Open Access Article
Open Access ArticleShort-range structure, the role of bismuth and property–structure correlations in bismuth borate glasses†
C. P. E.
Varsamis
*a,
N.
Makris
b,
C.
Valvi
c and
E. I.
Kamitsos
 *b
*b
aApplied Physics Laboratory, Faculty of Engineering, University of West Attica, 250 Thivon, 112 41 Egaleo, Attica, Greece. E-mail: cvars@uniwa.gr
bTheoretical and Physical Chemistry Institute, National Hellenic Research Foundation, 48 Vass. Constantinou Ave., 116 35 Athens, Greece. E-mail: eikam@eie.gr
cElectronic Devices and Materials Laboratory, Department of Electrical and Electronics Engineering, University of West Attica, 250 Thivon, 112 41 Egaleo, Attica, Greece
First published on 12th April 2021
Abstract
Bismuth-containing borate glasses, xBi2O3–(1 − x)B2O3, were synthesized in the broad composition range 0.20 ≤ x ≤ 0.80 by melting in Pt crucibles and splat-quenching between two metal blocks. Infrared reflectance spectra, measured in the range 30–5000 cm−1, were transformed into absorption coefficient spectra and then deconvoluted into component bands to probe the glass structure as a function of composition. Integrated intensities of bands above 800 cm−1 were used in combination with mass and charge balance equations to quantify the short-range borate structure in terms of the molar fractions X4m, X4o, X3, X2, X1 and X0 for borate units BØ4−, BØ2O23−, BØ3, BØ2O−, BØO22− and BO33−, where Ø and O− denote bridging and non-bridging oxygen atoms. Borate tetrahedral units were found to be present in both the meta-borate, BØ4−, and ortho-borate, BØ2O23−, forms with BØ4− constituting the dominating tetrahedral species for 0.20 ≤ x ≤ 0.70. The BØ2O23− units prevail at higher Bi2O3 levels (x > 0.7), and coexist with their isomeric triangular borate species BO33− (BØ2O23− ⇌ BO33−). The present IR results for the total molar fraction of borate tetrahedral units, X4 = X4m + X4o, are in very good agreement with reported NMR results for the fraction of boron atoms in four-fold coordination, N4. Besides evaluating X4m and X4o, the present work reports also for the first time the fractions of all types of triangular borate species X3−n with n = 0, 1, 2 and 3. The IR region below 550 cm−1 was found to be dominated by the Bi–O vibrational activity in coexisting ionic (160–230 cm−1) and distorted BiO6 sites (330–365 cm−1 and 475–510 cm−1), a result reflecting the dual role of Bi2O3 as glass-modifier and glass-former oxide. The latter role dominates in glasses exceeding 60 mol% Bi2O3, and is consistent with the extended glass formation in the bismuth-borate system. The structural results were used to calculate the average number of bridging B–Ø bonds per boron center, the average Bi–O and B–O single bond energy, and the atomic packing density of the studied glasses. These properties vary approximately linearly with Bi2O3 content in the three regimes 0.2 ≤ x ≤ 0.4, 0.4 < x ≤ 0.6 and 0.6 < x ≤ 0.83, and contribute collectively to the composition dependence of glass transition temperature.
1. Introduction
Bismuth borate glasses constitute a promising family of materials for contemporary technological applications. For more than two decades the majority of such applications involved the field of photonics,1–15 while recent investigations recognize that the non-toxic bismuth borate glasses may replace radiation shielding concretes and lead-based commercial glasses for gamma ray and neutron radiation shielding applications.16–18Early studies19 suggested that potential alternatives to semiconductor materials with nonlinear optical properties could be transparent glasses with high linear refractive index. Thus, stable glassy systems having a broad forming range and containing highly polarizable metal ions are desirable; one possible candidate is the bismuth-borate system. The pioneering work on the complete phase diagram of the Bi2O3–B2O3 system by Levin and McDaniel20 showed that stable glasses can be prepared with up to ca. 76 mol% Bi2O3, whereas five crystalline compounds also exist with molar ratios of bismuth oxide to boron oxide of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 3
3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 2
5, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 12
1 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Among these crystals, the monoclinic BiB3O6 shows exceptionally large linear and nonlinear optical coefficients.21,22 More recent studies on glass formation and properties in the bismuth-borate system extended the glass forming range up to ca. 88.2 mol% Bi2O3 by employing the twin roller quenching technique.23,24
1. Among these crystals, the monoclinic BiB3O6 shows exceptionally large linear and nonlinear optical coefficients.21,22 More recent studies on glass formation and properties in the bismuth-borate system extended the glass forming range up to ca. 88.2 mol% Bi2O3 by employing the twin roller quenching technique.23,24
Glasses xBi2O3–(1 − x)B2O3 continue attracting interest mostly because of their unique linear and nonlinear optical properties which can be tuned by varying the Bi2O3 content over a broad composition range.23–27 For example, the second order non-linear optical susceptibility induced by thermal poling was found to increase with increasing the Bi2O3 content,8 and the third order optical non-linearity is one order of magnitude larger than that of silica glass and has a response time faster than 200 fs.9 In addition, doping bismuth borate glasses with a variety of rare earth ions, like Dy3+, Eu3+, Tb3+ and Nd3+ makes them luminescence materials with potential applications in solid-state lasers and optical amplifiers.11,12,15 Recently, bismuth-borate glasses have been used as suitable matrices for the incorporation of gold nanoparticles resulting in materials with high refractive index suitable for photonic applications.14 Bismuth-borate glasses have also large and positive values of the thermo-optic coefficient (dn/dT), i.e. large changes of the refractive index with temperature, with dn/dT increasing with Bi2O3 content.28
The correlation of properties with composition and structure allows for the tuning of the glass response according to specific technological applications. Therefore, the investigation of chemical, physical/optical and structural properties of Bi2O3-containing borate glasses is of key importance and several efforts have been made in this direction.29–38 In previous studies, a multitude of characterization techniques have been employed including XPS,29,30 infrared,29,32,33,38 Raman,25,32–35 neutron diffraction,31 and 11B MAS NMR.25,37
It is widely documented that the structure of metal oxide-containing borate glasses consists of borate tetrahedral and triangular units; the number of non-bridging oxygen atoms on the latter units depends on the modification level of the borate network.39–55 In the absence of metal oxides, i.e. pure B2O3 glass, the borate structure contains only neutral BØ3 triangles (Ø denotes an oxygen atom bridging two boron centers), of which ca. 75% are arranged in planar six-membered boroxol rings.56 Upon addition of metal oxide to B2O3, BØ3 triangles are converted initially to charged tetrahedral units BØ4−. The further increase of metal oxide content results in the progressive depolymerization of the borate backbone, through the breaking of B–O–B linkages and the creation of non-bridging oxygen atoms (NBO, or O−). The latter are formed mainly on charged triangular borate units which change gradually from BØ2O− to BØO22− and to BO33−i.e. from metaborate to pyroborate and to ortho-borate triangular moieties, respectively.39–55
Previous investigations on xBi2O3–(1 − x)B2O3 glasses revealed the coexistence of borate tetrahedral and triangular units for modification levels up to ca. x = 0.70.25,31,34,37 For the quantification of the borate structure, the molar fraction of tetrahedral borate units, N4, was obtained by NMR25,37 and neutron diffraction31 techniques. The NMR results showed that N4 exhibits a maximum value at about x = 0.42, while neutron diffraction data indicated a gradual increase of N4 from N4 = 0.38 at x = 0.30 to N4 = 0.48 at x = 0.67. However, neither the nature of the borate triangles was unambiguously identified nor their molar fractions were obtained in previous studies;25–31,34,36–38 due mostly to two reasons. First, the employed NMR and neutron diffraction techniques could not easily distinguish the different types of triangular borate units. Second, when infrared spectroscopy was used, by which the vibrational modes of different triangular borate units can be resolved,44–50 the infrared spectra of bismuth-borate glasses were measured using the KBr pellet technique.29,32–38 While this method allows for the derivation of qualitative information, it leads usually to partial hydrolysis of the dispersed glass, to ion-exchange phenomena and to the mixing of transmission and reflection responses; these factors lead to spectral distortions and to non-reproducible intensities of the infrared absorption bands.45,57
The literature overview presented above shows that a detailed mapping of the borate network structure as a function of bismuth oxide content is still lacking. We present here for the first time, to the best of our knowledge, a systematic investigation in the bismuth-borate glass system by means of infrared reflectance spectroscopy. This technique has been successfully applied in the past to study different families of glasses, to quantify their network structure and to reveal the nature and distribution of sites hosting the metal ions.44–50,57 Specifically, the analysis of the mid-infrared spectra, i.e. above ca. 650 cm−1, can facilitate the quantitative mapping of the borate structural units; whereas information about the oxide sites hosting the Bi ions can be deduced from the analysis of far-infrared profiles. This will permit to identify the role of Bi2O3 in borate glasses either as modifier and/or as glass-former oxide. The quantification of structure allows discussing the evolution of glass transition temperature on the basis of glass rigidity, as viewed in terms of the average number of bridging boron–oxygen bonds per boron center, the average boron–oxygen and bismuth–oxygen single bond energy and the atomic packing density of the bismuth-borate glasses.
2. Experimental
2.1. Glass preparation
Bismuth-borate glasses with the nominal composition xBi2O3–(1 − x)B2O3 were prepared by mixing appropriate stoichiometric amounts of high purity (>99.5%) reagent-grade anhydrous Bi2O3 and B2O3 powders. Batches corresponding to approximately 5 g were thoroughly mixed, placed in Pt crucibles and melted for 15 minutes at temperatures between 800 and 1000 °C depending on composition. The use of Pt crucibles and low melting temperatures are crucial factors, because a previous investigation on glasses containing bismuth oxide showed that silica and porcelain crucibles are very strongly corroded when used for melting and this leads to alterations of glass composition.58 The melts of this study were homogenized by frequent stirring, and glass samples were prepared by the conventional splat-quenching technique by quenching the melt between two polished stainless-steel blocks. With this preparation method, nine bismuth-borate compositions were obtained in bulk glass forms in the range 0.2 ≤ x ≤ 0.8 (x = 0.2, 0.3, 0.4, 0.5, 0.6, 0.65, 0.7, 0.75 and 0.80). The glass specimens had thickness of about 1 mm with smooth and flat surfaces, suitable for specular reflectance measurements without any further treatment. The prepared glasses were light yellow to brownish with increasing the Bi2O3 content.2.2. Infrared spectroscopy
Infrared (IR) reflectance spectra were measured on a vacuum Fourier-transform spectrometer (Bruker IFS 113v) at room temperature. A quasi-normal incidence mode (11°) was employed with an aluminum mirror as reference. Different infrared sources (globar and Hg arc lamp) and detectors (DTGS with KBr and polyethylene windows) were used to cover effectively the 30–5000 cm−1 frequency range. Mid-infrared spectra (400–5000 cm−1) were measured with a KBr beam splitter, whereas far-infrared spectra at frequencies below 700 cm−1 were collected using five different mylar beam splitters with thickness 3.5–50 μm. Each spectrum, measured using a particular optic configuration, is the average of 200 scans with 2 cm−1 resolution. The final reflectance spectrum of each glass composition results from the appropriate merging of the six individual measured spectra to give a continuous spectrum in the 30–5000 cm−1 range.The measured reflectance spectra were analyzed by the Kramers–Kronig (KK) method to calculate the real, n(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ), and imaginary, k(
), and imaginary, k(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ), parts of the complex refractive index ñ(
), parts of the complex refractive index ñ(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) = n(
) = n(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) + ik(
) + ik(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ), where
), where ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) is the frequency in wavenumbers (cm−1). The KK procedure requires extrapolation of the measured reflectance spectrum at the two limits
is the frequency in wavenumbers (cm−1). The KK procedure requires extrapolation of the measured reflectance spectrum at the two limits ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) → 0 and
→ 0 and ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) → ∞, as has been described in previous studies.45,57 The results of this study are presented and discussed here in terms of the absorption coefficient spectra α(
→ ∞, as has been described in previous studies.45,57 The results of this study are presented and discussed here in terms of the absorption coefficient spectra α(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) calculated by a(
) calculated by a(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) = 4π
) = 4π![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) k(
k(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ). Then, the absorption coefficient spectra were deconvoluted into Gaussian component bands, Gi(
). Then, the absorption coefficient spectra were deconvoluted into Gaussian component bands, Gi(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ), according to the relationship:
), according to the relationship:
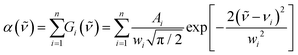 | (1) |
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) spectrum. The fitting procedure requires using, as a first step, the simplex algorithm followed by the application of the Levenberg–Marquardt algorithm, with a tolerance of 10−8.60 In the fitting procedure, the minimum number of component bands is used and, at the same time, the width values of the component bands that are assigned to the same vibrational mode of a particular structural unit are restricted to differ by less than 10% for different glass compositions. By imposing this constraint, it becomes meaningful to extract a quantitative description for the glass speciation.
) spectrum. The fitting procedure requires using, as a first step, the simplex algorithm followed by the application of the Levenberg–Marquardt algorithm, with a tolerance of 10−8.60 In the fitting procedure, the minimum number of component bands is used and, at the same time, the width values of the component bands that are assigned to the same vibrational mode of a particular structural unit are restricted to differ by less than 10% for different glass compositions. By imposing this constraint, it becomes meaningful to extract a quantitative description for the glass speciation.
3. Results
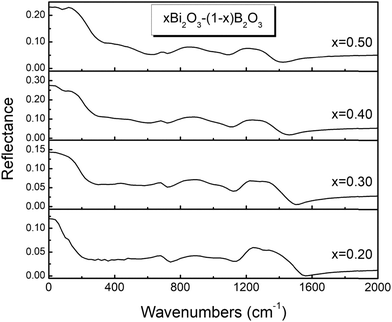
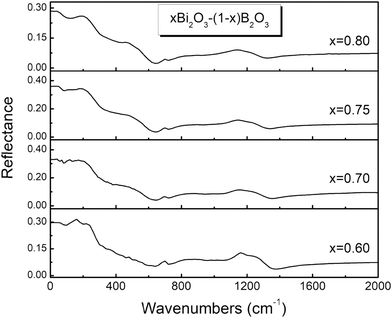
The reflectance spectra of glasses xBi2O3–(1 − x)B2O3 are depicted in Fig. 1 and 2 for 0.2 ≤ x ≤ 0.5 and 0.6 ≤ x ≤ 0.8 respectively. As the Bi2O3 content increases the far-IR activity shifts to higher frequencies and enhances relative to that in the mid-IR range. The far-infrared profiles of the studied glasses should result mainly from vibrational modes of Bi-containing oxide sites, while the mid-infrared activity arises from B–O vibrations in borate entities.44–52A detailed consideration of the spectral profiles and their composition dependence will be presented next in the absorption coefficient formalism, a(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ). The calculated α(
). The calculated α(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) spectra are depicted in Fig. 3 and 4 after being normalized to their band maxima and appropriately shifted for better visualization.
) spectra are depicted in Fig. 3 and 4 after being normalized to their band maxima and appropriately shifted for better visualization.
3.1. Mid-infrared spectra
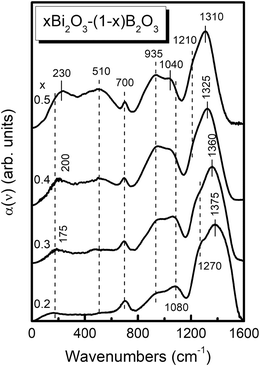
Inspection of Fig. 3 and 4 shows that all mid-infrared spectra exhibit three distinct absorption profiles, situated between ca. 600–750 cm−1, 750–1150 cm−1 and 1150–1600 cm−1, with shape and relative intensity depending strongly on glass composition. According to previous studies,44–52,57 the high-frequency IR envelope at ca. 1150–1600 cm−1 originates from asymmetric B–O stretching vibration modes of borate triangular units, while the corresponding modes of tetrahedral borate units are active at lower frequencies (750–1150 cm−1). Deformation modes of the borate units give rise to weaker absorption bands in the ca. 600–750 cm−1 range. For example, the relatively narrow peaks at 700 and 710 cm−1 in Fig. 3 and 4 can be attributed to the out-of-plane bend of borate triangular units.It is observed that the high-frequency profile exhibits a red shift upon increasing the Bi2O3 content, the peak maxima shifting from 1375 to 1310 cm−1 for 0.2 ≤ x ≤ 0.5 and from 1285 to 1240 cm−1 for 0.6 ≤ x ≤ 0.8. In addition, the bandwidth of the high-frequency profile decreases progressively upon increasing the modification level. It is noted that pronounced red shifts of the high-frequency IR absorption envelopes were observed also in recent studies of Sr,Eu borate glasses51 and highly modified Sr,Mn borate glasses.61 These trends have been attributed to the progressive depolymerization of the borate network as meta-borate triangles, BØ2O−, in super-structural units transform progressively to pyroborate dimers, B2O54−, and to ortho-borate monomers, BO33−, upon increasing the degree of modification.
For x = 0.20 and 0.30, the high-frequency envelope is characterized by the presence of a peak at 1375–1360 cm−1 and a shoulder at ca. 1270 cm−1, which signal the presence of meta-borate triangles, BØ2O−. In particular, the peak at 1375–1360 cm−1 can be attributed to the stretching of B–O− bonds and the lower-frequency shoulder to the stretching of B–Ø bonds in BØ2O− triangles. Previous studies of lithium borate45 and silver borate47 glasses showed that replacement of a bridging oxygen (Ø) by a non-bridging one (O−) in a BØ3 triangle of D3h symmetry to form a BØ2O− unit will reduce the symmetry to C2v, and this will split the doubly generate E′ asymmetric stretching mode under D3h into two IR-active components of A1 and B2 symmetry. This phenomenon is manifested in the infrared absorption spectra of Li- and Ag-borate glasses by the appearance of two component bands in the 1220–1250 cm−1 and 1435–1450 cm−1 frequency ranges, attributed to the stretching of B–Ø and B–O− bonds of BØ2O− units, respectively. Similar results were found in recent studies of highly modified borate glasses.51,52,61 The observed decrease in frequency of the B–O− stretching in BØ2O− units (1375–1360 cm−1) in the present study suggests stronger interactions between Bi3+ ions and non-bridging oxygens, which consequently weakens the strength in B–O− bonding and increases the degree of covalence in Bi–O bonding.
For compositions with x = 0.4 and 0.5, the progressive downshift of the peak frequency to 1325 and 1310 cm−1 is attributed to the formation of pyroborate dimers, B2O54−, in accordance with previous findings in highly modified borate glasses.45,51,52,61 As shown in Fig. 3, this downshift is accompanied by the presence of a shoulder at ca. 1210 cm−1. Such features are typical for the asymmetric stretching of the terminal B–O− bonds (1325–1310 cm−1) and the B–O–B bridges (1210 cm−1) in pyroborate units.45,51,52,61
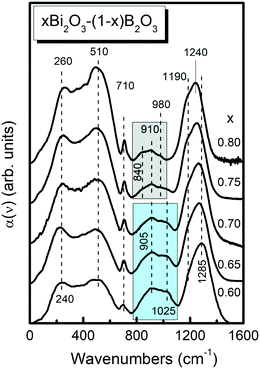
At large Bi2O3 contents (0.6 ≤ x ≤ 0.8, Fig. 4) the high frequency envelope consists of two distinct features, a peak at 1285–1240 cm−1 and a shoulder at ca. 1190 cm−1. These spectral characteristics signal the formation of ortho-borate units, BO33−,45,51,61 as they can be assigned to the splitting of the doubly degenerate ν3 asymmetric stretching mode of such units. The presence of BO33− units is also manifested by the well-defined peak around 710 cm−1 that is due to the their out-of-plane bending mode.45,51,61
The absorption envelope of BØ4− tetrahedral units, between ca. 750 to 1150 cm−1, appears to gain its maximum intensity relative to the high-frequency envelope for the composition x = 0.4 (Fig. 3), indicating a maximum fraction of BØ4− units. At higher Bi2O3 contents, the 750–1150 cm−1 envelop loses gradually intensity indicating the destruction of BØ4− units in favor of other structural entities. For x = 0.75 and 0.80, this mid-frequency absorption profile is substantially narrowed by almost 100 cm−1 and is characterized by the appearance of peaks at around 840, 910 and 980 cm−1. Previous studies of ortho-borate crystals YBO3,62 GdBO3,63 EuBO3, TbBO3 and DyBO364 having the vaterite type structure, that consists of BØ2O23− tetrahedra arranged in B3O99− rings, showed that the strongest infrared activity is in the 800–1150 cm−1 range with sharp peaks at ca. 870, 920 and 1010 cm−1. Along the same lines, previous studies of superionic AgI-containing ortho-borate glasses,65 Sr,Eu ortho-borate glasses51 and highly modified Sr,Mn borate glasses52 supported the existence of BØ2O23− tetrahedral units at very high modification levels. For convenience, the assignments of the infrared bands discussed here are summarized in Table 1 as a function of Bi2O3 content.
| Composition | Frequency range (cm−1) | Assignment |
|---|---|---|
| x = 0.2–0.8 | 150–230 | Bi–O stretch in ionic bismuth–oxygen sites |
| 325–380 | Asymmetric bend in distorted BiO6 sites | |
| 480–510 | Asymmetric stretch in distorted BiO6 sites | |
| 595–615 | In-plane bend of triangular borate units | |
| 695–710 | Out-of-plane bend of triangular borate units | |
| x = 0.20–0.75 | 820–850 | B–Ø stretch in BØ4− units |
| 910–960 | ||
| 1020–1100 | ||
| x = 0.60–0.80 | 810–820 | B–Ø ring and B–O− terminal stretch in [B3O9]9− rings of BØ2O23− tetrahedral units |
| 900–910 | ||
| 1025–1030 | ||
| x = 0.30–0.65 | 1170–1215 | B–O–B stretch in pyroborate units, BØO22− |
| x = 0.20–0.40 | 1215–1260 | B–Ø stretch in BØ2O− units |
| x = 0.70–0.80 | 1155–1200 | B–O− stretch in ortho-borate units, BO33− |
| 1240–1290 | ||
| x = 0.30–0.65 | 1270–1330 | B–O− stretch in pyroborate units, BØO22− |
| x = 0.20–0.40 | 1330–1390 | B–O− stretch in BØ2O− units |
| x = 0.20 | 1480 | B–Ø stretch in BØ3 units |
A key finding of this study is that the structure of bismuth-borate glasses with modification level higher than x = 0.70 contains ortho-borate tetrahedra BØ2O23− in coexistence with ortho-borate triangles BO33−. These species are present in equilibrium because they are chemically isomeric, i.e. BØ2O23−⇌ BO33−. It is noted also that the formation of BØ2O23− tetrahedral species, which have two bridging (Ø) and two non-bridging (O−) oxygen atoms, is compatible with the very broad glass-forming region 0.2 ≤ x ≤ 0.8 in xBi2O3–(1 − x)B2O3 glasses. While the nominal ortho-borate composition (O/B = 3/1) corresponds to x = 0.5 in such glasses, the glass-forming region extends well-above this limit and reaches x = 0.80 by splat-quenching and even x = 0.88 by twin roller quenching.23 This is in contrast to lithium-borate glasses xLi2O–(1 − x)B2O3 for which glass formation by splat-quenching was possible up to x = 0.73, i.e. the pure ortho-borate glass composition (x = 0.75) was not achieved due to partial crystallization at x = 0.73.45 This can be rationalized in terms of the main structural difference between these two glass systems; while BO33− triangles are the only borate species present in Li-ortho-borate, they coexists with their isomeric tetrahedral ortho-borate units BØ2O23− in highly-modified Bi-borate glasses and this appears to prevent crystallization.
The above findings are in line with reported structures and infrared spectra of crystalline bismuth-borate compounds of similar stoichiometry. Starting with the low Bi2O3 content x = 0.20, the structure of the Bi2B8O15 crystal consists of infinite chains of meta-borate rings B3O63− linked together by BiO6 octahedra, BØ3 triangles and BØ4− tetrahedra.66 The corresponding mid-IR transmittance spectrum35 exhibits the two characteristic bands of BØ2O− units centered at ca. 1250 and 1360 cm−1, and several peaks in the 800–1100 cm−1 range due to BØ4− tetrahedra. The BiB3O6 crystal, corresponding to x = 0.25 and having the meta-borate stoichiometry (O/B = 2/1), consists of infinite chains of alternating meta-borate triangles BØ2O− and meta-borate tetrahedra BØ4− in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.67 The mid-IR spectrum of BiB3O6 crystal35 resembles that of Bi2B8O15, with characteristic absorptions of meta-borate triangular and tetrahedral species.
1 ratio.67 The mid-IR spectrum of BiB3O6 crystal35 resembles that of Bi2B8O15, with characteristic absorptions of meta-borate triangular and tetrahedral species.
The Bi3B5O12 crystal structure, x = 0.375, contains Bi3+ cations, isolated pentaborate type anions B5O117− and O2− anions not bonded to any boron–oxygen group and situated in the centers of isolated OBi3 triangles.68 The isolated pentaborate type anions are formed by two condensed rings sharing a BØ4− tetrahedron; one ring consists of this BØ4− tetrahedron and two BØ2O− triangles and the other involves the common BØ4− tetrahedron bonded to a BØ2O− triangle and to a BØ2O23− tetrahedron.68 The mid-IR transmittance spectrum of the Bi3B5O12 crystal35 exhibits a broad band centered at ca. 1320 cm−1 characteristic of BØ2O− triangles, bands in the range 1030–1125 cm−1 for BØ4− tetrahedral units and well defined bands at 840 and 938 cm−1 consistent with the existence of BØ2O23− tetrahedra.
The structures of the crystalline compounds BiBO3 (ortho-borate, x = 0.50) and Bi4B2O9 (x = 0.67) contain discrete planar ortho-borate triangles, BO33−.69,70 The mid-IR transmittance spectra35 of these two crystals can be fully described in terms of bands attributed to BO33− vibrational modes; these include the B–O asymmetric stretch (broad envelope in the 1100–1300 cm−1 range), and out-of-plane bend at 690 cm−1. The presence of multiple bands in the 1100–1300 cm−1 envelope arises from the low positional symmetry and the existence of non-equivalent sites for the ortho-borate triangles in the crystal structure, which remove the degeneracy of the asymmetric stretching vibration mode of the BO33− units.
To summarize this section, our mid-IR structural analysis for four-fold coordinated B atoms in Bi-borate glasses suggests the presence of meta-borate tetrahedral units, BØ4−, for glasses with 0.2 ≤ x ≤ 0.70, with ortho-borate tetrahedral units, BØ2O23−, prevailing at higher modification levels. Units with three-fold coordinated B atoms include the meta-borate triangles, BØ2O−, dominating in glasses with x = 0.2 and 0.3, and pyroborate dimers, B2O54−, for x = 0.3 to x = 0.6, while the ortho-borate monomers, BO33−, constitute the majority of triangular borate species for x ≥ 0.50.
3.2. Far-infrared spectra
Information on the bonding of metal cation in glass can be deduced from the far-IR spectral region, where vibrations of metal ions against their sites in the glass network are active.44,45,52,57,61,65 As seen in Fig. 3 and 4, introduction of Bi2O3 in the borate glass causes the progressive evolution of a complex absorption profile which extends up to ca. 670 cm−1. The contribution of this profile to the entire infrared response increases with Bi2O3 addition, suggesting that its origin should be traced mainly to vibration modes involving Bi cations.Inspection of Fig. 3 shows that for the lower modification levels, x = 0.2–0.4, the far-IR profile appears to consist mainly of a low-frequency band with peak frequency increasing from about 160 cm−1 (x = 0.20) to 200 cm−1 (x = 0.40). For the x = 0.50 glass, the low-frequency band about 220 cm−1 is in co-existence with a higher-frequency component centered at about 510 cm−1. These two features dominate the far-infrared region for the rest of glass compositions, 0.60 ≤ x ≤ 0.80, as depicted in Fig. 4. In addition, the first peak at about 240 cm−1 appears to loss intensity at higher Bi2O3 contents in favor of the peak at about 510 cm−1, which at x = 0.80 becomes the dominant feature of the entire IR spectrum.
We note that an infrared and Raman study of crystalline α-Bi2O3 and bismuth oxide derivatives with sillenite structure showed strong infrared activity in the 400–600 cm−1 range, assigned to the stretching of Bi–O bonds in BiO6 polyhedra.71 Similarly, studies on multicomponent bismuthate glasses assigned the far-IR absorption centered around 480 cm−1 to the Bi–O stretching in strongly distorted BiO6 octahedral moieties.32,33,36,72,73 The existence of BiO6 octahedra was proposed also in Raman studies of unconventional lithium-bismuthate glasses74 and ternary Bi2O3-containing phosphate and borate glasses,75 in a neutron and X-ray diffraction study of Bi2O3–Ga2O3 glasses,76 and in a structural study of Bi2O3–Fe2O3 glasses by X-ray diffraction, IR, EPR and Mossbauer spectroscopies.77 On these grounds, we associate the band at ca. 500 cm−1 with the formation of BiO6 octahedral units in the bismuth-borate glasses of this study.
The lower-frequency peak in the 160–240 cm−1 range can be attributed to vibrations of Bi cations against their ionic sites formed by oxygen atoms of the glass network. This assignment is in line with a plethora of previous investigations on metal oxide-containing borate glasses by both experimental and molecular simulations techniques.44–52,57,61,65,78–81
4. Discussion
4.1. Quantification of the short-range borate structure
The previously described qualitative picture for the evolution of the borate structure with Bi2O3 content can be further elaborated to determine the borate speciation expressed as molar fractions of the various borate entities. To this aim, we proceed by considering the deconvolution of the absorption coefficient spectra α(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) according to eqn (1). Representative deconvoluted α(
) according to eqn (1). Representative deconvoluted α(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) spectra are depicted in Fig. 5 for x = 0.2 and x = 0.4 and in Fig. 6 for the x = 0.65 and x = 0.80 compositions, whereas the fitting parameters of the component bands are listed in Table 2 for all glass compositions.
) spectra are depicted in Fig. 5 for x = 0.2 and x = 0.4 and in Fig. 6 for the x = 0.65 and x = 0.80 compositions, whereas the fitting parameters of the component bands are listed in Table 2 for all glass compositions.
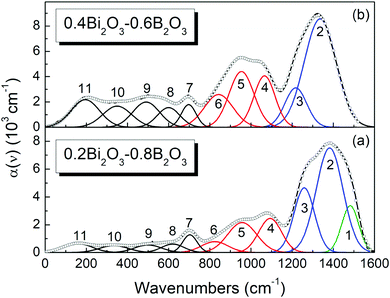 | ||
Fig. 5 Deconvolution into Gaussian component bands of the infrared absorption coefficient spectra, a(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ), of glasses xBi2O3–(1 − x)B2O3 with x = 0.2 (a) and 0.4 (b). Black solid lines represent the experimental spectra and circle symbols (○) the simulated spectra. Gaussian component bands are colored as follows; ), of glasses xBi2O3–(1 − x)B2O3 with x = 0.2 (a) and 0.4 (b). Black solid lines represent the experimental spectra and circle symbols (○) the simulated spectra. Gaussian component bands are colored as follows;  : band 1 attributed to BØ3 triangles (x = 0.2), : band 1 attributed to BØ3 triangles (x = 0.2),  : bands 2 and 3 attributed to BØ2O− (x = 0.2) or to BØO22− and BO33− triangles (x = 0.4), : bands 2 and 3 attributed to BØ2O− (x = 0.2) or to BØO22− and BO33− triangles (x = 0.4),  : bands 4–6 attributed to BØ4− tetrahedral units. For assignments of bands 7–11 see text and Table 2. : bands 4–6 attributed to BØ4− tetrahedral units. For assignments of bands 7–11 see text and Table 2. | ||
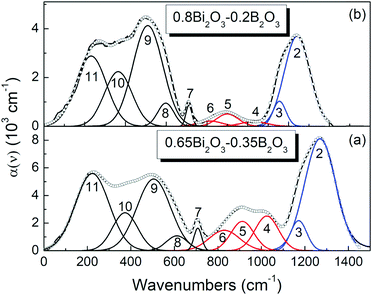 | ||
Fig. 6 Deconvolution into Gaussian component bands of the infrared absorption coefficient spectra, a(![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ), of glasses xBi2O3–(1 − x)B2O3 with x = 0.65 and 0.80. Black solid lines represent the experimental spectra and circle symbols (○) the simulated spectra. Gaussian component bands are colored as follows; ), of glasses xBi2O3–(1 − x)B2O3 with x = 0.65 and 0.80. Black solid lines represent the experimental spectra and circle symbols (○) the simulated spectra. Gaussian component bands are colored as follows;  : bands 2 and 3 attributed to BO33− triangles, : bands 2 and 3 attributed to BO33− triangles,  : bands 4–6 attributed to BØ4− tetrahedral units. For assignments of bands 7–11 see text and Table 2. : bands 4–6 attributed to BØ4− tetrahedral units. For assignments of bands 7–11 see text and Table 2. | ||
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) spectra of glasses xBi2O3–(1 − x)B2O3; resonance frequency νi (cm−1), width wi (cm−1) and integrated intensity Ai (104 cm−2)
) spectra of glasses xBi2O3–(1 − x)B2O3; resonance frequency νi (cm−1), width wi (cm−1) and integrated intensity Ai (104 cm−2)
| x | 0.20 | 0.30 | 0.40 | 0.50 | 0.60 | 0.65 | 0.70 | 0.75 | 0.80 |
|---|---|---|---|---|---|---|---|---|---|
| ν 1 | 1481.9 | ||||||||
| w 1 | 82.1 | ||||||||
| A 1 | 34.74 | ||||||||
| ν 2 | 1382.0 | 1393.0 | 1331.0 | 1315.3 | 1290.8 | 1270.0 | 1265.2 | 1251.2 | 1242.1 |
| w 2 | 130.0 | 136.3 | 159.6 | 143.9 | 137.1 | 142.1 | 135.4 | 134.6 | 126.2 |
| A 2 | 122.57 | 115.4 | 176.0 | 109.5 | 168.1 | 144.5 | 99.0 | 93.0 | 58.5 |
| ν 3 | 1258.8 | 1262.8 | 1213.9 | 1199.3 | 1185.0 | 1171.4 | 1166.6 | 1158.2 | 1155.5 |
| w 3 | 100.0 | 140.6 | 93.1 | 101.7 | 87.4 | 73.7 | 68.3 | 65.0 | 63.5 |
| A 3 | 58.4 | 85.5 | 30.0 | 27.4 | 33.6 | 20.2 | 13.8 | 12.9 | 8.2 |
| ν 4 | 1093.1 | 1078.8 | 1066.0 | 1060.5 | 1030.0 | 1025.0 | 1021.1 | 1024.4 | 1030.0 |
| w 4 | 110.0 | 90.2 | 107.1 | 88.5 | 109.9 | 107.9 | 109.5 | 105.2 | 130.0 |
| A 4 | 34.0 | 32.7 | 55.3 | 23.1 | 48.8 | 34.1 | 18.9 | 10.7 | 3.2 |
| ν 5 | 957.3 | 957.0 | 952.1 | 942.7 | 927.1 | 912.8 | 908.3 | 909.1 | 900.0 |
| w 5 | 140.0 | 140.0 | 120.0 | 147.0 | 99.1 | 104.9 | 103.7 | 114.0 | 107.3 |
| A 5 | 38.0 | 62.7 | 65.4 | 59.4 | 31.7 | 28.0 | 16.5 | 14.6 | 6.8 |
| ν 6 | 824.1 | 820.0 | 841.8 | 820.5 | 850.0 | 830.0 | 820.0 | 810.0 | 813.9 |
| w 6 | 130.0 | 110.0 | 140.0 | 130.0 | 126.0 | 140.0 | 150.0 | 139.7 | 119.1 |
| A 6 | 12.8 | 16.0 | 44.7 | 16.6 | 40.0 | 26.0 | 15.0 | 8.3 | 3.3 |
| ν 7 | 703.2 | 697.1 | 697.3 | 705.3 | 703.6 | 707.5 | 709.6 | 709.9 | 711.1 |
| w 7 | 69.2 | 70.6 | 70.6 | 54.3 | 59.9 | 35.9 | 32.2 | 30.0 | 29.7 |
| A 7 | 11.1 | 13.3 | 15.8 | 7.1 | 13.3 | 7.3 | 4.6 | 4.4 | 3.2 |
| ν 8 | 609.9 | 598.1 | 600.2 | 607.9 | 597.3 | 615.6 | 599.7 | 600.0 | 597.8 |
| w 8 | 110.0 | 108.6 | 110.0 | 120.0 | 90.0 | 92.1 | 100.8 | 84.3 | 79.2 |
| A 8 | 9.9 | 13.2 | 21.6 | 19.0 | 20.6 | 12.1 | 15.2 | 11.1 | 9.4 |
| ν 9 | 475.0 | 480.0 | 490.0 | 493.2 | 495.0 | 505.0 | 500.1 | 505.8 | 510.0 |
| w 9 | 120.0 | 110.7 | 130.0 | 140.0 | 150 | 155.0 | 150.0 | 151.7 | 147.0 |
| A 9 | 8.2 | 13.0 | 32.8 | 37.0 | 96.1 | 102.9 | 77.0 | 92.5 | 76.1 |
| ν 10 | 330.0 | 341.4 | 350.0 | 356.1 | 357.3 | 365.0 | 368.4 | 360.7 | 365.0 |
| w 10 | 120.8 | 140.0 | 137.5 | 140.0 | 125 | 120.0 | 130.0 | 130.0 | 135.0 |
| A 10 | 7.1 | 13.0 | 28.6 | 27.5 | 44.9 | 43.9 | 37.6 | 46.0 | 37.9 |
| ν 11 | 161.5 | 185.3 | 195.5 | 209.6 | 225.0 | 220.0 | 230.8 | 225.4 | 232.0 |
| w 11 | 145.2 | 130.0 | 139.0 | 140.0 | 137.5 | 150.0 | 150.0 | 140.0 | 150.0 |
| A 11 | 12.1 | 16.9 | 38.3 | 38.3 | 81.6 | 103.9 | 74.1 | 72.6 | 54.2 |
In the following, we focus on the bands 1–6 in the 750–1600 cm−1 range which originate from the stretching vibration modes of borate units and use the notation Xi for the molar fraction of the unit having i bridging oxygen atoms per boron. Thus, X4m denotes the molar fraction of meta-borate tetrahedral unit, BØ4−, and X3, X2, X1, X0 the molar fractions of BØ3, BØ2O−, BØO22− and BO33− triangular units, respectively.
For glass compositions 0.20 ≤ x ≤ 0.40, mass and charge balance considerations give:
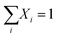 | (2) |
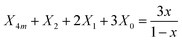 | (3) |
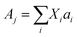 | (4) |
Starting with the x = 0.20 glass composition, Fig. 5 shows that the 750–1600 cm−1 range is best fitted with six component bands. According to Section 3.1, the three bands at 1093, 957 and 824 cm−1 are due to BØ4− tetrahedra, whereas the components at ca. 1382 and 1258 cm−1 are due to the stretching of B–O− and B–Ø bonds in BØ2O− units, respectively. The remaining band at 1482 cm−1 originates from the stretching of B–Ø bonds in neutral BØ3 triangles. This assignment is consistent with the infrared spectrum of bure B2O3 glass that exhibits a similar feature at ca. 1500 cm−1.45 Following these assignments, eqn (2) and (3) take the forms:
| X4m + X3 + X2 = 1 | (5) |
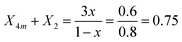 | (6) |
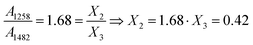 | (7) |
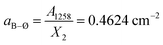 . The obtained values for X2 and X3 and eqn (5) give the molar fraction of meta-borate tetrahedral units X4m = 0.33.
. The obtained values for X2 and X3 and eqn (5) give the molar fraction of meta-borate tetrahedral units X4m = 0.33.
The fitting results in Table 2 indicate that for x = 0.30 the 800–1600 cm−1 range is best fitted with five component bands (Fig. 5, bands 2–6). The first three bands at 1079, 957 and 820 cm−1 are due to BØ4− tetrahedra, whereas the components at ca. 1393 and 1263 cm−1 are due to the stretching of B–O− and B–Ø bonds of BØ2O− and BØO2−2 units, respectively. For the x = 0.30 glass, eqn (2) and (3) give:
| X4m + X2 + X1 = 1 | (8) |
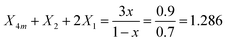 | (9) |
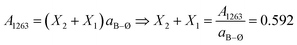 . Thus, the calculated molar fractions of the borate units are X1 = 0.286, X2 = 0.306, X4m = 0.408.
. Thus, the calculated molar fractions of the borate units are X1 = 0.286, X2 = 0.306, X4m = 0.408.
For the x = 0.40 composition, the 800–1600 cm−1 range is best fitted with five component bands. The first three at 1066, 952 and 842 cm−1 are typical for BØ4− tetrahedra, while the two remaining components at 1331 and 1214 cm−1 originate from the vibrations of both BØO22− and BO33− units. Eqn (2) and (3) take now the following forms:
| X4m + X1 + X0 = 1 | (10) |
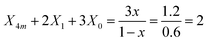 | (11) |
| X4m = X0 | (12) |
| X1 + 2X0 = 1 | (13) |
| B2O54− → B∅4− + BO33− | (14) |
To proceed further, we note that for compositions with x ≥ 0.65 the only borate triangles present are the BO33− units. Also, we observe that for the two component bands attributed to BO33− units, due to the splitting of their doubly degenerate ν3 asymmetric stretching mode, the results of Table 2 indicate that the ratio of the integrated intensities of the low (1155–1170 cm−1) vs. the high (1240–1270 cm−1) frequency component band attributed to BO33− units is constant and equal to 0.14 for x ≥ 0.65. This was a constraint imposed in the fitting procedure in order to minimize the residual between experimental and simulated data in the x = 0.65 up to x = 0.80 composition range. With this constraint in mind, we return to composition x = 0.40 for which eqn (4) gives for the two components at 1331 and 1214 cm−1 the relations:
| A1331 = (X1 + X0)aB–O− | (15a) |
| A1214 = X1aB–Ø + 0.14X0aB–O− | (15b) |
| X12aB–Ø + X1(aB–Ø − 0.14A1331 − A1214) + 0.14A1331 − A1214 = 0 | (16) |
As mentioned in Section 3.1 and shown in Fig. 4, the borate speciation for glasses with x = 0.50 and 0.60 involves BØO22− and BO33− trigonal units and borate tetrahedra in the form of either BØ2O23− or BØ4−. The evaluation of molar fractions is based now on the mass balance equation, eqn (3), and on eqn (15), by adopting the previously calculated values for aB–O− = 0.785 cm−2 and aB–Ø = 0.4624 cm−2 and the results of fitting for the normalized integrated intensities. In fact, a pair of equations similar to eqn (15) applied for the first two component bands, 1290–1315 cm−1 and 1185–1199 cm−1, can be solved to obtain first the molar fractions X1 and X0 of BØO22− and BO33− units. Then, the remaining molar fraction of borate tetrahedra results from the mass balance equation. With this procedure the calculated molar fractions for x = 0.50 are X1 = 0.145, X0 = 0.447 and X4 = 0.408, whereas for x = 0.60 the corresponding values are X1 = 0.089, X0 = 0.576 and X4 = 0.335. Details for the calculation of molar fraction of borate species for x = 0.50 and 0.60 are presented in the ESI.†
For the remaining glass compositions, x = 0.65–0.80, the molar fraction of BØO22− species vanishes and the molar fraction of BO33− units can be deduced from the normalized integrated intensity A2 of the highest-frequency component band with center frequency 1242–1270 cm−1 (see Table 2), as follows:
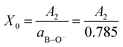 | (17) |
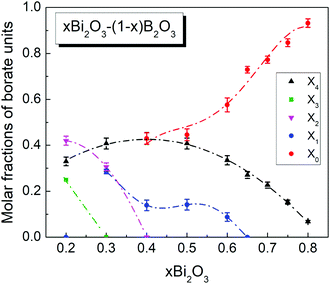 | ||
| Fig. 7 Molar fractions of the short-range order borate units in glasses xBi2O3–(1 − x)B2O3 as a function of composition, calculated from data of the deconvoluted absorption coefficient spectra (Fig. 5, 6 and Table 2). Lines are drawn to guide the eye. For details see text. | ||
The IR-derived molar fraction of borate tetrahedra X4 is compared in Fig. 8a with the reported N4 values from NMR spectroscopy.37 It is evident that in the common composition range, between x = 0.2 and x = 0.65, the IR and NMR data are in good agreement. The experimental X4 data are also compared in Fig. 8a with the theoretical N4th value obtained if all oxygen atoms introduced by Bi2O3 are forming BØ4− units, N4th = 3x/(1 − x). Clearly, the experimental rate for BØ4− formation is considerably lower than 3x/(1 − x). For x = 0.2 the experimental rate is 1.3x/(1 − x), indicating that only 43% of the introduced oxygen forms BØ4− units. For x = 0.3 the experimental N4 rate reduces to x/(1 − x), indicating that 33% of the available oxygen forms BØ4− units. Therefore, the rest of the added oxygen should participate in other structural arrangements, including the non-bridging oxygen containing BØ2O− and BØO22− units with molar fractions X2 and X1 (Fig. 7).
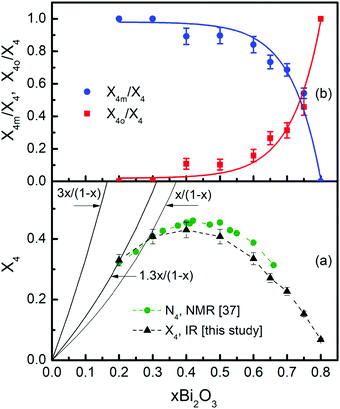 | ||
Fig. 8 (a) Comparison of the molar fractions of borate tetrahedral units in glasses xBi2O3–(1 − x)B2O3 obtained in the present IR study, X4 (▲), and in the NMR study of ref. 37, N4 ( ). Dashed lines are drawn as guides to the eye. The solid black lines present the theoretical value for the fraction of BØ4− units, N4th, formed at rates 3x/(1 − x), 1.3x/(1 − x) and x/(1 − x). (b) Fractional contributions of the meta-borate tetrahedral units BØ4−, X4m/X4, and of the ortho-borate tetrahedral units BØ2O23−, X4o/X4, to the total fraction X4 of borate tetrahedra in glasses xBi2O3–(1 − x)B2O3. Solid lines are the fitted exponential curves according to eqn (20). ). Dashed lines are drawn as guides to the eye. The solid black lines present the theoretical value for the fraction of BØ4− units, N4th, formed at rates 3x/(1 − x), 1.3x/(1 − x) and x/(1 − x). (b) Fractional contributions of the meta-borate tetrahedral units BØ4−, X4m/X4, and of the ortho-borate tetrahedral units BØ2O23−, X4o/X4, to the total fraction X4 of borate tetrahedra in glasses xBi2O3–(1 − x)B2O3. Solid lines are the fitted exponential curves according to eqn (20). | ||
The last demanding task to fully resolve the short-range borate structure is to separate the contributions of the BØ2O23− and BØ4− tetrahedral species to the X4 values, as resulted from the analysis of the IR spectra. Unfortunately, the characteristic bands of both tetrahedral borate species lie in the 800–1200 cm−1 range and overlap strongly; thus, the deconvolution procedure cannot be adopted in this case. Nevertheless, it is possible to evaluate the contributions of the BØ2O23− and BØ4− species by observing that at low Bi2O3 contents x = 0.2 and 0.3 only BØ4− species are present and at the highest content, x = 0.80, BØ2O23− units prevail as shown in Fig. 3 and 4 and discussed in Section 3.1. Based on the results of Table 2 and the calculated X4 values, the normalized absorption coefficients a4m and a4o for meta-borate BØ4− and ortho-borate BØ2O23− species can be evaluated: a4m = 0.865 cm−2 and a4o = 2.440 cm−2. Then, if we denote by X4m and X4o the contributions to the molar fraction X4 of the meta- and ortho-borate tetrahedra, the following equations result:
| X4m + X4o = X4 a4mX4m + a4oX4o = A4t | (18) |
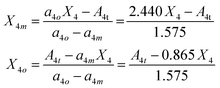 | (19) |
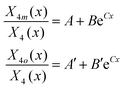 | (20) |
4.2. Bonding of bismuth in borate glasses
As observed in Fig. 5 and 6, the infrared profiles below 650 cm−1 can be described by four component bands. Table 2 shows that the frequencies of these components are in the ranges 162–232 cm−1 (band 11), 330–365 cm−1 (band 10), 475–510 cm−1 (band 9) and 597–615 cm−1 (band 8). The latter component (band 8) is related to borate species as it can be assigned to the in-plane bending mode of borate triangular units.45The composition dependence of frequency and relative integrated intensity of bands 9, 10 and 11 are shown in Fig. 9a and b, respectively; relative intensities were obtained by normalizing with the integrated intensity of the entire infrared spectrum. The integrated intensity of band 11 shows an increasing trend up to about x = 0.60 and then a tendency for saturation at higher Bi2O3 levels. As noted above, band 11 can be associated to bismuth–oxygen stretching, ν(Bi–O), in ionic sites formed by oxygen atoms provided by the borate network in analogy to previous findings for metal oxide-containing borate glasses.45,47,48,52,78–81
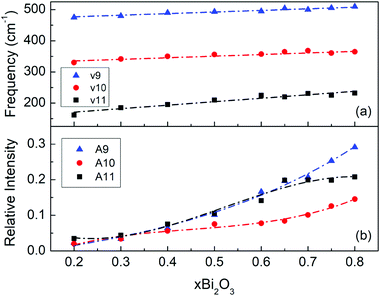 | ||
| Fig. 9 Composition dependence of frequency (a), and of relative integrated intensity (b) of component bands 9–11 obtained by deconvolution of the infrared spectra of glasses xBi2O3–(1 − x)B2O3 (see Fig. 5, 6 and Table 2). Lines are drawn to guide the eye. | ||
Bands 9 and 10 exhibit similar dependences on composition in terms of both frequency and relative intensity. This suggests that these bands can be associated to vibrations of the same bismuthate species, like the BiO6 octahedra discussed in Section 3.2. BiO6 octahedra would have two infrared active T1u modes under Oh point group; the asymmetric stretching (ω3) and the asymmetric bending (ω4) modes.82 Due to structural distortions, the degeneracy of the ω3 and ω4 modes will be removed in the glassy state and contribute to broadening of the corresponding bands. Along these lines, we attribute band 9 to the asymmetric stretch (ω3) and band 10 to the asymmetric bend (ω4) of distorted BiO6 octahedra in borate glasses. Using frequency data from Table 2 for the ω4 (ν10) and ω3 (ν9) modes of BiO6 octahedra we find an average value of 0.71 for the frequency ratio ω4/ω3, in good agreement with the average value ω4/ω3 = 0.69 for octahedral Bi3+ complexes.82
We note also that the relatively high-frequency value of the ω3 mode (average 495 cm−1) indicates considerable covalence in the Bi–O bonding. Nevertheless, the corresponding Bi–O bond strength is lower than the average B–O strength as suggested by the large difference in the so-called interaction parameter for the corresponding cation–anion pairs, Ath, as determined by Dimitrov and Komatsu;83i.e. Ath(Bi2O3) = 0.008 and Ath(B2O3) = 0.244. In addition to this approach, a comparison between the Bi–O and B–O bond strengths can be made by employing experimentally derived values for the weighted average frequency of the ω3 and ω4 modes for the BiO6 glass-forming sites, νBi–O(f) = 451 cm−1, and the weighted average νB–O frequency in 4-fold, νB–O(4) = 952 cm−1, and 3-fold, νB–O(3) = 1292 cm−1, coordinated boron–oxygen sites. For this, we used frequencies ν9 and ν10 from Table 2 to calculate the weighted average νBi–O(f) value, frequencies ν4, ν5 and ν6 for νB–O(4) and frequencies ν2 and ν3 for νB–O(3).
The harmonic oscillator model for the frequency of vibration of an M–O bond gives νM–O = (1/2πc)(kM–O/μM–O)1/2 where c is the speed of light and kM–O and μM–O are the force constant and reduced mass of vibration; this leading to the following expression for M = Bi and M = B:
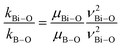 | (21) |
In conclusion, infrared spectroscopy gives values for the average Bi–O bond strength reflecting the dual role of Bi2O3 in borate glasses, i.e. as glass-forming and glass-modifying oxide with average Bi–O bond strengths reaching about 28% and 6% of the average B–O bond strength in triangular borate sites.
4.3. Property–structure correlations in bismuth-borate glasses
The strong effect of Bi2O3 on the nature and distribution of structural units constituting the glass, as shown in Fig. 7 and 8, can provide insights into the composition dependence of physical properties. Previous studies focused on the optical basicity and electronic polarizability of bismuth-borate glasses.28,30 We consider here the glass transition temperature, Tg, a property related to the rigidity of the glass network; the latter reflects the interatomic bonding energy and the atomic packing density of the glass.84,85The composition dependence of Tg is shown in Fig. 10a, based on data reported by Bajaj et al.37 for Bi2O3 contents up to 66 mol% and by George et al.24 for higher Bi2O3 contents (Table 3). Clearly, Tg exhibits a decreasing trend upon increasing Bi2O3 content and the rate of decreasing Tg appears to be composition dependent. In particular, three composition regimes can be distinguished where Tg exhibits approximate linear dependencies on the Bi2O3 content: (I) 0.2 ≤ x ≤ 0.4, where Tg decreases at a rather mild rate; (II) 0.4 < x ≤ 0.6, with a fast decrease of Tg; and (III) 0.6 < x ≤ 0.83, where Tg decreases at a reduced rate in comparison to regime II.
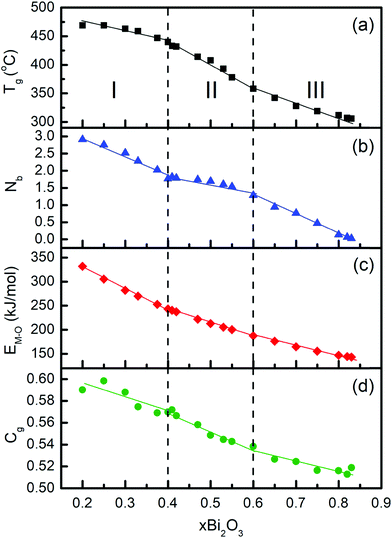 | ||
| Fig. 10 Composition dependence of properties in glasses xBi2O3–(1 − x)B2O3; (a) glass transition temperature Tg,24,37 (b) average number of bridging boron–oxygen bonds per boron center Nb, calculated from eqn (22), (c) average single bond strength EM–O, calculated from eqn (24), and (d) atomic packing density, Cg, calculated from eqn (26). Vertical dashed lines mark the Bi2O3 contents x = 0.4 and x = 0.6 which define three regimes (I, II and III) with different rates in the composition dependence of Tg. Straight lines are linear fits to data in the three composition regimes. For details see text. | ||
| xBi2O3 | d (g cm−3) | M W (g mol−1) | T g (°C) | N b | E M–O (kJ mol−1) | C g |
|---|---|---|---|---|---|---|
| a Density and glass transition temperature data are from Bajaj et al.37 for Bi2O3 contents up to 66 mol% and from George et al.24 for higher Bi2O3 contents. | ||||||
| 0.20 | 4.46 | 148.89 | 469 | 2.91 | 331.7 | 0.590 |
| 0.25 | 5.05 | 168.71 | 469 | 2.75 | 305.4 | 0.598 |
| 0.30 | 5.47 | 188.52 | 463 | 2.52 | 282.0 | 0.588 |
| 0.33 | 5.64 | 200.41 | 459 | 2.28 | 269.9 | 0.575 |
| 0.375 | 6.01 | 218.25 | 447 | 2.03 | 252.7 | 0.569 |
| 0.40 | 6.25 | 228.16 | 440 | 1.77 | 243.4 | 0.570 |
| 0.41 | 6.36 | 232.12 | 433 | 1.82 | 240.6 | 0.572 |
| 0.42 | 6.39 | 236.08 | 432 | 1.79 | 237.3 | 0.566 |
| 0.47 | 6.74 | 255.90 | 414 | 1.75 | 221.8 | 0.558 |
| 0.50 | 6.87 | 267.79 | 408 | 1.70 | 212.8 | 0.549 |
| 0.53 | 7.07 | 279.68 | 393 | 1.60 | 205.2 | 0.545 |
| 0.55 | 7.21 | 287.61 | 378 | 1.53 | 200.0 | 0.543 |
| 0.60 | 7.55 | 307.42 | 358 | 1.28 | 187.6 | 0.538 |
| 0.65 | 7.76 | 327.24 | 342 | 0.94 | 176.3 | 0.527 |
| 0.70 | 8.10 | 347.06 | 328 | 0.76 | 164.6 | 0.524 |
| 0.75 | 8.33 | 366.88 | 319 | 0.47 | 155.3 | 0.516 |
| 0.80 | 8.67 | 386.69 | 312 | 0.14 | 146.9 | 0.516 |
| 0.82 | 8.75 | 394.62 | 307 | 0.06 | 144.4 | 0.513 |
| 0.83 | 8.92 | 398.58 | 306 | 0.03 | 143.1 | 0.519 |
The variation of Tg with Bi2O3 content can be viewed in terms of the molar fractions of the short-range units constituting the borate structure (Fig. 7 and 8). The composition range I is characterized by decreasing fractions X3, X2 and X1, while X4 increases towards its maximum value at x = 0.4. The net effect of these structural rearrangements leads apparently to a decreasing connectivity of the borate network as manifested by a slightly decreasing Tg. In region II, the combined effect of decreasing X4 and the formation of the completely depolymerized ortho-borate triangular BO33− species (X0) cause a pronounced decrease of Tg. It is of interest to note that in region III the decrease of Tg slows down, despite the continuing decrease of X4 and the rise of X0 as observed in Fig. 7. This apparent discrepancy can be resolved by recalling the increasing formation of ortho-borate tetrahedral BØ2O23− species in region III (X4o in Fig. 8). This process restores some of the borate connectivity, because BØ2O23− provides two bridging boron–oxygen bonds (B–Ø) per boron in comparison to zero B–Ø bonds offered by BO33− species.
For alkali-borate glasses M2O–2B2O3 (M = Li–Cs), Tg was found to vary proportionally to the average number of bridging B–Ø bonds per boron center, Nb.86 Using the molar fraction data of this study, Nb can be calculated for bismuth-borate glasses as follows:
| Nb = 4X4m + 2X4o + 3X3 + 2X2 + X1 | (22) |
As observed in Fig. 10b, the slopes of the Nb variations show differences compared to those of Tg; for example, the decrease of Nb in regime II is not as steep as that of Tg. This indicates that the proportionality constant between Tg and Nb is composition dependent. Also, although Nb plays a crucial role on Tg, there are additional factors which influence Tg as well. They are the strength of the metal cation–oxygen interactions,87 and the effectiveness in packing of the glass constituents as quantified by the atomic packing density Cg.84,85
Previous studies on alkali-borate,87 alkaline-earth borate,48 and transition metal-borate52 glasses, having fixed metal oxide contents, demonstrated linear-dependences of Tg on the strength of the M–O interactions, which express the ability of the Mn+ cations to cross-link network segments through M–O bonding. Instead of looking separately at the effects of metal cation–oxygen (i.e., Bi–O) and B–O bonding on Tg, we propose to evaluate their combined effect on the interatomic bonding energy of the glass determining the rigidity of the glass network, in terms of the average single bond energy EM–O calculated as follows:
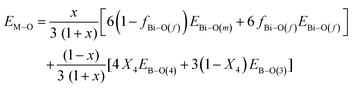 | (23) |
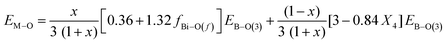 | (24) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 and apply eqn (24) to calculate the average single bond energy, EM–O, in glasses xBi2O3–(1 − x)B2O3. The results are given in Table 3 and plotted in Fig. 10c as a function of composition. It is found that consideration of both borate and bismuthate constituents leads to a rather smooth decrease in the average single bond energy with Bi2O3 content. Nevertheless, progressive changes in the slop of EM–Oversus Bi2O3 content can still be seen in the three composition regimes. In any case, the variation of EM–O with composition is in line with the general trends of both Tg and Nb.
28 and apply eqn (24) to calculate the average single bond energy, EM–O, in glasses xBi2O3–(1 − x)B2O3. The results are given in Table 3 and plotted in Fig. 10c as a function of composition. It is found that consideration of both borate and bismuthate constituents leads to a rather smooth decrease in the average single bond energy with Bi2O3 content. Nevertheless, progressive changes in the slop of EM–Oversus Bi2O3 content can still be seen in the three composition regimes. In any case, the variation of EM–O with composition is in line with the general trends of both Tg and Nb.
Finally, we evaluate the composition dependence of the atomic packing density Cg in bismuth-borate glasses. Cg is defined as the ratio between the theoretical volume occupied by the constituent ions and the real volume of glass. For multicomponent glasses Cg is given by the general equation:
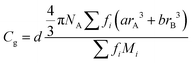 | (25) |
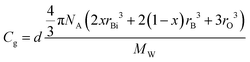 | (26) |
The calculated Cg values are given in Table 3, while the evolution of the atomic packing density with composition is demonstrated in Fig. 10d. Despite the fact that the Cg values seem to have relatively larger scattering than those for Nb and EM–O, all three properties are found to exhibit a general decreasing trend with Bi2O3 content. It is noted though that Cg shows a maximum at x = 0.25, which should reflect the minimum in Vm at the same composition as reported by George et al.24 In any case, it is found that Nb, EM–O and Cg all contribute to shaping the overall composition dependence of Tg (Fig. 10a). Considering that the glass structure affects directly Nb, EM–O and Cg as expressed by eqn (22), (24) and (26), the present results demonstrate the close correlation between Tg and glass structure in the broad glass-forming region of the bismuth-borate system.
5. Conclusions
Glasses in the system xBi2O3–(1 − x)B2O3 were synthesized by melting in Pt crucibles and subsequent splat-quenching between two stainless-steel blocks; this technique was found to give homogeneous glasses in the continuous range 0.2 ≤ x ≤ 0.8. The evolution of glass structure with composition (x) was studied by infrared (IR) reflectance spectroscopy in a broad and continuous spectral range, comprising well-separated far- (30–650 cm−1) and mid-IR (650–5000 cm−1) responses for bismuth-borate glasses. Absorption coefficient spectra were calculated from the measured reflectance spectra, and were deconvoluted into component bands attributed to vibration modes of specific bismuthate and borate species.The Bi–O vibrational activity was probed at low frequencies in the far-IR region. It involves a band at 160–230 cm−1 due to Bi–O stretch in ionic sites reflecting the glass-modifying role of Bi2O3, and bands at 330–365 cm−1 and 475–510 cm−1 assigned to the asymmetric bend and asymmetric stretch in distorted BiO6 sites. The relatively high frequency of the latter bands demonstrates considerable covalence in the Bi–O bonding in BiO6 sites and manifests the parallel glass-forming role of Bi2O3. Ionic Bi–O and relatively covalent BiO6 sites were found to coexist in the composition region 0.20 ≤ x ≤ 0.80. The latter sites prevail for Bi2O3 contents above 60 mol%, and account for the extended glass-forming range of the bismuth-borate system. Consideration of the corresponding IR frequencies allowed estimating the average Bi–O bond strengths in glass-modifying and glass-forming bismuthate sites; they reach ca. 6% and 28% of the average B–O bond strength in triangular borate sites.
Bands arising from B–O stretching modes were found in the 810–1090 cm−1 and 1155–1490 cm−1 range for borate units having tetrahedral and triangular B–O coordination, while their bending modes are active in the 595–710 cm−1 range. Comparison with previous IR studies on modified borate glasses and bismuth-borate crystalline compounds allowed the borate speciation as a function of composition. Thus, two kinds of borate tetrahedral species were identified having the meta-borate, BØ4−, and ortho-borate, BØ2O23−, stoichiometry where the oxygen atoms are all bridging (Ø) or two are bridging and two non-bridging (O−), respectively. In addition, all possible types of triangular borate units were identified in bismuth-borate glasses for the first time, i.e. units BØ3, BØ2O−, BØO22− and BO33− with increasing number of non-bridging oxygen atoms per boron. The IR results on the relative integrated intensity of bands characterizing the different borate species were combined with mass and charge balance equations to quantify for the first time all six type of borate species present in the bismuth-borate glass system. The complete mapping of the short-range borate structure as a function of composition was achieved in terms of the molar fractions X4m, X4o, X3, X2, X1 and X0 of units BØ4−, BØ2O23−, BØ3, BØ2O−, BØO22− and BO33−, respectively. The present IR results for the total molar fraction X4 of the borate tetrahedral species, X4 = X4m + X4o, were found in excellent agreement with reported N4 data from NMR spectroscopy.37 In addition to X4 or N4, the present work gives the X4m and X4o fractions as well as the fractions of all types of triangular borate species X3−n where n = 0, 1, 2 and 3.
The composition dependence of the glass transition temperature, Tg, of bismuth-borate glasses24,37 was discussed in terms of three glass properties calculated using the structural results of this work: the average number of bridging boron–oxygen bonds (B–Ø) per boron center, Nb, the average Bi–O and B–O single bond energy, EM–O, and the atomic packing density, Cg. It was found that Nb, EM–O and Cg all exhibit approximate linear dependencies on the Bi2O3 content in the three composition regimes 0.2 ≤ x ≤ 0.4, 0.4 < x ≤ 0.6 and 0.6 < x ≤ 0.83, and contribute to the overall composition dependence of Tg. In conclusion, Tg-structure correlations are determined by the combined effect of the borate cross-linking ability, the strength of the interatomic bonding energy and the effectiveness of atomic packing in the studied bismuth-borate glasses.
Author contributions
C. P. E. Varsamis: conceptualization, software, formal analysis, writing – original draft. N. Makris: investigation, formal analysis. C. Valvi: investigation, formal analysis. E. I. Kamitsos: conceptualization, supervision, funding acquisition, writing – review & editing, project administration.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
EIK acknowledges support by the project “National Infrastructure in Nanotechnology, Advanced Materials and Micro-/Nanoelectronics” (MIS 5002772) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund).References
- M. B. Saisudha, K. S. R. K. Rao, H. L. Bhat and J. Ramakrishna, J. Appl. Phys., 1996, 80, 4845–4853 CrossRef CAS.
- G. S. Murugan and K. B. R. Varma, J. Non-Cryst. Solids, 2001, 279, 1–13 CrossRef.
- P. Becker, Cryst. Res. Technol., 2003, 38, 74–82 CrossRef CAS.
- Y. Chen, Y. Huang, M. Huang, R. Chen and Z. Luo, Opt. Mater., 2004, 25, 271–278 CrossRef CAS.
- I. I. Oprea, H. Hesse and K. Betzler, Opt. Mater., 2004, 26, 235–237 CrossRef CAS.
- R. Ihara, T. Honma, Y. Benino, T. Fujiwara and T. Komatsu, Opt. Mater., 2004, 27, 403–408 CrossRef CAS.
- R. Ihara, Y. Benino, T. Fujiwara and T. Komatsu, Sci. Technol. Adv. Mater., 2005, 6, 138–142 CrossRef CAS.
- O. Deparis, F. P. Mezzapesa, C. Corbari, P. G. Kazansky and K. Sakaguchim, J. Non-Cryst. Solids, 2005, 351, 2166–2177 CrossRef CAS.
- A. S. L. Gomez, E. L. Falcão Filho, C. B. de Araújo, D. Rativa, R. E. de Araujo, K. Sakaguchi, F. P. Mezzapesa, I. C. S. Carvalho and P. G. Kazansky, J. Appl. Phys., 2007, 101, 033115 CrossRef.
- S. Insitipong, J. Kaewkhao, T. Ratana and P. Limsuwan, Procedia Eng., 2011, 8, 195–199 CrossRef CAS.
- K. Swapna, Sk Mahamuda, A. Srinivasa Rao, M. Jayasimhadri, T. Sasikala and L. Rama Moorthy, Ceram. Interfaces, 2013, 39, 8459–8465 CrossRef CAS.
- K. Swapna, S. Mahamuda, A. Srinivasa Rao, T. Sasikala, P. Packiyaraj, L. Rama Moorthy and G. V. Prakash, J. Luminescence, 2014, 156, 80–86 CrossRef CAS.
- K. Swapna, S. Mahamuda, A. Srinivasa Rao, M. Jayasimhadri, S. Shakya and G. Vijaya Prakash, J. Luminescence, 2014, 156, 180–187 CrossRef CAS.
- S. Singla, V. G. Achanta, N. Mahendru, S. S. Prabhu, M. Falconieri and G. Sharma, Opt. Mater., 2017, 72, 91–97 CrossRef CAS.
- B. Munisudhakar, C. Nageswara Raju, M. Reddi Babu, N. Manohar Reddy and L. Rama Moorthy, Mater. Today: Proc., 2020, 26, 5–10 CAS.
- M. G. Dong, M. I. Sayyed, G. Lakshminarayana, M. Çelikbilek Ersundu, A. E. Ersundu, P. Nayar and M. A. Mahdi, J. Non-Cryst. Solids, 2017, 468, 12–16 CrossRef CAS.
- M. I. Sayyed, G. Lakshminarayana, M. G. Dong, M. Çelikbilek Ersundu, A. E. Ersundu and I. V. Kityk, Radiat. Phys. Chem., 2018, 145, 26–33 CrossRef CAS.
- P. Kaur, K. J. Singh, S. Thakur, P. Singh and B. S. Bajwa, Spectrochim. Acta, Part A, 2019, 206, 367–377 CrossRef CAS PubMed.
- D. W. Hall, M. A. Newhouse, N. F. Borrelli, W. H. Dumbaugh and D. L. Weidman, Appl. Phys. Lett., 1989, 54, 1293–1295 CrossRef CAS.
- E. M. Levin and C. L. McDaniel, J. Am. Ceram. Soc., 1962, 45, 355–360 CrossRef CAS.
- H. Hellwig, J. Liebertz and L. Bohaty, Solid State Commun., 1999, 109, 249–251 CrossRef CAS.
- H. Hellwig, J. Liebertz and L. Bohaty, J. Appl. Phys., 2000, 88, 240–244 CrossRef CAS.
- C. Stehle, C. Vira, D. Hogan, S. Feller and M. Affatigato, Phys. Chem. Glasses, 1998, 39, 83–86 CAS.
- H. B. George, C. Vira, C. Stahle, J. Meyer, S. Evers, D. Hogan, S. Feller and M. Affatigato, Phys. Chem. Glasses, 1999, 40, 326–332 CAS.
- K. Terashima, T. H. Shimoto and T. Yoko, Phys. Chem. Glasses, 1997, 38, 211–217 CAS.
- P. Becker, Adv. Mater., 1998, 10, 979–992 CrossRef CAS.
- Y. Watanabe, S. Sakata, T. Watanabe and T. Tsuchiya, J. Non-Cryst. Solids, 1998, 240, 212–220 CrossRef CAS.
- T. Komatsu, N. Ito, T. Honma and V. Dimitrov, J. Non-Cryst. Solids, 2010, 356, 2310–2314 CrossRef CAS.
- B. V. R. Chowdari and Z. Rong, Solid State Ionics, 1996, 90, 151–160 CrossRef CAS.
- T. Honma, Y. Benino, T. Komatsu, R. Sato and V. Dimitrov, Phys. Chem. Glasses, 2002, 43, 32–40 CAS.
- C. E. Stone, A. C. Wright, R. N. Sinclair, S. A. Feller, M. Affatigato, D. L. Hogan, N. D. Nelson, C. Vira, Y. B. Dimitriev, E. M. Gattef and D. Ehrt, Phys. Chem. Glasses, 2000, 41, 409–412 CAS.
- L. Baia, R. Stefan, W. Kiefer, J. Popp and S. Simon, J. Non-Cryst. Solids, 2002, 303, 379–386 CrossRef CAS.
- L. Baia, R. Stefan, J. Popp, S. Simon and W. Kiefer, J. Non-Cryst. Solids, 2003, 324, 109–117 CrossRef CAS.
- B. Karthikeyan and S. Mohan, Phys. B, 2003, 334, 298–302 CrossRef CAS.
- A. V. Egorysheva, V. I. Burkov, Yu. F. Kargin, V. G. Plotnichenko and V. V. Koltashev, Crystallogr. Rep., 2005, 50, 127–136 CrossRef CAS.
- L. Baia, R. Stefan, W. Kiefer and S. Simon, J. Raman Spectrosc., 2005, 36, 262–266 CrossRef CAS.
- A. Bajaj, A. Khanna, B. Chen, J. G. Longstaffe, U. W. Zwanziger, J. W. Zwanziger, Y. Gomez and F. Gonzalez, J. Non-Cryst. Solids, 2009, 355, 45–53 CrossRef CAS.
- N. M. Bobkova, Glass Ceram., 2015, 72, 360–365 CrossRef.
- G. E. Jellison, S. A. Feller and P. J. Bray, Phys. Chem. Glasses, 1978, 19, 52 CAS.
- S. A. Feller, W. J. Dell and P. J. Bray, J. Non-Cryst. Solids, 1982, 51, 21–30 CrossRef CAS.
- R. E. Youngman and J. W. Zwanziger, J. Non-Cryst. Solids, 1994, 168, 293–297 CrossRef CAS.
- R. E. Youngman and J. W. Zwanziger, J. Phys. Chem., 1996, 100, 16720–16728 CrossRef CAS.
- S. A. Feller, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2018, 59, 153–167 Search PubMed.
- E. I. Kamitsos, M. A. Karakassides and G. D. Chryssikos, Phys. Chem. Glasses, 1989, 30, 229–234 CAS.
- E. I. Kamitsos, A. P. Patsis, M. A. Karakassides and G. D. Chryssikos, J. Non-Cryst. Solids, 1990, 126, 52–67 CrossRef CAS.
- E. I. Kamitsos and G. D. Chryssikos, J. Mol. Struct., 1991, 247, 1–16 CrossRef CAS.
- C. P. Varsamis, E. I. Kamitsos and G. D. Chryssikos, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 3885–3898 CrossRef CAS.
- Y. D. Yiannopoulos, G. D. Chryssikos and E. I. Kamitsos, Phys. Chem. Glasses, 2001, 42, 164–172 CAS.
- C. P. E. Varsamis, A. Vegiri and E. I. Kamitsos, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 104203 CrossRef.
- E. I. Kamitsos, Phys. Chem. Glasses, 2003, 44, 79–87 CAS.
- A. Winterstein-Beckmann, D. Möncke, D. Palles, E. I. Kamitsos and L. Wondraczek, J. Phys. Chem. B, 2015, 119, 3259–3272 CrossRef CAS PubMed.
- D. Möncke, E. I. Kamitsos, D. Palles, R. Limbach, A. Winterstein-Beckmann, T. Honma, Z. Yao, T. Rouxel and L. Wondraczek, J. Chem. Phys., 2016, 145, 124501 CrossRef PubMed.
- A. C. Wright, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2010, 51, 1–39 CAS.
- G. Lelong, L. Cormier, L. Hennet, F. Michel, J.-P. Rueff, J. M. Ablett and G. Monaco, J. Non-Cryst. Solids, 2017, 472, 1–8 CrossRef CAS.
- M. S. Bødker, J. C. Mauro, R. E. Youngman and M. M. Smedskjaer, J. Phys. Chem. B, 2019, 123, 1206–1213 CrossRef PubMed.
- G. Ferlat, T. Charpentier, A. P. Seitsonen, A. Takada, M. Lazzeri, L. Cormier, G. Calas and F. Mauri, Phys. Rev. Lett., 2008, 101, 065504 CrossRef PubMed.
- E. I. Kamitsos, Y. D. Yiannopoulos, C. P. Varsamis and H. Jain, J. Non-Cryst. Solids, 1997, 222, 59–68 CrossRef CAS.
- O. Sanz, E. Haro-Poniatowski, J. Gonzalo and J. M. Fernandez Navarro, J. Non-Cryst. Solids, 2006, 352, 761–768 CrossRef CAS.
- C. P. Varsamis, E. I. Kamitsos, N. Machida and T. Minami, J. Phys. Chem. B, 1997, 101, 3734–3741 CrossRef CAS.
- J. J. More, Lect. Notes Math., 1978, 630, 105–116 Search PubMed.
- A. Winterstein-Beckmann, D. Möncke, D. Palles, E. I. Kamitsos and L. Wondraczek, J. Non-Cryst. Solids, 2013, 376, 165–174 CrossRef CAS.
- G. Chadeyron, M. El-Ghozzi, R. Mahiou, A. Arbus and J. C. Cousseins, J. Solid State Chem., 1997, 128, 261–266 CrossRef CAS.
- M. Ren, J. H. Lin, Y. Dong, L. Q. Yang and M. Z. Su, Chem. Mater., 1999, 11, 1576–1580 CrossRef CAS.
- R. Velchuri, B. V. Kumar, V. R. Devi, G. Prasad, D. J. Prakash and M. Vithal, Mater. Res. Bull., 2011, 46, 1219–1226 CrossRef CAS.
- C. P. E. Varsamis, E. I. Kamitsos, M. Tatsumisago and T. Minami, J. Non-Cryst. Solids, 2004, 345&346, 93–98 Search PubMed.
- A. V. Egorysheva, A. S. Kanishcheva, Yu. F. Kargin, Yu. E. Gorbunova and Yu. N. Mikhailov, Z. Neorg. Chim., 2002, 47, 1961–1965 CAS.
- G. M. Kuzmicheva and T. I. Mel’nikova, Z. Neorg. Chim., 2009, 54, 74–81 CAS.
- S. Filatov, Y. Shepelev, R. Bubnova, N. Sennova, A. V. Egorysheva and Y. F. Kargin, J. Solid State Chem., 2004, 177, 515–522 CrossRef CAS.
- P. Becker and R. Frohlich, Z. Naturforsch., 2004, 59b, 256–258 CrossRef.
- A. Hyman and A. Perloff, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 2007–2011 CrossRef CAS.
- R. J. Betsch and W. B. White, Spectrochim. Acta, Part A, 1978, 34, 505–514 CrossRef.
- R. Iordanova, V. Dimitrov, Y. Dimitriev and D. Klissurski, J. Non-Cryst. Solids, 1994, 180, 58–65 CrossRef CAS.
- R. Iordanova, Y. Dimitriev, V. Dimitrov, S. Kassabov and D. Klissurski, J. Non-Cryst. Solids, 1996, 204, 141–150 CrossRef CAS.
- S. Hazra, S. Mandal and A. Ghosh, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 8021–8025 CrossRef CAS.
- N. Kitamura and K. Fukumi, J. Ceram. Soc. Jpn., 2013, 121, 355–360 CrossRef CAS.
- F. Miyaji, T. Yoko, J. Jin, S. Sakka, T. Fukunaga and M. Misawa, J. Non-Cryst. Solids, 1994, 175, 211–223 CrossRef CAS.
- Y. Dimitriev, Ch Petkov, E. Gattev, T. Stoilova and G. Gochev, J. Non-Cryst. Solids, 1989, 112, 120–125 CrossRef.
- J. A. Duffy, E. I. Kamitsos, G. D. Chryssikos and A. P. Patsis, Phys. Chem. Glasses, 1993, 34, 153–157 CAS.
- E. I. Kamitsos, G. D. Chryssikos, A. P. Patsis and J. A. Duffy, J. Non-Cryst. Solids, 1996, 196, 249–254 CrossRef CAS.
- Z. Y. Yao, D. Möncke, E. I. Kamitsos, P. Houizot, F. Célarié, T. Rouxel and L. Wondraczek, J. Non-Cryst. Solids, 2016, 435, 55–68 CrossRef CAS.
- E. I. Kamitsos, C. P. E. Varsamis and A. Vegiri, Phys. Chem. Glasses: Eur. J. Glass Sci. Technol., Part B, 2020, 61, 107–120 Search PubMed.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A, Theory and Applications in Inorganic Chemistry, Wiley, New York, 6th edn, 2009 Search PubMed.
- V. Dimitrov and T. Komatsu, J. Ceram. Soc. Jpn., 1999, 107, 1012–1018 CrossRef CAS.
- T. Rouxel, J. Am. Ceram. Soc., 2007, 90, 3019–3039 CrossRef CAS.
- A. Makishima and J. D. Mackenzie, J. Non-Cryst. Solids, 1973, 12, 35–45 CrossRef CAS.
- E. I. Kamitsos, A. P. Patsis and G. D. Chryssikos, J. Non-Cryst. Solids, 1993, 152, 246–257 CrossRef CAS.
- E. I. Kamitsos, G. D. Chryssikos and M. A. Karakassides, Phys. Chem. Glasses, 1988, 29, 121–126 CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Found. Crystallogr., 1976, 32, 751–767 CrossRef.
- N. S. Tagiara, E. Moayedi, A. Kyritsis, L. Wondraczek and E. I. Kamitsos, J. Phys. Chem. B, 2019, 123, 7905–7918 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Application of infrared spectroscopy for the calculation of the molar fractions of the borate species present in glass compositions x = 0.50 and 0.60 of the system xBi2O3–(1 − x)B2O3. See DOI: 10.1039/d1cp00301a |
| This journal is © the Owner Societies 2021 |