Ethylene oligomerization into linear olefins over cobalt oxide on carbon catalyst†
Abstract
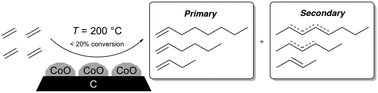
Here, we show that C4–C12 linear olefins, including linear alpha olefins, can be selectively produced from ethylene over a stable cobalt oxide on carbon catalyst. Both bulk and surface cobalt phases are CoO when the catalyst is stable, suggesting CoO is the stable cobalt phase for oligomerization. During the reaction, polyethylene forms in the catalyst pores which influences the product selectivity. The catalyst is more stable at higher temperatures (∼200 °C) likely due to reduction of Co3O4 to CoO while rapid deactivation is observed at lower temperatures (e.g., 80–140 °C). The product selectivity can be fit to two different Schulz Flory distributions, one from C4 to C10 olefins and one above C10 olefins, suggesting that transport restrictions influence product selectivity. At 48.3% conversion, product linearities up to C12 olefins are above 90%, making it the most selective heterogeneous catalyst to linear olefins to date in the absence of activators and/or solvents.


 Please wait while we load your content...
Please wait while we load your content...