Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mixed-dimensional membranes: chemistry and structure–property relationships
Yanan
Liu
ab,
Marc-Olivier
Coppens
 *b and
Zhongyi
Jiang
*b and
Zhongyi
Jiang
 *a
*a
aKey Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: zhyjiang@tju.edu.cn
bEPSRC “Frontier Engineering” Centre for Nature Inspired Engineering & Department of Chemical Engineering, University College London, London, WC1E 7JE, UK. E-mail: m.coppens@ucl.ac.uk
First published on 9th September 2021
Abstract
Tremendous progress in two-dimensional (2D) nanomaterial chemistry affords abundant opportunities for the sustainable development of membranes and membrane processes. In this review, we propose the concept of mixed dimensional membranes (MDMs), which are fabricated through the integration of 2D materials with nanomaterials of different dimensionality and chemistry. Complementing mixed matrix membranes or hybrid membranes, MDMs stimulate different conceptual thinking about designing advanced membranes from the angle of the dimensions of the building blocks as well as the final structures, including the nanochannels and the bulk structures. In this review, we survey MDMs (denoted nD/2D, where n is 0, 1 or 3) in terms of the dimensions of membrane-forming nanomaterials, as well as their fabrication methods. Subsequently, we highlight three kinds of nanochannels, which are 1D nanochannels within 1D/2D membranes, 2D nanochannels within 0D/2D membranes, and 3D nanochannels within 3D/2D membranes. Strategies to tune the physical and chemical microenvironments of the nanochannels as well as the bulk structures based on the size, type, structure and chemical character of nanomaterials are discussed. Some representative applications of MDMs are illustrated for gas molecular separations, liquid molecular separations, ionic separations and oil/water separation. Finally, current challenges and a future perspective on MDMs are presented.
Key learning points1. The concept of mixed-dimensional membranes (MDMs) and their application in molecular, ionic and oil/water separations.2. Common 2D nanomaterials and nanomaterials of different dimensions as the building blocks of MDMs. 3. Common methods for rational design and precise construction of MDMs. 4. Strategies to tune the physical and chemical microenvironments of the nanochannels as well as bulk structures in MDMs. 5. Relationships among synthetic chemistry, mixed-dimensional structure and separation performance of MDMs. |
1. Introduction
Membrane technology is playing an increasingly indispensable role in a broad range of applications to separate gas and liquid mixtures, affording sought-after outcomes without the drawbacks of many conventional technologies. These include continuous and automatic operation, high selectivity, easy scale up, low space requirement, as well as low consumption of chemicals and energy.1 Membrane technologies rely on membrane materials with selective permeability to achieve separation, purification, enrichment or concentration of components in feeds under a range of driving forces, including primarily chemical potential or concentration gradients, but also pressure, temperature, and electric potential gradients.2 Nevertheless, membranes often suffer from trade-offs between permeability and selectivity, permeability and mechanical strength, as well as permeability and antifouling properties.2,3 Hence, tailoring the structure of membrane materials, involving the physical/chemical microenvironments of the membrane surface, and of the pores or channels have attracted intense research focus in recent years.Owing to their extraordinary size-dependent properties, including high specific surface area, tunable pore or particle size and surface chemistry, nanomaterials have opened new avenues for fundamental research and industrial applications.4 Especially, in 2004, the first isolation of a single layer of graphene sparked a revolution in atomically thin materials, including a range of 2D nanomaterials, from graphene oxide to transition metal dichalcogenides (TMDs), MXenes, layered double hydroxides (LDHs), hexagonal boron nitride, zeolite nanosheets, metal–organic framework (MOF) nanosheets and covalent-organic framework (COF) nanosheets.5 The rapid development of 2D nanomaterials affords unlimited opportunities to design diverse mixed-dimensional nanomaterials, combining the distinct properties of nanomaterials with different dimensions and chemistries. Mixed-dimensional materials, also called mixed-dimensional van der Waals heterostructures in some areas – such as field-effect and logic devices, photodetectors, photovoltaics, light-emitting devices, and so on – can provide numerous approaches to implementing precise manipulation of carrier behaviour at the atomic interface, and exploiting unique devices with new functionalities.6 There are many excellent review papers about mixed-dimensional materials, particularly, mixed-dimensional materials based on 2D materials, which represent the most popular kind of mixed-dimensional materials, because any layered 2D material can be easily integrated with other materials by diverse interactions.7
Along with the booming development of mixed-dimensional materials, mixed-dimensional membranes (MDMs), also called heterostructure or heterojunction membranes, are attracting increasing research attention. They are composed of nanomaterials of different dimensions as well as chemistries, and combine the features of nanomaterials to exhibit unique composite structures with high controllability for selective mass transport. According to the combination of the dimensions of nanomaterials, MDMs can be divided into 0D/1D, 0D/2D, 1D/2D, 1D/3D and 2D/3D membranes. Considering the length of this tutorial review and contemporary research topics, we will focus on MDMs which are based on 2D materials, namely 0D/2D, 1D/2D and 3D/2D membranes. Ultrathin 2D materials are rapidly emerging as versatile building blocks for the design and development of advanced membranes.1,8 Also, 2D materials can be integrated into an ultrathin lamellar membrane with controllable nanochannels, which refer to channels between the layers and allow the target molecules to quickly pass through, thus leading to relatively high permeance with a high rejection rate; taken together, such method has potential for large-scale membrane fabrication.9,10 When the nanosheets are stacked into a membrane, the interlayer interaction and the intercalated compounds can create interlayer channels with hierarchical structures and different chemical microenvironments for mass transport.11,12 Depending on the materials used to intercalate into the space between the nanosheets, the selective nanochannels adopt different topologies and chemistries, which vary from 1D nanochannels based on 1D/2D membranes, 2D nanochannels based on 0D/2D membranes and 3D nanochannels based on 3D/2D membranes. 1D nanochannels are constructed by intercalating 1D nanomaterials, in which one dimension is usually much longer than the other two dimensions, between adjacent nanosheets via propping up the nanosheets. 2D nanochannels are constructed by intercalating 0D nanomaterials of very small size between adjacent nanosheets via the relatively strong interactions between 0D nanomaterials and nanosheets to adhere the nanosheets. 3D nanochannels are constructed by intercalating 3D nanomaterials, which usually have complex structures, including nanochannels, embossments and valleys, between adjacent nanosheets.
Up to now, there are many excellent reviews about nanomaterials, especially 2D nanomaterials, focusing on the assembly of membranes and their applications in molecular and ionic separations. However, as far as we know, there is no review with the theme of MDMs. Besides, a number of recent breakthroughs have been achieved that deserve a systematic summary of MDMs. Mixed matrix membranes (MMMs) are the most popular type of membranes, formed by blending nanomaterials into a polymer matrix, in which the interfacial compatibility between nanomaterials and polymers is the key point for selective mass transport. In contrast with MMMs, MDMs are fabricated by intercalating nanomaterials between adjacent 2D materials. For MDMs, the construction and manipulation of nanochannels as well as bulk structures are the key point for selective mass transport. In MDMs, the physical microenvironments of nanochannels for mass transport and separation can be controllably adjusted through the type, size and amount of the intercalated nanomaterials, leading to precise size-based sieving, and the chemical microenvironments of nanochannels can be tuned by the chemical nature of the nanomaterials, resulting in a tunable interaction with the species to be separated. Also, the bulk structures of membranes, including thickness, charge, as well as surface morphology and wettability can be constructed with high precision. They show a clearer, insight-based and straightforward strategy to construct membrane structures from the angle of the dimensions of the building blocks, as well as the final structures for target separations, thus stimulating novel and in-depth understanding on membrane structure–function relationships, particularly when combined with theoretical studies. Simultaneously, the MDMs with different channel structures can offer unique separation performance, guiding the choice of membrane materials for target separations. Accordingly, this tutorial review focuses on the significant progress in chemistry and structure–property relationships of MDMs, including nanochannels and bulk structures in MDMs, as well as the importance of structural design on membrane performance (Fig. 1). We classify and introduce preparation methods for the different classes of MDMs. As the core part of this review, the controlled construction of nanochannels and bulk structures are thoroughly discussed, pertaining to 1D nanochannels based on 1D/2D membranes, 2D nanochannels based on 0D/2D membranes and 3D nanochannels based on 3D/2D membranes. Then, the mass transport mechanism of MDMs is briefly described for different applications. Finally, current challenges and future research directions of MDMs are analysed.
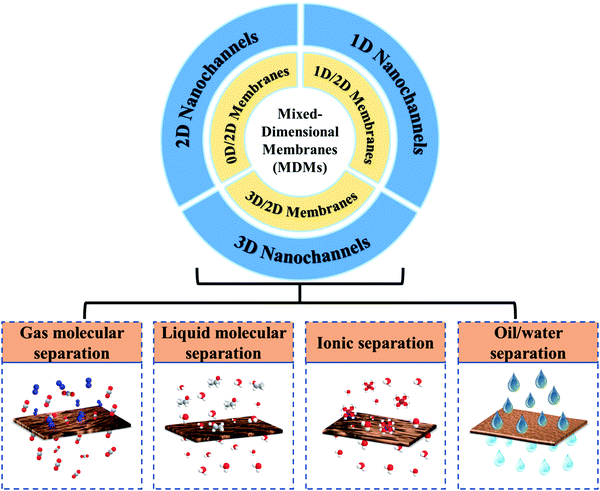 | ||
| Fig. 1 The scope of this review, including classification of MDMs, structures in MDMs and applications of MDMs. | ||
2. Classification and preparation methods of MDMs
Mixed-dimensional membrane is a new concept in the membrane field. Therefore, a classification of MDMs is presented first, based on 2D nanomaterials as important example. Meanwhile, high-performance MDMs call for high-quality nanomaterials and skilful methods to fabricate them for target separations. Being the major component, the defining parameters of the 2D materials, including their size, thickness, surface characteristics, and pores bear considerable influence on the ultimate membrane structure. There are comprehensive reviews on the synthesis of 2D materials, including top-down and bottom-up methods.13 Accordingly, in this section, the preparation methods of 2D nanomaterials are not covered, but we will introduce the classification of MDMs and their preparation methods, including coating, filtration and layer-by-layer assembly.2.1 Classification of MDMs
MDMs are fabricated from the combination of nanomaterials with different dimensions. When using 2D materials as the common constituent, MDMs can be divided into 0D/2D membranes, 1D/2D membranes and 3D/2D membranes, as shown in Fig. 2. 2D materials display unique features for the heterostructure design, arising from the interactions between 2D materials and other materials through covalent interactions, electrostatic interactions, hydration interactions, π–π interactions and van der Waals interactions based on their chemical characteristics.1,8 In consequence, it is possible to easily assemble 2D materials with other materials into functional membranes without sophisticated and sometimes impractical procedures. Also, 2D materials are recognized as promising candidates to fabricate high-performance membranes with superior permeability and selectivity by controlled construction of nanochannels, leading to low mass transport resistance and high selectivity.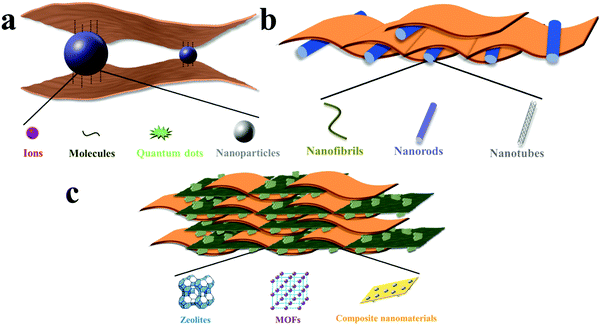 | ||
| Fig. 2 Schematic classification of MDMs: (a) 0D/2D membranes, (b) 1D/2D membranes, (c) 3D/2D membranes. | ||
As the most popular 2D materials, graphene-based 2D materials, including graphene, graphene oxide (GO) and reduced graphene oxide (rGO) have attracted most attention, due to their distinct advantages, such as high specific surface area and mechanical strength, excellent flexibility, super-low friction for water and CO2 transport, as well as progress toward large-scale production.10,14–16 Thus, they have been widely explored in membrane fabrication for various applications, such as water desalination, gas separation, fuel cells and oil/water separations.3,11,14 Graphene is a monolayer of graphite with a honeycomb-like lattice and carbon atoms that are sp2 hybridized, enabling both hydrophobic interactions and π–π interactions with other materials. Graphene can be prepared by bottom-up and top-down methods. Different from graphene, GO is usually fabricated by chemical oxidation and ultrasonic exfoliation of graphite. It is the oxidized state of graphene and has a high level of oxygen-containing groups, including hydroxyl, carboxyl and epoxy groups, which can impart covalent interactions, hydrogen bonding and complexation.
Meanwhile, some inorganic 2D materials like transition metal dichalcogenides (TMDs), MXenes and layered double hydroxides (LDHs) are usually more appropriate for membrane processes under harsh conditions, owing to their superior thermal and chemical stability.5,17–19 TMDs are obtained by establishing X–M–X structures, where the chalcogens in two hexagonal planes are separated by a plane formed by metal atoms. TMDs are usually fabricated by bottom-up chemical vapor deposition or top-down exfoliation. Because TMDs are nonporous materials, separation primarily occurs via the nanochannels between adjacent TMDs layers in TMD-assembled membranes. MXenes are a new class of 2D materials, containing transition metal carbides, nitrides and carbonitrides, which can only be obtained from the exfoliation of their bulk phases. After exfoliation, the surface of MXene nanosheets are covered with OH, F and O, which enable van der Waals and hydrogen bonds between the MXene surface and other materials. LDHs are typically ionic lamellar compounds that are composed of positively charged brucite-like layers, negatively charged compensating ions and solvated molecules. LDH nanosheets can be fabricated by either top-down delamination or bottom-up controlled nucleation. Moreover, some 2D materials of intrinsic nanoporosities, such as zeolite nanosheets, MOF nanosheets and COF nanosheets have emerged in recent years and increasingly utilized for membrane separations, especially for precise molecular and ionic separations, profiting from the tunability of the nanopores in those nanosheets.20,21
The nanopores in 2D materials make the structure of MDMs more complicated. Hence, in this review, we will not discuss the influence of nanopores in 2D materials on the construction of nanochannels. Materials with other dimensions, namely 0D materials (such as ions, molecules, quantum dots and nanoparticles), 1D materials (such as nanofibrils, nanorods and nanotubes) and 3D materials (such as zeolites, metal–organic frameworks and some composite nanomaterials) can be incorporated with 2D materials for controlled construction of nanochannels and bulk structures for a variety of applications.3,11,22 It should be also noted that blending 2D materials into a polymer matrix is not covered in this review.
2.2 Preparation methods of MDMs
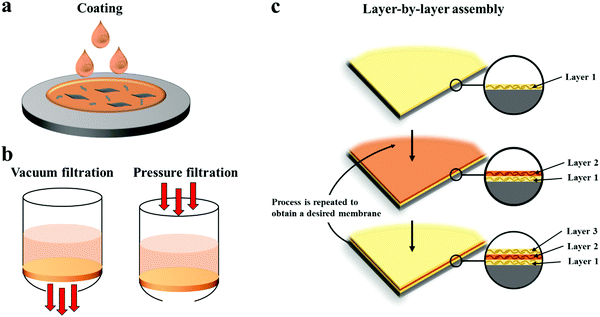 | ||
| Fig. 3 Representative preparation methods of MDMs: (a) coating, (b) vacuum/pressure filtration and (c) layer-by-layer assembly. | ||
Drop coating, as the simplest coating method, is perfectly suitable for 2D materials-based membranes; for example, GO-based membranes with cation-based control of the interlayer spacing for precise ionic separation were fabricated by drop coating.11 Spin coating is usually conducted by casting the solution onto a spinning substrate, or casting the solution on a stationary substrate and then spinning, normally leading to a thin and smooth film, which more easily produces a well-interlocked, organized lamellar structure, due to the centrifugal and shear forces during the spinning process.23 Spray coating produces homogenous membranes by spraying an aerosolized solution onto a rotating substrate; it has been applied in large scale applications, and, compared with other coating methods, spray coating can also be utilized to coat tubular membranes.8 Casting is a popular method to fabricate polymer membranes and has been used to fabricate highly ordered, uniform and continuous lamellar membranes at large scale, because the shear force generated by the transverse motion of the doctor blade favours alignment of 2D materials.24
3. Controlled construction of nanochannels and bulk structures
Both the nanochannels and bulk structures of the membrane influence the membrane performance. These structures can be tuned through the physical and chemical characteristics of the membrane forming materials. The characteristics of nanomaterials, including size, type and wettability, exert physical control over the nanochannels and bulk structures. Similarly, the characteristics of nanomaterials, including, the type, amount and distribution of functional groups on nanomaterials, impact the chemical properties of the ultimate structures. Laminated 2D material-based membranes assembled from micrometer-sized 2D materials have excellent features, including tunable chemical characteristics, high mechanical strength and good flexibility, which make 2D materials the superior candidate for filtration and sieving applications. In addition, the interlayer nanochannels in a lamellar membrane, formed by the stacking of 2D materials, play a crucial role in separations, especially arising from the size and chemical functionalities of these channels.In MDMs, the physical and chemical microenvironments of the nanochannels and the bulk structures can dramatically affect the membrane performance, including permeability, selectivity, stability and antifouling properties. In the following section, the controlled construction of nanochannels and the bulk structures, as well as control strategies will be discussed, along with structure–performance relationships.
3.1 1D nanochannels based on 1D/2D membranes
1D nanochannels, acting as “transport tunnels”, are usually fabricated in 1D/2D membranes by intercalating 1D materials, such as nanofibrils, nanorods and nanotubes, between adjacent 2D material layers to prop up the 2D materials. The features of the 1D materials influence geometrical, physical and chemical microenvironments of the nanochannels and the bulk structures, including size (length and diameter), wettability (hydrophobicity or hydrophilicity) and chemical characteristics. Meanwhile, the functional groups on 1D materials can have a decisive effect on the interactions between 1D materials and 2D materials, leading to distinct formation mechanisms for nanochannels. The 1D nanochannels could be tuned by adjusting the size and amount of 1D materials, as well as the interactions between 1D and 2D materials, which also govern the long-term stability of the nanochannels. Meanwhile, according to the structure of 1D materials, especially their broad size range, 1D nanochannels can be constructed in a wide size range as well, leading to extensive applications, such as precise ionic and liquid molecular separations, as well as oil/water separations.When 1D materials are intercalated between adjacent 2D materials, a 1D nanochannel can form between the 1D materials and the 2D materials, as 1D materials can prop up 2D materials. Such 1D nanochannels tend to become narrower further away from the 1D materials, due to the flexibility of 2D materials (Fig. 4(a)). The geometrical characteristics of 1D materials, such as their diameter and length, determine the size and continuity of 1D nanochannels, respectively. The size of 1D nanochannels increases with the diameter of the 1D materials, and longer 1D materials are more likely to form continuous 1D nanochannels. Meanwhile, owing to the flexibility of biomaterials, nanofibrils may not be suitable to precisely control 1D nanochannels, because they can undergo a shape change during the membrane fabrication process. On the other hand, some of them, like cellulose and silk nanofibrils, as well as CNTs, can form flexural 1D nanochannels, according to the flexibility of the 1D materials.
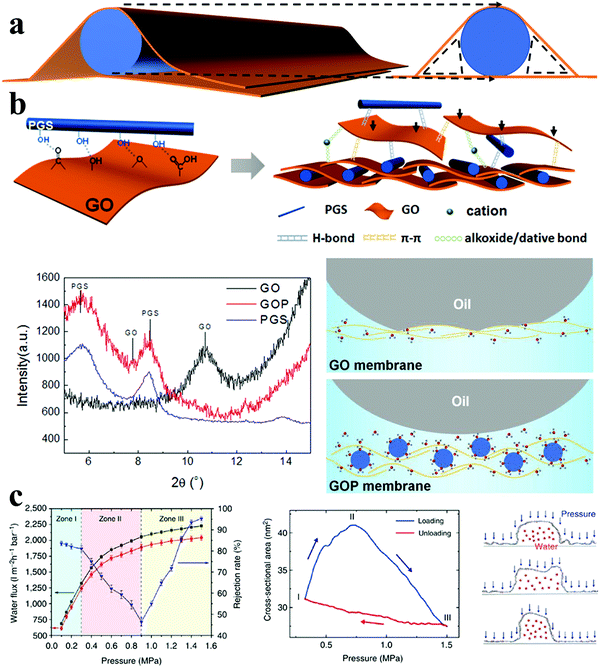 | ||
| Fig. 4 Microenvironments of 1D nanochannels and bulk structures in 1D (nanorods)/2D membranes. (a) Schematic diagram of 1D nanochannels in 1D/2D membranes. (b) Schematic diagram of the fabrication process of PGS/GO membranes and the interactions between 1D PGS and 2D GO (top), X-ray diffraction (XRD) patterns of GO membrane and PGS/GO membrane (denoted GOP) (left), schematic diagram of the oil/water/membrane interfaces of GO and PGS/GO membranes (denoted GOP) (right). Reproduced with permission from ref. 22. Copyright 2016, American Chemical Society. (c) Response of 1D nanochannels in nanostrand-channelled GO membranes to the applied pressure: pressure-dependent flux and rejection (left), the cross-sectional area of the 1D nanochannels with the change of applied pressure (middle), and the modelled response of half-cylindrical 1D nanochannels (right). Reproduced with permission from ref. 32 Copyright 2013, Springer Nature. | ||
In recent work by Jiang and collaborators, 1D nanochannels were created by intercalating 1D palygorskite (PGS) nanorods between adjacent 2D GO nanosheets, as shown in Fig. 4(b).22 Through hydrogen bond interactions between PGS nanorods and GO nanosheets, the microenvironments of nanochannels and bulk structures could be tailored, which endowed the resulting membranes with enhanced permeability and a hierarchical surface structure. By simply adjusting the mass ratio of PGS nanorods and GO nanosheets, the size of the nanochannels was increased from 0.82 nm to 1.13 nm. Moreover, the hierarchical structure of the membrane surface could be tuned, along with strong hydrophilicity, bonding water molecules to form a hydration layer, as well as strong superoleophobicity, with low oil adhesion under water. Consequently, through the synergistic control of the physical and chemical microenvironments of the nanochannels and membrane surface, a high-performance membrane with enhanced permeate flux and excellent antifouling property was fabricated for oil/water separation. In addition, molecular dynamics (MD) simulations can provide some theoretical insight into the structure of the 1D nanochannels in nanostrand-channelled GO membranes. When a water-filled GO nanochannel was loaded with pressure, its shape changed from round into a flattened rectangle. Then, as the pressure increased continuously, the flattened rectangle was compressed into round ripples (Fig. 4c). These simulation results qualitatively explain the abnormal rejection of Evans blue. Accordingly, the cross-sectional area morphology of the 1D nanochannels could be altered by the applied pressure, leading to controlled water permeability and rejection of dye.32
CNTs are often used to prepare graphene-based membranes, because of their excellent compatibility with graphene nanosheets. Single-walled carbon nanotubes (SWCNTs) with a bundle diameter of 5–20 nm were used to build 1D nanochannels by intercalating SWCNTs between adjacent GO nanosheets (Fig. 5(a)).33 1D nanochannels in SWCNT-intercalated GO membrane could increase the transport of water molecules, without sacrificing the rejection of molecules larger than 1.8 nm. They exhibited excellent pH-stability in wastewater treatment under extreme pH conditions. Furthermore, acid treated multi-walled carbon nanotubes (MWCNTs) with a diameter of 50 nm were intercalated between adjacent rGO nanosheets to prepare a nanofiltration membrane with high water flux and dye rejection, good rejection for salt ions, as well as antifouling properties (Fig. 5(b)).31 The uniform dispersion of MWCNTs between rGO nanosheets resulted in a larger number of 1D nanochannels for fast water transport without sacrificing the rejection of dye molecules. MWCNTs acted as a space holder through the interactions between MWCNTs and rGO nanosheets based on the chemical characteristics of MWCNTs, including hydrogen bond interactions and π–π interactions to avoid the shrinkage of 1D nanochannels at high driving pressure or high ionic strength. The negative charges on the membrane coming from the carboxyl groups on the rGO nanosheets could repel anions according to Donnan's exclusion theory. At the same time, cations could also be retained for reasons of electroneutrality, leading to high rejection for multivalent anion and monovalent cation salts. To avoid swelling, arising from the hydrophilicity of some 2D materials like MXenes, MWCNTs were utilized to intercalate between adjacent MXene nanosheets to countervail the close-fitting of MXene nanosheets by the strong π–π interactions and van der Waals forces.34 Accompanied by post-treatment, the membranes possessed enhanced interlayer spacing (from 13.9 Å to 15.1 Å), resisting swelling and pressure (Fig. 5c). Hence, intercalating CNTs between adjacent layers of 2D materials could synergistically affect the physical and chemical microenvironments of the nanochannels, as well as the bulk structures, leading to high-performance membranes.
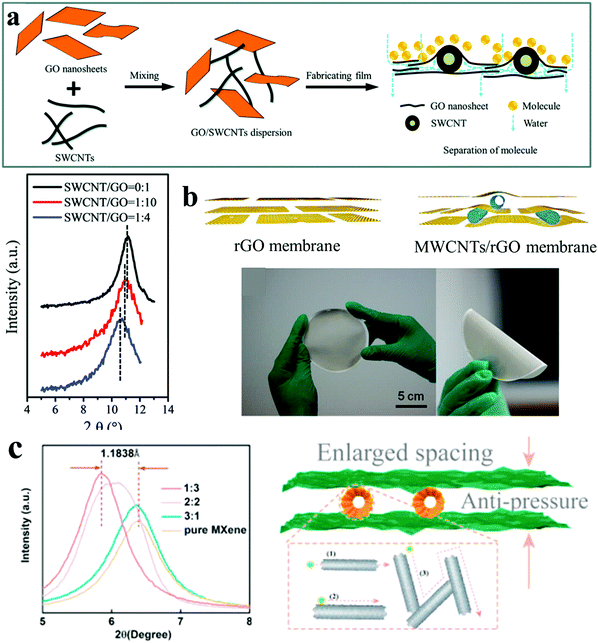 | ||
Fig. 5 Microenvironments of 1D nanochannels and bulk structures in 1D (CNT)/2D membranes. (a) Schematic diagram showing the fabrication process of SWCNT-intercalated GO ultrathin films and the process of the separation of other molecules from water (top), and XRD patterns of SWCNT/GO membranes. The d-spacings are 0.789 nm for the pure GO film, 0.807 nm for a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 0.823 nm for a mass ratio of 1 10, and 0.823 nm for a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (bottom). Reproduced with permission from ref. 33. Copyright 2015, Royal Society of Chemistry. (b) Schematic diagram of the structures of rGO and MWCNTs/rGO membrane (top), digital photos of a piece of MWCNTs/rGO membrane with a diameter of 100 mm (bottom). Reproduced with permission from ref. 31. Copyright 2015, American Chemical Society. (c) Interlayer spacing of MWCNTs/MXene membranes (left), and schematic diagram of transport mechanisms in MWCNTs/MXene membranes (right). Reproduced with permission from ref. 34. Copyright 2021, Elsevier. 4 (bottom). Reproduced with permission from ref. 33. Copyright 2015, Royal Society of Chemistry. (b) Schematic diagram of the structures of rGO and MWCNTs/rGO membrane (top), digital photos of a piece of MWCNTs/rGO membrane with a diameter of 100 mm (bottom). Reproduced with permission from ref. 31. Copyright 2015, American Chemical Society. (c) Interlayer spacing of MWCNTs/MXene membranes (left), and schematic diagram of transport mechanisms in MWCNTs/MXene membranes (right). Reproduced with permission from ref. 34. Copyright 2021, Elsevier. | ||
3.2 2D nanochannels based on 0D/2D membranes
2D nanochannels like the “archways in a bridge” are usually fabricated in 0D/2D membranes by intercalating 0D materials, such as ions, molecules, quantum dots and nanoparticles, between adjacent 2D materials to anchor the 2D materials. As with 1D materials, the features of 0D materials, including their size, type and chemical characteristics will influence the geometrical, physical and chemical microenvironments of the nanochannels and the bulk structures. Meanwhile, the interactions between 0D and 2D materials have a decisive effect on the formation mechanisms of nanochannels, which is determined by the chemical characteristics of the 0D materials. Thus, the 2D nanochannels could be tuned by the geometrical, physical and chemical characteristics of 0D materials, which determine the size, chemical microenvironment and stability of 2D nanochannels. Hence, the small size and precise size regulation of 0D nanomaterials can construct highly tunable 2D nanochannels, which is useful for precise separation requirements, in ionic, gas molecular and liquid molecular separations.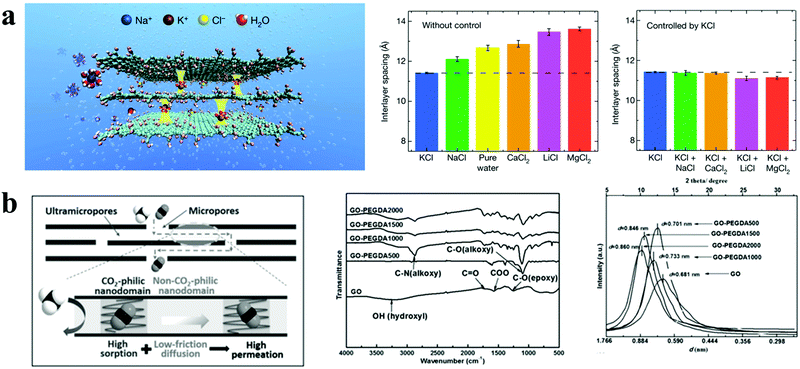 | ||
| Fig. 6 Microenvironments of 2D nanochannels and bulk structures in 0D (ions or molecules)/2D membranes. (a) Schematic of controlling the interlayer spacing of GO-based membrane using K+ ions to reject other ions (left), interlayer spacing of GO-based membranes immersed in various solutions (middle), and interlayer spacing of GO-based membranes that were soaked in KCl solution (0.25 M), followed by immersion in various salt solutions (right). Reproduced with permission from ref. 11. Copyright 2017, Springer Nature. (b) Schematic of gas molecular transport in 2D nanochannels with both CO2-philic and non-CO2-philic nanodomains (left), FTIR spectra of GO-based membranes (middle), and the interlayer spacing of GO-based membranes from XRD patterns (right). Reproduced with permission from ref. 35. Copyright 2017 John Wiley and Sons. | ||
In recent work by Jiang and collaborators, poly(ethylene glycol) diamines (PEGDA) with different chain lengths were used as the intercalated linker to tailor the physical and chemical microenvironments of the 2D nanochannels and bulk structures (Fig. 6(b)).35 Distinct CO2-philic and non-CO2-philic nanodomains resulted from the moderate CO2 affinity of PEG segments and the super-low friction between graphene nanosheets and CO2, respectively. More specifically, by modifying the GO epoxy groups with PEG units, CO2-philic nanodomains were constructed to accommodate higher CO2 molecular access in the 2D nanochannels, leading to high CO2 adsorption; the unreacted graphitic nanodomains with a function similar to frictionless carbon nanotube walls exhibited a nonpolar and non-CO2-philic function, leading to low-friction, fast diffusion of CO2. Meanwhile, the size of the 2D nanochannels could be controlled by changing the molecular weight of PEGDA, which afforded properly sized 2D nanochannels for gas transport and enabled precise size-sieving of gas molecules. Accordingly, the PEGDA intermediated GO membranes possessed high gas separation performance, including high CO2 permeance and CO2/CH4 selectivity.
Nanoparticles of size larger than simple ions and molecules can be used to create wider 2D nanochannels. A MXene membrane was fabricated by first intercalating colloidal Fe(OH)3 nanoparticles between adjacent MXene nanosheets and then removing the Fe(OH)3 nanoparticles to construct highly permeable 2D nanochannels (Fig. 7(a)).26 Through the electrostatic interaction between Fe(OH)3 nanoparticles and MXene nanosheets, the positively charged Fe(OH)3 nanoparticles with a diameter around 4–5 nm were bound to disperse well among the negatively charged MXene nanosheets. HCl was utilized to remove the intercalated Fe(OH)3 nanoparticles to form the 2D nanochannels with a size of 2–5 nm. The resulting MXene membrane exhibited excellent water permeance and a high rejection rate for molecules with sizes larger than 2.5 nm. Moreover, the functional nanoparticles could be intercalated between adjacent 2D materials to construct 2D nanochannels, thus endowing the membranes with additional functionalities. GO-based membranes with photocatalytic antifouling property were fabricated by incorporating TiO2 nanoparticles to treat dye solutions (Fig. 7(b)).38 TiO2 nanoparticles were utilized to decorate sporadically GO nanosheets to form GO-based composite sheets with low TiO2 loading, which could be assembled into membranes via filtration to construct 2D nanochannels, thus obtaining a desirable permeation flux and rejection rates. Then, additional TiO2 particles were coated onto the membrane surface to form a hierarchical filtration membrane, which could remove the intercepted dyes under UV light irradiation, affording excellent photocatalytic antifouling property.
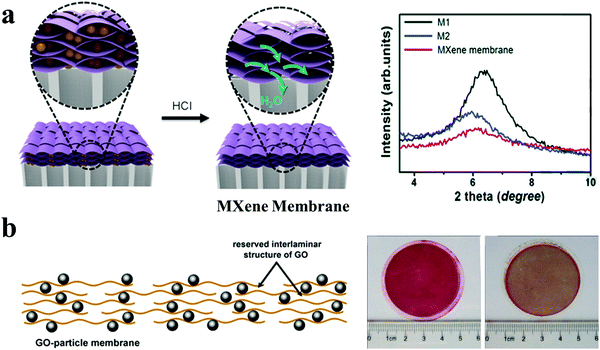 | ||
| Fig. 7 Microenvironments of 2D nanochannels and bulk structures in 0D (particles)/2D membranes. (a) Preparation method for a MXene membrane with an arched structure (left), and XRD patterns showing the enlarged interlayer spacing of the MXene-based membrane after intercalation (right). Reproduced with permission from ref. 26. Copyright 2017, John Wiley and Sons. (b) Schematic of TiO2/GO membranes (left), and photos of TiO2/GO membranes after filtration of a dye solution without (middle) and with (right) UV light irradiation. Reproduced with permission from ref. 38. Copyright 2014, American Chemical Society. | ||
3.3 3D nanochannels based on 3D/2D membranes
3D nanochannels like “transport networks” are usually fabricated in 3D/2D membranes by intercalating 3D nanoporous materials or 3D materials, such as MOFs, zeolites and some composite nanomaterials between adjacent 2D materials to form a pore network. The characteristics of the 3D materials influence the geometrical, physical and chemical microenvironments of the nanochannels and the bulk structures, through their size, pore structure and chemical characteristics; especially, the nanopores in the 3D materials can provide additional channels for mass transport. Meanwhile, the formation mechanism of the 3D nanochannels can be influenced by the chemical characteristics of the 3D materials, as they have a decisive effect on the interactions between them and the 2D materials, which also impact the long-term stability of the nanochannels. Moreover, based on the structure of the 3D nanomaterials, particularly, their own pore structure, 3D nanochannels can be constructed of different sizes, where, for example, small sizes are desired for gas molecular separation and large sizes for oil/water separation.Compared with graphene-based materials, which are typically made from graphite by a top-down method, MOFs are usually crystallized from small molecules by a bottom-up method, which offers excellent opportunities to construct 3D nanochannels with 2D materials, thus obtaining molecular sieving membranes with high performance.42 A MOF/graphene membrane for H2/CO2 separation was fabricated through in situ growth of ZIF-8 between rGO nanosheets using the polar oxygen groups on rGO nanosheets to construct 3D nanochannels; as a result, ZIF-8 exhibited a nanosheet structure as well (Fig. 8).39 The interlayer spacing of rGO nanosheets was bolstered up and anchored by MOFs to form 3D nanochannels that could be adjusted by the impregnation of MOFs with different chemical properties and pore size, thus elevating the membrane performance. Another kind of MOF (UIO-66-(COOH)2) with ball structure and high hydrophilicity was intercalated between adjacent GO nanosheets to construct 3D nanochannels, leading to membranes with a loose structure and excellent selective permeability for the dehydration of ethyl acetate (EA) aqueous solution.43 A suitable size of the MOF could avoid nonselective voids between MOFs and GO, and, at the same time, ensure the homogeneous dispersion of MOFs. Moreover, the hydrophilicity of MOFs was conducive to the construction of 3D nanochannels with high hydrophilicity, conferring fast water transport.
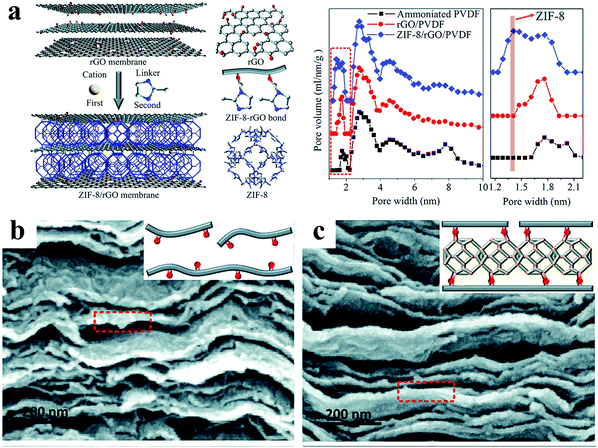 | ||
| Fig. 8 Microenvironments of 3D nanochannels and bulk structures in 3D(MOF)/2D membranes. (a) Schematic diagram of 3D nanochannels in ZIF-8/rGO membranes (left), pore size distributions of the ammoniated PVDF hollow fiber, rGO membrane and ZIF-8/rGO membrane (right). (b and c) Cross-sectional images of rGO and ZIF-8/rGO membranes. Reproduced with permission from ref. 39. Copyright 2016, Royal Society of Chemistry. | ||
Recently, Liu et al. fabricated several kinds of composite materials and intercalated them between adjacent 2D materials to construct 3D nanochannels for high-performance membranes. A photocatalytic 0D/2D heterojunction was synthesized by loading 0D TiO2 nanoparticles onto 2D graphitic carbon nitride (g-C3N4) nanosheets, followed by intercalating 3D composite nanomaterials between adjacent 2D GO nanosheets to fabricate a 3D/2D membrane for oil/water separation, as shown in Fig. 9(a).3 The intercalation of the composite materials could enlarge the interlayer spacing between GO nanosheets to construct 3D nanochannels; meanwhile, the hydrophilicity of the composite materials contributed to regulating the microenvironments of the 3D nanochannels and the bulk structures, resulting in high water permeability. Moreover, the hydrogen bonds between the composite materials and GO nanosheets could firmly bind those materials to increase the stability of the membranes during their use in an aqueous environment. Furthermore, the photocatalytic property of this composite material endowed the membrane with excellent antifouling properties by degrading the oil adhering on the membrane surface under sunlight. In addition, another kind of composite material formed by vertically growing COF nanosheets on GO nanosheets was intercalated between adjacent GO nanosheets to construct wide channels of 42.2 nm for water transport (Fig. 9(b)).41 Similarly, the functionality and wettability of this composite material could render membrane elevated stability of the membrane in aqueous environment, higher hydrophilicity, as well as superior antifouling property during an oil/water separation process.
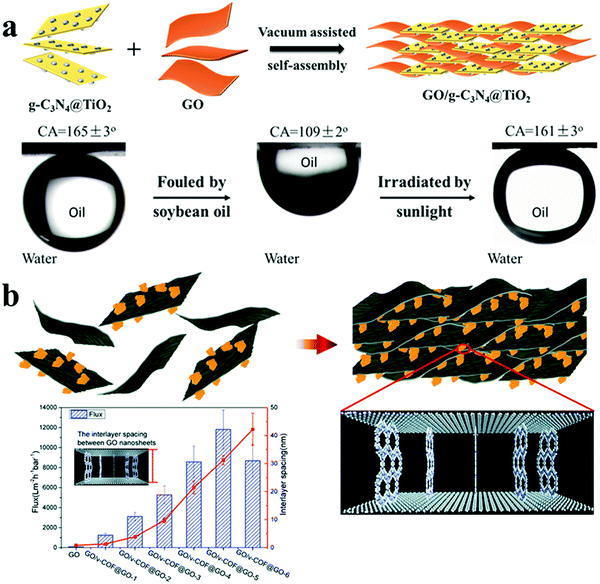 | ||
| Fig. 9 Microenvironments of 3D nanochannels and bulk structures in 3D(composites)/2D membranes. (a) Schematic of the preparation process of 3D nanochannels in g-C3N4@TiO2/GO membranes (top), the superoleophobicity underwater and photocatalytic degradation of GO-based membranes (down). Reproduced with permission from ref. 3. Copyright 2018, John Wiley and Sons. (b) The preparation process (top) and interlayer spacing (down) of v-COF@GO/GO membranes. Reproduced with permission from ref. 41. Copyright 2019, Royal Society of Chemistry. | ||
4. Applications
Due to their versatile, controllable structures, MDMs are drawing increasing attention for gas molecular separations, liquid molecular separations, ionic separations and oil/water separations. The microenvironment of the nanochannels plays a vital role in governing mass transport and separation of molecules and ions, and this environment can be particularly well controlled in MDMs, including channel size and chemistry, while maintaining a stable membrane structure during separation processes. Also, the surface architecture will influence the interactions between the membrane and the species in the mixture, hereby influencing the separation performance and antifouling property. Hence, fine-tuning of the nanochannels and the bulk structures could produce the desired microenvironment to enhance a particular separation process.4.1 Gas molecular separations
Gas molecular separation processes are important in refineries and chemical industries, including CO2 capture, air separation and hydrogen purification. Different from conventional gas separation unit operations (e.g., adsorption processes and cryogenic distillation), membrane-based gas separations do not require a phase change, which makes them attractive.44 The ideal gas molecular separation membrane should possess high permeability and selectivity for target gas molecules. Although membrane technology has made great strides over the last four decades, commercially available membranes still suffer from the well-known trade-off between membrane permeability and selectivity. Accordingly, the goal of simultaneously achieving high separation permeability and excellent selectivity draws continuous attention from both academia and industry. For membrane material development, an important goal is to control the nanochannel architecture with high precision and broad range.MDMs exhibit great potential for gas separation, due to precisely controlling the geometrical, physical and chemical microenvironments of the nanochannels. Since the discovery of graphene, a lot of effort has been devoted to 2D nanochannels in MDMs for gas separations. The first research about 2D nanochannels in GO membranes for gas separations was reported by Park's group.23 Subsequently, the intercalation of nanomaterials of other dimensions between 2D materials has been the focus of a lot of research to improve the separation performance. Because the size of 0D materials can be precisely controlled, 0D/2D membranes possess the ability to include nanochannels with highly precise characteristics; especially, the functional groups on 0D materials offer strong interactions with 2D materials to improve the stability of the membranes and can control the chemical microenvironments of the 2D nanochannels. Some 3D materials with tunable channels can provide additional channels in 3D/2D membranes for transport and separation. Nevertheless, there is no report about 1D/2D membranes used for gas separation, the reason being the difficulty to control the 1D nanochannels to the required high precision.
Molecules with different functional groups could be used to control the physical and chemical microenvironments of 2D nanochannels in 0D/2D membranes. It has been demonstrated that borate could cross-link GO nanosheets to achieve an ultrathin membrane (<10 nm) to enhance CO2 permeability and CO2/CH4 selectivity from 105 GPU and 16 to 650 GPU and 75, respectively (Fig. 10(a)).12 Then, piperazine, a brush-like CO2-philic agent, was introduced into 2D nanochannels through a simple coating process to achieve highly efficient CO2/N2 separation under wet conditions.45 The sub-20 nm thick layered GO-based hollow fiber membrane exhibited excellent separation performance with a CO2 permeance of 1020 GPU and a CO2/N2 selectivity as high as 680, thus showing its potential for CO2 capture from flue gas. Similarly, poly(ethylene glycol)diamines (PEGDA) with a CO2-philic segment of PEO were utilized to construct CO2-philic nanodomains, thus realizing a high adsorption capacity.35 Combined with the non-CO2-philic nanodomains formed from the low-friction with CO2 of unreacted GO surface, the membrane showed a high CO2 permeance of 175.5 GPU and a CO2/CH4 selectivity of 69.5 under dry conditions.
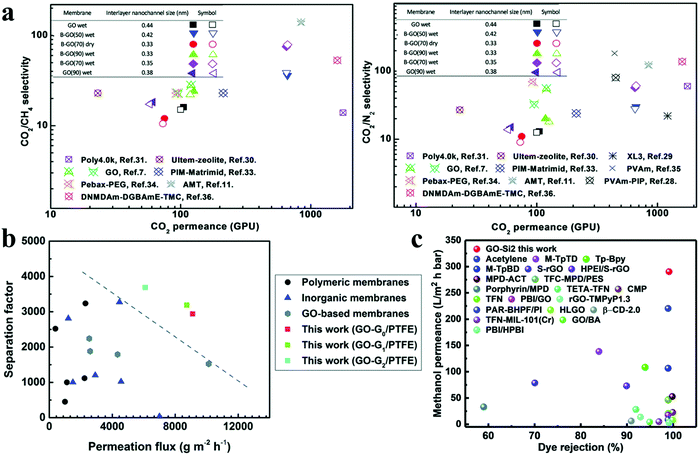 | ||
| Fig. 10 Separation performance of MDMs for gas and liquid molecular separations. (a) Comparison of borate/GO membranes (denoted B-GO) with state-of-the-art membranes for CO2/CH4 (left) and CO2/N2 (right) separation. Reproduced with permission from ref. 12. Copyright 2016, Royal Society of Chemistry. (b) Comparison of the butanol dehydration performance of PAMAM/GO membranes (denoted GO-G/PTTFE) including the water permeation flux and separation factor of butanol. Reproduced with permission from ref. 46. Copyright 2019, Royal Society of Chemistry. (c) Performance comparison of the SiO2/GO membrane (red dot: GO-Si2) with other membranes for dye and methanol separation. Reproduced with permission from ref. 47. Copyright 2019, Royal Society of Chemistry. | ||
MOFs with tunable pore size and channels for separation and transport have been assembled with graphene-based nanosheets by in situ crystallization of ZIF-8 between rGO nanosheets to synthesize 3D/2D membranes.39 The impregnation of ZIF-8 between layers of rGO could strongly anchor and bolster up rGO to form a porous bulk structure with uniform 3D nanochannels. Hence, the H2/CO2 selectivity of ZIF-8/rGO membranes reached about 26.4, and many other kinds of MOFs could be inserted between adjacent rGO nanosheets to achieve both high selectivity (>10.3) and superior permeability (in the range of 73–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112 Barrer).
112 Barrer).
4.2 Liquid molecular separations
Liquid molecular separations can be divided into liquid–liquid separations and liquid-molecular solute separations, which can be realized by a pervaporation membrane and by nanofiltration or organic solvent nanofiltration membranes, respectively. In a pervaporation process, a liquid mixture is placed in contact with one side of the membrane and a low-pressure vapor is formed as the permeate, which is removed from the other side of the membrane. In a liquid-molecular solute separation process, the solvent is usually recovered from the process streams through rejecting the molecular solutes based on sieving and electrostatic interactions. Compared with gas molecular separations, membranes play a more prominent role for liquid molecular separations in industry. However, the introduction of liquids could swell the membrane structure, and potentially deteriorate it. Accordingly, higher stability is required for liquid molecular separation membranes. Owing to the strong interactions between 0D materials and a 2D material, especially, especially where the functional groups on 0D materials react with the functional groups on 2D materials, 0D/2D membranes hold a dominant position in liquid molecular separation. However, to date, there are few 1D/2D membranes and no 3D/2D membranes used in liquid molecular separations, because of the limitation in required precision and stability of the channel size.Compared with distillation and adsorption, pervaporation appears as a significant alternative membrane separation process with the potential to separate liquid mixtures, because of the advantages of low energy consumption and easy operation. Especially, pervaporation membranes show potential in separating azeotropic mixtures, components with close boiling points, temperature-sensitive compounds and organic–organic mixtures. A range of molecules have been intercalated between adjacent 2D materials to improve the membrane performance and stability during liquid–liquid separation processes. Zhao et al. have used gelatin and GO to be alternately deposited on a membrane support through electrostatic attraction, hydrogen bonding, and hydrophobic interaction, leading to high performance for ethanol dehydration with a permeation flux of 2275 g m−2 h−1 and a water content in the permeate of 98.7 wt% at 350 K, for a water content in the feed of 20 wt%.28 Mimicking vein structures in nature, primary amine-terminated polyamidoamine (PAMAM) dendrimers with a medium molecular weight, possessing a regularly branched structure and multiple peripheral amine groups, were utilized in small quantities to tune the interlayer spacing in the range of 0.43–0.76 nm in the wet state.46 Accordingly, the resulting membranes exhibited a permeation flux of 9108 g m−2 h−1 and a separation factor of 2941 for butanol dehydration, which was far beyond the performance of state-of-the-art membrane counterparts (Fig. 10(b)). Moreover, thanks to crosslinking of supramolecular dendrimers, the membranes exhibited outstanding mechanical stability.
Liquid-molecular solute separation is one of the main applications of nanofiltration and organic solvent nanofiltration. An alternating dual-space channel GO-based membrane with locally tailored chemical microenvironments was fabricated by in situ intercalating and crosslinking scattered sub-5 nm silica nanoparticles between adjacent GO nanosheets. The intercalating nanoparticles widened the interlayer channels to enhance the permeance; simultaneously, the GO layers bended and the π–π interactions retained the narrow hydrophobic channels to improve the solute rejection. Hence, the resulting membranes showed methanol permeance of 290 L m−2 h−1 bar−1, with more than 90% rejection of dyes larger than 1.5 nm (Fig. 10(c)).47 Inspired by the structure of aquaporins for fast water transport and solutes separation, an octapeptide sequence RFRFRFRF (Arg–Phe–Arg–Phe–Arg–Phe–Arg–Phe), named RF8, was immobilized on the GO surface to produce a GO-RF8 membrane.48 Molecular dynamics simulations were utilized to reveal the unique configuration of RF8 peptides and the water transport in this membrane, demonstrating the core function of RF8 in aquaporins, and manifesting that this membrane possessed wide potential for liquid-molecular solutes separation.
4.3 Ionic separations
Currently, the leading technology for water desalination uses reverse osmosis (RO), which is considered to be the most promising membrane-based desalination process to meet the ever-increasing demand for fresh water. With the development of nanomaterials, especially the advent of 2D materials, an increasing range of RO membranes is synthesized for ionic separations. Especially, MDMs’ attraction is rising for selective water and ion transport, and this involves various effects and mechanisms. Owing to the high hydrophilicity of some 2D materials, the latter can attract water molecules into the interlayer space, which hydrates the 2D materials and increases the interlayer spacing; the resulting swelling of the 2D materials in aqueous environments is the main reason impairing the separation efficiency in ionic separations. One or several interactions can be involved to stabilize these 2D materials, forming MDMs by intercalating crosslinkers between adjacent layers, including chemical bonds, chelate bonds, π–π interactions, hydrogen bonds, and so on. In consequence, 0D/2D membranes are of interest for this application, due to the strong interactions between 0D and 2D materials. Meanwhile, some 1D materials possessing strong interactions with 2D materials can be used to fabricate 1D/2D membranes for ionic separations. However, 3D/2D membranes lose the potential for ionic separations, because the large size of 3D materials renders too large interlayer spacing between 2D materials.Until now, size exclusion and surface charge regulation, referring to the channel size and chemical environment of the membrane surface, respectively, have still been the main separation strategies. Size exclusion takes advantage of the size difference among the species in the mixture, when the nanochannels are wide enough to permit the target species to move through and small enough to block the unwanted species. For example, cations can be used to control the interlayer spacing of a GO membrane with Ångström precision, and interlayer spacing controlled by one type of cations can efficiently and selectively exclude other cations that have larger hydrated volumes (Fig. 11(a)).11 Surface charge control depends on electrostatic interactions between the membrane and the species in the mixture to isolate certain ions from water and other ions. An electroconductive CNT/rGO membrane was fabricated as a filter membrane and an electrode for remarkable ionic separation by the enhanced Donnan effect from more capacitive counterions onto the membrane under an applied bias (Fig. 11(b)).49
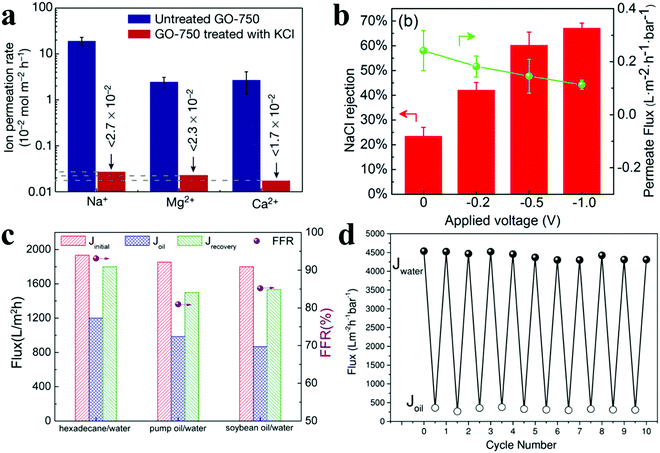 | ||
| Fig. 11 Separation performance of MDMs for ionic and oil/water separations. (a) Ion permeation rate of ions/GO membrane. Reproduced with permission from ref. 11. Copyright 2017, Springer Nature. (b) Permeation fluxes and NaCl rejection using CNT/rGO membranes in desalination. Reproduced with permission from ref. 49. Copyright 2018, Royal Society of Chemistry. (c) Permeation fluxes and flux recovery ratios of GPS/GO membranes. Reproduced with permission from ref. 22. Copyright 2016, American Chemical Society. (d) Permeation fluxes of g-C3N4@TiO2/GO membranes for cyclic separation experiment. Reproduced with permission from ref. 3. Copyright 2018, John Wiley and Sons. | ||
4.4 Oil/water separations
Oil contamination is a main source of water pollution. It can be classified into floating oil, dispersed oil, emulsified oil and dissolved oil, and has become a major environmental concern in our daily life and a wide range of industries. Among them, the diameter of emulsified oil drops is normally less than 10 μm, and mostly ranging from 0.1–2 μm. Due to stabilization by surfactants, emulsified oil droplets cannot aggregate, making physical oily wastewater treatment technologies (e.g., air flotation, centrifugal, flocculation) impossible or impractical. Membrane separation technology, by sieving oil droplets through nanopores, is recognized as the most effective and environmentally benign technology to cope with oily wastewater, and especially oil–water emulsions.The development of high-performance membranes for oil/water separations is confronted by two key problems: membrane fouling and low-permeation flux. For oil/water separation processes, the oil droplets always contact the membrane surface first; therefore, the complex interactions of oil droplets with the membrane surface, such as electrostatic interactions, hydrophobic interactions and van der Waals interactions, will result in flux decline and serious membrane fouling. Controlling the physical and chemical microenvironments of the membrane surface to weaken the interactions between the membrane surface and oil droplets is the key to reduce membrane fouling. Compared with other membrane separation processes, nanochannels in oil/water separation membranes are usually larger and there is a much less strict requirement to precisely regulate their size, because the droplets in oily wastewater are large as well, and their size distribution is wide. Coupled with the swelling of membranes in aqueous environment, a new issue about membrane stability appears in MDMs. Also, a large discharge of oily wastewater requires high-flux membranes to achieve a high treatment capacity, thus increasing the risk of serious membrane fouling. Hence, synergistically controlling the nanochannels in the membranes and the microenvironments on the membrane surface is essential to fabricate high-performance membranes with high permeate flux and excellent antifouling properties for oil/water separation. To achieve high permeability, a large interlayer spacing is needed, leading to the suitability of 1D/2D and 3D/2D membranes and the unsuitability of 0D/2D membranes for oil/water separation.
To improve membrane performance, a variety of materials have been used to fabricate MDMs. PGS nanorods with high hydrophilicity were used to intercalate GO nanosheets and endowed the resulting PGS/GO membrane with enhanced permeability and antifouling property; hence, the permeate fluxes increased from 267 L m−2 h−1 for a GO membrane to 1867 L m−2 h−1 for a PGS/GO membrane. By rationally imparting chemical and physical joint defense mechanisms, the PGS/GO membranes showed outstanding antifouling properties for various oil/water separations with different concentrations, pH, or oil species (Fig. 11(c)).22 When a 0D/2D heterojunction was intercalated into adjacent GO nanosheets to increase interlayer spacing, the initial permeate flux of the membrane reached 4536 L m−2 h−1 bar−1, which is more than 40-fold that of GO membranes (101 L m−2 h−1 bar−1), and, after being fouled by oil, a sunlight-driven self-cleaning process was achieved through the photocatalytic property of the heterojunction, maintaining a flux recovery ratio of 95% after ten cycles of filtration experiments (Fig. 11(d)).3
5. Conclusions and outlook
In summary, the blooming of nanoscience and technology has opened up a novel avenue to developing membranes from nanomaterials with different dimensions. Along with the exploitation of abundant synthesis methods, a number of nanomaterials with different structures, wettability and chemistry have been utilized to fabricate a variety of MDMs with well-ordered nanochannels and hierarchical structures for molecular, ionic and oil/water separations. Based on the diversity of nanomaterials and membrane fabrication methods, ideal architectures and favourable microenvironments have been created within the nanochannels and the bulk structures to endow MDMs with efficient mass transport and superior separation performance, as well as excellent antifouling properties in many cases. We envisage that the concept of MDMs can provide some new insights. By summarizing the existing research about graphene/GO-based membranes, we conclude that more efforts could be focused on incorporating other 2D materials into MDMs, and conclusions arising from graphene-based membrane in MDMs could also be applicable for MDMs based on other 2D materials. Meanwhile, studies involving other 2D materials could lead to a new breakthrough, like the ability to use them in harsh environment, due to the stability of these 2D materials. Table 1 summarizes the key features and challenges for MDMs. For gas molecular separation membranes, when there is a small difference in size between the molecules to be separated, the precise control of the nanochannels is key for membrane fabrication, hence, 0D materials of small size, such as molecules and ions, are frequently utilized. For liquid molecular separation and ionic separation membranes, liquid molecules inside nanochannels can increase the chance of membrane swelling; accordingly, some strong interactions like covalent interactions, chelate bonds and electrostatic interactions, are often chosen to crosslink the nanomaterials, thus enhancing membrane robustness and stability. For oil/water separation membranes, since the size of oil droplets in oil/water mixtures is very large, wide nanochannels are required for fast water transport; in addition, oil foulants contact the membrane surface first, so that the physical and chemical microenvironments of the membrane surface should be carefully designed to decrease the nonspecific interactions between oil foulants and membrane surface, achieving the desired antifouling property.| Membranes | 1D/2D membranes | 0D/2D membranes | 3D/2D membranes |
| Nanochannels | 1D nanochannels | 2D nanochannels | 3D nanochannels |
| Main materials intercalated between 2D materials | Nanofibrils, nanorods, nanotubes, etc. | Ions, molecules, quantum dots, nanoparticles, etc. | Zeolites, MOFs, composite nanomaterials, etc. |
| Interactions between intercalated materials and 2D materials | Covalent interactions, hydrogen bonds, π–π interactions, hydrophobic interactions, etc. | Covalent interactions, chelate bonds, electrostatic interactions, π–π interactions, hydrogen bonds, etc. | Covalent interactions, hydrogen bond interaction, hydrophobic interactions, etc. |
| Main applications | Liquid molecular separations, ionic separations and oil/water separation | Gas molecular separations, liquid molecular separations and ionic separations | Gas molecular separations and oil/water separation |
| Main challenges | Precise control and stability of nanochannels | Precise control and stability of nanochannels | Stability of membranes |
Despite significant progress during the past few years, MDMs are still in their infancy and almost no MDMs can be synthesized at large scale in an economically viable manner. In order to facilitate membrane research and development, several challenges from emerging materials should be addressed. Firstly, more kinds of nanomaterials with different dimensions should be employed in membrane fabrication. A huge number of nanomaterials have emerged, thanks to the fast development of modular chemistry and materials genomics; however, only few have been utilized to fabricate membranes. Secondly, research on MDMs is primarily focused on fundamental aspects and there is almost no report about MDMs in practical applications, because of the limited methods available for scalable, controlled synthesis of uniform and defect-free nanomaterials. Also, a facile approach to fabricate MDMs should be exploited to produce membranes at a large scale. Thirdly, efficient methods for regulating the geometrical, physical and chemical microenvironments of both nanochannels and bulk structures should be developed to elucidate and control the mass transport and separation mechanisms. A more uniform, well controlled and high-efficiency nanochannel in MDMs is beneficial to unravel the mass transport mechanism, which underpins membrane performance. For the same reasons, more efforts should be devoted to simulation methods, in order to acquire theoretical understanding about how the chemical and physical microenvironments affect the mass transport behaviour in nanochannels,50 which will guide the design and fine-tuning of membrane structures. Finally, based on the development of nanomaterials and membrane fabrication methods, the efficient control of nanochannels and bulk structures, as well as in-depth exploitation of the relevant mass transport mechanisms, MDMs with higher separation performance can be expected and utilized in a broader range of applications. Through the joint efforts from chemistry, nanoscience and technology, and chemical engineering, MDMs hold great promise in breaking through the bottleneck of conventional membranes and become an attractive candidate for new-generation membranes, playing a more critical role in molecular and ionic separations, as well as oil/water separation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for financial support from the National Natural Science Foundation of China (21961142013, 91934302, 21621004, and 21490583), National Key R&D Program of China (2016YFB0600503), Natural Science Foundation of Tianjin City (18JCZDJC36900), and the EPSRC via “Frontier Engineering” and “Frontier Engineering: Progression” Awards (EP/K038656/1, EP/S03305X/1).Notes and references
- S. Kim, H. T. Wang and Y. M. Lee, Angew. Chem., Int. Ed., 2019, 58, 17512–17527 CrossRef CAS PubMed.
- H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356, eaab0530 CrossRef PubMed.
- Y. Liu, Y. Su, J. Guan, J. Cao, R. Zhang, M. He, K. Gao, L. Zhou and Z. Jiang, Adv. Funct. Mater., 2018, 28, 1706545 CrossRef.
- M. Huang, S. Li, Z. Zhang, X. Xiong, X. Li and Y. Wu, Nat. Nanotechnol., 2017, 12, 1148 CrossRef CAS PubMed.
- M. Liu, P. A. Gurr, Q. Fu, P. A. Webley and G. G. Qiao, J. Mater. Chem. A, 2018, 6, 23169–23196 RSC.
- R. Frisenda, A. J. Molina-Mendoza, T. Mueller, A. Castellanos-Gomez and H. S. van der Zant, Chem. Soc. Rev., 2018, 47, 3339–3358 RSC.
- D. Jariwala, T. J. Marks and M. C. Hersam, Nat. Mater., 2017, 16, 170–181 CrossRef CAS PubMed.
- J. Zhu, J. Hou, A. Uliana, Y. Zhang, M. Tian and B. Van der Bruggen, J. Mater. Chem. A, 2018, 6, 3773–3792 RSC.
- Y. Kang, Y. Xia, H. T. Wang and X. W. Zhang, Adv. Funct. Mater., 2019, 29, 1902014 CrossRef.
- C. Cheng, G. Jiang, C. J. Garvey, Y. Wang, G. P. Simon, J. Z. Liu and D. Li, Sci. Adv., 2016, 2, e1501272 CrossRef PubMed.
- L. Chen, G. S. Shi, J. Shen, B. Q. Peng, B. W. Zhang, Y. Z. Wang, F. G. Bian, J. J. Wang, D. Y. Li, Z. Qian, G. Xu, G. P. Liu, J. R. Zeng, L. J. Zhang, Y. Z. Yang, G. Q. Zhou, M. H. Wu, W. Q. Jin, J. Y. Li and H. P. Fang, Nature, 2017, 550, 415–418 CrossRef PubMed.
- S. Wang, Y. Wu, N. Zhang, G. He, Q. Xin, X. Wu, H. Wu, X. Cao, M. D. Guiver and Z. Jiang, Energy Environ. Sci., 2016, 9, 3107–3112 RSC.
- S. Wang, L. Yang, G. He, B. Shi, Y. Li, H. Wu, R. Zhang, S. Nunes and Z. Jiang, Chem. Soc. Rev., 2020, 49, 1071–1089 RSC.
- G. He, M. Xu, J. Zhao, S. Jiang, S. Wang, Z. Li, X. He, T. Huang, M. Cao and H. Wu, Adv. Mater., 2017, 29, 1605898 CrossRef PubMed.
- Q. Yang, Y. Su, C. Chi, C. Cherian, K. Huang, V. Kravets, F. Wang, J. Zhang, A. Pratt and A. Grigorenko, Nat. Mater., 2017, 16, 1198–1202 CrossRef CAS PubMed.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS PubMed.
- X. He, L. Cao, G. He, A. Zhao, X. Mao, T. Huang, Y. Li, H. Wu, J. Sun and Z. Jiang, J. Mater. Chem. A, 2018, 6, 10277–10285 RSC.
- H. Li, T. J. Ko, M. Lee, H. S. Chung, S. S. Han, K. H. Oh, A. Sadmani, H. Kang and Y. Jung, Nano Lett., 2019, 19, 5194–5204 CrossRef CAS PubMed.
- L. Ding, Y. Wei, L. Li, T. Zhang, H. Wang, J. Xue, L.-X. Ding, S. Wang, J. Caro and Y. Gogotsi, Nat. Commun., 2018, 9, 1–7 CrossRef PubMed.
- G. Li, K. Zhang and T. Tsuru, ACS Appl. Mater. Interfaces, 2017, 9, 8433–8436 CrossRef CAS PubMed.
- Y. Wang, L. Li, Y. Wei, J. Xue, H. Chen, L. Ding, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2017, 56, 8974–8980 CrossRef CAS PubMed.
- X. Zhao, Y. Su, Y. Liu, Y. Li and Z. Jiang, ACS Appl. Mater. Interfaces, 2016, 8, 8247–8256 CrossRef CAS PubMed.
- H. W. Kim, H. W. Yoon, S.-M. Yoon, B. M. Yoo, B. K. Ahn, Y. H. Cho, H. J. Shin, H. Yang, U. Paik, S. Kwon, J.-Y. Choi and H. B. Park, Science, 2013, 342, 91–95 CrossRef CAS PubMed.
- A. Akbari, P. Sheath, S. T. Martin, D. B. Shinde, M. Shaibani, P. C. Banerjee, R. Tkacz, D. Bhattacharyya and M. Majumder, Nat. Commun., 2016, 7, 1–12 Search PubMed.
- C.-H. Tsou, Q.-F. An, S.-C. Lo, M. De Guzman, W.-S. Hung, C.-C. Hu, K.-R. Lee and J.-Y. Lai, J. Membr. Sci., 2015, 477, 93–100 CrossRef CAS.
- L. Ding, Y. Wei, Y. Wang, H. Chen, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2017, 56, 1825–1829 CrossRef CAS PubMed.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- J. Zhao, Y. Zhu, F. Pan, G. He, C. Fang, K. Cao, R. Xing and Z. Jiang, J. Membr. Sci., 2015, 487, 162–172 CrossRef CAS.
- J. J. Richardson, M. Bjornmalm and F. Caruso, Science, 2015, 348, aaa2491 CrossRef PubMed.
- H. Yang, L. Yang, H. Wang, Z. Xu, Y. Zhao, Y. Luo, N. Nasir, Y. Song, H. Wu and F. Pan, Nat. Commun., 2019, 10, 1–10 CrossRef PubMed.
- Y. Han, Y. Jiang and C. Gao, ACS Appl. Mater. Interfaces, 2015, 7, 8147–8155 CrossRef CAS PubMed.
- H. Huang, Z. Song, N. Wei, L. Shi, Y. Mao, Y. Ying, L. Sun, Z. Xu and X. Peng, Nat. Commun., 2013, 4, 1–9 Search PubMed.
- S. J. Gao, H. Qin, P. Liu and J. Jin, J. Mater. Chem. A, 2015, 3, 6649–6654 RSC.
- D.-D. Shao, Q. Zhang, L. Wang, Z.-Y. Wang, Y.-X. Jing, X.-L. Cao, F. Zhang and S.-P. Sun, J. Membr. Sci., 2021, 623, 119033 CrossRef CAS.
- S. Wang, Y. Xie, G. He, Q. Xin, J. Zhang, L. Yang, Y. Li, H. Wu, Y. Zhang and M. D. Guiver, Angew. Chem., Int. Ed., 2017, 56, 14246–14251 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- B. Shi, H. Wu, J. Shen, L. Cao, X. He, Y. Ma, Y. Li, J. Li, M. Xu and X. Mao, ACS Nano, 2019, 13, 10366–10375 CrossRef CAS PubMed.
- C. Xu, Y. Xu and J. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 16117–16123 CrossRef CAS PubMed.
- W. Li, Y. Zhang, P. Su, Z. Xu, G. Zhang, C. Shen and Q. Meng, J. Mater. Chem. A, 2016, 4, 18747–18752 RSC.
- D. Kim, M. Y. Jeon, B. L. Stottrup and M. Tsapatsis, Angew. Chem., Int. Ed., 2018, 130, 489–494 CrossRef.
- Y. Liu, J. Guan, Y. Su, R. Zhang, J. Cao, M. He, J. Yuan, F. Wang, X. You and Z. Jiang, J. Mater. Chem. A, 2019, 7, 25458–25466 RSC.
- X. Ma, P. Kumar, N. Mittal, A. Khlyustova, P. Daoutidis, K. A. Mkhoyan and M. Tsapatsis, Science, 2018, 361, 1008–1011 CrossRef CAS PubMed.
- Y. Ying, D. Liu, W. Zhang, J. Ma, H. Huang, Q. Yang and C. Zhong, ACS Appl. Mater. Interfaces, 2017, 9, 1710–1718 CrossRef CAS PubMed.
- J. L. Humphrey, Separation process technology, McGraw-Hill, Canada, 1997 Search PubMed.
- F. Zhou, H. N. Tien, W. L. Xu, J.-T. Chen, Q. Liu, E. Hicks, M. Fathizadeh, S. Li and M. Yu, Nat. Commun., 2017, 8, 1–8 CrossRef CAS PubMed.
- Y. Song, R. Li, F. Pan, Z. He, H. Yang, Y. Li, L. Yang, M. Wang, H. Wang and Z. Jiang, J. Mater. Chem. A, 2019, 7, 18642–18652 RSC.
- S. Wang, D. Mahalingam, B. Sutisna and S. P. Nunes, J. Mater. Chem. A, 2019, 7, 11673–11682 RSC.
- C. S. Lee, M. k. Choi, Y. Y. Hwang, H. Kim, M. K. Kim and Y. J. Lee, Adv. Mater., 2018, 30, 1705944 CrossRef PubMed.
- C. Hu, Z. Liu, X. Lu, J. Sun, H. Liu and J. Qu, J. Mater. Chem. A, 2018, 6, 4737–4745 RSC.
- B. C. Bukowski, F. J. Keil, P. I. Ravikovitch, G. Sastre, R. Q. Snurr and M.-O. Coppens, Adsorption, 2021, 27, 1–78 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |