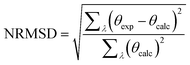Open Access Article
Open Access ArticleTools and methods for circular dichroism spectroscopy of proteins: a tutorial review
A. J.
Miles
 a,
Robert W.
Janes
a,
Robert W.
Janes
 b and
B. A.
Wallace
b and
B. A.
Wallace
 *a
*a
aInstitute of Structural and Molecular Biology, Birkbeck University of London, London WC1E 7HX, UK. E-mail: b.wallace@mail.cryst.bbk.ac.uk
bSchool of Biological and Chemical Sciences, Queen Mary University of London, London E1 4NS, UK
First published on 15th June 2021
Abstract
Circular dichroism (CD) spectroscopy is a widely-used method in biochemistry, structural biology and pharmaceutical chemistry. More than 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 papers published in the past decade have included CD characterisations of proteins; many of those studies have also included other complementary chemical, biophysical, and computational chemistry methods. This tutorial review describes the background to the technique of CD spectroscopy and good practice methods for high quality data collection. It specifically focuses on both established and new methods and tools available for experimental design and interpretation, data processing, visualisation, analysis, validation, archiving, and accession, including tools developed to enhance the complementarity of this method with other structural and chemical biology studies.
000 papers published in the past decade have included CD characterisations of proteins; many of those studies have also included other complementary chemical, biophysical, and computational chemistry methods. This tutorial review describes the background to the technique of CD spectroscopy and good practice methods for high quality data collection. It specifically focuses on both established and new methods and tools available for experimental design and interpretation, data processing, visualisation, analysis, validation, archiving, and accession, including tools developed to enhance the complementarity of this method with other structural and chemical biology studies.
Key learning points1. Circular dichoism (CD) spectroscopy, a widely-used method for examining the structures and conformational changes of proteins, provides complementary information to that obtainable by other biophysical, chemical, and structural biology techniques.2. Methods for good practice in measuring, processing, analysing, and interpreting CD spectra of proteins are described. 3. Means of accessing and utilising links to a wide range of online and downloadable tools for comparisons, secondary structure analyses, and predictions of CD spectra are presented. 4. Information is provided on how to access archived CD data sets and associated metadata in the Protein Circular Dichroism Data Bank (PCDDB), and its links to other data bases and validation protocols, and online information. 5. Examples of recent studies and developments utilising CD in novel studies of proteins exemplify its complementarity to other methods. |
1. Introduction to circular dichroism spectroscopy of proteins
Biomolecules such as proteins are built up of chiral subunits that produce signals when illuminated by circularly-polarised light in the near and far ultraviolet wavelength ranges where the amide and carbonyl groups of the polypeptide backbones absorb. Circular dichroism (CD) spectroscopy is an optical spectroscopic method which exploits the differential absorption of left- and right-circularly polarised light by such chromophores, and can be harnessed to derive structural information about protein conformations. It has been widely used to discern the secondary structure of proteins based on electronic transitions in the far ultraviolet (UV) wavelength region (∼240 to 170 nm) and to monitor the local tertiary structure environment of aromatic amino acid residues in the near UV region (∼300 to 260 nm), as a function of their physical or chemical environment, amino acid composition (i.e. mutations), or intermolecular interactions.1–4 In the past decade, more than 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 papers have been published using CD spectroscopy to characterise the structures of polypeptides and proteins. The information derived by CD spectroscopy, which can also include information on dynamic changes in solution or in environments such as membranes or films, is often complementary to that produced by other biophysical, computational and chemical methods such as crystallography, cryo-electron microscopy, NMR spectroscopy, FTIR spectroscopy, vibrational circular dichroism (VCD) spectroscopy,5 molecular dynamics simulations, and low angle scattering. Indeed, in the same 10 year time period, nearly half of the publications which included CD spectroscopy also included information derived from at least one of these other techniques.
000 papers have been published using CD spectroscopy to characterise the structures of polypeptides and proteins. The information derived by CD spectroscopy, which can also include information on dynamic changes in solution or in environments such as membranes or films, is often complementary to that produced by other biophysical, computational and chemical methods such as crystallography, cryo-electron microscopy, NMR spectroscopy, FTIR spectroscopy, vibrational circular dichroism (VCD) spectroscopy,5 molecular dynamics simulations, and low angle scattering. Indeed, in the same 10 year time period, nearly half of the publications which included CD spectroscopy also included information derived from at least one of these other techniques.
CD spectroscopy has a number of advantages with respect to the higher resolution structural techniques such as crystallography, electron microscopy, and NMR spectroscopy in that it requires relatively small amounts of sample under conditions (temperature, concentration and components present) that may be more comparable to those found in cells. This has resulted in its wide-spread use both by the biochemical and structural biology communities to complement the information derived by those other biophysical methods,4 as well as by the pharmaceutical industry,6,7 to assess whether a protein is correctly folded, to monitor structural changes induced by interactions with ligands including other proteins, and to determine protein stability under environmental stresses induced by, for example, changes in pH or temperature. In recent years circular dichroism beamlines have been developed at synchrotron light sources, taking advantage of both their high light flux, which enables faster collection of data from smaller amounts of protein, and the higher information content available due to the lower wavelengths that can be achieved at these high-intensity light sources.8
This tutorial review discusses not only methods and software currently available for obtaining, processing, validating, and interpreting high quality CD data obtained using lab-based (commercial) CD instruments (as well as synchrotron radiation circular dichroism (SRCD) beamlines) on a variety of samples types, but also the bioinformatics tools and resources available for determining novel details of the structure and function of proteins based on such data.
2. Methods, tools, and protocols for CD data collection, analyses, and display
2.1 Measuring CD spectra
To optimise the amount of high quality and reproducible CD data obtained from a given sample, it is essential to follow good practice protocols for data collection (see Table 1 for example).8,9 To accurately determine the secondary structure of a protein based on CD data, the data obtained must include a spectral range covering, at least, the wavelengths between 240 and 190 nm; more accurate results are obtained if data collected includes even lower wavelengths, because more electronic transitions (peaks) will be included, increasing the information content of the data. To achieve such measurements, conditions must be used so that the total absorbance of the sample (due to protein, buffer and other added components) is below ∼1.2 at all wavelengths. This may be challenging for wavelengths below 210 nm, where the absorbance tends to rise, sometimes precipitously, due to the cumulative effects of the buffer and the peptide chromophores along with contributions from water (or other solvents) and the composition material of the optical cell. In addition, light scattering effects from any undissolved protein or suspended particles in the solution, such as lipids present in membrane samples,9 may also contribute to this challenge.| Procedure | Notes |
|---|---|
| Prepare highly purified protein in low absorbing buffer | ≥95% of protein should be the protein of interest |
| Based on estimated concentration choose a cell pathlength so that the absorption is <1.2 at all wavelengths | If the absorbance is too high, choose a shorter pathlength cell or a different buffer |
| Verify cell pathlength | Cells with pathlengths <0.1 mm can be measured using the interference fringe method |
| Measure protein concentration accurately | If possible, measure concentration again immediately before measuring spectrum |
| Collect repeat CD spectra of sample and baseline | Monitor the HT signal. Make sure it does not exceed the linear range of the instrument |
| Average the sample data | Make sure there are no outliers due to un-equilibrated or leaking or light-sensitive sample or buffer components |
| Average the baseline data | |
| Subtract averaged baseline from averaged sample | Make sure the baseline and sample spectra overlay at wavelengths >250 nm (where there should not be a protein signal) |
| Calibrate the net (sample-baseline) spectrum (optional) | Important when comparing spectra measured on different instruments or after the lamp has been changed |
| Scale to standard units | Using the determined values of cell pathlength, protein concentration and mean residue weight |
The overall absorbance of the sample can be monitored by the dynode voltage or high tension (HT) signal produced during data collection. This is a measure of the voltage applied to the detector to amplify the small circular dichroism signal. The maximum HT cutoff values for individual CD instruments differ, but they correspond to the maximum dynode voltage reading above which the sample absorbance is too high for sufficient light to penetrate. Above this value the apparent CD signal and the intensity of the unpolarised light signal that emerges from the sample decrease to a level where the spectrum becomes noisy, and lead to distortions in both the magnitude and the shape of the measured peaks. Hence the maximum HT value for a given CD instrument needs to be determined.
For a given sample, minimisation of the total absorbance can be achieved by choosing buffer constituents with low absorbance in both the near and far UV wavelength region, and where this is not possible, using the lowest possible concentration of buffer and salts without compromising the stability of the protein. In addition a combination of protein and buffer concentrations, optical cell pathlength, optical cell material, and instrument parameters such as slit width and averaging time can be used to optimise the CD signal.8–10
Secondary structure analyses are also significantly affected by the accuracy of the protein concentration measurement, since this will have an effect on the magnitude of the CD spectrum when scaled to standard units.8,9 The most widely used colorimetric methods for determining protein concentrations, including Biuret, Lowry, bicinchoninic acid assays, and Coomassie blue staining; all produce different values/accuracies for proteins (depending on their amino acid compositions). Measuring the absorption of the sample at 280 nm (A280) is the most convenient and reliable method (although its accuracy can depend on the number of aromatic amino acids and to a lesser extent, their location (surface or buried) in the protein). Such measurements can be achieved (without wasting a great deal material) using micro-UV spectrometers such as Nanodrops.
Another important consideration is if there is a time lag between sample preparation, concentration determination, and CD measurements, or even during the course of a long series of CD measurements, there may be a change in the protein concentration, due to aggregation or precipitation, especially if the sample is unstable or sensitive to light. This issue can be obviated in part by measuring the concentration of the protein immediately before (or as near in time as possible to) measuring the CD spectrum. It can also be monitored by examining the HT measurements obtained during the course of the CD measurements. If the HT values decrease, this could be indicative of protein precipitation/aggregation, bubble formation, or even sample leakage, during the course of the measurement, and should indicate that a new sample needs to be used.
Using an inaccurate value for the optical cell pathlength will also have significant effects on spectral magnitude and therefore the accuracy of secondary structure analysis. This can be an issue when using demountable cells with pathlengths less 0.01 cm, as the manufacturer-reported values can have a wide margin of error. In addition, the loading and assembly of such cells can be non-reproducible.10 However, accurate pathlength measurements can be obtained for these cells using the interference fringe method,10 which requires use of a standard benchtop UV/Vis spectrophotometer.
Finally, as CD instruments are comprised of a number of optical components, there can be variations between instruments which lead to small differences in spectra of the same sample measured on two different instruments. Such disparities can be reduced or mitigated by obtaining calibration measurements with a standard reference material such camphorsulphonic acid (CSA) or ammonium camphour sulfonate (ACS) measured on the same instrument used for the sample measurements.7,11,12 Both of these compounds have two well-defined and well-separated peaks of known absolute magnitude, so comparisons between the calculated and the experimental values can be used to create a calibration curve over the wavelength range of the spectrum. Multiplying the measured CD values of the protein spectrum by the CD values of this curve at each wavelength, will adjust the spectrum so that it better matches the spectra measured on any other instrument where this procedure is carried out.
2.2 Processing methods and tools
CD spectra are usually produced by averaging a set of repeat scans (or accumulations) obtained for the sample, after which the averaged spectrum of the baseline (a solution that contains all components of the sample except the protein), is subtracted. The final net spectrum can then be calibrated against a solution (CSA or ACS) with a known signal, and normalised to standard units of mean residue ellipticity (MRE, degrees cm2 dmol−1 residue−1) or delta epsilon (Δε, M−1 cm−1) using the concentration, optical cell pathlength, and mean residue weight value for the protein. Each step in this process provides an opportunity to identify anomalies in the data. For example, there may be a systematic change in the data as a function of time that is apparent in repeated scans (possibly as a result of light-inducted conformational changes, or because the protein precipitates/aggregates with time). Processing data using a standard spreadsheet is possible but this method can be time-consuming; hence there are a number of possible alternatives described in this section.Instrument-processed data can be exported in ASCII format for external use including secondary structure analysis using the tools described in Section 3. However there is no consensus of file structure which would enable comparisons of data produced on different instruments, for example, data collected on a benchtop instrument and at an SRCD beamline, or two different bench-top instruments, which would enable simple comparisons. For this reason, the generic data processing software CDToolX,13 which is described in the following section, was created for use with the output of any CD or synchrotron radiation circular dichroism (SRCD) instrument, and provides formatted output results that are instrument-independent.
2.3 Methods and tools for analysing protein secondary structure from CD data
Protein CD spectra include two types of electronic transitions arising from peptide absorptions in the far UV.1 These include an n → π* transition that gives rise to a signal at ∼222 nm, and parallel and perpendicular π → π* transitions that give rise to signals at lower wavelengths in the range from ∼190–210 nm (illustrated in Fig. 3). In addition, an intra-amide charge transfer transition arising from through-space interactions occurs at around 180 nm, which is generally only accessible using synchrotron radiation as a light source.8 The different types of secondary structures that are adopted by polypeptide chains (for example, helix or beta strand) depend on the dihedral (Φ, Ψ) angles between adjacent residues in the polypeptide chain; the magnitudes and wavelengths of the CD signals that arise from the electronic transitions depend upon these angles and therefore upon the secondary structure of the protein. The overall shape and magnitude of a protein CD spectrum in the far ultraviolet (UV) wavelength region reflects a linear combination of its constituent secondary structural elements. Fig. 4 shows examples of spectra of proteins that are each dominated by one type of secondary structure, but most proteins contain multiple types of secondary structures that all contribute to their net CD spectrum.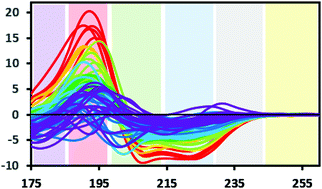 | ||
| Fig. 3 Bands defining the approximate wavelength range for electronic transitions responsible for the CD signals: Yellow: Signals due to aromatic residues (if present) may be detected in 100× pathlength cells; grey: occasionally a signal may be detected here due to the presence of stacked aromatic amino acids,1 blue: n → π* transition. Green and red: π → π* transition, this transition can give rise to two peaks due to exciton splitting, purple: charge transfer transition (generally only detectable when using synchrotron radiation source) (SRCD spectra). The depicted spectra are from the SP175 dataset21 and are available in the PCDDB35 with codes CD0000010000 to CD0000710000. They are shaded according to percentage of helix in the crystal structure of the protein as follows: red: 70% +, orange: 60–70%, yellow: 50–60%, light green: 40–50%, dark green: 30–40%, light blue: 20–30%, dark blue: 10–20%, purple: 0–10%. | ||
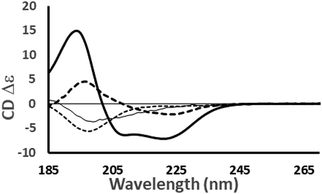 | ||
Fig. 4 Examples of the different shapes and magnitudes of CD spectra of proteins comprised primarily of different types of secondary structure: Predominantly helical [haemoglobin] ( ); predominantly anti-parallel beta sheet [concanavalin A] (- - -); predominantly right-hand twisted beta sheet [elastase] (—); predominantly disordered [HASPA] (⋯). The spectral data used in this figures are available in the PCDDB35 with accession codes: CD0000037000, CD0000020000, CD0000031000 and CD0005282000, respectively. The shape and intensity of each spectrum arises from the sum of all the secondary structure elements present in the protein (Fig. 3). There is no single band attributable to a certain secondary structure element, however, in general the magnitude of a negative peak at ∼222 nm is dependent on alpha helical content whereas a negative peak at ∼200 nm is indicative of disorder. ); predominantly anti-parallel beta sheet [concanavalin A] (- - -); predominantly right-hand twisted beta sheet [elastase] (—); predominantly disordered [HASPA] (⋯). The spectral data used in this figures are available in the PCDDB35 with accession codes: CD0000037000, CD0000020000, CD0000031000 and CD0005282000, respectively. The shape and intensity of each spectrum arises from the sum of all the secondary structure elements present in the protein (Fig. 3). There is no single band attributable to a certain secondary structure element, however, in general the magnitude of a negative peak at ∼222 nm is dependent on alpha helical content whereas a negative peak at ∼200 nm is indicative of disorder. | ||
There are a number of deconvolution methods available for obtaining quantitative secondary structural information from CD spectra,1,2,14 each of which requires a reference dataset of CD spectra produced from proteins with known (crystal) structures, in order to produce a calculated secondary structure spectrum that best matches the query (experimental) spectrum. These methods range from relatively simple least squares algorithms, to more complex ridge regression (RR) and singular value deconvolution (SVD) methods with variable selection (VS) functions, which fit the experimental spectrum to a weighted sum of the individual reference spectra. In general, a broader-base (more, and more varied) of components present in the reference database, allows more accurate identification of the protein structure that produced the query spectrum. However, the results of some types of analyses can be optimised by the use of specialised datasets, such as those designed for integral membrane proteins (which, due to their presence in low dielectric environments, tend to have transitions at somewhat different wavelengths than soluble proteins2,11). The characteristics and component types of current publically-available datasets are listed in Table 2, along with an indication as to which type of protein each database is best suited for.
| Reference dataset | Wavelength range (nm) | Number of proteins | Server | Types of proteins |
|---|---|---|---|---|
| SET125 | 178–260 | 29 | DichroWeb16 | soluble, globular |
| SET225 | 178–260 | 22 | DichroWeb | soluble, globular |
| SET325 | 185–240 | 37 | DichroWeb | soluble, globular |
| SET425 | 190–240 | 43 | DichroWeb | soluble, globular |
| SET525 | 178–260 | 17 | DichroWeb | soluble, globular |
| SET625 | 185–240 | 42 | DichroWeb | soluble, globular and denatured proteins |
| SET725 | 190–240 | 48 | DichroWeb | soluble, globular and denatured proteins |
| SP17521 | 175–240 | 71 | DichroWeb | soluble, globular (bioinformatics definitions) |
| SMP18022 | 180–240 | 129 | DichroWeb | membrane and soluble proteins |
| SP175+29 | 175–240 | 79 | BeStSel29 | soluble, globular especially β-sheet |
Alternative analysis methods15 use neural networks (NN) trained on sets of CD reference data. The accuracy of these depend upon both the suitability of the dataset used for training, on the breadth of protein spectra available, and the spectral wavelength range covered.
The dataset chosen for use will depend on the type of protein to be analysed. For example, to analyse the spectrum of a highly structured soluble protein, an appropriate choice may be SP175,21 which contains 71 high quality spectra of a bioinformatics-defined set of soluble globular proteins, covering all protein fold classes, which has a selectable wavelength range covering 175 nm to 240 nm. Alternatively, the SMP18022 dataset contains not only spectra of the soluble globular SP17521 proteins, but also 29 membrane protein spectra with low wavelength cutoffs of 180 nm, and hence is more suitable for analysing membrane proteins. The choice of dataset may be restricted by the low wavelength cutoff of the experimental data: for data which extend to a low wavelength of only 190 nm, truncated versions of SP17521 and SMP18022 are available. Spectral data that do not extend to wavelengths at least as low as 190 nm or below are not suitable for analysis by DichroWeb,16 as they do not have sufficient information content to enable detailed definitions of secondary structure.21 The secondary structure definitions used to create these reference datasets are those defined by the “Dictionary of Protein Secondary Structures” (DSSP) algorithm,23 based on conformational characteristics identified in crystal structures.
The DichroWeb16 server accepts file formats produced by most commercial and SRCD instruments, plus CDToolX13- and CDTool14-generated files, and simple two column (wavelength, value) text files. User files can be directly uploaded to the online server, and a number of parameters can be manually selected, including the high and low wavelengths of the data, the lowest wavelength to be considered in the analysis (which may differ from the lowest wavelength collected, if that wavelength resulted in an HT value that exceeded the cutoff limit of the instrument), and the spectral units, either mdeg or mean residue ellipticity (MRE). Input of the wavelength step size is also required. The algorithm and dataset to be used are selected from a dropdown box, and if the input spectrum has units of mdeg, the concentration in mg ml−1, optical pathlength in cm, and the protein mean residue weight are also required inputs so that the spectrum can be converted to MRE units prior to analysis.
The output pages (Fig. 5a and b) provide both a compact and an extended listing of the secondary structural results, and a plot of the back-calculated spectrum based on the secondary structure determined, which is overlaid on the experimental spectrum, as a visual indicator of the quality of the result (i.e. correspondence between the shapes and magnitudes of the calculated and measured spectra). The compact results table simply lists the predicted secondary structure fractions, whereas the extended results table (Fig. 5a) also provides a goodness-of-fit parameter known as the normalised root mean squared deviation (NRMSD24) which is an indication of the correspondence between the measured data and the back-calculated spectrum produced from the derived secondary structures, and is similar to an “R-factor” in crystallography. It is defined as:
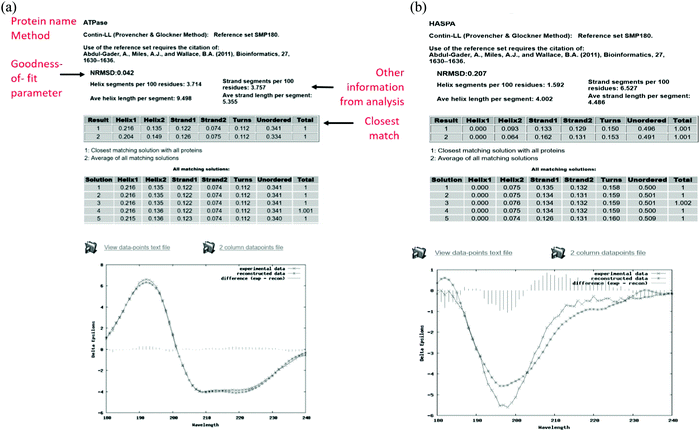 | ||
| Fig. 5 (a) Example results page obtained using the DichroWeb16 server for a “good quality analysis”. (top) The protein name (ATPase, PCDDB35 code (CD0004003000)) is displayed and the analysis method used [ContinLL15] is listed on the next line. Below this is the NRMSD24 “goodness-of-fit parameter”, which should optimally be <0.1 (as it is in this example), indicating a close correspondence between the back-calculated and measured spectra. If it is not, then another method, reference data set and/or scale factor should be used. (middle) Tables [shaded areas] of calculated secondary structure results obtained using the CONTINLL17 method and the appropriate (SMP180,22 membrane protein) reference data set. The arrow at the right of the top row indicates what is usually the closest/most suitable solution. The lower shaded box indicates other possible solutions obtained using other types of calculations. (bottom) Plot showing a comparison of the experimental spectrum (crosses), the back-calculated closest match spectrum (stars), and the difference spectrum (vertical bars) between the experimental and back-calculated spectra (vertical lines). The low NRMSD24 is consistent with the close match of the calculated and experimental spectra. These, plus the small magnitude difference spectrum indicate this is a “good quality” analysis. (b) Example results page obtained using the DichroWeb17 server for a “poor” quality analysis. This was obtained for an intrinsically disordered protein, HASPA (PCDDB code: CD0005282000). As in Fig. 5a, except in this case neither the (high) NRMSD27 value (>0.1) nor the correspondence between the calculated and experimental spectra, suggest that the best solution is an accurate reflection of the secondary structure. This is because this is an intrinsically disordered protein and is comprised of mostly unordered or disordered (not helical, sheet nor turn) secondary structures. As such it does not have well-defined phi, psi angles, and as the reference dataset does not contain many spectra of proteins with significant amounts of disorder (largely because this type of protein does not tend to crystallise), the NRMSD27 value is high. There is also a greater difference between the experimental and back-calculated spectra for this protein as compared to that for the well-ordered protein depicted in the Fig. 5a. | ||
The calculated results (Fig. 5) include the following secondary structure types: regular and distorted alpha helix, regular and distorted beta sheet, and turns.25 The distorted helix and sheet fractions include the residues at either end of an alpha helix and one residue at either end of a beta strand, which have slightly different dihedral angles than the corresponding canonical structures based on crystallographic data and thus have slightly different characteristic spectra. The ‘turn’ fraction includes beta turns, bends, and bridges as defined by DSSP.23 All other types of structure, including random coil, are classified as ‘unordered’. Two exceptions to these classifications are those produced by dataset 2, which uses as structural assignments α-helix, 310 helix, β-strand, turn, polyproline-II helix, unordered, and dataset 6 which uses the same structural definitions but combines the two types of helical fractions.26,27
The predicted average helix and strand lengths (which may or may not be accurate depending on the protein structural type, and hence should not be relied upon), and a list of solutions to all iterations of the calculations as the algorithm approaches the best fit back-calculated spectrum solution are also listed.
A further calculation option is that of a variable scale factor, which enables the user to multiply the input data by a small factor (<±0.1) to compensate for small experimental spectral magnitude errors28 (as illustrated in an example for the protein hemerythrin in Table 3a).
| (a) | ||||||
|---|---|---|---|---|---|---|
| Protein name (PDB ID) or PCDDB ID | Secondary structure | % Secondary structure calculated using | % Secondary structures from crystal structure | |||
| Server | ||||||
| METHOD | ||||||
| Dataset | ||||||
| DichroWeb16 | DichroWeb | BestSel29 | K2D332 | |||
| CONTINLL17 | SELCON317 | SP175+29 | ||||
| SP175t21 | SP175t | |||||
| Caletexin | Helix | 58 | 63 | 61 | 53 | 62 |
| CD0004676000 | Sheet | 5 | 5 | 0 | 10 | 2 |
| Antithrombin | Helix | 27 | 29 | 32 | 23 | 26 |
| CD0003889000 | Sheet | 26 | 22 | 18 | 25 | 27 |
| Bj-xtrIT | Helix | 27 | 28 | 25 | 15 | 26 |
| CD0004244000 | Sheet | 20 | 8 | 20 | 21 | 18 |
| Hemerythrin | Helix | 53 | 54 | 50 | 50 | 70 |
| (1HRT), scale1.0 | Sheet | 15 | 15 | 7 | 11 | 0 |
| NRMSD = 0.055 | ||||||
| Hemerythrin | Helix | 67 | 69 | 75 | 66 | 70 |
| NRMSD = 0.029 | Sheet | 7 | 4 | 7 | 2 | 0 |
| (1HRT), scale1.3 | ||||||
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protein name PCDDB ID | Secondary structure | % Secondary structure calculated using | Average % from Bioinformatics | |||||
| Server | ||||||||
| METHOD | ||||||||
| Dataset | ||||||||
| DichroWeb16 | DichroWeb | DichroWeb | DichroWeb | BestSel29 | K2D32 | |||
| CONTINLL17 | SELCON317 | CONTINLL | SELCON3 | SP175+29 | ||||
| SP175t21 | SP175t21 | SET625 | SET625 | |||||
| HASPA | Helix | 9 | 14 | 4 | 6 | 0 | 2 | 4 |
| CD0005282000 | Sheet | 30 | 27 | 15 | 13 | 34 | 23 | 1 |
| Other | 61 | 59 | 81 | 81 | 66 | 75 | 97 | |
| NRMSD | 0.104 | 0.399 | 0.145 | 0.214 | 0.015 | — | — | |
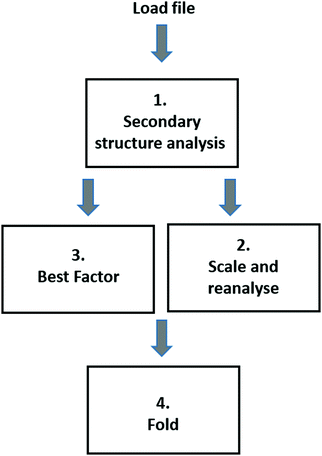 | ||
| Fig. 6 Workflow for analyses using the BeStSel server.29 (1) The initial secondary structure analysis displays the input spectrum and back-calculated spectrum, along with the predicted secondary structure fractions (displayed both as a table and a pie chart). (2) The experimental spectrum is rescaled by a user-defined factor and reanalysed, or (3) the spectrum is automatically scaled using the “best factor” function. The results of the latter provide a graph of the NRMSD value24 (same definition as in the DichroWeb16 server) as a function of scale factor, along with the associated values for the calculated secondary structure fractions. (4) Ribbon diagrams for proteins with similar secondary structure compositions (but not necessarily the same folds) as the proteins. | ||
Currently the SMP180 dataset22 available in DichroWeb16 is the only bespoke reference dataset available for analysing membrane proteins, which tend to exhibit peaks at slightly different wavelengths than soluble proteins with the same secondary structure content. Use of this reference data set may improve predictions for membrane proteins.
Despite the wide range of secondary structure analysis methods and dataset options, characterisations of proteins with high fractions of disordered structure are still challenging. Such proteins tend not to crystallise, so in the PDB30 there are relatively few crystal structures which are not primarily composed of canonical secondary structure types, although individual entries may include some disordered regions. As a result, all the analysis methods that rely upon reference to known protein structural types are less successful in defining the structures of such proteins, which are generally referred to as “intrinsically-disordered proteins”. Such spectra can often be identified visually as they tend to display only a single negative peak at ∼200 nm (Fig. 4 and 5b), and can sometimes resemble the spectra of some beta-rich proteins in both shape and magnitude (but with an altered peak position). Consequently, analyses using any of the existing datasets often assign significant amounts of beta structure to these spectra (Fig. 5b and Table 3b). Only datasets 6 and 7 in DichroWeb16 contain the spectra of any denatured proteins (which may or may not be similar to disordered proteins), but as disorder covers a wide range of (often flexible) secondary structural features, none of the presently available analyses are particularly suitable for this important class of proteins. Unfortunately, such proteins or regions of proteins appear to play important roles in the regulation of cell functions, and are also not well characterised by other (complementary) structural methods such crystallography, NMR and IR spectroscopies.
2.4 Identifying spectral artifacts and improving CD spectra
Quality control considerations for both the integrity of proteins under study and the analysis methods used to characterise them are important for producing suitable CD analyses. This has led to the development of ValiDichro34 software that can be used to identify some artifacts and irreproducibility in CD data, and/or errors in the parameters used for calculating secondary structures from CD data.ValiDichro34 tests CD spectral data quality, reproducibility, and completeness (wavelength range), as well as the associated metadata for consistency. Quality tests include maximum and minimum peak magnitudes (outside normal ranges for spectra when scaled to units of Δε or mean residue ellipticity). Outliers may indicate concentration or optical cell pathlength errors, or problems with the sample such as the flattening of peaks due to light scattering or when sample absorbance is too high. The HT signal is also tested to ensure that it does not exceed the instrument-dependent maximum (as discussed in Section 2.1), along with its gradient in the wavelength ranges of 240–260 nm where there should be minimal absorbance from the protein. Peak locations, which usually fall within a narrow range of wavelengths, are scrutinised since deviations may highlight absorbance issues or instrument error (although they can instead be indicative of interesting spectral features). If raw (unprocessed, unscaled) spectral data is provided, the standard deviations between the peak magnitudes of repeat scans are checked to determine if any outliers have been included which may distort the averaged spectrum. Excessive use of smoothing on the final processed spectrum leading to peak magnitude and wavelength artifacts, is also detected by comparing the raw data to the final spectrum. Finally, an overall assessment of the protein spectrum is made, comparing it to the first five basis spectra produced by the singular value decomposition of the SP17521 and SMP18022 datasets. Although the above is not an exhaustive list of tests carried out by ValiDichro,34 it provides an indication of how much detail is considered.
ValiDichro34 accepts many commonly-used file formats, including .pcd (PCDDB35) files or its equivalent XML version (.pcdXML), plus CDToolX.gen and CDTool.gen files. Instrument-specific formats (saved as ASCII files), and two- and three-column free formats, where the third column containing HT data is available. Some metadata are automatically extracted from the files and the rest are manually entered via text boxes and dropdown lists. The output includes a pass/flag/fail status for each test and suggestions designed to improve the data.
2.5 Public archive of CD spectra (the PCDDB35)
In 2006, with CD and (SRCD) having become ubiquitous methods employed in the molecular life sciences, there was an obvious need for a public repository where authors could store data and make it available to other researchers. Following extensive public consultations with the structural biology, spectroscopic and bioinformatics communities, the full resource, described below, was created and has evolved since then to include additional links to other databases, bioinformatics resources, and parameters for quality control assessments. To date, more than one million files have been downloaded, either as individual files or the full database contents.Depositions to the databank require depositor registration although accessions and downloads do not. When a deposition is to be made, the depositor requests an appropriate number of PCDDB ids (nine digit accession codes) be reserved. The spectra can be uploaded as ASCII text files, as generated by most CD instruments, or as CDToolX.gen or CDTool.gen files. Although only the fully-processed spectrum is required, spectra from all stages of the data processing, including the CSA or ACS spectrum used for instrument calibration, can be uploaded for completeness/good practice/tracing. Information including experimental conditions (including protein purity), instrument parameters, and protein sequence data are essential; links to other sites, including UNIPROT36 and the PDB,30 and other parameters such as enzyme classification, and citation details for the article in which data is presented, are strongly encouraged in order to create a comprehensive entry. Spectra are tested by a version of ValiDichro34 (see Section 2.4.1) and given a pass/flag/fail status before they can be committed to the database. Depositors can download a summary ValiDichro34 report that can be submitted to journals along with their manuscripts as an indication of data quality and accessibility.
The PCDDB35 provides an extensive list of searching criteria, including all of the above fields. The full contents of the database or just a single spectrum can be downloaded as .gen files, or as a two column (wavelength, CD) .pcd file.
2.6 Methods for comparing CD spectra
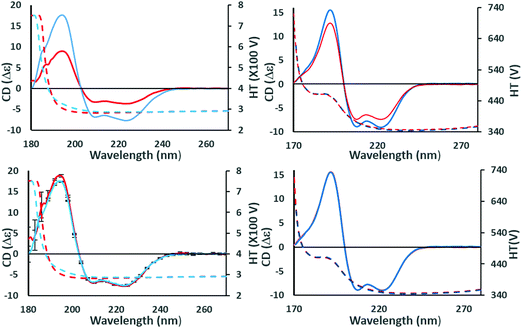
Spectra can be visually compared using CDToolX13 and CDTool14 software (see Section 2.2), in generic spreadsheet packages, or from the results of secondary structure analyses using the analysis programs described above. This can be important, for example, if spectra of two very similar proteins, wild type and mutant, have seemingly very similar characteristics but slightly different magnitudes and/or shapes. The difference may reflect a true difference or be an artifact due to concentration or incorrect optical cell pathlength values used, or other measurement errors. The ‘scale to value’ functions provided in CDToolX13 and CDTool14 software enable the facile scaling of the spectra so that they have the same magnitude at a selected peak wavelength, which will make any differences in the shapes of the spectra immediately apparent (Fig. 2).Alternatively, the DichroMatch37 function in the PCDDB35 may be useful for identifying spectral nearest-neighbours of a query unknown protein based on the spectra of known proteins, which can offer insights into the structure and function of the query protein.
DichroMatch37 accepts CDToolX.gen and CDTool.gen files, PCDDB.pcd files, and simple two column (wavelength, CD) ASCII text files. Alternatively a PCDDBid can be entered and the appropriate spectrum will be retrieved by the programme from the PCDDB.35 DichroMatch then searches all the component spectra in the PCDDB35 for similar protein spectra, with the output listing them in order of increasing NRMSD24 differences from the query spectrum.
2.7 Predictions of CD spectra
A number of methods have been developed for the prediction of a CD spectrum based on a protein's atomic coordinates. This37 can be useful for comparing two proteins when, for example, the X-ray diffraction, cryo-electron microscopy, or NMR structure of one is available, but only the CD spectrum of the other is available. Such comparisons may be useful to confirm homology, to determine if a mutant has folded correctly (and in a similar way to the wild-type protein), and for monitoring the effects of ligand binding or different environmental factors on conformation.The ab initio method of DichroCalc33 uses quantum mechanical calculations based on the averaged crystal structure to generate predicted spectra, whereas the PDB2CD38 and PDBMD2CD39 servers generate CD spectra using proteins present in a reference set with similar structural characteristics, by least squares fitting procedures. Both the PDBMD2CD server39 (based on structures produced using user-input molecular dynamic simulation results), and the downloadable programme SESCA40 generate predicted spectra using a principal component approach.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 or SMP180
21 or SMP180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 datasets. It achieves this using three different levels of structure-based information in the associated PDB30 files. The first level is the secondary structure content based on the DSSP23 definitions of alpha helix (H), beta-strand (E) and the other (O) category (which includes everything else). Then the localized topological features of the secondary structure components are considered by assessing the relative juxtaposition of secondary structural elements. Finally the overall structural similarity between the query and derived subset is determined. Following further refinement, the CD data of the remaining similar proteins are averaged to obtain the CD spectrum of the query protein.
22 datasets. It achieves this using three different levels of structure-based information in the associated PDB30 files. The first level is the secondary structure content based on the DSSP23 definitions of alpha helix (H), beta-strand (E) and the other (O) category (which includes everything else). Then the localized topological features of the secondary structure components are considered by assessing the relative juxtaposition of secondary structural elements. Finally the overall structural similarity between the query and derived subset is determined. Following further refinement, the CD data of the remaining similar proteins are averaged to obtain the CD spectrum of the query protein.
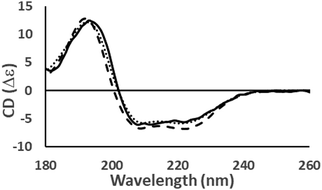
The user is required to choose between reference datasets SP17521 or SMP18022 before uploading a PDB30 file or entering the PDB30 code. In the output, the calculated spectrum is accompanied by a summary of the calculated secondary structure and a link to its PCDDB35 entry, if available. The website displays the spectrum calculated by PDB2CD38 superimposed on the experimental spectrum (Fig. 7).
Given coordinate data as input, the PDBMD2CD39 server creates basis spectra representing seven secondary structural types derived from a least squares regression of 83 spectra present in the SMP180 reference data set. A second set of basis spectra is also calculated from the PDB30 structures of proteins in the reference set with the closest secondary structure content to the query protein. A predicted spectrum is then derived from each basis set and the two are averaged to produce the calculated spectrum.
PDB30 format files can be uploaded to the server as archive files (.tar/.gz/.zip/.bz2) or as 4 digit PDB30 codes separated by commas. The input page also has an optional function ‘Split NMR models’, which can be selected when analysing multiple NMR structures, so that each structure can be considered individually. When the calculations are complete, the output is divided into three tabs: results, clustering and compare to experiment. The first of these displays a plot of all the predicted spectra and the averaged predicted spectrum with an interactive 3D representation of the most representative input structure. Other information produced includes the average RMSD between all the generated spectra and the average spectrum. The clustering tab displays k-mean clustering, a method of identifying different structural populations in the input data if >50 structures have been predicted. Finally, for the ‘compare to experiment tab’, an experimental spectrum can be uploaded as a two column text file so that it can be compared with the predicted spectra (Fig. 7).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm peaks, it is less accurate in assigning a value at 222 nm, leading to distortions in the overall shape of the spectra of helical proteins. This is not an issue for the spectra of beta-sheet rich proteins, which generally do not have a peak at 222 nm. PDB2CD38 produces more complete spectral matches to experimental data, but it requires similar proteins to the query protein be present in the comparison dataset in order to work accurately. PDBMD2CD39 and SESCA40 both enable prediction of CD spectra from multiple coordinate files as well as single structure files.
nm peaks, it is less accurate in assigning a value at 222 nm, leading to distortions in the overall shape of the spectra of helical proteins. This is not an issue for the spectra of beta-sheet rich proteins, which generally do not have a peak at 222 nm. PDB2CD38 produces more complete spectral matches to experimental data, but it requires similar proteins to the query protein be present in the comparison dataset in order to work accurately. PDBMD2CD39 and SESCA40 both enable prediction of CD spectra from multiple coordinate files as well as single structure files.
3. Applications demonstrating uses of CD spectroscopy for studying proteins
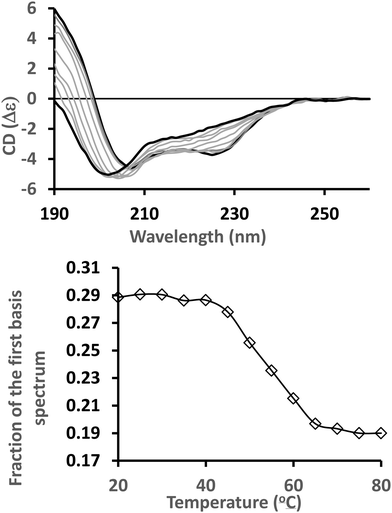
The above-described wide array of tools and resources now available for different types of analyses are now enabling novel applications of CD spectroscopy to answer specific biological questions that can complement other structural biology techniques.5 Some recent illustrative studies include the following selected examples which focus on the cross-overs/complementarity with other methods, as noted in Section 1.In an investigation of environmental effects on structure and stability,41 the secondary structures and thermal stabilities of the voltage-gated sodium channel NavMs (sodium channel from Magnetococcus marinus) in different environments were undertaken using CD and thermal melt CD studies. CD data was processed with CDTool14 and analysed using the DichroWeb16 server. SVD analysis of thermal melt data was performed using the CDToolX.13 Different amphipols and detergents were examined in order to identify amphipathic environments that stabilised the protein structure; indicating these could be suitable for cryo-electron microscopy studies.
In a study aimed at identifying drug binding sites42 not visible by crystallography, also for the NavMs channel, thermal melt circular dichroism spectroscopy was used to compare the stability of the full-length channel and a pore-only construct in the presence and absence of the anticonvulsant drug valproic acid. Deconvolution analyses and secondary structure determinations for apo- and drug-bound forms of both types of constructs at different temperatures using the DichroWeb16 server indicated that the interaction involved the pore-only domain, and not the voltage-sensor region, an unexpected and novel result for this type of drug. This study provided structural data in a system for which high resolution methods such as crystallography and cryo-electron microscopy have not yet been able to identify the binding sites.
The pharmaceutical industry relies on CD to ensure consistency between batches of drugs both during development and production. Perez et al.,43 characterised a novel monoclonal antibody by near UV CD, far UV CD, and fluorescence spectroscopies. Secondary structure analyses using the DichroWeb16 server, after minimizing the differences in protein concentration between batches by scaling, then enabled comparisons using DichroMatch.37
Another example is a study by Zheng et al.44 which illustrated the utility of predictive (computational) tools for evaluating the conformational preferences of the C-terminal peptide of the P66 domain of human immunodeficiency virus HIV-1 reverse transcriptase. CD spectroscopy was then used to confirm that the structure did indeed form a beta-sheet rich structure, as predicted, and the resulting spectrum compared favourably with the theoretical CD spectra generated by the computational server PDB2CD.38
4. Websites and downloadable tools for processing, analysing, interpreting, and comparing CD spectroscopic and other structural biology data
Data repository
The Protein Circular Dichroism Data Bank (PCDDB35), a databank of validated CD spectra and metadata, is accessible at: https://pcddb.cryst.bbk.ac.uk. It is an archive of published spectroscopic data, data collection parameters, and auxiliary information for each protein, and includes the amino acid sequence (including any construct-specific differences), the Enzyme Classification code (E. C. number) – if relevant, the CATH structure classification (if available), and cross-references to the corresponding entries in the Uniprot and Protein Data Banks.Tools for processing and validation of CD data
CDToolX13 and its predecessor CDTool14 are generic (instrument-independent) programmes for data processing and singular value deconvolution analyses which are downloadable at http://www.cdtools.cryst.bbk.ac.ukA video describing how to process data using CDToolX14 can be found on YouTube at: https://www.youtube.com/watch?v=ajNkfi9OzBU
Videos describing how to set up and use the CDToolX14 database are available on YouTube at:
https://www.youtube.com/watch?v=8Doy77UuO-s https://www.youtube.com/watch?v=u8Afbbmt6Jg
A detailed video describing how the SVD function in CDToolX13 can be used is available on the YouTube: https://www.youtube.com/watch?v=IymFg4hIfd4
The ValiDichro server34 which enables checking of spectra and metadata for quality and validity at https://pcddb.cryst.bbk.ac.uk/validichro/
Tools for secondary structure analyses
The DichroWeb server16 for secondary structure analyses is available at http://DichroWeb.cryst.bbk.ac.uk/html/home.shtml and includes a wide range of computational options, including reference databases, methods, and analysis tools.The BeStSel server,29 is accessible at http://bestsel.elte.hu/index.php for secondary structure analyses, and is especially focused on beta sheet-rich proteins.
The K2D3 secondary structure server,32 based on a neural network approach, is accessible at http://cbdm-01.zdv.uni-mainz.de/~andrade/k2d3/
The CONTINLL17 secondary structure analysis program is downloadable at http://s-provencher.com/contin.shtml
The SESCA analysis program40 requires a Python environment, and is downloadable at https://www.mpibpc.mpg.de/sesca
Reference data sets for secondary structure analyses
The component spectra of the SP175 dataset21 and associated secondary structure and other metadata are downloadable from the PCDDB35 (see above) as accession codes: CD0000001000 to CD0000071000.The component spectra of the SMP180 dataset22 (for analysis of membrane proteins) are downloadable from the PCDDB.35 This data set includes 29 membrane proteins (accession codes CD0000099000 to CD0000128000), the SP17521 proteins (see above), and 26 additional soluble proteins (accession codes CD0000072000 to CD0000098000).
Examples of tools for spectral prediction and comparisons
The DichroMatch server37 for identifying similar protein structures on the basis of related spectra is available at: https://pcddb.cryst.bbk.ac.uk/dichromatch.phpThe PDB2CD server38 for calculation of theoretical CD spectra for proteins based on their PDB coordinates is accessible at: https://pdb2cd.cryst.bbk.ac.uk
The PDBMD2CD server39 for calculation of spectra based structures derived from molecular dynamics simulations is accessible at: https://pdbmd2cd.cryst.bbk.ac.uk
The DichroCalc server33 for calculation of theoretical spectra from protein structures, is accessible at: https://comp.chem.nottingham.ac.uk/dichrocalc/index.html
Instrument-specific software tools
CD instruments are supplied with software for processing and analysing data collected on their specific instrument. Software manuals and/or tutorials can be accessed at:Jasco training videos are available at: https://jascoinc.com/training-video/video-category/spectra-manager-for-cd/
The APP Chirascan manual can be requested at: https://www.photophysics.com
The Olis Instruments website homepage is at: http://olisweb.com/
YouTube videos about CD procedures and software
Informational and training videos are available on the “PCDDB Channel” at: https://www.youtube.com/user/ThePcddb/videos?app=desktop&view=0&sort=dd&shelf_id=15. Conclusions
This tutorial review has described (and illustrated) methodologies for the collection and analysis of CD data. It includes extensive information on tools and resources available for analyses, validation, comparison and interpretation of CD spectroscopy data and the interoperability of CD spectroscopy with other structural biology techniques.Conflicts of interest
There are not conflicts of interest to declare.Acknowledgements
This work was supported by grants from the UK Biotechnology and Biological Sciences Research Council (BBSRC) Bioinformatics and Biological Resources Fund (most recently, BB/P024092 to BAW and BB/P024106 to RWJ).References
- Circular Dichroism and the Conformational Analysis of Biomolecules, ed. G. D. Fasman, Plenum Press, New York, 1996 Search PubMed.
- B. A. Wallace and R. W. Janes, in Modern Techniques for Circular Dichroism and Synchrotron Radiation Circular Dichroism Spectroscopy, ed. B. A. Wallace and R. W. Janes, IOS Press, Amsterdam, The Netherlands, 1st edn, 2009, ch. 1, pp. 1–18 Search PubMed.
- S. C. Goodchild, K. Jayasundera and A. Rodger, in Biomolecular and Bioanalytical Techniques: Theory, Methodology and Applications, ed. V. Ramesh, Wiley, New Jersey, USA, 1st edn, 2019, ch. 15, pp. 365–384 Search PubMed.
- B. A. Wallace, Curr. Opin. Struct. Biol., 2019, 58, 191 Search PubMed.
- L. A. Nafie, Chirality, 2020, 32, 667 Search PubMed.
- J. Ravi, A. E. Hills and A. E. Knight, in Modern Techniques for Circular Dichroism and Synchrotron Radiation Circular Dichroism Spectroscopy, ed. B. A. Wallace and R. W. Janes, IOS press, Amsterdam, Netherlands, 1st edn, 2009, ch. 6, pp. 125–140 Search PubMed.
- A. J. Miles and B. A. Wallace, in Biophysical Characterization of Proteins in Developing Biopharmaceuticals, ed. D. J. Houde and S. A. Berkowitz, Elsevier, Amsterdam, Netherlands, 2nd edn, 2020, ch. 6, pp. 123–152 Search PubMed.
- A. J. Miles and B. A. Wallace, Chem. Soc. Rev., 2006, 35, 39 Search PubMed.
- S. M. Kelly, T. J. Jess and N. C. Price, Biochim. Biophys. Acta, 2005, 1751, 119 Search PubMed.
- A. J. Miles, F. Wien, J. G. Lees and B. A. Wallace, Spectroscopy, 2005, 19, 43 Search PubMed.
- A. J. Miles and B. A. Wallace, Chem. Soc. Rev., 2016, 45, 4859 Search PubMed.
- A. J. Miles, F. Wien, J. G. Lees, A. Rodger, R. W. Janes and B. A. Wallace, Spectroscopy, 2003, 17, 653 Search PubMed.
- A. J. Miles and B. A. Wallace, Protein Sci., 2018, 27, 1717 Search PubMed.
- J. G. Lees, B. R. Smith, F. Wien, A. J. Miles and B. A. Wallace, Anal. Biochem., 2004, 332, 285 Search PubMed.
- C. Perez-Iratxeta and M. A. Andrade-Navarro, BMC Struct. Biol., 2008, 8, 25 Search PubMed.
- L. Whitmore and B. A. Wallace, Biopolymers, 2008, 89, 392 Search PubMed.
- S. W. Provencher and J. Glockner, Biochemistry, 1981, 20, 33 Search PubMed.
- N. Sreerama and R. W. Woody, Anal. Biochem., 1993, 209, 32 Search PubMed.
- L. A. Compton and W. C. Johnson, Jr., Anal. Biochem., 1986, 155, 155 Search PubMed.
- P. Manavalan and W. C. Johnson, Jr., Anal. Biochem., 1987, 167, 76 Search PubMed.
- J. G. Lees, A. J. Miles, F. Wien and B. A. Wallace, Bioinformatics, 2006, 22, 1955 Search PubMed.
- A. Abdul-Gader, A. J. Miles and B. A. Wallace, Bioinformatics, 2011, 27, 1630 Search PubMed.
- W. Kabsch and C. Sander, Biopolymers, 1983, 22, 2577 Search PubMed.
- D. Mao, E. Wachter and B. A. Wallace, Biochemistry, 1982, 21, 4960 Search PubMed.
- N. Sreerama, S. Y. Venyaminov and R. W. Woody, Protein Sci., 1999, 8, 370 Search PubMed.
- S. Y. Venyaminov, I. A. Baikalov, Z. M. Shen, C. S. C. Wu and J. T. Yang, Anal. Biochem., 1993, 214, 17 Search PubMed.
- N. Sreerama and R. W. Woody, Anal. Biochem., 2000, 287, 252 Search PubMed.
- A. J. Miles, L. Whitmore and B. A. Wallace, Protein Sci., 2005, 14, 368 Search PubMed.
- A. Micsonai, F. Wien, E. Bulyáki, J. Kun, E. Moussong, Y. H. Lee, Y. Goto, M. Réfrégier and J. Kardos, Nucleic Acids Res., 2018, 46, W315 Search PubMed.
- H. Berman, K. Henrick, H. Nakamura and J. L. Markley, Nucleic Acids Res., 2007, 35, D301 Search PubMed.
- N. L. Dawson, T. E. Lewis, S. Das, J. G. Lees, D. Lee, P. Ashford, C. A. Orengo and I. Sillitoe, Nucleic Acids Res., 2017, 45, D289 Search PubMed.
- C. Louis-Jeune, M. A. Andrade-Navarro and C. Perez-Iratxeta, Proteins: Struct., Funct., Bioinf., 2012, 80, 374 Search PubMed.
- S. B. Jasim, Z. Li, E. E. Guest and J. D. Hirst, J. Mol. Biol., 2018, 430, 2196 Search PubMed.
- B. Woollett, L. Whitmore, R. W. Janes and B. A. Wallace, Nucleic Acids Res., 2013, 41, W417 Search PubMed.
- L. Whitmore, A. J. Miles, L. Mavridis, R. W. Janes and B. A. Wallace, Nucleic Acids Res., 2017, 45, D303 Search PubMed.
- The UniProt Consortium, Nucleic Acids Res., 2019, 47, D506 Search PubMed.
- L. Whitmore, L. Mavridis, R. W. Janes and B. A. Wallace, Protein Sci., 2018, 27, 10 Search PubMed.
- L. Mavridis and R. W. Janes, Bioinformatics, 2017, 33, 56 Search PubMed.
- E. D. Drew and R. W. Janes, Nucleic Acids Res., 2020, 48, W17 Search PubMed.
- G. Nagy, M. Igaev, N. C. Jones, S. V. Hoffmann and H. Grubmüller, J. Chem. Theory Comput., 2019, 15, 5087 Search PubMed.
- S. M. Ireland, A. Sula and B. A. Wallace, Biopolymers, 2018, 109, e23067 Search PubMed.
- G. Zanatta, A. Sula, A. J. Miles, L. C. T. Ng, R. Torella, D. C. Pryde, P. G. DeCaen and B. A. Wallace, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 26549 Search PubMed.
- L. M. Pérez, A. D. R. Taño, L. R. M. Márquez, J. A. G. Pérez, A. V. Garay and R. B. Santana, PLoS One, 2019, 14, e0215442 Search PubMed.
- X. H. Zheng, G. A. Mueller, K. Kim, L. Perera, E. F. DeRose and R. E. London, Biochem. J., 2017, 474, 3321 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |