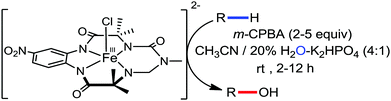
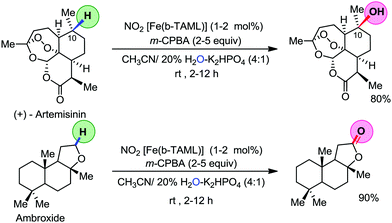
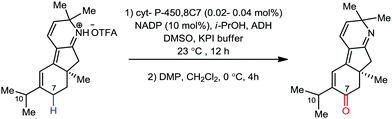
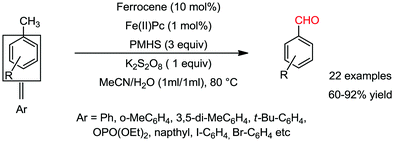
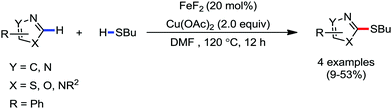
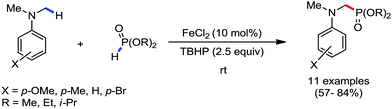
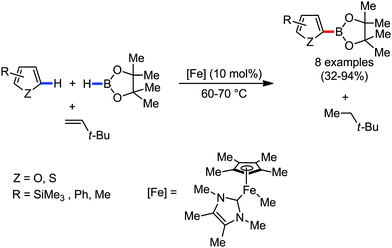
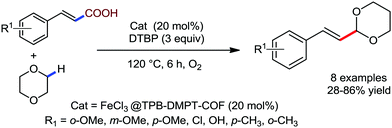
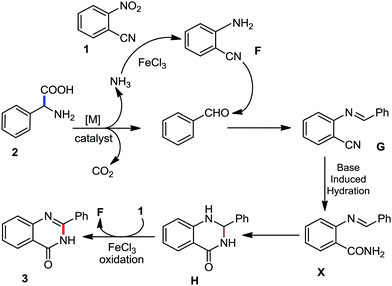
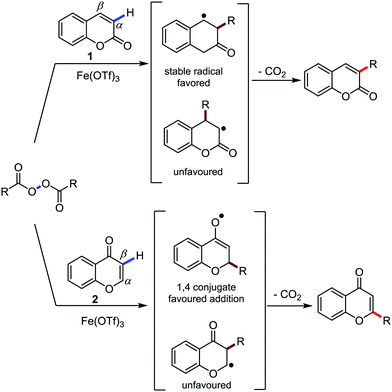
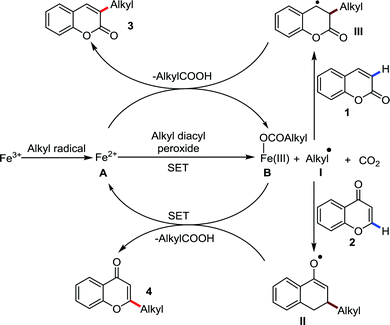
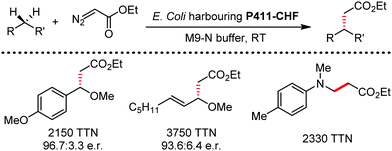
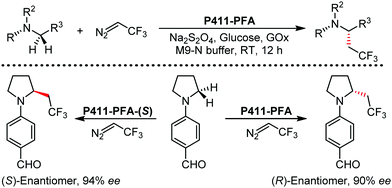
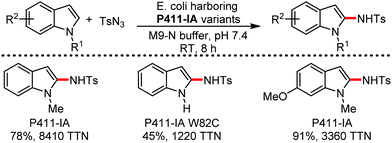
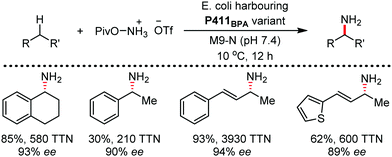
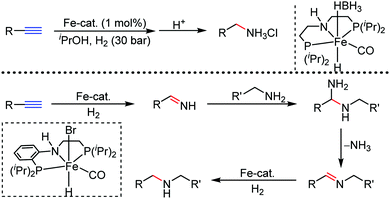
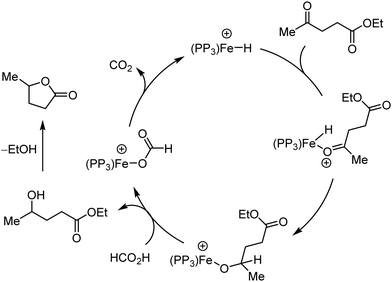
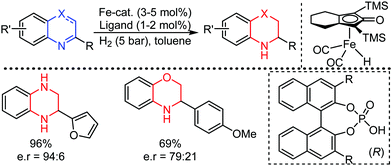
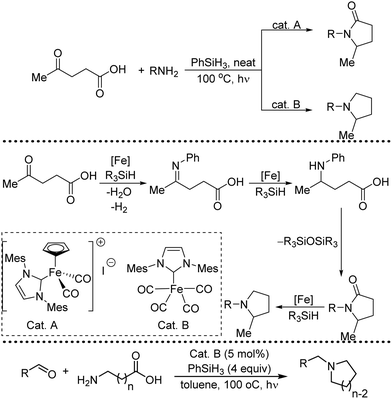
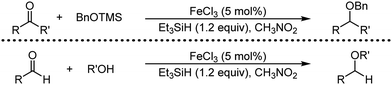
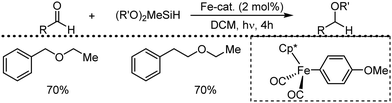
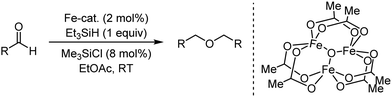
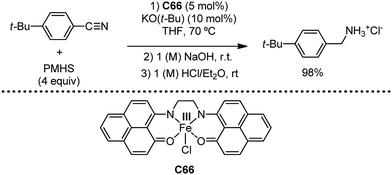
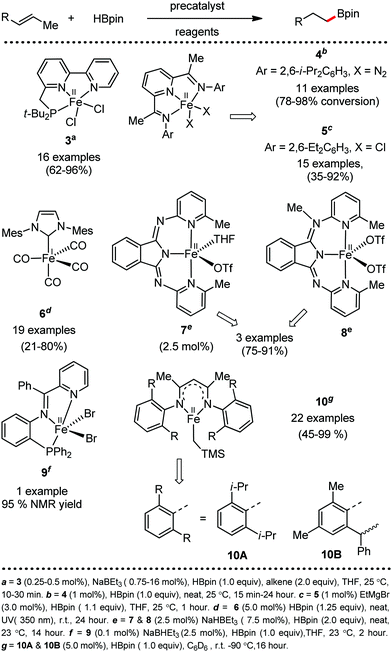
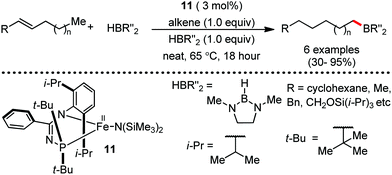
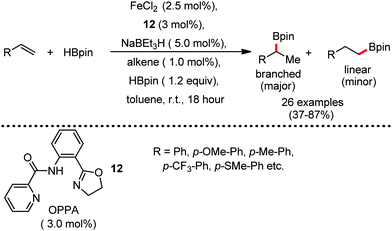
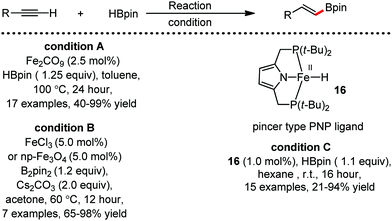
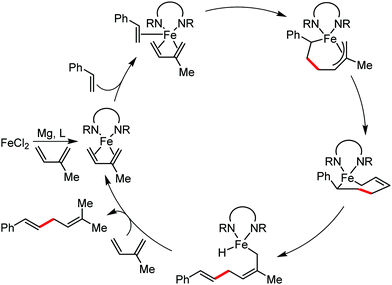
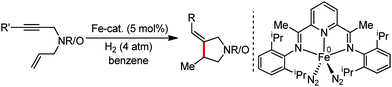
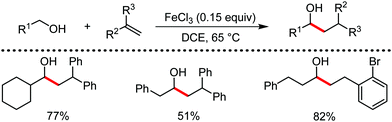
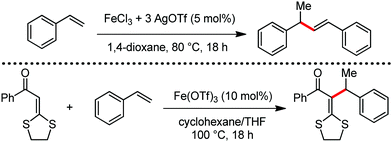
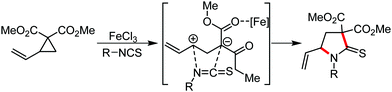
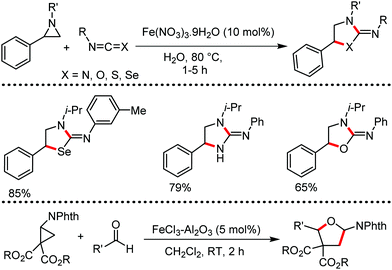
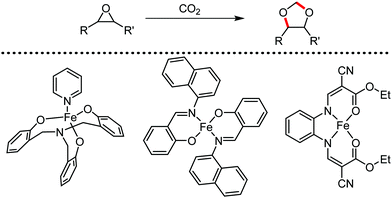
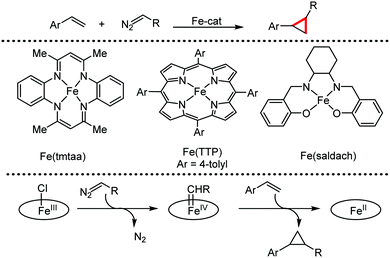
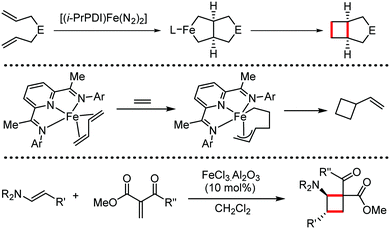
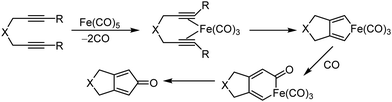
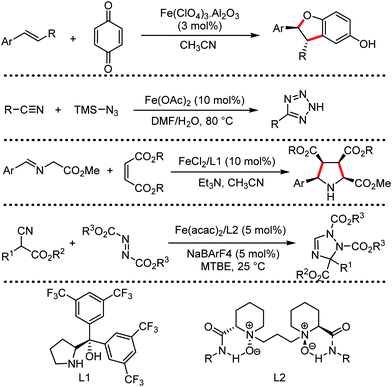
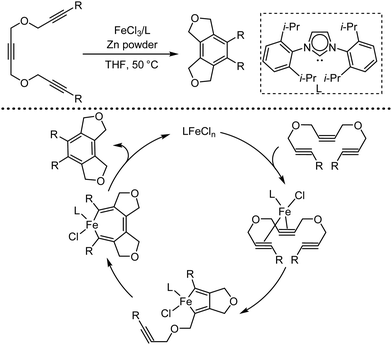
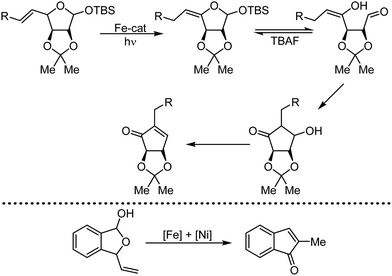
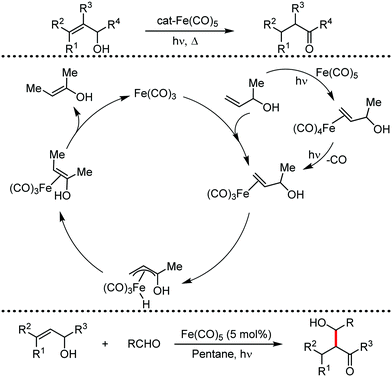
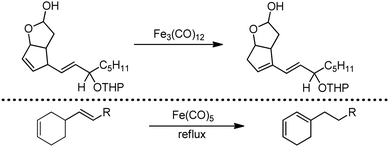
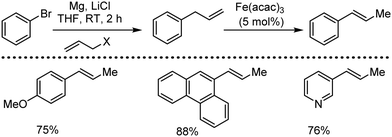
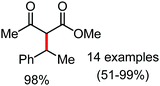
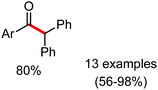
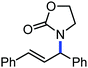
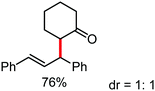
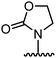
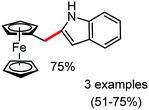
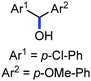
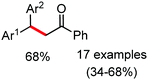
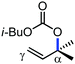
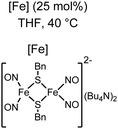
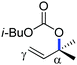
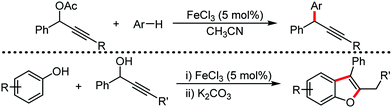
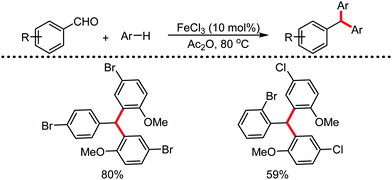
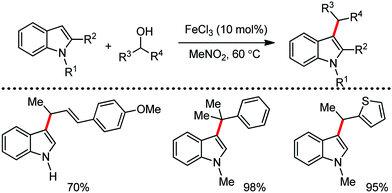
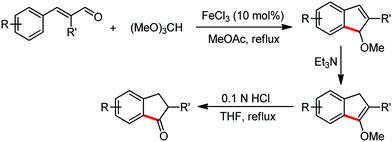
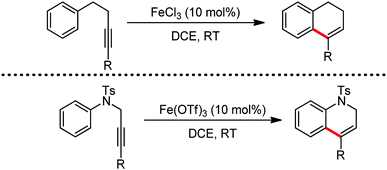
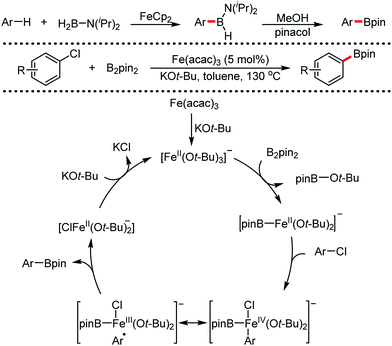
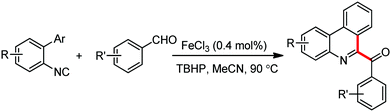
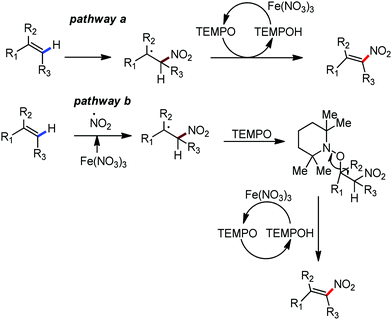
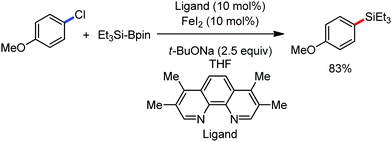
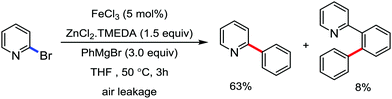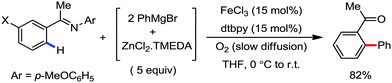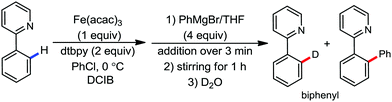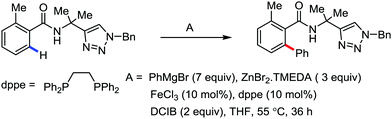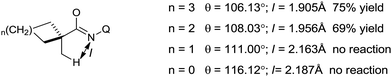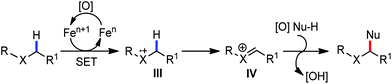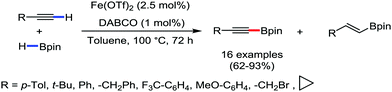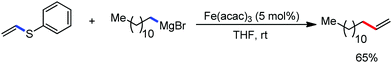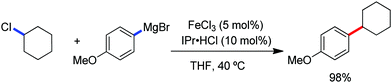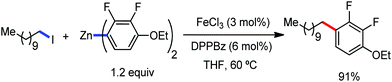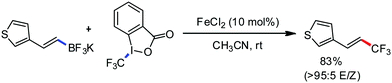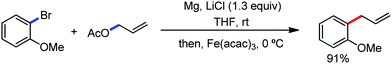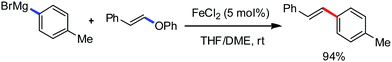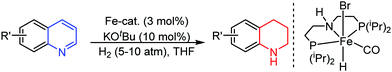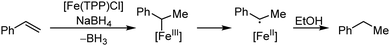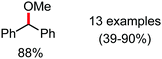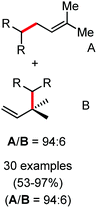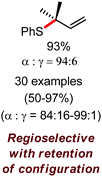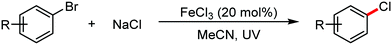Organic synthesis with the most abundant transition metal–iron: from rust to multitasking catalysts
Sujoy
Rana
 *a,
Jyoti Prasad
Biswas
b,
Sabarni
Paul
a,
Aniruddha
Paik
a and
Debabrata
Maiti
*a,
Jyoti Prasad
Biswas
b,
Sabarni
Paul
a,
Aniruddha
Paik
a and
Debabrata
Maiti
 *bc
*bc
aDepartment of Chemistry, University of North Bengal, Raja Rammohunpur, Darjeeling, West Bengal 734013, India. E-mail: sujoyran@gmail.com
bDepartment of Chemistry, IIT Bombay, Powai, Mumbai-400076, India. E-mail: dmaiti@chem.iitb.ac.in
cTokyo Tech World Research Hub Initiative (WRHI), Laboratory for Chemistry and Life Science, Tokyo Institute of Technology, Tokyo 152-8550, Japan
First published on 5th January 2021
Abstract
In industries and academic laboratories, several late transition metal-catalyzed prerequisite reactions are widely performed during single and multistep synthesis. However, besides the desired products, these reactions lead to the generation of numerous chemical waste materials, by-products, hazardous gases, and other poisonous materials, which are discarded in the environment. This is partly responsible for the creation of global warming, resulting in climate adversities. Thus, the development of environmentally benign, cheap, easily accessible, and earth-abundant metal catalysts is desirable to minimize these issues. Certainly, iron is one of the most important metals belonging to this family. The field of iron catalysis has been explored in the last two-three decades out of its rich chemistry depending on its oxidation states and ligand cooperation. Moreover, this field has been enriched by the promising development of iron-catalyzed reactions namely, C–H bond activation, including organometallic C–H activation and C–H functionalization via outer-sphere pathway, cross-dehydrogenative couplings, insertion reactions, cross-coupling reactions, hydrogenations including hydrogen borrowing reactions, hydrosilylation and hydroboration, addition reactions and substitution reactions. Thus, herein an inclusive overview of these reaction have been well documented.
1. Introduction
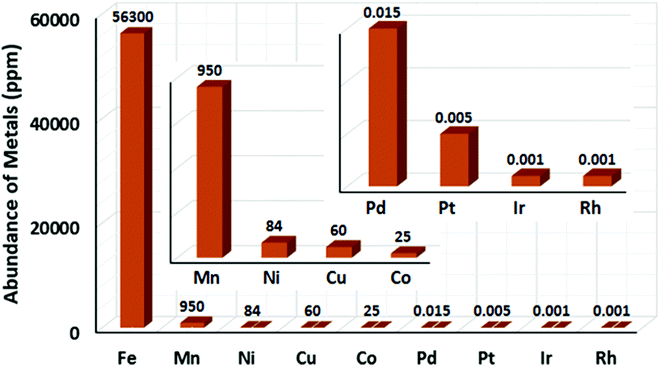
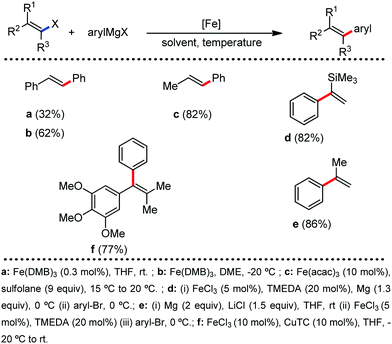
Catalysis is an important synthetic tool, which is widely used to accomplish the transformations required for the synthesis of drugs, natural products, and various fine chemicals. It enhances the atom economy of the reaction and reduces the energy consumption and cost of the production.1 A high percentage of catalysts used in organic synthesis comprises of transition metals (TMs) due to their variable oxidation states and co-ordination numbers.1a,2,3 Among the various TMs, the late transition metals (e.g. Ru,4 Pd,5 Rh,6 and Ir7) catalysts have been immensely used for the development of different synthetic methods since the 1960s. Especially, palladium catalysis has been extensively used in cross-coupling reactions for the formation of carbon–carbon and carbon–heteroatom bonds.8 Further, many C–H activation reactions, including both directed and non-directed reactions, are being pursued with the late TM catalysts of Ru, Pd, Rh, Ir, Ru, etc.9 However, the fundamental problem associated with late TM catalysts is their high price and toxicity to the environment.1b,10 The metal waste products from late TM catalysis contribute to the pollution in the Earth's crust and oceans. Therefore, chemists are concerned over the use of late transition metal catalysts in synthetic chemistry. Presently, many research groups focus on the development of alternative catalytic methods involving the Earth-abundant and less-toxic 3d metals for organic synthesis considering sustainable chemistry.1a–d,11,12 Contextually, the use of cheap, easily accessible, iron catalysts in organic synthesis can bring a renaissance in the field of catalysis. Notably, iron is the most abundant metal on the Earth's surface among the 3d, 4d and 5d metals (Fig. 1).1b,10c,13 Since the time of evolution, iron has become an indispensable part of different biological systems and metabolic processes. Similarly, it is found in numerous metalloenzymes and metalloproteins prevailing in nature and the human body.14 Thus, it is one of the most important congeners among the 3d metals. Moreover, if the cost of metallic iron is compared with the other late transition metals, it is found that iron is less expensive by several magnitudes (Fig. 2).10 Thus, the utilization of iron catalysts is certainly a sustainable approach.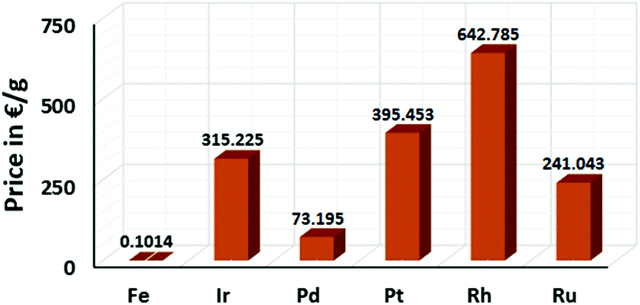 | ||
| Fig. 2 Price comparison among iron and other transition metals used in catalysis (Source: Sigma Aldrich). | ||
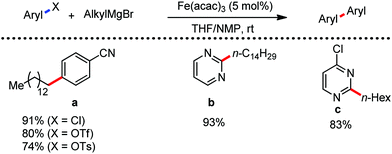
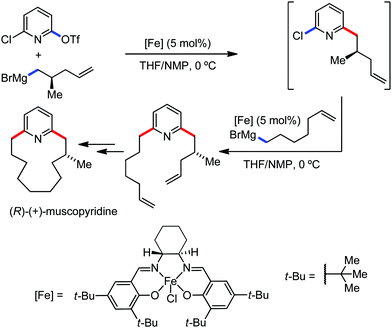
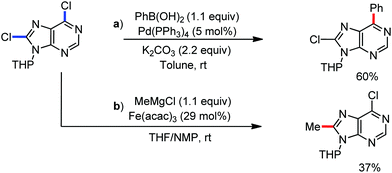
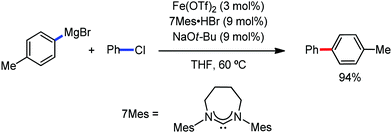
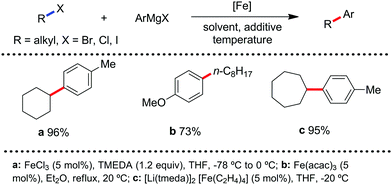
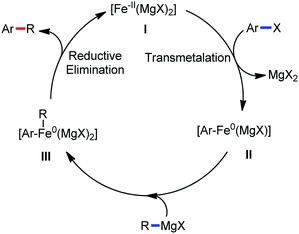
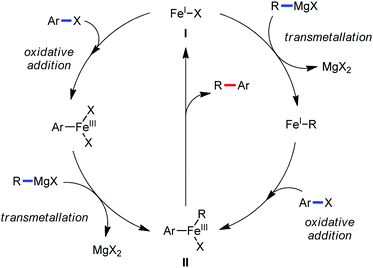
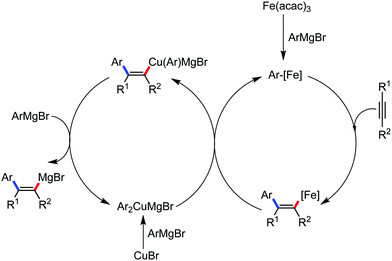
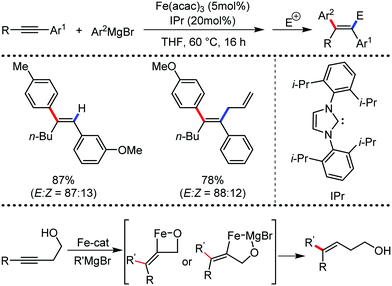
The use of iron salts as a precatalyst in synthetic chemistry was initiated a long time ago. In the 1940s, and 1970s, Kharash and Fields15 employed iron salts for the homocoupling of Grignard reagents, which was long before the discovery of the nickel16 and palladium17 catalyzed cross-coupling reactions in the late 1960s and early 1970s, respectively. Subsequently, in the 1970s, Kochi and Tamura utilized iron catalysts for carbon–carbon bond formation reactions.18 Unlike the late TMs, iron can have different oxidation states ranging from 2− to 6+. Hence, iron catalysis can be utilized to perform different types of reactions occurring via different elementary pathways by altering its oxidation states in the presence of suitable ligands.3k Evidently, it can participate in different reaction pathways such as single electron transfer (SET), oxidative addition (OA), transmetalation, reductive elimination, sigma bond metathesis, atom transfer reactions (ATR), hydrogen atom abstraction (HAA), and oxygen atom rebound (OAR). For example, high-valent iron intermediates with formal oxidation states of 4+ and 5+ can perform site selective C–H oxidation reactions involving the HAA and OAR pathways very well. On the other hand, iron intermediates with the 2−, 0, 1+, 2+ and 3+ oxidation states are responsible for C–C and C–X (X = N, O, S, etc.) bond formation reactions involving oxidative addition, transmetallation, reductive elimination, ATR, etc. Furthermore, iron-catalyzed C–H activation reactions occur with iron having the 2+ or 3+ oxidation state, involving the σ-bond metathesis pathway, transmetallation, and reductive elimination. Thus, many reactions are well performed using iron catalysts in the presence of different nitrogen and phosphorous ligands. Notably, the multitasking ability of iron in participating in these reactions arises from the metal–ligand cooperativity.
It is remarkable that in the last two-three decades iron catalysis has been extensively developed and is utilized in organic synthesis, which is evident from the publication of research articles, books and review articles.1–3,10 The recent discovery of iron-catalyzed distal C–H functionalization reactions has started a new dimension in iron catalysis. Furthermore, many current discoveries of iron-catalyzed C–H activation methods, cross-coupling reactions, cross-dehydrogenative coupling, C–H hydroxylation reactions, asymmetric transfer hydrogenations, etc. have initiated a new horizon in organic synthesis. Therefore, based on its rich chemistry, iron catalysis can possibly be utilized in every type of reaction executed by late and heavy transition metals. Notably, the iron-catalyzed reactions including C–H bond activations, C–H oxidations, C–H halogenations, insertion reactions, cross-dehydrogenative coupling (CDC), cross-coupling, reduction, addition, elimination, isomerization, and substitutions reactions are outlined chronologically together with detailed mechanistic discussions. Mainly, herein, we illustrate the iron-catalyzed reactions from the early stages to more recent developments.
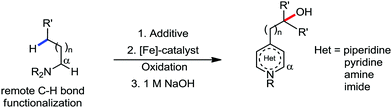
2. Iron-catalyzed C–H bond activation
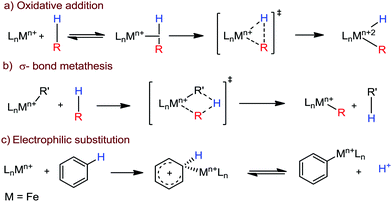
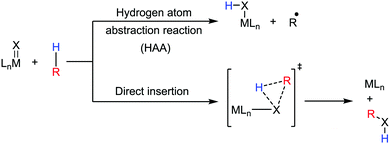
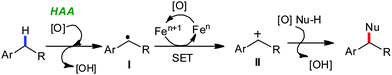
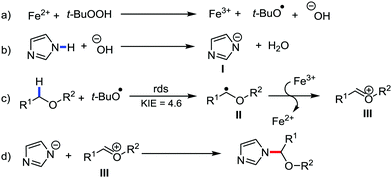
Transition metal-catalyzed C–H bond activation/functionalization reactions have emerged as a vital strategy in organic synthesis since the discovery of the first metal-mediated C–H bond activation with ruthenium in 1993.19 Thereafter, Pd, Rh and Ir-catalyzed C–H activation reactions have been explored to construct carbon–carbon and carbon–heteroatom bonds.4–9,20 Importantly, C–H bond activation is one of the sustainable approaches being utilized to diminish the number of steps required in multistep synthesis. Moreover, considering sustainable chemistry, the utilization of the earth abundant metal, iron-catalyzed C–H activation methods in synthetic chemistry is desirable. Contextually, herein, we depict the iron-catalyzed C–H bond activation methodologies in a chronological way together with a detailed mechanistic discussion.C–H bond activation can occur via two different elementary pathways, including organometallic C–H activations and outer sphere pathway-based C–H bond activation.21 These two approaches are discussed as follows: (i) organometallic C–H activation: the organometallic C–H activation proceeds via co-ordination of a C–H bond to the vacant site of a metal. Subsequently, it gets cleaved by different elementary pathways to create an organometallic intermediate. The elementary steps of organometallic C–H bond activation can be categorized into three types: (a) oxidative addition: the C–H bond adds to a low-valent iron complex for the formation a C–Fe–H species, and the oxidation state of the metal centre changes by two units (Scheme 1a). (b) σ-Bond metathesis: this particular elementary step occurs concertedly via a four-member transition state. The ligand/group (R′) of the iron-complex acts as a base to remove the hydrogen atom of the C–H bond, and concomitantly ligand exchange occurs, resulting in the formation of a C–H bond-activated organo-iron-complex. Here, the oxidation state of the metal centre does not change. This process is also called deprotonative metalation (Scheme 1b). The third elementary step is well known, (c) electrophilic substitution (Scheme 1c). The iron-catalyzed C–H activations through electrophilic substitutions are mainly discussed in the later part of this review (Section 10.3). (ii) Outer sphere pathway for C–H bond activation: outer sphere C–H bond activation generally proceeds through two different types of elementary pathways. The first is direct insertion of a C–H bond into the ligand sphere of a transition metal complex, where the ligand (group) of the catalyst recognises functionality in the substrate (Scheme 2) (mainly C–H bonds in the present discussion). On the other hand, the second pathway proceeds via hydrogen atom abstraction (HAA) from C–H bonds by high-valent metal species. We demonstrate these C–H functionalization reactions consecutively (Sections 2 and 3).
2.1 Organometallic C–H activation
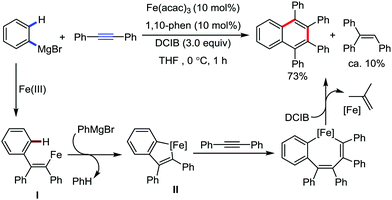
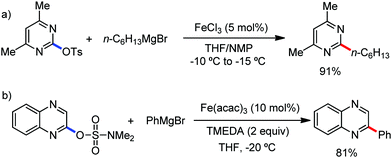
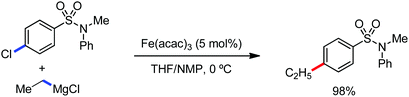
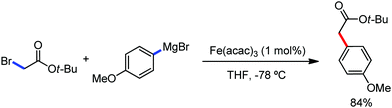
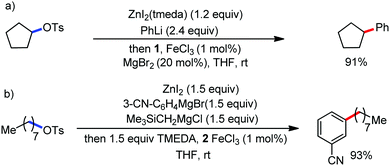
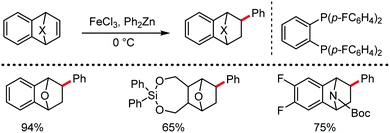
The fundamental investigation of iron-mediated C–H activation was started in 1968 by Hata et al.22 Subsequently, different research groups including Corriu et al.,23 Jesson et al.,24 Field et al.25 and Knochel et al.26 set up mechanistic foundations for iron-mediated C–H activation based on stoichiometric studies. In 1968, Hata et al. first reported C–H bond activation upon the irradiation of a 1,2-bis(diphenylphosphino)ethane-ligated complex, where the ortho-metalated iron(II)hydride complex was generated via the oxidative addition of an ortho C–H bond directly to the iron(0) complex.22 Later, in 2009, Camadalni and co-workers developed a method for C–H bond activation with an iron(II) complex via the σ-bond metathesis pathway.27 Notably, the iron-catalyzed C–H activation reactions have been explored with directing group and non-directing group-based approaches. In both strategies, C–H bonds act as hidden functional groups. In this subsection, we describe the C–H activation of different C–H bonds involving organoiron intermediates and classify them based on the elementary pathways such as the oxidative addition and σ-bond metathesis pathways.2.1.1.1 Iron-catalyzed arylation or heteroarylation of C(sp2)–H bonds using carbon nucleophiles. Organoiron complexes can activate various types of C–H bonds stoichiometrically. However, early attempts to develop iron-catalyzed C–H bond activation by Jones et al.28 have not been exploited for synthetic utility due to the low efficiency of the reactions. However, the serendipitous discovery by Nakamura and co-workers marked the beginning of C–H bond activation in 2008. In this reaction, they found an unexpected ternary coupling product in 8% yield during the optimization of the cross-coupling reaction between 2-bromopyridine and phenyl zinc reagent under an oxygen atmosphere (Scheme 3).29 The side product led to the discovery of a new research direction from the cross-coupling reaction to C–H activation. They tried to justify the reason for the formation of the side product and found that air leaked in the reaction flask. Thereafter, they discovered that dichloroisobutane (DCIB) should be used as oxidant for the formation of the desired C–H activation product and to reduce the formation of the diphenylated product (Scheme 4).29b In addition, the organozinc reagent (Ph2Zn) obtained from phenyl lithium or Mg-free organozinc reagent did not provide the desired product. Moreover, the steric effect of the substituents present in the aryl moiety of the organozinc reagent has an adverse effect on the formation of the reaction (2-tolylzinc reagent cannot deliver any product). Importantly, the reaction takes place at 0 °C, which is different from the other C–H bond activation reactions, requiring a temperature above 80 °C.30
Notably, benzoquinoline, 2-phenylpyridine, 2-phenylpyrimidine, and 4-phenylpyrimidine were well tolerated under the optimized condition of the reaction. Although, there was no solid evidence regarding the mechanistic pathway, they hypothesized that the 1,10-phenanthroline ligand and TMEDA coordinate with iron(III) and zinc, respectively, and it involves the redox cycle of iron in the presence of the oxidant DCIB. Thus, C–C bond formation via C–H bond activation by a new class of homogeneous iron catalysts has been well established.
Subsequently, in 2009, the same group developed a reaction methodology for ortho-C–H bond activation of aromatic imines using the aforementioned catalytic system, resulting in the formation of ortho-arylated ketones and aldehydes upon hydrolysis (Scheme 5).31 Interestingly, this iron(III)-containing catalytic system can perform ortho C–H bond activation with respect to the electrofugal leaving group, whereas with the same substrate, the para C–H bond is activated via the Pd-catalyzed Negishi coupling reaction. Notably, this reaction also occurs at a temperature of 0 °C and can tolerate a large number of reactive functional groups such as aryl bromides, chlorides, triflates, and sulphonates. Importantly, the mild oxidant DCIB is essential for the iron-catalyzed C–H arylation over C–X (X = Br and OTf) bond activation. Various aromatic ketimines and aromatic aldimines are also compatible in the reaction. Although the aromatic ketimines provided only mono-arylation products in presence of two accessible ortho C–H bonds, the aromatic aldimine resulted in the formation of a mixture of mono- and di-arylated products due to the higher conformational flexibility of the aldimines.
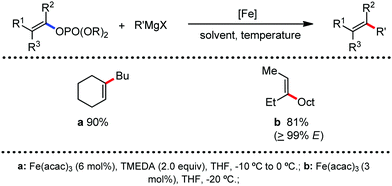
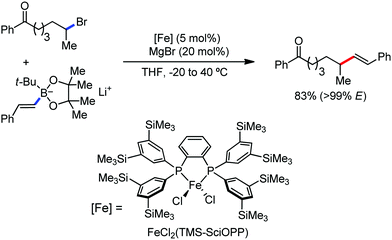
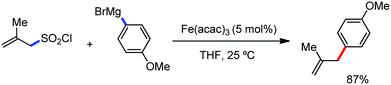
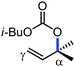
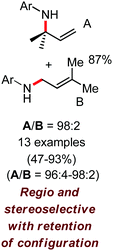
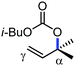
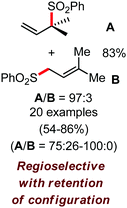
In 2010, they reported the iron(III)-catalyzed chelation-controlled arylation of olefins without the use of any external ligands.32 Importantly, this reaction methodology provides the substituted olefins with good regio- and stereoselectivity in high yield. Notably, this was the first application of oxidative Heck coupling in the presence of an iron catalyst using an organozinc reagent and Grignard reagent as the nucleophilic partner. In the same year, the same group achieved the arylation of imines and 2-phenyl pyridines under iron catalysis in the presence of molecular oxygen (Scheme 6) as the oxidant in place of DCIB.33 They introduced it in the reaction mixture through the process of slow diffusion. However, although molecular oxygen acts as a green and cost-effective oxidant, the presence of DCIB as an oxidant provides superior results.
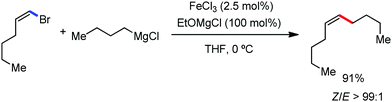
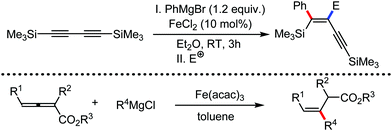
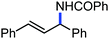
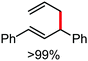
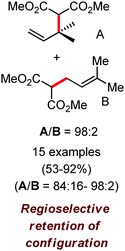
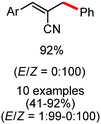
Further in 2011, they reported that cyclic and acyclic olefins having a directing group such as pyridine or imine provide only stereospecific arylation of the olefinic C–H bond to afford the syn-product with respect to the directing group.34 Moreover, they discovered that upon changing the aromatic co-solvent from tetrahydrofuran (THF) to chlorobenzene in Et2O, the ratio of the isomerization product of the starting material (Scheme 7) varied from 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (Z/E) to 97
5 (Z/E) to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Z/E). In addition, vinyl pyridine bearing the tert-butyl group at the 1 position undergoes the reaction to provide the exclusive formation of the Z-isomer (99% isolated yield) together with >99
3 (Z/E). In addition, vinyl pyridine bearing the tert-butyl group at the 1 position undergoes the reaction to provide the exclusive formation of the Z-isomer (99% isolated yield) together with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Z
1 ratio of Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E isomer. On the other hand, without the use of the oxidant, i.e. 1,2-dichloro-2-methyl propane, the olefin isomerisation product (E isomer) is obtained as the major product. Based on the two aforementioned facts, they hypothesized that the reduced iron species is involved in isomerization, which indeed established the role of the Grignard reagent as the reducing agent. Moreover, the aromatic cosolvent serves as a ligand for the low-valent iron species,35 which is formed during the course of the reaction and prevents the interaction with the olefin product to deliver the syn-product. Again, this low valent iron species is not the iron hydride because it did not give any hydrogenation product in the reaction (Scheme 7). Thus, they concluded that this reaction methodology (Scheme 7) is different from the previous one,32 which followed the oxidative Heck coupling reaction. In the oxidative Heck coupling, the generated five-membered metallacycle upon the addition of aryliron species to the olefinic bond undergoes β-hydride elimination and forms the desired anti-selective product (Scheme 8b) together with the formation of the reduced product of the starting material, which is indeed the major side reaction of the oxidative Heck coupling.32 On the other hand, for the reaction methodology (Scheme 7), the authors proposed the involvement of the five-member metallacycle, which undergoes reductive elimination upon interaction with the oxidant to provide the syn-selective product (Scheme 8a). Importantly, here, no reduced product of the starting material was obtained. It should be noted that the slow addition of the Grignard reagent is required to prevent the immediate iron-catalyzed homocoupling of PhMgBr and to increase the performance of the iron catalyst.36,37 The reaction was completed within 5 min of slow addition at a lower temperature (0 °C).
E isomer. On the other hand, without the use of the oxidant, i.e. 1,2-dichloro-2-methyl propane, the olefin isomerisation product (E isomer) is obtained as the major product. Based on the two aforementioned facts, they hypothesized that the reduced iron species is involved in isomerization, which indeed established the role of the Grignard reagent as the reducing agent. Moreover, the aromatic cosolvent serves as a ligand for the low-valent iron species,35 which is formed during the course of the reaction and prevents the interaction with the olefin product to deliver the syn-product. Again, this low valent iron species is not the iron hydride because it did not give any hydrogenation product in the reaction (Scheme 7). Thus, they concluded that this reaction methodology (Scheme 7) is different from the previous one,32 which followed the oxidative Heck coupling reaction. In the oxidative Heck coupling, the generated five-membered metallacycle upon the addition of aryliron species to the olefinic bond undergoes β-hydride elimination and forms the desired anti-selective product (Scheme 8b) together with the formation of the reduced product of the starting material, which is indeed the major side reaction of the oxidative Heck coupling.32 On the other hand, for the reaction methodology (Scheme 7), the authors proposed the involvement of the five-member metallacycle, which undergoes reductive elimination upon interaction with the oxidant to provide the syn-selective product (Scheme 8a). Importantly, here, no reduced product of the starting material was obtained. It should be noted that the slow addition of the Grignard reagent is required to prevent the immediate iron-catalyzed homocoupling of PhMgBr and to increase the performance of the iron catalyst.36,37 The reaction was completed within 5 min of slow addition at a lower temperature (0 °C).
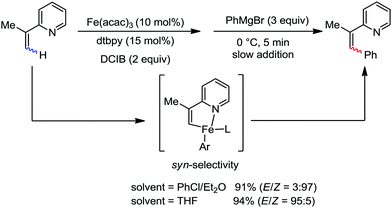 | ||
| Scheme 7 Iron-catalyzed stereospecific arylation of 1-substituted vinyl pyridine with Grignard reagents. | ||
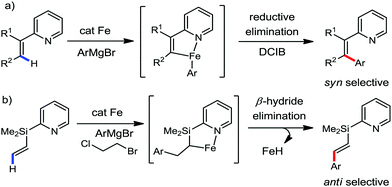 | ||
| Scheme 8 Iron-catalyzed C–H activation (a) during stereospecific syn-arylation and (b) oxidative Heck coupling reaction. | ||
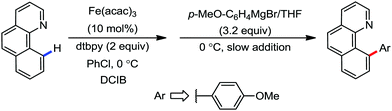
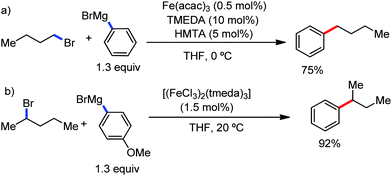
The reports regarding directed C–H bond activation published up to 2011 used a large amount of zinc salt in the presence of an aryl Grignard reagent to form the aryl zinc reagent. However, the attempts to use only the Grignard reagent (G.R.) failed due to the homocoupling of the aryl moiety of the G.R. Thus, in 2011, the Nakamura group modified the catalytic conditions of the reaction, i.e. slow addition of the G.R. to suppress the oxidative homocoupling and use of chlorobenzene as the cosolvent to stabilize the low-valent iron intermediate. These modifications created an approach to use the G.R. alone to provide the stereospecific arylation of the olefinic C–H bond with a high reaction rate (the reaction was completed within 5 min during the addition of the G.R. at 0 °C). Notably, both the N-heteroaromatic and the aromatic ketimines are well tolerated (Scheme 9).38
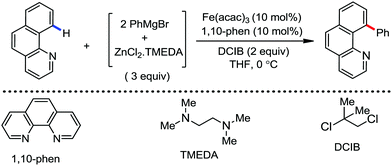
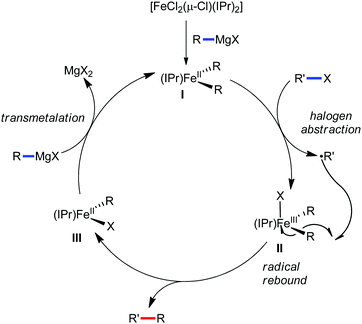
To gain to insight into the reaction intermediate and the mechanistic pathway, the Nakamura group performed several stoichiometric studies. The reaction between Fe(acac)3 and the dtbpy (4,4′-di-tert-butyl bipyridine) ligand together with the slow addition of PhMgBr resulted in the formation of an organometallic intermediate, which was quenched by D2O to deliver the deuterium-incorporated ortho product. This result indeed suggests the formation of the ortho-metalated ferracycle intermediate, which undergoes reductive elimination upon interaction with the mild oxidant DCIB (Scheme 10).
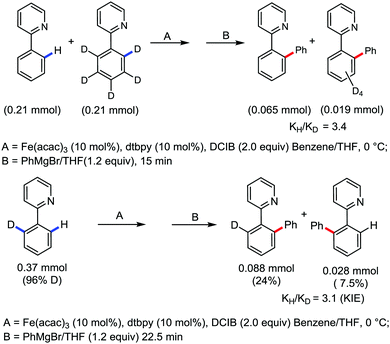
Moreover, the similarity in the large magnitude in both the intramolecular (KIE = 3.1) and intermolecular (KIE = 3.4) kinetic isotope effect value suggests the reversible coordination of the pyridyl nitrogen atom to the iron catalyst followed by C–H bond cleavage (the first irreversible step of the catalytic cycle) (Scheme 11). Based on the experiments and literature studies, they proposed a catalytic cycle consisting of four steps (Scheme 12). Initially, the reversible coordination between the pyridyl nitrogen atom and the iron centre generates species II followed by irreversible metalation of the ortho C–H bond associated with the elimination of the arene, resulting in the formation of active organoiron intermediate III. Thereafter, the formation of a carbon–carbon bond upon the interaction with DCIB via the reductive elimination from ortho-ferrated intermediate III produces the desired coupling product together with the formation of isobutene and dichloroiron species. Finally, the transmetalation of the dichloroiron species with the aryl Grignard reagent regenerates the active species I to complete the catalytic cycle. However, although the proposed mechanistic pathway is in accordance with the mechanistic information gained thus far, further studies were required to understand the nature of the reactive intermediate and the bond breaking and formation.
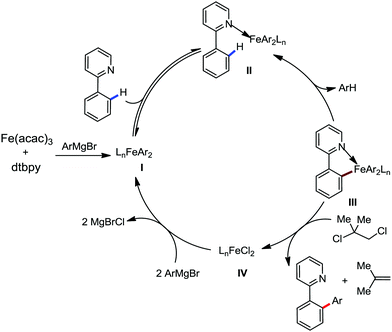 | ||
| Scheme 12 Proposed mechanistic pathway of iron-catalyzed-directed C–H bond arylation based on the experimental results. | ||
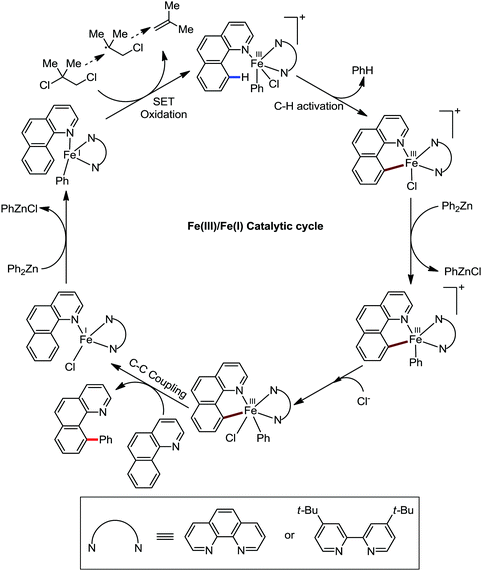
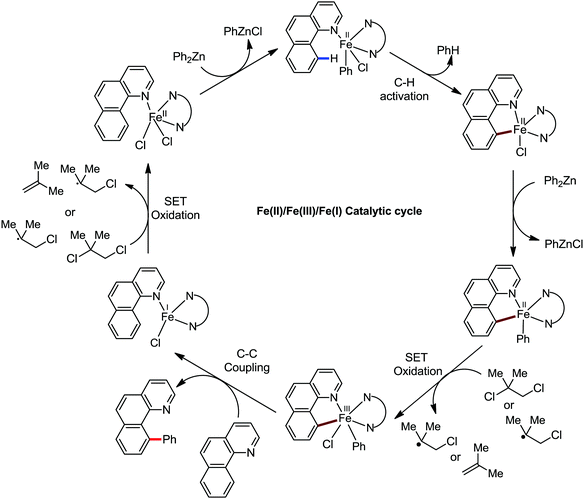
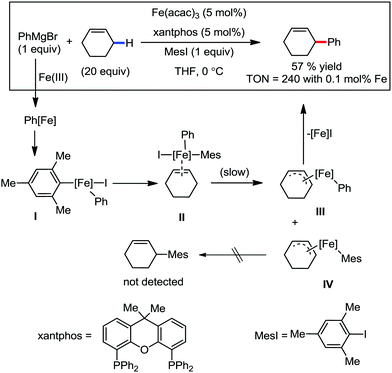
Accordingly, Chen and co-workers performed extensive theoretical studies to get better insight into the mechanism of the iron-mediated C–H functionalization reactions.39 They proposed that the two-state reactivity (TSR) of Fe(II) and Fe(III) causes the C–H activation. In this two-state reactivity condition, the high-spin ground state of Fe(II) or Fe(III) is crossed over by both the low-spin singlet and doublet state. However, this C–H activation is energetically less favourable for iron in the Fe(0) or Fe(I) oxidation state. Holland also demonstrated a similar spin acceleration phenomenon for various organometallic reactions.40 The Fe(II) formed due to the C–H cleavage via activation and transmetalation of the aryl moiety undergoes oxidation to Fe(III) via a one-electron transfer mechanism by the oxidant DCIB. Additionally, studies also revealed that the Fe(I) species formed after the C–C bond formation is also oxidised by DCIB to regenerate the active catalyst, Fe(II). Furthermore, calculations also confirmed the vital role of the ligand sphere in the stabilization of the low-spin state and assistance for the TSR mechanism during C–H activation. This observation was supported by the experimental findings by Nakamura and co-workers for C–H functionalization with arylboron compounds.41 Based on these observations, Chen's group proposed the involvement of one of the following two possible mechanistic cycles: the Fe(III)/Fe(I) cycle (Scheme 14) or Fe(II)/Fe(III)/Fe(I) cycle (Scheme 13).39,41
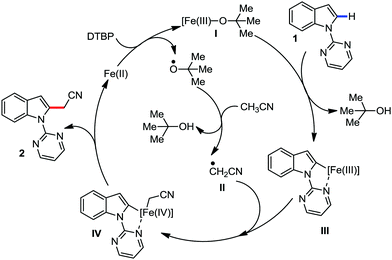
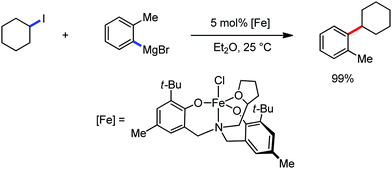
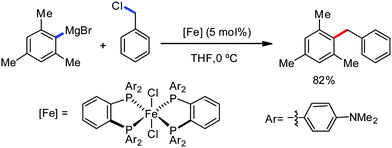
Recently, in 2019, Neidig and co-workers investigated the triazole-assisted iron-catalyzed C–H arylation reaction originally performed by Ackermann and co-workers42 using freeze-trapped 57Fe Mössbauer, MCD (magnetic circular dichroism) and EPR (electron paramagnetic resonance) spectroscopy together with synthetic and reaction studies, providing detailed structural insight into the reactive intermediates. They isolated and identified the in situ-formed iron intermediates, which represent a transformation pathway for the C–H activation reaction (Scheme 15).43 Based on spectroscopic studies, they suggested that the initial formation of the substrate-bound but non-activated Fe(II) intermediate I occurred through the reduction of Fe(III) to Fe(II) by Ar2Zn. Thereafter, upon the addition of dppbz and excess Ar2Zn, C–H activation occurs, producing low-spin cyclometalated iron(II) aryl complex II. Then it undergoes the rate-determining slow transmetalation step upon the addition of more Ar2Zn, forming complex III. This complex III is suggested to be the reactive species since it reacts with DCIB to form the product.
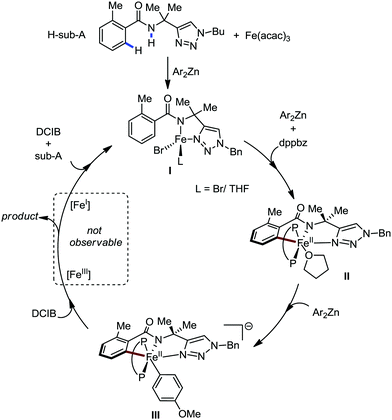 | ||
| Scheme 15 Reactivity transformations of the observed iron intermediates in triazole-assisted iron-catalyzed C–H activation. | ||
Notably, the formation of this iron species through transmetalation has been found to have a high activation barrier, and thus a high temperature is required for C–C bond formation. To elucidate the role of oxidants in the catalysis, they suggested that the oxidant DCIB oxidizes the off-cycle reduced iron species and the transient on-cycle Fe(I) species. In addition, DCIB promotes the reductive elimination of transmetalated complex III. Furthermore, based on the stoichiometric reaction, they suggested an Fe(II)/Fe(III)/Fe(I) cycle, which is consistent with the proposed mechanism using DFT studies.
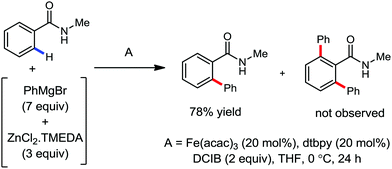
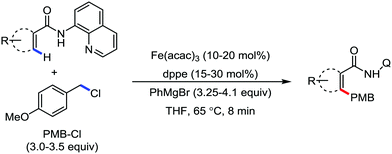
In 2012, Nakamura's group reported the iron-catalyzed monoselective ortho arylation of secondary benzamides with organozinc reagents in the presence of an aryl Grignard reagent and TMEDA (Scheme 16).44 Notably, primary benzamides and bulkier N-isopropylcarboxamides and N-phenylcarboxamides did not deliver the desired monoarylated C–H activation product, which indeed suggests the importance of the steric and electronic factor during the coordination of the substrate. Importantly, only the mono-arylated product was obtained with high selectivity. Mainly, ortho substitution of the mono-arylated product forces the amide anionic directing group out of the arene plane, preventing the formation of the second metallacycle. Thus, the reason for the formation of the mono-arylated product is rationalized based on the above steric factor.
This synthetic transformation has some disadvantages also. One of the disadvantages is the requirement of a preformed Grignard reagent or zinc reagent. Thus, it limits the substrate scope and the functional group tolerance. Hence, the nitrogen-directed C–H functionalization of arenes and alkenes with aryl bromide is promoted in the presence of metallic magnesium, which is a new approach to handle organometallic reagents.45
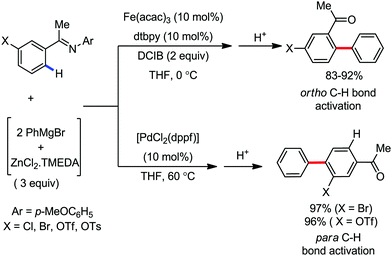
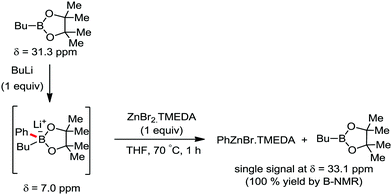
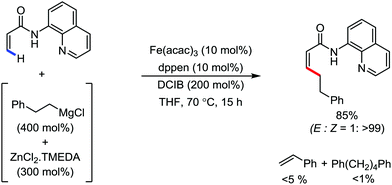
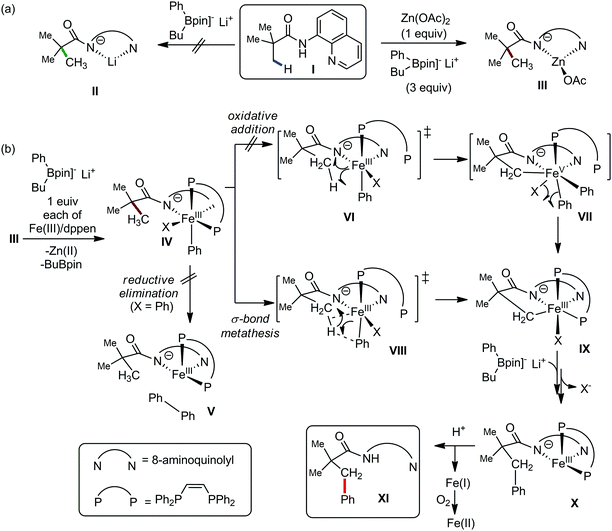
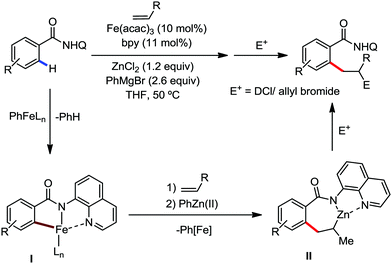
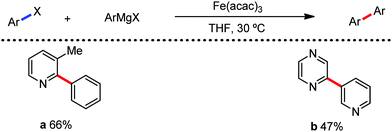
In 2014, Nakamura and co-workers reported the iron-catalyzed 8-quinolylamide-directed C–H arylation reaction of a variety of aromatic, heteroaromatic and olefinic substrates with aryl and alkenyl boron compounds together with the formation of <5% of side product (Scheme 17).41,46–49 The phenylboronic acid pinacol ester was activated in the presence of butyllithium followed by in situ boron to iron transmetalation catalyzed by zinc halide. The catalytic system consisted of iron(III) salt, zinc(II) salt, and a diphosphine ligand (dppen) having a conjugated backbone. Notably, borate acts as both a base to deprotonate the amide N–H and C–H bonds and a coupling partner. The synthetic transformation protocol shows much broader substrate scope compare to the Grignard or zinc reagent due to the lower nucleophilicity and better functional group tolerance of borate. Aryl fluoride, chloride, bromide, ether, amine, sulphide, trifluoromethyl, cyano and ester groups are well tolerated. Heteroaromatics such as thiophene and indoles can also be used as a substrate. In addition, mono-arylation takes place in the case of arylcarboxamide, possessing a meta-substituent, while the reaction with a simple benzamide and para-substituted benzamide provides a mixture of mono and diarylated product. Arenecarboxamide together with alkenecarboxamide was well explored under the reaction conditions. The amount of biphenyl formed is suppressed to a synthetically viable level by carrying out the reaction at lower temperature (30 °C) due to the lower stability of the corresponding ferracycle. Additionally, using tiglamide as the substrate, the arylation product is obtained with high Z selectivity, while the Z/E isomerization product is obtained in high yield using acrylamide as the substrate. During the investigation of the mechanism, they found some key factors regarding the mechanism. The stoichiometric reaction provides 95% yield of the C–H arylated product together with the formation of a small amount of biphenyl without the presence of the mild oxidant DCIB (Scheme 18). This result is an indication of the effective participation the phenyliron(III) intermediate in C–H cleavage and C–C bond formation through metallacycle I rather than being consumed by the oxidation of the phenyl ligand. Thus, the stoichiometric experiment supports the Fe(III)/Fe(I) mechanistic pathway, where the Fe(I) intermediate reacts with the mild oxidant DCIB to regenerate the Fe(III) species. The other two important factors of the reaction are the presence of the zinc co-catalyst and a bis(phosphine) ligand having a conjugated backbone (dppen). Here, the aryl group undergoes transmetalation from borate to zinc in the absence of the iron catalyst. This is supported by the 11B-NMR spectroscopic analysis (Scheme 19).
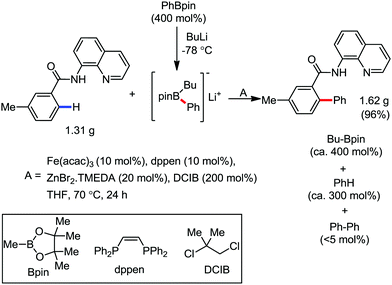 | ||
| Scheme 17 Iron(III)-catalyzed aryl–aryl coupling with arylboron compounds assisted by bidentate auxiliary. | ||
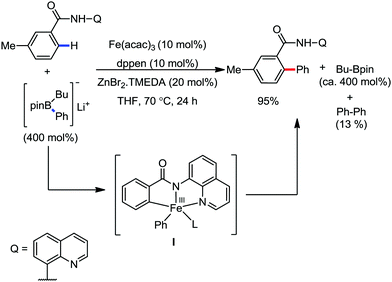 | ||
| Scheme 18 Experimental evidence of C–H bond cleavage by iron(III) species in the absence of DCIB oxidant. | ||
Moreover, the electron-donating ligand such as dppf or dppe prevented the reaction, while the electron-accepting ligand such as 1,10-phenanthroline or dppen promoted the reaction via the metal-to-ligand charge transfer (MLCT) in the case of ferracycle intermediate I. This suggests the involvement of the iron(I) intermediate, which is formed during C–C bond formation via reductive elimination of the initial iron(III) intermediate.
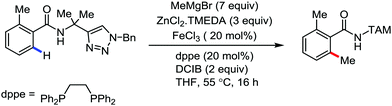
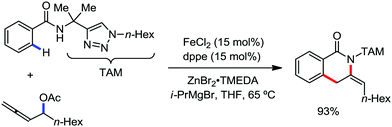
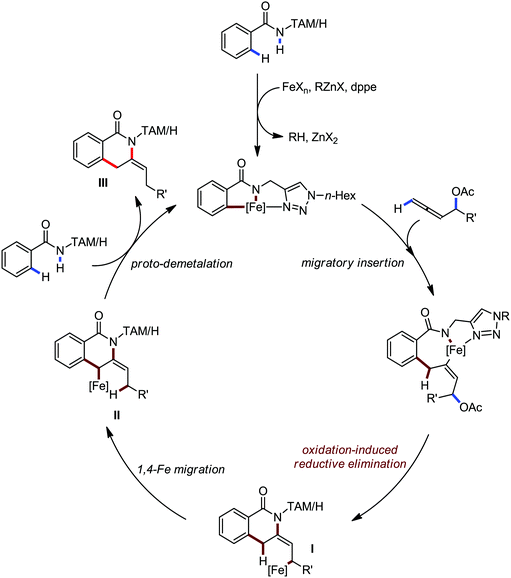
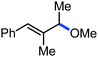
In 2014, Ackermann and co-workers reported the easily accessible novel 1,2,3-triazole-enabled iron-catalyzed C–H bond arylation of arenes and alkenes with excellent chemo and diastereoselectivities (Scheme 20).42 The directing group TAM showed a better result due to the stabilization of the low-valent iron intermediate in the presence of the dppe ligand in comparison to the 8-quinolylamine directing group (reported by Nakamura's group). Interestingly, the syn-selective arylation product of acrylamide was obtained in 57% yield without Z/E isomerization under the reaction conditions (Scheme 21).
In the same year, Deboef and co-workers reported the imine-directed iron catalyzed ortho arylation via C–H bond activation of aromatic heterocycles such as pyridines, furans and thiophenes (Scheme 22).50 The conversion of the starting material was complete within 15 min at 0 °C. Moreover, the GCMS analysis only demonstrated the existence of the unreacted starting material, the desired ortho arylated heterocycles as the desired product and aryl homocoupling product from the aryl Grignard reagent. Here, the additive KF was used to reduce the formation of the homocoupling product from the Grignard reagent.
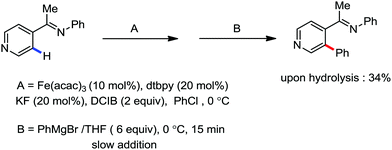 | ||
| Scheme 22 Iron-catalyzed arylation of heteroaromatics via directed C–H bond activation with Grignard reagent. | ||
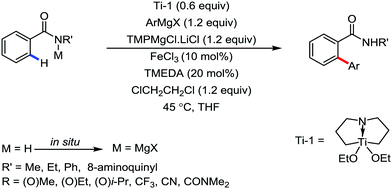
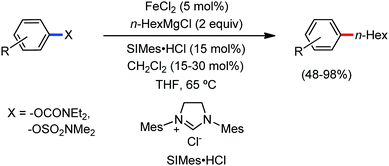
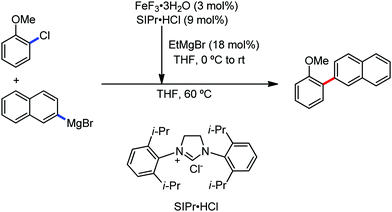
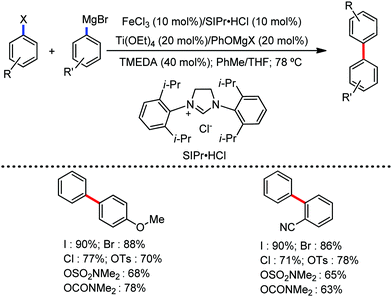
All the aforementioned ortho C–H bond arylation methods require a bi or tridentate amide as the directing group and a specific iron–ligand system. However, in 2019, for the first time, Duan and co-workers51 developed a method of ortho arylation of benzamides using a mixed titanate complex in the presence of a weakly co-ordinating amide group (CONHMe) and an iron catalyst (FeCl3/TMEDA) (Scheme 23). Here, the Ti-1 complex with ArMgBr and TMPMgCl·LiCl effectively deprotonate the amide and execute the ortho C–H bond arylation via ferration with amide. The synthetic reaction protocol shows high tolerance towards steric hindrance and sensitive functional groups under the catalytic condition of FeCl3/TMEDA in the absence of phosphine or NHC ligand. This synthetic transformation method using a Knochel-type Grignard reagent for ortho arylation accomplished the facile synthesis of series of valuable compounds such as urolithin B and crinasiadine. Moreover, highly hindered diarylation is also possible to afford the fully substituted thiophene.
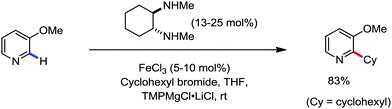
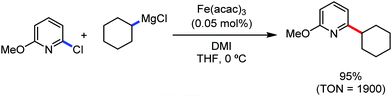
In 2010, Daugluis and co-workers reported the deprotonative alkylation of heteroarenes and electron-withdrawing group-containing arenes in the presence of a catalytic amount of iron(III) chloride together with the TMPMgCl·LiCl base, trans-N,N′-dimethylcyclohexane-1,2-diammine as the ligand and THF solvent (Scheme 24).52 Different heteroarenes such as furan, thiophene, pyridine and arenes with ester and cyano groups were efficiently alkylated using primary and secondary halides as the electrophiles. Further, the presence of radical intermediates was suggested from the observation of ring-opening product formation using cyclopropylmethyl bromide as the substrate.
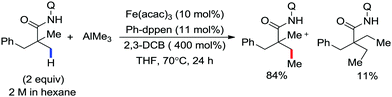
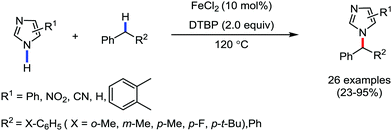
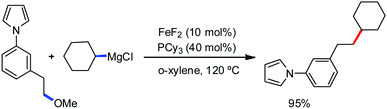
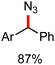
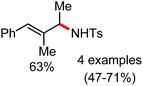
In 2017, Ackermann and co-workers designed a new trisubstituted 1,2,3-triazole (TST) as the directing group for the C–H bond functionalization of electron-rich benzyl and aryl amines by an iron catalyst.53 Notably, this TST group is easily removable by using aqueous HCl in 1,4-dioxane. Here, FeCl3/dppen in the presence of the mild oxidizing agent 2,3-dichlorobutane (DCB) was used to achieve the C–H methylation, ethylation and arylation product with chemoselectivity. The synthetic utility of this transformation is the racemization-free modification of an enantiomerically enriched benzyl amine (Scheme 25).
2.1.1.2 Iron-catalyzed alkylation of C(sp2)–H bonds using carbon nucleophiles. The alkylation of the C(sp2)–H bond with an alkyl metal compound is more challenging due to the possibility of fast β-hydride elimination compared to C–H bond arylation.54 However, although the ortho-alkylation of N-methylbenzamide and N-heterocyclic substrates has been achieved using cobalt catalysts,55–58 these results were not achieved using iron catalysts due to the decomposition of the alkyliron species. However, this reaction was successful by preventing the early reduction of the organoiron intermediate through saturation of the intermediate towards new coordination. Basically, the alkene cannot coordinate to the organoiron intermediate due to the absence of a vacant site, and thus the decomposition of intermediate can be delayed.
In 2015, Nakamura and co-workers reported the iron-catalyzed directed alkylation of alkenes and arenes possessing a bidentate directing group (e.g. 8-quinolylamide auxiliary) in combination with a bis(phosphine) ligand (dppen) together with a primary and secondary alkylzinc halide (Scheme 26).59 Monoalkylzinc halide was found to be a better alkyl donor than dialkylzinc or alkylmagnesium halide due to the weaker reducing ability of monoalkylzinc to stabilize the organoiron(III) active species. The desired alkylated product was obtained in high yield with high Z stereoselectivity. Homocoupling of the organozinc reagent and β-hydrogen elimination were significantly suppressed under the reaction conditions. Notably, primary alkyl and benzyl groups were introduced smoothly, while a mixture of linear and branched alkyl products (e.g. for i-PrMgBr, branched![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linear = 21
linear = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79) was obtained with acyclic secondary alkyl groups due to partial isomerization.
79) was obtained with acyclic secondary alkyl groups due to partial isomerization.
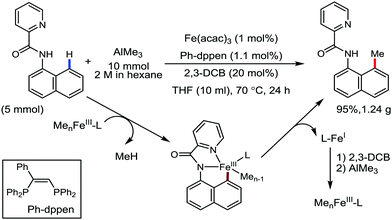
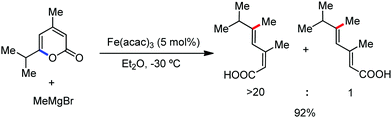
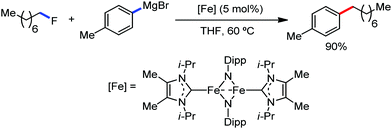
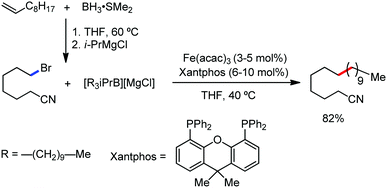
Earlier attempts for iron-catalyzed methylation proceeded with the formation of the desired product in low yield.32 In 2015, Nakamura and co-workers reported the conversion of the C(sp2)–H and C(sp3)–H bonds to the corresponding C–Me bond by using commercially available AlMe3 or its air-stable derivative, the bis(trimethylaluminium)-1,4-diazabicyclo-[2.2.2]octane adduct [DABCO·2AlMe3], as an effective methyl donor.60 The reaction progresses in the presence of a catalytic amount of an inorganic iron(III) salt and diphosphine ligand together with 2,3-dichlorobutane (DCIB) as the stoichiometric oxidant (Scheme 27). This reaction tolerates a variety of amide substrates bearing a piconyl or 8-aminoquinolyl-directing group, enabling the methylation of a variety of (hetero)aryl, alkenyl and alkyl amides. The authors also proposed that the mild reactive aluminium reagent prevented early reduction of organoiron(III) reactive species. Thus, the weaker reducing ability of AlMe3 than magnesium and zinc reagents is responsible for the very high turnover number of the catalyst, i.e. 6500. Notably, the C–H alkylation reaction using alkyl aluminium is sensitive to the size of the aluminium reagent. Consequently, triethyl aluminium undergoes the reaction smoothly, while large alkyl aluminium reagents such as triisobutyl aluminium and trioctyl aluminium cannot perform the reaction.
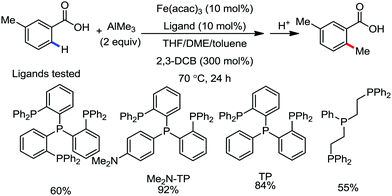
Furthermore in 2016, they discovered a combination of a mildly reactive methyl aluminium reagent and new tridentate phosphine ligand, 4-((bis-(2-diphenylphosphanyl)phenyl)phosphanyl)-N,N-dimethyl aniline (Me2N-TP), for ortho C–H bond functionalization of the C–Me bonds in aromatics and heteroaromatics bearing simple carbonyl groups under mild oxidative conditions without the need for any additional directing groups.61 Other ligands such as monodentate phosphine, bidentate phosphine and nitrogen ligands are ineffective (Scheme 28). Moreover, the reaction is chemoselective and was successfully used for the gram-scale synthesis of the mono-ortho-methylation product from arenecarboxylic acids without affecting the boronate moiety. Notably, the catalyst was not poisoned in the presence of coordinative heteroatoms such as nitrogen and sulfide. Aryl carboxylic acids having substituents at the meta-position undergo selective mono-methylation at the less hindered C–H site, while for 4-substituted benzoic acid and benzoic acid, a mono and dimethylated product is obtained. The reaction conditions also tolerate a variety of functional groups such as boronic ester, halide, sulphide, heterocycles and enolizable ketones. The catalytic system is powerful enough to methylate all four ortho C–H bonds of benzophenone. The exclusive monomethylated product was obtained in the case of tert-butyl phenyl ketone. Xanthone, dibenzosuberenone, and thioxanthone afford the corresponding products, while fluorenone does not provide any product due to the small ionic size of iron not permitting the formation of the ferracycle.
In 2015, Ackermann and co-workers reported the C–H bond methylation of (hetero)arenes and alkenes using TAM as the directing group.62 Here, FeCl3 showed the best catalytic efficiency with the dppe ligand in the presence of dimethylzinc as the methylating agent. They also reported the ethylation of arenes using diethylzinc. Notably, anilides also afforded the ortho-methylated product (Scheme 29).
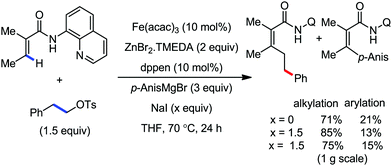
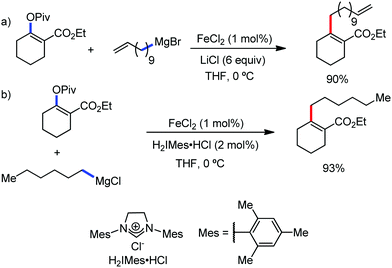
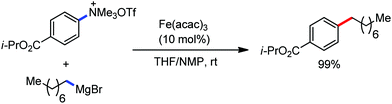
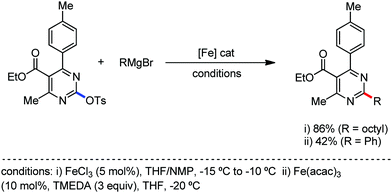
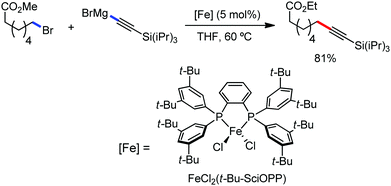
2.1.2.1 Iron-catalyzed alkylation of C(sp2)–H bond with carbon electrophiles. In 2014, Nakamura and co-workers reported the iron-catalyzed alkylation of alkenes, arenes and heteroarenes possessing an 8-quinolylamide group as the directing group with primary and secondary alkyl tosylates, mesylates and halides.65 Fe(acac)3/diphosphine catalyst/ligand and ArMgBr (as a base) were utilized to carry out the reaction (Scheme 30).
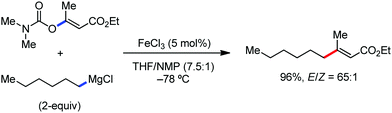
A notable feature of this reaction is the use of mesylates, tosylate derivatives, as a source of alkyl electrophiles, which were largely ignored before as alkyl donors for C–H functionalization. Here, specifically the 8-aminoquinoline directing group was essential for the formation of the product and dry NaI was added to suppress the amount of arylation side product. Moreover, the ligand plays an important role for the suppression of the undesired cross-coupling product together with the C–H arylation product. Diphosphine ligands having a π-bridge or conjugated backbone such as dppen or dppbz are effective for the formation of the product. Here, dppen showed the best efficacy. Notably, diphosphine ligands devoid of a π-bridge such as dppe or monodentate phosphine and bipyridine ligands are ineffective to produce the desired product. The reaction proceeds stereospecifically for alkene substrates and it provides only the syn-alkylated product. However, it takes place without the loss of the regiochemical integrity of the starting material, which indicates that β-elimination of an iron hydride does not interfere with the reaction. Moreover, the reaction proceeds with the loss of the stereochemistry of the chiral centre with a secondary tosylate. The loss of the stereochemistry of the chiral centre connected to a tosyl group indicates the radical-like character of the C–Fe bond in the intermediate, which is formed prior to C–C bond formation. Moreover, the radical nature of the alkyl iron intermediate was confirmed by the radical clock experiment using 5-hexenyl tosylate, affording mainly the cyclized product without the olefin isomerization, while the ring-opening product was obtained under the same reaction conditions with cyclopropylmethyl bromide (Scheme 31).68 In addition, the radical scavenger TEMPO completely inhibits the reaction. This indeed supports the radical character of the organoiron intermediate. A variety of functional groups such as alkyl chloride, aryl halide and ester are well tolerated under the reaction conditions. Interestingly, alcohols also act as an alkyl donor in this reaction protocol after in situ conversion of the alcohol into the corresponding mesylate. However, tertiary alkyl halides do not react under the reaction conditions.
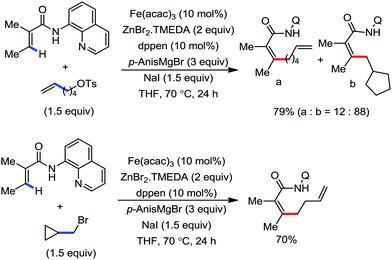 | ||
| Scheme 31 Radical-clock experiment for direct ortho alkylation of carboxamides with (pseudo) alkyl halides under iron catalysis. | ||
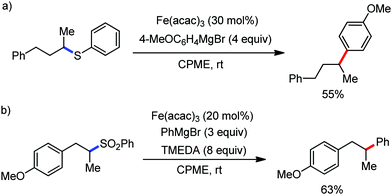
Cook and co-workers in 2014 reported an iron-catalyzed methodology for the ortho-alkylation of carboxamides using the aforementioned 8-aminoquinoline as the directing group (Scheme 32).69 Here, a catalytic amount of Fe(acac)3 was used in the presence of the dppe ligand instead of the dppen ligand used by Nakamura for a similar-type reaction. The base PhMgBr is added slowly in the reaction mixture.65 The reaction proceeds without over alkylation and provides high levels of regioselectivity. Moreover, the benzylation reaction is performed in air using reagent grade THF. Interestingly, secondary benzyl chlorides, e.g. 1-chloroethyl benzene, provided the benzylated product without isomerization in 11% yield together with a dimerized product in 33% yield.
This is the first example of directed C–H functionalization with a secondary benzylic electrophile. The reaction with secondary alkyl bromides provides over alkylation product. However, the overalkylation product can be suppressed in the presence of one equivalent of the radical scavenger 2,6-di-tert-butyl-4-methylphenol (BHT) by trapping the transient secondary alkyl radical. Interestingly, some secondary allyl bromides and iodides produce a mixture of linear and branched product. Moreover, the time (5–8 min) required for the reaction is much less, which indicates the fast generation of highly reactive organoiron species formed by the slow addition of Grignard reagent.
In continuation of the above report, they further reported the robust iron-catalyzed ortho arylation of alkyl amides with primary electrophiles (Scheme 33).70 The reaction condition is very similar to the aforementioned strategy. However, the addition time and amount of reagents were slightly modified. The reaction also provides the desired product in high yields with regioselectivity. Further, the reaction is completed within 10 min, and was performed in bio-derived 2-methyl tetrahydrofuran solvent during a gram-scale synthesis. Notably, aryl amides having electron-donating substituents decrease the energy of substrate co-ordination. Thus, they lead to a higher conversion to the product. On the other hand, aryl amides bearing electron-withdrawing substituents increase the energy of the substrate coordination and inhibit the rate of the conversion relative to the electron-rich substrate. A radical clock experiment showed a similar result in comparison to the report by Nakamura. Moreover, the authors performed intramolecular and intermolecular kinetic experimental studies. A small intermolecular KIE (KH/KD = 1.5, up to 6% yield) and a significant intramolecular KIE (KH/KD = 2.9) were observed. Based on these experiments, they proposed a plausible mechanistic explanation involving the turnover-limiting binding of the substrate to iron, followed by rapid and irreversible C–H cleavage.
 | ||
| Scheme 33 Iron-catalyzed alkylation of arenecarboxamide with primary alkyl halides using Fe(acac)3 together with the dppe ligand. | ||
In 2016, Ackermann and co-workers reported the robust iron-catalyzed C–H alkylation of arenes and heteroarenes by a triazole-directing group in the presence of Fe(acac)3/dppe as the catalytic system and phenylmagnesium bromide as the base additive (Scheme 34).71 The reaction protocol proceeds well in the presence of secondary and primary alkyl bromides. Notably, methyl iodide also undergoes the reaction. The robustness of the iron catalyst is reflected by the tolerance of the ether and ester functional groups. Notably, the yield of the product is suppressed in the presence of the radical scavenger TEMPO. This suggests that the reaction may proceed via a single electron-type reaction mechanism.
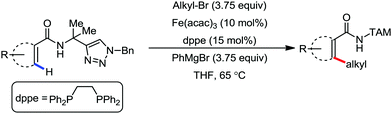 | ||
| Scheme 34 Triazole-assisted direct C(sp2)–H alkylation of arene carboxamides in the presence of an iron catalyst. | ||
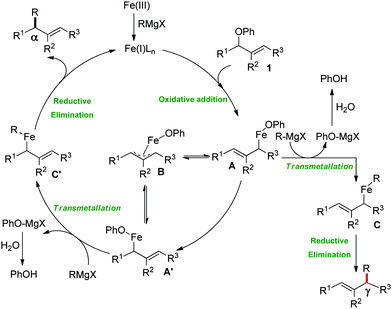
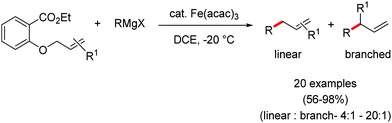
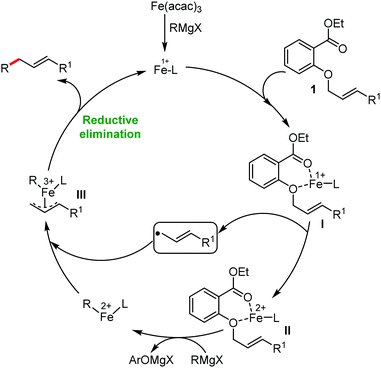
2.1.2.2 Iron-catalyzed allylation of C(sp2)–H bonds using carbon electrophiles. Nakamura and co-workers in 2013 reported the first example of the iron-catalyzed ortho allylation of aromatic carboxamide with allyl ethers.64 Arenes possessing N-(quionolin-8-yl)-amide as the directing group undergo ortho-allylation in the presence of iron(III)salt/dppen as the catalyst/ligand system and ZnCl2·TMEDA as an additive. Notably, 3.4 equiv. of tert-BuCH2MgBr is necessary to afford the desired allylated product. One equivalent of it is consumed to deprotonate the amide proton and other 2.4 equivalents form 1.2 equivalents of (t-BuCH2)2Zn. Notably, the potential side reaction of organozinc with allylphenyl ether and oxidative C–H functionalization with organometallic reagent were not observed in this case. Allylic substrates having a better leaving group such as allyl chloride, acetate and carbonate are less effective. Moreover, a variety of electron-rich and electron-deficient arenes are well tolerated to provide the allylated product with monoselectivity. Notably, iron-catalyzed isomerisation of the double bond to the styrene product was not observed (Scheme 35). The allyl phenyl ether having α-substitution afforded only the γ-product in high yield with a stereoisomeric mixture (E/Z = 59
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41). Notably, the E/Z ratio remained constant throughout the reaction, indicating that the stereomixture is not due to the isomerization of the product. Instead, it is due to the allylation step. Allyl phenyl ethers having β and γ substituents do not participate in the reaction. Additionally, an intermolecular KIE (KH/KD = 1.1) indicated that the reaction proceeds via fast iron-catalyzed C–H activation, followed by the reaction of the resulting intermediate with the allyl phenyl ethers.
41). Notably, the E/Z ratio remained constant throughout the reaction, indicating that the stereomixture is not due to the isomerization of the product. Instead, it is due to the allylation step. Allyl phenyl ethers having β and γ substituents do not participate in the reaction. Additionally, an intermolecular KIE (KH/KD = 1.1) indicated that the reaction proceeds via fast iron-catalyzed C–H activation, followed by the reaction of the resulting intermediate with the allyl phenyl ethers.
 | ||
| Scheme 35 Monoselective ortho C–H allylation of arenecarboxamide using phenyl allyl ether in the presence of an iron catalyst. | ||
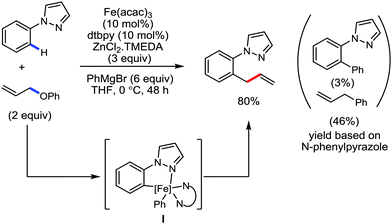
Later, ortho allylation of 1-arylpyrazoles with allyl phenyl ether via iron-catalyzed C–H activation was reported in 2014 (Scheme 36).66 The desired allylation is selectively obtained in the presence of iron(III)salt/dtbpy as the catalyst/ligand and phenylzinc reagent as a base additive. The catalytic system consisted of a bipyridine-type ligand and the diphenylzinc base acts as the key for favouring the formation of intermediate I. The undesired C–H phenylation is suppressed to less than 3%. Notably, the choice of the allylating reagent is the most important. Allylic electrophiles bearing a poor leaving group such as thiolate, amide, and siloxy are mostly recovered, while those having a better leaving group such as acetate, benzoate, carbonate and chloride are not effective for the formation of the desired product. However, the phenoxide anion generated during the reaction process causes catalytic deactivation. Thus, complete conversion of the reaction does not occur, and the starting material is recovered. Moreover, substrates having an electron-donating group at the para-position of the aryl group proceed well, while substrates containing an electron-withdrawing group at the para position of the aryl group are less reactive. The allylation of the meta-substituted substrates takes place selectively at the less hindered position in good yield. The reaction of N-arylpyrazole provides the desired allylated product with exclusive monoselectivity in a γ-selective fashion. Other N-heterocycles such as 2-phenylpyridine or benzoquinoline are unreactive or react poorly under the reaction conditions.
In 2016, Ackermann and co-workers reported the ortho C–H allylation of hetero(arenes) and alkenes bearing a triazole amide auxiliary (Scheme 37).71 They used Fe(acac)3/dppe as the catalytic/ligand system and phenylmagnesium bromide as the base additive. Allylic chlorides and secondary allylic chlorides were used as electrophile sources. This optimized catalyst was used for the first iron-catalyzed C–H allylation in alkenes. Acyclic alkenes are allylated with syn-selectivity without E/Z isomerization. Allyl chlorides having substitution at the α- and γ-position afford a mixture of linear and branched products relative to the high γ-regioselectivity, as reported by Nakamura and co-workers. Notably, here the branched product is the major product.
 | ||
| Scheme 37 Triazole-directing group-assisted ortho allylation of (hetero)arene and alkenecarboxamide. | ||
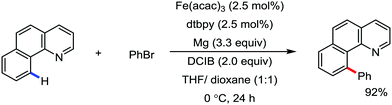
2.1.2.3 Iron-catalyzed arylation of C(sp2)–H bond using carbon electrophiles. Iron-catalyzed C–H bond arylation is quite challenging because of the higher bond energy of the Ar–X bond than the alkyl–X bond. Previously, one formal iron-catalyzed C–H coupling was reported with aryl bromides in the presence of metallic magnesium and diamine ligand and an organic dihalide.72 The use of a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of tetrahydrofuran and 1,4-dioxane is essential for the C–H bond arylation. The reaction probably proceeds via in situ formation of the Grignard reagent, followed by participation of the Grignard reagent in the iron-catalyzed oxidative C–H bond arylation (Scheme 38).
1 mixture of tetrahydrofuran and 1,4-dioxane is essential for the C–H bond arylation. The reaction probably proceeds via in situ formation of the Grignard reagent, followed by participation of the Grignard reagent in the iron-catalyzed oxidative C–H bond arylation (Scheme 38).
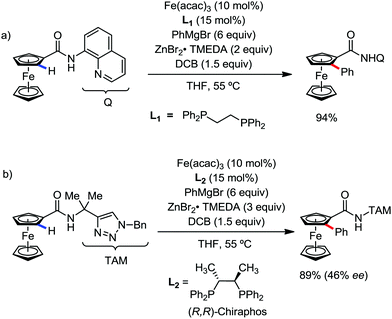
In 2017, Butenschön and Schmeil introduced the iron-catalyzed ortho-arylation and -alkylation of ferrocene substituted by an ortho-directing group (ODG) bearing a bidentate ODG, producing excellent yields of the ortho-substituted ferrocenes (Scheme 39a).73 The ortho-arylation reaction was found to give excellent yields of product, while the ortho-alkylation resulted in low to moderate yields. The moderate yields of the product for the methylation and ethylation reaction are found due to the formation of side products via the undesirable dehydrogenative dimerization of ferrocene derivatives. In addition, they presented the enantioselective iron-catalyzed C–H activation using (R,R)-ChiraPhos as the chiral ligand with iron(III) acetylacetonate forming ortho-phenylated ferrocenes in excellent yields and enantiomeric excess of up to 46% (Scheme 39b).
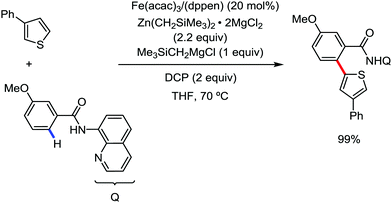
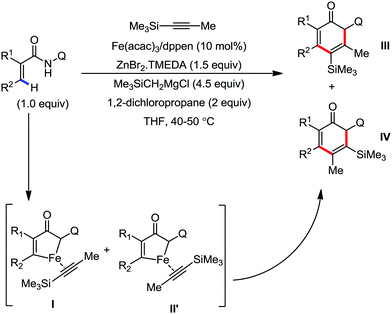
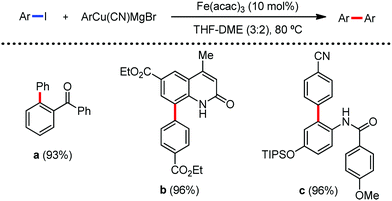
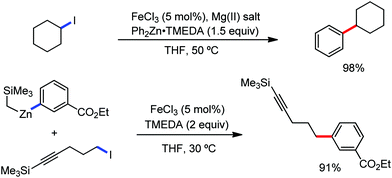
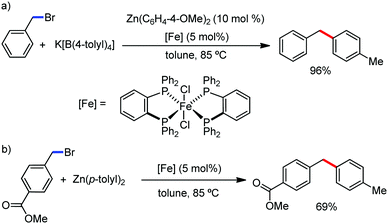
In 2019, Nakamura and co-workers reported the homocoupling-free iron-catalyzed reaction of (hetero)arene and (hetero)arene or olefin containing an N-(quinolin-8-yl)amide group, resulting in high yields of cross-coupled products (Scheme 40).74 The reaction is performed by using Fe(acac)3 combined with bisphosphine ligand, dppen as the catalyst in the presence of the organozinc reagent Zn(CH2SiMe3)2 as the base and dichloropropane (DCP) as a mild oxidizing agent.
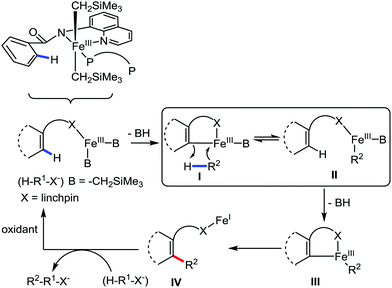
This reaction was found to be very efficient for coupling (hetero)arenes and alkene carboxamides with thiophene derivatives, giving excellent yields. Thiophenes with both electron-rich and electron-deficient substituents give good yields of monoarylated product. On the other hand, although arene carboxamides with electron-donating substituents react well, arene carboxamides bearing electron-withdrawing groups remain inactive. In addition, cyclic and acyclic alkenamides react stereospecifically, giving only the Z-products. The reaction conditions tolerate various functional groups such as ether, ketone, tertiary amine and aryl silane. Furthermore, benzene and halobenzenes provide low to moderate yields on reaction with arene carboxamides. This reaction has been suggested to proceed through two consecutive C–H activation steps (Scheme 41). The activation step involves amide group-assisted arene C–H deprotonation to form ferracycle I, followed by C–H deprotonation of the heteroarene by the ferracycle to form short-lived species II. In this species II, the two reactants remain connected via coordination of the amide anion with iron. Deuterium leveling experiments demonstrated the presence of fast equilibrium between species I and II. Then, iron species II undergoes slow irreversible deprotonation, generating diorganoiron(III) intermediate III. Thereafter, cross-coupled intermediate IV is formed by the reductive elimination of intermediate III, followed by the formation of the final product and subsequent oxidation to the active Fe(III) species.
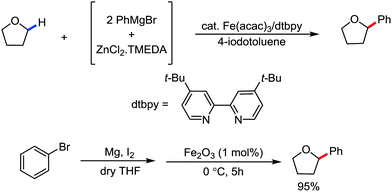
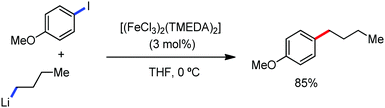
2.1.3.1 Iron-catalyzed arylation, heteroarylation and alkenylation of C(sp3)–H bonds with carbon nucleophiles. The C(sp3)–H bond functionalization can also be performed in the presence of an iron catalyst. In this process, metal coordination becomes much more difficult than that with the C(sp2)–H bond due to the absence of a π-system. Nakamura and co-workers in 2010 reported an iron-catalyzed C–H activation method for C–C bond formation at the α-position of aliphatic amines through intramolecular 1,5-hydrogen transfer.75 They discovered this method during the cross-coupling reaction between 4-iodotoluene with phenylzinc reagent in THF solvent (Scheme 42).
The same reaction also occurs during the preparation of phenylmagnesium bromide from bromobenzene in the presence of a catalytic amount of Fe2O3. This type of reaction proceeds via the reductive generation of an aryl radical from iodoarenes, followed by hydrogen abstraction by the aryl radical to form a radical at the 2-position of THF. Then, the recombination of the 2-tetrahydrofuranyl radical with iron and subsequent reductive elimination lead to the formation of the C–C bond.76 Subsequently, Nakamura and co-workers used the idea of THF arylation to examine a variety of pyrrolidine derivatives. They envisioned that the N-IBn group can serve as an internal trigger for the cleavage of the C–H bond next to the amine group to generate aryl radical I upon interaction with the organoiron species through single-electron transfer from iron. Aryl radical I intramolecularly abstracts the α-hydrogen via 1,5-hydrogen transfer to form α-aminoalkyl radical II, followed by C(sp3)–C(sp2) bond formation through reactive organoiron intermediate III to generate the desired product (Scheme 43).
The reaction proceeds within 15–30 min and the rate of the reaction is insensitive to the substituents of the aryl Grignard reagent. When an alkenyl Grignard reagent is used, the alkenylation product is obtained in good yield (Scheme 44). The reaction conditions were applied to a variety of aliphatic amines, including both cyclic and acyclic amines. However, the rate of the reaction is sluggish with anilides, affording the arylation product in low yield. Notably, a deuterium labelling experiment confirmed the intramolecular 1,5-hydrogen transfer and an intermolecular KIE experiment indicated that the 1,5 hydrogen-transfer step is not involved in the turnover-limiting step.
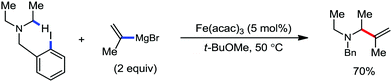
Further in 2013, they utilized this reactivity for the iron-catalyzed allylic arylation of olefins via C(sp3)–H activation.77 Mesityl iodide was used as the oxidant for the transformation. Mechanistically, PhMgBr in the presence of an iron(III) salt first generates Ph[Fe] species, which undergoes a reaction with mesityl iodide to generate reactive coordinatively unsaturated phenylmesityl iron intermediate I. Then, intermediate I coordinates with the olefin to form intermediate II, followed by the abstraction of the allylic hydrogen atom to generate two π-allylic iron species III and IV. The abstraction of the hydrogen atom is independent of the steric effect of the mesityl group. It should be noted that the reductive elimination occurs selectively from intermediate III to form the arylated product. The reductive elimination does not occur from IV due to the steric effect of the mesityl group (Scheme 45). The regio and stereoselectivity of the reaction together with the deuterium labelling experiment suggested that C–H bond activation is the slowest step of the catalytic cycle. Notably, the catalytic turnover number reaches up to 240. Under similar conditions, PhMgBr can arylate cyclohexane in 10% yield. This result indeed suggests the very high reactivity of the organoiron catalytic species and potential C–H bond activation strategy of simple hydrocarbons. The difference in reactivity between trans and cis-β-methyl styrene and the observed regioselectivity are in agreement with the formation of an allyl iron intermediate rather than a free radical pathway.
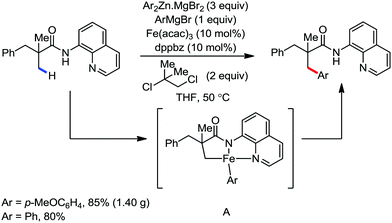
In the same year, they reported the β-arylation of carboxamides via iron-catalyzed C(sp3)–H bond activation.78 A 2,2-disubstituted propionamide possessing an 8-aminoquinolinyl group as the directing group was arylated at the β-methyl position with an organozinc reagent in the presence of the organic oxidant DCIB and a catalytic amount of iron(III) salt together with the bisphosphine ligand at 50 °C (Scheme 46).
This reaction is very sensitive towards the structure of the directing group, substrate and choice of ligand. The reaction shows a preference for C–H bond activation on the methyl group over the benzyl group present in the substrate. This suggests that the reaction has less radical character, which is consistent with the more organometallic character involving the formation of ferracycle A. The NH–Q-directing group and dppbz ligand are found to be the most effective for the reaction conditions. Further, investigation of the cyclic substrates revealed some interesting information. Evidently, cyclohexane and cyclopentanecarboxamide react well to provide the arylated product in moderate yields, while the corresponding cyclobutane and cyclopropanecarboxamide do not afford the desired product. The authors explained these observations based on the bond angle and the distance of CH3–C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) during the formation of the reactive ferracycle intermediate. The bond angle (θ) and distance between the β-hydrogen and amide nitrogen (l) are larger in the case of the unreactive substrate (Scheme 47), making the formation of the ferracycle intermediate less feasible. Here, the kinetic isotope effect (KIE) experiment indicated that the cleavage of the C(sp3)–H bond is involved in the rate-determining step.
O) during the formation of the reactive ferracycle intermediate. The bond angle (θ) and distance between the β-hydrogen and amide nitrogen (l) are larger in the case of the unreactive substrate (Scheme 47), making the formation of the ferracycle intermediate less feasible. Here, the kinetic isotope effect (KIE) experiment indicated that the cleavage of the C(sp3)–H bond is involved in the rate-determining step.
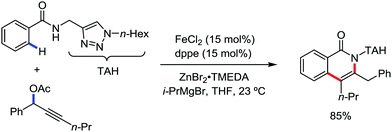
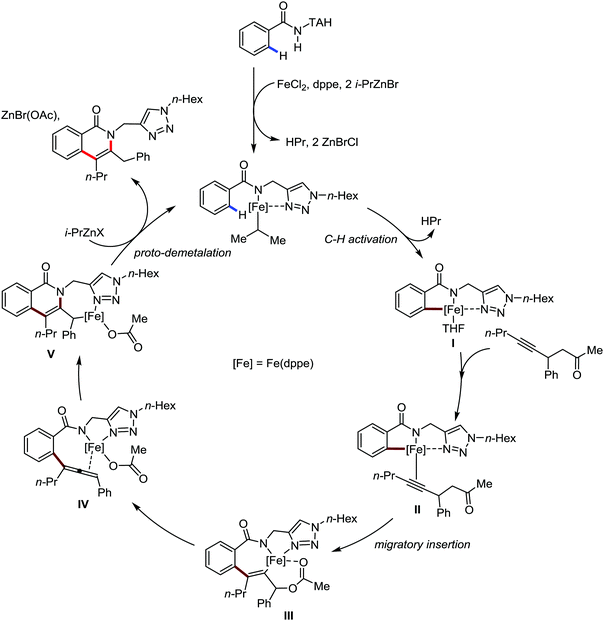
Ackermann and co-workers in 2014 extended this concept to the activation of the C(sp3)–H bond of aliphatic amides possessing the 1,2,3-triazole-based auxiliary TAM.42 Interestingly, the reaction conditions and selectivity using TAM as a directing group were the same as the reactions bearing 8-quinolyl amide as the directing group (Scheme 48). However, this reaction has some drawbacks since it provides the arylation product together with the formation of the biaryl homo-coupling product. Here, they suggested that two organic groups are transferred to the iron in the presence of an organozinc reagent, facilitating the reductive elimination pathway to a low-valent iron centre (Scheme 48).
 | ||
| Scheme 48 Triazole-directing group-assisted β-arylation of aliphatic carboxamide in the presence of an iron catalyst. | ||
In this context, in 2016, Nakamura and co-workers reported a method for the arylation, heteroarylation and alkenylation of C(sp3)–H bonds of propionamides possessing 8-quinolylamide as the directing group with an organoborane reagent (Scheme 50).79 Here, a zinc(II) salt (20 mol%) was used as the co-catalytic system in presence of iron(III) catalyst (10 mol%). This Zn(II) co-catalyst was found to assist the deprotonation of the amide and iron/boron transmetalation. Moreover, (Z)-1,2-bis-(diphenylphosphino)ethane (dppen) and its electron-rich congener (Z)-1,2-[bis(4-methoxyphenyl)phosphine]ethane (p-OMe-dppen) bearing a conjugated backbone were used due to the stabilization of low-valent organoiron species through spin delocalisation over the ligand. Several amide substrates that react very sluggishly with organozinc reagents (e.g. 2-phenyl-substituted propionamide) provided the product in good yield with borate. In the case of alkenylation, (E)-alkenyl borate reacted with 100% stereoretention, while the (Z)-alkenyl borate reacted with 83% stereoretention. Based on the previous results together with a related study on the stoichiometry and a density functional study (DFT), the authors proposed the reaction mechanistic pathway. Notably, in the presence of organoborane reagent, only one organic group is transferred to a high-valent iron-center in each step of the catalytic cycle, suppressing the formation of the by-product and catalytically inactive reduced species. The zinc-assisted deprotonation of the amide in the substrate generates zinc amide III (Scheme 49). Notably, the amide deprotonation by lithium borate is very slow in the absence of the zinc salt. Further, iron/boron transmetalation affords intermediate IV. However, C–H bond activation via the oxidative pathway is unlikely due to the unfavourable oxidation of Fe(III) to Fe(V). Thus, C–H activation occurs via the σ-bond metathesis pathway. Particularly, monophenyliron complex IV undergoes C–H activation through transition state VIIIvia σ-bond metathesis and generates intermediate metalacycle IX. Finally, ferracycle IX undergoes reductive elimination in the presence of phenyl anion to form the C–C bond, and apparently, unstable organoiron(I)-intermediate X (Scheme 49), which is stabilized in the presence of a ligand.
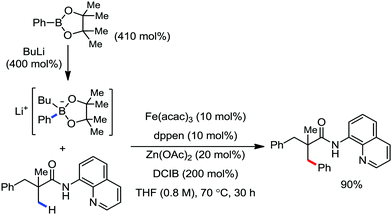 | ||
| Scheme 50 β-Arylation of aliphatic carboxamide with arylborate reagents in the presence of Fe/Zn co-catalytic system. | ||
2.1.3.2 Iron-catalyzed alkylation of C(sp3)–H bonds using carbon-nucleophiles. Construction of the C(sp3)–C(sp3) bond via iron-catalyzed C(sp3)–H activation is challenging due to the weak interaction between the iron catalyst and saturated C(sp3)–H bonds, and the poor stability of both the C(sp3)–H ferracycle and the alkyl iron species together with the high propensity of the alkyliron intermediate to undergo β-hydride elimination.
Nakamura and co-workers in 2010 first reported an example of the iron-catalyzed alkylation of C(sp3)–H bonds during their study on the α-functionalization of amines through 1,5-hydrogen transfer under iron catalysis.75 A similar mechanism involving hydrogen atom abstraction was discussed (see Scheme 43). Here, an alkylmagnesium halide is used for the alkylation at the α-position of the amine in moderate yield together with the formation of a large amount of homo-coupling product due to the dimerisation of hexylmagnesium bromide (Scheme 51).
 | ||
| Scheme 51 Iron-promoted α-alkylation of aliphatic amine with alkylmagnesium halides via a 1,5-hydrogen transfer pathway. | ||
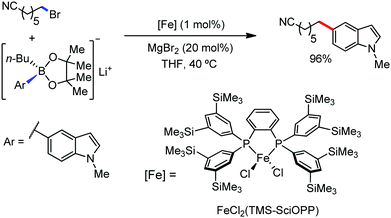
In 2018, Nakamura and co-workers developed a selective γ-C–H arylation method at the alkyl side chain of 2-iodoalkylarenes with diphenylzinc using a catalytic amount of Fe(acac)3 and N-heterocyclic carbene ligand in fluorobenzene solvent (Scheme 52).80 The aromatic halide is reported to direct the arylation of the γ-C–H bond of the alkylarene with high regioselectivity. It was proposed that initially the in situ-generated organoiron transfers one electron to the aryl iodide to form iron intermediate 1, followed by the transfer of γ-H through 1,5-hydrogen shift-generating species 2 (Scheme 52). Then, the recombination and reductive elimination of complex 2 result in the formation of the C–C bond. Notably, the involvement of the radical pathway in the formation of intermediate 1 was proven by the ring-closing product in the reaction with 1-(but-3-ene-1-yl)-2-iodobenzene. Further, the deuterium labelling experiment showed no incorporation of deuterium in the phenylated product, whereas nearly 97% deuterium was incorporated in the dehalogenated by-product. Various 2-haloalkylarenes reacted with diarylzinc reagents in this reaction protocol to afford moderate yields of the single regioisomer as the product formed via 1,5-hydrogen shift together with the dehalogenated by-product.
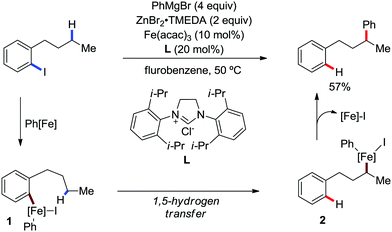 | ||
| Scheme 52 Remote arylation of 2-iodobutylbenzene catalyzed by iron and the proposed reaction intermediates. | ||
In 2015, Nakamura and co-workers reported the methylation of 2,2-disubstituted propionamide in the presence of Fe(acac)3/Ph-dppen as the catalyst/ligand system.60 Here, trimethylaluminium is used as the methylating agent and 2,3-dichlorobutane is used as the oxidant (Scheme 53).
Ackermann and co-workers in the same year reported the methylation of the β C–H bond of an aliphatic carboxamide bearing a triazole auxiliary (TAM) in the presence of dimethylzinc (Scheme 54).62 Notably, it follows the organometallic C–H activation pathway instead of cleavage of the C–H bond. Moreover, the activation of the primary C–H bond is preferred over the benzylic C–H bond and the methylation product is not obtained with the secondary C–H bond.
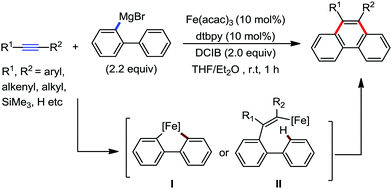 | ||
| Scheme 55 [4+2] Benzannulation of alkynes with biaryl or 2-alkenylphenyl Grignard reagents under iron catalysis. | ||
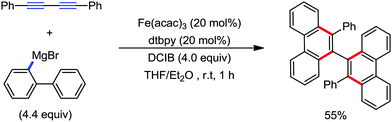 | ||
| Scheme 56 [4+2] Benzannulation of 1,2-diyne with biaryl Grignard reagents in the presence of an iron catalyst. | ||
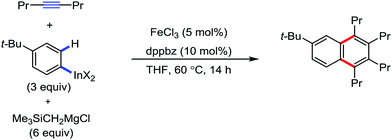
In 2012, they reported the synthesis of polysubstituted naphthalenes via the iron-catalyzed [2+2+2] oxidative annulation of arylmagnesium with two molecules of internal alkynes via C–H bond activation in the presence of 1,10-phenanthroline ligand.83 The reaction proceeds through the iron-catalyzed carbometalation of the alkynes with an aryl Grignard reagent to form alkenyl iron species I, followed by intramolecular C–H bond activation to form five-membered ferracycle II. Then the insertion of a second molecule of alkyne leads to the formation of III, followed by reductive elimination and oxidation by DCIB, which delivers the cyclization product and the iron catalyst is regenerated simultaneously. Both the diaryl alkynes and dialkyl alkynes are well tolerated. However, the yield of the product with dialkyl alkynes is poor. The main limitation of the present reaction is the lack of regioselectivity. Consequently, alkynes and Grignard reagents possessing different aryl groups provide a mixture of regioisomers because of the isomerization of intermediate I, leading to the generation of two different ferracycles II (Scheme 57).
Adak and Yoshikai in 2012 reported the iron–bisphosphine complex-catalyzed annulation reaction of arylindium reagents and alkynes to produce substituted naphthalenes in good yield (Scheme 58).84 Here, no additional oxidant is required for the catalytic turnover, although the overall transformation appears to be an oxidative coupling of the arylindium reagents and alkynes. They proposed the generation of an indium hydride species to regenerate the iron catalyst. Arylindium reagents possessing electron-donating substituents react faster than electron-deficient substituents. Further, substituted dialkyl alkynes, diaryl alkynes and aryl alkyl alkynes are tolerated, but result in the formation of a lower amount of product. The reaction progresses with high regioselectivity to produce 1,4-dialkyl-2,3-diphenylnapthalenes using 1-phenylpropyne or 1-phenyl-1-butyne as the substrate. The selectivity indeed suggests a mechanism involving the insertion of alkyne into ferracycle I formed after carbometallation, followed by C–H activation to form bis(alkenyl)iron species II. Then reductive elimination generates naphthalene instead of forming aryl alkenyl-ligated iron intermediate III (Scheme 59). Notably, unsymmetrical dialkyl alkenes produce an equimolar mixture of four regioisomers.
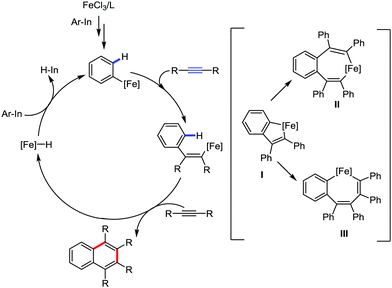 | ||
| Scheme 59 Probable mechanistic pathway for the iron-assisted [2+2+2] annulation reaction between arylindium and alkyne. | ||
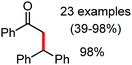
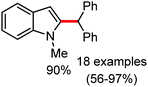
In 2014, Yoshikai and co-workers reported the iron-catalyzed imine-directed C-2 alkylation and alkenylation of indoles with vinylarenes and alkynes (Scheme 60).85 The reaction is initiated by the formation of an iron–N-heterocyclic carbene catalyst from an iron(III) salt and an imidazolinium salt together with Grignard reagent. Notably, this is the first example of an iron-catalyzed hydroarylation involving directed C–H activation. The cis-isomer of β-methylstyrene bearing aryl and silyl substituents reacts well, while trans-β-methylstyrene does not react significantly. This indeed suggests the importance of olefin coordination to the organoiron intermediate. Various alkynes including diaryl alkynes, dialkyl alkynes, and silyl alkynes are well tolerated under the reaction conditions. Further, the reaction results in good regio and stereoselective product formations. Using an unsymmetrical diaryl acetate bearing a mesityl and a phenyl group as the substrate, 70% yield of alkenylation product was obtained with exclusive regioselectivity. Similarly, high regioselectivity was observed with silyl alkynes and styrene, where the C–C bond is formed at the distal position to the silyl group. To gain mechanistic insight for this reaction protocol, a deuterium labelling experiment was performed. It revealed that the D atom of the C-2 position of indole is incorporated completely into the vinylic position (97% incorporation). Thus, based on the deuterium-labelling experiment, a plausible catalytic cycle was suggested. It involves chelation-assisted oxidative addition of the C–H bond to iron, followed by migratory insertion of the alkene or alkyne into the Fe–H bond. Subsequently, reductive elimination from the resulting diorganoiron species leads to the formation of the desired product together with regeneration of the iron catalyst (Scheme 61). Notably, the observation regarding the H/D scrambling experiment suggests that the oxidative addition and migratory insertion are reversible. Moreover, the rate of the reaction and the regioselectivity are controlled in the reductive elimination step.
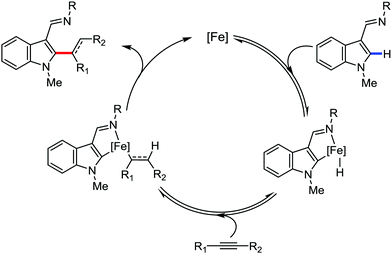 | ||
| Scheme 61 Proposed mechanistic pathway of iron-catalyzed imine-directed C2 alkenylation of indole with alkynes. | ||
In the case of a stoichiometric reaction, the iron(0) carbonyl complex is inserted into the arene C–H bond in the presence of an imine-directing group.86–88 Thus, based on this stoichiometric reaction, Wang and co-workers described an iron-catalyzed redox-neutral [4+2] cyclization reaction between internal alkynes and aromatic imines to afford cis-3,4-dihydroisoquinolines (Scheme 62).89 In 2016, Nakamura and co-workers reported an oxidative C–H activation strategy to synthesize pyridine and isoquinoline derivatives through the coupling of amides and alkynes.91 Here, an iron(III) salt and cis-1,2-bis(diphenylphosphino)ethylene (dppen) as the ligand are used together with the mild organic oxidant 1,2-dichloropropane (DCP) to promote both C–H bond cleavage and C–N bond formation. The dppen ligand helps to stabilize the unstable iron(I) intermediate via single-electron transfer (SET), which is generated after the formation of the C–C bond through the reductive elimination of the Fe(III) species.90
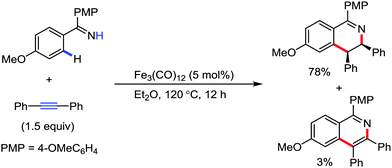 | ||
| Scheme 62 Annulations of N–H imines with internal alkynes through C–H activation under the catalytic activity of Fe3(CO)12. | ||
A broad range of internal alkynes including diaryl alkynes, dialkyl alkynes, silyl alkynes, 1,3 diyne and enynes are well tolerated under the reaction conditions. However, terminal alkynes do not afford the desired annulation product. Moreover, an unsymmetrical alkyne provides the pyridone derivative with high regioselectivity controlled by the β-substituents present on the alkeneamide substrate. The observed regioselectivity arises because of the steric effects present in the substrate (Scheme 63).
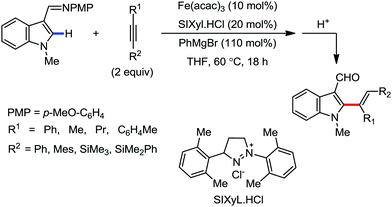
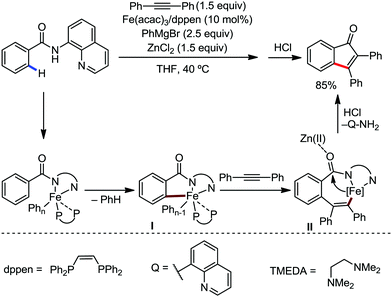
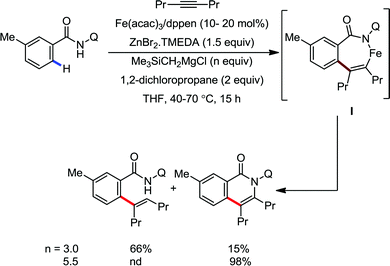
Notably, the nature of the organometallic base is very important to get achieve selectivity for the formation of the product between alkenylation and cyclization. Notably, using mono-organic zinc halide as the base, the alkenylation product was observed as the major product, regardless of the presence or absence of the oxidant. On the other hand, the cyclization product is obtained as the major product using diorganozinc as the base in the presence of DCP oxidant. This suggests the formation of the common ferrate intermediate IV for both pathways, and the strong nucleophilic diorganozinc reagent helps in the formation of a ferrate species, which immediately undergoes oxidation to promote C–N bond formation (Scheme 64). In 2018, they developed the iron-catalyzed annulation of a bidentate directing group bearing carboxamide with internal alkyne using Fe(acac)3 and cis-1,2-bis(diphenylphosphino)ethane (dppen) as the catalyst–ligand system in the presence of in situ-generated phenylzinc chloride from PhMgBr and ZnCl2 as the base (Scheme 65).91 Various electron-rich and electron-deficient benzamides and diphenylacetylenes were reacted under the reaction conditions, and high yields of the corresponding indenone derivatives were obtained. The reaction is initiated with ortho C–H activation of the carboxamide in the presence of an iron catalyst and organozinc chloride to produce complex I (Scheme 65). This is followed by insertion of alkyne to generate intermediate II. Thereafter, cyclization occurs in the presence of a Lewis acid, ZnCl2, forming indenone as the desired product.
 | ||
| Scheme 64 Organometallic base-regulated selective formation of oxidative annulations or alkenylation products. | ||
Further, they developed a directed alkylation method for carboxamides by olefins through a carbometalation pathway in the presence of an iron catalyst, giving high yields of the desired monoalkylated product (Scheme 66).92 This reaction is an alternative to the Murai-type reactions, which provide the alkylated product through C–H activation followed by the reaction between the metal intermediate and alkene via hydrometalation.93,94 Initially, ortho C–H activation of the carboxamides with a quinoline directing group is performed in the presence of iron(III) acetylacetonate together with a bipyridine ligand, forming iron intermediate I followed by its regioselective carbometalation to olefin using the in situ-generated phenylzinc base from ZnCl2 and PhMgBr to generate stable alkylzinc intermediate II (Scheme 66). Finally, this intermediate is either deuterated by DCl or trapped by allyl bromide, affording the corresponding linear alkylated product. Notably, a wide range of carboxamides and mono-substituted alkenes result in the formation of the corresponding linear alkylated products selectively in high yields without the presence of any branched alkyl products. The reaction conditions also tolerate different functional groups such as bromide, ester, silane and boron, demonstrating its synthetic utilities.
In 2018, Ackermann and co-workers reported the first iron-catalyzed allene annulations via a unique C–H/N–H/C–O/C–H functionalization, giving different substituted isoquinolones in high yields (Scheme 67).95 The allenyl acetates react with a variety of TAH-directed substituted benzamides in the presence of dppe (1,2-bis(diphenylphosphino)ethane)-ligated iron(III) acetylacetonate and in situ-generated diarylzinc as the base, resulting in high yields of site- and chemo-selective isoquinolones. Based on the higher reactivity of electron-deficient arenes in the intermolecular competition experiments, they suggested the involvement of the ligand-to-ligand hydrogen transfer (LLHT) mechanism for C–H activation. Also, based on the observation of the hydroarylation product for the reaction using the acetate-free allene, the presence of oxidation-induced reductive elimination was suggested. Mechanistically, the annulation is initiated with easy C–H metalation and subsequent allene migratory insertion, followed by oxidation-induced reductive elimination, generating iron allyl complex I (Scheme 68). Thereafter, allyl iron complex I was proposed to undergo unique intermolecular C–H activation by 1,4-iron migration, generating allyl-benzylic iron intermediate II. Then, intermediate II undergoes proto-demetalation with the amide motif of the TAM/H benzamide substrates, resulting in exo-methylene-3,4-dihydroisoquinoline III. Finally, the desired isoquinolone is produced by the isomerization of III.
In 2019, Mo et al. reported iron-catalyzed C–H/N–H alkyne annulations using triazole amide as the directing group (Scheme 69).96 A wide variety of TAH-benzamides (TAH = 1,2,3-triazole directing group) undergo the reaction, producing moderate to excellent yields of the corresponding isoquinolones. Notably, the mild reaction conditions tolerate different substituents on the aryl group of the TAH amides and on propargylic acetates.
Based on the obtained higher yields for the electron-deficient arenes in the intermolecular competition experiments, they proposed a ligand-to-ligand hydrogen transfer (LLHT) mechanism. Further, Mössbauer studies revealed the presence of high-spin iron(II) intermediates. Mechanistically, the C–H/N–H annulation catalytic cycle is initiated with C–H activation via LLHT, generating species I, followed by co-ordination of propargylic substrates to I, producing intermediate II (Scheme 70). Then, intermediate II undergoes rapid migratory insertion to form complex III. Thereafter, complex IV is formed by the cleavage of the C–O bond of the acetate leaving group of complex III. Then, the ligated allene moiety is inserted into the N–Fe bond of complex IV to form annulated iron complex V. Finally, proto-demetalation of V produces the desired isoquinolone with concomitant regeneration of the active catalyst.
2.1.5.1 Iron-catalyzed amination of C–H bonds. The first example of intramolecular amination was introduced by Berslow and Gellman in 1983. The intramolecular nitrogen functionalization of the saturated carbon atom of 2,5-diisopropylbenzenesulfonamide (I) was executed in the presence of the iron–porphyrin complex Fe(III)(TPP)(Cl) (TPP = tetraphenylporphyrin) in acetonitrile (Scheme 71).97 Initially, the starting material reacts with phenyliodone diacetate (PIDA) and KOH/MeOH to give the (imidoiodo)benzene derivative (II). This is followed by its decomposition in the presence of Fe(III)(TPP)Cl in CH3CN to generate the insertion product (III) together with a small amount of the unsaturated sufonamide (IV).
 | ||
| Scheme 71 Intramolecular nitrogen functionalization at the saturated carbon of 2,5-diisopropylbenzenesulfonamide. | ||
In 2014, Nakamura and co-workers reported a stoichiometric method with chloroamine to form an in situ-generated ferracycle intermediate, which affords the amination product (Scheme 72).98 Following this report, they developed the synthesis of anthranilic acid derivatives via the ortho amination of aromatic carboxamides with N-chloroamines. Aromatic carboxamides bearing 8-quinolinylamide as a directing group undergo ortho amination with N-chloroamines and N-benzylamines in the presence of iron(III) salt/dppbz ligand and an organometallic base (PhMgBr). Importantly, they employed the double slow addition strategy to develop a catalytic reaction. This strategy inhibits the direct reaction between N-chloroamine and PhMgBr, which prevents the catalytic turnover. Notably, the directing group and the bis(phosphine) ligand (dppbz) are crucial for the formation of the desired product with high yield and good selectivity.
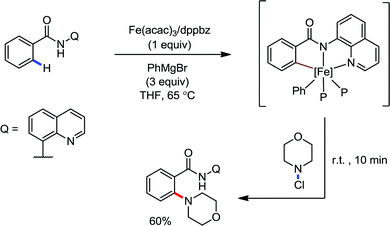 | ||
| Scheme 72 Iron-catalyzed ortho C–H functionalization of carboxamides with N-chloromorpholine via a ferracycle intermediate. | ||
They also showed that the electronic effect of the bis(phosphine) ligand plays an important role in dictating the product selectivity. A more electron-rich ligand favours the formation of the phenylation product, whereas a less electron-rich ligand favours the formation of the aminated product. Notably, a further increase in the number of fluorine atoms in the ligand decreases the yield of the product (Scheme 73). A variety of chloramines including cyclic and acyclic chloramines are well tolerated under the reaction conditions. Moreover, the reaction is very sensitive to the steric effect, and in all cases, only the monoaminated product is obtained selectively without diamination.
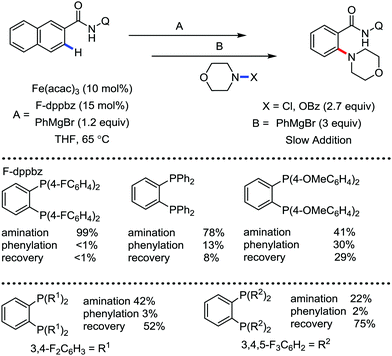 | ||
| Scheme 73 Iron-promoted C–H amination through double slow additions and reactivity of different ligands. | ||
In 2015, Nakamura and co-workers developed the iron-catalyzed intramolecular ring-closing C–H amination of o-phenylenediamines to synthesize 2,7-disubstituted 5,10-diaryl-5,10-dihydrophenazines (Scheme 74).99 The author suggested two possible mechanistic pathways. In the first pathway, C–H bond activation occurs, followed by reductive elimination to form the C–N bond. In the other mechanistic pathway, the reaction occurs via the electrophilic amination pathway. The substituted dihydrophenazine derivatives are used in hole-injection materials in organic electroluminescence (OEL) devices.
Very recently in 2020, Flack and co-workers developed the operationally simple and sustainable iron-catalyzed selective amination of the benzylic meta-C–H bond in the presence of picolinates as the directing group (Scheme 75).100 The reaction is performed using FeCl3 together with hydroxylamine-O-sulfonic acid (HOSA) in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) solvent and triethyl amine as the base. Based on the spectroscopic data, only the meta-aminated product was reported to be formed among the three possible regioisomeric amination products. Notably, the substrates with substituents at one or both ortho positions afford the desired products with high yields. Based on this observation, they suggested the direct addition reaction to the aromatic π system. Moreover, the kinetic isotope effect study revealed that C–H cleavage is not the rate-determining step, and thus the possibility of the C–H activation pathway was excluded. Thus, electrophilic aromatic substitution (SEAr) was suggested as the most probable pathway for the functionalization. This is consistent with the rapid reaction of the para-methoxy-substituted substrate, demonstrating the influence of electron-donating substituents on the SEAr functionalization.
2.1.5.2 Iron-catalyzed borylation and silylation of C–H bonds. In 2013, Mankad and co-workers developed a heterobimetallic Cu–Fe and Zn–Fe complex catalyzing C–H borylation. The C–H borylation of arenes was carried out previously using noble metal catalysts. The optimal catalyst, i.e. (IPr)Cu–FeCp(CO)2, requires only 5% loading under photochemical conditions to show efficient activity (Scheme 76).101 This bimetallic catalytic system is highly robust under the reaction conditions. Notably, it can be reused by adding some additional substrates to the reaction mixture.
 | ||
| Scheme 76 Fe–Cu cooperativity-induced C–H borylation under iron catalysis and its proposed mechanism. | ||
Notably, the reaction protocol is very sensitive to the steric bulk of arene systems. In the case of substituted toluene at the ortho position, no borylation product is obtained. The authors proposed a plausible mechanistic pathway consisting of three steps. Initially, B–H bimetallic oxidative addition undergoes via metal–metal bond cleavage, followed by photochemical C–H borylation by the resulting boryl iron intermediate. Finally, H–H bimetallic reductive elimination occurs to liberate hydrogen with the concomitant formation of a metal–metal bond.
The dehydrogenative C–H borylation with a bimetallic complex was well explored by Mankad and co-workers.101 Further, in 2015, the particular dehydrogenative C–H borylation by a mononuclear iron catalyst without any additive was developed by Darcel and co-workers.102 They reported the dehydrogenative borylation of a simple hetero(arene) with pinacolborane in the presence of iron/bis(diphosphine) (dmpe) ligand (Scheme 77). Both FeH2(dmpe)2 and FeMe2(dmpe)2 were proven to be good catalysts for the reaction protocol. The corresponding borylated compound is obtained in moderate to good yield with 5% catalyst loading under the photochemical condition (350 nm). Based on the stoichiometric reactivity study and isolation of the original trans-hydride(boryl) iron complex, [Fe(H)(Bpin)(dmpe)2], the authors proposed the mechanistic pathway. Initially, dmpe-ligated iron(0) complex 1 reacts with arenes the reversible oxidative addition of the C–H bond to iron to generate iron hydride intermediate 2. Then, the iron hydride complex undergoes a reaction with HBpin to give the borylated arene and to form iron dihydride precatalyst 3. Thereafter, precatalyst 3 reductively eliminates hydrogen via the regeneration of dmpe-ligated iron (0) complex 1. Notably, the reaction can also proceed via other pathways involving an iron boryl hydride complex.
 | ||
| Scheme 77 Iron-catalyzed C–H borylation of arenes under UV irradiation together with the proposed mechanism. | ||
Direct silylation of inert C–H bonds provide an easy approach for the synthesis of organosilane compounds having wide applications in organic synthesis,103 bioactive compounds,104 organic materials,105etc.106 There are a few methods for the synthesis of organosilicon compounds such as the traditional cross-coupling method107 for the introduction of a silyl group in organic molecules, involving the reaction between an organometallic compound and silicon electrophile,108 and other transition metal-catalyzed C–H silylation reactions.109 Iron-catalyzed methods for the silylation of the C–H bond involving organoiron species are very rare. In 2014, Nagashima and co-workers developed a disilaferracycledicarbonyl complex containing weakly coordinated η2-(H-Si) ligands for carrying out the C–H silylation reaction.110 The C-3 selective silylation of indole derivatives and stoichiometric C–H bond functionalization of arenes were achieved using the above-mentioned well-defined iron complex (Scheme 78). Similarly, Ito et al. reported the silylation of C–H bonds through an NCN-pincer-type iron–carbonyl–silyl complex.111
In 2017, Yoshigoe et al. presented an iron-catalyzed ortho-selective C–H borylation procedure for a 2-phenylpyridine derivative with the 9-bicycloboranonane (9-BBN) dimer in the presence of iron(III) bromide, resulting in ortho-borylated products in moderately high yields (Scheme 79).112 Both electron-withdrawing and donating groups on the phenyl and pyridyl ring of 2-phenylpyridine are well tolerated, giving good yields of borylated products. In addition, various substrates are efficiently borylated using this reaction protocol.
Mechanistically, the formation of complex I occurs initially from the reaction of 2-phenylpyridine and 9-BBN via a Lewis acid base interaction (Scheme 80). This is followed by the elimination of one molecule of HBr in the presence of FeBr3, forming complex II. Thereafter, successive elimination of HBr (first cycle) or H2 (after the first cycle) through iron-catalyzed C–H bond activation occurs, forming ferracycle III. Then, this ferracycle undergoes reductive elimination, producing the desired product together with the concomitant generation of iron(I) species.
In 2018, Yoshikai and co-workers reported the N–H imine-directed C–H silylation of pivalophenone N–H amine with hydrosilane in the presence of [Fe3(CO)12] as a catalyst and norbornene as a hydrogen acceptor in chlorobenzene solvent (Scheme 81).113 Various pivalophenone imines containing para-substituents such as Me, Ph, OMe, OCF3, NMe2, SMe and Cl undergo the reaction, giving moderate yields of the desired silylated products. Notably, this reaction provides a way for the formation of ortho-silylated benzonitrile under peroxide photolysis of the products, converting the imine group to a cyano group. Based on deuterium labelling experiments, it was suggested that the C–H activation undergoes through a reversible pathway.
2.1.5.3 Iron-catalyzed tritiation of C–H bonds. In 2016, Chirik and co-workers reported that an iron complex, [bis(arylimdazol-2-ylidene)pyridine iron bis(nitrogen)], can be used as a catalyst for the exchange of deuterium and tritium with the arene C–H bond using deuterium and tritium gas based on the concept of reversible oxidative addition of C–H bonds of arenes with dihydrogen via a low-valent iron complex.114 The authors proposed that the site selectivity of the iron catalyst is orthogonal to the currently used iridium catalysts (Scheme 82). Thus, the present method of tritiation of C–H bonds in orthogonal positions compared to Ir-catalyzed methods can be important means for drug discovery. Interestingly, this synthetic protocol has been used for the tritiation of commercial drug molecules such as Cinacalet.
2.1.5.4 Iron-catalyzed ethoxylation of C–H bonds. In 2018, Ding and co-workers developed the first iron-catalyzed ethoxylation of an aryl C–H bond-bearing DMEDA (DMEDA = 1,2-dimethylethylenediamine) directing group using iron(III) acetylacetonate in the presence of cobalt as a co-catalyst together with methenamine ligand, Ag2O additive and AgOPiv as the oxidant (Scheme 83).115 Various types of benzamide substrates containing electron-donating and electron-withdrawing groups on the phenyl ring undergo the reaction, giving good yields of the ethoxylated product. Notably, para- and meta-substituted benzamides give better results than the ortho-substituted derivatives. Also, meta-substituted benzamides give the ethoxylation products through the regioselective reaction at the less hindered C–H bond.
The plausible mechanism of the reaction starts with the formation of complex I through the reaction of benzamide, Co(OAc)2 and methenamine with the iron catalyst (Scheme 84). This is followed by basic pivalate anion-assisted C–H activation to produce intermediate II. Thereafter, reductive elimination from intermediate II generates another intermediate, III. Finally, this intermediate undergoes deprotonation to give the desired product together with the concomitant regeneration of iron by the oxidation of Ag+. Also, the Co(OAc)2 formed in the final step is recycled in this process.
2.1.5.5 Iron-catalyzed direct insertion to C–H bonds. Ge and co-workers in 2016 reported the iron-catalyzed E-selective dehydrogenative borylation of vinyl arenes with pinacolborane116 (Scheme 85). Here, they used an iron(0) Fe(PMe3)4 complex. Monosubstituted and disubstituted vinyl arenes undergo the synthetic transformation. Furthermore, this reaction protocol can be used for the further transformation of the resulting vinyl boronate esters to develop one-pot synthetic procedure for the functionalization of vinylic C–H bond of vinylarenes. Mechanistically, this iron-catalyzed reaction proceeds via the syn insertion of vinylarenes into the Fe-boryl species. Then the β-hydride elimination from the syn coplanar conformation of the borylalkyl iron intermediate leads to the formation of the desired product.
In 2016, Pérez and co-workers reported the first instance of the iron-catalyzed selective direct functionalization of aryl sp2 C–H bonds with ethyl diazoacetate using an iron complex containing the tetradentate PyTACN ligand (PyTACN = 1-(2-pyridylmethyl)-4,7-dimethyl-1,4,7-triazacyclonoane) together with 8 equiv. of NaBAr4F (BAr4F = tetrakis(bis-3′5-trifluoromethylphenyl)borate) as the Cl or OTf scavenger (Scheme 86).117 The reaction methodology affords the insertion products exclusively without the formation of the usually observed cycloheptatriene derivatives obtained by the Buchner addition reaction.118
Also, for alkylbenzenes, the C(sp2)–H bonds are selectively functionalized over the C(sp3)–H bonds. However, several monoalkyl-substituted benzenes undergo selective functionalization at the C(sp2)–H bonds to give the desired products in moderate to excellent yields. Substrates bearing electron-withdrawing groups such as Cl and CF3 were found to be inactive.
Based on the observations from kinetic studies and Hammett studies, they proposed the mechanistic pathway for the reaction (Scheme 87). It was suggested that the initial halide loss of the iron precatalyst in the presence of a halide scavenger forms dicationic species I, followed by its binding with the diazo compound in a dihapto manner to generate species II. Then, species II is converted to metallocarbene species III through the loss of an N2 molecule. Thereafter, the arene reacts with the carbene ligand of complex IIIvia an outer sphere mechanism, generating Wheland-type119 intermediate IV. Finally, a 1,5-hydrogen shift in intermediate IV results in the formation of the product together with the concomitant regeneration of I. Later, computational studies on the reaction supported the formation of the Wheland-type intermediate. They also revealed the formation of this intermediate via hydrogen atom transfer from the arene to the carbonyl group of the carbene, resulting in the formation of high chemoselectivity towards the insertion product.
2.2 C–H functionalisation via outer sphere pathway
In 1894, Fenton discovered a method using hydrogen peroxide and iron(II)–sulphate (FeSO4) in acidic condition to oxidise the C–H bonds present in a compound.124 After four decades of its discovery, Haber–Weiss proposed the mechanism of the Fenton reaction.125 Mechanistically, iron(II) (Fe2+) is oxidized by H2O2 to give iron(III) (Fe3+) and the hydroxyl radical (˙OH) and hydroxide ion (−OH) (Fig. 3). Then, the in situ-formed reactive hydroxyl radical carries out C–H bond oxidation. Notably, the hydroxyl radical is the second most reactive radical after the fluorine radical (based on the oxidation potential comparison). Notably, oxidation by the Fenton reagent is rapid and exothermic due to the formation of a strong bond (H–OH, BDE ∼ 119 kcal mol−1).126 Further, this reagent is a non-selective oxidant and provides a mixture of secondary and tertiary oxidized product (in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). However, the selectivity in C–H bond oxidation is very important in organic synthesis. In 1980, iron salts together with a monodentate pyridine ligand (used in solvent quantity) in acetic acid (AcOH) were used for C–H bond oxidation, which is known as GIF chemistry (Fig. 4). This method basically converts a saturated hydrocarbon into ketone at room temperature under mild conditions. This reaction has been proposed to proceed via the formation of a high-valent iron(V)–oxo intermediate. Notably, the hydroxyl radical does not form in this reaction similar to the Fenton chemistry. This method results in the formation of the secondary oxidized product in a greater amount than the tertiary oxidized product.127
1 ratio). However, the selectivity in C–H bond oxidation is very important in organic synthesis. In 1980, iron salts together with a monodentate pyridine ligand (used in solvent quantity) in acetic acid (AcOH) were used for C–H bond oxidation, which is known as GIF chemistry (Fig. 4). This method basically converts a saturated hydrocarbon into ketone at room temperature under mild conditions. This reaction has been proposed to proceed via the formation of a high-valent iron(V)–oxo intermediate. Notably, the hydroxyl radical does not form in this reaction similar to the Fenton chemistry. This method results in the formation of the secondary oxidized product in a greater amount than the tertiary oxidized product.127
In synthetic chemistry, stoichiometric electrophilic organic heterocyclic (e.g. dioxiranes and oxaziridines) oxidants are primarily used for tertiary C–H bond oxidation. In the early 20th century, most of the oxidation reactions were performed using 3-methyl (trifluoromethyl)dioxirane (TFDO) as the oxidizing agent. However, it is not a good oxidizing reagent due to its operational difficulties.123 Moreover, it cannot tolerate the structural diversity of different substrates. Biologically, non-heme iron enzymes such as methane monooxygenase128 (MMO) and rieske-dioxygenase129 can successfully catalyse C–H bond oxidation via intermediate diiron(IV)–oxo and iron(V)oxo species, respectively. Thus, these biological systems inspired chemists to develop synthetic models for selective C–H bond oxidation catalysts.
2.2.1.1 Bio-mimetic synthetic model for C–H bond oxidation. In 1997, Prof. Que Jr. and co-workers reported the first example of non-heme iron-catalyzed stereospecific alkane hydroxylation in the presence of an oxidant (H2O2).130 They employed tripodal a nitrogen ligand [(TPA) = (tris(2-methylpyridyl)amine)] in an iron catalyst system, providing excellent reactivity. Further modification in electronic and steric properties of this tripodal ligand system significantly improved its reactivity.131 Moreover, in this work, they showed evidence of iron(V)–oxo species as the key species executing C–H oxidation. Subsequently, they synthesised an FeII complex [FeII(Me2PyTACN)(CF3SO3)2] and characterised it via X-ray crystallography (Scheme 88).132 The X-ray diffraction structure showed the presence of a distorted octahedral low-spin iron(II) centre containing a tetradentate ligand (Me2PyTACN) together with two triflate anions in a cis fashion. This iron complex shows high catalytic activity in the presence of H2O2 as an oxidant for the oxidation of cyclohexane and other tertiary C–H bonds present in substrates such as dimethylcyclohexene, adamantane and 2,3-dimethyl butane. They proposed the formation of the active intermediate, an HO–FeV
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O complex, towards alkane oxidation with an iron complex. The reaction pathway using this non-heme Fe(II) catalyst is different from the former FeIII(TPA) complex.
O complex, towards alkane oxidation with an iron complex. The reaction pathway using this non-heme Fe(II) catalyst is different from the former FeIII(TPA) complex.
The reaction using TACN ligands was proposed to proceed via an unusual rebound-like mechanism (Scheme 89).133 Notably, the cis configuration of the two hydroxyl groups in the catalytic system is different from stereo-requirement of the trans-configuration in the heme complex. This is a unique feature in the non-heme catalytic system.
The aforementioned C–H bond oxidation protocol by Que and co-workers is the fundamental work on the development of selective iron-catalyzed oxidation reactions. This fundamental study actually helped chemists develop several other C–H bond oxidation catalysts. Selective iron-catalyzed C–H bond oxidation methods were pioneered by White and co-workers in 2007.134 Subsequently, selective catalysts towards C–H bond oxidation reactions were developed by several other groups. Here, we discuss these iron-catalyzed C–H bond oxidation methods in a chronological manner.
2.2.1.2 Catalytic methods for C–H bond oxidation and epoxidation. To accomplish the selective oxidation of aliphatic C(sp3)–H bonds, White and co-workers developed an iron-catalyzed aliphatic C–H bond oxidation protocol using an FeII(PDP) complex [PDP = 2-({(S)-1-(pyridine-2-yl-methyl)pyrrolidin-2-yl][pyrrolidin-1yl}methyl)pyridine] (Scheme 90).134 The higher rigidity present in the PDP ligand leads to an increase in selectivity in C–H bond oxidation. Here, site selectivity is dictated by different factors, i.e. electronic, steric, and stereo-electronic factors, of the substrate. Electron-rich tertiary C–H bonds show higher reactivity than primary and secondary C–H bonds. Thus, electronic factors rather than the steric effect of C–H bonds play the key role in this transformation.
Notably, the presence of an electron-withdrawing group at the α or β carbon of the unactivated C–H bond decreases its reactivity. Particularly, the presence of acetate, ester, carbonyl and halogen functional groups decreases the reactivity of adjacent C–H bonds. Consequently, the remote tertiary C–H bonds show better reactivity than the adjacent C–H bond (Scheme 91). The chemoselectivity and the stereoselectivity of the reaction can be due to concerted C–H bond oxidation by the electrophilic oxidant.135
The synthetic application of the reaction protocol has been extended to complex substrates where the selectivity rule determines the fate of C–H bond oxidation. For example, the most electron-rich and least sterically hindered 3° C–H bond at the C-10 position undergoes oxidation in the case of the natural product (+)-artemisinin (Scheme 92a). Interestingly, the iron-catalyzed process using the FeII(PDP) catalyst affords a higher yield of the desired product and requires a shorter time than the enzymatic reaction. Further, carboxylate-directed hydroxylation followed by in situ lactonization of tetrahydrogibberellic acid affords the lactone product in 52% yield with a requirement of recycling the starting material once (Scheme 92). Notably, the slow addition strategy results in a higher conversion without decreasing the site selectivity or chemoselectivity.136 More interestingly, the hydroxylation product of (+)-artemisinin and tetrahydrogibberellic acid is obtained in high yield without recycling the recovered starting material (Scheme 92b). Furthermore, this synthetic protocol has been applied for the diversification of natural products having potential application in drug discovery with high chemo and regioselectivity.
Encouraged by these initial results, they further worked on the selective oxidation of more abundant secondary C–H bonds.137 Notably, secondary C–H bonds have higher steric hindrance, but lower electron density compared to tertiary bonds. Therefore, the selective oxidation of secondary C–H bonds can be a useful method for methylene C–H functionalization. Here, the highly chemoselective and diastereoselective oxidation products are obtained in the case of complex molecules. The observed selectivity is governed by electronic, steric, and stereo-electronic factors. To clarify, a diterpenoid-derived dione having 14 secondary C–H bonds and two tertiary C–H bonds undergoes oxidation selectively at the C-2 position. In this system, two tertiary C–H bonds are electronically and sterically deactivated according to the selectivity rule. The side chain and the B ring are electronically deactivated due to the presence of the electron-withdrawing carbonyl functionality. Thus, the least bulky site (C-2 position) of the A ring is the active site for the oxidation. The C-2 and C-3 oxidized product were obtained in 52% and 28% (isolated yield), respectively. The starting material (12%) was recovered after completion of the reaction (Scheme 93). Similarly, another complex molecule, pleuromutilone, affords highly chemo, regioselective and stereoselective C-7 equatorial oxidized products (Scheme 94) under the optimized reaction conditions.
Inspired by these bioinspired iron catalysts for C–H oxidation, Ribas and Costas in 2009 jointly developed a catalyst that is structurally related to [FeII(MEP)(CH3CN)2]2+ and [FeII(BPBP)(CH3CN)2]2+ (reported by White and Chen) (Scheme 95).138
They introduced another non-heme iron catalyst, [FeII((S,S,R)-(MCPP))(CF3SO3)2], supported by a nitrogen-based chiral ligand (MCPP) containing the bulky pinene group at the remote position of the pyridine ring. Notably, this bulky and rigid ligand backbone protected the iron centre of the catalyst from external perturbation by oxygen and prevents the decomposition of the catalyst via bimolecular processes. Moreover, the chiral 1,2-cyclohexane diamine backbone imparts rigidity to the ligand, which allows the predetermination of topological chirality and determines the relative orientations of the robust pinene CH3 groups with respect to the metal sites.
The ellipsoid model of the solid-state molecular structure of [FeII(MCPP)(CF3SO3)2] shows that the distorted octahedral iron(II) centre contains a ligand with cis-α topology. The nitrogen atoms of the two pyridine rings are in a trans fashion and two aliphatic nitrogens are situated cis to each other. The catalyst exhibits high stereospecificity and regioselectivity during hydroxylation, suggesting the involvement of a highly selective high valent iron–oxo complex instead of any free radical-type intermediates (Scheme 96). Notably, the aforementioned catalyst shows good selectivity towards tertiary C–H bonds, although there is a presence of statistically more important secondary C–H bonds in the substrates. This preference for the tertiary position indicates that the strength of the C–H bond is a major factor in dictating the site selectivity. This catalyst shows better selectivity and efficiency compared with the results using the [FeII(MEP)(CF3SO3)2], [FeII(BPBP)(CF3SO3)2], and [FeII(PyTACN)(CF3SO3)2] catalysts. Both the electronic and steric factors play a major role to differentiate C–H bonds. For example, the regiospecific oxidation of the tertiary C–H bond of (−)-acetoxy-p-menthane was observed using [(S,S,R)-FeII(MCPP)(CF3SO3)2]/H2O2 as the catalyst/oxidant system in 62% yield with only 1% catalyst loading. Here, the hydroxylation occurs more readily at the more accessible (C-1)–H bond to give the corresponding product (Scheme 96). Further, it is believed that non-heme iron-complex I is oxidized by H2O2 to generate intermediate iron–oxo complex II, which reacts with C–H bonds via hydrogen atom transfer and the oxygen rebound pathway to provide the hydroxylation product (Scheme 97).
Substrate-controlled site-selective C–H bond oxidation protocols were developed using small molecule non-heme iron catalysts up to 2012. Later in 2013, White and co-workers developed another small molecule non-heme iron catalyst, [FeII(CF3PDP)]2+. This non-heme iron catalyst demonstrates the trajectory restriction governed strategy to provide predictable, catalyst-controlled site selectivity in simple and complex molecules.139 Basically, the presence of pendent aryl rings at the 5-position of the pyridine rings of PDP ligand having an ortho-CF3 group deactivates the ligand towards oxidation due to its electron withdrawing property. Further, steric bulk present in the ligand system enforces a perpendicular arrangement of the biaryl rings, where the CF3 groups extend towards the active sites of the catalyst and narrow the cone of approach trajectories to 76° (Scheme 98). Thus, site selectivity is primarily governed by the nonbonding interaction between the catalyst and substrate. It is observed that the FeII(PDP) catalyst can differentiate between 3° and 2° aliphatic C–H bonds based on electronic, steric and stereo-electronic factors. Consequently, the FeII(PDP) catalyst provides substrate control selectivity. Interestingly, using the FeII(CF3PDP) catalyst on the same substrate affords the secondary C–H bond oxidized product in a major amount. Thus, the FeII(CF3PDP) catalyst can alter the intrinsic site selectivity of the oxidation product obtained by the FeII(PDP) catalyst. Subsequently, a qualitative mathematical model was developed, which helps to relate the site selectivity of the aforementioned catalyst to the properties of the substrate. They also applied this model to (+)-artemisinin. This natural product has nine potential sites for oxidation, and among them, only C-10 and C-9 are the likely sites for oxidation based on the unfavourable electronic and steric factors at the other sites. They calculated the site selectivity ratio to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 (C-9
1.3 (C-9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-10) using the [(S),(S)-FeII(PDP)] catalyst and their model. Thus, C-10 is the most appropriate site for oxidation according to their model. Experimentally, 54% yield of the (+)-10-β-hydroxy-artemisinin was obtained together with 23% yield of (+)-9-oxo-artemisinin in a 2
C-10) using the [(S),(S)-FeII(PDP)] catalyst and their model. Thus, C-10 is the most appropriate site for oxidation according to their model. Experimentally, 54% yield of the (+)-10-β-hydroxy-artemisinin was obtained together with 23% yield of (+)-9-oxo-artemisinin in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (C-10
1 (C-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-9) ratio. On the other hand, the same reaction with the [(S),(S)-FeII(CF3PDP)] catalyst showed the calculated site selectivity ratio of 17
C-9) ratio. On the other hand, the same reaction with the [(S),(S)-FeII(CF3PDP)] catalyst showed the calculated site selectivity ratio of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (C-9
1 (C-9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C-10). Thus, it favours oxidation at the C-10 position based on the large steric difference parameter, affording 52% yield of (+)-9-oxo-artemisinin as the major product (Scheme 99).
C-10). Thus, it favours oxidation at the C-10 position based on the large steric difference parameter, affording 52% yield of (+)-9-oxo-artemisinin as the major product (Scheme 99).
Imide-containing heterocycles diminish the basicity of nitrogen due to the presence of two carbonyl moieties at both adjacent positions of nitrogen (Scheme 100).140 Consequently, they tolerate the oxidative condition and promote remote C(sp3)–H functionalization without the addition of any additives. The same reaction protocol with a strong Brønsted acid having a strong coordinating counterion (e.g. triofluoroacetic acid or sulphuric acid) is not an effective additive to provide the desired product. Both substituted piperidine and pyridine are well tolerated under the oxidative condition of the reaction. Selective oxidation of the remote 3° C(sp3)–H bond is observed with the FeII(PDP) catalyst in the presence of additives and H2O2/ACOH as the oxidant, without the detection of either the benzylic or methylenic oxidation product. On the other hand, the 2° C(sp3)–H oxidized product is obtained with high selectivity in the presence of the FeII(CF3PDP) catalyst and H2O2/ACOH as the oxidant system together with the aforementioned additives.
Although the yield of the isolated product decreases using HBF4 as an additive, it has advantages in the presence of sterically hindered substituents attached to nitrogen, causing retardation in effective BF3 co-ordination. The remote oxidation product was obtained in good yield with electron-rich pyridine, whereas a lower yield of product was obtained in the presence of electron-deficient substrates. Oxidation in the late-stage diversification of medicinally important complex molecules has been performed using the aforementioned nitrogen tolerance strategies in the presence of either [FeII(PDP)]2+ or [FeII(CF3PDP)]2+ catalyst. For instance, in general, streptovitacin is obtained via an eight-step de novo synthesis with 7% overall yield. However, it can be prepared via the direct one-step oxidation of cycloheximide in the presence of [FeII(S,S)-PDP]2+ catalyst in excellent yield (50%) using the above mentioned reaction protocol (Scheme 101).
Further in 2016, the oxidative diversification of amino acids and peptides by the aforementioned two small molecule catalysts, [FeII(PDP)]2+ and [FeII(CF3-PDP)]2+, was reported. These catalysts are capable of facilitating targeted C–H bond oxidative modification with the retention of the α-centre chirality (Scheme 102).141 Oxidation of proline to 5-hydroxyproline is furnished via the formation of versatile intermediates. These intermediates are transformed to rigid arylated derivatives or flexible linear carboxylic acids, olefins, alcohols and amines in both monomer and peptide forms. This C–H bond oxidation strategy has the capacity for generating diverse amino acids and peptide molecules. Subsequently, four ‘chiral pool’ amino acids were converted into twenty-one chiral unnatural amino acids. The newly formed unnatural amino-acids represent seven distinct functional group arrays. Moreover, late-stage C–H functionalization of single proline-containing tripeptide resulted in the formation of eight tripeptides. Each of the tripeptides belongs to different unnatural amino acids. Additionally, a macrocyclic pentapeptide containing a proline moiety was transformed to a product containing linear unnatural amino acids via late-stage C–H bond oxidation.
In 2016, Costas and Gebbink developed another set of chiral non-heme iron catalysts, [FeII(tipsMCP)(OTf)2] 1 and [FeII(tipsPDP)(OTf)2] 2, containing sterically bulky chiral ligands for executing the regioselective oxidation of alkyl C–H bonds in the presence of H2O2 as an oxidant.142 These bulky chiral ligands were synthesized by introducing the bulky tris(isopropyl)silyl (TIPS) moiety at the meta-position of two pyridine rings of chiral tetradentate aminopyridine ligands, PDP and MCP. The corresponding iron catalysts 1 and 2 and their enantiomeric analogues are employed for C–H oxidation reactions (Scheme 103). Notably, both iron catalysts have backbones with different natures and exhibit a well-defined and constrained envelope around the cis-labile position of the iron centre, where the reactive Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit is formed upon reaction with H2O2. Interestingly, while executing the C–H oxidation of different substrates, the bulkier TIPS-substituted catalysts, Λ-tips1 and Λ-tips2, provided better yields of the corresponding C–H oxidized product compared to the unsubstituted iron catalysts, Δ-1′ and Δ-2′. Further, they performed control experiments with two iron catalysts, 1 and 2, to examine the ability of the catalysts to differentiate between secondary and tertiary C–H bonds (2°/3°). Additionally, they envisaged the role of the ligand backbone of the catalysts in discriminating two different methylenic sites differing in steric hindrance (K3 and K2, Scheme 103). To elucidate the above cases, the C–H oxidation reaction between catalysts and trans-1,2-dimethylcyclohexane is discussed.
O unit is formed upon reaction with H2O2. Interestingly, while executing the C–H oxidation of different substrates, the bulkier TIPS-substituted catalysts, Λ-tips1 and Λ-tips2, provided better yields of the corresponding C–H oxidized product compared to the unsubstituted iron catalysts, Δ-1′ and Δ-2′. Further, they performed control experiments with two iron catalysts, 1 and 2, to examine the ability of the catalysts to differentiate between secondary and tertiary C–H bonds (2°/3°). Additionally, they envisaged the role of the ligand backbone of the catalysts in discriminating two different methylenic sites differing in steric hindrance (K3 and K2, Scheme 103). To elucidate the above cases, the C–H oxidation reaction between catalysts and trans-1,2-dimethylcyclohexane is discussed.
Interestingly, catalyst Λ-tips1 preferentially oxidises secondary C–H bonds over tertiary C–H bonds (with a ratio of C–H oxidation products of 2°/3° = 13) during the reaction with trans-1,2-dimethylcyclohexane, whereas catalyst Λ-1′ shows slow selectivity between secondary and tertiary C–H bonds (ratio of C–H oxidized products of 2°/3° = 3). Notably, the similar reactions with catalysts Λ-tips2 and Λ-2′ provide the secondary and tertiary C–H oxidized products with the ratio of 2°/3° = 4 and 2°/3° = 1, respectively (Scheme 103). Further, a better ratio of different methylenic sites, K2 and K3 oxidized products, is obtained with the bulkier catalyst, Λ-tips1 (K3/K2 = 3), compared to the unsubstituted catalyst, Λ-1′ (K3/K2 = 1.9). Thus, the discrimination between different methylenic sites is improved by the introduction of steric bulk in the catalyst, Λ-tips1. Here, the nature of the backbone and rigidity present in the system play a significant role in determining the selectivity. Moreover, they accomplished the site-selective oxidation between the methylenic sites of terpenoid and steroidal substrates using all the catalysts. Notably, they observed unprecedented site selectivity during C–H oxidation of trans-androsterone acetate (Scheme 104). In this substrate, the C6 and C12 methylenic sites are oxidized by employing the four chiral catalysts, Λ-tips1, Δ-tips1, Λ-tips2 and Δ-tips2. Interestingly, the Λ-tips1 and Λ-tips2 catalysts preferentially oxidise the C6 site, whereas Δ-tips1 and Δ-tips2 oxidize the C12 site. Thus, the present study revealed the role of the chirality of the ligand backbone present in the catalyst in determining the site selectivity during product formation.
Furthermore in 2016, Basallote, Company and Costas reported the reaction of [FeII(PyNMe3)(CF3SO3)2] with an excess amount of peracetic acid, resulting in the formation of a meta-stable compound existing as a pair of electromeric species, [FeII(PyNMe3)(OOAc)]2+ and [FeV(PyNMe3)(O)(OAc)]2+, in fast equilibrium (Scheme 105).143 Species 2 (2a and 2b) is kinetically competent for oxidising strong olefinic C–H bonds (with bond dissociation energies of up to 100 kcal mol−1) through the initial hydrogen atom transfer (HAT) mechanism. Notably, oxygen atom transfer (OAT) from the pair of electronic species occurs extremely fast in the olefinic substrate to generate epoxide. This was confirmed by stopped-flow UV-vis analysis. The rate of epoxidation of olefin depends on the electron density of the olefin substrate. More substituted double bonds show a faster rate due to the inductive effect (+I) of the substituents, causing the accumulation of more electron density at the double bond.
The above-mentioned statement also supports the electrophilic character of the FeV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species generated during oxygen atom transfer (OAT) in the course of epoxidation.
O species generated during oxygen atom transfer (OAT) in the course of epoxidation.
In 2017, Sengupta and co-workers reported a synthetic method describing a peroxidase-mimicking iron complex, [FeIII(b-TAML)(OH2)], which catalyzed the photocatalytic hydroxylation and epoxidation of alkanes and alkenes.144 Here, [RuII(bpy)3]Cl2 and [CoIII(NH3)Cl]Cl2 were used as the photosensitizer and one-electron acceptor in acetonitrile–phosphate buffer solution, respectively. Notably, water was used as the oxygen atom source (Scheme 106). This was confirmed by an H2O18 labelling experiment. More than 90% of the 18O-labelled oxygen atoms were incorporated in the hydroxylated and epoxide product. The high electron-donating tetra-anionic N-donors stabilised the high-valent iron–oxo species such as [FeV(b-TAML)(O)]− and [(b-TAML)FeIV(O)]2−, which selectively catalysed the oxidation of the unactivated alkanes and alkenes. The hydroxylation of unactivated alkanes having several substrates including natural products was mostly observed at the tertiary C–H bond with 3°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2° selectively up to ∼100
2° selectively up to ∼100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Mechanistically, an active [{(b-TAML)FeIV}2-μ-oxo]2− (2) dimer is generated from the starting complex (1) via one-electron and one-proton transfer (proton-coupled electron transfer, PCET) process. The subsequent disproportionation of 2 occurs upon the addition of the substrate, assisting the generation of FeV
1. Mechanistically, an active [{(b-TAML)FeIV}2-μ-oxo]2− (2) dimer is generated from the starting complex (1) via one-electron and one-proton transfer (proton-coupled electron transfer, PCET) process. The subsequent disproportionation of 2 occurs upon the addition of the substrate, assisting the generation of FeV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species via the regeneration of 1 (Scheme 107).
O species via the regeneration of 1 (Scheme 107).
In the same year, they developed an iron-catalyzed synthetic protocol describing peroxidase-mimicking chemistry using an iron catalyst-containing biuret-modified TAML macrocyclic ligand framework (NO2-b-TAML) (Scheme 108). The modified iron catalyst, NO2[FeIII-(b-TAML)(Cl)]2−, can carry out the selective oxidation of unactivated 3° C–H bonds and activated 2° C–H bonds with a low catalyst loading (1 mol%). This synthetic protocol provides high product yield with excellent mass balance and configuration retention using m-CPBA as an oxidant under near-neutral conditions.145 Notably, selective 3° C–H bond oxidation occurs at a faster rate in the case of the cis-isomer than the trans-isomer. This can be attributed to the reactivity of both isomers, including the strain present in the system, which is released in the transition state of the cis-isomer.
Moreover, the selectivity between aliphatic C–H bond oxidation of the 3° and 2° sites of any complex molecule is predicted based on the electronic, steric, and stereo-electronic effect. The high selectivity using this reaction protocol is due to the involvement of high-valent iron(IV)–oxo intermediates instead of organic radicals. For example, the complex molecule (+)-artemisinin having five 3° C–H bonds together with the fragile endoperoxide moiety affords (+)-10-β-hydroxyartemisinin in 38% yield. Further, the oxidation of the α-ethereal C–H bond (activated 2° C–H bond) occurs exclusively in the case of ambroxide, having nine possible sites of oxidation (Scheme 109). Notably, this is in contrast to the oxidation by PDP complexes reported by White and co-workers.137
Overall, a total of 18 substrates including arenes, N-heterocycles and polar functional groups are well tolerated under the optimized reaction conditions. However, although White and co-workers140 reported remote C–H bond oxidation having basic heteroaromatic nitrogen groups in the presence of Brønsted and Lewis acids as additives, the aforementioned method using the NO2[FeIII-(b-TAML)(Cl)]2− catalyst provides remote C–H bond oxidation (Scheme 110) without the use of Brønsted and Lewis acids as additives.
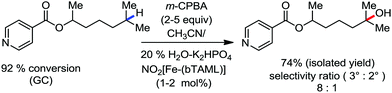 | ||
| Scheme 110 Oxidation of 3° C–H bonds in the presence of N-heterocycles with NO2[Fe-(b-TAML)] complex. | ||
Thus far, the discussed non-heme iron catalysts have been utilized for the selective C–H oxidation of natural products. Fascinatingly, besides this, some artificial enzymes have been developed for the selective oxidation of a highly conjugated norditerpene alkaloid isolated from the Nigella glandulifera plant. In 2017, Arnold and Stoltz reported the first enantioselective total synthesis of the norditerpenoid alkaloid nigelladine A through biocatalytic late-stage C–H bond oxidation using an iron co-factor containing artificial metalloenzymes, i.e. engineered cytochrome P-450 (8C7) enzyme, in 12 steps with an overall yield of 5%.146 This method leads to chemo as well as regioselective allylic 2° C–H bond oxidation even in the presence of four other oxidisable positions. Noticeably, this enzymatic technique provides a turnover of up to 1700 to the C-7 mono-oxygenation product (Scheme 111). Importantly, the other reported traditional chemical oxidation methods147,148 failed to give the desired C–H bond oxidation product.
In 2019, Han and co-workers reported an iron-catalyzed efficient methodology for the oxidation of methylarenes and alkylarenes to deliver arylaldehydes, arylketones and aryl esters efficiently.149 They employed a combination of iron(II) phthalocyanine (1 mol%) and ferrocene (10 mol%) as a catalyst in the presence of K2S2O8 (1 equiv.) as an oxidant, together with polymethylhydroxy silane (PMHS) as an activator. Moreover, the reaction was carried out in a CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixture under ambient air (1 atm of O2) at 80 °C (Scheme 112). Interestingly, toluenes bearing electron-donating groups such as methyl, polymethyl, 4-tert-butyl, and diethylphenyl phosphate produced the desired selective oxidized product in high yield (86–92% yield). Also, electron-withdrawing substituents (bromo, iodo, sulfonyl, etc.) containing toluene derivatives provided the desired arylaldehyde at a slow reaction rate with high yield (60–94% yield) in the presence of an increased amount of oxidant (3 equiv. of K2S2O8). Remarkably, the one-step oxidation of methylthiophenes to the corresponding thenaldehydes (90% yields) was achieved using this reaction protocol. Importantly, thenaldehydes are widely used for the synthesis of the chemotherapeutic medicine teniposide, a common hepatoprotectant tenylidone, and the insectifuge pyrantel.
1) solvent mixture under ambient air (1 atm of O2) at 80 °C (Scheme 112). Interestingly, toluenes bearing electron-donating groups such as methyl, polymethyl, 4-tert-butyl, and diethylphenyl phosphate produced the desired selective oxidized product in high yield (86–92% yield). Also, electron-withdrawing substituents (bromo, iodo, sulfonyl, etc.) containing toluene derivatives provided the desired arylaldehyde at a slow reaction rate with high yield (60–94% yield) in the presence of an increased amount of oxidant (3 equiv. of K2S2O8). Remarkably, the one-step oxidation of methylthiophenes to the corresponding thenaldehydes (90% yields) was achieved using this reaction protocol. Importantly, thenaldehydes are widely used for the synthesis of the chemotherapeutic medicine teniposide, a common hepatoprotectant tenylidone, and the insectifuge pyrantel.
Most intriguingly, the present methodology has been found to be compatible in the presence of unprotected boronic acids, which are very sensitive towards reagents such as bases, organic acids and oxidants due to unstable tricoordination nature of the boron centre.150 Thus, the present protocol chemoselectively oxidizes the aromatic methyl moiety in the presence of boronic acid to generate carbonyl compounds in good to excellent yields. Notably, for this particular type of reaction, they utilized FeCl2 (10 mol%) as the catalyst instead of the combination of ferrocene, iron(II) phthalocyanine and tetra-butyl ammonium bromide (TBAB), in the presence of the same oxidant/PMHS/solvent system. Notably, aldehydoarylboronic acids have high synthetic versatility due to the presence of both nucleophilic (C–B bond) and electrophilic (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond) moieties. The robustness of this reaction protocol leads to the late-stage oxidation of complex molecules such as dehydroabetic acid, gemfibrozil, and tocopherol nicotinate.
O bond) moieties. The robustness of this reaction protocol leads to the late-stage oxidation of complex molecules such as dehydroabetic acid, gemfibrozil, and tocopherol nicotinate.
Mechanistically, the reaction is initiated via oxidation of the Fe(II) center by persulfate to form Fe(III) species and sulfate radical anion I (Scheme 113). Notably, this Fe(III) species is further reduced for the regeneration of Fe(II) species in the presence of PMHS. Then, radical I reacts with alkylarenes via the single-electron transfer mechanism (SET) to generate alkylaromatic cation II. This cation is very acidic and readily eliminates benzylic hydrogen for the further formation of intermediate radical III. Notably, the formation of radical intermediate III was confirmed by trapping it with the radical scavenger 2,2,6,6-tetramerthylpiperodinooxy (TEMPO). Subsequently, radical intermediate III reacts with molecular oxygen (O2) to form benzyl peroxide radical IV followed by abstraction of hydrogen from PMHS. Thereafter, hydroperoxide V undergoes the elimination of water molecule for the formation of the desired aryl aldehydes.151 However, the possibility of self-reaction of two benzyl peroxide radicals IV cannot not be excluded during the formation of the aldehyde.152
Very recently in 2020, Costas and co-workers introduced another synthetic method describing an iron complex, (R, R)-FetipsPDP(OTf)2 (Λ-tips2), catalysed site and product chemoselective aliphatic C–H bond oxidation of polyfunctional substrates in the presence of hydrogen peroxide as a terminal oxidant and fluorinated alcohol as the solvent (Scheme 114).153 The reaction is performed in the presence of a fluorinated solvent such as 1,1,3,3,3,3-hexafluoro-2-propanol (HFIP), exhibiting a strong polarity reversal in the hydroxyl moiety of 1,2-diols due to hydrogen bonding. This hydrogen bonding strongly deactivates the proximal C–H bonds, and thus inhibits proximal C–H bond oxidation. Further, this methodology provides access to the predominant hydroxylation of remote and non-activated C–H bonds, providing orthogonal chemo-selectivity to existing alcohol oxidation methods (Scheme 114). Furthermore, this reaction protocol was successfully applied for executing C–H oxidation of sugars, steroids and other densely functionalized substrates without protecting the hydroxyl functionalities. For example, the anticancer drug capecitabine composed of a highly polar functional group-enriched core together with an oxidation-sensitive five-member cyclic ether possessing a tertiary C–H bond at the γ position and syn 1,2-diol at the α and β positions are well tolerated under the standard reaction conditions. Noteworthily, the cyclic ether moiety is connected to a fluorinated N-heterocyclic moiety connected to an alkyl carbamate moiety. Also, several 4, 5, 6-member metal chelating units are present, having the ability to deactivate the metal catalyst (Scheme 115). However, despite these complexities, capecitabine undergoes hydroxylation (50% yields) at the remote methylenic sites of the alkyl chain (68% conversion) together with the formation of 12% of the corresponding carbonyl compound, keeping the densely functional core intact.
2.2.1.3 Catalytic methods for the formation of C(sp3)–C bond via iron–carbene intermediate. Although, enzymatic and small molecule iron catalysts are capable of C(sp3)–H oxidation involving high valent iron–oxo complexes, the catalytic transformation of the C(sp3)–H bond to a C(sp3)–C bond via an iron–carbene intermediate has been a long-term challenge for chemists. However, in 2017, White and co-workers reported the iron phthalocyanine-catalyzed allylic and benzylic C(sp3)–H alkylation via an isoelectronic iron–carbene intermediate.154 The electrophilicity of a disubstituted diazo compound having a benzylic or allylic moiety enhances the reactivity by producing a strongly electrophilic metallocarbene. Subsequently, the metalocarbene is involved in the C–H bond insertion pathways. Notably, sulfonate ester diazo-precursors are found to be the best diazo compound, producing strongly electrophilic metallocarbene and increasing the C–H alkylation reactivity according to the results obtained via13C-NMR spectroscopy (Scheme 116).
Moreover, the catalyst [PcFeIII]Cl having π-accepting character in the presence of non-coordinating counterions make it more electrophilic, leading to a significant increase in the yield of the C–H insertion product with excellent selectivity. After precise investigation of the non-coordinating counterion to enhance the electrophilic character of the iron catalyst, NaBArF4 exhibits the best result as a coordinating counter ion to increase the yield of the insertion product. Mechanistically, an electrophilic iron–carbene intermediate initiates the homolytic C–H bond cleavage. Then the organo-iron intermediate undergoes the radical rebound mechanistic pathway to form the C–C bond. A stabilized allylic radical is preferred over the secondary aliphatic radical formed during the stepwise olefin oxidation process (Scheme 117). Interestingly, the reaction proceeds via a stepwise mechanistic pathway, which was concluded from the results of the kinetic isotope effect study. The stepwise mechanistic pathway actually improves the chemoselectivity for C–H insertion over cyclopropanation compared to the rhodium catalyst pathway. The intermolecular KIE study indicated C–H bond cleavage is involved in the rate-determining step of the reaction. Notably, scrambling of the olefin geometry is observed using [PcFeIII]Cl as the catalyst, which is consistent with the more stable allylic radical intermediate. The extent of olefin isomerisation is dependent on the electronic substitution of the ligand.
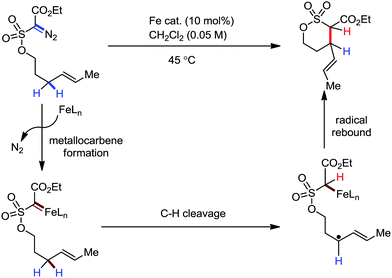 | ||
| Scheme 117 Mechanistic hypothesis of catalytic C(sp3)–H alkylation via an iron–carbene intermediate. | ||
2.2.1.4 Wacker-type oxidation. The palladium (Pd)-catalyzed oxidation of olefins to carbonyl compounds is known as Wacker–Tsuji oxidation. It involves substrates that are readily available, inexpensive and thermally-air and water stable. This oxidation protocol is one of the most straightforward strategies for the preparation of carbonyl compounds. It has wide application in the synthesis of natural products, pharmaceuticals, and commodity chemicals. However, this type of oxidation also has some limitations. The oxidation of electron-deficient internal alkenes cannot be achieved by this process due to the increase in congestion and decrease in electron density of the double bonds, leading to the strong reluctance to generate the η2-Pd olefin complex. Although, Pd-catalyzed Wacker oxidation for the formation of carbonyl compounds from olefin compounds is well known, the late transition metal Pd is very toxic, inexpensive and has low abundance in the Earth's crust. Therefore, the development of alternative catalysts based on the cheap, non-toxic, earth abundant transition metals is of immense importance. Although iron-catalyzed Wacker oxidations are very rare, Che and co-workers155 in 2011 reported the iron-catalyzed anti-Markovnikov oxidation of terminal alkenes to aldehydes using iodosylbenzene as an oxidant via a tandem epoxidation–isomerization (E–I pathway) pathway. The iron(III) porphyrin complex [(2,6-Cl2TPP) Fe(OTf)] was employed as the catalyst, delivering the desired product regioselectively in good yield (Scheme 118).
In 2012, Lahiri and co-workers reported an efficient method of iron-catalyzed regioselective oxidation of styrene with the anti-Markovnikov formation of acetal.156 This method utilizes an iron catalyst, Fe(BF4)2·6H2O, in the presence of pyridine-2,6-dicarboxylic acid as the supporting ligand and iodobenzenediacetate (PhI(OAc)2) as an oxidant in methanol under aerial circumstances (Scheme 119). Further, the use of a dehydrating agent, molecular sieves (3 Å), in the reaction is crucial to achieve better yield of the desired product by minimizing the rearranged product. A wide range of different aromatic and aliphatic cyclic olefins are well tolerated chemoselectively to provide the terminal acetals. In contrast to the anti-Markovnikov selectivity observed in the present method, the palladium-catalyzed acetilization reaction provides Markovnikov selectivity.157
Based on the literature, a plausible mechanism for this reaction was suggested. It is believed that the reaction proceeds via the generation of iron–oxo intermediate I, which reacts with the olefin in a side-way approach to generate intermediate II, followed by a 1,2-hydride shift and two successive solvent nucleophilic attacks at the β position from intermediate III, leading to the formation of the desired product (Scheme 120).
In the same year, they developed another method of regioselective oxidation of terminal alkenes to aldehyde using the same iron catalyst and supporting ligand in the presence of iodosyl benzene as the oxidant in chloroform.158 Different aromatic, aliphatic and terminal olefins together with alkenyl olefins were found to be compatible under the standard reaction conditions (Scheme 121). Based on the experimental results and literature reports, the tentative mechanism of the reaction was proposed, involving two possible pathways, namely tandem epoxidation–isomerization (pathway a) and pinacol-like rearrangement (pathway b) (Scheme 122).159,160
Earlier in 2011, Che and co-workers studied the iron–porphyrin catalyzed anti-Markovnikov oxidation of both terminal aryl and aliphatic olefins to aldehyde using PhIO as an oxidant.161 However, due to the shortcoming of using PhIO as an oxidant, in 2014, they synthesized an iron-catalyst, [FeIII(TF4DMAP)OTf], in the presence of H2O2 as the terminal oxidant for the anti-Markovnikov oxidation of terminal aryl alkenes to aldehydes (Scheme 123).162 It has been presumed that initially [Fe(Por)]+ is converted to the [Fe(O)(Por)] complex in the presence of H2O2, which takes part in the reaction with terminal aryl alkenes to afford the corresponding epoxide, followed by the regeneration of the [Fe(Por)]+ complex, inducing isomerization of the epoxide to the desired product.
Significantly, similar to C–H oxidation, iron co-factor-containing artificial metalloenzymes can also be used in the anti-Markovnikov oxidation of olefins. In 2017, Arnold and co-workers reported the high-valent metal–oxo-mediated anti-Markovnikov oxidation of alkenes using an engineered cytochrome P-450 (aMOx) enzyme in combination with alcohol dehydrogenase (ADH) and oxygen as a terminal oxidant.163 Generally, the high-valent metal–oxo-mediated reaction proceeds via the concerted epoxidation pathway having a low energy barrier, resulting in the formation of the epoxidation product. However, this reaction also provides the direct anti-Markovnikov oxidation product when it proceeds via the formation of the high energy carbocationic intermediate (Scheme 124). This unstable intermediate is stabilised via 1,2-hydride migration, which was also confirmed by an isotopic labelling study. Noteworthily, the asymmetric induction in the case of this methodology arises due to the 1,2-hydride migration. Moreover, the enantioselectivity originates from the locked arrangement of the substrates in a specific conformation, which aligns one of the C–H bonds coplanar to the empty p orbital of the carbocation intermediate. Notably, a wide variety of styrenes are oxidized to the anti-Markovnikov carbonyl product with a TTN of 3800 and 81% selectivity. The para-substituted styrenes are converted into the desired products with high selectivity, while that with meta and ortho substitutions exhibit a lower level of selectivity (Scheme 125). Accordingly, this suggests that the direct anti-Markovnikov oxidation is a catalyst-controlled process, which depends on the exact orientation of the substrate to prevail over epoxidation.
Subsequently, influenced by the chemistry of cytochrome P-450163,164 and the recent reports published by Barton and co-workers,165 Han et al. first developed an iron-catalyzed highly efficient and selective oxidation protocol. Here, polymethylhydroxysilane (PMHS) is used as the reductant in alcohol for the aerobic oxidation of styrene, internal olefins and electron-deficient aryl olefins to ketones (Scheme 126).166
Subsequently, the substrate scope was tested under the optimized reaction conditions. A series of styrenes that are normally challenging substrates for the Wacker oxidation were found to afford the desired product in good to excellent yield with excellent selectivity. Substrates having electron-donating substituents and electron-withdrawing substituents in the aryl rings give a comparative yield. Moreover, a wide range of functional groups such as halo groups (Cl, Br, and I), esters, benzyl chloride, nitro group and carboxylic acids are well tolerated. Particularly, the presence of several readily oxidizable groups such as aldehyde, phenol, silane, and arylboronic acids and strong coordinating pyridyl groups are well tolerated. Notably, under slightly modified conditions, i.e. using Fe(acac)2 as the catalyst and t-BuOH as the solvent, aliphatic terminal olefins undergo smooth conversion into the expected ketone with complete Markovnikov selectivity. In contrast to Pd-catalyzed Wacker-type oxidation, electron-deficient internal alkenes such as trans-benzylideneacetone and heterocyclic trans-4-(2-thienyl)-but-3-en-2-one undergo the reaction to produce the desired product. Additionally, an aliphatic internal alkene, (3Z)-hept-3-en-1-ol, which is completely unreactive, was reported by Sigman167 and co-workers to be an efficient substrate exhibiting sufficient reactivity. Moreover, this synthetic protocol is applied for the oxidation of natural products and synthetic compounds with high complexity. For instance, a derivative of the antibacterial drug pleuromutlin underwent the reaction protocol to deliver the desired oxidized product in 70% yield. The mechanistic pathway of the iron-catalyzed Wacker-type oxidation of olefins to ketones using molecular oxygen as the sole oxidant in the presence of reductive silane is shown in Scheme 127.
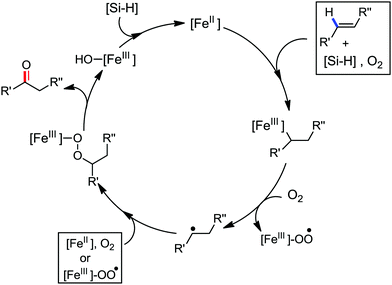 | ||
| Scheme 127 Proposed mechanistic pathway of iron-catalyzed aerobic Wacker-type oxidations of olefins. | ||
Initially, the olefin reacts with iron(III) hydride to generate the alkyl iron-intermediate. The iron–hydride is formed from the reaction between the iron(II) salt and hydrosilane. Notably, the best catalytic efficiency is shown by FeCl2 towards this transformation. The reaction intermediate with oxygen generates the carbon-centered radical, followed by the generation of an iron–peroxide complex. At this stage, the iron–peroxide complex undergoes a concerted pathway with simultaneous O–O and C–H bond breaking to afford the ketone product via the formation of the Fe(III)–OH species. The Fe(III)–OH species is reduced by hydrosilane to regenerate the Fe(II) catalyst. The intermediate carbon-centred radical was intercepted with the radical scavenger galvinoxyl. Thus, this suggests that the reaction proceeds via the radical pathway. Furthermore, no deuterium was incorporated in the product using EtOD as the solvent, while 96% deuterium was incorporated at the α-position of the ketone using (EtO)3SiD as the silane. This indicates the addition of hydrogen across the olefin from the hydrosilane.
The Wacker-type oxidation of 1-decene to 2-decanone by di-oxygen catalyzed by Pd(OAc)2, hydroquinone and iron(II) phthalocyanin (FePc) in acidic aqueous dimethylformamide was reported by Ford and co-workers in 1990.168 The transformation provides high yield at room temperature within 40 min. The factors controlling the activity of the multi-component catalyst are the structure and morphology of the FePc catalyst. The iron catalyst exists in the β-FePc form and dimeric-μ-oxo diiron FePc species. The dimeric μ-oxo form of the FePc catalyst is highly active due to its high surface area and less crystalline character than the other possible form. However, the scope for oxidation using this synthetic method is limited by the solubility of 1-decene in aqueous DMF.
Subsequently, in 2018, Knölker and co-workers developed a mild and operationally simple procedure with excellent chemoselectivity and functional group tolerance.169 In this reaction protocol, they used a hexadecafluorinated iron–phthalocyanine complex (PcFeF16) (Fig. 5) as the catalyst with a stoichiometric amount of triethylsilane as an additive in the presence of molecular oxygen for the oxidation of olefin into ketones with good to high yields (Scheme 128).
This catalytic system shows better reactivity than FePc due to its better solubility in ethanol and the electron-withdrawing effect of its fluorine substituents. Notably, the ketone product is obtained in up to 95% yield with 100% regioselectivity, while the corresponding alcohol is obtained as the side-product. Mechanistically, a μ-oxo-bridged diiron complex is initially formed from the parent iron–phthalocyanine complex (Scheme 129). The corresponding diiron complex was also detected by ESI mass-spectroscopy. This diiron complex undergoes formal dissociation to generate two iron complex fragments. Then, the nucleophilic attack of the anionic species at the silicon atom of the triethylsilane leads to the formation of a pentavalent silicon anion intermediate, providing an iron(III) hydride species via the hydride transfer to the cationic species. The Fe(III)–hydride complex transfers the hydrogen atom to the alkene. Thereafter, it follows the same mechanistic pathway mentioned in Scheme 127.
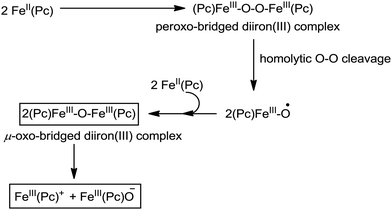 | ||
| Scheme 129 Proposed mechanistic pathway for the Fe(Pc)F16-catalyzed oxidation of olefins to ketones. | ||
2.2.1.5 Baeyer–Villiger-type oxidation. Adolf Baeyer and Victor Villiger discovered the oxidation of menthone to the corresponding lactone using a mixture of sodium persulfate and concentrated sulphuric acid. Thereafter, this persulfuric acid was replaced by other organic per-acids such as m-CPBA, trifluoroperacetic acid and perbenzoic acid. Thus, the conversion of ketone into the ester in the presence of per-acid as an oxidant has become one of the most well-known and widely applied organic reactions, which is known as Baeyer–Villiger (B–V) oxidation. However, the organic peracids used in the oxidation reactions are hazardous and expensive. Thus, using clean and inexpensive oxidants is extremely significant and attractive from an environmental and economic viewpoint. The aerobic Baeyer–Villiger (B–V) oxidation of ketones in the presence of aldehyde was reported using Fe2O3 and Fe–MCM-41 under heterogeneous conditions.170 However, the drawback of the MCM-41 system is its unidirectional pore system. Thus, to overcome this drawback, Koodali and co-workers in 2008 discovered a highly active mesoporous catalyst, Fe-MCM-48, for the Baeyer–Villiger oxidation of cyclohexanones and the bulky molecule 2-adamantanone system.171 The catalyst can be reused for at least three catalytic cycles without any loss in activity. Notably, cubic MCM-48 with its interwoven and continuous 3D regular pore system provides favourable mass-transfer kinetics and is a better candidate for catalytic applications. Powder XRD (X-ray diffraction) and DR (diffuse reflectance) studies indicated the incorporation of Fe3+ into the silica pore wall in a tetrahedral position. Moreover, this material has a large surface area (1979 m2 g−1) and large pore volume (1.2 cm3 g−1).
In biology, oxygenase enzymes such as cytochrome P-450 carry out the oxygenation of different bio-molecules.172 Inspired by these enzymes, Ji et al. in 2008 reported the highly efficient selective Baeyer–Villiger (B–V) oxidation for the first time using iron(III)–meso-tetraphenylporphyrin [(TPP)FeIIICl] with molecular oxygen in the presence of benzaldehyde for the conversion of ketones into lactones (Scheme 130).173 Notably, the oxidation of cyclohexanone catalyzed by the iron metalloporphyrin involves radical species. Thus, toluene was used as the solvent for the transformation due to its reluctance to undergo radical addition. However, although different metals (Ru, Mn, Fe, and Co) were tried with the TPP ligand, iron showed the best efficacy for the oxidation reaction. The reason for this is that the catalytic activity and selectivity of different metalloporphyrins depend on the stability of the different valences of their metal atoms and their electric potentials. Notably, cyclic ketones are more efficiently oxidized than acyclic ketones. Moreover, six-membered cyclic ketones are the most efficient among the cyclic ketones to generate the corresponding oxidized products. The turnover number (TON) of the (TPP)FeCl catalyst can be maximized to 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the large-scale oxidation of cyclohexanone.
000 for the large-scale oxidation of cyclohexanone.
Here, ε-caprolactone was obtained in 96% yield in the presence of benzaldehyde, while the use of isobutyraldehyde afforded the product in only 11% yield. To investigate this difference, they further studied the mechanistic pathway. Based on the results of in situ FTIR, UV-vis spectroscopy and starch/KI experiments, they proposed the mechanistic pathway (Scheme 131).174 In the initial step of the mechanistic pathway, the iron–porphyrin reacts with the aldehyde to generate the acyl radical, which reacts with the molecular oxygen to give the acyl peroxy radical. The resultant acyl peroxy radical as a carrier in the chain mechanism reacts with another molecule of aldehyde to generate peroxybenzoic acid. The peroxybenzoic acid is involved in two pathways, A and B, in the presence of benzaldehyde and isobutyraldehyde, respectively. In the presence of benzaldehyde (pathway A), it reacts with another iron–porphyrin molecule to generate a high-valent iron(V)–porphyrin intermediate via the elimination of benzoic acid. Then, the intermediate combines with cyclohexanone to take part in the oxygen transfer step, affording ε-caprolactone. However, in the presence of isobutyraldehyde (pathway B), peroxybenzoic acid attacks the activated ketone to generate a Criegee adduct, followed by protolysis to afford a lactone (Scheme 131).
2.2.2.1 Halogenations in Nature. Nature has the ability to synthesize all the useful bioactive molecules and valuable natural products containing carbon halogen bonds. There are several enzymes that perform halogenation in nature, such as (a) heme–iron peroxidaseses, (b) vanadium-dependent haloperoxidase, and (c) non-heme–iron halogenases.175 Interestingly, these enzymes contain first row transition metals as a metal co-factor in their active sites. Among these enzymes, the heme and vanadium-dependent haloperoxidase enzymes perform non-specific halogenations in the presence of hydrogen peroxide at multiple sites of bio-molecules due to the release of hypohalous acid.176 However, in the case of non heme–iron halogenase enzymes, molecular oxygen oxidizes the metal co-factor iron(II) centre and forms the high valent cis-iron(IV)–oxo–halide intermediate (S = 2), which performs regio- and stereospecific C–H halogenations of bio-molecules. For example, the α-keto glutarate (α-KG)-dependent non-heme halogenase enzyme performs C(sp3)–H halogenations by overriding hydroxylation reactions. This enzyme has been identified from SyrB2 during the biosynthesis of Syringomycin E.175 The active site of this enzyme contains iron(II), which is coordinated with two histidine moieties, one aspartate moiety, one chloride and one water molecule (Scheme 132). Mechanistically, the iron–oxo–halide intermediate abstracts the hydrogen atom (HAA) from the C–H bond to generate a nascent radical and iron(III) halide species. This nascent radical and iron(III) halide complex are placed in the enzyme in such a way that the radical selectively undergoes rebound with the halide and provides the selective halogenated product.177 The observed selectivity with halogenase enzymes via chloro rebound of the alkyl radical can be rationalised based on the following factors: (i) the relative differences in redox potential of the rebound site, i.e. between OH and X radical, (ii) the pattern of hydrogen bonding at the active site, (iii) the selectivity of enzyme due to the relative position of the substrate, (iv) dynamics in the rebound intermediate, and (v) deactivation of the hydroxyl group by protonation or CO2 interference. Among them, the relative positioning of the substrate in the protein cavity is widely accepted for favouring selective halogen transfer.178 However, the perfect positioning of the substrate between the halide and hydroxide in the absence of a protein chain is quite challenging, which is an unavoidable issue in the case of biomimetic synthetic analogues of non-heme iron(IV)–oxo halide complexes, resulting in the formation of a mixture of halogenation and hydroxylation products.
2.2.2.2 Bio-mimetic halogenations by synthetic non-heme iron complexes. Since the 1990s to the present, there have been extensive efforts to understand and mimic the active site structure and reactivity of halogenase enzymes. Thereafter, with the biomimetic synthesis mimic analogues of these enzymes, their functional reactivity have been tested. In this section, the attempts to mimic the structural and functional behaviour of iron-based non-heme halogenase enzymes are discussed.
In 1993, Que and co-workers first reported the synthesis and structure of biomimetic non-heme iron complexes, [FeIII(TPA)X2](ClO4) [X = Cl, Br, and N3] [TPA = tris(2-pyridylmethyl)amine]. Treatment of this complex in presence of stoichiometric amount of tert-butyl hydroperoxide, and subsequently cyclohexane affords cyclohexylbromide.179 The kinetic isotope effect (KIE) between cyclohexane and d12-cyclohexane (KH/KD = 7–10) suggests that a hydrogen atom abstraction (HAA) step is involved in the rate-determining step of this reaction. The observed values of KIE significantly vary with nature of the tripodal ligand system and halide groups. Thus, they proposed the involvement of iron(III)–alkyl peroxide I (Scheme 133) and high-valent iron(V)–oxo–halide intermediate II, where the iron centres coordinate with one halide atom. Initially they assumed that intermediates I and II can participate in the HAA step in the presence of the substrate (cyclohexane and adamantine) (Scheme 133). However, the experiments with different peracids having different R (R = tertiary-butyl, i.e. t-Bu, and cumyl group, i.e. [PhCMe2]) groups do not affect the fate of the reaction.
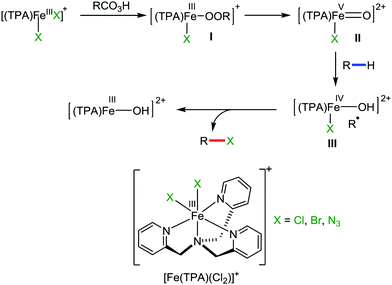 | ||
| Scheme 133 C–H halogenations by model high-valent non-heme iron–oxo model complex, [FeV(TPA)(O)(X2)]. | ||
Thus, it was concluded that intermediate II does HAA from the C–H bonds of cycloalkanes. Further, they observed that the high efficiency of the ligand (X) is transferred to the substrates with respect to ROOH. Additionally, the lack of formation of the alkyl radical homocoupling product together with the failure of 4-methyl-2,6-di-tert-butyl phenol to affect the reaction supports that iron(IV)–oxo–hydroxo–halide species III and the radical remain in the cage. Then, the nascent radical reacts via the halide rebound pathway, resulting in the major halogenated products together with minor hydroxylation product. The observed selectivity was rationalized based on the difference in redox potential between chloride and hydroxide during radical transfer from the intermediate C (ECl˙/Cl− = 1.36 V vs. NHE, EHO˙/HO− = 2.02 V vs. NHE).
In 2006, Que and co-workers synthesized and characterized Fe(IV)–oxo–halide complex 2, [FeIV(TPA)(O)X]+.180 The presence of N,N,N-tris(2-pyridylmethyl)amine (TPA) as a ligand provides a tripodal topology around the iron centre having a labile sixth coordination site adjacent to the oxo group. Treatment of peracid and 3 equivalents of NR4X (X = CF3CO2, Cl, and Br) with iron(II)-complex 1, [FeII(TPA)(NCMe)](OTf)2, in acetonitrile at −40 °C affords a series of metastable [FeIV(TPA)(O)X]+ complexes 2 (Scheme 134). Spectroscopic studies and DFT calculation confirmed the cis geometry of the MeCN group with respect to the oxo ligand, which can be substituted by a chloride or bromide ion in the presence of NR4X [X = Cl, and Br]. Later, they introduced the TMG2dien [2′,2′-(2-2′-(methylazanediyl)-bis(ethane-1,2-diyl))bis(1,1,3,3-tetramethylguanidine)] ligand with Fe(II) for the synthesis of cis-[FeIV(TMG2dien)(O)(X)]+5 (Fig. 6).181 In 2007, Noack and Seigbahn demonstrated that high valent [FeIV(TPA)(O)(Cl)]+2 (Scheme 134) is capable of stereospecific alkane chlorination involving a chloride anion rather than a chlorine radical182via computational studies. Moreover, they also reported that the HAA step is the rate-determining step of the oxidative chlorination reaction and this reaction proceeds via the concerted mechanistic pathway. However, Visser183 and co-workers reported that the stereochemistry significantly affects the selectivity based on a DFT study. The cis geometry of the oxo ligand with an amine-N ligand favours halogenation, while the trans geometry favours the hydroxylation reaction. Further, in 2010, Comba and co-workers developed the halogenation reaction of alkanes using a low molecular weight non-heme iron(II)-complex containing the bispidine ligand system (L = 3,7-dimethyl-9-oxo-2,4-bis(2-pyridyl)-3,7-diazabicyclo[3.3.1]nonane-1,5-dicarboxylate methyl ester).183 They observed that the KIE value and yield ratio between the halogenated and hydroxylated products are sensitive towards the nature of the oxidant. This is due to the involvement of different high-valent iron intermediates, e.g. FeIV and FeV, and unwanted alkoxide radical (OR/OH radical in the case of oxidant-like TBHP and H2O2). Here, [FeIV(bispidine)(O)(Cl)]2+3 (Fig. 6) was proposed as the key intermediate to carry out the reaction via HAA and the halide rebound pathway.183 The kinetic isotope effect value was determined to be (KIE = 14) between normal C–H- and C–D-containing substrates. Also, they found the preferential tertiary (by 3) over the secondary C–H bond abstraction via the rate-limiting HAA step.
Later, Costas and co-workers utilized the 1-(20-pyridylmethyl)-4,7-dimethyl-1,4,7-triazacyclononane (PyTACN) ligand to synthesize [FeIV(PyTACN)(O)(Cl)]+ complex 4 (Fig. 6), and subsequently employed it for C–H halogenations with different substrates.184 However, complex 4 did not provide any halogenation product during reaction with cycloalkanes. This is due to the strong coordination of the halide group to the metal centre, which provides higher stability to complex 4 (Fig. 6).
In 2014, Maiti and co-workers reported a method for the oxidative chlorination/bromination of electron-rich arenes and olefins in the presence of a biomimetic non-hemeiron(II)-complex as the catalyst, iodosyl benzene (as the oxidant) and halide sources (KCl and KBr) in methanol solvent (Scheme 135).185 Different electron-rich arenes and olefins were halogenated using a stoichiometric amount of non-heme iron(II)–halide complexes, [FeII(TPA)X2] (X = Cl and Br) in the presence of PhIO in acetonitrile solvent. Moreover, the catalytic halogenation reactions of arenes and olefins were performed using [FeII(TPA)(CH3CN)2](ClO4)2 as the catalyst in the presence of external halide sources in methanol solvent. Interestingly, under these reaction conditions, halo-methoxylation products were obtained with olefins.
Further studies were carried out to understand the mechanism of the reaction. Notably, no aliphatic halogenations were observed under the reaction conditions. Moreover, the low-temperature ESI-mass spectroscopy study at −40 °C from the reaction between [FeII(TPA)Br] 2 (Scheme 136) and iodosyl benzene enabled the detection of [FeIV(TPA)(O)(Br)]+ species I (Scheme 136). Additionally, the authors detected the iron(III)methoxy halide species [FeIII(OMe)(X)]+III from the reaction mixture by ESI-MS study. The EPR study of the same reaction mixture suggested that [FeIII(OMe)(X)]+ is possibly a high-spin species. Thus, based on the preliminary experiments and intermediates detected, a plausible mechanism involving the X+ equivalent was proposed. It was presumed that intermediate III undergoes oxidative transformation to generate reactive iron-bound hypohalite species IV, which possibly accomplishes the electrophilic halogenations (Scheme 136).
In 2016, Paine and co-workers developed a non-heme iron complex, [FeII(TpPh2)(benzilate)] (TpPh2 = hydrotris(3,5-diphenyl-pyrazol-1-yl)borate), and generated non-heme iron(IV)oxo–aquo complex I in the presence of a proton source and dioxygen molecule.186 Treatment of complex I with pyridinium perchlorate and tetrabutylammonium halide produced reactive iron(IV)–oxo–halide intermediate 6via the substitution of a water molecule with halide (Scheme 137). Subsequently, complex 6 (Fig. 6) reacted with different substrates and resulted in major halogenations together with minor hydroxylation products. Notably, this is the first example of a non-heme model complex involving oxygen (O2) as the oxidant to obtain a halogenated product. In the same year, Que and co-workers carried out C–H bond halogenation reactions using [FeIV(TQA)(O)(Cl)]+ (7) [TQA = tris-((quinolyl-2-methyl)amine)] and [FeIV(TQA)(O)(Br)]+ (7), which are generated via ligand exchange from [FeIV(TQA)(O)(MeCN)]2+ with halide sources at a low temperature (Fig. 6).187 Notably, this is the only synthetic complex to reproduce the Mössbauer parameters of the spin (S = 2) of the iron(IV)–oxo–halide intermediate of the α-KG-dependent halogenase enzymes. Thus, a higher selectivity (compare to other synthetic analogues) for the formation of the halogenated product was achieved together with the hydroxylation product. However, halogenations by this synthetic cis-iron(IV)–oxo–halide complex do not match the selectivity of the halogenase enzyme.
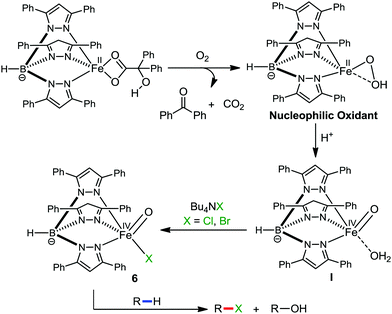 | ||
| Scheme 137 Mechanistic proposal for the formation of electrophilic iron–oxygen oxidant and its role in C–H bond halogenations. | ||
Recently, in 2018, Maiti and co-workers developed a selective C–H halogenation reaction using non-heme iron(IV)–oxo and iron(II)–halide complexes, where the former acted as a radical generator via HAA from the substrates and the latter as a halide atom donor (Scheme 134).183 Precisely, the authors utilized pentadentate ligand, 2PyN2Q [1,1-di(pyridin-2-yl)-N,N-bis(quinolin-2-ylmethyl)methanamine], supported non-heme iron(IV)–oxo 8 and iron(II)–halide complexes 9 and 10 (Scheme 138). After the generation of complex 8, i.e. [FeIV(2PyN2Q)(O)]2+ from [FeII(2PyN2Q)(OTf)2] in the presence of m-CPBA (meta-chloroperbenzoic acid) in acetonitrile at room temperature, different halide sources such as tetra-butyl ammonium chloride and bromide (Bu4N+Cl− or Bu4N+Br) were added to the reaction mixture together with cyclohexane.188 However, no halogenated products were obtained from this reaction. This observation can be explained due to the lack of formation of the [FeIV(2PyN2Q)(O)X]+ intermediate by the halide exchange reaction between the pentadentate ligand containing the iron(IV)–oxo complex and halide sources. Subsequently, the room temperature stable iron(IV)–oxo, [FeIV(2PyN2Q)(O)]2+8, was used for hydrogen atom abstraction (HAA) from the aliphatic substrates in conjunction with iron(II)–halide complexes [FeII(2PyN2Q)(X)]+ (X = Cl, Br) 9 and 10 to provide the halogen atom to the cage-escaped carbon centred radical.
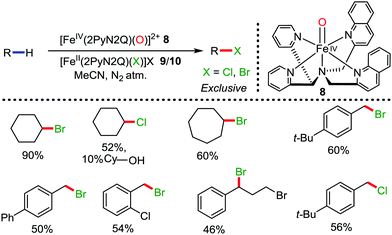 | ||
| Scheme 138 Selective halogenations by a non-heme iron(IV)–oxo, [FeIV(2PyN2Q)(O)]2+, in the presence of [FeII(2PyN2Q)X]2+ as the halide source. | ||
Interestingly, cyclohexane delivers the cyclohexylbromide derivative selectively in 90% yield (with respect to the limiting reagent [FeII(2PyN2Q)(Br)]Br) under the standard reaction conditions (Scheme 139). Moreover, various cycloalkanes and toluene derivatives are well tolerated under the reaction conditions to provide the selective halogenated product together with a minor amount of oxidized product in some cases (Scheme 136). A kinetic isotope effect was observed using toluene and d8-toluene under the halogenation reaction conditions (KIE = 13.5). Thus, this indicates that the initial HAA step is involved in the rate-determining step. Further, the radical trapping experiments during the reaction between 8 and cyclohexane in the presence of CCl3Br provided exclusively cyclohexylbromide. This suggests that the radical generated via the HAA pathway undergoes dissociation from the cage with iron(III)–hydroxide, which is trapped by the Br˙ radical coming from the cleavage of the C–Br bond of CCl3Br. Subsequently, the cage-escaped radical reacts selectively with the iron(III)–halide via the halide rebound pathway. Moreover, the feasibility of this particular halide rebound step was tested using pre-oxidized iron(III)–halide complexes (prepared from iron(II)–halide and m-CPBA) instead of iron(II)–halide complexes under the standard reaction conditions.
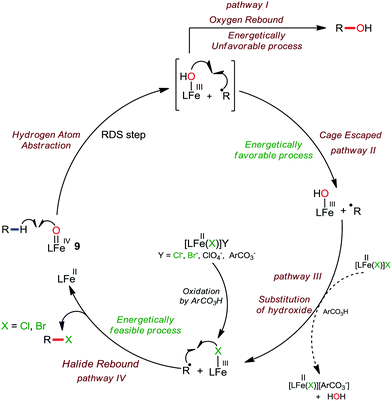 | ||
| Scheme 139 Proposed mechanism of non-heme iron(IV)–oxo [FeIV(2PyN2Q)(O)]2+-mediated C–H halogenation. | ||
Noticeably, the selective halogenation product was obtained from the above reaction. Furthermore, DFT calculation revealed that the sterically bulkier pentadentate ligand 2PyN2Q (compared to N4Py) exerted a large difference in the electronic structure of [Fe(2PyN2Q)(O)]2+. This difference in electronic structure exerts a high energy barrier in the oxygen rebound process (pathway I) and favours the low energetic cage-escaped pathway for the radical (pathway II). Further, the computational study revealed the reason for the selectivity in halogenation over hydroxylation. Firstly, it suggested that the cage-escaped radical preferentially penetrates the coordination sphere of the iron(III)–halide complex (due to its high electron affinity) since it is an energetically favourable pathway compared to oxygen rebound with iron(III)–hydroxide in the cage. Secondly, it also suggested that hydroxide substitution (pathway III) by the halide counter anions present in non-heme iron(II)–halide complexes 9 and 10 is a spontaneous process. Finally, the transition-state calculations demonstrated that the halide rebound (pathway IV) of the radical with the iron(III)–halide is an energetically downhill process due to the longer Fe–Cl bond compared to oxygen rebound with iron(III)–hydroxide, which has been found to be an uphill process due to the short Fe–O bond distance. Thus, based on the experimental and computational studies, a plausible mechanism was proposed involving the aforementioned pathways (Scheme 139).
The present bio-inspired stoichiometric halogenation reactions involving high-valent iron(IV)-complexes depict the different mechanistic aspects of C–H halogenation reactions. However, the present situation demands iron-catalyzed methods for the selective halogenation of aliphatic C–H bonds considering the importance carbon–halogen compounds. Contextually, Groves and co-workers developed manganese porphyrin catalyzed halogenation reactions including chlorination and fluorination, where selective halogenated products are obtained together with a minor amount of hydroxylation product.189 However, in the case of iron catalysis, there is no report on methods for catalytic aliphatic C–H halogenation. Thus, iron catalysis remains elusive and challenging for the development of catalytic aliphatic C–H halogenation reactions.
3. Cross dehydrogenative coupling (CDC) reaction
Transition metal or organic reagent-mediated carbon–carbon (C–C) and carbon–heteroatom (C–X) (X = N, O, P, S, Se, etc.) bond formation involving two different C–H bonds or one C–H and one X–H bond is formally known as the cross dehydrogenative coupling (CDC) reaction.190 In this reaction, the direct coupling of either two C–H bonds (to form a C–C bond) or one C–H bond and another nucleophilic coupling partner, X–H (to form C–X bond), requires a suitable oxidant.191 Moreover, in this process, two hydrogen atoms are eliminated. There are several benefits of the CDC reaction. Firstly, it does not require the prefunctionalization or preparation of any starting material. Secondly, the starting materials are easily available. Interestingly, this strategy improves the atom economy and the step economy of oxidative coupling.192,193 However, the CDC reaction has been found to be challenging due to the poor reactivity of C–H bonds, and thus the chemists have developed new methods to activate different types of C–H bonds for the formation of C–C and C–X bonds. Contextually, here, we elucidate the prospects of the most abundant transition metal, iron, catalyzed CDC reactions. We classify the iron-catalyzed CDC reactions into different topics based on the type of bond formed via CDC, e.g. C–C or C–X. Then each topic is further divided into sub-topics based on the hybridization and types of bonds participating in the reactions. Li et al. in 2007 reported the first example of an iron-catalyzed CDC reaction, describing the formation of the C–C bond.1943.1 CDC between C (sp3)–H and X (sp3)–H (X = C, N, O, S, etc.) bonds
Iron-catalyzed CDC of C(sp3)–H is quite challenging due to its low reactivity originating from the high bond energy and absence of suitable co-ordination sites for the iron catalyst compared to the oxidative coupling of C(sp2)–H or C(sp)–H bonds. In the iron-catalyzed CDC reaction, the oxidant abstracts a hydrogen atom from the C–H bond to generate carbon-centred radical I, followed by the generation of carbocationic intermediate IIvia the single-electron transfer (SET) process. Finally, the nucleophile attacks carbocation intermediate II, resulting in the formation of the desired product (Fig. 7).195Alternatively, the C–H bond adjacent to the heteroatom is abstracted via the SET mechanistic pathway and generates radical cation III, which is readily α-deprotonated by the oxidant to generate carbenium ion IV. Finally, it is attacked by an incoming nucleophile to give the final coupled product (Fig. 8).195 Notably, here the deprotonation of Nu–H occurs either before or after the attack to the carbocationic intermediate depending on the acidity of the Nu–H bond.
3.1.1.1 C–C bond formation with benzylic C(sp3)–H bonds. Based on Li's earlier investigation, diphenyl methane has been chosen as one of the standard substrates due to the presence of two activated C–H bonds at the benzylic position.194 The other active coupling partner is the 1,2-dicarbonyl compound. The best efficacy is shown by FeCl2 as a catalyst compared to Cu or Co salts. The use of the di-tert-butyl peroxide (DTBP) instead of tert-butyl hydro peroxide (TBHP) as an oxidant increases the yield of the product. Notably, this is the first example of an iron-catalyzed CDC reaction. The synthetic method is applicable to both cyclic and acyclic benzylic compounds. The electronic effect of the substituents shows very little effect on the yield of the product (Scheme 140).
Mechanistically, the initial formation of the tert-butoxyl radical occurs in the presence of ferrous chloride via homolytic cleavage of tert-butyl hydroperoxide (TBHP). Then the tert-butoxyl radical abstracts a hydrogen atom from the benzylic C(sp3)–H bond, which results in the formation of a benzylic radical (Scheme 141). On the other hand, the Fe(III) catalyst reacts with the 1,3-dicarbonyl compound to form a chelate Fe-enolate complex. Finally, the electrophilic radical is attacked by the benzylic radical in the chelate complex, affording the product via the regeneration iron(II). The isolated yield of the product is increased up to 80% upon increasing the reaction time. Notably, the reaction is also found to proceed efficiently at room temperature.
Later in 2007, Li and Zhang employed inactive cycloalkanes for the oxidative coupling reaction with active 1,3-diketone in the presence of di-tertiary butyl peroxide (DTBP) as an oxidant and iron salt as a catalyst.196 However, the active catalyst is the chelated Fe–enolate complex, which is formed from the reaction between iron(II) chloride and the dicarbonyl compound (Scheme 142). Notably, the same reaction protocol was also conducted on different types of cycloalkanes such as cyclohexane, cyclopentane, cycloheptane, norbornane, adamantane and n-hexane systems. Notably, the reaction with the norbornane system provides the alkylation product as a mixture of two diastereomers in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Here, the desired product was not obtained from the reaction of the bridgehead C–H bonds. Moreover, when the adamantane system was reacted with ethyl benzoylacetate, a mixture of the alkylation product and the coupling product on the more substituted carbon was obtained with a 4
1 ratio. Here, the desired product was not obtained from the reaction of the bridgehead C–H bonds. Moreover, when the adamantane system was reacted with ethyl benzoylacetate, a mixture of the alkylation product and the coupling product on the more substituted carbon was obtained with a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Interestingly, n-hexane also yields two regioisomeric alkylation products with β-ketoester (in 1
1 ratio. Interestingly, n-hexane also yields two regioisomeric alkylation products with β-ketoester (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio). The slightly higher ratio of the corresponding alkylation product is due to the formation of the more stable radical at the C3-methylene position rather than the alkylation at the C2-methylene position.
2 ratio). The slightly higher ratio of the corresponding alkylation product is due to the formation of the more stable radical at the C3-methylene position rather than the alkylation at the C2-methylene position.
Li et al. in 2012 described the oxidative coupling reaction of a 1,3-dicarbonyl compound with a toluene derivative in place of cycloalkanes196 in the presence of iron(II)-acetate/di-tert-butylperoxide (DTBP) as the catalyst/oxidant system (Scheme 143).197 Various substituted toluenes undergo the oxidative coupling efficiently with the 1,3-dicarbonyl compound under the optimized conditions. The mechanistic pathway suggests that the benzylic radical undergoes addition to the benzoyl methano-iron species in this reaction.
In 2015, Song et al. reported an iron-catalyzed tandem cross-dehydrogenative coupling reaction of toluene derivatives with 1,3-dicarbonyl compounds using 2,3-dicholoro-5,6-dicyano-1,4-benzoquinone as the oxidant.198 Notably, 1,3-dicarbonyl compounds bearing electron-donating groups such as alkyl (e.g. methyl and ethyl), aryl and alkoxide were found to react efficiently under the reaction conditions. Moreover, electron-withdrawing substituents (e.g. F) on the aromatic ring of aryl-β-ketone esters are also well tolerated. This reaction proceeds through initial sp3–sp2 coupling followed by sp3–sp3 oxidative coupling to afford the desired product. The reaction is proposed to be initiated by the homocoupling of two molecules of aryl methane to form a diarylmethylene intermediate. Then the intermediate participates in the CDC reaction with the 1,3-dicarbonyl compound to give the final desired product (Scheme 144).
In 2012, Xu et al. reported the iron-catalyzed benzylic C(sp3)–H vinylation of 2-methyl aza-arenes with N,N-dimethyl amides.199 Mainly, one of the carbon atoms present in the N,N-dimethyl moiety of N,N-dimethyl amides is transferred to the 2-methyl aza-arenes to give the desired product in good yield (Scheme 145). Thus, the N-methyl amide acts as the carbon source for vinylation. Notably, among the different amides, N,N-dimethyl formamide and N,N-dimethyl acetamide provide the desired product. Also, different electron-donating and electron-withdrawing aromatic rings provide the desired product in good to excellent yields. Further, they performed control experiments gain mechanistic insight. Notably, the reaction failed to give the vinylation product when it was carried out in the presence of 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) as a radical scavenger. Thus, this suggests that the reaction may occur via a radical pathway. Moreover, the observed primary kinetic isotope effect (KIE, KH/KD = 2) reveals that the cleavage of the C–H bond of the N,N-dimethyl amide is involved in the rate-determining step. Thus, based on these results, it was proposed that enamine intermediate I is generated from the model substrate 2-methyl-3-phenylquinoxaline 1 in the presence of iron(III) (Scheme 146). Subsequently, intermediate I attacks iminium ion intermediate III to generate the desired vinylated product Vvia elimination.
In 2013, a similar type of iron-catalyzed vinylation of 2-methyl quinolines with N,N-dimethyl formamide in the presence of TBHP was reported by Wang et al.200 Notably, based on the successful radical experiment and primary kinetic isotope effect study, the authors proposed a similar type of mechanism involving enamine and iminium intermediates as mentioned in Scheme 146. In 2015, Li et al. utilized N,N-dimethyl acetamide for the α-methylation of ketones in the presence of FeCl3 as a catalyst to form α,β unsaturated carbonyl compounds in moderate to excellent yield (Scheme 147).201 Various types of ketones such as aryl and alkyl ketones, enones and dicarbonyl compounds are well tolerated in the standard reaction protocol.
In 2016, Su and co-workers described the iron(III) catalyzed CDC of 3-benzylic indoles with active methylene or indoles groups via a mechanically induced high speed ball-milling process (HSBM) at room temperature in the absence of solvent.202 Notably, HSBM is a green mechanical technique for the formation of nanomaterials, which promotes chemical reactions with high efficacy, energy economy and even new reactivity. The reaction affords the desired 3-aryl methyl indole derivatives in moderate to high yield within 21 min of grinding. Here, silica gel is used as a grinding auxiliary for better substrate mixing (Scheme 148).
Although, iron(II) and iron(III) show catalytic activity in this method, iron(III) salts show better efficacy. Further, among the different iron salts, Fe(NO3)2·9H2O was chosen due to its ease of handling. The best result was obtained using DDQ as the oxidant. However, it is added in several portions to avoid radical polymerization. Both the electron-withdrawing substituents and electron-donating aryl substituents including the oxidation-sensitive OH group in the 3-aryl methyl of indoles are well tolerated to afford moderate to excellent yields of the corresponding product. Notably, electron-rich substituents give a better product yield. However, indoles are also taken as nucleophiles in place of the active methylene group. Here, both N-substituted and N-free indoles are tolerated under the standard optimized conditions to give the bis-indole derivative. The nucleophiles, indoles (R3 = indole), possessing an electron-donating groups provide a lower yield of product compared to that with electron-withdrawing groups.
In 2018, Oshima and Yazaki reported ligand-supported chemoselective cross-dehydrogenative coupling using hydrocarbon feedstock with non-tautomerizable 2-acylimidazole under mild conditions.203 The synthetic transformation is based on the simultaneous catalyst-controlled activation strategy, i.e. in situ catalytic activation of 2-acyl imidazole and catalyst control radical generation from the hydrocarbon to form the desired product. The tert-butoxy radical from DTBP is generated in the presence of FeCl2 as the catalyst and L1 as the ligand after screening of the conditions. This method using the catalyst/ligand system allows catalyst-control radical generation from hydrocarbons (Scheme 149). Notably, an undesired homo-coupling product of toluene (i.e. dibenzyl) was obtained under the optimized conditions. Several control experiments revealed that no product is obtained in the absence of either FeCl2 or ligand.
The reaction does not occur at 80 °C in the absence of ligand. However, a moderate yield of the desired product is obtained when the reaction is carried out at 120 °C. Evidently, the generation of tert-butoxy radical is facilitated by thermal heating at a higher temperature. Rationally, the utilization of a ligand in the presence of the FeCl2 catalyst can accelerate the homolytic cleavage of DTBP to generate the radical even at a lower temperature.
Based on a series of mechanistic studies, a plausible catalytic cycle was proposed (Scheme 150). Initially, iron(III)-based intermediate I is formed in the presence of FeCl2 and DTBP through the Fenton-type reaction with the help of the L1 ligand. Then 2-acyl imidazole coordinates with the iron intermediate to afford iron(III) and iron(III)-based intermediate II. Subsequently, enolization occurs in intermediate II, where two iron species act as a Lewis acid/Brønsted base co-operative catalyst system, facilitating the generation of enolate III and radical intermediate IV. On the other hand, the cleavage of the benzyl C–H bond (Ph-CH2-H: BDE= 89.6 ± 0.6 kcal mol−1) is accomplished by the tert-butoxy radical (t-BuO-H: BDE = 105.7 ± 0.7 kcal mol−1) to generate benzyl radical V. Notably, the cleavage of the benzyl C–H bond occurs with the help of the FeII/L1 system and not by thermal degradation. Then benzyl radical V undergoes coupling with enolate III rather than radical intermediate IV to afford intermediate VI. Finally, the iron(II) centre in VI is oxidized and delivers the desired product in good yield with the regeneration of active iron species I.
Recently in 2019, Cai et al. reported the regioselective ligand-supported α C–H alkylation of N-methyl anilines (without any directing group) by cross dehydrogenative coupling between two C(sp3)–H sources.204 Different types of C(sp3)–H bonds including cyclic alkanes, cyclic ethers and toluene derivatives are used as coupling partners. Notably, the desired product is not obtained when anisole is used as the substrate instead of amine. A variety of N-methylanilines having electron donating groups such as OMe and Me at the C(4) position of the phenyl ring and electron-withdrawing groups such as cyano, halogen, trifluoromethyl and phenyl groups provide moderate to excellent yield of the desired products. This indicates that the position of the substituents in the phenyl ring does not have any significant role in the reaction yield. Notably, the best catalytic efficacy was shown by FeCl2 in the presence of 3,4,7,8-tetramethyl-1,10-phenanthroline as the ligand (Scheme 151). Notably, the anion bound to the iron plays a crucial role in the catalysis. The yield of the desired product increased by using Na2CO3 as an additive. Interestingly, the formation of the desired product was not observed using radical scavengers such as TEMPO or BHT. Thus, this suggests that the reaction may occur via the radical pathway. Further, a trace amount of product is obtained in the absence of the iron catalyst, indicating the importance of the iron catalyst in electron transfer. Also, the product is not obtained in the absence of TBHP. Clearly, this suggests that TBHP plays the role of an oxidant and radical initiator. Moreover, the ligand acts as an activating agent for the catalyst to facilitate the reaction. The additive Na2CO3 acts as a base for deprotonation.
Interestingly, the reaction also occurs under an argon atmosphere, indicating no requirement of additional O2 for the reaction. Thus, based on these experiments, a plausible mechanism of the reaction was suggested, as shown in Scheme 152, where in situ generated radical II is formed from substrate 1via the SET process. Then, it undergoes reduction in the presence of a base additive. Finally, radical–radical coupling between radical II and the cyclohexyl radical provides the desired product III.
3.1.1.2 CDC with C(sp3)–H bond adjacent to heteroatom. C(sp3)–H bonds adjacent to a heteroatom are highly desirable for the direct functionalization and derivation of heteroatom-containing organic compounds. Inspired by iron-catalyzed CDC with biphenyl methane derivatives, Li et al. reported iron-catalyzed C–C bond formation via the direct oxidation of α-C–H bonds of ether with 1,3-dicarbonyl compounds.205 Among the different iron catalysts, FeCl2, FeBr2, Fe(OAc)2, and Fe2(CO)9 were found to be equally efficient for the CDC reaction between THF (tetrahydrofuran) and benzoylacetate. Both the cyclic and the linear ether derivatives with 1,3-dicarbonyl compounds are well tolerated to give the desired product in good yield. Notably, substrates containing sulfur and nitrogen as heteroatoms are also compatible under this C–H bond oxidative transformation (Scheme 153).
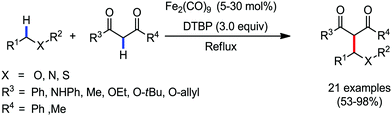 | ||
| Scheme 153 Iron-catalyzed oxidative coupling between C–H bonds adjacent to heteroatoms and 1,3-dicarbonyls. | ||
Further, Li and co-workers extended their reaction protocol with N,N-dimethyl aniline and two equivalents of 1,3-dicarbonyl compound to obtain a methylene-bridged bis-1,3-dicarbonyl compound (Scheme 154).206 Notably, they used N,N-dimethylaniline as the methylenic source for the first time in this type of reaction. The best catalytic efficiency was exhibited by Fe2(CO)9. Based on several experiments, a plausible reaction pathway was proposed. Initially, one molecule of N,N-dimethylaniline and another molecule of 1,3-dicarbonyl compound undergoes iron-catalyzed cross-dehydrogenative coupling to form intermediate I. Then, intermediate I undergoes substitution with another molecule of 1,3-dicarbonyl compound via the SN2 pathway to give the final product via the removal of N-methyl aniline as the leaving group. The other feasible mechanistic pathway is initiated by the Cope elimination of I to form intermediate II, which undergoes Michael addition with the second molecule of 1,3-dicarbonyl to afford the desired product (Scheme 155).
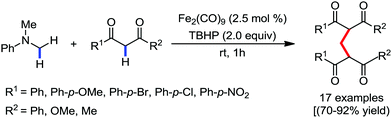 | ||
| Scheme 154 Neutral diiron-nonacarbonyl-catalyzed dialkylation of the methylene group with 1,3-dicarbonyls. | ||
Based on the same concept, Li et al. in 2011 used N-alkyl tertiary amines in place of N,N-dimethylamine as alkylating agents. The CDC reaction between two molecules of N-alkyl tertiary amines and 1,3-dicarbonyl compound in the presence of the Fe2(CO)9/TBHP system was reported.207 The reaction protocol affords various β-1,3-dicarbonyl aldehydes in moderate to good yields. Notably, the synthetic transformation is completed within 10 min at room temperature. Initially, the reaction starts via condensation between two molecules of N-alkyl tertiary amines to generate α-β-unsaturated aldehyde. Then, the unsaturated aldehyde undergoes Michael addition with the 1,3-dicarbonyl compound to afford the desired product (Scheme 156).
In 2011, Hayashi and Shirakawa reported the cross dehydrogenative coupling between tetrahydroisoquinolines with nitroalkanes in the presence of the FeCl3/DTBP catalyst/oxidant system (Scheme 157).208 This synthetic method results in the formation of various 1-nitroaldol-substituted tetrahydroisoquinolines in excellent yields.
Additionally, activated methylene compounds have also been successfully used as nucleophilic sources. Furthermore, Li et al. described a similar type of synthetic transformation using magnetic Fe3O4 or CuFe2O4 nanoparticles as a catalyst. Noteworthily, this catalyst can easily be recovered and used for at least nine runs without any loss in activity.209,210 Moreover, the CDC between isochromans with 1,3-dicarbonyl compounds was reported by Mancheño and Richter using the Fe(II)triflate/oxammonium salt of TEMPO as the catalyst/oxidant system.211 The reaction is very solvent dependent. No products are obtained by employing solvents such as dichloroethane (DCE), tetrahydrofuran (THF), acetonitrile (MeCN) and toluene. Surprisingly, further substitution at the benzylic position of isochroman inhibits the reaction. This can be rationalized based on the enhanced steric hindrance in the ether.
The iron(II)-chloride-catalyzed oxidative coupling of propargylic ethers with 1,3-dicarbonyl compounds was reported by Zhang and co-workers. This synthetic method affords various propargylated β-dicarbonyl compounds in moderate yield (Scheme 158).212 In 2015, the novel iron-catalyzed dual oxidative tandem annulation of a glycine derivative with tetrahydrofuran were reported by Huo's group.213 This synthetic methodology is performed under mild reaction conditions to afford various quinoline-fused lactones in moderate yield. Notably, various glycine esters having electron-withdrawing groups and electron-donating groups at the meta, para and ortho positions of the phenyl ring provide the desired product smoothly. However, when 2-methyl tetrahydrofuran or 2-5-dimethyl tetrahydrofuran is used as the substrate, lower yields of the desired quinoline-fused lactones are obtained. Based on several experiments, the authors suggested a possible reaction mechanistic pathway (Scheme 159). Initially, the glycine ester and THF are transformed into an aryl amine derivative and dihydrofuran (DHF), respectively, in the presence of FeCl2/TBHP. Then, the oxidative imino Diels–Alder reaction between them generates a tetrahydroquinazoline intermediate, followed by isomerization in the presence of 12 M hydrochloric acid (HCl), resulting in the formation of the final product.
3.1.2.1 Benzylic C(sp3)–H amidation reaction. In 2008, Fu and co-workers reported the first example of the iron-catalyzed amidation of the benzylic C(sp3)–H bond via the CDC reaction.217 The synthetic transformation is carried out in the presence of efficient, inexpensive and air stable FeCl2/NBS as the catalyst/oxidant system together with carboxamides and sulfonamides as a nitrogen source. The corresponding desired products are obtained in moderate to excellent yield under the optimized conditions. This iron-catalyzed benzylic C(sp3)–H bond amidation is initiated by the generation of bromosulfonamide or bromocarboximide intermediate Ivia the bromination of sulfonamide or carboximide with N-bromo succinamide (NBS) (Scheme 160). Then intermediate I reacts with FeCl2 to generate intermediate II. Thereafter, intermediate II undergoes conversion into iron-nitrene complex III, which reacts with benzylic C(sp3)–H via insertion into C–H (transition state IV) bonds, providing the desired product.
3.1.2.2 Amidation of C(sp3)–H bond adjacent to heteroatoms. The iron-nitrene complex can be used as a key intermediate during the synthesis of nitrogen-containing organic compounds. However, a new approach for the C–N bond formation process was established by Li et al. in 2010.218 The authors reported the iron-catalyzed oxidative C–N bond formation of azoles and ethers in good to excellent yield. Notably, imidazole and its derivatives undergo C–N bond formation with THF in the presence of FeCl3·6H2O/TBHP as the catalyst/oxidant system in good yield. Moreover, benzimidazole and various ethers are also amidated in good yields using the standard reaction protocol (Scheme 161). Mechanistically, the hydroxide ion is generated from the reaction between Fe2+ and t-BuOOH. Then, it abstracts the N–H proton from the imidazole system to give anion intermediate I.
On the other hand, the α-C–H bond of the ether is activated by the iron salt and tert-butoxyl radical to form radical intermediate II, which is oxidized by Fe3+ and generates oxonium intermediate III. Thereafter, the nucleophilic attack of anionic intermediate I to oxonium intermediate III provides the desired coupling product (Scheme 162).
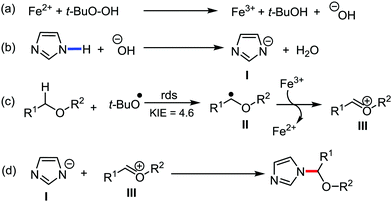 | ||
| Scheme 162 Probable mechanistic pathway of oxidative C–N bond formation of azole derivatives and ethers. | ||
In 2012, Zhu et al. described the FeCl2/TBHP reagent-catalyzed direct amination of aryl amides with N-substituted pyrolidin-2-one to afford the desired product in moderate to excellent yield in a regioselective manner (Scheme 163).219 Here, the methylenic C(sp3)–H bond adjacent to nitrogen is aminated. In 2014, Bao et al. reported a similar reaction with the FeCl3 catalyst and TBHP oxidant system in the absence of a ligand.220 Yang et al. in 2013 reported the iron-catalyzed direct C-1 amination of isochroman derivatives with aniline derivatives (Scheme 164).221 The C-1 position adjacent to the heteroatom oxygen is oxidized to form the oxonium ion intermediate. Subsequently, this intermediate is attacked by N-nucleophiles to yield a variety of hemiacetals selectively in good to excellent yield.
In 2014, Gu et al. developed an efficient method of iron-catalyzed one-pot oxidative tandem cyclization for the formation of benzoxazole from 2-(aryloxy)-anilines.222 Based on the experimental observations and previous results, a plausible mechanistic pathway was suggested. It is believed that the tert-butoxyl radical (t-BuO˙) (formed from the reaction between Fe2+ and DTBP) abstracts the hydrogen atom adjacent to the oxygen of 2-(aryloxy)anilines to form a carbon-centered radical, followed by oxidation to form the oxonium ion intermediate via the SET process. Subsequently, intramolecular attack of the nitrogen atom of aniline to the oxonium ion intermediate affords the desired product (Scheme 165).
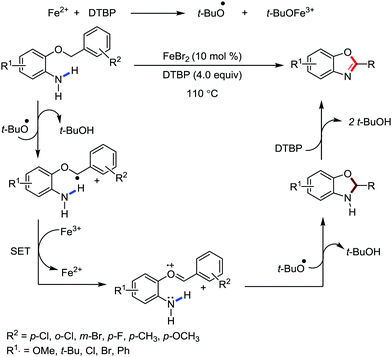 | ||
| Scheme 165 Iron-promoted tandem oxidative cyclization for the synthesis of substituted benzoxazoles. | ||
In 2018, Chikhalia and co-workers developed an iron-catalyzed cross dehydrogenative C–N coupling of thiohydantions with amines. The reaction is performed with 1,3-dibenzyl-2-thiohydantoin 1a and 2-aminopyridine 2a using FeCl3/TBHP as the catalyst/oxidant (in 1,4-di-oxane as the solvent) system at 80 °C under aerobic conditions (Scheme 166).223 The desired product is obtained in 86% yield under the optimized reaction conditions. Notably, both heteroaryl amines and anilines are well tolerated under the reaction conditions.
They proposed a possible mechanistic pathway for the C(sp3)–H amination of 1,3-dibenzyl-2-thiohydantoin (Scheme 167). Initially, tertiary-butyl hydroperoxide (TBHP) splits into a hydroxyl anion and tert-butoxy radical (t-BuO˙), followed by HAA from 1 and SET oxidation generates cation II (Scheme 167). On the other hand, anion III is formed via the deprotonation of 2-aminopyridine 2, which reacts with cation II to produce the desired product.
3.1.3.1 C–O bond formation with benzylic C(sp3)–H bond through CDC. In 2012, Urabe et al. reported a method for the Fe(acac)3-catalyzed synthesis of tert-butyl peroxyacetals via C–H bond oxygenations.224 Various olefinic and acetylenic tert-butyl peroxyacetals and unsaturated peroxy ortho-esters result in the formation of the desired product in good to excellent yields (Scheme 168).
A reaction mechanistic pathway was proposed for the formation of tert-butyl peroxyacetals (Scheme 169). Initially, the tert-butoxy radical abstracts a hydrogen atom from the ether to form the radical intermediate, which results in the formation of a carbocation intermediate via the SET mechanistic pathway in the presence of an iron salt. Thereafter, the nucleophilic attack by t-BuOOH provides the final product.
In 2015, Zhang and co-workers developed a straightforward strategy for the formation of various α-keto benzyl esters using benzyl derivatives with benzoylformic acids in the presence of FeF3/DTBP as the catalyst/oxidant system in an inert atmosphere (Scheme 170).225 Various benzyl derivatives having electron donating groups are well tolerated under the reaction conditions. However, benzyl derivatives with electron-withdrawing groups afford a poorer yield of the desired product. On the other hand, benzoylformic acids having both electron-withdrawing groups (e.g. F, Cl, Br, and CF3) and electron-donating groups (e.g. Me and OMe) are well tolerated under the reaction conditions. The plausible mechanistic pathway suggests that nucleophilic attack by benzoylformic acids on the benzyl carbocation, which is forms via the oxidation of the benzyl C–H bond, results in the synthesis of the product.
3.1.3.2 C–O bond formation with propargylic C(sp3)–H bond through CDC. In 2012, Jiao et al. reported an oxidative C(sp3)–O bond formation protocol between aryl propargyl azides and carboxylic acids in the presence of FeCl2/DDQ as the catalyst/oxidant system (Scheme 171).226 Noteworthily, a diverse range of aryl propyne substrates containing electron-withdrawing groups such as chloro and bromo and electron-donating groups (e.g. OMe and Me) are well tolerated. Moreover, a variety of carboxylic acids such as aliphatic acids, acids having aryl and double bonds, and even sterically hindered carboxylic acids are well tolerated under the reaction conditions. The reaction proceeds smoothly in dichloroethane (DCE) solvent to yield the product in 45–83% yield. The azides are derived in situ from the reaction between NaN3 and the corresponding chlorides. However, although propargyl chlorides are structurally similar to the azide group, they do not undergo the synthetic transformation. This suggests that the azide group can act as an assisting group to facilitate the synthetic transformation. The products formed in the reaction are further transformed into other useful products such as 4,5-disubstituted-1,2,3-triazoles, 3-alkoxyenols and benzotriazole.
Considering the mechanistic perspective, it was proposed that the reaction proceeds through the single-electron oxidation mechanism between propargyl azides and the FeCl2/DDQ system to form radical intermediate I, which is further oxidized to cationic intermediate II (Scheme 172). Successively, the nucleophilic attack of the carboxylic acid to carbocationic intermediate II produces the desired product with the regeneration of the active catalyst. Notably, cation II is stabilized due to the presence of the azido group.
3.1.3.3 C–O bond formation with a C(sp3)–H bond adjacent to a heteroatom through CDC. In 2005, Pearson and Kwak reported a reaction protocol demonstrating the iron-catalyzed oxidation of chiral 2-pyrrolidino-1-ethanol derivatives to oxazolopyrrolidines in a stereoselective manner (Scheme 173).227
This reaction protocol involves a tricarbonyl(cyclohexadiene)iron complex as the catalyst and trimethyl N-oxide (Me3NO) as the oxidant. According to the proposed mechanism, it was presumed that the trimethyl-N-oxide possibly removes one CO ligand from the metal carbonyl and also eases the demetallation of the diene–Fe(CO)3 complex.228 However, although the detailed mechanistic pathway of this reaction protocol is not clear, it is assumed that the coordination of the actual oxidant to the –OH group, followed by simultaneous intramolecular oxidation of amine gives the product. Notably, here the iminium intermediate reacts with the –OH group to generate an oxazolidine ring.
In 2011, Hayashi et al. depicted the FeCl3-catalyzed C–H bond oxidation of alkyl amides to α-(tert-butoxy)alkylamides in the presence of FeCl3/DTBP as the catalyst/oxidant system.229 Here, nucleophilic attack by the tert-butyl alcohol to the in situ generated acyliminium salt produces the desired α-(tert-butoxy)alkylamide (Scheme 174).
In the same year, Liang et al. reported a similar type of C–O bond formation methodology by the oxygenation of C(sp3)–H bonds adjacent to the nitrogen atom of unprotected aryl ureas in the presence of FeSO4/TBHP as the catalyst/oxidant combination in water (Scheme 175).230 Various N-arylpyrrolidine-1-carboxamides having electron-withdrawing groups such as p-methoxyphenyl and p-methylphenyl and electron-withdrawing groups are well tolerated under the reaction conditions. Noteworthily, 2-methyl-N-phenylpiperidine-1-carboxamide does not give any product due to the steric effect of the methyl group on the piperidine ring. The reaction protocol provides an efficient simultaneous dioxygenation of both α-positions with respect to the nitrogen atom, generating various tert-butoxylated and hydroxylated aryl ureas in good yield.
Mechanistically, initially, TBHP splits to form a tert-butoxyradical (t-BuO˙) in the presence of FeSO4. Then, t-BuO˙ reacts with another molecule of TBHP to generate the tert-butyl peroxy radical (t-BuOO˙). The peroxyl radical reacts with aminyl radical I (which is generated through the oxidation of aryl urea 1) to generate the unstable N-(tert-butylperoxyl)-urea II. Thereafter, elimination of the tert-butyl peroxy radical generates a new rearranged carbon radical IIIvia 1,4-hydrogen radical transfer. Notably, radical III is trapped by tert-butanol to give α-tert-butoxylated urea IV and regenerates TBHP. TBHP abstracts the other hydrogen atom adjacent to nitrogen. Thus, the hydroxyl group is introduced to the second α-position of IV (Scheme 176).
Tang et al. in 2015 developed an efficient methodology for the functionalization of pyrrolones with alcohol in the presence of an iron catalyst.231 This reaction protocol is carried out in the presence of Fe(OTf)3 as the catalyst. Notably, pyrrolones containing variety of electron-donating groups such as p-methoxyphenyl or p-methylphenyl and electron-donating groups such as p-chlorophenyl groups are well tolerated under the reaction conditions. Moreover, primary or allylic alcohols were found to be a good nucleophile for this reaction methodology. However, isopropanol and trifluoroethanol afford a lower yield of the desired product. Here, pyrrolones in the presence of the iron catalyst and molecular oxygen then form an acyliminium ion intermediate. Then, nucleophilic attack of alcohols to the intermediate affords the corresponding product (Scheme 177).
Chang and Wang in 2014 described the iron-catalyzed oxidative coupling between salicylaldehyde and cyclic ethers through direct α-C–H functionalization of ethers in the presence of labile –CHO group.232 The corresponding acetals are obtained in moderate to excellent yield in this transformation (Scheme 178). Notably, simple salicylaldehyde and its derivatives with electron-withdrawing groups such as 5-OCF3 and 5-Br together with electron-donating 5-CMe3 and 5-Me groups provide the desired product in good to excellent yield. However, 5-NO2-substituted salicylaldehyde does not result in the formation of the desired product.
In 2014, Han et al. reported a reaction method for the oxidative CDC-based esterification of the C–H bonds of ethers with carboxylic acid in the presence of Fe2(CO)9 and TBHP as the catalyst and oxidant system (Scheme 179).233 Mainly, aromatic acids and phenyl acetic acids together with symmetric and asymmetric ethers are well tolerated under the standard reaction protocol to give the desired product. Notably, only the methylenic C–H bond adjacent to one of the oxygen atoms is selectively esterified using non-equivalent ethers such as 1,3-dioxolane since the hydrogen atom abstraction from the C–H bond in between the oxygen atom generates the highly unstable bridge-head radical intermediate. Hence, the oxidative esterification reaction occurs regioselectively. Further, the decarboxylative alkenylation of cyclic ether was executed using cinnamic acid as the substrate under the same reaction conditions. The possible mechanistic pathway was described as follows. Initially, μ-oxo, μ-carboxylic diiron(III) intermediate I is formed upon the reaction between the iron catalyst and carboxylic acid in the presence of an oxidant. Then, intermediate I is oxidized to Fe(IV) intermediate II, followed by its reaction with the cyclic ether, generating intermediate III. Finally, the cross-coupling from intermediate III affords the desired product with concomitant regeneration of the Fe(III) species.
Additionally, the intermolecular competitive kinetic isotope effect (KIE = 3) suggests that cleavage of the C(sp3)–H bond adjacent to the oxygen atom may be involved in the rate-determining step (Scheme 180). Based on the same concept, in 2017, Liu and Ying reported an alternative method using FeIIITECPCl/DTBP as the catalyst/oxidant (Scheme 181) for the synthesis of ester derivatives.234 This reaction was suggested to follow a similar mechanistic pathway as mentioned above to give the regioselective product.
3.2 CDC between C (sp3)–H and X(sp2)–H bonds
3.2.1.1 Benzylic C(sp3)–H bond. Shi et al. in 2009 reported a method regarding the cross-dehydrogenative arylation (CDA) of benzylic C–H bonds of electron-rich arenes in the presence of FeCl2 and DDQ as the oxidant (Scheme 182).235 Various diarylmethanes and electron-rich arenes are well tolerated and the desired products are obtained in good yield regioselectively. Notably, an increase in the yield of the product was observed upon increasing the nucleophilicity of the electron-rich system. Thus, this suggests that the reaction proceeds via the Friedel–Crafts electrophilic substitution-type mechanistic pathway. Further, a double CDA product is obtained in high yield in the presence of more electron-rich arenes (i.e. 1,2,3-trimethoxy benzene).
Mechanistically, the diarylmethane undergoes the SET process to form the benzyl radical in the presence of an iron catalyst. This benzyl radical further undergoes another SET process to form the benzyl cation. Subsequently, Friedel–Crafts-type alkylation by the electron-rich arenes followed by abstraction of a proton by the reduced hydroquinone affords the desired product via the regeneration of the catalyst (Scheme 183). The large intramolecular kinetic isotope effect (KH/KD = 6.0) indicates that the cleavage of the benzylic C–H bond is involved in the rate-determining step of the reaction.
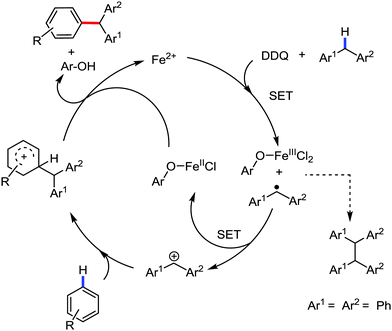 | ||
| Scheme 183 Probable reaction mechanistic pathway of iron-catalyzed cross-dehydrogenative arylation (CDA). | ||
In their subsequent studies, they considered that the electron-rich alkene as the nucleophile, which can react with the in situ generated carbocationic intermediate. However, in their initial studies, they found that styrene can be applied to construct the C–C bond with diphenylmethane by CDC via a Heck-type reaction. Unfortunately, styrene with electron-rich or electron-deficient substituents in its phenyl ring fails to afford the desired product. Subsequently, instead of styrene, they used 1-aryl vinyl acetate, which smoothly afforded the cross-coupling product in good to excellent yield in the presence of FeCl2/DTBP as the catalyst/oxidant system. Notably, the observed electronic feature of the substrate diarylmethane does not support the hypothesis of pathway B (Scheme 185).
The synthetic transformation produces the keto product with vinyl acetate via a pseudo C(sp3)–C(sp3) coupling (Scheme 184), different from the product obtained from styrene.236 Various vinyl acetates and diarylmethanes were tested under the optimized conditions. Both the electron-withdrawing groups and electron-donating groups on the phenyl ring of the diarylmethanes were well tolerated. Notably, the synthetic transformation also tolerates simple toluene and provides a moderate product yield.
According to their proposed hypothesis, the reaction is started with the abstraction the benzylic C(sp3)–H in presence of iron salt and DTBP and generates the radical intermediate I (Scheme 185). Then the radical intermediate is oxidized to generate the benzyl carbocation intermediate IIvia the single electron transfer (SET) process. The reaction may proceed through two possible pathways (A and B) involving the reactive intermediates I and II. In the pathway A, the radical intermediate I, iron catalyst and tert-butoxy radical (generated from di-tertiarybutyl peroxide, DTBP) undergoes electrophilic addition with aryl-vinyl acetate to form Fe coordinated radical intermediate III which subsequently results information of the desired product via the β-session with the regeneration of Fe(II) catalyst as well as AcOt-Bu as by-product. On the other hand, in pathway B, the benzyl cation intermediate II is intercepted by the vinyl acetate to produce the desired product. An intermolecular competitive kinetic isotopic effect (KH/KD = 2.4) have indicated involvement of proton abstraction in the rate-determining step.
Later, Li et al. reported another method describing FeBr3-catalyzed cross-dehydrogenative arylation (CDA) of 3-substituted oxindoles with electron-rich arenes and heteroaromatic compounds under aerobic conditions (Scheme 186).237 This reaction provides 3,3-disubstituted oxindoles in moderate to good yield.
Recently in 2019, Nishikata and co-workers reported a reaction protocol for the tertiary C–H alkylation of heteroarenes via the iron-enhanced reactivity of radicals for the generation of functionalized quaternary carbons (Scheme 187).238 Notably, bromides possessing alkyl, aryl, arylester, and arylamide groups are well tolerated under the reaction conditions. Moreover, substituted benzofurans, thiophenes and furan derivatives having alkyl, ketone and aldehyde groups were found to be well compatible. Here, the alkyl radical is generated through the abstraction of Br from the α-bromocarbonyl compound via the SET pathway with an iron(II) salt to generate the oxidised iron(III) species. Subsequently, iron(III) co-ordinates to the carbonyl group of the resulting alkyl radical having enhanced reactivity.
Actually, the co-ordination of FeIII species with the carbonyl group of the alkyl radical was confirmed by DFT studies. Interestingly, only tertiary C–H alkylation is possible using this reaction protocol. Neither the primary nor the secondary alkyl radical reacts with heteroarenes because of the lower stability of these radicals. Moreover, the desired product was not obtained in the presence of the radical scavenger BHT or TEMPO. Thus, this suggests that the reaction proceeds via the radical pathway. Initially, tertiary alkyl radical I is generated by the reaction between Fe(II) and the α-bromocarbonyl compound. Then, radical I undergoes addition to the C-2 position (highest nucleophilicity calculated by Fukui indices) of benzofuran to form II. Subsequently, intermediate II reacts with the iron bromide species to generate III together with the regeneration of Fe(II). Finally, intermediate III undergoes elimination in the presence of base to afford the desired product (Scheme 187).
3.2.1.2 CDC with C(sp3)–H bond adjacent to heteroatom. Tetrahydroisoquinolines (THIQs) and indoles are very common substructures found in medicinal products and natural products. In 2009, Li et al. developed a one-pot reaction protocol for the oxidation of C–H bonds adjacent to the oxygen atom with an indole derivative in the presence of FeCl2 as the catalyst and di-tertiary butyl peroxide (DTBP) as the oxidant.239 The reaction proceeds via C–O bond cleavage (Scheme 188). Mechanistically, the in situ-generated indole–ether adduct reacts effectively with another molecule of electron-rich indole to give the 1,1-biaryl product (Scheme 189). The synthetic transformation follows the Friedel–Crafts alkylation-type reaction. Only the mono indolation product of isochroman is selectively obtained with the electron-poor 4-nitro indole. Thus, this indicates that the first indolation step is easy and does not depend on the properties of indole, while the second indolation step is the rate-determining step and proceeds efficiently only with electron-rich indoles.
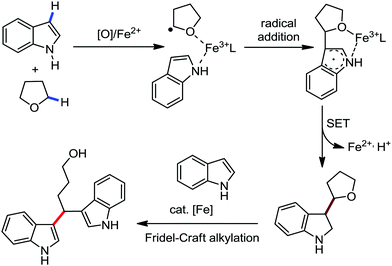 | ||
| Scheme 189 Possible mechanistic pathway for the iron-catalyzed synthesis of 1,1-bis-indolylmethanes. | ||
Later, in 2010, Schnürch and co-workers reported an efficient solvent- and ligand-free iron-catalyzed method for the indolation and arylation of N-Boc-protected tetrahydroisoquinolines (THIQs) and isochromans, respectively.240 Among the different iron salts such as FePO4, Fe2(SO4)3, and Fe(NO3)2·9H2O, the iron-nitrate salt exhibits the best catalytic activity (Scheme 190). Moreover, no desired product is obtained without the use of the oxidant TBHP. Notably, this reaction fails when molecular oxygen is used as the oxidant with or without the presence of the radical inhibitor azobisisobutyronitrile (AIBN). Various electron-withdrawing groups such as nitro and chloro and electron-donating groups (e.g. 5-OCH3) at the C-5 position of the indoles are well tolerated under the reaction conditions. However, the free amine and the ester functionality on the indole provide very poor yields of the desired product. Importantly, azaindoles and electron-rich arenes can also be used as suitable substrate materials besides indoles. Interestingly, isochromans can also be employed as the starting substrate in place of THIQs. Although the cross-dehydrogenative coupling between isochromans and indoles yielded the bis-indolation product,241 the coupling between the electron-rich 1,4-dimethoxy benzene and isochroman afforded the desired arylation product in 55% yield. In previous reports,242 the deprotection of the protecting groups (PG) after the indolation step failed. However, this report mentioned the effective cleavage of the Boc-protecting group under high-temperature microwave conditions within a few seconds to give the product in good yield.
Notably, this practical oxidative reaction is completely suppressed when 1.5 equivalents of the radical scavenger TEMPO is added. Thus, the reaction mechanism proceeds via the radical pathway. The most probable mechanistic pathway is depicted in Scheme 191. Initially, the iron catalyst coordinates to the NH of the indole and the carbonyl of the Boc-protecting group, forming complex A. Thereafter, the addition of THBP leads to iron-mediated cleavage of peroxide, which results in the formation of a water molecule via hydrogen abstraction from THIQ. Subsequently, coordination of the tert-butyl group to the iron centre occurs. Then radical addition leads to intermediate C, which undergoes oxidation to afford the desired product.
Afterwards, Ge and Zhou reported the CDC reaction of coumarins with ethers in the presence of an iron catalyst via the C(sp3)–H bond oxidation of ethers in 2015.243 Notably, this reaction is performed between different ethers and various coumarins having electron-withdrawing groups and electron-donating groups, which provides a moderate to good yield of ether-substituted coumarin derivatives. However, the reaction between electron-withdrawing nitro-substituted coumarins with 1,4-dioxane cannot afford the desired product. Mechanistically, the in situ-generated radical attacks the α-position of coumarin esters to form more a stable radical intermediate. Thus, α-ether-substituted coumarin derivatives are obtained in a regiospecific manner (Scheme 192). Here, DABCO is used as the ligand. A trace amount of desired product is obtained in the absence of the ligand.
In the same year, Correa et al. reported the direct α-arylation of ethers with azoles in the presence of FeF2/TBHP as the catalyst/oxidant combination.244 Here, FeF2 plays a major role in both heterolytic cleavage of the oxidant and oxidation of the carbon radical to the oxonium ion intermediate. Interestingly, several functional groups such as esters, amides, halides and ethers associated with benzothiazoles are well tolerated under the reaction conditions. However, substituted benzothiazoles with electron-deficient substituents provide a lower product yield. On the other hand, besides tetrahydrofuran, other cyclic and acyclic ethers such as 1,3-dioxalane and 1,4-dioxane are also used in the reaction. Moreover, this methodology is used for the synthesis of a variety of C2-alkylated products of (benzo)azoles constituting a straightforward strategy for the generation of medicinally important heterocyclic compounds (Scheme 193).
Itami and Wunsh in 2009 reported the iron-catalyzed oxidative coupling of N-methyl amines with different heteroarenes.245 The reaction mainly proceeds via the formation of the iminium ion intermediate by the abstraction of a hydrogen atom predominately from the methyl C–H bond of N-methyl. The transformation takes place selectively in the N-methyl C–H bond in the presence of other reactive C–H bonds such as acidic benzylic C–H bond and the C–H bond α to the carbonyl group in the substrate. This oxidative coupling transformation was applied to the intermolecular oxidative coupling between thiophene, furans and indoles with methylamines (Scheme 194). Further, this methodology was applied for intramolecular oxidative coupling for the formation of benzazepine-like bicyclic nitrogen heterocycles. However, a low GC yield (27%) of the product was recorded. The reason for the low yield of the product was attributed to the difficulty in the cyclization step to form seven-member rings. However, the product shows good binding affinity and selectivity towards σ1,246 σ2, and NMDA247 receptors (Scheme 195).
 | ||
| Scheme 195 Intramolecular oxidative coupling for the synthesis of benzazepine-like bicyclic nitrogen heterocycle. | ||
In 2012, Deng et al. developed a novel method for the tandem cross-dehydrogenative coupling (CDC) of terminal allylic C(sp3)–H with C(sp2)–H of styrene via benzoannulation in order to synthesize polysubstituted naphthalene (Scheme 196).248 They mainly utilized styrene (1.5 equivalents) and FeCl3 (2 equivalents) in nitromethane at room temperature to afford biologically and synthetically important polysubstituted naphthalenes. Initially, substrate 1 reacts with an allylic methyl group syn to the 1-phenyl group via CDC reaction in the presence of 20 mol% FeCl3 to afford the desired product. Further, the authors speculated about the possibility of C–C bond coupling between styrene and its derivative together with the in situ-generated carbocation species from substrate 1.
The substrate scope for the above-mentioned transformation has been tested. Substrates with strong electron-donating substituents on either Ar1 or Ar2 do not provide the desired product. Other weakly electron-donating and electron-withdrawing substituents on either Ar1 or Ar2 give moderate to good yield of the substituted naphthalene. Interestingly, in the presence of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO), no desired product is obtained. This implies that the reaction occurs via a radical intermediate (Scheme 197).
In 2013, You and co-workers described the oxidative coupling of N-(pyridine-2-ylcarbonyl)-substituted α-amino acid derivatives with indoles in the presence of FeCl3·6H2O/DTBP (Scheme 198).249 The 2-pyridine carbonyl group is an ideal substituent as the N-protecting group due to its high coordinating and better elimination ability. Other activating substituents at the amino group, i.e. imidazol-2-ylcarbonyl, 2-quinolin-2-ylcarbonyl, isoquinoline-1-ylcarbonyl and pyrimidin-2-ylcarbonyl groups, are found to be less effective. Also, non-nitrogen-containing substituents are not effective under this synthetic transformation. This method has been well explored with various functional groups on both α-amino acids and nucleophiles. It affords a series of α-quaternary α-amino acid derivatives in good yield. Mechanistically, it is presumed that the 2-picolinamido-α-tertiary amino acid coordinates with FeIII to form intermediate I. Then, the tert-butoxy radical (generated from DTBP) abstracts α-H from the intermediate to generate radical intermediate II. Radical intermediate II undergoes an SET process to form α-ketimine intermediate III. Thereafter, the addition of nucleophile to radical intermediate III is facilitated due to the coordination of Fe2+ with the picolin-amido group. Notably, this coordination mainly activates the α-ketimine to afford α-quaternary α-amino acid esters as the product. Interestingly, this reaction was also carried out under an N2 atmosphere, and the desired product was obtained in 39% yield. This suggests that the re-oxidation of the released Fe2+ occurs in the presence of air and di-tertiarybutyl peroxide (DTBP) to complete the catalytic cycle (Scheme 199).
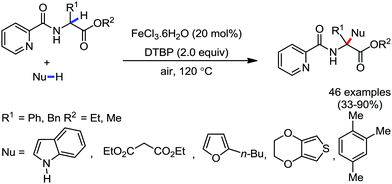 | ||
| Scheme 198 Iron-catalyzed oxidative coupling between the α-C(sp3)–H bonds of α-quaternary α-amino acid esters and arenes. | ||
Doyle and co-workers in the same year reported the FeCl3-catalyzed aerobic C–H bond functionalization of tertiary anilines including tetrahydroisoquinolines.250 The reaction protocol affords facile functionalization with siloxyfuran, nitroalkanes and other nucleophiles using the cheap FeCl3 as a the catalyst and the green oxidant O2. The oxidation of tertiary anilines under the Fe(III)/O2 system forms a reactive iminium ion intermediate. This intermediate undergoes an oxidative Mannich reaction to give the corresponding product with various nucleophiles. The addition of one equivalent of weak acid such as 1,1,1,3,3,3-hexafluro-2-propanol (HFIP) is required to promote the oxidative aza-Henry reaction in the presence of coupling partner nitroalkanes to inhibit the coordination of Fe(III) with the amine reactant or to activate the nitroalkanes (Scheme 200).
After exploring the substrate scope of the reaction, it was proposed that the electron transfer between FeCl3 and amine produced the radical cation and iron(II) chloride. This process may be feasible by considering the redox potential comparison between the Fe(III)/Fe(II) (+0.77 V vs. NHE) and amine/radical cation redox pairs (+0.79 V/+1.08 V vs. NHE). The abstraction of one hydrogen atom from the radical cation generates the iminium-ion intermediate through iron(II)-bound dioxygen. The resultant peroxo-species enters the Haber–Weiss or Fenton-like reaction to complete the process (Scheme 201).
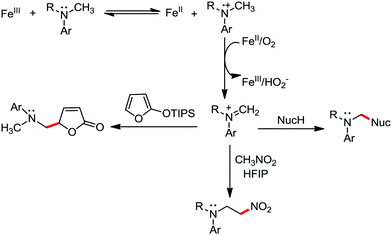 | ||
| Scheme 201 Possible reaction mechanism for the iron-catalyzed aerobic C–H functionalization of tertiary anilines. | ||
Later in 2018, Wan and Guo reported a synthetic method for the iron-catalyzed C-2 cyanomethylation of indoles and pyrroles via direct oxidative CDC with acetonitrile derivatives.251 The best yield of the product is obtained by performing the reaction under an argon atmosphere (Scheme 202). Notably, this synthetic method tolerates different C-3-substituted indoles and furnishes the desired product in good yield. This method is facilitated in the presence of pyrimidinyl as the directing group. Interestingly, changing the directing group from pyrimidinyl to pyridyl, Me or Ac group leads to a decrease in the yield. Notably, N-pivoloylindol as the N-protecting group delivers the C3-cyano methylation product instead of the desired C2-cyano methylation product. This method is also well compatible with aurin, tryptophan and melatonin analogues, leading to the formation of the corresponding products. The corresponding product of the tryptophan analogue is used for rapid diversification for the synthesis of non-natural amino acids. On the other hand, the product obtained from melatonin is utilized as a dietary supplement and neuro-hormone drug discovery.
Based on the experimental findings and literature studies, a plausible mechanistic pathway was proposed. Initially, the Fe(OAc)2 catalyst is oxidized by DTBP to give the active Fe(III) species I and tert-butoxy radical. Subsequently, the tert-butoxy radical abstracts the hydrogen atom from acetonitrile to form radical II. On the other hand, the coordination-based directed C–H insertion of N-(2-pyrimidyl)-indole 1 with Fe(III) species generates metallacycle intermediate III together with the formation of tert-butyl alcohol. Radical II reacts with intermediate III and generates another organoiron intermediate IV. Finally, the desired product 2 is obtained via reductive elimination together with the regeneration of the Fe(II) system, completing the catalytic cycle (Scheme 203).
3.2.2.1 C–N bond formation with benzylic C(sp3)–H bond. In 2011, Chen and Qiu reported an iron-catalyzed C–N bond formation method between the imidazole moiety and benzylic hydrocarbons (Scheme 204).252 This reaction was investigated with the starting material having no pre-functionalization. Thus, it is a technique for the direct amidation of benzylic C(sp3)–H bonds to form valuable nitrogen-containing compounds. Mechanistically, a benzyl cation intermediate undergoes nucleophilic attack by the imidazole moiety. In 2012, Chen et al. reported a similar synthetic method.253 In 2014, Bolm et al. developed an efficient method for C–N bond formation by hetero-CDC between sulfoximines and diarylmethanes.254 This reaction results in the formation of N-alkylated sulfoximines with α-branched substituents (Scheme 205).
3.2.2.2 C(sp3)–H bond adjacent to heteroatom. In 2010, Li et al. reported the N-alkylation of azoles via the oxidation of C(sp3)–H bonds adjacent to the oxygen atom of ether (Scheme 206).218 This reaction was performed with a variety of azoles and ethers after establishing the optimized conditions, affording the desired product in good to excellent yield. In 2015, Wang and He reported a similar type of method using aryl tetrazole as the coupling partner.255
The probable reaction mechanism is described in Scheme 207. Mechanistically, the nucleophilic attack of intermediate I (which is formed via the abstraction of a proton from azoles) to oxonium ion intermediate III affords the desired product. The electronic property of the azoles in this reaction was investigated by a competition experiment. The result of the competition experiment indicates that the reactivity of the azole is related to the nucleophilicity of its conjugate bases rather than its acidity. This is in good accordance with the nucleophilic reaction. Further, the observed primary kinetic isotope effect (KH/KD = 4.0) suggests that the cleavage of the α-C–H bond to the oxygen atom of ether is involved in the rate-determining step (Scheme 207).
In 2012, Chen et al. described another method for the N-alkylation of azole derivatives with amides and sulfonamides (Scheme 208).256 This reaction proceeds via the oxidative cleavage of the C(sp3)–H bond adjacent to the nitrogen atom of amides and sulfonamides to give a variety of azole derivatives. These azole derivatives are structural motifs in N-heterocyclic carbene (NHC) and ionic liquids. A similar reaction protocol was reported by Reddy et al. in 2015.257 In 2013, Bao et al. developed a reaction protocol demonstrating the intramolecular oxidative amination of aryl hydrazones for the generation of indazoles using FeBr3/O2 as the catalyst/oxidant system (Scheme 209).258 They also synthesized 1,3,5-trisubstituted pyrazoles of phenylhydrazones via intramolecular oxidative coupling.
In 2014, Hazra and co-workers reported an iron-catalyzed oxidative deamination procedure for the regioselective synthesis of functionalized imidazopyridine derivatives (Scheme 210).259 The coupling reaction is carried out between 2-aminopyridine and nitroalkane to afford the imidazopyridine derivative. Notably, 2-aminopyridine with methyl substitution irrespective of the position on the pyridine ring affords the desired the product in good yield. Moreover, nitroalkane and the other coupling partners of the reaction having different functionalities such as –Me, –SMe, –Cl, and –F, and having acid-sensitive groups (such as –CO2Me and oxymethylene) are well tolerated under the reaction conditions. Notably, aliphatic nitroalkanes such as 3-methyl-1-nitro-1-but-ene and (2-nitrovinyl)cyclohexane do not give any product. Further, the reaction protocol was applied to styrenes to react with 2-aminopyridine. The reaction with styrene requires an extra requirement of an oxidant (TEMPO) and provides good yields of the imidazopyridines. Based on preliminary observations, the authors presumed that the reaction starts with (Scheme 211) the Michael addition of 2-amino pyridine with nitroalkane, generating adduct compound I. Then, the oxidation of adduct compound I occurs by the iron catalyst through the SET process followed by cyclization and deprotonation, resulting the desired product.
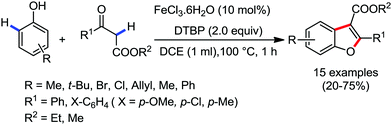 | ||
| Scheme 212 Iron-catalyzed tandem oxidative coupling and annulations between phenols and β-keto esters. | ||
Mechanistically, the reaction initiates with the formation of Fen+-chelated species I followed by reductive elimination to produce adduct II. Then, adduct II undergoes tautomerisation to form phenol III, which subsequently undergoes intramolecular condensation to afford the desired product. The iron catalyst in this method plays a dichotomous catalytic role. It acts as a transition metal catalyst in the oxidative coupling step and a Lewis acid in the condensation step (Scheme 213). This methodology was applied to the total synthesis of coumestrol.261 Particularly, it was utilized for the first stage of the two-step synthesis (Scheme 214). It mainly involves a modified aerobic oxidative cross-coupling reaction between 3-methoxy phenol and 2-(2,4-dimethoxy benzoyl)acetate in the presence of FeCl3/2,2 bipyridine/DTBP as the catalyst/ligand/oxidant system. The coupled product undergoes sequential deprotonation followed by lactonization, affording coumestrol in 59% overall yield.
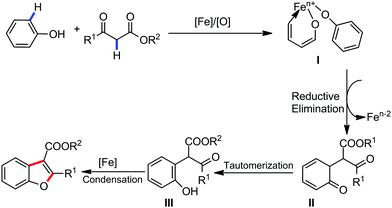 | ||
| Scheme 213 Probable mechanistic pathway for the iron-catalyzed oxidative reaction of phenols and β-keto esters. | ||
Recently in 2018, Xuan and co-workers carried out the formal synthesis of lithospermic acid via the CDC reaction between ortho-bromo phenol and β-keto ester (Scheme 215).262
3.3 CDC between C(sp3)–H and C(sp)–H bonds
In 2009, Vogel et al. developed a chemoselective method for the FeCl2-catalyzed oxidative coupling of silyl group-protected terminal alkynes with two different tertiary amines.263 Notably, the methyl amino group reacts much more rapidly than the other alkyl amino groups present in the substrate. Interestingly, the reaction protocol was successfully applied to a large variety of aromatic and aliphatic amines in the presence of FeCl2/DTBP as the catalyst/oxidant system. The chemoselectivity of the reaction originates from the steric hindrance of the substituents of the substrates. Mechanistically, the FeCl2 catalyst generates the iminium ion intermediate via the SET oxidation process (Scheme 216). Then, the intermediate is attacked by the nucleophile, the alkynyl carbanion, to form coupled product I. Thereafter, treatment with tetra-n-butylammonium fluoride (TBAF) removes the triethyl silyl group and generates aniline derivative II. Further, compound II reacts with another alkynyl moiety in the presence of an iron catalyst and DTBP, which results in the formation of asymmetric diamine via a double CDC process.Subsequently, Hu et al. reported an efficient route for the synthesis of quinolines via the FeCl3-catalyzed oxidative coupling of N-aryl glycine derivatives with alkynes.264 This methodology results in the synthesis of a series of substituted quinolines in good yield. Notably, C(sp3)–H/C(sp)–H coupling followed by intramolecular Friedel–Crafts reaction is responsible for the formation of the desired product (Scheme 217).
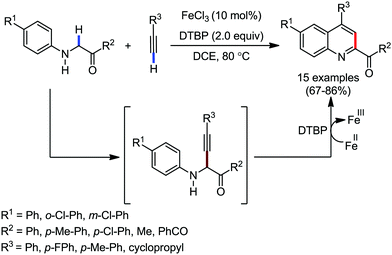 | ||
| Scheme 217 Synthesis of quinolines by iron-catalyzed oxidative coupling of N-arylglycine derivatives. | ||
3.4 CDC between C(sp2)–H and X(sp3)–H bonds
Subsequently, in the same year, Sun et al. reported an environmentally benign methodology for the amination of benzoxazoles with the green oxidant H2O2.265 This one-pot synthesis involves two steps. Initially, the ring opening of benzoxazole generates the amidine intermediate, followed by iron-catalyzed oxidative intramolecular cyclization to afford the desired product (Scheme 218).In 2014, Lei and co-workers developed an efficient method for the oxidative coupling of amides with aldehyde using FeBr2 as the catalyst and TBHP as the oxidant.266 A variety of amides and aldehydes were explored as substrates under the optimized conditions for the direct formation of imine product. Mechanistically, the aryl bromide intermediate is formed in situ from aldehyde and reacts with amides to provide the desired product (Scheme 219).
3.5 CDC between C(sp2)–H and X(sp2)–H bonds
Later, in 2010, Zhang et al. reported a method for the synthesis of 3,3′-biindolyl derivatives, implicating the intermolecular oxidative homocoupling of indoles using iron(III) chloride as the catalyst under aerobic conditions (Scheme 221).268 Additionally, Zheng et al. reported the synthesis of fused porphyrin dimers by intermolecular homo-coupling using Fe(OTf)3 as the catalyst.269 In 2011, Chandrasekharam and co-workers reported the iron-catalyzed cross-dehydrogenative coupling (CDC) of two aromatic compounds using TBHP as the oxidant (Scheme 222).270 This direct oxidative coupling is very selective for the formation of the C–C bond at the ortho position to the functional group of the substrate. Notably, various substitutions on anilines such as alkyl, halide, alkoxy and cyano groups are well tolerated under the reaction conditions. However, electron-donating alkoxy substituted anilines exhibited a reduction in yield. This C–C bond-forming methodology affords a variety of functionalized 2,2′-disubstituted biaryl compounds. Notably, this method does not give any homo-coupled product under the mild optimized conditions due to the sufficient difference in the oxidation potential between the two coupling partners (difference of 440 mV). Moreover, this methodology is not sensitive to air or moisture. In 2013, they synthesized various anilido-dibenzo furanols by applying the above methodology. Notably, these products have moderate to good antimicrobial activity.271
Later on, Pappo et al. described an efficient method for selective oxidative unsymmetrical biphenol coupling using FeCl3/DTBP as the catalyst/oxidant system in 1,1,1,3,3,3-hexafluoropropan-2-ol (HFIP) solvent (Scheme 223).272 The kinetic studies of the reaction protocol support the formation of the chelated radical-anion coupling mechanism, where both phenolic partners are attached to the catalyst during the C–C bond-forming step. The mechanistic pathway is shown in Scheme 224. Initially, two phenolic partners A and B undergo reversible binding with peroxide and iron salt to form complex I. The peroxide bond is cleaved from the metal, followed by an SET process to generate phenoxyl A˙ radical-bound complex II. This particular step is involved in the rate-limiting step. The latter electrophile reacts with chelated phenol(ate)complex B to form III or with a second chelated phenol(ate) A in a homo-coupling process. The desired products including biphenol and tert-butyl alcohol are obtained by the ligand exchange process.
The chemoselectivity of the reaction is determined by the phenol oxidation step and coupling step. Additionally, based on the mechanistic study, the authors proposed a general model (Scheme 225) regarding the feasibility of the cross-coupling reaction for the given pair of phenols. This is well supported by the electrochemical method and density functional theory calculations. They specifically proposed some key factors as follows: (i) phenol A undergoes selective oxidation to phenoxyl radical A in the presence of the other phenol partner B (EoxA < EoxB), (ii) the coupling reaction takes place via the radical-anion coupling pathway, and (iii) phenol B is a stronger nucleophile than phenol A (NA > NB) (where N is the theoretical global nucleophilicity).
Mechanistically, an iron–copper bimetallic chelate complex is believed to be formed via the coordination of nitrogen and the double bond (Scheme 226). This helps to enhance the reactivity of the enolates. Further, electrocyclic ring closing within the chelated complex results in the formation of key intermediate I. Finally, a proton is eliminated from I to give aromatically stable indole derivatives. The substrate scope for this method was well studied. It was found that the electron-donating group at the para position and electron-withdrawing group at the ortho position produce the desired product in high yield.
In 2015, Lei and co-workers described a novel chemoselective method related to the oxidative C(sp2)–H/C(sp2)–H cross-coupling reaction between electron-rich arenes and alkenes using FeCl3/DDQ as the catalyst/oxidant system (Scheme 227).274 In this method, the direct arylation product is obtained using diarylethylenes as the substrate, whereas the double arylation product is obtained using styrene derivatives as the substrate. Notably, various diarylethylenes such as 2-2′-(ethane-1,1-diyl)dinaphthalene and 1,1-dinapthalenes possessing electron-withdrawing and electron-donating groups are well tolerated under the reaction conditions. Further, the radical trapping experiment and EPR (electron paramagnetic resonance) study support that the reaction proceeds via the radical pathway. Notably, DDQ plays a key role in the formation of the aryl radical. Further, the XAFS (X-ray absorption fine structure) study revealed that the oxidation state of the iron catalyst does not change during the reaction. Thus, it can be proposed that FeCl3 acts as a Lewis acid catalyst. Mechanistically, electron-rich arenes are oxidized by DDQ to form the aryl radical. Then the aryl radical is intercepted by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (from olefins) to yield the radical intermediate, which then undergoes oxidation to form the cation, followed by the elimination of one proton to afford the desired triarylethylene product (Scheme 228).
C bond (from olefins) to yield the radical intermediate, which then undergoes oxidation to form the cation, followed by the elimination of one proton to afford the desired triarylethylene product (Scheme 228).
In 2015, Li et al. reported a reaction protocol regarding the oxidative coupling of alkenes with the C(sp3)–H bonds of formamide. They employed two sets of reaction conditions, depicted as pathways A and B (Scheme 231A and B, respectively). Firstly, the tert-butoxy peroxide radical is formed in the presence of tert-BuOK and TBHP. Then, this radical abstracts a hydrogen atom from the C(sp3)–H bond of formamide. However, the ability of alkenes to react with the C(sp3)–H bond of formamide decreases due to presence of FeCl3 and the tert-butoxy radical in pathway B.276 Noteworthily, the tert-butoxy radical is consumed by Fen+1 to form the Fen+(t-BuO) species. Thus, it suppresses the formation of the t-BuOO˙ radical. The reaction initiates upon the generation of carbonyl radical I by hydrogen atom abstraction from formamide by the tert-butoxy radical. Then, radical I reacts with alkenes to generate alkyl radical II. Subsequently, radical II is converted to Fe3+(Ot-Bu) and an alkyl cation(III) via the SET process in the presence of Fe3+. Finally, β-hydrogen elimination from alkyl cation III by the tert-butoxy anion and base, DABCO, gives the desired product and tert-butanol (Scheme 232).
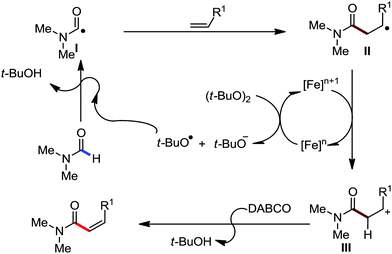 | ||
| Scheme 232 Probable mechanistic pathway for the direct oxidative coupling between alkenes and formamide. | ||
Later, in 2016, Hazra and co-workers reported a direct method for the 1,2-dicarbonylation of imidazoheterocycles using oxoaldehydes through cross-dehydrogenative coupling in ambient air using FeCl3 as the catalyst in toluene (Scheme 233).277 The present reaction protocol is also applicable to indolizines. Moreover, imidazopyridine produces bisimidazopyridine with arylaldehyde under the optimized reaction conditions.
Based on experimental evidence, they proposed the possible mechanism of the reaction. It was presumed that the reaction occurs via a non-radical pathway. Initially, FeCl3 catalyses the addition of phenylglyoxal to benzo[d]imidazo[2,1-b]thiazole at the C-3 position to generate Fe(III)-chelated benzo[d]imidazo[2,1-b]thiazole intermediate I. Then, the Fe(III)-based intermediate is oxidized to Fe(IV)oxo complex II through aerial oxidation. Subsequently, the desired product is obtained via reductive elimination. Simultaneously, Fe(II) is oxidized to Fe(III) in the presence of air to complete the catalytic cycle (Scheme 234).
3.6 CDC between C(sp3)–H with C(sp)–H
In 2009, Volla and Vogel et al. described the C(sp)–C(sp3) coupling reaction between terminal alkynes and tertiary amines (Scheme 235).278 Notably, FeCl2 shows the best catalytic activity under the optimized reaction conditions. The desired product is obtained under the solvent-free conditions using FeCl2/DTBP as the catalyst/oxidant system.In 2011, Bobade et al. reported the iron-catalyzed oxidative alkylation of azoles with terminal alkynes under ligand- and solvent-free conditions.279 The reaction of phenylacetylene bearing an electron-donating group affords a higher yield, while an electron-withdrawing group results in a lower yield of the product due to the dimerization of the alkynes. Notably, the reaction mechanism is insensitive to the steric hindrance of the substituent on the terminal alkyne group. A series of 2-alkynylated benzoxazoles and benzothiazoles was obtained in high yield with the help of this method (Scheme 236). Mechanistically, deprotonation of azole at the C(2)–H bond takes place in the presence of DTBP to form an azole radical, which reacts with an in situ-generated iron-acetylide complex to give the 2-alkynylzole product via the regeneration of the Fe(II) species.
Later, in 2012, Jiao et al. developed the oxidative coupling between terminal alkynes and benzylic ethers and alkanes.280 Notably, the Fe(OTf)2 and DDQ combination shows the best catalytic efficiency (Scheme 237).
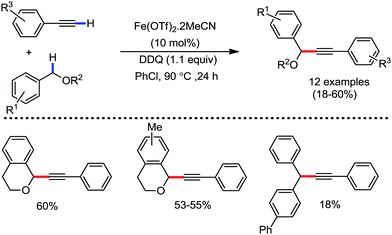 | ||
| Scheme 237 Fe-Catalyzed oxidative coupling between terminal alkynes with benzylic ethers and alkanes. | ||
3.7 Tandem carbon–carbon and C–Heteroatom oxidative coupling reaction
In 2012, Panda and Jena reported a synthetic method elucidating the tandem C–C and C–N oxidative coupling and subsequent annulation reaction for the synthesis of substituted pyrazoles.281 The present method provides a route for the regioselective synthesis of 1,3-di- and 1,3,5-tri-substituted pyrazoles between hydrazones and vicinal diols using FeCl3/TBHP as the catalyst/oxidant (Scheme 238). This reaction affords the desired product in good yields under aerobic conditions. Notably, the presence of an electron-donating group on the N-aryl group enhances the reactivity of hydrazone by increasing the nucleophilicity of the nitrogen. However, the presence of an electron-withdrawing group such as nitro on the N-phenyl ring retards the rate of the reaction. Furthermore, in this methodology, various electron-donating groups and electron-withdrawing groups at the C(3) position of the aryl ring are well tolerated under the reaction conditions.Further, the tandem C–C and C–N oxidative coupling and cyclization was reported in the same year by Hu and co-workers for the synthesis of quinolines between N-alkylaryl amines and alkenes (Scheme 239).282 The thorough optimization of the reaction showed that the combination of FeCl3 as the catalyst and di-tertiarybutylhydroperoxide (DTBP) as the oxidant provides the best yield. In 2015, Gopalaiah and Chandrudu reported the synthesis of various 1,3-benzazoles via the oxidative carbon–carbon, carbon–heteroatom coupling reaction (Scheme 240).283 The coupling reaction between benzylamines with 2-amino/hydroxyl/mercapto-anilines produces the desired substituted benzoxazoles, benzimidazoles, and benzothiazoles in moderate to good yields under aerobic conditions.
3.8 Different C–H functionalization reactions via CDC
Heteroaryl sulphides are important structural motifs in many pharmaceutically active compounds and advanced materials. Thus, the formation of heteroaryl thioethers via the direct thiolation of heteroarene C–H bonds with thiols is a very desirable method. In 2012, Gao and Yu reported a facile method for the copper(II)-mediated thiolation of the heteroarene C–H bond in the presence of a Lewis acid catalyst (i.e. FeF2) (Scheme 241).284 The Lewis acid FeF2 activates the heteroarenes, which are coupled with thiols in the presence of copper(II).Organophosphorus compounds are found in a wide range of nucleotides, pharmaceuticals and phosphine-containing ligands. They also play an important role in cell metabolism, growth, cell division and genetic processes. In 2009, Ofial et al. developed an efficient method for the iron-catalyzed oxidative α-phosphonation of tertiary amines with dialkyl H-phosphonates (Scheme 242).285 The reaction protocol affords the synthesis of new C–P bonds containing non-functionalized starting materials.
In 2010, Tatsumi and Ohki described a regioselective method involving dehydrogenative borylation of furans and thiophenes with pinacolborane using a coordinatively unsaturated NHC iron complex as the catalyst (Scheme 243).286 The reaction is carried out in the presence of tert-butylethylene to trap evolved hydrogen from the coupling partners during borylation. Further, it facilitates the reaction to proceed in the forward direction. Notably, the reaction protocol is regioselective due to the formation of the borylated product at the 2(5)-position of heterocycles in good yield.
In 2018, Darcel and co-workers reported a borylation method for the iron-catalyzed dehydrogenative borylation of terminal alkynes (Scheme 244).287 They used a catalytic system consisting of Fe(OTf)2 (2.5 mol%) and DABCO (1 mol%). This catalytic system selectively promotes the dehydrogenative borylation of aromatic as well as aliphatic terminal alkynes to provide the alkynylboronate derivatives in the presence of 1 equivalent of pinacolborane at 100 °C in toluene. Notably, the presence of para-electron-withdrawing substitution on the arylacetylene derivatives requires a shorter reaction time compared to arylacetylene derivatives having para-electron-donating substituents. This methodology is applicable to a variety of terminal alkynes. However, the reaction protocol is not compatible with terminal alkynes possessing primary amine, alcohol or carboxylic acid derivatives. Mechanistically, the Lewis acid Fe(OTf)2 activates the B–H bond of pinacolborane to enhance the electrophilicity of the boron centre to react with the acetylenic derivative. This process is accelerated due to the presence of DABCO, which helps to increase the nucleophilicity of the terminal alkylenic carbon.
Carbon–heteroatom especially carbon–sulphur (S) and selenium (Se) bonds are very common in natural products and pharmaceuticals. Recently, in 2019, Xu et al. established an iron-catalyzed CDC reaction protocol for the direct synthesis of C(sp3)–S/Se bonds from oxindoles, phenylacetamides, pyrazolones, phenylacetonitrile and ethyl cyanoacetate with thiols and selenols using FeCl3/K2CO3 as the catalyst/base system in DMSO solvent (Scheme 245).193 Here, molecular oxygen is employed as an ideal green oxidant. Notably, this reaction is broadly used in the chemical industry and in the modification of drugs.
After exploring the substrate scope, they performed a radical trapping experiment to gain mechanistic insight. Interestingly, in the presence of TEMPO, no desired product was obtained. Thus, this suggests that the reaction proceeds via the involvement of radical intermediates. Based on the preliminary observations, a plausible mechanistic pathway was hypothesized (Scheme 246). Initially, indolin-2-one is readily oxidized to the corresponding radical Ivia the single-electron transfer (SET) pathway in the presence of a base. Meanwhile, disulfide II is generated by the oxidative coupling of thiols in the presence of molecular oxygen. Finally, radical intermediate I attacks disulfides II to afford the desired product IV together with sulfide radical III. This sulfide radical is converted to disulfides in the presence of oxygen.
4. Decarboxylative coupling
Metal-mediated decarboxylative coupling is a powerful strategy to form carbon–carbon and carbon–heteroatom bonds, involving less expensive and commercially available carboxylic acids and their derivatives.288 Generally, in this strategy, the C–C bond next to carboxylate groups is cleaved, whereas a new C–C bond or C–heteroatom bond is formed. This decarboxylative coupling is an alternative strategy to traditional cross-coupling reactions involving organometallic reagents. In this section, we discuss iron-catalysed decarboxylative coupling reactions.The iron-catalyzed decarboxylation of a variety of allylic phenols to form allylic ethers was described by Trivedi and Tunge289 in 2009. The decarboxylative allylation of the allylic phenol is catalyzed by Bu4N[Fe(CO)3NO] catalyst in the presence of PPh3 using methyl tert-butyl ether (MTBE) as the solvent. Notably, this reaction takes place via the formation of a π-allylic iron intermediate instead of σ-allyl intermediate (Scheme 247). Moreover, the position of the oxygen nucleophile in the product is not dependent on the position of the leaving group in the reactant. Thus, this suggests that the reaction is regiospecific.
Notably, the observed regioselectivity is a kinetic phenomenon and is not due to the equilibrium shifting to a more stable product. A variety of phenols having electron-withdrawing and electron-donating groups are well tolerated under the reaction conditions, obtaining good to excellent yields of the decarboxylating coupling products. The reaction protocol is also effective for the sterically demanding ortho-substituted phenolates (Scheme 248).
In 2011, Li and co-workers reported the iron-catalyzed decarboxylative cross-coupling reaction between proline derivatives and substituted β-naphthols to form C(sp2)–C(sp3) bonds.290 For example, N-benzyl proline and β-naphthol in the presence of FeSO4/DTBP as the catalyst/oxidant system afford the desired product in moderate to excellent yield (Scheme 249). According to the proposed mechanism, initially, amino acid derivative 1 undergoes oxidative decarboxylation in the presence of iron(II) and oxidant to generate imine-type intermediate I (Scheme 250). Subsequently, the intermediate coordinates with iron, resulting in the formation of intermediate II. Finally, intermediate II undergoes coupling with the incoming nucleophile to afford the desired product via transient intermediate III. Concomitantly, the iron catalyst is regenerated.
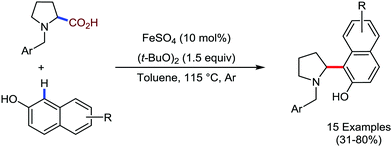 | ||
| Scheme 249 Iron-catalyzed decarboxylative C(sp3)–C(sp2) coupling of proline derivatives and β-naphthols. | ||
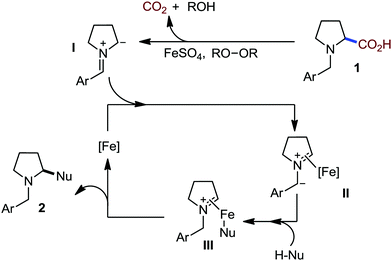 | ||
| Scheme 250 Proposed mechanism for iron-catalyzed decarboxylative coupling between proline and β-naphthol. | ||
Later, in 2013, Pan and co-workers reported the decarboxylative coupling of aryl vinyl carboxylic acid with cycloalkanes using Fe(acac)3/DTBP as the catalyst/oxidant system (Scheme 251).291 The electron-donating group and electron-withdrawing group at any position of the aryl ring provide the desired product in excellent yields (84–91%). However, there is a slight decrease in the yield of the product with the ortho-substituted substrate. Further, a lower yield of product was obtained due to steric hindrance when 2,6-disubstituted aryl vinyl carboxylic acid was employed as the substrate. The reaction exclusively provides the trans-product in a stereospecific manner. The radical intermediate was trapped in the presence of several radical scavengers such as TEMPO or AIBN.
Thus, it was presumed that the reaction may proceed through the radical pathway (Scheme 252). Initially, Fe(III) catalyses the cleavage of DTBP to generate the tert-butoxy radical in the presence of aryl vinyl carboxylic acid. Then, the tert-butoxy radical (t-BuO˙) abstracts the hydrogen from cyclohexane to generate a cyclohexane radical. Thereafter, the radical attacks the α-position of the double bond to generate a more stable radical intermediate. Finally, the radical intermediate undergoes oxidative decarboxylation in the presence of Fe(III) to deliver the product. The Fe(III) catalyst is regenerated in the presence of DTBP and cinnamic acid via oxidation.
Later, in the same year, they reported sp3(C)–sp2(C) coupling under similar reaction conditions by changing one of the coupling partners, i.e. cyclic ether instead of cycloalkane is used (Scheme 253).292 Moreover, the reaction follows the same mechanistic pathway as stated above. Further, the substrate scope was explored under the optimal conditions. It was observed that the electronic effect of cinnamic acid hardly affects the reactivity. The substrate scope of cyclic ether was also tested. Notably, the reactions are also carried with tetrahydrofuran and tetrahydropyran. A lower yield is obtained with tetrahydropyran. Then, different types of heterocyclic acrylic acids such as thiophene and furan were also tested, which all afforded the desired product in good yield. Besides, they also explored the reaction protocol with 1-naphthyl acrylic acid, which provided the desired product in 73% yield.
Following the same concept, Mao and co-workers developed the direct alkylation of the sp3(C)–H bond of toluene via the decarboxylation of cinnamic acid under ligand-free conditions in a regioselective manner.293 Notably, they utilized the less expensive ferrocene as the catalyst and DTBP as the oxidant at 110 °C (under an argon environment) (Scheme 254). Interestingly, a good result was also obtained by using Fe3O4 in the form of nanoparticles (70–80 nm) as a reusable catalyst for seven cycles in the absence of ligand. The substrate scope for both coupling partners was investigated. It was found that electron-rich and electron-deficient cinnamic acid together with heteroaryl acrylic acid afford the desired product in good yields. Moreover, both mesitylene and xylene are well tolerated under the reaction conditions and only the mono-coupled product is obtained. Further, the observed primary kinetic isotope effect (KIE = 5) suggests the involvement of sp3(C)–H bond cleavage in the rate-limiting step.
Notably, all the previous reactions were performed under inert conditions for 24 h to afford the product. Interestingly, when the reaction was carried out under aerobic conditions, it required a very short time (6 h) for completion. Recently, in 2018, following the aforementioned reaction protocol, Esteves and Buarque developed a cross-coupling reaction between cinnamic acid and 1,4-dioxane with excellent yield (86% yield) (Scheme 255).294 Here, FeCl3·6H2O is held in the pores of a layered crystalline imine-based mesoporous covalent organic framework (TPB-DMTP-COF) with the surface area of 1200 m2 g−1. The pores of 34 Å afford the material FeCl3@TPB-DMTP-COF, which works as an iron-based heterogeneous catalyst. Basically, the nanopores within the COF are entropically favoured. Further, they increase the vibrational breathing modes and the local pressure, which are beneficial for the rate of reaction. The reaction method provides the trans-product stereoselectively. This can be explained based on the presence of a common radical intermediate, which gets enough time to equilibrate into the suitable conformation via the elimination of molecular CO2 to afford the trans product.
In 2015, Guo and co-workers described the decarboxylative cross-coupling reaction between α-oxo carboxylic acid and acrylic acid under a nitrogen atmosphere using FeCl2 as the catalyst and K2S2O8 as the oxidant to give the α-β-unsaturated compound in high yield (84% yield) (Scheme 256).295 A broad range of substrates underwent this synthetic transformation with good functional group tolerance. Further, the yield of the reaction was improved by adding two equivalents of HCOO2Na·2H2O as a base. The best yield was obtained using a mixture of H2O and DMSO (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 17
DMSO = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as the solvent. Moreover, a trace amount of product was obtained in the presence of radical scavengers such as TEMPO or hydroquinone. Thus, it was suggested that the reaction may proceed via the radical pathway.
3) as the solvent. Moreover, a trace amount of product was obtained in the presence of radical scavengers such as TEMPO or hydroquinone. Thus, it was suggested that the reaction may proceed via the radical pathway.
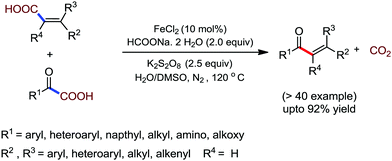 | ||
| Scheme 256 Iron-catalyzed oxidative radical decarboxylative cross-coupling of α-oxocarboxylic acids. | ||
Based on the literature and experimental observations, a plausible mechanistic pathway was proposed, involving radical intermediates (Scheme 257). Initially, acyl radical II is generated from α-oxocarboxylate 1 in the presence of FeCl2 and K2S2O8via oxidative radical decarboxylation. Notably, 21% yield of the desired product was obtained without the addition of FeCl2. This result suggests that α-oxocarboxylate 1 is activated only by K2S2O8 to produce intermediate II. Then, the addition of benzoyl radical II to the double bond of acrylic acid III results in a more stable radical intermediate IV. Then radical intermediate IV undergoes β-elimination to afford the desired product 2 together with the release of a second molecule of carbon dioxide (CO2). Further, a gram-scale synthesis was performed for the above-mentioned reaction protocol. The reaction of 1.5 g (10 mmol) of benzoylformic acid with 2.0 equivalents of cinnamic acid generated the desired product in 62% yield under the standard conditions.
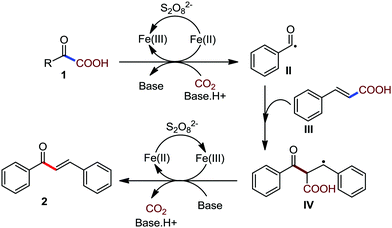 | ||
| Scheme 257 Mechanistic pathway of iron-catalyzed oxidative radical decarboxylative cross-coupling reaction. | ||
The quinazolinones are an important class of nitrogen heterocycles found in a large number of natural products having a broad range of biological activity. Generally, the condensation reaction between o-aminobenzimide and aldehyde/alcohol/carboxylic acid together with their derivatives are required for the synthesis of the quinazoline moiety. However, Kumar and co-workers reported the iron(III) chloride-catalyzed decarboxylative deaminative functionalization of phenylglycine.296 A one-pot synthetic route starting with less expensive nitroarenes for the formation of quinazolinones and the benzimidazole moiety was developed in this reaction protocol. Various 2-nitrobenzonitriles and phenyl glycine in the presence of 10 mol% of FeCl3 in toluene with 2 equivalents of K2CO3 as the base (at 120 °C) yielded the desired product in high yield (Scheme 258). Electron-withdrawing groups such as chloro, bromo, nitro, and trifluoromethyls are well tolerated.
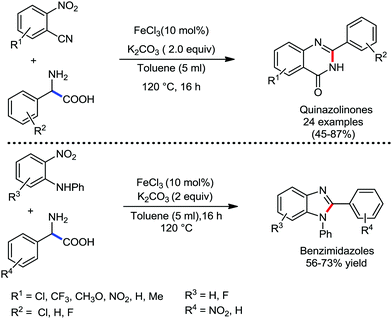 | ||
| Scheme 258 Iron-catalyzed decarboxylative–deaminative functionalization of phenyl glycine derivatives. | ||
Notably, benzimidazoles are obtained by the reaction between 2-nitro-N,N-diphenylamine and phenyl glycine under the same reaction conditions (Scheme 258). The reaction mechanism was proposed based on several experiments and a literature survey (Scheme 259). Firstly, phenyl glycine 2 undergoes decarboxylation and deamination constructively to convert into benzaldehyde in the presence of a metal catalyst. The ammonia generated during the deamination process reduces the nitro group of 2-nitrobenzonitrile 1 in the presence of FeCl3 to form 2-aminobenzonitrile F. Then, intermediate F and benzaldehyde condense to form imine G, which undergoes controlled base-induced hydration to form X. Then, intermediate X cyclises to provide 2,3-dihydroquinazoline-4(1H)one H. Intermediate H may also be beneficial for the conversion of 1 to Fvia the well-known transfer hydrogenation288 and is converted to the desired product 3.
Recently, in 2019, Jin and Sun reported the iron-catalyzed regioselective decarboxylative alkylation of coumarins and chromones with alkyl diacyl peroxides.297 The alkyl diacyl peroxide is easily synthesized from the corresponding carboxylic acid. This alkyl diacyl peroxide is easily decomposed to form an alkyl radical in the presence of an iron catalyst. The present reaction method is regioselective since the in situ-generated alkyl radical attacks the α-position of coumarin 1 having a higher electron density than the β-position. On the other hand, a similar electron-rich alkyl radical attacks the β-position of chromone 2 instead of the α-position since it possesses a higher electron density (Scheme 260).
Notably, coumarin having electron-withdrawing groups or electron-donating groups at the 6-position of the aromatic ring are well tolerated. Even strong electron-withdrawing groups such as nitrile (CN) and nitro (NO2) are also well tolerated in this decarboxylative coupling, affording the α-substituted product in good yield. Moreover, coumarins having an electron-donating group or electron-withdrawing group at the 7/8 position of the aromatic ring were found to be compatible with the above reaction method. This method was also explored for a variety of alkyl diacyl peroxides.
Based on controlled experiments and literature reports, a probable mechanism was proposed (Scheme 261). Initially, iron(III) in the presence of alkyl the radical is reduced to iron(II). Then, iron(II) is oxidized by alkyl diacyl peroxide to provide alkyl radical I by the extrusion of molecular CO2via an SET process. Alkyl radical I attacks the coumarin and chromone in the preferable position to form radicals II and III, respectively. Finally, radical intermediates II and III are converted to the desired products 3 and 4 through the elimination of alkyl-COOH by the SET process, respectively.
5. Iron-catalyzed insertion reaction
5.1 Carbene and nitrene insertion
Iron–porphyrin and related complexes are known to carry out numerous reactions in biological and synthetic systems and insertion reactions are not the exception. Gross and co-workers showed that both corrole and porphyrin-based iron complexes are effective towards carbene insertion in N–H, O–H and olefinic double bond (Scheme 262).298 Ethyl diazoacetate (EDA) was used as the carbene precursor for N–H insertion of various aniline derivatives and morpholine. Interestingly, in the presence of both aniline and olefin, insertion takes place completely in the N–H bond only with the iron–corrole complex, thus providing high selectivity. Subsequently, Woo's group reported iron(III)tetraphenylporphyrin chloride, Fe(TPP)Cl, as an effective catalyst for N–H carbene insertion.299 In addition to EDA, dimethyl diazomalonate and methyl phenyldiazoacetate were tested successfully as carbene sources. The Hammett study suggested that the transition state is partially cationic in nature, and is stabilized by the resonance effect to some extent. The nucleophilic attack by the amine nitrogen on the electron-deficient carbene carbon was also implied from the negative value of reaction constant (ρ). This is consistent with the reluctance of a second N–H insertion due to the decrease in the nucleophilicity of the nitrogen centre. Interestingly, the intermediate formed after nucleophilic attack from amine is trapped with β,γ-unsaturated α-keto esters to form three-component products (Scheme 263).300 This reaction uses mild conditions and only 1 mol% of the porphyrin catalyst to synthesise complex molecules.In the presence of a catalytic chiral bisoxazoline ligand, FeCl2·4H2O can catalyse the insertion of carbene into O–H bonds (Scheme 264).301 Not only various alcohols are compatible with this method, but α-diazoarylacetate-derived carbenes can be enantioselectively inserted to water O–H bonds with more than 90% yield and 90% ee in most cases. Structural rigidity of the ligand is presumed to be crucial in order to control the enantioselectivity. Moreover, a variety of structurally complex chiral molecules can be synthesized from the appropriate products including the drug clopidogrel. Similar chiral spirobisoxazoline ligands have also been used for carbene insertion into N-substituted indoles at the electronically favoured C-3 position, although with moderate ee.302 The KIE was determined to be 5.06, which is indicative of proton transfer in the rate determining step.
Che's group utilized the ligand 4,4′,4′′-trichloro-2,2′:6′,2′′-terpyridine for complexation with iron as a catalyst for nitrene transfer reaction to olefins. N-Tosyl-iodinanes (PhINTs) and N-nosyl-iodinanes (PhINNs) were used as the nitrene precursor to afford aziridines in excellent yields.303 The heterogenized version of the catalyst is derived by attaching the catalyst to poly(ethyl glycol), and similar result was obtained for aziridination. Also, 2-azidoarylimine obtained from 2-azidoaniline and aldehyde can undergo intramolecular nitrene insertion into the imine C(sp2)–H bond in the presence of catalytic FeBr2.304 The dearomatised intermediate formed (Scheme 265) undergoes tautomerization to form benzoimidazole derivatives. Various aryl aldehydes and 2-azidoaniline react successfully; however, aliphatic aldehydes do not produce any products.
The [FeIII(F20-tpp)Cl] (F20-tpp = meso-tetrakis(pentafluorophenyl)porphyrinatodianion) complex is also found to be effective towards nitrene insertion in the olefinic double bond and C–H bond (Scheme 266).305,306 In the presence of N3-R, mono olefinic substituted styrenes produce aziridines, whereas α-substituted styrenes yield allylic amines. In the case of alkylarenes, C–H insertion has been observed at the benzylic position. A plethora of substrates were reported by Che's group to undergo nitrene insertion by this method. They were also successful in synthesizing N-heterocycles via intramolecular insertion. Indole, indoline, tetrahydroquinoline and highly decorated quinazolinone and dihyroquinazoline can be synthesized by utilizing a single method. The indole synthesis of azidoacrylate was later simplified by Bolm's group using only Fe(OTf)2 as the catalyst and β-azido cinnamic esters as the substrate.307 The theoretical study on [FeIII(F20-tpp)Cl] revealed that an iron-nitrene intermediate indeed plays a crucial role and the N2 liberation step is the probable rate-determining step.308 When the indoline formation reaction was carried out with a substrate such that the product formed has a leaving group present at the C-2 position (e.g. –OR) (Scheme 267), an unexpected alkyl migration was observed, forming an 2,3-dialkyl-substituted indole derivative.309 This behaviour was presumed to occur via the formation of an iminium ion upon removal of the leaving group, and subsequent proton abstraction. The groups of both Chan and Che also independently documented nitrene insertion into the aldehyde C–H bond using FeCl2 in conjunction with a pyridine-based ligand (Scheme 268).310,311
Betley and co-workers described that iron dipyrromethene complexes are capable of amination at the benzylic position of toluene in the presence of alkylazide (Scheme 269).312 The very high kH/kD value of 12.8 indicates C–H activation as the rate-determining step. Moreover, the observation of 1,2-diphenylethane in the reaction mixture is indicative of radical formation. Also, the formation of an Fe-imide intermediate was confirmed by X-ray crystallography. Thus, the authors proposed the most plausible mechanistic cycle for the formation of the Fe-imide intermediate from the iron dipyrromethene complex and alkylazide, which abstracts a hydrogen from toluene. Then the benzyl radical rebounds with the amide ligand co-ordinated with iron to form the final amine product (Scheme 270). Further, the kinetic isotope effect study gave a high value of primary KIE = 12.8, excluding the possibility of an insertion pathway.
Che's group explored the scope of iron-imide mediated amination using the [Fe(qpy)(MeCN)2](ClO4)2 (qpy = 2,2′:6′,2′′:6′′,2′′′:6′′′,2′′′′-quinquepyridine) complex and PhINR.313 They were also able to synthesize various heterocycles through intramolecular amination. In another report, Betley's group used aliphatic alkylazides to form various pyrrolidine derivatives catalyzed by a similar system (Scheme 269).314 The famous pentadentate non-heme iron complex [Fe(N4Py)(CH3CN)](ClO4)2 (N4Py = N,N-bis(2-pyridyl-methyl)bis(2-pyridyl)methylamine) was used by Liu's group to achieve amination of the benzylic C(sp3)–H bond using TsNBrNa as the amine source (Scheme 272).315
The Lewis acidic Fe(OTf)2-catalyzed carbene insertion in the Si–H bond was reported by Ollevier's group (Scheme 271).316 Under the optimized conditions, α-aryl-α-diazoacetates were reacted with R3SiH in the presence of 5 mol% of iron catalyst in CH2Cl2 at 40 °C. The enantioselective version of the reaction was also developed using a chiral hexamethyl-1,1′-spirobiindane-based bisoxazoline ligand (HMSI-BOX) with excellent ee.317 In another report, diazoacetonitrile was used as the carbene source for insertion into N–H and S–H bonds, thus providing α-amino nitriles and α-mercapto nitriles as products.
In 2014, Betley and co-workers developed a chemoselective method depicting iron-catalyzed intermolecular C–H amination of olefinic substrates in the presence of dipyrrinato iron catalysts 1 and 2 and organic azides (Scheme 273).318 During the course of the reaction, the iron catalyst reacts with the organic azide and forms high-spin iron-imido radical intermediate 3, which actively executes the C–H amination reaction.
Notably, it was suggested that the unique electronic structure of iron-imido intermediate 3 governs the chemoselectivity of the reaction. The present synthetic method tolerates different benzylic substrates and styrenyl substrates to give C–H amination and aziridination products, respectively. More interestingly, the α-linear olefinic substrates (during reaction with catalyst 2) containing allylic C–H bonds exclusively provide the amination product at the terminal position with no formation of aziridine products.
Thus, the observed regioselectivity was rationalized based on the steric bulk between the ligand backbone and the adamantyl imido fragment. Further, the reaction between iron catalysts with cis-β-methyl styrene exclusively provides the linear allylic amination product, suggesting the involvement of H-atom abstraction followed by radical recombination to give the amination product.
Later in 2017, following their work in 2013, they reported another method for the iron-dipyrrinato complex-catalyzed diastereoselective synthesis of 2,5-disubstituted pyrrolidines from aliphatic azides (Scheme 274).319 Interestingly, in this method, the dipyrrinato–iron phenoxide complex shows the best diastereoselectivity among other variant of catalysts. Different aliphatic azides containing both electron-donating and electron-withdrawing substituents are well amenable to provide the desired products. Notably, among the iron alkoxide and aryloxide catalysts, iron phenoxide 4 was found to be the best to exhibit high diastereoselectivity. Moreover, from the mechanistic investigation with experimental and theoretical studies, it was suggested that the reaction may occur via a direct C–H amination pathway (as shown in Scheme 270).
Recently, Arnold's group reported a number of pioneering works on iron co-factor-containing artificial metalloenzyme-catalyzed C–H functionalization. In the very first example of carbene insertion into the C–H bond catalyzed by metalloenzymes, they utilized mutated P411s derived from P450BM3 of Bacillus megaterium (Scheme 275). After screening a variety of catalysts, P411-CHF was found to be best for the selective benzylic C–H alkylation via carbene insertion.320 An enantiomeric ratio of up to 98.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 and turnover number of up to 2150 were obtained with this methodology at ambient temperature. Various substituents at the benzene ring such as methoxy, bromo, and silyl are well tolerated under the reaction conditions. It was presumed that the reaction proceeds via the formation of a high-valent iron(enzyme)–carbene intermediate. Fascinatingly, this particular catalyst selectively delivers benzylic alkylation over cyclopropanation in the presence of an olefinic double bond. Moreover, they applied the method during the formal synthesis of lyngbic acid. This protocol was further extended to carbene insertion into allylic, propargylic and α-amino C–H bonds.
1.4 and turnover number of up to 2150 were obtained with this methodology at ambient temperature. Various substituents at the benzene ring such as methoxy, bromo, and silyl are well tolerated under the reaction conditions. It was presumed that the reaction proceeds via the formation of a high-valent iron(enzyme)–carbene intermediate. Fascinatingly, this particular catalyst selectively delivers benzylic alkylation over cyclopropanation in the presence of an olefinic double bond. Moreover, they applied the method during the formal synthesis of lyngbic acid. This protocol was further extended to carbene insertion into allylic, propargylic and α-amino C–H bonds.
In a subsequent report, Arnold's group found that the P411-PFA variant effectively catalyzes the α-amino C–H fluoroalkylation of aryl dialkyl amines (Scheme 276).321 Both diazotrifluoroethane and diazopentafluoropropane are compatible alkylating agents for various N-alkyl anilines to provide a turnover number of up to 2100. Notably, the use of aryl pyrolidines as the substrate yields R-enantiomer of the alkylated product with excellent ee with the present catalyst. Moreover, they also found that a quadruple mutant enzyme, P411-PFA-(S), was equally effective in producing the S-enantiomer. This strategy was also extended to (α)diazo-(γ)lactones as a carbene source with a linage of P411–C10 variants (Scheme 277).322
Meanwhile, they also explored nitrene insertion reactions into C–H bonds using mutated iron-heme enzymes. Upon testing various mutants, it was observed that P411-IA and a few of its variants were effective for the C2 amidation of indoles with sulfonyl azides (Scheme 278).323 Although the scope of the reaction was not studied in detail, promising numbers with respect to yields and turnovers were obtained.
The systematic mutation of the P411 enzyme provides extremely effective P411Diane variants for intramolecular nitrene insertion into C–H bonds (Scheme 279).324 Sulfamyl azide was used to produce the nitrene intermediate, which is inserted enantioselectively in the enzymatic environment into an activated C–H bond to form a chiral 5-6-membered diamine thiadiazolidine dioxide. Both secondary and tertiary C(sp3)–H bonds adjacent to arene or olefin can be subjected to insertion. Not only various substituents on the arene ring such as methyl, halide, and methoxy are compatible with the reaction protocol, but thiophene instead of arene also provided the desired product in 99% ee. Another variant of P411Diane (P411Diane1I327P) was then established to be effective for the same reaction with primary.
C–H bonds with excellent ee. Moreover, similar to carbene insertion, slight modification of the protein chain affords the reverse enantioselectivity. The computational study supports that the heme-ferrous cofactor first forms iron-nitrenoid species with the azide, which then abstracts hydrogen from the C–H bond to form a carbon-centered radical. The protein chain controls enantioselective rebound between the carbon radical and nitrogen provides the chiral insertion product.
Recently, Arnold's group established similar enzyme-catalyzed amination at the benzylic and allylic positions.325 They presumed that pivolylammonium triflate would provide an unprotected iron-nitrenoid intermediate with heme iron and deliver selective insertion with the help of a systematically mutated protein chain. Indeed, the P411–B2 variant was found to be excellent for this purpose. High yield and enantioselectivity were obtained under the reaction protocol for various cyclic and acyclic benzylic and allylic substrates.
6. Iron-catalyzed cross-coupling reaction
Metal-catalyzed cross-coupling is a standard tool for carbon–carbon bond formation. Its importance is due to its extensive application in the synthesis of many natural products, pharmaceutically active compounds, polymers and various fine chemicals. The cross-coupling between organic electrophiles and organometallic nucleophiles in various hybridization is catalyzed by transition metals. This field is dominated by the late transition metals such as palladium, iridium and rhodium.5–7 However, presently the world needs green and cheaper alternatives to late transition metals. Notably, iron has been found to be one of the best alternatives since it is abundant, cheap, non-toxic and environmentally benign. Overall, it presents a greener approach for carbon–carbon cross-coupling reactions. Here, we well document the iron-catalyzed cross-coupling reactions with detailed mechanistic discussions.The early reports of iron catalysis were found from the work of Kharash in 194115 and Kochi in 1971.18 However, despite these early reports, iron catalysis remained unexplored for a long time until Caheiz significantly highlighted its high synthetic potential in 1998.326 Since then, a renaissance commenced in this field with an immense increase in the number of reports and reviews.3h,3l,327–354
Here, we focus on presenting an overview of the thorough development of iron catalysis in carbon–carbon cross-coupling reactions from Kharash's and Kochi's early work to recent investigations. Generally, iron-catalyzed cross-coupling involves two different types of coupling partners, one is electrophilic and the other is nucleophilic. In this section, we divide this topic into subsections based on the type of electrophiles and nucleophiles used in the reaction.
6.1 Cross-coupling of alkenyl and alkynyl electrophiles with various nucleophilic partners
6.1.1.1 Cross-coupling with alkylmagnesium nucleophiles. The first iron-catalyzed cross-coupling between alkenyl halide and alkyl Grignard reagent was reported by Kochi and Tamura in 1971 (a, Scheme 280).18 This reaction proceeds stereospecifically, giving the E or Z cross-coupled product from the corresponding E or Z vinyl bromides.18b In this context, the use of an iron catalyst containing a diketone ligand such as Fe(dbm)3 (dbm = dibenzoylmethane) shows the highest conversion rate using less vinyl bromides.18d
While investigating Kochis's work, in 1998 Caheiz et al. found that using NMP (N-methylpyrrolidione) co-solvent increases the yield to a greater extent (b, Scheme 280).355,356 High stereoselectivity has also been reported for the reaction conditions. In addition, the dialkylation of 1,1-dichoro-1-alkenes in the presence of Fe(acac)3 catalyst was reported.357 Surprisingly, the presence of NMP in this dialkylation reaction decreases the yield. However, tetrahydrofuran (THF) as the solvent at −30 °C provides a good product yield.
In 2012, Caheiz et al. showed the use of iron(II) arylthiolates as a potent catalyst for the coupling of alkenyl halides with alkyl Grignard reagents (c, Scheme 280).358 Notably, the use of NMP as a co-solvent, which is classified as reprotoxic, is avoided in the reaction. The products are observed in high yields with complete retention of the configuration at the double bond.
Thomas and co-workers developed a cross-coupling reaction between alkenyl halides and Grignard reagents, affording alkanes in moderate to high yields (Scheme 281).359 This cross-coupling is performed using a dipyridyldiiminoiron(II) complex as the catalyst. The post-synthesis reduction of alkene to alkane is performed using N,N-dimethylaminoborohydride as the reducing agent.
Recently in 2019, Noël and co-workers presented a selective and practical method for the cross-coupling of alkynyl chlorides with alkylmagnesium halides in the presence of iron catalysts and NHC ligand (Scheme 282a).360 The catalytic system with iron(III) acetylacetonate and SIPr·HCl affords the best result. They also coupled styrenyl chlorides with Grignard reagents in the presence of the same iron catalyst without any ligand (Scheme 282b). Notably, various electro-neutral, electron-donating and electron-withdrawing substituents are well tolerated with both styrenyl and alkynyl chlorides, giving excellent yields of the desired coupled product. The stereochemistry of the β-chloro styrene substrates is retained in the coupled products. Further, they combined the formation of a Grignard reagent and iron-catalyzed cross-coupling reaction in a single continuous flow process. This uninterrupted flow process results in both the C(sp)–C(sp3)- and C(sp2)–C(sp3)-coupled product with the same stereoselectivity and yields in absence of a ligand. Additionally, around the same time, Caheiz et al. introduced a very potent method for the cross-coupling between alkenyl halides and alkyl Grignard reagents, giving high yields of coupled products on the gram scale (Scheme 283).361 The reaction is successfully performed using a catalytic amount of iron(III) chloride in the presence EtOMgCl in THF. The latter is prepared by the metalation of EtOH with isopropylmagnesium chloride in THF. A wide variety of Grignard reagents undergo the stereoselective cross-coupling reaction, producing high to excellent yields of desired olefins with complete retention of stereochemistry. Notably, the use of alkoxide magnesium salts provides a cheap and eco-friendly alternative to the toxic NMP additive or expensive ligand.
6.1.1.2 Cross-coupling with aryl magnesium nucleophiles. Kochi in his works demonstrated the first iron-catalyzed cross-coupling of phenylmagnesium bromide with an alkenyl halide, but the yield was poor (a, Scheme 284).18c Later, Molander et al. investigated on the same reaction and modified it.362 They reported that the use of DME and decreasing the temperature can significantly increase the yield (32% to 62%) (b, Scheme 284). Later, Cahiez et al. mentioned the use of sulfolane in placing of NMP as a cosolvent to increase the yield of the Fe(acac)3-catalyzed cross-coupling of alkenyl bromide and phenylmagnesium chloride (c, Scheme 284).355
In 2011, von Wangelin and co-workers reported a domino process involving the iron-catalyzed preformation of aryl Grignard reagent and subsequent cross-coupling with alkenyl bromides.363 The catalytic system consists of iron(III) chloride with TMEDA (N,N,N′,N′-tetramethylethelenediammine) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio. This catalytic solvent serves for both the aryl Grignard formation and the cross-coupling (d, Scheme 284). Notably electron-rich aryl bromides and heteroaryl bromides are incompatible in this method due to the incomplete formation of the Grignard reagent and side reaction. They require an iron-free preformation using Mg and LiCl (e, Scheme 284). Both procedures result in the formation of high yields of corresponding styrene derivatives. The iron-catalyzed stereoselective Kumada–Corriu cross-coupling was used as a key step in the synthesis of the anticancer agent combrestatin-A-4.364 Additionally, the large-scale synthesis of the calcium sensing receptor antagonist cinacalcet hydrochloride was reported using alkenyl chloride and aryl Grignard in the presence of Fe(acac)3.365
4 ratio. This catalytic solvent serves for both the aryl Grignard formation and the cross-coupling (d, Scheme 284). Notably electron-rich aryl bromides and heteroaryl bromides are incompatible in this method due to the incomplete formation of the Grignard reagent and side reaction. They require an iron-free preformation using Mg and LiCl (e, Scheme 284). Both procedures result in the formation of high yields of corresponding styrene derivatives. The iron-catalyzed stereoselective Kumada–Corriu cross-coupling was used as a key step in the synthesis of the anticancer agent combrestatin-A-4.364 Additionally, the large-scale synthesis of the calcium sensing receptor antagonist cinacalcet hydrochloride was reported using alkenyl chloride and aryl Grignard in the presence of Fe(acac)3.365
Further in 2012, synthetically important and biological motif 1,1-diarylethylenes were prepared via a cross-coupling reaction catalyzed by an iron–copper co-catalyst system. Polyoxygenated 1-halostyrenes are coupled with aryl Grignard reagents in the presence FeCl3 and copper(I) thiophene-2-carboxylate (CuTC) of the catalyst combination, affording good to excellent yields of the corresponding products (f, Scheme 284).366 However, the individual use of Cu and Fe catalysts gives unsatisfactory results. A large substrate scope was reported, ranging from electron-withdrawing group-substituted to electron-donating-substituted aryl Grignard reagents.
6.1.1.3 Cross-coupling with alkynyl magnesium nucleophiles. In 2008, Nakamura and co-workers demonstrated the iron(III) chloride-catalyzed reaction between alkynyl Grignard nucleophiles and alkenyl bromides (Scheme 285).367 The reaction provides a very efficient pathway for the synthesis of conjugated enynes. The enynes are significantly used in many bioactive molecules and drug intermediates. Moreover, lithium salts such as LiBr and LiCl accelerate the reaction significantly. An Fe(0)/Fe(II) catalytic cycle was proposed in this methodology.
6.1.1.4 Cross-coupling with organomanganese nucleophiles. In 1996, Caheiz and co-workers performed a reaction between alkenyl halides and alkylmanganese chlorides in the presence of iron catalysts. They obtained very efficient conversion using Fe(acac)3 in THF solvent together with NMP as the co-solvent. The substituted olefins were obtained with excellent stereoselectivity (Scheme 286).368
Recently in 2019, Knochel and co-workers reported a method for the iron-catalyzed cross-coupling of alkenyl iodides and bromides with benzylmanganese halides in the presence of iron(II) chloride in THF (Scheme 287).369 The benzylmanganese reagents are readily prepared from the corresponding benzyl halides by treatment with manganese turnings and MnCl2·2LiCl in THF. Various functionalized benzylmanganese reagents are added to the alkenyl electrophiles as and iodoacrylates derivatives with retention of the configuration at the double bond. Notably, high yields of polyfunctionalized alkenes are produced using this method. Through mechanistic studies, they suggested the formation of an iron(II) ate complex as the active species, which reacts with the alkenyl electrophile.
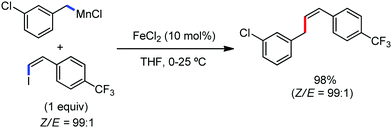 | ||
| Scheme 287 Cross-coupling of alkenyl iodides with benzylmanganese nucleophiles in the presence of an iron catalyst. | ||
6.1.1.5 Cross-coupling with organolithium nucleophiles. Recently in 2019, Wong et al. developed an efficient synthetic method involving an iron-mediated coupling reaction between organolithium compounds and alkenyl iodides (Scheme 288).370,371 A large variety of substrates undergo stereospecific coupling, providing moderate to good yields of the coupled products. The best results were obtained using iron(III) acetylacetonate precatalyst and DavePhos as the ligand. The reaction protocol is also efficient for gram-scale synthesis, providing the respective desired product in satisfactory yields.
6.1.2.1 Cross-coupling with organomagnesium nucleophiles. In cross-coupling reactions, enol triflates are preferred as the electrophilic partner due to their easy preparation from the corresponding ketones. Füstner et al. reported the cross-coupling of a large variety of alkenyl tosylates with alkyl and aryl Grignard reagent in moderate to high yields (a, Scheme 289).372 The reaction was found to be highly regioselective. Thus, a large number of functional groups such as esters, ethers, carbamates, acetals and lactones are tolerated on the electrophilic partners.
They proposed a low-valent Fe(II−) metal cluster having the formal composition of [Fe(MgX2)]n as the active site. This species is believed to be formed by the reduction of the iron precatalyst via β-hydride elimination. However, an effective reaction is also given by methylmagnesium bromide despite the absence of β-hydrogen. In this context, the presence of an iron–ate complex was predicted. Accordingly, successful coupling occurs by employing the ‘ate’ complex [Me4Fe][Li2(OEt)2], resulting in high product yields.373 Notably, the reaction protocol was applied to the synthesis of many natural products such as latrunculin A374,375 and latrunculin B.375 Also, the formal synthesis of latrunculins C, M and S was successfully performed using the same methodology. Fürstner et al. also demonstrated the formation and catalytic potential of a homoleptic organoferrate complex [(Me4Fe)(MeLi)][Li(OEt)2] in the iron-catalyzed cross-coupling of alkenyl triflate with methylmagnesium bromide.373
In 2008, Tam and co-workers developed an iron-catalyzed coupling reaction between a bicyclic alkenyl triflate and Grignard reagents. A wide range of aliphatic and aromatic Grignard reagents are coupled using Fe(acac)3 as the precatalyst. The best yields are obtained using DMPU (DMPU = N,N′-dimethylpropyleneurea) or THF/NMP as the solvent at −25 °C (b, Scheme 289).376,377 The disubstituted bicyclo cross-coupled product is also obtained in good yield (c, Scheme 289).
Furthermore, a successful method for the preparation of stereodefined β,β-disubstituted α,β-unsaturated esters was developed using an iron-catalyzed cross-coupling reaction (Scheme 290).378 Also, methoxy carbonyl group-substituted E- and Z-vinyl tosylates are efficiently coupled to Grignard reagents with complete stereoretention. The highest yields with E-enol tosylates are obtained using iron(III) chloride, whereas Z-enol tosylates prefer Fe(DMB)3 (DMB = dibenzoylmethane) as the precatalyst. Notably, tetrahydrofuran (THF) was found to be the most suitable solvent for this transformation.
6.1.2.2 Cross-coupling with organocopper nucleophiles. In 2005, Knochel and co-workers introduced an iron-mediated coupling of alkenyl sulfonates using functionalized arylcopper reagents as the nucleophilic partners (Scheme 291).379 The desired olefins are obtained in good yields using Fe(acac)3 as the catalyst in DME (DME = dimethoxyethane) solvent. Notably, arylcopper reagents are prepared from the corresponding arylmagnesium derivatives by treatment with a copper(I) cyanide–di(lithium chloride) complex.
In addition, a one-pot procedure was also reported for the preparation of enol phosphates from the corresponding ketone and subsequent cross-coupling to form the product. This method provides the desired coupled product in good yields (Scheme 293).380 Caheiz et al. in their following reports demonstrated a new route for the stereoselective preparation of terminal-conjugated dienes with significant yields. Purposefully, they performed the coupling between Grignard reagents and dienol phosphates in the presence of Fe(acac)3 as a precatalyst.381 This procedure was applied to the synthesis of the pheromone of Diparopsis castanea. Also, the synthesis of 4-substituted tetrahydropyridines was successfully carried out using vinyl phosphates produced from 4-piperidones via iron-catalyzed cross-coupling.382 Evans et al. synthesized a potent and selective k-opioid receptor agonist, salvinorin A.383,384 In the key step of this synthetic pathway, the iron-catalyzed cross-coupling reaction with alkenyl enol phosphates is used.
A unique example of iron-catalyzed ring opening and subsequent alkenyl-X/methylMgBr cross-coupling was developed by Fürstner and co-workers (Scheme 295).387 The ring opening of the 2-pyrone substrates occurs upon nucleophilic attack of the methyl Grignard reagent in the presence of iron(III) acetylacetonate. Mainly, dienyl carboxylates are obtained with the retention of configuration at the double bond. The authors proposed a conjugate addition mechanism rather than an oxidative addition/reductive elimination. This new methodology has been applied for the synthesis of several natural products such as the cytotoxic tryptamine derivative granulatamide B and marine macrolide pateamine A.384
Notably, the easily accessible alkenyl acetates were coupled with alkyl and arylmagnesium halides by von Wangelin and co-workers using simple iron salts under mild conditions (Scheme 296).388 Importantly, the thermodynamically favoured acylation and deprotonation reaction are suppressed under the reaction conditions. A wide substrate scope was presented with respect to both coupling partners.
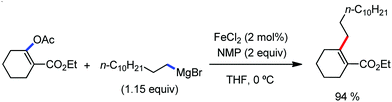 | ||
| Scheme 296 Cross-coupling of alkenyl acetates with alkyl magnesium halides in the presence of a simple iron catalyst. | ||
In 2016, Rivera et al. introduced a cross-coupling reaction of stereodefined N,N-dimethyl enol carbamates and organomagnesium reagents in the presence of simple iron(III) chloride in a THF/NMP solvent mixture, affording tri- and tetrasubstituted acrylates in high yields (Scheme 297).389 Although, a large number of alkyl Grignard reagents were found to give high yields of products, the phenyl and isopropyl Grignard reagents resulted in poor product yields. They also demonstrated the tolerance of highly reactive functional groups such as esters in this reaction.
In 2008, Vogel et al. reported the iron-catalyzed desulfinylative cross-coupling of alkenesulfonyl chlorides with alkyl or arylmagnesium halides (Scheme 299).391 The reaction was accomplished using Fe(acac)3 as the catalyst with perfect retention of the geometry of the double bond. Alkenyl selenides and tellurides were also found to be good electrophilic partners in the iron-catalyzed cross-coupling reaction (Scheme 300).392 The reaction proceeds with complete retention of the configuration at the double bond, giving good product yields. Particularly, the use of triethyl amine as the ligand is beneficial for the complete conversion of the reaction.
6.2 Cross coupling of aryl electrophiles with various nucleophilic partners
6.2.1.1 Using alkyl Grignard reagents. Pridgen et al. first reported the iron(III) acetylacetonate-catalyzed cross-coupling reaction of aryl halides with Grignard reagents.393 They efficiently coupled o-chloroarylimine with an alkyl Grignard reagent in the presence of an iron catalyst. The Fe(acac)3-catalyzed cross-coupling of alkyl Grignard with aryl and heteroaryl electrophiles was well investigated by Fürstner et al. (a and b, Scheme 301).394,395 A wide variety of functionalized aryl and heteroaryl chlorides, triflates and tosylates produce the cross-coupled products in high yields. Interestingly, aryl bromides and iodides afford lower yields compared to chloride, triflates and tosylates. Moreover, this method presents a complementary approach to the corresponding palladium catalysis.
The gram-scale synthesis of the liquid crystalline material 4-nonyl benzoic acid was reported by Fürstner et al. in 2005.396 The same method was utilized for the selective monoalkylation of dichloroarenes and heteroarenes, affording the corresponding product in good yields (c, Scheme 301).372 Notably, this methodology is superior to the corresponding palladium-catalyzed reaction. This procedure was successfully applied in the synthesis of the immunosuppressive agent FTY720. Both aryl chloride and triflate are efficient as a starting material for the synthesis of the same target.397,398
Furthermore, the iron-catalyzed monoalkylation procedure was utilized for the synthesis of the macrocyclic spermidine alkaloid (−)-isooncinotine.399 An iron–salen catalyst is used for the consecutive alkylation of two different alkyl groups with 2-chloro-6-trifloxypyridine. This alkylation is the key step in the synthesis of the odoriferous alkaloid (R)-(+)-muscopyridine (Scheme 302).400
In 2004, Hocek and co-workers reported a dichotomy in the regioselective cross-coupling reactions of 6,8-dichloropurines with palladium-catalyzed phenyl boronic acid and iron-catalyzed methyl magnesium chloride (Scheme 303).401 The palladium catalysis with one equivalent of phenyl boronic acid gives 8-chloro-6-phenylpurine (Scheme 303a),402 whereas iron catalysis affords the 6-chloro-8-methylpurine derivative (Scheme 303b)403 as the major product.
Similar types of iron-catalyzed selective monomethylation of dichloro- and trichloro-heterocyclic arenes were also reported.404–406 4-Substituted pyrrolo[3,2-c]quinolines were synthesized via the cross-coupling of alkyl and arylmagnesium halides with 4-chloro pyrrolo[3,2-c]quinoline in the presence of an iron catalyst.406 The reactions are completed in a short time of 30 min, resulting in moderate to excellent yields in the presence of a THF/NMP solvent mixture.
In addition to chlorides, heteroaryl sulfonates and phosphates were proven to be efficient electrophilic partners in iron-catalyzed cross-coupling. The best yields are obtained using iron(III) chloride in a THF/NMP solvent mixture at a low temperature (Scheme 304a).407 Furthermore, the cross-coupling of heteroaryl sulfamate with phenylmagnesium bromide was performed using Fe(acac)3 and TMEDA (Scheme 304b).
In 2011, Wolf et al. studied the catalytic properties of a low-oxidation state iron complex in the cross-coupling reaction.408 The catalytic efficacy of the complexes was investigated by using these complexes in the cross-coupling of aryl, alkenyl and alkyl electrophiles with alkyl and arylmagnesium bromides. They concluded that the oxidation state of the precatalyst has little significance in its catalytic performance. However, a labile coordination environment is necessary. Consequently, an anionic bis-anthracene iron complex affords good results.
The synthesis of 2-substituted furans was successfully accomplished in moderate yields using the iron-catalyzed cross-coupling reaction of 2-bromofuran with Grignard reagents. The reaction is catalyzed by Fe(acac)3 in DMPU (N,N′-dimethylpropyleneurea) solvent (Scheme 305).409 It is important to note that the corresponding palladium-catalyzed reaction did not afford the desired product.
N-Heterocyclic carbenes are significantly used as ligands in the iron-catalyzed cross-coupling of non-activated electron-rich aryl chlorides with alkyl Grignard reagents (Scheme 306).410 The best results are obtained using the tetrahydrate of iron(III) chloride as the catalyst. In this reaction protocol, primary Grignard reagents are coupled with excellent yields, whereas secondary alkyl nucleophiles give moderate yields. The above synthetic method of the iron-catalyzed cross-coupling of aryl chlorides with alkyl Grignard reagents was used for the construction of pentapodal ω-functional derivatives of corannulene.411
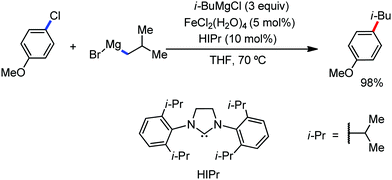 | ||
| Scheme 306 Cross-coupling of aryl halides and alkyl Grignard reagent in the presence of iron salt and NHC ligand. | ||
Later in 2013, a selective and simple iron-promoted cross-coupling reaction of aryl chlorides especially o-chlorostyrenes with alkyl Grignard reagents was developed by von Wangelin and co-workers (Scheme 307).412 Both electron-rich and electron-poor aryl chlorides give good yields in the presence of Fe(acac)3 in a THF/NMP (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixture.
1) solvent mixture.
In the same year, Fox et al. reported the use of TMEDA (tetramethylethylenediamine) as a cheap and easily removable ligand for the iron-catalyzed coupling of aryl chlorides with alkyl Grignard reagents.413 This method allows a very low catalyst loading (0.1–1 mol%) and high conversions for different substrates containing ester, amide and trifluoromethyl functional groups. In addition to alkenyl pivalates, Shi et al. accomplished the cross-coupling with 2-naphthyl pivalates in the presence of iron catalysts. Interestingly, the yield was found to increase dramatically from moderate to high by changing the substrate from 2-naphthyl pivalates to 2-naphthyl-N,N-dimethyl carbamate. Notably, both reactions are catalyzed by iron(III) chloride in the presence of NHC ligand (Scheme 308).385
Iron(II) arylthiolates are used as a precatalyst for the cross-coupling of aryl halides with alkyl Grignard reagents, giving good yields of the coupled product.355 Aryl sulfamates and carbamates are also coupled with arylmagnesium chlorides (Scheme 309).344,414 This reaction is performed using iron(II) chloride and NHC ligand in dichloromethane solvent. A wide scope of electrophiles ranging from electron-deficient arenes and heteroarenes to electron-rich arenes is observed. This method has been used for the synthesis of polyfunctionalized arenes due to its directing group ability and low reactivity compared to conventional cross-coupling.
Cook and Agarwal presented a similar reaction for the iron-catalyzed coupling of arylsulfamates and tosylates with primary and secondary alkyl Grignard reagents.415 In the case of isopropyl nucleophile, FeF3·3H2O is essential to slow down the isomerization and enhance the branched to linear selectivity.
Wang and Guo developed the cross-coupling reaction between trimethyl ammonium triflates and alkyl magnesium reagents in the presence of iron(III) acetylacetonate in a THF/NMP mixture (Scheme 310).416 A series of functional groups on both coupling partners are well tolerated. Secondary and primary Grignard reagents possessing β-hydrogens are found to be compatible with the reaction conditions, providing good yields. The adenosine A2A receptor antagonist ST1535 was synthesized via the cross-coupling of 2-chloropurine and n-butylmagnesium chloride in the presence of Fe(acac)3.417
Further in 2015, Wang and co-workers described the iron-promoted coupling reaction of N-heteroaromatic tosylates with alkyl and aryl Grignard reagents, producing high yields of the coupled products (Scheme 311).418 A large variety of tetrasubstituted pyrimidines are synthesized from the corresponding pyrimidin-2-yl tosylates using FeCl3 in a mixture of THF/NMP for alkyl Grignard reagents and Fe(acac)3 in TMEDA for aryl nucleophiles.
In 2017, Piontek and Szostak reported the iron-catalyzed C(sp2)–C(sp3) cross-coupling reaction between polyaromatic tosylates and alkyl Grignard reagents in the presence of Fe(acac)3 and NMP as an O-coordinating ligand (Scheme 312).419 This procedure affords high yields of alkylated polycyclic arenes having considerable applications as common motifs in a wide range of electronic materials, pharmaceuticals and high-performance fluids. Moreover, a large variety of polyaromatic substrates and β-hydrogen containing Grignard reagents are found to give high yields of products. The mild conditions of the reaction allow the coupling of polyaromatic substrates bearing sensitive carboxylic-derived functional groups.
Further in 2018, Bisz and Szostak reported the iron-catalyzed cross-coupling between chlorosulfonamides and alkyl Grignard reagents in the presence of mild iron(III) acetylacetonate as a precatalyst and NMP as a ligand in THF, producing good to excellent yields of alkylated benzosulfonamides (Scheme 313).420 The alkyl benzosulfonamides are synthetically very important due to their immense application for the production of a large number of pharmaceuticals, agrochemicals and plasticizers. Notably, a wide variety of functionalized benzosulfonamides are efficiently coupled with a large number of Grignard reagents including challenging β-hydrogen-containing Grignard reagents to produce excellent yields of desired products. Interestingly, there is no attack of the Grignard reagent on the electrophilic sulfonamide bond in any of the reported examples. The sulfonamides have been demonstrated as one of the most reactive activating groups for iron-promoted cross-coupling reactions.
They also reported the use of the green and eco-friendly 2-methyltetrahydrofuran (2-MeTHF) as a solvent for the iron-catalyzed cross-coupling reaction.421 They successfully coupled aryl chlorides and tosylates with β-hydrogen-possessing Grignard reagents in the presence of iron(III) acetylacetonate in 2-MeTHF, resulting in the formation of good to excellent yields of products. Notably, a wide range of aryl and heteroaryl chlorides and tosylates react with various Grignard reagents with high yields, demonstrating the high efficiency of the reaction. Further in 2019, they introduced N-methylcaprolactum as a non-toxic alternative to the carcinogenic N-methylpyrrolidine (NMP) used as a co-solvent for different iron-catalyzed cross-coupling reactions. The efficiency of the reaction was demonstrated through the iron-catalyzed alkylation of aryl chlorides and tosylates, providing excellent yields of products.422
Furthermore, they presented the first reactivity study on the effects of amides as O-coordinating ligands for the iron-mediated cross-coupling reaction of aryl and heteroaryl chlorides with β-hydrogen-containing Grignard reagents, finding some efficient alternatives to the reprotoxic NMP (Scheme 314).423 Several highly efficient ligands such as TMU (TMU = tetramethyl urea), N-cyclic and coordinating benzamides have been discovered through these studies. These investigations led the way towards eco-friendly and sustainable chemistry by replacing the carcinogenic NMP.
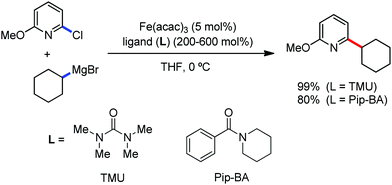 | ||
| Scheme 314 Iron-catalyzed cross-coupling of heteroaryl halides with alkyl Grignard reagents using newly introduced ligands. | ||
In 2019, the same group demonstrated an efficient protocol for the synthesis of alkyl substituted amides via the cross-coupling of chlorobenzamide derivatives and alkyl Grignard reagents in the presence of iron(III) acetylacetonate and O-coordinating ligands such as TMEDA and NMP (Scheme 315).424 The methodology affords high yields of the desired products, providing good tolerance to a large number of sensitive amide substituents. They also showed the method to be compatible with the challenging alkyl Grignard reagents possessing a β-hydrogen. Overall, a wide substrate scope was reported. Notably, the products have broad application in the fields of pharmaceuticals, agrochemicals and different biologically active molecules. This protocol was utilized for the synthesis of the 5-HT receptor antagonist.
Around the same time, Szostak and co-workers developed an efficient iron-catalyzed cross-coupling reaction for aryl and heteroaryl chlorides with alkyl Grignard reagents possessing β-hydrogen atoms using a low catalyst loading (Scheme 316).425 The reaction is performed in the presence of the environment-friendly cyclic urea ligand 1,3-dimethyl-2-imidazolidinone (DMI). A broad range of substrates with various functional groups are compatible with this reaction protocol, producing high yields of the corresponding coupling products.
6.2.1.2 Using aryl Grignard reagents. Unsymmetrical biaryl compounds are often found in natural products such as alkaloids and numerous biologically active components of pharmaceuticals and agrochemicals. Thus, aryl–aryl bond formation is one of the most important tools of modern synthetic chemistry.
Although the earliest efforts of iron-assisted aryl–aryl bond formation was made by Fürstner et al., they were unsuccessful in accomplishing an efficient coupling. The phenylmagnesium bromide mainly undergoes homocoupling to form biphenyl. Indeed, this phenomenon was reported by Kharash.15,426 Notably, they reported that in the presence of a catalytic amount of iron salt, homocoupling of the aryl Grignard reagent occurs when aryl halides are present as oxidizing agents.427,428 In 2002, Figadère et al. were the first to couple aryl moieties using the iron-catalyzed cross-coupling reaction.429 They coupled phenyl Grignard reagents with 2-halo and 3-halo quinolines together with substituted halopyridines in the presence of Fe(acac)3 in THF, obtaining the desired product in moderate to good yields (a, Scheme 317). Based on the same conditions, Ple and co-workers coupled substituted heteroaryl chlorides with phenylmagnesium chlorides and 3-pyridylmagnesium chlorides.430 However, the yields of the products obtained were moderate (b, Scheme 317).
The most efficient method for selective biaryl coupling was introduced by Hatekeyma and Nakamura in 2007, giving excellent yields of product. The substituted aryl halides are coupled with arylmagnesium halides in the presence of iron trifluoride trihydrate, ethylmagnesium bromide and NHC ligand (Scheme 318).431,432 The ethylmagnesium bromide added to the precursor catalyst helps in the generation of the active iron species via the reduction of the precursor. Most importantly, the iron fluorides in combination with the NHC ligand significantly suppress the homocoupling reaction. In comparison to the reactivity of aryl halides under Fürstner's conditions,394–396 the chlorides are more reactive than bromides and iodides. On the other hand, aryl fluorides were found to be inert to the conditions. The method also affords high yields of coupled products using sterically hindered mesityl magnesium bromide as the nucleophilic partner.
In 2012, von Wangalin et al. came up with a synthetic method for biaryl synthesis from o-chlorostyrenes and aryl Grignard reagents using iron(III) acetylacetonate as the catalyst (Scheme 319).433 The o-chlorostyrenes were found to be exceptionally efficient compared to m- or p-chlorostyrenes. Most interestingly, the substrate with methyl or phenyl substituents at the vinyl group of the m- and p-chlorostyrenes gave good yields of the coupled products. The less basic fluoro-substituted arylmagnesium bromides as the nucleophilic partner gave the best yields. Actually, the vinyl substituents undergo coordination to the iron catalyst, followed by haptotropic migration to the location of the C–Cl bond.
Further, Knochel et al. used iron(III) bromide to efficiently couple N-heterocyclic halides with aryl Grignard reagents (Scheme 320).434 The solvent combination of methyl tert-butyl ether (MTBE) and THF at room temperature gave the best results with improved yield. Moreover, a large variety of heteroaryl electrophiles extending from halopyridine, haloquinoline, and pyrimidine to diazines provide satisfactory results in good yields. Both the electron-withdrawing and electron donating-substituents on the aryl Grignard species are significantly tolerated. Later, the application of isoquinoline as a ligand was found to improve the yield and the rate for the reaction (Scheme 321).435 They coupled halogenated pyrimidine, triazine, and pyridines together with other heteroaryl halides with the functionalized aryl Grignard reagent, obtaining good yields of product. This method enhances the rate to a greater extent. Notably, they synthesized the challenging heteroaryl-heteroaryl coupling product by utilizing this methodology. However, the cross-coupling of 1,4-chlorobromobenzene or 1,4-dibromobenzene with phenylmagnesium bromide in the presence of iron(III) chloride yields mixtures of p-terphenyl and homo-coupled biphenyl as the products.436
Aryl chlorides were coupled with arylmagnesium reagents by Duong et al. in 2014 using iron(III) tert-butoxide in the presence of N-heterocyclic ligand and sodium tert-butoxide base.437 This method is very efficient for the formation of aryl–aryl cross-coupled products in excellent yields. Most importantly, the utilization of tert-butoxide in the reaction suppresses the homocoupling reaction by hindering the reduction of the iron precursors by the aryl Grignard reagents. Further in 2015, they reported the catalytic efficiency of iron(II) triflates together with N-heterocyclic carbene ligand for the cross-coupling of aryl chlorides and tosylates with aryl Grignard reagents, producing high yields of coupled products (Scheme 322).438 Both the electron-donating and electron-withdrawing substituents including the highly deactivating trifluoromethyl group on the coupling partners are well tolerated under the reaction conditions, producing significant yields. Also, a number of functional groups such as ether, alkene, tertiary amines and silyl protected phenols are found to be compatible in this reaction.
In 2017, Huynh and Duong studied the influence of the structural factors of the NHC ligands for the Fe(OTf)2/NHC-catalyzed cross-coupling of chlorobenzene and p-tolylmagnesium bromide.439 The yields of the products are dependent on a number of structural parameters. These factors include the bulk of the N-substituents, the counterions present in the carbene precursors and the degree of unsaturation of the NHC backbone. Notably, the stereo-electronic properties of the NHC ligands were evaluated using HEP (Huynh's electronic parameter) and %Vbur calculations. Through these extensive studies, SIPrNap was found to be the most efficient NHC ligand for the above-mentioned reaction protocol with both aryl chlorides and tosylates as the electrophilic partner. Recently, in 2018 they demonstrated the first structure–activity relationship (SAR) studies on expanded-ring N-heterocyclic carbenes as ligands in the iron-promoted cross-coupling of aryl halides and aryl Grignard reagents.440 NHC ligands with different ring sizes and different bulky N-aryl substituents were prepared and evaluated for their catalytic activity. The N-mesityl substituted seven-membered NHC, 7Mes·HCl, showed the best catalytic activity for aryl–aryl cross-coupling using Fe(OTf)2 and sodium tert-butoxide (Scheme 323). The catalyst system provides very good yields of product with a large number of substrates.
Around the same time, Duan and co-workers introduced an iron-based catalyzed cross-coupling reaction between aryl halides and aryl Grignard reagents (Scheme 324).441 The catalytic system consists of FeCl3/TMEDA together with Ti(OEt)4 as the co-catalyst in a THF/toluene solvent mixture in the presence of phenolate additive. The phenolate group induces a bimetallic cooperation between the two metals, and thus increases the catalytic activity of the iron system. Various aryl and heteroaryl halides bearing different substituents were coupled with the common Grignard reagents and with Knochel-type Grignard reagents.442 Moreover, high yields of the cross-coupled products are obtained together with the suppression of side products (homo-coupled and dehalogenated products). Notably, for the Knochel-type Grignard reagents, the yield of the products increases with the removal of the in situ formed isopropyl halides before the cross-coupling reaction. However, the aryl chlorides give poor yields of products under this condition.
Further in 2019, they reported that a similar Fe–Ti co-catalyst system with NHC ligand can be used for the efficient cross-coupling of various (hetero)aryl electrophiles and aryl Grignard reagents (Scheme 325).443 The weakly electrophilic aryl chlorides are efficiently coupled, affording good yields of product. In addition, oxygen-based aryl electrophiles such as ArOTs, ArOSO2NMe2, and ArOCONMe2 undergo chemoselective biaryl coupling in the presence of sensitive functional groups. The reaction shows good tolerance for a large number of functional groups on both coupling partners. Further, this reaction protocol was successfully exploited for the synthesis of the terphenyl intermediate anidulafungin.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solvent mixture, leading to coupled products in good yields. Interestingly, a contrast in the reactivity of aryl halides was observed for arylcopper reagents in comparison to arylmagnesium species. The aryl triflates showed poor reactivity, whereas the tosylates were completely unreactive. The presence of both electron-donating substituents is well tolerated with slight variations in yield.
2) solvent mixture, leading to coupled products in good yields. Interestingly, a contrast in the reactivity of aryl halides was observed for arylcopper reagents in comparison to arylmagnesium species. The aryl triflates showed poor reactivity, whereas the tosylates were completely unreactive. The presence of both electron-donating substituents is well tolerated with slight variations in yield.
On further investigation on arylcopper reagents, they revealed that the presence of the amide functionality on the aryl iodide made the reaction faster, resulting in high yields of the coupled products. Excellent yields were obtained by using iodoquinolines as substrates (b and c, Scheme 326).445 A range of functional groups on the benzamides and quinolines are well tolerated, leading to functionalized biphenyls and 8-phenylquinolin-29(1H)-ones, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bar).446 It was suggested that pressure accelerates the reduction of the metal precatalyst to the catalytically active oxidation state. Also, the catalytic decomposition is suppressed in the presence of pressure. Further in 2009, the Suzuki coupling reaction of heteroaryl halides with sodium tetraphenylborates using an iron-zinc catalyst was developed by Bedford et al.447 Hetero bi-phenyls were produced in good to high yields.
000 bar).446 It was suggested that pressure accelerates the reduction of the metal precatalyst to the catalytically active oxidation state. Also, the catalytic decomposition is suppressed in the presence of pressure. Further in 2009, the Suzuki coupling reaction of heteroaryl halides with sodium tetraphenylborates using an iron-zinc catalyst was developed by Bedford et al.447 Hetero bi-phenyls were produced in good to high yields.
6.3 Cross-coupling of acyl electrophiles with various nucleophilic partners
The earliest instance of iron catalysis in ketone synthesis by reaction between acyl halides and Grignard reagent was found in the reports by Cook and co-workers in 1953.451 The reaction with ferric chloride as the catalyst gives the highest yields compared to that of other metallic halides as the catalyst. They were successful in synthesizing straight-chain aliphatic ketones in good yields.Thereafter, thorough investigations on this iron-catalyzed ketone formation reaction were performed by Marchese et al.452–455 They demonstrated that a catalytic amount of iron(III) acetylacetonate helps in efficiently coupling acyl chlorides with Grignard reagents (Scheme 330a). Notably, the concomitant alcohol formation is stopped in the presence of an iron catalyst. Later, Fürstner and co-workers reinvestigated this reaction using the same catalytic system and showed that a wide variety of substrates were coupled efficiently, giving good to excellent yields of the desired products.372
Later, in 2004, Knochel and co-workers developed the reaction between arylacyl cyanides and organomagnesium reagents in the presence of Fe(acac)3 in THF (Scheme 330b).456 The polyfunctional benzophenone derivatives were formed in high yields. The acyl cyanides were evidenced to be better acylating agents in comparison to acyl chlorides since the cyano group increases the reactivity of the adjacent carbonyl group. In contrast, the chlorine of acyl chloride decreases the reactivity by acting as a donor atom. The iron-catalyzed reaction of Grignard reagents and acyl chloride is very useful from a synthetic point of view.384 Additionally, due to its extensive use in ketone synthesis, it was successfully employed during the synthesis of latrunculin A374,375 and B.375 It was also used in a key step for the synthesis of the musk odorant (3R)-(Z)-5-muscenone (Scheme 331).457
6.4 Cross-coupling of alkyl electrophiles with various nucleophilic partners
6.4.1.1 Coupling using organomagnesium reagents. The cross-coupling reaction with alkyl electrophiles is a challenging task for researchers due to the accompanying side reaction. The major side reaction together with the cross-coupling is β-hydride elimination, producing olefins. Another side reaction found is the dehalogenation reaction producing alkanes. However, two methods for efficient alkyl–aryl cross-coupling were developed by Nakamura458 and Hayashi.459 In 2004, Nakamura et al. described the reaction of alkyl halides with aryl Grignard reagents in the presence of iron(III) chloride and TMEDA in THF at low temperature. The synthetic procedure resulted in the formation of high yields of coupled product with much less homo-coupled product. Importantly, the use of TMEDA suppresses the side reactions such as olefin formation (a, Scheme 332).458 On the other hand, Hayashi et al. were able to couple aryl Grignard reagents with β-hydrogen-containing alkyl halides under the influence of Fe(acac)3 in diethyl ether (b, Scheme 332). Secondary and primary alkyl bromides were found to be good coupling partners, giving moderate to good yields.459
Fürstner et al. used the well-defined low-valent iron complex [Li(TMEDA)]2[Fe(C2H4)4] to achieve the coupling between alkyl halides and aryl Grignard reagent in excellent yields (c, Scheme 332).373,460 Among the halides, the bromides and iodides are more reactive compared to chlorides. A large variety of alkyl iodides and bromides are coupled efficiently, resulting in high product yields.
Later, mild reaction conditions using an iron–salen complex as the active catalyst were used by them to accomplish the cross-coupling of β-hydrogen-containing alkyl halides with aryl Grignard reagents (Scheme 333).461 The alkyl arenes were produced in good yields. Notably, a low level of the β-eliminated product and the dehalogenated product is maintained under the reaction conditions.
In 2005, Bedford et al. reported that an FeCl3 precatalyst and amine ligands efficiently catalyzed the coupling of alkyl halides bearing β-hydrogen with aryl Grignard reagents (a, Scheme 334).462 The highest conversion was obtained using triethylamine and DABCO together with a minor amount of elimination and hydrodehalogenated products. Additionally, the use of iron(III) chloride with phosphine, phosphite or arsine ligands is found to overcome the limitation of the slow addition procedure, as reported by Nakamura et al. (b, Scheme 334).463 The desired alkyl arenes are obtained in high yields. A radical mechanism was inferred from the radical clock experiment using methyl cyclopropane, resulting in the ring-opening product. They also reported that the use of N-heterocyclic ligands gives high product yields (c, Scheme 334).
An excellent yield of alkyl–aryl cross-coupled products was also obtained with the use of iron nanoparticles as the active catalyst.464 The iron nanoparticles are either prepared separately and then stabilized by polyethylene glycol (PEG) or formed in situ and stabilized by 1,6-bis(diphenylphosphino)hexane.
In 2011, the ortho-phenylene-tethered bisphosphine ligand-containing iron(II) chloride complex FeCl2(3,5-t-Bu2-SciOPP) was prepared by Nakamura et al. It is used in the Kumada–Corriu coupling between alkyl halides and aryl Grignard reagents (Scheme 335).465 High catalytic activity is shown by the complex since tertiary alkyl halides are also coupled together with primary and secondary halides, resulting in good yields. Notably, the ring-opening product for the reaction with (iodomethyl)cyclopropane in the catalytic condition confirms the presence of radical intermediates. Around the same time, Yamaguchi and Asami reported the synthesis of a tridentate β-aminoketonato iron complex and its application as an effective catalyst in the cross-coupling reaction of alkyl halides with aryl Grignard reagents.466
The chemoselective coupling of an α-bromocarboxylic acid derivative was performed by Nakamura and co-workers using the simple Fe(acac)3 precatalyst in THF at −78 °C (Scheme 336).467 The reaction proceeds efficiently, resulting in good to high yields of the corresponding phenylacetic acid derivatives as the coupled product. The bulky tert-butyl esters are used to reduce the nucleophilic attack of the Grignard reagents at the carbonyl centre.
Later, in 2015, they reported the first iron-catalyzed enantioselective coupling of α-chloro- and α-bromoalkanoates with aryl Grignard reagents in the presence of catalytic amounts of Fe(acac)3 and the P-chiral bisphosphine ligand BenzP* (Scheme 337).468 The optically active α-arylalkanoates are synthesized in high yields through this efficient protocol. Various electron-rich, electron-deficient and electron-neutral aryl Grignard reagents provide good yields of products, while the ortho-substituted aryl Grignard reagents undergo slow reaction, giving poor yields. The deprotected products are used as important molecules in pharmaceuticals as nonsteroidal anti-inflammatory analgesics and cyclooxygenase inhibitors.384
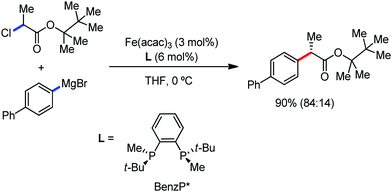 | ||
| Scheme 337 Enantioselective reaction of α-haloalkanoates with Grignard reagent in the presence of chiral ligand. | ||
In 2006, Gartner and co-workers demonstrated the use of the air-stable ionic liquid butylmethylimidazoliumtetrachoroferrate (bmim-FeCl4) as a catalyst to carry out the biphasic cross-coupling of alkyl halides with aryl Grignard reagents.469 Most importantly, the ionic liquid catalyst was recollected and reused after simple decantation of the ethereal layer containing the products. The catalyst can be recycled effectively for up to four times.
In 2007, two new catalytic systems for the cross-coupling of alkyl halides and aryl Grignard reagents were developed by Caheiz and co-workers.470 One of the systems consists of Fe(acac)3 together with an HMTA/TMEDA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (HMTA = hexamethylenetetramine) ligand mixture (Scheme 338a) and the other consists of the new complex [(FeCl3)2(TMEDA)3] (Scheme 338b). Both catalytic systems worked efficiently for the coupling reaction with secondary and primary alkyl halides, giving good product yields. Notably, this is the first use of HMTA in a cross-coupling reaction catalyzed by transition metals. This method is employed for the large-scale production of sec-butyl benzene. The catalyst system of iron salt and TMEDA is also used for the cross-coupling of the alkyl bromides and biphenyl magnesium bromides, resulting in high yields of the desired coupled products.471
2) (HMTA = hexamethylenetetramine) ligand mixture (Scheme 338a) and the other consists of the new complex [(FeCl3)2(TMEDA)3] (Scheme 338b). Both catalytic systems worked efficiently for the coupling reaction with secondary and primary alkyl halides, giving good product yields. Notably, this is the first use of HMTA in a cross-coupling reaction catalyzed by transition metals. This method is employed for the large-scale production of sec-butyl benzene. The catalyst system of iron salt and TMEDA is also used for the cross-coupling of the alkyl bromides and biphenyl magnesium bromides, resulting in high yields of the desired coupled products.471
In 2008, Kozak and co-workers synthesized an iron(III) amine-bis(phenolate) complex and used it as a catalyst in the coupling of β-hydrogen-containing primary and secondary alkyl halides with aryl Grignard reagents (Scheme 339).472 The yield varied from low to high depending on the substrate. Furthermore, the double cross-coupling reaction of dichloromethane with aryl Grignard reagents is performed using a similar but dimeric iron(III) bisphenolate complex, resulting in diaryl methane together with 1,2-diarylethane as the side product.473o-Tolylmagnesium bromide alone results in high yields of products. Also, FeCl3 is an effective catalyst for the coupling, giving moderate yields of product. A significant amount 1,2-diarylethane was also found as the side product.
In 2011, Knochel et al. demonstrated that the diastereoselective cross-coupling of cyclic iodohydrin derivatives with aryl Grignard reagents can be performed using a substoichiometric amount of FeCl2·LiCl. TBS-protected (TBS = tert-butyldimethylsilyl) iodohydrins undergo cross-coupling with aryl and heteroarylmagnesium halides, producing high yields of thermodynamically more stable trans products in excellent selectivity (Scheme 340).474
Alkanesulfonyl chlorides were found to undergo desulfinylative cross-coupling with Grignard reagents in the presence of simple Fe(acac)3 in a THF/NMP solvent mixture at an elevated temperature (Scheme 341).391 A wide variety of alkanesulfonyl chlorides and aryl Grignard reagents undergo this reaction with good yields.
Gao and co-workers reported the use of the NHC-ligand containing anionic iron(II) complex [Fe(IPr)Br3](HIPr)·PhMe as a highly efficient catalyst in the cross-coupling of p-tolylmagnesium bromide with cyclohexyl bromide.475 Successful conversion was obtained with a very low catalyst loading of 0.5–1 mol%, giving the product in very high yields. Together with cyclohexyl bromides, other alkyl halides such as cyclopentyl bromide, 2-bromohexane and 1-bromooctane afford high product yields. Thereafter, well characterized di- and tetra-N heterocyclic carbene-bearing Fe(II) complexes were found to be catalytically active in the cross-coupling reaction.476 Their catalytic activity was evaluated using the same standard cross-coupling reaction between cyclohexyl bromides and p-tolylmagnesium bromides. Both complexes were found to be efficient, affording good yields, but were less active compared to the abovementioned anionic iron(II) complex having two NHC ligands.
In 2012, Nakamura and co-workers developed a simple catalytic system consisting of an N-heterocyclic carbene ligand and iron(III) chloride, which gave high yields of the coupling product (Scheme 342).477 The cross-coupling of a wide range of primary, secondary and tertiary alkyl chlorides with aryl Grignard reagents occurs under this catalytic system. Most importantly, this method was efficiently applied to the arylation of the challenging polychloroalkanes, yielding high conversions.
Around the same time, Deng and co-workers came up with a method for the arylation of primary alkyl fluorides with aryl Grignard reagents under the catalytic activity of a dinuclear iron(II) complex having an NHC ligand (Scheme 343).478 A large variety of Grignard reagents and primary alkyl fluorides was cross-coupled with good to excellent yields. In the reaction with cyclopropylmethyl fluoride as the substrate, a ring-opened terminal alkene was formed together with the desired cross-coupled product and the aryl–aryl homocoupling product. This result indicates the presence of a radical intermediate in the reaction.
A series of iron(III)-containing imidazolium salts were synthesized and investigated for their catalytic efficiency in the benchmark cross-coupling reaction of cyclohexyl bromide with p-tolylmagnesium bromide.479 All six salts investigated were highly efficient precatalysts, resulting in good to excellent yields of the coupled products. Interestingly, the catalytic efficiency can be varied by varying the N-substituents in the imidazole ring.
In 2013, Denmark and Cresswell demonstrated the first systematic study of alkyl aryl thioethers as electrophiles in the iron-catalyzed cross-coupling reaction. After extensive optimization, they found that aryl–alkyl cross-coupling by the cleavage of the C(sp3)–S bond occurs in the presence of iron(III) acetylacetonate in cyclopentylmethyl ether (CPME) solvent at room temperature (Scheme 344).480 Also, the reaction of secondary alkyl sulfones is catalyzed under the same conditions together with 8 equiv. of TMEDA as the additive.
Bis(phenol)-functionalized benzimidazolium-based iron(III) complexes have been efficiently used for the coupling of primary and secondary alkyl halides containing β-hydrogens with aryl Grignard reagents.481 Compared to FeCl3 and FeBr3, which are highly hygroscopic, these complexes exist as non-hygroscopic and air-stable solids, making them easier to handle. One of the complexes used for coupling was found to be highly efficient, resulting in excellent yields with much less β-hydride elimination products. Also, the same complex was recycled and worked efficiently even after eight runs.
Thereafter in 2014, Fürstner et al. developed a new method for the arylation of alkyl halides with the bulky ortho-substituted aryl Grignard reagents using iron(II) chloride with bis(diethylphosphino)-ethane (depe) as a ligand (Scheme 345).482 High yields were obtained with the alkyl bromides and chlorides together with a large variety of iodides. Moreover, the procedure also allows the arylation of neopentylic electrophiles with mesitylmagnesium bromides. Notably, the in situ generated and isolated [(depe)Fe(Mes)2] was suggested as the active catalyst species for the coupling using mesitylmagnesium bromide. This complex is generated in situ from [(depe)2FeCl2] upon treatment with MesMgBr.
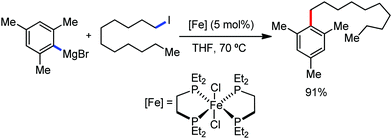 | ||
| Scheme 345 Iron-catalyzed cross-coupling with mesityl magnesium halides by bisphosphine iron complex. | ||
Recently in 2018, Zhang and co-workers introduced the first example of iron-promoted cross-coupling of arylmagnesium halides with difluoroalkyl bromides, producing biologically beneficial coupled products (Scheme 346).483 Iron(III) chloride is utilized together with modified TMEDA ligand having bulky butylene substitution at one of the carbon atoms of its ethylene backbone. Notably, this was the first report where substituted TMEDA was used deliberately in iron-catalyzed cross-coupling. The procedure affords the coupling of a wide range of difluoroalkyl bromides with a large number of substituted Grignard reagents, producing high yields of desired products. The reaction conditions also tolerate many important functional groups such as silyl ether, sulfuryl, ester, thiazole and ferrocenyl groups. Furthermore, the first coupling reaction of bromodifluoromethane with a Grignard reagent was successfully executed using this methodology.
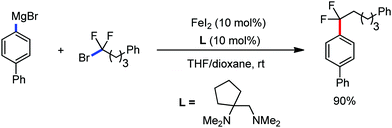 | ||
| Scheme 346 Cross-coupling of difluoroalkyl bromides and arylmagnesium halides using butylene-substituted TMEDA. | ||
6.4.1.2 Coupling using organozinc reagents. The iron-catalyzed coupling of primary and secondary alkyl halides with arylzinc reagents was demonstrated by Nakamura and co-workers in 2005. The reaction is performed in the presence of iron(III) chloride as the precatalyst and TMEDA as the ligand (Scheme 347).484 Most interestingly, the presence of magnesium is necessary for the reaction to occur. Further, an improvement in the functional group tolerance is observed compared to Grignard reagents due to the mild reactivity of arylzinc reagents. Diarylzinc and mixed diorganozinc containing trimethylsilyl methyl ligand are used to produce high yields of alkyl–aryl and alkyl–heteroaryl-coupled products. Also, they cross-coupled labile and low reactive polyfluorinated aryl metals with alkyl halides using 1,2-bis(diphenylphosphino)benzene (DPPBz) ligand instead of TMEDA with iron salt to produce the coupled products (Scheme 348).485 A variety of mono-, di- and triflorophenylzinc reagents are satisfactorily coupled with the secondary alkyl chlorides, primary bromides and chlorides. The formed polyfluorinated products are key structural units for a number of drugs, agrochemicals and dyes.
Later in 2009, the same group reported an efficient procedure for the iron-catalyzed cross-coupling of alkyl tosylates with arylzinc reagents in the presence of excess of tetramethylethylenediamine (TMEDA) and magnesium salts. The arylzinc reagents are prepared in situ from aryl Grignard reagents or aryl lithium compounds with zinc halide-TMEDA in the presence of magnesium dihalides (Scheme 349a).486 Notably, the use of ZnI2 as the zinc halide gives the best results since the cross-coupling reaction proceeds from the in situ-generated alkyl iodides rather than the sulfonates. Another method utilizes a mixed diorganozinc reagent bearing a non-transferable trimethylsilylmethyl ligand in the presence of TMEDA for the installation of functionalized arenes in alkyl tosylates (Scheme 349b).
In 2012, Quin and co-workers reported the first example of coupling of α-halo-β,β-difluoroethylene substrates with in situ-generated arylzinc reagents (Scheme 350).487 The catalyst system is comprised of iron(II) chloride and 1,3-bis(diphenylphosphino)propane (dppp) co-ligand together with the TMEDA ligand. They suggested the presence of radical intermediates in the reaction.
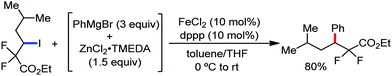 | ||
| Scheme 350 Iron-catalyzed cross-coupling of α-halo-β-β-difluroethylene substrate and arylzinc reagents. | ||
6.4.1.3 Coupling using organoboron nucleophile. In 2010, Nakamura and co-workers developed an efficient protocol for the iron-catalyzed Suzuki–Miyaura coupling of alkyl halides and aryl boronates using iron(II) chloride, diphosphine ligand and magnesium bromide (Scheme 351).488 They designed two novel iron diphosphine complexes bearing a sterically hindered o-phenylene-tethered diphosphine ligand and found that they were efficient in the abovementioned coupling reaction, resulting in good to excellent yields of products. The presence of magnesium bromide as a co-catalyst is also necessary. A variety of lithium arylboronates prepared in situ from the corresponding arylboronic acid pinacol ester and alkyllithium were found to participate in the reaction. It is noteworthy that the ring-opening product formed during the reaction of (bromomethyl)cyclopropane indicates the presence of alkyl radicals. In contrast to this method, Bedford et al. accomplished the same reaction using the simple iron(III) acetylacetonate or iron complexes containing inexpensive diphosphine ligands such as 1,2-bis(diphenylphosphino)ethane (dppe) and 1,3-bis(diphenylphosphino)propane (dppp) (Scheme 352).489 However, the procedure for the formation arylboronate and the necessity of magnesium bromide were same. A wide range of substrates gave satisfactorily high yields of products.
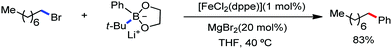 | ||
| Scheme 352 Alkyl–aryl cross-coupling of alkyl halides and aryl boronates in the presence of iron bis(phosphine) complex. | ||
6.4.1.4 Coupling using organoaluminum nucleophiles. Organoaluminum reagents have also been used as nucleophilic partners for the cross-coupling reaction in the presence of an iron catalyst. Nakamura and co-workers reported the first iron-catalyzed Negishi coupling of arylaluminum reagents and alkyl halides in the presence of the well-defined iron complex FeCl2(DPPBz) (DPPBz = 1,2-Bis(diphenylphosphino)benzene).490 The in situ generated aluminate complexes are the active species. Notably, the aluminate complex remains in dynamic equilibrium with the unreactive neutral aluminium species and the equilibrium is maintained by the in situ-generated MgX2 salts.
Later, they performed the cross-coupling reaction of non-protected chlorocyclohexanols with aryl aluminum, producing aryl alkanols in the presence of their previously synthesized bisphosphine complex FeCl2(SciOPP) (Scheme 353).491 The in situ-generated aluminum alkoxide offers high catalytic activity and enhanced diastereoselectivity with the formation of excellent yields of trans product.
 | ||
| Scheme 353 Diastereoselective cross-coupling of chlorocyclohexanols and aryl aluminium reagents using FeCl2(TMS-SciOPP) complex. | ||
Recently in 2019, Nakamura and co-workers developed a C(sp3)–C(sp3) Negishi cross-coupling reaction between alkyl halides and alkylaluminium reagents using a catalytic system consisting of Fe(OAc)2, bisphosphine ligand, and Bn-Nixantphos (Scheme 354).492 Notably, the addition of potassium fluoride is necessary for the conversion. This methodology efficiently produces moderate to good yields of products with complete suppression of the undesirable β-hydrogen elimination. The ring-opening products for the reaction of (bromomethyl)cyclopropane suggest the presence of radical intermediates.
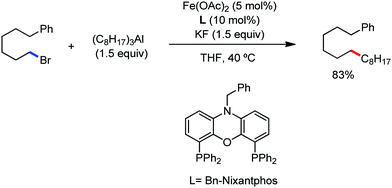 | ||
| Scheme 354 Cross-coupling reaction alkyl halides and alkylaluminium reagents using iron salts with Bn-Nixantphos. | ||
6.4.1.5 Coupling using organomanganese nucleophiles. In 2017, Knochel and co-workers developed the cross-coupling reaction between di(hetero)arylmanganese reagents and primary and secondary alkyl halides using iron(II) chloride in THF as the catalytic system (Scheme 355).493 A wide variety of functionalized di(hetero)aryl manganese substrates react with functionalized primary and secondary alkyl halides, producing moderate to high yields of the desired products. Notably, no rearrangement of the secondary alkyl halides is found in this reaction.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, respectively (Scheme 356).494 A large variety of primary and secondary alkyl bromides and iodides are coupled successfully and provide the desired products in excellent yields. Also, the chlorides are inefficient for coupling under the reaction conditions. The reaction is highly stereoselective with good retention of the E/Z ratio of the starting materials in the coupled product.
1 ratio, respectively (Scheme 356).494 A large variety of primary and secondary alkyl bromides and iodides are coupled successfully and provide the desired products in excellent yields. Also, the chlorides are inefficient for coupling under the reaction conditions. The reaction is highly stereoselective with good retention of the E/Z ratio of the starting materials in the coupled product.
Later in the same year, a very similar reaction was also reported by Cossy et al. using FeCl3 and TMEDA in THF.495 A wide substrate scope was presented with good tolerance of various functional groups. This method was applied for the introduction of an E-trisubstituted terminal double bond in the spiroketal core of spirangien A.496 It was also utilized for the formal synthesis of spirangien A, as reported by Rizzacasa et al.497 Moreover, the same reaction was used in the course of the synthesis of (+) allokainic acid (Scheme 357),498 the rennin inhibitor aliskiren,499 and ent-amphidinol.500
In 2009, Nakamura et al. utilized iron(III) chloride in the presence excess TMEDA ligand in THF for the selective cross-coupling of alkyl halides with alkenylzinc reagents (Scheme 358).501 Different functionalized alkyl bromides with cyano, acetoxy and carbamate functional groups are well tolerated, providing good yields of the products. The stereochemistry of the starting alkenylzinc is retained throughout the coupling reaction.
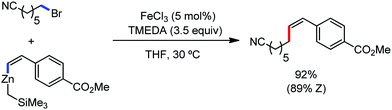 | ||
| Scheme 358 Cross-coupling of alkyl halides and alkenyl zinc reagents in the presence of an iron catalyst. | ||
Further, they reported the Suzuki–Miyaura cross-coupling of E- and Z-alkenylboronic acid pinacol esters with alkyl halides in the presence of sterically congested (bisphosphine)iron complexes as the active catalysts (Scheme 359).502 The reaction proceeds in a stereoselective manner, producing high yields of coupled products. Alkyl bromides and iodides possessing acetoxy, benzoyl, carbamate, ethoxycarbonyl and cyano groups are well tolerated. Notably, the presence of magnesium salts is essential for the transformation to proceed.
Togni's reagent was employed for the trifluoromethylation of terminal olefins using potassium vinyltrifluoroborates in the presence of a catalytic amount of FeCl2 (Scheme 360).503 Good yields of products are obtained using 2-arylvinyltrifluoroborates and 2-heteroarylvinyltrifluoroborates as the substrates. The yields can be increased by decreasing the catalyst loading in acetonitrile solvent. The E-configuration of substrates is retained with high selectivity, while pure Z-isomers result in stereoconvergence, providing the E product exclusively. Through these observations, the authors inferred the absence of the transmetalation/reductive elimination mechanism. Instead they suggested a mechanism involving radicals or carbocations.
In contrast to Nakamura's method, Hu et al. introduced a procedure for coupling alkynyl Grignard reagents to alkyl halides in the absence of any phosphine ligand. They used simple iron(II) bromides in a THF/NMP solvent mixture at room temperature to accomplish the reaction, affording good yields of substituted alkynes (Scheme 362).505 The mild conditions of this reaction facilitate the alkynylation of a wide variety of functionalized cyclic and acyclic secondary alkyl halides and primary alkyl halides.
The coupling between polychlorinated alkyl solvents and alkylmagnesium halides was developed using an iron catalytic system comprised of a pincer ligand bearing an iron(III) complex in THF.507 This process efficiently removes the toxic solvents dichloromethane, chloroform and carbon tetrachloride, with the subsequent formation of value-added products.
In 2012, Nakamura and co-workers developed the iron catalyzed alkyl–alkyl Suzuki–Miyaura cross-coupling of non-activated alkyl halides with alkylboron compounds (Scheme 363).508 The coupled products are formed in high yields due to the catalytic presence of iron(III) acetylacetonate and XantPhos ligand. It is noteworthy that alkyl borates are prepared by hydroboration of the corresponding alkenes followed by activation using isopropyl Grignard reagent. The reaction conditions show tolerance to different functional groups on both coupling partners, resulting in significant yields of the desired products. This reaction has been successfully utilized for the synthesis of long chain fatty acid derivates.
In 2013, Cárdenas et al. employed iron(II) acetate and an NHC ligand (IMes·HCl) to accomplish the alkyl–alkyl Kumada-type cross-coupling of alkyl iodides with alkyl Grignard reagents (Scheme 364).509 The β-elimination reaction is suppressed significantly with the formation of the desired coupled product in good yields. Based on mechanistic investigations, they suggested a radical pathway involving Fe(I) as the active species.
The iron-mediated cross-coupling reaction of homobenzylic methyl esters with alkyl Grignard reagents via C–O bond cleavage was reported by Shi and co-workers. Iron(II) fluorides with the tricyclohexylphosphine ligand provide the best results in o-xylene solvent (Scheme 365).510 They hypothesized that the reaction proceeds through dehydroalkoxylation, producing alcohol and the styrene derivative as intermediates, followed by carbometalation of the styrene intermediates with the Grignard reagent. A wide variety of methyl ethers containing different aryl groups at the β-position give good yields of C(sp3)–C(sp3)-coupled product. The importance of the counter anion of Grignard reagents is demonstrated by the different reactivities of the chlorides and bromides. Although n-hexylMgCl gives a good result, n-hexylMgBr remains unreacted.
In 2016, Fürstner introduced the first iron-promoted cross-coupling reaction of tert-alkyl electrophiles, 1-alkynylcyclopropyl tosylates, with alkyl Grignard reagents in the presence of a catalytic amount of Fe(acac)3, resulting in high yields of the tosylate-substituted alkylated product (Scheme 366).511 Most importantly, the usually observed allene side products are not obtained except for the reaction using branched alkyl- or arylmagnesium reagents. Notably, the produced (1-methylcyclopropyl)ethynyl group acts as an important structural motif for many bioactive compounds.
 | ||
| Scheme 366 Reaction of 1-alkynylcyclopropyl tosylates and alkyl Grignard reagents catalyzed by iron. | ||
Around the same time, Wong and co-workers utilized the iron complex [(FeCl3)2(TMEDA)3] for the cross-coupling of alkyl halides and alkyllithium reagents, obtaining good yields of products.363,440
6.5 Cross-coupling of benzyl, allyl and propargyl electrophiles with various nucleophilic partners
The early instances of iron-catalyzed reaction between Grignard reagent and propargyl halides were found in the reports by Pasto et al. in 1976.512,513 The use of a catalytic amount of iron(III) chloride yielded allenes as the major product. Later, Yamamoto and co-workers performed iron-catalyzed selective coupling between allylic phosphates and Grignard reagents. This synthetic protocol affords the SN2 products preferentially over the SN2′ products.514,515 High yields of product were reported using iron(III) acetylacetonate as the precatalyst. The tricyclo alkyl Grignard reagents are also coupled with propargylic halides and allylic halides in the presence of the same iron salt.516The structurally well-defined ferrate complex [Li(TMEDA)]2[Fe(C2H4)4] catalyzes the coupling reaction of propargylic, benzylic and allylic halides with phenyl Grignard reagents, providing excellent yields of the desired products (Scheme 367).373,460 In addition, the benzyl bromides are coupled in moderate yields with arylmagnesium halides in the presence of an amine bisphenolate iron complex prepared by Kozak et al.464
(E)-Styrenesulfonyl chlorides undergo desulfinylative cross-coupling with n-hexyl- and n-octylmagnesium bromides in the presence of Fe(acac)3, giving moderate yields of products.391 Also, allylsulfonyl chlorides are coupled similarly with aryl and alkyl Grignard reagents, providing high yields under the same reaction conditions (Scheme 368).517 Moreover, a wide range of substituted aromatic Grignard reagents together with one heteroaromatic Grignard reagent are well tolerated. Both but-2-ene-1-sulfonyl chloride and but-3-ene-2-sulfonyl chloride form the same ratio of regioisomers. The linear product is preferred over the branched product. Through this observation, it was suggested that a same type of intermediate is formed from both species followed by coupling with the Grignard reagent with the same regioselectivity.
A mixed iron–zinc catalytic system catalyzes the Suzuki coupling of different benzyl halides with sodium or potassium tetraarylborates, producing good to excellent yields of coupled product (Scheme 369a).447
The Negishi coupling between aryl zinc reagents and benzyl halides is performed using the same iron complex bearing the 1,2-bis(diphenylphosphino)benzene ligand (Scheme 369b).518 The complex shows good catalytic activity, giving excellent yield of selective-coupled products. Notably, the coupling occurs selectively at the benzylic halide in the presence of aryl halides.
A simple procedure for the iron-catalyzed cross-coupling of allyl electrophiles with arylmagnesium bromides was developed by von Wangelin and co-workers in 2010 (Scheme 370).519 This method employs a one-pot procedure for the formation of Grignard reagents and subsequent coupling of allyl electrophiles. The reaction is successfully accomplished in the presence of ligand-free iron(III) acetylacetonate. A wide range of allylic electrophiles act as suitable substrates together with allyl acetates. This simple method efficiently tolerates electron-withdrawing and electron-donating substituents on the aryl reagents, giving the best results with ortho-oxygen substituents. Prenyl acetate, crotyl acetate and cinnamyl acetate as electrophiles afford the linear and branched regioisomers via the SN and SN′ pathways, respectively. The linear allylarene is formed as the major product from prenyl acetate with 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 selectivity. Although crotylation gives lower regioselectivity, cinnamyl acetate provides the linear product exclusively.
1 selectivity. Although crotylation gives lower regioselectivity, cinnamyl acetate provides the linear product exclusively.
In 2013, Bedford et al. revealed the versatile activity of the simple bis(diphenylphosphino)ethane ligand containing an iron complex in the coupling of benzyl halides with organozinc, organoboron, organoaluminum and organoindium reagents.520 The complexes presented excellent activity, producing high yields of products in the reaction with all the above-mentioned nucleophilic partners. In the same year, benzyl chlorides were selectively coupled with aryl Grignard nucleophiles in the presence of iron(II) chloride and ortho-phenylenebisphosphine ligands by Nakamura and co-workers. Most importantly, the products are formed according to the electronic properties of the bisphosphine ligands (Scheme 371).521 It was found that electron-rich substituents on the ligand provide good yield of the coupled product. In contrast, electron-deficient substituents result in reductive homocoupling of the benzyl chlorides. The investigation of substrate scope using an iron(II) catalyst bearing electron-rich bisphosphine ligands revealed the feasibility of a wide range of aryl Grignard reagents for the reaction.
In the same year, an air-stable and non-hygroscopic iron(III) amine-bis(phenolate) complex was employed by Kozak et al. for the cross-coupling of benzyl chlorides and bromides with arylmagnesium halides, providing low to high yields of products (Scheme 372).522 In some instances, the homo-coupled products were formed in major amounts.
 | ||
| Scheme 372 Cross-coupling of benzyl halides and aryl Grignard reagents using iron(III) amine-bis(phenolate) complex. | ||
In 2014, 4-ethynyloxetan-2-ones were coupled with aryl and alkyl Grignard reagents by Zang et al. in the presence of iron(III) chloride hexahydrate (FeCl3·8H2O), resulting in the ring-opened 4-alkynoic acids in good yields (Scheme 373).523 The reaction occurs regioselectively to produce the alkynoic acid in a major amount together with a minor amount of allenes as by-products. The prevalence of SN2 substitution reaction at the β-carbon atom of the 4-ethyloxetan-2-one was inferred from the complete inversion of configuration at that site.
 | ||
| Scheme 373 Iron-catalyzed reaction of 4-(trimethylsilylethynyl)oxetan-2-one with Grignard reagents affording 4-alkynoic acids. | ||
An iron-catalyzed allyl–alkyl cross-coupling reaction was employed by von Wangelin et al. for the selective deallylation of allylphenyl ethers (Scheme 374).524 The best results were obtained using ethylmagnesium nucleophiles in the presence of iron(II) chloride in an m-xylene/THF solvent mixture. A wide variety of functionalized ethers efficiently produced the corresponding phenols or alcohols in high yields. Furthermore, allylic and benzylic halides were also used in the iron-mediated Suzuki–Miyaura coupling with arylboron compounds.481
In 2016, Bäckvall and co-workers introduced a reaction protocol for the synthesis of substituted α-allenols using the iron-catalyzed cross-coupling of propargyl carboxylates and Grignard reagents (Scheme 375).525 They investigated the process using two classes of propargyl substrates, A and B. Both classes of substrates provide high yields of the desired substituted α-allenols. The catalytic system consists of simple iron(III) acetylacetonate in diethyl ether solvent. A wide range of functional groups such as silyl ethers, carbamates and acetals, together with a large number of Grignard reagents are well tolerated under the mild conditions of the reaction. Interestingly, sterically congested Grignard reagents give the highest yields. Notably, the starting propargylic substrates are readily prepared via a one-pot synthesis.
6.6 Miscellaneous examples of cross-coupling with various coupling partners
The Sonogashira cross-coupling reaction between terminal phenylacetylenes with iodo-aryl derivates was reported in 2016 using the iron(II) complex FeCl2(bdmd) and co-catalyst [Cu(CH3CN)4]BF4 in 1,4-dioxane (bdmd = bis((diphenylphosphanyl)methyl)diphenylsilane) (Scheme 376).526 Different functional groups at the para position such as fluoro, methoxy, acetyl and phenyl are well tolerated, giving moderate to good yields of the desired coupling products. The ortho-amine or hydroxyl substituents are found to produce significantly higher yields. This result is either due to co-ordination of the functional groups to the iron centre or the ortho-directing effect. Various control experiments revealed that the presence of both iron and copper complexes is necessary for the transformation to occur.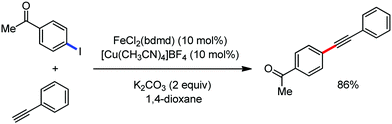 | ||
| Scheme 376 Iron-catalyzed Sonogashira cross-coupling of terminal phenyl acetylenes and iodoaryl derivatives. | ||
In the same year, Iwasaki et al. presented the iron-promoted cross-coupling reaction of aryl vinyl ethers with aryl Grignard reagents through the selective cleavage of the vinylic C–O bond in the presence of simple iron(II) chlorides, providing the styrene derivatives in good to excellent yields (Scheme 377).527 Both electron-withdrawing and donating substituents and other functional groups on the aryl nucleophiles are well tolerated.
In 2017, Nakamura and co-workers developed the first iron-catalyzed stereoselective cross-coupling of less reactive glycosyl halides with aryl metal reagents, producing high yields of a variety of aryl and vinyl C-glycosides (Scheme 378).528 This transformation occurs efficiently in the presence of a catalytic system consisting of iron(II) chloride and the bulky bisphosphine ligand TMS-SciOPP in THF. The reaction was suggested to proceed through a radical reaction, as confirmed by the radical clock reaction. This reaction was employed for the synthesis of a sodium-glucose co-transported 2 (SGLT2) inhibitor, Canagliflozin.
Recently in 2018, Cao and co-workers demonstrated the synthesis of pinacol esters of arylboronic acid via the iron-catalyzed cross-coupling between bis(pinacolato)diboron (B2pin2) and aryl fluorides (Scheme 379).529 The reaction is carried out in the presence of iron(II) chloride and LiHMDS (lithium hexamethyldisilazide) base in the solvent mixture of toluene and HMPA. Notably, the experiments are carried under an argon atmosphere. Only biaryl fluorides afford the desired borylation product in moderate to good yields, whereas other aryl fluorides provide unsatisfactory results.
6.7 Mechanistic elucidations of cross-coupling reactions
The mechanism of iron catalysis in cross-coupling was not well established compared to that of palladium- and nickel-catalyzed reactions until the recent studies by Neidig and co-workers. Since iron can exhibit various oxidation and spin states, the possible reaction pathway and reactive intermediates are highly dependent on the reaction conditions and the coupling partner. Thus, detailed mechanistic insight into iron-catalyzed cross-coupling reactions remained elusive for a long time until Fürstner et al. showed some reactive intermediates based on experimental evidence.373,460 Although Kochi proposed the initial mechanism based on experimental evidence, it lacked more reasonable evidence at that time.Since then, different mechanistic hypotheses have been reported starting from the early investigations by Kochi to the recent reports of iron-catalyzed cross-coupling reactions using different advanced spectroscopic techniques. In this section, the proposed mechanisms are summarized and classified into two broad categories based on the number of electrons transferred.
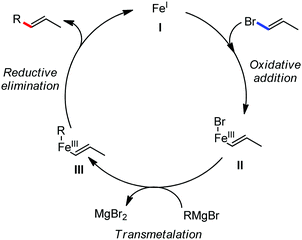 | ||
| Scheme 380 Mechanism of iron-catalyzed cross-coupling of alkenyl halides and Grignard reagents as proposed by Kochi. | ||
The proposal of the S = 1/2 iron(I) intermediate by Kochi remained a mystery for a long time until Neidig and co-workers identified it using advanced EPR (electron paramagnetic resonance) and MCD (magnetic circular dichroism) studies. In 2014, they synthesized and characterized a homoleptic tetramethyliron(III) ferrate complex [MgCl(THF)5][FeMe4] from the reaction of FeCl3 with methyl magnesium bromide in THF.532 It was reported that on warming this square planar complex, spin S = 3/2 is converted to S = 1/2 iron(I) species initially proposed by Kochi as the active intermediate. Also, the evolution of ethane during this process confirmed the intermediate of the complex during the reduction of FeCl3 to form the reduced species in the catalytic process. Later in 2016, they isolated a novel iron methyl cluster, [MgCl(THF)5][Fe8Me12], and characterized it as the species consistent with Kochi's S = 1/2 iron(I) species.533 Notably, the direct reaction of the iron(I) species with electrophile gave a negligible result. However, the combination of MeMgBr with the electrophile led to the fast and selective formation of the coupled product. Notably, the formation of Me12Fe8− cluster ions by the treatment of Fe(acac)3 with MeMgX (X = Cl, Br) was confirmed through the electrospray ionization (ESI) mass spectroscopy investigation by Koszinowski and Parchomyl in 2017.534 In addition, they reported the disappearance of the ferrate cluster in the presence of the chelating ligand TMEDA. However, Neidig and co-workers reported the formation of a trimethyliron(II) ferrate species, [Mg(NMP)6][FeMe3]2, instead of the [Fe8Me12]− cluster in the presence of NMP as a co-solvent in the reaction.535 Through the combination of GC analysis and Mössbauer spectroscopic studies they found that the complex was highly reactive for the selective formation of the cross-coupled products. They also demonstrated the interaction of the six NMP molecules with the magnesium through their carbonyl oxygens, which preferentially stabilized the complex over the [Fe8Me12]− cluster formation. Further, they investigated the effect of β-hydrogen atom-bearing Grignard reagents such as EtMgBr on the fate of iron salts under similar reaction conditions. They observed the presence of the iron species [Fe8Et12]− and [FeEt3]−, which are analogous to the species formed in the presence of NMP. These species also have similar catalytic competencies.536
The presence of low-valent iron active species was proposed by Fürstner et al. They proposed a catalytic cycle based on Fe(II−) and Fe(0) species for the coupling between aryl halides and Grignard reagents.373,394,395 In this process, the Fe(III) or Fe(II) salts are reduced by excess Grignard reagent to form a highly nucleophilic iron ensemble, [FeII−(MgX2)]n. This process is well established in the reports regarding the formation of “inorganic Grignard reagents” by Bogdanović et al. (Scheme 381).537,538 [Fe(MgX)2]n remains as a cluster containing iron and magnesium centres connected via covalent intermetallic bonds. This nucleophilic cluster undergoes a catalytic cycle similar to the well-established pathway for cross-coupling reactions catalyzed by nickel and palladium (Scheme 382).394,395
The Fe(II−) species I oxidatively inserts into the aryl halides owing to its high nucleophilic character with the release of magnesium halide. It was proposed that the resulting Fe(0) complex II is alkylated by a Grignard reagent to form complex III. Finally, reductive elimination of III occurs to form the desired coupled product with the regeneration of the catalytically active Fe–Mg species (Scheme 382). Notably, the reduction of the iron precatalyst by the alkyl Grignard reagent occurs with the concomitant β-hydride elimination of the alkyl substituent of the Grignard reagent. Therefore, the presence of β-hydrogen is a necessary condition for the occurrence of the above mechanism. This fact was evidenced from the inactivity of methylmagnesium halides, which did not provide any product compared to higher alkyl Grignard towards aryl halides in the presence of suitable precatalysts.378,379 Accordingly, Fürstner et al. demonstrated the formation of the homoleptic organoferrate complexes [(Me4Fe)(MeLi)][Li(OEt2)]2 and [Ph4Fe][Li(Et2O)2][Li(1,4-dioxane)] from the reaction of methyllithium and phenyllithium with FeCl3, respectively.
It was observed that these complexes are moderately nucleophilic and react efficiently with activated electrophiles such as acyl chlorides and alkenyl triflates.373,539 They also synthesized several lithium ferrate complexes having low formal oxidation states, which showed successful reactivity with aryl chlorides and allyl halides. Alkyl, allyl, benzyl and propargyl halides were also coupled with organomagnesium reagents in the presence of the above-mentioned ferrate complexes. However, in accordance with the experimental observations, they suggested the presence of interconnected catalytic cycles with the formal oxidation states of Fe(I)/Fe(III), Fe(0)/Fe(II), and the previously mentioned Fe(II−)/Fe(0).
In addition, in 2016, Parchomyk et al. utilized electrospray-ionization (ESI) mass spectroscopy for the identification and characterization of in situ-formed ionic species as intermediates during the reactions of iron-catalyzed cross-coupling reactions.540 They studied the Fe(acac)3-catalyzed cross-coupling reaction of PhMgCl with i-PrCl in THF and investigated the effects of various ligands and additives on the reaction. They reported the formation of different anionic iron ‘ate’ complexes having various nuclearity and oxidation states such as mononuclear phenylferrates [Ph3FeII]− and [Ph4FeIII]− formed by the addition of excess of PhMgCl to Fe(acac)3. Moreover, the gas-phase fragmentation experiment showed that the addition of i-PrCl to this reaction results in the formation of the heteroleptic complex [Ph3Fei-Pr]−. This heteroleptic complex undergoes reductive elimination to form the cross-coupled product. Further, in 2018 have reported studies with a wide range of simple iron precursors and Grignard reagents in tetrahydrofuran.541 All the iron precursors resulted in the formation of organoferrates RnFem−, with large variations in aggregation (1 ≤ m ≤ 8) and oxidation state (I to IV) depending on the nature of the organic counterpart. Several organoferrates react with organyl halides and pseudo halides, forming heteroleptic complexes. These heteroleptic complexes upon gas-phase fragmentation result in reductive elimination to produce the cross-coupled products. Notably, the aggregation and the oxidation states are influence by the presence of additive and ligands such as TMEDA and dppbz.
In 2008, Nakamura et al. proposed a mechanism involving Fe(0) and Fe(II) as the on-cycle species for the cross-coupling of alkenyl halides with alkynyl Grignard reagents to form enynes (Scheme 383).367 The iron(III) chlorides are initially reduced to low-valent Fe(0) alkynyl species I together with the formation of diyne as the side product from the Grignard reagent.
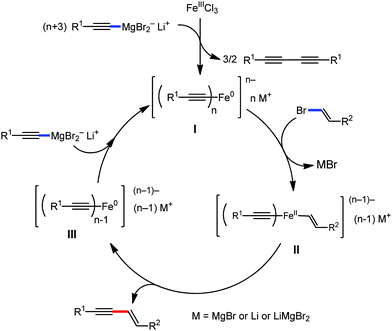 | ||
| Scheme 383 Mechanism for the iron-catalyzed cross-coupling of alkenyl halides and alkynyl Grignard reagents. | ||
Importantly, the presence of lithium salt accelerates the initial reduction process. The alkenyl halide is oxidatively added to the Fe(0) active species. This is followed by reductive elimination of high-valent ferrate complex II to produce the corresponding enyne together with the formation of complex III. A molecule to alkynyl Grignard reagent is added to complex III to regenerate active iron species I.
A unique metalate mechanism with the Fe(II)/Fe(IV) catalytic cycle was proposed by Nakamura et al. for the coupling reaction between aryl chlorides and aryl Grignard reagents in the presence of iron fluorides and NHC ligands (Scheme 384).432 The mechanism proceeds with the initial formation of heteroleptic metalate complex I from the aryl magnesium reagent and iron(II) fluorides followed by oxidative addition to an aryl halide, producing higher valent Fe(IV) species II. Thereafter, this species undergoes reductive elimination to give the asymmetrical biaryl product with subsequent formation of Fe(II) complex III. In the final step of the cycle, transmetalation of species III with the Grignard reagent results in the regeneration of reactive intermediate I. Through density functional theory (DFT) calculation and controlled experiments they suggested that the initial transmetalation and homocoupling are suppressed in the presence of the strong coordination of the fluoride ions.
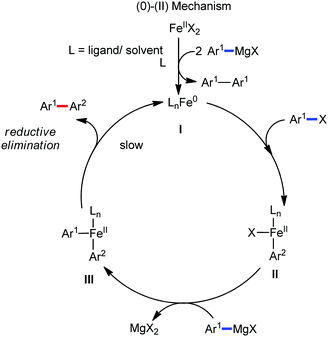 | ||
| Scheme 384 Fe(0)/Fe(II) mechanism for iron-catalyzed cross-coupling between aryl halides and aryl Grignard reagent. | ||
Consequently, the metalate mechanism is favoured in comparison to the well-established Fe(0)/Fe(II) mechanism (Scheme 385) of palladium- and nickel-catalyzed cross-coupling reactions.
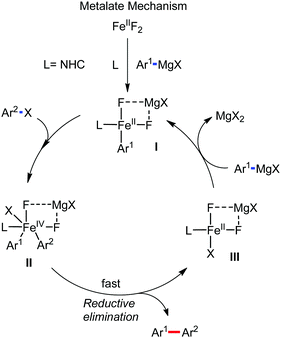 | ||
| Scheme 385 Proposed metalate mechanism for iron-catalyzed cross-coupling between aryl halides and aryl Grignard reagent. | ||
Further, Norrby et al. investigated the cross-coupling of aryl electrophiles and alkyl Grignard nucleophiles using Hammett and computational studies.542,543 They proposed the presence of Fe(I) complex I as the active species. This species can undergo transmetalation before or after oxidative addition, forming Fe(III) complex II (Scheme 386). Fe(III) complex II in turn undergoes reductive elimination, resulting in the coupled product and a new Fe(I) complex. The oxidative addition is the rate-limiting step and transmetalation is the fast step. The cycles containing oxidation states lower than Fe(I), that is Fe(II−)/Fe(0), Fe(I−)/Fe(0) and Fe(0)/Fe(II), are disfavoured from a thermodynamic point of view. Mainly, the low-valent iron species can act as an active precatalyst but cannot be regenerated in the catalytic cycle due to the high energy barrier for the required reductive elimination process. The low temperature kinetic investigation of the reaction also supported the Fe(I)–Fe(III) catalytic cycle.544 Quantification of the product showed that Grignard reagents alone can reduce the iron precatalyst to Fe(I) as the lowest oxidation state.545 Further, the computational study revealed the presence of the S = 3/2 spin state for the active catalyst even though Fe(III) salts having the S = 5/2 spin state were used as the precatalyst.
In contrast, Ren et al. performed theoretical analysis of the mechanism of the in situ-generated low-valent iron species, [Fe(MgX)2], as postulated by Fürstner. They performed density functional theory (DFT) calculations for the iron-catalyzed coupling of 4-chlorobenzoic acid methyl ester and n-hexylmagnesium chloride.546 They generalized the Fe(II−)/Fe(0) catalytic cycle into three steps, consisting of the oxidation of [Fe(MgBr)2] to form the arylated species followed by the addition of the Grignard reagent, forming the aryl-alkyl Fe–Mg cluster, and finally reductive elimination, resulting in the coupled product with the regeneration of [Fe(MgX)2]. Notably, according to Fürstner's mechanism, the oxidative addition is associated with the removal of magnesium dihalide followed by the addition of the Grignard reagent. In relation to this, it was concluded that the addition of Grignard reagent undergoes a low energy barrier when it occurs before the removal of MgX2. The final reductive elimination was found to be the rate-limiting step.
Recently in 2018, Neidig and co-workers reported the formation of iron-phenyl species [FePh2(μ-Ph)]22− from the reaction of 4 equivalents PhMgBr and Fe(acac)3 in THF at −80 °C, which is stabilized in the presence of NMP.547 On performing the reaction at −30 °C, they isolated a tetranuclear iron-phenyl cluster Fe4(μ-Ph)6(THF)4. This tetranuclear species was found to be reactive to bromocyclohexane, giving the coupled product rapidly, whereas the species [FePh2(μ-Ph)]22− remained unreactive to electrophiles. Further synthetic studies revealed the formation of analogous tetranuclear iron species using both 4-F-PhMgBr and p-tolylMgBr.
In 2012, Bedford and co-workers demonstrated the influence of Fe(I) complexes in the Negishi cross-coupling between benzyl halides and diarylzinc reagents in the presence of diphenylphosphinobenzene (dpbz)-containing iron(III) complexes.548 The early formation of the homocoupling product in the reaction indicated the reduction of the Fe(III) precatalyst to an Fe(I) intermediate species. In this context, they synthesized and characterized three Fe(I)(dpbz)2 complexes (I, II, and III) to gain better insight into the catalytic efficacy of Fe(I) intermediates in the cross-coupling reaction (Scheme 386). The complexes were found to be very efficient as catalytic species on application to the cross-coupling of diarylzinc with benzyl electrophiles and 2-pyridyl halides, giving high yields of products (Scheme 387). Through this comparable efficiency of the Fe(I) complexes to that of the Fe(III) precatalyst, Fe(I) was suggested as the lowest kinetically relevant species. Through further investigation on the same reaction using XAFS spectroscopy, they recently reported that during the turnover, the diphosphine ligand co-ordinates predominantly to the Zn(II) ions instead of iron.549 Their studies also revealed the presence of homoleptic arylferrates formed by the reaction of the iron precursors with excess diarylzinc reagents. Furthermore, they proposed the formation of a mixed Fe–Zn(dpbz) species, having crucial role in the catalytic activity. The presence of Fe(I) as the active species was also confirmed by Bauer and co-workers through X-ray absorption studies.550
The role of the TMEDA ligand in the iron-promoted cross-coupling reaction of alkyl halides and bulky aryl Grignard reagents was thoroughly investigated by Bedford and co-workers in 2013.551 In these reactions, the homoleptic organo-iron is formed as the catalytically active species. The neutral complexes containing TMEDA react more slowly than that of the homoleptic ligand. However, TMEDA plays a role in the suppression of the competing reaction by trapping the corresponding off-cycle intermediates. Notably, Fe(II) appears to be the lowest oxidation state for the system containing bulky aryl Grignard reagents due to the resistance in reductive elimination. However, lower oxidation states below Fe(II) are feasible in the presence of smaller and less bulky aryl Grignard reagents. The formation of the Fe(I) complex for smaller aryl Grignard such as benzyl and tolylmagnesium halides was confirmed by the presence of the S = 1/2 spin state in EPR measurements.
In 2014, Lefèvre and Jutand carefully studied the coupling reactions of aryl and heteroaryl halides and aryl Grignard reagents catalyzed by iron(III) acetylacetonate using 1HNMR, cyclic voltammetry, EPR and DFT.552 They reported the formation of two reduced complexes produced via the reduction of Fe(acac)3 in the presence of excess of PhMgBr.460 One of them was identified as [PhFeII(acac)(thf)n] and the other as [PhFeI(acac)(thf)]−. The Fe(II) species remains inert to aryl or heteroaryl halides. In contrast, the Fe(I) complex reacts in two different pathways depending on the substrates. The aryl halides are reduced by the Fe(I) species in a monoelectronic inner-sphere reduction pathway, resulting in the corresponding arenes and homocoupling products. On the other hand, the heteroaryl chlorides react with [PhFeI(acac)(thf)]−via the oxidative addition/reductive elimination pathway to afford the coupled products.
Although several possible mechanistic pathways have been proposed, recent studies using advanced spectroscopic methods are more consistent with the Fe(I)/Fe(II) catalytic cycle.
This alkyl radical remains associated with the iron centre during the transmetalation step. Iron-aryl complex III formed through transmetalation with the Grignard reagent is attacked by an alkyl radical, followed by elimination of the coupled product with concomitant regeneration active species I.
A similar mechanism was proposed by Caheiz et al. for the alkylation of aryl Grignard reagents having 0, I+ and II+ as the on-cycle oxidation states (Scheme 389).470 Initially, the Fe(III) precatalyst is reduced by arylmagnesium halides, producing Fe–Mg cluster-type active species I having iron in the zero oxidation state. The transmetalation step was suggested to precede the electron transfer process. Thereafter, the oxidative addition of the alkyl halide occurs in two consecutive steps, consisting of the generation of alkyl radical III and its subsequent addition to the iron centre with the concomitant release of MgX2. Species IV thus formed undergoes reductive elimination, forming the coupled product together with the regeneration of the active Fe(0) complex.
In 2009, Nagashima and co-workers presented a mechanistic overview of the cross-coupling of alkyl halides and aryl Grignard reagents, elucidating the role of the TMEDA ligand in the reaction (Scheme 390).554 The mechanism initiates with the formation of an Fe(II) complex (TMEDA)Fe(Ar)2 (I) from FeCl3 and the aryl Grignard reagent, followed by the addition of an alkyl halide to it. In this addition process, diaryliron(II) complex I undergoes one-electron oxidation to form diaryl(halogeno)iron(III) complex II together with the formation of an alkyl radical. The alkyl radical remains in close association to the iron(III) complex. Next, this alkyl radical abstracts one aryl radical from the diaryliron(III) complex, resulting in the alkylated arene and aryl(halogeno)iron(II) complex III. Thereafter, this Fe(II) species undergoes transmetalation to regenerate the initial Fe(II) species, I. Notably the active diaryliron(II)–TMEDA complex was isolated and its structure was determined by X-ray crystallography. Also, the lifetime of the radical intermediate was estimated using radical clock experiments by applying the reaction conditions separately to 1-bromo-5-hexene (a) and bromomethyl cyclopropane (b) (Scheme 391). Although the latter gave fast ring-opening products, no cyclopentyl product was observed for 1-bromo-5-hexene.
In 2010, Nakamura and co-workers demonstrated a similar Fe(II)/Fe(III) catalytic cycle for the iron-catalyzed Suzuki–Miyaura coupling between lithium borates and alkyl halides using iron(II) diphosphine complexes (Scheme 392).488 The effect of the bisphosphine ligand-containing iron complexes, FeCl2(SciOPP), in this reaction and in the Kumada reaction was investigated by Neidig and co-workers using Mössbauer spectroscopy, magnetic dichroism (MCD), and DFT studies.555,556 They have identified in situ FeMes2(SciOPP) as the active catalyst for the cross-coupling reaction of mesitylmagnesium bromide (MesMgBr) with primary alkyl halides in the presence of the bisphosphine iron complex FeCl2(SciOPP). The advanced spectroscopic techniques also revealed the formation of the FeXMes(SciOPP) (X = halides) complex together with the mesityldecane product for the reaction of primary alkyl halides with FeMes2(SciOPP). This observation was in accordance with the previous proposals of Fe(II)/Fe(III) catalytic mechanisms.
Further spectroscopic studies were performed by the Neidig group on the Suzuki–Miyaura and Kumada coupling of phenyl nucleophiles with secondary alkyl halides in the presence of the same iron–SciOPP complexes.557 They reported the formation of an Fe(0)(η6-biphenyl)(SciOPP) complex by the reaction of FeCl2(SciOPP) and phenyl nucleophiles. Although the reactivity of low-valent species in the cross-coupling reaction is known, the iron(0) complex was found to be catalytically incompetent species towards electrophiles. In contrast, the mono- and bis-phenylated Fe(II) species produced prior to the reductive elimination were identified and found to be much more reactive toward electrophiles, giving the cross-coupled product in catalytically relevant rates. Furthermore, the monophenylated iron(II) species has higher selectivity towards coupled product formation for both the Suzuki–Miyaura and Kumada coupling reactions. Additionally, the presence of a significant amount of under transmetalated iron for both reactions indicated that the mono-phenylated iron complex Fe(Ph)X(SciOPP) (X = Br and Cl) is the prevalent active species.
The same FeCl2(SciOPP) complex was utilized for the alkynylation of non-activated alkyl halides by Nakamura and co-workers.504 A single-electron transfer mechanism was proposed analogous to the other mechanisms for the reaction using the same iron–SciOPP complex. Hu and co-workers also suggested a similar mechanism in their alkyl–alkynyl cross-coupling using a phosphine-free iron catalyst.505 Accordingly, in 2017, Neidig and co-workers provided detailed insight into the iron–SciOPP catalyzed alkynylation reaction of alkyl halides and alkynyl Grignard reagents using different inorganic spectroscopic methods and thorough reaction studies.558 They studied the reaction using (triisopropylsilyl)ethynylmagnesium bromide as the nucleophile with cycloheptyl bromide electrophile using FeBr2(SciOPP) as the catalyst. They demonstrated the in situ formation of mono-, bis- and tris-alkynylated iron(II) SciOPP complexes upon the initial addition of the iron–SciOPP catalyst to the Grignard reagent. The rapid redistribution of the alkynyl ligand attached to these species made them inaccessible in THF solvent. However, these species were found to be stable in non-polar solvents such as toluene and were assessed for their reactivity towards electrophiles. The tris-alkynylated iron(II)–SciOPP species was catalytically inefficient, remaining unreactive to the cycloheptyl bromide electrophile. On the other hand, the neutral mono- and bis-alkynylated iron(II)–SciOPP species readily reacted with the electrophile to generate the coupled products at catalytically relevant rates. Also, the rate of addition of the Grignard reagent was found to have a significant effect on the yields and selectivity of product formation. Furthermore, the role of the steric bulk of the Grignard reagent was well demonstrated in this study.
Nakamura and co-workers proposed a combined mechanistic cycle for their first iron-catalyzed enantioselective and chemoselective cross-coupling between alkyl halides and Grignard reagents using a chiral–bisphosphine–iron complex.559 They suggested the occurrence of either the Fe(II)/Fe(III)/Fe(I) (in-cage) or Fe(II)/Fe(III) mechanistic (out-of-cage) pathway, having a common organoiron(II) intermediate I as the active species (Scheme 393). Notably, the coupling reaction initially undergoes iron-promoted C–Cl activation to generate complex III together with alkyl radical formation. This is followed by C–C bond formation via the in-cage or out-of-cage pathway. They re-evaluated the mechanistic pathways in 2017 using DFT and MC-AFIR methods.560 The mechanistic cycle proposed after computational studies consists of initial C–Cl bond activation of active species I, generating complex II. Thereafter, metal-radical coordination occurs via transmetalation, forming intermediate III. Then, coordination between iron centre III and the radical occurs to generate species IV. Finally, C–C coupling occurs via reductive elimination in species IV to give the desired product together with the regeneration of the active species (Scheme 394). It was suggested that both the iron(I) and iron(II) species are generated in the reaction before the addition of the electrophile. Notably, the iron(I) species initiates the C–Cl activation process. Furthermore, the C–C bond formation by the reaction of the iron(II) species and radical species via the inner-sphere mechanism is the selectivity-determining step of the cycle. On the other hand, Gutierrez and co-workers on employing quantum mechanical calculations demonstrated that the formation of the product occurs via the low-energy quartet spin state of iron instead of the high-energy sextet spin state.561 The stereoselectivity is observed due to the presence of key non-covalent interactions such as π-donor/acceptor and C–H–π interactions.
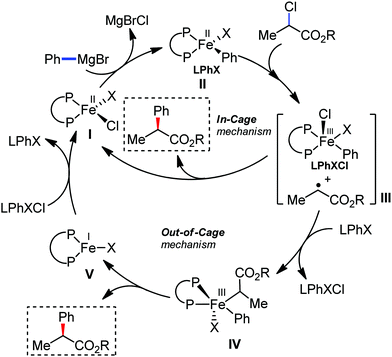 | ||
| Scheme 393 Originally proposed combined mechanistic cycle for iron-catalyzed enantioselective cross-coupling. | ||
Nakamura and co-workers also proposed a similar radical mechanism for their iron–SciOPP-catalyzed stereoselective anomeric arylation of glucosyl halides and aryl metal reagents.562 Radical clock experiments confirmed the presence of glycosyl radical intermediates. Further, DFT studies revealed that the relative stability of the glycosyl radical conformers results in the high diastereoselective nature of the reaction.
A plausible mechanism involving a radical intermediate was presented by them for the cross-coupling of non-activated chloroalkanes with aryl Grignard reagents in the presence of iron N-heterocyclic carbene catalysts (Scheme 395).467 Initially, iron(III) chloride is reduced to NHC-bearing iron(II) intermediate I on reaction with the Grignard reagent and imidazolium salt in the induction step. Thereafter, ferrate(II) intermediate complex II is formed, which has been suggested as the active species of the catalytic cycle. Then, an SET mechanism operates via the homolytic cleavage of the C–Cl bond of the alkyl chloride, generating the alkyl radical and iron(III) complex III. The radical remains bound to the iron centre in the solvent cage and it recombines with the aryl ligand of the iron centre to form the desired coupled product. Deng and co-workers also investigated the mechanistic aspects of the iron-catalyzed cross-coupling reaction with NHC ligands.563 They reported the synthesis and characterization of the NHC-bearing ferrous phenyl complex [(IPr2Me2)2FePh2] and studied its reactivity towards C–C bond formation. The complex prepared from the reaction of ferrous chloride with PhMgBr and IPr2Me2(IPr2Me2 = 1,3-diisopropyl-4,5-dimethylimidazole-2-ylidene) was found to react with alkyl halides, affording the cross-coupling products together with the formation of the monophenyliron(II) complex (IPr2Me2)2FePhX. They suggested the involvement of the single-electron transfer (SET) mechanism for the C–X bond cleavage in alkyl halides through the observation of the ring-opening product for the reaction with cyclopropylmethyl bromide. Also, the formation of alkenes and alkanes as side products confirmed the presence of radical intermediates in the reaction.
Further in 2015, Tonzetich and co-workers studied the mechanism of cross-coupling reactions catalyzed by H-heterocyclic carbene ligand-containing iron complexes.564 They employed the iron precatalyst [FeCl2(μ-Cl)2(IPr)2] to perform detailed mechanistic studies by means of radical clock, radical trapping and single turnover experiments. Through these studies, they proposed a radical-based catalytic cycle involving an Fe(II)/Fe(III) redox couple (Scheme 396). The proposed cycle begins with the transmetalation of the iron(II) precursor, forming arylated iron(II) active species I. This is followed by halogen atom abstraction of the alkyl halide to form alkyl radical and iron(III) intermediate II. This alkyl radical after dissociation from the metal solvent cage reacts with the aryl group present on II, forming the coupled product together with the concomitant regeneration of Fe(II) complex III. Finally, transmetalation of the formed Fe(II) complex results in the regeneration of active species I.
Notably, Càrdenas and co-workers proposed an Fe(I)/Fe(II)/Fe(III) catalytic cycle with Fe(I) as the active species for the cross-coupling between alkyl iodides and acetyl group-containing alkyl Grignard reagents using Fe(OAc)2 together with the NHC ligand IMes·HCl.509 The existence of Fe(I) species in the reaction medium was confirmed by EPR spectroscopy, showing the presence of the S = 1/2 species. In addition, the radical clock experiments suggested the participation of radical intermediates. Thus, the two-step oxidative addition of the alkyl halide was suggested, consisting of homolytic cleavage of the alkyl halide forming an alkyl radical and Fe(II) species (II/III′), followed by collapse of the alkyl radical with the Fe(II) complex, resulting in the Fe(III) complex (IV) (Scheme 397). This Fe(III) complex undergoes reductive elimination to form the coupled product. The Fe(I) active species (I) can be evolved either through oxidative addition of the alkyl halide (cycle A) or through transmetalation of the Grignard reagent (cycle B) (Scheme 397). However, both cycles afford the coupled product via reductive elimination of iron(III) species II.
Accordingly, in 2018, Neidig and co-workers reinvestigated this reaction to gain better insight into the reactive species using a series of inorganic spectroscopic techniques together with inorganic synthesis of model complexes or reactive intermediates and reaction studies.565 Employing the iron–NHC-catalyzed reaction of (2-(1,3-dioxan-2-yl)ethyl)magnesium bromide with 1-iodo-3-phenylpropane, they identified and demonstrated the in situ formed [(IMes)Fe((1,3-dioxan-2-yl)ethyl)2] (X) as the reactive species (Scheme 398). In contrast to previous proposals, the S = 1/2 iron species were found to be minor off-cycle species. The high reactivity of the bis-alkylated species [(IMes)Fe((1,3-dioxan-2-yl)ethyl)2] towards electrophiles was demonstrated by its two turnovers with respect to iron, producing the cross-coupled product with selectivity similar to a catalytic reaction. The reason behind the much lower β-hydride elimination from the alkyl nucleophile in the Càrdenas reaction conditions was disclosed through this investigation. The resistance to β-hydride elimination is due to the presence of a five-membered chelate ring in iron(II) of the bis-alkylated species, which is formed by the chelation of the ligated alkyl group through its carbon and one oxygen of the acetyl moiety.
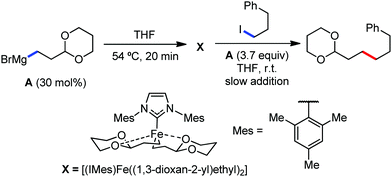 | ||
| Scheme 398 Iron-catalyzed cross-coupling of (2-(1,3-dioxan-2-yl)ethyl)magnesium bromide with 1-iodo-3-phenylpropane in the presence of isolated active catalyst X. | ||
Moreover, other bulkier NHC ligands were found to be less effective in the catalysis due to the inhibition of this chelate formation as result of their bulkiness.
Nakamura and co-workers also proposed a two-step oxidative addition analogous to the proposals of Càrdenas for their iron catalyzed alkyl–alkyl Suzuki–Miyaura cross-coupling (Scheme 399).508 The catalytic cycle proceeds with initial homolytic cleavage of the alkyl halide, producing the alkyl radical with concomitant first-step oxidation of the Fe(n) species (I) to Fe(n + 1) species (II). This is followed by the addition of the alkyl radical to the iron centre, causing the next one-electron oxidation, forming the intermediate Fe(n + 2) species (III). Finally, reductive elimination of this intermediate Fe(n + 2) species produces the coupled product together with regeneration to the initial reactive species (I) via transmetalation to intermediate IV.
In addition, in 2015, Hu and co-workers reported a unique bimetallic and radical oxidative addition in their proposed catalytic cycle for the alkyl–aryl Kumada coupling using a pincer ligand-bearing iron complex.566 They also established the presence of an Fe(II) aryl ‘ate’ complex as the active species.
Nakamaura and co-workers proposed a radical mechanism for their iron-catalyzed coupling of halohydrins with aryl aluminium reagents, which was very similar to the mechanism for their cross-coupling using iron–NHC complexes.491 Further, Bedford et al. depicted a radical pathway for describing the iron-mediated Negishi coupling of benzyl halides and phosphates.518 Furthermore, the DFT calculation and competitive kinetic experiments by Norrby and co-workers in 2015 justified the radical nature of iron-catalyzed cross-coupling reactions. In their studies with cross-coupling of Grignard reagents and alkyl halides, they suggested that the reaction of the alkyl halides goes through an atom-transfer-initiated radical pathway.567,568
7. Iron-catalyzed reduction reactions
7.1 Hydrogenation
This intermediate further undergoes stepwise reduction to monoene and then methyl stearate. Various dienes are expected to be formed in the first reduction step when triene is subjected to the reaction conditions, which indeed was observed.570 However, further hydrogenation was detected only for the conjugated dienes, while the non-conjugatable dienes remained unreacted. Although these reports showed great potential in iron-catalyzed hydrogenation, little exploration has been found until the start of 21st century. In 2004, Chirik's group synthesized Fe(0) bis(imino)pyridine complexes (iPrPDI)Fe(N2)2 [iPrPDI = ((2,6-CHMe2)2C6H3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)2C5H3N] with two N2 co-ordination, which showed promising reactivity towards the hydrogenation of alkenes (Scheme 401).571
CMe)2C5H3N] with two N2 co-ordination, which showed promising reactivity towards the hydrogenation of alkenes (Scheme 401).571
A TOF of 1814 mol h−1 was observed for the hydrogenation of 1-hexene with 0.3 mol% of catalyst under 1 atm hydrogen pressure. However, the reactivity was found to be highly substrate dependent. A year later, the same group developed various iron(0)α-diimine complexes and tested them for hydrogenation (Scheme 401).572 Interestingly, when cyclooctadiene (COD) co-ordinated [ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)C(Me)
C(Me)C(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr]Fe(η2:η2-1,5-C8H12) was treated with H2 in C6D12, it reduced one double bond of COD; however, the use of C6D6 gave a thermodynamically more stable [ArN
NAr]Fe(η2:η2-1,5-C8H12) was treated with H2 in C6D12, it reduced one double bond of COD; however, the use of C6D6 gave a thermodynamically more stable [ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)C(Me)
C(Me)C(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NAr]Fe(η6-C6D6) complex instead of hydrogenation. Both the COD and cyclooctene complexes were also found to be competent in hydrogenating 1-hexene to hexane, but with a relatively lower TOF.
NAr]Fe(η6-C6D6) complex instead of hydrogenation. Both the COD and cyclooctene complexes were also found to be competent in hydrogenating 1-hexene to hexane, but with a relatively lower TOF.
Further experimental as and computational studies revealed that indeed co-ordination of the arene group deactivates both the bis(imino)pyridine and α-diimine complexes (Scheme 401).573
Thus, as expected, the reactivity of the bis(imino)pyridine improved upon changing the substitution on the imine nitrogen to an isopropyl group.574 Upon optimization of the various parameters, a range of functional group tolerance was also found.575–577 The reaction is believed to proceed via the oxidative addition of H2 to the metal center upon ligand dissociation of labile N2. Then migratory insertion into the olefin gives an alkyl iron intermediate, followed by reductive elimination to give the final product. Wangelin's group synthesized a series of diamine-based BIAN-Fe complexes and found that (dipp2BIAN)FeCl2 [dipp2BIAN = 2,6-diisopropylphenyl bis(imino)acenaphthene] was a very good candidate for alkene hydrogenation; however, it requires a strong reducing agent such as n-BuLi.578
In 2009, de Vries co-workers carried out hydrogenation using iron nanoparticles synthesized by reducing FeCl3 with a Grignard reagent.579,580 The Fe-NPs with a size of 2.67 ± 0.60 nm were presumed to be coated with MgCl and THF. The NPs were excellent candidates for the hydrogenation of alkenes and alkynes, and the controlled experiment showed that none of the starting materials such FeCl3 or Grignard reagents were responsible for this reactivity. The kinetic experiments revealed that alkynes react better with these NPs than alkenes. Moreover, both the alkane and cis-alkene formed during the hydrogenation of alkynes and the ratio could be controlled to obtain one major form by optimizing the parameters. Later, in 2011, Breit's group synthesized Fe-NPs deposited on chemically-derived graphene from Fe(CO)5.581 Initially, 0.9 mol% of catalyst converted 43% of 1-hexene to hexane under 20 bar H2 pressure and 100 °C. However, the use of 5 mol% EtMgCl resulted in near full conversion, which is possibly due to surface activation by the Grignard reagent. Wangelin's group improvised a strategy and used FeCl3 and EtMgCl to generate an in situ-reduced catalyst which can carry out hydrogenation of aromatic alkenes (Scheme 402).582 Thomas's group further improved this strategy by using FeCl2 as a precatalyst, iPrMgCl as a reducing agent and additional tetradentate iminopyridine as a ligand (Scheme 402).583 Chaudret's group synthesized ultra-small Fe nanoparticles with a size of 1.5 ± 0.2 via the decomposition of {Fe(N[Si(CH3)3]2)2}2, which also proved to be an excellent catalyst towards the hydrogenation of alkenes and alkynes to alkane in >99% yield in most cases.584
Beller and co-workers utilized Fe(BF4)2·6H2O in conjunction with the [P(CH2CH2PPh2)3] [PP3] ligand to achieve the partial reduction of arylacetylenes to the corresponding styrene derivatives (Scheme 403).585 Interestingly, formic acid was used as the reducing agent at 40 °C to deliver of the styrene product with >99% yield in most cases. It was believed that the triphosphine co-ordinated iron forms a dihydride complex upon reaction with HCOOH, eliminating CO2. Subsequent hydrogen transfer to the co-ordinated alkene followed by reductive elimination affords the final product. Similarly, a ferraboratrane ligated with triphosphine-borane (TPB) underwent heterolytic H2 addition to form (TPB)(μ-H)Fe(L)(H), which in turn could hydrogenate styrene across the double bond (Scheme 404).586 Milstein and co-workers synthesized a pincer-based [(HACRPNP)Fe(CH3CN)(η2-CH3CHCNBH3)] [HACRPNP = 4,5-bis((diphenylphosphino)-9H-acridine-10-ide)] complex to carry out the hydrogenation of aromatic alkynes (Scheme 404).587 They were able to optimize the reaction in favour of selective E-alkene formation, although the selectivity is attributed to the isomerization of the Z-isomer into more stable form under the reaction conditions.
Complementary to this, FeCl3/EtMgCl-derived Fe-NPs stabilized by an ionic liquid selectively provided the Z-isomer upon the hydrogenation of alkynes with molecular hydrogen. In 2016, Jones and co-workers utilized a PNP pincer-based (PNPiPr)Fe(H)(CO) [PNPiPr = N(CH2CH2PiPr2)2] complex to hydrogenate styrenes and activated olefins (Scheme 404). Under 1 atm H2 pressure, they obtained 100% NMR yields for many styrene derivatives including 2-vinylpyridine. The reaction is believed to proceed in a stepwise manner, where hydride transfer from the metal is followed by proton transfer. Recently, a carbazolide-based PNP ligand co-ordinated ferroushydrido complex was reported by Gade's group, which also showed similar alkene hydrogenation reactivity.588
In 2014, Nakazawa and co-workers synthesized[2,5-bis(triphenylsilyl)-3,4-butylene-(η5-C4COEEt2H)]Fe(CO)2H (E = Si, Ge) complexes bearing Si–H and Ge–H bonds.589 These complexes could perform transfer hydrogenation of p-tolylacetylene in the presence of IPA, although with poor yield and selectivity (Scheme 405). The mechanism described by the authors involves CO substitution by the alkyne and subsequent insertion into the Fe–H bond. The Si–H bond distantly bound with the Cp ring adds oxidatively to form another Fe–H bond. Finally, reductive elimination gives the product and the catalyst is regenerated by taking two hydrogens from IPA, leaving acetone as the by-product.
With the aim of achieving chiral hydrogenation, Chirik and co-workers synthesized a number of bis(phosphine)iron(II)dialkyl complexes with bidentate chiral phosphine ligands.590 Although high yields for the hydrogenation of pro chiral alkenes were obtained, the enantioselectivity was non-detectable. It was concluded that under the reaction conditions, iron dissociates from the ligand and forms an Fe(0) amorphous species, which is responsible for the observed reactivity; however, the ligand cannot participate to induce stereoselectivity.
The groups of Wolf and Wangelin documented hydrogenation with bis(η4-anthracene)ferrate complexes (Scheme 406). Interestingly, the metal is in the 1− oxidation state and termed as “quasi naked” metal species.591,592 The reactivity of these 18-electron complex species probably starts by losing an arene ligand to undergo H2 oxidative addition. The ferrate complex showed decent reactivity; however, the cobalt analogue was found to be better catalyst.
In 2017, Wangelin and co-workers reported that treating Fe[N(SiMe3)2]2 with diBAL-H formed different sizes of Fe4–7 nanostructures (Scheme 407).593 The metal ions with the oxidation states of 0, 1+, and 2+ arrange themselves in a planner geometry, while the ligand is situated in the periphery. Except for the Fe4 clusters, bridging hydrogens were found in the crystal structures. These nano-clusters are responsible for the excellent reactivity of Fe[N(SiMe3)2]2/DIBAL-H in the hydrogenation of alkenes, particularly tetrasubstituted alkenes. Recently, disilaferracyclic isocyanide complexes were also shown to be very effective for the hydrogenation of tri- and tetra-substituted alkenes with H2.594 The reaction initiates with the removal of one ligand and co-ordination of two H2. Successive Si–Fe/H–H metathesis forms two Si–H bonds, which further undergo two-fold metathesis with Fe–C(alkene) to provide the final product (Scheme 408).
In 2018, a Knölker iron complex modified at the Cp ring and one CO substituted by the PPh3 ligand showed selective hydrogenation of α,β-unsaturated ketones. Firstly, cesium isopropoxide is formed in situ from IPA and cesium carbonate and reacts with the metal complex to give the hydride complex. In the key step, both the substrate oxygen and Cp-ligand oxygen interact with Cs+ simultaneously; thus, only olefin can interact with the metal centre. Hence, highly selective olefin hydrogenation is observed even in the presence of carbonyl.
Two different hydrogens sources, i.e. one proton from [Et3NH]+ and the other from iron carbon-hydride, are essential for the reaction to proceed (Scheme 409). Later, they found that the direct use of molecular hydrogen as a hydrogenating agent enhances the reactivity and provides evidence in favour of nucleophilic attack on the carbonyl by [HFe(CO)4]−.596
Beller's group reported the transfer hydrogenation of ketones to secondary alcohols using the [Fe3(CO)12]/terpy/PPh3 catalytic system and isopropanol as the hydrogenating agent (Scheme 410).597 Deuterium labelling studies revealed the involvement of the monohydride transfer mechanism, that is the metal hydride species is involved only in the transfer of hydrogen from the donor carbon centre to the ketone carbon; however, the dihydride mechanism cannot be ruled out completely. The same reaction could also be carried out with an in situ-generated iron–porphyrin complex simply by replacing the terpy and PPh3 ligands with porphyrin.598 Later, this iron–porphyrin transfer hydrogenation chemistry was further extended to the reduction of α-hydroxyprotected ketones.599
Casey and co-workers utilized a cyclopentadienone tricarbonyl iron complex (Knölker's iron complex) to achieve the hydrogenation of ketones (Scheme 411).600,601 In the presence of sufficient H2 pressure, the catalyst shuttles between the hydrogenated and dehydrogenated forms, where the former hydrogenates the ketone to form dehydrogenated species, which in turn goes back to the hydrogenated form upon the addition of H2.602 The reaction presumably starts with H-bond formation between the catalyst –OH and ketone group, followed by insertion into the Fe–H bond. Then, one molecule of H2 oxidatively adds to the metal, while one of these two hydrogen atoms transfers to the cyclopentadienone to regenerate the catalyst. However, the computation study suggested that the hydrogenation proceeds via an outer-sphere concerted mechanism, that is simultaneous transfer of both hydrogens to the substrate.603–607
The asymmetric version of this reaction was carried out by Gao's group in 2004 using [Et3NH]+[HFe3(CO)11]− in conjunction with the chiral diaminodiphosphine ligand; however, the yield and enantiomeric excess were inconsistent with the variation in substrate.608 In 2008, Morris’ group utilized the [Fe(NCMe)(CO){(R,R)-cyP2N2}](BF4)2 complex equipped with the chiral tetradentate PNNP ligand system (R,R)-cyP2N2 [(R,R)-{PPh2(o-C6H4)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H10N
NC6H10N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(o-C6H4)PPh2}] for this purpose (Scheme 412).609,610 In contrast to Gao's diaminodiphosphine, they used a diiminodiphosphine system together with potassium tertiarybutoxide as the base; however, the imine was presumed to undergo hydrogenation into amine under the reaction conditions. Nonetheless, no improvement in enantiomeric excess was observed. Therefore, a series of diiminodiphosphine ligands was synthesized with 1,2-diphenyldiiminoethane in their backbone instead of cyclohexanediimine to give better reactivity towards the asymmetric transfer hydrogenation of ketones and imines.611–613 Interestingly, ligands with Cy or iPr substitution at the phosphorus center and acetonitrile co-ordination at the two-axial position of the iron were inactive.
CH(o-C6H4)PPh2}] for this purpose (Scheme 412).609,610 In contrast to Gao's diaminodiphosphine, they used a diiminodiphosphine system together with potassium tertiarybutoxide as the base; however, the imine was presumed to undergo hydrogenation into amine under the reaction conditions. Nonetheless, no improvement in enantiomeric excess was observed. Therefore, a series of diiminodiphosphine ligands was synthesized with 1,2-diphenyldiiminoethane in their backbone instead of cyclohexanediimine to give better reactivity towards the asymmetric transfer hydrogenation of ketones and imines.611–613 Interestingly, ligands with Cy or iPr substitution at the phosphorus center and acetonitrile co-ordination at the two-axial position of the iron were inactive.
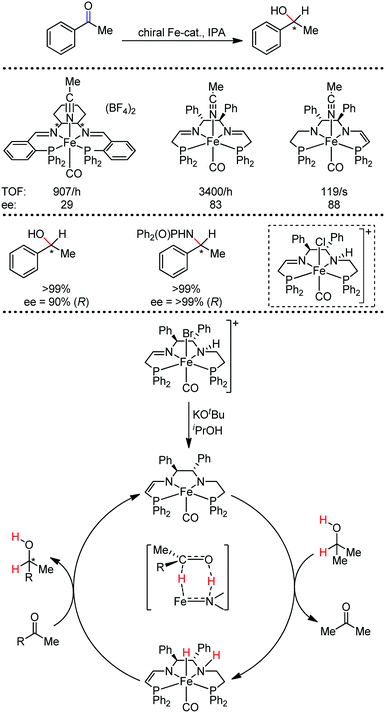 | ||
| Scheme 412 PNNP-based iron catalyst-mediated asymmetric transfer hydrogenation of carbonyl compounds. | ||
Further improvement revealed that bis(eneamido)-based PNNP ligands are also potentially good candidates.614–617
After optimization of various substituents on the imine backbone and phosphorus center and axial ligands, the 5,5,5-fused membered PNNP-based bis(eneamido)diphosphine and PNNHP-based amine(imine)diphosphine were found to be the best, having phenyl substitution at the phosphorus and CO and halide as the axial ligands. For instance, (S,S)-[Fe(CO)(Br)(PAr2CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCHPhCHPhN
NCHPhCHPhN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2PAr2)]BPh4 can provide enantiomeric excess and TON of up to 99% and 6000, respectively.618–627
CHCH2PAr2)]BPh4 can provide enantiomeric excess and TON of up to 99% and 6000, respectively.618–627
Experiments and computations suggest that the hydrogen from the –OH of IPA transfers to the imine-nitrogen and the hydrogen from –CHMe2 to iron, probably in a concerted pathway (Scheme 412). The hydride complex then interacts with the –CO bond of the substrate in similar but reverse step for hydrogen transfer from N to O and Fe to C, forming the alcohol product. However, careful observation revealed that the first generation 6,5,6-PNNP-based complexes follows a different mechanism. The formation of Fe-NPs with a size of 4.5 nm was detected in those cases, where the chiral ligands were found to be surface bound, and hence enantioselectivity was observed.628,629 Moreover, a mechanism for catalyst deactivation occurs via isomerisation of the ligand double bonds, which is dependent on the ligand architecture.630 Thus, it has a major contribution towards varying reactivity with respect to different substituents.
Milstein and co-workers synthesized a tridentate PNP pincer ligand-based iron hydride catalyst to achieve the hydrogenation of ketones.631 The complex [(iPrPNP)FeH(CO)Br] [(i-PrPNP = 2,6-bis(diisopropylphosphinomethyl)pyridine)] synthesized from the dibromo complex by treatment with NaHBEt3 could catalyze various ketones in the presence of H2 with a TON of up to 1880. Interestingly, the mechanism is believed to involve a dearomatized intermediate stabilized by ethanol (Scheme 413). The parent complex upon treatment with NaBH4 gives an iron-borohydride complex, which is also an equally active hydrogenating catalyst.632 The combination of experimental and computational studies suggested that ethanol co-ordination rearomatizes the ligand. Subsequently, dual hydrogen atom transfer occurs to the ketone, one from the ligand arm to the carbonyl carbon, and the other from co-ordinated EtOH to the carbonyl oxygen. The addition of H2 to the resultant complex gives back the native catalyst.633 However, in a more recent report, Kirchner indicates that the insertion of the carbonyl –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group into the Fe–H bond is more probable, and thus the reaction proceeds via the inner-sphere mechanism.634 In 2015, PONOP ligand-based complexes were reported by Hu's group.635 The complexes are structurally similar to that of PNP with no Fe–O co-ordination, and thus similar reactivity was observed towards hydrogenation.
O group into the Fe–H bond is more probable, and thus the reaction proceeds via the inner-sphere mechanism.634 In 2015, PONOP ligand-based complexes were reported by Hu's group.635 The complexes are structurally similar to that of PNP with no Fe–O co-ordination, and thus similar reactivity was observed towards hydrogenation.
Morris's group synthesized a chiral version of a PNP′ pincer ligand-based complex (S,S)-[Fe(Ph2PCH(Ph)CH(Me)N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2PCy2)(NCCH3)3]2+ (Scheme 414).636 It could effectively catalyze ketone to aldehyde in the presence of LiAlH4, t-AmylOH and KO(t-Bu) in excellent yields and decent enantiomeric excess (ee). Moreover, using (diphenylphosphinoyl)-propiophenoneimine as the substrate, they obtained the corresponding amine in 90% ee. Kirchner synthesized another asymmetric version of the complex using the chiral BINEPINE group at one phosphorus end; however, the two diastereomeric forms were not isolated, and therefore it cannot be used for asymmetric reactions.637 A plethora of other chiral PNP′ and PNHP′ pincer ligands with various backbone substituents were synthesized and tested for the reduction of ketones and imines to obtain enantiomeric excess as good as 96%.638–646 Although the mechanism varies from one catalyst to another, these non-aromatic ligand-based complexes are believed to show dual hydrogen transfer from iron to the carbonyl carbon and nitrogen to the carbonyl oxygen. Recently, the ionic liquid 1-butyl-2,3-dimethyl-imidazolium-bis(trifluoromethylsulfonyl) ([bm2im][NTf2]) supported on silica was used as a biphasic medium for catalysis with a PNP-based complex.647 In the presence of DBU at 50 bar H2 pressure, the system provided >98% yield of various aldehydes.
CHCH2PCy2)(NCCH3)3]2+ (Scheme 414).636 It could effectively catalyze ketone to aldehyde in the presence of LiAlH4, t-AmylOH and KO(t-Bu) in excellent yields and decent enantiomeric excess (ee). Moreover, using (diphenylphosphinoyl)-propiophenoneimine as the substrate, they obtained the corresponding amine in 90% ee. Kirchner synthesized another asymmetric version of the complex using the chiral BINEPINE group at one phosphorus end; however, the two diastereomeric forms were not isolated, and therefore it cannot be used for asymmetric reactions.637 A plethora of other chiral PNP′ and PNHP′ pincer ligands with various backbone substituents were synthesized and tested for the reduction of ketones and imines to obtain enantiomeric excess as good as 96%.638–646 Although the mechanism varies from one catalyst to another, these non-aromatic ligand-based complexes are believed to show dual hydrogen transfer from iron to the carbonyl carbon and nitrogen to the carbonyl oxygen. Recently, the ionic liquid 1-butyl-2,3-dimethyl-imidazolium-bis(trifluoromethylsulfonyl) ([bm2im][NTf2]) supported on silica was used as a biphasic medium for catalysis with a PNP-based complex.647 In the presence of DBU at 50 bar H2 pressure, the system provided >98% yield of various aldehydes.
A PNN-based complex [Fe(H)(CO)(MeCN)LPNN](BF4) [LPNN = 2-{(di-tert-butylphosphino)methyl}-6-{1-((mesitylimino)ethyl}pyridine)] with one imine arm and one phosphine arm in the ligand was reported by Milstein's group.648 A similar dearomatization mechanism was proposed for hydrogenation, although it was found to be less reactive than pyridine-based PNP systems.
In 2010, Royo's group synthesized Fe(II) complexes with cyclopentadiene-substituted NHC ligands (Scheme 415).649 The four-legged piano-stool geometric complexes showed excellent reactivity towards the transfer hydrogenation of ketones with IPA. An iron(II)–bis(isonitrile) complex was studied for asymmetric transfer hydrogenation, but achieved only moderate enantiomeric excess.650 The authors proposed that one of the isocyanide groups is involved in transferring hydrogen to the carbonyl carbon in a Meerwein–Ponndorf–Verley (MPV) mechanism. In 2012, Funk and co-workers modified Knölker's iron catalyst by one CO substitution with alkylnitriles, and the acetonitrile co-ordinated complex was found to be an excellent catalyst towards the transfer hydrogenation of aldehydes and ketones.651 Simultaneously, Berkessel's group synthesized a chiral version of the complex by replacing one of the CO ligands with a chiral phosphoramidite ligand (Scheme 416).652 The complex converted 90% of acetophenone to 1-phenylethanol under UV radiation; however, only 31% ee of the (S) isomer was observed. In the subsequent year, the groups of Beller and Renaud also modified the Knölker's-type complex with various substitutions at the Cp ring to catalyze the reaction in water.653,654 The tetrahedral bis-(N-heterocyclic carbene)–iron complex reported by Glorius's group was found to be equally effective.655 Mono NHC substitution by Bala's group656 and alkyl/aryl substitution at the cyclodipentanone by Funk's group were also explored.657 In 2016 Wills et al. carried out further experiments on an approach similar to that of Berkessel, although no improvement was obtained.658 On the contrary, Gennari synthesized chiral BINOL-based (cyclopentadienone)iron complexes (Scheme 416).659 They proposed that preferential Re-face attack on the metal hydride would form the S isomer as the major form; however, the maximum enantiomeric excess obtained was 54%.
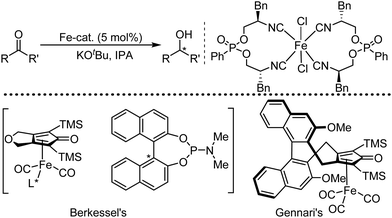 | ||
| Scheme 416 Asymmetric transfer hydrogenation with bi(diisocyanide) and modified Knölker's iron complexes. | ||
In 2017, Piarulli's group introduced another modification in the form of [bis(hexamethylene)cyclopentadienone]irontricarbonyl, which was found to be very effective for carbonyl reduction in the presence of Me3NO activator.660 Wu and co-workers used Knölker's iron catalyst in a water gas-shift reaction to achieve the hydrogenation of aromatic aldehydes.661 A combination of paraformaldehyde and water/DMSO was used to generate formic acid in situ, which further degraded to liberate H2. The H2 reacts with carbonyls in the presence of the iron catalyst to form the corresponding alcohols.
Due to the lower enantioselectivity obtained in their first attempt, Gao's group shifted their attention from PNNP ligands to P2N4-type macrocyclic ligands (Scheme 417).662,663 The 22-membered cyclic ligand showed exceptional reactivity for asymmetric hydrogenation with 0.5 mol% Fe3(CO)12 under 50 bar H2 pressure. The scope of the substrate included hydrogenation of aryl ketones, heterocyclic ketones and β-ketoesters, all with excellent yields and ee. The experimental results suggest the formation of NPs embedded in a ligand environment; thus, the heterogeneous nature of the catalysis was proposed.
A series of N2P2 macrocyclic ligands was introduced by Mezzetti's group (Scheme 417).664 The well-defined cyclic diimine–diphosphine iron complexes were active in the asymmetric transfer hydrogenation of ketones with IPA; however, could only induce moderate enantioselectivity. The axial ligands have a significant effect on the reactivity and tert-butyl nitrile was found to be the best. Later, they shifted to the saturated version (NH)2P2, which gives excellent enantioselectivity for both carbonyls and imines.665–669 An increase in the P–Fe–P bite angles has a positive impact on stereoselectivity, although it led to harsh conditions for sterically bulky substrates. These complexes are also effective towards the conversion of benzil to chiral benzoin.
Beller and co-workers developed [FeF(L)][BF4] [L = tris{2-(diphenylphosphino)phenyl}phosphine or PP3] using the TetraPhos ligand for the selective carbonyl reduction of α,β-unsaturated aldehydes (Scheme 418).670,671 They optimized the reaction to favour the formation of vinyl alcohol with >99% selectivity. Since the active catalyst is hexa-co-ordinated, one of the phosphines must dissociate for aldehyde to co-ordinate. Thus, the steric factor plays a crucial role in the observed selectivity. In addition, the catalyst also performs the reduction of various other aldehydes effectively. In another report by the same group, they showed that the catalytic NADH model and H2 combination is an effective hydrogenating agent in the presence of an Fe3(CO)12 and Fe(OTf)3 mixture for the selective ketone reduction of β-ketoesters (Scheme 418).672 Both iron catalysts are proposed to play a well-defined role. However, the Fe3(CO)12-catalyzed hydrogenation NAD model was ineffective in promoting transfer hydrogenation. On the contrary, due to its higher Lewis acidity, the Fe(III) salt could reduce the LUMO of the substrate, easing hydrogenation by the NADH model.
Recently, Sun and co-workers synthesized a series of PSiP-based Fe(II)–hydride complexes by adding the ligand Si–H bond oxidatively to the metal (Scheme 419).673 These complexes were found to be effective in the transfer hydrogenation of various aldehydes with IPA. It is believed that the co-ordinated aldehyde is first inserted into the Fe–H bond and takes a proton from the IPA to give the product. The hydrogen of the alcohol carbon is abstracted by the metal to regenerate the active catalyst.
In 2019, Li's group utilized three selenophenolatohydridoiron(II) complexes, cis-[(H)(SeAr)Fe(PMe3)4], for the transfer hydrogenation of aldehydes (Scheme 420).674 For Ar = Ph, the best results were obtained and various benzaldehydes, alkenyl and alkynyl aldehydes could be transformed to the corresponding alcohols using this methodology.
Knölker's iron complex has been reported to be the active catalyst in the hydrogenation of imines. Beller's group used chiral 3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate (TRIP) in conjunction with Knölker's complex to achieve amines from the corresponding imines with excellent enantioselectivity (Scheme 421).675 The authors proposed from NMR experiments that the chiral ligand interacts simultaneously with both the oxygen atom of the complex ligand and iron-bound N–H bond to control the stereoselectivity. This protocol was further extended to the synthesis of chiral 1-arylethyl amines from arylacetylenes and anilines.676 The in situ-generated imine from the hydroamination of alkenes was treated with the above combination of catalyst and ligand to obtain chiral secondary amines with decent enantiomeric excess (Scheme 421). On the other hand, the catalyst was utilized by Benaglia's group for the diastereoselective hydrogenation of chiral imines.677 The [bis(hexamethylene)cyclopentadienone]irontricarbonyl complex synthesized by Piarulli's group was also an equally effective catalyst for the hydrogenation of imines.678
The iron-catalyzed reduction of CO2 was demonstrated with [FeH(PP3)]BF4 in the presence of molecular hydrogen (Scheme 422).679 Later, the TON of the transformation was improved by approximately three-fold by changing the complex to [FeF(PP3)]BF4.680 The reaction proceeds via H2 coordination to form [FeH(H2)(PP3)](BF4), which is converted to the dihydride complex in the presence of a base. Subsequently, CO2 inserts into the Fe–H bond, giving the co-ordinated iron-formate species. The base present in the medium abstracts the formate and the cycle continues. The PNP ligand-based complex developed by Milstein's group also catalyzed the reduction of CO2 with a lower TON (Scheme 423).681 However, the important fact is that the latter reaction can be carried out in water medium. The pyrazine-based PNP ligand also developed by Milstein's group showed similar reactivity.682 In contrast, Bernskoetter and co-workers presented a non-aromatic pincer ligand HN{CH2CH2(PR2)}-based complex to improve the CO2 hydrogenation tremendously and obtained a high turnover number (58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990), although reactivity is dependent on the ancillary ligand (Scheme 423).683,684
990), although reactivity is dependent on the ancillary ligand (Scheme 423).683,684
Further, 2,6-diaminopyridyl-bis(diisopropylphosphine)iron(II) carbonylbromide is another complex reported for promoting the reaction.
The water-soluble version of the PP3 ligand was synthesized using sulphate substituents on the phenyl rings of –PPh2·Fe(BF4)2 in the presence of a hydrophilic ligand, which was found to be suitable for the reduction of CO2 with H2 in aqueous medium.685 The rac-isomer of a linear tetraphosphine iron(II) complex was reported by Gonsalvi in favour of bicarbonate hydrogenation.686 The iron–hydride complex was believed to be the key intermediate, as was in Beller's work. The computation study predicted that the most favourable reaction pathway involves the disproportionation of two bicarbonates into carbonate, water and CO2, which in turn react with the Fe–H complex.687 An iron(II) chloride complex of hydrotris(1-pyrazolyl)methane was employed by Martins’ group, which could deliver methanol from carbon-dioxide (Scheme 424).688 It was presumed that the iron–hydride bond is formed with the help of the ligand and CO2 inserts there, producing formaldehyde and water. A second hydrogenation of formaldehyde at the metal center releases methanol.
The (SiPR3)Fe(L)(H) [SiPR3 = [Si(o-C6H4PR2)3]−] complex was reported by Peters and co-workers for the reduction of CO2 (Scheme 425).689 The use of triethylammonium chloride was necessary in the reaction, which underwent metathesis with Fe-formate to form triethylammonium formate. Similarly, Knölker's catalyst was investigated by Zhou's group to obtain a TON of only up to 307.690
The PNP pincer ligands have been found to be effective towards hydrogenation of esters (Scheme 426). Milstein's catalyst showed promising results to catalyze the hydrogenation of alkyl trifluoroacetate to form two alcohol products.691 The groups of Beller and Guan individually used non-aromatic PNP ligands and could diversify the protocol to various other esters.692,693 In the first step, hemiacetal is formed upon reduction of the carbonyl group, which degrades into alcohol and aldehyde. The aldehyde undergoes hydrogenation for a second time to yield the final product. Guan extended this methodology to coconut oil-derived samples containing mostly methyl laurate and other ester derivatives.694 This methodology is not only scalable, but can be used in the direct reduction of coconut oil. Pignataro presented that the (cyclopentadienone)iron complexes were equally effective.
Similar to alkyl trifluoroacetate, the corresponding amides, i.e. N-substituted trifluoroacetamides, also underwent hydrogenation with [(iPr-PNP)Fe(H)(Br(CO))].695 The initial hydrogenation of the carbonyl group formed a hemiaminal, which forms an equilibrium with aldehyde and amine. Under the reaction conditions, the aldehyde further hydrogenates to alcohol, and thus the equilibrium shifts towards the right. The non-aromatic PNP-based iron–carbonyl complexes were used by Sanford and Bernskoetter with small variations at the phosphorus center, whereas Langer's group used the corresponding iron-borohydride complex.696,697 All these complexes show excellent reactivity for amide hydrogenation to amines and alcohols.
Beller's group also optimized the reduction of nitriles to amines with the PNP-based iron-borohydride catalyst in IPA (Scheme 427).698,699 The two-fold transfer hydrogenation starts with the elimination of BH3 and concerted dual hydrogenation to form imine. The imine undergoes hydrogenation for a second time to finally produce amine. On the other hand, Milstein's group employed bis(2-diisopropylphosphinobenzyl)amine as the PNP ligand to obtain up to 99% yield with various aromatic and aliphatic nitriles.700 They also extended the protocol to hydrogenative cross-coupling of nitriles with amines.701 The partially hydrogenated nitriles to imine are trapped by an amine to form a gem-diamine, which eliminates an NH3 moiety to produce the secondary imine.
γ-Valerolactone (GVL) is of high industrial importance due to its manifold application, particularly because it can be used as biofuel. The easiest way to synthesize GVL is via the hydrogenation of the bioproduct levulinic acid or its ester (Scheme 428). To replace the precious metal catalyst for this transformation, Fu's group optimized the reaction with iron(II) catalysts in conjunction with multidentate phosphine ligands.702 It was found that Fe(OTf)2 in the presence of the tris[(2-diphenylphosphino)ethyl]phosphine ligand can provide GVL from levuliniate ethyl ester in 99% yield. Formic acid was used as the hydrogen source at 140 °C. A cheaper catalyst, [Fe3(CO)12], in the presence of imidazole as a base was used by Burtoloso's group to obtain 92% yield at the cost of an elevated temperature of 180 °C.703 Upon further optimization, Fu's group found that Casey's catalyst is more effective and can provide GVL in 95% yield in a transfer hydrogenation reaction with IPA at 100 °C.704
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of the resultant 1-phenyl-1,2,3,4-tetrahydroquinoxaline was obtained. The reaction proceeds via the formation of diamine first, which undergoes twofold hydrogenation by the iron catalyst, and the phosphate ligand is believed to control the stereochemistry via hydrogen bonding.
5 of the resultant 1-phenyl-1,2,3,4-tetrahydroquinoxaline was obtained. The reaction proceeds via the formation of diamine first, which undergoes twofold hydrogenation by the iron catalyst, and the phosphate ligand is believed to control the stereochemistry via hydrogen bonding.
A year later, Jones and co-workers reported the hydrogenation of quinolines, substituted indole and pyridine with a PNP-based iron borohydride catalyst and potassium tertiary-butoxide base (Scheme 430).706 The authors presented that the same catalyst is capable of performing the reverse reaction in the absence of the base.
7.2 Reduction with other reagents
In 2012, Prabhu and co-workers developed the FeCl3-catalyzed reduction of alkenes with hydrazine (Scheme 432).711 In the optimized conditions, they utilized 5 mol% of the catalyst and 1.5 equivalent of hydrazine in EtOH under aerobic conditions to obtain reduced alkene with up to 99% yield. Both the internal and terminal olefins together with the terminal alkynes could be reduced under the reaction conditions. Most promisingly, various other functional groups such as acid, ester, and nitro are well tolerated under the reaction conditions. Experimental evidence suggest that FeCl2(H2NNH2) is probably the active catalyst for the transformation.
Thomas’ group reported alkene reduction with the Fe(OTf)2 and NaHBEt3 (or NaBH4) combination (Scheme 433).712 A variety of substrates including alkynes and a mixture of alkene and alkane are well tolerated. The mechanism of the reaction was reported to unclear; however, a radical pathway was suggested due to the stoppage of the reaction with a radical scavenger. In 2015, Wangelin and coworkers optimized the FeCl3-catalyzed hydrogenation with LiAlH4 and excellent yields were obtained for various styrene derivatives.713 It was suggested that initially the reaction proceeds in a homogeneous manner; however, the formation of a nanocluster over time is possible, and hence a decrease in reactivity occurs.
Renaud's group used Knölker's iron complex for this transformation, which was found to be quite effective (Scheme 436).717 After detailed experimental and computational studies, the authors recommended a plausible mechanism.718 In the first step, one CO is eliminated as CO2 and H2 is added to form Fe–H and –OH on the Cp ring. Then the imine or iminium formed from the carbonyl and amine undergoes outer-sphere two-fold hydrogen transfer to yield the product.
Sodium borohydride was used as the reducing agent in a Fe(OTf)3-catalyzed reaction.719 However, iron plays a different role in this process by activating carbonyl through its Lewis acidity (Scheme 437). Then the imine formed is reduced by NaBH4 to amine. Chusov et al. developed the hydrogen-free reductive amination of carbonyls with super stoichiometric iron pentacarbonyl (Scheme 438).720 Under solvent-free conditions, various cyclic amines were used at a temperature of 130 °C. Another approach was described by Taddei's group, where a mixture of IPA/water was utilized as the hydrogen source (Scheme 439).721 The NaOH and DMAP-promoted reaction was best catalyzed by Fe2(CO)9. It was proposed that NaOH reacts with the iron complex to form Na[HFe(CO)4], eliminating carbon-dioxide. The hydride complex transfers hydride to the imine carbon, while the nitrogen takes a proton from water. The DMAP-Fe species formed in this process is transformed to a hydride complex by NaOH and 4-dimethylaminopyridineoxide is formed, which in turn is converted to DMAP in the presence of IPA, releasing acetone and water.
Recently, Darcel and co-workers reported two different iron–carbene complexes and achieved two different amination products from levulinic acid and its esters (Scheme 440).722 The use of [CpFe(CO)2(IMes)][I] [IMes = 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene] as the catalyst gave pyrrolidones, whereas [Fe(CO)4(IMes)] selectively produced pyrrolidine in the presence of PhSiH3. Fascinatingly, 2-formylbenzoic acid also underwent cyclic amination to produce isoindolines. Mechanistically, the imine is hydrosilylated by the metal catalyst, followed by cyclization to pyrrolidone. Depending on the conditions, the pyrrolidone undergoes further reduction to cyclic amine. This protocol was further extended to the synthesis of 5, 6 and 7-membered cyclic amines from 4-aminobutanoic acid, 5-aminopentanoic acid and 6-aminohexanoic acid, respectively, using the latter complex, which followed a similar reaction pathway.723
The Cp*Fe(CO)2(4-C6H4CH3) complex was utilized by Argouarch et al. for the reductive etherification of aldehydes with dialkoxymethylsilane (Scheme 442).726 UV irradiation is required for the reaction in order to activate the catalyst through CO ligand dissociation. The 16-electron complex is believed to act as a Lewis acid and activates the carbonyl compound. The reaction proceeds via the formation of hemiacetal.
In 2015, Leino's group introduced Fe(III) oxo acetate in conjunction with Et3SiH and Me3SiCl to form self-condensed ether from various aldehydes (Scheme 443).727 They also extended the etherification with alkoxy trimethylsilyl. The reaction possibly proceeds via initial hydrosilylation of one carbonyl molecule followed by the addition of another aldehyde in an SN2 fashion. The acetal is further reduced to generate the ether molecule.
7.2.5.1 Nitro reduction. In 1972, while studying the complexation of Fe3(CO)12 with p-nitrophenylbutadiene, Landesberg and co-workers obtained a complex with the corresponding amine instead (Scheme 444).728 Further, they found that the reduction is also possible for nitrobenzene with an equivalent amount of Fe3(CO)12. The source of hydrogen was explained to be from methanol solvent, whereas the oxygen atoms of the nitro group combined with the carbon-monoxide to liberate carbon-dioxide.
In 1975, during the optimization nitroarene reduction with H2, Knifton showed the potential of Fe(CO)3(PPh3)2 to catalyze the reaction;729 however, three decades later, Chaudhari's group reported ferrous sulphate heptahydrate as an efficient catalyst for the hydrogenation of nitroarenes (Scheme 445).730 The substrate–aqueous biphasic system in the presence of EDTANa2 was developed to provide the corresponding anilines at a TOF of up to 529 h−1 under 27.2 atm H2 pressure. Purposefully, Beller's group synthesized Fe2O3 nanoparticles deposited on nitrogen-doped graphene (Scheme 446).731 The size of the of the oxide particles was found to be 20–80 nm, which consist of smaller particles with a size of 2–5 nm. The heterogeneous catalyst was instrumental in carrying out the conversion of nitroarene to aniline under 50 bar H2 pressure in an H2O–THF solvent system.
Various carbonyl, ester, amide and heterocyclic substituents on the substrates remained untouched during the hydrogenation. The same group later employed the P(PhPPh2)3 ligand system to synthesize the corresponding iron(II)tetrafluoroborate complex (Scheme 447), which is an excellent catalyst for the hydrogenation of the –NO2 group.732 Mechanistically, it was proposed that first hydrogenation of the nitro group eliminates a water molecule to form nitrosoarene. Further hydrogenation of nitrosoarene gives hydroxylamine and then aniline. An alternate pathway is the formation azobenzene, which undergoes two-fold hydrogenation to give aniline.
The iron-catalyzed reduction of nitroarenes via hydrosilylation was reported in 2010 by Beller's group.733 FeBr2 in combination with PPh3 was found to be the best catalyst for this transformation in the presence of PhSiH3. Later, it was found that 1,1,3,3-tetramethyldisiloxane (TMDS) also serves the purpose of reduction using Fe(acac)3 as the catalyst. Various functional group tolerance was observed including cyano, ester and acid.734,735
Most interestingly, when dinitrobenzene was subjected to the reaction, only one of the nitro groups was reduced.
Hydrazine has also been used in many instances as a reductant for this transformation. Lu's group used resin-supported hydrazine hydrate in the presence iron oxide hydroxide as a catalyst.736 The reactions were also performed on the gram scale under reflux conditions in isopropanol to obtain more than 95% yield within minutes. It was proposed that Fe(III) is first reduced by hydrazine to Fe(II), which in turn is oxidised by the nitro group followed by hydrogen transfer. In contrast, Singh's group utilized iron(II) phthalocyanine (FePc) as the catalyst in combination with free hydrazine.737 Magnetically separable Fe3O4 nanoparticles were employed for the hydrogenation of nitroarenes with hydrazine (Scheme 448).738 However, nitroalkanes gave a very poor yield, which is attributed to the weaker co-ordination of the aliphatic nitro group to the NPs. Interestingly, the production of nitrogen and water as the only biproduct allowed the catalyst to be reused by simply washing with ethanol. Later, the in situ generation of Fe3O4 nanocrystals was realized by reducing Fe(acac)3 with hydrazine, which in turn catalyzed the nitro reduction.739 Iron-phenanthroline deposited over carbon powder synthesized via the pyrolysis of an Fe(OAc)2/1,10-phenanthroline mixture with Vulcan XC72R is an excellent effective catalyst also. In the presence of 4 equivalents hydrazine amine, a large number of aromatic and heterocyclic nitro compounds were reduced in more than 90% yield. γ-Fe2O3–polymer porous composites were also reported by Su's group to be effective towards the reduction with hydrazine.740
The first transfer hydrogenation of nitroarenes with IPA was obtained in the presence of γ-Fe2O3 (Scheme 449).741 In the presence of the strong base KOH, moderate yields were obtained under reflux conditions. The reaction is believed to proceed in a pathway similar to the Meerwin–Ponderof–Verley reaction. The catalyst-adsorbed alkoxide transfers the hydride to the nitro group in the MPV mechanism, while the proton is transferred from the catalyst surface. On the contrary, formic acid is the most suitable hydrogen transfer agent with Beller's Fe(BF4)2·6H2O/PP3 catalyst.742 However this reaction follows a similar mechanism proposed for the reduction with molecular hydrogen. In 2014, Thomas’ group realised this transformation using NaBH4 as the reducing agent in an Fe(OTf)3-catalyzed reaction (Scheme 450).743 The authors achieved a wide substrate scope, having an important advantage of room temperature reaction.
7.2.5.2 Azide reduction. The bis(imino)pyridineiron complex (iPrPDI)Fe(N2)2 [(iPrPDI = (2,6-iPr2C6H3N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)2C5H3N)] synthesized by Chirik's group was shown to be effective for the reduction of aryl azide to aniline.744 The authors proposed that the reaction proceeds via H2 addition across the Fe–N bond, then reductive elimination of –H and –NHAr finally provides the reduced product (Scheme 451).
CMe)2C5H3N)] synthesized by Chirik's group was shown to be effective for the reduction of aryl azide to aniline.744 The authors proposed that the reaction proceeds via H2 addition across the Fe–N bond, then reductive elimination of –H and –NHAr finally provides the reduced product (Scheme 451).
Dash's group utilized Fe3O4 nanoparticles in the presence of hydrazine as a reducing agent (Scheme 452).745 Various aryl azides and sulfonyl azides were transformed into amines using the catalyst in water medium. The reaction possibly starts with the oxidation of hydrazine to diimide, which transfers two hydrogens to the adjacent internal nitrogens in a concerted pathway. Subsequent hydrogen transfer from the second nitrogen eliminates N2 and gives the aniline.
7.2.5.3 Sulfoxide reduction. In 2011, Enthaler reported the iron-catalyzed reduction of sulfoxides to sulfides.746 Fe2(CO)9 was used as the catalyst in combination with polymethylhydrosiloxane or PhSiH3 as the reducing agent (Scheme 453). Various aryl sulfoxides including cyano and nitro-substituted sulfoxides and a few aliphatic sulfoxides undergo reduction to the corresponding sulfides. It is believed that the –SO group first co-ordinates to the metal center and gets activated. Subsequent interaction with the reductant provides the final product.
Royo's group synthesized an NHC substituted cyclopentadienyl-based iron(II) complex and utilized it for the reduction of sulfoxides (Scheme 454).747 Both aliphatic and aromatic sulfides were prepared in the presence of PhSiH3. The control experiments showed that the yield of the reaction is highly affected by radical trapping reagents, and thus a radical pathway was proposed.
In 2014, Barta and co-workers utilized Knölker's iron catalyst for the hydrogen borrowing coupling of aromatic and aliphatic amines with various alcohols (Scheme 457).750 The catalyst is believed to behave identically as in transfer hydrogenation.
The two hydrogens from the alcohol is first taken by the catalyst, one by the metal the other by cyclopentadienone, which are later transferred to the imine. Remarkably, the use of diols provides N-heterocycles of various sizes via consecutive intramolecular coupling. In a subsequent report, they also presented one-pot hetero dialkylation with two different alcohols based on their reactivity towards primary and secondary amines.751 They also showed that unprotected amino acids undergo N-alkylation with alcohols, although the yields are poor compared to the ruthenium catalyst counterpart.752
Knölker's catalyst was further employed by Sundararaju's group to obtain allylamines from allyl alcohols and various other unsaturated amines by Wills’ group.753,754
However, in all these reports, only primary alcohols were utilized, whereas secondary alcohols remained hardly explored probably due to their lower reactivity. Zhao and co-workers visualized that one explanation for this low reactivity is the slow imine formation with a ketone (Scheme 458).755 Thus, they employed a Lewis acid, specifically AgF, and consequently drastic enhancement of the reactivity of secondary alcohols was observed. The authors attributed this enhancement to the faster Lewis acid co-ordination-assisted imine formation pathway.
Darcel's group pioneered α-alkylation of ketones by benzyl alcohol derivatives using Knölker's-type complex (Scheme 459).756 Initially, the formation of the desired coupling product is accompanied with transfer hydrogenation from alcohol to ketone. Thorough optimization revealed that the use of the PPh3 ligand and Cs2CO3 base together with catalyst selectively produced α-alkylated ketones. This reaction follows a similar mechanism, where the alcohol transfers two hydrogens to the complex and undergoes aldol condensation with ketone and subsequent olefin reduction. The computational study revealed that enone reduction proceeds via the formation of enol followed by tautomerization.757 Fascinatingly, 2-amino benzylalcohol undergoes two-fold coupling with ketones and produces quinoline without getting reduced.
In 2017, utilizing the concept of borrowing hydrogen, Piersanti and co-workers performed C-3 benzylation of indoles with benzyl alcohol (Scheme 460).758 Iron–phthalocyanine was found to be the best catalyst in the presence of cesium carbonate base. The control experiments showed that the dehydrogenated alcohol condenses with indole, forming benzylideneindolenine, which in turn undergoes reduction to the desired product. In addition to benzaldehyde derivatives, different heterocyclic aldehydes are also compatible under the reaction conditions.
In 2004, Takahashi and co-workers developed the FeCl3-catalyzed dechlorination of arylchlorides with Grignard reagents.760 The authors proposed that in situ-generated [Fe(MgCl)2] reacts with Ar–Cl to produce [ArFeMgCl], which in turn forms [(Ar)(Bu)Fe(MgCl)2] with BuMgCl (Scheme 462). This intermediate is more prone towards reductive elimination and can only be diverted to β-hydride elimination by increasing the electron density on the metal center, which they achieved by electron-donating group substitution on the arene ring. Wangelin's group reoptimized the reaction and found that catalytic Fe(acac)3 in combination with tBuMgCl reduced this discrepancy to some extent.761 Both electron-withdrawing and donating and ortho-substituted aryl halides undergo dehalogenation. Although, C–I > C–Br > C–Cl selectivity was observed for the same substrate, individually all three types of bonds can be reduced when present in different substrates. Interestingly, a few alkyl bromides also produced debromination under the reaction conditions. Later, this strategy was extended to the mono dehalogenation of 2,2-dibromocyclopropylarenes and 2,2-dichlorocyclopropylarenes (Scheme 463).762 The diastereoselectivities of the reactions were moderate; however, no ring-opening product was obtained.
Recently, Rasappan's group demonstrated the dehalogenation of aromatic and aliphatic halides with PhSiH3 in the presence of FeCl3 catalyst (Scheme 464).763 Mechanistically, PhSiH3 promotes the formation of metal hydride species to which R–X oxidatively adds to form a metal-alkyl-hydride intermediate. Then, reductive elimination of –R and –H provides the desired product.
7.3 Iron-catalyzed hydrosilylation reactions
The first instance of iron-catalyzed hydrosilylation of alkenes was introduced by Nesmeyanov et al. in 1962.766 The reaction was performed using [Fe(CO)5] or colloidal iron as the catalyst. A mixture of alkylsilanes (hydrosilylation reaction product) and vinylsilanes (dehydrogenative silylation product) was obtained depending on the amount of trialkylsilanes used. Notably, the reaction of ethylene with 0.2 equiv. of triethylsilane specifically produced the triethylvinylsilane in 92% yield, whereas, 3 equiv. of triethylsilane resulted in the formation of tetraethylsilane with 79% yield (Scheme 465).
Further, the formation of alkanes, alkylsilanes and alkenylsilanes upon the photoirradiation of Fe(CO)5 in the presence of alkene and trialkylsilanes was reported by Schroeder et al.767
In 1993, Murai and co-workers achieved the selective dehydrogenative hydrosilylation of styrenes with triethylsilane by using Fe3(CO)12 as the catalyst.768 The corresponding (E)-β-(triethylsilyl)styrene derivatives were obtained in high yields with complete selectivity. The complexes bearing multivinylsilicon ligands efficiently catalyzed the hydrosilylation and dehydrogenative silylation of styrene with trisubstituted silanes and hydrosiloxanes (Scheme 466).766,769
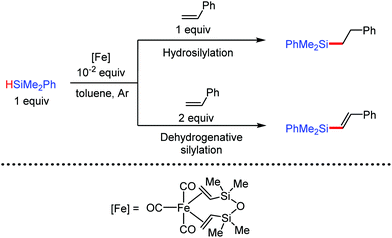 | ||
| Scheme 466 Hydrosilylation and dehydrogenative silylation of styrene using multivinylsilicon ligand-bearing iron complex. | ||
In 2004, Chirik and co-workers synthesized and characterized diiminopyridineiron(0) dinitrogen complexes, which catalyze the hydrosilylation of alkenes and alkynes. Both bis(nitrogen), (i-PrPDI)Fe(N2)2 (i-PrPDI = ((2,6-CHMe2)2C6H3N = CMe)2C5H3N), and its mono(dinitrogen) analogue, (i-PrPDI)Fe(N2), were found to catalyze the hydrosilylation of terminal and cyclic alkenes, giving the anti-Markovnikov product exclusively with high TOFs (TOF = turn over frequency) (Scheme 467).770–772 Also, the reactions were efficiently performed with a low catalyst loading (≤0.3 mol%) in a non-polar solvent at ambient temperature. The terminal alkenes react most rapidly followed by the gem-disubstituted olefins and other internal olefins. Moreover, in this reaction, phenyl silane is added to diphenylacetylene in the presence of the complexes, resulting in the selective formation of the corresponding ‘E’-vinyl silane. Notably, the isolated intermediate iron(0) bis(silane) complex, (i-PrPDI)Fe(η2-SiH3Ph)2, was identified as a competent catalyst for the hydrosilylation of 1-hexene. Further, in comparison to the methyl-substituted precursor, (i-PrPDI)Fe(N2)2, the phenyl-substituted complex (i-PrPhPDI)Fe(N2)2 showed superior catalytic activity towards the hydrosilylation of 1-hexene. On the other hand, the reverse is true for hindered substrates such as cyclohexene and (+)-(R)-limonene.772
Recently in 2017, Lam et al. elucidated the mechanism of the bis(imino)pyridine iron complex ((PDI)Fe)-catalyzed hydrosilylation of olefins using DFT calculations (Scheme 468).773 A mechanism similar to the Chalk–Harrod mechanism774 was proposed. The DFT calculation also supported the formation of the regioselective anti-Markovnikov product based on the requirement of a lower activation energy for anti-Markovnikov migration compared to Markovnikov migration. The mechanism initiates with the co-ordination of the olefin to active catalytic species A to form complex B. This is followed by co-ordination of hydrosilane to complex B to generate C. Most importantly, in contrast to the actual Chalk–Harrod mechanism, direct anti-Markovnikov hydride migration occurs from the co-ordinated silane into the Fe-bound olefin without early oxidation addition of Si–H at the metal centre. Thereafter, Si–C reductive elimination of (PDI)Fe(alkyl)(SiR3) (D) produces the desired alkylsilane with the concomitant regeneration of the active catalytic species. It is worth mentioning that the iron center can adopt a variety of spin states due to the presence of the redox non-innocent bis(imino)pyridine (PDI) ligand. Consequently, a variation occurs in the electronic configuration in the complexes involved in the mechanism.
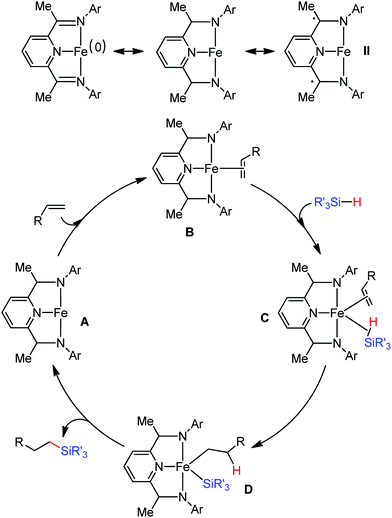 | ||
| Scheme 468 Plausible mechanism of hydrosilylation of alkenes using bis(imino)pyridine iron catalyst. | ||
In 2013, Thomas and co-workers reported an efficient iron-catalyzed procedure for the hydrosilylation of alkenes and alkynes using an FeCl2 precatalyst and bis(imino)pyridine ligand L1 in the presence of ethylmagnesium bromide (Scheme 469).775 The in situ-generated active iron catalyst affords the hydrosilylation products in excellent yields with high chemo, regio and stereoselectivity. A wide variety of styrene derivatives containing electron-withdrawing and electron-donating groups undergo this reaction. Various functional groups such as aniline, ester, ketone, nitrile and aldimine are well tolerated under the reaction conditions. Notably, hydrosilylation of internal alkynes gives (E)-vinylsilanes in high yield via the syn-addition of Si–H. However, the trans-addition of the Si–H bond leads to the formation of (Z)-vinylsilanes from the terminal alkynes.
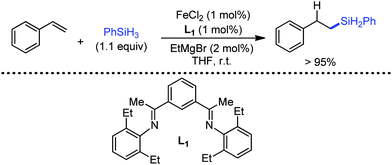 | ||
| Scheme 469 Iron-catalyzed hydrosilylation of alkene with bis(imino)pyridine ligand-containing complex. | ||
Sterically hindered tertiary silanes were added to terminal alkenes using bis(imino)pyridine(dinitrogen) iron complexes, [(EtPDI)Fe(N2)]2(μ2-N2) and [(MePDI)Fe(N2)]2(μ2-N2) (Scheme 470).776 Hydrosilylation occurred very efficiently under mild reaction conditions using very low catalyst loadings (∼0.004–0.05 mol%). The anti-Markovnikov hydrosilylation products were obtained regioselectively with high yields.
In 2016, Thomas and co-workers introduced a very efficient procedure for the hydrosilylation of alkenes and alkynes using an air-and moisture-stable iron(II) pre-catalyst, [EtBIPFe(OTf)2] (EtBip = 2,6-bis[1-(2,6-diethylphenyliminoethyl]pyridine)), in the presence of iPr2NEt as the activator for the catalyst.777 A wide range of aliphatic and aromatic terminal alkenes undergo efficient anti-Markovnikov hydrosilylation to give the desired linear silanes in excellent yields with complete regioselectivity (Scheme 471a). Hydrosilylation occurs chemoselectively at the terminal alkenes in the presence of various functional groups such as ketone, ester and imine. Also, terminal alkynes afford the (Z)-vinylsilanes with excellent selectivity under the reaction conditions (Scheme 471b). Notably, the practicability of the reaction was proven by the completion of the gram-scale hydrosilylation of 1-octene in 10 min using a very low catalyst loading of 0.25 mol% under an air atmosphere. Importantly, the catalytic system is equally effective in an air and inert atmosphere.
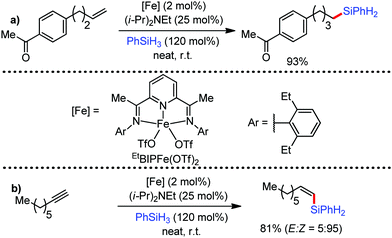 | ||
| Scheme 471 Iron-catalyzed hydrosilylation of alkenes and alkynes in the presence of air- and moisture-stable iron complex. | ||
The monohydrosilylation of the C4 alkene of 1,2,4-trivinylcyclohexane (TVCH) with tertiary alkoxysilanes was achieved under the catalytic activity of various aryl-substituted bis(imino)pyridine iron dinitrogen complexes.778 Notably, the hydrosilylation reaction is utilized for the manufacture of tires having a low rolling resistance. Among the iron complexes, (iPrPDI)Fe(N2)2 and (4-Me2N-iPrPDI)Fe(N2)2 (4-Me2N-iPrPDI = 2,6-(2,6-iPr2–C6H3N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe)2-4-Me2N–C5H2N) showed the highest selectivity (>98%) towards monohydrosilylation using MD′M (MD′M = (Me3SiO)2MeSiH) at the desired 4-vinyl position of the substrates (Scheme 472). Notably, the bis(imino)pyridine iron dinitrogen complexes exhibited better catalytic efficiency compared to the commercially utilized platinum catalysts.779
CMe)2-4-Me2N–C5H2N) showed the highest selectivity (>98%) towards monohydrosilylation using MD′M (MD′M = (Me3SiO)2MeSiH) at the desired 4-vinyl position of the substrates (Scheme 472). Notably, the bis(imino)pyridine iron dinitrogen complexes exhibited better catalytic efficiency compared to the commercially utilized platinum catalysts.779
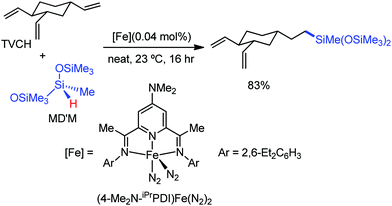 | ||
| Scheme 472 Selective hydrosilylation of 1,2,4-trivinylcyclohexane (TVCH) in the presence of bis(imino)pyridine iron complexes. | ||
Nakazawa and co-workers reported the synthesis of a number of iron complexes containing substituted terpyridine ligands. Some of these complexes exhibited catalytic activity in the hydrosilylation of alkenes in the presence of NaHBEt3 as the activator of the catalyst. The unsymmetrically disubstituted terpyridine complexes showed high efficiency with turnover numbers of up to 1533 for the hydrosilylation of 1-octene and 1-hexene using diphenylsilane, phenylsilane and methylphenylsilane. Selective single- and double-hydrosilylation of the alkenes occurs depending on the amount of catalyst used (Scheme 473).780
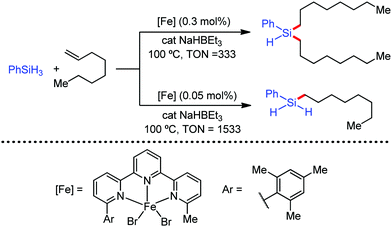 | ||
| Scheme 473 Hydrosilylation of 1-octene in the presence of iron complexes containing substituted terpyridine ligands. | ||
Around the same time, the Chirik group established the catalytic efficiency of a 2,2′:6′,2′′-terpyridine iron dialkyl complex, (terpy)Fe(CH2SiMe3)2, for the hydrosilylation of 1-octene with tertiary hydrosilanes (Scheme 474).781 In addition, complete hydrosilylation (>95%) of vinylcyclohexene oxide (VCHO) was achieved using (terpy)Fe(CH2SiMe3)2 as the catalyst to give the synthetically important anti-Markovnikov product with the oxirane moiety remaining intact. However, the conversion was unsuccessful using bis(imino)pyridine iron complexes and a platinum catalyst.771
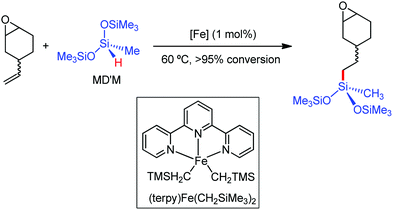 | ||
| Scheme 474 Hydrosilylation of vinylcyclohexene oxide (VCHO) in the presence of (terpy)Fe(CH2SiMe3)2 as the catalyst. | ||
Electron-donating phosphinite-iminopyridine (PNN) ligand-bearing pincer iron complexes were prepared and characterized by Huang and co-workers in 2013.782 These iron complexes on treatment with NaHBEt3, catalyze the hydrosilylation of a wide range of alkenes using alkylsilanes such as PhSiH3 and Ph2SiH2. Complex C1 performs most efficiently for the hydrosilylation of various alkenes with good functional group tolerance (Scheme 475). High yields of anti-Markovnikov products were obtained by the reaction conditions under the catalysis of this complex. Significantly, the hydrosilylation methodology was applied for the synthesis of the insecticide silafluofen.
In 2016, Nakazawa and co-workers reported the synthesis of iminobipyridine iron(II) complexes A–D and demonstrated their catalytic efficiency towards the hydrosilylation of alkenes by silanes such as PhSiH3, Ph2SiH2, PhMeSiH2 and Ph2MeSiH in the presence of NaBHEt3 as the activating agent.783 Hydrosilylation of 1-octene with Ph2SiH2 occurred very efficiently in the presence of a catalytic amount (0.008 mol%) of complex B to give Ph2(octyl)SiH in 96% yield with a TON as high as 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 038 (Scheme 476). Notably, the iminobipyridine complexes with NaBHEt3 resulted in the formation of dialkylated and trialkylated silanes together with monoalkylated silanes as the hydrosilylation product. Further, ketimine-type iminobipyridine (BPI) iron complexes were also synthesized, which exhibited high catalytic activity in the hydrosilylation reaction of alkenes using primary, secondary and tertiary silanes to afford the corresponding monoalkylated and dialkylated silanes.784 Among the complexes, (HBPIMes,Me)FeBr2 (E) showed the highest TON of 42
038 (Scheme 476). Notably, the iminobipyridine complexes with NaBHEt3 resulted in the formation of dialkylated and trialkylated silanes together with monoalkylated silanes as the hydrosilylation product. Further, ketimine-type iminobipyridine (BPI) iron complexes were also synthesized, which exhibited high catalytic activity in the hydrosilylation reaction of alkenes using primary, secondary and tertiary silanes to afford the corresponding monoalkylated and dialkylated silanes.784 Among the complexes, (HBPIMes,Me)FeBr2 (E) showed the highest TON of 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the iron-catalyzed hydrosilylation of 1-octene with Ph2SiH2 using only 0.001 mol% catalyst loading (A–D, E in Scheme 476). However, analogous to the aldimine-type iminobipyridine, these complexes also need NaBHEt3 for activation.
000 for the iron-catalyzed hydrosilylation of 1-octene with Ph2SiH2 using only 0.001 mol% catalyst loading (A–D, E in Scheme 476). However, analogous to the aldimine-type iminobipyridine, these complexes also need NaBHEt3 for activation.
In 2015, Lu and co-workers reported the first enantioselective iron-catalyzed asymmetric hydrosilylation reaction of 1,1-disubstituted aryl alkenes to give the anti-Markovnikov product using iron complexes bearing iminopyridineoxazoline ligands.785 High yields of hydrosilylation products were obtained with high enantioselectivity in the presence of NaBHEt3 as the catalyst activator. Chiral oxazoline-substituted iminopyridine iron complex C2 gave the best result on activation with NaBHEt3 (Scheme 477). The electron-rich and electron-deficient α-alkylstyrenes undergo the reaction with moderate chemoselectivity to give good to excellent yields of products. Although, fluoro, methoxy, trifluoromethyl, chloro and ketimine groups are tolerated, functional groups such as ketone, aldehyde, ester and alcohols react with silanes.
Recently in 2018, they introduced an iron-catalyzed enantioselective hydrosilylation procedure for terminal aliphatic alkenes, resulting in the formation of Markovnikov addition products selectively. This reaction was conducted in the presence of a catalytic amount of chiral oxazolineiminopyridine (OIP) iron complex C3 together with sodium tert-butoxide as the activator (Scheme 478).786 A wide range of aliphatic alkenes gave high yields of the branched product with high regioselectivity (branched/linear: >97/3) and high enantioselectivity (>99% ee). The reaction condition tolerates various functional groups such as ether, halide, silyl, protected alcohol, ester, amide, acetal and amine. Further, heterocycles such as 2-naphthalene, pyridine, indole and furan are also tolerated.
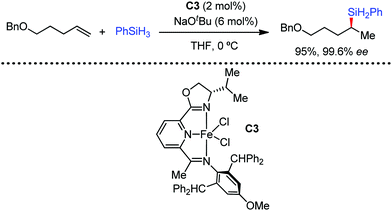 | ||
| Scheme 478 Markovnikov hydrosilylation of olefins in the presence of chiral oxazolineiminopyridine (OIP) iron complex. | ||
Iron complexes bearing 2,9-diaryl-1,10-phenanthroline ligands have been found to exhibit efficient catalytic activity for the hydrosilylation of internal and terminal alkenes with high selectivities. Zhu and co-workers synthesized these complexes and demonstrated their reactivity in the hydrosilylation of alkenes.787 Iron complex C4 catalyzes the reaction of internal alkenes in the presence of EtMgBr as a reducing agent to afford the benzylic hydrosilylation product selectivity in moderate to high yields (Scheme 479). Various β-methyl styrenes form high yield of the corresponding branched silanes. The reaction conditions tolerate various functional groups on β-methyl styrenes. In addition, these catalysts show high Markovnikov selectivity for the hydrosilylation of terminal alkenes such as terminal styrenes and also for 1,3-dienes. The mechanistic studies were performed through deuteration experiment studies, kinetic isotopic effect experiments and density functional theory (DFT) calculations. Based on the mechanism, they rationalized the high benzylic selectivity for the hydrosilylation of alkenes by the π–π interaction between the phenanthroline of the ligand and the phenyl group of the alkene.
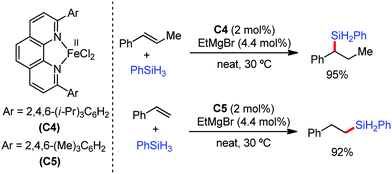 | ||
| Scheme 479 Hydrosilylation of terminal and internal alkenes in the presence of 1,10-phenanthroline ligand-bearing iron complexes. | ||
In 2016, Nagashima and co-workers used the combination of Fe(OPv)2 (Pv = pivolyl) and 1-adamantyl isocyanide (CNAd) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio for the hydrosilylation of styrenes with PMDS (PMDS = 1,1,3,3,3-pentamethyldisiloxane). This reaction afforded the corresponding anti-Markovnikov products in high yields.788
2 ratio for the hydrosilylation of styrenes with PMDS (PMDS = 1,1,3,3,3-pentamethyldisiloxane). This reaction afforded the corresponding anti-Markovnikov products in high yields.788
In 2010, Ritter and coworkers synthesized well-defined bis(aryl) iron(II) precatalyst C6 and used it in the 1,4-hydrosilylation of 1,3-dienes by triethoxysilane to give the corresponding linear allylsilanes predominantly (Scheme 480).789 The active catalyst formed in situ by using iminopyridine ligand L controls the high regioselectivity towards the predominant formation of the linear products (linear/branched: >94/6). Additionally, the allylsilanes were obtained with high stereoselectivity (E/Z ratio >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
The hydrosilylation of alkynes to vinylsilanes in the presence of Fe2(CO)9 and tributylphosphine was reported by Enthlaler and co-workers in 2011.790 A number of substituted diphenylacetylenes were hydrosilylated by (EtO)3SiH to afford high yields of (Z)-stilbenes with excellent selectivity at the alkynes in the presence of various functional groups such as aldehydes, esters, amides, epoxide and alkenes.791
Plietker and co-workers reported the synthesis and use of the iron hydride complex Fe(H)(CO)(NO)(Ph3P)2 in the stereodivergent hydrosilylation of internal alkynes.792 Most importantly, the iron catalyst together with triethylamine additives afforded either the E- or Z-selective hydrosilylation product depending on the nature of the silane. The stereoselectivity is directed by the steric demand. The sterically hindered silane PhMe(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)SiH resulted in the selective formation of ‘E’-vinylsilanes. Contradictorily, the less hindered silane PhSiH3 generated the ‘Z’-vinylsilanes selectively in high yields. However, the selectivity is also dependent on the substituents on the alkynes (Scheme 481). Although the diaryl and aryl/alkyl acetylenes give either the ‘Z’- or ‘E’-vinylsilanes according to the used silanes, the dialkyl acetylenes affords the ‘E’-configured vinylsilanes independent of the used silanes.
CH)SiH resulted in the selective formation of ‘E’-vinylsilanes. Contradictorily, the less hindered silane PhSiH3 generated the ‘Z’-vinylsilanes selectively in high yields. However, the selectivity is also dependent on the substituents on the alkynes (Scheme 481). Although the diaryl and aryl/alkyl acetylenes give either the ‘Z’- or ‘E’-vinylsilanes according to the used silanes, the dialkyl acetylenes affords the ‘E’-configured vinylsilanes independent of the used silanes.
The early instance of iron-catalyzed hydrosilylation was shown by Brunner and Fisch in 1990.792 The corresponding silylated ether was obtained from the reaction of acetophenone and diphenylsilane in the presence of a catalytic amount of the iron complex [Fe(Cp)(CO)(X)(L)] (X = COMe, Me; L = (S)-(+)-Ph2P[N(Me)CH(Me)Ph]) (Scheme 482). Notably, silylated enol ethers are not formed in this reaction.
Later in 2007, Beller and co-workers employed Fe(OAc)2 in combination with the tricyclohexylphosphine (PCy3) ligand for the hydrosilylation of aldehydes with polymethylhydrosliloxane (PMHS) (Scheme 483).793 Basically, the aldehydes are converted to the corresponding silylether intermediates followed by basic workup to form the corresponding alcohols. Various substituted benzaldehydes are reduced chemoselectively in the presence of functional groups such as ester, cyano, chloro, bromo, nitro, fluoro, and o-benzyl to give the corresponding benzyl alcohols in high yields. Heteroaryl aldehydes and aliphatic α and β-unsaturated aldehydes are also hydrosilylated in moderate to high yields. Again, this procedure is also used for the hydrosilylation of ketones using PMHS.794
The substituted arylalkyl ketones give good to excellent yields (81–96%) of benzyl alcohols, whereas dialkyl ketones give moderate yields (50–87%) of corresponding alcohols. Further, the enantioselective reduction of ketones is achieved in the presence of Fe(OAc)2 and the chiral diphosphine ligand, (S,S)-Me-DuPhos (Scheme 484). The aryl ketones are reduced efficiently to give the corresponding alcohols with high enantioselectivity (up to 99%).795
On the other hand, Nishiyama and co-workers reported the hydrosilylation of ketones with (EtO)2MeSiH using Fe(OAc)2 in combination with nitrogen-based ligands such as N,N,N′,N′-tetramethylethylene diamine (TMEDA), bis(oxazolinyl)pyridine (pybox-dm) and bis-tert-butyl-bipyridiene (bipy-tb) (Scheme 485). This methodology provides moderate to excellent yields (50–90%) of the corresponding alcohols.796 Also, completely selective reduction of aromatic and aliphatic ketones with (EtO)2MeSiH is achieved by employing Fe(OAc)2 together with sodium thiophene-2-carboxylate (STC) (Scheme 485).797
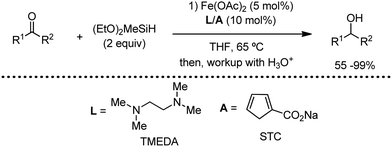 | ||
| Scheme 485 Iron-catalyzed hydrosilylation of ketones in the presence Fe(OAc)2 with TMEDA ligand or STC as an additive. | ||
The iron catalyst obtained from the Fe(OAc)2 precatalyst and chiral bis(oxazolinylphenyl)amine (Bopa) ligands was very efficient in the asymmetric hydrosilylation of phenyl ketones with high enantioselectivity.796,798 The corresponding ‘R’-configured alcohols were obtained with enantioselectivity of up to 88% ee using the iron Bopa-dpm (L) catalyst. In contrast, the ‘S’-configured alcohols were provided with high enantioselectivity (up to 95% ee) by the (S,S)-Bopa/FeCl2 (C6) complexes in the presence of zinc powder as the additive (Scheme 486).799
In 2011, Theil and co-workers reported the iron-catalyzed hydrosilylation of aldehydes and ketones with PMHS using the catalyst precursors prepared from the combination of N1-alkylated 2-(3(5)-pyrazolylpyridine) ligands and iron octanoate [Fe(O2C8H5)2] (Scheme 487).800 The practicability of this catalytic system was demonstrated by the efficient reduction of aromatic aldehydes and ketones, and aliphatic ketones with excellent yields of up to 99%.
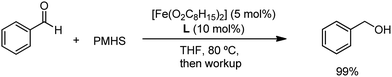 | ||
| Scheme 487 Iron-catalyzed hydrosilylation of aldehydes and ketones using iron octanoate together with N1-alkylated 2-(3(5)-pyrazolylpyridine) ligands. | ||
Around the same time, Plietker and co-workers used Bu4N[Fe(CO)3(NO)] (TBAFe) together with the PCy3 ligand for the hydrosilylation of aldehydes and ketones to give moderate to high yields of the corresponding alcohols.801,802 Notably, the reactions occur with low catalyst loadings (1.0–2.5 mol%) (Scheme 488).
In 2010, Tilley and co-workers utilized the low-valent, cost-effective, highly active catalyst [Fe{N(SiMe3)2}2] for the hydrosilylation of ketones and aldehydes at 23 °C (Scheme 489).803 Various functional groups such as ether, cyclopropyl, olefin bromoaryl, dimethylamino and nitrile groups are well tolerated under the reaction conditions.
Further, in 2013, Turenlet and co-workers reported the synthesis and utilization of N-phosphinoamidinate ligand-containing iron(II) bis(trimethylsilyl)amide complex C7 as a precatalyst in the room temperature hydrosilylation of carbonyl compounds with phenylsilane (Scheme 490).804 This catalytic system is very efficient in reducing a wide range of carbonyl compounds including substituted ketones, enones, and esters. The reaction occurs in the presence of a very low catalyst concentration (0.01–1.0 mol% Fe) with only one equiv. of phenylsilane relative to the carbonyl compound. Togni and co-workers performed the hydrosilylative reduction of acetophenone with phenylsilane using a pentacoordinated cyclopentadienyl-containing stereogenic diamine ligand (L) together with the iron precursor Fe(OAc)2. Although high conversion is provided by the catalytic system, the enantioselectivity is low (37%).805
In 2008, Chirik and co-workers developed catalytically efficient bis(imino)pyridine (PDI) iron complexes for the hydrosilylation of aldehydes and ketones with PhSiH3 and Ph2SiH2 (Scheme 491).806 The complex (iPrPDI)Fe(N2)2 (C8) was found to catalyze the hydrosilylation of p-tolualdehyde and acetophenone to give the corresponding silyl ethers in less than one hour. Further, complexes C9 and C10 are highly efficient in the hydrosilylative reduction of a broad range of aromatic ketones and aliphatic ketones. Notably, the α,β-unsaturated ketones are chemoselectively reduced to the corresponding unsaturated alcohols.
In 2011, Guan and co-workers synthesized the phosphonite-based pincer ligand (POCOP)-bearing iron–hydride complex [2,6-(iPr2PO)2C6H3]Fe(H)(PMe3)2 (C11) and applied it in the hydrosilylation of aldehydes and ketones (Scheme 492).807 A wide range of carbonyls are efficiently reduced by (EtO)3SiH in the presence of a catalytic amount of the iron–hydride complex. The corresponding alcohols are obtained in high yields without affecting the substituted functional groups such as –F, Me2N–, MeO–, –NH2 and pyridyl groups. From a mechanistic point of view, they suggested that the active catalytic species is generated by the dissociation of PMe3 of CO in the initial step. Further, the cationic iron POCOP-pincer complexes C12 and C13 show superior catalytic efficacy compared to their hydride counterparts.808,809
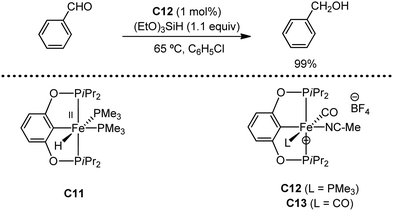 | ||
| Scheme 492 Iron-catalyzed hydrosilylation of aldehydes and ketones in the presence of iron POCOP-pincer complex. | ||
In 2014, Sun and co-workers reported the synthesis of tridentate bis(phosphine)silyl [PSiP] ligand-containing hydrido iron(II) complex C14 and used it for the hydrosilylation of aldehydes and ketones.810 Excellent catalytic efficiency was shown by the complex, giving 100% yield of benzyl alcohol in the reduction of benzaldehydes with (EtO)3SiH in the presence of 1 mol% of catalyst (Scheme 493). Further, diphosphinito PCP ligand-based iron complexes C15 and C16 demonstrated catalytic efficiency towards the hydrosilylation of ketones and aldehydes.811,812 Complex C15 showed slightly higher activity compared to C14. High yields of corresponding alcohols were obtained by reducing ketones with (EtO)3SiH using only 0.3% catalyst loading. In 2016, iron complexes with a similar structure such as (2,6-bis(di-tert-butyl-phosphinomethyl)pyridine) [(t-BuPNP)FeCl2] (C17) and (2,6-bis(di-tert-butyl-phosphinito)pyridine) [(t-BuPONOP)FeCl2] (C18) were synthesized by Findlater and co-workers. These complexes exhibited higher activities for the room temperature hydrosilylation of ketones and aldehydes with (EtO)3SiH.813
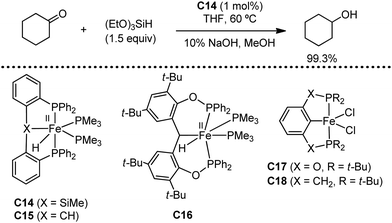 | ||
| Scheme 493 Hydrosilylation of ketones and aldehydes in the presence of various iron-pincer complexes. | ||
In 2014, Driess and co-workers synthesized a new bis(silyl)pyridine pincer-type [SiNSi] ligand-containing iron(0) complex, [SiNSi]Fe(PMe)2 (C19) (Scheme 494), and demonstrated it as an efficient catalyst for the hydrosilylation of ketones.814 Further, through combined experimental and theoretical studies, they revealed that the activation of carbonyl species and silane occurs at the ligand sphere (silyl ligand) instead of the direct involvement of the iron(II) site.815 Significantly, all the processes throughout the reduction, that is activation of the reactant, bond forming, and bond breaking, occur at the periphery of the iron centre.
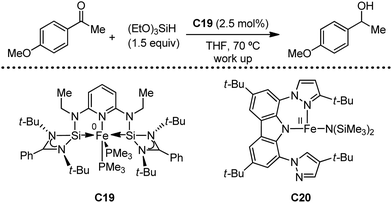 | ||
| Scheme 494 Iron-catalyzed hydrosilylation of aldehydes and ketones by bis(silyl)pyridine pincer-type iron complex and NNN-pincer ligand-bearing iron complex. | ||
Lee and co-workers utilized an NNN-pincer ligand-bearing low co-ordinate iron(II) complex (C20) (Scheme 494) for the hydrosilylation of carbonyl compounds with PhSiH3 at room temperature.816 In 2008, Gade and co-workers reported the synthesis of the chiral bis(pyridylimino)isoindole (bpi) pincer ligand-containing iron complex [Fe(tertapheny-carbpi)(OAc)] (C21) and achieved the asymmetric hydrosilylation of ketones under its catalytic activity.817 The ketones are hydrosilylated using PMHS or (EtO)2SiH followed by basic workup to give the corresponding alcohols with stereoselectivities of up to 93% ee (Scheme 495).
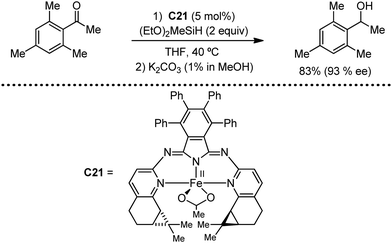 | ||
| Scheme 495 Iron-catalyzed asymmetric hydrosilylation of ketones to alcohol using chiral bpi-pincer iron complex. | ||
Further, the asymmetric hydrosilylation of ketones with PhSiH3 was performed by Chirik and co-workers using the enantiopure pyridine bis(oxazoline)iron complex (iPrPybox)Fe(CH2SiMe3)2 (C22) together with B(C6H5)3 as an activator in diethyl ether.818 This catalytic system provides the corresponding alcohols with moderate enantioselectivity up to 54% ee. Notably the bis-oxazoline iron complex (iPrBox)Fe(CH2SiMe3)2 also shows similar reactivity (Scheme 496). In 2010, Nishiyama and co-workers utilized a similar bis(oxazolinyl)-phenyl ligated iron complex, (S,S)-(Phebox-ip)FeBr(CO)2 (C23), together with sodium acetate for the asymmetric hydrosilylation of 4-phenylacetophenone with (EtO)2MeSiH as the hydride source. A high yield (99%) of R alcohol was obtained with enantioselectivity of 66% ee under the reaction conditions (Scheme 496).819 On the other hand, the complex (Phebox-ip)Fe(CO)2(SiMe3) (C24) alone gave the ‘R’-alcohol with 32% ee under similar reaction conditions with toluene as the solvent.820
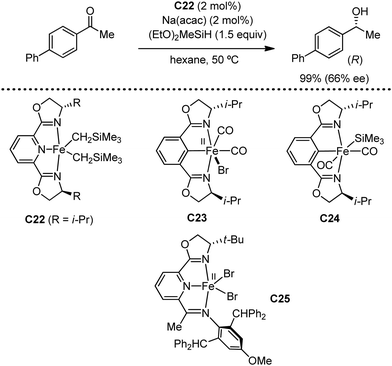 | ||
| Scheme 496 Asymmetric hydrosilylation of aldehydes and ketones in the presence of chiral iron complexes. | ||
In 2015, Huang and co-workers synthesized unsymmetrical iminopyridine-oxazoline (IPO) iron complex C25 having a structure similar to the pybox ligand with one arm modified.821 They utilized this chiral complex together with NaBHEt3 for the asymmetric hydrosilylation of ketones. Various aryl-alkyl ketones and alkyl ketones were hydrosilylated stereoselectively, giving the desired alcohols in high yields (up to 99%) with up to 93% ee.
Around the same time, Gade and co-workers applied chiral boxmi (boxmi = bis(oxazolinylmethylidene)isoindoline) NNN-pincer ligated iron alkoxide complex C26 for the asymmetric hydrosilylation of ketones with (EtO)2MeSiH (Scheme 497).822 This complex was highly efficient as a catalyst to give high yield of the corresponding alcohols with enantioselectivity as high as 99% ee. Notably, a TOF (turnover frequency) of 240 h−1 at −40 °C was shown by the catalyst.
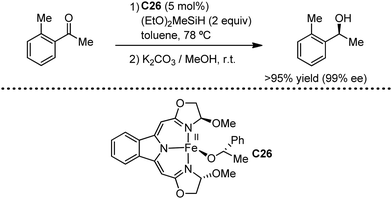 | ||
| Scheme 497 Iron-catalyzed asymmetric hydrosilylation of ketones in the presence of chiral boxmi pincer iron alkoxide complex. | ||
Mechanistically, active alkoxide complex I undergoes σ-bond metathesis with the silane in the initial step to form iron hydrido complex II together with the generation of silyl ether (Scheme 498).823 Next, species II undergoes co-ordination to ketone. This is followed by the subsequent insertion of the ketone to the Fe–H bond to regenerate active complex I. Notably, this step is the stereoselectivity-determining step of the reaction.
After the initial use of piano stool iron complexes in the hydrosilylation of ketone by Brunner in 1990,792 Nikonov and Vyboishchikov performed the hydrosilylation of benzaldehyde with PhMeSiH2 in the presence of dihydride silyl complex C28 (Scheme 499).824 This complex was prepared from cationic phosphine complex C27. Notably, complex C27 also catalyzes the hydrosilylation of benzaldehyde using PhSiH3 as the hydride donor.
A series of phosphine-containing cyclopentadienyl iron carbonyl complexes (C29–C33) was utilized by Darcel and co-workers for the hydrosilylation of aldehydes and ketones.825 The cationic complexes with PF6 as the counter anion (C29–C31) under irradiation of visible light gave better results towards the hydrosilylation of aldehydes using PMHS of phenylsilane as the hydride source (Scheme 500). On the other hand, the hydrosilylation of ketones was successfully performed using neutral complex C33. Moderate to high yields of corresponding alcohols were obtained from both processes.
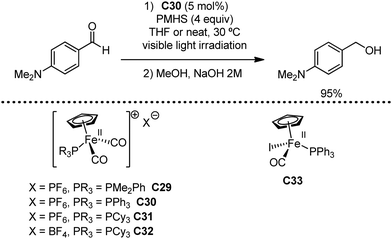 | ||
| Scheme 500 Hydrosilylation of aldehydes in the presence of piano stool iron complexes prepared by Darcel and co-workers. | ||
Further, N-heterocyclic carbene (NHC)-bearing cyclopentadienyl stool complexes were applied as suitable catalysts for the hydrosilylation of ketones and aldehydes.826 In 2010, Royo and co-workers reported the hydrosilylation of aldehydes with (EtO)2MeSiH under the catalytic activity of iron complex C34, bearing a chelating NHC-cyclopentadienyl ligand (Scheme 501).827 Thereafter, in 2011, Darcel and co-workers used the NHC-containing piano stool iron complex [CpFe(CO)2(IMes)]+I− (C35) for the catalytic hydrosilylation of aldehydes of aldehydes and ketones.828 Complex C36 was prepared by photoirradiation of C35 by visible light (Scheme 501). Aldehydes and ketones were reduced efficiently with PhSiH3 in the presence of complex C35 under visible light irradiation. On the other hand, no such activation was necessary for the catalytic hydrosilylation with complex C36. The corresponding alcohols were obtained in moderate to high yields. Furthermore, the conversion rate and yield of the reaction were found to increase on performing the reaction in solvent-free conditions.829 Also, a lower temperature (50 °C) is required compared to the temperature in toluene (70 °C).
Cationic cyclopentadienyl piano-stool complex C37 bearing 1,3-disubstituted imidazolidin-2-ylidene as the NHC ligand was exploited as a suitable catalyst for the hydrosilylation of aldehydes and ketones with phenylsilane under neat conditions (Scheme 502).830 The aldehydes were reduced very efficiently at 100 °C giving the full conversion in much less time (5 min to 1 h). However, the full conversion of ketones to their corresponding alcohols took slightly more time (4 h). Around the same time, zwitterionic piano-stool NHC–iron complexes were employed for the hydrosilylation of aldehydes and ketones.831 The complex having an NHC ligand with a malonate backbone, C38, was the most efficient complex among the complexes to catalyze the reduction of aromatic aldehydes in the presence of visible light irradiation by diphenylsilane with high chemoselectivity (Scheme 502). Similar visible light-activated catalytic activities were also shown by piano-stool-structured iron complexes bearing benzamidazole (e.g.C39) and imidazole (e.g.C40)-derived NHC ligands (Scheme 502).832 The activity of the benzamidazole-derived NHC iron complexes was more efficient compared to the imidazole-based analogues towards the hydrosilylation of benzaldehyde and acetophenone with phenylsilane. Substituents such as bromo, nitro, nitrile, dimethylamino, methoxy and methane on the aldehyde substrates were well tolerated under this reaction condition. Furthermore, piano-stool complexes bearing monodentate triazolylidene ligands such as C41 were developed and used by Albrecht and co-workers as active catalysts in the hydrosilylation of aldehydes and ketones (Scheme 502).833 Very high efficiency was exhibited by complex C41 in the hydrosilylation of aldehydes with phenylsilane, giving a high TOF of up to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 h−1 using a very low amount of catalyst (0.1 mol%) in 1,2-dichloromethane solvent. However, the hydrosilylation reaction rate for ketones is lower than that for aldehydes. It is noteworthy that an induction period of 20–30 min was observed before a sharp increase in activity during the course of hydrosilylation of aldehydes.
400 h−1 using a very low amount of catalyst (0.1 mol%) in 1,2-dichloromethane solvent. However, the hydrosilylation reaction rate for ketones is lower than that for aldehydes. It is noteworthy that an induction period of 20–30 min was observed before a sharp increase in activity during the course of hydrosilylation of aldehydes.
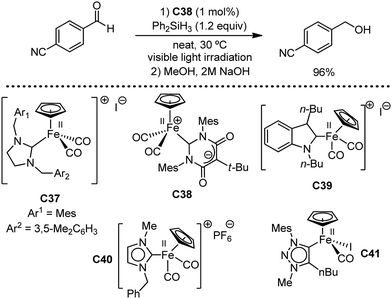 | ||
| Scheme 502 Iron-catalyzed hydrosilylation of ketones and aldehydes in the presence of piano-stool NHC iron complexes. | ||
In addition to the NHC-ligated iron-stool complexes, various other iron complexes containing NHC ligands were developed, which are efficient in hydrosilylation of aldehydes and ketones. In 2012, Glorius and coworkers reported the use of the square planar NHC iron(II) complex, (IMes)2FeMe2 (C42), as an effective catalyst for the hydrosilylation of 2′-acetonapthone with hydride donors such as (EtO)3SiH and Ph2SiH2 (Scheme 503).834 They also synthesized diamine-bridged bis-NHC iron dichloride complex C43 and applied it for the catalytic hydrosilylation of acetophenone with Ph2SiH2.835 This complex in combination with methyllithium in THF catalyzes the hydrosilylation reaction to give the corresponding alcohol with a high yield of 95%. Further, the iron(0) NHC complex Fe(IMes)(CO)4 (C44) prepared by Royo and co-workers was effective as a catalyst in the hydrosilylation of benzaldehyde derivatives and acetophenone (Scheme 503).836 High yields of corresponding benzyl alcohols were obtained using phenylsilane as the reducing agent in the presence of a catalytic amount of C44 in THF solvent at room temperature. Recently, in 2018, Song and co-workers reported the synthesis of N-picolyl N-heterocyclic ligand bearing iron complex C45.837 This complex was used for the catalytic hydrosilylation of ketones with excess PMHS to give alcohols in high yields. Notably, the high catalytic efficiency of this complex was proved by complete conversion in the presence of a low catalyst loading of 0.02 mol%.
In 2011, Adolfsson and co-workers performed the hydrosilylation of ketones with PMHS using an in situ catalyst formed from Fe(OAc)2 and NHC precursor IMes·HCl together with nBuLi as a base in THF at 65 °C (Scheme 504).838 Various ketones including arylalky-, dialkyl- and heteroarylketones were reduced to the desired alcohols in high yields (>99%) without affecting the other functional groups. A similar in situ-formed catalyst using 1-(2-hydroxyethyl)-3-methylimidazolium triflate, [HEMIM][OTf], as the NHC precursor catalyzed the hydrosilylation of ketones and aldehydes with a greater efficiency than IMes·HCl (Scheme 504).839 The catalytic system using [HEMIM][OTf] showed a high TOF of 200 h−1 with ketones compared to the TOF of 10 h−1 using IMes·HCl.
The combination of PHMS and FeCl3 in DCE (DCE = 1,2-dichloroethane) was used by Campagne for the reduction of aldehydes and ketones to the corresponding alkanes in the presence of microwave irradiation (Scheme 505).840 This simple and inexpensive procedure provides good to excellent yields of the methylene derivatives.
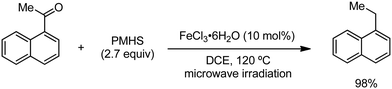 | ||
| Scheme 505 Reduction of aldehydes and ketones to methylene compounds in the presence of an iron catalyst. | ||
A large number of iron hydride complexes from Fe(PMe)4 were synthesized and used for the catalytic hydrosilylation of aldehydes and ketones by Sun, Li and co-workers. Starting from complex C46 in 2014,841 followed by complexes C47 in 2015,842C48 in 2016,843C49 in 2017844 and C50 in 2018,845 they were demonstrated to be very efficient catalysts for the hydrosilylation of aromatic and aliphatic aldehydes and ketones with (EtO)3SiH (Scheme 506). Notably, the selective reduction of α,β-unsaturated aldehydes and ketones to the corresponding α,β-unsaturated alcohols was performed in the presence of C48, C49, and C50. Additionally, the selenophenolato analog of C49 was reported as an effective catalyst for hydrosilylation reactions.846
The iron-bearing diphenylphosphinoethane (DPPE) ligand, [(DPPE)2Fe(H)2] (C51), was reported by Darcel and coworkers as a suitable pre-catalyst for the reduction of aldehydes and ketones with PMHS together with the presence of NaB(OEt)4 as a co-catalyst (Scheme 507).847 This system provides the corresponding alcohols in high yields of up to 99% upon activation with visible light. The aromatic and heteroaromatic aldehydes and ketones with bromo-, methoxy-, cyano- and amino-functional groups give high yields of the desired products.
In 2015, Findlater and co-workers used the iron complex with a bis(arylimino)acenapthene framework, BIAN-Fe(C7H8), for the hydrosilylation of aldehydes and ketones with Ph2SiH2 under neat conditions (Scheme 508).848 Various substituted aldehydes and ketones undergo efficient chemoselective hydrosilylation to form the desired alcohols in high yields with good functional group tolerance.
Around the same time, Thomas and co-workers reported the hydrosilylation of ketones and aldehydes with (EtO)3SiH using air-moisture-stable amine bis(phinolate)iron complex C52 in CD3CN solvent (Scheme 509).849 This catalytic system can efficiently reduce a large number of aldehydes and ketones, including α,β-unsaturated aldehydes and ketones, to their corresponding alcohols. The mild reaction conditions tolerate various functional groups such as CN, OMe, CF3, Cl, F and OCF3. In 2015, Peters and co-workers employed the DPB ligated iron complex [(DPB)Fe]2(N2) (C53) (DPB = bis(o-diisopropylphosphinophenyl)phenylborane) (Scheme 509) for the reduction of aldehydes and ketones via hydrosilylation with Ph2SiH2.850 Most aldehydes and ketone were hydrosilylated in excellent yields, except for the carbonyl compounds with high steric hindrance at the carbonyl site (e.g. tert-butyl methyl ketone and cyclopentanone). Further in 2016, Fernanadez and coworkers reported the synthesis and use of anthraquinonic ligand-bearing iron complex C54 as an active catalyst for the hydrosilylation of ketones at low catalyst loadings (0.25 mol%).851 Another similar complex, C55, also catalyzes the reduction of a wide range of aldehydes and ketones to the corresponding alcohols using (EtO)3SiH as the reductant (Scheme 510).852 Full conversions of the reactions were obtained with only 0.25–0.5 mol% catalyst loadings.
Then, in 2016, Mandal and co-workers designed a unique NHC iron(0) complex, C57, and applied it in the hydrosilylation of imines with phenylsilane (Scheme 512a).854 This complex promotes the reduction of aldemines and ketimines to high yields of corresponding amines at room temperature with a very low catalyst loading as low as 0.005 mol% in DMSO, giving a high TOF of 17000. The mild reaction conditions provide hydrosilylation with high chemoselectivity, tolerating a large number of functional groups such as nitro, ketone, ester, nitrile, unsaturated double bond and acid group. Additionally, imines containing sugar derivatives were efficiently reduced to the biologically important N-alkylated sugar derivatives using this catalytic system (Scheme 512b).
They investigated the mechanism of the reaction and proposed a Chalk–Harrod mechanistic pathway based on DFT calculations. Initially, iron precatalyst C57 undergoes dissociation of a CO molecule to form active species I, having 16 electrons (Scheme 513). Subsequently, the phenylsilane undergoes oxidative addition to this electron-deficient active species, generating intermediate II. Then this intermediate undergoes another dissociation of the CO ligand together with concomitant co-ordination of the imine derivative to form III. Thereafter, species IV is formed by hydride migration from the iron centre of III to the coordinated imine together with the attachment of a CO ligand to the iron centre. Finally, reductive elimination occurs to form the silylated amine together with the regeneration of active species I.
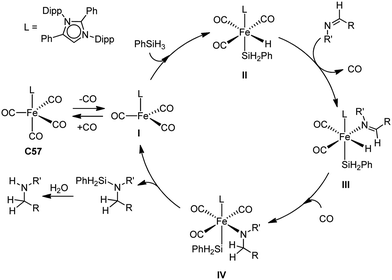 | ||
| Scheme 513 Proposed mechanism for the iron-catalyzed hydrosilylation of imines in the presence of iron complex C57. | ||
The direct reductive amination (DRA) of aldehydes and ketones was performed via the hydrosilylation reaction in the presence of iron catalysts. In 2010, Enthler first demonstrated a procedure for the formation of secondary amines by the DRA of aldehydes with anilines in the presence of FeCl3 together with excess PHMS (Scheme 514).855 A wide range of aldehydes and substituted anilines underwent the reaction, forming the desired secondary amines in high yields. Then in 2012, Darcel and co-workers reported the DRA of substituted benzaldehydes with secondary amines under hydrosilylation conditions using cyclopentadienyl piano-stool iron complex C58 bearing a phosphanyl-pyridine chelating ligand (Scheme 514).856 The reaction is performed by employing PMHS in dimethylcarbonate solvent at 40 °C.
Further, in 2014, N-alkylated anilines were synthesized from homoallylic or allylic alcohols and anilines (both primary and secondary) through DRA catalyzed by Fe(cod)(CO)3 (cod = cycloocta-1,5-diene) in ethanol under visible light irradiation (Scheme 515).857 A large number of allylic and homoallylic alcohols underwent efficient reaction with various primary and secondary amines to give moderate to high yields of desired N-alkylated anilines. Notably, the hydrosilylation is performed with hydrosilane, PMHS. Most importantly, the reaction occurs via a three-step one sequence consisting of isomerization of allylic alcohol, condensation and hydrosilylation.
Around the same time, Lemaire and co-workers performed the reduction of aromatic nitro derivatives to amine derivatives utilizing a mixture of Fe(acac)3 and tetramethyldisiloxane (TMDS) at 60 °C.860,861 This reaction procedure tolerates functional groups such as cyano, bromo, ester, acid and aldehydes. Further, in 2016, Thomas and co-workers applied amine-bis(phenolate) ligand-bearing complex C59 for the similar reduction of nitroarenes to the corresponding amines (Scheme 517).862 A wide range of substituted nitroarenes were chemoselectively reduced by (EtO)3SiH in acetonitrile solvent at 80 °C in the presence of a catalytic amount of the iron(III) complex. The efficiency of the procedure was demonstrated by the yields of the corresponding amines and tolerance to carbonyl, imine, ester, cyano and sulfonyl functional groups.
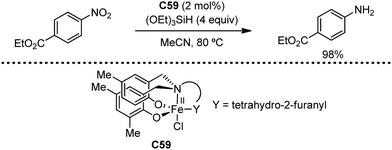 | ||
| Scheme 517 Reduction of nitroarenes to amines in the presence of amine bis(phenolate) ligand-bearing iron complex. | ||
In 2015, Baran and co-workers demonstrated a unique procedure for the synthesis of hindered amines from nitroarenes and alkenes via a reductive hydroamination reaction. The olefin hydroamination reaction was performed under the catalytic activity of Fe(acac)3 in the presence of PhSiH3 as the reductant in ethanol.863 Notably, the desired amines were obtained from the N,O-alkylated adduct via its reductive cleavage using Zn powder (Scheme 518). A huge number of substituted amines were produced in moderate yields (24–80%) together with tolerance to a large number of functional groups. From a mechanistic point of view, it was proposed that nitroarenes are initially reduced to the corresponding nitrosoarenes followed by adduct formation with alkyl radicals from alkenes. Thereafter, the adduct undergoes cleavage at the N–O bond to give the corresponding hindered amines.
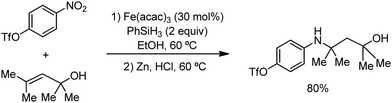 | ||
| Scheme 518 Synthesis of hindered amine from nitroarene and alkenes in the presence of an iron catalyst. | ||
Recently in 2017, indoles were prepared via the reductive cyclization of ortho-nitrostyrenes in the presence of Fe(OAc)2 in combination with 4,7-(MeO)2phen as the ligand and phenylsilane as the reducing agent (Scheme 519).864 The reaction occurs via the formation of nitrosostyrenes as the intermediate, followed by electrocyclization to generate N-hydroxyindone and its subsequent reduction by phenylsilane to produce the desired indoles. Notably, active iron–hydride species is formed by the reduction of the iron precatalyst by phenylsilane. This procedure affords high yields of indoles from their corresponding nitrostyrenes.
In 2009, Beller and co-workers reported the first instance of iron-mediated hydrosilylation of amides with PMHS using a catalytic amount of [Fe3(CO)12] to form the corresponding amines (Scheme 521).869 A wide range of aliphatic, aromatic, heterocyclic and heteroaromatic tertiary amines undergo efficient reduction with high functional group tolerance. The secondary amides are also reduced in high yields under this reaction condition but only in the presence of PhSiH3 as the reducing agent.
Around the same time, Nagashima and co-workers also reported the use of [Fe3(CO)12] together with [Fe(CO)5] as efficient catalysts for the reduction of carboxamides to amines with TMDS as the hydrosilane (Scheme 522a).858 The reduction was also successfully performed under photoirradiation of the reaction system by a high pressure mercury lamp for 9 h at room temperature (Scheme 522b). Both the thermal- and photo-assisted procedures afforded good yields of the corresponding amines from the amides. Further, in 2011, they introduced a new procedure for carboxamide hydrosilylation using the heptanuclear iron carbonyl cluster [Fe3(CO)11(μ-H)]2Fe(DMF)4 and BDSB (BDSB = 1,2-bis-(dimethylsilyl)benzene) as a reductant to give the products in less time and at a lower catalyst loading (0.5 mol%).870
In 2011, Darcel and Sortais utilized the well-defined NHC-bearing piano-stool iron complex [Fe(Cp)(CO)2(IMes)]I (C60) as a very effective catalyst in hydrosilylation of amides with phenylsilane under visible light activation (Scheme 523).871 Tertiary and secondary amides were efficiently reduced to the corresponding amides with moderate yield in neat conditions at 100 °C.
In situ-formed NHC–iron complexes have also been applied for the hydrosilylation of carboxamides. In 2013, Adolfsson and co-workers performed the hydrosilylation of tertiary amides with PMHS as the hydrosilane using the NHC–iron catalyst developed in situ from Fe(OAc)2 and [PhHEMIM][OTf] in the presence of LiCl and nBuLi (Scheme 524).872 Notably, the chemoselectivity of the reaction increased in the presence of the LiCl additive. Additionally, it also increased the rate of the reaction by increasing the activity of the catalytic system.
Also, the chelated bis-NHC ligand-containing iron(0) complex [Fe{(DippC:)2CH2}(η6-C6H6)] (C61) (DippC: = bis-(N-Dipp-imidazole-ylidene)methylene) was shown to be an efficient catalyst for amide reduction with the reductant Ph2SiH2 at 70 °C (Scheme 524).873 This catalytic system afforded the desired amines in good to excellent yields.
Recently, in 2019, Turculet and co-workers synthesized two low-coordinate iron(II) complexes C62 and C63 bearing N-(phosphinoaryl)anilide as a P,N ligand and demonstrated their catalytic activity in the reduction of tertiary amides via hydrosilylation with PhSiMe3 to form the corresponding amines.874 Although three-coordinate complex C62 and four-coordinate complex C63 showed similar activity at 80 °C, C62 was equally efficient at room temperature (Scheme 525). Various tertiary amines including sterically hindered N,N-dibenzylbenzamide were efficiently reduced to their corresponding amines with high yields. Notably, in contrast to the previously reported iron catalysts, this catalytic system does not require any photochemical activation. In addition, the N-phosphinoamidinate-ligated iron complex (P,N)Fe(N(SiMe)2) (C64) was found to catalyze the hydrosilylative reduction of N,N-dibenzylbenzamide to dibenzylamine using PhSiH3 (Scheme 525).875
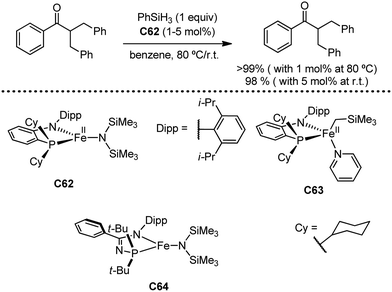 | ||
| Scheme 525 Iron-catalyzed hydrosilylation reaction of carboxamides in the presence of N-(phosphinoaryl)anilido iron complexes. | ||
The troublesome reduction of primary amides to primary amines was successfully performed by Beller and co-workers by the consecutive use of two different iron complexes.876 The reaction was performed in two steps starting from dehydration of primary amide to the corresponding nitrile using the complex [NHEt3][Fe3H(CO)11] together with (EtO)2MeSiH in toluene. This was followed by reduction of the nitrile to primary amine by (EtO)2SiH in the presence of Fe(OAc)2 with terpyridine ligand L (Scheme 526). This methodology produced the primary amines in moderate yields.
Recently in 2019, Mandal and co-workers employed phenalenyl ligand-bearing iron(III) complex C66 for the reduction of nitrile to primary amine with PMHS in the presence of KO(t-Bu) in THF at 70 °C (Scheme 528).878 Various aromatic, heteroaromatic and aliphatic nitriles were reduced to the corresponding primary amines via hydrosilylation under this reaction condition. The reaction procedure chemoselectively reduces the substituted nitriles in the presence of functional groups such as –OCH3, –F, –Cl, –Br, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and –CF3. Notably, all the primary amines were isolated as their hydrochlorinated salts by treating them with methanolic HCl solution in Et2O.
C, and –CF3. Notably, all the primary amines were isolated as their hydrochlorinated salts by treating them with methanolic HCl solution in Et2O.
In 2012, Darcel and Sortais reported the first ester hydrosilylation reaction catalyzed by a well-defined iron complex, [Fe(Cp)(CO)2(PCy)3][PF6] (C67), in neat conditions and under visible light activation at 100 °C (Scheme 530).879 The complex catalyzes a large variety of esters including aliphatic esters, aromatic esters, alkanoates and acetates to give the corresponding primary alcohols in good yields. In 2013, a similar hydroslilyation of carboxylic esters to alcohols by PMHS was performed using Fe(stearate)2 together with the ligand NH2CH2CH2NH2 at 100 °C (Scheme 531a).880 Around the same time, N-phosphinoamidinate ligand-bearing three coordinate iron complex C68 was applied by Turculet and co-workers for a similar conversion at room temperature (Scheme 531b).804 Notably, the alcohols were obtained via the hydrosilylation of esters with phenylsilane at low catalyst loadings.
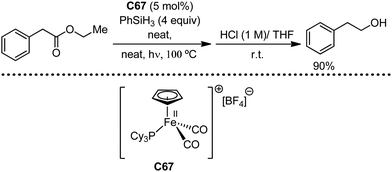 | ||
| Scheme 530 Iron-catalyzed hydrosilylation of carboxylic esters by cyclopentadienyl iron complex C67. | ||
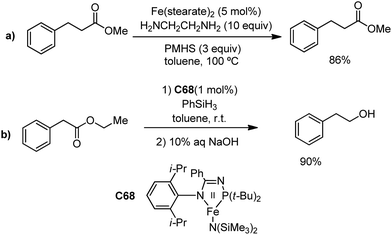 | ||
| Scheme 531 Hydrosilylation of esters to alcohols in the presence of different iron catalyst systems. | ||
On the other hand, the selective reduction of esters to ethers was achieved by Beller and co-workers in 2012. The reaction was performed at 100 °C using 10 mol% of [Fe3(CO)12] in combination with 3 equiv. of TMDS in toluene (Scheme 532).881 A broad range of aromatic and aliphatic esters were reduced via hydrosilylation to their corresponding ethers in high yields.
Again, esters were specifically reduced to aldehydes using the fully characterized882 NHC–iron complex [(IMes)Fe(CO)4] (C69) and hydrosilanes under UV irradiation at room temperature (Scheme 533).883 The reaction methodology chemoselectively reduces various esters, ranging from methyl, benzyl ester, substituted methyl acetates, aliphatic esters and lactones to the corresponding aldehydes and lactols in high yields. The diethylsilane and diphenylsilane give the best results depending on the substrate ester.
From a mechanistic point of view, the reaction is initiated with the release of a CO ligand through UV activation to form 16-electron complex I (Scheme 534).883 This is followed by the formation of intermediate complex IIvia the oxidative addition of hydrosilane to I. Then, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the ester is either inserted in the Fe–H or Fe–Si bond, forming the corresponding complex III or IV. Finally, the silylated acetal is produced via reductive elimination from complex III or IV together with the regeneration of active iron(0) species I.
O bond of the ester is either inserted in the Fe–H or Fe–Si bond, forming the corresponding complex III or IV. Finally, the silylated acetal is produced via reductive elimination from complex III or IV together with the regeneration of active iron(0) species I.
In addition to esters, acid chlorides were also reduced to aldehydes by employing the catalytic system consisting of iron oxide (FeO) and tris(2,4,6-trimethylphenyl)phosphine together with PhSiH3,884 and moderate yields of the desired aldehydes were obtained.
The selective reduction of carboxylic acids to aldehydes or alcohols was reported by Darcel and co-workers. The alcohols were selectively obtained from carboxylic acids via a one-pot procedure using the (COD)Fe(CO)3 (C70) catalyst together with phenylsilane under UV radiation at room temperature. The carboxylic acids were specifically converted to the corresponding alcohols in good yields (Scheme 536).885 In contrast, the aldehydes were selectively produced by performing the reaction in the presence of (t-PBO)Fe(CO)3 (C71) and TMDS at 50 °C.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of urea is very difficult. Basically, formamidines undergo over-reduction to form aminals, amines and methanol. However, Cantat and co-workers overcame this problem by employing a mixture of Fe(acac)2 and tetraphos (L) in THF as the catalytic system (Scheme 537).886 The urea derivatives were reduced via hydrosilylation with PhSiH3 to give moderate to high yields of formamidines (A) together with the formation of a lower amount of carboimides (B). Further, the same catalytic system at room temperature was able to reduce CO2 selectively to form formamide and methylamine derivatives.887
O bond of urea is very difficult. Basically, formamidines undergo over-reduction to form aminals, amines and methanol. However, Cantat and co-workers overcame this problem by employing a mixture of Fe(acac)2 and tetraphos (L) in THF as the catalytic system (Scheme 537).886 The urea derivatives were reduced via hydrosilylation with PhSiH3 to give moderate to high yields of formamidines (A) together with the formation of a lower amount of carboimides (B). Further, the same catalytic system at room temperature was able to reduce CO2 selectively to form formamide and methylamine derivatives.887
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the presence of various reducible functional groups such as esters, cyano, sulfonyl, epoxides, alkenyl and aldehyde groups to give excellent yields of the sulfides.
O bond in the presence of various reducible functional groups such as esters, cyano, sulfonyl, epoxides, alkenyl and aldehyde groups to give excellent yields of the sulfides.
Thereafter in 2012, Royo and co-workers applied well-defined iron complex-bearing NHC-functionalized cyclopentadienyl-chelated complex C72 in together with AgBF4 for the similar hydrosilylation of sulfoxides with PhSiH3 in toluene (Scheme 539).889 This reaction condition also afforded the desired sulfides in high yields.
7.4 Hydroboration
Metal-mediated hydroboration reaction is a very straightforward and efficient method for the synthesis of organic boronates.890 These boronates are widely used as intermediates in organic synthesis, particularly as nucleophilic partners in Suzuki-Miyaura891 cross-coupling and C–H activation reactions to construct carbon–carbon bonds or carbon–heteroatom bonds.892 Moreover, hydroboration reactions are very selective and atom-economical reactions.892 In these reactions, pinacolborane or catecholboranes are employed as the boron source due to their stability towards atmospheric oxidation. Previously, different heavy transition metal complexes (Rh and Ir) have been used efficiently in homogeneous hydroboration reaction.893 Throughout the last two-three decades, significant progress has been achieved in iron-catalyzed hydroboration reactions, which are discussed chronologically in the following section.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and ester group are well tolerated under the reaction conditions to afford only the 1,4-hydroboration product. Moreover, the reaction only delivers the E-alkenes exclusively even with unsymmetrical dienes.
C bond and ester group are well tolerated under the reaction conditions to afford only the 1,4-hydroboration product. Moreover, the reaction only delivers the E-alkenes exclusively even with unsymmetrical dienes.
Similarly, in 2014, Huang and co-workers reported the 1,4-hydroboration of 1-aryl substituted 1,3-diene for the regio and stereoselective synthesis of secondary benzylic Z-allylboronates using a series of new imino-bypyridine iron complexes having bulky diphenylphosphinomethylketimine substituents (Scheme 541).897 The iron complex was used as a precatalyst which, formed an active catalyst898in situ upon the addition of NaBEt3H (two equivalents relative to Fe). Interestingly, both the electron-withdrawing group and the electron-donating group present at the meta- and para-position of the aryl group afforded the desired branched products in high yield. Moreover, the 1,4-disubstituted internal dienes also delivered the benzylic allylic boronate with good selectivity.
Throughout the last few years, the hydroboration of olefins has been performed using bidentate ligand-containing iron complexes. Later, Huang et al. first introduced the idea of using a tridentate ligand, which was found to be essential for iron-catalyzed hydroboration.899 The use of the tridentate ligand generates a less electrophilic iron centre, which helps in the easy formation of the olefin-ligated FeII-boryl intermediate during the catalytic cycle of alkene hydroboration. Purposefully, they employed an electron-rich iron complex bearing a bipyridyl-based phosphine ligand (PNN)900 (catalyst 3) in the presence of NaBEt3H and pinacolborane (Scheme 543). The reaction regioselectively provides the anti-Markovnikov hydroboration product together with the chemoselective hydroboration of the terminal alkenes in the presence of multiple C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in a polyene system. This methodology works well for simple acyclic α-olefins, 1,1-disubstituted α-olefins and alkenes bearing functional groups such as silyl, ether, acetal, and amine. Notably, the reaction protocol does not result in the formation of the desired products during the hydroboration with internal alkenes. Furthermore, when the reaction is conducted with styrene in the presence of HBpin in THF, it does not only produce the linear hydroboration product, but also generates the dehydrogenative hydroboration product and ethylbenzene as side products. However, the selectivity during the formation of the product is solvent dependent. Changing the solvent from tetrahydrofuran to acetonitrile increased the yield of the linear hydroboration product. Interestingly, in presence of 40 mol% of acetonitrile, side product formation was inhibited.
C bonds in a polyene system. This methodology works well for simple acyclic α-olefins, 1,1-disubstituted α-olefins and alkenes bearing functional groups such as silyl, ether, acetal, and amine. Notably, the reaction protocol does not result in the formation of the desired products during the hydroboration with internal alkenes. Furthermore, when the reaction is conducted with styrene in the presence of HBpin in THF, it does not only produce the linear hydroboration product, but also generates the dehydrogenative hydroboration product and ethylbenzene as side products. However, the selectivity during the formation of the product is solvent dependent. Changing the solvent from tetrahydrofuran to acetonitrile increased the yield of the linear hydroboration product. Interestingly, in presence of 40 mol% of acetonitrile, side product formation was inhibited.
Based on the preliminary data, the authors proposed the mechanistic cycle. Initially, in presence of NaHBEt3, the alkene coordinates to the iron centre to produce complex A, followed by the oxidative addition of HBpin to complex A, generating formal 18-electron Fe(II)-boryl hydride species B. Thereafter, the terminal alkene (except vinylarenes) undergoes selective 1,2-insertion into the Fe–H bond (pathway I) to generate linear alkyl complex C, followed by reductive elimination to deliver the desired anti-Markovnikov product. On the other hand, vinyl arenes having no α-substituents generate the dehydrogenative borylation (pathway II) product via the insertion of vinyl arenes into the Fe–B bond rather than the Fe–H bond of complex B. The dehydrogenative borylation proceeds via the formation of η3-benzyl Fe(II) species D, a formal 18-electron complex, which undergoes η3-η1 rearrangements followed by β-hydride elimination to form the vinylboronate ester (Scheme 542).
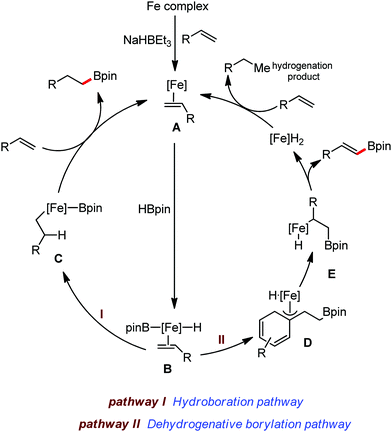 | ||
| Scheme 542 Proposed mechanistic pathway for the iron-catalyzed hydroboration and dehydrogenative borylation. | ||
Later, Chirik and co-workers used a bis(imino)pyridine iron dinitrogen complex (Scheme 543, catalyst 4) for the anti-Markovnikov synthesis of alkyl boronates.901 Noteworthily, the reaction was performed in the absence of organic solvent, which extended the substrate scope of this methodology. Consequently, the reaction promotes the hydroboration of terminal, internal, cyclic and geminal alkenes with high activity and selectivity using a catalytic mixture of 1 mol% of [i-Pr(PDI)Fe(N2)2] (catalyst 4) and HBpin. Particularly, for internal alkenes, e.g. cis-4-octene, a mixture of 1- and 4-octylboronate products was obtained in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio within 24 h of reaction, indicating an isomerization process. Notably, the reaction methodology is also performed with styrene to obtain the selective anti-Markovnikov hydroboration product in the presence of 1 mol% of catalyst 4. Furthermore, 1-phenyl propylboronate ester and 2-phenyl propylboronate ester are obtained from the catalytic hydroboration of α- and cis-β-methyl styrene after 24 h of reaction.
1 ratio within 24 h of reaction, indicating an isomerization process. Notably, the reaction methodology is also performed with styrene to obtain the selective anti-Markovnikov hydroboration product in the presence of 1 mol% of catalyst 4. Furthermore, 1-phenyl propylboronate ester and 2-phenyl propylboronate ester are obtained from the catalytic hydroboration of α- and cis-β-methyl styrene after 24 h of reaction.
Successively, Thomas and co-workers reported the regio, chemo and stereoselective iron-catalyzed hydroboration of alkenes and alkynes using a catalytic combination of dichloro iron(II)complex (Scheme 543, catalyst 5) or in situ formation of 1 mol% of precatalyst from FeCl2 and free bis(imino)pyridine ligand in the presence of EtMgBr with pinacolborane in THF at room temperature.902 Terminal alkenes substituted with aryl chloride, fluoride and bromide and substrates having more than one unsaturated group such as esters, alcohols, amide, imines, and amines are well tolerated under the standard reaction conditions. In addition, this synthetic methodology was also applied for the hydroboration of internal alkenes, e.g. cyclooctene, and the hydrogermylation of styrene, obtaining the selective linear hydrogermylated product in 86% yield. Interestingly, this is the first reported example of an iron-catalyzed hydrogermylation reaction.
Later, Darcel and Sortais used a molecular-defined iron NHC complex [(MesI)Fe(CO)4] (Scheme 543, catalyst 6) for the hydroboration of functionalized terminal alkenes at room temperature.903 The reaction was performed under UV irradiation (350 nm) in the presence of HBpin and absence of organic solvent. Various functional groups such as ester, acetal, ether, silylether, epoxide, and nitrile are well tolerated under the reaction conditions.
In 2014, Szymczak and co-workers synthesized aminobispyridine (N,N,N) Fe(II) complexes, [FeII(bMepi)(THF)]OTf (Scheme 543, catalyst 7) and [Fe(bMepiMe)(OTf)2] (Scheme 543, catalyst 8) to catalyze the hydroboration reaction of terminal linear and cyclic olefins together with HBpin and NaBEt3H at room temperature under neat conditions.904 Their idea of introducing an alkyl group at the remote site of the ligand backbone modifies the electronic environment of the secondary coordination sphere of the ligand by tuning the electrophilicity of the iron complex. Consequently, this characteristic of the ligand helps to catalyze the hydroboration reaction at an increased rate, affording the corresponding vinyl boronate esters as the desired product with distinct regioselectivity from internal and terminal alkynes under the same reaction conditions.
Additionally, when the hydroboration reaction of styrene was performed in presence of the [Fe(bMepi)(THF)]OTf (Scheme 543, catalyst 7) catalyst, the anti-Markovnikov hydroboration product was obtained as the major product (75% yield) together with the formation of the dehydrogenative borylation and hydrogenation product (11% yield of side products). On the other hand, under the same reaction conditions, using [Fe(bMepiMe)(OTf)2] as the catalyst (catalyst 8) afforded only 81% yield of the anti-Markovnikov hydroboration product. However, when cis-4-octene was subjected to [Fe(bMepiMe)(OTf)2], a mixture of alkyl boronates was delivered as the product, whereas only linear boronates were obtained using [Fe(bMepi)(THF)]OTf as the catalyst, indicating the isomerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond followed by hydroborylation.
C bond followed by hydroborylation.
In 2015, Rauchfuss and co-workers developed a series of new phosphine-diimine ligands via the condensation reaction between 2-(diphenylphosphino)aniline (PNH2) and a variety of formyl and ketopyridine derivatives.905 Noteworthily, the authors synthesized a low-spin [Fe PNHPyR]2+ (R = Me, H) complex (Scheme 543, complex 9) using a combination of ferrous halide and the ligand generated from the condensation reaction between PNH2 and pyridine carboxylate and its 6-methyl derivatives. This low-spin complex was used for the hydroboration of 1-octene in together with HBpin and NaBEt3H at room temperature.
In 2016, Webstar and co-workers reported two iron(II) β-diketiminate complexes (Scheme 543, complex 10) for the regio and chemoselective hydroboration of alkenes and alkynes in the presence of HBpin or HBcat in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reagent ratio without any requirement of external reducing agents or activator.906 With the first catalyst 10A, terminal linear olefins delivered the anti-Markovnikov product (45–99% yield) using HBpin in C6D6 solvent for 16 h at room temperature. Moreover, functional groups such as epoxide, ketone, and phosphine are well tolerated under the reaction conditions. Interestingly, only terminal double bonds are hydroborylated, keeping the other multiple double bonds present in the molecule intact. Using the same catalyst at 60 °C, styrene also undergoes the reaction to give the exclusive formation of the anti-Markovnikov hydroborylated product. Importantly, the specific formation of 2-phenylpropyl boronate esters and 1-phenylpropyl boronate esters was obtained during the hydroboration reaction of α- and cis-β-methylstyrene, respectively. Furthermore, second catalyst 10B having different steric and electronic characteristics in its ligand structure can change the reactivity of styrene, i.e. only the Markovnikov hydroborylated product is obtained without any formation of the dehydrogenative hydroborylation and hydrogenation product. Notably, double hydroborylation of diphenylacetylene occurs to afford the geminal dipinacolborane product.
1 reagent ratio without any requirement of external reducing agents or activator.906 With the first catalyst 10A, terminal linear olefins delivered the anti-Markovnikov product (45–99% yield) using HBpin in C6D6 solvent for 16 h at room temperature. Moreover, functional groups such as epoxide, ketone, and phosphine are well tolerated under the reaction conditions. Interestingly, only terminal double bonds are hydroborylated, keeping the other multiple double bonds present in the molecule intact. Using the same catalyst at 60 °C, styrene also undergoes the reaction to give the exclusive formation of the anti-Markovnikov hydroborylated product. Importantly, the specific formation of 2-phenylpropyl boronate esters and 1-phenylpropyl boronate esters was obtained during the hydroboration reaction of α- and cis-β-methylstyrene, respectively. Furthermore, second catalyst 10B having different steric and electronic characteristics in its ligand structure can change the reactivity of styrene, i.e. only the Markovnikov hydroborylated product is obtained without any formation of the dehydrogenative hydroborylation and hydrogenation product. Notably, double hydroborylation of diphenylacetylene occurs to afford the geminal dipinacolborane product.
In the same year, Turculet and co-workers reported an N-phosphinoamidinate-coordinated iron catalyst for the isomerization–hydroboration of terminal, germinal and internal alkenes using 1,3-dimethyl-1,3-diaza-2-boracyclopentane at 65 °C (Scheme 544, complex 11).907 Evidently, more challenging substrates such as trans-4-octene provide the terminal hydroboration product selectively under the reaction conditions. Notably, 1,3-dimethyl-1,3-diaza-2-boracyclopentane is a better hydroboron reagent source than HBpin or benzo-1,3,2-diazaborolane for the above-mentioned methodology. Further, Zhou and co-workers reported the hydroboration of styrenes and heteroaryl alkenes in the presence of a simple catalytic system having bis(pinacolato)diboron, FeCl2 (0.1–10 mol%), K-O(t-Bu) (1.2 equivalent) and t-BuOH (1 equivalent) in THF at 65 °C for 12 h.908 This transformation provides the selective anti-Markovnikov product with 64–99% yield.
The catalytic systems discussed thus far performing the hydroboration of styrene generated the selective anti-Markovnikov product. However, the regioselective formation of the Markovnikov hydroborylation product using an iron-based catalytic system was not discovered until 2016. However, in 2017, Lu and co-workers reported the highly-Markovnikov-selective hydroboration of styrenes using a catalytic system containing an iron catalyst containing an oxazolinyl phenyl picolinamide (OPPA) (12) ligand, HBpin, and NaBEt3H in toluene at 30 °C for 18 h to deliver the branched hydroboration product with up to >50 > 1 (branched > linear product) (Scheme 545).909 The electronic nature of the substitution present in styrene does not affect in the selective formation of the desired product in good yield. Furthermore, the late-stage synthesis of bioactive molecules possessing styrene such as (+) menthol and vitamin E have also been exclusively converted to the corresponding selective branched products in moderate yield.
Furthermore, Thomas and co-workers reported two series of structurally related alkoxytethered NHC iron(II) complexes, which performed the activator-free regioselective hydroboration of terminal alkene systems (Scheme 546).910 Notably, using iron complex 14 and HBpin under neat conditions at room temperature with styrene, the selective branched hydroboration product was obtained with the improved selectivity of branched: linear ratio from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 37
1 to 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. On the other hand, the use of styrene derivatives with HBCat and iron complex 13 led to the formation of the anti-Markovnikov (linear) hydroboration product. However, because of the difficulties in the purification of catechol boronic ester, these products were oxidised in the presence of H2O2/NaOH to the linear alcoholic product or transesterified with pinacol to provide the primary alkyl boronic ester. Although enantioselective hydroboration of alkenes is very rare, Lu and co-workers in 2014 first reported asymmetric hydroboration using imino-pyridine oxazoline (IPO) iron catalyst 15 for the formation of the anti-Markovnikov hydroboration product of 1,1-disubstituted aryl arenes with pinacolborane (Scheme 547).911 Notably, the imino-pyridine group stabilized the iron catalyst and the chiral oxazoline group controlled the enantioselectivity. The reaction was also well proceeded in the presence of a variety of substituted styrenes including electron-rich and electron-deficient substituents. Notably, silylether, tertiaryamines and methylsulphides are also well tolerated under the reaction conditions. In addition, the isolated and bench-stable chiral alkyl boronic esters were also transformed into a variety of chiral compounds such as alcohols, amines and fluorinated boronic derivatives.
1. On the other hand, the use of styrene derivatives with HBCat and iron complex 13 led to the formation of the anti-Markovnikov (linear) hydroboration product. However, because of the difficulties in the purification of catechol boronic ester, these products were oxidised in the presence of H2O2/NaOH to the linear alcoholic product or transesterified with pinacol to provide the primary alkyl boronic ester. Although enantioselective hydroboration of alkenes is very rare, Lu and co-workers in 2014 first reported asymmetric hydroboration using imino-pyridine oxazoline (IPO) iron catalyst 15 for the formation of the anti-Markovnikov hydroboration product of 1,1-disubstituted aryl arenes with pinacolborane (Scheme 547).911 Notably, the imino-pyridine group stabilized the iron catalyst and the chiral oxazoline group controlled the enantioselectivity. The reaction was also well proceeded in the presence of a variety of substituted styrenes including electron-rich and electron-deficient substituents. Notably, silylether, tertiaryamines and methylsulphides are also well tolerated under the reaction conditions. In addition, the isolated and bench-stable chiral alkyl boronic esters were also transformed into a variety of chiral compounds such as alcohols, amines and fluorinated boronic derivatives.
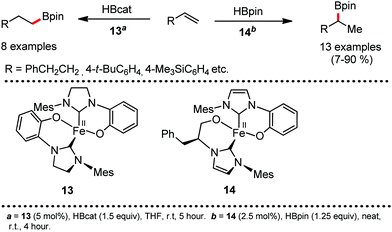 | ||
| Scheme 546 Iron-catalyzed systems for the selective anti-Markovnikov and Markovnikov hydroborylation of styrenes. | ||
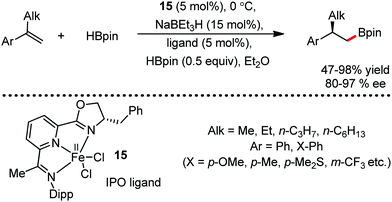 | ||
| Scheme 547 Iminopyridineoxazoline iron-catalyzed asymmetric hydroboration of 1,1-disubstituted aryl alkenes. | ||
Simultaneously, the hydroboration of alkynes was reported by Enthaler using [Fe2(CO)9] as a catalyst in the presence of pinacolborane to afford vinyl boronate esters (Scheme 548, condition A).912 Although E-vinyl boronate derivatives were obtained selectively from the terminal alkynes, a more complex mixture of regioisomers was obtained from the internal non-symmetric alkynes. Furthermore, iron-nanoparticles (np-Fe3O4) or anhydrous FeCl3 were also used as a catalyst for the monoborylation of alkynes using bis(pinacolato)diboron (Scheme 548, condition B).913 The E-vinyl boronates were obtained as the product with high regio and stereoselectivity in good to excellent yield. Notably, the catalyst was used for six consecutive cycles without loss in its activity.
In 2017, Nishibayashi and co-workers synthesized an iron catalyst possessing a pyrrolidine-based PNP pincer ligand to catalyze the hydroboration of alkynes with pinacolborane at room temperature to provide E-alkenyl boranes under ambient conditions (Scheme 548, catalyst 16).914 Although meta and para-tolylacetylenes provide the alkenyl borane in good yield, the ortho analogue does not afford any desired product in reasonable yield, indicating the role of steric hindrance in inhibiting the reaction. Both aromatic and aliphatic alkynes were converted to the desired product under the reaction conditions. However, internal alkynes such as diphenylacetylene did not afford any desired product. Importantly, the reaction methodology only provides the complete selective hydroboration reaction of alkynes, even in the presence of the alkene moiety. Interestingly, the turnover number (TON) of the catalyst was found to be 710 for the hydroboration reaction between alkynes and hydroborane at high temperature (60 °C). Notably, this is the highest TON reported to date for the iron-catalyzed hydroboration reaction.
In the same year, Kirchner and co-workers synthesized a non-classical iron(II) polyhydride compound bearing a tridentate PNP pincer-type ligand with the general formula [Fe(PNP)(H)2(η2-H2)] (Scheme 549).915 As soon as this compound is added to excess BH3·THF, it results in the formation of a complex for the chemo, regio and stereoselective hydroboration reaction of terminal alkynes to generate the respective vinyl boronate with high Z-selectivity.916 However, internal alkynes do not provide any type of desired product. Further, when the reaction was performed in C6D6, vinylboronates were obtained having the E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio of 29
Z ratio of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 to 1
71 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 within 3 to 16 h.
99 within 3 to 16 h.
In 2019, Findlater and co-workers reported two iron complexes, (BIAN)Fe(I)-η6-toluene (Scheme 550, catalyst 18) and FeCl2(BIAN) (Scheme 550, catalyst 19) [BIAN = bis(2,6-diisopropylaniline)acenaphthrene], which catalyzed the regioselective hydroboration of alkenes and alkynes.917 Linear vinyl and alkyl boronic esters were obtained respectively in the presence of pinacolborane and an activator (Nat-BuO) at 70 °C under neat conditions. Notably, the first catalyst, [(BIAN)Fe(I)η6-toluene] (Scheme 550, catalyst 18), was used for the hydroboration of terminal alkynes. Various terminal phenylacetylenes having electron-withdrawing and electron-donating substituents were hydroborated in good yields. On the other hand, the second catalyst, FeCl2(BIAN) (Scheme 550, catalyst 19) was used for the hydroboration of internal alkynes. This is due to the increased steric characteristics of the internal alkynes, which destabilise the close approach of the substrates to the iron centre rather than the co-ordination of the η6-toluene group. To rationalize the reaction, bis(trimethylsilyl)acetylene provided a mixture of isomers containing both the hydrogenated (GC yield = 33%) and non-hydrogenated products. Moreover, styrene bearing both electron-donating and electron-withdrawing substituents delivered the selective anti-Markovnikov hydroboration product in good yield with a ratio of linear: branched of >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
Recently, Plietker and co-workers have reported a structurally-defined iron–hydride complex, [FeH(CO)(NO)(PPh3)2], which catalyzes the stereoselective hydroboration of internal alkynes (Scheme 551).918 Both aliphatic and aromatic alkynes undergo the reaction to provide the corresponding vinylboronates with very high Z/E selectivity in moderate to good yields in the presence of either HBpin or B2pin2 as a boron source. Importantly, in the case of asymmetric internal alkynes, the regioselectivity of the product solely depends on the boron source. Using HBpin as the boron source, the Markovnikov product is obtained with good regioselectivity, while the complementary anti-Markovnikov product is obtained in comparable regioselectivity using B2pin2/MeOH as the boron source. This type of regioselectivity indicates the involvement of different catalytic species during the catalytic cycle depending on the boron source.
Vinylcyclopropane (VCP) is a very useful synthon, which has been used particularly for the making of all carbon rings. In 2017, Lu and co-workers reported the Markovnikov-type hydroboration of VCP using the iron complex IPO·FeCl2 (Scheme 552, catalyst 20) in the presence of HBpin and NaBEt3H in toluene at room temperature for 0.5 h.919 Iron catalyst 20 provides regioselective C–C bond cleavage of VCP, affording homoallylic organoboronic esters with E-selectivity. Notably, a variety of functional groups such as amino, sulphide, silylether, halides, remote C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and thienyl are well tolerated under the reaction conditions. To implement the asymmetric transformation, the reaction between various racemic vinylcyclopropanes and HBpin using complex 21 was carried out to afford various chiral homoallylic boronic esters in 39–97% yield with 72–99% ee. These chiral homoallylic boronate esters were also converted to the chiral poly-substituted tetrahydrofuran and tetrahydropyran.
C bond and thienyl are well tolerated under the reaction conditions. To implement the asymmetric transformation, the reaction between various racemic vinylcyclopropanes and HBpin using complex 21 was carried out to afford various chiral homoallylic boronic esters in 39–97% yield with 72–99% ee. These chiral homoallylic boronate esters were also converted to the chiral poly-substituted tetrahydrofuran and tetrahydropyran.
Based on the preliminary studies, the authors proposed a reaction mechanistic pathway. Initially, iron–hydride complex A is generated from IPO·FeCl2 and NaBEt3H. Then, complex A undergoes coordination with the alkene part of vinylcyclopropane to form complex B. Thereafter, complex B undergoes alkene insertion into the iron–hydride bond followed by selective β-carbon elimination to generate complex D. Finally, complex D undergoes ligand exchange with HBpin to generate the homoallylic boronate ester with simultaneous regeneration of iron–hydride complex A (Scheme 553).
Later, Wang and co-workers reported the catalytic hydroboration of N-heteroarenes using the N2-bridged diiron complex [Cp*-(Ph2PC6H4S)Fe]2[(μ-N2)] (22) in the presence of pinacolborane in C6D6 at 50 °C.920N-Borylated-1,2-reduced derivatives (61–99% yield) were obtained using this reaction strategy in high regioselectivity (Scheme 554, catalyst 22).
In the same year, Bai and co-workers reported a low-coordinated homoleptic tri-tert-butylphosphoranimido iron(II) dimer for the hydroboration of carbonyl compounds at room temperature (Scheme 555, catalyst 23).934 Various aldehydes and ketones were converted to the respective alcohol in 54–90% yield in the presence of the iron(II) catalyst (used in low catalytic amount) with HBpin in a solution of benzene for 13 h after simple acidic quenching. Simultaneously, Tong and Wang also reported the room temperature hydroboration of carbonyl compounds using an iron(II) hydride complex bearing a tetradentate PSNP ligand in combination with pinacolborane in a solution of C6D6 for 20–40 min (Scheme 555, catalyst 24, condition E).935
In 2018, Hein and Baker synthesized a five-coordinated, paramagnetic imine-coupled Fe(II) complex, [Fe (N2S2)2] (used in low catalytic amount (0.1 mol%)), which catalyzed the selective hydroboration reaction of carbonyl compounds in the presence of pinacolborane at room temperature to deliver the borate ester in 26–99% yield (Scheme 555, catalyst 25, condition F).936 Noteworthily, this iron(II) complex is prepared from the readily available benzothiazolidine ligand [SMeNHS] having a potentially labile thioether donor. The advantage of this reaction methodology is the chemoselectivity of the aldehyde group in the presence of ketones. Various mono and disubstituted aldehydes are well tolerated under the reaction conditions, even in the presence of various functional groups such as nitriles, alkenes, amines and halides.
Subsequently, Geetharani and co-workers developed easily synthesized iron(II) amide complexes that catalyze the selective hydroboration of carbonyl compounds in the presence of pinacolborane without any additive (Scheme 555, condition B).937 Various aldehydes having substituents such as methoxy, cyano, nitro, hydroxyl, and halides are well tolerated under the reaction conditions. Interestingly, the wide functional group tolerance of this reaction methodology is much better than the methodologies of Bai and co-workers934 using Fe2(NPtBu3)4 (do not contain any examples having cyano and hydroxyl groups) and Findlater and co-workers933 using Fe(acac)3 (do not contain any examples having cyano, nitro and hydroxy groups). Interestingly, this methodology is also chemoselective. Clearly, α-unsaturated aldehyde reacts with only the carbonyl functionality, keeping the alkene moiety intact.
Concurrently, Zhang and co-workers reported the catalytic hydroboration of ketones with pinacolborane using the 2D iron(II) coordination polymer (CP) of a divergent 4,2′,6′,4′′-terpyridine(tpy) derivative (Scheme 555, condition C).938 Various ketones having different functional groups such as nitro, nitrile, alkene, alkynes and amides are well tolerated under the reaction conditions to provide the boronate ester in good to excellent yield. These boronate esters on hydrolysis via silica gel column chromatography are converted to the corresponding alcohols. Notably, this solid iron catalytic system is more active towards the hydroboration reaction of ketones compared to aldehydes, exhibiting the opposite trend from the other iron-catalyzed methods for hydroboration.932,936
In 2018, Gade and co-workers reported an ultrafast iron-catalyzed reduction of functionalized ketones using a molecularly defined chiral boxmi iron alkyl complex (used in a low catalytic amount of 0.5 mol%) (Scheme 556, complex 26).939 This reaction strategy led to the enantioselective generation (ee up to >99%) of halohydrins, oxaheterocycles (oxiranes, oxetanes, tetrahydrofuran and dioxiranes) and amino alcohols via the boronate ester intermediate. Interestingly, the gram-scale synthesis of 3-chloro-1-phenylpropan-1-ol was successfully executed using this reaction methodology. Moreover, this chemical is the basis for the formation of the anti-depression drug (R)-fluroxetine following the original synthesis by Corey and co-workers.940
Around the same time, Darcel and co-workers synthesized a series of dicarbonyl PCP iron hydride complexes for the selective dehydrogenative borylation of styrene derivatives in combination with HBpin (Scheme 557, 27a or 28, condition B).942 Vinyl boronate esters were produced in high yield (up to 80% isolated yield) from styrene derivatives using a combination of either catalyst 27 or 28 with pinacolborane under neat conditions and UV irradiation (350 nm) for 24 h at room temperature.
8. Addition reaction
8.1 Carbometalation
The iron-catalyzed carbometalation across strained olefin was first introduced by Nakamura and co-workers (Scheme 558).943 Utilizing FeCl3 as the catalyst, the carbometalation of cyclopopenone acetal was achieved with both organomagnesium and organozinc reagents. They trapped the organometallic intermediate. Notably, in the absence of the iron catalyst, the reaction do not give the product. More interestingly, the asymmetric version was developed with the aid of phosphine- and amine-based chiral ligands. This opened up a new direction for iron-catalyzed asymmetric synthesis. Various aryl, alkyl and alkenyl organometallic reagents are compatible under the reaction conditions. In 2007, Kotora's group found that a specifically designed iron catalyst accomplished the selective alkylative cyclisation of 2-chloro-1,7-dienes in the presence of organoaluminium compounds (Scheme 559).944 Although no evidence was provided in support of carboalumination, a high probability of the addition of AlR3 across the double bond can be considered.Subsequently, Lam and co-workers used trialkylaluminium reagent to carbometalate electron-poor cyclopropenes, which undergo ring opening (Scheme 560).945 Expectedly, the regioselectivity is dictated by substitution on the alkene, where the more substituted carbon is preferentially attacked by the alkyl nucleophile. A plethora of different electron-poor cyclopropenes are tolerated under the reaction conditions. In a few instances, even lower than an equivalent amount of trialkylaluminium produced a good product yield, indicating the possibility of multiple alkyl group transfer.
The plausible mechanism suggests that the presence of ester groups in the ring is essential to stabilize the intermediate during the reaction. Later, it was shown that tuning of the ligand can suppress ring cleavage through electrophilic trapping.946 Heterobicyclic alkenes undergo carbometalation in the presence of FeCl3 and ZnR2 but do not show ring opening in the presence of electron-deficient phosphine-based ligands (Scheme 561). A suitable electrophile then replaces the metal from the organometallic intermediate to provide doubly substituted bicyclic heterocycles. A number of possible electrophilic substitutions such as deuteration, iodination, alkylation, and acylation make it a versatile methodology. More promisingly, the asymmetric version was also achieved by using chiral phosphine ligand systems.
The iron-promoted carbometalation of alkynes was demonstrated by Hayashi using arylmagnesium bromide (Scheme 562).947 Simple 4-octyne upon reaction with a Grignard reagent in the presence of Fe(acac)3 provides a carbometalated intermediate, which can be trapped by various electrophiles such as H2O, D2O, and PhCHO to generate the corresponding substituted alkenes. Although CuBr is required to increase the yield, it does not take part in addition across the triple bond, rather it promotes the transmetalation step. Later, this reaction was improved with the aid of a catalytic carbene ligand.948 Fe(acac)3/carbene not only enhanced the reactivity, but also diversified the substrate scope of the reaction, including electrophilic substitution such as iodination and allylation. The authors attributed this enhancement to the ease of the transmetalation step with the Fe(carbene) complex, which is believed to be the bottleneck with Fe/Cu catalysis. This protocol can also be extended to propargyl and homopropargyl alcohol to obtain substituted allyl and homoallyl alcohols.949 However, only a phosphine ligand is sufficient in conjunction with an iron catalyst to promote alkylmagnesiation. High regioselectivity is observed due to coordinating nature of the –OH group. Two intermediates (Scheme 563), viz. (vinyl)Fe-coordinated with alcohol and magnesium-included cyclic intermediate, are presumed to be possible and can lead to the formation of the final product via transmetalation. Later, the Fe–Cu cooperative reaction was further extended to perform sequential reactions merging with alkyl exchange under the same conditions to provide diversely substituted olefins.950 Conjugative dialkynes can also be carbometalated across one of the triple bonds to provide vinylalkynes.951 Interestingly, when phenyl-silyl substituted unsymmetrical diyne is used, selective addition at the phenyl terminal is observed.
Alkyllithium reagents also behave in a similar fashion. Hosomi and co-workers found that butyllithium can be added across a triple bond using Fe(acac)3 catalyst in toluene at −20 °C (Scheme 563).952 Several electrophiles can also be trapped during the reaction; however, only heteroatom-containing substrates undergo carbolithiation. This preference is due to the chelation effect that can be administered by the heteroatom to the catalyst. This discrepancy was resolved later using a different condition using FeCl3 in diethyl ether (Scheme 564).953,954 It has been found that the iron catalyst was first reduced by alkyllithium, which acted as the real catalyst. Thus, the efficiency of the reaction was enhanced by pre-treatment with zinc to reduce iron. Inspired by the catalytic system for carbomagnesiation, carbolithiation was further extended to aryl and vinyl lithiation as well. In the case of aryllithium addition, the reaction proceeds with the substituent at different positions even with silyl substitution.
Urabe and co-workers found that if enynes are used as substrates, intramolecular cyclization occurs to provide five- and six-membered products depending on the distance between the double and triple bond, with an exogenous double bond as a residue of alkyne (Scheme 565).955 The reagent combination of FeCl2/t-BuMgCl is the best combination for this transformation. The substrates invariably require electron-withdrawing groups such as ester, amide, and nitrile at the terminal position of both the alkene and alkyne. However, when it is applied to dienes, unexpectedly fused cyclic ketones are observed as the final product due to rapid Dickmann condensation to provide a thermodynamically more stable product. An analogous protocol was reported specifically for the synthesis of pyrrole and THF derivatives containing one exocyclic double bond from amines and ethers.956 However, diethyl zinc was mentioned to be essential, which probably assists the elimination step in order to obtain final product.
Allenes when subjected to Grignard reagents in the presence of Fe(acac)3 afforded α,β-unsaturated alkenoates with predominant Z-selectivity (Scheme 566).957–959 The dienolate intermediate can be trapped with acyl chloride to form the Z-isomer of the α-acylated product. However, the same reaction selectively provides the E-isomer when allyl carboxylic acid ester is used. Moreover, using allylic acetate in conjunction with Pd catalyst affords α-allylated alk-3(Z)-enoate and allyl halide with CuI selectively gives the E-version of the product. The same strategy can be utilized to synthesize allyl silanes by employing silyl substituted allenes.
In 2009, Tu and co-workers developed FeCl3-catalyzed addition of the α-C–H bond of primary alcohols across olefinic double bonds (Scheme 571).964 It was observed that the alkyl group selectively coupled at the less hindered side of the alkene. This behaviour and other experiments support the mechanism in which the metal abstracts a hydrogen from the α-position of the alcohol to form FeIV–H species and an alkyl radical. Since the addition of the radical to the less hindered side of the olefin will produce the more stable intermediate, the above-mentioned selectivity was observed. Various aliphatic alcohols including phenethyl alcohols could be coupled with a number of styrene derivatives.
Iron-catalyzed E-selective head-to-tail styrene dimerization was demonstrated by Corma et al. in a regioselective manner.965 In this respect, it was observed that a comparatively higher acidic iron catalyst Fe(NTf)3 works best in 1,4-dioxane. Although various aryl substituted styrenes yield products, their internal analogues do not produce any product. Through controlled reactions, it was suggested that a benzylic cationic Fe(III) species is formed, which is attacked by another co-ordinated styrene molecule, producing the coupled product, and the catalyst is regenerated simultaneously. Moreover, α-EWG ketene dithioacetals can also be added to styrene molecules effectively in an iron-catalyzed reaction.966
Although the possibility of forming three different products including styrene dimerization exists, Yu and co-workers optimized the reaction in favor of the formation of R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SR′2 with Fe(OTf)3 (Scheme 572). A related mechanism was been suggested, where the stabilization by two –SR groups towards the cationic intermediate could be attributed as the driving force for heteromolecular coupling over styrene dimerization.
SR′2 with Fe(OTf)3 (Scheme 572). A related mechanism was been suggested, where the stabilization by two –SR groups towards the cationic intermediate could be attributed as the driving force for heteromolecular coupling over styrene dimerization.
It was found that treating olefins with iodobenzene in the presence of FeCl2 and NaBH4 can yield the same result as hydroalkylation. The intramolecular version of the reaction produces the cyclized product with diastereoselectivity upon treatment with FeCl2/NaBH4 in acetonitrile (Scheme 573).967 The use of deuterated NaBD4 confirmed that the hydrogen of the other end of the alkene is transferred from the reducing agent. However, a different mechanism was proposed, where the iron catalyst is majorly involved in the electron transfer process to generate a radical from iodoalkane. The cyclized radical then abstracts a hydrogen radical from BH4−.
The hydroalkynylation of norbornene with simple phenyl acetylene was first reported by Sakakura and co-workers with limited diversity (Scheme 574).968 The yield of the reaction is highly dependent on hardness of the metal ion, and in turn the acidity.
Thus, Fe3+ was found to be the best catalyst among many others, which is also consistent with the ease of the formation of the cationic intermediate. The co-ordinated acetylene then reacts with the cationic species quite similar to the other two versions to provide the hydroalkynylated product. FeCl3 also catalyzes the dimerization of terminal alkynes to provide enynes in the presence of potassium alkoxide.969 Although the yields are moderate, the E/Z selectivity is poor and aliphatic alkynes do not produce any results. Improved selectivity was realised later upon changing the ligand DMEDA to dppe. Not only different substituents such as halide, methoxy and amine on the arene ring were tolerated under this protocol, but a thiophene-substituted terminal alkyne also gave a promising yield.970 It was observed that the yield decreases drastically in the presence of a radical quencher. Thus, a radical pathway was suggested with Fe in the 4+ oxidation state as one of the key intermediates.
Li and co-workers reported the iron-catalyzed 1,2-alkylarylation of activated alkene.973 Different substituted oxindoles were synthesized from N-alkyl–N-aryl acrylamide by alkylation with heteroatom-containing alkanes followed by intramolecular arylation (Scheme 576). The reaction was best realized with catalytic FeCl3 in conjunction with DBU as the ligand and TBHP as the oxidant. The kinetic isotope effect (kH/kD = 1) and radical trapping experiment suggested a radical mechanism may occur. The hydroperoxide in the presence of a, iron catalyst abstracts a hydrogen atom from the alkane adjacent to the heteroatom. Thus, the alkane radical attacks acrylamide and subsequent ring formation occurs. Later, it was observed that the alkyl radical can also be generated from the peroxides to carry out 1,2-methylarylation in a mechanistically similar fashion.974,975 The alkoxy radical rearranges itself to form a methyl radical, leaving carbonyl, which can enter the reaction cycle. Further, alcohols and PhSO2CF2I instead of ethers were also established to be compatible with slight modification of the reaction conditions.976,977 The latter case uses hydrogen peroxide to generate a radical from PhSO2CF2I, and thus provides fluorinated heterocycles.
The iron-catalyzed reaction of 2-biarylmagnesium bromide with alkyne and 1,2-diyne to synthesize phenanthrene and bis-phenanthrene derivatives via [4+2] benzannulation was described earlier in Section 2 (Schemes 55 and 56).81,82 Also, the reaction of aryl Grignard reagents and arylindiums with alkynes to form naphthalene derivatives via [2+2+2] benzannulation was depicted in the same section (Schemes 55 and 56, respectively).81,82
The N-alkyl aniline can form an iminium ion in the presence of a base, and thus can also undergo annulation with alkyne in the presence of FeCl3 (Scheme 577). Indeed, a variety of substituted quinoline derivatives were reported by Liu's group.978
Electrophilic addition of this cation to alkyne results in the formation of a vinyl cation. The halide is then transferred from XFeCl3− to yield the alkenyl halide (Scheme 579). The styrene double bond can also be decorated with C–Cl and C–C bond formation in an FeCl3-catalyzed reaction.980 Mechanistically, 1,3-dithiane in the presence of NCS is halogenated at the 2-position (Scheme 580), which forms two radicals in the presence of Fe(III). Sequential coupling of these radicals with styrene forms the product. The formation of the favoured benzylic radical possibly drives is towards observed regioselectivity.
Similarly, the addition of perfluoroiodides across terminal alkynes can be achieved (Scheme 581).981 FeBr2 has been used as a catalyst in this particular transformation in dioxane solvent at 60 °C. A comparable radical mechanism was speculated for this transformation. A series of cross-coupling reactions was performed by the authors to show the applicability of the transformation. FeBr2 catalyzes the addition of acid chloride to alkynes and the formation of β-chloro vinyl ketones.982 However a concerted mechanism has been proposed instead of radical. The chloroacylation reaction was further extended by Cheng's group with a broad substrate scope.983 The authors indeed support the concerted mechanism (Scheme 582) and predict that the favoured intermediate dictates the regioselectivity being halide substitution at the benzylic position.
The FeCl2-catalyzed carbonylation–peroxidation of alkenes by alkylperoxide and aldehydes was reported by Li and co-workers (Scheme 583).984 An alkoxy radical is catalyzed by Fe(II), consequently forming a carbonyl radical, which is trapped by TEMPO. Thus, radical attack on the alkene and subsequent interaction with another peroxide molecule drive the reaction forward. In addition to the diverse substrate scope, authors showed the transformation of a selected substrate to epoxide by treatment with DBU.
A porphyrin-based diiron complex was employed by Sorokin's group to achieve the hydroacylation of olefins in the presence of peroxide (Scheme 584).985 The electronic interplay between the two iron atoms the causes facile formation of an alkoxy radical, and subsequent radical additions result in hydroacylation.
Similarly, Plietker's group showed that C–O and C–C bond formation can be realized in the alkoxy allylation of highly activated olefins (Scheme 585).986 An allylalkyl carbonate was used for this purpose in presence of Bu4N[Fe(CO)3(NO)] catalyst. The interaction of the allylalkyl carbonate with the catalyst eliminates CO2 and forms an [Fe]-allyl complex together with an alkoxide ion. Nucleophilic attack of the alkoxide onto the activated olefin generates another carbanion, which reacts with [Fe]-allyl to form the product. However, this protocol fails with substituted allyls. A three-component reaction was then developed, where Boc-protected allyl alcohols were used as the allylic source and simple alcohols as the alkoxide source to obtain identical products. This methodology was also extended to phosphono-allylation using (MeO)(O)PH instead of alcohol.987
The synthesis of dihydrobenzofuran derivatives was reported by the groups of Pappo and Lei separately from phenol and olefins (Scheme 586).988,989 In the latter case, the reaction shuts down in the presence of TEMPO and in the absence of DDQ, and thus the formation of a phenoxy radical was proposed. The phenoxy radical adds to the olefin aided by the iron catalyst to form the cyclized product, giving different substituted dihydrobenzofurans.
N-Aryl-N-(2-alkynyl)toluenesulfonamides undergo cyclization in the presence of FeCl3 under reflux conditions in DCE (Scheme 587).990 The alkyne activated by the iron catalyst undergoes 6-endo-dig cyclization to form an ionic species, from which the tosyl group leaves to yield the quinoline moiety. A variety of quinolines have been shown to be prepared using this method. Interestingly, when a structurally similar alkynyl amide was treated under similar conditions, spirocyclic pyrimidine was observed instead of the formation of a six-membered cycle (Scheme 587).991 The iron-catalyzed synthesis of oxindoles has been achieved via the formation of C–S and C–C bonds in the reaction between N-alkyl–N-arylacrylamide and arenesulphinic acids.992 The reaction proceeds through the formation of an ˙SO2Ar radical under aerobic conditions. Subsequent attack on the acrylamide and cyclization construct differently substituted oxindoles. 1,4-Diphenyl-2-trifluoromethylbut-1-en-3-yne upon reaction with (RX)2 (X = S, Se) provides poly-substituted naphthalenes (Scheme 588).993 Since (RX)2 is known to be a good candidate as a radical generatorit was assumed that it can cyclize similar to the formation of quinoline. However, a radical route is expected instead of an ionic pathway, which was indeed observed as the reaction is quenched upon the addition of TEMPO.
Deng and co-workers reported the synthesis of vinyl sulfides by reacting alkynes with sodium salts of arylsulphinates (Scheme 589).994 Fascinatingly, in the formation of tetra-substituted vinyl thioethers, both aryl and thioaryl groups are provided solely from sodium sulphinates; however, with uncontrolled stereoselectivity.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and then to the other carbonyl (Scheme 591). An optimum CO pressure is essential since high concentration deactivates the catalyst. A number of alkynes and primary amines are presented to be suitable for this transformation. The scope of this reaction was further extended to the synthesis of highly complex succinimides by combining it with Sonogashira coupling.996 Moreover, oxidative dehydrogenation of succinimides with DDQ give the corresponding maleimides also. In this regard, the synthesis of Himanimide A was performed with improved efficiency, and the first synthesis of Himanimide B was also reported.997
O bonds and then to the other carbonyl (Scheme 591). An optimum CO pressure is essential since high concentration deactivates the catalyst. A number of alkynes and primary amines are presented to be suitable for this transformation. The scope of this reaction was further extended to the synthesis of highly complex succinimides by combining it with Sonogashira coupling.996 Moreover, oxidative dehydrogenation of succinimides with DDQ give the corresponding maleimides also. In this regard, the synthesis of Himanimide A was performed with improved efficiency, and the first synthesis of Himanimide B was also reported.997
The same group carried out extensive optimization to achieve mono-carbonylation.998 Indeed, it was found that selective mono-carbonylation is controlled by a specific ligand (Scheme 592) and reduced CO pressure to 10 atm. The scope of cinnamides are diversified with respect to both N-substitution and olefin substitution.
The iron-catalyzed synthesis of β-hydroxyesters was reported by Taniguchi and co-workers from alkenes and carbazates (Scheme 593).999 The hydrazine undergoes N2 elimination to form an alkoxycarbonyl radical, the addition of which to alkene generates a tertiary radical. Trapping of this radical by atmospheric oxygen results in the desired β-hydroxyesters. Various aryl, alkynyl, bicyclic and heterocyclic substituted olefins undergo product formation. The iron-mediated carboxylation of styrenes can be achieved from CO2 insertion (Scheme 594).1000 The addition of a bis(arylimine)pyridine ligand gives the best result together with Grignard reagent. The Grignard reagent (EtMgX) helps to activate the styrene molecule and forms an α-Grignard reagent with the substrate via transmetalation. Subsequent CO2 insertion gives the desired α-acid product. Surprisingly, reversal of selectivity is observed for ortho-methyl-substituted styrenes, presumably because of steric factors.
Both Yu's and Du's groups independently reported the synthesis of alkylester-substituted oxindole from N-alkyl N-aryl acrylamides and carbazates via arylalkoxycarbonylation (Scheme 595).1001,1002 This reaction uses FeCl2·4H2O together with TBHP in MeCN. A similar radical pathway is followed as that described for β-hydroxyester formation by carbazates; however, the termination step involves ring closing with the ortho-carbon center of the N-aryl group. Carbazates when react with 2-isocyanobiphenyl in the presence of Fe(acac)2 and TBHP to produce phenanthridine-6-carboxylates.1003 The use of a radical trapping agent stopped the reaction, and kH/kD was shown to be unity. Therefore, a similar radical mechanism can be proposed for this transformation also.
Asymmetric ring opening of meso-epoxides with anilines was reported by Ollevier's group for the enantioselective synthesis of 2-aminols.1007,1008 An iron(II)-per chlorate [Fe(ClO4)2] catalyst together with Bolm's ligand is effective for this transformation to obtain up to 90–96% ee (Scheme 596). Characterization of the active catalyst shows that the bipyridine-bound iron probably co-ordinates with the epoxide-oxygen and dictates the addition of amine through steric interference.
Meso-aziridines react with amines in the presence of FeCl2(mep) and AgSbF6 to give diamines. Various N-substituted aziridines and anilines are compatible with this reaction, providing excellent yields (Scheme 597).1009
A very interesting iron-catalyzed synthesis of quinoline from aniline and styrene oxide via C–O and C–C bond cleavage in tandem was presented by Wang and co-workers (Scheme 600).1010 It was proposed that the amine attacks the epoxide, which results in C–O bond breakage to form an enamine. Addition of another epoxide followed by ortho C–H bond activation of aniline and C–C bond cleavage forms a metallacycle. Reductive elimination and subsequent dehydrogenation give the final product. Despite the complicated mechanism of this reaction, is believed to be operational, and a good number of quinoline derivatives can be synthesized.
Propargyl epoxides react with Grignard reagent in the presence of 5 mol% Fe(acac)3 to provide allenols (Scheme 598).1011 Both terminal and internal alkynes show similar reactivity. It is evident that this reaction is consistent towards forming syn allenoles, which is opposite to that observed for Cu catalysis in the literature.
This is probably because of the metal co-ordination with oxygen during the transfer of the nucleophile. This reaction was also effectively utilized in the total synthesis of amphidinolide X.1012,1013
The activated vinyl cyclopropane ring can be opened in the presence of a suitable Grignard reagent (Scheme 599).1014 A secondary alkylmagnesium bromide works best for the transformation. The carbanion generated in the process can also be trapped by allyl bromide or propargyl bromide. Although reasonable yields are obtained, the E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio is not consistent. Amusingly, alkynyl cyclopropane also provides allene under the conditions. Plietker et al. also observed similar reactivity of the allylic C–C bond of vinylic cyclopropanes; however, they utilized the combination of TBAFe and NHC ligands (Scheme 601).1015 The iron-catalyzed ring opening of 7-oxabicyclo[2.2.1]hept-2-enes was performed by Irie's group to obtain 5,6-cis-disubstituted cyclohexa-1,3-dienes (Scheme 602).1016 The monoene product obtained after ring opening in the iron(III) hydroxide oxide-catalyzed reaction was further transformed to the diene by treatment with Zn. The FeCl3-catalyzed ring opening of tetrahydrofuran derivatives was documented by Sajiki's group (Scheme 603).1017 They have trapped the ring opening intermediate with different nucleophiles to obtain the corresponding substituted alcohols.
Z ratio is not consistent. Amusingly, alkynyl cyclopropane also provides allene under the conditions. Plietker et al. also observed similar reactivity of the allylic C–C bond of vinylic cyclopropanes; however, they utilized the combination of TBAFe and NHC ligands (Scheme 601).1015 The iron-catalyzed ring opening of 7-oxabicyclo[2.2.1]hept-2-enes was performed by Irie's group to obtain 5,6-cis-disubstituted cyclohexa-1,3-dienes (Scheme 602).1016 The monoene product obtained after ring opening in the iron(III) hydroxide oxide-catalyzed reaction was further transformed to the diene by treatment with Zn. The FeCl3-catalyzed ring opening of tetrahydrofuran derivatives was documented by Sajiki's group (Scheme 603).1017 They have trapped the ring opening intermediate with different nucleophiles to obtain the corresponding substituted alcohols.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S to the zwitterionic intermediate of the cyclopropane (Scheme 606).
S to the zwitterionic intermediate of the cyclopropane (Scheme 606).
Yoon and co-workers presented that iron-catalyzed ring expansion is also feasible with oxaziridines (Scheme 607).1023 Styrenes react with N-sulfonyl oxaziridines in the presence of Fe(acac)3 in acetonitrile to form oxazolidines in excellent yields. It was found that the use of Fe(NTf2)2 in conjunction with a chiral 4,5-dinaphthyl-substituted bis(oxazoline) ligand provides enantioselective transformation with high enantiomeric excess.1024 Moreover, chiral 1,2-hydroxyamine can be obtained by the straightforward ring opening of these oxazolidines.
Iron-catalyzed ring expansion of aziridines with isoselenocyanates provides iminoazoselenolidine in water medium (Scheme 608).1025 This reaction is also believed to proceed via the [3+2] cycloaddition mechanism. A plethora of differently substituted iminoazoselenolidines have been synthesized. Fascinatingly, substitution of Se with N, O or S does not alter the reactivity and different classes of heterocycles can be obtained. In another report, Waser and co-workers established the [3+2] annulation of aldehydes to donor–acceptor cyclopropanes (Scheme 608).1026 The use of phthalimide and gem-diester as the donor–acceptor combination was observed to be the best for the formation of tetrahydrofurans.
Various iron complexes have been reported for the synthesis of cyclic carbonates from epoxides with carbondioxide.1027–1033 Among them, triphenylamine-based iron complexes have been extensively studied and successful towards the catalytic ring expansion of epoxides with CO2. Kleij and co-workers synthesized an iminophenol-based complex, which in conjunction with NBu4X catalyzes the formation of dioxolanones. Döring and co-workers showed that the well characterized tetradentate ligated complex can also carry out this transformation with a high turnover number in solvent-free conditions (Scheme 609).
8.2 Cycloaddition
The iron-catalyzed cyclopropanation reaction was first investigated with iron(II)porphyrin systems.1034 Styrenes were found to be highly susceptible towards [2+1] cycloaddition with diazo compounds such as EDA (Scheme 610). It is believed that the reaction proceeds via the formation of Fe-carbene species that interacts with olefin to form a radical intermediate, which was also supported by secondary KIE. The free rotation of the radical allows the opportunity to minimize steric hindrance, and hence the trans-product is obtained in a major amount, although a few anomalies are also observed. For obvious reasons, the ratio depends on the steric factors of substituents on the porphyrin ring and diazo compound.1035 Lowering the temperature also significantly enhances the trans-selectivity. For instance, in the cyclopropanation of styrene with EDA with (TTP)Fe, the trans/cis ratio significantly changes from 8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 29 ± 2 upon changing the temperature from room temperature to −78 °C. In a comparative study, Woo and co-workers found that (tmtaa)Fe and (saldach)Fe show poorer reactivity compared to (TTP)Fe; however, the result for (tmtaa)Fe slightly improved with p-tolyldiazomethane.1036
1 to 29 ± 2 upon changing the temperature from room temperature to −78 °C. In a comparative study, Woo and co-workers found that (tmtaa)Fe and (saldach)Fe show poorer reactivity compared to (TTP)Fe; however, the result for (tmtaa)Fe slightly improved with p-tolyldiazomethane.1036
Nguyen and co-workers developed μ-oxo-bis[(salen)iron(III)] complexes to catalyze the cyclopropanation reaction from diazo compounds (Scheme 611).1037,1038 These air-stable catalysts do not require an inert atmosphere like the previous catalysts. A fraction of the diazo reagent first reduces the iron(III)–salen dimer to iron(II)–salen species, which again reacts with diazoalkane to form an Fe–carbene intermediate. The formation of ethyl glyoxylate in the process of catalyst reduction with EDA can be spectroscopically detected. It was observed that substitution on the ligand does not have an adverse effect of the reactivity, but the use of an internal styrene causes the yields to decrease drastically compared to terminal styrenes.
Lai and co-workers used the Halterman iron porphyrin [P*Fe(Cl)] to achieve asymmetric cyclopropanation (Scheme 612).1039 The catalyst provides moderate trans-selectivity and reasonable enantiomeric excess of the trans-product. The presence of the carbene intermediate can be detected spectroscopically. The Hammett study suggested that the carbene intermediate may be slightly electropositive at the carbene center and the benzylic position may be partially cationic in the transition state. Interestingly, donor solvents such as THF increases the trans-selectivity probably through co-ordinating to the iron center at the sixth position. A similar asymmetric reaction was reported by Simonneaux's group with 2,2,2-trifluorodiazoethane to obtain trans-1-trifluoromethyl-2-arylcyclopropane.1040 The catalyst was also transformed into a water-soluble catalyst by sulphonate substitution on the ligand and a comparable result was obtained.1041
Chiral C1 and C2-2,2′:6′,2′′-terpyridine iron complexes can catalyze asymmetric cyclopropanation reactions effectively.1042 The native chloro-co-ordinated complex is reluctant towards catalysis, and thus requires treatment with AgOTf prior to the reaction. The bulkiness of the substitution on the ligand has a positive effect only up to a certain extent. However, unlike previous cases, a U-shaped Hammett plot was observed. A DFT study on cyclopropanation with Cp(CO)(L)Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHR revealed that there are two possible pathways that the metal-carbene can add to the olefin: (1) co-ordination of ethene to iron, gradual interaction with the iron–carbene bond to form a 4-membered metallacycle and subsequent reductive elimination, and (2) direct attack of the ethene double bond on the carbene carbon (Scheme 613).1043,1044
CHR revealed that there are two possible pathways that the metal-carbene can add to the olefin: (1) co-ordination of ethene to iron, gradual interaction with the iron–carbene bond to form a 4-membered metallacycle and subsequent reductive elimination, and (2) direct attack of the ethene double bond on the carbene carbon (Scheme 613).1043,1044
Carreira's group in 2010 developed the cyclopropanation of styrenes by in situ-generated F3CCH carbene from F3CCH2NH2·HCl in aqueous medium (Scheme 614).1045 The reaction is best catalyzed by 3 mol% of [Fe(TPP)Cl] in water to obtain trans-1-trifluoromethyl-2-arylcyclopropanes in excellent yields and exclusively trans-product. Later, this protocol was extended to synthesize trifluoromethyl-substituted alkynyl and vinylcyclopropanes.1046 Remarkably, only the terminal double bond undergoes cyclopropanation and a single trans-isomer is obtained. A similar approach has been also utilized to synthesize arylcyclopropyl esters via in situ carbene formation from EtO2CCH2NH3Cl.1047 However, only the maximum of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 trans-selectivity has been obtained in this instance.
1 trans-selectivity has been obtained in this instance.
N-Methyl-N-nitroso-p-toluenesulfonamide in 6 M KOH can form diazomethane, which was used as an advantage by Carreira's group to add methylene across the double bond to synthesize simple cyclopropanes.1048 Although the use of 6 M KOH left limited choices for catalyst, the previous best catalyst [Fe(TPP)Cl] is also efficient for this transformation. A similar strategy was also reported by Werz and co-workers using N-methyl-N-nitroso-m-carboxyphenylsulfonamide as the diazomethane precursor (Scheme 614).1049
To improve the stereoselectivity, Gallo and co-workers synthesized an exotic iron–porphyrin complex with C2 symmetry.1050 A TOF of 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 was obtained with mere 0.005 mol% catalyst loading for the cyclopropanation of α-methyl styrene with EDA. Moreover, >99
000 h−1 was obtained with mere 0.005 mol% catalyst loading for the cyclopropanation of α-methyl styrene with EDA. Moreover, >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 trans-selectivity was observed with an enantiomeric excess of up to 87%.
1 trans-selectivity was observed with an enantiomeric excess of up to 87%.
An analogous iron-catalyzed aziridination reaction via [2+1] cycloaddition of nitrene and alkene by [Fe(TPP)Cl] was documented by Mansuy and co-workers in 1984.1051 However they showed the superiority of the corresponding Mn complex over the iron complex in most instances. [Fe(TPP)Cl]-catalyzed aziridination was thoroughly catalyzed by Zhang's group using bromamine-T as the nitrene source (Scheme 615).1052 The reaction is not restricted to only styrenes, but different cyclic and acyclic olefins have also been shown to undergo the nitrene transfer reaction. It is believed that bromamine-T first reacts with [Fe(TPP)Cl] to form Fe = nitrene, which interacts with olefin to provide the final product. Interestingly, structurally similar iron–phthalocyanines have also been found to be equally effective in carrying out the aziridination of styrene derivatives and cyclohexene.
Hossain and co-workers introduced a chiral iron-pybox complex in order to achieve chiral aziridination.1053 Interestingly, they used N-benzylideneaniline as a substrate and EDA as a carbine source for the formation of aziridine (Scheme 616). Although they could induce chirality, the yields and enantiomeric excess were poor. The experimental and DFT studies carried out by Halfen's group on nitrene transfer aziridination by various non-heme iron complexes revealed that there is more than one pathway involved, and the most prominent one may be the formation of a four-membered metallacycle similar to carbene chemistry.1054
Recently, Latour, Maldivi and Maiti reported a rigorous mechanistic investigation on the non-heme iron complex-mediated aziridination of styrenes by means of both experiment and computation.1055 It was found that the reactivity of these iron-nitrene complexes is dependent on the electron affinity of the active species, where the higher the electron affinity, the higher tendency for attack on the double bond. Thus, [(2PyN2Q)FeIV(NTs)]2+ was found to be a better catalyst than [(N4Py)FeIV(NTs)]2+ for this purpose. The reason being presence of quinoline in the former results in weaker ligation by the nitrogen, and hence increases the electron affinity of iron.
Bolm and co-workers were able to optimize the aziridination of olefins using iron triflate as a catalyst (Scheme 617).1056 In the presence of 5 mol% of Fe(OTf)2, various styrenes and cyclooctene provide up to 90% yield in MeCN. Later, it was found that the use of ethylmethylimidazolium bis[(trifluoromethyl)sulfonyl]amide improved the reaction drastically, for instance styrene yielded >95% of the product compared to 82%.1057 In this case, the chiral pybox ligands also show potential chiral inductivity; however, with very low ee.
The iron-catalyzed [2+2] cycloaddition of imines with ketenes was demonstrated by Fu's group in 2005 (Scheme 618).1058 A chiral iron complex was used to obtain enantioselective β-lactams. However, the most intriguing part is that the cis and trans selectivity were highly dependent of the substitution at the imine nitrogen, and while the N-tosyl imine gave the cis product majorly, N-triflyl showed higher cis selectivity. Thus, two different mechanisms are thought to occur, originating from the different stabilities of the tautomerized forms of the imines. Since N-tosyl imine prefers the neutral form, the ketene attacks the imine, and because the more stable conformation of the N-triflyl imine is the ionic form, the reverse attack may be operative.
Chirick and co-workers developed the iron complex (iPrPDI)Fe(N2)2 {iPrPDI = 2,6-(2,6-iPr2C6H3NCMe)2C5H3N}, which catalyzed the [2+2] cycloaddition of α,ω-dienes to obtain the corresponding bicyclic compounds (Scheme 619).1059 A similar type of complex was later employed for the formation of vinyl cyclobutane from the cycloaddition of butadiene and ethylene.1060 It was proposed that to the co-ordinated butadiene, ethylene adds oxidatively to form crystallographically characterized intermediate X. Subsequent reductive elimination yields the final product. In another report, Waser and co-workers documented the FeCl3·Al2O3-catalyzed cycloaddition of enimides and alkylidene malonates to obtain β-amino acid cyclobutane derivatives.1061
The synthesis of cyclopentenones from alkenes and/or alkynes via [2+2+1] cycloaddition catalyzed by Fe(CO)5 was first reported in 1953. Since then, numerous reports have been published on studies centred on this reaction and its application towards organic synthesis.1062–1068 The reaction proceeds via ligand substitution by two alkynes (Scheme 620). Oxidative coupling of the co-ordinated alkynes then forms a five-membered metallacycle. Insertion of one CO group expands the ring size to six, and subsequent reductive elimination provides the desired product. [2+2+1] cycloaddition can also be applied to the cyclization between ketamine, alkene and carbon monoxide.
Itoh's group reported the [3+2] cycloaddition of 1,4-quinones and styrenes to obtain 2,3-dihydrobenzofuran derivatives (Scheme 621).1069 The most suitable catalyst was alumina-supported Fe(ClO4)3 in acetonitrile. Later, it was observed that ionic liquids have an adverse effect on the reaction, and accordingly improved the efficiency significantly. The authors proposed a mechanism for the transformation, where the Fe3+ ion oxidizes the styrene moiety to form a radical cation and Fe2+. This Fe2+ in turn reduces quinone to deliver a radical anion, which reacts with the previous intermediate to form a new 5-membered ring. The ionic behaviour of the intermediate is also supported by the anion dependency of the ionic liquid. An asymmetric [3+2] cycloaddition between diphenylnitrone and methacrolein was reported with a chiral iron-cyclopentadienyl base catalyst.1070 Although isoxazolidines are formed in decent yields, the regioselectivity is poor in most cases. In another excellent method, Bolm and co-workers reported the synthesis of tetrazoles from Ar-CN and TMS-N3.1071 Fe(OAc)2 catalyzed the reaction in DMF/H2O at 80 °C to provide excellent yields of tetrazole derivatives.
Wang and co-workers documented the cycloaddition of azomethine ylides and dialkyl maleates in the presence of FeCl2 and a chiral bidentate ligand.1072 The imino ester is believed to co-ordinate with the metal center to form an azomethine ylide dipole followed by attack from maleates. The chiral ligand controls the orientation of the second edition to provide chiral pyrrolidines. Interestingly, maleimides can also be reacted under the protocol to form bicyclic chiral heterocycles. A similar asymmetric [3+2] cyclization of isocyanoesters with azodicarboxylates was also observed in the presence of an Fe(acac)3 catalyst.1073
Okamoto's group extensively optimized the cyclotrimerization of triynes (Scheme 622).1074–1076 Both FeCl3 and FeCl2 showed similar reactivity towards the [2+2+2] cycloaddition in the presence of a suitable NHC ligand. The reaction initiates with the oxidative cyclization of two alkynes followed by insertion to the last alkyne. Finally, reductive elimination provides the aromatic end product. This procedure can be extended towards the synthesis of biaryls from hexaynes.
9. Elimination and Isomerization
9.1 Elimination
The first iron-catalyzed elimination reaction was reported by Beller's group.1077 [Et3NH][HFe3(CO)11] in the presence of silanes catalyzes the formation of nitriles from amides (Scheme 623). Silane swaps amide hydrogens to form bis(silyl)amide, which is in equilibrium with N,O-bis(silyl)imidate, dominated towards the latter. Cyanide then forms upon elimination of disiloxane. Different substituted aryl amides, benzothiophenes, furans and even vinyl amides are transformed to their corresponding nitrile products efficiently. Later, Enthaler drastically simplified the reaction conditions and showed that the reaction proceeds excellently in the presence of FeCl2·4H2O and N-methyl-N-(trimethylsilyl)trifluoroacetamide at a lower temperature.1078The dehydration of fructose and sucrose has also been shown to be catalyzed by FeCl3–Et4NBr to give 5-hydroxymethylfurfural (Scheme 624).1079 However the same method results in very poor yield for glucose. This indicates ketose is more susceptible to undergo dehydration than aldose under the reaction conditions. For the production of furfural from xylose, a biphasic system was used. FeCl3·6H2O, NaCl and xylose were employed in aqueous medium combined with 2-methyltetrahydrofuran as the second phase to obtain a yield of up to 70%.1080 Most interestingly, crude lignocellulose provided furfural in the organic phase at a rate of 3.5 g L−1 h−1. Later, the synthesis of 5-hydroxymethyl-2-furfural from fructose was scaled up using an imidazolium-attached polystyrene resin-supported NHC ligand and iron salt.1081 The bilayer extraction technique was utilized to obtain up to 73% yield and the catalyst was found to be reusable also.
Ryu's group in 2012 developed the iron-catalyzed decarbonylation of aliphatic carboxylic acid to form olefins (Scheme 625).1082 It was observed that FeCl3 in the presence of KI, Ac2O, 1,5-bis(diphenylphosphino)pentane (DPPPent) and CO catalyzes the reaction. However, although FeI2 produces a similar result, it fails in the absence of KI. Moreover, 20 atm pressure of CO is also required for the reaction to proceed. Thus, Fe(CO)mIn has been proposed to be the active species, which interacts with anhydride formed from Ac2O and the substrate. Subsequent CO removal and β-hydride elimination result in α-olefins. The dehydration of propargyl alcohols can be achieved in an FeCl3-catalyzed reaction in the presence of a 1,2,3-triazole ligand to form enynes (Scheme 626).1083 The chemoselectivity is controlled by the specific ligand by tuning the electronics of the iron center. Remarkably, they also synthesized very fancy 1,4-enediynes using this methodology.
9.2 Isomerization
In 1962, while working with π-ally iron carbonyl complexes, Pettit and co-workers found that iron carbonyl complexes can convert allylic alcohol to carbonyl compounds via the formation of an allyl-Fe intermediate.1084 Logan and co-workers in 1967 showed several examples of allyl alcohol isomerization using Fe(CO)5 as the catalyst under the combination of heating and radiation (Scheme 627).1085 Various groups studied the mechanism in-depth and showed different substrate compatibility using iron–carbonyl complexes under UV-irradiation.1086,1087 Among them, Grée's group pioneered not only Fe(CO)5-catalyzed enol–carbonyl isomerization, but various synthetic utilizations of this reaction.1088–1090 As described by Grée et al., the pentacarbonyliron complex undergoes two-CO ligand dissociation in the presence of light to form an olefin co-ordinated complex. Then a shift in the double bond takes place via the formation of a metal–hydride–allyl complex. The resultant α-enol undergoes rapid tautomerization into the corresponding saturated carbonyl compound. Both primary and secondary cinnamyl alcohols, and conjugated allyl alcohols can be converted to carbonyls.The formation of the enol intermediate triggered investigation of aldol-condensation.1091,1092 Indeed it was found that in the presence of an external aldehyde, the enol intermediate can be trapped to produce the aldol product in the presence of Fe(CO)5 catalyst, although the regioselectivity has been found to be substrate specific. A computational study showed that the reaction between the free enol with the carbonyl is the least energy demanding. Both 1,2- and 1,1-di-substituted disubstituted alcohols are compatible under the reaction conditions; however, sterically hindered carbonyls gave lower yields. Thus, the catalyst was modified to (bda)Fe(CO)3 and (COT)Fe(CO)3, which are superior to the pentacarbonyliorn complex. Moreover, carbonyls containing attached protected amine groups and isolated double bonds could also be treated for condensation with the above-mentioned catalysts.
In addition, Fe(CO)5 can catalyze intramolecular condensation following isomerization. For example, vinylic furanose derivatives can be converted into cyclopentenones.1093 It is unclear whether isomerization or ring opening takes place first (Scheme 628), but both paths lead to the formation of an acyclic enol-aldehyde intermediate, which undergoes intramolecular aldol reaction, followed by crotonization, leading to the product. Similarly, 3-vinyl-1,3-dihydroisobenzofuran-1-ol can undergo ring opening, isomerization and condensation to form indenone.1094 Moreover, this strategy has also been utilized to synthesize gabosines and 4-methyl-3-piperidones.1095,1096 Renaud and co-workers also showed that a tetra(isonitrile)-based iron complex can catalyze trifluoromethylated allyl alcohols into the corresponding carbonyl compounds.1097
In 1966, Davison and co-workers showed that in polyunsaturated fatty acids with isolated double bonds, when treated with Fe(CO)5 under high temperature of around 180 °C, isomerization takes place to form conjugated di and triene co-ordinated to Fe(CO)3.1098 The conjugated polyenes can be isolated upon decomplexation. Similarly, A-prostaglandins with separate double bonds can be converted into C-prostaglandins via triiron dodecacarbonyl-catalyzed isomerization (Scheme 629).1099 Moreover, the reactivity of the diene carbonyl-iron can be utilized to realize various other derivatives. Waegell and co-workers found that 4-vinylcyclohexenes can be converted to cyclohexadienes in an Fe(CO)5-catalyzed reaction.1100 Periasamy et al. reported that the combination of Na2Fe(CO)4/CuCl or Na2Fe(CO)4/BrCH2CH2Br can isomerize terminal olefins into the corresponding internal olefins at room temperature.1101 The deuterium scrambling pattern of α-deuterated 3-ethyl-1-pentene with Fe3(CO)12 revealed that both metal hydride addition-elimination and π-allyl metal hydride are involved in the isomerization; however, the extent of each mechanism depends on the catalyst system.1102
Wangelin and co-workers developed another method to isomerize alkenes to the corresponding styrene derivatives using 5 mol% Fe(acac)3 in the presence of PhMgBr as the reducing agent (Scheme 630).1103 3-Allyl pyridine and terminal aliphatic alkenes are also transformed into internal isomers. Treatment of cis-stilbene under the same reaction conditions gives trans-stilbene in 97% yield. More interestingly, allylated products formed from arylbromides can be in situ isomerized. Another reaction condition was developed for similar reactivity, where Fe3(CO)12 was used as a catalyst in the presence of KOH/H2O.1104
Hayashi and co-workers reported that Fe–Cu cooperative catalysis can isomerize secondary Grignard reagents into primary Grignard reagents (Scheme 631).1105 When 2-hexyl magnesium bromide was treated with FeCl3/CuBr/PBu3, followed by chloro(phenyl)silane treatment, silylation at the 1-position occurs in 83% with >99% selectivity. The reaction almost stops in the absence of both catalysts. It was proposed that oxidative addition followed by β-hydride elimination and subsequent olefin insertion form alkyliron species, which undergoes trans metalation with CuRMgBr. Finally, reductive elimination from the Cu center gives the isomerized product.
The FeBr2-catalyzed [1,2]-shift of α-arylaldehydes was reported by Martin's group (Scheme 632).1106 It was found that for tertiary α-arylaldehydes upon treatment with FeBr2 in toluene under heating condition, a [1,2]-shift is observed. However, the nature of the shifting group is highly dependent on the nature of the aryl group. If the aryl group can sufficiently stabilize a carbocation, an aliphatic group migrates, otherwise migration of the aryl group itself is observed.
9.3 Cycloisomerization
Takacs’ group and a few others extensively studied the iron-catalyzed intramolecular carbocyclization of triene ethers.1107–1114 It was observed that both the nature of cyclization and stereochemistry are highly substrate dependent. For instance, the triene substrate (Scheme 633) in the presence of 10 mol% bpy·Fe(0) in benzene gives the five-membered product in a major amount; however, the substrate triene diether under the same conditions gives the 6-membered product. Similarly, depending on the substituent R2 being hydrogen or alkyl, cis or trans selectivity is observed. Several natural products such as mitsugashiwalactone, isoirkdomyrmecin, and protoemetinoi have also been synthesized using this protocol as one of the key steps.1110,1112,1114 Mechanistically it was proposed that all three alkenes co-ordinate to the iron center followed by oxidative cyclization. Finally, β-hydride elimination and subsequent reductive elimination provide the end product (Scheme 634). Interestingly, the amine substrate also undergoes similar cyclization to form a bicyclic compound in the presence of catalytic Fe(acac)3 and bioxazoline ligand (Scheme 635).1103Fürstner and co-workers developed the Fe(0)-catalyzed cycloisomerization of enynes in 2005.1115 [CpFe(C2H4)2][Li(tmeda)] was found to be best for the transformation, giving excellent yields. A series of 5, n (n = 5, 6, 7, 8, 10, 12) bicyclic molecules were synthesized together with a few exocyclic cyclopentane derivatives. Another method for the synthesis of exocyclic double bond-substituted cyclopentanes was described by Lee and co-workers via the Conia-ene type cyclization of 2-alkynic 1,3-dicarbonyls catalyzed by FeCl3 (Scheme 636).1116 The mechanism was presumed to proceed via the formation of an enol-alkyne iron complex and subsequent stereospecific 5-exo-dig cyclization.
The asymmetric version was later reported by White's group using a well-designed iron–salen complex.1117 Various α-alkynyl-β-keto esters were converted into Conia-ene cyclized product with excellent ee. Thiophenes substituted at the 3-position give fused heterocycles with α,β-unsaturated ketone via Nazarov cyclization in the presence the FeCl3 catalyst (Scheme 637).1118 Similar nitrogen-containing heterocycles were also reported by Itoh's group; however, 2-substituted pyrroles were used as the substrate.1119 Later, it was found that 2-substituted indoles, benzofurans and benzothiophenes showed similar reactivity.1120 The asymmetric version of the iron-catalyzed Nazarov catalyst was developed by the same group using a chiral 2,6-dioxazoline-substituted pyridine ligand.1121 The cyclization reaction was effectively utilized in the total synthesis of xanthocidin.1122
9.4 Intramolecular ring expansion
In the transformation of epoxide to tetrahydrofuran via the addition of an external olefin,1123 epoxyalkenes are supposed to provide bicyclic compounds upon intramolecular ring expansion. It was found that Fe(salen) catalyzes the cyclization of simple epoxyalkenes separated by three –CH2– groups to form hexahydrocyclopenta[c]furans, whereas incorporation of an oxygen atom in between provides tetrahydrofuro[3,4-c]furans (Scheme 638).1124,1125 Electron-rich arene substitution at the epoxide end shows higher reactivity, while enhanced diastereoselectivity is observed for ketone and ester substitution.In 2009, Jiao and co-workers developed the iron-catalyzed ring expansion of alkynylcyclopropyl alkanols to cyclobutanols (Scheme 639).1126 The catalyst loading of 10 mol% of FeCl2 in O2 atmosphere works best for this transformation. It is presumed that is Fe(II) oxidized to Fe(III) in the presence of molecular oxygen, which co-ordinates with alkyne and abstracts hydroxide to form a cyclopropylmethyl cation. Subsequent 1,2-alkyl migration and recombination of the hydroxide provides the final product. Although to a lesser extent, the trans geometry is dominant, this is probably due to attack of hydroxide from the less hindered side.
Zheng's group found that the synthesis of indole derivatives from vinyl nitrenes via direct amination to an aromatic C–H bond can be effected with the FeCl2 catalyst compared to previously reported Pd and Rh catalysis (Scheme 640).1127 The Fe(II) species first co-ordinates with vinyl nitrene to form an iron-azirine complex. Subsequently, C–N bond cleavage results in an Fe-nitrene complex, which attacks the aromatic C–H bond and indole formation is realized via five-centered 6-π electrocyclization. Ketone-substituted vinyl nitrenes also undergo cyclization in the presence of FeCl3 under an inert atmosphere to form isoxazoles.1128 A similar mechanism can explain this reaction, where the Fe–nitrene interacts with the carbonyl group instead of the carbocation.
10. Iron-catalyzed substitution reactions
10.1 Nucleophilic substitution reaction
Nucleophilic substitution is one of the fundamental reactions in organic chemistry. It is an essential tool to make carbon–carbon and carbon–heteroatom bonds in a regio/stereo/chemo and diastereoselective manner.1129 In the classical organic chemistry, stoichiometric organic/inorganic reagents were used for SN reactions. However, the use of transition metal catalysts has revolutionized this field. It has enabled to achieve the chemo, regio and stereoselective synthesis in a predictable manner. The earliest example of iron-mediated SN reaction was the iron-catalyzed Friedel–Crafts alkylation.1130 The initial developments of iron-promoted SN reactions were well documented by Bolm in 2004.3h Thereafter, a comprehensive outline of the iron-catalyzed SN reactions was documented by Knölker in 2015.3l Thus, herein we have summarized the iron-catalyzed benzylic and allylic substitutions up to the Knölker reports in a tabular way and then demonstrate the recent reports elaborately. The SN reactions require one electrophile-bearing leaving group and a nucleophile. The incoming nucleophile in general attacks the nucleophilic carbon of the electrophile and forms a bond with the concomitant elimination of the leaving group. Depending on the steric and electronic properties of the electrophile and the conditions, the reaction proceeds via three different pathways, namely SN1, SN2 and SN2′.1129a In the SN1 pathway, the substrates undergo ionization in the presence of strongly acidic condition and generate a stable carbocation intermediate, which is attacked by the nucleophile to form C–C/C–heteroatom bonds. Notably, this reaction results in the formation of a mixture of enantiomers when it is executed with a pure enantiomeric substrate (Scheme 641).On the other hand, the reaction occurring via the SN2 pathway proceeds through a concerted pathway and results in the formation of the desired products in a stereospecific way. Similarly, in the SN2′ pathway, the reaction goes through conjugate addition with the electrophile in a concerted pathway (Scheme 642). Notably, Lewis acidic iron-catalyzed reactions can occur through the above-mentioned three pathways. The iron center of these catalysts coordinates with the leaving group and polarizes the electron density and enhance the partial positive charge on the carbon to facilitate the substitution reaction. The iron-catalyzed SN reactions are mostly limited to electrophiles in which their leaving groups have π-conjugation with benzyl, allyl and propargyl groups. Among them, benzylic substitution reactions are mostly catalyzed by Lewis acidic iron catalysts and have been found to proceed majorly through the SN1/SN2 pathways. Additionally, these catalysts, can promote the allylic and propargylic substitutions reactions.
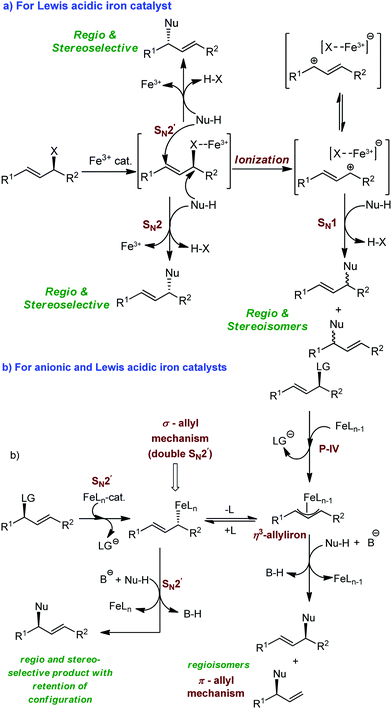 | ||
| Scheme 642 General reaction pathways for allylic substitution by Lewis acidic and anionic iron catalysts. | ||
The iron-catalyzed SN reactions at sp3 allylic carbon are widely used for C/N/O/S-alkylation reactions. Importantly, the regio/stereo-chemical outcome of the allylic substitution reactions depends on the reaction pathways involved, depending on the nature of the catalysts, ligands and electrophiles. The possible pathways for Lewis acid-catalyzed reactions were previously shown (Scheme 642a and b).1129a The reaction may proceed through concerted SN2 or SN2′ pathways to result in the formation of stereo and regioselective products. Also, it can occur via an allylic cationic intermediate, producing a mixture of stereo/regioisomeric products (Scheme 642a). Besides these, some neutral, anionic (ferrates) and Lewis acidic iron catalysts have been employed for the allylic substitution reactions. These reactions generally occur through either σ-allyl (double SN2′ pathway) or π-allyl pathways (through the formation of η3-allyl-iron intermediate). Notably, these two possible pathways govern the selectivity in product formation. Mainly, when the reaction occurs through the σ-allyl pathway, it results in the formation of regio/stereoselective products with the retention of the configuration at stereogenic center. On the other hand, the reaction occurring through the π-allyl pathway produces regioisomeric products and the observed regioselectivity in this case depends on the substituents present in the substrates. In the following sections, the benzylic, allylic and propargylic substitution reactions are summarized consecutively. We classify these reactions based on the types of electrophiles and type of bond formed.
10.1.1.1 Carbon–carbon/carbon–heteroatom bond formation. The benzylic SN reactions are generally employed as a standard tool to construct carbon–carbon and carbon–heteroatom bonds by different synthetic chemistry groups across the globe. Lewis acidic iron catalysts have been extensively used for these reactions with benzylic electrophiles. Numerous benzylic electrophiles including benzylic alcohols, benzyl methyl ethers, carboxamides containing vinylogous benzylic alcohols, N-benzyl sulfonamides, secondary and tertiary silyl ethers and vinylogous benzylic ethers are utilized for SN reactions to form carbon–carbon (Tables 1 and 2) and carbon–heteroatom bonds (Tables 3 and 4). Particularly, the iron-catalyzed SN reactions proceeding through the SN1 pathway in the presence of Lewis acidic iron catalysts such as iron(III)–chloride (FeCl3) and iron(III)-triflate (Fe(Otf)3) have been utilized to form carbon–carbon bonds with different carbon-centered nucleophiles.1131–1140 However, the possibility of the SN2 pathway for these reactions cannot be completely excluded. Moreover, a carbon-supported iron-ionic liquid was also used for the benzylation of 1,3-dicarbonyls.1134 Also, the Lewis acidic iron(III) chloride has been applied for the decarboxylative alkylation of β-keto acids with benzyl alcohols.1135 Similarly, carbon–carbon bond formation reactions occurring through benzylic substitutions have been described (Table 2).1141–1148 Among these reactions, the β-alkylation of secondary benzylic alcohols by primary alcohols (Table 2, entry 3) proceeds through the three successive steps, including oxidation, aldol condensation and reduction in the presence of ferrocene carboxaldehyde as the catalyst.1143 According to the proposed mechanism, initially both alcohols are oxidized to carbonyl compounds via hydride transfer to the ferrocene catalyst, generating the iron–hydride species. Then, the base-mediated aldol condensation between these two carbonyl compounds results in the formation of an α,β-unsaturated ketone. Finally, this ketone is reduced by iron–hydride species to generate the alkylation product. It is interesting to note that the initial oxidation of alcohols via hydrogen transfer and the final reduction of unsaturated ketones by iron–hydride species follow the well-known hydrogen auto transfer or hydrogen borrowing reactions.748–758,1144 However, despite the abundance of normal organic compounds as C-centered nucleophiles, allyl trimethyl silanes have been used as C-based nucleophiles with benzylic electrophiles (Table 1, entries 8 and 11, and Table 2, entries 7 and 8).1139,1140,1148 Furthermore, an organo-silane derivative has been used as an electrophile linked to benzylic-propargylic alcohol (Table 2, entry 4).1145 More intriguingly, ferrocenylmethanol is used as electrophile for C-alkylations (Table 2, entry 5).1145 Furthermore, in addition to C-centered nucleophiles, different N/S-centered nucleophiles have been employed to construct carbon–heteroatom bonds (Tables 3 and 4).1149–1159 Importantly, an iron-catalyzed Ritter-type reaction has been performed to make C–N bond-containing compounds (Table 3, entry 2).1150 Also, together with the concept of SN reactions, the hydrogen-borrowing reactions have been applied to make C–N bonds in the presence of magnetite (Fe3O4) and the mix-metal oxide NiCuFeOx as catalysts, respectively (Table 3, entries 3–6).1151,1152 Additionally, sulfur-centered nucleophiles are also used to synthesize C–S bond-containing compounds (Table 3, entries 7–9).1153 Also, the homoallylic alcohols participate as electrophiles to make C–heteroatom bonds, where the nucleophile attacks the terminal double bond, followed by the formation of π-an allyl-iron intermediate, resulting in the generation of double-bond-isomerized products (Table 3, entry 9).1153 Notably, in Table 4, besides the Lewis acidic iron catalysts such as [FeCl3 and Fe(Otf)3], the iron-cyclopentadienyl carbonyl complex[Fp][Otf] catalyst has been used for the benzylic substitutions (Table 4, entries 3 and 4).1156–1159 Thus far, the described N-alkylation reactions in Table 3 proceed through the hydrogen auto transfer pathway. However, recently in 2016, the simple SN reaction has been applied for N-alkylations by Maurya and coworkers. They developed a method for the ligand-free iron(II)-catalyzed N-alkylations of hindered secondary and primary alcohols with different amines.1160 The Lewis acid iron(II)-bromide was found to be the best catalyst in the presence of potassium hydrogen sulfate in toluene solvent (Scheme 643). Notably, different amines including primary, secondary and heterocyclic amines together with various benzyl alcohols and heteroaryl methanol are well tolerated for N-alkylation under the optimized conditions to produce the desired products in good to excellent yields. Further, this methodology has been utilized for the amination of different naturally occurring alcohols such as nerol, geraniol and phytol in good yields. Based on control experiments, a simple carbocation pathway has been suggested for this reaction (Scheme 644). It is believed that initially the benzylic alcohol coordinates with iron(II)-bromide to generate intermediate a, which reacts with another molecule of FeBr2to form intermediate ether b. Then, the stable intermediate b reacts with FeBr2 to generate carbocationic species d, which is trapped by the different aryl amines to provide N-alkylated products.
10.1.2.1 Carbon–carbon/carbon–heteroatom bond formation. Similar to benzylic sp3 substitutions, allylic substitutions have been developed for the formation of carbon–carbon and carbon–heteroatom bonds (Tables 5 and 7 and Tables 8 and 9 respectively). Interestingly, this field of allylic substitutions has been chronologically established with different iron carbonyl complex-mediated reactions. Initially, it was started with the neutral diiron nonacarbonyl [Fe2(CO)9] as a catalyst by Nicholas et al.1161 Thereafter, different anionic iron carbonyl complexes such as tricarbonyl nitrosyl ferrates, [Fe(CO)3NO]Na, [Fe(CO)3NO](Nbu4) (TBAFe), were utilized by the groups of Nicholas, Roustan, Xu and Plietker for the development of regio/stereoselective allylic SN reactions.1162–1168 Notably, the selectivity depends on the nature of the electrophiles, nucleophiles and catalysts. Mainly, the Heiber-type (TBAFe) and other anionic complex-catalyzed regio/stereoselective C-alkylation reactions are described (Table 5).1163–1165,1168 These reactions have been found to proceed via the σ-allyl pathway (Table 5, entries 1–4), and consequently result in the formation of regio/stereoselective products.1163–1165,1168 The role of ligands is vital for the successful implementation of allylic substitutions. Generally, phosphorus, nitrogen and carbene-based ligands are employed in the presence of TBAFe and other anionic complexes (Table 5, entries 3 and 4). These ligands generally enhance the stability and reactivity of iron complexes. Thus, the role of ligands in allylic substitutions was illustrated (Tables 5–7).
Particularly, Plietker and coworkers employed NHC ligands to improve the substrate scope and selectivity in allylic substitutions.1167 Interestingly, the different substituents such as tert-butyl and mesityl groups on the NHC ligand alter the reaction pathway. Moreover, a structurally well-characterized π-allyliron complex has been employed for this substitution reaction in the presence of an N-heterocyclic carbene ligand (Table 5, entry 6).1168 Later, the allylic substitution of allylic alcohols with arenes was developed in the presence of Lewis acid FeCl3-contaminated K-10 montmorillonite clay, providing the stereoselective formation of E- or Z-olefins (Table 5, entries 5 and 6).1169 Notably, in these reactions, the starting alcohols are prepared by Baylis–Hillman reaction. The reaction has been presumed to proceed through the Friedel–Crafts alkylation pathway via conjugate addition of arenes to allylic alcohols. Besides phosphines and NHC ligands, nitrogen ligands (TMEDA) are also used for the allylic substitutions (Table 6, entry 1) in the presence of [Bu4N][Fe(CO)3NO] as the catalyst, affording regioselective products.1170 The neutral diiron-nonacarbonyl complex has also been used for diastereoselective C-alkylation reactions (Table 6, entry 2).1171 Moreover, besides the neutral and anionic iron catalysts, the Lewis acid iron catalyst FeCl3 was used for intra-molecular allylic substitution to synthesize indene rings (Table 6, entry 3), which is proposed to proceed through cationic cyclisation.1172 This synthetic protocol has been utilized for the synthesis of (±)-junginol and epi-junginol. Later, ferrate-catalyzed C-alkylation was developed, where N, O and S-centered nucleophiles react with the allylic electrophile to form carbon–heteroatom bonds (Table 7, entries 1, 2, and 5 and Table 8, entries 1–7). The allylation of primary amines has been reported with allylic carbonates in a regio/stereoselective manner with retained configurations at the stereogenic center in case of enantiopure allyl carbonates (Table 7, entry 1).1173,1174 Similarly, sulfonylation of allylic carbonates have also been developed employing different aryl sulfinates as nucleophiles (Table 7, entry 2).1175 Also, the Lewis acidic iron-catalyzed intramolecular diastereoselective synthesis has been utilized to synthesize substituted piperidines and tetrahydropyrans (Table 7, entries 3 and 4).1176,1177 This methodology is applied for the diastereoselective synthesis of cis-isooxazolidines.1178 Further, the binuclear ferrate complex is employed as a catalyst for regioselective allylic sulfenylation reactions (Table 7, entry 5).1179
Afterwards, O-alkylation reactions were reported (Table 8, entries 1 and 2). The α-sulfonyl succinimides are used as nucleophile sources with allylic carbonates as electrophiles, resulting in the formation of the ipso-substitution (α-substitution) product in good selectivity (Table 8, entry 1).1180 Furthermore, allyl phenyl ethers were synthesized regioselectively starting with phenyl–allyl-carbonates in the presence of the ferrate complex PPh3 in MTBE solvent (Table 8, entry 2).1181 This reaction has been proposed to proceed through decarboxylative arylation via the formation of an η3-allyliron intermediate (π-allyl pathway) and produces the regioselective linear product. Lewis acid iron catalysts are also used for intramolecular SN2′ allylic aminations (Table 8, entry 3).1182 Further, the O-allylation of phenol was carried out using allyl carbonates as substrates in the presence TBAFe (catalyst) and a triazololium-based abnormal NHC ligand in THF solvent (Table 8, entry 4).1183 The reaction provides completely ipso-substitution and the regioselective O-allylation product and substantiates previous reports (Table 8, entry 2). Moreover, this method shows reverse selectivity in comparison to the general C-alkylation reactions, which is significantly enhanced at the allylic position in the presence of increased steric bulk. Besides, FeCl3·6H2O-catalyzed allylic substitutions have been developed with N/C-nucleophiles with free allylic alcohols (Table 8, entries 5–7).1184
Recently, in 2016, Pastor and co-workers developed an efficient protocol for the synthesis of quinolines, 2 and 4-allylanilines by allylic substitution (Scheme 645a–c).1185 Interestingly, they employed a Lewis acid iron(III)-based ionic liquid (A) as the catalyst for the reaction. The present method provides the desired products through the selective C-allylation pathways, which are unlike other iron-catalyzed allylation reactions, where N-alkylation occurs preferentially (as shown in Tables 1 and 2). Based on preliminary mechanistic investigations, a cationic pathway has been assumed for the formation of the desired products. In the same year, the iron-catalyzed allylic amination directly involving allylic alcohols and amines was demonstrated by Sundararaju and coworkers based on the hypothesis of hydrogen borrowing reactions (Scheme 646).753 They used a molecular iron catalyst, i.e. Knölker's complex A, in the presence of trimethyl N-oxide (Me3NO) as an additive in toluene solvent.
Notably, a variety of amines including cyclic secondary amines, aliphatic amines, benzylic amines as well as aromatic amines are well reacted with different terminally substituted allylic alcohols to produce allylic amination products in good to moderate yields under the optimized reaction conditions. Further, this method has been successfully employed for the one-pot synthesis of common drugs such as cinnarizine and naftifine, involving cinnamyl alcohol the as substrate. Based on the mechanistic investigations and literature reports on the hydrogen borrowing redox process, a possible mechanism was suggested (Scheme 647). It is presumed that catalyst A initially reacts with Me3NO to generate intermediate B (a 16e− species) via the removal of CO. Then, intermediate B coordinates with allylic alcohol 1 and generates intermediate C, in which alcohol is activated through hydrogen bonding. Thereafter one hydrogen atom is transferred from the alcohol to cyclopentadienone moiety with a concomitant change in the oxidation state of iron to generate intermediate D, which undergoes β-hydride elimination and forms iron–hydride intermediate Evia the elimination of aldehyde. Then, the aldehyde undergoes condensation with amine 2, resulting in the formation of imine, which is reduced by E (through outer-sphere hydrogenation) to produce the desired product 3.
In 2018, Nakata and coworkers developed the chiral auxiliary-controlled diastereo-convergent allylation reactions of allyl trimethyl silane with diastereomeric mixtures of diarylmethanols in the presence of FeCl3 as a Lewis acid catalyst (Scheme 648).1186 Further, the present methodology was successfully applied to a variety of substrates, irrespective of the electronic and steric factors of the substituents present in the aromatic ring. The synthetic method successfully delivered the desired product in the key step during the synthesis of (R)-tolterodine. Based on the experimental evidence, a plausible reaction pathway and transition state were proposed for the reaction. It is presumed that iron initially coordinates with the hydroxyl oxygen of diarylmethanol A and generates intermediate I, which subsequently forms carbocationic intermediate II. Finally, the allyltrimethylsilane reacts with II from the sterically less hindered path b (shown in the TS) to produce the desired allylated product in a diastereoconvergent manner (Scheme 649).
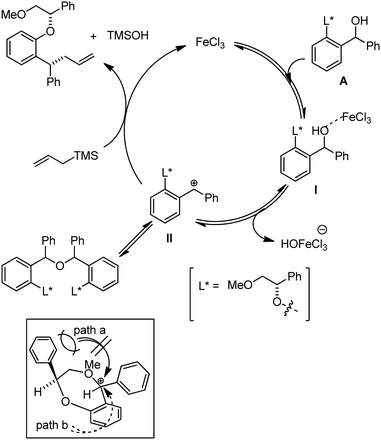 | ||
| Scheme 649 Proposed mechanism of iron-catalyzed diastereo-convergent allylation reactions of diarylmethanols. | ||
10.1.2.2 Allylic substitutions with Grignard reagents. The above-described reactions deal with normal organic compounds/inorganic salts as nucleophiles. However, iron catalysts can also promote allylic substitution with Grignard reagents. The SN2-selective iron-catalyzed allylic substitution was developed by reacting allylic phosphates with Grignard reagents, producing C-alkylated products in a stereoselective and regioselective manner (Table 9, entry 1).514,515 Further, similar reactions have been applied for the diastereoselective synthesis of methyl cyclopropanes (Table 9, entry 2).1187 Further this concept is applied for the ring-opening reactions of allylic epoxides.
In 2016, Li and coworkers described the Lewis acid iron-catalyzed α-allylic substitution of allyl ether with Grignard reagents (Scheme 650).1188 They utilized Fe(acac)3 as the catalyst in a DCE/NMP solvent mixture at −15 °C. Interestingly, the present protocol utilized different aryloxy allyl ethers as the substrate, where phenoxy groups act as leaving groups. Moreover, different aryl and aliphatic Grignard reagents containing electronically biased substituents are well tolerated under the optimized reaction conditions, providing good to excellent yield of the allylic substitution products. Further, they carried out a deuterium labeling study to gain mechanistic insight into reaction. The labeling study with a D-labeled allyl ether provided mixtures of regioisomeric products (α and γ substituted product).
Thus, this suggests the involvement of σ–π–σ equilibrium between the iron catalysts and substrates. Based on this preliminary mechanistic investigation, a tentative mechanism was proposed (Scheme 651). Initially, iron(III) is reduced to iron(I) in the presence of Grignard reagents (similar to the cross-coupling reactions). Thereafter, iron(I) takes part in the oxidative addition with the substrate to generate intermediate A, which remains in σ–π–σ equilibrium with B and C. Then, the transmetalation and final reductive elimination result in the formation of the desired products.
Moreover, the formation of the selective α-substituted products has been rationalized based on the steric effects of the substituents in the substrates. Besides the aforesaid mechanism, the authors suggested that the reaction may also proceed through the SN2 and SN2′ pathways. Later, in 2018, they developed the directed iron-catalyzed allylic substitution with Grignard reagents through selective cleavage of the C–O bond using ethyl salicylate ester as a directing leaving group in the presence of a catalytic amount of iron(III)-acetyl acetonate and Grignard reagents in DCE at −20 °C (Scheme 652).1189 The reaction is started with the chelation of the phenolic group of the salicylate ester through the generation of a π-allyl iron intermediate. Notably, the present method well tolerated a variety of differently substituted phenolic crotyl ethers and salicylate O-allyl ethers in the presence of different aryls and aliphatic Grignard reagents and provided good to excellent yields of the regioselective allylic substitution products. Based on the experimental evidence, a plausible mechanism was suggested, involving sequential steps such as initial reduction of iron(III) to iron(I) in the presence of Grignard reagents, coordination with the substrate, homolytic cleavage to generate the allylic radical and the final reductive elimination from a π-allylic iron intermediate to produce the desired products (Scheme 653).
10.1.3.1 Carbon–carbon bond formation. In 2006, Zhan and co-workers reported the iron-catalyzed substitution of propargylic acetates with phenols in the presence of FeCl3 in acetonitrile at room temperature to synthesize C–C coupling products via the Friedel–Crafts arylation reaction (Scheme 654).1190 The propargylic acetates react with various phenols, naphthols, and electron-rich aromatics, affording the corresponding substituted products in high yields. In addition, the propargylic alcohols also undergo iron-catalyzed nucleophilic ipso-substitution with allyl trimethylsilane under the same reaction conditions to give 1,5-enynes in high yields (Scheme 655a).1191 Further, heteroaromatic compounds and electron-rich arenes undergo nucleophilic substitution to the propargylated arenes under the catalytic activity of FeCl3 in acetonitrile at room temperature (Scheme 655b).1191 Notably, in all the cases, high regioselectivity is maintained to give the desired propargylated products in the presence of various reactive functional groups. Also, the reaction conditions are not sensitive to air and moisture.
In 2010, Jana and co-workers employed nucleophilic substitution of propargylic alcohols for the preparation of propargyl-substituted β-dicarbonyl compounds using FeCl3 in dichloromethane as an efficient catalytic system (Scheme 656).1192 A variety of 1,3-dicarbonyl compounds undergo the substitution reaction with different propargylic alcohols at room temperature to give the desired substituted products in high yields.
Around the same time, Zhan and co-workers reported the use of iron-catalyzed nucleophilic substitution of propargylic alcohols with alkynylsilanes for the synthesis of 1,4-diynes (Scheme 657).1193 High yields of various substituted 1,4-diynes are obtained under the catalytic activity of iron(III) chloride in nitromethane solvent at room temperature. In 2013, Bi and co-workers demonstrated a methodology for the synthesis of gem-bis(alkylthio)vinylallenes from tertiary propargylic alcohols and α-oxo ketene dithioacetals in the presence of FeBr3 at room temperature (Scheme 658).1194 The reaction proceeds through dehydration and C(sp2)–C(sp2) coupling between the substrates under iron catalysis, affording the corresponding vinylallenes in excellent yields.
10.1.3.2 Carbon–heteroatom bond formation. Zhan and co-workers also reported the iron-promoted nucleophilic substitution of propargylic acetates with heteroatomic nucleophiles, and alcohols together with carbon nucleophiles (Scheme 659).1190 The reaction was achieved in the presence of FeCl3 in acetonitrile at room temperate to afford the corresponding propargylic ethers in high yields with high regioselectivity. Various propargylic acetates undergo substitution with a wide range of primary, secondary and tertiary alcohols to give the desired ethers. Additionally, the same catalyst system also catalyzes the nucleophilic substitution of propargylic alcohols with different heteroaromatic nucleophiles such as alcohols, thiols and amides, forming the corresponding C–O, C–S and C–N-bonded propargylic products, respectively (Scheme 660).1191 Notably, the FeCl3 catalyst provides a cheap, environmentally benign and overall advantageous alternative to the other transition metal catalysts such as ruthenium, rhenium, cobalt and gold complexes.
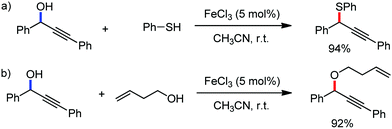 | ||
| Scheme 660 Nucleophilic substitution reaction of propargylic alcohols with different heteroatomic nucleophiles under iron catalysis. | ||
In 2009, Campagne and co-workers utilized the iron-catalyzed nucleophilic substitution of propargylic alcohols with Cbz-protected hydroxylamine together with gold-catalyzed cyclization for the one-pot synthesis of 2,3-dihydroisoxazoles (isoxazolines) (Scheme 661a).1195 Initially, the N-protected hydroxylamine is propargylated in the presence of FeCl3. This is followed by subsequent cyclization upon the addition of NaAuCl4·2H2O and DMAP as a base in the same reaction mixture to give poor to good yields of the corresponding isoxazolines. Further, the sulfonyl-protected hydroxyl amines also gives high yields of isoxazolines under similar iron(III)- and gold(III)-catalyzed one-pot reaction procedure, but with the presence of pyridine as a co-catalyst (Scheme 661b).1196 Additionally, a wide range of isoxazolines have been prepared from sulfonyl-protected hydroxylamines and propargylic alcohols via a one-pot procedure under the catalytic activity of FeCl3 in dichloromethane and triethylamine as a base (Scheme 662). Notably, the isolable N-sulfonyl-protected propargylated hydroxylamine I is initially formed in this process. Thereafter, the hydroxylamine intermediate is converted to oxime intermediate IIvia the β-elimination of the sulfonyl species. Then, this oxime intermediate undergoes cyclization to give the isoxazoles. Furthermore, 4-iodoisoxazoles and 4-iodoisoxazolines have been obtained from I in a one-pot procedure via iodocyclization using iodine monochloride.
In 2011, they devised an uninterrupted one-pot procedure consisting of substitution/annulations/cross-coupling/hydrogenolysis/oxidation in sequence for the preparation of trisubstituted isoxazoles from propargylic alcohols using a palladium(0) catalyst together with an iron(III) catalyst (Scheme 663).1197 After the initial propargylic substitution of Cbz-protected hydroxylamine under iron catalysis, the palladium(0) catalyst extends the sequence by promoting the annulation/cross-coupling reactions. This is followed by hydrogenolysis of Cbz and final aerobic oxidation in the same sequence to give the corresponding isoxazoles. High yields of isoxazoles are obtained through this novel one-pot procedure. Propargylic alcohols also undergo nucleophilic substitution with triazoles in the presence of FeCl3 to afford excellent yields of propargylated triazoles.1198 The N-1 or N-2 substitution products are formed regioselectively depending on the nature of the triazole nucleophile employed in the reaction. The benzotriazole reacts through the N-1 position (Scheme 664a), whereas the 4-phenyl-substituted and 4-benzoyl-5-phenyl-disubstituted triazoles give the N-2 substitution products predominantly (Scheme 664b).
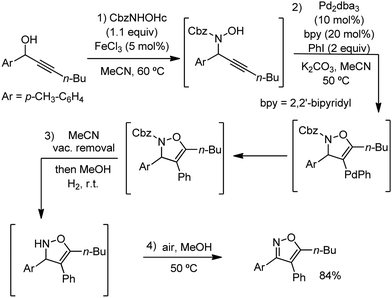 | ||
| Scheme 663 Preparation of trisubstituted isoxazole via an iron–palladium-catalyzed one-pot reaction. | ||
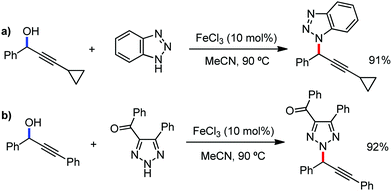 | ||
| Scheme 664 Iron-catalyzed nucleophilic substitution of propargylic alcohols with triazoles to form propargylated triazoles. | ||
In 2012, Shi and co-workers exploited the iron-catalyzed nucleophilic substitution of tertiary propargylic alcohols with 1,2,3-triazoles for the synthesis of allene triazoles in high yields.1199 The substitution occurs via an SN2′ pathway, giving the corresponding allene triazoles. The N-1 allenyl-substituted products are obtained by the use of benzotriazole as the nucleophile (Scheme 665a). On the other hand, the 4-substituted 1,2,3-triazoles afford a mixture of N-1 and N-2-allene triazoles with a major amount of N-1-allenyl-substituted product (Scheme 665b).
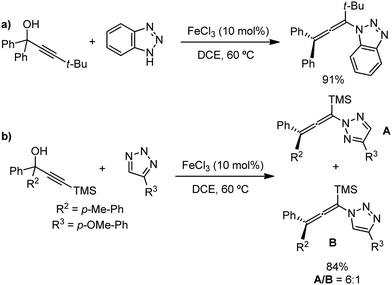 | ||
| Scheme 665 Nucleophilic substitution of tertiary propargylic alcohols by 1,2,3-triazoles in the presence of FeCl3. | ||
Zanotti and co-workers reported the dehydrogenative etherification of ferrocenylmethanol with various propargylic alcohols using [Fp][Otf] (Fp = [Fe(CO)2(Cp)]+) as the catalyst in toluene at room temperature (Scheme 666a).1200 The reaction provides good yields of the desired propargylated ferrocenylmethyl ethers. Additionally, propargylic alcohols also undergo dehydrative etherification with different alcohols such as benzyl alcohols, isopropyl alcohol, ethanol and phenol under the same reaction conditions to give moderate yields of the corresponding ethers (Scheme 666b).
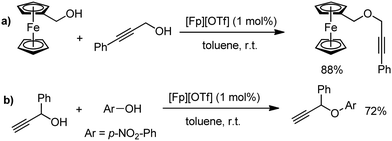 | ||
| Scheme 666 Iron-catalyzed dehydrogenative etherification of propargylic alcohol in the presence of [Fp][Otf]. | ||
In 2012, Zhan and co-workers introduced a new strategy for the synthesis of acrylonitrile from 3-(trimethylsilyl)propargylic alcohols and para-tolylsulfonohydrazide via iron-catalyzed propargylic substitution/aza-Meyer–Schuster rearrangement and elimination reaction in a domino process (Scheme 667).1201 The reaction occurs in the presence of FeCl3 in nitromethane at 60 °C to afford a variety of acrylonitriles in moderate to high yields. A wide range of symmetric and asymmetric propargylic alcohols undergo this reaction with good functional group tolerance. Notably, this is the first example of aza-Meyer–Schuster rearrangement with the migration of the nitrogen atom. The practicability of this procedure was demonstrated by the gram-scale synthesis of acrylonitriles.
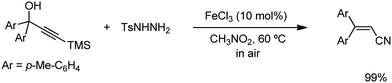 | ||
| Scheme 667 Iron-catalyzed synthesis of acrylonitrile via a domino propargylic substitution/aza-Meyer–Schuster rearrangement. | ||
Further, the same reaction conditions were utilized for the preparation of 3,4,5-substituted and 1,3,5-substituted pyrazoles from propargylic alcohols with aryl or alkyl substitution at the 3-position via a one-pot cascade reaction.1202 The one-pot procedure consists of four reactions in sequence, i.e. iron-catalyzed propargylic substitution/aza-Meyer–Schuster rearrangement/base-induced electrocyclization/thermal [1,5] sigmatropic shift. Notably, the propargylic alcohols with an aryl substituent in the C1 position form the 3,4,5-pyrazoles via migration of the aryl group to the adjacent carbon atom (Scheme 668a). On the other hand, the alkyl group of 1-alkyl substituted propargylic alcohols undergoes migration to the adjacent nitrogen atom to give the corresponding 1,3,5-substituted pyrazoles (Scheme 668b).
In 2015, Bauer and co-workers reported the use of ferrocenium hexafluorophosphate ([Fc]PF6) for the nucleophilic substitution of terminal tertiary propargylic alcohols with different alcohols (Scheme 669a).1203 Moderate to high yields of the propargylic ethers are obtained in the presence of only 5 mol% of [Fc]PF6 with an equimolar amount of propargylic alcohols and alcohol nucleophiles. Notably, the mixed alkyl–aryl propargylic alcohols give superior results compared to purely aromatic propargylic alcohols. Recently, in 2019, they reported the [Fc]PF6-catalyzed etherification of cyclopropyl-substituted tertiary propargylic alcohols with primary and secondary alcohols as nuclophiles.1204 The tertiary propargylic alcohols with a cyclopropyl group in the α-position provide the rearranged product, i.e. the E-configured ene-ynes containing ethers in moderate isolated yields (Scheme 669b). This rearrangement occurs through the cyclopropyl ring opening. However, the cyclobutyl-substituted propargylic alcohols (Scheme 669c) and thiophene-possessing cyclopropyl-substituted propargylic alcohols (Scheme 669d) give the corresponding propargylic ethers without any rearrangement.
In 2016, Wang and co-workers employed propargylic alcohols with aryl amines for the synthesis of indole and 1,2-dihydroquinoline via an iron-catalyzed cascade reaction consisting of Friedel–Crafts reaction/intramolecular hydroamination reaction in sequence (Scheme 670).1205 The reaction was performed in the presence of iron(III) chloride hexahydrate as the catalyst. Various substituted arylamines undergo reaction with α-diarylated propargylic alcohols to give moderate to high yields of the 1,2-dihydroquinolines. However, the propargylic alcohols with an alkyl substituent at the α-position afford indoles together with the 1,2-dihydroquinolines.
 | ||
| Scheme 671 Nucleophilic substitution of alkyl chlorides with iodides in the presence of FeCl3 as the catalyst. | ||
In 2009, Cossey and co-workers developed a procedure for the Ritter reaction1150b using FeCl3·6H2O at 150 °C.1150 The tert-butyl acetate undergoes reaction with nitriles and water to give good yields of the tert-butyl amides (Scheme 672a). Later, in 2014, Cook and coworkers introduced a superior iron-catalyzed Ritter reaction procedure for the synthesis of carboxamides by employing a catalytic system consisting of FeCl3 and AgSbF6 in dichloroethane (DCE) solvent at 100 °C.1137 Cyclic secondary alcohols react with acetonitrile under the reaction conditions, affording the corresponding amides in moderate yields (Scheme 672b).
In 2011, Martín and co-workers demonstrated the iron-catalyzed halogenations of alkyl sulphonates by employing trimethysilyl halides in the presence of iron(III) salts such as Fe(acac)3 and FeCl3 (Scheme 673).1207 The stereochemistry of the substituted products depends on the reaction conditions and nature of the substrates. However, some of the alkyl mesylates undergo substitution with complete retention of the stereochemistry in the produced halides. Significantly, the quisylates (8-quinolinesulfonate) and pysylates (2-pyridienesulfonates) were found to be superior leaving groups compared to the mesylates under the same reaction conditions. Notably, the quisylates are halogenated with the formation of completely inverted products. Additionally, the secondary mesylates are chemoselectively halogenated in the presence of primary mesylates in the substrate under stoichiometric conditions using FeCl3 or FeBr3.
In 2012, He and co-workers reported a three-coupling reaction procedure for the synthesis of propargylamines from alkynes, dichloromethane and amines under the catalytic activity of iron(III) chloride in CH3CN together with 1,1,3,3-tetramethylguanidine (TMG) as a base (Scheme 674).1208 Various substituted alkynes undergo reaction with piperidine and other secondary aliphatic amines together with dichloromethane to give moderate to excellent yields of the corresponding propargylic amines. However, aromatic amines and primary amines do not give any results under the reaction conditions.
In 2013, Shi and co-workers developed an air- and moisture-stable catalyst, NiCuFeOx, and used it for the N-alkylation of ammonia and amines with alcohols and primary amines.1152 A large number of primary and secondary alkylamines, and various substituted anilines are N-alkylated with a wide range of alcohols and other amines in the presence of the catalyst in xylene, giving high yields of the corresponding amines. Primary amines are also prepared in high yields from ammonia and alcohols. In addition, N-heterocyclic amines can be prepared from diols via intramolecular double alkylation (Scheme 675). In 2012, Enthaler et al. introduced an efficient procedure for the depolymerization of polyether with benzoyl chloride nucleophile under the catalytic activity of FeCl4·4H2O at 100 °C in neat conditions (Scheme 676a).1209 A wide range of polymeric ethers are depolymerized under the reaction conditions to give high yields of corresponding chloroethers. Significantly, the process was also efficient in scale-up experiments for the depolymerization reaction of 45.0 mmol polyethylene glycol by benzoyl chloride, affording the desired 2-chloroethyl benzoate in 87% isolated yield. Chloroethers are generally converted to monomers for the formation of new polymers. Also, good yields of chloroethers were produced by employing fatty acids as nucleophiles under the same reaction protocol.1210
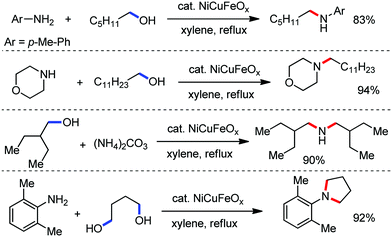 | ||
| Scheme 675 N-Alkylation of ammonia and amines with alcohols in the presence of NiCuFeOx as a catalyst. | ||
Further in 2013, they reported a ring-closing depolymerization of polyTHF under iron catalysis to form good yields of highly pure tetrahydrofuran (THF) (Scheme 676b).1211 This conversion is performed using FeCl3 as the catalyst at in the temperature range of 100 °C to 180 °C.
Around the same time, Taniguchi and co-workers developed an alternative methodology for the Mitsunobu reaction1212 using ethyl 2-(3,4-dichlorophenyl)hydrazinecarboxylate in place of DEAD (DEAD = diethyl azodicarboxylate) together with iron phthalocyanine (Fe(Pc)) as the catalyst (Scheme 677).1213 Moderate to good yields of inverted esters with high enantiomeric excess were obtained from the reaction of various chiral alcohols with different substituted carboxylic acids. Additionally, the reaction conditions allow the substitution of alcohols with nitrogen nucleophiles in moderate yields. Most importantly, the hydrazinecarboxylate undergoes aerobic oxidation to azocarboxylate within the reaction system in the presence of the iron catalyst.
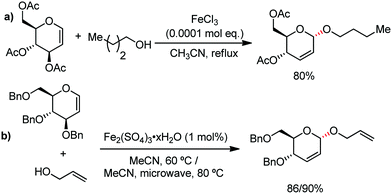 | ||
| Scheme 678 Glycosylation of peracetyl glycals and perbenzyl glycals in the presence of iron catalysts. | ||
10.2 Electrophilic substitution
Chen's group studied the thiolation of 1,3,5-trimethoxybenzene with disulfides in an FeBr3-catalyzed reaction (Scheme 681).1220 Different aryl, alkyl, allyl and heterocyclic sulfides could be substituted on the substrate in the presence of 20 mol% of catalyst in DMF at 145 °C, although the yields were poor in many cases. This protocol was extended by Zhong's group towards the thiolation of 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-3-cyanopyrazole at the 4-position.1221 However, a catalytic amount of iodine is required for the transformation to occur under milder conditions. The authors proposed that disulfide first reacts with iodine to form iodo-alkyl sulfide (RSI), and subsequently electrophilic substitution occurs at the C-4 position of the substrate (Scheme 681).
In 2010, the iron-catalyzed bromination of aryl azides was reported by Kuang's group.1222 Ferric chloride was found to be the best catalyst for the transformation in conjunction with NBS as the brominating agent. In most of the cases, bromo-substitution occurred at the ortho-position to the azide. However, it also depends on the other substituents (Scheme 682). On the other hand, iodination of electron-rich arenes was achieved using Fe(NO3)3, I2 and in situ-generated alkyl carbonic acid.1223 It has been proposed that carbon dioxide and ethylene glycol generate a proton in the reaction medium, which reacts with nitrate and iodine to produce an iodinium ion; however, the specific role of iron is not clear.
The synthesis of aryl azides with azidocarboxylates was reported by Fang's group (Scheme 683).1224 The electron-rich arene ring was found to undergo proton substitution with azidocarboxylates in the presence of 5 mol% FeCl3. Notably, ferric chloride catalyzes the thioarylation of phenols (Scheme 684).1225 In the absence of influence by other groups, 1-(arylthio)pyrrolidine-2,5-dione delivers substitution at the para-position to the hydroxyl group.
It is believed that co-ordination of the Lewis acid with succinimide triggers the generation of the arylthionium ion, which undergoes substitution at the electron-rich positions. Various thioaryls and phenol derivatives can be coupled under the reaction protocol with decent yields.
The iron-catalyzed Friedel–Crafts acylation in a pyridine-based ionic liquid was achieved by Malhotra's group in 2005.1231 They found that FeCl3-[EtPy]+[CF3COO]− is the best combination for the acylation with acetic anhydride. Li's group reported the synthesis of xanthenes in the FeCl3-catalyzed benzylation of phenols followed by a cyclization reaction (Scheme 687).1232 Although o-bromo benzyl acetates are used mostly, the corresponding o-bromo benzyl bromides and carbonates can also be used. In the first step, benzylation takes place at the ortho-position to the phenol and subsequently undergoes intra-molecular C–O coupling.
A propargylation reaction of arenes and heteroarenes was achieved with the corresponding propargyl acetate using the same Lewis acid catalyst (Scheme 688).1233 High yields for the transformation was obtained with electronically driven complete regioselectivity. In 2013, Han's group developed the electrophilic ortho-substitution of various phenols with propargyl alcohol.1234 The resultant product undergoes rapid cyclization aided by a base present in the reaction to produce benzofurans. Noteworthily, an aryl group is invariably required at the α-position of the alcohol probably to stabilize the electropositive intermediate.
Wu's group synthesized triaryl methane from benzaldehydes and two arene molecules in a similar alkylation manner (Scheme 689).1235 The benzaldehyde first attacks one of the arene rings and forms acetate in the presence of acetic anhydride, which in turn substitutes a proton from another arene. A similar triaryl methane synthesis was also reported by Shao-Hong in 2012. However, indolizines are used as arene coupling partners and no additives such as acetic anhydride was utilized.1236 The FeCl3-catalyzed Friedel–Crafts C-3 alkylation of indoles with alcohols was reported by Jana's group in 2007 (Scheme 690).1237 Remarkably, in addition to allyl, aryl and heteroaryl secondary alcohols, their tertiary counterparts also undergo smooth reaction with different indole derivatives. The FeCl3/AgSbF6 combination was found to be effective for the alkylation of simple arenes with unactivated secondary alcohols.1238 The same condition was also applicable for intramolecular alkylation to yield cyclic products such as tetrahydronaphthalene and other higher benzoannulene analogues.
Womak and co-workers reported that 2-alkylcinnamaldehydes could undergo intramolecular Friedel–Crafts reaction in the presence of FeCl3 and (MeO)3CH to deliver 1-alkoxy-2-alkyl-1H-indenes (Scheme 691).1239 These indenes are further transformed into 2-alkylindanones through stepwise treatment with trimethylamine and aqueous HCl respectively. Later they found that acetic anhydride instead of (MeO)3CH simplified the synthesis of indanones, which follows a similar reaction pathway.1240
An unusual way of iron-catalyzed benzylation of aromatics was reported from benzyl ethers.1241 Shi's group optimized the benzyl C–O bond breakage and subsequent Friedel–Crafts addition to arenes to obtain di- and tri-aryl methanes. Benzylation can also be achieved with N-protected imines, forming diaryl methylamine; however, highly electron-rich arenes are required (Scheme 692).1242 On the other hand, the use of aryl aziridines instead of imines under the same reaction conditions react faster to produce 2,2-diaryl-ethylamine. Similar benzylation can also be achieved with benzyl thiocyanate via unsymmetrical C–S bond cleavage in the presence of ferric bromide.1243
Intramolecular Friedel–Crafts alkylation with epoxides was reported by Pericas’ group.1244 Co-ordination of the epoxide oxygen of aryl glycidyl ethers to a Lewis acid (FeBr3) allows the formation of a carbocation, which undergoes cyclization to yield 3-chromanols or tetrahydrobenzo[c]oxepin-4-ols, depending on the chain length. Another example of intramolecular electrophilic alkylation was reported Zhou's group by synthesizing indenes from 1,1,3-triarylallyl alcohols (Scheme 693).1245 Noteworthily, FeCl3 catalyzes the formation of the tertiary carbocation; however, it is reluctant to undergo cyclization due to the steric interaction between the three aryl groups. Thus, rearrangement leads to a less substituted carbocation, which undergoes intramolecular aromatic substitution, resulting in the production of indenes. During the evaluation of the reaction scope, it was observed that two aryl groups, one at the 1-position and the other at the 3-position could be substituted with aliphatic groups or hydrogen. Moreover, the reaction condition is tolerated by various functional groups such chloro, nitro, and methoxy. Present on arene rings. Similarly, FeCl3 is reported to catalyze the intramolecular alkylation of 2-aryl benzylalcohol1246 and o-aryloxybenzaldehydes1247 to produce fluorine and xanthone derivatives, respectively.
4-(Arylamino)propargyl alcohols in the presence of a Lewis acidic iron catalyst form propargyl cation and isomerizes to an allenyl cation.1248 The addition of this cation to the ortho-position of the arene ring produces allene-substituted tetrahydroisoquinolines. Under suitable conditions, it undergoes further isomerization to olefin-substituted dihydroisoquinoline. However, Zhou's group found that the formation of the major product is highly substrate dependent. When nitrogen was replaced with a –C(CH2OR) group, the corresponding tetrahydronaphthalene and dihydronaphthalene were obtained at 15 °C and 60 °C, respectively (Scheme 694).1249 Bandini and co-workers showed that the use of allyl alcohol instead of propargyl alcohol also produces the cyclic product in a similar pathway.1250
Li and co-workers developed the synthesis of xanthenes from 2-aryloxybenzaldehydes and electron-rich arenes and heteroarenes (Scheme 695).1251 Interestingly, they proposed that the initially formed triaryl methane undergoes C–C bond cleavage to eliminate one of the external arenes and reacts intramolecularly with the arene ring of aryloxybenzaldehyde.
Specially designed α,α-dialkyl acetoacetanilides upon treatment with 10 mol% FeCl3 at 60 °C in acetonitrile undergo intramolecular alkenylation to deliver 4-alkylene quinolin-2-ones (Scheme 696).1252 The quaternary carbon center plays a vital role by exerting enough strain to undergo cyclization. The authors proposed that co-ordination of the Lewis acid forms a carbocation at the terminal carbonyl center, and subsequently electrophilic substitution occurs at the arene ring.
During the synthesis of the prostaglandin EP4 receptor antagonist, Gauvreau and co-workers optimized the C-3 benzylation of 2,4-dimethylthiophene with a benzyl alcohol derivative and found FeCl3 to be the best catalyst.1253 In contrast, Lei's group demonstrated the condensation of aldehydes with 2-naphthols (Scheme 697).1254 The metal co-ordinates with aldehyde first, then undergoes two-fold electrophilic substitution at the 1-position of both naphthols to form triarylmethane. Then the two hydroxyl groups of naphthols eliminate a water molecule to produce 14-aryl(alkyl)-14-H-dibenzo-[a,j]xanthenes. Later, Zhang's group developed a solvent-free version of this reaction using an Fe(Otf)3 catalyst.1255
2-Benzhydryl-1,3-diphenylpropane-1,3-dione is known to form a diphenylmethyl cation in the presence of an iron catalyst.1256 Li's group utilized this concept, and found that indole derivatives undergo electrophilic substitution at the C-3 position with the cations (Scheme 698). The use of various olefins, alkynes, cycloalkyl arenes and aryl groups instead of phenyl also works well under the reaction conditions.
In 2010, Hayashi and co-workers reported the iron-catalyzed substitution of alkyl amides into an aryl C–H bond (Scheme 699).1257 The tert-butoxide radical selectively abstracts a hydrogen from the α-C–H of the alkylamide and forms α-(tert-butoxy)alkylamides, which in turn interact with the metal catalyst to form a carbocation stabilised by the amide group. The following substitution of the cation in arene results in the desired product. Under the reaction conditions, both arenes and heteroarenes substituted with halide, ester, and methoxy groups can be coupled with cyclic and acyclic amides. In a subsequent report, they showed that this strategy is also applicable towards the alkylamination of heteroarenes and electron-rich arenes.1258
The synthesis of 5,6-dihydro-indolo[1,2-a]quinoxaline derivatives was reported by Xu's group via Pictet–Spengler cyclization (Scheme 700).1259 The imine formed between 2-(indol-1-yl)arylamine and benzaldehyde undergoes substitution at the 2-position of indole in the iron-catalyzed reaction to deliver the product. Interestingly, furfuraldehyde also showed high reactivity and produced the corresponding product.
Yu and co-workers developed the C-3 alkenylation of indoles in 2012 (Scheme 701).1260 In the first step, iron promotes the formation of phenylacetaldehyde diethylacetal with ethanol, which disintegrates to an oxocarbenium cation. Due to electronic preferences, this cation undergoes electrophilic substitution at the 3-position. The authors also proposed an alternate pathway, where direct attack of the aldehyde is followed by dehydration to produce the alkene. A variety of substituted indoles including N-allyl indole underwent alkenylation at the C-3 position under the reaction conditions.
The iron-catalyzed asymmetric alkylation of indoles was achieved with the combination of Fe(ClO4)2 and a chiral bipyridyl-diol-based ligand.1261 A diaryl meso-epoxide was employed as the alkylating agent to obtain chiral 1,2-diaryl-2-(3-indolyl) alcohols in excellent yield and >95% ee in all instances (Scheme 702). Interestingly, structure elucidation of the pre-catalyst showed a rare pentagonal bipyramidal geometry around the metal center co-ordinated with the ligand, two THF and one water molecule.
Cinnamyl alcohol undergoes dehydrative alkylation to produce 1,3-diaryl propene.1262 A cationic Fe(III)-porphyrin complex was employed by Matsubara to generate an allylic, cation which in turn underwent electrophilic substitution (Scheme 703). Regioselective C-5 allylation of protected 8-aminoquinolines with cinnamyl alcohol in an FeCl3-catalyzed reaction was reported by Zeng's group in 2013 (Scheme 703).1263 Both amine and pyridine nitrogens are capable of dictating the selectivity in their favour, and therefore the authors postulated that metal chelation withdraws the lone-pair electron density from N-pyridine, thus the C-5 product was observed.
In 2014, Leino and coworkers developed an iron-catalyzed reductive alkylation of ketones and aldehydes with arenes in the presence of catalytic FeCl3/Fe(acac)3 (4 mol%) and stoichiometric chlorotrimethylsilane/triethylsilane together with triethylsilylhydride.1264 Similarly, in 2017, Maji and co-workers showed that cinnamyl esters and related α,β-unsaturated carbonyl compounds can also be employed for the electrophilic alkylation of arenes with the Fe(Otf)3 catalyst.1265
Although Saidi's group found an iron catalyst to be very effective towards alkylation at the C-3 position of indole only as an optimization entry,1266 Itoh's group performed extensive study on the reaction. It was observed that Fe(BF4)3 serves as the best catalyst (Scheme 704).1267,1268 Noteworthily, a slight change in the reaction conditions also allowed the formation of 2,3-dialkyl indoles. This strategy could also be extended for the mono and di-alkylation of pyrrole derivatives.1269 Similar C-3 substitution of indole derivatives was also observed with enamides and nitroalkenes.1270,1271 FeCl3 helped in the formation of the iminium ion in case of enamide, whereas co-ordination to the nitro group activated nitroalkenes. Interestingly, indolynitroalkenes also underwent substitution at the para-position of ortho-haloanilines.1272 Huang's group utilized β-aryl α′-hydroxy enones to achieve the asymmetric alkylation of indoles (Scheme 705).1273 The metal co-ordinates with the alkylating agent in a bidentate fashion and a chiral phosphoric acid ligand simultaneously. In the transition state, the ligand is likely to be hydrogen bonded with the indole nitrogen as well, and thus chirality is induced.
In 2006, Beller's group introduced the FeCl3-catalyzed substitution of (het)arenes with styrene (Scheme 706).1274 A plethora of styrenes and arenes and heteroarenes form 1-aryl-1-(het)arylalkanes via the electrophilic alkylation reaction. In the subsequent year, Lu's group established the reaction with arylacetylenes, i.e. alkenylation of arenes (Scheme 706).1275 The authors proposed that FeCl3 reacts with the alkyne and produces a carbocation adjacent to the aryl group, followed by electrophilic attack on the arene ring. The observed kinetic isotope effect value of 1 supports this mechanism instead of C–H activation. Evaluation of the substrate scope revealed that the formation of the Z-isomer was predominant with internal alkynes. Remarkably, propiolic acids were also found to produce cinnamic acids in a similar fashion (Scheme 707).1276,1277 Indoles can be employed with propiolic acid also; however, di-heteroarylation is observed, leading to bis(indol-3-yl)alkanoic acids.1278 Interestingly, the reaction between propiolic acid and phenol results in the formation of coumarin.1279 The authors proposed two different pathways for this observation: (i) hydroarylation followed by cyclization or (ii) esterification followed by intramolecular electrophilic substitution. On the other hand, benzyl alcohols react with aromatic alkynes, yielding indenes.1280 Later, Chen and co-workers simplified the strategy by in situ generating benzyl bromides through the bromination of the benzylic C–H bond with NBS.1281 In both reactions, the benzyl cation probably attacks the alkyne and the resultant alkenyl cation undergoes electrophilic substitution on the arene ring.
The intramolecular version of the hydroarylation of alkynes was reported by Campagne for 4-arylalkynes and by Takaki for N-propergylanilines to obtain dihydronaphthalenes and dihydroquinolines, respectively (Scheme 708).1282,1283 Similarly, arylalkynyl phenyl sulfides and sulphonamides produce cyclic vinyl sulphides and vinylamines, respectively.1284
The concept of electrophilic alkenylation was also utilized by Tian's group in a 3-component reaction.1285 Initially, FeCl3 catalyzes the generation of the carbonium ion from benzyl alcohol, which attacks a terminal alkyne to form an alkenyl cation. Subsequently, substitution occurs at the arene ring to produce various trisubstituted alkenes.
10.3 Radical substitution
In 2013, Pucheault's group reported the iron-catalyzed borylation of aryldiazonium salts via a radical pathway.1286 In the presence of ferrocene, the diazonium salt eliminates nitrogen to produce an aryl radical, which reacts with aminoborane, yielding aryl(amino)borane (Scheme 709). This intermediate upon sequential treatment with methanol and pinacol produces aryl pinacolatoborane. Various substituted aryldiazonium salts were converted to the corresponding borylated product in a decent yield using this protocol. In 2017, Nakamura achieved the direct borylation of aryl and heteroaryl chlorides with bispinacolatoborane.1287 Catalytic ferric acetylacetonate was used as the catalyst in the presence of potassium tertiary-butoxide to oxidatively add aryl chloride to the metal. This intermediate is believed to be in equilibrium with the aryl radical, which coupled with the iron-co-ordinated Bpin. Recently, Feng's group discovered that 2-aryloxypyridines underwent homolytic C–O cleavage with catalytic Fe(Oac)2, and recoupled with Bpin to produce the corresponding borylated product.1288Intramolecular dehydrogenative aromatic radical substitution with aldehydes was documented by Studer and co-workers (see Scheme 229).269 Under the reaction protocol, 2-arylbenzaldehydes form fluorenones, while 2-aryloxybenzaldehydes yield xanthones. The reaction initiates via the ferrocene-promoted generation of the tert-butoxy radical from t-BuOOH and subsequent HAA of the aldehyde hydrogen (see Scheme 230). The carbon radical undergoes typical base-assisted radical substitution on the arene to complete cyclization. They have also established that the radical generated from a benzaldehyde in a similar fashion reacts with 2-isocyanobiphenyls (Scheme 710).1289 The resultant imidoyl radical undergoes cyclization via substitution, forming phenanthridines.
Chen and co-workers developed a halogen exchange methodology via radical substitution (Scheme 711).1290 Aryl bromides upon treatment with sodium chloride and a catalytic amount of FeCl3 were converted to aryl chloride under UV radiation. The chlorine radical is believed to be generated by the homolytic dissociation of the Fe–Cl bond. The Fe(II) species formed in the process is oxidised back to Fe(III) by molecular oxygen and takes up a Cl−, completing the catalytic cycle. Not only aryl bromides, various heteroaryl bromides also undergo the halogen exchange effectively.
11. Miscellaneous
11.1 Radical-mediated functionalizations
In 2013, Maiti and co-workers reported the regio and stereoselective nitration of olefins in the presence of ferric nitrate and a catalytic amount of 2,2,6,6-tetramethylpiperidine (TEMPO) following their earlier works on radical-based nitrations.1291 The reaction provides nitro-olefins in good yields with excellent E-selectivity (Scheme 712). Notably, the nature of the solvent has a significant effect on the nitration. Non-polar solvents were found to be better solvents compared with polar solvents. A wide variety of olefins including aromatic, aliphatic and heteroaromatic olefins are well tolerated under the reaction conditions. Both the electron-withdrawing group and electron-donating group present in the substrate provide the desired nitration product in good to excellent yield. Notably, this reaction protocol can tolerate the presence of the chloromethyl group together with olefin.Further, a proposed mechanistic pathway has been suggested based on the experimental evidence. Initially, Fe(NO3)3, ferric nitrate under thermal conditions produces the nitro radical. This nitro radical then undergoes the reaction at the less hindered side of the olefin in order to generate the secondary or benzylic radical. Subsequently, this nitroalkane radical can be converted to the desired product via two possible pathways. In the first pathway (pathway a), TEMPO abstracts the hydrogen atom, whereas in the second pathway (pathway b), TEMPO is trapped and oxidises the benzylic radical to form the desired product stereoselectively (Scheme 713).
In the next year, they reported the generation of arylated quiniones by the iron-catalyzed oxidative addition of phenols (Scheme 714).1292 Mechanistically, the phenoxy radical is generated by the homolytic cleavage of persulphate. Subsequently, the phenoxy radical undergoes oxidation in the presence of oxygen to form quinone. The aryl radical generated from ArB(OH)2 attacks the more electrophilic quinone due to the coordination with Fe(III)/Fe(IV) under the oxygen atmosphere to afford the quinoline-arylation product. Notably, the homo-coupled product is also generated together with the quinoline-arylation product. The generation of the homo-coupled product confirms the radical nature of the reaction. Further, the observed kinetic isotope effect study (KIE = 2.96) confirms the conversion of ArOH to quinone in the rate-determining step (Scheme 715).
This synthetic transformation technique was used for the total synthesis of natural products such as phellodonin, sarcodonin ε, leucomelone and betulinan A. In 2014, they developed an iron-catalyzed regioselective method for the direct arylation at the C-3 position of N-alkyl-2-pyridone (Scheme 716).1293 Using this reaction protocol, 3-(2-methoxyphenyl)-one-2(1H)-one was synthesized, which can be further utilized as a precursor for the synthesis of the pharmaceutically relevant benzofuro[2,3-b]quinoline moiety via the successive cyclization reaction.
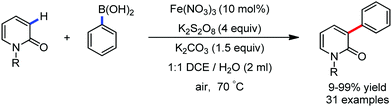 | ||
| Scheme 716 Iron-catalyzed regioselective direct arylation at the C-3 position of N-alkyl-2-pyridone. | ||
Based on the literature survey and experimental observations, a possible mechanistic pathway has been suggested (Scheme 717). Initially, the sulphate radical is generated thermally or in the presence of iron(II). Then, the sulphate radical reacts with arylboronic acid to form the aryl radical. Subsequently, this aryl radical reacts selectively at the C-3 position of the 2-pyridone moiety. The selective attack of the aryl radical at the C-3 position of pyridone can be rationalised due to the large coefficient of HOMO and LUMO of the C-3 atom. This results in the formation of resonance-stabilized radical adduct I. The iron(III) or sulphate radical abstracts one electron from I to generate cation II, followed by aromatisation, leading to the formation of the desired product. Under aerobic conditions, iron(II) is oxidised to iron(III).
In 2018, Zhong and co-workers reported the iron-catalyzed regiospecific intermolecular radical cyclization of anilines.1294 The protocol does not require a ligand and directing group. The synthetic protocol allows the formation of the electrophilic anilido radical, which undergoes intermolecular [3+2] cyclization with nucleophilic α-substituted alkenes to form gem-disubstituted indulines (Scheme 718).
Mechanistically, N-centered neutral radical I is generated via N–H hydrogen bond abstraction by the oxidant DDQ (Scheme 719). This was confirmed by an EPR study. On the other hand, the comparison of the redox potential (EP/2vs. SCE in MeCN) between FeCl3 and α-substituted alkene 1 (1.85 V) rules out the pathway involving the radical cation of 1via single-electron oxidation. The electrophilicity of anilide radical I is not sufficient to attack α-substituted alkene 1. However, the coordination by the iron catalyst activates carbon-centered radical II. Here, FeCl3 acts as a Lewis acid. Then, the cyclization takes place via the stepwise addition involving tertiary carbon radical IV. This was confirmed by a radical clock experimental study. Further, carbon-centered radical V is generated, and subsequent cleavage of the ortho C–H bond affords the desired product by aromatization of the phenyl ring with the simultaneous release of FeCl3. The observed kinetic isotope effect (KIE, KH/KD = 5.7) suggests that the cleavage of the ortho C–H bond may be involved in the rate-determining step.
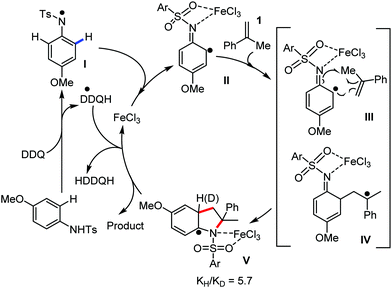 | ||
| Scheme 719 Proposed mechanistic pathway of iron-catalyzed regiospecific intermolecular radical cyclization of anilines. | ||
In the same year, Hazra1295 and co-workers reported an iron-catalyzed and potassium persulfate-mediated thiocyanation of a wide range of 2H-indazoles at room temperature to obtain 2-aryl-3-thiocyanato-2H-indazoles as the desired product (Scheme 720). However, 2H-indazole with an electron-withdrawing group (–NO2) in its phenyl ring and having aliphatic substituents at the N-2 position does not afford the desired product. Mechanistically, the reaction proceeds via the radical mechanistic pathway. This was confirmed by adding a radical scavenger to the reaction mixture.
Based on the literature studies and controlled experiments, they proposed a mechanistic pathway (Scheme 721). Initially, ferric thiocyanate I complex is formed via ligand exchange (SCN−), and then it is oxidised to form the SCN radical in the presence of K2S2O8. Then, the co-ordination of Fe(III) with 2-phenyl-2H-indazole results in the formation of intermediate II. Subsequently, the SCN radical reacts with intermediate II selectively at the C-3 position to form radical intermediate III. The coordination of Fe(III) with the substrate is very important to stabilize the radical in resonating structure III′. The co-ordination of the N atom of the III′ radical with Fe(III) reduces the charge density of the nitrogen atom through σ-bonding and pπ–dπ overlapping. Then, radical intermediate III is further oxidised to give carbocation IV followed by elimination of H+ to afford the desired product.
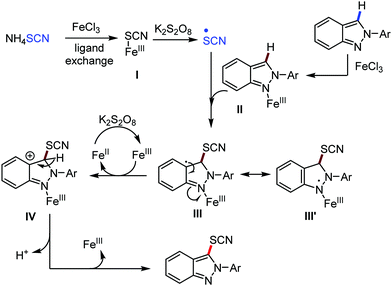 | ||
| Scheme 721 Possible mechanistic pathway of iron-catalyzed potassium persulphate-mediated thiocyanation of 2H-indazole. | ||
In 2019, Punniyamurthy and co-workers reported the iron-catalyzed regioselective remote C(sp3)–H carboxylation of naphthyl and quinoline amides using CBr4 and alcohol via the co-ordination activation strategy and SET process (Scheme 722).1296 The reaction in the presence of a radical scavenger such as TEMPO or BHT provides a trace amount of the desired product. Thus, the present observation suggests that reaction may involve a radical pathway. It was believed that Fe(acac)3 reacts with PivOH to generate the active Fe(III) species, which undergoes the reaction with substrate I (Scheme 723). Simultaneously, the radical ˙CBr3 is generated in the reaction via the SET mechanistic pathway from CBr4 in the presence of iron(II) (iron(II) is regenerated in situ through the reduction of Fe(III) with alcohol). The ˙CBr3 radical reacts at the electrophilic carbon atom to produce radical intermediate II, which is converted to IIIvia another SET mechanistic pathway. Subsequently, the deprotonation of III generates species IV and Fe(II) species, which is reoxidized to Fe(III) by the excess CBr4 present under the reaction conditions. Then the deprotonation of IV by a bromide ion generates the V intermediate, which undergoes protodemetalation through the regeneration of the active Fe(III) catalyst to form intermediate VI. Finally, alcoholysis of VII provides the desired product. Notably, the scope of this synthetic protocol can be extended for the remote C-5 carboxylation of analogous 8-aminoquinolimides.
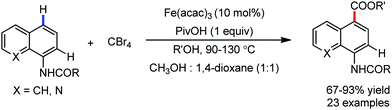 | ||
| Scheme 722 Iron-catalyzed regioselective remote C(sp2)–H carboxylation of naphthyl and quinolone amides. | ||
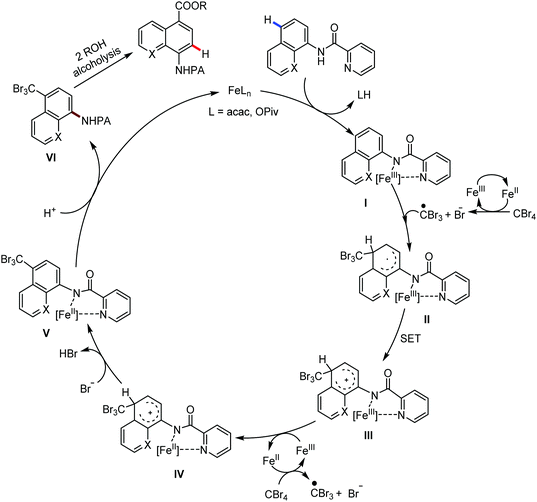 | ||
| Scheme 723 Possible mechanistic pathway for the iron-catalyzed regioselective remote C(sp2)–H carboxylation of naphthyl and quinolone amides. | ||
In 2017, the electron-deficient (hetero)arenes were ortho-preferentially alkylated by Bao and co-workers using the Fe(Ots)3 iron catalyst under an N2 atmosphere at 100 °C via an alkyl radical nucleophilic aromatic substitution reaction (Scheme 724).1297 Alkyl diacyl peroxides were used as the alkyl radical source. Several electron-deficient aryl rings were ortho-alkylated under the reaction conditions to give moderate to high yields of products. Further, various alkyl diacyl peroxides give good yields of the ortho-products by reacting with ortho- and para-dichlorobenzenes. Also, heteroarenes give good to excellent yields of products via this radical alkylation. The DFT studies and HOMO–LUMO analysis also supported the observed regioselectivity of the reaction.
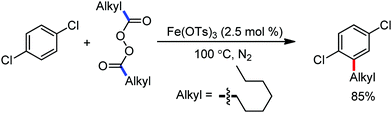 | ||
| Scheme 724 ortho-Preferred alkylation of electron-deficient arenes in the presence of iron catalysts. | ||
They proposed a hypothetical mechanism for the alkyl radical nucleophilic aromatic substitution (Scheme 725). Initially, the alkyl diacyl peroxide undergoes homolytic cleavage in the presence of iron(II) catalyst I at high temperature to give alkyl free radical III together with the release of CO2. Simultaneously, Fe(II) species I undergoes single-electron transfer (SET) to form iron(III) species II. Then, delocalized radical species IV is formed via the nucleophilic attack of alkyl radical III on the electron-deficient arene. This is followed by the oxidation of IV by iron(III) species II to give delocalized carbocation species V with the concomitant regeneration of active Fe(II) species I. The carboxylate anion released in this process abstracts a proton from V to give the desired ortho-alkylated product.
In 2019, Guo and co-workers demonstrated that α,β-unsaturated carboxylic acids can undergo decarboxylative ketoalkylation in the presence of an iron catalyst via alkoxy radical-promoted unstrained C–C bond cleavage to produce alkenyl ketones (Scheme 726).1298 This reaction has been performed successfully in the presence of ferrocene as the iron catalyst together with Bphen as the ligand in EtOH at room temperature. A broad range of substituted cinnamic acids with electron-withdrawing and electron-donating substituents react with various cycloalkylsilyl peroxides to give good yields of alkenylated product with excellent stereoselectivity towards E-alkene. The reaction conditions tolerate a large number of functional groups such as halogens, hydroxy, methoxy, trifluoromethyl and cyano groups. Moreover, seven-, eight- and twelve-membered peroxides also provided the desired products with good to excellent yields. They suggested the presence of a radical intermediate based on the observation of the products for the reactions using TEMPO.
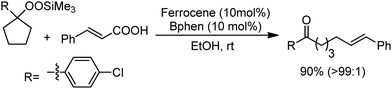 | ||
| Scheme 726 Decarboxylative keto alkylation of α,β-unsaturated carboxylic acids in the presence of an iron catalyst. | ||
Their proposed mechanism initiates with the one-electron reduction of cyclopentyl silyl peroxide by the in situ-generated Fen complex from ferrocene and Bphen to give alkoxy radical I (Scheme 727). Alkoxy radical I undergoes subsequent β-fragmentation to form radical II, followed by its attack on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of cinnamic acid to give radical III. Thereafter, trimethyl silanoate deprotonates III to generate IV. This is followed the oxidation of IV by Fen+1, leading to the formation of diradical intermediate V. Finally, this intermediate undergoes decarboxylation, forming the desired product.
C of cinnamic acid to give radical III. Thereafter, trimethyl silanoate deprotonates III to generate IV. This is followed the oxidation of IV by Fen+1, leading to the formation of diradical intermediate V. Finally, this intermediate undergoes decarboxylation, forming the desired product.
Recently, in 2020, Feng and co-workers reported an iron-catalyzed methodology for the silylation of (hetero)aryl chlorides (Scheme 728).1299 The reaction can be fruitfully performed in the presence of the silylborane reagent Et3Si-Bpin under the catalytic activity of FeI2 together with 3,4,7,8-tetra-Me-phen as the ligand. A large number of aryl and heteroaryl chlorides undergo the reaction to give good to moderate yields of the silylated product. Further, the reaction condition provides good tolerance to a large number of functional groups such as hydroxyl, amine, Boc, Bpin, BnO, morpholinyl, CF3, and alkenyl groups. This reaction has been employed in pharmaceutical chemistry for the late functionalization of molecules in the synthetic process. Pharmaceuticals such as the antitussive and antihistamine, cloperastine, and the antiemetic, buclizine and clomipramine have been successfully silylated to the corresponding products in good yields. Notably, they suggested a radical mechanism based on the results of radical clock experiments and reaction with the radical scavenger TEMPO and radical inhibitor BHT.
12. Conclusion and perspective
Over the past few decades, considerable developments have been accomplished in the field of iron catalysis to find green, non-toxic, cheap and overall sustainable alternatives to precious metal catalysts. A large number of promising and efficient iron-catalyzed reactions have been thoroughly discussed in this review. Different organic reactions such as C–H bond oxidation, cross-coupling reaction, C–H activation reaction, cross dehydrogenative coupling reactions, halogenations, hydrogenations, additions, insertion and substitution reactions are successfully performed using iron catalysts. Among them, significant progress has been made in the iron-catalyzed cross-coupling reactions. Despite the predominance of palladium catalysts in this field, iron catalysts have been proven to be competent by their ability to couple aryl, alkenyl and alkyl electrophiles with various organometallic nucleophiles with high efficiency. Also, the application of these methodologies for the total synthesis of various natural products shows the versatility of iron catalysis in cross-coupling. Furthermore, the recent elucidation of the mechanism by the in situ-generated intermediates of the iron-catalyzed cross-coupling reactions using advanced physical inorganic spectroscopic techniques has enabled researchers to gain better insight into the reaction, which will help them to develop novel reaction methods in the future. In addition to cross-coupling, C–H bond activation using iron catalysts has been investigated and developed in the last decade. Highly efficient iron-mediated procedures for the ortho C–H activation and very recently for the meta C–H activation of substrates with suitable directing groups have been well established. Although para C–H activation has been performed using noble metal catalysts such as palladium, it is yet to be achieved by iron catalysts. Moreover, several future developments with iron catalysis are yet to come including C–H activation without the use of a directing group and activation of methylene C(sp3)–H bonds. Bio-inspired non-heme iron catalysts have been synthesized and utilized in site-selective C–H oxidations, which can be predicted by the substrates and catalysts. Further, the recent discoveries have revealed the role of ligand chirality of the catalysts in tuning the site selectivity. Similarly, bio-inspired non-heme iron catalysts have been developed for selective halogenations for aliphatic C–H bonds in a stoichiometric manner. Thus, this has opened up a new scope to develop catalytic halogenation methods. On the other hand, cross-dehydrogenative coupling has emerged as an efficient tool for C–C bond formation due to the formation of the bond without the need for any pre-functionalization of the substrates. In this context, the iron-catalyzed cross dehydrogenative coupling shows a sustainable variant to the dominating late transition metals such as rhodium, iridium, ruthenium, and copper catalysis. Most intriguingly, the increase in the number of iron catalysts for the asymmetric transfer hydrogenation of prochiral ketones for the synthesis of valuable alcohols and imines has brought this to a new level.Finally, the iron-catalyzed reactions depicted herein are very useful in organic synthesis, and some of them are found to be superior to the precious late transition metals catalysts. Indeed, the modification of different synthesized iron catalysts helps us to reduce the steps of various organic transformations. Optimistically, green and cheap iron catalysts will possibly give every possible reaction usually implemented by the toxic and precious noble metals catalysts.
List of abbreviations
| PMP | Polymethylpentene |
| DCIB | Dichloroisobutane |
| TMEDA | Tetramethyethylenediamine |
| THF | Tetrahydrofuran |
| dtbpy | 4,4′-Di-tert-butyl bipyridine |
| acac | Acetylacetonato |
| dppf | 1,1′-Bis(diphenylphosphino)ferrocene |
| dppen | (Z)-1,2-Bis-(diphenylphosphino) ethane |
| dppe | 1,2-Bis(diphenylphosphino)ethane |
| DCB | 2,3-Dichlorobutane |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| DME | Dimethoxyethane |
| dppbz | 1,2-Bis(diphenylphosphino)benzene |
| TEMPO | (2,2,6,6-Tetramethylpiperidin-1-yl)oxidanyl |
| anis | Anisole |
| BHT | 2,6-Di-tert-butyl-4-methylphenol |
| DCP | 1,2-Dichloropropane |
| HOSA | Hydroxylamine-O-sulfonic acid |
| HFIP | 1,1,1,3,3,3-Hexafluroisopropanol |
| dmpe | 1,2-Bis(dimethylphosphino)ethane |
| 9-BBN | 9-Bicycloboranonane |
| DMEDA | 1,2-Dimethylethylenediamine |
| PyTACN | 1-(2-Pyridylmethyl)-4,7-dimethyl-1,4,7-triazacyclonoane |
| Bar4F | Tetrakis(bis-3′5-trifluoromethylphenyl)borate |
| CYP | Cytochrome P-450 |
| TFDO | (Trifluoromethyl)dioxirane |
| MMO | Methane monooxygenase |
| TPA | Tris(2-methylpyridyl)amine |
| PDP | 2-({(S)-1-[(Pyridine-2-yl-methyl)pyrrolidin-2-yl]pyrrolidin-1yl}methyl)pyridine |
| PMHS | Polymethyl hydroxy silane |
| TBAB | Tetra-butyl ammonium bromide |
| TMG | 1,1,3,3-Tetramethylguanidine |
| TpPh2 | Hydrotris(3,5-diphenyl-pyrazol-1-yl)borate |
| TQA | Tris-((quinolyl-2-methyl)amine) |
| DTBP | Di-tert-butyl peroxide |
| TBHP | tert-Butyl hydro peroxide |
| DDQ | 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone |
| DCE | 1,2-Dichloroethane |
| DHF | Dihydrofuran |
| NBS | N-Bromo succinamide |
| THIQs | Tetrahydroisoquinolines |
| AIBN | Azobisisobutyronitrile |
| NMDA | N-Methyl-D-aspartate |
| TBAF | tetra-n-Butylammonium fluoride |
| DMSO | Dimethyl sulfoxide |
| MTBE | Methyl tert-butyl ether |
| EDA | Ethyl diazoacetate |
| F20-tpp | meso-Tetrakis(pentafluorophenyl)porphyrinatodianion |
| qpy | 2,2′:6′,2′′:6′′,2′′′:6′′′,2′′′′-Quinquepyridine |
| N4Py | N,N-Bis(2-pyridyl-methyl)bis(2-pyridyl)methylamine |
| HMSI-BOX | Hexamethyl-1,1′-spirobiindane-based bisoxazoline ligand (HMSI-BOX) |
| dbm | Dibenzoylmethane |
| NMP | N-Nethylpyrrolidione |
| DMPU | N,N′-Dimethylpropyleneurea |
| DMB | Dibenzoylmethane |
| LDA | Lithium diisopropylamide |
| TMU | Tetramethyl urea |
| DMI | 1,3-Dimethyl-2-imidazolidinone |
| dppy | 2-Diphenylphosphinopyridine |
| HMTA | Hexamethylenetetramine |
| TBS | tert-Butyldimethylsilyl |
| CPME | Cyclopentylmethyl ether |
| depe | Bis(diethylphosphino)-ethane |
| dppp | 1,3-Bis(diphenylphosphino)propane |
| bdmd | Bis((diphenylphosphanyl)methyl)diphenylsilane |
| LiHMDS | Lithium hexamethyldisilazide |
| COD | Cyclooctadiene |
| dipp2BIAN | 2,6-Diisopropylphenyl bis(imino)acenaphthene |
| TPB | Triphosphine-borane |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| IPA | Isopropyl alcohol |
| TRIP | 3,3′-Bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate |
| GVL | γ-Valerolactone |
| TPP | Triphenylphosphine |
| Imes | 1,3-Bis(2,4,6-trimethylphenyl)imidazol-2-ylidene |
| TVCH | 1,2,4-Trivinylcyclohexane |
| VCHO | Vinylcyclohexene oxide |
| PNN | Phosphinite-iminopyridine |
| OIP | Oxazolineiminopyridine |
| PMDS | 1,1,3,3,3-Pentamethyldisiloxane |
| pybox-dm | Bis(oxazolinyl)pyridine |
| bioy-tb | Bis-tert-butyl-bipyridiene |
| STC | Sodium thiophene-2-carboxylate |
| Bopa | Bis(oxazolinylphenyl)amine |
| PsiP | Bis(phosphine)silyl |
| bpi | Bis(pyridylimino)isoindole |
| IPO | Iminopyridine-oxazoline |
| boxmi | Bis(oxazolinylmethylidene)isoindoline |
| HEMIM | 1-(2-Hydroxyethyl)-3-methylimidazolium |
| DPB | Bis(o-diisopropylphosphinophenyl)phenylborane |
| TMDS | 1,1,3,3-Tetramethyldisiloxane |
| BDSB | 1,2-Bis-(dimethylsilyl)benzene |
| DippC: | Bis-(N-Dipp-imidazole-ylidene)methylene |
| Hbpin | Pinacolborane |
| BIAN | Bis(2,6-diisopropylaniline)acenaphthrene |
| VCP | Vinylcyclopropane |
| NCS | N-Chlorosuccinimide |
| TBA | Tertiary butyl alcohol |
| TFA | Trifluoroacetic acid |
| tmtaa | Dibenzotetramethyltetraaza[14]annulene |
| DPPPent | 1,5-Bis(diphenylphosphino)pentane (DPPPent) |
| OTBDMS | tert-Butyldimethylsilyl ethers |
| DEAD | Diethyl azodicarboxylate |
| Bmim | 1-Butyl-3-methylimidazolium |
| Omim | 1-Methyl-3-octylimidazolium |
| BINAM | 1,1′-Binaphthyl-2,2′-diamine (FeCl2–BINAM) |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge BRNS-India (RD/0118-BRNS000-002) (DM) and SERB-India (SRG/2019/000310) (SR) for financial support. CSIR-India is acknowledged for fellowship (JP). AP thanks SERB-India for fellowship (Project assistant fellowship from SRG/2019/000310).References
- (a) J. D. Hayler, D. K. Leahy and E. M. Simmons, Organometallics, 2019, 38, 36–46 CrossRef CAS; (b) K. S. Egorova and V. P. Ananikov, Angew. Chem., Int. Ed., 2016, 55, 12150–12162 CrossRef CAS; (c) Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, Weinheim, Germany, 2008 Search PubMed; (d) Topics in Organometallic Chemistry-Iron Catalysis: Fundamentals and Applications, ed. B. Plietker, Springer Verlag, Berlin, 2011, vol. 33 Search PubMed; (e) A. Correa, O. García Mancheno and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (f) A. A. O. Sarhan and C. Bolm, Chem. Soc. Rev., 2009, 38, 2730 RSC; (g) C.-J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197 CrossRef CAS; (h) C. H. Lam, V. Escande, K. E. Mellor, J. B. Zimmerman and P. T. Anastas, J. Chem. Educ., 2019, 96, 761–765 CrossRef CAS; (i) I. Eilks and F. Rauch, Chem. Educ. Res. Pract., 2012, 13, 57–58 RSC; (j) M. M. Kirchhoff, Resour., Conserv. Recycl., 2005, 44, 237–243 CrossRef; (k) R. A. Sheldon, J. R. Soc., Interface, 2016, 13, 20160087 CrossRef.
- (a) A. R. Muci and S. L. Buchwald, in Cross-coupling reactions, ed. N. Miyaura, Springer, Berlin, Heidelberg, 2002, pp. 131–209 Search PubMed; (b) J. Hartwig, Organotransition metal chemistry: from bonding to catalysis, University Science Books, Sausalito, 2009 Search PubMed; (c) Hydrofunctionalization, ed. V. P. Ananikov and M. Tanaka, Springer, Berlin, 2013 Search PubMed; (d) Metal catalyzed cross-coupling reactions and more, ed. A. de Meijere, S. Brase and M. Oestreich, Wiley-VCH, Weinhem, 2013 Search PubMed; (e) Olefin metathesis: theory and practice, ed. K. Grela, Wiley, Hoboken, 2014 Search PubMed; (f) Homogeneous catalysis for unreactive bond activation, ed. Z.-J. Shi, Wiley, Hoboken, 2015 Search PubMed; (g) J. M. Thomas and W. J. Thomas, Principles and practice of heterogeneous catalysis, Wiley-VCH, Weinheim, 2015 Search PubMed.
- (a) H. M. L. Davies and D. Morton, J. Org. Chem., 2016, 81, 343–350 CrossRef CAS; (b) F. Roudesly, J. Oble and G. Poli, J. Mol. Catal. A: Chem., 2017, 426, 275–296 CrossRef CAS; (c) F. F. Khan, S. K. Sinha, G. K. Lahiri and D. Maiti, Chem. – Asian J., 2018, 13, 2243–2256 CrossRef CAS; (d) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900–2936 RSC; (e) D. E. Stephens and O. V. Larionov, Tetrahedron, 2015, 71, 8683–8716 CrossRef CAS; (f) R. Sharma and U. Sharma, Catal. Rev., 2018, 60, 497–565 CrossRef CAS; (g) S. Rana, A. Modak, S. Maity, T. Patra and D. Maiti, Progress in Inorganic Chemistry, 2014, vol. 59, pp. 1–188 Search PubMed; (h) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217–6254 CrossRef CAS; (i) W. B. Motherwell, Appl. Organomet. Chem., 2000, 14, 170 CrossRef CAS; (j) D. Wei and C. Darcel, Chem. Rev., 2019, 119, 2550–2610 CrossRef CAS; (k) A. Fürstner, ACS Cent. Sci., 2016, 2, 778–789 CrossRef; (l) I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS; (m) R. Shang, L. Ilies and E. Nakamura, Chem. Rev., 2017, 117, 9086–9139 CrossRef CAS; (n) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS; (o) J.-L. Renaud, ChemSusChem, 2009, 2, 351 CrossRef CAS; (p) A. Jacobi von Wangelin, Angew. Chem., Int. Ed., 2009, 48, 1533–1534 CrossRef; (q) A. Dey, S. K. Sinha, T. K. Achar and D. Maiti, Angew. Chem., Int. Ed., 2019, 58, 10820–10843 CrossRef CAS; (r) N. Goswami and D. Maiti, Isr. J. Chem., 2020, 60, 303–312 CrossRef CAS; (s) A. Dey, S. Maity and D. Maiti, Chem. Commun., 2016, 52, 12398–12414 RSC; (t) S. K. Sinha, G. Zanoni and D. Maiti, Asian J. Org. Chem., 2018, 7, 1178–1192 CrossRef CAS; (u) T. Bhattacharya, S. Pimparkar and D. Maiti, RSC Adv., 2018, 8, 19456–19464 RSC; (v) N. Thrimurtulu, A. Dey, D. Maiti and C. M. R. Volla, in Strategies for Palladium-Catalyzed Non-Directed and Directed C-H Bond Functionalization, ed. A. R. Kapdi and D. Maiti, Elsevier, 2017, pp. 453–470 Search PubMed; (w) A. Correa, O. García Mancheno and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (x) A. A. O. Sarhan and C. Bolm, Chem. Soc. Rev., 2009, 38, 2730 RSC.
- (a) P. Nareddy, F. Jordan and M. Szostak, ACS Catal., 2017, 7, 5721–5745 CAS; (b) J. A. Leitch and C. G. Frost, Chem. Soc. Rev., 2017, 46, 7145–7153 RSC; (c) G.-F. Zha, H.-L. Qin and E. A. B. Kantchev, RSC Adv., 2016, 6, 30875–30885 RSC; (d) S. Ruiz, P. Villuendas and E. P. Urriolabeitia, Tetrahedron Lett., 2016, 57, 3413–3432 CrossRef CAS; (e) R. Manikandan and M. Jeganmohan, Org. Biomol. Chem., 2015, 13, 10420–10436 RSC; (f) V. S. Thirunavukkarasu, S. I. Kozhushkov and L. Ackermann, Chem. Commun., 2014, 50, 29–39 RSC; (g) B. Li and P. H. Dixneuf, Top. Organomet. Chem., 2014, 48, 119–193 CrossRef; (h) S. De Sarkar, W. Liu, S. I. Kozhushkov and L. Ackermann, Adv. Synth. Catal., 2014, 356, 1461–1479 CrossRef CAS; (i) S. I. Kozhushkov and L. Ackermann, Chem. Sci., 2013, 4, 886–896 RSC; (j) L. Ackermann, Acc. Chem. Res., 2014, 47, 281–295 CrossRef CAS; (k) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS.
- (a) P. Y. Choy, S. M. Wong, A. Kapdi and F. Y. Kwong, Org. Chem. Front., 2018, 5, 288–321 RSC; (b) O. Baudo, Acc. Chem. Res., 2017, 50, 1114–1123 CrossRef; (c) N. Della Ca’, M. Fontana, E. Motti and M. Catellani, Acc. Chem. Res., 2016, 49, 1389–1400 CrossRef; (d) J. Le Bras and J. Muzart, Eur. J. Org. Chem., 2018, 1176–1203 CrossRef CAS; (e) F. Kakiuchi and T. Kochi, Isr. J. Chem., 2017, 57, 953–963 CrossRef CAS; (f) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 46, 677–685 RSC; (g) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS; (h) S. R. Neufeldt and M. S. Sanford, Acc. Chem. Res., 2012, 45, 936–946 CrossRef CAS; (i) J. Ye and M. Lautens, Nat. Chem., 2015, 7, 863–870 CrossRef CAS; (j) M. Catellani, E. Motti and N. Della Ca, Acc. Chem. Res., 2008, 41, 1512–1522 CrossRef CAS.
- (a) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624–655 CrossRef CAS; (b) T. Piou and T. Rovis, Acc. Chem. Res., 2018, 51, 170–180 CrossRef CAS; (c) Y. Yang, K. Li, Y. Cheng, D. Wan, M. Li and J. You, Chem. Commun., 2016, 52, 2872–2884 RSC; (d) B. Ye and N. Cramer, Acc. Chem. Res., 2015, 48, 1308–1318 CrossRef CAS; (e) J. W. Delord, F. W. Patureau and F. Glorius, Top. Organomet. Chem., 2015, 55, 1–27 CrossRef; (f) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651–3678 RSC; (g) J. Du Bois, Org. Process Res. Dev., 2011, 15, 758–762 CrossRef CAS; (h) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814–825 CrossRef CAS; (i) T. Satoh and M. Miura, Chem. – Eur. J., 2010, 16, 11212–11222 CrossRef CAS.
- (a) M. Nagamoto and T. Nishimura, ACS Catal., 2017, 7, 833–847 CrossRef CAS; (b) C. Yuan and B. Liu, Org. Chem. Front., 2018, 5, 106–131 RSC; (c) C. Haldar, M. E. Hoque, R. Bisht and B. Chattopadhyay, Tetrahedron Lett., 2018, 59, 1269–1277 CrossRef CAS; (d) L. S. Sharninghausen and R. H. Crabtree, Isr. J. Chem., 2017, 57, 937–944 CrossRef CAS; (e) S. Pan and T. Shibata, ACS Catal., 2013, 3, 704–712 CrossRef CAS; (f) T. Suzuki, Chem. Rev., 2011, 111, 1825–1845 CrossRef CAS; (g) J. Choi and A. S. Goldman, Top. Organomet. Chem., 2011, 34, 139–167 CrossRef CAS; (h) T. Satoh, K. Ueura and M. Miura, Pure Appl. Chem., 2008, 80, 1127–1134 CAS; (i) J. Kim and S. Chang, Angew. Chem., Int. Ed., 2014, 53, 2203–2207 CrossRef CAS.
- (a) A. Suzuki, (Noble lecture), Angew. Chem., Int. Ed., 2011, 50, 6722–6737 CrossRef; (b) E. Negishi, (Noble lecture), Angew. Chem., Int. Ed., 2011, 50, 6738–6764 CrossRef; (c) C. C. C. Johansson Seechurn, O. M. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS; (d) A. Biffis, P. Centomo, A. Del Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249–2295 CrossRef CAS; (e) F. Christoffel and T. R. Ward, Catal. Lett., 2018, 148, 489–511 CrossRef CAS; (f) D. Roy and Y. Uzomi, Adv. Synth. Catal., 2018, 360, 602–605 CrossRef CAS; (g) P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS.
- (a) H. Chen and J. F. Hartwig, Angew. Chem., Int. Ed., 1999, 38, 3391–3393 CrossRef CAS; (b) A. M. Suess, M. Z. Ertem, C. J. Cramer and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 9797–9804 CrossRef CAS; (c) Z. Ding and N. Yoshikai, Org. Lett., 2010, 12, 4180–4183 CrossRef CAS; (d) Z. Ding and N. Yoshikai, Synthesis, 2011, 2561–2566 CAS; (e) J. Y. Kim, S. H. Cho, J. Joseph and S. Chang, Angew. Chem., Int. Ed., 2010, 49, 9899–9903 CrossRef CAS; (f) S. Zhang, P. Qian, M. Zhang, M. Hu and J. Cheng, J. Org. Chem., 2010, 75, 6732–6735 CrossRef CAS.
- (a) K. S. Egorova and V. P. Ananikov, Organometallics, 2017, 36, 4071–4090 CrossRef CAS; (b) http://mineralsprices.com/default.aspx#rar, http://www.infomine.com/investment/metal-prices/ and http://original.metal.com/metals/productsinfo/201102250232; (c) S. Enthaler, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3317–3321 CrossRef CAS; (d) S.-S. Wang and G.-Y. Yang, Catal. Sci. Technol., 2016, 6, 2862–2876 RSC; (e) Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, Weinheim, Germany, 2008 Search PubMed; (f) Topics in Organometallic Chemistry-Iron Catalysis: Fundamentals and Applications, ed. B. Plietker, Springer Verlag, Berlin, 2011, vol. 33 Search PubMed.
- (a) N. C. Andrews, N. Engl. J. Med., 1999, 341, 1986–1995 CrossRef CAS; (b) A. L. Feig and S. J. Lippard, Chem. Rev., 1994, 94, 759–805 CrossRef CAS; (c) K. S. Egorova and V. P. Ananikov, Organometallics, 2017, 36, 4071–4090 CrossRef CAS; (d) F. Kallmeier and R. Kempe, Angew. Chem., Int. Ed., 2018, 57, 46–60 CrossRef CAS; (e) A. Rosas-Hernandez, C. Steinlechner, H. Junge and M. Beller, Top. Curr. Chem., 2018, 376, 1 CrossRef CAS; (f) P. T. Wolczanski, Organometallics, 2018, 37, 505–516 CrossRef CAS; (g) N. Rajesh, N. Barsu and B. Sundararaju, Tetrahedron Lett., 2018, 59, 862–868 CrossRef CAS; (h) H. Nagae, A. Kundu, M. Inoue, H. Tsurugi and K. Mashima, Asian J. Org. Chem., 2018, 7, 1256–1269 CrossRef CAS; (i) Y. Li, Y. Hu and X.-F. Wu, Chem. Soc. Rev., 2018, 5, 260–272 Search PubMed; (j) S. Fukuzumi, Y.-M. Lee and W. Nam, Coord. Chem. Rev., 2018, 355, 54–73 CrossRef CAS; (k) J. Chen and Z. Lu, Org. Chem. Front., 2018, 5, 260–272 RSC; (l) J. E. Zweig, D. E. Kim and T. R. Newhouse, Chem. Rev., 2017, 117, 11680–11752 CrossRef CAS; (m) R. L. Webster, Dalton Trans., 2017, 46, 4483–4498 RSC; (n) G. Potosching, N. Maulide and M. Schnurch, Chem. – Eur. J., 2017, 23, 9206–9232 CrossRef; (o) P. J. Chirik, Angew. Chem., Int. Ed., 2017, 56, 5170–5181 CrossRef CAS; (p) S. Bezzenine-Lafollee, R. Gil, D. Prim and J. Hannedouche, Molecules, 2017, 22, 1901 CrossRef; (q) S. W. M. Crossley, C. Obradors, R. M. Martinez and R. Shenvi, Chem. Rev., 2016, 116, 8912–9000 CrossRef CAS; (r) B. Su, Z.-C. Cao and Z.-J. Shi, Acc. Chem. Res., 2015, 48, 886–896 CrossRef CAS; (s) J. Mao and H. Ge, Eur. J. Org. Chem., 2015, 7859–7868 CrossRef; (t) A. Kulkarni and O. Dauglis, Synthesis, 2009, 4087–4109 CAS.
- E. Nakamura and K. Sato, Nat. Mater., 2011, 10, 158–161 CrossRef CAS.
- (a) E. M. Burbidge, G. R. Burbidge, W. A. Fowler and F. Hoyle, Rev. Mod. Phys., 1957, 29, 547–650 CrossRef; (b) M. Halka and B. Nordstrom, Transition Metals; Facts on file, London, 2011 Search PubMed.
- J. Müller and M. Bröring, Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, Weinheim, Germany, 2008, pp. 29–72 Search PubMed.
- M. S. Kharasch and E. K. Fields, J. Am. Chem. Soc., 1941, 63, 2316 CrossRef CAS.
- K. Tamao, K. Sumitani and M. Kumada, J. Am. Chem. Soc., 1972, 94, 4374–4376 CrossRef CAS.
- E. Negishi, N. Okukado, A. O. King, D. E. Van Horn and B. I. Spiegel, J. Am. Chem. Soc., 1978, 100, 2254–2256 CrossRef CAS.
- (a) M. Tamura and J. Kochi, Synthesis, 1971, 303 CrossRef CAS; (b) M. Tamura and J. K. Kochi, J. Am. Chem. Soc., 1971, 93, 1487 CrossRef CAS; (c) J. K. Kochi, Acc. Chem. Res., 1974, 7, 351 CrossRef CAS; (d) S. M. Neumann and J. K. Kochi, J. Org. Chem., 1975, 40, 599 CrossRef CAS.
- (a) F. Roudesly, J. Oble and G. Poli, J. Mol. Catal. A: Chem., 2017, 426, 275 CrossRef CAS; (b) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900 RSC.
- D. E. Stephens and O. V. Larionov, Tetrahedron, 2015, 71(46), 8683 CrossRef CAS.
- P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192 CrossRef CAS.
- G. Hata, H. Kondo and A. Miyake, J. Am. Chem. Soc., 1968, 90, 2278 CrossRef CAS.
- G. Cerveau, E. Colomer and R. J. P. Corriu, J. Organomet. Chem., 1977, 136, 349 CrossRef CAS.
- (a) C. A. Tolman, S. D. Ittel, A. D. English and J. P. Jesson, J. Am. Chem. Soc., 1978, 100, 4080 CrossRef CAS; (b) S. D. Ittel, C. A. Tolman, A. D. English and J. P. Jesson, J. Am. Chem. Soc., 1978, 100, 7577 CrossRef CAS; (c) C. A. Tolman, S. D. Ittel, A. D. English and J. P. Jesson, J. Am. Chem. Soc., 1979, 101, 1742 CrossRef CAS.
- (a) M. V. Bakerand and L. D. Field, J. Am. Chem. Soc., 1986, 108, 7433 CrossRef; (b) M. K. Whittlesey, R. J. Mawby, R. Osman, R. N. Perutz, L. D. Field, M. P. Wilkinson and M. W. George, J. Am. Chem. Soc., 1993, 115, 8627 CrossRef CAS.
- S. H. Wunderlich and P. Knochel, Angew. Chem., Int. Ed., 2009, 48, 9717 CrossRef CAS.
- S. Camadanli, R. Beck, U. Flörke and H.-F. Klein, Organometallics, 2009, 28, 2300 CrossRef CAS.
- W. D. Jones, G. P. Foster and J. M. Putinas, J. Am. Chem. Soc., 1987, 109, 5047 CrossRef CAS.
- (a) R. Shang, L. Ilies and E. Nakamura, Chem. Rev., 2017, 117, 9086–9139 CrossRef CAS; (b) J. Norinder, A. Matsumoto, N. Yoshikai and E. Nakamura, J. Am. Chem. Soc., 2008, 130, 5858–5859 CrossRef CAS.
- (a) F. Kakiuchi and S. Murai, in Topics in Organometallic Chemistry, ed. S. Murai, Springer-Verlag, Berlin, 1999, vol. 3, pp. 47–79 Search PubMed; (b) F. Kakiuchi and S. Murai, Acc. Chem. Res., 2002, 35, 826 CrossRef CAS; (c) V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731 CrossRef CAS; (d) F. Kakiuchi and N. Chatani, Adv. Synth. Catal., 2003, 345, 1077 CrossRef CAS; (e) Handbook of C-H Transformations, ed. G. Dyker, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed; (f) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS; (g) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (h) L. Ackermann, in Topics in Organometallic Chemistry, ed. N. Chatani, Springer-Verlag, Berlin, 2007, vol. 24, pp. 35–60 Search PubMed; (i) S. Oi, S. Fukita and Y. Inoue, Chem. Commun., 1998, 2439 RSC; (j) F. Kakiuchi, S. Kan, K. Igi, N. Chatani and S. Murai, J. Am. Chem. Soc., 2003, 125, 1698 CrossRef CAS; (k) F. Kakiuchi, Y. Matsuura, S. Kan and N. Chatani, J. Am. Chem. Soc., 2005, 127, 5936 CrossRef CAS; (l) X. Chen, J.-J. Li, X.-S. Hao, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 78 CrossRef CAS; (m) X. Chen, C. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634 CrossRef CAS; (n) S. Oi, S. Fukita, N. Hirata, N. Watanuki, S. Miyano and Y. Inoue, Org. Lett., 2001, 3, 2579 CrossRef CAS; (o) D. Kalyani, N. R. Deprez, L. V. Desai and M. Sanford, J. Am. Chem. Soc., 2005, 127, 7330 CrossRef CAS; (p) L. Ackermann, Org. Lett., 2005, 7, 3123 CrossRef CAS; (q) D. Shabashov and O. Daugulis, Org. Lett., 2005, 7, 3657 CrossRef CAS; (r) L. Ackermann, A. Althammer and R. Born, Angew. Chem., Int. Ed., 2006, 45, 2619–2622 CrossRef CAS.
- N. Yoshikai, A. Matsumoto, J. Norinder and E. Nakamura, Angew. Chem., Int. Ed., 2009, 48, 2925 CrossRef CAS.
- L. Ilies, J. Okabe, N. Yoshikai and E. Nakamura, Org. Lett., 2010, 12, 2838 CrossRef CAS.
- N. Yoshikai, A. Matsumoto, J. Norinder and E. Nakamura, Synlett, 2010, 313–316 CAS.
- L. Ilies, S. Asako and E. Nakamura, J. Am. Chem. Soc., 2011, 133, 7672 CrossRef CAS.
- S. C. Bart, E. J. Hawrelak, E. Lobkovsky and P. J. Chirik, Organometallics, 2005, 24, 5518 CrossRef CAS.
- G. Cahiez, C. Chaboche, F.-B. Mahuteau and M. Ahr, Org. Lett., 2005, 7, 1943 CrossRef CAS.
- T. Nagano and T. Hayashi, Org. Lett., 2005, 7, 491 CrossRef CAS.
- N. Yoshikai, S. Asako, T. Yamakawa, L. Ilies and E. Nakamura, Chem. – Asian J., 2011, 6, 3059 CrossRef CAS.
- Y. Sun, H. Tang, K. Chen, L. Hu, J. Yao, S. Shaik and H. Chen, J. Am. Chem. Soc., 2016, 138, 3715 CrossRef CAS.
- P. L. Holland, Acc. Chem. Res., 2015, 48, 1696 CrossRef CAS.
- R. Shang, L. Ilies, S. Asako and E. Nakamura, J. Am. Chem. Soc., 2014, 136, 14349 CrossRef CAS.
- Q. Gu, H. H. Al Mamari, K. Graczyk, E. Diers and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 3868 CrossRef CAS.
- T. E. Boddie, S. H. Carpenter, T. M. Baker, J. C. DeMuth, G. Cera, W. W. Brennessel, L. Ackermann and M. L. Neidig, J. Am. Chem. Soc., 2019, 141, 12338 CrossRef CAS.
- L. Ilies, E. Konno, Q. Chen and E. Nakamura, Asian J. Org. Chem., 2012, 1, 142 CrossRef CAS.
- L. Ilies, M. Kobayashi, A. Matsumoto, N. Yoshikai and E. Nakamura, Adv. Synth. Catal., 2012, 354, 593–596 CrossRef CAS.
- Y. Kobayashi and R. Mizojiri, Tetrahedron Lett., 1996, 37, 8531 CrossRef CAS.
- T. Hatakeyama, T. Hashimoto, Y. Kondo, Y. Fujiwara, H. Seike, H. Takaya, Y. Tamada, T. Ono and M. Nakamura, J. Am. Chem. Soc., 2010, 132, 10674 CrossRef CAS.
- O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053 CrossRef CAS.
- G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726–11743 CrossRef CAS.
- J. J. Sirois, R. Davis and B. DeBoef, Org. Lett., 2014, 16, 868 CrossRef CAS.
- Y.-M. Wei, M.-F. Wang and X.-F. Duan, Org. Lett., 2019, 21(16), 6471 CrossRef CAS.
- L. D. Tran and O. Daugulis, Org. Lett., 2010, 12, 4277–4279 CrossRef CAS.
- Z. Shen, G. Cera, T. Haven and L. Ackermann, Org. Lett., 2017, 19, 3795 CrossRef CAS.
- R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS.
- G. Cahiez and A. Moyeux, Chem. Rev., 2010, 110, 1435 CrossRef CAS.
- K. Gao and N. Yoshikai, Acc. Chem. Res., 2014, 47, 1208 CrossRef CAS.
- M. Moselage, J. Li and L. Ackermann, ACS Catal., 2016, 6, 498 CrossRef CAS.
- Q. Chen, L. Ilies, N. Yoshikai and E. Nakamura, Org. Lett., 2011, 13, 3232 CrossRef CAS.
- L. Ilies, S. Ichikawa, S. Asako, T. Matsubara and E. Nakamura, Adv. Synth. Catal., 2015, 357, 2175 CrossRef CAS.
- R. Shang, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2015, 137, 7660 CrossRef CAS.
- R. Shang, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2016, 138, 10132 CrossRef CAS.
- K. Graczyk, T. Haven and L. Ackermann, Chem. – Eur. J., 2015, 21, 8812 CrossRef CAS.
- W. Shi, C. Liu and A. Lei, Chem. Soc. Rev., 2011, 40, 2761 RSC.
- S. Asako, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2013, 135, 17755–17757 CrossRef CAS.
- L. Ilies, T. Matsubara, S. Ichikawa, S. Asako and E. Nakamura, J. Am. Chem. Soc., 2014, 136, 13126 CrossRef CAS.
- S. Asako, J. Norinder, L. Ilies, N. Yoshikai and E. Nakamura, Adv. Synth. Catal., 2014, 356, 1481 CrossRef CAS.
- T. Matsubara, S. Asako, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2014, 136, 646 CrossRef CAS.
- D. Griller and K. U. Ingold, Acc. Chem. Res., 1980, 13, 317 CrossRef CAS.
- E. R. Fruchey, B. M. Monks and S. P. Cook, J. Am. Chem. Soc., 2014, 136, 13130 CrossRef CAS.
- B. M. Monks, E. R. Fruchey and S. P. Cook, Angew. Chem., Int. Ed., 2014, 53, 11065 CrossRef CAS.
- G. Cera, T. Haven and L. Ackermann, Angew. Chem., Int. Ed., 2016, 55, 1484 CrossRef CAS.
- L. Ilies, M. Kobayashi, A. Matsumoto, N. Yoshikai and E. Nakamura, Adv. Synth. Catal., 2012, 354, 593 CrossRef CAS.
- D. Schmiel and H. Butenschön, Organometallics, 2017, 36, 4979 CrossRef CAS.
- T. Doba, T. Matsubara, L. Ilies, R. Shang and E. Nakamura, Nat. Catal., 2019, 2, 400 CrossRef CAS.
- N. Yoshikai, A. Mieczkowski, A. Matsumoto, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2010, 132, 5568 CrossRef CAS.
- P. P. Singh, S. Gudup, S. Ambala, U. Singh, S. Dadhwal, B. Singh, S. D. Sawant and R. A. Vishwakarma, Chem. Commun., 2011, 47, 5852 RSC.
- M. Sekine, L. Ilies and E. Nakamura, Org. Lett., 2013, 15, 714 CrossRef CAS.
- R. Shang, L. Ilies, A. Matsumoto and E. Nakamura, J. Am. Chem. Soc., 2013, 135, 6030 CrossRef CAS.
- L. Ilies, Y. Itabashi, R. Shang and E. Nakamura, ACS Catal., 2017, 7, 89 CrossRef CAS.
- B. Zhou, H. Sato, L. Ilies and E. Nakamura, ACS Catal., 2018, 8, 8 CrossRef CAS.
- A. Matsumoto, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2011, 133, 6557 CrossRef CAS.
- X. Zhang, X. Jiang, J. Luo, C. Chi, H. Chen and J. A. Wu, Chem. – Eur. J., 2010, 16, 464 CrossRef CAS.
- L. Ilies, A. Matsumoto, M. Kobayashi, N. Yoshikai and E. Nakamura, Synlett, 2012, 2381 CAS.
- L. Adak and N. Yoshikai, Tetrahedron, 2012, 68, 5167 CrossRef CAS.
- M. Y. Wong, T. Yamakawa and N. Yoshikai, Org. Lett., 2015, 17, 442 CrossRef CAS.
- P. E. Baikie and O. S. Mills, Chem. Commun., 1966, 707 RSC.
- W. T. Flannigan, G. R. Knox and P. L. Pauson, J. Chem. Soc. C, 1969, 2077 RSC.
- M. M. Bagga, P. L. Pauson, F. J. Preston and R. I. Reed, Chem. Commun., 1965, 543 RSC.
- T. Jia, C. Zhao, R. He, H. Chen and C. Wang, Angew. Chem., Int. Ed., 2016, 55, 5268 CrossRef CAS.
- T. Matsubara, L. Ilies and E. Nakamura, Chem. – Asian J., 2016, 11, 380 CrossRef CAS.
- L. Ilies, Y. Arslanoglu, T. Matsubara and E. Nakamura, Asian J. Org. Chem., 2018, 7, 1327 CrossRef CAS.
- L. Ilies, Y. Zhou, H. Yang, T. Matsubara, R. Shang and E. Nakamura, ACS Catal., 2018, 8, 11478 CrossRef CAS.
- S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Nature, 1993, 366, 529 CrossRef CAS.
- F. Kakiuchi and S. Murai, Acc. Chem. Res., 2002, 35, 826 CrossRef CAS.
- J. Mo, T. Müller, J. C. A. Oliveira and L. Ackermann, Angew. Chem., Int. Ed., 2018, 57, 7719 CrossRef CAS.
- J. Mo, T. Mgller, J. C. A. Oliveira, S. Demeshko, F. Meyer and L. Ackermann, Angew. Chem., Int. Ed., 2019, 58, 12874 CrossRef CAS.
- R. Breslow and S. H. Gellman, J. Am. Chem. Soc., 1983, 105, 6728–6729 CrossRef CAS.
- T. Hatakeyama, Y. Yoshimoto, S. K. Ghorai and M. Nakamura, Org. Lett., 2010, 12, 1516 CrossRef CAS.
- Y. Aoki, R. Imayoshi, T. Hatakeyama, H. Takaya and M. Nakamura, Heterocycles, 2015, 90, 893 CrossRef CAS.
- R. R. Anugu, S. Munnuri and J. R. Falck, J. Am. Chem. Soc., 2020, 142(11), 5266–5271 CrossRef CAS.
- T. J. Mazzacano and N. P. Mankad, J. Am. Chem. Soc., 2013, 135, 17258 CrossRef CAS.
- T. Dombray, C. G. Werncke, S. Jiang, M. Grellier, L. Vendier, S. Bontemps, J.-B. Sortais, S. S. Etienne and C. Darcel, J. Am. Chem. Soc., 2015, 137, 4062 CrossRef CAS.
- (a) F. Diederich and P. J. Stang, Metal-catalyzed cross-coupling reactions, Wiley-VCH, 1998, p. 421 CrossRef; (b) S. E. Denmark and R. F. Sweis, Acc. Chem. Res., 2002, 35, 835 CrossRef CAS.
- (a) M. Elbing and G. C. Bazan, Angew. Chem., Int. Ed., 2008, 47, 834 CrossRef CAS; (b) T. Kumagai and S. Itsuno, Macromolecules, 2002, 35, 5323 CrossRef CAS.
- A. K. Franz, Curr. Opin. Drug Discovery Dev., 2007, 10, 654 CAS.
- J. B. Gluyas, C. Burschka, S. Dorrich, J. Vallet, H. Gronemeyer and R. Tacke, Org. Biomol. Chem., 2012, 10, 6914 RSC.
- Y. Yang and C. Wang, Sci. China: Chem., 2015, 58, 1266 CrossRef CAS.
- S. Bähr, W. Xue and M. Oestreich, ACS Catal., 2019, 9, 16 CrossRef.
- C. Cheng and J. F. Hartwig, Chem. Rev., 2015, 115(17), 8946 CrossRef CAS.
- Y. Sunada, H. Soejima and H. Nagashima, Organometallics, 2014, 33, 5936 CrossRef CAS.
- J. Ito, S. Hosokawa, H.-B. Khalid and H. Nishiyama, Organometallics, 2015, 34, 1377–1383 CrossRef CAS.
- Y. Yoshigoe and Y. Kuninobu, Org. Lett., 2017, 19, 3450 CrossRef CAS.
- W. Xu, J. H. Pek and N. Yoshikai, Asian J. Org. Chem., 2018, 7, 1351 CrossRef CAS.
- R. P. Yu, D. Hesk, N. Rivera, I. Pelczer and P. J. Chirik, Nature, 2016, 529, 195 CrossRef.
- G. Zhang, J. Zhu and C. Ding, ChemistrySelect, 2018, 3, 9803 CrossRef CAS.
- C. Wang, C. Wu and S. Ge, ACS Catal., 2016, 6, 7585 CrossRef CAS.
- V. Postils, M. Rodríguez, G. Sabenya, A. Conde, M. M. Díaz-Requejo, P. J. Pérez, M. Costas, M. Sola and J. M. Luis, ACS Catal., 2018, 8, 4313 CrossRef CAS.
- E. Buchner and T. Curtius, Ber. Dtsch. Chem. Ges., 1885, 18, 2371 CrossRef.
- G. W. Wheland, J. Am. Chem. Soc., 1942, 64(4), 900 CrossRef CAS.
- (a) K. Ray, F. F. Pfaff, B. Wang and W. Nam, J. Am. Chem. Soc., 2014, 136, 13942–13958 CrossRef CAS; (b) M. Sankaralingam, Y.-M. Lee, W. Nam and S. Fukuzumi, Coord. Chem. Rev., 2017, 334, 25–42 CrossRef; (c) X. Huang and J. T. Groves, Chem. Rev., 2018, 118, 2491–2553 CrossRef CAS; (d) A. J. Jasniewski and L. Que, Jr., Chem. Rev., 2018, 118, 2554–2592 CrossRef CAS; (e) S. Kal and L. Que, JBIC, J. Biol. Inorg. Chem., 2017, 22, 339–365 CrossRef CAS; (f) X. Huang and J. T. Groves, JBIC, J. Biol. Inorg. Chem., 2017, 22, 185–207 CrossRef CAS; (g) S. Sahu and D. P. Goldberg, J. Am. Chem. Soc., 2016, 138, 11410–11428 CrossRef CAS; (h) C. P. Rizquez, A. R. Otero and J. M. Palomo, Org. Biomol. Chem., 2019, 17, 7114–7123 RSC; (i) R. V. K. Cochrane and J. C. Vederas, Acc. Chem. Res., 2014, 47(10), 3148–3161 CrossRef CAS.
- W. Jiang, R. A. Cacho, G. Chiou, N. K. Garg, Y. Tang and C. T. Walsh, J. Am. Chem. Soc., 2013, 135, 4457 CrossRef CAS.
- Y. R. Luo and J. P. Cheng, Bond Dissociation Energies, in CRC Handbook of Chemistry and Physics, ed. J. R. Rumble, CRC Press/Taylor & Francis, Boca Raton, FL, 99th edn, Internet Version 2018, 2018, pp. 9–72 Search PubMed.
- (a) R. Mello, M. Fiorentino, C. Fusco and R. Curci, J. Am. Chem. Soc., 1989, 111, 6749 CrossRef CAS; (b) W. Adam, R. Curci, M. E. G. Nunez and R. Mello, J. Am. Chem. Soc., 1991, 113, 7654 CrossRef CAS; (c) L. D’Accolti, A. Dinoi, C. Fusco, A. Russo and R. Curci, J. Org. Chem., 2003, 68, 7806 CrossRef; (d) P. Bovicelli, A. Gambacorta, P. Lupattelli and E. Mincione, Tetrahedron Lett., 1992, 33, 7411 CrossRef CAS; (e) P. Bovicelli, P. Lupattelli, E. Mincione, T. Prencipe and R. J. Curci, Org. Chem., 1992, 57, 5052 CrossRef CAS.
- (a) H. J. H. Fenton, J. Chem. Soc., Dalton Trans., 1894, 65, 899 RSC; (b) C. Walling, Acc. Chem. Res., 1975, 8, 125 CrossRef CAS.
- F. Haber and J. Weiss, Proc. R. Soc. London, Ser. A, 1934, 147, 332 CAS.
- J. Zhau and M. C. White, J. Am. Chem. Soc., 2018, 140(43), 13988–14009 CrossRef.
- (a) C. Rüchardt, Angew. Chem., Int. Ed. Engl., 1970, 9, 830 CrossRef; (b) D. H. R. Barton, F. Halley, N. Ozbalik, M. Schmitt, E. Young and G. Balavoine, J. Am. Chem. Soc., 1989, 111, 7144 CrossRef CAS; (c) M. J. Perkins, Chem. Soc. Rev., 1996, 25, 229 RSC.
- (a) R. M. Buchanan, S. Chen, J. F. Richardson, M. Bressan, L. Forti, A. Monillo and R. H. Fish, Inorg. Chem., 1994, 33, 3208–3209 CrossRef CAS; (b) R. Banerjee, Y. Proshlyakov, J. D. Lipscomb and D. A. Proshlyakov, Nature, 2015, 518, 431–434 CrossRef CAS.
- (a) S. Barry and G. L. Challis, ACS Catal., 2013, 3, 10 CrossRef; (b) W. Jiang, M. A. Wilson and D. P. Weeks, ACS Chem. Biol., 2013, 8, 1687–1691 CrossRef CAS; (c) L. P. Wackett, Enzyme Microb. Technol., 2002, 31, 577–587 CrossRef CAS.
- C. Kim, K. Chen, J. Kim and L. Que, Jr., J. Am. Chem. Soc., 1997, 119, 5964 CrossRef CAS.
- K. Chen and L. Que, Jr., J. Am. Chem. Soc., 2001, 123, 6327 CrossRef CAS.
- A. Company, L. Gómez, M. Güell, X. Ribas, J. M. Luis, L. Que, Jr. and M. Costas, J. Am. Chem. Soc., 2007, 129, 15766 CrossRef CAS.
- (a) K.-B. Cho, H. Hirao, S. Shaik and W. Nam, Chem. Soc. Rev., 2015, 45(5), 1197–1210 RSC; (b) S. Rana, A. Dey and D. Maiti, Chem. Commun., 2015, 51, 14469 RSC.
- M. S. Chen and M. C. White, Science, 2007, 318, 783 CrossRef CAS.
- C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293–1314 CrossRef CAS.
- N. A. Vermeulen, M. S. Chen and M. C. White, Tetrahedron, 2009, 65, 3078 CrossRef CAS.
- M. S. Chen and M. C. White, Science, 2010, 327, 566 CrossRef CAS.
- L. Gómez, I. Garcia-Bosch, A. Company, J. Benet-Buchholz, A. Polo, X. Sala, X. Ribas and M. Costas, Angew. Chem., Int. Ed., 2009, 121, 5830–5833 CrossRef.
- P. E. Gormisky and M. C. White, J. Am. Chem. Soc., 2013, 135(38), 14052–14055 CrossRef CAS.
- J. M. Howell, K. Feng, J. R. Clark, L. J. Trzepkowski and M. C. White, J. Am. Chem. Soc., 2015, 137(46), 14590–14593 CrossRef CAS.
- T. J. Osberger, D. C. Rogness, J. T. Kohrt, A. F. Stepan and M. C. White, Nature, 2016, 537, 214–219 CrossRef CAS.
- D. Font, M. Canta, M. Milan, O. Cuss, X. Ribas, R. J. M. K. Gebbink and M. Costas, Angew. Chem., Int. Ed., 2016, 55, 5776–5779 CrossRef CAS.
- J. Serrano-Plana, A. Aguinaco, R. Belda, E. García-España, M. G. Basallote, A. Company and M. Costas, Angew. Chem., Int. Ed., 2016, 55, 6310–6314 CrossRef CAS.
- B. Chandra, K. K. Singh and S. S. Gupta, Chem. Sci., 2017, 8, 7545–7551 RSC.
- S. Jana, M. Ghosh, M. Ambule and S. S. Gupta, Org. Lett., 2017, 19(4), 746–749 CrossRef CAS.
- S. A. Loskot, D. K. Romney, F. H. Arnold and B. M. Stoltz, J. Am. Chem. Soc., 2017, 139, 10196–10199 CrossRef CAS.
- W. G. Salmond, M. A. Barta and J. L. Havens, J. Org. Chem., 1978, 43, 2057–2059 CrossRef CAS.
- (a) M. s. Chen, N. Prabagaran, N. A. Labenz and C. M. White, J. Am. Chem. Soc., 2005, 127, 6970–6971 CrossRef CAS; (b) J.-Q. Yu and E. J. Corey, Org. Lett., 2002, 4, 2727–2730 CrossRef CAS; (c) X. Xing, N. R. O’Connor and B. M. Stoltz, Angew. Chem., Int. Ed., 2015, 54, 11186–11190 CrossRef CAS; (d) B. A. Sparling, D. C. Moebius and M. D. Shair, J. Am. Chem. Soc., 2013, 135, 644–647 CrossRef CAS; (e) N. R. Schmuff and B. M. Trost, J. Org. Chem., 1983, 48, 1404–1412 CrossRef CAS; (f) Y. Zhao and Y.-Y. Yeung, Org. Lett., 2010, 12, 2128–2131 CrossRef CAS; (g) Y. Wang, Y. Kuang and Y. Wang, Chem. Commun., 2015, 51, 5852–5855 RSC; (h) N. C. Wilde, M. Isomura, A. Mendoza and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 4909–4912 CrossRef CAS; (i) W. B. Young, J. J. Masters and S. Danishefsky, J. Am. Chem. Soc., 1995, 117, 5228–5234 CrossRef CAS; (j) J. Jasiczak, J. Chem. Soc., Perkin Trans. 1, 1988, 2687–2692 RSC; (k) E. J. Horn, B. R. Rosen, Y. Chen, J. Tang, K. Chen, M. D. Eastgate and P. S. Baran, Nature, 2016, 533, 77–81 CrossRef CAS; (l) J. G. Allen and S. J. Danishefsky, J. Am. Chem. Soc., 2001, 123, 351–352 CrossRef CAS; (m) R. K. Quinn, Z. A. Könst, S. E. Michalak, Y. Schmidt, A. R. Szklarski, A. R. Flores, S. Nam, D. A. Horne, C. D. Vanderwal and E. Alexanian, J. Am. Chem. Soc., 2016, 138, 696–702 CrossRef CAS.
- P. Hu, M. Tan, L. Cheng, H. Zhao, R. Feng, W.-J. Gu and W. Han, Nat. Commun., 2019, 10, 2425 CrossRef.
- G. A. Molander and N. Ellis, Acc. Chem. Res., 2007, 40, 275–286 CrossRef CAS.
- (a) Y. Ishii, S. Sakaguchi and T. Iwahama, Adv. Synth. Catal., 2001, 343, 393–427 CrossRef CAS; (b) E. Gaster, S. Kozuch and D. Pappo, Angew. Chem., Int. Ed., 2017, 56, 5912–5915 CrossRef CAS.
- G. A. Russell, J. Am. Chem. Soc., 1957, 79, 3871–3877 CrossRef CAS.
- M. Borrell, S. García-Caballero, M. Bietti and M. Costas, ACS Catal., 2020, 10, 4702–4709 CrossRef CAS.
- J. R. Griffin, C. I. Wendell, J. A. Garwin and M. C. White, J. Am. Chem. Soc., 2017, 139, 13624–13627 CrossRef CAS.
- G.-Q. Chen, Z.-J. Xu, C.-Y. Zhou and C.-M. Che, Chem. Commun., 2011, 47, 10963 RSC.
- A. Dutta Chowdhury and G. K. Lahiri, Chem. Commun., 2012, 48, 3448–3450 RSC.
- A. M. Balija, K. J. Stowers, M. J. Schultz and M. S. Sigman, Org. Lett., 2006, 8, 1121 CrossRef CAS.
- A. Dutta Chowdhury, R. Ray and G. K. Lahiri, Chem. Commun., 2012, 48, 5497–5499 RSC.
- (a) J. Chen and C.-M. Che, Angew. Chem., Int. Ed., 2004, 43, 4950 CrossRef CAS; (b) G. Jiang, J. Chen, H.-Y. Thu, J.-S. Huang, N. Zhu and C.-M. Che, Angew. Chem., Int. Ed., 2008, 47, 6638 CrossRef CAS; (c) G.-Q. Chen, Z.-J. Xu, C.-Y. Zhou and C.-M. Che, Chem. Commun., 2011, 47, 10963 RSC.
- (a) J. T. Groves and T. S. Myers, J. Am. Chem. Soc., 1983, 105, 5791 CrossRef CAS; (b) J. P. Collman, T. Kodadek and J. I. Brauman, J. Am. Chem. Soc., 1986, 108, 2588 CrossRef CAS.
- G.-Q. Chen, Z.-J. Xu, C.-Y. Zhou and C.-M. Che, Chem. Commun., 2011, 47, 10963 RSC.
- Y.-D. Du, C.-W. Tse, Z.-J. Xu, Y. Liu and C.-M. Che, Chem. Commun., 2014, 50, 12669 RSC.
- S. C. Hammer, G. Kubik, E. Watkins, S. Huang, H. Minges and F. H. Arnold, Science, 2017, 358, 215–218 CrossRef CAS.
- (a) P. R. Ortiz de Montellano, Cytochrome P-450 structure, mechanism and biochemistry, Plenum Press, New York, 1986 CrossRef; (b) D. Mansuy, Pure Appl. Chem., 1987, 59, 759–770 CAS; (c) B. Meunier, S. P. De Visser and S. Shaik, Chem. Rev., 2004, 104, 3947–3980 CrossRef CAS; (d) I. Tabushi, M. Kodera and M. Yokoyama, J. Am. Chem. Soc., 1985, 107, 4466–4473 CrossRef CAS.
- (a) J. Gui, C.-M. Pan, Y. Jin, T. Qin, J. C. Lo, B. J. Lee, S. H. Spergel, M. E. Mertzman, W. J. Pitts, T. E. La Cruz, M. A. Schmidt, N. Darvatkar, S. R. Natarajan and P. S. Baran, Science, 2015, 348, 886–891 CrossRef CAS; (b) J. C. Lo, J. Gui, Y. Yabe, C.-M. Pan and P. S. Baran, Nature, 2014, 516, 343–348 CrossRef CAS; (c) J. C. Lo, D. Y. Kim, C.-M. Pan, J. T. Edwards, Y. Yabe, J. H. Gui, T. Qin, S. Guti8rrez, J. Giacoboni, M. W. Smith, P. L. Holland and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 2484–2503 CrossRef CAS; (d) J. C. Lo, Y. Yabe and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 1304–1307 CrossRef CAS; (e) H. T. Dao, C. Li, Q. Michaudel, B. D. Maxwell and P. S. Baran, J. Am. Chem. Soc., 2015, 137, 8046–8049 CrossRef CAS; (f) S. W. M. Crossley, C. Obradors, R. M. Martinez and R. A. Shenvi, Chem. Rev., 2016, 116, 8912–9000 CrossRef CAS; (g) T. Hashimoto, D. Hirose and T. Taniguchi, Angew. Chem., Int. Ed., 2014, 53, 2730–2734 CrossRef CAS; (h) E. K. Leggans, T. J. Barker, K. K. Duncan and D. L. Boger, Org. Lett., 2012, 14, 1428–1431 CrossRef CAS; (i) T. Taniguchi, N. Goto, A. Nishibata and H. Ishibashi, Org. Lett., 2010, 12, 112–115 CrossRef CAS; (j) T. Sugimori, S.-I. Horike, S. Tsumura, M. Handa and K. Kasuga, Inorg. Chim. Acta, 1998, 283, 275–278 CrossRef CAS; (k) M. Takeuchi, M. Kodera, K. Kano and Z. Yoshida, J. Mol. Catal. A: Chem., 1996, 113, 51–57 CrossRef CAS.
- B. Liu, F. Jin, T. Wang, X. Yuan and W. Han, Angew. Chem., Int. Ed., 2017, 56, 12712–12717 CrossRef CAS.
- (a) R. J. DeLuca, J. L. Edwards, L. D. Steffens, B. W. Michel, X. Qiao, C. Zhu, S. P. Cook and M. S. Sigman, J. Org. Chem., 2013, 78, 1682 CrossRef CAS; (b) B. Liu and W. Han, Synlett, 2018, 383–387 CAS.
- S. Srinivasan and W. T. Ford, J. Mol. Catal., 1991, 64, 291303 CrossRef.
- F. Puls and H.-J. Knölker, Angew. Chem., Int. Ed., 2018, 57, 1222–1226 CrossRef CAS.
- H. Subramanian and R. T. Koodali, React. Kinet. Catal. Lett., 2008, 95, 239 CrossRef CAS.
- H. Subramanian and R. T. Koodali, React. Kinet. Catal. Lett., 2008, 95(2), 239–245 CrossRef CAS.
- (a) B. Meunier, Biomimetic Oxidations Mediated by Metal Complexes, Imperial College Press, London, 2000 Search PubMed; (b) X. T. Zhou, H. B. Ji, L. X. Pei, Y. B. She, J. C. Xu and L. F. Wang, Chin. J. Org. Chem., 2007, 27, 1039 CAS.
- X.-T. Zhou, H.-B. Ji and Q.-L. Yuan, J. Porphyrins Phthalocyanines, 2008, 12, 94–100 CrossRef CAS.
- H.-Y. Lan, X.-T. Zhou and H.-B. Ji, Tetrahedron, 2013, 69, 4241–4246 CrossRef CAS.
- (a) Halogenated Organic Compounds – A Global Perspective, in Dehalogenation, ed. M. M. Häggblom and I. D. Bossert, Springer, Boston, MA, 2003 Search PubMed; (b) C. Schnepel and N. Sewald, Chem. – Eur. J., 2017, 23, 12064–12086 CrossRef CAS.
- (a) Ullmann's Encyclopedia of Industrial Chemistry, 6th edn, Wiley-VCH, 2002 Search PubMed; (b) S. G. Davos, Organotransition Metal Chemistry: Application to Organic Synthesis, Pergamon Press, Oxford, 1982 Search PubMed; (c) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS; (d) F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef; (e) R. Stürmer, Angew. Chem., Int. Ed., 1999, 38, 3307–3308 CrossRef; (f) A. Butler and J. V. Walker, Chem. Rev., 1993, 93, 1937–1944 CrossRef CAS; (g) R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239 CrossRef CAS; (h) S. A. Cook, E. A. Hill and A. S. Borovik, Biochemistry, 2015, 54, 4167 CrossRef CAS; (i) L. C. Blasiak and C. L. Drennan, Acc. Chem. Res., 2009, 42, 147 CrossRef CAS.
- (a) C. Krebs, D. G. Fujimori, C. T. Walsh and J. M. Bollinger, Acc. Chem. Res., 2007, 40, 484 CrossRef CAS; (b) L. C. Blasiak, F. H. Vaillancourt, C. T. Walsh and C. L. Drennan, Nature, 2006, 440, 368 CrossRef CAS; (c) C. Wong, D. G. Fujimori, C. T. Walsh and C. L. Drennan, J. Am. Chem. Soc., 2009, 131, 4872 CrossRef CAS; (d) F. H. Vaillancourt, J. Yin and C. T. Walsh, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10111 CrossRef CAS; (e) C. T. Walsh and J. M. Bollinger, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 17723 CrossRef; (f) M. Srnec and E. I. Solomon, J. Am. Chem. Soc., 2017, 139, 2396 CrossRef CAS.
- P. Comba and S. Wunderlich, Chem. – Eur. J., 2010, 16, 7293 CrossRef CAS.
- T. Kojima, R. A. Leising, S. Yan and L. Que, J. Am. Chem. Soc., 1993, 115, 11328 CrossRef CAS.
- J.-U. Rohde, A. Stubna, E. L. Bominaar, E. Münck, W. Nam and L. Que, Inorg. Chem., 2006, 45, 6435 CrossRef CAS.
- J. England, Y. Guo, K. M. V. Heuvelen, M. A. Cranswick, G. T. Rohde, E. L. Bominaar, E. Münck and L. Que, J. Am. Chem. Soc., 2011, 133, 11880 CrossRef CAS.
- H. Noack and P. E. M. Siegbahn, J. Biol. Inorg. Chem., 2007, 12, 1151 CrossRef CAS.
- M. G. Quesne and S. P. de Visser, J. Biol. Inorg. Chem., 2012, 17, 841 CrossRef CAS.
- O. Planas, M. Clémancey, J.-M. Latour, A. Company and M. Costas, Chem. Commun., 2014, 50, 10887 RSC.
- S. Rana, S. Bag, T. Patra and D. Maiti, Adv. Synth. Catal., 2014, 356, 2453–2458 CrossRef CAS.
- S. Chatterjee and T. K. Paine, Angew. Chem., Int. Ed., 2016, 55, 7717 CrossRef CAS.
- M. Puri, A. N. Biswas, R. Fan, Y. Guo and L. Que, J. Am. Chem. Soc., 2016, 138, 2484 CrossRef CAS.
- S. Rana, J. P. Biswas, A. Sen, M. Clémency, G. Blondin, J.-M. Latour, G. Rajaraman and D. Maiti, Chem. Sci., 2018, 9, 7843 RSC.
- (a) W. Liu and J. T. Groves, J. Am. Chem. Soc., 2010, 132, 12847 CrossRef CAS; (b) W. Liu and J. T. Groves, Acc. Chem. Res., 2015, 48, 1727 CrossRef CAS; (c) W. Liu, X. Huang, M.-J. Cheng, R. J. Nielsen, W. A. Goddard and J. T. Groves, Science, 2012, 337, 1322 CrossRef CAS; X. Huang, W. Liu, J. M. Hooker and J. T. Groves, Angew. Chem., Int. Ed., 2015, 54, 5241 Search PubMed.
- (a) C.-J. Li, Cross-dehydrogenative coupling (CDC): Exploring C–C bond formations beyond functional group transformations, Acc. Chem. Res., 2009, 42, 335–344 CrossRef CAS; (b) A. S.-K. Tsang, S. J. Park and M. H. Todd, From C-H to C-C bonds: dehydrogenative coupling, 2014, ch. 11, pp. 254–294, 10.1039/9781782620082-00254.
- (a) A. A. O. Sarhan and C. Bolm, Chem. Soc. Rev., 2009, 38, 2730–2744 RSC; (b) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293–1314 CrossRef CAS.
- (a) Z. Li, D. S. Bohle and C. J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS; (b) C. J. Li and Z. Li, Pure Appl. Chem., 2006, 78, 935 CAS; (c) W. J. Yoo and C. J. Li, Top. Curr. Chem., 2009, 292, 281 CrossRef; (d) C. J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS; (e) S. A. Girard, T. Knauber and C. J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS; (f) C. J. Li, From C–H to C–C bonds cross-dehydrogenative-coupling, RSC, London, 2014 RSC.
- L.-S. Huang, D.-Y. Han and D.-Z. Xu, Adv. Synth. Catal., 2019, 361, 4016–4040 CrossRef CAS.
- Z. Li, L. Cao and C. J. Li, Angew. Chem., Int. Ed., 2007, 46, 6505 CrossRef CAS.
- L. Lv and Z. Li, Top. Curr. Chem. (Z), 2016, 374, 38 CrossRef.
- Y. Zhang and C. J. Li, Eur. J. Org. Chem., 2007, 4654 CrossRef CAS.
- S. Pan, J. Liu, Y. Li and Z. Li, Chin. Sci. Bull., 2012, 57, 2382 CrossRef CAS.
- K. Yang and Q. Song, Org. Lett., 2015, 17, 548 CrossRef CAS.
- S. J. Lou, D. Q. Xu, D. F. Shen, Y. F. Wang, Y. K. Liu and Z. Y. Xu, Chem. Commun., 2012, 48, 11993 RSC.
- Y. Li, F. Guo, Z. Zha and Z. Wan, Chem. – Asian J., 2013, 8, 534 CrossRef CAS.
- Y. M. Li, S. J. Lou, Q. H. Zhou, L. W. Zhu, L. F. Zhu and L. Li, Eur. J. Org. Chem., 2015, 3044 CrossRef CAS.
- J. B. Yu, Y. Zhang, Z. J. Jiang and W. K. Su, J. Org. Chem., 2016, 81, 11514–11520 CrossRef CAS.
- K. Hashiguchi, T. Tanaka, R. Yazaki and T. Ohshima, ACS Catal., 2018, 8(9), 8430–8440 CrossRef.
- Z. L. Li, K. K. Sun, P. Y. Wuand and C. Cai, J. Org. Chem., 2019, 84, 6830–6839 CrossRef CAS.
- Z. Li, R. Yu and H. Li, Angew. Chem., Int. Ed., 2008, 47, 7497 CrossRef CAS.
- H. Li, Z. He, X. Goo, W. Li, X. Zhao and Z. Li, Org. Lett., 2009, 11, 4176 CrossRef CAS.
- W. Liu, J. Liu, D. Ogawa, Y. Nishihara, X. Guo and Z. Li, Org. Lett., 2011, 13, 6272 CrossRef CAS.
- E. Shirakawa, T. Yoneda, K. Moriya, K. Ota, N. Uchiyama, R. Nishikawa and T. Hayashi, Chem. Lett., 2011, 40, 1041 CrossRef CAS.
- T. Zeng, G. Song, A. Moores and C. J. Li, Synlett, 2010, 2002 CAS.
- R. Hudson, S. Ishikawa, C. J. Li and A. Moores, Synlett, 2013, 1637 CAS.
- H. Richter and O. G. Mancheno, Eur. J. Org. Chem., 2010, 4460 CAS.
- Y. Xie, M. Yu and Y. Zhang, Synthesis, 2011, 2803 CAS.
- Y. Wang, F. Chen, Y. Yuan, H. Xie and C. Huo, Org. Lett., 2015, 17, 5028 CrossRef.
- (a) D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338 CrossRef CAS; (b) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS.
- (a) J. Hassan, M. Sevingnon, C. Gozzi, E. Shulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS; (b) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS.
- (a) A. Klapars, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727 CrossRef CAS; (b) A. Klapars, J. C. Antila, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421 CrossRef CAS.
- Z. Wang, Y. Zhang, Y. Zhao, Y. Jiang and H. Fu, Org. Lett., 2008, 10, 1863 CrossRef CAS.
- S. Pan, J. Liu, H. Li, Z. Wang, X. Guo and Z. Li, Org. Lett., 2010, 12, 1932 CrossRef CAS.
- X. Mao, Y. Wu, X. Jiang, X. Liu, Y. Cheng and C. Zhu, RSC Adv., 2012, 2, 6733 RSC.
- M. Sun, T. Zhang and W. Bao, Tetrahedron Lett., 2014, 55, 893 CrossRef CAS.
- D. Chen, F. Pan, J. Gao and J. Yang, Synlett, 2013, 2085 CAS.
- L. Gu, W. Wang, Y. Xiong, X. Huang and G. Li, Eur. J. Org. Chem., 2014, 319 CrossRef CAS.
- S. S. Mudaliar, A. P. Patel, J. J. Patel and K. H. Chikhalia, Tetrahedron Lett., 2018, 59, 734–738 CrossRef CAS.
- S. Iwata, T. Hata and H. Urabe, Adv. Synth. Catal., 2012, 354, 3480 CrossRef CAS.
- Y. He, J. Mao, G. Rong, H. Yan and G. Zhang, Adv. Synth. Catal., 2015, 357, 2125 CrossRef CAS.
- T. Wang, W. Zhou, H. Yin, J. A. Ma and N. Jiao, Angew. Chem., Int. Ed., 2012, 51, 10823 CrossRef CAS.
- A. J. Pearson and Y. Kwak, Tetrahedron Lett., 2005, 46, 3407 CrossRef CAS.
- (a) Y. Shvo and E. Hazum, Chem. Commun., 1974, 336–337 RSC; (b) J. H. Eekhof, H. Hogeveen and R. M. Kellogg, Chem. Commun., 1976, 657 RSC; (c) J. Elzinga and H. Hogeveen, Chem. Commun., 1977, 705–706 RSC.
- E. Shirakawa, N. Uchiyama and T. Hayashi, J. Org. Chem., 2011, 76, 25 CrossRef CAS.
- Y. Wei, H. Ding, S. Lin and F. Liang, Org. Lett., 2011, 13, 1674 CrossRef CAS.
- L. W. Liu, Z. Z. Wang, H. H. Zhang, W. S. Zhang, J. Z. Wang and Y. Tang, Chem. Commun., 2015, 51, 9531 RSC.
- B. D. Barve, Y. C. Wu, M. El-Shazly, M. Korinek, Y. B. Cheng, J. J. Wang and F. R. Chang, Org. Lett., 2014, 16, 1912 CrossRef CAS.
- J. Zhao, H. Fang, W. Zhou, Y. Pan and J. Han, J. Org. Chem., 2014, 79, 3847 CrossRef CAS.
- W.-H. Wen, A.-N. Xie, H.-H. Wang, D.-X. Zhang, A. Ali, X. Ying and H.-Y. Liu, Tetrahedron, 2017, 73, 7169–7176 CrossRef CAS.
- Y. Z. Li, B. J. Li, X. Y. Lu, S. Lin and Z. J. Shi, Angew. Chem., Int. Ed., 2009, 48, 3817 CrossRef CAS.
- C.-X. Song, G.-X. Cai, T. R. Farrell, Z.-P. Jiang, H. Li, L.-B. Gan and Z. J. Shi, Chem. Commun., 2009, 6002–6004 RSC.
- H. R. Wu, H. Y. Huang, C. L. Ren, L. Liu, D. Wang and C. J. Li, Chem. – Eur. J., 2015, 21, 16744 CrossRef CAS.
- Y. Yamane, K. Yoshinaga, M. Sumimoto and T. Nishikata, ACS Catal., 2019, 9, 1757–1762 CrossRef CAS.
- X. Guo, S. Pan, J. Liu and Z. Li, J. Org. Chem., 2009, 74, 8848 CrossRef CAS.
- M. Ghobrial, K. Harhammer, M. D. Mihovilovic and M. Schnürch, Chem. Commun., 2010, 46, 8836–8838 RSC.
- X. Guo, S. Pan, J. Liu and Z. Li, J. Org. Chem., 2009, 74, 8848 CrossRef CAS.
- (a) C. Z. Li, private communication, 2010; (b) P. M. Chincholkar, A. S. Kale, V. K. Gumaste and A. R. A. S. Deshmukh, Tetrahedron, 2009, 65, 2605 CrossRef CAS; (c) C. J. Abraham, D. H. Paull, C. Dogo-Isonagie and T. Lectka, Synlett, 2009, 1651 CAS; (d) X. Guo, S. Pan, J. Liu and Z. Li, J. Org. Chem., 2009, 74, 8848 CrossRef CAS; (e) T. Lectka, Synlett, 2009, 1651 CrossRef.
- B. Niu, W. Zhao, Y. Ding, Z. Bian, C. U. Pittman, A. Zhou and H. Ge, J. Org. Chem., 2015, 80, 7251 CrossRef CAS.
- A. Correa, B. Fiser and B. E. Gómez, Chem. Commun., 2015, 51, 13365 RSC.
- M. Ohta, M. P. Quick, J. Yamaguchi, B. Wünsch and K. Itami, Chem. – Asian J., 2009, 4, 1416 CrossRef CAS.
- (a) O. Krull and B. Wnsch, Bioorg. Med. Chem., 2004, 12, 1439 CrossRef CAS; (b) U. Wirt, D. Schepmann and B. Wnsch, Eur. J. Org. Chem., 2007, 462 CrossRef CAS; (c) S. M. Husain, R. Frçhlich, D. Schepmann and B. Wnsch, J. Org. Chem., 2009, 74, 2788 CrossRef CAS; (d) T. Schläger, D. Schepmann, E.-U. Würthwein and B. Wünch, Bioorg. Med. Chem., 2008, 16(6), 2992–3001 CrossRef.
- H. Liu, L. Cao, J. Sun, J. S. Fossey and W. P. Deng, Chem. Commun., 2012, 48, 2674–2676 RSC.
- K. Li, G. Tan, J. Huang, F. Song and J. You, Angew. Chem., Int. Ed., 2013, 52, 12942 CrossRef CAS.
- M. O. Ratnikov, X. Xu and M. P. Doyle, J. Am. Chem. Soc., 2013, 135, 9475 CrossRef CAS.
- K. Qiao, D. Zhang, K. Zhang, X. Yuan, M.-W. Zheng, T.-F. Guo, Z. Fang, L. Wan and K. Guo, Org. Chem. Front., 2018, 5, 1129 RSC.
- Q. Xia, W. Chen and H. Qiu, J. Org. Chem., 2011, 76, 7577 CrossRef CAS.
- X. Liu, Y. Chen, K. Li, D. Wang and B. Chen, Chin. J. Chem., 2012, 30, 2285 CrossRef CAS.
- Y. Cheng, W. Dong, L. Wang, K. Parthasarathy and C. Bolm, Org. Lett., 2014, 16, 2000 CrossRef CAS.
- S. Pan, J. Liu, H. Li, Z. Wang, X. Guo and Z. Li, Org. Lett., 2010, 12, 1932 CrossRef CAS.
- K. Q. Zhu, L. Wang, Q. Chen and M. Y. He, Tetrahedron Lett., 2015, 56, 4943 CrossRef CAS.
- Q. Xia and W. Chen, J. Org. Chem., 2012, 77, 9366 CrossRef CAS.
- G. Saidulu, R. A. Kumar and K. R. Reddy, Tetrahedron Lett., 2015, 56, 4200 CrossRef CAS.
- (a) T. Zhang and W. Bao, J. Org. Chem., 2013, 78, 1317 CrossRef CAS; (b) T. Zhang and W. Bao, J. Org. Chem., 2013, 78, 1317 CrossRef CAS.
- K. Monir, A. K. Bagdi, M. Ghosh and A. Hajra, Org. Lett., 2014, 16, 4630 CrossRef CAS.
- X. Guo, R. Yu, H. Li and Z. Li, J. Am. Chem. Soc., 2009, 131, 17387 CrossRef CAS.
- U. A. Kshirsagar, R. Parnes, H. Goldshtein, R. Ofir, R. Zarivach and D. Pappo, Chem. – Eur. J., 2013, 19, 13575 CrossRef CAS.
- Y. Zheng, W. Song, Y. Zhu, B. Wei and L. Xuan, Tetrahedron, 2018, 74, 5950–5954 CrossRef CAS.
- C. M. R. Volla and P. Vogel, Org. Lett., 2009, 11, 1701 CrossRef CAS.
- P. Liu, Z. Wang, J. Lin and X. Hu, Eur. J. Org. Chem., 2012, 1583 CrossRef CAS.
- D. Xu, W. Wang, C. Miao, Q. Zhang, C. Xia and W. Sun, Green Chem., 2013, 15, 2975 RSC.
- J. Wang, C. Liu, J. Yuan and A. Lei, Chem. Commun., 2014, 50, 4736 RSC.
- K. L. Wang, M. Y. Lv, Q. M. Wang and R. Q. Huang, Tetrahedron, 2008, 64, 7504 CrossRef CAS.
- T. Niu and Y. Zhang, Tetrahedron Lett., 2010, 51, 6847 CrossRef CAS.
- C. M. Feng, Y. Z. Zhu, S. C. Zhang, Y. Zang and J. Y. Zheng, Org. Biomol. Chem., 2015, 13, 2566 RSC.
- M. Chandrasekharam, B. Chiranjeevi, K. S. V. Gupta and B. Sridhar, J. Org. Chem., 2011, 76, 10229 CrossRef CAS.
- B. Chiranjeevi, G. Koyyada, S. Prabusreenivasan, V. Kumar, P. Sujitha, C. G. Kumar, B. Sridhar, S. Shaik and M. Chandrasekharam, RSC Adv., 2013, 3, 16475 RSC.
- A. Libman, H. Shalit, Y. Vainer, S. Narute, S. Kozuch and D. Pappo, J. Am. Chem. Soc., 2015, 137, 11453 CrossRef CAS.
- Z. H. Guan, Z. Y. Yan, Z. H. Ren, X. Y. Liu and Y. M. Liang, Chem. Commun., 2010, 46, 2823 RSC.
- Y. Ma, D. Zhang, Z. Yan, M. Wang, C. Bian, X. Gao, E. E. Bunel and A. Lei, Org. Lett., 2015, 17, 2174 CrossRef CAS.
- S. Wertz, D. Leifert and A. Studer, Org. Lett., 2013, 15, 928 CrossRef CAS.
- X. H. Yang, W. T. Wei, H. B. Li, R. J. Song and J. H. Li, Chem. Commun., 2014, 50, 12867 RSC.
- S. Samanta, S. Mondal, S. Santra, G. Kibriya and A. Hazra, J. Org. Chem., 2016, 81, 10088–10093 CrossRef CAS.
- C. M. R. Volla and P. Vogel, Org. Lett., 2009, 11, 1701 CrossRef CAS.
- S. S. Patil, R. P. Jadhav, S. V. Patil and V. D. Bobade, Tetrahedron Lett., 2011, 52, 5617 CrossRef CAS.
- S. K. Xiang, B. Zhang, L. H. Zhang, Y. X. Cui and N. Jiao, Sci. China: Chem., 2012, 55, 50 CrossRef CAS.
- N. Panda and A. K. Jena, J. Org. Chem., 2012, 77, 9401 CrossRef CAS.
- P. Liu, Y. Li, H. Wang, Z. Wang and X. Hu, Tetrahedron Lett., 2012, 53, 6654 CrossRef CAS.
- K. Gopalaiah and S. N. Chandrudu, RSC Adv., 2015, 5, 5015 RSC.
- C. Dai, Z. Xu, F. Huang, Z. Yu and Y. F. Gao, J. Org. Chem., 2012, 77, 4414 CrossRef CAS.
- (a) W. Han and A. R. Ofial, Chem. Commun., 2009, 6023 RSC; (b) W. Han, P. Mayer and A. R. Ofial, Adv. Synth. Catal., 2010, 352, 1667 CrossRef CAS.
- T. Hatanaka, Y. Ohki and K. Tatsumi, Chem. – Asian J., 2010, 5, 1657 CrossRef CAS.
- D. Wei, B. Carboni, J.-B. Sortais and C. Darcela, Adv. Synth. Catal., 2018, 360, 3649–3654 CrossRef CAS.
- (a) R. Shang and L. Lu, Sci. China: Chem., 2011, 54, 1670–1687 CrossRef CAS; (b) L. J. Gooβen and K. Gooβen, Decarboxylative cross coupling, Topics in organometallic chemistry, 2012, vol. 44, pp. 121–141, https://link.springer.com/chapter/10.1007/3418_2012_44 Search PubMed.
- R. Trivedi and J. A. Tunge, Org. Lett., 2009, 11(24), 5650–5652 CrossRef CAS.
- H. P. Bi, W. W. Chen, Y. M. Liang and C. J. Li, Org. Lett., 2009, 11, 3246 CrossRef CAS.
- J. Zhao, H. Fang, J. Han and Y. Pan, Beilstein J. Org. Chem., 2013, 9, 1718–1723 CrossRef.
- J. Zhao, W. Zhou, J. Han, G. Li and Y. Pan, Tetrahedron Lett., 2013, 54(48), 6507–6510 CrossRef CAS.
- H. Yang, H. Yan, P. Sun, Y. Zhu, L. Lu, D. Liu, G. Rong and J. Mao, Green Chem., 2013, 15, 976 RSC.
- J. M. C. Cifuentes, B. X. Ferreira, P. M. Esteves and C. D. Buarque, Top. Catal., 2018, 61, 689–698 CrossRef CAS.
- Q. Jiang, J. Jia, B. Xu, A. Zhao and C.-C. Guo, J. Org. Chem., 2015, 80, 3586–3596 CrossRef CAS.
- (a) M. Kumar, Richa, S. Sharma, V. Bhatt and N. Kumar, Adv. Synth. Catal., 2015, 357, 2862–2868 CrossRef CAS; (b) H. Wang, X. Cao, F. Xiao, S. Liu and G. J. Deng, Org. Lett., 2013, 15, 4900–4903 CrossRef CAS.
- C. Jin, X. Zhang, B. Sun, Z. Yan and T. Xu, Synlett, 2019, 1585 CAS.
- I. Aviv and Z. Gross, Synlett, 2006, 951–953 CAS.
- L. K. Baumann, H. M. Mbuvi, G. Du and L. K. Woo, Organometallics, 2007, 26, 3995–4002 CrossRef CAS.
- C. Ma, D. Xing, C. Zhai, J. Che, S. Liu, J. Wang and W. Hu, Org. Lett., 2013, 15, 6140–6143 CrossRef CAS.
- S.-F. Zhu, Y. Cai, H.-X. Mao, J.-H. Xie and Q.-L. Zhou, Nat. Chem., 2010, 2, 546–550 CrossRef CAS.
- Y. Cai, S.-F. Zhu, G.-P. Wang and Q.-L. Zhoua, Adv. Synth. Catal., 2011, 353, 2939–2944 CrossRef CAS.
- P. Liu, E. L.-M. Wong, A. W.-H. Yuen and C.-M. Che, Org. Lett., 2008, 10, 3275–3278 CrossRef CAS.
- B. J. Stokes, C. V. Vogel, L. K. Urnezis, M. Pan and T. G. Driver, Org. Lett., 2008, 10, 2884–2887 Search PubMed.
- Y. Liu and C.-M. Che, Chem. – Eur. J., 2010, 16, 10494–10501 CrossRef CAS.
- Y. Liu, J. Wei and C.-M. Che, Chem. Commun., 2010, 46, 6926–6928 RSC.
- J. Bonnamour and C. Bolm, Org. Lett., 2011, 13, 2012–2014 CrossRef CAS.
- J. Li, C. Wua, Q. Zhanga and B. Yan, Dalton Trans., 2013, 42, 14369–14373 RSC.
- Q. Nguyen, T. Nguyen and T. G. Driver, J. Am. Chem. Soc., 2013, 135, 620–623 CrossRef CAS.
- Q. Chen, Z.-J. Xu, Y. Liu, C.-Y. Zhou and C.-M. Che, Synlett, 2011, 1174–1178 Search PubMed.
- T. M. U. Ton, C. Tejo, S. Tania, J. W. W. Chang and P. W. H. Chan, J. Org. Chem., 2011, 76, 4894–4904 CrossRef CAS.
- E. R. King, E. T. Hennessy and T. A. Betley, J. Am. Chem. Soc., 2011, 133, 4917–4923 CrossRef CAS.
- Y. Liu, X. Guan, E. L.-M. Wong, P. Liu, J.-S. Huang and C.-M. Che, J. Am. Chem. Soc., 2013, 135, 7194–7204 CrossRef CAS.
- E. T. Hennessy and T. A. Betley, Science, 2013, 340, 591–595 CrossRef CAS.
- H. Wang, Y. Li, Z. Wang, J. Lou, Y. Xiao, G. Qiu, X. Hu, H.-J. Altenbach and P. Liu, RSC Adv., 2014, 4, 25287–25290 RSC.
- H. Keipour and T. Ollevier, Org. Lett., 2017, 19, 5736–5739 CrossRef CAS.
- H. Gu, Z. Han, H. Xie and X. Lin, Org. Lett., 2018, 20, 6544–6549 CrossRef CAS.
- E. T. Hennessy, R. Y. Liu, D. A. Iovan, R. A. Duncana and T. A. Betley, Chem. Sci., 2014, 5, 1526–1532 RSC.
- D. A. Lovan, M. J. T. Wilding, Y. Baek, E. T. Hennessy and T. A. Betley, Angew. Chem., Int. Ed., 2017, 56, 15599 CrossRef.
- R. K. Zhang, K. Chen, X. Huang, L. Wohlschlager, H. Renata and F. H. Arnold, Nature, 2019, 565, 67–72 CrossRef CAS.
- J. Zhang, X. Huang, R. K. Zhang and F. H. Arnold, J. Am. Chem. Soc., 2019, 141, 9798–9802 CrossRef CAS.
- A. Z. Zhou, K. Chen and F. H. Arnold, ACS Catal., 2020, 10, 5393–5398 CrossRef CAS.
- O. F. Brandenberg, D. C. Miller, U. Markel, A. O. Chaib and F. H. Arnold, ACS Catal., 2019, 9, 8271–8275 CrossRef CAS.
- Y. Yang, I. Cho, X. Qi, P. Liu and F. H. Arnold, Nat. Chem., 2019, 11, 987–993 CrossRef CAS.
- Z.-J. Jia, S. Gao and F. H. Arnold, J. Am. Chem. Soc., 2020, 142, 10279–10283 CrossRef CAS.
- G. Cahiez and H. Avedissian, Synthesis, 1998, 1199 CrossRef CAS.
- D. Necas and M. Kotora, Chem. Listy, 2006, 100, 967 CAS.
- H.-J. Knölker, in Organometallics in Synthesis—Third Manual, ed. M. Schlosser, Wiley, Hoboken, NJ, 2013, p. 545 Search PubMed.
- E. B. Bauer, Curr. Org. Chem., 2008, 12, 1341 CrossRef CAS.
- M. Jegelka and B. Plietker, Top. Organomet. Chem., 2011, 33, 177 CrossRef CAS.
- H. Shinokubo and K. Oshima, Eur. J. Org. Chem., 2004, 2081 CrossRef CAS.
- M. Hocek and D. Hocková, Collect. Symp. Ser., 2005, 7, 213 CAS.
- M. Oestreich, Nachr. Chem., 2004, 52, 446 CrossRef CAS.
- A. Fürstner and R. Martin, Chem. Lett., 2005, 34, 624 CrossRef.
- A. Leitner, in Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, Weinheim, Germany, 2008, p. 147 Search PubMed.
- G. Cahiez and C. Duplais, in Chemistry of OrganomagnesiumCompounds, ed. Z. Rappoport and I. Marek, John Wiley & Sons Ltd, Chichester, UK, 2008, p. 595 Search PubMed.
- L. Ackermann and A. Althammer, Chem. Unserer Zeit, 2009, 43, 74 CrossRef CAS.
- B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef CAS.
- A. Rudolph and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 2656 CrossRef CAS.
- M. Nakamura and S. Ito, in Modern Arylation Methods, ed. L. Ackermann, Wiley-VCH, Weinheim, Germany, 2009, p. 155 Search PubMed.
- A. Fürstner, Angew. Chem., Int. Ed., 2009, 48, 1364 CrossRef.
- W. M. Czaplik, M. Mayer, J. Cvengros and A. J. von Wangelin, ChemSusChem, 2009, 2, 396 CrossRef CAS.
- E. Nakamura and N. Yoshikai, J. Org. Chem., 2010, 75, 6061 CrossRef CAS.
- T. Mesganaw and N. K. Garg, Org. Process Res. Dev., 2013, 17, 29 CrossRef CAS.
- R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS.
- W. M. Czaplik, M. Mayer, S. Grupe and A. J. von Wangelin, Pure Appl. Chem., 2010, 82, 1545 CAS.
- S. Asako, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2013, 135, 17755 CrossRef CAS.
- A. T. Lindhardt, T. M. Goegsig, D. Gauthier, D. Lupp, M. L. H. Mantel, K. M. Bjerglund and T. Skrydstrup, Isr. J. Chem., 2010, 50, 558 CrossRef CAS.
- L. Ilies and E. Nakamura, PATAI’S Chemistry of Functional Groups, John Wiley & Sons, Ltd, Chichester, UK, 2014, p. 539 Search PubMed.
- E. Nakamura, T. Hatakeyama, S. Ito, K. Ishizuka, L. Ilies and M. Nakamura, in Organic Reactions, ed. S. E. Denmark, John Wiley & Sons, Ltd, Chichester, UK, 2014, vol. 83, p. 1 Search PubMed.
- A. Guérinot and J. Cossy, Top. Curr. Chem. (Z), 2016, 374, 49 CrossRef.
- T. L. Mako and J. A. Byers, Inorg. Chem. Front., 2016, 3, 766 RSC.
- R. B. Bedford and P. B. Brenner, in The Development of Iron Catalysts for Cross-Coupling Reactions, ed. E. Bauer, Iron Catalysis II. Topics in Organometallic Chemistry, Springer, Cham, 2015, vol. 50 Search PubMed.
- O. M. Kuzminaa, A. K. Steiba, A. Moyeux, G. Cahiez and P. Knochel, Synthesis, 2015, 1696 Search PubMed.
- G. Cahiez and H. Avedissian, Synthesis, 1998, 1199 CrossRef CAS.
- W. Dohle, F. Kopp, G. Cahiez and P. Knochel, Synlett, 2001, 1901 CrossRef CAS.
- S. M. Dos, X. Franck, R. Hocquemiller, B. Figadère, J.-F. Peyrat, O. Provot, J.-D. Brionand and M. Alami, Synlett, 2004, 2697 Search PubMed.
- G. Cahiez, O. Gager, J. Buendia and C. Patinote, Chem. – Eur. J., 2012, 18, 5860 CrossRef CAS.
- B. A. F. Le Bailly, M. D. Greenhalgh and S. P. Thomas, Chem. Commun., 2012, 48, 1580 RSC.
- Y. Deng, X.-J. Wei, X. Wang, Y. Sun and T. Nöel, Chem. – Eur. J., 2019, 25, 14532 CrossRef CAS.
- G. Cahiez, G. Lefèvre, A. Moyeux, O. Guerret, E. Gayon, L. Guillonneau, N. Lefèvre, Q. Gu and E. Zhou, Org. Lett., 2019, 21, 2679 CrossRef CAS.
- G. A. Molander, B. J. Rahn, D. C. Shubert and S. E. Bonde, Tetrahedron Lett., 1983, 24, 5449 CrossRef CAS.
- W. M. Czaplik, M. Mayer and A. J. von Wangelin, ChemCatChem, 2011, 3, 135 CrossRef CAS.
- A. A. Camacho-Davila, Synth. Commun., 2008, 38, 3823 CrossRef CAS.
- N. Tewari, N. Maheshwari, R. Medhane, H. Nizar and M. Prasad, Org. Process Res. Dev., 2012, 16, 1566 CrossRef CAS.
- A. Hamze, J.-D. Brion and M. Alami, Org. Lett., 2012, 14, 2782 CrossRef CAS.
- T. Hatakeyama, Y. Yoshimoto, T. Gabriel and M. Nakamura, Org. Lett., 2008, 10, 5341 CrossRef CAS.
- G. Cahiez and S. Marquais, Tetrahedron Lett., 1996, 37, 1773 CrossRef CAS.
- A. Desaintjean, S. Belrhomari, L. Rousseau, G. Lefèvre and P. Knochel, Org. Lett., 2019, 21, 8684 CrossRef CAS.
- X.-L. Lu, M. Shannon, X.-S. Peng and H. N. C. Wong, Org. Lett., 2019, 21(8), 2546 CrossRef CAS.
- Z. Zhong, X.-S. Peng and H. N. C. Wong, Chin. Chem. Lett., 2019, 30, 1463 CrossRef CAS.
- B. Scheiper, M. Bonnekessel, H. Krause and A. Fürstner, Org. Chem., 2004, 69, 3943 CrossRef CAS.
- A. Fürstner, R. Martin, H. Krause, G. Seidel, R. Goddard and C. W. Lehmann, J. Am. Chem. Soc., 2008, 130, 8773 CrossRef.
- A. Fürstner and L. Turet, Angew. Chem., Int. Ed., 2005, 44, 3462 CrossRef.
- A. Fürstner, D. De Souza, L. Turet, M. D. B. Fenster, L. Parra-Rapado, C. Wirtz, R. Mynott and C. W. Lehmann, Chem. – Eur. J., 2007, 13, 115 CrossRef.
- M. P. Le, G. C. Tsui, J. C. C. Whitney and W. Tam, J. Org. Chem., 2008, 73, 7829 CrossRef.
- G. C. Tsui, M. P. Le, A. Allen and W. Tam, Synthesis, 2009, 609 CAS.
- H. Nishikado, H. Nakatsuji, K. Ueno, R. Nagase and Y. Tanabe, Synlett, 2010, 2087 CAS.
- G. Dunet and P. Knochel, Synlett, 2006, 407 CAS.
- G. Cahiez, O. Gager and V. Habiak, Synthesis, 2008, 2636 CrossRef CAS.
- G. Cahiez, V. Habiak and O. Gager, Org. Lett., 2008, 10, 2389 CrossRef CAS.
- U. S. Larsen, L. Martiny and M. Begtrup, Tetrahedron Lett., 2005, 46, 4261 CrossRef CAS.
- J. R. Scheerer, J. F. Lawrence, G. C. Wang and D. A. Evans, J. Am. Chem. Soc., 2007, 129, 8968 CrossRef CAS.
- A. Piontek, E. Bisz and M. Szostak, Angew. Chem., Int. Ed., 2018, 57, 11116 CrossRef CAS.
- B.-J. Li, L. Xu, Z.-H. Wu, B.-T. Guan, C.-L. Sun, B.-Q. Wang and Z.-J. Shi, J. Am. Chem. Soc., 2009, 131, 14656 CrossRef CAS.
- B.-J. Li, X.-S. Zhang and Z.-J. Shi, Org. Synth., 2014, 91, 83 CrossRef CAS.
- C.-L. Sun and A. Fürstner, Angew. Chem., Int. Ed., 2013, 52, 13071 CrossRef CAS.
- D. Gärtner, A. L. Stein, S. Grupe, J. Arp and A. J. von Wangelin, Angew. Chem., Int. Ed., 2015, 54, 10545 CrossRef.
- A. C. P. Rivera, R. Still and D. E. Frantz, Angew. Chem., Int. Ed., 2016, 55, 6689 CrossRef CAS.
- K. Itami, S. Higashi, M. Mineno and J.-I. Yoshida, Org. Lett., 2005, 7, 1219 CrossRef CAS.
- C. M. R. Vollaand and P. Vogel, Angew. Chem., Int. Ed., 2008, 47, 1305 CrossRef.
- C. C. Silveira, S. R. Mendes and L. Wolf, J. Braz. Chem. Soc., 2010, 21, 2138 CrossRef CAS.
- L. N. Pridgen, L. Snyder and J. Prol, J. Org. Chem., 1989, 54, 1523 CrossRef CAS.
- A. Furstner and A. Leitner, Angew. Chem., Int. Ed., 2002, 41, 609 CrossRef.
- A. Fürstner, A. Leitner, M. Méndez and H. Krause, J. Am. Chem. Soc., 2002, 124, 13856 CrossRef.
- A. Fürstner, A. Leitner and G. Seidel, Org. Synth., 2005, 81, 33 CrossRef.
- G. Seidel, D. Laurich and A. Fürstner, J. Org. Chem., 2004, 69, 3950 CrossRef CAS.
- X. Feng, Y. Mei, Y. Luo and W. Lu, Monatsh. Chem., 2012, 143, 161 CrossRef CAS.
- B. Scheiper, F. Glorius, A. Leitner and A. Fürstner, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11960 CrossRef CAS.
- A. Fürstner and A. Leitner, Angew. Chem., Int. Ed., 2003, 42, 308 CrossRef.
- M. Hocek, D. Hocková and H. Dvořáková, Synthesis, 2004, 889 CrossRef CAS.
- M. Hocek and R. Pohl, Synthesis, 2004, 2869 CrossRef CAS.
- T. Kubelka, L. Slavetinska, B. Klepetarova and M. Hocek, Eur. J. Org. Chem., 2010, 2666 CrossRef CAS.
- M. Hocek, R. Pohl and I. Císařová, Eur. J. Org. Chem., 2005, 3026 CrossRef CAS.
- S. Malhotra, P. S. Seng, S. G. Koenig, A. J. Deese and K. A. Ford, Org. Lett., 2013, 15, 3698 CrossRef CAS.
- E. Colacino, H. Benakki, F. Guenoun, J. Martinez and F. Lamaty, Synth. Commun., 2009, 39, 1583 CrossRef CAS.
- T. M. Gøgsig, A. T. Lindhardt and T. Skrydstrup, Org. Lett., 2009, 11, 4886 CrossRef.
- K. Weber, E.-M. Schnöckelborg and R. Wolf, ChemCatChem, 2011, 3, 1572 CrossRef CAS.
- J. Haner, K. Jack, J. Nagireddy, R. M. Abdul, R. Durham and W. Tam, Synthesis, 2011, 731 CAS.
- M. C. Perry, A. N. Gillett and T. C. Law, Tetrahedron Lett., 2012, 53, 4436 CrossRef CAS.
- M. Mattarella and J. S. Siegel, Org. Biomol. Chem., 2012, 10, 5799 RSC.
- S. Gülak, T. N. Gieshoff and A. J. von Wangelin, Adv. Synth. Catal., 2013, 355, 2197 CrossRef.
- P. J. Rushworth, D. G. Hulcoop and D. J. Fox, J. Org. Chem., 2013, 78, 9517 CrossRef CAS.
- A. L. Silberstein, S. D. Ramgren and N. K. Garg, Org. Lett., 2012, 14, 3796 CrossRef CAS.
- T. Agrawal and S. P. Cook, Org. Lett., 2013, 15, 96 CrossRef CAS.
- W.-J. Guo and Z.-X. Wang, Tetrahedron, 2013, 69, 9580 CrossRef CAS.
- F. Bartoccini, G. Piersanti, S. Armaroli, A. Cerri and W. Cabri, Tetrahedron Lett., 2014, 55, 1376 CrossRef CAS.
- X. Chen, Z.-J. Quan and X.-C. Wang, Appl. Organomet. Chem., 2015, 29, 296 CrossRef CAS.
- A. Piontek and M. Szostak, Eur. J. Org. Chem., 2017, 7271 CrossRef CAS.
- E. Biszand and M. Szostak, J. Org. Chem., 2019, 84, 1640 CrossRef.
- E. Bisz and M. Szostak, ChemSusChem, 2018, 11, 1290 CrossRef CAS.
- E. Bisz, P. Podchorodecka and M. Szostak, ChemCatChem, 2019, 11, 1196 CrossRef CAS.
- E. Bisz, M. Kardela and M. Szostak, ChemCatChem, 2019, 11, 5733 CrossRef CAS.
- E. Bisza and M. Szostak, Adv. Synth. Catal., 2019, 361, 85 CrossRef.
- E. Bisza, M. Kardela, A. Piontek and M. Szostak, Catal. Sci. Technol., 2019, 9, 1092 RSC.
- M. S. Kharasch, W. Nudenberg and S. Archer, J. Am. Chem. Soc., 1943, 65, 495 CrossRef CAS.
- A. Fürstner, H. Krause and C. W. Lehmann, Angew. Chem., Int. Ed., 2006, 45, 440 CrossRef.
- G. Cahiez, C. Chaboche, F. Mahuteau-Betzer and M. Ahr, Org. Lett., 2005, 7, 1943 CrossRef CAS.
- J. Quintin, X. Franck, R. Hocquemiller and B. Figadère, Tetrahedron Lett., 2002, 43, 3547 CrossRef CAS.
- L. Boully, M. Darabantu, A. Turck and N. Plé, J. Heterocycl. Chem., 2005, 42, 1423 CrossRef CAS.
- T. Hatakeyama and M. Nakamura, J. Am. Chem. Soc., 2007, 129, 9844 CrossRef CAS.
- T. Hatakeyama, S. Hashimoto, K. Ishizuka and M. Nakamura, J. Am. Chem. Soc., 2009, 131, 11949 CrossRef CAS.
- S. Güak and A. J. von Wangelin, Angew. Chem., Int. Ed., 2012, 51, 1357 CrossRef.
- O. M. Kuzmina, A. K. Steib, D. Flubacher and P. Knochel, Org. Lett., 2012, 14, 4818 CrossRef CAS.
- O. M. Kuzmina, A. K. Steib, J. T. Markiewicz, D. Flubacher and P. Knochel, Angew. Chem., Int. Ed., 2013, 52, 4945 CrossRef CAS.
- M. J. Rahman, R. Pervin, R. Hasan and D. Debnath, J. Sci. Res., 2013, 5, 127 CrossRef CAS.
- Y.-Y. Chua and H. A. Duong, Chem. Commun., 2014, 50, 8424 RSC.
- Y.-Y. Chua and H. A. Duong, Chem. Commun., 2016, 52, 1466 RSC.
- W. Wu, Q. Teng, Y.-Y. Chua, H. V. Huynh and H. A. Duong, Organometallics, 2017, 36, 2293 CrossRef CAS.
- Q. Teng, W. Wu, H. A. Duong and H. V. Huynh, Chem. Commun., 2018, 54, 6044 RSC.
- R. Zhang, Y. Zhao, K.-M. Liu and X.-F. Duan, Org. Lett., 2018, 20, 7942 CrossRef CAS.
- R. L.-Y. Bao, R. Zhao and L. Shi, Chem. Commun., 2015, 51, 6884 RSC.
- L. Wang, Y.-M. Wei, Y. Zhao and X.-F. Duan, J. Org. Chem., 2019, 84, 5176 CrossRef CAS.
- I. Sapountzis, W. Lin, C. C. Kofink, C. Despotopoulou and P. Knochel, Angew. Chem., Int. Ed., 2005, 44, 1654 CrossRef CAS.
- C. C. Kofink, B. Blank, S. Pagano, N. Götz and P. Knochel, Chem. Commun., 2007, 1954 RSC.
- Y. Guo, D. J. Young and T. S. A. Hor, Tetrahedron Lett., 2008, 49, 5620 CrossRef CAS.
- R. B. Bedford, M. A. Hall, G. R. Hodges, M. Huwe and M. C. Wilkinson, Chem. Commun., 2009, 6430 RSC.
- Z. Jia, Q. Liu, X.-S. Peng and H. N. C. Wong, Nat. Commun., 2016, 7, 10614 CrossRef.
- A. D. Benischke, A. J. A. Breuillac, A. Moyeux, G. Cahiez and P. Knochel, Synlett, 2016, 471 CAS.
- T.-T. Hung, C.-M. Huang and F.-Y. Tsai, ChemCatChem, 2012, 4, 540 CrossRef CAS.
- W. C. Percival, R. B. Wagner and N. C. Cook, J. Am. Chem. Soc., 1953, 75, 3731 CrossRef CAS.
- C. Cardellicchio, V. Fiandanese, G. Marchese and L. Ronzini, Tetrahedron Lett., 1987, 28, 2053 CrossRef CAS.
- F. Babudri, A. D’Ettole, V. Fiandanese, G. Marchese and F. Naso, J. Organomet. Chem., 1991, 405, 53 CrossRef CAS.
- V. Fiandanese, G. Marchese, V. Martina and L. Ronzini, Tetrahedron Lett., 1984, 25, 4805 CrossRef CAS.
- M. M. Dell’Anna, P. Mastrorilli, C. F. Nobile, G. Marchese and M. R. Taurino, J. Mol. Catal. A: Chem., 2000, 161, 239 CrossRef.
- C. Duplais, F. Bures, I. Sapountzis, T. J. Korn, G. Cahiez and P. Knochel, Angew. Chem., Int. Ed., 2004, 43, 2968 CrossRef CAS.
- H. H. Choi, Y. H. Son, M. S. Jung and E. J. Kang, Tetrahedron Lett., 2011, 52, 2312 CrossRef CAS.
- M. Nakamura, K. Matsuo, S. Ito and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 3686 CrossRef CAS.
- T. Nagano and T. Hayashi, Org. Lett., 2004, 6, 1297 CrossRef CAS.
- R. Martin and A. Fürstner, Angew. Chem., Int. Ed., 2004, 43, 3955 CrossRef CAS.
- R. B. Bedford, D. W. Bruce, R. M. Frost, J. W. Goodby and M. Hird, Chem. Commun., 2004, 2822 RSC.
- R. B. Bedford, D. W. Bruce, R. M. Frost and M. Hird, Chem. Commun., 2005, 4161 RSC.
- R. B. Bedford, M. Betham, D. W. Bruce and A. A. Danopoulos, J. Org. Chem., 2006, 71, 1104 CrossRef CAS.
- R. B. Bedford, M. Betham, D. W. Bruce, S. A. Davis, R. M. Frost and M. Hird, Chem. Commun., 2006, 1398 RSC.
- T. Hatakeyama, Y.-i. Fujiwara, Y. Okada, T. Itoh, T. Hashimoto, S. Kawamura, K. Ogata, H. Takaya and M. Nakamura, Chem. Lett., 2011, 40, 1030 CrossRef CAS.
- Y. Yamaguchi, H. Ando, M. Nagaya, H. Hinago, T. Ito and M. Asami, Chem. Lett., 2011, 40, 983 CrossRef CAS.
- M. Jinand and M. Nakamura, Chem. Lett., 2011, 40, 1012 CrossRef.
- M. Jin, L. Adak and M. Nakamura, J. Am. Chem. Soc., 2015, 137, 7128 CrossRef CAS.
- K. Bica and P. Gaertner, Org. Lett., 2006, 8, 733 CrossRef CAS.
- G. Cahiez, V. Habiak, C. Duplais and A. Moyeux, Angew. Chem., Int. Ed., 2007, 46, 4364 CrossRef CAS.
- Z. Q. Dai, K. Q. Liu, Z. Y. Zhang, B. M. Wei and J. T. Guan, Asian J. Chem., 2013, 25, 6303 CrossRef CAS.
- R. R. Chowdhury, A. K. Crane, C. Fowler, P. Kwong and C. M. Kozak, Chem. Commun., 2008, 94 RSC.
- X. Qian and C. M. Kozak, Synlett, 2011, 852 CAS.
- A. K. Steib, T. Thaler, K. Komeyama, P. Mayer and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 3303 CrossRef CAS.
- H.-h. Gao, C.-h. Yan, X.-P. Tao, Y. Xia, H.-M. Sun, Q. Shen and Y. Zhang, Organometallics, 2010, 29, 4189 CrossRef CAS.
- S. Meyer, C. M. Orben, S. Demeshko, S. Dechert and F. Meyer, Organometallics, 2011, 30, 6692 CrossRef CAS.
- S. K. Ghorai, M. Jin, T. Hatakeyama and M. Nakamura, Org. Lett., 2012, 14, 1066 CrossRef CAS.
- Z. Mo, Q. Zhang and L. Deng, Organometallics, 2012, 31, 6518 CrossRef CAS.
- Y. Xia, C. H. Yan, Z. Li, H. H. Gao, H. M. Sun, Q. Shen and Y. Zhang, Chin. Sci. Bull., 2013, 58, 493 CrossRef CAS.
- S. E. Denmark and A. J. Cresswell, J. Org. Chem., 2013, 78, 12593 CrossRef CAS.
- C.-L. Xia, C.-F. Xie, Y.-F. Wu, H.-M. Sun, Q. Shen and Y. Zhang, Org. Biomol. Chem., 2013, 11, 8135 RSC.
- C.-L. Sun, H. Krause and A. Fürstner, Adv. Synth. Catal., 2014, 356, 1281 CrossRef CAS.
- L. An, Y.-L. Xiao, S. Zhang and X. Zhang, Angew. Chem., Int. Ed., 2018, 57, 6921 CrossRef CAS.
- M. Nakamura, S. Ito, K. Matsuo and E. Nakamura, Synlett, 2005, 1794 CrossRef CAS.
- T. Hatakeyama, Y. Kondo, Y.-i. Fujiwara, H. Takaya, S. Ito, E. Nakamura and M. Nakamura, Chem. Commun., 2009, 1216 RSC.
- S. Ito, Y.-i. Fujiwara, E. Nakamura and M. Nakamura, Org. Lett., 2009, 11, 4306 CrossRef CAS.
- X. Lin, F. Zheng and F.-L. Qing, Organometallics, 2012, 31, 1578 CrossRef CAS.
- T. Hatakeyama, T. Hashimoto, Y. Kondo, Y. Fujiwara, H. Seike, H. Takaya, Y. Tamada, T. Ono and M. Nakamura, J. Am. Chem. Soc., 2010, 132, 10674 CrossRef CAS.
- R. B. Bedford, P. B. Brenner, E. Carter, T. W. Carvell, P. M. Cogswell, T. Gallagher, J. N. Harvey, D. M. Murphy, E. C. Neeve, J. Nunn and D. R. Pye, Chem. – Eur. J., 2014, 20, 7935 CrossRef CAS.
- S. Kawamura, K. Ishizuka, H. Takaya and M. Nakamura, Chem. Commun., 2010, 46, 6054 RSC.
- S. Kawamura, T. Kawabata, K. Ishizuka and M. Nakamura, Chem. Commun., 2012, 48, 9376 RSC.
- R. Agata, S. Kawamura, K. Isozaki and M. Nakamura, Chem. Lett., 2019, 48, 238 CrossRef CAS.
- M. S. Hofmayera, J. M. Hammanna, G. Cahiezb and P. Knochel, Synlett, 2018, 65 Search PubMed.
- G. Cahiez, C. Duplais and A. Moyeux, Org. Lett., 2007, 9, 3253 CrossRef CAS.
- A. Guérinot, S. Reymond and J. Cossy, Angew. Chem., Int. Ed., 2007, 46, 6521 CrossRef.
- A. Guerinot, G. Lepesqueux, S. Sable, S. Reymond and J. Cossy, J. Org. Chem., 2010, 75, 5151 CrossRef CAS.
- C. Gregg, C. Gunawan, A. W. Y. Ng, S. Wimala, S. Wickremasinghe and M. A. Rizzacasa, Org. Lett., 2013, 15, 516 CrossRef CAS.
- K.-i. Yamada, T. Sato, M. Hosoi, Y. Yamamoto and K. Tomioka, Chem. Pharm. Bull., 2010, 58, 1511 CrossRef CAS.
- U. K. Neelam, S. Gangula, V. P. Reddy and R. Bandichhor, Chem. Biol. Interface, 2013, 3, 14 CAS.
- C. Bensoussan, N. Rival, G. Hanquet, F. Colobert, S. Reymond and J. Cossy, Tetrahedron, 2013, 69, 7759 CrossRef CAS.
- T. Hatakeyama, N. Nakagawa and M. Nakamura, Org. Lett., 2009, 11, 4496 CrossRef CAS.
- T. Hashimoto, T. Hatakeyama and M. Nakamura, J. Org. Chem., 2012, 77, 1168 CrossRef CAS.
- A. T. Parsons, T. D. Senecal and S. L. Buchwald, Angew. Chem., Int. Ed., 2012, 51, 2947 CrossRef CAS.
- T. Hatakeyama, Y. Okada, Y. Yoshimoto and M. Nakamura, Angew. Chem., Int. Ed., 2011, 50, 10973 CrossRef CAS.
- C. W. Cheung, P. Ren and X. Hu, Org. Lett., 2014, 16, 2566 CrossRef CAS.
- K. G. Dongol, H. Koh, M. Sau and C. L. L. Chai, Adv. Synth. Catal., 2007, 349, 1015 CrossRef CAS.
- Y. Gartia, S. Pulla, P. Ramidi, C. C. Farris, Z. Nima, D. E. Jones, A. S. Biris and A. Ghosh, Catal. Lett., 2012, 142, 1397 CrossRef CAS.
- T. Hatakeyama, T. Hashimoto, K. K. A. D. S. Kathriarachchi, T. Zenmyo, H. Seike and M. Nakamura, Angew. Chem., Int. Ed., 2012, 51, 8834 CrossRef CAS.
- M. Guisán-Ceinos, F. Tato, E. Buñuel, P. Calle and D. Cárdenas, J. Chem. Sci., 2013, 4, 1098 Search PubMed.
- S. Luo, D.-G. Yu, R.-Y. Zhu, X. Wang, L. Wang and Z.-J. Shi, Chem. Commun., 2013, 49, 7794 RSC.
- D. J. Tindall, H. Krause and A. Fürstner, Adv. Synth. Catal., 2016, 358, 2398 CrossRef CAS.
- D. J. Pasto, S.-K. Chou, A. Waterhouse, R. H. Shults and G. F. Hennion, J. Org. Chem., 1978, 43, 1385 CrossRef CAS.
- D. J. Pasto, G. F. Hennion, R. H. Shults, A. Waterhouse and S.-K. Chou, J. Org. Chem., 1976, 41, 3496 CrossRef CAS.
- A. Yanagisawa, N. Nomura and H. Yamamoto, Tetrahedron, 1994, 50, 6017 CrossRef CAS.
- A. Yanagisawa, N. Nomura and H. Yamamoto, Synlett, 1991, 513 CrossRef CAS.
- A. S. K. Hashmi and G. Szeimies, Chem. Ber., 1994, 127, 1075 CrossRef.
- C. M. R. Volla, D. Marković, S. R. Dubbaka and P. Vogel, Eur. J. Org. Chem., 2009, 6281 CrossRef CAS.
- R. B. Bedford, M. Huwe and M. C. Wilkinson, Chem. Commun., 2009, 600 RSC.
- M. Mayer, W. M. Czaplik and A. J. von Wangelin, Adv. Synth. Catal., 2010, 352, 2147 CrossRef CAS.
- R. B. Bedford, E. Carter, P. M. Cogswell, N. J. Gower, M. F. Haddow, J. N. Harvey, D. M. Murphy, E. C. Neeve and J. Nunn, Angew. Chem., Int. Ed., 2013, 52, 1285 CrossRef CAS.
- S. Kawamura and M. Nakamura, Chem. Lett., 2013, 42, 183 CrossRef CAS.
- E. F. Chard, L. N. Dawe and C. M. Kozak, J. Organomet. Chem., 2013, 737, 32 CrossRef CAS.
- X. Zhang, Y. Qiu, C. Fu and S. Ma, Org. Chem. Front., 2014, 1, 247 RSC.
- D. Gärtner, H. Konnerth and J. A. von Wangelin, Catal. Sci. Technol., 2013, 3, 2541 RSC.
- S. N. Kessler, F. Hundemer and J.-E. Bäckvall, ACS Catal., 2016, 6, 7448 CrossRef CAS.
- A. Petuker, M. EI-Tokhey, M. L. Reback, B. Mallick and U.-P. Apfel, ChemistrySelect, 2016, 1, 2717 CrossRef CAS.
- T. Iwasaki, R. Akimoto, H. Kuniyasu and N. Kambe, Chem. – Asian J., 2016, 11, 2834 CrossRef CAS.
- L. Adak, S. Kawamura, G. Toma, T. Takenaka, K. Isozaki, H. Takaya, A. Orita, H. C. Li, T. K. M. Shing and M. Nakamura, J. Am. Chem. Soc., 2017, 139, 10693 CrossRef CAS.
- X. Zhao, M. Wu, Y. Liu and S. Cao, Org. Lett., 2018, 20, 5564 CrossRef CAS.
- J. K. Kochi, J. Organomet. Chem., 2002, 653, 11 CrossRef CAS.
- R. S. Smith and J. K. Kochi, J. Org. Chem., 1976, 41, 502 CrossRef CAS.
- M. H. Al-Afyouni, K. L. Fillman, W. W. Brennessel and M. L. Neidig, J. Am. Chem. Soc., 2014, 136, 15457 CrossRef CAS.
- S. B. Muñoz, S. L. Daifuku, W. W. Brennessel and M. L. Neidig, J. Am. Chem. Soc., 2016, 138, 7492 CrossRef.
- T. Parchomyk and K. Koszinowski, Chem. – Eur. J., 2017, 23, 3213 CrossRef CAS.
- S. B. Muñoz, S. L. Daifuku, J. D. Sears, T. M. Baker, S. H. Carpenter, W. W. Brennessel and M. L. Neidig, Angew. Chem., Int. Ed., 2018, 57, 6496 CrossRef.
- J. D. Sears, S. B. Muñoz, S. L. Daifuku, A. A. Shaps, S. H. Carpenter, W. W. Brennessel and M. L. Neidig, Angew. Chem., Int. Ed., 2019, 58, 2769 CrossRef CAS.
- B. Bogdanović and M. Schwickardi, Angew. Chem., Int. Ed., 2000, 39, 4610 CrossRef.
- L. E. Aleandri, B. Bogdanović, P. Bons, C. Duerr, A. Gaidies, T. Hartwig, S. C. Huckett, M. Lagarden, U. Wilczok and R. A. Brand, Chem. Mater., 1995, 7, 1153 CrossRef CAS.
- A. Fürstner, H. Krause and C. W. Lehmann, Angew. Chem., Int. Ed., 2006, 45, 440 CrossRef.
- T. Parchomyk and K. Koszinowski, Chem. – Eur. J., 2016, 22, 15609 CrossRef CAS.
- T. Parchomyk, S. Demeshko, F. Meyerand and K. Koszinowski, J. Am. Chem. Soc., 2018, 140, 9709 CrossRef CAS.
- J. Kleimark, A. Hedström, P.-F. Larsson, C. Johansson and P.-O. Norrby, ChemCatChem, 2009, 1, 152 CrossRef CAS.
- A. Hedström, U. Bollmann, J. Bravidor and P.-O. Norrby, Chem. – Eur. J., 2011, 17, 11991 CrossRef.
- J. Kleimark, P.-F. Larsson, P. Emamy, A. Hedström and P.-O. Norrby, Adv. Synth. Catal., 2012, 354, 448 CrossRef CAS.
- A. Hedström, E. Lindstedt and P.-O. Norrby, J. Organomet. Chem., 2013, 748, 51 CrossRef.
- Q. Ren, S. Guan, F. Jiang and J. Fang, J. Phys. Chem. A, 2013, 117, 756 CrossRef CAS.
- S. H. Carpenter, T. M. Baker, S. B. Muñoz, W. W. Brennessel and M. L. Neidig, Chem. Sci., 2018, 9, 7931 RSC.
- C. J. Adams, R. B. Bedford, E. Carter, N. J. Gower, M. F. Haddow, J. N. Harvey, M. Huwe, M. A. Cartes, S. M. Mansell, C. Mendoza, D. M. Murphy, E. C. Neeve and J. Nunn, J. Am. Chem. Soc., 2012, 134, 10333 CrossRef CAS.
- A. M. Messinis, S. L. J. Luckham, P. P. Wells, D. Gianolio, E. K. Gibson, H. M. O’Brien, H. A. Sparkes, S. A. Davis, J. Callison, D. Elorriaga, O. Hernandez-Fajardo and R. B. Bedford, Nat. Catal., 2019, 2, 123 CrossRef CAS.
- R. Schoch, W. Desens, T. Werner and M. Bauer, Chem. – Eur. J., 2013, 19, 15816 CrossRef CAS.
- R. B. Bedford, P. B. Brenner, E. Carter, P. M. Cogswell, M. F. Haddow, J. N. Harvey, D. M. Murphy, J. Nunn and C. H. Woodall, Angew. Chem., Int. Ed., 2014, 53, 1804 CrossRef CAS.
- G. Lefèvre and A. Jutand, Chem. – Eur. J., 2014, 20, 4796 CrossRef.
- R. B. Bedford, M. Betham, D. W. Bruce, A. A. Danopoulos, R. M. Frost and M. Hird, J. Org. Chem., 2006, 71, 1104 CrossRef CAS.
- D. Noda, Y. Sunada, T. Hatakeyama, M. Nakamura and H. Nagashima, J. Am. Chem. Soc., 2009, 131, 6078 CrossRef CAS.
- S. L. Daifuku, M. H. Al-Afyouni, B. E. R. Snyder, J. L. Kneebone and M. L. Neidig, J. Am. Chem. Soc., 2014, 136, 9132 CrossRef CAS.
- J. L. Kneebone, V. E. Fleischauer, S. L. Daifuku, A. A. Shaps, J. M. Bailey, T. E. Iannuzzi and M. L. Neidig, Inorg. Chem., 2016, 55, 272 CrossRef CAS.
- S. L. Daifuku, J. L. Kneebone, B. E. R. Snyder and M. L. Neidig, J. Am. Chem. Soc., 2015, 137, 11432 CrossRef CAS.
- J. L. Kneebone, W. W. Brennessel and M. L. Neidig, J. Am. Chem. Soc., 2017, 139, 6988 CrossRef CAS.
- M. Jin, L. Adak and M. Nakamura, J. Am. Chem. Soc., 2015, 137, 7128 CrossRef CAS.
- A. K. Sharma, W. M. C. Sameera, M. Jin, L. Adak, C. Okuzono, T. Iwamoto, M. Kato, M. Nakamura and K. Morokuma, J. Am. Chem. Soc., 2017, 139, 16117 CrossRef CAS.
- W. Lee, J. Zhou and O. Gutierrez, J. Am. Chem. Soc., 2017, 139, 16126 CrossRef CAS.
- L. Adak, S. Kawamura, G. Toma, T. Takenaka, K. Isozaki, H. Takaya, A. Orita, H. C. Li, T. K. M. Shingand and M. Nakamura, J. Am. Chem. Soc., 2017, 139, 10693 CrossRef CAS.
- Y. Liu, J. Xiao, L. Wang, Y. Song and L. Deng, Organometallics, 2015, 34, 599 CrossRef.
- J. A. Przyojski, K. P. Veggeberg, H. D. Arman and Z. J. Tonzetich, ACS Catal., 2015, 5, 5938 CrossRef CAS.
- V. E. Fleischauer, S. B. Muñoz, P. G. N. Neate, W. W. Brennessel and M. L. Neidig, Chem. Sci., 2018, 9, 1878 RSC.
- G. Bauer, M. D. Wodrich, R. Scopelliti and X. Hu, Organometallics, 2015, 34, 289 CrossRef CAS.
- A. Hedstrçm, Z. Izakian, I. Vreto, C.-J. Wallentin and P.-O. Norrby, Chem. – Eur. J., 2015, 21, 5946 CrossRef.
- A. Bekhradnia and P.-O. Norrby, Dalton Trans., 2015, 44, 3959–3962 RSC.
- E. N. Frankel, E. A. Emken, H. M. Peters, V. L. Davison and R. O. Butterfield, J. Org. Chem., 1964, 29, 3292–3297 CrossRef CAS.
- E. N. Frankel, E. A. Emken and V. L. Davison, J. Org. Chem., 1965, 30, 2739–2745 CrossRef CAS.
- S. C. Bart, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2004, 126, 13794–13807 CrossRef CAS.
- S. C. Bart, E. J. Hawrelak, E. Lobkovsky and P. J. Chirik, Organometallics, 2005, 24, 5518–5527 CrossRef CAS.
- A. M. Archer, M. W. Bouwkamp, M.-P. Cortez, E. Lobkovsky and P. J. Chirik, Organometallics, 2006, 25, 4269–4278 CrossRef CAS.
- R. J. Trovitch, E. Lobkovsky and P. J. Chirik, Inorg. Chem., 2006, 45, 7252–7260 CrossRef CAS.
- R. J. Trovitch, E. Lobkovsky, E. Bill and P. J. Chirik, Organometallics, 2008, 27, 1470–1478 CrossRef CAS.
- S. K. Russell, J. M. Darmon, E. Lobkovsky and P. J. Chirik, Inorg. Chem., 2010, 49, 2782–2792 CrossRef CAS.
- S. K. Russell, C. Milsmann, E. Lobkovsky, T. Weyhermuller and P. J. Chirik, Inorg. Chem., 2011, 50, 3159–3169 CrossRef CAS.
- M. Villa, D. Miesel, A. Hildebrandt, F. Ragaini, D. Schaarschmidt and A. J. von Wangelin, ChemCatChem, 2007, 9, 3203–3209 CrossRef.
- P.-H. Phua, L. Lefort, J. A. F. Boogers, M. Tristany and J. G. de Vries, Chem. Commun., 2009, 3747–3749 RSC.
- C. Rangheard, C. de J. Fernandez, P.-H. Phua, J. Hoorn, L. Lefort and J. G. de Vries, Dalton Trans., 2010, 39, 8464–8471 RSC.
- M. Stein, J. Wieland, P. Steurer, F. Tolle, R. Mulhaupt and B. Breit, Adv. Synth. Catal., 2011, 353, 523–527 CrossRef CAS.
- A. Welther, M. Bauer, M. Mayer and A. J. von Wangelin, ChemCatChem, 2012, 4, 1088–1093 CrossRef CAS.
- D. J. Frank, L. Guiet, A. Kaslin, E. Murphy and S. P. Thomas, RSC Adv., 2013, 3, 25698–25701 RSC.
- V. Kelsen, B. Wendt, S. Werkmeister, K. Junge, M. Beller and B. Chaudret, Chem. Commun., 2013, 49, 3416–3418 RSC.
- G. Wienhofer, F. A. Westerhaus, R. V. Jagadeesh, K. Junge, H. Junge and M. Beller, Chem. Commun., 2012, 48, 4827–4829 RSC.
- H. Fong, M.-E. Moret, Y. Lee and J. C. Peters, Organometallics, 2013, 32, 3053–3062 CrossRef CAS.
- D. Srimani, Y. Diskin-Posner, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2013, 52, 14131–14134 CrossRef CAS.
- J. C. Ott, C. K. Blasius, H. Wadepohl and L. H. Gade, Inorg. Chem., 2018, 57, 3183–3191 CrossRef CAS.
- M. Kamitani, Y. Nishiguchi, R. Tada, M. Itazaki and H. Nakazawa, Organometallics, 2014, 33, 1532–1535 CrossRef CAS.
- J. M. Hoyt, M. l. Shevlin, G. W. Margulieux, S. W. Krska, M. T. Tudge and P. J. Chirik, Organometallics, 2014, 33, 5781–5790 CrossRef CAS.
- D. Gartner, A. Welther, B. R. Rad, R. Wolf and A. J. von Wangelin, Angew. Chem., Int. Ed., 2014, 53, 3722–3726 CrossRef.
- P. Büschelberger, D. Gärtner, E. Reyes-Rodriguez, F. Kreyenschmidt, K. Koszinowski, A. J. von Wangelin and R. Wolf, Chem. – Eur. J., 2017, 23, 3139–3151 CrossRef.
- T. N. Gieshoff, U. Chakraborty, M. Villa and A. J. von Wangelin, Angew. Chem., Int. Ed., 2017, 56, 3585–3589 CrossRef CAS.
- Y. Sunada, H. Ogushi, T. Yamamoto, S. Uto, M. Sawano, A. Tahara, H. Tanaka, Y. Shiota, K. Yoshizawa and H. Nagashima, J. Am. Chem. Soc., 2018, 140, 4119–4134 CrossRef CAS.
- L. Markó, M. A. Radhi and I. Ötvös, J. Organomet. Chem., 1981, 281, 369–376 CrossRef.
- L. Markó and J. Palágyi, Transition Met. Chem., 1983, 8, 207–209 CrossRef.
- S. Enthaler, B. Hagemann, G. Erre, K. Junge and M. Beller, Chem. – Asian J., 2006, 1, 598–604 CrossRef CAS.
- S. Enthaler, G. Erre, M. K. Tse, K. Junge and M. Beller, Tetrahedron Lett., 2006, 47, 8095–8099 CrossRef CAS.
- S. Enthaler, B. Spilker, G. Erre, K. Junge, M. K. Tse and M. Beller, Tetrahedron, 2008, 64, 3867–3876 CrossRef CAS.
- C. P. Casey and H. Guan, J. Am. Chem. Soc., 2007, 129, 5816–5817 CrossRef CAS.
- C. P. Casey and H. Guan, J. Am. Chem. Soc., 2009, 131, 2499–2507 CrossRef CAS.
- R. M. Bullock, Angew. Chem., Int. Ed., 2007, 46, 7360–7363 CrossRef CAS.
- H. Zhang, D. Chen, Y. Zhang, G. Zhang and J. Liu, Dalton Trans., 2010, 39, 1972–1978 RSC.
- X. Lu, Y. Zhang, P. Yun, M. Zhang and T. Li, Org. Biomol. Chem., 2013, 11, 5264–5277 RSC.
- X. Lu, Y. Zhang, M. Zhang and T. Li, J. Organomet. Chem., 2014, 749, 69–74 CrossRef CAS.
- X. Lu, Y. Zhang, N. Turner, M. Zhang and T. Li, Org. Biomol. Chem., 2014, 12, 4361–4371 RSC.
- A. Quintard and J. Rodriguez, Angew. Chem., Int. Ed., 2014, 53, 4044–4055 CrossRef CAS.
- J. S. Chen, L. L. Chen, Y. Xing, G. Chen, W. Y. Shen, Z. R. Dong, Y. Y. Li and J. X. Gao, Acta Chim. Sin., 2004, 62, 1745–1750 CAS.
- C. Sui-Seng, F. Freutel, A. J. Lough and R. H. Morris, Angew. Chem., Int. Ed., 2008, 47, 940–943 CrossRef CAS.
- A. Mikhailine, A. J. Lough and R. H. Morris, J. Am. Chem. Soc., 2009, 131, 1394–1395 CrossRef CAS.
- N. Meyer, A. J. Lough and R. H. Morris, Chem. – Eur. J., 2009, 15, 5605–5610 CrossRef CAS.
- D. E. Prokopchuk, J. F. Sonnenberg, N. Meyer, M. Z.-D. Iuliis, A. J. Lough and R. H. Morris, Organometallics, 2012, 31, 3056–3064 CrossRef CAS.
- C. Sui-Seng, F. N. Haque, A. Hadzovic, A.-M. Putz, V. Reuss, N. Meyer, A. J. Lough, M. Z.-D. Iuliis and R. H. Morris, Inorg. Chem., 2009, 48, 735–743 CrossRef CAS.
- P. O. Lagaditis, A. J. Lough and R. H. Morris, Inorg. Chem., 2010, 49, 10057–10066 CrossRef CAS.
- A. A. Mikhailine and R. H. Morris, Inorg. Chem., 2010, 49, 11039–11044 CrossRef CAS.
- P. E. Sues, A. J. Lough and R. H. Morris, Organometallics, 2011, 30, 4418–4431 CrossRef CAS.
- P. O. Lagaditis, A. J. Lough and R. H. Morris, J. Am. Chem. Soc., 2011, 133, 9662–9665 CrossRef CAS.
- A. A. Mikhailine, M. I. Maishan, A. J. Lough and R. H. Morris, J. Am. Chem. Soc., 2012, 134, 12266–12280 CrossRef CAS.
- D. E. Prokopchuk and R. H. Morris, Organometallics, 2012, 31, 7375–7385 CrossRef CAS.
- W. Zuo, A. J. Lough, Y. F. Li and R. H. Morris, Science, 2013, 342, 1080–1083 CrossRef CAS.
- W. Zuo, S. Tauer, D. E. Prokopchuk and R. H. Morris, Organometallics, 2014, 33, 5791–5801 CrossRef CAS.
- W. Zuo and R. H. Morris, Nat. Protoc., 2015, 10, 241–257 CrossRef CAS.
- S. A. M. Smith and R. H. Morris, Synthesis, 2015, 1775–1779 CAS.
- W. Zuo, D. E. Prokopchuk, A. J. Lough and R. H. Morris, ACS Catal., 2016, 6, 301–314 CrossRef CAS.
- K. Z. Demmans, O. W. K. Ko and R. H. Morris, RSC Adv., 2016, 6, 88580–88587 RSC.
- K. Z. Demmans, C. S. G. Seo, A. J. Lougha and R. H. Morris, Chem. Sci., 2017, 8, 6531–6541 RSC.
- S. A. M. Smith, D. E. Prokopchuk and R. H. Morris, Isr. J. Chem., 2017, 57, 1204–1215 CrossRef CAS.
- J. F. Sonnenberg, N. Coombs, P. A. Dube and R. H. Morris, J. Am. Chem. Soc., 2012, 134, 5893–5899 CrossRef CAS.
- J. F. Sonnenberg and R. H. Morris, Catal. Sci. Technol., 2014, 4, 3426–3438 RSC.
- P. O. Lagaditis, P. E. Sues, A. J. Lough and R. H. Morris, Dalton Trans., 2015, 44, 12119–12127 RSC.
- R. Langer, G. Leitus, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2011, 50, 2120–2124 CrossRef CAS.
- R. Langer, M. A. Iron, L. Konstantinovski, Y. Diskin-Posner, G. Leitus, Y. Ben-David and D. Milstein, Chem. – Eur. J., 2012, 18, 7196–7209 CrossRef CAS.
- X. Yang, Inorg. Chem., 2011, 50, 12836–12843 CrossRef CAS.
- N. Gorgas, B. Stöger, L. F. Veiros, E. Pittenauer, G. Allmaier and K. Kirchner, Organometallics, 2014, 33, 6905–6914 CrossRef CAS.
- S. Mazza, R. Scopelliti and X. Hu, Organometallics, 2015, 34, 1538–1545 CrossRef CAS.
- P. O. Lagaditis, P. E. Sues, J. F. Sonnenberg, K. Y. Wan, A. J. Lough and R. H. Morris, J. Am. Chem. Soc., 2014, 136, 1367–1380 CrossRef CAS.
- C. Schroder-Holzhacker, N. Gorgas, B. Stoger and K. Kirchner, Monatsh. Chem., 2016, 147, 1023–1030 CrossRef.
- J. F. Sonnenberg, A. J. Lough and R. H. Morris, Organometallics, 2014, 33, 6452–6465 CrossRef CAS.
- W. Zuo, D. E. Prokopchuk, A. J. Lough and R. H. Morris, ACS Catal., 2016, 6, 301–314 CrossRef CAS.
- A. Zirakzadeh, K. Kirchner, A. Roller, B. Stöger, M. Widhalm and R. H. Morris, Organometallics, 2016, 35, 3781–3787 CrossRef CAS.
- J. F. Sonnenberg, K. Y. Wan, P. E. Sues and R. H. Morris, ACS Catal., 2017, 7, 316–326 CrossRef CAS.
- S. A. M. Smith, P. O. Lagaditis, A. Lüpke, A. J. Lough and R. H. Morris, Chem. – Eur. J., 2017, 23, 7212–7216 CrossRef CAS.
- C. S. G. Seo, T. Tannoux, S. A. M. Smith, A. J. Lough and R. H. Morris, J. Org. Chem., 2019, 84, 12040–12049 CrossRef CAS.
- R. Huber, A. Passera and A. Mezzetti, Organometallics, 2018, 37, 396–405 CrossRef CAS.
- R. Huber, A. Passera, E. Gubler and A. Mezzetti, Adv. Synth. Catal., 2018, 360, 2900–2913 CrossRef CAS.
- S. Chakraborty, P. O. Lagaditis, M. Förster, E. A. Bielinski, N. Hazari, M. C. Holthausen, W. D. Jones and S. Schneider, ACS Catal., 2014, 4, 3994–4003 CrossRef CAS.
- J. Brünig, Z. Csendes, S. Weber, N. Gorgas, R. W. Bittner, A. Limbeck, K. Bica, H. Hoffmann and K. Kirchner, ACS Catal., 2018, 8, 1048–1051 CrossRef.
- B. Butschke, M. Feller, Y. Diskin-Posner and D. Milstein, Catal. Sci. Technol., 2016, 6, 4428–4437 RSC.
- V. V. K. M. Kandepi, J. M. S. Cardoso, E. Peris and B. Royo, Organometallics, 2010, 29, 2777–2782 CrossRef CAS.
- A. Naik, T. Maji and O. Reiser, Chem. Commun., 2010, 46, 4475–4477 RSC.
- T. N. Plank, J. L. Drake, D. K. Kim and T. W. Funk, Adv. Synth. Catal., 2012, 354, 597–601 CrossRef CAS.
- A. Berkessel, S. Reichau, A. von der Hoh, N. Leconte and J.-M. Neudorfl, Organometallics, 2011, 30, 3880–3887 CrossRef CAS.
- S. Fleischer, S. Zhou, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 5120–5124 CrossRef CAS.
- D. S. Merel, M. Elie, J.-F. Lohier, S. Gaillard and J.-L. Renaud, ChemCatChem, 2013, 5, 2939–2945 CrossRef CAS.
- T. Hashimoto, S. Urban, R. Hoshino, Y. Ohki, K. Tatsumi and F. Glorius, Organometallics, 2012, 31, 4474–4479 CrossRef CAS.
- M. D. Bala and M. I. Ikhile, J. Mol. Catal. A: Chem., 2014, 385, 98–105 CrossRef CAS.
- T. W. Funk, A. R. Mahoney, R. A. Sponenburg, K. P. Zimmerman, D. K. Kim and E. E. Harrison, Organometallics, 2018, 37, 1133–1140 CrossRef CAS.
- R. Hodgkinson, A. D. Grosso, G. Clarkson and M. Wills, Dalton Trans., 2016, 45, 3992–4005 RSC.
- P. Gajewski, M. Renom-Carrasco, S. V. Facchini, L. Pignataro, L. Lefort, J. G. de Vries, R. Ferraccioli, A. Forni, U. Piarulli and C. Gennari, Eur. J. Org. Chem., 2015, 1887–1893 CrossRef CAS.
- S. V. Facchini, J.-M. Neudörfl, L. Pignataro, M. Cettolin, C. Gennari, A. Berkessel and U. Piarulli, ChemCatChem, 2017, 9, 1461–1468 CrossRef.
- K. Natte, W. Li, S. Zhou, H. Neumann and X.-F. Wu, Tetrahedron Lett., 2015, 56, 1118–1121 CrossRef CAS.
- S. Yu, W. Shen, Y. Li, Z. Dong, Y. Xu, Q. Li, J. Zhang and J. Gao, Adv. Synth. Catal., 2012, 354, 818–822 CrossRef CAS.
- Y. Li, S. Yu, X. Wu, J. Xiao, W. Shen, Z. Dong and J. Gao, J. Am. Chem. Soc., 2014, 136, 4031–4039 CrossRef CAS.
- R. Bigler and A. Mezzetti, Org. Lett., 2014, 16, 6460–6463 CrossRef CAS.
- R. Bigler, R. Huber and A. Mezzetti, Angew. Chem., Int. Ed., 2015, 54, 5171–5174 CrossRef CAS.
- R. Bigler and A. Mezzetti, Org. Process Res. Dev., 2016, 20, 253–261 CrossRef CAS.
- R. Bigler, R. Huber, M. Stöckli and A. Mezzetti, ACS Catal., 2016, 6, 6455–6464 CrossRef CAS.
- L. D. Luca and A. Mezzetti, Angew. Chem., Int. Ed., 2017, 56, 11949–11953 CrossRef.
- R. Bigler, R. Huber and A. Mezzetti, Synlett, 2016, 831–847 CAS.
- G. Wienhçfer, F. A. Westerhaus, K. Junge, R. Ludwig and M. Beller, Chem. – Eur. J., 2013, 19, 7701–7707 CrossRef.
- G. Wienhöfer, F. A. Westerhaus, K. Junge and M. Beller, J. Organomet. Chem., 2013, 744, 156–159 CrossRef.
- L.-Q. Lu, Y. Li, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 8382–8386 CrossRef CAS.
- P. Zhang, X. Li, X. Qi, H. Sun, O. Fuhr and D. Fenske, RSC Adv., 2018, 8, 14092–14099 RSC.
- Y. Wanga, Z. Dua, T. Zheng, H. Sun and X. Li, Catal. Commun., 2019, 124, 32–35 CrossRef.
- S. Zhou, S. Fleischer, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 5120–5124 CrossRef CAS.
- S. Fleischer, S. Werkmeister, S. Zhou, K. Junge and M. Beller, Chem. – Eur. J., 2012, 18, 9005–9010 CrossRef CAS.
- D. Brenna, S. Rossi, F. Cozzi and M. Benaglia, Org. Biomol. Chem., 2017, 15, 5685–5688 RSC.
- S. V. Facchini, M. Cettolin, X. Bai, G. Casamassima, L. Pignataro, C. Gennari and U. Piarulli, Adv. Synth. Catal., 2018, 360, 1054–1059 CrossRef CAS.
- C. Federsel, A. Boddien, R. Jackstell, R. Jennerjahn, P. J. Dyson, R. Scopelliti, G. Laurenczy and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9777–9780 CrossRef CAS.
- C. Ziebart, C. Federsel, P. Anbarasan, R. Jackstell, W. Baumann, A. Spannenberg and M. Beller, J. Am. Chem. Soc., 2012, 134, 20701–20704 CrossRef CAS.
- R. Langer, Y. Diskin-Posner, G. Leitus, L. J. W. Shimon, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2011, 50, 9948–9952 CrossRef CAS.
- O. Rivada-Wheelaghan, A. Dauth, G. Leitus, Y. Diskin-Posner and D. Milstein, Inorg. Chem., 2015, 54, 4526–4538 CrossRef CAS.
- Y. Zhang, A. D. MacIntosh, J. L. Wong, E. A. Bielinski, P. G. Williard, B. Q. Mercado, N. Hazari and W. H. Bernskoetter, Chem. Sci., 2015, 6, 4291–4299 RSC.
- N. E. Smith, W. H. Bernskoetter, N. Hazari and B. Q. Mercado, Organometallics, 2017, 36, 3995–4004 CrossRef CAS.
- M. Montandon-Clerc and G. Laurenczy, J. Catal., 2018, 362, 78–84 CrossRef CAS.
- F. Bertini, I. Mellone, A. Ienco, M. Peruzzini and L. Gonsalvi, ACS Catal., 2015, 5, 1254–1265 CrossRef CAS.
- R. Marcos, F. Bertini, Z. Rinkevicius, M. Peruzzini, L. Gonsalvi and M. S. G. Ahlquist, Chem. – Eur. J., 2018, 24, 5366–5372 CrossRef CAS.
- A. P. C. Ribeiro, L. M. D. R. S. Martins and A. J. L. Pombeiro, Green Chem., 2017, 19, 4811–4815 RSC.
- H. Fong and J. C. Peters, Inorg. Chem., 2015, 54, 5124–5135 CrossRef CAS.
- F. Zhu, L. Zhu-Ge, G. Yang and S. Zhou, ChemSusChem, 2015, 8, 609–612 CrossRef CAS.
- T. Zell, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2014, 53, 4685–4689 CrossRef CAS.
- S. Werkmeister, K. Junge, B. Wendt, E. Alberico, H. Jiao, W. Baumann, H. Junge, F. Gallou and M. Beller, Angew. Chem., Int. Ed., 2014, 53, 8722–8726 CrossRef CAS.
- S. Chakraborty, H. Dai, P. Bhattacharya, N. T. Fairweather, M. S. Gibson, J. A. Krause and H. Guan, J. Am. Chem. Soc., 2014, 136, 7869–7872 CrossRef CAS.
- N. T. Fairweather, M. S. Gibson and H. Guan, Organometallics, 2015, 34, 335–339 CrossRef CAS.
- J. A. Garg, S. Chakraborty, Y. Ben-David and D. Milstein, Chem. Commun., 2016, 52, 5285–5288 RSC.
- N. M. Rezayee, D. C. Samblanet and M. S. Sanford, ACS Catal., 2016, 6, 6377–6383 CrossRef CAS.
- U. Jayarathne, Y. Zhang, N. Hazari and W. H. Bernskoetter, Organometallics, 2017, 36, 409–416 CrossRef CAS.
- C. Bornschein, S. Werkmeister, B. Wendt, H. Jiao, E. Alberico, W. Baumann, H. Junge, K. Junge and M. Beller, Nat. Commun., 2014, 5, 4111 CrossRef CAS.
- S. Lange, S. Elangovan, C. Cordes, A. Spannenberg, H. Jiao, H. Junge, S. Bachmann, M. Scalone, C. Topf, A. K. Jungea and M. Beller, Catal. Sci. Technol., 2016, 6, 4768–4772 RSC.
- S. Chakraborty, G. Leitus and D. Milstein, Chem. Commun., 2016, 52, 1812–1815 RSC.
- S. Chakraborty, G. Leitus and D. Milstein, Angew. Chem., Int. Ed., 2017, 56, 2074–2078 CrossRef CAS.
- M.-C. Fu, R. Shang, Z. Huang and Y. Fu, Synlett, 2014, 2748–2752 CrossRef CAS.
- G. Metzkera and A. C. B. Burtoloso, Chem. Commun., 2015, 51, 14199–14202 RSC.
- N. Dai, R. Shang, M. Fu and Y. Fu, Chin. J. Chem., 2015, 33, 405–408 CrossRef CAS.
- S. Fleischer, S. Zhou, S. Werkmeister, K. Junge and M. Beller, Chem. – Eur. J., 2013, 19, 4997–5003 CrossRef CAS.
- S. Chakraborty, W. W. Brennessel and W. D. Jones, J. Am. Chem. Soc., 2014, 136, 8564–8567 CrossRef CAS.
- E. C. Ashby and J. J. Lin, Tetrahedron Lett., 1977, 51, 4481–4484 CrossRef.
- E. C. Ashby and J. J. Lin, J. Org. Chem., 1978, 43, 2567–2572 CrossRef CAS.
- K. Kano, M. Takeuchi, S. Hashimoto and Z.-I. Yoshida, J. Chem. Soc., Chem. Commun., 1991, 1728–1729 RSC.
- M. Takeuchi and K. Kano, Organometallics, 1993, 12, 2059–2064 CrossRef CAS.
- M. Lamani, G. S. Ravikumara and K. R. Prabhu, Adv. Synth. Catal., 2012, 354, 1437–1442 CrossRef CAS.
- T. S. Carter, L. Guiet, D. J. Frank, J. West and S. P. Thomas, Adv. Synth. Catal., 2013, 355, 880–884 CrossRef CAS.
- T. N. Gieshoff, M. Villa, A. Welther, M. Plois, U. Chakraborty, R. Wolf and A. J. von Wangelin, Green Chem., 2015, 17, 1408–1413 RSC.
- Y. Moglie, F. Alonso, C. Vitale, M. Yusb and G. Radivoy, Tetrahedron, 2006, 62, 2812–2819 CrossRef CAS.
- M. D. Bhor, M. J. Bhanushali, N. S. Nandurkar and B. M. Bhanage, Tetrahedron Lett., 2008, 49, 965–969 CrossRef CAS.
- S. Fleischer, S. Zhou, K. Junge and M. Beller, Chem. – Asian J., 2011, 6, 2240–2245 CrossRef CAS.
- A. Pagnoux-Ozherelyeva, N. Pannetier, M. D. Mbaye, S. Gaillard and J.-L. Renaud, Angew. Chem., Int. Ed., 2012, 51, 4976–4980 CrossRef CAS.
- S. Moulin, H. Dentel, A. Pagnoux-Ozherelyeva, S. Gaillard, A. Poater, L. Cavallo, J.-F. Lohier and J.-L. Renaud, Chem. – Eur. J., 2013, 19, 17881–17890 CrossRef CAS.
- N. U. Kumar, B. S. Reddy, V. P. Reddy and R. Bandichhor, Tetrahedron Lett., 2012, 53, 4354–4356 CrossRef.
- O. I. Afanasyev, D. L. Usanov and D. Chusov, Org. Biomol. Chem., 2017, 15, 10164–10166 RSC.
- E. Petricci, N. Santillo, D. Castagnolo, E. Cini and M. Taddeia, Adv. Synth. Catal., 2018, 360, 2560–2565 CrossRef CAS.
- D. Wei, C. Netkaew and C. Darcel, Adv. Synth. Catal., 2019, 361, 1781–1786 CrossRef CAS.
- D. Wei, C. Netkaew, V. Carré and C. Darcel, ChemSusChem, 2019, 12, 3008 CrossRef CAS.
- K. Iwanami, H. Seo, Y. Tobita and T. Oriyama, Synthesis, 2005, 183–186 CAS.
- K. Iwanami, K. Yano and T. Oriyama, Chem. Lett., 2007, 36, 38–39 CrossRef CAS.
- G. Argouarch, G. Grelaud, T. Roisnel, M. G. Humphrey and F. Paul, Tetrahedron Lett., 2012, 53, 5015–5018 CrossRef CAS.
- R. Savela and R. Leino, Synthesis, 2015, 1749–1760 CAS.
- J. M. Landesberg, L. Katz and C. Olsen, J. Org. Chem., 1972, 37, 930–936 CrossRef CAS.
- J. F. Knifton, J. Org. Chem., 1976, 41, 1200–1206 CrossRef CAS.
- R. M. Deshpande, A. N. Mahajan, M. M. Diwakar, P. S. Ozarde and R. V. Chaudhari, J. Org. Chem., 2004, 69, 4835–4838 CrossRef CAS.
- R. V. Jagadeesh, A.-E. Surkus, H. Junge, M.-M. Pohl, J. Radnik, J. Rabeah, H. Huan, V. Schünemann, A. Brückner and M. Beller, Science, 2013, 342, 1073–1076 CrossRef CAS.
- G. Wienhofer, M. Baseda-Kruger, C. Ziebart, F. A. Westerhaus, W. Baumann, R. Jackstell, K. Junge and M. Beller, Chem. Commun., 2013, 49, 9089–9091 RSC.
- K. Junge, B. Wendt, N. Shaikh and M. Beller, Chem. Commun., 2010, 46, 1769–1771 RSC.
- L. Pehlivan, E. Métay, S. Laval, W. Dayoub, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron Lett., 2010, 51, 1939–1941 CrossRef CAS.
- L. Pehlivan, E. Metay, S. Laval, W. Dayoub, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron, 2011, 67, 1971–1976 CrossRef CAS.
- Q. Shi, R. Lu, K. Jin, Z. Zhang and D. Zhao, Green Chem., 2006, 8, 868–870 RSC.
- U. Sharma, P. K. Verma, N. Kumar, V. Kumar, M. Bala and B. Singh, Chem. – Eur. J., 2011, 17, 5903–5907 CrossRef CAS.
- S. Kim, E. Kim and B. M. Kim, Chem. – Asian J., 2011, 6, 1921–1925 CrossRef CAS.
- D. Cantillo, M. Baghbanzadeh and C. O. Kappe, Angew. Chem., Int. Ed., 2012, 51, 10190–10193 CrossRef CAS.
- X. Gu, Z. Sun, S. Wu, W. Qi, H. Wang, X. Xu and D. Su, Chem. Commun., 2013, 49, 10088–10090 RSC.
- S. U. Sonavane, M. B. Gawande, S. S. Deshpande, A. Venkataraman and R. V. Jayaram, Catal. Commun., 2007, 8, 1803–1806 CrossRef CAS.
- G. Wienhofer, I. Sorribes, A. Boddien, F. Westerhaus, K. Junge, H. Junge, R. Llusar and M. Beller, J. Am. Chem. Soc, 2011, 133, 12875–12879 CrossRef.
- A. J. MacNair, M.-M. Tran, J. E. Nelson, G. U. Sloan, A. Ironmonger and S. P. Thomas, Org. Biomol. Chem., 2014, 12, 5082–5088 RSC.
- S. C. Bart, E. Lobkovsky, E. Bill and P. J. Chirik, J. Am. Chem. Soc., 2006, 128, 5302–5303 CrossRef CAS.
- S. Pagoti, S. Surana, A. Chauhan, B. Parasara and J. Dash, Catal. Sci. Technol., 2013, 3, 584–588 RSC.
- S. Enthaler, ChemCatChem, 2011, 3, 666–670 CrossRef CAS.
- J. M. S. Cardoso and B. Royo, Chem. Commun., 2012, 48, 4944–4946 RSC.
- X. Cui, F. Shi, Y. Zhang and Y. Deng, Tetrahedron Lett., 2010, 51, 2048–2051 CrossRef CAS.
- M. Bala, P. K. Verma, U. Sharma, N. Kumar and B. Singh, Green Chem., 2013, 15, 1687–1693 RSC.
- T. Yan, B. L. Feringa and K. Barta, Nat. Commun., 2014, 5, 5602 CrossRef CAS.
- T. Yan, B. L. Feringa and K. Barta, ACS Catal., 2016, 6, 381–388 CrossRef CAS.
- T. Yan, B. L. Feringa and K. Barta, Sci. Adv., 2017, 3, eaao6494 CrossRef.
- B. Emayavaramban, M. Roy and B. Sundararaju, Chem. – Eur. J., 2016, 22, 3952–3955 CrossRef CAS.
- T. J. Brown, M. Cumbes, L. J. Diorazio, G. J. Clarkson and M. Wills, J. Org. Chem., 2017, 82, 10489–10503 CrossRef CAS.
- H.-J. Pan, T. W. Ng and Y. Zhao, Chem. Commun., 2015, 51, 11907–11910 RSC.
- S. Elangovan, J.-B. Sortais, M. Beller and C. Darcel, Angew. Chem., Int. Ed., 2015, 54, 14483–14486 CrossRef CAS.
- C. Seck, M. D. Mbaye, S. Coufourier, A. Lator, J.-F. Lohier, A. Poater, T. R. Ward, S. Gaillard and J.-L. Renaud, ChemCatChem, 2017, 9, 4410–4416 CrossRef CAS.
- G. D. Gregorio, M. Mari, F. Bartoccini and G. Piersanti, J. Org. Chem., 2017, 82, 8769–8775 CrossRef.
- J.-J. Brunet and M. Taillefer, J. Organomet. Chem., 1988, 348, C5–C8 CrossRef CAS.
- H. Guo, K.-i. Kanno and T. Takahashi, Chem. Lett., 2004, 33, 1356–1357 CrossRef CAS.
- W. M. Czaplik, S. Grupe, M. Mayer and A. J. von Wangelin, Chem. Commun., 2010, 46, 6350–6352 RSC.
- S. Grupe and A. J. von Wangelin, ChemCatChem, 2013, 5, 706–710 CrossRef CAS.
- R. Pilli, V. Balakrishnan, R. Chandrasekaran and R. Rasappan, Org. Biomol. Chem., 2019, 17, 1749–1753 RSC.
- S. A. Ponomarenko and S. Kirchmeyer, in Conjugated organosilicon materials for organic electronics and photonics, ed. A. M. Muzafarov, Silicon Polymers, Springer-Verlag, Heidelberg, 2010, pp. 33–110 Search PubMed.
- B. Marciniec, Hydrosilylation of carbon-carbon multiple bonds in organic synthesis, Hydrosilylation, Springer Netherlands, Dordrecht, 2009, pp. 87–123 Search PubMed.
- A. Nesmeyanov, R. K. Freidlina, E. Chukovskaya, R. Petrova and A. Belyavsky, Tetrahedron, 1962, 17, 61–68 CrossRef CAS.
- M. A. Schroeder and M. S. Wrighton, J. Organomet. Chem., 1977, 128, 345–358 CrossRef CAS.
- F. Kakiuchi, Y. Tanaka, N. Chatani and S. Murai, J. Organomet. Chem., 1993, 456, 45–47 CrossRef CAS.
- B. Marciniec, A. Kownacka, I. Kownacki and R. Taylor, Appl. Catal., A, 2014, 486, 230–238 CrossRef CAS.
- S. C. Bart, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2004, 126, 13794–13807 CrossRef CAS.
- S. C. Bart, E. J. Hawrelak, E. Lobkovsky and P. J. Chirik, Organometallics, 2005, 24, 5518–5527 CrossRef CAS.
- A. M. Archer, M. W. Bouwkamp, M.-P. Cortez, E. Lobkovsky and P. J. Chirik, Organometallics, 2006, 25, 4269–4278 CrossRef CAS.
- Y. C. Lam, R. J. Nielsen, W. A. Goddard III and A. K. Dash, Dalton Trans., 2017, 46, 12507 RSC.
- S. Sakaki, N. Mizoe and M. Sugimoto, Organometallics, 1998, 17(12), 2510 CrossRef CAS.
- M. D. Greenhalgh, D. J. Frank and S. P. Thomas, Adv. Synth. Catal., 2014, 356, 584–590 CrossRef CAS.
- A. M. Tondreau, C. C. H. Atienza, K. J. Weller, S. A. Nye, K. M. Lewis, J. G. Delis and P. J. Chirik, Science, 2012, 335, 567–570 CrossRef CAS.
- A. J. Challinor, M. Calin, G. S. Nichol, N. B. Carter and S. P. Thomas, Adv. Synth. Catal., 2016, 358, 2404–2409 CrossRef CAS.
- C. C. H. Atienza, A. M. Tondreau, K. J. Weller, K. M. Lewis, R. W. Cruse, S. A. Nye, J. L. Boyer, J. G. Delis and P. J. Chirik, ACS Catal., 2012, 2, 2169–2172 CrossRef.
- (a) I. E. Markó, S. Steŕin, O. Buisine, G. Berthon, G. Michaud, B. Tinanat and J.-P. Declercq, Adv. Synth. Catal., 2004, 346, 1429 CrossRef; (b) J. V. Obligacion and P. J. Chirik, Nat. Rev. Chem., 2018, 2, 15 CrossRef CAS; (c) L. N. Lewis, K. G. Sy, G. L. Bryant and P. E. Donahue, Organometallics, 1991, 10(10), 3750 CrossRef CAS.
- K. Kamata, A. Suzuki, Y. Nakai and H. Nakazawa, Organometallics, 2012, 31, 3825–3828 CrossRef CAS.
- A. M. Tondreau, C. C. H. Atienza, J. M. Darmon, C. Milsmann, H. M. Hoyt, K. J. Weller, S. A. Nye, K. M. Lewis, J. Boyer and J. G. Delis, Organometallics, 2012, 31, 4886 CrossRef CAS.
- D. Peng, Y. Zhang, X. Du, L. Zhang, X. Leng, M. D. Walter and Z. Huang, J. Am. Chem. Soc., 2013, 135, 19154–19166 CrossRef CAS.
- K. Hayasaka, K. Kamata and H. Nakazawa, Bull. Chem. Soc. Jpn., 2016, 89, 394–404 CrossRef CAS.
- Y. Toya, K. Hayasaka and H. Nakazawa, Organometallics, 2017, 36, 1727–1735 CrossRef CAS.
- J. Nakazawa, B. Cheng, M. Cao and Z. Lu, Angew. Chem., Int. Ed., 2015, 54, 4661–4664 CrossRef.
- B. Cheng, W. Liu and Z. Lu, J. Am. Chem. Soc., 2018, 140, 5014–5017 CrossRef CAS.
- M.-Y. Hu, Q. He, S.-J. Fan, Z.-C. Wang, L.-Y. Liu, Y.-J. Mu, Q. Peng and S.-F. Zhu, Nat. Commun., 2018, 9, 221 CrossRef.
- D. Noda, A. Tahara, Y. Sunada and H. Nagashima, J. Am. Chem. Soc., 2016, 138, 2480–2483 CrossRef CAS.
- J. Y. Wu, B. N. Stanzl and T. Ritter, J. Am. Chem. Soc., 2010, 132, 13214–13216 CrossRef CAS.
- S. Enthaler, M. Haberberger and E. Irran, Chem. – Asian J., 2011, 6, 1613–1623 CrossRef CAS.
- C. Belger and B. Plietker, Chem. Commun., 2012, 48, 5419–5421 RSC.
- H. Brunner and K. Fisch, Angew. Chem., Int. Ed. Engl., 1990, 29, 1131–1132 CrossRef.
- N. S. Shaikh, K. Junge and M. Beller, Org. Lett., 2007, 9, 5429–5432 CrossRef CAS.
- D. Addis, N. Shaikh, S. Zhou, S. Das, K. Junge and M. Beller, Chem. – Asian J., 2010, 5, 1687–1691 CrossRef CAS.
- N. S. Shaikh, S. Enthaler, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 2497–2501 CrossRef CAS.
- H. Nishiyama and A. Furuta, Chem. Commun., 2007, 760–762 RSC.
- A. Furuta and H. Nishiyama, Tetrahedron Lett., 2008, 49, 110–113 CrossRef CAS.
- T. Inagaki, L. T. Phong, A. Furuta, J.-i. Ito and H. Nishiyama, Chem. – Eur. J., 2010, 16, 3090–3096 CrossRef CAS.
- T. Inagaki, A. Ito, J.-i. Ito and H. Nishiyama, Angew. Chem., Int. Ed., 2010, 49, 9384–9387 CrossRef CAS.
- K. Muller, A. Muller, T. Jozak, A. Ahrens-Botzong, V. Schünemann and W. R. Thiel, ChemCatChem, 2011, 3, 887–892 CrossRef CAS.
- A. P. Dieskau, J. M. Begouin and B. Plietker, Eur. J. Org. Chem., 2011, 5291–5296 CrossRef CAS.
- B. Plietker and A. Dieskau, Eur. J. Org. Chem., 2009, 775–787 CrossRef CAS.
- J. Yang and T. D. Tilley, Angew. Chem., Int. Ed., 2010, 49, 10186–10188 CrossRef CAS.
- A. J. Ruddy, C. M. Kelly, S. M. Crawford, C. A. Wheaton, O. L. Sydora, B. L. Small, M. Stradiotto and L. Turculet, Organometallics, 2013, 32, 5581–5588 CrossRef CAS.
- M. Flückiger and A. Togni, Eur. J. Org. Chem., 2011, 4353 CrossRef.
- A. M. Togni, E. Lobkovsky and P. J. Chirik, Org. Lett., 2008, 10, 2789–2792 CrossRef.
- P. Bhattacharya, J. A. Krause and H. Guan, Organometallics, 2011, 30, 4720–4729 CrossRef CAS.
- P. Bhattacharya, J. A. Krause and H. Guan, Organometallics, 2014, 33, 6113–6121 CrossRef CAS.
- S. Chakraborty, P. Bhattacharya, H. Dai and H. Guan, Acc. Chem. Res., 2015, 48, 1995–2003 CrossRef CAS.
- S. Wu, X. Li, Z. Xiong, W. Xu, Y. Lu and H. Sun, Organometallics, 2013, 32, 3227–3237 CrossRef CAS.
- H. Zhao, H. Sun and X. Li, Organometallics, 2014, 33, 3535–3539 CrossRef CAS.
- S. Huang, H. Zhao, X. Li, L. Wang and H. Sun, RSC Adv., 2015, 5, 15660–15667 RSC.
- A. D. Smith, A. Saini, L. M. Singer, N. Phadke and M. Findlater, Polyhedron, 2016, 114, 286 CrossRef CAS.
- D. Gallego, S. Inoue, B. Blom and M. Driess, Organometallics, 2014, 33, 6885–6897 CrossRef CAS.
- T. T. Metsänen, D. Gallego, T. Szilvási, M. Driess and M. Oestreich, Chem. Sci., 2015, 6, 7143–7149 RSC.
- H.-J. Lin, S. Lutz, C. O’Kane, M. Zeller, C.-H. Chen, T. Chen and W.-T. Lee, Dalton Trans., 2018, 47, 3243–3247 RSC.
- B. K. Langlotz, H. Wadepohl and L. H. Gade, Angew. Chem., Int. Ed., 2008, 47, 4670–4674 CrossRef CAS.
- A. M. Tondreau, J. M. Darmon, B. M. Wile, S. K. Floyd, E. Lobkovsky and P. J. Chirik, Organometallics, 2009, 28, 3928 CrossRef CAS.
- S. Hosokawa, J.-I. Ito and H. Nishiyama, Organometallics, 2010, 29, 5773 CrossRef CAS.
- J.-I. Ito, S. Hosokawa, H. B. Khalid and H. Nishiyama, Organometallics, 2015, 34, 1377 CrossRef CAS.
- Z. Zuo, L. Zhang, X. Leng and Z. Huang, Chem. Commun., 2015, 51, 5073 RSC.
- T. Bleith, H. Wadepohl and L. H. Gade, J. Am. Chem. Soc., 2015, 137, 2456 CrossRef CAS.
- T. Bleith and L. H. Gade, J. Am. Chem. Soc., 2016, 138, 4972–4983 CrossRef CAS.
- D. V. Gutsulyak, L. G. Kuzmina, J. A. Howard, S. F. Vyboishchikov and G. I. Nikonov, J. Am. Chem. Soc., 2008, 130, 3732 CrossRef CAS.
- J. Zheng, L. C. Misal Castro, T. Roisnel, C. Darcel and J.-B. Sortais, Inorg. Chim. Acta, 2012, 380, 301 CrossRef CAS.
- D. Bézier, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2013, 355, 19 CrossRef.
- V. V. K. M. Kandepi, J. M. S. Cardoso, E. Peris and B. Royo, Organometallics, 2010, 29, 2777 CrossRef CAS.
- F. Jiang, D. Bezier, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2011, 353, 239 CrossRef CAS.
- D. Bézier, F. Jiang, T. Roisnel, J.-B. Sortais and C. Darcel, Eur. J. Inorg. Chem., 2012, 1333 CrossRef.
- S. Demir, Y. Gökçe, N. Kaloğlu, J.-B. Sortais, C. Darcel and I. Özdemir, Appl. Organomet. Chem., 2013, 27, 459 CrossRef CAS.
- V. César, L. C. Misal Castro, T. Dombray, J.-B. Sortais, C. Darcel, S. Labat, K. Miqueu, J.-M. Sotiropoulos, R. Brousses and N. Lugan, Organometallics, 2013, 32, 4643 CrossRef.
- D. Kumar, A. Prakasham, L. P. Bheeter, J.-B. Sortais, M. Gangwar, T. Roisnel, A. C. Kalita, C. Darcel and P. Ghosh, J. Organomet. Chem., 2014, 762, 81 CrossRef CAS.
- C. Johnson and M. Albrecht, Organometallics, 2017, 36, 2902 CrossRef CAS.
- T. Hashimoto, S. Urban, R. Hoshino, Y. Ohki, K. Tatsumi and F. Glorius, Organometallics, 2012, 31, 4474 CrossRef CAS.
- C. Grohmann, T. Hashimoto, R. Fröhlich, Y. Ohki, K. Tatsumi and F. Glorius, Organometallics, 2012, 31, 8047 CrossRef CAS.
- S. Warratz, L. Postigo and B. Royo, Organometallics, 2013, 32, 893 CrossRef CAS.
- Q. Liang, N. J. Liu and D. Song, Dalton Trans., 2018, 47, 9889 RSC.
- E. Buitrago, L. Zani and H. Adolfsson, Appl. Organomet. Chem., 2011, 25, 748 CrossRef CAS.
- E. Buitrago, F. Tinnis and H. Adolfsson, Adv. Synth. Catal., 2012, 354, 217 CrossRef CAS.
- C. Dal Zotto, D. Virieux and J.-M. Campagne, Synlett, 2009, 276 CAS.
- Z. Zuo, H. Sun, L. Wang and X. Li, Dalton Trans., 2014, 43, 11716 RSC.
- B. Xue, H. Sun and X. Li, RSC Adv., 2015, 5, 52000 RSC.
- T. Zheng, J. Li, S. Zhang, B. Xue, H. Sun, X. Li, O. Fuhr and D. Fenske, Organometallics, 2016, 35, 3538 CrossRef CAS.
- B. Xue, H. Sun, Q. Niu, X. Li, O. Fuhr and D. Fenske, Catal. Commun., 2017, 94, 23 CrossRef CAS.
- S. Ren, S. Xie, T. Zheng, Y. Wang, S. Xu, B. Xue, X. Li, H. Sun, O. Fuhr and D. Fenske, Dalton Trans., 2018, 47, 4352 RSC.
- Y. Wang, S. Ren, W. Zhang, B. Xue, X. Qi, H. Sun, X. Li, O. Fuhr and D. Fenske, Catal. Commun., 2018, 115, 1 CrossRef CAS.
- L. C. Misal Castro, D. Bézier, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2011, 353, 1279 CrossRef.
- F. S. Wekesa, R. Arias-Ugarte, L. Kong, Z. Sumner, G. P. McGovern and M. Findlater, Organometallics, 2015, 34, 5051 CrossRef CAS.
- K. Zhu, M. P. Shaver and S. P. Thomas, Eur. J. Org. Chem., 2015, 2119 CrossRef CAS.
- M. A. Nesbit, D. L. Suess and J. C. Peters, Organometallics, 2015, 34, 4741 CrossRef CAS.
- Á. Raya-Barón, M. A. Ortuño, P. Oña-Burgos, A. Rodríguez-Diéguez, R. Langer, C. J. Cramer, I. Kuzu and I. Fernández, Organometallics, 2016, 35, 4083 CrossRef.
- Á. Raya-Barón, C. P. Galdeano-Ruano, P. Oña-Burgos, A. Rodríguez-Diéguez, R. Langer, R. López-Ruiz, R. Romero-González, I. Kuzu and I. Fernández, Dalton Trans., 2018, 47, 7272 RSC.
- L. C. Misal Castro, J.-B. Sortais and C. Darcel, Chem. Commun., 2012, 48, 151 RSC.
- M. Bhunia, P. K. Hota, G. Vijaykumar, D. Adhikari and S. K. Mandal, Organometallics, 2016, 35, 2930 CrossRef CAS.
- S. Enthaler, ChemCatChem, 2010, 2, 1411 CrossRef CAS.
- H. Jaafar, H. Li, L. C. Misal Castro, J. Zheng, T. Roisnel, V. Dorcet, J. B. Sortais and C. Darcel, Eur. J. Inorg. Chem., 2012, 3546 CrossRef CAS.
- H. Li, M. Achard, C. Bruneau, J.-B. Sortais and C. Darcel, RSC Adv., 2014, 4, 25892 RSC.
- Y. Sunada, H. Kawakami, T. Imaoka, Y. Motoyama and H. Nagashima, Angew. Chem., Int. Ed., 2009, 48, 9511 CrossRef CAS.
- K. Junge, B. Wendt, N. Shaikh and M. Beller, Chem. Commun., 2010, 46, 1769 RSC.
- L. Pehlivan, E. Métay, S. Laval, W. Dayoub, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron Lett., 2010, 51, 1939 CrossRef CAS.
- L. Pehlivan, E. Métay, S. Laval, W. Dayoub, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron, 2011, 67, 1971 CrossRef CAS.
- K. Zhu, M. P. Shaver and S. P. Thomas, Chem. Sci., 2016, 7, 3031 RSC.
- J. Gui, C.-M. Pan, Y. Jin, T. Qin, J. C. Lo, B. J. Lee, S. H. Spergel, M. E. Mertzman, W. J. Pitts and T. E. La Cruz, Science, 2015, 348, 886 CrossRef CAS.
- M. Shevlin, X. Guan and T. G. Driver, ACS Catal., 2017, 7, 5518 CrossRef CAS.
- S. Werkmeister, K. Junge and M. Beller, Org. Process Res. Dev., 2014, 18, 289 CrossRef CAS.
- B. Xue, H. Sun, Y. Wang, T. Zheng, X. Li, O. Fuhr and D. Fenske, Catal. Commun., 2016, 86, 148 CrossRef CAS.
- S. Zhou, D. Addis, S. Das, K. Junge and M. Beller, Chem. Commun., 2009, 4883–4885 RSC.
- D. Bezier, G. T. Venkanna, J.-B. Sortais and C. Darcel, ChemCatChem, 2011, 3, 1747 CrossRef CAS.
- S. Zhou, K. Junge, D. Addis, S. Das and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 9507 CrossRef CAS.
- H. Tsutsumi, Y. Sunada and H. Nagashima, Chem. Commun., 2011, 47, 6581 RSC.
- D. Bezier, G. T. Venkanna, J.-B. Sortais and C. Darcel, ChemCatChem, 2011, 3, 1747 CrossRef CAS.
- A. Volkov, E. Buitrago and H. Adolfsson, Eur. J. Org. Chem., 2013, 2066 CrossRef CAS.
- B. Blom, G. Tan, S. Enthaler, S. Inoue, J. D. Epping and M. Driess, J. Am. Chem. Soc., 2013, 135, 18108 CrossRef CAS.
- D. J. Hale, L. J. Murphy, R. McDonald, M. J. Ferguson and L. Turculet, ChemCatChem, 2019, 11, 3818 CrossRef CAS.
- C. M. Macaulay, T. Ogawa, R. McDonald, O. L. Sydora, M. Stradiotto and L. Turculet, Dalton Trans., 2019, 48, 9581 RSC.
- S. Das, B. Wendt, K. Möller, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 1662–1666 CrossRef CAS.
- M. Ito, M. Itazaki and H. Nakazawa, ChemCatChem, 2016, 8, 3323 CrossRef CAS.
- S. Das, H. S. Das, B. Singh, R. K. Haridasan, A. Das and S. K. Mandal, Inorg. Chem., 2019, 58(17), 11274 CrossRef CAS.
- D. Bézier, G. T. Venkanna, L. C. Misal Castro, J. Zheng, T. Roisnel, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2012, 354, 1879 CrossRef.
- K. Junge, B. Wendt, S. Zhou and M. Beller, Eur. J. Org. Chem., 2013, 2061 CrossRef CAS.
- S. Das, Y. Li, K. Junge and M. Beller, Chem. Commun., 2012, 48, 10742 RSC.
- S. Quintero-Duque, H. Li, L. C. Misal Castro, V. Dorcet, T. Roisnel, E. Clot, M. Grellier, J.-B. Sortais and C. Darcel, Isr. J. Chem., 2017, 57, 1216 CrossRef CAS.
- H. Li, L. C. Misal Castro, J. Zheng, T. Roisnel, V. Dorcet, J.-B. Sortais and C. Darcel, Angew. Chem., Int. Ed., 2013, 52, 8045 CrossRef CAS.
- C. Cong, T. Fujihara, J. Terao and Y. Tsuji, Catal. Commun., 2014, 50, 25 CrossRef CAS.
- L. C. Misal Castro, H. Li, J.-B. Sortais and C. Darcel, Chem. Commun., 2012, 48, 10514 RSC.
- J. Pouessel, O. Jacquet and T. Cantat, ChemCatChem, 2013, 5, 3552 CrossRef CAS.
- X. Frogneux, O. Jacquet and T. Cantat, Catal. Sci. Technol., 2014, 4, 1529 RSC.
- S. Enthaler, ChemCatChem, 2011, 3, 666 CrossRef CAS.
- J. M. Cardoso and B. Royo, Chem. Commun., 2012, 48, 4944 RSC.
- (a) K. Burgess and M. J. Ohlmeyer, Chem. Rev., 1991, 91, 1179–1191 CrossRef CAS; (b) J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992–2002 RSC; (c) N. Miyaura, Hydroboration, Diboration, Silylboration, and Stannylboration, in Catalytic Heterofunctionalization, ed. A. Togni and H. Grützmacher, Wiley-VCH Verlag GmbH, Weinheim, 2001, pp. 1–45 Search PubMed; (d) I. Mkhalid, J. Barnard, T. Marder, J. Murphy and J. Hartwig, Chem. Rev., 2010, 110, 890–931 CrossRef CAS.
- (a) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS; (b) S. R. Chemier, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544 CrossRef.
- (a) H. Doucet, Eur. J. Org. Chem., 2008, 2013–2030 CrossRef CAS; (b) D. G. Hall, Boronic Acids: Preparation, Applications in OrganicSynthesis and Medicine, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, 2006 Search PubMed; (c) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS; (d) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; S. P. Thomas and V. K. Aggarwal, Angew. Chem., Int. Ed., 2009, 48, 1896 Search PubMed; (e) A. M. Carroll, T. P. O’Sullivan and P. J. Guiry, Adv. Synth. Catal., 2005, 347, 609 CrossRef CAS; (f) C. M. Crudden and D. Edwards, Eur. J. Org. Chem., 2003, 4695 CrossRef CAS; (g) K. Burgess and M. J. Ohlmeyer, Chem. Rev., 1991, 91, 1179 CrossRef CAS.
- (a) K. Oshima, T. Ohmura and M. Suginome, J. Am. Chem. Soc., 2012, 134, 3699 CrossRef CAS; (b) J. Cid, J. J. Carbo and E. Fernandez, Eur. J. Chem., 2012, 18, 1512 CrossRef CAS; (c) W. J. Fleming, H. Bunz-Müller and P. J. Guiry, Eur. J. Inorg. Chem., 2010, 5996 CrossRef CAS; (d) S. M. Smith and J. M. Takacs, Org. Lett., 2010, 12, 4612 CrossRef CAS; (e) S. M. Smith and J. M. Takacs, J. Am. Chem. Soc., 2010, 132, 1740 CrossRef CAS; (f) C. J. Lata and C. M. Crudden, J. Am. Chem. Soc., 2010, 132, 131 CrossRef CAS; (g) B. Nguyen and J. M. Brown, Adv. Synth. Catal., 2009, 351, 1333 CAS; (h) C. M. Crudden, Y. B. Hleba and A. C. Chen, J. Am. Chem. Soc., 2004, 126, 9200 CAS; (i) C. M. Crudden and D. Edwards, Eur. J. Org. Chem., 2003, 4695 CrossRef CAS; (j) S. Pereira and M. Srebnik, J. Am. Chem. Soc., 1996, 118, 909 CrossRef CAS; (k) D. A. Evans, G. C. Fu and A. H. Hoveyda, J. Am. Chem. Soc., 1992, 114, 6671 CrossRef CAS; (l) C. E. Tucker, J. Davidson and P. J. Knochel, Org. Chem., 1992, 57, 3482 CrossRef CAS; (m) D. Männing and H. Nöth, Angew. Chem., Int. Ed. Engl., 1985, 24, 879 ( Angew. Chem. , 1985 , 97 , 854 ) CrossRef; (n) G. M. Lee, C. M. Vogels, A. D. Decken and S. A. Westcott, Eur. J. Inorg. Chem., 2011, 2433 CrossRef CAS; (o) N. M. Hunter, C. M. Vogels, A. Decken, A. Bell and S. A. Westcott, Inorg. Chim. Acta, 2011, 365, 408 CrossRef CAS; (p) C. Mazet and D. Gerard, Chem. Commun., 2011, 47, 298 RSC; (q) J. A. Melanson, C. M. Vogels, A. Decken and S. A. Westcott, Inorg. Chem. Commun., 2010, 13, 1396 CrossRef CAS; (r) J. Chipot, C. M. Vogels, R. McDonald, S. A. Westcott and M. Stradiotto, Organometallics, 2006, 25, 5965 CrossRef; (s) A. Black, J. M. Brown and C. Pichon, Chem. Commun., 2005, 5284 RSC; (t) Y. Yamamoto, R. Fujikawa, T. Umemoto and N. Miyaura, Tetrahedron, 2004, 60, 10695 CrossRef CAS; (u) A. P. Luna, M. Bonin, L. Micouin and H. P. Husson, J. Am. Chem. Soc., 2002, 124, 12098 CrossRef; (v) J. A. Brinkman, T. T. Nguyen and J. R. Sowa, Org. Lett., 2000, 2, 981 CrossRef CAS; (w) A. H. Hoveyda, D. A. Evans and G. C. Fu, Chem. Rev., 1993, 93, 1307 CrossRef CAS.
- J. Y. Wu, B. Moreau and T. Ritter, J. Am. Chem. Soc., 2009, 131, 12915–12917 CrossRef CAS.
- X. Chen, Z. Cheng and Z. Lu, Org. Lett., 2017, 19, 969–971 CrossRef CAS.
- J. Chen, T. Xi and Z. Lu, Org. Lett., 2014, 16, 6452–6455 CAS.
- C. Chen, X. Shen, J. Chen, X. Hong and Z. Lu, Org. Lett., 2017, 19, 5422–5425 CAS.
- J. F. Hartwig and S. Huber, J. Am. Chem. Soc., 1993, 115, 4908–4909 CrossRef CAS.
- K. M. Waltz, X. He, C. Muhoro and J. F. Hartwig, J. Am. Chem. Soc., 1995, 117, 11357–11358 CrossRef CAS.
- Y. Cao, Y. Zhang, L. Zhang, D. Zhang, X. Leng and Z. Huang, Org. Chem. Front., 2014, 1, 1101–1106 RSC.
- (a) M. W. Bouwkamp, A. C. Bowman, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2006, 128, 13340 CrossRef CAS; (b) K. Kamata, A. Suzuki, Y. Nakaiand and H. Nakazawa, Organometallics, 2012, 31, 3825 CrossRef CAS.
- L. Zhang, D. Peng, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2013, 125, 3764–3768 CrossRef.
- E. Balaraman, B. Gnanaprakasam, L. J. W. Shimon and D. Milstein, J. Am. Chem. Soc., 2010, 132, 16756 CrossRef CAS.
- J. V. Obligacíon and P. J. Chirik, Org. Lett., 2013, 15, 2680–2683 CrossRef.
- M. D. Greenhalgh and S. P. Thomas, Chem. Commun., 2013, 49, 11230–11232 RSC.
- J. Zheng, J.-B. Sortais and C. Darcel, ChemCatChem, 2014, 6, 763–766 CrossRef CAS.
- K.-N. T. Tseng, J. W. Kampf and N. K. Szymczak, ACS Catal., 2015, 5, 411–415 CrossRef CAS.
- R. Gilbert-Wilson, W.-Y. Chu and T. B. Rauchfuss, Inorg. Chem., 2015, 54, 5596–5603 CrossRef CAS.
- M. Espinal-Viguri, C. R. Woof and R. L. Webster, Chem. – Eur. J., 2016, 22, 11605–11608 CrossRef CAS.
- T. Ogawa, A. J. Ruddy, O. L. Sydora, M. Stradiotto and L. Turculet, Organometallics, 2017, 36, 417–423 CrossRef CAS.
- Y. Liu, Y. Zhou, H. Wang and J. Qu, RSC Adv., 2015, 5, 73705–73713 RSC.
- A. J. MacNair, C. R. Millet, G. S. Nichol, A. Ironmonger and S. P. Thomas, ACS Catal., 2016, 6, 7217–7221 CrossRef CAS.
- M. Haberberger and S. Enthaler, Chem. – Asian J., 2013, 8, 50–54 CrossRef CAS.
- V. S. Rawat and B. Sreedhar, Synlett, 2014, 1132–1136 Search PubMed.
- K. Nakajima, T. Kato and Y. Nishibayashi, Org. Lett., 2017, 19, 4323–4326 CrossRef CAS.
- N. Gorgas, L. G. Alves, B. Stöger, A. M. Martins, L. F. Veiros and K. Kirchner, J. Am. Chem. Soc., 2017, 139, 8130–8133 CrossRef CAS.
- (a) C. Gunanathan, M. Hölscher, F. Pan and W. Leitner, J. Am. Chem. Soc., 2012, 134, 14349–14352 CrossRef CAS; (b) T. Ohmura, Y. Yamamoto and N. Miyaura, J. Am. Chem. Soc., 2000, 122, 4990–4991 CrossRef CAS.
- A. Singh, S. Shafiei-Haghighi, C. R. Smith, D. K. Unruh and M. Findlater, Asian J. Org. Chem., 2018, 47, 9231–9236 Search PubMed.
- F. Rami, F. Bächtleand and B. J. Plietker, Catal. Sci. Technol., 2020, 10, 1492–1497 RSC.
- F. Zhang, H. Song, X. Zhuang, C.-H. Tung and W. Wang, J. Am. Chem. Soc., 2017, 139, 17775–17778 CrossRef CAS.
- S. Bagherzadeh and N. P. Mankad, Chem. Commun., 2016, 52, 3844–3846 RSC.
- G. Zhang, H. Zeng, J. Wu, Z. Yin, S. Zheng and J. C. Fettinger, Angew. Chem., Int. Ed., 2016, 128, 14581–14584 CrossRef.
- (a) C. W. Lindsley and M. DiMare, Tetrahedron Lett., 1994, 35, 5141–5144 CrossRef CAS; (b) G. Giffels, C. Dreisbach, U. Kragl, M. Weigerding, H. Waldmann and C. Wandrey, Angew. Chem., Int. Ed. Engl., 1995, 34, 2005–2006 CrossRef CAS; (c) F. Almqvist, L. Torstensson, A. Gudmundsson and T. Frejd, Angew. Chem., Int. Ed. Engl., 1997, 36, 376–377 CrossRef CAS; (d) I. Sarvary, F. Almqvist and T. Frejd, Chem. – Eur. J., 2001, 7, 2158–2166 CrossRef CAS; (e) A. A. Oluyadi, S. Ma and C. N. Muhoro, Organometallics, 2013, 32, 70–78 CrossRef CAS.
- (a) M. Bandini, P. G. Cozzi, M. de Angelis and A. Umani-Ronchi, Tetrahedron Lett., 2000, 41, 1601–1605 CrossRef CAS; (b) S.-G. Roh, Y.-C. Park, D.-K. Park, T.-J. Kim and J. H. Jeong, Polyhedron, 2001, 20, 1961–1965 CrossRef CAS; (c) M. Locatelli and P. G. Cozzi, Angew. Chem., Int. Ed., 2003, 42, 4928–4930 CrossRef CAS; (d) L. M. Fleury and B. L. Ashfeld, Org. Lett., 2009, 11, 5670–5673 CrossRef CAS; (e) P. A. Lummis, M. R. Momeni, M. W. Lui, R. McDonald, M. J. Ferguson, M. Miskolzie, A. Brown and E. Rivard, Angew. Chem., Int. Ed., 2014, 53, 9347–9351 CrossRef CAS.
- S. Yadav, S. Pahar and S. S. Sen, Chem. Commun., 2017, 53, 4562–4564 RSC.
- (a) M. Arrowsmith, T. J. Hadlington, M. S. Hill and G. Kociok-Kohn, Chem. Commun., 2012, 48, 4567–4569 RSC; (b) L. Fohlmeister and A. Stasch, Chem. – Eur. J., 2016, 22, 10235–10246 CrossRef CAS; (c) K. Manna, P. Ji, F. X. Greene and W. Lin, J. Am. Chem. Soc., 2016, 138, 7488–7491 CrossRef CAS; (d) D. Mukherjee, H. Osseili, T. P. Spaniol and J. Okuda, J. Am. Chem. Soc., 2016, 138, 10790–10793 CrossRef CAS; (e) D. Mukherjee, S. Shirase, T. P. Spaniol, K. Mashima and J. Okuda, Chem. Commun., 2016, 52, 13155–13158 RSC; (f) H. Osseili, D. Mukherjee, K. Beckerle, T. P. Spaniol and J. Okuda, Organometallics, 2017, 36, 3029–3034 CrossRef CAS.
- (a) Z. Yang, M. Zhong, X. Ma, S. De, C. Anusha, P. Parameswaran and H. W. Roesky, Angew. Chem., Int. Ed., 2015, 54, 10225–10229 CrossRef CAS; (b) V. K. Jakhar, M. K. Barman and S. Nembenna, Org. Lett., 2016, 18, 4710–4713 CrossRef CAS.
- T. J. Hadlington, M. Hermann, G. Frenking and C. Jones, J. Am. Chem. Soc., 2014, 136, 3028–3031 CrossRef CAS.
- C. C. Chong, H. Hirao and R. Kinjo, Angew. Chem., Int. Ed., 2015, 54, 190–194 CrossRef CAS.
- A. J. Blake, A. Cunningham, A. Ford, S. J. Teat and S. Woodward, Chem. – Eur. J., 2000, 6, 3586–3594 CrossRef CAS.
- Y. Wu, C. Shan, Y. Sun, P. Chen, J. Ying, J. Zhu, L. Liu and Y. Zhao, Chem. Commun., 2016, 52, 13799–13802 RSC.
- S. Chen, D. Yan, M. Xue, Y. Hong, Y. Yao and Q. Shen, Org. Lett., 2017, 19, 3382–3385 CrossRef CAS.
- S. R. Tamang and M. Findlater, J. Org. Chem., 2017, 82, 12857–12862 CrossRef CAS.
- T. Bai, T. Janes and D. Song, Dalton Trans., 2017, 46, 12408–12412 RSC.
- J. Liu, A. Zhang, H. Song, Q. Tong, C.-H. Tung and W. Wang, Chin. Chem. Lett., 2018, 29, 949–953 CrossRef CAS.
- U. K. Das, C. S. Higman, B. Gabidullin, J. E. Hein and R. T. Baker, ACS Catal., 2018, 8, 1076–1081 CrossRef CAS.
- A. Baisha, S. Baruah and K. Geerthani, Dalton Trans., 2018, 47, 9231–9236 RSC.
- D. Wei, B. Carboni, J.-B. Sortais and C. Darcel, Adv. Synth. Catal., 2018, 360, 3649 CrossRef CAS.
- C. K. Blasius, V. Vasilenko and L. H. Gade, Angew. Chem., Int. Ed., 2018, 57, 10231–10235 CrossRef CAS.
- E. J. Corey and G. A. Reichard, Tetrahedron Lett., 1989, 30, 5207–5210 CrossRef CAS.
- C. Wang, C. Wu and S. Ge, ACS Catal., 2016, 6, 7585–7589 CrossRef CAS.
- S. Jiang, S. Quintero-Duque, T. Roisnel, V. Dorcet, M. Grellier, S. Sabo-Etienne, C. Darcel and J.-B. Sortais, Dalton Trans., 2016, 45, 11101–11108 RSC.
- M. Nakamura, A. Hirai and E. Nakamura, J. Am. Chem. Soc., 2000, 122, 978–979 CrossRef CAS.
- D. Necas, P. Drabina, M. Sedlak and M. Kotora, Tetrahedron Lett., 2007, 48, 4539–4541 CrossRef CAS.
- Y. Wang, E. A. F. Fordyce, F. Y. Chen and H. W. Lam, Angew. Chem., Int. Ed., 2008, 47, 7350–7353 CrossRef.
- S. Ito, T. Itoh and M. Nakamura, Angew. Chem., Int. Ed., 2011, 50, 454–457 CrossRef CAS.
- E. Shirakawa, T. Yamagami, T. Kimura, S. Yamaguchi and T. Hayashi, J. Am. Chem. Soc., 2005, 127, 17164–17165 CrossRef CAS.
- T. Yamagami, R. Shintani, E. Shirakawa and T. Hayashi, Org. Lett., 2007, 9, 1045–1048 CrossRef CAS.
- D. Zhang and J. M. Ready, J. Am. Chem. Soc., 2006, 128, 15050–15051 CrossRef CAS.
- E. Shirakawa, D. Ikeda, T. Ozawa, S. Watanabe and T. Hayashi, Chem. Commun., 2009, 1885–1887 RSC.
- L. Ilies, T. Yoshida and E. Nakamura, Synlett, 2014, 527–530 CAS.
- M. Hojo, Y. Murakami, H. Aihara, R. Sakuragi, Y. Baba and A. Hosomi, Angew. Chem., Int. Ed., 2001, 40, 621–623 CrossRef CAS.
- E. Shirakawa, S. Masui, R. Narui, R. Watabe, D. Ikeda and T. Hayashi, Chem. Commun., 2011, 47, 9714–9716 RSC.
- E. Shirakawa, D. Ikeda, S. Masui, M. Yoshida and T. Hayashi, J. Am. Chem. Soc., 2012, 134, 272–279 CrossRef CAS.
- T. Hata, S. Sujaku, N. Hirone, K. Nakano, J. Imoto, H. Imade and H. Urabe, Chem. – Eur. J., 2011, 17, 14593–14602 CrossRef CAS.
- A. Lin, Z.-W. Zhang and J. Yang, Org. Lett., 2014, 16, 386–389 CrossRef CAS.
- Z. Lu, G. Chai and S. Ma, J. Am. Chem. Soc., 2007, 129, 14546–14547 CrossRef CAS.
- Z. Lu, G. Chai, X. Zhang and S. Ma, Org. Lett., 2008, 10, 3517–3520 CrossRef CAS.
- G. Chai, R. Zeng, C. Fu and S. Ma, Eur. J. Org. Chem., 2013, 148–154 CrossRef CAS.
- J. Kischel, D. Michalik, A. Zapf and M. Beller, Chem. – Asian J., 2007, 2, 909–914 CrossRef CAS.
- C. D. Zotto, J. Michaux, A. Zarate-Ruiz, E. Gayon, D. Virieux, J.-M. Campagne, V. Terrasson, G. Pieters, A. Gaucher and D. Prim, J. Organomet. Chem., 2011, 696, 296–304 CrossRef.
- B. Moreau, J. Y. Wu and T. Ritter, Org. Lett., 2009, 11, 337–339 CrossRef CAS.
- (a) K. T. Sylvester and P. J. Chirik, J. Am. Chem. Soc., 2009, 131, 8772–8774 CrossRef CAS; (b) J. M. Hoyt, K. T. Sylvester, S. P. Semproni and P. J. Chirik, J. Am. Chem. Soc., 2013, 135, 4862–4877 CrossRef CAS.
- S.-Y. Zhang, Y.-Q. Tu, C.-A. Fan, F.-M. Zhang and L. Shi, Angew. Chem., Int. Ed., 2009, 48, 8761–8765 CrossRef CAS.
- J. R. Cabrero-Antonino, A. Leyva-Parez and A. Corma, Adv. Synth. Catal., 2010, 352, 1571–1576 CrossRef CAS.
- Q. Yang, P. Wu, J. Chen and Z. Yu, Chem. Commun., 2014, 50, 6337–6339 RSC.
- A. Ekomie, G. Lefevre, L. Fensterbank, E. Lacote, M. Malacria, C. Ollivier and A. Jutand, Angew. Chem., Int. Ed., 2012, 51, 6942–6946 CrossRef CAS.
- K. Kohno, K. Nakagawa, T. Yahagi, J.-C. Choi, H. Yasuda and T. Sakakura, J. Am. Chem. Soc., 2009, 131, 2784–2785 CrossRef CAS.
- G. C. Midya, S. Paladhi, K. Dhara and J. Dash, Chem. Commun., 2011, 47, 6698–6700 RSC.
- G. C. Midya, B. Parasar, K. Dharab and J. Dash, Org. Biomol. Chem., 2014, 12, 1812–1822 RSC.
- H. Li, W. Li, W. Liu, Z. He and Z. Li, Angew. Chem., Int. Ed., 2011, 50, 2975–2978 CrossRef CAS.
- Y. Chen, K. Li, X. Liu, J. Zhu and B. Chen, Synlett, 2013, 130–134 Search PubMed.
- W.-T. Wei, M.-B. Zhou, J.-H. Fan, W. Liu, R.-J. Song, Y. Liu, M. Hu, P. Xie and J.-H. Li, Angew. Chem., Int. Ed., 2013, 52, 3638–3641 CrossRef CAS.
- J.-H. Fan, M.-B. Zhou, Y. Liu, W.-T. Wei, X.-H. Ouyang, R.-J. Song and J.-H. Li, Synlett, 2014, 657–660 CAS.
- Q. Dai, J. Yu, Y. Jiang, S. Guo, H. Yanga and J. Cheng, Chem. Commun., 2014, 50, 3865–3867 RSC.
- X.-H. Ouyang, R.-J. Song and J.-H. Li, Eur. J. Org. Chem., 2014, 3395–3401 CrossRef CAS.
- J.-Y. Wang, X. Zhang, Y. Bao, Y.-M. Xu, X.-F. Cheng and X.-S. Wang, Org. Biomol. Chem., 2014, 12, 5582–5585 RSC.
- P. Liu, Y. Li, H. Wang, Z. Wang and X. Hu, Tetrahedron Lett., 2012, 53, 6654–6656 CrossRef CAS.
- Z. Liu, J. Wang, Y. Zhao and B. Zhou, Adv. Synth. Catal., 2009, 351, 371–374 CrossRef CAS.
- W. Du, L. Tian, J. Lai, X. Huo, X. Xie, X. She and S. Tang, Org. Lett., 2014, 16, 2470–2473 CrossRef CAS.
- T. Xu, C. W. Cheung and X. Hu, Angew. Chem., Int. Ed., 2014, 53, 4910–4914 CrossRef CAS.
- B. Wang, S. Wang, P. Li and L. Wang, Chem. Commun., 2010, 46, 5891–5893 RSC.
- P. Gandeepan, K. Parthasarathy, T.-H. Su and C.-H. Cheng, Adv. Synth. Catal., 2012, 354, 457–468 CrossRef CAS.
- W. Liu, Y. Li, K. Liu and Z. Li, J. Am. Chem. Soc., 2011, 133, 10756–10759 CrossRef CAS.
- L. X. Alvarez, E. V. Kudrik and A. B. Sorokin, Chem. – Eur. J., 2011, 17, 9298–9301 CrossRef CAS.
- A. P. Dieskau, M. S. Holzwarth and B. Plietker, Chem. – Eur. J., 2012, 18, 2423–2429 CrossRef CAS.
- S. Rommel, A. P. Dieskau and B. Plietker, Eur. J. Org. Chem., 2013, 1790–1795 CrossRef CAS.
- U. A. Kshirsagar, C. Regev, R. Parnesu and D. Pappo, Org. Lett., 2013, 15, 3174–3177 CrossRef CAS.
- Z. Huang, L. Jin, Y. Feng, P. Peng, H. Yi and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 7151–7155 CrossRef CAS.
- B. Roy, I. Ansary, S. Samanta and K. C. Majumdar, Tetrahedron Lett., 2012, 53, 5119–5122 CrossRef CAS.
- K. C. Majumdar, S. Ponra and T. Ghosh, Synthesis, 2013, 3164–3172 CrossRef CAS.
- T. Shen, Y. Yuan, S. Songa and N. Jiao, Chem. Commun., 2014, 50, 4115–4118 RSC.
- Z.-J. Yang, B.-L. Hu, C.-L. Deng and X.-G. Zhang, Adv. Synth. Catal., 2014, 356, 1962–1966 CrossRef CAS.
- S. Liu, L. Tang, H. Chen, F. Zhao and G.-J. Deng, Org. Biomol. Chem., 2014, 12, 6076–6079 RSC.
- K. M. Driller, H. Klein, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 6041–6044 CrossRef CAS.
- S. Prateeptongkum, K. M. Driller, R. Jackstell, A. Spannenberg and M. Beller, Chem. – Eur. J., 2010, 16, 9606–9615 CrossRef CAS.
- S. Prateeptongkum, K. M. Driller, R. Jackstell and M. Beller, Chem. – Asian J., 2010, 5, 2173–2176 CrossRef CAS.
- K. M. Driller, S. Prateeptongkum, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 537–541 CrossRef CAS.
- T. Taniguchi, Y. Sugiura, H. Zaimoku and H. Ishibashi, Angew. Chem., Int. Ed., 2010, 49, 10154–10157 CrossRef CAS.
- M. D. Greenhalgh and S. P. Thomas, J. Am. Chem. Soc., 2012, 134, 11900–11903 CrossRef CAS.
- X. Xu, Y. Tang, X. Li, G. Hong, M. Fang and X. Du, J. Org. Chem., 2014, 79, 446–451 CrossRef CAS.
- G. Wang, S. Wang, J. Wang, S.-Y. Chen and X.-Q. Yu, Tetrahedron, 2014, 70, 3466–3470 CrossRef CAS.
- C. Pan, J. Han, H. Zhang and C. Zhu, J. Org. Chem., 2014, 79, 5374–5378 CrossRef CAS.
- H. Yamashita, Bull. Chem. Soc. Jpn., 1988, 61, 1213–1220 CrossRef CAS.
- N. Iranpoor and P. Salehi, Synthesis, 1994, 1152–1154 CrossRef CAS.
- N. Iranpoor, T. Tarrian and Z. Movahedi, Synthesis, 1996, 1473–1475 CrossRef CAS.
- B. Plancq and T. Ollevier, Chem. Commun., 2012, 48, 3806–3808 RSC.
- B. Plancq and T. Ollevier, Aust. J. Chem., 2012, 65(12), 1564–1572 CrossRef CAS.
- A. Marti, L. Richter and C. Schneider, Synlett, 2011, 2513–2516 CAS.
- Y. Zhang, M. Wang, P. Li and L. Wang, Org. Lett., 2012, 14, 2206–2209 CrossRef CAS.
- A. Furstner and M. Mendez, Angew. Chem., Int. Ed., 2003, 42, 5355–5357 CrossRef.
- O. Lepage, E. Kattnig and A. Furstner, J. Am. Chem. Soc., 2004, 126, 15970–15971 CrossRef CAS.
- A. Furstner, E. Kattnig and O. Lepage, J. Am. Chem. Soc., 2006, 128, 9194–9204 CrossRef.
- B. D. Sherry and A. Furstner, Chem. Commun., 2009, 7116–7118 RSC.
- A. P. Dieskau, M. S. Holzwarth and B. Plietker, J. Am. Chem. Soc., 2012, 134, 5048–5051 CrossRef CAS.
- T. Yano, T. Fujishima and R. Irie, Synthesis, 2010, 818–822 CAS.
- Y. Sawama, K. Shibata, Y. Sawama, M. Takubo, Y. Monguchi, N. Krause and H. Sajiki, Org. Lett., 2013, 15, 5282–5285 CrossRef CAS.
- G. Hilt, P. Bolze and I. Kieltsch, Chem. Commun., 2005, 1996–1998 RSC.
- G. Hilt, C. Walter and P. Bolze, Adv. Synth. Catal., 2006, 348, 1241–1247 CrossRef CAS.
- G. Hilt, P. Bolze and K. Harms, Chem. – Eur. J., 2007, 13, 4312–4325 CrossRef CAS.
- J. Fan, L. Gao and Z. Wang, Chem. Commun., 2009, 5021–5023 RSC.
- H. Wang, W. Yang, H. Liu, W. Wang and H. Li, Org. Biomol. Chem., 2012, 10, 5032–5035 RSC.
- K. S. Williamson and T. P. Yoon, J. Am. Chem. Soc., 2010, 132, 4570–4571 CrossRef CAS.
- K. S. Williamson and T. P. Yoon, J. Am. Chem. Soc., 2012, 134(30), 12370–12373 CrossRef CAS.
- M. Sengoden and T. Punniyamurthy, Angew. Chem., Int. Ed., 2013, 52, 572–575 CrossRef CAS.
- F. Benfatti, F. de Nanteuil and J. Waser, Org. Lett., 2012, 14, 386–389 CrossRef CAS.
- A. Buchard, M. R. Kember, K. G. Sandeman and C. K. Williams, Chem. Commun., 2011, 47, 212–214 RSC.
- J. E. Dengler, M. W. Lehenmeier, S. Klaus, C. E. Anderson, E. Herdtweck and B. Rieger, Eur. J. Inorg. Chem., 2011, 336–343 CrossRef CAS.
- C. J. Whiteoak, E. Martin, M. M. Belmonte, J. Benet-Buchholz and A. W. Kleija, Adv. Synth. Catal., 2012, 354, 469–476 CrossRef CAS.
- C. J. Whiteoak, E. Martin, E. Escudero-Adan and A. W. Kleija, Adv. Synth. Catal., 2013, 355, 2233–2239 CrossRef CAS.
- M. Taherimehr, S. M. Al-Amsyar, C. J. Whiteoak, A. W. Kleij and P. P. Pescarmona, Green Chem., 2013, 15, 3083–3090 RSC.
- M. Sunjuk, A. S. A.-S. E. Al-Ramahi and A. K. Q. A. Saleh, Transition Met. Chem., 2013, 38, 253–257 CrossRef CAS.
- M. A. Fuchs, T. A. Zevaco, E. Ember, O. Walter, I. Held, E. Dinjusa and M. Döring, Dalton Trans., 2013, 42, 5322–5329 RSC.
- J. R. Wolf, C. G. Hamaker, J.-P. Djukic, T. Kodadek and L. K. Woo, J. Am. Chem. Soc., 1995, 117, 9194–9199 CrossRef CAS.
- P. Tagliatesta and A. Pastorini, J. Mol. Catal. A: Chem., 2003, 198, 57–61 CrossRef CAS.
- C. G. Hamaker, G. A. Mirafzal and L. K. Woo, Organometallics, 2001, 20, 5171–5176 CrossRef CAS.
- S. K. Edulji and S. T. Nguyen, Organometallics, 2003, 22, 3374–3381 CrossRef CAS.
- S. K. Edulji and S. T. Nguyen, Pure Appl. Chem., 2004, 76, 645–649 CAS.
- T.-S. Lai, F.-Y. Chan, P.-K. So, D.-L. Ma, K.-Y. Wong and C.-M. Che, Dalton Trans., 2006, 4845–4851 RSC.
- P. Le Maux, S. Juillard and G. Simonneaux, Synthesis, 2006, 1701–1704 CAS.
- I. Nicolas, P. LeMaux and G. Simonneaux, Tetrahedron Lett., 2008, 49, 5793–5795 CrossRef CAS.
- C.-T. Yeung, K.-C. Sham, W.-S. Lee, W.-T. Wong, W.-Y. Wong and H.-L. Kwong, Inorg. Chim. Acta, 2009, 362, 3267–3273 CrossRef CAS.
- D. J. Casper, A. V. Sklyarov, S. Hardcastle, T. L. Barr, F. H. Forsterling, K. F. Surerus and M. M. Hossain, Inorg. Chim. Acta, 2006, 359, 3129–3138 CrossRef CAS.
- F. Wang, Q. Meng and M. Li, Int. J. Quantum Chem., 2007, 108, 945–953 CrossRef.
- B. Morandi and E. M. Carreira, Angew. Chem., Int. Ed., 2010, 49, 938–941 CrossRef CAS.
- B. Morandi, J. Cheang and E. M. Carreira, Org. Lett., 2011, 13, 3080–3081 CrossRef CAS.
- B. Morandi, A. Dolva and E. M. Carreira, Org. Lett., 2012, 14, 2162–2163 CrossRef CAS.
- B. Morandi and E. M. Carreira, Science, 2012, 335, 1471–1474 CrossRef CAS.
- J. Kaschel, T. F. Schneider and D. B. Werz, Angew. Chem., Int. Ed., 2012, 51, 7085–7086 CrossRef CAS.
- D. Intrieri, S. Le Gac, A. Caselli, E. Rose, B. Boitrel and E. Gallo, Chem. Commun., 2014, 50, 1811–1813 RSC.
- D. Mansuy, J.-P. Mahy, A. Dureault, G. Bedi and P. Battioni, J. Chem. Soc., Chem. Commun., 1984, 1161–1163 RSC.
- R. Vyas, G.-Y. Gao, J. D. Harden and X. P. Zhang, Org. Lett., 2004, 6, 1907–1910 CrossRef CAS.
- M. Redlich and M. M. Hossain, Tetrahedron Lett., 2004, 45, 8987–8990 CrossRef CAS.
- (a) K. L. Klotz, L. M. Slominski, A. V. Hull, V. M. Gottsacker, R. Mas-Balleste, L. Que, Jr. and J. A. Halfen, Chem. Commun., 2007, 2063–2065 RSC; (b) K. L. Klotz, L. M. Slominski, M. E. Riemer, J. A. Phillips and J. A. Halfen, Inorg. Chem., 2009, 48, 801–803 CrossRef CAS.
- G. Coin, R. Parta, S. Rana, J. P. Biswas, P. Dubourdeaux, M. Clémancey, S. P. de Visser, D. Maiti, P. Maldivi and J.-M. Latour, ACS Catal., 2020, 10(17), 10010–10020 CrossRef CAS.
- M. Nakanishi, A.-F. Salit and C. Bolm, Adv. Synth. Catal., 2008, 350, 1835–1840 CrossRef CAS.
- A. C. Mayer, A.-F. Salit and C. Bolm, Chem. Commun., 2008, 5975–5977 RSC.
- E. C. Lee, B. L. Hodous, E. Bergin, C. Shih and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 11586–11587 CrossRef CAS.
- M. W. Bouwkamp, A. C. Bowman, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2006, 128, 13340–13341 CrossRef CAS.
- S. K. Russell, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2011, 133, 8858–8861 CrossRef CAS.
- F. de Nanteuil and J. Waser, Angew. Chem., Int. Ed., 2013, 52, 9009–9013 CrossRef CAS.
- H.-J. Knölker, J. Heber and C. H. Mahler, Synlett, 1992, 1002–1004 CrossRef.
- H.-J. Knölker and J. Heber, Synlett, 1993, 924–926 CrossRef.
- J. Blanco-Urgoiti, L. Añorbe, L. Pérez-Serrano, G. Domínguez and J. Pérez-Castells, Chem. Soc. Rev., 2004, 33, 32–42 RSC.
- H.-J. Knölker, J. Prakt. Chem., 1994, 336, 277–279 CrossRef.
- H.-J. Knölker, E. Baum and J. Heber, Tetrahedron Lett., 1995, 36, 1647–1650 Search PubMed.
- H.-J. Knölker, Chem. Soc. Rev., 1999, 28, 151–157 RSC.
- H.-J. Knölker, A. Braier, D. J. Bröcher, S. Cämmerer, W. Fröhner, P. Gonser, H. Hermann, D. Herzberg, K. R. Reddy and G. Rohde, Pure Appl. Chem., 2001, 73, 1075–1086 Search PubMed.
- H. Ohara, H. Kiyokaneb and T. Itoh, Tetrahedron Lett., 2002, 43, 3041–3044 CrossRef CAS.
- A. Badoiu, G. Bernardinelli, J. Mareda, E. P. Kundig and F. Viton, Chem. – Asian J., 2008, 3, 1298–1311 CrossRef CAS.
- J. Bonnamour and C. Bolm, Chem. – Eur. J., 2009, 15, 4543–4545 CrossRef CAS.
- H. Wu, B. Wang, H. Liu and L. Wang, Tetrahedron, 2011, 67, 1210–1215 CrossRef CAS.
- M. Wang, X. Liu, P. He, L. Lin and X. Feng, Chem. Commun., 2013, 49, 2572–2574 RSC.
- N. Saino, D. Kogure and S. Okamoto, Org. Lett., 2005, 7, 3056–3057 CrossRef.
- N. Saino, D. Kogure, K. Kase and S. Okamoto, J. Organomet. Chem., 2006, 691, 3129–3136 CrossRef CAS.
- S. Okamoto and Y.-K. Sugiyama, Synlett, 2013, 1044–1060 CrossRef CAS.
- S. Zhou, D. Addis, S. Das, K. Junge and M. Beller, Chem. Commun., 2009, 4883–4885 RSC.
- S. Enthaler, Eur. J. Org. Chem., 2011, 4760–4763 CAS.
- X. Tonga, M. Li, N. Yanb, Y. Maa, P. J. Dysonb and Y. Li, Catalysis, 2011, 175, 524–527 Search PubMed.
- T. vom Stein, P. M. Grande, W. Leitner and P. D. de Maria, ChemSusChem, 2011, 4, 1592–1594 CrossRef CAS.
- Y.-H. Kim, S. Shin, H.-J. Yoon, J. W. Kim, J. K. Cho and Y.-S. Lee, Catal. Commun., 2013, 40, 18–22 CrossRef CAS.
- S. Maetani, T. Fukuyama, N. Suzuki, D. Ishiharab and I. Ryu, Chem. Commun., 2012, 48, 2552–2554 RSC.
- W. Yan, X. Ye, N. G. Akhmedov, J. L. Petersen and X. Shi, Org. Lett., 2012, 14, 2358–2361 CrossRef CAS.
- G. F. Emerson and R. Pettit, J. Am. Chem. Soc., 1962, 84, 4591–4592 CrossRef CAS.
- R. Damico and T. Logan, J. Org. Chem., 1967, 32, 2356–2358 CrossRef CAS.
- F. G. Cowherd and J. L. Von Rosenberg, J. Am. Chem. Soc., 1969, 91, 2157–2158 CrossRef CAS.
- J. U. Strauss and P. W. Ford, Tetrahedron Lett., 1975, 33, 2917–2918 CrossRef.
- H. Cherkaoui, M. Soufiaoui and R. Grée, Tetrahedron, 2001, 57, 2379–2383 CrossRef CAS.
- C. Crevisy, M. Wietrich, V. Le Boulaire, R. Uma and R. Grée, Tetrahedron Lett., 2001, 42, 395–398 CrossRef CAS.
- V. Branchadell, C. Crevisy and R. Grée, Chem. – Eur. J., 2003, 9, 2062–2067 CrossRef CAS.
- R. Uma, N. Gouault, C. Crevisy and R. Grée, Tetrahedron Lett., 2003, 44, 6187–6190 CrossRef CAS.
- V. Branchadell, C. Crevisy and R. Grée, Chem. – Eur. J., 2004, 10, 5795–5803 CrossRef CAS.
- J. Petrignet, I. Prathap, S. Chandrasekhar, J. S. Yadav and R. Grée, Angew. Chem., Int. Ed., 2007, 46, 6297–6300 CrossRef CAS.
- J. Petrignet, T. Roisnel and R. Grée, Chem. – Eur. J., 2007, 13, 7374–7384 CrossRef CAS.
- D. H. Mac, R. Samineni, A. Sattar, S. Chandrasekhar, J. S. Yadav and R. Grée, Tetrahedron, 2011, 67, 9305–9310 CrossRef CAS.
- D. H. Mac, A. Sattar, S. Chandrasekhar, J. S. Yadav and R. Grée, Tetrahedron, 2012, 68, 8863–8868 CrossRef CAS.
- D. Cahard, V. Bizet, X. Dai, S. Gaillard and J.-L. Renaud, J. Fluorine Chem., 2013, 155, 78–82 CrossRef CAS.
- E. N. Frankel, E. A. Emken and V. L. Davison, J. Am. Oil Chem. Soc., 1996, 43, 307–311 CrossRef.
- E. J. Corey and G. Moinet, J. Am. Chem. Soc., 1973, 95, 7185–7186 CrossRef CAS.
- J. Rodriguez, P. Brun and B. Waegell, Tetrahedron Lett., 1986, 27, 835–836 CrossRef.
- M. R. Reddy and M. Periasamy, J. Organomet. Chem., 1995, 491, 263–266 CrossRef.
- C. P. Casey and C. R. Cyr, J. Am. Chem. Soc., 1973, 95, 2248–2253 CrossRef CAS.
- M. Mayer, A. Welther and A. J. von Wangelin, ChemCatChem, 2011, 3, 1567–1571 CrossRef CAS.
- R. Jennerjahn, R. Jackstell, I. Piras, R. Franke, H. Jiao, M. Bauer and M. Beller, ChemSusChem, 2012, 5, 734–739 CrossRef CAS.
- E. Shirakawa, D. Ikeda, S. Yamaguchi and T. Hayashi, Chem. Commun., 2008, 1214–1216 RSC.
- Á. Gutiérrez-Bonet, A. Flores-Gaspar and R. Martin, J. Am. Chem. Soc., 2013, 135, 12576–12579 CrossRef.
- J. M. Takacs and L. G. Anderson, J. Am. Chem. Soc., 1987, 109, 2200–2202 CrossRef CAS.
- J. M. Takacs, L. G. Anderson, M. W. Creswell and B. E. Takacs, Tetrahedron Lett., 1987, 28, 5627–5630 CrossRef CAS.
- J. M. Takacs, P. W. Newsome and C. Kuehn, Tetrahedron, 1990, 46, 5507–5522 CrossRef CAS.
- J. M. Takacs and Y. C. Myoung, Tetrahedron Lett., 1992, 33, 317–320 CrossRef CAS.
- J. M. Takacs, J. J. Weidner and B. E. Takacs, Tetrahedron Lett., 1993, 34, 6219–6222 CrossRef CAS.
- J. M. Takacs and S. C. Boito, Tetrahedron Lett., 1995, 36, 2941–2944 CrossRef CAS.
- J. M. Takacs, J. J. Weidner, P. W. Newsome, B. E. Takacs, R. Chidambaram and R. Shoemaker, J. Org. Chem., 1995, 60, 3473–3486 CrossRef CAS.
- J. M. Takacs, S. Vayalakkada, S. J. Mehrman and C. L. Kingsbury, Tetrahedron Lett., 2002, 43, 8417–8420 CrossRef CAS.
- A. Fürstner, R. Martin and K. Majima, J. Am. Chem. Soc., 2005, 127, 12236–12237 CrossRef.
- L. Y. Chan, S. Kim, Y. Park and P. H. Lee, J. Org. Chem., 2012, 77, 5239–5244 CrossRef CAS.
- S. Shaw and J. D. White, J. Am. Chem. Soc., 2014, 136, 13578–13581 CrossRef CAS.
- M. Kawatsura, Y. Higuchi, S. Hayase, M. Nanjo and T. Itoh, Synlett, 2008, 1009–1012 CAS.
- M. Fujiwara, M. Kawatsura, S. Hayase, M. Nanjo and T. Itoh, Adv. Synth. Catal., 2009, 351, 123–128 CrossRef CAS.
- M. Sakae, S. Oshitani, C. Ibara, M. Natsuyama, T. Nokami and T. Itoh, Heteroat. Chem., 2014, 25, 482–491 CrossRef CAS.
- K. Yaji and M. Shindo, Tetrahedron Lett., 2010, 51, 5469–5472 CrossRef CAS.
- K. Yaji and M. Shindo, Tetrahedron, 2010, 66, 9808–9813 CrossRef CAS.
- G. Hilt, P. Bolze and I. Kieltsch, Chem. Commun., 2005, 1996–1998 RSC.
- G. Hilt, C. Walter and P. Bolze, Adv. Synth. Catal., 2006, 348, 1241–1247 CrossRef CAS.
- G. Hilt, P. Bolze, M. Heitbaum, K. Hasse, K. Harms and W. Massa, Adv. Synth. Catal., 2007, 349, 2018–2026 CrossRef CAS.
- A. Chen, R. Lin, Q. Liu and N. Jiao, Chem. Commun., 2009, 6842–6844 RSC.
- S. Jana, M. D. Clements, B. K. Sharp and N. Zheng, Org. Lett., 2010, 12, 3736–3739 CrossRef CAS.
- Y. Zheng, C. Yang, D. Zhang-Negrerie, Y. Du and K. Zhao, Tetrahedron Lett., 2003, 54, 6157–6160 CrossRef.
- (a) B. Plietker, in Iron Catalysis in Organic Chemistry, ed. B. Plietker, Wiley-VCH, 2008, 197 CrossRef; (b) M. Jegelka and B. Plietker, Top. Organomet. Chem., 2011, 33, 177 CrossRef CAS; (c) X. J. Shang and Z. Q. Liu, Chin. Sci. Bull., 2012, 57, 2335 CrossRef CAS.
- R. Taylor, Electrophilic Aromatic Substitution, John Wiley & Sons, Chichester, UK, 1990 Search PubMed.
- V. V. Namboodiri and R. S. Varma, Tetrahedron Lett., 2002, 43, 4593 CrossRef CAS.
- J. Kischel, K. Mertins, D. Michalik, A. Zapf and M. Beller, Adv. Synth. Catal., 2007, 349, 865 CrossRef CAS.
- U. Jana, S. Biswas and S. Maiti, Tetrahedron Lett., 2007, 48, 4065 CrossRef CAS.
- P. Moriel and A. B. García, Green Chem., 2014, 15, 4306 RSC.
- C. F. Yang, C. Shen, H. H. Li and S. K. Tian, Chin. Sci. Bull., 2012, 57, 2377 CrossRef CAS.
- U. Jana, S. Biswas and S. Maiti, Eur. J. Org. Chem., 2008, 5798 CrossRef CAS.
- L. R. Jefferies and S. P. Cook, Tetrahedron, 2014, 70, 4204 CrossRef CAS.
- S.-K. Xiang, L.-H. Zhang and N. Jiao, Chem. Commun., 2009, 6487 RSC.
- J. Han, Z. Cui, J. Wang and Z. Liu, Synth. Commun., 2010, 40, 2042 CrossRef CAS.
- Z.-Y. Jiang, C.-H. Zhang, F.-L. Gu, K.-F. Yang, G.-Q. Lai, L.-W. Xu and C.-G. Xia, Synlett, 2010, 1251 CAS.
- C. F. Yang, J.-Y. Wang and S.-K. Tian, Chem. Commun., 2011, 47, 8343 RSC.
- L. Y. Chan, S. Kim, W. T. Chung, C. Long and S. Kim, Synlett, 2011, 415 CAS.
- J. Yang, X. Liu, D.-L. Meng, H.-Y. Chen, Z.-H. Zong, T.-T. Feng and K. Sun, Adv. Synth. Catal., 2012, 354, 328 CrossRef CAS.
- (a) A. J. Rawlings, L. J. Diorazio and M. Wills, Org. Lett., 2015, 17, 1086 CrossRef CAS; (b) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555 CrossRef CAS; (c) G. E. Dobereinerand and R. H. Crabtree, Chem. Rev., 2010, 110, 681–1703 CrossRef; (d) Q. Yang, Q. Wang and Z. Yu, Chem. Soc. Rev., 2015, 44, 2305 RSC.
- A. Macé, F. Tripoteau, Q. Zhao, E. Gayon, E. Vrancken, J.-M. Campagne and B. Carboni, Org. Lett., 2013, 15, 906 CrossRef.
- R. Mazzoni, M. Salmi, S. Zacchini and V. Zanotti, Eur. J. Inorg. Chem., 2013, 3710 CrossRef CAS.
- X. Pan, M. Li and Y. Gu, Chem. – Asian J., 2014, 9, 268 CrossRef CAS.
- X. Fan, X.-M. Cui, Y.-H. Guan, L.-A. Fu, H. Lv, K. Guo and H.-B. Zhu, Eur. J. Org. Chem., 2014, 498 CrossRef CAS.
- U. Jana, S. Maiti and S. Biswas, Tetrahedron Lett., 2008, 49, 858 CrossRef CAS.
- (a) B. Anxionnat, A. Guérinot, S. Reymond and J. Cossy, Tetrahedron Lett., 2009, 50, 3470 CrossRef CAS; (b) J. J. Ritter and P. P. Minieri, J. Am. Chem. Soc., 1948, 70, 4045 CrossRef CAS.
- R. Martínez, D. J. Ramón and M. Yus, Org. Biomol. Chem., 2009, 7, 2176 RSC.
- X. Cui, X. Dai, Y. Deng and F. Shi, Chem. – Eur. J., 2013, 19, 3665 CrossRef CAS.
- M. A. Reddy, P. S. Reddy and B. Sreedhar, Adv. Synth. Catal., 2010, 352, 1861 CrossRef CAS.
- H. Basavaprabhu and V. V. Sureshbabu, Org. Biomol. Chem., 2012, 10, 2528 RSC.
- Y. Sawama, S. Nagata, Y. Yabe, K. Morita, Y. Monguchi and H. Sajiki, Chem. – Eur. J., 2012, 18, 16608 CrossRef CAS.
- Y. Sawama, R. Goto, S. Nagata, Y. Shishido, Y. Monguchi and H. Sajiki, Chem. – Eur. J., 2014, 20, 2631 CrossRef CAS.
- R. Mazzoni, M. Salmi, S. Zacchini and V. Zanotti, Eur. J. Inorg. Chem., 2013, 3710 CrossRef CAS.
- (a) W. Li, X. Zheng and Z. Li, Adv. Synth. Catal., 2013, 355, 181 CrossRef CAS; (b) J. E. M. N. Klein and B. Plietker, Org. Biomol. Chem., 2013, 11, 1271 RSC.
- X. Fan, L.-A. Fu, N. Li, H. Lv, X.-M. Cui and Y. Qi, Org. Biomol. Chem., 2013, 11, 2147 RSC.
- O. S. Nayal, M. S. Thakur, M. Kumar, N. Kumar and S. K. Maurya, Adv. Synth. Catal., 2018, 360(4), 730–737 CrossRef CAS.
- J. Dieter and K. M. Nicholas, J. Organomet. Chem., 1981, 212, 107 CrossRef CAS.
- (a) S. J. Ladoulis and J. Nicholas, J. Organomet. Chem., 1985, 285, C13 CrossRef CAS; (b) G. S. Silverman and S. Nicholas, Organometallics, 1986, 5, 2117 CrossRef CAS.
- (a) J. L. Roustan, J. Y. Mérour and F. Houlihan, Tetrahedron Lett., 1979, 39, 3721 CrossRef; (b) J. L. Roustan, J. Y. Mérour and F. Houlihan, Tetrahedron Lett., 1979, 20, 3721 CrossRef; (c) J.-L. Roustan, M. Abedini and H. H. J. Baer, Organomet. Chem., 1989, 376, C20 CrossRef CAS.
- (a) Y. Xu and B. Zhou, J. Org. Chem., 1987, 52, 974 CrossRef CAS; (b) B. Zhou and Y. Xu, J. Org. Chem., 1988, 53, 4419 CrossRef CAS.
- B. Plietker, Angew. Chem., Int. Ed., 2006, 45, 1469 CrossRef CAS.
- J. E. M. N. Klein, B. Miehlich, M. S. Holzwarth, M. Bauer, M. Milek, M. M. Khusniyarov, G. Knizia, H.-J. Werner and B. Plietker, Angew. Chem., Int. Ed., 2014, 53, 1790 CrossRef CAS.
- B. Plietker, A. Dieskau, K. Möws and A. Jatsch, Angew. Chem., Int. Ed., 2008, 47, 198 CrossRef CAS.
- M. Holzwarth, A. Dieskau, M. Tabassam and B. Plietker, Angew. Chem., Int. Ed., 2009, 48, 7251 CrossRef CAS.
- B. Das, A. Majhi, J. Banerjee, N. Chowdhury and K. Venkateswarlu, Chem. Lett., 2005, 34, 1492 CrossRef CAS.
- C. Yu, A. Zhou and J. He, RSC Adv., 2012, 2, 8627 RSC.
- G. K. Jarugumilli and S. P. Cook, Org. Lett., 2011, 13, 1904 CrossRef CAS.
- D. H. Dethe and G. Murhade, Org. Lett., 2013, 15, 429 CrossRef CAS.
- B. Plietker, Angew. Chem., Int. Ed., 2006, 45, 6053 CrossRef CAS.
- A. Streitwieser, E. G. Jayasree, F. Hasanayn and S. S.-H. Leung, J. Org. Chem., 2008, 73, 9426 CrossRef CAS.
- M. Jegelka and B. Plietker, Org. Lett., 2009, 11, 3462 CrossRef CAS.
- A. Guérinot, A. Serra-Muns, C. Gnamm, C. Bensoussan, S. Reymond and J. Cossy, Org. Lett., 2010, 12, 1808 CrossRef.
- A. Guérinot, A. Serra-Muns, C. Bensoussan, S. Reymond and J. Cossy, Tetrahedron, 2011, 67, 5024 CrossRef.
- J. Cornil, A. Guérinot, S. Reymond and J. Cossy, J. Org. Chem., 2013, 78, 10273 CrossRef CAS.
- M. S. Holzwarth, W. Frey and B. Plietker, Chem. Commun., 2011, 47, 11113 RSC.
- (a) M. Jegelka and B. Plietker, Chem. – Eur. J., 2011, 17, 10417 CrossRef CAS; (b) M. Jegelka and B. Plietker, ChemCatChem, 2012, 4, 329 CrossRef CAS.
- R. Trivedi and J. A. Tunge, Org. Lett., 2009, 11, 5650 CrossRef CAS.
- Z. Wang, S. Li, B. Yu, H. Wu, Y. Wang and X. J. Sun, Org. Chem., 2012, 77, 8615 CrossRef CAS.
- J. E. M. N. Klein, M. S. Holzwarth, S. Hohloch, B. Sarkar and B. Plietker, Eur. J. Org. Chem., 2013, 6310 CrossRef CAS.
- P. Trillo, A. Baeza and C. Nájera, Eur. J. Org. Chem., 2012, 2929 CrossRef CAS.
- P. Trillo and I. M. Pastor, Adv. Synth. Catal., 2016, 358(18), 2929–2939 CrossRef CAS.
- R. Fujihara and K. Nakata, Eur. J. Org. Chem., 2018, 6566 CrossRef CAS.
- (a) X. Xie and J. M. Fox, Synthesis, 2013, 1807 CAS; (b) M. Nakamura, K. Matsuo, T. Inoue and E. Nakamura, Org. Lett., 2003, 5, 1373 CrossRef CAS; (c) T. Hata, R. Bannai, M. Otskui and H. Urabe, Org. Lett., 2010, 12, 1012 CrossRef CAS.
- Q. Longying, M. Enlu, J. Fan and L. Zhiping, Tetrahedron Lett., 2016, 57, 2211 CrossRef.
- M. Enlu, J. Yifan, C. Yuanjin, Q. Longying, Y. Xiaoyu and L. Zhiping, Asian J. Org. Chem., 2018, 7, 914 CrossRef.
- Z.-P. Zhan and H.-J. Liu, Synlett, 2006, 2278 CrossRef CAS.
- Z.-P. Zhan, J.-L. Yu, H.-J. Liu, Y.-Y. Cui, R.-F. Yang, W.-Z. Yang and J.-P. Li, J. Org. Chem., 2006, 71, 8298 CrossRef CAS.
- S. Maiti, S. Biswas and U. Jana, Synth. Commun., 2010, 41, 243 CrossRef.
- M. Lin, X.-L. Chen, T. Wang, P. Yan, S.-X. Xu and Z.-P. Zhan, Chem. Lett., 2011, 40, 111 CrossRef CAS.
- Q. Li, Y. Wang, Z. Fang, P. Liao, B.-D. Barry, G. Che and X. Bi, Synthesis, 2013, 609 CAS.
- O. Debleds, Z. C. Dal, E. Vrancken, J.-M. Campagne and P. Retailleau, Adv. Synth. Catal., 2009, 351, 1991 CrossRef CAS.
- O. Debleds, E. Gayon, E. Ostaszuk, E. Vrancken and J.-M. Campagne, Chem. – Eur. J., 2010, 16, 12207 CrossRef CAS.
- E. Gayon, O. Quinonero, S. Lemouzy, E. Vrancken and J.-M. Campagne, Org. Lett., 2011, 13, 6418 CrossRef CAS.
- W. Yan, Q. Wang, Y. Chen, J. L. Petersen and X. Shi, Org. Lett., 2010, 12, 3308 CrossRef CAS.
- W. Yan, X. Ye, K. Weise, J. L. Petersen and X. Shi, Chem. Commun., 2012, 48, 3521 RSC.
- L. Busetto, R. Mazzoni, M. Salmi, S. Zacchini and V. Zanotti, RSC Adv., 2012, 2, 6810 RSC.
- L. Hao, F. Wu, Z.-C. Ding, S.-X. Xu, Y.-L. Ma, L. Chen and Z.-P. Zhan, Chem. – Eur. J., 2012, 18, 6453 CrossRef CAS.
- L. Hao, J.-J. Hong, J. Zhu and Z.-P. Zhan, Chem. – Eur. J., 2013, 19, 5715 CrossRef CAS.
- M. J. Queensen, J. M. Rabus and E. B. Bauer, J. Mol. Catal. A: Chem., 2015, 407, 221–229 CrossRef CAS.
- D. S. Talasila, M. J. Queensen, M. B. Flaspoler, K. Jurkowski, E. Stephenson, J. M. Rabus and E. B. Bauer, Eur. J. Org. Chem., 2019, 2019(44), 7348–7358 CrossRef CAS.
- M. Shao, Y. Wu, Z. Feng, X. Gu and S. Wang, Org. Biomol. Chem., 2016, 14, 2515 RSC.
- J. A. Miller and M. J. Nunn, J. Chem. Soc., Perkin Trans. 1, 1976, 416 RSC.
- N. Ortega, A. Feher-Voelger, M. Brovetto, J. I. Padron, V. S. Martin and T. Martin, Adv. Synth. Catal., 2011, 353, 963 CrossRef CAS.
- J. Gao, Q.-W. Song, L.-N. He, Z.-Z. Yang and X.-Y. Dou, Chem. Commun., 2012, 48, 2024 RSC.
- S. Enthaler and M. Weidauer, ChemSusChem, 2012, 5, 1195 CrossRef CAS.
- S. Enthaler, Eur. J. Lipid Sci. Technol., 2013, 115, 239 CrossRef CAS.
- S. Enthaler and A. Trautner, ChemSusChem, 2013, 6, 1334 CrossRef CAS.
- (a) O. Mitsunobu, M. Yamada and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1967, 40, 935–939 CrossRef CAS; (b) O. Mitsunobu and M. T. Yamada, Bull. Chem. Soc. Jpn., 1967, 40, 2380–2382 CrossRef CAS.
- D. Hirose, T. Taniguchi and H. Ishibashi, Angew. Chem., Int. Ed., 2013, 52, 4613 CrossRef CAS.
- C. Masson, J. Soto and M. Bessodes, Synlett, 2000, 1281 CAS.
- K. Krohn, U. Flörke and D. J. Gehle, Carbohydr. Chem., 2002, 21, 431 CrossRef CAS.
- R. D. Tilve, M. V. Alexander, A. C. Khandekar, S. D. Samant and V. R. Kanetkar, J. Mol. Catal. A: Chem., 2004, 223, 237 CrossRef CAS.
- G. Zhang, Q. Liu, L. Shi and J. Wang, Tetrahedron, 2008, 64, 339 CrossRef CAS.
- P. Chen and S. Wang, Tetrahedron, 2012, 68, 5356 CrossRef CAS.
- M. V. Alexander, A. C. Khandekar and S. D. Samant, J. Mol. Catal. A: Chem., 2004, 223, 75–83 CrossRef CAS.
- M. Zhang, S. Zhang, C. Pan and F. Chen, Synth. Commun., 2012, 42, 2844–2853 CrossRef CAS.
- M. Xu, X. H. Zhang and P. Zhong, Synth. Commun., 2012, 42, 3472–3481 CrossRef CAS.
- H. Jin, Z. D. Huang, C. X. Kuang and X. K. Wang, Chin. Chem. Lett., 2011, 22, 310–313 CrossRef CAS.
- R. Ma, C.-B. Huang, A.-H. Liu, X.-D. Li and L.-N. He, Catal. Sci. Technol., 2014, 4, 4308–4312 RSC.
- Z. Wang, X. Xing, L. Xue, Y. Xiong and L. Fang, Synthesis, 2014, 0757–0760 CrossRef CAS.
- H. Tian, C. Zhu, H. Yanga and H. Fu, Chem. Commun., 2014, 50, 8875–8877 RSC.
- S.-J. Ji, M.-F. Zhou, D.-G. Gu, Z.-Q. Jiang and T.-P. Loh, Eur. J. Org. Chem., 2004, 1584–1587 CrossRef CAS.
- S. Palaniappan and A. John, J. Mol. Catal. A: Chem., 2005, 242, 168–172 CrossRef CAS.
- N. Seyedi, Transition. Met. Chem., 2013, 38, 93–103 CrossRef CAS.
- H. Veisi, B. Maleki, F. H. Eshbala, H. Veisi, R. Masti, S. S. Ashrafi and M. Baghayeri, RSC Adv., 2014, 4, 30683–30688 RSC.
- S. Badigenchala, D. Ganapathy, A. Das, R. Singh and G. Sekar, Synthesis, 2014, 101–109 Search PubMed.
- Y. Xiao and S. V. Malhotra, J. Organomet. Chem., 2005, 690, 3609–3613 CrossRef CAS.
- X. Xu, X. Xu, H. Li, X. Xie and Y. Li, Org. Lett., 2010, 12, 100–103 CrossRef CAS.
- Z.-P. Zhan, Y.-Y. Cui and H.-J. Liu, Tetrahedron Lett., 2006, 47, 9143–9146 CrossRef CAS.
- F.-Q. Yuana and F.-S. Han, Adv. Synth. Catal., 2013, 355, 537–547 Search PubMed.
- Z. Li, Z. Duan, J. Kang, H. Wang, L. Yu and Y. Wu, Tetrahedron, 2008, 64, 1924–1930 CrossRef CAS.
- X. Shao-Hong, J. Chem. Res., 2012, 36, 441–443 CrossRef.
- U. Jana, S. Maiti and S. Biswas, Tetrahedron Lett., 2007, 48, 7160–7163 CrossRef CAS.
- L. R. Jefferies and S. P. Cook, Org. Lett., 2014, 16(7), 2026–2029 CrossRef CAS.
- G. B. Womack, J. G. Angeles, V. E. Fanelli and C. A. Heyer, J. Org. Chem., 2007, 72, 7046–7049 CrossRef CAS.
- G. B. Womack, J. G. Angeles, V. E. Fanelli, B. Indradas, R. L. Snowden and P. Sonnay, J. Org. Chem., 2009, 74, 5738–5741 CrossRef CAS.
- B.-Q. Wang, S.-K. Xiang, Z.-P. Sun, B.-T. Guan, P. Hu, K.-Q. Zhao and Z.-J. Shi, Tetrahedron Lett., 2008, 49, 4310–4312 CrossRef CAS.
- Z. Wang, X. Sun and J. Wu, Tetrahedron, 2008, 64, 5013–5018 CrossRef CAS.
- X.-K. Guo, D.-Y. Zhao, J.-H. Li, X.-G. Zhang, C.-L. Deng and R.-Y. Tang, Synlett, 2012, 627–631 CAS.
- R. Marcos, C. Rodriguez-Escrich, C. I. Herrerias and M. A. Pericas, J. Am. Chem. Soc., 2008, 130, 16838–16839 CrossRef CAS.
- J. Wang, L. Zhang, Y. Jing, W. Huang and X. Zhou, Tetrahedron Lett., 2009, 50, 4978–4982 CrossRef CAS.
- S. Sarkar, S. Maiti, K. Bera, S. Jalal and U. Jana, Tetrahedron Lett., 2012, 53, 5544–5547 CrossRef CAS.
- Y. Jing Yu, D. Chun Yi, L. Hai Yan, L. Hong Feng and L. Yan Zhong, Chin. Sci. Bull., 2012, 57, 2364–2367 CrossRef.
- W. Huang, Q. Shen, J. Wang and X. Zhou, J. Org. Chem., 2008, 73, 1586–1589 CrossRef CAS.
- W. Huang, L. Hong, P. Zheng, R. Liu and X. Zhou, Tetrahedron, 2009, 65, 3603–3610 CrossRef CAS.
- M. Bandini, M. Tragni and A. Umani-Ronchi, Adv. Synth. Catal., 2009, 351, 2521–2524 CrossRef CAS.
- H. Li, J. Yang, Y. Liu and Y. Li, J. Org. Chem., 2009, 74, 6797–6801 CrossRef CAS.
- Y. Wang, W.-Q. Li, G. Che, X. Bi, P. Liao, Q. Zhanga and Q. Liu, Chem. Commun., 2010, 46, 6843–6845 RSC.
- D. Gauvreau, S. J. Dolman, G. Hughes, P. D. O’Shea and I. W. Davies, J. Org. Chem., 2010, 75, 4078–4085 CrossRef CAS.
- W. Boa, L. Pinhua, Z. Yichenga and W. Lei, Chin. J. Chem., 2010, 28, 2463–2468 CrossRef.
- Y. Cao, C. Yao, B. Qin and H. Zhang, Res. Chem. Intermed., 2013, 39, 3055–3062 CrossRef CAS.
- H. Li, W. Li, W. Liu, Z. He and Z. Li, Angew. Chem., Int. Ed., 2011, 50, 2975–2978 CrossRef CAS.
- E. Shirakawa, N. Uchiyama and T. Hayashi, J. Org. Chem., 2011, 76, 25–34 CrossRef CAS.
- E. Shirakawa, T. Yoneda, K. Moriya, K. Ota, N. Uchiyama, R. Nishikawa and T. Hayashi, Chem. Lett., 2011, 40, 1041–1043 CrossRef CAS.
- H. Xu and L.-l. Fan, Eur. J. Med. Chem., 2011, 46, 1919–1925 CrossRef CAS.
- Q. Yang, L. Wang, T. Guo and Z. Yu, J. Org. Chem., 2012, 77, 8355–8361 CrossRef CAS.
- B. Plancq, M. Lafantaisie, S. Companys, C. Maroun and T. Ollevier, Org. Biomol. Chem., 2013, 11, 7463–7466 RSC.
- S. Teranishi, T. Kurahashi and S. Matsubara, Synlett, 2013, 2148–2152 CAS.
- X. Cong and X. Zeng, Org. Lett., 2014, 16, 3716–3719 CrossRef CAS.
- R. Savela, M. Majewski and R. Leino, Eur. J. Org. Chem., 2014, 4137–4147 CrossRef CAS.
- A. Bhattacharya, P. M. Shukla and B. Maji, R. Soc. Open Sci., 2017, 4, 170748 CrossRef.
- N. Azizi, F. Arynasaba and M. R. Saidi, Org. Biomol. Chem., 2006, 4, 4275–4277 RSC.
- T. Itoh, H. Uehara, K. Ogiso, S. Nomura, S. Hayase and M. Kawatsura, Chem. Lett., 2007, 36, 50–51 CrossRef CAS.
- J.-K. Kobayashi, S.-I. Matsui, K. Ogiso, S. Hayase, M. Kawatsura and T. Itoh, Tetrahedron, 2010, 66, 3917–3922 CrossRef CAS.
- M. Kawatsura, M. Fujiwara, H. Uehara, S. Nomura, S. Hayase and T. Itoh, Chem. Lett., 2008, 37, 794–795 CrossRef CAS.
- T. Niu, L. Huang, T. Wu and Y. Zhang, Org. Biomol. Chem., 2011, 9, 273–277 RSC.
- L. Liang, Q. Liu, J. Zhang, F. Wang and Y. Yuan, Res. Chem. Intermed., 2013, 39, 1957–1962 CrossRef CAS.
- M. R. Zanwar, V. Kavala, S. D. Gawande, C.-W. Kuo, W.-C. Huang, T.-S. Kuo, H.-N. Huang, C.-H. He and C.-F. Yao, J. Org. Chem., 2014, 79, 1842–1849 CrossRef CAS.
- L. Yang, Q. Zhu, S. Guo, B. Qian, C. Xia and H. Huang, Chem. – Eur. J., 2010, 16, 1638–1645 CrossRef CAS.
- J. Kischel, I. Jovel, K. Mertins, A. Zapf and M. Beller, Org. Lett., 2006, 8, 19–22 CrossRef CAS.
- R. Li, S. R. Wang and W. Lu, Org. Lett., 2007, 9, 2219–2222 CrossRef CAS.
- T. Hashimoto, T. Izumi, Md. S. Kutubi and T. Kitamura, Tetrahedron Lett., 2010, 51, 761–763 CrossRef CAS.
- T. Hashimoto, S. Kutubi, T. Izumi, A. Rahman and T. Kitamura, J. Organomet. Chem., 2011, 696, 99–105 CrossRef CAS.
- Md. S. Kutubi and T. Kitamura, Tetrahedron, 2011, 67, 8140–8145 CrossRef CAS.
- Md. S. Kutubi, T. Hashimoto and T. Kitamura, Synthesis, 2011, 1283–1289 CAS.
- X. Bu, J. Hong and X. Zhou, Adv. Synth. Catal., 2011, 353, 2111–2118 CrossRef CAS.
- Y. Chen, K. Li, X. Liu, J. Zhu and B. Chen, Synlett, 2013, 0130–0134 Search PubMed.
- C. D. Zotto, J. Wehbe, D. Virieux and J.-M. Campagne, Synlett, 2008, 2033–2035 Search PubMed.
- K. Komeyama, R. Igawa and K. Takaki, Chem. Commun., 2010, 46, 1748–1750 RSC.
- D. Eom, J. Mo, P. H. Lee, Z. Gao and S. Kim, Eur. J. Org. Chem., 2013, 533–540 CrossRef CAS.
- H.-H. Li, Y.-H. Jin, J.-Q. Wang and S.-K. Tian, Org. Biomol. Chem., 2009, 7, 3219–3221 RSC.
- L. D. Marciasini, N. Richy, M. Vaultier and M. Pucheault, Adv. Synth. Catal., 2013, 355, 1083–1088 CrossRef CAS.
- T. Yoshida, L. Ilies and E. Nakamura, ACS Catal., 2017, 7, 3199–3203 CrossRef CAS.
- X. Zeng, Y. Zhang, Z. Liu, S. Geng, Y. He and Z. Feng, Org. Lett., 2020, 22, 2950–2955 CrossRef CAS.
- D. Leifert, C. G. Daniliuc and A. Studer, Org. Lett., 2013, 15, 6286–6289 CrossRef CAS.
- Y. Wang, L. Li, H. Ji, W. Ma, C. Chen and J. Zhao, Chem. Commun., 2014, 50, 2344–2346 RSC.
- T. Naveen, S. Maity, U. Sharma and D. Maiti, J. Org. Chem., 2013, 78(12), 5949–5954 CrossRef CAS.
- A. Deb, S. Agasti, T. Saboo and D. Maiti, Adv. Synth. Catal., 2014, 356, 705–710 CrossRef CAS.
- A. Modak, S. Rana and D. Maiti, J. Org. Chem., 2015, 80, 296–303 CrossRef CAS.
- Y. Ni, Q. Yu, Q. Liu, H. Zuo, H.-B. Yu, W.-J. Wei, R.-Z. Liao and F. Zhong, Org. Lett., 2018, 20, 1404–1408 CrossRef CAS.
- A. Deya and A. Hajraa, Adv. Synth. Catal., 2019, 361, 842–849 CrossRef.
- S. Kumar, S. Pradhan, S. Roy, P. B. De and T. Punniyamurthy, J. Org. Chem., 2019, 84, 10481–10489 CrossRef CAS.
- F. Yu, T. Wang, H. Zhou, Y. Li, X. Zhang and H. Bao, Org. Lett., 2017, 19(24), 6538–6541 CrossRef CAS.
- P. Gao, H. Wu, J.-C. Yang and L.-N. Guo, Org. Lett., 2019, 21(17), 7104–7108 CrossRef CAS.
- J. Jia, X. Zeng, Z. Liu, L. Zhao, C.-Y. He, X.-F. Li and Z. Feng, Org. Lett., 2020, 22(7), 2816–2821 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |