzin, respectively. Water concentrations of bentazone and metribuzin were strongly and positively correlated to those in biofilms, especially in the high-concentration treatments (i.e., r = 0.84 and 0.87, p = 0.0006 and 0.0002, respectively), despite low sorption, whereas no correlation was found for metazachlor, when looking at the different concentration levels separately.
Kow) decreased their average sorption to biofilms, albeit not significantly (r = 0.99, p = 0.0591). The largest fraction (i.e., 8% of total loss) was recorded in metazachlor-high on day 8 of the experiment. By the end of the experiment (day 16), biofilm sorption accounted for at most 0.01, 0.08 and 0.09% of the total loss of bentazone, metazachlor and metribuzin, respectively. Water concentrations of bentazone and metribuzin were strongly and positively correlated to those in biofilms, especially in the high-concentration treatments (i.e., r = 0.84 and 0.87, p = 0.0006 and 0.0002, respectively), despite low sorption, whereas no correlation was found for metazachlor, when looking at the different concentration levels separately.
Discussion
Our study shows a more than 78% decrease in herbicide water concentrations in all treatments after 8 days. These rapid declines were explained only to a small extent (<8% of the total herbicide loss) by sorption and accumulation in biofilms. Instead, more than 94% of the added compounds were lost from the experimental vessels by day 16. We conjecture that this was due to mineralization and subsequent evasion of 14CO2, driven by enzymatic action from heterotrophic microbes on herbicide molecules when labile organic C-sources became limiting for their growth (cf.29). Modern pesticides are relatively small organic molecules that can be readily used by heterotrophic microbes (e.g.30,31). Our 14C-labeled herbicides had one or multiple 14C-atoms well integrated in their molecules (Table 1), leaving mineralization as the single option for the observed large loss of label from our experimental units. Likewise, and for similar time frames as applied in our study, Bohuss et al.32 concluded that biodegradation was the main removal pathway and that sorption to biofilms explained less than 0.6% of the total loss of the herbicides atrazine and acetochlor, whereas Lawrence et al.33 showed similar sorption for triazine herbicides in river biofilms. As sorption is a prerequisite for biodegradation, it is likely that even the low sorption observed in our biofilms was sufficient to induce a rapid microbial degradation of the herbicides and ultimately their elimination from the microcosms as CO2.The observed rapid degradation/mineralization of herbicides in the biofilms implies a rapid turnover of sorbed herbicides and little accumulation. Also other studies34,35 concluded that microbial degradation, rather than sorption, was the primary fate of herbicides (carbamates and diazinon) in 10–14 d experiments with river biofilms. Possibly, the architecture of biofilms may have changed with herbicide exposure,36 thus altering the availability of sorption sites and allowing rapid degradation of herbicides in the biofilms. Interestingly, the rapid mineralization of herbicide molecules in our study occurred when only two of four tiles remained in our experimental units, implying an increase in degradation rates per surface area of biofilm, further stressing the adaptation of biofilm microbiota towards a high efficiency in herbicide degradation/mineralization. If similar degradation/mineralization rates occur under field conditions in summer, then monitoring programs may seriously underestimate the run-off and/or leakage of pesticides from agricultural soils.
Beside C also herbicide-N and -S were likely readily taken up by microbes, as these are key elements in the synthesis of proteins and specific amino acids37 and frequently more limiting than C. However, although N makes up 11.6–26.1% (by weight) of the three herbicides tested, while S makes up 14.9% in metribuzin and 40.0% in bentazone, the contributions of herbicide-N and -S were a negligible share of the total-N and -S in the algal growth medium, i.e. less than 0.008% and 0.030%, respectively, in the high-exposure treatments. These low numbers, however, should be seen as underestimates of their relative importance, as N and S originating from herbicides occur in organic molecules and will have a higher bioavailability than the nitrate and sulphate molecules in the medium. Comparisons of herbicide-N and -S with organic molecules in the periphyton biofilms (e.g. originating from algal exudates/decay and microfaunal excretions) would give a more justified estimate of the relative role of herbicide-associated N and S for the metabolism of heterotrophic microbes. Unfortunately, such data were not available from our study.
The water N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio (by weight) on day 8 was 16
P ratio (by weight) on day 8 was 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showing conditions for algal growth that are close to optimal, i.e. close to the Redfield ratio,38 whereas the continuous aeration of the test vessels guaranteed a constant supply of atmospheric CO2. Earlier studies have also shown that recovery from herbicide-induced photosynthetic inhibition can be fast,15i.e. through rapid, adaptive evolution (cf.39). Moreover, algae have short generation times, which also facilitates fast recovery. It is possible, however, that biofilm-associated bacteria were limited by low-molecular organic C-sources and used added herbicides as a C-source. While most studies address herbicide effects on algae (e.g., inhibition of photosynthesis), much less is known about their effects on and interactions with heterotrophic bacteria in aquatic systems.40
1, showing conditions for algal growth that are close to optimal, i.e. close to the Redfield ratio,38 whereas the continuous aeration of the test vessels guaranteed a constant supply of atmospheric CO2. Earlier studies have also shown that recovery from herbicide-induced photosynthetic inhibition can be fast,15i.e. through rapid, adaptive evolution (cf.39). Moreover, algae have short generation times, which also facilitates fast recovery. It is possible, however, that biofilm-associated bacteria were limited by low-molecular organic C-sources and used added herbicides as a C-source. While most studies address herbicide effects on algae (e.g., inhibition of photosynthesis), much less is known about their effects on and interactions with heterotrophic bacteria in aquatic systems.40
Herbicide loss through volatilization and photolysis is likely negligible in our study. First, the selected herbicides are classified as ‘non-volatile’ according to Henry's law constants (Table 1), and it is thus unlikely that they partitioned from the water into the air phase. In line with this, the known metabolites of the investigated herbicides (Table S1†), are also generally less lipophilic than their parent compounds (except for metazachlor oxalic acid), and hence their sorption is not expected to be higher. Second, photolysis was likely not quantitatively important, because the lights in our experiments did not cover the UV spectrum. Despite differences in light intensity (629 vs. 924 lux) between our main experiment and additional run to test for abiotic degradation, the lack of UV-range wavelengths in both light sources, and thus the lack of energy necessary to break the chemical bonds within the herbicide molecules41 should have prevented direct photolysis. The latter is further supported by our observation that herbicide concentrations in light and dark treatments without biofilms were similar (Fig S1†) and did not show a decrease in herbicide concentration after 16 days (Fig S2†). Also, observed herbicide declines were between 3 and 18 times faster than expected from their documented hydrolysis half-life only (Table 1), thus stressing the role of biofilms in this process. This further supports our conclusion that the observed rapid declines in herbicide concentrations most likely were due to microbial degradation in the biofilms and subsequent evasion of 14CO2.
Potential growth inhibition from herbicide action can lead to a decline in the excretion rates of low-molecular compounds by algae and limit the growth of microbial heterotrophs in the biofilms. The equivalent ratios between the EC50 for algae and the high exposure concentrations for metribuzin and bentazone were 2.3× and 0.014×, implying that negative effects likely were negligible. The EC50 values are based on tests with single planktonic species, where the compounds' bioavailability likely is much higher than in the complex biofilms in our experiment. Also should the multispecies assemblages of our biofilms likely have a higher resilience than single-species populations of plankton algae in standardized tests, further alleviating herbicide effects.
Spiking concentration affected sorption of metribuzin and metazachlor to biofilms in our study (Table 2). In particular, the fact that metribuzin sorbed to biofilms to a larger extent (i.e., 5-fold more) in low than in high concentrations is an important finding, as it illustrates a high uptake at low environmental levels, which can affect phototrophic biofilm community structure42 and pose a risk for transfer to higher trophic levels.43 For bentazone, sorption was similar for the low and high concentration treatments (Table S2†), suggesting saturation at the lowest concentration due to saturation of algal kinetic uptake rates.
Conclusions and outlook
Our study highlights the importance of biofilms for self-depuration of aquatic ecosystems, and suggests that biodegradation is the main degradation pathway for herbicides, mass balance-wise (sensu44). Our findings illustrate a rapid removal of herbicides from the water phase, with more than 94% of the amount eliminated after 16 days. As biofilm sorption only explained a small fraction of herbicide loss from the water, and as photolysis and volatilization of herbicides were judged negligible, we conclude that biodegradation was the main pathway of herbicide loss from our experimental units. This implies that modern herbicides likely are short-lived in surface waters during the growing season, where microbes compete for low-molecular carbon-substrata (including herbicides). Such degradation, including complete mineralization, contributes to the valuable ecosystem service of self-purification of surface water that biofilm microbes provide and contribute to a systematic underestimation of pesticide run-off/leakage from agricultural soils based on water concentrations.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was funded by the Swedish Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS, Dnr. 2014-01026) and by SLU's Centre for Pesticides in the Environment (CKB). The authors thank Dr Steffi Gottschalk for laboratory assistance and the staff at our pesticide lab for support in herbicide analyses.References
- Aquatic biofilms: ecology, water quality and wastewater treatment, ed. A. M. Romaní Cornet, H. Guasch and M. D. Balaguer, Caister Academic Press, Norfolk, UK, 2016 Search PubMed.
- D. C. P. Lau, I. Sundh, T. Vrede, J. Pickova and W. Goedkoop, Autochthonous resources are the main driver of consumer production in dystrophic boreal lakes, Ecology, 2014, 95, 1506–1519 CrossRef PubMed.
- T. J. Battin, K. Besemer, M. M. Bengtsson, A. M. Romani and A. I. Packmann, The ecology and biogeochemistry of stream biofilms, Nat. Rev. Microbiol., 2016, 14, 251–263 CrossRef CAS PubMed.
- H.-C. Flemming and J. Wingender, The biofilm matrix, Nat. Rev. Microbiol., 2010, 8, 623–633 CrossRef CAS PubMed.
- P.-Y. Ancion, G. Lear, A. Dopheide and G. D. Lewis, Metal concentrations in stream biofilm and sediments and their potential to explain biofilm microbial community structure, Environ. Pollut., 2013, 173, 117–124 CrossRef CAS PubMed.
- A. W. Decho, Microbial biofilms in intertidal systems: an overview, Cont. Shelf Res., 2000, 20, 1257–1273 CrossRef.
- M. Tsezos and J. P. Bell, Comparison of the biosorption and desorption of hazardous organic pollutants by live and dead biomass, Water Res., 1989, 23, 561–568 CrossRef CAS.
- R. C. Rooney, C. Davy, J. Gilbert, R. Prosser, C. Robichaud and C. Sheedy, Periphyton bioconcentrates pesticides downstream of catchment dominated by agricultural land use, Sci. Total Environ., 2020, 702, 134472 CrossRef CAS PubMed.
- C.-J. Tien and C. S. Chen, Patterns of Metal Accumulation by Natural River Biofilms During Their Growth and Seasonal Succession, Arch. Environ. Contam. Toxicol., 2013, 64, 605–616 CrossRef CAS PubMed.
- B. J. Mahler, T. S. Schmidt, L. H. Nowell, S. L. Qi, P. C. Van Metre, M. L. Hladik, D. M. Carlisle, M. D. Munn and J. May, Biofilms Provide New Insight into Pesticide Occurrence in Streams and Links to Aquatic Ecological Communities, Environ. Sci. Technol., 2020, 54, 5509–5519 CrossRef CAS PubMed.
- FAO, http://www.fao.org/faostat/en/#data/RP, (accessed 1 October 2019).
- C. Leu, H. Singer, C. Stamm, S. R. Müller and R. P. Schwarzenbach, Simultaneous Assessment of Sources, Processes, and Factors Influencing Herbicide Losses to Surface Waters in a Small Agricultural Catchment, Environ. Sci. Technol., 2004, 38, 3827–3834 CrossRef CAS PubMed.
- J. P. Bray, S. J. Nichols, A. Keely-Smith, R. Thompson, S. Bhattacharyya, S. Gupta, A. Gupta, J. Gao, X. Wang, S. Kaserzon, J. F. Mueller, A. Chou and B. J. Kefford, Stressor dominance and sensitivity-dependent antagonism: disentangling the freshwater effects of an insecticide among co-occuring agricultural stressors, J. Appl. Ecol., 2019, 1365–2664 Search PubMed.
- S. Kim Tiam, S. Morin, S. Pesce, A. Feurtet-Mazel, A. Moreira, P. Gonzalez and N. Mazzella, Environmental effects of realistic pesticide mixtures on natural biofilm communities with different exposure histories, Sci. Total Environ., 2014, 473–474, 496–506 CrossRef PubMed.
- M. A. Bighiu, S. Gottschalk, Å. Arrhenius and W. Goedkoop, Pesticide mixtures cause short-term, reversible effects on the function of autotrophic periphyton assemblages: pesticide mixture effects on biofilm structure and function, Environ. Toxicol. Chem., 2020, 39(7), 1367–1374 CrossRef CAS PubMed.
- M. A. Bighiu, S. Höss, W. Traunspurger, M. Kahlert and W. Goedkoop, Limited effects of pesticides on stream macroinvertebrates, biofilm nematodes, and algae in intensive agricultural landscapes in Sweden, Water Res., 2020, 174, 115640 CrossRef CAS PubMed.
- V. C. Schreiner, E. Szöcs, A. K. Bhowmik, M. G. Vijver and R. B. Schäfer, Pesticide mixtures in streams of several European countries and the USA, Sci. Total Environ., 2016, 573, 680–689 CrossRef CAS PubMed.
- G. Matthess, Fate of Pesticides in Aquatic Environments, 1994, 56 Search PubMed.
- T. Katagi, Aerobic microbial transformation of pesticides in surface water, J. Pestic. Sci., 2013, 38, 10–26 CrossRef CAS.
- M. Kattwinkel, J.-V. Kühne, K. Foit and M. Liess, Climate change, agricultural insecticide exposure, and risk for freshwater communities, Ecol. Appl., 2011, 21, 2068–2081 CrossRef PubMed.
- H. C. J. Godfray, J. R. Beddington, I. R. Crute, L. Haddad, D. Lawrence, J. F. Muir, J. Pretty, S. Robinson, S. M. Thomas and C. Toulmin, Food Security: The Challenge of Feeding 9 Billion People, Science, 2010, 327, 812 CrossRef CAS PubMed.
- W. Fang, Y. Peng, D. Muir, J. Lin and X. Zhang, A critical review of synthetic chemicals in surface waters of the US, the EU and China, Environ. Int., 2019, 131, 104994 CrossRef CAS PubMed.
- Pesticide Properties Database, http://sitem.herts.ac.uk/aeru/ppdb/index.htm, (accessed 18 May 2020) Search PubMed.
- US Environmental Protection Agency, https://comptox.epa.gov/dashboard, (accessed 18 May 2020).
- K. Lindström, Nutrient requirements of the dinoflagellate Peridinium gatunense, J. Phycol., 1991, 207–219 CrossRef.
- G. Ahlgren, L. Lundstedt, M. Brett and C. Forsberg, Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters, J. Plankton Res., 1990, 12, 809–818 CrossRef CAS.
- J. A. Shoemaker and M. V. Bassett, National Exposure Research Laboratory Office of Research and Development, U. S. Environmental Protection Agency, Cincinnati, Ohio, 2005, vol. 45268, p. 61 Search PubMed.
- US EPA, Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS, 2007, vol. 77 Search PubMed.
- G. M. Wolfaardt, J. R. Lawrence, R. D. Robarts and D. E. Caldwell, Bioaccumulation of the Herbicide Diclofop in Extracellular Polymers and Its Utilization by a Biofilm Community during Starvation, Appl. Environ. Microbiol., 1995, 61, 7 CrossRef PubMed.
- P. Larsson, L. Okla and L. Tranvik, Microbial degradation of xenobiotic, aromatic pollutants in humic water, Appl. Environ. Microbiol., 1988, 54, 1864–1867 CrossRef CAS PubMed.
- I. J. Allan, W. A. House, A. Parker and J. E. Carter, Transport and distribution of lindane and simazine in a riverine environment: measurements in bed sediments and modelling, Pest Manage. Sci., 2004, 60, 417–433 CrossRef CAS PubMed.
- I. Bohuss, T. Rékasi, S. Szikora, K. Barkács, G. Záray and É. Ács, Interaction of acetochlor and atrazine with natural freshwater biofilms grown on polycarbonate substrate in lake Velence (Hungary), Microchem. J., 2005, 79, 201–205 CrossRef CAS.
- J. R. Lawrence, G. Kopf, J. V. Headley and T. R. Neu, Sorption and metabolism of selected herbicides in river biofilm communities, Can. J. Microbiol., 2001, 47, 634–641 CrossRef CAS PubMed.
- C.-J. Tien, T.-L. Chuang and C. S. Chen, The Role of Naturally Occurring River Biofilms on the Degradation Kinetics of Diazinon, Clean: Soil, Air, Water, 2011, 39, 931–938 CAS.
- C.-J. Tien, M.-C. Lin, W.-H. Chiu and C. S. Chen, Biodegradation of carbamate pesticides by natural river biofilms in different seasons and their effects on biofilm community structure, Environ. Pollut., 2013, 179, 95–104 CrossRef CAS PubMed.
- G. M. Wolfaardt, J. R. Lawrence, R. D. Robarts, S. J. Caldwell and D. E. Caldwell, Multicellular Organization in a Degradative Biofilm Community, 1994, vol. 13 Search PubMed.
- C. J. Hurst, The Structure and Function of Aquatic Microbial Communities, Springer International Publishing Imprint, Springer, Cham, 2019 Search PubMed.
- A. C. Redfield, The biological control of chemical factors in the environment, Am. Sci., 1958, 46, 19 Search PubMed.
- I. E. Huertas, M. Rouco, V. López-Rodas and E. Costas, Warming will affect phytoplankton differently: evidence through a mechanistic approach, Proc. R. Soc. B, 2011, 278, 3534–3543 CrossRef PubMed.
- Z. R. Staley, V. J. Harwood and J. R. Rohr, A synthesis of the effects of pesticides on microbial persistence in aquatic ecosystems, Crit. Rev. Toxicol., 2015, 45, 813–836 CrossRef CAS PubMed.
- T. Nawaz and S. Sengupta, in Advances in Water Purification Techniques, Elsevier, 2019, pp. 67–114 Search PubMed.
- K. Gustavson, F. Møhlenberg and L. Schlüter, Effects of Exposure Duration of Herbicides on Natural Stream Periphyton Communities and Recovery, Arch. Environ. Contam. Toxicol., 2003, 45, 48–58 CrossRef CAS PubMed.
- C. Bonnineau, J. Artigas, B. Chaumet, A. Dabrin, J. Faburé, B. J. D. Ferrari, J. D. Lebrun, C. Margoum, N. Mazzella, C. Miège, S. Morin, E. Uher, M. Babut and S. Pesce, Role of Biofilms in Contaminant Bioaccumulation and Trophic Transfer in Aquatic Ecosystems, Current State of Knowledge and Future Challenges, 2020, vol. 39 Search PubMed.
- K. Fenner, S. Canonica, L. P. Wackett and M. Elsner, Evaluating pesticide degradation in the environment: blind spots and emerging opportunities, Science, 2013, 341, 752–758 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0em00394h |
| This journal is © The Royal Society of Chemistry 2021 |