Characterizing an allosteric inhibitor-induced inactive state in with-no-lysine kinase 1 using Gaussian accelerated molecular dynamics simulations†
Abstract
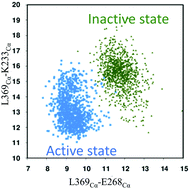
The With-No-Lysine (WNK) kinase plays a significant role in controlling blood pressure and body fluid homeostasis. Consequently, WNK1 is considered a potential target for treating hypertension. However, the highly conserved ATP-binding pocket in human isoforms WNK1/2/3/4 poses an immense challenge in designing competitive inhibitors. In contrast, allosteric inhibitors that bind to a non-conserved site provide a promising approach. To better understand how the allosteric inhibitors induce an inactive state in WNK1, we have performed 1 μs long Gaussian accelerated molecular dynamics simulations (GaMD) of the apo and complex systems along with free energy calculations and structural analyses. Our results indicate that major structural variations come from the activation loop and αC-helix. Our studies suggest that the inactive state is characterized by an open catalytic cleft between the N- and C-lobe, outward movement of the αC-helix, open P-loop, distorted αC-helix, and an extended activation loop that rearranges with a vanished short helix in its N-terminal. The outward movement of the αC-helix breaks the salt-bridge between Glu268 and R348 and renders the kinase domain inactive. Overall, our study provides detailed insights into the inhibitor-induced allosteric mechanisms and may help design specific allosteric inhibitors against WNK1 for treating hypertension.


 Please wait while we load your content...
Please wait while we load your content...