Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparing pathways for electricity-based production of dimethoxymethane as a sustainable fuel†
Jannik
Burre
 a,
Dominik
Bongartz
a,
Dominik
Bongartz
 a,
Sarah
Deutz
a,
Sarah
Deutz
 b,
Chalachew
Mebrahtu
b,
Chalachew
Mebrahtu
 c,
Ole
Osterthun
c,
Ole
Osterthun
 c,
Ruiyan
Sun
c,
Ruiyan
Sun
 c,
Simon
Völker
c,
Simon
Völker
 b,
André
Bardow
b,
André
Bardow
 bdef,
Jürgen
Klankermayer
bdef,
Jürgen
Klankermayer
 c,
Regina
Palkovits
c,
Regina
Palkovits
 c and
Alexander
Mitsos
c and
Alexander
Mitsos
 *ade
*ade
aProcess Systems Engineering (AVT.SVT), RWTH Aachen University, Forckenbeckstraße 51, 52074 Aachen, Germany. E-mail: amitsos@alum.mit.edu; Tel: +49 241 80 94704
bInstitute of Technical Thermodynamics, RWTH Aachen University, Schinkelstraße 8, 52062 Aachen, Germany
cInstitute of Technical and Macromolecular Chemistry, RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany
dJARA-ENERGY, Templergraben 55, 52056 Aachen, Germany
eInstitute of Energy and Climate Research: Energy Systems Engineering (IEK-10), Forschungszentrum Jülich GmbH, Wilhelm-Johnen-Straße, 52425 Jülich, Germany
fDepartment of Mechanical and Process Engineering, ETH Zurich, Tannenstraße 3, 8092 Zurich, Switzerland
First published on 18th June 2021
Abstract
Synthetic dimethoxymethane (DMM) is a promising fuel or blend component as it offers outstanding combustion characteristics. DMM production from hydrogen (H2) and carbon dioxide (CO2) is technically feasible with established technology but results in a low overall process efficiency. Recent research in catalyst development has increased DMM yield significantly and new reaction pathways have been proposed. Yet, it remains unknown how the achievements in catalyst development affect process performance. To close this gap, we analyze processes based on five reaction pathways regarding exergy efficiency, production cost, and climate impact. As the pathways have different technology readiness levels, we develop a methodology that ensures consistent boundary conditions and model detail between pathways. The methodology enables a hierarchical optimization-based process design and evaluation. The results show that the non-oxidative (i.e., reductive, dehydrogenative, and transfer-hydrogenative) pathways consume stoichiometrically less H2 not only than the established and oxidative pathway, but also less than most other electricity-based fuels (e-fuels). The higher resource efficiency of these pathways increases process exergy efficiency from 75% to 84%; production cost (2.1$ Ldiesel-eq.−1) becomes competitive to other e-fuels; and the impact on climate change reduces by up to 92% compared to fossil diesel, if renewable electricity is utilized. Whereas the reductive pathway may already enable a sustainable production of DMM with only little catalyst improvements, the dehydrogenative and transfer-hydrogenative pathways still require a higher DMM selectivity and methanol conversion, respectively. With considerable catalyst improvements, a maximum exergy efficiency of 92% and minimum production cost of 2.0$ Ldiesel-eq.−1 are achievable. Our analyses show: With the non-oxidative pathways, the high potential of DMM is no longer restricted to its outstanding combustion characteristics but extended to its production.
Broader contextThe urgent need for introducing renewable energy into the mobility sector and the low energy density of state-of-the-art batteries call for alternative solutions to meet climate targets. Chemical energy carriers produced from renewable electricity-so called e-fuels-may contribute substantially to such a solution. Oxymethylene ethers (OMEn) are particularly promising as they can not only be produced from carbon dioxide and renewable hydrogen. They can also drastically reduce hazardous emissions during combustion (such as nitrogen oxide and soot emissions) compared to fossil diesel. The commercial production of OMEn is however not sustainable and prevents its broad introduction into the transportation sector. Major inefficiencies are caused by the multitude of involved process steps already towards the first member of OMEn, dimethoxymethane (DMM). To improve process performance, new catalysts have been developed enabling more direct and potentially sustainable pathways for DMM production. In order to evaluate how much these achievements in catalyst development improve sustainability-and finally estimate whether DMM can become a sustainable e-fuel-a combined techno-economic analysis (TEA) and life cycle assessment (LCA) of the these pathways is inevitable. |
1 Introduction
Greenhouse gas (GHG) emissions from transportation make up about 20% of the global GHG emissions and have been increasing continuously during the past decades. Efficiency improvements have been insufficient to outweigh this increase, which is mainly caused by the growing transportation demand.1 Additionally, air pollution from transportation has become an increasing cause for health concerns, especially in urban areas. Both aspects motivate the development of transportation options with low emissions based on renewable energy.One approach to introduce renewable energy into the transportation sector is its direct electrification by battery electric vehicles (BEV). While BEV might be beneficial for short-distance and light-duty transportation,2 their wide application-particularly for long-distance and heavy-duty transportation-is questionable in the short- to medium-term.3 For such applications, liquid fuels produced from biomass (biofuels) and/or renewable electricity (e-fuels) can be advantageous due to their comparatively high energy density.
Recently, oxymethylene ethers (OMEn, CH3O(CH2O)nCH3) have received considerable attention as full substitutes4–6 or blend components7–10 for diesel fuels. Currently, fossil diesel largely dominates fuel consumption in long-distance and heavy-duty transportation and will maintain its crucial role in the next decades, as highlighted by the International Energy Agency.11 The addressable market for OMEn as a fuel alternative is therefore enormous and ideally complements the one for gasoline fuel alternatives such as ethanol from renewable resources.12 OMEn can be produced from renewable syngas via biomass gasification,13–15 or from renewable hydrogen (H2) and carbon dioxide (CO2)16–20 potentially achieving carbon neutrality over their entire life cycle. Their volumetric energy density (∼20 MJ L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21) is about 40% lower than that of diesel, but it is similar to that of other e-fuels22 and about one order of magnitude higher than that of Li-ion batteries for BEV.23 This makes OMEn particularly suitable for long-distance and heavy-duty transportation. Both OME1 (methylal or dimethoxymethane, hereinafter referred to as DMM) and OME3–5 offer outstanding combustion characteristics (e.g., high thermodynamic efficiency,7,9,10 low pollutant emissions5,7–10) but differ in production, infrastructure, and engine compatibility. Whereas OME3–5 has more diesel-like properties and can be combusted in conventional diesel engines, DMM needs to be either mixed with additives to gain engine compatibility7 or blended with diesel.8,24 However, engine modifications seem to remain indispensable for both DMM and OME3–5.4,5,25 In addition to the potential direct application in internal combustion engines, DMM is a key intermediate in OME3–5 production via paraformaldehyde,26 trioxane,27,28 or in novel routes via gaseous formaldehyde.29,30
21) is about 40% lower than that of diesel, but it is similar to that of other e-fuels22 and about one order of magnitude higher than that of Li-ion batteries for BEV.23 This makes OMEn particularly suitable for long-distance and heavy-duty transportation. Both OME1 (methylal or dimethoxymethane, hereinafter referred to as DMM) and OME3–5 offer outstanding combustion characteristics (e.g., high thermodynamic efficiency,7,9,10 low pollutant emissions5,7–10) but differ in production, infrastructure, and engine compatibility. Whereas OME3–5 has more diesel-like properties and can be combusted in conventional diesel engines, DMM needs to be either mixed with additives to gain engine compatibility7 or blended with diesel.8,24 However, engine modifications seem to remain indispensable for both DMM and OME3–5.4,5,25 In addition to the potential direct application in internal combustion engines, DMM is a key intermediate in OME3–5 production via paraformaldehyde,26 trioxane,27,28 or in novel routes via gaseous formaldehyde.29,30
Commercial DMM production takes place via the condensation reaction of methanol and aqueous formaldehyde (FA).31 The major drawback of the underlying reaction pathway is the reaction of H2 to water during upstream FA production increasing H2 demand considerably. Moreover, the more water is present in the system, the more undesired side products are formed.32
To overcome the limitations of commercial DMM production, promising process alternatives have been proposed. A few authors suggest DMM purification via extractive33,34 and pressure swing distillation35 intending to increase energy efficiency. Other studies propose reactive distillation to shift FA conversion towards DMM36 and combine this approach with pressure swing distillation.37 This combined process concept has been used to develop and analyze the entire process chain for DMM production from renewable H2 and CO2.17 In all of these processes, aqueous FA production still represents a key process step. Although FA production is a highly established and simple process step,26 its exergy efficiency is rather low (73%).17
The inherent weaknesses of the reaction pathway containing FA production have spurned research activity toward pathways avoiding FA production. Significant improvements in catalyst performance have been achieved38 and novel DMM synthesis pathways proposed (cf. Section 2). However, no process concepts have been developed and analyzed so far. Instead, the similar thermodynamic properties of the multi-component system within a novel pathway (the direct reduction of CO239) to those within an established one have been used to estimate the process performance and the impact on climate change of DMM production avoiding FA formation. An increased exergy efficiency of 86% for DMM production from renewable H2 and CO2 and the possibility of significant cradle-to-grave CO2-equivalent emission reductions compared to fossil diesel was reported.20 Given the environmental benefits of DMM and the promising estimates on process performance, the use of more detailed process models in a comparative process analysis alongside the development of novel reaction pathways is essential for sustainable DMM production in the short- to medium-term.
A fair comparison of pathway alternatives is however difficult to perform due to their wide range of technology readiness levels (TRL) and data availability. The comparison of technologies with different TRL has recently received increasing attention, especially in the context of emerging CO2 utilization technologies.40 Comprehensive literature reviews about methods, guidelines, and frameworks for techno-economic analysis (TEA),41 life cycle assessment (LCA),42 and combined TEA and LCA43 dedicated to technologies with varying TRL have recently been published. None of them combines process evaluation based on TEA and LCA with optimization-based process design for technologies with different TRL to explicitly provide feedback for catalyst development.
This article has the major goal to evaluate all reaction pathways from methanol towards DMM in order to provide feedback on the current and prospective process performance for catalyst development. Given the different maturities of the reaction pathways, we introduce a hierarchical process development and evaluation methodology to ensure a fair comparison across these pathways. We perform TEA and LCA on each level to determine the key performance indicators (KPIs) relevant for sustainable e-fuel production. As several pathways are still at an early stage of development, we identify their bottlenecks and derive performance goals that are necessary for process sustainability in the near future.
In Section 2, we summarize all reaction pathways and corresponding key characteristics for DMM production from methanol. Section 3 provides the methodology for process development and evaluation that enables a fair comparison of these pathways. On the basis of the methodology, Section 4 presents the results of the comparison and discusses perspective improvement potential. In Section 5, we conclude our findings.
2 Pathways for DMM synthesis
Commercial DMM production is based on the established pathway (Section 2.1), which has been investigated extensively. In contrast, most of the direct pathways (Sections 2.2–2.5) have been proposed recently. Herein, we present the key ideas of these pathways. More detailed information about catalysis and reaction conditions can be found in ESI,† Section S1.2.1 Established pathway
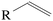
Currently, DMM is produced by the reaction of methanol with (typically aqueous) FA:| 2CH3OH + CH2O ⇌ DMM + H2O. | (R1) |
Thus, two intermediates are required: methanol and FA. Methanol can be produced directly from H2 and CO2 according to the reaction
| 3H2 + CO2 ⇌ CH3OH + H2O. | (R2) |
| CH3OH + 1/2O2 → CH2O + H2O. | (R3) |
| 9H2 + 3CO2 + 1/2O2 ⇌ DMM + 5H2O. | (R4) |
2.2 Oxidation of methanol
The direct oxidation of methanol to DMM involves two sequential reactions occurring in the same reactor: in situ methanol oxidation to FA and subsequent FA acetalization with methanol to DMM. Both add up to the overall reaction equation| 3CH3OH + 1/2O2 → DMM + 2H2O, | (R5) |
In contrast to the established pathway, these water molecules are not bound to FA (resulting in methylene glycols), such that the formed water can be more easily removed from the reaction mixture and purged from the process.
2.3 Reduction of CO2
The direct reduction of CO2 to DMM incorporates CO2 and H2 into methanol following the overall reaction equation| 2CH3OH + CO2 + 2H2 ⇌ DMM + 2H2O. | (R6) |
In contrast to the aforementioned pathways, where FA is produced in a redox-inefficient oxidation step either in a dedicated process step (cf. established pathway) or in situ (cf. oxidative pathway), FA formation is avoided in the reductive pathway. This results in a lower overall H2 consumption-the main cost driver of e-fuels-following the overall reaction equation starting from H2 and CO2
| 8H2 + 3CO2 ⇌ DMM + 4H2O. | (R7) |
2.4 Dehydrogenation of methanol
A further non-oxidative pathway for DMM synthesis can be achieved by coupling the dehydrogenation of methanol to FA with the acetalization of FA with methanol:47| 3CH3OH ⇌ DMM + H2O + H2. | (R8) |
2.5 Transfer-hydrogenation of methanol
Similar to methanol dehydrogenation, methanol transfer-hydrogenation releases one mole H2 per mole DMM produced following the overall reaction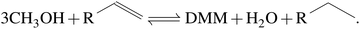 | (R9) |
 ). Thus, downstream purification of the off gas to access molecular hydrogen is omitted. A subsequent reaction between the FA molecule produced from methanol dehydrogenation and the second equivalent of methanol takes place to form MM. A third equivalent of methanol can then undergo a condensation reaction with MM leading to the desired product DMM.48
). Thus, downstream purification of the off gas to access molecular hydrogen is omitted. A subsequent reaction between the FA molecule produced from methanol dehydrogenation and the second equivalent of methanol takes place to form MM. A third equivalent of methanol can then undergo a condensation reaction with MM leading to the desired product DMM.48
Similar to the other non-oxidative pathways (reductive and dehydrogenative pathway), the transfer-hydrogenation of methanol toward DMM benefits from the same savings in overall H2 consumption (cf.reaction (R7)). These savings are enabled by a beneficial H2 management via the regeneration of H2 from its carrier substance and its recycling.
3 Process design and evaluation accounting for TRL
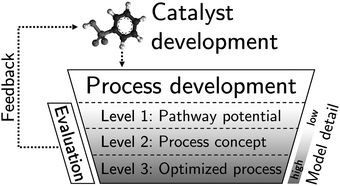
The TRLs of the DMM reaction pathways differ significantly, and so does the data availability for process design and evaluation: Whereas the established pathway has been investigated extensively over the last three decades (Section 2.1), the majority of direct DMM pathways has been discovered during the last years (Sections 2.2–2.5). To still ensure a fair comparison despite the different stages of development and to ultimately lead the way towards sustainable DMM production, we introduce a hierarchical process development and evaluation methodology (Fig. 1). Within this methodology, process development (Section 3.1) and evaluation (Section 3.2) take place on up to three hierarchy levels depending on the data availability for each considered pathway. On Level 1, only reaction equations are required for calculating the maximum pathway potential. On Level 2, thermodynamic data and at least experimental data on reactions showing a sufficiently high performance need to be available to develop first process concepts. On Level 3, more detailed models are required for process optimization. On each level, three KPIs are calculated: process efficiency, production cost, and impact on climate change. We use these performance indicators to derive pathway-specific feedback for catalyst development to effectively benefit from both process and catalyst development (Section 4.4).By applying the methodology to each pathway, we attain two important achievements: First, the pathways become comparable up to their highest common level despite their discrepancies in maturity. A fair comparison is ensured by using the same boundary conditions, model detail, and input data. Second, the gradual refinement of models and input data throughout the levels reveals information about the most relevant parameters and the gap between current and highest possible process performance. This information is not only essential for process optimization but also for catalyst optimization.
3.1 Process development
We develop the process for each pathway sequentially from Level 1, containing only the most essential pathway characteristics, up to Level 3, containing enough information about the process for process optimization using rigorous models. In this section, we explicitly distinguish between levels, because the models and methods used for process development differ significantly between levels. Each level addresses an individual question:• Level 1: What is the maximum potential of the pathway?
• Level 2: Which performance can we expect from a corresponding process?
• Level 3: What is the actual performance of an optimized process?
3.2 Pathway evaluation
The results obtained on each level in process development (cf. Section 3.1) are inputs for the pathway evaluation. In accordance with the levels considered for process development, we evaluate each pathway up to their highest level always considering the three KPIs: process efficiency, production cost, and impact on climate change. The analyses between levels are dependent from one another only in such a way that the optimized process on Level 3 results from the least energy-intensive process concept on Level 2. The evaluation models and methods are the same for each level and differ only in their input data (cf. Table S5, ESI†).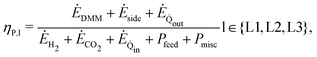 | (1) |
![[Q with combining dot above]](https://www.rsc.org/images/entities/i_char_0051_0307.gif) out and Ė
out and Ė![[Q with combining dot above]](https://www.rsc.org/images/entities/i_char_0051_0307.gif) in is the DMM-specific exergy of excess heat and heat demand of the process, respectively (Tambient = 298.15 K); and Pfeed and Pmisc is the DMM-specific electricity demand for feed compression and miscellaneous compression and pumping within the entire process, respectively. We distinguish between two types of exergy efficiency: process exergy efficiency (cf.eqn (1)) and system exergy efficiency (cf. eqn (S2), ESI†). Process exergy efficiency refers only to the process for DMM production from H2 and CO2, thus decoupling the provision of raw materials from the process. In contrast, system exergy efficiency refers to the entire system including the provision of raw materials. For the provision of raw materials, we consider a best and a worst case scenario (Table 1). In the best case scenario, H2 is provided by a solid oxide electrolyzer cell (SOEC) and CO2 is provided by a high purity (∼100%) industrial point source. In the worst case scenario, H2 is provided by a polymer electrolyte membrane (PEM) electrolysis and CO2 is provided by direct air capture (DAC). The parameters for the best and worst case scenario are summarized in Table S8 (ESI†).
in is the DMM-specific exergy of excess heat and heat demand of the process, respectively (Tambient = 298.15 K); and Pfeed and Pmisc is the DMM-specific electricity demand for feed compression and miscellaneous compression and pumping within the entire process, respectively. We distinguish between two types of exergy efficiency: process exergy efficiency (cf.eqn (1)) and system exergy efficiency (cf. eqn (S2), ESI†). Process exergy efficiency refers only to the process for DMM production from H2 and CO2, thus decoupling the provision of raw materials from the process. In contrast, system exergy efficiency refers to the entire system including the provision of raw materials. For the provision of raw materials, we consider a best and a worst case scenario (Table 1). In the best case scenario, H2 is provided by a solid oxide electrolyzer cell (SOEC) and CO2 is provided by a high purity (∼100%) industrial point source. In the worst case scenario, H2 is provided by a polymer electrolyte membrane (PEM) electrolysis and CO2 is provided by direct air capture (DAC). The parameters for the best and worst case scenario are summarized in Table S8 (ESI†).
| Best case scenario | Worst case scenario | |
|---|---|---|
| H2 provision | SOEC | PEM electrolysis |
| CO2 provision | Ideal point source | DAC |
| Electricity | Onshore wind park (Germany) | Power grid mix today (Germany) |
| Heat (T < 90 °C) | Heat pump | Steam |
| Heat (90 °C < T < 250 °C) | Electrode boiler | Steam |
| Heat (T > 250 °C) | Electrode boiler | Natural gas boiler |
| Cooling | Vapor compression refrigeration system |
The goal of this study is to compare DMM pathways from a climate point of view. As DMM intends to substitute fossil fuels particularly in long-distance and heavy-duty transportation, we also include fossil diesel to this comparison. Due to the different combustion characteristics of these two fuels, we consider their combustion at the end-of-life to cover the cradle-to-grave perspective. The functional unit “the provision of 1 MJ of enthalpy of combustion” for both DMM and fossil diesel enables a consistent comparison between the investigated product systems. To ensure consistency also with the TEA, we do not consider environmental credits (so-called avoided burdens, i.e., an environmental credit to account for the avoided burden of the conventional production) for co-produced side products (MF and dimethyl ether (DME)) and heat. The influence of these avoided burdens on the climate impact of DMM is however investigated in Section S6 (ESI†). We focus on the impact category climate change (heat radiation absorption of the atmosphere caused by anthropogenic emissions, measured in kg of CO2-equivalents57) as e-fuels mainly aim at reducing the climate impact of transportation. For a holistic assessment of DMM, further impact categories are also important but beyond the scope of this work. The same best and worst case scenarios as for the exergy efficiency analyses are used and extended according to Table 1. All assumptions and datasets are summarized in ESI,† Section S3.3.
4 Results on key performance indicators for DMM production
On the basis of the models and assumptions presented in Section 3, each pathway is analyzed up to a certain level given their data availability. Thermodynamic data is available for all considered pathways in open literature (cf. Section 3.1). In contrast, reaction data differs significantly between pathways. For the established pathway, reaction kinetics are available58,59 and enable the propagation of the pathway through all three levels. For the oxidative pathway, a comprehensive experimental data base on reaction performance has been accumulated,38 yet a kinetic model has not been derived. Notwithstanding, the vast amount of data makes the propagation through all three levels possible. For the reductive pathway, considerable achievements in catalyst optimization39,46 and the successful application of an alternative catalytic system45 make process development and evaluation on Level 2 possible. For process optimization on Level 3, however, a more extensive data base or (ideally) reaction kinetics are required. For the dehydrogenative pathway, only little experimental data has been reported.47 Nevertheless, the achieved reaction performance is high enough to develop a first process concept on Level 2. For the transfer-hydrogenative pathway, turnover numbers (TON) of the same magnitude as for the reductive pathway have been reported.48 A low catalyst loading compared to the reductive pathway however results in lower DMM single-pass yields with respect to methanol. Thus, the evaluation of a process concept for the transfer-hydrogenative pathway on Level 2 would systematically underestimate its performance.On the basis of these classifications, we developed process concepts for the reductive and dehydrogenative pathway (Fig. 2) following the procedure presented in Section 3.1. For the established and oxidative pathway, we adapted processes from the literature17,60 and calculated corresponding mass and energy balances (Tables S11–S13, ESI†) for the analyses on both Level 2 and 3.
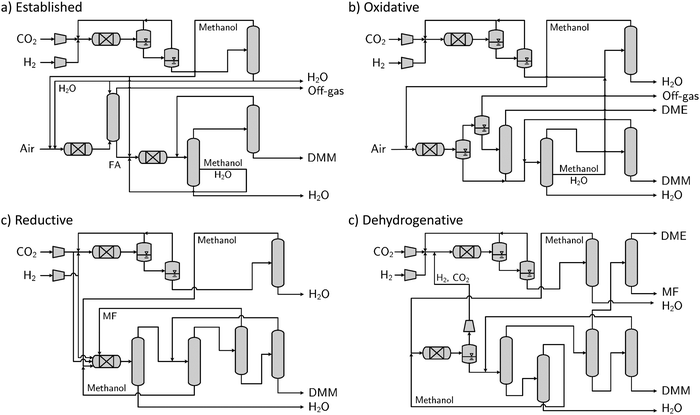 | ||
| Fig. 2 Process concepts for all considered pathways on Level 2. All possible distillation sequences have been screened and the least energy-intensive sequence has been chosen. | ||
In the following, we present the results of the analyses structured by KPI to highlight both how they differ between pathways and how they evolve through the levels for each pathway.
4.1 Exergy efficiency
On Level 1, we show that the benefit of the lower stoichiometric H2 consumption for the reductive, dehydrogenative, and transfer-hydrogenative pathway corresponds to an increase in process exergy efficiency of 11% (Fig. 3). These non-oxidative pathways consume 8 mol H2 per 1 mol DMM formed instead of 9 mol H2 (established and oxidative pathway). The benefit of saving 1 mol H2 per 1 mol DMM produced enables a maximum process exergy efficiency of almost 97%. A comparison with the maximum process exergy efficiency of other e-fuels (ethanol: 90%; methanol: 95%; methane: 83%; DME: 97%; all based on HHV) and their higher stoichiometric H2 consumption relative to their heating values (Table S14, ESI†) demonstrate the great potential of the non-oxidative DMM reaction pathways. Considering the experimental selectivities of the entire value chain on Level 1 instead of perfect selectivity, the gap between experimental exergy efficiencies and their theoretical limit become clear (Fig. 3). The negative effects caused by a low reaction conversion, however, is captured only by Level 2 and 3 evaluations.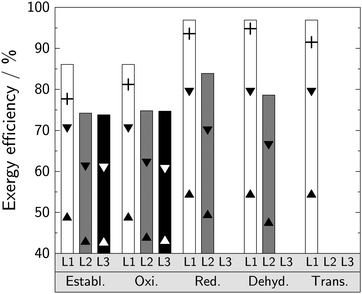 | ||
| Fig. 3 Exergy efficiencies for all evaluated levels and pathways. The bars correspond to the process exergy efficiency (DMM production starting from H2 and CO2), whereas the triangles correspond to the best (▼) and worst (▲) case scenario for the system exergy efficiency (DMM production starting from electricity, water, and a CO2 source). The two scenarios are specified in Table 1. The crosses correspond to Level 1 evaluations considering the experimental selectivities of the entire value chain instead of perfect selectivity. | ||
On Level 2, by considering the actual reaction performance from lab-scale experiments or pilot plants (Table S1, ESI†) and process concepts (cf.Fig. 2), the exergy efficiency decreases significantly for all pathways. One common reason for this decrease is that up to 6% of the exergy content of DMM is required for H2 and CO2 feed compression to maximum operating pressures of 70 to 80 bar. Furthermore, main exergy losses in the established pathway are due to methanol combustion in the FA process step17 and a comparably low overall carbon-based DMM yield (90%). The overall carbon-based DMM yield of the oxidative pathway (94%) is considerably higher and only little external heating is required (cf. Table S13, ESI†). Yet, exergy efficiency is only about the same as that of the established pathway, because the product removal from a highly diluted gaseous reactor effluent requires more than 5% of the DMM exergy. The overall carbon-based DMM yield of the reductive pathway (97%) is even higher than that of the oxidative pathway as the only reported side product is the intermediate MF and assumed to be recycled back into the reactor. However, a relatively low methanol conversion of 10% results in high recycle streams and a high heat demand for separation (8% of the DMM exergy). This bottleneck-the most relevant one of the reductive pathway-has not been captured by the process estimations in preceding studies, where process efficiency has been overestimated (86%).20 The methanol conversion of the dehydrogenative pathway is only 3.6%, thus resulting in a six times higher methanol recycle stream compared to the reductive pathway and in a heat demand for product separation of 16% of the DMM exergy. This increased heat demand reduces exergy efficiency considerably. Also the overall carbon-based DMM yield of the associated process is significantly lower (77%) as MF and small amounts of DME are formed as side products. In contrast to the reductive pathway, MF can not be converted further to DMM in the dehydrogenative pathway. As MF and DME are valuable side products, the comparatively low DMM selectivity has only negligible effect on exergy efficiency.
On Level 3, the exergy efficiency of both the established and oxidative pathway do not differ significantly from those of Level 2. Performance gains due to optimization are balanced by accounting for more detailed models (e.g., tray-to-tray distillation models) and the power demand for additional process units (e.g., pumps and compressors for recycle streams). However, we still benefit from the analyses on Level 3 in two ways: First, we obtain information on equipment sizing, which enables costing. Second, we gain more reliability on the results as more rigorous models are used. These two outcomes are highly relevant for industrial implementation.
4.2 Production cost
As is typical for e-fuels, the H2 price dominates DMM production cost for all pathways and levels (Fig. 4).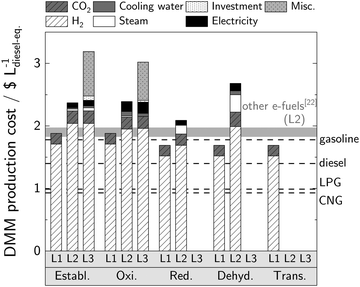 | ||
Fig. 4 Production cost for each synthesis route for all evaluated levels. Perspective H2 and CO2 prices of 5$ kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 and 70$ t−1 61 and 70$ t−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 are considered for PEM electrolysis and CO2 capture from post-combustion, respectively. A price of 7.78$ GJ−1 for steam at 160 °C;63 0.02$ t−1 and 1.2$ t−1 for cooling water at 25 °C and 5 °C, respectively;64 and 2.40$ t−1 for refrigeration at −35 °C 62 are considered for PEM electrolysis and CO2 capture from post-combustion, respectively. A price of 7.78$ GJ−1 for steam at 160 °C;63 0.02$ t−1 and 1.2$ t−1 for cooling water at 25 °C and 5 °C, respectively;64 and 2.40$ t−1 for refrigeration at −35 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 are considered. Investment cost and all miscellaneous costs are calculated after Guthrie65 and Turton et al.,66 respectively. Corresponding cost parameters can be found in Section S3.2 (ESI†). The production cost of other e-fuels refer to methane, methanol, and DME and correspond to the assumptions and model detail on Level 2.22 The consumer prices of the conventional fossil fuels gasoline, diesel, LPG, and CNG include all taxes. 64 are considered. Investment cost and all miscellaneous costs are calculated after Guthrie65 and Turton et al.,66 respectively. Corresponding cost parameters can be found in Section S3.2 (ESI†). The production cost of other e-fuels refer to methane, methanol, and DME and correspond to the assumptions and model detail on Level 2.22 The consumer prices of the conventional fossil fuels gasoline, diesel, LPG, and CNG include all taxes. | ||
On Level 1, the analyses reveal that the H2 savings of the non-oxidative pathways correspond to cost reductions of 11% of total raw material costs. As transportation is highly price sensitive, these savings in H2 consumption are paramount for DMM production.
On Level 2, an imperfect selectivity toward DMM results in additional costs. These are most pronounced for the dehydrogenative pathway due to the significant co-production of MF and DME, for which we do not consider a monetary credit given the lack of reliable data. For this pathway, also the costs for steam are the highest among the pathways due to its low methanol conversion (cf. Table S1, ESI†) and thus high recycle streams. Whereas conversion improvements by catalyst modifications (cf. Section 4.4) are limited by the chemical equilibrium of the dehydrogenative pathway, process modifications have potential to elevate equilibrium conversion. For instance, membrane reactors with molecular-sieves can be used for selective and in situ H2 removal.67 This might increase methanol conversion and reduce DMM production cost significantly. With the currently achievable reaction performance, however, the burdens of the dehydrogenative pathway result in considerably higher DMM production cost compared to other e-fuels (methane, methanol, and DME), which were evaluated on Level 2 using the same boundary conditions and model depth.22 In contrast, production cost via the reductive pathway are in the same range as that of other e-fuels already with the current catalyst and can be further reduced by an optimized reactor design and/or co-solvent facilitating the dissolution of H2 and CO2 into the reaction phase. For the oxidative pathway, the product removal from the gaseous reactor effluent by a refrigeration machine causes the highest electricity costs among the pathways and should be avoided in an industrial application. Therefore, the application of a less energy-intensive unit operation for product removal (e.g., adsorption) need to be investigated in future work to further reduce DMM production cost. Beyond that, the product dilution in the reactor effluent can be reduced by using pure oxygen from the electrolyzer instead of air for the oxidation reaction. An increased product concentration in the reactor effluent would directly reduce energy demand for product purification.
On Level 3, the economic evaluation for the established and oxidative pathway shows that investment cost for the DMM plant can be neglected (less than 1%) considering a plant life time of 10 years. It is important to note, however, that investment cost for the electrolyzer are considered in the H2 price already. The consideration of miscellaneous costs on Level 3 results in an increase of production cost of about 20% compared to Level 2 for the established and oxidative pathway. As these costs mainly constitute general manufacturing expenses (cf. Turton et al.66), a similar increase is expected for the non-oxidative pathways.
The H2 price is the largest cost driver for DMM production having a major influence on whether an e-fuel like DMM will become a relevant contributor in a future mobility concept or not. Unfortunately, it is also one of the most uncertain ones, which makes an analysis of its influence on DMM production cost indispensable.
Fig. 5 reveals clearly: only at low H2 prices (below 3.7$ kg−1), DMM has the chance to become cost competitive with its main competitor fossil diesel. For the extensive application of DMM in individual transportation, where fuel acceptance is driven by fuel cost,68 a competitiveness with cheap fossil diesel and thus low H2 prices might be an essential precondition. The target consumer price of DMM should therefore lie within the region of the consumer price of fossil diesel (1.4$ L−1). Without the non-oxidative pathways, DMM production cost will hardly fall below this target price as H2 prices would need to fall below 0.5$ kg−1 for the established pathway.
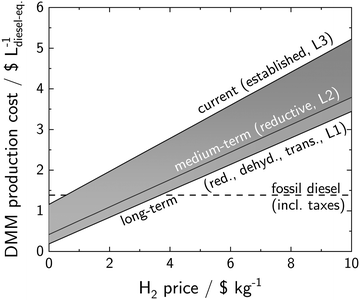 | ||
| Fig. 5 DMM production cost dependence on H2 price. The base case CO2 price is 70$ t−1.62 | ||
In terms of CO2 supply, the technology for CO2 provision determines whether CO2 contributes significantly to DMM production cost or not. Considering industrial point sources for CO2 provision (0$ t−1 to 200$ t−1,62 Fig. S1, ESI†), the share of CO2 on total DMM production cost is comparably low. Technologies with a higher cost for CO2 capture (e.g., 600$ t−1 for DAC69) may however become a limiting factor for a successful implementation of DMM in a future mobility concept.
4.3 Impact on climate change
The results of the LCA for the best case scenario show clearly: If renewable electricity for DMM production is used exclusively (according to the power-to-X concept53), the cradle-to-grave impact on climate change of DMM does not differ significantly between pathways and levels (Fig. 6). Compared to that of fossil diesel (86 gCO2-eq. MJ−1), all pathways for DMM production enable considerable CO2-eq. emission reductions.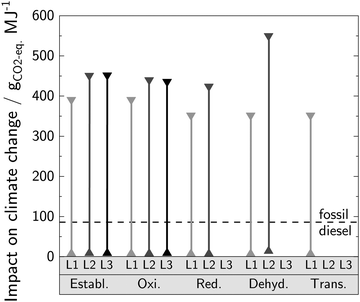 | ||
| Fig. 6 Ranges of cradle-to-grave impact on climate change of different DMM synthesis routes. The routes are evaluated on different levels for both the best (▲) and worst (▼) case scenario and are compared to fossil diesel. The functional unit is “the provision of 1 MJ of enthalpy of combustion”. The combustion of the co-products MF and DME at their end-of-life is included for the dehydrogenation and oxidative route on Level 2 and 3 to cover the cradle-to-grave perspective. The two scenarios are specified in Table 1. | ||
For the worst case scenario, the impact on climate change of the different routes is in the range of 350 gCO2-eq. MJ−1 to 550 gCO2-eq. MJ−1 and, by this, exceeds that of fossil diesel considerably. With the current German electricity mix,70 it is therefore not climate friendly to produce and use DMM as an alternative to fossil diesel. The different impacts on climate change between the pathways and levels are caused by the same reasons as those for the differences in exergy efficiencies (cf. Section 4.1). A detailed contribution analysis for both the best and worst case scenario are presented in Fig. S2 and S3 (ESI†), respectively.
From the analyses of the worst and best case scenario regarding the impact on climate change of DMM, we can draw two main conclusions: First, the carbon footprint of electricity supply needs to be low as cradle-to-grave CO2-eq. emissions of DMM exceed those of fossil diesel considerably if the current German electricity mix is utilized. Second, the higher the carbon footprint of the electricity mix is, the more important becomes the choice for the pathway in order to yield a low impact on climate change of DMM.
Fig. 7 therefore shows the impact on climate change of DMM for the different pathways on Level 2 as a function of the impact on climate change of electricity supply. The intersections of the graphs with the solid black line are the break-even points, where the CO2-eq. emissions of DMM fall below those of fossil diesel. The impact on climate change of the reductive DMM pathway depends the least on that of the electricity supply. Below the break-even point of 190 gCO2-eq. kW h−1, DMM produced by the reductive pathway is more favorable compared to fossil diesel. This break-even point has a rigorous bound at about 225 gCO2-eq. kW h−1 (Fig. S4, ESI†). At this bound, only stoichiometric H2 and CO2 consumption is considered (Level 1) such that no process can exceed this bound. The established and oxidative pathways have a break-even point of 170 gCO2-eq. kW h−1 and are thus less favorable compared to the reductive pathway. For these three pathways, the grid mixes of Norway, France, and in contrast to DMM/fossil diesel blends with 35 vol% DMM,20 also of Switzerland and Finland, are sufficient for producing and using neat DMM with less impact on climate change than fossil diesel already today. In this range, it does matter which pathway is used for DMM production from a climate impact perspective. The carbon footprint of the dehydrogenative pathway depends most strongly on the impact on climate change of electricity supply as the selectivity towards DMM is the lowest. The co-production of MF and DME requires additional electricity and the low methanol conversion results in a high heat demand for product separation (cf. Tables S11–S13, ESI†). The impacts on climate change evaluated on Level 1 and 3 are presented in Fig. S4 and S5 (ESI†), respectively.
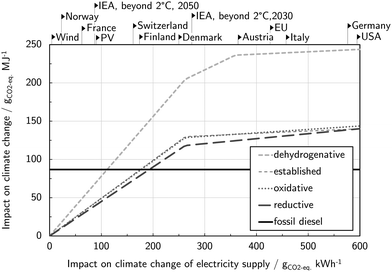 | ||
| Fig. 7 Sensitivity of the cradle-to-grave impact on climate change of DMM with respect to the impact on climate change of electricity supply for different DMM synthesis pathways on Level 2 and fossil diesel. The impact on climate change is calculated using the current catalyst performance of each pathway. Avoided burdens for co-produced MF, DME, and excess heat are not considered. Below 360 gCO2-eq. kW h−1, heat between 90 °C to 250 °C is provided by electrode boilers instead of steam production (relevant only for the dehydrogenative pathway). H2 is supplied by SOEC instead of conventional steam methane reforming below 260 gCO2-eq. kW h−1. At lower carbon-intensities of electricity supply, both the electrode boilers and the SOEC allow for lower climate change impacts compared to their conventional counterparts. The solid black lines at the top of the graph represent the impact on climate change of country-specific grid mixes and two forecasts for the global grid mix of 2030 and 2050 that are based on the “beyond 2 °C scenario” of the International Energy Agency.71 | ||
4.4 Catalyst improvement potential
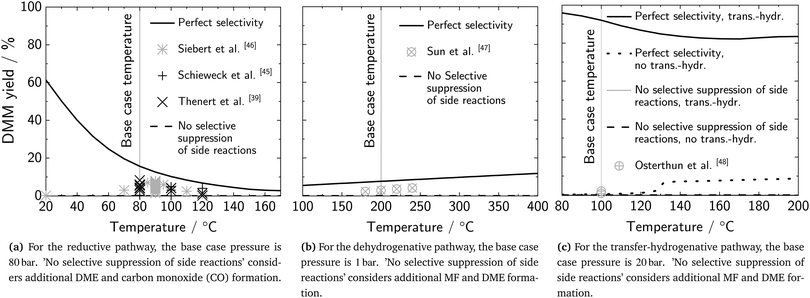
The non-oxidative pathways have been proposed just recently and may offer catalyst improvement potential that has not been captured with the analyses on Level 2 so far. To consider this improvement potential, we analyze the theoretically achievable reaction performance of the reductive, dehydrogenative, and transfer-hydrogenative pathway by assuming restricted equilibrium conversion. Restricted equilibrium considers only the desired reaction (perfect DMM selectivity).72 We calculate the equilibrium DMM yield using the stoichiometric chemical and phase equilibrium (REquil reactor model in Aspen Plus v11) and evaluate the current gap to this performance. We then use these theoretical reaction conditions to evaluate the maximum process efficiency, minimum production cost, and minimum impact on climate change on Level 2 for all non-oxidative pathways.At equilibrium yield, the heat demand required for product separation is reduced by 81% compared to the current state of the catalyst (Tables S17 and Fig. S7, ESI†). As the heat demand of the reductive pathway causes the main exergy losses, this reduction is highly beneficial and increases exergy efficiency by 5 percentage points to an overall process efficiency of 89%. Production costs are reduced by only 0.1$ Ldiesel-eq.−1 as fossil-based heating is cheap compared to renewable H2. A major benefit in reducing fossil-based heating rather lies in associated CO2-eq. emission reductions, which are about 10% (44 gCO2-eq. MJ−1) in the worst case scenario. The best case scenario utilizes almost CO2-neutral heating sources, such that a reduced heat demand has only a negligible effect on climate impact (Fig. S10, ESI†).
Similar to the reductive pathway, heat demand decreases by about 80% at equilibrium yield at 300 °C and exergy efficiency increases by 6 percentage points to an overall process efficiency of 85% (Table S17 and Fig. S8, ESI†). The maximum cost reduction potential is 0.6$ Ldiesel-eq.−1 or 22% of the production costs considering the current state of the catalyst. This reduction is enabled mainly by the assumed perfect selectivity (0.3$ Ldiesel-eq.−1) and by the lower heat demand (0.2$ Ldiesel-eq.−1). As the absolute reduction in heat demand is even higher than that of the reductive pathway, the associated reduction in CO2-eq. emissions in the worst case scenario is higher as well (26% or 140 gCO2-eq. MJ−1). Although the best case scenario utilizes almost CO2-neutral heating sources, significant CO2-eq. emission reductions can be achieved there as well due to the higher selectivity (cf. Fig. S10, ESI†).
The equilibrium reaction calculations reveal that experimentally achieved DMM yields (up to 2.1%) are well below restricted equilibrium conditions (92.1%) at 100 °C assuming the concurrent transfer-hydrogenation reaction of styrene to ethylbenzene (EB) (Fig. 8c). Whereas the experimentally achieved maximum selectivity is almost perfect (98.2%), methanol conversion is still low (0.9%). Without the H2 removal by the transfer-hydrogenative reaction (‘no trans.-hydr.’ in Fig. 8c), equilibrium yield would be limited to only 0.3%, which is about one third of the experimentally achieved yield. The strong effect of in situ H2 removal on DMM yield for the transfer-hydrogenative pathway indicates similar benefits for the dehydrogenative pathway and should therefore be investigated in future research. Irrespective of a concurrent transfer-hydrogenation reaction, if the catalyst would not suppress MF and DME formation selectively, no DMM would be formed but exclusively MF and DME with selectivities of 83.1% and 16.9%, respectively.
To evaluate the maximum potential of the transfer-hydrogenative pathway, we assume equilibrium yield at 100 °C (92.1%) and the concurrent transfer-hydrogenation of styrene to EB. In contrast to the other non-oxidative pathways, the evaluated process concept for the transfer-hydrogenative pathway contains the following additional steps: the transfer-hydrogenation of styrene, the subsequent removal of its hydrogenated molecule EB, the dehydrogenation back to styrene and H2, and the recycling of styrene into the reactor. The resulting process concept and model assumptions are given in ESI,† Fig. S13.
If EB dehydrogenation and H2 recycling is considered at no cost (decoupling the pathway evaluation from the choice of the model hydrogen carrier substance), a maximum process exergy efficiency of 91% can be achieved (Fig. S12a, ESI†). This is slightly higher than that of the reductive pathway (89%) and significantly higher than that of the dehydrogenative pathway (85%) due to a higher equilibrium conversion and, in turn, a lower heat demand. Considering commercial EB dehydrogenation and H2 recycling, the efficiency drops to 72% due to its massive heat demand at high temperature (cf. Table S17, ESI†). It should be noted that the use of styrene as a hydrogen acceptor is not industrially viable, since styrene itself is produced industrially by EB dehydrogenation. The hydrogen carrier couple styrene-EB was nevertheless selected for the experiments due to its ease of hydrogenation and analytical simplifications in the experimental development of a transition-metal catalyst that could catalyze the conversion of methanol to DMM. For industrial applications, a hydrogen carrier couple allowing for a more energy efficient H2 recycling has to be found, which is subject to ongoing research. Alternatively, the co-production of a high value hydrogenated by-product could be envisioned as well.
The minimum production cost for the transfer-hydrogenative pathway is identical to that of the reductive pathway (2.0$ Ldiesel-eq.−1) (Fig. S12b, ESI†). The same amount of H2 and CO2 is consumed as well as compressed to operating pressure. DMM production cost by the dehydrogenative pathway is slightly more expensive (2.1$ Ldiesel-eq.−1) as a considerable amount of external heat is required for product separation despite equilibrium conversion.
In terms of impact on climate change, the transfer-hydrogenation pathway has the lowest climate impact among the alternative pathways in the worst case scenario (370 gCO2-eq. MJ−1) if EB dehydrogenation and H2 recycling at no cost is considered (Fig. S12c, ESI†). In the other cases, the climate impact slightly exceeds that of the dehydrogenative pathway. For the best case scenario, the impact on climate change is below 10 gCO2-eq. MJ−1 and thus, similar to the alternative pathways, well below that of fossil diesel (86 gCO2-eq. MJ−1) (Fig. S10, ESI†).
In total, methanol transfer-hydrogenation toward DMM can represent a promising pathway alternative if two prerequisites can be satisfied: significant catalyst improvements allowing a higher single-pass DMM yield and a more promising H2 carrier substance. Such a substance can be more promising either in terms of a higher efficiency regarding H2 management, or in terms of value as a final by-product.
A summary of the existing bottlenecks connected to each reaction pathway is given in ESI,† Table S18. To support future research in DMM synthesis, we therein estimate the significance of each bottleneck's impact on KPIs and propose improvement measures correspondingly.
5 Conclusions
Extensive effort in catalyst development for dimethoxymethane (DMM) production during the last years have resulted in remarkably improved reaction performances and completely new reaction pathways. Yet, the achievements in catalyst development have not been assessed from a process perspective such that their actual performance and sustainability for industrial applications still remain unknown. In this paper, we demonstrate that DMM produced via different reaction pathways offers considerable benefits compared to fossil diesel if requirements on cost and carbon footprint of raw material provision are met. However, for each individual pathway special aspects for their further development need to be addressed if those requirements can not be fulfilled to an arbitrary large extent.Our hierarchical process evaluation shows that comparable process efficiencies to other e-fuels (e.g., dimethyl ether (DME)) can only be achieved if H2 consumption is low. The non-oxidative pathways (reductive, dehydrogenative, and transfer-hydrogenative pathway) have a maximum exergy efficiency of 97% (Level 1), which is significantly higher than that of the established and oxidative pathway and most other e-fuels. More detailed process analyses (Level 2 and 3) of all pathways reveal that exergy losses mainly result from an energy-intensive product separation and a low DMM single-pass yield of current catalysts. Although these losses are more pronounced for the non-oxidative reaction pathways, their exergy efficiency is higher than that of the established and oxidative one. Their comparably low H2 consumption is key for efficient DMM production-especially in a world with limited renewable electricity.
The production cost for DMM may fall below the price of fossil diesel only if H2 can be produced for less than 4$ kg−1 and if a low H2 consumption can be ensured. Cost competitiveness with fossil diesel is most likely achievable for the reductive pathway already for the current catalyst performance. It is also the only pathway that is currently cost-competitive with the production of other e-fuels.
In terms of the impact on climate change of DMM, the pathways do not differ significantly from one another if exclusively renewable electricity is utilized. In this case, DMM has the potential to reduce its climate impact by up to 92% compared to fossil diesel. In contrast, if the impact on climate change of electricity supply increases to that of Finland or Switzerland (up to 190 gCO2-eq. kW h−1), it does matter which pathway is used for DMM production from a climate impact perspective. In this range, the currently high heat demand and co-production of side products make the impact on climate change of the dehydrogenative pathway being most dependent on the impact on climate change of electricity supply. The climate impact of the reductive pathway is least dependent on the electricity carbon-intensity and is lower than that of fossil diesel below 190 gCO2-eq. kW h−1. For an even higher carbon-intensity of electricity supply, DMM should not be considered as an alternative fuel since its impacts on climate change would exceed those of fossil diesel.
In contrast to the established and oxidative pathway, the non-oxidative pathways have been proposed just recently such that their catalyst performances are expected to still improve significantly. For exploiting the full potential of these pathways and ultimately deciding which one is suited most for sustainable DMM production, more research need to be conducted. For the dehydrogenative pathway, future catalyst development need to aim at suppressing side reactions even more effectively. Alternatively, a systematic exploitation of the co-products in a multi-product plant for producing different e-fuels (e.g., DMM, DME, and methyl formate) should be evaluated. For the transfer-hydrogenative pathway, future experimental studies should aim not exclusively at increasing turnover numbers, but also increasing single-pass DMM yield. Together with the identification of an industrially viable hydrogen carrier substance, comparable performance indicators to those of the reductive pathway can be achieved. The reductive pathway is the most developed one among the non-oxidative pathways. We have shown that already with a catalyst at its current state, an efficient DMM production at competitive cost and low climate impact is possible in some countries. To reach the next stage of development, the handling of the homogeneous catalyst needs to be analyzed.
Author contributions
J. Burre developed the concept of the study and proposed the process development and evaluation methodology. J. Burre and D. Bongartz developed the processes and performed process optimization under the supervision of A. Mitsos. J. Burre and D. Bongartz evaluated the processes regarding efficiency. J. Burre calculated production costs. S. Deutz and S. Völker calculated the impact on climate change supervised by A. Bardow and wrote corresponding sections of the manuscript. C. Mebrahtu, O. Osterthun, and R. Sun wrote the descriptions of the novel reaction pathways and provided fundamental information for the discussions regarding catalyst improvement potential under the supervision of J. Klankermayer and R. Palkovits. J. Burre wrote the rest of the manuscript. All authors contributed to the discussions and revisions of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge funding by the German Federal Ministry of Education and Research (BMBF) within the Kopernikus Project P2X: Flexible use of renewable resources – exploration, validation and implementation of “Power-to-X” concepts (FKZ 03SFK2A) and the project NAMOSYN: Nachhaltige Mobilität durch synthetische Kraftstoffe (FKZ 03SF0566P0). This work is further funded by Deutsche Forschungsgesellschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – Cluster of Excellence 2186 “The Fuel Science Center” – ID: 390919832.References
- BP, BP Energy Outlook: 2019 edition, 2019, https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2019.pdf, Accessed: 2020-01-21.
- M. Contestabile, G. J. Offer, R. Slade, F. Jaeger and M. Thoennes, Energy Environ. Sci., 2011, 4, 3754–3772 Search PubMed.
- E. Çabukoglu, G. Georges, L. Küng, G. Pareschi and K. Boulouchos, Transp. Res. Part C: Emerging Technol., 2018, 88, 107–123 Search PubMed.
- D. Pélerin, K. Gaukel, M. Härtl, E. Jacob and G. Wachtmeister, Fuel, 2020, 259, 116231 Search PubMed.
- M. Härtl, K. Gaukel, D. Pélerin and G. Wachtmeister, MTZ - Motortech. Z., 2017, 78, 52–59 Search PubMed.
- A. García, J. Monsalve-Serrano, D. Villalta, R. L. Sari, V. G. Zavaleta and P. Gaillard, Appl. Energy, 2019, 253, 113622 Search PubMed.
- M. Härtl, P. Seidenspinner, E. Jacob and G. Wachtmeister, Fuel, 2015, 153, 328–335 Search PubMed.
- A. Omari, B. Heuser and S. Pischinger, Fuel, 2017, 209, 232–237 Search PubMed.
- S. E. Iannuzzi, C. Barro, K. Boulouchos and J. Burger, Fuel, 2017, 203, 57–67 Search PubMed.
- A. Omari, B. Heuser, S. Pischinger and C. Rüdinger, Appl. Energy, 2019, 239, 1242–1249 Search PubMed.
- IEA, International Energy Agency, Energy Efficiency Indicators - Highlights, 2020.
- L. Ding, T. Shi, J. Gu, Y. Cui, Z. Zhang, C. Yang, T. Chen, M. Lin, P. Wang, N. Xue, L. Peng, X. Guo, Y. Zhu, Z. Chen and W. Ding, Chem, 2020, 6, 2673–2689 Search PubMed.
- N. Mahbub, A. O. Oyedun, A. Kumar, D. Oestreich, U. Arnold and J. Sauer, J. Cleaner Prod., 2017, 165, 1249–1262 Search PubMed.
- A. O. Oyedun, A. Kumar, D. Oestreich, U. Arnold and J. Sauer, Biofuels, Bioprod. Biorefin., 2018, 12, 694–710 Search PubMed.
- X. Zhang, A. O. Oyedun, A. Kumar, D. Oestreich, U. Arnold and J. Sauer, Biomass Bioenergy, 2016, 90, 7–14 Search PubMed.
- S. Schemme, R. C. Samsun, R. Peters and D. Stolten, Fuel, 2017, 205, 198–221 Search PubMed.
- D. Bongartz, J. Burre and A. Mitsos, Ind. Eng. Chem. Res., 2019, 58, 4881–4889 Search PubMed.
- J. Burre, D. Bongartz and A. Mitsos, Ind. Eng. Chem. Res., 2019, 58, 5567–5578 Search PubMed.
- M. Held, Y. Tönges, D. Pélerin, M. Härtl, G. Wachtmeister and J. Burger, Energy Environ. Sci., 2019, 12, 1019–1034 Search PubMed.
- S. Deutz, D. Bongartz, B. Heuser, A. Kätelhön, L. S. Langenhorst, A. Omari, M. Walters, J. Klankermayer, W. Leitner, A. Mitsos, S. Pischinger and A. Bardow, Energy Environ. Sci., 2018, 11, 331–343 Search PubMed.
- L. Lautenschütz, D. Oestreich, P. Seidenspinner, U. Arnold, E. Dinjus and J. Sauer, Fuel, 2016, 173, 129–137 Search PubMed.
- D. Bongartz, L. Doré, K. Eichler, T. Grube, B. Heuser, L. E. Hombach, M. Robinius, S. Pischinger, D. Stolten, G. Walther and A. Mitsos, Appl. Energy, 2018, 231, 757–767 Search PubMed.
- Z. P. Cano, D. Banham, S. Ye, A. Hintennach, J. Lu, M. Fowler and Z. Chen, Nat. Energy, 2018, 3, 279–289 Search PubMed.
- K. D. Vertin, J. M. Ohi, D. W. Naegeli, K. H. Childress, G. P. Hagen, C. I. McCarthy, A. S. Cheng and R. W. Dibble, Methylal and Methylal-Diesel Blended Fuels for Use in Compression-Ignition Engines, SAE Technical Paper Series, 1999 Search PubMed.
- J. Schröder and K. Görsch, Energy Fuels, 2020, 34, 450–459 Search PubMed.
- G. Reuss, W. Disteldorf, A. Gamer and A. Hilt, Formaldehyde, Ullmann's Encyclopedia of Industrial Chemistry, 2000 Search PubMed.
- J. Burger, E. Ströfer and H. Hasse, Chem. Eng. Res. Des., 2013, 91, 2648–2662 Search PubMed.
- N. Schmitz, J. Burger, E. Ströfer and H. Hasse, Fuel, 2016, 185, 67–72 Search PubMed.
- A. Peter, S. M. Fehr, V. Dybbert, D. Himmel, I. Lindner, E. Jacob, M. Ouda, A. Schaadt, R. J. White, H. Scherer and I. Krossing, Angew. Chem., 2018, 130, 9605–9608 Search PubMed.
- A. Peter, G. Stebens, J. F. Baumgärtner, E. Jacob, F. K. Mantei, M. Ouda and I. Krossing, ChemCatChem, 2020, 12, 2416–2420 Search PubMed.
- J. Walker, Formaldehyde, Reinhold Publishing, 1944 Search PubMed.
- C. Kuhnert, M. Albert, S. Breyer, I. Hahnenstein, H. Hasse and G. Maurer, Ind. Eng. Chem. Res., 2006, 45, 5155–5164 Search PubMed.
- H. Liu, H. Gao, Y. Ma, Z. Gao and W. Eli, Chem. Eng. Technol., 2012, 35, 841–846 Search PubMed.
- Q. Wang, B. Yu and C. Xu, Ind. Eng. Chem. Res., 2012, 51, 1281–1292 Search PubMed.
- B. Yu, Q. Wang and C. Xu, Ind. Eng. Chem. Res., 2012, 51, 1293–1310 Search PubMed.
- X. Zhang, S. Zhang and C. Jian, Chem. Eng. Res. Des., 2011, 89, 573–580 Search PubMed.
- J.-O. Weidert, J. Burger, M. Renner, S. Blagov and H. Hasse, Ind. Eng. Chem. Res., 2017, 56, 575–582 Search PubMed.
- R. Sun, I. Delidovich and R. Palkovits, ACS Catal., 2019, 9, 1298–1318 Search PubMed.
- K. Thenert, K. Beydoun, J. Wiesenthal, W. Leitner and J. Klankermayer, Angew. Chem., 2016, 128, 12454–12457 Search PubMed.
- K. Roh, A. Bardow, D. Bongartz, J. Burre, W. Chung, S. Deutz, D. Han, M. Heßelmann, Y. Kohlhaas, A. König, J. S. Lee, R. Meys, S. Völker, M. Wessling, J. H. Lee and A. Mitsos, Green Chem., 2020, 22, 3842–3859 Search PubMed.
- A. W. Zimmermann and R. Schomäcker, Energy Technol., 2017, 5, 850–860 Search PubMed.
- J. A. Bergerson, A. Brandt, J. Cresko, M. Carbajales-Dale, H. L. MacLean, H. S. Matthews, S. McCoy, M. McManus, S. A. Miller, W. R. Morrow, I. D. Posen, T. Seager, T. Skone and S. Sleep, J. Ind. Ecol., 2019, 24, 11–25 Search PubMed.
- G. Thomassen, M. V. Dael, S. V. Passel and F. You, Green Chem., 2019, 21, 4868–4886 Search PubMed.
- S. Su, P. Zaza and A. Renken, Chem. Eng. Technol., 1994, 17, 34–40 Search PubMed.
- B. G. Schieweck and J. Klankermayer, Angew. Chem., 2017, 129, 10994–10997 Search PubMed.
- M. Siebert, M. Seibicke, A. F. Siegle, S. Kräh and O. Trapp, J. Am. Chem. Soc., 2019, 141, 334–341 Search PubMed.
- R. Sun, C. Mebrahtu, J. P. Hofmann, D. Bongartz, J. Burre, C. H. Gierlich, P. J. Hausoul, A. Mitsos and R. Palkovits, Sustainable Energy Fuels, 2021, 5(1), 117–126 Search PubMed.
- O. Osterthun and J. Klankermayer, Angew. Chem. Search PubMed , submitted.
- K. Kraemer, S. Kossack and W. Marquardt, Ind. Eng. Chem. Res., 2009, 48, 6749–6764 Search PubMed.
- J. Bausa, R. v. Watzdorf and W. Marquardt, AIChE J., 1998, 44, 2181–2198 Search PubMed.
- Process Systems Engineering (AVT.SVT), EE-Toolbox, 2017, https://www.avt.rwth-aachen.de/cms/AVT/Forschung/Software/iptv/Shortcut-Toolbox/?lidx=1, Accessed: 2020-03-04.
- S. Kossack, K. Kraemer and W. Marquardt, Ind. Eng. Chem. Res., 2006, 45, 8492–8502 Search PubMed.
- J. Burre, D. Bongartz, L. Brée, K. Roh and A. Mitsos, Chem. Ing. Tech., 2019, 92, 74–84 Search PubMed.
- International Organization for Standardization, ISO 14040: Environmental Management: Life Cycle Assessment: Principles and Framework, ISO, 2006.
- International Organization for Standardization, ISO 14044: Environmental Management: Life Cycle Assessment: Requirements and Guidelines, ISO, 2006.
- L. J. Müller, A. Kätelhön, M. Bachmann, A. Zimmermann, A. Sternberg and A. Bardow, Front. Energy Res., 2020, 8, 15 Search PubMed.
- J. Guinée, Int. J. Life Cycle Assess., 2001, 6, 255 Search PubMed.
- É. S. Van-Dal and C. Bouallou, J. Cleaner Prod., 2013, 57, 38–45 Search PubMed.
- J.-O. Drunsel, M. Renner and H. Hasse, Chem. Eng. Res. Des., 2012, 90, 696–703 Search PubMed.
- D. Bongartz, J. Burre, A. Ziegler and A. Mitsos, in Computer Aided Chemical Engineering: 31st European Symposium on Computer Aided Process Engineering (ESCAPE 31), ed. M. Türkay and R. Gani, Power-to-OME1via Direct Oxidation of Methanol: Process Design and Global Flowsheet Optimization, 2021, in press Search PubMed.
- T. Grube, L. Doré, A. Hoffrichter, L. E. Hombach, S. Raths, M. Robinius, M. Nobis, S. Schiebahn, V. Tietze, A. Schnettler, G. Walther and D. Stolten, Sustainable Energy Fuels, 2018, 2, 1500–1515 Search PubMed.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. M. Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 Search PubMed.
- W. L. Luyben, AIChE J., 2010, 57, 655–670 Search PubMed.
- W. D. Seider, J. D. Seader and D. R. Lewin, Product & Process Design Principles: Synthesis, Analysis and Evaluation, John Wiley & Sons, 2009 Search PubMed.
- K. M. Guthrie, Chem. Eng., 1969, 24, 114–142 Search PubMed.
- R. Turton, R. C. Bailie, W. B. Whiting and J. A. Shaeiwitz, Analysis, synthesis and design of chemical processes, Pearson Education, 2008 Search PubMed.
- H. Li, Z. Song, X. Zhang, Y. Huang, S. Li, Y. Mao, H. J. Ploehn, Y. Bao and M. Yu, Science, 2013, 342, 95–98 Search PubMed.
- A. Linzenich, K. Arning, D. Bongartz, A. Mitsos and M. Ziefle, Appl. Energy, 2019, 249, 222–236 Search PubMed.
- R. Socolow, M. Desmond, R. Aines, J. Blackstock, O. Bolland, T. Kaarsberg, N. Lewis, M. Mazzotti, A. Pfeffer, K. Sawyeret al.Direct air capture of CO2 with chemicals: a technology assessment for the APS Panel on Public Affairs, American physical society technical report, 2011.
- ecoinvent, ecoinvent Database Version 3.01, https://ecoquery.ecoinvent.org/Search/Index, Accessed: 2020-07-09.
- IEA, International Energy Agency, Energy Technology Perspectives 2017: Catalysing Energy Technology Transformation, 2017.
- A. Mitsos, I. Palou-Rivera and P. I. Barton, Ind. Eng. Chem. Res., 2004, 43, 74–84 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ee00689d |
| This journal is © The Royal Society of Chemistry 2021 |