Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A ruthenium cis-dihydride with 2-phosphinophosphinine ligands catalyses the acceptorless dehydrogenation of benzyl alcohol†‡
Elizabeth C.
Trodden
ab,
Matthew P.
Delve
b,
Christian
Luz
b,
Robert J.
Newland
b,
John M.
Andresen
*a and
Stephen M.
Mansell
 *b
*b
aResearch Centre for Carbon Solutions (RCCS), Heriot-Watt University, Edinburgh, EH14 4AS, UK
bInstitute of Chemical Sciences, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, UK. E-mail: j.andresen@hw.ac.uk; s.mansell@hw.ac.uk
First published on 3rd September 2021
Abstract
The first ruthenium dihydride complex featuring a phosphinine ligand cis-[Ru(H)2(2-PPh2-3-Me-6-SiMe3-PC5H2)2] was synthesised exclusively as the cis-isomer. When formed in situ from the reaction of cis-[Ru(Cl)2(2-PPh2-3-Me-6-SiMe3-PC5H2)2] with two equivalents of Na[BHEt3], as demonstrated by 31P and 1H NMR spectroscopy, the catalysed acceptorless dehydrogenation of benzyl alcohol was observed leading to benzyl benzoate in up to 70% yield.
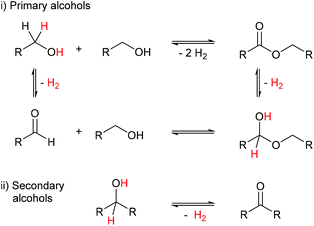
Acceptorless dehydrogenation (AD) is a growing area of significance for hydrogen production and storage, and liquid organic hydrogen carriers (LOHCs) are attractive for hydrogen storage since alcohols can be stored under ambient temperature and pressure.1–4 The H2 that is generated is a clean alternative fuel burning to produce solely water vapour, or H2 can be utilised in fuel cells.5,6 AD has additional applications in chemical synthesis because catalytic AD reactions do not require toxic stoichiometric reagents, thus minimising harmful waste.7–9 AD coupling reactions of alcohols can provide a cleaner, more atom efficient synthesis of esters, for example, without the need for acid chloride intermediates.7 Esters are used in the production of perfumes, paints and varnishes, and are therefore high value commodities.10
Alcohols are important starting materials that can be bio-sourced, offering complementary synthetic routes to conventional routes based on fossil-fuel-derived starting materials, although alcohols tend to be quite unreactive.11,12 The AD of secondary alcohols leads to ketones, whereas primary alcohols can give aldehydes and esters (Scheme 1). Ru catalysts are important in these processes but can be deactivated by decarbonylation of the aldehydes formed.7 Early Ru catalysts for AD include [Ru(OCOCF3)2(CO)(PPh3)2] alongside an acid promotor,13 related ruthenium(II) trifluoroacetate diphosphine complexes,14 [Ru(H)2(PPh3)4], at 180 °C,15,16 and Shvo's catalyst at 145 °C for the conversion of PhCH2OH to benzyl benzoate.17
Pioneering studies by Milstein and coworkers have shown that metal–ligand cooperation (MLC), the active participation of the ligand in bond-making and bond-breaking steps in homogeneous catalytic reactions,18–21 between a dearomatised pincer ligand and a Ru centre gave highly effective catalysts for ester formation from alcohols.22 For benzyl alcohol at 115 °C, a 92% yield of benzyl benzoate was achieved in 4 h.22 Pincer-ligand systems23 involving both precious10,24–31 and first row18,32–38 transition metals have been the focus of recent investigations into AD.
Homogeneous ruthenium catalysts with phosphine ligands have proven extremely useful over many decades including those with the small bite-angle ligand bis(diphenylphosphinomethane) (dppm).39 For example, the pre-catalyst cis-[Ru(Cl)2(dppm)2] achieved an initial TOF of 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 for the reaction of CO2 with hydrogen in an amine solution leading to the production of an amine-formate adduct, thus demonstrating facile CO2 conversion.40 The development of phosphinines, the phosphorus analogue of pyridine, as effective ligands for homogeneous catalysis has grown over the last 25 years,41–43 and now includes applications such as Rh-catalysed hydroformylation44 and hydrogenation,45,46 Au-catalysed cycloisomerisations,47 Cr-catalysed ethylene oligomerisation48 and the hydroboration of carbonyls.49 The application of homogeneous metal-phosphinine catalysts to confront energy challenges has also been explored, including in water oxidation reactions50 and the catalytic upgrading of alcohols to advanced biofuels.46 Previous work in our group has demonstrated that Ru catalysts based on the bidentate chelating phosphinophosphinine ligand 2-PPh2-3-Me-6-SiMe3-PC5H2 (PP′) catalysed the room temperature transfer hydrogenation of acetophenone and the H-borrowing upgrading of alcohol fuels.46,51 Ruthenium hydrides are implicated in many catalytic reactions, yet the first Ru complex comprising of both a hydride and phosphinine ligand has only been described recently by us.52 Herein, we describe the first ruthenium phosphinine dihydride complex and compare its catalytic activity to that of classical diphosphine-supported complexes for AD reactions.
000 h−1 for the reaction of CO2 with hydrogen in an amine solution leading to the production of an amine-formate adduct, thus demonstrating facile CO2 conversion.40 The development of phosphinines, the phosphorus analogue of pyridine, as effective ligands for homogeneous catalysis has grown over the last 25 years,41–43 and now includes applications such as Rh-catalysed hydroformylation44 and hydrogenation,45,46 Au-catalysed cycloisomerisations,47 Cr-catalysed ethylene oligomerisation48 and the hydroboration of carbonyls.49 The application of homogeneous metal-phosphinine catalysts to confront energy challenges has also been explored, including in water oxidation reactions50 and the catalytic upgrading of alcohols to advanced biofuels.46 Previous work in our group has demonstrated that Ru catalysts based on the bidentate chelating phosphinophosphinine ligand 2-PPh2-3-Me-6-SiMe3-PC5H2 (PP′) catalysed the room temperature transfer hydrogenation of acetophenone and the H-borrowing upgrading of alcohol fuels.46,51 Ruthenium hydrides are implicated in many catalytic reactions, yet the first Ru complex comprising of both a hydride and phosphinine ligand has only been described recently by us.52 Herein, we describe the first ruthenium phosphinine dihydride complex and compare its catalytic activity to that of classical diphosphine-supported complexes for AD reactions.
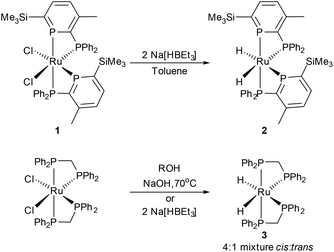
Ruthenium dihydride fragments supported by phosphine ligands are well known, and have proven to be successful catalysts for numerous reactions, as well as suitable precursors for photochemical C–H activation.53,54 [Ru(H)2(dppm)2] exists as a mixture of cis and trans isomers55 and can be synthesised from [Ru(Cl)2(dppm)2]56 through reaction with primary or secondary alcohols and NaOH,57 or by reaction with Na[BHEt3] (Scheme 2). Building on recent work describing a [RuCp*(H)] fragment supported by a bis(phosphinine) ligand,52 the ruthenium dihydride cis-[Ru(H)2(PP′)2] (2) was synthesised from the dichloro precursor cis-[Ru(Cl)2(PP′)2] (1) by reaction with two equivalents of Na[BHEt3] (Scheme 2).§
The formation of cis-[Ru(H)2(PP′)2] (2) was evident by an immediate colour change of orange to bright red upon the addition of Na[HBEt3] to 1. 31P{1H} NMR spectroscopy confirmed the presence of only four resonances, two at high chemical shift indicative of phosphinine groups, and two at lower chemical shift from the diphenylphosphino groups. Two doublet-of-doublet-of-doublets resonances were observed with a large trans-coupling (261 Hz), which has decreased compared to the trans2JPP coupling in 1 (425 Hz). The 1H NMR spectrum displayed resonances for two inequivalent PP′ ligands as well as two multiplet resonances at −6.55 and −7.04 ppm for the two Ru–H.§
Slow evaporation of a petroleum ether solution of 2 in a glovebox yielded crystals suitable for structural characterisation using X-ray diffraction.¶ The crystal structure of 2, Fig. 1, shows Ru(1) in a slightly distorted octahedral geometry with two phosphinophosphinine and cis-dihydride ligands, which were located in the Fourier difference map and refined. The phosphinine donors are cis-disposed, due to their strong π-accepting character, and this explains why only one isomer of 2 was formed (as a racemic mixture). The bond lengths of the phosphinine phosphorus atoms P(1) and P(3) to Ru(1) (2.2795(5) and 2.2634(5) Å respectively) are shorter than the distances from the diphenylphosphino phosphorus atoms P(2) and P(4) to Ru(1) (2.3071(5) and 2.3889(5) Å respectively). The angles between phosphorus atoms on the same ligand, e.g. P(1)–Ru(1)–P(2) (70.37(2)°), are smaller than that of phosphorus atoms on different ligands (e.g. P(1)–Ru(1)–P(4): 109.05(2)°), showing the distortion from octahedral geometry.
Despite the clean synthetic route, attempts to isolate 2 were problematic whenever 2 was dried under vacuum. Upon exposure to vacuum, the resulting 31P{1H} NMR spectra displayed many resonances of weak intensity instead of the anticipated product. Analysis by mass spectrometry (APCI) corroborated these results with only very low intensity signals for masses above 300 Da. This was indicative that drying under vacuum led to the decomposition of 2. Isolation of 2 by evaporation of the solvent under a stream of nitrogen led to the successful isolation of 2 in modest yields (25 mg, 27%). The mass spectrum now displayed a mass envelope centred at 835 Da with the correct isotopic distribution and accurate mass for [C42H49P496RuSi2]+, [M − H]+.
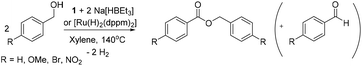
Complex 1 was used as a precatalyst for 2 in the acceptorless dehydrogenation of benzyl alcohols (Scheme 3) (Table 1).
| Entry | Cat. | Substrate R = | Temp (°C) | Loading (mol%) | Time (h) | Yield (%) ester | Yield (%) aldehyde | TON (TOF/h−1) |
|---|---|---|---|---|---|---|---|---|
a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2 equivalents Na[HBEt3]. Yields: average of two runs, quantified using 1,3,5-trimethoxy benzene as an internal standard. TON = (2× mmol ester + mmol aldehyde if ≥1%)/mmol cat. 1 + 2 equivalents Na[HBEt3]. Yields: average of two runs, quantified using 1,3,5-trimethoxy benzene as an internal standard. TON = (2× mmol ester + mmol aldehyde if ≥1%)/mmol cat.
|
||||||||
| 1a | 2 | H | 140 | 1 | 45 | 28 | 1 | 29 (0.6) |
| 2a | 2 | H | 140 | 2 | 45 | 35 | 1 | 18 (0.4) |
| 3a | 2 | H | 140 | 2 | 94 | 61 | <1 | 31 (0.3) |
| 4a | 2 | H | 140 | 2 | 140 | 70 | <1 | 35 (0.3) |
| 5a | 2 | H | 140 | 5 | 45 | 38 | <1 | 8 (0.2) |
| 6a | 2 | OMe | 140 | 2 | 45 | 5 | 30 | 18 (0.4) |
| 7a | 2 | Br | 140 | 2 | 45 | 11 | 11 | 11 (0.2) |
| 8a | 2 | NO2 | 140 | 2 | 45 | 12 | 2 | 7 (0.2) |
| 9 | 3 | H | 140 | 1 | 45 | 19 | <1 | 19 (0.4) |
| 10 | 3 | H | 140 | 2 | 45 | 27 | 1 | 14 (0.3) |
| 11 | 3 | H | 140 | 2 | 94 | 38 | <1 | 19 (0.2) |
| 12 | 3 | H | 140 | 2 | 140 | 64 | <1 | 32 (0.2) |
| 13 | 3 | OMe | 140 | 2 | 45 | 16 | 20 | 18 (0.4) |
| 14 | 3 | Br | 140 | 2 | 45 | 7 | 9 | 8 (0.2) |
| 15 | 3 | NO2 | 140 | 2 | 45 | 10 | 5 | 8 (0.2) |
Four benzyl alcohols with different electron donating and withdrawing substituents were screened to test the reactivity of 2. These reactions were compared to the catalytic activity of [Ru(H)2(dppm)2] (3). Base line reactions with no Ru catalyst gave no formation of benzyl benzoate, and when only Na[HBEt3] was added, there was only a small amount of benzaldehyde produced (0.5%). Using 1 mol% of 2, a 28% yield of ester was evident after 45 hours at 140 °C (run 1), which is higher than for 3 (19%, run 9). Doubling the catalyst concentration and increasing the reaction time led to better conversions, thereby increasing yields (run 3: 61% for 2, run 11: 38% for 3 after 94 hours) up to 70% benzyl benzoate for 2. The addition of base (NaOtBu) did not increase yields. Substitution of the benzyl alcohol with a para-methoxy group led to significant production of the substituted benzaldehyde as the favoured product (30%, run 6). Electron withdrawing substituents inhibit the acceptorless dehydrogenation reaction (para-NO2: 12%, run 8; para-Br: 11%, run 7), although the Br-substituted aldehyde by-product is also observed to a considerable extent (11%). In comparison [RuH2(dppm)2] produced similar yields and outcomes for substituted benzaldehydes.
Comparisons between different catalysts can be made for the coupling of benzyl alcohol. The mixture of [RuCl2(p-cymene)(IiPr)] (2.5 mol%), KOH (10 mol%) and PCy3 (4.5 mol%) at 163 °C for 18 h gave a yield of 31% benzyl benzoate; benzyl alcohols with para-OMe and Me groups led to lower yields.58 A RuCl2 complex with an aryl-tethered phosphine ligand in combination with 2 equiv. NaOtBu gave benzaldehyde as the product at 110 °C (2 mol%, 36 h, 80% yield).59 [RuH2(PPh3)4] (2 mol%) at 180 °C in refluxing mesitylene gave 60% benzyl benzoate.15 We note that the use of [RuCl2(PPh3)3] did not lead to substantial amounts of ester formation (2.4%) even with the addition of KOtBu.60 Yields were found to be similar to 2 after 120 h using an imidazolyl phosphine ruthenium dichloride complex with the addition of base (KOtBu).60 Morton and Cole-Hamilton achieved high rates of hydrogen production from aliphatic alcohols using [RuH2(N2)(PPh3)3], albeit at 150 °C.61 Aldehydes were synthesised from primary alcohols in moderately high yields with ruthenium triazolylidene dichloride complexes.62 In our work, an electron donating OMe substituent produced mixtures of ester![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aldehyde products (run 6, 1
aldehyde products (run 6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6; run 13, 1.6
6; run 13, 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in a higher ratio of aldehyde compared to an Ir-phosphine pincer catalyst (3
2) in a higher ratio of aldehyde compared to an Ir-phosphine pincer catalyst (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).24
1).24
In summary, we have prepared cis-[Ru(H)2(PP′)2], the first metal dihydride supported by phosphinophosphinine ligands. When formed in situ from the dichloride complex and Na[BHEt3], the catalysed acceptorless dehydrogenative coupling of benzyl alcohols to esters was observed. While cis-[Ru(H)2(PP′)2] showed improved catalytic activity compared to [Ru(H)2(dppm)2], its broadly similar activity to conventional Ru complexes in promoting the AD of benzyl alcohol into benzyl benzoate implies typical supporting ligand behaviour as opposed to metal–ligand cooperative pathways.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the UK National Mass Spectrometry Facility at Swansea University for sample analysis and the Engineering and Physical Sciences Research Council and the EPSRC Centre for Doctoral Training in Critical Resource Catalysis (CRITICAT) for financial support [E. C. T.; grant code: EP/L016419/1].Notes and references
- M. Trincado, D. Banerjee and H. Grützmacher, Energy Environ. Sci., 2014, 7, 2464 RSC.
- J. Campos, Phys. Sci. Rev., 2018, 3, 20170017 Search PubMed.
- R. H. Crabtree, Chem. Rev., 2017, 117, 9228 CrossRef CAS PubMed.
- K. Sordakis, C. H. Tang, L. K. Vogt, H. Junge, P. J. Dyson, M. Beller and G. Laurenczy, Chem. Rev., 2018, 118, 372 CrossRef CAS PubMed.
- H. A. Gasteiger and N. M. Marković, Science, 2009, 324, 48 CrossRef CAS PubMed.
- O. Z. Sharaf and M. F. Orhan, Renewable Sustainable Energy Rev., 2014, 32, 810 CrossRef CAS.
- C. Gunanathan and D. Milstein, Science, 2013, 341, 249 CrossRef CAS PubMed.
- G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681 CrossRef CAS PubMed.
- A. C. Marr, Catal. Sci. Technol., 2012, 2, 279 RSC.
- M. Nielsen, H. Junge, A. Kammer and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 5711 CrossRef CAS PubMed.
- A. J. A. Watson and J. M. J. Williams, Science, 2010, 329, 635 CrossRef CAS PubMed.
- P. Pandey, I. Dutta and J. K. Bera, Proc. Natl. Acad. Sci., India, Sect. A, 2016, 86, 561 CrossRef CAS.
- A. Dobson and S. D. Robinson, Inorg. Chem., 1977, 16, 137 CrossRef CAS.
- C. W. Jung and P. E. Garrou, Organometallics, 1982, 1, 658 CrossRef CAS.
- S. Murahashi, T. Naota, K. Ito, Y. Maeda and H. Taki, J. Org. Chem., 1987, 52, 4319 CrossRef CAS.
- S.-I. Murahashi, K.-i. Ito, T. Naota and Y. Maeda, Tetrahedron Lett., 1981, 22, 5327 CrossRef CAS.
- Y. Blum and Y. Shvo, J. Organomet. Chem., 1985, 282, C7 CrossRef CAS.
- M. R. Elsby and R. T. Baker, Chem. Soc. Rev., 2020, 49, 8933 RSC.
- C. Gunanathan and D. Milstein, Acc. Chem. Res., 2011, 44, 588 CrossRef CAS PubMed.
- J. R. Khusnutdinova and D. Milstein, Angew. Chem., Int. Ed., 2015, 54, 12236 CrossRef CAS PubMed.
- T. P. Gonçalves, I. Dutta and K.-W. Huang, Chem. Commun., 2021, 57, 3070 RSC.
- J. Zhang, G. Leitus, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2005, 127, 10840 CrossRef CAS PubMed.
- S. Werkmeister, J. Neumann, K. Junge and M. Beller, Chem. – Eur. J., 2015, 21, 12226 CrossRef CAS PubMed.
- S. Musa, I. Shaposhnikov, S. Cohen and D. Gelman, Angew. Chem., Int. Ed., 2011, 50, 3533 CrossRef CAS PubMed.
- D. Spasyuk, C. Vicent and D. G. Gusev, J. Am. Chem. Soc., 2015, 137, 3743 CrossRef CAS PubMed.
- E. Fogler, J. A. Garg, P. Hu, G. Leitus, L. J. W. Shimon and D. Milstein, Chem. – Eur. J., 2014, 20, 15727 CrossRef CAS PubMed.
- M. Nielsen, A. Kammer, D. Cozzula, H. Junge, S. Gladiali and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 9593 CrossRef CAS PubMed.
- K.-N. T. Tseng, J. W. Kampf and N. K. Szymczak, Organometallics, 2013, 32, 2046 CrossRef CAS.
- C. Gunanathan, L. J. W. Shimon and D. Milstein, J. Am. Chem. Soc., 2009, 131, 3146 CrossRef CAS PubMed.
- X. He, Y. Li, H. Fu, X. Zheng, H. Chen, R. Li and X. Yu, Organometallics, 2019, 38, 1750 CrossRef CAS.
- J. Schörgenhumer, A. Zimmermann and M. Waser, Org. Process Res. Dev., 2018, 22, 862 CrossRef.
- S. Chakraborty, P. O. Lagaditis, M. Förster, E. A. Bielinski, N. Hazari, M. C. Holthausen, W. D. Jones and S. Schneider, ACS Catal., 2014, 4, 3994 CrossRef CAS.
- N. A. Eberhardt, N. P. N. Wellala, Y. Li, J. A. Krause and H. Guan, Organometallics, 2019, 38, 1468 CrossRef CAS.
- D. H. Nguyen, X. Trivelli, F. Capet, J.-F. Paul, F. Dumeignil and R. M. Gauvin, ACS Catal., 2017, 7, 2022 CrossRef CAS.
- Z. Wei, A. de Aguirre, K. Junge, M. Beller and H. Jiao, Eur. J. Inorg. Chem., 2018, 2018, 4643 CrossRef CAS.
- G. Zhang, K. V. Vasudevan, B. L. Scott and S. K. Hanson, J. Am. Chem. Soc., 2013, 135, 8668 CrossRef CAS PubMed.
- U. K. Das, Y. Ben-David, G. Leitus, Y. Diskin-Posner and D. Milstein, ACS Catal., 2019, 9, 479 CrossRef CAS.
- F. Kallmeier and R. Kempe, Angew. Chem., Int. Ed., 2018, 57, 46 CrossRef CAS PubMed.
- S. M. Mansell, Dalton Trans., 2017, 46, 15157 RSC.
- M. Scott, B. B. Molinos, C. Westhues, G. Franciò and W. Leitner, ChemSusChem, 2017, 10, 1085 CrossRef CAS PubMed.
- C. Müller and D. Vogt, Dalton Trans., 2007, 5505 RSC.
- N. T. Coles, A. S. Abels, J. Leitl, R. Wolf, H. Grützmacher and C. Müller, Coord. Chem. Rev., 2021, 433, 213729 CrossRef CAS.
- C. Müller and D. Vogt, in Phosphorus Compounds. Catalysis by Metal Complexes, ed. M. Peruzzini and L. Gonsalvi, Springer, Dordrecht, 2011, vol. 37 Search PubMed.
- B. Breit, Chem. Commun., 1996, 2071 RSC.
- C. Müller, L. G. López, H. Kooijman, A. L. Spek and D. Vogt, Tetrahedron Lett., 2006, 47, 2017 CrossRef.
- R. J. Newland, M. F. Wyatt, R. L. Wingad and S. M. Mansell, Dalton Trans., 2017, 46, 6172 RSC.
- M. Rigo, L. Hettmanczyk, F. J. L. Heutz, S. Hohloch, M. Lutz, B. Sarkar and C. Müller, Dalton Trans., 2017, 46, 86 RSC.
- R. J. Newland, A. Smith, D. M. Smith, N. Fey, M. J. Hanton and S. M. Mansell, Organometallics, 2018, 37, 1062 CrossRef CAS.
- R. J. Newland, J. M. Lynam and S. M. Mansell, Chem. Commun., 2018, 54, 5482 RSC.
- L. E. E. Broeckx, A. Bucci, C. Zuccaccia, M. Lutz, A. Macchioni and C. Müller, Organometallics, 2015, 34, 2943 CrossRef CAS.
- R. J. Newland, M. P. Delve, R. L. Wingad and S. M. Mansell, New J. Chem., 2018, 42, 19625 RSC.
- P. A. Cleaves and S. M. Mansell, Organometallics, 2019, 38, 1595 CrossRef CAS.
- L. Cronin, M. C. Nicasio, R. N. Perutz, R. G. Peters, D. M. Roddick and M. K. Whittlesey, J. Am. Chem. Soc., 1995, 117, 10047 CrossRef CAS.
- R. N. Perutz and B. Procacci, Chem. Rev., 2016, 116, 8506 CrossRef CAS PubMed.
- B. Chaudret, G. Commenges and R. Poilblanc, J. Chem. Soc., Dalton Trans., 1984, 1635 RSC.
- A. Keller, B. Jasionka, T. Głowiak, A. Ershov and R. Matusiak, Inorg. Chim. Acta, 2003, 344, 49 CrossRef CAS.
- M. Khorasani-Motlagh, N. Safari, C. B. Pamplin, B. O. Patrick and B. R. James, Inorg. Chim. Acta, 2001, 320, 184 CrossRef CAS.
- A. Sølvhøj and R. Madsen, Organometallics, 2011, 30, 6044 CrossRef.
- J. Yuan, Y. Sun, G.-A. Yu, C. Zhao, N.-F. She, S.-L. Mao, P.-S. Huang, Z.-J. Han, J. Yin and S.-H. Liu, Dalton Trans., 2012, 41, 10309 RSC.
- R. Langer, A. Gese, D. Gesevičius, M. Jost, B. R. Langer, F. Schneck, A. Venker and W. Xu, Eur. J. Inorg. Chem., 2015, 696 CrossRef CAS.
- D. Morton and D. J. Cole-Hamilton, J. Chem. Soc., Chem. Commun., 1988, 1154 RSC.
- A. Prades, E. Peris and M. Albrecht, Organometallics, 2011, 30, 1162 CrossRef CAS.
Footnotes |
| † This paper is in memory of Prof. Paul Kamer. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2083014. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1dt02508b |
| § Analytical data for 2: 1H-NMR (400 MHz, C6D6, 298 K): δ = 8.26 (m, 2H, CHarom.), 7.78 (m, 2H, CHarom.), 7.57 (ddd, 1H, CHarom.), 7.48 (m, 3H, CHarom.), 7.16 (m overlapping with C6D5H), 7.05–6.89 (m, 7H, CHarom.), 6.87 (m, 3H, CHarom.), 6.77 (m, 2H, CHarom.), 6.37 (dd, 2H, CHarom), 1.64 (s, 3H, CH3), 1.61 (s, 3H, CH3), 0.62 (s, 9H, SiMe3), 0.27 (s, 9H, SiMe3), −6.55 (m, 1H, Hhydride), −7.04 (m, 1H, Hhydride) ppm; 31P{1H}-NMR (162 MHz, C6D6, 298 K): δ = 253.3 (ddd, 2JP2–P4 = 291 Hz, P2), 250.4 (m, P1), 31.6 (ddd, 2JP4–P2 = 291 Hz, P4), 15.7 (m, P3) ppm; HRMS (APCI/QTof) m/z: calcd for [C42H49P496RuSi2]+ 829.1400 [M − H]+, found 829.1404; calcd for [C30H40P3RuSi2]+ 651.0919 [M − HPPh2]+, found 651.0930. |
¶ Crystal data for C42H50P4RuSi2 (M = 835.95 g mol−1): monoclinic, space group P21/n (no. 14), a = 14.5408(5) Å, b = 10.5871(3) Å, c = 27.8908(9) Å, β = 104.1980(10)°, V = 4162.5(2) Å3, Z = 4, T = 100.0 K, μ(Cu-Kα) = 5.263 mm−1, Dcalc = 1.334 g cm−3, 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865 reflections measured (6.538° ≤ 2Θ ≤ 156.18°), 8484 unique (Rint = 0.0386, Rsigma = 0.0152). The final R1 was 0.0250 (I > 2σ(I)) and wR2 was 0.0606. CCDC: 2083014.‡ 865 reflections measured (6.538° ≤ 2Θ ≤ 156.18°), 8484 unique (Rint = 0.0386, Rsigma = 0.0152). The final R1 was 0.0250 (I > 2σ(I)) and wR2 was 0.0606. CCDC: 2083014.‡ |
| This journal is © The Royal Society of Chemistry 2021 |