A kinetics study on hydrogen abstraction reactions of cyclopentane by hydrogen, methyl, and ethyl radicals†
Abstract
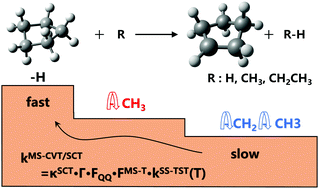
Hydrogen abstraction reactions of (cyclo)alkanes by radicals play a fundamental role in both combustion and atmospheric chemistry. In this work, we select three common radicals in the pyrolysis of hydrocarbon fuels: hydrogen radical (Ḣ), methyl radical (ĊH3), and ethyl radical (ĊH2CH3) to investigate the kinetics of their hydrogen abstraction reactions with cyclopentane. The rate constants over a broad temperature range of 150–3000 K are calculated by using the multi-structural variational transition state theory in the small-curvature tunneling approximation (MS-CVT/SCT), by which the multi-structural torsional (MS-T) anharmonicity of partition functions, variational effects, and corner-cutting tunneling are all included in dynamics calculations. We stress the particular importance of considering the MS-T anharmonicity in the rate constant calculation for the reaction with the ethyl radical compared to those with hydrogen and methyl radicals. The MS-T anharmonicity significantly accelerates the reaction with the ethyl radical in the whole temperature range, and in particular, it increases the rate constant by a factor of >−9 at 1000 K. We also found that the tunneling effect drastically increases the rate constants at low-temperatures by up to 3–5 orders of magnitudes. The calculated reaction rate constants have an order of 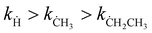 .
.


 Please wait while we load your content...
Please wait while we load your content...