Open Access Article
Open Access ArticleInfluence of temperature on the equilibria of oxidovanadium(IV) complexes in solution†
Daniele
Sanna
 a,
Giuseppe
Lubinu
b,
Valeria
Ugone
a,
Giuseppe
Lubinu
b,
Valeria
Ugone
 *a and
Eugenio
Garribba
*a and
Eugenio
Garribba
 b
b
aIstituto di Chimica Biomolecolare, Consiglio Nazionale delle Ricerche, Trav. La Crucca 3, I-07100 Sassari, Italy. E-mail: valeria.ugone@cnr.it
bDipartimento di Chimica e Farmacia, Università di Sassari, Via Vienna 2, I-07100 Sassari, Italy
First published on 18th October 2021
Abstract
The equilibria in the solution of three different oxidovanadium(IV) complexes, VO(dhp)2 (dhp = 1,2-dimethyl-3-hydroxy-4(1H)-pyridinonato), VO(ma)2 (ma = maltolato) and VO(pic)2(H2O) (pic = picolinato), were examined in the temperature range of 120–352 K through a combination of instrumental (EPR spectroscopy) and computational techniques (DFT methods). The results revealed that a general equilibrium exists: VOL2 + H2O ⇄ cis-VOL2(H2O) ⇄ trans-VOL2(H2O), where cis and trans refer to the relative position of H2O and the oxido ligand. The equilibrium is more or less shifted to the right depending on the ligand, the temperature, the ionic strength and the coordinating properties of the solvent. With VO(dhp)2, only the square pyramidal species exists at 298 K in aqueous solution, while at 120 K the cis- and trans-VO(dhp)2(H2O) species are also present. The complex of maltol exists almost exclusively in the form cis-VO(ma)2(H2O) in aqueous solution at 298 K, while the trans species can be revealed only at higher temperatures, where the EPR linewidth significantly decreases. The equilibria involving 1-methylimidazole (MeIm), a model for the side chain His coordination, are also influenced by temperature, with its coordination being favored by decreasing the temperature. The implications of these results in the study of the (vanadium complex)–protein systems are discussed and the interaction with myoglobin (Mb) is examined as a representative example.
1. Introduction
Vanadium compounds have been shown to be a new class of potential metallodrugs due to their promising pharmacological properties as antidiabetic, antitumoral and antiviral drugs.1–10The main problem with orally administered V compounds is their poor absorption in the gastrointestinal tract.11,12 To overcome this limitation, neutral VIVOL2 complexes were proposed and the benchmark compound for these studies is VO(ma)2, bis(maltolato)oxidovanadium(IV) or BMOV.13,14
After absorption in the gastrointestinal tract, VIVOL2 complexes reach the bloodstream where they encounter high and low molecular mass components. Therefore, the vanadium–protein interaction has been intensively studied for several years in order to understand their role in metal transport.15–31 Several instrumental tools have been applied to study the binding of VIVOL2−protein, such as X-ray diffraction analysis, UV-Vis spectroscopy, circular dichroism, mass spectrometry, ENDOR and ESEEM, but surely Electron Paramagnetic Resonance (EPR) spectroscopy is one of the most used techniques to characterize the interaction of VIV species with biomolecules.32 Usually, EPR spectra are recorded at low temperatures (77 or 120 K) since anisotropic spectra allow one to get information on the symmetry and the coordination geometry of the VIVO complexes, the identity of the equatorial ligands through the Az value (more sensitive to the equatorial donors than isotropic A0), and the presence of minor species in solution.32,33 In contrast, EPR spectra are often little informative at 298 K because the lines are not narrow enough to allow for distinguishing the presence of more than one species in solution with similar A0 values. However, spectra recorded at 298 K can give some important information about the V–protein interaction; indeed, an anisotropic spectrum is expected when the binding is strong enough to block the metal species on the protein surface (hindering the rotational motion in the EPR time scale), while an isotropic spectrum is expected when the complexes are free to rotate in solution and no covalent binding or weak non-covalent interaction occurs.32
During the last few years, EPR spectroscopy allowed for demonstrating that the interaction with proteins depends on the thermodynamic stability and geometry assumed by VIVOL2 complexes in aqueous solution.32 In fact, a non-covalent interaction or a weak axial binding is expected for stable VIVOL2 complexes,15,34 an equatorial binding for cis-octahedral VIVOL2(H2O) species after the replacement of the water molecule with an amino acid side-chain,15,18,35,36 and the interaction with two adjacent positions of the moiety VIVOL+ for bis-chelated complexes with low stability.25–27
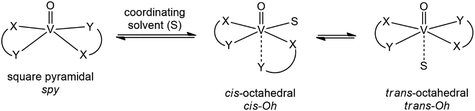
Depending on the ligand, the equilibria shown in Scheme 1 are possible for a VIVOL2 complex.37–39 It has been already shown that these equilibria can be affected by temperature40 and this should be considered when the spectral data are collected at low temperatures. In other words, the type of interaction and the composition and structure of the adducts could depend on the temperature.
In this study, the influence of temperature on the solution equilibria of oxidovanadium(IV) complexes was studied. In particular, VO(dhp)2 (dhp = 1,2-dimethyl-3-hydroxy-4(1H)-pyridinonato), VO(ma)2 (ma = maltolato) and VO(pic)2(H2O) (pic = picolinato) were examined in the temperature range of 120–352 K through a combination of instrumental (EPR spectroscopy) and computational techniques (DFT methods). The results were applied to the analysis of the systems formed by VIVOL2 and myoglobin (Mb).
2. Experimental and computational section
2.1. Chemicals
Water was deionized prior to use through a Millipore Milli-Q Academic purification system. Methanol, chloroform, and toluene were purchased from Sigma-Aldrich. The chemicals oxidovanadium(IV) sulfate trihydrate (VOSO4·3H2O), pyridine-2-carboxylic acid (picolinic acid), 3-hydroxy-2-methyl-4H-pyran-4-one (maltol), 1,2-dimethyl-3-hydroxy-4(1H)-pyridinone (deferiprone), 1-methylimidazole (MeIm), myoglobin from equine heart (Mb, M1882) and 4-(2-hydroxyethyl)-piperazine-1-ethanesulfonic acid (HEPES) were Sigma-Aldrich products of the highest grade available and used as received.The complexes [VIVO(dhp)2], [VIVO(ma)2] and [VIVO(pic)2(H2O)] were synthesized following the procedure established in the literature.41–43 [VO(dhp)2]: yield 86%; C, 48.79; H, 4.94; N, 7.96 (calc. for C14H16N2O5V: C, 48.99; H, 4.70; N, 8.16); ESI-MS(+), [VO(dhp)2 + H]+ 344.06 m/z. [VO(ma)2]: yield 88%; C, 45.45; H 3.18 (calc. for C12H10O7V: C, 45.47; H, 3.16); ESI-MS(+), [VO(ma)2 + H]+ 317.99 m/z. [VO(pic)2(H2O)]: yield 60%; C, 43.72; H, 3.18; N, 8.42 (calc. for C12H10N2O6V, 43.79; H, 3.06; N, 8.51); ESI-MS(+), [VO(pic)2 + H]+ 311.99 m/z; ESI-MS(−), [VO(pic)2(OH)]− 326.98 m/z.
2.2. Analytical and spectroscopic measurements
Elemental analysis (C, H, and N) was carried out using a PerkinElmer 240 B elemental analyser. ESI-MS spectra were recorded using a high-resolution Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ mass spectrometer (Thermo Fisher Scientific). The solutions, obtained by dissolving the solid compounds in LC-MS H2O (V concentration 5 × 10−6 M), were infused at a flow rate of 5.00 μL min−1 into the ESI chamber. The spectra were recorded in the range of m/z 50–750 with a resolution of 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The instrumental conditions for the ESI-MS(+) measurements were: a spray voltage of 2300 V, a capillary temperature of 250 °C, sheath gas 5 (arbitrary units), auxiliary gas 3 (arbitrary units), sweep gas 0 (arbitrary units), and a probe heater temperature of 50 °C. The instrumental conditions for the ESI-MS(−) measurements were: a spray voltage of −1900 V, a capillary temperature of 250 °C, sheath gas 20 (arbitrary units), auxiliary gas 5 (arbitrary units), sweep gas 0 (arbitrary units), and a probe heater temperature of 14 °C. The mass spectra were analysed by using the Thermo Xcalibur 3.0.63 software (Thermo Fisher Scientific).
000. The instrumental conditions for the ESI-MS(+) measurements were: a spray voltage of 2300 V, a capillary temperature of 250 °C, sheath gas 5 (arbitrary units), auxiliary gas 3 (arbitrary units), sweep gas 0 (arbitrary units), and a probe heater temperature of 50 °C. The instrumental conditions for the ESI-MS(−) measurements were: a spray voltage of −1900 V, a capillary temperature of 250 °C, sheath gas 20 (arbitrary units), auxiliary gas 5 (arbitrary units), sweep gas 0 (arbitrary units), and a probe heater temperature of 14 °C. The mass spectra were analysed by using the Thermo Xcalibur 3.0.63 software (Thermo Fisher Scientific).
The aqueous solutions for EPR measurements were prepared by dissolving VOSO4·3H2O and the ligand (L = ma, dhp, and pic) in ultrapure water to get a VIVO2+ concentration of 1.0 × 10−3 M and a metal-to-ligand molar ratio of 1/2. HEPES buffer of 0.1 M concentration was added and the pH value was adjusted to the desired value. In the ternary systems, an appropriate amount of Mb or MeIm was added to the V–L solutions to obtain a VIVO2+/L/Mb molar ratio of 1/2/1 or VIVO2+/L/MeIm 1/2/4, respectively.
The solutions prepared in the absence of the HEPES buffer were obtained by dissolving an appropriate amount of the solid compound in the solvent or solvent mixture (CHCl3/toluene 6/4 v/v, MeOH or H2O/MeOH 9/1 v/v) to obtain a concentration of 1.0 × 10−3 M.
Argon was bubbled through all the solutions to ensure the absence of oxygen and avoid the oxidation of VIVO2+ ions.
For frozen solution EPR measurements, DMSO is usually added to the aqueous samples to get a better resolution of the spectra. However, as the addition of this solvent can influence the equilibria in solution and denature the proteins, HEPES was used for EPR measurements at 120 K, which allowed us to achieve a good resolution of the spectra.
HEPES solutions were also used for measurements at 298 K or higher temperatures in order to have the same solvent composition at different temperatures. The use of a buffer instead of organic solvents is a good strategy to have more significant results from a biological point of view and can be extended to different proteins.
The EPR spectra were recorded using an X-band Bruker EMX spectrometer equipped with an HP 53150A microwave frequency counter. The instrumental parameters were as follows: microwave frequency, 9.40–9.41 GHz at 120 K and 9.83–9.84 GHz at 298–352 K; microwave power, 20 mW; time constant, 81.92 ms; modulation frequency, 100 kHz; modulation amplitude, 0.4 mT; and resolution, 4096 points. When the samples were transferred into the EPR tubes (120 K) or a Bruker AquaX cell (298 K), the spectra were immediately measured. Signal averaging was used to increase the signal-to-noise ratio.44 The EPR spectra at variable temperatures (298–352 K) were measured in capillary tubes and the temperature was controlled using a variable temperature unit ER4131VT. The recorded spectra were simulated with the software Bruker WINEPR SimFonia (version 1.26 (beta), Bruker Analytik GmbH, 1997).
2.3. DFT calculations
DFT calculations were carried out with Gaussian 09 (revision C.01).45 The VIVO complex geometries and their relative stability were computed at the level of theory B3P86/6-311g(d,p); this method guarantees a good degree of accuracy in the structural optimization of first-row transition metal complexes46,47 and, particularly, of vanadium compounds.48 Water was simulated within the framework of the SMD model.49For dhp complexes, the relative stability of the bis-chelated species at 298 and 120 K was determined by calculating the value of ΔGaq for the equilibrium in solution:
| [VOL2] + H2O ⇌ cis-[VOL2(H2O)] | (1) |
All the different possible geometries of the square pyramidal and cis-octahedral isomers were considered (Scheme S1†).
In the case of ma and pic species, only the most stable cis-octahedral structures were considered in the DFT calculations (Schemes S2 and S3†), as suggested by previous studies36 and references therein.
The Gibbs energy in aqueous solution (Gaq) for each species can be separated into the electronic plus nuclear repulsion energy (Eele), the thermal contribution (Gtherm) and the solvation energy (ΔGsolv): Gaq = Eele + Gtherm + ΔGsolv. The term RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(24.46) was considered to account for the standard state correction from the gas phase to the aqueous solution. The thermal contribution was estimated using the ideal gas model and the calculated harmonic vibrational frequencies to determine the correction due to the zero-point energy and the thermal population of the vibrational levels.
ln(24.46) was considered to account for the standard state correction from the gas phase to the aqueous solution. The thermal contribution was estimated using the ideal gas model and the calculated harmonic vibrational frequencies to determine the correction due to the zero-point energy and the thermal population of the vibrational levels.
The trans-Oh isomers were not considered as it was noticed that, using this level of calculation with the SMD model for water, they are distorted toward the spy species. Furthermore, experimental evidence suggests that these species are less stable than cis-Oh, being present in solution in small amounts.
For the evaluation of protein binding, the structures of the model complex [VOL2(MeIm)] (with L = ma, pic, and dhp) were computed by considering all the possible orientations of 1-methylimidazole. The latter has been used since it is a good model for histidine coordination.15 A relaxed scan calculation was performed by changing the dihedral angle O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) V–NMeIm–CMeIm from 0° to 360° (with the ligand parallel to V
V–NMeIm–CMeIm from 0° to 360° (with the ligand parallel to V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O when the angle is 0 or 180° and perpendicular when the angle is 90 or 270°), with an increment of 10° in the case of dhp complexes. The structures of the most stable isomers, namely those corresponding to the minima Eaq (electronic energy in solution) in the scan calculation, were subsequently optimized and used in the calculation of thermodynamic parameters. The Gibbs energy in aqueous solution (ΔGaq) was computed considering the following reactions:
O when the angle is 0 or 180° and perpendicular when the angle is 90 or 270°), with an increment of 10° in the case of dhp complexes. The structures of the most stable isomers, namely those corresponding to the minima Eaq (electronic energy in solution) in the scan calculation, were subsequently optimized and used in the calculation of thermodynamic parameters. The Gibbs energy in aqueous solution (ΔGaq) was computed considering the following reactions:
| [VOL2] + Melm ⇌ cis-[VOL2(Melm)] | (2) |
| cis-[VOL2(H2O)] + Melm ⇌ cis-[VOL2(Melm)] + H2O | (3) |
In the systems with ma and pic, instead of doing a scan calculation, the structures of cis-[VOL2(MeIm)] with dihedral angles θ = 0, 90, 180, and 270° were optimized. All the frequency calculations were done both at 298.15 K, the default temperature in Gaussian 09, and at 120 K.
The ΔGaq values obtained from the DFT calculations are not necessarily coincident with the real values as they were calculated with the SMD model instead of using explicit solvent molecules; therefore, only the relative values at 298 and 120 K are relevant for the purposes of this study.
For the optimized structures of both binary and ternary species, the 51V hyperfine coupling constants (A) were calculated using the half-and-half hybrid functional BHandHLYP and the basis set 6-311+g(d), according to the procedures previously published.50–55 It must be taken into account that for a VIVO2+ species, Az is usually negative; however, in the literature its absolute value is often reported and this formalism was also used in this study. The theoretical background is described in detail in ref. 56–58. The percent deviation (PD) of the absolute calculated value, Acalcdz, from the absolute experimental value, Az, was obtained as follows: 100 × [(Acalcdz − Az)/Az].
3. Results and discussion
3.1. Systems with dhp
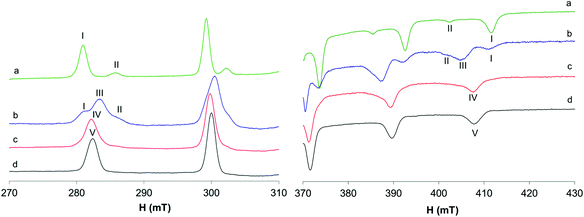
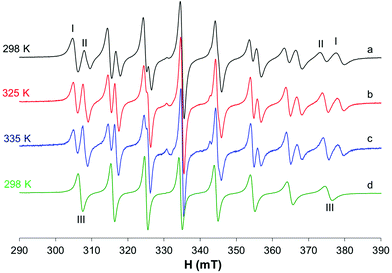
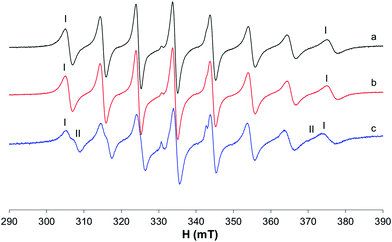
The behavior of the [VO(dhp)2] complex in solution has been already studied by the pH-potentiometric, EPR and DFT methods.39,41 The EPR spectra recorded at 120 K suggest that, in the pH range 5–8, the bis-chelated species is present in solution as a mixture of the square pyramidal [VO(dhp)2] and octahedral cis-[VO(dhp)2(H2O)] in equilibrium between each other, together with a small amount of trans-[VO(dhp)2(H2O)],39 this latter species revealed when the spectra were recorded at 1 mM concentration.In the present study, the results at low temperatures were reanalyzed and it was confirmed that the resonances of the third species with lower Az (which corresponds to trans-[VO(dhp)2(H2O)] where the water molecule is coordinated in the axial position) are present when the spectrum is recorded in HEPES buffer (0.1 M) at physiological pH (Fig. 1b, II). To evaluate if the buffer and other ions (as SO42− from VOSO4 salt) can influence the equilibrium between the species in solution, the spectrum was recorded by dissolving the solid complex VO(dhp)2 in a mixture H2O/MeOH 9/1 v/v (Fig. 1a). At these experimental conditions, the predominant species is that with the highest Az value (gz 1.940, Az 169.0 × 10−4 cm−1, I) which corresponds to cis-[VO(dhp)2(H2O)], together with a small amount of that with the lowest Az (gz 1.953, Az 151.9 × 10−4 cm−1, II) which is the isomer trans-[VO(dhp)2(H2O)]. No evidence of the square pyramidal [VO(dhp)2] are present in the mixture H2O/MeOH. This behavior can be explained considering that all the three species are in equilibrium, and this can be shifted by changing the media in which the spectra are recorded. Moreover, the formation of [VO(dhp)2] is favored by an increase in the ionic strength, since this condition decreases the number of solvent molecules in solution “available” for metal centre coordination; on the other hand, cis/trans-[VO(dhp)2(H2O)], with a water molecule directly coordinated, are favored at low ionic strengths, when the solvent is able to coordinate the metal in the equatorial (cis) or axial (trans) position.
When the spectra are recorded in the presence of myoglobin (Fig. 1c), a new series of bands with Az = 162.7 × 10−4 cm−1 are detected, which can be attributed to the formation of a mixed species with the composition VO(dhp)2(Mb) (IV in Fig. 1), where an accessible His-N binds equatorially to the VO(dhp)2 moiety.26 The Az value is intermediate to those of cis-[VO(dhp)2(H2O)] and [VO(dhp)2] and is practically coincident with that of cis-[VO(dhp)2(MeIm)] (163.0 × 10−4 cm−1, V in Fig. 1d), which is in agreement with the literature data.15 Probably, a small amount of binary complex remains in solution as the signals of the two isomers, spy and cis-Oh, can be noticed in the spectrum (see Fig. 1c). The experimental EPR parameters of both the binary and ternary species and the comparison with those calculated by the DFT methods are reported in Table S1.†
The EPR spectra recorded at 298 K with HEPES 0.1 M show that in the binary system only one species is present in solution and the same signals are detected in the system with 1-methylimidazole and Mb (Fig. 2). The same species was detected at temperatures higher than 298 K (Fig. S1†) and, even if the signals appear narrower, no appreciable differences were observed after the addition of MeIm to the solution. The different behavior at 298 K compared with that at 120 K can be explained by considering that only the spy species exists in solution at room temperature (g0 1.976, A0 84.1 × 10−4 cm−1) and MeIm does not bind to V. The experimental A0 value, measured at 298 K, was compared with that calculated from frozen solution spectrum using the relationship Aexpct0 = (Ax + Ay + Az)/3; indeed, the value obtained from the simulated spectra (Fig S2,†Aexpct0 = 90.9 × 10−4 cm−1) is quite different from that measured at 298 K (A0 84.1 × 10−4 cm−1), confirming that the mixed species cis-[VO(dhp)2(MeIm)] is formed only at 120 K.
Moreover, at 298 K, the eventual anisotropic signals of the adduct formed by the cis-VO(dhp)2 moiety with Mb are not detected or exist in such small amounts that cannot be revealed by EPR spectroscopy. This finding indicates that there is no strong covalent interaction with the protein because the only free site, that in the axial position, is not prone to the binding of the amino acid side-chains34 and confirms that the most stable species is, at these experimental conditions, the spy isomer.
To evaluate if by decreasing the ionic strength it is possible to shift the equilibrium toward the formation of cis-[VO(dhp)2(H2O)], the spectrum of the binary system was also recorded without adding the HEPES buffer; as it can be noticed from Fig. 2a, no spectral changes were observed thus confirming that at 298 K the complex present in solution is [VO(dhp)2].
The different behavior at 120 K can be explained considering that there is a certain percentage of the cis complex in solution which, in the presence of 1-methylimidazole, transforms into cis-[VO(dhp)2(MeIm)] where the water molecule coordinated in the equatorial plane is replaced by MeIm. At the same time, the formation of this species influences the spy + H2O ⇄ cis-Oh equilibrium, which is shifted toward the formation of the octahedral complex thus leading to the complete transformation into cis-[VO(dhp)2(MeIm)] and the detection of a single signal in the EPR spectrum.
The equilibrium between the square pyramidal VO(dhp)2 and the cis-octahedral VO(dhp)2(H2O) was studied by DFT calculations considering the structures of all possible isomers (Scheme S1†). The results are collected in Table S2† and it can be noticed that, both at 298 and 120 K, the values of Gibbs energy calculated for the cis-Oh isomers are quite close to each other with differences of less than 1 kcal mol−1; therefore, it can be concluded that a mixture of the possible isomers may be present in solution. The energy of the two spy complexes (SPY-5-12 and SPY-5-13) is also similar.
To evaluate the influence of temperature in the spy/cis-Oh equilibrium, the ΔGaq for the reaction VOL2 + H2O ⇄ cis-[VOL2(H2O)] has been calculated considering the corresponding average G values for the spy and cis-Oh forms and the contribution of the water molecule. The results suggest that the equilibrium is always shifted toward the spy isomer but more at 298 K where the mean value of ΔGaq calculated is 10.1 kcal mol−1vs. 5.5 kcal mol−1 at 120 K (see Table 1). This is in agreement with the EPR results which show that at 298 K only the [VO(dhp)2] species is present in the solution, while at 120 K both square pyramidal and octahedral complexes exist, with the spy isomer being the predominant one.
| Reaction | ΔGaq (298.15 K) | ΔGaq (120 K) |
|---|---|---|
| a Values reported in kcal mol−1. b Calculations performed at the B3P86/6-311g(d,p) level of theory using the SMD model for water. c G aq mean value of all SPY-5 isomers was considered. d G aq mean value of all OC-6 isomers was considered. e G aq mean value of the OC-6–34 isomer formed by MeIm with the two dihedral angles in Table S3† was considered. f G aq mean value of the OC-6–23 isomer formed by MeIm with the two dihedral angles in Table S3† was considered. g G aq value of the OC-6–34 isomer was considered. h G aq value of the OC-6–23 isomer was considered. | ||
| [VO(dhp)2]c + H2O ⇄ cis-[VO(dhp)2(H2O)]d | 10.12 | 5.52 |
| [VO(dhp)2]c + MeIm ⇄ cis-[VO(dhp)2(MeIm)]e | 9.12 | 2.70 |
| [VO(dhp)2]c + MeIm ⇄ cis-[VO(dhp)2(MeIm)]f | 8.43 | 2.31 |
| cis-[VO(dhp)2(H2O)]g + MeIm ⇄ cis-[VO(dhp)2(MeIm)]e + H2O | −1.69 | −3.26 |
| cis-[VO(dhp)2(H2O)]h + MeIm ⇄ cis-[VO(dhp)2(MeIm)]f + H2O | −1.34 | −2.93 |
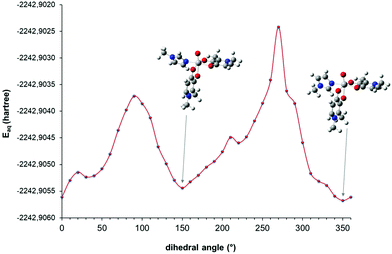
In order to determine the most stable conformation(s) of the mixed complex cis-[VO(dhp)2(MeIm)] at 298 and 120 K as a function of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) V–NMeIm–CMeIm dihedral angle, a series of DFT calculations were performed. Since the G values of the cis-Oh isomers are close to each other, only OC-6–34 and OC-6–23 were chosen as starting points to build the corresponding mixed complex, being the structures with the lowest and the highest energy (see Table S2†). A relaxed scan calculation was performed by changing the orientation of MeIm with respect to the V
V–NMeIm–CMeIm dihedral angle, a series of DFT calculations were performed. Since the G values of the cis-Oh isomers are close to each other, only OC-6–34 and OC-6–23 were chosen as starting points to build the corresponding mixed complex, being the structures with the lowest and the highest energy (see Table S2†). A relaxed scan calculation was performed by changing the orientation of MeIm with respect to the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of cis-VO(dhp)2 moiety and the results are shown in Fig. 3 and S3.† It can be noticed that the structures corresponding to the minimum points in the scan calculation are those in which the dihedral angle is 150 and 340–350°—for the OC-6–34 and OC-6–23 isomer, respectively—which were further optimized and used in the determination of Gaq.
O bond of cis-VO(dhp)2 moiety and the results are shown in Fig. 3 and S3.† It can be noticed that the structures corresponding to the minimum points in the scan calculation are those in which the dihedral angle is 150 and 340–350°—for the OC-6–34 and OC-6–23 isomer, respectively—which were further optimized and used in the determination of Gaq.
The dihedral angle and Gibbs energy values calculated for the optimized structures of cis-[VO(dhp)2(MeIm)] isomers are listed in Table S3† and their relative stability was determined considering that the starting complex can assume the spy geometry or the cis-Oh configuration. The data obtained (Table 1) suggest that the equilibrium [VO(dhp)2] + MeIm ⇄ cis-[VO(dhp)2(MeIm)] is always shifted toward the binary complex (with the positive values of ΔGaq both at 298 and 120 K) in agreement with what was observed in the EPR experiment which does not show the formation of a mixed species when only the spy complex is present in solution, namely at 298 K. On the other hand, when the substitution reaction cis-[VO(dhp)2(H2O)] + MeIm ⇄ cis-[VO(dhp)2(MeIm)] + H2O is taken into account, negative values of ΔGaq were obtained and the results suggest that the temperature decrease favours the coordination of MeIm, as confirmed by the experimental results. The latest results explain why protein binding was detected clearly only at low temperatures.26
3.2. Systems with ma
The bis-chelated VIVO complex formed by maltol exists in aqueous solution as a cis-octahedral structure [VO(ma)2(H2O)].37,38,59The broad EPR signal obtained at 120 K (Fig. S4a†), in HEPES 0.1 M solution, suggests that different isomers can be present at physiological pH with slightly different Az values. Among the possible geometric isomers, DFT calculations suggested that the most probable structure is that with the coordination mode [(Oket, Ophen); (Oket, Oaxphen); H2O] (Scheme S2†).38
In the presence of MeIm, cis-[VO(ma)2(H2O)] completely converts into the mixed complex cis-[VO(ma)2(MeIm)] where the water molecule is replaced by the model ligand (Fig. S4c†). In the system with Mb, the EPR signal is similar to that of cis-[VO(ma)2(MeIm)], suggesting that the species VO(ma)2(Mb) is formed, with the protein coordinating the vanadium ion through an imidazole group of a histidine residue.26
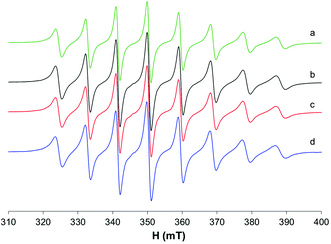
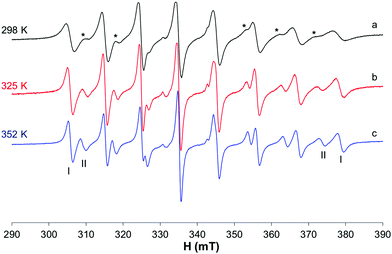
At 298 K (Fig. 4a), when the spectra are recorded in HEPES 0.1 M, together with the predominant cis-Oh complex (I), a small amount of another species with a lower value of Az is present in solution (whose signals are indicated with the asterisks in Fig. 4). It was noticed that at 325 and 352 K the amount of this species increases suggesting that the equilibrium depends on temperature (Fig. 4b and c). Moreover, at high temperatures the resonances are narrower, and it is possible to extract the spin Hamiltonian EPR parameters (Ig0 = 1.969, A0 = 95.7 × 10−4 cm−1 and IIg0 = 1.974, A0 = 84.9 × 10−4 cm−1). The formation of species I was confirmed by the accordance with the experimental A0 and that calculated from Ax, Ay and Az measured at 120 K (Fig. S5†), where the cis-Oh species is the only one present in solution (A0 = 95.7 × 10−4 cm−1vs. Aexpct0 = (Ax + Ay + Az)/3 = 96.3 × 10−4 cm−1).
The results are very similar to those previously obtained by Orvig and coworkers, who examined the temperature range 296–352 K.37
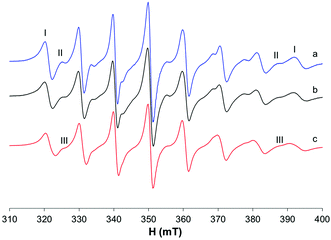
The EPR spectra were also recorded in MeOH (Fig. 5a–c); in this situation, two species are in equilibrium and their ratio changes with increasing temperature: at 335 K, the species characterized by g0 1.974 and A0 88.0 × 10−4 cm−1 is predominant, while at 298 K the major species is that with g0 1.970 and A0 96.3 × 10−4 cm−1. In non-coordinating solvents (CHCl3/toluene 6/4 v/v, Fig. 5d), only one species is observed with g0 1.975 and A0 90.4 × 10−4 cm−1. Similar results were obtained by Orvig and coworkers;37 these authors interpreted the equilibrium between different species, the square pyramidal and the two octahedral ones, cis and trans, with a solvent molecule coordinated to the metal complex. However, they were not able to distinguish between the EPR signals due to VO(ma)2 and those of trans-VO(ma)2(H2O); it is now clear from the results in the literature that the effect of the solvent coordination in the axial position, trans to the oxido group, causes a noticeable decrease of the hyperfine coupling constant, A0 or Az, due to a significant decrease of the Fermi contact term.39,43 Therefore, the two species in equilibrium in methanol solution are the cis-Oh and the trans-Oh isomers (A0 96.3 and 88.0 × 10−4 cm−1, I and II in Fig. 5, respectively), while the only species in non-coordinating solvents is the spy isomer (III, A0 90.4 × 10−4 cm−1).
In the ternary system with MeIm, small differences in the EPR spectra recorded at 298 K suggest that a small amount of the model complex cis-[VO(ma)2(MeIm)] could be formed (Fig. 6c); this was confirmed by the results obtained at higher temperatures (325 and 352 K) where it is possible to appreciate the presence of a new signal because of the narrowing of the bands (Fig. S6†).
On the other hand, in presence of myoglobin a similar isotropic spectrum is obtained which suggests that no binding or a very weak binding with the protein occurs (Fig. 6b).
The experimental and calculated EPR parameters of the binary and ternary species mentioned above are listed in Table S1.†
DFT calculations were performed considering four different orientations of MeIm (dihedral angle θ = 0, 90, 180, and 270°) and only the most stable cis-octahedral isomers where the water ligand was replaced by MeIm (Scheme S2†). The Gaq values at 298 and 120 K for each species are reported in Table S4† and the results of the thermodynamic calculations in Table 2. The data in Table 2 allow us to explain that the behavior of the ma complexes is probably due to the lower stability at 298 K of the mixed species. Even if the cis-Oh complex is formed both at 298 and 120 K — contrary to what was observed with the dhp ligand where only spy is present at 298 K — the calculated ΔGaq values for the reaction cis-[VO(ma)2(H2O)] + MeIm ⇄ cis-[VO(ma)2(MeIm)] + H2O are more negative at 120 K (see Table 2), suggesting that the formation of the mixed complex is favored at low temperatures.
| Reaction | ΔGaq (298.15 K) | ΔGaq (120 K) |
|---|---|---|
| a Values reported in kcal mol−1. b Calculations performed at the B3P86/6-311g(d,p) level of theory using the SMD model for water. c G aq value of the OC-6–32 isomer was considered. d G aq mean value of the OC-6–32 isomer formed by MeIm with the two dihedral angles in Table S4† was considered. e G aq value of the OC-6–34 isomer was considered. f G aq mean value of the OC-6–34 isomer formed by MeIm with the two dihedral angles in Table S4† was considered. | ||
| cis-[VO(ma)2(H2O)]c + MeIm ⇄ cis-[VO(ma)2(MeIm)]d + H2O | −1.30 | −2.89 |
| cis-[VO(ma)2(H2O)]e + MeIm ⇄ cis-[VO(ma)2(MeIm)]f + H2O | −3.79 | −4.50 |
3.3. Systems with pic
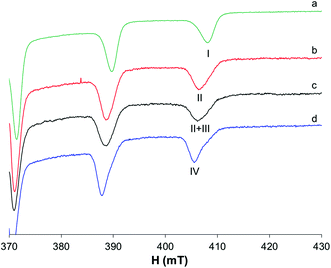
The results previously published60,61 show that in the binary system VO2+/pic 1/2, vanadium is present in solution at physiological pH as a mixture of cis-[VO(pic)2(OH)]− and cis-[VO(pic)2(H2O)] (Fig. 7b); the latter becomes the predominant species at lower pH (Fig. 7a) with a pK of 6.98.To evaluate if protein coordination occurs in the system with Mb, it should be taken into account that the replacement of the equatorial OH− in cis-[VO(pic)2(OH)]− with an imidazole nitrogen atom does not significantly change the EPR parameters, as already confirmed in the literature.15 Therefore, even if a comparison of the spectra of the systems VO2+/pic/Mb and VO2+/pic at physiological pH shows very small differences (Fig. 7c), it can be supposed that an imidazole donor from Mb replaces, at least partly, the equatorial OH− ion in the hydroxido species leading to the formation of the mixed complex VO(pic)2(Mb).
The differences are more visible upon comparing the resonances of the binary system with those of VO2+/pic/MeIm (Fig. 7d) where, despite the similar Az values (Table S1†), the different band shapes suggest that the coordination of MeIm to V can partially occur.
The differences are slightly visible at 298 K (Fig. S7†) also, where the neutral complex cis-[VO(pic)2(H2O)] coexists with the hydroxido species. When MeIm is added to the solution, an increase in the intensity of the internal resonances suggests that cis-[VO(pic)2(H2O)] transforms in part to cis-[VO(pic)2(MeIm)]. The EPR spectra recorded with Mb show no clear evidence of protein binding as only isotropic signals were obtained (Fig. 8).
DFT calculations suggest that the formation of the mixed complex is thermodynamically favored since the calculated values of ΔGaq for the reaction cis-[VO(pic)2(H2O)] + MeIm ⇄ cis-[VO(pic)2(MeIm)] + H2O are negative both at 298 and 120 K; as observed with the other ligands, the decrease in temperature favors the coordination of MeIm (Table 3).
| Reaction | ΔGaq (298.15 K) | ΔGaq (120 K) |
|---|---|---|
| a Values reported in kcal mol−1. b Calculations performed at the B3P86/6-311g(d,p) level of theory using the SMD model for water. c G aq value of the OC-6–24 isomer was considered. d G aq mean value of the OC-6–24 isomer formed by MeIm with the two dihedral angles in Table S5† was considered. e G aq value of the OC-6–23 isomer was considered. f G aq mean value of the OC-6–23 isomer formed by MeIm with the two dihedral angles in Table S5† was considered. | ||
| cis-[VO(pic)2(H2O)]c + MeIm ⇄ cis-[VO(pic)2(MeIm)]d + H2O | −4.39 | −5.26 |
| cis-[VO(pic)2(H2O)]e + MeIm ⇄ cis-[VO(pic)2(MeIm)]f + H2O | −3.67 | −4.86 |
4. Conclusions
In this study, the influence of different parameters, such as temperature, ionic strength, solvent and the type of ligand, on the equilibria of some oxidovanadium(IV) complexes in solution has been investigated in order to elucidate their interaction with proteins.EPR spectroscopy is one of the most used techniques to study VIVO2+ complexes, their equilibria and transformations in organisms after the interaction with the blood serum bioligands and cellular components. Of course, these processes take place at 37 °C (310 K) and in the liquid phase. The interaction of vanadium complexes with proteins has been mainly studied by frozen solution EPR spectroscopy (120 K) because the oxidation to VV is minimized and anisotropic spectra can give information about the symmetry and the coordination geometry of the VIVO complexes. Often, EPR studies are accompanied by UV-vis and pH-potentiometric measurements that are necessarily performed at 298 K. Therefore, in order to better analyze the results and refer them to the physiological conditions, it is important to understand how the equilibria in solution can be affected by changing the temperature and other experimental conditions.
The results of this study showed that for the potential metallodrugs VO(dhp)2, VO(ma)2 and VO(pic)2(H2O), a general equilibrium between different geometries exists in solution: VOL2 + H2O ⇄ cis-VOL2(H2O) ⇄ trans-VOL2(H2O). As first observed, the strength of the ligand determines the preference of penta- or hexa-coordinated structures; in particular, for the complex VO(dhp)2 at 298 K only the square pyramidal species exists in aqueous solution, while the complexes VO(ma)2 and VO(pic)2 prefer the octahedral geometry, cis-VOL2(H2O), where the water molecule bound in the equatorial plane can deprotonate producing the corresponding mono-hydroxido species cis-[VOL2(OH)]−. This is in agreement with the data reported in the literature.38,60 When the complex exists as cis-VOL2(H2O), the increase in temperature allows us to reveal the presence of a minor species, trans-Oh, due to the significant decrease in the EPR linewidth. The presence of the trans-Oh complex is indicated by the small value of A0 or Az.39,43 It must be pointed out that the equilibria between the spy, cis-Oh and trans-Oh species could be more common than what is believed in the literature. They have been discussed some years ago for picolinate derivatives,39,43 observed for dhp39 and for maltol, even if in the latter case the authors were not able to distinguish between VO(ma)2 and trans-[VO(ma)2(H2O)] species.37
The amount of solvent-coordinated complexes depends also on the ionic strength of the solution, which can be changed when buffers or salt are added. On the other hand, cooling the solutions down to 120 K causes the shift of the equilibria towards the octahedral species, which has been already observed with other VIVO2+ complexes.40,60
When the interaction with 1-methylimidazole, a model for the side chain His coordination, was investigated, it was observed that no coordination occurs at 298 K when the V complex has a spy geometry, such as VO(dhp)2, while the formation of the mixed species cis-[VOL2(MeIm)] seems always to be favored by decreasing the temperature, where the complexes are present as cis-[VOL2(H2O)] species and MeIm can easily replace the coordinated water molecule.
These effects can be related to the type of interaction observed with a protein (Mb in this study). In particular, the results obtained with all the three examined complexes provide strong evidence that the interaction with Mb is favored at low temperatures. This must be considered in the interpretation of the data. However, depending on the ligand, protein and stabilization through secondary interactions, such as hydrogen bonds and van der Waals contacts, a small amount of the adducts can also be formed at room temperature; for example, the adduct formed by cis-VO(pic)2 with lysozyme was crystallized at 293 K and its structure is solved with the binding of Asp52-COO in one of the equatorial sites.35
DFT calculations permit us to evaluate the relative stability of different species in solution at different temperatures and can represent a valuable method to demonstrate this transformation, even if the stabilization of the amino acid side chains must be taken into account. The results confirm that this behavior is probably due to the lower stability at 298 K of the mixed species compared to 120 K.
Therefore, even if EPR spectra at 120 K are more easily interpretable, the lowering of the temperature can shift the equilibria and stabilize species which do not exist or exist in low amounts under physiological conditions (310 K). Therefore, EPR spectra at 298 K (or at 310 K) are surely helpful to have a more complete and realistic picture of the interaction of V complexes with proteins or other bioligands.
Considering these results, often EPR spectroscopy is not enough to characterize completely a VIVOL2–protein system and an integrated approach, based on the application of other techniques, such as ESI-MS, CD and ESEEM/ENDOR spectroscopy, XRD plus several computational techniques, such as docking, QM/MM and MD methods, is strongly recommended.23,25,26,29,31,32,62
The importance of this study is not limited to the vanadium solution chemistry since the same considerations can be extended to other metal complexes, such as Cu(II) and other first-row transition elements. Therefore, it is essential to consider the behavior of potential metallodrugs under different experimental conditions (including temperature, ionic strength, solvents, etc.) when their interaction with biological molecules is investigated. This fact becomes fundamental even when the results of various techniques are compared, especially when obtained under different experimental conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Regione Autonoma della Sardegna (grant RASSR79857), Fondazione di Sardegna (grant FdS2017Garribba) and Università di Sassari (fondo di Ateneo per la ricerca 2020, Lubinu).References
- D. Gambino, Coord. Chem. Rev., 2011, 255, 2193–2203 CrossRef CAS.
- D. Rehder, Future Med. Chem., 2012, 4, 1823–1837 CrossRef CAS PubMed.
- J. Costa Pessoa, S. Etcheverry and D. Gambino, Coord. Chem. Rev., 2015, 301–302, 24–48 CrossRef PubMed.
- D. Rehder, Future Med. Chem., 2016, 8, 325–338 CrossRef CAS PubMed.
- D. Rehder, ChemTexts, 2018, 4, 20 CrossRef.
- D. Crans, C. L. Yang, A. Haase and X. Yang, in Metallo-Drugs: Development and Action of Anticancer Agents, ed. A. Sigel, H. Sigel, E. Freisinger and R. K. O. Sigel, De Gruyter GmbH, Berlin, 2018, vol. 18, pp. 251–280 Search PubMed.
- S. Treviño, A. Díaz, E. Sánchez-Lara, B. L. Sanchez-Gaytan, J. M. Perez-Aguilar and E. González-Vergara, Biol. Trace Elem. Res., 2019, 188, 68–98 CrossRef PubMed.
- D. C. Crans, L. Henry, G. Cardiff and B. I. Posner, in Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic, ed. P. L. Carver, De Gruyter GmbH, Berlin, 2019, pp. 203–230 Search PubMed.
- A. Ścibior, Ł. Pietrzyk, Z. Plewa and A. Skiba, J. Trace Elem. Med. Biol., 2020, 61, 126508 CrossRef PubMed.
- D. Rehder, Inorg. Chim. Acta, 2020, 504, 119445 CrossRef CAS.
- F. H. Nielsen, in Handbook of Nutritionally Essential Mineral Elements, ed. B. l. O'Dell and R. A. Sunde, Marcel Dekker, Inc., New York, 1997, ch. 22, pp. 619–360 Search PubMed.
- S. S. Gropper, J. L. Smith and J. L. Groff, Advanced Nutrition and Human Metabolism, Cengage Learning, Wadsworth, 5th edn, 2009 Search PubMed.
- K. H. Thompson and C. Orvig, J. Inorg. Biochem., 2006, 100, 1925–1935 CrossRef CAS PubMed.
- K. H. Thompson, J. Lichter, C. LeBel, M. C. Scaife, J. H. McNeill and C. Orvig, J. Inorg. Biochem., 2009, 103, 554–558 CrossRef CAS PubMed.
- D. Sanna, G. Micera and E. Garribba, Inorg. Chem., 2010, 49, 174–187 CrossRef CAS PubMed.
- D. Sanna, P. Buglyo, G. Micera and E. Garribba, J. Biol. Inorg. Chem., 2010, 15, 825–839 CrossRef CAS PubMed.
- D. Sanna, G. Micera and E. Garribba, Inorg. Chem., 2011, 50, 3717–3728 CrossRef CAS PubMed.
- D. Sanna, L. Bíró, P. Buglyó, G. Micera and E. Garribba, Metallomics, 2012, 4, 33–36 CrossRef CAS PubMed.
- I. Correia, T. Jakusch, E. Cobbinna, S. Mehtab, I. Tomaz, N. V. Nagy, A. Rockenbauer, J. Costa Pessoa and T. Kiss, Dalton Trans., 2012, 41, 6477–6487 RSC.
- D. Sanna, G. Micera and E. Garribba, Inorg. Chem., 2013, 52, 11975–11985 CrossRef CAS PubMed.
- J. Costa Pessoa, E. Garribba, M. F. A. Santos and T. Santos-Silva, Coord. Chem. Rev., 2015, 301–302, 49–86 CrossRef CAS.
- T. Jakusch and T. Kiss, Coord. Chem. Rev., 2017, 351, 118–126 CrossRef CAS.
- I. Correia, I. Chorna, I. Cavaco, S. Roy, M. L. Kuznetsov, N. Ribeiro, G. Justino, F. Marques, T. Santos-Silva, M. F. A. Santos, H. M. Santos, J. L. Capelo, J. Doutch and J. Costa Pessoa, Chem. - Asian J., 2017, 12, 2062–2084 CrossRef CAS PubMed.
- D. Sanna and E. Garribba, in An Introduction to Vanadium: Chemistry, Occurrence and Applications, ed. R. Bowell, Nova Science Publishers, Inc., Hauppage, NY, 2019, ch. 6, pp. 141–183 Search PubMed.
- V. Ugone, D. Sanna, G. Sciortino, J. D. Marechal and E. Garribba, Inorg. Chem., 2019, 58, 8064–8078 CrossRef PubMed.
- G. Sciortino, D. Sanna, V. Ugone, J. D. Maréchal and E. Garribba, Inorg. Chem. Front., 2019, 6, 1561–1578 RSC.
- V. Ugone, D. Sanna, G. Sciortino, D. C. Crans and E. Garribba, Inorg. Chem., 2020, 59, 9739–9755 CrossRef CAS PubMed.
- G. Sciortino and E. Garribba, Chem. Commun., 2020, 56, 12218–12221 RSC.
- A. Banerjee, S. P. Dash, M. Mohanty, G. Sahu, G. Sciortino, E. Garribba, M. F. N. N. Carvalho, F. Marques, J. Costa Pessoa, W. Kaminsky, K. Brzezinski and R. Dinda, Inorg. Chem., 2020, 59, 14042–14057 CrossRef CAS PubMed.
- A. Levina and P. A. Lay, Inorg. Chem., 2020, 59, 16143–16153 CrossRef CAS PubMed.
- V. Ugone, D. Sanna, S. Ruggiu, G. Sciortino and E. Garribba, Inorg. Chem. Front., 2021, 8, 1189–1196 RSC.
- J. Costa Pessoa, M. F. A. Santos, I. Correia, D. Sanna, G. Sciortino and E. Garribba, Coord. Chem. Rev., 2021, 449, 214192 CrossRef.
- E. Garribba, G. Micera and D. Sanna, Inorg. Chim. Acta, 2006, 359, 4470–4476 CrossRef CAS.
- G. Sciortino, V. Ugone, D. Sanna, G. Lubinu, S. Ruggiu, J.-D. Maréchal and E. Garribba, Front. Chem., 2020, 8, 345 CrossRef CAS PubMed.
- M. F. A. Santos, I. Correia, A. R. Oliveira, E. Garribba, J. Costa Pessoa and T. Santos-Silva, Eur. J. Inorg. Chem., 2014, 3293–3297 CrossRef CAS.
- G. Sciortino, D. Sanna, V. Ugone, G. Micera, A. Lledós, J.-D. Maréchal and E. Garribba, Inorg. Chem., 2017, 56, 12938–12951 CrossRef CAS PubMed.
- G. R. Hanson, Y. Sun and C. Orvig, Inorg. Chem., 1996, 35, 6507–6512 CrossRef CAS PubMed.
- D. Sanna, P. Buglyó, L. Bíró, G. Micera and E. Garribba, Eur. J. Inorg. Chem., 2012, 1079–1092 CrossRef CAS.
- S. Gorelsky, G. Micera and E. Garribba, Chem. – Eur. J., 2010, 16, 8167–8180 CrossRef CAS PubMed.
- D. Sanna, V. Ugone, G. Micera and E. Garribba, Dalton Trans., 2012, 41, 7304–7318 RSC.
- P. Buglyó, T. Kiss, E. Kiss, D. Sanna, E. Garribba and G. Micera, J. Chem. Soc., Dalton Trans., 2002, 2275–2282 RSC.
- C. Orvig, P. Caravan, L. Gelmini, N. Glover, F. G. Herring, H. Li, J. H. McNeill, S. J. Rettig and I. A. Setyawati, J. Am. Chem. Soc., 1995, 117, 12759–12770 CrossRef CAS.
- E. Lodyga-Chruscinska, G. Micera and E. Garribba, Inorg. Chem., 2011, 50, 883–899 CrossRef CAS PubMed.
- D. Sanna, E. Garribba and G. Micera, J. Inorg. Biochem., 2009, 103, 648–655 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. L. Caricato, X. H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. J. Adamo, J. R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, revision C.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- M. Bühl and H. Kabrede, J. Chem. Theory Comput., 2006, 2, 1282–1290 CrossRef PubMed.
- M. Bühl, C. Reimann, D. A. Pantazis, T. Bredow and F. Neese, J. Chem. Theory Comput., 2008, 4, 1449–1459 CrossRef PubMed.
- G. Micera and E. Garribba, Int. J. Quantum Chem., 2012, 112, 2486–2498 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- G. Micera, V. L. Pecoraro and E. Garribba, Inorg. Chem., 2009, 48, 5790–5796 CrossRef CAS PubMed.
- D. Sanna, K. Varnágy, S. Timári, G. Micera and E. Garribba, Inorg. Chem., 2011, 50, 10328–10341 CrossRef CAS PubMed.
- G. Micera and E. Garribba, Eur. J. Inorg. Chem., 2011, 2011, 3768–3780 CrossRef CAS.
- D. Sanna, V. L. Pecoraro, G. Micera and E. Garribba, J. Biol. Inorg. Chem., 2012, 17, 773–790 CrossRef CAS PubMed.
- S. Kundu, D. Mondal, K. Bhattacharya, A. Endo, D. Sanna, E. Garribba and M. Chaudhury, Inorg. Chem., 2015, 54, 6203–6215 CrossRef CAS PubMed.
- S. P. Dash, S. Majumder, A. Banerjee, M. F. N. N. Carvalho, P. Adão, J. Costa Pessoa, K. Brzezinski, E. Garribba, H. Reuter and R. Dinda, Inorg. Chem., 2016, 55, 1165–1182 CrossRef CAS PubMed.
- G. Micera and E. Garribba, Dalton Trans., 2009, 1914–1918 RSC.
- G. Micera and E. Garribba, J. Comput. Chem., 2011, 32, 2822–2835 CrossRef CAS PubMed.
- D. Sanna, G. Sciortino, V. Ugone, G. Micera and E. Garribba, Inorg. Chem., 2016, 55, 7373–7387 CrossRef CAS PubMed.
- D. Sanna, L. Bíró, P. Buglyó, G. Micera and E. Garribba, J. Inorg. Biochem., 2012, 115, 87–99 CrossRef CAS PubMed.
- E. Kiss, E. Garribba, G. Micera, T. Kiss and H. Sakurai, J. Inorg. Biochem., 2000, 78, 97–108 CrossRef CAS PubMed.
- E. Kiss, K. Petrohán, D. Sanna, E. Garribba, G. Micera and T. Kiss, Polyhedron, 2000, 19, 55–61 CrossRef CAS.
- G. Sciortino, J. D. Maréchal and E. Garribba, Inorg. Chem. Front., 2021, 8, 1951–1974 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1dt02680a |
| This journal is © The Royal Society of Chemistry 2021 |