Electron deficiency but semiconductive diamond-like B2CN originated from three-center bonds
Abstract
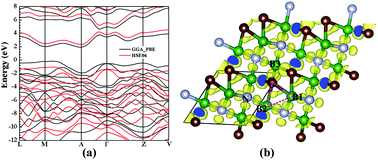
B2CN was one of the synthesized light element compounds, which was expected to be superhard material with a metallic character due to its electron deficienct nature. However, in this work, we discovered two novel semiconducting superhard B2CN phases using particle swarm intelligence technique and first-principles calculations, which were reported to have three-dimensional and four coordinated covalent diamond-like structures. These two new phases were calculated to be dynamically stable at zero and high pressures, and can be deduced from the previously reported Pmma phase by pressure-induced structural phase transitions. More importantly, unlike the previously proposed metallic B2CN structures, these two new phases combine superhard (the calculated Vickers hardness reached ∼55 GPa) and semiconducting character. The semiconducting behavior of the newly predicted B2CN phases breaks the traditional view of the metallic character of the electron deficient diamond-like B–C–N ternary compounds. By a detail analyzation of the electron localization functions of these two new phases, three-center bonds were reported between some B, C and B atoms, which were suggested to be the primary mechanism that helps the compound overcome its electron-deficient nature and finally exhibit a semiconducting behavior.


 Please wait while we load your content...
Please wait while we load your content...